- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
MedWatch: The FDA Safety Information and Adverse Event Reporting Program
MedWatch , the FDA’s medical product safety reporting program for health professionals, patients and consumers.
MedWatch receives reports from the public and when appropriate, publishes safety alerts for FDA-regulated products such as:
- Prescription and over-the-counter medicines
- Biologics such as blood components, blood/plasma derivatives and gene therapies.
- Medical devices such as hearing aids, breast pumps, and pacemakers.
- Combination products such as pre-filled drug syringe, metered-dose inhalers and nasal spray.
- Special nutritional products such as medical foods and infant formulas.
- Cosmetics such as moisturizers, makeup, shampoos, hair dyes and tattoos.
- Food such as beverages and ingredients added to foods.
Other products that the FDA regulates include tobacco products , vaccines , and animal drug, device, pet food and livestock feed . These products use different reporting pathways and it is recommended that reports concerning these products be submitted directly to the appropriate portals.
MedWatch - The FDA Safety Information and Adverse Event Reporting Program
Your FDA gateway for clinically important safety information and reporting serious problems with human medical products.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Increasing adverse drug reaction reporting—How can we do better?
Contributed equally to this work with: Miri Potlog Shchory
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft
* E-mail: [email protected]
Affiliations Department of Public Health, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel, Assaf Harofeh School of Nursing, Faculty of Medicine, The Hebrew University of Jerusalem, Jerusalem, Israel
Roles Conceptualization, Methodology
Affiliation Clinical Pharmacology Unit, Haemek Medical Center, Afula affiliated to The Bruce Rapapport School of Medicine, Technion, Haifa, Israel
Affiliation Clinical Pharmacology Unit, Kaplan Medical Center, Hebrew University and Hadassah Medical School, Rehovot, Jerusalem, Israel
Roles Conceptualization, Data curation, Methodology, Writing – review & editing
¶ ‡ These authors also contributed equally to this work.
Affiliation Clinical Pharmacology Unit, Shamir Medical Center (Assaf Harofeh), Zerifin, Affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel
Roles Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing
Affiliation Department of Public Health, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel
- Miri Potlog Shchory,
- Lee H. Goldstein,
- Lidia Arcavi,
- Renata Shihmanter,
- Matitiahu Berkovitch,
- Amalia Levy

- Published: August 13, 2020
- https://doi.org/10.1371/journal.pone.0235591
- Peer Review
- Reader Comments
Adverse drug reactions (ADRs) are associated with morbidity and mortality worldwide. Although national systems for reporting ADRs exist there is a low reporting rate. The aim of the current study was to evaluate an intervention plan for improving ADRs reporting among medical professionals (physicians and nurses). A multicentre intervention study was conducted, in which one medical centre was randomly assigned to the intervention group and two medical centres to the control group. The study consisted of 3 phases: baseline data collection, intervention and follow-up of the reporting rate. The questionnaire that was filled in at base line and at the end of study, contained questions about personal/professional demographic variables, and statements regarding knowledge of and behaviour toward ADRs reporting. The intervention program consisted of posters, lectures, distant electronic learning and reminders. An increase in the number of ADRs reports was noted in the intervention group (74 times higher than in the control group) during the intervention period, which was gradually decreased with as the study progressed (adjusted O.R = 74.1, 95% CI = 21.11–260.1, p<0.001). The changes in the "knowledge related to behaviour” (p = 0.01) and in the "behaviour related to reporting" (p<0.001) score was significantly higher in the intervention group. Specialist physicians and nurses (p<0.001), fulfilling additional positions (p<0.001) and those working in other places (p = 0.05) demonstrated a high rate of report. Lectures were preferable as a method to encourage ADRs reporting. The most convenient reporting tools were telephone and online reporting. Thus, implementation and maintenance of a continuous intervention program, by a pharmacovigilance specialist staff member, will improve ADRs reporting rates.
Citation: Potlog Shchory M, Goldstein LH, Arcavi L, Shihmanter R, Berkovitch M, Levy A (2020) Increasing adverse drug reaction reporting—How can we do better? PLoS ONE 15(8): e0235591. https://doi.org/10.1371/journal.pone.0235591
Editor: Karen Cohen, University of Cape Town, SOUTH AFRICA
Received: September 1, 2019; Accepted: June 19, 2020; Published: August 13, 2020
Copyright: © 2020 Potlog Shchory et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data files are available from the ICPSR database - project ID-openicpsr-119582.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
An integral part of drug therapy is the adverse reactions associated with the drug [ 1 ]. These reactions can cause personal injury, hospitalization overload and increase in health costs, thereby creating a heavy burden on national healthcare systems [ 2 – 5 ]. Studies around the world have demonstrated that 3–7% of all hospitalizations are a result of adverse drug reactions (ADRs) and 10–20% of inpatients suffer from drug-related adverse reactions [ 3 , 6 – 8 ]. Serious ADRs were found to be the fourth to sixth causes of death in hospitalized patients in the US, leading to extended hospitalization and doubling of the cost of treating these patients [ 9 , 10 ]. Therefore, both healthcare teams and patients share a common goal of early detection and prevention of ADRs.
Pharmacovigilance is defined by the WHO as the science and activities relating to the detection, assessment, understanding and prevention of adverse reactions or any other drug-related problem. National systems for reporting drugs' adverse reactions can be found in almost every country [ 10 – 12 ]. Spontaneous reporting of medical staff regarding the occurrence of adverse reactions is the major source for monitoring and investigating adverse reactions of marketed drugs. However, only 1 in 20 adverse reactions is actually detected and defined as a real side effect; this leads to the erroneous assumption that the incidence of adverse reactions is much lower than it actually is [ 13 – 15 ]. Inadequate ADR reporting may lead to loss of clinical information that could prevent substantial damage to patients and consequently minimize health costs.
Hence, it is very important to encourage medical staff to report any definite or suspected ADR, as well as to establish and maintain an accurate database which can be used for analyzing and processing of accumulated data, drawing conclusions and providing further recommendations. This series of actions could optimize patients’ wellbeing and safety and improve the functioning of the healthcare system.
Israel has been an official member of the World Health Organization international program for monitoring drugs since 1973. In August 2012, the Israeli ministry of health (MOH) published guidelines for reporting adverse events and new safety information. This document specifies the type of information that the Marketing Authorization Holder (MAH) requires reporting and enables the MAH to appoint a pharmacist to serve as a qualified person responsible for matters related to the reports included in this standard operating procedure, according to the standard worldwide practice. Those regulations were update at February 2013 in order to clarify the work processes related to reporting ADRs and new safety information, to update the definitions of the SOP and to update the types of information requiring reporting by the MAH. The aim of additional update from May, 2013 was to adjust the SOP to the Pharmacists Regulations [ 16 ]. A new regulation was launched at October, 2014, in which a reporting system in medical institutions for both common and severe ADRs was established. In July 2019, a new portal for reporting adverse events to the Risk Management Department was established. The purpose of all these regulations was to raise the awareness of the healthcare teams to the importance of reporting ADRs [ 17 ]. Nevertheless, the reporting rate in Israel, as is worldwide, is still quite low. Schwartzberg el al., identified 16,409 of Individual Case Safety Reports (ICSRs) submitted to the MOH’s ADRs central database between September 2014 and August 2016. However, of these reports, only 5.5% were submitted by health care professionals from medical institutions, while 94.3% were submitted by pharmaceutical companies (MAH and importers) and only 0.2% of the reports have been submitted by patients and the general public [ 18 ].
The purpose of the present study was to establish and evaluate an intervention plan in order to improve the reporting norms of ADRs among medical professionals (physicians and nurses).
Materials and methods
Study design.
The study was conducted over a period of 17 months (August 2012 to December 2013) and included the healthcare teams (physicians and nurses) of internal medicine divisions from three public medical centers in Israel: "A", "B" and "C", while each division served as a cluster. The medical centers selected for the study were public hospitals serving an urban and rural population of 0.5–1 million people each. In Israel, a high percentage of physicians and nurses are from the Commonwealth States and Russian is their first language. In order to make it easier for them to complete the questionnaire and to increase the response rate it was translated into Russian. Staff members who lacked sufficient knowledge of Hebrew or Russian to fill in the questionnaires were excluded from the study. Medical Center "A" was randomly selected to be the intervention group. Medical centers "B" and "C" were merged and served as the control group. The randomization among the centers was raffled by an external person who was not related to the study. ADRs reports were collected from the Israeli Ministry of Health computerized website for all three medical centers, and reports from medical center "A" (the intervention group) were also collected from the physical reporting binders available in the departments. The study was approved by the institutional review board of each medical center respectively. "A"—Ethics ("Helsinki") Committee; (protocol Number 180/10); "B"—Committee Helsinki; (protocol Number EMC-0107-10); "C"—Ethics ("Helsinki") Committee (protocol Number ver:1 KAP 1). In the introduction to the questionnaire a detailed explanation regarding the research and its rational was provided (See Supplement 1 in the S1 File ). In this section the participant was required to give consent to be included in the research. Verbal consent was received during a meeting in which the research was presented to each participant. As part of the consent procedure the interviewer explained to each medical staff member (physicians and nurses) about the study. Potential participants were informed that taking part in the study was voluntary. Those who agreed to participate gave their oral consent. A record of all participants who provided oral consent was kept by the principal investigator. Every local IRB approved the use of verbal consent. Written consent was not obtained from the participants because the participants were staff members and not patients.
The study consisted of 3 phases: The first phase of baseline data collection lasted three months and included handing out a questionnaire to the healthcare teams. The questionnaire contained questions about personal and professional demographic data, and statements regarding the knowledge of and behavior toward ADRs reporting.
The second phase of the study was a 5-month intervention phase. The purpose of the intervention plan was to improve the staff reporting rate of ADRs. Site "A" was randomly selected as the medical center for intervention. The intervention program consisted of the following: a) posters for raising the awareness of the medical staff; these were placed at various locations throughout the departments, such as: physicians’/ nurses’ rooms, medication rooms, and dining areas; b) Forty-five minute lectures that were given during divisional meetings of physicians and nurses separately. The lectures included: definitions of ADRs and pharmacovigilance, an explanation about the importance of the issue, data from international studies in the field, information about the relationship between adverse drug events and morbidity/mortality rates, incidence and prevalence of ADRs during hospital admission, the costs of ADRs for the healthcare system and the patients, presenting the reasons of ADR under reporting and a description of the current practices in Israel and around the world; c) Program promotion. This included: visiting the departments and talking with the medical staff twice weekly, presenting the program in the medical center portal and homepage and sending text messages to the participants on their mobile phones every two weeks (a total of 8 text messages were sent); d) introduction of distant electronic learning into the medical center portal.
The third phase of the study lasted 9 months and included following up on the monthly reporting rates. ADRs were reported through the Ministry of Health computerized website (for both the intervention and the control group) or documented in binders available only in the departments of the intervention group. At the end of this phase the participants from both groups were asked to fill in the same questionnaire again.
The questionnaire (Supplement 1 in S1 File )
The questionnaire design was based on the causes for underreporting of ADRs among professionals, known as the “seven deadly sins” and on the combined theoretical model of the factors affecting the conditions for ADR reporting by healthcare professionals [ 17 , 19 – 32 ].
Demographic data included profession and degree, date and place of birth, gender, country of professional training, years of experience, expertise, as well as additional roles and positions in other medical institutions.
"Knowledge related to behavior" was intended to explore the knowledge of the participants about the ADR reporting procedure and its importance. It was investigated using the following general question which dealt with identification of ADRs and the reasons for reporting/not reporting: "You may notice an irregular adverse reaction from drug treatment and not report it since:" Then the participants were prompted to choose one of 5 statements: "A. I know the adverse drug reaction has already been documented by the pharmaceutical company"; "B. I do not know that there is a center for reporting adverse drug reactions"; "C. I am not aware of the need for reporting adverse drug reactions"; "D. I don't know how to report adverse drug reactions"; and "E. Reporting one adverse drug reaction does not significantly contribute to the reporting mechanism". The answers were ranged on a scale from 1 point—no reason for not reporting to 10 points—good reason for not reporting. This means that a staff member who reports ADRs will receive a lower score.
The score of "behavior related to reporting" was constructed from two items that analyzed the patterns of reporting to the National Center of pharmacovigilance and to pharmaceutical companies. The statements were: "A. I spoke with pharmaceutical companies about the possibility of adverse drug reactions with their drugs"; and "B. Have you ever reported adverse drug reactions to a national reporting center?". The average of this score ranged from 1 point—non reporting behavior—through 10 points—reporting behavior. The higher rate of reporting achieved a higher score.
The main hypothesis: after the intervention program there would be more ADRs reporting among medical professionals (physicians and nurses) in the intervention group compared to the control group and to ADRs reporting base line.
Data analysis
Data analysis was carried out using the SPSS 21 software (PASW inc., USA). The statistical analysis was conducted according to the phases of the study. Means and standard deviation were used for continuous variables and examined by T or One-Way ANOVA/Mann Whitney tests based on the variables distribution. The score of "Knowledge related to behavior" and the score of "behavior related to reporting" didn’t distribute normally, thus we used a- parametric test (Mann Whitney). Percentages and numbers were used for categorical variables and were tested by chi square or Fisher’s exact tests as appropriate. Statistical significance was established as p≤0.05. The multi-variable models examined the independent effect of the intervention program on reporting. The difference (change) was viewed as the dependent variable, and factors forecasting change were examined through multi-variable linear regression models. Identifying independent predicting factors of reporting adverse reactions was done through multi-variable logistic regression models.
Quantitative variables, such as the differences between groups, comparison between physicians and nurses and between medical centers, were analyzed by chi square or Fisher’s exact tests. The differences and changes between the various parameters (knowledge and reporting patterns) were calculated by comparing the data collected after the intervention phase with the baseline data (change). The differences in knowledge and reporting among the study groups were compared separately by T tests with independent samples or through One-Way ANOVA. The building strategy for multivariable models was forced all the independent variables to one block. Both statistical and clinical justifications were considered. The models included all the variables that were found to be significant (p<0.05) in the univariate data analysis, and covariates that were important to controlling for, as a baseline characteristic, according to the research questions. All the independent variables that were included in the analyses were presented in the results of the multivariable models. The multivariate models examined the effect of the intervention program on knowledge and reporting. The difference (change) was defined as the dependent variable and factors forecasting change were examined through linear regression. The adjusted factors of reporting adverse reactions (with 95% confidence intervals, 95% CI) were performed through multivariate logistic regression models. The independent effect of every measure index of the questionnaire was adjusted to demographic and professional variables.
433 (81.5%) medical staff members, physicians and nurses, completed the questionnaire twice, before and after the intervention. 47.8% of the participants were from the "A" medical center, 28.4% from "B" and 23.8% from "C". Distribution by gender was 69.1% females and 30.9% males. 73% were nurses and 27% physicians. No selection bias was found between the staff members completed the questionnaire the first time and those completed it twice. No differences in personal or professional variables were found between the intervention group ("A" medical center) and control group ("B" and "C" medical centers), except for the ratio between physicians and nurses and the subjects country of origin and average age."
During the research period, 336 ADRs were reported, of which 288 (85.7%) were reported in Medical Center “A”, with 285 ADRs from the reporting binders and 3 ADRs from the Ministry of Health’s computerized website. The ADRs reports from the control groups comprised 10 reports (3%) in center “B” and 38 reports (11.3%) in center C; these were reported to the Health Ministry’s computerized website. The reports were checked and there were no duplicate reports by the staff members on both reporting channels in the intervention group.
The number of ADRs reports in the intervention vs. the control groups during the study period is presented in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
A rapid and substantial increase in the number of ADR reports was noted in the intervention group during the 5-month intervention period, which gradually decreased toward the end of the study.
https://doi.org/10.1371/journal.pone.0235591.g001
A rapid and substantial increase in the number of ADR reports was noted in the intervention group during the 5-month intervention period, which gradually decreased toward the end of the study. The reporting in the intervention group went nearly back to baseline and was even lower than in the control group. On the other hand, there was almost no change in the number of ADR reports in the control group during the entire study duration. After the intervention period the reporting rate in the intervention group reverted to almost baseline and was lower than the control group, similarly to the trend that was observed in the baseline.
Comparison of the rate of ADRs reports received from physicians vs. nurses indicated that in both groups a substantial increase in the number of ADR reports was observed during the intervention period, which gradually decreased toward the end of the study.
Comparison of the score of "knowledge related to behavior" showed that before any intervention the mean score of the control group was significantly lower than that observed in the intervention group (3.84±2.20 and 4.37±2.27 respectively, p = 0.02), demonstrating that the control group was more aware of ADRs reporting. Nevertheless, the changes in the "knowledge related to behavior” score was significantly higher in the intervention than in the control group (a change of -0.69±2.58 in the intervention group vs. -0.11±2.19 in the control group, P = 0.01)) Table 1 ).
The changes in the "knowledge related to behavior” score was significantly higher in the intervention than in the control group.
https://doi.org/10.1371/journal.pone.0235591.t001
When the score of "behavior related to reporting" was compared between the intervention and control groups upon intervention, a statistically significant increase in the score "behavior related to reporting" was observed in the intervention group, with a mean change of 0.65±2.22 (2.21±1.88 before intervention and 2.37±2.87 after intervention, P<0.001). No significant difference in the score of "behavior related to reporting" was noted in the control group ( Table 2 ).
https://doi.org/10.1371/journal.pone.0235591.t002
Comparison of patterns in "behavior related to reporting" was conducted according to various demographic and professional-related variables. The results revealed that the nurses demonstrated less changes in "behavior related to reporting" than physicians (P = 0.003). A significant positive correlation was found between the numbers of patients treated per day by the medical staff (nurses and physicians) and "behavior related to reporting", i.e. as the number of patients per caregiver increased, the change in "behavior related to reporting" score was higher (P = 0.02). In addition, the results revealed that the more aware the caregiver is of the fact that the patients are consuming more than one medication per day, a larger change in "behavior related to reporting" score is observed (P = 0.02, r = 0.13) ( Table 3 ).
Nurses demonstrated less changes in "behavior related to reporting" than physicians A significant positive correlation was found between the numbers of patients treated per day by the medical staff (nurses and physicians) and "behavior related to reporting", as well as between the awareness of the caregiver that the patients are consuming more than one medication per day, and the change in "behavior related to reporting" score.
https://doi.org/10.1371/journal.pone.0235591.t003
The demographic and the professional variables and reporting/non reporting behavior were later examined within the intervention group. As seen in Table 4 , physicians reported more than nurses (56.9% vs. 36.5%, p = 0.009). Specialists, both nurses and physicians, reported more than non-specialists (60.6% vs. 32.6%, p<0.001). Those (both physicians and nurses) fulfilling additional positions and those working in other places beside the hospital demonstrated high rates of reports (66.7% vs. 33.6%, p<0.001 and 60.0% vs. 39.8%, p = 0.05, respectively) ( Table 4 ).
Physicians reported more than nurses; specialists, reported more than non-specialists; those fulfilling additional positions and those working in other places beside the hospital demonstrated high rates of reports.
https://doi.org/10.1371/journal.pone.0235591.t004
Further analysis of the previous data to physicians and nurses revealed that the demographic and professional variables among the physicians did not have any effect on the percentage of ADRs reports. However, among the nurses, specialty (56.1% vs 29.6%, p = 0.02) and fulfilling additional positions (63.3% vs. 28.8%, p<0.0001) indeed increased reporting rates, while no difference was observed with regard to working in other places besides the hospital (37.5% vs. 36.6%, p = 0.96).
The intervention plan had a strong, independent and statistically significant effect on "behavior related to reporting" (p = 0.008). In addition, profession and number of patients treated per day by the caregiver also had a significant effect on the "behavior related to reporting" (p = 0.01 and p = 0.02, respectively) ( Table 5 ).
The intervention plan had a strong, independent and statistically significant effect on "behavior related to reporting". Profession and number of patients treated per day by the caregiver also had a significant effect on the "behavior related to reporting".
https://doi.org/10.1371/journal.pone.0235591.t005
In order to examine the independent effect of the intervention plan on reporting (yes / no), a logistic model was constructed. We found that the intervention plan had a strong, independent, statistically significant effect on the staffs’ actual ADRs reporting. After standardization for specialist, expertise, holding managerial positions and those who work in other places, subjects in the intervention group reported 74 times higher than their counterparts in the control group (O.R = 74.1, 95% CI = 21.11–260.1, p<0.001) ( Table 6 ).
A logistic model revealed that the intervention plan had a strong, independent, statistically significant effect on the staffs’ actual ADRs reporting.
https://doi.org/10.1371/journal.pone.0235591.t006
Education and lectures were preferable, while payment for reporting was the least desirable method for encouraging medical staff to report side effects. The most convenient reporting tools were found to be the telephone and an internet site.
The purpose of this study was to establish and evaluate an intervention plan for increasing ADR reporting rate among physicians and nurses. This study demonstrates that the rate of ADR reporting increased significantly during the intervention period, and declined gradually thereafter. However, almost no change in the numbers of reports was observed in the control group during the entire duration of the study. The trend presented in the data that the rate of reports decreased over time after the intervention program was discontinued implies that continued intervention may be required to maintain the high rate of reports. Interestingly, the reporting rate in the control group was higher than in the intervention group at base line, though it was not statistically significant. The intervention plan increased the reporting rate and the differences between the control and the intervention rose and were statistically significant throughout the intervention period. However, as distance from the intervention period increased, the amount of reports decreased eventually reverting to the trend that was observed at the baseline. The impact of the intervention waned with time. An example to this trend was demonstrated also in an educational intervention program to improve physicians' reporting of ADRs. In this study the reporting rate in the intervention group increased during the intervention, while it gradually decreased through 13 months of follow-up [ 9 ].
The compliance rate of the participants in the present study for filling in both questionnaires was rather high (81.5%). A lower rate of compliance was reported in similar studies, in which 22.8% (Biagi 2013) or 47% (Passier 2009) of the physicians answered questionnaires regarding ADRs reporting [ 33 , 34 ]. Another study from Venezuela (Garciani 2011) reported higher compliance rate of 65.4% among physicians and pharmacists and 60% among nurses [ 35 ]. Interestingly, a much lower compliance rate was found in studies in which the questionnaires were sent to the participants by e-mail or regular mail [ 36 ]. The relatively high rate of compliance in the current study may be associated with the constant presence of the investigator at the medical centers and the personal contact with the study participants.
According to the results of the current study, physicians reported ADRs more than nurses. In addition, the change in the “Behavior related to reporting” score in the intervention group was higher among physicians compared to the nurses. The changes in the “Behavior related to reporting” is also demonstrated by the increase in the rate of ADRs in the intervention group compared the control group. This finding is consistent with other studies [ 1 , 37 – 39 ]. In a study that took place in Korea, spontaneous reports of ADRs by e-mail were 13% among nurses vs. 53% reports by physicians. Some studies demonstrated that nurses mostly tend to report an ADR to a physician. Hanafi et al. have shown that 89% of the nurses who participated in the study said that they reported the ADR to the physician [ 40 ]. The Hajebi’s study found that 56% of the nurses that come across a drug-related side effect reported to the department physician, 26% to the head nurse and 13% to a pharmacist [ 41 ]. The availability and accessibility of physicians to nurses who work in hospitals probably encouraged the reporting to physicians. This tendency could explain the difference in ADR reporting rates between nurses and physicians. Contrarily, in a research that was conducted in an Israeli public hospital where the medical staff was encouraged to report ADRs to the clinical pharmacology unit, the nurses were found to have reported more ADRs than the physicians [ 42 ]. In the present study, despite the fact that the nurses reported less than the physicians, their rate of reports peaked more quickly and decreased more slowly than that of the physicians.
We also found that professionals who fulfill additional positions in the department or in other health institutions (such as: community health services or treating senior citizens at nursing homes) demonstrated higher rates of ADR reporting. In addition, the results of the present study showed that specialists reported more ADRs than non-specialists and that the rate of ADR reporting was associated with the number of patients treated per day by the caregiver. A contrary observation was reported by a study in Ireland, which investigated the rate of ADR reporting among 118 hospital-based physicians. This study found a higher rate of ADR reporting among general physicians than among surgeons [ 43 ].
The present study demonstrated that the preferred method for increasing the rate of ADR reporting was lectures and education A study that compared telephone-interview intervention with a workshop intervention showed that the latter increased ADR reporting rate by a fourfold on average compared to the control group over 20 months post-intervention. However, no significant difference vs. the control group in ADR reporting was found in the telephone-interview intervention [ 44 ]. Other studies have shown that improved communication with fellow physicians and involvement of pharmacists might be the best ways to improve ADR reporting [ 21 , 34 ]. Regular newsletter on current awareness in drug safety, information on new ADRs, and international drug safety information were also identified as tools or methods that may motivate ADR reporting in a study conducted by Santosh et al. among 450 healthcare professionals working at Regional Pharmacovigilance Centers in Nepal [ 21 ].
Our research shows that the preferred means of reporting were telephone or website. Other studies also report these methods as preferential. A study of 500 nurses from a teaching hospital in Teheran showed that among the 10% who reported an ADR, the majority of the nurses preferred using the telephone [ 45 ]. Among physicians in India and the Netherlands, most of the ADRs were reported using the computerized system [ 34 , 46 ].
Payment for reporting was found to be the least favored method to encourage ADR reporting in the current study. A survey of 91 practice nurses, health visitors, school nurses and general physicians conducted by Pulford et al., has shown that payment for ADR reporting was indeed the least acceptable out of 14 other options of gratuity [ 47 ].
Conclusions
The results of this study indicate that training and educating medical practitioners and providing them with relevant knowledge regarding ADR reporting is essential. Due to the observation that the reporting rate decreased with time upon the finalization of the intervention period, it seems that maintaining a program to encourage reporting, is necessary. Regular implementation of such a program in the healthcare system will increase the awareness of the medical staff and improve reporting rates. Maintaining the intervention program could be carried out by nomination of a pharmacovigilance specialist trustee to administer a routine intervention program. This expert could be a physician, a nurse or a pharmacist. Visits, personal discussions, posters, lectures about the importance of ADRs reporting and how to carry it out and sending text messages to the medical staff on their mobile phones with reminders and relevant information should be used in order to continuously raise their awareness reporting about ADRs. In addition, the personal contact of the medical staff with the trustee will encourage their commitment to report about ADRs. This will probably improve monitoring of medication use, decrease morbidity/mortality rates and hospitalization duration.
Limitations
The study was carried out in internal divisions of only 3 public medical centers, and therefore may not have an external validation in other hospitals.
The study included only physicians and nurses, therefore the results cannot be applied to additional medical professionals.
There were some differences in the basic characteristics between the clusters (hospitals) which may have affected the quality of the intervention to a certain extent. However, after adjusting for the demographic variables, we can assume that the results of the study are indeed due to the effect of the intervention.
We cannot assume from this study that the effect of the intervention would improve safety of medicine use in the long term and reduce health costs.
Supporting information
https://doi.org/10.1371/journal.pone.0235591.s001
https://doi.org/10.1371/journal.pone.0235591.s002
https://doi.org/10.1371/journal.pone.0235591.s003
- View Article
- Google Scholar
- 2. WHO, Geneva. Safety of Medicines: A guide to detecting and reporting adverse drug reactions. 2002; URL: http://www.who.int/medicuned/en/d/jh2992e/
- PubMed/NCBI
- 4. Atkinson AJ, Abernethy DR, Daniels CE, FASHP RP, Robert L. Dedrick RL, et al. Principles of Clinical pharmacology. second edition (2007) Chapter 25 Clinical analysis of adverse drug reaction 389–402.
- 16. https://www.health.gov.il/hozer/DR_6E.pdf
- 22. Inman WHW. Assessment of drug safety problems. In: Gent M, Shigmatsu I, editors. Epidemiological issues in reported drug-induced illnesses. Honolulu (ON): McMaster University Library Press, 1976:17–24.
- 23. Inman WHW, Weber JCT. The United Kingdom. In Inman WHW, editor. Monitoring for drug safety. 2nd ed. Lancaster: MTP Press Ltd, 1986: 13–47.
- 37. Guidelines for reporting side effects to medication by a registry (June 1998) Ministry of Health.
Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update
- Systematic Review
- Open access
- Published: 06 June 2023
- Volume 46 , pages 625–636, ( 2023 )
Cite this article
You have full access to this open access article

