WHO World Malaria Report 2020: India continues to make Impressive Gains in reduction of Malaria Burden India the only high endemic country which has reported a decline of 17.6% in 2019 over 2018 India has sustained Annual Parasitic Incidence (API) of less than one since 2012
The World Malaria Report (WMR) 2020 released by WHO, which gives the estimated cases for malaria across the world, based on mathematical projections, indicates that India has made considerable progress in reducing its malaria burden. India is the only high endemic country which has reported a decline of 17.6% in 2019 as compared to 2018. The Annual Parasitic Incidence (API) reduced by 27.6% in 2018 compared to 2017 and by 18.4% in 2019 as compared to 2018. India has sustained API less than one since year 2012.
India has also contributed to the largest drop in cases region-wide, from approximately 20 million to about 6 million. The percentage drop in the malaria cases was 71.8% and deaths was 73.9% between 2000 to 2019.
India achieved a reduction of 83.34% in malaria morbidity and 92% in malaria mortality between the year 2000 (20,31,790 cases, 932 deaths) and 2019 (3,38,494 cases, 77 deaths), thereby achieving Goal 6 of the Millennium Development Goals (50-75% decrease in case incidence between 2000 and 2019).
Figure 1: Epidemiological trends of Malaria in India (2000-2019) Pv; Plasmodium Vivax & pf; Plasmodium Falciparum
Decrease in incidence of Malaria cases is also exhibited in the year-on-year tally. The cases and fatalities have declined significantly by 21.27% and 20% in the year 2019 (3,38,494 cases, 77 deaths) as compared to 2018 (4,29,928 cases, 96 deaths). The total number of malaria cases reported in 2020, till October, (1,57,284) has further decreased by 45.02 percent as compared to corresponding period of 2019 (2,86,091).
Malaria Elimination efforts were initiated in the country in 2015 and were intensified after the launch of National Framework for Malaria Elimination (NFME) in 2016 by the Ministry of Health and Family Welfare. National Strategic Plan for Malaria Elimination (2017-22) was launched by the Health Ministry in July, 2017 which laid down strategies for the next five years.
Figure 2: Epidemiological situation of Malaria in India (2015 – 2019)
The first two years saw a 27.7% decline in cases and 49.5% reduction in fatalities; 11,69,261 cases and 385 deaths in 2015to 8,44,558 cases and 194 deaths in 2017.
States of Odisha, Chhattisgarh, Jharkhand, Meghalaya and Madhya Pradesh disproportionately accounted for nearly 45.47 percent (1,53,909 cases out of India’s 3,38,494 cases) of malaria cases and 70.54 percent (1,10,708 cases out of India’s 1,56,940 cases) of falciparum Malaria cases in 2019. 63.64% (49 out of 77) of malaria deaths were also reported from these states.
Due to the efforts made by the Government of India in provision of microscopes, rapid diagnostics Long Lasting Insecticidal Nets (LLINs) – about 5 crores have been distributed in 7 North-East States, Chhattisgarh, Jharkhand, Madhya Pradesh and Odisha up to 2018-19 and another 2.25 crore LLINs are being supplied/distributed during current financial year to high burden areas leading to reduction in endemicity in these otherwise very high endemic states. Additional procurement of 2.52 crore LLINs is initiated.Use of LLINs has been accepted by the community at large and has been one of the main contributors to the drastic malaria decline in the country.
Decline of API in HBHI (High Burden High Impact) Regions of India (2016-2019)
WHO has initiated the High Burden to High Impact (HBHI) initiative in 11 high malaria burden countries, including India. Implementation of “High Burden to High Impact (HBHI)” initiative has been started in four states i.e. West Bengal and Jharkhand, Chhattisgarh and Madhya Pradesh in July, 2019. A key strategy to reignite progress is the “High burden to high impact” (HBHI) response, catalyzed in 2018 by WHO and the RBM Partnership to End Malaria continued to make impressive gains in India, with 18% reductions in cases and 20% reductions in death respectively, over the last 2 years.
Malaria has been made notifiable in 31 states/UTs (Andhra Pradesh, Arunachal Pradesh, Assam, Chhattisgarh, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Manipur, Mizoram, Nagaland, Odisha, Punjab, Rajasthan, , Sikkim, Tamil Nadu, Telangana, Tripura Uttar Pradesh, Uttarakhand, West Bengal, Pudducherry Chandigarh, Daman & Diu, D&N Haveli and Lakshadweep) and decline has been observed in the hitherto high endemic states. Percentage of decline in the year 2019 as compared to 2018 is as follows: Odisha – 40.35%, Meghalaya- 59.10%, Jharkhand – 34.96%, Madhya Pradesh –36.50% and Chhattisgarh –23.20%.
The figures and trends between last two decades clearly show the drastic decline in malaria. The malaria elimination target of 2030 looks achievable building on the Union Government’s strategic interventions in this regard.
