
- Previous Article
- Next Article

Presentation
Clinical pearls, case study: treating hypertension in patients with diabetes.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Evan M. Benjamin; Case Study: Treating Hypertension in Patients With Diabetes. Clin Diabetes 1 July 2004; 22 (3): 137–138. https://doi.org/10.2337/diaclin.22.3.137
Download citation file:
- Ris (Zotero)
- Reference Manager
L.N. is a 49-year-old white woman with a history of type 2 diabetes,obesity, hypertension, and migraine headaches. The patient was diagnosed with type 2 diabetes 9 years ago when she presented with mild polyuria and polydipsia. L.N. is 5′4″ and has always been on the large side,with her weight fluctuating between 165 and 185 lb.
Initial treatment for her diabetes consisted of an oral sulfonylurea with the rapid addition of metformin. Her diabetes has been under fair control with a most recent hemoglobin A 1c of 7.4%.
Hypertension was diagnosed 5 years ago when blood pressure (BP) measured in the office was noted to be consistently elevated in the range of 160/90 mmHg on three occasions. L.N. was initially treated with lisinopril, starting at 10 mg daily and increasing to 20 mg daily, yet her BP control has fluctuated.
One year ago, microalbuminuria was detected on an annual urine screen, with 1,943 mg/dl of microalbumin identified on a spot urine sample. L.N. comes into the office today for her usual follow-up visit for diabetes. Physical examination reveals an obese woman with a BP of 154/86 mmHg and a pulse of 78 bpm.
What are the effects of controlling BP in people with diabetes?
What is the target BP for patients with diabetes and hypertension?
Which antihypertensive agents are recommended for patients with diabetes?
Diabetes mellitus is a major risk factor for cardiovascular disease (CVD). Approximately two-thirds of people with diabetes die from complications of CVD. Nearly half of middle-aged people with diabetes have evidence of coronary artery disease (CAD), compared with only one-fourth of people without diabetes in similar populations.
Patients with diabetes are prone to a number of cardiovascular risk factors beyond hyperglycemia. These risk factors, including hypertension,dyslipidemia, and a sedentary lifestyle, are particularly prevalent among patients with diabetes. To reduce the mortality and morbidity from CVD among patients with diabetes, aggressive treatment of glycemic control as well as other cardiovascular risk factors must be initiated.
Studies that have compared antihypertensive treatment in patients with diabetes versus placebo have shown reduced cardiovascular events. The United Kingdom Prospective Diabetes Study (UKPDS), which followed patients with diabetes for an average of 8.5 years, found that patients with tight BP control (< 150/< 85 mmHg) versus less tight control (< 180/< 105 mmHg) had lower rates of myocardial infarction (MI), stroke, and peripheral vascular events. In the UKPDS, each 10-mmHg decrease in mean systolic BP was associated with a 12% reduction in risk for any complication related to diabetes, a 15% reduction for death related to diabetes, and an 11% reduction for MI. Another trial followed patients for 2 years and compared calcium-channel blockers and angiotensin-converting enzyme (ACE) inhibitors,with or without hydrochlorothiazide against placebo and found a significant reduction in acute MI, congestive heart failure, and sudden cardiac death in the intervention group compared to placebo.
The Hypertension Optimal Treatment (HOT) trial has shown that patients assigned to lower BP targets have improved outcomes. In the HOT trial,patients who achieved a diastolic BP of < 80 mmHg benefited the most in terms of reduction of cardiovascular events. Other epidemiological studies have shown that BPs > 120/70 mmHg are associated with increased cardiovascular morbidity and mortality in people with diabetes. The American Diabetes Association has recommended a target BP goal of < 130/80 mmHg. Studies have shown that there is no lower threshold value for BP and that the risk of morbidity and mortality will continue to decrease well into the normal range.
Many classes of drugs have been used in numerous trials to treat patients with hypertension. All classes of drugs have been shown to be superior to placebo in terms of reducing morbidity and mortality. Often, numerous agents(three or more) are needed to achieve specific target levels of BP. Use of almost any drug therapy to reduce hypertension in patients with diabetes has been shown to be effective in decreasing cardiovascular risk. Keeping in mind that numerous agents are often required to achieve the target level of BP control, recommending specific agents becomes a not-so-simple task. The literature continues to evolve, and individual patient conditions and preferences also must come into play.
While lowering BP by any means will help to reduce cardiovascular morbidity, there is evidence that may help guide the selection of an antihypertensive regimen. The UKPDS showed no significant differences in outcomes for treatment for hypertension using an ACE inhibitor or aβ-blocker. In addition, both ACE inhibitors and angiotensin II receptor blockers (ARBs) have been shown to slow the development and progression of diabetic nephropathy. In the Heart Outcomes Prevention Evaluation (HOPE)trial, ACE inhibitors were found to have a favorable effect in reducing cardiovascular morbidity and mortality, whereas recent trials have shown a renal protective benefit from both ACE inhibitors and ARBs. ACE inhibitors andβ-blockers seem to be better than dihydropyridine calcium-channel blockers to reduce MI and heart failure. However, trials using dihydropyridine calcium-channel blockers in combination with ACE inhibitors andβ-blockers do not appear to show any increased morbidity or mortality in CVD, as has been implicated in the past for dihydropyridine calcium-channel blockers alone. Recently, the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) in high-risk hypertensive patients,including those with diabetes, demonstrated that chlorthalidone, a thiazide-type diuretic, was superior to an ACE inhibitor, lisinopril, in preventing one or more forms of CVD.
L.N. is a typical patient with obesity, diabetes, and hypertension. Her BP control can be improved. To achieve the target BP goal of < 130/80 mmHg, it may be necessary to maximize the dose of the ACE inhibitor and to add a second and perhaps even a third agent.
Diuretics have been shown to have synergistic effects with ACE inhibitors,and one could be added. Because L.N. has migraine headaches as well as diabetic nephropathy, it may be necessary to individualize her treatment. Adding a β-blocker to the ACE inhibitor will certainly help lower her BP and is associated with good evidence to reduce cardiovascular morbidity. Theβ-blocker may also help to reduce the burden caused by her migraine headaches. Because of the presence of microalbuminuria, the combination of ARBs and ACE inhibitors could also be considered to help reduce BP as well as retard the progression of diabetic nephropathy. Overall, more aggressive treatment to control L.N.'s hypertension will be necessary. Information obtained from recent trials and emerging new pharmacological agents now make it easier to achieve BP control targets.
Hypertension is a risk factor for cardiovascular complications of diabetes.
Clinical trials demonstrate that drug therapy versus placebo will reduce cardiovascular events when treating patients with hypertension and diabetes.
A target BP goal of < 130/80 mmHg is recommended.
Pharmacological therapy needs to be individualized to fit patients'needs.
ACE inhibitors, ARBs, diuretics, and β-blockers have all been documented to be effective pharmacological treatment.
Combinations of drugs are often necessary to achieve target levels of BP control.
ACE inhibitors and ARBs are agents best suited to retard progression of nephropathy.
Evan M. Benjamin, MD, FACP, is an assistant professor of medicine and Vice President of Healthcare Quality at Baystate Medical Center in Springfield, Mass.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Case Studies: BP Evaluation and Treatment in Patients with Prediabetes or Diabetes
—the new acc/aha blood pressure guidelines call for a more aggressive diagnostic and treatment approach in most situations..
By Kevin O. Hwang, MD, MPH, Associate Professor, McGovern Medical School, Houston, TX
The following case studies illustrate how the new ACC/AHA guideline specifies a shift in the definition of BP categories and treatment targets.

A 59-year-old man with type 2 diabetes presents with concerns about high blood pressure (BP). At a recent visit to his dentist he was told his BP was high. He was reclining in the dentist’s chair when his BP was taken, but he doesn’t remember the exact reading. He has no symptoms. He has never taken medications for high BP. He takes metformin for type 2 diabetes.
His BP is measured once at 146/95 mm Hg in the left arm while sitting. Physical exam is unremarkable except for obesity. EKG is unremarkable.
BP Measurement
Controlling BP in patients with diabetes reduces the risk of cardiovascular events, but the available data are not sufficient to classify this patient with respect to BP status. The reading taken while reclining in the dentist’s chair was likely inaccurate. A single reading in the medical clinic, even with correct technique, is not adequate for clinical decision-making because individual BP measurements vary in unpredictable or random ways.
The accuracy of BP measurement is affected by patient preparation and positioning, technique, and timing. Before the first reading, the patient should avoid smoking, caffeine, and exercise for at least 30 minutes and should sit quietly in a chair for at least 5 minutes with back supported and feet flat on the floor. An appropriately sized cuff should be placed on the bare upper arm and with the arm supported at heart level. For the first encounter, BP should be recorded in both arms. The arm with the higher reading should be used for subsequent measurements.
It is recommended that one use an average of 2 to 3 readings, separated by 1 to 2 minutes, obtained on 2 to 3 separate visits. Some of those readings should be performed outside of the clinical setting, either with home BP self-monitoring or 24-hour ambulatory BP monitoring, especially when confirming the diagnosis of sustained hypertension. Note that a clinic BP of 140/90 corresponds to home BP values of 135/85. Multiple BP readings in the clinic and at home allow for classification into one of the following categories.
The BP is measured in the office with the correct technique and timing referenced above. The patient is educated on how on to measure BP at home with a validated monitor. He should take at least 2 readings 1 minute apart in the morning and in the evening before supper (4 readings per day). The optimal schedule is to measure BP every day for a week before the next clinic visit, which is set for a month from now. Obtaining multiple clinic and home BP readings on multiple days will support a well-informed assessment of the patient’s BP status and subsequent treatment decisions.
A 62 year old African-American woman with prediabetes presents for her annual physical. She has no complaints. The average of 2 BP readings in her right arm is BP 143/88. Her physical exam is unremarkable except for obesity. She has no history of myocardial infarction, stroke, kidney disease, or heart failure. After the visit, she measures her BP at home and returns 1 month later. The average BP from multiple clinic and home readings is 138/86.
Her total cholesterol is 260 mg/dL, HDL 42 mg/dL, and LDL 165 mg/dL. She does not smoke.
Stage 1 Hypertension
Under the 2017 ACC/AHA guideline, she has stage 1 hypertension (HTN). This guideline uses a uniform BP definition for HTN without regard to patient age or comorbid illnesses, such as diabetes or chronic kidney disease.
In patients with stage 1 HTN and no known atherosclerotic cardiovascular disease (ASCVD) , the new guideline recommends treating with BP-lowering medications if the 10-year risk for ASCVD risk is 10% or greater. With input such as her age, gender, race, lipid profile, and other risk factors, the ACC/AHA Pooled Cohort Equations tool estimates her 10-year risk to be approximately 10.5%.
With stage 1 HTN and 10-year ASCVD risk of 10% or higher, she would benefit from a BP-lowering medication. Thiazide diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and calcium channel blockers are first-line agents for HTN because they reduce the risk of clinical events. In African-Americans, thiazide diuretics and calcium channel blockers are more effective for lowering BP and preventing cardiovascular events compared to ACE inhibitors or ARBs.
Patient-specific factors, such as age, comorbidities, concurrent medications, drug adherence, and out-of-pocket costs should be considered. Shared decision making should drive the ultimate choice of antihypertensive medication(s).
Nonpharmacologic strategies for prediabetes and HTN include dietary changes, physical activity, and weight loss. If clinically appropriate, she should also avoid agents which could elevate BP, such as NSAIDs, oral steroids, stimulants, and decongestants.
A goal BP of 130/80 is recommended. After starting the new BP medication, she should monitor BP at home and return to the clinic in 1 month. If the BP goal is not met at that time despite adherence to treatment, consideration should be given to intensifying treatment by increasing the dose of the first medication or adding a second agent.
A 63 year old man with type 2 diabetes has an average BP of 151/92 over the span of several weeks of measuring at home and in the clinic. He also has albuminuria.
Stage 2 Hypertension:
The BP treatment goal patients with diabetes and HTN is less than 130/80. While some patients can be effectively treated with a single agent, serious consideration should be given to starting with 2 drugs of different classes, especially if BP is more than 20/10 mm Hg above their BP target. Giving both medications as a fixed-dose combination may improve adherence.
In this man with diabetes and HTN, any of the first-line classes of antihypertensive agents (diuretics, ACE inhibitors, ARBs, and CCBs) would be reasonable choices. Given the presence of albuminuria, an ACE inhibitor or ARB would be beneficial for slowing progression of kidney disease. However, an ACE inhibitor and ARB should not be used simultaneously due to an increase in cardiovascular and renal risk observed in clinical trials.
He is started on a fixed-dose combination of an ACE-inhibitor and thiazide diuretic. He purchases a validated BP monitor which can transmit BP readings to his provider’s electronic health records system. Direct transmission of BP data to the provider has been shown to help patients achieve greater reductions in BP compared to self-monitoring without transmission of data. One month follow-up is recommended to determine if the treatment goal has been met.
Published: April 30, 2018
- 2. Final Recommendation Statement: High Blood Pressure in Adults: Screening. U.S. Preventive Services Task Force. September 2017.
Does cabg owe its success more to sag--or to mag, dvt treatment: home versus hospital, perioperative thromboembolic complications predict long-term vte risk, coronary plaque in people with well-controlled hiv and low ascvd risk, poor neighborhood, poor mi outcome, stroke risk at age 66 to 74 years—with atrial fibrillation but without other risk factors, using cardiovascular biomarkers to diagnose type 2 mi: yea or nay, cardiac rehabilitation: outcomes in south asian patients.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 February 2021
The interaction on hypertension between family history and diabetes and other risk factors
- An-le Li 1 ,
- Qian Peng 1 ,
- Yue-qin Shao 1 ,
- Xiang Fang 1 &
- Yi-ying Zhang 1
Scientific Reports volume 11 , Article number: 4716 ( 2021 ) Cite this article
7450 Accesses
24 Citations
Metrics details
- Medical research
- Risk factors
To explore the individual effect and interaction of diabetes and family history and other risk factors on hypertension in Han in Shanghai China. The method of case–control study with l:l matched pairs was used, 342 cases of hypertension and 342 controls were selected and investigate their exposed factors with face-to-face. The method of epidemiology research was used to explore the individual effect and interaction of diabetes and family history and other risk factors on hypertension. The individual effect of family history (OR = 4.103, 95%CI 2.660–6.330), diabetes (OR = 4.219, 95%CI 2.926–6.083), personal taste (OR = 1.256, 95%CI 1.091–1.593), drinking behavior (OR = 1.391, 95%CI 1.010–1.914) and smoking behavior (OR = 1.057, 95%CI 1.00–1.117) were significant (p < 0.05). But individual effect of sex, education, occupation, work/life pressure, environmental noise, sleeping time and sports habit were not significant (p > 0.05). The OR of interaction between FH and DM to hypertension was 16.537 (95%CI 10.070–21.157), between FH and drinking behavior was 4.0 (95%CI 2.461–6.502), FH and sport habit was 7.668 (95%CI 3.598–16.344), FH and personal taste was 6.521 (95%CI 3.858–11.024), FH and smoking behavior was 5.526 (95%CI 3.404–8.972), FH and work/life pressure was 4.087 (95%CI 2.144–7.788). The SI of FH and DM was 2.27, RERI was 8.68, AP was 52.48% and PAP was 55.86%. FH and DM, personal taste, smoking behavior had positive interaction on hypertension, but FH and sport habits, drinking behavior, work/life pressure had reverse interaction on hypertension. FH and diabetes were very important risk factors with significant effect for hypertension. FH and diabetes, personal taste, smoking behavior had positive interaction on hypertension, but FH and sport habits, drinking behavior, work/life pressure had reverse interaction on hypertension.
Similar content being viewed by others
Socio-demographic and lifestyle factors associated with hypertension in Nigeria: results from a country-wide survey
Development of a risk prediction model for incident hypertension in Japanese individuals: the Hisayama Study
Impact of hypertension stratified by diabetes on the lifetime risk of cardiovascular disease mortality in Japan: a pooled analysis of data from the Evidence for Cardiovascular Prevention from Observational Cohorts in Japan study
Introduction.
Hypertension is a multifactorial disease caused by genetic and acquired environmental factors, the role of gene and gene, as well as between gene and acquired environmental factors, including among acquired risk factors, leads to increased risk of hypertension and disease among different populations. Unhealthy lifestyle including obesity and lack of exercise can significantly increase hypertension incidence 1 , 2 , 3 , 4 . The study result from familial aggregation showed that the prevalence rate of brothers and sisters in offspring was from 20 to 66% in positive population of parents, the estimated possibility of hereditary was over 50% in a plurality of twin studies 5 , 6 . It showed that more than half of the blood pressure changed could be attributed to the accumulation of genetic effects.
In fact, the individual effect of single factor on the result could not truly reflect the actual effect of factors, because there was interaction between risk factors, which might weaken the role of single factor, or enhance the role of single factor. Therefore, the interaction between factors may be more able to reflect the real relationship between factors and results. There were many studies on the influencing factors of hypertension at home and abroad, but there were few reports on the interaction between the influencing factors of hypertension.
There were two models to analyze biological interaction: addition and multiplication. To explore the risk factors and etiological factors of hypertension from the perspective of environment and genetics, and to carry out effective etiological prevention are the fundamental countermeasures and measures to reduce the incidence of hypertension. It might be of public health significance to explore the interaction with additive model. Therefore, this study selected additive model to analyze the interaction between family history of hypertension and diabetes and other exposed factors on the incidence of hypertension.
Source of cases and controls
All cases were randomly selected from hypertension registry and follow-up management system in Jiading district in Shanghai, and all controls were randomly selected from the community population. The cases of this study were patients with hypertension who have been definitely diagnosed in the hospital and had been using antihypertensive drugs (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg without using antihypertensive drugs). The controls of this study was non hypertensive patients, their blood pressure were SBP < 140 mmHg and DBP < 100 mmHg and unused antihypertensive drugs. According to l:l matched pairs design, all controls had no hypertension, and controls were required the same sex, same race, living in the same community as cases, and the difference of age was not more than 5 years old and at the same age group. Every case or control gave informed consent to participate in the study which was approved by the local ethics committee (JD-2016-KY-18). They were able to correctly respond to the investigators for the health information of themselves and their nuclear family members.
Investigation method and content
Investigation was conducted by trained public health investigators, using a unified questionnaire. Using direct survey method, the contents of the questionnaire mainly include: age, sex, age of onset, diagnosis time, hospital name, diabetes history and so on. The criteria for judging whether all the respondents had essential hypertension and diabetes (all relatives of cases and controls): whether they had been diagnosed with essential hypertension or diabetes in the hospital before this investigation. If they had been diagnosed with essential hypertension in the hospital, it is “Yes”; if they had not been diagnosed, it is “No”.
Statistical analysis
Statistical analyses were performed using the statistical software package (IBM SPSS statistics version 21). When P values < 0.05, the difference was considered statistically significant. Mean and standard deviation (SD) were used to compute for quantitative variables (age and so on), and comparisons between groups were performed by t-test. Number (n) and percentage (%)) were computed for the categorical data, comparisons between groups were performed by the chi-square ( χ 2 ) test. Multivariate logistic regression analyses were conducted for investigated risk factors, odds ratios (OR) and 95% confidence intervals (CI) were calculated. In multivariate analysis, OR were adjusted by sex. The additive model was used by cross analysis to calculate the additive interaction effect 1 . The calculated indicators were SI (synergistic effect index), RERI (relative excess risk due to interaction), AP (attributable proportion due to interaction) and PAP (the percentage of the interaction between the pure factors).
All methods were carried out in accordance with relevant guidelines and regulations. The investigated object, content and methods of study were implemented according to the design scheme and technical route.
Ethics approval and consent to participate
Ethical approval was granted by Jiading district center for disease control and prevention research ethics committee. All subjects gave informed consent to participate in the study, they would like to participate in investigation and answer all the related questions in the questionnaire.
Individual effect analysis
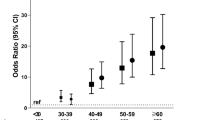
Among 684 investigated participants (342 hypertension cases and 342 controls) aged 28–87 years old in this study, male was 50.73%, female was 49.27%. 76.17% participants had family history of hypertension, 23.83% had not. Between case group and control group, the statistical test results showed that the difference of sex, education level, work and life pressure, living environmental noise, person's taste, sleeping time, sports habit, drinking behavior and smoking behavior was no significant (p > 0.05). But the difference of family history (FH) and occupation between case group and control group was significant (p < 0.05). The difference of mean age between case group and control group was no significant (t = 0.894, p = 0.372).
The result of logistic regression analysis showed that individual effect of family history of hypertension, diabetes history, personal taste, drinking behavior and smoking behavior were significant (p < 0.05). But individual effect of sex, education, occupation, work/life pressure, environmental noise, sleeping time and sports habit were not significant (p > 0.05). See Table 1 .
The OR result showed that family history of hypertension, diabetes history, drinking behavior and smoking behavior were important risk factors to hypertension. The OR between family history and hypertension was 4.103 (95%CI 2.660–6.330); the OR between diabetes history and hypertension was 4.219 (95%CI 2.926–6.083); the OR between drinking behavior and hypertension was 1.391 (95%CI 1.010–1.914); the OR between smoking behavior and hypertension was 1.057 (95%CI 1.000–1.117). The OR between personal taste and hypertension was 1.256 (95%CI 1.091–1.593). See Table 1 .
Interaction analysis
Interaction of family history and diabetes.
The effect of risk factors on the occurrence of hypertension was often not independent; they often interacted with each other and promoted the occurrence of hypertension through interaction. In order to explore the interaction between family history and diabetes, the methods of interactive effects analysis was used. Table 2 shows the result of interactive effects between family history and diabetes. The OR of interaction between family history and diabetes to hypertension was 16.537, the OR of family history and diabetes to hypertension were respectively 4.505 and 4.354. OR (FH+DM) > OR FH + OR DM . It was showed that family history and diabetes have positive interaction with hypertension.
According the result of Table 3 , additive model was used to calculate the additive interaction effect: the synergistic effect index (SI) of family history and diabetes to hypertension was 2.27; relative excess risk due to interaction (RERI) was 8.68; Attributable proportion due to interaction (AP) was 52.48%; and the percentage of the interaction between the pure factors (PAP) was 55.86%. The result of PAP indicated that 55.86% of hypertension was attributable to the interaction of them, when exposed to both family history and diabetes risk factors.
Interaction of FH and other risk factors
In order to better observe the interaction between family history (FH) and other risk factors (drinking behavior, sport habits, personal taste, smoking behavior and work/life pressure), we changed behavior from three categories (no, occasionally and regular) to two categories (yes or no), the combination of occasionally drinking and regular drinking was yes (have drink behavior); occasionally sport and regular sport was yes (have sport habits). The same as, balance taste and light taste was no salty; occasionally and regular smoking behavior was yes (smoking), little and more work/life pressure was have pressure.
The result showed OR of interaction between FH and drinking behavior to hypertension was 4.0, the OR of family history and smoking behavior to hypertension were respectively 4.942 and 0.741. OR (FH+Dr) < OR FH + OR Dr . It was showed that FH and drinking behavior have reverse interaction on hypertension. See Table 3 .
The result showed OR of interaction between FH and sport habits to hypertension was 7.668, the OR of family history and sport habits to hypertension were respectively 8.571 and 1.773. OR (FH+S) < OR FH + OR S . It was showed that FH and sport habits have reverse interaction on hypertension. See Table 4 .
The result showed OR of interaction between FH and taste preference to hypertension was 6.521, the OR of family history and taste preference to hypertension were respectively 4.840 and 1.386. OR (FH+T) > OR FH + OR T . It was showed that FH and taste preference (salty) have positive interaction on hypertension. See Table 5 .
The result showed OR of interaction between FH and smoking behavior to hypertension was 5.526, the OR of family history and smoking behavior to hypertension were respectively 4.359 and 0.871. OR (FH+Sm) > OR FH + OR Sm . It was showed that FH and smoking behavior have positive interaction on hypertension. See Table 6 .
The result showed OR of interaction between FH and work/life pressure to hypertension was 4.087, the OR of family history and work/life pressure to hypertension were respectively 5.217 and 2.229. OR (FH+P) < OR FH + OR P . It was showed that FH and work/life pressure have reverse interaction on hypertension. See Table 7 .
The factors influencing the occurrence of hypertension include congenital factors and natural factors, congenital factors refer to hereditary factors such as genes or family history, acquired factors mainly include bad living habits, overweight / obesity, etc. Body mass index was a comprehensive indicator of the outcome of acquired lifestyle, and closely related to the occurrence of hypertension 7 , 8 , 9 , 10 , 11 , 12 . Family history of hypertension was an important marker of genetic factors, it was often used as an alternative indicator to study the relationship between genetic factors and diseases 13 , 14 , 15 , 16 . Previous studies had not been considered the interaction between genetic and environmental factors. More attention needed to pay to the related research, to evaluate the relationship between polymorphic gene and exposed factors. Hypertension and diabetes usually occurred successively, due to the hardening of blood vessels diabetes could induce hypertension 17 , 18 , 19 .
There were many risk factors for hypertension, such as smoking, drinking, mental tension, lack of exercise, family genetics and so on 20 , 21 . In this study, the result showed that effect of family history of hypertension, diabetes history, personal taste, drinking behavior and smoking behavior were significant (p < 0.05). But effect of education, occupation, work/ life pressure, environmental noise, sleeping time and sports habit were not significant (p > 0.05). The OR result showed that family history of hypertension, diabetes history and drinking behavior were important risk factors to hypertension. The OR of family history of hypertension was 4.103, diabetes history was 4.219, drinking behavior was 1.391, smoking behavior was 1.057. This result showed that family history of hypertension, diabetes history, drinking behavior and smoking behavior were important factors of hypertension, especially family history and diabetes.
Hypertension was one of the common complications of diabetes; the incidence of hypertension in domestic diabetes patients with hypertension was 20–30% 22 , 23 . The OR of interaction between family history and diabetes to hypertension was 16.537. It was showed that FH and DM have positive interaction with hypertension. The percentage of the interaction between the pure factors (PAP) was 55.86%, it indicated that 55.86% of hypertension was attributable to the interaction of them, when exposed to both family history and diabetes risk factors. Because the disorder of glucose metabolism could accelerate the hardening of renal artery and systemic arteriole, increase the peripheral resistance and blood pressure, hyperglycemia can increase blood volume, overload the kidneys, retention of water and sodium, and eventually raise blood pressure. The increase of blood pressure was related to cardiac output and peripheral resistance. The increase of cardiac output without peripheral change could lead to the rise of blood pressure; the increase of peripheral resistance without the change of cardiac output or blood volume could also lead to the rise of blood pressure, and both changes of diabetic patients led to the rapid rise of blood pressure and serious complications.
Alcohol was one of the risk factors of hypertension 24 , 25 , 26 , 27 . Long term small amount of alcohol could increase blood pressure; small amount of alcohol could increase blood pressure, heart rate and heart load of patients with hypertension. In this study, the result of the logistic regression analysis showed that drinking behavior were risk factor to hypertension, the OR of drinking behavior was 1.391. Family history of hypertension and drinking behavior had reverse interaction on hypertension. This might be due to the interference of occasional drinking behavior, small amount of alcohol may have vascular protection. It needs further study.
High salt intake in salt sensitive individuals could lead to elevated blood pressure by affecting water and sodium metabolism, vascular function and sympathetic nervous system 28 , 29 . In this study, the result of the logistic regression analysis showed that personal taste were risk factor to hypertension, the OR of personal taste was 1.256. Family history of hypertension and drinking behavior had positive interaction on hypertension. The lower of 95%CI of OR < 1 (0.991), perhaps it was related to the fact that Shanghai residents generally like light food. It needs further study.
Smoking is a risk factor for cardiovascular disease, and smoking is associated with hypertension 30 , 31 . In this study, the result of the logistic regression analysis showed that smoking behavior were risk factor to hypertension, the OR of personal taste was 1.256. Family history and smoking behavior had positive interaction on hypertension.
Due to space limitation, only the interaction between family history and several common acquired factors were analyzed in this study. In fact, there were also interactions among acquired factors, and the interaction among multiple factors may be even more different. In short, the individual effect of single factor was strong did not mean that it must be very important role in the outcome of disease, the individual effect of single factor was weak did not mean that it must be very unimportant role in the outcome of disease. Pay attention to the interaction between factors, and expect more and better research results appear.
Family history and diabetes were very important risk factors with significant effect for hypertension. FH and DM, taste preference, smoking behavior had positive interaction with hypertension, but FH and sport habits, drinking behavior, work/life pressure had reverse interaction with hypertension.
Data availability
The questionnaire and database supporting the conclusions of this article are available, through contact with [email protected].
Li, A. L., Peng, Q., Shao, Y. Q., Fang, X. & Zhang, Y. Y. The effect of body mass index and its interaction with family history on hypertension: A case-control study. Clin. Hypertens. 25 (6), 1–8 (2019).
Google Scholar
Zaw, K. K. et al. Prevalence of hypertension and its associated factors in the adult population in Yangon Division, Myanmar. Asia Pac. J. Public Health 23 (4), 496–506 (2011).
Article Google Scholar
Center for chronic non communicable diseases control and prevention of China Center for Disease Control and Prevention. Surveillance report of chronic diseases and their risk factors in China (2010) (Military Medical Science Press, Beijing, 2012).
Ehrel, G. B. Genome-wide association studies: Contribution of genomic to understanding blood pressure and essential hypertension. Curr. Hypertens. Rep. 12 , 17–25 (2010).
Li, A. L., Fang, X., Zhang, Y. Y., Peng, Q. & Yin, X. H. Familial aggregation and heritability of hypertension in Han population in Shanghai China: A case-control study. Clin. Hypertens. 25 (17), 1–7 (2019).
Levy, D. et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 41 (6), 677–687 (2009).
Article CAS Google Scholar
Millar, S., Perry, I. J. & Phillips, C. M. Surrogate measures of adiposity and cardio- metabolic risk—why the uncertainty? A review of recent meta-analytic studies. J. Diabetes Metab. S11 , 004 (2013).
Tadic, M. et al. The association between obesity, blood pressure variability and right ventricular function and mechanics in hypertensive patients. J. Am. Soc. Echo Cardiogr. 29 (8), 802–811 (2016).
Tadic, M. et al. The interaction between blood pressure variability, obesity, and left ventricular mechanics: Findings from the hypertensive population. J. Hypertens. 34 (4), 772–780 (2016).
Manimunda, S. P. et al. Association of hypertension with risk factors & hypertension related behaviour among the aboriginal Nicobarese tribe living in Car Nicobar Island, India. Indian J. Med. Res. 133 (3), 287–293 (2011).
PubMed PubMed Central Google Scholar
Kotchen, T. A. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 23 (11), 1170–1178 (2010).
Modesti, P. A. et al. Cardiovascular risk assessment in low-resource settings: A consensus document of the European Society of Hypertension Working Group on Hypertension and Cardiovascular Risk in Low Resource Settings. J. Hypertens. 32 (5), 951–960 (2014).
Ehret, G. B. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 (7367), 103–109 (2011).
Article ADS Google Scholar
Kato, N. et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat. Genet. 43 (6), 531–538 (2011).
Zhang, Y. et al. A rare variant at the KYNU gene is associated with kynurenines activity and essential hypertension in the Han Chinese population. Circ. Cardiovasc. Genet. 4 (6), 687–694 (2011).
Rafiq, S., Anand, S. & Roberts, R. Genome-wide association studies of hypertension: Have they been fruitful?. Cardiovasc. Transl. Res. 3 (3), 189–196 (2010).
Raikou, V. D. & Gavriil, S. Body-mass index and the risk of albuminuria in hypertensive patients with a poor estimated glomerular filtration rate and the potential role of diabetes mellitus. Diabetes Metab. Syndr. 13 (2), 1041–1046 (2019).
Ren, Q., Ma, C.-S., Wang, J.-G., Guo, X.-H. & Jia, L.-N. Albuminuria and other target organ damage in Chinese patients with hypertension and diabetes: A data analysis based on the ATTEND study. J. Diabetes Complicat. 34 (1), 1070–1074 (2020).
Shibata, D. et al. Vascular risk factors and findings on brain MRI of elderly adult American Indians: The strong heart study. Neuro Epidemiol. 52 (3–4), 173–180 (2019).
Liu, L. S. et al. 2018 Chinese guidelines for prevention and treatment of hypertension—a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J. Geriatr. Cardiol. 16 (3), 182–241 (2019).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Article PubMed PubMed Central Google Scholar
Wang, L., Li, J., Li, Y., Yao, S., Zhao, M. & Wang, C. The effects of hypertension and diabetes on new-onset chronic kidney disease: A prospective cohort study [J]. J. Clin. Hypertens. (2019).
Liu, J. et al. Prevalence of diabetes mellitus in out patients with essential hypertension in China: A cross-sectional study. BMJ Open. 3 (11), e003798 (2013).
Jung, M. H. et al. The effect of alcohol dose on the development of hypertension in Asian and Western men: Systematic review and meta-analysis. Korean J. Intern. Med. https://doi.org/10.3904/kjim.2019.016 (2019).
Tatsumi, Y. et al. Hyperuricemia predicts the risk for developing hypertension independent of alcohol drinking status in men and women: The Saku study. Hypertens. Res. https://doi.org/10.1038/s41440-019-0361-0 (2019).
Article PubMed Google Scholar
Puddey, I. B., Mori, T. A., Barden, A. E. & Beilin, L. J. Alcohol and hypertension-new insights and lingering controversies. Curr. Hypertens. Rep. 21 (10), 79 (2019).
Arnold, C., Ullrich, C., Wensing, M. & Pfinder, M. Prenatal alcohol exposure and the associated risk of elevated blood pressure: A cross-sectional analysis of 3- to 17-year-olds in Germany. Am. J. Hypertens. 32 (11), 1118–1125 (2019).
Bkaily, G., Simon, Y., Menkovic, I., Bkaily, C. & Jacques, D. High salt-induced hypertrophy of human vascular smooth muscle cells associated with a decrease in glycocalyx. J. Mol. Cell. Cardiol. https://doi.org/10.1016/j.yjmcc.2018.10.006 (2018).
Clemmer, J. S., Hester, R. L. & Pruett, W. A. Simulating a virtual population’s sensitivity to salt and uninephrectomy. J. R. Soc. Int. https://doi.org/10.1098/rsfs.2016.0134 (2018).
Ding, L. et al. Smoking, heavy drinking, physical inactivity, and obesity among middle-aged and older adults in China: Cross-sectional findings from the baseline survey of CHARLS 2011–2012. BMC Public Health https://doi.org/10.1186/s12889-020-08625-5 (2020).
Holanger, M., Kjeldsen, S. E., Jamerson, K. & Julius, S. Smoking and overweight associated with masked uncontrolled hypertension: A Hypertension Optimal Treatment (HOT) Sub-Study. Blood Press. https://doi.org/10.1080/08037051.2020.1787815 (2020).
Download references
Acknowledgements
Heartfelt thanks to all doctors, nurses and public health workers in 13 community health service centers in Jiading district in Shanghai for their hard work. Thank for some advice of the experts!
This study was funded by Jiading district health and family planning commission research project in Shanghai (No: 2016-KY-18).
Author information
Authors and affiliations.
Jiading District Center for Disease Control and Prevention, Shanghai, China
An-le Li, Qian Peng, Yue-qin Shao, Xiang Fang & Yi-ying Zhang
You can also search for this author in PubMed Google Scholar
Contributions
The original idea for the project was conceived by A.L.; Q.P., X.F. and Y.Z. participated in the collection of early data, quality control and gave a lot of administrative support. A.L. conceptualized the paper, analyzed data and wrote a first draft of the manuscript. All authors contributed to subsequent drafts and approved the final manuscript.
Corresponding author
Correspondence to An-le Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, Al., Peng, Q., Shao, Yq. et al. The interaction on hypertension between family history and diabetes and other risk factors. Sci Rep 11 , 4716 (2021). https://doi.org/10.1038/s41598-021-83589-z
Download citation
Received : 21 May 2020
Accepted : 19 January 2021
Published : 25 February 2021
DOI : https://doi.org/10.1038/s41598-021-83589-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The interaction between general or abdominal obesity and hypertension on the risk of type 2 diabetes mellitus: a cross-sectional analysis in iranian adults from the rancd cohort study.
- Yahya Pasdar
- Shahab Rezaeian
- Mitra Darbandi
BMC Public Health (2024)
The association of periodontal disease and oral health with hypertension, NHANES 2009–2018
- Xiaojing Yuan
- Qingsong Chen
BMC Public Health (2023)
Socioeconomic and behavioral determinants of non-compliance with physician referrals following community screening for diabetes, hypertension and hyperlipidemia: a mixed-methods study
- Sungwon Yoon
- Lian Leng Low
Scientific Reports (2023)
Effects of additive interactions among obesity, visceral adiposity, and sarcopenia on nonalcoholic fatty liver disease
- Goh Eun Chung
- Ji Bong Jeong
Risk probability and influencing factors of stroke in followed-up hypertension patients
- Xin-zhi Jian
BMC Cardiovascular Disorders (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Developing a toolkit for implementing evidence-based guidelines to manage hypertension and diabetes in Cambodia: a descriptive case study
Affiliations.
- 1 Programme in Health Services and Systems Research, Duke-NUS Medical School, 8 College Road, Singapore, 169857, Singapore. [email protected].
- 2 Programme in Health Services and Systems Research, Duke-NUS Medical School, 8 College Road, Singapore, 169857, Singapore.
- 3 Duke University Medical Center, Duke University, Durham, NC, United States of America.
- 4 KHANA Center for Population Health Research, Phnom Penh, Cambodia.
- 5 Department of Preventive Medicine, Ministry of Health, Phnom Penh, Cambodia.
- 6 Case Western Reserve University, Cleveland, OH, United States of America.
- 7 Center for Global Health Research, Touro University California, Vallejo, CA, United States of America.
- 8 Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore.
- PMID: 36443781
- PMCID: PMC9706829
- DOI: 10.1186/s12961-022-00912-4
Background: In Cambodia, economic development accompanied by health reforms has led to a rapidly ageing population and an increasing incidence and prevalence of noncommunicable diseases. National strategic plans recognize primary care health centres as the focal points of care for treating and managing chronic conditions, particularly hypertension and type 2 diabetes. However, health centres have limited experience in providing such services. This case study describes the process of developing a toolkit to facilitate the use of evidence-based guidelines to manage hypertension and type 2 diabetes at the health-centre level.
Methods: We developed and revised a preliminary toolkit based on the feedback received from key stakeholders. We gathered feedback through an iterative process of group and one-to-one consultations with representatives of the Ministry of Health, provincial health department, health centres and nongovernmental organizations between April 2019 and March 2021.
Results: A toolkit was developed and organized according to the core tasks required to treat and manage hypertension and type 2 diabetes patients. The main tools included patient identification and treatment cards, risk screening forms, a treatment flowchart, referral forms, and patient education material on risk factors and lifestyle recommendations on diet, exercise, and smoking cessation. The toolkit supplements existing guidelines by incorporating context-specific features, including drug availability and the types of medication and dosage guidelines recommended by the Ministry of Health. Referral forms can be extended to incorporate engagement with community health workers and patient education material adapted to the local context. All tools were translated into Khmer and can be modified as needed based on available resources and arrangements with other institutions.
Conclusions: Our study demonstrates how a toolkit can be developed through iterative engagement with relevant stakeholders individually and in groups to support the implementation of evidence-based guidelines. Such toolkits can help strengthen the function and capacity of the primary care system to provide care for noncommunicable diseases, serving as the first step towards developing a more comprehensive and sustainable health system in the context of population ageing and caring for patients with chronic diseases.
Keywords: Asia; Low- and middle-income countries; Noncommunicable diseases; Primary care; Toolkits.
© 2022. The Author(s).
- Diabetes Mellitus, Type 2* / therapy
- Health Facilities
- Hypertension* / therapy
- Noncommunicable Diseases*
Grants and funding
- K18018/World Health Organization Centre for Health Development
- Diabetes & Primary Care
- Vol:24 | No:01
Interactive case study: Managing hypertension in diabetes – tricky cases
Share this article + Add to reading list – Remove from reading list ↓ Download pdf

Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of type 2 diabetes.
The three mini-case studies created for this issue of the journal cover various aspects relating to the management of complex cases of hypertension with type 2 diabetes.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in type 2 diabetes by encouraging us to make evidence-based decisions in the context of individual cases.
You are invited to respond to the questions by typing in your answers. In this way, you are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Winston, a 67-year-old Afro-Caribbean man, has had type 2 diabetes for 15 years. Despite triple therapy, his BP is 155/78 mmHg, with an eGFR of 65 mL/min/1.73 m 2 and ACR of 2.2 mg/mmol.
What could you do in this next consultation?
79-year-old Lily, who lives alone and has osteoarthritis, has type 2 diabetes that is well controlled. With triple antihypertensive therapy, her BP is 155/70 mmHg. There are concerns about her renal function and she has bilateral ankle oedema.
What would be your concerns over intensifying Lily’s antihypertensive therapy?
Mar k, 47 years old, has persistent genital thrush. His blood glucose is 14.3 mmol/L, his BMI is 36.4 kg/m 2 and there is a family history of type 2 diabetes. BP of 194/126 mmHg is recorded.
How would you respond to Mark’s raised BP?
By working through these interactive cases, we will consider the following issues and more:
- The options available if triple antihypertensive therapy is proving to be inadequate.
- Treating hypertension in an older person who has significant comorbidities.
- When to refer an individual with high blood pressure immediately to hospital.
Click here to access the new interactive case study.
Diabetes Distilled: Optimising sleep – simple questions and goals
Q&a: lipid management – part 1: measuring lipids and lipid targets, q&a: lipid management – part 2: use of statins, editorial: updated guidance on prescribing incretin-based therapy, cardiovascular risk reduction and the wider uptake of cgm, updated guidance from the pcds and abcd: managing the national glp-1 ra shortage, at a glance factsheet: tirzepatide for management of type 2 diabetes, how to diagnose and treat hypertension in adults with type 2 diabetes.

