- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies

Case Study: 29-Year-Old Female with Postpartum Hemorrhage
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 29-year-old female (G1P1) is readmitted two weeks post–vaginal delivery due to increased vaginal bleeding. She reports that the bleeding began on the tenth day after delivery and has increased in severity each subsequent day. The delivery was uncomplicated with minimal blood loss and the patient did not receive any epidural anesthesia. She has taken 200 mg of ibuprofen daily since delivery. The patient reports a medical history of iron deficient anemia due to menorrhagia. She is adopted and does not know her family history.
Since admission, the patient has received three units of blood. The obstetrics team has ruled out retained placental tissue and uterine atony as the cause of bleeding. Her laboratory values are as follows:
What hematologic disease is most likely contributing to her bleeding?
- NSAID-induced platelet dysfunction
- Acquired factor VIII inhibitor
- von Willebrand disease
- Disseminated intravascular coagulation
- Microangiopathy hemolytic anemia
Explanation
This patient is presenting with secondary postpartum hemorrhage with a clinical and laboratory history suggestive of von Willebrand disease (vWD). Overall, there is a 20 percent risk of peripartum bleeding with vWD, and 75 percent of women with moderate to severe vWD can experience severe bleeding. Bleeding can be seen from any type of vWD. For women with mild forms of vWD, the peripartum period can be the first manifestation of the disease. 1 Furthermore, since peripartum bleeding unrelated to a bleeding disorder is common, the diagnosis of an underlying bleeding diathesis may not be considered.
During pregnancy both factor VIII and von Willebrand factor (vWF) levels increase, with peaks at 29 to 32 weeks gestation and at 35 weeks gestation, respectively. 2 Following delivery, vWF levels may fall precipitously within the first few weeks resulting in delayed uterine bleeding. 3 Management of women with known vWD includes monitoring vWF levels during pregnancy and for three to four weeks post-partum to ensure return to baseline. Prophylaxis and treatment of vWD during pregnancy is based upon the severity of disease and specific type of vWD.
Acquired factor VIII inhibitors are a rare but serious cause of secondary postpartum hemorrhage. 4 The activated partial thromboplastin time (aPTT) is typically significantly prolonged if there is a factor VIII inhibitor. Disseminated intravascular coagulation is an important cause of post-partum hemorrhage. Significant prolongations of the aPTT and PT as well as low fibrinogen levels are necessary to make the diagnosis. Microangiopathic hemolytic anemia may occur in the peripartum period and is associated with low hemoglobin and platelets. However, this diagnosis results in manifestations of microvascular thrombosis rather than hemorrhage.
Case study submitted by James N. Cooper, MD, of the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD.
- Ragni MV, Bontemp FA, Hassett AC. von Willebrand disease and bleeding in women . Haemophilia. 1999; 5:313-317
- Sié P, Caron C, Azam J, et el. Reassessment of von Willebrand factor (VWF), VWF propeptide, factor VIII:C and plasminogen activator inhibitors 1 and 2 during normal pregnancy . Br J Haematol. 2003; 121:897-903.
- Kujovich JL. von Willebrand disease and pregnancy . J Thromb Haemost. 2005; 3:246-253.
- Paidas MJ, Hossain N. Unexpected postpartum hemorrhage due to an acquired factor VIII inhibitor . Am J Perinatol. 2014 31:645-654
American Society of Hematology. (1). Case Study: 29-Year-Old Female with Postpartum Hemorrhage. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/female-postpartum-hemorrhage .
American Society of Hematology. "Case Study: 29-Year-Old Female with Postpartum Hemorrhage." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/female-postpartum-hemorrhage (label-accessed May 25, 2024).
"American Society of Hematology." Case Study: 29-Year-Old Female with Postpartum Hemorrhage, 25 May. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/female-postpartum-hemorrhage .
Citation Manager Formats

Postpartum Hemorrhage (PPH): Prevention & Management
Nov 17, 2014
870 likes | 1.62k Views
Postpartum Hemorrhage (PPH): Prevention & Management. Evidence and Action. Objectives. Describe the global mortality burden of PPH Present current evidence and action to prevent PPH Share key evidence and action to manage PPH
Share Presentation
- maternal deaths
- pph prevention
- severe pph 7
- emerging pph prevention innovations

Presentation Transcript
Postpartum Hemorrhage (PPH): Prevention & Management Evidence and Action
Objectives • Describe the global mortality burden of PPH • Present current evidence and action to prevent PPH • Share key evidence and action to manage PPH • Discuss key elements in a comprehensive program to reduce deaths from PPH
PPH: Leading Cause of Maternal Mortality • Hemorrhage is a leading cause of maternal deaths • 35% of global maternal deaths • estimated 132,000 maternal deaths • 14 million women in developing countries experience PPH—26 women every minute Sources: Khan et al., 2006; POPPHI, 2009; Taking Stock of Maternal, Newborn and Child Survival, 2000–2010 Decade Report
Other direct causes include embolism, ectopic pregnancy, anesthesia-related. Indirect causes include: malaria, heart disease. Source: Adapted from " WHO Analysis of causes of maternal deaths: A systematic review.” The Lancet, vol 367, April 1, 2006.
Maternal & Newborn Health: Scope of Problem • 180–200 million pregnancies per year • 75 million unwanted pregnancies • 50 million induced abortions • 20 million unsafe abortions (same as above) • 342,900 maternal deaths (2008) • 1 maternal death = 30 maternal morbidities • 3 million neonatal deaths (first week of life) • 3 million stillbirths Source: Hogan et al., 2010
Where is Motherhood Less Safe? Deaths of Women from Pregnancy and Childbirth: 99% in developing world World Map in Proportion to Maternal Mortality Source: worldmapper.org
What is PPH? • Blood loss >500mL in the first 24 hours after delivery • Severe PPH is loss of 1000mL or more. • Accurately quantifying blood loss is difficult in most clinical or home settings. • Many severely anemic women cannot tolerate even 500 mL blood loss Graphic credit: ??? Source: Making Pregnancy Safer, through promoting Evidence-based Care, Global Health Council Technical Report, 2002
Incidence of PPH Source: Goudar, Eldavitch, Bellad, 2003
Why Do Women Die From Postpartum Hemorrhage? • We cannot predict who will get PPH. • Almost 50% of women deliver without a skilled birth attendant (SBA). • 50% of maternal deaths occur in the first 24 hours following birth, mostly due to PPH • PPH can kill in as little as 2 hours • Anemia increases the risk of dying from PPH • Timely referral and transport to facilities are often not available or affordable. • Emergency obstetric care is available to less than 20% of women. Source: Taking Stock of Maternal, Newborn and Child Survival, 2000–2010 Decade Report
Prevention Management What Can Be Done? Photo credit: Lauren Goldsmith Photo credit: ??? POPPHI Source: World Health Organization, IMPAC: MCPC 2003
PPH Prevention • In the facility: Active management of the third stage of labor (AMTSL) • During deliveries with a skilled provider • Prevents immediate PPH • Associated with almost 60% reduction in PPH occurrence • In the home/community: Misoprostol • During home births without a skilled provider • Community-based counseling and distribution of misoprostol Source: Begley et al., 2010, WHO Recommendations for the Prevention of Postpartum Hemorrhage, 2007
PPH Prevention & Management
Risk of PPH Source: Prendiville et al., BMJ 1988. Villar et al., 2002
Active vs. Expectant Management of Third Stage 4 studies 4,829 women Source: Begley et al., Cochrane Review 2010
Active Management of the Third Stage of Labor (AMTSL) • Administration of a uterotonic agent within one minute after the baby is born (oxytocin is the uterotonic of choice); • Controlled cord traction while supporting and stabilizing the uterus by applying counter traction; • Uterine massage after delivery of the placenta. Source: AMTSL: A Demonstration, Jhpiego, 2005
AMTSL • More effective than physiologic management • 60% decrease in PPH and severe PPH • Decreased need for blood transfusion • Decreased anemia (<9 g/dl) • Uterotonic agent = most effective component • Choice depends on cost, stability, safety, side effects, type of birth attendant, cold chain availability Source: WHO guidelines for the management of postpartum haemorrhage and retained placenta, 2009
Choice of Uterotonic Drug • Oxytocin preferred • Fast-acting, inexpensive, no contraindications for use in the third stage of labor, relatively few side effects • Requires refrigeration to maintain potency, requires injection (safety) • Misoprostol • Does not require refrigeration or injection, no contraindications for use in the third stage of labor • Common side effects include shivering and elevated temperature, is less effective than oxytocin Source: WHO guidelines for the management of postpartum haemorrhage and retained placenta, 2009
Choice of Uterotonics Source: IMPAC, MCPC 2006, Hofmeyr et al., 2009
More Evidence • Double-blind placebo controlled WHO multi-center RCT: Oxytocin vs. Misoprostol in hospital1 • 8 countries • Oxytocin (n = 9266); Misoprostol (n = 9264) • Severe PPH (1000cc): 3% vs. 4% • Misoprostol—higher incidence of shivering • Conclusion: Oxytocin preferred over Misoprostol • Double blind placebo controlled RCT in rural Guinea Bissau: Misoprostol vs. Placebo • Misoprostol alone reduces severe PPH (1000mls+) 11% vs. 17% RR 0.66 (0.44–0.98) Source: Gulmezoglu, et al., Lancet 2001, Høj BMJ 2005
Misoprostol: Evidence • Clinical demonstration study1 • Oral Misoprostol reduced PPH incidence to 6% • Double-blind placebo controlled study2 • Oral Misoprostol reduced need for treatment of PPH from 8.4% 2.8% • Rectal Misoprostol vs. Syntometrin for 3rd stage3 • Similar reduction in length of 3rd stage, postpartum blood loss and postpartum hemoglobin; Higher BP with Syntometrin • Oral Misoprostol vs. Placebo4 • PPH: 7% vs. 15% • Need for therapeutic Oxytocin: 16% vs. 38% Source: 1: O’Brien, 1997; 2: Hofmeyr, 1998; 3: Bamigboye, 1998; 4: Surbek, 1999
A Randomized Placebo-Controlled Trial of Oral Misoprostol 600 mcg for Prevention of PPH at Four Primary Health Center Areas of Belgaum District, Karnataka India Source: Derman et al., Lancet 2006
Misoprostol at Home Births: 2006 • Oral misoprostol can be delivered with efficacy and feasibility in a rural home delivery setting. • Reduced acute PPH by almost 50% (compared to placebo) • Associated with an 80% reduction in acute severe PPH Source: Derman et al., 2006
Blood Loss Distribution 95th Percentile M: 500 ml P: 800 ml Source: Derman et al, 2006
Completed programs Indonesia, Gambia, Guinea Bissau New programs underway Pakistan, Nepal, Bangladesh, Kenya, Uganda, Afghanistan Feasibility for Misoprostol use at Homebirth • INDONESIA PROGRAM • Safety: No women took medication at wrong time • Acceptability: users said they would recommend it and purchase drug for future births • Feasibility: 94% coverage with PPH prevention method achieved • Effectiveness: • 25% reduction in perceived excessive bleeding OR 0.76 (0.55– 1.05) • 45% reduction in need for referral for PPH 0.53 (0.24–1.12) Source: Prevention of Postpartum Hemorrhage Study, 2004 Jhpiego
WHO Recommendations for the Prevention of PPH (WHO 2007) 7. In the absence of AMTSL, should uterotonics be used alone for prevention of PPH? Recommendation: • In the absence of AMTSL, a uterotonic drug (oxytocin or misoprostol) should be offeredby a health worker trained in its use for prevention of PPH (strong recommendation, moderate quality evidence) Source: WHO Recommendations for the Prevention of Postpartum Hemorrhage, 2007
Uterotonic in 3rd Stage Reduces PPH Source: WHO Recommendations for the Prevention of Postpartum Hemorrhage, 2007
Emerging PPH Prevention Innovations • Oxytocin Uniject™ for simpler dosing and improved infection prevention during AMTSL • Angola study compared Uniject with expectant management • Intervention group experienced significantly decreased PPH (40.4% vs. 8.2%), severe PPH (7.5% vs. 1%) andblood loss (447 vs. 239mL). • Shortened the interval between birth of the baby and delivery of the placenta to less than 10 minutes for 89.4% vs. 5.4% of women in the expectant managment group • No significant difference in manual removal of the placenta between the two groups • Some evidence from Mali • Midwives preferred Uniject over standard injection practices at home births • Uniject simplifies AMTSL practice significantly to expand uterotonic coverage and allow task-shifting to auxiliary nurse midwives Photo credit: PATH • Sources: Strand RT, et al., ActaObstetGynecol Scand. 2005; Tsu VD et al., 2003
PPH Management: A Comprehensive Approach Source: WHO handbook: Monitoring emergency obstetric care 2009
Survey Results: Universal Uterotonic Use • 10 countries surveyed • Use of uterotonic high • Correct use of AMTSL was low: only 0.5 to 32 percent of observed deliveries • Findings suggest that AMTSL was not used at 1.4 million deliveries per year Source: POPPHI, 2009
All SBAs authorized to practice AMTSL and use oxytocin for AMTSL AMTSL integrated into preservice: doctors, nurses, midwives Oxytocin and ergomterine on National Essential Drugs List for PPH prevention and treatment; not misoprostol Ergometrine first line drug 58% of selected facilities have oxytocin in stock Results: Improved Policy Environment to Support Evidence-based Practice—Uganda
Cumulative % coverage of eligible pregnant women Results: Increased Uterotonic Coverage in Afghanistan Source: Sanghvi H et al., 2010
Results: Increased Uterotonic Coverage in Indonesia Uterotonic coverage: Oxytocin or misoprostol tablets Source: Sanghvi, et al., Prevention of Postpartum Hemorrhage Study, Jhpiego 2004
Results: Increased Uterotonic Coverage in Nepal Estimated total pregnancies—16,000 100% 73% Received miso—11,700 22% 53% Took miso—8,616 SBA Received oxytocic 75% Source: Nepal Family Health Program Technical Brief #11: Community-based Postpartum Hemorrhage Prevention
Results: Increased Attendance with SBA in Indonesia Source: Prevention of Postpartum Hemorrhage Study, Jhpiego 2004
Results: Reduced PPH Rate in Niger • Promotion of AMTSL, 33 government facilities • Increased AMTSL coverage from 5% to 98% of births • Dropped the PPH rate from 2.5% to 0.2% Source: URC, 2009
Results: Reduced Cases & Costs in Afghanistan • Training TBAs to administer misoprostol to treat PPH, 2 hypothetical cohorts of 10,000 women: • TBA referral after blood loss ≥500 ml • Administer 1,000 μg of misoprostol at blood loss ≥500 ml • Misoprostol strategy could: • Prevent 1647 cases of severe PPH (range: 810–2920) • Save $115,335 in costs of referral, IV therapy and transfusions (range: $13,991–$1,563,593) per 10,000 births. Source: S.E.K. Bradley et al., IJOG, 2006
Results: Anecdotal Mortality Impact • Indonesia: 1 district • Before program (2004): 19 PPH cases; 7 maternal deaths • During program (2005): 8 PPH cases; 2 maternal deaths • Nepal: 1 district • Expected # maternal deaths for the period: 45 • Observed # maternal deaths for the period: 29 • Afghanistan: • Expected # maternal deaths in intervention area: 27 • Actual # maternal deaths: 1 (postpartum eclampsia)
Results: PPH Reduction Modeling • Sub-Saharan Africa • Comprehensive intervention package (health facility strengthening and community-based services) reduces deaths due to PPH or sepsis after delivery by 32%—compared to just health facility strengthening alone (12% reduction) Source: Pagel et al., Lancet 2009
PPH Management: WHO Guidelines • WHO (2009) provides countries with evidence-based guidelines on the safety, quality and usefulness of interventions related to PPH management • These guidelines are focused on facility-based care and PPH management in faciliites with CEmONC capacity. Source: World Health Organization. WHO Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. 2009.
Management of PPH • Early detection—rapid management • Severe bleeding after birth is NOT normal • Monitor bleeding regularly during the PP period • Most emergency measures can be managed by a nurse or midwife (who has been trained) • Uterine massage • Administer uterotonics • Bimanual compression • Manual removal of placenta • Suture tears • Uterine/ovarian artery ligation or hysterectomy (by MD) Source: World Health Organization. WHO Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. 2009.
Atonic Uterus!First Action Is Massage Uterus
Source: IMPAC MCPC, 2003, Begley et al., 2010 Atonic Uterus (continued)
Manual Removal of Placenta • Wearing HLD gloves, insert hand into vagina along the cord. • Locate edge of placenta and slowly using edge of hand with fingers tightly together detach placenta from placental bed. • Hold on to placenta while providing counter-traction with other hand, and remove it. Source: IMPAC MCPC, 2003
Source: IMPAC MCPC, 2003 Bimanual Compression of the Uterus • Wearing HLD gloves, insert hand into vagina; form fist. • Place fist into anterior fornix and apply pressure against anterior wall of uterus. • With other hand, press deeply into abdomen behind uterus, applying pressure against posterior wall of uterus. • Maintain compression until bleeding is controlled and uterus contracts.
Source: IMPAC MCPC, 2003 Compression of Abdominal Aorta • Apply downward pressure with closed fist over abdominal aorta through abdominal wall (just above umbilicus slightly to patient’s left) • With other hand, palpate femoral pulse to check adequacy of compression • Pulse palpable = inadequate • Pulse not palpable = adequate • Maintain compression until bleeding is controlled
Emerging PPH Management Innovations • Use of misoprostol for treatment of PPH that occurs at home • A non-pneumatic anti-shock garment (NASG) to stabilize and prevent/treat shock during transport and management of PPH • Condom tamponade to treat PPH at facilities Source: Georgiou et al., 2009, IMPAC MCPC 2003, Ojengbede et al., 2011
Misoprostol for PPH Treatment in Home A 2005 study in Kigoma, Tanzania demonstrated that: • Traditional birth attendants (TBAs) can correctly diagnose and treat PPH with misoprostol after home births. • Only 2% of women in the intervention area (compared to 19% in the control group) were referred for further PPH treatment. • Of those referred, only 1% from the intervention area but 95% from the non-intervention area needed additional PPH treatment. Source: Prata N, et al., Int J Gynaecol Obstet. 2005
Treatment with Misoprostol vs. Oxytocin A study of PPH treatment options compared misoprostol (800 μg sublingual) with intravenous oxytocin (40 IU) to treat PPH in women who were not exposed to oxytocin prophalactically in three countries: • In both groups over 90% of women had active bleeding was controlled within 20 minutes (90% misoprostol, 96% oxytocin) • Oxytocin more effective at reducing median additional blood loss • Women receiving misoprostol more frequently needed additional uterotonic drugs or blood transfusion and experienced shivering and fever • Conclusion: Intravenous oxytocin should be used when available, with misoprostol as treatment alternative when oxytocin is not available. Source: Winikoff B, et al., Lancet. 2010 Jan 16;375(9710): 210–6.
Doing it Right: Technologies that can expedite care for PPH where it occurs Technologies appropriate for peripheral level services, even for homebirth Sources: Tsu, V; Coffey, P. BJOG 2009, Carroli, G et al., 2001
PPH Intervention: Anti-shock Garment • The Non-pneumatic Anti-Shock Garment (NASG) applies circumferential counter pressure to the lower body, legs, pelvis and stomach with pressure limits • Study among 1442 women in Egypt and Nigeria • Use of the NASG reduced median blood loss (from 400 mL in the pre-intervention phase to 200 mL) • Halved emergency hysterectomies (8.9% to 4.0%) • Decreased mortality (from 6.3% to 3.5%). Source: Miller S, et al,. BMC Pregnancy and Childbirth 2010, 10:64.
- More by User

Postpartum Hemorrhage (PPH) and abnormalities of the Third Stage
Postpartum Hemorrhage (PPH) and abnormalities of the Third Stage. Sept 12 – Dr. Z. Malewski. Definition. Excessive bleeding from the genital tract after the birth of the child Conventionally defined as a loss of more than 500ml of blood
1.41k views • 11 slides

Postpartum Hemorrhage
Postpartum Hemorrhage. Dr. Yasir Katib MB BS, FRCSC. Postpartum Haemorrhage. Introduction Risk Factors Prevention Treatment Pelvic Haematoma Umbrella Pack Uterine Inversion. PPH - Introduction. Acute blood loss – most common cause of hypotension in obstetrics
1.26k views • 31 slides

Postpartum Hypopituitarism Overt Diabetes Insipidus after Massive Postpartum Hemorrhage
. A 39-year-old lady delivered a normal term male infant weighting 3500g, about 3 hours ago without any instrument and uterine pressure. However, massive vaginal bleeding occurred one hour after this delivery. Oxytocin and methylergonovin were prescribed, but in vain. . . Massive
481 views • 36 slides

Postpartum Hemorrhage. HEE HEE That’s the only fake blood I could manage!!! Too messy. Jessi Goldstein MD MCH Fellow September 7, 2011. Objectives. We will discuss: Prevention of PPH. Causes of PPH. Initial and secondary interventions in PPH in both vaginal and Cesarean delivery.
1.04k views • 33 slides

Postpartum Hemorrhage(PPH)
Postpartum Hemorrhage(PPH). 产后出血 林建华. Major causes of death for pregnancy women ( maternal mortality). Postpartum hemorrhage ( 28%) heart diseases pregnancy-induced hypertension (or Amniotic fluid embolism ) infection. Definition of PPH.
1.32k views • 33 slides

Third Trimester Bleeding, Postpartum Hemorrhage, Shock Management
1.16k views • 46 slides

Routine postpartum care for the woman Name of presenter: Prevention of Postpartum Hemorrhage Initiative (POPPHI) Proj
Routine postpartum care for the woman Name of presenter: Prevention of Postpartum Hemorrhage Initiative (POPPHI) Project BASICS. By the end of this session, participants will be able to: Describe essential care to provide to the postpartum woman.
1.04k views • 37 slides

POSTPARTUM MANAGEMENT
POSTPARTUM MANAGEMENT. Out Line:. Introduction of post partum care Physical and psychosocial post partum care Examples of physical &psychosocial midwifery diagnosis in the post partum care Expected out comes for post partum care Physiological needs during post partum
1.57k views • 88 slides

Misoprostol for the Prevention of Postpartum Hemorrhage
Misoprostol for the Prevention of Postpartum Hemorrhage. Lisa J. Thomas, MD, FACOG Women’s Commission for Refugee Women and Children. Misoprostol . Background PGE1, heat stable, inexpensive Indications, Dosage, and Routes Abortion, Incomplete Abortion, Induction of Labor
491 views • 8 slides

Postpartum Hemorrhage. Postpartum Hemorrhage. รองศาสตราจารย์วราภรณ์ เชื้ออินทร์ ภาควิชาวิสัญญีวิทยา คณะแพทยศาสตร์ มหาวิทยาลัยขอนแก่น. การประชุมวิชาการวิสัญญีวิทยามอ.-มข.-มช. ครั้งที่ 3 17 กรกฎาคม 2551 ณ. รร.ป่าตอง รีสอร์ท จ.ภูเก็ต.
1.03k views • 22 slides

Postpartum Hemorrhage . Obsterics & Gynecology Hospital of Fudan University Weirong Gu. Postpartum Hemorrhage(PPH). Blood loss in excess of 500 ml following birth within the first 24 hours of delivery Serious intrapartum complication
3.79k views • 58 slides

Postpartum Hemorrhage. Learning Objectives. Define and differentiate between early and late PPH. Identify causes and risk factors of PPH. Identify symptoms and signs of PPH and list the laboratory investigations.
1.87k views • 47 slides

Postpartum Hemorrhage. Dr.Suresh Babu Chaduvula Professor Dept. of Obstetrics & Gynecology College of Medicine, Abha , KKU, Saudi Arabia. POSTPARTUM HEMORRHAGE [ PPH ]. Definition:
1.54k views • 34 slides
Simulation Training Assessment Tool (STAT )– Postpartum Hemorrhage
Simulation Training Assessment Tool (STAT )– Postpartum Hemorrhage. Learning Objectives: Recognize postpartum hemorrhage Recognize source from uterus and not due to cervical/vaginal laceration Recognize appropriate treatment and therapeutic doses of medications. Date: . Instructor(s): .
456 views • 1 slides
POSTPARTUM HEMORRHAGE
POSTPARTUM HEMORRHAGE. OBJECTIVES. To understand the importance of prompt and appropriate management in saving lives from PPH Define PPH List the causes and risk factors for PPH Discuss the steps taken in managing PPH. Recognizing Postpartum Hemorrhage. Bleeding >500 ml after childbirth
2.02k views • 18 slides

Scaling Up Misoprostol for Community-Based Prevention of Postpartum Hemorrhage in Bangladesh
Scaling Up Misoprostol for Community-Based Prevention of Postpartum Hemorrhage in Bangladesh Dr. Tapash Ranjan Das PM (MCH) & Deputy Director (MCH), DGFP & Dr. Abu Jamil Faisel Project Director, Mayer Hashi project (an Associate Award of the RESPOND project) &
269 views • 11 slides

Third Trimester Bleeding, Postpartum Hemorrhage, & Shock Management
Third Trimester Bleeding, Postpartum Hemorrhage, & Shock Management. UNC School of Medicine Obstetrics and Gynecology Clerkship Case Based Seminar Series. Objectives for Third Trimester Bleeding. List the causes of third trimester bleeding
887 views • 46 slides

Antepartum and Postpartum Hemorrhage
Antepartum and Postpartum Hemorrhage. Dr. Megha Jain. University College of Medical Sciences & GTB Hospital, Delhi. email: [email protected]. www.anaesthesia.co.in. Antepartum hemorrhage. Bleeding from or into genital tract after 28 th week of gestation.
1.89k views • 36 slides

Postpartum Hemorrhage. Dr.B Khani MD. Postpartum Hemorrhage. EBL > 500 cc 10% of deliveries If within 24 hrs. pp = 1 pp hemorrhage If 24 hrs. - 6 wks. pp = 2 pp hemorrhage Causes uterine atony – genital trauma retained placenta – placenta accreta uterine inversion. Uterine Atony.
926 views • 12 slides

Prevention and Management of TURP-Related Hemorrhage
Dr. Abdullah Ahmad Ghazi (R5) KSMC 8 May 2012. Prevention and Management of TURP-Related Hemorrhage. TURP gold standard in BPH Using of A-Cog & A-Plt is increasing. 4% on A-Cog 37% on A-plt. Introduction. The most common perioperative complication in TURP is hemorrhage.
668 views • 32 slides

Postpartum Hemorrhage. Christopher R. Graber, MD Salina Women’s Clinic 21 Feb 2012. Overview. Background Etiology of postpartum hemorrhage Primary Secondary Risk factors Evaluation and management Medical Surgical. Background. Severe bleeding is #1 worldwide cause of maternal death
702 views • 19 slides

Reducing Maternal Mortality Due to Postpartum Hemorrhage (PPH)
Reducing Maternal Mortality Due to Postpartum Hemorrhage (PPH). Objectives. Present PPH as a public health priority Define interventions available for PPH prevention and management Share country experiences and expected results. What Is Safe Motherhood?.
640 views • 34 slides

NurseStudy.Net
Nursing Education Site

Postpartum Hemorrhage Nursing Diagnosis and Nursing Care Plan
Last updated on April 29th, 2023 at 11:45 pm
Postpartum Hemorrhage Nursing Care Plans Diagnosis and Interventions
Postpartum Hemorrhage NCLEX Review and Nursing Care Plans
Postpartum hemorrhage (PPH) is a medical emergency that involves the abnormal or excessive vaginal bleeding of the mother after the birth of her baby.
It is important to note that vaginal bleeding called lochia is normally heavy from just after delivery until the next few hours and may not stop until the next few days.
The color of blood will usually change from bright red to brown over a couple of weeks. The full stoppage of lochia normally occurs no more than 12 weeks after delivery.
However, in postpartum hemorrhage there is either a heavy vaginal bleeding of at least 500 mL in the first 24 hours of delivery or between 23 hours and 12 weeks of delivery.
Types of Lochia
Postpartum hemorrhage may involve excessive bleeding and abnormality of lochia or postpartum vaginal discharge. It is especially important to take note of the duration of lochia rubra to help in the diagnosis of PPH. The following are the normal characteristics of the types or stages of lochia:
- Lochia rubra – refers to the first vaginal discharge; rubra means red in color; usually happens from Day 1 to Day 5 after birth
- Lochia serosa – the vaginal discharge appears either brownish or pinkish; typically occurs until Day 10 after birth
- Lochia alba – the vaginal discharge appears whitish or yellowish; typically happens from the 2 nd week to the 6 th week after birth, but may also extend to 12 weeks postpartum
Types of Postpartum Hemorrhage
- Primary PPH – occurs when the mother loses at least 500 mL or more of blood within the first 24 hours of delivering the baby.
- Major Primary PPH – losing 500 mL to 1000 mL of blood
- Minor Primary PPH – losing more than 1000 mL of blood
- Secondary PPH – occurs when the mother has heavy or abnormal vaginal bleeding between 24 hours and 12 weeks of delivering the baby.
Signs and Symptoms of Postpartum Hemorrhage
- Uncontrolled bleeding
- Hypotension – decreased blood pressure
- Tachycardia – increased heart rate
- Anemia – decrease in the red blood cell count or hemoglobin level
- Edema or hematoma – swelling and pain in or around the vaginal area
- Fatigue – extreme tiredness
The patient should also be educated on the following warning signs that would indicate the need to inform their healthcare provider either during hospital stay or after discharge:
- Excessive or increased vaginal bleeding – if the patient needs a new sanitary pad after an hour, or if she passes large blood clots
- Blurry vision or other visual disturbances
- Light-headedness or dizziness
- New or worsening stomach pain
- Tachycardia
Causes and Risk Factors of Postpartum Hemorrhage
The 4 T’s is a mnemonic that can be used to remember the 4 common causes of postpartum hemorrhage:
- Tone – uterine atony is the most common cause of PPH; overstretched uterus may cause a soft and boggy tone
- Trauma – rupture, inversion, hematoma, and/or laceration
- Tissue – retained or invasive placenta
- Thrombin – coagulopathy; bleeding disorders or blood clotting problems
The following are risk factors of postpartum hemorrhage:
A. Before Delivery
- Placenta previa – a condition wherein the placenta is situated low near the neck of the uterus
- Abruptio placentae – a condition wherein the placenta separates from the uterus earlier than expected
- Multiple pregnancies – carrying twins or more
- History of postpartum hemorrhage
- Pre-eclampsia – high blood pressure
- Obesity or having a BMI of greater than 35
- Thrombocytopenia or other blood clotting problems
- On anticoagulant therapy
B. After Delivery
- Delivery by Cesarean section
- Forceps delivery
- Induction of labor
- Delayed delivery of placenta or retained placenta – not passing the placenta within the hour after birth of the baby
- Tear in the perineum (lacerations) or episiotomy
- Fetal macrosomia – having a baby that weighs more than 9 lbs or 4 kg
- Hyperthermia during labor
- Having had a long labor – more than 12 hours
- Age of the mother – having the first baby at age 40 years or above
- Use of general anesthetic during delivery
Complications of Postpartum Hemorrhage
- Hypovolemic shock
- Failure of major organs, such as the lungs and kidneys
- Postpartum fatigue
Diagnosis of Postpartum Hemorrhage
- Measurement of blood loss – PPH is defined as blood loss of more than 500 mL in the first 24 hours post delivery
- Blood tests – include full blood count (particularly hemoglobin and hematocrit), clotting factors, and factor essays
- Pelvic exam – pregnant women who are at risk for PPH will undergo pelvic exam which checks the vagina, uterus, and cervix
- Imaging – ultrasound is the first imaging choice to visualize the baby and the pelvic organs
Prevention of Postpartum Hemorrhage
The following measures can be undertaken to prevent the likelihood of postpartum hemorrhage:
- Early recognition of the risk for PPH. Stopping or reducing anticoagulants, oral iron supplementation, coagulation tests, and regular antenatal check-ups are helpful in preventing PPH.
Treatment for Postpartum Hemorrhage
- Medications. Several medications may be prescribed to treat PPH:
- Uterotonic agents – utilized to prevent or control PPH. Oxytocin is the first-line prevention and treatment for PPH. It is used to decrease the blood flow through the uterus after the delivery of the baby.
- Adjuvant therapies – anti-bleeding drugs can be administered within the first 3 hours of the start of PPH
- Antibiotics – may be required if a bacterial infection has caused or contributed to PPH based on the culture results of the lochia
- Intravenous fluid replacement
- Uterine massage
- Transfusion – low hemoglobin /hematocrit level and excessive blood loss may require transfusion of blood and plasma products.
- Application of pressure on labial or perineal lacerations
- Episiotomy Repair – timely repair of lacerations and episiotomy is important in controlling PPH
- Reduction of uterine inversion – the Johnson method is a manual procedure wherein the protruding uterus is returned in the normal position by pushing it inside toward the direction of the umbilicus
- Manual removal of retained placental tissues
- Surgery- hysterectomy (removal of the uterus) or laparatomy may be needed if the other treatments are not effective in stopping PPH
Nursing Diagnosis for Postpartum Hemorrhage
Nursing care plan for postpartum hemorrhage 1.
Nursing Diagnosis: Fluid Volume Deficit related to blood volume loss secondary to postpartum hemorrhage as evidenced by lochia rubia of 500 mL in the first 24 hours post-delivery, decrease in red blood cell count/ hemoglobin/ hematocrit levels, skin pallor, heart rate of 120 bpm, blood pressure level of 85/50, and lightheadedness
Desired Outcome: The patient will have a lochia flow of less than one saturated pad per hour, a hemoglobin (HB) level of over 100, blood pressure and heart rate levels within normal range, full level of consciousness, and normal skin color
Nursing Care Plan for Postpartum Hemorrhage 2
Nursing Diagnosis: Risk for Bleeding related to C-section delivery of the baby
Desired Outcome: To prevent any bleeding episode after C-section delivery of the baby.
Nursing Care Plan for Postpartum Hemorrhage 3
Ineffective Tissue Perfusion
Diagnosis: Ineffective Tissue Perfusion related to hypovolemia secondary to postpartum hemorrhage as demonstrated by reduced arterial pulsations, cold and pale color skin at the extremities, increased perspiration, lesser capillary refill, reduced milk production, changes in vital signs, and altered neurologic status.
Desired Outcomes:
- The patient will exhibit vital signs within the normal range.
- The patient laboratory result of arterial blood gases, hematocrit, and hemoglobin levels are acceptable findings.
- The patient will show signs of desired hormonal changes such as a sufficient supply of breastmilk for lactation, and resumption of normal menstruation cycle.
Nursing Care Plan for Postpartum Hemorrhage 4
Risk for Infection
Nursing Diagnosis: Risk for Infection related to the stasis of body fluids and traumatized tissues secondary to postpartum hemorrhage.
- The patient will express an understanding of the causative, and risk factors.
- The patient’s vital signs will be maintained within normal ranges.
- The patient’s will exhibit lochia free from foul smelling odor.
- The patient’s laborataory values will improve and within normal levels.
Nursing Care Plan for Postpartum Hemorrhage 5
Risk for Impaired Attachment
Nursing Diagnosis: Risk for Impaired Attachment related to anxiety associated with the parent role secondary to postpartum hemorrhage.
- The patient will verbalize a feeling of happiness with the role as a parent.
- The patient will take the duty for the physical and emotional well-being of the infant.
- The patient will show proper behavior related to positive attachment to the infant.
- The patient will participate in mutually satisfying contact with the child.

More Postpartum Hemorrhage Nursing Diagnosis
- Risk for Shock (Hypovolemic)
- Risk for Injury
- Ineffective Coping
Nursing References
Ackley, B. J., Ladwig, G. B., Makic, M. B., Martinez-Kratz, M. R., & Zanotti, M. (2020). Nursing diagnoses handbook: An evidence-based guide to planning care . St. Louis, MO: Elsevier. Buy on Amazon
Gulanick, M., & Myers, J. L. (2022). Nursing care plans: Diagnoses, interventions, & outcomes . St. Louis, MO: Elsevier. Buy on Amazon
Ignatavicius, D. D., Workman, M. L., Rebar, C. R., & Heimgartner, N. M. (2020). Medical-surgical nursing: Concepts for interprofessional collaborative care . St. Louis, MO: Elsevier. Buy on Amazon
Silvestri, L. A. (2020). Saunders comprehensive review for the NCLEX-RN examination . St. Louis, MO: Elsevier. Buy on Amazon
Disclaimer:
Please follow your facilities guidelines, policies, and procedures.
The medical information on this site is provided as an information resource only and is not to be used or relied on for any diagnostic or treatment purposes.
This information is intended to be nursing education and should not be used as a substitute for professional diagnosis and treatment.
Anna Curran. RN, BSN, PHN
Leave a Comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .


ANN EVENSEN, MD, JANICE M. ANDERSON, MD, AND PATRICIA FONTAINE, MD, MS
This is an updated version of the article that appeared in print.
Am Fam Physician. 2017;95(7):442-449
Author disclosure: No relevant financial affiliations.
Postpartum hemorrhage is common and can occur in patients without risk factors for hemorrhage. Active management of the third stage of labor should be used routinely to reduce its incidence. Use of oxytocin after delivery of the anterior shoulder is the most important and effective component of this practice. Oxytocin is more effective than misoprostol for prevention and treatment of uterine atony and has fewer adverse effects. Routine episiotomy should be avoided to decrease blood loss and the risk of anal laceration. Appropriate management of postpartum hemorrhage requires prompt diagnosis and treatment. The Four T's mnemonic can be used to identify and address the four most common causes of postpartum hemorrhage (uterine atony [Tone]; laceration, hematoma, inversion, rupture [Trauma]; retained tissue or invasive placenta [Tissue]; and coagulopathy [Thrombin]). Rapid team-based care minimizes morbidity and mortality associated with postpartum hemorrhage, regardless of cause. Massive transfusion protocols allow for rapid and appropriate response to hemorrhages exceeding 1,500 mL of blood loss. The National Partnership for Maternal Safety has developed an obstetric hemorrhage consensus bundle of 13 patient- and systems-level recommendations to reduce morbidity and mortality from postpartum hemorrhage.
Approximately 3% to 5% of obstetric patients will experience postpartum hemorrhage. 1 Annually, these preventable events are the cause of one-fourth of maternal deaths worldwide and 12% of maternal deaths in the United States. 2 , 3 The American College of Obstetricians and Gynecologists defines early postpartum hemorrhage as at least 1,000 mL total blood loss or loss of blood coinciding with signs and symptoms of hypovolemia within 24 hours after delivery of the fetus or intrapartum loss. 4 , 5 Primary postpartum hemorrhage may occur before delivery of the placenta and up to 24 hours after delivery of the fetus. Complications of postpartum hemorrhage are listed in Table 1 3 , 6 , 7 ; these range from worsening of common postpartum symptoms such as fatigue and depressed mood, to death from cardiovascular collapse.
This review presents evidence-based recommendations for the prevention of and appropriate response to postpartum hemorrhage and is intended for physicians who provide antenatal, intrapartum, and postpartum care.
Risk factors for postpartum hemorrhage are listed in Table 2 . 8 However, 20% of postpartum hemorrhage occurs in women with no risk factors, so physicians must be prepared to manage this condition at every delivery. 9 Strategies for decreasing the morbidity and mortality associated with postpartum hemorrhage are listed in Table 3 , 6 , 10 – 14 including the choice to deliver infants in women at high risk of hemorrhage at facilities with immediately available surgical, intensive care, and blood bank services.
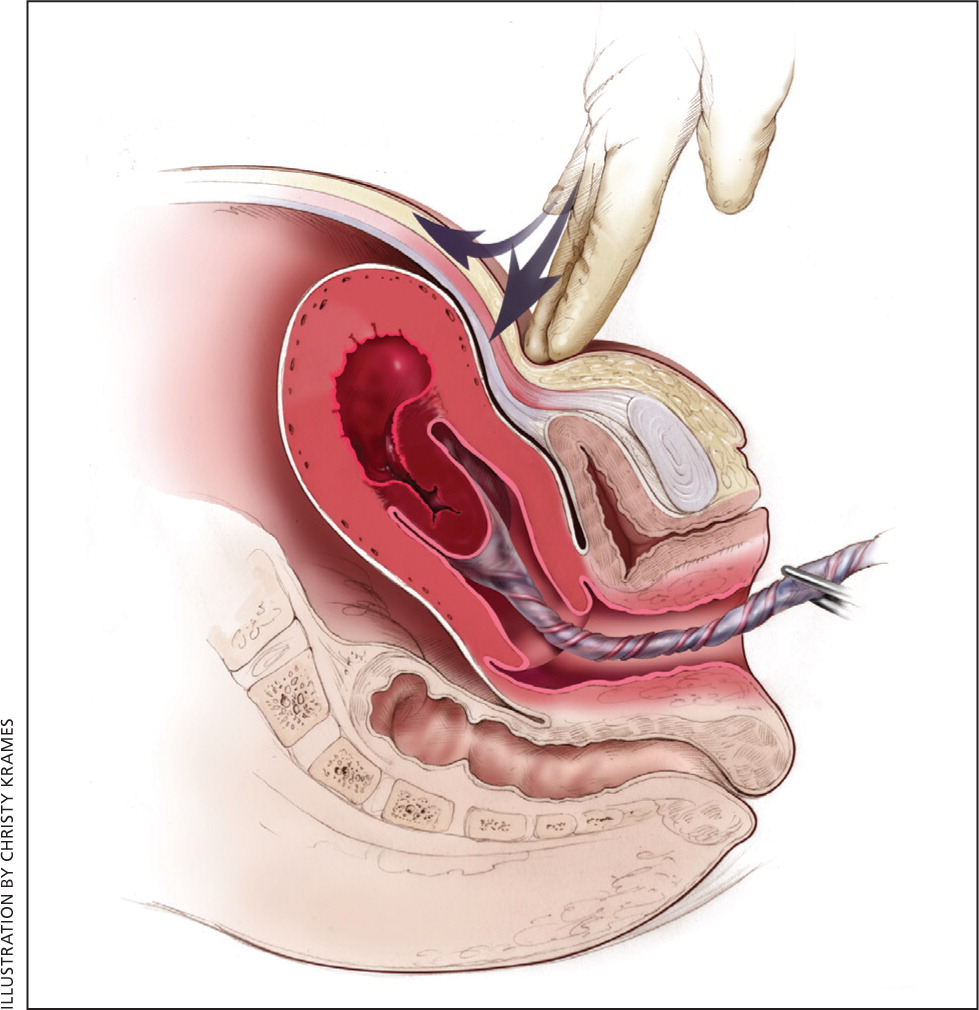
The most effective strategy to prevent postpartum hemorrhage is active management of the third stage of labor (AMTSL). AMTSL also reduces the risk of a postpartum maternal hemoglobin level lower than 9 g per dL (90 g per L) and the need for manual removal of the placenta. 11 Components of this practice include: (1) administering oxytocin (Pitocin) with or soon after the delivery of the anterior shoulder; (2) controlled cord traction (Brandt-Andrews maneuver) to deliver the placenta; and (3) uterine massage after delivery of the placenta. 11 Placental delivery can be achieved using the Brandt-Andrews maneuver, in which firm traction on the umbilical cord is applied with one hand while the other applies suprapubic counterpressure 15 ( eFigure A ).
The individual components of AMTSL have been evaluated and compared. Based on existing evidence, the most important component is administration of a uterotonic drug, preferably oxytocin. 12 , 16 The number needed to treat to prevent one case of hemorrhage 500 mL or greater is 7 for oxytocin administered after delivery of the fetal anterior shoulder or after delivery of the neonate compared with placebo. 16 The risk of postpartum hemorrhage is also reduced if oxytocin is administered after placental delivery instead of at the time of delivery of the anterior shoulder. 17 Dosing instructions are provided in Table 4 . 6
An alternative to oxytocin is misoprostol (Cytotec), an inexpensive medication that does not require injection and is more effective than placebo in preventing postpartum hemorrhage. 12 However, most studies have shown that oxytocin is superior to misoprostol. 12 , 18 Misoprostol also causes more adverse effects than oxytocin—commonly nausea, diarrhea, and fever within three hours of birth. 12 , 18
The benefits of controlled cord traction and uterine massage in preventing postpartum hemorrhage are less clear, but these strategies may be helpful. 15 , 19 , 20 Controlled cord traction does not prevent severe postpartum hemorrhage, but reduces the incidence of less severe blood loss (500 to 1,000 mL) and reduces the need for manual extraction of the placenta. 21
Diagnosis and Management
Diagnosis of postpartum hemorrhage begins with recognition of excessive bleeding and targeted examination to determine its cause ( Figure 1 6 ). Cumulative blood loss should be monitored throughout labor and delivery and postpartum with quantitative measurement, if possible. 22 Although some important sources of blood loss may occur intrapartum (e.g., episiotomy, uterine rupture), most of the fluid expelled during delivery of the infant is urine or amniotic fluid. Quantitative measurement of postpartum bleeding begins immediately after the birth of the infant and entails measuring cumulative blood loss with a calibrated underbuttocks drape, or by weighing blood-soaked pads, sponges, and clots; combined use of these methods is also appropriate for obtaining an accurate measurement. 22 Healthy pregnant women can typically tolerate 500 to 1,000 mL of blood loss without having signs or symptoms. 9 Tachycardia may be the earliest sign of postpartum hemorrhage. Orthostasis, hypotension, nausea, dyspnea, oliguria, and chest pain may indicate hypovolemia from significant hemorrhage. If excess bleeding is diagnosed, the Four T's mnemonic (uterine atony [Tone]; laceration, hematoma, inversion, rupture [Trauma]; retained tissue or invasive placenta [Tissue]; and coagulopathy [Thrombin]) can be used to identify specific causes ( Table 5 6 ). Regardless of the cause of bleeding, physicians should immediately summon additional personnel and begin appropriate emergency hemorrhage protocols.
TONE (UTERINE ATONY)
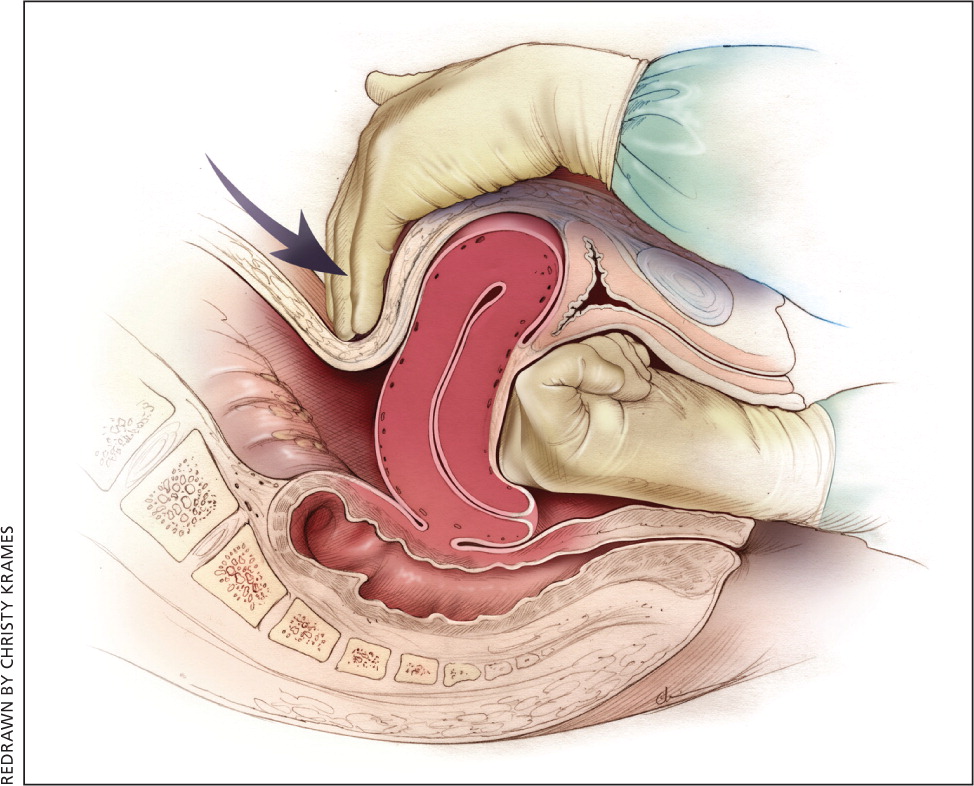
Uterine atony is the most common cause of postpartum hemorrhage. 9 Brisk blood flow after delivery of the placenta unresponsive to transabdominal massage should prompt immediate action including bimanual compression of the uterus and use of uterotonic medications ( Table 4 6 ). Massage is performed by placing one hand in the vagina and pushing against the body of the uterus while the other hand compresses the fundus from above through the abdominal wall ( eFigure B ).
Uterotonic agents include oxytocin, ergot alkaloids, and prostaglandins. Oxytocin is the most effective treatment for postpartum hemorrhage, even if already used for labor induction or augmentation or as part of AMTSL. 8 , 23 , 24 The choice of a second-line uterotonic should be based on patient-specific factors such as hypertension, asthma, or use of protease inhibitors. Although it is not a uterotonic, tranexamic acid (Cyklokapron) may reduce mortality due to bleeding from postpartum hemorrhage (but not overall mortality) when given within the first three hours and may be considered as an adjuvant therapy. 25 [updated] Table 4 outlines dosages, cautions, contraindications, and common adverse effects of uterotonic medications and tranexamic acid. 6
Lacerations and hematomas due to birth trauma can cause significant blood loss that can be lessened by hemostasis and timely repair. Episiotomy increases the risk of blood loss and anal sphincter tears; this procedure should be avoided unless urgent delivery is necessary and the perineum is thought to be a limiting factor. 26
Vaginal and vulvar hematomas can present as pain or as a change in vital signs disproportionate to the amount of blood loss. Small hematomas can be managed with ice packs, analgesia, and observation. Patients with persistent signs of volume loss despite fluid replacement, as well as those with large (greater than 3 to 4 cm) or enlarging hematomas, require incision and evacuation of the clot. 27 The involved area should be irrigated and hemostasis achieved by ligating bleeding vessels, placing figure-of-eight sutures, and creating a layered closure, or by using any of these methods alone.
Uterine inversion is rare, occurring in only 0.04% of deliveries, and is a potential cause of postpartum hemorrhage. 27 AMTSL does not appear to increase the incidence of uterine inversion, but invasive placenta does. 27 , 28 The contributions of other conditions such as fundal implantation of the placenta, fundal pressure, and undue cord traction are unclear. 27 The inverted uterus usually appears as a bluish-gray mass protruding from the vagina. Patients with uterine inversion may have signs of shock without excess blood loss. If the placenta is attached, it should be left in place until after reduction to limit hemorrhage. 27 Every attempt should be made to quickly replace the uterus. The Johnson method of reduction begins with grasping the protruding fundus with the palm of the hand, directing the fingers toward the posterior fornix. 27 The uterus is returned to position by lifting it up through the pelvis and into the abdomen ( eFigure C ). Once the uterus is reverted, uterotonic agents can promote uterine tone and prevent recurrence. If initial attempts to replace the uterus fail or contraction of the lower uterine segment (contraction ring) develops, the use of magnesium sulfate, terbutaline, nitroglycerin, or general anesthesia may allow sufficient uterine relaxation for manipulation. 28
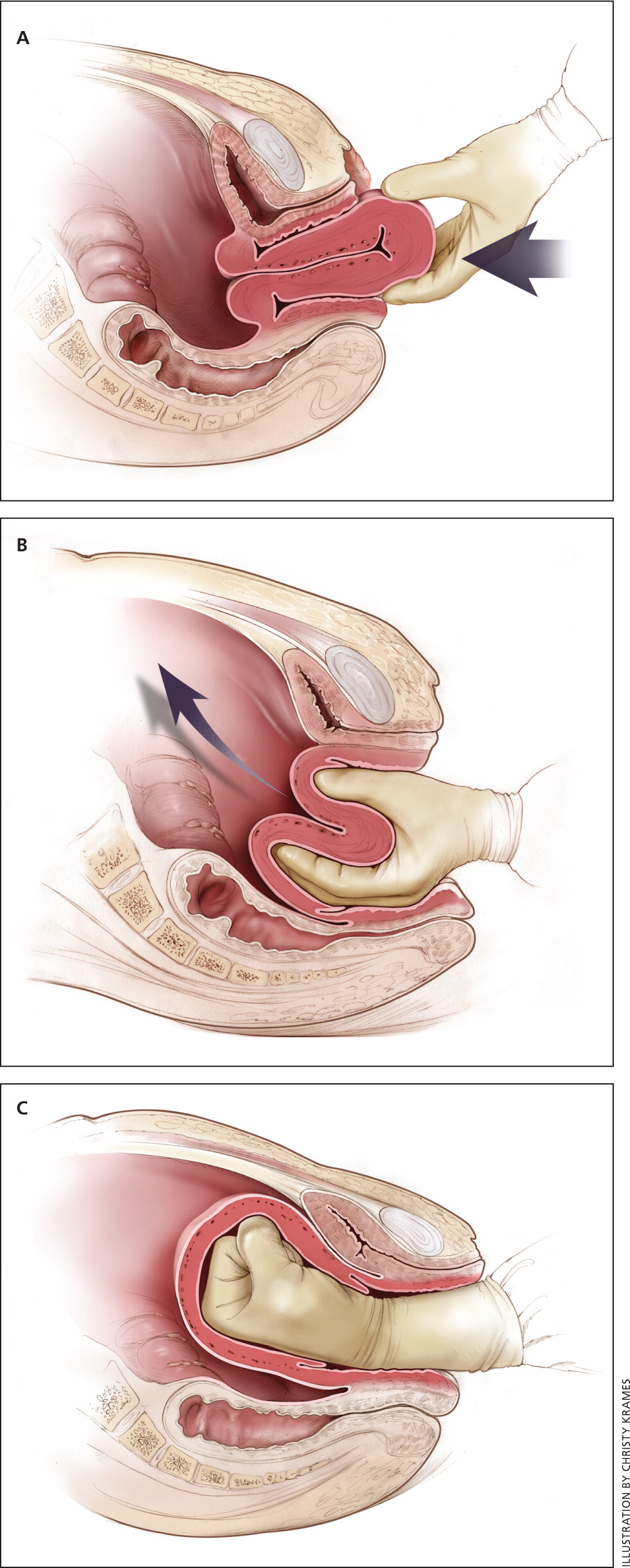
Uterine rupture can cause intrapartum and postpartum hemorrhage. 29 Although rare in an unscarred uterus, clinically significant uterine rupture occurs in 0.8% of vaginal births after cesarean delivery via low transverse uterine incision. 30 Induction and augmentation increase the risk of uterine rupture, especially for patients with prior cesarean delivery. 31 Before delivery, the primary sign of uterine rupture is fetal bradycardia. 31 , 32 Other signs and symptoms of uterine rupture are listed in eTable A .
Retained tissue (i.e., placenta, placental fragments, or blood clots) prevents the uterus from contracting enough to achieve optimal tone. Classic signs of placental separation include a small gush of blood, lengthening of the umbilical cord, and a slight rise of the uterus. The mean time from delivery to placental expulsion is eight to nine minutes. 33 Longer intervals are associated with an increased risk of postpartum hemorrhage, with rates doubling after 10 minutes. 33 Retained placenta (i.e., failure of the placenta to deliver within 30 minutes) occurs in less than 3% of vaginal deliveries. 34 , 35 If the placenta is retained, consider manual removal using appropriate analgesia. 35 Injecting the umbilical vein with saline and oxytocin does not clearly reduce the need for manual removal. 35 – 37 If blunt dissection with the edge of the gloved hand does not reveal the tissue plane between the uterine wall and placenta, invasive placenta should be considered.
Invasive placenta (placenta accreta, increta, or percreta) can cause life-threatening postpartum hemorrhage. 13 , 34 , 35 The incidence has increased with time, mirroring the increase in cesarean deliveries. 13 , 34 In addition to prior cesarean delivery, other risk factors for invasive placenta include placenta previa, advanced maternal age, high parity, and previous invasive placenta. 13 , 34 Treatment of invasive placenta can require hysterectomy or, in select cases, conservative management (i.e., leaving the placenta in place or giving weekly oral methotrexate). 13
THROMBIN (COAGULATION DEFECTS)
Coagulation defects can cause a hemorrhage or be the result of one. These defects should be suspected in patients who have not responded to the usual measures to treat postpartum hemorrhage or who are oozing from puncture sites. A coagulation defect should also be suspected if blood does not clot in bedside receptacles or red-top (no additives) laboratory collection tubes within five to 10 minutes. Coagulation defects may be congenital or acquired ( eTable B ). Evaluation should include a platelet count and measurement of prothrombin time, partial thromboplastin time, fibrinogen level, fibrin split products, and quantitative d-dimer assay. Physicians should treat the underlying disease process, if known, and support intravascular volume, serially evaluate coagulation status, and replace appropriate blood components using an emergency release protocol to improve response time and decrease risk of dilutional coagulopathy. 7 , 38 , 39 [updated]
Ongoing or Severe Hemorrhage
Significant blood loss from any cause requires immediate resuscitation measures using an interdisciplinary, stage-based team approach. Physicians should perform a primary maternal survey and institute care based on American Heart Association standards and an assessment of blood loss. 14 , 40 Patients should be given oxygen, ventilated as needed, and provided intravenous fluid and blood replacement with normal saline or other crystalloid fluids administered through two large-bore intravenous needles. Fluid replacement volume should initially be given as a bolus infusion and subsequently adjusted based on frequent reevaluation of the patient's vital signs and symptoms. The use of O negative blood may be needed while waiting for type-specific blood.
Elevating the patient's legs will improve venous return. Draining the bladder with a Foley catheter may improve uterine atony and will allow monitoring of urine output. Massive transfusion protocols to decrease the risk of dilutional coagulopathy and other postpartum hemorrhage complications have been established. These protocols typically recommend the use of four units of fresh frozen plasma and one unit of platelets for every four to six units of packed red blood cells used. 7 , 39
Uterus-conserving treatments include uterine packing (plain gauze or gauze soaked with vasopressin, chitosan, or carboprost [Hemabate]), artery ligation, uterine artery embolization, B-lynch compression sutures, and balloon tamponade. 7 , 41 – 43 Balloon tamponade (in which direct pressure is applied to potential bleeding sites via a balloon that is inserted through the vagina and cervix and inflated with sterile water or saline), uterine packing, aortic compression, and nonpneumatic antishock garments may be used to limit bleeding while definitive treatment or transport is arranged. 7 , 41 , 44 Hysterectomy is the definitive treatment in women with severe, intractable hemorrhage.
Follow-up of postpartum hemorrhage includes monitoring for ongoing blood loss and vital signs, assessing for signs of anemia (fatigue, shortness of breath, chest pain, or lactation problems), and debriefing with patients and staff. Many patients experience acute and posttraumatic stress disorders after a traumatic delivery. Individual, trauma-focused cognitive behavior therapy can be offered to reduce acute traumatic stress symptoms. 45 Debriefing with staff may identify necessary systems-level changes ( Table 3 ). 6 , 10 – 14
Systems Approach to Prevention and Treatment
Complications of postpartum hemorrhage are common, even in high-resource countries and well-staffed delivery suites. Based on an analysis of systems errors identified in The Joint Commission's 2010 Sentinel Event Alert, the commission recommended that hospitals establish protocols to enable an optimal response to changes in maternal vital signs and clinical condition. These protocols should be tested in drills, and systems problems that interfere with care should be fixed through their continual refinement. 46 In response, The Council on Patient Safety in Women's Health Care outlined essential steps that delivery units should take to decrease the incidence and severity of postpartum hemorrhage 14 ( Table 3 6 , 10 – 14 ). The creation of a hemorrhage cart with supplies, and the use of huddles, rapid response teams, and massive transfusion protocols are among the recommendations. Advanced Life Support in Obstetrics (ALSO) training can be part of a systems approach to improving patient care. The use of interdisciplinary team training with in situ simulation, available through the ALSO program and from TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), has been shown to improve perinatal safety. 47 , 48
This article updates previous articles on this topic by Maughan, et al. , 49 and by Anderson and Etches . 50
Data Sources: A PubMed search was completed in Clinical Queries using the key term postpartum hemorrhage. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Cochrane Database of Systematic Reviews, Essential Evidence Plus, National Institute for Health and Care Excellence guidelines, Agency for Healthcare Research and Quality evidence reports, the Institute for Clinical Systems Improvement, and the National Guideline Clearinghouse. Search dates: October 12, 2015, and January 19, 2016.
This article is one in a series on “Advanced Life Support in Obstetrics (ALSO),” initially established by Mark Deutchman, MD, Denver, Colo. The coordinator of this series is Larry Leeman, MD, MPH, ALSO Managing Editor, Albuquerque, N.M.
Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55.
Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323-e333.
Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199(1):36.e1-36.e5.
Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124(1):150-153.
American College of Obstetricians and Gynecologists. Obstetric data definitions (version 1.0). Revitalize. https://www.acog.org/-/media/Departments/Patient-Safety-and-Quality-Improvement/2014reVITALizeObstetricDataDefinitionsV10.pdf . Accessed October 2, 2016.
Evensen A, Anderson J. Chapter J. Postpartum hemorrhage: third stage pregnancy. In: Leeman L, Quinlan J, Dresang LT, eds. Advanced Life Support in Obstetrics: Provider Syllabus. 5th ed. Leawood, Kan.: American Academy of Family Physicians; 2014.
Guidelines and Audit Committee of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg52/ . Accessed March 23, 2017. [updated]
Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2014(2):CD003249.
Magann EF, Evans S, Hutchinson M, Collins R, Howard BC, Morrison JC. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J. 2005;98(4):419-422.
Council on Patient Safety in Women's Health Care. Obstetric hemorrhage patient safety bundle. http://safehealthcareforeverywoman.org/patient-safety-bundles/obstetric-hemorrhage/ [login required]. Accessed October 16, 2016.
Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015(3):CD007412.
Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012(8):CD000494.
Committee on Obstetric Practice. Committee opinion no. 529: placenta accreta. Obstet Gynecol. 2012;120(1):207-211.
Main EK, Goffman D, Scavone BM, et al.; National Partnership for Maternal Safety; Council on Patient Safety in Women's Health Care. National partnership for maternal safety: consensus bundle on obstetric hemorrhage [published correction appears in Obstet Gynecol. 2015;126(5):1111]. Obstet Gynecol. 2015;126(1):155-162.
Deneux-Tharaux C, Sentilhes L, Maillard F, et al. Effect of routine controlled cord traction as part of the active management of the third stage of labour on postpartum haemorrhage: multicentre randomized controlled trial (TRACOR) [published corrections appear in BMJ. 2013;347:f6619 and BMJ. 2013;346:f2542]. BMJ. 2013;346:f1541.
Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013(10):CD001808.
Soltani H, Hutchon DR, Poulose TA. Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database Syst Rev. 2010(8):CD006173.
Bellad MB, Tara D, Ganachari MS, et al. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG. 2012;119(8):975-982 , discussion 982–986.
Hofmeyr GJ, Abdel-Aleem H, Abdel-Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2013(7):CD006431.
Chen M, Chang Q, Duan T, He J, Zhang L, Liu X. Uterine massage to reduce blood loss after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2013;122(2 pt 1):290-295.
Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labour. Cochrane Database Syst Rev. 2015(1):CD008020.
Quantification of blood loss: AWHONN practice brief number 1. J Obstet Gynecol Neonatal Nurs. 2015;44(1):158-160.
American College of Obstetricians and Gynecologists. Postpartum hemorrhage from vaginal delivery. Patient safety checklist no. 10. Obstet Gynecol. 2013;121:1151-1152.
Sosa CG, Althabe F, Belizan JM, Buekens P. Use of oxytocin during early stages of labor and its effect on active management of third stage of labor. Am J Obstet Gynecol. 2011;204(3):238.e1-238.e5.
WOMAN Trial Collaborators.
Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009(1):CD000081.
You WB, Zahn CM. Postpartum hemorrhage: abnormally adherent placenta, uterine inversion, and puerperal hematomas. Clin Obstet Gynecol. 2006;49(1):184-197.
Baskett TF. Acute uterine inversion: a review of 40 cases. J Obstet Gynaecol Can. 2002;24(12):953-956.
Guiliano M, Closset E, Therby D, LeGoueff F, Deruelle P, Subtil D. Signs, symptoms and complications of complete and partial uterine ruptures during pregnancy and delivery. Eur J Obstet Gynecol Reprod Biol. 2014;179:130-134.
National Institutes of Health Consensus Development Conference Panel. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010. Obstet Gynecol. 2010;115(6):1279-1295.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 115: vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
Guise JM, McDonagh MS, Osterweil P, Nygren P, Chan BK, Helfand M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ. 2004;329(7456):19-25.
Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105(2):290-293.
Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192(5):1458-1461.
Weeks AD. The retained placenta. Best Pract Res Clin Obstet Gynaecol. 2008;22(6):1103-1117.
Nardin JM, Weeks A, Carroli G. Umbilical vein injection for management of retained placenta. Cochrane Database Syst Rev. 2011(5):CD001337.
Güngördük K, Asicioglu O, Besimoglu B, et al. Using intraumbilical vein injection of oxytocin in routine practice with active management of the third stage of labor: a randomized controlled trial. Obstet Gynecol. 2010;116(3):619-624.
Cunningham FG, Nelson DB. Disseminated intravascular coagulation syndromes in obstetrics. Obstet Gynecol. 2015;126(5):999-1011.
Lyndon A, Lagrew D, Shields LE, Main E, Cape V. Improving health care response to obstetric hemorrhage. (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11–10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, 3/17/15.
Neumar RW, Shuster M, Callaway CW, et al. Part 1: Executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
World Health Organization. WHO guidelines for the management of postpartum haemorrhage and retained placenta. http://apps.who.int/iris/bitstream/10665/44171/1/9789241598514_eng.pdf . Accessed September 29, 2016.
Grönvall M, Tikkanen M, Tallberg E, Paavonen J, Stefanovic V. Use of Bakri balloon tamponade in the treatment of postpartum hemorrhage: a series of 50 cases from a tertiary teaching hospital. Acta Obstet Gynecol Scand. 2013;92(4):433-438.
American College of Obstetricians and Gynecologists. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
Miller S, Martin HB, Morris JL. Anti-shock garment in postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2008;22(6):1057-1074.
Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database Syst Rev. 2010(3):CD007944.
Preventing maternal death. Jt Comm Perspect. 2010;30(3):7-9.
Riley W, Davis S, Miller K, Hansen H, Sainfort F, Sweet R. Didactic and simulation nontechnical skills team training to improve perinatal patient outcomes in a community hospital. Jt Comm J Qual Patient Saf. 2011;37(8):357-364.
American College of Obstetricians and Gynecologists Committee on Patient Safety and Quality Improvement. Committee opinion no. 590: preparing for clinical emergencies in obstetrics and gynecology. Obstet Gynecol. 2014;123(3):722-725.
Maughan K, Heim SW, Galazka SS. Preventing postpartum hemorrhage: managing the third stage of labor. Am Fam Physician. 2006;73(6):1025-1028.
Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75(6):875-882.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2017 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Diagnosis and management of postpartum hemorrhage and intrapartum asphyxia in a quality improvement initiative using nurse-mentoring and simulation in Bihar, India
Rakesh ghosh.
1 Institute for Global Health Sciences, University of California, San Francisco, San Francisco, CA, United States of America
Hilary Spindler
Melissa c. morgan.
2 Department of Pediatrics, University of California, San Francisco, San Francisco, CA, United States of America
3 Maternal, Adolescent, Reproductive, and Child Health Centre, London School of Hygiene and Tropical Medicine, London, United Kingdom
Susanna R. Cohen
4 College of Nursing, University of Utah, Salt Lake City, UT, United States of America
Nilophor Begum
5 CARE India, Patna, Bihar, India
Tanmay Mahapatra
Dilys m. walker.
6 Department of Obstetrics and Gynecology and Reproductive Services, University of California, San Francisco, San Francisco, CA, United States of America
Associated Data
Minimal data set is within the paper or Supporting Information files.
In the state of Bihar, India a multi-faceted quality improvement nurse-mentoring program was implemented to improve provider skills in normal and complicated deliveries. The objective of this analysis was to examine changes in diagnosis and management of postpartum hemorrhage (PPH) of the mother and intrapartum asphyxia of the infant in primary care facilities in Bihar, during the program.
During the program, mentor pairs visited each facility for one week, covering four facilities over a four-week period and returned for subsequent week-long visits once every month for seven to nine consecutive months. Between- and within-facility comparisons were made using a quasi-experimental and a longitudinal design over time, respectively, to measure change due to the intervention. The proportions of PPH and intrapartum asphyxia among all births as well as the proportions of PPH and intrapartum asphyxia cases that were effectively managed were examined. Zero-inflated negative binomial models and marginal structural methodology were used to assess change in diagnosis and management of complications after accounting for clustering of deliveries within facilities as well as time varying confounding.
This analysis included 55,938 deliveries from 320 facilities. About 2% of all deliveries, were complicated with PPH and 3% with intrapartum asphyxia. Between-facility comparisons across phases demonstrated diagnosis was always higher in the final week of intervention (PPH: 2.5–5.4%, intrapartum asphyxia: 4.2–5.6%) relative to the first week (PPH: 1.2–2.1%, intrapartum asphyxia: 0.7–3.3%). Within-facility comparisons showed PPH diagnosis increased from week 1 through 5 (from 1.6% to 4.4%), after which it decreased through week 7 (3.1%). A similar trend was observed for intrapartum asphyxia. For both outcomes, the proportion of diagnosed cases where selected evidence-based practices were used for management either remained stable or increased over time.
Conclusions
The nurse-mentoring program appears to have built providers’ capacity to identify PPH and intrapartum asphyxia cases but diagnosis levels are still not on par with levels observed in Southeast Asia and globally.
Introduction
Globally, an estimated 275,000 maternal deaths and 2.7 million neonatal deaths occur annually, a quarter of which occurs in India [ 1 , 2 ]. Hemorrhage, the leading cause of maternal mortality accounted for 27% of all deaths globally and 38% in India [ 3 , 4 ]. Intrapartum asphyxia is the second important cause, accounting for 11% and 19%, of all neonatal deaths globally and in India, respectively [ 2 , 5 ]. Further, a third of all neonatal deaths globally [ 6 ] and in India [ 7 ] occur within 24-hours of birth. Thus, interventions aimed at improving intrapartum and immediate postnatal care could significantly impact neonatal and maternal survival.
A critical step towards preventing maternal and neonatal mortality is timely diagnosis and management of postpartum hemorrhage (PPH) and intrapartum asphyxia, which remains largely underdiagnosed in primary care facilities in India [ 8 , 9 ]. Skilled health personnel, who attend 71% of all deliveries worldwide and 79% in India, need to be able to identify and manage such complications [ 10 , 11 ]. In fact, estimates suggest that basic neonatal resuscitation (NR) including drying and stimulating, repositioning, clearing airways and positive pressure ventilation (PPV), could prevent about 30% of intrapartum-related neonatal deaths [ 12 ].
The Government of India initiated a program in 2005 to increase institutional deliveries with the expectation that skilled attendants are better able to identify and manage maternal and neonatal complications, thereby saving lives [ 13 ]. However, in the state of Bihar, where the population is predominantly rural [ 14 ], despite an increase in institutional delivery, concomitant reduction in neonatal mortality was not observed [ 2 ], suggesting sub-optimal quality of care in these facilities. Indeed, studies from Bihar report that providers lack essential clinical skills, and facilities lack trained staff and adequate infrastructure [ 15 , 16 ].
A nurse-mentoring program including integrated simulation training targeting individual and team performance was implemented in Bihar with the overall aim of improving the quality of facility-based care [ 17 ]. Previous reports have demonstrated effectiveness of this intervention, implemented on a smaller scale, to increase use of evidence-based practices (EBP) for both intrapartum and neonatal care among normal deliveries [ 18 , 19 ]. We hypothesized that the nurse-mentoring program also built the providers’ capacity to identify and manage maternal and neonatal complications. The objective of this analysis was to examine changes in diagnosis and management of PPH and intrapartum asphyxia during a mobile nurse-mentoring program in 320 Basic Emergency Obstetric and Neonatal Care (BEmONC) facilities in Bihar, India. The SQUIRE 2.0 guidelines to report quality improvement studies were used [ 20 ].
In 2011, CARE India, a non-governmental organization, collaborated with the Government of Bihar to implement a pilot program in eight districts [ 18 ]. Promising results from the pilot phase [ 18 , 19 ] led to scale-up to all 38 districts in Bihar, covering an estimated 110 million population, as Apatkaleen Matritva evam Navjat Tatparta (AMANAT), meaning ‘emergency obstetrical and neonatal readiness’ in Hindi. AMANAT was a multi-faceted quality improvement nurse-mentoring program to reduce maternal and neonatal mortality by improving provider skills in normal and complicated deliveries. Other key components included support for positive changes in infrastructure and management, infection control, hazardous waste disposal, and creating and maintaining a newborn care corner in public health facilities.
The AMANAT program was implemented in four phases between May 2015 and January 2017 at 320 high volume, BEmONC facilities at the primary care level (80 facilities per phase (P), P1 –May to October 2015, P2 –September 2015 to May 2016, P3 –Nov 2015 to June 2016 and P4 –June 2016 to Jan 2017). Due to administrative limitations, only facilities with adequate readiness in terms of infrastructure and management were included. In Bihar, BEmONC facilities serve twice as many people than federally mandated, often with limited resources to effectively diagnose and manage obstetric and neonatal emergencies [ 14 ]. Only vaginal deliveries are conducted in these facilities, attended by auxiliary or general nurse midwives (ANMs and GNMs).
Intervention
In each phase, a pair of nurses (mentors) were assigned four facilities to conduct on-site mentoring of labor room nurse mentees [ 21 ]. Mentor pairs visited each facility for one week, covering four assigned facilities over a four-week period. The mentor pairs returned for subsequent week-long visits once every month for seven to nine consecutive months. In other words, facilities received one week of mentoring in a month for 7–9 consecutive months. The mentors engaged in a variety of activities including skill demonstrations, didactic sessions, high-fidelity simulation and bedside mentoring during actual patient care.
An integral part of the nurse-mentoring program was PRONTO International’s ( http://prontointernational.org ) simulation and team training. The simulation and team training curriculum was tailored to address local contextual needs and incorporated in the AMANAT program since the outset. The three components of the PRONTO curriculum were: (1) realistic human-centered in-situ simulation scenarios of normal and complicated deliveries, including scenarios with simultaneously occurring emergencies, to promote use of EBPs, (2) efficient teamwork and communication (T&C) among providers and (3) increasing provider awareness around person-centered maternity care [ 21 , 22 ]. Simulations were conducted in providers’ usual work settings utilizing a maternal actor wearing PartoPants (a hybrid low-tech birth simulator) [ 22 ], and a NeoNatalie infant mannequin, nurses from the facilities acted as the patient to gain in-sight into the patient experience. A unique aspect of PRONTO’s simulation is use of a maternal actor instead of a mannequin. PRONTO trained all nurses to facilitate and video-record simulations, conduct video-aided debriefings after simulations and perform rapid debriefings after live deliveries.
The T&C component focused on building collaborative environment among mentees. It included structured team-building activities as well as integration of specific communication techniques, including ‘think out loud,’ ‘call back,’ ‘call out,’ ‘SBAR’ (Situation, Background, Assessment, Recommendation), and debriefing (adapted from the TeamSTEPPS curriculum) [ 23 ]. These activities provided mentees with an opportunity to practice technical and non-technical competencies required to manage a variety of obstetric and neonatal complications as a team, even as a very small team.
The Institutional Review Board of the Indian Institute of Health Management Research in Jaipur, India (date–June 27, 2015) and the Committee for Human Research at the University of California San Francisco approved the study (date–May 20, 2015). Study ID# 14–15446.
Data collection systems
We used two data sources, which were collected and maintained by CARE India- the Facility Information System (FIS) and direct observation of deliveries (DOD). The FIS system was used to record data for the weeks of mentoring and DOD was conducted before and after mentoring. FIS was a web-based system, which provided information on deliveries and mentoring activities. Data were collected daily by the mentors during each of their week-long visits and entered directly into the system. The mentors obtained the daily data on all deliveries that occurred during the day using observation and facility registers and cross-checked with the staff when necessary. FIS data were collected only for the weeks when mentors were present in the facilities for mentoring and not for the other weeks. Delivery data included patient demographics, delivery mode, obstetric and neonatal complications, intrapartum management and discharge dispositions. Mentoring data included date, time and topics covered in each session, number and characteristics of simulations performed and staff attendance. The second source of data was DOD, collected by clinically trained nurses who observed deliveries between 9 am and 5 pm over the week immediately before and after the intervention. At baseline, when mentoring had not yet started, nurses observed deliveries in the facilities they later mentored, but for endline, they observed deliveries in different facilities. DOD data were used to generate facility-specific clinical practice scores for intrapartum and newborn care [ 24 ]. As only daytime deliveries were used, it might have overestimated the performance scores.
Clinical outcomes and covariates
We used two clinical outcomes–PPH and intrapartum asphyxia. In the setting of a BEmONC facility in Bihar, where there are limitations in equipment and clinician competency, the providers used the accepted definition of PPH as blood loss associated with obstetric labor or childbirth of more than 500ml for a vaginal delivery. However, the operational definition of PPH in this setting was, “a provider observing persistent trickling of more than expected blood, or a blood clot that was the size of a fist, or changing pads every 5–15 minutes.” For PPH management, we examined specific steps of fluid or uterotonics administration.
For intrapartum (or birth) asphyxia, we used the WHO definition of “failure to initiate or sustain breathing at birth”. However, for operational purposes the intervention emphasized to identify neonates who did not breathe within the first 30 seconds, with prompt initiation of PPV to make best use of the first minute after birth. The authors recognize that this a departure from standard recommendations, but we adapted it to instill a sense of urgency. For intrapartum asphyxia management, we examined specific steps of drying, warming, clearing airways and PPV as recommended by ILCOR [ 25 ]. Facilities in the sample did not have the equipment or laboratory capacity to assist in the diagnosis of asphyxia.
The terminology pertaining to intrapartum birth asphyxia has evolved to objectively define the condition and correctly identify neonates with the condition. The WHO definition is neither predictive of outcome nor does it imply any causation. The ICD-10 categories of P20 “intrauterine hypoxia” and P21 “birth asphyxia” are classified by onset characteristics [ 26 ] but do not provide clear diagnostic criteria or threshold values and APGAR scores, fetal acidosis and fetal distress lack specificity. The terms “post-asphyxial encephalopathy” or “hypoxic ischemic encephalopathy” are also used to describe encephalopathy caused due to intrapartum injury [ 26 , 27 ]. However, recent guidelines from the American Academy of Pediatrics, American College of Obstetrics and Gynecology, and International Cerebral Palsy Task Force recommend against the use of these terms unless intrapartum-related causation can be established [ 27 ]. Instead, the term “neonatal encephalopathy” is recommended. In low and middle income countries, where advanced facilities necessary to ascertain intrapartum causation are rarely available in public health centers, and where a sizeable proportion of the births happen without skilled birth attendants, the chances of neonatal encephalopathy occurring as a result of intrapartum hypoxia are much higher [ 27 ]. In keeping with the recommendation, and following other studies from very similar settings [ 28 , 29 ], we used a clinical symptom-based indicator to determine intrapartum asphyxia because it was the most feasible method of diagnosis that could be implemented in the study setting.
Number of weeks of mentoring per facility was the key variable of interest. There were two sets of covariates: time-dependent and time-independent ( Fig 1 ). Time-independent covariates included phase of intervention, number of complication simulations and T&C activities performed, which accounted for the mentor’s prioritization of activities during mentoring. As this analysis pertained complicated deliveries, only simulation scenarios that involved complications were considered ( S1 Appendix ). The time-dependent covariates included physician availability during a delivery (in-person or by phone), proportion of total mentee-sessions attended, facility-level practice scores, number of days of mentoring per week, and number of births per week. We calculated availability of a physician as deliveries per mentoring week when a doctor attended a mother or a neonate (or consulted by phone). We measured participation in mentoring activities through the proportion of mentee-sessions that were marked as present ( S1 Appendix ). The ‘facility level practice scores’ covariate was generated using twenty-three EBPs from DOD data collected before (baseline) and after (endline) intervention ( S1 Appendix ). As the highest diagnosis was observed around week 5, we assigned the baseline scores to the first 3 weeks and the endline scores from week 4 onwards.