GIS maps – Shrinking malaria endemicity (District level)
HFW/World Malaria Report/2ndDecember2020/1

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Supplements
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 7, Issue 6
- What India can learn from globally successful malaria elimination programmes
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Sachin Sharma 1 ,
- Reena Verma 1 ,
- Bhawna Yadav 1 ,
- Amit Kumar 1 ,
- Manju Rahi 2 ,
- http://orcid.org/0000-0002-3305-0034 Amit Sharma 1 , 3
- 1 ICMR-National Institute of Malaria Research , New Delhi , Delhi , India
- 2 Division of Epidemiology and Communicable Diseases , Indian Council of Medical Research , Delhi , Delhi , India
- 3 International Centre for Genetic Engineering and Biotechnology , New Delhi , Delhi , India
- Correspondence to Dr Manju Rahi; drmanjurahi{at}gmail.com ; Dr Amit Sharma; directornimr{at}gmail.com
India is targeting malaria elimination by 2030. Understanding and adopting the strategies employed by countries that have successfully eliminated malaria can serve as a crucial thrust in this direction for a geographically diverse country like India. This analysis is based on extensive literature search on malaria elimination policies, strategies and programmes adopted by nine countries (China, El Salvador, Algeria, Argentina, Uzbekistan, Paraguay, Sri Lanka, Maldives and Armenia) which have attained malaria-free status over the past decade. The key points which India can learn from their journey are mandatory time-bound response in the form of case reporting and management, rapid vector control response, continuous epidemiological and entomological surveillance, elevated community participation, more training and capacity building, private sector involvement, use of quality diagnostics, cross-border collaborations, inclusion of prevention of re-establishment programmes into the elimination plans, higher investment in research, and uninterrupted funds for successful implementation of malaria elimination programmes. These learnings would help India and other South Asian countries steer their programmes by devising tailor-made strategies for their own regions.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjgh-2022-008431
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
SUMMARY BOX
By 2021, 40 countries have been certified by the WHO as malaria-free. There are 85 malaria-endemic countries, with an estimated 241 million cases in 2020.
India continues to show a sustained decline in overall malaria but faces several challenges in its malaria elimination journey.
An overview of malaria elimination strategies which were central to the success achieved by nine countries in the past decade is presented here. Some of these can be emulated by India and other South Asian countries to overcome the challenges in their elimination drive.
Barriers to smooth adoption of these strategies in the Indian programme have also been described.
Introduction
The 2021 World Malaria Report (WMR) estimated 241 million cases with approximately 0.63 million malaria deaths from 85 malaria-endemic countries ( figure 1 ). Most of the deaths were reported in children under 5 years of age (~77%) and majority were in African nations (~96%). By 2021, 40 countries have been certified by the World Health Organization (WHO) as malaria-free, 1 two (Malaysia and the Islamic Republic of Iran) have achieved zero indigenous case status, and Azerbaijan and Tajikistan have applied for certification. 2 The world’s 11 highest malaria burden countries (India being one of them), accounting for 70% of global cases, have adopted the WHO’s High Burden to High Impact initiative to formulate a country-led response for malaria elimination. 3
- Download figure
- Open in new tab
- Download powerpoint
World map depicting country-wise contribution of global malaria cases in 2020 and WHO-certified malaria-free countries so far. 1 2
India is slated for malaria elimination by 2030. 4 It contributed 83% of the estimated malaria cases and 82% of malaria deaths in South-East Asia Region (SEAR) in 2020, according to the 2021 WMR. Plasmodium falciparum and P. vivax are the major prevalent parasites in India. The country contributed 51% of the global P. vivax cases in 2016, when the country launched the National Framework for Malaria Elimination, outlining the goals, strategies, guidelines and time-bound targets to eliminate malaria in India by 2030. 4 Through concerted efforts, India has managed to sustain the decline in overall burden, with 186 532 cases and 93 deaths reported in 2020 by India’s national programme ( figure 2 ). Malaria endemicity in India is heterogeneous, with the highest endemicity regions being tribal and forested areas of the country. Analysis of epidemiological data from 2000 to 2019 revealed that forested districts contributed ~32% of malaria cases and 42% of mortality due to malaria, while harbouring ~6.6% of the country’s population. 5 The different topographies, climatic conditions and ecosystems support breeding and survival of Anopheles species. The major challenges towards malaria elimination in India are large population size, population movement across regions of different endemicities (the risk of parasite carriers moving from high endemic to low endemic areas), undetected asymptomatic and subpatent malaria cases, multiple vectors, threat of drug and insecticide resistance, shortage of skilled human resources, lack of reporting from the private sector, and unplanned expansion of urban and semi-urban areas. These factors add to the complexity of malaria transmission and make malaria elimination challenging in India.
Reported number of malaria cases and deaths in India and species-wise break-up of cases in India in the last 10 years (2011–2020). Data source: Directorate of the National Centre for Vector Borne Diseases Control, Government of India, and WHO World Malaria Report 2021. 2
Several countries share the above challenges associated with malaria control and prevention but have successfully eliminated malaria. Here, we have selected nine countries as they have successfully eliminated malaria in the last decade (2011–2021). We chose these countries for the reason that in these 10 years the most impactful interventions, namely insecticide impregnated bed nets, rapid diagnostics and artemisinin-based drug therapy, were deployed and they made a major dent in the burden of malaria in endemic countries. 6 Moreover, molecular surveillance of parasites and systematic periodic vector surveillance studies were established as the monitoring mechanisms during this decade itself. Additionally, some of these countries share a similar ecological and epidemiological scenario, such as the dominance of P. vivax cases in Sri Lanka and the southern part of India.
