- En español – ExME
- Em português – EME

Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
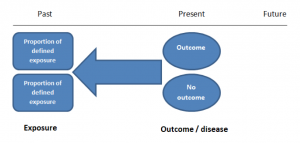
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
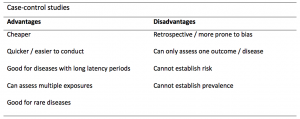
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
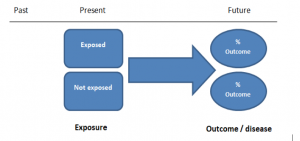
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
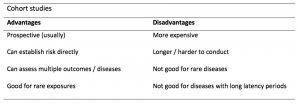
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.
Case Cohort Study
- Reference work entry
- Cite this reference work entry

6570 Accesses
In a case-cohort study, cases are defined as those participants of the cohort who developed the disease of interest, but controls are identified before the cases develop. This means that controls are randomly chosen from all cohort participants regardless of whether they have the disease of interest or not, and that baseline data can be collected early in the study.
Case-cohort studies are very similar to nested case-control studies . The main difference between a nested case-control study and a case-cohort study is the way in which controls are chosen. Generally, the main advantage of case-cohort design over nested case-control design is that the same control group can be used for comparison with different case groups in a case-cohort study. The main disadvantages of the case-cohort design is that it requires a more complicated statistical analysis and it can be less efficient than a nested case-control study under some circumstances (e. g.,...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Editor information
Editors and affiliations.
Network EUROlifestyle Research Association Public Health Saxony-Saxony Anhalt e.V. Medical Faculty, University of Technology, Fiedlerstr. 27, 01307, Dresden, Germany
Wilhelm Kirch ( Professor Dr. Dr. ) ( Professor Dr. Dr. )
Rights and permissions
Reprints and permissions
Copyright information
© 2008 Springer-Verlag
About this entry
Cite this entry.
(2008). Case Cohort Study . In: Kirch, W. (eds) Encyclopedia of Public Health. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-5614-7_323
Download citation
DOI : https://doi.org/10.1007/978-1-4020-5614-7_323
Publisher Name : Springer, Dordrecht
Print ISBN : 978-1-4020-5613-0
Online ISBN : 978-1-4020-5614-7
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Designing and Conducting Analytic Studies in the Field
Brendan R. Jackson And Patricia M. Griffin
Analytic studies can be a key component of field investigations, but beware of an impulse to begin one too quickly. Studies can be time- and resource-intensive, and a hastily constructed study might not answer the correct questions. For example, in a foodborne disease outbreak investigation, if the culprit food is not on your study’s questionnaire, you probably will not be able to implicate it. Analytic studies typically should be used to test hypotheses, not generate them. However, in certain situations, collecting data quickly about patients and a comparison group can be a way to explore multiple hypotheses. In almost all situations, generating hypotheses before designing a study will help you clarify your study objectives and ask better questions.
- Generating Hypotheses
- Study Designs for Testing Hypotheses
- Types of Observational Studies for Testing Hypotheses
- Selection of Controls in Case–Control Studies
- Matching in Case–Control Studies
- Example: Using an Analytic Study to Solve an Outbreak at a Church Potluck Dinner (But Not That Church Potluck)
- Outbreaks with Universal Exposure
The initial steps of an investigation, described in previous chapters, are some of your best sources of hypotheses. Key activities include the following:
- By examining the sex distribution among persons in outbreaks, US enteric disease investigators have learned to suspect a vegetable as the source when most patients are women. (Of course, generalizations do not always hold true!)
- In an outbreak of bloodstream infections caused by Serratia marcescens among patients receiving parenteral nutrition (food administered through an intravenous catheter), investigators had a difficult time finding the source until they noted that none of the 19 cases were among children. Further investigation of the parenteral nutrition administered to adults but not children in that hospital identified contaminated amino acid solution as the source ( 1 ).
- Focus on outliers. Give extra attention to the earliest and latest cases on an epidemic curve and to persons who recently visited the neighborhood where the outbreak is occurring. Interviews with these patients can yield important clues (e.g., by identifying the index case, secondary case, or a narrowed list of common exposures).
- Determine sources of similar outbreaks. Consult health department records, review the literature, and consult experts to learn about previous sources. Be mindful that new sources frequently occur, given ever-changing social, behavioral, and commercial trends.
- Conduct a small number of in-depth, open-ended interviews. When a likely source is not quickly evident, conducting in-depth (often >1 hour), open-ended interviews with a subset of patients (usually 5 to 10) or their caregivers can be the best way to identify possible sources. It helps to begin with a semistructured list of questions designed to help the patient recall the events and exposures of every day during the incubation period. The interview can end with a “shotgun” questionnaire (see activity 6) ( Box 7.1 ). A key component of this technique is that one investigator ideally conducts, or at least participates in, as many interviews as possible (five or more) because reading notes from others’ interviews is no substitute for soliciting and hearing the information first-hand. For example, in a 2009 Escherichia coli O157 outbreak, investigators were initially unable to find the source through general and targeted questionnaires. During open-ended interviews with five patients, the interviewer noted that most reported having eaten strawberries, a particular type of candy, and uncooked prepackaged cookie dough. An analytic study was then conducted that included questions about these exposures; it confirmed cookie dough as the source ( 3 ).
- Ask patients what they think. Patients can have helpful thoughts about the source of their illness. However, be aware that patients often associate their most recent food exposure (e.g., a meal) with illness, whereas the inciting exposure might have been long before.
- Consider administering a shotgun questionnaire. Such questionnaires, which typically ask about hundreds of possible exposures, are best used on a limited number of patients as part of hypothesis-generating interviews. After generating hypotheses, investigators can create a questionnaire targeted to that investigation. Although not an ideal method, shotgun questionnaires can be used by multiple interviewers to obtain data about large numbers of patients ( Box 7.1 ).
In November 2014, a US surveillance system for foodborne diseases (PulseNet) detected a cluster (i.e., a possible outbreak) of listeriosis cases based on similar-appearing Listeria monocytogenes isolates by pulsed-field gel electrophoresis of the isolates. No suspected foods were identified through routine patient interviews by using a Listeria -specific questionnaire with approximately 40 common food sources of listeriosis (e.g., soft cheese and deli meat). The outbreak’s descriptive epidemiology offered no clear leads: the sex distribution was nearly even, the age spectrum was wide, and the case-fatality rate of approximately 20% was typical. Notably, however, 3 of the 35 cases occurred among previously healthy school-aged children, which is highly unusual for listeriosis. Most cases occurred during late October and early November.
Investigators began reinterviewing patients by using a hypothesis-generating shotgun questionnaire with more than 500 foods, but it did not include caramel apples. By comparing the first nine patient responses with data from a published survey of food consumption, strawberries and ice cream emerged as hypotheses. However, several interviewed patients denied having eaten these foods during the month before illness. An investigator then conducted lengthy, open-ended interviews with patients and their family members. During one interview, he asked about special foods eaten during recent holidays, and the patient’s wife replied that her husband had eaten prepackaged caramel apples around Halloween. Although produce items had been implicated in past listeriosis outbreaks, caramel apples seemed an unlikely source. However, the interviewer took note of this connection because he had previously interviewed another patient who reported having eaten caramel apples. This event underscores the importance of one person conducting multiple interviews because that person might make subtle mental connections that may be missed when reviewing other interviewers’ notes. In fact, several other investigators listening to the interview noted this exposure—among hundreds of others—but thought little of it.
In this investigation, the finding of high strawberry and ice cream consumption among patients, coupled with the timing of the outbreak during a holiday period, helped make a sweet food (i.e., caramel apples) seem more plausible as the possible source.
To explore the caramel apple hypothesis, investigators asked five other patients about this exposure, and four reported having eaten them. On the basis of these initial results, investigators designed and administered a targeted questionnaire to patients involved in the outbreak, as well as to patients infected with unrelated strains of L. monocytogenes (i.e., a case–case study). This study, combined with testing of apples and the apple packing facility, confirmed that caramel apples were the source (2). Had a single interviewer performed multiple open-ended interviews to generate hypotheses before the shotgun questionnaire, the outbreak might have been solved sooner.
As evident in public health and clinical guidelines, randomized controlled trials (e.g., trials of drugs, vaccines, and community-level interventions) are the reference standard for epidemiology, providing the highest level of evidence. However, such studies are not possible in certain situations, including outbreak investigations. Instead, investigators must rely on observational studies, which can provide sufficient evidence for public health action. In observational studies, the epidemiologist documents rather than determines the exposures, quantifying the statistical association between exposure and disease. Here again, the key when designing such studies is to obtain a relevant comparison group for the patients ( Box 7.2 ).
Because field analytic studies are used to quantify the association between exposure and disease, defining what is meant by exposure and disease is essential. Exposure is used broadly, meaning demographic characteristics, genetic or immunologic makeup, behaviors, environmental exposures, and other factors that might influence a person’s risk for disease. Because precise information can help accurately estimate an exposure’s effect on disease, exposure measures should be as objective and standard as possible. Developing a measure of exposure can be conceptually straightforward for an exposure that is a relatively discrete event or characteristic—for example, whether a person received a spinal injection with steroid medication compounded at a specific pharmacy or whether a person received a typhoid vaccination during the year before international travel. Although these exposures might be straightforward in theory, they can be subject to interpretation in practice. Should a patient injected with a medication from an unknown pharmacy be considered exposed? Whatever decision is made should be documented and applied consistently.
Additionally, exposures often are subject to the whims of memory. Memory aids (e.g., restaurant menus, vaccination cards, credit card receipts, and shopper cards) can be helpful. More than just a binary yes or no, the dose of an exposure can also be enlightening. For example, in an outbreak of fungal bloodstream infections linked to contaminated intravenous saline flushes administered at an oncology clinic, affected patients had received a greater number of flushes than unaffected patients ( 4 ). Similarly, in an outbreak of Listeria monocytogenes infections, the association with deli meat became apparent only when the exposure evaluated was consumption of deli meat more than twice a week ( 5 ).
Defining disease (e.g., does a person have botulism?) might sound simple, but often it is not; read more about making and applying disease case definitions in Chapter 3 .
Three types of observational studies are commonly used in the field. All are best performed by using a standard questionnaire specific for that investigation, developed on the basis of hypothesis-generating interviews.
