An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Implementation of the Mental Capacity Act: a national observational study comparing resultant trends in place of death for older heart failure decedents with or without comorbid dementia
James m. beattie.
1 Cicely Saunders Institute of Palliative Care and Rehabilitation, King’s College London, London, UK
2 School of Cardiovascular Medicine and Sciences, King’s College London, London, UK
Irene J. Higginson
Theresa a. mcdonagh, associated data.
Additional file 1 :Supplementary files S-1, S-2, S-3, and S-4 are available online.
Heart failure (HF) is increasingly prevalent in the growing elderly population and commonly associated with cognitive impairment. We compared trends in place of death (PoD) of HF patients with/without comorbid dementia around the implementation period of the Mental Capacity Act (MCA) in October 2007, this legislation supporting patient-centred decision making for those with reduced agency.
Analyses of death certification data for England between January 2001 and December 2018, describing the PoD and sociodemographic characteristics of all people ≥ 65 years registered with HF as the underlying cause of death, with/without a mention of comorbid dementia. We used modified Poisson regression with robust error variance to determine the prevalence ratio (PR) of the outcome in dying at home, in care homes or hospices compared to dying in hospital. Covariates included year of death, age, gender, marital status, comorbidity burden, index of multiple deprivation and urban/rural settings.
One hundred twenty thousand sixty-eight HF-related death records were included of which 8199 mentioned dementia as a contributory cause. The overall prevalence proportion of dementia was 6.8%, the trend significantly increasing from 5.6 to 8.0% pre- and post-MCA (Cochran-Armitage trend test p < 0.0001). Dementia was coded as unspecified (78.2%), Alzheimer’s disease (13.5%) and vascular (8.3%). Demented decedents were commonly older, female, and with more comorbidities. Pre-MCA, PoD for non-demented HF patients was hospital 68.2%, care homes 20.2% and 10.7% dying at home. Corresponding figures for those with comorbid dementia were 47.6%, 48.0% and 4.2%, respectively. Following MCA enforcement, PoD for those without dementia shifted from hospital to home, 62.5% and 17.2%, respectively; PR: 1.026 [95%CI: 1.024–1.029]. While home deaths also rose to 10.0% for those with dementia, with hospital deaths increasing to 50.4%, this trend was insignificant, PR: 1.001 [0.988–1.015]. Care home deaths reduced for all, with/without dementia, PR: 0.959 [0.949–0.969] and PR: 0.996 [0.993–0.998], respectively. Hospice as PoD was rare for both groups with no appreciable change over the study period.
Conclusions
Our analyses suggest the MCA did not materially affect the PoD of HF decedents with comorbid dementia, likely reflecting difficulties implementing this legislation in real-life clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-021-02210-2.
The incidence and prevalence of heart failure varies across the world reflecting regional differences in cardiovascular disease burden, ethnic and socioeconomic diversity. While the age-adjusted incidence and prevalence of heart failure may be declining in Westernised countries, absolute rates of these indices are increasing in parallel with societal ageing [ 1 ]. In the United Kingdom (UK), about 900,000 people are living with heart failure. The prevalence is 1–2% in the general population, rising to at least 10% in those ≥70 years of age [ 2 , 3 ]. The lifetime risk of developing heart failure is about 20% at 40 years, but for each age decile between 65 and 85 years, the incidence doubles for men and trebles for women [ 4 ].
Crafted by international consensus [ 5 ], a universal definition of heart failure has recently emerged as
…a clinical syndrome with symptoms and / or signs caused by a structural and / or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and / or objective evidence of pulmonary or systemic congestion.
Increasingly accurate diagnostic protocols have been established, and based on the left ventricular ejection fraction (EF), the percentage volume of the diastolic blood pool ejected during systole, an updated classification system linked to the above definition has characterised four clinical phenotypes: heart failure with a reduced EF [< 40%] (HFrEF); heart failure with a mildly reduced EF [40–49%] (HFmrEF); heart failure with a preserved EF [≥50%] (HFpEF); and heart failure with an improved EF [baseline EF ≤40%, a ≥10% point increase from baseline EF, the improved EF > 40%] (HFimpEF) [ 5 ]. Advances in heart failure therapy, particularly those for HFrEF, have enabled some patients to live longer, more comfortable lives, but for many, heart failure remains a life-limiting condition, the 5- and 10-year case-fatality rates being about 50% and 75%, respectively, similar to outcomes for common cancers [ 3 , 6 ]. As well as being encumbered with an unpredictable disease trajectory, this burgeoning and increasingly elderly clinical cohort usually exhibit several comorbidities which add to the complexity and challenge the coordination required of their care [ 2 ].
Cognitive impairment, ranging from mild forms to severe as manifest in dementia, is relatively common, affecting between 25 and 70% of those with heart failure across a series of studies, and estimated at 40% overall in a meta-analysis [ 7 – 9 ]. Disordered cognition is heterogeneous and demonstrable across a range of higher cortical domains including attention, memory, speech and language processing, learning and executive function [ 10 ], such deficits beyond those arising from normative ageing of the brain. Cognitive impairment may be transitory, sometimes occurring as delirium in patients presenting with acute heart failure [ 11 ], but often presents as a long-term progressive condition, more frequently encountered in older people with chronic heart failure compared to their age-matched healthy counterparts [ 12 ]. The impact of these persistent features tends to fluctuate over time, but even when mild, may impact heart failure patients’ self-care behaviours and treatment adherence resulting in greater rates of hospital admission and mortality [ 13 , 14 ]. There appears to be no direct correlation between the severity of these two conditions [ 15 ], but dilemmas may arise when treating cognitively impaired heart failure patients, sometimes relating to the continued efficacy of established treatment modalities, ceilings of care, and resuscitation issues [ 16 ]. These confront not only professional healthcare providers but also their informal carers, usually family members, who take responsibility for much day-to-day practical support. In assisting those affected by dementia-related loss of intellectual capacity, such carers may be called upon to act as decisional proxies and offer insight into patients previously voiced values and preferences for treatment.
The Mental Capacity Act (MCA) of 2005, applicable to residents in England and Wales aged ≥16 years, sets out a statutory framework to foster person-centred decision making and advance care planning for those who may lack capacity due to a lifelong learning disability, or as a consequence of the transient or permanent effects of acute or long-term illnesses [ 17 ]. For people with cognitive impairment and a progressive, ultimately fatal condition such as heart failure, the MCA may be particularly important in fulfilling goals of care close to the end of life. Achieving care in appropriate settings and the preferred place of death (PoD) are generally accepted as benchmarks of good quality end-of-life care, death at home or customary place of residence usually regarded as the desired option [ 18 ]. Given the relatively frequent association of cognitive impairment with heart failure, it might be expected that the decision-making processes legally constituted within the MCA would drive changes in the final place of care and death during the terminal phase of this condition. The code of practice setting out the standards required to comply with the MCA came fully into force on October 1, 2007 [ 19 ]. Thus, we undertook a comparative trend analysis covering the period of implementation of this legislation to discern any resultant variation in the PoD of heart failure decedents rendered vulnerable by comorbid dementia.
Application of the Mental Capacity Act
As outlined above, the MCA and associated code of practice offer legislative protection to promote patient empowerment and safeguard their autonomy. Prepared when mental capacity is intact, patients may formulate an advance decision such as one to refuse life-sustaining treatment or, if ≥18 years, appoint a close person as a personal welfare lasting power of attorney (LPA) to undertake decisions on their behalf if agency is later lost. Thereafter, any clinical treatment protocol, where possible, should be in accordance with their previously documented choices and values, or these as expressed through their nominated personal welfare LPA. For the purposes of the Act, a two-stage capacity test is applicable. To qualify through Stage 1, the individual must exhibit a demonstrable functional impairment of the mind or brain. For Stage 2, capacity is deemed to be lost if they lack the ability to fulfil any of the following: (a) understand the information pertinent to the decision, (b) retain the information, (c) deliberate on that information as part of the decision-making process and (d) communicate their decision by any means possible. In the context of the study, we must emphasise that many people diagnosed with dementia can still make decisions about many aspects of their care, and loss of capacity should not be regarded as an all-or-none phenomenon based on that diagnostic label. Indeed, under the terms of the MCA, retention of capacity is assumed, and capacity is both decision and time specific.
Study design
This was a national population-based observational study examining anonymised individual-level death registration data collated by the Office for National Statistics (ONS) from 2001 to 2018, provided to us under license, and relating to heart failure decedents resident in England.
Data source and study cohort
In the UK, the death certificate is completed by the responsible clinician, civil registration of the cause of death by a relative or another qualified informant being legally required within 5 days of medical certification. Sometimes a coroner assumes this role after a post-mortem examination or inquest. Following transcription of the information on the death certificate by the recording registrar, this is digitised and uploaded to the ONS for subsequent diagnostic coding in accordance with the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This study dataset comprised all deaths registered in England from January 2001 through December 2018 of people aged ≥ 65 years for whom heart failure was recorded as the underlying cause of death. We elected to study those aged ≥ 65 years at death a priori, perceiving this subset to include the vast majority of heart failure decedents with or without dementia as a marker of cognitive impairment. Heart failure as the primary cause of death was determined by the allocation of any ICD-10: I50 code by the ONS. Designation of diminished intellectual capacity for this cohort was determined when dementia of any aetiology was mentioned as a contributory cause of death. The presence of comorbid dementia was indicated by the application of ICD-10 codes G30 [Alzheimer’s disease]; F00 [dementia in Alzheimer’s disease with late onset, atypical or mixed type, and unspecified]; F01 [vascular dementia]; F02 [dementia in other diseases classified elsewhere]; or F03 [unspecified dementia]. ONS death data acquisition and coding processes are subject to regular quality assurance. Pertinent to this study period, it should be noted that in 2011 there was a change in ONS mortality data coding practice, the previous coding of unspecified cerebrovascular disease when registered as a contributory cause of death being reclassified as vascular dementia [ 20 ].
The outcome variable was PoD as recorded on death certificates and codified by the ONS. Characterisation of PoD for this study was based on the classification system defined by the National End of Life Care Intelligence Network, part of Public Health England [ 21 ]. This specifies 5 groupings of PoD: (a) Hospital, which incorporates all acute, specialist, and community hospitals whether they be National Health Service (NHS) or private, but not psychiatric hospitals; (b) Care home, including residential and nursing homes; (c) Own residence, the decedent’s usual place of abode, but excludes communal living arrangements such as convents, monasteries, hostels or prisons; (d) Hospice, commonly standalone NHS or independent establishments; (e) Other places, covering psychiatric hospitals, other people’s homes, communal living institutions as described above, workplaces, public spaces or roads. This grouping also applies to those declared dead on arrival at hospital, potentially relevant to some heart failure patients who succumb to sudden death. Where the PoD was unknown or unspecified, these data were incorporated in descriptive statistics but not considered further in multiple adjusted analyses.
Period of death as the independent variable of interest incorporated a binary indicator for the year of death pre- and post-enforcement of the MCA [0: 2001–2007; 1: 2008–2018]. Covariates included age at death, number of mentioned contributory causes, gender, marital status, socioeconomic position as measured by the Index of Multiple Deprivation, and categorisation of the location of decedents’ usual residence as urban or rural based on the relevant postcode as archived on the ONS classification system [ 22 , 23 ].
Statistical analysis
Categorical and continuous variables were described using count (percentages) and means (standard deviation [SD]) as appropriate. The proportion of hospital deaths among heart failure decedents with or without comorbid dementia and the number of patients who died from heart failure with comorbid dementia were plotted to visually determine temporal trends, the latter also assessed statistically using a two-tailed Cochran-Armitage trend test.
We used modified Poisson regression with robust error variance [ 24 ] to evaluate the independent association between enforcement of the MCA and PoD. Three models were constructed separately for heart failure patients who died with or without comorbid dementia: home (1) versus hospital (0); care home (1) versus hospital (0); hospice (1) versus hospital (0). All covariates were forced to stay in the models to control their effects. The prevalence ratio (PR) was derived from the respective model to quantify the magnitude of association.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). To control for Type 1 error, we applied Bonferroni correction to the alpha level. A two-sided p value of 0.008 (0.05/6) was considered statistically significant.
Study sample
Between 2001 and 2018, 120,068 people aged ≥65 years whose deaths were registered as directly due to heart failure were identified, their data subsequently included in this analysis. Table Table1 1 describes the characteristics of these heart failure decedents. Overall, 8199 (6.8% [confidence intervals (CI) 6.7 to 7.0]) of these registrations were documented with dementia as a contributory cause, this being classified as unspecified dementia in 78.2%, Alzheimer’s disease in 13.5%, and as vascular dementia in 8.3%. No other dementia subtypes were denoted. For the periods 2001–2007 and 2008–2018, pre- and post-enforcement of the MCA, the numbers of heart failure decedents with dementia were 3427 (5.6%) and 4772 (8.0%) respectively. The prevalence proportion of dementia gradually increased on an annual basis, this trend being statistically significant ( Z =18.87, p < 0.0001) as shown on Fig. Fig.1. 1 . There was no discernible artefactual change in the general rate of dementia mentioned as a contributory cause of death associated with the 2011 change in ONS coding practice. However, as shown in Table Table2, 2 , there was a contemporaneous and statistically significant reduction ( p < 0.0001) in the coding of ‘ unspecified dementia ’ with an equivalent increase in coding for ‘ vascular dementia ’.
Characteristics of heart failure decedents with or without comorbid dementia, n (column %), England 2001–2018
| Variable | Value | With dementia | Without dementia |
|---|---|---|---|
| All | All | 8199 (6.8) | 111,869 (93.2) |
| Age at death (years) | 65–74 | 207 (2.5) | 9293 (8.3) |
| 75–84 | 2082 (25.5) | 33,920 (29.9) | |
| 85+ | 5910 (72.0) | 68,656 (61.8) | |
| Gender | Female | 5498 (67.2) | 67,474 (60.0) |
| Male | 2701 (32.8) | 44,395 (40.0) | |
| Marital status | Divorced | 1945 (23.7) | 30,118 (27.1) |
| Single | 365 (4.4) | 5343 (4.9) | |
| Widowed | 553 (6.8) | 8481 (7.5) | |
| Married | 5309 (64.8) | 67,497 (60.1) | |
| Unknown | 27 (0.3) | 430 (0.4) | |
| Year of death | 2001–2007 | 3427 (87.2) | 57,240 (88.5) |
| 2008–2018 | 4772 (56.4) | 54,629 (55.7) | |
| No. comorbidities | 0 | -- | 15,139 (13.4) |
| 1 | 1595 (19.4) | 44,661 (39.3) | |
| 2 | 3474 (42.5) | 31,048 (27.9) | |
| 3 | 1922 (23.4) | 13,750 (12.6) | |
| 4+ | 1208 (14.6) | 7271 (6.8) | |
| Deprivation* | Most deprived | 1445 (17.7) | 20,352 (18.1) |
| 2 | 1685 (20.5) | 22,250 (19.8) | |
| 3 | 1836 (22.4) | 24,227 (21.7) | |
| 4 | 1748 (21.3) | 23,918 (21.4) | |
| 5 | 1485 (18.1) | 21,122 (19.0) | |
| Rural/urban indicator* | Urban | 6653 (81.1) | 89,986 (80.4) |
| Rural | 1546 (18.9) | 21,883 (19.6) | |
| Place of death | Hospital | 4033 (49.2) | 73,177 (64.9) |
| Care home | 3497 (42.8) | 21,850 (19.5) | |
| Home | 623 (7.5) | 15,541 (14.4) | |
| Hospice | 22 (0.3) | 606 (0.6) | |
| Other places | 24 (0.3) | 695 (0.6) |
* p values for the difference between the two groups = 0.12. For all other inter-group comparisons, p < 0.0001

Percentages of heart failure decedents ( n =120,068) with comorbid dementia ( n =8199) by year of death, England, 2001–2018
Distribution of dementia subtypes [ n (%)] following the ONS change in dementia coding practice of 2011
| Coding | Pre- coding change | Post-coding change | Total |
|---|---|---|---|
| 642 (12.8) | 465 (14.6) | 1107 (13.5) | |
| 4287 (85.5) | 2128 (66.8) | 6415 (78.2) | |
| 84 (1.7) | 593 (18.6) | 677 (8.3) | |
| 5013 (61.1) | 3186 (38.9) | 8199 (100) |
AD Alzheimer’s disease, UD unspecified dementia, VD vascular dementia
The p values for comparison of the proportions of UD and VD pre- and post-coding change were statistically significant ( p < 0.0001)
Most heart failure decedents were ≥85 years old at the time of death (62.1%). The mean age at death increased between the two study periods (2001–2007: 85.7 years [SD 7.4]; 2008–2018: 86.6 years [SD 7.5], the age at death being greater for those with dementia for both intervals at 87.1 [SD 6.2] and 88.1 years [SD 6.0], respectively. A relatively higher level of multimorbidity was noted for heart failure decedents with dementia. Most of those dying from heart failure were female, this proportionately greater at 67.2% for the dementia group compared to 60.0% for those without dementia. Across the totality of heart failure decedents, there was no significant difference in marital status between those with or without dementia, Most heart failure patients lived and died in urban environments and deprivation quintiles were similar for both study populations.
Trends in place of death
Over the period of implementation of the MCA, comparative outcomes in PoD for these heart failure decedents are shown in Fig. Fig.2. 2 . For the period 2001–2007, hospital was the most common PoD for the non-demented heart failure group at 68.2%, 20.2% dying in a care home, and 10.7% dying at home. This changed a little for the latter period 2008–2018, reducing to 62.5% and 18.8% for hospital and care homes respectively, the proportion of home deaths increasing to 17.2%. In contrast, for heart failure decedents with dementia, there was a small increase in the proportion of hospital deaths, this rising from 47.6 to 50.4%. For this group, there was a reduction in care home deaths from 48.0 to 38.8%, with a modest rise in home deaths, 4.2% to 10.0%. Hospice as the PoD was rare for both clinical cohorts and declined over the study period. The time trend in hospital deaths is shown in Fig. Fig.3. 3 . There was a statistically significant reduction in hospital deaths for heart failure decedents without dementia ( p < 0.001). On the other hand, the marginal increase in hospital deaths for those with dementia was not significant ( p =0.97). Adjusted PRs following implementation of the MCA confirm increased PoD at home compared to hospital for non-dementia patients, PR: 1.026 [CI: 1.024–1.029] ( p < 0.0001), this trend not significant for those with dementia, PR: 1.001 [CI 0.988–1.015] ( p =0.83). Care home deaths reduced for both groups, PR: 0.959 [CI 0.949–0.969] ( p < 0.0001), and PR: 0.995 [CI 0.993–0.998] ( p < 0.0001) for those with and without dementia, respectively. Starting from an already small base, adjusted PRs for hospice rather than hospital as PoD declined significantly for both non-demented and demented heart failure decedents being 0.979 [CI 0.977–0.980 ( p < 0.0001) and 0.946 [CI 0.934–0.959] ( p < 0.0001), respectively. A summary of the adjusted PRs for dying in a premise other than hospital following MCA enforcement is shown in Table Table3. 3 . Fully detailed results for all three model sets are available in Additional file 1 : Supplementary Tables S-2 to S-4.

