
Home » Education » Difference Between Introduction and Literature Review

Difference Between Introduction and Literature Review
Main difference – introduction vs literature review.
Although introduction and literature review are found towards the beginning of a text, there is a difference between them in terms of their function and purpose. The main difference between introduction and literature review is their purpose; the purpose of an introduction is to briefly introduce the text to the readers whereas the purpose of a literature review is to review and critically evaluate the existing research on a selected research area.
In this article, we will be discussing,
1. What is an Introduction? – Definition, Features, Characteristics
2. What is a Literature Review? – Definition, Features, Characteristics

What is an Introduction
An introduction is the first part of an article, paper, book or a study that briefly introduces what will be found in the following sections. An introduction basically introduces the text to the readers. It may contain various types of information, but given below some common elements that can be found in the introduction section.
- Background/context to the paper
- Outline of key issues
- Thesis statement
- Aims and purpose of the paper
- Definition of terms and concepts
Note that some introductions may not have all these elements. For example, an introduction to a short essay will only have several lines. Introductions can be found in nonfiction books, essays, research articles, thesis, etc. There can be slight variations in these various genres, but all these introductions will provide a basic outline of the whole text.
Introduction of a thesis or dissertation will describe the background of the research, your rationale for the thesis topic, what exactly are you trying to answer, and the importance of your research.

What is a Literature Review
A literature review, which is written at the start of a research study, is essential to a research project. A literature review is an evaluation of the existing research material on a selected research area. This involves reading the major published work (both printed and online work) in a chosen research area and reviewing and critically evaluating them. A literature review should show the researcher’s awareness and insight of contrasting arguments, theories, and approaches. According to Caulley (1992) a good literature review should do the following:
- Compare and contrast different researchers’ views
- Identify areas in which researchers are in disagreement
- Group researchers who have similar conclusions
- Criticize the research methodology
- Highlight exemplary studies
- Highlight gaps in research
- Indicate the connection between your study and previous studies
- Indicate how your study will contribute to the literature in general
- Conclude by summarizing what the literature says
Literature reviews help researchers to evaluate the existing literature, to identify a gap in the research area, to place their study in the existing research and identify future research.

Introduction is at the beginning of a text.
Literature Review is located after the introduction or background.
Introduction introduces the main text to the readers.
Literature Review critically evaluates the existing research on the selected research area and identifies the research gap.
Introduction will have information such as background/context to the paper, outline of key issues, thesis statement, aims, and purpose of the paper and definition of terms and concepts.
Literature Review will have summaries, reviews, critical evaluations, and comparisons of selected research studies.
Image Courtesy: Pixabay
About the Author: Hasa
Hasanthi is a seasoned content writer and editor with over 8 years of experience. Armed with a BA degree in English and a knack for digital marketing, she explores her passions for literature, history, culture, and food through her engaging and informative writing.
You May Also Like These
Leave a reply cancel reply.
How to write a literature review introduction (+ examples)
The introduction to a literature review serves as your reader’s guide through your academic work and thought process. Explore the significance of literature review introductions in review papers, academic papers, essays, theses, and dissertations. We delve into the purpose and necessity of these introductions, explore the essential components of literature review introductions, and provide step-by-step guidance on how to craft your own, along with examples.
Why you need an introduction for a literature review
When you need an introduction for a literature review, what to include in a literature review introduction, examples of literature review introductions, steps to write your own literature review introduction.
A literature review is a comprehensive examination of the international academic literature concerning a particular topic. It involves summarizing published works, theories, and concepts while also highlighting gaps and offering critical reflections.
In academic writing , the introduction for a literature review is an indispensable component. Effective academic writing requires proper paragraph structuring to guide your reader through your argumentation. This includes providing an introduction to your literature review.
It is imperative to remember that you should never start sharing your findings abruptly. Even if there isn’t a dedicated introduction section .
Instead, you should always offer some form of introduction to orient the reader and clarify what they can expect.
There are three main scenarios in which you need an introduction for a literature review:
- Academic literature review papers: When your literature review constitutes the entirety of an academic review paper, a more substantial introduction is necessary. This introduction should resemble the standard introduction found in regular academic papers.
- Literature review section in an academic paper or essay: While this section tends to be brief, it’s important to precede the detailed literature review with a few introductory sentences. This helps orient the reader before delving into the literature itself.
- Literature review chapter or section in your thesis/dissertation: Every thesis and dissertation includes a literature review component, which also requires a concise introduction to set the stage for the subsequent review.
You may also like: How to write a fantastic thesis introduction (+15 examples)
It is crucial to customize the content and depth of your literature review introduction according to the specific format of your academic work.
In practical terms, this implies, for instance, that the introduction in an academic literature review paper, especially one derived from a systematic literature review , is quite comprehensive. Particularly compared to the rather brief one or two introductory sentences that are often found at the beginning of a literature review section in a standard academic paper. The introduction to the literature review chapter in a thesis or dissertation again adheres to different standards.
Here’s a structured breakdown based on length and the necessary information:
Academic literature review paper
The introduction of an academic literature review paper, which does not rely on empirical data, often necessitates a more extensive introduction than the brief literature review introductions typically found in empirical papers. It should encompass:
- The research problem: Clearly articulate the problem or question that your literature review aims to address.
- The research gap: Highlight the existing gaps, limitations, or unresolved aspects within the current body of literature related to the research problem.
- The research relevance: Explain why the chosen research problem and its subsequent investigation through a literature review are significant and relevant in your academic field.
- The literature review method: If applicable, describe the methodology employed in your literature review, especially if it is a systematic review or follows a specific research framework.
- The main findings or insights of the literature review: Summarize the key discoveries, insights, or trends that have emerged from your comprehensive review of the literature.
- The main argument of the literature review: Conclude the introduction by outlining the primary argument or statement that your literature review will substantiate, linking it to the research problem and relevance you’ve established.
- Preview of the literature review’s structure: Offer a glimpse into the organization of the literature review paper, acting as a guide for the reader. This overview outlines the subsequent sections of the paper and provides an understanding of what to anticipate.
By addressing these elements, your introduction will provide a clear and structured overview of what readers can expect in your literature review paper.
Regular literature review section in an academic article or essay
Most academic articles or essays incorporate regular literature review sections, often placed after the introduction. These sections serve to establish a scholarly basis for the research or discussion within the paper.
In a standard 8000-word journal article, the literature review section typically spans between 750 and 1250 words. The first few sentences or the first paragraph within this section often serve as an introduction. It should encompass:
- An introduction to the topic: When delving into the academic literature on a specific topic, it’s important to provide a smooth transition that aids the reader in comprehending why certain aspects will be discussed within your literature review.
- The core argument: While literature review sections primarily synthesize the work of other scholars, they should consistently connect to your central argument. This central argument serves as the crux of your message or the key takeaway you want your readers to retain. By positioning it at the outset of the literature review section and systematically substantiating it with evidence, you not only enhance reader comprehension but also elevate overall readability. This primary argument can typically be distilled into 1-2 succinct sentences.
In some cases, you might include:
- Methodology: Details about the methodology used, but only if your literature review employed a specialized method. If your approach involved a broader overview without a systematic methodology, you can omit this section, thereby conserving word count.
By addressing these elements, your introduction will effectively integrate your literature review into the broader context of your academic paper or essay. This will, in turn, assist your reader in seamlessly following your overarching line of argumentation.
Introduction to a literature review chapter in thesis or dissertation
The literature review typically constitutes a distinct chapter within a thesis or dissertation. Often, it is Chapter 2 of a thesis or dissertation.
Some students choose to incorporate a brief introductory section at the beginning of each chapter, including the literature review chapter. Alternatively, others opt to seamlessly integrate the introduction into the initial sentences of the literature review itself. Both approaches are acceptable, provided that you incorporate the following elements:
- Purpose of the literature review and its relevance to the thesis/dissertation research: Explain the broader objectives of the literature review within the context of your research and how it contributes to your thesis or dissertation. Essentially, you’re telling the reader why this literature review is important and how it fits into the larger scope of your academic work.
- Primary argument: Succinctly communicate what you aim to prove, explain, or explore through the review of existing literature. This statement helps guide the reader’s understanding of the review’s purpose and what to expect from it.
- Preview of the literature review’s content: Provide a brief overview of the topics or themes that your literature review will cover. It’s like a roadmap for the reader, outlining the main areas of focus within the review. This preview can help the reader anticipate the structure and organization of your literature review.
- Methodology: If your literature review involved a specific research method, such as a systematic review or meta-analysis, you should briefly describe that methodology. However, this is not always necessary, especially if your literature review is more of a narrative synthesis without a distinct research method.
By addressing these elements, your introduction will empower your literature review to play a pivotal role in your thesis or dissertation research. It will accomplish this by integrating your research into the broader academic literature and providing a solid theoretical foundation for your work.
Comprehending the art of crafting your own literature review introduction becomes significantly more accessible when you have concrete examples to examine. Here, you will find several examples that meet, or in most cases, adhere to the criteria described earlier.
Example 1: An effective introduction for an academic literature review paper
To begin, let’s delve into the introduction of an academic literature review paper. We will examine the paper “How does culture influence innovation? A systematic literature review”, which was published in 2018 in the journal Management Decision.

The entire introduction spans 611 words and is divided into five paragraphs. In this introduction, the authors accomplish the following:
- In the first paragraph, the authors introduce the broader topic of the literature review, which focuses on innovation and its significance in the context of economic competition. They underscore the importance of this topic, highlighting its relevance for both researchers and policymakers.
- In the second paragraph, the authors narrow down their focus to emphasize the specific role of culture in relation to innovation.
- In the third paragraph, the authors identify research gaps, noting that existing studies are often fragmented and disconnected. They then emphasize the value of conducting a systematic literature review to enhance our understanding of the topic.
- In the fourth paragraph, the authors introduce their specific objectives and explain how their insights can benefit other researchers and business practitioners.
- In the fifth and final paragraph, the authors provide an overview of the paper’s organization and structure.
In summary, this introduction stands as a solid example. While the authors deviate from previewing their key findings (which is a common practice at least in the social sciences), they do effectively cover all the other previously mentioned points.
Example 2: An effective introduction to a literature review section in an academic paper
The second example represents a typical academic paper, encompassing not only a literature review section but also empirical data, a case study, and other elements. We will closely examine the introduction to the literature review section in the paper “The environmentalism of the subalterns: a case study of environmental activism in Eastern Kurdistan/Rojhelat”, which was published in 2021 in the journal Local Environment.

The paper begins with a general introduction and then proceeds to the literature review, designated by the authors as their conceptual framework. Of particular interest is the first paragraph of this conceptual framework, comprising 142 words across five sentences:
“ A peripheral and marginalised nationality within a multinational though-Persian dominated Iranian society, the Kurdish people of Iranian Kurdistan (a region referred by the Kurds as Rojhelat/Eastern Kurdi-stan) have since the early twentieth century been subject to multifaceted and systematic discriminatory and exclusionary state policy in Iran. This condition has left a population of 12–15 million Kurds in Iran suffering from structural inequalities, disenfranchisement and deprivation. Mismanagement of Kurdistan’s natural resources and the degradation of its natural environmental are among examples of this disenfranchisement. As asserted by Julian Agyeman (2005), structural inequalities that sustain the domination of political and economic elites often simultaneously result in environmental degradation, injustice and discrimination against subaltern communities. This study argues that the environmental struggle in Eastern Kurdistan can be asserted as a (sub)element of the Kurdish liberation movement in Iran. Conceptually this research is inspired by and has been conducted through the lens of ‘subalternity’ ” ( Hassaniyan, 2021, p. 931 ).
In this first paragraph, the author is doing the following:
- The author contextualises the research
- The author links the research focus to the international literature on structural inequalities
- The author clearly presents the argument of the research
- The author clarifies how the research is inspired by and uses the concept of ‘subalternity’.
Thus, the author successfully introduces the literature review, from which point onward it dives into the main concept (‘subalternity’) of the research, and reviews the literature on socio-economic justice and environmental degradation.
While introductions to a literature review section aren’t always required to offer the same level of study context detail as demonstrated here, this introduction serves as a commendable model for orienting the reader within the literature review. It effectively underscores the literature review’s significance within the context of the study being conducted.
Examples 3-5: Effective introductions to literature review chapters
The introduction to a literature review chapter can vary in length, depending largely on the overall length of the literature review chapter itself. For example, a master’s thesis typically features a more concise literature review, thus necessitating a shorter introduction. In contrast, a Ph.D. thesis, with its more extensive literature review, often includes a more detailed introduction.
Numerous universities offer online repositories where you can access theses and dissertations from previous years, serving as valuable sources of reference. Many of these repositories, however, may require you to log in through your university account. Nevertheless, a few open-access repositories are accessible to anyone, such as the one by the University of Manchester . It’s important to note though that copyright restrictions apply to these resources, just as they would with published papers.
Master’s thesis literature review introduction
The first example is “Benchmarking Asymmetrical Heating Models of Spider Pulsar Companions” by P. Sun, a master’s thesis completed at the University of Manchester on January 9, 2024. The author, P. Sun, introduces the literature review chapter very briefly but effectively:
PhD thesis literature review chapter introduction
The second example is Deep Learning on Semi-Structured Data and its Applications to Video-Game AI, Woof, W. (Author). 31 Dec 2020, a PhD thesis completed at the University of Manchester . In Chapter 2, the author offers a comprehensive introduction to the topic in four paragraphs, with the final paragraph serving as an overview of the chapter’s structure:
PhD thesis literature review introduction
The last example is the doctoral thesis Metacognitive strategies and beliefs: Child correlates and early experiences Chan, K. Y. M. (Author). 31 Dec 2020 . The author clearly conducted a systematic literature review, commencing the review section with a discussion of the methodology and approach employed in locating and analyzing the selected records.

Having absorbed all of this information, let’s recap the essential steps and offer a succinct guide on how to proceed with creating your literature review introduction:
- Contextualize your review : Begin by clearly identifying the academic context in which your literature review resides and determining the necessary information to include.
- Outline your structure : Develop a structured outline for your literature review, highlighting the essential information you plan to incorporate in your introduction.
- Literature review process : Conduct a rigorous literature review, reviewing and analyzing relevant sources.
- Summarize and abstract : After completing the review, synthesize the findings and abstract key insights, trends, and knowledge gaps from the literature.
- Craft the introduction : Write your literature review introduction with meticulous attention to the seamless integration of your review into the larger context of your work. Ensure that your introduction effectively elucidates your rationale for the chosen review topics and the underlying reasons guiding your selection.
Master Academia
Get new content delivered directly to your inbox.
Subscribe and receive Master Academia's quarterly newsletter.
The best answers to "What are your plans for the future?"
10 tips for engaging your audience in academic writing, related articles.

Introduce yourself in a PhD interview (4 simple steps + examples)

37 creative ways to get motivation to study

Types of editorial decisions after peer review (+ how to react)

Minor revisions: Sample peer review comments and examples
Introductions and Literature Reviews
- Author By Troy Mikanovich
- Publication date December 16, 2022
- Categories: Academic Publication , Research Writing
- Categories: academic journal , CARS , introduction , literature review , research , research question
Writing literature reviews is one of the trickiest things you’ll have to do in graduate school. It is even more tricky because a lot of professors will want you to do things that are pedagogically valuable but so tailored to the specific class they are teaching that it can be hard to generalize the lessons you are meant to take away.
This page is meant to be a general overview to the goals and purposes of introductions and literature reviews (or an introduction that contains a literature review–we’ll talk about that), so even if it doesn’t exactly match what you have been asked to do in an assignment, I hope it’ll be helpful.
What is the difference between an introduction and a literature review?
As of writing this, the year is 2022 and words mean nothing. Rather than getting caught up on what these things are in some kind of objective sense, let’s look at what they are supposed to do.
The introduction and the literature review of your paper have the same job. Both are supposed to justify the question(s) you are asking about your topic and to demonstrate to your audience that the thing you are writing about is interesting and of some importance. However, while they have the same job, they do it in two different ways.
An introduction should demonstrate that there is some broader real-world significance to the thing that you are writing about. You can do this by establishing a problem or a puzzle or by giving some background information on your topic to show why it is important. Here’s an example from Brian E. Bride’s “Prevalence of Secondary Traumatic Stress among Social Workers” (2007, link below), where he begins by establishing a problem:
“ In the United States, the lifetime prevalence of exposure to traumatic events ranges from 40 percent to 81 percent, with 60.7 percent of men and 51.2 percent of women having been exposed to one or more traumas and 19.7 percent of men and 11.4 percent of women reporting exposure to three or more such events (Breslau, Davis, Peter-son, & Schultz, 1997; Kessler, Sonnega, Bromet, & Nelson, 1995; Stein, walker, Hazen, & Forde, 1997). Although exposure to traumatic events is high in the general population, it is even higher in subpopulations to whom social workers are likely to provide services…
Although not exhaustive of the populations with whom social workers practice, these examples illustrate that social workers face a high rate of professional contact with traumatized people. Social workers are increasingly being called on to assist survivors of childhood abuse, domestic violence, violent crime, disasters, and war and terrorism. It has become increasingly apparent that the psychological effects of traumatic events extend beyond those directly affected.”
So, Bride (2007) starts with a broad problem (lots of people with exposure to traumatic events) and narrows it to a more specific problem (social workers who work with those people are exposed to secondary trauma as they assist them) .
A literature review should demonstrate that there is some academic significance to the thing you are writing about. You can do this by establishing a scholarly problem (i.e. a “research gap”) and by demonstrating that the state of the existing scholarship on your topic needs to develop in a particular way.
As Bride (2007) transitions to talking about the scholarship on the topic of social workers and secondary trauma, he establishes what scholarship has done and identifies what it has not done .
“Figley (1999) defined secondary traumatic stress as “the natural, consequent behaviors and emotions resulting from knowledge about a traumatizing event experienced by a significant other. It is the stress resulting from helping or wanting to help a traumatized or suffering person” (p. 10). Chrestman (1999) noted that secondary traumatization includes symptoms parallel to those observed in people di-rectly exposed to trauma such as intrusive imagery related to clients’ traumatic disclosures (Courtois, 1988; Danieli, 1988; Herman, 1992; McCann & Pearlman, 1990); avoidant responses (Courtois; Haley, 1974); and physiological arousal (Figley, 1995; McCann & Pearlman, 1990). Thus, STS is a syndrome of symptoms identical to those of PTSD, the characteristic symptoms of which are intrusion, avoidance, and arousal (Figley, 1999)…
Collectively, these studies have provided empirical evidence that individuals who provide services to traumatized populations are at risk of experiencing symptoms of traumatic stress (Bride). However, the extant literature fails to document the prevalence of individual STS symptoms and the extent to which diagnostic criteria for PTSD are met as a result of work with traumatized populations.”
Taken together, Bride (2007) justifies its existence–the research that the author has undertaken in order to read the article that you are now reading–like this:
Broad real world background: Lots of people are suffering from traumatic stress.
Narrowed real world background: People who have suffered traumatic experiences often work with social workers.
Real world problem: Many social workers may through their work suffer from secondary exposure to traumatic experiences.
Broad academic background: There has been a lot of research on secondary traumatic stress
Narrowed academic background: Particularly, this research has shown that social workers are at risk of experiencing symptoms of secondary traumatic stress.
Academic problem/gap : We don’t know how prevalent individual symptoms of secondary traumatic stress are.
Introductions, then, give you space to explain why you are writing about the thing you are writing about, and literature reviews are where you explain what prior scholarship has said about the topic and what the consequences of that prior scholarship are. In an introduction you are writing about the topic; in a literature review you are writing about people writing about the topic.
So does a literature review need to be a separate section from an introduction? Or is a literature review part of an introduction?
It depends on your field, tbh. And on the expectations of the assignment/journal/outlet that you are writing for.
For instance, in the above example (Bride, 2007) the literature review is a part of the introduction. Here’s that paper and some other examples of other places where this is the case. Notice that they do not differentiate between an introductory section and a distinct “Literature Review” as they outline their topic/questions before describing their methodology:
Bride, B. E. (2007) Prevalence of secondary traumatic stress among social workers. Social Work, 52 (1), 63-70. https://doi.org/10.1093/sw/52.1.63
Wei, X., Teng, X., Bai, J., & Ren, F. (2022). Intergenerational transmission of depression during adolescence: The mediating roles of hostile attribution bias, empathetic concern, and social self-concept. The Journal of Psychology, 157 (1), 13-31. https://doi.org/10.1080/00223980.2022.2134276
Stephens, R., Dowber, H., Barrie, A., Sannida, A., & Atkins, K. 2022) Effect of swearing on strength: Disinhibition as a potential mediator. Quarterly Journal of Experimental Psychology . Advance online publication. https://doi.org/10.1177/17470218221082657
However, plenty of other articles have distinct “Literature Review” sections separate from their introductions. The first two examples name it as such, while the third organizes its literature review with thematic sub-sections:
Schraedley, M.K., & Dougherty, D.S. (2021). Creating and disrupting othering during policymaking in a polarized context. Journal of Communication, 72 (1), 111-140. https://doi.org/10.1093/joc/jqab042
Gil de Zúñiga, H., Cheng, Z., & González-González, P. (2022). Effects of the news finds me perception on algorithmic news attitudes and social media political homophily. Journal of Communication, 72 (5), 578-591. https://doi.org/10.1093/joc/jqac025
Brandão, T., Brites, R., Hipólito, J., & Nunes O. (2022) Attachment orientations and family functioning: The mediating role of emotion regulation. The Journal of Psychology , 157 (1), 1-12. https://doi.org/10.1080/00223980.2022.2128284
Whether you separate your literature review into its own distinct section is mostly a function of what you’ve been asked to do (if you are writing for a class) or what the conventions and constraints are of your field.
- UConn Library
- Literature Review: The What, Why and How-to Guide
- Introduction
Literature Review: The What, Why and How-to Guide — Introduction
- Getting Started
- How to Pick a Topic
- Strategies to Find Sources
- Evaluating Sources & Lit. Reviews
- Tips for Writing Literature Reviews
- Writing Literature Review: Useful Sites
- Citation Resources
- Other Academic Writings
What are Literature Reviews?
So, what is a literature review? "A literature review is an account of what has been published on a topic by accredited scholars and researchers. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries." Taylor, D. The literature review: A few tips on conducting it . University of Toronto Health Sciences Writing Centre.
Goals of Literature Reviews
What are the goals of creating a Literature Review? A literature could be written to accomplish different aims:
- To develop a theory or evaluate an existing theory
- To summarize the historical or existing state of a research topic
- Identify a problem in a field of research
Baumeister, R. F., & Leary, M. R. (1997). Writing narrative literature reviews . Review of General Psychology , 1 (3), 311-320.
What kinds of sources require a Literature Review?
- A research paper assigned in a course
- A thesis or dissertation
- A grant proposal
- An article intended for publication in a journal
All these instances require you to collect what has been written about your research topic so that you can demonstrate how your own research sheds new light on the topic.
Types of Literature Reviews
What kinds of literature reviews are written?
Narrative review: The purpose of this type of review is to describe the current state of the research on a specific topic/research and to offer a critical analysis of the literature reviewed. Studies are grouped by research/theoretical categories, and themes and trends, strengths and weakness, and gaps are identified. The review ends with a conclusion section which summarizes the findings regarding the state of the research of the specific study, the gaps identify and if applicable, explains how the author's research will address gaps identify in the review and expand the knowledge on the topic reviewed.
- Example : Predictors and Outcomes of U.S. Quality Maternity Leave: A Review and Conceptual Framework: 10.1177/08948453211037398
Systematic review : "The authors of a systematic review use a specific procedure to search the research literature, select the studies to include in their review, and critically evaluate the studies they find." (p. 139). Nelson, L. K. (2013). Research in Communication Sciences and Disorders . Plural Publishing.
- Example : The effect of leave policies on increasing fertility: a systematic review: 10.1057/s41599-022-01270-w
Meta-analysis : "Meta-analysis is a method of reviewing research findings in a quantitative fashion by transforming the data from individual studies into what is called an effect size and then pooling and analyzing this information. The basic goal in meta-analysis is to explain why different outcomes have occurred in different studies." (p. 197). Roberts, M. C., & Ilardi, S. S. (2003). Handbook of Research Methods in Clinical Psychology . Blackwell Publishing.
- Example : Employment Instability and Fertility in Europe: A Meta-Analysis: 10.1215/00703370-9164737
Meta-synthesis : "Qualitative meta-synthesis is a type of qualitative study that uses as data the findings from other qualitative studies linked by the same or related topic." (p.312). Zimmer, L. (2006). Qualitative meta-synthesis: A question of dialoguing with texts . Journal of Advanced Nursing , 53 (3), 311-318.
- Example : Women’s perspectives on career successes and barriers: A qualitative meta-synthesis: 10.1177/05390184221113735
Literature Reviews in the Health Sciences
- UConn Health subject guide on systematic reviews Explanation of the different review types used in health sciences literature as well as tools to help you find the right review type
- << Previous: Getting Started
- Next: How to Pick a Topic >>
- Last Updated: Sep 21, 2022 2:16 PM
- URL: https://guides.lib.uconn.edu/literaturereview

