- Research article
- Open access
- Published: 13 July 2020

What is the role of the practice nurse in the care of people living with dementia, or cognitive impairment, and their support person(s)?: a systematic review
- Caroline Gibson ORCID: orcid.org/0000-0002-3774-2601 1 ,
- Dianne Goeman 1 , 2 &
- Dimity Pond 1
BMC Family Practice volume 21 , Article number: 141 ( 2020 ) Cite this article
13k Accesses
16 Citations
4 Altmetric
Metrics details
The potential value of expanding the Practice Nurse role to include the recognition and management of dementia has been acknowledged. Practice Nurses are well-positioned to provide comprehensive dementia information and support so that people living with dementia are better equipped to self-manage their health and live well with dementia. The purpose of this review was to systematically examine published literature to identify existing and potential roles of Practice Nurse’s in the delivery of care to people affected by dementia and to describe the characteristics and effectiveness of nurse interventions in dementia models of care.
The PRISMA statement guided the systematic review of the quantitative and qualitative evidence for roles and characteristics of the Practice Nurse in the delivery of dementia care. A comprehensive literature search of seven electronic databases and Google scholar identified relevant original research published in English between January 2000 and January 2019. Thirteen articles met the inclusion criteria and were extracted into the Covidence software for analysis.
The heterogeneity of the included studies purpose, design and outcomes measures and the diversity in health systems and primary care nurses scope of practice made it difficult to synthesise the findings and draw conclusions. The heterogeneity did, however, provide important insights into the characteristics of roles undertaken by nurses working in the general practice setting, which were potentially beneficial to people living with dementia and their support person. These included patient accessibility to the Practice Nurse, early recognition and management of cognitive changes, care management and collaboration with the General Practitioner. Limitations of the provision of dementia care by Practice Nurses included a lack of definition of the role, inadequate dementia specific training, time constraints and poor communication with General Practitioners.
Conclusions
Embedding an evidence-based model that describes the role of the Practice Nurse in dementia care provision has the potential to increase early recognition of cognitive impairment and more appropriate primary care management of dementia.
Systematic review registration
PROSPERO 2018 CRD42018088191.
Peer Review reports
Introduction
Australian and international literature [ 1 , 2 ] reveals a significant gap in the delivery of dementia care in the general practice setting. In one study, 66% of participants (people with memory concerns) reported that they would like a memory test and 81% reported that they would speak with their General Practitioner (GP) if they thought they had dementia [ 3 ]. However, despite people’s intent to report their concerns with their GP, there is a significant gap in the delivery of dementia care in the general practice setting [ 1 ]. Barriers to the identification, diagnosis and management of dementia are multiple and complex, and in some cases include a perception by the GP that nothing can be done and that support options are lacking [ 4 ]. Dementia is the second leading cause of death in Australia and currently more than 400 000 Australians are living with dementia (5). This number is expected to increase three-fold by 2056 [ 5 ]. Around 83% of all males with dementia and 71% of females with dementia live in the community [ 5 ] with 50 percent of dementia cases remaining undiagnosed [ 6 ]. When combining these figures with the approximately 200 000 unpaid care-givers involved in supporting a person living with dementia [ 5 ] a significant number of people are likely to be attending general practices and not having their health and social care needs met. Exploring new ways to improve the identification and management of dementia in the primary care setting is needed.
Approximately two thirds of Australian general practices employ a nurse [ 7 ] and nurse-led clinics are known to maximise patient health outcomes in primary care [ 8 , 9 ]. The Practice Nurse (PN) is a primary health care nurse employed in General Practice. As described by the Australian Primary Health Care Nurse Association (APNA) the role of the PN can include women’s health, men’s health, aged care, chronic disease management, immunisation, wound management, health promotion and population health. Given that co-morbidity in people living with dementia is high [ 8 , 10 ] the PN is likely to have established a therapeutic relationship with people with cognitive decline through routine primary care treatment, health assessment, chronic disease management and health promotion activities.
The potential value of expanding the PN role to include the recognition and management of dementia has been acknowledged [ 4 , 11 , 12 ]. However, there is limited research on the role of the PN in dementia care delivery in Australian or in international literature. A significant barrier to GP’s discussing dementia with their patients is the perception that nothing can be done and that support options are lacking [ 4 ]. Developing a model of dementia care that incorporates a flexible clinical pathway to guide the PN, along with a compendium of resources that can be used to draw upon additional knowledge to assist in providing appropriate care for people with dementia, could help to overcome these barriers. The PN could offer the GP a means of providing immediate support to patients and their families, following a discussion about dementia that includes a conversation about their concerns and referral on to further supports as needed.
In summary, a PN model of dementia care has the potential to assist with the identification of cognition concerns and understanding of the impact of dementia on the health and well-being of an individual. Such a model is not only likely to lead to increased identification of dementia but also to more appropriate primary care treatment, chronic disease management, and, care planning for people with existing or emerging cognitive impairment or dementia and the people supporting them.
There has been no systematic review of the evidence on the role of the PN in dementia care delivery to date, therefore the aim of this review is to examine published literature to investigate the Practice Nurse role in the delivery of care to people affected by dementia.
This paper systematically reviews published literature to answer the review questions:
What are the existing and potential roles performed by the PN in the care of people living with dementia or cognitive impairment and their informal caregivers in General Practice?
What are the characteristics of any existing nurse interventions that provide care to people living with dementia, or cognitive impairment, and their informal caregivers in the General Practice setting?
The 27 item PRISMA-P Checklist [ 13 ] was used to guide this systematic review. The checklist includes items deemed essential for systematic review reporting [ 14 ].
Eligibility criteria
All published literature that described a role in care of a person with dementia and/ or their caregiver performed by a nurse in a General Practice setting published between the dates 1 January 2000 and 1 January 2019 were eligible for inclusion. Studies were limited to those published in English language.
Information sources
A search strategy was developed to identify published peer-reviewed studies describing the role of the PN in the care of people living with dementia, or cognitive impairment, and their informal caregivers in general practice.
Seven electronic databases (Cochrane Library, EMBASE, CINHAHL (EBSCO), OVID MEDLINE (PubMed), Scopus, INFORMIT HEALTH and PsycINFO) and Google Scholar were searched.
A review of the included paper’s reference lists and citations was undertaken to identify any additional studies that may not have been identified in the primary search.
Search strategy
Original searches were carried out on the 24 th February, 2018. Automatic search strategies for all included electronic databases were set up with weekly email alerts to identify eligible studies published from the date of the original search to 1 st January 2019. Search terms used included:
Practice Nurse, Primary Health Care Nurse , Primary Care Nurse, General Practice Nurse, General Practice Nurse (MeSH Nurse)
Dementia, Cognitive impairment, Cognitive deficit, Alzheimer’s disease, Memory loss, Vascular dementia, Lewy body dementia, Frontotemporal dementia, Younger onset dementia (MeSH Dementia) Cognitive impairment, Cognitive deficit, Cognitive decline, Cognitive dysfunction (MeSH Cognitive dysfunction)
Example of a search query
(Practice Nurs* or Primary Health Care Nurs* or Primary Care Nurs* or General Practice Nurs* or GP Nurs*).af.
(Dementia, or Cognitive impairment or Cognitive dysfunction or cognitive deficit or cognitive decline or alzheimer* or memory impairment or memory loss).af.
Study selection
All records from searches were retrieved in Endnote reference management software, and transferred to Covidence, the on-line standard production platform for Cochrane Reviews ( https://www.covidence.org/home ). Using Covidence, all records were independently screened for eligibility using the identified inclusion criteria by two authors (CG and DG). Any discrepancies were resolved by a consensus meeting with the third author (DP).
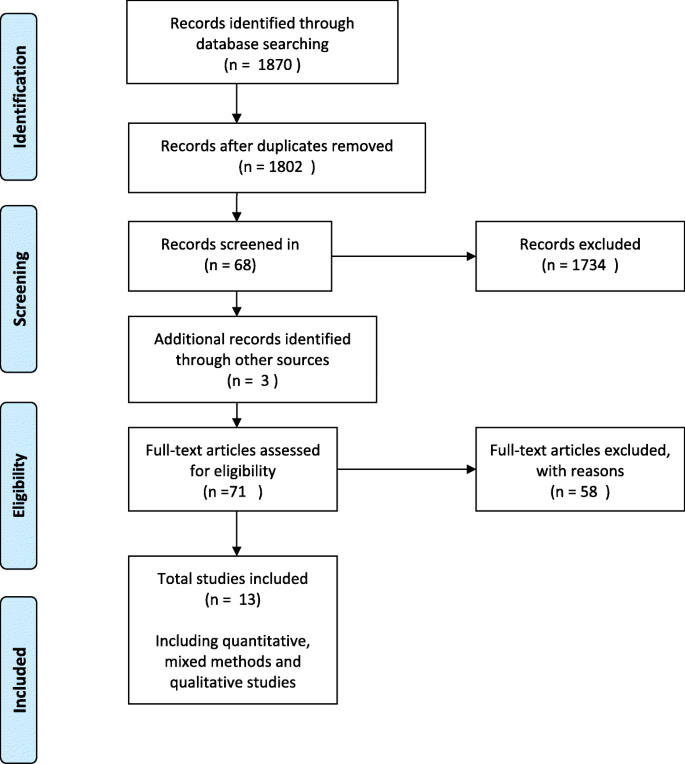
The steps taken for paper selection were an initial screening for relevance using the titles of identified references. Papers considered to be irrelevant were removed from the selection process. A conservative approach was taken. Abstracts of remaining titles were reviewed based on inclusion criteria. The abstracts were coded relevant, irrelevant or unsure. The irrelevant papers were discarded from the selection process. Published papers were retrieved for abstracts categorised as relevant or unsure. The retrieved papers were then reviewed and those deemed as meeting the selection criteria were included in the systematic review (see Fig. 1 ).
Where the findings of a study have been published as separate papers due to the reporting of different outcome measures the paper with the most detailed analysis relevant to the aims of this systematic review was included. The other papers adding information to the paper included in this systematic review were described as supplementary papers.
Data Collection processes
Data extraction for all study types included: author, year, country; aim; research design; instruments; sample and size; intervention type; analysis methods and outcomes. This information is described in Tables 5 , 6 , 7 and 8 .
Quality and risk of bias assessment
Two reviewers (CG, DG) independently assessed the studies for quality and risk of bias according to their specific study types. Any disagreements between the reviewers were resolved by discussion, with involvement of a third reviewer (DP).
Randomised Controlled Trial (RCT) studies were assessed for risk of bias using the Cochrane Risk of Bias Tool [ 44 ]. The CEBM Critical Appraisal tool [ 45 ] was used to assess the risk of bias in methodology, analysis and outcomes in cross-sectional studies. Mixed methods data was appraised using the Mixed Methods Appraisal Tool (MMAT) Version 2018 [ 46 ]. Risk of bias in qualitative studies was appraised using a tool based on the Critical Appraisal Skills Programme (CASP) Qualitative checklist [ 47 ]. The assessment criteria for each of the quality appraisal tools used is described in Tables 1 , 2 , 3 and 4 .
Synthesis of results
Synthesis of data from studies so diverse in research questions, methodologies, nurse scope of practice and health systems is inherently problematic and it was not possible to sensibly categorise findings into themes.
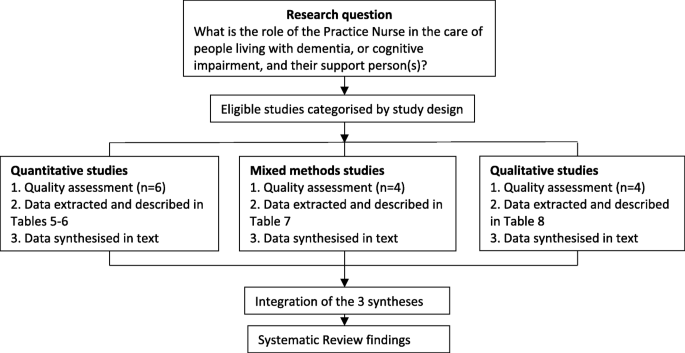
In this systematic review a rigorous and transparent method was utilised to organise, describe, explore and interpret the findings and generate new insights [ 48 , 49 ]. Eligible studies were selected using the defined inclusion criteria and then categorised into groups according to study design. Quality and risk of bias assessment was carried out according to their specific study types. Following quality assessment, data were extracted from the studies and tabulated under the headings: research aim; study design; instruments; sample characteristics; intervention type; analysis and outcomes. (Tables 5 , 6 , 7 and 8 ). The data were synthesised according to the three study types; quantitative, qualitative and mixed methods. The three syntheses were then integrated into one synthesis which informed the findings of this systematic review. (Refer Fig. 2 ).
Stages of the review
This approach provided an analysis of the published academic literature and enabled the exploration of relationships within and between studies and a description of themes across the included studies.
The search strategy identified 1870 references (Fig. 1 ). After removal of duplicates 1802 abstracts were examined for relevance and 68 full text references were obtained for full text screening. Hand-searching of references lists of included articles yielded an additional three articles. In total 71 articles were assessed for eligibility, of which 13 articles were selected for data extraction and analysis.
Fifty-eight studies assessed for eligibility were excluded. Eighteen were grey literature, 17 did not include the primary health care nurse, six were poster abstracts and the studies not published, and 17 papers were removed as they were multiple publications reporting on the same intervention and were included as supplementary papers. Three were duplicate studies [ 18 , 22 , 23 ] and two studies [ 16 , 50 ] were excluded as the outcomes had not been published. The authors of these studies were contacted. Bryans et al., [ 50 ] did not publish the outcomes of a survey study on primary health care nurses and dementia care due to significant loss to follow-up. For similar reasons, Perry et al., [ 16 ] did not publish the outcomes of the dementia training programme on diagnostic assessment and management of dementia by primary care nurses.
Study characteristics
Of the 13 included studies, six were quantitative studies: three RCTs and three survey questionnaires, four were mixed-method studies and three were qualitative studies using interviews.
The studies were conducted in the Netherlands (n=1), Germany (n=1), United States of America (n=1), The United Kingdom (n=5), Australia (n=4) and one was conducted across the Netherlands and the United Kingdom (n=1).
Four studies [ 15 , 18 , 19 , 35 ] evaluated dementia care management in primary health care. Exploring dementia care knowledge and attitudes of primary health care practitioners was the focus of three studies [ 3 , 11 , 12 ]. Two studies [ 39 , 41 ] explored participant experiences of dementia care delivery in primary health care and one study [ 29 ] explored service use and reported unmet needs of people with dementia and support person(s). Investigating the implications of early recognition of dementia for the roles of the primary health care team was the focus of one study [ 43 ] The authors of one study [ 34 ] developed quality indicators for dementia care in primary health care settings and one study investigated the value and useability of an online dementia management tool for health professionals [ 32 ]. The study interventions and outcomes are described in Tables 5 , 6 , 7 and 8 .
Quantitative Studies
Randomised controlled trials.
Three studies utilised an RCT [ 15 , 18 , 19 ] to investigate the impact of collaborative care on quality of life for people with dementia and their caregivers. The study by Van den Dungen et al., [ 15 ] also included an evaluation of family practitioner training on diagnosis of mild cognitive impairment.
