We will keep fighting for all libraries - stand with us!

Internet Archive Audio

- This Just In
- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
Solving problems with NMR spectroscopy
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
![[WorldCat (this item)] [WorldCat (this item)]](https://archive.org/images/worldcat-small.png)
plus-circle Add Review comment Reviews
Download options.
No suitable files to display here.
IN COLLECTIONS
Uploaded by station44.cebu on May 19, 2020
Solving Problems with NMR Spectroscopy (2nd Ed.) By Atta-ur-Rahman, Muhammad Iqbal Choudhary and Atia-tul-Wahab
- Post author By theSpectroscopy
- Post date February 20, 2023
- Categories In Books
- No Comments on Solving Problems with NMR Spectroscopy (2nd Ed.) By Atta-ur-Rahman, Muhammad Iqbal Choudhary and Atia-tul-Wahab

Free download Solving Problems with NMR Spectroscopy (2nd edition) authored by Atta-ur-Rahman, Muhammad Iqbal Choudhary and Atia-tul-Wahab in pdf.
The second edition of the book Solving Problems with NMR Spectroscopy is aimed to strengthen the understanding of how an NMR spectrometer functions. This revised version of the book takes the same problem-solving approach as the highly praised first edition, published in 1996. The book focuses on describing the basic principles of NMR spectroscopy and explains in detail the functioning of an NMR spectrometer.
The optimum use of this powerful technique is introduced step by step, and common problems encountered by the practitioners and users of NMR spectroscopy are described in an easy-to-understand manner. The real strength of the book is its highly practical approach in describing both the concepts and applications of NMR spectroscopy.
The second edition introduces a number of new topics, including developments in NMR hardware, such as cryogenically cooled probes, new probeheads, high-field magnets, and DNP–NMR, as well as innovative pulse sequences, such as DOSY, concatenated NMR techniques, and PANSY.
Particularly interesting is a new chapter on sensitivity issues in NMR spectroscopy and their currently available applications, which have driven most of the developments in this field. Another chapter on recent developments in NMR spectroscopy updates the readers about the changing landscape in this field.
Over 180 penetrating problems and their well-described solutions help to reinforce and test the understanding of the readers about various aspects of modern NMR spectroscopy. Many of these problems focus on developing the interpretation skills of the readers in various types of NMR spectra toward structure determination.
- The Basics of Modern NMR Spectroscopy
- Creating NMR Signals
- Sensitivity Enhancement
- Spin-Echo and Polarization Transfer
- The Second Dimension
- Nuclear Overhauser Effect
- Important 2D NMR Experiments
- Playing with Dimensions in NMR Spectroscopy
- Some Key Developments in NMR Spectroscopy
- Logical Approach for Solving Structural Problems
Free download Solving Problems with NMR Spectroscopy (2nd edition) authored by Atta-ur-Rahman, Muhammad Iqbal Choudhary and Atia-tul-Wahab in pdf from following download link(s).
Sometime download link(s) is/are not visible on mobile devices, so if you face this issue, kindly do visit this page via laptop/desktop computer.
Password for Download Links: thespectroscopy.com
File Size: 49.1 MB | Pages: 521 | Download Instructions | Disclaimer
You may also like to free download:
- NMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural Biology authored by Neil E. Jacobsen in pdf
- NMR Spectroscopy Basic Principles, Concepts and Applications in Chemistry (3rd edition) written by Harald Günther in pdf
- Modern Spectroscopy (4th edition) authored by J. Michael Hollas in pdf
Free download hundreds of best-selling chemistry books in pdf from HERE .
P.S: If the download link(s) is/are not working, kindly drop a comment below, so we’ll update the download link for you.
Happy Reading!
- Tags Chemistry Books , NMR Spectroscopy , Spectroscopy Books
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Organic Spectroscopy
Chem 203 Professor James S. Nowick
Problems from Previous Years' Exams
This archive includes six types of problems from the midterm and final exams of my Chem 203 Organic Spectroscopy class. The first three focus on infrared spectroscopy , mass spectrometry , and 1D NMR spectroscopy . The next focuses on using these three techniques together to determine the structures of organic compounds . The last two categories incorporate 2D NMR spectroscopy and are thus considered "advanced." The advanced spectral analysis problems focusing on analyzing 1- and 2D NMR spectra to address questions of stereochemistry. The advanced structure determination problems focus on using all of these techniques to determine the structures of organic compounds.
INFRARED (IR) SPECTROSCOPY PROBLEMS
2014 Midterm Exam Part I.1. ( 2014-MT-I.1.pdf ) Problem Type: Match aromatic compounds with IR spectra. Techniques: IR spectroscopy . Notes: A set of compounds with unusual functional groups. One of my favorites.
2013 Midterm Exam Part I.1. ( 2013-MT-I.1.pdf ) Problem Type: Match aromatic compounds with IR spectra. Techniques: IR spectroscopy . Notes: A set of aromatic compounds with similar structures but different functional groups.
2012 Midterm Exam Part I.1. ( 2012-MT-I.1.pdf ) Problem Type: Match aromatic compounds with IR spectra. Techniques: IR spectroscopy . Notes: A set of aromatic compounds with carbonyl and other functional groups.
2011 (fall) Midterm Exam Part I.1. ( 2011f-MT-I.1.pdf ) Problem Type: Match aromatic compounds with IR spectra. Techniques: IR spectroscopy . Notes: A set of aromatic compounds bearing different functional groups.
MASS SPECTROMETRY (MS) PROBLEMS
2014 Midterm Exam Part I.2. ( 2014-MT-I.2.pdf ) Problem Type: Interpret peaks of a large molecule, maitotoxin, in negative and positive ion modes Techniques: Negative ion FAB and ESI mass spectrometry . Notes: Concepts in mass, charge, and isotopomers. One of my favorites
2013 Midterm Exam Part I.2. ( 2013-MT-I.2.pdf ) Problem Type: Interpret peaks in an ESI mass spectrum. Techniques: ESI mass spectrometry . Notes: This is modern ESI MS problem that focuses on the concepts of mass, charge, and molecular formula.
2012 Midterm Exam Part I.2. ( 2012-MT-I.2.pdf ) Problem Type: Interpret peaks in EI and ESI mass spectra. Techniques: EI and ESI mass spectrometry . Notes: Concepts in fragmentation, isotope patterns, and molecular ions.
2011 (fall) Midterm Exam Part I.3. ( 2011f-MT-I.3.pdf ) Problem Type: Identify the transition metal complex from the isotope pattern. Techniques: EI mass spectrometry . Notes: Electron ionization (EI) mass spectra are shown for five transition metal acetylacetonate (acac) complexes.
NUCLEAR MAGNETIC RESONANCE (NMR) SPECTROSCOPY PROBLEMS
2014 Midterm Exam Part I.3. ( 2014-MT-I.3.pdf ) Problem Type: Interpret the 1 H NMR spectrum of ( S )-glycidyl benzyl ether. Techniques: 1 H NMR spectroscopy . Notes: This problem gets to the heart of coupling and diastereotopicity. It is one of my all-time favorites.
2013 Midterm Exam Part I.3. ( 2013-MT-I.3.pdf ) Problem Type: Match regioisomeric aromatic compounds with 1 H NMR spectra. Techniques: 1 H NMR spectroscopy . Notes: This is a great little matching problem that gets to the heart of pattern recognition, coupling, and symmetry in 1 H NMR spectroscopy.
2013 Midterm Exam Part I.4. ( 2013-MT-I.4.pdf ) Problem Type: Stereochemical determination by 1 H NMR spectroscopy. Techniques: 1 H NMR spectroscopy . Notes: This problem builds on some of my favorite concepts in sterochemical determination in a cyclohexane ring system (2-phenyl-1-cyclohexanol) from the coupling pattern.
2012 Midterm Exam Part I.3. ( 2012-MT-I.3.pdf ) Problem Type: Match the eight constitutional isomeric alcohols C 5 H 12 O with 1 H NMR and 13 C NMR spectra. Techniques: 1 H NMR and 13 C NMR spectroscopy . Notes: A challenging matching problem that probes concepts of chemical equivalence and symmetry in 1 H NMR spectroscopy. One of my favorites.
2011 (fall) Midterm Exam Part I.2a. ( 2011f-MT-I.2a.pdf ) Problem Type: Match the regioisomers of dinitrophenol with 1 H NMR spectra. Techniques: 1 H NMR and 13 C NMR spectroscopy . Notes: A matching problem that probes concepts of chemical equivalence and symmetry in 1 H NMR spectroscopy.
2011 (fall) Midterm Exam Part I.2b. ( 2011f-MT-I.2b.pdf ) Problem Type: Match the regioisomers of methylpyridine with 1 H NMR spectra. Techniques: 1 H NMR and 13 C NMR spectroscopy . Notes: A matching problem that probes concepts of chemical equivalence and symmetry in 1 H NMR spectroscopy.
BASIC STRUCTURE DETERMINATION PROBLEMS
2014 Midterm Exam Part II.1. ( 2014-MT-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS; IR (thin film on NaCl plates); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: A small but challenging molecule. One of my favorites.
2014 Midterm Exam Part II.2. ( 2014-MT-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS; IR (thin film on NaCl plates); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: This problem was designed to build on concepts of 1 H NMR non-first-order coupling pattern recogntion and symmetry.
2014 Midterm Exam Part II.3. ( 2014-MT-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS; IR (thin film on NaCl plates); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: A simple but challenging molecule with a rich 1 H NMR spectrum.
2014 Midterm Exam Part II.4. ( 2014-MT-II.4.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS; IR (KBr pellet); 500 MHz 1 H NMR in CD 3 SOCD 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CD 3 SOCD 3 . Notes: This problem was designed to build on concepts of 1 H NMR coupling pattern recogntion and symmetry.
2013 Midterm Exam Part II.1. ( 2013-MT-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; CI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in C 6 D 6 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in C 6 D 6 . Notes: We designed this molecule to illustrate principles of coupling patterns in the 1 H NMR spectrum and isotope patterns in the mass spectrum.