The importance of sleep in type 2 diabetes management.
22 May 2024

Claire Davies and Patrick Wainwright answer questions on lipid monitoring, triglycerides, familial hypercholesterolaemia and more.
21 May 2024

Claire Davies answers questions on statin prescribing and monitoring.

Jane Diggle highlights advice on preventing eye damage when initiating new incretin-based therapies.
20 May 2024
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Adherence to the dietary approaches to stop hypertension diet reduces the risk of diabetes mellitus: a systematic review and dose-response meta-analysis
- Meta-Analysis
- Open access
- Published: 30 May 2024
Cite this article
You have full access to this open access article

- Xiyan Quan 1 ,
- Xiaoming Shen 1 ,
- Chun Li 1 ,
- Yayuan Li 1 ,
- Tiangang Li 1 &
- Baifan Chen 1
131 Accesses
Explore all metrics
Despite several epidemiological studies reporting a significant association between adherence to the Dietary Approaches to Stop Hypertension (DASH) diet and the risk of diabetes mellitus, the results remain controversial. In this systematic review and meta-analysis, we aimed to summarize the existing evidence from published observational studies and evaluate the dose-response relationship between adherence to the DASH diet and diabetes mellitus risk.
We performed a systematic search for relevant articles published up to September 2023 using electronic databases of PubMed, Embase, Scopus, and China National Knowledge Infrastructure (CNKI). A random-effects model was applied to calculate the combined relative risks (RR) with 95% confidence intervals (CIs) for the highest compared to the lowest categories of DASH score in relation to diabetes mellitus risk. Heterogeneity among the included studies was assessed using the Cochran’s Q test and I-squared ( I 2 ) statistic. Literature search, study selection, data extraction, and quality assessment were performed by two independent reviewers.
Fifteen studies involving 557,475 participants and 57,064 diabetes mellitus cases were eligible for our analyses. Pooled analyses from included studies showed that high adherence to the DASH diet was significantly associated with a reduced risk of diabetes mellitus (RR: 0.82; 95% CI: 0.76–0.90, P < 0.001). Moreover, the dose-response meta-analysis revealed a linear trend between adherence to the DASH diet and diabetes mellitus (RR:0.99; 95%CI: 0.97–1.02, P dose-response = 0.546, P nonlinearity = 0.701). Subgroup analyses further revealed a significant inverse association between adherence to the DASH diet and diabetes mellitus risk in case-control studies (RR: 0.65; 95%CI: 0.29–1.43, P < 0.001), with a marginal inverse association in cohort studies (RR:0.83; 95%CI: 0.76–0.91, P < 0.001). Additionally, we conducted analyses separately by comparison and found a significant inverse association between DASH diet and diabetes mellitus risk in T3 vs T1 comparison studies (RR = 0.74; 95%CI: 0.64–0.86, P = 0.012).
The findings of this study demonstrate a protective association between adherence to the DASH diet and risk of diabetes mellitus. However, further prospective cohort studies and randomized controlled trials are needed to validate these findings.
Similar content being viewed by others

Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies

The Effects of Magnesium Supplementation on Blood Pressure and Obesity Measure Among Type 2 Diabetes Patient: a Systematic Review and Meta-analysis of Randomized Controlled Trials

Mediterranean dietary pattern and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective cohort studies
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (DM) represents a major global health problem, which has become a severe, chronic, non-communicable disease after cardio-cerebrovascular diseases [ 1 ]. According to the statistics reported by the International Diabetes Federation (IDF) in 2021, approximately 537 million adults aged 20–79 years worldwide were affected by Type 2 DM, and this number is projected to 784 million by 2045 [ 2 ]. Additionally, the latest release global DM map reveals that China alone had 140.9 million people with DM in 2021, accounting for a quarter of the total global DM patients [ 3 ]. It is well-known that lifestyle risk factors, such as obesity, cigarette smoking, sedentary behavior, and unhealthy eating habits, play an important role in the prevention of DM [ 4 ]. Therefore, making appropriate food choices and adopting a healthy diet are essential strategies to prevent or delay the onset of DM [ 5 ].
In the past several decades, a growing body of evidence supports the potential role of diet in the prevention of DM [ 6 , 7 , 8 ]. Many epidemiological studies have specifically reported the links between the intakes of specific nutrients or foods, such as vegetables, fruit, and whole grains, and the risk of DM [ 9 , 10 ]. However, due to the complex interactions between dietary components and the potential synergistic effects of nutrients and foods consumed together, previous studies have revealed only a limited influence of diet on DM risk [ 11 ]. Given that, dietary pattern analysis, which takes into account the complexity of whole diet, has been widely applied in nutritional epidemiology [ 12 ]. Consistently, the results of dietary pattern analysis could be more easily translated into national dietary guidelines [ 7 ].
For instance, the Dietary Approaches to Stop Hypertension (DASH) diet, originally developed for the management of high blood pressure, has been proposed to potentially lower blood glucose levels [ 13 ]. The DASH diet, a well-established healthy dietary pattern, emphasizes high consumption of fruits, vegetables, whole grains, nuts, and legumes, moderate consumption of low-fat dairy products, as well as low consumption of sodium, sweetened beverages, and red and processed meats [ 14 ]. Notably, the DASH diet has been recommended as one of three healthy dietary patterns in the 2020–2025 United States Dietary Guidelines for the public [ 15 ].
To date, many epidemiological studies have found that adherence to the DASH diet is significantly associated with several non-communicable diseases, such as hypertension, cardiovascular disease, chronic kidney disease, and certain types of cancer [ 16 , 17 , 18 ]. Notably, less is known about the influence of the DASH diet on the risk of DM. In 2009, Liese et al. published the first study reporting the association between adherence to the DASH diet and type 2 DM in the multiethnic Insulin Resistance Atherosclerosis Study [ 8 ]. Since then, there have been considerable attentions in medical research on the relationship between greater adherence to the DASH diet and risk of DM [ 7 , 8 , 19 , 20 ]. However, the results are not entirely consistent. Although some epidemiological studies have shown the protective role of the DASH diet in the development of DM [ 7 , 8 , 19 , 21 ], others have found no association [ 20 , 22 ]. Moreover, an earlier meta-analysis of 20 randomized controlled trials (RCTs) on the DASH diet and its impact on type 2 DM risk demonstrated that the DASH diet could significantly reduce fasting insulin concentration [ 23 ]. Furthermore, to the best of our current knowledge, there is no published systematic review and dose-response meta-analysis evaluating the effect of adherence to the DASH diet on DM risk. Therefore, we performed a systematic review and dose-response meta-analysis of observational studies published from inception until September 2023 to assess the potential impact of adherence to the DASH diet on DM risk.
Materials and methods
We followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for reporting this study [ 24 ]. A protocol has been registered in the International Prospective Register of Systematic Reviews (CRD42023465848).
Search strategy
An electronic literature search via four databases, including PubMed, Embase, Scopus, and CNKI was performed to find related publications written in the English or Chinese languages published from their dates of inception up to September 2023, with the following terms: {[“diabetes” (all fields) OR “diabetes mellitus” (all fields)] AND [“DASH score” (all fields) OR “DASH diet” (all fields) OR “DASH” (all fields) OR “Dietary Approaches To Stop Hypertension” (all fields) OR “Dietary Approaches To Stop Hypertension” (MeSH)]}. Moreover, we also found potentially related articles by manually searching the reference lists of retrieved articles and previously published reviews. The current search strategy was carried out by two independent reviewers (B.-F.C and X.-Y.Q).
Study selection
Two reviewers (B.-F.C and X.-Y.Q) independently screened the titles and abstracts of potential articles retrieved during the initial search, and ascertained studies exploring the relationship between adherence to the DASH diet and DM risk. After all reviewers agreed, the full-text versions of articles were reviewed according to the inclusion and exclusion criteria for the present systematic review and meta-analysis. The following studies were eligible for our analyses: (1) observational studies (e.g., case-control, cohort, or cross-sectional studies) performed in participants aged ≥18 years; (2) reported the data on the association between adherence to the DASH diet and DM risk; (3) provided the multivariable adjusted RRs, HRs, ORs with their corresponding 95%CIs of DM for the highest versus the lowest categories of DASH diet score (or sufficient data to calculate them); (4) If the data in the retrieved article lacked sufficient detail, the corresponding author of the original study is contacted via email; (5) DM diagnoses were confirmed by clinical interviews or self-report on a previous physician- made diagnosis of DM. Also, studies were excluded if they met one of the following criteria: (1) non-observational studies, e.g., letters, editorials, conference abstracts, reviews, or case reports; (2) studies written in non-English or non-Chinese; (3) irrelevant articles; (4) HRs, RRs or ORs with 95%CIs were not reported in the study. Any disagreements were settled by discussion or in consultation with the third reviewer (X.-M.S). The study population, exposure, comparison, outcome, and study design (PEICO) are shown in Table S1 .
Data extraction
Two independent reviewers (B.-F.C and X.-Y.Q) extracted the following information from the selected studies: the first author’s last name, publication year, location, study design, total number of participants, number of DM cases, mean age/age range for cases and participants, dietary assessment method, the factors that were adjusted or matched for in analyses, and reported risk estimates (HRs/ ORs/RRs) and their corresponding 95%CIs for highest versus lowest categories of DASH diet score.
Quality assessment
The Newcastle-Ottawa Quality Scale(NOS) was applied by two reviewers to independently assess the quality of included studies in this meta-analysis [ 25 ]. According to this scale, each study could be assigned a maximum score of 9 points for three main domains: selection(range 0–4 points), comparability (range 0–2 points), and assessment of outcomes (range 0–3 points). Studies scoring 7–9 points, 4–6 points, and 0–3 points, were identified as being high, medium, and low quality, respectively [ 26 ]. Disagreements were resolved by discussion to reach a consensus.
Statistical analysis
In this meta-analysis, we used RRs and 95%CIs as the effect size for the main analyses. Besides, ORs were converted into RRs using the following formula: RR = OR/[(1 − P 0 ) + (P 0 *OR)], in which P 0 shows the incidence of DM in the non-exposed group [ 27 ]. Subsequently, the RRs and corresponding 95% CIs for comparing incident DM between the highest and the lowest categories of DASH diet scores, were used to calculate log-transformed RRs with their corresponding standard errors (SEs). The Cochran’s Q test and I 2 statistic were utilized to evaluate the potential sources of between-study heterogeneity. P -values of Cochran’s Q test >0.10 or I 2 > 50% were considered to indicate high heterogeneity among the included studies, and then a random-effects model(DerSimonnian and Laird method) was used to pool the RRs. Otherwise, a fixed-effects model was used to calculate the pooled RRs [ 28 ]. If high heterogeneity was present, sensitivity and subgroup analyses were used to explore potential sources of heterogeneity. Subgroup analyses were performed based on comparison (Q5 vs Q1/Q4 vs. Q1/T3 vs. T1), mean age (>50 y/<50 y), country (Western countries/Asian countries), and study design (cohort/case-control studies). If more than 10 studies were available, publication bias was assessed through the visual inspection of the funnel plots and quantified by both Begg’s and Egger’s tests [ 29 ]. Sensitivity analyses were performed to explore the extent to which the pooled RRs might be affected by a single study or a group of studies. More importantly, we also performed a dose-response meta-analysis to estimate the RRs for each 1-score increase in DASH diet adherence. A two-stage GLST model based on generalized least squares was used to examine potential linear or non-linear dose-response relationship between adherence to the DASH diet and DM risk. All data analyses were carried out using STATA, version 11.2 (Stata Corp, College Station, TX). A 2-sided P value < 0.05 was considered statistically significant.
Search results
In the initial search, we retrieved 1373 articles through four database search and the reference lists of included studies and previously published reviews. After removing 563 duplicates, 810 articles were left for further screening. Consistently, reading titles and abstracts of these articles led to the exclusion of 773 articles because they didn’t report the relationship between adherence to DASH diet and risk of DM. Accordingly, the remaining thirty-seven full-text articles were examined for eligibility, and 22 were excluded for the following reasons: 5 did not evaluated DM risk; 1 lacked sufficient data and the corresponding author of this study could not be contacted; 16 did not mention DASH diet score. Finally, fifteen studies(13 cohort and 2 case-control studies) with 557,475 participants and 57,064 cases of DM were included for this systematic review and meta-analysis [ 6 , 7 , 8 , 19 , 21 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 35 , 36 , 37 ]. Figure 1 . indicated the flow chart of article selection process.

Flow chart of the process of study selection
Study characteristics
Characteristics of each eligible study are shown in Table 1 . Of these included studies, thirteen were cohort studies [ 7 , 8 , 19 , 21 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 35 , 36 ], and two were case-control studies [ 6 , 37 ]. The publication dates of these studies mentioned above varied between 2009 and 2023. Age of study participants ranged from 18 to 84 years. Sample size ranged from 334 to 166500. Eight of the included studies were performed in the United States [ 7 , 8 , 19 , 21 , 30 , 22 , 32 , 33 ], three in Iran [ 6 , 20 , 34 ], one in Taiwan China [ 35 ], one in Singapore [ 31 ], one in Brazil [ 36 ], and one study in Europe [ 37 ]. Fourteen of included studies used food frequency questionnaires(FFQs) to collect dietary data [ 7 , 8 , 19 , 21 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 35 , 36 , 37 ], and remaining one study used 24-h dietary recall [ 6 ]. In addition, all included articles used methods designed by Fung et al. (7 food groups and sodium) [ 7 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 36 ], Dixon et al. (7 food groups, saturated fat and alcohol) [ 6 ], Günther et al. (8 food groups) [ 16 ], Sacks et al. (7 food groups and sodium) [ 19 , 21 , 35 , 37 ] to extract DASH diet. Finally, based on the NOS scores, all of the included studies were considered to be of high-quality studies [ 6 , 7 , 8 , 19 , 21 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 35 , 36 , 37 ].
Adherence to the DASH diet and DM
Combining 17 effect sizes fSacksrom fifteen articles (557,475 participants and 57,064 cases of DM) were included to evaluate the relationship between adherence to the DASH diet and risk of DM in this study. Figure 2 showed the evidence of a reduced risk of DM in the highest compared with lowest categories of DASH diet (RR:0.82; 95% CI: 0.76, 0.90, P < 0.0001). The high heterogeneity was found among the included studies ( P < 0.0001; I 2 = 89.1%) and hence the effect was assessed using a random-effects model.

Forest plot of the association between adherence to the DASH diet and risk of DM
Dose-response analysis
Twelve studies [ 6 , 8 , 19 , 20 , 21 , 31 , 32 , 34 , 35 , 36 , 37 ] involving 11 cohort studies with DASH diet scores, were included in this dose-response analysis for DM risk. The dose-response meta-analysis showed a linear trend association between adherence to the DASH diet and DM risk (RR:0.99; 95%CI: 0.97–1.02, P dose-response = 0.546, P nonlinearity = 0.701) (Fig. 3 ). There was also a linear trend association between DASH diet and DM risk in the analysis of cohort studies ( P nonlinearity = 0.482, P dose-response = 0.599) (Fig. 4 ).

Dose-response association between adherence to the DASH diet and risk of DM in the analysis of twelve studies

Dose-response association between adherence to the DASH diet and risk of DM in the analysis of cohort studies
Subgroup analyses
Considering the high heterogeneity of the present study ( P < 0.0001; I 2 = 89.1%), subgroup analyses were performed to further explore the potential sources of heterogeneity among included studies (Table 2 ). Subgroup analyses was stratified basing on study design (cohort vs. case-control studies), country (Western vs. Asian countries), age (>50 y vs. <50 y), and comparison (Q5 vs.Q1/Q4 vs.Q1/T3 vs.T1). When we conducted analyses separately by study design (Fig. 5 ), we found a significant inverse association between adherence to the DASH diet and DM risk in case-control studies (RR = 0.65; 95% CI: 0.29–1.43, P < 0.001). In the cohort studies, there was a marginally significant association between the DASH diet and risk of DM (RR = 0.83; 95% CI: 0.76–0.91, P < 0.001). The stratified association between the DASH diet and risk of DM according to country (based on the random-effects model) is provided in Fig. 6 . There was significant heterogeneity in Asian countries, where a decreased risk of DM was shown (RR = 0.83; 95% CI: 0.66–1.05, P < 0.001). Similarly, in Western countries, the risk is similarly reduced (RR = 0.81; 95% CI: 0.77–0.85, P = 0.011). When we conducted analyses separately by age (Fig. 7 ), we found a significant inverse association between the DASH diet and DM risk in all age groups (<50 y: RR = 0.80; 95% CI: 0.66–0.97, P < 0.001 and >50 y: RR = 0.81; 95% CI: 0.77–0.86, P = 0.003). Similarly, we also performed stratified analysis based on comparison in Fig. 8 . Among comparison studies, there was more heterogeneity in T3 vs T1 ( P = 0.012, I 2 = 72.5%), and a significantly decreased risk of DM was shown (RR = 0.74; 95%CI: 0.64, 0.86; P = 0.012). In Q5 vs Q1 and Q4 vs Q1, a marginally significant association between DASH diet and risk of DM was shown.