- University of Texas Libraries
Literature Reviews
- What is a literature review?
- Steps in the Literature Review Process
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Analyze Results
- Librarian Support
What is a Literature Review?
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important past and current research and practices. It provides background and context, and shows how your research will contribute to the field.
A literature review should:
- Provide a comprehensive and updated review of the literature;
- Explain why this review has taken place;
- Articulate a position or hypothesis;
- Acknowledge and account for conflicting and corroborating points of view
From S age Research Methods
Purpose of a Literature Review
A literature review can be written as an introduction to a study to:
- Demonstrate how a study fills a gap in research
- Compare a study with other research that's been done
Or it can be a separate work (a research article on its own) which:
- Organizes or describes a topic
- Describes variables within a particular issue/problem
Limitations of a Literature Review
Some of the limitations of a literature review are:
- It's a snapshot in time. Unlike other reviews, this one has beginning, a middle and an end. There may be future developments that could make your work less relevant.
- It may be too focused. Some niche studies may miss the bigger picture.
- It can be difficult to be comprehensive. There is no way to make sure all the literature on a topic was considered.
- It is easy to be biased if you stick to top tier journals. There may be other places where people are publishing exemplary research. Look to open access publications and conferences to reflect a more inclusive collection. Also, make sure to include opposing views (and not just supporting evidence).
Source: Grant, Maria J., and Andrew Booth. “A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies.” Health Information & Libraries Journal, vol. 26, no. 2, June 2009, pp. 91–108. Wiley Online Library, doi:10.1111/j.1471-1842.2009.00848.x.
Meryl Brodsky : Communication and Information Studies
Hannah Chapman Tripp : Biology, Neuroscience
Carolyn Cunningham : Human Development & Family Sciences, Psychology, Sociology
Larayne Dallas : Engineering
Janelle Hedstrom : Special Education, Curriculum & Instruction, Ed Leadership & Policy
Susan Macicak : Linguistics
Imelda Vetter : Dell Medical School
For help in other subject areas, please see the guide to library specialists by subject .
Periodically, UT Libraries runs a workshop covering the basics and library support for literature reviews. While we try to offer these once per academic year, we find providing the recording to be helpful to community members who have missed the session. Following is the most recent recording of the workshop, Conducting a Literature Review. To view the recording, a UT login is required.
- October 26, 2022 recording
- Last Updated: Oct 26, 2022 2:49 PM
- URL: https://guides.lib.utexas.edu/literaturereviews

Research Skills
Introduction and literature review.
This section is the beginning of the article, but don’t expect it to contain any sort of position or argument. In academic articles, this section has one, overarching purpose: to demonstrate that the authors are familiar with all previous relevant research on the issue they are writing about. Therefore, this section is usually the most “citation-heavy” section of the paper. It is not uncommon to have one or more citations at the end of each sentence. You will likely also encounter a number of compound citations: parentheticals in which not one source, but two or more are cited at one time. Each sentence that precedes a citation in this section is typically a very brief paraphrase of the relevant methods or applicable findings of the other articles that have come before. This review of prior studies is a very important exercise for scholars because it demonstrates the depth of their understanding. None of the articles you read occur in a vacuum; they are usually part of an evolving web of scholarship. Each new article picks up the thread (or, usually, several threads) left by articles published recently. Another important thing to realize is that, in a very real sense, the authors have not really begun; they do not make an argument or say much that is new in this section. It is designed to provide an academic history and theoretical context for the topic of discussion.
At the very end of every literature review section, however, the authors do something important. After having demonstrated their familiarity with previous research, authors indicate that, even though much research has been done, there are still gaps in the research that need filling. You should try to find language such as, “While many studies have examined this subject, no one has looked at this particular issue in this way.” The authors then announce their intention to address that gap in knowledge with the research that follows. This rhetorical move always appears at the end of this section, and often gives the reader the clearest and most detailed description of what exactly the authors are looking at—and why. This is not a thesis, however. Academic articles are not like the essays you may be used to writing, in which the thesis appears at the end of the introduction. The research gap is more akin to a hypothesis than a thesis. It does not make an argument, which comes much later—usually in the discussion or conclusion.
There are also articles that are stand-alone literature reviews; these are sometimes called “Review Articles” or “Meta-analyses.” Rather than engaging in original research, these articles, if they are recent and on point, can provide you with the bibliographic information of all the important, recent sources on your topic. There are many ways to find sources that don’t involve a search engine of any kind. Look at your articles’ references lists to see if they contain any relevant-sounding articles that you haven’t found by other means. You can save a great deal of time this way.
- Parts of An Article. Authored by : Kerry Bowers. Provided by : The University of Mississippi. Project : WRIT 250 Committee OER Project. License : CC BY-SA: Attribution-ShareAlike

Privacy Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Comput Biol
- v.9(7); 2013 Jul

Ten Simple Rules for Writing a Literature Review
Marco pautasso.
1 Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France
2 Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,

The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
Funding Statement
This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.

Introduction vs. Literature Review — What's the Difference?
Difference Between Introduction and Literature Review
Table of contents, key differences, comparison chart, role in paper, relation to other research, compare with definitions, introduction, literature review, common curiosities, how does a literature review differ from an introduction in terms of content, what should i include in the introduction of my paper, how long should an introduction be, is it essential for a research paper to have both an introduction and a literature review, how detailed should a literature review be, should an introduction be captivating, what's the main purpose of an introduction in academic writing, can a literature review identify gaps in existing research, do all research papers have a literature review section, is the introduction the first section of a research paper, what's the primary goal of a literature review, can an introduction mention previous studies, is the literature review limited to academic articles, is a literature review subjective, can a literature review include recent research, share your discovery.

Author Spotlight
Popular Comparisons

Trending Comparisons

New Comparisons

Trending Terms

Believe in yourself, Prove yourself, Be yourself
Introduction vs Literature Review
Introduction is at the beginning of a text while literature review is located after the introduction or background.
Introduction is the part that introduces the main text to the readers while literature review critically evaluates the existing research on the selected research area and identifies the research gap.
Introduction have elements such as background, outline of key issues, thesis statement, aims and purpose of the paper and definition of terms and concepts while literature review have summaries, reviews, critical evaluations, and comparisons of selected research studies.
Recent Posts
- Experience as reviewer (Year 2024) April 14, 2024
Academic Staff Personal Site

Although I am just someone who does some teaching, some research, and some writing but my dream still alive which is to live in the simplest way, in the way you like with fully appreciation and love.
“Believe in yourself, Prove yourself, Be yourself”
Latest News
- Experience as reviewer (Year 2024)
- Experience as reviewer (Year 2023)
- Post Viva Thesis’ Correction
- Research Method and Research Design
- Research Gap
- WoS or Scopus?
- Professional Certification Received
- Experiences as Coach of Certification and Workshop
- Experiences as Panel of Competition/Booth
- ICICyTA 2022
- As Coach/Trainer
- As Conference Committee
- As Moderator/speaker
- As Reviewer
- As Viva Chair/Co-chair/Examiner (main/co)
- Certification
- News and Knowledge
- RG VicubeLab
- Teaching Courses (Current Semester)
- Teaching Courses (Previous Semesters)
- Course Materials
- Research Grants
- Current Research
- Completed research
Publication
- Journal Papers
- Conference Proceedings
- Books and Book Chapters
- Technical Reports and Other Publications

Supervision
- Undergraduate Supervision
- Main Supervisor (Master/PhD)
- Co-supervisor (Master/PhD)
Useful Links

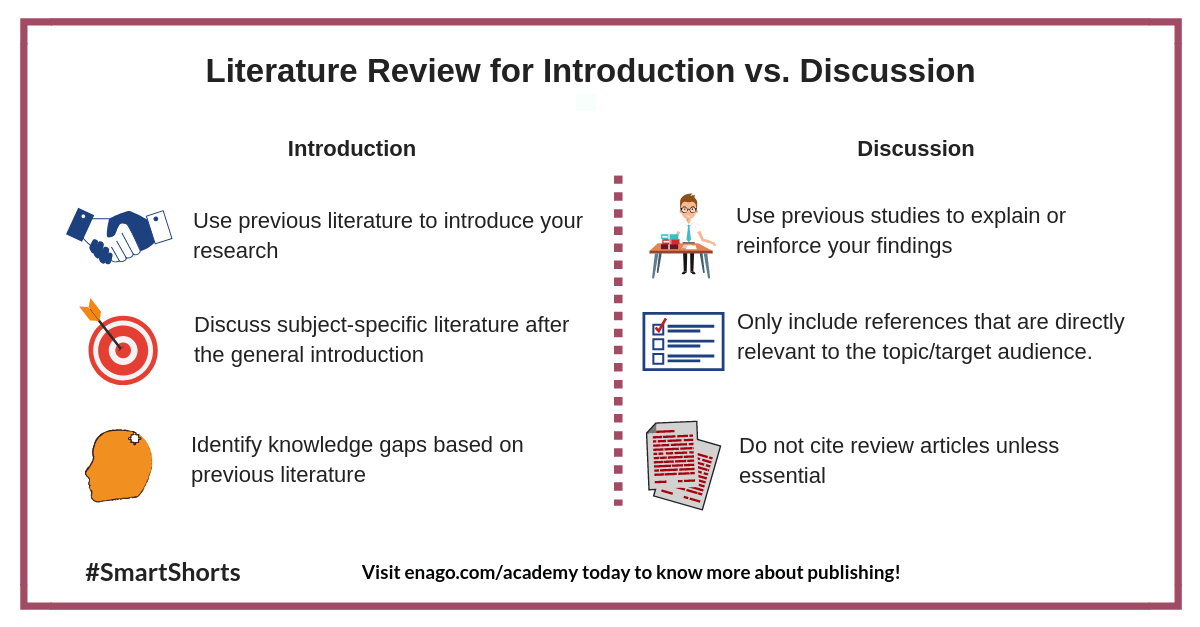
Literature Review for Introduction Vs. Discussion
A literature review presents a summary of studies related to a particular area of research. It identifies and summarizes all the relevant research conducted on a particular topic. Literature reviews are used in the introduction and discussion sections of your manuscripts . However, there are differences in how you can present literature reviews in each section. This smartshort describes how to effectively use literature reviews in these sections. You can also read a detailed article on this topic here.
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

Top 4 Tools for Keyword Selection
Keywords play an important role in making research discoverable. It helps researchers discover articles relevant…

Top 4 Tools to Create Scientific Images and Figures
A good image or figure can go a long way in effectively communicating your results…

Tips to Effectively Present Your Work
Presenting your work is an important part of scientific communication and is very important for…

Tips to Tackle Procrastination
You can end up wasting a lot of time procrastinating. Procrastination leads you to a…

Rules of Capitalization
Using too much capitalization or using it incorrectly can undermine, clutter, and confuse your writing…