2013 Midterm Exam Part II.2. ( 2013-MT-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS and CI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in C 6 D 6 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in C 6 D 6 .
2013 Midterm Exam Part II.3. ( 2013-MT-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in C 6 D 6 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in C 6 D 6 . Notes: A pretty spectrum with interesting coupling patterns. One of my favorites.
2013 Midterm Exam Part II.4. ( 2013-MT-II.4.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: A small but challenging molecule. One of my favorites.
2012 Midterm Exam Part II.1. ( 2012-MT-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; EI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: Concepts in pattern recognition, symmetry, and diastereotopicity.
2012 Midterm Exam Part II.2. ( 2012-MT-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; EI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in CD 3 SOCD 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CD 3 SOCD 3 . Notes: We designed this molecule to illustrate principles of coupling patterns in the 1 H NMR spectrum and isotope patterns in the mass spectrum.
2012 Midterm Exam Part II.3. ( 2012-MT-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; EI-MS (low resolution/accuracy); IR (solution in CHCl 3 in a 0.1 mm CaF 2 cell); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: A small but challenging molecule.
2012 Midterm Exam Part II.4. ( 2012-MT-II.4.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; EI-MS (low resolution/accuracy); IR (thin film on NaCl plates); 500 MHz 1 H NMR in CDCl 3 ; 125.8 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: Concepts in pattern recognition, symmetry, and diastereotopicity.
2011 (fall) Midterm Exam Part II.1. ( 2011f-MT-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; EI-MS (low resolution/accuracy); IR (thin film on salt plates); 500 MHz 1 H NMR in CDCl 3 ; 125.6 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: Concepts in pattern recognition and spin-spin coupling.
2011 (fall) Midterm Exam Part II.2. ( 2011f-MT-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: Exact mass; ESI-MS (low resolution/accuracy); IR (solution in CH 2 Cl 2 in a 0.1 mm CaF 2 cell); 400 MHz 1 H NMR in CDCl 3 ; 100.6 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: Concepts in pattern recognition and spin-spin coupling.
2011 (fall) Midterm Exam Part II.3. ( 2011f-MT-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Techniques: EI-MS (low resolution/accuracy); IR (thin film on salt plates); 500 MHz 1 H NMR in CDCl 3 ; 125.6 MHz 13 C NMR, DEPT 90, and DEPT 135 in CDCl 3 . Notes: A small molecule with interesting IR and NMR spectra.
ADVANCED SPECTRAL ANALYSIS PROBLEMS
2014 Final Exam Part I. ( 2014-F-I.pdf ) Problem Type: Assignment of NMR resonances and stereochemical analysis. Compound Information: Two diastereomeric L-hexopyranose pentaacetates. Techniques: 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT, COSY, HMQC, NOESY, and a 1D NOE experiment . Notes: This problem focuses on conformational and stereochemical analysis in two diastereomeric L-hexopyranose pentaacetates.
2013 Final Exam Part I. ( 2013-F-I.pdf ) Problem Type: Assignment of NMR resonances and stereochemical analysis. Compound Information: A pentacyclic compound. Techniques: 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT, COSY, TOCSY (20 ms mixing time), HMQC, HMBC, NOESY, and HSQC-TOCSY spectra with 5-, 10-, 25-, and 50-ms mixing times . Notes: This problem focuses on conformational and stereochemical analysis in a system of fused cyclohexane rings.
2012 Final Exam Part I. ( 2012-F-I.pdf ) Problem Type: Assignment of NMR resonances and stereochemical analysis. Compound Information: A tricyclic compound. Techniques: 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT, COSY, TOCSY, HMQC, HMBC, NOESY, and HSQC-TOCSY spectra with 5-, 10-, 20-, and 100-ms mixing times . Notes: This problem focuses on conformational and stereochemical analysis in a fused 5,6 ring system. It is a great showcase for HSQC-TOCSY, which helps tremendously in assignment of the resonances.
2011 (fall) Final Exam Part I. ( 2011-F-I.pdf ) Problem Type: Assignment of NMR resonances and stereochemical analysis. Compound Information: A tricyclic compound. Techniques: 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT, COSY, TOCSY, HMQC, HMBC, NOESY, and HSQC-TOCSY spectra with 5-, 10-, 20-, and 100-ms mixing times. . Notes: This problem focuses on conformational and stereochemical analysis in a fused 5,6 ring system. It is a great showcase for HSQC-TOCSY, which helps tremendously in assignment of the resonances.
ADVANCED STRUCTURE DETERMINATION PROBLEMS
2014 Final Exam Part II.1. ( 2014-F-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 10 H 17 NO 2 . Techniques: IR (thin film from CHCl 3 on salt plates), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, TOCSY (100 ms mixing time), HMQC, HMBC, and NOESY, and a 1D NOE experiment . Notes: This problem proved surprisingly challenging in spite of the small size of the molecule. It was the most popular problem of the 2014 final exam Part II problems.
2014 Final Exam Part II.2. ( 2014-F-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 18 H 21 NO 4 . Techniques: IR (KBr pellet), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, TOCSY (60 ms mixing time), HMQC, HMBC, NOESY, and HSQC-TOCSY spectra with increasing mixing times (5, 10, 25, and 50 ms) . Notes: This was the hardest and least popular of the 2014 final exam Part II problems. It is an beautiful and complex molecule with a disperse 1 H NMR spectrum with interesting resonances.
2014 Final Exam Part II.3. ( 2014-F-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 17 H 22 O 3 . Techniques: IR (KBr pellet), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, TOCSY (150 ms mixing time), HMQC, HMBC, NOESY, and select regions of the HSQC-TOCSY spectra with increasing mixing times (5, 10, 25, and 50 ms) . Notes: This problem was the second most popular of the 2014 final exam Part II problems. There is moderate overlap of the 1 H NMR resonances. The TOCSY and 50-ms HSQC-TOCSY spectra nicely illuminate the major spin systems.
2013 Final Exam Part II.1. ( 2013-F-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 18 H 25 NO. Related to codeine. Techniques: IR (solution in CHCl 3 ), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY (150 ms mixing time) and NOESY . Notes: The easiest of the 2013 final exam Part II problems. It is also my favorite. Although the molecule is large the problem is very workable and satisfying. I had been wanting to introduce it for a number of years, but it was only in 2013 that we were able implement it.
2013 Final Exam Part II.2. ( 2013-F-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 16 H 25 NO 2 . Related to codeine. Techniques: IR (solution in CHCl 3 ), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY (150 ms mixing time) and NOESY. Also included are select regions of the HSQC-TOCSY spectra with increasing mixing times . Notes: The hardest and least popular of the 2013 final exam Part II problems.
2013 Final Exam Part II.3. ( 2013-F-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 16 H 24 O. Techniques: IR (solution in CHCl 3 ), 500 MHz 1 H NMR, 125.8 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, TOCSY (20 ms mixing time), HMQC, HMBC, 1D NOE (irradiation at 3.36 ppm), and NOESY . Notes: This problem was the most popular of the 2013 final exam Part II problems.
2012 Final Exam Part II.1. ( 2012-F-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 17 H 19 ClO. Techniques: IR (solution in CHCl 3 ), 600 MHz 1 H NMR, 150.9 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY and NOESY . Notes: This was the second most popular of the 2012 final exam Part II problems. In spite of the larger size of the molecule (compared to the other two problems) it is manageable.
2012 Final Exam Part II.2. ( 2012-F-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 9 H 10 O 4 . Techniques: IR (thin film from CHCl 3 solution on NaCl plates), 500 MHz 1 H NMR, 125.7 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY and NOESY . Notes: The hardest and least popular of the 2012 final exam Part II problems. Although the molecule is small, it is challenging.
2012 Final Exam Part II.3. ( 2012-F-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 10 H 19 N. Techniques: IR (thin film on NaCl plates), 500 MHz 1 H NMR, 125.7 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY and NOESY . Notes: This was the most popular of the 2012 final exam Part II problems. In spite of the small size of the molecule, its structure is actually quite challenging, in part due to W-coupling seen in the COSY.
2011 (fall) Final Exam Part II.1. ( 2011f-F-II.1.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 15 H 22 O 2 . Techniques: IR (Thin Film on NaCl), 500 MHz 1 H NMR, 125.7 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY and NOESY . Notes: This was the easiest and most popular of the 2011 fall final exam Part II problems.
2011 (fall) Final Exam Part II.2. ( 2011f-F-II.2.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 16 H 22 O. Techniques: IR (Thin film on NaCl), 400 and 500 MHz 1 H NMR, 125.7 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY, and NOESY . Notes: This was my favorite among the 2011 fall final exam Part II problems.
2011 (fall) Final Exam Part II.3. ( 2011f-F-II.3.pdf ) Problem Type: Structure determination and assignment of NMR resonances. Compound Information: Molecular formula C 10 H 16 O. Techniques: IR (Thin Film on NaCl), 500 MHz 1 H NMR, 125.7 MHz 13 C NMR, DEPT-90, DEPT-135, COSY, HMQC, HMBC, TOCSY, and NOESY . Notes: In spite of the small size of this molecule, it was the hardest and most frustrating of the 2011 fall final exam Part II problems Only a few of those who attempted it got the correct structure and stereochemistry.