Observational Study Type 1: Cohort
In concept, a cohort study, like an experimental study, begins with a group of persons without the disease under study, but with different exposure experiences, and follows them over time to find out whether they experience the disease or health condition of interest. However, in a cohort study, each person’s exposure is merely recorded rather than assigned randomly by the investigator. Then the occurrence of disease among persons with different exposures is compared to assess whether the exposures are associated with increased risk for disease. Cohort studies can be prospective or retrospective.
Prospective Cohort Studies
A prospective cohort study enrolls participants before they experience the disease or condition of interest. The enrollees are then followed over time for occurrence of the disease or condition. The unexposed or lowest exposure group serves as the comparison group, providing an estimate of the baseline or expected amount of disease. An example of a prospective cohort study is the Framingham Heart Study. By assessing the exposures of an original cohort of more than 5,000 adults without cardiovascular disease (CVD), beginning in 1948 and following them over time, the study was the first to identify common CVD risk factors ( 6 ). Each case of CVD identified after enrollment was counted as an incident case. Incidence was then quantified as the number of cases divided by the sum of time that each person was followed (incidence rate) or as the number of cases divided by the number of participants being followed (attack rate or risk or i ncidence proportion). In field epidemiology, prospective cohort studies also often involve a group of persons who have had a known exposure (e.g., survived the World Trade Center attack on September 11, 2001 [ 7 ]) and who are then followed to examine the risk for subsequent illnesses with long incubation or latency periods.
Retrospective Cohort Studies
A retrospective cohort study enrolls a defined participant group after the disease or condition of interest has occurred. In field epidemiology, these studies are more common than prospective studies. The population affected is often well-defined (e.g., banquet attendees, a particular school’s students, or workers in a certain industry). Investigators elicit exposure histories and compare disease incidence among persons with different exposures or exposure levels.
Observational Study Type 2: Case–Control
In a case–control study, the investigator must identify a comparison group of control persons who have had similar opportunities for exposure as the case-patients. Case–control studies are commonly performed in field epidemiology when a cohort study is impractical (e.g., no defined cohort or too many non-ill persons in the group to interview). Whereas a cohort study proceeds conceptually from exposure to disease or condition, a case–control study begins conceptually with the disease or condition and looks backward at exposures. Excluding controls by symptoms alone might not guarantee that they do not have mild cases of the illness under investigation. Table 7.1 presents selected key differences between a case–control and retrospective cohort study.
Observational Study Type 3: Case–Case
In case–case studies, a group of patients with the same or similar disease serve as a comparison group (8). This method might require molecular subtyping of the suspected pathogen to distinguish outbreak-associated cases from other cases and is especially useful when relevant controls are difficult to identify. For example, controls for an investigation of Listeria illnesses typically are patients with immunocompromising conditions (e.g., cancer or corticosteroid use) who might be difficult to identify among the general population. Patients with Listeria isolates of a different subtype than the outbreak strain can serve as comparisons to help reduce bias when comparing food exposures. However, patients with similar illnesses can have similar exposures, which can introduce a bias, making identifying the source more difficult. Moreover, other considerations should influence the choice of a comparison group. If most outbreak-associated case-patients are from a single neighborhood or are of a certain race/ethnicity, other patients with listeriosis from across the country will serve as an inadequate comparison group.
Considerations for Selecting Controls
Selecting relevant controls is one of the most important considerations when designing a case–control study. Several key considerations are presented here; consult other resources for in-depth discussion ( 9,10 ). Ideally, controls should
- Thoroughly reflect the source population from which case-patients arose, and
- Provide a good estimate of the level of exposure one would expect from that population. Sometimes the source population is not so obvious, and a case–control study using controls from the general population might be needed to implicate a general exposure (e.g., visiting a specific clinic, restaurant, or fair). The investigation can then focus on specific exposures among persons with the general exposure (see also next section).
Controls should be chosen independently of any specific exposure under evaluation. If you select controls on the basis of lack of exposure, you are likely to find an association between illness and that exposure regardless of whether one exists. Also important is selecting controls from a source population in a way that minimizes confounding (see Chapter 8 ), which is the existence of a factor (e.g., annual income) that, by being associated with both exposure and disease, can affect the associations you are trying to examine.
When trying to enroll controls who reflect the source population, try to avoid overmatching (i.e., enrolling controls who are too similar to case-patients, resulting in fewer differences among case-patients and controls than ought to exist and decreased ability to identify exposure–disease associations). When conducting case–control studies in hospitals and other healthcare settings, ensure that controls do not have other diseases linked to the exposure under study.
Commonly Used Control Selection Methods
When an outbreak does not affect a defined population (e.g., potluck dinner attendees) but rather the community at large, a range of options can be used to determine how to select controls from a large group of persons.
- Random-digit dialing . This method, which involves selecting controls by using a system that randomly selects telephone numbers from a directory, has been a staple of US outbreak investigations. In recent years, however, declining response rates because of increasing use of caller identification and cellular phones and lack of readily available directory listings of cellular phone numbers by geographic area have made this method increasingly difficult. Even when this method was most useful, often 50 or more numbers needed to be dialed to reach one household or person who both answered and provided a usable match for the case-patient. Commercial databases that include cellular phone numbers have been used successfully to partially address this problem, but the method remains time-consuming ( 11 ).
- Random or systematic sampling from a list . For investigations in settings where a roster is available (e.g., attendees at a resort on certain dates), controls can be selected by either random or systematic sampling. Government records (e.g., motor vehicle, voter, or tax records) can provide lists of possible controls, but they might not be representative of the population being studied ( 11 ). For random sampling, a table or computer-generated list of random numbers can be used to select every n th persons to contact (e.g., every 12th or 13th).
- Neighborhood . Recruiting controls from the same neighborhood as case-patients (i.e., neighborhood matching) has commonly been used during case–control studies, particularly in low-and middle-income countries. For example, during an outbreak of typhoid fever in Tajikistan ( 12 ), investigators recruited controls by going door-to-door down a street, starting at a case-patient’s house; a study of cholera in Haiti used a similar method ( 13 ). Typically, the immediately neighboring households are skipped to prevent overmatching.
- Patients’ friends or relatives . Using friends and relatives as controls can be an effective technique when the characteristics of case-patients (e.g., very young children) make finding controls by a random method difficult. Typically, the investigator interviews a patient or his or her parent, then asks for the names and contact information for more friends or relatives who are needed as controls. One advantage is that the friends of an ill person are usually willing to participate, knowing their cooperation can help solve the puzzle. However, because they can have similar personal habits and preferences as patients, their exposures might be similar. Such overmatching can decrease the likelihood of finding the source of the illness or condition.
- Databases of persons with exposure information . Sources of data on persons with exposure information include survey data (e.g., FoodNet Population Survey [ 14 ]), public health databases of patients with other illnesses or a different subtype of the same illness, and previous studies. ( Chapter 4 describes additional sources.)
When considering outside data sources, investigators must determine whether those data provide an appropriate comparison group. For example, persons in surveys might differ from case-patients in ways that are impossible to determine. Other patients might be so similar to case-patients that risky exposures are unidentifiable, or they might be so different that exposures identified as risks are not true risks.
To help control for confounding, controls can be matched to case-patients on characteristics specified by investigators, including age group, sex, race/ethnicity, and neighborhood. Such matching does not itself reduce confounding, but it enables greater efficiency when matched analyses are performed that do ( 15 ). When deciding to match, however, be judicious. Matching on too many characteristics can make controls difficult to find (making a tough process even harder). Imagine calling hundreds of random telephone numbers trying to find a man of a particular ethnicity aged 50–54 years who is then willing to answer your questions. Also, remember not to match on the exposure of interest or on any other characteristic you wish to examine. Matched case–control study data typically necessitate a matched analysis (e.g., conditional logistic regression) ( 15 ).
Matching Types
The two main types of matching are pair matching and frequency matching.
Pair Matching
In pair matching, each control is matched to a specific case-patient. This method can be helpful logistically because it allows matching by friends or relatives, neighborhood, or telephone exchange, but finding controls who meet specific criteria can be burdensome.
Frequency Matching
In frequency matching, also called category matching , controls are matched to case-patients in proportion to the distribution of a characteristic among case-patients. For example, if 20% of case-patients are children aged 5–18 years, 50% are adults aged 19–49 years, and 30% are adults 50 years or older, controls should be enrolled in similar proportions. This method works best when most case-patients have been identified before control selection begins. It is more efficient than pair matching because a person identified as a possible control who might not meet the criteria for matching a particular case-patient might meet criteria for one of the case-patient groups.
Number of Controls
Most field case–control studies use control-to-case-patient ratios of 1:1, 2:1, or 3:1. Enrolling more than one control per case-patient can increase study power, which might be needed to detect a statistically significant difference in exposure between case-patients and controls, particularly when an outbreak involves a limited number of cases. The incremental gain of adding more controls beyond three or four is small because study power begins to plateau. Note that not all case-patients need to have the same number of controls. Sample size calculations can help in estimating a target number of controls to enroll, although sample sizes in certain field investigations are limited more by time and resource constraints. Still, estimating study power under a range of scenarios is wise because an analytic study might not be worth doing if you have little chance of detecting a statistically significant association. Sample size calculators for unmatched case–control studies are available at http://www.openepi.com and in the StatCalc function of Epi Info ( https://www.cdc.gov/epiinfo ).
More than One Control Group
Sometimes the choice of a control group is so vexing that investigators decide to use more than one type of control group (e.g., a hospital-based group and a community group). If the two control groups provide similar results and conclusions about risk factors for disease, the credibility of the findings is increased. In contrast, if the two control groups yield conflicting results, interpretation becomes more difficult.
Since the 1940s, field epidemiology students have studied a classic outbreak of gastrointestinal illness at a church potluck dinner in Oswego, New York ( 16 ). However, the case study presented here, used to illustrate study designs, is a different potluck dinner.
In April 2015, an astute neurologist in Lancaster, Ohio, contacted the local health department about a patient in the emergency department with a suspected case of botulism. Within 2 hours, four more patients arrived with similar symptoms, including blurred vision and shortness of breath. Health officials immediately recognized this as a botulism outbreak.
- If the source is a widely distributed commercial product, then the population to study is persons across the United States and possibly abroad.
- If the source is airborne, then the population to study is residents of a single city or area.