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

As a researcher, what do you consider most when choosing an image manipulation detector?
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
A Message From the Assistant Director of Content Development
The Purdue OWL® is committed to supporting students, instructors, and writers by offering a wide range of resources that are developed and revised with them in mind. To do this, the OWL team is always exploring possibilties for a better design, allowing accessibility and user experience to guide our process. As the OWL undergoes some changes, we welcome your feedback and suggestions by email at any time.
Please don't hesitate to contact us via our contact page if you have any questions or comments.
All the best,
Social Media
Facebook twitter.
- Open access
- Published: 02 June 2024
Neuroleptic malignant syndrome and serotonin syndrome: a comparative bibliometric analysis
- Waleed M. Sweileh 1
Orphanet Journal of Rare Diseases volume 19 , Article number: 221 ( 2024 ) Cite this article
149 Accesses
1 Altmetric
Metrics details
This study aimed to analyze and map scientific literature on Neuroleptic Malignant Syndrome (NMS) and Serotonin Syndrome (SS) from prestigious, internationally indexed journals. The objective was to identify key topics, impactful articles, prominent journals, research output, growth patterns, hotspots, and leading countries in the field, providing valuable insights for scholars, medical students, and international funding agencies.
A systematic search strategy was implemented in the PubMed MeSH database using specific keywords for NMS and SS. The search was conducted in the Scopus database, renowned for its extensive coverage of scholarly publications. Inclusion criteria comprised articles published from 1950 to December 31st, 2022, restricted to journal research and review articles written in English. Data were analyzed using Microsoft Excel for descriptive analysis, and VOSviewer was employed for bibliometric mapping.
The search yielded 1150 articles on NMS and 587 on SS, with the majority being case reports. Growth patterns revealed a surge in NMS research between 1981 and 1991, while SS research increased notably between 1993 and 1997. Active countries and journals differed between NMS and SS, with psychiatry journals predominating for NMS and pharmacology/toxicology journals for SS. Authorship analysis indicated higher multi-authored articles for NMS. Top impactful articles focused on review articles and pathogenic mechanisms. Research hotspots included antipsychotics and catatonia for NMS, while SS highlighted drug interactions and specific medications like linezolid and tramadol.
Conclusions
NMS and SS represent rare but life-threatening conditions, requiring detailed clinical and scientific understanding. Differential diagnosis and management necessitate caution in prescribing medications affecting central serotonin or dopamine systems, with awareness of potential drug interactions. International diagnostic tools and genetic screening tests may aid in safe diagnosis and prevention. Reporting rare cases and utilizing bibliometric analysis enhance knowledge dissemination and research exploration in the field of rare drug-induced medical conditions.
Introduction
Neuroleptic malignant syndrome (NMS) and serotonin syndrome (SS) are drug-induced, potentially life-threatening conditions that are infrequently encountered in medical practice, necessitating prompt intervention [ 1 , 2 , 3 , 4 ]. Neuroleptic Malignant Syndrome is characterized by a decrease in dopamine activity in the brain, often associated with the use of dopamine antagonists, primarily neuroleptic or antipsychotic medications [ 5 , 6 ]. While the exact pathophysiology of NMS remains incompletely understood, it is believed to involve dopamine dysregulation in the basal ganglia and hypothalamus. This dysregulation, particularly the blockade of dopamine receptors, especially D2 receptors, leads to a state of dopamine deficiency, manifesting in symptoms such as muscle rigidity, hyperthermia, and autonomic instability. Furthermore, withdrawal from dopamine agonists, such as L-Dopa, can also precipitate NMS in susceptible individuals. Serotonin Syndrome is characterized by an excess of serotonin (5-HT) in the central nervous system, typically stemming from the use of serotonergic medications or substances that elevate serotonin levels [ 7 , 8 ]. These drugs encompass antidepressants, notably selective serotonin reuptake inhibitors (SSRIs), opioids, specific psychedelics, serotonin agonists, and herbal supplements. The pathophysiology of SS revolves around the excessive stimulation of serotonin receptors, particularly the 5-HT2A receptors. This heightened stimulation precipitates a spectrum of symptoms, ranging from agitation, confusion, hyperthermia, muscle rigidity, to autonomic dysfunction. The severity of SS can vary widely, from mild manifestations to life-threatening conditions, contingent upon the extent of serotonin excess and individual susceptibility factors.
Both NMS and SS exhibit shared clinical manifestations, including hyperthermia, hypertension, hypersalivation, diaphoresis, and altered mental status [ 4 ], with instances of coexistence reported in some patients [ 9 ]. However, they diverge in their etiologies and clinical presentations. For instance, individuals with NMS typically display hyporeflexia, normal pupil size, and normal bowel sounds, contrasting with SS patients who often present with hyperreflexia, dilated pupils, and hyperactive bowel activity [ 10 ]. NMS is typified by lead-pipe muscle rigidity, whereas SS manifests with increased muscle tone, particularly in the lower extremities [ 11 , 12 ]. Given these distinctions, treatment strategies for NMS and SS diverge based on their underlying causes [ 2 ]. The mechanisms driving these syndromes differ significantly; while NMS involves diminished dopamine activity in the brain, SS is characterized by elevated serotonin levels [ 13 ]. Dopamine antagonists, such as neuroleptics or antipsychotics, are commonly implicated in NMS [ 14 , 15 , 16 ], although other triggers like withdrawal from dopamine agonists, like L-Dopa, can also induce NMS [ 17 , 18 ]. Conversely, SS can result from various drug classes, including antidepressants, opioids, psychedelics, serotonin agonists, and certain herbs [ 7 , 19 , 20 , 21 , 22 , 23 ]. Consequently, distinct medications are employed for their management; benzodiazepines and serotonin antagonists are standard therapy for SS, whereas dopaminergic agents and dantrolene are preferred for NMS [ 10 ]. While the incidence of NMS remains low, particularly among patients receiving newer generation antipsychotics [ 24 , 25 ], recent studies on SS incidence are lacking. However, a 1999 study reported an incidence of 0.4 cases per 1000 patient-months with nefazodone [ 26 ], while SS incidence reaches 14–16% in cases of selective serotonin reuptake inhibitor (SSRI) overdose [ 27 ].
Research context and objectives
The landscape of psychiatric pharmacotherapy has evolved over time, witnessing a surge in the number of approved drugs and the introduction of novel classes into clinical practice [ 28 , 29 , 30 , 31 ]. This trend is particularly notable in the treatment of depression and schizophrenia, where the absence of universally safe and effective drugs persists [ 32 , 33 , 34 , 35 , 36 ]. Additionally, off-label utilization of antidepressants and antipsychotics has been observed among patients with dementia and other neuro-cognitive disorders [ 37 , 38 , 39 , 40 , 41 ], contributing to an upward trajectory in psychiatric drug consumption [ 42 , 43 ]. The risk of SS is linked to any medication or herb augmenting the central serotonergic pathway, necessitating vigilant monitoring by healthcare professionals due to the potential for adverse effects, whether as a primary mechanism or side effect [ 20 ]. A concerning trend of unsupported polypharmacy in psychiatric medications has also emerged [ 44 ], along with significant prescribing of antidepressants and antipsychotics to dementia patients without documented indications of depression or psychosis [ 45 , 46 ], mirroring similar trends among individuals with intellectual disabilities [ 47 ]. The escalating demand for psychiatric therapy raises apprehensions regarding the likelihood of adverse medication effects [ 48 ], exacerbated by increased prescribing rates, polypharmacy, and off-label usage, which heighten the incidence of drug-induced toxicities, including NMS and SS. Analyzing published literature on drug-induced NMS and SS provides valuable insights into these rare yet severe toxicities, aligning with the pressing global public health burden of depression, schizophrenia, and related conditions, accentuated by the fatal toxicities associated with specific psychiatric medications. This scientific literature on NMS and SS is ripe for analysis and mapping to delineate current research hotspots [ 49 , 50 , 51 , 52 , 53 , 54 , 55 ], addressing the gap in the literature. Accordingly, the present study aims to analyze and map scientific research on NMS and SS published in prestigious, internationally indexed journals. Through this analysis, the study seeks to identify key topics, impactful articles, prominent journals, research output, growth patterns, hotspots, and leading countries in the field, providing valuable insights for scholars, medical students, and international funding agencies to discern research trajectories, bibliographic trends, and knowledge structures pertaining to NMS and SS. Ultimately, this endeavor aims to invigorate scholarly discourse and inform clinical practice in the field.
Database and keywords
In this study, we employed a systematic search strategy to extract relevant scientific literature on NMS and SS from the PubMed MeSH database. Specifically, we utilized the following keywords:
Malignant neuroleptic syndrome: “malignant neuroleptic syndrome”.
Serotonin syndrome: “serotonin syndrome” or “serotonin toxicity”.
To ensure comprehensive coverage, we conducted our search in Scopus, a prestigious scientific database owned by Elsevier, which has previously been utilized for analyzing research in psychiatry [ 56 , 57 ]. Scopus is renowned for its extensive coverage, encompassing a vast array of scholarly publications in the field. Notably, Scopus encompasses over 95% of the content included in other databases such as PubMed and Web of Science, rendering it an ideal platform for our study [ 58 ].
Inclusion and exclusion criteria
We restricted our search to articles published from 1950 to December 31st, 2022, and focused exclusively on journal research and review articles written in English. Excluded from our analysis were editorials, notes, letters, and conference abstracts. Additionally, articles pertaining to non-human subjects were excluded, ensuring the relevance of our findings. We meticulously reviewed the titles and abstracts of over 100 articles to eliminate irrelevant publications, such as those mentioning NMS or SS only marginally, thereby refining the scope of our analysis.
Our search strategy yielded results indicative of its validity, as evidenced by the prominent presence of leading scientists and journals in the fields of psychiatry and pharmacology. This reaffirmed the robustness of our search criteria and the relevance of the retrieved literature to our study objectives.
Data management, analysis, and mapping
The dataset comprising the retrieved articles was subjected to descriptive analysis using Microsoft Excel. Subsequently, we employed VOSviewer, a freely available online tool, for bibliometric mapping purposes [ 59 ]. VOSviewer maps offer researchers a visual tool for exploring bibliometric data, revealing patterns, relationships, and trends within a dataset. Interpretation of these maps involves understanding several key elements. Firstly, node size indicates the prominence or frequency of an item, with larger nodes representing more significant themes or influential publications. Secondly, node color categorizes items into clusters, with similar colors indicating thematic groupings. Thirdly, the thickness of connecting lines between nodes signifies the strength of associations, with thicker lines indicating stronger connections. Lastly, the distance between nodes reflects the similarity or dissimilarity between items, with closer nodes indicating stronger relationships. Overall, VOSviewer maps provide a comprehensive visual overview of bibliometric data, enabling researchers to identify clusters, influential publications, and emerging trends within their field of study by considering the interplay between node size, color, line thickness, and spatial relationships. Within the descriptive analysis, we presented lists of active countries and journals, alongside a linear graph illustrating the growth of publications over time. In the keyword visualization map generated using VOSviewer, node size corresponded to the frequency of occurrence of each keyword, enabling visual identification of prominent themes. Similarly, in the journal visualization map, node size reflected the normalized citation count received by each journal, providing insights into publication impact within the field.
Number of publications
The search strategy yielded a total of 1150 articles on NMS and 587 on SS. Among the articles on NMS, 791 (68.8%) were case reports, while 384 (65.4%) of the articles on SS took the form of case reports.
Growth of publications
The earliest scientific publication on NMS dates back to 1973 [ 60 ], while publications on SS emerged in 1979 [ 61 ]. Research on NMS experienced a notable surge between 1981 and 1991, followed by a fluctuating decline. Conversely, research on SS saw a steep increase between 1993 and 1997, followed by a fluctuating rise. Figure 1 illustrates the growth trends of research on NMS and SS.
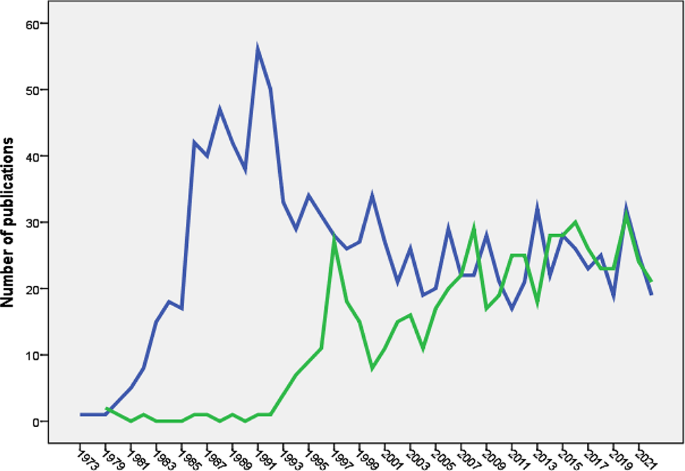
Annual growth of publications of NMS (blue line) and SS (green line). The Figure was created by SPSS program
Active countries and journals
Table 1 outlines the top five countries contributing articles on NMS and SS. Japan ranked second in NMS publications but fifth in SS publications. Table 2 presents the top five active journals for both NMS and SS, with NMS publications primarily within psychiatry journals and SS publications within pharmacology/toxicology journals.
Authorship analysis
Articles on NMS involved 3820 authors (mean = 3.1 authors per article), with 89 (7.3%) single-authored and 171 (14.1%) multi-authored articles. Similarly, articles on SS included 2105 authors (mean = 3.0 authors per article), with 102 (16.0%) single-authored and 41 (7.1%) multi-authored articles.
Most impactful articles
The top five impactful articles on NMS comprised mainly review articles and a research article focusing on the pathogenic role of dopamine antagonists [ 62 ]. For SS, the top five impactful articles included review articles and research articles discussing the Hunter diagnostic criteria [ 63 ] and the role of monoamine oxidase inhibitors (MAO-I) and opioid analgesics in serotonin toxicity [ 64 ].
Research hotspots
Research hotspots were identified by mapping author keywords with a minimum occurrence of five times (Figs. 2 and 3 ). Notable hotspots for SS included antidepressants, SSRIs, tramadol, linezolid, cyproheptadine, and drug interactions. For NMS, hotspots included antipsychotics (various drug names), catatonia, and rhabdomyolysis.
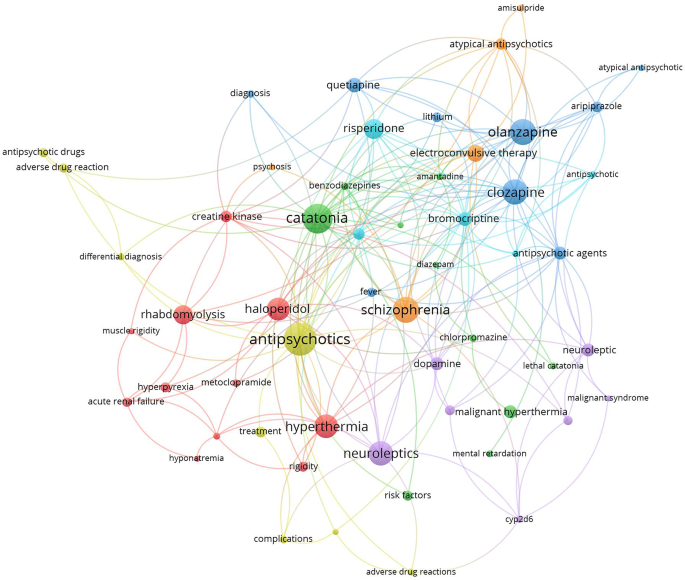
Network visualization map of author keywords with minimum occurrences of five times. Large nodes represent research hotspots on NMS. The term NMS was not shown to make other keywords more visible
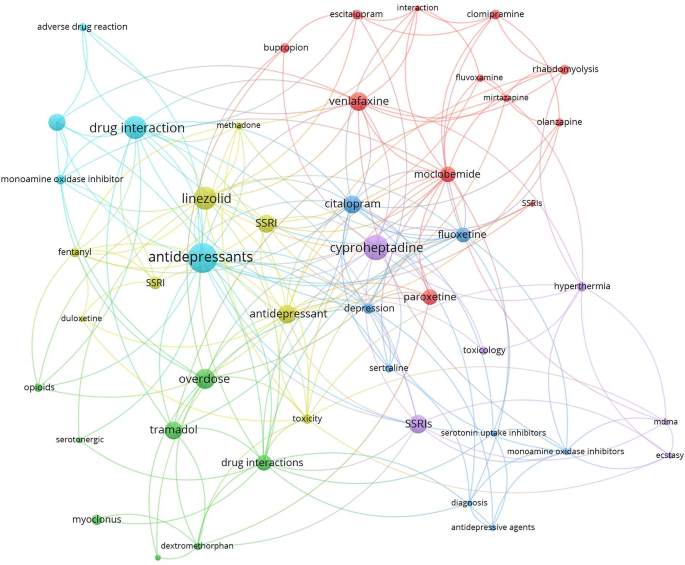
Network visualization map of author keywords with minimum occurrences of five times. Large nodes represent research hotspots on SS. The term SS was not shown to make other keywords more visible
Journal citation analysis
The top 15 active journals in publishing articles on NMS and SS were mapped (Figs. 4 and 5 ). Notably, articles on NMS published in the American Journal of Psychiatry and the Journal of Clinical Psychiatry received the highest number of citations per article. Similarly, articles on SS published in Clinical Toxicology and the Annals of Pharmacotherapy garnered the most citations per article.
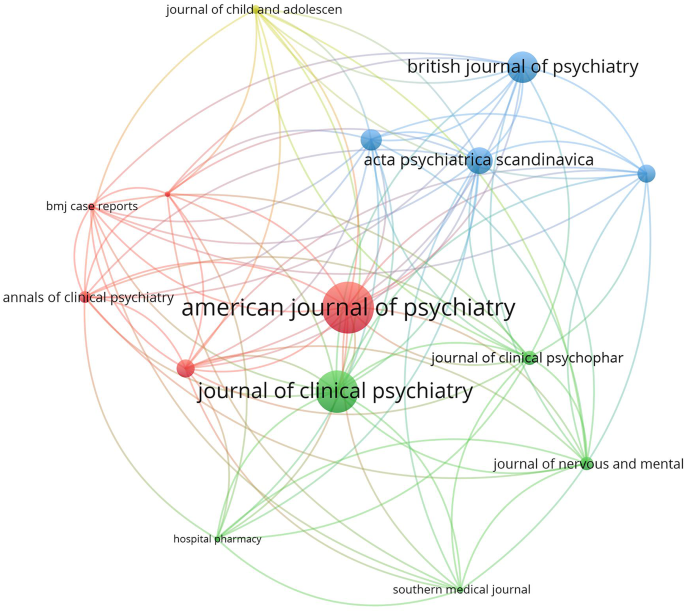
Network visualization map of the top 15 journals in the field of NMS. Large node sized indicates higher normalized citation count
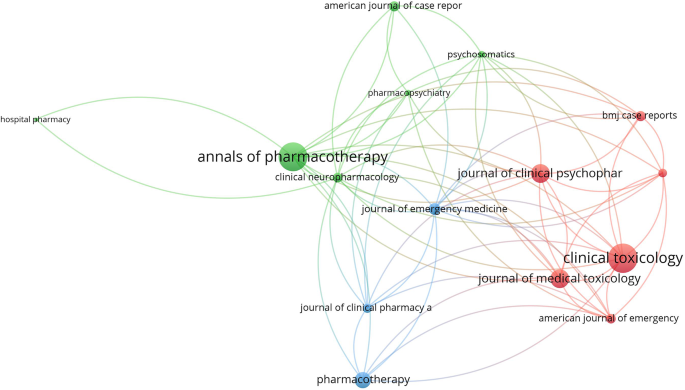
Network visualization map of the top 15 journals in the field of SS. Large node sized indicates higher normalized citation count
Geographic mapping
The geographic distribution of research publications on NMS and SS was illustrated on a worldwide map (Fig. 6 ), with the majority of contributions originating from the US. Several countries in specific regions showed minimal to no research output on either NMS or SS.
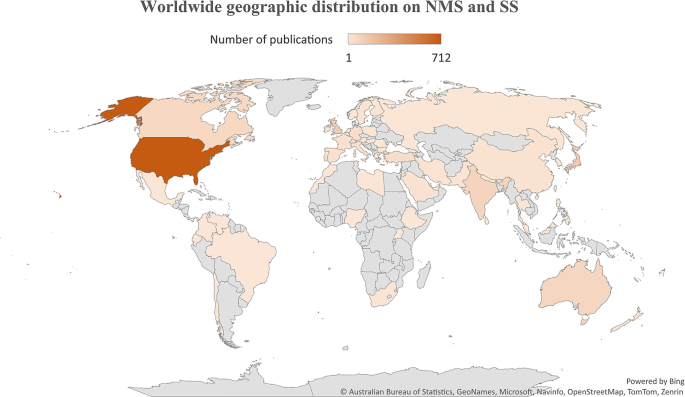
Worldwide distribution of research publications on NMS and SS. Figure was created by Microsoft Excel
Molecular genetics
The retrieved literature on NMS has 20 articles that discussed the potential link between NMS and certain genetics. Ten articles discussed the potential linkage between Cytochrome 2D6 and potential risk for NMS [ 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ]. Five articles discussed the potential linkage between dopamine receptor 2 gene polymorphism and NMS [ 75 , 76 , 77 , 78 , 79 ]. Four articles discussed the linkage between ryanodine receptor gene mutations and susceptibility to NMS [ 80 , 81 , 82 , 83 ]. No association was found between NMS and serotonin receptor gene variation [ 84 ]. The literature on SS has few articles that discussed the genetic predisposition of patients to SS such as the 5-HT receptor gene or the CYP 2D6 gene polymorphism [ 85 , 86 ].
Drug interactions
Serious drug-drug interactions leading to NMS were mentioned in a limited number of articles and involved the administration of two dopamine antagonists [ 87 ] or two atypical antipsychotic drugs [ 88 ]. However, there were many articles discussing potential SS caused by drug-drug interactions, which included SSRI–methylene blue [ 89 ], SSRI–metoclopramide [ 89 ], sertraline–phenelzine [ 90 ], anti-depressants–opioids [ 91 ], citalopram-fentanyl [ 92 ], a combination of two anti-depressants [ 93 ], SSRI-linezolid [ 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 ], sertraline–phenelzine [ 90 ], citalopram-buspirone [ 103 ], venlafaxine-tranylcypromine [ 104 ], and many others [ 92 , 105 , 106 , 107 , 108 , 109 ].
Non-psychiatric causative agents
The retrieved literature on SS, showed that several drugs and drug classes not related to antidepressants can induce SS. Examples of such drugs included Linezolid, CNS stimulants (amphetamine), hallucinogens (LSD), opioids (fentanyl), ondansetron, sumatriptan, and certain herbs (St. John’s wort), metoclopramide, ritonavir, and others [ 5 , 20 , 110 , 111 ]. The retrieved literature on NMS showed that drug-induced NMS is limited to antipsychotics and withdrawal of dopamine agonists [ 112 , 113 , 114 ].
Diagnostic criteria
For NMS, there were 30 articles that discussed issues related to diagnosis. In 2011, an international panel tried to develop NMS diagnostic criteria [ 115 , 116 ]. The neutrophil-lymphocyte ratio was suggested by certain researchers as a diagnostic test for NMS [ 117 , 118 ]. The differential diagnosis for NMS compared to SS and catatonia was also published [ 13 , 118 , 119 ]. For SS, there were 17 articles that discussed issues related to diagnosis of SS. The Hunter diagnostic criteria was one of these articles [ 63 ]. Other articles discussed controversies and the importance of differential diagnosis in SS [ 120 ].
The current study analyzed and compared the scientific literature on two rare drug-induced conditions with certain overlapping clinical features. Both syndromes are mainly caused by medications used in psychiatry, such as those for schizophrenia and depression. The name “NMS” implies that the syndrome is correlated with the use of neuroleptic medications, while the name “SS” implies that it is correlated with any medication or herb that raises serotonin centrally.
The analysis showed that the volume of research publications on NMS was larger and started earlier than research publications on SS. The NMS is associated with the use of dopamine antagonists (neuroleptics). The history of using old-generation antipsychotics for the treatment of schizophrenia dates back to the 1950s [ 121 , 122 , 123 , 124 , 125 ]. On the other hand, the introduction of the SSRI drug class, the main causative agent of SS, dates back to the late 1980s [ 126 ]. The difference in the history of introduction into clinical practice explains the differences between SS and NMS in growth patterns. The difference in the volume of literature between the two syndromes could be due to diagnostic uncertainty [ 127 ] for NMS versus SS, the seriousness of medical complications, or debate regarding whether an atypical antipsychotic drug class causes NMS in a similar way to conventional antipsychotics [ 13 , 30 , 128 , 129 , 130 ]. The current study showed that the number of research publications on NMS started to decline after 1991 but the number of publications on SS started to increase after 1997. The introduction of atypical antipsychotics with lesser dopaminergic side effects than conventional antipsychotics decreased the incidence of NMS and therefore decreased the number of publications with time. On the other hand, the increased number of SS publications after 1997 could be explained by the many reported drug interactions at serotonin level leading to more cases of SS with time.
The current study showed that journals in the field of psychiatry ranked highest in publishing articles on NMS, while those in the field of pharmacology/toxicology ranked highest in publishing articles on SS. The reason for this difference is difficult to explain. However, NMS is primarily limited to schizophrenia patients taking antipsychotic drugs, while SS might occur in normal people taking SSRIs for depression or any other condition. Furthermore, the potentially large numbers of drug- or drug-herb interactions make the SS interesting to pharmacology/toxicology journals [ 22 ]. Actually, SS has been termed “serotonin toxicity” implying relatedness to toxicology [ 131 ].
The findings of the current study regarding active countries were not surprising. The English-speaking countries, the US, the UK, Australia, and Canada showed leading roles in many scientific disciplines and ranked first in several studies that analyzed research activity [ 132 , 133 , 134 , 135 ]. This is due to advancements in technology, medicine, clinical practice, and research funding in high-income countries relative to other countries. However, there are also reasons related to the nature of journals indexed in Scopus. The vast majority of Scopus-indexed journals publish articles in English, and the vast majority of the journals are issued by publishers and institutions based in the US, Europe, or Australia. Therefore, Scopus might be biased toward scholars in English-speaking countries. The finding that research articles on NMS tend to be multi-authored while those on SS are not is not easy to explain. However, it is possible that cases of NMS tend to involve a larger medical team due to the nature of complications that might involve renal and blood complications. Furthermore, the treatment of NMS requires medications and follow-up. All this makes the number of authors in a case study of NMS higher than those involved in SS cases [ 13 , 136 , 137 ].
Of the retrieved articles on SS and NMS, the research article “The hunter serotonin toxicity criteria: Simple and accurate diagnostic decision rules for serotonin toxicity” [ 63 ] received the highest number of citations excluding the review articles. The diagnosis of SS is based on the clinical symptoms and the medical history of the patient. Harvey Sternbach introduced the first diagnostic criteria for SS in 1991 and the Hunter Diagnostic Criteria tool was introduced in 2003 [ 63 , 138 ].
Mapping the retrieved literature on NMS showed that rhabdomyolysis and catatonia constituted distinct research hotspots in addition to those related to antipsychotic medications and schizophrenia. However, mapping the author keywords of SS research publications showed that linezolid, drug interactions, and tramadol constituted research hotspots in addition to antidepressants and SSRIs. Rhabdomyolysis has been reported as a consequence of NMS even among children and adolescents [ 139 , 140 ]. However, reports of rhabdomyolysis among patients taking antipsychotics were published, suggesting that rhabdomyolysis could be a side effect of antipsychotics even in the absence of NMS [ 139 , 140 ]. Catatonia is, as NMS, a consequence of neuroleptic drugs, and there is an overlap in clinical features between the symptoms of catatonia and those of NMS, which makes the distinction between them difficult [ 141 ]. Linezolid is an antibiotic that was originally designed to be used as an anti-depressant by virtue of its MAO enzyme inhibition property [ 142 ]. This explains the many cases of SS induced by drug interactions with Linezolid [ 141 ]. The relatively higher number of research articles on drug/herb interactions leading to SS is attributed to the presence of many and different drug classes that affect and increase serotonergic pathways in the brain [ 90 , 105 , 109 , 143 ]. The scientific controversy about the potential ability of tramadol to cause SS received a high number of citations. Current scientific evidence supports the ability of tramadol to cause SS due to its molecular pharmacological effects on both the opioid and serotonergic systems [ 105 , 107 , 144 , 145 , 146 , 147 ]. Cyproheptadine was also a research hotspot in the field of SS. Cyproheptadine has anti-histaminic, anticholinergic, and anti-serotonergic properties and that is why it has been used to counter the symptoms of SS [ 148 , 149 , 150 ].