Chemistry Steps

Organic Chemistry
Organic structure determination, nmr practice problems .
In the following examples, we will learn how to solve NMR practice problems step-by-step in over 100 min video solutions which is essential for organic structure determination.
The emphasis is on the 1 H proton NMR and most problems are based on understanding its key principles such as the number of NMR signals , integration , signal splitting (multiplicity) , and, of course, the strategies of putting all of these together to come up with the correct structure.
Aside from the 1 H NMR , we will also go over determining the hydrogen deficiency index (HDI), solving problems where the 13 C NMR, DEPT , and IR are given along with the 1 H NMR spectrum.
The problems are chosen to demonstrate the most common patterns in 1 H NMR spectroscopy , as well as, the situations where you need to consider the possibility of signal overlapping , incorrect absolute values of integrations , as the instrument measures only the relative area for each peak, examples where fairly large molecules give rise to spectra with few signals because of the symmetry elements . We will also discuss the purpose of shaking the sample with deuterated solvents.
How to determine the structure of an unknown compound using NMR spectroscopy
Two things you will need to do very often:
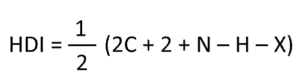
- IR – It will be very beneficial for you to remember key signals in IR spectroscopy so that you can identify the functional groups. Solving IR problems – here .
Before you start working on the first problem or getting stuck on one, let’s go ahead and summarize the most common patterns that you need to recognize.

Common Splitting Patterns in NMR spectroscopy
Start by analyzing the aliphatic region. Many molecules have at least one methyl group and depending on its environment, common splitting patterns are observed.
A triplet with an integration of three represents a methyl with an adjacent CH 2 group. So, look for a triplet and a quartet with integrations 3 and 2 which indicates an ethyl group ( Figure 1 a ).