Comparative outcomes (%) in place of death for heart failure decedents with and without comorbid dementia pre- (2001–2007) and post- (2008–2018) implementation of the Mental Capacity Act (MCA). Chi-square test p value < 0.0001

The time trend of hospital deaths among patients who died from heart failure with or without comorbid dementia, England 2001–2018
The adjusted prevalence ratios* (95% confidence intervals) of dying in a specific type of premise (compared to a hospital death) after implementation of MCA in heart failure decedents with or without comorbid dementia, England 2001–2018
| With dementia | Without dementia | |||
|---|---|---|---|---|
| Home | 1.001 (0.988 to 1.015) | =0.83 | 1.026 (1.024 to 1.029) | < 0.0001 |
| Care home | 0.959 (0.949 to 0.969) | < 0.0001 | 0.995 (0.993 to 0.998) | < 0.0001 |
| Hospice | 0.946 (0.934 to 0.959) | < 0.0001 | 0.979 (0.977 to 0.980) | < 0.0001 |
Hospital (Reference) | 1.000 | 1.000 | ||
*> 1 indicates a higher chance of death in the corresponding type of premise
In this first national study examining variation in the PoD for heart failure patients over the period of enactment and implementation of the MCA in England, our results suggest that the decision-making and advance care planning processes enshrined in this legislation had little material effect on the ultimate site of care provision determining PoD for those with cognitive impairment manifest as dementia. In the later years of this study period when the code of practice for this legislation was operational, trend data for heart failure decedents certified with comorbid dementia show a modest rise in hospital deaths with fewer care home deaths. Conversely, for this study phase, there was a small reduction in hospital deaths for those without dementia who were younger and with fewer comorbidities.
The reasons behind the apparent lack of impact of this legislation may be complex, but it has been proposed that the MCA is relatively poorly applied in clinical practice. While a variety of training models have been developed to disseminate information to health professionals on the five principles underpinning these regulations, recent reviews suggest poor understanding of when and how the provisions of the Act should be employed, with the need for clarity on the process of designating the role of surrogate decision-makers to properly ensure patients’ best interests are maintained [ 25 – 27 ]. A lack of confidence of those working in acute care settings has been particularly highlighted, specifically citing decision-making with respect to hospital discharge [ 28 ]. This issue may be especially relevant to our study observations given that we have demonstrated that the majority of those dying with heart failure in England do so in hospital, similar data emerging from the United States (US) [ 29 ].
At variance with the trends evident in this study, previous work has suggested a decline in the frequency of hospital deaths for those with dementia in recent years, with more people dying in care homes [ 30 ]. These findings were also based on ONS death certification data but included all individuals for whom dementia was mentioned as either the underlying or as a contributory cause of death. In contrast, the current study specifies heart failure as the primary cause of death, differentiating the comparator groups by the presence or absence of dementia mentioned only as a contributory cause. It is well established that the principal diagnosis is the main determinant of the site of clinical care [ 31 ], and community-based primary care practitioners appear to be relatively incognizant of patients’ preferences for place of care or PoD, particularly when dealing with non-cancer diagnoses [ 32 ]. Unless policies for comfort care are clearly outlined, should people with chronic heart failure living at home or in a nursing home suddenly deteriorate, the reactive response of professional staff may be to arrange emergency hospital admission by default. However, we have no information on any care transitions prior to the terminal phase for this study cohort, or whether this possible course of action had a bearing on the results of this study.
The completeness of death certification in the UK is regarded as relatively robust with proportionately fewer ‘ garbage codes ’ than data from many other countries [ 33 ]. However, dementia as recorded on death certification likely underestimates the true prevalence, and it has been suggested that studies using death certification alone may fail to account for 16-18% of dementia cases [ 34 ]. A variety of factors may influence such documentation. Rates of inclusion of dementia are generally increased in those who die in institutions such as care homes compared to those dying at home, particularly if dementia is at the severe end of the clinical spectrum and includes agitation [ 35 ]. While heart failure guidelines draw attention to cognitive impairment as a comorbidity, describing all grades of this by hospital-based clinicians is reportedly poor [ 14 ], and there are diverging views on whether dying in hospital positively or negatively affects the rate of recording of dementia at the time of death certification [ 35 , 36 ]. Recent initiatives to heighten clinicians’ awareness of dementia may improve matters. In 2012, NHS England introduced a quality improvement scheme through the Commissioning for Quality and Innovation (CQUIN) payment framework [ 37 ]. Acute healthcare providers were incentivised with the assurance of increased remuneration if 90% of all patients aged ≥75 years and whose emergency hospital admission lasted > 72 h were screened for dementia. Further financial gain was available if those patients whose initial assessment indicated dementia or was inconclusive were referred on for specialist review. While this dementia assessment and referral exercise was retired as a CQUIN indicator in April 2016, these conditions have been retained within the standard contract for English hospitals providing acute clinical services. It is possible that these administrative processes may have contributed in some measure to the increased mentions of dementia as certified for hospital decedents evident in the latter course of this study.
The relatively frequent concurrence of cognitive impairment and heart failure likely stems from various pathophysiologic features related to the latter condition combined with shared cardiovascular risk factors such as hypertension, hyperlipidaemia or dysglycaemia [ 7 , 38 , 39 ]. The haemodynamic and risk factor profiles for HFrEF and HFpEF clearly differ, but very few investigations have compared the spectrum of cognitive impairment across the range of ejection fraction phenotypes. There is a suggestion that affected domains of cognitive function may vary, but data is limited with inconsistent results [ 40 , 41 ].
To date, there is no evidence that evidence-based guideline-directed medical therapy (GDMT) for heart failure drives neurocognitive dysfunction [ 42 ], and indeed it has been posited that centrally acting angiotensin-converting enzyme inhibitors (ACEIs) such as perindopril or captopril, which cross the blood-brain barrier, may slow the progression of cognitive impairment in those with dementia [ 43 ]. Following the positive results of the PARADIGM-HF study demonstrating the benefits of sacubitril/valsartan, the first of a new class of drugs termed ARNIs (angiotensin receptor-neprilysin inhibitors) [ 44 ], this therapeutic option for HFrEF has been widely adopted. Neprilysin is a soluble metalloprotease which catalyses the degradation of natriuretic peptides (NPs), downregulation of this enzymatic activity likely increasing endogenous NP mediated natriuresis and vasodilation. However, such neprilysin inhibition might also interfere with the clearance of amyloid-β protein, vascular deposition of which results in cerebral amyloid angiopathy, a distinctive feature of Alzheimer’s disease. Dementia-related adverse events were not overrepresented through 4.3 years follow-up of the relevant PARADIGM-HF study arm compared to similar populations [ 45 ]. Nonetheless, as required by the Food and Drug Administration in the US, this potential hazard is currently being evaluated in the PERSPECTIVE study ( ClinicalTrials.gov ID {"type":"clinical-trial","attrs":{"text":"NCT02884206","term_id":"NCT02884206"}} NCT02884206 ). Due to report in 2022, this trial includes a battery of neurocognitive testing and sequential 18 F-labelled florbetaben positron emission tomography to assess any longitudinal changes in cerebral amyloid plaque burden. Importantly, recent evidence shows that the hearts of some patients with Alzheimer’s disease exhibit diastolic dysfunction and thickening of the interventricular septum. These features are characteristic of cardiac amyloidosis suggesting that in some individuals, amyloid-β protein may also accumulate in tissues other than the brain [ 46 ].
As inferred above, in recent years GDMT for those affected by heart failure has become increasingly effective [ 47 ], but heart failure is an ambulatory care sensitive condition and remains the commonest cause of acute hospitalisation in those > 65 years [ 48 ]. Following an index heart failure admission in England, the 1-year mortality for patients discharged alive is 39.6% with a 30-day all-cause readmission rate of 19.8% [ 49 ]. Readmissions for heart failure tend to follow a tri-phasic pattern. This was apparent in a study of 8543 heart failure patients in Toronto monitored for 10 years following their first hospital admission, by which time 98.8% had died, the median survival after heart failure diagnosis being 1.75 years [ 50 ]. About 30% of all readmissions occurred within 2-months of initial hospital discharge, 50% during the 2-month period leading up to death, with 15-20% taking place in the intervening ‘ plateau phase ’ of the heart failure disease trajectories. A sentinel clustering of admissions in the terminal phase of heart failure has been well described [ 51 ]. It is uncertain if the presence of dementia as a comorbidity influences the readmission rate. Rao and colleagues followed 10,317 patients for 5 years subsequent to their diagnosis with heart failure between April 2008 and March 2009 using the primary care-based Clinical Practice Research Datalink combined with Hospital Episode Statistics and ONS death registration data [ 52 ]. Their analysis indicated that comorbid dementia was a factor significantly affecting emergency hospital readmissions in only 3 of 8 regions across England.
Comparable to the epidemiological trends for heart failure, the age-adjusted prevalence and incidence of dementia may also be declining in high-income countries. The Medical Research Council Cognitive Function and Ageing Studies (CFAS 1 and II) of populations living in rural Cambridgeshire and the urban environments of Newcastle and Nottingham demonstrated a 24% reduction in the prevalence of dementia in those ≥65 years between 1989 and 2011 [ 53 ]. Consistent with our observations, the CFAS studies also suggested that women were more commonly affected, and while the prevalence of dementia in care home residents had increased from 56 to 70%, most people with dementia were still living at home. Similarly, dementia events have been continuously surveyed in the US-based Framingham Heart Study since 1975. Monitoring of this community cohort living in Massachusetts, predominantly of white European ancestry, has implied a 20% stepwise decline in the incidence of dementia each decade over the last 30 years [ 54 ]. The background to these cumulative decrements remains to be determined, but both the CFAS and Framingham study groups cited potential mechanisms in higher early educational attainment and attenuated vascular morbidity.
The Framingham Heart Study showed a non-significant reduction in Alzheimer’s disease with a more overt decrease in vascular dementia. In Westernised societies, Alzheimer’s is the most commonly encountered manifestation of dementia, but as an isolated pathophysiological process, this affects < 20% of those with heart failure. Rather, vascular dementia has been proposed as the likeliest associated variant, followed by mixed forms, then Alzheimer’s and other specific dementias [ 39 ]. This is at odds with the distribution of dementia subtypes noted in this study where unspecified dementia was most frequently mentioned on death certificates and coded as the dominant category. A similar finding was described in a Danish study of 324,418 patients admitted with incident heart failure and tracked for 35 years against an age- and sex-matched population without heart failure selected from the Danish Civil Registration System [ 55 ]. Adelborg and colleagues found a clear association between all-cause dementia and heart failure. This was relatively weak for Alzheimer’s disease, and while the vascular variant was represented, this was predominantly determined by the reported development of unspecified dementia. These authors proposed that some patients ostensibly exhibiting unspecified dementia may have been misclassified. However, in combining death certification data from sequential CFAS studies in England, all mentions of dementia as the underlying or a contributory cause of death showed a percentage distribution of subtypes of unspecified dementia, Alzheimer’s disease and vascular dementia as 69.3%, 21.6% and 8.6%, respectively [ 37 ]. These results are very similar to those noted in the current study and suggest that this proportional distribution of dementia variants is not specific to the heart failure population.
Advance care planning offers patients the potential to receive medical treatment consistent with their expressed preferences, values, and goals of care against the possibility of subsequent loss of decision-making capacity. This may help prevent needless hospital admissions and better achieve consensus on appropriate ceilings of care, avoiding exposure of patients and families to the distressing harms which sometimes accompany futile treatment escalation and burdensome invasive interventions close to the end of life. It is notable that a recent audit of end-of-life care in hospitals in England demonstrated that10% of heart failure patients were receiving mechanical ventilatory support within 24 h of death [ 56 ].
Given the unpredictability intrinsic to the heart failure disease trajectory which challenges individual prognostication even in the late stages of this disease, and the associated multimorbidity including cognitive impairment, it might be expected that advance care planning would be central to the care of people with this condition. However, advance care planning is not routinely incorporated within heart failure care and tends to be limited to the possible withdrawal of any implanted electronic or mechanical devices, or sometimes offered as one component of the still uncommon provision of palliative care [ 57 , 58 ]. A review and meta-analysis of 14 randomised controlled trials of advance care planning in heart failure, mostly US-based and involving 2924 individuals across a range of care structures, showed this to moderately improve the primary outcome measure in patients’ quality of life, together with similarly weighted favourable effects on secondary outcomes including communication about, and satisfaction with, end-of-life care [ 59 ].
Advance care planning for dementia was featured as a specific domain in the European Association of Palliative Care white paper on this condition [ 60 ], and the challenges taking this forward have been comprehensively reviewed [ 61 , 62 ]. Emerging themes suggest that while there is some disparity in the readiness of older people to engage in advance care planning, and the means to take this forward may also vary across the spectrum of healthcare delivery, the use of these instruments may be effective in promoting shared decision-making between patients, informal carers and professionals [ 63 ]. However, it should be noted that, in the UK, an advance care plan is not legally binding but merely an advisory statement of preferences and wishes in relation to general care and medical therapy [ 64 ].
Dialogue between the patient, family and clinician is basic to shared decision-making. However, triangulation of information between this triad does not necessarily mean equitably weighted opinions, and at times patients’ voices are marginalised, a dyadic interchange conducted between clinicians and family members. Such a discourse may be justified if the patient cannot physically contribute to meaningful shared decision-making, if capacity is deemed to be lost, legally binding instruments are not in place, and their preferences are unknown. Then, the opinion of the closest relative should be sought, their views respected and used to inform a care plan constructed in the best interests of the patient. However, it should also be remembered that the involvement of relatives as decisional proxies may be emotionally demanding, particularly if they are already distressed and experiencing anticipatory grief. Further, the assumption that there is always congruence between the opinions of family surrogate decision-makers and those perceived of patients is flawed. Rather, these are often misaligned, with at best moderate concordance between dementia/carer dyads when assessed within a hospital setting [ 65 ]. Qualitative studies examining difficulties in decision making during clinical encounters for those with dementia have cited tension between family members and conflicts between families and health professionals, some of the latter reluctant to undertake decisions on patients unfamiliar to them, particularly when there is poor information exchange following transfer from another care setting [ 66 ]. Even if decisional consensus is achieved, the discharge of hospital inpatients home may be subject to practical limitations depending on the social context. The caregiver burdens associated with heart failure and dementia have been well characterised. Informal caregivers may be unwilling or unable to reframe or enhance their roles, and it should be noted that the cohabitees of people living and dying with dementia tend to be of a similar advanced age [ 67 ]. Further, it has been proposed that there may be a gendering issue relating to care at home in that older women are more likely to have outlived their male partners and be devoid of spousal support [ 68 ]. However, in this study, the marital status of heart failure decedents with or without dementia appeared to be similar. Following the implementation of the MCA, our analysis suggests that proportionately more women with dementia died at home compared to those without dementia. The reason for this disparity is unclear, and a variety of other intersectional stressors which might constrain the social capital of older women close to the end of life may have been in play [ 69 , 70 ].
Strengths and weaknesses
To our knowledge, this is the first empirical study to systematically examine national data for England describing the PoD of people dying of heart failure with dementia documented as a contributory cause of death. A major strength is that our work is based on comprehensive data collated from the gamut of clinical practice over an 18-year period, but we acknowledge that we are dependent on the clinicians responsible for these heart failure decedents having made the correct diagnoses and accurately completing later death certification, over which there is no means of independent adjudication. Irrespective of the PoD, we have no access to information on the underlying aetiologies of heart failure, or the proportional distribution of resultant ejection fraction variants. Similarly, it is not possible to ascertain the duration of heart failure or nature of any care provision prior to the terminal phase, and whether this fatal outcome related to incident acute heart failure, worsening of chronic heart failure, or heart failure-related sudden cardiac death. It has been suggested that decisions to include dementia on death certificates rely on medical staff regarding this as clinically significant [ 71 ]. This is considered more likely if dementia is relatively severe, reaffirming our judgement in using the certified mention of dementia as a contributory cause of death to represent a valid marker of significant cognitive dysfunction, and therefore relevant to the aegis of the MCA. Regarding generalisability, the results of this study are germane to similar clinical populations, models of care delivery, and legal constructs. While the legal status of the MCA 2005 applies to England and Wales, beyond this jurisdiction but within the UK, this legislation is closely aligned to the Adults with Incapacity (Scotland) Act 2000, and the Mental Capacity Act (Northern Ireland) 2016. There is also some global resonance through Article 12 of the United Nations Convention on the Rights of Persons with Disabilities (CRPD) of 2006. While the pertinence of specific aspects of the CRPD have been subject to legal argument [ 72 ], both this and the MCA have informed the development of mental capacity policies and legislation in Canada, Australia, and New Zealand [ 73 , 74 ].
In this hypothesis-generating study, we have investigated the impact of the MCA on the PoD of heart failure decedents aged ≥65 years resident in England whom we have shown to be increasingly affected by comorbid dementia, dying at home or usual place of residence customarily accepted as the preferred option. Our analyses of trends over the period of enactment and implementation of the code of practice relating to this legislation show little to suggest any significant influence on PoD for this relatively vulnerable clinical cohort. The background to this somewhat neutral outcome is multifaceted, administration of the Act clearly challenging for a prognostically ambiguous population subject to flux in the often-nuanced scenarios typical of real-life clinical practice, a milieu dominated by the treatment imperative. Further, even if the precepts of the MCA are correctly applied, this course of action may be ineffectual in isolation. Recent evidence suggests that achieving a good death at home requires patients and their informal carers to feel secure in that setting, effected by the provision of a 24/7 responsive palliative care service, staffed by those competent in symptom relief and with good communication skills [ 75 ]. Fulfilling the preferred PoD of those with heart failure and dementia might be better achieved by embedding application of the MCA within a system of anticipatory sympathetic clinical navigation across all care sectors, contingent upon effective upskilling of the relevant professionals, with good inter-agency and multidisciplinary collaboration to support and maintain appropriately configured community-based palliative care.
Acknowledgements
The GUIDE_Care Services project (NIHR HS&DR: 14/19/22 ) and The GUIDE_Care project (NIHR HS&DR: 09/2000/58).
Pre-publication history
Presented in part at the 7th International Advance Care Planning Conference, Rotterdam, 2019.
Abbreviations
| ACEI | Angiotensin-converting enzyme inhibitor |
| ARNI | Angiotensin receptor-neprilysin inhibitor |
| CFAS | Cognitive Function and Ageing Study |
| CQUIN | Commissioning for Quality and Innovation |
| EF | Ejection fraction |
| GDMT | Guideline directed medical therapy |
| HF | Heart failure |
| HFimpEF | Heart Failure with improved Ejection Fraction |
| HFmrEF | Heart Failure with mildly reduced Ejection Fraction |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| ICD-10 | International Classification of Diseases 10 Revision |
| ID | Identifier |
| LPA | Lasting Power of Attorney |
| MCA | Mental Capacity Act |
| NHS | National Health Service |
| NP | Natriuretic peptide |
| ONS | Office for National Statistics |
| PoD | Place of death |
| SD | Standard deviation |
| UK | United Kingdom |
| US | United States |
Authors’ contributions
JMB: conception and design; organisation of the conduct of the study; interpretation of results; primary drafting and revision of the manuscript.
IJH and TAMcD: interpretation of the results; critical review and revision of the manuscript.
WG: conception and design; organisation of the conduct of the study; data acquisition and management; statistical analysis of the data; interpretation of results; drafting and revision of the manuscript. WG is guarantor of the data.
All authors approved the final version of the paper prior to submission.
Wei Gao and Irene J Higginson at King’s College London are supported by the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. These funding bodies had no role in the design of the study, the collection, analysis, and interpretation of the data, or in the writing of the manuscript.
Availability of data and materials
Declarations.
In accordance with General Data Protection Regulations arising from the UK Data Protection Act (2018), as all patient level data was fully anonymised, no ethical approval was required. Wei Gao is accredited to analyse this data through the ONS Approved Researcher Scheme.
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Challenges and expectations of the Mental Capacity Act 2005: an interview-based study of community-based specialist nurses working in dementia care
Affiliation.
- 1 Social Care Workforce Research Unit, King's College London, London, UK.
- PMID: 22098493
- DOI: 10.1111/j.1365-2702.2011.03912.x
Aims: This study aimed to explore experiences of specialist community nurses providing information about the Mental Capacity Act and supporting people with dementia and carers.
Background: The role of specialist community nurses and case managers, such as Admiral Nurses, suggests that providing information about the recent Mental Capacity Act (2005) in England and Wales would be appreciated by people with dementia and carers and would assist in assessment and support.
Design: In-depth qualitative methodology was adopted to explore experiences and opinions of Admiral Nurses using the Mental Capacity Act.
Method: A volunteer sample of 15 Admiral Nurses were interviewed in 2008 about their experiences of explaining the legal framework to carers and people with dementia and expectations of the Act. Thematic analysis identified textual consistencies in the interviews.
Results: Most participants reported positively about the Mental Capacity Act and considered it beneficial when working with people with dementia and carers. Specific themes included knowledge acquisition and training, alongside limited confidence with implementation; practice experiences in the community and the empowering nature of the Mental Capacity Act; practice expectations and challenges with implementation.
Conclusion: The Mental Capacity Act has potential for supporting the safeguarding and empowerment role of community nurses. However, not all participants felt confident using it and speculated this would improve with greater familiarity and use, which should be facilitated by refresher training and supervision.
Relevance to clinical practice: The article concludes that nurses providing support to carers and of people with dementia may need greater familiarity about legal provisions. This may assist them in providing general information, making timely referrals to sources of specialist legal advice, and in using the Act to reduce anxiety, conflict and disputes.
© 2011 Blackwell Publishing Ltd.
PubMed Disclaimer
Similar articles
- How do Admiral Nurses and care home staff help people living with dementia and their family carers prepare for end-of-life? Moore KJ, Crawley S, Cooper C, Sampson EL, Harrison-Dening K. Moore KJ, et al. Int J Geriatr Psychiatry. 2020 Apr;35(4):405-413. doi: 10.1002/gps.5256. Epub 2020 Jan 19. Int J Geriatr Psychiatry. 2020. PMID: 31894598
- Specialist nursing support for unpaid carers of people with dementia: a mixed-methods feasibility study. Gridley K, Aspinal F, Parker G, Weatherly H, Faria R, Longo F, van den Berg B. Gridley K, et al. Southampton (UK): NIHR Journals Library; 2019 Mar. Southampton (UK): NIHR Journals Library; 2019 Mar. PMID: 30916917 Free Books & Documents. Review.
- Transcultural nursing strategies for carers of people with dementia. Bunting M, Jenkins C. Bunting M, et al. Nurs Older People. 2016 Apr;28(3):21-5. doi: 10.7748/nop.28.3.21.s23. Nurs Older People. 2016. PMID: 27029989 Review.
- Specialist nursing and community support for the carers of people with dementia living at home: an evidence synthesis. Bunn F, Goodman C, Pinkney E, Drennan VM. Bunn F, et al. Health Soc Care Community. 2016 Jan;24(1):48-67. doi: 10.1111/hsc.12189. Epub 2015 Feb 16. Health Soc Care Community. 2016. PMID: 25684210
- Dementia nurses' experience of the Mental Capacity Act 2005: a follow-up study. Manthorpe J, Samsi K, Rapaport J. Manthorpe J, et al. Dementia (London). 2014 Jan;13(1):131-43. doi: 10.1177/1471301212454354. Epub 2012 Jul 11. Dementia (London). 2014. PMID: 24381044
- Advance decisions to refuse treatment and suicidal behaviour in emergency care: 'it's very much a step into the unknown'. Quinlivan L, Nowland R, Steeg S, Cooper J, Meehan D, Godfrey J, Robertson D, Longson D, Potokar J, Davies R, Allen N, Huxtable R, Mackway-Jones K, Hawton K, Gunnell D, Kapur N. Quinlivan L, et al. BJPsych Open. 2019 Jun 13;5(4):e50. doi: 10.1192/bjo.2019.42. BJPsych Open. 2019. PMID: 31530303 Free PMC article.
- The Assisted Decision-Making (Capacity) Bill 2013: content, commentary, controversy. Kelly BD. Kelly BD. Ir J Med Sci. 2015 Mar;184(1):31-46. doi: 10.1007/s11845-014-1096-1. Epub 2014 Mar 12. Ir J Med Sci. 2015. PMID: 24619367 Review.
Publication types
- Search in MeSH
Related information
- Cited in Books
Grants and funding
- RP-PG-0606-1005/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full text sources.
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Assessing the mental capacity of a person with dementia
The Mental Capacity Act gives guidance on assessing mental capacity – when it should be done and who should do it. This can be used when assessing the mental capacity of a person with dementia.
- Dementia and the Mental Capacity Act 2005
- You are here: Assessing the mental capacity of a person with dementia
- Making decisions for a person with dementia who lacks mental capacity
- Planning ahead using the Mental Capacity Act
- Mental Capacity Act – other resources
Mental Capacity Act
When should mental capacity be assessed in a person with dementia.
You must always assume that a person is able to make a decision for themselves, until it is proved that they can’t.
A person’s capacity may be questioned if there is doubt about whether they can make a particular decision. This could happen if:
- the person’s behaviour or circumstances are making those around them doubt whether the person has capacity to make a particular decision
- a professional says they have doubts about the person’s ability to make the decision – this could be a social worker or the person’s GP
- the person has previously been unable to make a decision for themselves.
To work out whether a person has capacity to make a decision, the law says you must do a test (often called an assessment) to find out whether they have the ability to make the particular decision at the particular time.
Before the person is tested, they should be given as much help as possible to make the decision for themselves. Those who are supporting the person to make the decision should find the most helpful way to communicate with the person. This may mean:
- trying to explain the information to them in a different way
- helping them to understand the ideas that are involved in making the decision
- breaking down information into small chunks.
Not all decisions need to be made immediately. It is sometimes possible to delay a decision until a person has capacity to make it. However, this won’t be possible for every decision.
Tips on communicating with a person with dementia
The person with dementia should be offered different ways of communicating their wishes and decisions. Better communication can make it easier to meet the needs of the person with dementia.
Who can assess mental capacity in a person with dementia?
In general, whoever is with the person when a decision is being made will assess their capacity. However, this will differ depending on the decision that needs to be made – for example:
- Everyday decisions (such as what someone will eat or wear) – whoever is with them at the time can assess the person’s capacity to make the decision. This is likely to be the person’s family member, carer or care worker.
- More complicated decisions (such as where someone will live or decisions about treatment) – a professional will assess the person’s capacity to make the decision.
In general, family members and carers know the person with dementia best. They can often tell when the person is or is not able to make a decision. When a person has dementia, it’s likely that they will have to do this more often as the person's condition progresses.
If the decision is complicated, the person's carer or family members can consult a professional, such as a solicitor or a health or social care professional. Note that certain professionals may charge for advice.
Whether it is someone close to the person or a professional, they must firmly believe that the person with dementia can’t make their own decision before taking action to make the decision for them. The steps below can help with this assessment.
How is mental capacity assessed in a person with dementia?
If you need to decide whether a person has the mental capacity to make a specific decision, follow the steps below.
Always try to use your knowledge of the person to help you decide. You can also ask other people for advice – such as the person’s GP, community nurse or social worker.
Are you concerned that a person with dementia is unable to make a certain decision?
Yes : Move to Step 2.
No : The person has mental capacity. Let them make their own decision.
Can the person make the decision with help and support – for example, if they are given the right information, given more time, and communicated with appropriately?
Yes : The person has mental capacity. Let them make their own decision.
No : Move to Step 3.
Does the person meet all of the following requirements?
- They understand all the information they need to make the decision.
- They can keep the information in their mind for long enough to make the decision.
- They can weigh up the information that is available in order to make a decision.
- They can communicate the decision in some way – for example, squeezing someone’s hand or blinking their eyes.
No : For this decision, at this time, the person lacks capacity. This means they cannot make the decision for themselves and someone will need to make it for them. For decisions about everyday things such as food and clothes, this may be a carer or relative. For a more complex decision, for example about treatment, a health or social care professional may be involved.
Challenges to mental capacity assessments
The outcome of a capacity assessment is sometimes challenged. This can happen for the following reasons:
- If someone else feels that a person had the mental capacity to make a decision, but they were not allowed to do so.
- If someone feels the person did not have the capacity to make a decision, but they were allowed to make one.
The person can challenge a capacity assessment themselves, or it could be challenged by their family member, friend or even a professional.
If you want to challenge a mental capacity assessment
- Start by speaking to the person who did the assessment. Ask why they made the decision they did and explain why you disagree with their assessment of the person’s capacity.
- If this doesn’t help, you can ask for the decision to be reviewed, either by the person who first made the assessment or by the organisation involved. This may be social services or a hospital.
- If you are still not satisfied, you can make a formal complaint. For example, if you disagree with a GP or a care home manager, the surgery or care home will have its own complaints procedure that you can follow. Ask them for information about how to make a complaint.
If you challenge a capacity assessment it could harm your relationship with the person who did the assessment. Therefore, before you challenge it, think about speaking to a local advice agency, a carers’ service or a solicitor.
If you contact a solicitor make sure you ask them at the start of your conversation how much they will charge. You can also speak to Alzheimer’s Society by calling our support line.
Dementia Support Line
If someone challenges a mental capacity assessment that you have made.
If this happens, try to stay calm. Take your time to explain why you believe the person could or couldn’t make the decision for themselves.
Carers and family members are not expected to write down each time they have to make a judgement about a person’s capacity and what their reasons were, especially when they are making decisions every day.
However, if you are asked, you should be able to give examples to show why you made the decision. This doesn’t happen often. Most family members and carers will never be challenged about the capacity assessments they make.
But it is something you should think about when you are judging whether the person has capacity to make a decision. The law says you must have a ‘reasonable belief’ that the person lacks capacity, so you would need to show that you had this belief.
- Email this page to a friend.
Previous Section
Next section, further reading.
What kind of information would you like to read? Use the button below to choose between help, advice and real stories.
Choose one or more options
- Information
- Real stories
- Dementia directory
Can people with dementia vote?