The current study showed that SS has a wide range of possible drug/herb interactions due to the many drugs that affect the serotonin system. Of particular interest is the one with opioid analgesics, since they are commonly used in hospital settings. Opioids, including fentanyl and even dextromethorphan in cough syrups, were reported to increase serotonin levels, and therefore caution should be practiced when given to patients with SSRIs in their medical records [ 19 , 22 , 109 , 151 ].
Limitations
Limitations arise in this study from various factors. Firstly, the reliance on the Scopus database for literature retrieval could potentially limit the inclusivity of articles from low- and middle-income countries. Although Scopus offers extensive coverage, the possibility exists that some relevant journals from these regions may not be indexed, thereby leading to a potential underestimation of publications from certain geographic areas. Secondly, despite efforts to employ a comprehensive search strategy, the use of a title-abstract search method might have resulted in the retrieval of some false-positive results. While validation tests were conducted to mitigate this issue, the possibility of false positives cannot be entirely ruled out. Thirdly, the analysis focused solely on articles published in English-language journals, which could introduce a language bias and limit the generalizability of findings. This exclusion of literature published in other languages may have led to the omission of relevant data from non-English sources. Lastly, diagnostic uncertainty poses a challenge in distinguishing between neuroleptic malignant syndrome (NMS) and serotonin syndrome (SS) due to overlapping clinical features and the absence of definitive diagnostic tests. Misdiagnosis or underreporting of cases may have occurred, potentially impacting the accuracy of the literature analysis and conclusions drawn from it.
In conclusion, NMS and SS represent rare but potentially life-threatening conditions associated with drug-induced dysregulation of dopamine and serotonin systems, respectively. The study analyzed and compared the scientific literature on these syndromes, revealing distinct growth patterns, research hotspots, and publication trends. The findings underscored the evolving landscape of psychiatric pharmacotherapy and the complexities involved in diagnosing and managing NMS and SS. While NMS research exhibited a longer history and a decline in publications over time, SS research witnessed a notable increase in publications, reflecting advancements in pharmacological understanding and the recognition of SS as a significant clinical entity. Identified research hotspots provided valuable insights into emerging areas of interest, including drug interactions, molecular genetics, and diagnostic criteria. Understanding these trends is essential for informing clinical practice, guiding future research endeavors, and promoting collaboration among scholars and healthcare professionals. Despite the study’s contributions, several limitations warrant consideration, including database restrictions, potential publication bias, and diagnostic uncertainties. Addressing these limitations through expanded literature search strategies, international collaboration, and improved diagnostic tools is crucial for advancing knowledge and enhancing patient care in the field of rare drug-induced syndromes. Moving forward, efforts to develop standardized diagnostic criteria, genetic screening tools, and international reporting mechanisms for NMS and SS are warranted. Additionally, continued bibliometric analysis and mapping of literature on rare medical conditions can facilitate ongoing research and contribute to the dissemination of knowledge across global healthcare communities.
Data availability
All data present in this article can be retrieved from Scopus using keywords listed in the methodology.
Abbreviations
- Neuroleptic malignant syndrome
Serotonin Syndrome/ Serotonin toxicity
Darracq MA, Comment. Neuroleptic malignant syndrome versus serotonin syndrome: the search for a diagnostic tool. Ann Pharmacother. 2012;46(4):611–2. https://doi.org/10.1345/aph.1P787a . author reply 3.
Article PubMed Google Scholar
Sokoro AA, Zivot J, Ariano RE. Neuroleptic malignant syndrome versus serotonin syndrome: the search for a diagnostic tool. Ann Pharmacother. 2011;45(9):e50. https://doi.org/10.1345/aph.1P787 .
Fernández M, Lago L, Alonso MG, Guede A, Benavente JL, Olivares JM. Serotonin syndrome versus neuroleptic malignant syndrome: a case report. Actas Esp Psiquiatr. 2018;46(2):68–74.
PubMed Google Scholar
Dosi R, Ambaliya A, Joshi H, Patell R. Serotonin syndrome versus neuroleptic malignant syndrome: a challenging clinical quandary. BMJ Case Rep. 2014. https://doi.org/10.1136/bcr-2014-204154 . 2014.
Article PubMed PubMed Central Google Scholar
Tormoehlen LM, Rusyniak DE. Neuroleptic malignant syndrome and serotonin syndrome. Handb Clin Neurol. 2018;157:663–75. https://doi.org/10.1016/b978-0-444-64074-1.00039-2 .
Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1). https://doi.org/10.4088/PCC.17r02185 .
Maitland S, Baker M. Serotonin syndrome. Drug Ther Bull. 2022;60(6):88–91. https://doi.org/10.1136/dtb.2021.000032 .
Mikkelsen N, Damkier P, Pedersen SA. Serotonin syndrome-A focused review. Basic Clin Pharmacol Toxicol. 2023;133(2):124–9. https://doi.org/10.1111/bcpt.13912 .
Article CAS PubMed Google Scholar
Prakash S, Lodha D, Rawat KS. Coexistence of serotonin syndrome and neuroleptic malignant syndrome: does it exist? BMJ Case Rep. 2021;14(8). https://doi.org/10.1136/bcr-2021-241578 .
Katus LE, Frucht SJ. Management of Serotonin Syndrome and neuroleptic malignant syndrome. Curr Treat Options Neurol. 2016;18(9):39. https://doi.org/10.1007/s11940-016-0423-4 .
Hadad E, Weinbroum AA, Ben-Abraham R. Drug-induced hyperthermia and muscle rigidity: a practical approach. Eur J Emerg Med. 2003;10(2):149–54. https://doi.org/10.1097/00063110-200306000-00018 .
Haddad PM, Dursun SM. Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol. 2008;23(Suppl 1):15–26. https://doi.org/10.1002/hup.918 .
Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155–62.
Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973–81. https://doi.org/10.1177/1060028016657553 .
Hasan S, Buckley P. Novel antipsychotics and the neuroleptic malignant syndrome: a review and critique. Am J Psychiatry. 1998;155(8):1113–6. https://doi.org/10.1176/ajp.155.8.1113 .
Belvederi Murri M, Guaglianone A, Bugliani M, Calcagno P, Respino M, Serafini G, et al. Second-generation antipsychotics and neuroleptic malignant syndrome: systematic review and case report analysis. Drugs R D. 2015;15(1):45–62. https://doi.org/10.1007/s40268-014-0078-0 .
Article CAS PubMed PubMed Central Google Scholar
Mizuno Y, Takubo H, Mizuta E, Kuno S. Malignant syndrome in Parkinson’s disease: concept and review of the literature. Parkinsonism Relat Disord. 2003;9(Suppl 1). https://doi.org/10.1016/s1353-8020(02)00125-6 . :S3-9.
Rainer C, Scheinost NA, Lefeber EJ. Neuroleptic malignant syndrome. When levodopa withdrawal is the cause. Postgrad Med. 1991;89(5):175–8. https://doi.org/10.1080/00325481.1991.11700900 .
Baldo BA, Rose MA. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: a mechanistic and clinical review. Br J Anaesth. 2020;124(1):44–62. https://doi.org/10.1016/j.bja.2019.08.010 .
Bartlett D. Drug-Induced Serotonin Syndrome. Crit Care Nurse. 2017;37(1):49–54. https://doi.org/10.4037/ccn2017169 .
Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, et al. Herb-drug interactions: a literature review. Drugs. 2005;65(9):1239–82. https://doi.org/10.2165/00003495-200565090-00005 .
Baldo BA. Opioid analgesic drugs and serotonin toxicity (syndrome): mechanisms, animal models, and links to clinical effects. Arch Toxicol. 2018;92(8):2457–73. https://doi.org/10.1007/s00204-018-2244-6 .
Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1178646919873925. https://doi.org/10.1177/1178646919873925 .
Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf. 1998;19(1):73–82. https://doi.org/10.2165/00002018-199819010-00006 .
Berman BD. Neuroleptic malignant syndrome: a review for neurohospitalists. Neurohospitalist. 2011;1(1):41–7. https://doi.org/10.1177/1941875210386491 .
Mackay FJ, Dunn NR, Mann RD. Antidepressants and the serotonin syndrome in general practice. Br J Gen Pract. 1999;49(448):871–4.
CAS PubMed PubMed Central Google Scholar
Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277–85. https://doi.org/10.1081/clt-120037428 .
Adell A. Brain NMDA receptors in Schizophrenia and Depression. Biomolecules. 2020;10(6). https://doi.org/10.3390/biom10060947 .
Laszlovszky I, Barabássy Á, Németh G, Cariprazine. A broad-spectrum antipsychotic for the treatment of Schizophrenia: Pharmacology, Efficacy, and Safety. Adv Ther. 2021;38(7):3652–73. https://doi.org/10.1007/s12325-021-01797-5 .
Orzelska-Górka J, Mikulska J, Wiszniewska A, Biała G. New atypical antipsychotics in the treatment of Schizophrenia and Depression. Int J Mol Sci. 2022;23(18). https://doi.org/10.3390/ijms231810624 .
Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401(10371):141–53. https://doi.org/10.1016/s0140-6736(22)02080-3 .
Preda A, Shapiro BB. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin Drug Saf. 2020;19(12):1529–38. https://doi.org/10.1080/14740338.2020.1832990 .
Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–77. https://doi.org/10.2147/tcrm.S117321 .
Cheniaux E, Nardi AE. Evaluating the efficacy and safety of antidepressants in patients with bipolar disorder. Expert Opin Drug Saf. 2019;18(10):893–913. https://doi.org/10.1080/14740338.2019.1651291 .
Sommer BR, Fenn H, Pompei P, DeBattista C, Lembke A, Wang P, et al. Safety of antidepressants in the elderly. Expert Opin Drug Saf. 2003;2(4):367–83. https://doi.org/10.1517/14740338.2.4.367 .
Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The Safety, Tolerability and Risks Associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–88. https://doi.org/10.1159/000447034 .
Yunusa I, Rashid N, Demos GN, Mahadik BS, Abler VC, Rajagopalan K. Comparative outcomes of commonly used off-label atypical antipsychotics in the treatment of dementia-related psychosis: a Network Meta-analysis. Adv Ther. 2022;39(5):1993–2008. https://doi.org/10.1007/s12325-022-02075-8 .
Scheltema Beduin A, de Haan L. Off-label second generation antipsychotics for impulse regulation disorders: a review. Psychopharmacol Bull. 2010;43(3):45–81.
Hefner G, Wolff J, Toto S, Reißner P, Klimke A. Off-label use of antidepressants, antipsychotics, and mood-stabilizers in psychiatry. J Neural Transm (Vienna). 2022;129(11):1353–65. https://doi.org/10.1007/s00702-022-02542-0 .
Haw C, Stubbs J. Off-label use of antipsychotics: are we mad? Expert Opin Drug Saf. 2007;6(5):533–45. https://doi.org/10.1517/14740338.6.5.533 .
Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J Manag Care Pharm. 2012;18(5 Suppl B):S1–20. https://doi.org/10.18553/jmcp.2012.18.s5-b.1 .
González-López MDC, Díaz-Calvo V, Ruíz-González C, Nievas-Soriano BJ, Rebollo-Lavado B, Parrón-Carreño T. Consumption of Psychiatric drugs in Primary Care during the COVID-19 pandemic. Int J Environ Res Public Health. 2022;19(8). https://doi.org/10.3390/ijerph19084782 .
Heald AH, Stedman M, Farman S, Khine C, Davies M, De Hert M, et al. Links between the amount of antipsychotic medication prescribed per population at general practice level, local demographic factors and medication selection. BMC Psychiatry. 2020;20(1):528. https://doi.org/10.1186/s12888-020-02915-3 .
Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry. 2010;67(1):26–36. https://doi.org/10.1001/archgenpsychiatry.2009.175 .
Drummond N, McCleary L, Freiheit E, Molnar F, Dalziel W, Cohen C, et al. Antidepressant and antipsychotic prescribing in primary care for people with dementia. Can Fam Physician. 2018;64(11):e488–97.
PubMed PubMed Central Google Scholar
Luo H, Lau WCY, Chai Y, Torre CO, Howard R, Liu KY, et al. Rates of antipsychotic drug prescribing among people living with Dementia during the COVID-19 pandemic. JAMA Psychiatry. 2023;80(3):211–9. https://doi.org/10.1001/jamapsychiatry.2022.4448 .
Henderson A, McSkimming P, Kinnear D, McCowan C, McIntosh A, Allan L, et al. Changes over a decade in psychotropic prescribing for people with intellectual disabilities: prospective cohort study. BMJ Open. 2020;10(9):e036862. https://doi.org/10.1136/bmjopen-2020-036862 .
Rogers K, Spring B. Mental Health professionals are in high demand as the pandemic enters a Second Year. CNBC; 2022.
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40. https://doi.org/10.1016/j.jpsychires.2019.08.002 .
Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. https://doi.org/10.1016/j.jad.2017.05.003 .
Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–73. https://doi.org/10.2147/ndt.S96649 .
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global Epidemiology and burden of Schizophrenia: findings from the global burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–203. https://doi.org/10.1093/schbul/sby058 .
Global, regional, and, national, burden, of, 12, mental, disorders, in, 204, countries, and, territories, 1990–2019, a, systematic, analysis, for, the, Global, Burden, of, Disease, Study, 2019. Lancet Psychiatry. 2022;9(2):137–50. https://doi.org/10.1016/s2215-0366(21)00395-3 .
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41. https://doi.org/10.1001/jamapsychiatry.2014.2502 .
Hany M, Rehman B, Azhar Y, Chapman J. Schizophrenia. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC.; 2023.
Sweileh WM. Analysis of global research output on diabetes depression and suicide. Ann Gen Psychiatry. 2018;17:44. https://doi.org/10.1186/s12991-018-0214-2 .
Sweileh WM. Contribution of researchers in the arab region to peer-reviewed literature on mental health and well-being of university students. Int J Ment Health Syst. 2021;15(1):50. https://doi.org/10.1186/s13033-021-00477-9 .
Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal: Official Publication Federation Am Soc Experimental Biology. 2008;22(2):338–42. https://doi.org/10.1096/fj.07-9492LSF .
Article CAS Google Scholar
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. https://doi.org/10.1007/s11192-009-0146-3 .
Meltzer HY. Rigidity, hyperpyrexia and coma following fluphenazine enanthate. Psychopharmacologia. 1973;29(4):337–46. https://doi.org/10.1007/bf00429281 .
Silbergeld EK, Hruska RE. Lisuride and LSD: dopaminergic and serotonergic interactions in the serotonin syndrome. Psychopharmacology. 1979;65(3):233–7. https://doi.org/10.1007/bf00492209 .
Henderson VW, Wooten GF. Neuroleptic malignant syndrome: a pathogenetic role for dopamine receptor blockade? Neurology. 1981;31(2):132–7. https://doi.org/10.1212/wnl.31.2.132 .
Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635–42. https://doi.org/10.1093/qjmed/hcg109 .
Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95(4):434–41. https://doi.org/10.1093/bja/aei210 .
Ueno S, Otani K, Kaneko S, Koshiro K, Kondoh K, Kotani Y, et al. Cytochrome P-450 2D6 gene polymorphism is not associated with neuroleptic malignant syndrome. Biol Psychiatry. 1996;40(1):72–4. https://doi.org/10.1016/0006-3223(95)00427-0 .
Ochi S, Kawasoe K, Abe M, Fukuhara R, Sonobe K, Kawabe K, et al. A case study: neuroleptic malignant syndrome with risperidone and CYP2D6 gene variation. Gen Hosp Psychiatry. 2011;33(6):640. https://doi.org/10.1016/j.genhosppsych.2011.03.003 . .e1-2.
Article Google Scholar
Kato D, Kawanishi C, Kishida I, Furuno T, Matsumura T, Hasegawa H, et al. CYP2D6 gene deletion allele in patients with neuroleptic malignant syndrome: preliminary report. Psychiatry Clin Neurosci. 2005;59(4):504–7. https://doi.org/10.1111/j.1440-1819.2005.01405.x .
Iwahashi K, Yoshihara E, Nakamura K, Ameno K, Watanabe M, Tsuneoka Y, et al. CYP2D6 HhaI genotype and the neuroleptic malignant syndrome. Neuropsychobiology. 1999;39(1):33–7. https://doi.org/10.1159/000026557 .
Iwahashi K, Nakamura K, Suwaki H, Tsuneoka Y, Ichikawa Y. CYP2D6 Hhal genotype and the neuroleptic malignant syndrome (NMS). Clin Chim Acta. 1997;265(1):143–4. https://doi.org/10.1016/s0009-8981(97)00113-7 .
Kato D, Kawanishi C, Kishida I, Furuno T, Suzuki K, Onishi H, et al. Effects of CYP2D6 polymorphisms on neuroleptic malignant syndrome. Eur J Clin Pharmacol. 2007;63(11):991–6. https://doi.org/10.1007/s00228-007-0355-8 .
Kawanishi C, Shimoda Y, Fujimaki J, Onishi H, Suzuki K, Hanihara T, et al. Mutation involving cytochrome P450IID6 in two Japanese patients with neuroleptic malignant syndrome. J Neurol Sci. 1998;160(1):102–4. https://doi.org/10.1016/s0022-510x(98)00238-x .
Kawanishi C, Hanihara T, Maruyama Y, Matsumura T, Onishi H, Inoue K, et al. Neuroleptic malignant syndrome and hydroxylase gene mutations: no association with CYP2D6A or CYP2D6B. Psychiatr Genet. 1997;7(3):127–9. https://doi.org/10.1097/00041444-199723000-00007 .
Butwicka A, Krystyna S, Retka W, Wolańczyk T. Neuroleptic malignant syndrome in an adolescent with CYP2D6 deficiency. Eur J Pediatr. 2014;173(12):1639–42. https://doi.org/10.1007/s00431-013-2208-z .
Zivković M, Mihaljević-Peles A, Sagud M, Silić A, Mihanović M. The role of CYP2D6 and TaqI a polymorphisms in malignant neuroleptic syndrome: two case reports with three episodes. Psychiatr Danub. 2010;22(1):112–6.
Mihara K, Kondo T, Suzuki A, Yasui-Furukori N, Ono S, Sano A, et al. Relationship between functional dopamine D2 and D3 receptors gene polymorphisms and neuroleptic malignant syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;117b(1):57–60. https://doi.org/10.1002/ajmg.b.10025 .
Kishida I, Kawanishi C, Furuno T, Kato D, Ishigami T, Kosaka K. Association in Japanese patients between neuroleptic malignant syndrome and functional polymorphisms of the dopamine D(2) receptor gene. Mol Psychiatry. 2004;9(3):293–8. https://doi.org/10.1038/sj.mp.4001422 .
Kishida I, Kawanishi C, Furuno T, Matsumura T, Hasegawa H, Sugiyama N, et al. Lack of association in Japanese patients between neuroleptic malignant syndrome and the TaqI a polymorphism of the dopamine D2 receptor gene. Psychiatr Genet. 2003;13(1):55–7. https://doi.org/10.1097/00041444-200303000-00010 .
Ram A, Cao Q, Keck PE Jr., Pope HG Jr., Otani K, Addonizio G, et al. Structural change in dopamine D2 receptor gene in a patient with neuroleptic malignant syndrome. Am J Med Genet. 1995;60(3):228–30. https://doi.org/10.1002/ajmg.1320600311 .
Akihito Suzuki MD, Tsuyoshi Kondo PD,, MDPD, Koichi Otani MD,, PD, Kazuo Mihara MD,, PD, Norio, Yasui-Furukori MD,, PD, Sano A,, MDPD et al. Association of the TaqI A Polymorphism of the Dopamine D2 Receptor Gene With Predisposition to Neuroleptic Malignant Syndrome. 2001;158(10):1714-6. https://doi.org/10.1176/appi.ajp.158.10.1714 .
Matsusue A, Hara K, Kageura M, Kashiwagi M, Lu W, Ishigami A, et al. Genetic analysis of ryanodine receptor 1 gene and carnitine palmitoyltransferase II gene: an autopsy case of neuroleptic malignant syndrome related to vegetamin. Leg Med (Tokyo). 2009;11(Suppl 1):S570–2. https://doi.org/10.1016/j.legalmed.2009.01.074 .
Miyatake R, Iwahashi K, Matsushita M, Nakamura K, Suwaki H. No association between the neuroleptic malignant syndrome and mutations in the RYR1 gene associated malignant hyperthermia. J Neurol Sci. 1996;143(1–2):161–5. https://doi.org/10.1016/s0022-510x(96)00015-9 .
Sato T, Nishio H, Iwata M, Kentotsuboi, Tamura A, Miyazaki T, et al. Postmortem molecular screening for mutations in ryanodine receptor type 1 (RYR1) gene in psychiatric patients suspected of having died of neuroleptic malignant syndrome. Forensic Sci Int. 2010;194(1–3):77–9. https://doi.org/10.1016/j.forsciint.2009.10.014 .
Russell T, Riazi S, Kraeva N, Steel AC, Hawryluck LA. Ecstacy-induced delayed rhabdomyolysis and neuroleptic malignant syndrome in a patient with a novel variant in the ryanodine receptor type 1 gene. Anaesthesia. 2012;67(9):1021–4. https://doi.org/10.1111/j.1365-2044.2012.07226.x .
Kawanishi C, Hanihara T, Shimoda Y, Suzuki K, Sugiyama N, Onishi H, et al. Lack of association between neuroleptic malignant syndrome and polymorphisms in the 5-HT1A and 5-HT2A receptor genes. Am J Psychiatry. 1998;155(9):1275–7. https://doi.org/10.1176/ajp.155.9.1275 .
Kaneda Y, Kawamura I, Fujii A, Ohmori T. Serotonin syndrome - ‘potential’ role of the CYP2D6 genetic polymorphism in asians. Int J Neuropsychopharmacol. 2002;5(1):105–6. https://doi.org/10.1017/s1461145701002723 .
Calmy KIL, Ambrosioni A, Assouline J, Daali B, Fathi Y. Serotonin syndrome following drug-drug interactions and CYP2D6 and CYP2C19 genetic polymorphisms in an HIV-infected patient. Aids. 2012;26(18):2417–8. https://doi.org/10.1097/QAD.0b013e32835a11ba .
Agarwal P, Omoruyi A, Perai KG, MacDaid K, Burton A. Neuroleptic malignant syndrome (NMS) on clozapine with a potential atypical Interaction with Paliperidone. Case Rep Psychiatry. 2021;2021:5584104. https://doi.org/10.1155/2021/5584104 .
Mazhar F, Akram S, Haider N, Ahmed R. Overlapping of Serotonin Syndrome with neuroleptic malignant syndrome due to Linezolid-Fluoxetine and Olanzapine-Metoclopramide interactions: a Case Report of two serious adverse drug effects caused by Medication Reconciliation failure on Hospital Admission. Case Rep Med. 2016;2016:7128909. https://doi.org/10.1155/2016/7128909 .
Gillman PK. CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J Psychopharmacol. 2011;25(3):429–36. https://doi.org/10.1177/0269881109359098 .
Graber MA, Hoehns TB, Perry PJ. Sertraline-phenelzine drug interaction: a serotonin syndrome reaction. Ann Pharmacother. 1994;28(6):732–5. https://doi.org/10.1177/106002809402800610 .
Gnanadesigan N, Espinoza RT, Smith R, Israel M, Reuben DB. Interaction of serotonergic antidepressants and opioid analgesics: is serotonin syndrome going undetected? J Am Med Dir Assoc. 2005;6(4):265–9. https://doi.org/10.1016/j.jamda.2005.04.012 .
Ailawadhi S, Sung KW, Carlson LA, Baer MR. Serotonin syndrome caused by interaction between citalopram and fentanyl. J Clin Pharm Ther. 2007;32(2):199–202. https://doi.org/10.1111/j.1365-2710.2007.00813.x .
Chan BS, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG. Serotonin syndrome resulting from drug interactions. Med J Aust. 1998;169(10):523–5. https://doi.org/10.5694/j.1326-5377.1998.tb123399.x .
Clark DB, Andrus MR, Byrd DC. Drug interactions between linezolid and selective serotonin reuptake inhibitors: case report involving sertraline and review of the literature. Pharmacotherapy. 2006;26(2):269–76. https://doi.org/10.1592/phco.26.2.269 .
Bai AD, McKenna S, Wise H, Loeb M, Gill SS. Association of Linezolid with Risk of Serotonin Syndrome in patients receiving antidepressants. JAMA Netw Open. 2022;5(12):e2247426. https://doi.org/10.1001/jamanetworkopen.2022.47426 .
Karkow DC, Kauer JF, Ernst EJ. Incidence of Serotonin Syndrome with Combined Use of Linezolid and Serotonin Reuptake inhibitors compared with Linezolid Monotherapy. J Clin Psychopharmacol. 2017;37(5):518–23. https://doi.org/10.1097/jcp.0000000000000751 .
Gupta V, Karnik ND, Deshpande R, Patil MA. Linezolid-induced serotonin syndrome. BMJ Case Rep. 2013;2013. https://doi.org/10.1136/bcr-2012-008199 .
Mitwally H, Saad MO, Alkhiyami D, Fahmi AM, Mahmoud S, Hmoud EA, et al. Risk of serotonin syndrome in acutely ill patients receiving linezolid and opioids concomitantly: a retrospective cohort study. IJID Reg. 2022;5:137–40. https://doi.org/10.1016/j.ijregi.2022.09.008 .
Huang V, Gortney JS. Risk of serotonin syndrome with concomitant administration of linezolid and serotonin agonists. Pharmacotherapy. 2006;26(12):1784–93. https://doi.org/10.1592/phco.26.12.1784 .
Gatti M, Raschi E, De Ponti F. Serotonin syndrome by drug interactions with linezolid: clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2021;77(2):233–9. https://doi.org/10.1007/s00228-020-02990-1 .
Masbough F, Roshanzamiri S, Rahimi M, Sahraei Z, Evini PET. Serotonin syndrome due to concomitant use of linezolid and methadone. Clin Case Rep. 2022;10(11):e6341. https://doi.org/10.1002/ccr3.6341 .
Essakow J, Jin L, Marupudi N, Wattier R, McQuillen P, Franzon D. Serotonin Syndrome in an infant Associated with Linezolid and Opioid Use. J Pediatr Pharmacol Ther. 2022;27(6):564–8. https://doi.org/10.5863/1551-6776-27.6.564 .