If there is a singlet with an integration of 3, this represents an isolated methyl group ( b ) . On the other hand, if the singlet integrates to 9 protons, this indicates a tert-Butyl group ( c )
Another common fragment is the combination of a doublet (6H) and a septet ( d ) . This indicates an isopropyl group where the methyl protons are split into a doublet by one adjacent proton and the six equivalent protons of the methyl groups split the signal of that one proton into a septet.
If you see a bunch of triplets each integrating to two protons, think about the -CH 2 -CH 2 – ethylene group ( e ) .
Other Regions and Splitting Patterns
Alkene protons appear at ~5-6 ppm and you can recognize them by the small integration (1 or 2) and complex splitting. This is because all the protons on a double bond are capable of splitting each other and the coupling constants vary anywhere from 5-18 Hz.

Aromatic protons appear at the ~7-8 ppm region. If you have a signal there, the first thing is to check how many groups (or how many protons) are on the aromatic ring. And this is done simply by looking at the integration:

One common splitting pattern in the aromatic region is the presence of two doublets each integrating to two protons. This indicates a symmetrically substituted ring with two groups (1, 4- or para – substituted).

Most other substitution patterns give complex splitting and the easiest for you will be looking at the total integration of all the aromatic signals.
Next functional group recognizable in 1 H NMR spectroscopy is the ~10 ppm signal of aldehydes. Usually, it shows up as a singlet, though splitting with adjacent protons is not uncommon either:

Another way of identifying aldehydes and ketones as well as checking for a ~200 ppm signal on the 13 C NMR. Esters and carboxylic acids appear less downfield – 160-180 ppm.
The OH peak
Like for the IR spectroscopy, the OH peak is a good indicator here as well. Look for a broad peak anywhere from 1-6 ppm. Most often though it will be in the 4-6 range.

Remember also that the OH signal is not split by adjacent protons unless the sample is very dry.
Other groups that give broad, and sometimes, deuterium-exchangeable signals are the amines, amides, and thiols.
Carboxylic acids
If you see a broad signal at 12 ppm, 90% of the time it tells you about a carboxylic acid.

In the following NMR practice problems, we will go over the best strategies you can use for identifying the structure of unknown compounds. As a Chemistry Steps Prime member, you will also get access to the Spectroscopy Summary Sheets in addition to these over 100 min videos of solving NMR problems.
NMR and IR Spectroscopy Summary Sheets
1. How to Solve IR Problems in 3 steps!
2. NMR Spectroscopy – 5 pages that include
- Chemical Shift and Integration
- Number of NMR signals
- Spin-Spin Splitting
- 13 C NMR Spectroscopy
- Determining the Structure of an Unknown
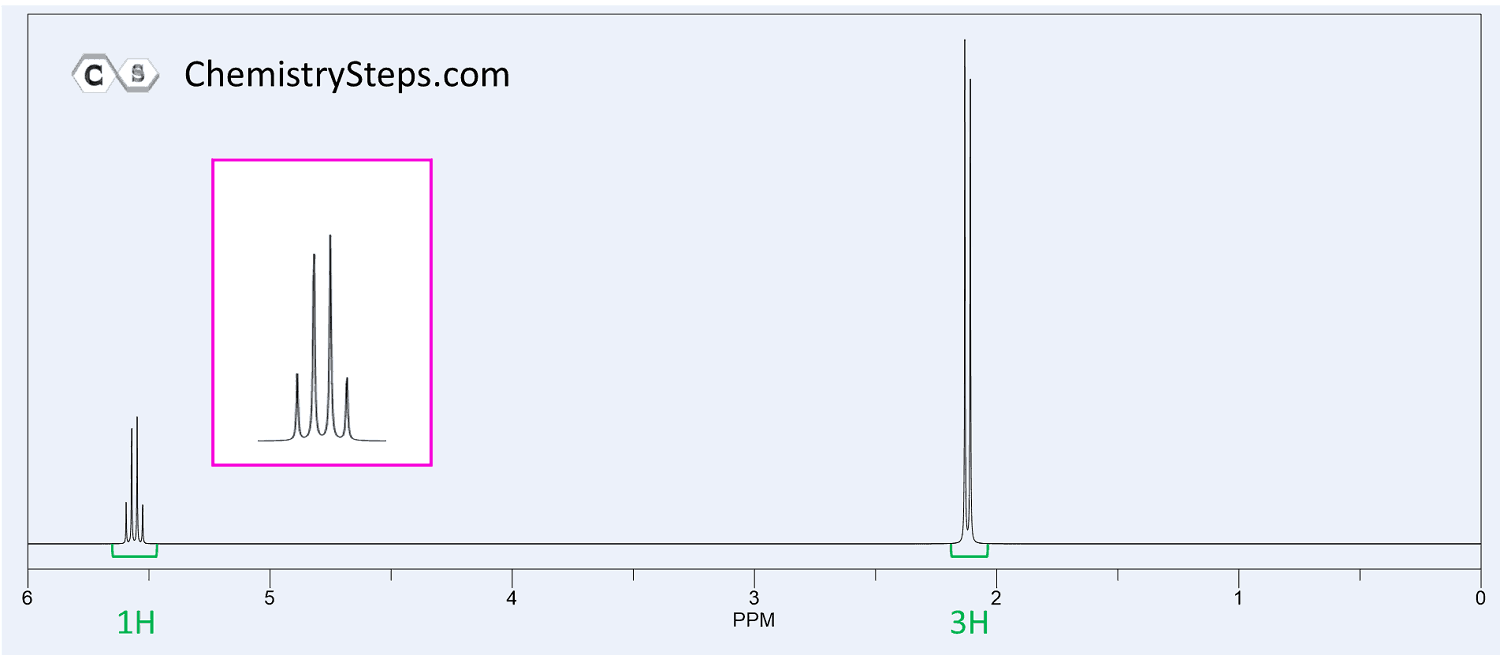
The 1 H NMR spectrum of compound X ( C 4 H 8 O 2 ) is shown below. It also shows a strong IR absorption band near 1730 cm −1 . Propose a structure for X .

This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and t he powerful set of Organic Chemistry 1 and 2 Summary Study Guides .
The 1 H NMR spectrum of compound X ( C 2 H 3 Cl 3 ) is shown below. Propose a structure for X.