How to claim Pension Credit and qualify for a free TV licence

Retirement benefits for people affected by dementia
Help for people affected by dementia on a low income, benefits for people affected by dementia of working age, carer's allowance, disability and mobility benefits for people living with dementia, sign up for dementia support by email.
Our regular support email includes the latest dementia advice, resources, real stories and more.
You can change what you receive at any time and we will never sell your details to third parties. Here’s our Privacy Policy .

You are here:
- Information and support
- Financial and legal support
Mental capacity and decision-making for people with dementia
- Publication date: May 2024
- Review date: May 2026
- Download the Mental capacity and decision making leaflet / 1mb
- (opens in a new window)
Mental capacity is a legal term that refers to whether someone is capable of making informed decisions. A person with dementia is likely to lose capacity over time, so it is important to know what to do in this situation.
Loss of mental capacity in a person with dementia
Because dementia is a progressive condition, most people with the diagnosis reach a point where they cannot make their own decisions, for example about their health, care, finances and living arrangements. This is called loss of capacity.
To have capacity, a person must be able to:
- understand the information relevant to the decision they are making
- retain that information for long enough to make the decision
- weigh up the information as part of their decision-making process
- communicate their decision to others – this does not have to be verbal; for example, nodding, blinking or hand gestures may all count
When a person loses capacity, family members, friends or professionals such as a doctor, social worker or solicitor may need to make decisions for them.
Capacity can fluctuate – for example, a person might lose capacity due to an illness like delirium (sudden, intense confusion) but regain it when they recover. They may also have capacity to make some decisions (eg what to buy from the shops) but not others (eg whether to sell their home).
How to tell if a person with dementia has capacity
As a family member, you can assess whether the person with dementia has capacity, but you must follow the Mental Capacity Act Code of Practice , which says:
- The person must be assumed to have capacity unless it is established that they do not have capacity
- The person must not be treated as unable to make a decision unless all practicable steps to help them do so have been taken without success
- The person is not to be treated as unable to make a decision simply because they make an unwise decision
- Any decision made for a person who lacks capacity must be in their best interests
- The decision must be made in the way that is least restrictive of the person’s rights and freedom
You might have an opinion about whether the person is able to make informed and safe decisions, but this is not a legal assessment of capacity; and making a decision that you disagree with does not necessarily mean they lack capacity. You must have ‘reasonable belief’ that they lack capacity according to the Mental Capacity Act , and be able to objectively explain your reasons to anyone who queries it.
Formal assessments of mental capacity for a person with dementia
If you are in any doubt about a person’s capacity, you can ask a professional to carry out a formal assessment of mental capacity. This is particularly important for major decisions like whether the person should move into a care home or sell their own home.
A formal assessment of mental capacity can be carried out by a professional such as a GP, social worker for decisions about health or care; or a solicitor for legal or financial decisions. They must consider two questions:
- Does the person have an impairment or disturbance in the functioning of their mind or brain?
- Does that impairment or disturbance mean they are unable to make the specific decision in question?
A formal assessment of mental capacity only covers the specific decision being made at that time – eg whether the person should receive an immediate medical treatment. If there are further decisions to be made, each will need a separate assessment.
The professional carrying out the assessment should keep a written record of the assessment and outcome.
Planning for a time when the person with dementia lacks capacity
When someone is diagnosed with dementia, they should be encouraged to start planning for the future as soon as possible. If the person has only recently been diagnosed or has young onset dementia (where symptoms develop before the age of 65) this may not seem urgent, but it is impossible to predict how quickly their condition will progress. Considering future plans early will make managing their care and finances less complicated and ensure their wishes are considered if they later lose capacity.
These plans should include:
An advance care plan (ACP) : this sets out the person’s wishes for their medical and personal care, including long-term care like moving into a nursing home. It is not legally binding but will help the people involved in the person’s care to make decisions in their best interests.
An advance decision : a legally binding document where a person decides to refuse certain medical treatments in the future if they cannot communicate their wishes at that time. It is also known as an advance decision to refuse treatment (ADRT) or a living Will and includes life-sustaining treatment like CPR, ventilation and antibiotics.
Lasting power of attorney (LPA) : a legal process where the person appoints someone trusted to make decisions on their behalf. There are separate LPAs for health and welfare, and property and financial affairs. Without an LPA, you may not legally be allowed to make decisions on the person’s behalf – even if you are their next of kin. You may have to apply to the Court of Protection to become the person’s ‘ deputy ’, which can be a complicated process.
A Will : this ensures the person’s money and other assets like property are left to the people and causes of their choosing after their death. It can be very difficult to make or change a Will on behalf of someone who has lost capacity, so it is important for them to make their Will as soon as possible. Dementia UK has free Will-writing offers for anyone wishing to make or amend a Will.
Who can make decisions for a person with dementia who lacks capacity?
If a person with dementia has lost capacity, other people may have to make decisions on their behalf in their best interests. A ‘best interest meeting’ should be arranged which includes the person themselves if possible, their family/friends, health and social care professionals and anyone else who is actively involved with supporting them. Family and friends can only legally make decisions if they have been nominated in the person’s LPA.
Every attempt should be made to find out the wishes of the person with dementia. If they have an ACP, this should be taken into account.
Best interests decisions should always be the least restrictive option possible. For example, if the person wishes to go out for walks but they are vulnerable and would be at risk, the least restrictive option would be for someone to accompany them, rather than deciding they cannot go out at all.
Some decisions, such as selling the person’s home or moving into residential care, can be very difficult and may cause disagreements with the person with dementia and/or family members. The best outcome is where everyone involved comes to a consensus about the best interests of the person with dementia.
Where there is a dispute, the person can access an independent mental capacity advocate (IMCA) to support them to communicate their wishes.
Deprivation of Liberty Safeguards (DoLS) for a person with dementia
Deprivation of liberty refers to a person having their freedom restricted for their own safety and being under continual supervision and control – for example in hospital or a care home. Deprivation of Liberty Safeguards (DoLS) ensure that the restrictions are appropriate and proportionate.
It is only legal to deprive an individual of their liberty by placement in a care home or hospital if:
- it is in their best interests and necessary to protect them from harm
- there are no alternative less restrictive care options
Before someone is deprived of liberty, a mental health assessor must check if they lack capacity. They and a best interests assessor (usually a social worker, nurse, psychologist or occupational therapist) will then discuss whether deprivation of liberty is in the person’s best interests. The outcome can be challenged by anyone who feels the decision is wrong.
Sources of support
To speak to a dementia specialist Admiral Nurse about capacity and decision-making or any other aspect of dementia, please call our Helpline on 0800 888 6678 (Monday-Friday 9am-9pm, Saturday and Sunday 9am-5pm, every day except 25th December) or email [email protected] .
Alternatively, you can book a phone or video appointment in our virtual clinic.
- Advance care planning
- Delirium (confusion)
- Dementia UK free Will offers
- Lasting power of attorney
- Advance decisions
- Deprivation of Liberty Safeguards (DoLS)
- Deputies: make decisions for someone who lacks capacity
- Independent mental capacity advocates
- Making decisions: who decides when you can’t?
- Making a statutory Will on behalf of someone else
- Mental Capacity Act Code of Practice
- NHS: the Mental Capacity Act
Call the Dementia UK Helpline
Our free, confidential Dementia Helpline is staffed by our dementia specialist Admiral Nurses who provide information, advice and support with any aspect of dementia.

It took me 40 years to hold my husband’s hand without fear – Mike’s story
Mike Parish, Founder of the LGBTQ+ Dementia Advisory Group, shares his journey caring for husband Tom and overcoming the fear of stigma.

“I can’t see how any family navigates dementia without an Admiral Nurse” – Elliott’s story
When Elliott’s father was diagnosed with young onset Alzheimer’s disease in his fifties, family life was changed forever.

Mohinder’s story – Reducing stigma in the Sikh community
Kaur talks about caring for her dad, Mohinder, since his mixed dementia diagnosis, and how community awareness needs to be improved.
What are the Five Key Principles of the Mental Capacity Act?
Last updated on 20th December 2023

In this article
The Mental Capacity Act contains five key principles, which must be applied at any time when the Act is being used for individuals who lack capacity.
It is useful for practitioners if they consider the principles in chronological order; principles 1 to 3 support the process before or at the point in identifying if someone lacks capacity. Once this has been ascertained, principles 4 and 5 support the subsequent decision-making process.
Anyone who is involved in applying the Act must be aware of each principle, what it means and, vitally, how it applies in each individual’s own circumstances. Although the principles must be applied lawfully, every person who is being assessed for capacity must be treated as a unique individual; two people who live with the same illness, for example dementia , will experience it in completely different ways and so coming to a decision about application of the principles will not be the same and assumptions about individuals’ capacity should never be made.
The five key principles are:
- Principle 1 – A presumption of capacity.
- Principle 2 – The right to be supported when making decisions.
- Principle 3 – An unwise decision cannot be seen as a wrong decision.
- Principle 4 – Best interests must be at the heart of all decision making.
- Principle 5 – Any intervention must be with the least restriction possible.
Principle 1 – A presumption of capacity
Every adult has a right to make their own decisions and they must be assumed to have the capacity to do so unless it is proven otherwise. Practitioners cannot assume that someone cannot make a decision for themselves just because they have a particular illness or disability, either physical or mental.
For example, if an individual has experienced a stroke, which has caused impairment to their ability to communicate, it is not acceptable to assume that this means that they lack capacity to make a decision, even if they might have some difficulties in communicating it.
Furthermore, in relation to this Principle, an assessment must only be sought if there is evidence to suggest that capacity might be an issue in terms of the decision that is being made.
When Principle 1 might be applied
- When the individual’s behavior or circumstances cause doubt about their capacity to make a decision.
- When someone is concerned about an individual’s capacity.
- When the individual has a previous diagnosis of an impairment that might impact their capacity to make some decisions.

Principle 2 – The right to be supported when making decisions
All individuals must be given every opportunity in terms of support and information before it is decided that they cannot decide for themselves. This means that every effort to encourage and support people to make decisions for themselves must be given, for example giving information in an alternative format to make it easier to understand – this is often referred to as ‘maximizing capacity’.
Other examples of ways in which capacity might be maximized include:
- Use of a signed language.
- Use of an interpreter (for verbal information).
- Use of a translator (for written information).
- Use of communication aids such as picture cards.
- Using an alternative communication format such as writing instead of speaking.
- Meeting with an individual on several different occasions to assess or review capacity.
- Changing a meeting’s location to one that is more suited to the individual’s needs.
- Changing a meeting’s time to one that is more suited to the individual’s needs.
When Principle 2 might be applied
If a lack of capacity is established then the individual must still be involved, as far as possible, in any decision that are made on their behalf.
This will apply when:
- Alternative forms of communication to ensure understanding have not been successful.
- Consistent attempts to change locations or times of a meeting have not been successful.
- Several meetings have all concluded that the individual’s level of capacity remains the same and that they are unable to make decisions on their own behalf.
Principle 2 Case study
Niko used to be a security guard working nightshifts at nightclubs and bars; after suffering a severe stroke however, he now requires full time care and supervision in a care home. Niko requires invasive surgery to fix a shunt in his brain to help alleviate pressure and reduce the likelihood of future strokes.
Initially the decision maker (DM) spoke to Niko about this decision around midday after a meeting at the care home; the DM suspected Niko lacked the capacity to make this decision as he presented as lethargic, withdrawn and disengaged.
However, the care home staff informed the DM that he is much more alert in the evenings as this was his routine for most of his working career. Upon returning out of hours, the DM was able to communicate more easily with Niko who was then able to make an informed decision.
Meeting with the individual at a time when they are more alert (including out of ‘office hours’), or by using aids that help them understand or communicate, is taking practicable steps to help the person make a decision.
Principle 3 – An unwise decision cannot be seen as a wrong decision
Individuals must retain the right to make decisions, which others may deem eccentric or unwise. Such a decision must not be used as a reason to establish lack of capacity, as each person has their own beliefs and preferences and these may be in complete contrast to those who would otherwise determine them as lacking in capacity.
Principle 3 enables a person’s culture and individual characteristics to be taken into account when they are making decisions and it is clear that any decision that an individual makes, where they have capacity to do so, must be respected no matter how odd it may seem.
The Principle also enables health and social care professionals to consider if the individual has capacity but also to determine if the individual is under pressure from someone else to make a decision. For example, if an individual suddenly decided to change their will and leave all of their money to a person they almost never see but who has recently been to visit them, there may be cause for concern that the individual has been coerced into the decision.
However, it must be taken into account that when an individual repeatedly makes unwise decisions which leave them at significant risk or when they make a decision that is out of character, this should warrant further investigation.

When Principle 3 might be applied
The following are examples of decisions that must be respected in line with this Principle, as long as the individual retains capacity:
- When an individual bases a decision on their religious beliefs, such as leaving money to a church or charity.
- When an individual decides to wear clothing that might be considered socially inappropriate.
- When an individual decides to stop taking some of their medication, which they do not believe is benefitting them.
- When an individual takes up a hobby or activity that others believe is too risky for them.
Principle 3 Case Study
Ingrid is in the early stages of dementia and is currently receiving support in her own home from professional care workers. She has recently been meeting up with her 38-year-old grandson every week in a local restaurant and has been paying for lunch each time.
Her support worker is concerned that the grandson is exploiting Ingrid for money and free lunches and considers whether the situation needs to be formally investigated. Ingrid, however, is able to recognise the concerns that the support worker has and explains that her grandson is the only relative she has contact with and is happy to spend her disposable money on him as it maintains their contact and makes her feel like she still has a purpose and identity as a grandmother.
Although this decision may appear unwise, and does cause some alarm, where the individual has capacity, their right to make unwise decisions should be protected.
Principle 4 – Best interests must be at the heart of all decision making
Decisions can only be made on behalf of an individual once it has been completely established that they lack capacity.
Any decision made on behalf of individuals who lack capacity must be done in their best interests. This means that what they would decide, if they had the capacity to do so should be the basis of the decision that is made. This can be very complex and depends on many factors that will maintain the individual’s wellbeing in a holistic manner, which means that all of their needs are taken into account.
It must also take into account the individual’s past wishes or actions, which includes an form of advance statement or directive that has been put into place.
When Principle 4 might be applied:
- When it has been full established that the individual lacks capacity.
- When it has been determined what is in the individual’s best interests.
- When previous wishes, wants and factors relating to wellbeing have been established.
Principle 4 Case study
Robert has been involved in a road traffic accident as a pedestrian and has an internal bleed. He is unconscious at the point he arrives at the accident and emergency department, although his wife is with him.
When she is informed he has lost some blood and requires a blood transfusion, she protests and states that it goes against Robert’s firmly held beliefs as a Jehovah’s Witness as blood represents life and only God can give life. She states he would rather die if this is God’s wish.
It would save Robert’s life to receive blood, however this would cause him significant emotional and psychological distress as it goes against his religious beliefs; taking Robert’s religious beliefs into consideration, it would not be in his best interests to receive a blood transfusion. However, alternative forms of medical support could be explored.
Principle 5 – Any intervention must be with the least restriction possible
Any decision made on behalf of individuals who lack capacity must be done in a manner which is least restrictive to their freedom and their basic rights. Any intervention should be assessed given the circumstances of that particular case.
The decision make must, according to this Principle, consider all options available to them and always consider if there is a less restrictive option to the one proposed.
Sometimes, it is determined that what is in an individual’s best interests is not necessarily that which is the least restrictive. In such instances, consideration must be given to amendment to forms of care and support which are less restrictive but which do not put the individual at risk and which still ensure that their needs are met.

When Principle 5 might be applied
- When all options have been considered and the least restrictive one chosen.
- When alternate forms of care have been considered if the least restrictive option may not be in the individual’s best interests for example if a person cannot go outside alone but to restrict them from doing this would be in deprivation of their liberty.
Principle 5 Case study
Ahmed has advanced stage dementia and has been found to lack the capacity to make a decision on his care and treatment; he currently requires full-time support and supervision. The care and treatment could be provided in a specialist dementia psychiatric unit, which would be highly restrictive.
The least restrictive option would be to receive care in his own home; however, this may not in the Ahmed’s best interests owing to concerns for his welfare and safety when his carers are not with him.
The less restrictive option could be a nursing home, which allows him more freedom and a less restrictive regime than a psychiatric unit, but has more supervision than he would receive at home.
Best interests
The principle of always acting in a person’s best interests is a vital part of the Mental Capacity Act and should be pivotal in any action that it taken on behalf of someone who has been identified as lacking capacity.
The Act gives a checklist of key factors that must be taken into account when trying to determine what someone’s best interests will be.
Checking someone’s best interests, includes the following:
- The wishes and feelings of the individual must be considered and these must be those, which are current and those, which may have been expressed prior to the loss of capacity.
- All relevant circumstances must be taken into account, for example the individual’s mental and physical health, how long this might be likely to last, their age, if they would be normally be able to make a decision for themselves and if a recovery is likely in the near future.
- If possible, the individual’s circumstances should be considered in terms of whether capacity to make a decision, which is non-urgent, can be postponed and made a time when their capacity might return. For example, someone experiencing a mental health crisis might not be able to make a decision in the immediate short term but they may be able to do so at a later time.
- Discrimination should be avoided; best interests should not take into account a person’s appearance, their age, condition or their behaviour, as none of these are relevant to what is best for them. The opinions of others who know the individual well might be useful in determining their best interests but such opinions should never prejudice a decision against the individual.
- The views of others should be taken into account if there is evidence that they have an interest in the welfare of the individual. The might include family, friends, carers and advocates.
- If an individual has previously put their wishes and feelings into a written statement, these must be taken into account and respected wherever this is possible.
- The individual must be encouraged to remain in control as far as this is possible and they must be involved in the decision-making process wherever they can.
There is no exhaustive list of factors to be taken into account when an individual’s best interests are being considered. However, those who are helping to ascertain what the individual’s best interests are must consider as many relevant factors as possible in order to bring about the best outcome.