- If the source is food from a restaurant, then the population to study is predominantly local residents and some travelers.
- If the source is a meal at a workplace or social setting, then the population to study is meal attendees.
- If the source is a meal at home, then the population to study is household members and any guests.
Descriptive epidemiology and questioning of the case-patients revealed that all had eaten at the same church potluck dinner and had no other common exposures, making the potluck the likely exposure site and attendees the likely source population. Thus, an analytic study would be targeted at potluck attendees, although investigators must remain alert to case-patients among nonattendees. As initial interviews were conducted, more cases of botulism were being diagnosed, quickly increasing to more than 25. The source of the outbreak needed to be identified rapidly to halt further exposure and illness.
- List of foods served at the potluck.
- Approximate number of attendees.
- A case definition.
- Information from 5–10 hypothesis-generating interviews with a few case-patients or their family members.
- A cohort study would be a reasonable option because a defined group exists (i.e., a cohort) of exposed persons who could be interviewed in a reasonable amount of time. The study would be retrospective because the outcome (i.e., botulism) has already occurred, and investigators could assess exposures retrospectively (i.e., foods eaten at the potluck) by interviewing attendees.
- In a cohort study, investigators can calculate the attack rate for botulism among potluck attendees who reported having eaten each food and for those who had not. For example, if 20 of the 30 attendees who had eaten a particular food (e.g., potato salad) had botulism, you would calculate the attack rate by dividing 20 (corresponding to cell a in Handout 7.1 ) by 30 (total exposed, or a + b), yielding approximately 67%. If 5 of the 45 attendees who had not eaten potato salad had botulism, the attack rate among the unexposed—5 / 45, corresponding to c/ (c + d)—would be approximately 11%. The risk ratio would be 6, which is calculated by dividing the attack rate among the exposed (67%) by the attack rate among the unexposed (11%).
- A case–control study would be the most feasible option because the entire cohort could not be identified and because the large number of attendees could make interviewing them all difficult. Rather than interview all non-ill persons, a subset could be interviewed as control subjects.
- The method of control subject selection should be considered carefully. If all attendees are not interviewed, determining the risk for botulism among the exposed and unexposed is impossible because investigators would not know the exposures for all non-ill attendees. Instead of risk, investigators calculate the odds of exposure, which can approximate risk. For example, if 20 (80%) of 25 case-patients had eaten potato salad, the odds of potato salad exposure among case-patients would be 20/ 5 = 4 (exposed/ unexposed, or a/ c in Handout 7.2 ). If 10 (20%) of 50 selected controls had eaten potato salad, the odds of exposure among control subjects would be 10/ 40 = 0.25 (or b/ d in Handout 7.2). Dividing the odds of exposure among the case-patients (a/ c) by the odds of exposure among control subjects (b / d) yields an odds ratio of 16 (4/ 0.25). The odds ratio is not a true measure of risk, but it can be used to implicate a food. An odds ratio can approximate a risk ratio when the outcome or disease is rare (e.g., roughly <5% of a population). In such cases, a/ b is similar to a/ (a + b). The odds ratio is typically higher than the risk ratio when >5% of exposed persons in the analysis have the illness.
In the actual outbreak, 29 (38%) of 77 potluck attendees had botulism. The investigators performed a cohort study, interviewing 75 of the 77 attendees about 52 foods served ( 17 ). The attack rate among persons who had eaten potato salad was significantly and substantially higher than the attack rate among those who had not, with a risk ratio of 14 (95% confidence interval 5–42). One of the potato salads served was made with incorrectly home-canned potatoes (a known source of botulinum toxin), and samples of discarded potato salad tested positive for botulinum toxin, supporting the findings of the analytic study. (Of note, persons often blame potato salad for causing illness when, in fact, it rarely is a source. This outbreak was a notable exception.)
In field epidemiology, the link between exposure and illness is often so strong that it is evident despite such inherent study limitations as small sample size and exposure misclassification. In this outbreak, a few of the patients with botulism reported not having eaten potato salad, and some of the attendees without botulism reported having eaten it. In epidemiologic studies, you rarely find 100% concordance between exposure and outcome for various reasons, including incomplete or erroneous recall because remembering everything eaten is difficult. Here, cross-contamination of potato salad with other foods might have helped explain cases among patients who had not eaten potato salad because only a small amount of botulinum toxin is needed to produce illness.
Two-by-Two Table to Calculate the Relative Risk, or Risk Ratio, in Cohort Studies
Two- by- two tables are covered in more detail in Chapter 8 .

Two-by-Two Table to Calculate the Odds Ratio in Case–Control Studies
A risk ratio cannot be calculated from a case–control study because true attack rates cannot be calculated.

What kind of study would you design if your hypothesis-generating interviews lead you to believe that everyone, or nearly everyone, was exposed to the same suspected infection source? How would you test hypotheses if all barbecue attendees, ill and non-ill, had eaten the chicken or if all town residents had drunk municipal tap water, and no unexposed group exists for comparison? A few factors that might be of help are the exposure timing (e.g., a particularly undercooked batch of barbeque), the exposure place (e.g., a section of the water system more contaminated than others), and the exposure dose (e.g., number of chicken pieces eaten or glasses of water drunk). Including questions about the time, place, and frequency of highly suspected exposures in a questionnaire can improve the chances of detecting a difference ( 18 ).
Cohort, case–control, and case–case studies are the types of analytic studies that field epidemiologists use most often. They are best used as mechanisms for evaluating—quantifying and testing—hypotheses identified in earlier phases of the investigation. Cohort studies, which are oriented conceptually from exposure to disease, are appropriate in settings in which an entire population is well-defined and available for enrollment (e.g., guests at a wedding reception). Cohort studies are also appropriate when well-defined groups can be enrolled by exposure status (e.g., employees working in different parts of a manufacturing plant). Case–control studies, in contrast, are useful when the population is less clearly defined. Case–control studies, oriented from disease to exposure, identify persons with disease and a comparable group of persons without disease (controls). Then the exposure experiences of the two groups are compared. Case–case studies are similar to case–control studies, except that controls have an illness not linked to the outbreak. Case–control studies are probably the type most often appropriate for field investigations. Although conceptually straightforward, the design of an effective epidemiologic study requires many careful decisions. Taking the time needed to develop good hypotheses can result in a questionnaire that is useful for identifying risk factors. The choice of an appropriate comparison group, how many controls per case-patient to enroll, whether to match, and how best to avoid potential biases are all crucial decisions for a successful study.
This chapter relies heavily on the work of Richard C. Dicker, who authored this chapter in the previous edition.
- Gupta N, Hocevar SN, Moulton-Meissner HA, et al. Outbreak of Serratia marcescens bloodstream infections in patients receiving parenteral nutrition prepared by a compounding pharmacy. Clin Infect Dis. 2014;59:1–8.
- Angelo K, Conrad A, Saupe A, et al. Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014–2015. Epidemiol Infect. 2017;145:848–56.
- Neil KP, Biggerstaff G, MacDonald JK, et al. A novel vehicle for transmission of Escherichia coli O157: H7 to humans: multistate outbreak of E. coli O157: H7 infections associated with consumption of ready-to-bake commercial prepackaged cookie dough—United States, 2009. Clin Infect Dis. 2012;54:511–8.
- Vasquez AM, Lake J, Ngai S, et al. Notes from the field: fungal bloodstream infections associated with a compounded intravenous medication at an outpatient oncology clinic—New York City, 2016. MMWR. 2016;65:1274–5.
- Gottlieb SL, Newbern EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis. 2006;42:29–36.
- Framingham Heart Study: A Project of the National Heart, Lung, and Blood Institute and Boston University. Framingham, MA: Framingham Heart Study; 2017. https://www.framinghamheartstudy.org/
- Jordan HT, Brackbill RM, Cone JE, et al. Mortality among survivors of the Sept 11, 2001, World Trade Center disaster: results from the World Trade Center Health Registry cohort. Lancet. 2011;378:879–87.
- McCarthy N, Giesecke J. Case– case comparisons to study causation of common infectious diseases. Int J Epidemiol. 1999;28:764–8.
- Rothman KJ, Greenland S. Modern epidemiology . 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case–control studies. I. Principles. Am J Epidemiol. 1992;135:1019–28.
- Chintapalli S, Goodman M, Allen M, et al. Assessment of a commercial searchable population directory as a means of selecting controls for case–control studies. Public Health Rep. 2009;124:378–83.
- Centers for Disease Control and Prevention. Epidemiologic case studies: typhoid in Tajikistan. http://www.cdc.gov/epicasestudies/classroom_typhoid.html
- Dunkle SE, Mba-Jonas A, Loharikar A, Fouche B, Peck M, Ayers T. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis. 2011;17:2143–6.
- Centers for Disease Control and Prevention. Foodborne Diseases Active Surveillance Network (FoodNet): population survey. http://www.cdc.gov/foodnet/surveys/population.html
- Pearce N. Analysis of matched case–control studies. BMJ. 2016;352:1969.
- Centers for Disease Control and Prevention. Case studies in applied epidemiology: Oswego: an outbreak of gastrointestinal illness following a church supper. http://www.cdc.gov/eis/casestudies.html
- McCarty CL, Angelo K, Beer KD, et al. Notes from the field.: large outbreak of botulism associated with a church potluck meal—Ohio, 2015. MMWR. 2015;64:802–3.
- Tostmann A, Bousema JT, Oliver I. Investigation of outbreaks complicated by universal exposure. Emerg Infect Dis. 2012;18:1717–22.
< Previous Chapter 6: Describing Epidemiologic Data
Next Chapter 8: Analayzing and Interpreting Data >
The fellowship application period is open now through June 5, 2024.

The host site application period is now closed.
For questions, please contact the EIS program directly at [email protected] .
- Laboratory Leadership Service (LLS)
- Fellowships and Training Opportunities
- Division of Workforce Development
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Cohort studies vs case control studies
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Stefan Tigges had no financial relationships to ineligible companies to disclose.
At the time the article was last revised Stefan Tigges had no financial relationships to ineligible companies to disclose.
- Case control studies vs cohort studies
Case control studies
Cohort studies.