Therefore, we feel that studying these countries’ practices and policies would be insightful for Indian malaria programme managers and malariologists. For example, the diverse mosquito species that caused malaria in Maldives and Uzbekistan and their elimination strategies for vector control could be additional lessons for India. Environmental engineering methods as adopted by Argentina could be helpful in tackling forest malaria in India. El Salvador had successful strategies for active surveillance of migrant populations searching for employment. In India, the migrant population is a threat for reintroducing malaria in states which have reached almost zero indigenous cases. The strategy of Algeria in combating P. falciparum and the involvement of non-governmental organisations (NGOs) and private sectors in Sri Lanka and Paraguay could be inspiring strategies for India to imbibe. It is essential to reach population groups even in the hard-to-reach and conflict-ridden areas to achieve elimination. In this regard, India could learn from Sri Lanka’s elimination drive even during a civil war. If we consider China, its population size is similar to India. Also, its battle, like India, has been a long one but successful, and therefore these countries’ strategies in eliminating malaria are a great learning lesson for us.
We also realise that it is difficult to emulate all the strategies and practices of the victorious countries due to diversity in the epidemiological and entomological picture and the different population scale in India. However, we believe that India can imbibe many of the best practices followed by these countries by closely studying the factors and influences behind the successful elimination of malaria, and if possible replicate them at the appropriate level in India. In this paper, we have analysed the strategies/policies used by these countries in their fight against malaria and have listed them in the following sections, which can serve as torchbearers for India.
Key strategies adopted by some selected countries
In 2021, China, in the WHO Western Pacific Region (WHO WPR), was declared malaria-free after reporting the last indigenous malaria case in 2016 7 —this was a culmination of efforts of over ~70 years. P. vivax was the major parasite species of concern in China. At the time of transition of their programme from control to elimination in 2011, P. vivax malaria cases (2118 cases) were ~1.5 times of P. falciparum (1269 cases). 8 The policies and strategies which became the cornerstone of malaria elimination programme in China were the following: (1) In 1967, China launched a national effort called ‘the 523 project’, which resulted in the discovery of the artemisinin group of highly effective antimalarial drugs, which are the most potent antimalarial drugs to date. (2) Within the control strategy of malaria, mass drug administration (MDA) was used on a large scale (1973–1983). (3) During the decline phase (1981–2000), the country implemented the strategy of environmental management as well as protective measures for exposed population {early distribution of insecticide impregnated bednets and indoor residual spray (IRS) for vector control}, and then a foci-based response (2000-2009) to reduce the high burden of malaria in different provinces by stratification based on transmission risk and incidence. Under this phase they also introduced the National Malaria Elimination Action Plan that combined surveillance and response with real-time reporting. 9–11 (4) During the malaria elimination phase (2011–2020), the country adopted local, tailor-made, pragmatic approaches with deployment of the ‘1-3-7’ surveillance strategy, which meant prompt reporting of confirmed cases within a day to a web-based national case reporting system, further investigation within 3 days and genome sequencing to distinguish imported and indigenous cases, treatment within 3 days, and foci response and adopted reactive case detection (RACD) within 7 days to prevent further transmission. 12 Imported malaria was tackled by the ‘1-3-7’ strategy and by the collaborative approaches of health professionals at the border, with polymerase chain reaction (PCR) as an additional diagnostic tool used by the reference laboratories at the counties. For prevention of re-establishment (POR), they restructured the 1-3-7 approach to 3-3-7, where the diagnosis of case is completed within 3 days, reconfirmation and epidemiological investigation are done within another 3 days, and foci investigation and response completion is done within 7 days after diagnosis. Additionally, China in 2017 initiated a subnational malaria elimination drive for individual provinces which was in tandem with the WHO 2017 Malaria Elimination Programme. They invested in building systems such as the National Institute of Parasitic Diseases and the Chinese Center for Disease Control and Prevention (China CDC), supported by capacity building and web-based reporting system at the grass-roots level. They also collaborated with the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM), which contributed to global malaria elimination efforts through collaborative projects which created an opportunity for mutual learning. 12
El Salvador
In 2021, El Salvador became the first country in the Central America of the WHO Region of the Americas (WHO AMR) to be certified as malaria-free. The last indigenous malaria case of P. falciparum in El Salvador was reported in 1995, while the last P. vivax cases were reported in 2016. 13 The key programmatic activities which possibly paved the way for elimination were the following: (1) geographical stratification using altitude and slide positivity rate data; (2) decentralisation for diagnosis facilities and data reporting; (3) weekly reporting systems and analysis; (4) computerised malaria information system; (5) decision on MDA and IRS at the local and regional level; (6) mandatory reporting by the private sector and (7) outbreak response on detection of two or more cases. 14