In all three models of care the nurse was the care manager who worked in collaboration with the primary care doctor. All care management models followed a structured assessment and care planning protocol. Care management ranged in duration from six [ 19 ] to twelve months [ 15 , 18 ]. In two studies [ 15 , 19 ] the care managers were registered nurses, with Van den Dungen et al., [ 15 ] specifying the nurse as a primary care nurse who acted as the study nurse. In the third study [ 18 ] the care manager was a geriatric nurse practitioner. All the nurses received dementia specific training and were integrated into the primary care team with only one care manager providing the dementia care management within the patients’ home [ 19 ]. In addition to training, in the model of care described in Callahan et al., [ 18 ] the nurse received weekly support from a geriatrician, geriatric psychiatrist and a psychologist.
Callahan et al., [ 18 ] and Thyrian et al., [ 19 ] reported a significant decrease in behavioural and psychological symptoms of dementia and caregiver stress with dementia care management, however, Thyrian et al., [ 19 ] reported there was no significant improvement in quality of life overall. Despite reporting that dementia care management had no impact on quality of life measures for patients or their care-givers, Van den Dungen et al.,[ 15 ] recommend that collaborative care with nurses in primary care deserves further exploration.
Survey Questionnaire studies
Three studies reported survey results [ 11 , 12 , 29 ]. Manthorpe et al., [ 12 ] and Trickey et al., [ 11 ] investigated dementia knowledge and attitudes of community nurses (CN), health visitors, community mental health care nurses (CMHN) and PNs in the provision of care for people living with dementia. The third study [ 29 ] explored service use and unmet needs of people with dementia recruited a decade apart.
Manthorpe et al., [ 12 ] reported all groups of primary health care nurses had similar knowledge related to the early signs and symptoms of dementia. However, PNs were less confident in providing advice and support than CMHNs. In the study undertaken by Trickey et al., [ 11 ], PNs completing the Over-75 year health check were less likely than other nurse groups to take any action, other than to refer to the GP, when presented with a person living with dementia and their support person. The Over-75 year health check is an annual health check including a mental assessment for people aged over 75 years [ 11 ].
Gilbert et al., [ 29 ] reported that support person(s) were increasingly contacting a PN for support with less evident use of CNs, health visitors and CMHNs. This may in part be attributed to greater access to a PN and the changing nature of the PN role with an increased focus on chronic disease management. Support person(s) reported that they were still not getting the advice and support they needed.
Authors of all three studies identified a need to improve PN knowledge of dementia and its management. In the study by Trickey et al., [ 11 ] participants reported guidelines would be helpful to address gaps in knowledge and to standardise practice.
Mixed method studies
Four studies reported mixed-methods research results [ 3 , 32 , 34 , 35 ].
Perry et al., [ 34 ] used a RAND modified Delphi method to construct a set of quality indicators for dementia diagnosis and management in primary care in the Netherlands. PNs were involved in the selection and validation process of the quality indicators. Of the final 23 quality indicators, two explicitly describe collaboration between the GP and the PN, an area in which the authors suggest improvement is highly recommended. A further three quality indicators emphasise the importance of developing and reviewing individualised care plans. This is commonly a PN role that is established and accepted in primary care settings [ 34 ]. Millard et al., [ 3 ] explored dementia literacy in a general practice setting. In this study two-thirds of the PNs reported a lack of dementia training. Despite this self-perceived lack of training, three-quarters of the PNs reported that the primary care doctor or nurse was the appropriate person to discuss dementia with patients. Ollerenshaw et al., [ 32 ] suggest that PNs may find an on-line dementia management support tool useful. Iliffe et al., [ 35 ] adapted a US model of primary care based care management (PREVENT) for people with dementia and tested its implementation in UK general practice. Despite case managers, patients and support person(s) reporting a positive experience and perceiving benefits of case management, Iliffe et al., [ 35 ] suggest that case management does not fit easily into practice routines and that it was not substantially beneficial for patients and support person(s).
Qualitative studies
All three qualitative studies [ 39 , 41 , 43 ] used interviews to explore experiences of primary health care practitioners, patients and support person(s), of dementia care. Dodd et al., [ 39 ] used semi-structured face-to-face interviews to contrast study participants’ experiences of a new primary care led dementia service with existing secondary care based memory services in Bristol, UK. Dodd et al., [ 41 ] used a semi-structured face-to-face interview to investigate participant’s experiences of a new primary care led dementia service in South Gloustershire, UK. In both these studies [ 39 , 41 ] the nurses were seconded from secondary care dementia services, with each nurse working with a group of primary health care clinics. Patients and support person(s) reported primary care led services to be positive and there was uniform praise for the work by the memory nurse. GPs reported they valued the advisory role provided by the memory nurse. Manthorpe et al., [ 43 ] explored implications of the early recognition of dementia for inter-professional working using focus group interviews. In this study the PN was identified as the practitioner most appropriate to take on screening for dementia and monitoring, however community mental health care nurses were considered to have the skills and capacity to take on long-term and complex cases.
Risk of bias
The methodological quality varied across the studies (Tables 1 , 2 , 3 and 4 ). The qualitative studies and all but one of the mixed methods studies rated high according to the quality appraisal criteria. Of the quantitative studies two of the three RCT studies lacked allocation concealment, blinding and presented incomplete outcome data which compromised their quality. The survey studies were of mixed quality with two of the three studies introducing selection bias and no sample size was based on consideration of statistical power.
In addition to these limitations, Callahan et al., [ 18 ] describe their study as unable to identify which of the subcomponents of the intervention were most effective in achieving the outcomes. Van den Dungen et al., [ 15 ] reported the rates of MCI or dementia identified were lower than expected. The authors state the reasons for this may have included a type 2 error with a low sensitivity of the cognitive tests performed by PN. In addition, there was sub-optimal implementation of the intervention with the family practitioner not always performing further diagnostic assessments on all persons referred by the PN [ 15 ]. Thyrian et al., [ 19 ] describe limitations of the study including potential selection bias as screening and recruitment were part of routine care. The intervention and control groups had an uneven number of participants; the GPs in the control group had fewer patients. In addition, the GPs may have become aware of their assignment to the control or intervention group [ 19 ].
Trickey et al., [ 11 ] describe a methodological limitation of using a vignette that may more correctly explore current practice rather than knowledge and attitudes [ 11 ]. Iliffe et al., [ 35 ] report time constraints for the case management role of the PNs may have meant there was insufficient time to show the potential of case management.
This systematic review of the published literature, available in English, on the current and potential role of the PN in the delivery of care to people living with dementia or cognitive impairment and their support person(s) evaluated thirteen studies.
There has been no previous systematic reviews of the role or potential role for the PN in the delivery of care to people living with dementia or cognitive impairment and their support person(s). The results from this review are therefore novel and should be used to inform the role of the PN in the provision of dementia care and also future research on this topic.
The heterogeneity of studies’ purpose, design, and outcomes measures make it difficult to synthesise the findings and draw conclusions. However, the heterogeneity did provide important insights into the different roles of nurses and advances understanding about the intervention itself rather than just its effectiveness. The only clearly defined role that was examined was that of the primary care based nurse as a care manager [ 15 , 18 , 19 , 35 ]. There were mixed findings regarding the effectiveness of the nurse-led care management model of care in improving quality of life measures for people living with dementia and their support person(s). However, no studies dismissed the potential of this model, with further research recommended. Callahan et al., [ 18 ] was assessed as the highest quality RCT study. The authors reported that a care management model of care can be implemented in primary care and that the effectiveness of the intervention depended on the key role of the nurse. All the nurses in these care management studies were registered nurses with dementia specific training, however in the Callahan et al., [ 18 ] study the care manager was a geriatric nurse practitioner. All health practitioners in the care manager studies described the experience as positive and perceived there to be benefits to the patient. Nurses did describe the role as time consuming and liaising with the primary care medical practitioner as cumbersome [ 15 , 39 ]. However, the care manager role was considered resource intensive, which could prove a challenge in its integration with practice routines that often operate, with limited time for consultations and budgetary constraints. The care management model described in Callahan et al., [ 18 ] was particularly resource intensive with one year of care management, weekly mentoring for the care manager, weekly then monthly patient contacts, and monthly care-giver support groups with concurrent exercise groups for the person living with dementia.
The other studies [ 3 , 11 , 12 , 29 , 32 , 34 , 39 , 41 , 43 ] explored characteristics of the role of the primary care based nurse in the care of people living with dementia and the support person. These studies were of variable quality but consistent in their outcomes. The PN was described as having an increasing profile in primary health care and being more accessible to patients, partly as a result of their changing role to include chronic disease management. There was recognition of the PN as the appropriate professional to take on the role of screening for cognitive impairment and monitoring, with the medical practitioner being responsible for diagnosis. The PN is usually responsible for the Over 75 health check which is currently underutilised [ 11 ] and provides an opportunity to identify people with cognitive impairment. A common issue in the studies was the poor recording of diagnosis or outcome of cognitive testing in electronic medical records. Several studies identified that post-diagnostic support and carer support were lacking in current dementia care provision in primary health care [ 29 , 35 , 39 ]. Patients with memory concerns reported that they would welcome the opportunity to discuss dementia risk reduction with the GP however the GP was not meeting this need [ 3 ]. This responsibility was reported as potentially within the scope of the primary care nurse role [ 3 ].
Developing good working relationships with the medical practitioner, familiarity with the primary care setting, perception of autonomy, dementia specific education and the embedding dementia care provision in primary health care were seen as essential to the success of the primary care nurse in dementia care provision. A consistent finding across the studies was that primary care nurses reported a lack of confidence in dementia care provision and the rating of their knowledge and skills as inadequate. This is despite the perception that nurses include themselves as an appropriate professional to discuss dementia with a patient. The need for education and training was stressed in all studies as necessary for successful dementia care provision. The use of guidelines was perceived as valuable by nurses to improve knowledge and standardise practice. Nurses in the care management models used detailed standardised protocols for dementia care provision.
Implications for practice and research
There is justification for the involvement of the PN in the recognition and care of people living with dementia and their support person(s). However, there is little evidence on the scope of practice and framework of primary care nurse models of dementia care provision. The different studies examined different aspects of the PNs role in relation to dementia. Differences in scopes of nurse practice and health systems mean one model of care may not be appropriate. However this systematic review provides insights into what components of a model of care may be effective. These roles included care management, identification and/ or management of behavioural and psychological symptoms of dementia. Some nurses were seconded from secondary care memory clinics, some were registered nurses working in general practice and one was a geriatric nurse practitioner. Dementia training for the nurses also greatly varied across studies from several hours to months and the types of training differed in breadth and intensity.
More high quality studies are required to establish the scope of practice, effectiveness, cost implications and the applicability of the PN role in the care of people living with dementia, or cognitive impairment, and their support person(s) in general practice.
Strengths and limitations
This is the first systematic review to investigate the role of the PN in the care of people living with dementia, or cognitive impairment, and their support person(s) in general practice. An explicit, systematic methodology was followed to review the published peer-reviewed literature relevant to the topic. National and international literature was reviewed and the studies utilised a variety of methodologies including qualitative, quantitative and mixed methods. It was not possible to conduct a meta-analysis due to the heterogeneous nature of the interventions. The studies included in this review were published in English only and grey or white literature was not included. Some studies may not have been identified by the search terms used in each database.
The aim of this systematic review was to investigate the role of the PN in the care of people living with dementia, or cognitive impairment, and their support person(s) in general practice. The potential value of the PN in the recognition and management of dementia has been acknowledged. However, the findings of this review revealed that there is limited evidence on the role of the PN in dementia care provision. The strength of this review is the identification of benefits of roles fulfilled by nurses in the general practice setting for people living with dementia and their support person(s). These included increased patient accessibility to the PN, early recognition and management of cognitive changes, care management and collaboration with the GP. Limitations of the provision of dementia care by the PN included a lack of definition of the role, inadequate dementia specific training, time constraints and poor communication with GPs.
Models of dementia care provision with mechanisms to support the practice nurse role and the embedding of it into usual general practice care have the potential to increase early recognition of cognitive impairment and more appropriate primary care management of dementia.
Availability of data and materials
Not applicable.
Abbreviations
Australian primary health nurse association
Community mental health nurse
Community nurse
Dementia care management
Family practitioner
General practitioner
Mini mental state examination
National health service
Quality of life
Randomised controlled trial
Koch T, Iliffe S. Dementia diagnosis and management: a narrative review of changing practice. Br J Gen Pract. 2011. https://doi.org/10.3399/bjp11X588493 .
Strivens E, Craig D. Managing dementia – related cognitive decline in patients and their caregivers. Aust Fam Physician. 2014;43(4):170–4.
PubMed Google Scholar
Millard FB, Kennedy RL, Baune BT. Dementia: Opportunities for risk reduction and early detection in general practice. Aust J Prim Health. 2011. https://doi.org/10.1071/PY10037 .
Phillips J, Pond D, Goode S. Timely diagnosis of Dementia: Can we do better? A report for Alzheimers Australia. NSW: Alzheimer’s Australia; 2011. p. 42. Paper 24.
Google Scholar
Brown L, Hansnata E, Anh LH. Economic cost of dementia in Australia 2016-2056. Canberra: Institute for Governance and Policy Analysis; 2017. p. 66.
Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000 Oct;160(19):2964–8.
Article CAS Google Scholar
Young J, Eley D, Patterson E, Turner C. A nurse-led model of chronic disease management in general practice - patients' perspectives. Aust Fam Pract. 2016;45(12):912–6.
Bunn F, Burn A, Goodman C, Robinson L, Rait G, Norton S, et al. Comorbidity and dementia: a mixed-method study on improving health care for people with dementia (CoDem). Health Serv Delivery Res. 2016;4(8).
Randall S, Crawford T, Currie J, River J, Betihavas V. Impact of community based nurse-led clinics on patient outcomes, patient satisfaction, patient access and cost-effectiveness - a systematic review. Int J Nurs Stud. 2017;73:24–33.
Article Google Scholar
Ibrahim JE, Anderson LJ, MacPhail A, Lovell JJ, Davis M, Winbolt M. Chronic disease self-management support for persons with dementia in a clinical setting. J Multidiscip Healthc. 2017;10:49–58.
Trickey H, Turton P, Harvey I, Wilcock G, Sharp D. Dementia and the Over-75 Check: the role of the primary care nurse. Health Soc Care Commun. 2000;8(1):9–16.
Manthorpe J, Iliffe S, Eden A. Early recognition of dementia by nurses. J Adv Nurs. 2003;44(2):183–91.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati L, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P). 2015: elaboration and explanation. BMJ. 2015. https://doi.org/10.1136/bmj.g7647 .
Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Research Methods and Reporting. BMJ. 2009. https://doi.org/10.1136/bmj.b2700 .
Van den Dungen P, Moll van Charante EP, van de Ven PM, van Marwijk HW, van der Horst HE, van Hout HPJ. Case Finding of Mild Cognitive Impairment and Dementia and Subsequent Care; Results of a Cluster RCT in Primary Care. PLoS One. 2016. https://doi.org/10.1371/journal.pone.0156958 .
Perry M, Draskovic I, van Achterberg T, Borm G, van Eijken M, Lucassen P, et al. Can an EASYcare based dementia training programme improve diagnostic assessment and management of dementia by GPs and primary care nurses? The design of a randomised controlled trial. BMC Health Serv Res. 2008. https://doi.org/10.1186/1472-6963-8-71 .