The 1 H NMR spectrum of compound X ( C 2 H 4 Cl 2 ) is shown below. Propose a structure for X.
The 1 H NMR and 13 C spectra of compound X ( C 5 H 10 Cl 2 ) are shown below. Propose a structure for X.
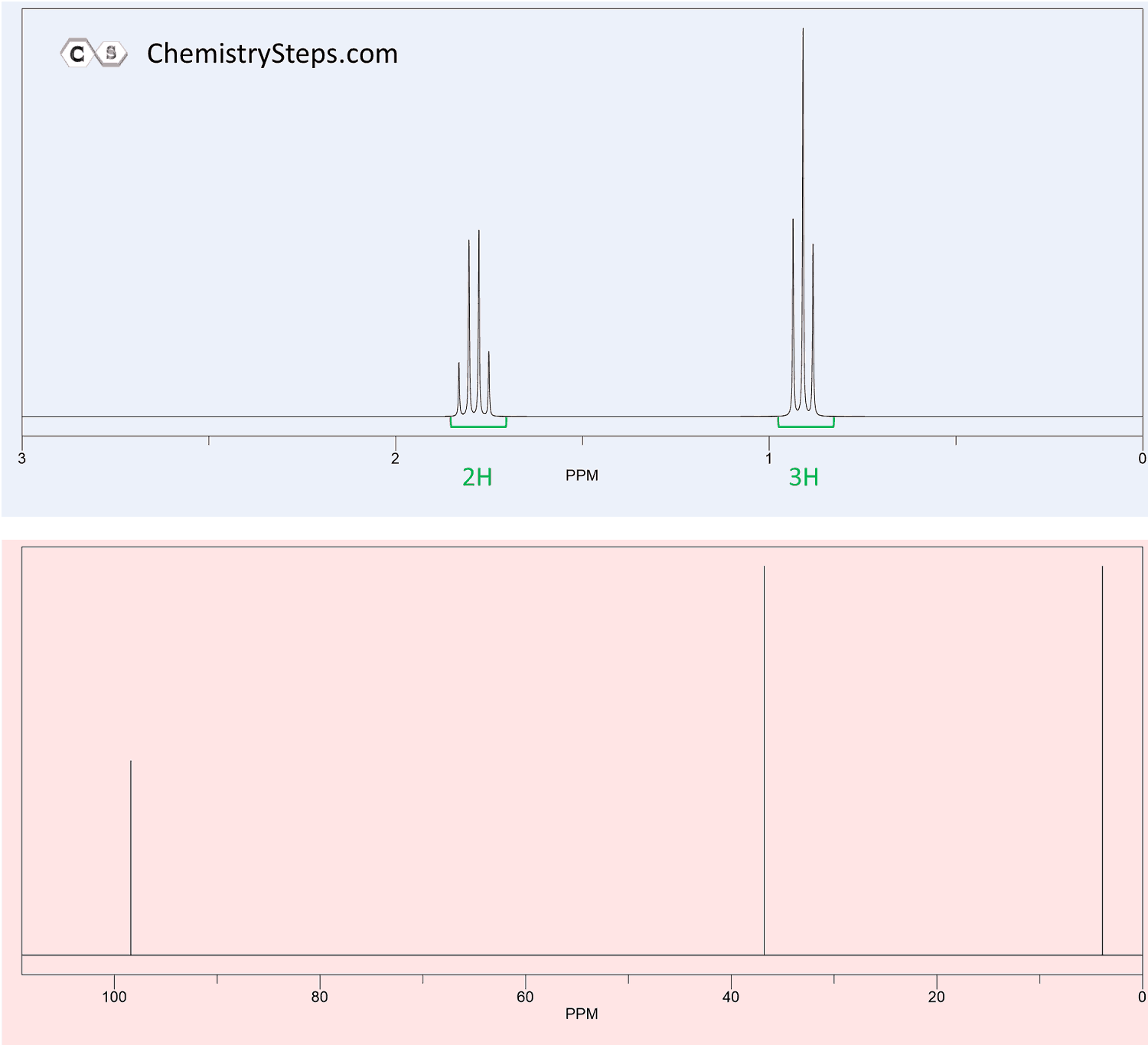
The 1 H NMR and 13 C spectra of compound X (C 6 H 12 O 2 ) are shown below. Propose a structure for X .

The 1 H NMR and 13 C spectra of compound X ( C 10 H 12 O 2 ) are shown below. The 13 C DEPT techniques were also used to identify the carbon types. Propose a structure for X.

The 1 H NMR of compound X ( C 9 H 10 O 2 ) is shown below. Propose a structure for X.

The 1 H NMR and 13 C spectra of compound X ( C 5 H 10 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR and 13 C spectra of compound X ( C 4 H 6 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR of compound X ( C 5 H 8 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR of compound X ( C 5 H 10 O 2 ) are shown below. Propose a structure for X.