- Patricia García-Abeijon 1 ,
- Catarina Costa ORCID: orcid.org/0000-0002-8247-0422 2 ,
- Margarita Taracido ORCID: orcid.org/0000-0003-1243-8352 1 , 3 , 4 ,
- Maria Teresa Herdeiro ORCID: orcid.org/0000-0002-0500-4049 5 ,
- Carla Torre ORCID: orcid.org/0000-0002-5542-9993 2 , 6 &
- Adolfo Figueiras ORCID: orcid.org/0000-0002-5766-8672 1 , 3 , 4
4363 Accesses
16 Citations
12 Altmetric
Explore all metrics
Introduction
Underreporting is a major limitation of the voluntary reporting system of adverse drug reactions (ADRs). A 2009 systematic review showed the knowledge and attitudes of health professionals were strongly related with underreporting of ADRs.
Our aim was to update our previous systematic review to determine factors (sociodemographic, knowledge and attitudes) associated with the underreporting of ADRs by healthcare professionals.
We searched the MEDLINE and EMBASE databases for studies published between 2007 and 2021 that met the following inclusion criteria: (1) published in English, French, Portuguese or Spanish; (2) involving health professionals; and (3) the goal was to evaluate factors associated with underreporting of ADRs through spontaneous reporting.
Overall, 65 papers were included. While health professional sociodemographic characteristics did not influence underreporting, knowledge and attitudes continue to show a significant effect: (1) ignorance (only serious ADRs need to be reported) in 86.2%; (2) lethargy (procrastination, lack of interest, and other excuses) in 84.6%; (3) complacency (the belief that only well tolerated drugs are allowed on the market) in 46.2%; (4) diffidence (fear of appearing ridiculous for reporting merely suspected ADRs) in 44.6%; and (5) insecurity (it is nearly impossible to determine whether or not a drug is responsible for a specific adverse reaction) in 33.8%, and the absence of feedback in 9.2%. In this review, the non-obligation to reporting and confidentiality emerge as new reasons for underreporting.
Conclusions
Attitudes regarding the reporting of adverse reactions continue to be the main determinants of underreporting. Even though these are potentially modifiable factors through educational interventions, minimal changes have been observed since 2009.
Clinical Trials Registration
PROSPERO registration number CRD42021227944.
Similar content being viewed by others
Factors associated with underreporting of adverse drug reactions by patients: a systematic review
Factors that motivate healthcare professionals to report adverse drug events: a systematic review.