Van den Dungen P, Moll van Charante EP, van Marwijk HW, van der Horst HE, van de Ven PM, van Hout HP. Case-finding of dementia in general practice and effects of subsequent collaborative care; design of a cluster RCT. BMC Public Health. 2012;12:609.
Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: A randomized controlled trial. JAMA. 2006. https://doi.org/10.1001/jama.295.18.2148 .
Thyrian JR, Hertel J, Wucherer D, Eichler T, Michalowsky B, Dreier-Wolfgramm A, et al. Effectiveness and Safety of Dementia Care Management in Primary Care: A Randomized Clinical Trial. JAMA Psychiatry. 2017. https://doi.org/10.1001/jamapsychiatry.2017.2124 .
Drier A, Thyrian JR, Eichler T, Hoffman W. Qualifications for nurses for the care of patients with dementia and support to their caregivers: A pilot evaluation of the dementia care management curriculum. Nurse Educ Today. 2016. https://doi.org/10.1016/j.nedt.2015.07.024 .
Thyrian JA, Fib T, Dreier A, Bowing G, Angel A, Lueke S, et al. Life- and person-centred help in Mecklenburg - Western Pomerania, Germany (DelpHi): study protocol for a randomised controlled trial. Trials. 2013;13:56.
Austrom MG, Hartwell C, Moore PS, Boustani M, Hendrie HC, Callahan CM. A care management model for enhancing physician practice for Alzheimer disease in primary care. Clin Gerontol. 2005. https://doi.org/10.1300/J018v29n0205 .
Austrom MG, Hartwell C, Moore P, Perkins AJ, Damush T, Unverzagt FW, et al. An integrated model of comprehensive care for people with Alzheimer's disease and their caregivers in a primary care setting. Dementia. 2006;5(3):339–52.
Austrom MG, Damush TM, Hartwell CW, Perkins T, Unverzagt FW, Boustani M, et al. Development and Implementation of Nonpharmacological Protocols for the Management of Patients with Alzheimer's Disease and their Families in a Multiracial Primary Care Setting. Gerontologist. 2004;44(4):548–53.
Boustani M, Callahan CM, Unverzagt FW, Austrom MG, Perkins AJ, Fultz BA, et al. Implementing a Screening and Diagnosis Program for Demenia in Primary Care. J Gen Intern Med. 2005. https://doi.org/10.1111/j.1525-1497.2005.0126.x .
Downs M, Rae C. General practitioners' approach to establishing and communicating a diagnosis of dementia. In: Annual Conference of the British Society of Gerontology, Liverpool University; 1996.
Iliffe S, Eden A, Downs M, Rae C. The diagnosis and management of dementia in primary care: development, implementation and evaluation of a national training program. Aging Ment Health. 1999;3(2):129–35.
Iliffe S, Manthorpe J, Eden A. Sooner or later? Issues in the early diagnosis of dementia in general practice: A qualitative study. Fam Pract. 2003. https://doi.org/10.1093/fampra/cmg407 .
Gilbert C, Wilcock J, Thuné-Boyle I, Iliffe S. A comparison of service use by people with dementia in two samples a decade apart. Dementia. 2017;2017. https://doi.org/10.1177/1471301215581504 .
Downs M, Turner S, Bryans M, Wilcock J, Keady J, Leven E, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomised controlled study. BMJ. 2006;332:692.
Iliffe S, Wilcock J, Griffin M, Jain P, Thune-Boyle I, Koch T, et al. Evidence-based interventions in dementia: A pragmatic cluster-randomised trial of an educational intervention to promote earlier recognition and response to dementia in primary care (EVIDEM-ED). Trials. 2010;11:13.
Ollerenshaw A, Wong Shee A, Yates M. Towards good dementia care: Awareness and uptake of an online Dementia Pathways tool for rural and regional primary health practitioners. Aust J Rural Health. 2017. https://doi.org/10.1111/ajr.12376 .
Millard FB, Baune BT. Dementia - who cares? A comparison of community needs and primary care services. Aust Fam Physician. 2009;38(6):642–9.
Perry M, Draskovic I, Van Achterberg T, Van Eijken M, Lucassen P, Vernooij-Dassen M, et al. Development and validation of quality indicators for dementia diagnosis and management in a primary care setting. J Am Geriatr Soc. 2010. https://doi.org/10.1111/j.1532-5415.2010.02726.x .
Iliffe S, Robinson L, Bamford C, Waugh A, Fox C, Livingston G, et al. Introducing case management for people with dementia in primary care: a mixed-methods study. Br J Gen Pract. 2014. https://doi.org/10.3399/bjgp14X682333 .
Bamford C, Poole M, Brittain K, Chew-Graham C, Fox C, Iliffe S, et al. Understanding the challenges to implementing case management for people with dementia in primary care in England: a qualitative study using Normalization Process Theory. BMC Health Serv Res. 2014;14:549.
Iliffe S, Waugh A, Poole M, Bamford C, Brittain K, Chew-Graham C, et al. The effectiveness of collaborative care for people with memory problems in primary care: Results of the CAREDEM case management modelling and feasibility study. Health Technol Assess. 2014. https://doi.org/10.3310/hta18520 .
Waugh A, Austin A, Manthorpe J, Fox C, Stephens B, Robinson L, Iliffe S. Designing a complex intervention for dementia case management in primary care. BMC Fam Pract. 2013;14:101.
Dodd E, Cheston R, Fear T, Brown E, Fox C, Morley C, et al. An evaluation of primary care led dementia diagnostic services in Bristol. BMC Health Serv Res. 2014. https://doi.org/10.1186/s12913-014-0592-3 .
Clarke JP. How to peer review a qualitative manuscript. In: Godlee F, Jefferson T, editors. Peer Review in Health Sciences. 2nd ed. London: BMJ Books; 2003. p. 219–35.
Dodd E, Cheston R, Cullum S, Jefferies R, Ismail K, Gatting L, et al. Primary care-led dementia diagnosis services in South Gloustershire: Themes from people and families living with dementia and health care professionals. Dementia. 2016. https://doi.org/10.1177/1471301214566476 .
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Manthorpe J, Iliffe S, Eden A. The implications of the early recognition of dementia for multiprofessional teamworking. Dementia. 2003;2(2):163–79.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011. https://doi.org/10.1136/bmj.d5928 .
CEBMa. Critical Appraisal of a Survey. In: Crombie, The Pocket Guide to Critical Appraisal: the critical appraisal approach used by the Oxford Centre for Evidence Medicine, checklists of the Dutch Cochrane Centre, BMJ editor's checklists of the EPPI Centre. 2018. Available at: https://www.cebma.org/wp-content/uploads/Critical-Appraisal-Questions-for-a-Survey.pdf . Accessed 14 Jul 2019.
Hong Q, Pluye P, Fabregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT). User Guide. Canada: Canadian Intellectual Property Office, Industry Canada; 2018.
Critical Appraisal Skills Programme. CASP Qualitative Checklist. UK: Oxford. 2018. Available from: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf . .
Goeman D, Renehan E, Koch S. What is the effectiveness of the support worker role for people with dementia and their carers? A systematic review. BMC Health Serv Res. 2016. https://doi.org/10.1186/s12913-016-1531-2 .
Thomas J, Harden A, Oakley A, Oliver S, Suttcliffe K, Rees R, et al. Integrating qualitative research with trials in systematic reviews. BMJ. 2004;328:7447.
Bryans M, Keady J, Turner S, Wilcock J, Down M, Iliffe S. An exploratory study into primary care nurses and dementia care. Brit J Nurs. 2003;12(17):1029–37.
Download references
Acknowledgements
This research contributes to a larger program of work being conducted by the Australian Community of Practice in Research in Dementia (ACcORD), which is funded by a Dementia Research Team Grant from the National Health and Medical Research Council. Caroline Gibson is supported by a University of Newcastle Postgraduate Research Scholarship from the Faculty of Health and Medicine.
Author information
Authors and affiliations.
Faculty of Health and Medicine, School of Medicine and Public Health, University of Newcastle, Callaghan, Australia
Caroline Gibson, Dianne Goeman & Dimity Pond
Central Clinical School, Monash University; Kolling Institute, the University of Sydney, Sydney, Australia
Dianne Goeman
You can also search for this author in PubMed Google Scholar
Contributions
CG, DG and DP developed the review question, designed the review and developed the search strategy. CG and DG conducted the selection, undertook the data extraction and conducted the quality assessment. DP helped resolve any discrepancies in the quality assessment. CG and DG wrote the manuscript and DP provided critical commentary on initial versions of the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Correspondence to Caroline Gibson .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declared no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gibson, C., Goeman, D. & Pond, D. What is the role of the practice nurse in the care of people living with dementia, or cognitive impairment, and their support person(s)?: a systematic review. BMC Fam Pract 21 , 141 (2020). https://doi.org/10.1186/s12875-020-01177-y
Download citation
Received : 25 December 2019
Accepted : 28 May 2020
Published : 13 July 2020
DOI : https://doi.org/10.1186/s12875-020-01177-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Practice nurse
- Primary health care nurse
- Cognitive impairment
BMC Primary Care
ISSN: 2731-4553
- General enquiries: [email protected]

- Advanced Life Support
- Endocrinology
- Gastroenterology
- Infectious disease
- Intensive care
- Palliative Care
- Respiratory
- Rheumatology
- Haematology
- Endocrine surgery
- General surgery
- Neurosurgery
- Ophthalmology
- Plastic surgery
- Vascular surgery
- Abdo examination
- Cardio examination
- Neurolo examination
- Resp examination
- Rheum examination
- Vasc exacmination
- Other examinations
- Clinical Cases
- Communication skills
- Prescribing

Dementia case study with questions and answers

Dementia case study with questions and answers
Common dementia exam questions for medical finals, OSCEs and MRCP PACES
The case below illustrates the key features in the assessment of a patient with dementia or undiagnosed memory decline. It works through history, examination and investigations – click on the plus symbols to see the answers to each question
Part 1: Mavis
- Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law.
- On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
- The patient herself acknowledges that there have been memory problems, but feels it is just her short term memory that is an issue.
Question 1.
- To begin the history, start broadly. Build rapport and establish both the patient’s view on memory impairment (if any) and the family’s (or other collateral history).
- Patient’s (and collateral) view of memory decline
- Biographical history
- Objective view of memory decline (e.g. knowledge of current affairs)
- Impact of memory decline on day-to-day living and hobbies
- Social history, including safety and driving
- General medical history (especially medications)
- See below for details on these…
Question 2.
- Is it for everything or are specific details missed out/glossed over?
- Try to pin down specific details (e.g. names of people/places).
- At what time in chronological order do things start to get hazy?
Question 3.
- If under 12 years this will lead to additional point being awarded on some cognitive tests
- Ask about long term memories, e.g. wedding day or different jobs
- Then move on to more recent memories, e.g. last holiday
Question 4.
- If your patient watches the news/read newspapers on a regular basis, ask them to recount the headlines from the past few days.
- Be sure to look for specifics to prevent your patient masking memory deficiencies with broad statements. For example: “The government are incompetent, aren’t they?!” should be clarified by pinning down exactly why they are incompetent, for example: “Jeremy Hunt”.
- If they like to read, can they recall plotlines from current books or items from magazines?
- If they watch TV, can they recount recent plot lines from soaps, or formats of quiz shows?
Question 5.
- Ask about hobbies and other daily activities, and whether or not these have declined recently.
- If your patient no longer participates in a particular hobby, find out why: is it as a result of a physical impairment (e.g. arthritis making cooking difficult), or as the result of a loss of interest/ability to complete tasks (e.g. no longer able to complete crosswords/puzzles).
- Once you have a good idea of the memory decline itself, begin to ask about other features. Including a social and general medical history.

Question 6.
- Review their social history and current set-up, and also subjective assessments from both patient and family over whether or not the current arrangements are safe and sustainable as they are.
- Previous and ongoing alcohol intake
- Smoking history
- Still driving (and if so, how safe that is considered to be from collateral history)
- Who else is at home
- Any package of care
- Upstairs/downstairs living
- Meal arrangements (and whether weight is being sustained).
- Of all these issues, that of driving is perhaps one of the most important, as any ultimate diagnosis of dementia must be informed (by law) to both the DVLA and also the patient’s insurers. If you feel they are still safe to drive despite the diagnosis, you may be asked to provide a report to the DVLA to support this viewpoint.
Now perform a more generalised history, to include past medical history and – more importantly – a drug history.
Question 7.
- Oxybutynin, commonly used in primary care for overactive bladder (anticholinergic side effects)
- Also see how the medications are given (e.g. Dossett box)
- Are lots of full packets found around the house?
Part 2: The History
On taking a history you have found:
- Mavis was able to give a moderately detailed biographical history, but struggled with details extending as far back as the location of her wedding, and also her main jobs throughout her life.
- After prompting from her family, she was able to supply more information, but it was not always entirely accurate.
- Her main hobby was knitting, and it was noted that she had been able to successfully knit a bobble hat for her great-grand child as recently as last month, although it had taken her considerably longer to complete than it might have done a few years previously, and it was a comparatively basic design compared to what she has been able to create previously.
- She has a few children living in the area, who would frequently pop in with shopping, but there had been times when they arrived to find that she was packed and in her coat, stating that she was “just getting ready to go home again”.
- She had been helping occasionally with the school run, but then a couple of weekends ago she had called up one of her sons – just before she was due to drive over for Sunday lunch – and said that she could not remember how to drive to his house.
- Ever since then, they had confiscated her keys to make sure she couldn’t drive. Although she liked to read the paper every day, she could not recall any recent major news events. Before proceeding to examine her, you note that the GP referral letter has stated that her dementia screen investigations have been completed.
Question 8.
- Raised WCC suggests infection as a cause of acute confusion
- Uraemia and other electrolyte disturbances can cause a persistent confusion.
- Again, to help rule out acute infection/inflammatory conditions
- Liver failure can cause hyperammonaemia, which can cause a persistent confusion.
- Hyper- or hypothyroidism can cause confusion.
- B12 deficiency is an easily missed and reversible cause of dementia.
- This looks for space occupying lesions/hydrocephalus which may cause confusion.
- This can also help to determine the degree of any vascular component of an ultimately diagnosed dementia.
Part 3: Examination
- With the exception of age-related involutional changes on the CT head (noted to have minimal white matter changes/small vessel disease), all the dementia screen bloods are reassuring.
- You next decide to perform a physical examination of Mavis.
Question 9.
- Important physical findings that are of particular relevance to dementia, are looking for other diseases that may have an effect on cognition.
- To look for evidence of stroke – unlikely in this case given the CT head
- Gait (shuffling) and limb movements (tremor, rigidity, bradykinesia)
- Affect is also important here and may also point to underlying depression
- Pay attention to vertical gaze palsy, as in the context of Parkinsonism this may represent a Parkinson plus condition (e.g. progressive supranuclear palsy).
- It is also useful to look at observations including blood pressure (may be overmedicated and at risk of falls from syncope) and postural blood pressure (again, may indicate overmedication but is also associated with Parkinson plus syndromes e.g. MSA)
Part 4: Cognitive Testing
- On examination she is alert and well, mobilising independently around the clinic waiting room area. A neurological examination was normal throughout, and there were no other major pathologies found on a general examination.
- You now proceed to cognitive testing:
Question 10.
