An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Case Rep


A Case of Plasmodium falciparum Malaria Treated with Artesunate in a 55-Year-Old Woman on Return to Florida from a Visit to Ghana
Jose a. rodriguez.
1 Department of Internal Medicine, Memorial Healthcare System, Pembroke Pines, FL, U.S.A.
Alejandra A. Roa
Ana-alicia leonso-bravo, pratik khatiwada, paula eckardt.
2 Division of Infectious Disease, Memorial Regional Hospital, Memorial Healthcare System, Hollywood, FL, U.S.A.
Juan Lemos-Ramirez
Patient: Female, 55-year-old
Final Diagnosis: Severe malaria
Symptoms: Altered mental status • dyspnea • fever
Medication: —
Clinical Procedure: —
Specialty: Critical Care Medicine • Infectious Diseases
Management of emergency care
Background:
Malaria is the infection caused by inoculation with the mostly obligate intraerythrocytic protozoa of the genus Plasmodium. Severe malaria manifests as multiple organ dysfunction with high parasitemia counts characterized by coma, stupor, and severe metabolic acidosis. Physicians in the United States do not frequently encounter patients with malaria, and the drugs are only available through the Centers for Disease Control and Prevention, which makes the management of this disease somewhat complicated. In 2019, the marketing of quinine for malaria was discontinued. In May 2020, the US Food and Drug Administration approved the use of intravenous artesunate for the treatment of adults and children with severe malaria. This case report describes a case of Plasmodium falciparum malaria in a 55-year-old woman who returned home to Florida from a visit to Ghana.
Case Report:
A previously healthy 55-year-old woman with no significant past medical history presented to the Emergency Department (ED) of a hospital in south Florida due to cyclic fever for 7 days. The patient’s family reported mental status changes since symptom onset. The patient had returned from a 10-day trip to Ghana 18 days prior to admission. On arrival to the ED, the patient appeared lethargic and within hours was in respiratory distress. She was intubated and mechanically ventilated in the ED for acute hypoxemic respiratory failure. A malaria smear was positive with 25% parasitemia, and a diagnosis of severe malaria was made, consistent with P. falciparum infection complicated by multi-organ failure. Infectious disease consultation was obtained and an infusion of intravenous (IV) quinidine and IV doxycycline was emergently started due to the anticipated delay in obtaining artesunate. During the second day of admission, the patient had QTc prolongation, so quinidine was switched to IV artesunate. The parasitemia and acidosis started improving by the third day of therapy.
Conclusions:
Given that artesunate is more effective, easier to dose, and more tolerable than quinidine, it is now the treatment of choice for severe malaria in the United States.
Malaria is the infection caused by the mostly obligate intraerythrocytic protozoa of the genus Plasmodium , which is spread to people by the inoculation from infected female Anopheles mosquitoes. Plasmodium falciparum is the major cause of severe malaria, which manifests as multiple organ dysfunction with high parasitemia counts that is characterized by coma, stupor, and severe metabolic acidosis [ 1 ].
Malaria occurs primarily in tropical and some subtropical regions of Africa, Central and South America, Asia, and Oceania, with an estimated 228 million cases worldwide in 2018 (93% of them occurring in Africa) [ 2 ]. About 2000 cases of malaria are diagnosed in the United States each year. Almost all these cases are imported by returning travelers or immigrants from endemic regions, with a limited number possibly occurring through local mosquito-borne transmission [ 2 ]. The US Centers for Disease Control and Prevention (CDC) has reported that among the 2000 cases of malaria diagnosed in the United States each year, about 300 cases are severe. The majority of these severe cases involve travelers returning from sub-Saharan Africa and South Asia [ 3 ].
In 2019, the marketing of quinine for malaria was discontinued in the United States. In May 2020, the US Food and Drug Administration (FDA) approved the use of intravenous artesunate for the treatment of severe malaria, with the recommendation that it should be followed by a full course of oral antimalarial treatments [ 4 ].
Physicians in the United States do not encounter patients with malaria frequently, and the drugs are only available through the CDC, which makes the management of this disease somewhat complicated. Among patients with unexplained fever or clinical deterioration who have traveled to an endemic area, malaria must be included in the differential diagnosis to avoid delays in appropriate treatment of malaria that would increase morbidity and mortality [ 5 , 6 ]. Therefore, it is imperative to have a better understanding of this disease and also to inform readers about the management and ways of improving it.
Case Report
A previously healthy 55-year-old woman with no significant past medical history presented to the Emergency Department (ED) of a hospital in south Florida owing to a fever for 7 days. Fever was quantified with readings of 40°C coming every 48 h, associated with various nonspecific symptoms such as malaise, fatigue, decreased appetite, productive cough for 2–3 days, and abdominal pain with associated watery diarrhea for 2–3 days. The patient’s family reported mental status changes (nonresponsive to her name, visual hallucinations) since symptom onset. The patient denied headache, loss of consciousness, neck rigidity, seizure, focal neurological symptoms, chest pain, hemoptysis, and difficulty breathing at the time of presentation. She denied any similar episodes in the past. She reported allergy (rash) to penicillin. No pertinent family history was noted. She lived with her son, who was asymptomatic, and worked as a biomedical engineer. The patient had returned from a 10-day trip to Ghana 18 days prior to admission, and she had also traveled to California 1 week before admission. She denied receiving malaria prophylaxis or vaccination against yellow fever and hepatitis A virus. She developed symptoms while in California and went to a hospital there, but she was discharged following unremarkable examination and normal laboratory results.
A few hours after arrival to the ED, the patient became lethargic and was found to be in respiratory distress. Vital signs were an oral temperature of 38.6°C, heart rate 121 beats/min, blood pressure 100/51 mmHg, respiratory rate 45 breaths/min, and SpO 2 93% on room air. She had dry mucous membranes, decreased bilateral breath sounds, and tenderness to palpation of the left lower quadrant abdomen. No obvious jaundice, enlarged lymph nodes, or splenomegaly was observed. On neurological examination, she was lethargic and confused with Glasgow coma scale (GCS) score of 14 (E4V4M6); her pupils were equal, round, and reactive to light; she had no cranial nerve or sensory deficit; and her neck was supple, with Brudzinski’s and Kernig’s signs both being negative.
Initial laboratory studies revealed leukocytosis with white cell count of 19 800/µL with 44% neutrophils, 22% lymphocytes, and 1% eosinophil; platelets 51 000/µL; hemoglobin 11.8 g/dL; hematocrit 35.3%; red blood cell distribution width 16.9%; lactate dehydrogenase 2714 U/L; haptoglobin <8 mg/dL; prothrombin time/international normalized ratio 14.9/1.4; troponin 0.161 ng/mL; blood glucose 26 mg/dL; blood urea nitrogen 157 mg/dL; creatinine 7.52 mg/dL; estimated glomerular filtration rate 6 mL/min; bicarbonate 5 mmol/L; anion gap 36; lactic acid 16.2 mmol/L; sodium 131 mmol/L; potassium 5.5 mmol/L; chloride 91 mmol/L; alanine transaminase 542 U/L; aspartate transaminase 1328 U/L; total bilirubin 14.5 mg/dL; and albumin 2.2 mg/dL. A malaria smear was positive with 25% parasitemia initially, and ring forms/trophozoites and a few elongated structures suggestive of developing gametocytes were reported ( Figure 1 ). An arterial blood gas obtained in the ED on 3 L of oxygen via nasal cannula showed pH of 7.03 and pCO 2 of 20 and HCO 3 of 0.

Numerous malaria organisms are present, affecting approximately 25% of red blood cells. Ring forms/ trophozoites have 1 or 2 chromatin dots. Multiply-infected red cells are not uncommon (2–4 trophozoites). A few elongated structures suggestive of developing gametocytes are seen.
The patient’s mental status gradually deteriorated while she was being evaluated at the ED. Her GCS score after 3 h of presentation was 10/15 (E2V3M3), and she had to be intubated and mechanically ventilated in the ED for acute hypoxemic respiratory failure and transferred to the Intensive Care Unit (ICU). Severe malaria was diagnosed, consistent with Plasmodium falciparum malaria complicated by multi-organ failure including respiratory, renal, and hepatic failure; septic shock; and disseminated intravascular coagulation. The acute toxic-metabolic encephalopathy was most likely due to cerebral malaria. The acute hypoxemic respiratory failure evolved into acute respiratory distress syndrome driven by the shock and severe acidemia, so vasopressors and steroids were started. Infectious disease consultation was obtained and an infusion of intravenous (IV) quinidine at a rate of 115.2 mg/h and IV doxycycline 100 mg every 12 h was emergently started due to the anticipated delay in obtaining artesunate. This was done with close monitoring of QTc, blood glucose, and parasitemia, while the CDC was contacted to request the emergent release of the artesunate. Empiric broad-spectrum antibiotic coverage was started with IV meropenem 1 g every 12 h and intermittently dosed IV vancomycin. Exchange transfusion was considered as a salvage therapy in case of nonresponse to medical therapy.
During the second day of admission, the patient had QTc prolongation, so quinidine was switched to IV artesunate every 24 h. The parasitemia and acidosis started improving and the positive end-expiratory pressure and FiO 2 requirements decreased. Computed tomography of the brain was unremarkable, and a lumbar puncture showed 3 white blood cells per high-power field, 12% neutrophils, 48% lymphocytes, 38% monocytes, and no organisms on gram stain. Vancomycin and meropenem were discontinued due to no evidence of bacterial meningitis. By the third day of therapy, the parasitemia decreased to 0.3% and was negative on day 6 ( Table 1 ). The multi-organ failure and septic shock were treated with supportive care including renal replacement therapy and platelet transfusions, and the patient was clinically improving. A 5-day course of IV artesunate and doxycycline was completed with an additional 7-day course of oral doxycycline at discharge to a rehabilitation facility with a favorable outcome and recuperation.
Daily parasitemia percentage.
This case illustrates the need to recognize severe malaria, especially cerebral malaria, and the need for more readily available parenteral artesunate in the United States. Severe malaria is defined as P. falciparum parasitemia >10% and signs of major organ dysfunction including impaired consciousness, prostration, 2 or more convulsive episodes in 24 h, acidosis (bicarbonate <15 mmol/L), hypoglycemia (glucose <40 mg/dL), severe anemia (hemoglobin <6 g/dL), recurrent or prolonged bleeding, renal impairment (creatinine >3 mg/dL), pulmonary edema, and shock. Less commonly, severe malaria can be caused by other Plasmodium species. Severe malaria tends to occur in young children in endemic areas and adults traveling to endemic countries owing to their lack of immunity; these populations are also at the highest risk for cerebral malaria for the same reasons [ 7 , 8 ]. The incubation period for P. falciparum is 12–14 days (range of 7–30 days). Severe malaria can cause many complications, with cerebral malaria being one of the most important to recognize due to its poor prognosis. It presents as impaired consciousness, delirium, and/or seizure [ 9 ].
Treatment of severe malaria requires prompt antimalarial therapy with supportive care and management of complications because mortality is highest in the first 24 h of presentation [ 8 , 10 , 11 ]. Prompt treatment is especially critical if cerebral malaria is suspected because it has a 15–20% mortality rate when treated and above 30% in cases with multiple vital organ dysfunction [ 9 , 12 ]. For severe malaria, the WHO recommends parenteral artesunate (if the artesunate of reliable quality is available); otherwise, treatment with quinidine is recommended. Quinidine was used in the United States because artesu-nate was neither approved by the FDA nor was it was commercially available before May 2020 [ 13 ].
Treatment is generally parenteral initially. It is then completed with oral antimalarial if the patient is tolerating oral intake and parasitemia is ≤1% when using artesunate. Completion of oral antimalarial therapy is 3–7 days afterward depending on the regimen, which can include doxycycline, clindamycin, quinidine, atovaquone-proguanil, and mefloquine (refer to Table 2 for dosing and duration of therapy) [ 12 , 14 ]. Mefloquine should be avoided if the patient has cerebral malaria due to the increased risk of neuropsychiatric effects, and it is not recommended if malaria was acquired in Southeast Asia due to drug resistance [ 8 , 12 , 14 ].
Antimalarial therapy.
FDA approval for the use of intravenous artesunate was based on evidence from multiple randomized controlled trials abroad, including Europe, as well as trials in adults and children from endemic areas in Asia and Africa [ 8 , 10 – 13 ]. The benefits tend to be most pronounced in patients with hyperparasitemia in endemic/nonendemic areas (reduced ICU and hospitalization length of stays). The patients also tend to have faster parasite clearance from the blood by about 1–2 days when treated with artesunate. The difference in outcomes is less pronounced with parasitemia less than 5% [ 10 – 14 ]; nonetheless, adult travelers to endemic areas have a higher likelihood of developing hyperparasitemia, so artesunate would likely still provide a benefit over quinidine. The mechanism of action for artesunate is incompletely understood, but it is hypothesized to involve the formation of free radicals that interfere with parasitic function and it has a broader spectrum of action against ring-stage parasites. By preventing maturation and sequestration of infected erythrocytes, artesunate improves removal by the spleen and allows for less microvascular obstruction and subsequent organ damage. This may explain why the benefits of artesunate are the most profound in patients with hyperparasitemia [ 10 , 11 , 15 , 16 ].
The WHO recommends artesunate for the treatment of severe malaria because it has been shown to reduce the adult mortality rate by about 39% relative to quinidine (24% greater reduction in the child mortality rate) [ 6 ], has fewer adverse effects and drug-drug interactions, and is easier to dose [ 9 – 11 ]. Quinidine is known to cause QTc prolongation, ototoxic effects, and hyperinsulinemic hypoglycemia. Artesunate is relatively quick acting and tolerated, but patients on this treatment must be monitored for delayed hemolytic anemia at 7, 14, and 30 days after completion of therapy [ 8 , 12 , 14 ].
The patient presented in this case had severe malaria, specifically cerebral malaria, 18 days after returning to the United States from a 10-day trip to Ghana. Other causes for the patient’s symptoms were excluded, including viral infection, meningitis, bacteremia, and so forth. When the patient was started on quinidine, there was minimal effect on the parasitemia (25% to 23.43% in 24 h). Once treatment was switched to artesunate due to QTc prolongation, the parasitemia dropped from 23.43% to 6.8% in 24 h, and the smear was negative within 5 days. This patient had complications from severe malaria but likely benefited from the faster clearance of malaria from the blood with artesunate, which the quinidine did not provide.
Ideally, this patient could have prevented contracting malaria with mosquito bite prevention and by receiving prophylaxis from a travel clinic, which can provide detailed, individualized, and effective travel counseling. Prophylaxis with atovaquoneproguanil, doxycycline, mefloquine, primaquine, tafenoquine, or rarely chloroquine (due to high rates of resistance) is started prior to travel, and it is continued during the trip and for a period of time after returning home. The regimen depends on the region of travel, length of stay, and local malaria resistance patterns [ 6 , 17 ].
Conclusions
This report presents a case of severe P. falciparum malaria treated with artesunate in a 55-year-old woman who returned to Florida from a visit to Ghana. The case highlights the importance of early diagnosis of malaria, particularly in patients who have returned from travel to countries where malaria is endemic. It also underscores the use of current diagnostic guidelines and regulatory approved treatment, which now includes IV artesunate.
Conflict of interest
References:

- Earn HPCSA and SACNASP CPD Points
- I have spots and my skin burns
- A case of a 10 year old boy with a 3 week history of diarrhoea, vomiting and cough
- A case of fever and general malaise
- A case of persistant hectic fever
- A case of sudden rapid neurological deterioration in an HIV positive 27 year old female
- A case of swollen hands
- An unusual cause of fulminant hepatitis
- Case of a right axillary swelling
- Case of giant wart
- Case of recurrent meningitis
- Case of repeated apnoea and infections in a premature infant
- Case of sudden onset of fever, rash and neck pain
- Doctor, my sister is confused
- Eight month old boy with recurrent infections
- Enlarged Testicles
- Failure to thrive despite appropriate treatment
- Right Axillary Swelling
- Severe anaemia in HIV positive child
- The case of a floppy infant
Two year old with spiking fevers and depressed level of consciousness
- 17 year old male with fever and decreased level of consciousness
- 3 TB Vignettes
- A 10 year old girl with a hard palate defect
- A case of decreased joint function, fever and rash
- Keep up while the storm is raging
- Fireworks of autoimmunity from birth
- My hands swell and are painful
- My eyes cross at twilight
- A case of a 3 month old infant with bloody urine and stools
- A case of scaly annular plaques
- Case of eye injury and decreased vision
- My head hurts and I cannot speak?
- TB or not TB: a confusing case
- A 7 year old with severe muscle weakness and difficulty walking
- Why can I not walk today?
- 14 year old with severe hip pain
- A 9 year old girl presents with body swelling, shortness of breath and backache
- A sudden turn of events after successful therapy
- Declining CD4 count, despite viral suppression?
- Defaulted treatment
- 25 year old female presents with persistent flu-like symptoms
- A case of persistent bloody diarrhoea
- I’ve been coughing for so long
- A case of acute fever, rash and vomiting
- Adverse event following routine vaccination
- A case of cough, wasting and lymphadenopathy
- A case of lymphadenopathy and night sweats
- Case of enlarged hard tongue
- A high risk pregnancy
- A four year old with immunodeficiency
- Young girl with recurrent history of mycobacterial disease
- Immunodeficiency and failure to thrive
- Case of recurrent infections
- An 8 year old boy with recurrent respiratory infections
- 4 year old boy with recurrent bacterial infections
- Is this treatment failure or malnutrition
- 1. A Snapshot of the Immune System
- 2. Ontogeny of the Immune System
- 3. The Innate Immune System
- 4. MHC & Antigen Presentation
- 5. Overview of T Cell Subsets
- 6. Thymic T Cell Development
- 7. gamma/delta T Cells
- 8. B Cell Activation and Plasma Cell Differentiation
- 9. Antibody Structure and Classes
- 10. Central and Peripheral Tolerance
- Immuno-Mexico 2024 Introduction
- Modulation of Peripheral Tolerance
- Metabolic Adaptation to Pathologic Milieu
- T Cell Exhaustion
- Suppression in the Context of Disease
- Redirecting Cytotoxicity
- Novel Therapeutic Strategies
- ImmunoInformatics
- Grant Writing
- Introduction to Immuno-Chile 2023
- Core Modules
- Gut Mucosal Immunity
- The Microbiome
- Gut Inflammation
- Viral Infections and Mucosal Immunity
- Colorectal Cancer
- Inflammatory Bowel Disease
- Equity, Diversity, Inclusion in Academia
- Immuno-India 2023 Introduction
- Principles of Epigenetic Regulation
- Epigenetics Research in Systems Immunology
- Epigenetic (De)regulation in Non-Malignant Diseases
- Epigenetic (De)regulation in Immunodeficiency and Malignant Diseases
- Immunometabolism and Therapeutic Applications of Epigenetic Modifiers
- Immuno-Morocco 2023 Introduction
- Cancer Cellular Therapies
- Cancer Antibody Therapies
- Cancer Vaccines
- Immunobiology of Leukemia & Therapies
- Immune Landscape of the Tumour
- Targeting the Tumour Microenvironment
- Flow Cytometry
- Immuno-Zambia 2022 Introduction
- Immunity to Viral Infections
- Immunity to SARS-CoV2
- Basic Immunology of HIV
- Immunity to Tuberculosis
- Immunity to Malaria
- Immunity to Schistosomiasis
- Immunity to Helminths
- Equity, Diversity and Inclusion in Academia
- Immuno-Argentina 2022 Introduction
- Dendritic Cells
- Trained Innate Immunity
- Gamma-Delta T cells
- Natural Killer Cell Memory
- Innate Immunity in Viral Infections
- Lectures – Innate Immunity
- T cells and Beyond
- Lectures – Cellular Immunity
- Strategies for Vaccine Design
- Lectures – Humoral Immunity
- Lectures – Vaccine development
- Lectures – Panel and Posters
- Immuno-Cuba 2022 Introduction
- Poster and Abstract Examples
- Immuno-Tunisia 2021 Introduction
- Basics of Anti-infectious Immunity
- Inborn Errors of Immunity and Infections
- Infection and Auto-Immunity
- Pathogen-Induced Immune Dysregulation & Cancer
- Understanding of Host-Pathogen Interaction & Applications (SARS-CoV-2)
- Day 1 – Basics of Anti-infectious Immunity
- Day 2 – Inborn Errors of Immunity and Infections
- Day 3 – Infection and Auto-immunity
- Day 4 – Pathogen-induced Immune Dysregulation and Cancer
- Day 5 – Understanding of Host-Pathogen Interaction and Applications
- Student Presentations
- Roundtable Discussions
- Orientation Meeting
- Poster Information
- Immuno-Colombia Introduction
- Core Modules Meeting
- Overview of Immunotherapy
- Check-Points Blockade Based Therapies
- Cancer Immunotherapy with γδ T cells
- CAR-T, armored CARs and CAR-NK therapies
- Anti-cytokines Therapies
- Tumor-infiltrating Lymphocytes (TIL)
- MDSC Promote Tumor Growth and Escape
- Immunological lab methods for patient’s follow-up
- Student Orientation Meeting
- Lectures – Week 1
- Lectures – Week 2
- Research Project
- Closing and Social
- Introduction to Immuno-Algeria 2020
- Hypersensitivity Reactions
- Immuno-Algeria Programme
- Online Lectures – Week 1
- Online Lectures – Week 2
- Student Presentations – Week 1
- Student Presentations – Week 2
- Introduction to Immuno-Ethiopia 2020
- Neutrophils
- Leishmaniasis – Transmission and Epidemiology
- Leishmaniasis – Immune Responses
- Leishmaniasis – Treatment and Vaccines
- Immunity to Helminth Infections
- Helminth immunomodulation on co-infections
- Malaria Vaccine Progress
- Immunity to Fungal Infections
- How to be successful scientist
- How to prepare a good academic CV
- Introduction to Immuno-Benin
- Immune Regulation in Pregnancy
- Immunity in infants and consequence of preeclampsia
- Schistosome infections and impact on Pregnancy
- Infant Immunity and Vaccines
- Regulation of Immunity & the Microbiome
- TGF-beta superfamily in infections and diseases
- Infectious Diseases in the Global Health era
- Immunity to Toxoplasma gondii
- A. melegueta inhibits inflammatory responses during Helminth Infections
- Host immune modulation by Helminth-induced products
- Immunity to HIV
- Immunity to Ebola
- Immunity to TB
- Genetic susceptibility in Tuberculosis
- Plant Extract Treatment for Diabetes
- Introduction to Immuno-South Africa 2019
- Models for Testing Vaccines
- Immune Responses to Vaccination
- IDA 2019 Quiz
- Introduction to Immuno-Jaipur
- Inflammation and autoinflammation
- Central and Peripheral Tolerance
- Autoimmunity and Chronic Inflammatory Diseases
- Autoimmunity & Dysregulation
- Novel Therapeutic strategies for Autoimmune Diseases
- Strategies to apply gamma/delta T cells for Immunotherapy
- Immune Responses to Cancer
- Tumour Microenvironment
- Cancer Immunotherapy
- Origin and perspectives of CAR T cells
- Metabolic checkpoints regulating immune responses
- Transplantation
- Primary Immunodeficiencies
- Growing up with Herpes virus
- Introduction to IUIS-ALAI-Mexico-ImmunoInformatics
- Introduction to Immunization Strategies
- Introduction to Immunoinformatics
- Omics Technologies
- Computational Modeling
- Machine Learning Methods
- Introduction to Immuno-Kenya
- Viruses hijacking host immune responses
- IFNs as 1st responders to virus infections
- HBV/HCV & Hepatocellular Carcinoma
- Cytokines as biomarkers for HCV
- HTLV & T cell Leukemia
- HCMV and Cancers
- HPV and Cancers
- EBV-induced Oncogenesis
- Adenoviruses
- KSHV and HIV
- Ethics in Cancer Research
- Sex and gender in Immunity
- Introduction to Immuno-Iran
- Immunity to Leishmaniasis
- Breaking Tolerance: Autoimmunity & Dysregulation
- Introduction to Immuno-Morocco
- Cancer Epidemiology and Aetiology
- Pathogens and Cancer
- Immunodeficiency and Cancer
- Introduction to Immuno-Brazil
- 1. Systems Vaccinology
- 2. Vaccine Development
- 3. Adjuvants
- 4. DNA Vaccines
- 5. Mucosal Vaccines
- 6. Vaccines for Neurodegenerative Diseases
- Introduction to Immuno-Gambia
- Immuno-Gambia Photos
- 1. Infant Immunity and Vaccines
- 2. Dendritic Cells
- 3. Conventional T Cells
- 4. gamma/delta T Cells
- 5. Immunity to Viral Infections
- 6. Immunity to Helminth Infections
- 7. Immunity to TB
- 8. Immunity to Malaria
- 9. Flow Cytometry
- Introduction to Immuno-South Africa
- 1. Introduction to Immunization Strategies
- 2. Immune Responses to Vaccination
- 3. Models for Testing Vaccines
- 4. Immune Escape
- 5. Grant Writing
- Introduction to Immuno-Ethiopia
- 1. Neutrophils
- 3. Exosomes
- 5. Immunity to Leishmania
- 6. Immunity to HIV
- 7. Immunity to Helminth Infections
- 8. Immunity to TB
- 9. Grant Writing
- Introduction to ONCOIMMUNOLOGY-MEXICO
- ONCOIMMUNOLOGY-MEXICO Photos
- 1. Cancer Epidemiology and Etiology
- 2. T lymphocyte mediated immunity
- 3. Immune Responses to Cancer
- 4. Cancer Stem Cells and Tumor-initiating cells.
- 5. Tumor Microenvironment
- 6. Pathogens and Cancer
- 7. Cancer Immunotherapy
- 8. Flow cytometry approaches in cancer
- Introduction to the Immunology Course
- Immuno-Tunisia Photo
- 1. Overview of the Immune System
- 2. Role of cytokines in Immunity
- 3. Tolerance and autoimmunity
- 4. Genetics, Epigenetics and immunoregulation
- 5. Microbes and immunoregulation
- 6. Inflammation and autoinflammation
- 7. T cell mediated autoimmune diseases
- 8. Antibody-mediated autoimmune diseases
- Introduction to the Immunology Symposium
- Immuno-South Africa Photo
- 1. Antibody Generation by B cells
- 2. Mucosal Immunity
- 3. Immunity to TB
- 4. Immunity to Malaria
- 5. Immunity to HIV
- 6. Defining a Biomarker
- 7. Grant Writing Exercise
- Immuno-Colombia Photo
- 1. Overview of Complement
- 2. Transplantation
- 3. Immune Regulation in Pregnancy
- 4. Breaking Tolerance: Autoimmunity & Dysregulation
- 5. Mucosal Immunity & Immunopathology
- 6. Regulation of Immunity & the Microbiome
- 7. Epigenetics & Modulation of Immunity
- 8. Primary Immunodeficiencies
- 9. Anti-tumour Immunity
- 10. Cancer Immunotherapy
- Introduction
- Immune Cells
- NCDs and Multimorbidity
- Mosquito Vector Biology
- Vaccines and Other Interventions
- Autoimmunity
- Career Development
- SUN Honours Introduction
- A Snapshot of the Immune System
- Ontogeny of the Immune System
- The Innate Immune System
- MHC & Antigen Presentation
- Overview of T Cell Subsets
- B Cell Activation and Plasma Cell Differentiation
- Antibody Structure and Classes
- Cellular Immunity and Immunological Memory
- Infectious Diseases Immunology
- Vaccinology
- Mucosal Immunity & Immunopathology
- Central & Peripheral Tolerance
- Epigenetics & Modulation of Immunity
- T cell and Ab-mediated autoimmune diseases
- Immunology of COVID-19 Vaccines
- 11th IDA 2022 Introduction
- Immunity to COVID-19
- Fundamentals of Immunology
- Fundamentals of Infection
- Integrating Immunology & Infection
- Infectious Diseases Symposium
- EULAR Symposium
- Thymic T Cell Development
- Immune Escape
- Genetics, Epigenetics and immunoregulation
- AfriBop 2021 Introduction
- Adaptive Immunity
- Fundamentals of Infection 2
- Fundamentals of Infection 3
- Host pathogen Interaction 1
- Host pathogen Interaction 2
- Student 3 minute Presentations
- 10th IDA 2021 Introduction
- Day 1 – Lectures
- Day 2 – Lectures
- Day 3 – Lectures
- Day 4 – Lectures
- Afribop 2020 Introduction
- WT PhD School Lectures 1
- EULAR symposium
- WT PhD School Lectures 2
- Host pathogen interaction 1
- Host pathogen interaction 2
- Bioinformatics
- Introduction to VACFA Vaccinology 2020
- Overview of Vaccinology
- Basic Principles of Immunity
- Adverse Events Following Immunization
- Targeted Immunization
- Challenges Facing Vaccination
- Vaccine Stakeholders
- Vaccination Questions Answered
- Malaria Vaccines
- IDA 2018 Introduction
- Vaccine Development
- Immune Escape by Pathogens
- Immunity to Viral Infections Introduction
- Flu, Ebola & SARS
- Antiretroviral Drug Treatments
- Responsible Conduct in Research
- Methods for Enhancing Reproducibility
- 6. B Cell Activation and Plasma Cell Differentiation
- 7. Antibody Structure and Classes
- CD Nomenclature
- 1. Transplantation
- 2. Central & Peripheral Tolerance
- 8. Inflammation and autoinflammation
- 9. T cell mediated autoimmune diseases
- 10. Antibody-mediated autoimmune diseases
- 1. Primary Immunodeficiencies
- Cancer Stem Cells and Tumour-initiating Cells
- 6. Tolerance and Autoimmunity
- Discovery of the Thymus as a central immunological organ
- History of Immune Response
- History of Immunoglobulin molecules
- History of MHC – 1901 – 1970
- History of MHC – 1971 – 2011
- SAIS/Immunopaedia Webinars 2022
- Metabolic control of T cell differentiation during immune responses to cancer
- Microbiome control of host immunity
- Shaping of anti-tumor immunity in the tumor microenvironment
- The unusual COVID-19 pandemic: the African story
- Immune responses to SARS-CoV-2
- Adaptive Immunity and Immune Memory to SARS-CoV-2 after COVID-19
- HIV prevention- antibodies and vaccine development (part 2)
- HIV prevention- antibodies and vaccine development (part 1)
- Immunopathology of COVID 19 lessons from pregnancy and from ageing
- Clinical representation of hyperinflammation
- In-depth characterisation of immune cells in Ebola virus
- Getting to the “bottom” of arthritis
- Immunoregulation and the tumor microenvironment
- Harnessing innate immunity from cancer therapy to COVID-19
- Flynn Webinar: Immune features associated natural infection
- Flynn Webinar: What immune cells play a role in protection against M.tb re-infection?
- JoAnne Flynn: BCG IV vaccination induces sterilising M.tb immunity
- IUIS-Immunopaedia-Frontiers Webinar on Immunology taught by P. falciparum
- COVID-19 Cytokine Storm & Paediatric COVID-19
- Immunothrombosis & COVID-19
- Severe vs mild COVID-19 immunity and Nicotinamide pathway
- BCG & COVID-19
- COVID-19 Vaccines
- Antibody responses and serology testing
- Flow Cytometry Part 1
- Flow Cytometry Part 2
- Flow Cytometry Part 3
- Lateral Flow
- Diagnostic Tools
- Diagnostic Tests
- HIV Life Cycle
- ARV Drug Information
- ARV Mode of Action
- ARV Drug Resistance
- Declining CD4 count
- Ambassador of the Month – 2024
- North America
- South America
- Ambassador of the Month – 2023
- Ambassador of the Month – 2022
- The Day of Immunology 2022
- AMBASSADOR SCI-TALKS
- The Day of Immunology 2021
- Ambassador of the Month – 2021
- Ambassador of the Month-2020
- Ambassador of the Month – 2019
- Ambassador of the Month – 2018
- Ambassador of the Month – 2017
- Host an IUIS Course in 2025
- Immuno-South Africa 2024
- COLLABORATIONS
We will not share your details

Patient Presentation
Differential diagnosis, examination, investigations, final outcome.
- Evaluation - Questions & answers
A 2 year old boy presented to a district hospital with decreased oral intake, listlessness and fever. On arrival he was adequately resuscitated but continued to have spiking fevers and a depressed level of consciousness.
Acknowledgement This case study was kindly provided by Barclay Stewart, Medical University of South Carolina, Fogarty International Clinical Research Scholar, Nairobi, Keny a
Six months ago the patient presented to the hospital with a two day history of irritability, decreased appetite, discomfort on lying down, recurrent fever, profuse sweating and diarrhea, no vomiting. On admission he was lethargic and dehydrated which worsened over a few hours and culminated in a seizure. He had no prior history of seizures. He was then diagnosed with severe malaria. He was treated appropriately and discharged 2 weeks later with no residual effects.
Past medical and surgical history
- There is no additional significant medical or surgical history.
- Road to health card shows all growth parameters to be within normal limits, with all vaccinations up to date.
Family and social history
- He lives with his mother, father, and two older siblings who are all healthy.
- His mother was recently tested and is HIV negative; his father has not been tested.
- Their home, which has electricity and water, is located in a low-lying area near Musina, a town in South Africa’s Limpopo province. This is the country’s most northerly located town, with a seasonal high rate of malaria transmission from October through May.
Travel History No travel outside of Musina since birth.
- Encephalitis
- Gastroenteritis with severe dehydration
- Toxic Shock Syndrome
- Typhoid Fever
- Brucellosis
- Relapsing Fever
- Katayama Fever
- Urinary tract infection
- Bacteraemia
On appearance the child is miserable and toxic looking.
- Pulse – 166
- Respiratory Rate – 34
- Temperature – 39.8
- Pulse-Oxygen – 95%
Height and weight were in the 65 percentile
- Eyes were sunken and jaundiced.
- No lymphadenopathy
- Erythematous, non bulging tympanic membranes.
- Non-inflamed nasal passage, no discharge.
- Pale oral mucosa
- No papilloedema
- No retinal heamorrhages
- Midline trachea
- Chest shape normal in appearance, tachypnoea present
- Mild subcostal retractions.
- Clear on auscultation bilaterally.
Cardiovascular
- Tachycardia with a regular rhythm.
- Normal S1 and S2 with a 2/6 mid systolic murmur best auscultated over the upper left sternal border with minimal radiation.
- Bounding pulses felt radially, femorally and dorsalis pedis
- Capillary refill within 2 seconds.
- Normal on inspection.
- Bowel sounds diminished but present.
- No hepatomegaly.
- 4cm splenomegaly.
Neurological
- Child listless though attempts to follow commands.
- Not resisting or crying in response to aggravating stimuli.
Human malaria infection is caused by four protozoa species of the genus Plasmodium. These are P.falciparum, P. malariae, P. vivax, and P. ovalae , of which the preponderance of severe malaria and mortality is due to P.falciparum . Children living in endemic areas typically have a primary malaria episode during their first few years of life and most toddlers and juveniles develop some degree of acquired immunity against severe disease but still experience periodic clinical episodes. Those who survive to adulthood are often clinically immune, however, low grade parasitaemia is often present but causes few symptoms. Adults in endemic areas maintain low-grade infections throughout the transmission season. Endemicity is typically defined as parasitaemia rates or palpable spleens in children aged 2-9 years. The categories include holoendemic where the rate is >75% (transmission of infection is year round and the bulk of mortality is seen in infants), hyperendemic where the rate is 51-75% (mortality is also mostly seen in infants), mesoendemic where the rate is 11-50% (regular seasonal transmission affecting infants, toddlers and adults who develop chronic ill health) and hypoendemic which is <10% (occasional epidemics, whole population is susceptible to severe and fatal disease). Clinical immunity also fails if a person moves away from an endemic area and during pregnancy.
The female Anopheles mosquito inoculates the host with 10 to 100 malaria sporozoites from her salivary glands during a blood meal. These microscopic motile forms of the malaria parasite are carried via the bloodstream to the liver. Within 30 minutes, those sporozoites not bound by previously formed antibodies, invade and begin replicating in hepatocytes. Parasites not destroyed by cytotoxic T lymphocytes in the liver replicate for 2-10 days creating merozoites. Tens of thousands of merozoites are released into the bloodstream as the hepatocyte bursts. Each merozoite is then able to bind, invade, and infect erythrocytes. After red blood cell (RBC) infection, each merozoite matures to form a highly metabolically active trophozoite, which replicates asexually to become multinucleate schizonts. As the schizonts enlarge they rupture erythrocytes 48 hours after their formation which results in 20-30 new merozoites which continue the cycle. Some sexual forms of the parasite develop during this erythrocytic stage; these gametocytes are responsible for infecting the salivary glands of female Anopheles mosquitoes. The gametes mature into ookinetes then into an oocyst. The oocyst ruptures and releases sporozoites which can then infect another host during a blood meal.
A person’s first infection usually creates no symptoms for 7-10 days, which is followed first by nonspecific symptoms such as headache, fatigue, abdominal discomfort and muscle aches. This is then followed by fever. During this latent period, parasite maturation occurs in the liver and parasites undergo a cycle of blood stage replication. Symptoms begin when the parasites undergoing an asexual blood cycle, reach threshold density sufficient to initiate the host’s pathogenic immune response process. Fever, malaria’s hallmark, is due to parasite-derived molecules released from ruptured host cells. These molecules activate host inflammatory cells, such as macrophages, which secrete pro-inflammatory pyrogenic cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)–α. As parasites synchronise their replication cycles the fever becomes periodic. Although childhood febrile convulsions can occur, generalised seizures are typically associated with P.falciparum infections and may herald cerebral malaria. Splenomegaly results from massive reticuloendothelial system activation to clear parasitised erythrocytes. Mild hepatomegally is common in young children, while mild jaundice is more common in adults. Anaemia is also common and is partly due to the phasic rupture of RBCs by mature schizonts, splenic sequestration of red blood cells and ineffective erythropoiesis.
Cerebral Malaria Onset may be gradual or sudden following a convulsion. Features include obtundation, delirium, abnormal behaviour and coma. Focal neurologic signs and meningism do not typically occur. Fifteen percent of children who survive cerebral malaria, especially when associated with hypoglycaemia, coma and anaemia will have some residual neurologic deficit.
Hypoglycaemia Common complication that is associated with a poor prognosis, particularly in children and pregnant women. Hypoglycaemia is due to a failure of hepatic gluconeogenesis and an increase in glucose consumption by host and parasite. This may manifest as an added complication during treatment as Quinine is also a potent stimulator of insulin secretion.
Haematologic Pathology Anaemia due to increased destruction and removal or red blood cells and dyserythropoesis. Mild thrombocytopaenia Mild coagulation abnormalities Bleeding and DIC in more severe cases
Renal pathology Interference in microcirculation resulting in tubular necrosis and acute renal failure, more common in adults.
Host Response-Immunology
- Antibody responses are induced during the sporozoite stage. Antibody bound sporozoites are prevented from invading hepatocytes.
- CD8 + T cells have been shown to be cytotoxic against maturing sporozoite infected liver cells.
- Both of these responses are potentially able to terminate the infection before the onset of clinical disease caused by the release of merozoites from hepatocytes and subsequent RBC invasion and rupture.
- CD4 + T cells are a requisite for the production of merozoite neutralising antibodies by B cells and the activation of macrophages which secrete interferon (INF) –γ to enhance parasitized RBC.
- The host is also able to develop transmission-blocking antibodies directed to gametocyte specific antigens. These antibodies hinder the development of the parasite within the mosquito vector, thereby preventing further infections. Though this immune response is not particularly valuable to the infected host, it does assist in reducing population level transmission.
Download images for this case
Plasmodium falciparum malaria.
It is recommended that patients receive prompt and effective treatment. Ideally, treatment should be initiated in a hospital setting. The choice of chemotherapy for malaria is dependent on the severity of disease, the known or suspected resistance pattern of the parasite in the area where the malaria infection was acquired, the species of parasite, patient characteristics (age, pregnancy, co-morbidity, allergies, other medications) and the presence or absence of vomiting. In South Africa, malaria treatment varies in the different provinces due to differences in the resistance patterns. These treatment guidelines may not be appropriate for infections contracted in other countries with high levels of multi-drug resistance.
The patient was treated with IV artesunate and anti-pyretics for 3 days. IV antibiotics were started on admission as there was no confirmatory diagnosis at the time and culture results were not yet available. On the third day the child was markedly improved. He was started on a full course of mefloquine on receiving laboratory results which confirmed infection with P.falciparum. Upon discharge there were no neurologic sequelae. He and his family were counseled on the use of insecticide-treated bed nets and indoor residual spraying.
Guerin, P.J., et al. (2002). Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect Dis. 2(9): p. 564-73.
Link to Abstract Ferreira, M.U et al. (2004). Antigenic diversity and immune evasion by malaria parasites. Clin Diagn Lab Immunol. 11(6): p. 987-95.
Link to Abstract May, J. et al. (1999). High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 61(2): p. 339-43.
Link to Abstract Rosenberg, R. et al. (1990). An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 84(2): p. 209-12.
Link to Abstract Ponnudurai, T. et al. (1982). Mosquito transmission of cultured Plasmodium falciparum. Trans R Soc Trop Med Hyg. 76(2): p. 278-9.
Link to Abstract Ferreira, M.U et al. (1998). The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann Trop Med Parasitol. 92(3): p. 245-56.
Link to Abstract Nardin, E.H et al. (1993). T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 11: p. 687-727.
Link to Abstract Nardin, E.H. et al. (1982). Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 156(1): p. 20-30.
Link to Abstract Inselburg, J. (1983). Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. J Parasitol. 69(3): p. 584-91.
Link to Abstract Aitman, T.J. et al. (2000). Malaria susceptibility and CD36 mutation. Nature. 405(6790): p. 1015-6.
Link to Abstract Jenkins, N. et al. (2007). Plasmodium falciparum intercellular adhesion molecule-1-based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 196(2): p. 321-7.
Link to Abstract McCormick, C.J. et al. (1997). Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest. 100(10): p. 2521-9.
Link to Abstract
Miller, L.H. et al. (2002). The pathogenic basis of malaria. Nature. 415(6872): p. 673-9.
Abdel-Latif, M.S. et al. (2003). Antibodies to Plasmodium falciparum rifin proteins are associated with rapid parasite clearance and asymptomatic infections. Infect Immun. 71(11): p. 6229-33.
Good, M.F. et al. (1998). Pathways and strategies for developing a malaria blood-stage vaccine. Annu Rev Immunol. 16: p. 57-87.
Hoffman, S.L. et al. (1998). Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 244(4908): p. 1078-81.
Link to Abstract Snewin, V.A et al. (1995). Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 181(1): p. 357-62.
Link to Abstract Hisaeda, H. et al. (2005). Malaria: immune evasion by parasites. Int J Biochem Cell Biol. 37(4): p. 700-6.
Link to Abstract Qari, S.H. et al. (1998). Predicted and observed alleles of Plasmodium falciparum merozoite surface protein-1 (MSP-1), a potential malaria vaccine antigen. Mol Biochem Parasitol. 92(2): p. 241-52.
Link to Abstract Burns, J.M. et al. (1989). A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 142(8): p. 2835-40.
Link to Abstract Smith, J.D. et al. (1995). Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 82(1): p. 101-10.
Link to Abstract Flick, K. et al. (2004). var genes, PfEMP1 and the human host. Mol Biochem Parasitol. 134(1): p. 3-9.
Link to Abstract Williamson, W.A. et al. (1978). Impairment of the immune response to vaccination after acute malaria. Lancet. 1(8078): p. 1328-9.
Link to Abstract Takeda, K. et al. (2003). Toll-like receptors. Annu Rev Immunol. 21: p. 335-76.
Link to Abstract Urban, B.C. et al. (1999). Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 400(6739): p. 73-7.
Link to Abstract Ocana-Morgner, C. et al. (2003). Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 197(2): p. 143-51.
Link to Abstract Omer, F.M et al. (2003). Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol. 171(10): p. 5430-6.
Link to Abstract Shevach, E.M. (2002). CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2(6): p. 389-400.
Hisaeda, H. et al. (2004). Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 10(1): p. 29-30.
Evaluation – Questions & answers
What is the diagnosis?
With regards to parasitized erythrocytes which endothelial receptors do they bind to resulting in occlusion of microvessels?
What are the three ways that infected erythrocytes can bind to occlude microvessels?
What is the benefit of occlusion of microvessels?
Which organs are most affected by occlusion of microvessels?
Describe the immune response required to neutralize malaria parasites at each stage during their development.

© 2004 - 2024 Immunopaedia.org.za Sitemap - Privacy Policy - Cookie Policy - PAIA - Terms & Conditions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .

- CLINICAL CASES
- ONLINE COURSES
- AMBASSADORS
- TREATMENT & DIAGNOSTICS
- Case report
- Open access
- Published: 06 May 2022
Case series of three malaria patients from Thailand infected with the simian parasite, Plasmodium cynomolgi
- Piyaporn Sai-ngam 1 na1 ,
- Kingkan Pidtana 1 na1 ,
- Preeyaporn Suida 2 ,
- Kamonporn Poramathikul 1 ,
- Paphavee Lertsethtakarn 1 ,
- Worachet Kuntawunginn 1 ,
- Sarayut Tadsaichol 3 ,
- Montri Arsanok 1 ,
- Siriporn Sornsakrin 1 ,
- Chaiyaporn Chaisatit 1 ,
- Chaiyawat Mathavarat 1 ,
- Sasikanya Thaloengsok 1 ,
- Parat Boonyarangka 1 ,
- Chadin Thongpiam 1 ,
- Samandra Demons 1 ,
- Brian Vesely 1 ,
- Norman C. Waters 4 ,
- Aungkana Saejeng 5 ,
- Mariusz Wojnarski 1 ,
- Sutchana Tabprasit 6 ,
- Chokchai Kwanpichit 7 ,
- John S. Griesenbeck 1 &
- Michele Spring ORCID: orcid.org/0000-0002-2921-9677 1 , 8
Malaria Journal volume 21 , Article number: 142 ( 2022 ) Cite this article
2383 Accesses
10 Citations
9 Altmetric
Metrics details
While human cases of Plasmodium knowlesi are now regularly recognized in Southeast Asia, infections with other simian malaria species, such as Plasmodium cynomolgi , are still rare. There has been a handful of clinical cases described, all from Malaysia, and retrospective studies of archived blood samples in Thailand and Cambodia have discovered the presence P. cynomolgi in isolates using polymerase chain reaction (PCR) assays.
Case presentation
In Thailand, an ongoing malaria surveillance study enrolled two patients from Yala Province diagnosed with Plasmodium vivax by blood smear, but who were subsequently found to be negative by PCR. Expanded PCR testing of these isolates detected mono-infection with P. cynomolgi , the first time this has been reported in Thailand. Upon re-testing of 60 isolates collected from Yala, one other case was identified, a co-infection of P. cynomolgi and P. vivax . The clinical course for all three was relatively mild, with symptoms commonly seen in malaria: fever, chills and headaches. All infections were cured with a course of chloroquine and primaquine.
In malaria-endemic areas with macaque populations, cases of simian malaria in humans are being reported at an increasing rate, although still comprise a very small percentage of total cases. Plasmodium cynomolgi and P. vivax are challenging to distinguish by blood smear; therefore, PCR can be employed when infections are suspected or as part of systematic malaria surveillance. As Thai MoPH policy schedules regular follow-up visits after each malaria infection, identifying those with P. cynomolgi will allow for monitoring of treatment efficacy, although at this time P. cynomolgi appears to have an uncomplicated clinical course and good response to commonly used anti-malarials.
The first naturally-acquired human infection of the simian malaria parasite, Plasmodium cynomolgi , was reported from Malaysia in 2014 [ 1 ]. Clinical cases have continued to be reported from Malaysia, and P. cynomolgi has been retrospectively detected in stored isolates from Malaysia, Cambodia and Thailand [ 2 , 3 , 4 , 5 , 6 , 7 , 8 ]. An ongoing malaria surveillance study in Thailand has been enrolling malaria patients to monitor transmission in border provinces and determine resistance patterns in order to better manage and predict effectiveness of anti-malarial treatments. As malaria cases continue to decrease in Thailand, it will become important for such surveillance studies to more actively monitor for human infections by simian malaria parasites.
Malaria case presentations
This minimal risk malaria surveillance study in Thailand has been enrolling individuals diagnosed with malaria by rapid diagnostic test (RDT) and/or microscopy since March 2019. The study operates in several border provinces: Yala (by Malaysia), Sisaket and Ubon Ratchathani (by Cambodia), and Ratchaburi (by Myanmar). After consent, a single venous blood sample is drawn, with a complete blood count (CBC), glucose 6-phosphate dehydrogenase (G6PD) CareStart™ RDT (Access Bio, Inc., USA) and fluorescent spot testing (R&D Diagnostics Ltd., Greece) performed by local Ministry of Public Health (MoPH) or Royal Thai Army (RTA) staff. The remaining blood sample shipped to US Armed Forces Research Institute of Medical Sciences (AFRIMS) in Bangkok, Thailand. There, speciation is verified by blood smears that are made and read by AFRIMS staff, and by conducting multiplex real time polymerase chain reaction (RT-PCR) on isolated parasite DNA. In addition, quantitative G6PD testing (Pointe Scientific, USA), PCR for molecular markers of resistance and submicroscopic gametocytaemia as well as ex-vivo and in-vitro drug susceptibility assays are performed. At the time of writing, 149 malaria patients have been enrolled: 128 Plasmodium vivax cases, 14 Plasmodium falciparum and four Plasmodium knowlesi cases . Three infections with P. cynomolgi were also detected. A short description of these, and the locations within Yala Province, Thailand (Fig. 1 ), follows.
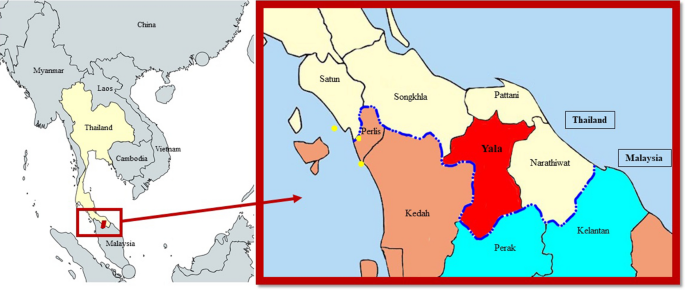
Location of human P. cynomolgi cases in Thailand. Map of Yala Province, Thailand with location of detected human P. cynomolgi cases (yellow dots). The royal blue dotted line indicates the border between Thailand and Malaysia. Provinces in light yellow and red are located in Thailand, and those that are brown and light blue are in Malaysia, with the two states of Perak and Kelantan being two areas with previously reported human P. cynomolgi cases [ 7 ]
A 53-year-old woman presented at a malaria clinic in Ban Nang Sata District, in March 2021 with 38 °C fever, headache, and chills for five days. The haematological assessment showed white blood count (WBC) at 4,200/mm 3 , haemoglobin at 10.9 g/dL, and platelets at 191,000/mm 3 . She reported working at a rubber plantation, and that her husband had recently been diagnosed and treated for P. vivax infection.
A 55-year-old female rubber plantation worker was part of a malaria active case detection investigation by malaria clinic staff from Ka Bang District, in February 2021. The patient reported a history of headache and fever for eight days, although on the day of examination, the subject’s tympanic temperature was 37 °C. Laboratory examination revealed WBC at 4800/mm 3 , haemoglobin at 11.7 g/dL, and platelet count at 330,000/mm 3 .
In June 2021, a 25-year-old male on active duty in the Royal Thai Army presented at a malaria clinic in Yala District, with a complaint of five days of fever and nighttime chills. His temperature was 37.8 °C. Haematology findings showed slight thrombocytopenia at 123,000/mm 3 , WBC at 6900/mm 3 , and haemoglobin at 12.5 g/dL. The patient stated he had been stationed in Yala District for at least 20 months, going out on daily patrols and sleeping overnight in the forest. He reported using mosquito repellent and mosquito coils for personal protection.
Using microscopy, all three subjects were diagnosed with P. vivax ; all presented with uncomplicated illness, had normal G6PD activity and reported no prior history of malaria. Each patient was treated by local health care staff with three days of chloroquine and a 2-week radical cure course of primaquine, as per Thai national treatment guidelines. All were found to be clinically well within 5 days of initiating the anti-malarials, with no recurrences at subsequent follow-up visits required by the Thai MoPH scheduled at 14-, 28-, 60- and 90-days post-treatment.
Laboratory investigations
Blood smears were prepared and read by two World Health Organization (WHO)-certified microscopists at the AFRIMS labs in Bangkok, Thailand. In brief, thick and thin smears were prepared on the same glass slide and air-dried and fixed in methanol, stained for 45 min (min) in 3% diluted Giemsa stain, and examined at an oil immersion magnification of × 100. Parasite counting was done per 500 white blood cells (WBC) in thick films, and percent parasitaemia was calculated based on the actual WBC count. Parasites resembling P. vivax were detected, with densities of 25, 10, and 2718 parasites/µL blood for Case A, B, and C, respectively. Only Case C had gametocytaemia, with four gametocytes per 200 WBCs, or 138 gametocytes/µL. Malaria parasite morphologies in Giemsa-stained thick blood smears are shown in Fig. 2 A–H, demonstrating growing trophozoite stages with amoeboid-shaped cytoplasm (red arrows). No ring forms were detected in any slide. Single (Panels A–D, F), double (blue arrow, Panel E), and triple (Panel H) chromatin dots were seen on examination. There was yellowish-brown pigment dispersed within the cytoplasm in some infected cells. In thin films, parasites were found only in Case C (Fig. 2 I), the individual with mixed infection and higher parasite count. The erythrocytes were not clearly enlarged or distorted, and Schüffner's stippling was prominently visible.
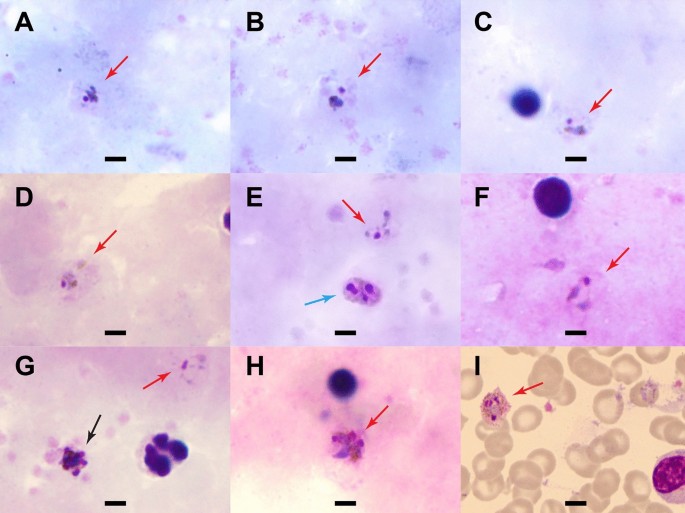
Parasite morphology in Giemsa-stained blood smears from the three malaria patients. Shown are malaria parasites detected in Giemsa-stained films at a magnification of 100x. A - E Case A (thick film) showing growing trophozoite stages with amoeboid-shaped cytoplasm (red arrows). Yellowish-brown pigments were visible ( A - D ) with double chromatin dots in E (blue arrow). F Case B (thick film) with growing trophozoite stages. G and H Case C (thick film). Parasites resembling P. vivax were found in the field of view ( G , red arrow). Early schizont with merozoites was also seen in G (black arrow) and triple chromatin dots in H . I Case C (thin film) with dominant Schüffner’s stippling (pink, scattered dots) and yellowish-brown pigments in a trophozoite. Erythrocytes did not appear enlarged. Scale bar indicates 5 µm
The PCR testing performed at AFRIMS is designed to detect five Plasmodium species: P. falciparum , P. vivax , Plasmodium malariae , Plasmodium ovale and P. knowlesi . Briefly, parasite genomic DNA is extracted from whole blood collected in ethylenediaminetetraacetic acid (EDTA) using EZ1 DNA blood kit with automated EZ1 Advanced XL purification system (QIAGEN, Valencia, CA, USA), and Plasmodium speciation confirmed by multiplex RT- PCR, using species-specific primers and probes [ 9 , 10 ]. Two of the study patients (A and B) were found to be negative by multiplex RT-PCR, with P. vivax reported for Case C.
Since asexual parasites had been observed on blood smear for Cases A and B, further investigations were undertaken to identify the species. The 5-species multiplex RT- PCR was re-run as well as a singleplex RT-PCR testing for P. cynomolgi. Primers and probes specific to small subunit rRNA, S-type (Genbank accession number L08242.1 were selected, with sequences as follows: Forward: 5′-ATTGCGGTCGCAAATAATGAAG-3′, Reverse: 5′-GGTATGATAAGCCAGGGAAGTG-3′ and Probe: 5′-FAM-TACTCGCTCCTTCTGTTCCCTGGA-BHQ1′). The reaction was carried out in a 25 µl reaction using Rotor-Gene Multiplex PCR kit (QIAGEN, Hilden, Germany) with cycling conditions consisting of an initial activation step at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 15 s and annealing /extension at 60 °C for 15 s. Blood from a macaque infected with P. cynomolgi was used as a positive control. Mono-infection with P. cynomolgi was confirmed by PCR in Cases A and B, with Case C having co-infection with P. vivax . All remaining Yala samples (n = 60) were then tested for P. cynomolgi by singleplex RT-PCR and were negative.
Plasmodium cynomolgi is a malaria species with Southeast Asian macaques as a natural host, transmitted through the bites of the forest-dwelling, Leucosphyrus Group of Anopheles mosquitoes, which exhibits relapses upon activation of hypnozoites similar to P. vivax [ 4 , 7 , 11 , 12 ]. This report describes three individuals enrolled in a malaria surveillance study in Thailand who were found to have P. cynomolgi infection, although after an initial microscopic diagnosis of and treatment for P. vivax . The morphologic characteristics shown on the blood films in Fig. 2 are present in both species, with similarities also evident at the structural level as described by Kosaisavee et al. [ 13 ]. For Case C, who harboured co-infection with P. cynomolgi and P. vivax , it was not possible to identify individual parasite species accurately, even in the thin film, and the parasitaemias in Cases A and B were too low to confidently locate parasites and characterize morphology. Malaria RDTs currently in use are not adequate diagnostic tools for P. cynomolgi . Test antigens are either pan- Plasmodium (e.g., aldolase or lactate dehydrogenase (LDH)) or P. falciparum or P. vivax specific, and the sensitivity in pan- Plasmodium RDTs detecting non-falciparum or non-vivax species of malaria is quite variable [ 14 ]. Cross- reactivity between P. vivax and P. cynomolgi LDH in laboratory setting has recently been demonstrated [ 15 ], but it is not clear this would translate to accuracy in a field-deployed RDT. In addition, the low parasitaemias seen in P. cynomolgi may further reduce RDT sensitivity. With the difficulties in diagnosis by blood smear even for qualified/experienced microscopists, and the lack of utility for RDTs, diagnostic testing by PCR or other molecular methods is likely to be required.
The only other publication on P. cynomolgi prevalence in Thailand conducted PCR assays on 1152 archived samples from malaria patients in Tak, Ubon Ratchathani, Chanthaburi, Yala, and Narathiwat Provinces during the period of 2007 to 2017 [ 8 ]. There were nine P. cynomolgi infections detected, all co-infections: P. cynomolgi with P. vivax (n = 7), with P. falciparum (n = 1), or with both P. vivax and P. knowlesi (n = 1). Cases were distributed across various years, diagnosed between April and December (rainy season is May–October), and found in all provinces, although Yala had five of the nine cases (55%). In these P. cynomolgi clinical cases from 2021, two of the three were mono-infections, which is the first time this has been reported in Thailand. There is one case report of P. cynomolgi mono-infection from a European tourist traveling through Thailand (Surat Thani Province) and Malaysia [ 3 ]. However, the origin of infection could not be confirmed.
With an initial microscopic diagnosis of P. vivax , the patients were not questioned for a history of contact with macaques. At the follow-up visits by the Yala study team, Case A and B did report the presence of macaques near their homes. In Thailand, the main hosts of P. cynomolgi , P. knowlesi , Plasmodium inui , and Plasmodium coatneyi are Macaca fascicularis and Macaca nemestrina , with recent reports in stump-tailed macaques, Macaca arctoides [ 16 ]. Co-infections of simian malaria are not uncommon in macaques, with the presence of two or three species simultaneously detected in 18% to 40% of monkeys [ 16 , 17 ], which may explain why some human studies report co-infections more than mono-infections [ 2 , 5 ]. Plasmodium cynomolgi was first reported as a mono-infection in a Malaysian woman in 2014 [ 1 ], and up to now, cases have been shown to exist in both peninsular Malaysia and Borneo Malaysia, the latter where P. knowlesi , another simian malaria is endemic [ 5 , 7 ]. There have been six other studies reporting the prevalence of P. cynomolgi in humans in Southeast Asia, shown in Table 1 .
To date, most of the publications reporting on human P. cynomolgi infections are retrospective testing of blood samples. In the two clinical case reports of mono-infection, and past experimental infections in humans [ 1 , 2 , 18 ], undifferentiated flu-like symptoms have been present, with symptoms occurring at very low parasitaemias and not progressing in severity. In humans, the anti-malarial treatment required for P. cynomolgi is not well studied, but macaques in P. cynomolgi drug and vaccine studies respond well to chloroquine and primaquine, the regimen for P. vivax in Thailand [ 19 ] . All the patients from Yala recovered rapidly, and there were no recurrences over three months of active follow-up. The low prevalence of simian malarias infecting humans means the parasites are not under frequent anti-malarial drug selection pressure and should remain susceptible to treatment [ 6 ]. In the study by Imwong et al. [ 6 ], two Cambodian individuals were found to have P. cynomolgi again three months after the initial diagnosis, but it was not possible to conclude whether it was a relapse, new infection, or persistent blood-stage infection.
The P. cynomolgi survey by Putaporntip et al. [ 8 ] demonstrated that P. cynomolgi has been infecting humans in Thailand for the last 15 years and is likely underdiagnosed. However among the published studies reviewed here, the prevalence of P. cynomolgi has been less than 1.5% in samples tested. In Thailand, the first clinical case P. knowlesi was reported in 2004, and by 2017, cases began to be regularly reported by the Thailand MoPH, peaking at 53 cases in 2021 [ 20 , 21 ]. It is not yet understood if the increases in human simian malaria infections are due to better detection methods, the result of human encroachment into macaque habitats, or both. The three Yala patients were diagnosed separately in time and space, although Yala province borders with Perak and Kelantan States in Malaysia where P. cynomolgi has been documented [ 7 , Fig. 1 ]. Whole-genome sequencing of the isolates is planned, which will allow lineage comparisons among these three cases as well as with data available from cases in the neighboring Malaysian states [ 7 ]. To mitigate the potential spread of P. cynomolgi and P. knowlesi and remain on track for malaria elimination, increased vigilance will be required for any signs of increased transmission in Yala and other areas in Thailand where exposure to macaques is possible.
Conclusions
This cases series is the second time human P. cynomolgi infections have been documented in Thailand and the first report of mono-infections, along with a description of the clinical course of each. P. cynomolgi is quite challenging to distinguish from P. vivax microscopically, and while this may lead to underdiagnosis, the disease course is usually mild and should be adequately and rapidly treated using antimalarial regimens for P. vivax . Molecular characterization is the most accurate way to detect these rare infections, but the capabilities may not reach the areas that need it most. Going forward, for all samples collected during this malaria surveillance study, primers for P. cynomolgi will be included for 6-species real time PCR verification. Although the diagnoses may not be available before treatment is administered, the results will allow for a more accurate estimation of infection prevalence in Thailand and evaluation of treatment efficacy during the 90-day Thai MoPH follow-up period.
Availability of data and materials
The majority of the data generated is presented in this article, but requests may be made to the corresponding author. Permission from Thai MoPH and Royal Thai Army will also be required.
Abbreviations
Armed Forces Research Institute of Medical Sciences
Deoxyribonucleic acid
Ethylenediaminetetraacetic acid
Glucose 6-phosphate dehydrogenase
Ministry of Public Health
Polymerase chain reaction
Ribosomal ribonucleic acid
Royal Thai Army
White blood cell
World Health Organization
Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi . Malar J. 2014;13:68.
Article Google Scholar
Singh B, Kadir KA, Hu TH, Raja TN, Mohamad DS, Lin LW, et al. Naturally acquired human infections with the simian malaria parasite, Plasmodium cynomolgi , in Sarawak, Malaysian Borneo. Internat J Infect Dis. 2018;73(Suppl):68.
Hartmeyer G, Stensvold CR, Fabricius T, Marmolin E, Høgh S, Nielsen H, et al. Plasmodium cynomolgi as cause of malaria in tourist to Southeast Asia, 2018. Emerg Infect Dis. 2019;25:1936–9.
Grignard L, Shah S, Chua TH, William T, Drakeley CJ, Fornace KM. Natural human infections with Plasmodium cynomolgi and other malaria species in an Elimination Setting in Sabah. Malaysia J Infect Dis. 2019;220:1946–9.
Raja TN, Hu TH, Kadir KA, Mohamad DSA, Rosli N, Wong LL, et al. Naturally acquired human Plasmodium cynomolgi and P knowlesi infections. Malaysian Borneo Emerg Infect Dis. 2020;26:1801–9.
Imwong M, Madmanee W, Suwannasin K, Kunasol C, Peto TJ, Tripura R, et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi . J Infect Dis. 2019;219:695–702.
Article CAS Google Scholar
Yap NJ, Hossain H, Nada-Raja T, Ngui R, Muslim A, Hoh BP, et al. Natural human infections with Plasmodium cynomolgi, P inui, and 4 other simian malaria parasites. Malaysia Emerg Infect Dis. 2021;27:2187–91.
Putaporntip C, Kuamsab N, Pattanawong U, Yanmanee S, Seethamchai S, Jongwutiwes S. Plasmodium cynomolgi co-infections among symptomatic malaria patients. Thailand Emerg Infect Dis. 2021;27:590–3.
Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS ONE. 2013;8: e71539.
Reller ME, Chen WH, Dalton J, Lichay MA, Dumler JS. Multiplex 5’ nuclease quantitative real-time PCR for clinical diagnosis of malaria and species-level identification and epidemiologic evaluation of malaria-causing parasites, including Plasmodium knowlesi . J Clin Microbiol. 2013;51:2931–8.
Vythilingam I, Chua TH, Liew JWK, Manin BO, Ferguson HM. The vectors of Plasmodium knowlesi and other simian malarias Southeast Asia: challenges in malaria elimination. Adv Parasitol. 2021;113:131–89.
Krotoski WA, Bray RS, Garnham PC, Gwadz RW, Killick-Kendrick R, Draper CC, et al. Observations on early and late post-sporozoite tissue stages in primate malaria. II. The hypnozoite of Plasmodium cynomolgi bastianellii from 3 to 105 days after infection, and detection of 36- to 40-hour pre-erythrocytic forms. Am J Trop Med Hyg. 1982;31:211–25.
Kosaisavee V, Suwanarusk R, Chua ACY, Kyle DE, Malleret B, Zhang R, et al. Strict tropism for CD71 + /CD234 + human reticulocytes limits the zoonotic potential of Plasmodium cynomolgi . Blood. 2017;130:1357–63.
Yerlikaya S, Campillo A, Gonzalez IJ. A Systematic Review: Performance of rapid diagnostic tests for the detection of Plasmodium knowlesi , Plasmodium malariae , and Plasmodium ovale monoinfections in human blood. J Infect Dis. 2018;218:265–76.
Barney R, Velasco M, Cooper CA, Rashid A, Kyle DE, Moon RW, et al. Diagnostic characteristics of lactate dehydrogenase on a multiplex assay for malaria detection including the zoonotic parasite Plasmodium knowlesi . Am J Trop Med Hyg. 2022;106:275–82.
Fungfuang W, Udom C, Tongthainan D, Abdul Kadir K, Singh B. Malaria parasites in macaques in Thailand: stump-tailed macaques ( Macaca arctoides ) are new natural hosts for Plasmodium knowlesi , Plasmoidum inui , Plasmodium coatneyi and Plasmodium fieldi . Malar J. 2020;19:350.
Zhang X, Kadir KA, Quintanilla-Zariñan LF, Villano J, Houghton P, Du H, et al. Distribution and prevalence of malaria parasites among long-tailed macaques ( Macaca fascicularis ) in regional populations across Southeast Asia. Malar J. 2016;15:450.
Coatney GR, Elder HA, Contacos PG, Getz ME, Greenland R, Rossan RN, et al. Transmission of the M strain of Plasmodium cynomolgi to man. Am J Trop Med Hyg. 1961;10:673–8.
Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J, et al. Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi -infected Rhesus monkeys ( Macaca mulatta ). Malar J. 2011;10:212.
Ngernna S, Rachaphaew N, Thammapalo S, Prikchoo P, Kaewnah O, Manopwisedjaroen K, et al. Case report: Case series of human Plasmodium knowlesi infection on the Southern Border of Thailand. Am J Trop Med Hyg. 2019;101:1397–401.
Thailand Malaria Elimination Programme, Ministry of Public Health, Thailand. http://malaria.ddc/ma.moph.go.th/malariar10/index_newversion.php . Accessed 17 December 2021.
Download references
Acknowledgements
We would like to thank all the malaria patients who have joined this study as well as the members Yala malaria study team who help recruit, diagnose, transport, translate, and follow-up volunteers: Chalermpol Osodpromma (Director of The Office of Disease Prevention and Control 12 Songkhla), Pathomporn Prikchoo, Suwich Thammapalo, Wanwisa Chunkaew, Sub Lieutenant Wijai Sakoolkaew, Salida Yama. We also would like AFRIMS staff and former staff who supported the surveillance project: Krisada Jongsakul, Nicholas Martin, Mark Fukuda, Kittijarankon Phontham, Saowaluk Wongarunkochakorn, Ladaporn Bodhidatta and Phimphan Pisutsan, as well those from the Ministry of Public Health Office of Disease Control and Prevention, Ubon Ratchathani: Danai Jaerakul and Chatree Raseebut, and our colleagues from the Royal Thai Army: Nithinart Chaitaveep and Darunee Utennam.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
The malaria surveillance study is supported by funding obtained through US Department of Defense Global Emerging Infections Surveillance (PROMIS ID P0055_22_AF) and Defense Malaria Assistance Programs.
Author information
Piyaporn Sai-ngam and Kingkan Pidtana Co-authors; both contributed equally to this work
Authors and Affiliations
US Army Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand
Piyaporn Sai-ngam, Kingkan Pidtana, Kamonporn Poramathikul, Paphavee Lertsethtakarn, Worachet Kuntawunginn, Montri Arsanok, Siriporn Sornsakrin, Chaiyaporn Chaisatit, Chaiyawat Mathavarat, Sasikanya Thaloengsok, Parat Boonyarangka, Chadin Thongpiam, Samandra Demons, Brian Vesely, Mariusz Wojnarski, John S. Griesenbeck & Michele Spring
Ministry of Public Health (MoPH), Vector Borne Disease Control Center 12.1, Yala, Thailand
Preeyaporn Suida
Southern Border Provinces Medical Center, Yala, Thailand
Sarayut Tadsaichol
US Army Medical Materiel Development Activity, Fort Detrick, MD, USA
Norman C. Waters
Ministry of Public Health, Division of Vector Borne Diseases, Nonthaburi, Thailand
Aungkana Saejeng
Royal Thai Army-Army Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand
Sutchana Tabprasit
Royal Thai Army-Forward Internal Security Operation Command Region 4, Yala, Thailand
Chokchai Kwanpichit
The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc, Bethesda, MD, USA
Michele Spring
You can also search for this author in PubMed Google Scholar
Contributions
Study concept, design and support: MS, JSG, MW, NW, BV, SD, WK, PL, BV, ST, AS Study execution and collection of samples/data: PS, KP, KP, MS, WK, PL, SS, PS, CK, SS, CC, MA, PB, CM, CT, ST Performed assays and interpreted data: PS, KP, KP, WK, PL, SS, PS, SS, CC, MA, PB, CM, CT, ST Drafting of the manuscript: PS, KP, KP, MS, PL, WK, CC, MW. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Michele Spring .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval for the conduct of this malaria study was obtained from the Walter Reed Army Institute of Research Institutional Review Board (WRAIR IRB) #00000794 in Silver Spring, Maryland, US on 12 September 2018 and Institute for Development of Human Research Protection (IHRP) IRB #00006539 in Bangkok, Thailand on 22 October 2018. Signed informed consent was obtained from all individuals prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sai-ngam, P., Pidtana, K., Suida, P. et al. Case series of three malaria patients from Thailand infected with the simian parasite, Plasmodium cynomolgi . Malar J 21 , 142 (2022). https://doi.org/10.1186/s12936-022-04167-w
Download citation
Received : 05 January 2022
Accepted : 20 April 2022
Published : 06 May 2022
DOI : https://doi.org/10.1186/s12936-022-04167-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

This nursing study guide provides an overview of malaria including the five species of the malaria parasite, treatment, preventive options, nursing interventions, and nursing care planning, nursing diagnosis , and management.
Malaria is one of the most common infectious diseases known to mankind and is among the leading causes of morbidity and mortality in the world. It predominantly occurs in tropical and subtropical areas such as in sub-Saharan Africa, Asia, and Latin America where the mosquitos that carry the parasite live.
Table of Contents
What is malaria, pathophysiology, statistics and incidences, clinical manifestations, assessment and diagnostic findings, pharmacologic management, nursing assessment, nursing diagnosis, nursing care planning and goals, nursing interventions, documentation guidelines.
Malaria , is a potentially life-threatening disease caused by infection with Plasmodium protozoa transmitted by an infective female Anopheles mosquito vector.
- Malaria is a serious and sometimes fatal disease caused by a parasite that commonly infects a certain type of mosquito which feeds on humans.
- People who get malaria are typically very sick with high fevers, shaking chills, and flu -like illness.
- The 5 Plasmodium species known to cause malaria in humans are P falciparum , P vivax , P ovale , P malariae , and P knowlesi .
- Timely identification of the infecting species is extremely important, as P falciparum infection can be fatal and is often resistant to standard chloroquine treatment.
- Plasmodium falciparum is distinguished from the rest of the plasmodia by its high level of parasitemia and the banana shape of its gametocytes.
The types (species) of Anopheles present in an area at a given time will influence the intensity of malaria transmission.
- Plasmodium falciparum. The most malignant form of malaria is caused by this species; P falciparum is able to infect RBCs of all ages, resulting in high levels of parasitemia; sequestration is a specific property of P falciparum; as it develops through its 48-hour life cycle, the organism demonstrates adherence properties, which result in the sequestration of the parasite in small postcapillary vessels.
- Plasmodium vivax. If this kind of infection goes untreated, it usually lasts for 2-3 months with diminishing frequency and intensity of paroxysms; of patients infected with P vivax, 50% experience a relapse within a few weeks to 5 years after the initial illness;P vivax infects only immature RBCs, leading to limited parasitemia.
- Plasmodium ovale. These infections are similar to P vivax infections, although they are usually less severe; P ovale infection often resolves without treatment; similar to P vivax, P ovale infects only immature RBCs, and parasitemia is usually less than that seen in P falciparum.
- Plasmodium malariae. Persons infected with this species of Plasmodium remain asymptomatic for a much longer period of time than do those infected with P vivax or P ovale; recrudescence is common in persons infected with P malariae.
- Plasmodium knowlesi. Autochthonous cases have been documented in Malaysian Borneo, Thailand, Myanmar, Singapore, the Philippines, and other neighboring countries; it is thought that simian malaria cases probably also occur in Central America and South America; patients infected with this, or other simian species, should be treated as aggressively as those infected with falciparum malaria, as P knowlesi may cause fatal disease.
The natural history of malaria involves cyclical infection of humans and female Anopheles mosquitoes.
- In humans, the parasites grow and multiply first in the liver cells and then in the red cells of the blood .
- In the blood, successive broods of parasites grow inside the red cells and destroy them, releasing daughter parasites (“merozoites”) that continue the cycle by invading other red cells.
- The blood-stage parasites are those that cause the symptoms of malaria; when certain forms of blood stage parasites (gametocytes, which occur in male and female forms) are ingested during blood feeding by a female Anopheles mosquito, they mate in the gut of the mosquito and begin a cycle of growth and multiplication in the mosquito.
- After 10-18 days, a form of the parasite called a sporozoite migrates to the mosquito’s salivary glands .
- When the Anopheles mosquito takes a blood meal on another human, anticoagulant saliva is injected together with the sporozoites, which migrate to the liver, thereby beginning a new cycle.
- Thus the infected mosquito carries the disease from one human to another (acting as a “vector”), while infected humans transmit the parasite to the mosquito.
- In contrast to the human host, the mosquito vector does not suffer from the presence of the parasites.
Malaria is one of the most severe public health problems worldwide
- Almost all US cases of malaria are imported from patients traveling from endemic areas.
- Outbreaks of locally transmitted cases of malaria in the United States have been small and relatively isolated, but the potential risk for the disease to re-emerge is present due to the abundance of competent vectors, especially in the southern states.
- In 2016, an estimated 445,000 people died of malaria—most were young children in sub-Saharan Africa.
- Within the last decade, increasing numbers of partners and resources have rapidly increased malaria control efforts.
- his scale-up of interventions has saved millions of lives globally and cut malaria mortality by 25% from 2010 to 2016, leading to hopes and plans for elimination and ultimately eradication.
- In areas with high transmission, the most vulnerable groups are young children, who have not developed immunity to malaria yet, and pregnant women, whose immunity has been decreased by pregnancy.
- Nearly half the world’s population lives in areas at risk of malaria transmission in 91 countries and territories.
- In 2016, malaria caused an estimated 216 million clinical episodes, and 445,000 deaths; an estimated 90% of deaths in 2016 were in the WHO African Region.
Causes of malaria may include the following:
- Endemic areas. Individuals with malaria typically acquired the infection in an endemic area following a mosquito bite.
- Transfusion. Cases of infection secondary to transfusion of infected blood are extremely rare.
- Poor immunity. The outcome of infection depends on host immunity; individuals with immunity can spontaneously clear the parasites; in those without immunity, the parasites continue to expand the infection.
- Climate. Climate is a key determinant of both the geographic distribution and the seasonality of malaria; without sufficient rainfall, mosquitoes cannot survive, and if not sufficiently warm, parasites cannot survive in the mosquito.
The classical malaria attack lasts 6–10 hours. It consists of:
- Cold stage. A sensation of cold and shivering.
- Hot stage. There is fever , headaches, vomiting ; and seizures in young children.
- Sweating stage. Patient experiences sweat, return to normal temperature, and tiredness .
Rapid and accurate diagnosis of malaria is integral to the appropriate treatment of affected individuals and in preventing the further spread of infection in the community.
- Blood smears. A diagnosis of malaria should be supported by the identification of the parasites on a thin or thick blood smear; thick smears are 20 times more sensitive than thin smears, but speciation may be more difficult; thin smears are less sensitive than thick smears, but they allow identification of the different species.
- Rapid diagnostic tests. Immunochromatographic tests based on antibody to histidine-rich protein-2 (PfHRP2), parasite LDH (pLDH), or Plasmodium aldolase appear to be very sensitive and specific; some RDTs may be able to detect P falciparum in parasitemias that are below the threshold of reliable microscopic species identification; only one RDT (BinaxNOW) has been approved to date for the diagnosis of malaria in the United State
- Other tests. In addition to the RDT listed above, new molecular techniques, such as PCR assay testing and nucleic acid sequence-based amplification (NASBA), are also available for diagnosis; they are more sensitive than thick smears but are expensive and unavailable in most developing countries.
Medical Management
Treatment of malaria depends on many factors including disease severity, the species of malaria parasite causing the infection, and the part of the world in which the infection was acquired.
- Inpatient. Patients with elevated parasitemia (>5% of RBCs infected), CNS infection, or otherwise severe symptoms and those with P falciparum infection should be considered for inpatient treatment to ensure that medicines are tolerated; obtain blood smears every day to demonstrate a response to treatment.
- Prevention. Avoid mosquitoes by limiting exposure during times of typical blood meals (ie, dawn, dusk); wearing long-sleeved clothing and using insect repellants may also prevent infection; avoid wearing perfumes and colognes.
- Consultations. Consider consulting an infectious disease specialist for assistance with malaria diagnosis, treatment, and disease management.
The 4 major drug classes currently used to treat malaria include quinoline-related compounds, antifolates, artemisinin derivatives, and antimicrobials; no single drug that can eradicate all forms of the parasite’s life cycle has been discovered or manufactured yet.
- Antimalarials. These agents inhibit growth by concentrating within acid vesicles of the parasite, increasing the internal pH of the organism; they also inhibit hemoglobin utilization and parasite metabolism.
Nursing Management
The nursing management of a patient with malaria may include the following:
Assessment of a patient with malaria include:
- History. In patients with suspected malaria, obtaining a history of recent or remote travel to an endemic area is critical; asking explicitly if they traveled to a tropical area at anytime in their life may enhance recall; maintain a high index of suspicion for malaria in any patient exhibiting any malarial symptoms and having a history of travel to endemic areas.
- Demographic data. Also determine the patient’s immune status, age, and pregnancy status; allergies or other medical conditions that he or she may have; and medications that he or she may be using.
Based on the assessment data, the major nursing diagnosis for a patient with malaria may include:
- Risk for infection related to weakened immune system.
- Hyperthermia related to increased metabolic rate and dehydration .
- Impaired tissue perfusion related to a decrease in the cellular components needed for the delivery of oxygen and nutrients in the body.
- Fluid volume deficit related to excessive sweating and dehydration .
- Knowledge deficit related to lack of exposure and information about the disease process, its treatment, and prognosis.
The nursing care plan goals for a patient with malaria are:
- Prevent infection.
- Reduce increase in and regain normal body temperature.
- Improve tissue perfusion.
- Improve fluid volume of the body.
- Gain information on malarial disease process, treatment, and prognosis.
Nursing interventions for a patient with malaria include the following:
- Improve body temperature. Warm water compress on forehead and both axilla (not more than 15 minutes each time); maintain warm environment by using warm blankets, adequate clothing); patient may sweat excessively, make sure to avoid exposing patient to wet clothes and linens; administration of antipyretic drugs as ordered.
- Improve tissue perfusion. Patient may need supplemental oxygen if condition is severe; maintain a well-ventilated room; head of the bed at 30º.; lessen activities that require moderate to high exertion.
- Improve fluid volume. Expect loss of fluid through sweat; provide information about fluid balance and guideline for fluid replacement; encourage increase in oral fluid intake; administer parenteral fluids as ordered.
- Educate the patient and family. Review the disease process and therapy, focusing on patient’s concerns; discuss importance of adhering to therapy; go over medication, purpose, frequency, dosage , and side effects; have a family member or trusted individual listen to and understand guideline of treatment as the patient chooses.
Nursing evaluation of patients with malaria includes meeting the following goals:
- Prevention of infection.
- Reduced increase in body temperature.
- Improved tissue perfusion.
- Improved fluid volume of the body.
- Gained and retained information on malarial disease process, treatment, and prognosis.
Nursing documentation in a patient with malaria include:
- Individual findings, including factors affecting, interactions, nature of social exchanges, specifics of individual behavior.
- Cultural and religious beliefs, and expectations.
- Plan of care.
- Teaching plan.
- Responses to interventions, teaching, and actions performed.
- Attainment or progress toward the desired outcome.
Sources and references for malaria nursing study guide:
- Black, J. M., & Hawks, J. H. (2005). Medical- surgical nursing . Elsevier Saunders,. [ Link ]
- Kimberlin, D. W. (2018). Red Book: 2018-2021 report of the committee on infectious diseases (No. Ed. 31). American academy of pediatrics.
- Oshinsky, D. M. (2005). Polio : an American story . Oxford University Press. [ Link ]
- Willis, L. (2019). Professional guide to diseases . Lippincott Williams & Wilkins. [ Link ]
- WHO Expert Committee on Malaria, & World Health Organization. (2000). WHO expert committee on malaria: twentieth report (No. 892). World Health Organization. [ Link ]
6 thoughts on “Malaria”
I love the content of this site.
The content is well explained
Well detailed information 😉 I like it 👍
Well explained and easy to understand.
Hi Cynthia, Thanks for the feedback! It’s great to hear that the malaria study guide was clear and easy to grasp. If there’s more you’d like to learn about malaria or any other topic, just give a shout. Always here to make learning as straightforward as possible!
very good, well explained and easy to understand. Good job.
Leave a Comment Cancel reply
The prevalence of Pfk13 polymorphism in malaria patients treated with artemisinin-based therapy: a systematic review and meta-analysis
- Published: 14 May 2024
- Volume 123 , article number 209 , ( 2024 )
Cite this article

- Dang The Hung ORCID: orcid.org/0000-0001-6109-5208 1 , 2 na1 ,
- Linh Tran ORCID: orcid.org/0000-0001-8667-082X 3 , 4 na1 ,
- Dao Ngoc Hien Tam ORCID: orcid.org/0000-0003-0162-2373 2 , 5 na1 ,
- Ghada Elshafei ORCID: orcid.org/0000-0001-6626-8972 2 , 6 ,
- Nguyen The Ky Cuong ORCID: orcid.org/0000-0002-8465-6896 2 , 7 ,
- Nam Xuan Ha ORCID: orcid.org/0000-0003-2202-2916 2 , 8 ,
- Sarah Abd Elaziz Khader ORCID: orcid.org/0000-0002-4397-3852 2 , 9 ,
- Loc Le Quang ORCID: orcid.org/0000-0001-6363-9862 2 , 10 ,
- Hosam Waleed Shaikhkhalil ORCID: orcid.org/0000-0003-1384-3886 2 , 11 ,
- Abdallfatah Abdallfatah ORCID: orcid.org/0000-0002-5670-2234 2 , 12 ,
- Jeza M Abdul Aziz ORCID: orcid.org/0000-0002-1522-1880 13 , 14 ,
- Kenji Hirayama ORCID: orcid.org/0000-0001-9467-1777 15 &
- Nguyen Tien Huy ORCID: orcid.org/0000-0002-9543-9440 15
43 Accesses
2 Altmetric
Explore all metrics
Artemisinin (ART) combination therapy is the main treatment for malaria. Pfk13 mutations (or K13 mutations, Kelch 13) are associated with ART resistance. This study aims to conduct a systematic review and meta-analysis of the prevalence of K13 mutations with ART resistance in malaria-endemic countries. An electronic search of studies in 2018 and a manual search in 2020 were performed to identify relevant studies. The risk of bias was assessed using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies. Data analysis was performed using R 4.1.0. Heterogeneity was estimated using the statistic I 2 and Cochran Q test. A total of 170 studies were included in our review. Of these, 55 studies investigated the prevalence of K13 mutations in Southeast Asia. The meta-analysis showed that Southeast Asia had the highest prevalence of K13 mutations, whereas Africa, South America, Oceania, and other Asian countries outside Southeast Asia had a low prevalence of K13 mutations. The C580Y mutation was the most common in Southeast Asia with 35.5% (95%CI: 25.4–46.4%), whereas the dominant mutation in Africa was K189T (22.8%, 95%CI: 7.6–43.2%). This study revealed the emergence of ART resistance associated with K13 mutations in Southeast Asia. The diversity of each type of K13 mutation in other regions was also reported.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Current Status of Malaria Control and Elimination in Africa: Epidemiology, Diagnosis, Treatment, Progress and Challenges

Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology

Updates on Malaria Epidemiology and Prevention Strategies
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ahouidi A, Oliveira R, Lobo L, Diedhiou C, Mboup S, Nogueira F (2021) Prevalence of pfk13 and pfmdr1 polymorphisms in Bounkiling. Southern Senegal Plos One 16(3):e0249357. https://doi.org/10.1371/journal.pone.0249357
Article CAS PubMed Google Scholar
Ariey F et al (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505(7481):50–55. https://doi.org/10.1038/nature12876
Arya A, Kojom Foko LP, Chaudhry S, Sharma A, Singh V (2021) Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: a systematic review of clinical studies from two malaria endemic regions - India and sub-Saharan Africa. Int J Parasitol Drugs Drug Resist 15:43–56. https://doi.org/10.1016/j.ijpddr.2020.11.006
Arzika II et al (2023) Plasmodium falciparum kelch13 polymorphisms identified after treatment failure with artemisinin-based combination therapy in Niger. Malar J 22(1):142. https://doi.org/10.1186/s12936-023-04571-w
Article CAS PubMed PubMed Central Google Scholar
Aung Pyae P, François N (2018) The artemisinin resistance in southeast asia: an imminent global threat to malaria elimination. In: Sylvie M, Vas D (eds) Towards Malaria Elimination. IntechOpen, Rijeka, p Ch. 2. https://doi.org/10.5772/intechopen.76519
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22:153–160
Article PubMed PubMed Central Google Scholar
Balikagala B et al (2021) Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385(13):1163–1171. https://doi.org/10.1056/NEJMoa2101746
Boualam MA, Pradines B, Drancourt M, Barbieri R (2021) Malaria in Europe: a historical perspective. Front Med (lausanne) 8:691095. https://doi.org/10.3389/fmed.2021.691095
Article PubMed Google Scholar
Daily JP, Minuti A, Khan N (2022) Diagnosis, Treatment, and Prevention of Malaria in the US: a review. JAMA 328(5):460–471. https://doi.org/10.1001/jama.2022.12366
Doolan DL (2021) Malaria research in Australia: looking through the lens of the past towards the future. Int J Parasitol 51(13–14):1255–1263. https://doi.org/10.1016/j.ijpara.2021.11.005
Fola AA et al (2023) Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia. Nat Microbiol 8(10):1911–1919. https://doi.org/10.1038/s41564-023-01461-4
Ghanchi NK, Qurashi B, Raees H, Beg MA (2021) Molecular surveillance of drug resistance: Plasmodium falciparum artemisinin resistance single nucleotide polymorphisms in Kelch protein propeller (K13) domain from Southern Pakistan. Malar J 20(1):176. https://doi.org/10.1186/s12936-021-03715-0
Hanboonkunupakarn B, White NJ (2016) The threat of artemisinin resistant malaria in Southeast Asia. Travel Med Infect Dis 14(6):548–550. https://doi.org/10.1016/j.tmaid.2016.11.016
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Mathenge PG et al (2020) Efficacy and resistance of different artemisinin-based combination therapies: a systematic review and network meta-analysis. Parasitol Int 74:101919. https://doi.org/10.1016/j.parint.2019.04.016
Mok S et al (2021) Artemisinin-resistant K13 mutations rewire Plasmodium falciparum’s intra-erythrocytic metabolic program to enhance survival. Nat Commun 12(1):530. https://doi.org/10.1038/s41467-020-20805-w
NIH Tool (2020) National heart lung and blood institute study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
Ndwiga L et al (2021) A review of the frequencies of Plasmodium falciparum Kelch 13 artemisinin resistance mutations in Africa. Int J Parasitol Drugs Drug Resist 16:155–161. https://doi.org/10.1016/j.ijpddr.2021.06.001
Niba PTN et al (2023) Evolution of Plasmodium falciparum antimalarial drug resistance markers post-adoption of artemisinin-based combination therapies in Yaounde, Cameroon. Int J Infect Dis 132:108–117. https://doi.org/10.1016/j.ijid.2023.03.050
Ocan M et al (2019) K13-propeller gene polymorphisms in Plasmodium falciparum parasite population in malaria affected countries: a systematic review of prevalence and risk factors. Malar J 18(1):60. https://doi.org/10.1186/s12936-019-2701-6
Owoloye A, Olufemi M, Idowu ET, Oyebola KM (2021) Prevalence of potential mediators of artemisinin resistance in African isolates of Plasmodium falciparum. Malar J 20(1):451. https://doi.org/10.1186/s12936-021-03987-6
Pai MP, Cottrell ML, Kashuba ADM, Bertino JSJ (2014) Pharmacokinetics and pharmacodynamics of anti-infective agents. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases pp 252–262. https://doi.org/10.1016/B978-1-4557-4801-3.00019-9
Peto TJ et al (2022) Triple therapy with artemether-lumefantrine plus amodiaquine versus artemether-lumefantrine alone for artemisinin-resistant, uncomplicated falciparum malaria: an open-label, randomised, multicentre trial. Lancet Infect Dis 22(6):867–878. https://doi.org/10.1016/s1473-3099(21)00692-7
Plucinski MM et al (2017) Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 16(1):186. https://doi.org/10.1186/s12936-017-1843-7
Rasmussen C, Alonso P, Ringwald P (2022) Current and emerging strategies to combat antimalarial resistance. Expert Rev Anti Infect Ther 20(3):353–372. https://doi.org/10.1080/14787210.2021.1962291
Schmedes SE PD, Dhal S, Kelley J, Svigel SS, Dimbu PR, et al. (2021) Plasmodium falciparum kelch 13 Mutations, 9 Countries in Africa, 2014-2018. Emerging Infect Dis Plasmodium falciparum kelch 13 mutations, 9 countries in Africa, 2014–2018. Emerging Infectious Diseases 27(7):1902-08. https://doi.org/10.3201/eid2707.203230
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Tran L et al (2022) A systematic review of antimalarial activities of Morinda species. S Afr J Bot 148:396–406. https://doi.org/10.1016/j.sajb.2022.05.007
Article CAS Google Scholar
Uwimana A et al (2021) Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 21(8):1120–1128. https://doi.org/10.1016/s1473-3099(21)00142-0
Vassar M, Atakpo P, Kash MJ (2016) Manual search approaches used by systematic reviewers in dermatology. J Med Libr Assoc 104(4):302–304. https://doi.org/10.3163/1536-5050.104.4.009
Witmer K et al (2020) Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob Agents Chemother 65(1):e00898–20. https://doi.org/10.1128/aac.00898-20
World Health Organization (2020) World malaria report 2020: 20 years of global progress and challenges. https://iris.who.int/handle/10665/337660 . Accessed 14 Oct 2023
World Health Organization (2022) WHO guidelines for malaria. https://iris.who.int/ . Accessed 14 Oct 2023
Yang C et al (2017) Polymorphisms of Plasmodium falciparum k13-propeller gene among migrant workers returning to Henan Province. China from Africa BMC Infect Dis 17(1):560. https://doi.org/10.1186/s12879-017-2634-z
Download references
Acknowledgements
We would like to thank Kirellos Said Abbas (Faculty of Medicine, Alexandria University, Alexandria, Egypt) and Gehad Mohamed Tawfik (Department of Otorhinolaryngology, Faculty of Medicine, Ain Shams University, Cairo, Egypt) for their contributions during the initial stages of the study. We also thank Peterson Gtionga Mathenge (Harvard Medical School, USA) for proofreading the manuscript.
Author information
Dang The Hung, Linh Tran, And Dao Ngoc Hien Tam share equal contribution to this work.
Authors and Affiliations
School of Biomedical Engineering & Imaging Sciences, Faculty of life Sciences & Medicine, Kings College London, London, WC2R 2LS, UK
Dang The Hung
Online Research Club, Nagasaki, 852-8523, Japan
Dang The Hung, Dao Ngoc Hien Tam, Ghada Elshafei, Nguyen The Ky Cuong, Nam Xuan Ha, Sarah Abd Elaziz Khader, Loc Le Quang, Hosam Waleed Shaikhkhalil & Abdallfatah Abdallfatah
Institute of Fundamental and Applied Sciences, Duy Tan University, Ho Chi Minh City, 700000, Vietnam
Faculty of Natural Sciences, Duy Tan University, Da Nang City, 550000, Vietnam
Asia Shine Trading & Service Co., Ltd, Ho Chi Minh City, 700000, Vietnam
Dao Ngoc Hien Tam
Faculty of Medicine, Modern University for Technology and Information, Cairo, 4236044, Egypt
Ghada Elshafei
International Cancer Specialists, Ho Chi Minh City, 70000, Vietnam
Nguyen The Ky Cuong
Hue University of Medicine and Pharmacy, Hue University, Hue City, 49000, Vietnam
Nam Xuan Ha
Faculty of Medicine, Ain Shams University, Cairo, 11591, Egypt
Sarah Abd Elaziz Khader
Faculty of Medicine, University of Medicine and Pharmacy, Ho Chi Minh City, 7000, Vietnam
Loc Le Quang
Faculty of Medicine, Islamic University of Gaza, Gaza Strip P840, Palestine
Hosam Waleed Shaikhkhalil
Faculty of Medicine, October 6 University, Giza, Egypt
Abdallfatah Abdallfatah
Biomedical Sciences, Komar University of Science and Technology, Sulaymaniyah, Iraq
Jeza M Abdul Aziz
Baxshin Research Center, Baxshin Hospital, Sulaymaniyah, Iraq
School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, 852-8523, Japan
Kenji Hirayama & Nguyen Tien Huy
You can also search for this author in PubMed Google Scholar
Contributions
KH and NTH developed the idea, conceived, and designed the study. DTH, LT, and DNHT performed literature search and data collection. DTH analyzed and synthesized the data. DTH assessed the quality of the included studies. DTH, LT, and AA interpreted the results and wrote the manuscript. All authors critically checked the data, reviewed and edited the manuscript. KH, NTH provided supervision and project administration. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Nguyen Tien Huy .
Ethics declarations
Ethical approval.
Ethical approval is not applicable for this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Alexander Maier
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 376 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Hung, D.T., Tran, L., Tam, D.N.H. et al. The prevalence of Pfk13 polymorphism in malaria patients treated with artemisinin-based therapy: a systematic review and meta-analysis. Parasitol Res 123 , 209 (2024). https://doi.org/10.1007/s00436-024-08203-3
Download citation
Received : 14 October 2023
Accepted : 04 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1007/s00436-024-08203-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- K13 mutations
- Artemisinin
- Find a journal
- Publish with us
- Track your research

East African Medical Journal Journal / East African Medical Journal / Vol. 103 No. 4 (2024): UON Supplement / Articles (function() { function async_load(){ var s = document.createElement('script'); s.type = 'text/javascript'; s.async = true; var theUrl = 'https://www.journalquality.info/journalquality/ratings/2405-www-ajol-info-eamj'; s.src = theUrl + ( theUrl.indexOf("?") >= 0 ? "&" : "?") + 'ref=' + encodeURIComponent(window.location.href); var embedder = document.getElementById('jpps-embedder-ajol-eamj'); embedder.parentNode.insertBefore(s, embedder); } if (window.attachEvent) window.attachEvent('onload', async_load); else window.addEventListener('load', async_load, false); })();
Article sidebar, article details, main article content, effects of community malaria case management to the overall malaria incidence in busia county, kenya, 2022, f.o. odhiambo, j.g. murangiri, r.j. kosgei, a.b. kihara.
Objectives : The objective of the present study was to determine whether Case Management of Malaria (CCMm) by Community Health Volunteers (CHVs) affect the trends of malaria incidence in Busia Kenya, 2018-2023. Specifically, the study aimed at determining the proportion and trends per year for those tested and treated for malaria in health facilities and Community Units and to correlate the trends with annual malaria incidence, out-patient malaria cases, weather patterns, climate change and commodity availability at the community level.
Methods : The research involved a retrospective cross-sectional study encompassing for Busia County as the study site involved analysis of routinely collected malaria program data which was abstracted online from the Kenya Health Information Systems.
Results : The proportion of Suspected Malaria Cases being tested in the community by the Community Health Volunteers compared to those tested at Health facilities increased from 11% in 2019 to 45% in 2022. The rate of malaria infections per month has remained almost constant, with peak infections occurring in May every year, except May 2020. Over time, the contribution of CCMm in overall malaria case management and incidence has increased, with more Malaria cases being treated at the Community as from mid-2022. The incidence of Malaria has remained high over the years.
Conclusion : Community Case Management of Malaria improves access to Malaria treatment services but does not in itself reduce the Annual Malaria Incidence in Busia County.
AJOL is a Non Profit Organisation that cannot function without donations. AJOL and the millions of African and international researchers who rely on our free services are deeply grateful for your contribution. AJOL is annually audited and was also independently assessed in 2019 by E&Y.
Your donation is guaranteed to directly contribute to Africans sharing their research output with a global readership.
- For annual AJOL Supporter contributions, please view our Supporters page.
Journal Identifiers

