Simplifying Organic Chemistry
Orgosolver provides study tools to help students with their organic chemistry homework and preparation for quizzes, exams, or even the MCAT. Our tools, quizzes, and study guides are designed to help students test every reaction or mechanism with any molecule they draw!

Need help studying for your next exam?
Check out our study guides and our dynamic reaction quizzes, 📋 orgo 1 final exam study guide, 📋 orgo 2 final exam study guide, 📋 reaction quizzes, who we are..
Two former organic chemistry students from the University of Maryland.
While taking Organic Chemistry back in 2009, we often discussed the lack of readily available resources on the web for this course. With the challenges presented by 2020 and the pandemic, we found ourselves again thinking about the ideas that we had discussed in our undergraduate days. Putting our professional skills together, we developed OrgoSolver; the first dynamic Organic Chemistry reaction solver.
Testimonials
Stockton university.
"It helped me with the mechanisms I needed. It allowed me to visualize what reactions I was working with!"
San Francisco State University
"The reaction solver is a great help for both learning reactions and solving retrosynthesis problems."
UC Berkeley
"Orgosolver helped me when nothing else could!"
Questions, Comments, Feedback? Email us at [email protected]

Chemistry Steps
Organic Chemistry
Doing practice problems is the only way to master organic chemistry! At Chemistry Steps, you can find all the topics of Organic 1 and 2 and their associated practice problems . There are more than 1000 practice questions and you can find them after each article listed below.
Here are some examples from the topics shown below.
Note : These are sample practice problems for the new users. Please go to the topics and where you can practice problems after the articles or in a separate post.
Registered members can access all the quizzes and their solutions too.
Organic Chemistry 1 Practice Problems
Structure and bonding practice problems.
Convert the following condensed structures into Bond-line structures :
a) CH 3 CONHCH 2 OCH 3
b) O(CH 2 ) 3 CHCH 3
c) CH 3 CHCHN(CH 3 )CH 2 CH 3
d) (CH 3 ) 3 CCH 2 CH 2 COOCH 2 CH 3
e) CH 3 CH 2 CCCH 2 CO(CH 2 ) 2 N(CH 3 ) 2
f) CH 3 CH 2 C(CH 3 )CHCH(CH 2 ) 4
g) (CH 3 ) 2 CCHCH 2 OCH(CH 3 )CH 2 CN
h) CH 3 CH 2 CHClCHBrCH 2 CONHCH(CH 3 ) 2
i) (CH 3 ) 2 CHCCCH 2 OCH(CH 2 Br)CH 2 CH 3
j) CH 3 NHCHCCH 3 CHClCON(C 2 H 5 ) 2
Answers and Solutiuons
Molecular Representations
Practice Problems
Identify the delocalized lone pairs of electrons for each of the following compounds and draw at least one resonance structure using the delocalized lone pairs.

Answers and Solutions
Acids and Bases Practice Problems
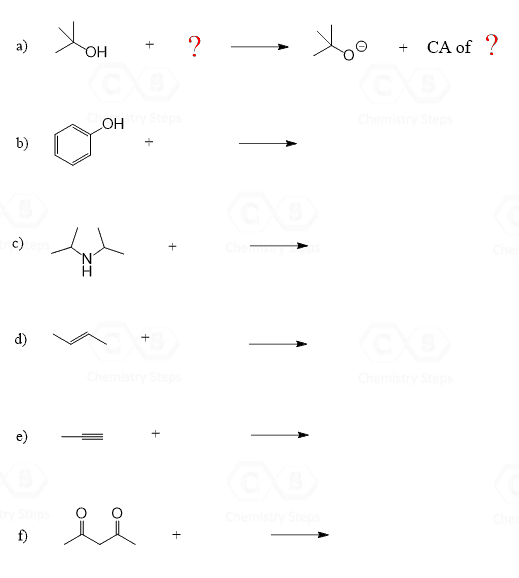
Alkanes and Cycloalkanes Practice Problems
Draw both chair conformation (ring-flip) and use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds:

Stereochemistry Practice Problems
Determine if each of the following alkenes has an E or Z configuration:

Determine the relationship in each of the following pairs. Do the drawings represent constitutional isomers or stereoisomers, or are they just different ways of drawing the same compound? If they are stereoisomers, are they enantiomers or diastereomers? Explain your answer by converting the drawings into the same representation, i.e. if you are comparing a Newman projection to a Fischer projection , you need to convert both into either a Newman or Fischer projection. Assign all the absolute configurations as R or S if you hesitate.

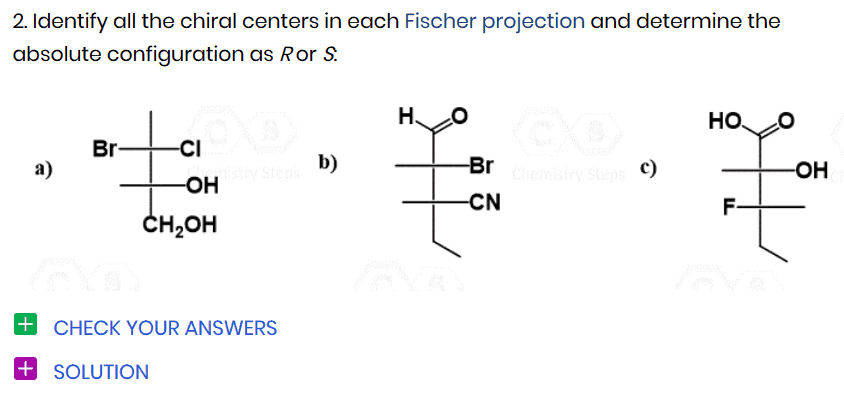
Identify all the chiral centers in each molecule and determine the absolute configuration as R or S :

Nucleophilic Substitution and Elimination Reactions
Predict the mechanism as SN1, SN2, E1 or E2 and draw the major organic product formed in each reaction. Consider any regioselectivity and stereoselectivity where applicable:

Reactions of Alkenes Practice Problems
Identify the reagents for each of the following addition reaction to an alkene:

Predict the product(s) that are formed after each step for reactions A-G . In each case, consider formation of any chiral center(s) and draw all expected stereoisomers.
This is a comprehensive problem that covers the following topics and will serve as a review of all of them:
Substitution and elimination reactions. Particularly, substitution and elimination reactions of alcohols , the regio – and stereochemistry of E2 reactions and E2 reaction of cyclohexanes .
Mesylation and tosylation in Substitution and elimination reactions.
Hydrohalogenation of alkenes according to the Markovnikov’s rule .
Radical hydrohalogenation of alkenes .
Hydroboration-Oxidation of Alkenes .
Halogenation of alkenes through halohydrin formation .
Syn and anti dihydroxilation of alkenes.
Elimination reactions: Zaitsev and Hoffman products .
Ozonoloysis of Alkenes.

Attempt solving the entire problem before accessing the answers!
The answers will give you the structure of the final product(s) only . Use this as a hint to determine the compounds formed after the first and second reactions.
Reactions of Alkynes Practice Problems
Identify the reagents for each of the following reactions of internal and terminal alkynes:

On the following synthetic scheme, identify the reagents, in the correct order, that you would use to achieve the following synthetic transformations. Determine the structure of compounds A and B and the major organic products resulting from the alkyne.

Organic Chemistry 2 Practice Problems
Nmr spectroscopy practice problems.
The 1 H NMR spectrum of compound X ( C 4 H 8 O 2 ) is shown below. It also shows a strong IR absorption band near 1730 cm −1 . Propose a structure for X .

IR Spectroscopy
Label the functional groups and identify the correct compound based on the IR spectrum.

Radicals Practice Problems
Predict the products when each of the following compounds is treated with NBS under UV light:

Alcohols Practice Problems
Predict the major organic product(s) for the following Grignard reactions of a ketone, aldehyde, ester, carbon dioxide and an epoxide:

Predict the major organic product when the following alcohol is treated with each oxidizing agent:

The Diels-Alder Reaction Practice Problems
For each Diels–Alder reaction, predict the major product(s) with correct stereochemistry when each cyclic diene is reacted with a dienophile:

Aromatic Substitution Practice Problems
Show how each compound can be synthesized from benzene and any other organic or inorganic reagents.
The order of reactions is very important! So, before every step, consider the ortho – , para – , or meta directing effect of the current group on the aromatic ring.
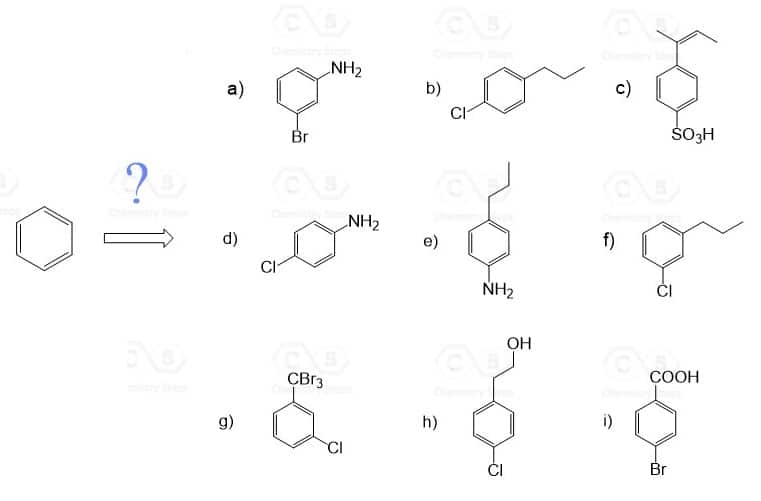
Devise a synthesis of each of the following compounds using an arene diazonium salt. They all require more than one step and you may select the desired regioisomer (for example the para product from an ortho, para mixture) when needed.

Aldehydes and Ketones Practice Problems
Predict the major product(s) obtained when each of the following compounds undergoes hydrolysis in the presence of an acid:
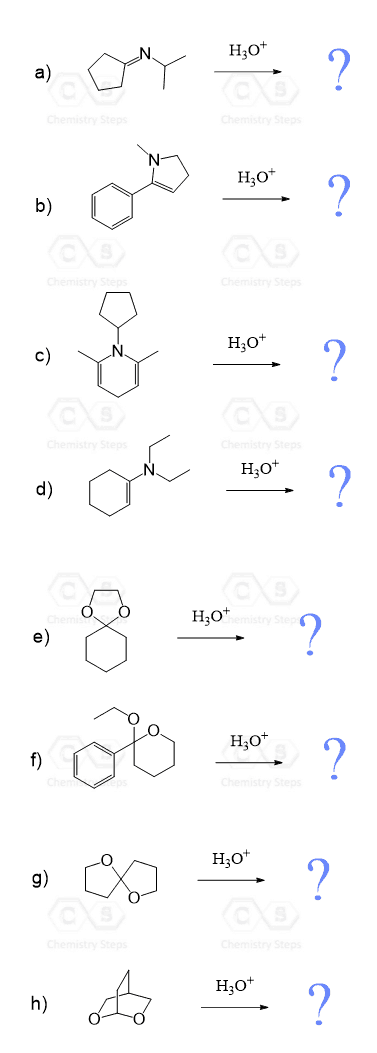
Carboxylic Acids and Their Derivatives Practice Problems
Predict the major organic product(s) for each of the following reactions. They all involve carboxylic acid derivatives such as esters, acid chlorides, nitriles, anhydrides, and amides . You may also need to go over the reactions covered in earlier chapters, particularly, the Grignard and Gilman reagents, oxidizing and reducing agents and electrophilic aromatic substitutions .
A link to each topic encountered in a given problem will be provided in the answer tab.

Alpha Carbon Chemistry – Enols and Enolates Practice Problems
his is a comprehensive practice problem on the alpha carbon chemistry. The topics covered range from the simple halogenation reactions of enols to multistep synthetic transformation .
To correctly answer these questions, you need to review the main principles of enolate chemistry – direct enolate alkylation, aldol condensation, crossed aldol condensation, alkylation using acetoacetic ester synthesis, malonic ester synthesis, the Stork enamine synthesis, Claisen condensation, Michael addition, and Robinson annulation.

Check out our new synthesis puzzles!

CS Prime membership will also grant you access to multiple-choice quizzes!

General Chemistry Overview Quiz
Molecular representations quiz, organic acids and bases quiz, alkanes and cycloalkanes practice quiz, stereochemistry practice problems quiz, nucleophilic substitution and elimination practice quiz, alkene addition reactions practice quiz, alkyne naming and reactions practice quiz, organic chemistry final practice quiz.
Aromatic Compounds
Alcohols Quiz – Naming, Preparation, and Reactions
Aldehydes and ketones reactions practice quiz, carboxylic acids and their derivatives quiz, carbohydrates practice problem quiz, iupac nomenclature summary quiz.
Table of Content
Structure and Bonding
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2 and sp Hybridization
- Molecular and Electron Geometry of Organic Molecules with Practice Problems
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry
- Converting between Bond-line, Lewis and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
Acids and Bases
Organic Acids and Bases
- Organic acid-base mechanisms
- Acid Strength and pKa
- How to Determine the Position of Equilibrium for an Acid–Base Reaction
- Factors That Determine the pKa and Acid Strength
- How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound
- Lewis Acids and Bases
Alkanes and Cycloalkanes
- Naming Alkanes by IUPAC nomenclature Rules Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary Secondary and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- Newman Projections with Practice Problems
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
Stereochemistry
- Enantiomers Diastereomers the Same or Constitutional Isomers with Practice Problems
- Chirality and Enantiomers: Determine if Enantiomers or Identical based on R and S Configuration
- Cis and Trans Stereoisomerism in Alkenes
- E and Z Alkene Configuration with Practice Problems
- How to Determine the R and S configuration
- The R and S Configuration Practice Problems
- Determine the Relationship – Enantiomers Diastereomers or Constitutional Isomers
- Optical Activity
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Calculating Enantiomeric Excess from Optical Activity
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers
Nucleophilic Substitution Reactions
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Is it SN1 SN2 E1 or E2 Mechanism With the Largest Collection of Practice Problems
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Introduction to Substitution Reactions
- All You Need to Know About the S N 2 Reaction Mechanism
- The SN2 Mechanism: Kinetcis, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- Mechanism and Stereochemistry of SN2 Reactions with Practice Problems
- Mesylates and Tosylates as Good Leaving Groups
- SOCl 2 and PBr 3 for Conversion of Alcohols to Alkyl Halides
- The SN1 Nucleophilic Substitution Reaction
- The SN1 Mechanism: Kinetcis, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- The Role of the Solvent in SN1, SN2, E1 and E2 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- When Is the Mechanism SN1 or SN2?
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Alcohols in Substitution Reactions with Tons of Practice Problems
Alkenes: Structure, Stability and Nomenclature
- Naming Alkenes by IUPAC Nomenclature Rules
Elimination Reactions
- General Features of Elimination
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl 3 for Dehydration of Alcohols
- The E1 Mechanism: Kinetcis, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- Dehydration of Alcohols by E1 and E2 Elimination
- Stereoselectivity of E1 Reactions
- Nucleophilic Substitution vs Elimination Reactions
- Regioselectivity of E1 Reactions
- E2 vs. E1 Elimination Mechanism with Practice Problems
Addition Reactions of Alkenes
- Markovnikov’s Rule with Practice Problems
- The Stereochemistry of Alkene Addition Reactions
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Oxymercuration-Demercuration
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Cis product in an anti Addition Reaction of Alkenes
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Addition of Alcohols to Alkenes
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Acid Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
Nuclear Magnetic Resonance (NMR) Spectroscopy
- NMR spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13 C Carbon NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
Organic Structure Determination
- Infrared (IR) Spectroscopy with Lots of Real Spectrum Practice Problems
Radical Reactions
- Initiation Propagation Termination in Radical Reactions
- Selectivity in Radical Halogenation
- Stability of Radicals
- Resonance Structures of Radicals
- Stereochemistry of Radical Halogenation with Practice Problems
- Allylic Bromination by NBS with Practice Problems
- Radical Halogenation in Organic Synthesis
Reactions of Alcohols
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr and HI Acids
- The Williamson Ether Synthesis
- POCl 3 for Dehydration of Alcohols Alcohols in Substitution Reactions with Tons of Practice Problems
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols and Their Use in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern and All of That
- NaIO4 Oxidative Cleavage of Diols
- Preparation of Epoxides
- Reactions of Epoxides Practice Problems
Conjugated Systems
- Resonance and Conjugated Dienes
- Allylic Carbocations
- 1,2 and 1,4 Electrophilic Addition to Dienes
- Kinetic vs Thermodynamic Control of Electrophilic Addition to Dienes
The Diels-Alder Reaction
- Diels Alder Reaction: Dienes and Dienophiles
- Predict the Products of the Diels-Alder Reaction with Practice Problems
- Endo and Exo products of Diels-Alder Reaction with Practice Problems
- Regioselectivity of the Diels–Alder Reaction with Practice Problems
- Identify the Diene and Dienophile of the Diels–Alder Reaction with Practice Problems
- Diels Alder Reaction in Organic Synthesis Practice Problems
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
- Aromaticity and Huckel’s Rule
- Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution – The Mechanism
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta Directors in Electrophilic Aromatic Substitution with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators ?
- Limitations on Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Electrophilic Aromatic Substitution with Arenediazonium Salts
- Reactions at the Benzylic Position
Nucleophilic Aromatic Substitution
Aldehydes and Ketones
- Nomenclature of Aldehydes and Ketones
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- The Addition-Elimination Mechanism
- Reduction of Carbonyl Compounds by Hydride Ion
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- Reactions of Aldehydes and Ketones with Amines-Practice Problems
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- The reaction of Aldehydes and Ketones with CN Cyanohydrin Formation
- Hydrolysis of Acetals, Imines and Enamines-Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction-Practice Problems
Carboxylic Acids and Their Derivatives-Nucleophilic Acyl Substitution
- Preparation of Carboxylic Acids
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids and Their Derivatives Practice Problems
Alpha Carbon Chemistry: Enols and Enolates
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
Organic Synthesis Problems
- Organic Chemistry Multistep Synthesis Practice Problems
All the practice problems are open to everyone for free! I have seen and prepared hundreds of exams for organic chemistry and these practice problems are the types that you will find in your exams . Their difficulty varies from one-step to more advanced ones including step organic synthesis problems.
After working on each question, you have the possibility to first check your answer and then refer to the step-by-step solution .
The practice problems are the only ones you get to do.
Multiple choice quizzes might be the “easy” way of glancing through the key concepts and getting feedback on what you need to work more.
These are also included in your Chemistry Steps membership.
Energy Changes In Organic Chemistry
NMR Spectroscopy
Infrared (IR) Spectroscopy
Ethers and Epoxides
Carboxylic Acids and Their Derivatives
Enolates in Organic Synthesis
Reactions of Amines
Synthesis Maps
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
Have we been helpful? Please let us know in the Reviews section here.
WassUp 1.9.4.5 timestamp: 2024-05-26 01:16:55AM UTC (08:16PM) If above timestamp is not current time, this page is cached.
Ace Your Next Organic Chemistry Exam.
With the moc membership.
1500+ Real-World exam quizzes
180+ Reactions and Mechanisms
200+ Downloadable Flashcards
Organic chemistry is awesome.
Over 400+ blog posts to guide you through introductory Organic Chemistry, organized by subject.
00 General Chemistry Review
- Lewis Structures
- Ionic and Covalent Bonding
- Chemical Kinetics
- Chemical Equilibria
- Valence Electrons of the First Row Elements
- How Concepts Build Up In Org 1 ("The Pyramid")
01 Bonding, Structure, and Resonance
- How Do We Know Methane (CH4) Is Tetrahedral?
- Hybrid Orbitals and Hybridization
- How To Determine Hybridization: A Shortcut
- Orbital Hybridization And Bond Strengths
- Sigma bonds come in six varieties: Pi bonds come in one
- A Key Skill: How to Calculate Formal Charge
- The Four Intermolecular Forces and How They Affect Boiling Points
- 3 Trends That Affect Boiling Points
- How To Use Electronegativity To Determine Electron Density (and why NOT to trust formal charge)
- Introduction to Resonance
- How To Use Curved Arrows To Interchange Resonance Forms
- Evaluating Resonance Forms (1) - The Rule of Least Charges
- How To Find The Best Resonance Structure By Applying Electronegativity
- Evaluating Resonance Structures With Negative Charges
- Evaluating Resonance Structures With Positive Charge
- Exploring Resonance: Pi-Donation
- Exploring Resonance: Pi-acceptors
- In Summary: Evaluating Resonance Structures
- Drawing Resonance Structures: 3 Common Mistakes To Avoid
- How to apply electronegativity and resonance to understand reactivity
- Bond Hybridization Practice
- Structure and Bonding Practice Quizzes
- Resonance Structures Practice
02 Acid Base Reactions
- Introduction to Acid-Base Reactions
- Acid Base Reactions In Organic Chemistry
- The Stronger The Acid, The Weaker The Conjugate Base
- Walkthrough of Acid-Base Reactions (3) - Acidity Trends
- Five Key Factors That Influence Acidity
- Acid-Base Reactions: Introducing Ka and pKa
- How to Use a pKa Table
- The pKa Table Is Your Friend
- A Handy Rule of Thumb for Acid-Base Reactions
- Acid Base Reactions Are Fast
- pKa Values Span 60 Orders Of Magnitude
- How Protonation and Deprotonation Affect Reactivity
- Acid Base Practice Problems
03 Alkanes and Nomenclature
- Meet the (Most Important) Functional Groups
- Condensed Formulas: Deciphering What the Brackets Mean
- Hidden Hydrogens, Hidden Lone Pairs, Hidden Counterions
- Don't Be Futyl, Learn The Butyls
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Branching, and Its Affect On Melting and Boiling Points
- The Many, Many Ways of Drawing Butane
- Wedge And Dash Convention For Tetrahedral Carbon
- Common Mistakes in Organic Chemistry: Pentavalent Carbon
- Table of Functional Group Priorities for Nomenclature
- Summary Sheet - Alkane Nomenclature
- Organic Chemistry IUPAC Nomenclature Demystified With A Simple Puzzle Piece Approach
- Boiling Point Quizzes
- Organic Chemistry Nomenclature Quizzes
04 Conformations and Cycloalkanes
- Staggered vs Eclipsed Conformations of Ethane
- Conformational Isomers of Propane
- Newman Projection of Butane (and Gauche Conformation)
- Introduction to Cycloalkanes (1)
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Calculation of Ring Strain In Cycloalkanes
- Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
- Cyclohexane Conformations
- Cyclohexane Chair Conformation: An Aerial Tour
- How To Draw The Cyclohexane Chair Conformation
- The Cyclohexane Chair Flip
- The Cyclohexane Chair Flip - Energy Diagram
- Substituted Cyclohexanes - Axial vs Equatorial
- Ranking The Bulkiness Of Substituents On Cyclohexanes: "A-Values"
- Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
- Fused Rings - Cis-Decalin and Trans-Decalin
- Naming Bicyclic Compounds - Fused, Bridged, and Spiro
- Bredt's Rule (And Summary of Cycloalkanes)
- Newman Projection Practice
- Cycloalkanes Practice Problems
05 A Primer On Organic Reactions
- The Most Important Question To Ask When Learning a New Reaction
- Learning New Reactions: How Do The Electrons Move?
- The Third Most Important Question to Ask When Learning A New Reaction
- 7 Factors that stabilize negative charge in organic chemistry
- 7 Factors That Stabilize Positive Charge in Organic Chemistry
- Nucleophiles and Electrophiles
- Curved Arrows (for reactions)
- Curved Arrows (2): Initial Tails and Final Heads
- Nucleophilicity vs. Basicity
- The Three Classes of Nucleophiles
- What Makes A Good Nucleophile?
- What makes a good leaving group?
- 3 Factors That Stabilize Carbocations
- Equilibrium and Energy Relationships
- What's a Transition State?
- Hammond's Postulate
- Learning Organic Chemistry Reactions: A Checklist (PDF)
- Introduction to Free Radical Substitution Reactions
- Introduction to Oxidative Cleavage Reactions
06 Free Radical Reactions
- Bond Dissociation Energies = Homolytic Cleavage
- Free Radical Reactions
- 3 Factors That Stabilize Free Radicals
- What Factors Destabilize Free Radicals?
- Bond Strengths And Radical Stability
- Free Radical Initiation: Why Is "Light" Or "Heat" Required?
- Initiation, Propagation, Termination
- Monochlorination Products Of Propane, Pentane, And Other Alkanes
- Selectivity In Free Radical Reactions
- Selectivity in Free Radical Reactions: Bromination vs. Chlorination
- Halogenation At Tiffany's
- Allylic Bromination
- Bonus Topic: Allylic Rearrangements
- In Summary: Free Radicals
- Synthesis (2) - Reactions of Alkanes
- Free Radicals Practice Quizzes
07 Stereochemistry and Chirality
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- How To Draw The Enantiomer Of A Chiral Molecule
- How To Draw A Bond Rotation
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- Assigning R/S To Newman Projections (And Converting Newman To Line Diagrams)
- How To Determine R and S Configurations On A Fischer Projection
- The Meso Trap
- Optical Rotation, Optical Activity, and Specific Rotation
- Optical Purity and Enantiomeric Excess
- What's a Racemic Mixture?
- Chiral Allenes And Chiral Axes
- Stereochemistry Practice Problems and Quizzes
08 Substitution Reactions
- Introduction to Nucleophilic Substitution Reactions
- Walkthrough of Substitution Reactions (1) - Introduction
- Two Types of Nucleophilic Substitution Reactions
- The SN2 Mechanism
- Why the SN2 Reaction Is Powerful
- The SN1 Mechanism
- The Conjugate Acid Is A Better Leaving Group
- Comparing the SN1 and SN2 Reactions
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- Common Blind Spot: Intramolecular Reactions
- The Conjugate Base is Always a Stronger Nucleophile
- Substitution Practice - SN1
- Substitution Practice - SN2
09 Elimination Reactions
- Elimination Reactions (1): Introduction And The Key Pattern
- Elimination Reactions (2): The Zaitsev Rule
- Elimination Reactions Are Favored By Heat
- Two Elimination Reaction Patterns
- The E1 Reaction
- The E2 Mechanism
- E1 vs E2: Comparing the E1 and E2 Reactions
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- Bulky Bases in Elimination Reactions
- Comparing the E1 vs SN1 Reactions
- Elimination (E1) Reactions With Rearrangements
- E1cB - Elimination (Unimolecular) Conjugate Base
- Elimination (E1) Practice Problems And Solutions
- Elimination (E2) Practice Problems and Solutions
10 Rearrangements
- Introduction to Rearrangement Reactions
- Rearrangement Reactions (1) - Hydride Shifts
- Carbocation Rearrangement Reactions (2) - Alkyl Shifts
- Pinacol Rearrangement
- The SN1, E1, and Alkene Addition Reactions All Pass Through A Carbocation Intermediate
11 SN1/SN2/E1/E2 Decision
- Identifying Where Substitution and Elimination Reactions Happen
- Deciding SN1/SN2/E1/E2 (1) - The Substrate
- Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
- SN1 vs E1 and SN2 vs E2 : The Temperature
- Deciding SN1/SN2/E1/E2 - The Solvent
- Wrapup: The Key Factors For Determining SN1/SN2/E1/E2
- Alkyl Halide Reaction Map And Summary
- SN1 SN2 E1 E2 Practice Problems
12 Alkene Reactions
- E and Z Notation For Alkenes (+ Cis/Trans)
- Alkene Stability
- Alkene Addition Reactions: "Regioselectivity" and "Stereoselectivity" (Syn/Anti)
- Stereoselective and Stereospecific Reactions
- Hydrohalogenation of Alkenes and Markovnikov's Rule
- Hydration of Alkenes With Aqueous Acid
- Rearrangements in Alkene Addition Reactions
- Halogenation of Alkenes and Halohydrin Formation
- Oxymercuration Demercuration of Alkenes
- Hydroboration Oxidation of Alkenes
- m-CPBA (meta-chloroperoxybenzoic acid)
- OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes
- Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
- Cyclopropanation of Alkenes
- A Fourth Alkene Addition Pattern - Free Radical Addition
- Alkene Reactions: Ozonolysis
- Summary: Three Key Families Of Alkene Reaction Mechanisms
- Synthesis (4) - Alkene Reaction Map, Including Alkyl Halide Reactions
- Alkene Reactions Practice Problems
13 Alkyne Reactions
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- Partial Reduction of Alkynes With Lindlar's Catalyst
- Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
- Alkyne Hydroboration With "R2BH"
- Hydration and Oxymercuration of Alkynes
- Hydrohalogenation of Alkynes
- Alkyne Halogenation: Bromination, Chlorination, and Iodination of Alkynes
- Alkyne Reactions - The "Concerted" Pathway
- Alkenes To Alkynes Via Halogenation And Elimination Reactions
- Alkynes Are A Blank Canvas
- Synthesis (5) - Reactions of Alkynes
- Alkyne Reactions Practice Problems With Answers
14 Alcohols, Epoxides and Ethers
- Alcohols - Nomenclature and Properties
- Alcohols Can Act As Acids Or Bases (And Why It Matters)
- Alcohols - Acidity and Basicity
- The Williamson Ether Synthesis
- Ethers From Alkenes, Tertiary Alkyl Halides and Alkoxymercuration
- Alcohols To Ethers via Acid Catalysis
- Cleavage Of Ethers With Acid
- Epoxides - The Outlier Of The Ether Family
- Opening of Epoxides With Acid
- Epoxide Ring Opening With Base
- Making Alkyl Halides From Alcohols
- Tosylates And Mesylates
- PBr3 and SOCl2
- Elimination Reactions of Alcohols
- Elimination of Alcohols To Alkenes With POCl3
- Alcohol Oxidation: "Strong" and "Weak" Oxidants
- Demystifying The Mechanisms of Alcohol Oxidations
- Protecting Groups For Alcohols
- Thiols And Thioethers
- Calculating the oxidation state of a carbon
- Oxidation and Reduction in Organic Chemistry
- Oxidation Ladders
- SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi
- Alcohol Reactions Roadmap (PDF)
- Alcohol Reaction Practice Problems
- Epoxide Reaction Quizzes
- Oxidation and Reduction Practice Quizzes
15 Organometallics
- What's An Organometallic?
- Formation of Grignard and Organolithium Reagents
- Organometallics Are Strong Bases
- Reactions of Grignard Reagents
- Protecting Groups In Grignard Reactions
- Synthesis Problems Involving Grignard Reagents
- Grignard Reactions And Synthesis (2)
- Organocuprates (Gilman Reagents): How They're Made
- Gilman Reagents (Organocuprates): What They're Used For
- The Heck, Suzuki, and Olefin Metathesis Reactions (And Why They Don't Belong In Most Introductory Organic Chemistry Courses)
- Reaction Map: Reactions of Organometallics
- Grignard Practice Problems
16 Spectroscopy
- Degrees of Unsaturation (or IHD, Index of Hydrogen Deficiency)
- Conjugation And Color (+ How Bleach Works)
- Introduction To UV-Vis Spectroscopy
- UV-Vis Spectroscopy: Absorbance of Carbonyls
- UV-Vis Spectroscopy: Practice Questions
- Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model
- Infrared Spectroscopy: A Quick Primer On Interpreting Spectra
- IR Spectroscopy: 4 Practice Problems
- 1H NMR: How Many Signals?
- Homotopic, Enantiotopic, Diastereotopic
- Diastereotopic Protons in 1H NMR Spectroscopy: Examples
- C13 NMR - How Many Signals
- Liquid Gold: Pheromones In Doe Urine
- Natural Product Isolation (1) - Extraction
- Natural Product Isolation (2) - Purification Techniques, An Overview
- Structure Determination Case Study: Deer Tarsal Gland Pheromone
17 Dienes and MO Theory
- What To Expect In Organic Chemistry 2
- Are these molecules conjugated?
- Conjugation And Resonance In Organic Chemistry
- Bonding And Antibonding Pi Orbitals
- Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion
- Pi Molecular Orbitals of Butadiene
- Reactions of Dienes: 1,2 and 1,4 Addition
- Thermodynamic and Kinetic Products
- More On 1,2 and 1,4 Additions To Dienes
- s-cis and s-trans
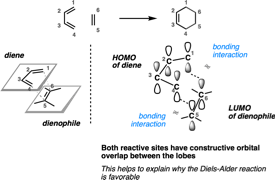
- The Diels-Alder Reaction
- Cyclic Dienes and Dienophiles in the Diels-Alder Reaction
- Stereochemistry of the Diels-Alder Reaction
- Exo vs Endo Products In The Diels Alder: How To Tell Them Apart
- HOMO and LUMO In the Diels Alder Reaction
- Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
- Diels-Alder Reaction: Kinetic and Thermodynamic Control
- The Retro Diels-Alder Reaction
- The Intramolecular Diels Alder Reaction
- Regiochemistry In The Diels-Alder Reaction
- The Cope and Claisen Rearrangements
- Electrocyclic Reactions
- Electrocyclic Ring Opening And Closure (2) - Six (or Eight) Pi Electrons
- Diels Alder Practice Problems
- Molecular Orbital Theory Practice
18 Aromaticity
- Introduction To Aromaticity
- Rules For Aromaticity
- Huckel's Rule: What Does 4n+2 Mean?
- Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems
- Antiaromatic Compounds and Antiaromaticity
- The Pi Molecular Orbitals of Benzene
- The Pi Molecular Orbitals of Cyclobutadiene
- Frost Circles
- Aromaticity Practice Quizzes
19 Reactions of Aromatic Molecules
- Electrophilic Aromatic Substitution: Introduction
- Activating and Deactivating Groups In Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution - The Mechanism
- Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution
- Understanding Ortho, Para, and Meta Directors
- Why are halogens ortho- para- directors?
- Disubstituted Benzenes: The Strongest Electron-Donor "Wins"
- Electrophilic Aromatic Substitutions (1) - Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) - Nitration and Sulfonation
- EAS Reactions (3) - Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Nucleophilic Aromatic Substitution (NAS)
- Nucleophilic Aromatic Substitution (2) - The Benzyne Mechanism
- Reactions on the "Benzylic" Carbon: Bromination And Oxidation
- The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions
- More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger
- Aromatic Synthesis (1) - "Order Of Operations"
- Synthesis of Benzene Derivatives (2) - Polarity Reversal
- Aromatic Synthesis (3) - Sulfonyl Blocking Groups
- Birch Reduction
- Synthesis (7): Reaction Map of Benzene and Related Aromatic Compounds
- Aromatic Reactions and Synthesis Practice
- Electrophilic Aromatic Substitution Practice Problems
20 Aldehydes and Ketones
- What's The Alpha Carbon In Carbonyl Compounds?
- Nucleophilic Addition To Carbonyls
- Aldehydes and Ketones: 14 Reactions With The Same Mechanism
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones
- Grignard Reagents For Addition To Aldehydes and Ketones
- Wittig Reaction
- Hydrates, Hemiacetals, and Acetals
- Imines - Properties, Formation, Reactions, and Mechanisms
- All About Enamines
- Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles (Part 2)
- Aldehydes Ketones Reaction Practice
21 Carboxylic Acid Derivatives
- Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)
- Addition-Elimination Mechanisms With Neutral Nucleophiles (Including Acid Catalysis)
- Basic Hydrolysis of Esters - Saponification
- Transesterification
- Proton Transfer
- Fischer Esterification - Carboxylic Acid to Ester Under Acidic Conditions
- Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
- LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes
- Di-isobutyl Aluminum Hydride (DIBAL) For The Partial Reduction of Esters and Nitriles
- Amide Hydrolysis
- Thionyl Chloride (SOCl2)
- Diazomethane (CH2N2)
- Carbonyl Chemistry: Learn Six Mechanisms For the Price Of One
- Making Music With Mechanisms (PADPED)
- Carboxylic Acid Derivatives Practice Questions
22 Enols and Enolates
- Keto-Enol Tautomerism
- Enolates - Formation, Stability, and Simple Reactions
- Kinetic Versus Thermodynamic Enolates
- Aldol Addition and Condensation Reactions
- Reactions of Enols - Acid-Catalyzed Aldol, Halogenation, and Mannich Reactions
- Claisen Condensation and Dieckmann Condensation
- Decarboxylation
- The Malonic Ester and Acetoacetic Ester Synthesis
- The Michael Addition Reaction and Conjugate Addition
- The Robinson Annulation
- Haloform Reaction
- The Hell–Volhard–Zelinsky Reaction
- Enols and Enolates Practice Quizzes
- The Amide Functional Group: Properties, Synthesis, and Nomenclature
- Basicity of Amines And pKaH
- 5 Key Basicity Trends of Amines
- The Mesomeric Effect And Aromatic Amines
- Nucleophilicity of Amines
- Alkylation of Amines (Sucks!)
- Reductive Amination
- The Gabriel Synthesis
- Some Reactions of Azides
- The Hofmann Elimination
- The Hofmann and Curtius Rearrangements
- The Cope Elimination
- Protecting Groups for Amines - Carbamates
- The Strecker Synthesis of Amino Acids
- Introduction to Peptide Synthesis
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions
- Amine Practice Questions
24 Carbohydrates
- D and L Notation For Sugars
- Pyranoses and Furanoses: Ring-Chain Tautomerism In Sugars
- What is Mutarotation?
- Reducing Sugars
- The Big Damn Post Of Carbohydrate-Related Chemistry Definitions
- The Haworth Projection
- Converting a Fischer Projection To A Haworth (And Vice Versa)
- Reactions of Sugars: Glycosylation and Protection
- The Ruff Degradation and Kiliani-Fischer Synthesis
- Isoelectric Points of Amino Acids (and How To Calculate Them)
- Carbohydrates Practice
- Amino Acid Quizzes
25 Fun and Miscellaneous
- A Gallery of Some Interesting Molecules From Nature
- Screw Organic Chemistry, I'm Just Going To Write About Cats
- On Cats, Part 1: Conformations and Configurations
- On Cats, Part 2: Cat Line Diagrams
- On Cats, Part 4: Enantiocats
- On Cats, Part 6: Stereocenters
- Organic Chemistry Is Shit
- The Organic Chemistry Behind "The Pill"
- Maybe they should call them, "Formal Wins" ?
- Why Do Organic Chemists Use Kilocalories?
- The Principle of Least Effort
- Organic Chemistry GIFS - Resonance Forms
- Reproducibility In Organic Chemistry
- What Holds The Nucleus Together?
- How Reactions Are Like Music
- Organic Chemistry and the New MCAT
26 Organic Chemistry Tips and Tricks
- Common Mistakes: Formal Charges Can Mislead
- Partial Charges Give Clues About Electron Flow
- Draw The Ugly Version First
- Organic Chemistry Study Tips: Learn the Trends
- The 8 Types of Arrows In Organic Chemistry, Explained
- Top 10 Skills To Master Before An Organic Chemistry 2 Final
- Common Mistakes with Carbonyls: Carboxylic Acids... Are Acids!
- Planning Organic Synthesis With "Reaction Maps"
- Alkene Addition Pattern #1: The "Carbocation Pathway"
- Alkene Addition Pattern #2: The "Three-Membered Ring" Pathway
- Alkene Addition Pattern #3: The "Concerted" Pathway
- Number Your Carbons!
- The 4 Major Classes of Reactions in Org 1
- How (and why) electrons flow
- Grossman's Rule
- Three Exam Tips
- A 3-Step Method For Thinking Through Synthesis Problems
- Putting It Together
- Putting Diels-Alder Products in Perspective
- The Ups and Downs of Cyclohexanes
- The Most Annoying Exceptions in Org 1 (Part 1)
- The Most Annoying Exceptions in Org 1 (Part 2)
- The Marriage May Be Bad, But the Divorce Still Costs Money
- 9 Nomenclature Conventions To Know
- Nucleophile attacks Electrophile

27 Case Studies of Successful O-Chem Students
- Success Stories: How Corina Got The The "Hard" Professor - And Got An A+ Anyway
- How Helena Aced Organic Chemistry
- From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around
- How Serge Aced Organic Chemistry
- Success Stories: How Zach Aced Organic Chemistry 1
- Success Stories: How Kari Went From C– to B+
- How Esther Bounced Back From a "C" To Get A's In Organic Chemistry 1 And 2
- How Tyrell Got The Highest Grade In Her Organic Chemistry Course
- This Is Why Students Use Flashcards
- Success Stories: How Stu Aced Organic Chemistry
- How John Pulled Up His Organic Chemistry Exam Grades
- Success Stories: How Nathan Aced Organic Chemistry (Without It Taking Over His Life)
- How Chris Aced Org 1 and Org 2
- Interview: How Jay Got an A+ In Organic Chemistry
- How to Do Well in Organic Chemistry: One Student's Advice
- "America's Top TA" Shares His Secrets For Teaching O-Chem
- "Organic Chemistry Is Like..." - A Few Metaphors
- How To Do Well In Organic Chemistry: Advice From A Tutor
- Guest post: "I went from being afraid of tests to actually looking forward to them".
See what’s inside
Always rigorous....
Often Irreverent...

Occasionally Profane
Saint Louis University
I had a test for orgo in exactly one week. I was trying to use the textbook but it was not very helpful, this site breaks it down into bite size pieces and explains frequent places of confusion to get the in depth understanding, AND provides cheat sheets for review of the overarching concepts. This is just awesome.

Courtney E.
Central Washington University
I stumbled across this website a couple years ago, seeking help in preparation for my general chemistry ACS exam, and it was helpful then so I've remembered it again and again for other classes, and am glad I can use it again now for organic chemistry.

Utah State University
I love everything I've used so far. I used the reaction guide for O Chem 1, and it saved my butt.

Wright State University
We had final exams this week and I just thought I would let you know that with the help of your fantastic website and the summary sheets I purchased, I finished organic chemistry 1 with a 99.5 average . Classmates were upset with me for being the curve buster. I blamed it on you and derected many of them to check out your website!

University of Pittsburgh
MOC was my best friend this past semester. Wouldn’t have gotten an A- without it.

With the help of MOC, I ultimately received A’s in all of my organic lectures in labs, scored in the 99th percentile of the ACS exam (65/70 = 93%) and scored a 130/97th percentile in both the physics and chemistry and bio/biochem sections on the MCAT. Thank you.

McMurdo Station, Antarctica
The site has been especially helpful for me in teaching basic O-chem and medicinal chemistry down here in Antarctica. (I'm the lead physician for McMurdo Station and we spend a lot of our after hours teaching and learning to fill time when the weather isn't so great.) From the bottom of the world - thanks!

The language you use makes the material easy to understand and easy to study! Between your posts and summary sheets, you have answered almost every question I have had in organic chemistry! MOC is such a lifesaver (and grade-saver)!

Cincinnati Public High School
I am a high school teacher in Cincinnati. I always start my class on MOC.

St. Joseph's University
I'm a biologist who has worked in chem labs most of my career. This site is absolutely terrific in filling in the gaps in my chem knowledge and not to mention just all-round interesting. Keep up the good work!

California State University Long Beach
This website has become my savior. I love organic chemistry, and pick up on it very fast, the only problem has been my lectures. Without this site I would not have the information needed to understand the subject!

Chemistry Olympiad
I was selected to represent my country in the International Chemistry Olympiad in 2017 and in 2018. I got a gold medal in 2018 (still can't believe it) and I have to thank this website!

LaMar University
The cheat sheets have been a great reference for my studies, as well as the reaction guide. Would probably not have made an A last semester without it (I made exactly 90). I did it with the more difficult organic professor too!

Thank you so much for such an awesome site! This is the reason I got an A in ochem one and two! Thank you!

West Chester University
I recently purchased the organic chemistry guide. I finally understand why things are doing what they are doing. I wish I would've had this numerous organic chemistry attempts ago.

Binghampton U.
These guides are awesome. Clear and concise. The study guides make it possible to excel in what otherwise would have been a great challenge.

Cal State East Bay
I have recommended your site to many of my classmates who have asked me "what my secret was"! Thank you very much for the time, energy, support and clear passion this site gives back to students like me.

TaraRochfordNutrition.com
The study materials on MOC were a lifesaver. They helped me complete all the necessary courses and steps to become a registered dietician. Thank you so much!

Penn State University
A friend at my university informed me about this website and said it was the only reason he passed organic chemistry!

Wayne State University
Thank you, not only for preventing OChem from being a "weeder" class for me, but also for making it an enjoyable journey. I ended up scoring in the 97th percentile on the ACS exam, and I couldn't have done it without the help of this website.

Christian M.
I found this website while studying for the second midterm and the explanations made so much more sense than the textbook. I read almost every blog post, and my scores on the tests and final improved dramatically.

Manuel E. (with Nobel Laureate Kip Thorne)
East Los Angeles College
The study guides have helped me in so many angles that helped me improve my grades, lessen my anxiety, and improved my overall confidence. Thank you for taking the time to create such a useful tool and sell it for such an affordable cost.

University of Buffalo
For a night owl who really only studies at night, it is often impossible to find a professor or TA who is awake to extinguish my burning questions, and even difficult to find a knowledgable friend. This site is that friend.

University of Connecticut
I went from a 40 on exam 2 to a 90 on exam 3 as a result of focusing on the big picture and applying the concepts to the questions. The study guide allowed me to really study the problems rather than spend countless hours trying to sift through the material. I also ended up with a B+ in the class!

Michelle T.
Simon Fraser University
Master organic chemistry literally taught me everything I needed to know for my ochem 1 course. I just wish I found it earlier! Would've helped me so much on my midterm.

Virginia Commonwealth University
The summary sheets let me quickly review everything I needed for my final exam. I was able to score a 54/70 on a final where the class average was 20/70.

University of South Florida
Thank you so much for this service! I finished Orgo 2 with a B+ because of these guides!

Florida Atlantic U.
Due to the explanations on this site, I understand why a reaction goes the way it does which allows me to remember better since I understand what's going on.

Lee University
I made an A in my Organic 2 class - this website was an invaluable resource!! Thanks so much.

Colorado State University
By the way I wish I would have made the move on your joining your site earlier in the semester. It would have made things a little bit easier. I joined about 2 weeks before finals and ended up getting an A in the class!
Case Studies Of Successful Students
Last semester I had the pleasure of working with “Chris” (a pseudonym) on second-semester organic chemistry topics. Before we met, Chris had already obtained a
How Helena Got 93 In Organic Chemistry An Australian reader, Helena, recently wrote to say she’d earned a 93 in her organic chemistry class as
Over the past few weeks I’ve been corresponding with loyal reader Serge, who was happy to report to me recently that his exam grades are
From The Blog
Reaction maps now available.

350 Examples of Classic Org 1/Org 2 Reactions From “Organic Syntheses”

Organic Chemistry GIFS – Resonance Forms
James Ashenhurst
Founder, master organic chemistry.
After doing a Ph. D. in organic synthesis at McGill and a postdoc at MIT, I applied for faculty positions at universities during the Great Recession. It didn’t work out. But having seen first-hand how many people struggled with the subject (including myself when I took it as an undergraduate) I thought there was a need for an online organic chemistry resource that had all the rigor of a traditional textbook, but was more approachable and accessible.
Drawing on the experience of thousands of hours spent tutoring students 1-on-1, as well as dozens of case studies, Master Organic Chemistry aims to fill in some of the conceptual gaps that aren’t traditionally covered by textbooks, and provide a friendly, logical and thorough pathway for learning introductory organic chemistry.

The following problems are meant to be useful study tools for students involved in most undergraduate organic chemistry courses. The problems have been color-coded to indicate whether they are:
1. Generally useful , 2. Most likely to be useful to students in year long, rather than survey courses , 3. Most likely to be useful only to students in courses for chemistry majors and/or honors students .
Some of these problems make use of a Molecular Editor drawing application. To practice using this editor Click Here .
Most of these Interactive Organic Chemistry Practice Problems have been developed by Professor William Reusch. �1999 William Reusch, All rights reserved. Comments, questions and errors should be sent to [email protected] . The Virtual Text and some of the problems make use of either the CHIME plugin , or Jmol . Click on the name for information and a free copy. If possible, monitor resolutions of 1024 x 768 or 1152 x 870 should be used.
Department of Chemistry Michigan State University East Lansing, MI 48824
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Organic chemistry
A brief introduction to organic chemistry, unit 1: structure and bonding, unit 2: resonance and acid-base chemistry, unit 3: alkanes, cycloalkanes, and functional groups, unit 4: stereochemistry, unit 5: substitution and elimination reactions, unit 6: alkenes and alkynes, unit 7: alcohols, ethers, epoxides, sulfides, unit 8: conjugated systems and pericyclic reactions, unit 9: aromatic compounds, unit 10: aldehydes and ketones, unit 11: carboxylic acids and derivatives, unit 12: alpha carbon chemistry, unit 13: amines, unit 14: spectroscopy.

This section contains various syntheses problems suitable for sophomore (introductory) level organic chemistry students. The synthesis complexity will generally increase down to list but the complexity level increase is not linear, so you might find some syntheses more challenging than the other ones. This list generally follows the reactions you would expect to see in a typical organic chemistry course in the approximate sequence of how they are typically taught. Of course, the curricula are not universal across the schools, so you might find that some that you haven’s seen before are used early on. Don’t get discouraged though. I have a writeup of all reactions you’ll see in these pages if you want to refresh your memory or learn a new reaction!
How to Practice Synthesis
I provide a possible answer for each synthesis here. But I encourage you to start the video for each problem and then pause it right after the synthesis problem is introduced. Give it a try and don’t get tempted by the answer below the video! Better, if you minimize the window as soon as you copy the problem onto your paper.
Once you have your answer, compare it to what I give for each problem. Feel free to share your ideas and alternatives in the comments below. I love the amazing discussions I have with you on YouTube and I encourage you to do the same here!
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Username or Email Address
Remember Me

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.13.2. Practice Problems
- Last updated
- Save as PDF
- Page ID 35009
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Propose a substitution mechanism for the following reactions. Pay special attention to stereochemistry if indicated. Look at the conditions given to determine if the substitution is unimolecular or bimolecular (SN 1 or SN 2 ).
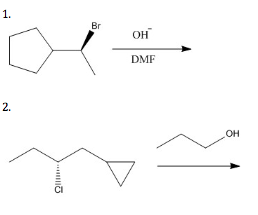
Propose an elimination mechanism for the following reactions. Pay special attention to stereochemistry if indicated. Look at the conditions given to determine if the elimination is unimolecular or bimolecular (E 1 or E 2 ).
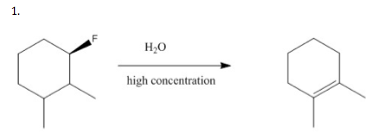
Show the major elimination product and draw the mechanism for each of the following reactions.
Additional Resources
Michigan State Virtual Textbook of Organic Chemistry
Practice Problems
Carey 4 th Edition On-Line Activity
Good set of problems
Practice problems
Additional Problems
17 • Additional Problems
Visualizing Chemistry
Predict the product from reaction of the following substance (reddish brown = Br) with:
Predict the product from reaction of the following substance with:
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethylmagnesium bromide. Is the product chiral? Is it optically active? Explain.
Mechanism Problems
Evidence for the intermediate carbocations in the acid-catalyzed dehydration of alcohols comes from the observation that rearrangements sometimes occur. Propose a mechanism to account for the formation of 2,3-dimethyl-2-butene from 3,3-dimethyl-2-butanol.
Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields a mixture of 1,2-dimethylcyclohexene and isopropylidenecyclopentane. Propose a mechanism to account for the formation of both products.
Epoxides react with Grignard reagents to yield alcohols. Propose a mechanism.
Treatment of the following epoxide with aqueous acid produces a carbocation intermediate that reacts with water to give a diol product. Show the structure of the carbocation, and propose a mechanism for the second step.
When the following alcohol is treated with POCl 3 and pyridine, the expected elimination product is formed. However, when the same alcohol is treated with H 2 SO 4 , the elimination product is 1,2-dimethylcyclopentene. Propose a mechanism for each pathway to account for these differences.
Naming Alcohols
Carvacrol is a naturally occurring substance isolated from oregano, thyme, and marjoram. What is its IUPAC name?
Synthesizing Alcohols
Reactions of Alcohols
Spectroscopy
The following 1 H NMR spectrum is that of an alcohol, C 8 H 10 O. Propose a structure.
Propose a structure consistent with the following spectral data for a compound C 8 H 18 O 2 :
- IR: 3350 cm –1
- 1 H NMR: 1.24 δ (12 H, singlet); 1.56 δ (4 H, singlet); 1.95 δ (2 H, singlet)
The 1 H NMR spectrum shown is that of 3-methyl-3-buten-1-ol. Assign all the observed resonance peaks to specific protons, and account for the splitting patterns.
A compound of unknown structure gave the following spectroscopic data:
- Mass spectrum: M + = 88.1
- IR: 3600 cm –1
- 1 H NMR: 1.4 δ (2 H, quartet, J = 7 Hz); 1.2 δ (6 H, singlet); 1.0 δ (1 H, singlet); 0.9 δ (3 H, triplet, J = 7 Hz)
- 13 C NMR: 74, 35, 27, 25 δ
Propose a structure for a compound C 15 H 24 O that has the following 1 H NMR spectrum. The peak marked by an asterisk disappears when D 2 O is added to the sample.
General Problems
Rank the following substituted phenols in order of increasing acidity, and explain your answer:
Benzyl chloride can be converted into benzaldehyde by treatment with nitromethane and base. The reaction involves initial conversion of nitromethane into its anion, followed by S N 2 reaction of the anion with benzyl chloride and subsequent E2 reaction. Write the mechanism in detail, using curved arrows to indicate the electron flow in each step.
Reaction of ( S )-3-methyl-2-pentanone with methylmagnesium bromide followed by acidification yields 2,3-dimethyl-2-pentanol. What is the stereochemistry of the product? Is the product optically active?
Testosterone is one of the most important male steroid hormones. When testosterone is dehydrated by treatment with acid, rearrangement occurs to yield the product shown. Propose a mechanism to account for this reaction.
p -Nitrophenol and 2,6-dimethyl-4-nitrophenol both have p K a = 7.15, but 3,5-dimethyl-4-nitrophenol has p K a = 8.25. Why is 3,5-dimethyl-4-nitrophenol so much less acidic?
Compound A , C 5 H 10 O, is one of the basic building blocks of nature. All steroids and many other naturally occurring compounds are built from compound A . Spectroscopic analysis of A yields the following information:
- IR: 3400 cm –1 ; 1640 cm –1
- 1 H NMR: 1.63 δ (3 H, singlet); 1.70 δ (3 H, singlet); 3.83 δ (1 H, broad singlet); 4.15 δ (2 H, doublet, J = 7 Hz); 5.70 δ (1 H, triplet, J = 7 Hz)
2,3-Dimethyl-2,3-butanediol has the common name pinacol. On heating with aqueous acid, pinacol rearranges to pinacolone, 3,3-dimethyl-2-butanone. Suggest a mechanism for this reaction.
Propose a synthesis of bicyclohexylidene, starting from cyclohexanone as the only source of carbon.
A problem often encountered in the oxidation of primary alcohols to carboxylic acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate:
Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:
Identify the reagents a – f in the following scheme:
Galactose, a constituent of the disaccharide lactose found in dairy products, is metabolized by a pathway that includes the isomerization of UDP-galactose to UDP-glucose, where UDP = uridylyl diphosphate. The enzyme responsible for the transformation uses NAD + as cofactor. Propose a mechanism.
C 8 H 10 O 2
Compound A , C 8 H 10 O, has the IR and 1 H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 δ disappears when D 2 O is added.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution-NonCommercial-ShareAlike License and you must attribute OpenStax.
Access for free at https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Authors: John McMurry, Professor Emeritus
- Publisher/website: OpenStax
- Book title: Organic Chemistry
- Publication date: Sep 20, 2023
- Location: Houston, Texas
- Book URL: https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Section URL: https://openstax.org/books/organic-chemistry/pages/17-additional-problems
© Jan 9, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

Organic Chemistry Problems

Welcome to Organic Chemistry Problems!
This web page is intended to be a resource for students, instructors, and practitioners of organic synthesis. Primarily, this resource is intended to provide extra example problems for students at the introductory graduate student level (i.e. first year graduate students who are in an organic synthesis course who have prior experience with organic chemistry) or advanced undergraduate level. The provided problems are intended to cover a number of key concepts including complex arrow pushing mechanisms, diastereoselective synthesis, and the uses of common reagents. This web page IS NOT intended to be a repository of named reactions, total syntheses, or reagents. Other such resources exist and links are provided to them on “external links” page. There are a number of features of this page that you may find useful.
Reaction Problems
The Additions, Reactions on Rings, and Other tabs serve contain the questions for the “quiz” portion for students who would like to work through the problem in detail on their own. Several variety of questions can be accessed by clicking on the title on the top of the page or by scrolling through the examples. The general question is to determine the product of the reaction(s) - including stereochemistry. For ease, questions are categorized by the functional group or motif undergoing the reaction. For most of these reactions, an established predictive model exists, such as A1,3 strain, Felkin-Ahn model, Cram’s chelation control model, directing group effects, or concave-convex differentiation. Alternatively, a number of reactions are provided with the corresponding product and students can attempt to provide a reasonable arrow pushing mechanism by which the product could be formed. The final class of questions provides students with a starting material and a sequence of common reagents/reactions. Students can attempt to predict the product of each subsequent step of these common synthetic reactions in a “road map” style, similar to what would appear in a journal article.
Reaction Answers
Answers are provided for all of the reactions can be found by clicking on the question's image. Each product is drawn with a predictive model that could be used to rationalize the formation of that compound. The primary reference containing the reaction is linked and can be accessed by clicking “Reference”. One additional feature that will likely be useful for instructors or practitioners is that the ChemDraw file is freely accessible and can be downloaded by clicking on “ChemDraw” and then the “download” button from the pop up page.
External Links
A number of wonderful web sites already have repositories for total syntheses, named reactions, reagents, or other information. Links to a wide range of organic chemistry related sites are provided on this page.
Contact Info
If you find an error, typo, or have other questions or comments about the site, please contact [email protected] . If you would like to submit a reaction, sequence, or mechanism please do so to the same email. All that is needed is your name and a chem draw file containing the full sequence, DOI of the primary source, and your name (so that we can add “contributed by ____” to the file). Please keep in mind that the problems are intended for first year graduate students. Obscure reagents, unduly complex mechanisms, or reactions involving multiple predictive models will not be suitable for this audience. That is about it! Please enjoy the site and all of the wonders and challenges that organic synthesis has to offer!
External Links
Send e-mail: [email protected].

- Science & Math

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Follow the author

Image Unavailable

- To view this video download Flash Player

The Art of Problem Solving in Organic Chemistry 2nd Edition
There is a newer edition of this item:.

- Teaches organic chemists structured and logical techniques to solve reaction problems and uses a unique, systematic approach.
- Stresses the logic and strategy of mechanistic problem solving -- a key piece of success for organic chemistry, beyond just specific reactions and facts
- Has a conversational tone and acts as a readable and approachable workbook allowing reader involvement instead of simply straightforward text
- Uses 60 solved and worked-through problems and reaction schemes for students to practice with, along with updated organic reactions and illustrated examples
- Includes website with supplementary material for chapters and problems: tapsoc.yolasite.com
- ISBN-10 9781118530214
- ISBN-13 978-1118530214
- Edition 2nd
- Publisher Wiley
- Publication date August 25, 2014
- Language English
- Dimensions 6.15 x 0.95 x 9.3 inches
- Print length 480 pages
- See all details

Editorial Reviews
“Summing Up: Highly recommended. Graduate students and above.” ( Choice , 1 May 2015)
From the Inside Flap
Multiple solutions are put forth for each problem, with related experimental evidence and critical reviews of organic chemistry methods. In addition, the book also includes supplementary material for the chapters and problems, along with useful links that can be found at http://tapsoc.yolasite. com. Advanced undergraduate and graduate students, as well as professionals in chemistry will benefit from this new edition.
From the Back Cover
About the author.
Miguel E. Alonso-Amelot is a Professor of Organic and Ecological Chemistry at the Universidad de Los Andes in Venezuela. With over 40 years of teaching experience, he has also led courses on these topics in the US, Europe, and Latin America. His previous research interests include the theory and application of metal carbenoids in cyclopropanes and heterocycles. Currently, he focuses on chemical ecology of tropical mountain ecosystems, and is a consultant on organic chemistry supporting plant natural product bioactivities. Among his publications, Dr. Alonso has written over 90 research articles, five book chapters, and four books, including the First Edition of The Art of Problem Solving in Organic Chemistry , published by Wiley.
Product details
- ASIN : 1118530217
- Publisher : Wiley; 2nd edition (August 25, 2014)
- Language : English
- Paperback : 480 pages
- ISBN-10 : 9781118530214
- ISBN-13 : 978-1118530214
- Item Weight : 1.5 pounds
- Dimensions : 6.15 x 0.95 x 9.3 inches
- #1,615 in Organic Chemistry (Books)
- #1,708 in Physical & Theoretical Chemistry (Books)
- #5,239 in Chemistry (Books)
About the author
Miguel e. alonso-amelot.
M. Alonso's (Caracas, 1946) life has moved along two parallel lines: one as scientist (organic chemistry of natural systems and ecology —PhD from Indiana University— and the arts, thanks to a balanced combination of his four chief passions: family, classic piano, trekking, and scientific research. He has lived in various countries and lives in Spain since 2008. He has published nearly 120 scientific papers and 4 books as a university professor, whereas a rather large collection of short stories compose his narrative expression. Ten of these have been awarded in as many international literature contests, and have been published in anthology books. The novel, “Un dragon en el convento” (A dragon in the convent)(2021), now available in Amazon, is the most recent addition to his narrative production. Details about Alonso can be expanded in:
Science: http://alonso-amelot.yolasite.com, and Literature: https://mea465.wixsite.com/miguel-e-alonso as well as the blog http://menriqlacroix.wordpress.com written under his alias Menriq La Croix
Customer reviews
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
- Sort reviews by Top reviews Most recent Top reviews
Top reviews from the United States
There was a problem filtering reviews right now. please try again later..
- Amazon Newsletter
- About Amazon
- Accessibility
- Sustainability
- Press Center
- Investor Relations
- Amazon Devices
- Amazon Science
- Sell on Amazon
- Sell apps on Amazon
- Supply to Amazon
- Protect & Build Your Brand
- Become an Affiliate
- Become a Delivery Driver
- Start a Package Delivery Business
- Advertise Your Products
- Self-Publish with Us
- Become an Amazon Hub Partner
- › See More Ways to Make Money
- Amazon Visa
- Amazon Store Card
- Amazon Secured Card
- Amazon Business Card
- Shop with Points
- Credit Card Marketplace
- Reload Your Balance
- Amazon Currency Converter
- Your Account
- Your Orders
- Shipping Rates & Policies
- Amazon Prime
- Returns & Replacements
- Manage Your Content and Devices
- Recalls and Product Safety Alerts
- Conditions of Use
- Privacy Notice
- Consumer Health Data Privacy Disclosure
- Your Ads Privacy Choices
- Skip to Guides Search
- Skip to breadcrumb
- Skip to main content
- Skip to footer
- Skip to chat link
- Report accessibility issues and get help
- Go to Penn Libraries Home
- Go to Franklin catalog
Practice Problems in Math and Science: Organic Chemistry
- Practice problems
Organic Chemistry 241 and 242 Books on Reserve
Meet your librarian

Chemistry Library
- Chemistry Library Homepage

Fall and Spring Semesters
Monday-Thursday: 9 a.m. – 10 p.m.
Friday: 9 a.m. – 7 p.m.
Saturday: 10 a.m. – 4 p.m.
Sunday: 12 a.m. – 7 p.m.
Summer Sessions
Monday-Thursday: 9 a.m. – 7 p.m.
Friday: 9 a.m. – 5 p.m.
Saturday: 12 a.m. – 5 p.m.
Sunday: Closed
Intersessions
Monday-Friday: 9 a.m. – 5 p.m.
Saturday –Sunday: Closed
- << Previous: Practice problems
- Next: Practice problems >>
- Last Updated: Feb 16, 2023 3:27 PM
- URL: https://guides.library.upenn.edu/supplementalresources

- ‹ Prev
- Next ›
1.1 Problems and Problem Solving
1.2 types and kinds of problems, 1.3 novice versus expert problem solvers/problem solving heuristics, 1.4 chemistry problems, 1.4.1 problems in stoichiometry, 1.4.2 problems in organic chemistry, 1.5 the present volume, 1.5.1 general issues in problem solving in chemistry education, 1.5.2 problem solving in organic chemistry and biochemistry, 1.5.3 chemistry problem solving under specific contexts, 1.5.4 new technologies in problem solving in chemistry, 1.5.5 new perspectives for problem solving in chemistry education, chapter 1: introduction − the many types and kinds of chemistry problems.
- Published: 17 May 2021
- Special Collection: 2021 ebook collection Series: Advances in Chemistry Education Research
- Open the Chapter PDF for in another window
- Get permissions
- Cite Icon Cite
G. Tsaparlis, in Problems and Problem Solving in Chemistry Education: Analysing Data, Looking for Patterns and Making Deductions, ed. G. Tsaparlis, The Royal Society of Chemistry, 2021, ch. 1, pp. 1-14.
Download citation file:
- Ris (Zotero)
- Reference Manager
Problem solving is a ubiquitous skill in the practice of chemistry, contributing to synthesis, spectroscopy, theory, analysis, and the characterization of compounds, and remains a major goal in chemistry education. A fundamental distinction should be drawn, on the one hand, between real problems and algorithmic exercises, and the differences in approach to problem solving exhibited between experts and novices on the other. This chapter outlines the many types and kinds of chemistry problems, placing particular emphasis on studies in quantitative stoichiometry problems and on qualitative organic chemistry problems (reaction mechanisms, synthesis, and spectroscopic identification of structure). The chapter concludes with a brief look at the contents of this book, which we hope will act as an appetizer for more systematic study.
According to the ancient Greeks, “The beginning of education is the study of names”, meaning the “examination of terminology”. 1 The word “problem” (in Greek: «πρόβλημα » /“ problēma” ) derives from the Greek verb “ proballein ” (“pro + ballein”), meaning “to throw forward” ( cf. ballistic and ballistics ), and also “to suggest”, “to argue” etc. Hence, the initial meaning of a “ problēma ” was “something that stands out”, from which various other meanings followed, for instance that of “a question” or of “a state of embarrassment”, which are very close to the current meaning of a problem . Among the works of Aristotle is that of “ Problēmata ”, which is a collection of “why” questions/problems and answers on “medical”, “mathematical”, “astronomical”, and other issues, e.g. , “Why do the changes of seasons and the winds intensify or pause and decide and cause the diseases?” 1
Problem solving is a complex set of activities, processes, and behaviors for which various models have been used at various times. Specifically, “problem solving is a process by which the learner discovers a combination of previously learned rules that they can apply to achieve a solution to a new situation (that is, the problem)”. 2 Zoller identifies problem solving, along with critical thinking and decision making, as high-order cognitive skills, assuming these capabilities to be the most important learning outcomes of good teaching. 3 Accordingly, problem solving is an integral component in students’ education in science and Eylon and Linn have considered problem solving as one of the major research perspectives in science education. 4
Bodner made a fundamental distinction between problems and exercises, which should be emphasized from the outset (see also the Foreword to this book). 5–7 For example, many problems in science can be simply solved by the application of well-defined procedures ( algorithms ), thus turning the problems into routine/algorithmic exercises. On the other hand, a real/novel/authentic problem is likely to require, for its solution, the contribution of a number of mental resources. 8
According to Sternberg, intelligence can best be understood through the study of nonentrenched ( i.e. , novel) tasks that require students to use concepts or form strategies that differ from those they are accustomed to. 9 Further, it was suggested that the limited success of the cognitive-correlates and cognitive-components approaches to measuring intelligence are due in part to the use of tasks that are more entrenched (familiar) than would be optimal for the study of intelligence.
The division of cognitive or thinking skills into Higher-Order (HOCS/HOTS) and Lower-Order (LOCS/LOTS) 3,10 is very relevant. Students are found to perform considerably better on questions requiring LOTS than on those requiring HOTS. Interestingly, performance on questions requiring HOTS often does not correlate with that on questions requiring LOTS. 10 In a school context, a task can be an exercise or a real problem depending on the subject's expertise and on what had been taught. A task may then be an exercise for one student, but a problem for another student. 11 I return to the issue of HOT/LOTS in Chapters 17 and 18.
Johnstone has provided a systematic classification of problem types, which is reproduced in Table 1.1 . 8 Types 1 and 2 are the “normal” problems usually encountered in academic situations. Type 1 is of the algorithmic exercise nature. Type 2 can become algorithmic with experience or teaching. Types 3 and 4 are more complex, with type 4 requiring very different reasoning from that used in types 1 and 2. Types 5–8 have open outcomes and/or goals, and can be very demanding. Type 8 is the nearest to real-life, everyday problems.
Classification of problems. Reproduced from ref. 8 with permission from the Royal Society of Chemistry.
Problem solving in chemistry, as in any other domain, is a huge field, so one cannot really be an expert in all aspects of it. Complementary to Johnstone's classification scheme, one can also identify the following forms: quantitative problems that involve mathematical formulas and computations, and qualitative ones; problems with missing or extraordinary data, with a unique solution/answer, or open problems with more than one solution; problems that cannot be solved exactly but need mathematical approximations; problems that need a laboratory experiment or a computer or a data bank; theoretical/thought problems or real-life ones; problems that can be answered through a literature search, or need the collaboration of specific experts, etc.
According to Bodner and Herron, “Problem solving is what chemists do, regardless of whether they work in the area of synthesis, spectroscopy, theory, analysis, or the characterization of compounds”. 12 Hancock et al. comment that: “The objective of much of chemistry teaching is to equip learners with knowledge they then apply to solve problems”, 13 and Cooper and Stowe ascertain that “historically, problem solving has been a major goal of chemistry education”. 14 The latter authors argue further that problem solving is not a monolithic activity, so the following activities “could all be (and have been) described as problem solving:
solving numerical problems using a provided equation
proposing organic syntheses of target compounds
constructing mechanisms of reactions
identifying patterns in data and making deductions from them
modeling chemical phenomena by computation
identifying an unknown compound from its spectroscopic properties
However, these activities require different patterns of thought, background knowledge, skills, and different types of evidence of student mastery” 14 (p. 6063).
Central among problem solving models have been those dealing with the differences in problem solving between experts and novices. Experts ( e.g., school and university teachers) are as a rule fluent in solving problems in their own field, but often fail to communicate to their students the required principles, strategies, and techniques for problem solving. It is then no surprise that the differences between experts and novices have been a central theme in problem solving education research. Mathematics came first, in 1945, with the publication of George Polya's classic book “ How to solve it: A new aspect of mathematical method ”: 15
“The teacher should put himself in the student's place, he should see the student's case, he should try to understand what is going on in the student's mind, and ask a question or indicate a step that could have occurred to the student himself ”.
Polya provided advice on teaching problem solving and proposed a four-stage model that included a detailed list of problem solving heuristics. The four stages are: understand the problem, devise a plan, carry out the plan, and look back . In 1979, Bourne, Dominowski, and Loftus modeled a three-stage process, consisting of preparation, production , and evaluation . 16 Then came the physicists. According to Larkin and Reif, novices look for an algorithm, while experts tend to think conceptually and use general strategies . Other basic differences are: (a) the comprehensive and more complete scheme employed by experts, in contrast to the sketchy one used by novices; and (b) the extra qualitative analysis step usually applied by experts, before embarking on detailed and quantitative means of solution. 17,18 Reif (1981, 1983) suggested further that in order for one to be able to solve problems one must have available: (a) a strategy for problem solving; (b) the right knowledge base, and (c) a good organization of the knowledge base. 18,19
Chemistry problem solving followed suit providing its own heuristics. Pilot and co-workers proposed useful procedures that include the steps that characterize expert solvers. 20–22 They developed an ordered system of heuristics, which is applicable to quantitative problem solving in many fields of science and technology. In particular, they devised a “ Program of Actions and Methods ”, which consists of four phases, as follows: Phase 1, analysis of the problem; Phase 2, transformation of the problem; Phase 3, execution of routine operations; Phase 4, checking the answer and interpretation of the results. Genya proposed the use of “sequences” of problems of gradually increasing complexity , with qualitative problems being used at the beginning. 23
Randles and Overton compared novice students with expert chemists in the approaches they used when solving open-ended problems. 24 Open-ended problems are defined as problems where not all the required data are given, where there is no one single possible strategy and where there is no single correct answer to the problem. It was found that: undergraduates adopted a greater number of novice-like approaches and produced poorer quality solutions; academics exhibited expert-like approaches and produced higher quality solutions; the approaches taken by industrial chemists were described as transitional.
Finally, one can justify the differences between novices and experts by employing the concept of working memory (see Chapter 5). Experienced learners can group ideas together to see much information as one ‘ chunk ’, while novice learners see all the separate pieces of information, causing an overload of working memory, which then cannot handle all the separate pieces at once. 25,26
Chemistry is unique in the diversity of its problems, some of which, such as problems in physical and analytical chemistry, are similar to problems in physics, while others, such problems in stoichiometry, in organic chemistry (especially in reaction mechanisms and synthesis), and in the spectroscopic identification of compounds and of molecular structure, are idiosyncratic to chemistry. We will have more to say about stoichiometry and organic chemistry below, but before that there is a need to refer to three figures whom we consider the originators of the field of chemistry education research: the Americans J. Dudley Herron and Dorothy L. Gabel and the Scot Alex H. Johnstone, for it is not a coincidence that all three dealt with chemistry problem solving.
For Herron, successful problem solvers have a good command of basic facts and principles; construct appropriate representations; have general reasoning strategies that permit logical connections among elements of the problem; and apply a number of verification strategies to ensure that the representation of the problem is consistent with the facts given, the solution is logically sound, the computations are error-free, and the problem solved is the problem presented. 27–29 Gabel has also carried out fundamental work on problem solving in chemistry. 30 For instance, she determined students’ skills and concepts that are prerequisites for solving problems on moles, through the use of analog tasks, and identified specific conceptual and mathematical difficulties. 31 She also studied how problem categorization enhances problem solving achievement. 32 Finally, Johnstone studied the connection of problem solving ability in chemistry (but also in physics and biology) with working memory and information processing. We will deal extensively with his relevant work in this book (see Chapter 5). In the rest of this section, reference will be made to some further foundational research work on problem solving in chemistry.
Working with German 16-year-old students in 1988, Sumfleth found that the knowledge of chemical terms is a necessary but not sufficient prerequisite for successful problem solving in structure-properties relationships and in stoichiometry. 33 In the U.S. it was realized quite early (in 1984) that students often use algorithmic methods without understanding the relevant underlying concepts. 32 Indeed, Nakhleh and Mitchell confirmed later (1993) that little connection existed between algorithmic problem solving skills and conceptual understanding. 34 These authors provided ways to evaluate students along a continuum of low-high algorithmic and conceptual problem solving skills, and admitted that the lecture method teaches students to solve algorithms rather than teaching chemistry concepts. Gabel and Bunce also emphasized that students who have not sufficiently grasped the chemistry behind a problem tend to use a memorized formula, manipulate the formula and plug in numbers until they fit. 30 Niaz compared student performance on conceptual and computational problems of chemical equilibrium and reported that students who perform better on problems requiring conceptual understanding also perform significantly better on problems requiring manipulation of data, that is, computational problems; he further suggested that solving computational problems before conceptual problems would be more conducive to learning, so it is plausible to suggest that students’ ability to solve computational/algorithmic problems is an essential prerequisite for a “progressive transition” leading to a resolution of novel problems that require conceptual understanding. 35–37
Stoichiometry problems are unique to chemistry and at the same time constitute a stumbling block for many students in introductory chemistry courses, with students often relying on algorithms. A review of some fundamental studies follows.
Hans-Jürgen Schmid carried out large scale studies in 1994 and 1997 in Germany and found that when working on easy-to-calculate problems students tended to invent/create a “non-mathematical” strategy of their own, but changed their strategy when moving from an easy-to-calculate problem to a more difficult one. 38,39 Swedish students were also found to behave in a similar manner. 40 A recent (2016) study with junior pre-service chemistry teachers in the Philippines reported that the most prominent strategy was the (algorithmic) mole method, while very few used the proportionality method and none the logical method. 41
Lorenzo developed, implemented, and evaluated a useful problem solving heuristic in the case of quantitative problems on stoichiometry and solutions. 42 The heuristic works as a metacognitive tool by helping students to understand the steps involved in problem solving, and further to tackle problems in a systematic way. The approach guides students by means of logical reasoning to make a qualitative representation of the solution to a problem before undertaking calculations, thus using a ‘backwards strategy’.
The problem format can serve to make a problem easier or more difficult. A large scale study with 16-year-old students in the UK examined three stoichiometry problems in a number of ways. 43 In Test A the questions were presented as they had previously appeared on National School Examinations, while in Test B each of the questions on Test A was presented in a structured sequence of four parts. An example of one of the questions from both Test A and Test B is given below.
- Test A. Silver chloride (AgCl) is formed in the following reaction: AgNO 3 + HCl → AgCl + HNO 3 Calculate the maximum yield of solid silver chloride that can be obtained from reacting 25 cm 3 of 2.0 M hydrochloric acid with excess silver nitrate. (AgCl = 143.5)
Test B. Silver chloride (AgCl) is formed in the following reaction:
(a) How many moles of silver chloride can be made from 1 mole of hydrochloric acid?
(b) How many moles are there in 25 cm 3 of 2.0 M hydrochloric acid?
(c) How many moles of silver chloride can be made from the number of moles of acid in (b)?
(d) What is the mass of the number of moles of silver chloride in (c)? (AgCl = 143.5)
Student scores on Test B were significantly higher than those on Test A, both overall and on each of the individual questions, showing that structuring serves to make the questions easier.
Drummond and Selvaratnam examined students’ competence in intellectual strategies needed for solving chemistry problems. 44 They gave students problems in two forms, the ‘standard’ one and one with ‘hint’ questions that suggested the strategies which should be used to solve the problems. Although performance in all test items was poor, it improved for the ‘hint’ questions.
Finally, Gulacar and colleagues studied the differences in general cognitive abilities and domain specific skills of higher- and lower-achieving students in stoichiometry problems and in addition they proposed a novel code system for revealing sources of students’ difficulties with stoichiometry. 45,46 The latter topic is tackled in Chapter 4 by Gulacar, Cox, and Fynewever.
Stoichiometry problems have also a place in organic chemistry, but non-mathematical problem solving in organic chemistry is quite a different story. 47 Studying the mechanisms of organic reactions is a challenging activity. The spectroscopic identification of the structure of organic molecules also requires high expertise and a lot of experience. On the other hand, an organic synthesis problem can be complex and difficult for the students, because the number of pathways by which students could synthesise target substance “X” from starting substance “A” may be numerous. The problem is then very demanding in terms of information processing. In addition, students find it difficult to accept that one starting compound treated with only one set of reagents could lead to more than one correct product. A number of studies have dealt with organic synthesis. 48–50 The following comments from two students echo the difficulties faced by many students (pp. 209–210): 50
“… having to do a synthesis problem is one of the more difficult things. Having to put everything together and sort of use your creativity, and knowing that I know everything solid to come up with a synthesis problem is difficult… it's just you can remember… you can use H 2 and nickel to add hydrogen to a bond but then there's like four other ways so if you're just looking for like what you react with, you can remember just that one but if you need five options just in case it's one of the other options that's given on the test… So, you have to know like multiple ways… and some things are used to maybe reduce… for example, something is used to reduce like a carboxylic acid and something else, the same thing, is used to reduce an aldehyde but then something else is used to like oxidize”.
Qualitative organic chemistry problems are dealt with in Chapters 6 and 7.
The present volume is the result of contributions from many experts in the field of chemistry education, with a clear focus on what can be identified as problem solving research. We are particularly fortunate that George Bodner , an authority in chemistry problem solving, has written the foreword to this book. (George has also published a review of research on problem solving in chemistry. 8 )
The book consists of eighteen chapters that cover many aspects of problem solving in chemistry and are organized under the following themes: (I) General issues in problem solving in chemistry education; (II) Problem solving in organic chemistry and biochemistry; (III) Chemistry problem solving in specific contexts; (IV) New technologies in problem solving in chemistry, and (V) New perspectives for problem solving in chemistry education. In the rest of this introductory chapter, I present a brief preview of the following contents.
The book starts with a discussion of qualitative reasoning in problem solving in chemistry. This type of reasoning helps us build inferences based on the analysis of qualitative values ( e.g. , high, low, weak, and strong) of the properties and behaviors of the components of a system, and the application of structure–property relationships. In Chapter 2, Talanquer summarizes core findings from research in chemistry education on the challenges that students face when engaging in this type of reasoning, and the strategies that support their learning in this area.
For Graulich, Langner, Vo , and Yuriev (Chapter 3), chemical problem solving relies on conceptual knowledge and the deployment of metacognitive problem solving processes, but novice problem solvers often grapple with both challenges simultaneously. Multiple scaffolding approaches have been developed to support student problem solving, often designed to address specific aspects or content area. The authors present a continuum of scaffolding so that a blending of prompts can be used to achieve specific goals. Providing students with opportunities to reflect on the problem solving work of others – peers or experts – can also be of benefit in deepening students’ conceptual reasoning skills.
A central theme in Gulacar, Cox and Fynewever 's chapter (Chapter 4) is the multitude of ways in which students can be unsuccessful when trying to solve problems. Each step of a multi-step problem can be labeled as a subproblem and represents content that students need to understand and use to be successful with the problem. The authors have developed a set of codes to categorize each student's attempted solution for every subproblem as either successful or not, and if unsuccessful, identifying why, thus providing a better understanding of common barriers to success, illustrated in the context of stoichiometry.
In Chapter 5, Tsaparlis re-examines the “working memory overload hypothesis” and associated with it the Johnstone–El Banna predictive model of problem solving. This famous predictive model is based on the effect of information processing, especially of working-memory capacity on problem solving. Other factors include mental capacity or M -capacity, degree of field dependence/independence, and developmental level/scientific reasoning. The Johnstone–El Banna model is re-examined and situations are explored where the model is valid, but also its limitations. A further examination of the role of the above cognitive factors in problem solving in chemistry is also made.
Proposing reaction mechanisms using the electron-pushing formalism, which is central to the practice and teaching of organic chemistry, is the subject of Chapter 6 by Bahttacharyya . The author argues that MR (Mechanistic Reasoning) using the EPF (Electron-Pushing Formalism) incorporates several other forms of reasoning, and is also considered as a useful transferrable skill for the biomedical sciences and allied fields.
Flynn considers synthesis problems as among the most challenging questions for students in organic chemistry courses. In Chapter 7, she describes the strategies used by students who have been successful in solving synthetic problems. Associated classroom and problem set activities are also described.
We all know that the determination of chemical identity is a fundamental chemistry practice that now depends almost exclusively on the characterization of molecular structure through spectroscopic analysis. This analysis is a day-to-day task for practicing organic chemists, and instruction in modern organic chemistry aims to cultivate such expertise. Accordingly, in Chapter 8, Connor and Shultz review studies that have investigated reasoning and problem solving approaches used to evaluate NMR and IR spectroscopic data for organic structural determination, and they provide a foundation for understanding how this problem solving expertise develops and how instruction may facilitate such learning. The aim is to present the current state of research, empirical insights into teaching and learning this practice, and trends in instructional innovations.
The idea that variation exists within a system and the varied population schema described by Talanquer are the theoretical tools for the study by Rodriguez, Hux, Philips, and Towns , which is reported in Chapter 9. The subject of the study is chemical kinetics in biochemistry, and especially of the action and mechanisms of inhibition agents in enzyme catalysis, where a sophisticated understanding requires students to learn to reason using probability-based reasoning.
In Chapter 10, Phelps, Hawkins and Hunter consider the purpose of the academic chemistry laboratory, with emphasis on the practice of problem solving skills beyond those of an algorithmic mathematical nature. The purpose represents a departure from the procedural skills training often associated with the reason we engage in laboratory work (learning to titrate for example). While technical skills are of course important, if part of what we are doing in undergraduate chemistry courses is to prepare students to go on to undertake research, somewhere in the curriculum there should be opportunities to practice solving problems that are both open-ended and laboratory-based. The history of academic chemistry laboratory practice is reviewed and its current state considered.
Chapter 11 by Broman focuses on chemistry problems and problem solving by employing context-based learning approaches, where open-ended problems focusing on higher-order thinking are explored. Chemistry teachers suggested contexts that they thought their students would find interesting and relevant, e.g. , chocolate, doping, and dietary supplements. The chapter analyses students’ interviews after they worked with the problems and discusses how to enhance student interest and perceived relevance in chemistry, and how students’ learning can be improved.
Team Based Learning (TBL) is the theme of Chapter 12 by Capel, Hancock, Howe, Jones, Phillips , and Plana . TBL is a structured small group collaborative form of learning, where learners are required to prepare for sessions in advance, then discuss and debate potential solutions to problems with their peers. It has been found to be highly effective at facilitating active learning. The authors describe their experience with embedding TBL into their chemistry curricula at all levels, including a transnational degree program with a Chinese university.
The ability of students to learn and value aspects of the chemistry curriculum that delve into the molecular basis of chemical events relies on the use of models/molecular representations, and enhanced awareness of how these models connect to chemical observations. Molecular representations in chemistry is the topic of Chapter 13 by Polifka, Baluyut and Holme , which focuses on technology solutions that enhance student understanding and learning of these conceptual aspects of chemistry.
In Chapter 14, Limniou, Papadopoulos, Gavril, Touni , and Chatziapostolidou present an IR spectra simulation. The software includes a wide range of chemical compounds supported by real IR spectra, allowing students to learn how to interpret an IR spectrum, via a step by step process. The chapter includes a report on a pilot trial with a small-scale face-to-face learning environment. The software is available on the Internet for everyone to download and use.
In Chapter 15, Sigalas explores chemistry problems with computational quantum chemistry tools in the undergraduate chemistry curriculum, the use of computational chemistry for the study of chemical phenomena, and the prediction and interpretation of experimental data from thermodynamics and isomerism to reaction mechanisms and spectroscopy. The pros and cons of a series of software tools for building molecular models, preparation of input data for standard software, and visualization of computational results are discussed.
In Chapter 16, Stamovlasis and Vaiopoulou address methodological and epistemological issues concerning research in chemistry problem solving. Following a short review of the relevant literature with emphasis on methodology and the statistical modeling used, the weak points of the traditional approaches are discussed and a novel epistemological framework based on complex dynamical system theory is described. Notably, research using catastrophe theory provides empirical evidence for these phenomena by modeling and explaining mental overload effects and students’ failures. Examples of the application of this theory to chemistry problem solving is reviewed.
Chapter 17 provides extended summaries of the chapters, including a commentary on the chapters. The chapter also provides a brief coverage of various important issues and topics related to chemistry problem solving that are not covered by other chapters in the book.
Finally, in Chapter 18, a Postscript address two specific problem solving issues: (a) the potential synergy between higher and lower-order thinking skills (HOTS and LOTS,) and (b) When problem solving might descend to chaos dynamics. The synergy between HOTS and LOTS is demonstrated by looking at the contribution of chemistry and biochemistry to overcoming the current coronavirus (COVID-19) pandemic. One the other hand, chaos theory provides an analogy with the time span of the predictive power of problem solving models.
«Ἀρχὴ παιδεύσεως ἡ τῶν ὀνομάτων ἐπίσκεψις» (Archē paedeuseōs hē tōn onomatōn episkepsis). By Antisthenes (ancient Greek philosopher), translated by W. A. Oldfather (1925).
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
This Feature Is Available To Subscribers Only
Sign In or Create an Account
MCAT and Organic Chemistry Study Guides, Videos, Cheat Sheets, tutoring and more
How to Tackle Organic Chemistry Synthesis Questions
November 10, 2016 By Leah4sci 4 Comments

It's not so scary at first, think about the simple acid/base deprotonation, an alkene reaction here, another there. Before you know it, you’re drowning in dozens upon dozens of reactions!
You’re asked to ‘memorize’ each one, to know what every reactant and reagent will do. And once you have it all down, don’t forget the dozen or so exceptions to the rule.
And once you have all THAT down, let’s put them all together: when you’re given molecule A and asked to come up with all 20 steps to produce product Z!

Ok, perhaps I’m exaggerating a bit…but really, just a tiny bit!
The average Organic Chemistry 1 or 2 exam synthesis question will range from two to five steps with intermediates.
How do you keep everything you learned straight?
And more importantly, how do you sift through the hundreds of data points in your head to produce the exact steps required to achieve the desired outcome?
As a kid, I was proud to call myself a nerd. Perhaps with a bit of OCD. I always looked for trends and found patterns where they didn’t exist. Perhaps that’s why I enjoy Orgo so much.
The SECRET to synthesis is simple:
A) Look for patterns, as we’ll explain below. B) when you get stuck, remember there’s likely more than one way to reach your desired product.
Coming up with a proper synthesis requires a combination of forward and reverse thinking!
We’ll cover the reverse thinking in the Retrosynthesis tutorial . For this tutorial, we’ll focus on the shorter and simpler synthesis.
Let’s start by looking for patterns.
The following questions will help you understand what to pay close attention to.
Look out for the following:
- What functional group is present on the reactant ?
- What functional group is present on the product ?
- Which reactions do I know to convert from one to the other?
- Do I know of a reaction that produces an intermediate to the above product?

“What do I have in the reactant?” A reactive pi bond!
“ What do I have in the product?” An alcohol.
“Which reaction do I know can help me convert from an alkene to an alcohol?”
Well, there are many if you think about it. Let’s go in order from most obvious to less obvious:
- Acid catalyzed hydration
- Oxymercuration-demercuration
- Hydroboration-oxidation

But what if you weren’t given the alkene, or didn’t think of these alkene reactions? What about putting a halogen leaving group on the alkene through hydrohalogenation ? Or a radical halogenation if starting with an alkane?

What if it wasn’t that easy?
What if you’re asked to start with an alkyne?

This is where we introduce many options: –> If you reduce the alkyne to an alkene, you may use one of the following as already discussed above:

–> If you go from the alkyne to a carbonyl (ketone or aldehyde) you can follow up by reduction:

Do you see where we’re going with this?
A) Identify the patterns B) Ask yourself, “What do I know?” to recognize these patterns C) Go from there
Let’s start with a simple example for concept, then apply the same logic to something more complicated.

- What functional group is present on the reactant ? Reactive terminal alkyne
- What functional group is present on the product ? Ketone on carbon #2
- Which reactions do I know to convert from one to the other? Acid catalyzed hydration of alkynes will yield a ketone following Markovnikov’s Rule .
HOWEVER , in this example we’re starting with a 3-carbon chain yet ending with a 4-carbon chain.
4. Do I know of a reaction that produces an intermediate to the above product? Yes! Terminal alkynes easily undergo chain elongation via SN2.
We'll start with an acid/base reaction to deprotonate the terminal alkyne forming a good nucleophile.

But wait, the product is an enol, not a ketone!!

And there we have it!
Even scarier than a synthesis by itself is the following exam question/request:
“Propose a reasonable mechanism to carry out the following synthesis.”
This is where you’re given one reactant, one product, and ONE or TWO sets of reagents. In other words, you’re given all the steps but asked to show how the different molecules work together.
While the question is completely different, the concept is the same .
We’ll modify the initial questions slightly:
- WHERE IS THE REACTIVITY ON THE STARTING MOLECULE? As in, where do I start the mechanism?
A student recently showed me this exam question for which not a single student in his class got full credit.
Let me preface this by saying YES, this is a tough question. BUT, if you think through it logically, you’ll realize that if you studied the individual steps and recognize them, you should be able to follow along.
In fact, I challenge you to give this a try and see how far you get before reading on.
Propose a reasonable mechanism for the following reaction. show all intermediates and formal charges. is this reaction sn1 sn2 e1 e2 .

What do you think? Did you get it?
Here's a video with a step by step solution:
No matter how well you prepare, you'll still get caught off-guard by one tricky step or another.
And when you get stuck?
Chances are, there’s more than one way to derive your product!
When I took my first weekly Organic Chemistry 2 quiz, I inadvertently set a trend of scoring top of the class, but it was a fluke. I didn’t realize we had weekly quizzes…
We were asked to outline a step by step procedure to separate two similar molecules with different functional groups. The process involved a series of reactions to prepare one molecule for extraction.
I remembered that there were about six steps, but I could only confidently answer four. I’d already bombed, then aced Orgo 1, and didn’t want to do that again!
No matter what I did, I couldn’t come up with the other steps. So instead, I crossed out my four and a half steps and wrote a detailed step by step procedure for carrying out fractional distillation.
My TA very reluctantly gave me full credit with an amused warning. No one else came close.
Am I asking you to outsmart the question?
Just recognize that the more reactions you learn, the more options you have for creating a single functional group.
If you're stuck on a certain pathway or can’t fully describe the steps, Ask yourself, “Is there another way to create the same functional group?”
Think back to the many alcohol formation reactions we discussed above. If you forgot one option, simply use another.
Here are a few interesting patterns and alternates to consider.
–> Chain Elongation ‘Go-To’ reactions
- Use alkynes in Orgo 1
- Use grignards or condensation in Orgo 2
–> Adding carboxylic acids or carbonyls
- Alcohol -> oxidation
- Oxidative cleavage using KMnO4 or O3 (smaller chain)
- Grignard and CO2 (longer chain)

–> ‘Moving’ Reactivity so that you can start/react at a different portion of the molecule compared to the current location of the active group.
Active groups include leaving groups, pi bonds, nucleophilic centers susceptible to attack and more.

- Move the Pi bond intermediate then apply a Markovnikov or Anti-Markovnikov addition .
- No pi bond? Radical halogenation to introduce a leaving group and THEN eliminate.
These are just SOME of the tricks you can utilize.
Maximizing Partial Credit On Your Exam
Gaining bonus points on exams is one thing, but here’s the best part:
The average synthesis question is worth anywhere from 10-30 points. And the average professor WILL give partial credit. So, if you can only remember four of five steps, DO NOT leave it blank to receive zero points!
Instead, write the four steps and add in as much relevant information as possible. Then VERY CONFIDENTLY fake the fifth step.
Do not write “ Magic! ” (yes, I’ve seen this on a student exam).
Make up something that appears to be just a ‘careless’ mistake. Your professor will be impressed by your work and hopefully give you an 80% for the question.
This also applies to reagents!
If you only remember the steps, but don’t remember which reagents will get you there, start by drawing out the molecules:
A –> B –> C
This has two benefits:
- It helps you think without distractions so you can clearly see how the molecule and functional groups change/evolve from step to step.
- Now that you have a clear picture of where you’re doing, you should be able to retrace your steps and fill in the required reagents!
Of course if Reagents are giving you trouble with this, here's a video on ‘Memorizing’ Organic Chemistry Reagents .
And if you can’t remember them, invent something rather than leave it blank. try to use a reagent that has the groups you’re adding..

Are they correct? Not quite! Both will cleave the alkyne.
BUT, I’ve seen enough students use this as a legitimate mistake that your professor may think the same and hopefully give you partial credit.
Again, I’m not asking you to invent on your exam.
But, guessing logically on a multi-step problem where you’ve already earned sufficient points will help you get closer to full credit.
I’ve done this successfully on my orgo exams and come out top of the class, despite missing half a point here and there on multiple questions. My Study Hall members and tutoring clients do the same thing to squeeze in a few extra points, bringing them to the top.
Bottom line, make sure you learn and UNDERSTAND all of the required reactions!
But even the best of us forget some things under pressure.
That’s when you can utilize the tips above to help you try your best and you’ll have an advantage with most professors. They'll hopefully give you the benefit of the doubt when answers are questionable or not exactly what they were seeking.
Ready to start thinking backwards? That's where the more difficult topic of Retrosynthetic Analysis or simply retrosynthesis comes into play.
I’d love to hear from you
Do you feel better about synthesis and knowing what to do when you are stuck? Let me know in the comments below
September 26, 2018 at 12:42 pm
its too much difficult topic I can’t understand….infact i can’t thnk too much like that to propose a mechanism………Wt should I do? paper b ha?
March 1, 2018 at 8:23 am
why in this case the pi bond forms in C1 and C2 and not in C2 and C3 (more substitued alkene) https://leah4sci.com/wp-content/uploads/2016/11/Moving-Reactivity-can-help-react-a-different-portion-of-the-molecule-using-intermediates-and-then-Mark-or-Anti-Markovnikov-addition.jpg
December 23, 2017 at 1:21 pm
Great post!!This article will be extremely helpful for students. Thank you for sharing this great guide it is really well written and very easy to understand with some great tips for chemistry. Nice work!
April 23, 2017 at 12:18 pm
I can’t explain how much this just helped me for my Ochem exam this thursday! THANK YOU
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Organic Chemistry Reference Material and Cheat Sheets

Alkene Reactions Overview Cheat Sheet – Organic Chemistry
The true key to successful mastery of alkene reactions lies in practice practice practice. However, … [Read More...]
Click for additional cheat sheets
MCAT Tutorials

Introduction To MCAT Math Without A Calculator
While the pre-2015 MCAT only tests you on science and verbal, you are still required to perform … [Read More...]
Click for additional MCAT tutorials
Organic Chemistry Tutorial Videos

Keto Enol Tautomerization Reaction and Mechanism
Keto Enol Tautomerization or KET, is an organic chemistry reaction in which ketone and enol … [Read More...]
Click for additional orgo tutorial videos

Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
Lesson plan maker, ai notes generator, verbose text enhancer.

IMAGES
VIDEO
COMMENTS
Simplifying Organic Chemistry. Orgosolver provides study tools to help students with their organic chemistry homework and preparation for quizzes, exams, or even the MCAT. Our tools, quizzes, and study guides are designed to help students test every reaction or mechanism with any molecule they draw!
Doing practice problems is the only way to master organic chemistry! At Chemistry Steps, you can find all the topics of Organic 1 and 2 and their associated practice problems. There are more than 1000 practice questions and you can find them after each article listed below. Here are some examples from the topics shown below.
Organic Chemistry Test 2! Google Classroom. Microsoft Teams. Consider the following reaction: Identify the correct order of reagents that will most likely carry out the reaction. Choose all answers that apply: 1. HBr, peroxide. 2. a l c. KOH + Δ.
Organic Chemistry is Awesome. Over 400+ blog posts to guide you through introductory Organic Chemistry, organized by subject. 00 General Chemistry Review. 01 Bonding, Structure, and Resonance. 02 Acid Base Reactions. 03 Alkanes and Nomenclature. 04 Conformations and Cycloalkanes. 05 A Primer On Organic Reactions. 06 Free Radical Reactions.
Since problem solving is essential to achieving an effective mastery of the subject, it is recommended that many more problems be worked. Most organic chemistry textbooks contain a broad assortment of suitable problems, and paperback collections of practice problems are also available.
A brief introduction to organic chemistry. Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them.
Practice. Synthesis Practice. This section contains various syntheses problems suitable for sophomore (introductory) level organic chemistry students. The synthesis complexity will generally increase down to list but the complexity level increase is not linear, so you might find some syntheses more challenging than the other ones.
6.13.2. Practice Problems. Propose a substitution mechanism for the following reactions. Pay special attention to stereochemistry if indicated. Look at the conditions given to determine if the substitution is unimolecular or bimolecular (SN1 or SN2). Solution. Propose an elimination mechanism for the following reactions.
Welcome to Real Organic Chemistry This site hosts a collection of ~300 real organic chemistry reactions taken from the scientific literature, with the intent of providing real-life practice problems for introductory organic chemistry students. The examples are curated to cover both "easy" to "hard", covering both concept-learning and exam-level ...
This playlist is full of fun practice problems that you can try once you've made it through the organic chemistry lecture series. Test your knowledge on spec...
The Art of Problem Solving in Organic Chemistry, Third Edition is an essential volume for advanced undergraduates, graduate students, lecturers, and professionals looking to improve their performance in finding solutions to organic reaction problems. It is an ideal textbook for courses on organic reactions and problem analysis, as well as an ...
This organic chemistry tutorial video provides practice solving organic synthesis problems using retrosynthetic analysis.
Why This Chapter? 6.1 Kinds of Organic Reactions; 6.2 How Organic Reactions Occur: Mechanisms; 6.3 Polar Reactions; 6.4 An Example of a Polar Reaction: Addition of HBr to Ethylene; 6.5 Using Curved Arrows in Polar Reaction Mechanisms; 6.6 Radical Reactions; 6.7 Describing a Reaction: Equilibria, Rates, and Energy Changes; 6.8 Describing a Reaction: Bond Dissociation Energies
Problem 17-26. Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields a mixture of 1,2-dimethylcyclohexene and isopropylidenecyclopentane. Propose a mechanism to account for the formation of both products. Problem 17-27. Epoxides react with Grignard reagents to yield alcohols.
Welcome to Organic Chemistry Problems! This web page is intended to be a resource for students, instructors, and practitioners of organic synthesis. Primarily, this resource is intended to provide extra example problems for students at the introductory graduate student level (i.e. first year graduate students who are in an organic synthesis ...
Brush up on Organic Chemistry Topics". Problem Solving. "HELP ME! I forgot. How do I solve this problem?" Applications of Organic Chemistry. "Why is Organic Chemistry important to ME?" Reaction Mechanisms.
The Art of Problem Solving in Organic Chemistry Second Edition. This long-awaited and rewritten new edition includes updated organic reactions and helps students understand and solve the complex mechanistic problems that organic chemists face. Using 60 new, solved, and worked-through problems the author uses a step-by-step approach to discuss ...
ISBN: 9780393935004. Publication Date: 2010-04-26. This guide provides students with fully worked solutions to all un-worked problems that appear in the text. In addition to the solutions presented for each specific problem, the authors present strategies for solving organic chemistry problems in general.
Stoichiometry problems have also a place in organic chemistry, but non-mathematical problem solving in organic chemistry is quite a different story. 47 Studying the mechanisms of organic reactions is a challenging activity. The spectroscopic identification of the structure of organic molecules also requires high expertise and a lot of experience.
The SECRET to synthesis is simple: A) Look for patterns, as we'll explain below. B) when you get stuck, remember there's likely more than one way to reach your desired product. Coming up with a proper synthesis requires a combination of forward and reverse thinking! We'll cover the reverse thinking in the Retrosynthesis tutorial.
Solving problems in organic chemistry often requires the consideration of reaction mechanisms. As such, much research has been devoted to the examination of how students consider mechanisms when solving various types of organic chemistry problems, such as predicting the products of a reaction or proposing a synthesis. This article provides a scoping review of this body of research, with a ...
The problems are all solved and fully explained. Every single step of each problem is shown in a logical and easy-to-follow format, with constant reference to theory. In addition to solved problems, the book provides methods, strategies, and explanations that help students master organic chemistry problems. The Guide includes the following ...
This AI tool helps answer chemistry questions and solve chemistry problems. HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.
Organic Chemistry as a Second Language: Second Semester Topics, 6e teaches students how to ask the right questions to solve problems, study more efficiently, and learn to speak the language of organic chemistry. Like its first-semester companion title, it is an essential 'guide on the side' for any organic chemistry student no matter what ...
There are various examples of the use of flowcharts in chemistry, such as point group classification, naming inorganic compounds, and problem-solving on the first law of thermodynamics. (22) Flowcharts providing only basic knowledge to summarize a subject can be found in organic chemistry books, and they are often located at the end of the ...