In 2019, Algeria, in the WHO African Region (AFR), was declared malaria-free by the WHO, with the last case of indigenous malaria reported in 2015. P. falciparum was the dominant parasite species. Geographical information system (GIS) mapping to identify imported cases of malaria, epidemiological surveys around each positive case and entomological surveillance to document the movement of mosquito vectors helped in curtailing imported malaria. 15
Argentina, in the WHO AMR, was certified by the WHO as a malaria-free country in 2019. In this South American country, the last indigenous case was reported in 2010. The most prevalent parasites in the country were P. falciparum and P. vivax . The key elements were IRS including at border areas, collaboration between border countries, prompt IRS by brigades on diagnosis of a malaria case and surveillance within the 500 meter radius of the identified case. Management of estuaries as breeding sites via infrastructural development and reintroduction of vertical vegetation, removal of green algae, and IRS with dichloro-diphenyl-trichloroethane (DDT) were especially helpful. 16
Uzbekistan, in the WHO European Region (WHO EUR), was declared malaria-free in 2018. The last locally acquired malaria case in Uzbekistan was reported in 2010. Both P. falciparum and P. vivax infections were prevalent in the country. Private sector involvement, supervised treatment for P. vivax cases, MDA, larvivorous fish Gambusia for vector control, and annual surveys to identify and liquidate water bodies acting as mosquito breeding grounds helped the country achieve malaria-free status in 2018. 17
Paraguay, in the WHO AMR, was declared malaria-free by the WHO in 2018, with the last indigenous case reported in 2011. 18 P. falciparum was the major reported parasite. Reporting of cases within 24 hours of detection and timely treatment, investigation of outbreaks within 24 hours of a case, GIS and establishment of a behavioural change communications plan for at-risk populations were some of the key steps.
Sri Lanka, which belongs to the WHO SEAR, was declared malaria-free in 2016, with the last case of indigenous P. vivax infection reported in 2012. 19 20 P. vivax and P. falciparum were the most prevalent parasites. Some of the crucial steps taken were the following: (1) the ‘1, 2, 3 approach’ that is confirmation within 24 hours (1 day) of malaria case by either a public or a private facility, investigation within 48 hours (2 days) and RACD within 72 hours (3 days); (2) close and periodic follow-up upto one year to tackle the resurgence of P. vivax cases in malaria camps and prevention of relapse due to lack of treatment compliance by directly observed primaquine (PQ) treatment was adopted 20 21 ; (3) public–private partnerships; (4) mobile clinics and (5) stringent vigilance on imported malaria, which is key to POR of malaria in Sri Lanka. 22 23 Despite facing a civil war, Sri Lanka achieved the elimination of malaria. The realisation that malaria is deadly but can be prevented and cured, the motivation and cooperation of conflicting groups to work with the government to protect the populations, and the involvement of neutral organisations played crucial roles. 24
Maldives became the first country within the WHO SEAR to eliminate malaria in 2015. The last case of indigenous P. vivax was reported in 1984, after which the reported cases were only from imported malaria. There are a total of 1200 islands in Maldives with large forested areas, out of which 198 are habitable. Important interventions which helped the country were the following: (1) hospital boat, called Golden Ray , which was equipped with medicines moved between the islands to treat patients (2) epidemiological and entomological surveys along with efforts to wipe out malaria vectors and (3) vigilance of imported malaria cases and vectors. 25
Armenia, in the WHO EUR, attained malaria-free status in 2011. The country reported its last indigenous case in 2009. P. vivax was the dominant parasite species and P. falciparum was via imported malaria. Mandatory notification, hospitalisation (in no later than 1–3 days), treatment of asymptomatic cases by supervised treatment, prophylaxis among military personnel and follow-up of patients for a period of 3 years for monitoring relapses were important steps. 26 27
Current challenges to India’s malaria elimination programme
India has managed to sustain the decline in overall malaria burden, but some of the significant current challenges which make malaria intractable in India are the following:
Incomplete understanding of the actual burden of malaria as the private sector is not involved in data reporting, although it caters to the healthcare needs of a large section of the population in India.
Inaccessible and remote areas of India are malaria-endemic (with persistent malaria), and providing health services to these communities becomes extremely difficult especially during monsoon and post-monsoon (transmission seasons) when these areas are cut off from the usual mode of communication and transportation.
Uncertain contribution of asymptomatic and low-density malaria infections to continued transmission of malaria.
Inadequate coverage and use of vector control products due to huge target population and time lag in replacements through a single channel of national control programme.
Cross-border malaria and internal migration.
Substantial burden of P. vivax malaria and weak mitigation policies and tools, such as missed diagnosis by the current methods, poor compliance to PQ’s 2-week course and lack of monitoring of relapses.
Emergence of drug and insecticide resistance in India’s neighbouring countries and border areas poses a threat of introduction of resistant parasites and vectors in the country.
Lack of skilled human resource in the national programme has been a long-standing challenge and more so with the integration of malaria programme with the general health services. Grass-root-level workers and healthcare staff are shared between several healthcare schemes and programmes and malaria may not be given the prioritisation it needs, more so when the target to report the last indigenous case is 2027 and elimination certification by 2030.
Lessons India can learn in its programme to accelerate malaria elimination
The national strategies for malaria elimination in the nine countries were mainly based on WHO guidelines and included intensified surveillance, vector control programmes, early diagnosis, rigorous case investigations, free and prompt treatment of patients based on malaria cases, and follow-ups. The key strategies of the nine countries are summarised in table 1 .
- View inline
Countries certified as malaria-free by the WHO from 2011 to 2021 and their key elimination strategies
India, in its national programme for malaria elimination, has all the major and essential elements well documented, and perhaps most of the steps are in the right direction to make malaria elimination possible. Additionally, certain strategies/policies/activities of the successful countries can be emulated in the following facets of the Indian elimination programme:
Strengthening of surveillance
Time-bound response by countries, such as the 1-3-7 strategy of China and the 1, 2, 3 strategy of Sri Lanka: a strong surveillance system was the most important pillar that played a crucial role in malaria elimination for most countries. The pivotal strategy, which could be adopted in our context, could be similar to those used in China and Sri Lanka. This will need to be supported by a strong web-based system that connects the public and private sectors at the level of healthcare to a central portal system. In India, at present, there is no time-bound strategy for mitigation of malaria cases on detection of a case. Hence, adopting some time frame would be useful in early management and thus in curtailing transmission. 28
Modernisation of surveillance system, that is, digital near real-time surveillance and smart surveillance systems: in China, a smart web-based health information system called the Chinese information system for disease control and prevention used time-bound alert Short Message Service (SMS) for follow-up and control measures. El Salvador also used computerised management information system to overcome delays in manual reporting system. 14 29 Such strong real-time surveillance and data-based decision making at the local level were also applied in Uzbekistan and Maldives. Countries like China and El Salvador overcame barriers by integrating digital technology and mobile SMS systems in their malaria elimination programme. Although surveillance has been strengthened in the malaria elimination drive in India, it still needs to be more inclusive and comprehensive. Therefore, this is the right time for India to adopt smart digital tools for surveillance, 30 and discussions at the national malaria elimination programme have already begun in this direction. Following the same, the ICMR-National Institute of Malaria Research has developed a Malaria Dashboard that is ready for data reporting, collation, visualisation and research. Case-based and foci-based examinations are much required, particularly in low transmission areas to achieve elimination in India. 31 Hence, implementation of a robust surveillance system (digital methods such as electronic dashboards) is very important. 30 32 It is therefore an opportunity for India to revamp its surveillance strategies from the archaic paper-based and aggregated systems to near real-time, digital and technology-backed integrated systems. 33 Such changes may fasten the process of malaria elimination.
Annual Parasite Index (API)-based stratification: India has stratified the states and districts (unit of implementation) based on their API. The subnational plan for elimination, although adopted by India, is yet to be fully deployed to certify states as malaria-free as and when they achieve this status.
Focus on high transmission areas: India, in its national strategic plan, has focused on high-burden endemic regions. Regular process monitoring, innovative research and prompt translation in policy along with increased community mobilisation will play an important role in reducing malaria cases.
Involvement of the private sector in reporting of malaria case data: Sri, Lanka, El Salvador and Paraguay have led by example and allowed active involvement of the private sector in the mainstream of malaria surveillance and management. In India, participation of the private sector is crucial because it provides 60%–70% of healthcare. The inclusion of this sector in India is in the nascent stages as there is no concrete roadmap towards this aim. We have suggested ways to involve the private sector in our previous work. 34 Additionally, India could gain insights from the national programmes of Sri Lanka, El Salvador and Paraguay which have actively involved private providers. India has made malaria a notifiable disease in 31 states, but it is only an initial step towards tackling under-reporting and underestimation of malaria burden in the country. Rapid diagnostics as field diagnostics have been the cornerstone of early identification and thus timely management of cases. Over-the-counter availability of rapid diagnostic tests (RDTs) in the commercial sector can empower people to self-diagnosis and reporting to the healthcare system. 34
Use of molecular tools of diagnosis and national reference laboratories
Prompt and accurate diagnosis of malaria cases, including the hidden burden of asymptomatic and subpatent infections, is important as it will help in treatment and thus cessation of transmission. Adoption of molecular methods for diagnosis in the national programme would be a way forward. The WHO recommends microscopy as the gold standard and RDT as field diagnostics. Use of molecular tools like PCR and loop-mediated isothermal amplification for RACD, as adopted by China, was useful in the identification and resolution of all malaria cases. India suffers from considerable burden of low-density infections which escape detection by routine diagnostics. 31 The development of field-friendly, point-of-care/collection molecular tests could help bring out the burden and management of subpatent malaria. 35 District-level healthcare facilities have been empowered in terms of infrastructure and expertise owing to the COVID-19 pandemic and these can be co-opted for diagnosis of submicroscopic malaria. 36 37 In India, the barrier to adopting molecular tools in its routine programme would be the prohibitive cost of infrastructure and training of the laboratory workforce. However, countries like China and Sri Lanka have shown the way that it is possible to use these more sensitive tools as routine diagnostics. The establishment of the National Reference Laboratory for quality assurance was adopted by China, El Salvador, Uzbekistan, Paraguay and Armenia. Having such central hub that connects all states and district-level laboratories can step up India’s centralized diagnostic structure.