Cohort and case control studies are two different types of observational studies used to determine if there is an association between exposure and a health outcome 1 . The studies are observational and not experimental because, unlike a randomized clinical trial, the investigators do not assign subjects to the exposed and unexposed groups. The lack of randomization makes observational studies more vulnerable to a type of bias called confounding . Exposures may be harmful (e.g. smoking) or protective (e.g. vaccines) and outcomes include the development of disease (e.g. lung cancer) or the avoidance of disease (e.g. infection). Both studies are also considered longitudinal because study subjects are followed for a specified length of time.
On this page:
Practical points.
Cohort studies are usually carried out prospectively and begin by defining a population at risk for a disease, then establishing which members of the cohort are exposed and which are unexposed. The cohort is then followed and the number of cases of disease is determined for the exposed and unexposed groups. If the study period is short (e.g. flu season) and the losses to follow-up are negligible, the probability or risk of developing the disease in question is calculated for both groups:
risk in exposed = (number of exposed with the outcome of interest) / (total number of exposed)
risk in unexposed = (number of unexposed with the outcome of interest) / (total number of unexposed)
The measure of association in a cohort study is the risk ratio:
risk ratio = risk in exposed / risk in unexposed
If the probability of disease is higher among the exposed, then the exposure is harmful and the risk ratio is >1. If the probability of disease is lower among the exposed, then the exposure is protective and the risk ratio is <1.
If a cohort study is lengthy, patients may be lost to follow up and it may be impossible to accurately calculate risks in the exposed and unexposed groups and thus a risk ratio. For cohort studies that go on for many years, an incidence rate is calculated, where instead of using the total number of exposed or unexposed in the denominator the number of person-years contributed by all of the subjects in the exposed or unexposed groups make up the denominator:
incidence rate in exposed = (number of exposed with the outcome of interest) / (person-years among exposed)
incidence rate in unexposed = (number of unexposed with the outcome of interest) / (person-years among unexposed)
The measure of association then calculated is the rate ratio :
rate ratio = incidence rate in exposed / incidence rate in unexposed
Doll and Hill’s 1956 cohort study comparing the rate at which smokers and non-smokers developed lung cancer found an incidence rate among smokers of 0.84 lung cancer deaths/1,000 person-years and for non-smokers 0.07 lung cancer deaths/1,000 person-years 2 . This yields a rate ratio of 0.84/0.07 or 12. This means that smokers developed lung cancer at a 12 times greater rate than non-smokers.
If the rate of disease is higher among the exposed, then the exposure is harmful and the rate ratio is >1. If the rate of disease is lower among the exposed, then the exposure is protective and the rate ratio is <1.
Case control studies are usually carried out retrospectively and begin by identifying subjects who have a particular disease (cases), then establishing which of the cases have the exposure of interest. Next, a group of subjects without the disease (controls) is chosen from the underlying population and the number of controls who have the exposure of interest is determined. Because we sample subjects with and without the disease, the proportion of subjects with the disease depends on how the subjects were sampled and not on the probability (risk) of developing the disease. Calculating risks and risk ratios is therefore incorrect. Instead, an odds ratio is calculated and is the measure of association for a case control study 3 .
The odds of being exposed among the cases and controls are calculated:
odds of exposure among cases = (number of cases exposed) / (number of cases unexposed)
odds of exposure among controls = (number of controls exposed) / (number of controls unexposed)
Next, the odds ratio is calculated:
odds ratio = odds of exposure among cases/odds of exposure among controls
Doll and Hill’s 1950 case control study of smoking and lung cancer found 688 smokers and 21 non-smokers in their cases for an odds of exposure of 688/21=32.8 4 . Among the controls, there were 650 smokers and 59 non-smokers for an odds of exposure of 650/59=11.0. This yields an odds ratio of 32.8/11.0=2.98, meaning that the odds of smoking among patients with lung cancer are nearly 3 times greater than the odds of smoking among patients without lung cancer.
If the odds ratio is >1, the odds of exposure are higher among the cases than the controls and the exposure is harmful. If the odds ratio is <1, the odds of exposure are lower among the cases than the controls and the exposure is protective.
because most cohort studies are prospective and require meticulous follow-up, cohort studies tend to be more expensive and take longer to complete than case control studies
because case control studies start with cases, they are efficient for studying rare diseases compared to cohort studies which would require enrolling many subjects to ensure that some subjects develop a rare disease
if a disease is rare, the odds ratio approximates the risk ratio
Quiz questions
- 1. Dettori J, Norvell D, Chapman J. Risks, Rates and Odds: What’s the Difference and Why Does It Matter? Global Spine Journal. 2021;11(7):1156-8. doi:10.1177/21925682211029640 - Pubmed
- 2. Doll R & Hill A. Lung Cancer and Other Causes of Death in Relation to Smoking. BMJ. 1956;2(5001):1071-81. doi:10.1136/bmj.2.5001.1071 - Pubmed
- 3. Szumilas M. Explaining Odds Ratios. J Can Acad Child Adolesc Psychiatry. 2010;19(3):227-9. PMC2938757 - Pubmed
- 4. Doll R & Hill A. Smoking and Carcinoma of the Lung. BMJ. 1950;2(4682):739-48. doi:10.1136/bmj.2.4682.739 - Pubmed
Incoming Links
- Confounding
- Question 2922
- Question 2919
- Question 2918
- Question 2917
- Question 2916
- Question 2915
- Question 2913

Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

Introduction to Epidemiological Studies
Affiliations.
- 1 Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece. [email protected].
- 2 Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece.
- PMID: 29876887
- DOI: 10.1007/978-1-4939-7868-7_1
The basic epidemiological study designs are cross-sectional, case-control, and cohort studies. Cross-sectional studies provide a snapshot of a population by determining both exposures and outcomes at one time point. Cohort studies identify the study groups based on the exposure and, then, the researchers follow up study participants to measure outcomes. Case-control studies identify the study groups based on the outcome, and the researchers retrospectively collect the exposure of interest. The present chapter discusses the basic concepts, the advantages, and disadvantages of epidemiological study designs and their systematic biases, including selection bias, information bias, and confounding.
Keywords: Bias; Case-control study; Cohort study; Confounding; Information bias; Observational studies; Selection bias; Study design.
Publication types
- Case-Control Studies
- Cohort Studies
- Cross-Sectional Studies
- Epidemiologic Research Design*
- Follow-Up Studies
User Preferences
Content preview.
Arcu felis bibendum ut tristique et egestas quis:
- Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris
- Duis aute irure dolor in reprehenderit in voluptate
- Excepteur sint occaecat cupidatat non proident
Keyboard Shortcuts
7.2.1 - case-cohort study design.
A case-cohort study is similar to a nested case-control study in that the cases and non-cases are within a parent cohort; cases and non-cases are identified at time \(t_1\), after baseline. In a case-cohort study, the cohort members were assessed for risk factors at any time prior to \(t_1\). Non-cases are randomly selected from the parent cohort, forming a subcohort. No matching is performed.
Advantages of Case-Cohort Study:
Similar to nested case-control study design:
- Efficient– not all members of the parent cohort require diagnostic testing
- Flexible– allows testing hypotheses not anticipated when the cohort was drawn \((t_0)\)
- Reduces selection bias – cases and noncases sampled from the same population
- Reduced information bias – risk factor exposure can be assessed with investigator blind to case status
Other advantages, as compared to nested case-control study design:
- The subcohort can be used to study multiple outcomes
- Risk can be measured at any time up to \(t_1\) (e.g. elapsed time from a variable event, such as menopause, birth)
- Subcohort can be used to calculate person-time risk
Disadvantages of Case-Cohort Study:
As compared to nested case-control study design:
- subcohort may have been established after \(t_0\)
- exposure information collected at different times (e.g. potential for sample deterioration)
Statistical Analysis for Case-Cohort Study:
Weighted Cox proportional hazards regression model (we will look at proportional hazards regression later in this course)
- Open access
- Published: 03 May 2024
Does depressurization of the portal vein before liver transplantation affect the recurrence of HCC? A nested case-control study
- Guo Wei 1 ,
- Yong Zhao 1 ,
- Shifeng Feng 1 ,
- Jingsheng Yuan 3 ,
- Gang Xu 3 ,
- Jian Yang 2 ,
- Lingxiang Kong 2 , 3 &
- Jiayin Yang 2
BMC Cancer volume 24 , Article number: 558 ( 2024 ) Cite this article
212 Accesses
Metrics details
Portal hypertension (PHT) has been proven to be closely related to the development of hepatocellular carcinoma (HCC). Whether PHT before liver transplantation (LT) will affect the recurrence of HCC is not clear.
110 patients with depressurization of the portal vein (DPV) operations (Transjugular Intrahepatic Portosystemic Shunt—TIPS, surgical portosystemic shunt or/and splenectomy) before LT from a HCC LT cohort, matched with 330 preoperative non-DPV patients; this constituted a nested case-control study. Subgroup analysis was based on the order of DPV before or after the occurrence of HCC.
The incidence of acute kidney injury and intra-abdominal bleeding after LT in the DPV group was significantly higher than that in non-DPV group. The 5-year survival rates in the DPV and non-DPV group were 83.4% and 82.7% respectively ( P = 0.930). In subgroup analysis, patients in the DPV prior to HCC subgroup may have a lower recurrence rate (4.7% vs.16.8%, P = 0.045) and a higher tumor free survival rate (88.9% vs.74.4%, P = 0.044) after LT under the up-to-date TNMI–II stage, while in TNM III stage, there was no difference for DPV prior to HCC subgroup compared with the DPV after HCC subgroup or the non-DPV group.
Compared with DPV after HCC, DPV treatment before HCC can reduce the recurrence rate of HCC after early transplantation (TNM I-II). DPV before LT can reduce the recurrence of early HCC.
Peer Review reports
Introduction
At present, liver transplantation (LT) is still the best choice for hepatocellular carcinoma (HCC) patients with liver cirrhosis with a better long-term survival rate and lower recurrence rate of HCC [ 1 , 2 ]. Gastrointestinal bleeding and refractory ascites caused by portal hypertension (PHT) are the most common complications that occur during the transplantation waiting period [ 3 , 4 , 5 ]. Once gastrointestinal hemorrhage occurs, the mortality in Child-Pugh C patients is as high as 40% within the first 6 weeks of bleeding [ 6 ]. Depressurization of the portal vein (DPV), not only controls gastrointestinal bleeding, but also reduces ascites relieving abdominal discomfort, and significantly reduces hepatorenal syndrome caused by replenishing insufficient volume [ 3 , 7 , 8 , 9 ]. DPV practices and preferences vary throughout the world, the main operative methods for DPV include splenectomy and portosystemic shunt and particular radiological DPV. DPV has become a frequently-used bridging therapy for LT [ 10 , 11 , 12 , 13 , 14 ].