Spigset O, Adielsson G. Combined serotonin syndrome and hyponatraemia caused by a citalopram-buspirone interaction. Int Clin Psychopharmacol. 1997;12(1):61–3. https://doi.org/10.1097/00004850-199701000-00010 .
Brubacher JR, Hoffman RS, Lurin MJ. Serotonin syndrome from venlafaxine-tranylcypromine interaction. Vet Hum Toxicol. 1996;38(5):358–61.
CAS PubMed Google Scholar
Nelson EM, Philbrick AM. Avoiding serotonin syndrome: the nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann Pharmacother. 2012;46(12):1712–6. https://doi.org/10.1345/aph.1Q748 .
Aboukarr A, Giudice M. Interaction between Monoamine oxidase B inhibitors and selective serotonin reuptake inhibitors. Can J Hosp Pharm. 2018;71(3):196–207.
Gray R, Moore Iii A, Berry F, Afroze F, Cherukupalli D. Serotonin Syndrome after PACU Administration of Tramadol and Meperidine. Turk J Anaesthesiol Reanim. 2022;50(4):309–11. https://doi.org/10.5152/tjar.2022.21355 .
Mateo-Carrasco H, Muñoz-Aguilera EM, García-Torrecillas JM, Abu Al-Robb H. Serotonin syndrome probably triggered by a morphine-phenelzine interaction. Pharmacotherapy. 2015;35(6):e102–5. https://doi.org/10.1002/phar.1581 .
Declercq PL, Eraldi JP, Beuzelin M, Gélinotte S, Marchalot A, Bougerol F, et al. Severe serotonin syndrome caused by an interaction between an antidepressant and a cough syrup. Therapie. 2021;76(3):249–52. https://doi.org/10.1016/j.therap.2020.02.020 .
Foong AL, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720–7.
Sun-Edelstein C, Tepper SJ, Shapiro RE. Drug-induced serotonin syndrome: a review. Expert Opin Drug Saf. 2008;7(5):587–96. https://doi.org/10.1517/14740338.7.5.587 .
Chan TC, Evans SD, Clark RF. Drug-induced hyperthermia. Crit Care Clin. 1997;13(4):785–808. https://doi.org/10.1016/s0749-0704(05)70369-9 .
Horseman M, Panahi L, Udeani G, Tenpas AS, Verduzco R Jr., Patel PH, et al. Drug-Induced Hyperth Rev Cureus. 2022;14(7):e27278. https://doi.org/10.7759/cureus.27278 .
Savvidou A, Jennions E, Wikström S, Olsson-Engman M, Sofou K, Darin N. Drug-induced hyperthermia with rhabdomyolysis in CLN3 disease. Eur J Paediatr Neurol. 2022;39:74–8. https://doi.org/10.1016/j.ejpn.2022.06.007 .
Gurrera RJ, Caroff SN, Cohen A, Carroll BT, DeRoos F, Francis A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222–8. https://doi.org/10.4088/JCP.10m06438 .
Gurrera RJ, Mortillaro G, Velamoor V, Caroff SN. A validation study of the International Consensus Diagnostic Criteria for neuroleptic malignant syndrome. J Clin Psychopharmacol. 2017;37(1):67–71. https://doi.org/10.1097/jcp.0000000000000640 .
Karamustafalioglu N, Kalelioglu T, Celikel G, Genc A, Emul M. Clinical utility of neutrophil-lymphocyte ratio in the diagnosis of neuroleptic malignant syndrome. Nord J Psychiatry. 2019;73(4–5):288–92. https://doi.org/10.1080/08039488.2019.1623315 .
Kalelioglu T, Celikel G, Balaban OD, Karamustafalioglu N, Penberthy JK. Can Neutrophil-Lymphocyte ratio be a useful Criterion for neuroleptic malignant syndrome in the absence of Leukocytosis? Iran J Psychiatry. 2021;16(3):370–3. https://doi.org/10.18502/ijps.v16i3.6264 .
Védie C, Poinso F, Hemmi F, Rivet B. Major symptoms and differential diagnosis of neuroleptic malignant syndrome: three case reports. Eur Psychiatry. 2000;15(5):334–7. https://doi.org/10.1016/s0924-9338(00)00403-x .
Uddin MF, Alweis R, Shah SR, Lateef N, Shahnawaz W, Ochani RK, et al. Controversies in serotonin syndrome diagnosis and management: a review. J Clin Diagn Res. 2017;11(9):Oe05–7. https://doi.org/10.7860/jcdr/2017/29473.10696 .
Jašović-Gašić M, Vuković O, Pantović M, Cvetić T, Marić-Bojović N. Antipsychotics–history of development and field of indication, new wine–old glassess. Psychiatr Danub. 2012;24(Suppl 3):S342–4.
Danilov DS. [A current view on the history of atypical antipsychotics]. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(5):85–93. https://doi.org/10.17116/jnevro20171175185-93 .
De Risio A, Lang AP. History and therapeutic rationale of long acting antipsychotics. Curr Clin Pharmacol. 2014;9(1):39–52. https://doi.org/10.2174/15748847113089990057 .
Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407–14. https://doi.org/10.1016/s0010-440x(99)90082-2 .
Ramachandraiah CT, Subramaniam N, Tancer M. The story of antipsychotics: past and present. Indian J Psychiatry. 2009;51(4):324–6. https://doi.org/10.4103/0019-5545.58304 .
Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA, Berrocoso E. Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discov. 2014;9(5):567–78. https://doi.org/10.1517/17460441.2014.907790 .
Margetić B, Aukst-Margetić B. Neuroleptic malignant syndrome and its controversies. Pharmacoepidemiol Drug Saf. 2010;19(5):429–35. https://doi.org/10.1002/pds.1937 .
Saha KB, Bo L, Zhao S, Xia J, Sampson S, Zaman RU. Chlorpromazine versus atypical antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2016;4(4):Cd010631. https://doi.org/10.1002/14651858.CD010631.pub2 .
Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370. https://doi.org/10.1155/2014/656370 .
Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. https://doi.org/10.1146/annurev-med-050911-161504 .
Talton CW. Serotonin Syndrome/Serotonin toxicity. Fed Pract. 2020;37(10):452–9. https://doi.org/10.12788/fp.0042 .
Sweileh WM, Sawalha AF, Al-Jabi SW, Zyoud SH, Shraim NY, Abu-Taha AS. A bibliometric analysis of literature on malaria vector resistance: (1996–2015). Globalization Health. 2016;12(1):76. https://doi.org/10.1186/s12992-016-0214-4 .
Zyoud SH, Shakhshir M, Abushanab AS, Al-Jabi SW, Koni A, Shahwan M, et al. Mapping the global research landscape on nutrition and the gut microbiota: visualization and bibliometric analysis. World J Gastroenterol. 2022;28(25):2981–93. https://doi.org/10.3748/wjg.v28.i25.2981 .
Sweileh WM. Bibliometric analysis of peer-reviewed literature on climate change and human health with an emphasis on infectious diseases. Globalization Health. 2020;16(1):44. https://doi.org/10.1186/s12992-020-00576-1 .
Sweileh WM. Global research trends of World Health Organization’s top eight emerging pathogens. Globalization Health. 2017;13(1):9. https://doi.org/10.1186/s12992-017-0233-9 .
Bilanakis N, Peritogiannis V, Kalampokis G. Infections as complications of neuroleptic malignant syndrome. World J Biol Psychiatry. 2009;10(4 Pt 3):973–6. https://doi.org/10.1080/15622970801935578 .
Taniguchi N, Tanii H, Nishikawa T, Miyamae Y, Shinozaki K, Inoue Y, et al. Classification system of complications in neuroleptic malignant syndrome. Methods Find Exp Clin Pharmacol. 1997;19(3):193–9.
Hegerl U, Bottlender R, Gallinat J, Kuss HJ, Ackenheil M, Möller HJ. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci. 1998;248(2):96–103. https://doi.org/10.1007/s004060050024 .
Modi S, Dharaiya D, Schultz L, Varelas P. Neuroleptic malignant syndrome: complications, outcomes, and Mortality. Neurocrit Care. 2016;24(1):97–103. https://doi.org/10.1007/s12028-015-0162-5 .
Park JI, Park TW. Rhabdomyolysis and Neuroleptic Malignant Syndrome Associated with very low-dose antipsychotics in children and adolescent. Clin Psychopharmacol Neurosci. 2019;17(3):450–2. https://doi.org/10.9758/cpn.2019.17.3.450 .
Desai S, Hirachan T, Toma A, Gerolemou A. Malignant Catatonia Versus neuroleptic malignant syndrome. Cureus. 2021;13(6):e15818. https://doi.org/10.7759/cureus.15818 .
Shouan A, Kumar R, Lal V, Grover S. Linezolid-induced serotonin syndrome. Ind Psychiatry J. 2020;29(2):345–8. https://doi.org/10.4103/ipj.ipj_37_19 .
Keltner N, Harris CP. Serotonin syndrome: a case of fatal SSRI/MAOI interaction. Perspect Psychiatr Care. 1994;30(4):26–31. https://doi.org/10.1111/j.1744-6163.1994.tb00446.x .
Park SH, Wackernah RC, Stimmel GL. Serotonin syndrome: is it a reason to avoid the use of tramadol with antidepressants? J Pharm Pract. 2014;27(1):71–8. https://doi.org/10.1177/0897190013504957 .
Beakley BD, Kaye AM, Kaye AD, Tramadol. Pharmacology, Side effects, and Serotonin Syndrome: a review. Pain Physician. 2015;18(4):395–400.
Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382. e1-.e6 https://doi.org/10.1016/j.amjmed.2018.04.025 .
Ruiz de Villa A, Jones T, Lleshi A, Macahuachi M, Lamar K, Bazikian Y. Serotonin toxicity precipitated by Tramadol in the setting of polypharmacy: a case of Serotonin Syndrome. Cureus. 2021;13(11):e20059. https://doi.org/10.7759/cureus.20059 .
Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615–9. https://doi.org/10.1016/s0736-4679(98)00057-2 .
Deardorff OG, Khan T, Kulkarni G, Doisy R, Loehr C. Serotonin Syndrome: Prophylactic Treatment with Cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16br01966 .
Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021–2. https://doi.org/10.1056/nejm199410133311514 .
Baldo BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. 2021;95(8):2627–42. https://doi.org/10.1007/s00204-021-03068-2 .
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, An-Najah National University, Nablus, Palestine
Waleed M. Sweileh
You can also search for this author in PubMed Google Scholar
Contributions
W.S initiated the idea, did the analysis, writing, and submission.
Corresponding author
Correspondence to Waleed M. Sweileh .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that he has no financial or non-financial competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sweileh, W.M. Neuroleptic malignant syndrome and serotonin syndrome: a comparative bibliometric analysis. Orphanet J Rare Dis 19 , 221 (2024). https://doi.org/10.1186/s13023-024-03227-5
Download citation
Received : 20 April 2023
Accepted : 27 May 2024
Published : 02 June 2024
DOI : https://doi.org/10.1186/s13023-024-03227-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Serotonin syndrome
- Research publications, comparative analysis
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 07 June 2024
Cost-effectiveness and threshold analysis of deep brain stimulation vs. treatment-as-usual for treatment-resistant depression
- Katherine E. Kabotyanski ORCID: orcid.org/0000-0001-9003-1588 1 ,
- Ricardo A. Najera 2 ,
- Garrett P. Banks ORCID: orcid.org/0000-0001-6292-977X 1 ,
- Himanshu Sharma 1 ,
- Nicole R. Provenza ORCID: orcid.org/0000-0002-6952-5417 1 ,
- Benjamin Y. Hayden 1 ,
- Sanjay J. Mathew ORCID: orcid.org/0000-0002-2715-6641 3 &
- Sameer A. Sheth ORCID: orcid.org/0000-0001-8770-8965 1
Translational Psychiatry volume 14 , Article number: 243 ( 2024 ) Cite this article
Metrics details
- Scientific community
Treatment-resistant depression (TRD) affects approximately 2.8 million people in the U.S. with estimated annual healthcare costs of $43.8 billion. Deep brain stimulation (DBS) is currently an investigational intervention for TRD. We used a decision-analytic model to compare cost-effectiveness of DBS to treatment-as-usual (TAU) for TRD. Because this therapy is not FDA approved or in common use, our goal was to establish an effectiveness threshold that trials would need to demonstrate for this therapy to be cost-effective. Remission and complication rates were determined from review of relevant studies. We used published utility scores to reflect quality of life after treatment. Medicare reimbursement rates and health economics data were used to approximate costs. We performed Monte Carlo (MC) simulations and probabilistic sensitivity analyses to estimate incremental cost-effectiveness ratios (ICER; USD/quality-adjusted life year [QALY]) at a 5-year time horizon. Cost-effectiveness was defined using willingness-to-pay (WTP) thresholds of $100,000/QALY and $50,000/QALY for moderate and definitive cost-effectiveness, respectively. We included 274 patients across 16 studies from 2009–2021 who underwent DBS for TRD and had ≥12 months follow-up in our model inputs. From a healthcare sector perspective, DBS using non-rechargeable devices (DBS-pc) would require 55% and 85% remission, while DBS using rechargeable devices (DBS-rc) would require 11% and 19% remission for moderate and definitive cost-effectiveness, respectively. From a societal perspective, DBS-pc would require 35% and 46% remission, while DBS-rc would require 8% and 10% remission for moderate and definitive cost-effectiveness, respectively. DBS-pc will unlikely be cost-effective at any time horizon without transformative improvements in battery longevity. If remission rates ≥8–19% are achieved, DBS-rc will likely be more cost-effective than TAU for TRD, with further increasing cost-effectiveness beyond 5 years.
Introduction
Neuropsychiatric disorders are a major cause of morbidity and mortality worldwide, yet treatment options for many of these conditions are limited in their specificity and long-term efficacy. Major depressive disorder (MDD), in particular, has an estimated annual prevalence of 8.3% among US adults [ 1 ] and is the leading cause of disability as well as death from suicide, globally [ 2 , 3 ]. MDD is characterized by the presence of a persistently depressed mood and/or anhedonia, as well as a number of debilitating somatic and psychological symptoms [ 4 ]. Clinical severity is measured using a variety of questionnaires, including the Hamilton Depression Rating Scale (HDRS) [ 5 ] and the Montgomery-Åsberg Depression Rating Scale (MADRS) [ 6 ]. The treatment of MDD is complicated by its multifactorial nature, high degree of comorbidity, and phenotypic heterogeneity [ 7 ].
Conventional treatment for MDD consists of psychotherapy and pharmacotherapy, but a significant proportion (up to 30%) of patients fail to respond to these treatment modalities [ 8 ]. While the definition of treatment-resistant depression (TRD) has not been standardized [ 9 ], patients whose symptoms do not adequately improve after multiple treatment trials are classified as having TRD [ 10 , 11 ]. TRD is associated with increased health care costs, reduced quality of life (QoL), high rates of unemployment, and high suicidality [ 12 ]. The poor prognosis and limited recourse for patients with TRD demonstrates a need for the development of new therapeutic methods.
In the last decade, more targeted treatment approaches that modulate specific networks in the brain have emerged as promising therapeutic candidates for decreasing symptom burden and improving QoL in individuals with TRD. In some developed countries, additional treatment options for TRD now include transcranial magnetic stimulation (TMS), electroconvulsive therapy (ECT), and vagus nerve stimulation (VNS). These non-invasive (TMS, ECT) and invasive (VNS) neurmodulatory treatments have been shown to be highly effective and even reduce medical costs for TRD [ 13 , 14 , 15 ]. However, some patients with depression do not attain meaningful improvement even with these additional therapies. Deep brain stimulation (DBS) is a neurosurgical intervention that involves stereotactically implanting electrodes to deliver continuous, yet adjustable, electrical stimulation to specific anatomic targets in the brain. The procedure has gained FDA approval for movement disorders, epilepsy, and obsessive-compulsive disorder, but is still experimental for TRD. Several DBS targets for TRD have been studied, including the subcallosal cingulate (SCC) [ 16 , 17 , 18 , 19 , 20 , 21 ], nucleus accumbens (NAcc) [ 22 , 23 ], ventral capsule/ventral striatum (VC/VS) or anterior limb of the internal capsule (ALIC) [ 24 , 25 , 26 ], bed nucleus of the stria terminalis (BNST) [ 27 , 28 ], and medial forebrain bundle (MFB) [ 29 , 30 , 31 ]. Although open-label trials showed encouraging response rates (20–92%), two randomized-controlled trials (RCTs) [ 17 , 32 ] were aborted by their industry sponsors out of concern that they would fail to demonstrate effectiveness relative to sham stimulation. Developments in targeting strategies since these trials have demonstrated significant promise in more recent open-label studies [ 33 , 34 ] and a pivotal new industry-sponsored RCT is being planned with FDA breakthrough therapy designation [ 35 ].
As this therapy is further investigated, it is worth considering its potential position within the health economic marketplace. We therefore used existing literature to conduct a cost-effectiveness analysis (CEA) of DBS compared to treatment-as-usual (TAU) for TRD. This CEA is unique in that DBS for TRD is not yet approved or commonly utilized, so our analyses determined effectiveness thresholds that would need to be demonstrated by future trials in order for the therapy to be cost-effective should it receive FDA approval. Specifically, we calculated the remission rates necessary to achieve acceptable incremental cost-effectiveness ratios (ICER) relative to TAU and propose future improvements that could increase its cost-effectiveness.
Using a decision analytic model [ 36 , 37 ], we compared DBS to TAU, which was defined as maintenance antidepressant medication (with or without augmentation therapies) along with concomitant non-pharmacologic treatments (i.e. psychotherapy, TMS, and/or ECT). Our base case for the model is an adult (age 22–70) diagnosed with a severe major depressive episode (HDRS-17 ≥ 18 or MADRS > 22), who has received at least 2 years of TAU without treatment response. Since patients who are eligible for DBS represent a particularly severe form of TRD, we chose to follow more thorough criteria for treatment-resistance compared to the standard definition including: lack of antidepressant response to 1) at least 3 medications from 3 different classes, 2) an adequate course of psychotherapy (>6 weeks), and 3) an adequate trial (>6 bilateral sessions) of ECT. The time horizon is 5 years following DBS, as is typical for novel surgical cost-effectiveness analyses [ 38 , 39 ]. All model inputs were derived from a retrospective review of relevant literature.
Literature review – efficacy
A PubMed search to identify clinical trials establishing the efficacy of DBS for TRD was conducted using the following terms: “Deep Brain Stimulation” AND “Treatment Resistant” AND “Depression” AND “Trial”. The search was completed in March 2023. We selected studies with original patient data that had at least 1 year of follow-up with response, remission, and complication rates and excluded any studies that were not either patient-blinded, sham-controlled, or included target-optimization. Open-label trials with fewer than 3 patients were also excluded. Data collected from selected studies included study design, patient inclusion/exclusion criteria, sample size, patient sex, patient age, follow-up time, patient-level pre-operative HDRS-17 or MADRS scores, 12- and 24-month post-operative HDRS-17 or MADRS scores, response/remission criteria, response/remission rates, and complication rates.
We presumed that the probability of remission from TAU in our model patients would be particularly low, given the rigorous eligibility criteria for classification as extremely treatment-resistant and for consideration as a neurosurgical candidate. In the patient sample we analyzed, for example, the average duration of TRD was approximately 20 years without clinical benefit, making spontaneous remission highly unlikely. However, in an effort to make as rigorous a model as possible, we sought to include some non-zero probability in the TAU treatment arm. As such, we conducted a general search of the literature for longitudinal studies (≥5 years follow-up) focusing on outcomes of patients with TRD on TAU [ 40 , 41 , 42 ].
Complications
Serious adverse events were limited to those related to DBS and were divided into three categories based on management strategy: lead revision/replacement, implantable pulse generator (IPG) revision/replacement, and short-hospitalization (e.g., 3–7 days for infection, skin erosion, etc.). Only complications that significantly added to costs or detracted from effectiveness were considered for our model, so self-limited complications were not included. No complications were considered for the TAU arm as these would fall into the self-limited category and would not affect model outputs.
Effectiveness – the utility model
Utility is a quantitative measure of a patient’s subjective improvement in QoL and ranges from 1 (perfect health) to 0 (death). In cost-effectiveness analyses, effectiveness is calculated by multiplying the net gain in utility by the duration (in years). The product is reported in quality-adjusted life-years (QALYs), where 1 QALY equals 1 year in perfect health [ 43 ]. Because the HDRS and MADRS are not designed to specifically measure QoL, and due to the paucity of available QoL data in our selected studies, we employed a utility model. Using published utility values for remission and non-remission in TRD [ 44 ], remission status was converted to a utility value for each patient in our sample and averaged to reflect mean QoL after treatment. A separate disutility value from the literature was assigned to complications to approximate the negative impact of post-operative DBS complications on QoL [ 45 ].
We conducted our analysis from both healthcare sector and societal perspectives. The healthcare sector approach accounts for all monetary costs of healthcare associated with an intervention (DBS or TAU), regardless of who bears the cost: the third-party payer (i.e., Medicare), the hospital, or the patient (out-of-pocket expenses). It does not consider costs of transportation, patient and caregiver time, productivity loss, or non-monetary costs—all of which are instead included in the societal perspective. While the societal perspective was previously considered the gold standard for cost-effectiveness analyses, recommendations from the Second Panel on Cost-Effectiveness in Health and Medicine have moved to include analyses from two perspectives [ 46 ]. Additionally, we created separate models to account for the varying costs of DBS using non-rechargeable (DBS-pc) versus rechargeable (DBS-rc) devices. While there was considerable variability between specific protocols, most of our selected studies used DBS-pc at initial implantation and switched patients to DBS-rc at the first IPG replacement. To estimate cost-effectiveness with this combined approach, we ran additional analyses using costs of DBS-pc with one battery replacement for the first 18 months and DBS-rc for the following 42 months.
The aggregate cost of DBS was defined as a sum of the cost of one pre-operative assessment, one pre-operative magnetic resonance imaging (MRI), one pre- and one post-operative computed tomography (CT), one bilateral stereotactic DBS lead and neurostimulator device implantation procedure, fifty-four follow-up programming office visits, and any hospital (facility) fees or additional out-of-pocket expenses. Additionally, the 5-year cost for DBS-pc included three IPG replacements. Costs associated with TAU included costs of pharmacotherapy (e.g. selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], tricyclic antidepressants, monoamine oxidase inhibitors, atypical antipsychotics, esketamine), cognitive-behavioral therapy (CBT), and TMS or ECT with a wide range and standard error to account for variability in individual treatment plans. Under the assumption that most remitters with either DBS or TAU would remain on some form of pharmacological therapy while discontinuing additional neuromodulation therapies, we defined a separate variable for the cost of pharmacotherapy alone and designated it as an incremental cost each year after remission. All cost data, including costs associated with complications, were collected from the 2023 Centers for Medicare & Medicaid Services (CMS) Physician Fee Schedule (based on HCPCS/CPT codes) [ 47 ] and from published health economics literature [ 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ].
Decision analytic model
We created our model using TreeAge Pro Healthcare 2023 (TreeAge Software, Williamstown, MA). The model placed patients in one of two treatment arms: DBS or TAU. Patients undergoing DBS had the opportunity to become remitters at 1, 2, 3, 4, and 5 years. The 5-year time horizon was chosen to capture the longer-term trajectory of DBS treatment response in our patient sample, including an initial 6–12 month optimization phase, 1–3 years of varying response, and a period of follow-up beyond 3 years with relatively sustained remission rates (see Fig. 1 for the time to remission in our analyzed sample). When a DBS patient remitted, their subsequent incremental cost per year after surgery transitioned from the costs of TAU to the cost of pharmacotherapy alone. Those who did not respond to DBS within the first year remained on TAU, thus accumulating the costs of TAU for each year of non-remission in addition to the initial costs of DBS. On the other hand, patients on TAU alone were given 1 year to remit. This decision was made based on prior studies, which showed that ~80–90% of patients with MDD who recover with TAU do so within the first 2 years of initiating treatment [ 61 , 62 , 63 , 64 , 65 ], with earlier response (typically within the first 4 weeks of initial treatment) predicting positive treatment outcomes [ 66 , 67 ]. Previous prospective studies found that after 5 years, the probability of MDD recovery with each additional year is only between 2% and 15% [ 40 , 68 ]. In TRD patients, particularly the severely treatment-resistant population eligible for DBS, the likelihood of response to TAU is inevitably even lower with each passing year [ 41 , 69 ].