Planning ahead – advance statements
A person’s best interests might be best determined by an ‘advance statement’ which is a written statement that outlines a person’s preferences, wishes, beliefs and values regarding their future care. Such a statement is important but it is not legally binding.
The aim of such a statement is to provide anyone who might make a decision in the individual’s best interests with a guide of what decision they would have made, had they have had the capacity to do so.
An advance statement can include any aspect of future health and social care, such as:
- How their religious or spiritual beliefs might affect the care that they are given.
- Where they would like to be cared for, such as at home or in a residential care setting.
- How they would like to do things, such as showering instead of bathing and following a vegetarian diet.
- Anything that concerns a practical issue such as caring for a pet or selling possessions.
Planning ahead – advance decisions
An advance decision, sometimes referred to, as a ‘living will’ is a legally binding decision that allows people who are aged 18 and over, whilst they are still capable, to refuse specified medical treatment for a time in the future when they may no longer be able to make such a decision.
An advance decision must remain valid and be applicable to an individual’s current circumstances; when it is so, healthcare professionals must follow the decision without question.
If the decision refuses life-sustaining treatment, it must:
- Be in writing, signed and witnessed.
- State clearly that the decision applies, even if it will put their life at risk.
Those individuals who make an advance decision should make their family, friends, carers and advocates know about it.


Mental Capacity Act
Study online and gain a full CPD certificate posted out to you the very next working day.
Take a look at this course
About the author

Megan Huziej
Megan has worked with CPD Online College since August 2020, she is in charge of content production, as well as planning, managing and delegating tasks. Megan works closely with our writers, voice artists, companies and individuals to create the most appropriate and relevant content as well as also using and managing SEO. She gained her Business Administration Level 3 qualification over the duration of being at CPD Online College as well. Outside of work Megan loves to venture to different places and eateries as well as spending quality time with friends and family.
Similar posts

Addressing and Learning from Repeat Customer Complaints

Case Study: Inspiring Recovery Stories from Brain Injury Survivors

The Link Between Mental Health and Cardiovascular Diseases

How Social Media Influences Adolescent Mental Health
Celebrating our clients and partners.

Privacy Overview

- Adults & Carers
Case Scenarios: Mental Capacity / Best Interests Decisions
Introduction, mary: real life outcome, joe: real life outcome, shobna: real life outcome, cerise: real life outcome.
These scenarios are intended to provoke thought and discussion with respect to issues related to mental capacity / best interests decisions and associated social work themes and topics.
It is recommended that the student is given the initial scenario and suggested questions, and then asked to consider the relevant issues and what action they might take. Following this the real life outcome can be shared and discussed further.
The scenarios are based on real life situations (with a few amendments) and the outcomes are the actual turn of events. However, it is possible to interpret the scenarios differently to open up discussion and there are some suggestions to support further debate and reflection. Students might feel that the actual outcome was not the correct one and should be encouraged to express and analyse their views in an evidence based manner.
All names have been changed to maintain confidentiality.
Mary has been admitted to hospital following a fall at home. She is 85 years old and lives on her own. Up until this point she has managed independently with some support from her daughter (who lives 50 miles away) and her neighbours. The fall has resulted in a marked reduction in Mary’s mobility and currently she is unable to weight bear, requiring full support with her personal care needs. At times she is also getting confused. Mary has been assessed by a physiotherapist who feels that the best option would be for her to move into a nursing home. Her daughter supports this recommendation as she feels that she would not be able to offer her mother the support she needs if she returned home. However, at this stage Mary has not been assessed by a social worker and there is the potential for her needs to be met at home with a package of care. Mary has stated that she does not want to go into a nursing home.
- What are the issues?
- Do you think Mary has capacity to make this decision?
- If you do, why?
- If no/not sure – why not, what are the concerns?
- Who should take responsibility for the assessment of capacity?
- Who else should be involved?
Joe is a 42 year old man with Down’s Syndrome. He has a supported living tenancy and shares his home with one other person. Joe and his housemate receive support from a provider during the day but not in the evenings and at night. Recently Joe has been frequenting a local pub where he has made friends with a group of men. He has become involved in their business and delivers packages for them during the day for which he receives a small amount of money. Joe does not know what these packages contain but believes that they are washing machine spares. Joe’s support staff have spoken to him about these activities and have told him that he must stop. They have tried to ban him from going to the pub. Joe says that the men from the pub are his friends and he can do what he likes.
- Do you think that Joe has capacity to decide whether to be involved with these men?
- If not, why not and how have you reached this decision?
- What other concerns might you have about Joe’s situation and how might you address these?
- Who would you involve?
Shobna is an older woman with a severe learning disability who has recently moved from long term hospital accommodation to her own home in the community. Following her move it is discovered that she has been suffering from an untreated problem with her throat for many years, which has led to issues with swallowing and reflux. In order to investigate further it is necessary for Shobna to have a gastroscopy. She does not communicate verbally and needs total support in all areas of her life to make her needs known. She has no contact with any family members. The consultant in charge of Shobna’s care has asked her support staff to sign a consent form for this treatment.
- Do you think that Shobna has the capacity to consent to this treatment?
- If yes, explain how you have reached this decision?
- If not, why not?
- Who should be involved in any best interests decision and who should the decision maker be?
- Have you any other concerns about this scenario?
Cerise has been admitted to hospital for the fourth time in a three month period. She has been suffering from health problems exasperated by her heavy smoking. She has some mental health issues which impact on her ability to manage her self-care needs. During her recent hospital stay it has come to light that Cerise has handed responsibility for her finances to a neighbour. There are concerns that this person is also giving Cerise medication that she has not been prescribed, to help her sleep. Cerise has admitted taking this medication and that she knows it was wrong to do so. However, she has not changed this behaviour following each discharge and is adamant that the neighbour is helping her and is her friend.
- Do you think that Cerise has capacity with respect to deciding who looks after her money?
- If you do, why do you think that?
- Do you think that the neighbour has Cerise’s interests at heart?
Case study outcomes
Mary did have the capacity to decide where she lived. However, she was not able to move home immediately as she was not well enough. A period of time in rehab was identified for further assessment of her needs and to identify whether a package of care at home would be successful. The assessor in this case could be the social worker (considering future accommodation) or the consultant (identifying health needs) but actually was a combination of both. Mary’s daughter was involved but it was made clear to her that, as her mother had capacity, she could not dictate circumstances. However, it was necessary to consider the daughter’s input as a potential future carer for Mary. Other professionals were also involved: nursing staff, physiotherapist etc. Mrs Smith’s fluctuating capacity was found to be the result of a urine infection.
Issues for further discussion:
- Input from physiotherapist – had he/she jumped to conclusions, were there pressures to discharge Mary which impacted on the recommendation to move her to a nursing home?
- Daughter – had she been swayed by the physio’s advice and has she got legitimate concerns about her mother’s ability to cope?
- Mary – did she have all the information she needed to make an informed decision (i.e. was she expecting to return home and life return to the way it was – this may be unrealistic)?
- Confusion – if someone’s capacity is fluctuating how might this affect the assessment?
Joe has a learning disability so there were concerns about his ability to make the best decision. However, he was able to identify the issues involved and showed some understanding of the consequences of his actions. The Mental Capacity Act advises that individuals are able to make ‘unwise decisions’ and ones that we would not always agree with. It was decided that Joe did have capacity and was making an unwise decision in this case. Support staff disagreed and, although their opinions were taken into account, it was felt that their actions were designed to limit Joe’s independence rather than protect him. It is important to recognise that Joe might be placing himself at risk and even involving himself in illegal activities. It was necessary, therefore, to consider the issues under safeguarding procedures and to involve the Police. Joe was visited by a social worker and police officer who talked to him about the risks he might be placing himself under and that he might be arrested for his actions. Joe agreed to stop delivering the parcels but wanted to remain friends with the men in the pub.
- Joe’s right to make ‘unwise decisions’ – what does this mean and who decides what they are?
- Support staff – what are their motivators, why might they disagree with the decision?
- Conflicting views – what might be the consequences for Joe?
- Safeguarding – how might this be approached, do you think it was appropriate to involve the Police?
Shobna did not have capacity to make this decision due to her severe learning disability and inability to understand the information required. The decision maker was the consultant (reluctantly!) as he/she was responsible for administering the treatment. However, he/she should not have asked support staff to sign a consent form as no person is legally able to do this for another adult. The consultant must make a best interests decision and proceed accordingly after consulting all parties. In this case Shobna had no family so it was necessary to consult with those that knew her well (staff, advocate, community nurse). It might be appropriate to refer to an Independent Mental Capacity Advocate (IMCA) but in this case it was not necessary as the decision was minor in nature.
- Communication – what happens if a person cannot communicate verbally, does this automatically mean that they do not have capacity?
- Signing consent forms – who is able to do this and what happens when the person themselves cannot?
- IMCA – what do they do and when would you consider making a referral for their input?
- Shobna’s health condition – in her previous accommodation this had gone unnoticed (ignored?) for many years – should this be investigated?
Cerise did have capacity with respect to deciding to give her neighbour responsibility to look after her money. She was well aware of the risks involved and prepared to accept that this person might misuse her trust. Cerise valued the relationship she had with the neighbour even though they could not really be considered friends. There were some concerns about the neighbour spending Cerise’s money but she herself did not want to change this arrangement so, although there was a safeguarding planning meeting, no further action was taken. From Cerise’s numerous admissions to hospital, however, it was identified that she was struggling to look after herself so alternative housing options were discussed with her. She was keen to live somewhere with more support and eventually moved to an extra care housing tenancy. The added benefit was that the neighbour then decided to no longer offer his/her services and Cerise received tenancy support which helped her to manage her money independently.
- Unwise decision – was Cerise making one, do you agree with the outcome of the assessment?
- Safeguarding – do you agree with the action taken, if not what else could have been done?
- Professional judgements – did you make any assumptions, what was behind these, how might they impact on your actions?
– End –

- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Making decisions for...
Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK
- Related content
- Peer review
- Gill Livingston , professor of older people’s mental health 1 ,
- Gerard Leavey , director of research 2 ,
- Monica Manela , research doctor 1 ,
- Deborah Livingston , research fellow 1 ,
- Greta Rait , senior clinical scientist 3 ,
- Elizabeth Sampson , senior lecturer in psychiatric and supportive care of the elderly 1 ,
- Shilpa Bavishi , research assistant 1 ,
- Khodayar Shahriyarmolki , research assistant 1 ,
- Claudia Cooper , senior clinical lecturer in old age psychiatry 1
- 1 Department of Mental Health Sciences, University College London, London W1W 7EJ
- 2 Northern Ireland Association for Mental Health (NIAMH) and University of Ulster, Belfast BT7 1HE
- 3 MRC General Practice Research Framework, London NW1 2ND
- Correspondence to: G Livingston g.livingston{at}ucl.ac.uk
- Accepted 10 June 2010
Objective To identify common difficult decisions made by family carers on behalf of people with dementia, and facilitators of and barriers to such decisions, in order to produce information for family carers about overcoming barriers.
Design Qualitative study to delineate decision areas through focus groups and complexity of decision making in individual interviews.
Setting Community settings in London.
Participants 43 family carers of people with dementia in focus groups and 46 carers who had already made such decisions in individual interviews.
Results Family carers identified five core problematic areas of decision making: accessing dementia related health and social services; care homes; legal-financial matters; non-dementia related health care; and making plans for the person with dementia if the carer became too ill to care for them. They highlighted the difficulties in making proxy decisions, especially against active resistance, and their altered role of patient manager while still a family member. Families devised strategies to gain agreement in order to ensure that the person with dementia retained dignity.
Conclusions The following strategies helped with implementation of decisions: introducing change slowly; organising legal changes for the carer as well as the patient; involving a professional to persuade the patient to accept services; and emphasising that services optimised, not impeded, independence. To access services, carers made patients’ general practice appointments, accompanied them to the surgery, pointed out symptoms, gained permission to receive confidential information, asked for referral to specialist services, and used professionals’ authority to gain patients’ agreement. End of life decisions were particularly difficult. They were helped by knowledge of the person with dementia’s previous views, clear prognostic information, and family support. Information sheets to help carers to overcome barriers to proxy decision making have been developed; their impact in practice has yet to be evaluated.
Introduction
As the population ages, a large and increasing number of people are caring for a relative or friend with dementia. 1 Decisions often have to be made on behalf of people without capacity, which includes many people with dementia, and relatives often do this, either by themselves or by acting as an advocate and giving advice to professionals for the person for whom they care. They report major barriers to doing so, including difficulties in deciding what to do and the family member experiencing distress in making a decision. 2 Other obstacles to proxy decision making include having insufficient information about the possible alternatives and their effect. 2 3 In addition, lack of emotional support for families of people with early dementia who still have capacity acts as a barrier to the difficult discussion of future care options, including placement in residential institutions with 24 hour care (care homes), and makes reaching a decision more difficult. 4 5 Decision making will differ according to previous experiences, education, and social and cultural background. 6 Some people seek information, whereas others do not. 7 All are facilitated in making decisions if they have access to good information and support. 8
Some countries have legislation about proxy decision making, enabling family carers to make decisions on behalf of people without capacity, but this is not universally the case, and legislation differs. 5 9 In some countries, including the United Kingdom, mental capacity legislation means that proxy decision making by family members is likely to be more broadly used in future. In the UK, if a person lacks capacity to make their own medical or social decisions, the Mental Capacity Act mandates that (except when a valid advanced directive is in place) a relative who has been given lasting power of attorney makes such decisions. If there is no lasting power of attorney, the closest relative must be consulted and his or her views only disregarded for a very good reason, such as if they do not seem to be in the patient’s best interest or are impossible. Relatives therefore have a high level of involvement in decision making, and this heightens the need for information about barriers and facilitators in family carers’ experience of making such decisions; however, research has so far concentrated on professionals. 10 The first decision is often about obtaining a diagnosis, and this is often difficult to make, particularly in the early stages. The general practitioner may make the diagnosis or refer the patient to a specialist. Assessments include a history and mental state and cognitive tests from the person, a history from an informant, and physical investigations.
Previous studies have asked carers’ views about theoretical scenarios of medical decisions at the end of life, such as whether life sustaining treatment and resuscitation should be offered. 3 11 This study is, to our knowledge, the first to ask carers about the decisions that they have made.
Our objectives were to identify common difficult decisions made by family carers on behalf of people with dementia, to identify facilitators of and barriers to decision making in order to be able to provide appropriate care and overcome such barriers, and to use this information to assist in such decisions in future by making information available about barriers and how to overcome them in printed leaflets and online. This information is for family carers and patients and also a wider readership of doctors and other professionals from all specialties who encounter relatives and patients with these difficulties and who help them to make such decisions.
Participants were from healthcare settings in inner and outer London. These comprised four general practices, five community clinics and three memory clinics in mental health services, and a specialist neurology dementia clinic for people with atypical or young onset dementia.
The study had two phases: an initial focus group phase to generate a list of the most common areas in which family carers reported making difficult decisions on behalf of recipients of care, and a second phase in which these domains were discussed during in-depth individual interviews with carers who had experienced making a decision in those particular areas. In the second phase, we stopped recruiting once we had agreed that new or different subjects and perspectives were unlikely to be provided by additional participants. We recorded sociodemographic characteristics of all participants.
We used slightly different methods of identifying and approaching patients in primary and secondary care in each phase. In primary care, general practices identified people with dementia and wrote to their family carer if known to explain the study and ask if they would agree to be approached by researchers. If they wrote or phoned agreeing to this approach, we sent an information sheet.
In secondary care, one of the research clinicians wrote directly to the carers they knew who had previously agreed to be approached about studies. The letter specified that unless the carer contacted the clinician’s team a researcher would phone to ask if they wished to discuss the study further or take part. Alternatively, the clinician spoke to the carer during a consultation. The clinician explained the study briefly and gave an information sheet. In clinics, a DeNDroN (research network) staff member was available to explain the study further.
In all settings, a researcher phoned carers a few days after the information sheet had been sent, asking if they wanted to meet the researcher. Those who agreed to this were seen, and the study was discussed with them; those who gave informed consent then participated.
Participants
We defined a carer as an adult family member or friend who gave unpaid support for the person with dementia and who regarded themselves as a family carer. We selected for individual interviews those carers who had made decisions about the care of a person with dementia. All were currently caring or recently bereaved. To cover the range of experiences, we selected our purposive sample for people with diverse socioeconomic characteristics (sex, age, level of education, religion, and ethnicity) and those caring for people at different stages of dementia. Some were newly referred, and others had been known to services for a considerable time; we also included bereaved carers in an attempt to cover the spectrum of experiences and views. The aim of the purposive sampling was to maximise the validity of our findings by ensuring that we included carers from a range of sociodemographic groups and achieved maximum variation.
Phase 1: focus groups
We allocated participants to focus groups on the basis of shared or similar experiences, to ensure that membership remained sufficiently homogenous. The groups comprised people caring for parents (five participants), spouses (14), people now living in care homes (eight), people with young onset dementias (six), and those seen in primary care settings (10). We facilitated discussions by using a topic guide about carers’ experiences, attitudes, feelings, and beliefs.
Phase 2: individual interviews
We reviewed the transcripts of focus groups to ensure that the interview schedules covered the subjects raised in focus groups relating to each decision area. We then discussed participants’ personal accounts of making decisions in the five areas identified by the focus groups as most common and problematic in individual interviews by using semi-structured schedules particular to each area. Seventeen of the participants in focus groups who had made one of the decisions in question also participated in individual interviews. Interview schedules covered choices, barriers, and facilitators in decision making; cultural, religious, and spiritual beliefs and practices; and dilemmas, consequences, and advice. Interviews continued until analysis indicated that saturation of data had been achieved.
We digitally recorded discussions and interviews, transcribed them verbatim, and removed identifying information to preserve anonymity. We used the qualitative research software programme Atlas.ti 5.2 to assist in coding, management, and analysis of data. 12 We sent the transcripts of individual interviews to the participants for comments and alterations. Participants reading transcripts of their interviews is considered helpful in some qualitative studies as a method of quality control and validation. Participants can ensure that the transcript is a true record of what they intended to say or, where necessary, can elaborate or provide a more nuanced perspective.
Carers all gave informed consent to the study, including recording and anonymous quotation. They have read and approved their transcripts and been given the materials with all the quotes in. We do not think that a carer could be identified, as we have deliberately given non-specific demographic information.
In both the focus group and individual interview stages, the interviews and analysis were part of an iterative process in which the study team agreed on a preliminary coding frame by using initial interviews and a broadly thematic content analytic approach. 13 We analysed the focus group data to yield the full range of views on current and required provision of information. We began analysis after completing the first two focus groups and at a similarly early stage in analysis of the individual interviews. This is consistent with grounded theory techniques.
In the individual interviews, we developed our coding frame to cover the predetermined subjects and new and emergent themes. Two raters coded all data independently to ensure reliability. 14 Disagreements between the raters were resolved through discussion with each other after they had completed their independent rating and by discussion with GL and CC. The team met periodically to refine the interviews in accordance with emergent themes and frame as the coding progressed 13 ; for example, we added prompts about research as this was raised by interviewees. A thorough engagement with the texts and the consensus of several researchers helped to ensure that we did not end recruitment prematurely.
Tables 1 ⇓ and 2 ⇓ describe the sociodemographic characteristics of the 43 focus group participants and the 46 individual interviewees and their care recipients. As many individual interviewees had made more than one of the identified decisions, we had a total of 107 interviews covering the five decisions.
Sociodemographic characteristics of family carers and recipients of care. Values are numbers (percentages) unless stated otherwise
- View inline
Number, relationship to care recipient, sex, and ethnicity of 46 carers taking part in topic specific individual interviews
Focus groups
From the focus data, we identified five core problematic areas of decision making in dementia care and some of the factors affecting them (table 3 ⇓ ).
Problematic areas of decision making for family members in dementia care
Individual interviews
Help seeking and decision making were seldom clear cut. Participants consistently described difficulties with the responsibility of making a decision for another adult, denial and resistance by the person with memory problems, and barriers to accessing services. In circumstances in which long time roles and patterns of authority are reversed and confidences are sometimes breached, making decisions for a family member is burdened with difficulty. The ease of decision making was often determined by factors related to all the protagonists. Generally, these are the patient, the primary carer, other family members, the healthcare and social care professionals, and sometimes the voluntary sector. These relationships are not always comfortable, encompassing love, duty, and bewildered resignation. In many cases, the journey towards a decision was directed by a mixture of fatigue and a lack of obvious or available alternatives. The participants in the study related a complex, often distressing journey in negotiating an appropriate mixture of care and control within the care system.
Financial and legal decisions may be made in the context of family and professional mistrust and scepticism. Thus, in addition to service related factors, the quality of family relationships and the ensuing dynamics often determined the speed and direction of advice and support sought and the outcomes in terms of decisions made. The caring experience varied widely in terms of the relationship of patient and carer, accommodation arrangements, and the (associated) level of formal care provided. Some of this complexity is exemplified in the following sections. The box outlines barriers to and facilitators of decision making. We illustrate below some of the complexity of decision making with reference to key issues and situations.
Barriers to and facilitators of decision making by carers
Denial of problem
Rejection of help
Professional
Not recognising problems
Late diagnosis
Timing and quantity of information giving
Confidentiality and data protection
Bureaucracy and rigidity (sticking to protocols)
Psychological
Role conflict
Carer guilt
Family conflict
Rigidity (solution fixed when circumstances change)
Facilitators
Deference to authority
Suggesting interventions to facilitate agreement
Quality and timing of information
Ensuring that the patient is asked to give permission for information to be given to carers
Access to legal advice
Psychological—coping strategies
Carer accompanying patient to professionals
Social support (extended family, voluntary and community networks)
Resources for carer (financial and social)
Family cohesion
Re-conceptualisation of services as optimising independence
Allowing services to develop slowly (rather than “all or nothing”)
Knowledge of what the patient wanted when competent
Sharing—for example, power of attorney being made for both the carer and the person with dementia
Accessing healthcare and social care help for dementia
Once the carer had decided to seek help, the first point of contact was usually the general practitioner, often despite opposition from the recipient of care:
“The hardest decision that I’ve had to make was to convince my wife there was something wrong with her, she didn’t want to know . . . she wouldn’t talk to no one about it.” (husband of early onset patient)
Carers tackled this problem in a variety of ways. Going to see the general practitioner together helped, for example, as did the doctor writing to the patient. In some case, families’ strategies included manipulation, albeit benign:
“I used to [be] a bit conniving, say I’m coming to see the doctor, for me; that’s the only way I could get her to the surgery, and then you start talking . . . she loved the doctor.” (husband of early onset patient)
Professional encounters
Once at the doctors, carers often described difficulties in obtaining the correct diagnosis, with problems either discounted or attributed incorrectly, or a reluctance to refer to specialist services. The patient’s lack of insight often contributed to this, and sometimes they failed to receive a diagnosis until their relative’s behaviour was very high risk:
“He was sort of in denial. . . He convinced the doctor there was nothing wrong.” (wife)
“It took me about eighteen months to get [the doctor] to . . . give her a test . . . later . . . my daughter . . . found the gas turned on and it’s not alight, so . . . when I left for work I had to turn the gas off, then I really pushed the doctor.” (husband of early onset patient)
They commonly found that confidentiality impeded them in receiving information, but if it was clear that the care recipient gave permission then this improved:
“On the phone the people would say ‘well we’d have to speak to your mother first to get permission to talk about her issues’ because you know they couldn’t say anything to me. . . I have to get my mother’s permission to represent her.” (daughter)
Introducing services
After carers had made a decision, they had to negotiate this with the person for whom they cared. Strategies such as introducing services a little at a time or enlisting the doctor’s medical “authority” helped. If the patients still refused, this sometimes led to greater restriction:
“She wasn’t washing herself, she kept saying ‘no, I don’t want [carers].’ She [healthcare professional] said ‘you can try and help slowly.’ I said ‘yes we will try it once a week.’ They started a care package and it is every day now.” (son)
“So long as you say . . . ‘doctor’ in the sentence . . . she will go along with that, she will listen to that authority so that’s been good actually.” (daughter)
“He refused all help. He wouldn’t let anyone come into the house, no form of carer. But then, he was wandering, a danger to himself . . . he was out all night, no idea where he had been.” (wife)
Information for carers
Information was key to making decisions, but after diagnosis the quality, quantity, and timing of information about dementia provided by professional services was sometimes considered unhelpful. Carers wanted information but not all at once:
“We didn’t realise what dementia meant, the implications. . . I think that people who are carers should receive some training . . . told what to expect and what to do, before it happens, not when it happens.” (widower)
“I found, when he was first diagnosed, it was an awful lot to take in, you’re given all this information on what you should be doing, you don’t really want to know it.” (wife of early onset patient)
“The advice that I would give is get as much information as possible, because information is really hard to get . . . but . . . is there.” (wife)
Decisions about care homes
The decision that a close relative should go into a care home provoked considerable difficulties. An acknowledgment exists, albeit often tacit, that this represents a major rupture in the relationship between carer and patient. In this context, the sense of relief at care being provided is often accompanied by feelings of failure or betrayal. For example, although carers often knew that the person with dementia would never have wanted to live in a care home, as circumstances changed they felt compelled to act against this knowledge. Most families decided to keep someone at home as long as possible. The sense of guilt and failure seems to be particularly distressing for people obliged to cope alone, and although the timing and appropriateness of placement in a care home is not always agreed, carers generally found it helpful to hear the perspectives of other members of the family or professionals. This “gave permission,” alleviated guilt, and re-conceptualised care homes as providing safety, either for the carer or the person with dementia, particularly if homecare services were refused. The following quotes illustrate these concerns:
“And my husband said ‘promise me one thing, you’d never put me into a home,’ and I said, ‘I promise’.” (wife)
“He has to be at the day centre six days a week . . . just one day a week when he’s home on Sunday, it’s very difficult, so it’s better than him being in a nursing home.” (wife of young onset patient)
“My husband couldn’t continue as he was, and he refused to have any one looking after him at home.” (wife)
“And because I had . . . somebody [brother] close to me saying [a care home], he could see it from a different angle to me and . . . that’s when I decided.” (daughter)
“The GP thought that it was quite irresponsible, the idea that we should wait until my husband had an accident or something very serious happened.” (wife)
“At the end of the four weeks of respite, the man in charge in the home said to me ‘how can you take him home? It always needs two people to see to him.’ So, after that, I decided to leave him there.” (wife)
When choosing a care home, carers looked for safety, good relationships with the staff, pleasant smell, cleanliness, good atmosphere, geographical proximity to family/friends, cultural and religious identity, activities, communal areas, garden and lift access, and previous respite experiences. Many used religious and voluntary organisations to which they were connected. Carers were concerned about financial implications. Internet sites were helpful, as were voluntary sector organisations such as the Alzheimer’s Society. Although these concerns added to the pressure in making decisions, carers agreed that feeling strongly involved in the decision and that it was the right thing to do was essential, as indicated by this participant:
“I think whatever you do, you’ve got to do it with a relatively good grace. If you feel that you’ve been pushed into it, or you’re obliged to do it, then I think it won’t work.” (widow)
Legal matters
Finances were a major concern for many carers. In part, taking over their management represented a milestone in deterioration and role change and, when the patient had managed the family finances, a role reversal. Joint accounts made the logistics easier. For family carers, a pressing concern about the person’s vulnerability to exploitation exists, along with a worry that they are being perceived as exploitative. However, power of attorney for financial decisions, as opposed to health and social care, seemed easier. Many participants related the importance of making decisions about wills and power of attorney when the patient retained capacity. This was sometimes recognised only with hindsight:
“I realised he couldn’t, no longer sign cheques and things like that, and then we just put everything into joint, all our financial things are joint.” (wife)
“The only thing that could happen now is Court of Protection . . . because my wife can’t sign.” (husband of early onset patient)
“I made wills, my advice is to get it done sooner rather than later.” (husband of early onset patient)
Various strategies were used to alleviate the difficulties. For instance, organising legal changes for the family carer, as well as the person with dementia, was often more acceptable than to do it for the person with dementia alone. Leaving manageable amounts of money for the person with dementia ensured that they retained as much financial independence and dignity as possible:
“I only did property and financial affairs—that took so long. And to have done health and welfare would have raised too many awkward questions . . . doing financial affairs fitted in with all our discussions about . . . our money.” (wife)
“. . . if we did [lasting power of attorney] for both of them [parents], it wouldn’t feel like it was just for my mum because she’s dementing.” (son)
“. . . what I did was I opened a separate account with my name on his behalf but left his account open and every Friday £30 went into [it].” (niece)
Some people completed the forms themselves, on paper or through the internet, or with help from Age Concern, whereas most needed a solicitor to deal with the complexity:
“The best thing I. did . . . was to get a good solicitor and leave it [power of attorney] to him.”(widower)
Families were often able to persuade someone to stop driving, sometimes with a doctor’s help, rather than reporting them to the Driver and Vehicle Licensing Agency:
“My husband was a little unhappy about the driving, but he accepted it; I think he was both unhappy and relieved.” (wife)
“I was already getting worried about it [driving], we saw one of the doctors and [when I asked] he said, ‘well, if he doesn’t have to drive, I don’t think he should’ and he agreed. . . That’s one of the few decisions . . . I pushed him into.” (wife)
Making decisions about non-dementia related health care
Several carers mentioned the need to weigh up the impact of a general anaesthetic on cognition, as well as how dementia would affect recovery:
“She has also arthritis on her knees, but . . . she won’t understand how to do physiotherapy . . . we (sisters) discussed [the decision to have a knee replacement] . . . it’s not worth it.” (daughter)
“She had previously been to have an extraction . . . they did try [local anaesthetic] . . . she won’t keep her mouth open . . . she really has to have the anaesthetic. It is bad enough how she is; I don’t want her to be in pain.” (sister)
“We weren’t in agreement with each other . . . whether to have a heart operation. . . . with hindsight . . . it was the right decision . . . he decided . . . it made his mental abilities much worse, but physically, he’s much better.” (wife)
Non-dementia health decisions
End of life care and resuscitation.
Discussions about end of life care were often influenced by experiences with other people they had known with dementia or other illnesses:
“My mum was talking about when she was going to die before she even got unwell. . . I wasn’t to have her resuscitated.” (daughter)
“My brother-in-law fell . . . and they said to his wife ‘do you want us to resuscitate him?’ and she said ‘I can’t tell you to let him die!’ So they kept him going . . . he came to at one stage . . . and he shook his head like that and my sister-in-law said ‘I’m so sorry, I should have said to them don’t resuscitate him’ and I thought, ‘I’m not going to let my husband suffer in that way’.” (wife)
Some talked about the helpfulness of consulting other family members, or the difficulties when they did not agree:
“Resuscitation was the biggest decision. . . I consulted with my children and my wife’s sisters and they were all in agreement . . . she has gone through enough.” (husband of young onset patient)
“My brother and sister . . . wanted the drip, antibiotics and the oxygen reinstated . . . and the doctor said ‘it will flood her heart’ and she died a horrific death . . . but my brother always said, ‘oh where there’s hope’ . . . There was no hope.” (daughter)
Quality of life was important to the decision:
“I would not like my sister to be resuscitated . . . she has got no quality of life . . . so why put her in the same predicament and for us to be in sorrow . . . longer?” (sister)
“Mum’s body . . . just didn’t function. . . The doctor said, ‘would you like us to resuscitate,’ we thought another couple of days of suffering like this, why?” (son)
At the end of life, the decision about whether instituting artificial nutrition would keep the person most comfortable was very difficult and complex:
“When it got very close to the end of [his] life, they did ask me whether I wanted him to be fed through his stomach . . . the doctor . . . gave me the facts and didn’t try to influence me . . . but it seemed . . . that . . . to prolong his life would be cruelty.” (wife)
“In the last few weeks she virtually stopped eating . . . they . . . spoon fed her . . . it was all done in a very calm, serene way. I’d be against it if it was forced.” (son)
“When he finally was coming home, we still had to feed him artificially through the abdomen, and . . . he was never quite the same man again.” (widow)
Only one participant had made a written advanced decision (of doubtful legality, as it asked for life to be shortened):
“Both of us have written living wills . . . there are certain circumstances under which we prefer . . . actually to have our life shortened . . . we did give the draft . . . to our GP . . . he told us that we had to reformulate it because . . . it has got a different name . . . advanced decisions.” (wife)
Taking part in research
Carers considered the person with dementia’s previous wishes when deciding about participation in research. They also considered potential benefits to the person with dementia and to others or thought that they derived personal benefit:
“I know he would volunteer partly because . . . he did [participate in research].” (wife)
“When we talked about donating organs before [he] got ill, he’d always said he didn’t want it . . . so it was quite simple.” (wife of young onset patient)
“If they ever came out with a drug I don’t mind . . . making that decision for my sister to be given a chance.” (sister)
“I really wanted to be part of the research, because my own experience . . . was horrible, anything that can be done to stop other people having that same experience . . . has to be worthwhile.” (daughter)
“. . . having the assessment through the research was very useful . . . one learns a little also because . . . you don’t realise sometimes how you are feeling.” (widow)
Plans for future if carer was too ill to care
Several carers had made plans for the care of the person with dementia if they were no longer able to provide care, whereas others thought that things should be sorted out as they happened:
“What if something happened to me? . . . I’ve even spoken to my children about that . . . they would not be responsible for their dad, they’d be there for him, but other arrangements would be made for his care. I’ve set everything out . . . I want to make things easy for my two children.”(wife)
“You have to decide [everything]; and it’s not a matter of it being easy, or not easy, you just have to do it because that’s the practicalities of life.” (wife of young onset patient)
“When I read and see things about how people go through . . . that is a bit worrying. But then I decided I will just take each day as it comes. You can’t look too far in the future.” (wife)
Family carers of people with dementia have to make difficult decisions throughout the course of dementia from onset until the end of life. This is the first study to ask a purposively sampled, diverse range of people caring for someone with dementia what were the most difficult decisions and what were the barriers and solutions. Participants consistently described particular problems, emphasising the resistance of the person with dementia and changing their long time family role. They became a patient manager, while remaining a family member. Even with the legal authority to make and enforce decisions for the person without capacity, in practice families nearly always need to gain agreement to ensure that the person with dementia retains dignity. To acquire a diagnosis or a referral for diagnosis, for example, carers had to make the transition from seeing their relative as an autonomous adult to being their spokesperson. Family cohesion alleviated emotional conflicts and facilitated decisions, as did professionals’ support, information sharing, and use of their “authority” to advocate helpful interventions. Carers emphasised the practical and emotional importance of the fresh perspective of other family members and support from professionals and voluntary organisations in making decisions. Some carers had found sources of support and information, but others had not. Many of the carers said that this was the first time they had had the opportunity to discuss the difficulties around making decisions; they valued this and questioned why they had not been offered it earlier. Problems were aggravated by carers’ role conflict and guilt. Family disagreements compounded decision making difficulties. This was particularly evident in end of life decision making, when the need for decisions to be unanimous was, perhaps, particularly strong. Those who opted for the more life prolonging treatment seemed to be more likely to express regret. Only one person cared for someone with dementia who had made an advance directive, and circumstances had not occurred in which it could be used.
Strengths and weaknesses of study
Our carers came from a wide range of settings and sociodemographic backgrounds, and we achieved theoretical saturation. All those who took part in the research were willing to do so, lived in a city, recognised that their relative had dementia, and for the purposes of our study were willing to define themselves as family carers. Most of the participants were known to secondary care. Thus, we may have missed people who did not see themselves as carers or whose relative had not received a diagnosis. Nevertheless, some of the participants had originally not accepted that their relative had dementia, or their relative had refused to go to doctors, and some were known only to primary care. We did not make any assessment of the patients’ ability to make the decisions the carers mentioned, and some may not have lacked capacity in the situation specified. All of the people with dementia were at least 50 years old. Their respect for doctors’ authority may be a generational effect, and attitudes may be different in decades to come. Younger carers (five were in their 30s), however, also emphasised how professionals’ authority helped emotionally as well as practically. Carers and people with dementia may have differing views about difficulties and what to do in a given situation, and this is not always related to the dementia. They may have separate interests, or their views may have diverged. 15
Comparison with other studies
Other studies have, like ours, found that many patients are not diagnosed as having dementia until late in the illness, often at a time when a crisis occurs, which could have been avoided with earlier diagnosis and intervention. 16 Late diagnosis often comes too late for the person with dementia to be able to make most choices; earlier diagnosis and management of dementia facilitates patients’ choice and improves the quality of life of the person with dementia and their relatives, as well as being cost effective. 16
Our study adds some strategies to facilitate such diagnosis. In common with a recent ethical report, we found that doctors have an important role as either barriers or facilitators and should, for example, actively encourage the person with dementia to allow sharing of information with family carers. 15 Professionals should deliver information in chunks, as carers valued information but, as in previous studies, found it overwhelming if given all together at diagnosis. 17 As in previous studies, interviewees found that professionals’ views and advice reduced guilt. 15
Clinical and policy implications
Supporting carers to make decisions is an important and urgent next step. We have, therefore, devised a series of factsheets covering barriers and facilitators for family carers based on the findings of this study and covering these key decisions, including carers’ experiences in their own words and with a summary “Things to think about” section. These set out the strategies that enabled carers to cope. Providing information for carers is a critical component of high quality dementia care, but carers do not want it all at once. 17 We have therefore designed our factsheets so that carers can be provided with information currently relevant to them or that they would like to think about. These are available on bmj.com, and we are also planning to make them available in non-clinical settings, as they discuss accessing help and should therefore be useful before diagnosis.
We recommend several important strategies to help to overcome the difficulties described (summarised in the box). Professionals can recommend the following successful strategies to aid the implementation of most decisions: introducing change slowly, organising legal changes for the family carer as well as the person with dementia, and involving a professional to persuade the person with dementia to accept services and emphasising that services optimise rather than impede independence. When dealing with services, carers often needed to ask for what they felt was needed rather than waiting for it to be offered.
To access diagnosis and care, the carer can make appointments with the general practitioner, accompany the patient to the surgery, point out symptoms, gain permission to receive confidential information, ask for a referral to specialist services, and use the professionals’ authority to persuade the patient to agree to this.
People found decisions around end of life care very difficult. They were helped by knowing the views that the person with dementia held before losing capacity about what they would want in this situation, as well as by clear prognostic information, knowledge about future quality of life, and family support.
Services need to be appropriate for age and culture. Many people referred to their own ethnic or family culture as being one in which, for example, people would not use care homes. Carers often wanted a care home or day centre specific to their beliefs and cultures, so their relative would feel more at home. Similarly, they made decisions about end of life care on the basis of the person’s previous wishes, religious views, and quality of life.
Although considering previous discussions with the person with dementia to understand their wishes was often helpful, carers found that circumstances changed. For example, the decision never to allow a relative to move to a care home was revised because of unacceptable risks if they continued to live at home. They then needed to make decisions that had previously been unthinkable, and they often felt guilty.
Unanswered questions and future research
We have identified that family conflict makes decisions more difficult, but family counselling has not previously been found to be of benefit for carers and future research should perhaps concentrate on using specific coping strategies that have previously helped carers to manage. 18 Although we hypothesised before collecting the data that we would find ethno-cultural differences related to help seeking, in fact such differences were minor and mainly reflected dietary and language choices. Although social class differences may exist, they did not emerge strongly from the data.
Our study raises several unanswered questions. Should all doctors routinely be asking patients whether they can discuss their medical details with their closest relative in all circumstances or in specified circumstances in the future? Our family carers often did not think that they had been informed or understood what might happen if an attempt was made to resuscitate someone with advanced dementia. How can this be communicated better? Although we have produced leaflets to help with the concerns that were raised by relatives, the impact of the leaflets has yet to be evaluated in real life. We do not know whether they will help if delivered by themselves or if they need to be delivered by a professional who can provide the back-up care and help carers to access the services suggested. Further work should evaluate this.
What is already known on this topic
A large and increasing number of family carers make proxy decisions for relatives or friends with dementia
They find this distressing, and barriers to making such decisions include lack of information and emotional support
Better information faciltiates such decisions
What this study adds
Difficulties in decision making for people without capacity are often aggravated by their active resistance
Legal authority is not enough; families had to devise strategies to overcome barriers and to gain agreement
Support for carers to make these decisions is important; the strategies from this study will be made available to carers and professionals
Cite this as: BMJ 2010;341:c4184
We thank the carers who gave us their time and talked of their experiences; North Thames DeNDRoN, PCRN-GL, and clinicians in Camden and Islington NHS Foundation Trust, Barnet, Enfield and Haringey Mental Health Trust, Camden Primary Care Trust, and the Dementia Research Centre, UCLH, for helping to identify participants; the Alzheimer’s Society Islington for helping us to design the study; and Camden and Islington NHS Foundation Trust for allowing us to use Camden Mews day hospital as the venue for the focus groups.
Contributors: GLivingston, CC, GLeavey, S Nurock, ES, and GR contributed to the design of the study. GL, ES, K Judd, DL, and SB identified the participants. GLivingston, GLeavey, CC, ES, and GR facilitated the focus groups. MM, DL, SB, KS, and R Li did the individual interviews. DL, MM, SB, and KS analysed the data for themes and, with GLivingston and CC, agreed on a coding frame. All authors contributed substantially to conception and design, or analysis and interpretation of data, and to drafting the article or revising it critically for important intellectual content. GLivingston is the guarantor.
Funding: The study was funded by BUPA Foundation, which had no role in the study design; the collection, analysis, and interpretation of data; or the writing of the article and the decision to submit it for publication. All the researchers are independent of the funders.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that GLivingston, GLeavey, GR, and CC have support from BUPA Foundation for the submitted work; (2) SB, KS, DL, and MM have no relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all authors have no non-financial interests that may be relevant to the submitted work.
Ethical approval: The relevant research ethics and research and development committees approved the study. Participating family carers gave signed informed consent.
Data sharing: No additional data available.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode .
- ↵ Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005 ; 366 : 2112 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Hirschman K, Kapo J, Karlawish J. Why doesn’t a family member of a person with advanced dementia use a substituted judgment when making a decision for that person? Am J Geriatr Psychiatry 2006 ; 14 : 659 -67. OpenUrl CrossRef PubMed Web of Science
- ↵ Mezey M, Kluger M, Maislin G, Mittelman M. Life sustaining treatment decisions by spouses of patients with Alzheimer’s disease. J Am Geriatr Soc 1996 ; 44 : 144 -50. OpenUrl PubMed Web of Science
- ↵ Davies S, Nolan MR. ‘Making the best of things’: relatives’ experiences of decisions about care-home entry. Ageing Soc 2003 ; 23 : 429 -50. OpenUrl CrossRef
- ↵ Alzheimer Research Forum. AD research participation: informed consent complicates trials, part 1. 2008. www.alzforum.org/new/detail.asp?id=1867 .
- ↵ Butcher HK, Holkup PA, Park M, Maas M. Thematic analysis of the experience of making a decision to place a family member with Alzheimer’s disease in a special care unit. Res Nurs Health 2001 ; 24 : 470 -80. OpenUrl CrossRef PubMed Web of Science
- ↵ Wackerbarth S. The Alzheimer’s family caregiver as decision maker: a typology of decision styles. Gerontologist 2002 ; 42 : 340 . OpenUrl
- ↵ Hansen L, Archbold PG, Stewart BJ. Role strain and ease in decision-making to withdraw or withhold life support for elderly relatives. J Nurs Scholarsh 2004 ; 36 : 233 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Department of Health. The Mental Capacity Act 2005. 2005. www.opsi.gov.uk/acts/acts2005/ukpga_20050009_en_1 .
- ↵ Shah AK, Heginbotham C, Fulford B, Banner N. The application of the Mental Capacity Act 2005 among geriatric psychiatry patients: a pilot study. Int Psychogeriatr 2009 ; 5 : 922 -30. OpenUrl
- ↵ Potkins D, Bradley S, Shrimanker J, O’Brien J, Swann A, Ballard C. End of life treatment decisions in people with dementia: carers’ views and the factors which influence them. Int J Geriatr Psychiatry 2000 ; 15 : 1005 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Coffey A, Attkinson P. Making sense of qualitative data; complementary research strategies. Sage, 1996.
- ↵ Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Sage, 1994.
- ↵ Smith JA. Beyond the divide between cognition and discourse: using interpretative phenomenological analysis in health psychology. Psychology and Health 1996 ; 11 : 261 -71. OpenUrl CrossRef Web of Science
- ↵ Nuffield Council on Bioethics. Dementia: ethical issues. Nuffield Council on Bioethics, 2009.
- ↵ Department of Health. Living well with dementia: a national dementia strategy. DH, 2009.
- ↵ Wald C, Fahy M, Livingston G, Walker Z. What to tell dementia caregivers—the rule of threes. Int J Geriatr Psychiatry 2003 ; 18 : 313 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Selwood A, Katona C, Lyketsos C, Johnston K, Livingston G. A systematic review of the effect of psychological approaches to family caregivers of people with dementia. J Affect Disord 2007 ; 101 : 75 -89. OpenUrl CrossRef PubMed Web of Science
- Research article
- Open access
- Published: 20 January 2022
Implementation of the Mental Capacity Act: a national observational study comparing resultant trends in place of death for older heart failure decedents with or without comorbid dementia
- James M. Beattie ORCID: orcid.org/0000-0001-7370-3485 1 , 2 ,
- Irene J. Higginson ORCID: orcid.org/0000-0002-3687-1313 1 ,
- Theresa A. McDonagh ORCID: orcid.org/0000-0003-1305-9602 2 &
- Wei Gao ORCID: orcid.org/0000-0001-8298-3415 1
BMC Medicine volume 20 , Article number: 30 ( 2022 ) Cite this article
2436 Accesses
1 Citations
2 Altmetric
Metrics details
Heart failure (HF) is increasingly prevalent in the growing elderly population and commonly associated with cognitive impairment. We compared trends in place of death (PoD) of HF patients with/without comorbid dementia around the implementation period of the Mental Capacity Act (MCA) in October 2007, this legislation supporting patient-centred decision making for those with reduced agency.
Analyses of death certification data for England between January 2001 and December 2018, describing the PoD and sociodemographic characteristics of all people ≥ 65 years registered with HF as the underlying cause of death, with/without a mention of comorbid dementia. We used modified Poisson regression with robust error variance to determine the prevalence ratio (PR) of the outcome in dying at home, in care homes or hospices compared to dying in hospital. Covariates included year of death, age, gender, marital status, comorbidity burden, index of multiple deprivation and urban/rural settings.
One hundred twenty thousand sixty-eight HF-related death records were included of which 8199 mentioned dementia as a contributory cause. The overall prevalence proportion of dementia was 6.8%, the trend significantly increasing from 5.6 to 8.0% pre- and post-MCA (Cochran-Armitage trend test p < 0.0001). Dementia was coded as unspecified (78.2%), Alzheimer’s disease (13.5%) and vascular (8.3%). Demented decedents were commonly older, female, and with more comorbidities. Pre-MCA, PoD for non-demented HF patients was hospital 68.2%, care homes 20.2% and 10.7% dying at home. Corresponding figures for those with comorbid dementia were 47.6%, 48.0% and 4.2%, respectively. Following MCA enforcement, PoD for those without dementia shifted from hospital to home, 62.5% and 17.2%, respectively; PR: 1.026 [95%CI: 1.024–1.029]. While home deaths also rose to 10.0% for those with dementia, with hospital deaths increasing to 50.4%, this trend was insignificant, PR: 1.001 [0.988–1.015]. Care home deaths reduced for all, with/without dementia, PR: 0.959 [0.949–0.969] and PR: 0.996 [0.993–0.998], respectively. Hospice as PoD was rare for both groups with no appreciable change over the study period.
Conclusions
Our analyses suggest the MCA did not materially affect the PoD of HF decedents with comorbid dementia, likely reflecting difficulties implementing this legislation in real-life clinical practice.
Peer Review reports
The incidence and prevalence of heart failure varies across the world reflecting regional differences in cardiovascular disease burden, ethnic and socioeconomic diversity. While the age-adjusted incidence and prevalence of heart failure may be declining in Westernised countries, absolute rates of these indices are increasing in parallel with societal ageing [ 1 ]. In the United Kingdom (UK), about 900,000 people are living with heart failure. The prevalence is 1–2% in the general population, rising to at least 10% in those ≥70 years of age [ 2 , 3 ]. The lifetime risk of developing heart failure is about 20% at 40 years, but for each age decile between 65 and 85 years, the incidence doubles for men and trebles for women [ 4 ].
Crafted by international consensus [ 5 ], a universal definition of heart failure has recently emerged as
…a clinical syndrome with symptoms and / or signs caused by a structural and / or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and / or objective evidence of pulmonary or systemic congestion.
Increasingly accurate diagnostic protocols have been established, and based on the left ventricular ejection fraction (EF), the percentage volume of the diastolic blood pool ejected during systole, an updated classification system linked to the above definition has characterised four clinical phenotypes: heart failure with a reduced EF [< 40%] (HFrEF); heart failure with a mildly reduced EF [40–49%] (HFmrEF); heart failure with a preserved EF [≥50%] (HFpEF); and heart failure with an improved EF [baseline EF ≤40%, a ≥10% point increase from baseline EF, the improved EF > 40%] (HFimpEF) [ 5 ]. Advances in heart failure therapy, particularly those for HFrEF, have enabled some patients to live longer, more comfortable lives, but for many, heart failure remains a life-limiting condition, the 5- and 10-year case-fatality rates being about 50% and 75%, respectively, similar to outcomes for common cancers [ 3 , 6 ]. As well as being encumbered with an unpredictable disease trajectory, this burgeoning and increasingly elderly clinical cohort usually exhibit several comorbidities which add to the complexity and challenge the coordination required of their care [ 2 ].
Cognitive impairment, ranging from mild forms to severe as manifest in dementia, is relatively common, affecting between 25 and 70% of those with heart failure across a series of studies, and estimated at 40% overall in a meta-analysis [ 7 , 8 , 9 ]. Disordered cognition is heterogeneous and demonstrable across a range of higher cortical domains including attention, memory, speech and language processing, learning and executive function [ 10 ], such deficits beyond those arising from normative ageing of the brain. Cognitive impairment may be transitory, sometimes occurring as delirium in patients presenting with acute heart failure [ 11 ], but often presents as a long-term progressive condition, more frequently encountered in older people with chronic heart failure compared to their age-matched healthy counterparts [ 12 ]. The impact of these persistent features tends to fluctuate over time, but even when mild, may impact heart failure patients’ self-care behaviours and treatment adherence resulting in greater rates of hospital admission and mortality [ 13 , 14 ]. There appears to be no direct correlation between the severity of these two conditions [ 15 ], but dilemmas may arise when treating cognitively impaired heart failure patients, sometimes relating to the continued efficacy of established treatment modalities, ceilings of care, and resuscitation issues [ 16 ]. These confront not only professional healthcare providers but also their informal carers, usually family members, who take responsibility for much day-to-day practical support. In assisting those affected by dementia-related loss of intellectual capacity, such carers may be called upon to act as decisional proxies and offer insight into patients previously voiced values and preferences for treatment.
The Mental Capacity Act (MCA) of 2005, applicable to residents in England and Wales aged ≥16 years, sets out a statutory framework to foster person-centred decision making and advance care planning for those who may lack capacity due to a lifelong learning disability, or as a consequence of the transient or permanent effects of acute or long-term illnesses [ 17 ]. For people with cognitive impairment and a progressive, ultimately fatal condition such as heart failure, the MCA may be particularly important in fulfilling goals of care close to the end of life. Achieving care in appropriate settings and the preferred place of death (PoD) are generally accepted as benchmarks of good quality end-of-life care, death at home or customary place of residence usually regarded as the desired option [ 18 ]. Given the relatively frequent association of cognitive impairment with heart failure, it might be expected that the decision-making processes legally constituted within the MCA would drive changes in the final place of care and death during the terminal phase of this condition. The code of practice setting out the standards required to comply with the MCA came fully into force on October 1, 2007 [ 19 ]. Thus, we undertook a comparative trend analysis covering the period of implementation of this legislation to discern any resultant variation in the PoD of heart failure decedents rendered vulnerable by comorbid dementia.
Application of the Mental Capacity Act
As outlined above, the MCA and associated code of practice offer legislative protection to promote patient empowerment and safeguard their autonomy. Prepared when mental capacity is intact, patients may formulate an advance decision such as one to refuse life-sustaining treatment or, if ≥18 years, appoint a close person as a personal welfare lasting power of attorney (LPA) to undertake decisions on their behalf if agency is later lost. Thereafter, any clinical treatment protocol, where possible, should be in accordance with their previously documented choices and values, or these as expressed through their nominated personal welfare LPA. For the purposes of the Act, a two-stage capacity test is applicable. To qualify through Stage 1, the individual must exhibit a demonstrable functional impairment of the mind or brain. For Stage 2, capacity is deemed to be lost if they lack the ability to fulfil any of the following: (a) understand the information pertinent to the decision, (b) retain the information, (c) deliberate on that information as part of the decision-making process and (d) communicate their decision by any means possible. In the context of the study, we must emphasise that many people diagnosed with dementia can still make decisions about many aspects of their care, and loss of capacity should not be regarded as an all-or-none phenomenon based on that diagnostic label. Indeed, under the terms of the MCA, retention of capacity is assumed, and capacity is both decision and time specific.
Study design
This was a national population-based observational study examining anonymised individual-level death registration data collated by the Office for National Statistics (ONS) from 2001 to 2018, provided to us under license, and relating to heart failure decedents resident in England.
Data source and study cohort
In the UK, the death certificate is completed by the responsible clinician, civil registration of the cause of death by a relative or another qualified informant being legally required within 5 days of medical certification. Sometimes a coroner assumes this role after a post-mortem examination or inquest. Following transcription of the information on the death certificate by the recording registrar, this is digitised and uploaded to the ONS for subsequent diagnostic coding in accordance with the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This study dataset comprised all deaths registered in England from January 2001 through December 2018 of people aged ≥ 65 years for whom heart failure was recorded as the underlying cause of death. We elected to study those aged ≥ 65 years at death a priori, perceiving this subset to include the vast majority of heart failure decedents with or without dementia as a marker of cognitive impairment. Heart failure as the primary cause of death was determined by the allocation of any ICD-10: I50 code by the ONS. Designation of diminished intellectual capacity for this cohort was determined when dementia of any aetiology was mentioned as a contributory cause of death. The presence of comorbid dementia was indicated by the application of ICD-10 codes G30 [Alzheimer’s disease]; F00 [dementia in Alzheimer’s disease with late onset, atypical or mixed type, and unspecified]; F01 [vascular dementia]; F02 [dementia in other diseases classified elsewhere]; or F03 [unspecified dementia]. ONS death data acquisition and coding processes are subject to regular quality assurance. Pertinent to this study period, it should be noted that in 2011 there was a change in ONS mortality data coding practice, the previous coding of unspecified cerebrovascular disease when registered as a contributory cause of death being reclassified as vascular dementia [ 20 ].
The outcome variable was PoD as recorded on death certificates and codified by the ONS. Characterisation of PoD for this study was based on the classification system defined by the National End of Life Care Intelligence Network, part of Public Health England [ 21 ]. This specifies 5 groupings of PoD: (a) Hospital, which incorporates all acute, specialist, and community hospitals whether they be National Health Service (NHS) or private, but not psychiatric hospitals; (b) Care home, including residential and nursing homes; (c) Own residence, the decedent’s usual place of abode, but excludes communal living arrangements such as convents, monasteries, hostels or prisons; (d) Hospice, commonly standalone NHS or independent establishments; (e) Other places, covering psychiatric hospitals, other people’s homes, communal living institutions as described above, workplaces, public spaces or roads. This grouping also applies to those declared dead on arrival at hospital, potentially relevant to some heart failure patients who succumb to sudden death. Where the PoD was unknown or unspecified, these data were incorporated in descriptive statistics but not considered further in multiple adjusted analyses.
Period of death as the independent variable of interest incorporated a binary indicator for the year of death pre- and post-enforcement of the MCA [0: 2001–2007; 1: 2008–2018]. Covariates included age at death, number of mentioned contributory causes, gender, marital status, socioeconomic position as measured by the Index of Multiple Deprivation, and categorisation of the location of decedents’ usual residence as urban or rural based on the relevant postcode as archived on the ONS classification system [ 22 , 23 ].
Statistical analysis
Categorical and continuous variables were described using count (percentages) and means (standard deviation [SD]) as appropriate. The proportion of hospital deaths among heart failure decedents with or without comorbid dementia and the number of patients who died from heart failure with comorbid dementia were plotted to visually determine temporal trends, the latter also assessed statistically using a two-tailed Cochran-Armitage trend test.
We used modified Poisson regression with robust error variance [ 24 ] to evaluate the independent association between enforcement of the MCA and PoD. Three models were constructed separately for heart failure patients who died with or without comorbid dementia: home (1) versus hospital (0); care home (1) versus hospital (0); hospice (1) versus hospital (0). All covariates were forced to stay in the models to control their effects. The prevalence ratio (PR) was derived from the respective model to quantify the magnitude of association.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). To control for Type 1 error, we applied Bonferroni correction to the alpha level. A two-sided p value of 0.008 (0.05/6) was considered statistically significant.
Study sample
Between 2001 and 2018, 120,068 people aged ≥65 years whose deaths were registered as directly due to heart failure were identified, their data subsequently included in this analysis. Table 1 describes the characteristics of these heart failure decedents. Overall, 8199 (6.8% [confidence intervals (CI) 6.7 to 7.0]) of these registrations were documented with dementia as a contributory cause, this being classified as unspecified dementia in 78.2%, Alzheimer’s disease in 13.5%, and as vascular dementia in 8.3%. No other dementia subtypes were denoted. For the periods 2001–2007 and 2008–2018, pre- and post-enforcement of the MCA, the numbers of heart failure decedents with dementia were 3427 (5.6%) and 4772 (8.0%) respectively. The prevalence proportion of dementia gradually increased on an annual basis, this trend being statistically significant ( Z =18.87, p < 0.0001) as shown on Fig. 1 . There was no discernible artefactual change in the general rate of dementia mentioned as a contributory cause of death associated with the 2011 change in ONS coding practice. However, as shown in Table 2 , there was a contemporaneous and statistically significant reduction ( p < 0.0001) in the coding of ‘ unspecified dementia ’ with an equivalent increase in coding for ‘ vascular dementia ’.

Percentages of heart failure decedents ( n =120,068) with comorbid dementia ( n =8199) by year of death, England, 2001–2018
Most heart failure decedents were ≥85 years old at the time of death (62.1%). The mean age at death increased between the two study periods (2001–2007: 85.7 years [SD 7.4]; 2008–2018: 86.6 years [SD 7.5], the age at death being greater for those with dementia for both intervals at 87.1 [SD 6.2] and 88.1 years [SD 6.0], respectively. A relatively higher level of multimorbidity was noted for heart failure decedents with dementia. Most of those dying from heart failure were female, this proportionately greater at 67.2% for the dementia group compared to 60.0% for those without dementia. Across the totality of heart failure decedents, there was no significant difference in marital status between those with or without dementia, Most heart failure patients lived and died in urban environments and deprivation quintiles were similar for both study populations.
Trends in place of death
Over the period of implementation of the MCA, comparative outcomes in PoD for these heart failure decedents are shown in Fig. 2 . For the period 2001–2007, hospital was the most common PoD for the non-demented heart failure group at 68.2%, 20.2% dying in a care home, and 10.7% dying at home. This changed a little for the latter period 2008–2018, reducing to 62.5% and 18.8% for hospital and care homes respectively, the proportion of home deaths increasing to 17.2%. In contrast, for heart failure decedents with dementia, there was a small increase in the proportion of hospital deaths, this rising from 47.6 to 50.4%. For this group, there was a reduction in care home deaths from 48.0 to 38.8%, with a modest rise in home deaths, 4.2% to 10.0%. Hospice as the PoD was rare for both clinical cohorts and declined over the study period. The time trend in hospital deaths is shown in Fig. 3 . There was a statistically significant reduction in hospital deaths for heart failure decedents without dementia ( p < 0.001). On the other hand, the marginal increase in hospital deaths for those with dementia was not significant ( p =0.97). Adjusted PRs following implementation of the MCA confirm increased PoD at home compared to hospital for non-dementia patients, PR: 1.026 [CI: 1.024–1.029] ( p < 0.0001), this trend not significant for those with dementia, PR: 1.001 [CI 0.988–1.015] ( p =0.83). Care home deaths reduced for both groups, PR: 0.959 [CI 0.949–0.969] ( p < 0.0001), and PR: 0.995 [CI 0.993–0.998] ( p < 0.0001) for those with and without dementia, respectively. Starting from an already small base, adjusted PRs for hospice rather than hospital as PoD declined significantly for both non-demented and demented heart failure decedents being 0.979 [CI 0.977–0.980 ( p < 0.0001) and 0.946 [CI 0.934–0.959] ( p < 0.0001), respectively. A summary of the adjusted PRs for dying in a premise other than hospital following MCA enforcement is shown in Table 3 . Fully detailed results for all three model sets are available in Additional file 1 : Supplementary Tables S-2 to S-4.
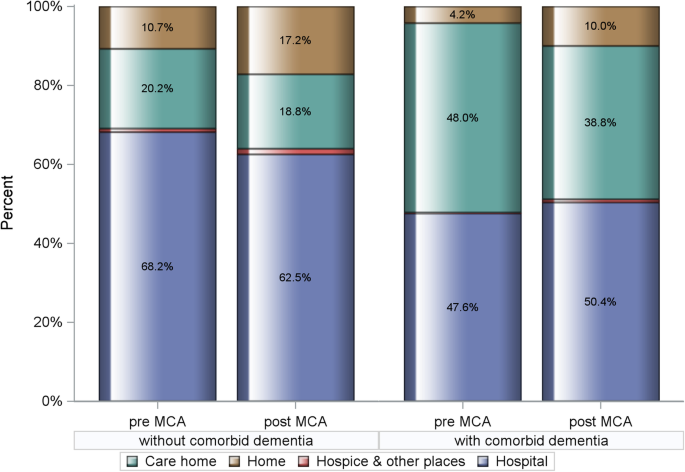
Comparative outcomes (%) in place of death for heart failure decedents with and without comorbid dementia pre- (2001–2007) and post- (2008–2018) implementation of the Mental Capacity Act (MCA). Chi-square test p value < 0.0001
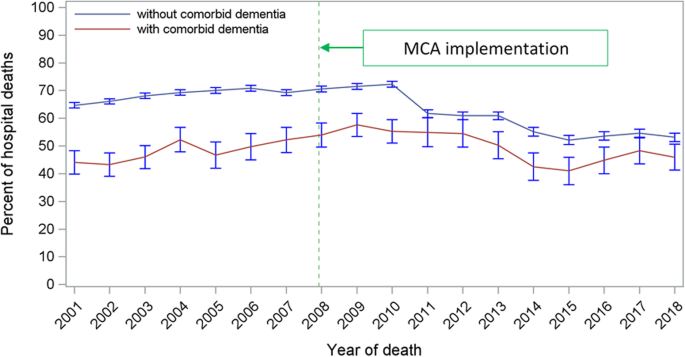
The time trend of hospital deaths among patients who died from heart failure with or without comorbid dementia, England 2001–2018
In this first national study examining variation in the PoD for heart failure patients over the period of enactment and implementation of the MCA in England, our results suggest that the decision-making and advance care planning processes enshrined in this legislation had little material effect on the ultimate site of care provision determining PoD for those with cognitive impairment manifest as dementia. In the later years of this study period when the code of practice for this legislation was operational, trend data for heart failure decedents certified with comorbid dementia show a modest rise in hospital deaths with fewer care home deaths. Conversely, for this study phase, there was a small reduction in hospital deaths for those without dementia who were younger and with fewer comorbidities.
The reasons behind the apparent lack of impact of this legislation may be complex, but it has been proposed that the MCA is relatively poorly applied in clinical practice. While a variety of training models have been developed to disseminate information to health professionals on the five principles underpinning these regulations, recent reviews suggest poor understanding of when and how the provisions of the Act should be employed, with the need for clarity on the process of designating the role of surrogate decision-makers to properly ensure patients’ best interests are maintained [ 25 , 26 , 27 ]. A lack of confidence of those working in acute care settings has been particularly highlighted, specifically citing decision-making with respect to hospital discharge [ 28 ]. This issue may be especially relevant to our study observations given that we have demonstrated that the majority of those dying with heart failure in England do so in hospital, similar data emerging from the United States (US) [ 29 ].
At variance with the trends evident in this study, previous work has suggested a decline in the frequency of hospital deaths for those with dementia in recent years, with more people dying in care homes [ 30 ]. These findings were also based on ONS death certification data but included all individuals for whom dementia was mentioned as either the underlying or as a contributory cause of death. In contrast, the current study specifies heart failure as the primary cause of death, differentiating the comparator groups by the presence or absence of dementia mentioned only as a contributory cause. It is well established that the principal diagnosis is the main determinant of the site of clinical care [ 31 ], and community-based primary care practitioners appear to be relatively incognizant of patients’ preferences for place of care or PoD, particularly when dealing with non-cancer diagnoses [ 32 ]. Unless policies for comfort care are clearly outlined, should people with chronic heart failure living at home or in a nursing home suddenly deteriorate, the reactive response of professional staff may be to arrange emergency hospital admission by default. However, we have no information on any care transitions prior to the terminal phase for this study cohort, or whether this possible course of action had a bearing on the results of this study.
The completeness of death certification in the UK is regarded as relatively robust with proportionately fewer ‘ garbage codes ’ than data from many other countries [ 33 ]. However, dementia as recorded on death certification likely underestimates the true prevalence, and it has been suggested that studies using death certification alone may fail to account for 16-18% of dementia cases [ 34 ]. A variety of factors may influence such documentation. Rates of inclusion of dementia are generally increased in those who die in institutions such as care homes compared to those dying at home, particularly if dementia is at the severe end of the clinical spectrum and includes agitation [ 35 ]. While heart failure guidelines draw attention to cognitive impairment as a comorbidity, describing all grades of this by hospital-based clinicians is reportedly poor [ 14 ], and there are diverging views on whether dying in hospital positively or negatively affects the rate of recording of dementia at the time of death certification [ 35 , 36 ]. Recent initiatives to heighten clinicians’ awareness of dementia may improve matters. In 2012, NHS England introduced a quality improvement scheme through the Commissioning for Quality and Innovation (CQUIN) payment framework [ 37 ]. Acute healthcare providers were incentivised with the assurance of increased remuneration if 90% of all patients aged ≥75 years and whose emergency hospital admission lasted > 72 h were screened for dementia. Further financial gain was available if those patients whose initial assessment indicated dementia or was inconclusive were referred on for specialist review. While this dementia assessment and referral exercise was retired as a CQUIN indicator in April 2016, these conditions have been retained within the standard contract for English hospitals providing acute clinical services. It is possible that these administrative processes may have contributed in some measure to the increased mentions of dementia as certified for hospital decedents evident in the latter course of this study.
The relatively frequent concurrence of cognitive impairment and heart failure likely stems from various pathophysiologic features related to the latter condition combined with shared cardiovascular risk factors such as hypertension, hyperlipidaemia or dysglycaemia [ 7 , 38 , 39 ]. The haemodynamic and risk factor profiles for HFrEF and HFpEF clearly differ, but very few investigations have compared the spectrum of cognitive impairment across the range of ejection fraction phenotypes. There is a suggestion that affected domains of cognitive function may vary, but data is limited with inconsistent results [ 40 , 41 ].
To date, there is no evidence that evidence-based guideline-directed medical therapy (GDMT) for heart failure drives neurocognitive dysfunction [ 42 ], and indeed it has been posited that centrally acting angiotensin-converting enzyme inhibitors (ACEIs) such as perindopril or captopril, which cross the blood-brain barrier, may slow the progression of cognitive impairment in those with dementia [ 43 ]. Following the positive results of the PARADIGM-HF study demonstrating the benefits of sacubitril/valsartan, the first of a new class of drugs termed ARNIs (angiotensin receptor-neprilysin inhibitors) [ 44 ], this therapeutic option for HFrEF has been widely adopted. Neprilysin is a soluble metalloprotease which catalyses the degradation of natriuretic peptides (NPs), downregulation of this enzymatic activity likely increasing endogenous NP mediated natriuresis and vasodilation. However, such neprilysin inhibition might also interfere with the clearance of amyloid-β protein, vascular deposition of which results in cerebral amyloid angiopathy, a distinctive feature of Alzheimer’s disease. Dementia-related adverse events were not overrepresented through 4.3 years follow-up of the relevant PARADIGM-HF study arm compared to similar populations [ 45 ]. Nonetheless, as required by the Food and Drug Administration in the US, this potential hazard is currently being evaluated in the PERSPECTIVE study ( ClinicalTrials.gov ID NCT02884206). Due to report in 2022, this trial includes a battery of neurocognitive testing and sequential 18 F-labelled florbetaben positron emission tomography to assess any longitudinal changes in cerebral amyloid plaque burden. Importantly, recent evidence shows that the hearts of some patients with Alzheimer’s disease exhibit diastolic dysfunction and thickening of the interventricular septum. These features are characteristic of cardiac amyloidosis suggesting that in some individuals, amyloid-β protein may also accumulate in tissues other than the brain [ 46 ].
As inferred above, in recent years GDMT for those affected by heart failure has become increasingly effective [ 47 ], but heart failure is an ambulatory care sensitive condition and remains the commonest cause of acute hospitalisation in those > 65 years [ 48 ]. Following an index heart failure admission in England, the 1-year mortality for patients discharged alive is 39.6% with a 30-day all-cause readmission rate of 19.8% [ 49 ]. Readmissions for heart failure tend to follow a tri-phasic pattern. This was apparent in a study of 8543 heart failure patients in Toronto monitored for 10 years following their first hospital admission, by which time 98.8% had died, the median survival after heart failure diagnosis being 1.75 years [ 50 ]. About 30% of all readmissions occurred within 2-months of initial hospital discharge, 50% during the 2-month period leading up to death, with 15-20% taking place in the intervening ‘ plateau phase ’ of the heart failure disease trajectories. A sentinel clustering of admissions in the terminal phase of heart failure has been well described [ 51 ]. It is uncertain if the presence of dementia as a comorbidity influences the readmission rate. Rao and colleagues followed 10,317 patients for 5 years subsequent to their diagnosis with heart failure between April 2008 and March 2009 using the primary care-based Clinical Practice Research Datalink combined with Hospital Episode Statistics and ONS death registration data [ 52 ]. Their analysis indicated that comorbid dementia was a factor significantly affecting emergency hospital readmissions in only 3 of 8 regions across England.
Comparable to the epidemiological trends for heart failure, the age-adjusted prevalence and incidence of dementia may also be declining in high-income countries. The Medical Research Council Cognitive Function and Ageing Studies (CFAS 1 and II) of populations living in rural Cambridgeshire and the urban environments of Newcastle and Nottingham demonstrated a 24% reduction in the prevalence of dementia in those ≥65 years between 1989 and 2011 [ 53 ]. Consistent with our observations, the CFAS studies also suggested that women were more commonly affected, and while the prevalence of dementia in care home residents had increased from 56 to 70%, most people with dementia were still living at home. Similarly, dementia events have been continuously surveyed in the US-based Framingham Heart Study since 1975. Monitoring of this community cohort living in Massachusetts, predominantly of white European ancestry, has implied a 20% stepwise decline in the incidence of dementia each decade over the last 30 years [ 54 ]. The background to these cumulative decrements remains to be determined, but both the CFAS and Framingham study groups cited potential mechanisms in higher early educational attainment and attenuated vascular morbidity.
The Framingham Heart Study showed a non-significant reduction in Alzheimer’s disease with a more overt decrease in vascular dementia. In Westernised societies, Alzheimer’s is the most commonly encountered manifestation of dementia, but as an isolated pathophysiological process, this affects < 20% of those with heart failure. Rather, vascular dementia has been proposed as the likeliest associated variant, followed by mixed forms, then Alzheimer’s and other specific dementias [ 39 ]. This is at odds with the distribution of dementia subtypes noted in this study where unspecified dementia was most frequently mentioned on death certificates and coded as the dominant category. A similar finding was described in a Danish study of 324,418 patients admitted with incident heart failure and tracked for 35 years against an age- and sex-matched population without heart failure selected from the Danish Civil Registration System [ 55 ]. Adelborg and colleagues found a clear association between all-cause dementia and heart failure. This was relatively weak for Alzheimer’s disease, and while the vascular variant was represented, this was predominantly determined by the reported development of unspecified dementia. These authors proposed that some patients ostensibly exhibiting unspecified dementia may have been misclassified. However, in combining death certification data from sequential CFAS studies in England, all mentions of dementia as the underlying or a contributory cause of death showed a percentage distribution of subtypes of unspecified dementia, Alzheimer’s disease and vascular dementia as 69.3%, 21.6% and 8.6%, respectively [ 37 ]. These results are very similar to those noted in the current study and suggest that this proportional distribution of dementia variants is not specific to the heart failure population.
Advance care planning offers patients the potential to receive medical treatment consistent with their expressed preferences, values, and goals of care against the possibility of subsequent loss of decision-making capacity. This may help prevent needless hospital admissions and better achieve consensus on appropriate ceilings of care, avoiding exposure of patients and families to the distressing harms which sometimes accompany futile treatment escalation and burdensome invasive interventions close to the end of life. It is notable that a recent audit of end-of-life care in hospitals in England demonstrated that10% of heart failure patients were receiving mechanical ventilatory support within 24 h of death [ 56 ].
Given the unpredictability intrinsic to the heart failure disease trajectory which challenges individual prognostication even in the late stages of this disease, and the associated multimorbidity including cognitive impairment, it might be expected that advance care planning would be central to the care of people with this condition. However, advance care planning is not routinely incorporated within heart failure care and tends to be limited to the possible withdrawal of any implanted electronic or mechanical devices, or sometimes offered as one component of the still uncommon provision of palliative care [ 57 , 58 ]. A review and meta-analysis of 14 randomised controlled trials of advance care planning in heart failure, mostly US-based and involving 2924 individuals across a range of care structures, showed this to moderately improve the primary outcome measure in patients’ quality of life, together with similarly weighted favourable effects on secondary outcomes including communication about, and satisfaction with, end-of-life care [ 59 ].
Advance care planning for dementia was featured as a specific domain in the European Association of Palliative Care white paper on this condition [ 60 ], and the challenges taking this forward have been comprehensively reviewed [ 61 , 62 ]. Emerging themes suggest that while there is some disparity in the readiness of older people to engage in advance care planning, and the means to take this forward may also vary across the spectrum of healthcare delivery, the use of these instruments may be effective in promoting shared decision-making between patients, informal carers and professionals [ 63 ]. However, it should be noted that, in the UK, an advance care plan is not legally binding but merely an advisory statement of preferences and wishes in relation to general care and medical therapy [ 64 ].
Dialogue between the patient, family and clinician is basic to shared decision-making. However, triangulation of information between this triad does not necessarily mean equitably weighted opinions, and at times patients’ voices are marginalised, a dyadic interchange conducted between clinicians and family members. Such a discourse may be justified if the patient cannot physically contribute to meaningful shared decision-making, if capacity is deemed to be lost, legally binding instruments are not in place, and their preferences are unknown. Then, the opinion of the closest relative should be sought, their views respected and used to inform a care plan constructed in the best interests of the patient. However, it should also be remembered that the involvement of relatives as decisional proxies may be emotionally demanding, particularly if they are already distressed and experiencing anticipatory grief. Further, the assumption that there is always congruence between the opinions of family surrogate decision-makers and those perceived of patients is flawed. Rather, these are often misaligned, with at best moderate concordance between dementia/carer dyads when assessed within a hospital setting [ 65 ]. Qualitative studies examining difficulties in decision making during clinical encounters for those with dementia have cited tension between family members and conflicts between families and health professionals, some of the latter reluctant to undertake decisions on patients unfamiliar to them, particularly when there is poor information exchange following transfer from another care setting [ 66 ]. Even if decisional consensus is achieved, the discharge of hospital inpatients home may be subject to practical limitations depending on the social context. The caregiver burdens associated with heart failure and dementia have been well characterised. Informal caregivers may be unwilling or unable to reframe or enhance their roles, and it should be noted that the cohabitees of people living and dying with dementia tend to be of a similar advanced age [ 67 ]. Further, it has been proposed that there may be a gendering issue relating to care at home in that older women are more likely to have outlived their male partners and be devoid of spousal support [ 68 ]. However, in this study, the marital status of heart failure decedents with or without dementia appeared to be similar. Following the implementation of the MCA, our analysis suggests that proportionately more women with dementia died at home compared to those without dementia. The reason for this disparity is unclear, and a variety of other intersectional stressors which might constrain the social capital of older women close to the end of life may have been in play [ 69 , 70 ].
Strengths and weaknesses
To our knowledge, this is the first empirical study to systematically examine national data for England describing the PoD of people dying of heart failure with dementia documented as a contributory cause of death. A major strength is that our work is based on comprehensive data collated from the gamut of clinical practice over an 18-year period, but we acknowledge that we are dependent on the clinicians responsible for these heart failure decedents having made the correct diagnoses and accurately completing later death certification, over which there is no means of independent adjudication. Irrespective of the PoD, we have no access to information on the underlying aetiologies of heart failure, or the proportional distribution of resultant ejection fraction variants. Similarly, it is not possible to ascertain the duration of heart failure or nature of any care provision prior to the terminal phase, and whether this fatal outcome related to incident acute heart failure, worsening of chronic heart failure, or heart failure-related sudden cardiac death. It has been suggested that decisions to include dementia on death certificates rely on medical staff regarding this as clinically significant [ 71 ]. This is considered more likely if dementia is relatively severe, reaffirming our judgement in using the certified mention of dementia as a contributory cause of death to represent a valid marker of significant cognitive dysfunction, and therefore relevant to the aegis of the MCA. Regarding generalisability, the results of this study are germane to similar clinical populations, models of care delivery, and legal constructs. While the legal status of the MCA 2005 applies to England and Wales, beyond this jurisdiction but within the UK, this legislation is closely aligned to the Adults with Incapacity (Scotland) Act 2000, and the Mental Capacity Act (Northern Ireland) 2016. There is also some global resonance through Article 12 of the United Nations Convention on the Rights of Persons with Disabilities (CRPD) of 2006. While the pertinence of specific aspects of the CRPD have been subject to legal argument [ 72 ], both this and the MCA have informed the development of mental capacity policies and legislation in Canada, Australia, and New Zealand [ 73 , 74 ].
In this hypothesis-generating study, we have investigated the impact of the MCA on the PoD of heart failure decedents aged ≥65 years resident in England whom we have shown to be increasingly affected by comorbid dementia, dying at home or usual place of residence customarily accepted as the preferred option. Our analyses of trends over the period of enactment and implementation of the code of practice relating to this legislation show little to suggest any significant influence on PoD for this relatively vulnerable clinical cohort. The background to this somewhat neutral outcome is multifaceted, administration of the Act clearly challenging for a prognostically ambiguous population subject to flux in the often-nuanced scenarios typical of real-life clinical practice, a milieu dominated by the treatment imperative. Further, even if the precepts of the MCA are correctly applied, this course of action may be ineffectual in isolation. Recent evidence suggests that achieving a good death at home requires patients and their informal carers to feel secure in that setting, effected by the provision of a 24/7 responsive palliative care service, staffed by those competent in symptom relief and with good communication skills [ 75 ]. Fulfilling the preferred PoD of those with heart failure and dementia might be better achieved by embedding application of the MCA within a system of anticipatory sympathetic clinical navigation across all care sectors, contingent upon effective upskilling of the relevant professionals, with good inter-agency and multidisciplinary collaboration to support and maintain appropriately configured community-based palliative care.
Availability of data and materials
Additional file 1 :Supplementary files S-1, S-2, S-3, and S-4 are available online.
Abbreviations
Angiotensin-converting enzyme inhibitor
Angiotensin receptor-neprilysin inhibitor
Cognitive Function and Ageing Study
Commissioning for Quality and Innovation
Ejection fraction
Guideline directed medical therapy
- Heart failure
Heart Failure with improved Ejection Fraction
Heart Failure with mildly reduced Ejection Fraction
Heart Failure with preserved Ejection Fraction
Heart Failure with reduced Ejection Fraction
International Classification of Diseases 10 th Revision
Lasting Power of Attorney
- Mental Capacity Act
National Health Service
Natriuretic peptide
Office for National Statistics
- Place of death
Standard deviation
United Kingdom
United States
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–78. https://doi.org/10.1038/nrcardio.2016.25 .
Article Google Scholar
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–80. https://doi.org/10.1016/S0140-6736(17)32520-5 .
Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: Population based cohort study. BMJ. 2019;364:l223. https://doi.org/10.1136/bmj.l223 .
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, et al. Levy D; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72. https://doi.org/10.1161/01.cir.0000039105.49749.6f .
Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;S1071-9164(21):00050–6. https://doi.org/10.1016/j.cardfail.2021.01.022 .
Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1,162,309 hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes. 2010;3(6):573–80. https://doi.org/10.1161/CIRCOUTCOMES.110.957571 .
Witt LS, Rotter J, Stearns SC, Gottesman RF, Kucharska-Newton AM, Richey Sharrett A, Wruck LM, Bressler J, Sueta CA, Chang PP. Heart failure and cognitive impairment in the Atherosclerosis Risk in Communities (ARIC) Study. J Gen Intern Med 2018;33(10):1721-1728. https://doi.org/10.1007/s11606-018-4556-x .
Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. https://doi.org/10.1016/j.ijcard.2014.10.087 .
Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23(6):464–75. https://doi.org/10.1016/j.cardfail.2017.04.007 .
Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neurosci. 2019;21(3):227–37. https://doi.org/10.31887/DCNS.2019.21.3/pharvey .
Correale M, Altamura M, Carnevale R, Tricarico L, Malerba S, Gallotta AM, et al. Delirium in heart failure. Heart Fail Rev. 2020;25(5):713–23. https://doi.org/10.1007/s10741-019-09842-w .
Hammond CA, Blades NJ, Chaudhry SI, Dodson JA, Longstreth WT Jr, Heckbert SR, et al. Long-term cognitive decline after newly diagnosed heart failure: longitudinal analysis in the CHS (Cardiovascular Health Study). Circ Heart Fail. 2018;11(3):e004476. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004476 .
Uchmanowicz I, Jankowska-Polańska B, Mazur G, Sivarajan FE. Cognitive deficits and self-care behaviors in elderly adults with heart failure. Clin Interv Aging. 2017;12:1565–72. https://doi.org/10.2147/CIA.S140309 .
Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126(2):120–6. https://doi.org/10.1016/j.amjmed.2012.05.029 Erratum in: e25.
Huijts M, van Oostenbrugge RJ, Duits A, Burkard T, Muzzarelli S, Maeder MT, et al. Cognitive impairment in heart failure: results from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) randomized trial. Eur J Heart Fail. 2013;15(6):699–707. https://doi.org/10.1093/eurjhf/hft020 .
Article CAS Google Scholar
Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, et al. American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928–52. https://doi.org/10.1161/CIR.0b013e31824f2173 .
Her Majesty’s Government (UK). Mental Capacity Act 2005. London: The Stationary Office Limited; 2005.
Google Scholar
Gomes B, Calanzani N, Higginson IJ. Local preferences and place of death in regions within England 2010: Cicely Saunders International; 2011. https://www.kcl.ac.uk/cicelysaunders/attachments/keyreport-Local-preferences-and-place-of-death-in-regions-within-England.pdf Accessed 15 Feb 2021
Mental Capacity Act 2005. Code of Practice. London: The Stationary Office Limited; 2007. https://www.gov.uk/government/publications/mental-capacity-act-code-of-practice Accessed 5 Jan 2021
Dying with Dementia. National Dementia Intelligence Network and National End of Life Care Intelligence Network briefing: Public Health England; 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733064/Dying_with_dementia_briefing.pdf Accessed 18 Dec 2020
Public Health England. Classification of Place of Death. A technical bulletin from the National End of Life Intelligence Network. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/831853/Classification_Place_Death_report.pdf Accessed 12 Nov 2020.
Department for Communities and Local Government. The English Indices of Multiple Deprivation 2015. London; 2015. https://www.gov.uk/government/statistics/english-indices-ofdeprivation-2015 Accessed 20 Dec 2020
Office for National Statistics. Rural / Urban classifications: The classification systems used to produce a rural / urban view from government statistics. 2011 https://www.ons.gov.uk/methodology/geography/geographicalproducts/ruralurbanclassifications/2011ruralurbanclassification Accessed 21 Dec 2020.
Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. https://doi.org/10.1093/aje/kwh090 PMID: 15033648.
Hinsliff-Smith K, Feakes R, Whitworth G, Seymour J, Moghaddam N, Dening T, et al. What do we know about the application of the Mental Capacity Act (2005) in healthcare practice regarding decision-making for frail and older people? A systematic literature review. Health Soc Care Community. 2017;25(2):295–308. https://doi.org/10.1111/hsc.12310 .
Jenkins C, Webster N, Smythe A, Cowdell F. What is the nature of Mental Capacity Act training and how do health and social care practitioners change their practice post-training? A narrative review. J Clin Nurs. 2020;29(13-14):2093–106. https://doi.org/10.1111/jocn.15256 .
Wade DT, Kitzinger C. Making healthcare decisions in a person's best interests when they lack capacity: clinical guidance based on a review of evidence. Clin Rehabil. 2019;33(10):1571–85. https://doi.org/10.1177/0269215519852987 .
Poole M, Bond J, Emmett C, Greener H, Louw SJ, Robinson L, et al. Going home? An ethnographic study of assessment of capacity and best interests in people with dementia being discharged from hospital. BMC Geriatr. 2014;14:56. https://doi.org/10.1186/1471-2318-14-56 .
Chuzi S, Molsberry R, Ogunseitan A, Warraich HJ, Wilcox JE, Grady KL, et al. Trends in place of death for cardiovascular mortality related to heart failure in the United States from 2003 to 2017. Circ Heart Fail. 2020;13(2):e006587. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006587 .
Sleeman KE, Ho YK, Verne J, Gao W, Higginson IJ. GUIDE_Care project. Reversal of English trend towards hospital death in dementia: a population-based study of place of death and associated individual and regional factors, 2001-2010. BMC Neurol. 2014;14:59. https://doi.org/10.1186/1471-2377-14-59 .
Murtagh FEM, Bausewein C, Petkova H, Sleeman KE, Dodd RH, Johnston B, et al. Understanding place of death for patients with non-malignant conditions: a systematic literature review. NIHR Service Delivery and Organisation programme: Final report; 2012.
Abarshi E, Onwuteaka-Philipsen B, Donker G, Echteld M, Van den Block L, Deliens L. General practitioner awareness of preferred place of death and correlates of dying in a preferred place: a nationwide mortality follow-back study in the Netherlands. J Pain Symptom Manag. 2009;38(4):568–77. https://doi.org/10.1016/j.jpainsymman.2008.12.007 .
Iburg KM, Mikkelsen L, Adair T, Lopez AD. Are cause of death data fit for purpose? Evidence from 20 countries at different levels of socio-economic development. PLoS ONE. 2020;15(8):e0237539. https://doi.org/10.1371/journal.pone.0237539 .
Russ TC, Gatz M, Pedersen NL, Hannah J, Wyper G, Batty GD, et al. Geographical variation in dementia: examining the role of environmental factors in Sweden and Scotland. Epidemiology. 2015;26(2):263–70. https://doi.org/10.1097/EDE.0000000000000230 .
Perera G, Stewart R, Higginson IJ, Sleeman KE. Reporting of clinically diagnosed dementia on death certificates: retrospective cohort study. Age Ageing. 2016;45(5):668–73. https://doi.org/10.1093/ageing/afw077 .
Gao L, Calloway R, Zhao E, Brayne C, Matthews FE. Medical Research Council Cognitive Function and Ageing Collaboration. Accuracy of death certification of dementia in population-based samples of older people: analysis over time. Age Ageing. 2018;47(4):589–94. https://doi.org/10.1093/ageing/afy068 .
Harrison JR. Improving inpatient care for older adults: Implementing dementia Commissioning for Quality and Innovation (CQUIN). BMJ Qual Improv Rep. 2017;6(w4875):u212202. https://doi.org/10.1136/bmjquality.u212202.w4875 https://bmjopenquality.bmj.com/content/6/1/u212202.w4875 .
van der Velpen IF, Feleus S, Bertens AS, Sabayan B. Hemodynamic and serum cardiac markers and risk of cognitive impairment and dementia. Alzheimers Dement. 2017;13(4):441–53. https://doi.org/10.1016/j.jalz.2016.09.004 .
Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 2010;7(4):433–7. https://doi.org/10.1016/j.hrthm.2009.12.004 .
Cermakova P, Lund LH, Fereshtehnejad SM, Johnell K, Winblad B, Dahlström U, et al. Heart failure and dementia: survival in relation to types of heart failure and different dementia disorders. Eur J Heart Fail. 2015;17(6):612–9. https://doi.org/10.1002/ejhf.222 .
Athilingam P, D'Aoust RF, Miller L, Chen L. Cognitive profile in persons with systolic and diastolic heart failure. Congest Heart Fail. 2013;19(1):44–50. https://doi.org/10.1111/chf.12001 .
Bratzke LC, Moser DK, Pelter MM, Paul SM, Nesbitt TS, Cooper LS, et al. Evidence-based heart failure medications and cognition. J Cardiovasc Nurs. 2016;31(1):62–8. https://doi.org/10.1097/JCN.0000000000000216 .
Gao Y, O'Caoimh R, Healy L, Kerins DM, Eustace J, Guyatt G, et al. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open. 2013;3(7):e002881. https://doi.org/10.1136/bmjopen-2013-002881 .
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077 .
Cannon JA, Shen L, Jhund PS, Kristensen SL, Køber L, Chen F, et al. PARADIGM-HF Investigators and Committees. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur J Heart Fail. 2017;19(1):129–37. https://doi.org/10.1002/ejhf.687 .
Sanna GD, Nusdeo G, Piras MR, Forteleoni A, Murru MR, Saba PS, et al. Cardiac abnormalities in Alzheimer Disease: clinical relevance beyond pathophysiological rationale and instrumental findings. JACC Heart Fail. 2019;7(2):121–8. https://doi.org/10.1016/j.jchf.2018.10.022 .
Committee W, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, et al. Update to the 2017 ACC Expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;2021(20) 37867-0:S0735–1097. https://doi.org/10.1016/j.jacc.2020.11.022 .
Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385(9970):812–24. https://doi.org/10.1016/S0140-6736(14)61889-4 .
Bottle A, Honeyford K, Chowdhury F, Bell D, Aylin P. Factors associated with hospital emergency readmission and mortality rates in patients with heart failure or chronic obstructive pulmonary disease: a national observational study. Southampton (UK) NIHR J Libr. 2018; https://www.ncbi.nlm.nih.gov/books/NBK513479/ Accessed 28 Feb 2021.
Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5(4):414–21. https://doi.org/10.1161/CIRCHEARTFAILURE.111.964791 .
Udelson JE, Stevenson LW. The future of heart failure diagnosis, therapy, and management. Circulation. 2016;133(25):2671–86. https://doi.org/10.1161/CIRCULATIONAHA.116.023518 .
Rao A, Kim D, Darzi A, Majeed A, Aylin P, Bottle A. Regional variations in trajectories of long-term readmission rates among patients in England with heart failure. BMC Cardiovasc Disord. 2019;19(1):86. https://doi.org/10.1186/s12872-019-1057-8 .
Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. Medical Research Council Cognitive Function and Ageing Collaboration. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–12. https://doi.org/10.1016/S0140-6736(13)61570-6 .
Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med. 2016;374(6):523–32. https://doi.org/10.1056/NEJMoa1504327 .
Adelborg K, Horváth-Puhó E, Ording A, Pedersen L, Sørensen HT, Henderson VW. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail. 2017;19(2):253–60. https://doi.org/10.1002/ejhf.631 .
Royal College of Physicians. End of Life Care Audit – Dying in Hospital . London: Royal College of Physicians; 2016. https://www.rcplondon.ac.uk/projects/outputs/end-life-care-audit-dying-hospital-national-report-england-2016 Accessed 17 Feb 2021
Butler J, Binney Z, Kalogeropoulos A, Owen M, Clevenger C, Gunter D, et al. Advance directives among hospitalized patients with heart failure. JACC Heart Fail. 2015;3(2):112–21. https://doi.org/10.1016/j.jchf.2014.07.016 .
Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017;70(3):331–41. https://doi.org/10.1016/j.jacc.2017.05.030 .
Schichtel M, Wee B, Perera R, Onakpoya I. The effect of advance care planning on heart failure: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(3):874–84. https://doi.org/10.1007/s11606-019-05482-w .
van der Steen JT, Radbruch L, Hertogh CM, de Boer ME, Hughes JC, Larkin P, et al. European Association for Palliative Care (EAPC). White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28(3):197–209. https://doi.org/10.1177/0269216313493685 .
Harrison Dening K, Sampson EL, De Vries K. Advance care planning in dementia: recommendations for healthcare professionals. Palliat Care. 2019;12:1178224219826579. https://doi.org/10.1177/1178224219826579 .
Jones K, Birchley G, Huxtable R, Clare L, Walter T, Dixon J. End of life care: A scoping review of experiences of advance care planning for people with dementia. Dementia (London). 2019;18(3):825–45. https://doi.org/10.1177/1471301216676121 .
Fried TR, Redding CA, Robbins ML, Paiva A, O'Leary JR, Iannone L. Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc. 2010;58(12):2329–36. https://doi.org/10.1111/j.1532-5415.2010.03184.x .
Birchley G, Jones K, Huxtable R, Dixon J, Kitzinger J, Clare L. Dying well with reduced agency: a scoping review and thematic synthesis of the decision-making process in dementia, traumatic brain injury and frailty. BMC Med Ethics. 2016;17(1):46. https://doi.org/10.1186/s12910-016-0129-x .
Miller LM, Whitlatch CJ, Lee CS, Lyons KS. Incongruent perceptions of the care values of hospitalized persons with dementia: a pilot study of patient-family caregiver dyads. Aging Ment Health. 2018;22(4):489–96. https://doi.org/10.1080/13607863.2017.1280766 .
Lamahewa K, Mathew R, Iliffe S, Wilcock J, Manthorpe J, Sampson EL, et al. A qualitative study exploring the difficulties influencing decision making at the end of life for people with dementia. Health Expect. 2018;21(1):118–27. https://doi.org/10.1111/hex.12593 .
Sampson EL, Lodwick R, Rait G, Candy B, Low J, King M, et al. Living with an older person dying from cancer, lung disease, or dementia: health outcomes from a general practice cohort study. J Pain Symptom Manage. 2016;51(5):839–48. https://doi.org/10.1016/j.jpainsymman.2015.12.319 .
Gott M, Morgan T, Williams L. Gender and palliative care: a call to arms. Palliat Care Soc Pract. 2020;14:2632352420957997. https://doi.org/10.1177/2632352420957997 .
Lewis JM, DiGiacomo M, Luckett T, Davidson PM, Currow DC. A social capital framework for palliative care: supporting health and well-being for people with life-limiting illness and their carers through social relations and networks. J Pain Symptom Manag. 2013;45(1):92–103. https://doi.org/10.1016/j.jpainsymman.2011.12.283 .
Sawyer JM, Sallnow L, Kupeli N, Stone P, Sampson EL. Social networks, social capital and end-of-life care for people with dementia: a realist review. BMJ Open. 2019;9(12):e030703. https://doi.org/10.1136/bmjopen-2019-030703 .
Sibbett RA, Russ TC, Deary IJ, Starr JM. Dementia ascertainment using existing data in UK longitudinal and cohort studies: a systematic review of methodology. BMC Psychiatry. 2017;17(1):239. https://doi.org/10.1186/s12888-017-1401-4 .
Bartlett P. At the interface between paradigms: English mental capacity law and the CRPD. Front Psychiatry. 2020;11:570735. https://doi.org/10.3389/fpsyt.2020.570735 .
Davidson G, Brophy L, Campbell J, Farrell SJ, Gooding P, O'Brien AM. An international comparison of legal frameworks for supported and substitute decision-making in mental health services. Int J Law Psychiatry. 2016;44:30–40. https://doi.org/10.1016/j.ijlp.2015.08.029 .
Douglass A. Mental Capacity: Updating New Zealand’s Law and Practice (Report for the New Zealand Law Foundation, Dunedin, July 2016) http://www.lawfoundation.org.nz/ Accessed 18 May 2021.
Sarmento VP, Gysels M, Higginson IJ, Gomes B. Home palliative care works: but how? A meta-ethnography of the experiences of patients and family caregivers. BMJ Support Palliat Care. 2017;7:390–403. https://doi.org/10.1136/bmjspcare-2016-001141 .
Download references
Acknowledgements
The GUIDE_Care Services project (NIHR HS&DR: 14/19/22 ) and The GUIDE_Care project (NIHR HS&DR: 09/2000/58).
Pre-publication history
Presented in part at the 7th International Advance Care Planning Conference, Rotterdam, 2019.
Wei Gao and Irene J Higginson at King’s College London are supported by the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. These funding bodies had no role in the design of the study, the collection, analysis, and interpretation of the data, or in the writing of the manuscript.
Author information
Authors and affiliations.
Cicely Saunders Institute of Palliative Care and Rehabilitation, King’s College London, London, UK
James M. Beattie, Irene J. Higginson & Wei Gao
School of Cardiovascular Medicine and Sciences, King’s College London, London, UK
James M. Beattie & Theresa A. McDonagh
You can also search for this author in PubMed Google Scholar
Contributions
JMB: conception and design; organisation of the conduct of the study; interpretation of results; primary drafting and revision of the manuscript.
IJH and TAMcD: interpretation of the results; critical review and revision of the manuscript.
WG: conception and design; organisation of the conduct of the study; data acquisition and management; statistical analysis of the data; interpretation of results; drafting and revision of the manuscript. WG is guarantor of the data.
All authors approved the final version of the paper prior to submission.
Corresponding author
Correspondence to Wei Gao .
Ethics declarations
Ethics approval and consent for participation.
In accordance with General Data Protection Regulations arising from the UK Data Protection Act (2018), as all patient level data was fully anonymised, no ethical approval was required. Wei Gao is accredited to analyse this data through the ONS Approved Researcher Scheme.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Supplementary Table S-1. Characteristics of heart failure decedents, with or without comorbid dementia, before and after MCA implementation, n (column %), England 2001-2018. Supplementary Table S-2. Multiple adjusted prevalence ratios for the factors associated with home death compared to hospital death among patients who died from heart failure with or without dementia, England 2001-2018. Supplementary Table S-3. Multiple adjusted prevalence ratios for the factors associated with care home death compared to hospital death among patients who died from heart failure with or without dementia, England 2001-2018. Supplementary Table S-4. Multiple adjusted prevalence ratios for the factors associated with hospice death compared to hospital death among patients who died from heart failure with or without dementia, England 2001-2018
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Beattie, J.M., Higginson, I.J., McDonagh, T.A. et al. Implementation of the Mental Capacity Act: a national observational study comparing resultant trends in place of death for older heart failure decedents with or without comorbid dementia. BMC Med 20 , 30 (2022). https://doi.org/10.1186/s12916-021-02210-2
Download citation
Received : 17 June 2021
Accepted : 07 December 2021
Published : 20 January 2022
DOI : https://doi.org/10.1186/s12916-021-02210-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Comorbidity
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- The Mental Capacity Act (2005)
- What questions should I ask?
- Best Interest Decision
- Deprivation of Liberty Safeguards (DoLS)
- Liberty Protection Safeguards (LPS)
- Lasting Power of Attorney (LPA)
- Glossary of Terms
- Mental Capacity Training
- Mental Capacity Assessments
- Best Interest Assessor
- Consultancy
- Testimonials
- Mental Capacity in Practice
Case study: What do we mean by ‘decision specific’?
- 1 January 2024 22 December 2023
- Case Study , Mental Capacity in Practice

We all make many thousands of micro-decisions on a daily basis, from the way we make a cup of tea to what we watch on TV. However, when it comes to decision making, the Mental Capacity Act (2005) is ‘decision specific’. This means that it relates to a specific decision made at a specific time. It does not refer to decision-making more broadly. This raises the question: how specific do we need to be when we assess capacity?
A familiar character
To help us unpack some of the issues around this question, I’d like to introduce you to a familiar character…
- Wheezy the Penguin is 92-year-old and lives in a nursing home near his family.
- He has a diagnosis of Chronic Obstructive Pulmonary Disorder (COPD) and has had several Cerebral Vascular Accidents (CVAs), also known as strokes. This has left him with significantly reduced mobility, and he is reliant on staff to support his movements between bed, chair and toilet.
- Following his most recent stroke, staff have noted a significant decline in Wheezy’s short-term memory and processing skills. This has been formerly assessed by the professional teams, who have diagnosed Vascular Dementia, presenting with moderate cognitive impairment.
- Wheezy has been observed by team members to be increasingly confused during times of manual handling, from which the team discussed whether he can provide informed consent on what support he needs to move about.
At this stage, the team recognise that a Mental Capacity Assessment is required for Wheezy, as there is a reasonable belief that he is not able to provide informed consent for manual handling support. The initial decision to be addressed is: ‘can Wheezy make an informed decision regarding support for manual handling, including the use of a hoist?’
Broadly speaking, an unspoken ‘rule of thumb’ to support practitioners in daily practice is to assess for a commonly grouped cluster of decisions, such as:
- Manual handling
- Consent for photography
- Personal care support
- Management of ‘small’ finances
- Household hygiene
This approach can be found within the Mental Capacity Act (2005) itself in the ‘clustering’, or grouping of decisions for Lasting Powers of Attorney (LPA) and Deputyships to either ‘Property and Finance’ or ‘Health and Welfare’. Although it is equally recognised that it could be argued that this does undermine the element of ‘decision specific’.
Assessment questions should be carefully tailored to that individual’s needs and preferences around that ‘cluster’ of decisions. For example, if regarding ‘personal care’, it may encompass teeth, toileting, and bathing. Another example may be ‘household hygiene’, which may include laundry, hoovering, polishing, and dusting, cleaning kitchen items following use and disinfecting surfaces.
However, caution is required. While it can be useful in certain cases to group together related elements for the purpose of certain Mental Capacity Assessments, we should never standardise this approach, or apply a ‘one size fits all’ philosophy that presumes incapacity, counter to the five core principles of the Act .
Our response to the case scenario
As each person and their circumstances are unique, including what they may or may not be able to provide consent for, allowances need to be made in the assessment with appropriate graded support :
- If Wheezy was able to provide informed consent for manual handling in terms of repositioning in bed and associated movements for personal care needs, this should be a consent form, or signature of agreement to the care plan section.
- Equally, this would be the same if Wheezy was able to provide informed consent for use of his wheelchair, including the lap strap for safety when moving.
- However, in our case study example, if he was not able to give informed consent for the use of a hoist to transfer from his bed to his wheelchair for activities, appointments, or other needs, then this would need a specific mental capacity assessment only for the use of the hoist, not for manual handling as a whole.
Find out more
For help and support with Mental Capacity Assessments, as well as training for your team , please do get in touch .
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
Leave a Reply Cancel reply

IMAGES
VIDEO
COMMENTS
case study he has hypothetically explored what actions may have been required if the circumstances were different. The analysis of how the case was handled is provided by Steven Richards, director of Edge Training & Consultancy, who provides training on the Mental Capacity Act and deprivation of liberty safeguards. The legal analysis at the end ...
The MCA 200517 safeguards the rights of people with dementia and carers, enshrining principles of capacity, decision-making and best interests. Our study, designed in four phases (pre diagnosis, immediately after diagnosis, living with dementia, end of life), explored the MCA's implementation and impact.
Capacity to make one's own decisions is fundamental to the autonomy of the individual. Capacity is a functional assessment made by a clinician to determine if a patient is capable of making a specific decision. Competency is a global assessment and legal determination made by a judge in court. Capacity evaluation for a patient with dementia is ...
Abstract. Mental capacity is the ability to retain, process, and weigh up information to provide a completely objective decision independent of any cognitive impairment present. Awareness of an individual's mental capacity is important for all healthcare professionals. Mental capacity and capacity assessments are guided by the legal framework ...
Step 1 - starting with the presumption of capacity. Step 2 - remembering that capacity is decision/issue and time speci c. Step 3 - preparation for assessments. Step 4 - practicable steps. Step 5 - applying the test for capacity. Stage 1: the functional test. Stage 2: the diagnostic test.
Dementia and the Mental Capacity Act 2005. People with dementia may lose mental capacity and become unable to make some decisions. The Mental Capacity Act is the law in England and Wales that protects people who lack capacity to make a decision. Dementia and the Mental Capacity Act 2005. Assessing the mental capacity of a person with dementia.
The Mental Capacity Act (MCA) of 2005, applicable to residents in England and Wales aged ≥16 years, sets out a statutory framework to foster person-centred decision making and advance care planning for those who may lack capacity due to a lifelong learning disability, or as a consequence of the transient or permanent effects of acute or long ...
Undertaking capacity assessments for people with dementia in general hospitals Nurs Older People. 2016 Aug;28(7) :28-33. doi ... There is variation in practice despite the legal framework provided by the Mental Capacity Act (MCA) 2005, covering England and Wales, which raises questions about adherence to the legislation. Using a case study ...
For research to have an impact on people with dementia, they need to be involved in studies. However, undertaking research with participants who may lack capacity to consent poses many challenges for researchers. This article explores the practical application of the Mental Capacity Act 2005 in the process and provides realistic advice on ...
Aims: This study aimed to explore experiences of specialist community nurses providing information about the Mental Capacity Act and supporting people with dementia and carers. Background: The role of specialist community nurses and case managers, such as Admiral Nurses, suggests that providing information about the recent Mental Capacity Act (2005) in England and Wales would be appreciated by ...
Mental Capacity Act 2005: An interview-based study of community-based specialist nurses working in dementia care. Journal of Clinical Nursing , 21 (11-12), 1697-1705.
As part of a 5-year research program investigating the implementation and adoption of the Mental Capacity Act in dementia practice, we interviewed staff working in different care homes at two time points (32 staff at Time 1 in 2008 and 27 staff at Time 2 in 2012) in South East England. ... Kitchen G., Manela M., Livingston G. (2005) A ...
If you need to decide whether a person has the mental capacity to make a specific decision, follow the steps below. Always try to use your knowledge of the person to help you decide. You can also ask other people for advice - such as the person's GP, community nurse or social worker. Step 1 Step 2 Step 3.
Mental Capacity Act Scemarios - May 2016 Page 1 of 7 Mental Capacity Act Scenarios Individuals 1. A person with declining capacity The story Mrs Ali lives at home on her own. She owns the house. She and her family had been told that she has dementia. She gets quite a lot of help from her son, Javid, who lives nearby
Sources of support. To speak to a dementia specialist Admiral Nurse about capacity and decision-making or any other aspect of dementia, please call our Helpline on 0800 888 6678 (Monday-Friday 9am-9pm, Saturday and Sunday 9am-5pm, every day except 25th December) or email [email protected].
The five key principles are: Principle 1 - A presumption of capacity. Principle 2 - The right to be supported when making decisions. Principle 3 - An unwise decision cannot be seen as a wrong decision. Principle 4 - Best interests must be at the heart of all decision making. Principle 5 - Any intervention must be with the least ...
Introduction. These scenarios are intended to provoke thought and discussion with respect to issues related to mental capacity / best interests decisions and associated social work themes and topics. It is recommended that the student is given the initial scenario and suggested questions, and then asked to consider the relevant issues and what ...
Objective To identify common difficult decisions made by family carers on behalf of people with dementia, and facilitators of and barriers to such decisions, in order to produce information for family carers about overcoming barriers. Design Qualitative study to delineate decision areas through focus groups and complexity of decision making in individual interviews. Setting Community settings ...
Case study: Presumption of capacity in practice. The first principle of the Mental Capacity Act (2005) states that we must always presume capacity in the first instance. This means that we should always start from a position where we presume that an individual has the ability to make a decision (i.e. has capacity), rather than the other way round.
Mental Capacity Act (Northern Ireland) 2016 training, FAQs and other resources . Maximising capacity. ... My patient has mild dementia. If the patient can't tell you, ask those who know them what time of day is best for a consultation. ... Case study . Mental capacity: Supporting patients to make decisions about their care. Case study .
Background Heart failure (HF) is increasingly prevalent in the growing elderly population and commonly associated with cognitive impairment. We compared trends in place of death (PoD) of HF patients with/without comorbid dementia around the implementation period of the Mental Capacity Act (MCA) in October 2007, this legislation supporting patient-centred decision making for those with reduced ...
Case study: Presumption of capacity in practice. The first principle of the Mental Capacity Act (2005) states that we must always presume capacity in the first instance. This means that we should always start from a position where we presume that an individual has the ability to make a decision (i.e. has capacity), rather than the other way round.
Case Study, Mental Capacity in Practice. We all make many thousands of micro-decisions on a daily basis, from the way we make a cup of tea to what we watch on TV. However, when it comes to decision making, the Mental Capacity Act (2005) is 'decision specific'. This means that it relates to a specific decision made at a specific time.