India, in its national guidelines, has a special emphasis on P. vivax elimination. The foreseeable barriers for India are poor compliance of PQ treatment, inadequate follow-up of patients with P. vivax malaria, cross-border and migration issues. Compliance to antimalarials, especially for P. vivax malaria, which needs 14-day treatment with PQ is a daunting challenge in India. Almost half of India’s malaria burden is P. vivax malaria. It is widely acknowledged that it will be difficult to achieve elimination of P. vivax as compared with P. falciparum . Adoption of single-day treatment with tafenoquine may be considered by India after due regulatory consideration. 38 The issue of compliance can be overcome if the P. vivax antimalarial therapy is administered as directly observed treatment (DOT). We could follow the Sri Lankan example which to tackle the P. vivax resurgence in army camps adopted PQ directly observed treatment for infected army personnel. 20 21 El Salvador and Uzbekistan supervised the PQ treatment. Similarly, Armenia had also introduced DOTs for both P. falciparum and P. vivax control in their national programme. Good compliance to 14-day radical treatment with primaquine along with estimation of glucose-6-phosphate dehydrogenase (G6PD) deficiency in the population, addressing low-density infections and tackling asymptomatics will play pivotal role in P. vivax elimination.
Follow-up of P. vivax cases to capture relapse cases: P. vivax malaria is characterised by latent hypnozoites which can get activated in variable durations. Therefore, it is important to follow these cases so as to capture relapse cases and treat them on time. In the national guidelines of Sri Lanka, El Salvador, Armenia, Algeria and Maldives, at least 6 months to 1 year of follow-up was practised.
Cross-border malaria: countries which have achieved malaria elimination have robust mitigation strategies against imported malaria. Cross-country cooperation is at the centre of the elimination programme. Strict surveillance of cross-border transmission as embraced by China, Algeria, El Salvador, Sri Lanka, Armenia, Paraguay and Uzbekistan with deployment of mobile teams and examination of travellers from malaria-endemic countries through RACD could be possible steps which need induction in our control guidelines. India has porous borders with many neighbouring malaria-endemic countries and also poses a threat to the neighbouring countries which are at the cusp of elimination such as Bhutan. 39 Therefore, India should institute strong parasite and vector surveillance programmes to curtail exchange. Cross-border malaria issues have been handled well by China, Algeria, El Salvador, Sri Lanka, Armenia, Paraguay and Uzbekistan by intense surveillance of migrants and travellers.
Internal migration: El Salvador had carried out active surveillance and chloroquine+primaquine (CQ+PQ) single-dose prophylaxis for migrant populations in employments such as cotton production, coffee fields or factories. 13 In India, the interstate movement of people is high for employment, tourism and other purposes. Special attention is needed in areas where malaria elimination status has progressed from control to pre-elimination phase. For example, the state of Punjab has shown a drastic decline in the number of malaria cases. 40 However, there is always a risk of re-establishment owing to the constant influx of migrant labourers for construction/agriculture activities in the state. This mobile population is very often from malaria-endemic states such as Bihar, Chhattisgarh and Jharkhand and thus could act as reservoirs for the parasite resulting in re-establishment of infection. Thus, India could also consider devising plans for screening, treating and reporting of malaria cases among such migrant populations.
Many malaria elimination demonstration projects have successfully shown that adopting certain strategies can dent the malaria endemicity situation. One of the programmes, the Comprehensive Case Management Plan (CCMP), has been adopted by the government of Odisha as the Durgama Anchalare Malaria Nirakarana (DAMaN) programme for mitigation of malaria in inaccessible areas and has contributed to a remarkable decline in malaria. Biannual screening of malaria in mass surveys and subsequent treatment has been adopted as a programme strategy in this state’s malaria plan. 41
In order to implement public–private partnerships, Indian policy makers should devise a well-defined strategy to work locally and focally at panchayat/district levels with emphasis on common platform for reporting, regular communication and assessment of progress.
Vector control and management of resistance
Robust vector surveillance using smart tools: China, Algeria and Sri Lanka adopted a robust vector surveillance with the use of GIS and spatiotemporal analysis. Similar to these platforms, a web-based database of vector surveillance should be adopted in India.
Insecticide resistance management (IRM): Resistance to routinely used insecticides (DDT and some synthetic pyrethroids) has been reported in the malaria-endemic areas of India. Frequency of insecticide resistance should be monitored at sentinel sites periodically. IRM with rotational or mosaic pattern with insecticides of different mechanisms of action has been deployed by Sri Lanka. Focal IRS in areas such as plantations, factories and along countries’ border with high endemicity regions have been adopted by China, Sri Lanka and El Salvador. Environmental engineering methods were adopted by Argentina. All these strategies and tools could be useful to India with its diverse geographical regions. Laboratory studies conducted in India are indicative that newer tools like attractive toxic sugar baits (ATSBs) could be a promising vector control. 42
Integrated vector management (IVM): IRM and IVM, although discussed in India, are not holistically implemented. In Maldives, elimination of malaria vectors was one of the key contributing factors to maintaining a malaria-free status since 1984. El Salvador and Armenia implemented water management projects to reduce mosquito breeding sites and also planted neem trees to prevent mosquitoes in the surrounding areas. India should consider IVM as an umbrella vector management programme.
Role of partners
Currently, the different partners and stakeholders in India are working in silos towards the common goal of malaria elimination. There is a lack of cohesion at the central level and thus at the peripheral levels. Experiences of other countries can be leveraged on using the strengths of partner organisations and taking them in the fold of the national programme. Sri Lanka’s successful elimination was achieved despite facing a civil war. Indian policy makers could involve NGOs, private partners and voluntary collaborative network, as adopted by Sri Lanka, El Salvador, Armenia, Paraguay and Algeria, to enhance successful deployment of all components of malaria elimination. Involvement of intersectoral ministries and uninterrupted funding were adopted in Sri Lanka to eliminate the disease. National and international partners are crucial to achieving malaria elimination, especially for overcoming the last-mile challenges. 39
Prevention of re-establishment strategies
POR of malaria transmission in a malaria-free country is a daunting task. After elimination, active case detection (ACD) with mobile malaria clinics is still maintained in Sri Lanka. Algeria has taken stern steps in quickly identifying any imported malaria, followed by appropriate POR actions. Obligatory notification and reporting of malaria and timely epidemiological investigation of each imported case and focus are followed by Paraguay, Uzbekistan, El Salvador and Maldives. These activities would need to be undertaken by the states which have eliminated malaria to prevent its re-establishment from other states. India would need robust alert systems and prompt surveillance and diagnostics to mitigate the threat of imported malaria, as done by other countries.
Domestic funding and sustained political commitment
In addition to global and international funding {GFATM, World Bank, President’s Malaria Initiative (PMI)/United States Agency for International Development (USAID)}, China, Sri Lanka, Algeria, Armenia, Uzbekistan and El Salvador allotted heavy domestic funding for malaria elimination. 2 In India, the national programme is mainly funded by the Government of India, but the Global Fund is the major financial source for procurement of long-lasting insecticidal nets (LLINs) in India. From 2016 to 2018, ~80% (~40 million) of the LLINs distributed in the country were procured by the Global Fund financial resources. Political and financial commitment from the government is vital to maintain the momentum of the malaria elimination programme in India. 43 India needs to pledge substantial funds for sustenance of the national programme for malaria elimination and beyond. Provision of funds in the scenario of withdrawal of the Global Fund also needs to be created.
Promoting research
Research and Development (R&D) need constant thrust as the limitations of the currently available tools can hinder the achievement of malaria elimination, and threats like drug and vector resistance need investment in research to discover newer tools. Malaria-free countries have invested in research to identify the most optimum strategies, tools and operations. China invested in R&D and artemisinin derivatives have been the cornerstone of malaria treatment. In 1967, the CDC Division of Parasitic Diseases was set up in San Salvador. 44 Efforts should be put in to develop and validate non-histidine-rich protein (non-HRP) 2/3-based RDTs, feasibility of a single dose of tafenoquine for P. vivax malaria 38 , field-friendly molecular tools, and robust research to assess the impact of climate change on malaria transmission. 45 Research into the possible zoonotic transmission of malaria parasites in India should also be considered. Innovative approaches to mosquito control like ATSB, 42 insecticide impregnated clothing, hammocks etc could be explored to address the challenges associated with forest malaria, 5 outdoor biting and insecticide resistance. Concerted efforts have been initiated in this direction by a nodal research body of the Government of India. 46 Consolidation and implementation of the research findings into policy and practice would provide the necessary thrust to malaria elimination in India. However, poor investment and lack of priority to continue research once elimination is near or achieved can derail research programmes on promising tools. Advocacy for continued and breakthrough research needs to be made at the highest level.
India’s track record in elimination of other infectious diseases
Despite enormous population and diverse geographical conditions, India has successfully eliminated polio (2014), 47 smallpox (1977) 48 and guinea worm disease (2000) 49 50 and is possibly on track to eliminate tuberculosis by 2025.
India was certified polio-free in 2014. Considering the scale at which the polio vaccination drive was conducted, it can be considered as one of the biggest success stories. However, the path was not easy for India. The challenges ranged from arranging logistics for a large population and reaching even the hard-to-reach areas, building trust, changing perceptions and convincing the communities to accept vaccines amidst anti-vaccine movements. 51 This was achieved by enhanced communication within the community, by involving local representatives and religious leaders, and by providing basic health packages to address immediate concerns regarding other health issues. Screening of migrant and mobile populations and international travellers played a crucial role in identifying potential sources of continued transmission. In addition to government officials, several partners worked collectively in the polio elimination drive. Defining the role and accountability of each of the public and private stakeholder was also done to avoid redundancy in the tasks performed. Further, the accuracy of data was monitored to get reliable information on disease prevalence. The need for training healthcare workers was identified and addressed by capacity building and repeated trainings. 52
As India is hurtling towards malaria elimination, it is the most appropriate time to review and assess the strategies and practices by countries that have been successful in achieving malaria elimination. The same can be tailored according to India and neighbouring South Asian countries as they share several commonalities in the context of environmental conditions, vectors, parasites, community behaviour and health infrastructures. These shared features could be the basis of cross-learnings and can help India and others steer their malaria elimination programme.