Subgroup analyses of DASH diet and risk of DM according to study design

Subgroup analyses of DASH diet and risk of DM according to country

Subgroup analyses of DASH diet and risk of DM according to age

Subgroup analyses of DASH diet and risk of DM according to comparison
Publication bias
The funnel plots showed little evidence of asymmetry (Supplementary Fig. 1 ), and there is no evidence of publication bias (highest versus lowest categories of DASH diet score: Egger’s test, P = 0.088; Begg’s test, P = 0.711).
The quality of included studies was shown in Appendix 1. All included studies received an NOS score ≥7, and they were considered to be of high-quality studies [ 6 , 7 , 8 , 19 , 30 , 31 , 32 , 33 , 22 , 20 , 34 , 35 , 36 , 37 ].
Sensitivity analyses
Based on the results of sensitivity analysis (Supplementary Fig. 2 ), a cohort study performed by Mirmiran et al. [ 10 ] was outside the limit and might be the source of heterogeneity. When this study was excluded in the repeat analysis (Supplementary Fig. 3 ), the results showed a slight reduction in the pooled RRs of the association between adherence to the DASH diet and DM risk (RR = 0.81; 95%CI: 0.76–0.86, P < 0.001). Meanwhile, heterogeneity of included studies has decreased from 89.1% to 71.0%.
To our current knowledge, this is the first systematic review and meta-analysis to quantitatively summarize the published evidence on the association between greater adherence to the DASH diet and the risk of developing DM. Our analysis includes data from fifteen studies, involving 57,064 diabetes cases and 557,475 participants. The results demonstrated that high adherence to the DASH diet was associated with an 18% reduction in the risk of DM. Furthermore, the dose-response analysis showed a linear trend between DASH diet and DM risk. Sensitivity analysis also showed that excluding a certain study did not significantly alter the pooled effect of DASH diet adherence on DM risk. Overall, our findings add to the evidence of an inverse association between adherence to the DASH diet and DM risk and support the adoption of DASH diet adherence as a primary prevention strategy for DM.
Over the past four decades, the global epidemic of DM has continued to escalate [ 38 ]. As of 2021, an estimated 537 million adults worldwide are living with diabetes, with 80% of them residing in low- and middle-income countries [ 3 ]. This continued upward trend underscores the urgency of preventive measures. Among the various risk factors for DM, dietary factors, especially overall dietary patterns have garnered considerable attention [ 5 ]. Researchers have identified the whole dietary patterns using either a priori or a posteriori methods in nutritional epidemiology studies [ 12 ]. For example, the DASH diet, as a priori dietary pattern, emphasizes high consumption of fruits, vegetables, whole grains, nuts, and legumes, moderate consumption of low-fat dairy products, as well as low consumption of sodium, sweetened beverages, and red and processed meats [ 14 ]. Despite numerous epidemiological studies have investigated the relationship between adherence to the DASH diet and DM risk [ 20 , 22 , 33 , 34 , 35 , 36 , 37 ], the findings remain inconsistent. For instance, the Iran cohort study by Esfandiar and colleagues reported a 13% increased risk of DM associated with adherence to the DASH diet (RR = 1.13, 95%CI: 0.88–1.46) [ 34 ]. Conversely, the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) found that greater adherence to the DASH diet was not significantly related to the risk of newly diagnosed DM (OR:1.07; 95%CI: 0.84–1.36) [ 36 ]. The observed inconsistencies in published studies might be attributed to sociodemographic disparities among study populations and differences in the adjustment for potential confounders. For instance, in a previous systematic literature review and meta-analysis, Jannasch et al found that adherence to the DASH diet may change over time due to shifts in socioeconomic factors including poverty, globalization, and increased access to high-calorie foods [ 39 ]. Among the 15 studies included, most adjusted for critical confounding variables, such as age, BMI, physical activity, smoking status, and energy intake. Besides, some studies also adjusted for family history of DM and sex [ 39 ]. In our study, nine of the included studies were conducted in Western countries, while six studies were conducted in Asian countries and elsewhere. It is widely recognized that there exist significant differences between Eastern and Western diets [ 40 ]. Furthermore, to date, the relationship between adherence to the DASH diet and risk of DM has not yet been studied in a systematic review and dose-response meta-analysis. In this context, identifying the association between adherence to the DASH diet and DM risk through a systematic review and meta-analysis would seem to hold significant value.
Our analyses revealed a significant inverse association between adherence to the DASH diet and DM risk. Consitent with our results, several prior studies have reported an inverse association between “healthy” dietary patterns, which share some similar components with the DASH diet, and the risk of DM [ 41 ]. Consistently, a previous meta-analysis of 10 prospective cohort studies, involving 404,528 participants, found that high adherence to “healthy” dietary patterns significantly reduced the risk of type 2 DM (RR:0.86; 95%CI:0.82–0.90) [ 42 ]. However, contrary to our results, another studies did not find a significant association between adherence to the DASH diet and DM risk [ 35 , 36 ]. Although evidence associating DASH diet to DM remains somewhat inconsistent, some possible mechanisms have been reported to explain the observed inverse association. First, the DASH diet emphasizes increased consumption of fruits, vegetables, whole grains, and legumes, all of which are rich sources of dietary fiber. Increasing evidence has suggested that higher intake of dietary fiber was associated with a reduced risk of type 2 DM [ 43 ]. Moreover, Lattimer and Haub’s findings underscored that high intake of dietary fiber, particularly soluble fiber, could delay gastric emptying and limit carbohydrate absorption, reducing in lower postprandibular blood glucose and insulin levels [ 44 ]. Second, vegetables and whole grains are the most important foods in the DASH diet, both of which have a low glycaemic index and load. A recent systematic review and updated meta-analyses of prospective cohort studies showed a robust association between diets high in glycemic index and glycemic load and reduced risk of type 2 DM [ 45 ]. Third, whole grains consumption has been linked to a decrease the risk of overweight and obesity [ 46 ], both of which are recognized risk factors for DM [ 47 ]. Fourth, numerous studies have suggested that antioxidants, including vitamin C and carotenoids abundant in vegetables and fruits, are associated with the lower risk of obesity and hypertension, all of which are critical risk factors for type 2 DM [ 48 , 49 ]. Notably, Evans and colleagues proposed that dietary antioxidants could counteract oxidative stress, thereby enhancing insulin sensitivity and improving insulin secretion [ 50 ]. Fifth, adherence to the DASH diet’s beneficial effect on DM risk may stem from the consumption of low-fat dairy products. Prori studies demonstrated that higher dairy intake, particularly low-fat dairy, were correlated with a reduced risk of type 2 DM in men [ 51 ]. Lastly, the DASH diet, characterized by its emphasis on minimizing red and processed meats consumption, has been associated with DM risk. Shu et al. reported that high consumption of red meat was associated with an increased risk of type 2 DM [ 40 ]. As far as we know, red and processed meats are also rich sources of dietary iron. Epidemiological studies have shown that excessive iron stores in the body can promote insulin resistance, thereby increasing the risk of type 2 DM [ 52 ]. Additionally, processed meats often contain high levels of nitrates, nitrites, and nitrosamines, which are suspected to increase the risk of type 2 DM [ 40 ]. In summary, the aforementioned these mechanisms support the beneficial association between high adherence to the DASH diet and reduced risk of DM.
While a significant inverse relationship existed between adherence to the DASH diet and DM, our study also found a high heterogeneity (I 2 = 89.1%; P < 0.001). To address this, we performed subgroup analyses based on study design(cohort vs case-control studies), country (Western vs Asian countries), age groups (>50 y vs <50 y), and comparison (Q5 vs.Q1/Q4 vs.Q1/T3 vs.T1). The results suggested that significant heterogeneity might be mainly due to the differences in age and country. Specifically, when the results were stratified by age and country, the heterogeneity decreased from 89.1% to 64.6%, and 55.0%, respectively. Although significant heterogeneity cannot be fully explored, there are several possible explanations for the high heterogeneity. First, Considering the variations in DASH diet between Eastern and Western populations, it is important to note that while RRs/HRs/ORs were calculated using the highest category of DASH diet (with the lowest category as a reference), the definition of DASH diet may exhibit slight differences across different studies. These variations contribute to the observed significant heterogeneity. Second, the results were pooled from different populations with different dietary habits, which might result in significant heterogeneity. Additionally, fourteen of included studies used FFQs to collect dietary data. Thus, recall bias about dietary intakes is inevitable. Third, despite adjustments for potential confounders have been included in all the included studies, a certain degree of residual or unmeasured confounding factors may exist. Ultimately, substantial heterogeneity remained in the subgroup analyses, indicating the existence of unmeasured confounding factors.
Strengths and limitations
This systematic review and meta-analysis has several strengths and limitations. First, to our knowledge, this is the first comprehensive systematic review and dose-repsonse meta-analysis to clarify the association between adherence to the DASH diet and risk of DM. Our findings add the existing evidence regarding the beneficial effect of high adherence to the DASH diet on DM. Second, the rigorous selection of articles was carried out according to the pre-determined inclusion and exclusion criteria. Third, there were no obvious signs of publication bias in the funnel plot, and the Egger’s and Begg’s tests for publication bias were non-significant. Fourth, the quality assessment showed that the majority of studies included in the present meta-analysis were of high quality. Fifth, subgroup and sensitivity analyses were used to further explore the potential sources of heterogeneity, thereby improving the accuracy of the study results. Finally, the reported ORs/RRs /HRs were multivariate and all included studies had adjusted for some potential confounders, including age, physical activity, and total energy intake, which can affect the relationship between adherence to the DASH diet and DM risk. Despite these strengths, several limitations should be taken into account when interpreting our findings. First, in our analyses, two of the included studies used a case-control design, which is more susceptible to recall and selection bias than a cohort design. Additionally, potential confounding by pre-existing and undiagnosed diseases should be acknowledged. Thus, prospective cohort studies or randomized controlled trials are needed to further confirm the exact association between DASH diet and DM risk. Second, fourteen of the included studies used a FFQ to collect dietary intake data. However, this method may have caused misclassification and resulted in the under-or overestimation of DASH diet consumption. Furthermore, dietary intake data were self-reported, which might also lead to recall and selection biases. Third, the levels of the highest and the lowest categories of DASH diet scores were inconsistent across the included studies, which might have attenuated the true association between adherence to the DASH diet and DM risk. Also, adherence to the DASH diet may have altered over the follow-up period. Studies have reported that adherence to the DASH diet can fluctuate due to changes in socioeconomic factors such as poverty, globalization, and increased access to energy-dense foods. Fourth, significant heterogeneity was found in this study. Although we performed subgroup and sensitivity analyses to explore potential sources of heterogeneity, we could not fully ascertain and explain the sources of inter-study heterogeneity. Fifth, although high adherence to the DASH diet was associated with a decreased risk of DM, the results should be interpreted with caution. Meanwhile, there was also inconsistent adjustment for potential confounders in the included studies. As a result, the data included in our analyses may suffer from varying degrees of completeness and accuracy. Finally, it’s important to note that this study had a geographical restriction. The majority of included studies were performed in the United States, where dietary intakes markedly differ from those in Asian countries. This limitation might reduce the heterogeneity of this meta-analysis. Hence, further large prospective studies and randomized controlled trials are needed to confirm our findings across different regions and populations.
Conclusions
In conclusion, our study showed a significant inverse association between adherence to the DASH diet and the risk of DM. Our findings contribute to the available evidence supporting the protective role of adherence to the DASH diet against DM and highlight the importance of greater adherence to the DASH diet in the prevention of DM. However, future research, particularly well-designed prospective studies or randomized controlled trials, is needed to further confirm these findings across different geographic regions.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
H. Guo, H. Wu, Z. Li, The pathogenesis of diabetes. Int. J. Mol. Sci. 24 (8), 6978 (2023). https://doi.org/10.3390/ijms24086978
Article CAS PubMed PubMed Central Google Scholar
International Diabetes Federation. Diabetes facts & figures. https://idf.org/aboutdiabetes/what-is-diabetes/factsfigures.html .
International Diabetes Federation. IDF Diabetes Atla. 10th ed. (2023). http://www.diabetesatlas.org .
W.C. Knowler, E. Barrett-Connor, S.E. Fowler, R.F. Hamman, J.M. Lachin, E.A. Walker et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 (6), 393–403 (2002). https://doi.org/10.1056/NEJMoa012512
Article CAS PubMed Google Scholar
M. Neuenschwander, A. Ballon, K.S. Weber, T. Norat, D. Aune, L. Schwingshackl et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ 366 , l2368 (2019). https://doi.org/10.1136/bmj.l2368
Article PubMed PubMed Central Google Scholar
V. Izadi, H. Tehrani, F. Haghighatdoost, A. Dehghan, P.J. Surkan, L. Azadbakht, Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 32 (10), 1092–1096 (2016). https://doi.org/10.1016/j.nut.2016.03.006
S. Jacobs, B.E. Harmon, C.J. Boushey, Y. Morimoto, L.R. Wilkens, L. Le Marchand et al. A priori-defined diet quality indexes and risk of type 2 diabetes: the Multiethnic Cohort. Diabetologia 58 (1), 98–112 (2015). https://doi.org/10.1007/s00125-014-3404-8
A.D. Liese, M. Nichols, X. Sun, R.B. D’Agostino Jr, S.M. Haffner, Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 32 (8), 1434–1436 (2009). https://doi.org/10.2337/dc09-0228
E.S. Ford, A.H. Mokdad, Fruit and vegetable consumption and diabetes mellitus incidence among U.S. adults. Prev. Med. 32 (1), 33–39 (2001). https://doi.org/10.1006/pmed.2000.0772
F. Ghanbari-Gohari, S.M. Mousavi, A. Esmaillzadeh, Consumption of whole grains and risk of type 2 diabetes: A comprehensive systematic review and dose-response meta-analysis of prospective cohort studies. Food Sci. Nutr. 10 (6), 1950–1960 (2022). https://doi.org/10.1002/fsn3.2811
K. Esposito, D. Giugliano, Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care 29 (4), 954 (2006). https://doi.org/10.2337/diacare.29.04.06.dc05-2541
Article PubMed Google Scholar
F.B. Hu, Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 13 (1), 3–9 (2002). https://doi.org/10.1097/00041433-200202000-00002
A. Mahdavi, H. Mohammadi, S. Foshati, N. Shokri-Mashhadi, C.C.T. Clark, A. Moafi et al. Effects of the dietary approach to stop hypertension (DASH) diet on blood pressure, blood glucose, and lipid profile in adolescents with hemophilia: a randomized clinical trial. Food Sci. Nutr. 9 (1), 145–153 (2020). https://doi.org/10.1002/fsn3.1972
F.M. Sacks, L.P. Svetkey, W.M. Vollmer, L.J. Appel, G.A. Bray, D. Harsha et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 344 (1), 3–10 (2001). https://doi.org/10.1056/NEJM200101043440101
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. (2020). https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf .
X. Theodoridis, M. Chourdakis, L. Chrysoula, V. Chroni, I. Tirodimos, K. Dipla et al. Adherence to the DASH diet and risk of hypertension: a systematic review and meta-analysis. Nutrients 15 (14), 3261 (2023). https://doi.org/10.3390/nu15143261
A. Salehi-Abargouei, Z. Maghsoudi, F. Shirani, L. Azadbakht, Effects of dietary approaches to stop hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition 29 (4), 611–618 (2013). https://doi.org/10.1016/j.nut.2012.12.018
L. Shu, Y.Q. Huang, X.Y. Zhang, P.F. Zheng, Q. Zhu, J.Y. Zhou, Adherence to the dietary approaches to stop hypertension diet reduces the risk of breast cancer: a systematic review and meta-analysis. Front. Nutr. 9 , 1032654 (2023). https://doi.org/10.3389/fnut.2022.1032654
D.K. Tobias, F.B. Hu, J. Chavarro, B. Rosner, D. Mozaffarian, C. Zhang, Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch. Intern. Med. 172 (20), 1566–1572 (2012). https://doi.org/10.1001/archinternmed.2012.3747
P. Mirmiran, S. Hosseini, Z. Bahadoran, F. Azizi, Dietary pattern scores in relation to pre-diabetes regression to normal glycemia or progression to type 2 diabetes: a 9-year follow-up. BMC Endocr. Disord. 23 (1), 20 (2023). https://doi.org/10.1186/s12902-023-01275-9
D.K. Tobias, C. Zhang, J. Chavarro, K. Bowers, J. Rich-Edwards, B. Rosner et al. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 96 (2), 289–295 (2012). https://doi.org/10.3945/ajcn.111.028266
M.C. Otto, N.S. Padhye, A.G. Bertoni, D.R. Jacobs Jr, D. Mozaffarian, Everything in Moderation-Dietary Diversity and Quality, Central Obesity and Risk of Diabetes. PLoS One 10 (10), e0141341 (2015). https://doi.org/10.1371/journal.pone.0141341
F. Shirani, A. Salehi-Abargouei, L. Azadbakht, Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 29 (7-8), 939–947 (2013). https://doi.org/10.1016/j.nut.2012.12.021
M.D.F. McInnes, D. Moher, B.D. Thombs, T.A. McGrath, P.M. Bossuyt, the PRISMA-DTA Group, et al. Matic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA. 2018;319(4):388–396 https://doi.org/10.1001/jama.2017.19163
G.A. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos, et al. The Newcastle-Ottawa Scale(NOS) for assessing the quality of nonrandomized studies in meta-analysis; Ottawa Health Research Institute: Ottawa, ON, Canada, 2023
Y. Zhong, Y. Zhu, Q. Li, F. Wang, X. Ge, G. Zhou et al. Association between Mediterranean diet adherence and colorectal cancer: a dose-response meta-analysis. Am. J. Clin. Nutr. 111 (6), 1214–1225 (2020). https://doi.org/10.1093/ajcn/nqaa083
R.L. Grant, Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 348 , f7450 (2014). https://doi.org/10.1136/bmj.f7450
J.P. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560 (2003). https://doi.org/10.1136/bmj.327.7414.557
C.B. Begg, M. Mazumdar, Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4), 1088–1101 (1994)
A.J. Glenn, J. Li, K. Lo, D.J.A. Jenkins, B.A. Boucher, A.J. Hanley et al. The portfolio diet and incident type 2 diabetes: findings from the Women’s Health Initiative Prospective Cohort Study. Diabetes Care 46 (1), 28–37 (2023). https://doi.org/10.2337/dc22-1029
G.C. Chen, W.P. Koh, N. Neelakantan, J.M. Yuan, L.Q. Qin, R.M. van Dam, Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am. J. Epidemiol. 187 (12), 2651–2661 (2018). https://doi.org/10.1093/aje/kwy183
L. de Koning, S.E. Chiuve, T.T. Fung, W.C. Willett, E.B. Rimm, F.B. Hu, Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 34 (5), 1150–1156 (2011). https://doi.org/10.2337/dc10-2352
S. Jacobs, C.J. Boushey, A.A. Franke, Y.B. Shvetsov, K.R. Monroe, C.A. Haiman et al. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br. J. Nutr. 118 (4), 312–320 (2017). https://doi.org/10.1017/S0007114517002033
Z. Esfandiar, F. Hosseini-Esfahani, P. Mirmiran, F. Azizi, Diet quality indices and the risk of type 2 diabetes in the Tehran Lipid and Glucose Study. BMJ Open Diabetes Res. Care 10 (5), e002818 (2022). https://doi.org/10.1136/bmjdrc-2022-002818
R. Lin, K.L. Chien, M.C. Tsai, Y.J. Wang, L.Y. Hsu, Association between a priori and a posteriori dietary patterns and the risk of type 2 diabetes: a representative cohort study in Taiwan. J. Nutr. Sci. 12 , e16 (2023). https://doi.org/10.1017/jns.2023.8
M. Drehmer, A.O. Odegaard, M.I. Schmidt, B.B. Duncan, L.O. Cardoso, S.M.A. Matos et al. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 9 , 13 (2017). https://doi.org/10.1186/s13098-017-0211-7
InterAct Consortium, Adherence to predefined dietary patterns and incident type 2 diabetes in European populations: EPIC-InterAct Study. Diabetologia 57 (2), 321–333 (2014). https://doi.org/10.1007/s00125-013-3092-9
Article CAS Google Scholar
J. Pearson-Stuttard, Y.J. Cheng, J. Bennett, E.P. Vamos, B. Zhou, J. Valabhji et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10 (1), 46–57 (2022). https://doi.org/10.1016/S2213-8587(21)00288-6
F. Jannasch, J. Kröger, M.B. Schulze, Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J. Nutr. 147 (6), 1174–1182 (2017). https://doi.org/10.3945/jn.116.242552 . Epub 2017 Apr 19
L. Shu, X.M. Shen, C. Li, X.Y. Zhang, P.F. Zheng, Dietary patterns are associated with type 2 diabetes mellitus among middle-aged adults in Zhejiang Province, China. Nutr. J. 16 (1), 81 (2017). https://doi.org/10.1186/s12937-017-0303-0
S. Zeraattalab-Motlagh, A. Jayedi, S. Shab-Bidar, Mediterranean dietary pattern and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 61 (4), 1735–1748 (2022). https://doi.org/10.1007/s00394-021-02761-3
Z. Maghsoudi, R. Ghiasvand, A. Salehi-Abargouei, Empirically derived dietary patterns and incident type 2 diabetes mellitus: a systematic review and meta-analysis on prospective observational studies. Public Health Nutr. 19 (2), 230–241 (2016). https://doi.org/10.1017/S1368980015001251
P.Y. Wang, J.C. Fang, Z.H. Gao, C. Zhang, S.Y. Xie, Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J. Diabetes Investig. 7 (1), 56–69 (2016). https://doi.org/10.1111/jdi.12376
J.M. Lattimer, M.D. Haub, Effects of dietary fiber and its components on metabolic health. Nutrients 2 (12), 1266–1289 (2010). https://doi.org/10.3390/nu2121266
G. Livesey, R. Taylor, H.F. Livesey, A.E. Buyken, D.J.A. Jenkins, L.S.A. Augustin et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 11 (6), 1280 (2019). https://doi.org/10.3390/nu11061280
S. Schlesinger, M. Neuenschwander, C. Schwedhelm, G. Hoffmann, A. Bechthold, H. Boeing et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 10 (2), 205–218 (2019). https://doi.org/10.1093/advances/nmy092
S.E. Kahn, R.L. Hull, K.M. Utzschneider, Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 (7121), 840–846 (2006). https://doi.org/10.1038/nature05482
J.M. Nuñez-Cordoba, A. Alonso, J.J. Beunza, S. Palma, E. Gomez-Gracia, M.A. Martinez-Gonzalez, Role of vegetables and fruits in Mediterranean diets to prevent hypertension. Eur. J. Clin. Nutr. 63 (5), 605–612 (2009). https://doi.org/10.1038/ejcn.2008.22
X.M. Shen, L. Shu, Y.Q. Huang, X.Y. Zhang, P.F. Zheng, Q. Zhu, Association between dietary patterns and glycaemic control in a middle-aged Chinese population. Public Health Nutr. 25 (8), 1–9 (2021). https://doi.org/10.1017/S1368980021003931
J.L. Evans, I.D. Goldfine, B.A. Maddux, G.M. Grodsky, Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23 (5), 599–622 (2002). https://doi.org/10.1210/er.2001-0039
H.K. Choi, W.C. Willett, M.J. Stampfer, E. Rimm, F.B. Hu, Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch. Intern. Med. 165 (9), 997–1003 (2005). https://doi.org/10.1001/archinte.165.9.997
R. Jiang, J. Ma, A. Ascherio, M.J. Stampfer, W.C. Willett, F.B. Hu, Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am. J. Clin. Nutr. 79 (1), 70–75 (2004). https://doi.org/10.1093/ajcn/79.1.70
Download references
Author information
Authors and affiliations.
Department of Endocrinology, Pinghu First People’s Hospital, Pinghu, 314200, Zhejiang, Republic of China
Xiyan Quan, Xiaoming Shen, Chun Li, Yayuan Li, Tiangang Li & Baifan Chen
You can also search for this author in PubMed Google Scholar

Contributions
The authors’ responsibilities were as follows: B.-F.C and X.-M.S were responsible for study concept and design; B.-F.C conducted the statistical analysis; X.-Y.Q and B.-F.C were responsible for literature search and screening; X.-Y.Q and Y.-Y.L conducted the data extraction; C.L and T.-G.L performed the quality assessment; B.-F.C and X.-Y.Q were responsible for analysis and interpretation of the data. X.-Y.Q drafted the manuscript. B.-F.C and X.-M.S critically revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Baifan Chen .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary figure 1, supplementary figure 2, supplementary figure 3, supplementary tables1, supplementary figures caption, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Quan, X., Shen, X., Li, C. et al. Adherence to the dietary approaches to stop hypertension diet reduces the risk of diabetes mellitus: a systematic review and dose-response meta-analysis. Endocrine (2024). https://doi.org/10.1007/s12020-024-03882-5
Download citation
Received : 07 April 2024
Accepted : 16 May 2024
Published : 30 May 2024
DOI : https://doi.org/10.1007/s12020-024-03882-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetes mellitus
- Dietary Approaches to Stop Hypertension
- Systematic review
- Meta-analysis
- Epidemiology
- Find a journal
- Publish with us
- Track your research
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Case Presentation
Mr. E.A. is a 40-year-old black male who presented to his Primary Care Provider for a diabetes follow up on October 14th, 2019. The patient complains of a general constant headache that has lasted the past week, with no relieving factors. He also reports an unusual increase in fatigue and general muscle ache without any change in his daily routine. Patient also reports occasional numbness and tingling of face and arms. He is concerned that these symptoms could potentially be a result of his new diabetes medication that he began roughly a week ago. Patient states that he has not had any caffeine or smoked tobacco in the last thirty minutes. During assessment vital signs read BP 165/87, Temp 97.5 , RR 16, O 98%, and HR 86. E.A states he has not lost or gained any weight. After 10 mins, the vital signs were retaken BP 170/90, Temp 97.8, RR 15, O 99% and HR 82. Hg A1c 7.8%, three months prior Hg A1c was 8.0%. Glucose 180 mg/dL (fasting). FAST test done; negative for stroke. CT test, Chem 7 and CBC have been ordered.
Past medical history
Diagnosed with diabetes (type 2) at 32 years old
Overweight, BMI of 31
Had a cholecystomy at 38 years old
Diagnosed with dyslipidemia at 32 years old
Past family history
Mother alive, diagnosed diabetic at 42 years old
Father alive with Hypertension diagnosed at 55 years old
Brother alive and well at 45 years old
Sister alive and obese at 34 years old
Pertinent social history
Social drinker on occasion
Smokes a pack of cigarettes per day
Works full time as an IT technician and is in graduate school
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Type 2 Diabetes and Hypertension: A Study on Bidirectional Causality
Dianjianyi sun.
1 Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA
2 Department of Public Health Laboratory Sciences, West China School of Public Health, Sichuan University, Chengdu 610041, Sichuan Province, China
Yoriko Heianza
3 Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China
Vivian A. Fonseca
4 Section of Endocrinology and Metabolism, Tulane University School of Medicine, New Orleans, LA
5 Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA
6 Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA
AUTHOR CONTRIBUTIONS
Associated Data
In observational studies, type 2 diabetes (T2D) has been associated with an increased risk of hypertension (HTN), and vice versa; however, the causality between these conditions remains to be determined.
Objectives:
This population-based prospective cohort study sought to investigate the bidirectional causal relations of T2D with HTN, systolic and diastolic blood pressure (SBP and DBP) using Mendelian randomization (MR) analysis.
Methods and Results:
After exclusion of participants free of a history of heart failure, cardiovascular disease, cardiac procedures, and non T2D diabetes, a total of 318,664 unrelated individuals with qualified genotyping data of European descent aged 37–73 from UK Biobank were included. The genetically instrumented T2D and HTN were constructed using 134 and 233 single nucleotide polymorphisms (SNPs), respectively. Seven complementary MR methods were applied, including inverse variance weighted method (IVW), two median-based methods (simple and weighted), MR-Egger, MR-RAPS, MR-PRESSO, and multivariate MR. The genetically instrumented T2D was associated with risk of HTN (OR 1.07 [95% CI, 1.04–1.10], P =3.4×10 −7 ), whereas the genetically determined HTN showed no relationship with T2D (OR 0.96 [0.88–1.04], P =0.34). Our MR estimates from T2D to BP showed that the genetically instrumented T2D was associated with a 0.67 mm Hg higher SBP (95% CI 0.41–0.93, P =5.75×10 −7 ), but not with a higher DBP. There was no clear evidence showing a causal effect of elevated SBP or DBP on T2D risk. Positive pleiotropic bias was indicated in the HTN→T2D relation (OR of MR-Egger intercept 1.010 [1.004–1.016], P =0.001), but not from T2D to HTN (1.001 [0.998–1.004], P =0.556).
Conclusions:
T2D may causally affect HTN, whereas the relationship from HTN to T2D is unlikely to be causal. These findings suggest the importance of keeping an optimal glycemic profile in general populations, and BP screening and monitoring, especially SBP, in patients with T2D.
INTRODUCTION
Type 2 diabetes (T2D) and hypertension (HTN), the two leading components of the global burden of disease, are commonly found to coexist 1 – 3 . The co-existence of T2D and HTN confers a dramatically increased risk (2~4 fold) of cardiovascular disease, end-stage kidney disease, and death, compared to the normotensive and nondiabetic adults 3 . Hence, understanding the bidirectional relations between T2D and HTN is of significant public health importance regarding disease prevention and management of complications.
A large group of prospective studies has associated T2D with an increased HTN risk 4 – 6 , and a similar amount of evidence has been reported on the positive relationship between blood pressure and incident T2D 7 . Nevertheless, these prior observational data were limited for causal inference due to the potential bias introduced by confounding factors and/or reverse causality.
In recent years, Mendelian randomization (MR) analysis, a form of instrumental variable (IV) analysis that leverages the random assortment of genetic variants during gamete formation and therefore minimizes the influence of confounding and reverse causation, has been increasingly used in estimating causal inference between exposures and outcomes 8 . In the present study, we performed bidirectional MR analyses for causal inference between T2D and HTN among 318,664 participants from the UK Biobank, as well as bidirectional MR association analysis of T2D with systolic and diastolic blood pressure (SBP and DBP).
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to UK Biobank & the Access Team at ku.ca.knaboibku@ssecca
Data sources and study participants.
The UK Biobank is a large prospective study of over half a million participants aged 37–73 years living in the United Kingdom. Participant recruitment was conducted in 22 centers across the UK between 2006 and 2010, with a variety of individual-level health information obtained from self-administrated questionnaires, physical measurements, biological sample tests, and linked health records 9 . In the present study, we firstly excluded participants who withdrew from the cohort till Oct 16, 2018 (n=73), had a history of heart disease and procedures (n=61,827) or diabetes other than T2D 10 (n=5989), non-European (n=29815), and without validated genotyping data (n=110,670), leaving 318,664 unrelated European participants for the final analyses (a flow chart of selection of study participants was shown in details in Figure 1 ). Definitions of above-mentioned diseases and heart procedures are presented in the Online Table I and Table II , respectively. All participants provided electronic informed consent, and the study was approved by the NHS National Research Ethics Service (Ref: 11/NW/0382), and Institutional Review Board of Tulane University Health Sciences Center (Study number: 2018–1872).

ASCVD, atherosclerotic cardiovascular disease; T2D, type 2 diabetes; QC, quality control; PCA, principle component analysis.
Ascertainment of T2D and HTN.
Information on prevalent and incident T2D and HTN was regularly collected through cumulative medical records of hospital diagnoses and was supplemented by survey data from questionnaires and physical measures at baseline and in two repeated surveys. The International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) was used in death records; whereas both ICD-10-CM and ICD-9-CM were used in medical records. T2D was defined using a validated algorithm according to self-report diabetes data 10 , as well as ICD-9/10-CM coded hospital records ( Online Table I ). We defined HTN cases using ICD-10-CM code “I10” and ICD 9-CM”250” and “401”, self-reported physician-diagnosed HTN, and the use of blood pressure (BP) lowering medication not due to heart disease (including angiotensin-converting-enzyme inhibitor, angiotensin II receptor blocker, beta blocker, calcium channel blocker, and diuretics). For the BP measures in each survey, systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg was used to define HTN.
Measurements.
Sociodemographic characteristics at recruitment were obtained from local NHS Primary Care Trust registries before arrival at the Assessment Centre, including age (measured in years), sex (female/male), ethnicity (British/Irish/Any other white ethnicity), and Townsend deprivation index (a proxy for the socio-economic position). Information on lifestyles including physical activity (metabolic equivalents (METs) minutes per week calculated on the basis of the International Physical Activity Questionnaire short form 11 ), smoking, and alcohol drinking status (never/previous/current/missing) were obtained using a touch-screen questionnaire. Body mass index (BMI) was derived from weight in kilograms (BC-418MA body composition analyzer) divided by standing height in meters (Seca 202 stadiometer) squared. 9 SBP/DBP (mm Hg) were averaged over two repeated automated measurements (OMRON Healthcare Europe, NA, Hoofddorp) 9 . For participants who reported to be taking BP medication (19.08% of individuals) at baseline and two repeated surveys, we adjusted for medication use by adding 15 and 10 mm Hg to SBP and DBP, respectively. For a small portion (4~5%) of participants who participated in more than one follow-up visits, an average value of above mentioned quantitative measures (including PA METs minutes, BMI, and BP) was used in our analyses. For participants with missing data on BMI (0.29%), SBP (5.46%), DBP (5.46%), Townsend deprivation index (0.12%), and physical activity (0.69%), a “predictive mean matching” multiple imputation approach 12 was applied.
Genotype and imputed data.
UK Biobank genotyping was conducted by the Affymetrix Research Services Laboratory in Santa Clara, California, USA, using two similar custom-designed chips (UK BiLEVE array and UK Biobank Axiom array). General quality control procedures (P for Hardy–Weinberg equilibrium test≥1.0×10 −6 , call rate ≥90%, and imputation R 2 ≥0.3) were employed in the UK Biobank genetic data analysis. Forty genetic principal components (PCs) were calculated, accounting for the effects of the population structure and batch-based genotyping. Genotype imputation was further performed using the 1000 Genomes Phase 3 reference panel, resulting in a dataset of 92,693,895 variants in 487,409 individuals.
Genetic instrument variables for T2D and HTN.
Given the results of four genome-wide association studies (GWAS) in Europeans from DIAGRAM consortium 13 – 16 , we identified 134 out of 193 T2D-related single nucleotide polymorphisms (SNPs) that passed genotyping QC, and restricted to a set of only biallelic SNPs on 22 auto-chromosomes not in a linkage disequilibrium (LD) clumping (R 2 <0.01 within 1 Mb using European population genotype data originated from Phase 3 (Version 5) of the 1000 Genomes Project) as reference. Similarly, 233 out of 262 novel and previously reported SNPs from a recent GWAS of BP conducted by Warren et al 17 were satisfied with our inclusion criteria. Thus, a total of 367 SNPs were selected for genetic IVs ( Online Table III ).
Mendelian randomization analysis.
A diagram for MR was presented by using the genetic variants as IVs ( Online Figure I ). As recommended, six complementary MR approaches were adopted in our analyses to assess the causal effect of the exposure on the outcome and its robustness, including inverse variance weighted method (IVW), two median-based methods (simple and weighted), MR-Egger regression, MR-RAPS (Mendelian randomization using the robust adjusted profile score) 18 , and MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) 19 . We conducted heterogeneity tests in MR analyses using IVW and MR-Egger, sensitivity analyses were performed using the weighted median method and Leave-one-out analysis 20 ( Online Figure II-V ). If there was no evidence of directional pleiotropy ( P for MR-Egger intercept>0.05 8 , 21 ), the estimate from the inverse-variance weighted (IVW) method was considered as the most reliable indicator. Two types of pleiotropy-corrected MR estimates were also reported, including the MR-RAPS estimate (a method for correcting for pleiotropy using robust adjusted profile scores) 18 and the MR-PRESSO estimate (a method for correcting for outliers in IVW) 19 . A consistent MR effect across the six methods might indicate a true causal effect. 22 In addition, multivariable MR (MMR) analysis was performed by considering BMI and dyslipidemia as potential confounders or intermediators ( Online Table IV ).
A multivariate logistic or linear regression model was fitted for binary traits or continuous traits, respectively, as well as odds ratios (OR) and regression coefficients from two models used as the MR estimates. In the IV-exposure association analyses, we controlled for age, sex, Townsend deprivation index, assessment center (22 centers), batch effects (106 batches), and the first ten genetic PCs as covariates (model 1). In the IV-outcome association analyses, we further adjusted for BMI, dyslipidemia, smoking status, alcohol drinking status, and PA METs minutes per week (model 2).
All data analyses were conducted using R version 3.4.4. Missing data imputation was performed using the “ MICE ” R package, and MR analyses were conducted using the “ Two sample Mendelian randomization ”, “ MR-RAPS ” and “ MR-PRESSO ” R packages. The threshold of statistical significance was P<0.05 (2-sided α=0.05).
The average age of the 318,664 UK Biobank participants included was 56.2 years, 44.8% were men, and 93.1% were British ( Table 1 ). Two-thirds of the participants were overweight or obese (BMI≥25.0). Current smokers and drinkers accounted for 10.2% and 93.7%, respectively. There were 13,931 (4.4%) and 172,344 (54.1%) participants with T2D and HTN, respectively. Of note, 85.1% of T2D patients had HTN, while 6.9% of hypertensive participants were diabetic.
Characteristics of Participants from UK Biobank Used in the Analysis
SD, standard deviation; IQR, interquartile range; BMI, body mass index; MET, metabolic equivalent; SBP, systolic blood pressure; DBP, diastolic blood pressure.
In our bidirectional MR analysis, a total of 134 and 233 SNPs were included as genetic IVs for T2D and HTN, respectively ( Figure 1 ). In the T2D→HTN MR analysis by using IVW method, the genetically instrumented T2D increased HTN risk (OR: 1.07; 95% confidence interval [CI], 1.04–1.10, P =3.4×10 −7 ) without detected pleiotropy bias ( P =0.56). In contrast, pleiotropy bias was indicated in the HTN→T2D MR analysis ( P for MR-Egger intercept =0.001), and the IVW estimate showed there was no association between the genetically instrumented HTN and T2D (OR: 0.98; 95% CI, 0.90–1.08, P =0.70). Even after correcting for pleiotropy, our MR results still demonstrated that there was no causal effect of HTN on T2D (OR 0.96 [95%CI: 0.88–1.04], P =0.34 in MR-RAPS; and OR 0.95 [95%CI: 0.88–1.02], P =0.14 in MR-PRESSO). When analyzed using different MR methods, the causal effect of T2D on HTN was quite robust and consistent (ORs ranged from 1.06 to 1.09, all P <0.01), whereas the genetically instrumented HTN was not associated with T2D (ORs ranged from 0.95 to 1.05, all P>0.05 except for MR-Egger). Of note, in our MMR analysis, unbalanced horizontal pleiotropy owing to BMI but not dyslipidemia in the T2D→HTN relation was detected (OR, 0.94 [94% CI, 0.90–0.98], P =0.004), the causal effect of T2D on HTN was further enhanced after controlling for such negative bias (OR: 1.08; 95% confidence interval [CI], 1.05–1.11, P =8.7×10 −8 ) ( Online Table IV ). No horizontal pleiotropy due to BMI or dyslipidemia was detected in our HTN→T2D MR analyses ( Online Table IV ).
Furthermore, our MR analyses showed that the genetically instrumented T2D was consistently associated with a higher SBP (regression coefficients [β] in mm Hg ranged from 0.39 to 0.83, all P <0.01 except for P for MR-Egger =0.066), but not with a higher DBP (β ranged from −0.07 to 0.32) ( Table 2 ). When SBP/DBP→T2D relations were analyzed, neither the genetically instrumented SBP nor DBP increased T2D risk (OR ranged from 0.976 to 1.003 for the SBP→T2D relation, and OR ranged from 0.961 to 1.013 for the DBP→T2D relation) ( Table 3 ).
Mendelian Randomization Associations of Type 2 Diabetes with Systolic and Diastolic Blood Pressure using Genetic Instrument Variables
The effect size was presented as a regression coefficient and its 95% confidence interval. CI, confidence interval; T2D, type 2 diabetes; SBP, systolic blood pressure; DBP, diastolic blood pressure; IVW, the inverse-variance weighted (IVW) method; IV, instrument variables; MR, Mendelian randomization; MR-RAPS, an MR method for correcting for horizontal pleiotropy using robust adjusted profile scores; MR-PRESSO, an MR method for correcting for pleiotropy residual sum and outlier.
Mendelian Randomization Associations of Systolic and Diastolic Blood Pressure with Type 2 Diabetes using Genetic Instrument Variables
The effect size was presented as odds ratio and its 95% confidence interval. CI, confidence interval; T2D, type 2 diabetes; SBP, systolic blood pressure; DBP, diastolic blood pressure; IVW, the inverse-variance weighted (IVW) method; IV, instrument variables; MR, Mendelian randomization; MR-RAPS, an MR method for correcting for horizontal pleiotropy using robust adjusted profile scores; MR-PRESSO, an MR method for correcting for pleiotropy residual sum and outlier.
By using individual-level data for 318,664 UK Biobank participants, our bidirectional MR analyses showed consistent evidence that the genetically instrumented T2D increased HTN risk, whereas the MR estimates for the HTN→T2D relation were unlikely to be causal. In addition, the genetically instrumented T2D was strongly associated with a higher SBP rather than a higher DBP.
Our study is the first to provide strong evidence for a causal relationship of T2D with HTN risk, dominantly driven by the causal effect of T2D on a higher SBP instead of DBP. Goharian et al 23 , however, didn’t find a causal relationship between fasting glucose and SBP/DBP in healthy children and adolescents, likely due to insufficient statistical power (n=1506), narrowed variance of BP in the young population, a lack of cumulative impact of raised glucose level on BP in childhood, or pleiotropy bias. However, our findings are in line with prospective cohort studies showing T2D and hyperglycemia were associated with incident HTN 4 – 6 , as well as two MR studies conducted in general adults showing that higher glucose level 24 and greater genetic predisposition to T2D 25 were associated with increased arterial stiffness, which coincided with the development of HTN. The precise mechanisms for our findings of a causal relationship of T2D with a higher SBP but not a higher DBP are largely unknown. We speculate that an accelerated arterial stiffness resulting from T2D was associated with a greater increase in SBP instead of a higher DBP during the aging process 1 , 26 , 27 . Furthermore, a recent meta-analysis of 49 trials demonstrated that the antihypertensive treatment for lowering SBP rather than DBP in patients with diabetes reduced the risk of all-cause and cardiovascular mortality substantially, as well as incident myocardial infarction, stroke, heart failure, and end-stage renal disease 28 .
The T2D→HTN causality is biologically plausible. T2D shared broad cardiometabolic disorders, including obesity, insulin resistance (IR), β-cell dysfunction, inflammation, oxidative stress, vascular dysfunction, sodium retention, sympathetic excitation, renin-angiotensin-aldosterone system activation, and kidney damage, which has been widely proposed in the initiation and maintenance of HTN 3 , 29 . However, the magnitude of our genetic association of T2D on HTN (ORs ranged from 1.06 to 1.09) were much lower compared to the observational associations in the current study (multivariate-adjusted OR, 2.42 [95% CI, 2.32–2.53] in Online Table V ) and in Framingham Offspring Study (3.14 [95% CI, 2.17–4.54]) 30 . First, observational associations might be over-estimated due to various residual confounding and other bias (e.g., subtle arterial stiffness and kidney disease ahead of T2D onset 3 , 5 ). Second, the genetically instrumented T2D in our MR analyses might not be comprehensively characterized by a complex network of T2D pathophysiologic mechanisms 31 , 32 on HTN development. Third, the role of detection bias in inflating the observational estimates should also be considered, where patients with diagnosed and treated diabetes will be much more likely to have close surveillance of their BP and start on antihypertensive medications for reasons (e.g., heart failure, left ventricular hypertrophy, and chronic kidney disease) rather than elevated BP 5 . Additionally, as our MMR analysis showing a negative association between T2D-IVs and BMI, we speculated that the lower MR estimate might be as a result of undetected negative bias due to unbalanced pleiotropic effects of T2D-IVs on above mentioned biological pathways 8 . Further studies are warranted to elucidate the precise mechanisms.
Previous MR studies for a causal relationship from elevated BP to an increased risk of T2D have yielded inconsistent results. Aikens et al 33 reported that 1-mm Hg genetic increase in SBP was associated with a 2% increased risk of T2D, by adopting a 2-sample MR approach integrating summary-level GWAS data from 37,293 T2D cases and 125,686 controls. In contrast, a more recent MR study conducted by Zhu et al 34 using data from two community-based studies (n=162,030) showed that there were no causal relations from BP to T2D (OR, 1.07 [95%CI, 0.89–1.29]; P =0.44 for SBP→T2D; OR, 1.12 [95%CI, 0.94–1.33]; P =0.20 for DBP→T2D), which was in line with our findings. In a case-control setting, selection bias might be inferred as T2D patients were more likely to take BP lowering medications compared with controls, in which the use of β blocker and diuretics was associated with the increased risk of T2D 35 . Hence, MR estimates based on a general population would be less biased for causal inference between HTN and T2D risk 36 . Moreover, in compared with 29 BP-related SNPs accounted for only 2.2% of BP variance 37 were adopted as IVs in the above two MR studies 33 , 34 , a total number of over 200 SNPs here we used as IVs for BP might be more indicative as over 3.56% of the variance could be explained 17 .
Previous reported observational associations of HTN with T2D risk were unlikely to be causal, and instead, might be the results of two of bias ― collider bias or pleiotropy 8 . As mentioned previously 33 , a false association between HTN and T2D might occur when the study sample comprised an excess number of undiagnosed coronary artery disease cases in T2D patients (collider stratification) 38 . Our MR estimates, however, were less likely to be affected by collider bias as we excluded patients with prevalent and incident cardiovascular diseases before running MR analyses. But still, the causal HTN→T2D relationship can be true in terms of recently proposed biologic mechanisms that HTN manifests as vasoconstriction 39 , IR 40 , and inflammation 33 , which increase the risk of T2D.
Our study is the first to use a bidirectional MR approach to investigate the causal relationship between T2D and HTN/SBP/DBP among over 300 thousand adults, with a variety of sensitivity analyses and MR diagnostics performed for evaluating the robustness of our MR estimates. However, there are several limitations. First, the lack of data on glycemic traits (e.g., fasting glucose, insulin, and HbA1c) might have led to a small number of undiagnosed T2D cases, who were misclassified into controls. However, the information on the linked health records provided us with an alternative way to identify such cases. Second, the lack of data on insulin resistance, β cell function, chronic inflammation, and renal function limited further investigation of the precise mechanisms underlying the observed bidirectional associations between T2D and HTN. Third, the present MR analyses conducted in participants of European descent might limit the generalization of our findings in other ancestry groups.
In summary, our comprehensively bidirectional MR results suggest a potentially causal T2D→HTN relationship, especially a causal relationship of T2D with a higher SBP but not with a higher DBP. In contrast, the HTN→T2D association was unlikely to be causal. Our findings have clinical significance for maintaining an optimal glycemic profile for the general populations, and the importance of BP screening and monitoring, especially SBP, in patients with T2D.

T2D, type 2 diabetes; HTN, hypertension; IVs, instrument variables; IVW, the inverse-variance weighted (IVW) method; MR, Mendelian randomization; OR, odds ratio; CI, confidence interval; MR RAPS, an MR method for correcting for horizontal pleiotropy using robust adjusted profile scores.
a , MR-RAPS estimates were given after pruning two SNPs (rs73455744 and rs7041847) with extraordinarily large direct effects 18 ;
b , MR-RAPS estimates were given after pruning six SNPs (rs10922502, rs2760061, rs17477177, rs12628032, rs10948071, and rs449789) with extraordinarily large direct effects 18 ;
c , MR-PRESSO IV outlier detected was rs12899811;
d , MR-PRESSO IV outliers detected: rs4660293, rs62270945, rs2071518, rs2782980, rs8059962, and rs76326501.
NOVELTY AND SIGNIFICANCE
What is known.
- Type 2 diabetes (T2D) is associated with an increased risk of hypertension (HTN), and vice versa, in observational studies.
What New Information Does This Article Contribute?
- T2D may causally affect hypertension, whereas the HTN→T2D relation is unlikely to be causal.
In this bidirectional Mendelian randomization analysis, the genetic predisposition to T2D is associated with the development of HTN and elevated SBP, but not with elevated diastolic BP; whereas the “HTN to T2D” relation is unlikely to be causal. BP control, especially for SBP control, in patients with T2D is essential in clinical practice and self-management, and it is of great importance for general populations to maintain an optimal glycemic profile and a normal BP level.
Supplementary Material
314487 online, acknowledgments.
The authors thank the participants, the members, the project development and management teams in the present study in the UK for their outstanding commitment and cooperation. This research has been conducted using the UK Biobank Resource, approved project number 29256.
SOURCES OF FUNDING
Dr. Qi is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation Grant2011036. This study has been conducted using the UK Biobank Resource, approved project number 29256.
Nonstandard Abbreviations and Acronyms:
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
- Insulin, Medicines, & Other Diabetes Treatments
- Español
Insulin, Medicines, & Other Diabetes Treatments
On this page:
What medicines might I take for diabetes?
What type of diabetes do i have, what are the different types of insulin, what are the different ways to take insulin, what oral medicines treat type 2 diabetes, what other injectable medicines treat diabetes, what should i know about side effects of diabetes medicines.
- What questions should I ask about my diabetes medicines?
Do I have other treatment options for my diabetes?
Clinical trials for insulin, medicines, & other diabetes treatments.
Taking insulin or other diabetes medicines is often part of treating diabetes. In addition to making healthy food and beverage choices, getting physical activity, getting enough sleep, and managing stress, medicines can help you manage the disease. Some other treatment options are also available.
The medicine you take depends on the type of diabetes you have and how well the medicine controls your blood glucose levels, also called blood sugar levels. Other factors, such as any other health conditions you may have, medication costs, your insurance coverage and copays, access to care, and your lifestyle, may affect what diabetes medicine you take.
Type 1 diabetes
If you have type 1 diabetes , you must take insulin because your pancreas does not make it. You will need to take insulin several times during the day, including when you eat and drink, to control your blood glucose level.
There are different ways to take insulin . You can use a needle and syringe , an insulin pen , or an insulin pump . An artificial pancreas —also called an automated insulin delivery system—may be another option for some people.
Type 2 diabetes
Some people with type 2 diabetes can control their blood glucose level by making lifestyle changes. These lifestyle changes include consuming healthy meals and beverages, limiting calories if they have overweight or obesity , and getting physical activity.
Many people with type 2 diabetes need to take diabetes medicines as well. These medicines may include diabetes pills or medicines you inject, such as insulin. Over time, you may need more than one diabetes medicine to control your blood glucose level. Even if you do not take insulin, you may need it at special times, such as if you are pregnant or if you are in the hospital for treatment.
Gestational diabetes
If you have gestational diabetes , you can manage your blood glucose level by following a healthy eating plan and doing a moderate-intensity physical activity, such as brisk walking for 150 minutes, each week. If consuming healthy food and beverages and getting regular physical activity aren’t enough to keep your blood glucose level in your target range, a doctor will work with you and may recommend you take insulin. Insulin is safe to take while you are pregnant.
No matter what type of diabetes you have, taking diabetes medicines every day can feel like a burden sometimes. New medications and improved delivery systems can help make it easier to manage your blood glucose levels. Talk with your doctor to find out which medications and delivery systems will work best for you and fit into your lifestyle.
Several types of insulin are available. Each type starts to work at a different speed, known as “onset,” and its effects last a different length of time, known as “duration.” Most types of insulin reach a peak, which is when they have the strongest effect. After the peak, the effects of the insulin wear off over the next few hours or so. Table 1 lists the different types of insulin, how fast they start to work, when they peak, and how long they last.
Table 1. Types of insulin and how they work 1,2
Another type of insulin, called premixed insulin, is a combination of insulins listed in Table 1. Premixed insulin starts to work in 15 to 60 minutes and can last from 10 to 16 hours. The peak time varies depending on which insulins are mixed.
Your doctor will work with you to review your medication options. Talk with your doctor about your activity level, what you eat and drink, how well you manage your blood glucose levels, your age and lifestyle, and how long your body takes to absorb insulin.
Follow your doctor’s advice on when and how to take your insulin. If you're worried about the cost, talk with your doctor. Some types of insulin cost more than others. You can also find resources to get financial help for diabetes care .
The way you take insulin may depend on your lifestyle, insurance plan, and preferences. Talk with your doctor about the options and which one is best for you. Most people with diabetes take insulin using a needle and syringe, insulin pen, or insulin pump. Inhalers and insulin jet injectors are less common ways to take insulin. Artificial pancreas systems are now approved by the U.S. Food and Drug Administration (FDA). Talk with your doctor to see if an artificial pancreas is an option for you.
Needle and syringe
You can give yourself insulin shots using a needle and syringe . You draw up your dose of insulin from the vial—or bottle—through the needle into the syringe. Insulin works fastest when you inject it in your belly, but your doctor may recommend alternating the spot where you inject it. Injecting insulin in the same spot repeatedly could cause the tissue to harden, making it harder to take shots in that area over time. Other spots you can inject insulin include your thigh, buttocks, or upper arm, but it may take longer for the insulin to work from those areas. Some people with diabetes who take insulin need 2 to 4 shots a day to reach their blood glucose targets. Others can take a single shot. Injection aids can help you give yourself the shots.

An insulin pen looks like a writing pen but has a needle for its point. Some insulin pens come filled with insulin and are disposable. Others have room for an insulin cartridge that you insert and replace after use. Many people find insulin pens easier to use, but they cost more than needles and syringes. You may want to consider using an insulin pen if you find it hard to fill the syringe while holding the vial or cannot read the markings on the syringe. Different pen types have features that can help with your injections. Some reusable pens have a memory function, which can recall dose amounts and timing. Other types of “connected” insulin pens can be programmed to calculate insulin doses and provide downloadable data reports, which can help you and your doctor adjust your insulin doses.

An insulin pump is a small machine that gives you steady doses of insulin throughout the day. You wear one type of pump outside your body on a belt or in a pocket or pouch. The insulin pump connects to a small plastic tube and a very small needle. You insert the plastic tube with a needle under your skin, then take out the needle. The plastic tube will stay inserted for several days while attached to the insulin pump. The machine pumps insulin through the tube into your body 24 hours a day and can be programmed to give you more or less insulin based on your needs. You can also give yourself doses of insulin through the pump at mealtimes.
Another type of pump has no tubes. This pump attaches directly to your skin with a self-adhesive pad and is controlled by a hand-held device. The plastic tube and pump device are changed every several days.

Another way to take insulin is by breathing powdered insulin into your mouth from an inhaler device. The insulin goes into your lungs and moves quickly into your blood. You may want to use an insulin inhaler to avoid using needles. Inhaled insulin is only for adults with type 1 or type 2 diabetes. Taking insulin with an inhaler is less common than using a needle and syringe.
Jet injector
A jet injector is a device that sends a fine spray of insulin into the skin at high pressure instead of using a needle to deliver the insulin. It is used less commonly than a needle and syringe or a pen.
Artificial pancreas
An artificial pancreas is a system of three devices that work together to mimic how a healthy pancreas controls blood glucose in the body. A continuous glucose monitor (CGM) tracks blood glucose levels every few minutes using a small sensor inserted under the skin that is held in place with an adhesive pad. The CGM wirelessly sends the information to a program on a smartphone or an insulin infusion pump. The program calculates how much insulin you need. The insulin infusion pump will adjust how much insulin is given from minute to minute to help keep your blood glucose level in your target range. An artificial pancreas is mainly used to help people with type 1 diabetes.
You may need to take medicines to manage your type 2 diabetes, in addition to consuming healthy foods and beverages and being physically active. You can take many diabetes medicines by mouth. These medicines are called oral medicines.
Most people with type 2 diabetes start with metformin pills. Metformin also comes as a liquid. Metformin helps your liver make less glucose and helps your body use insulin better. This drug may help you lose a small amount of weight.
Other oral medicines act in different ways to lower blood glucose levels. Combining two or three kinds of diabetes medicines can lower blood glucose levels better than taking just one medicine.
Read about different kinds of diabetes medicines (PDF, 2.8 MB) from the FDA.
If you have type 1 diabetes, your doctor may recommend you take other medicines, in addition to insulin, to help control your blood glucose. Some of these medicines work to slow how fast food and beverages move through your stomach . These medicines also slow down how quickly and how high your blood glucose levels rise after eating. Other medicines work to block certain hormones in your digestive system that raise blood glucose levels after meals or help the kidneys to remove more glucose from your blood.
Besides insulin, other types of injected medicines (PDF, 2.8 MB) are available that will keep your blood glucose level from rising too high after you eat or drink. These medicines, known as glucagon-like peptide-1 (GLP-1) receptor agonists, 3 may make you feel less hungry and help you lose some weight. GLP-1 medicines are not substitutes for insulin.
Side effects are problems that result from taking a medicine. Some diabetes medicines can cause hypoglycemia , also called low blood glucose, if you don’t balance your medicines with food and activity.
Ask your doctor whether your diabetes medicine can cause hypoglycemia or other side effects, such as upset stomach and weight gain. Aim to take your diabetes medicines as your doctor instructs you, to help prevent side effects and diabetes problems.
If medicines and lifestyle changes are not enough to manage your diabetes, there are other treatments that might help you. These treatments include weight-loss (bariatric) surgery for certain people with type 1 or type 2 diabetes, or pancreatic islet transplantation for some people with type 1 diabetes.
Weight-loss surgery
Weight-loss surgery are operations that help you lose weight by making changes to your digestive system. Weight-loss surgery is also called bariatric or metabolic surgery.
This type of surgery may help some people who have obesity and type 2 diabetes lose a large amount of weight and bring their blood glucose levels back to a healthy range. How long the improved response lasts can vary by patient, type of weight-loss surgery, and the amount of weight the person lost. Other factors include how long a person had diabetes and whether the person used insulin. Some people with type 2 diabetes may no longer need to use diabetes medicines after weight-loss surgery . 4
Researchers are studying whether weight-loss surgery can help control blood glucose levels in people with type 1 diabetes who have obesity. 5
Pancreatic islet transplantation
Pancreatic islet transplantation is an experimental treatment for people with type 1 diabetes who have trouble controlling their blood glucose levels. Pancreatic islets are clusters of cells in the pancreas that make the hormone insulin. In type 1 diabetes, the body’s immune system attacks these cells. A pancreatic islet transplantation replaces destroyed islets with new islets from organ donors. The new islets make and release insulin. Because researchers are still studying pancreatic islet transplantation , the procedure is only available to people enrolled in research studies.
The NIDDK conducts and supports clinical trials in many diseases and conditions, including diabetes. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for insulin, medicines, and other diabetes treatments?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Find out if clinical trials are right for you .
Researchers are studying many aspects of diabetes medicines, including
- new types of insulin
- the most effective times to take diabetes medicines
- new types of monitoring devices and delivery systems
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical trials for insulin, medicines, and other diabetes treatments are looking for participants?
You can view a filtered list of clinical studies on insulin, medicines, and other diabetes treatments covered in this health topic that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank Stuart A. Weinzimer, M.D., Yale University School of Medicine
- Open access
- Published: 27 May 2024
Postoperative pneumonia after femoral fracture surgery: an in-depth retrospective analysis
- Mohammad Hamdan ORCID: orcid.org/0000-0003-2382-5585 1 ,
- Bassem I. Haddad ORCID: orcid.org/0000-0001-7246-2384 1 ,
- Jamil Almohtasib ORCID: orcid.org/0000-0002-5055-674X 2 ,
- Mira Eid ORCID: orcid.org/0000-0002-6371-2174 2 ,
- Tasneem Jamal Al-Din ORCID: orcid.org/0009-0004-6882-9066 2 ,
- Hashem A. Rayyan ORCID: orcid.org/0009-0003-7211-7224 2 ,
- Ahmad M. Altantawi 2 ,
- Abdussalam S. Akaheal 1 &
- Mohammad Ali Alshrouf ORCID: orcid.org/0000-0003-3806-2859 2
BMC Musculoskeletal Disorders volume 25 , Article number: 413 ( 2024 ) Cite this article
243 Accesses
Metrics details
Femoral fractures significantly contribute to disability, predominantly in the elderly. Despite this, data on postoperative pneumonia following femoral fracture surgeries remains sparse. Our study sought to explore the incidence and impact of postoperative pneumonia on outcomes following such surgeries.
A retrospective study analyzed femoral fracture patients hospitalized from 2016 to 2022. We scrutinized postoperative outcomes, including pneumonia, hospital stay duration, intensive care unit (ICU) admissions, and in-hospital mortality. We established stringent diagnostic criteria for postoperative pneumonia, incorporating both clinical signs and radiological evidence, excluding patients with prior infections or those discharged within 24 h post-surgery. Statistical analyses involved Chi-square and t-tests, linear regression, and logestic regression using SPSS.
Out of 636 patients, 10.8% were diagnosed with postoperative pneumonia. The average age was 79.55 ± 8.57 years, with a male prevalence of 47.8%. Common comorbidities were hypertension (78.3%), diabetes (60.9%), and cardiovascular diseases (40.6%). Surgical interventions were categorized as intramedullary nailing (40.6%), partial hip replacement (37.7%), and dynamic hip screw (21.7%). Postoperative pneumonia was associated with older age (AOR = 1.053, 95% CI 1.020 to 1.087, p = 0.002), ICU admission (AOR = 2.283, 95% CI 1.256 to 4.148, p = 0.007), and longer length of hospital stay (AOR = 1.079, 95% CI 1.030 to 1.130, p = 0.001). The presence of pneumonia was associated with a 2.621-day increase in hospitalization after adjusting for other variables ( p < 0.001, 95% CI: 1.454 to 3.789).
This study accentuates the clinical significance of postoperative pneumonia in femoral fracture patients, with a noted incidence of 10.8%. A notable association with older age, prolonged hospital stays, and ICU admissions was observed, underscoring the necessity of addressing this complication to improve patient outcomes and healthcare resource allocation.
Peer Review reports
Introduction
Femoral fractures are recognized as a serious, debilitating problem worldwide, especially concerning the geriatric population. As this issue continues to rise, with an annual estimate of 1.6 million patients with a hip fracture hospitalized [ 1 ], the number of hip fracture surgeries simultaneously expands alongside their associated complications, like postoperative pneumonia. It has been estimated that the total annual incidence of geriatric hip fractures in the Middle East in general is between 60 and 150 per 100,000 [ 2 , 3 , 4 , 5 ]. There is a scarcity of research examining the incidence and complications of hip fractures among the Jordanian population. According to recent research in Jordan, it was estimated that the annual incidence of hip fracture patients above the age of 55 in 2021 was approximately 96 cases per 100,000 individuals [ 6 ].
Factors such as advanced age, anemia, diabetes, prior stroke, the number of comorbidities, an American society of anesthesiologists (ASA) score ≥ III, general anesthesia, and delay in surgery were positively correlated to acquiring pneumonia after surgery [ 7 , 8 ]. On the other hand, many elements, regardless of pneumonia, were found to affect the length-of-stay (LOS) following a hip fracture surgery. These included advanced age, higher ASA physical status scores, comorbid burden, with the addition of female gender, severe obesity with a body mass index (BMI) exceeding 40, the use of a cemented implant in the total hip replacement, previous hip fractures, acute renal failure, diabetes, cerebrovascular disease, smokers, and others [ 9 , 10 , 11 ]. Others were linked with death after these surgeries. For instance, longer LOS, age over 80, poor mobility prior to the surgery, inability to return to baseline mobility, the presence of 3 or more comorbidities, an ASA over III or IV, chest infection, and heart failure [ 10 , 12 , 13 ]. In the management of hip fractures, particularly among elderly patients, postoperative complications significantly influence outcomes and mortality rates [ 14 , 15 , 16 ]. Among these complications, pneumonia stands out as a critical risk factor. The incidence of pneumonia following surgery not only complicates recovery but also markedly increases the risk of mortality [ 14 , 16 , 17 ]. This relationship is particularly pronounced in geriatric patients, who may already present with compromised health status pre-surgery.
While many of the aforementioned factors overlap, very limited data is available regarding the complications associated with the development of postoperative pneumonia after femoral fracture surgeries. Some evidence suggests that postoperative pneumonia significantly increased the 30-day mortality to be 27–43%, prolonged the hospital stay by 56%, increased the rate of sepsis by about 10%, and increased the risk of readmission by eightfold [ 18 , 19 ].The exact relationship between the incidence of pneumonia and these numerous complications is not well understood, and there are variable trends in this regard, which calls for further investigation. Moreover, studies suggest that the 30-day mortality was higher in hip fracture patients with coronavirus disease (COVID-19) infection; however, vaccinated patients with COVID-19 infection had a comparable mortality risk to those without the virus, indicating that the illness was less severe [ 20 , 21 ].
The incidence and implications of postoperative pneumonia have been a worthy topic of discussion, and scholars have attempted to investigate the potential risk factors for postoperative pneumonia following surgically treated femoral fractures in elderly patients [ 7 , 18 , 19 , 22 , 23 ]. Due to the lack of such data in this population, this study aims to determine the incidence and effects of postoperative pneumonia after femoral fracture surgery on the length-of-stay in the hospital, as well as the mortality rate and factors associated with postoperative pneumonia in the hospital in the Jordanian population. We hypothesize that the effects of pneumonia on our sample would increase both factors involved. Therefore, the results emerging from this study are expected to help optimize the care provided to these patients and eventually improve their quality of life.
Methodology
Study design.
We conducted a retrospective cohort study using data that was prospectively collected from patients diagnosed with femur fractures who have undergone surgical treatment at Jordan University Hospital between the years 2016 and 2022. Prior to the study’s beginning, the protocol was evaluated and approved by the Jordan University Hospital ethics committee, and the appropriate institutional review board (IRB) approved the study proposal (approval number 101,202,315,854; 2/3/2023). The Code of Ethics of the World Medical Association (Declaration of Helsinki) was followed while conducting the study.
Study population
The research includes patients diagnosed with femoral fractures, confirmed through imaging, and admitted to the University of Jordan Hospital within the time frame of 2016 to 2022. There are no specific limitations regarding the time frame between the occurrence of the fracture and admission. Inclusion criteria cover patients who underwent femoral fracture surgery as confirmed through imaging (e.g., X-rays, computed tomography (CT) scans), patients admitted to the University of Jordan Hospital within the specified time frame of 2016 to 2022, and had complete medical records for analysis. Exclusion criteria involve patients discharged within 24 h of admission, to ensure that we could accurately capture cases of postoperative pneumonia, which typically do not manifest immediately after surgery, and patients who were diagnosed with other infectious diseases (such as respiratory infections and urinary tract infections). The diagnosis was based on the presence of any symptoms or signs and additional diagnostic tests that were necessary, and this was done to avoid any possible confounding effects and focus only on the incidence and effects of postoperative pneumonia related to the surgery.
Data collection
The data were systematically retrieved from electronic medical records at Jordan University Hospital. The data encompassed vital patient information, such as gender and age. A comprehensive evaluation of previous medical history was done to identify conditions like diabetes, hypertension, and malignancies, along with any other cardiovascular, pulmonary, renal, and neurological diseases. Pulmonary co-morbidity was based on the patient having one of the following: asthma, COPD, or pulmonary fibrosis. Factors related to surgery, including if the surgery was performed within 48 h, type of fracture (classified according to the International Classification of Diseases, Tenth Revision (ICD-10) codes). These included femur neck fractures (S72.0), pertrochanteric fracture (S72.1), intertrochanteric fractures (S72.14), subtrochanteric fractures (S72.2), and femoral shaft fractures (S72.3)), type of surgical procedure (dynamic hip screw (DHS), intramedullary nailing (IMN), partial hip replacement (PHR), proximal femoral nail antirotation (PFNA)), and type of anesthesia (general vs. spinal) were also scrutinized. The hemoglobin level upon admission was also collected as a standard procedure aimed at evaluating patients’ overall health status and identifying potential risks associated with surgery, such as anemia or other hematological conditions. Additionally, the study took into account post-operative outcomes by assessing the occurrence of post-operative pneumonia, length of hospital-stay, and in-hospital mortality. In our study, strict hospital guidelines were implemented to control COVID-19 infections among patients. These guidelines mandate that all patients undergo a COVID-19 polymerase chain reaction (PCR) test within 48 h prior to the operation, and positive cases were quarantined and the surgery delayed.
Diagnosis of pneumonia
Post-operative pneumonia was diagnosed by the respiratory team in the hospital, depending on the clinical findings of the patient and an examination of the chest X-ray findings after surgery. The assessment of chest X-ray involved an examination by experienced radiologists, with specific attention to the presence of infiltrates, consolidations, or other abnormalities indicative of pneumonia. In addition, the clinical assessment included a thorough examination, which included the evaluation of temperature, respiration rate, white blood cell count, chest physical examination, and other relevant indicators.
Statistical analysis
All collected data was cleaned, coded, and analyzed on SPSS version 27. Categorical variables (e.g., gender) were presented as frequencies n (%), while continuous variables (e.g., age) were presented as means ± standard deviations. Mean differences in responses and domain scores were examined using the independent t-test and Chi-square test. Femoral fracture patients were split according to their diagnosis of pneumonia; mean differences were examined using the independent t-test. Variables that showed univariate analysis with a p < 0.1 were included in the logistic regression model in order to control for possible confounding factors for the predictors of post-operative pneumonia, which were summarized using adjusted odds ratio (AOR). A linear regression model was conducted to test the effect of post-operative pneumonia on the length of stay, adjusted for variables with p < 0.1 in univariant analysis. All statistical tests are conducted with a 95% confidence interval and a 5% error margin. A p-value of less than 0.05 is considered statistically significant.
A total of 636 patients were included in our study between 2016 and 2022. Of which, 69 patients (10.8%) were treated for post-operative pneumonia. The mean age for these patients was 79.55 ± 8.57, with 47.8% being male. Hypertension (78.3%), diabetes (60.9%), and cardiovascular disease (40.6%) were the most prevalent co-morbidities. Table 1 demonstrates the patient characteristics and clinical information in relation to the diagnosis of pneumonia.
In a logistic regression to investigate the influence of postoperative pneumonia on variables with a p-value less than 0.1, the results revealed positive statistically significant predictors including older age, length of stay, and ICU admission. Table 2 showcases the outcomes of the regression model, examining the influence of pneumonia on various clinical parameters.
We used linear regression to analyze the predictors of length of hospital stay. Relevant variables with a p-value < 0.1 in univariate analysis were entered into the model. Our analysis revealed that post-operative pneumonia and ICU admission were positively associated with the length of hospital stay, indicating longer stays. Conversely, higher hemoglobin levels and surgery performed within 48 h of admission were negatively associated with the length of hospital stay, indicating shorter stays (Table 3 ). Figure 1 represents a boxplot overlaid on top of a violin plot, illustrating the influence of postoperative pneumonia on the length of hospital stay, stratified by age groups. It demonstrates a significant increase in the length of hospital stay for patients diagnosed with pneumonia within the age groups of 71–80 and + 81 years ( p < 0.05).

Boxplot on top of the violin plot to demonstrate the impact of postoperative pneumonia on length of hospital stay with a subgroup analysis by age groups
Our retrospective study, which included 636 patients who had surgical procedures for femoral fractures from 2016 to 2022, provides insights into the prevalence and outcomes of postoperative pneumonia in this specific population. Significantly, 10.8% of patients were diagnosed with post-operative pneumonia. The participants had a mean age of 79.55 ± 8.57 and a balanced male-to-female ratio. Postoperative pneumonia was associated with older age, ICU admission, and a longer length of hospital stay. The presence of pneumonia was associated with a 2.621-day increase in hospitalization after adjusting for other variables. In addition, prolonged hospital stay was associated with surgery not being performed within 48 h of admission, lower hemoglobin levels upon admission, and ICU admission.
The effect of postoperative pneumonia (POP) on the duration of hospitalization following femoral fracture surgeries is a crucial aspect to consider when assessing patient care. Consistent with the existing literature, our study revealed that patients diagnosed with POP experience a longer hospital stay and are more likely to be admitted to the ICU [ 1 ]. This extended stay not only bears repercussions for individual patients but also exerts an effect on healthcare institutions by increasing the cost of care and utilizing scarce resources inefficiently. Therefore, it is essential to minimize the risk of POP by identifying high-risk patients and implementing strategies for the early detection and treatment of pneumonia. In a cohort study designed to evaluate a standardized POP prevention program, Kazaure et al. showcased a notable decrease of 43.6% in POP rates post-implementation [ 24 ]. Additionally, in a non-randomized, quasi-experimental study, Chang et al. highlighted the benefits of pulmonary rehabilitation in elderly patients with hip fractures, demonstrating a significantly lower incidence of POP and shorter hospital stay in patients receiving chest physiotherapy on the first post-surgery day in comparison to the control group [ 25 ]. Therefore, a multifaceted approach focused on identifying high-risk patients and adopting comprehensive preventive and management strategies emerges as a critical factor in mitigating the risk of POP and subsequently reducing the duration of hospitalization and ICU admissions.
On the other hand, the lack of a significant relationship between mortality and pneumonia in our population warrants further investigation and exploration, as it contradicts previous literature [ 19 ]. Jang et al. studied the effect of pneumonia on all-cause mortality after elderly hip fractures, which suggested an increase in mortality in pneumonia patients at 30 days to 1 year compared to non-pneumonia patients [ 26 ]. This could be attributed to the fact that the population used in their study is composed of patients over the age of 65, compared to the population in our study, which included patients from a wider range of age groups. The impact of COVID-19 pneumonia on overall mortality among hip fracture patients, which Fessler et al. studied, is another factor worth mentioning [ 27 ]. Several meta-analysis studies suggested a significant increase in mortality among patients with femoral or hip fractures who had a perioperative or concomitant COVID-19 infection [ 27 , 28 , 29 ]. In addition, in national research involving 3303 adults who underwent hip fracture surgery, the all-cause mortality for individuals who tested positive for COVID-19 was 27.0%, compared to 12.4% for those who tested negative for COVID-19 [ 30 ]. On the other hand, COVID-19 infection did not significantly modify 30-day and 6-month mortality, and in another study, they found no significant difference in 120-day mortality [ 31 , 32 ]. This information is critical for orthopedic surgeons to consider when managing patients with femoral fractures and concomitant COVID-19 infection. This could have an effect on our mortality results because our population includes patients from before and after the pandemic.
In our study, older age was a significant predictor of postoperative pneumonia in patients after femoral fracture. Similar to our study results, in a study including 3147 patients, they found a postoperative pneumonia rate of 5.8%, and they found age to be an independent risk factor for postoperative pneumonia [ 18 ].n In a multicenter retrospective study, they found that older age was associated with a higher risk for aspiration pneumonia in patients with hip fractures [ 33 ]. Moreover, low hemoglobin levels on admission have been linked to increased severity and adverse outcomes in various infectious diseases [ 34 , 35 ]. In the context of pneumonia, reduced hemoglobin levels may reflect compromised oxygen-carrying capacity, potentially exacerbating tissue hypoxia and impairing the immune responses that combat infection [ 35 ]. Furthermore, a previous study found that low hemoglobin levels upon admission were significantly associated with 6-month mortality in hip fracture patients [ 36 ]. In our study, lower hemoglobin levels were associated with prolonged hospital stays in the linear regression, but there was no significant difference in the hemoglobin level between the patients with postoperative pneumonia and those without.
The data in this paper ascertains that there are comorbidities more prevalent than others in those patients who developed postoperative pneumonia. These include hypertension, diabetes, and cardiovascular disease. However, only cardiovascular disease was significantly more prevalent in patients with prolonged LOS, regardless of the pneumonia diagnosis. While the specific comorbidities involved are not extensively discussed, a retrospective multi-center cohort study has found that broadly, the presence of preoperative comorbidities has been associated with a rise in LOS [ 7 , 10 ]. In addition, evidence suggests an increased prevalence of comorbidities coinciding with the initial incidence of hip fractures. A study by Yu Jiang et al., which involved patients undergoing surgical treatment for hip fractures, found hypertension as the most prevalent comorbidity at 52.0% (67.8% in our study), followed by 23.6% with type 2 diabetes (49.4% in our study), coronary heart disease (20.9%), stroke (18.7%), and arrhythmia (11.2%) (combined cardiovascular disease prevalence in our study was 29.1%) [ 37 ].
Previous studies have linked hypertension to respiratory infections, specifically pneumonia [ 38 ]. Chronic hypertension commonly coexists with endothelial dysfunction, immunological dysregulation, and altered inflammatory responses, suggesting a complicated interaction [ 39 , 40 ]. The higher prevalence (78.3%) of hypertension among patients with postoperative pneumonia in our study, warrants consideration. Also, hypertension has an established role in immune modulation and a potential impact on lung function [ 38 , 39 , 40 ]. Furthermore, similar to the previously mentioned effects of hypertension, diabetes also had several mechanisms that could increase their risk of infection, including increased altered immune cell function, bacterial proliferation, and changes in vascular permeability and endothelial cells, which was attributed to an increase in the incidence of postoperative pneumonia after an array of surgeries [ 41 ]. This element of immunosuppression may explain why diabetes was more prevalent (60.9%) in our cases of postoperative pneumonia. Nonetheless, its effects on increased LOS could be explained by an increase in other postoperative complications [ 42 ], which negatively impact surgical outcomes and require further interventions post-operatively. Moreover, it is well known that diabetes influences wound healing due to its deleterious effects on microcirculation and the metabolic pathway [ 43 ]. Cardiovascular diseases (CVD) were found to be a trigger for hemodynamic instability, which contributes to pulmonary congestion and edema that could result in an infection, possibly pneumonia [ 44 ]. Moreover, Lee et al. reported that for geriatric patients with femoral neck fractures undergoing hemiarthroplasty procedures, congestive heart failure doubled the chances of developing POP [ 45 ]. This further confirms our findings of increased POP and LOS in patients with cardiovascular diseases on univariate analysis. Preoperative cardiac evaluation guidelines set out by the American College of Cardiology/American Heart Association (ACC/AHA) categorize any orthopedic procedure, including femoral fracture repair, as “intermediate risk.” [ 46 ]. Specifically, heart failure has been previously found to increase LOS following hip fracture surgeries, which goes hand in hand with our findings [ 47 ].
A Danish study in 2019 confirmed our findings regarding postoperative pneumonia. It suggested that a delay of 12 h was associated with an increased risk of pneumonia in patients with no comorbidities, a delay of 24 h was associated with an increased risk of pneumonia in patients with a medium level of comorbidity, and a delay of 48 h was associated with an increased risk of reoperation due to infection in patients with a high level of comorbidity. In conclusion, a delay in surgery was associated with an increased risk of hospital-treated pneumonia and reoperations due to infection within 30 days of surgery [ 48 ]. Many articles have confirmed that a delay in surgery over 48 h is concurrent with worsening outcomes, hence increased LOS, reasoning that a delay in the performance of surgery is linked to major medical complications, minor medical complications, and pressure sores [ 49 , 50 ]. Furthermore, prior research involving polytrauma patients has demonstrated that early stabilization of femur fractures is linked to a reduced risk of acute respiratory distress syndrome and mortality [ 51 ]. Interestingly, a retrospective review conducted in 2018 revealed that increasing time to surgery was associated with longer postoperative lengths of stay but not with adverse outcomes of surgery [ 52 ].
The retrospective study investigating the impact of pneumonia on the length of hospital stay and mortality in elderly femoral fracture patients exhibits several notable strengths. The study addressed a clinically significant issue by investigating the impact of pneumonia, specifically in femoral fracture patients. In addition, understanding the interplay between these two conditions can inform healthcare strategies and improve patient care. Furthermore, a larger sample increases the likelihood of detecting true associations, strengthens the study’s external validity, and utilizes multivariate analysis controlled for potential confounding variables. However, certain limitations warrant consideration. The study’s retrospective design is inherently limited by its reliance on existing medical records, which may lack some critical information. Moreover, conducting the study at a single healthcare center may limit the generalizability of the findings. Also, failure to account for nosocomial cases could underestimate the true impact of hospital-acquired infections on the studied outcomes. Future studies could benefit from incorporating ASA grades and utilizing the CURB-65 scoring system, which could potentially enrich the analysis and provide deeper insights into the prognostic factors influencing postoperative outcomes.
In light of our findings, this study underscores the significant impact of postoperative pneumonia on the outcomes of patients undergoing femur fracture surgery. With a notable incidence of 10.8%, postoperative pneumonia was associated with older age, prolonged hospital stay, and intensive care unit (ICU) admissions, though it did not significantly affect mortality rates. In addition, prolonged hospital stay was associated with surgery not being performed within 48 h of admission, lower hemoglobin levels upon admission, and ICU admission. For clinicians, our study emphasizes the importance of early identification and management of risk factors for postoperative pneumonia. Implementing targeted interventions, such as preoperative optimization, timely surgical intervention, and enhanced postoperative care protocols, could mitigate the risk of developing pneumonia, improve overall outcomes, and lower the incidence of postoperative pneumonia in patients with femur fractures.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the sake of patient privacy but are available from the corresponding author on reasonable request.
Salarbaks AM, Lindeboom R, Nijmeijer W. Pneumonia in hospitalized elderly hip fracture patients: the effects on length of hospital-stay, in-hospital and thirty-day mortality and a search for potential predictors. Injury. 2020;51:1846–50.
Article CAS PubMed Google Scholar
Saleh YAL, Sulimani RA, Alomary S, Alnajjar YI, Vandenput L, Liu E, et al. Incidence of hip fracture in Saudi Arabia and the development of a FRAX model. Arch Osteoporos. 2022;17:56.
Article PubMed PubMed Central Google Scholar
Abdulla N, Alsaed OS, Lutf A, Alam F, Abdulmomen I, Al Emadi S, et al. Epidemiology of hip fracture in Qatar and development of a country specific FRAX model. Arch Osteoporos. 2022;17:49.
Maalouf G, Bachour F, Hlais S, Maalouf NM, Yazbeck P, Yaghi Y, et al. Epidemiology of hip fractures in Lebanon: a nationwide survey. Orthop Traumatol Surg Res. 2013;99:675–80.
Azizieh FY. Incidence of hip fracture in Kuwait: a national registry-based study. Arch Osteoporos. 2015;10:40.
Article PubMed Google Scholar
Dawod MS, Alisi MS, Saber YO, Abdel-Hay QA, Al-Aktam BM, Alfaouri Y, et al. Characteristics of Elderly hip fracture patients in Jordan: a Multicenter Epidemiological Study. Int J Gen Med. 2022;15:6591–8.
Lv H, Yin P, Long A, Gao Y, Zhao Z, Li J, et al. Clinical characteristics and risk factors of postoperative pneumonia after hip fracture surgery: a prospective cohort study. Osteoporos Int. 2016;27:3001–9.
Flikweert ER, Wendt KW, Diercks RL, Izaks GJ, Landsheer D, Stevens M, et al. Complications after hip fracture surgery: are they preventable? Eur J Trauma Emerg Surg. 2018;44:573–80.
Bilal DM, Niazi DNS, Mann B. Factors affecting the length of stay after total hip replacement; a retrospective analysis. Int J Orthop Sci. 2018;4:236–9.
Article Google Scholar
Lari A, Haidar A, AlRumaidhi Y, Awad M, AlMutairi O. Predictors of mortality and length of stay after hip fractures - a multicenter retrospective analysis. J Clin Orthop Trauma. 2022;28:101853.
Venishetty N, Beale J, Martinez J, Mounasamy V, Sambandam S. Understanding factors that impact the length of stay after total hip arthroplasty – A national in-patient sample-based study. J Clin Orthop Trauma. 2023;46.
Tjiang GC, Koppert CL, Hermans ET, Poelhekke LM, Dawson I. [Replacement of the femoral head due to fracture of the hip: prognostic factors for the duration of hospitalisation, institutionalisation and mortality]. Ned Tijdschr Geneeskd. 2003;147:2483–7.
CAS PubMed Google Scholar
Roche JJW, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374.
Article CAS PubMed PubMed Central Google Scholar
Gao Y, Zhang Y, Shi L, Gao W, Li Y, Chen H, et al. What are risk factors of postoperative pneumonia in geriatric individuals after hip fracture surgery: a systematic review and Meta-analysis. Orthop Surg. 2022;15:38–52.
Xiang B, Jiao S, Si Y, Yao Y, Yuan F, Chen R. Risk factors for postoperative pneumonia: a case-control study. Front Public Health. 2022;10:913897.
Lee SH, Kim KU. Risk factors for postoperative pneumonia in the Elderly following hip fracture surgery: a systematic review and Meta-analysis. Geriatr Orthop Surg Rehabil. 2022;13:21514593221083825.
Zhao K, Zhang J, Li J, Guo J, Meng H, Zhu Y, et al. In-Hospital postoperative pneumonia following geriatric intertrochanteric fracture surgery: incidence and risk factors. Clin Interv Aging. 2020;15:1599–609.
Tian Y, Zhu Y, Zhang K, Tian M, Qin S, Li X, et al. Incidence and risk factors for postoperative pneumonia following surgically treated hip fracture in geriatric patients: a retrospective cohort study. J Orthop Surg Res. 2022;17:179.
Bohl DD, Sershon RA, Saltzman BM, Darrith B, Della Valle CJ, Incidence. Risk factors, and clinical implications of Pneumonia after surgery for geriatric hip fracture. J Arthroplasty. 2018;33:1552–e15561.
Hall AJ, Clement ND, MacLullich AMJ, White TO, Duckworth AD. Vaccination against COVID-19 reduced the mortality risk of COVID-positive hip fracture patients to baseline levels: the nationwide data-linked IMPACT protect study. Osteoporos Int. 2024;35:353–63.
Ding L, Wei J, Wang B. The impact of COVID-19 on the prevalence, mortality, and Associated Risk factors for mortality in patients with hip fractures: a Meta-analysis. J Am Med Dir Assoc. 2023;24:846–54.
Shin K-H, Kim J-J, Son S-W, Hwang K-S, Han S-B. Early postoperative hypoalbuminaemia as a risk factor for postoperative pneumonia following hip fracture surgery. Clin Interv Aging. 2020;15:1907–15.
Yu Y, Zheng P. Determination of risk factors of postoperative pneumonia in elderly patients with hip fracture: what can we do? PLoS ONE. 2022;17:e0273350.
Kazaure HS, Martin M, Yoon JK, Wren SM. Long-term results of a postoperative pneumonia prevention program for the inpatient surgical ward. JAMA Surg. 2014;149:914–8.
Chang S-C, Lai J-I, Lu M-C, Lin K-H, Wang W-S, Lo S-S, et al. Reduction in the incidence of pneumonia in elderly patients after hip fracture surgery. Med (Baltim). 2018;97:e11845.
Jang S-Y, Cha Y, Yoo J-I, Yu Y-T, Kim J-T, Park C-H, et al. Effect of Pneumonia on all-cause Mortality after Elderly Hip fracture: a Korean Nationwide Cohort Study. J Korean Med Sci. 2019;35:e9.
Article PubMed Central Google Scholar
Fessler J, Jacobsen T, Lauritzen JB, Jørgensen HL. Mortality among hip fracture patients infected with COVID-19 perioperatively. Eur J Trauma Emerg Surg. 2021;47:659–64.
Patralekh MK, Jain VK, Iyengar KP, Upadhyaya GK, Vaishya R. Mortality escalates in patients of proximal femoral fractures with COVID-19: a systematic review and meta-analysis of 35 studies on 4255 patients. J Clin Orthop Trauma. 2021;18:80–93.
Freitas T, Ibrahim A, Lourenço A, Chen-Xu J. Mortality in COVID-19 patients after proximal femur fracture surgery: a systematic review and meta-analysis. Hip Int. 2023;33:762–70.
Levitt EB, Patch DA, Mabry S, Terrero A, Jaeger B, Haendel MA et al. Association Between COVID-19 and Mortality in Hip Fracture Surgery in the National COVID Cohort Collaborative (N3C): A Retrospective Cohort Study. J Am Acad Orthop Surg Glob Res Rev. 2022;6:e21.00282.
Boukebous B, Maillot C, Neouze A, Esnault H, Gao F, Biau D, et al. Excess mortality after hip fracture during COVID-19 pandemic: more about disruption, less about virulence—lesson from a trauma center. PLoS ONE. 2022;17:e0263680.
Chan G, Narang A, Aframian A, Ali Z, Bridgeman J, Carr A, et al. Medium-term mortality after hip fractures and COVID-19: a prospective multi-centre UK study. Chin J Traumatol. 2022;25:161–5.
Byun S-E, Shon H-C, Kim JW, Kim HK, Sim Y. Risk factors and prognostic implications of aspiration pneumonia in older hip fracture patients: a multicenter retrospective analysis. Geriatr Gerontol Int. 2019;19:119–23.
Jung SM, Kim Y-J, Ryoo SM, Kim WY. Relationship between low hemoglobin levels and mortality in patients with septic shock. Acute Crit Care. 2019;34:141–7.
Veronese N, Segala FV, Carruba L, La Carrubba A, Pollicino F, Di Franco G, et al. Anemia as a risk factor for disease progression in patients admitted for COVID-19: data from a large, multicenter cohort study. Sci Rep. 2023;13:9035.
Haddad BI, Hamdan M, Alshrouf MA, Alzubi A, Khirsheh A, Al-Oleimat A, et al. Preoperative hemoglobin levels and mortality outcomes after hip fracture patients. BMC Surg. 2023;23:266.
Jiang Y, Luo Y, Lyu H, Li Y, Gao Y, Fu X, et al. Trends in Comorbidities and Postoperative complications of geriatric hip fracture patients from 2000 to 2019: results from a hip fracture cohort in a Tertiary Hospital. Orthop Surg. 2021;13:1890–8.
Zekavat SM, Honigberg M, Pirruccello JP, Kohli P, Karlson EW, Newton-Cheh C, et al. Elevated blood pressure increases Pneumonia Risk: Epidemiological Association and mendelian randomization in the UK Biobank. Med. 2021;2:137–e1484.
Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–55.
Su S, Chen R, Zhang S, Shu H, Luo J. Immune system changes in those with hypertension when infected with SARS-CoV-2. Cell Immunol. 2022;378:104562.
López-de-Andrés A, Perez-Farinos N, de Miguel-Díez J, Hernández-Barrera V, Jiménez-Trujillo I, Méndez-Bailón M, et al. Type 2 diabetes and postoperative pneumonia: an observational, population-based study using the Spanish Hospital Discharge Database, 2001–2015. PLoS ONE. 2019;14:e0211230.
Merrell LA, Esper GW, Ganta A, Egol KA, Konda SR. Impact of poorly controlled diabetes and Glycosylated Hemoglobin Values in geriatric hip fracture Mortality Risk Assessment. Cureus. 2023;15:e36422.
PubMed PubMed Central Google Scholar
Barman PK, Koh TJ. Macrophage dysregulation and impaired skin Wound Healing in Diabetes. Front Cell Dev Biol. 2020;8:528.
Ramalho SHR, Shah AM. Lung function and cardiovascular disease: a link. Trends Cardiovasc Med. 2021;31:93–8.
Lee R, Lee D, Gowda NB, Probasco WV, Ibrahim G, Falk DP, et al. Surgical complications associated with congestive heart failure in elderly patients following primary hip hemiarthroplasty for femoral neck fractures. Eur J Orthop Surg Traumatol. 2019;29:1253–61.
Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 2002 guidelines on Perioperative Cardiovascular evaluation for noncardiac surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–499.
PubMed Google Scholar
Cullen MW, Gullerud RE, Larson DR, Melton LJ, Huddleston JM. Impact of heart failure on hip fracture outcomes: a population-based study. J Hosp Med. 2011;6:507–12.
Glassou EN, Kjørholt KK, Hansen TB, Pedersen AB. Delay in surgery, risk of hospital-treated infections and the prognostic impact of comorbidity in hip fracture patients. A Danish nationwide cohort study, 2005–2016. Clin Epidemiol. 2019;11:383–95.
Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246.
Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91:922–7.
Blair JA, Kusnezov N, Fisher T, Prabhakar G, Bader JO, Belmont PJ. Early Stabilization of Femur Fractures in the setting of Polytrauma is Associated with decreased risk of Pulmonary complications and Mortality. J Surg Orthop Adv. 2019;28:137–43.
Mitchell SM, Chung AS, Walker JB, Hustedt JW, Russell GV, Jones CB. Delay in hip fracture surgery prolongs postoperative hospital length of Stay but does not adversely affect outcomes at 30 days. J Orthop Trauma. 2018;32:629–33.
Download references
Acknowledgements
Not applicable.
No funding was received for conducting this study.
Author information
Authors and affiliations.
Department of Special Surgery, Division of Orthopedics, School of Medicine, The University of Jordan, Amman, 11942, Jordan
Mohammad Hamdan, Bassem I. Haddad & Abdussalam S. Akaheal
School of Medicine, The University of Jordan, Amman, 11942, Jordan
Jamil Almohtasib, Mira Eid, Tasneem Jamal Al-Din, Hashem A. Rayyan, Ahmad M. Altantawi & Mohammad Ali Alshrouf
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, MH and BH; methodology, TA and AMA; validation, MH, BIH and HAR; formal analysis, MAA and JA; investigation, JA, HAR, ME , AMA, and TA; resources, JA and MAA; data curation, JA, AMA, ME, TA, HAR, and MAA; writing—original draft preparation, MAA, and JA, HAR, ME , AMA, and TA; writing—review and editing, MAA, MH, BH, JA, HAR, ME , AMA, TA, and MAA; visualization, MAA and JA; supervision, MH, BH and MAA; project administration, MH. All authors made substantial contributions to conception and design and have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad Ali Alshrouf .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Institutional Review Board of the University of Jordan (approval number 101202315854; 2/3/2023). Informed written consent was obtained from the patients.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hamdan, M., Haddad, B.I., Almohtasib, J. et al. Postoperative pneumonia after femoral fracture surgery: an in-depth retrospective analysis. BMC Musculoskelet Disord 25 , 413 (2024). https://doi.org/10.1186/s12891-024-07529-4
Download citation
Received : 30 December 2023
Accepted : 17 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12891-024-07529-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Femoral fracture
- Postoperative complications
- Orthopedic procedures
BMC Musculoskeletal Disorders
ISSN: 1471-2474
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Hypertension is a risk factor for cardiovascular complications of diabetes. Clinical trials demonstrate that drug therapy versus placebo will reduce cardiovascular events when treating patients with hypertension and diabetes. A target BP goal of < 130/80 mmHg is recommended. Pharmacological therapy needs to be individualized to fit patients'needs.
Further assessment of this case study shows that the patient has poorly controlled hypertension, despite multiple medications. The patient also has metabolic syndrome complicated by diabetes, microalbuminuria and peripheral arterial disease. The patient's hypertensive treatment options must be evaluated in light of the fact that polypharmacy ...
Epidemiological studies indicate that there is a very high incidence of hypertension, including increases in resistant hypertension and associated CVD in patients with type 2 diabetes. 1 In the Framingham Heart Study, type 2 diabetes was associated with a 2- to 4-fold increased risk of hypertension, peripheral arterial disease, and myocardial ...
Type 2 diabetes mellitus and hypertension overlap in the population. In many subjects, development of diabetes mellitus is characterized by a relatively rapid increase in plasma glucose values. Wheth ... A Pulse Wave Analysis-Based Case-Control Study from an Urban Area of West India, Pulse, 10.1159/000519357, 9:3-4, (89-98), .
2.1.2 Clinical History. The patient's history is relatively ordinary for an overweight, diabetic, and hypertensive subject. However, during the last period (5-6 months), she has diurnal tiredness and perceives a disturbed sleep, even if her husband states that she sleeps regularly without snoring nor awakenings.
The case study created for this issue of the journal covers various aspects relating to the diagnosis and management of hypertension in type 2 diabetes. The format uses a typical clinical scenario as a tool for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Introduction. Hypertension (HTN) is present in more than 50% of patients with diabetes mellitus (DM) and contributes significantly to both micro and macrovascular disease in DM ( 1 - 4) ( Fig 1 ). Indeed, the risk for cardiovascular disease (CVD) is four-fold higher in patients with both DM and HTN as compared to the normotensive non-diabetic ...
A 45-year-old woman presented with hypertension, fatigue, and episodic confusion. After medications were administered, the blood pressure decreased but fatigue and confusion persisted. Four ...
Hypertension, obesity, and diabetes mellitus commonly co-exist, and all 3 conditions are associated with increased insulin resistance. ... Antihypertensive drug class interactions and risk for incident diabetes: a nested case-control study. J Am Heart Assoc. 2013; 2:e000125. doi: 10.1161/JAHA.113.000125 Link Google Scholar; 25.
OBJECTIVE: To review a case study of a patient with poorly controlled hyper-tension and diabetes. SUMMARY: Further assessment of this case study shows that the patient has poorly controlled hypertension, despite multiple medications. The patient also has metabolic syndrome complicated.by diabetes, microalbuminuria and peripheral arterial disease.
Stage 1 hypertension (HTN) is now defined as 130-139/80-89. In patients with stage 1 HTN, BP-lowering meds are recommended for those with ASCVD, diabetes, chronic kidney disease, or estimated 10 ...
Diabetes and hypertension often coexist and enhance the risk of CVD events synergistically. ... a more recent case-control study showed that fibrate use was associated with a reduced risk of ...
Background: Many diabetics develop hypertension, and it is a major risk factor for cardiovascular and microvascular complications. Objective: To review a case study of a patient with poorly controlled hypertension and diabetes. Summary: Further assessment of this case study shows that the patient has poorly controlled hypertension, despite multiple medications.
Cumulative incidence of diabetes by hypertension and arterial stiffness status. ... There is no consensus on the associations of AS and hypertension with the risk of diabetes. Some studies showed a positive association between AS and diabetes, 9-12 demonstrating that ... (CASE-J) trial. Diabetes Care. 2010; 33:1122-1127. doi: 10.2337/dc09 ...
The combined impact of diabetes and high blood pressure can increase the risk of cardiovascular disease, kidney disease, and other health issues. Without treatment, diabetes and high blood ...
Hypertension and type 2 diabetes are common comorbidities. Hypertension is twice as frequent in patients with diabetes compared with those who do not have diabetes. ... For example, in the San Antonio Heart Study, 85% of those with T2D had hypertension by the fifth decade of life, whereas 50% of those with hypertension experienced impaired ...
To explore the individual effect and interaction of diabetes and family history and other risk factors on hypertension in Han in Shanghai China. The method of case-control study with l:l matched ...
This case study describes the process of developing a toolkit to facilitate the use of evidence-based guidelines to manage hypertension and type 2 diabetes at the health-centre level. Methods: We developed and revised a preliminary toolkit based on the feedback received from key stakeholders.
The three mini-case studies presented here take you through the considerations of managing complex cases of hypertension in type 2 diabetes. Diabetes & Primary Care 's series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of type 2 diabetes.
Background Despite several epidemiological studies reporting a significant association between adherence to the Dietary Approaches to Stop Hypertension (DASH) diet and the risk of diabetes mellitus, the results remain controversial. In this systematic review and meta-analysis, we aimed to summarize the existing evidence from published observational studies and evaluate the dose-response ...
Patient Case Presentation. Mr. E.A. is a 40-year-old black male who presented to his Primary Care Provider for a diabetes follow up on October 14th, 2019. The patient complains of a general constant headache that has lasted the past week, with no relieving factors. He also reports an unusual increase in fatigue and general muscle ache without ...
Type 2 diabetes (T2D) and hypertension (HTN), the two leading components of the global burden of disease, are commonly found to coexist 1 - 3. The co-existence of T2D and HTN confers a dramatically increased risk (2~4 fold) of cardiovascular disease, end-stage kidney disease, and death, compared to the normotensive and nondiabetic adults 3.
Citation 1 A similar study topic was previously reported by Deravi et al Citation 2 in a systematic review, which also showed that the most frequent comorbidities in patients with severe COVID treated in the intensive care unit (ICU) were diabetes mellitus and hypertension. Diabetes can cause neutrophils and T cells to operate less well, which ...
We thank Songtao Cai and colleagues for their Correspondence. Our study's aim was to compare integrated management with standard vertical management, with both models of care delivered by health services staff in close to normal conditions. We showed that integrated management resulted in a very high level of retention in care for participants living with diabetes, hypertension, or both, which ...
In type 1 diabetes, the body's immune system attacks these cells. A pancreatic islet transplantation replaces destroyed islets with new islets from organ donors. The new islets make and release insulin. Because researchers are still studying pancreatic islet transplantation, the procedure is only available to people enrolled in research studies.
A study by Yu Jiang et al., which involved patients undergoing surgical treatment for hip fractures, found hypertension as the most prevalent comorbidity at 52.0% (67.8% in our study), followed by 23.6% with type 2 diabetes (49.4% in our study), coronary heart disease (20.9%), stroke (18.7%), and arrhythmia (11.2%) (combined cardiovascular ...