- Click here for details on the MOCA
- Click here for details on the MMSE
- Click here for details on the CLOX test
Part 5: Diagnosis
- Mavis scores 14/30 on a MOCA, losing marks throughout multiple domains of cognition.
Question 11.
- Given the progressive nature of symptoms described by the family, the impairment over multiple domains on cognitive testing, and the impact on daily living that this is starting to have (e.g. packing and getting ready to leave her own home, mistakenly believing she is somewhere else), coupled with the results from her dementia screen, this is most likely an Alzheimer’s type dementia .
Question 12.
- You should proceed by establishing whether or not Mavis would like to be given a formal diagnosis, and if so, explain the above.
- You should review her lying and standing BP and ECG, and – if these give no contraindications – suggest a trial of treatment with an acetylcholinesterase inhibitor, such as donepezil.
- It is important to note the potential side effects – the most distressing of which are related to issues of incontinence.
- If available, put her in touch with support groups
- Given the history of forgetting routes before even getting into the care, advise the patient that she should stop driving and that they need to inform the DVLA of this (for now, we will skip over the depravation of liberty issues that the premature confiscation of keys performed by the family has caused…)
- The GP should be informed of the new diagnosis, and if there are concerns over safety, review by social services for potential support should be arranged.
- Follow-up is advisable over the next few months to see whether the trial of treatment has been beneficial, and whether side effects have been well-tolerated.
Now click here to learn more about dementia
Perfect revision for medical students, finals, osces and mrcp paces, …or click here to learn about the diagnosis and management of delirium.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Case Study 3: A 58-Year-Old Woman Referred for Evaluation of Suspected Alzheimer Dementia
Case presentation.
A 58-year-old left-handed woman with 12 years of education was referred for further evaluation and management of progressive cognitive dysfunction. A diagnosis of Alzheimer disease (AD) was suspected by the referring neurologist on the basis of an MRI demonstrating mild temporal atrophy, EEG demonstrating bitemporal slowing, positive cerebrospinal fluid (CSF) AD biomarkers, and apolipoprotein E ( APOE ) ε4, ε4 genotype. The patient had a 4-year history of progressive cognitive symptoms. Early symptoms included difficulties recalling recent events and information, sustaining attention, and learning new tasks. She was repeatedly disoriented to the time of day; for example, she would get dressed for the day in the middle of the night. She had difficulties finding words and expressing her thoughts, with a decline in the richness of her vocabulary.
An initial clinical interview revealed that the patient had become increasingly withdrawn from family and friends. Over the year prior to evaluation at our center, she developed an array of neuropsychiatric symptoms, including depression, anxiety, and recurrent, well-formed visual hallucinations of unfamiliar people in her house. She did not feel threatened or bothered by these people and retained insight that others could not see them.
The patient had last worked as an administrative assistant approximately 3 years prior to evaluation at our center and was unable to obtain new work because of her cognitive symptoms. More recently, her husband assumed responsibility for over-seeing administration of her medications and for shopping and most meal planning and preparation. She continued to drive intermittently and to prepare breakfast independently. She remained independent in basic activities of daily living, including showering, dressing, eating, toileting, and mobility.
The patient’s mother and father lived into their 70s and 80s, respectively, and died from cancer, without any history of progressive cognitive impairment or dementia. A maternal aunt who died in her 70s had progressive cognitive impairment starting in her late 50s.
- Questions: What are the diagnostic considerations based on the history? Is this presentation suggestive of AD? Would additional history be helpful?
Insidious onset and gradual progression of cognitive symptoms over the course of 4 years raises concern for a neurodegenerative disorder. In this type of scenario, it is useful to derive a three-tiered diagnostic formulation comprising neurodegenerative clinical syndrome, severity, and suspected underlying neuropathology ( 1 , 2 ). Setting aside the reports of the neuroimaging, CSF, and genetic results, aspects of the patient’s history suggesting changes in episodic memory, attention, executive function, and word retrieval could suggest a multidomain, amnesic syndrome as frequently occurs in the context of AD neuropathology. However, recurrent, well-formed visual hallucinations are very uncommon with isolated AD neuropathology and suggestive of contributions from Lewy body disease (LBD) neuropathology, as occurs in association with syndromic dementia with Lewy bodies (DLB), Parkinson disease (PD), or PD with dementia (PDD) ( 3 ). It would be useful to know whether this patient has additional history indicating other core clinical features of a DLB syndrome, including fluctuating cognition with pronounced variations in attention and alertness; REM sleep behavior disorder (RBD), suggested by dream enactment behaviors; and motor symptoms potentially reflecting parkinsonism ( 4 , 5 ). Instruments such as the Mayo Fluctuations Scale and the Queen Square Visual Hallucination Inventory provide useful questions for assessing the range of fluctuation phenomena and minor and major visual hallucinations and illusions possible in DLB ( 6 , 7 ) ( Figure 1 ). Additional supportive clinical features would include various other symptoms in the domains of motor function, sleep, neuropsychiatric function, sensory processing, and autonomic function ( 8 – 11 ).

Clinical features of mild cognitive impairment and dementia with Lewy bodies a
a Asterisks denote essential (***), core (**), and supportive (*) clinical features per consensus diagnostic criteria ( 4 , 5 ). †, item from the Mayo Fluctuations Scale ( 6 ); ‡, item from the Queen Square Visual Hallucination Inventory ( 7 ); MCI, mild cognitive impairment; OSA, obstructive sleep apnea; PLMS, periodic limb movements of sleep; REM, rapid eye movement; RLS, restless legs syndrome.
Additional history revealed that the patient’s husband observed her to have periods of being in a “trance-like” state, as well as drowsiness and an increased tendency to sleep in the daytime. She was noted to have recurrent episodes of “acting out her nightmares,” at times kicking and screaming, dating back to the onset of her cognitive symptoms. Her walking had slowed, and her voice had become softer. She was slower and less coordinated when using her hands, her handwriting became smaller, and she developed intermittent tremors of her hands and arms when using them. She experienced constipation with increasing frequency and severity.
- Question: What are the aims of the cognitive and neurological examinations in this context?
In the context of a history highly suggestive of DLB, one can increase diagnostic confidence by establishing a suggestive neuropsychological profile or by confirming features of parkinsonism on examination. DLB is frequently associated with early impairments in attention, executive function, and visuospatial processing ( 12 – 14 ). To assess parkinsonism, it is useful to gain familiarity with elements of the motor examination section of the Movement Disorders Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale, including assessment of speech, facial expression, rigidity, finger tapping, hand movements (opening and closing), hand pronation-supination, toe tapping, leg agility (foot stomping), arising from chair, gait, posture, postural stability, global spontaneity of movement, and presence or absence of postural, kinetic, or rest tremors of the hands ( 15 ). PD syndrome is more likely to be associated with typical parkinsonism, i.e., early asymmetrical “pill-rolling” resting tremor, limb bradykinesia and rigidity that tend to be responsive to levodopa, and minimal to no early postural instability. Although typical parkinsonism can occur in DLB, atypical features occur more frequently, including the absence of resting tremor, the presence of postural-kinetic or mixed tremor, less prominent and more symmetrical early bradykinesia and rigidity, more prominent early postural instability, and reduced responsiveness to levodopa ( 16 – 18 ). Despite these distinctions, it is noteworthy that all symptomatic features of DLB can occur in PDD and vice versa, with the arbitrary distinguishing feature between the syndromes being whether cognitive dysfunction develops prior to or concurrently with parkinsonism (as in DLB and as was observed in this patient) or following parkinsonism (as in PDD) ( 4 ). Considering PD, PDD, and DLB as syndromes under the umbrella term “Lewy body disorders” allows for variability in presentations along a spectrum, from individuals with predominantly motor symptoms to those with predominantly cognitive symptoms ( 19 ). These disorders all share a common neuropathological substrate that is indistinguishable at the microscopic level but appears to differ in terms of topographical distribution and spreading patterns ( 20 ).
This patient’s mental status examination revealed grossly apparent psychomotor slowing. She scored a 24/30 on the Mini-Mental State Examination, missing 2 points on orientation to year and day of the week, 2 points on the three-item delayed word recall test (obtaining both with a category cue), and 1 point for poor pentagon copy ( 21 ). She was given a subset of the Hooper Visual Organization Test, on which she correctly identified seven of 13 objects represented in line drawings as puzzle pieces (suggesting a moderate level of visuospatial impairment) ( 22 ).
On elemental neurological examination, the patient had hypomimia, saccadic intrusions during smooth-pursuit eye movements, and slow, hypophonic speech. Strength was full in the proximal and distal muscles of the arms and legs. There was a postural-kinetic tremor of both hands, mild left-greater-than-right bradykinesia apparent on finger tapping and hand movements, and mild left-greater-than-right cogwheel rigidity in the arms. She rose from a chair easily, without the use of her arms. Her gait was mildly slow, with a narrow base and left-greater-than-right reduced arm swing. There was no retropulsion on pull testing.
Taken together, these examination results provided additional support for a DLB syndrome. The cognitive examination, though limited and nonspecific, provided some evidence of slow processing speed, impaired memory (at least at the level of retrieval), and visuospatial dysfunction. The motor examination provided unequivocal evidence of parkinsonism, some features that were typical, such as asymmetrical limb bradykinesia and cogwheel rigidity, and other features that were atypical, such as postural-kinetic tremor of the hands.
- Question: What initial tests and studies are indicated?
Structural neuroimaging of the brain, preferably with MRI, is a recommended component in the initial evaluation of suspected dementia ( 23 , 24 ). When specific neurodegenerative causes of dementia are under consideration, imaging serves at least two purposes. First, it helps to assess for evidence of alternative (nondegenerative) conditions that might account for or contribute to symptoms. Second, it helps to assess for atrophy in a topographical distribution suggestive of a neurodegenerative syndrome and/or neuropathology, which can be useful in cases with possible underlying AD or frontotemporal lobar degeneration (FTLD) neuropathological changes ( 25 ). In a case such as this one, with features suggesting a DLB syndrome, imaging can be useful to assess for alternative causes of parkinsonism, such as vascular disease. While relative preservation of medial temporal lobe (MTL) regions on structural neuroimaging represents a supportive biomarker for DLB, evidence of MTL atrophy does not preclude a diagnosis of DLB, particularly considering that a DLB clinical syndrome may be associated with mixed LBD and AD neuropathological changes ( 4 , 26 ).
In cases of suspected DLB with profound fluctuations in attention, focal dyscognitive seizures are included in the differential diagnosis. Here, an EEG can be useful to distinguish between epileptiform activity (which is consistent with seizures) and prominent posterior slow-wave activity with periodic fluctuations in the pre-alpha/theta range (which is another supportive biomarker for DLB) ( 4 ).
Otherwise, a standard laboratory evaluation including comprehensive metabolic profile (CMP), vitamin B12 level, TSH, and complete blood counts (CBC) would add value in screening for potential contributing factors.
This patient’s MRI, completed approximately 9 months prior to referral, demonstrated a mild degree of T2 hyper-intense signal changes in the periventricular, subcortical and juxtacortical white matter. There was no diffusion restriction, evidence of atrophy, or abnormal enhancement ( Figure 2 ). A routine EEG demonstrated “mild intermittent bitemporal irregular slowing with no focal or generalized epileptiform features.” There were no pertinent lab result abnormalities.
- Question: Are additional tests and studies indicated?
Given the presence of all four core clinical features, multiple supportive clinical features (constipation, anxiety, and depression), and a supportive biomarker (relative preservation of MTL structures on MRI) for DLB in this case, there was no strong rationale to obtain additional data to confirm the diagnosis. In cases in which a diagnosis of DLB is less clear, consideration may be given to obtaining what have been designated indicative biomarkers, including dopamine transporter SPECT or PET imaging for evidence of reduced uptake in the basal ganglia, 123 iodine-metaiodobenzylguanidine (MIBG) myocardial scintigraphy for evidence of low uptake, or polysomnography for confirmation of REM sleep without atonia ( 4 ).

MRI of the patient’s brain obtained 9 months prior to referral and approximately 3 years after the onset of symptoms
At the time of the patient’s initial presentation, when Lewy body features were less apparent, the referring neurologist had a higher level of suspicion for an amnesic syndrome with underlying AD neuropathology than for a DLB syndrome with underlying LBD or mixed LBD-AD neuropathology. Concern for AD in a patient with mild cognitive impairment or dementia at a young age at onset (<65 years) represents an appropriate indication for obtaining CSF beta-amyloid-42 (Aβ42), total tau, and phosphorylated tau levels, as occurred in this case ( 27 ). Molecular biomarkers of AD neuropathology can also provide value in etiologically mixed presentations in which AD might contribute to an illness in which other factors are also suspected, e.g., LBD, vascular neuropathology, or both ( 28 ). Indeed, CSF evidence of underlying AD in patients with DLB has been associated with more rapid cognitive decline ( 29 ).
CSF results were suggestive of AD, because Aβ42 was low at 437.2 pg/ml, total tau was elevated at 639.7 pg/ml, the Aβ42-to-total tau index was low at 0.44, and phosphorylated tau was elevated at 83 pg/ml.
- Question: Is genetic testing indicated?
Experts do not recommend routine clinical genetic testing for patients with DLB or patients with AD who lack a family history suggesting autosomal-dominant inheritance ( 4 , 30 ). In cases in which a familial autosomal-dominant neurodegenerative disorder is suggested by the clinical presentation and family history, consideration may be given for referral to a genetics counselor and potential testing for mutations in selected genes. Families with an autosomal-dominant disorder typically contain at least three affected individuals in two or more generations, with two of the individuals being first-degree relatives of the third individual, and the disorder usually involves an early age at onset. Although the presence of the APOE ε4 allele is a risk factor for both AD and DLB, its presence is neither sensitive to nor specific for either condition, and APOE genotyping is not recommended as part of a diagnostic evaluation ( 30 , 31 ). Although the utility of APOE genotyping for purposes other than diagnosis, such as clinical prognostication, has not been studied as extensively, the presence of an APOE ε4 allele in patients with DLB has been associated with greater severity of LBD neuropathology, independent of AD neuropathology, and shorter mean time between onset of cognitive symptoms and death ( 32 , 33 ).
Prior to referral, this patient tested negative for mutations in PSEN1 , PSEN2 , and APP . She was found to be a homozygous carrier of the APOE ε4 allele.
- Question: What would be an appropriate diagnostic formulation?
As reviewed above, ample evidence supported a syndromic diagnosis of DLB. The patient’s loss of independence in selected instrumental activities of daily living supported staging at a level of mild dementia. Regarding suspected neuropathology, several factors supported a prediction of mixed LBD and AD neuropathology. A clinical diagnosis of DLB has greater than 95% specificity for pathological confirmation of diffuse neocortical Lewy bodies ( 34 ). Clinical and neuropathological correlation studies likewise suggest that 60%–70% of cases with a clinical diagnosis of DLB have intermediate- or high-level AD copathology ( 26 ).
- Question: What are reasonable therapeutic considerations?