Malaria cases in Texas and Florida are the first US spread in 20 years, CDC says

The United States has seen five cases of malaria spread by mosquitos in the past two months − the first time there has been local spread in 20 years − prompting authorities to issue a public health alert warning doctors, public health authorities and the public about the risk.
Four cases were identified in southwest Florida and one in southern Texas, the Centers for Disease Control and Prevention said. The five cases are the first in 20 years to be caught locally in the United States.
"Malaria is a medical emergency and should be treated accordingly," the CDC said. "Patients suspected of having malaria should be urgently evaluated in a facility that is able to provide rapid diagnosis and treatment, within 24 hours of presentation."
Malaria is a serious disease transmitted through the bite of an infective female anopheline mosquito, according to the CDC. Although malaria can be fatal, the CDC said, illness and death from the disease can usually be prevented.
There is no evidence the five cases in the two states are related, the CDC said. The four cases in Florida were identified in Sarasota County, and the Florida Department of Health issued a statewide mosquito-borne illness advisory Monday.
Only one case was identified in a Texas resident who spent time working outdoors in Cameron County, according to the Texas Department of State Health Services .
Both departments in Florida and Texas said public health authorities were monitoring local mosquito populations and surveilling their regions for other cases. The Florida Department of Health said it was also working to control the mosquito population in Sarasota County.
The CDC said all five patients have been treated and were improving. Cases of locally acquired malaria have not occurred in the United States since 2003, when eight cases were identified in Palm Beach County, Florida.
Malaria cases are rare in US
Even with the five identified cases, the CDC said, the risk of catching malaria in the United States "remains extremely low."
But the health agency warned that female anopheline mosquitoes can be found throughout many regions in the country and can spread malaria if they feed on a person already infected with the disease.
"The risk is higher in areas where local climatic conditions allow the Anopheles mosquito to survive during most of or the entire year and where travelers from malaria-endemic areas are found," the CDC said.
More than 240 million cases of malaria occur each year worldwide, and 95% of cases are in Africa, according to the CDC. And a majority of cases in the United States are from people who travel from countries with malaria transmission.
Before the COVID-19 pandemic, the CDC said, there were about 2,000 cases of mostly travel-related malaria in the United States each year, and about 300 people experienced severe disease.
Although rare, malaria can also spread through blood transfusions, organ transplants, unsafe needle-sharing practice and from mother to fetus, according to the CDC.
The CDC warned that more people could bring the disease into the United States with summer international travel increasing to pre-pandemic levels and advised people to use bug spray during the warmer months.
Symptoms of malaria include fever, chills, headache, muscle aches and fatigue. People may also experience nausea, vomiting and diarrhea. Though symptoms generally start about 10 days to four weeks after infection, people may feel sick as late as a year after infection.
Experts report increase in 'mosquito days'
The number of "mosquito days," or periods where mosquitoes thrive in warm and humid weather, has increased in more than 170 U.S. locations over the past several decades, according to a report in May 2023 from the nonprofit climate science research organization Climate Central.
According to the report, a mosquito day has an average relative humidity of 42% or higher in addition to daily temperatures of 50 to 95 degrees. From 1979 to 2022, the report said, 173 U.S. locations saw annual mosquito days increase by 16 days on average.
The report warned that as the climate warms, especially during the spring and fall, many regions are becoming "more hospitable to mosquitoes," which allow the flying insects to arrive earlier and survive later into the year.
More mosquitoes also means a possible increase in health risks. "More mosquito days mean more opportunities for mosquitoes to bite people and potentially transmit disease," the report said.
How to get rid of mosquitoes
Mosquitoes flock toward dark, humid places like under the sink, in showers, closets and laundry rooms, and under furniture, according to the CDC. Once they’re inside, they may start laying eggs in your home.
The first step you can take to minimize mosquitoes in or around your home is to check for and eliminate any standing water. One of the most common examples are trays under potted plants to catch excess water, said Elmer Gray , a public health extension specialist at the University of Georgia.
“If you have house plants on your deck and you have mosquitoes on your deck, you might be growing them right there,” Gray said.
Check your house and yard for areas that might be gathering water. That could be old tires collecting rainwater, dog dishes left outside, tree holes, rain barrels, gutters or garbage cans.
Contributing: Clare Mulroy
Sex work in bars linked to rapid mpox spread in DR Congo hot spot
Interactions involving sex workers in bars is likely driving rapid mpox transmission in densely populated areas of the Democratic Republic of Congo (DRC), researchers reported in an observational preprint study of hospitalized patients in Kamituga health zone.
The outbreak involves a novel clade 1 mpox lineage that is more virulent and deadly than the clade 2 virus mpox virus spreading globally since 2022.
Researchers examined the demographic and clinical characteristics of 371 patients with suspected mpox infections who were admitted to the hospital between September 2023 and April 2024. Slightly more than half were women, and cases were reported from 15 health areas.
Four cases were fatal, and four of eight pregnant women experienced fetal loss. Three healthcare workers were infected while caring for patients.
Overwhelming majority had connections to sex workers in bars
Data revealed that 88.4% had recently visited bars for professional sexual interactions, which researchers said was the likely source of infection.
Expanding case numbers and spillover to other health zones points to a need for cross-border surveillance, as well as vaccination and health education to curb the spread of the virus, the group wrote.
Korean study shows high broad-spectrum antibiotic exposure at end of life
A study of patients at the end of life in South Korea found high rates of exposure to broad-spectrum antibiotics, particularly among those with cancer, researchers reported today in Antimicrobial Stewardship & Healthcare Epidemiology.
Using data from the Korean National Health Insurance Database, researchers from Seoul National University analyzed antibiotic consumption during the final month, 6 months, and year of life in patients with and without cancer from 2006 to 2018.
Although smaller studies have found that antibiotic use during end-of-life (EOL) care is common, especially in cancer patients who are at risk for infection, the evidence supporting its use is limited. The study authors note that since cancer is the second-leading cause of death in most Organization for Economic Cooperation and Development countries, antibiotic use among terminal cancer patients is an important target for antimicrobial stewardship.
Increased use of broad-spectrum antibiotics
Of the more than 3.4 million decedents in the study population, 28.1% had cancer. Overall antibiotic consumption rates decreased slightly among decedents in their final month, with a less pronounced annual decrease rate among cancer decedents compared with non-cancer controls (0.4% vs 2.3%).
But over the study period, while narrow-spectrum antibiotics were used less, use and prescription of broad-spectrum antibiotics (beta-lactam/beta-lactamase inhibitor combinations, carbapenems, and polymyxins) steadily increased, and prescription rates were higher in cancer decedents than in non-cancer controls.
Specifically, carbapenem prescription rates increased from 5.6% to 18.5% (rate ratio [RR], 1.087; 95% confidence interval [CI], 1.085 to 1.088) in cancer decedents and from 2.9% to 13.2% (RR, 1.115; 95% CI, 1.113 to 1.116) in non-cancer decedents.
"Our findings suggest that patients at EOL, particularly those with cancer, are increasingly and heavily exposed to broad-spectrum antibiotics, which, although likely a consequence of increased AMR [antimicrobial resistance] over time, poses a great threat for further AMR emergence and spread," the study authors wrote. "There is a need to critically assess the tangible benefits of antibiotic use in EOL care and reconsider the perceptions of its noninvasive nature."
Report urges G7 countries to commit to incentives for antibiotic development
The Global Coalition on Aging (GCOA) last week issued a report calling on G7 countries to commit to funding pull incentives and making other investments in antibiotic innovation.
The report, which summarizes a GCOA-convened April meeting that included experts and government officials from Japan, the United States, Canada, the United Kingdom, Italy, and the European Union, asserts that the lack of new antibiotics and rising resistance to current antibiotics is having a severe impact on those most at risk of infection and that the current antibiotic pipeline is inadequate and must be prioritized.
Therefore, the experts concluded, incentive structures to develop new antibiotics, such as the subscription-based model adopted in the United Kingdom, must be supported, and other countries in the G7 must "rise to the challenge" and complement the UK model with their own incentives. To date, the United Kingdom is the only country to follow up on pledges to incentivize new antibiotic research and development made at the 2022 G7 meeting.
Call for 'fair share' contributions
Noting that the current healthcare burden of antimicrobial resistance (AMR) is estimated at $1 trillion globally, the experts agreed that if all G7 countries contributed their "fair share" to a $4.5 billion pull incentive to fund a single new antibiotic, the return on investment would be 5:1.
The report urged G7 countries to use the opportunity presented by the upcoming United Nations High-Level Meeting on AMR and demonstrate their commitment to solving the problem.
"There will never be more political momentum on AMR than this year," the report states. "All countries must take this opportunity to address AMR through collaborative multi-stakeholder action and commitments to invest a fair share in our most vital healthcare infrastructure."

BSE reported in Scottish breeding cow
Scotland's government last week reported a classical bovine spongiform encephalopathy (BSE) case involving a cow that died on a farm in Ayrshire, its fifth such case since 2014.

In a statement, officials said the fallen cow was tested as part of its BSE surveillance program and did not enter the food chain. The animal died after showing clinical signs consistent with BSE. The cow had been used for breeding. Ayrshire is in southwest Scotland.
A notification today from the World Organization for Animal Health (WOAH) said the cow was 7-and-a-half-years old and was close to calving. Over a 2-week period, the indigenous cow displayed illness symptoms and was recumbent and aggressive before it died. There are 206 cows on the breeding farm.
BSE is not contagious and comes in two forms, classical and atypical. Classical BSE is associated with the outbreak in the United Kingdom in the 1980s and Creutzfeldt-Jakob disease (mad cow disease).
Precautionary culling for cow's farm cohorts
Restrictions remain in place for the cow's cohorts and offspring as a precaution, and those born in the last 2 years have been identified, will be humanely culled, and will be tested for BSE.
The United Kingdom's Animal and Plant Health Agency has launched an epidemiological investigation to determine the cause of the case.
BSE is a fatal neurogenerative disease that is part of a group of prion-related illnesses that includes chronic wasting disease (CWD), which affects deer and other cervids.
CDC reports 41% more imported malaria cases in 3 southern border cities in 2023 than 2022
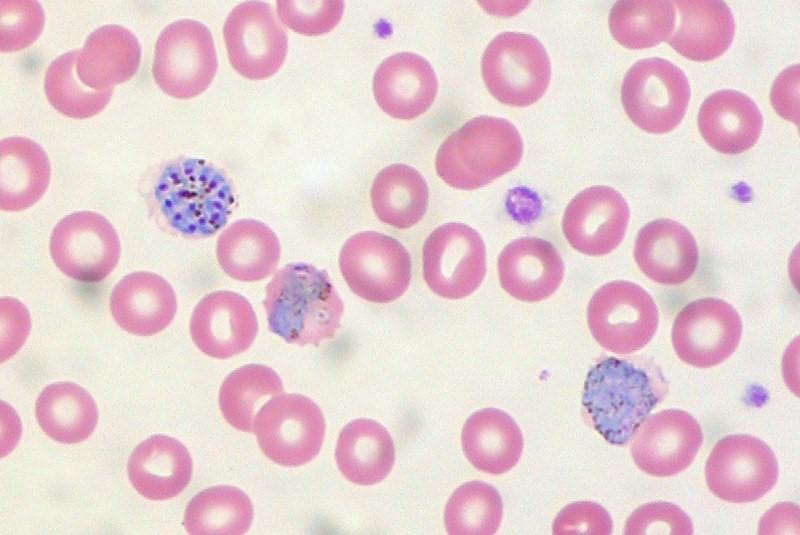
A higher proportion of people who trekked through at least one country with endemic malaria on their way to three southern US border cities arrived with cases of the mosquito-borne illness—nearly a third of them with severe disease—in 2023 than in 2022, finds a study published in Morbidity and Mortality Weekly Report .
Researchers from the Centers for Disease Control and Prevention (CDC) and local health departments conducted enhanced imported malaria case investigations from January to December 2023.
P eople born outside the United States who arrived in the three cities within the past 6 months were classified as newly arrived refugees officially admitted through the US Refugee Admissions Program, other new arrivals such as asylum seekers, or people of unknown immigration status.
72% of cases in other newly arrived migrants
Sixty-eight imported malaria cases were identified in Pima, Arizona (18 cases); San Diego, California (27); and El Paso, Texas (23), compared with 28 cases in 2022 in Pima (3), San Diego (12), and El Paso (13). Of the 68 cases in 2023, 22% occurred in US residents, 3% among newly arrived refugees, 72% in other newly arrived migrants, and 3% in travelers of unknown immigration status.
Outreach and education about malaria directed to local health care professionals and to new arrivals with recent travel in areas with endemic malaria are crucial.
US residents and refugees had traveled directly from another country with endemic malaria. Of the 49 other newly arrived migrants, 94% had traveled through at least one endemic country, including the country of origin.
The median length of travel was 29 days, and 73% of travelers said they had crossed land borders. Thirty-one percent of malaria patients were severely ill, and severe disease was more common in other newly arrived migrants (37%) than in US residents (7%). A total of 91% were hospitalized; none died.
" Outreach and education about malaria directed to local health care professionals and to new arrivals with recent travel in areas with endemic malaria are crucial because prompt care seeking, diagnosis, and treatment of malaria will reduce morbidity in this population, " the researchers concluded.
In case you missed it
This week's top reads, wastewater testing finds h5n1 avian flu in 9 texas cities.
Wastewater detections began in early March, and so far sequencing hasn't found any mutations linked to human adaptation.

CDC launches new influenza A wastewater dashboard; states report more H5N1 in dairy herds
The tracker will help with surveillance, but it doesn't distinguish the influenza A subtype or determine the source of the virus.

USDA experiments suggest H5N1 not viable in properly cooked ground beef
Federal officials said it's unclear if dairy cow outbreaks are peaking, and they haven't made much headway testing farm workers for avian flu virus.

Feds announces assistance for US farmers affected by H5N1 avian flu
The assistance is meant to both improve on-site biosecurity and address financial losses connected to lost milk production in herds affected by H5N1.

USDA confirms 3 more H5N1 outbreaks in dairy herds
Of the three newly confirmed outbreaks, two are in Michigan and one is in Idaho.
WHO describes MERS cluster in Saudi Arabia
The index case-patient, who died from his infection, shared a hospital room with the second patient identified.
Study: Before vaccines, 44% of COVID-19 patients in ICU died
The overall case-hospitalization rate among patients was 5.7%.

Global meta-analysis estimates 43% rate of multidrug resistance in COVID patients
A total of 76% of patients were prescribed antibiotics.
Data: Heart-failure patients have 82% better odds of living longer if vaccinated against COVID
Vaccinated patients also had a 47% lower risk of hospitalization for heart failure and a 13% reduced risk of infection over 6 months.
Hospital COVID patients 35% more likely to die than flu patients last winter, study suggests
The results should be interpreted in the context of twice as many hospitalizations for COVID than flu during the study period, the authors say.

Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
Severe outcomes of malaria in children under time-varying exposure
- Pablo M. De Salazar ORCID: orcid.org/0000-0002-8096-2001 1 , 2 ,
- Alice Kamau 3 ,
- Aurelien Cavelan 1 , 2 ,
- Samuel Akech 3 ,
- Arthur Mpimbaza 4 ,
- Robert W. Snow ORCID: orcid.org/0000-0003-3725-6088 3 , 5 na1 &
- Melissa A. Penny 1 , 2 , 6 , 7 na1
Nature Communications volume 15 , Article number: 4069 ( 2024 ) Cite this article
70 Accesses
Metrics details
- Epidemiology
- Policy and public health in microbiology
In malaria epidemiology, interpolation frameworks based on available observations are critical for policy decisions and interpreting disease burden. Updating our understanding of the empirical evidence across different populations, settings, and timeframes is crucial to improving inference for supporting public health. Here, via individual-based modeling, we evaluate a large, multicountry, contemporary Plasmodium falciparum severe malaria dataset to better understand the relationship between prevalence and incidence of malaria pediatric hospitalizations - a proxy of malaria severe outcomes- in East-Africa. We find that life-long exposure dynamics, and subsequent protection patterns in children, substantially determine the likelihood of malaria hospitalizations relative to ongoing prevalence at the population level. Unsteady transmission patterns over a lifetime in children -increasing or decreasing- lead to an exponential relationship of hospitalization rates versus prevalence rather than the asymptotic pattern observed under steady transmission. Addressing this increase in the complexity of malaria epidemiology is crucial to update burden assessments via inference models that guide current and future policy decisions.
Similar content being viewed by others

No evidence of sustained nonzoonotic Plasmodium knowlesi transmission in Malaysia from modelling malaria case data
Bayesian Spatiotemporal Modeling of Routinely Collected Data to Assess the Effect of Health Programs in Malaria Incidence During Pregnancy in Burkina Faso
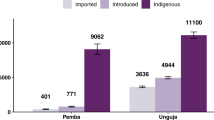
Modelling the impact of interventions on imported, introduced and indigenous malaria infections in Zanzibar, Tanzania
Introduction.
Assessing the burden of malaria life-threatening outcomes in populations at risk is a critically important step in evaluating and improving control efforts. Malaria mortality is challenging to measure accurately in the community 1 but remains a fundamental component of statistical-based interpolation from prevalence estimates, resulting in high uncertainty 2 . Inference of disease burden has been approached with different grades of sophistication, ranging from purely data-driven fits to multi-level mechanistic microsimulations 3 , 4 , 5 , 6 . Independent of the complexity of the approach, the ability of a model to generate accurate, robust, and valid malaria disease outcomes using exposure predictors, such as prevalence, requires (1) high-quality data as input from real-world observations, and (2) a comprehensive understanding and identification of the key factors determining the relationship between exposure and clinical outcomes.
Severe, life-threatening malaria syndromes presenting to hospitals are a valuable proxy for malaria-related death among communities. High exposure rates in children at a very young age are known to offset the risk of severe clinical outcomes at older ages 7 . This leads to a characteristic asymptotic pattern between exposure and disease risk at the population level, consistent with consensual malaria theory and historical observations 4 , 7 , 8 , 9 . Recent work has assessed the empirical relationship between community prevalence and the risk of severe malaria syndromes, namely severe anemia, cerebral malaria, and respiratory distress among children in East Africa, based on the largest standardized Plasmodium falciparum malaria pediatric dataset available to date 10 . Findings show that the occurrence of these severe malaria outcomes in the population may relate differently to increasing community prevalence. Particularly, an asymptotic relationship was observed when predicting severe anemia over community prevalence, while an exponential relationship was favored when predicting a combined outcome comprising the three syndromes. Evaluating new sources of standardized data, such as this contemporary dataset, contextualized with historical sources of data 9 , 11 improves our understanding of the accuracy, robustness, and validity of the inference frameworks.
Here, we use a previously validated multi-level individual-based malaria model 12 , OpenMalaria ( https://github.com/SwissTPH/openmalaria/wiki ), to systematically investigate clinical and epidemiological factors influencing the relationship between potentially life-threatening hospital malaria admissions among children upon a given observed community prevalence. We use community-based malaria hospitalization incidence rates in small catchment populations and adjusted for case under-ascertainment as an empirical proxy of the incidence of severe malaria outcomes. Our analysis framework interrogates standardized malaria data obtained in Sub-Saharan African time-sites within the 1990s throughout 2020 9 , 10 , 11 , 13 , aiming to improve and update our understanding of the dynamics from P. falciparum malaria infection to the occurrence of severe outcomes in children.
Malaria admissions were assembled from individual records of 21 hospitals representing 35 time-sites in Kenya, Uganda, and Tanzania, among children resident in specific catchment areas -within a defined distance radius from the hospital where surveillance took place- excluding urban settings where it was possible to estimate single-year censused population estimates. We assume that the hospitalization rates per time-site represent a lower limit of the hospitalization incidence and can be reasonably comparable when further adjusting for case ascertainment. Cases were included if malaria was the primary cause of hospitalization, and those with underlying conditions were excluded 10 , 13 . We further used data on malaria hospitalization incidence obtained with similar approaches between 1992 and 1997 at seven hospital time-site locations in Kenya, The Gambia, and Malawi 9 , 11 . This allows us to compare and interpret our findings with a dataset that has been classically used for informing malaria inference models 4 , 8 , 14 aiming to estimate severe outcomes of malaria across populations.
For each of the time-sites in the above datasets, the average number of hospitalizations due to malaria among children three months to 9 years old per 1000 children per year were paired with age-diagnostic method standardized community prevalence estimates, as empirical P. falciparum Parasite Rates among children 2–10 years old ( ePf PR 2–10 ). The data was obtained from community and school surveys undertaken during the period of hospital surveillance within the same catchment areas 9 , 10 , 11 . Further, we assessed the past exposure dynamics using catchment site-specific time-series of modeled age- and test-standardized parasite rates estimates, herein referred to as mPf PR 2–10 . For each time-site of the contemporary dataset, annual mPf PR 2–10 estimates were obtained using a Bayesian hierarchical geospatial model detailed elsewhere 13 , 15 . In those time sites where modeled estimates were available for at least 7 past years ( n = 27), and up to a maximum of 9 years, we computed the median mPf PR 2–10 of the time series. We assume that the median prevalence across the past 7–9 years roughly represents the cumulative past transmission to which the population of children up to 10 years of age have been long-life, which can then be compared to the empirical prevalence at the time of the survey to evaluate the gap between past and present-day transmission
Visual inspection of the empirical relationship between prevalence and hospitalization rates for the 35 time-sites included in the contemporary dataset does not suggest an asymptotic relationship of malaria hospitalization incidence across the ePf PR 2–10 range within the full dataset (Fig. 1a .). For illustration, we highlight four representative time-sites in Fig. 1a and alongside the mPf PR 2–10 time-series of these time-sites for the years prior to the collection of the empirical data (Fig. 1b ). Three major patterns of time-varying transmission are depicted, showing (1) a substantial increase in the mPf PR 2–10 (Apac A), (2) relative constant mPf PR 2–10 (e.g, Busia) (3) steady increase (Mubende B) and 4) substantial decrease in the Pf PR 2–10 (e.g., Jinja B). As depicted in Fig. 1c , there is substantial change between the ePf PR 2–10 and the median value of the mPf PR 2–10 over the previous years for each of the time-sites comparing at least seven years and up to ten years of modeled past exposure estimates. The difference between the ePf PR 2–10 and the median value of past mPf PR 2–10 can be interpreted as the gap in past exposure relative to ongoing exposure. For those time-sites at the higher end of the current ePf PR 2–10 range (i.e., higher than the median empirical prevalence, 20%), exposure had primarily substantially increased or remained relatively stable (12 out of 14 sites). For those time sites at the lower end of the range (lower than 20%), exposure had decreased or remained relatively stable (13 out of 13 sites). All available mPf PR 2–10 time series for the 35-time sites are shown in Fig. S1 13 , 15 .
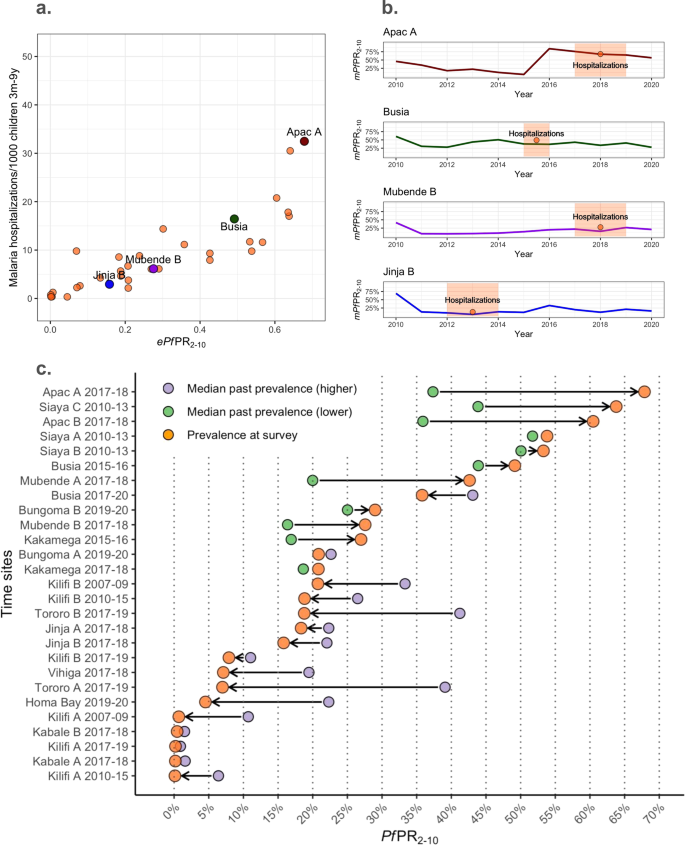
a The ePf PR 2–10 -severe malaria incidence empirical relationships highlighting four representative time-sites (red, green, purple, and blue colored dots) within all time-sites (orange dots). b Present day mPf PR 2–10 over time in four representative time-sites (red, green, purple, and blue colored lines) and time-site ePf PR 2–10 (orange) with highlighted time periods for which hospitalization incidence estimates were available for the empirical relations in ( a ). c Summarizing the prevalence trends over time estimated for each time-site as an increasing trend -when the estimated median mPf PR 2–10 in the past 7 to 9 years is lower than the ePf PR 2–10 at the time of assessment of severe outcomes incidence- or decreasing trend the estimated median mPf PR 2–10 in the past 7–9 years is lower than the ePf PR 2–10 at the time of assessment of severe outcomes incidence. The median past mPf PR 2–10 per time-site is plotted as yellow (for those with increasing trends) or red (for those time-sites with reducing trends), and ePf PR 2–10 per time-site is plotted in orange. Estimates have been computed among the 27-time sites with at least seven years of available past mPf PR 2–10 estimates.
Understanding the complex relationship between malaria exposure, immunity, and clinical outcomes across populations and time requires causal analytical frameworks that (a) can combine empirical observations with theory (b) can address multiple interacting causal effects, threshold dynamics, and interference (c) have generally accepted principles to build the models, populate and calibrate their parameters and test their predictions for avoiding misspecification. Individual-based models are amongst the few modeling tools that fulfill these requirements 1 , 2 .
Open-Malaria ( https://github.com/SwissTPH/openmalaria/wiki ) is an multi-level individual-based model that includes several key features that allow to generate counterfactuals of the effect of malaria exposure on clinical disease under different scenarios of population structure, changing transmission, health-access, diagnostic thresholds, drug-efficacy, and other major malaria control and prevention interventions 3 . Random effects can be incorporated into the modeled processes and allow the inclusion of uncertainty and heterogeneity in the simulations. Relevant to our analyses, the framework encompasses submodels specifically parameterized to empirical data including (1) population structure 4 , 5 ; (2) within-host dynamics of parasite burden and addressing the effect on single individuals of repeated infections in developing immunity and subsequent infections 6 , 7 , 8 ; (3) disease progression 6 , 7 and health-seeking behavior including rates of individuals accessing health services, as well as time to diagnostic and treatment 4 ; (4) efficacy of case-management including diagnostic sensitivity and specificity, first- and second-line treatment effectiveness and efficacy of hospitalization 4 ; and (5) the effect of age-structured comorbidities on severe malaria outcomes upon infection 5 . Further details are provided in the Supplementary Note 2 .
We iteratively interrogated the data under different sets of plausible parameterizations of our individual-based model, hereafter referred to as scenario analysis. The scenario analysis explores hypotheses of the impact of well-known determinants on disease risk and changes in contemporary disease risk compared to historical observations, including the deployment and availability of artemisinin-derivatives in primary- and hospital-care, improved treatment adherence, the reduction in the occurrence and progression of malaria-associated comorbidities. In addition to addressing the detailed major key changes between the historical and contemporary datasets, we further address the life-long exposure dynamics evidenced by the modeled community prevalence estimates (Fig. 1b and Fig. S1 ). The risk of malaria disease outcomes, including those severe, depends on immunity, which in turn depends on previous exposure dynamics. We hypothesize that the year-to-year variability of PfPR could strongly influence the risk of hospitalization. Thus, we further assessed the impact of this variability on malaria exposure on the ongoing hospital admission risk estimated from our individual-based model scenarios. Here, we define steady exposure as the exposure of a specific population that does not change substantially over the years, and we define unsteady exposure as an exposure that shows substantial increasing or decreasing dynamics over time. We performed simulations that included generic step-up and step-down exposure dynamics with differences between pre- and intra-survey prevalence within the range of those observed empirically. Further details are found in the Supplementary Note 3 . For each scenario analysis, we performed over 1000 individual-based simulations. We tested the model outputs— Pf PR 2–10 and incidence rate of malaria admissions—against three regression models, namely an intercept-only, a log-logistic, and a log-linear model, which was previously used to evaluate the prevalence-hospitalization relationship 10 .
The historical dataset shows higher levels of hospitalization rates at similar prevalence, consistent with the expected reduction of comorbidities and improved management effectiveness in the contemporary dataset (Fig. 2 and Supplementary Note 5 ). Figure 2a, b shows (1) the historical (Fig. 2a ) and contemporary (Fig. 2b ) empirical estimates, (2) their respective time-frame-specific simulations under the assumption of steady exposure, and (3) the regression-based predicted relationship, shown as the median and 50% and 95% prediction intervals. For both the historical and the contemporary predictions, the log-logistic regression model provides a better fit to the model outputs, with the asymptote reaching around 60% and 40% Pf PR 2–10 , respectively. However, while the prediction model based on steady transmission is consistent with the historical empirical data, it fails to recover the contemporary dataset relationship, with a substantial number of time-sites outlying from the predictions range, particularly for the highest ePf PR 2–10 values (i.e., over 60%).
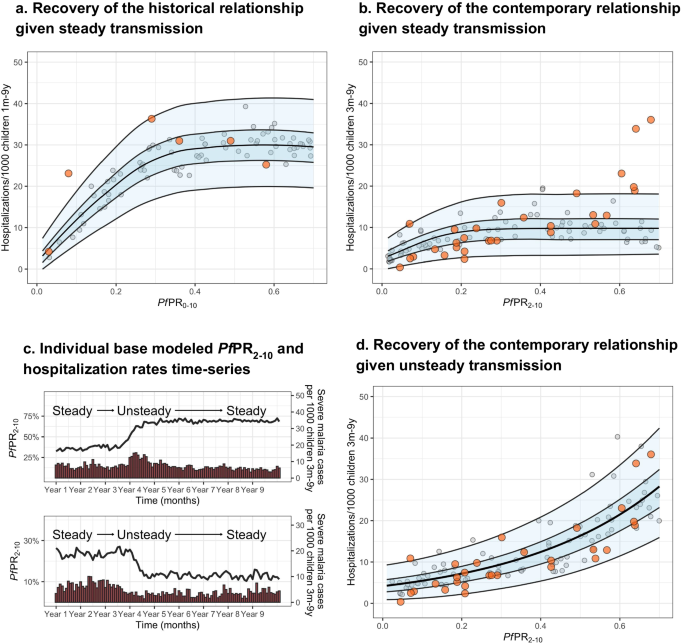
Showing the empirical Pf PR 2–10 -hospitalization rates relationship obtained from a the historical dataset (orange dots), and b the contemporary dataset (orange dots) overlapping respective Pf PR-hospitalization model-based estimates obtained through simulations consistent with steady transmission (gray dots, n = 100) and respective levels of health care access, treatment and comorbidities ( b ), and the best-fit model-based log-logistic regressions (median black line, blue ribbons 50% and 95% prediction intervals). c Representative simulation patterns of Pf PR 2–10 (black lines, increasing at the top and decreasing at the bottom) and subsequent malaria hospitalization incidence over time (red columns). d The empirical Pf PR 2–10 -hospitalization rates relationship obtained from the contemporary dataset (orange dots) overlapping Pf PR 2–10 -severity model-based estimates obtained through simulations scenarios consistent with time-varying exposure (gray dots, n = 100) and levels in health care access, treatment, and comorbidities, and the best-fit model-based log-linear regression (median black line, blue ribbons 50% and 95% prediction intervals).
Given that model predictions based on steady exposure do not capture the pattern of the empirical contemporary data, we further evaluated scenarios with unsteady past malaria transmission (either increasing or decreasing dynamics), and how these different trends affect the Pf PR 2–10 -hospitalization rate incidence relationship. Specifically, we performed simulations that included step-up and step-down exposure dynamics with differences between pre- and intra-survey prevalence within the range of those observed empirically (see Methods). Simulations captured representative patterns of time-varying exposure, with decreasing, steady or increasing transmission before computing severe disease incidence (Fig. 2c ) over the range of Pf PR 2–10 values. The best fit to a regression model is then obtained using the log-linear model (Fig. 2d ). Based on performance metrics 16 , simulations under the assumption of unsteady patterns of past exposure predict the relationship of the contemporary dataset more accurately than those based on steady exposure. Further, modeled predictions under unsteady exposure show hospitalization rates in different age groups increase towards higher ePf PR 2–10 in a similar way as observed in the empirical estimates (Fig. S2 ). However, this is opposite to the pattern in historical observations under steady state exposure; severe disease incidence among youngest children is typically higher in high transmission settings than in low transmission settings, whereas the opposite occurs among older children 4 , 7 , 8 , 9 . Consistent with these results, our model recovers more accurately the observed hospitalization age structure (Fig. S3 , left column for representative time-sites) under the unsteady transmission assumption (Fig. S3 , middle column) than under the steady-state assumption (Fig. S3 , right column). Further, when assessing the model estimates of hospitalization risk later in time (i.e., allowing the scenarios to maintain a steady-state level transmission over 5 years), the prevalence-hospitalization rates relationship transitions to the asymptotic pattern expected for steady transmission (Fig. S4 ).
Via scenario analysis, we have systematically evaluated the major clinical and epidemiological determinants influencing the occurrence of malaria hospitalization upon infection at the population level and thus proved a contemporary characterization of relationship trends and changes between malaria community prevalence and life-threatening disease risk in children. We found that the asymptotic relationship between prevalence and hospitalization disease risk, expected under a relatively steady transmission, is lost when children have been exposed to unstable, time-varying past malaria transmission. Overall, our analyses support the assumption that substantial fluctuations in malaria transmission over the years have led to a particular prevalence-hospitalization relationship observed among the East-African settings 10 , where increasing prevalence does not necessarily lead to saturating disease risk but increases toward the highest rates in an exponential manner. However, our analyses show that if transmission is further maintained at a steady-state level over sufficient time, disease risk would also eventually re-equilibrate back to the asymptotic relationship relative to the parasite rates (fig. S4 ). To date, inference frameworks aiming to estimate or predict severe outcomes of malaria used for policy decisions and public health action do not explicitly include past exposure as an independent variable 5 , 17 . Our analysis framework is capable of reconciling historical and contemporary observations encompassing three decades in sub-Saharan Africa and underscores the importance of taking the variability of past malaria exposure among children into account when predicting severe disease risk.
Our analyses have several limitations that need to be acknowledged. First, we use community-based hospitalization in non-urban settings as an empirical proxy of the incidence of severe outcomes of malaria. While these estimates could under- or over-estimate the true number of severe outcomes, the data was obtained aiming to standardized the under-ascertainment of cases, estimates would be affected by site specific treatment-seeking behaviors and therefore represent a lower limit for hospitalization rate at each time site. Also, the empirical contemporary dataset does not necessarily represent urban settings, where the referral pathways from infection to hospitalization might be more complex to understand or subject to other potential biases. Further, the curated data does not include cases where malaria was not the major syndrome for hospitalization. Our modeling approach allows access to health (i.e., access to diagnosis, treatment, and/or hospitalization) to randomly vary within the range of the rates estimated for the three countries, with sensitivity analysis showing that deviations from this assumption do not influence the overall relationship between prevalence and hospitalization. Nevertheless, if the data represents stronger deviations from these assumptions regarding case identification but remains relatively similar across time sites, the overall prevalence-severe disease trend will still hold. Second, the empirical Pf PR estimates obtained through community and school surveys might not necessarily reflect the underlying prevalence dynamics for the full catchment population, given how heterogeneous malaria exposure can be at a very granular spatial level. However, we obtain a similar prevalence-hospitalization relationship using estimates computed using the geospatial model (see Section “Discussion”, Fig. S10 ), thus supporting the assumption of the empirical values being a representative summarizing value for the catchment populations. Also, the time series of Pf PR values used to compute exposure steadiness can bear high uncertainty on the precise estimates. Nevertheless, given that we did not aim to replicate the prevalence changes over time but addressed this matter focusing on the relative change, our framework will remain well informed if the estimates approximate true trends. Third, our model OpenMalaria simulations are based on spatially homogeneous malaria transmission because the catchment populations in the empirical data are small. Assuming a heterogeneous transmission structure can affect the magnitude of the clinical outcomes, central estimates will not change 18 . Fourth, while parameterization of the hospitalization rates, efficacy of treatment, and comorbidities are consistent with the literature, for simplicity, we assumed similar ranges of values across time-sites with stochastic variation. In Supplementary Note 4 , we provide an uncertainty analysis of these mechanistic parameters, showing that our results hold under no major deviations from tenable assumptions. Last, we have applied parametric regression models to simulation data, which likely misspecifies the mechanical interpretation of the model. Still, we believe this is justified given this approach was originally used to determine the empirical relationship 10 and we aimed to replicate the trends under potential plausible mechanistic scenarios.
We have provided evidence that variation in malaria transmission and subsequent disease protection after life-long exposure can strongly influence severe disease risk estimates under otherwise equivalent ongoing force of transmission. Notably, frequent implementation and withdrawal of infection prevention strategies can strongly contribute to unsteady malaria exposure patterns and thus increase severe disease risk. Other potential sources of variability in the exposure include substantial movement of individuals from areas with different community prevalence (i.e., via increasing or reducing the overall population-level susceptibility to severe disease) or strong environmental changes influencing entomological inoculation rates such as urbanization or climatic drivers (i.e., prolonged drought or excessive rainfall).
Under constant, similar malaria exposure and health access rates, a population of children with higher immunity will substantially reduce their risk of severe malaria and, therefore, the number of severe cases. However, if malaria transmission has marked changes, either sudden reductions or increases, the resulting severe disease will be significantly lower or higher, respectively, than if the population had remained under constant transmission. This understanding is critical to evaluate the effects of interventions, and such mechanistic processes need to be included in future analytical approaches providing predictions of malaria disease burden. The processes that must be incorporated into disease burden estimates are best defined through differences in the age–structure patterns of the risk conditional to past exposure. For example, it is expected that following a strong reduction in malaria transmission, severe cases among age groups of children at a certain prevalence will be reduced earlier in time but will likely increase in later periods if malaria prevalence reaches steady levels. Similarly, the withdrawal of effective prevention and control strategies will lead to a higher number of severe cases than those expected when malaria prevalence has remained unchanged over time. In short, if past exposure and the dynamics described here are not accounted for in burden estimates, it will lead to long-term overestimation of severe malaria risk in places with recent effective interventions. And conversely, it will lead to long-term underestimation of severe malaria in places with deterioration of interventions. Finally, our findings underpin the need to build back rigorous clinical surveillance of severe malaria under the changing landscape of parasite exposure in Africa. It is striking that only two longitudinal clinical series exist since the launch of the Roll Back Malaria initiative in 2000 19 , 20 .
Overall, our findings provide evidence that inference in malaria epidemiology, such as the generation of counterfactual scenarios with predictions on clinical outcomes for policy decisions, should account from now on past exposure and subsequent protection to avoid substantial bias in such risk predictions and highlight the increase in the complexity of malaria epidemiology arising from unsteady transmission dynamics.
Contemporary malaria hospitalization incidence data and paired community prevalence estimates
The contemporary P. falciparum malaria hospitalization incidence data has been obtained from 35 time-sites in Kenya ( n = 18), Uganda ( n = 14), and Tanzania ( n = 3) between 2006 and 2020 and has been previously described elsewhere 10 . The data was collated from individual records of 21 hospitals, including hospitalized malaria cases in children resident in specific catchment areas within a 30 km range and excluding urban settings. Given potential differences in treatment-seeking behaviors at the different sites, the computed estimates represent the lower limit for hospitalization incidence, thus requiring further assumptions for adjusting to under ascertainment (see model parameterization in the Supplementary Text). for In the present analysis, cases were included if malaria was deemed the primary cause of hospitalization. Per each time-site, the number of hospitalizations among children 3 months to 9 years old per 1000 children per year was computed as a proxy of life-threatening disease incidence from the catchment population, obtained from census data and census data projections. Hospitalization data was paired with community surveys performed at the same periods of the hospital surveys for each time-site, adjusting for diagnostic accuracy (i.e., microscopy vs. rapid test) and standardized by computing the Pf PR 2–10 as described elsewhere 21 , 22 . Further details on hospitalization data, community prevalence, and estimates of hospital catchment population can be found in Supplementary Note 1 .
Modeled community prevalence time-series
We assessed the past exposure dynamics using catchment site-specific time series of modeled age- and test-standardized parasite rates estimates, referred to as mPf PR 2–10 13 , 15 . Modeled mPf PR 2–10 estimates were explicitly obtained for each time-site catchment population using a geospatial model detailed elsewhere 13 , 15 , a Bayesian hierarchical geostatistical framework based on more than 180000 geo-coded empirical prevalence survey data points from East Africa, interpolated in time to 1 × 1 km resolutions using climatic and ecological covariates. For a set of sites ( n = 11), annual mPf PR 2–10 estimates are available since 2000, while for the rest ( n = 15) available data begin in 2010. Time series of all the available mPf PR 2–10 per site are shown in Fig. S1 . Thus, for 27 out of the 35 present-day time sites, mPf PR 2–10 time series included at least seven-time points of past annual estimates. Further, to estimate the gap between past and present transmission, we first computed the median value of the annual mPf PR 2–10 time-series for each of the time-sites, under the assumption that it roughly represents the life-long transmission at which the surveyed population of children have been exposed in the previous years previous. This median mPf PR 2–10 can then be compared to the empirical Pf PR 2–10 that is estimated at the time of surveying the malaria hospitalization incidence.
Historical malaria hospitalization incidence data and paired community prevalence estimates
As an alternative source of data, we analyzed a historical dataset obtained between 1992 and 1997, encompassing the relation between severe disease incidence measured as malaria hospitalization rates and the Pf PR 2–10 up to 70% and published elsewhere 9 . Similar inclusion and exclusion criteria had been used to obtain both datasets explicitly to allow comparison. Thus, comparable approaches to those used in the contemporary dataset were used to estimate malaria hospitalization incidence at six hospital time-site locations in The Gambia ( n = 3), Kenya ( n = 2), and Malawi ( n = 1) and paired community prevalence estimates 7 , 9 , 11 . Further details can be found in Supplementary Note 1 .
Agent-based model of malaria transmission, immunity, and disease dynamics
To recover the relationship between hospitalization rates and Pf PR 2–10 , we used an individual-based model of P. falciparum malaria transmission and disease dynamics, OpenMalaria, previously described and calibrated elsewhere 12 . Briefly, OpenMalaria features within-host parasite dynamics, the progression of clinical disease, development of immunity, individual care-seeking behavior, vector exposure, and pharmaceutical and non-pharmaceutical antimalarial interventions at vector and human level ( https://github.com/SwissTPH/openmalaria/wiki ) 14 , 23 , 24 . The full model is calibrated to fit 23 parameters to 11 objectives representing different epidemiological outcomes, including age-specific prevalence and incidence patterns, age-specific mortality rates, and hospitalization rates, using a Bayesian optimization approach 12 . A detailed description of the model and references for the key technical details are provided in Supplementary Note 2 .
Model parameterization on disease management, comorbidities, and health access
In order to compare model predictions to empirical data, we first parameterized several key inputs of the individual-based model to be consistent with both historical and contemporary datasets and the corresponding existing clinical and epidemiological knowledge, including different sets of variables addressing (a) the individual probability of malaria exposure per time-step to cover a range of Pf PR 2–10 up to 70%, (b) the effectiveness in malaria case management, including the rate of access of uncomplicated and severe malaria cases to health-care (i.e., rate of accurate diagnostic of true occurrence and subsequent timely treatment) and the efficacy of the available malaria drugs at each period (e.g., the efficacy of artemisinin derivatives combination therapies in clearing malaria), and (c) the co-occurrence of other diseases with influence on malaria that affect the severe progression of malaria across age-groups.
To evaluate the contemporary dataset, model parameterization for the main analysis was as follows: (1) Pf PR 2–10 estimates ranged up to 70%; (2) drug efficacy ranged between 85 and 100% 25 , 26 ; (3) the rates of individuals suffering from malaria that required hospitalization who received diagnostic and treatment ranged from 60 to 90% while the rate of individuals with uncomplicated malaria accessing diagnostic and treatment ranged 20–60% 27 ; and (4) co-occurrence of diseases contributing to malaria hospitalization was substantially reduced by 60–80% since the 1990s 28 , 29 , 30 . For (2–4), we parameterized the model by randomly sampling values from a uniform distribution defined by the respective ranges. Sensitivity analysis for these assumptions can be found in the Supplementary Note 3 . To evaluate the historical dataset, we include the following: (1) Pf PR 2–10 estimates ranged up to 70% (2) first-line drug efficacy for uncomplicated malaria was negligible 31 ; (3) access rates of malaria requiring hospitalization ranged from 40–60% 8 , 32 , and (4) co-occurrence of diseases contributing to malaria hospitalization approximated a hyperbolic decay distribution over age-groups 8 . Details on key model parameters and submodels are provided in Supplementary Note 3 .
Time-varying malaria exposure on severity estimates
To evaluate how time-varying malaria exposure rates influence the ongoing Pf PR 2–10 -hospitalization rates relationship, we produced a set of plausible malaria-transmission scenarios consistent with time-varying exposure as for those obtained from a Bayesian hierarchical geostatistical framework 13 , 15 across all our time-sites. Particularly, we evaluated how a rapid reduction or increase of the Pf PR 2–10 level over 6 months (e.g., Pf PR 2–10 increasing from 10% to 70%) could affect the severe malaria incidence relative to a steady-state transmission assumption described before. This was performed by setting (1) the initial exposure rate (i.e., the individual probability of exposure to infectious bites pre-survey as a single rate over simulation time-steps), independent from (2) the final exposure rate (i.e., the probability of exposure during the survey period, also as a single rate over time steps). For simplicity, we set constant initial and final exposure rates. The relationship between the initial and final exposure rate was parameterized to reflect archetypical mPf PR 2–10 patterns,—depicted in Fig. 1b —showing (1) a substantial increase in the mPf PR 2–10 (e.g., Apac A), (2) relative constant mPf PR 2–10 (e.g., Busia), and (3) substantial decrease in the Pf PR 2–10 (e.g., Jinja B). Thus, our definition of time-varying (unsteady) exposure comprises both substantial increasing and decreasing dynamics. For each time site with retrospective data encompassing at least 7 years, we computed the average mPf PR 2–10 available up to 9 years prior to the date when the hospitalization data was available, used it as a proxy of the initial exposure rate, and computed the fold-change Pf PR 2–10 . We then performed a local polynomial regression model to obtain predictions of the relationship between the final exposure rate and the corresponding relative change per time site. See fig. S5 in Supplementary depicting the relationship between the contemporary community prevalence as ePf PR 2–10 , and estimated relative change at survey computed from the median value of mPf PR 2–10 of the past 7–9 years for each time-site. We then used these inputs of exposure to produce simulations using a combination of initial and final exposure rates to map a range of simulation-based Pf PR 2–10 -hospitalization rates.
Scenario analysis to recover the empirical relationship
To evaluate under which scenarios OpenMalaria can recover the empirical prevalence-hospitalization, we implemented an iterative 4-step procedure to explore hypotheses of the impact of the determinants on disease risk and changes in disease risk—see sections above for details. The procedure is represented schematically in the Supplementary (Fig. S6 ). For each scenario analysis, we performed four iterative steps as follows:
Using a high-performance computing framework ( http://scicore.unibas.ch/ ) we performed 1000 population-level individual-based model simulations of malaria transmission at a steady-state (i.e., same entomological inoculation rate for each simulation) over long periods of time (i.e., over 90 years which ensures that lifelong malaria exposure has occurred all the generations evaluated prospectively); computing hospitalization rates among children 3 months- 9 years per 1000 persons-year across values of Pf PR 2–10 within the input range. In each of the simulations, we parameterized the model, mapping the range of values set up for the major epidemiological determinants described in the previous section, namely disease management (which includes rates and efficacy of diagnostic and treatment of severe and uncomplicated malaria) and co-occurrence of comorbidities. We set the parameters according to the empirical dataset we were evaluating (i.e., historical or contemporary). At the same time, we evaluated the model outcomes simulating steady and unsteady transmission.
In order to assess how accurately the model simulations represent the empirical data, we evaluated the performance of two regression models to recover the prevalence-hospitalization relationship obtained via scenario analysis, namely a log-linear model and a log-logistic model. The approach was chosen to be consistent with the previous analysis framework of the contemporary dataset 10 , which analyses severe malaria syndrome-specific cases against prevalence. See Supplementary Note 3 for details on the regression models, model selection criteria, and computation of uncertainty estimates.
We used the best-fit regression model among the above to evaluate how the modeling framework predicted the empirical ePf PR 2–10 -hospitalization incidence relationship. Specifically, we evaluated the accuracy of the model predictions using prediction performance metrics 16 (see Supplementary Note 3 and Table S2 ).
We updated model assumptions based on the evaluation above and reparametrized the scenario(/s) accordingly.
Evaluation of key assumptions for scenario modeling
To evaluate the robustness of the simulation-based predictions of the Pf PR 2–10 -hospitalization relationship under time-varying exposure, we performed three major sensitivity analyses: (a) how increased or reduced rates of both uncomplicated case management and hospitalizations at the population level influence the severe disease incidence and prevalence relationship; (b) how the relationship changes over higher or lower drug efficacy estimates; (c) how changes on the incidence of comorbidities influence the severe disease risk. Details of the evaluation are provided in Supplementary Note 4 and Figs. S7 and S8 .
The age structure of the severity estimates and the effect of time-varying malaria
Last, to assess if the model framework recovers hospitalization risk over different age groups, we compared both empirical and simulation-based estimates disaggregated by age. We performed the 4-steps iterative analysis described above for the contemporary dataset under the steady and time-varying transmission assumptions and evaluated the Pf PR 2–10 -hospitalization relationship by age. More specifically, we computed hospitalization incidence over age groups at values of Pf PR2–10 equal to those estimated in the for four representative time sites Apac A, Busia, Mubende B, and Jinja B. Further details on the age-structured analysis are provided in Supplementary Note 5 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Empirical contemporary data used in this analysis have been curated and uploaded to the Harvard Dataverse: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/XGDB3K . Correspondence and requests for materials should be addressed to the KEMRI Wellcome Data Governance Committee ([email protected]). These data are available through a formal requesting process to the KEMRI Institutional Data Access/Ethics Committee. Guideline details can be found on the KEMRI Wellcome website: https://kemri-wellcome.org/about-us/#ChildVerticalTab_15 .
Code availability
Model code, plotting code, and simulation data are available at https://github.com/PDeSalazarSwissTPH/SevereMalaria .
Snow, R. W. Sixty years trying to define the malaria burden in Africa: have we made any progress? BMC Med. 12 , 227 (2014).
Article PubMed PubMed Central Google Scholar
White, N. J., Day, N. P. J., Ashley, E. A., Smithuis, F. M. & Nosten, F. H. Have we really failed to roll back malaria? Lancet 399 , 799–800 (2022).
Camponovo, F., Bever, C. A., Galactionova, K., Smith, T. & Penny, M. A. Incidence and admission rates for severe malaria and their impact on mortality in Africa. Malar. J. 16 , 1 (2017).
Griffin, J. T. et al. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. Biol. Sci. 282 , 20142657 (2015).
PubMed PubMed Central Google Scholar
Weiss, D. J. et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum , 2000-17: a spatial and temporal modelling study. Lancet 394 , 322–331 (2019).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 , 207–211 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Snow, R. & Marsh, K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv. Parasitol. https://doi.org/10.1016/s0065-308x(02)52013-3 (2002).
Ross, A., Maire, N., Molineaux, L. & Smith, T. An epidemiologic model of severe morbidity and mortality caused by Plasmodium falciparum . Am. J. Trop. Med. Hyg. 75 , 63–73 (2006).
Article PubMed Google Scholar
Marsh, K. & Snow, R. W. Malaria transmission and morbidity. Parassitologia 41 , 241–246 (1999).
Paton, R. S. et al. Malaria infection and severe disease risks in Africa. Science 373 , 926–931 (2021).
Snow, R. W. et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. The Lancet https://doi.org/10.1016/s0140-6736(97)02038-2 (1997).
Reiker, T. et al. Emulator-based Bayesian optimization for efficient multi-objective calibration of an individual-based model of malaria. Nat. Commun. 12 , 7212 (2021).
Kamau, A. et al. Malaria hospitalisation in East Africa: age, phenotype and transmission intensity. BMC Med. 20 , 28 (2022).
Smith, T. et al. Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: overview. Am. J. Trop. Med. Hyg. 75 , 1–10 (2006).
Alegana, V. A. et al. Plasmodium falciparum parasite prevalence in East Africa: Updating data for malaria stratification. PLOS Global Public Health https://doi.org/10.1371/journal.pgph.0000014 (2021).
Dziak, J. J., Coffman, D. L., Lanza, S. T., Li, R. & Jermiin, L. S. Sensitivity and specificity of information criteria. Brief. Bioinform. 21 , 553–565 (2020).
World malaria report. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (2023).
Penny, M. A. et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet 387 , 367–375 (2016).
Njuguna, P. et al. Observational study: 27 years of severe malaria surveillance in Kilifi, Kenya. BMC Med. 17 , 124 (2019).
Guinovart, C. et al. The epidemiology of severe malaria at Manhiça District Hospital, Mozambique: a retrospective analysis of 20 years of malaria admissions surveillance data. Lancet Glob. Health 10 , e873–e881 (2022).
Article CAS PubMed Google Scholar
Smith, D. L., Guerra, C. A., Snow, R. W. & Hay, S. I. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar. J. 6 , 1–10 (2007).
Article Google Scholar
Mappin, B. et al. Standardizing Plasmodium falciparum infection prevalence measured via microscopy versus rapid diagnostic test. Malar. J. 14 , 460 (2015).
Smith, T. et al. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology 135 , 1507–1516 (2008).
Smith, T. et al. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 9 , e1001157 (2012).
World Health Organization. Guidelines for the Treatment of Malaria . (World Health Organization, 2010).
World Health Organization. Guidelines for the Treatment of Malaria . Third Edition. (World Health Organization, 2015).
The DHS Program. https://dhsprogram.com/methodology/survey-types/mis.cfm .
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390 , 1151–1210 (2017).
Reiner, R. C. et al. Identifying residual hotspots and mapping lower respiratory infection morbidity and mortality in African children from 2000 to 2017. Nat. Microbiol. 4 , 2310–2318 (2019).
Reiner, R. C. Jr et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000-2015. N. Engl. J. Med. 379 , 1128–1138 (2018).
Amin, A. A. et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar. J. 6 , 72 (2007).
Goodman, C. A., Coleman, P. G. & Mills, A. Economic analysis of malaria control in sub-Saharan Africa. (Global Forum for Health Research, Geneva, 2000).
Download references
Acknowledgements
Calculations were performed at sciCORE ( http://scicore.unibas.ch/ ) scientific computing center at the University of Basel. Funding : This study was partly funded by Bill & Melinda Gates Foundation (INV- 025569 to MAP) supporting M.A.P. and P.M.S. M.A.P. also received support via a Swiss National Science Foundation Professorship (PP00P3_203450 to M.A.P.); R.W.S. is supported under a Wellcome Trust Principal Research Fellowship (212176/Z/18/Z), which also provided support for A.K.; R.W.S., A.K. and S.A. are grateful for the support from the Wellcome Trust to the Core Award for the East Africa Major Overseas Program (203077/Z/16/Z). S.A. was supported by the CDC Foundation through funding from the World Health Organization for the RTS,S evaluation (project requisition number 2018/854999).
Author information
These authors contributed equally: Robert W. Snow, Melissa A. Penny.
Authors and Affiliations
Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
Pablo M. De Salazar, Aurelien Cavelan & Melissa A. Penny
University of Basel, Basel, Switzerland
Kenya Medical Research Institute (KEMRI)-Wellcome Trust Research Programme, Nairobi, Kenya
Alice Kamau, Samuel Akech & Robert W. Snow
Child Health and Development Centre, College of Health Sciences, Makerere University, Kampala, Uganda
Arthur Mpimbaza
Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK
Robert W. Snow
Telethon Kids Institute, Nedlands, WA, Australia
Melissa A. Penny
Centre for Child Health Research, University of Western Australia, Crawley, WA, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: P.M.S., M.A.P., A.K., R.W.S.; methodology: P.M.S., M.A.P., R.W.S.; investigation: P.M.S., S.A., A.M., A.K., A.C., R.W.S., M.A.P.; visualization: P.M.S., M.A.P.; funding acquisition: M.A.P., R.W.S.; writing—original draft: P.M.S., M.A.P., R.W.S.; writing—review & editing: P.M.S., M.A.P., R.W.S., A.K., A.C., S.A., A.M.
Corresponding authors
Correspondence to Pablo M. De Salazar or Melissa A. Penny .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics declaration
Kenya hospital surveillance: Kenya Medical Research Institute/ Scientific and Ethics Review Unit (KEMRI SERU) IRB numbers 1433, 3057, 3149; 1801, 2558, 2465, 3459, and 3771; Uganda hospital surveillance approved by the CDC as public health surveillance/non-research (NCEZID 031416), Tanzania hospital surveillance approved by the ethics committees of the National Institute for Medical Research, Tanzania, and the London School of Hygiene and Tropical Medicine. Kenya community parasite surveys KEMRI SERU IRB numbers 3149, 3592, 1801, 2558, 2675, 2801, and 3822; Uganda community prevalence surveys Vector Control Division Research Ethics Committee, MoH, Kampala: VCDREC/117.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
De Salazar, P.M., Kamau, A., Cavelan, A. et al. Severe outcomes of malaria in children under time-varying exposure. Nat Commun 15 , 4069 (2024). https://doi.org/10.1038/s41467-024-48191-7
Download citation
Received : 31 August 2023
Accepted : 22 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41467-024-48191-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.

Contact | Patient Info | Foundation | AASM Engage JOIN Today Login with CSICloud
- AASM Scoring Manual
- Artificial Intelligence
- COVID-19 Resources
- EHR Integration
- Emerging Technology
- Patient Information
- Practice Promotion Resources
- Provider Fact Sheets
- #SleepTechnology
- Telemedicine

- Annual Meeting
- Career Center
- Case Study of the Month
- Change Agents Submission Winners
- Compensation Survey
- Conference Support
- Continuing Medical Education (CME)
- Maintenance of Certification (MOC)
- State Sleep Societies
- Talking Sleep Podcast
- Young Investigators Research Forum (YIRF)

- Leadership Election
- Board Nomination Process
- Membership Directory
- Volunteer Opportunities
- International Assembly

- Accreditation News
- Accreditation Verification
- Program Changes

AASM accreditation demonstrates a sleep medicine provider’s commitment to high quality, patient-centered care through adherence to these standards.
- AASM Social Media Ambassador
- Advertising
- Affiliated Sites
- Autoscoring Certification
- Diversity, Equity and Inclusion
- Event Code of Conduct Policy
- Guiding Principles for Industry Support
- CMSS Financial Disclosure
- IEP Sponsors
- Industry Programs
- Newsletters
- Patient Advocacy Roundtable
- President’s Report
- Social Media
- Strategic Plan
- Working at AASM
- Practice Standards
- Coding and Reimbursement
- Choose Sleep
- Advanced Practice Registered Nurses and Physician Assistants (APRN PA)
- Accredited Sleep Technologist Education Program (A-STEP)
- Inter-scorer Reliability (ISR)
- Coding Education Program (A-CEP)
- Individual Member – Benefits
- Individual Member – Categories
- Members-Only Resources
- Apply for AASM Fellow
- Individual Member – FAQs
- Facility Member – Benefits
- Facility Member – FAQs
- Sleep Team Assemblies
- Types of Accreditation
- Choose AASM Accreditation
- Special Application Types
- Apply or Renew

Case Study of the Month – May 2024
Members only resource, share this story, choose your platform, related posts.

Case Study of the Month – April 2024

Case Study of the Month – March 2024
- Open access
- Published: 15 May 2024
Learning together for better health using an evidence-based Learning Health System framework: a case study in stroke
- Helena Teede 1 , 2 na1 ,
- Dominique A. Cadilhac 3 , 4 na1 ,
- Tara Purvis 3 ,
- Monique F. Kilkenny 3 , 4 ,
- Bruce C.V. Campbell 4 , 5 , 6 ,
- Coralie English 7 ,
- Alison Johnson 2 ,
- Emily Callander 1 ,
- Rohan S. Grimley 8 , 9 ,
- Christopher Levi 10 ,
- Sandy Middleton 11 , 12 ,
- Kelvin Hill 13 &
- Joanne Enticott ORCID: orcid.org/0000-0002-4480-5690 1
BMC Medicine volume 22 , Article number: 198 ( 2024 ) Cite this article
2 Altmetric
Metrics details
In the context of expanding digital health tools, the health system is ready for Learning Health System (LHS) models. These models, with proper governance and stakeholder engagement, enable the integration of digital infrastructure to provide feedback to all relevant parties including clinicians and consumers on performance against best practice standards, as well as fostering innovation and aligning healthcare with patient needs. The LHS literature primarily includes opinion or consensus-based frameworks and lacks validation or evidence of benefit. Our aim was to outline a rigorously codesigned, evidence-based LHS framework and present a national case study of an LHS-aligned national stroke program that has delivered clinical benefit.
Current core components of a LHS involve capturing evidence from communities and stakeholders (quadrant 1), integrating evidence from research findings (quadrant 2), leveraging evidence from data and practice (quadrant 3), and generating evidence from implementation (quadrant 4) for iterative system-level improvement. The Australian Stroke program was selected as the case study as it provides an exemplar of how an iterative LHS works in practice at a national level encompassing and integrating evidence from all four LHS quadrants. Using this case study, we demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world research for optimising healthcare improvement. We emphasize the transition from research as an endpoint, to research as an enabler and a solution for impact in healthcare improvement.
Conclusions
The Australian Stroke program has nationally improved stroke care since 2007, showcasing the value of integrated LHS-aligned approaches for tangible impact on outcomes. This LHS case study is a practical example for other health conditions and settings to follow suit.
Peer Review reports
Internationally, health systems are facing a crisis, driven by an ageing population, increasing complexity, multi-morbidity, rapidly advancing health technology and rising costs that threaten sustainability and mandate transformation and improvement [ 1 , 2 ]. Although research has generated solutions to healthcare challenges, and the advent of big data and digital health holds great promise, entrenched siloes and poor integration of knowledge generation, knowledge implementation and healthcare delivery between stakeholders, curtails momentum towards, and consistent attainment of, evidence-and value-based care [ 3 ]. This is compounded by the short supply of research and innovation leadership within the healthcare sector, and poorly integrated and often inaccessible health data systems, which have crippled the potential to deliver on digital-driven innovation [ 4 ]. Current approaches to healthcare improvement are also often isolated with limited sustainability, scale-up and impact [ 5 ].
Evidence suggests that integration and partnership across academic and healthcare delivery stakeholders are key to progress, including those with lived experience and their families (referred to here as consumers and community), diverse disciplines (both research and clinical), policy makers and funders. Utilization of evidence from research and evidence from practice including data from routine care, supported by implementation research, are key to sustainably embedding improvement and optimising health care and outcomes. A strategy to achieve this integration is through the Learning Health System (LHS) (Fig. 1 ) [ 2 , 6 , 7 , 8 ]. Although there are numerous publications on LHS approaches [ 9 , 10 , 11 , 12 ], many focus on research perspectives and data, most do not demonstrate tangible healthcare improvement or better health outcomes. [ 6 ]
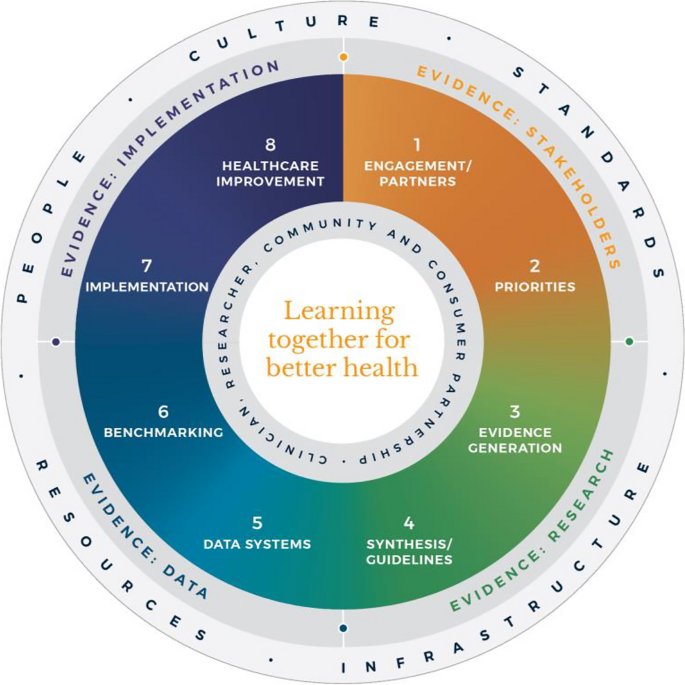
Monash Learning Health System: The Learn Together for Better Health Framework developed by Monash Partners and Monash University (from Enticott et al. 2021 [ 7 ]). Four evidence quadrants: Q1 (orange) is evidence from stakeholders; Q2 (green) is evidence from research; Q3 (light blue) is evidence from data; and, Q4 (dark blue) is evidence from implementation and healthcare improvement
In developed nations, it has been estimated that 60% of care provided aligns with the evidence base, 30% is low value and 10% is potentially harmful [ 13 ]. In some areas, clinical advances have been rapid and research and evidence have paved the way for dramatic improvement in outcomes, mandating rapid implementation of evidence into healthcare (e.g. polio and COVID-19 vaccines). However, healthcare improvement is challenging and slow [ 5 ]. Health systems are highly complex in their design, networks and interacting components, and change is difficult to enact, sustain and scale up. [ 3 ] New effective strategies are needed to meet community needs and deliver evidence-based and value-based care, which reorients care from serving the provider, services and system, towards serving community needs, based on evidence and quality. It goes beyond cost to encompass patient and provider experience, quality care and outcomes, efficiency and sustainability [ 2 , 6 ].
The costs of stroke care are expected to rise rapidly in the next decades, unless improvements in stroke care to reduce the disabling effects of strokes can be successfully developed and implemented [ 14 ]. Here, we briefly describe the Monash LHS framework (Fig. 1 ) [ 2 , 6 , 7 ] and outline an exemplar case in order to demonstrate how to apply evidence-based processes to healthcare improvement and embed real-world research for optimising healthcare. The Australian LHS exemplar in stroke care has driven nationwide improvement in stroke care since 2007.
An evidence-based Learning Health System framework
In Australia, members of this author group (HT, AJ, JE) have rigorously co-developed an evidence-based LHS framework, known simply as the Monash LHS [ 7 ]. The Monash LHS was designed to support sustainable, iterative and continuous robust benefit of improved clinical outcomes. It was created with national engagement in order to be applicable to Australian settings. Through this rigorous approach, core LHS principles and components have been established (Fig. 1 ). Evidence shows that people/workforce, culture, standards, governance and resources were all key to an effective LHS [ 2 , 6 ]. Culture is vital including trust, transparency, partnership and co-design. Key processes include legally compliant data sharing, linkage and governance, resources, and infrastructure [ 4 ]. The Monash LHS integrates disparate and often siloed stakeholders, infrastructure and expertise to ‘Learn Together for Better Health’ [ 7 ] (Fig. 1 ). This integrates (i) evidence from community and stakeholders including priority areas and outcomes; (ii) evidence from research and guidelines; (iii) evidence from practice (from data) with advanced analytics and benchmarking; and (iv) evidence from implementation science and health economics. Importantly, it starts with the problem and priorities of key stakeholders including the community, health professionals and services and creates an iterative learning system to address these. The following case study was chosen as it is an exemplar of how a Monash LHS-aligned national stroke program has delivered clinical benefit.
Australian Stroke Learning Health System
Internationally, the application of LHS approaches in stroke has resulted in improved stroke care and outcomes [ 12 ]. For example, in Canada a sustained decrease in 30-day in-hospital mortality has been found commensurate with an increase in resources to establish the multifactorial stroke system intervention for stroke treatment and prevention [ 15 ]. Arguably, with rapid advances in evidence and in the context of an ageing population with high cost and care burden and substantive impacts on quality of life, stroke is an area with a need for rapid research translation into evidence-based and value-based healthcare improvement. However, a recent systematic review found that the existing literature had few comprehensive examples of LHS adoption [ 12 ]. Although healthcare improvement systems and approaches were described, less is known about patient-clinician and stakeholder engagement, governance and culture, or embedding of data informatics into everyday practice to inform and drive improvement [ 12 ]. For example, in a recent review of quality improvement collaborations, it was found that although clinical processes in stroke care are improved, their short-term nature means there is uncertainty about sustainability and impacts on patient outcomes [ 16 ]. Table 1 provides the main features of the Australian Stroke LHS based on the four core domains and eight elements of the Learning Together for Better Health Framework described in Fig. 1 . The features are further expanded on in the following sections.
Evidence from stakeholders (LHS quadrant 1, Fig. 1 )
Engagement, partners and priorities.
Within the stroke field, there have been various support mechanisms to facilitate an LHS approach including partnership and broad stakeholder engagement that includes clinical networks and policy makers from different jurisdictions. Since 2008, the Australian Stroke Coalition has been co-led by the Stroke Foundation, a charitable consumer advocacy organisation, and Stroke Society of Australasia a professional society with membership covering academics and multidisciplinary clinician networks, that are collectively working to improve stroke care ( https://australianstrokecoalition.org.au/ ). Surveys, focus groups and workshops have been used for identifying priorities from stakeholders. Recent agreed priorities have been to improve stroke care and strengthen the voice for stroke care at a national ( https://strokefoundation.org.au/ ) and international level ( https://www.world-stroke.org/news-and-blog/news/world-stroke-organization-tackle-gaps-in-access-to-quality-stroke-care ), as well as reduce duplication amongst stakeholders. This activity is built on a foundation and culture of research and innovation embedded within the stroke ‘community of practice’. Consumers, as people with lived experience of stroke are important members of the Australian Stroke Coalition, as well as representatives from different clinical colleges. Consumers also provide critical input to a range of LHS activities via the Stroke Foundation Consumer Council, Stroke Living Guidelines committees, and the Australian Stroke Clinical Registry (AuSCR) Steering Committee (described below).
Evidence from research (LHS quadrant 2, Fig. 1 )
Advancement of the evidence for stroke interventions and synthesis into clinical guidelines.
To implement best practice, it is crucial to distil the large volume of scientific and trial literature into actionable recommendations for clinicians to use in practice [ 24 ]. The first Australian clinical guidelines for acute stroke were produced in 2003 following the increasing evidence emerging for prevention interventions (e.g. carotid endarterectomy, blood pressure lowering), acute medical treatments (intravenous thrombolysis, aspirin within 48 h of ischemic stroke), and optimised hospital management (care in dedicated stroke units by a specialised and coordinated multidisciplinary team) [ 25 ]. Importantly, a number of the innovations were developed, researched and proven effective by key opinion leaders embedded in the Australian stroke care community. In 2005, the clinical guidelines for Stroke Rehabilitation and Recovery [ 26 ] were produced, with subsequent merged guidelines periodically updated. However, the traditional process of periodic guideline updates is challenging for end users when new research can render recommendations redundant and this lack of currency erodes stakeholder trust [ 27 ]. In response to this challenge the Stroke Foundation and Cochrane Australia entered a pioneering project to produce the first electronic ‘living’ guidelines globally [ 20 ]. Major shifts in the evidence for reperfusion therapies (e.g. extended time-window intravenous thrombolysis and endovascular clot retrieval), among other advances, were able to be converted into new recommendations, approved by the Australian National Health and Medical Research Council within a few months of publication. Feedback on this process confirmed the increased use and trust in the guidelines by clinicians. The process informed other living guidelines programs, including the successful COVID-19 clinical guidelines [ 28 ].
However, best practice clinical guideline recommendations are necessary but insufficient for healthcare improvement and nesting these within an LHS with stakeholder partnership, enables implementation via a range of proven methods, including audit and feedback strategies [ 29 ].
Evidence from data and practice (LHS quadrant 3, Fig. 1 )
Data systems and benchmarking : revealing the disparities in care between health services. A national system for standardized stroke data collection was established as the National Stroke Audit program in 2007 by the Stroke Foundation [ 30 ] following various state-level programs (e.g. New South Wales Audit) [ 31 ] to identify evidence-practice gaps and prioritise improvement efforts to increase access to stroke units and other acute treatments [ 32 ]. The Audit program alternates each year between acute (commencing in 2007) and rehabilitation in-patient services (commencing in 2008). The Audit program provides a ‘deep dive’ on the majority of recommendations in the clinical guidelines whereby participating hospitals provide audits of up to 40 consecutive patient medical records and respond to a survey about organizational resources to manage stroke. In 2009, the AuSCR was established to provide information on patients managed in acute hospitals based on a small subset of quality processes of care linked to benchmarked reports of performance (Fig. 2 ) [ 33 ]. In this way, the continuous collection of high-priority processes of stroke care could be regularly collected and reviewed to guide improvement to care [ 34 ]. Plus clinical quality registry programs within Australia have shown a meaningful return on investment attributed to enhanced survival, improvements in quality of life and avoided costs of treatment or hospital stay [ 35 ].
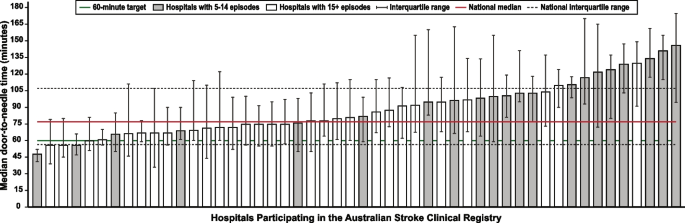
Example performance report from the Australian Stroke Clinical Registry: average door-to-needle time in providing intravenous thrombolysis by different hospitals in 2021 [ 36 ]. Each bar in the figure represents a single hospital
The Australian Stroke Coalition endorsed the creation of an integrated technological solution for collecting data through a single portal for multiple programs in 2013. In 2015, the Stroke Foundation, AuSCR consortium, and other relevant groups cooperated to design an integrated data management platform (the Australian Stroke Data Tool) to reduce duplication of effort for hospital staff in the collection of overlapping variables in the same patients [ 19 ]. Importantly, a national data dictionary then provided the common data definitions to facilitate standardized data capture. Another important feature of AuSCR is the collection of patient-reported outcome surveys between 90 and 180 days after stroke, and annual linkage with national death records to ascertain survival status [ 33 ]. To support a LHS approach, hospitals that participate in AuSCR have access to a range of real-time performance reports. In efforts to minimize the burden of data collection in the AuSCR, interoperability approaches to import data directly from hospital or state-level managed stroke databases have been established (Fig. 3 ); however, the application has been variable and 41% of hospitals still manually enter all their data.
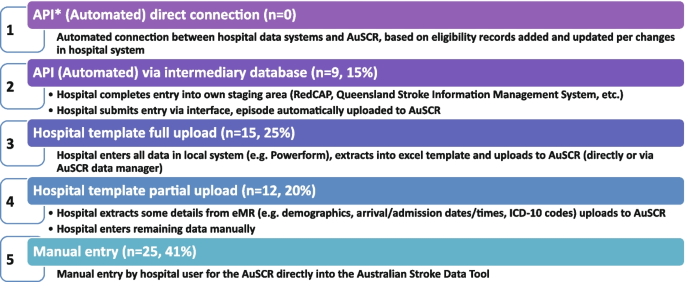
Current status of automated data importing solutions in the Australian Stroke Clinical Registry, 2022, with ‘ n ’ representing the number of hospitals. AuSCR, Australian Stroke Clinical Registry; AuSDaT, Australian Stroke Data Tool; API, Application Programming Interface; ICD, International Classification of Diseases; RedCAP, Research Electronic Data Capture; eMR, electronic medical records
For acute stroke care, the Australian Commission on Quality and Safety in Health Care facilitated the co-design (clinicians, academics, consumers) and publication of the national Acute Stroke Clinical Care Standard in 2015 [ 17 ], and subsequent review [ 18 ]. The indicator set for the Acute Stroke Standard then informed the expansion of the minimum dataset for AuSCR so that hospitals could routinely track their performance. The national Audit program enabled hospitals not involved in the AuSCR to assess their performance every two years against the Acute Stroke Standard. Complementing these efforts, the Stroke Foundation, working with the sector, developed the Acute and Rehabilitation Stroke Services Frameworks to outline the principles, essential elements, models of care and staffing recommendations for stroke services ( https://informme.org.au/guidelines/national-stroke-services-frameworks ). The Frameworks are intended to guide where stroke services should be developed, and monitor their uptake with the organizational survey component of the Audit program.
Evidence from implementation and healthcare improvement (LHS quadrant 4, Fig. 1 )
Research to better utilize and augment data from registries through linkage [ 37 , 38 , 39 , 40 ] and to ensure presentation of hospital or service level data are understood by clinicians has ensured advancement in the field for the Australian Stroke LHS [ 41 ]. Importantly, greater insights into whole patient journeys, before and after a stroke, can now enable exploration of value-based care. The LHS and stroke data platform have enabled focused and time-limited projects to create a better understanding of the quality of care in acute or rehabilitation settings [ 22 , 42 , 43 ]. Within stroke, all the elements of an LHS culminate into the ready availability of benchmarked performance data and support for implementation of strategies to address gaps in care.
Implementation research to grow the evidence base for effective improvement interventions has also been a key pillar in the Australian context. These include multi-component implementation interventions to achieve behaviour change for particular aspects of stroke care, [ 22 , 23 , 44 , 45 ] and real-world approaches to augmenting access to hyperacute interventions in stroke through the use of technology and telehealth [ 46 , 47 , 48 , 49 ]. The evidence from these studies feeds into the living guidelines program and the data collection systems, such as the Audit program or AuSCR, which are then amended to ensure data aligns to recommended care. For example, the use of ‘hyperacute aspirin within the first 48 h of ischemic stroke’ was modified to be ‘hyperacute antiplatelet…’ to incorporate new evidence that other medications or combinations are appropriate to use. Additionally, new datasets have been developed to align with evidence such as the Fever, Sugar, and Swallow variables [ 42 ]. Evidence on improvements in access to best practice care from the acute Audit program [ 50 ] and AuSCR is emerging [ 36 ]. For example, between 2007 and 2017, the odds of receiving intravenous thrombolysis after ischemic stroke increased by 16% 9OR 1.06 95% CI 1.13–1.18) and being managed in a stroke unit by 18% (OR 1.18 95% CI 1.17–1.20). Over this period, the median length of hospital stay for all patients decreased from 6.3 days in 2007 to 5.0 days in 2017 [ 51 ]. When considering the number of additional patients who would receive treatment in 2017 in comparison to 2007 it was estimated that without this additional treatment, over 17,000 healthy years of life would be lost in 2017 (17,786 disability-adjusted life years) [ 51 ]. There is evidence on the cost-effectiveness of different system-focussed strategies to augment treatment access for acute ischemic stroke (e.g. Victorian Stroke Telemedicine program [ 52 ] and Melbourne Mobile Stroke Unit ambulance [ 53 ]). Reciprocally, evidence from the national Rehabilitation Audit, where the LHS approach has been less complete or embedded, has shown fewer areas of healthcare improvement over time [ 51 , 54 ].
Within the field of stroke in Australia, there is indirect evidence that the collective efforts that align to establishing the components of a LHS have had an impact. Overall, the age-standardised rate of stroke events has reduced by 27% between 2001 and 2020, from 169 to 124 events per 100,000 population. Substantial declines in mortality rates have been reported since 1980. Commensurate with national clinical guidelines being updated in 2007 and the first National Stroke Audit being undertaken in 2007, the mortality rates for men (37.4 deaths per 100,000) and women (36.1 deaths per 100,0000 has declined to 23.8 and 23.9 per 100,000, respectively in 2021 [ 55 ].
Underpinning the LHS with the integration of the four quadrants of evidence from stakeholders, research and guidelines, practice and implementation, and core LHS principles have been addressed. Leadership and governance have been important, and programs have been established to augment workforce training and capacity building in best practice professional development. Medical practitioners are able to undertake courses and mentoring through the Australasian Stroke Academy ( http://www.strokeacademy.com.au/ ) while nurses (and other health professionals) can access teaching modules in stroke care from the Acute Stroke Nurses Education Network ( https://asnen.org/ ). The Association of Neurovascular Clinicians offers distance-accessible education and certification to develop stroke expertise for interdisciplinary professionals, including advanced stroke co-ordinator certification ( www.anvc.org ). Consumer initiative interventions are also used in the design of the AuSCR Public Summary Annual reports (available at https://auscr.com.au/about/annual-reports/ ) and consumer-related resources related to the Living Guidelines ( https://enableme.org.au/resources ).
The important success factors and lessons from stroke as a national exemplar LHS in Australia include leadership, culture, workforce and resources integrated with (1) established and broad partnerships across the academic-clinical sector divide and stakeholder engagement; (2) the living guidelines program; (3) national data infrastructure, including a national data dictionary that provides the common data framework to support standardized data capture; (4) various implementation strategies including benchmarking and feedback as well as engagement strategies targeting different levels of the health system; and (5) implementation and improvement research to advance stroke systems of care and reduce unwarranted variation in practice (Fig. 1 ). Priority opportunities now include the advancement of interoperability with electronic medical records as an area all clinical quality registry’s programs needs to be addressed, as well as providing more dynamic and interactive data dashboards tailored to the need of clinicians and health service executives.
There is a clear mandate to optimise healthcare improvement with big data offering major opportunities for change. However, we have lacked the approaches to capture evidence from the community and stakeholders, to integrate evidence from research, to capture and leverage data or evidence from practice and to generate and build on evidence from implementation using iterative system-level improvement. The LHS provides this opportunity and is shown to deliver impact. Here, we have outlined the process applied to generate an evidence-based LHS and provide a leading exemplar in stroke care. This highlights the value of moving from single-focus isolated approaches/initiatives to healthcare improvement and the benefit of integration to deliver demonstrable outcomes for our funders and key stakeholders — our community. This work provides insight into strategies that can both apply evidence-based processes to healthcare improvement as well as implementing evidence-based practices into care, moving beyond research as an endpoint, to research as an enabler, underpinning delivery of better healthcare.
Availability of data and materials
Not applicable
Abbreviations
Australian Stroke Clinical Registry
Confidence interval
- Learning Health System
World Health Organization. Delivering quality health services . OECD Publishing; 2018.
Enticott J, Braaf S, Johnson A, Jones A, Teede HJ. Leaders’ perspectives on learning health systems: A qualitative study. BMC Health Serv Res. 2020;20:1087.
Article PubMed PubMed Central Google Scholar
Melder A, Robinson T, McLoughlin I, Iedema R, Teede H. An overview of healthcare improvement: Unpacking the complexity for clinicians and managers in a learning health system. Intern Med J. 2020;50:1174–84.
Article PubMed Google Scholar
Alberto IRI, Alberto NRI, Ghosh AK, Jain B, Jayakumar S, Martinez-Martin N, et al. The impact of commercial health datasets on medical research and health-care algorithms. Lancet Digit Health. 2023;5:e288–94.
Article CAS PubMed PubMed Central Google Scholar
Dixon-Woods M. How to improve healthcare improvement—an essay by Mary Dixon-Woods. BMJ. 2019;367: l5514.
Enticott J, Johnson A, Teede H. Learning health systems using data to drive healthcare improvement and impact: A systematic review. BMC Health Serv Res. 2021;21:200.
Enticott JC, Melder A, Johnson A, Jones A, Shaw T, Keech W, et al. A learning health system framework to operationalize health data to improve quality care: An Australian perspective. Front Med (Lausanne). 2021;8:730021.
Dammery G, Ellis LA, Churruca K, Mahadeva J, Lopez F, Carrigan A, et al. The journey to a learning health system in primary care: A qualitative case study utilising an embedded research approach. BMC Prim Care. 2023;24:22.
Foley T, Horwitz L, Zahran R. The learning healthcare project: Realising the potential of learning health systems. 2021. Available from https://learninghealthcareproject.org/wp-content/uploads/2021/05/LHS2021report.pdf . Accessed Jan 2024.
Institute of Medicine. Best care at lower cost: The path to continuously learning health care in America. Washington: The National Academies Press; 2013.
Google Scholar
Zurynski Y, Smith CL, Vedovi A, Ellis LA, Knaggs G, Meulenbroeks I, et al. Mapping the learning health system: A scoping review of current evidence - a white paper. 2020:63
Cadilhac DA, Bravata DM, Bettger J, Mikulik R, Norrving B, Uvere E, et al. Stroke learning health systems: A topical narrative review with case examples. Stroke. 2023;54:1148–59.
Braithwaite J, Glasziou P, Westbrook J. The three numbers you need to know about healthcare: The 60–30-10 challenge. BMC Med. 2020;18:1–8.
Article Google Scholar
King D, Wittenberg R, Patel A, Quayyum Z, Berdunov V, Knapp M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing. 2020;49:277–82.
Ganesh A, Lindsay P, Fang J, Kapral MK, Cote R, Joiner I, et al. Integrated systems of stroke care and reduction in 30-day mortality: A retrospective analysis. Neurology. 2016;86:898–904.
Lowther HJ, Harrison J, Hill JE, Gaskins NJ, Lazo KC, Clegg AJ, et al. The effectiveness of quality improvement collaboratives in improving stroke care and the facilitators and barriers to their implementation: A systematic review. Implement Sci. 2021;16:16.
Australian Commission on Safety and Quality in Health Care. Acute stroke clinical care standard. 2015. Available from https://www.safetyandquality.gov.au/our-work/clinical-care-standards/acute-stroke-clinical-care-standard . Accessed Jan 2024.
Australian Commission on Safety and Quality in Health Care. Acute stroke clinical care standard. Sydney: ACSQHC; 2019. Available from https://www.safetyandquality.gov.au/publications-and-resources/resource-library/acute-stroke-clinical-care-standard-evidence-sources . Accessed Jan 2024.
Ryan O, Ghuliani J, Grabsch B, Hill K, G CC, Breen S, et al. Development, implementation, and evaluation of the Australian Stroke Data Tool (AuSDaT): Comprehensive data capturing for multiple uses. Health Inf Manag. 2022:18333583221117184.
English C, Bayley M, Hill K, Langhorne P, Molag M, Ranta A, et al. Bringing stroke clinical guidelines to life. Int J Stroke. 2019;14:337–9.
English C, Hill K, Cadilhac DA, Hackett ML, Lannin NA, Middleton S, et al. Living clinical guidelines for stroke: Updates, challenges and opportunities. Med J Aust. 2022;216:510–4.
Cadilhac DA, Grimley R, Kilkenny MF, Andrew NE, Lannin NA, Hill K, et al. Multicenter, prospective, controlled, before-and-after, quality improvement study (Stroke123) of acute stroke care. Stroke. 2019;50:1525–30.
Cadilhac DA, Marion V, Andrew NE, Breen SJ, Grabsch B, Purvis T, et al. A stepped-wedge cluster-randomized trial to improve adherence to evidence-based practices for acute stroke management. Jt Comm J Qual Patient Saf. 2022.
Elliott J, Lawrence R, Minx JC, Oladapo OT, Ravaud P, Jeppesen BT, et al. Decision makers need constantly updated evidence synthesis. Nature. 2021;600:383–5.
Article CAS PubMed Google Scholar
National Stroke Foundation. National guidelines for acute stroke management. Melbourne: National Stroke Foundation; 2003.
National Stroke Foundation. Clinical guidelines for stroke rehabilitation and recovery. Melbourne: National Stroke Foundation; 2005.
Phan TG, Thrift A, Cadilhac D, Srikanth V. A plea for the use of systematic review methodology when writing guidelines and timely publication of guidelines. Intern Med J . 2012;42:1369–1371; author reply 1371–1362
Tendal B, Vogel JP, McDonald S, Norris S, Cumpston M, White H, et al. Weekly updates of national living evidence-based guidelines: Methods for the Australian living guidelines for care of people with COVID-19. J Clin Epidemiol. 2021;131:11–21.
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50.
Harris D, Cadilhac D, Hankey GJ, Hillier S, Kilkenny M, Lalor E. National stroke audit: The Australian experience. Clin Audit. 2010;2:25–31.
Cadilhac DA, Purvis T, Kilkenny MF, Longworth M, Mohr K, Pollack M, et al. Evaluation of rural stroke services: Does implementation of coordinators and pathways improve care in rural hospitals? Stroke. 2013;44:2848–53.
Cadilhac DA, Moss KM, Price CJ, Lannin NA, Lim JY, Anderson CS. Pathways to enhancing the quality of stroke care through national data monitoring systems for hospitals. Med J Aust. 2013;199:650–1.
Cadilhac DA, Lannin NA, Anderson CS, Levi CR, Faux S, Price C, et al. Protocol and pilot data for establishing the Australian Stroke Clinical Registry. Int J Stroke. 2010;5:217–26.
Ivers N, Jamtvedt G, Flottorp S, Young J, Odgaard-Jensen J, French S, et al. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev . 2012
Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries. Final report. . 2016:79
Cadilhac DA, Dalli LL, Morrison J, Lester M, Paice K, Moss K, et al. The Australian Stroke Clinical Registry annual report 2021. Melbourne; 2022. Available from https://auscr.com.au/about/annual-reports/ . Accessed 6 May 2024.
Kilkenny MF, Kim J, Andrew NE, Sundararajan V, Thrift AG, Katzenellenbogen JM, et al. Maximising data value and avoiding data waste: A validation study in stroke research. Med J Aust. 2019;210:27–31.
Eliakundu AL, Smith K, Kilkenny MF, Kim J, Bagot KL, Andrew E, et al. Linking data from the Australian Stroke Clinical Registry with ambulance and emergency administrative data in Victoria. Inquiry. 2022;59:469580221102200.
PubMed Google Scholar
Andrew NE, Kim J, Cadilhac DA, Sundararajan V, Thrift AG, Churilov L, et al. Protocol for evaluation of enhanced models of primary care in the management of stroke and other chronic disease (PRECISE): A data linkage healthcare evaluation study. Int J Popul Data Sci. 2019;4:1097.
CAS PubMed PubMed Central Google Scholar
Mosalski S, Shiner CT, Lannin NA, Cadilhac DA, Faux SG, Kim J, et al. Increased relative functional gain and improved stroke outcomes: A linked registry study of the impact of rehabilitation. J Stroke Cerebrovasc Dis. 2021;30: 106015.
Ryan OF, Hancock SL, Marion V, Kelly P, Kilkenny MF, Clissold B, et al. Feedback of aggregate patient-reported outcomes (PROs) data to clinicians and hospital end users: Findings from an Australian codesign workshop process. BMJ Open. 2022;12:e055999.
Grimley RS, Rosbergen IC, Gustafsson L, Horton E, Green T, Cadigan G, et al. Dose and setting of rehabilitation received after stroke in Queensland, Australia: A prospective cohort study. Clin Rehabil. 2020;34:812–23.
Purvis T, Middleton S, Craig LE, Kilkenny MF, Dale S, Hill K, et al. Inclusion of a care bundle for fever, hyperglycaemia and swallow management in a national audit for acute stroke: Evidence of upscale and spread. Implement Sci. 2019;14:87.
Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D’Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomised controlled trial. Lancet. 2011;378:1699–706.
Middleton S, Dale S, Cheung NW, Cadilhac DA, Grimshaw JM, Levi C, et al. Nurse-initiated acute stroke care in emergency departments. Stroke. 2019:STROKEAHA118020701.
Hood RJ, Maltby S, Keynes A, Kluge MG, Nalivaiko E, Ryan A, et al. Development and pilot implementation of TACTICS VR: A virtual reality-based stroke management workflow training application and training framework. Front Neurol. 2021;12:665808.
Bladin CF, Kim J, Bagot KL, Vu M, Moloczij N, Denisenko S, et al. Improving acute stroke care in regional hospitals: Clinical evaluation of the Victorian Stroke Telemedicine program. Med J Aust. 2020;212:371–7.
Bladin CF, Bagot KL, Vu M, Kim J, Bernard S, Smith K, et al. Real-world, feasibility study to investigate the use of a multidisciplinary app (Pulsara) to improve prehospital communication and timelines for acute stroke/STEMI care. BMJ Open. 2022;12:e052332.
Zhao H, Coote S, Easton D, Langenberg F, Stephenson M, Smith K, et al. Melbourne mobile stroke unit and reperfusion therapy: Greater clinical impact of thrombectomy than thrombolysis. Stroke. 2020;51:922–30.
Purvis T, Cadilhac DA, Hill K, Reyneke M, Olaiya MT, Dalli LL, et al. Twenty years of monitoring acute stroke care in Australia from the national stroke audit program (1999–2019): Achievements and areas of future focus. J Health Serv Res Policy. 2023.
Cadilhac DA, Purvis T, Reyneke M, Dalli LL, Kim J, Kilkenny MF. Evaluation of the national stroke audit program: 20-year report. Melbourne; 2019.
Kim J, Tan E, Gao L, Moodie M, Dewey HM, Bagot KL, et al. Cost-effectiveness of the Victorian Stroke Telemedicine program. Aust Health Rev. 2022;46:294–301.
Kim J, Easton D, Zhao H, Coote S, Sookram G, Smith K, et al. Economic evaluation of the Melbourne mobile stroke unit. Int J Stroke. 2021;16:466–75.
Stroke Foundation. National stroke audit – rehabilitation services report 2020. Melbourne; 2020.
Australian Institute of Health and Welfare. Heart, stroke and vascular disease: Australian facts. 2023. Webpage https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/hsvd-facts/contents/about (accessed Jan 2024).
Download references
Acknowledgements
The following authors hold National Health and Medical Research Council Research Fellowships: HT (#2009326), DAC (#1154273), SM (#1196352), MFK Future Leader Research Fellowship (National Heart Foundation #105737). The Funders of this work did not have any direct role in the design of the study, its execution, analyses, interpretation of the data, or decision to submit results for publication.
Author information
Helena Teede and Dominique A. Cadilhac contributed equally.
Authors and Affiliations
Monash Centre for Health Research and Implementation, 43-51 Kanooka Grove, Clayton, VIC, Australia
Helena Teede, Emily Callander & Joanne Enticott
Monash Partners Academic Health Science Centre, 43-51 Kanooka Grove, Clayton, VIC, Australia
Helena Teede & Alison Johnson
Stroke and Ageing Research, Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Level 2 Monash University Research, Victorian Heart Hospital, 631 Blackburn Rd, Clayton, VIC, Australia
Dominique A. Cadilhac, Tara Purvis & Monique F. Kilkenny
Stroke Theme, The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Heidelberg, VIC, Australia
Dominique A. Cadilhac, Monique F. Kilkenny & Bruce C.V. Campbell
Department of Neurology, Melbourne Brain Centre, Royal Melbourne Hospital, Parkville, VIC, Australia
Bruce C.V. Campbell
Department of Medicine, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Victoria, Australia
School of Health Sciences, Heart and Stroke Program, University of Newcastle, Hunter Medical Research Institute, University Drive, Callaghan, NSW, Australia
Coralie English
School of Medicine and Dentistry, Griffith University, Birtinya, QLD, Australia
Rohan S. Grimley
Clinical Excellence Division, Queensland Health, Brisbane, Australia
John Hunter Hospital, Hunter New England Local Health District and University of Newcastle, Sydney, NSW, Australia
Christopher Levi
School of Nursing, Midwifery and Paramedicine, Australian Catholic University, Sydney, NSW, Australia
Sandy Middleton
Nursing Research Institute, St Vincent’s Health Network Sydney and and Australian Catholic University, Sydney, NSW, Australia
Stroke Foundation, Level 7, 461 Bourke St, Melbourne, VIC, Australia
Kelvin Hill
You can also search for this author in PubMed Google Scholar
Contributions
HT: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. DAC: conception, design and initial draft, provided essential literature and case study examples, approved the submitted version. TP: revised the manuscript critically for important intellectual content, approved the submitted version. MFK: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. BC: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. CE: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. AJ: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. EC: revised the manuscript critically for important intellectual content, approved the submitted version. RSG: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. CL: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. SM: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. KH: revised the manuscript critically for important intellectual content, provided essential literature and case study examples, approved the submitted version. JE: conception, design and initial draft, developed the theoretical formalism for learning health system framework, approved the submitted version. All authors read and approved the final manuscript.
Authors’ Twitter handles
@HelenaTeede
@DominiqueCad
@Coralie_English
@EmilyCallander
@EnticottJo
Corresponding authors
Correspondence to Helena Teede or Dominique A. Cadilhac .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Teede, H., Cadilhac, D.A., Purvis, T. et al. Learning together for better health using an evidence-based Learning Health System framework: a case study in stroke. BMC Med 22 , 198 (2024). https://doi.org/10.1186/s12916-024-03416-w
Download citation
Received : 23 July 2023
Accepted : 30 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s12916-024-03416-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Evidence-based medicine
- Person-centred care
- Models of care
- Healthcare improvement
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]

COMMENTS
The patient presented in this case had severe malaria, specifically cerebral malaria, 18 days after returning to the United States from a 10-day trip to Ghana. Other causes for the patient's symptoms were excluded, including viral infection, meningitis, bacteremia, and so forth.
Clinical Case Study 1: Fever 6 months after a visit to Pakistan. A 44-year-old man is seen at a physician's office in the United States, during a week-end, for suspected malaria. The patient was born in Pakistan but has lived in the United States for the past 12 years. He travels frequently back to Pakistan to visit friends and relatives.
presenting to Lincoln Hospitals Emergency Department with fevers and fatigue. The patients travel history to Togo, along with her symptoms, resulted in a differential diagnosis which included Ebola as well as Malaria. New York City's Department of Health and Mental Hygiene was contacted for further clarification of presence of Ebola in Togo. The present case report is meant to educate about ...
Most cases of malaria diagnosed in the United States occur during the U.S. summer months among patients who have traveled to sub-Saharan Africa. 3 The majority of cases are diagnosed in patients ...
Plasmodium falciparum malaria especially in older patients is a potentially serious disease and should be treated as a medical emergency. When complications develop, such as cerebral malaria and hemolysis, an aggressive treatment with an intravenous regimen can be life saving. Interactive clinical case-study dealing with a 65 year old woman ...
The malaria elimination case‑study series ... parasitological examination of all febrile patients or as parasitological examination of the target population without prior fever screening. annual blood examination rate The number of examinations of blood slides for malaria by microscopy per 100 population per year.
Diagnostic procedures for detecting active malaria infection are, in order of increasing sensitivity: thin blood smear, thick blood smear, and PCR. Serology does not detect active infection, but measures past exposure to malaria. Interactive clinical case-study dealing with a 30 year-old woman who is immunocompromised.
A meta-analysis of 14 studies demonstrated that patients with mixed Plasmodium infection (1,999/35,755) and patients with P. falciparum malaria (9,249/294,397) had an equal risk of developing ...
Malaria Case Management Clinical Case In the absence of parasitological confirmation of suspected malaria case, the patient may be treated as having vivax malaria. Chloroquine 25mg/kg base over 3 days (10 mg base/kg on Day 1, 10mg base/kg on Day 2 and 5mg base/kg on Day 3) should be prescribed. Patient must be called for follow-up after 48 hours.
Human malaria infection is caused by four protozoa species of the genus Plasmodium. These are P.falciparum, P. malariae, P. vivax, and P. ovalae, of which the preponderance of severe malaria and mortality is due to P.falciparum. Children living in endemic areas typically have a primary malaria episode during their first few years of life and ...
The first naturally-acquired human infection of the simian malaria parasite, Plasmodium cynomolgi, was reported from Malaysia in 2014 [].Clinical cases have continued to be reported from Malaysia, and P. cynomolgi has been retrospectively detected in stored isolates from Malaysia, Cambodia and Thailand [2,3,4,5,6,7,8].An ongoing malaria surveillance study in Thailand has been enrolling malaria ...
A community-based case-control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. ... The number of malaria patients steadily increased and peaked in ...
An unmatched case-control study was conducted among 322 individuals, comprising 161 cases and 161 controls, who were randomly selected malaria patients visiting public health facilities in South ...
Suspected malaria case in which malaria parasites have been demonstrated in a patient's blood by microscopy or a rapid diagnostic test. presumed malaria Suspected malaria case with no diagnostic test to confirm malaria but nevertheless treated presumptively as malaria. suspected malaria Patient illness suspected by a health worker to be due
Public Health Case Study 2: Malaria in Pennsylvania. Plasmodium species found in patient PH-2. (Image not from this patient.) A 49-year-old man from Pennsylvania receives 4 units of packed red blood cells (PRBCs) on January 15 while undergoing hip replacement surgery. He is again hospitalized on February 1 with fever, hypotension, and renal ...
Updated on May 9, 2024. By Marianne Belleza, R.N. This nursing study guide provides an overview of malaria including the five species of the malaria parasite, treatment, preventive options, nursing interventions, and nursing care planning, nursing diagnosis, and management. Malaria is one of the most common infectious diseases known to mankind ...
Artemisinin (ART) combination therapy is the main treatment for malaria. Pfk13 mutations (or K13 mutations, Kelch 13) are associated with ART resistance. This study aims to conduct a systematic review and meta-analysis of the prevalence of K13 mutations with ART resistance in malaria-endemic countries. An electronic search of studies in 2018 and a manual search in 2020 were performed to ...
Main outcome: Level of adherence to malaria treatment guidelines in the treatment of uncomplicated malaria. Results: Overall, 170 health facilities offering outpatient services were recruited in the study, 223 health workers and 567 febrile patients were interviewed. Malaria parasitological diagnosis was provided in 86.5% of the facilities.
Objectives: The objective of the present study was to determine whether Case Management of Malaria (CCMm) by Community Health Volunteers (CHVs) affect the trends of malaria incidence in Busia Kenya, 2018-2023. Specifically, the study aimed at determining the proportion and trends per year for those tested and treated for malaria in health facilities and Community Units and to correlate the ...
The United States has seen five cases of malaria spread by mosquitos in the past two months − the first time there has been local spread in 20 years − prompting authorities to issue a public ...
A study of patients at the end of life in South Korea found high rates of exposure to broad-spectrum antibiotics, particularly among those with cancer, researchers reported today in Antimicrobial Stewardship & Healthcare Epidemiology.. wuwhanfoto / iStock. Using data from the Korean National Health Insurance Database, researchers from Seoul National University analyzed antibiotic consumption ...
We use community-based malaria hospitalization incidence rates in small catchment populations and adjusted for case under-ascertainment as an empirical proxy of the incidence of severe malaria ...
The May case study is about a 27-year-old male veteran with EDS six months after he was injured in a bomb blast.
In developed nations, it has been estimated that 60% of care provided aligns with the evidence base, 30% is low value and 10% is potentially harmful [].In some areas, clinical advances have been rapid and research and evidence have paved the way for dramatic improvement in outcomes, mandating rapid implementation of evidence into healthcare (e.g. polio and COVID-19 vaccines).