Shows the cumulative percentage of total patients in our sample that achieved remission over 5 years of follow-up. The majority (79.7%) of TRD patients who achieved remission with DBS did so within the first year. Only 1% of patients who achieved remission within 5 years of DBS did so after year 3.
For DBS patients, all post-operative complications throughout the 5-year treatment period were assigned prior to year 1 on the model for simplicity. By extension, costs and disutilities associated with complications were factored into final calculations once and did not accumulate over time. For both treatment arms, there was no incremental utility or disutility for continuing as a non-remitter, as the utility of non-remission was applied to those who failed to remit at the end of each treatment arm. Finally, mortality rate was not considered in our model given the relatively short time horizon and negligible added mortality risk of either treatment [ 70 , 71 , 72 , 73 , 74 , 75 ].
We analyzed our model using TreeAge Pro Healthcare 2023 (TreeAge Software, Williamstown, MA). To account for uncertainty and variability, model inputs were parametrized using pooled means and standard deviations, and a Monte Carlo (MC) simulation ( n = 100,000) was performed. We examined the primary model output, the incremental cost-effectiveness ratio (ICER), using a willingness-to-pay (WTP) threshold approach. The ICER ($/QALY) is calculated by dividing incremental cost – the difference in net cost ($) between two treatment arms – by incremental effectiveness – the difference in QALYs gained between two treatment arms. Based on current accepted definitions of cost-effectiveness, unequivocal cost-effectiveness was considered an ICER less than $0/QALY, definitive cost-effectiveness was achieved at less than $50,000/QALY, moderate cost-effectiveness between $50,000 and $100,000/QALY, and cost-ineffectiveness at greater than $100,000/QALY gained [ 76 ]. Results of the MC simulation were further analyzed using both probabilistic and deterministic sensitivity analyses. Finally, using one-way sensitivity analyses, we analyzed the minimum 1-year remission rate necessary to achieve cost-effectiveness at each WTP threshold.
Literature review
Our PubMed search yielded 76 initial results (see Fig. 2 for our PRISMA [ 77 ] flowchart). From the 16 studies selected for data collection (published 2009–2021), we found a total of 274 unique patients who underwent DBS for TRD [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. Six additional studies (three narrative reviews [ 25 , 78 , 79 ] and three systematic reviews and meta-analyses [ 80 , 81 , 82 ]) were used to cross-reference data and eliminate duplicates. Across all patients, mean baseline (pre-operative) MADRS and HDRS-17 scores were 34.09 (±5.11) and 23.68 (±3.96), respectively. Based on the established remission criteria of MADRS score ≤10 or HDRS-17 score ≤7, overall remission rate was 24.2% at 1 year (n = 260). Mean follow-up time was 33.17 (±26.65) months. Using observed-case analysis from studies that included long-term follow-up data, remission rates were 23.7% at 2 years (n = 177), 40% at 3 years (n = 60), 33.3% at 4 years (n = 39), and 36.7% at 5 years (n = 30). Comprehensive outcomes and complications data from our systematic review can be found in Table 1 .

Data added to the PRISMA template (from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6[7]:e1000097) under the terms of the Creative Commons Attribution License.
For an overview of our completed model, see Fig. 3 . All base case model inputs and distributions are included in Table 2 . The average 5-year cost of DBS-rc, which included one pre-operative office visit ($167.40), one pre-operative MRI ($160.63), one pre-operative and one post-operative CT ($81.34), electrodes and neurostimulator device ($2,731.31), IPG implant ($20,378.78), estimated hospital fees ($2,607.00), and post-operative follow-up ($3,397.59) was $29,524.05 (±$817.66). The average 5-year cost of DBS-pc, which included all of the same costs as DBS-rc plus 3 IPG replacements ($91,042.80), was $120,566.85 (±$817.66). The standard deviation represents the variability in hospital fees and number of follow-up programming visits required (other values in this calculation are fixed and based on Medicare reimbursement amounts). Estimated costs from complications were $27,066.84 (lead revision/replacement), $30,347.60 (IPG revision/replacement), and $13,035.00 (short hospitalization). Mean yearly cost of TAU, which was calculated as the average of either psychotherapy and medications alone ($7,721.84), psychotherapy and medications with ECT ($17,097.84) and psychotherapy and medications with ECT and TMS ($23,277.84) was $16,032.51 (±$7,832.53) from a healthcare sector perspective and $38,575.86 (±$5,722.14) from a societal perspective. The annual cost of pharmacotherapy alone was $1,576 (±$1,174.00). Using published utility scores [ 44 ], we included the following utility values in our model: 0.84 (±0.15) for remission, and 0.54 (±0.25) for non-remission.
Compares cost-effectiveness of DBS versus TAU for TRD over a 5-year time horizon. Variable definitions, probabilities, and individual payoff formulas at terminal branches were omitted for simplicity.
MC simulation
Based on the results of our MC simulation (n = 100,000), after 5 years DBS-pc was less cost-effective than TAU from both healthcare sector (ICER = $254,719.81/QALY) and societal (ICER = $178,949.98/QALY) perspectives. DBS-rc, however, was definitively more cost-effective than TAU from a healthcare sector perspective (ICER = $31,878.61/QALY) and unequivocally more cost-effective than TAU from a societal perspective (ICER = −$43,924.23/QALY). See Table 3 for a summary of cost-effectiveness rankings. A combined approach (DBS-pc switched to DBS-rc at first IPG replacement) was just shy of being moderately more cost-effective than TAU (ICER = $105,831.71/QALY) from the healthcare sector perspective and was definitively more cost-effective than TAU (ICER = $30,195.12/QALY) from the societal perspective.
Net cost for TAU over 5 years was approximately $79,500.00 from the healthcare sector perspective and $191,000.00 from the societal perspective. Net cost of DBS-pc over 5 years was $183,529.00 from the healthcare sector perspective and $264,039.00 from the societal perspective. Net cost of DBS-rc over 5 years was $92,549.00 from the healthcare sector perspective and $173,065.00 from the societal perspective. Net effectiveness of DBS over 5 years was 3.12 QALYs, compared to 2.71 QALYs for TAU. The incremental effectiveness of DBS was therefore 0.41 QALYs.
Sensitivity analyses
Using probabilistic sensitivity analysis, outputs from the MC simulation were plotted on cost-effectiveness acceptability curves (Fig. 4 ). MC simulation revealed that TAU was more cost-effective than DBS-pc (Fig. 4A ) in 100% (at $50,000/QALY) and 100% (at $100,000/QALY) of iterations from the healthcare sector perspective and in 100% (at $50,000/QALY) and 97% (at $100,000/QALY) of iterations from the societal perspective. Conversely, DBS-rc (Fig. 4B ) was more cost-effective than TAU in 72.8% (at $50,000/QALY) and 100% (at $100,000/QALY) of iterations from the healthcare sector perspective and in 100% (at $50,000/QALY) and 100% (at $100,000/QALY) of iterations from the societal perspective.

Show results from Monte Carlo simulation (n = 100,000) and probabilistic sensitivity analysis. For DBS-pc ( A ), the TAU (red) curves dominate the DBS (blue) curves. TAU is thus shown to be more cost-effective than DBS-pc in 100% of iterations from both the healthcare sector (dark red) and societal sector (light red) perspectives at $50,000/QALY. For DBS-rc ( B ), the DBS (blue) curves dominate the TAU (red) curves. DBS-rc is thus shown to be more cost-effective than TAU in 72.8% of iterations from the healthcare sector perspective (dark blue) and 100% of iterations from the societal sector perspective (light blue) at $50,000/QALY. The black vertical line marks the broadly-accepted WTP threshold of $50,000 per QALY.
Using 1-way sensitivity analyses, we determined the minimum 1-year remission rates needed to achieve acceptable ICERs at WTP thresholds of $50,000 (for definitive cost-effectiveness) and $100,000 (for moderate cost effectiveness) per QALY (Fig. 5 ). DBS-pc (Fig. 5A ) requires 55% remission for moderate cost-effectiveness and 85% remission for definitive cost-effectiveness from a healthcare sector perspective. From a societal perspective, DBS-pc requires 35% and 46% remission for moderate and definitive cost-effectiveness, respectively. DBS-rc (Fig. 5B ) requires 11% and 19% remission for moderate and definitive cost-effectiveness, respectively, from a healthcare sector perspective. From a societal perspective, DBS-rc requires 8% and 10% remission for moderate and definitive cost-effectiveness, respectively. A combined approach (DBS-pc switched to DBS-rc at first IPG replacement) requires 26% and 41% remission for moderate and definitive cost-effectiveness, respectively, from a healthcare sector perspective. From a societal perspective, DBS with this approach requires 16% and 21% remission for moderate and definitive cost-effectiveness, respectively.

Show minimum 1-year remission rates necessary for DBS for TRD to achieve acceptable incremental cost-effectiveness ratios (ICER) at accepted willingness-to-pay (WTP) thresholds of $50,000 (for moderate cost-effectiveness) and $100,000 (for definitive cost-effectiveness) per QALY. DBS-pc ( A ) requires 55% remission for moderate cost-effectiveness and 85% remission for definitive cost-effectiveness from a healthcare sector perspective (dark blue), and 35% remission for moderate cost-effectiveness and 46% remission for definitive cost-effectiveness from a societal perspective (light blue). DBS-rc ( B ) requires 11% remission for moderate cost-effectiveness and 19% remission for definitive cost-effectiveness from a healthcare sector perspective (dark blue), and 8% remission for moderate cost-effectiveness and 10% remission for definitive cost-effectiveness from a societal perspective (light blue).
Finally, deterministic sensitivity analyses were used to create tornado diagrams (Fig. 6 ) that illustrate the effects of varying each parametrized input in our decision analytic model on the overall ICER for non-rechargeable (Fig. 6A ) and rechargeable (Fig. 6B ) DBS for TRD, from both healthcare sector and societal perspectives. All costs, probabilities, and utilities were varied within sensitivity ranges 20% above and below mean values. For all four scenarios, the parameter (with sensitivity ranges) that had the greatest impact on ICER variance was utility of remission (0.67 to 0.99). Other parameters with the greatest impact for all four scenarios included: utility of non-remission (0.43 to 0.65), cost of DBS ($96,453.48 to $144,680.22 for non-rechargeable; $23,619.24 to $35,428.86 for rechargeable), probability of remission in year 1 (0.19 to 0.29), and cost of TAU ($12,826.01 to $19,239.01 for the healthcare sector perspective; $30,860.69 to $46,291.03 for the societal perspective). For utility of remission, cost of TAU, and probability of remission in year 1, lower values increased the ICER. Conversely, for utility of non-remission and cost of DBS, higher values increased the ICER.
Displays the effect of varying each parametrized input on the ICER for DBS with non-rechargeable ( A ) and rechargeable ( B ) devices from both the healthcare sector (top) and societal sector (bottom) perspectives. All costs, probabilities, and utilities were varied within a sensitivity range 20% above and below mean values. Red bars show the impact of an increase and blue bars show the impact of a decrease in the variable value on overall ICER.
Importantly, none of the parameters in any of the scenarios affected the ICER in a way that impacted the cost-effectiveness of DBS at a WTP threshold of $100 K. Only in the scenario of DBS-rc from the healthcare sector perspective, a utility of remission lower than 0.735 and a utility of non-remission above 0.648 would increase the ICER beyond a WTP threshold of $50 K. Based on previously published literature [ 44 ], the lower limit for utility of remission was 0.76, while the upper limit for utility of non-remission was 0.58, so these parameter values would be outside the expected range and are unlikely to impact our cost-effectiveness results. Overall, DBS-pc did not reach cost-effectiveness compared to TAU, while DBS-rc remained cost-effective compared to TAU (from both healthcare sector and societal perspectives) under a broad range of cost and effectiveness values.
This study, which to our knowledge is the first economic evaluation comparing DBS to TAU for TRD, shows that regardless of economic perspective, DBS-pc would not be more cost-effective than TAU. However, with a 1-year remission rate of at least 20%, DBS-rc would be more cost-effective than TAU for TRD, particularly when considering the large societal costs of TRD. With a mean 1-year remission of 24.2% in our sample, DBS-rc had a higher probability of being cost-effective, even despite having higher upfront costs compared to TAU from either economic perspective. From a healthcare sector perspective, under the broadly accepted WTP threshold of $50,000/QALY, DBS-rc had a 72.8% probability of being cost-effective after 5 years, reaching 100% probability of cost-effectiveness at a WTP of $100,000/QALY. From a societal perspective, DBS-rc had a 100% probability of being cost-effective after 5 years at both WTP thresholds.
As this is the first cost-effectiveness study of DBS for TRD, there are no directly comparable analyses, however the cost-effectiveness of DBS for non-psychiatric indications has been explored in several studies. In a cost-utility analysis of DBS vs long-term medical management for Parkinson’s disease, Dams et al. [ 83 ] found the average 5-year cost of treatment with DBS to be €67,374.12 (~$75,693.81) with an average 5-year incremental cost-utility ratio (ICUR) of €36,065.82/QALY (~$40,483.70/QALY) from a healthcare provider perspective, adapted to 2023 values. It is likely that the total DBS costs presented in that study are lower than those we used for DBS-pc because battery replacement costs were only included every 4 years, rather than every 1.5 years as in our study. DBS battery depletion is assumed to be quicker in TRD due to the higher amplitudes typically required for TRD than for Parkinson’s disease [ 84 ], but if battery life for non-rechargeable IPGs can be extended to 4 years or more, it is likely that the cost-effectiveness of DBS-pc would increase substantially.
Chan et al. [ 85 ] compared the cost-effectiveness of DBS vs. VNS for patients with refractory epilepsy and found the cost of DBS over 5 years to be €72,251 (~$81,129.93) with an expected effectiveness of 3.42 QALYs (5-year ICER = €21,126.02, ~$23,728.53) from a healthcare sector perspective. In this study, the authors also only accounted for costs of IPG replacement every 5 years (compared to 1.5 years in our study) and used response (defined as ≥50% reduction in seizure frequency) rather than remission status as the primary outcome, resulting in both lower costs and higher effectiveness for DBS-pc. As previously discussed, a longer battery life would improve the cost-effectiveness of DBS-pc for any indication. While we chose to employ remission as a more stringent outcome metric, it is likely that the threshold for cost-effectiveness of DBS for TRD would also be lower if response (defined as ≥50% reduction in symptom severity) was considered instead.
Growing evidence has suggested the potential of DBS for treating other highly debilitating and costly medical conditions such as chronic pain, obesity, and dementia, with several clinical trials now underway [ 86 ]. Mahajan et al. [ 38 ] examined the cost-effectiveness of DBS compared to laparoscopic Roux-en-Y gastric bypass (LRYGB) for obesity using a societal perspective and calculated the overall 5-year cost of DBS to be $29,951. While the study did not specify between rechargeable and non-rechargeable devices, this value is very similar to our average 5-year cost of $29,524.05 for DBS-rc.
DBS has been used off-label for a variety of other psychiatric conditions including Tourette’s, bipolar disorder, post-traumatic stress disorder, eating disorders, and substance use disorders, yet economic evaluations have been limited by low case numbers and lack of published clinical trial data. Taking a societal perspective and using Medicare reimbursement as a proxy for direct costs, Kuijper et al. [ 39 ] conducted a threshold and cost-effectiveness analysis of DBS compared to contingency management for cocaine use disorder. The 1-year cost of DBS-rc for cocaine use disorder was calculated to be $27,988.45, which is very close to our 1-year cost of DBS-rc at $28,071.09.
Finally, cost-effectiveness analyses of DBS vs TAU for treatment-resistant obsessive-compulsive disorder (TROCD) offer perhaps the most relevant comparison for our findings. Separate studies from Ooms et al. [ 87 ] and Moon et al. [ 88 ] both compared the cost-effectiveness of DBS vs TAU for patients with TROCD. While each of these models were applied to countries outside of the United States (the Netherlands, Korea, and the U.K.) and used different time horizons (4, 10, and 2 years) as well as different effect measures (area under the curve analysis and response status) than our study, their results are nonetheless informative and allow for a reasonable comparison of direct and indirect costs. Ooms et al. [ 87 ]. found the cost of DBS-pc (including battery changes every 14 months) over 4 years to be €127,112.74 from a societal perspective. Moon et al. [ 88 ] found the cost of DBS-pc over 10 years (including battery replacements every 3 years) to be $44,672 in Korea and $42,322 in the U.K., from a healthcare payer perspective. In the former study, the costs for DBS-pc are very close to those in our study, likely due to a similar frequency of battery replacements. In the latter study, a lower frequency (every 3 years) and substantially lower cost ($2,232 in Korea and $760 in the U.K.) of battery replacements account for the overall lower costs of DBS-pc compared to our findings. The longer time horizon of 10 years in the Moon et al. [ 88 ] study highlights the increasing cost-effectiveness of DBS over time relative to TAU. Unlike Parkinson’s patients, those receiving DBS for psychiatric conditions are relatively young with fewer surgical risk factors [ 72 , 89 , 90 ] and therefore have a longer time horizon to benefit from DBS treatment. Extending the time horizon for economic analyses of DBS for psychiatric indications would better capture the true cost-effectiveness of this intervention. Relatedly, the area under the curve (AUC) approach for measuring antidepressant effect over time in the Ooms et al. [ 87 ] study may better capture overall symptom improvement throughout the course of treatment and is likely more representative of the patient’s subjective gain in QoL [ 31 ]. While our selected studies lacked sufficient patient-level QoL data for this approach, this method of calculating QALYs would improve future economic evaluations and underscores the need to include QoL measures in clinical trials.
Considering the lack of consistency in reported costs for DBS across multiple indications, our study highlights the need for greater public access to healthcare economics data. Though societal costs are highly variable and difficult to estimate, and exact healthcare costs may not be readily accessible in a complex system such as that in the U.S., accurate cost data are nonetheless imperative for future cost-effectiveness analyses to capture the full economic burden of disease. Ideally, in addition to safety and efficacy data, large prospective trials would collect cost and utility data across multiple institutions to account for cost differences. For DBS, in particular, it is important for studies to also provide patient-level data on specific stimulation targets and parameters, as well as effects on battery life to most accurately compare costs and efficacy across studies. This approach would enable more generalizable conclusions, and results of these studies would influence medical decision making not only at the physician and patient levels, but also for hospital administrators, insurance companies, and government healthcare programs such as Medicare and Medicaid [ 91 ]. Despite promising preliminary results for multiple conditions, public acceptance of and insurance coverage for surgical treatment of psychiatric illness is still disproportionately lower than for other non-psychiatric indications [ 92 , 93 , 94 ]. As the field of psychiatric neurosurgery develops, there is a growing need for high-quality cost-effectiveness analyses.
It is important to remember that TRD patients who meet eligibility criteria for DBS are a smaller percentage of all patients with TRD, as they must have also failed to respond to additional treatments such as augmentation strategies with ketamine and non-invasive neuromodulation. Furthermore, considering the high incidence of treatment-resistance in MDD and the low probability of response to TAU after 3 years in this population, it is possible that the criteria for surgical treatment of TRD are overly stringent in terms of disease duration. At least 5 years since MDD onset is commonly required for DBS eligibility, but there is evidence that it would be reasonable to enroll patients sooner, as long as they have significant symptom severity and have proven to be treatment-refractory (multiple failed SSRIs, adjunctive medication trials, CBT, and either TMS or ECT). Multiple studies have found that longer duration of untreated disease is a major prognostic factor for poor treatment response and worse long-term outcomes [ 95 , 96 , 97 , 98 ]. Compared to MDD, TRD patients utilize twice the number of outpatient healthcare resources, triple the number of inpatient stays, and have 23% higher all-cause mortality [ 99 ]. Additionally, rates of intentional self-harm and suicide are significantly higher in TRD [ 100 ], further increasing the risk of negative outcomes in these refractory patients while they wait to fulfill DBS eligibility criteria. Thus, continued TAU may unnecessarily prolong suffering with severe disease and simultaneously increase cost burden to patients, caregivers, healthcare systems, and society at large. Given these results, earlier treatment of TRD with DBS may be both clinically and economically more effective.
With rising incidence and high rates of untreated depression [ 101 , 102 ], the economic impact of TRD on society at large cannot be overstated. In addition to decreased quality of life, higher healthcare resource utilization, and increased all-cause mortality for patients themselves [ 12 ], TRD also has a wide sphere of impact on others including family members, caregivers, and employers [ 103 ]. Mrazek et al. [ 49 ]. found that the societal burden of major depressive disorder for the United States in 2012 was $188 billion-- $57 billion dollars more than the societal cost of cancer and $15 billion dollars more than that of diabetes. Providing new cost-effective treatment options to patients with TRD would provide substantial benefit in diminishing not only their TRD-related healthcare costs, but also other non-TRD healthcare costs, while also allowing them to return to work and reengage in society in meaningful ways. According to society preferences, willingness-to-pay is higher for patients with a higher level of disease, younger age, and larger QOL gains [ 104 ]. Since TRD patients have a high disease burden, are relatively young in age, and benefit from substantial increases in QOL with remission, raising WTP thresholds in accordance to society preferences would make DBS for TRD more cost-effective even with higher total costs and lower remission rates. As new treatments for highly prevalent and debilitating conditions like TRD become available and their safety is established, the economic threshold for their approval should be adjusted in the broader context of societal impact.
Several important limitations apply to this study. One limitation was the high variability of TAU costs between patients. These costs vary widely depending on the specific combination of medications, psychotherapy, TMS, and ECT that any given patient may be using prior to DBS, as well as on the high variability in costs attributed to transportation, lost productivity, and healthcare utilization unrelated to depression for each patient. To account for this variability, we included very large standard deviations for these parameters.
Second, we chose to only include remission and non-remission as outcomes, as response rates likely do not accurately reflect meaningful patient outcomes due to the arbitrary nature of a 50% reduction criterium and high rates of return to non-response status [ 31 ]. Further, it is unlikely that a single utility value could accurately represent the range in QoL changes associated with varying levels of treatment response. For refractory illnesses, such as TRD, it is likely that even small sub-threshold reductions in symptoms could still provide considerable improvement in QoL. As such, response status may likely underestimate effectiveness and therefore inflate ICERs.
Additionally, we did not include relapse as a possible outcome in our model, though up to 80% of TRD patients experience relapse within 5 years of response despite continued maintenance treatment [ 63 , 105 , 106 , 107 ]. Remission, on the other hand, is the ideal outcome of depression treatment with a low risk of relapse [ 108 , 109 , 110 ]. Thus, we assumed that remitters in either treatment arm would remain remitters for the duration of the model. This was supported by previous literature and the data reported in our selected studies. Although most patients in our sample who achieved remission with DBS did so by year 3, we still included non-zero probability for remission with DBS in years 4 and 5, the same as was used to represent a non-zero probability of remission with TAU. This small but non-zero value reflects stability of remission status in either treatment arm. Nevertheless, it is possible that some patients, particularly those with residual symptoms, would relapse despite remission [ 111 , 112 ]. Due to a lack of longitudinal data on residual symptoms or utility values associates with these symptoms, it is impossible to include these variables in this analysis. While considerably more complex, a Markov model that accounts for patient response, relapse, and remission at each year of the decision tree could more fully account for every possible outcome in depression treatment and would allow for a more thorough analysis. At this time, a paucity of detailed individual patient-level data reflecting the transitions between these outcomes limits the feasibility of accurately using such an approach.
Third, our utility model is based on prior prospective cohort studies and cost-effectiveness analyses that collected direct measures of health-related quality-of-life (HRQoL) scores and health utilities. Thus, utilities associated with remission status and disutilities associated with complications are indirect derivations for our cohort and should be interpreted as approximations of true utility. Importantly, two main sources from which utility values in these studies were derived are based on MDD [ 113 , 114 ]. For TRD, the transition from non-remission to remission is likely associated with an even greater improvement in health utility, so our utility values are likely conservative. Further, we assumed that non-remission in this context did not necessarily mean non-response, so we included a wider range of values for our estimate—from severe depression (~0.2–0.55) on the lower end to response without remission (0.67–0.72) on the higher end [ 44 ]. As there is no prior literature defining the disutility of DBS complications in TRD specifically, we assumed that the disutility of complications related to DBS would be similar across psychiatric disorders. Clinical trials for TRD should move to include direct measures of QoL (e.g., EQ-5D, SF-36, etc.) in addition to standard efficacy measures (e.g., HDRS, MADRS, etc.) to assess for these potential differences between indications and ensure that future health economics studies have sufficient data to support more generalizable claims of cost-effectiveness.
Our 5-year time horizon restricts complications to those occurring up to 60 months after surgery. In our aggregate sample, this captured greater than 99% of all complications. Previous work has found that the majority of DBS-related complications occur within the first 5 years and complication rates with DBS do not increase in the long-term [ 115 , 116 ]. While recent reports have published outcomes of DBS for TRD for up to 8 years of follow-up, we found that available data were still insufficient to carry the model past 5 years without adding significant uncertainty. Additionally, there was significant variation in the timepoint of the last observation (range = 0.50–142.92 months) where such an analysis would inappropriately represent the efficacy of DBS treatment and the confidence interval would be much too large for meaningful interpretation. As more data become available, it will be important to assess cost-effectiveness with this additional efficacy and complication information in the long-term. In our study, we assumed a non-staged approach for initial implantation and that all complications occurred after initial implantation for model simplicity. Although we did not include the probability of additional post-operative complications for each IPG replacement, it is important to note that non-rechargeable devices incur extra costs, not only for the new IPG itself, but also for the associated surgical procedure, hospitalization, and any post-operative complications, such as infection or hemorrhage, which further increase the incremental cost of DBS-pc each year.
Finally, though there is a well-documented risk of suicidality in TRD patients, this outcome was not included in our model for several reasons. First, estimates of the monetary cost or specific disutility associated with either suicidal ideation or suicide attempt (e.g., emergency psychiatric hospitalization, medication changes, intensive inpatient therapy, various medical and neurologic sequelae, etc.) are limited by lack of available data. Second, while 11% (n = 29) of patients in our DBS sample experienced suicidality and 4% (n = 12) completed suicide, there is no evidence to support that these adverse events were directly related to DBS. Suicide is unfortunately common in TRD, regardless of treatment approach, so it was assumed that any additional costs incurred due to suicide would be the same for each treatment arm and would therefore not affect the model.
While the efficacy of DBS for TRD has not yet been definitively established, prior studies report remission rates of approximately 25–45% [ 20 , 21 , 26 , 32 , 80 , 81 , 107 , 117 , 118 , 119 , 120 , 121 ]. From our analyses, DBS-pc is unlikely to be cost-effective compared to TAU even at remission rates at the higher end of this range. DBS-rc, however, would only need remission rates in the 10–20% range to reach cost-effectiveness compared to TAU. Based on outcomes from 274 patients with TRD across 16 studies showing an average 1-year remission rate of 24.2%, we found that DBS with a rechargeable device would be more cost-effective than treatment as usual for treatment-resistant depression, under a range of possible cost and effectiveness values.
DBS with rechargeable devices is particularly well suited for psychiatric indications. Patients report increased satisfaction with rechargeable IPGs [ 122 ], psychiatric indications require high amplitude stimulation settings for treatment compared to other DBS conditions and therefore more quickly deplete battery life [ 84 ], and the longer time horizon of treatment in these relatively young patients allows for the extended benefits of DBS to outweigh high upfront costs. Our analyses show that an approach of initial implantation with a non-rechargeable device and subsequent transition to a rechargeable IPG in patients who experience response at the time of first battery replacement would reach both moderate and definitive cost-effectiveness at 5 years, with increasing cost-effectiveness each following year.
Our results demonstrate that, using current estimates of treatment efficacy, DBS with rechargeable devices for TRD already represents a cost-effective approach compared to current treatments. As DBS strategies continue to improve, so will justification for its use as an effective treatment for patients with TRD.
Major Depression [Internet]. National Institute of Mental Health (NIMH). Available from: https://www.nimh.nih.gov/health/statistics/major-depression
Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517.
PubMed Google Scholar
World Health Organization. Mental and behavioural disorders team. Preventing suicide: a resource for primary health care workers [Internet]. World Health Organization; 2000. Report No.: WHO/MNH/MBD/00.4. Available from: https://apps.who.int/iris/handle/10665/67603
American psychiatric association and american psychiatric association - 2013 diagnostic and statistical manual of mental disorders (2013).
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
CAS PubMed PubMed Central Google Scholar
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
CAS PubMed Google Scholar
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:1–20.
Google Scholar
Voineskos D, Daskalakis ZJ, Blumberger DM. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. 2020;16:221–34.
Keller MB. Issues in treatment-resistant depression. J Clin Psychiatry. 2005;66 Suppl 8:5–12.
Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88.
PubMed PubMed Central Google Scholar
Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–59.
Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210.
Noda Y, Miyashita C, Komatsu Y, Kito S, Mimura M. Cost-effectiveness analysis comparing repetitive transcranial magnetic stimulation therapy with antidepressant treatment in patients with treatment-resistant depression in Japan. Psychiatry Res. 2023;330:115573.
Fitzgibbon KP, Plett D, Chan BCF, Hancock-Howard R, Coyte PC, Blumberger DM. Cost–utility analysis of electroconvulsive therapy and repetitive transcranial magnetic stimulation for treatment-resistant depression in Ontario. Can J Psychiatry. 2020;65:164–73.
Cohen LJ, Allen JC Jr. Estimating the potential savings with vagus nerve stimulation for treatment-resistant depression: a payer perspective*. Curr Med Res Opin. 2008;24:2203–17.
Ramasubbu R, Clark DL, Golding S, Dobson KS, Mackie A, Haffenden A, et al. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. Lancet Psychiatry. 2020;7:29–40.
Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, et al. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. AJP. 2019;176:949–56.
Merkl A, Aust S, Schneider GH, Visser-Vandewalle V, Horn A, Kühn AA, et al. Deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a double-blinded randomized controlled study and long-term follow-up in eight patients. J Affect Disord. 2018;227:521–9.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49.
Puigdemont D, Pérez-Egea R, Portella MJ, Molet J, de Diego-Adeliño J, Gironell A, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol. 2012;15:121–33.
Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–10.
Millet B, Jaafari N, Polosan M, Baup N, Giordana B, Haegelen C, et al. Limbic versus cognitive target for deep brain stimulation in treatment-resistant depression: accumbens more promising than caudate. Eur Neuropsychopharmacol. 2014;24:1229–39.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacol. 2012;37:1975–85.
CAS Google Scholar
Hitti FL, Cristancho MA, Yang AI, O’Reardon JP, Bhati MT, Baltuch GH. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression: a decade of clinical follow-up. J Clin Psychiatry. 2021;82:37487.
van der Wal JM, Bergfeld IO, Lok A, Mantione M, Figee M, Notten P, et al. Long-term deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. J Neurol Neurosurg Psychiatry. 2020;91:189–95.
Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75.
Fitzgerald PB, Segrave R, Richardson KE, Knox LA, Herring S, Daskalakis ZJ, et al. A pilot study of bed nucleus of the stria terminalis deep brain stimulation in treatment-resistant depression. Brain Stimul. 2018;11:921–8.
Raymaekers S, Luyten L, Bervoets C, Gabriëls L, Nuttin B. Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and long-term follow-up. Transl Psychiatry. 2017;7:e1251–e1251.
Coenen VA, Bewernick BH, Kayser S, Kilian H, Boström J, Greschus S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacol. 2019;44:1224–32.
Fenoy AJ, Schulz PE, Selvaraj S, Burrows CL, Zunta-Soares G, Durkin K, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8:1–11.
Bewernick BH, Kayser S, Gippert SM, Switala C, Coenen VA, Schlaepfer TE. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimul. 2017;10:664–71.
Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78:240–8.
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23:843–9.
Riva-Posse P, Crowell AL, Wright K, Waters AC, Choi K, Garlow SJ, et al. Rapid antidepressant effects of deep brain stimulation and their relation to surgical protocol. Biol Psychiatry. 2020;88:e37–9.
Abbott receives FDA’s breakthrough device designation to explore use of deep brain stimulation to manage severe depression [internet]. abbott media room. Available from: https://abbott.mediaroom.com/2022-07-12-Abbott-Receives-FDAs-Breakthrough-Device-Designation-to-Explore-Use-of-Deep-Brain-Stimulation-to-Manage-Severe-Depression
Grutters JPC, Joore MA, van der Horst F, Stokroos RJ, Anteunis LJC. Decision-analytic modeling to assist decision making in organizational innovation: the case of shared care in hearing aid provision. Health Serv Res. 2008;43:1662–73.
Najera RA, Gregory ST, Shofty B, Anand A, Gadot R, Youngerman BE, et al. Cost-effectiveness analysis of radiosurgical capsulotomy versus treatment as usual for treatment-resistant obsessive-compulsive disorder. J Neurosurg. 2022;138:347–57.
Mahajan UV, Ojukwu DI, Azagury DE, Safer DL, Cunningham T, Halpern CH. Can responsive deep brain stimulation be a cost-effective treatment for severe obesity? Obes (Silver Spring). 2022;30:338–46.
Kuijper FM, Mahajan UV, Ku S, Barbosa DAN, Alessi SM, Stein SC, et al. Deep brain stimulation compared with contingency management for the treatment of cocaine use disorders: a threshold and cost-effectiveness analysis. Neuromodulation. 2022;25:253–62.
Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RMA, et al. Time to recovery, chronicity, and levels of psychopathology in major depression: a 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–16.
Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. AJP. 2017;174:640–8.
Gronemann FH, Jorgensen MB, Nordentoft M, Andersen PK, Osler M. Incidence of, risk factors for, and changes over time in treatment-resistant depression in denmark: a register-based cohort study. J Clin Psychiatry. 2018;79:21247.
Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–8.
Hannah LA, Walsh CM, Jopling L, Perez J, Cardinal RN, Cameron RA Economic evaluation of interventions for treatment-resistant depression: a systematic review. Front Psychiatry. 2023;14:1056210.
Kumar KK, Appelboom G, Lamsam L, Caplan AL, Williams NR, Bhati MT, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90:469–73.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093.
CMS 2019 medicare physician fee schedule [Internet]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files-Items/RVU19A
Sussman M, O’sullivan AK, Shah A, Olfson M, Menzin J. Economic burden of treatment-resistant depression on the U.S. health care system. J Manag Care Spec Pharm. 2019;25:823–35.
Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65:977–87.
Ross EL, Zivin K, Maixner DF. Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry. 2018;75:713–22.
Voigt J, Carpenter L, Leuchter A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients – a lifetime analysis. PLOS ONE. 2017;12:e0186950.
Amos TB, Tandon N, Lefebvre P, Pilon D, Kamstra RL, Pivneva I, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79:5360.
Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of medicaid beneficiaries with treatment-resistant depression. JMCP. 2018;24:226–36.
Olchanski N, McInnis Myers M, Halseth M, Cyr PL, Bockstedt L, Goss TF, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35:512–22.
Taneja C, Papakostas GI, Jing Y, Baker RA, Forbes RA, Oster G. Cost-effectiveness of adjunctive therapy with atypical antipsychotics for acute treatment of major depressive disorder. annals of pharmacotherapy. 2012;46:642–49.
Ivanova JI, Birnbaum HG, Kidolezi Y, Subramanian G, Khan SA, Stensland MD. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26:2475–84.
Teresa B, Gibson P, Yonghua Jing P, Ginger Smith Carls P, Edward Kim MD, Erin Bagalman J, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16:370–7.
Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65:341–7.
Corey-Lisle PK, Birnbaum HG, Greenberg PE, Claxton AJ. Identification of a claims data. J Clin Psychiatry. 2002;63:8441.
Russell WHCJM. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:8690.
Keller MB, Shapiro RW, Lavori PW, Wolfe N. Recovery in major depressive disorder: analysis with the life table and regression models. Arch Gen Psychiatry. 1982;39:905–10.
Kennedy N, Abbott R, Paykel ES. Longitudinal syndromal and sub-syndromal symptoms after severe depression: 10-year follow-up study. Br J Psychiatry. 2004;184:330–6.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. AJP. 2006;163:1905–17.
Verduijn J, Verhoeven JE, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman ATF, et al. Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: full recovery is the exception rather than the rule. BMC Med. 2017;15:215.
Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D. CNS Drugs. 2009;23:627–47.
Beard JIL, Delgadillo J. Early response to psychological therapy as a predictor of depression and anxiety treatment outcomes: a systematic review and meta-analysis. Depress Anxiety. 2019;36:866–78.
Henkel V, Seemüller F, Obermeier M, Adli M, Bauer M, Mundt C, et al. Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115:439–49.
Mueller TI, Keller MB, Leon AC, Solomon DA, Shea MT, Coryell W, et al. Recovery after 5 years of unremitting major depressive disorder. Arch Gen Psychiatry. 1996;53:794–9.
Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67:15790.
Kennedy SH, Lam RW. Enhancing outcomes in the management of treatment resistant depression: a focus on atypical antipsychotics. Bipolar Disord. 2003;5:36–47.
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16.
Saleh C, Fontaine D. Deep brain stimulation for psychiatric diseases: what are the risks? Curr Psychiatry Rep. 2015;17:33.
Tørring N, Sanghani SN, Petrides G, Kellner CH, Østergaard SD. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135:388–97.
Watts BV, Peltzman T, Shiner B. Mortality after electroconvulsive therapy. Br J Psychiatry. 2021;219:588–93.
Voges J, Waerzeggers Y, Maarouf M, Lehrke R, Koulousakis A, Lenartz D, et al. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery—experiences from a single centre. J Neurol Neurosurg Psychiatry. 2006;77:868–72.
ICER_2020_2023_VAF_013120-4-2.pdf [Internet]. Available from: https://icer.org/wp-content/uploads/2021/03/ICER_2020_2023_VAF_013120-4-2.pdf
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Roet M, Boonstra J, Sahin E, Mulders AEP, Leentjens AFG, Jahanshahi A. Deep brain stimulation for treatment-resistant depression: towards a more personalized treatment approach. J Clin Med. 2020;9:2729.
Dandekar MP, Diaz AP, Rahman Z, Silva RH, Nahas Z, Aaronson S, et al. A narrative review on invasive brain stimulation for treatment-resistant depression. Braz J Psychiatry. 44:317–30.
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:224–32.
Wu Y, Mo J, Sui L, Zhang J, Hu W, Zhang C, et al. Deep brain stimulation in treatment-resistant depression: a systematic review and meta-analysis on efficacy and safety. Front Neurosci. 2021;15:655412.
Hitti FL, Yang AI, Cristancho MA, Baltuch GH. Deep brain stimulation is effective for treatment-resistant depression: a meta-analysis and meta-regression. J Clin Med. 2020;9:2796.
Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, et al. Cost-effectiveness of deep brain stimulation in patients with Parkinson’s disease. Mov Disord. 2013;28:763–71.
van Riesen C, Tsironis G, Gruber D, Klostermann F, Krause P, Schneider GH, et al. Disease-specific longevity of impulse generators in deep brain stimulation and review of the literature. J Neural Transm. 2016;123:621–30.
Chan HY, Wijnen BFM, Majoie MHJM, Evers SMAA, Hiligsmann M. Economic evaluation of deep brain stimulation compared with vagus nerve stimulation and usual care for patients with refractory epilepsy: a lifetime decision analytic model. Epilepsia. 2022;63:641–51.
Harmsen IE, Elias GJB, Beyn ME, Boutet A, Pancholi A, Germann J, et al. Clinical trials for deep brain stimulation: current state of affairs. Brain Stimul. 2020;13:378–85.
Ooms P, Blankers M, Figee M, Bergfeld IO, van den Munckhof P, Schuurman PR, et al. Cost-effectiveness of deep brain stimulation versus treatment as usual for obsessive-compulsive disorder. Brain Stimul. 2017;10:836–42.
Moon W, Kim SN, Park S, Paek SH, Kwon JS. The cost-effectiveness of deep brain stimulation for patients with treatment-resistant obsessive-compulsive disorder. Med (Baltim). 2017;96:e7397.
Alonso P, Cuadras D, Gabriëls L, Denys D, Goodman W, Greenberg BD, et al. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLOS One. 2015;10:e0133591.
Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: a meta-analysis of ten years’ experience. Mov Disord. 2007;22:1722–8.
Kim DD, Basu A. How does cost-effectiveness analysis inform health care decisions? AMA J Ethics. 2021;23:639–47.
Hitti FL, Widge AS, Riva-Posse P, Malone DA, Okun MS, Shanechi MM, et al. Future directions in psychiatric neurosurgery: proceedings of the 2022 American Society for Stereotactic and Functional Neurosurgery meeting on surgical neuromodulation for psychiatric disorders. Brain Stimul. 2023;16:867–78.
Davis RA, Giordano J, Hufford DB, Sheth SA, Warnke P, Widge AS, et al. Restriction of access to deep brain stimulation for refractory OCD: failure to apply the federal parity act. Front Psychiatry. 2021;12:706181.
Visser-Vandewalle V, Andrade P, Mosley PE, Greenberg BD, Schuurman R, McLaughlin NC, et al. Deep brain stimulation for obsessive-compulsive disorder: a crisis of access. Nat Med. 2022;28:1529–32.
Ghio L, Gotelli S, Marcenaro M, Amore M, Natta W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J Affect Disord. 2014;152-4:45–51.
Hung CI, Liu CY, Yang CH. Untreated duration predicted the severity of depression at the two-year follow-up point. PLOS One. 2017;12:e0185119.
Kautzky A, Dold M, Bartova L, Spies M, Kranz GS, Souery D, et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr Scand. 2019;139:78–88.
Kraus C, Kadriu B, Lanzenberger R, Zarate CA Jr., Kasper S. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 2019;9:1–17.
Lundberg J, Cars T, Lööv SÅ, Söderling J, Sundström J, Tiihonen J, et al. Association of treatment-resistant depression with patient outcomes and health care resource utilization in a population-wide study. JAMA Psychiatry. 2023;80:167–75.
Gronemann FH, Jørgensen MB, Nordentoft M, Andersen PK, Osler M. Treatment-resistant depression and risk of all-cause mortality and suicidality in Danish patients with major depression. J Psychiatr Res. 2021;135:197–202.
Goodwin RD, Dierker LC, Wu M, Galea S, Hoven CW, Weinberger AH. Trends in U.S. depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63:726–33.
Moreno-Agostino D, Wu YT, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression: a systematic review and meta-analysis. J Affect Disord. 2021;281:235–43.
Greenberg PE, Birnbaum HG. The economic burden of depression in the US: societal and patient perspectives. Expert Opin Pharmacother. 2005;6:369–76.
Reckers-Droog V, van Exel J, Brouwer W. Willingness to pay for health-related quality of life gains in relation to disease severity and the age of patients. Value Health. 2021;24:1182–92.
Nierenberg AA, DeSecco L. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62:5–9.
Nierenberg AA. Long-term management of chronic depression. Prim Care Companion CNS Disord. 2001;3:10692.
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8.
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45.
Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression remission and beyond. JAMA. 2003;289:3152–60.
Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59.
Paykel ES. Partial remission, residual symptoms, and relapse in depression. Dialogues Clin Neurosci. 2008;10:431–7.
Cowen PJ, Anderson IM. New approaches to treating resistant depression. BJPsych Adv. 2015;21:315–23.
Sapin C, Fantino B, Nowicki ML, Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2:20.
Revicki DA, Wood M. Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. J Affect Disord. 1998;48:25–36.
Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir (Wien). 2005;147:1061–4.
Jung IH, Chang KW, Park SH, Chang WS, Jung HH, Chang JW. Complications after deep brain stimulation: a 21-year experience in 426 patients. Front Aging Neurosci. 2022;14:819730.
Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7.
Merkl A, Schneider GH, Schönecker T, Aust S, Kühl KP, Kupsch A, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol. 2013;249:160–8.
Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–12.
Accolla EA, Aust S, Merkl A, Schneider GH, Kühn AA, Bajbouj M, et al. Deep brain stimulation of the posterior gyrus rectus region for treatment resistant depression. J Affect Disord. 2016;194:33–7.
Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73:456–64.
Hitti FL, Vaughan KA, Ramayya AG, McShane BJ, Baltuch GH. Reduced long-term cost and increased patient satisfaction with rechargeable implantable pulse generators for deep brain stimulation. J Neurosurg. 2018;131:799–806.
Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZHT. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J Psychiatry Neurosci. 2013;38:325–32.
Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–77.
Download references
Acknowledgements
This work was funded by the McNair Foundation (SAS) as well as the National Institutes of Health (NIH) under award number T32GM136611 and by BRASS: Baylor Research Advocates for Student Scientists (KEK).
Author information
Authors and affiliations.
Department of Neurosurgery, Baylor College of Medicine, Houston, TX, USA
Katherine E. Kabotyanski, Garrett P. Banks, Himanshu Sharma, Nicole R. Provenza, Benjamin Y. Hayden & Sameer A. Sheth
Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL, USA
Ricardo A. Najera
Menninger Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine, Houston, TX, USA
Sanjay J. Mathew
You can also search for this author in PubMed Google Scholar
Contributions
KK, RN, and SS contributed to conceptualization of the study. KK and RN contributed to article selection and data collection. KK conducted quantitative analysis and figure preparation. KK prepared the initial manuscript. All authors contributed to manuscript editing and critical review.
Corresponding author
Correspondence to Sameer A. Sheth .
Ethics declarations
Competing interests.
Dr. Sheth is a consultant for Boston Scientific, Neuropace, Zimmer Biomet, Koh Young, Sensoria Therapeutics, and Varian Medican and is co-founder of Motif Neurotech. Dr. Mathew has served as a consultant for Abbott, Almatica Pharma, Biohaven, BioXcel Therapeutics, Boehringer-Ingelheim, Brii Biosciences, Clexio Biosciences, COMPASS Pathways, Delix Therapeutics, Douglas Pharmaceuticals, Eleusis, Engrail Therapeutics, Freedom Biosciences, Janssen, Liva Nova, Levo Therapeutics, Merck, Neumora, Neurocrine, Perception Neurosciences, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Seelos Therapeutics, Signant Health, Sunovion, Xenon Pharmaceuticals, and XW Pharma.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Prisma checklist, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kabotyanski, K.E., Najera, R.A., Banks, G.P. et al. Cost-effectiveness and threshold analysis of deep brain stimulation vs. treatment-as-usual for treatment-resistant depression. Transl Psychiatry 14 , 243 (2024). https://doi.org/10.1038/s41398-024-02951-7
Download citation
Received : 13 November 2023
Revised : 14 May 2024
Accepted : 20 May 2024
Published : 07 June 2024
DOI : https://doi.org/10.1038/s41398-024-02951-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Difference Between Introduction and Literature Review Order. Introduction is at the beginning of a text. Literature Review is located after the introduction or background. Function. Introduction introduces the main text to the readers. Literature Review critically evaluates the existing research on the selected research area and identifies the ...
Learn the purpose, components, and steps of writing a literature review introduction for different academic formats. See examples of effective introductions for literature review papers, sections, and chapters.
Learn the difference between introductions and literature reviews in academic writing, and how to write them effectively. See examples from a social work article and other sources.
Learn how to conduct a literature review for your thesis, dissertation, or research paper. Find out the purpose, steps, and tips for writing a literature review, with examples and templates.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays).
What are the goals of creating a Literature Review? A literature could be written to accomplish different aims: To develop a theory or evaluate an existing theory; To summarize the historical or existing state of a research topic; Identify a problem in a field of research ; Baumeister, R. F., & Leary, M. R. (1997). Writing narrative literature ...
Learn the differences, purposes, and tips for writing an introduction and a literature review for your dissertation or thesis. Find out how to structure, synthesise, and justify your research based on the current literature.
Learn the differences, purposes, and tips for writing an introduction and a literature review for your dissertation or thesis. Find out how to structure, synthesise, and justify your research topic and question using relevant literature.
The key here is to focus first on the literature relevant to the puzzle. In this example, the tokenism literature sets up a puzzle derived from a theory and contradictory empirical evidence. Let's consider what each of these means... The literature(s) from which you develop the theoretical/empirical puzzle that drives your research question.
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
Whatever stage you are at in your academic life, you will have to review the literature and write about it. You will be asked to do this as a student when you write essays, dissertations and theses. Later, whenever you write an academic paper, there will usually be some element of literature review in the introduction. And if you have to
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important ...
Neither introduction nor the conclusion can outline anything in detail: both act as guides to the content within the rest of the dissertation. Literature Reviews: Every dissertation will have a literature review in some shape or form. A literature review needs to show that you understand the literature currently on or around your topic.
Literature Reviews Amber Huett, David MacMillan, Katie Crum, and Dr. R. T. Koch July 2011 UNA Center for Writing Excellence 2 Conclusion Depending on the purpose(s) of your literature review, your conclusion may include the following: Introduction to further research: The conclusion of your literature review can be used to explain
Academic articles are not like the essays you may be used to writing, in which the thesis appears at the end of the introduction. The research gap is more akin to a hypothesis than a thesis. It does not make an argument, which comes much later—usually in the discussion or conclusion. There are also articles that are stand-alone literature ...
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications .For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively .Given such mountains of papers, scientists cannot be expected to examine in detail every ...
Learn the differences between a literature review and a research paper introduction!Get my Scientific Research Paper Checklist: https://www.sciencegradschool...
The Introduction provides a general overview of the topic, setting the stage for readers. In contrast, the Literature Review delves deeper, examining existing research on the topic. Introduction sections are typically brief, aiming to hook the reader and outline the main points the paper will address. On the other hand, the Literature Review ...
This is essentially a summary of the literature review section (which is why the literature review should be written before the introduction, or else you don't know what to summarize). Briefly but clearly present my research objective or research question. Summarize the methodology and procedures that I used to carry out the research objective.
Following an introduction to a broader topic, the literature review directs readers to relevant studies that are significant for the objectives of the present study. The authors are advised to present the information thematically, preferably chronologically, for a better understanding of the readers from a wide range of disciplines.
Posted on May 18, 2022 ·. Introduction is at the beginning of a text while literature review is located after the introduction or background. Introduction is the part that introduces the main text to the readers while literature review critically evaluates the existing research on the selected research area and identifies the research gap.
Avoid these 3 Common Mistakes made in Introductions and Literature Reviews.Get My Scientific Paper Checklist to Write Successful Research Articles:https://ww...
Literature Review for Introduction Vs. Discussion. < 1 . min read . A literature review presents a summary of studies related to a particular area of research. It identifies and summarizes all the relevant research conducted on a particular topic. Literature reviews are used in the introduction and discussion sections of your manuscripts.
Mission. The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives.
Objective This study aimed to analyze and map scientific literature on Neuroleptic Malignant Syndrome (NMS) and Serotonin Syndrome (SS) from prestigious, internationally indexed journals. The objective was to identify key topics, impactful articles, prominent journals, research output, growth patterns, hotspots, and leading countries in the field, providing valuable insights for scholars ...
Literature review. Our PubMed search yielded 76 initial results (see Fig. 2 for our PRISMA [] flowchart).From the 16 studies selected for data collection (published 2009-2021), we found a total ...