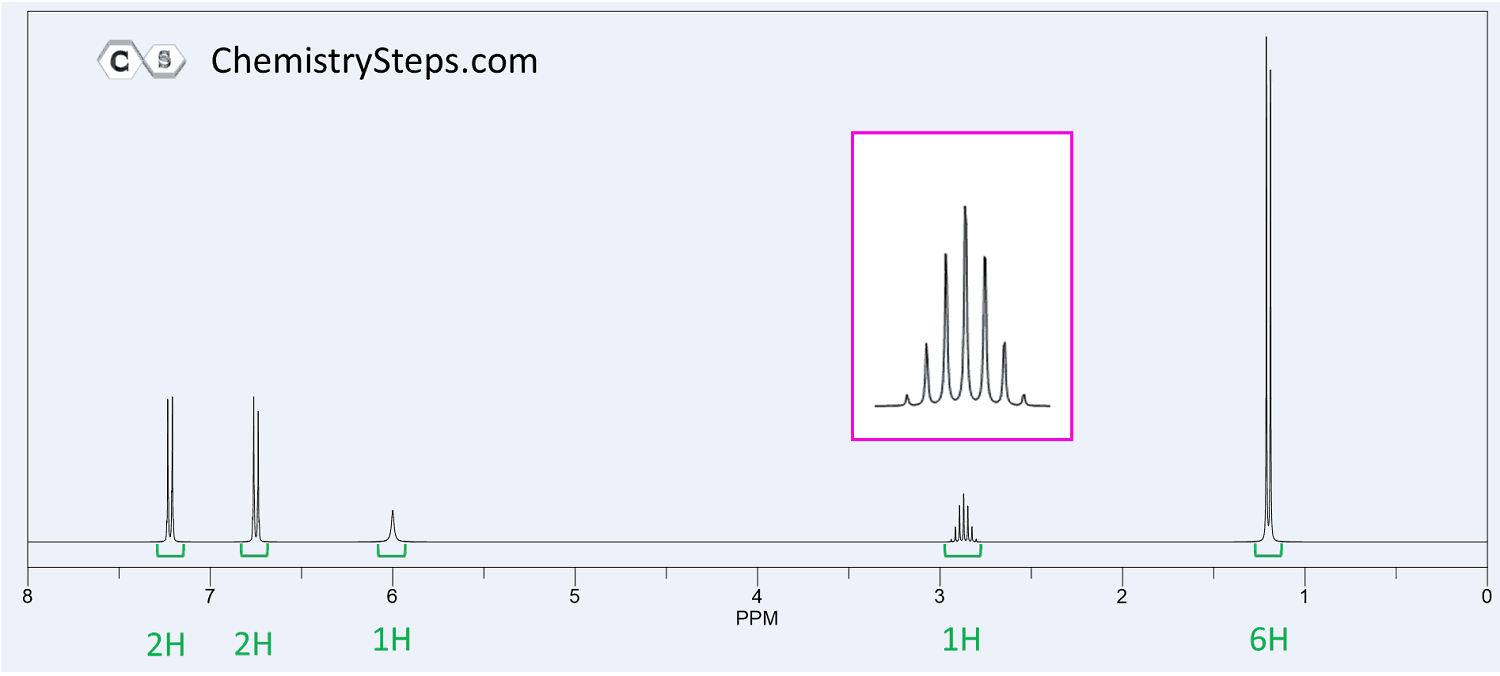
The 1 H NMR of compound X ( C 9 H 12 O ) are shown below. When the sample is mixed and shaken with an excess of deuterium oxide, the signal at 6 ppm disappears. Propose a structure for X.
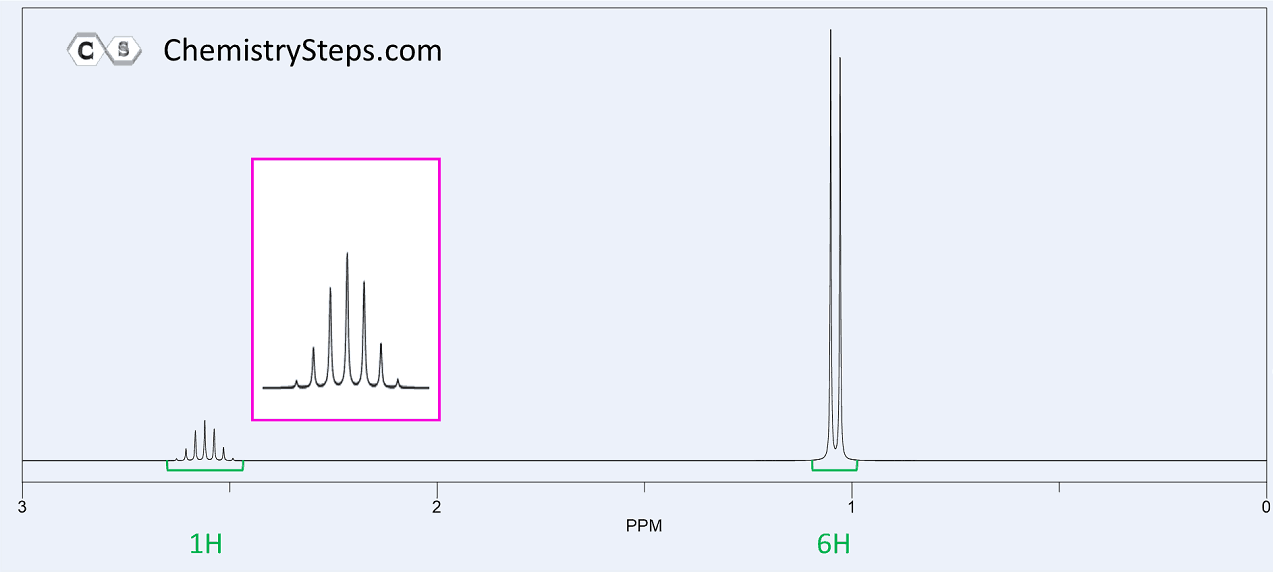
The 1 H NMR of compound X ( C 7 H 14 O ) are shown below. Propose a structure for X.
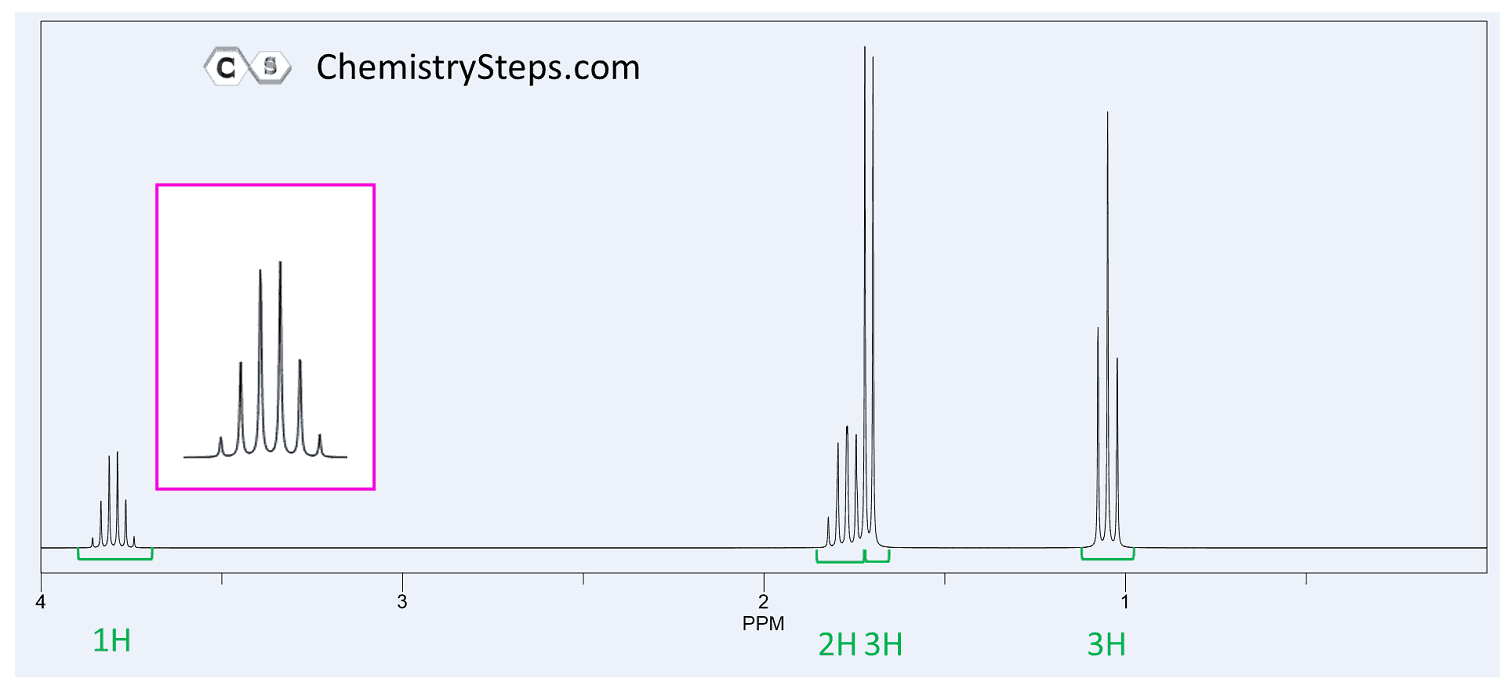
The 1 H NMR and 13 C spectra of compound X ( C 8 H 10 O ) together with its IR spectrum are shown below. Propose a structure for X.

The 1 H NMR of compound X ( C 4 H 9 Br ) are shown below. Propose a structure for X.
The 1 H NMR spectra of three isomers of Butanol are shown below. Draw the structures of all the isomers for butanol and assign each spectrum to the correct isomer.