Why hospital-based healthcare professionals do not report adverse drug reactions: a mixed methods study using the Theoretical Domains Framework
Avoid common mistakes on your manuscript.
1 Introduction
Adverse drug reactions (ADRs) are responsible for 10% of outpatient appointments [ 1 , 2 ] and 3.5–10% of hospital admissions [ 1 , 2 ], and are the fifth leading cause of death in hospitalized patients, in addition to prolonging stays and presenting a high economic impact [ 3 , 4 ]. The spontaneous reporting of suspected ADRs by health professionals allows continuous determination of the benefit–risk ratio of a given drug and is one of the best methods to generate signals regarding unexpected events and rare ADRs [ 1 , 5 ].
Underreporting is a major limitation of spontaneous notification systems, as it is estimated that only 6–10% of all ADRs are reported [ 6 , 7 ]. On the one hand, this high underreporting rate prevents ADRs from being quantified in order to calculate their impact in terms of incidence and risk [ 6 ], and on the other hand, it delays the activation of warning signals, with the consequent repercussions on public health [ 5 , 6 , 8 ]. These delays in decisions to restrict a drug’s use or to withdraw it may result in many more patients being affected [ 5 ].
In 2009, our group carried out a systematic review on factors associated with the underreporting of ADRs [ 8 ], which showed that a high proportion of studies found that the main factors related to underreporting are the knowledge and attitudes of health professionals. We believe that an update of this review may be of interest for several reasons: (1) numerous studies have been published since 2006; (2) we wanted to check whether these studies have identified new factors related to underreporting; and (3) in our 2009 review, methodological problems had been identified (many studies do not specify the type of design, or have a low percentage of participation and response) and we consider that it would be of interest to assess whether the methodological quality has improved. In the current manuscript, we update our previous review [ 8 ] on the factors (socioprofessional characteristics, knowledges, attitudes) associated with underreporting of ADRs by health professionals.
2 Methods and Data Extraction
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020, 27-item checklist guideline, and its research protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (registry number CRD42021227944).
2.1 Bibliographic Search Method
A bibliographic search of studies published between January 2007 and February 2021 was performed in the MEDLINE (Pubmed) and EMBASE scientific databases. The search terms used were (‘attitude*’ OR ‘knowledge*’ OR ‘barrier*’ OR ‘facilitators*’) AND (‘adverse drug reaction reporting systems’ [MeSH] OR ‘drug related side effects and adverse reactions’ [MeSH]) AND ‘report*’.
Studies were required to meet the following inclusion criteria: (1) they were published in English, French, Portuguese or Spanish; (2) the study population was defined as healthcare professionals (doctors, pharmacists, nurses, administrators, residents, and other health professionals); (3) articles whose objective was to evaluate the factors associated with ADR underreporting; and (4) articles that address ADR reporting through spontaneous reporting.
Studies were excluded if they met any of the following criteria: (1) did not have an abstract and/or full text; (2) conference abstracts, thesis, comments, letters, abstracts, or editorials; (3) systematic reviews and/or meta-analyses; (4) articles on a specific pathology and/or treatment; (5) were carried out on patients and/or consumers; (6) did not focus on the study objective; (7) identified attitudes and knowledge but were not directly associated with the reasons for underreporting.
2.2 Quality Evaluation
An assessment of the quality and risk of bias of the articles was performed using the AXIS cross-sectional study assessment tool [ 9 ]. This tool contains 20 questions, 10 related to the methods, 5 related to the results,which indicates the importance of these sections in quality, 1 to the introduction, and 4 to the discussion (Table 1 ).
Of the 20 questions, 7 evaluated the quality of reporting (1, 4, 10, 11, 12, 16 and 18), 7 evaluated the quality of the study design (2, 3, 5, 8, 17, 19 and 20), and 6 evaluated the possible introduction of biases in the study (6, 7, 9, 13, 14 and 15) [ 9 ].
Two reviewers (CC and PGA) independently conducted the critical assessment for each included study. In the case of disagreement, a third reviewer (CT, MTH, or AF) was responsible for resolving discrepancies.
2.3 Data Extraction
All articles retrieved were independently screened by two reviewers (CC and PGA), who further independently conducted the full-text analysis to assess suitability for the inclusion of each potentially eligible study. In the case of any divergent decisions, a third reviewer (CT, MTH, or AF) acted as referee to reach consensus.
The data extracted from the articles were entered into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) and two tables were created. In the first table, the author (publication year), country, study population, workplace, sample size, survey distribution, survey scale and AXIS not fulfilled criteria were included (Online Resource Table S1).
The second table contained the author (publication year), response rate, personal and professional factors associated with ADR reporting, and reasons for not reporting the ADR (Online Resource Table S2). All the reasons cited have been extracted from the results of the studies and have been assigned to one of Inman’s ‘deadly sins’ [ 10 , 11 , 12 ]. In 1976, Inman proposed a theoretical model to explain the reasons for ADR underreporting by health professionals. This model was called Inman’s ‘seven deadly sins’ and included complacency diffidence, indifference, ignorance, ambition, financial reimbursement, legal aspects, fear, and feedback. The model has been modified and expanded, including insecurity, unavailability of notification form, and lethargy [ 10 , 11 , 12 ]. These reasons can be grouped into attitudes related to the professional activity (legal fears, economic interests, and ambition to publish), ADR-related knowledge and attitudes (complacency, diffidence, indifference, ignorance, and insecurity), and related to excuses (lethargy) [ 8 ].
The median of respondents who stated that this attitude determines their ADR reporting was calculated for each of the attitudes (Online Resource Table S3).
2.4 Sensitivity Analysis
In order to assess the impact of the quality of the articles included in the results of the review, a sensitivity analysis was performed. This subanalysis included articles whose AXIS score was above the median of the total studies.
3.1 Article Selection
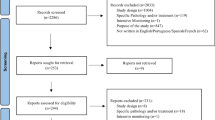
Using the chosen search strategy, 1758 articles were located—1706 in MEDLINE and 52 in EMBASE (see Fig. 1 ). After reading the abstracts, it was considered that 203 articles met the inclusion criteria, and a complete full-text analysis of these was carried out. After a full-text reading, 138 articles were excluded because they did not meet the inclusion criteria, and 65 articles were selected—57 from MEDLINE and 8 from EMBASE (Fig. 1 )
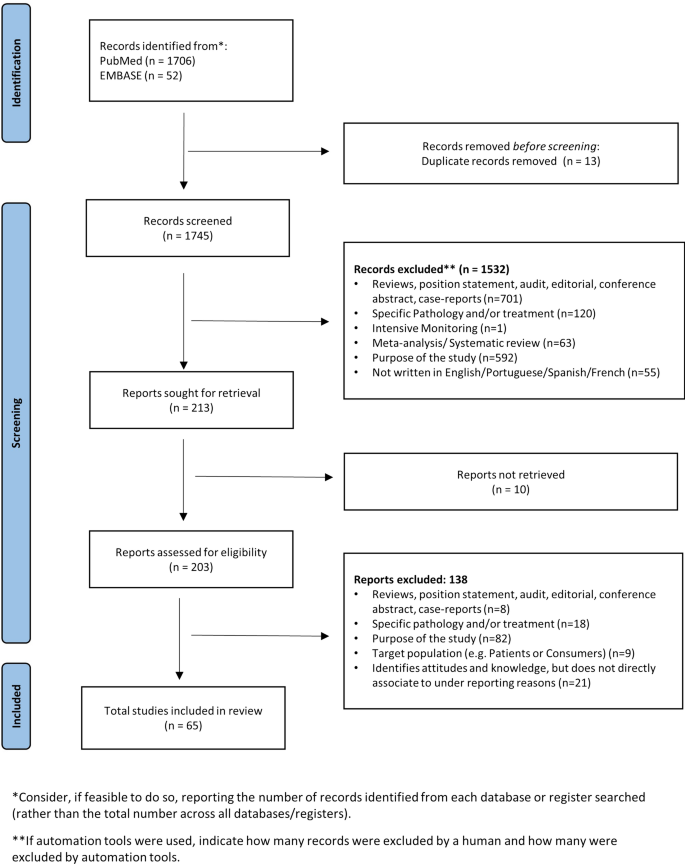
Study identification process
3.2 Characteristics of the Selected Articles
More than half of the articles (45/65, 69.2%) were cross-sectional studies [ 13 , 14 , 16 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 44 , 45 , 47 , 48 , 49 , 50 , 51 , 54 , 55 , 56 , 59 , 60 , 61 , 64 , 65 , 69 , 70 , 71 , 74 ], 21.5% did not indicate the design type used [ 20 , 25 , 52 , 57 , 58 , 62 , 63 , 66 , 67 , 68 , 72 , 73 , 75 , 76 ], 4.6% were case-control studies [ 40 , 46 , 77 ] (during the same period, the cases had reported ADR and the controls had not), 3.1% (2/65) were mixed studies [ 15 , 43 ] (only data from the quantitative part were used), and one (1.5%) was an intervention study [ 53 ] (pre-intervention data were extracted). Two studies were conducted before a pharmacovigilance system was in place [ 17 , 31 ].
According to geographical distribution, we located studies carried out in 36 countries: 38.5% were carried out in Asia [ 19 , 22 , 28 , 29 , 31 , 32 , 35 , 37 , 38 , 39 , 44 , 46 , 47 , 50 , 51 , 52 , 53 , 56 , 58 , 59 , 61 , 63 , 64 , 65 , 72 ], 27.7% in Africa [ 13 , 14 , 15 , 17 , 18 , 23 , 27 , 30 , 34 , 42 , 45 , 48 , 49 , 65 , 69 , 70 , 71 , 74 ], 24.6% in Europe [ 20 , 21 , 24 , 25 , 33 , 40 , 43 , 54 , 55 , 57 , 60 , 62 , 66 , 73 , 75 , 77 ], 7.7% in America [ 16 , 36 , 41 , 67 , 76 ], and one in Australia [ 27 ].
Regarding the setting where the study was performed, 43.1% took place in hospitals [ 14 , 15 , 17 , 18 , 19 , 22 , 24 , 30 , 32 , 35 , 41 , 42 , 43 , 44 , 46 , 47 , 48 , 53 , 56 , 59 , 63 , 64 , 66 , 68 , 69 , 70 , 73 , 74 ], 21.5% in pharmacies [ 20 , 21 , 26 , 31 , 33 , 38 , 39 , 51 , 61 , 65 , 72 , 75 , 76 , 77 ], 9.2% did not mention the place [ 16 , 25 , 52 , 55 , 57 , 62 ], 6.2% in hospitals and pharmacies [ 28 , 50 , 67 , 71 ], and 6.2% in primary care [ 13 , 23 , 27 , 29 ]. In 10 articles, the results of surveys carried out in two different health facilities are combined [ 28 , 34 , 36 , 37 , 40 , 49 , 50 , 54 , 67 , 71 ].
Regarding the study population, pharmacists were the most studied [ 20 , 21 , 26 , 28 , 31 , 33 , 39 , 46 , 50 , 51 , 52 , 61 , 65 , 67 , 68 , 72 , 75 , 76 , 77 ], followed by doctors, pharmacists and nurses together [ 13 , 14 , 19 , 22 , 27 , 30 , 36 , 41 , 44 , 45 , 49 , 56 , 64 ], and lastly, doctors [ 15 , 37 , 48 , 54 , 55 , 58 , 60 , 63 , 66 , 70 , 73 ]. In 30 articles, the survey was conducted on two or more types of professionals [ 13 , 14 , 16 , 17 , 18 , 19 , 22 , 23 , 24 , 25 , 27 , 29 , 30 , 32 , 34 , 35 , 36 , 38 , 41 , 42 , 44 , 45 , 47 , 49 , 53 , 56 , 57 , 62 , 64 , 71 ] and in one study the survey was conducted on health professionals and patients [ 16 ].
3.3 Sample Size and Response Rate
The sample size (see Online Resource Table S1) varied between 80 [ 23 , 77 ] and 3351 [ 57 ]. A total of 41 articles presented a sample of more than 200 subjects [ 13 , 14 , 15 , 19 , 20 , 21 , 22 , 24 , 26 , 28 , 29 , 30 , 31 , 32 , 33 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 47 , 48 , 49 , 51 , 52 , 53 , 55 , 56 , 57 , 58 , 60 , 61 , 62 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 73 , 74 , 75 ], 9 had more than 1000 subjects [ 19 , 39 , 40 , 49 , 57 , 62 , 67 , 73 ], and 3 did not mention the sample size [ 16 , 25 , 44 ].
In the articles that mentioned response rate, this varied between 16.4% [ 67 ] and 100% [ 17 , 22 , 23 , 34 , 63 , 76 ] (see Online Resource Table S2). More than half of the articles had response rates > 50% [ 13 , 14 , 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 41 , 42 , 43 , 44 , 46 , 48 , 49 , 50 , 54 , 56 , 58 , 59 , 61 , 63 , 64 , 65 , 68 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 ], and six reached 100% [ 17 , 22 , 23 , 34 , 63 , 76 ]; nine articles did not mention the response rate [ 16 , 26 , 27 , 39 , 45 , 47 , 62 , 66 , 69 ].
3.4 Survey Distribution and Scale
The survey was distributed personally in more than half of the articles [ 13 , 14 , 15 , 17 , 18 , 19 , 20 , 22 , 23 , 24 , 27 , 28 , 29 , 30 , 31 , 37 , 38 , 39 , 41 , 42 , 44 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 56 , 58 , 59 , 61 , 62 , 64 , 65 , 72 , 74 , 76 , 77 ], and the remainder were sent by internet [ 16 , 25 , 26 , 33 , 35 , 36 , 39 , 52 , 57 , 62 ] or post [ 21 , 33 , 40 , 46 , 55 , 60 , 67 , 73 ], while 10.8% did not mention how the survey was distributed [ 32 , 34 , 43 , 45 , 63 , 69 , 71 ]. In four articles, two or more distribution types were combined [ 33 , 35 , 39 , 62 ] (see Online Resource Table S1)
The most used scale (Online Resource Table S1) to answer the survey questions was the Likert scale [ 15 , 17 , 18 , 19 , 21 , 22 , 23 , 26 , 27 , 28 , 32 , 36 , 38 , 39 , 43 , 46 , 49 , 51 , 55 , 56 , 57 , 65 , 66 , 75 , 77 ], followed by multiple-choice and free text [ 14 , 24 , 30 , 37 , 41 , 50 ], while one article used the visual analog scale [ 40 ]. It is noteworthy that almost half (28/65) of the articles did not mention the scale used [ 13 , 16 , 20 , 25 , 29 , 31 , 34 , 35 , 42 , 44 , 45 , 47 , 48 , 52 , 53 , 54 , 58 , 59 , 60 , 62 , 64 , 69 , 70 , 71 , 72 , 73 , 74 , 76 ]. In 19 articles, multiple scales were used [ 14 , 18 , 21 , 24 , 26 , 27 , 28 , 30 , 37 , 41 , 43 , 46 , 50 , 51 , 55 , 57 , 66 , 75 , 77 ].
3.5 Factors Identified as Influential
Each of the selected articles was evaluated to identify the factors said to influence ADR underreporting.
3.5.1 Personal and Professional Factors
Regarding the personal and professional factors that influence ADR reporting (see Online Resource Table S2), years of experience stands out, with 12.3% (8 articles), training and profession were cited in 10.8% of the articles (7/65), age and workplace in 9.2% (6/65), qualification in 7.7% (5/65), and sex in 6.2% (4/65).
3.5.2 Influence of Knowledge and Attitudes
Table 1 , Online Resource Table S2, and Online Resource Table S3 show that the most reported reasons by professionals for not reporting ADRs were lethargy and ignorance, followed by other reasons, such as lack of confidence and complacency: (1) ignorance in 56 (86.2%) articles, with an average of respondents who stated that attitude determines their ADR reporting (respondents average of 37.8%); (2) lethargy is described in 55 (84.6%) articles (respondents average of 33.3%); (3) other reasons (lack of internet access, lack of employer support, patient management is more important) in 38 (58.5%) articles (respondents average of 16.3%); (4) complacency (respondents average of 28.6%) in 30 (46.2%) articles; (5) diffidence (respondents average of 26.9%) in 29 (44.6%) articles; (6) unavailability of the reporting form (respondents average of 37.4%) and insecurity in 22 (33.8%) articles (respondents average of 34.4%); (7) legal aspects in 19 (29.2%) articles (respondents average of 19.5%); (8) indifference in 18 (27.7%) articles (respondents average of 23.5%); (9) financial reimbursement in 14 (21.5%) articles (respondents average of 22.2%); (10) lack of information in 13 (20.0%) articles (respondents average of 23.3%); (11) obligation or duty to inform in 12 (18.5%) articles (respondents average of 20.9%); (12) fear in 7 (10.8%) articles (respondents average of 24.5%); (13) confidentiality of both the patient and the professional (respondents average of 13.4%) and feedback in 6 (9.2%) articles (respondents average of 29.4%); and (14) ambition in 2 (3.1%) articles (respondents average of 15.9%).
3.6 AXIS Tool
The results of AXIS tool evaluation (see Online Resource Table S1, and Fig. 2 ) indicated that 29.2% (19/65) of the studies met more than 17 AXIS criteria (see Table S1), almost half of the studies (29/65, 44.6%) met 14–16 criteria, and about one-quarter of the studies (17/65, 26.2%) met 13 or fewer criteria.
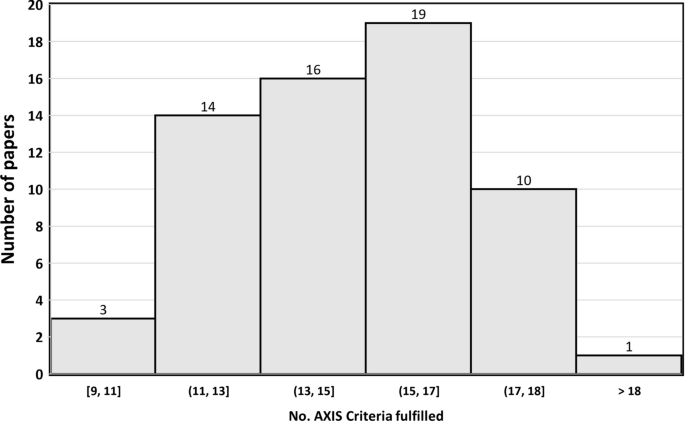
Distribution of papers by the number of AXIS criteria fulfilled
The criteria “Were the aims/objectives of the study clear?”, “Were the risk factor and outcome variables measured appropriate to the aim of the study”, and “Were the basic data adequately described?” were fulfilled in all articles.
In contrast, no articles met the criteria “If appropriate, was information about non-responders described?”, only 13 met the criteria “Were measures undertaken to address and categorize non-responders?”, and 34 met the criteria “Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation?”.
3.7 Sensitivity Analysis: Results for the Highest-Quality Studies
The median AXIS score was 16 criteria. Table 1 shows the most frequent criteria among the 32 articles with the highest degree of evidence. It is observed that ignorance (90.6%), lethargy (81.3%), complacency (43.8%), and difference (40.6%) were the most named attitudes on the part of health professionals, results very similar to the total of the studies.
4 Discussion
This review was based on more studies than our 2009 review (65 vs. 45) [ 8 ] and has confirmed and extended the previous findings: health professionals’ attitudes to ADRs, modeled through Inman’s ‘seven deadly sins’, continue to be the most important determinants of underreporting, and personal and professional characteristics continue to show little influence. In addition, the new information included in the current review allowed to associate the perception of absence of an obligation to notify and the lack of information and confidentiality with the underreporting. These results can help design interventions that improve ADR reporting, since the main reasons found in this review are potentially modifiable.
Sociodemographic and professional characteristics are only cited as factors associated with the reporting of ADRs in fewer than half of the articles included and yet attitudes and knowledge are cited in one way or another in almost all the articles. These factors can be grouped into four categories [ 8 , 78 ]: (1) factors associated with ADR-related knowledge and attitudes (fear, complacency, insecurity, diffidence, indifference, and ignorance); (2) factors relating to the professional activity (financial incentives, legal aspects, and ambition to publish); (3) excuses made by professionals (lethargy, unavailability of the reporting form); and (4) there is another group of factors, such as the absence of an obligation to notify, lack of information or confidentiality, which emerge in this review as being associated with underreporting.
4.1 Adverse Drug Reaction-Related Knowledge and Attitudes
Inman’s knowledge/attitude related to ‘sins’ show associations with underreporting in a high proportion of studies. Thus, ignorance about pharmacovigilance, how to recognize ADRs, which types and how they should be reported, as well as the requirements needed and what has to be done with the submitted report, was the attitude that most conditioned underreporting in our review (52/65), which is consistent with other systematic reviews [ 8 , 79 , 80 ]. Related to ignorance is complacency , which is based on the conviction that adverse reactions of medication are known when the drug comes on to the market and that only well tolerated medications are marketed, and appears to be associated with reporting of a figure very similar to that of the previous revision of 2009. This seems to indicate that there have been no significant advances in the training of health professionals on ADR since ignorance and complacency are potentially modified through training on undergraduate[ 81 , 82 ] and postgraduate pharmacovigilance education [ 81 ]. In this sense, educational sessions (workshops or lectures) [ 83 , 84 , 85 , 86 , 87 ] showed a positive impact on ADR reporting. Nonetheless, the increased rate of SR originated was shown to decrease over time [ 83 , 84 , 88 ]. Consequently, interventions should be carried out periodically and not only reinforce what has been learned but also provide information on possible updates that may have occurred. It could also be interesting to try nudge strategies, which have been shown to be effective in improving the behaviors of health professionals in other fields [ 89 ].
A diffidence attitude results in the lack of confidence and fear of appearing ridiculous (a sentiment of ‘foolish’) either by the diagnosis made or for reporting a suspicious ADR, hence the thought to only report if the health professional is totally sure the ADR was due to a given medicine. This reason can be related to the thought that it is impossible to have certainty and the concern that the report may be wrong ( insecurity ). Once again, we would like to emphasize the importance of education and practical training with constant contact with ADR cases to further improve confidence and certainty.
4.2 Factors Related to Professional Activity
Compared with the 2009 review, a lower proportion of legal aspects have been reported (29/45 vs. 19/65). However, in this review, a new factor related to legal aspects that had not been detected in the previous review has emerged: patient confidentiality , which appears in 9% of the articles. On the other hand, ‘ Financial reimbursement’ is reported in a higher proportion (21% of articles) as a reason for not reporting an ADR compared with the previous review (11%). In fact, interventions based on economic incentives have been shown to be effective [ 90 , 91 , 92 ], which is consistent with the perception that reporting is not the health professionals professional duty, reported in 18% of articles.
4.3 Excuses
The third group encompasses attitudes related to excuses, such as lethargy (procrastination and postponing the notification, lack of time or motivation, effort or interest to report, need for an easier method, the report will generate extra work, and forgetfulness), which is the most frequent reason for not reporting (53/65; 81%). This can be combated by providing health professionals with training (emphasizing the sort time needed to report [ 84 ]), facilitating the notification process, or eliminating the barriers they have to notify ADRs [ 8 ]. Another frequent excuse is the unavailability of a yellow card/ADR form or a shipping address. In this sense, interventions based on the use of information systems (active or passive) and use of an electronic reporting tool has been seen to increase notification. Strategies such as web-based software, hyperlinks, telephone applications, online reporting form, and electronic health records are examples that have emerged with this advance [ 93 , 94 , 95 , 96 , 97 , 98 , 99 ].
4.4 Other Reasons
This was the third most cited reason by healthcare professionals and includes statements related to lack of feedback from the national regulatory authorities on the submitted form, lack of training, and lack of enough information about the patient to make the report. We believe this is understandable, i.e. the lack of feedback can discourage reporting as people expect to receive feedback to know if what they did was correct. Receiving personalized feedback on the reporting procedure has been described as a motive for reporting ADRs [ 21 , 73 , 100 , 101 ]. The lack of information may, for example, be tackled by increasing the consultation time, sharing information from different databases, or even registering a telephone or email contact to clarify doubts at any time.
4.5 Methodology Discussion of the Included Studies
From the methodological point of view and compared with the previous review of 2009, a significant methodological improvement is observed in many aspects, fundamentally in terms of specification of the design (64/65 vs. 3/45 in the 2009 review) and sample size (41/65 articles have more than 200 subjects vs. 25/45 in the 2009 review). The percentage of responses is also higher among the articles included in this review (average of 75% vs. 64% in the 2009 review). Nevertheless, some AXIS factors, especially those linked to non-responders are still not sufficiently described. However sensitivity analysis to assess whether the results of the current review were influenced by the quality of the articles showed that the results for half of the studies that met more AXIS criteria were very similar to the overall review. Finally, the geographical distribution of our review focuses more on Asian and African countries, while the 2009 review focused on European countries. Despite this difference, the findings on the influence of attitudes are similar.
4.6 Limitations
This systematic review has several limitations. First, the great variety of methods used in the articles. The study population, the selected place/setting, the survey distribution, or the scale are very heterogeneous. In two articles, the study was carried out before the implementation of the pharmacovigilance system.
Second, it is common that when the study population includes various groups of health professionals, the results are not shown stratified by health professional type, which prevents us from detecting which factors are associated with each profession and makes comparison between studies difficult.
Third, regarding the reasons cited for underreporting, very few articles conducted the survey using Inman’s reasons as items. The definition of ‘sins’ was not exactly the same in all the articles and therefore the interpretations, both of the authors and those made in this systematic review, may differ from the reasons cited by Inman.
Finally, although an improvement in methodological quality is observed with respect to the 2009 review, great heterogeneity is observed in the fullfilment of the AXIS tool criteria.
5 Conclusion
The results of this review show that, as seen in the 2009 review, low ADR reporting is still associated with a series of attitudes (ignorance, lack of confidence, or complacency) and excuses that are potentially modifiable through training or by facilitating the notification process. This suggests that over the last decade there have been no significant advances in the training of health professionals on ADR reporting and that it is necessary for health systems and undergraduate (academy/training levels, or courses) centers to deepen the training of health professionals on the subject of drug safety. This would provide a greater capacity to activate early warnings to the pharmacovigilance systems and thus allow the health authorities to react more quickly to ensure that the least possible number of patients are affected.
Montané E, Santesmases J. Adverse drug reaction. Med Clin (Bar). 2020;154(5):178–1842.
Article Google Scholar
Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–89. https://doi.org/10.1345/aph.1P627 .
Article PubMed Google Scholar
Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–53. https://doi.org/10.1007/s40264-015-0281-0 .
Article CAS PubMed PubMed Central Google Scholar
Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483–5. https://doi.org/10.1001/jama.284.4.483 .
Article CAS PubMed Google Scholar
Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998. https://doi.org/10.1016/S0149-2918(98)80007-6 .
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions. A systematic review. Drug Saf. 2006;29(5):385–96. https://doi.org/10.2165/00002018-200932010-00002 .
Herdeiro MT, Figueiras A, Polónia J, et al. Physicians’ attitudes and adverse drug reaction reporting. Drug Saf. 2005;28:825–33. https://doi.org/10.2165/00002018-200528090-00007 .
Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12): e011458.
Article PubMed PubMed Central Google Scholar
Inman WHW, Weber JCT. The United Kingdom. In: Inman WHW, editor. Monitoring for drug safety. 2nd ed. Lancaster: MTP Press Ltd; 1986. p. 13–47.
Google Scholar
Inman WHW. Assessment of drug safety problems. In: Gent M, Shigmatsu I, editors. Epidemiological issues in reported drug-induced illnesses. Honolulu: McMaster University Library Press; 1976. p. 17–24.
Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41(5):434–5.
Haines HM, Meyer JC, Summers RS, Godman BB. Knowledge, attitudes and practices of health care professionals towards adverse drug reaction reporting in public sector primary health care facilities in a South African district. Eur J Clin Pharmacol. 2020;76(7):991–1001.
Gidey K, Seifu M, Hailu BY, Asgedom SW, Niriayo YL. Healthcare professionals knowledge, attitude and practice of adverse drug reactions reporting in Ethiopia: a cross-sectional study. BMJ Open. 2020;10(2): e034553.
Nadew SS, Beyene KG, Beza SW. Adverse drug reaction reporting practice and associated factors among medical doctors in government hospitals in Addis Ababa, Ethiopia. PLoS ONE. 2020;15(1):1–19.
Melo JRR, Duarte EC, de Araújo FK, et al. Under-reporting of adverse drug reactions among healthcare professionals in Brazil: an estimate based on national pharmacovigilance survey. J Young Pharm. 2020;12(4):360–5.
Braiki R, Douville F, Hasine AB, Souli I. Facteurs liés au signalement des évènements indésirables associés aux soins dans un hôpital Tunisien. Sante Publique. 2019;31(4):553–9.
Kassa Alemu B, Biru TT. Health care professionals' knowledge, attitude, and practice towards adverse drug reaction reporting and associated factors at selected public hospitals in Northeast Ethiopia: a cross-sectional study. Biomed Res Int. 2019; 8690546.
Le TT, Nguyen TTH, Nguyen C, Tran NH, Tran LA, Nguyen TB, Nguyen N, Nguyen HA. Factors associated with spontaneous adverse drug reaction reporting among healthcare professionals in Vietnam. J Clin Pharm Ther. 2020;45(1):122–7.
Kopciuch D, Zaprutko T, Paczkowska A, Ratajczak P, Zielińska-Tomczak Ł, Kus K, Nowakowska E. Safety of medicines-pharmacists’ knowledge, practice, and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Pharmacoepidemiol Drug Saf. 2019;28(12):1543–51.
Hughes ML, Weiss M. Adverse drug reaction reporting by community pharmacists—the barriers and facilitators. Pharmacoepidemiol Drug Saf. 2019;28(12):1552–9.
Danekhu K, Shrestha S, Aryal S, Shankar PR. Health-care professionals’ knowledge and perception of adverse drug reaction reporting and pharmacovigilance in a tertiary care teaching hospital of Nepal. Hosp Pharm. 2021;56(3):178–86.
Adisa R, Omitogun TI. Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC Health Serv Res. 2019;19(1):926.
Ergün Y, Ergün TB, Toker E, Ünal E, Akben M. Knowledge attitude and practice of Turkish health professionals towards pharmacovigilance in a university hospital. Int Health. 2019;11(3):177–84.
Thompson A, Randall C, Howard J, Barker C, Bowden D, Mooney P, et al. Nonmedical prescriber experiences of training and competence to report adverse drug reactions in the UK. J Clin Pharm Ther. 2019;44(1):78–83.
Li R, Curtain C, Bereznicki L, Zaidi STR. Community pharmacists’ knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018;40(4):878–89.
Seid MA, Kasahun AE, Mante BM, Gebremariam SN. Healthcare professionals’ knowledge, attitude and practice towards adverse drug reaction (ADR) reporting at the health center level in Ethiopia. Int J Clin Pharm. 2018;40(4):895–902.
Bahnassi A, Al-Harbi F. Syrian pharmacovigilance system: a survey of pharmacists’ knowledge, attitudes and practices. East Mediterr Health J. 2018;24(6):569–78.
Lemay J, Alsaleh FM, Al-Buresli L, Al-Mutairi M, Abahussain EA, Bayoud T. Reporting of Adverse Drug Reactions in Primary Care Settings in Kuwait: A Comparative Study of Physicians and Pharmacists. Med Princ Pract. 2018;27(1):30–8.
Terblanche A, Meyer JC, Godman B, Summers RS. Knowledge, attitudes and perspective on adverse drug reaction reporting in a public sector hospital in South Africa: baseline analysis. Hosp Pract (1995). 2017;45(5):238–45.
Hajj A, Hallit S, Ramia E, Salameh P, Order of Pharmacists Scientific Committee – Medication Safety Subcommittee. Medication safety knowledge, attitudes and practices among community pharmacists in Lebanon. Curr Med Res Opin. 2018;34(1):149–56.
Abu Hammour K, El-Dahiyat F, Abu FR. Health care professionals knowledge and perception of pharmacovigilance in a tertiary care teaching hospital in Amman, Jordan. J Eval Clin Pract. 2017;23(3):608–13.
Cheema E, Haseeb A, Khan TM, Sutcliffe P, Singer DR. Barriers to reporting of adverse drugs reactions: a cross sectional study among community pharmacists in United Kingdom. Pharm Pract. 2017;15(3):931.
Gurmesa LT, Dedefo MG. Factors affecting adverse drug reaction reporting of healthcare professionals and their knowledge, attitude, and practice towards ADR reporting in Nekemte Town, West Ethiopia. Biomed Res Int. 2016;2016:5728462.
Almandil NB. Healthcare professionals’ awareness and knowledge of adverse drug reactions and pharmacovigilance. Saudi Med J. 2016;37(12):1359–64.
Stergiopoulos S, Brown CA, Felix T, Grampp G, Getz KA. A survey of adverse event reporting practices among US healthcare professionals. Drug Saf. 2016;39(11):1117–27.
Peymani P, Tabrizi R, Afifi S, Namazi S, Heydari ST, Shirazi MK, et al. Knowledge, attitude and practice of General Practitioners towards adverse drug reaction reporting in South of Iran, Shiraz (Pharmacoepidemiology report). Int J Risk Saf Med. 2016;28(1):25–31.
Amin MN, Khan TM, Dewan SM, Islam MS, Moghal MR, Ming LC. Cross-sectional study exploring barriers to adverse drug reactions reporting in community pharmacy settings in Dhaka, Bangladesh. BMJ Open. 2016;6(8): e010912.
Yu YM, Lee E, Koo BS, Jeong KH, Choi KH, Kang LK, Lee MS, Choi KH, Oh JM, Shin WG. Predictive factors of spontaneous reporting of adverse drug reactions among community pharmacists. PLoS ONE. 2016;11(5): e0155517.
Mendes Marques JI, Polónia JM, Figueiras AG, Costa Santos CM, Herdeiro MT. Nurses’ attitudes and spontaneous adverse drug reaction reporting: a case-control study in Portugal. J Nurs Manag. 2016;24(3):409–16.
Cerruti L, Lebel D, Bussières JF. Perception de la pharmacovigilance par les pharmaciens hospitaliers québécois [Hospital pharmacists’ perception of pharmacovigilance in Quebec]. Ann Pharm Fr. 2016;74(2):137–45.
Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. Afr Health Sci. 2015;15(4):1308–17.
De Angelis A, Giusti A, Colaceci S, Vellone E, Alvaro R. Nurses’ reporting of suspect adverse drug reactions: a mixed-methods study. Ann Ist Super Sanita. 2015;51(4):277–83.
PubMed Google Scholar
Alshammari TM, Alamri KK, Ghawa YA, Alohali NF, Abualkol SA, Aljadhey HS. Knowledge and attitude of health-care professionals in hospitals towards pharmacovigilance in Saudi Arabia. Int J Clin Pharm. 2015;37(6):1104–10.
Nde F, Fah AB, Simo FA, Wouessidjewe D. State of knowledge of Cameroonian drug prescribers on pharmacovigilance. Pan Afr Med J. 2015;20:70.
Liu J, Zhou Z, Yang S, Feng B, Zhao J, Liu H, Huang H, Fang Y. Factors that affect adverse drug reaction reporting among hospital pharmacists in Western China. Int J Clin Pharm. 2015;37(3):457–64.
Tandon VR, Mahajan V, Khajuria V, Gillani Z. Under-reporting of adverse drug reactions: a challenge for pharmacovigilance in India. Indian J Pharmacol. 2015;47(1):65–71.
Sabblah GT, Akweongo P, Darko D, Dodoo AN, Sulley AM. Adverse drug reaction reporting by doctors in a developing country: a case study from Ghana. Ghana Med J. 2014;48(4):189–93.
Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;4(11): e005869.
Afifi S, Maharloui N, Peymani P, Namazi S, Gharaei AG, Jahani P, Lankarani KB. Adverse drug reactions reporting: pharmacists’ knowledge, attitude and practice in Shiraz. Iran Int J Risk Saf Med. 2014;26(3):139–45.
Elkalmi RM, Hassali MA, Ibrahim MI, Jamshed SQ, Al-Lela OQ. Community pharmacists’ attitudes, perceptions, and barriers toward adverse drug reaction reporting in Malaysia: a quantitative insight. J Patient Saf. 2014;10(2):81–7.
Wilbur K. Pharmacovigilance in Qatar: a survey of pharmacists. East Mediterr Health J. 2013;19(11):930–5.
Sanghavi DR, Dhande PP, Pandit VA. Perception of pharmacovigilance among doctors in a tertiary care hospital: influence of an interventional lecture. Int J Risk Saf Med. 2013;25(4):197–204.
Stoynova V, Getov IN, Naseva EK, Lebanova HV, Grigorov EE. Physicians’ knowledge and attitude towards adverse event reporting system and result to intervention-randomized nested trial among Bulgarian physicians. Med Glas (Zenica). 2013;10(2):365–72.
Yip J, Radford DR, Brown D. How do UK dentists deal with adverse drug reaction reporting? Br Dent J. 2013;214(8):E22.
Santosh KC, Tragulpiankit P, Gorsanan S, Edwards IR. Attitudes among healthcare professionals to the reporting of adverse drug reactions in Nepal. BMC Pharmacol Toxicol. 2013;14:16.
Stewart D, MacLure K, Paudyal V, Hughes C, Courtenay M, McLay J. Non-medical prescribers and pharmacovigilance: participation, competence and future needs. Int J Clin Pharm. 2013;35(2):268–74.
Agarwal R, Daher AM, Mohd IN. Knowledge, practices and attitudes towards adverse drug reaction reporting by private practitioners from Klang Valley in Malaysia. Malays J Med Sci. 2013;20(2):52–61.
PubMed PubMed Central Google Scholar
Pimpalkhute SA, Jaiswal KM, Sontakke SD, Bajait CS, Gaikwad A. Evaluation of awareness about pharmacovigilance and adverse drug reaction monitoring in resident doctors of a tertiary care teaching hospital. Indian J Med Sci. 2012;66(3–4):55–61.
Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region). Eur J Clin Pharmacol. 2013;69(2):237–44.
Prakasam A, Nidamanuri A, Kumar S. Knowledge, perception and practice of pharmacovigilance among community pharmacists in South India. Pharm Pract (Internet). 2012;10(4):222–6.
Giofrè C, Scicchitano F, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety in Calabria (Italy): 2012 adverse events analysis. J Pharmacol Pharmacother. 2013;4(Suppl 1):S55-60.
Chopra D, Wardhan N, Rehan HS. Knowledge, attitude and practices associated with adverse drug reaction reporting amongst doctors in a teaching hospital. Int J Risk Saf Med. 2011;23(4):227–32.
Palaian S, Ibrahim MI, Mishra P. Health professionals’ knowledge, attitude and practices towards pharmacovigilance in Nepal. Pharm Pract (Granada). 2011;9(4):228–35.
Oreagba IA, Ogunleye OJ, Olayemi SO. The knowledge, perceptions and practice of pharmacovigilance amongst community pharmacists in Lagos state, South West Nigeria. Pharmacoepidemiol Drug Saf. 2011;20(1):30–5.
Moumtzoglou A. Factors that prevent physicians reporting adverse events. Int J Health Care Qual Assur. 2010;23(1):51–8.
Gavaza P, Brown CM, Khoza S. Texas pharmacists’ opinions on reporting serious adverse drug events to the Food and Drug Administration: a qualitative study. Pharm World Sci. 2010;32(5):651–7.
Su C, Ji H, Su Y. Hospital pharmacists’ knowledge and opinions regarding adverse drug reaction reporting in Northern China. Pharmacoepidemiol Drug Saf. 2010;19(3):217–22.
Ohaju-Obodo JO, Iribhogbe OI. Extent of pharmacovigilance among resident doctors in Edo and Lagos states of Nigeria. Pharmacoepidemiol Drug Saf. 2010;19(2):191–5.
Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14.
Elnour AA, Ahmed AD, Yousif MA, Shehab A. Awareness and reporting of adverse drug reactions among health care professionals in Sudan. Jt Comm J Qual Patient Saf. 2009;35(6):324–9.
Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31(2):183–7.
Ekman E, Bäckström M. Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol. 2009;65(1):43–6.
Okezie EO, Olufunmilayo F. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008;17(5):517–22.
Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–62.
Gossell-Williams M, Adebayo SA. The Pharmwatch Programme: challenges to engaging the community pharmacists in Jamaica. Pharm Pract. 2008;6(4):187–90.
Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30(11):1073–82.
Herdeiro MT, Polonia J, Gestal-Otero JJ, Figueiras A. Factors that influence spontaneous reporting of adverse drug reactions: a model centralized in the medical professional. J Eval Clin Pract. 2004;10(4):483–9. https://doi.org/10.1111/j.1365-2753.2003.00456.x .
Varallo FR, Guimarães SOP, Abjaude SAR, Mastroianni PC. Causes for the underreporting of adverse drug events by health professionals: A systematic review. Rev da Esc Enferm. 2014;48(4):739–47. https://doi.org/10.1590/S0080-623420140000400023 .
Hailu AD, Mohammed SA. Adverse drug reaction reporting in Ethiopia: systematic review. Biomed Res Int. 2020. https://doi.org/10.1155/2020/8569314 .
Herrera CR. Undergraduate and postgraduate pharmacovigilance education: a proposal for appropriate curriculum content. Br J Clin Pharmacol. 2020;86(4):779–90. https://doi.org/10.1111/bcp.14179 .
Tripathi RK, Jalgaonkar SV, Sarkate PV, Rege NN. Implementation of a module to promote competency in adverse drug reaction reporting in undergraduate medical students. Indian J Pharmacol. 2016;48(Suppl 1):S69–73. https://doi.org/10.4103/0253-7613.193314 .
Shchory MP, Goldstein LH, Arcavi L, Shihmanter R, Berkovitch M, Levy A. Increasing adverse drug reaction reporting-how can we do better? PLoS ONE. 2020;15:1–15. https://doi.org/10.1371/journal.pone.0235591 .
Article CAS Google Scholar
Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296(9):1086–93.
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polnia J, Falco A, Figueiras A. Workshop-and telephone-based interventions to improve adverse drug reaction reporting: a cluster-randomized trial in Portugal. Drug Saf. 2012;35(8):655–65. https://doi.org/10.2165/11599750-000000000-00000 .
Shrestha S, Sharma S, Bhasima R, Kunwor P, Adhikari B, Sapkota B. Impact of an educational intervention on pharmacovigilance knowledge and attitudes among health professionals in a Nepal cancer hospital. BMC Med Educ. 2020;20(1):1–10. https://doi.org/10.1186/s12909-020-02084-7 .
Shrestha S, Shrestha R, Abidi A, et al. Workshop on adverse drug reaction reporting, pharmacovigilance and its implementation in cancer hospital in Nepal: an event report. Adv Med Educ Pract. 2020;11:9–14. https://doi.org/10.2147/AMEP.S225208 .
Richards D, Toop L, Graham P. Do clinical practice education groups result in sustained change in GP prescribing? Fam Pr. 2003;20(2):199–206. https://doi.org/10.1093/fampra/20.2.199 .
Yoong SL, Hall A, Stacey F, Grady A, Sutherland R, Wyse R, et al. Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci. 2020;15(1):50. https://doi.org/10.1186/s13012-020-01011-0 .
Feely J, Moriarty S, O’Connor P. Pharmacy-coordinated program that encourages physician reporting of adverse drug reactions. Am J Hosp Pharm. 1990;47(6):1327–33.
Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ (Clinical Res ed). 1990;300(6716):22–3. https://doi.org/10.1136/bmj.300.6716.22 .
Bäckström M, Mjörndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions—a way of dealing with an old problem? Eur J Clin Pharmacol. 2006;62(5):381–5. https://doi.org/10.1007/s00228-005-0072-0 .
Ribeiro-Vaz I, Santos C, Da Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Saf. 2012;35(5):387–94. https://doi.org/10.2165/11597190-000000000-00000 .
Ribeiro-Vaz I, Silva AM, Costa Santos C, Cruz-Correia R. How to promote adverse drug reaction reports using information systems—a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2016;16(1):1–10. https://doi.org/10.1186/s12911-016-0265-8 .
Maguire A, Douglas I, Smeeth L, Thompson M. Secondary use of electronic health record data: spontaneous triggered adverse drug event reporting. Pharmacoepidemiol Drug Saf. 2010;19(12):1211–5. https://doi.org/10.1002/pds.2027 .
Li R, Zaidi STR, Chen T, Castelino R. Effectiveness of interventions to improve adverse drug reaction reporting by healthcare professionals over the last decade: a systematic review. Pharmacoepidemiol Drug Saf. 2020;29(1):1–8. https://doi.org/10.1002/pds.4906 .
Lander AR, Blicher TM, Jimenez-solem E, Jespersen M, Kampmann JP, Christensen HR. Introducing an adverse drug event manager. Eur J Hosp Pharm Sci Pract. 2013;20(2):78–81. https://doi.org/10.1136/ejhpharm-2012-000171 .
Johansson M, Brunlöf G, Edward C, Wallerstedt SM. Effects of e-mails containing ADR information and a current case report on ADR reporting rate and quality of reports. Eur J Clin Pharmacol. 2009;65(5):511–4. https://doi.org/10.1007/s00228-008-0603-6 .
Abadie D, Chebane L, Bert M, Durrieu G, Montastruc J. Online reporting of adverse drug reactions: a study from a French Regional Pharmacovigilance Center. Therapie. 2014;69(5):395–400. https://doi.org/10.2515/therapie/2014035 .
Oosterhuis I, Van Hunsel FPAM, Van Puijenbroek EP. Expectations for feedback in adverse drug reporting by healthcare professionals in the Netherlands. Drug Saf. 2012;35(3):221–32. https://doi.org/10.2165/11594910-000000000-00000 .
Aldryhim AY, Alomair A, Alqhtani M, et al. Factors that facilitate reporting of adverse drug reactions by pharmacists in Saudi Arabia. Expert Opin Drug Saf. 2019;18(8):745–52. https://doi.org/10.1080/14740338.2019.1632287 .
Download references
Acknowledgments
The authors would like to thank Elena Lopez-Gonzalez for her commentaries, and Michael Benedict for reviewing and revising the English.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and affiliations.
Department of Public Health, Faculty of Farmacy, University of Santiago de Compostela, Praza do Seminario de Estudos Galegos, s/n, 15705, Santiago de Compostela, Spain
Patricia García-Abeijon, Margarita Taracido & Adolfo Figueiras
Faculdade de Farmácia da, Universidade de Lisboa, Lisbon, Portugal
Catarina Costa & Carla Torre
Health Research Institute of Santiago de Compostela (IDIS), University of Santiago de Compostela, Santiago de Compostela, Spain
Margarita Taracido & Adolfo Figueiras
Consortium for Biomedical Research in Epidemiology and Public Health (CIBER Epidemiology and Public Health-CIBERESP), Madrid, Spain
Department of Medical Sciences, Institute of Biomedicine-iBiMED, University of Aveiro, Aveiro, Portugal
Maria Teresa Herdeiro
Laboratory of Systems Integration Pharmacology, Clinical and Regulatory Science, Research Institute for Medicines (iMED.ULisboa), Lisbon, Portugal
Carla Torre
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Adolfo Figueiras .
Ethics declarations
This study has been funded in part by the Instituto de Salud Carlos III (ISCIII) through the project PI19/01006, co-financed by FEDER, European Union.
Conflict of interest
Patricia García-Abeijon, Catarina Costa, Margarita Taracido, Maria Teresa Herdeiro, Carla Torre, and Adolfo Figueiras have no conflicts of interest that are directly relevant to the contents of this article.
Ethics approval and consent to participate
Not applicable.
Consent to participate
Consent for publication, availability of data and materials, code availability, author contributions.
Conceptualization: AF, CT, MTH, MT. Article screening: PGA, CC. Writing—original draft: PGA, CC. Writing—review and editing: MT, MTH, CT, AF. Final approval: PGA, CC, MT, MTH, CT, AF. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 251 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
García-Abeijon, P., Costa, C., Taracido, M. et al. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf 46 , 625–636 (2023). https://doi.org/10.1007/s40264-023-01302-7
Download citation
Accepted : 26 March 2023
Published : 06 June 2023
Issue Date : July 2023
DOI : https://doi.org/10.1007/s40264-023-01302-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 18 August 2022
Perceptions, practices and barriers to reporting of adverse drug reactions among HIV infected patients and their doctors in 3 public sector hospitals of the Ethekwini Metropolitan, Kwa-Zulu Natal: a cross sectional and retrospective analysis
- Sindiswa Zondi 1 &
- Panjasaram Naidoo 1
BMC Health Services Research volume 22 , Article number: 1054 ( 2022 ) Cite this article
1299 Accesses
1 Citations
Metrics details
Adverse drug reactions (ADRs) remain a global public health concern. Pharmacovigilance practises are essential in ensuring patients safety and post drug marketing surveillance. This study aimed to describe practices, perceptions and barriers towards ADR reporting practices amongst People Living with HIV/AIDS (PLWHA), who are on Highly Active Anti-Retroviral Therapy (HAART) and their doctors.
The study took place at 3 public sector hospitals. The first phase of the study was a quantitative cross-sectional study using a closed ended questionnaire that was given to PLWHA. Phase two was a retrospective analysis of these patients’ medical files, whilst phase 3 included a descriptive statistics to determine the frequencies and percentages for variables such as ADR reporting practices by doctors .
Spontaneous reporting, was evident with 202 patients (48%) indicating that they reported experiencing ADRs to their doctors. Ten doctors (77%) indicated that they received PV training. Eight (62%) doctors indicated that the completed ADR reporting forms were submitted to the pharmacy manager in the hospital for forwarding to the regulatory authority, with 2 (15%) indicating that they submitted directly to the South African Health Products Regulatory Authority. Four (31%) doctors stated that the system of reporting ADRs is ineffective with the majority of the doctors 12 (92%) responding that the reporting of ADRs is very important/critical. A barrier cited by 4 patients (0.9%) for non-reporting of their ADRs was transport cost. Whilst doctors' barriers included reporting being time consuming (31%), and a lack of availability of reporting forms (31%).
Patients and doctors are reporting ADRs but more education and easier reporting process should be available to strengthen the knowledge and reporting of ADRs. Doctors agree that it is critical to report ADRs. Electronic reporting should be encouraged to lessen the time it takes to report ADRs.
Peer Review reports
The 2020 Joint United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (UNAIDS) report states that there are 37.7 million people living with Human Immunodeficiency Virus (HIV) globally [ 1 ]. The Eastern and Southern African region has the highest number of people living with HIV i.e. 20.7 million [ 1 ]. Furthermore, there are 7.5 million people living with HIV and Acquired Immunodeficiency Syndrome (AIDS) in South Africa [ 2 ]. Despite the efficacy of Highly Active Anti-Retroviral therapy (HAART) to treat HIV disease, like all other medicines, it is also associated with risk and in the case of HAART, findings indicate that it is also associated with Adverse Drug Reactions (ADRs) [ 3 , 4 ]. ADRs are a cause of mortality and morbidity globally [ 5 ]. World Health Organization (WHO) defines ADRs as “any response to a drug which is noxious and unintended, and occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiologic function” [ 5 ].
The detection of rare ADRs is often unachievable in clinical trials and some ADRs only present post marketing, this is due to the presence of co-morbidities, lack of long-term use, concomitant use of medicines, minimal number of patient exposure and diversity in the patient population [ 6 , 7 ]. For this reason, Health Care Professionals (HCPs) and patients ought to report ADRs detected post marketing. WHO defines Pharmacovigilance (PV) as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug related problems” [ 5 ]. One of the challenges experienced by HCP is feedback. This was confirmed in a South African study where a pharmacist was reported to say “you report ADRs in a vacuum, you give it to somebody and you never hear again and it’s nice to get feedback, from whoever is collecting these ADRs to say, look, this is what we’re looking for, this is not what we’re looking for” [ 8 ].
The three main key role players in the South African PV system are HCPs, pharmaceutical industry, and the public [ 9 ]. The South African Health Products Regulatory Authority (SAHPRA) is the medicines regulatory body of South Africa [ 3 ]. SAHPRA has a pharmacovigilance unit that monitors ADRs known as the National Adverse Drug Event Monitoring Centre (NADEMC). NADEMC collates and manages ADRs voluntarily reported by HCPs [ 3 ]. The reported ADRs are then relayed from NADEMC to SAHPRA [ 10 ]. Data collected by the national ADR database in South Africa is further relayed to the Uppsala Monitoring Centre’s VigiBase [ 11 ]. South Africa has collected only 28,609 ADRs which were reported to VigiBase since it joined WHO International Drug Monitoring Programme in 1992 [ 12 ]. This indicates low reporting as it was found that there have been 27 ADR reporting forms per million per year collected [ 13 ]. Some of the barriers to the proper management of data relayed to SAHPRA is underreporting, poor data base compatibility, malfunction of the electronic system, no public relations officers and HCPs not receiving feedback [ 10 , 14 ].
Spontaneous reporting which is also called passive surveillance, targeted spontaneous reporting and cohort event monitoring are PV surveillance methods that have been implemented and are practiced in South Africa [ 15 ]. Spontaneous reporting is defined as “an unsolicited communication by health care professionals or consumers that describes one or more adverse drug reactions in a patient who was given one or more medicinal products and that does not derive from a study or any organized data collection scheme” [ 16 ]. Spontaneous reporting has been identified as an easy and an ideal form of reporting ADRs used by HCPs in many PV systems [ 5 , 9 , 17 ]. The spontaneous reporting of ADRs by HCPs is low and it is not mandatory in most countries [ 18 , 19 ].
Cohort event monitoring is an active surveillance tool that is used to monitor and document ADRs experienced by patients who have been enrolled to investigate the effects of the prescribed medication, furthermore in this method, there is no interference despite the severity of an ADR [ 20 ]. Targeted spontaneous reporting is a novel PV tool that conjoins elements of spontaneous reporting and cohort event monitoring [ 21 ]. There is an identification of a specific group of patients who have been described a specific drug or regimen and the subsequent ADRs are monitored in a cohort setting [ 21 ].
Enhancing drug safety to alleviate patient harm ought to be a shared responsibility of amongst key holders. Drug safety practices should not be limited to new medicines but rather all medicines should be monitored and reported for associated ADRs in order for new knowledge to be constantly generated regarding disease and medicine [ 5 ]. The responsibility of the ongoing collection of ADRs associated with medicines mainly lies with the pharmaceutical industry however post marketing, consumers play an important part. They are most likely to report ADRs to their HCPs compared to communicating them directly to the pharmaceutical industry. Therefore HCPs play a critical role in reporting ADRs to ensure a proper PV system is available and functional [ 22 , 23 ]. The aim of this study was to gauge and describe practices, perceptions and barriers towards the reporting of ADRs by PLWHA on HAART and their doctors.
Methodology
The study was carried out at 3 public sector hospitals situated in the eThekwini Metro area of Kwa-Zulu Natal, South Africa. These hospitals are all similar in size and are categorized as regional hospitals by the South African Health Care System. The hospitals had to have an antiretroviral (ARV) outpatient clinic, attached to it in order to qualify as a research site. They were coded as Facility A, B and C. Facility A and B are in residential areas and can be accessed by residents through walking. Facility C is in a tourist/commercial area that is accessible by means of transportation. All three facilities have qualified nurses who do administration. Nurses also take vitals such as weight and draw blood for biochemical assays. The clinics also have doctors who manage patients and prescribe ARVs. The facilities also have pharmacists who dispense ARVs and counsel patients on the proper use of their medication as well as give information to doctors and other health care providers on medications.
Study design and instrument
The study was divided into three phases. The first phase was the quantitative cross sectional study which entailed the distribution of close ended questionnaires to patients on HAART. The second phase was a retrospective analysis of these patients' medical files. The purpose of the retrospective analysis was to describe the ADRs associated with HAART experienced by PLWHA. Another purpose of the retrospective analysis was to correlate and confirm if the ADRs stated on the questionnaire were really experienced by patients. Therefore the medical files were transcribed to describe experienced ADRs from the period 1991 to 2019. ADRs are unexpected therefore retrospective analysis was appropriate to document past experienced ADRS. Phase 3 of the study included descriptive statistics to determine the frequencies and percentages for variables such as ADR reporting practices by doctors, education of patients by doctors and barriers to reporting. The closed and open ended questionnaires were distributed to doctors who manage these cohort of patients described in phase 1. The cross sectional study took place between the 2 nd of December 2019 and the 31 st of January 2020, whilst the retrospective study analysis dated back from 1991–2019. The questionnaire was developed by looking at questions from similar studies, looking at other questionnaires and mostly from an in depth literature review [ 24 , 25 , 26 , 27 , 28 , 29 ]. The questions were constructed to achieve information on the objectives of the study.
The pretesting of the questionnaire was done by giving the questionnaire to 3 doctors in the facilities where the study was carried out, but these doctors were not involved in the ARV clinic, hence were not part of the sample population. A total of 3 doctors participated in the pilot study and gave their input. With regards to the patient questionnaire post graduate students at the university were invited to participate in the pilot study and give their input. Suggestions from the participants in the pilot study were adopted, therefore the questionnaires were further amended to exclude race as a variable.
Study population
All PLWHA who attended these selected sites and their respective doctors who managed them formed the study population. PLWHA who had been on HAART for more than 6 months and were 18 years and older during the study period were included. All HCPs who managed these patients were included in the study. The study only targeted doctors who managed outpatient HIV/AIDS patients. HIV patients younger than 18 years and those less than 6 months on HAART were excluded.
Sampling techniques and calculation of sample size
The total target population of patients using HAART in the Metro was 383,869 during the time of the study [ 30 ]. Sample size was calculated using single population proportion formula \({\varvec{n}}=\frac{\frac{{{\varvec{Z}}}^{2}{\varvec{P}}(1-{\varvec{P}})}{{{\varvec{d}}}^{2}}}{1+\frac{{{\varvec{Z}}}^{2}{\varvec{P}}(1-{\varvec{P}})}{{{\varvec{N}}{\varvec{d}}}^{2}}}\) , using 95% confidence level, 5% degree of precision, 50% of expected number of patients with the number of ADRs [ 31 ]. The sample size was calculated to be 384, the possible dropout was considered, and therefore 10% was added to make the sample size 423.
The sample size was distributed amongst three ARV facilities hence the sample size for each hospital was 141.
Prior to commencing the study, permission was sought from the hospitals and ethical approval was obtained from the Institution. All the HCPs who prescribe HAART in the three facilities were asked to participate in the study. The ethics approval number being BE053/19.
Data collection procedure
In each study facility, about 400 patients came to the clinic daily. During the study period, patients awaiting consultation with a doctor were approached as a group and briefed on the study to obtain their consent to participate in the study. The informed consent, anonymity, voluntary participation, inclusion criteria were communicated with the patients. Patients who consented to enroll in the study were given a coded closed ended, anonymous questionnaire to fill. The purpose of the coding was to match the questionnaire to the patient’s clinical file which was the second phase of the study. The questionnaire was available in both English and IsiZulu, the latter being the most common language spoken in the eThekwini Metro and South Africa generally [ 32 ]. The questionnaire required information on their demographics, confirmation of ADRs experienced and the reporting of ADRs. The questionnaires were collected immediately after completion. The researcher ascertained that the questionnaires are returned after completed by encouraging the participants to return the questionnaires. This was done by informing the participants that they can continue with the questionnaire even after the consultation with doctor whilst waiting for medicine to be dispensed by the pharmacist, if the completion was of the questionnaire was disturbed by the doctor’s consultation. However it is not all the approached patients who returned the questionnaires. All the data collected was treated confidentially.
The HCPs were approached, the study explained, and consent obtained before the coded questionnaire was given to them. The HCPs were given a week to complete the questionnaires.
Data analysis
All analysis was conducted using SPSS version 25. Continuous and categorical variables such as patients and doctors demographics, years of experience post intern, and the number of ADRs reported were compared using the descriptive analysis to determine frequencies and percentages.
Response rate
Socio-demographic information of patient participants.
Table 1 describes the socio-demographic information of the PLWHA participants. Of the 426 respondents, 296 (69%) respondents were females, 126 (30%) were males and 4 (1%) were transgender respondents. Two hundred and three (52%) patients had secondary school education, whilst 113 (27%) had primary school level of education, 71 (17%) having tertiary education and 12 (3%) patients having no educational background. The respondents ranged from 18 to 69, with the median age of 41 years (IQR 39.5 to 41.7). Two hundred and twenty (52%) patients were unemployed, 126 (30%) were employed with 61 (14%) being patients that were self-employed while 16 (4%) were receiving a pension grant. Three hundred and fifteen (74%) patients travelled by taxi, 49 (12%) walked, 18 (10%) travelled by bus, 37 (9%) used own car and 4 (0.9%) travelled by train. Two hundred and forty four (57%) patients lived in the township, 82 (19%) urban area, 70 (16%) rural area, 25 (6%) semi-urban.

ADRs’ reporting practices amongst patient participants
In facility B, 4 patients indicated that they lacked funds as a barrier to reporting an ADR (Table 2 ). Two of whom matriculated, whilst one had primary school education and 1 had no educational background. Three of these patients were unemployed whilst the one participant who was employed was a matriculant. All four patients resided in a township and had to use a taxi to travel to the facility.
In facility B, non-reporting by 3 patients was due to them consulting a traditional healer. Of these 3 patients, two resided in a township whilst one resided in a rural area.
One patient from Facility B and 2 patients from Facility C did not report an ADR as they found it was not necessary to do so even though one patient from Facility B and 1 from Facility C were informed by their doctor of possible ADRs and to report.
ADR reporting practices amongst patient participants hospitalized due to ADRs
Table 3 describes the reporting practices of the 36 patient participants who were hospitalised due to experiencing an ADR.
Two hospitalized patients that did not report an ADR due to transport costs whilst the other 2 consulted a traditional healer.
Mortality rate was zero for the 36 patients who were hospitalized due to ADRs.
Retrospective analysis of patient participants’ medical files yielded the following results
Three hundred six (72%) of patients experienced an ADR as recorded on their files.
Demographic characteristics of doctor participants
The response rate was 100%. HCPs who responded were 13 in number and were all medical doctors that managed the patient participants.
The demographics of the doctor participants are described in Table 4 .
In Facility C there was no doctor who had more than 10 years’ experience of working with HIV infected patients.
Training of doctor participants on pharmacovigilance
In Facility A, all 6 doctors indicated that they received PV training in their undergraduate studies.
In Facility B, there were 3 doctors who indicated that they received PV training in their undergraduate study, of these 3 doctors, 1 doctor did a postgraduate supplementary course on HIV and PV.
In Facility C, there was 1 doctor who received PV training in the undergraduate years, whilst 2 doctors did postgraduate supplementary training.
Open ended responses by doctor participants on training:
1. Have more workshops educating HCP on PV.
2. Have seminars for intern doctors regarding ADRs and PV.
ADR diagnosis and form submission practice of the doctor participants and education of patients by doctors
In facility A, 2 (33%) doctors did not indicate where they submit completed ADR forms, of these two doctors one indicated that he or she never fills in an ADR form and another indicated to sometimes completing an ADR form (Table 5 ).
The doctor participant in facility A, who does not use laboratory results to diagnose ADRs, had less than 10 years’ experience post intern and working with HIV patients, furthermore the same doctor did not respond to where the ADR form ought to be submitted. The same doctor indicated that it is critical to report an ADR however indicated to never completing an ADR form when patients experience an ADR (Table 5 ).
There were 9 (69%) doctor participants who responded that they always educate patients about ADRs (Table 5 ).
Only 8 (62%) doctors indicated they report an ADR whether it is serious or not serious with in a day. However if it is a new drug, 10 doctors indicated that they would report the ADR within a day or 2 days, however 1 doctor indicated to reporting ADRs associated with new medicines in a month (Table 5 ).
There were 330 (77%) patients who were warned by doctors that they might experience an ADR. There were 306 (72%) patients who experienced an ADR, 202 (47%) of which reported experienced ADRs at their facilities. There were 104 (24%) patients who did not report experienced ADRs despite having 9 (69%) doctors in the 3 facilities indicating that they always educate patients regarding ADRs.
Perceptions towards ADR practices among doctor participants
All doctors stated that electronic spontaneous reporting of ADRs would be more effective than the manual submission of ADRs, and that SAHPRA/Department of Health/NADEMC’ should host more pharmacovigilance workshops as they felt it would improve the practice of pharmacovigilance and reporting;
They also perceived PV procedures as being essential for medicine safety.
There were 6 (46%) doctors who responded that it is critical to carry out spontaneous reporting of ADRs. The 1 doctor who indicated that it was difficult to fill in an ADR reporting form was a 10 to 15 years post intern doctor and had between 5 to 10 years’ experience of working with HIV infected patients. This doctor also did not have PV supplementary training.
Barriers to reporting ADRs and doctor participants
Doctors were asked to indicate barriers that limit their ADR reporting practices. Table 8 describes the barriers as stated by the doctor participants in the different facilities.
The 2 doctors who did not find time as a barrier had over 21 years’ experience as doctors. The doctor in facility A, who indicated to being afraid of taking responsibility, did not respond as to whether the ADR form is completed or not. The doctor in facility C who also responded to being afraid of taking responsibility in reporting ADRs also responded that the current PV system is inefficient.
This study gave a fair overview on the reporting practices of both PLWHA and their doctors. In addition, it also gave some information on doctor’s perceptions and barriers experienced by doctors in the reporting of ADRs.
It is evident that even though patients had been informed by their doctors to report an ADR, they did not do so (Table 6 ) (77% were warned that they might experience an ADR versus 47% reported ADRs at their facilities). They cited various reasons such as lack of funds to go back to the clinic, or used alternative healers such as traditional healers to manage their ADR or did not find it necessary to do so (Table 2 ). These findings were similar to a study that was conducted in Nigeria whereby 89 (24.7%) patient participants experienced at least one ADR and 38 (39%) reported experienced ADRs to HCPs [ 33 ]. In a study that was conducted in Malaysia there were 240 (72%) patient participants that were informed by doctors of the possibility of experiencing ADRs, 233 (66.8%) reported experienced ADRs to the doctors [ 34 ]. Furthermore, in this study, where no self-reporting of an experienced ADR was stated, it was found that ADRs were recorded on the patients' files. This could possibly be due to the patients not being able to differentiate between an ADR and an Adverse Drug Effect (ADE) or the seriousness of the former. Poor knowledge could have led to this behaviour.
Reasons for some patients not reporting ADRs in our study, included patients in facility B and C, who indicated that they did not find it necessary to report an ADR at their facilities, this might be due to these patients not deeming the reporting of ADRs as important and might have concluded that the experienced ADRs as non-serious. Another reason cited by patients for not reporting was the management of ADRs by traditional healers. The consultation of traditional healers to treat ADRs that were caused by western medication is a concern and it can be assumed that these patients sought treatment from a traditional healer because these traditional healers were easily accessible and lived nearby to these patients. Of these 3 patients, 2 were eventually hospitalized. In addition, 8% of the patients in our study were hospitalized due to ADRs (Table 3 ). The timeous reporting of ADRs could lead to ADRs’ preventability and reduction in the financial burden and hospital costs associated with ADRs. As cited in studies, 1 in 10 adult admissions are due to ADRs [ 35 ]. South Africa in multi-ethnic base country and the use of traditional medicines is common, furthermore an Ethekwini based study showed that its participants used HAART concurrently with traditional medicines [ 36 ]. A study conducted by Abdel-Latif and Abdel-Wahab indicated that in a multi-ethnic country it is essential for HCPs to monitor and report ADRs [ 37 ]. The lack of funds cited by some participants who experienced an ADR but did not go to the clinic for management poses a serious threat to the achievement of positive health outcomes. All patients who indicated, that they did not have money to go to the clinic, were from low income areas, with 75% of them being unemployed (Table 1 ). However of concern is that the location of these hospitals are not remotely situated, are within residential suburbs and can be accessed by foot. It can therefore be assumed that these patients preferred to use transportation to go to the clinic rather than to walk to the clinic if funding was an issue. This however leads to further detriment of their health and increased health costs as ADR is a “response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or for the modification of physiologic function” and needs urgent attention [ 38 ].
Further concern noted in this study was that a third of the patients stated that they reported ADRs after a month with almost 50% of the hospitalized patients indicating that they reported their ADRs after a month. Fortunately there were no deaths from the ADR experienced. This begs the question as to whether patients were informed that they should report an ADR immediately at the clinic. This late reporting is not acceptable as a serious ADR could have led to death. This study further found that the incidence of ADR was high 306 (72%). A finding similar to a study that was conducted by Hagos et al ., where by 62.8% patients experienced at least one ADR [ 39 ].
Therefore educating patients about ADRs and the importance of reporting immediately and its subsequent management is vital. In addition, it is essential that patients are informed in layman’s terms for better understanding. Knowing the difference between an ADR and an ADE should form part of the education programme for patients. In order to confirm that patients understood what information was given to them, is to ask patients to repeat the information given to them. One of the doctor participant suggested that there ought to be constant PV workshops and in these workshops it should be highlighted that it is crucial for doctors to continuously educate patients about ADRs and also to educate them about the importance of immediate reporting of ADRs.
With regards to doctors’ training, the doctor participants in this study either received PV training during their undergraduate or underwent PV training through supplementary courses, thus the doctors in this study at a minimum had basic PV knowledge to carry out ADR reporting. Maigetter et al., reported that HCPs ought to receive PV training in order to encourage them to participate in PV activities such as the reporting of ADRs [ 10 ]. Even though facility C had more patients and fewer doctors, the doctors were all proactive in educating patients regarding ADRs (Table 6 ). Facility A had more doctors compared to other facilities and it is concerning that only one doctor responded to sometimes educating patients regarding ADRs. Normally shortage of human resources does not allow for much education of patients, but surprisingly in this facility there were more doctors, education of patients was done sometimes.
The practice of these doctor participants was evidenced by ten doctors of the 13 reporting serious and non-serious ADRs within a day or 2 days which is optimum practice however it was concerning that one doctor in facility C indicated that an ADR from a newly registered medicine would be reported within a month (Table 5 ). In terms of post marketing surveillance, it is important that all ADRs or undocumented ADEs should be reported immediately to the pharmacovigilance unit and/or the manufacturer. This could be once again related to shortage of staff as facility C had fewer doctors managing more patients than facility A and facility B. Half of the doctors in facility A indicated that they never report an ADR which is concerning as the SAHPRA ADR reporting guideline states that HCPs are encouraged to report ADRs even in cases whereby HCPs are uncertain regarding whether a drug caused an ADR or not [ 40 ]. The reporting of ADRs associated with new medicines should be done immediately to SAHPRA as this is a safety issue and depending on the severity of an ADR, it ought to be removed from the market. Information about newly registered medicines’ safety and effectiveness is made available after clinical trials [ 40 ]. PV is therefore an important tool in the post marketing surveillance and it is critical after clinical trials as post marketing populations have different genetic predispositions and pharmacokinetics and have comorbidities, use traditional medicines which affects the drugs thereby generating undocumented ADRs [ 9 , 41 ].
A study conducted by Boguluva et al ., indicated that 109 (46.8%) of HCPs did not know where to submit the ADR form. This was similar to the findings in this study whereby 23% doctors (2 in facility A and 1 in facility B) did not indicate where the form ought to be submitted, this might due to doctors not knowing where the ADR form ought to be submitted or not being proactive in PV practices [ 13 ]. This is concerning as facility B had more patients who experienced ADRs than the other facilities, therefore all doctors in facility B should have been more proactive in reporting ADRs. In spite of these shortcomings the majority of doctors (77%) in this study were compliant with the reporting of ADRs by either submitting the form to the pharmacy manager or directly to SAHPRA (Table 5 ). The submission of the ADR forms to the pharmacy manager is due to following the hospital protocol for ADR submissions.
One of the open ended responses received stated “I am more interested in the feedback from the data collected during spontaneous reporting” this statement is similar to the findings from a study conducted by Joubert and Naidoo, where all participants indicated that they would like to receive more communication between them and the PV centres [ 42 ]. Studies have shown that HCPs not receiving feedback from PV centres discourages HCPs from completing reporting ADRs in the future [ 43 , 44 ]. As stated in the South African National Development Plan for HIV, TB and STIs 2017–2022, that there ought to the transparency amongst stakeholders and that all stakeholders ought to have access amongst shared data such as data on community response [ 45 ].
In this study doctors perceived the reporting of ADR to be either important, very important or very critical practice. This coincides with a South African study which showed that 177 (76%) of the participants indicated that it was very important to report ADRs [ 13 ]. A study conducted by Adisa and Omitogun showed that 76 (95%) of their HCPs indicated that the reporting of ADRs as being important [ 33 ].
With respect to the PV system, 31% of doctor participants indicated that the system of reporting ADRs is ineffective (Table 7 ) which was similar to the findings of Joubert and Naidoo where HCPs who participated in the study were lowly satisfied with the PV systems in South Africa. [ 42 ]. Some of the barriers cited that affected optimum PV practices in this study included the unavailability of forms and that it was time consuming to report an ADR expressed by almost 50% of the doctor participants (Table 8 ). In contrast to another study by Khan 2013, which stated that 32% of HCPs agreed to the reporting of ADRs being time consuming with 18% of HCPs strongly agreeing on the unavailability of the ADR reporting forms [ 46 ].
All the doctors who participated in this study, indicated, that an electronic reporting of ADRs would be more ideal for the South African PV system to be efficient, the electronic reporting of ADRs would therefore lessen the time it takes to complete a hard copy ADR form and walking to submitting it to the pharmacy manager. It is evident that most African countries utilize the manual system of reporting ADRs [ 47 ]. SAHPRA has implemented an electronic reporting of ADRs through an eReporting link which is available at the SAHPRA website [ 40 ]. Furthermore SAHPRA has also implemented a MedSafety App which can be downloadable to a cellphone and can be accessed by both HCPs and the public to report ADRs associated with medicines [ 48 ]. This therefore suggests that doctors were not knowledgeable with the current electronic reporting tools during the study period as the communications were made in March 2020 and again in April 2021. Even though eReporting has been implemented in South Africa, doctors in Facilities A and B, who indicated they do not have Wi-Fi access may not be able to carry out electronic reporting which will once again pose a barrier to effective and timeous reporting of ADR.
South Africa is a third world country with limited recourses and a burden of HIV/AIDS epidemic. South Africa has one of the largest HAART programmes globally [ 2 ]. Continuous research on ADRs associated with HAART is crucial, as ADRs are 'an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product’ [ 49 ]. This makes ADRs a global challenge as they are unpredictable and idiosyncratic and ought to be quantified. This study provides knowledge on the current barriers, perceptions in ADR reporting. Such research is a useful tool in SAHPRA and the Department of Health in order to strategize effective measures to ensure timeous and on-going reporting of ADRs. Reporting of ADRs in pharmaceutical industries is important in order to alleviate risks associated with medicines. However the study carried limitations such as loss of patient files in facilities which posed a barrier in recording experienced ADRs, other limitations included a small sample size of the doctors as the study only targeted doctors who managed outpatient HIV/AIDS patients. The population size of doctors managing outpatient HIV/AIDS patients is smaller compared to the doctors in the main regional hospitals to which the ARV clinics are attached. The non-response bias was among other limitations in this study. It can be assumed that those who did not respond may have had poor knowledge on the objectives of this study. It can also be assumed that when variable under investigation is one that comprises health related variables or is socially undesirable. Some patients did not respond to questions such as whether they experienced diarrhea and depression, as these conditions are socially undesirable. People with risk behaviour are less likely to respond on the questionnaire. The non-response bias affected the study’s generalizability therefore due to the small sample size, the study cannot be generalizable to all PLWHA and doctors who manage PLWHA.
Conclusion and recommendations
ADRs are associated with HAART, with patients experiencing them at different levels of severity and patienst are aware of ADRs and are report them to doctors; however some patients were still not clear about what to report, ADEs have been incorrectly reported as ADRs in some instances. Doctors have been trained in PV and they report ADRs and the reporting is perceived as being very important to critical. However policies in the hospitals determine where an ADR report should go to, pharmacy managers first before being submitted to SAHPRA. Patients' barriers have also been noted such as lack of travel funds to go to the clinic, whilst the lack of education by doctors resulted in some patients seeking traditional healers to manage their ADR. The doctor barriers that appeared to prevent optimum reporting practices were it being time consuming and more importantly lack of feedback which does not augur well for an efficient PV system. Electronic reporting should be pursued earnestly in order to overcome these barriers.
Availability of data and materials
All the data that was generated or analyzed during this study is included in this published article.
Abbreviations
Acquired Immunodeficiency Syndrome
- Adverse Drug Reactions
Adverse Drug Effects
Health Care Professionals
Highly Active Anti-Retroviral therapy
Human Immunodeficiency Virus
National Adverse Drug Event Monitoring Centre
People living with HIV/AIDS
- Pharmacovigilance
South African Health Products Regulatory Authority
World Health Organization
Global report: UNAIDS report on the global AIDS epidemic 2020. Geneva: UNAIDS; 2020.
Avert. Global information and education on HIV and AIDS. HIV and AIDS in South Africa. 2020.
Google Scholar
Terblanche A. Pharmacovigilance and the reporting of adverse drug reactions. S Afr Pharm J. 2018;85(6):65–8.
Isaac OA, Ncube NBQ, Bradley HA, Agbaji OO, Kanki P. Antiretroviral Therapy-associated Adverse Drug Reactions and their Effects on Virologic Failure- A Retrospective Cohort Study in Nigeria. Curr HIV Res. 2018;16:436–46. https://doi.org/10.2174/1389450120666190214144609 .
Article CAS Google Scholar
World Health Organization. The Importance of Pharmacovigilance: Safety Monitoring of Medicinal Products. 2002. Available from: http://apps.who.int/iris/bitstream/handle/10665/67378/WHO_EDM_QSM_2002.2.pdf;jsessionid=3FC18F13D20E2ABBC97001C97E386FC5?sequence= .
World Health Organization. Pharmacovigilance: Ensuring the Safe Use of Medicines — WHO Policy Perspectives on Medicines. 2004. Available at http://whqlibdoc.who.int/hq/2004/WHO_EDM_2004.8.pdf .
Smith MI, Wertheimer AI, Fincham JE. Pharmacy and the US health care system. London: Pharmaceutical Press; 2013;(4):299-302 ISBN 9780857110220.
Ruud KW, Srinivas SC, Toverud EL. Addressing gaps in PV practices in the antiretroviral therapy program in the Eastern Cape Province, South Africa. Res Social Adm Pharm. 2010;6(4):345–53.
Article Google Scholar
Mehta U, Kalk E, Boulle A, Nkambule P, Gouws J, Rees H. Cohen K. Pharmacovigilance: A public health priority for South Africa. S Afr Health Rev; 2017. p. 125–33.
Maigetter K, Pollock AM, Kadam A, Ward K, Weiss MG. Pharmacovigilance in India, Uganda and South Africa with reference to WHO’s minimum requirements. Int J Health Policy Manag. 2015;4(5):295–305. https://doi.org/10.15171/ijhpm.2015.55 Published 2015 Mar 9.
Mehta U. Pharmacovigilance: the devastating consequences of not thinking about adverse drug reactions. Continuing Medical Education. 2011;29(6):247–51.
Ampadu HH, Hoekman J, de Bruin ML, Pal SN, Olsson S, Sartori D, Leufkens HG, Dodoo AN. Adverse Drug Reaction Reporting in Africa and a Comparison of Individual Case Safety Report Characteristics Between Africa and the Rest of the World: Analyses of Spontaneous Reports in VigiBase®. Drug Saf. 2016;39(4):335–45. https://doi.org/10.1007/s40264-015-0387-4 .
Article CAS PubMed PubMed Central Google Scholar
Bogolubova S, Padayachee N, Schellack N. Knowledge, attitudes and practices of nurses and pharmacists towards adverse drug reaction reporting in the South African private hospital sector. Health SA Gesondheid. 2018;23:a1064. https://doi.org/10.4102/hsag.v23i0.1064 .
Lalvani P, Milstein J. Access to new health products in low income countries and the challenges of pharmacovigilance. Chris Duncombe, Priorities and Initiatives to advance pharmacovigilance in HIV program: presentation at WHO/GF stakeholder’s meeting. Accra: Empowers School of Health; 2010.
Mehta U, Dheda M, Steel G, Blockman M, Ntilivamunda A, Maartens G, Pillay Y, Cohen K. Strengthening pharmacovigilance in South Africa. SAMJ. 2014;104(2):104–6. https://doi.org/10.7196/samj .
Article CAS PubMed Google Scholar
Food and Drug Administration. Post-approval safety data management: definitions and standards for expedited reporting E2D. European Union International Conference on Harmonisation. USA: ICH; 2003.
Hartigan-Go K. Pharmacovigilance and the pursuit of rational drug use: The Philippines experience. Toxicology. 2003;181–182(1–3):103–7.
British Medical Association Board of Science: Reporting adverse drug reactions—a guide for healthcare professionals May 2006. http://www.isoponline.org/wpcontent/uploads/2015/01/BMAreport.pdf .
Balidemaj F. Adverse drug reactions: problems with spontaneous reporting systems and communicating information to providers to improve reporting rate globally. Eur J Public Health. 2017:27(3). https://doi.org/10.1093/eurpub/ckx186.064 .
Layton D, Shakir SAW. Specialist Cohort Event Monitoring Studies: A New Study Method for Risk Management in Pharmacovigilance. Drug Safety. 2015;38(2):153–63. https://doi.org/10.1007/s40264-014-0260-x .
Rachlis B, Karwa R, Chema C, Pastakia S, Olsson S, Wools-Kaloustian K, Jakait B, Maina M, Yotebieng M, Kumarasamy N, Freeman A, de Redeneire N, Duda SN, Davies M, Braitstein P. Targeted Spontaneous Reporting: Assessing Opportunities to Conduct Routine Pharmacovigilance for Antiretroviral Treatment on an International Scale. Drug Saf. 2016;39(10):959–76. https://doi.org/10.1007/s40264-016-0434-9 .
Li R, Curtain C, Bereznicki L, Zaidi STR. Community pharmacists’ knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018;40:878–89. https://doi.org/10.1007/s11096-018-0700-2 .
Article PubMed PubMed Central Google Scholar
Australian Government Department of Health and Aging. Australian requirements and recommendations for pharmacovigilance responsibilities of sponsors of medicines version 1.3. Canberra: Therapeutic Goods Administration; 2014.
Lohit K, Vidya R, Manjunath N. Development and Validation of Questionnaire to Assess The Knowledge, Attitude and Practice towards Adverse Drug Reactions Reporting among Healthcare Professionals. J Int Med Dent. 2016;3:63–72. https://doi.org/10.18320/JIMD/201603.0263 .
Motheral B, Brooks J, Clark M, Crown WH, Davey P, Hutchins D, Martin BC, Stang P. A Checklist for Retrospective Database Studies—Report of the ISPOR Task Force on Retrospective Databases. Value Health. 2003;6:3.
Upadhyaya HB, Vora MB, Nagar JG, Patel PB. Knowledge, attitude and practices toward pharmacovigilance and adverse drug reactions in postgraduate students of Tertiary Care Hospital in Gujarat. J Adv Pharm Technol Res. 2015;6(1):29–34. https://doi.org/10.4103/2231-4040.150369 .
Gupta SK, Nayak RP, Shivaranjani R, Vidyarthi SK. A questionnaire study on the knowledge, attitude, and the practice of pharmacovigilance among the healthcare professionals in a teaching hospital in South India. Perspect Clin Res. 2015;6(1):45–52. https://doi.org/10.4103/2229-3485.148816 PMID: 25657902; PMCID: PMC4314847.
Hardeep, Bajaj JK, Rakesh K. A survey on the knowledge, attitude and the practice of pharmacovigilance among the health care professionals in a teaching hospital in northern India. J Clin Diagn Res. 2013;7(1):97–9. https://doi.org/10.7860/JCDR/2012/4883.2680
Ganesan S, Sandhiya S, Reddy KC, Subrahmanyam DK, Adithan C. The Impact of the Educational Intervention on Knowledge, Attitude, and Practice of Pharmacovigilance toward Adverse Drug Reactions Reporting among Health-care Professionals in a Tertiary Care Hospital in South India. J Nat Sci Biol Med. 2017;8(2):203–9. https://doi.org/10.4103/0976-9668.210014 PMID: 28781488; PMCID: PMC5523529.
Gumede CZ. EThekwini District AIDS Council Quarter 1, 2017/2018 report. Durban: Ethekwini Municipality; http://www.kznonline.gov.za/hivaids/councils/Provincial-Councils-on-AIDS/2017/September/eThekwini%20presentation.pdf . Accessed 6 Mar 2019.
Cochran WG. Sampling techniques. Chichester: Wiley; 2007.
StatsSA. 2021. https://www.statssa.gov.za/census/census_2011/census_products/Census_2011_Census_in_brief.pdf . Accessed 28 July 2021.
Adisa R, Omitogun TI. Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, south western Nigeria. BMC Health Serv Res. 2019;19:926. https://doi.org/10.1186/s12913-019-4775-9 .
Elkalmi R, Hassali MA, Al-Lela OQ, Jawad Awadh AI, Al-Shami AK, Jamshed SQ. Adverse drug reactions reporting: Knowledge and opinion of general public in Penang. Malaysia J Pharm Bioallied Sci. 2013;5(3):224–8. https://doi.org/10.4103/0975-7406.116824 .
Article PubMed Google Scholar
Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73(6):759–70. https://doi.org/10.1007/s00228-017-2225-3 .
Babb DA, Pemba L, Seatlanyane P, Charalambous S, Churchyard GJ, Alison D. Grant Use of traditional medicine by HIV-infected individuals in South Africa in the era of antiretroviral therapy. Psychol Health Med. 2007;12(3):314–20. https://doi.org/10.1080/13548500600621511 .
Abdel-Latif MMM, Abdel-Wahab BA. Knowledge and awareness of adverse drug reactions and pharmacovigilance practices among healthcare professionals in Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia. SPJ. 2015;23(2):154–61. https://doi.org/10.1016/j.jsps.2014.07.005 .
Nebeker J, Barach P, Samore M. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801. https://doi.org/10.7326/0003-4819-140-10-200405180-00009 .
Hagos L, Fessehaye S, Anand IS. Nature and prevalence of adverse drug reaction of antiretroviral medications in Halibet National Referral Hospital: a retrospective study. BMC Pharmacol Toxicol. 2019;20:24. https://doi.org/10.1186/s40360-019-0307-9 .
South African Health Products Regulatory Authority. Communication to Health Care professionals. Guideline for Adverse Drug Reactions Reporting for Health Care Professionals. 2020.
Agrawal P. Drug Discovery and Development: An Insight into Pharmacovigilance. J Pharmacovigilance. 2014;2(3):e120. https://doi.org/10.4172/2329-6887.1000e120 .
Joubert MC, Naidoo P. Knowledge, perceptions and practices of pharmacovigilance amongst community and hospital pharmacists in a selected district of North West Province South Africa. Health SA Gesondheid. 2016;21:238e244. https://doi.org/10.1016/j.hsag.2016.04.005 .
Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol. 2013;75:1109–17.
Avery A, Anderson C, Bond C, Fortnum H, Gifford A, Hannaford P, et al. Evaluation of patient reporting of adverse drug reactions to the UK ’Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 2011;15:1–234.
National Development Agency. South Africa national development plan 2017–2022. Parktown: National Development Agency; 2017. ISBN: 978-0-621-45121-4.
Khan TM. 0Community pharmacists’ knowledge and perceptions about adverse drug reactions and barriers towards their reporting in Eastern region, Alahsa. Saudi Arabia Ther Adv Drug Saf. 2013;4(2):45–51. https://doi.org/10.1177/2042098612474292 .
Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;4(11):e005869. https://doi.org/10.1136/bmjopen-2014-005869 .
South African Health Products Regulatory Authority. SAHPRA Launches the Med Safety App for self-reporting of suspected adverse drug reactions by the public and healthcare professionals. 2021.
Edwards R, Aronson J. Adverse drug reactions: definitions, diagnosis and management. The lancet. 2000;356:1255–9.
Download references
Acknowledgements
The authors would like to thank all the patients and health care workers who participated in this study. The authors would like to thank Dr. Zelalem Dessie (statistician) who guided with statistical analysis and interpretation.
The authors would like to the thank the Department of Pharmaceutical Sciences under the College of Health Sciences at the University of Kwa-Zulu Natal for covering the cost of research instruments and the travelling allowance.
Author information
Authors and affiliations.
Discipline of Pharmaceutical Sciences, School of Health Sciences, University of Kwa-Zulu Natal, P.O. Box X5401, Durban, 4000, South Africa
Sindiswa Zondi & Panjasaram Naidoo
You can also search for this author in PubMed Google Scholar
Contributions
SZ and PN formulated the study design. SZ collected data, conducted statistical analysis and wrote the manuscript. PN read, amended and approved the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Sindiswa Zondi .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from the University of Kwa-Zulu Natal, Biomedical Research Ethics Committee with approval number being BE053/19. All methods were carried out in accordance the Biomedical Research Ethics Committee. Gatekeeper’s permissions were obtained from the 3 study sites and the South African Department of Health. After the study was explained to possible participants, the consent from the participants was only obtained only after the participants had competed and signed the informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zondi, S., Naidoo, P. Perceptions, practices and barriers to reporting of adverse drug reactions among HIV infected patients and their doctors in 3 public sector hospitals of the Ethekwini Metropolitan, Kwa-Zulu Natal: a cross sectional and retrospective analysis. BMC Health Serv Res 22 , 1054 (2022). https://doi.org/10.1186/s12913-022-08395-3
Download citation
Received : 05 February 2022
Accepted : 29 July 2022
Published : 18 August 2022
DOI : https://doi.org/10.1186/s12913-022-08395-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Perceptions
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Reporting of adverse drug reactions in India: A review of the current scenario, obstacles and possible solutions
Affiliations.
- 1 Indian Institute of Public Health, Gurgaon, India.
- 2 Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- PMID: 30175985
- DOI: 10.3233/JRS-180025
Pharmacovigilance is a practice aimed to monitor drug safety in real life conditions and capture adverse drug events during the post marketing phase of drug's life cycle. But under reporting of adverse reactions is a major cause of concern and a threat to the pharmacovigilance systems. The present article looks into the major obstacles affecting the spontaneous reporting of adverse drug reactions (ADRs) in India and the possible solutions. As per available scientific literature, the major impediments to ADR reporting are inadequate knowledge and awareness among health professionals, clinicians' perceptions towards reporting, problems with establishing reporting systems in hospitals and insufficient training to recognize ADRs. Measures to improve the situation include greater involvement of nurses, pharmacists as well as consumers in the reporting of ADRs, making the process simpler and faster through electronic means, introducing educational interventions and training programs for health care providers and spreading awareness about the reporting system amongst caregivers and receivers alike. Providing a momentum to the pharmacovigilance system and ensuring a robust reporting process is a challenge but proper planning, feasible solutions and focussed efforts can help bring about the change ensuring patient safety - the ultimate goal of pharmacovigilance.
Keywords: Adverse drug reactions; spontaneous reporting; underreporting.
Publication types
- Adverse Drug Reaction Reporting Systems / statistics & numerical data*
- Attitude of Health Personnel
- Drug-Related Side Effects and Adverse Reactions*
- Health Knowledge, Attitudes, Practice
- Health Personnel / psychology*
- Mandatory Reporting*
- Middle Aged
- Pharmacists / psychology*
- Pharmacovigilance*
- Risk Management / statistics & numerical data*
- Surveys and Questionnaires

- News and Community
Reporting adverse events
Everyone can play an important role in monitoring the safety of therapeutic goods in Australia
If you think you may be experiencing a side effect after using a medicine or vaccine or a problem involving the use of a medical device, seek advice from a health professional as soon as possible.
Overview of adverse events
Adverse events are unintended and sometimes harmful occurrences associated with the use of a medicine, vaccine or medical device (collectively known as therapeutic goods). Adverse events include side effects to medicines and vaccines, and problems or incidents involving medical devices.
Examples of adverse events are any unfavourable and unintended sign, symptom or disease associated with the use of a therapeutic good. An abnormal laboratory finding could be one example of an unfavourable and intended sign.
In the case of medical devices, an adverse event can also be a problem or incident that has caused, or could cause, harm to patients, caregivers, health professionals or others. These can include 'near misses' – events that might have led to a death or serious injury. It may be that timely intervention from a health professional prevented an adverse event.
Importantly, an adverse event is not always caused by the therapeutic good itself. An adverse event could be a result of incorrect user interaction or other circumstances such as two properly functioning devices that do not operate as intended when used in combination. The occurrence of an adverse event does not necessarily mean that there is something wrong with the therapeutic good.
Everyone can play an important role in monitoring the safety of therapeutic goods in Australia by reporting suspected adverse events to the TGA .
Frequently asked questions
Why report adverse events to the TGA?
When a therapeutic good is first registered and made available in Australia, information about its safety and effectiveness is usually only available from clinical trials.
Clinical trials provide information about many of the possible adverse events associated with a therapeutic good, but do not detect all possible adverse events because they:
- usually do not continue for long enough to detect adverse events that take a long time to develop
- do not include enough patients to detect adverse events that occur rarely
- do not include all of the different types of people who might eventually use the product and who might be more susceptible to some adverse events, such as older people, children, pregnant women or people with other medical conditions.
The TGA, like other regulatory agencies around the world, monitors the safety of therapeutic goods to contribute to a better understanding of their possible adverse events when they are used outside the controlled conditions of clinical trials.
Reports by consumers and health professionals provide important information for the TGA's safety monitoring program.
Who can report an adverse event?
Anyone can.
Information about the number of adverse event reports received each year by the TGA can be found at Adverse events: Australian statistics on medicines and Adverse events: Australian statistics on medical devices .
Most adverse event reports are made by sponsors (e.g. pharmaceutical companies and medical device suppliers), but many are also made by state and territory health departments, hospitals, health professionals and consumers.
If you have any concerns about an adverse event it is important to also speak to a health professional.
Reporting medicine or vaccine adverse events
Consumers: report a side effect of a medicine or vaccine using the online form.
Health professionals:
- report an adverse event of a medicine or vaccine
- report via email, fax or mail using the National Adverse Events Following Immunisation (AEFI) reporting form
All medicines and vaccines can cause side effects or other adverse events.
Medicines include:
- prescription medicines
- over-the-counter medicines that are purchased without a prescription
- herbal medicines
- naturopathic or homeopathic preparations
- nutritional supplements, like vitamins and minerals.
For further information on the features and functionality of the online adverse event reporting forms, users should refer to the Adverse Event Management System (AEMS) Guidance for
- Health Professionals , and
Reporting medical device adverse events
Medical device consumers: report problems or incidents through the consumer online Medical Device Incident Report form .
Health professionals: report problems or incidents online through the health professional online Medical Device Incident Report form .
Sponsors and manufacturers: report using the TGA's online reporting system .
The TGA's medical device Incident Reporting and Investigation Scheme (IRIS) is responsible for the management of all reports of adverse events or problems associated with medical devices.
Medical devices range from a bandage that you would put on a scratch to high risk products such as pacemakers that are implanted in your body. Other examples of medical devices include:
- artificial hips
- blood pressure monitors
- breast implants
- lubricating eyedrops
- MRI scanners
- orthodontics, such as braces and fillings
- tongue depressors.
Typical problems with medical devices include:
- deficiencies in labelling, instructions or packaging
- defective components
- performance failures
- poor construction or design.
Which events should I report?
You don't need to be certain, just suspicious!
Every report counts. While an individual report may not be enough to determine whether a particular therapeutic good caused an adverse event. All reports help to build a picture of the safety profile of a product and assist with the TGA's safety monitoring program.
The work of the TGA is based on applying scientific and clinical expertise to decision making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines, medical devices and other therapeutic goods.
The TGA particularly needs to know:
- all suspected adverse events to new therapeutic goods
- all suspected medicine and/or vaccine interactions
- unexpected adverse events (that is, adverse events that do not appear in the Product Information , Consumer Medicine Information and/or product labelling)
- danger to life
- admission to hospital
- prolongation of hospitalisation
- absence from productive activity
- increased investigational or treatment costs
- birth defects.
What information do I need to report?
Reporters are encouraged to provide as much detail as possible, but at bare minimum are asked to provide:
- contact details for the reporter (name, address, phone number)
- patient identifier (such as initials, date of birth or age, but not their full name)
- details of the product involved
- details of the suspected adverse event.
By providing all information relevant to a specific adverse event, you can help TGA staff to assess the possible role of the product in causing the adverse event.
The TGA asks for contact details from people making reports so that it can seek further information about suspected adverse events - we cannot accept anonymous reports.
Providing as much information as possible will reduce the need for the TGA to follow up. However, it is important not to delay reporting an adverse event if some information is not available. If we need more information, we will contact you.
Any information identifying the reporter or patient is kept confidential. See the 'Privacy information' section below for further information.
What happens to reports
Medicine and vaccine adverse event reports that the TGA receives are entered into the TGA Adverse Event Management System (AEMS) . Medical device incident reports are recorded in the Incident Reporting and Investigation Scheme (IRIS) database.
Information recorded in the database includes the adverse event, the therapeutic good involved, and other relevant information, such as relevant medical history, laboratory results and how the adverse event was treated.
Serious reports are usually entered into the AEMS within two working days and a letter of acknowledgement is sent to the reporter. Each report is given a unique ID number. If you need to add more information about the case you can use the ID number to have it added to your existing report.
All adverse events are risk assessed and entered into the appropriate database for future reference. The information is used by TGA staff to help identify safety signals. A safety signal is a 'flag' for a possible safety concern. When the TGA identifies a signal, it undertakes a detailed evaluation to establish the possible role of the therapeutic good in causing the adverse event.
After a report has been entered into the AEMS (14 days) or IRIS database (3 months), information is transferred to the publicly accessible and searchable Database of Adverse Event Notifications . This time lag enables TGA staff to check and analyse the information in the report.
What can the TGA do in response to a safety concern
If the TGA identifies a safety concern relating to a therapeutic good, it can take regulatory action to ensure that the product continues to have acceptable safety, efficacy/performance and quality for its intended use. The TGA also seeks to ensure that health professionals and the public are aware of the safety concern and any changes to the availability and recommended use of the product.
Actions the TGA can take in response to a safety concern include:
- informing health professionals and consumers through alerts and articles in publications such as Medicines Safety Update and Medical Devices Safety Update (see the 'Publications' page for further information)
- requiring changes to product labelling, or adding warnings, precautions and adverse event information to the Product Information and Consumer Medicine Information
- cancelling the registration of the product, or limiting the population in which it can be used
- requiring the sponsor to undertake post-marketing studies to investigate the safety concern if more information is needed before a judgment can be made about the need for further action.
Safety publications
For safety-related publications, see the ' Safety of medicines and medical devices ' section of the TGA's publications page.
Reporting adverse events using General Practitioner software
Medical practices using the Best Practice or Medical Director software can download and install templates to their software to create Adverse Drug Reaction (ADR) reports. Completed reports can be emailed, faxed or posted to the TGA.
Note: the RTF documents below should not be opened using Word or they may become corrupted. Please read the 'How to install the ADR template' guidance before opening and downloading the template documents.
Best Practice
How to save a document to your own computer
- Best Practice: How to install the ADR template
- Best Practice: How to use the ADR template
- Best Practice: Adverse Drug Reaction report template (rtf,116kb)
Medical Director
- Medical Director: How to install the ADR template
- Medical Director: How to use the ADR template
- Medical Director: Adverse Drug Reaction Report template (rtf,542kb)
Privacy statement
The TGA collects a variety of personal information in the course of performing its functions.
Information about how the TGA handles personal information under the Privacy Act 1988 can be found on the Privacy web page.
Supporting documents
Medical Director Adverse Drug Reaction Report template [application/rtf, 541.88 KB]
Best Practice Adverse Drug Reaction report template [application/rtf, 116.02 KB]
- Biological medicines
- Over the counter (OTC) medicines
Help us improve the Therapeutic Goods Administration site
The Department of Health and Aged Care acknowledges First Nations peoples as the Traditional Owners of Country throughout Australia, and their continuing connection to land, sea and community. We pay our respects to them and their cultures, and to all Elders both past and present.
© Commonwealth of Australia
IRS update: Boosting alternative dispute resolution, and more
The Internal Revenue Service is concerned that not enough taxpayers are taking advantage of its alternative dispute resolution process for resolving disputes.
Finding ways to boost use of the ADR process was one of several topics discussed at the IRS's National Public Liaison May meeting in Washington, D.C. Among them were promoter investigations at the Office of Fraud & Enforcement, filing season processing, and stakeholder liaison.
Alternate dispute resolution
The ADR process was lauded for its ability to resolve disputes much more quickly than the standard appeals process. It is a fundamentally different path that shows promise to both taxpayers and the IRS. Moreover, studies have shown that the process ends with both parties being more compliant. The fact that it reaches a shared resolution changes the perspective on both sides, according to officials. Both parties need to be willing to compromise in order to reach an agreed outcome.
However, ADR usage has been lower than expected and continues to decrease, according to officials. The IRS has convened a cross-functional team to study and provide changes going forward, according to Stephen Mankowski, tax chair at the National Conference of CPA Practitioners, who was at the meeting.

"Some were procedural tweaks, while others were more sweeping recommendations that can improve the system," he said. An example is when the taxpayer is denied without explanation. "Now, all denials are required to have details on the results. Also, tentative denial gets reviewed prior to submission to the taxpayer. Public education and internal training will need to occur to encourage taxpayer usage."
The Office of Fraud Enforcement
A goal of the Office of Fraud Enforcement is to work with the practitioner community, as well as alternate channels, to make referrals. It has partnered with Small Business/Self-Employed Division research to get information at Tax Forums. Nearly all practitioners surveyed were familiar with the term "abusive transactions."
"It can be a fine line between fraud and tax planning, and clarification is needed on the website to illustrate this better, according to respondents in a poll," Mankowski explained. "Practitioners felt that simple online submission was important, as well as the IRS releasing statistics. Both help to hold people accountable."
Dirty Dozen " tax scams. Overall, practitioners want more and better means for reporting. A common view was the desire to see a "perp walk" upon arrest.
Tax season wrap-up
Filing season was a success, according to the IRS. There were no huge system issues; although there were some slow transmissions, they were quickly cleared up. Program completion dates for refunds are normal. More extensions were filed this season than last year, and returns are continuing to arrive. The IRS just began digitizing Form 709 from prior years, and will continue to digitize old returns and ultimately current-year returns. The service continues to struggle with hiring, with Kansas City being hit the hardest.
Direct File Pilot was available in 12 states this year. Its goal was to give the IRS the ability to provide simple tax preparation and filing for free. It became generally available to the public in those states in mid-March.
Stakeholder Liaison
The goal of Stakeholder Liaison is to help practitioners navigate the IRS. It is planning on involvement in more events in 2024 than at any time in the past. It presents 50-60 webinars annually, with subject matter experts addressing the majority of topics.
The Financial Accounting Foundation commemorated the 40th anniversary of the creation of the Governmental Accounting Standards Board in its annual report and also referenced the 50th anniversary last year of the Financial Accounting Standards Board.

The International Sustainability Standards Board and the IFRS Foundation published an inaugural guide to using their standards in different countries.

The proposed rules on the Clean Electricity Production Credit and the Clean Electricity Investment Credit aim to spur renewable energy development.

There's a new method of counting defined contribution retirement plan participants that can make a big difference for clients.

Gen Z accounting interns believe the biggest misconception about their generation is that they don't want to work hard, according to a new KPMG survey.

Big Four firm PwC announced it has made a deal with OpenAI to become the first reseller for ChatGPT Enterprise.

Harding Loevner International Equity ADR Composite Q1 2024 Report
- Harding Loevner is an investment manager that invests primarily in publicly traded global equities. We were founded in 1989 by former managers for the Rockefeller family. Harding Loevner is a Delaware limited partnership. We operate independently of Affiliated Managers Group.
The International Equity ADR composite rose 2.26% in the quarter gross of fees, trailing the 4.81% gain for the MSCI ACWI ex US Index.
- The portfolio lagged across most regions and sectors, with over half of this attributed to Japan, a market comprising just one-seventh of the index.
- The IT sector has delivered very strong returns over the last decade, and TSMC has enabled our IT holdings to keep up with those returns.

Market Review
Stock markets gained in the quarter despite rising bond yields, due in no small part to ongoing interest in the prospects for artificial intelligence (AI). Most sectors and all regions except Pacific ex Japan finished in positive territory.
Monetary policies in global developed markets, which had previously moved together toward higher rates to curb inflation, began to diverge as central bankers addressed varied inflationary trends. In the US, the Federal Reserve kept its benchmark rate steady at 5.25–5.5% for the fifth consecutive meeting, as higher- than-expected Consumer Price Index figures largely caused by rising housing expenses dashed hopes for an early rate cut. Nevertheless, the Fed continued to signal three rate cuts this year. Longer-term interest rates in the US such as mortgage rates actually increased as investors adjusted their expectations for timing and magnitude of future rate cuts from the Fed; expectations began the year at seven cuts of 25 basis points (bps), well ahead of guidance. Both the Bank of England and European Central Bank also kept rates unchanged, in contrast to the Swiss National Bank’s unexpected reduction of 25 bps—the first cut by a major central bank cut since the pandemic’s end—triggered by inflation there returning to the bank’s target range.
MSCI ACWI ex US Index Performance (USD %)
Source: FactSet, MSCI Inc. Data as of March 31, 2024.
In a landmark move, the Bank of Japan (BOJ) raised short-term interest rates, bringing to a close the country’s decade-long era of negative interest rates. The BOJ also announced an end to both its yield curve control policy, which had capped long-term Japanese government bond yields, and its asset-purchase program, which had encompassed not only government bonds but also stock ETFs and real estate investment trusts, in a sustained effort to offset negative wealth effects from deflation. As a result, yields on Japanese 10-year bonds increased, though they remain well below comparable yields in other developed markets. In contrast, the People’s Bank of China continued efforts to stimulate the real estate sector and broader economic activity and introduced measures to invigorate its moribund economy, including reducing the cash-reserve requirements for banks—freeing up more funds for lending—and enhancing credit availability to eligible developers.
The Bloomberg Commodity Index, which tracks a broad set of commodity prices, rose slightly. Strong gains in energy prices and precious metals were offset by weakness in agricultural commodities such as corn and wheat and certain industrial metals such as nickel, which fell due to fears of weakening battery demand and increased supply from Indonesia, a key global producer. Brent crude oil, a major global benchmark, rose almost $10 to reach $85 per barrel, largely due to OPEC+ supply cuts and disruptions in Russian refining capacity.
The US dollar appreciated against most major currencies, buoyed by flows into the US equity market and the curtailed number of expected rate cuts, a distinct reversal from the dollar’s broad decline last quarter.
The Information Technology (IT) sector was once again the top performer, closing the period with an 11% increase, led by substantial gains in semiconductor stocks. Shares of AI beneficiaries such as TSMC ( TSM ) — the exclusive manufacturer of NVIDIA’s ( NVDA ) GPU chips—and Dutch lithography specialist ASML ( ASML ) both surged. Industrials also performed well, largely attributable to solid returns from Capital Goods stocks. In contrast, interest-rate-sensitive sectors such as Real Estate and Utilities lagged, pulled down by the decline in bond prices, while Consumer Staples trailed as investors favored faster-growing sectors. The Materials sector suffered from declines in the shares of large mining companies like BHP ( BHP ) and Rio Tinto ( RIO ) , both of which tumbled with the slump in prices for industrial metals.
Of major regions, Japan spearheaded performance. The Pacific ex Japan region underperformed, hurt by poor returns in Hong Kong, which was weighed down by ongoing economic weakness on the Chinese mainland. Emerging Markets also lagged, with poor returns for Chinese stocks partly offset by strong performance in India and Taiwan.
Shares of faster-growing companies outperformed those of slower-growing companies this quarter, though the margin between the top quintile of growth and the bottom was not as extreme as seen in the US. The fastest-growing cohort came out on top in just three sectors—Health Care, IT, and Utilities—the former of which was buoyed by a very strong returns from Danish pharmaceutical company Novo Nordisk ( NVO ). Higher-quality companies, characterized by lower debt levels and more consistent earnings, also performed better than those of lower quality. In Japan, last year’s value rally continued, as the stocks of lower-quality companies and those in the cheapest cohorts significantly outperformed.
Performance and Attribution
The portfolio lagged across most regions and sectors, with over half of this attributed to Japan, a market comprising just one-seventh of the index. In Japan, investors once again favored the cheapest stocks, typically associated with the least-profitable and slower-growing companies. The stocks from the two cheapest quintiles of value outperformed the other three quintiles by 1300 bps. Our holdings there, typically priced at a premium due to their superior proven quality and growth track records, fell afoul of this trend. Adding injury to that insult, Sony ( SONY ) shares fell after the company released quarterly results that featured fewer sales of its PlayStation 5 console than expected.
In Europe, relative performance suffered due to a few poor stocks combined with the narrow segment of market-leading large-cap stocks we’ve been reluctant to own for valuation reasons. As for the latter, Danish obesity drugmaker Novo Nordisk, Dutch semiconductor equipment manufacturer ASML, and French luxury goods conglomerate LVMH ( OTCPK:LVMHF ) added nearly a full percentage point to the index return, but none to our portfolio. We do own Infineon Technologies ( OTCQX:IFNNY )( OTCQX:IFNNF ) , the German power semiconductor manufacturer, whose shares fell and hurt returns the most. The firm faced headwinds as its industrial and automotive customers postponed purchases in efforts to deplete inventories they had accumulated previously. Decelerating sales of electric vehicles (EVs) slashed expectations of faster growth from that source for its power management chips.
Unlike last year, Emerging Markets in general, and China in particular, were not a drag on relative performance. There were some stocks elsewhere that suffered from their connection to China. In Hong Kong, insurer AIA Group ( OTCPK:AAGIY ) ( OTCPK:AAIGF ) lagged even as its COVID-19-constrained new business production began to rebound. Further afield in Australia and the UK, shares of mining giants BHP and Rio Tinto declined in parallel with the falling price of iron ore, reflecting continued concerns about Chinese steel production.
Viewed by sector, weak stocks in Health Care, IT, Consumer Discretionary, and Industrials were the biggest drag on performance. In Health Care, Novo Nordisk’s strong performance and large market cap single-handedly delivered nearly all that sector’s contribution to the index. Among the stocks we owned instead, Roche ( OTCQX:RHHBY ) ( OTCQX:RHHBF ) ( OTCPK:RHHVF ) and BioNTech ( BNTX ) suffered due to waning COVID-related sales, with contributions from their drug pipelines seemingly too remote to attract the attention of Health Care investors captivated by weight-loss drug makers.
First Quarter 2024 Performance Attribution
International Equity ADR Composite vs. MSCI ACWI ex US Index
“OTHER”: Includes companies classified in countries outside the index. (Source: Harding Loevner International Equity ADR composite, FactSet, MSCI Inc. The total effect shown here may differ from the variance of the composite performance and benchmark performance shown on the first page of this report due to the way in which FactSet calculates performance attribution. This information is supplemental to the composite GIPS Presentation.)
Within IT, the drag from Infineon shares, along with the absence of ASML, hurt returns. Dassault Systèmes ( OTCPK:DASTY ) ( OTCPK:DASTF ) , little-exposed to the AI craze, also hurt returns, as demand from Life Sciences customers fell short of investor expectations, causing management to issue disappointing guidance due to expected continued weakness. This offset gains from SAP ( SAP ) , which does tout the AI features of its enterprise software. Strong returns from TSMC, the primary manufacturer of NVIDIA’s GPU chips, partially made up the lost ground; we sold a slice off our holding as it approached our single security limit of 5%. In Industrials, our four Swedish multinationals, Atlas Copco ( OTCPK:ATLKY )( OTCPK:ATLCY )( OTCPK:ATLKF ) , ASSA ABLOY ( OTCPK:ASAZF ) ( OTCPK:ASAZY ) , Epiroc ( OTCPK:EPOKY ) , and Alfa Laval ( OTCPK:ALFVF ) ( OTCPK:ALFVY ) , all suffered modest share price reversals and collectively contributed to the sector’s underperformance. For Consumer Discretionary, Sony was the biggest drag on returns, compounded by the absence of Toyota Motors ( TM ) and LVMH from our portfolio. These latter two companies alone accounted for about half the sector’s overall contribution to the index.
Financials were a bright spot in the quarter, especially shares of [[ BBVA]] . The banks’ strong net interest income growth in both Spain and Mexico will allow it to boost its dividends and share repurchase programs. Additionally, the Dutch payments specialist, Adyen ( OTCPK:ADYEY )( OTCPK:ADYYF ) , continued its path to recovery reporting a 23% year-over-year increase in revenue for the latest quarter, which has significantly bolstered investor confidence in its market position. Offsetting this was continued weakness in shares of HDFC Bank ( HDB ) following its merger with its parent HDFC Corp last year, with its results suffering from a slower-than-expected pace of replacing higher-cost wholesale funding with lower-cost retail bank deposits.
Perspective and Outlook
As global markets increasingly align to capitalize on the AI theme, driving up a diverse array of beneficiaries, our decision to own, or not, various companies tied to this theme is rooted in a deliberate process of bottom-up decision-making that spans years and occasionally decades. We recognize and can elucidate the ways in which our portfolio of high-quality, growth-oriented companies stand to benefit from this burgeoning area of growth. But our investment in these companies is not predicated on some specific target for AI exposure, despite the pain caused by missing out on some companies that are tied to that theme during certain times. While we have significant portfolio-level exposure to the growth AI may bring, it comes via a diverse set of companies that are already fundamentally strong.
The companies that investors anticipate will benefit from AI fall into two broad categories. The first includes companies that provide goods and services enhanced by AI—enabling them, improving them or delivering them more efficiently. This includes internet giants such as Meta Platforms and Alphabet, and other large service providers like Salesforce and SAP. Many of these companies are focused on the potential of generative AI, which is capable of processing complex language prompts to perform a variety of tasks, mimicking human-like creativity and problem-solving skills across a broad spectrum of applications.
The second group that is drawing investor interest consists of companies developing the underlying technology that powers AI. Leading this group are the designers and manufacturers of the advanced semiconductors necessary to run AI algorithms, but the interest also extends to the producers of semiconductor manufacturing equipment and providers of the critical computing infrastructure required by AI systems.
NVIDIA, headquartered in the United States, has dominated headlines as the leading designer and vendor of AI-focused semiconductors and software. Another US-based chip designer, AMD ( AMD ), has also ventured into the AI arena. But neither company undertakes the manufacturing of their AI-centric semiconductors. Instead, the production of virtually all high-performance AI chips designed by these firms is carried out by TSMC, a company we’ve owned for decades.
The AI chips produced for NVIDIA and AMD include Central Processing Units (CPUs) and Graphics Processing Units (GPUs) fabricated using some of TSMC’s most sophisticated manufacturing technologies, resulting in chips at the pinnacle of the industry in terms of transistor density, speed, and energy efficiency per computing unit. These attributes are critical for AI applications, where processing power and efficiency are paramount. NVIDIA and AMD’s reliance on TSMC extends beyond chip manufacturing. They also increasingly depend on TSMC’s innovative capabilities in integrating and packaging the logic, memory, and input/output components.
In January, TSMC CEO C.C. Wei highlighted the company’s pivotal role in the AI sector, stating “We are a key enabler for AI applications. So far today, everything you saw for AI comes from TSMC.” He also projected that AI-specific chips would constitute nearly 20% of TSMC’s total revenue in 2027. Rising use of AI applications may also spur demand for other types of semiconductors manufactured by TSMC, as chips used in networking devices and smartphones grow ever-more advanced, underscoring the company’s integral role in the global semiconductor ecosystem.
After growing at a 6% annualized rate over the last quarter century, total global semiconductor sales plateaued at close to US$500B per year over the last three years, recent projections suggest the surging demand for all things AI-related could result in an incremental US$400B of annual semiconductor sales between 2027 and 2030. That would power a return to double-digit revenue growth rates that the industry has not seen since before the year 2000.
The anticipated growth in semiconductor demand is boosting expectations for related suppliers. As giants like TSMC and Samsung increase investments, suppliers such as ASML, known for its advanced EUV lithography machines essential for cutting-edge chips, stand to benefit. This dynamic extends to a broad set of companies involved in various facets of semiconductor production.
Daifuku ( OTCPK:DFKCY ) ( OTCPK:DAIUF ) , a Japanese firm specializing in automated handling systems, particularly for ultra-low vibration transport, is also key supplier for TSMC. Cleanroom automation systems for semiconductor and display makers represent about one-third of Daifuku’s sales. Along with the rising demand for its cutting-edge cleanroom technologies, there’s also plenty of demand for the company’s legacy products, especially in China. Daifuku’s business remains strong in Taiwan and Korea, while efforts to revitalize semiconductor manufacturing in Japan and the US present further growth opportunities.
Data centers are also likely to see increased investment to accommodate the expanding range of AI services. That expansion will create opportunities for companies that supply essential infrastructure components for datacenters, including Schneider Electric ( OTCPK:SBGSF ) ( OTCPK:SBGSY ) and Infineon, which are key suppliers of some of these critical elements, including cooling and power management.
Data centers are notable energy hogs, using 10 to 50 times more energy per square foot than a typical office building; in the US, data centers already account for 4% of the total electricity usage. Schneider’s expertise in power management for IT infrastructure has positioned it as a leader in this space, offering a range of services that include electricity usage management, infrastructure management software, cooling systems, uninterruptible power supplies, and rack systems. The company’s recent projections call for organic growth between 7% and 10% annually through 2027, along with consistent margin improvement.
Infineon, widely recognized for its automotive semiconductors, particularly for electric and hybrid vehicles, also plays a crucial role in data centers and AI computing. The high power demands and the necessity for power density is a good match for Infineon’s switched mode power supplies, conversion solutions, and voltage regulator designs that power high performance CPU’s, GPUs, and memory products, helping to reduce power consumption.
While current forecasts predict a swift expansion of AI computing, and corresponding investments in AI-related capex, the range of these forecasts varies significantly. While investors are currently focused on growth and rewards, there are risks and uncertainties worth keeping in mind. For instance, the semiconductor wafer fabrication equipment industry has grown massively over the past 30 years, from US$10B in annual sales in 1990 to over US$60B per year in 2020. But that growth has been highly variable, with the industry taking 10 years to surpass a US$40B sales peak in 2007, and twice seeing peak to trough declines of over 40%. Should AI turn out to be more evolution than revolution, or if the surge in capex investment falls short of estimates, or if the industry’s path is as variable as fabrication’s, we remain confident in the resilience and long-term growth potential of the companies within our portfolio, as none of them are wholly dependent on AI associated predictions to grow.
Portfolio Highlights
The idea that themes in the portfolio emerge from a collection of disparate individual holdings revealed to share exposure to growing trends, and that sector allocations are merely the result of an accumulation of specific stock decisions, is illustrated well by our long-term stance in favor of the Information Technology and Consumer Staples sectors. We’ve owned TSMC continuously since 1999 in this strategy, and the holding has been a cornerstone of our IT weight, leading to a persistent overweight to the semiconductor industry group. This description, written for our commentary nearly a quarter-century ago, still rings true: “The global trend toward outsourcing is not confined to the services sector. Capital requirements in the semiconductor manufacturing industry are so high that many electronic equipment companies have gradually withdrawn from manufacturing, choosing to fulfill their semiconductor requirements from external sources. By taking orders from a broad base of such OEM customers, TSMC has become the world’s most efficient chipmaker, and can generate high returns on the large capital investment required.” Management has redoubled investment to reinforce that efficiency and retain its technology lead, when others balked at the audacious commitment required, and that sustained advantage has enabled growth that has rewarded investors handsomely over time.
The IT sector has delivered very strong returns over the last decade, and TSMC has enabled our IT holdings to keep up with those returns. But our outsized allocation to IT overall, partly a result of this longstanding holding, has contributed significantly to relative performance over the past five and ten years.
The semiconductor industry has historically been highly cyclical, characterized by frequent revenue downturns of 10% or more, with even larger profit declines especially for the weakest competitors. Recognizing our limitations in forecasting these cycles, we’ve aimed to mitigate the cyclical volatility of our substantial semiconductor investments by diversifying with investments in less-cyclical sectors, such as Consumer Staples. This balance allows us to manage risk while maintaining exposure to the growth potential of the semiconductor industry. This approach has resulted in our overweight in Consumer Staples for most of the past 15 years.
The companies within Consumer Staples, which include food, beverage, and personal household goods makers, typically exhibit more moderate growth rates compared to many of our other holdings. However, the stability of their profitability, as reflected in rock-steady Cash Flow Return on Investment (CFROI) over many years, has resulted in their share prices being significantly less volatile than the rest of the market. This lower volatility, along with lower market sensitivity (as measured by their beta) provides substantial portfolio diversification benefits, particularly during market downturns. It creates opportunities to rebalance our holdings, shifting away from stable stocks towards more-cyclical and more-volatile segments when other investors are seeking safety in the predictability of Consumer Staples.
This approach of seizing opportunities amid market fear— implicitly heeding Warren Buffett’s advice to “Be greedy when others are fearful”— has been successfully implemented repeatedly over the years, such as our early battle against the rising valuations afforded high-quality companies in 2015-16, then during the COVID-induced market plunge of 2020, and later amid the sharp downturn in shares of high-growth companies in 2022. By reducing our allocation to Consumer Staples during these periods, we capitalized on the enhanced potential of more volatile market segments. This contrarian behavior, which demands not only the fortitude to trade against the crowd, but also the patience to hold less-glamorous investments during more risk-friendly environments, has been pivotal. On average, our approach has resulted in Consumer Staples contributing to our relative performance over the last decade.
A word about our view on Novo Nordisk is in order. Currently, its drugs Ozempic and Wegovy for diabetes and obesity are the only engines of the company’s growth. By 2030, the drugs are expected to account for 70% of the company’s revenue, but then the patents on the drugs will expire in 2031 and 2032. The price and volume pressure that will ensue at that point is a substantial challenge to Novo’s long-term earnings and thus how we view the current valuation of its shares.
Further, there are nearer risks to Novo’s market position than the eventual patent expiries. The current duopoly between Novo and Lilly ( LLY ) for anti-obesity drugs is not a foregone conclusion; there is a proliferation of candidate drugs from other companies, many with early data that’s as compelling as Novo and Lilly’s drugs. And because commercial payors (insurers and Medicare) haven’t normalized the coverage of obesity drugs, their bargaining power could curb the growth of (or the prices commanded for) GLP-1 drugs for obesity overall. Those risks to Novo’s revenue and profits aren’t reflected in the forecasts used to justify Novo’s current share price.

Original Post
Editor's Note: The summary bullets for this article were chosen by Seeking Alpha editors.
This article was written by
Recommended For You
About hlmix ticker, more on hlmix, related stocks, trending analysis, trending news.

191 BJP, 143 Cong LS poll candidates face criminal cases: ADR
New Delhi, May 29 (PTI) Out of the 440 BJP candidates contesting the Lok Sabha polls, 191 have declared criminal cases in their election affidavits, according to an analysis by a poll rights body.
For the Congress, out of its 327 candidates, 143, or 44 per cent, have declared criminal cases, the Association for Democratic Reforms (ADR) said in a report based on data compiled from the affidavits on the Election Commission's website.
The cases, it said, include those related to murder, attempted murder, crimes against women and hate speech.
The ADR said the data obtained from the poll panel's website reveals that a "significant portion of candidates contesting in the elections have self-declared criminal cases against them".
The analysis presents a breakdown of candidates with declared criminal cases party-wise, it said. The report stated that among the major parties, the Bharatiya Janata Party has 191 (43 per cent) of candidates who have declared criminal cases, while the Congress has 143 (44 per cent) of such candidates.
The report also delves into the number of candidates facing serious criminal cases.
One hundred and thirty of the total BJP candidates in the poll fray have declared serious criminal cases, while for the Congress it is 88 candidates (27 per cent of the total candidates it has fielded), according to the report.
The Samajwadi Party has 71 candidates in the elections and 40 have declared criminal cases, with 30 of them named in serious crimes.
Thirty-three of the 52 candidates of the Communist Party of India (Marxist) have declared criminal cases, with 18 facing serious charges, and in the Trinamool Congress, 20 of 48 candidates have declared criminal cases with 12 facing serious charges.
The state-wise analysis also provides insights into the prevalence of candidates with criminal backgrounds.
For instance, in Maharashtra, 266 of the 1,119 contesting candidates have declared criminal cases, with 183 names serious crimes. Tamil Nadu has 137 out of 945 candidates with criminal cases, with 83 facing serious charges.
In Uttar Pradesh, 213 out of 851 candidates have declared criminal cases against themselves with 179 of them being registered against serious offences.
In Telangana it is 104 of 524 candidates, 112 of 507 candidates in West Bengal and 115 of 496 candidates in Bihar. For Jharkhand it is 69 of 242 candidates, Odisha it is 52 of 207 candidates and Kerala it is 67 of 189 candidates.
In Punjab and Andhra Pradesh, 69 of 328 candidates and 88 of 450 candidates, respectively, have declared criminal cases. In Delhi, 25 of 162 candidates have declared criminal cases, the report stated.
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springerplus

Adverse drug reactions (ADRS) reporting: awareness and reasons of under-reporting among health care professionals, a challenge for pharmacists
Sumbul shamim.
1 Department of Pharmacology, Faculty of Pharmaceutical Sciences, Dow College of Pharmacy, Dow University of Health Sciences, Ojha Campus, Karachi, Pakistan
Syed Muhammad Sharib
2 Dow College of Pharmacy, Dow University of Health Sciences, Ojha Campus, Karachi, Pakistan
Saima Mahmood Malhi
Sidrat-ul muntaha, hassan raza, ali salman farooq, mehwish hussain.
3 Department of Research, Dow University of Health Sciences, Ojha Campus, Karachi, Pakistan
To measure awareness about adverse drug reaction (ADRs) reporting among doctors, pharmacists and nurses and to determine reasons of ADRs under-reporting in Pakistan.
In present study, a self-administered questionnaire was used to measure the awareness level about ADRs reporting among health care professionals (HCPs) of Pakistan. This was a cross sectional study.
Out of the respondents 51 % were physicians, 29.7 % pharmacists and 19.3 % were nurses. 65.5 % of HCP population observed ADRs, out of which only 57.4 % reported these in their respective hospitals. About 77.3 % of population understood the importance of reporting ADRs while 67.3 % of population agrees that pharmacists are chief personnel for the development of system. 71.8 % of HCPs agrees that ADRs are not reported because Community pharmacy lacks legally qualified pharmacists. Only 14.3 % of HCPs population knows that there is any ADR reporting organization in Pakistan.
The study recommends the need of such reporting system and more than half of the studied population agreed that pharmacists are required in developing such system.
As per definition of Pharmacovigilance (PV) it is not only science but also actions which are for the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. The Thalidomide disaster in 1961 was the start of establishing the WHO Program for International Drug Monitoring, WHO promotes PV at the country level by working in collaboration with the Monitoring centre at Uppsala. More than 135 countries are the part of this program. This program not only enhances patient safety for use of medicines but also gives information about safe use and prevention and treatment of any Adverse Drug Reactions (ADRs) ( http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/ ).
WHO’s definition of ADRs, which has been in use for about 30 years, is “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function” (WHO 1972 ).
The birth defects were caused by thalidomide in 1961–1962 in about ten thousand children in different regions of the world, when the pregnant mothers used it for nausea and vomiting. As a result in 1968 the WHO started the Program for International Drug Monitoring (PIDM) for early detection of ADRs. This activity is now called as Pharmacovigilance. Uppsala Monitoring Centre (UMC) in Sweden is responsible to monitor and manage the WHO-PIDM activities (Collet 2000 ; Blenkinsopp et al. 2007 ; Gregg and Stuart 2010 ).
The inclination should be there to not only observe but also report unwanted and unexpected medical events in all areas where medicines are being used. At any dosage and by an overdose or by misuse or abuse of a medicine the adverse drug reactions or adverse events can occur (WHO 2002a ). Pharmacovigilance is applied throughout the life cycle of a medicine that is from the pre-approval phase to the end use by the patients. These lead to burden on patients not only in disease form by prolonged stay in hospital but also the financial burden it creates immensely (Johnson and Bootman 1997 ). Pharmacovigilance focuses on not only effectiveness and benefits but also on safety and risk analysis with the aim to improve patient care (Cipolle et al. 2004 ; www.fda.com ). The safety of patients is totally related with the safety of medicines. ADR monitoring is an integral part of quality assurance department in developed countries but unfortunately Pakistan has limited accountability system for medicines (Scurti et al. 2012 ; Rollins 2013 ).
Healthcare systems rely mainly on the detection and reporting of suspected ADRs to identify new reactions, record the frequency with which they are reported, evaluate factors that may increase risk and provide information to prescribers with a view to preventing future ADRs, shows that adverse drug reaction are by anyway causing deaths (Collet 2000 ). The actual statistics of ADR related death in Pakistan is not available because of underdevelopment of such system throughout Pakistan few of the hospitals like AGHA KHAN and DOW UNIVERSITY HOSPITAL practices reporting at their own level but system in just a few health care sectors in a large population is not sufficient obviously. Efforts are increasing to ensure that resource poor countries, which bear almost 90 % of the global disease burden, have access to effective medicines (Mahmood et al. 2011 ). Pakistan Pharmacist Federation has launched a campaign to implement the directions of Health Department, Government of Punjab, Pakistan to establish pharmacovigilance centre, adverse drug reporting, drug information and poison control centre at provincial and hospital level (Davies et al. 2009 ). Medication errors are usually not reported for the reason that the errors are considered as not very much significant by the prescriber that they should be reported ( www.pharmacistfed.wordpress.com ). Under reporting of ADRs influenced by prescriber’s and reporter’s medical knowledge and their approach to give significance to any types of ADR. This under reporting creates a negative impact on Public Health (Pirmohamed et al. 2007 ).
The purpose of the study is to evaluate the knowledge and concerns of the health care professionals about adverse drug reaction and its reporting which claims the development and incorporation of system including adverse drug reaction reporting and training to health care professionals about detection, assessment and control adverse drug reaction. Even though in countries like UK where pharmacovigilance activities are being practiced, the occurrence rate of ADR is 6.7 % with overall fatality rate is 0.32 % (Kazeem and Jacob 2009 ). Our study acknowledges the importance of ADR reporting and steps must be taken at national level to ensure the incorporation of pharmacovigilance centre in the health care sector.
A cross sectional study was conducted from June, 2013 to August, 2014 in Karachi, a metropolitan city of Pakistan. Target responders consisted of different health care professionals including nurses, pharmacist and physicians/doctors working in different health care services of the city.
The questionnaire being addressed was adapted from studies regarding concerns of health care professionals about adverse drug reaction reporting and reasons of underreporting these reactions, information and knowledge about reporting of ADR. Health care professionals mostly addressed were physicians, pharmacists and nurses working at public sector hospitals. (WHO Program for International Drug Monitoring 2010 )
Institutional Research Review Committee has approved this study and has found it exempted from any IRB.
Sample size
At 99 % confidence interval with 5 % bound of error of unawareness rate of 84 % of Irish physicians (Williams and Feely 1999 ). The calculated sample size was 357 HCPs which was calculated by the population size of HCPs in Karachi, Pakistan.
Study population
According to sample size calculated 357 questionnaire forms were distributed to health care professionals of different Tertiary Health Care sectors of Karachi, including Dow University Hospital, Civil Hospital, Abbasi Shaheed Hospital, Liaquat National Hospital, Orthopedics and Medical Institute, Patel Hospital. Questionnaire forms were self addressed to HCPs.Of the respondents 51 % were physicians including house officers, RMO’s, surgeons, consultant doctors, and 29.7 % were pharmacist working at inpatient pharmacy services and 19.3 % nurses.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 21. Descriptive statistics were employed to report the response of respondents in terms of frequency and percentage. Since, responses were ordinal scaled, therefore, gradient effect Chi-square test was executed to measure association of knowledge, attitude, perception, practices and reasons of non-compliance with different HCPs. P value less than 0.05 was considered to show significant association.
A total of 357 responses were compiled in the data file. Out of the respondents 51 % (n = 182) were physicians, 29.7 % (n = 106) were pharmacists and 19.3 % (n = 69) were nurses. The findings depicted only 43.4 % (n = 155) HCPs knew the term Pharmacovigilance and ADR reporting. The frequency of knowledge of term Pharmacovigilance was significantly more among pharmacists followed by physicians (P < 0.0001). Only 31.7 % of respondents know that there is any ADR reporting form at the website of Drug Regulatory Authority of Pakistan (DRAP) while DRAP is established since 2012. Pharmacist followed by nurses confirmed the knowledge of DRAP webpage significantly more than physicians (P = 0.017). Furthermore only 14.3 % of HCPs respondents knows that there is any ADR reporting organization in Pakistan. Though, the linear association of the knowledge of ADR reporting organization in Pakistan was not significant showing similar knowledge among three types of HCPs (Table 1 ) indicating that there is a problem about national reporting in Pakistan across all the professionals.
Table 1
Knowledge about ADR reporting from three different HCPs
About 67.3 % of respondents agreed that pharmacists are chief personnel for the development of system (Table 2 ). The agreement of relating pharmacists as chief personnel for reporting ADR was proportionally least among physicians followed by nurses (P = 0.0002). The proportion of agreement to collaborate pharmacist with other HCPs was high among pharmacist. The requirement of drug utilization review and ADR reporting system is highly acknowledged by all three health care professionals (P = 0.308).
Table 2
Attitude for ADR reporting from three different HCPs
About 77.3 % of respondents understood the importance of reporting ADRs. Though, only 38.9 % confessed presence of ADR reporting system in their respective health care system (Table 3 ). There was no significant association of owning the responsibility of ADR reporting among these three professionals. While asking about presence of ADR reporting system in respective health care setup, the proportion of denying the same was significantly higher from physicians, followed by pharmacists (P < 0.0001).
Table 3
Perception about ADR reporting from three different HCPs
Nearly half percent (n = 186) HCPs were found to be trained for detecting, reporting and controlling ADR. The training of detection and reporting of ADR was found significantly more among nurses (P = 0.006). Around 65.5 % of HCP respondents observed ADR whereas only 57.4 % report these in their respective hospitals. Observation of ADR was significantly not different among these three HCPs. Though, reporting the same was found significantly least among physicians followed by nurses (P = 0.001). Reporting of ADR to any pharmaceutical industry was not in higher proportion (Table 4 ).
Table 4
Practices for ADR reporting from three different HCPs
Furthermore, our study also highlighted various reasons of underreporting. HCPs (84.6 %) have uncertainty whether the ADRs occurred due to drugs, unavailability of reporting forms (e.g. yellow cards), 71.8 % of HCPs agrees that ADRs are not reported because Community pharmacy lacks legally qualified pharmacists (Table 5 ). Physicians were significantly least agreed of ADR underreporting due to shortage of time (P = 0.013), complacency (P = 0.011) and belief of safe marketed drugs (P = 0.006).
Table 5
Reasons for ADR misreporting from three different HCPs
Because of a counterfeit antihypertensive medicine at the Punjab Institute of Cardiology (PIC) hospital at Lahore, Pakistan, the lives of over 100 heart patients were taken away in January 2012 (Kazeem and Jacob 2009 ). The counterfeit medicine(s) deposited in the bone marrow of patients and lost the patient’s resistances. The drug caused the suppression of generation of white blood cells after being deposited in bone marrow. Which lead to change in skin pigmentation, severe chest infection, decreased platelet count and blood vomiting (Thenews.com.pk 2012 ).
As it has been reported that the infer drugs include isotab (isosorbide nitrate), lipitor (atorvastatin calcium), soloprin (aspirin), cardiovestin (simvastatin), concort (amlodipine), and alfagril (clopidogrel) (Desk 2012 ).
Import of medicines from Pakistan was banned by Sri Lanka as a precautionary measure (Usman 2012 ). As a consequence, Efroze Pharma was called by the WHO for increased vigilance on the use of isotab, for which it released a global drug safety alert (no. 125) (Wasif 2012 ).
Considering the importance of ADRs reporting, our study showed inadequate knowledge of physician and nurses about an adverse drug reaction and reporting. Study also reveals the point of enhancing awareness among the HCPs also increase in DRAP’s role for this purpose. (WHO 2002b )
Doctors, pharmacists and nurses as well as patients can report ADRs. This may become more rapid and advanced by the use of new softwares and internet. The way in which companies and governments handle patients data may become safe by law now is passed by the member states will enable the security and privacy of patient data (The News Tribe 2012 ; MedWatch 2015 ; Chanda 2007 ; Strengthening Pharmaceutical Systems (SPS) 2009 ; Cobert and Silvey 1999 ).
Because of absence of appropriate clinical trials in the paediatric population, drugs prescription in children has a high risk of developing unknown or rare adverse drug reactions (ADRs). The spontaneous reporting of suspected ADRs is an important way to promote reasonable warning signals. The family paediatricians (FPs) play a crucial role in this reporting ( http://www.isoponline.org ).
Pharmacists can be the chief personnel for the development of Pharmacovigilance system as 59.89 % (n = 109) Physicians, 66.66 % (n = 46) nurses and 80.1 % (n = 85) Pharmacists agrees upon the point. In 60.1 % of HCPs opinion there is no any Pharmacovigilance system exists in their respective health care sectors.
As per study results, HCPs observe ADRs during their clinical practice but the reporting of those ADRs are very much limited to the concerning authorities whether Pharmaceutical Industry or Government concerned department (Khurshid et al. 2008 ).
Furthermore, study reveals the reasons that could be looked into for the betterment of reporting of ADRs. According to the study, reasons mainly causing underreporting of ADR’s are, Uncertainty of whether ADRs occur due to drug or not, community pharmacy lacking legally qualified pharmacist, Unavailability of ADR reporting form (e.g. Yellow cards), and awareness regarding system (Pellegrino et al. 2013 ). Financial issues can be resolved if the steps are taken at governmental level. Physicians generally are not very much responsive to ADR reporting program mainly because of the time that they prefer to reserve for patients rather to spare time for reporting (Table 5 ). Other reasons also contributes but to a lesser extent in underreporting of ADRs (WHO 2002b ).
Study also force on the point of having Drug Utilization Review or ADR reporting system within the Health Care Sectors to reduce the rate of adverse drug events ( http://www.who-umc.org ).
Incorporation of pharmacists in health care sectors for making policies regarding the system and acceptability among other health care professionals for the pharmacists to detect report and control ADRs is necessary. As has been mentioned that physicians are not very cooperative to report ADRs so, to increase reporting many countries allowed pharmacists working in hospital and community, nurses and even patients to report ADR (Olsson 1998 ).
There is also presence of nurses throughout in all hospitals especially in public sectors hospitals who even do not know about the presence of such reactions even most of the At least 60 % of ADRs are preventable ( http://www.isoponline.org ).
Medical practitioners are the primary component of ADR reporting system but every healthcare professional who is having knowledge, attitudes and perceptions about ADR can play its part in reporting ADRs (Khurshid et al. 2008 ). As 77.47 % (n = 141) Physicians, 82.01 % (n = 57) nurses and 91.51 % (n = 97) pharmacists agrees upon the point (Table 2 ).
Adverse drug events are preventable most of the time. Even then it is reported to be the 8 th -leading cause of death which exceeds the deaths attributable to motor vehicle accidents, breast cancer or AIDS (Shin et al. 2011 ). These results shows that even in developed country where expertise are practicing such system, the ADR related deaths are a matter of serious concerns.
Pharmacovigilance system implementation is the need which is possible by collaboration between academia, health care providers including pharmacist, patient, manufacturer, government, media, and civil society, Uppsala Monitoring Center (UMC), Sweden operating under (WHO), FDA, Isop and other international organization working on drug safety. To enhance the patients trust it is great opportunity for Pharmacists to built Pharmacovigilance system in Pakistan ( http://www.ppma.org.pk/PPMAIndustry.aspx ). Reporting of any doubtful ADR event by health care professional is of concern because seemingly the relationship between the medicine and its reaction is not very much clear but analysis after reporting can evaluate its importance. Further, such PVsystem on national level can work with help of coordinator and a core committee to make plans and to take decision for the national centre to maintain the quality (National Health Policy 2001 ). Focus group interviews can be beneficial retrieving the reasons of underreporting than deliberately action upon them can lead to appropriate reporting leading to better patient health care ( http://www.dcomoh.gov.pk/ ). Fear of reaction from the nurse managers and coworkers, fear of termination from job are found to be the reasons of underreporting of medication errors by nurses. With the help of continuous medical education programs the nurses knowledge regarding medications can be enhanced ( http://www.pharma-iq.com/regulatorylegal/articles/building-a-harmonisedpharmacovigilanceframework/ ).
Authors’ contributions
SS: She actually conceived the idea and designed the study. She was also involved in acquisition of data, analysis and interpretation of data. During questionnaire filling any query sent by Health care professional was also answered by her. In addition she performed the drafting of manuscript and revised it critically as per requirement. She was the key person for giving final approval of the version to be published. SMS: He contributed in questionnaire development and data collection. He was the key person for coordination with different health care professionals in Health care setups. The queries and difficulties in questionnaire filling which could not be answered by him, were coordinated to main author by him. SMM: She participated in designing of study and development of questionnaire. In addition during conduct of study she was checking the proper filling of questionnaire, and if some queries were there, she was answering them also. SM: She was involved in questionnaire development, data collection and was the responsible person for coordination with different health care professionals in Health care setups. HR: He was involved in questionnaire development, data collection and was the responsible person for coordination with different health care professionals in Health care setups. SA: He was involved in questionnaire development, data collection and was the responsible person for coordination with different health care professionals in Health care setups. ASF: He was involved in questionnaire development, data collection and was the responsible person for coordination with different health care professionals in Health care setups. MH: She was the key person for statistical analysis and revising the manuscript for statistical part of Results. All authors read and approved the final manuscript.
Acknowledgements
Authors are very much thankful to all the physicians, pharmacists and nurses for their participation and cooperation in this study. This work would remain incomplete without high cooperation from administration of Dow University Hospital, Civil Hospital, Abbasi Shaheed Hospital, Liaquat National Hospital, Orthopedics and Medical Institute and Patel Hospital, Karachi Pakistan. We are highly indebted to these hospitals’ administration.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Sumbul Shamim, Email: [email protected] .
Syed Muhammad Sharib, Email: moc.liamtoh@deys_birahs .
Saima Mahmood Malhi, Email: moc.liamg@jehmms .
Sidrat-ul Muntaha, Email: moc.liamtoh@5nawzirardis .
Hassan Raza, Email: [email protected] .
Saniya Ata, Email: moc.llimtoh@ataayinas .
Ali Salman Farooq, Email: moc.liamtoh@2102_namlasila .
Mehwish Hussain, Email: [email protected] .
- Adverse drug reaction reporting form Islamabad, Drugs Control Organization, Ministry of Health of Pakistan, Islamabad. http://www.dcomoh.gov.pk/ . Accessed 23 May 5 2011
- Being a member of the WHO Program for International Drug Monitoring (2010) Published and printed by the Uppsala Monitoring Centre
- Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007; 63 (2):148–156. doi: 10.1111/j.1365-2125.2006.02746.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Building a Harmonised Pharmacovigilance Framework in Asia. http://www.pharma-iq.com/regulatorylegal/articles/building-a-harmonisedpharmacovigilanceframework/ . Accessed 23 May 2011
- Chanda K. Need to be ‘vigilant’ about pharmacovigilance studies. JMSR. 2007; 2 :11–12. doi: 10.1186/1750-2187-2-11. [ CrossRef ] [ Google Scholar ]
- Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the clinicianûs guide. New York: McGraw-Hill; 2004. [ Google Scholar ]
- Cobert B, Silvey J. The internet and drug safety: what are the implications for pharmacovigilance? Drug Saf. 1999; 20 (2):95–107. doi: 10.2165/00002018-199920020-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Collet JP. Limitations of clinical trials. Rev Rrat. 2000; 50 (8):833–837. [ PubMed ] [ Google Scholar ]
- Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLOS One. 2009; 4 (2):e4439. doi: 10.1371/journal.pone.0004439. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Desk W (2012) Fake medicine: unofficial death toll reaches 112—The Express Tribune. Tribune.com.pk. Retrieved 2012-01-31
- FDA Act 1938 www.fda.com . Accessed 14 Act 2011
- Gregg WS, Stuart JP. Randomized trials, statistics, and clinical inference. J Am Coll Cardiol. 2010; 55 (5):428–431. doi: 10.1016/j.jacc.2009.06.066. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- International Society of Pharmacovigilance. http://www.isoponline.org . Special Interest Group (SIG). Accessed 1 June 2015
- Johnson JA, Bootman JL. Drug-related morbidity and mortality and the economic impact of pharmaceutical care. Am J Health Syst Pharm. 1997; 54 :554–558. [ PubMed ] [ Google Scholar ]
- Kazeem AO, Jacob OA. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Pharmacol Toxicol. 2009; 9 (1):14. doi: 10.1186/1471-2210-9-14. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khurshid K, Rozmin N, Rashida JM, Jacqueline D, Bustamante-Gavino I, Amina M. A systematic approach of tracking and reporting medication errors at a tertiary care university hospital,Karachi, Pakistan. Ther Clin Risk Manag. 2008; 4 (4):673–679. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mahmood KT, Amin F, Tahir M, Haq IUl. Pharmacovigilance—a need for best patient care in Pakistan. A review. Pharm Sci Res. 2011; 3 (11):1566–1584. [ Google Scholar ]
- MedWatch (2015) FDA's MedWatch Safety Alerts for Consumers: August 2015, Section Drugs
- National Health Policy 2001 (2001) The way forward. Islamabad, Ministry of Health, Government of Pakistan. http://siteresources.worldbank.org/PAKISTANEXTN/Resources/PakistanDevelopmentForum/NationalHealthPolicy.pdf . Accessed 23 May 2011
- Olsson S. The role of the WHO program on international drug monitoring in coordinating worldwide drug safety efforts. Drug Saf. 1998; 19 (1):1–10. doi: 10.2165/00002018-199819010-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pharmaceutical Review: ISSN 2220-5187 Professional Publication of Pharmacist Federation of Pakistan (Registered). www.pharmacistfed.wordpress.com
- Pharmacovigilance. UMC_WHO. http://www.who-umc.org . Accessed 14 July 2011
- Pellegrino P, et al. Pharmacovigilance knowledge in family paediatricians. A survey study in Italy. Health Policy. 2013; 113 (1–2):216–220. doi: 10.1016/j.healthpol.2013.08.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pirmohamed M, Atuah KN, Dodoo ANO, Winstanley P. Pharmacovigilance in developing countries. BMJ. 2007; 335 (7618):462. doi: 10.1136/bmj.39323.586123.BE. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Profile Pakistan Pharmaceutical Industry available at Pakistan Pharmaceutical Manufacturing Association. http://www.ppma.org.pk/PPMAIndustry.aspx . Accessed 15 Nov 2011
- Rollins DE (2013) Adverse drug reactions and clinical toxicology. Chapter 61 Remington pharmacy practice, 21st edn. Mack Publishing Company, USA
- Scurti V, Romero M, Tognoni G. A plea for a more epidemiological and patient-oriented pharmacovigilance. Eur J Clin Pharmacol. 2012; 68 (1):11–19. doi: 10.1007/s00228-011-1096-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shin YS, Park J-W, Lee Y-W, Kim C-W, Dhong H-J, Park H-S, Cho Y-J, Cho S-H, Pyun BY, Lee KH, Lee HR, Hong C-S. Current status of oriental medicine in treating Korean allergy patients. Pharmacoepidemiol Drug Saf. 2011; 20 :99–104. doi: 10.1002/pds.1947. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Strengthening Pharmaceutical Systems (SPS) (2009) Supporting pharmacovigilance in developing countries: the systems perspective. Submitted to the US Agency for International Development by the SPS Program. Management Sciences for Health, Arlington, VA
- The News Tribe (2012) WHO bans Pakistani medicine Isotab. Retrieved 2012-02-04
- Thenews.com.pk (2012-01-24) About 100 people have died from spurious antihypertensives in Lahore. Retrieved 2012-01-31
- Usman A (2012) PIC free medicine: as deaths soar past 80, authorities remained clueless—The Express Tribune. Tribune.com.pk. Retrieved 2012-01-31
- Wasif S (2012) Sri Lanka bans import of Pakistani medicines–The Express Tribune. Tribune.com.pk. Retrieved 2012-02-04
- WHO (1972) International drug monitoring: the role of national centres. Technical report series WHO 1972, no 498 [ PubMed ]
- WHO (2002a) The importance of pharmacovigilance—safety monitoring of medicinal products. http://apps.who.int/medicinedocs/en/d/Js4893e/8.html . Accessed 4 Sep 2011
- WHO (2002b) The importance of pharmacovigilance. www.who.org . Accessed 14 July 2011
- Williams D, Feely J. Underreporting of adverse drug reactions: attitudes of Irish doctors. Ir J Med Sci. 1999; 168 (4):257–261. doi: 10.1007/BF02944353. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- WHO Web site. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/

IMAGES
VIDEO
COMMENTS
Introducing measures to facilitate ADR reporting for both consumer and patients and HCPs can be beneficial. A 2020 systematic review of interventions to improve ADR reporting also concluded that there was scope to include community pharmacies to improve ADR reporting . These findings were also reported in other reviews [20,21].
state reporting requirements (Figure 2). a. Sequester as much evidence and clinical information as possible prior to reporting. b. FDA offers 2 reporting tools, MedWatch27 and the Vaccine Adverse Event Reporting System (VAERS),28 which should be used to report events and allow for tracking of national safety signals. 11. ADR reports should be ...
An adverse drug reaction (ADR) refers to an untoward reaction to a medication. ADRs are common and constitute a significant healthcare burden. The most robust database of ADRs available is the U.S. Food and Drug Administration's Adverse Event Reporting System (FAERS). In 2022, there were over 1.25 million serious adverse events reported and nearly 175,000 deaths.[1] There are 6 emergency ...
FDA Adverse Event Reporting System supports the FDA's post-marketing safety surveillance program for all marketed drug and therapeutic biologic products. It contains adverse event reports FDA has ...
MedWatch is made up of voluntary and mandatory reporting on prescription medicines, over-the-counter medicines, non-vaccine biologicals, medical devices, special nutritional products, cosmetics ...
How to Report. Suspected ADR reporting forms [Figure 1] for healthcare professionals and consumers are available on the website of IPC to report ADR.To remove language barrier in ADR reporting, the consumer reporting form [Figure 2] are made available in 10 vernacular languages (Hindi, Tamil, Telugu, Kannada, Bengali, Gujarati, Assamese, Marathi, Oriya, and Malayalam).
Adverse Drug Reaction Reporting 549 Evaluation of causality determines the medication(s) suspected of causing the ADR. Assessment of the prob-ability of a medication causing an ADR depends on evaluation of six criteria: 1. Event is a documented, known response to the suspected agent 2. Event is not explained by the disease state 3. Timing of ...
We evaluated methods to improve ADR reporting via a systematic literature review. We searched Medline, the Cochrane Library, Embase and the National Library of Health for studies examining methods of improving the reporting of adverse drug reactions published between January 1997 and August 2007. The search strategy used the key MeSH terms and ...
Promote ADR Reporting Culture: Awareness campaigns targeting healthcare professionals, patients, and the general public should be conducted to promote a culture of ADR reporting. These campaigns should emphasize the importance of reporting ADRs, clarify reporting procedures, and raise awareness about the role of pharmacovigilance in ensuring ...
Adverse drug reactions (ADRs) are associated with morbidity and mortality worldwide. Although national systems for reporting ADRs exist there is a low reporting rate. The aim of the current study was to evaluate an intervention plan for improving ADRs reporting among medical professionals (physicians and nurses). A multicentre intervention study was conducted, in which one medical centre was ...
An adverse drug reaction, is distinguished from the adverse event by; the former has a suspicion of a causal relationship between the medicinal product and the reaction, i.e. judged as being at least possibly related to the reaction by the reporting or the reviewing health professional, while
1 Briefing Note Safety of medicines - adverse drug reactions Key facts Unintended, harmful reactions to medicines (known as adverse drug reactions) are among the leading causes of death in many countries. The majority of adverse drug reactions (ADR) are preventable. People in every country are affected by ADRs. In some countries, ADR-related costs such as hospitalization, surgery and lost
Introduction Underreporting is a major limitation of the voluntary reporting system of adverse drug reactions (ADRs). A 2009 systematic review showed the knowledge and attitudes of health professionals were strongly related with underreporting of ADRs. Objective Our aim was to update our previous systematic review to determine factors (sociodemographic, knowledge and attitudes) associated with ...
2.63_ADR_reporting_HCPs_May_2021_v1 Page 6 2 DEFINITIONS These terms may have other meaning under different context but the definitions below are applicable to the context of this document. 2.1 Adverse Drug Reaction (ADR) or Adverse Reaction An adverse drug reaction (ADR) means a noxious and unintended response to a medicine, including lack of
What is an adverse drug reaction? According to information published by the Medicines and Healthcare products Regulatory Agency (MHRA) 'an adverse drug reaction (ADR) is an unwanted or harmful reaction experienced following the administration of a drug or combination of drugs, and is suspected to be related to the drug.
Appendix S1 Search strategies for adverse drug event (ADE) reporting systems. Appendix S2 Combined WHO 'Minimum Recommended Dataset' for ADE reporting and the assessment criteria for spontaneous ADR reporting forms developed by Bandeker for quality assessment of reporting systems used for quality assessment data extraction. Appendix S3 Characteristics of publicly funded reporting systems
Adverse drug reactions (ADRs) remain a global public health concern. Pharmacovigilance practises are essential in ensuring patients safety and post drug marketing surveillance. This study aimed to describe practices, perceptions and barriers towards ADR reporting practices amongst People Living with HIV/AIDS (PLWHA), who are on Highly Active Anti-Retroviral Therapy (HAART) and their doctors.
As per available scientific literature, the major impediments to ADR reporting are inadequate knowledge and awareness among health professionals, clinicians' perceptions towards reporting, problems with establishing reporting systems in hospitals and insufficient training to recognize ADRs. Measures to improve the situation include greater ...
ADR reporting - patient guideline. Did you. know? You can report side effects yourself. As a patient, you have the right to report unwanted side effects of medicines directly to the authorities. You can also report a side effect on behalf of someone in your care, such as a child or relative. Remember to speak to your doctor or pharmacist if you ...
Reporting adverse events using General Practitioner software. Medical practices using the Best Practice or Medical Director software can download and install templates to their software to create Adverse Drug Reaction (ADR) reports. Completed reports can be emailed, faxed or posted to the TGA. Note: the RTF documents below should not be opened ...
All suspected ADR should be reported as soon as possible because over reporting is always better than under reporting. Death event must be reported as soon as possible, while all other serious ADR/event needs to report within 7 days only. All non-serious cases must be reported within 30 days. Reporting delay may create serious problem.
The Internal Revenue Service is concerned that not enough taxpayers are taking advantage of its alternative dispute resolution process for resolving disputes. ... Overall, practitioners want more and better means for reporting. A common view was the desire to see a "perp walk" upon arrest. Tax season wrap-up.
The International Equity ADR composite rose 2.26% in the quarter gross of fees, trailing the 4.81% gain for the MSCI ACWI ex US Index. The portfolio lagged across most regions and sectors, with ...
Our research shows that the preferred means of reporting were telephone or website. Other studies also report these methods as preferential. A study of 500 nurses from a teaching hospital in Teheran showed that among the 10% who reported an ADR, the majority of the nurses preferred using the telephone [ 45 ].
For the Congress, out of its 327 candidates, 143, or 44 per cent, have declared criminal cases, the Association for Democratic Reforms (ADR) said in a report based on data compiled from the ...
Daqo New Energy Corp (ADR)'s ( DQ) price is currently up 10.83% so far this month. During the month of May, Daqo New Energy Corp (ADR)'s stock price has reached a high of $23.00 and a low of $17.93. Over the last year, Daqo New Energy Corp (ADR) has hit prices as high as $43.66 and as low as $17.30. Year to date, Daqo New Energy Corp (ADR ...
The report said the average increase in assets of 16 re-contesting MPs of the Trinamool Congress was 53.84 per cent (Rs 15.69 crore to Rs 24.15 crore), 35.54 per cent for 11 Janata Dal (United ...
1:54. Indonesia's antitrust authorities are investigating Southeast Asia's e-commerce leader Sea Ltd. for potentially unfairly favoring its own delivery service over other alternatives. Online ...
The questionnaire being addressed was adapted from studies regarding concerns of health care professionals about adverse drug reaction reporting and reasons of underreporting these reactions, information and knowledge about reporting of ADR. Health care professionals mostly addressed were physicians, pharmacists and nurses working at public ...
For the sixth phase of the Lok Sabha polls, the Association for Democratic Reforms (ADR) and The National Election Watch analysed the self-sworn affidavits of all 866 contenting candidates and ...