Because many different types of symptoms can arise in the context of DLB, it is helpful to take a systematic approach to therapeutic planning by reviewing the nature and severity of disease impact on cognition, neuropsychiatric health (e.g., mood, anxiety, and psychosis), sleep, motor function, and autonomic function. Considering the impact of symptoms within each of these domains on the patient’s quality of life and ability to carry out intended activities helps to identify and prioritize targets for pharmacological intervention ( 35 – 50 ) ( Table 1 ). Additional research is needed to establish stronger evidence for many symptomatic treatments ( 35 , 45 , 51 ). Importantly, nontrivial symptomatic benefits can frequently be obtained from discontinuing and avoiding non-essential medications with anticholinergic or dopamine receptor–blocking properties. Neuroleptic medications in particular should be avoided, given their propensity to precipitate severe, potentially life-threatening reactions ( 52 ).
Symptomatic pharmacotherapeutic considerations in dementia with Lewy bodies (DLB) a
At the time of referral, the symptoms with the greatest impact on this patient’s level of function and quality of life were those involving her cognitive function, mood, anxiety, and sleep. Although meaningful in terms of diagnosis, the patient’s formed visual hallucinations and motor symptoms were not causing significant distress at presentation and therefore did not warrant pharmacological treatment. Evidence from randomized controlled trials and meta-analyses supports the efficacy of cholinesterase inhibitors for cognitive and potentially for neuropsychiatric symptoms (including anxiety, delusions, and hallucinations) in DLB patients ( 53 , 54 ). Rivastigmine and donepezil have been studied more extensively than galantamine, and the results with rivastigmine and donepezil have been comparable ( 35 , 36 ). Either would be a reasonable choice for this patient, with monitoring for sleep-related and gastrointestinal side effects ( Table 1 ).
No systematic studies of antidepressants or anxiolytics have been conducted for treatment of depression or anxiety among patients with DLB ( 55 ). Selected selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, such as sertraline, escitalopram, and venlafaxine, may provide benefit, although they should be used carefully (i.e., “start low and go slow”) given their potential to cause or exacerbate gastrointestinal and sleep-related problems in this population. Patients with treatment-refractory depression may benefit from repetitive transcranial magnetic stimulation or electroconvulsive therapy ( 56 , 57 ).
In cases of RBD involving frequent, disruptive, or injurious behaviors, melatonin is often well tolerated and effective in reducing dream enactment behaviors ( 58 ). Standard practice is to start at 3 mg or 5 mg and to titrate weekly in increments of 3 mg or 5 mg as needed, up to 15 mg to 18 mg nightly ( 59 ). Clonazepam may be used as a second-line treatment in severe cases; however, the potential to exacerbate cognitive dysfunction and obstructive sleep apnea should be noted.
This patient was started on transdermal rivastigmine, titrated from 4.6 mg daily to 9.5 mg daily after 1 month, and there were notable reductions in her visual hallucinations and fluctuations in attention and alertness. She did not tolerate a further increase in dose to 13.3 mg daily on account of insomnia. She derived a moderate benefit from melatonin 6 mg nightly, with respect to reducing dream enactment behaviors. Sertraline, started approximately 6 months later, was effective in reducing her anxiety at a dose of 50 mg daily. Insomnia, initially nonresponsive to medications, including trazodone and mirtazapine, improved later with an increase in the dose of melatonin to 15 mg nightly.
The patient’s clinical status deteriorated steadily despite these early symptomatic improvements. Two years after her initial behavioral neurology evaluation and 6 years after onset of symptoms, she required full-time supervision and assistance to pick out clothes and dress, and she appeared frequently frustrated and agitated. Her husband derived benefit from visits with a social worker in the behavioral neurology and neuropsychiatry unit, who provided disease-specific counseling and education, assistance with completion of medical and financial legal documents, and recommendations on care arrangements and reviewed behavioral and environment-based strategies for managing neuropsychiatric symptoms. In-home services and support from additional family members allowed her husband to continue to work on a part-time basis.
In subsequent months, the patient’s course was complicated by intermittent problems, such as dehydration, constipation, and emotional lability, and by chronic problems, including the loss of communicative abilities and mobility. She was transitioned to a memory care unit and later to skilled nursing. She died at age 63, 5 years after the initial behavioral neurology presentation and 9 years after the onset of symptoms.
NEUROPATHOLOGY
The weight of the patient’s brain was 1,160 g, which is at the lower end of the normal range for an adult female. On gross examination of the right cerebral hemisphere (the left hemisphere was banked for future research), there was mild atrophy of the anterior temporal lobe and superior temporal gyrus and no significant atrophy of the frontal, parietal, and occipital lobes. Cut sections of the midbrain revealed pallor of the substantia nigra. Similar gross depigmentation was noted in the region of the locus coeruleus in the pons.
Histologically, microscopy with hematoxylin and eosin staining confirmed a loss of pigmented neurons in the substantia nigra, as well as abundant extraneuronal pigment. There were frequent small, round eosinophilic inclusions in the neurons of the substantia nigra, representing Lewy bodies ( Figure 3 ). These were also identified with synuclein immunohistochemistry (IHC) as higher-intensity brown areas, with horseradish peroxidase chromagen–reflecting, synuclein-positive intraneuronal inclusions, which are distinct from the smaller punctate pigmented endogenous neuromelanin granules. Numerous synuclein-positive neurites were also apparent. Moderate or frequent Lewy bodies were likewise seen in the medulla, locus coeruleus, nucleus basalis of Meynert, amygdala, hippocampus, and anterior cingulate cortex.

Histopathological findings for a patient with mixed Lewy body dementia and Alzheimer disease neuropathology a
a Microscopic views of the midbrain substantia nigra with hematoxylin and eosin (panel A) and synuclein immunohistochemistry (panel B) demonstrate Lewy bodies (arrows) and extraneuronal pigment (arrow-head). Tau immunohistochemistry on Ammon’s horn of the hippocampus indicates the presence of neurofibrillary tangles (panel C). β-amyloid immunohistochemistry of the frontal cortex indicates the presence of β-amyloid plaques (arrow) and cerebral amyloid angiopathy (arrowhead) (panel D).
LBD can be classified into one of three neuropathological types—brainstem predominant, limbic (transitional), or diffuse neocortical—by semiquantitative grading of Lewy body severity in selected regions in the brainstem, basal forebrain and limbic systems, and neocortex ( 60 ). In this case, the presence of moderate numbers of synuclein-positive inclusions in the frontal, temporal, and parietal cortices reflected relatively severe disease and merited the designation of diffuse neocortical LBD.
In the hippocampus, mild loss of pyramidal neurons was noted in the CA1 and CA2 regions. Higher-power microscopic examination of neurons demonstrated vacuolated cytoplasm with small eosinophilic granules, a finding that is termed granulovacuolar neurodegeneration. β-amyloid IHC revealed moderate to frequent plaques in the amygdala and hippocampus, as well as in the anterior cingulate, frontal, temporal, parietal, and occipital cortices. Tau IHC revealed frequent tau-positive neurofibrillary tangles (NFTs) and neurites within the hippocampus and amygdala; less frequent NFTs in the anterior cingulate, frontal, temporal, and parietal cortices; and rare NFTs in the occipital cortex. Taken together, these findings were consistent with stage IV neurofibrillary pathology according to the Braak and Braak ( 61 ) scheme and an intermediate level of AD neuropathological change per the National Institute on Aging–Alzheimer’s Association guidelines for the neuropathological assessment of AD ( 62 ).
β-amyloid deposition in the walls of scattered cerebral blood vessels in the hippocampus and temporal neocortex led to the additional neuropathological diagnosis of cerebral amyloid angiopathy of mild to moderate severity.
This case highlights the complexity of DLB as a multisystem disorder affecting cognition, neuropsychiatric function, sleep, motor function, and autonomic function. Symptoms in these different domains are variably present at varying levels of severity across individuals and at different time points along the disease course, which may lead to confusion in distinguishing DLB from PD in some cases (i.e., in patients who have prominent early parkinsonism) and from amnesic mild cognitive impairment or dementia due to suspected AD in others (i.e., in patients who do not have early parkinsonism). Recognizing prominent early impairments in attention and in executive and visuospatial function and proactively reviewing for fluctuations in arousal and attention, formed and minor visual hallucinations, dream enactment, and autonomic dysfunction promotes early consideration of DLB in cases without salient parkinsonism at presentation. It is useful for cognitive neurologists and neuropsychiatrists to develop a time-efficient examination of motor systems for elements of parkinsonism, to identify and characterize typical versus atypical features, and to grade severity. A systematic approach to reviewing symptom severity and the influence of symptoms on daily life across the domains potentially affected by DLB can help the clinician identify and prioritize potential targets for therapeutic intervention. Neuropsychiatric symptoms are very common, independent of motor symptoms, and tied to alterations in monoaminergic neurotransmitters ( 63 ). Published case series have suggested high rates of delusions (>75%), anxiety (65%–70%), depression (60%–65%), and apathy (55%–60%), with some symptoms tending to worsen with disease progression (delusions, hallucinations, and anxiety) and others tending to remain stable (depression) ( 8 , 52 ).
Neuropathologically, this case highlights the principle that the presence of multiple underlying neuropathologies is more the rule than the exception and that pathologies can have additive effects contributing to the severity and rate of progression of cognitive dysfunction ( 64 ). Patients with mixed LBD-AD neuropathology are more likely to have parkinsonism, visual hallucinations, RBD, and cognitive fluctuations, as well as faster rates of cognitive and functional decline than those with pure AD neuropathology ( 65 – 67 ). Patients with mixed LBD-AD neuropathology are more likely to have memory loss and less likely to have autonomic dysfunction than those with pure LBD ( 65 ). Important topics for ongoing research include understanding what factors contribute to the high coprevalence of AD and diffuse neocortical LBD pathology and understanding more fully how APOE ε4 might influence both.
Acknowledgments
This work was supported in part by NIH grants K08 AG065502 and T32 HL007627 (to Dr. Miller).
The authors report no financial relationships with commercial interests.
The authors have confirmed that details of the case have been disguised to protect patient privacy.
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

Make an Impact in the Fight to End Alzheimer's
- Models of Care Case Studies
The Alzheimer’s Association is committed to connecting clinicians to effective, evidence-based models of care that can be replicated in community settings. Two of these models — the UCLA Alzheimer’s and Dementia Care program and the Age-Friendly Health Systems initiative — are detailed below.
UCLA Alzheimer's and Dementia Care program Age-Friendly Health Systems initiative UCLA Alzheimer’s and Dementia Care program
A dementia-specific model of care that significantly improved the experience for caregivers and people living with the disease.
About the program
The Alzheimer’s Association has partnered with UCLA to replicate the UCLA Alzheimer’s and Dementia Care (ADC) program through a grant from the John A. Hartford Foundation. The program follows a co-management model within the UCLA health system and partners with community-based organizations (CBOs) to provide comprehensive, coordinated, individualized care for people living with Alzheimer’s disease and other dementias.
The goals of the program are to:
- Maximize function, independence and dignity for people living with dementia.
- Minimize caregiver strain and burnout.
- Reduce unnecessary costs through improved care.
To qualify for the program, participants must have a diagnosis of dementia and live outside of a nursing home. The mean age of the first program participants was 82 years old. Almost all of the caregivers were the children (59%) or spouses (41%) of individuals living with Alzheimer’s or other dementias.
Comprehensive care
The ADC program utilizes a co-management model in which a nurse practitioner Dementia Care Specialist (DCS) partners with the participant’s primary care doctor to develop and implement a personalized care plan. The DCS provides support via four key components:
- Conducting in-person needs assessments of individuals living with Alzheimer’s and their caregivers.
- Creating and implementing individualized dementia care plans.
- Monitoring and revising care plans, as needed.
- Providing access 24/7, 365 days a year for assistance and advice to help avoid Emergency Department (ED) visits and hospitalizations.
Community resources
The ADC program also connects caregivers with resources provided by CBOs, including:
- Adult day care.
- Counseling.
- Case management.
- Legal and financial advice.
- Workforce development focused on training families and caregivers.
Program effectiveness
At one year, the quality of care provided by the program as measured by nationally accepted quality measures for dementia was exceedingly high — 92% compared to a benchmark of 38%. As a result, the improvements experienced by both caregivers and patients were significant:
- Ninety-four percent of caregivers felt that their role was supported.
- Ninety-two percent would recommend the program to others.
- Confidence in handling problems and complications of Alzheimer’s and other dementias improved by 79%.
- Caregiver distress related to behavioral symptoms, depression scores and strain improved by 31%, 24% and 15%, respectively.
- Despite disease progression, behavioral symptoms like agitation, irritability, apathy and nighttime behaviors in people living with dementia improved by 22%.
- Depressive symptoms experienced by individuals living with the disease were reduced by 34%.
Cost benefits of the program
An external evaluator compared utilization and cost outcomes and determined that over the course of 3 1/2 years, participants in UCLA’s program had lower total Medicare costs of care ($2,404 per year) relative to those receiving usual care.
In addition to cost savings for individuals and their families, the ADC program reports several financial benefits for health systems, including:
- Hospitalizations: 12% reduction
- ED visits: 20% reduction
- ICU stays: 21% reduction
- Hospital days: 26% reduction
- Hospice in last six months: 60% increase
- Nursing home placement: 40% reduction
UCLA finds that a care program following the ADC model may be able to pay for itself depending on local labor costs, comprehensiveness of billing and local overhead applied to clinical revenue.
To learn more or to contact UCLA about training and replication of the program, visit the UCLA Alzheimer’s and Dementia Care Program website.
Age-Friendly Health Systems initiative
A model of care that incorporates person-centered dementia care into a broader framework for the care of older adults.
About the initiative
Age-Friendly Health Systems is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association (AHA) and the Catholic Health Association of the United States (CHA). Together in 2017, they set a bold vision to build a social movement so all care with older adults is age-friendly care, that:
- Follows an essential set of evidence-based practices.
- Causes no harm.
- Aligns with “What Matters” to the older adult and their family caregivers.
The Age-Friendly Health Systems initiative defines “What Matters” as knowing and aligning care with each older adult’s specific health outcome goals and care preferences including, but not limited to, end-of-life care, and across settings of care.
- Health outcome goals relate to the values and activities that matter most to an individual, help motivate the individual to sustain and improve health, and could be impacted by a decline in health — for example, babysitting a grandchild, walking with friends in the morning, or volunteering in the community. When identified in a specific, actionable, and reliable manner, patients’ health outcome goals can guide decision making.
- Care preferences include the health care activities (e.g., medications, self-management tasks, health care visits, testing, and procedures) that patients are willing and able (or not willing or able) to do or receive.
The 4Ms framework of an Age-Friendly Health System
The 4Ms are not a program, but a framework to guide how care is provided to older adults through every interaction with a health system’s care and services. The 4Ms — What Matters, Medication, Mentation, and Mobility — make the complex care of older adults more manageable because they:
- Identify the core issues that should drive all care and decision making with the care of older adults.
- Organize care and focus on the older adult’s wellness and strengths rather than solely on disease.
- Are relevant regardless of an older adult’s individual disease(s).
- Apply regardless of the number of functional problems an older adult may have, or that person’s cultural, ethnic or religious background.
The 4Ms framework is most effective when all 4Ms are implemented together and are practiced reliably (i.e., for all older adults, in all settings and across settings, in every interaction).
The intention is to incorporate the 4Ms into existing care — rather than layering them on top —to organize the efficient delivery of effective care. This is achieved primarily through redeploying existing health system resources. Many health systems have found they already provide care aligned with one or more of the 4Ms for many of their older adult patients. Much of the effort, then, is to incorporate the other elements and organize care so all 4Ms guide every encounter with an older adult and their family caregivers.
Cost benefits of the initiative
The business case for becoming an Age-Friendly Health System focuses on its financial returns and is stronger when:
- The financial benefits are captured by the health system that is making the investment.
- Utilization and associated expenses of “usual” care are especially burdensome.
- The health system is effective in mitigating those costs.
- The added expense of becoming age-friendly is lower.
See the IHI report, The Business Case for Becoming an Age-Friendly Health System , for guidance on how to make the business case for your health system.
To learn more or to contact IHI about joining the initiative, visit the IHI Age-Friendly Health Systems website.
Health Systems and Medical Professionals Menu
- Alzheimer’s Association Innovation Roundtable (AAIR)
- Prescribing Lecanemab
- Billing Codes for Alzheimer’s and Related Dementia
- Cognitive Screening and Assessment
- Dementia Care Navigation Guiding Principles
- Dementia Care Navigation Roundtable
- Differential Diagnosis of Alzheimer's Disease
- Differential Diagnosis of Creutzfeldt-Jakob Disease
- Differential Diagnosis of Lewy Body Dementia
- Differential Diagnosis of Frontotemporal Dementia
- Differential Diagnosis of Huntington's Disease
- Differential Diagnosis of Korsakoff Syndrome
- Differential Diagnosis of Mixed Dementia
- Differential Diagnosis of Normal Pressure Hydrocephalus
- Differential Diagnosis of Parkinson's Disease Dementia
- Differential Diagnosis of Vascular Dementia
- Disclosure of Diagnosis
- Advanced Imaging and Biomarkers
- Dementia Care Delivery and Accreditation
- Care Planning Visit
- Advanced Care Planning
- Alzheimer's Network (ALZ-NET)
- Guidelines Index
- Clinical Trials Recruiting
- Downloadable Resources for Patients and Caregivers
- I Have Alzheimer's
- Clinical Trials
- Instructional Videos
- Cognitive Assessment Tools
- ISTAART Membership
- Alzheimer's & Dementia Journal
- Continuing Education on Alzheimer's and Dementia
- The Alzheimer’s and Dementia Care ECHO® Program for Health Systems and Medical Professionals
- Risk Reduction
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Call for papers
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 34, Issue 1
- Case of early-onset Alzheimer’s disease with atypical manifestation
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1224-4320 Lin Zhu 1 ,
- Limin Sun 2 ,
- Lin Sun 3 , 4 and
- Shifu Xiao 3 , 4
- 1 Department of Rehabilitation Medicine , Shanghai No.3 Rehabilitation Hospital , Shanghai , China
- 2 Department of Rehabilitation Medicine , Huashan Hospital Fudan University , Shanghai , China
- 3 Department of Geriatric Psychiatry , Shanghai Mental Health Center , Shanghai , China
- 4 Alzheimer's Disease and Related Disorders Center, Shanghai Jiao Tong University , Shanghai , China
- Correspondence to Professor Shifu Xiao; xiaoshifu{at}msn.com
Short-term memory decline is the typical clinical manifestation of Alzheimer’s disease (AD). However, early-onset AD usually has atypical symptoms and may get misdiagnosed. In the present case study, we reported a patient who experienced symptoms of memory loss with progressive non-fluent aphasia accompanied by gradual social withdrawal. He did not meet the diagnostic criteria of AD based on the clinical manifestation and brain MRI. However, his cerebrospinal fluid examination showed a decreased level of beta-amyloid 42, and increased total tau and phosphorylated tau. Massive amyloid β-protein deposition by 11C-Pittsburgh positron emission tomography confirmed the diagnosis of frontal variant AD. This case indicated that early-onset AD may have progressive non-fluent aphasia as the core manifestation. The combination of individual and precision diagnosis would be beneficial for similar cases.
- dual (psychiatry)
- cognition disorders
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/gpsych-2020-100283
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Clinical report and methods.
Early-onset Alzheimer’s disease (EOAD), which comprises 5% of Alzheimer’s disease (AD), shows a 1.6-year average delay in diagnosis compared with late-onset AD. 1 2 The clinical phenotype of atypical EOAD is heterogeneous, and primary progressive aphasia (PPA) is rarely the initial manifestation of related dementia syndromes. Compared with the progressive non-fluent aphasia (PNFA) related to the language variant phenotype of frontotemporal lobar degeneration (FTLD), molecular imaging studies in patients with primary progressive aphasia suggest the pathological basis of AD. 3 Neurodegeneration uaually starts in a specific neural anatomic networks. The clinical phenotype of PPA can usually infer the type of protein degeneration, which can be used to infer gene mutation. With the development of biomarkers such as genetics, molecular biology, neuroimaging and positron emission tomography (PET), accurate diagnosis can be gradually achieved. In this case study, we describe an AD patient with PNFA as the first symptom.
The patient was a 63-year-old married man, a right-handed businessman, native of Shanghai, with 12 years of school education. He has memory loss and non-fluent speech for 7 years combined with personality changes for 5 years. The patient recovered from hepatitis A 32 years ago and has well-controlled hypertension for 30 years.
The patient’s caregiver described that the patient showed forgetfulness and developed poor pronunciation at the age of 56. His short-term memory has gradually declined as noticed that he repeatedly gave money to customers while selling clothes. He frequently forgot where he parked his bicycle, and it was hard for him to speak a full sentence; his language was vague and short. He was impatient when being asked to repeat a word. Over time, he could only say some single syllables. He evolved into fully aphasia gradually, and his personality also changed gradually. At the age of 59, he could not recognise himself in the mirror and he often hid his shoes because he was worried that they would be stolen. Therefore, his wife had accompanied him to see a neurologist. The physical and neurological examination revealed no remarkable signs. His brain MRI showed mild atrophy in the bilateral frontal lobe ( figure 1A at the age of 59). Fluorodeoxyglucose positron emission tomography (FDG-PET) revealed that glucose metabolism in the bilateral frontal and parietal lobe was declined, and the left side was significant ( figure 1B at the age of 59). The Mini-Mental State Examination (MMSE) score was 18 out of 30 (18/30). At that point, he was diagnosed with cognitive impairment and treated with rivastigmine. After the treatment, his memory improved slightly. In 2017, the neurologist gave him quetiapine and donepezil due to developing visual hallucinations and irritability. The second brain MRI scan revealed increased frontal and temporal atrophy compared with the first one ( figure 1C at the age of 61). The FDG-PET revealed that the cerebral cortical glucose metabolism was further reduced, especially the bilateral frontal and parietal lobes were obvious ( figure 1D at the age of 61).
- Download figure
- Open in new tab
- Download powerpoint
Brain imaging and cognitive score of the patient. (A) The patient’s MRI in May 2015 revealed mild atrophy of the bilateral frontal lobe (at the age of 59). (B) The patient’s FDG-PET in May 2015 revealed that glucose metabolism in the bilateral frontal and parietal lobe was reduced, and the left side was significant (at the age of 59). (C) The patient’s MRI in July 2017 (2 years after the first scan), revealed more atrophy of the bilateral frontal lobe and temporal lobe atrophy occurred (at the age of 61). (D) The patient’s FDG-PET in August 2017 revealed that the cerebral cortical glucose metabolism was reduced more, bilateral frontal and parietal lobes obvious in particular (at the age of 61). (E) The patient’s third MRI in May 2019 (2 years after the second scan) revealed atrophy of the whole cerebral cortex with bilateral frontal lobes, temporal lobe and hippocampus more affected (at the age of 63). (F) The patient’s 11C-PIB PET in May 2019 revealed saliently amyloid deposition in diffuse cortical areas, particularly in the bilateral frontal, parietal, temporal cortices and posterior cingulated gyrus (at the age of 63). (G) Mini-Mental State Examination (MMSE) of the patient. MMSE in May 2015 revealed a total score was 18/30 (at the age of 59). MMSE in May and December 2019 revealed a total score were 3/30 and 2/30; the results showed severe impairments in language and other cognitive areas (at the age of 63). 11C-PIB PET, 11C-Pittsburgh compound B positron emission tomography; FDG-PET, fluorodeoxyglucose positron emission tomography.
In May 2019, the patient’s symptoms aggravated further, which included bad temper, crying often and being more difficult to be looked after. His wife brought him to seek help from a psychiatrist, and he was admitted into the Department of Geriatric Psychiatry of Shanghai Mental Health Center. He underwent routine laboratory tests to exclude non-neurodegenerative and dementia. His neurological examination showed gait abnormality, negative Babinski’s sign, muscular tension hyperactivity, knee jerk reflex hyperactivity and a weak positive right palmar jaw reflex. The MMSE score was 3/30. The patient exhibited severe impairments in orientation (2/10), attention and calculation (1/5), recall (0/6), language (0/8) and visual construction (0/1). The Montreal Cognitive Assessment score was 0 (0/30), which was significantly lower than it was in 2015( figure 1G ). The third brain MRI demonstrated atrophy of the cerebral cortex, especially in the bilateral frontal lobes and hippocampus. The medial temporal lobe atrophy scale was at grade 3 ( figure 1E at the age of 63).
In addition, we tested three pathogenic genes for early-onset AD including amyloid precursor protein, presenilin-1, presenilin-2 genes related to neurocognitive disorders, but no mutation was found. Apolipoprotein E (APOE) genotyping showed APOE ε3/ε3 type. In order to reach a definite diagnosis, the patient underwent 11C-Pittsburgh compound B positron emission tomography (11C-PIB PET) and cerebrospinal fluid (CSF) examination. 11C-PIB PET revealed noticeable amyloid deposition in diffuse cortical areas, particularly in the bilateral frontal, parietal, temporal cortices and posterior cingulated gyrus ( figure 1F at the age of 63). The measured CSF biomarkers showed decreased amyloid β-protein (Aβ) 42 (462 pg/ml; cut-off >562 pg/ml), increased total tau (754 pg/ml; cut-off <370 pg/ml) and increased phosphorylated tau (87.40 pg/ml; cut-off <66.26 pg/ml). Eventually, the diagnosis of frontal variant EOAD was reconfirmed considering the early onset of dementia, the slow progression of symptoms, the absence of focal neurological damage signs and the exclusion of other systemic or brain diseases that could cause dementia. Due to the gastrointestinal adverse reactions of the patient, rivastigmine was suspended. We used memantine 10 mg b.i.d. and donepezil 5 mg q.d. to improve cognition and to control psychobehavioural symptoms and vortioxetine 10 mg q.d. to improve mood. After the treatment and follow-up for 7 months, the patient’s behaviour and mood was improvved significally, and his language expression improved slightly ( figure 1G at the age of 63).
The initial clinical manifestations of the patient included short-term memory decline, poor pronunciation and personality changes at an early stage, followed by behavioural and psychological symptoms of dementia, including hallucinations, delusions of theft, gradual decline in self-care as well as depression. The patient’s brain MRI initially showed mild atrophy of the bilateral frontal lobe. With the progress of the disease, more severe atrophy of the cerebral cortex, temporal lobe and hippocampus appeared besides the further atrophy of the bilateral frontal lobe. The atypical manifestation such as early aphasia, frontal lobe atrophy and personality changes can mislead clinicians in diagnosing frontotemporal lobar degeneration. This is the main reason leading to the misdiagnosis of this patient, which should be taken as a lesson or future reference for clinicians.
According to the current classification schemes, the clinical symptoms were in line with PNFA, which are halting speech by speech sound errors with spared content word comprehension and atrophy of the left frontal lobe. 4 PNFA is one of the primary progressive aphasias. 4 This patient met the diagnostic criteria of frontotemporal dementia, consistent with the early personality changes and cognitive abnormalities. 5 In the past 7 years, the patient’s speech fluency and cognitive function decreased continuously and rapidly. The clinical manifestations could not be explained by typical AD. The CSF phosphorylated tau was slightly higher, and no gene mutations associated with AD were found, which further made it harder to reach the diagnosis. However, the 11C-PIB PET showed heavy and extensive Aβ-amyloid depositions and provided definite pathological evidence of AD. A retrospective study found PNFA with 13%– 31% of cases might have the pathology of AD. 6 The patient met the research diagnostic AT(N) framework of AD, with A: (11C-PIB PET revealed amyloid depositions, CSF Aβ42 decreased), T: (CSF phosphorylated microtubule-associated protein tau increased) and N: (cortical atrophy on MRI, glucose hypometabolism in the bilateral frontal parietal lobe and CSF total microtubule-associated protein tau increased). 7 We use the AD pathological markers as the gold standard to exclude other types of dementia and reach an earlier and more accurate diagnosis. It’s worth pointing out that the patient might have mixed neuropathology. Santos-Santos 6 found that 75% of PNFA or PPA cases may have mixed pathological changes of FTLD and AD. This poses a new challenge for clinicians, suggesting that verified, reliable and accessible biomarkers for diagnosis of FTLD should be developed urgently. Otherwise, the comorbid pathological cases would only be accurately diagnosed after autopsy.
After reaching a clear diagnosis, and according to the China guidelines for the diagnosis and treatment of dementia and cognitive impairment in 2018 and the guidelines for the diagnosis and treatment of AD, 8 the patient was treated with cholinesterase inhibitors and excitatory amino acid receptor antagonists to enhance cognition, and antidepressants were given to relieve his mood. After the treatment, the patient’s symptoms were improved, and his mood was stable. Additionally, the biopsychosocial medical model has become more and more accepted. We should treat the patients with medication and non-drug intervention for patients and their caregivers. Spouses and caregivers of patients with early-onset dementia bear a greater burden and higher depression rates. 9 The speech impairments of this patient appeared early. He was emotionally unstable, grumpy and easy to be tearful, which was alleviated when his wife comforted him. Two weeks later, he was released from the hospital and continued to receive comprehensive rehabilitation treatments. Anyway, providing individualised psychosocial support for patients and their caregivers is very important for improving symptoms and quality of life. 10
Some of PNFAs are due to the underlying pathology of AD, which is more common in EOAD. In the present case, neither clinical examination nor MRI could definitively differentiate FTLD from EOAD. According to AT(N) research framework, we could eventually confirm the neuropathy diagnosis of AD or frontal-variant AD (fvAD), but the previous misdiagnoses were significant. FvAD can lead to social withdrawal and depression. These patients should benefit from accurate diagnosis, medication treatment and individualised psychosocial intervention.
- van Vliet D ,
- de Vugt ME ,
- Bakker C , et al
- Rabinovici GD ,
- Jagust WJ ,
- Furst AJ , et al
- Gorno-Tempini ML ,
- Hillis AE ,
- Weintraub S , et al
- McKhann GM ,
- Albert MS ,
- Grossman M , et al
- Santos-Santos MA ,
- Iaccarino L , et al
- Bennett DA ,
- Blennow K , et al
- Ducharme F ,
- Kergoat M-J ,
- Antoine P , et al
- Ferriero DM ,
- Frisoni GB , et al
Lin Zhu obtained a bachelor’s degree in clinical medicine from Shanghai Jiao Tong University School of Medicine, Shanghai, China in 2006. She is currently working as an attending doctor and psychotherapist at the Neurorehabilitation Department of Shanghai Third Rehabilitation Hospital. After completing clinical training, she started a two-year master program and was certified by the Institute of Psychology of the Chinese Academy of Sciences. In addition, she has also been trained and actively involved in clinical neurological research for half year in the Department of Geriatric Psychiatry of Shanghai Mental Health Center, Shanghai, China. Her main research interest includes the rehabilitation of elderly with psychiatric disorders.
Contributors LZ drafted the case report and manuscript; LMS performed the literature search; LS and SX supervised and revised the manuscript. All authors approved the final manuscript.
Funding This study was supported by a grant of Clinical Research Centre Project of Shanghai Mental Health Centre (CRC2017ZD02) and Scientific Research Program of Shanghai Jing an District Health Committee (2020MS16).
Competing interests None declared.
Patient consent for publication Parental/guardian consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

Case studies
These three case studies help you to consider different situations that people with dementia face. They are:
- Raj , a 52 year old with a job and family, who has early onset dementia
- Bob and Edith , an older married couple who both have dementia and are struggling to cope, along with their family
- Joan , an older woman, who lives alone and has just been diagnosed with dementia
Each case study contains:
- A vignette setting out the situation
- An ecogram showing who is involved
- An assessment which gives essential information about what is happening and the social worker’s conclusion
- A care and support plan which says what actions will be taken to achieve outcomes
You can use the practice guidance to think about how you would respond in these situations.

- Equal opportunities
- Complaints procedure
- Terms and conditions
- Privacy policy
- Cookie policy
- Accessibility

- Introduction
- Conclusions
- Article Information
Plot shows dementia incidence rate ratios (IRRs) for infection (reference, no infection). Model 1 IRRs were adjusted for age, sex, calendar year, and highest attained educational level. Model 2 IRRs were further adjusted for hypertension, diabetes, hypercholesteremia, myocardial infarction, and stroke. The fully adjusted IRRs were further adjusted for autoimmune diseases. The forest plot presents the fully adjusted estimates. NA indicates not applicable.
Plot shows dementia incidence rate ratios (IRRs) for each infection site when stratified by time since first infection: dementia within 5 years and greater than 5 years of first infection (reference, no infection of each site). Model 2 IRRs were adjusted for age, sex, calendar year, highest attained educational level, hypertension, diabetes, hypercholesteremia, myocardial infarction, and stroke. The fully adjusted IRRs were further adjusted for autoimmune diseases. The forest plot presents the fully adjusted estimates.
Plot shows dementia incidence rate ratios (IRRs) for any autoimmune disease (reference, no disease). Model 1 IRRs were adjusted for age, sex, calendar year, and highest attained educational level. Model 2 IRRs were further adjusted for hypertension, diabetes, hypercholesteremia, myocardial infarction, and stroke. Fully adjusted IRRs were further adjusted for infections. The forest plot presents the fully adjusted estimates. The systemic autoimmune diseases group includes seropositive rheumatoid arthritis, Wegener granulomatosis, dermatopolymyositis, polymyalgia rheumatica, systemic sclerosis, systemic lupus erythematosis, Sjogren syndrome, and ankylosing spondylitis. Anemia is autoimmune pernicious anemia.
eAppendix. Supplemental Methods
eTable 1. ICD Codes for Infections Definition, by Infection Site
eTable 2. ICD Codes for Autoimmune Diseases Definition, by Disease Type
eTable 3. ICD and ATC Codes for Dementia Definition
eTable 4. ICD and ATC Codes for Chosen Comorbidities
eFigure 1. Data Analysis Illustration
eFigure 2. Population Flow Chart
eTable 5. Infection Sites and Subsequent Dementia (Common Reference = No Infection)
eTable 6. Autoimmune Disease Types and Subsequent Dementia (Common Reference = No Disease)
eFigure 3. Sensitivity Analyses
eReferences
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Janbek J , Laursen TM , Frimodt-Møller N, et al. Hospital-Diagnosed Infections, Autoimmune Diseases, and Subsequent Dementia Incidence. JAMA Netw Open. 2023;6(9):e2332635. doi:10.1001/jamanetworkopen.2023.32635
Manage citations:
© 2024
- Permissions
Hospital-Diagnosed Infections, Autoimmune Diseases, and Subsequent Dementia Incidence
- 1 Danish Dementia Research Centre, Department of Neurology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark
- 2 National Centre for Register-Based Research, Department of Economics and Business Economics, Aarhus BSS, Aarhus University, Aarhus, Denmark
- 3 Department of Clinical Microbiology, Copenhagen University Hospital– Rigshospitalet, Copenhagen, Denmark
- 4 Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
- 5 Danish Multiple Sclerosis Center, Department of Neurology, Copenhagen University Hospital–Rigshospitalet, Glostrup, Denmark
- 6 Division of Infection Medicine, University of Edinburgh, Edinburgh, United Kingdom
Question Is exposure to infections and/or autoimmune diseases associated with dementia incidence?
Findings In this nationwide cohort study of 1 493 896 individuals, infections were associated with a statistically significant 1.49-fold increased dementia incidence in a dose-dependent manner, particularly in the short term. Associations were less clear for autoimmune diseases.
Meaning The observed associations of infections with dementia and the lack of such associations for autoimmune disease may point toward a role for infection-specific processes, rather than general systemic inflammation, as recently hypothesized.
Importance Systemic inflammation has been suggested to explain reported associations between infections and dementia. Associations between autoimmune diseases and dementia also suggest a role for peripheral systemic inflammation.
Objective To investigate the associations of infections and autoimmune diseases with subsequent dementia incidence and to explore potential shared signals presented by the immune system in the 2 conditions.
Design, Setting, and Participants This nationwide, population-based, registry-based cohort study was conducted between 1978 and 2018 (40-year study period). All Danish residents born 1928 to 1953, alive and in Denmark on January 1, 1978, and at age 65 years were included. Persons with prior registered dementia and those with HIV infections were excluded. Data were analyzed between May 2022 and January 2023.
Exposures Hospital-diagnosed infections and autoimmune diseases.
Main Outcomes and Measures All-cause dementia, defined as the date of a first registered dementia diagnosis after age 65 years in the registries. Poisson regression with person-years at risk as an offset variable was used to analyze time to first dementia diagnosis.
Results A total of 1 493 896 individuals (763 987 women [51%]) were followed for 14 093 303 person-years (677 147 [45%] with infections, 127 721 [9%] with autoimmune diseases, and 75 543 [5%] with dementia). Among individuals with infections, 343 504 (51%) were men, whereas among those with autoimmune diseases, 77 466 (61%) were women. The dementia incidence rate ratio (IRR) following any infection was 1.49 (95% CI, 1.47-1.52) and increased along with increasing numbers of infections in a dose-dependent manner. Dementia rates were increased for all infection sites in the short term, but not always in the long term. The dementia IRR following any autoimmune disease was 1.04 (95% CI, 1.01-1.06), but no dose-dependent increase was observed, and only a few autoimmune conditions showed increased IRRs for dementia.
Conclusions and Relevance These findings may point toward a role for infection-specific processes in the development of dementia, rather than general systemic inflammation, as previously hypothesized. Assessing these 2 conditions in a single setting may allow for additional insights into their roles in dementia and for hypotheses on possible underlying mechanisms.
There has been extensive debate about whether infections might be causally linked to dementia, particularly Alzheimer disease (AD). 1 Evidence from epidemiological and other studies has several limitations. First, studies have generally focused on specific infections and/or pathogens; therefore, it is unclear whether specific infectious agents might be involved or whether inflammation induced by infection could underlie the reported observed risks. Second, most studies investigated associations in postmortem brains and were, hence, challenged by temporality. Third, most studies had short follow-up periods and were based on selected populations. Four recent population-based studies 2 - 5 showed an increased risk of dementia following different types of infection (hospital and/or primary care diagnoses). Two reported an increased risk of dementia and/or AD following any infection 3 , 5 and suggested that systemic inflammation, rather than specific infections or pathogens, might explain the observed increase in risk.
A large body of literature supports a role for systemic inflammation in increased dementia risk and includes epidemiological studies on diseases that induce a proinflammatory state. 6 Epidemiological studies assessing links between autoimmune diseases and dementia share a common hypothesis that peripheral systemic inflammation may increase the risk of dementia. Most studies investigated individual autoimmune diseases (or groups) and found mixed results. 7 The evidence, therefore, remains inconclusive.
By investigating infections and autoimmune diseases together, we aimed to explore potential shared signals presented by the immune system in these different but mainly inflammatory conditions that could advance knowledge on their roles as dementia risk factors and on the possible underlying mechanisms. The aim was, therefore, to investigate the association between infections and autoimmune diseases and dementia incidence.
We used population-based Danish national registries. The National Patient Register (NPR) and the Psychiatric Central Research Register hold records from somatic (from 1977) and psychiatric (from 1969) wards, respectively, with outpatient data added in 1995. The National Prescription Register contains all prescription drugs dispensed at Danish pharmacies from 1995. 8 No diagnostic codes from primary care are directly recorded in the health registers. We defined our variables using either hospital diagnostic codes or prescriptions or their combination. Danish law does not require ethical approval or informed consent for registry-based studies. The study followed Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guidelines for cohort studies.
This population-based cohort study was conducted from 1978 to 2018. Included were all individuals born from 1928 to 1953 who were alive and in Denmark on January 1, 1978, and on the date of their 65th birthday. Persons with prior recorded dementia and those with HIV infections were excluded.
Exposure was defined as inpatient, outpatient, or emergency hospital contacts with a primary or secondary discharge diagnosis of an infection or autoimmune disease (codes are shown in eTable 1 and eTable 2 in Supplement 1 ) in the NPR from age 50 years onward (based on inpatient data only before 1995). Infections were assessed as follows: any (ie, presence of the date of a first registered diagnosis; reference, no infection); burden (detailed in the eAppendix in Supplement 1 ), defined as the number of new infections and of inpatient admissions (reference, no infection); time since first infection within 5 years and greater than 5 years (reference, no infection) to test for associations in the short and long term, respectively; and infection site (ie, date of a first registered diagnosis of an infection of each site as defined in eTable 1 in Supplement 1 ; reference, no infection of the assessed site). Each person could have infections in multiple sites.
Autoimmune diseases were assessed as follows: any (ie, presence of a date of a first registered diagnosis; reference, no autoimmune disease); burden (detailed in the eAppendix in Supplement 1 ), defined as the number of different types of autoimmune disease and of inpatient admissions (reference, no autoimmune disease); and type of disease, defined as the presence of the date of a first registered diagnosis of an autoimmune disease of each category (eTable 2 in Supplement 1 ) (reference, no disease of the type assessed). Each person could have multiple diseases.
All-cause dementia was defined as the date of a first registered dementia diagnosis after age 65 years in the NPR and Psychiatric Central Research Registry (validated previously 9 ), or the date of a first redeemed antidementia prescription from the National Prescription Register (codes in eTable 3 in Supplement 1 ). The latter was used to identify dementia cases diagnosed in the primary care setting (used as disease proxies). 10 , 11
Covariates were sex, age, calendar year, highest attained education at age 50 years, and selected comorbidities (ie, diabetes, hypertension, stroke, myocardial infarction, and hypercholesteremia). Comorbidities were defined using hospital inpatient and outpatient diagnoses or medication prescriptions (eTable 4 in Supplement 1 ).
Poisson regression with person-years at risk as an offset variable was used to analyze time to first dementia diagnosis (approximation to Cox regression) using SAS statistical software version 9.4 (SAS Institute) and estimating incidence rate ratios (IRRs). 12 , 13 Constant rates were assumed in the used periods only (age in 5-year intervals and calendar year in 1-year intervals), which were small enough periods to ensure that the constant rates were fulfilled. 12 , 13 Statistical significance was determined using 2-sided P < .05. Individuals were followed from their 50th birthday (risk time from age 65 years). Follow-up terminated on the date of the outcome, emigration, death, or December 31, 2018, whichever came first. Exposure dates, comorbidities, age, and calendar time were analyzed time dependently (eFigure 1 in Supplement 1 ).
Exposures were analyzed as defined. To test the comparability of infection sites and/or autoimmune disease types with each other, we also analyzed IRRs with a common reference to no infection or no disease. Model 1 was adjusted for age, sex, calendar year, and highest attained education; model 2 was further adjusted for selected comorbidities; and the fully adjusted model was further adjusted for infections when analyzing autoimmune diseases and vice versa to test whether one was associated with the risk of the other.
Post hoc, we analyzed dementia incidence since the first infection for each infection site. Four sensitivity analyses were defined a priori to test the robustness of results. First, we analyzed IRRs in 2 calendar periods to test risk variation. Second, we estimated mortality rate ratios following exposures. Third, we removed codes that were judged to be uncertain for infection (eTable 1 in Supplement 1 ). Fourth, we included exposures before age 50 years. Data were analyzed between May 2022 and January 2023.
In total 1 493 896 individuals (763 987 women [51%]) were followed-up for 14 093 303 person-years (eFigure 2 in Supplement 1 ). During the study period, 677 147 people (45%) were registered with infections, and 127 721 (9%) were registered with autoimmune diseases, from age 50 years onward. A total of 75 543 persons (5%) were registered with all-cause dementia (from age 65 years onward; median [IQR] age at dementia incidence, 77 [72-81] years). Among individuals with infections, 343 504 (51%) were men, whereas among those with autoimmune diseases, 77 466 (61%) were women. On the date of dementia diagnosis, people with infections or autoimmune diseases were slightly older than those without ( Table ).
The median (IQR) age at first exposure was 66 (59-72) years for infection and 65 (57-71) years for autoimmune disease. Respiratory infections were the most common, followed by gastrointestinal and urinary infections. The most common autoimmune diseases were rheumatoid arthritis and polymyalgia rheumatica.
Figure 1 presents dementia IRRs following infections. The IRR was increased for persons with any infection vs those without infection (fully adjusted IRR, 1.49; 95% CI, 1.47-1.52). IRRs were similar in men and women and increased in a dose-dependent manner along with increasing burden of infection (fully adjusted IRR for ≥3 infections, 1.81; 95% CI, 1.77-1.86). The IRR was increased both within 5 years (fully adjusted IRR, 1.83; 95% CI, 1.80-1.87) and more than 5 years (fully adjusted IRR, 1.34; 95% CI, 1.31-1.36) after infection. Significantly increased IRRs were seen across all infection sites except for cardiovascular infections, with the highest for urinary infections (fully adjusted IRR, 1.81; 95% CI, 1.78-1.85).
Figure 2 presents post hoc analysis of the time since the first infection at each site. Dementia IRRs were highest within 5 years and were significantly increased for all sites except for ear and cardiovascular infections. At more than 5 years after infection, IRRs were smaller but remained statistically significant across some sites.
Figure 3 presents dementia IRRs following autoimmune disease. The IRR for persons with any autoimmune disease was increased vs those without disease but was very small, especially after adjustment for infections (fully adjusted IRR, 1.04; 95% CI, 1.01-1.06). The IRR for women was slightly higher (fully adjusted IRR, 1.05; 95% CI, 1.02-1.08). No dose-response association was seen for autoimmune disease burden. Statistically significant IRRs were seen in a few disease categories, but were very small, particularly after adjustment for infections.
eTable 5 and eTable 6 in Supplement 1 show IRRs for each site or disease type when a common reference group was set. Trends were similar to those presented in Figure 1 .
Sensitivity analyses showed increased mortality rate ratios following infection and autoimmune disease, but they were higher for infections than for autoimmune disease. Other analyses showed the robustness of estimates to any definitions we made (eFigure 3 in Supplement 1 ).
In this nationwide cohort study of approximately 1.5 million individuals over a period of 40 years (risk time for up to 25 years), we found that hospital-diagnosed infections were associated with increased IRRs for subsequent all-cause dementia, whereas IRRs were much smaller for autoimmune diseases. Infections were associated with a 1.49-fold increased rate of dementia. We observed a dose-response association, and dementia rates were increased in both the short and long term, although the increase was greater in the short term. Dementia rates were increased for all infection sites (except for cardiovascular) in the short term, but not always in the long term. By contrast, autoimmune diseases were associated with only a 1.04-fold increased rate of dementia, but no dose-response association was seen and only a few autoimmune disease types were associated with any increase in dementia IRR. In addition, the effect sizes were small, particularly after adjustment for infections.
Our findings are consistent with previous population-based studies evaluating dementia risks after hospital-diagnosed infections. Sipilä et al 3 reported a 1.5-fold increased dementia risk in Finland and a 2.6-fold increased risk in the UK biobank, Sun et al 5 reported a 1.16-fold increased AD risk in Sweden, and Muzambi et al 2 found a 1.99-fold increased dementia rate in the UK (all observed dose-response associations). Muzambi et al 2 also assessed infections diagnosed in the primary care setting and found a significant but small association with dementia risk, consistent with another previous study. 4 In all studies, as with ours, associations persisted in the long term but were much lower than in the short term, as also reported in the recent study by Levine et al. 14
The infection sites analyzed in our study were not similarly analyzed in the previous studies. Sun et al 5 assessed central nervous system, gastrointestinal, respiratory, genitourinary, and skin infections, whereas Muzambi et al 2 assessed sepsis, pneumonia and other respiratory infections, and urinary and skin infections. These were also the sites that had the highest short-term and long-term IRRs in our study. Our data also showed increased rates for all other infection sites except for herpesvirus, musculoskeletal, and genital infections, which were no longer significant in the long term (in addition to already insignificant estimates for cardiovascular infections). We also observed overall smaller effect sizes in the long term across most sites, suggesting potential reverse causality. The short-term effects could indicate a role for infections in triggering, accelerating, or unmasking already existing dementia pathology and is, thus, an important clinical and public health avenue for interventions. In addition, as shown in our and the previous studies, the associations are probably not specific for central nervous system infection and do not appear to be organ, system, or pathogen specific.
Other previous studies investigated specific infections and reported increased dementia risks associated with gastrointestinal infections, 15 sepsis, 16 and pneumonia. 17 , 18 Herpesvirus infections have gained the most attention but with mixed results, and all recent large studies except one showed no increased dementia risk. 19 - 24 In our study, for any herpesvirus infection, only short-term estimates were significant.
The evidence to date from epidemiological studies is mixed. Most previous studies that investigated specific autoimmune diseases were based on small and selected populations. Population-based studies on specific autoimmune diseases (eg, inflammatory bowel diseases, 7 , 25 , 26 psoriasis, 27 rheumatic diseases, 28 , 29 and others 7 ) have reported either no or a small increase in dementia risk, especially in the long term (where assessed), although with some heterogeneity. Three previous studies investigated several types of autoimmune diseases in the UK 29 , 30 and Sweden. 31 These found increased dementia risk following only some autoimmune diseases. However, estimates generally attenuated toward the null in the long term, and any positive effect sizes were small, as in our study, and were generally higher in the Swedish study. 31 In our study, we further noted the lack of a dose-response association, which was not investigated previously. This, together with the small IRRs, provides little evidence for an association of autoimmune disease with subsequent dementia development, especially after adjustment for infections. Autoimmune disease diagnosis may also be preceded by infections (multiple sclerosis is a recent example 32 ), thus explaining the overall change in estimates after adjustment for infections. The small effect sizes reported may reflect ascertainment bias because patients with autoimmune conditions probably receive more thorough medical follow-up, which could increase the likelihood of receiving a dementia diagnosis.
Previous studies 3 , 5 have suggested that systemic inflammation, rather than specific pathogens, could underlie the association between infections and dementia. The likely mechanisms include a role for proinflammatory and anti-inflammatory cytokines, blood-brain barrier dysfunction, 33 and peripheral-central immune system crosstalk. 6 Similar mechanisms involving peripheral systemic inflammation have also been hypothesized to link some autoimmune diseases with dementia. 7 , 29 Recent data from genomewide association studies 34 suggest that autoimmunity may be associated with dementia pathology. Overall, it is increasingly suggested that immune system dysregulation leading to an overactive, underactive, and/or chronic inflammatory response may play a role in the development of dementia.
Our data and the observational nature of our study do not directly support or refute any proposed mechanism, nor can we make firm conclusions about the role of inflammation in dementia on the basis of our findings. However, the associations of infection with dementia found in our study, together with the very small IRRs for autoimmune disease, may point toward a role for infection-specific processes rather than general systemic inflammation.
Several findings suggest that a possible explanation is that a weakened immune system may predispose not only to severe infections (that require hospitalization) but also to dementia development. First, previous reports 2 , 4 showed that infections that did not require hospitalization (diagnosed in the primary care setting) were not associated with increased dementia risk. Second, we have previously shown that people with dementia have higher rates of infection hospitalization, 35 demonstrating a bidirectional association and the vulnerability to infection hospitalization. In the present study, the increased short-term but not long-term dementia risks for some infection sites lead us to theorize that the observed infection and dementia associations could be related to reverse causality. Along with the small IRRs for autoimmune disease, the evidence could possibly point away from an association between systemic inflammation and dementia development and instead could suggest that severe infections resulting from a weakened immune system, rather than inflammation per se, underlie the association with dementia. Finally, it is possible that some of the observed risks might be attributed to delirium, 36 and we encourage future studies to assess its potential role.
The major strengths of our study lie in the comprehensive investigation of both conditions, infections and autoimmune disease. To our knowledge, both conditions have been evaluated for the first time in one population and one single setting, which allows us to compare them with each other and draw important mechanistic conclusions. Further strengths of this study are its prospective and nationwide coverage, with negligible loss to follow-up or selection bias, as well as the long study period.
This study also has limitations that should be mentioned. First, we were unable to explore dementia subtypes because of diagnostic uncertainties. In studies 3 , 17 , 29 , 30 that assessed subtypes, associations with autoimmune disease and/or infections were more prominent for vascular dementia than for other subtypes. These findings, if based on valid subtype definitions, are of special interest because associations between infections and dementia have principally been directed toward AD pathology, prompted by findings that Aβ peptide, the deposition of which is a hallmark of AD, has antimicrobial properties. 1 Another major limitation was the inability to isolate associations attributed to treatment vs the conditions themselves, in part because of a lack of comprehensive treatment data. Attempts to isolate such associations should be conducted in future studies.
Importantly, we note the limitations of our data for drawing conclusions on the associations of autoimmune diseases with dementia. In most cases, autoimmune diseases are treated with long-term anti-inflammatory or immunosuppressive medications. This may mean that the small IRRs observed in our study are masked by the effect of medications either by suppressing severe inflammation (hence, inflammation per se was not assessed in our study) or reducing dementia risk, as suggested in some studies 34 , 37 , 38 (albeit with inconclusive evidence). To acknowledge this, we assessed autoimmune disease exposure using inpatient admissions (to proxy active disease and/or inflammation). No clear dose-response association was observed because the 95% CIs overlapped between exposure groups. However, we did not have data on the severity of inflammation or disease and on treatments dispensed at hospitals. In addition, we concluded that the association of autoimmune disease with dementia is tenuous, albeit statistically significant, especially in model 1. We further point out the potential for interaction between autoimmune diseases and infections, which we did not assess.
Other limitations common to observational and registry-based studies include residual confounding from lifestyle differences and validity of diagnostic codes. We also did not have genetic data, which are likely to play an important role in the associations under investigation. 34 Furthermore, it is not possible to conclude whether the dose-response association is present in both the short and long term, because we assessed this regardless of the timing.
In this cohort study of approximately 1.5 million people over a period of 40 years, hospital-diagnosed infections, at all but 1 infection site, were associated with increased rates of dementia (particularly in the short term), and we found a dose-response association. The IRRs were much smaller for autoimmune disease. To our knowledge, this study is the first to assess all sites of infection, the long-term and short-term risks across all sites, and the burden of infection and autoimmune disease using multiple measures. Importantly, this is the first study, to our knowledge, to assess the 2 exposures in 1 nationwide cohort, allowing us to draw insights that will advance knowledge on their roles as risk factors for dementia development and on the possible underlying mechanisms.
Accepted for Publication: August 1, 2023.
Published: September 7, 2023. doi:10.1001/jamanetworkopen.2023.32635
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Janbek J et al. JAMA Network Open .
Corresponding Author: Janet Janbek, PhD, Danish Dementia Research Centre, Department of Neurology, Copenhagen University Hospital–Rigshospitalet, Blegdamsvej 9, Entrance 8, 2100 Copenhagen Ø, Denmark ( [email protected] ).
Author Contributions: Drs Janbek and Laursen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Janbek, Laursen, Frimodt-Møller, Magyari, Waldemar.
Acquisition, analysis, or interpretation of data: Janbek, Magyari, Haas, Lathe, Waldemar.
Drafting of the manuscript: Janbek, Frimodt-Møller.
Critical review of the manuscript for important intellectual content: Janbek, Laursen, Magyari, Haas, Lathe, Waldemar.
Statistical analysis: Janbek, Laursen.
Obtained funding: Waldemar.
Administrative, technical, or material support: Janbek, Haas, Lathe.
Supervision: Laursen, Frimodt-Møller, Magyari, Waldemar.
Conflict of Interest Disclosures: Dr Magyari reported receiving grants and personal fees from Biogen, Roche, Novartis, Merck, and Sanofi and nonfinancial support from Merck (funding of conference participation) outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by grants from the Danish Ministry of Health to the Danish Dementia Research Centre to Dr Waldemar.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like Betty is upset about visiting the HPP and insists her memory problems are nothing to worry about.. Betty states, "This is all really silly. I don't need to be here."Which response by the PN is the best?, Which information about Betty, obtained by the PN, is a risk factor(s) for Alzheimer's disease? (Select all that apply.), The ...
CASE PRESENTATION. A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties.
Presentation of Case. Dr. David L. Perez: A 62-year-old, left-handed man was seen in the memory disorders clinic of this hospital because of memory loss, personality changes, and odd behavior ...
In this study the PN was identified as the practitioner most appropriate to take on screening for dementia and monitoring, however community mental health care nurses were considered to have the skills and capacity to take on long-term and complex cases. Risk of bias. The methodological quality varied across the studies (Tables 1, 2, 3 and 4 ...
There was no first-degree family history of presenile dementia. Neurocognitive assessment at the first clinic visit revealed a Mini Mental State Examination (MMSE) score of 14/30; poor verbal fluency (patient was able to produce only 5 animal names and 1 F-word in 1 min) as well as poor visuospatial and executive skills ( Fig. 1 ).
Part 1: Mavis. Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law. On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
The US Reducing Disability in Dementia study 209 implemented an at-home multicomponent intervention including exercise education, training to increase pleasant events, and activator-behaviour-consequence problem-solving approach over 6 weeks by case managers in 255 community dwelling people with dementia older than 60 years and their family ...
One study adopted 18F-florbetaben PET imaging in Down syndrome patients, suggesting potential role of amyloid imaging in identifying population at risk of dementia. 17 Similar study was conducted on patients with Down syndrome, but with 18F-florbetapir tracer. 18 An attempt to differentiate Down Syndrome pathology from AD has also been made ...
1 INTRODUCTION. Shared decision-making (SDM) is widely recognized as the optimal approach for patients, including those with dementia, to receive care and treatment consistent with their individual preferences, choices and goals (Goossens et al., 2020; Mariani et al., 2017).Dementia is a syndrome commonly associated with chronic or progressive conditions resulting from various brain disorders ...
In a 6-week trial of pimavanserin for the treatment of hallucinations and delusions associated with Parkinson's disease-related psychosis, 15 a subgroup of patients with cognitive impairment ...
PN Dementia Case Study Quiz Individual Name: SOPHIA D MARTIN Student Number: dw2174ad Institution: Northwest Tech College Bemidji PN Program Type: PN Test Date: 3/28/ Individual Score: 100% Practice Time: 1 min Individual Performance in the Major Content Areas # Individual Individual Score (% Correct) Sub-Scale Points Score. Cognition 5 100% ...
CASE PRESENTATION. A 58-year-old left-handed woman with 12 years of education was referred for further evaluation and management of progressive cognitive dysfunction. A diagnosis of Alzheimer disease (AD) was suspected by the referring neurologist on the basis of an MRI demonstrating mild temporal atrophy, EEG demonstrating bitemporal slowing ...
Case management. Legal and financial advice. Workforce development focused on training families and caregivers. Program effectiveness. At one year, the quality of care provided by the program as measured by nationally accepted quality measures for dementia was exceedingly high — 92% compared to a benchmark of 38%.
• Case Study - Mr. Y's Story (Facilitator's Version) (see Appendix Bin this guide, pg. 27) • Discussion Guide content o Background on Delirium, Dementia, and Depression o Section A: Questions about Differentiating Delirium, Dementia, and Depression o Section B: Questions about Delirium and Care Strategies
Short-term memory decline is the typical clinical manifestation of Alzheimer's disease (AD). However, early-onset AD usually has atypical symptoms and may get misdiagnosed. In the present case study, we reported a patient who experienced symptoms of memory loss with progressive non-fluent aphasia accompanied by gradual social withdrawal. He did not meet the diagnostic criteria of AD based on ...
Individual Performance Profile PN Dementia Case Study Quiz Individual Name: SARAH B KRAFT Student Number: Institution: MN West Community and Tech College Online PN Program Type: PN Test Date: 3/26/2022 Individual Score: 100.0% Practice Time: 2 min Individual Performance in the Major Content Areas # Individual Individual Score (% Correct) Sub ...
These three case studies help you to consider different situations that people with dementia face. They are: Raj, a 52 year old with a job and family, who has early onset dementia. Bob and Edith, an older married couple who both have dementia and are struggling to cope, along with their family. Joan, an older woman, who lives alone and has just ...
Sipilä PN, Heikkilä N, Lindbohm JV, et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. ... Herpes zoster does not increase the risk of neurodegenerative dementia: a case-control study. Am J Alzheimers Dis Other Demen. Published online April 22 ...
Everyday life in rural places can be hampered by many different infrastructural challenges for which technology is often considered a panacea. However, little is known about technological interactions among people living rurally with dementia, who more often live alone and make up a larger proportion of the rural population in England compared to urban. Assemblage theory in a multiple case ...
Monitoring induced vibrations caused by blasting works is becoming an increasingly common form of preventive activity conducted in open-pit mines. Measurement stations also record other events unrelated to blasting works. This article presents a comparison of the intensity of vibrations induced by blasting works in an open-pit mine and mining tremors in an underground mine. The recorded data ...
2. Patient will follow simple 1 step directions utilizing compensatory strategies with 80% accuracy. 3. Patient will be oriented x4 utilizing compensatory strategies with 80% accuracy. 4. Patient will express wants/needs when presented with two verbal options with 80% accuracy. 5.