- NMR spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13 C NMR NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
6 thoughts on “NMR Practice Problems – Solving Strategies”
Thank you! These strategies are great and I feel more optimistic about NMR questions. Hopefully I won’t get them though… : )
I hope you got the NMR right on the test.
Super helpful for understanding NMR. Thank you!
Great to hear, Pokharel.
This was great! Thank you
You are welcome, Andrew.
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
Have we been helpful? Please let us know in the Reviews section here.
WassUp 1.9.4.5 timestamp: 2024-05-14 01:19:22AM UTC (08:19PM) If above timestamp is not current time, this page is cached.
When you look at an NMR spectrum, do you see only a bunch of disordered lines or peaks? Then you have come to the right place. This site was established to provide people interested in NMR with a library of NMR spectroscopy problems. Interpretation of spectra is a skill that requires pattern recognition and/or practice to master the chaos. This site provides one dimensional spectra of different nuclei, COSY, HSQC, HMBC and some less common spectra of various compounds for you to interpret, together with worked solutions. Hopefully, these problems will provide a useful resource to help you better understand NMR spectral interpretation.
A series of about 50 problems is available in printable form. The print version will not be developed further. The PDF documents are still available (german only). Get them here:
The step-by-step approach works nearly perfectly on the desktop and most tablets. On cellular phones, PowerPoint documents are sometimes displayed incorrectly. There is a workaround, but it is unfortunately much too complicated to be practical. As an alternative, all documents can be saved locally. Feel free to follow the download links. A free PowerPoint viewer is available in both the iOS and Android AppStores. As a bonus, there would be no need for a permanent internet connection. Of course, this offline method also works with desktop computers.
The author is always grateful for criticism, suggestions, references to errors and information about the design via email:
The OChem Whisperer
Guide to Solving NMR Questions
How to solve any nmr question.
Solving NMR questions is easier than you think. All you need is a step-by-step process to help guide you through each question. And here it is…
Most NMR questions on an exam involve determining a specific structure rather than memorizing and repeating various NMR values. Typically, you will be given an NMR spectra and a molecular formula (sometimes an IR spectra will be provided). I have put together a few ideas that might make this process a bit easier. I am in the process of putting together a more concise document than this as a study aid. This post is meant to walk you through the thought process of how to tackle this type of problem. The description is a bit long (….so hold on!), but once you get it, you can just use the algorithm to solve your NMR problems. Here are some reference values and a couple of proton NMR spectra:
Proton NMR Reference Values

(cem.msu.edu)

(process-nmr.com)

(Our example 1H NMR spectra for this post; unknown source)
Start with an algorithm to get you on track
When staring an NMR question, you can use the following algorithm to help guide you through the thought process:

(above should say C2H5Cl = C2H6)
Calculate Degrees of Freedom
We notice the first thing says calculate degrees of unsaturation… what is that ? This allows us to determine if there are any double bonds or rings (cyclic structures) in the compound. Let’s look at an example; the formula is C 4 H 8 O 2 . Now, we need to compare this formula with the formula of a completely saturated hydrocarbon (all single bonds…no double bonds):
This formula tells us how many hydrogens we need to have a carbon compound with NO double bonds or rings. Let’s look at our example…
If I have a compound with 4 carbons, this 4 refers to n. Now, if we plug this in the formula, we get
C 4 H 2(4)+2 = C 4 H 10
This says that if we have a compound with only 4 carbons, we need 10 hydrogens to have a compound with no double bonds or rings. If we look at our example, we have C 4 H 8 O 2 . For now, all we need to look at is the C 4 H 8 when dealing with degrees of unsaturation (we will discuss what to do with heteroatoms a bit later). What we now want to do is subtract the hydrogens in our example ( C 4 H 8 ) from the saturated formula ( C 4 H 10 ):
C 4 H 10 – C 4 H 8 = 2 hydrogens
This leaves us with a value of 2. Now, the last things we do to get our degrees of unsaturation is divide this number by 2:
How to use the degrees of unsaturation to get the answer
This leaves us with 1; therefore, we have 1° of unsaturation. So, what does this mean? Each degree of unsaturation equates to a double bond or ring. Here are a few examples to further clarify:
1° of unsaturation = 1 double bond or 1 cyclic structure
2° of unsaturation = 2 double bonds; 1 alkyne; 1 double bond and 1 cyclic structure; 2 cyclic structures
3° of unsaturation = 3 double bonds; 2 double bonds and 1 cyclic structure; etc…
When you see 4° of unsaturation, think benzene; 3° of unsaturation for the 3 double bonds and 1° of unsaturation for the ring.
In our example, it means we have one double bond or one cyclic structure in our compound. Let’s look at few ring systems:

The larger the ring, the more stable the ring (with this series). Three and four-membered rings are rare. Usually, you will hear more about 5- and 6-membered rings. Since this is the case, we more than likely have a double bond (but never rule out a ring until you have looked at the NMR spectra).
Next, if we look at the algorithm, we need to consider the other atoms (other than carbon and hydrogen) in our formula…oxygen. Since we have degrees of freedom…think carbonyl. That is all we can do for now with our algorithm. Let’s now look at our spectra and see if we can start to determine the structure from the peaks:
Interpreting the spectra – splitting patterns

We have three peaks: a quartet around 4 ppm; a singlet around 2 ppm; and a triplet around 1 ppm. At this point, let’s review singlet, doublet, triplet, quartet, and multiplet:

What do these peaks refer to and how to we get the specific peak pattern? Well, we use the n+1 rule to figure out the pattern:

Putting the fragments together
Once you have your fragments, it is a matter of figuring out how to put them together. By looking at the spectra and where the peaks show up (ppm), you can figure out how the fragments go together.

Click NMR pictures to see the images as a PDF.
Don’t forget IR Spectroscopy!
Here is a simple guide showing you the most common IR values
Share this:
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Notify me of new posts by email.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.7: 2-D NMR Problems
- Last updated
- Save as PDF
- Page ID 432217
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Solve unknown problems using a variety of spectra and the molecular formula.
Exercise \(\PageIndex{1}\)
Propose a structure using the spectral data below for C 10 H 14 O. Note: You may need to check for solvent peaks. This sample was dissolved in CDCl 3 .

COSY:

NOESY:

13 C:

HSQC:

HMBC:

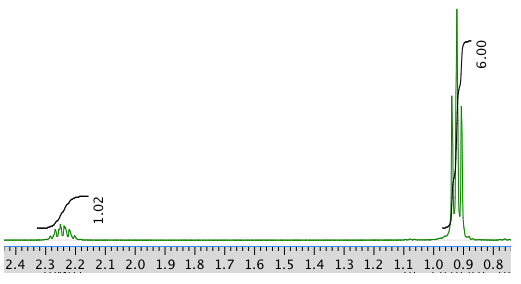
Exercise \(\PageIndex{2}\)
Present an analysis of the following data and propose a structure.
MW: 131 amu
The full 1 H NMR spectrum in D 2 O:
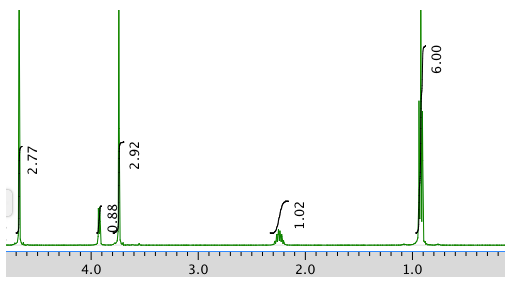
An expansion:
Example \(\PageIndex{3}\)
Propose a structure using the spectral data below for C 10 H 14 O. Note: You may need to check for solvent peaks. This sample was dissolved in CDCl 3 .
Mass Spectrum:


IMAGES
VIDEO
COMMENTS
Solving Problems with NMR Spectroscopy presents the basic principles and applications of NMR spectroscopy with only as much math as is necessary. It shows how to solve chemical structures with NMR by giving clear examples and solutions. This text will enable organic chemistry students to choose the most appropriate NMR techniques to solve ...
More emphasis on practical aspects of NMR spectroscopy, such as the use of Shigemi tubes and various types of cryogenic probes. Over 100 new problems and questions addressing the key concepts in NMR spectroscopy. Improved figures and diagrams. More than 180 example problems to solve, with detailed solutions provided at the end of each chapter.
Save as PDF Page ID 23530 ... Molecular skeleton is built up using 2-dimensional NMR spectroscopy. Relative configuration is predicted by coupling constant (3 J). ... Step by Step guide to solving NMR problems. 12.08 Solving NMR spectra is shared under a CC BY-NC-SA 4.0 license and was authored, ...
Organic Chemistry 307 - Solving NMR Problems - H. D. Roth. Now we go to high field (right): there is a triplet (3 H) at 0.9 ppm; 3 H at high field is almost always a methyl group. The signal is a triplet (n + 1 = 3); therefore, the methyl group must have (n =) 2 1H neighbors; that must be a CH2group. The CH2 signal is a quartet (n + 1 = 4 ...
Determine the degree of unsaturation for the compound. Assign the five pertinent peaks in the IR spectrum. Suggest a structure for compound W based on the spectra given. Show all your work. Place your final answer in the box provided below. Only a molecule placed is this box will receive credit! 5. 2.
1. Compound W has an empirical formula of C11H10O2. Given are the following spectra. Show all your work (= label peaks in the spectra!) Determine the degree of unsaturation for the compound. Assign the six pertinent peaks in the infrared spectrum. Suggest a structure for compound W based on the spectra given. Place your final answer in the box ...
2017. TLDR. An overview of the critical 1H-, 13C- and 31P-NMR parameters for structural and analytical investigations and selected analytical and structural studies with emphasis in the identification of major and minor unsaturated fatty acids in complex lipid extracts without the need for the isolation of the individual components. Expand.
Solving Problems with NMR Spectroscopy, Second Edition, is a fully updated and revised version of the best-selling book. This new edition still clearly presents the basic principles and applications of NMR spectroscopy with only as much math as is necessary. It shows how to solve chemical structures with NMR by giving many new, clear examples for readers to understand and try, with new ...
Solving Problems with NMR Spectroscopy Atta-ur-Rahman and Muhammad Iqbal Choudhary H.E.J. Research Institute of Chemistry ... 7.1 SELECTrVE PULSES IN MODERN NMR SPECTROSCOPY 365 7.2 ONE-DIMENSIONAL EXPERIMENTS USING SOFT PULSES 366 7.2.1 1D COSY 367 7.2.2 1D Relayed COSY 368 7.2.3 SELINQUATE 369
Description. Solving Problems with NMR Spectroscopy presents the basic principles and applications of NMR spectroscopy with only as much math as is necessary. It shows how to solve chemical structures with NMR by giving clear examples and solutions. This text will enable organic chemistry students to choose the most appropriate NMR techniques ...
Free download Solving Problems with NMR Spectroscopy (2nd edition) authored by Atta-ur-Rahman, Muhammad Iqbal Choudhary and Atia-tul-Wahab in pdf from following download link(s). Sometime download link(s) is/are not visible on mobile devices, so if you face this issue, kindly do visit this page via laptop/desktop computer.
Chapter 13 Problem Set 7/8 9) For each of the compounds below, draw a rough estimation of its expected 1H NMR spectrum. Challenge Problems: 10)Use the spectral data provided to predict the product of the reaction shown below. 11) The vinyl proton chemical shift in the two compounds shown below is substantially different.
Techniques: 1 H NMR spectroscopy. Notes: This is a great little matching problem that gets to the heart of pattern recognition, coupling, and symmetry in 1 H NMR spectroscopy. 2013 Midterm Exam Part I.4. (2013-MT-I.4.pdf) Problem Type: Stereochemical determination by 1 H NMR spectroscopy. Techniques: 1 H NMR spectroscopy.
Nuclear magnetic resonance (NMR) spectroscopy is a very powerful analytical method that has found many applications in physics, chemistry, biology, and medicine. It is an indispensable tool particularly for synthetic chemists because it can be used for a rapid elucidation of the structures of organic compounds. For this reason, NMR spectral interpretation is a part of the core knowledge of all ...
Welcome to WebSpectra - This site was established to provide chemistry students with a library of spectroscopy problems. Interpretation of spectra is a technique that requires practice - this site provides 1 H NMR and 13 C NMR, DEPT, COSY and IR spectra of various compounds for students to interpret. Hopefully, these problems will provide a useful resource to better understand spectroscopy.
The following exercises are designed to help you become familiar with predicting the 1H NMR spectra of simple organic molecules. For each example you should find the number of signals you expect, where they should show on the scale (chemical shift), and what shape they should be (splitting patterns). Use the spectroscopy sheet to become ...
In the following NMR practice problems, we will go over the best strategies you can use for identifying the structure of unknown compounds. As a Chemistry Steps Prime member, you will also get access to the Spectroscopy Summary Sheets in addition to these over 100 min videos of solving NMR problems.
Save as PDF Page ID 432208 ... Solve unknown problems using 13 C and 1 H NMR spectra and molecular formula. Exercise \(\PageIndex{1}\) ... 6.6: Uses of C-13 NMR Spectroscopy; 6.S: Summary; Was this article helpful? Yes; No; Recommended articles. Article type Section or Page; Tags. author@Lauren Reutenauer
Campus Bookshelves. Purdue University. Purdue: Chem 26200: Organic Chemistry II (Wenthold) Chapter 12. Nuclear Magnetic Resonance. 12.08. Solving NMR Spectra. 12.08.1 Proton NMR Practice Problems. Expand/collapse global location.
This site was established to provide interested people in NMR with a library of NMR spectroscopy problems. Interpretation of spectra is a technique that requires pattern recognition and/or practice to order the chaos. This site provides one dimensional spectra of different nucleus, COSY, HSQC, HMBC and some less common spectra of various compounds to interprete.
Campus Bookshelves. Purdue University. Purdue: Chem 26200: Organic Chemistry II (Wenthold) Chapter 12. Nuclear Magnetic Resonance. 12.10 Integrated Spectroscopy Problems. 12.10.2 MS, IR and NMR Problems. Expand/collapse global location.
The description is a bit long (….so hold on!), but once you get it, you can just use the algorithm to solve your NMR problems. Here are some reference values and a couple of proton NMR spectra: Proton NMR Reference Values (cem.msu.edu) (mhhe.com) (process-nmr.com) (1H NMR of Taxol; unknown source) (Our example 1H NMR spectra for this post ...
Example 7.7.3 7.7. 3. Propose a structure using the spectral data below for C 10 H 14 O. Note: You may need to check for solvent peaks. This sample was dissolved in CDCl 3. Mass Spectrum: 1 H: COSY: 13 C: HSQC: HMBC: