Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Working with sources
- How to Write a Summary | Guide & Examples

How to Write a Summary | Guide & Examples
Published on November 23, 2020 by Shona McCombes . Revised on May 31, 2023.
Summarizing , or writing a summary, means giving a concise overview of a text’s main points in your own words. A summary is always much shorter than the original text.
There are five key steps that can help you to write a summary:
- Read the text
- Break it down into sections
- Identify the key points in each section
- Write the summary
- Check the summary against the article
Writing a summary does not involve critiquing or evaluating the source . You should simply provide an accurate account of the most important information and ideas (without copying any text from the original).
Table of contents
When to write a summary, step 1: read the text, step 2: break the text down into sections, step 3: identify the key points in each section, step 4: write the summary, step 5: check the summary against the article, other interesting articles, frequently asked questions about summarizing.
There are many situations in which you might have to summarize an article or other source:
- As a stand-alone assignment to show you’ve understood the material
- To keep notes that will help you remember what you’ve read
- To give an overview of other researchers’ work in a literature review
When you’re writing an academic text like an essay , research paper , or dissertation , you’ll integrate sources in a variety of ways. You might use a brief quote to support your point, or paraphrase a few sentences or paragraphs.
But it’s often appropriate to summarize a whole article or chapter if it is especially relevant to your own research, or to provide an overview of a source before you analyze or critique it.
In any case, the goal of summarizing is to give your reader a clear understanding of the original source. Follow the five steps outlined below to write a good summary.
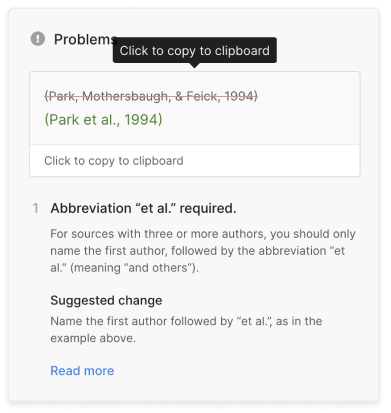
Scribbr Citation Checker New
The AI-powered Citation Checker helps you avoid common mistakes such as:
- Missing commas and periods
- Incorrect usage of “et al.”
- Ampersands (&) in narrative citations
- Missing reference entries
You should read the article more than once to make sure you’ve thoroughly understood it. It’s often effective to read in three stages:
- Scan the article quickly to get a sense of its topic and overall shape.
- Read the article carefully, highlighting important points and taking notes as you read.
- Skim the article again to confirm you’ve understood the key points, and reread any particularly important or difficult passages.
There are some tricks you can use to identify the key points as you read:
- Start by reading the abstract . This already contains the author’s own summary of their work, and it tells you what to expect from the article.
- Pay attention to headings and subheadings . These should give you a good sense of what each part is about.
- Read the introduction and the conclusion together and compare them: What did the author set out to do, and what was the outcome?
To make the text more manageable and understand its sub-points, break it down into smaller sections.
If the text is a scientific paper that follows a standard empirical structure, it is probably already organized into clearly marked sections, usually including an introduction , methods , results , and discussion .
Other types of articles may not be explicitly divided into sections. But most articles and essays will be structured around a series of sub-points or themes.
Now it’s time go through each section and pick out its most important points. What does your reader need to know to understand the overall argument or conclusion of the article?
Keep in mind that a summary does not involve paraphrasing every single paragraph of the article. Your goal is to extract the essential points, leaving out anything that can be considered background information or supplementary detail.
In a scientific article, there are some easy questions you can ask to identify the key points in each part.
| Introduction | or problem was addressed? |
|---|---|
| Methods | |
| Results | supported? |
| Discussion/conclusion |
If the article takes a different form, you might have to think more carefully about what points are most important for the reader to understand its argument.
In that case, pay particular attention to the thesis statement —the central claim that the author wants us to accept, which usually appears in the introduction—and the topic sentences that signal the main idea of each paragraph.
Prevent plagiarism. Run a free check.
Now that you know the key points that the article aims to communicate, you need to put them in your own words.
To avoid plagiarism and show you’ve understood the article, it’s essential to properly paraphrase the author’s ideas. Do not copy and paste parts of the article, not even just a sentence or two.
The best way to do this is to put the article aside and write out your own understanding of the author’s key points.
Examples of article summaries
Let’s take a look at an example. Below, we summarize this article , which scientifically investigates the old saying “an apple a day keeps the doctor away.”
Davis et al. (2015) set out to empirically test the popular saying “an apple a day keeps the doctor away.” Apples are often used to represent a healthy lifestyle, and research has shown their nutritional properties could be beneficial for various aspects of health. The authors’ unique approach is to take the saying literally and ask: do people who eat apples use healthcare services less frequently? If there is indeed such a relationship, they suggest, promoting apple consumption could help reduce healthcare costs.
The study used publicly available cross-sectional data from the National Health and Nutrition Examination Survey. Participants were categorized as either apple eaters or non-apple eaters based on their self-reported apple consumption in an average 24-hour period. They were also categorized as either avoiding or not avoiding the use of healthcare services in the past year. The data was statistically analyzed to test whether there was an association between apple consumption and several dependent variables: physician visits, hospital stays, use of mental health services, and use of prescription medication.
Although apple eaters were slightly more likely to have avoided physician visits, this relationship was not statistically significant after adjusting for various relevant factors. No association was found between apple consumption and hospital stays or mental health service use. However, apple eaters were found to be slightly more likely to have avoided using prescription medication. Based on these results, the authors conclude that an apple a day does not keep the doctor away, but it may keep the pharmacist away. They suggest that this finding could have implications for reducing healthcare costs, considering the high annual costs of prescription medication and the inexpensiveness of apples.
However, the authors also note several limitations of the study: most importantly, that apple eaters are likely to differ from non-apple eaters in ways that may have confounded the results (for example, apple eaters may be more likely to be health-conscious). To establish any causal relationship between apple consumption and avoidance of medication, they recommend experimental research.
An article summary like the above would be appropriate for a stand-alone summary assignment. However, you’ll often want to give an even more concise summary of an article.
For example, in a literature review or meta analysis you may want to briefly summarize this study as part of a wider discussion of various sources. In this case, we can boil our summary down even further to include only the most relevant information.
Using national survey data, Davis et al. (2015) tested the assertion that “an apple a day keeps the doctor away” and did not find statistically significant evidence to support this hypothesis. While people who consumed apples were slightly less likely to use prescription medications, the study was unable to demonstrate a causal relationship between these variables.
Citing the source you’re summarizing
When including a summary as part of a larger text, it’s essential to properly cite the source you’re summarizing. The exact format depends on your citation style , but it usually includes an in-text citation and a full reference at the end of your paper.
You can easily create your citations and references in APA or MLA using our free citation generators.
APA Citation Generator MLA Citation Generator
Finally, read through the article once more to ensure that:
- You’ve accurately represented the author’s work
- You haven’t missed any essential information
- The phrasing is not too similar to any sentences in the original.
If you’re summarizing many articles as part of your own work, it may be a good idea to use a plagiarism checker to double-check that your text is completely original and properly cited. Just be sure to use one that’s safe and reliable.
If you want to know more about ChatGPT, AI tools , citation , and plagiarism , make sure to check out some of our other articles with explanations and examples.
- ChatGPT vs human editor
- ChatGPT citations
- Is ChatGPT trustworthy?
- Using ChatGPT for your studies
- What is ChatGPT?
- Chicago style
- Paraphrasing
Plagiarism
- Types of plagiarism
- Self-plagiarism
- Avoiding plagiarism
- Academic integrity
- Consequences of plagiarism
- Common knowledge
A summary is a short overview of the main points of an article or other source, written entirely in your own words. Want to make your life super easy? Try our free text summarizer today!
A summary is always much shorter than the original text. The length of a summary can range from just a few sentences to several paragraphs; it depends on the length of the article you’re summarizing, and on the purpose of the summary.
You might have to write a summary of a source:
- As a stand-alone assignment to prove you understand the material
- For your own use, to keep notes on your reading
- To provide an overview of other researchers’ work in a literature review
- In a paper , to summarize or introduce a relevant study
To avoid plagiarism when summarizing an article or other source, follow these two rules:
- Write the summary entirely in your own words by paraphrasing the author’s ideas.
- Cite the source with an in-text citation and a full reference so your reader can easily find the original text.
An abstract concisely explains all the key points of an academic text such as a thesis , dissertation or journal article. It should summarize the whole text, not just introduce it.
An abstract is a type of summary , but summaries are also written elsewhere in academic writing . For example, you might summarize a source in a paper , in a literature review , or as a standalone assignment.
All can be done within seconds with our free text summarizer .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, May 31). How to Write a Summary | Guide & Examples. Scribbr. Retrieved July 2, 2024, from https://www.scribbr.com/working-with-sources/how-to-summarize/
Is this article helpful?
Shona McCombes
Other students also liked, how to paraphrase | step-by-step guide & examples, how to quote | citing quotes in apa, mla & chicago, the basics of in-text citation | apa & mla examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Article review writing format, steps, examples and illustration PDF Compiled by Mohammed Yismaw

2021, Article review writing format, steps, examples and illustration PDF Compiled by Mohammed Yismaw
The purpose of this document is to help students and researchers understand how a review of an academic journal is conducted and reported in different fields of study. Review articles in academic journals that analyze or discuss researches previously published by others, rather than reporting new research results or findings. Summaries and critiques are two ways to write a review of a scientific journal article. Both types of writing ask you first to read and understand an article from the primary literature about your topic. The summary involves briefly but accurately stating the key points of the article for a reader who has not read the original article. The critique begins by summarizing the article and then analyzes and evaluates the author’s research. Summaries and critiques help you learn to synthesize information from different sources and are usually limited to two pages maximum.
Related Papers
Harald von Kortzfleisch , Christoph Kahle
Neue Technologien und Innovationen stellen heutzutage wichtige Schlüsselelemente der Wachstums und Erfolgssicherung von Unternehmen dar. Durch einen in Geschwindigkeit und Intensität immer schneller zunehmenden Wettbewerb nehmen Innovationen eine immer zentralere Rolle im Praxisalltag von Unternehmen ein. Dieser technische Fortschritt treibt auch in der Wissenschaft das Thema des Innovationsmanagements in den letzten Jahrzehnten immer stärker voran und wird dort ausgiebig diskutiert. Die Bedeutung von Innovationen wächst dabei ebenfalls aus der Sicht der Kunden, welche heutzutage viel differenzierter als früher Produkte und Dienste nachfragen und somit Unternehmen vor neue Herausforderungen stellen. Ãberdies stellen Innovationen heute ein entscheidendes Bindeglied zwischen Marktorientierung und erhofften Unternehmenserfolg dar. Seit einigen Jahren lässt sich eine Ãffnung der Unternehmensgrenzen für externe Quellen wie Kunden, Zulieferer, Universitäten oder teilweise auch M...
SSRN Electronic Journal
Helmut Krcmar
Dominic Lindner
Alexandra Waluszewski
Research Policy
Nuria Gonzalez Alvarez
Creativity and Innovation Management
Matti Pihlajamaa
Firms tap into user knowledge to learn about the users’ needs. While users have been recognized as a valuable source of knowledge for innovation, few studies have investigated how their knowledge is integrated into innovation processes in the context of complex products and systems (CoPS). The purpose of this study is to reveal the practices of CoPS manufacturers to facilitate user knowledge utilization for innovation. We investigate two case companies, a medical device manufacturer and an aircraft manufacturer, and report on seven managerial practices for utilizing user knowledge. We adopt the absorptive capacity model in structuring our findings and elaborate three of the model's sub-capabilities (recognition of the value of user knowledge, acquisition of user knowledge, and assimilation/transformation of user knowledge) by proposing that each is associated with a distinct managerial goal and related practices: (1) Sensitizing the organization to the innovation potential of user knowledge, (2) identifying and gaining access to suitable user knowledge, and (3) analyzing and interpreting user knowledge and integrating it into product development. Our study contributes to the innovation management literature by analyzing the capabilities required to utilize user knowledge throughout the CoPS innovation process.
Information & Management
Diffusion of digital technologies into the manufacturing industry has created new opportunities for innovation that firms must address to remain competitive. We investigate the role of customer and user knowledge in the digital innovation processes of three global B2B manufacturing companies. We find that the B2B manufacturing industry's characteristics influence how users and customers may be leveraged. Customers making the purchasing decisions are considered for knowledge about short-term changes in market needs, while users working directly with the products provide long-term guidance for digital innovation. We identify practices for acquiring, distributing, and using customer and user knowledge for digital innovation.
Journal of business market management
Patricia Sandmeier
Journal of Entrepreneurship, Management and Innovation
Journal of Entrepreneurship, Management and Innovation JEMI
Given the rising role of users in innovation processes and the increasing amount of research in this field the aim of this paper is to explore the limits of our understanding of the user innovation (UI) concept. In doing so, the study addresses four basic questions: (1) Why do users create and share innovation? (2) Who is the user-innovator? (3) What type of innovation do users create? (4) How do users innovate? The results of a systematic literature review identified the main research streams on user innovation, together with weaknesses of past research and perspectives for future studies.
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Gernot Grabher
Journal of Computer‐ …
Petra Schubert , Kathrin Möslein
Mossimo Sesom
Shahab Zare
Arthur Shulman
International Journal of Technology Management
Richard Farr
European Journal of Dental Education
Y.P. CHANDRA
Chandra Yanto
Management Science
John Roberts
Maria Antikainen
Johanna Bragge
intechopen.com
Ivona Vrdoljak Raguz
Service Science
Tuure Tuunanen
Jouni K Juntunen
Benji Decker
Eva Heiskanen
Handbook of Marketing
Jerome Hauser
Service Industries Journal
Christian Kowalkowski
Journal of Engineering Design
Anna Rönnbäck , Ola Isaksson
Journal of Management
Bettina Bastian
International Journal of Innovation Management
Harald von Kortzfleisch
Guido H Baltes
Technology Analysis & Strategic Management
Raimo Lovio
Marco Bertoni , Christian Johansson
Dominik Walcher
Managing Service Quality
Tor W. Andreassen
Journal of Product Innovation Management
Gary Schirr
System Sciences, 2004. …
Dominik Walcher , Ralf Reichwald
Edina Vadovics
Jouni Similä
Luis Cancino Muñoz
Shell Artillery
Ralf Reichwald
Journal of the Academy of …
Ian Wilkinson , Subroto Roy
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- All eBooks & Audiobooks
- Academic eBook Collection
- Home Grown eBook Collection
- Off-Campus Access
- Literature Resource Center
- Opposing Viewpoints
- ProQuest Central
- Course Guides
- Citing Sources
- Library Research
- Websites by Topic
- Book-a-Librarian
- Research Tutorials
- Use the Catalog
- Use Databases
- Use Films on Demand
- Use Home Grown eBooks
- Use NC LIVE
- Evaluating Sources
- Primary vs. Secondary
- Scholarly vs. Popular
- Make an Appointment
- Writing Tools
- Annotated Bibliographies
- Summaries, Reviews & Critiques
- Writing Center
Service Alert

Article Summaries, Reviews & Critiques
Writing an article summary.
- Writing an article REVIEW
- Writing an article CRITIQUE
- Citing Sources This link opens in a new window
- About RCC Library
Text: 336-308-8801
Email: [email protected]
Call: 336-633-0204
Schedule: Book-a-Librarian
Like us on Facebook
Links on this guide may go to external web sites not connected with Randolph Community College. Their inclusion is not an endorsement by Randolph Community College and the College is not responsible for the accuracy of their content or the security of their site.
When writing a summary, the goal is to compose a concise and objective overview of the original article. The summary should focus only on the article's main ideas and important details that support those ideas.
Guidelines for summarizing an article:
- State the main ideas.
- Identify the most important details that support the main ideas.
- Summarize in your own words.
- Do not copy phrases or sentences unless they are being used as direct quotations.
- Express the underlying meaning of the article, but do not critique or analyze.
- The summary should be about one third the length of the original article.
Your summary should include:
- Give an overview of the article, including the title and the name of the author.
- Provide a thesis statement that states the main idea of the article.
- Use the body paragraphs to explain the supporting ideas of your thesis statement.
- One-paragraph summary - one sentence per supporting detail, providing 1-2 examples for each.
- Multi-paragraph summary - one paragraph per supporting detail, providing 2-3 examples for each.
- Start each paragraph with a topic sentence.
- Use transitional words and phrases to connect ideas.
- Summarize your thesis statement and the underlying meaning of the article.
Adapted from "Guidelines for Using In-Text Citations in a Summary (or Research Paper)" by Christine Bauer-Ramazani, 2020
Additional Resources
All links open in a new window.
How to Write a Summary - Guide & Examples (from Scribbr.com)
Writing a Summary (from The University of Arizona Global Campus Writing Center)
- Next: Writing an article REVIEW >>
- Last Updated: Mar 15, 2024 9:32 AM
- URL: https://libguides.randolph.edu/summaries

- Find Nursing Sources
- CRAAP - Evaluate Sources
APA Article Summary
Writing center.
- Qualitative vs Quantitative
Visit & Contact Us

Text: 51 8.21 2.7685
Email: [email protected]
Phone: 518.736.3622 x8058
Think of an article summary in APA format as the formal version of telling your best friend about the great movie you saw last night or talking to your mom about the awesome book you just finished reading. In each case you're relying on your insights as to what details are important, necessary and enticing to your audience.
An APA summary has four crucial components:
1. The original research article ( click here for an example ) - make sure you have the full-text of the article.
2. Your summary ( click here for an example ) of the orginal research article.
3. The APA citation of the original research article ( click here for example on page 2 ) .
4. An outside reader - use FM's Writing Center. Hours are listed below.
Need more tips and strategies for writing your summary? This link is a great place to start.
- APA Article Summary - Tips

Writing Center
2023-2024 Hours:
Monday - Friday 9am - 4pm

- << Previous: APA Style
- Next: Writing Center >>
- Last Updated: Feb 23, 2024 11:18 AM
- URL: https://library.fmcc.edu/nursing