Cirrhosis is the most significant independent risk factor for HCC [ 15 , 16 ]. Although PHT is mainly caused by cirrhosis, it has also been shown to be a risk factor of HCC independent of cirrhosis [ 17 ]. In addition, HCC recurrence may be related to PHT in HCC radiofrequency ablation treatment [ 18 ]. According to the theory of soil and seeds [ 19 ], LT is different from other surgical treatments that remove seeds (HCC). LT completely removes the soil (sick liver) and seeds together, in theory, eliminating the possibility of recurrence. However, 5-year recurrence rates are still 7.8%, and can even reach up to 40% in HCC LT beyond the Milan standard [ 20 ]. A 2018 retrospective analysis by Mazzaferro et al. found that the 5-year cumulative mortality rate related to the recurrence of HCC after LT was 8.1%, accounting for 1/3 of the total death of the LT recipients [ 21 ]. Therefore, HCC recurrence following LT is still an important research area. Based on the evidence that PHT is closely related to HCC; the purpose of this study was to observe whether reducing the portal pressure limits the recurrence of HCC after LT.
This work has been reported in line with the STROCSS criteria [ 22 ]. The unique identifying number of this retrospective research is ChiCTR2000032141(date of first registration 20/04/2020, http://www.chictr.org.cn/showproj.aspx?proj=52598 ). Sichuan University West China Hospital and the Public Health Clinical Center of Chengdu Hospital are cooperative hospitals. The Public Health Clinical Center of Chengdu provides initial outpatient and follow-up services for some liver disease patients, but all surgeries are completed by West China Hospital. The two closely cooperate to form a medical whole, so patients are not distinguished.
Inclusion and exclusion criteria
The inclusion criteria of the open cohort were as follows: HCC patients 18 years or older without invasion of the main branch of the portal vein or the hepatic vein; HCC that did not directly invade the adjacent organs (except the gallbladder) or penetrate the peritoneum; There was no extrahepatic metastasis (TNM ≤ III A). The patients all had liver cirrhosis and portal hypertension. The diameter of portal vein was measured by ultrasound to determine whether the diameter was increased and to detect liver cirrhosis. Exclusion criteria included retransplantation, multiple organ transplantation, domino LT, double donor LT, or treatment with mTOR inhibitors (e.g., Everolimus/Rapamune) after transplantation. LT grafts were donated by patients who suffered cardiac death ( n = 494) or brain death ( n = 298). All the HCC were examined by pathology after undergoing a DPV operation. All included tumors were simple HCC, other tumor types such as cholangiocarcinoma or other special types of liver tumors were excluded. Patients with portal hypertension related complications, such as severe gastrointestinal varices, were informed of the risk of gastrointestinal bleeding and voluntarily decided to undergo DPV treatment.
Diagnostic criteria and follow up for HCC LT
TNM classification of HCC was conducted following the American Joint Committee of Cancer (AJCC) AJCC Cancer Staging Manual 8th edition in 2017 ( [ 23 ]). Acute renal injury (AKI) was assessed using the 2015 edition of the International Club of Ascites: the increase of sCr in 48 h was ≥ 0.3 mg/dl (26.5 μmol/L); or the increase of sCr in 48 h was ≥ 1.5 times of baseline value; or the urine volume lasted for 6 h < 0.5 ml·kg − 1 ·H− 1 [ 24 , 25 , 26 ]. Follow-up in the outpatient clinic was conducted routinely. Measurements of alpha fetoprotein and hepatitis B virus deoxyribonucleic acid (DNA), and abdominal ultrasonography were done every 3 months, and a CT scan was performed every 6 months. All hepatitis B virus DNA-positive patients were treated with anti-viral therapy before and after surgery. When intrahepatic recurrence was difficult to ascertain, MRI or contrast-enhanced ultrasonography was performed. Tumor recurrence was determined mainly based on radiographic evidence and/or AFP level. Patients who showed tumor recurrence after surgery were treated with the following options: resection, radio frequency ablation, re-LT, transcatheter arterial chemoembolization, or sorafenib. Patients were monitored until October 2019 or until their death, and their medical records were retrospectively reviewed. Our center requires standard treatment guidelines be followed for HCC patients with elevated HCV RNA. Patients are treated with direct-acting antiviral drugs (DAAS) while waiting for transplantation. If HCV recurrence occurs after transplantation, DAAS should be performed as soon as possible. Additionally, the use of glucocorticoids should be withdrawn as soon as possible after LT, and calcineurin inhibitor maintenance therapy (e.g., tacrolimus) should be minimized. No patients included in this study received any downstage therapy prior to LT. Downstaging refers to methods of treating HCC, such as radiation therapy, chemotherapy, and molecular targeted therapy. Patients with PHT are diagnosed through a comprehensive assessment, which includes a medical history review of liver disease, radiological findings indicative of liver fibrosis or sclerosis, endoscopic identification of gastric varices, and portal vein color Doppler ultrasound revealing portal vein dilatation or collateral circulation formation.
Case-control study composition
A total of 792 HCC patients underwent their first LT between September 2007 and January 2022. From these patients, we conducted a propensity score matching (PSM) analysis of 114 patients who underwent DPV (TIPS, surgical portosystemic shunt or/and splenectomy) before LT (32 Transjugular Intrahepatic Portosystemic Shunt-TIPS, 82 surgical portosystemic shunt or/and splenectomy), and 342 matched non-DPV control patients based on their PSM score on the day of LT but prior to surgery (Fig. S1 ).
Demographic and disease characteristics
Baseline characteristics of the DPV and non-DPV groups, matched prior to surgery on the day of LT, are shown in Table 1 . There was no significant difference between the DPV and non-DPV groups with respect to disease or population characteristics before transplantation.
Intraoperative and postoperative outcomes between the DPV and non-DPV groups
The postoperative complication data for the DPV and non-DPV groups are shown in Fig. 1 A. Intra-abdominal bleeding, incidence of postoperative AKI, and Clavien–Dindo Grade III–V complications were significantly higher in the DPV group than those in the non-DPV group. There was no significant difference in cumulative survival rate between the DPV and non-DPV groups within the different TNM stages (Fig. 2 ).
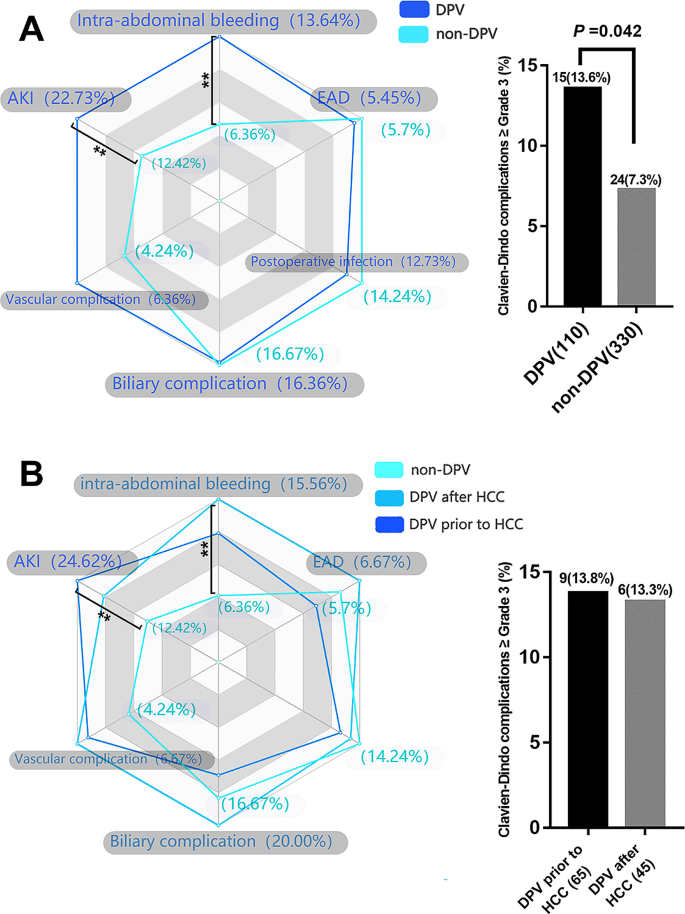
Analysis of main postoperative complications by radar chart and histogram. ** P < 0.05 ( A ) The incidence of AKI (22.7% vs. 12.4, P = 0.011) and intra-abdominal bleeding (13.6% vs. 6.4%, P = 0.012) in DPV group was significantly higher than that in non-DPV group, while the incidence of Clavien–Dindo III-V complication in DPV group was higher than that in non-DPV group. ( B ) In DPV subgroup comparison (DPV prior to HCC and DPV after HCC), there was no significant difference between the two subgroups in the incidence of specific major complications and the incidence of Clavien–Dindo III-V overall complications
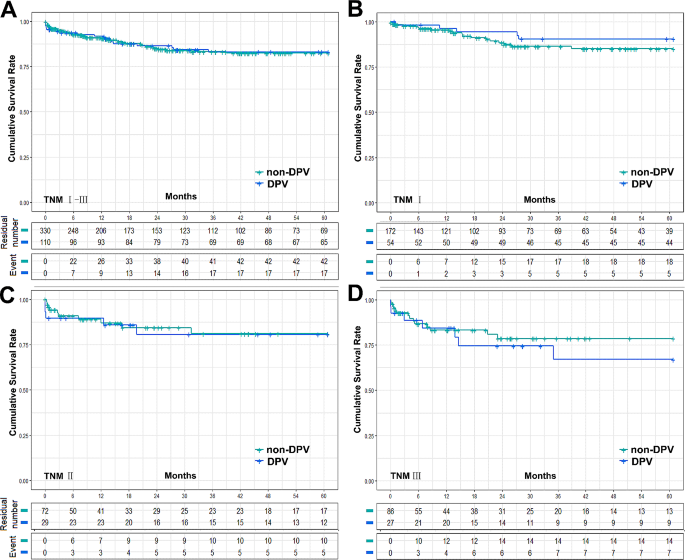
Cumulative survival rate between DPV and non-DPV group. ( A ) The 5-year survival rates of DPV ( n = 110) and non-DPV ( n = 330) group were 83.0% and 82.4% respectively, log-rank P = 0.934, and the 5-year median survival times were 52.52 ± 1.87 (95% Cl 48.85 to 56.19) and 52.30 ± 1.21 (95% Cl 49.93 to 54.68), respectively. ( B ) In HCC TNMIpatients, the 5-year survival rates of DPV ( n = 54) and non-DPV ( n = 172) were 90.4% and 85.1% respectively, log-rank P = 0.342, and the 5-year median survival times were 56.55 ± 1.87 (95% Cl 52.89 to 60.22) and 54.18 ± 1.47 (95% Cl 51.31 to 57.05), respectively. ( C ) In HCC TNMIIpatients, The 5-year survival rates of DPV ( n = 29) and non DPV ( n = 72) group were 80.7% and 81.0% respectively, log-rank P = 0.862, and the 5-year median survival times were 50.59 ± 4.15 (95% Cl 42.45 to 58.73) and 51.28 ± 2.77 (95% Cl 45.85 to 56.72), respectively. ( D ) In HCC TNM III patients, The 5-year survival rates of DPV ( n = 27) and non DPV ( n = 86) group were 67.0% and 78.5% respectively, log-rank P = 0.483, and the 5-year median survival times were 45.17 ± 5.00 (95% Cl 35.38 to 54.97) and 49.37 ± 2.77 (95% Cl 43.95 to 54.79), respectively
HCC and DPV subgroups
DPV patients were divided into two subgroups according to the sequence of their DPV treatment: DPV prior to HCC ( n = 65) and DPV after HCC ( n = 45). Subgroup analysis found no significant differences between these groups in postoperative complications (Fig. 1 B). However, patients with TNM I–II HCC had a significantly better cumulative recurrence rate and tumor-free survival rate when undergoing DPV prior to HCC compared to the non-DPV group. However, there was no significant difference between the subgroup that received DPV after HCC and the non-DPV group. In TNM III HCC patients, there was no significant difference in tumor recurrence rate or tumor-free survival rate between the non-DPV group and either DPV subgroup (Fig. 3 ).
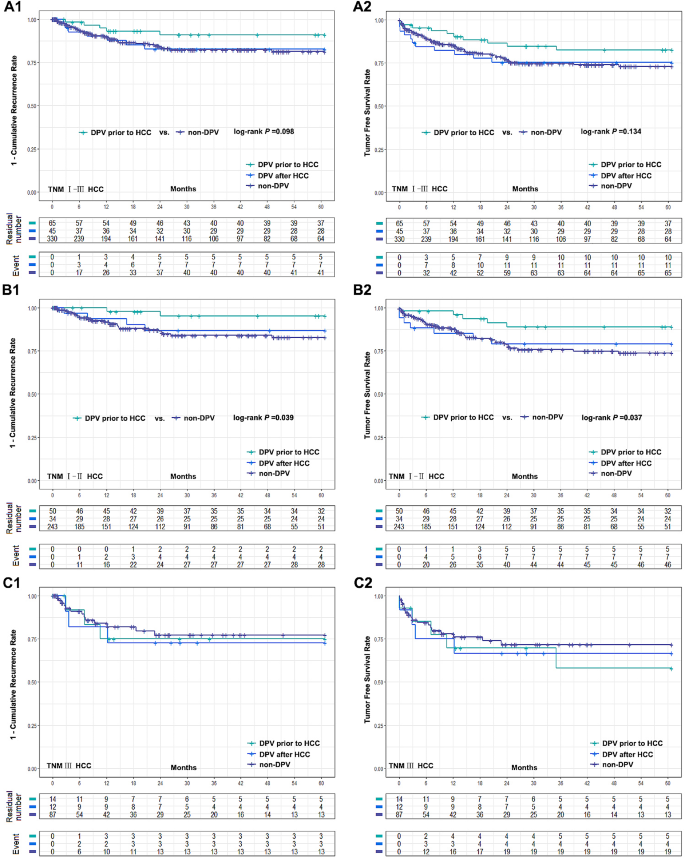
Comparison of cumulative recurrence rate and tumor free survival rate between two DPV subgroups (DPV prior to HCC ( n = 65) or DPV after HCC ( n = 45)) and non-DPV ( n = 330) group after LT. (A1-A2) The 5-year cumulative recurrence rate and tumor free survival rate of DPV after HCC subgroup and non-DPV group were 16.4% vs. 18.7%, log-rank P = 0.928, 75.3% vs. 73.0% log-rank P = 0.979, respectively. The 5-year cumulative recurrence rate and tumor free survival rate of DPV prior to HCC subgroup and non-DPV group were 9.0% vs. 18.7%, log-rank P = 0.098, 82.5% vs. 73.0%, log-rank P = 0.134. (B1-B2) In HCC TNM I-II patients, DPV prior to HCC subgroup were significantly better than non-DPV group in 5-year recurrence rate and tumor free survival rate (4.7% vs. 17.3%, log-rank P = 0.039 and 88.9% vs. 73.8%, log-rank P = 0.037). There was no significant difference between DPV after HCC subgroup and non-DPV group (13.2% vs. 17.3%, log-rank P = 0.619, 79.1% vs. 73.8%, log-rank P = 0.671). (C1-C2) The 5-year cumulative recurrence rate and tumor free survival rate of DPV prior to HCC, DPV after HCC and non-DPV were 25.0%, 27.3%, 22.8%, and 58.0%, 66.7%, 71.6%, respectively. There was no significant difference between groups (log-rank P > 0.05)
At present, HCC recurrence remains the main problem affecting the LT prognosis ( [ 27 ]). Although PHT has been shown to be closely associated with the development of HCC ( [ 28 ]) and to significantly affect prognosis following other non-LT radical treatments for HCC ( [ 18 , 29 , 30 ]), whether preoperative DPV can affect the prognosis of LT for HCC has not been reported. Our main finding is that patients who underwent DPV prior to HCC had a lower cumulative recurrence rate and higher tumor-free survival rate after LT in the AJCC TNM I–II stages, while in TNM III stage, DPV prior to HCC resulted in no difference between DPV after HCC or non-DPV patients.
The AJCC levels of evidence were established by the AJCC 8th Edition cancer staging system. Most of the confirmed risk factors related to the prognosis of LT for HCC were controlled in the latest TNM stage ( [ 31 , 32 ]). It is possible that there are relevant HCC risk factors that have not yet been reported due to the uncontrolled nature of even multiple-center studies and the difficulty of performing epidemiological studies on HCC ( [ 33 ]). One of the potential limitations of this study is the fact that DPV consists of three different procedures. Although there is no theoretical and clinical evidence suggesting that surgical portosystemic shunt and/or splenectomy will affect HCC recurrence after LT or other radical treatment, TIPS may increase the risk of metastasis caused by intrahepatic shunt through HCC ( [ 34 , 35 ]). If TIPS after HCC could lead to HCC extrahepatic metastasis, there would be a selection bias in our study. Since LT is not recommended for patients with extrahepatic metastasis, patients who developed extrahepatic metastasis due to TIPS after HCC will be excluded from LT. Wallace et al. reported that 2 of 9 patients developed lung metastases from TIPS crossing through a hepatic malignancy ( [ 35 ]). However, Bettinger et al. ( [ 36 ]). reported no metastasis was observed in 40 patients with centrally located tumors in the liver (segment VIII, V, and IV). A recent case-control study observing 217 patients found that TIPS is safe for PHT in patients with HCC ( [ 37 ]). Although we were unable to confirm that DPV after HCC does not increase the risk of HCC metastasis after transplantation, our results showed there is no increase in HCC recurrence or metastasis in patients who met the criteria for LT and underwent DPV after HCC. Therefore, early LT (before metastasis) for patients undergoing DPV after HCC may prevent this possibility of postoperative potential HCC metastasis.
As early as 1985, Bjørneboe et al. demonstrated that portal-systemic shunt may increase the risk of primary HCC in cirrhosis of the liver based on an observational cohort study of 201 people ( [ 38 ]). Banares et al. observed that TIPS may lead to an increase in 5-year cumulative incidence of HCC to 34%, significantly higher than the 25% observed in the control group ( [ 33 ]). However, this view has not been unanimously accepted ( [ 39 ]). A series of related studies shows that DPV may cause nodular regenerative hyperplasia, but it does not increase the incidence of HCC ( [ 40 , 41 , 42 ]) and a recent meta-analysis also found that the incidence of HCC was not increased ( [ 43 ]). Moreover, in contrast with other radical treatments, multiple HCC in the liver is not an absolute contraindication for LT. At present, there is still a lack of relevant research reports on this topic. In our study we found no difference between the DPV prior to HCC subgroup and the non-DPV group in the recurrence rate of HCC.
Although we did not observe a difference between patients who received DPV prior to HCC and non-DPV patients in the overall cohort, subgroup analysis highlighted differences between the different tumor TNM stages. Our results found that patients who met the criteria for AJCC TNMI–II stage who underwent DPV prior to HCC had a lower recurrence rate after LT, while patients in TNM III stage, showed no difference between DPV after HCC and non-DPV patients. Relatively low portal pressure may have a protective effect in early HCC patients, but when HCC develops past a certain point this may no longer be effective and this threshold is likely to be between TNM II and III stages. According to the theory by Tanaka et al., portal vein pressure is positively correlated with the pressure of both liver tissue and HCC, and the greater the pressure difference between the tumor and the adjacent tissue, the more likely the tumor is to invade tissue with relatively low pressure ( [ 44 ]). Therefore, it is possible that reducing the pressure of the portal vein simultaneously reduces the pressure of the liver tissue. HCC occurs in the liver tissue with relatively normal pressure compared to PHT, which makes the pressure in HCC also relatively low. Therefore, HCC with lower internal pressure is less likely spread into the adjacent tissue. However, with the development of HCC, and an increase in HCC pressure, the pressure difference between the tumor and surrounding tissue begins to be significant. In this scenario, the relatively low pressure of the liver tissue would no longer play a protective role but instead may allow HCC to reach the hepatic vein and enter the circulation easily, eventually leading to an increased risk of recurrence. However, it is worth noting that this interpretation is only a reasonable deduction based on our results and the theory suggested by Tanaka et al. and there is still a lack of direct evidence to support this.
PHT results from elevated intrahepatic vascular resistance due to sinusoidal alterations and liver fibrosis. This condition is compounded by the opening of portosystemic collateral vessels and the formation of new vessels, driven by vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) ( [ 45 ]). HCC, a common complication of cirrhosis, shares similar underlying factors with PHT, increasing the complexity of liver pathology. Inhibition of VEGF receptor 2 signaling has shown potential in reducing collateral vessel formation in experimental models ( [ 46 ]). VEGF plays a crucial role in neovascularization and fibrosis within the liver parenchyma, involving hepatocytes and hepatic stellate cells (HSCs). Activated HSCs, stimulated by VEGF, contribute to fibrosis, while portal myofibroblasts aid angiogenesis through collagen production. Liver stiffness influences sinusoidal angiogenesis and fibrosis progression. The resultant intrahepatic neovessels Increased hypoxia, inflammatory cytokines (IL-6, IL-1α), growth factors (epidermal growth factor, transforming growth factor-α and -β, fibroblast growth factor, PDGF) ( [ 46 , 47 ]). Anti-VEGF therapies like Sorafenib offer promise in mitigating PHT ( [ 48 ]). Chronic inflammation and angiogenesis triggered by PHT play a significant role in hepatocarcinogenesis, implying that early intervention targeting angiogenesis in liver disease could hold therapeutic promise.
Since DPV prior to HCC followed by LT can significantly reduce the recurrence rate in the early stage of the tumor, we wondered whether DPV might be applicable to all patients with PHT. However, we are still unsure if early DPV will increase the incidence of HCC and the postoperative complications of DPV are still worthy of consideration. Portal vein thrombosis (PVT) induced by DPV can progress to cavernous transformation, escalating the challenges of LT and amplifying the likelihood of intraoperative and short-term postoperative complications ( [ 49 ]). Vigilance is paramount when employing Tips, ensuring they do not penetrate the inferior vena cava excessively, particularly into the right atrium. Misplaced Tips can precipitate severe bleeding and jeopardize patient survival post-LT. Nevertheless, conventional pre-transplant TIPS placement primarily correlates with heightened portal flow gradient and does not significantly impede subsequent LT outcomes ( [ 50 ]). . Our study shows that DPV patients had a higher incidence of complications including AKI and postoperative blood loss in HCC stages III–V than non-DPV patients in all TNM stages, but there was no significant difference in the long-term survival rate. At present, there have been few reports about the complications of DPV after LT. Tripathi et al. found that patients who underwent LT after TIPS had higher dialysis rates and the long post-operative hospital stages, but that the overall survival rate is not affected ( [ 51 ]). Other associated reports did not focus on postoperative complications, but they all reported that there was still no significant difference in survival between their DPV and non DPV group ( [ 52 ]). Therefore, DPV is still a safe and feasible bridging treatment for LT, but it may be accompanied by relatively high complications after LT. It can also be reasonably inferred that the corresponding treatment costs will be increased. Altogether, we believe that it is still necessary to be cautious before deeming DPV applicable to all patients with PHT, especially for those without gastrointestinal hemorrhage or refractory ascites caused by PHT.
The retrospective nature of our work should be acknowledged as a key limitation, even when using a case-control study design based on PSM. However, PSM may lead to other biases due to unmeasured patient characteristics which may have influenced outcomes. Furthermore, because it is difficult to measure, we were unable to directly measure the portal vein pressure. We could only infer a reduction in portal vein pressure through demonstrated methods such as TIPS and obvious postoperative improvement in symptoms. However, we do not know the relationship between the degree of reduction and our results. At present, noninvasive methods of detecting portal vein pressure are indirect and cannot directly reflect the accurate pressure value. Therefore, a technical breakthrough that is able to provide a more in-depth demonstration of the relationship between portal pressure and tumor recurrence is necessary. In addition, our data source was a single center which limits the study’s scope. In HCC TNM III patients, there were only 14 cases of DPV prior to HCC and 12 cases of DPV after HCC. Further multi-center studies are required to verify these findings.
Based on our results, we propose that non-HCC patients with current DPV indications (such as patients with severe gastrointestinal bleeding and irreversible ascites) should be treated more actively with DPV. If HCC occurred in such patients and LT performed early (TNM I–II), such patients may have relatively lower recurrence rates than patients in non-DPV or DPV after HCC patients. DPV before LT can reduce the recurrence of early HCC.
Data availability
All related data of our center are stored in the Chinese Liver Transplant Registry, a platform for unified management of LT centers in mainland China (CLTR: http://cltr.cotr.cn ). The data that support the findings of this study are available from the Chinese Liver Transplant Registry (CLTR: http://cltr.cotr.cn ), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. But all related data in this study are available from the corresponding author on reasonable request.
Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, et al. Liver transplantation for hepatocellular carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1136–42.
Article PubMed Google Scholar
de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21(39):11185–98.
Article PubMed PubMed Central Google Scholar
Lee EW, Shahrouki P, Alanis L, Ding P, Kee ST. Management options for gastric variceal hemorrhage. JAMA Surg. 2019;154(6):540–8.
Rajwani K, Fortune BE, Brown RS, Jr. Critical care management of gastrointestinal bleeding and ascites in liver failure. Semin Respir Crit Care Med. 2018;39(5):566–77.
Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 2021;56(7):593–619.
Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Th Adv Gastroenterol. 2014;7(5):206–16.
Article Google Scholar
Wong F, Sniderman K, Liu P, Blendis L. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology. 1997;112(3):899–907.
Article CAS PubMed Google Scholar
Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536.
Lesmana CRA, Raharjo M, Gani RA. Managing liver cirrhotic complications: overview of esophageal and gastric varices. Clin Mol Hepatol. 2020;26(4):444–60.
Sellers CM, Nezami N, Schilsky ML, Kim HS. Transjugular intrahepatic portosystemic shunt as a bridge to liver transplant: current state and future directions. Transplantation Reviews (Orlando Fla). 2019;33(2):64–71.
Kong L, Li M, Li L, Jiang L, Yang J, Yan L. Splenectomy before adult liver transplantation: a retrospective study. BMC Surg. 2017;17(1):44.
de Ville J, D’Ambrosio G, Grimaldi C. Surgical management of portal hypertension in children. Semin Pediatr Surg. 2012;21(3):219–32.
Bai D-S, Zhou B-H, Qian J-J, Zhang C, Jin S-J, Jiang G-Q. Effects of laparoscopic splenectomy and azygoportal disconnection on liver synthesis function and cirrhosis: a 2-year prospective study. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-7307-7
Yoshizumi T, Itoh S, Shimokawa M, Inokuchi S, Harada N, Takeishi K, et al. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J Hepatol. 2021;74(2):372–9.
Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128–39.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50.
Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50(5):923–8.
Kim R, Jeong WK, Kang TW, Song KD, Lee MW, Ahn SH et al. Intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma: relationship with portal hypertension. Acta radiologica (Stockholm, Sweden: 1987). 2019;60(12):1609-18.
Mancuso A, Perricone G. Hepatocellular carcinoma and liver transplantation: state of the art. J Clin Translational Hepatol. 2014;2(3):176–81.
Google Scholar
Gundlach JP, Ellrichmann M, van Rosmalen M, Vogelaar S, Eimer C, Rheinbay C, et al. Liver transplantation for HCC in cirrhosis: are Milan criteria outdated? Z Gastroenterol. 2024;62(1):43–9.
Article CAS PubMed PubMed Central Google Scholar
Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–39.
Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–65.
Amin MBGD. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.
Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62(4):968–74.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204.
Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. 2022;157(9):779–88.
PubMed PubMed Central Google Scholar
Thabut D, Kudo M. Treatment of portal hypertension in patients with HCC in the era of Baveno VII. J Hepatol. 2023;78(3):658–62.
Jung KS, Kim JH, Kim SU, Song K, Kim BK, Park JY, et al. Liver stiffness value-based risk estimation of late recurrence after curative resection of hepatocellular carcinoma: development and validation of a predictive model. PLoS ONE. 2014;9(6):e99167–e.
Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology (Baltimore MD). 2015;61(2):526–36.
Liu Z, Liu Y, Zhang W, Hong Y, Meng J, Wang J, et al. Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation: a discovery and validation study. Hepatol Int. 2022;16(3):577–89.
Vauthey J-N, Ribero D, Abdalla EK, Jonas S, Bharat A, Schumacher G, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg. 2007;204(5):1016–28.
Bañares R, Núñez O, Escudero M, Fernández C, Vaquero J, Beceiro I, et al. Patients with cirrhosis and bare-stent TIPS may have increased risk of hepatocellular carcinoma. Hepatology (Baltimore MD). 2005;41(3):566–71.
Fichtl A, Seufferlein T, Zizer E. Risks and benefits of TIPS in HCC and other liver malignancies: a literature review. BMC Gastroenterol. 2023;23(1):403.
Wallace M, Swaim M. Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J Vascular Interventional Radiology: JVIR. 2003;14(4):501–7.
Bettinger D, Knüppel E, Euringer W, Spangenberg HC, Rössle M, Thimme R, et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2015;41(1):126–36.
Luo S-H, Chu J-G, Huang H, Yao K-C. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7(13):1599–610.
Bjørneboe M, Andersen JR, Christensen U, Skinhøj P, Jensen OM. Does a portal-systemic shunt increase the risk of primary hepatic carcinoma in cirrhosis of the liver? Scand J Gastroenterol. 1985;20(1):59–64.
Libbrecht L, Maleux G, Verslype C, Nevens F, Roskams T. Influence of TIPS on development of hepatocellular carcinoma in cirrhosis. Hepatology (Baltimore MD). 2005;42(1):236–7.
Cazals-Hatem D, Vilgrain V, Genin P, Denninger M-H, Durand F, Belghiti J, et al. Arterial and portal circulation and parenchymal changes in Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology (Baltimore MD). 2003;37(3):510–9.
De Santis A, Iegri C, Kondili L, Riggio O, Salvatori FM, Catalano C, et al. Hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt: a retrospective case-control study. Dig Liver Disease: Official J Italian Soc Gastroenterol Italian Association Study Liver. 2014;46(8):726–30.
Borentain P, Garcia S, Gregoire E, Vidal V, Ananian P, Ressiot E, et al. Transjugular intrahepatic porto-systemic shunt is a risk factor for liver dysplasia but not hepatocellular carcinoma: a retrospective study of explanted livers. Dig Liver Disease: Official J Italian Soc Gastroenterol Italian Association Study Liver. 2015;47(1):57–61.
Chen B, Pang L, Chen H-B, Wu D-B, Wang Y-H, Chen E-Q. TIPS is not associated with a higher risk of developing HCC in Cirrhotic patients: a systematic review and meta-analysis. J Clin Translational Hepatol. 2019;7(3):232–7.
Tanaka T, Yamanaka N, Oriyama T, Furukawa K, Okamoto E. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26(2):283–7.
Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61(4):1406–15.
Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses?. Liver Int. 2021;41(8):1734–43.
Kong L, Zhou Y, Bu H, Lv T, Shi Y, Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35(1):131.
Ma R, Chen J, Liang Y, Lin S, Zhu L, Liang X, et al. Sorafenib: a potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed Pharmacother. 2017;88:459–68.
Kurata N, Ogura Y, Ogiso S, Onishi Y, Kamei H, Kodera Y. Splenectomy in living donor liver transplantation and risk factors of portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2019;18(4):337–42.
Matsushima H, Fujiki M, Sasaki K, Cywinski JB, D’Amico G, Uso TD, et al. Can pretransplant TIPS be harmful in liver transplantation? A propensity score matching analysis. Surgery. 2020;168(1):33–9.
Tripathi D, Therapondos G, Redhead DN, Madhavan KK, Hayes PC. Transjugular intrahepatic portosystemic stent-shunt and its effects on orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2002;14(8):827–32.
Barbier L, Hardwigsen J, Borentain P, Biance N, Daghfous A, Louis G, et al. Impact of transjugular intrahepatic portosystemic shunting on liver transplantation: 12-year single-center experience. Clin Res Hepatol Gastroenterol. 2014;38(2):155–63.
Huang J, Millis JM, Mao Y, Millis MA, Sang X, Zhong S. A pilot programme of organ donation after cardiac death in China. Lancet. 2012;379(9818):862–5.
Download references
Acknowledgements
Not Applicable.
This study was supported by grants from Chengdu Science and Technology Projects (2022-YF05-01253-SN); Sichuan Natural Science Foundation (2022NSFSC0805); China Postdoctoral Science Foundation (2022M720102); Sichuan Medical Association (2021HR58); Research Project of Chengdu Health Commission (2023179).
Author information
Authors and affiliations.
Department of General Surgery, Public health clinical center of chengdu, Chengdu, Sichuan Province, China
Guo Wei, Yong Zhao & Shifeng Feng
Department of Liver transplantation center, West China Hospital of Sichuan University, Chengdu, Sichuan Province, China
Tao Lv, Jian Yang, Lingxiang Kong & Jiayin Yang
Department of Liver transplantation Laboratory, West China Hospital of Sichuan University, Chengdu, Sichuan Province, China
Jingsheng Yuan, Gang Xu & Lingxiang Kong
You can also search for this author in PubMed Google Scholar
Contributions
LK, WG and Jiayin Y designed the study; WG, GX, Jiayin Y, YZ, SF, Jian Y, TL, LK performed the research and collected the data; LK, SF, YZ analyzed and interpreted the data; WG, LK wrote the first draft of the manuscript; All authors edited the manuscript and approved the final draft; The acquisition of funding is from Jiayin Y.
Corresponding authors
Correspondence to Lingxiang Kong or Jiayin Yang .
Ethics declarations
Ethical approval and source of grafts.
In our study, the liver grafts utilized for LT since 2007 were obtained from deceased citizens in accordance with a new organ procurement and allocation policy implemented in China after 2012 ([ 53 ]). No prisoners were included as donors. The protocol was approved by the Ethics Committee of the West China Hospital of Sichuan University West China Hospital. Written informed consent was obtained from all the recipients prior to their surgery, and all of donations were voluntary and altruistic in all cases, and were in accordance with the ethical guidelines of the Declaration of Helsinki. The written informed consents from the next of kin of the dead citizen are kept at the Red Cross Society of Sichuan Province, China. ( www.scredcross.org.cn ).
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wei, G., Zhao, Y., Feng, S. et al. Does depressurization of the portal vein before liver transplantation affect the recurrence of HCC? A nested case-control study. BMC Cancer 24 , 558 (2024). https://doi.org/10.1186/s12885-024-12322-6
Download citation
Received : 25 April 2023
Accepted : 30 April 2024
Published : 03 May 2024
DOI : https://doi.org/10.1186/s12885-024-12322-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hepatocellular carcinoma (HCC)
- Liver transplantation (LT)
- Tumor recurrence
- Portal hypertension (PHT)
- Portosystemic Shunt (PSS)
- Splenectomy
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction. Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence. These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as ...
For example, imagine a study of 20 consecutive patients with a certain disease that can be treated in two different ways. A study that divides the 20 patients into two groups according to the treatment received and compares the outcomes of these groups (e.g., provides aggregated absolute risks per group or a risk ratio) would be probably classified as a cohort study (the example used in the ...
Design. In a case-control study, a number of cases and noncases (controls) are identified, and the occurrence of one or more prior exposures is compared between groups to evaluate drug-outcome associations ( Figure 1 ). A case-control study runs in reverse relative to a cohort study. 21 As such, study inception occurs when a patient ...
Cohort studies and case-control studies are two primary types of observational studies that aid in evaluating associations between diseases and exposures. In this review article, we describe these study designs, methodological issues, and provide examples from the plastic surgery literature. Keywords: observational studies, case-control study ...
A case-cohort study is similar to a nested case-control study in that the cases and non-cases are within a parent cohort; cases and non-cases are identified at time t1, after baseline. In a case-cohort study, the cohort members were assessed for risk factors at any time prior to t1. Non-cases are randomly selected from the parent cohort ...
A case series may be a study that samples patients with both a specific outcome and a specific exposure, or one that samples patients with a specific outcome and includes patients regardless of whether they have specific exposures. Whereas a cohort study, in principle, enables the calculation of an absolute risk or a rate for the outcome, such ...
The case-control study can be subcategorized into four different subtypes based on how the control group is selected and when the cases develop the disease of interest as described in the following sections. Nested Case-Control Study When a case-control study is performed within a cohort study, it is called a nested case-control study. In a nested
Cohort studies are prospective in nature. You suspect that, for example: • Exposure to a particular chemical, water from a particular source, etc. seems to lead to a particular disease state, or; ... Case-control studies are retrospective in nature. You have patients that are sick with a new disease. There are others from the same area that ...
Case-cohort studies are very similar to nested case-control studies . The main difference between a nested case-control study and a case-cohort study is the way in which controls are chosen. Generally, the main advantage of case-cohort design over nested case-control design is that the same control group can be used for comparison with ...
A case-cohort study has all the previously mentioned advantages that are present in a nested case-control study. In addition, a major advantage of the case-cohort design is the ability to study several disease outcomes using the same subcohort. For example, if we want to evaluate the role of a specific risk factor for two different diseases ...
Cohort studies of multiple exposures/more than one group. This idea can be easily extended to studies with more than one exposure. In this case, all studies with exposure-based sampling gathering multiple exposures (i.e., at least two different exposures, manifestations of exposures or levels of exposures) can be considered as (comparative) cohort studies (Fig. 3).
Cohort Design. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an identified study population (Mann, 2012; Song & Chung, 2010).Since participants do not have the outcome or disease at study entry, the temporal causality between exposure and outcome(s) can be assessed using this design (Hulley, 2013; Song & Chung, 2010).
Case-control studies are commonly performed in field epidemiology when a cohort study is impractical (e.g., no defined cohort or too many non-ill persons in the group to interview). Whereas a cohort study proceeds conceptually from exposure to disease or condition, a case-control study begins conceptually with the disease or condition and ...
Cohort and case control studies are two different types of observational studies used to determine if there is an association between exposure and a health outcome 1. The studies are observational and not experimental because, unlike a randomized clinical trial, the investigators do not assign subjects to the exposed and unexposed groups. ...
ance for distinguishing cohort studies from case series be-cause in many definitions, they share a main design feature of having a follow-up period examining the ex-posed individuals over time [2, 3]. The only difference be-tween cohort studies and case series in many definitions is that cohort studies compare different groups (i.e., examine
Abstract. The basic epidemiological study designs are cross-sectional, case-control, and cohort studies. Cross-sectional studies provide a snapshot of a population by determining both exposures and outcomes at one time point. Cohort studies identify the study groups based on the exposure and, then, the researchers follow up study participants ...
A case-cohort study is similar to a nested case-control study in that the cases and non-cases are within a parent cohort; cases and non-cases are identified at time t 1, after baseline. In a case-cohort study, the cohort members were assessed for risk factors at any time prior to t 1. Non-cases are randomly selected from the parent cohort ...
A case-control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group. While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are ...
Visualization of case-cohort designs assuming a time-on-study time scale. (A) The case-cohort study includes (1) a sample of individuals from the cohort who have experienced the outcome of interest ("cases") and (2) a sample of individuals randomly selected from among the members of the full cohort observed at baseline (the "sub-cohort
A case-control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group. While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are ...
It has been demonstrated that the case-cohort study design, for a single disease outcome, is more efficient than a nested case-control study design; however, the difference is very small [1]. Compared to the nested case-control studies, a major advantage of the case-cohort design is the ability to study several disease outcomes using the same
Amber S Gordon. Beverly Claire Walters. Case-control (case-control, case-controlled) studies are beginning to appear more frequently in the neurosurgical literature. They can be more robust, if ...
In the traditional hierarchy of study designs, the randomized controlled trial (RCT) is placed on top, followed by cohort studies, case‐control studies, case reports and case series. 2 However, the foremost consideration for the choice of study design should be the research question. For some research questions, an RCT might be the most ...
Case-control study composition. A total of 792 HCC patients underwent their first LT between September 2007 and January 2022. From these patients, we conducted a propensity score matching (PSM) analysis of 114 patients who underwent DPV (TIPS, surgical portosystemic shunt or/and splenectomy) before LT (32 Transjugular Intrahepatic Portosystemic Shunt-TIPS, 82 surgical portosystemic shunt or ...