A key driver diagram is also included that gives a broader overview of the overall AMANAT program.
Statistical analysis
Due to the statewide coverage of the program true controls were not available. To examine the intervention effect, we made a quasi-experimental comparison between-facilities as well as a longitudinal comparison within-facilities over time. For the between-facilities comparison using distinct sets across phases, the proportions in the final week of intervention (intervention effect) were contrasted with the proportions in the first week of the subsequent phase (surrogate controls) that was proximal in time. The respective first and final week comparisons between phases 2 and 1, as well as phases 4 and 3 were concurrent, while for phases 3 and 2 they were five months apart. We estimated the facility specific proportions of diagnosed or managed cases for the first and the final week, which were then averaged across all facilities in that phase.
For the within-facility longitudinal comparison the unit of analysis was facility-week. Using the start and end date of each mentoring week for each facility we aggregated the number of births and complications, and converted the individual-level birth dataset to a repeated observation facility-week longitudinal dataset. As the outcomes were counts with the overall incidences small (<3%) and their variances were greater than the mean, the negative binomial model was preferred. Further, there were many facility-weeks with no complications, when either there were no complications or complications were undiagnosed. In other words, zero counts can be divided into true counts (no complication occurred) and identification errors (complications not diagnosed). These two sets of zeros are statistically identical but generated through two different processes. A facility that fails to identify any complication will always have zero count. However, a facility that identifies complications, will have zero and non-zero counts depending on occurrence. Thus, the number of facilities with zero complications for a facility-week cannot be explained in the same manner as other facility-weeks with one or more identified complications. A standard model would not distinguish between the two processes. Therefore, we utilized the zero-inflated negative binomial model, which includes a binary logistic model to predict the odds that a facility will diagnose or manage complications, while the negative binomial model generated the incidence rate ratios (IRR) for diagnosis or management of complications, per week of mentoring. To account for correlation in the outcomes given that deliveries are clustered in both time and space, we used the sandwich variance estimator, which provides correct standard error for zero inflation models regardless of the correlation [ 30 ]. For the diagnosis of complications, we used a one-knot linear spline to model the increasing and decreasing trend ( S1 Appendix ). Temporal trend in management was linear and was modelled linearly.
Fig 1 shows the complex relationships of exposure-outcome with time-dependent as well as time-independent confounding ( S1 Appendix ). The thick black arrows from training to diagnosis represent the direct effects of training on diagnosis and management of complications in the concurrent (black) and subsequent (grey) weeks ( Fig 1 ). Mentees performance in week 1 influenced focus of the training in week 2 (e.g. correct diagnosis shifted focus of the training to management), shown by the solid red arrows. These directed paths represent past outcome influencing future exposure. Content of trainings in each week is pre-determined with some flexibility to modify as required, represented by the directed paths from week 1 training to week 2 and so on. Delivery load in the index week influenced time available for training in that week, represented by the directed paths from total births to training weeks. Further, the number of complications identified in a week was also dependent on the number of deliveries in that week, represented by the directed path from total births to diagnosis. Total deliveries in week 1 likely influenced diagnosis and management in week 2 through two pathways: (a) conditional on training time in week 1, which likely affected diagnosis and management in subsequent weeks, and (b) through diagnosis and management in week 1, conditional on training time in week 2. The AMANAT program through infrastructure strengthening likely improved the standing of the facilities in the community and mentoring likely improved service delivery by mentees. The overall improvement in care provided by the facility may increase the delivery load after additional weeks of training, as shown by broken red arrows. The number of complicated simulations run in week 1 likely influenced diagnosis rates in week 1 as well as in subsequent weeks. Furthermore, future diagnosis rates were conditional on learning from past performance of simulations of PPH or intrapartum asphyxia complications. Many of these relationships justify the use of marginal structural models (MSM) to account for time-varying confounding because conventional models will be inadequate [ 31 ]. As the results from the MSMs were similar ( S1 Table ) and the AIC values from MSMs were larger than the individual variable adjusted models ( S2 Table ), main tables reported the latter.
In a sensitivity analysis, we adjusted the final models with additional confounders, particularly those that are considered risk factors of intrapartum asphyxia, such as premature rupture of membranes, multiple births, preterm birth, low birth weight, obstructed or prolonged labor, cord prolapse, breech presentation, and anemia. The longitudinal trends of these risk factors over the months of mentoring could have varied due to mentoring, thus fulfilling the conditions of confounders.
Two-tailed significance was examined at the 5% level. The final models were restricted to week 7 because the number of facilities receiving >7 weeks of mentoring reduced drastically ( Table 1 ). Comparing weekly proportions from a much smaller subset of facilities with that of the entire pool is misleading. S3 Table presents results without exclusion. We checked the final models for outliers and regression assumptions. We analyzed data using Stata 14.2 (Stata Corp., TX). The Indian Institute of Health Management Research University and the Committee for Human Research at the University of California, San Francisco approved this study.
1 Percentage of total births unless the denominator is mentioned alongside the indicator.
2 If a delivery had both PPH and intrapartum asphyxia, it was counted in both of these categories, i.e., the categories are not mutually exclusive.
3 Observed: all stages of delivery occurred when a mentor was present in the facility; partially observed: only part of the delivery occurred when a mentor was present in the facility; unobserved: delivery occurred in the absence of a mentor.
4 A physician was either physically present or available on call.
5 The numbers are cumulative.
A total of 55,938 deliveries were recorded in 320 facilities during the mentoring period. Of these, 1,291 (2%) had PPH (half of which were atonic) and 1,631 (3%) had intrapartum asphyxia ( Table 1 ). Few had preeclampsia/eclampsia [n = 302 (0.5%)] or sepsis [n = 83 (0.2%)]. More than a quarter of deliveries occurred when mentors were present (observed) in facilities, and 58% occurred outside work hours (not observed). Eighty-five percent of facilities received at least seven weeks of mentoring.
The total number of deliveries occurring in individual facilities over the entire mentoring weeks ranged from 23 to 642, with a median of 159 [interquartile range (IQR): 100, 223] ( Table 2 ). The average number of mentoring days per facility was 39 (SD: 5). On average, facilities performed 19 (SD: 10) maternal, 10 (SD: 5) neonatal simulations and 7 (SD: 6) T&C activities. Average staff attendance in mentoring sessions was 81% (SD: 11%). Facility level intrapartum and newborn practice scores improved from baseline to endline.
1 SD–Standard Deviation, IQR–Interquartile Range i.e. 25 th percentile and 75 th percentile.
2 For example, if there were 4 mentees in a facility and there were 10 training sessions during a mentoring week, the total mentee-sessions for the week was 40. Therefore, 80% attendance would mean 4 mentees were present for 8 of the 10 sessions (= 32 mentee-sessions).
3 A set of 11 and 12 evidence-based practice indicators were used to generate intrapartum or newborn scores, respectively that range from 0 to 100. Zero indicates none of the scores were performed in the facility and 100 means all of those were performed.
In the between-facility comparisons across phases, diagnosis was always higher in the final week of intervention (PPH: 2.5–5.4%, intrapartum asphyxia: 4.2–5.6%) relative to the first week (PPH: 1.2–2.1%, intrapartum asphyxia: 0.7–3.3%), which tended to be significant, except in a few cases ( Table 3 ). In general, proportions of PPH or intrapartum asphyxia cases that were managed using selected EBPs were also higher after intervention but these are based on small numbers and may not be stable estimates ( S4 Table ).
A comparison between facilities across phases.
1 Number of facilities from which the proportion was estimated. Distinct set of facilities were covered in each phase.
2 Unpaired t-test was used to compare the overall mean proportions of complications by phase.
3 The number is less than 320 as there are three comparisons of approximately 80 pairs of facilities. There was nothing previous to phase 1 where the first week of phase 1 can be compared with.
The final longitudinal models had 52,099 deliveries with 1,239 PPH cases, after excluding deliveries with dates inconsistent with arrival and discharge dates and those that occurred outside the days of mentoring ( Table 4 ). The within-facility investigation shows PPH diagnosis among all deliveries increased up to week 5 (from 1.6% to 4.4%), after which they decreased through week 7 (3.1%) and diagnosis was frequent when a mentor was present ( Fig 2A ). Adjusted IRR demonstrated a 17% increase in PPH incidence [1.17, 95% confidence interval (CI) 1.05, 1.31] associated with each additional week of mentoring up to week 5 and a 14% decline (IRR 0.86, 95% CI: 0.77, 0.97) for weeks 5 through 7 ( Table 4 ). MSM models produced similar IRRs ( S1 Table ). The odds that a facility will identify a PPH case increased per one-week increase in mentoring, (OR 1.25, 95% CI: 2.17, 3.70).

Temporal trend in proportions of diagnosed postpartum hemorrhage (2a) and intrapartum asphyxia (2b) cases and management practices as a proportion of diagnosed cases during the AMANAT mentoring program in Bihar, 2015–2017.
1 Adjusted for days per week of nurse-mentoring, total number of births per week, phase of program, physician available, proportion of mentee-sessions attended, facility level practice scores, number of postpartum hemorrhage simulations performed, number of neonatal resuscitation simulations performed, and number of teamwork and communication activities performed. Additionally, the models for management practices were also adjusted for the counts of the respective complications.
2 Number of diagnosed cases/Total number of deliveries included in the final model.
3 Increase in incidence rate ratios (IRR, 95% confidence interval) for diagnosis of complications, per additional week of mentoring, from the negative binomial part of the zero-inflated negative binomial model.
4 Odds ratios (OR) from the logistic part of the zero-inflated negative binomial model, give the odds that a facility will identify complications, per additional week of mentoring.
5 Specific management practices relevant for postpartum hemorrhage.
6 Specific management practices relevant for intrapartum asphyxia.
7 The point estimate for Positive Pressure Ventilation (PPV) is too small [2×10 5 (95% CI: (1.4×10 4 , 3.3×10 6 )] and the CI is too wide to be of any interpretable importance.
Among all PPH cases, 96% and 84% received IV fluids or uterotonics, respectively. From week 1 through 7, these proportions changed little and in the adjusted models changes per week were not significant ( Fig 2A and Table 4 ).
The diagnosis of intrapartum asphyxia among all livebirths increased from 2.5% in week 1 to 4.8% in week 5, after which it reduced to 4.0% through week 7 ( Fig 2B ). When a mentor was present, diagnosis generally tended to increase from week to week. Adjusted IRR was 1.21 (95% CI: 1.13, 1.29) for week 1 through 5, followed by non-significant decline (IRR 0.91, 95% CI: 0.82, 1.01), associated with each additional week of mentoring ( Table 4 ). IRRs from the MSM models were similar ( S1 Table ). In sensitivity analyses, results were practically unchanged to adjustment with other risk factors that are mentioned in the methods. The odds that a facility will diagnose an intrapartum asphyxia case increased with each week of mentoring (OR 6.67, 95% CI: 1.52, 33.33), though the CI was too wide and should be interpreted cautiously.
Seventy-eight percent of the asphyxiated newborns were taken to a radiant warmer, 92% were dried or stimulated, 81% were suctioned and 41% received PPV. From week 1 through 7 of mentoring, asphyxia management improved and adjusted models showed a 5–9 percentage-points increase in radiant warmer use, drying/stimulation and PPV with each additional week of mentoring ( Fig 2B , Table 4 ).
This investigation identified some improvement in the diagnosis of PPH and intrapartum asphyxia in both between- and within-facility comparisons. Comparison between facilities within similar geographies and time generally suggests improvement in diagnosis. Within-facilities over time, diagnosis of PPH and intrapartum asphyxia among all deliveries increased up to week 5, after which it began trending downward. Despite the overall increase in proportions of PPH and intrapartum asphyxia, these were still not on par with levels observed in Southeast Asia and globally, suggesting some complicated deliveries remain undiagnosed [ 32 – 34 ]. For both outcomes, the proportion of diagnosed cases where selected EBPs were used for management either remained stable or increased as diagnosis increased, demonstrating that the absolute number of cases with acceptable management practices kept pace or increased with increased diagnosis. The results also suggest that, among facilities that did not diagnose any PPH or intrapartum asphyxia initially, mentoring enabled providers to begin diagnosing complications. Thus, the nurse-mentoring program appears to have built provider’s capacity to identify PPH and/or intrapartum asphyxia. Once identified, providers seem to be relatively well poised to manage these complications.
Studies from the United States and Canada showed temporal increase in PPH incidence, driven by an increase in uterine atony, changes in demography, maternal comorbidities, or delivery mode [ 35 , 36 ]. Our results are unlikely to be explained by these factors. We found both increasing and decreasing trends within a relatively short period. It is unlikely that demographic factors reversed directions in this large (>100 million) population [ 14 ], in the absence of major events (epidemic, migration etc.). Increase in Caesarean sections cannot explain the results, which are based on vaginal deliveries, nor can delivery load, as the models adjusted for this. Multifetal pregnancy or treatment with magnesium sulfate can overdistend the uterus and compromise contractility, leading to atonic PPH [ 35 ]. In this dataset, there were five twin deliveries and three women received magnesium sulfate among those with PPH. Thus, improvements observed in this investigation is likely due to the intervention, although the potential for other explanations remain as we did not have true controls and intervention was not assigned randomly.
Overall proportions of PPH in this study are consistent with another report on Helping Mothers Survive (HMS) Bleeding after Birth [ 37 ]. That study assessed blood loss subjectively and reported a decrease in proportion of patients that lost between 500 and 1000 ml of blood but found an increase in the proportion that lost <500 ml, after relative to before training, which could be due to a more accurate assessment after training that shifted patients into different categories [ 37 ]. This could be a potential explanation for the downward trend we observed in PPH diagnosis when providers were “over” sensitized to identifying complications in the early weeks, which then normalized to a more accurate assessment over the last couple of weeks. It could also be because routine administration of uterotonics for active management of the third stage of labor (AMTSL) may not have reached the peak by week 5, and may have increased further thereby actually reducing PPH incidence. DOD data on uterotonics use for AMTSL supports this observation (38% at baseline to 71% at endline), though the data to track usage by week were not available. A systematic review reported insufficient evidence to suggest simulation training improves NR [ 38 ]. However, other reports from Helping Babies Breathe simulation training reported improved knowledge and skills; clinical performance of stimulation, suction, and bag-mask ventilation; and demonstrated positive impact on fresh stillbirth and mortality on the first day of life [ 39 – 42 ]. Evidence on retention of knowledge and skills after training is mixed [ 41 , 43 ]. The rigorously conducted Better Birth trial in India and HMS program in Tanzania reported decrease in skills after 9–12 months [ 44 , 45 ], another very small study of physicians suggests retention of PPH-related skills for up to two years [ 46 ]. The management of complications, including uterotonic use and NR, observed in this study was comparable to those observed post-intervention in other settings [ 37 , 47 , 48 ]. Given that mentors collected data, we cannot completely rule out systematic overreporting (bias) of outcomes. However, comparability of our results with that of other studies give confidence against such occurrence. Furthermore, if mentors were systematically overreporting complications, it would be unlikely to see a consistent decline precisely timed at week 5 for both of the outcomes.
Among the strengths are the large statewide coverage powering the investigation and lending limited external validity to facilities in similar low-resource settings and readiness. The longitudinal comparison within-facilities enabled examination of trends over time, which a pre-post design would have missed. The analytical strategy minimizes the possibility of residual confounding and strengthens causal inference because several models and a range of covariate adjustment yielded robust results.
Given the operational limits in Bihar we were unable to use objective measures for the diagnosis of PPH (blood loss) and intrapartum asphyxia (APGAR score, umbilical cord pH, neuroimaging, etc.). We tried using a calibrated obstetric drape to quantify blood loss; however, we could not support universal use, as there were concerns about cleaning, re-use and infections. To establish intrapartum causation of asphyxia more accurately, postnatal neuroimaging or blood gas analysis are needed, which were not available in Bihar, and we acknowledge this as a limitation.
Additionally, we did not have true control facilities and addressed this limitation by using both between- and within-facility comparisons. Another challenge we had was related to measurement of time, which is critical for an asphyxiated infant. Simply noting a specific step to resuscitate an infant was performed with no reference to time portrays an incomplete picture of case management. A related study identified several barriers to clinical urgency among mentees, including poor understanding of the indications (e.g., immediate versus delayed cord clamping, significance of effective ventilation within 60 seconds) [ 49 ]. There is also a possibility of reporting bias, as FIS data were collected by nurse mentors, and may not reflect adoption of EBPs by mentees, exclusively, the chances of which are minimal for reasons discussed above. Overreporting will bias the results if it is differential. In other words, overreporting has to be only in certain type of facilities, i.e., those with more or less weeks of mentoring, not both. If overreporting is randomly distributed across all facilities (i.e., non-differential), it will affect significance, but not point estimates [ 50 ]. Likewise, non-identification of complications is unlikely to be restricted to facilities with zero counts for all mentoring weeks but scattered across all 320 facilities as it does not depend on facilities but specific provider skills as well as case severity. If non-identification was spread across all facilities and it was non-differential by exposure, significance and not the point estimates will be affected [ 50 ]. Finally, we did not have adequate numbers for other important complications such as preeclampsia and sepsis. Global estimates suggest these are also severely underreported.
During the AMANAT program there was an increase in the diagnosis of PPH, which decreased somewhat during the last two weeks. At baseline, the majority of the PPH cases were managed using selected EBPs, which remained largely unchanged throughout the program. Diagnosis and management of intrapartum asphyxia using selected EBPs improved with duration of mentoring. Diagnosis of PPH and intrapartum asphyxia in public facilities in Bihar is still not on par with regional or international levels. Thus, continued efforts to improve providers’ ability to recognize and act on these important causes of maternal and newborn mortality are needed. In order to sustain the gains achieved through this program, in the next phase of intervention, champion mentees were identified from facilities, then trained to serve as mentors and continue these activities in their respective facilities. This study also provides empirical evidence that, following identification, providers demonstrated the capacity to appropriately manage PPH and intrapartum asphyxia.
Supporting information
The results are for weeks 1 through 9 of mentoring without any exclusion.
S1 Appendix
Acknowledgments.
The authors would like to thank the Database Management System development team of CARE India and the staff members of the PRONTO office in Patna, India. We also thank all of the mentors, mentees, facility in charge, Regional Clinical Capacity Building Specialists for their tireless efforts in implementing the AMANAT program throughout the state of Bihar as well as the CMAI management. Additionally, we are thankful to the CARE India team for their active support and engagement in integrating simulation and team training into the AMANAT program. Finally, we would like to thank the countless women in Bihar for their participation in this program.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study was funded by the Bill and Melinda Gates Foundation (OPP1112431) to DW.
Data Availability
Postpartum Hemorrhage (PPH) [NextGen]
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
- Severe bleeding post delivery
- Can be up to 2 weeks after delivery
- A major cause of maternal mortality
Nursing Points
- Previous hemorrhage
- Large fetus
- Multiple pregnancies
- Preeclampsia
- Prolonged labor
- Precipitous labor
- Assisted delivery
- Placenta previa
- Placental abruption
- Number 1 cause
- Injury to the birth canal during delivery
- Retention of tissue from the placenta or fetus
- Bleeding disorders (coagulopathies) – the most dangerous being DIC
- Early: first 24 hours
- Late: after the first 24 hours
- Loss of 500 ml of blood for vaginal delivery
- Loss of 1000 ml of blood for c-section
- Boggy uterus on assessment or puddle of blood or constant ooze or trickle
- Remember that chucks pad under the patient
- Restlessness and tachycardia are early signs
- Hypotension is a late sign
Therapeutic Management
- Every 15 minutes for first hour
- Every 30 minutes x 2
- Every hour times 4
- Assessment of location and bleeding.
- Can weigh pads – 1 g = 1 mL
- Labs: H/H – 6 hours after to see effects
- Methylergonovine
- Carpropost Theramine
- Blood products may be indicated, depending on severity
- D&C or hysterectomy
Nursing Concepts
Patient education.
- S/s to report to provider (bleeding)
- Can occur up to 2 weeks postpartum
Nursing Care Plan (NCP) for Postpartum Hemorrhage (PPH)
12.07 meds for pph (postpartum hemorrhage), view the full outline.
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
View the FULL Transcript
**nextgen practice and understanding.

Welcome to the NURSING.com NextGen Practice Course. This FREE course is designed to provide an understanding of the forthcoming new Nextgen question types and a chance to practice taking them. As you go through these lessons, take a practice quiz to experience what these new formats will look and feel like on the NCLEX, starting April 2023. You can do this!
NextGen Practice Lessons
- 10 Questions

IMAGES
VIDEO
COMMENTS
Postpartum hemorrhage. Postpartum hemorrhage (PPH) is excessive bleeding following childbirth. It is a leading cause of maternal mortality, accounting for nearly one quarter of maternal deaths worldwide. The most common cause is uterine atony, or failure of the uterus to contract after delivery. Other causes include retained placenta, trauma ...
Postpartum haemorrhage (pph) Jun 26, 2012 • Download as PPTX, PDF •. 33 likes • 43,678 views. Ezmeer Emiral.
WHO Statement regarding the use of misoprostol for postpartum haemorrhage prevention and treatment. 2009. Ref No: WHO/RHR/09.22 In the absence of personnel to offer active management of the 3rd stage of labour, it is recommended that the trained health worker should offer Misoprostol 600mcg orally immediately after the birth of the baby.
Postpartum hemorrhage - Download as a PDF or view online for free. ... of PPH, Obstretical Nursing, By Devanshi, B.Sc. Honours in Nursing, 3rd year, Rajkumari Amrit Kaur College of Nursing Nursing Management of Postpartum Haemorrhage by Devanshi. ... Postpartum Haemorrhage "A Study of Case" Norseen Hosameldeen Lotfy 221101448 2.
A 29-year-old female (G1P1) is readmitted two weeks post-vaginal delivery due to increased vaginal bleeding. She reports that the bleeding began on the tenth day after delivery and has increased in severity each subsequent day. The delivery was uncomplicated with minimal blood loss and the patient did not receive any epidural anesthesia.
Postpartum Hemorrhage (PPH): Prevention & Management Evidence and Action. Objectives • Describe the global mortality burden of PPH • Present current evidence and action to prevent PPH • Share key evidence and action to manage PPH • Discuss key elements in a comprehensive program to reduce deaths from PPH. PPH: Leading Cause of Maternal Mortality • Hemorrhage is a leading cause of ...
Postpartum hemorrhage (PPH) is the leading cause of maternal mortality with the greatest opportunity for prevention. PPH is increasing because of lack of recognition and timely intervention. Gap analysis showed a failure to accurately recognize PPH because of the absence of standardized methods to quantify blood loss. The IOWA Model of Evidence-Based Practice was used to identify the problem ...
Identify postpartum hemorrhage due to uterine atony and be able to treat with appropriate medical management. Demonstrate teamwork and communication skills during a simulated postpartum hemorrhage. Planned Completion Points To successfully complete this scenario, the care team should successfully do the following: Recognize uterine atony as the ...
UNFOLDING Reasoning Case Study: Postpartum Hemorrhage (PPH) History of Present Problem: Brenda Jackson is a 22-year-old African American, G-1, now T-1 P -0 A- 0 L-1 who is Group B strep positive and was treated with four doses of penicillin G. She had a vaginal delivery over an intact perineum after 19 hours of labor at 39 weeks gestation.
Types of Postpartum Hemorrhage. Primary PPH - occurs when the mother loses at least 500 mL or more of blood within the first 24 hours of delivering the baby. Major Primary PPH - losing 500 mL to 1000 mL of blood. Minor Primary PPH - losing more than 1000 mL of blood. Secondary PPH - occurs when the mother has heavy or abnormal vaginal ...
11, 12, 16, 18. Oxytocin is the most effective treatment for postpartum hemorrhage, even if already used for labor induction or augmentation or as part of active management of the third stage of ...
The WOMAN study is a placebo-controlled trial conducted in 21 countries that assessed the impact of tranexamic acid in 20 021 women with PPH >500 mL after vaginal birth or >1000 mL after CS. 18 The maternal mortality rate was 2.4% (n = 483) with 72% (n = 346) of deaths caused by hemorrhage. Administration of 1 g of tranexamic acid (with a ...
CAUSES. The causes of postpartum hemorrhage can be classified by the 4 Ts mnemonic: tone, trauma, tissue, and thrombin ().Uterine atony is the most common cause of postpartum hemorrhage, causing up to 80% of all cases. 1 Uterine atony is caused by dysfunctional hypocontractility of the myometrium during the immediate puerperium. Uterine atony can develop in women with leiomyomata, multifetal ...
Postpartum hemorrhage (PPH) is defined as a blood loss of more than 500 mL after vaginal delivery or more than 1000 mL after a cesarean section, occurring within 24 hours of birth. PPH can be classified into primary, occurring within the first 24 hours post-delivery, and secondary, occurring from 24 hours to 12 weeks postpartum.
Introduction. Globally, an estimated 275,000 maternal deaths and 2.7 million neonatal deaths occur annually, a quarter of which occurs in India [1, 2].Hemorrhage, the leading cause of maternal mortality accounted for 27% of all deaths globally and 38% in India [3, 4].Intrapartum asphyxia is the second important cause, accounting for 11% and 19%, of all neonatal deaths globally and in India ...
ated by AI for the management of postpartum hemorrhage (PPH). Methods: This cross-sectional exploratory study involved creating a scenario for an imaginary patient with PPH. Information was put into 3 AI platforms (GPT-4, LaMDA, Med-PaLM) on consecutive days without prior conversation. Care plans were evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE ...
Study with Quizlet and memorize flashcards containing terms like Review the "Nurses Notes" below, then click to select the assessment data that requires follow-up by the nurse., Review the new updates in both "Provider Orders" and "Nureses' Notes" tabs, then click to specify if each of the client's findings in the left column is consistent with the fact that the client is deteriorating or the ...
Uterine atony is the number one cause of postpartum hemorrhage. It is classified as blood loss of 500 ml or more of blood for a vaginal delivery and 1000 ml or more of a c-section. Symptoms will be of hypovolemia so there is blood loss, tachycardia, and hypotension. It will be treated with medications such as Oxytocin, Methylergonovine and ...
Study with Quizlet and memorize flashcards containing terms like Review the "Nurses Notes" below, then click to select the assessment data that requires follow-up by the nurse. 09:20, 19-year-old gravida 1, para 0 (G1 P0) female, admitted in active labor at 39 weeks with 3 cm dilation after an uncomplicated pregnancy. 12:45, Client has normal progress in cervical dilation with a prolonged ...
RH emergencies are related conditions that frequently occur during pregnancy and in the immediate postpartum period 3 and threaten the lives of both the mother and the fetus/newborn baby. 4 These emergencies are normally unpredictable and create an ethical dilemma 5 leading to mismanagement which is common in low-income settings such as Uganda ...