- Peterborough

Writing Article Summaries
- Understanding Article Summaries
Common Problems in Article Summaries
Read carefully and closely, structure of the summary, writing the summary.
- Sample Outlines and Paragraphs
Understanding Article Summaries
An article summary is a short, focused paper about one scholarly article that is informed by a critical reading of that article. For argumentative articles, the summary identifies, explains, and analyses the thesis and supporting arguments; for empirical articles, the summary identifies, explains, and analyses the research questions, methods, findings, and implications of the study.
Although article summaries are often short and rarely account for a large portion of your grade, they are a strong indicator of your reading and writing skills. Professors ask you to write article summaries to help you to develop essential skills in critical reading, summarizing, and clear, organized writing. Furthermore, an article summary requires you to read a scholarly article quite closely, which provides a useful introduction to the conventions of writing in your discipline (e.g. Political Studies, Biology, or Anthropology).
The most common problem that students have when writing an article summary is that they misunderstand the goal of the assignment. In an article summary, your job is to write about the article, not about the actual topic of the article. For example, if you are summarizing Smith’s article about the causes of the Bubonic plague in Europe, your summary should be about Smith’s article: What does she want to find out about the plague? What evidence does she use? What is her argument? You are not writing a paper about the actual causes of Bubonic plague in Europe.
Further, as a part of critical reading, you will often consider your own position on a topic or an argument; it is tempting to include an assessment or opinion about the thesis or findings, but this is not the goal of an article summary. Rather, you must identify, explain, and analyse the main point and how it is supported.
Your key to success in writing an article summary is your understanding of the article; therefore, it is essential to read carefully and closely. The Academic Skills Centre offers helpful instruction on the steps for critical reading: pre-reading, active and analytical reading, and reflection.
Argumentative Articles
As you read an argumentative article, consider the following questions:
- What is the topic?
- What is the research question? In other words, what is the author trying to find out about that topic?
- How does the author position his/her article in relation to other studies of the topic?
- What is the thesis or position? What are the supporting arguments?
- How are supporting arguments developed? What kind of evidence is used?
- What is the significance of the author’s thesis? What does it help you to understand about the topic?
Empirical Articles
As you read an empirical article, consider the following questions:
- What is the research question?
- What are the predictions and the rationale for these predictions?
- What methods were used (participants, sampling, materials, procedure)? What were the variables and controls?
- What were the main results?
- Are the findings supported by previous research?
- What are the limitations of the study?
- What are the implications or applications of the findings?
Create a Reverse Outline
Creating a reverse outline is one way to ensure that you fully understand the article. Pre-read the article (read the abstract, introduction, and/or conclusion). Summarize the main question(s) and thesis or findings. Skim subheadings and topic sentences to understand the organization; make notes in the margins about each section. Read each paragraph within a section; make short notes about the main idea or purpose of each paragraph. This strategy will help you to see how parts of the article connect to the main idea or the whole of the article.
A summary is written in paragraph form and generally does not include subheadings. An introduction is important to clearly identify the article, the topic, the question or purpose of the article, and its thesis or findings. The body paragraphs for a summary of an argumentative article will explain how arguments and evidence support the thesis. Alternatively, the body paragraphs of an empirical article summary may explain the methods and findings, making connections to predictions. The conclusion explains the significance of the argument or implications of the findings. This structure ensures that your summary is focused and clear.
Professors will often give you a list of required topics to include in your summary and/or explain how they want you to organize your summary. Make sure you read the assignment sheet with care and adapt the sample outlines below accordingly.
One significant challenge in writing an article summary is deciding what information or examples from the article to include. Remember, article summaries are much shorter than the article itself. You do not have the space to explain every point the author makes. Instead, you will need to explain the author’s main points and find a few excellent examples that illustrate these points.
You should also keep in mind that article summaries need to be written in your own words. Scholarly writing can use complex terminology to explain complicated ideas, which makes it difficult to understand and to summarize correctly. In the face of difficult text, many students tend to use direct quotations, saving them the time and energy required to understand and reword it. However, a summary requires you to summarize, which means “to state briefly or succinctly” (Oxford English Dictionary) the main ideas presented in a text. The brevity must come from you, in your own words, which demonstrates that you understand the article.
Sample Outlines and Paragraph
Sample outline for an argumentative article summary.
- General topic of article
- Author’s research question or approach to the topic
- Author’s thesis
- Explain some key points and how they support the thesis
- Provide a key example or two that the author uses as evidence to support these points
- Review how the main points work together to support the thesis?
- How does the author explain the significance or implications of his/her article?
Sample Outline for an Empirical Article Summary
- General topic of study
- Author’s research question
- Variables and hypotheses
- Participants
- Experiment design
- Materials used
- Key results
- Did the results support the hypotheses?
- Implications or applications of the study
- Major limitations of the study
Sample Paragraph
The paragraph below is an example of an introductory paragraph from a summary of an empirical article:
Tavernier and Willoughby’s (2014) study explored the relationships between university students’ sleep and their intrapersonal, interpersonal, and educational development. While the authors cited many scholars who have explored these relationships, they pointed out that most of these studies focused on unidirectional correlations over a short period of time. In contrast, Tavernier and Willoughby tested whether there was a bidirectional or unidirectional association between participants’ sleep quality and duration and several psychosocial factors including intrapersonal adjustment, friendship quality, and academic achievement. Further they conducted a longitudinal study over a period of three years in order to determine whether there were changes in the strength or direction of these associations over time. They predicted that sleep quality would correlate with measures of intrapersonal adjustment, friendship quality, and academic achievement; they further hypothesized that this correlation would be bidirectional: sleep quality would predict psychosocial measures and at the same time, psychosocial measures would predict sleep quality.
MigrationConfirmed set by Tish
Courtesy the Odegaard Writing & Research Center
http://depts.washington.edu/owrc
- Business Templates
FREE 16+ Article Summary Samples in PDF | MS Word
This article summary is usually seen in research or in academy in which the essential information of their sample report or discovery is written in one or two paragraph that contains all the main data of it. This shortening tool can be printed for the reader’s interest of reading the whole article or not a sit hands out its importance and uniqueness compared to others. This Professional Summary Templates is free in the internet where you can use the sample format in making any rundown of article with different topics, you can also have it in Microsoft word or excel. Article summary template is the miniature version of the long and detailed content of a document.
Article Summary Template
Article summary template - 8+ samples , examples & formats, book summary template - 6+ samples, examples & format, sample business summary template - 8+ free documents in pdf ..., magazine article summary template.

- Google Docs
Journal Article Summary Template

College Article Summary Template

Research Article Summary Template

Newspaper Article Summary Template

Free Article Summary Template

Academic Article Summary Template

Free APA Article Summary Template

Science News Article Summary Template

Distance Learning Article Summary Template

Newspaper Article Summary Questions Template

If you are looking to summarize the article from the newspaper and also want to raise questions about the same then the template is ideal for that. A newspaper article can be easily summarized with the help of this template as it gives the separate space for each point of the article and anyone can have a fair idea about the main article and its content. Even you can use the same template to ask questions about the article from the author or from anyone as it also provides the space to write the questions about the main points of the article.
Journal Article Summary Format
This is the sample about how to write the summary of a long article or research paper. This template is very useful if you want to summarize a big article or research paper into one page. This template is designed by professionals and contains everything which is required to summarize the article in effectively. This template starts from giving the details about the authors and while giving the information about the study plan , procedures and the outcomes it also states the personal comments. All this details makes this template very impressive and easy to use.

This template is designed by the professionals who help the person who is writing the summary of an article. This template is step by step guide about the summary writing . This template contains the complete procedure of summary writing and helps the person who is writing the summer. It starts from the basic details like Name, Date and Title of the article and also contains the key points and major ideas of the article.

If you looking to summarize a research journal in one page then this is the best template you can take sample reference from. This template is both the guide and the format for summarization of a article. This template also guide about what are the key points to remember while summarizing the article. If you are a beginner and looking for genuine guidelines about the summarization work then you just need to go through this template.
Science Article Summary Template

Article Summary Example

Newspaper Article Summary Questions Outline Template
Why we Need Article Summary?
An article summary is required to get the essence of an article without going through the complete article. It is required to save time and the efforts of the reader. To summarize the article there must be a good template and the templates given here are the best for it. These templates not only provide the format for summarizing but they also help in writing the summary for an article writing .
When we Need Article Summary Template?
To summarize the long article it is very important to have a professionally designed template so that each important detail about the article can be captured effectively.
Templates given here are designed by professionals to help the person who is summarizing the article. These templates also act as the guide for the person as they contain the points and guidelines about summation. You may like Project Summary .
How these “Article summary Template” will help you?
It is tough task for anyone to summarize the long article in just one page without losing its essence or main points. Templates given here are best way to perform this work without a hassle. These templates are best guide for a person who is struggling to summarize the article in one page. Be it a newspaper article of a book, with the help of these templates you can easily summarize them. You can also see Case Summary .
To summarize a big article in one page you just need to follow the instructions given in these templates. These templates will provide end to end help in summarizing the article.
If you have any DMCA issues on this post, please contact us!
Related Posts
Sample police reports, sample cover page for research paper templates, sample news report templates, sample discharge summary templates, sample cashier job descriptions, travel budget templates, questionnaire samples, journal article samples & templates, newspaper article samples, sample welcome speech, sample balancing equations worksheet templates, sample sign language alphabet chart templates, sample morse code alphabet chart templates, sample phonics alphabet chart templates, sample army height and weight chart templates, evaluation essay example - 7+ samples in word, pdf, examples of invitation letters, sample cornell notes paper template - 7+ free documents in pdf ..., sample physical therapist resume - 8+ examples in word, pdf.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 July 2024
Association of Crohn’s disease and ulcerative colitis with the risk of neurological diseases: a large-scale Mendelian randomization study
- Yinan Wang 1 , 2 na1 ,
- Yiming Jia 1 na1 ,
- Qingyun Xu 1 ,
- Pinni Yang 1 ,
- Lulu Sun 1 ,
- Xinyue Chang 1 ,
- Mengyao Shi 1 ,
- Daoxia Guo 1 , 3 ,
- Yonghong Zhang 1 &
- Zhengbao Zhu 1
Journal of Human Genetics ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Epidemiology
- Risk factors
Observational studies suggested increased risks of Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) in patients with Crohn’s disease (CD) and ulcerative colitis (UC). We aimed to assess the causality for the associations of CD and UC with the risks of AD, PD, and MS through a two-sample Mendelian randomization (MR) study. Independent single nucleotide polymorphisms associated with CD (17,897 cases and 33,977 controls) and UC (13,768 cases and 33,977 controls) were identified as genetic instruments based on a European-descent genome-wide association study (GWAS) released by the International Inflammatory Bowel Disease Genetics Consortium. Summary statistics for AD (combined: 25,881 cases and 256,837 controls), PD (combined: 35,836 cases and 665,686 controls), and MS (combined: 48,477 cases and 285,515 controls) were obtained from the largest GWASs and FinnGen study of European ancestry, respectively. MR estimates were generated using the inverse-variance weighted method in the main analysis with a series of sensitivity analyses. MR analyses were conducted per outcome database and were subsequently meta-analyzed to generate combined estimates. Genetically predicted UC was significantly associated with increased risks of AD (combined: OR, 1.03; 95% CI, 1.01–1.05; P = 1.80 × 10 −3 ) and MS (combined: OR, 1.37; 95% CI, 1.23–1.53; P = 1.18 × 10 −8 ), while there was no association between genetically predicted UC and the risk of PD. In contrast, no significant associations were observed for genetically predicted CD with AD, PD, and MS. MR-Egger regression showed no directional pleiotropy for the identified associations, and sensitivity analyses with different MR methods further confirmed these findings. This study suggested significant adverse effects of UC on AD and MS, highlighting that UC patients should receive early intervention with optimal adjunctive medical therapy to reduce the risks of AD and MS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Association between inflammatory bowel disease and Parkinson’s disease: A Mendelian randomization study

COVID-19 and risk of neurodegenerative disorders: A Mendelian randomization study

Rheumatoid arthritis decreases risk for Parkinson’s disease: a Mendelian randomization study
Hodson R. Inflammatory bowel disease. Nature. 2016;540:S97.
Article CAS PubMed Google Scholar
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17.
Article CAS PubMed PubMed Central Google Scholar
Collaborators GIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. lancet Gastroenterol Hepatol. 2020;5:17–30.
Article Google Scholar
Ferro JM, Oliveira Santos M. Neurology of inflammatory bowel disease. J Neurological Sci. 2021;424:117426.
Article CAS Google Scholar
Niesler B, Kuerten S, Demir IE, Schäfer KH. Disorders of the enteric nervous system - a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18:393–410.
Article PubMed Google Scholar
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80.
Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70:85–91.
Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, et al. Inflammatory Bowel Disease and Parkinson’s Disease: A Nationwide Swedish Cohort Study. Inflamm Bowel Dis. 2019;25:111–23.
Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:819–26.
Fu P, Gao M, Yung KKL. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem Neurosci. 2020;11:395–405.
Wang X, Wan J, Wang M, Zhang Y, Wu K, Yang F. Multiple sclerosis and inflammatory bowel disease: A systematic review and meta-analysis. Ann Clin Transl Neurol. 2022;9:132–40.
Article PubMed PubMed Central Google Scholar
Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4:330–45.
Lee K, Lim CY. Mendelian Randomization Analysis in Observational Epidemiology. J Lipid Atherosclerosis. 2019;8:67–77.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98.
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–86.
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30.
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–102.
Patsopoulos NA, Baranzini SE, Santaniello A, Shoostari P, Cotsapas C, Wong G, et al. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365:eaav7188.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv. 2022. https://www.medrxiv.org/content/10.1101/2022.03.03.22271360v1 .
Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304–14.
Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–26.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408.
Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner’s perspective. Genet Epidemiol. 2019;43:609–16.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30–42.
Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081.
Kim GH, Lee YC, Kim TJ, Kim ER, Hong SN, Chang DK, et al. Risk of Neurodegenerative Diseases in Patients with Inflammatory Bowel Disease: A Nationwide Population-based Cohort Study. J Crohns Colitis. 2022;16:436–43.
Sand JR, Troelsen FS, Horváth-Puhó E, Henderson VW, Sørensen HT, Erichsen R. Risk of dementia in patients with inflammatory bowel disease: a Danish population-based study. Aliment Pharmacol Ther. 2022;56: 831–43.
Zhu Y, Yuan M, Liu Y, Yang F, Chen WZ, Xu ZZ, et al. Association between inflammatory bowel diseases and Parkinson’s disease: systematic review and meta-analysis. Neural Regen Res. 2022;17:344–53.
Kosmidou M, Katsanos AH, Katsanos KH, Kyritsis AP, Tsivgoulis G, Christodoulou D, et al. Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J Neurol. 2017;264:254–9.
Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–36.
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384–413.
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol. 2018;113:481–517.
Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582–97.
Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dementia. 2016;12:719–32.
Lee HS, Lobbestael E, Vermeire S, Sabino J, Cleynen I. Inflammatory bowel disease and Parkinson’s disease: common pathophysiological links. Gut. 2021;70:408–17.
CAS PubMed Google Scholar
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–36.
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74:3769–87.
Kesika P, Suganthy N, Sivamaruthi BS, Chaiyasut C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci 2021;264:118627.
Zac-Varghese S, Tan T, Bloom SR. Hormonal interactions between gut and brain. Discov Med. 2010;10:543–52.
PubMed Google Scholar
Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20:1165–79.
Green C, Elliott L, Beaudoin C, Bernstein CN. A population-based ecologic study of inflammatory bowel disease: searching for etiologic clues. Am J Epidemiol. 2006;164:615–23.
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24.
Yang Y, Musco H, Simpson-Yap S, Zhu Z, Wang Y, Lin X, et al. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat Commun. 2021;12:5641.
Brumpton B, Sanderson E, Heilbron K, Hartwig FP, Harrison S, Vie G, et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat Commun. 2020;11:3519.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608.
Download references
Acknowledgements
We thank the authors and participants of all GWASs used, for making their results publicly available. We also acknowledge the participants and investigators of the FinnGen study. Full acknowledgement and funding statements for each of these resources are available via the relevant cited reports.
This study was supported by the National Natural Science Foundation of China (grant: 82103921 and 82020108028) and the Natural Science Research Project of Jiangsu Provincial Higher Education (grant: 21KJB330006).
Author information
These authors contributed equally: Yinan Wang, Yiming Jia.
Authors and Affiliations
Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Major Chronic Non-communicable Diseases, Suzhou Medical College of Soochow University, Suzhou, China
Yinan Wang, Yiming Jia, Qingyun Xu, Pinni Yang, Lulu Sun, Yi Liu, Xinyue Chang, Yu He, Mengyao Shi, Daoxia Guo, Yonghong Zhang & Zhengbao Zhu
Ningbo Center for Disease Control and Prevention, Ningbo, China
School of Nursing, Suzhou Medical College of Soochow University, Suzhou, China
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Daoxia Guo or Zhengbao Zhu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary information
Supplementary materials, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Wang, Y., Jia, Y., Xu, Q. et al. Association of Crohn’s disease and ulcerative colitis with the risk of neurological diseases: a large-scale Mendelian randomization study. J Hum Genet (2024). https://doi.org/10.1038/s10038-024-01271-4
Download citation
Received : 15 August 2023
Revised : 05 June 2024
Accepted : 23 June 2024
Published : 01 July 2024
DOI : https://doi.org/10.1038/s10038-024-01271-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Search Menu
- Sign in through your institution
- Editor's Choice
- Advance articles
- Review Series
- Virtual Issues
- Clinical & Experimental Treatment of…
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Call for Papers
- About Clinical & Experimental Immunology
- About the British Society for Immunology
- Editorial Board
- Early Career Researcher Editorial Board
- Advertising & Corporate Services
- Publishing with us
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, approaches to overcome rituximab resistance in aid, car t-cell therapy, tce: clinical trial experience and technical aspects, employing t cells to disrupt the b–t collaboration: car t and tce, developing personalized b cell targeting regimens, conclusions, acknowledgements, ethical approval, conflict of interests, author contributions.
- < Previous
Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?
- Article contents
- Figures & tables
- Supplementary Data
Kavina Shah, Maria Leandro, Mark Cragg, Florian Kollert, Franz Schuler, Christian Klein, Venkat Reddy, Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?, Clinical and Experimental Immunology , Volume 217, Issue 1, July 2024, Pages 15–30, https://doi.org/10.1093/cei/uxae031
- Permissions Icon Permissions
B and T cells collaborate to drive autoimmune disease (AID). Historically, B- and T-cell (B–T cell) co-interaction was targeted through different pathways such as alemtuzumab, abatacept, and dapirolizumab with variable impact on B-cell depletion (BCD), whereas the majority of patients with AID including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and organ transplantation benefit from targeted BCD with anti-CD20 monoclonal antibodies such as rituximab, ocrelizumab, or ofatumumab. Refractory AID is a significant problem for patients with incomplete BCD with a greater frequency of IgD − CD27 + switched memory B cells, CD19 + CD20 − B cells, and plasma cells that are not directly targeted by anti-CD20 antibodies, whereas most lymphoid tissue plasma cells express CD19. Furthermore, B–T-cell collaboration is predominant in lymphoid tissues and at sites of inflammation such as the joint and kidney, where BCD may be inefficient, due to limited access to key effector cells. In the treatment of cancer, chimeric antigen receptor (CAR) T-cell therapy and T-cell engagers (TCE) that recruit T cells to induce B-cell cytotoxicity have delivered promising results for anti-CD19 CAR T-cell therapies, the CD19 TCE blinatumomab and CD20 TCE such as mosunetuzumab, glofitamab, or epcoritamab. Limited evidence suggests that anti-CD19 CAR T-cell therapy may be effective in managing refractory AID whereas we await evaluation of TCE for use in non-oncological indications. Therefore, here, we discuss the potential mechanistic advantages of novel therapies that rely on T cells as effector cells to disrupt B–T-cell collaboration toward overcoming rituximab-resistant AID.

B–T-cell collaboration in the pathogenesis of autoimmune disease
B- and T-cell (B–T-cell) collaboration perpetuates chronic inflammation in a range of autoimmune diseases (AID) including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS) [ 1 , 2 ]. This cellular collaboration may occur through contact-dependent or -independent pathways through cytokines and other immune stimuli. Within lymphoid aggregates and the germinal center, B–T-cell interactions involve an array of molecular pairings [ 3 ], summarized in Fig. 1 and Table 1 . These signals stimulate T-cell secretion of cytokines and promote differentiation of naïve to memory B cells and plasma cells (PCs), Fig. 1 . Some of these pathways have been targeted, as discussed later, whereas others are the subject of novel therapeutic strategies.
Overview of CD antigens and other molecules involved in B- and T-cell collaboration along with their function/utility
| Marker (± ligand/receptor) . | Meaning/function/application . |
|---|---|
| CD3 (TCR) | T-cell activation signaling and regulation of TCR expression |
| CD4 (MHC II) | T-helper cell |
| CD8 (MHC I) | Cytotoxic T cell |
| CD19 (co-receptor for BCR) | Pan B cell marker. Regulates B-cell development, activation, and differentiation |
| CD20 | B-cell activation and proliferation. Also present on a minority of T cells |
| CD27 (CD70) | Marker of B- and T-cell memory |
| CD28 (CD80/86) | Co-stimulation between B and T cells |
| CD40 (CD40L) | Co-stimulation between B and T cells |
| BAFF-R (BAFF) or BLyS | B-cell activating factor enhances B-cell survival |
| PD-1 (PD-L1 and PD-L2) | Programmed cell death, down-regulates the immune response |
| CXCL-10 (CXCR3) | Recruitment of monocytes, T cells, NK cells |
| CXCL-13 (CXCR5) | B-cell chemoattractant |
| CCR2 (CCL-2 also known as MCP-1) | Trafficking of monocytes to inflammatory sites |
| ICOS-ICOSL | ICOS part of the CD28 superfamily, provides co-stimulatory signal to activated T cells upon binding to ICOS-L |
| IL21-IL21R | Promotes proliferation and function of T and B cells, enhances cytotoxicity of CD8 T cells and NK cells |
| TCR-MHCII | MHC displays peptides to the TCR, and TCR can discriminate foreign from self-peptides |
| Marker (± ligand/receptor) . | Meaning/function/application . |
|---|---|
| CD3 (TCR) | T-cell activation signaling and regulation of TCR expression |
| CD4 (MHC II) | T-helper cell |
| CD8 (MHC I) | Cytotoxic T cell |
| CD19 (co-receptor for BCR) | Pan B cell marker. Regulates B-cell development, activation, and differentiation |
| CD20 | B-cell activation and proliferation. Also present on a minority of T cells |
| CD27 (CD70) | Marker of B- and T-cell memory |
| CD28 (CD80/86) | Co-stimulation between B and T cells |
| CD40 (CD40L) | Co-stimulation between B and T cells |
| BAFF-R (BAFF) or BLyS | B-cell activating factor enhances B-cell survival |
| PD-1 (PD-L1 and PD-L2) | Programmed cell death, down-regulates the immune response |
| CXCL-10 (CXCR3) | Recruitment of monocytes, T cells, NK cells |
| CXCL-13 (CXCR5) | B-cell chemoattractant |
| CCR2 (CCL-2 also known as MCP-1) | Trafficking of monocytes to inflammatory sites |
| ICOS-ICOSL | ICOS part of the CD28 superfamily, provides co-stimulatory signal to activated T cells upon binding to ICOS-L |
| IL21-IL21R | Promotes proliferation and function of T and B cells, enhances cytotoxicity of CD8 T cells and NK cells |
| TCR-MHCII | MHC displays peptides to the TCR, and TCR can discriminate foreign from self-peptides |
CXCL: CXC chemokine ligand; CCR: C-C motif chemokine receptor; ICOS, MCP: monocyte chemoattractant protein; MHC: major histocompatibility complex; TCR: T-cell receptor.
![sample of article summary pdf Pathways of B–T-cell co-stimulation and trials of therapeutic agents. Molecular pairings are explained in Table 1. Drugs that target co-stimulation are outlined here. Dapirolizumab is an anti-CD40L mAb, currently in phase III study in SLE (NCT04294667). Bleslumab is an IgG4 mAb that targets CD40 which underwent phase II trial in plaque psoriasis with no clinical improvement compared to placebo [4], and demonstrated non-inferiority compared with standard of care for acute rejection in renal transplant recipients [5]. Iscalimab is another anti-CD40 mAb which is undergoing phase II trial in SLE and Sjogren’s Syndrome (NCT03656562, NCT04541589). Abatacept inhibits CD80/86 to prevent engagement with CD28 and is approved for use in RA but failed to meet the primary endpoint in the lupus nephritis phase III trial. AMG 557, anti-ICOSL antibody, underwent phase II trial in SLE and a newer therapy inhibiting ICOSL and BAFF is undergoing phase II trial (NCT04058028). PD-1 agonist, Peresolimab demonstrated modest improvement in disease activity in a phase II trial for patients with RA. Image created using Biorender.com](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cei/217/1/10.1093_cei_uxae031/1/m_uxae031_fig1.jpeg?Expires=1722904047&Signature=xqwcWsVr55ZrVaEKF9RPn~JEWbuff~ULgMSxHevAEMmAgPpBYmzLABYoOmZebCb4v87v7scnbOaX8VESTGqLM1kr7eQ0vmgP4PBHdaU9lRxWQGVIgvmP7IKC4fHPl6ZcmqlE1MumrghlkS0~7mxU8DTa5QFF1qj4OX1LZIkqOxYgiDSSNp1MT7HeCEGEJZ2X2yUE1fykhnt~Gwb~IKOIw3wP-LUKkCZnbmGKr8YmNh8sxLhvwicEC87tXgiXFgU16dqt-bfs-FX7X9CEXCS4KQWqZXtnNvO9B7~S01fBBcoC8zCOo91GuohlYSoZHZofBB9qpZTK1B6qhREcenUw-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pathways of B–T-cell co-stimulation and trials of therapeutic agents. Molecular pairings are explained in Table 1 . Drugs that target co-stimulation are outlined here. Dapirolizumab is an anti-CD40L mAb, currently in phase III study in SLE (NCT04294667). Bleslumab is an IgG4 mAb that targets CD40 which underwent phase II trial in plaque psoriasis with no clinical improvement compared to placebo [ 4 ], and demonstrated non-inferiority compared with standard of care for acute rejection in renal transplant recipients [ 5 ]. Iscalimab is another anti-CD40 mAb which is undergoing phase II trial in SLE and Sjogren’s Syndrome (NCT03656562, NCT04541589). Abatacept inhibits CD80/86 to prevent engagement with CD28 and is approved for use in RA but failed to meet the primary endpoint in the lupus nephritis phase III trial. AMG 557, anti-ICOSL antibody, underwent phase II trial in SLE and a newer therapy inhibiting ICOSL and BAFF is undergoing phase II trial (NCT04058028). PD-1 agonist, Peresolimab demonstrated modest improvement in disease activity in a phase II trial for patients with RA. Image created using Biorender.com
In this context of an ongoing immune response, an appreciation of B-cell biology is helpful. B cells originate from hematopoietic stem cells in the bone marrow and undergo differentiation in secondary lymphoid organs [ 6 ]. Differential expression of various cell surface markers, including cluster of differentiation (CD) molecules and immunoglobulin isotypes help to define classical subpopulations including naïve B cells (IgD+CD27−), unswitched memory B cells (IgD+CD27+), switched memory B cells (IgD−CD27+) and double negative memory B cells (IgD−CD27−) [ 6 ]. Naïve B cells have not yet encountered antigen, whereas switched memory B cells are primed to respond to antigen and double negative memory B cells increase with aging, autoimmunity, and chronic infectious diseases [ 7 ]. Until recently, the focus of B-cell depletion therapy has been on rituximab, an anti-CD20 monoclonal antibody that is widely used in hematological malignancies and AID (discussed in more detail below). The first FDA approved targeted biologic therapy for SLE was Belimumab, a mAb directed at B-cell activating factor (BAFF, also known as BLyS) [ 8 ], however, real-world data demonstrates variable success [ 9 , 10 ]. BAFF is a B-cell survival and differentiation factor and is elevated in the serum of patients with SLE [ 11 ].
B–T-cell interactions in the peripheral inflammatory sites of various AID including RA SLE, type I diabetes mellitus, and celiac disease exhibit a population of T cells which are termed T-peripheral helper cells [ 1 , 12 , 13 ]. Rao et al . identified these cells, adjacent to B cells in lymphoid aggregates of the synovium in patients with RA as PD-1hiCXCR5 − CD4 + which lack Bcl6 but produce IL-21 and CXCL13, resulting in B-cell differentiation into plasmablasts (PBs) [ 14 ]. This perpetuates B–T-cell networking in inflamed tissues, where ectopic lymphoid structures [ 15 ] are formed. Thus, B–T-cell collaboration occurs in both lymphoid tissues and at sites of inflammation.
Disrupting the B–T-cell networking in AID, historical perspectives
B–T-cell collaboration is a dominant source of chronic inflammation in AID. Hence, disrupting this network is an appealing therapeutic strategy. Over the past four decades, B–T-cell co-stimulation was targeted through different pathways such as alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H), abatacept (cytotoxic T-lymphocyte antigen 4 immunoglobulin), and dapirolizumab (anti-CD40L) with variable impact on B-cell depletion (BCD), Fig. 2 . In the 1980s, alemtuzumab was used to deplete CD52 expressing cells including B and T cells, providing the first insights into disrupting B–T-cell networking. The 1990s trials of alemtuzumab in RA were terminated due to suboptimal therapeutic index probably owing to prolonged depletion of regulatory T cells [ 16 ], although it continues to be used to treat MS (albeit at lower doses). Abatacept inhibits the co-stimulatory CD28-CD80/86 pathway and is approved for RA [ 17 ] although the ALLURE trial of abatacept in lupus nephritis (LN) did not meet its primary endpoint [ 18 ]. Attempts have been made to block other key co-stimulatory signaling pathways including the CD40-CD40L axis. Second-generation agents have been developed including dapirolizumab-pegol which had favorable biomarker and safety response in SLE [ 19 ]; phase III results are awaited (NCT04294667). Therefore, despite these advances, there remains a great unmet need for disrupting B–T-cell collaboration in refractory patients with AID.

Historical timeline of therapies that target B–T-cell collaboration in autoimmune disease. These agents were designed either to deplete B cells and/or disrupt the B–T-cell collaboration. The top row denotes the target antigen, the second row demonstrates the drugs that have undergone clinical trial (later two, t are yet to undergo clinical trial in AID). The third row represents therapies that interrupt B–T-cell networking and the fourth row represents treatments that employ T cells as effector cells. Text in italics under CD20 represents other approved anti-CD20 mAbs, *denotes pending approval
BCD with rituximab in RA and SLE; why is it suboptimal?
In the past three decades, BCD therapy with the CD20 monoclonal antibody rituximab, has revolutionized the treatment of severe or refractory AID and has been approved for use in RA [ 20 ], ANCA vasculitis [ 21 ], and pemphigus vulgaris (PV) [ 22 ] and is prescribed widely “off-licence” in SLE [ 23 ] and in immune thrombocytopenic purpura (ITP) [ 24 ]. Data from the Lupus Nephritis Assessment with Rituximab (LUNAR) study reported complete BCD with complete response, as defined in the study [ 25 ]. However, there remains a significant proportion of patients, up to 30%, who have disease refractory to rituximab, particularly in the context of incomplete BCD [ 23 ] and/or repopulation with PB and switched memory B cells (IgD − CD27 + , SwMBC) [ 26 ].
How do memory B cells and CD19 + CD20 − PBs evade rituximab?
B cells can evade rituximab’s effects either through intrinsic mechanisms (lacking CD20 expression and antigenic modulation) or extrinsic mechanisms such as restricted vascular access to effector cells as discussed previously [ 27 ]. Upon activation, naïve B cells solicit T-cell co-stimulation in lymphoid tissues and at sites of inflammation such as the joint and the kidney to differentiate into memory B cells and antibody-secreting cells including short-lived CD19 + CD20 − PBs and long-lived CD20 − PCs [ 14 , 28 ]. In RA, rituximab fails to completely deplete SwMBC and CD19 + CD20 − PCs in lymphoid tissues [ 29 ], joints, and bone marrow [ 30–32 ] contributing to poor response. In patients with ITP with poor response to rituximab, autoreactive splenic memory B cells down-regulate their BCR and up-regulate anti-apoptotic proteins and evades rituximab while retaining the capacity to reactivate and differentiate into autoantibody secreting CD19 + CD20 − PBs [ 24 ]. In muscle-specific kinase myasthenia gravis, autoreactive SwMBC evades rituximab and differentiate into autoantibody secreting CD19 + CD20 − PBs contributing to relapse [ 33 ]. Further, rituximab has no direct effect on CD19 + CD20 − PBs and PCs, as they do not express CD20 [ 34 , 35 ]. Thus, SwMBCs, CD19 + CD20 − PBs and CD19 + CD20 − PCs evade rituximab through distinct mechanisms, Fig. 3 .

Life cycle of B lineage cells. B cells originate in the bone marrow and migrate through peripheral circulation into lymphoid tissues such as lymph nodes and the spleen. Naïve B cells mature into memory B cells which then differentiate into switched memory B cells, SwMBC (IgD − ,CD27+), or double negative memory B cells (DN MBC; IgD − , CD27 − ) entering the peripheral circulation or plasma blasts (PBs) and plasma cells (PCs) a majority of which reside in the bone marrow, tissues, and inflammatory sites. Proportions of CD19 + CD20 + versus CD19 + CD20 − B cells are demonstrated pictorially within each subpopulation. Anti-CD20 monoclonal antibodies such as rituximab may not completely deplete CD19 + CD20 + B cells in tissue and do not target CD19 + CD20 − B cells, therefore, alternative strategies of depletion including CD19 targeting approaches may help to overcome rituximab resistance in autoimmunity
Broadly, anti-CD20 mAbs can be grouped into types I and II, where type I mAbs such as rituximab, are more efficient at clustering CD20 compared to type II anti-CD20 mAbs [ 36 ]. This enables efficient complement activation and therefore enhanced complement-dependent cytotoxicity (CDC), however, it also increases the propensity for internalization of CD20:CD20 mAb complexes by B cells [ 37 ]. In addition, incomplete BCD with rituximab may be related to its internalization of rituximab [ 38 ]. Type II anti-CD20 mAbs such as obinutuzumab may, at least in part, overcome this resistance mechanism [ 27 ]. In a pivotal phase II study, obinutuzumab was shown to improve clinical response in LN [ 39 ] and phase III studies are ongoing. However, CD19 + CD20 − PBs and CD19 + CD20 − PCs are still not directly targeted. Furthermore, disease-associated macrophage phagocytic defects [ 40 ] and vascular access limitations may compromise the ability of anti-CD20 mAbs (and other B-cell depleting mAbs, such as those directed to CD19) to evoke antibody-dependent cellular phagocytosis (ADCP) [ 27 , 41 ] as they rely on FcγR-bearing effector cells. In addition, NK cells are also scarce in tissues, limiting antibody dependent cellular cytotoxicity (ADCC). For example, we have previously reported that incomplete depletion and/ or persistent infiltration of B cells in the kidneys was associated with active LN refractory to rituximab [ 42 ].
Through histological analysis of kidney [ 43 ] and skin [ 44 ] of patients with AID, and the synovium in patients with RA [ 14 ], we know that B cells interact with T cells in lymphoid tissues and at sites of inflammation, to differentiate into autoantibody secreting PBs and PCs. At these sites, limited access to rituximab’s key effector cells, macrophages, and NK cells, may compromise depletion. Thus, antigen expression, modulation, and access to effector cells influence the efficiency of rituximab-mediated BCD. Therefore, it is important to consider both alternative target antigens and therapies that recruit other effector cells to improve BCD.
Is CD19 an ideal target?
CD19 regulates the threshold for B-cell activation as a co-receptor of the BCR complex [ 45 ] with consequent implications for influencing autoimmunity [ 46 ]. CD19 deficiency impairs humoral immunity, at least in part, due to an increased threshold for B-cell activation [ 47 ] whereas overexpression is associated with AID such as SLE [ 28 ]. When compared with CD19 − CD20 − PCs, CD19 + CD20 − PCs accumulate more mutations and retain greater proliferative capacity, at least in vitro [ 34 ]. These observations implicate a significant role for CD19 in B-cell differentiation and activation.
When compared with CD20, B lineage cells express CD19 at an earlier stage in development and retain expression through all stages of differentiation into CD19 + CD20 − PBs and some CD19 + CD20 − PCs [ 28 ]. CD19 hi CD11c + memory B cells in humans were shown to respond robustly to antigen challenge, in vitro [ 48 ]. More recent evidence suggests that double negative (IgD − CD27 − ) DN B cells which express the transcription factor T-box expressed in T cells (T-bet) encoded by Tbx21 , termed DN-T-bet + B cells are expanded in aging, are associated with higher mortality from COVID-19 infection and disease activity in SLE as well as disease pathogenesis in RA. Therefore they are of great interest in the field of B-cell research [ 49 ].
Further, they demonstrate increased expression of CD19 which strengthens the argument to target CD19 in AID (Shah et al ., in preparation). Considering the availability of newer therapies that target CD19, particularly in the field of oncology, we reappraise the concept of targeting CD19, put forward over a decade ago, to treat AID [ 28 ]. In addition, evidence from oncology highlights that cancers refractory to monoclonal antibodies have been effectively treated with CD19-targeted chimeric antigen receptor (CAR) T cells, probably owing to the deeper depletion of B cells which provides promise for patients with AID resistant to current mAb therapy, highlighted by the published case series in SLE [ 50 ]. These mechanistic considerations indicate that targeting CD19, particularly in AID, may overcome anti-CD20 mAb resistance.
How to target CD19-T-cell engagement as a mechanism of action?
Therapeutic options to target CD19 + B cells and PCs include (i) anti-CD19 mAbs; (ii) CD19-targeted CAR T cells; and (iii) CD19-directed T-cell engagers (TCE). The anti-CD19 mAb inebilizuzmab is approved for the treatment of neuromyelitis optica spectrum disorder [ 51 ] and showed initial promising results in a clinical trial in systemic sclerosis [ 52 ]. BCD with inebilizumab was greater in transgenic mice blood and spleen as well as in an in vitro ADCC assay using human PBMCs when compared to rituximab [ 53 ]. However, similar to rituximab, anti-CD19 mAbs are also disposed to internalization [ 54 ] and would be limited by disease-associated macrophage phagocytic defects [ 40 ] and vascular access limitations. Therefore, CD19-directed CAR T cells and CD19 TCE may be of greater utility in AID and will be discussed in the following sections.
The introduction of CAR T cells to treat cancer has been instrumental in providing individualized, targeted treatment through genetically engineered T cells that express a CAR specific to a tumor-associated antigen, such as CD19 in B cell [ 55 ] malignancies. Recognition of the target antigen-bearing B cells activates CAR T cells to proliferate and selectively eliminate the target B cells. The basic structure of a CAR includes an extracellular surface domain for antigen recognition (typically derived from an antibody fragment), a transmembrane domain, and an intracellular signaling domain that activates T cells (typically derived from CD3z chain). The evolution of CAR from first to fourth generation includes the addition of co-stimulatory domains (one in second generation and two in third generation CARs) as well as co-expression of additional transgenes for cytokine secretion (fourth generation) [ 56 ], Fig. 4 .

Evolution of CARs across the generations. All CARs have a single chain variable region of a mAb. ( A ) first-generation CARs contain an intracellular signaling domain of CD3 zeta chain alone; ( B ) second-generation includes a single co-stimulatory domain (CD28 or 4-1BB); ( C ) third-generation CARs combine two of the above co-stimulatory domains; and ( D ) fourth-generation CARs are diversified in that they can express cytokines. Image created using BioRender.com
Once administered, CAR T cells can also expand and establish immune memory, thus providing long-term surveillance of disease as described in malignancy [ 57 ]. CAR T-cell therapy has been approved for the treatment of B-cell acute lymphoblastic leukemia (ALL), lymphoma, and multiple myeloma [ 55 ]. Factors such as antigen overload are considered to contribute to undesirable effects including cytokine release syndrome (CRS) and neurotoxicity, leading to newer generation therapies with fewer toxicities being developed [ 58 ]. Complete remission for at least 3 years, of various relapsed B-cell malignancies was demonstrated in 51% of patients treated with CAR T-cell therapies, with few late-onset side effects [ 59 ]. This success led to CAR T cells being explored for treating refractory AID.
CAR T-cell therapy in AID
The success of using CAR T-cell therapy for the management of B-cell malignancies inspired its research in a range of AID including SLE, myasthenia gravis, and type 1 diabetes mellitus, as outlined in Table 1 . In animal models of SLE, anti-CD19 CAR T-cell treatment resulted in profound and sustained BCD with low circulating PCs and increased survival rates [ 60 ]. This data provided the basis for the use of anti-CD19 CAR T-cell therapy in the treatment of five patients with refractory multiorgan lupus which was well tolerated leading to serological and clinical remission at relatively short follow-up [ 50 ]. Probably owing to lower antigen load, the first cohort of patients with SLE-treated with anti-CD19 CAR T-cell therapy experienced only low-grade CRS [ 61 ], of which tocilizumab (anti-IL-6 receptor mAb) was used (successfully) in only one patient owing to persistent fevers for 3 days [ 50 ]. Thus, current preliminary evidence suggests that CD19 targeting CAR T-cell therapy seems a safe and effective therapeutic strategy in AID such as SLE. Anti-CD19 CAR T-cell therapy was associated with a reduction in autoantibodies and pro-inflammatory cytokines including IL-6 and TNF-α [ 62 ]. Intriguingly, despite excellent clinical responses, the authors demonstrated an increase in serum BAFF levels.
With regard to other autoimmune diseases, single case studies of anti-CD19 CAR T-cell therapy indicate a potential use of the approach also in anti-synthetase syndrome [ 63 ] and systemic sclerosis [ 64 ]. To note, an important potential confounder when appraising the mechanisms of response to CAR T-cell therapy is the use of lymphocyte depletion with fludarabine that may have contributed to the response. Several studies exploring the safety, tolerability, and preliminary efficacy of anti-CD19 CAR T-cell therapy in AID have been initiated (NCT05938725, NCT05869955, NCT03030976, NCT05798117, and NCT05930314).
Limitations of CAR T-cell therapy
Although the case examples of anti-CAR T cells in AID are promising, it is also important to understand the limitations. Two of the five patients treated with anti-CD19 CAR T-cell therapy had persistence of clonotypic IgG in follow-up samples, demonstrating suboptimal depletion and/or rapid repopulation of memory B cells [ 50 ]. Remarkably, despite lower antigen overload, three of five SLE patients treated with anti-CD19 CAR T-cell therapy repopulated their B cells by day 50 after treatment [ 50 ] when compared with prolonged BCD achieved in B-cell malignancies up to several years post-infusion [ 55 ]. Potential explanations for incomplete depletion and/or relatively early repopulation of B cells include (i) complete depletion of target cells removing the sustained stimulus needed to maintain an optimal pool of CAR T cells, as CAR T cells had disappeared at week 4 after treatment; (ii) higher proportion of senescent and/or exhausted SLE CAR T cells; and (iii) potential inhibition of CAR T-cell expansion due to the persistent effects of immunosuppression such as mycophenolate mofetil beyond cessation of therapy [ 65 ].
Implications of lymphodepletion in AID
Patients with AID, particularly SLE, are often lymphopenic owing to the underlying disease process and the effects of immunosuppression, which may impact the process of leukapheresis required to generate the CAR T cells. Nevertheless, patients with active SLE in the previously discussed case series [ 50 ] were successfully leukapheresed before CAR T-cell therapy and concurrent treatment with steroids and immunosuppressive agents [ 66 ]. The process of lymphodepletion itself increases the likelihood of infections and is an additional step preceding CAR T-cell therapy, compared to “off the shelf” TCE therapy.
Risks of hypogammaglobulinemia
A major consideration with CAR T-cell therapy is the risk of hypogammaglobulinemia; this may be observed with TCE but likely to a lesser extent. In the treatment of cancer, approximately a third of patients develop hypogammaglobulinemia following CAR T-cell infusion [ 67 ], owing to potent and persistent depletion of normal CD19 + B cells. Very low IgG levels can arise from 9 weeks after treatment and continue beyond 4 years [ 67 ]. This poses a risk of serious life-threatening infections, necessitating intravenous immunoglobulin infusions as a prevention strategy, as per the majority of trials [ 68 ], however, this can be expensive and not readily accessible for all patients.
Importantly, B-cell aplasia and hypogammaglobulinemia result in suboptimal vaccine responses, which is also a significant concern especially in the current era of SARS-CoV-2 infection with only 29% of patients who receive CAR T-cell therapy for lymphoma/myeloma mounting a clinically relevant antibody response to vaccination [ 69 ]. Reassuringly, vaccine responses were stable following CAR T-cell therapy in the SLE case series [ 50 ], likely related to the remaining pool of CD19 − plasma cells which are able to secrete antibodies 2 years post-treatment [ 70 ]. These aspects also need to be accounted for during TCE trial design in AID.
Logistical limitations of CAR T cell therapy
Logistical limitations are also considerable. For example, in patients with rapidly progressing cancer or AID, the practical feasibility of CAR T-cell therapy may be limited as there is typically a protracted vein-to-vein time of approximately 6–8 weeks, due to the time required for producing, transporting, and ensuring quality control of the personalized cell therapy, as illustrated in the graphical abstract. This process is typical for most CAR T-cell therapies, although the novel YTB323 omits the ex vivo expansion stage (NCT05798117).
Further disadvantages of CAR T-cell therapy include the high cost involved with engineering and storage of CAR T cells and the specialist training required to administer treatment as detailed in Table 2 . Therefore, readily available and effective novel treatments are required while awaiting CAR T-cell therapy [ 79 ]. One approach to obviate the limiting factor of individual custom-made CAR T cells is the generation of “universal CAR T cells” as reviewed by Zhao et al . [ 56 ]. These can serve as “off the shelf” therapies to treat a wide range of clinical indications as they are engineered to target multiple antigens. Further gene editing work is underway to ensure universal CAR T cells are not depleted by the recipient’s immune system and are able to expand without causing harmful effects [ 80 ].
Evidence for the use of CAR T-cell therapies in non-malignant settings
| Specialty . | Indication . | Study phase/type . | Outcome . | Ref . |
|---|---|---|---|---|
| Neurology | Multiple sclerosis (murine model = experimental autoimmune encephalomyelitis) | Murine model | Depleted B cells in peripheral blood and CNS Improved clinical scores of EAE | [ ] |
| Myasthenia Gravis (using anti-B-cell maturation antigen CAR T cells) | Phase 1b/2a (human) | Safe, well-tolerated, and clinical improvement Phase IIb ongoing (NCT04146051) | [ ] | |
| Transplant medicine | Post-transplant lymphoproliferative disorder (PTLD) post-renal transplant | Case series ( = 3) (human) | Demonstrated safety and feasibility (with regard to stopping immunosuppression) however only one of three patients maintained in remission at 3 months follow-up | [ ] |
| Case series of three patients with refractory PTLD post solid organ transplants (cardiac transplant, kidney transplant, and pancreas transplant) | Case series ( = 3) (human) | Poor outcomes, multiple complications including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), acute kidney injury, lack of response to CAR T-cell therapy, and mortality | [ ] | |
| Refractory PTLD post heart and kidney transplant | Case report (human) | Six months post CAR T-cell infusion, clinically well, and normal ejection fraction on echocardiography | [ ] | |
| Rheumatology | Systemic lupus erythematosus | Case series ( = 5) (human) | Deep depletion of B cells, clinical improvement, normalization of anti-ds-DNA antibodies and all achieved remission after 3 months. Three patients repopulated B cells less than 50 days post CAR T-cell therapy (although mainly naïve B cells) | [ ] |
| Systemic sclerosis (diffuse cutaneous) | Case report (human) | Extensive fibrosis (skin, heart, and lung)—all showing improvement post treatment Well tolerated, mild CRS (Grade 1), no signs of ICANS. | [ ] | |
| Anti-synthetase syndrome (myositis and interstitial lung disease) | Case report ( = 2) (human) | Treated with CD19-targeting CAR T cells. Excellent outcome with biochemical, serological, and radiological resolution of myositis and improvement in pulmonary function tests/CT chest. | [ , ] | |
| Dermatology | Pemphigus vulgaris—target antigen desmoglein 3 | Preclinical study, (human) | Depletion of Dsg3 cells and antibodies in human pemphigus vulgaris model | [ ] |
| Endocrinology | Type I diabetes Mellitus—target antigen Insulin | Murine model | Delayed onset of diabetes but no long-term protection | [ ] |
| Specialty . | Indication . | Study phase/type . | Outcome . | Ref . |
|---|---|---|---|---|
| Neurology | Multiple sclerosis (murine model = experimental autoimmune encephalomyelitis) | Murine model | Depleted B cells in peripheral blood and CNS Improved clinical scores of EAE | [ ] |
| Myasthenia Gravis (using anti-B-cell maturation antigen CAR T cells) | Phase 1b/2a (human) | Safe, well-tolerated, and clinical improvement Phase IIb ongoing (NCT04146051) | [ ] | |
| Transplant medicine | Post-transplant lymphoproliferative disorder (PTLD) post-renal transplant | Case series ( = 3) (human) | Demonstrated safety and feasibility (with regard to stopping immunosuppression) however only one of three patients maintained in remission at 3 months follow-up | [ ] |
| Case series of three patients with refractory PTLD post solid organ transplants (cardiac transplant, kidney transplant, and pancreas transplant) | Case series ( = 3) (human) | Poor outcomes, multiple complications including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), acute kidney injury, lack of response to CAR T-cell therapy, and mortality | [ ] | |
| Refractory PTLD post heart and kidney transplant | Case report (human) | Six months post CAR T-cell infusion, clinically well, and normal ejection fraction on echocardiography | [ ] | |
| Rheumatology | Systemic lupus erythematosus | Case series ( = 5) (human) | Deep depletion of B cells, clinical improvement, normalization of anti-ds-DNA antibodies and all achieved remission after 3 months. Three patients repopulated B cells less than 50 days post CAR T-cell therapy (although mainly naïve B cells) | [ ] |
| Systemic sclerosis (diffuse cutaneous) | Case report (human) | Extensive fibrosis (skin, heart, and lung)—all showing improvement post treatment Well tolerated, mild CRS (Grade 1), no signs of ICANS. | [ ] | |
| Anti-synthetase syndrome (myositis and interstitial lung disease) | Case report ( = 2) (human) | Treated with CD19-targeting CAR T cells. Excellent outcome with biochemical, serological, and radiological resolution of myositis and improvement in pulmonary function tests/CT chest. | [ , ] | |
| Dermatology | Pemphigus vulgaris—target antigen desmoglein 3 | Preclinical study, (human) | Depletion of Dsg3 cells and antibodies in human pemphigus vulgaris model | [ ] |
| Endocrinology | Type I diabetes Mellitus—target antigen Insulin | Murine model | Delayed onset of diabetes but no long-term protection | [ ] |
To this end, we consider alternative strategies, with the potential of TCE bispecific antibodies as a novel therapeutic option to disrupt B-T cell collaboration in AID. Table 2 outlines the major differences and similarities of using CAR T-cell therapy and TCEs.
TCE represents a novel class of targeted therapeutics that recruit T cells [ 81 ]. From a clinical perspective, in the late 1990s, the potential for bispecific antibodies as therapeutic interventions became clearer for cancers such as breast, leukemia, and lung [ 82 ], which led to a surge of interest in their use and FDA approval of catumaxomab for malignant ascites [ 83 ] and blinatumomab for refractory B-ALL [ 84 ] More recently, three CD20 T-cell engagers, mosunetuzumab, glofitamab, and epcoritamab have been approved for treatment of refractory/relapsed follicular lymphoma and refractory/relapsed diffuse large B-cell lymphoma [ 85 ]. Technological advancements over time have enabled a range of modifications to enhance the flexibility and number of binding sites, half-life, production yield, and potency of these therapeutics [ 86 ].
TCE technologies
TCEs can be broadly categorized into (i) small, short half-life bispecific antibody fragments (single chain variable fragments) such as bispecific T-cell engagers (BiTE ® s) which require repeated administration ( Fig. 5A ); and (ii) larger IgG-based T-cell bispecific antibodies (TCBs) with extended half-lives ( Fig. 5B and C ). The development of TCBs has evolved from single chain variable fragments in the early 1990s [ 87 ], to the development of “knobs into holes” (KiH) technology in the late 1990s [ 88 ] to the more advanced technologies including CrossMab to engineer bispecific antibodies [ 89 , 90 ], Fig. 5 .

Selected TCE formats in a schematic representation used for T-cell redirecting therapies. ( A ) Blinatumomab, tandem scFv (single chain variable fragment) (BiTE) format. ( B ) Mosunetuzumab, IgG-based-TCE with monovalent binding using a native antibody structure with 1 Fab arm to bind CD20 (target antigen) and 1 Fab arm to bind CD3 on T cells, combined with the KiH technology as demonstrated in the CH3 domain to achieve heavy chain heterodimerization. ( C ) Epcoritamab, IgG-based TCE with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange, termed DuoBody®. ( D ) Glofitamab, bivalent binding to increase the avidity of TCE binding to the target antigen, CD20, with additional KiH and CrossMab VH-VL with charge interactions using variable regions. Image created using Biorender.com
Blinatumomab, a BiTE ® composed of two single-chain antibodies targeting CD19 on B cells and CD3ε on T cells fused via a flexible linker ( Fig. 5A ), is approved for B-cell ALL [ 85 ]. It is engineered to have a short half-life of 2 h to enable tight control of serum levels in case of adverse events. Blinatumomab relies on the presence of CD19 + target cells to activate T cells, with sensitive response from CD8 + T cells to induce lysis of tumor cells as demonstrated in video-assisted microscopy studies [ 91 ]. In vitro studies of human B-lymphoma cells demonstrated a higher degree of tumor cell elimination with blinatumomab compared to rituximab [ 92 ]. Interestingly, the combination of blinatumomab and rituximab was synergistically more efficient, especially at low effector-to-target cell ratios and low Blinatumomab concentrations [ 92 ]. This combined effect was found to be due to potent activation of pro-caspases 3 and 7 in target cells, which is instrumental in triggering granzyme-mediated apoptosis. The BiTE subtype is potent with regard to target cell killing. Regardless, the requirement for repeat dosing of Blinatumomab may limit its routine use in clinical practice.
Three CD20 TCE have been approved for refractory B cell lymphomas: mosunetuzumab, glofitamab, and epcoritamab [ 85 ], Fig. 5 . Mosunetuzumab is an IgG-based TCE with 1:1 binding to CD20 and CD3; it uses KiH technology and in vitro assembly to overcome incorrect light chain association [ 93 ]. Epcoritamab is also IgG-based, although employs the unique DuoBody® technology with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange [ 94 ]. Recent IgG-based TCEs have been developed for increased avidity. Glofitamab has two Fab regions which bind CD20, one Fab region which binds CD3 (so-called 2:1 format), and a longer half-life of 10 days, owing to its Fc region and interaction with FcRn [ 90 ]. The Fc also includes the P329G LALA mutations [ 81 ], which abolish conventional effector functions and therefore it employ a different mechanism of action compared to rituximab. The 2:1 format ( Fig. 5C ) enables greater potency with regard to B-cell cytotoxicity compared to 1:1 antibodies, thought to be due to the close proximity of the CD20 binder and CD3 binder, resulting in a more stable T cell to target B-cell synapse induced by the head-to-tail fusion design [ 95 ].
Effector mechanisms of TCEs: lessons learnt from treating malignant disease
Bispecific antibodies can redirect the effector function of various immune cells. T cells are promising as effector cells as they are abundant, able to expand rapidly, and have potent cytotoxic capacity. TCE are designed to by-pass the normal major histocompatibility complex–T-cell receptor (MHC–TCR) interaction usually required between antigen presenting cells and T cells, and instead co-engage the CD3 molecules on the T cell and form an immunological synapse via the target antigen such as CD19 or CD20 on the surface of B cells that helps redirect co-stimulation to cytotoxicity [ 96 , 97 ], Fig. 6 . This synapse is similar to that formed during cytotoxicity with CAR T cells. The CD20-TCE recruitment of T cells is evident in in vitro culture assays demonstrating that tumor lysis is dependent on T-cell recruitment, activation, and expansion of CD4 + and more profoundly CD8 + subsets [ 81 ]. Importantly, CD20-TCE depleted B cells in the spleen and lymph nodes, efficiently [ 81 ]. These findings may be of relevance to AID where inefficient BCD in lymphoid tissues and inflammatory sites, as discussed earlier, contributes to refractory disease.

The potential effect of immunosuppressive treatments on T-cell effector function. Mycophenolate mofetil (MMF) as per the bottom panel, results in fewer T cells to serve as effector cells for therapies such as CD19 TCE and CD19 CAR T cells. MMF can directly reduce the number of T cells and impair their activation and reduce their cytotoxicity against target B cells with lower release of perforin and granzyme molecules. Image created using Biorender.com
As discussed above, in AID, B and T cells colocalize in lymphoid tissues and at inflammatory sites. Therefore, using CAR T cells or TCE that employ T cells as effector cells to deplete B cells may provide a distinct advantage over rituximab-mediated BCD that relies on macrophages and/or NK cells as the dominant effector mechanism. The key differences and similarities between CAR T-cell therapy and TCE therapy are described in Table 3 .
Mechanistic differences and similarities between CAR T and TCE: experience in oncology
| . | CAR T-cell therapy . | TCE . |
|---|---|---|
| Side effect profile | Variable between CAR T regimens. In some oncological indications, about 80% suffer CRS, longer lasting and at a higher grade Neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in approximately 13–21% of patients, lasting 4–5 times longer than with TCE. | Variable between different TCE and indications. Approx. 50% suffer CRS, earlier onset but shorter duration. Obinutuzumab (anti-CD20mAb) pre-treatment limits CRS Neurologic side effects e.g. headache but less severe than ICANS, much less frequent than CAR T cells. |
| Efficacy | Higher rates of complete response in hematological malignancies | Dose-dependent response, but can be up to 30% less effective than CAR T cell therapy |
| Pre-conditioning | Leukodepletion so higher rates of infection and risk of rejection in transplant patients. | No preconditioning, but pre-medication with dexamethasone to reduce cytokine production and with obinutuzumab for glofitamab |
| Hypogammaglobulinemia | Persistence of engineered T cells resulting in sustained B-cell aplasia and hypogammaglobulinemia may require IVIg | TCB can deplete normal B cells and plasma precursor cells leading to a higher risk of hypogammaglobulinemia, but therapeutic regimen could be personalized according to clinical need |
| Effector cell type | Engineered T cells Less differentiated T cells (naïve and memory) show better efficacy than effector T cells | Endogenous T cells Antigen-experienced T cells mediate TCE-induced cell death, whereas naïve T cells are not activated |
| Cost | +++ (~£300 000 in the UK) [ ] | ++ (~£56,000 per cycle UK) [ ] |
| Production | Personalized therapy requiring individual engineering of patient’s T cells—labor intensive, time-consuming (resulting in disease progression), and higher risk of a production error. Also requires the patient to have sufficient peripheral T-cell counts for successful isolation of T cells from leukapheresis. | “Off the shelf” medication, so technically less delay to administration than CAR T-cell therapy. Can be manufactured in large quantities. Can be used independently of peripheral lymphocyte counts |
| Administration | Single intravenous administration, however, from decision to treat to administering therapy can be 6–8 weeks when disease may progress. Specialist training of staff required to administer CAR T-cell therapy and monitor for complications during infusion | Shorter half-life so may need repeat dosing. Quick to administer so can treat patient promptly and halt progression of disease. No additional specialist training required, similar administration to routine mAbs used such as rituximab. |
| Approval for use | ALL, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma (FDA approval) | Blinatumomab (CD3-CD19) for ALL, epcoritamab-bysp and glofitamab (CD3-CD20) for DLBCL (FDA approval), mosunetuzumab (CD3-CD20) for follicular lymphoma |
| Repeat treatment | Complicated due to maintenance of T-cell pool, patient factors (risk of infection). | More convenient and standardized |
| . | CAR T-cell therapy . | TCE . |
|---|---|---|
| Side effect profile | Variable between CAR T regimens. In some oncological indications, about 80% suffer CRS, longer lasting and at a higher grade Neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in approximately 13–21% of patients, lasting 4–5 times longer than with TCE. | Variable between different TCE and indications. Approx. 50% suffer CRS, earlier onset but shorter duration. Obinutuzumab (anti-CD20mAb) pre-treatment limits CRS Neurologic side effects e.g. headache but less severe than ICANS, much less frequent than CAR T cells. |
| Efficacy | Higher rates of complete response in hematological malignancies | Dose-dependent response, but can be up to 30% less effective than CAR T cell therapy |
| Pre-conditioning | Leukodepletion so higher rates of infection and risk of rejection in transplant patients. | No preconditioning, but pre-medication with dexamethasone to reduce cytokine production and with obinutuzumab for glofitamab |
| Hypogammaglobulinemia | Persistence of engineered T cells resulting in sustained B-cell aplasia and hypogammaglobulinemia may require IVIg | TCB can deplete normal B cells and plasma precursor cells leading to a higher risk of hypogammaglobulinemia, but therapeutic regimen could be personalized according to clinical need |
| Effector cell type | Engineered T cells Less differentiated T cells (naïve and memory) show better efficacy than effector T cells | Endogenous T cells Antigen-experienced T cells mediate TCE-induced cell death, whereas naïve T cells are not activated |
| Cost | +++ (~£300 000 in the UK) [ ] | ++ (~£56,000 per cycle UK) [ ] |
| Production | Personalized therapy requiring individual engineering of patient’s T cells—labor intensive, time-consuming (resulting in disease progression), and higher risk of a production error. Also requires the patient to have sufficient peripheral T-cell counts for successful isolation of T cells from leukapheresis. | “Off the shelf” medication, so technically less delay to administration than CAR T-cell therapy. Can be manufactured in large quantities. Can be used independently of peripheral lymphocyte counts |
| Administration | Single intravenous administration, however, from decision to treat to administering therapy can be 6–8 weeks when disease may progress. Specialist training of staff required to administer CAR T-cell therapy and monitor for complications during infusion | Shorter half-life so may need repeat dosing. Quick to administer so can treat patient promptly and halt progression of disease. No additional specialist training required, similar administration to routine mAbs used such as rituximab. |
| Approval for use | ALL, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma (FDA approval) | Blinatumomab (CD3-CD19) for ALL, epcoritamab-bysp and glofitamab (CD3-CD20) for DLBCL (FDA approval), mosunetuzumab (CD3-CD20) for follicular lymphoma |
| Repeat treatment | Complicated due to maintenance of T-cell pool, patient factors (risk of infection). | More convenient and standardized |
Aside from requiring lymphodepletion, an important aspect to highlight is that the expansion of CARs in vivo cannot be controlled, demonstrated by the rapid rise in circulating CARs, reaching up to 59% by day nine post-infusion [ 50 ].
In addition, the expansion and duration of CAR T-cell action is not easily controlled, whereas a TCE can be given at a specific dose and the half-life of the molecule is expected to determine its duration of action. Overall, treatment with TCE may potentially overcome some of these limitations of CAR T-cell therapy such as a lag time from decision to treatment to allow for engineering of CAR T cells, prior leukapheresis, and requirement for specialist centers with experience of cell-based immunotherapies.
Immunological/biological pitfalls in recruiting T cells as effector cells
Despite the undoubted promise of CAR T cells and TCE, there remain potential hurdles. Both CAR T cells and TCE may evoke “bystander killing” of antigen-negative cells directly in contact with antigen-positive cells [ 100 ]. While this local bystander effect is desirable in the treatment of solid tumors to prevent the escape of antigen-negative cancer cells, the potential implications of this in AID are unknown.
More recently, there are an increasing number of reports of macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH) as a complication of CAR T-cell therapy given for hematological malignancies, possibly as a distinct variant of CRS [ 101 ]. MAS/HLH is a serious condition of hyperinflammation, fevers, and cytopenias, and can be life-threatening. Patients with autoimmune disease such as SLE are already predisposed to developing secondary MAS/HLH [ 102 ], therefore initiation of CAR T-cell therapy in this cohort needs careful consideration.
Another potential pitfall with recruiting T cells as effector cells is a possible reduction in T-cell counts, which may increase the risk of infection, due to apoptosis noted with first-generation CAR T-cell treatments [ 103 ]. Reassuringly, in studies with CD20-TCB, peripheral T-cell counts decreased in the first 24 h of drug administration before returning to baseline by 72 h [ 81 ], considered to reflect an activation-induced marginalization. Therefore, the risk in the short term with these agents seems low but will need monitoring in the long term.
Impact of the tissue microenvironment
An additional consideration is the tissue microenvironment, which is known to influence T-cell cytotoxicity. AID-related T-cell subpopulations with features of anergy, exhaustion, and senescence may compromise the efficiency of TCE [ 104 ]. In addition, resistance to TCEs may arise from immune escape, through the expression of immune checkpoint molecules such as PD-1. In this context, combination treatment with checkpoint inhibitors, already explored in cancer immunotherapy may be limited by the potential activation of autoreactive T cells [ 105 ]. Alternatively, next generation trispecific TCEs to additionally provide co-stimulation may be beneficial [ 106 ]. As CD3 is a pan T-cell marker, TCEs can recruit all T-cell populations including naïve, regulatory T cells, and exhausted T cells as effector cells. In AID, regulatory and exhausted T cells are associated with disease remission and improved prognosis [ 107 ]. Mechanistic clinical studies will help us understand the clinical relevance of these potential limitations.
Clinical adverse effects of recruiting T cells as effector cells
The main adverse effect associated with both types of T-cell therapy is CRS, which is the rapid systemic release of pro-inflammatory cytokines including IL-6, IL-10, TNF-α, and IFN-γ, upon activation of the T cells [ 108 ]. CRS manifests as fever, fatigue, and vasodilation, and can lead to multi-organ failure. Pre-treatment with corticosteroids such as dexamethasone may reduce the risk of CRS. Anti-IL-6 receptor antibody, tocilizumab, has been approved for use prior to CAR T-cell therapy to attenuate CRS [ 109 ]. In murine models, combination treatment with Janus Kinase (JAK) inhibitors or mammalian target of rapamycin (mTOR) inhibitor, restricted CD19-TCB-related CRS while retaining their efficacy [ 110 ].
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another dose-dependent unwanted side effect unique to patients receiving T-cell engaging treatments, through adherence of T cells to cerebral microvascular endothelium and migration across the blood-brain barrier [ 111 ]. In ALL, ICANS, characterized by headache, dizziness, tremor, confusion, and encephalopathy, was associated with high-dose blinatumomab given in the first treatment cycle, probably owing to the higher tumor burden. As the target cell load is much lower in AID, the required dose of TCEs will be lower, consequently, the risk of CRS and ICANS should be lower than that reported for cancer immunotherapy.
What is the impact of immunosuppressive therapy on T-cell cytotoxicity in the context of TCE and CAR T cells?
Other important considerations include AID-specific concurrent drug regimens. For example, transplant recipients and patients with AID and transplant recipients receive immunosuppressants to regulate immune response. In the context of T-cell-based therapy, concurrent use of immunosuppressants may inhibit the effector function of the T cells, thereby, compromising the efficiency of CAR T cells and TCEs. For example, mycophenolate mofetil (MMF) can induce apoptosis in activated human T cells [ 112 ]; and in a murine model, mycophenolic acid, the active form of MMF has shown dose-dependent reduction in the generation of cytotoxic T cells [ 113 ]. Fig. 6 illustrates the potential impact of immunosuppressants on T-cell cytotoxicity in the context of TCE and CAR T-cell therapies. Therefore withholding immunosuppressants for a period of time to allow for T-cell recovery to enhance performance may be considered in prospective trial design [ 114 ].
In a case series of renal transplant recipients requiring CAR T-cell therapy for post-transplant lymphoproliferative disorders (PTLD), MMF was discontinued at the time of PTLD diagnosis (with DLBCL), and tacrolimus was stopped 2 weeks prior to leukapheresis for production of CAR T cells [ 73 ]. Similarly, a report of CAR T-cell infusion for anti-synthetase syndrome involved tapering azathioprine and steroids 7 days before leukapheresis and starting MMF 35 days after CAR T-cell infusion [ 76 ], which allowed for harvesting of fully functional T cells. This aligns with our proposition of correct sequencing of immunosuppressive treatments including the use of corticosteroids to allow full efficacy of TCE and/or CAR T therapies.
Where pathogenic B-cell identity is well described, CAR T therapy can potentially enhance the prospects for personalized therapy. For example, desmoglein 3 targeting CAR T cells were engineered to selectively eliminate Dsg3 specific B cells, in vitro and in vivo in animal models [ 115 ] toward developing therapies for PV. Currently, a phase I study of BCMA CAR T therapy (NCT04561557) is ongoing for the treatment of neurological disorders including Aquaporin-related neuromyelitis optica spectrum disorder (NMOSD). However, the identity of pathogenic B cells remains elusive for the majority of AID, where non-selective BCD therapy remains the current standard strategy.
In routine practice of managing AID, rituximab induction therapy incorporates two doses of 1 g, given 2 weeks apart. Retreatment with the same or lower dose of rituximab, is usually at 6 months or longer for optimal management of disease activity [ 17 ]. Current evidence highlights that response can be improved with better depletion with a lower frequency of memory B cells and PB in RA and SLE [ 27 ]. As discussed previously, presumably due to more efficient BCD, obinutuzumab treatment seems to be effective in LN [ 39 ]. To this end, targeting CD19 and disrupting the B–T-cell networking in AID, with CD19/CD3 TCEs or CAR T cells would be expected to provide mechanistic advantages. For example, targeting CD19, expressed on memory B cells, CD19 + CD20 − PBs, and CD19 + CD20 − PCs should help deplete these “rituximab-resistant cells” whereas the use of TCEs would help direct T cells from B-cell “co-stimulation to cytotoxicity” to disrupt B–T networking. Key lessons from previous SLE rituximab trials include (i) patient selection with regard to disease manifestations, severity of disease activity, serological parameters, and previous treatment are important to consider so as not to exclude the most active patients, (ii) defining standard concomitant therapy in the comparator and placebo arms as variable usage of glucocorticoid and immunosuppressants such as MMF can impact outcomes, (iii) defining endpoints in particular the steroid sparing effect, (iv) selecting the right disease activity index, and (v) defining follow-up duration and side effects. These serve as a reminder of the importance of optimal trial design to evaluate the “real” potential of TCE [ 25 , 116 ].
Optimizing co-therapies with immunosuppressants, and sequential therapy with rituximab
Co-therapy with immunosuppressants and/or rituximab therapy may influence the efficacy and safety of TCEs. As demonstrated in Fig. 6 , patients with AID are often being treated with immunosuppression such as MMF and corticosteroids. Therefore, considering discontinuation of MMF for 3–6 weeks [ 50 ] may optimize the effector function of T cells to disrupt the B–T-cell network in AID. Thereafter, a delayed introduction of MMF may be considered as needed for optimal control of disease activity.
Sequential therapy with rituximab, which is already competitively priced as a biosimilar, followed by CD19-TCE will enable targeting of B–T-cell networks in ectopic lymphoid tissue within peripherally inflamed tissues in AID, Fig. 3 . A potential limitation of this sequence is that rituximab therapy may result in lower expression of CD19 [ 24 ], probably through internalization as shown in vitro [ 38 ], thus, compromising the efficiency of CD19-TCE or CD19-CAR T therapy. Therefore, treatment with CD19-TCE first followed by rituximab, as needed, could be considered as an alternative strategy for those with poor depletion with CD19-TCE alone. In this context, it would be important to have strategies to detect B cells using novel antibodies that bind an alternative epitope to the therapeutic mAbs, less challenging for CD19 as it is a bigger antigen than CD20.
CD19 CAR T-cell or CD19-TCE therapy to convert B- and T-cell co-stimulation into conflict and disrupt their networking could prove to be a paradigm shift in treating AID. TCE, designed and developed through advanced antibody engineering methods, offers a mechanistically sound, logistically convenient, and favorable alternative therapeutic strategy in the management of refractory AID. To this end, mechanistic studies of TCE in AID, particularly during early-phase clinical trials, are of critical importance to optimize the use of TCE in combination with standard-of-care therapy as an alternative strategy to deplete B-lineage cells to improve outcomes for people with refractory AID.
Not applicable.
None declared.
Funders include Cancer Research UK (DRCRPG-May23/100001), VR's work is funded by MRC-CARP Fellowship from the UK Research and Innovation, Medical Research Council (MR/T024968/1), research grant from UCLH Biomedical Research Centre, National Institute for Health and Care Research, and Roche Innovation Center, Zurich.
Florian Kollert, Roche: Employment and Stock Ownership; Franz Schuler, Roche: Employment, patents, stock ownership; Christian Klein, Roche: Employment, patents, stock ownership; Venkat R Reddy, Research grants from Roche.
Rao DA. The rise of peripheral T helper cells in autoimmune disease . Nat Rev Rheumatol 2019 , 15 , 453 – 4 . doi: 10.1038/s41584-019-0241-7
Google Scholar
van Langelaar J , Rijvers L , Smolders J , van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers . Front Immunol 2020 , 11 , 760 . doi: 10.3389/fimmu.2020.00760
Petersone L , Edner NM , Ovcinnikovs V , Heuts F , Ross EM , Ntavli E , et al. . T cell/B cell collaboration and autoimmunity: an intimate relationship . Front Immunol 2018 , 9 , 1941 . doi: 10.3389/fimmu.2018.01941
Anil Kumar MS , Papp K , Tainaka R , Valluri U , Wang X , Zhu T , et al. . Randomized, controlled study of bleselumab (ASKP1240) pharmacokinetics and safety in patients with moderate-to-severe plaque psoriasis . Biopharm Drug Dispos 2018 , 39 , 245 – 55 . doi: 10.1002/bdd.2130
Harland RC , Klintmalm G , Jensik S , Yang H , Bromberg J , Holman J , et al. . Efficacy and safety of bleselumab in kidney transplant recipients: a phase 2, randomized, open-label, noninferiority study . Am J Transplant 2020 , 20 , 159 – 71 . doi: 10.1111/ajt.15591
Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies . Arthritis Res Therapy 2013 , 15 , S3 . doi: 10.1186/ar3908
Sachinidis A , Garyfallos A. Double negative (DN) B cells: a connecting bridge between rheumatic diseases and COVID-19 ? Mediterr J Rheumatol 2021 , 32 , 192 – 9 . doi: 10.31138/mjr.32.3.192
Horowitz DL , Furie R. Belimumab is approved by the FDA: what more do we need to know to optimize decision making ? Curr Rheumatol Rep 2012 , 14 , 318 – 23 . doi: 10.1007/s11926-012-0256-4
Venturelli V , Isenberg DA. Targeted therapy for SLE-what works, what doesn’t, what’s next . J Clin Med 2023 , 12 , 3198 . doi: 10.3390/jcm12093198
Parodis I , Vital EM , Hassan S-U , Jönsen A , Bengtsson AA , Eriksson P , et al. . De novo lupus nephritis during treatment with belimumab . Rheumatology 2020 , 60 , 4348 – 54 . doi: 10.1093/rheumatology/keaa796
Guerreiro Castro S , Isenberg DA. Belimumab in systemic lupus erythematosus (SLE): evidence-to-date and clinical usefulness . Ther Adv Musculoskelet Dis 2017 , 9 , 75 – 85 . doi: 10.1177/1759720X17690474
Christophersen A , Lund EG , Snir O , Sola E , Kanduri C , Dahal-Koirala S , et al. . Distinct phenotype of CD4(+) T cells driving celiac disease identified in multiple autoimmune conditions . Nat Med 2019 , 25 , 734 – 7 . doi: 10.1038/s41591-019-0403-9
Ekman I , Ihantola E-L , Viisanen T , Rao DA , Näntö-Salonen K , Knip M , et al. . Circulating CXCR5(-)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes . Diabetologia 2019 , 62 , 1681 – 8 . doi: 10.1007/s00125-019-4936-8
Rao DA , Gurish MF , Marshall JL , Slowikowski K , Fonseka CY , Liu Y , et al. . Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis . Nature 2017 , 542 , 110 – 4 . doi: 10.1038/nature20810
Pitzalis C , Jones GW , Bombardieri M , Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity . Nat Rev Immunol 2014 , 14 , 447 – 62 . doi: 10.1038/nri3700
Cooles FAH , Anderson AE , Drayton T , Harry RA , Diboll J , Munro L , et al. . Immune reconstitution 20 years after treatment with alemtuzumab in a rheumatoid arthritis cohort: implications for lymphocyte depleting therapies . Arthritis Res Ther 2016 , 18 , 302 . doi: 10.1186/s13075-016-1188-6
NICE . Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor . Technology Appraisal Guidance [TA195] 2010 .
Dooley MA , Appel G , Furie R , Wofsy D , Takeuchi T , Malvar A , Doria A , et al. . A phase III randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept or placebo on standard of care in patients with active class III or IV lupus nephritis . Arthritis Rheumatol . 2018 , 70 , 1 – 3584 .
Furie RA , Bruce IN , Dörner T , Leon MG , Leszczyński P , Urowitz M , et al. . Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus . Rheumatology (Oxford, England) 2021 , 60 , 5397 – 407 . doi: 10.1093/rheumatology/keab381
Norris-Grey C , Cambridge G , Moore S , Reddy V , Leandro M. Long-term persistence of rituximab in patients with rheumatoid arthritis: an evaluation of the UCL cohort from 1998 to 2020 . Rheumatology 2021 , 61 , 591 – 6 . doi: 10.1093/rheumatology/keab248
Smith KGC , Jones RB , Burns SM , Jayne DRW. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment . Arthritis Rheumatism 2006 , 54 , 2970 – 82 . doi: 10.1002/art.22046
Werth VP , Joly P , Mimouni D , Maverakis E , Caux F , Lehane P , et al. .; PEMPHIX Study Group . Rituximab versus mycophenolate mofetil in patients with Pemphigus vulgaris . N Engl J Med 2021 , 384 , 2295 – 305 . doi: 10.1056/NEJMoa2028564
Shah K , Cragg M , Leandro M , Reddy V. Anti-CD20 monoclonal antibodies in systemic lupus erythematosus . Biologicals 2021 , 69 , 1 – 14 . doi: 10.1016/j.biologicals.2020.11.002
Crickx E , Chappert P , Sokal A , Weller S , Azzaoui I , Vandenberghe A , et al. . Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia . Sci Transl Med 2021 , 13 , eabc3961 . doi: 10.1126/scitranslmed.abc3961
Gomez Mendez LM , Cascino MD , Garg J , Katsumoto TR , Brakeman P , Dall’Era M , et al. . Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis . Clin J Am Soc Nephrol 2018 , 13 , 1502 – 9 . doi: 10.2215/CJN.01070118
Vital EM , Dass S , Buch MH , Henshaw K , Pease CT , Martin MF , et al. . B cell biomarkers of rituximab responses in systemic lupus erythematosus . Arthritis Rheum 2011 , 63 , 3038 – 47 . doi: 10.1002/art.30466
Reddy V , Dahal LN , Cragg MS , Leandro M. Optimising B-cell depletion in autoimmune disease: is obinutuzumab the answer ? Drug Discov Today 2016 , 21 , 1330 – 8 . doi: 10.1016/j.drudis.2016.06.009
Mei HE , Schmidt S , Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity . Arthritis Res Ther 2012 , 14 , S1 . doi: 10.1186/ar3909
Ramwadhdoebe TH , van Baarsen LGM , Boumans MJH , Bruijnen STG , Safy M , Berger FH , et al. . Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis . Rheumatology (Oxford) 2019 , 58 , 1075 – 85 . doi: 10.1093/rheumatology/key428
Thurlings RM , Vos K , Wijbrandts CA , Zwinderman AH , Gerlag DM , Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response . Ann Rheum Dis 2008 , 67 , 917 – 25 . doi: 10.1136/ard.2007.080960
Teng YK , Levarht EW , Toes RE , Huizinga TW , van Laar JM. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation . Ann Rheum Dis 2009 , 68 , 1011 – 6 . doi: 10.1136/ard.2008.092791
Nakou M , Katsikas G , Sidiropoulos P , Bertsias G , Papadimitraki E , Raptopoulou A , et al. . Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response . Arthritis Res Ther 2009 , 11 , R131 . doi: 10.1186/ar2798
Stathopoulos P , Kumar A , Nowak RJ , O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis . JCI Insight 2017 , 2 , e94263 . doi: 10.1172/jci.insight.94263
Mei HE , Wirries I , Frolich D , Brisslert M , Giesecke C , Grun JR , et al. . A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow . Blood 2015 , 125 , 1739 – 48 . doi: 10.1182/blood-2014-02-555169
Halliley JL , Tipton CM , Liesveld J , Rosenberg AF , Darce J , Gregoretti IV , et al. . Long-lived plasma cells are contained within the CD19(−)CD38(hi)CD138(+) subset in human bone marrow . Immunity 2015 , 43 , 132 – 45 . doi: 10.1016/j.immuni.2015.06.016
Cragg MS , Morgan SM , Chan HTC , Morgan BP , Filatov AV , Johnson PWM , et al. . Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts . Blood 2003 , 101 , 1045 – 52 . doi: 10.1182/blood-2002-06-1761
Lim SH , Vaughan AT , Ashton-Key M , Williams EL , Dixon SV , Chan HTC , et al. . Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy . Blood 2011 , 118 , 2530 – 40 . doi: 10.1182/blood-2011-01-330357
Reddy V , Cambridge G , Isenberg DA , Glennie MJ , Cragg MS , Leandro M. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus . Arthritis Rheumatol 2015 , 67 , 2046 – 55 . doi: 10.1002/art.39167
Furie RA , Aroca G , Cascino MD , Garg JP , Rovin BH , Alvarez A , et al. . B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial . Ann Rheum Dis 2022 , 81 , 100 – 7 . doi: 10.1136/annrheumdis-2021-220920
Mok CC , Lau CS. Pathogenesis of systemic lupus erythematosus . J Clin Pathol 2003 , 56 , 481 – 90 . doi: 10.1136/jcp.56.7.481
Gong Q , Ou Q , Ye S , Lee WP , Cornelius J , Diehl L , et al. . Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy . J Immunol 2005 , 174 , 817 – 26 . doi: 10.4049/jimmunol.174.2.817
Reddy VR , Pepper RJ , Shah K , Cambridge G , Henderson SR , Klein C , et al. . Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab ? Rheumatology 2021 , 61 , 2894 – 904 . doi: 10.1093/rheumatology/keab827
Chang A , Henderson SG , Brandt D , Liu N , Guttikonda R , Hsieh C , et al. . In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis . J Immunol (Baltimore, Md. : 1950) 2011 , 186 , 1849 – 60 . doi: 10.4049/jimmunol.1001983
Fetter T , Niebel D , Braegelmann C , Wenzel J. Skin-associated B cells in the pathogenesis of cutaneous autoimmune diseases—implications for therapeutic approaches . Cells 2020 , 9 , 2627 . doi: 10.3390/cells9122627
Carter RH , Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes . Science 1992 , 256 , 105 – 7 . doi: 10.1126/science.1373518
Tedder TF , Inaoki M , Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity . Immunity 1997 , 6 , 107 – 18 . doi: 10.1016/s1074-7613(00)80418-5
van Zelm MC , Reisli I , van der Burg M , Castaño D , van Noesel CJM , van Tol MJD , et al. . An antibody-deficiency syndrome due to mutations in the CD19 gene . N Engl J Med 2006 , 354 , 1901 – 12 . doi: 10.1056/NEJMoa051568
Glass DR , Tsai AG , Oliveria JP , Hartmann FJ , Kimmey SC , Calderon AA , et al. . An integrated multi-omic single-cell atlas of human B cell identity . Immunity 2020 , 53 , 217 – 32.e5 . doi: 10.1016/j.immuni.2020.06.013
Wing E , Sutherland C , Miles K , Gray D , Goodyear CS , Otto TD , et al. . Double-negative-2 B cells are the major synovial plasma cell precursor in rheumatoid arthritis . Front Immunol 2023 , 14 , 1241474 . doi: 10.3389/fimmu.2023.1241474
Mackensen A , Müller F , Mougiakakos D , Böltz S , Wilhelm A , Aigner M , et al. . Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus . Nat Med 2022 , 28 , 2124 – 32 . doi: 10.1038/s41591-022-02017-5
Cree BAC , Bennett JL , Kim HJ , Weinshenker BG , Pittock SJ , Wingerchuk DM , et al. .; N-MOmentum study investigators . Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial . Lancet 2019 , 394 , 1352 – 63 . doi: 10.1016/S0140-6736(19)31817-3
Schiopu E , Chatterjee S , Hsu V , Flor A , Cimbora D , Patra K , et al. . Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study . Arthritis Res Ther 2016 , 18 , 131 . doi: 10.1186/s13075-016-1021-2
Herbst R , Wang Y , Gallagher S , Mittereder N , Kuta E , Damschroder M , et al. . B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody . J Pharmacol Exp Ther 2010 , 335 , 213 – 22 . doi: 10.1124/jpet.110.168062
Reddy V , Cambridge G , Isenberg DA , Glennie MJ , Cragg MS , Leandro M. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus . Arthritis Rheumatol 2015 , 67 , 2046 – 55 .
Cappell KM , Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far . Nat Rev Clin Oncol 2023 , 20 , 359 – 71 . doi: 10.1038/s41571-023-00754-1
Zhao J , Lin Q , Song Y , Liu D. Universal CARs, universal T cells, and universal CAR T cells . J Hematol Oncol 2018 , 11 , 132 . doi: 10.1186/s13045-018-0677-2
Feins S , Kong W , Williams EF , Milone MC , Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer . Am J Hematol 2019 , 94 , S3 – 9 . doi: 10.1002/ajh.25418
Mullard A. FDA approves fourth CAR-T cell therapy . Nat Rev Drug Discov 2021 , 20 , 166 . doi: 10.1038/d41573-021-00030-w
Cappell KM , Sherry RM , Yang JC , Goff SL , Vanasse DA , McIntyre L , et al. . Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy . J Clin Oncol: Off J Am Soc Clin Oncol 2020 , 38 , 3805 – 15 . doi: 10.1200/JCO.20.01467
Kansal R , Richardson N , Neeli I , Khawaja S , Chamberlain D , Ghani M , et al. . Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus . Sci Transl Med 2019 , 11 , eaav1648 . doi: 10.1126/scitranslmed.aav1648
Taubmann J , Müller F , Boeltz S , Völkl S , Aigner M , Kleyer A , et al. . OP0141 Long term safety and efficacy of CAR-T cell treatment in refractory systemic lupus erythematosus—data from the first seven patients . Ann Rheum Dis 2023 , 82 , 93 – 4 .
Nunez D , Patel D , Volkov J , Wong S , Vorndran Z , Müller F , et al. . Cytokine and reactivity profiles in SLE patients following anti-CD19 CART therapy . Mol Ther Methods Clin Dev 2023 , 31 , 101104 . doi: 10.1016/j.omtm.2023.08.023
Müller F , Boeltz S , Knitza J , Aigner M , Völkl S , Kharboutli S , et al. . CD19-targeted CAR T cells in refractory antisynthetase syndrome . Lancet (London, England) 2023 , 401 , 815 – 8 . doi: 10.1016/S0140-6736(23)00023-5
Bergmann C , Müller F , Distler JHW , Györfi A-H , Völkl S , Aigner M , et al. . Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells . Ann Rheum Dis 2023 , 82 , 1117 – 20 . doi: 10.1136/ard-2023-223952
Griffin J , Xue S , Carpenter B , Velica P , Holler A , Nicholson E , et al. . T cells engineered for resistance to mycophenolate mofetil demonstrate enhanced expansion and improved tumour control in immunosuppressed hosts . Mol Ther 2014 , 22 , S58 .
Kretschmann S , Völkl S , Reimann H , Krönke G , Schett G , Achenbach S , et al. . Successful generation of CD19 chimeric antigen receptor T cells from patients with advanced systemic lupus erythematosus . Transplant Cell Ther 2023 , 29 , 27 – 33 . doi: 10.1016/j.jtct.2022.10.004
Wat J , Barmettler S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions . J Allergy Clin Immunol Pract 2022 , 10 , 460 – 6 . doi: 10.1016/j.jaip.2021.10.037
Hill JA , Giralt S , Torgerson TR , Lazarus HM. CAR-T—and a side order of IgG, to go? Immunoglobulin replacement in patients receiving CAR-T cell therapy . Blood Rev 2019 , 38 , 100596 . doi: 10.1016/j.blre.2019.100596
Wiedmeier-Nutor JE , Iqbal M , Rosenthal AC , Bezerra ED , Garcia-Robledo JE , Bansal R , et al. . Response to COVID-19 vaccination post-CAR T therapy in patients with non-hodgkin lymphoma and multiple myeloma . Clin Lymphoma Myeloma Leuk 2023 , 23 , 456 – 62 . doi: 10.1016/j.clml.2023.03.002
Bhoj VG , Arhontoulis D , Wertheim G , Capobianchi J , Callahan CA , Ellebrecht CT , et al. . Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy . Blood 2016 , 128 , 360 – 70 . doi: 10.1182/blood-2016-01-694356
Gupta S , Simic M , Sagan SA , Shepherd C , Duecker J , Sobel RA , et al. . CAR-T cell–mediated B-cell depletion in central nervous system autoimmunity . Neurology - Neuroimmunology Neuroinflammation 2023 , 10 , e200080 .
Granit V , Benatar M , Kurtoglu M , Miljković MD , Chahin N , Sahagian G , et al. .; MG-001 Study Team . Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study . Lancet Neurol 2023 , 22 , 578 – 90 . doi: 10.1016/S1474-4422(23)00194-1
Mamlouk O , Nair R , Iyer SP , Edwards A , Neelapu SS , Steiner RE , et al. . Safety of CAR T-cell therapy in kidney transplant recipients . Blood 2021 , 137 , 2558 – 62 . doi: 10.1182/blood.2020008759
Krishnamoorthy S , Ghobadi A , Santos RD , Schilling JD , Malone AF , Murad H , et al. . CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder . Am J Transplant 2021 , 21 , 809 – 14 . doi: 10.1111/ajt.16367
Oren D , DeFilippis EM , Lotan D , Clerkin KJ , Fried J , Reshef R , et al. . Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD . JACC CardioOncol 2022 , 4 , 713 – 6 . doi: 10.1016/j.jaccao.2022.09.002
Pecher AC , Hensen L , Klein R , Schairer R , Lutz K , Atar D , et al. . CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome . JAMA 2023 , 329 , 2154 – 62 . doi: 10.1001/jama.2023.8753
Lee J , Lundgren DK , Mao X , Manfredo-Vieira S , Nunez-Cruz S , Williams EF , et al. . Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris . J Clin Invest 2020 , 130 , 6317 – 24 . doi: 10.1172/JCI138416
Zhang L , Sosinowski T , Cox AR , Cepeda JR , Sekhar NS , Hartig SM , et al. . Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes . J Autoimmun 2019 , 96 , 50 – 8 . doi: 10.1016/j.jaut.2018.08.004
Guidetti A , Perrone G , Coluccia P , Fumagalli L , Dodero A , Farina L , et al. . The real life accessibility to CAR T-cell therapy: current experience in the only active center in Italy . Blood 2019 , 134 , 5619 – 5619 . doi: 10.1182/blood-2019-125286
Jo S , Das S , Williams A , Chretien A-S , Pagliardini T , Le Roy A , et al. . Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing . Nat Commun 2022 , 13 , 3453 . doi: 10.1038/s41467-022-30896-2
Bacac M , Colombetti S , Herter S , Sam J , Perro M , Chen S , et al. . CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies . Clin Cancer Res 2018 , 24 , 4785 – 97 . doi: 10.1158/1078-0432.CCR-18-0455
Ball ED , Guyre PM , Mills L , Fisher J , Dinces NB , Fanger MW. Initial trial of bispecific antibody-mediated immunotherapy of CD15-bearing tumors: cytotoxicity of human tumor cells using a bispecific antibody comprised of anti-CD15 (MoAb PM81) and anti-CD64/Fc gamma RI (MoAb 32) . J Hematother 1992 , 1 , 85 – 94 . doi: 10.1089/scd.1.1992.1.85
Seimetz D , Lindhofer H , Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy . Cancer Treat Rev 2010 , 36 , 458 – 67 . doi: 10.1016/j.ctrv.2010.03.001
Przepiorka D , Ko CW , Deisseroth A , Yancey CL , Candau-Chacon R , Chiu HJ , et al. . FDA approval: blinatumomab . Clin Cancer Res 2015 , 21 , 4035 – 9 . doi: 10.1158/1078-0432.CCR-15-0612
Tapia-Galisteo A , Álvarez-Vallina L , Sanz L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies . J Hematol Oncol 2023 , 16 , 83 . doi: 10.1186/s13045-023-01482-w
Kontermann RE , Brinkmann U. Bispecific antibodies . Drug Discov Today 2015 , 20 , 838 – 47 . doi: 10.1016/j.drudis.2015.02.008
Gruber M , Schodin BA , Wilson ER , Kranz DM. Efficient tumor cell lysis mediated by a bispecific single chain antibody expressed in Escherichia coli . J Immunol 1994 , 152 , 5368 – 74 .
Ridgway JB , Presta LG , Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization . Protein Eng 1996 , 9 , 617 – 21 . doi: 10.1093/protein/9.7.617
Klein C , Sustmann C , Thomas M , Stubenrauch K , Croasdale R , Schanzer J , et al. . Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies . MAbs 2012 , 4 , 653 – 63 . doi: 10.4161/mabs.21379
Surowka M , Schaefer W , Klein C. Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins . mAbs 2021 , 13 , 1967714 . doi: 10.1080/19420862.2021.1967714
Hoffmann P , Hofmeister R , Brischwein K , Brandl C , Crommer S , Bargou R , et al. . Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct . Int J Cancer 2005 , 115 , 98 – 104 . doi: 10.1002/ijc.20908
d’Argouges S , Wissing S , Brandl C , Prang N , Lutterbuese R , Kozhich A , et al. . Combination of rituximab with blinatumomab (MT103/MEDI-538), a T cell-engaging CD19-/CD3-bispecific antibody, for highly efficient lysis of human B lymphoma cells . Leuk Res 2009 , 33 , 465 – 73 . doi: 10.1016/j.leukres.2008.08.025
Sun LL , Ellerman D , Mathieu M , Hristopoulos M , Chen X , Li Y , et al. . Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies . Sci Transl Med 2015 , 7 , 287ra70 – ra70 . doi: 10.1126/scitranslmed.aaa4802
Engelberts PJ , Hiemstra IH , de Jong B , Schuurhuis DH , Meesters J , Beltran Hernandez I , et al. . DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing . EBioMedicine 2020 , 52 , 102625 . doi: 10.1016/j.ebiom.2019.102625
Klein C , Neumann C , Fauti T , Weinzierl T , Freimoser-Grundschober A , Waldhauer I , et al. . Abstract 3629: Engineering a novel asymmetric head-to-tail 2 + 1 T-cell bispecific (2 + 1 TCB) IgG antibody platform with superior T-cell killing compared to 1 + 1 asymmetric TCBs . Cancer Res 2017 , 77 , 3629 – 3629 . doi: 10.1158/1538-7445.am2017-3629
Grakoui A , Bromley SK , Sumen C , Davis MM , Shaw AS , Allen PM , et al. . The immunological synapse: a molecular machine controlling T cell activation . Science 1999 , 285 , 221 – 7 . doi: 10.1126/science.285.5425.221
Martz E. Multiple target cell killing by the cytolytic T lymphocyte and the mechanism of cytotoxicity . Transplantation 1976 , 21 , 5 – 11 . doi: 10.1097/00007890-197601000-00002
NICE. Axicabtagene ciloleucel for treating relapsed or refractory diffuse large B-cell lymphoma after first-line chemoimmunotherapy . Technology appraisal guidance [TA895]. 2023 , 1 – 24 .
NICE . Blinatumomab for treating acute lymphoblastic leukaemia in remission with minimal residual disease activity . Technology appraisal guidance [TA589] . 2019 , 1 – 24 .
Ross SL , Sherman M , McElroy PL , Lofgren JA , Moody G , Baeuerle PA , et al. . Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing . PLoS One 2017 , 12 , e0183390 . doi: 10.1371/journal.pone.0183390
Rainone M , Ngo D , Baird JH , Budde LE , Htut M , Aldoss I , et al. . Interferon-γ blockade in CAR T-cell therapy-associated macrophage activation syndrome/hemophagocytic lymphohistiocytosis . Blood Adv 2023 , 7 , 533 – 6 . doi: 10.1182/bloodadvances.2022008256
Lerkvaleekul B , Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key . Open Access Rheumatol 2018 , 10 , 117 – 28 . doi: 10.2147/OARRR.S151013
Zhang C , Liu J , Zhong JF , Zhang X. Engineering CAR-T cells . Biomarker Res 2017 , 5 , 1 – 6 .
Crespo J , Sun H , Welling TH , Tian Z , Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment . Curr Opin Immunol 2013 , 25 , 214 – 21 . doi: 10.1016/j.coi.2012.12.003
Zhang K , Kong X , Li Y , Wang Z , Zhang L , Xuan L. PD-1/PD-L1 Inhibitors in patients with preexisting autoimmune diseases . Front Pharmacol 2022 , 13 , 854967 . doi: 10.3389/fphar.2022.854967
Wu L , Seung E , Xu L , Rao E , Lord DM , Wei RR , et al. . Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation . Nat Cancer 2020 , 1 , 86 – 98 . doi: 10.1038/s43018-019-0004-z
McKinney EF , Lee JC , Jayne DRW , Lyons PA , Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection . Nature 2015 , 523 , 612 – 6 . doi: 10.1038/nature14468
Singh A , Dees S , Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer . Br J Cancer 2021 , 124 , 1037 – 48 . doi: 10.1038/s41416-020-01225-5
Le RQ , Li L , Yuan W , Shord SS , Nie L , Habtemariam BA , et al. . FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome . Oncologist 2018 , 23 , 943 – 7 . doi: 10.1634/theoncologist.2018-0028
Leclercq G , Haegel H , Toso A , Zimmermann T , Green L , Steinhoff N , et al. . JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy . J ImmunoTher Cancer 2022 , 10 , e003766 . doi: 10.1136/jitc-2021-003766
Zhou S , Liu M , Ren F , Meng X , Yu J. The landscape of bispecific T cell engager in cancer treatment . Biomarker Res 2021 , 9 , 1 – 23 .
Nakamura M , Ogawa N , Shalabi A , Maley WR , Longo D , Burdick JF. Positive effect on T-cell regulatory apoptosis by mycophenolate mofetil . Clin Transplant 2001 , 15 , 36 – 40 . doi: 10.1034/j.1399-0012.2001.00006.x
Eugui EM , Mirkovich A , Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice . Scand J Immunol 1991 , 33 , 175 – 83 . doi: 10.1111/j.1365-3083.1991.tb03747.x
Prémaud A , Rousseau A , Johnson G , Canivet C , Gandia P , Muscari F , et al. . Inhibition of T-cell activation and proliferation by mycophenolic acid in patients awaiting liver transplantation: PK/PD relationships . Pharmacol Res 2011 , 63 , 432 – 8 . doi: 10.1016/j.phrs.2011.01.005
Ellebrecht CT , Bhoj VG , Nace A , Choi EJ , Mao X , Cho MJ , et al. . Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease . Science 2016 , 353 , 179 – 84 . doi: 10.1126/science.aaf6756
Reddy V , Jayne D , Close D , Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design . Arthritis Res Ther 2013 , 15 , S2 – 16 . doi: 10.1186/ar3910
| Month: | Total Views: |
|---|---|
| April 2024 | 561 |
| May 2024 | 1,003 |
| June 2024 | 1,058 |
Email alerts
Citing articles via.
- About Clinical & Experimental Immunology
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1365-2249
- Copyright © 2024 British Society for Immunology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Get started with Survey123 reports
One of the features of Survey123 is exporting custom PDF or Word format reports based on survey data. If you are new to Survey123 or haven’t explored Survey123 reports yet, this blog will help you get started with creating reports.
Reports are generated based on a report template associated with the survey. A report template is a Microsoft Word document that provides the report formatting and includes placeholders to indicate where survey data should be inserted when the report is exported. Since the template is a Word document, almost any formatting options that are possible in Word can be included in your template. This could include images or logos, text formatting, tables, headers, and footers. This customizability is useful for designing visually appealing reports, but it is also useful when adhering to a required report format, for example when submitting data to a government agency. See below for examples of report templates and an example of an exported report.
The Survey123 website is the central hub for viewing submitted data and managing various survey settings and features, including reports. The report template is added to the survey by uploading the template file in the Manage templates option on the Reports tab on the survey’s Data tab. Once at least one report template is uploaded, reports for one or multiple records can be exported.
Report types – individual vs. summary
There are two types of reports in Survey123: individual reports and summary reports. It’s also possible to include both in the same report, in which case they would be individual and summary sections.
An individual report displays the data for a single survey record, while a summary report displays data for multiple records in one report. Summary reports often aggregate the data using statistics. For example, if we have a survey that documents a water violation, an individual report will show details for a single specific water violation. A summary report, on the other hand, would display statistics for multiple violations, for example the total count of violations or the total count of each type of violation.
The report template’s syntax determines whether a report will be a summary report or an individual report. A summary report has a designated start tag and end tag, and everything between the two tags will be treated as a summary section.
Report templates
Report templates support a wide array of options for displaying your survey data, but there are a few ideas that are the core components to even the most complex reports:
• Question placeholders – Returns the value for the question name indicated. For example: ${question_name}
• Repeat start/end tags – Accesses questions within a repeat in a survey. Questions from the repeat can be displayed by including question placeholders between the start and end tags.
• Methods and parameters – Methods and parameters are additional syntax appended to start tag or question placeholder that modifies the returned value in some way. For example, the syntax ${geopoint1 | getValue:"x"} will return this geopoint’s x-coordinate because the getValue method is applied to the question placeholder and the parameter is set to x.
• Summary sections – Aggregates the question values for multiple survey records. A summary section is designated with a summary section start tag ( $<$summary> ) and end tag ( $</> ).
These are the foundational concepts for creating a report template, but it’s not necessary to create report templates from scratch. There are several resources to help you get started with a report template quickly.
Quick reference
The first resource for getting started with building a template is the quick reference. This option provides examples and descriptions for various methods and parameters that can be used for each question in your survey.
These syntax snippets include the question names from your survey, so they can be copied and pasted directly into your report template. The quick reference section is a good way to discover the different report syntax options that are available.
Sample templates
Another resource for getting started with report templates is the sample template option in the Survey123 website. This option generates a report template that includes the labels and placeholder syntax for all questions and repeats in your survey. You can then modify this template by removing unwanted questions, reordering questions, adding methods, and so on.
Esri templates gallery
If you’d like to see examples of completed report templates, some of the Esri templates available in Survey123 Connect include report templates that can be used with the samples. You can find these by opening Connect, clicking Esri Templates, and searching for “report template”. The survey template description includes a hyperlink to the report template.
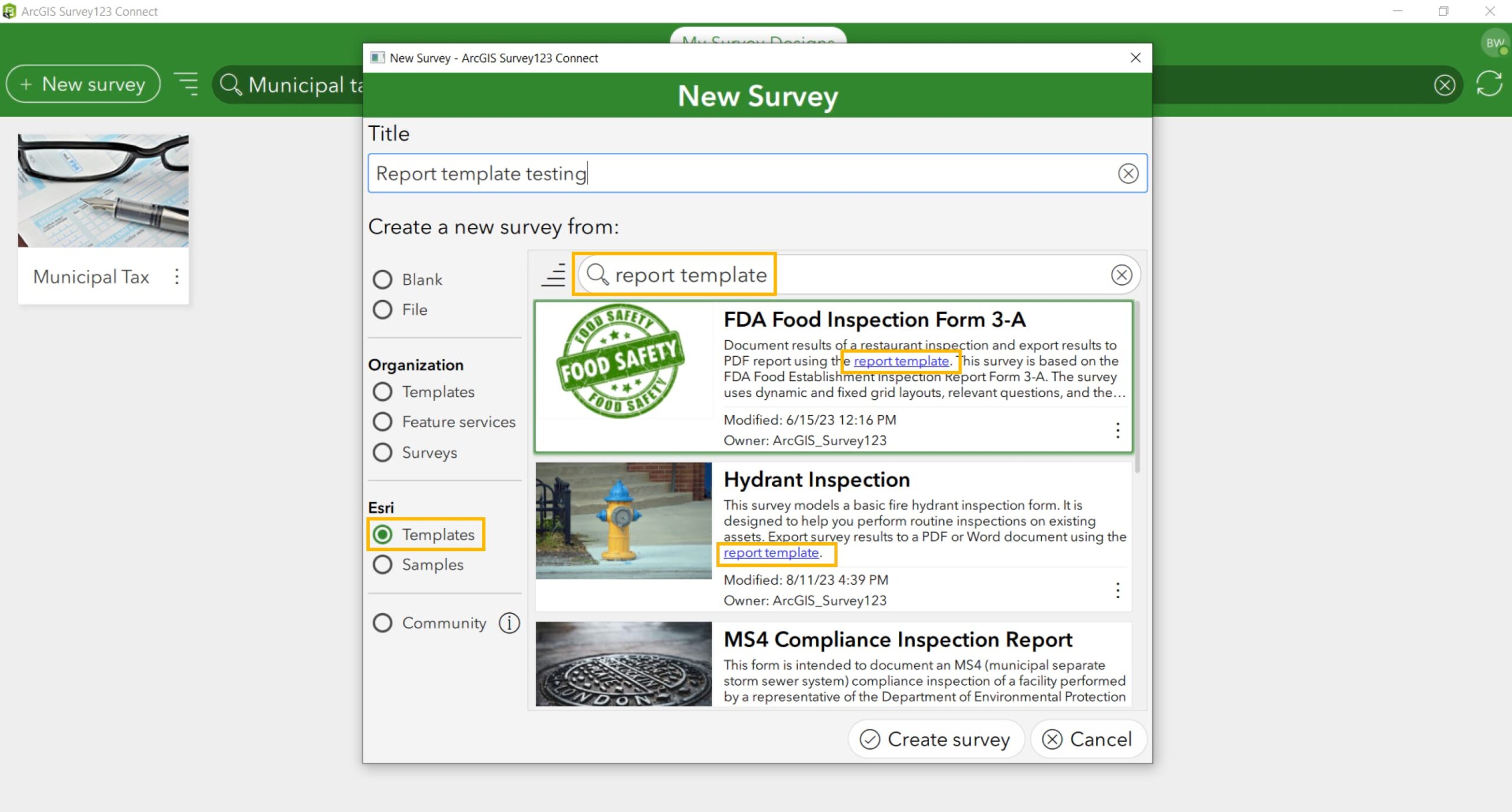
Documentation
The final resource for exploring report templates is the Survey123 Report templates documentation . This documentation describes all the supported report functionality, and includes a helpful table of all the methods and parameters that can be used in reports.
What are the requirements for authoring and generating a Survey123 report?
Surveys in ArcGIS Online are generated by an ArcGIS Online premium service. If your survey is hosted in ArcGIS Online, each report will cost 0.5 credits plus 0.5 credits for each additional survey record included in the report (except summary reports).
Survey123 reports can be used with ArcGIS Enterprise with some exceptions. The following list of exceptions was written by Ismael Chivite when the report functionality was originally released in 2019 but is still applicable.
- Your ArcGIS Enterprise instance must be accessible to the Survey123 ArcGIS Online report service. That is, you must configure your ArcGIS Enterprise web adaptor to expose access to your Enterprise instance from the internet. Please note that portals with Integrated Windows Authentication will not work with the report service.
- Use the Upgrade Attachments tool in ArcGIS Pro.
- Use the following syntax in your report template:

Make sure you the above as three separate lines in your template. This expression will basically show all attachments found in your feature.
If you are using ArcGIS Enterprise 10.8.1 or newer, as long as you do not use a feature service published from ArcMap, you can use images in your report template normally.
Bearing in mind the limitations above, the use of the Survey123 report service from your ArcGIS Enterprise instance will not consume ArcGIS Online credit costs.
User types, privileges, and sharing
In order to upload a report template , a user must be either the survey owner or the organization administrator.
In order to export a report , a user must:
- Have the feature report privilege enabled.
- Have at least a ‘User’ level user type.
- Have had the survey results shared with them in the Survey123 website collaborate tab. This shares the survey form item, results feature layer, and report template.
It’s also possible to automate your report. For example, if you want a report to be exported at the same time every week, this is possible. One of the most common methods of automating reports is with third-party automation software like Microsoft Power Automate or Make , which have drag-and-drop interfaces to set up automations. It’s also possible to automate reports using a code-based approach such as the ArcGIS API for Python or the ArcGIS Rest API .
If you’re interested in learning more about Survey123 reports, also see these webinars on report automation and creating report templates:
Survey123 Tricks of the Trade (Live): Automate Reports
Survey123 Tricks of the Trade (Live): Create Report Templates
Article Discussion:
Related content:.
- sharing and collaboration
- arcgis survey123
- report template

2023 Esri User Conference: ArcGIS Survey123 Team's Top Picks
Christie Roland | ArcGIS Survey123 | August 23, 2023
Dive into the ArcGIS Survey123 team's curated picks from the 2023 Esri User Conference. Learn new tools and tips to help elevate your work.
Read this article

Share With Us Your Survey Designs
Christie Roland | ArcGIS Survey123 | November 7, 2022
Share with us your survey designs to become a featured data collection template in the ArcGIS Survey123 community gallery.

Barcode Scanning in ArcGIS Survey123
ismael | ArcGIS Survey123 | October 24, 2016
Learn how to add a barcode question into a Survey123 smart form.
Next Article
What’s new in the ArcGIS Business Analyst data browser (June 2024)
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.
Print MS Word DOCX Documents in .NET using C# Without Interop

This article shows how to print MS Word DOCX documents in .NET using C#. It uses TX Text Control to load Office Open XML documents and print them to any printer, PDF or image file.
Although there are libraries such as the Open XML SDK for creating DOCX files, printing is a different story. The only way to print a DOCX file is to open it in Microsoft Word and print it manually. For a web application that needs to print a lot of documents, or for a server-side application that needs to print documents automatically, this is not a good solution.
TX Text Control provides not only the ability to programmatically create DOCX files, but also the ability to print them without using MS Word or Microsoft Office Interop. This article shows the different ways to print a DOCX file using TX Text Control:
- Direct printing with full printer access
- Creating a PDF file
- Creating SVG images from the document pages
- Creating images from the document pages
- Preparing the Application
A .NET 8 console application is created for the purposes of this demo.
Prerequisites The following tutorial requires a trial version of TX Text Control .NET Server for ASP.NET. Download Trial Version
In Visual Studio, create a new Console App using .NET 8.
In the Solution Explorer , select your created project and choose Manage NuGet Packages... from the Project main menu.
Select Text Control Offline Packages from the Package source drop-down.
Install the latest versions of the following package:
- TXTextControl.TextControl.ASP.SDK
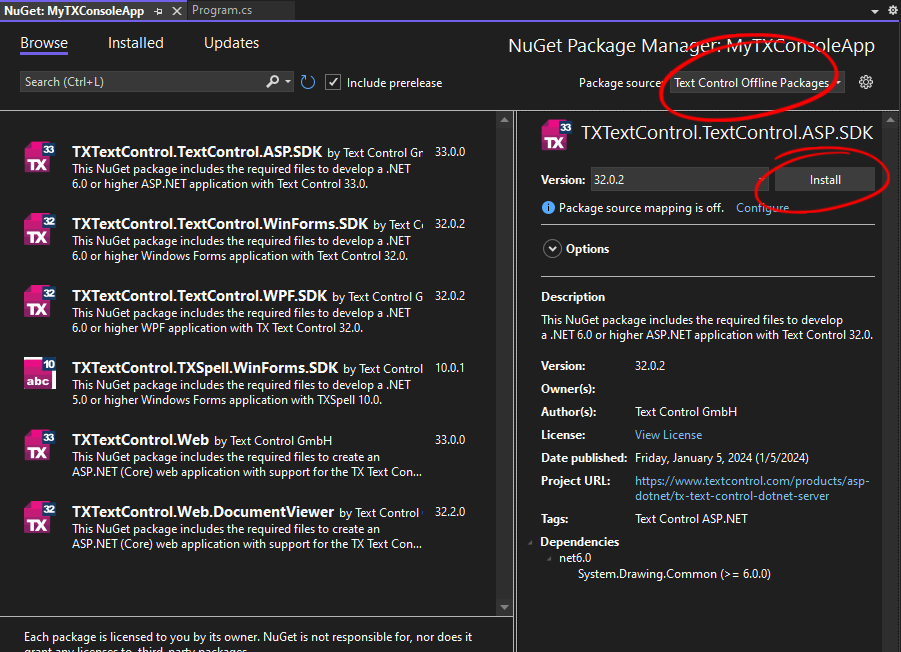
- Direct Printing
| using System.Drawing.Printing; | |
| using (TXTextControl.ServerTextControl tx = new TXTextControl.ServerTextControl()) | |
| { | |
| tx.Create(); | |
| tx.Load("test.docx", TXTextControl.StreamType.WordprocessingML); | |
| PrintDocument printDocument = new PrintDocument(); | |
| printDocument.PrinterSettings.PrinterName = "Microsoft Print to PDF"; | |
| tx.Print(printDocument); | |
| } |
TX Text Control allows you to load and print not only DOCX files, but also binary MS Word format DOC and RTF files.
You can also print to a specific printer by passing the printer name to the PrinterSettings object. In this case the printer name is Microsoft Print to PDF and the output is a PDF file.
| using System.Drawing.Printing; | |
| using (TXTextControl.ServerTextControl tx = new TXTextControl.ServerTextControl()) | |
| { | |
| tx.Create(); | |
| tx.Load("test.docx", TXTextControl.StreamType.WordprocessingML); | |
| PrintDocument printDocument = new PrintDocument(); | |
| printDocument.PrinterSettings.PrinterName = "Microsoft Print to PDF"; | |
| printDocument.PrinterSettings.PrintToFile = true; | |
| printDocument.PrinterSettings.PrintFileName = "test.pdf"; | |
| tx.Print(printDocument); | |
| } |
- Creating a PDF File
| using (TXTextControl.ServerTextControl tx = new TXTextControl.ServerTextControl()) | |
| { | |
| tx.Create(); | |
| tx.Load("test.docx", TXTextControl.StreamType.WordprocessingML); | |
| tx.Save("test.pdf", TXTextControl.StreamType.AdobePDF); | |
| } |
This code uses the ServerTextControl to directly generate the binary PDF document without using a printer driver. The PDF file is stored in the file system. The Save method can be used to save the document in various formats including DOCX, DOC, RTF, and PDF.
- Creating SVG Images
TX Text Control can also be used to create SVG images from the document pages. The advantage of SVG is that it's a standard that's supported by all browsers, and because it's a vector graphics format, there's no loss of quality for text content.
| string svgSources = tx.GetPages()[1].GetImage(TXTextControl.Page.PageContent.All, 300); |
The first parameter PageContent defines the content to be returned by the method. The second parameter defines the resolution of embedded bitmap images.
The following method creates SVG images from all pages of a document and saves them to the file system.
| public string[] CreateSVG(ServerTextControl TextControl, | |
| bool GlyphOutlines = false, | |
| int FromPage = 1, | |
| int ToPage = -1) | |
| { | |
| // create array for SVGs | |
| string[] svgPages = new string[(ToPage == -1 ? TextControl.Pages : ToPage)]; | |
| // set page content | |
| TXTextControl.Page.PageContent pageContent = | |
| GlyphOutlines ? TXTextControl.Page.PageContent.All | TXTextControl.Page.PageContent.GlyphOutlines | |
| : TXTextControl.Page.PageContent.All; | |
| for (int i = FromPage; i <= (ToPage == -1 ? TextControl.Pages : ToPage); i++) | |
| { | |
| // get SVG from page | |
| svgPages[i - 1] = TextControl.GetPages()[i].GetImage(pageContent, 96); | |
| } | |
| return svgPages; | |
| } |
- Creating Images
TX Text Control can also be used to create images such as JPG or PNG from the document pages. The following code shows how to create images from the document pages in a Console application.
| using System.Collections; | |
| using System.Drawing.Imaging; | |
| using System.Drawing; | |
| using TXTextControl; | |
| using (TXTextControl.ServerTextControl tx = new TXTextControl.ServerTextControl()) | |
| { | |
| tx.Create(); | |
| tx.Load("test.docx", TXTextControl.StreamType.WordprocessingML); | |
| ArrayList inputImages = new ArrayList(); | |
| foreach (Page page in tx.GetPages()) | |
| { | |
| MemoryStream image = new MemoryStream(); | |
| Bitmap mf = page.GetImage(100, TXTextControl.Page.PageContent.All); | |
| mf.Save(image, ImageFormat.Png); | |
| inputImages.Add(image); | |
| } | |
| // save images as files | |
| int i = 0; | |
| foreach (MemoryStream ms in inputImages) | |
| { | |
| FileStream file = new FileStream("image" + i.ToString() + ".png", FileMode.Create, FileAccess.Write); | |
| ms.WriteTo(file); | |
| file.Close(); | |
| i++; | |
| } | |
| } |
The GetImage method has an implementation that returns a Bitmap object of the page which are stored in an array of Bitmap objects. The second part shows how to save these images to the file system.
TX Text Control provides the ability to programmatically create DOCX files, and also the ability to print them without using MS Word or Microsoft Office Interop. This article showed the various ways to print a DOCX file using TX Text Control.
ASP.NET Core .NET 6 .NET 7 .NET 8 Angular Blazor React JavaScript
Integrate document processing into your applications to create documents such as PDFs and MS Word documents, including client-side document editing, viewing, and electronic signatures.
Download Trial
- Trial Access Token
Getting started with:
- ASP.NET Core
Related Posts
Text to table and table to text in tx text control and c#.
TX Text Control provides powerful table features and also full access to text formatting which can be used to create tables from text and vice versa. This article shows how to convert text to tables and tables to text.
Extract Data from PDF Documents with C#
Learn how to extract text from PDF documents using the TX Text Control PDF import feature in C#. This article shows how to extract text, attachments, form field values and metadata from PDF documents.
Inject JavaScript to PDF Documents in C#
Learn how to inject JavaScript into PDF documents using TX Text Control .NET Server for ASP.NET. This article shows how to add JavaScript to a PDF document to execute code when the document is opened.
Document Templates Tip: Say No to Forced Page Breaks
This article explains why to avoid forced page breaks in document templates and how to create templates that automatically adjust to the content using other word processing features.
- Prerequisites
Popular Products
- TX Text Control .NET Server for ASP.NET
- Client-Side Packages (Angular, React)
- TX Text Control .NET for Windows Forms
- TX Text Control .NET for WPF
Technologies
- Document Editing
- PDF Processing
- Electronic Signatures
- Document Viewing
Get Products
- Free Trials
- Online Store
- Documentation
Getting Started
- Windows Forms
- Open Support Case
- Support FAQ
Ready To Talk?
- USA: +1 704-544-7445
- Germany: +49 421 42706710
Text Control is an award-winning vendor of document processing and reporting components for Windows, web, cloud and mobile development technologies.
We ♥ documents.
Copyright © 2024 Text Control GmbH. All rights reserved. Impressum .
TX Text Control, DS Server, ReportingCloud and other product names used herein might be trademarks or registered trademarks of Text Control GmbH and/or one of its subsidiaries or affiliates in the U.S. and/or other countries.
Our partners and we may store and access personal data such as cookies , device identifiers or other similar technologies on your device and process such data to personalize content and ads, provide social media features and analyze our traffic.
Cookies, device identifiers, or other information can be stored or accessed on your device for the purposes presented to you.
Ads and content can be personalized based on a profile. More data can be added to better personalize ads and content. Ad and content performance can be measured. Insights about audiences who saw the ads and content can be derived. Data can be used to build or improve user experience, systems and software. The information may be transferred, stored and processed to countries outside the EU, including the United States.
By clicking on I AGREE , you allow the use of these cookies and agree to the privacy policy . You can withdraw your consent at any time, by clicking I DISAGREE . To reopen this dialog, click on Consent in the footer of any page.
I Agree I Disagree Impressum

Six Takeaways From the First Presidential Debate
In a testy, personal clash, President Biden failed to ease worries about his age, Donald Trump forcefully made his case (with wild claims and exaggerations) and the moderators held their fact-checking fire.
The debate stage in Atlanta on Thursday night. Credit... Kenny Holston/The New York Times
Supported by
- Share full article

By Shane Goldmacher and Jonathan Swan
Shane Goldmacher reported from the debate in Atlanta, and Jonathan Swan from Washington.
- June 28, 2024
President Biden struggled through his first debate of the 2024 campaign against Donald J. Trump, meandering and mumbling through answers as the former president pressed his case for a second term with limited resistance from his rival.
They disagreed on abortion, inflation, climate change, foreign affairs and immigration. But the sharpest contrast was in their presentation.
Mr. Trump was confident and forceful, even as he let loose a stream of misleading attacks and falsehoods. Mr. Biden spoke with a hoarse and halting voice, closing his eyes occasionally to gather thoughts that sometimes couldn’t be corralled. Democratic anxiety rose by the minute. About halfway through, people close to Mr. Biden put out word that he had a cold.
Mr. Trump relentlessly hammered Mr. Biden on areas of vulnerability, sending exaggerations and embellishments — he was the “greatest” and his opponent the “worst” — flying unchecked through the audience-free CNN studio in Atlanta.
Here are six takeaways:
Biden stumbled over his words as he answered a question on the national debt.
“We’d be able to wipe out his debt. We’d be able to help make sure that all those things we need to do child care, elder care, making sure that we continue to strengthen our health care system, making sure that we’re able to make every single solitary person eligible for what I’ve been able to do with the — with, with the Covid, excuse me. With dealing with everything we have to do with — look, if — we finally beat Medicare.” “Thank you, President Biden. President Trump.” “He was right. He did beat Medicaid, beat it to death, and he’s destroying Medicare.”

The debate exposed Biden’s biggest weakness.
Mr. Biden’s allies desperately hoped he could turn in a commanding performance to calm voters’ persistent concerns about his age.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Advertisement

COMMENTS
The analysis shows that you can evaluate the evidence presented in the research and explain why the research could be important. Summary. The summary portion of the paper should be written with enough detail so that a reader would not have to look at the original research to understand all the main points. At the same time, the summary section ...
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
Summaries, like abstracts, follow the structure of the original article: Introduction (and Background), Methods, Results, and Discussion. They frequently require an APA citation. Summaries generally do not make use of direct quotes. DIRECTIONS: To compose your summary, follow prompts 1- 6. Use the questions after each prompt to guide your writing.
You must include a SEPARATE TITLE. PAGE that includes your instructor's name, section, time/day the class meets, your name, student id #. Danielle Wilson. Psych 100 Section 005. Tuesday Thursday 1:00PM. Ms. Trich Kremer. 913553226. Your Name. /Day the class meetsStudent ID NumberThe paper must be typed, double-spaced.
Writing the Article Summary Like an abstract in a published research article, the purpose of an article summary is give the reader a brief, structured overview of the study that was done. It is important that you understand the writing an article summary is a low-stress activity. By using these tips, the task becomes very easy.
Introduction. Writing a summary or abstract teaches you how to condense information and how to read an article more effectively and with better understanding. Research articles usually contain these parts: Title/Author Information, Abstract, Introduction, Methodology, Result or Findings, Discussion or Conclusion, and References.
the writer has structured the information in her article, you are well on your way to summarizing it. Read the abstract at the beginning of the article if there is one. The abstract is an even more concise summary of the article than the summary you will do. Read the article through once to capture the gists of the article, its main ideas. You are
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
Summarize the main question(s) and thesis or findings. Skim subheadings and topic sentences to understand the organization; make notes in the margins about each section. Read each paragraph within a section; make short notes about the main idea or purpose of each paragraph.
Article Review Definition of Genre Summaries and critiques are two ways to write a review of a scientific journal article. Both types of writing ask you first to read and understand an article from the primary literature about your topic. The summary involves briefly but accurately stating the key points of the article for a reader who has
Write the literature review in the past tense; the research has already been completed. The article cannot "do", "find", or "say" anything. The authors are the people who conducted the study. The above format is a guideline. It may be necessary to change the verbs or to expand an idea. Sample format, Page 2 of 2.
4. Before you begin the first draft of your summary: Try to describe the article in your own words first. Try to distill the article down to its "scientific essence." Include all the key points and be accurate. A reader who has not read the original article should be able to understand your summary. 5.
• A summary is typically one-quarter to one-third the length of the original and is written in third person. • The summary may sometimes quote a particularly effective word or phrase from the original, which should be placed in quotation marks. • Identify the original work by title and author in the first sentence of the SUMMARY.
A summary paragraph works the same way. A summary should contain: - the title of the article (or whatever work) you are summarizing and the author's full name. - the author's main ideas and supporting details. - a brief, objective, and concise retelling. - your own words. A summary should NOT contain: - minor or excessive details.
When writing a summary, the goal is to compose a concise and objective overview of the original article. The summary should focus only on the article's main ideas and important details that support those ideas. Guidelines for summarizing an article: State the main ideas. Identify the most important details that support the main ideas.
The original research article ( click here for an example) - make sure you have the full-text of the article. 2. Your summary ( click here for an example) of the orginal research article. 3. The APA citation of the original research article ( click here for example on page 2). 4. An outside reader - use FM's Writing Center. Hours are listed below.
Notice that this summary condenses a seven-page paper and translates it into a cogent and clear synopsis. Note the absence of technical jargon, direct quotations and dense sentences. The student has distilled this paper down into clear statements of the main purpose of the study, experimental method, main findings and interpretation of the results.
Microsoft Word - summarizing.doc. Summarizing a Research Article. Research articles use a standard format to clearly communicate information about an experiment. A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. Sometimes there are minor variations, such as a ...
Pre-read the article (read the abstract, introduction, and/or conclusion). Summarize the main question (s) and thesis or findings. Skim subheadings and topic sentences to understand the organization; make notes in the margins about each section. Read each paragraph within a section; make short notes about the main idea or purpose of each paragraph.
Preparing to Write: To write a good summary it is important to thoroughly understand the material you are working with. Here are some preliminary steps in writing a summary. Skim the text, noting in your mind the subheadings. If there are no subheadings, try to divide the text into sections. Consider why you have been assigned the text. Try to.
This Professional Summary Templates is free in the internet where you can use the sample format in making any rundown of article with different topics, you can also have it in Microsoft word or excel. Article summary template is the miniature version of the long and detailed content of a document. Sample Business Summary Template - 8+ Free ...
PDF | On Nov 20, 2014, Katherine Myers-Coffman published Research Article Summary | Find, read and cite all the research you need on ResearchGate
Study design. In this study, a two-sample MR approach was exploited to evaluate the effects of CD and UC on the risks of AD, PD, and MS. A schematic overview of the study design and data sources ...
Summary. B and T cells collaborate to drive autoimmune disease (AID). Historically, B- and T-cell (B-T cell) co-interaction was targeted through different pathways such as alemtuzumab, abatacept, and dapirolizumab with variable impact on B-cell depletion (BCD), whereas the majority of patients with AID including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and ...
The Atlanta Hawks own the No. 1 overall pick for one of the most uncertain and unpredictable NBA drafts in recent memory. For the first time ever, the 2024 NBA draft is utilizing a two-night ...
There are two types of reports in Survey123: individual reports and summary reports. It's also possible to include both in the same report, in which case they would be individual and summary sections. An individual report displays the data for a single survey record, while a summary report displays data for multiple records in one report ...
The US Supreme Court ruled that Donald Trump has some immunity from criminal charges for trying overturn the 2020 election results. The decision all but ensures that a trial won't happen before ...
This code uses the ServerTextControl to directly generate the binary PDF document without using a printer driver. The PDF file is stored in the file system. The Save method can be used to save the document in various formats including DOCX, DOC, RTF, and PDF.. Creating SVG Images. TX Text Control can also be used to create SVG images from the document pages.
Below is a summary of the API actions and sample their sample responses. 1. Get recommendations . ... Sample workbook. If you need a sample for reference or to get started, you can refer to the Microsoft Sentinel Optimization Workbook as mentioned earlier. Install the workbook from the content hub, save the template, and launch the workbook.
In a testy, personal clash, President Biden failed to ease worries about his age, Donald Trump forcefully made his case (with wild claims and exaggerations) and the moderators held their fact ...