Ethics statements
Patient consent for publication.
Not required.
Acknowledgments
We acknowledge Bhabani Shankar Muduli and Mansi Arora for their contribution and help in generating the world map depicting country-wise contribution to global malaria cases (figure 1).
- ↵ Countries and territories certified malaria-free by who . Available: https://www.who.int/teams/control-of-neglected-tropical-diseases/yaws/diagnosis-and-treatment/global-malaria-programme [Accessed 16 Dec 2021 ].
- World Health Organization
- ↵ High burden to high impact: a targeted malaria response. , 2021 . Available: https://www.who.int/publications-detail-redirect/WHO-CDS-GMP-2018.25 [Accessed 17 Dec 2021 ].
- India CO for, Organization WH
- ↵ World malaria report 2020. 2021 . Available: https://www.who.int/publications-detail-redirect/9789240015791 [Accessed 17 Dec 2021 ].
- Yin J-hai ,
- Yang M-ni ,
- Zhou S-sen ,
- Zhou S , et al
- Ruktanonchai NW , et al
- Beiersmann C , et al
- Huang F , et al
- Liang X-H ,
- Lu S-N , et al
- ↵ Malaria elimination in El Salvador: a historical and epidemiological perspective . Available: https://www.path.org/resources/malaria-elimination-in-el-salvador-a-historical-and-epidemiological-perspective/ [Accessed 17 Dec 2021 ].
- Burton RA ,
- Chévez JER ,
- Sauerbrey M , et al
- Nasir SMI ,
- Amarasekara S ,
- Wickremasinghe R , et al
- Alba Soto C
- Razakov SA ,
- Shakhgunova GS
- ↵ Paraguay now malaria-free . Available: https://www.downtoearth.org.in/news/health/paraguay-now-malaria-free-60829 [Accessed 16 Dec 2021 ].
- Abeyasinghe RR ,
- Galappaththy GNL ,
- Smith Gueye C , et al
- Fernando SD ,
- Rodrigo C ,
- de Silva N , et al
- Premaratne R ,
- Wickremasinghe R ,
- Ranaweera D , et al
- Gosling R ,
- Abeyasinghe R
- Fernando D ,
- Wijeyaratne P ,
- Hounsell KG ,
- Sadiq T , et al
- ↵ Asia Ro for S-E, organization WH. Malaria-free Maldives. who regional office for south-east Asia , 2016 . Available: https://apps.who.int/iris/handle/10665/250397 [Accessed 17 Dec 2021 ].
- Grigorian G ,
- Solkhomonian L
- Davidyants VA ,
- Kondrashin AV ,
- Vanyan AV , et al
- Sturrock HJW ,
- Cotter C , et al
- Herrera S ,
- Ochoa-Orozco SA ,
- González IJ , et al
- Gahtori R , et al
- Pal Bhowmick I ,
- Chouhan A , et al
- Saroha P , et al
- Baharia RK ,
- Das P , et al
- Singh K , et al
- ↵ Monthly malaria information system (MMIS): national center for vector borne diseases control (NCVBDC) . Available: https://nvbdcp.gov.in/index1.php?lang=1&level=1&sublinkid=5879&lid=3957 [Accessed 17 Dec 2021 ].
- Ghosal J , et al
- ↵ Laboratory evaluation of the efficacy of boric acid containing toxic sugar baits against Anopheles culicifacies, an. stephensi and Aedes aegypti mosquitoes . Available: https://www.jvbd.org/preprintarticle.asp?id=331414;type=0 [Accessed 2 Jan 2022 ].
- ↵ Malaria : National Center for Vector Borne Diseases Control (NCVBDC) . Available: https://nvbdcp.gov.in/index1.php?lang=1&level=1&sublinkid=5784&lid=3689 [Accessed 29 Dec 2021 ].
- Caminade C ,
- Rocklov J , et al
- Vashishtha VM
- ↵ Guinea Worm Eradication Programme (GWEP) : National Centre for Disease Control (NCDC) . Available: https://ncdc.gov.in/index1.php?lang=1&level=1&sublinkid=142&lid=73 [Accessed 24 Feb 2022 ].
- Bellatin A ,
- Rao S , et al
Handling editor Seye Abimbola
Contributors MR and AS conceived the idea and framed the manuscript. SS wrote the initial draft. RV, BY and AK did review of literature, analysis and graphics. All authors read and approved the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Open access
- Published: 16 April 2020
Surveillance based estimation of burden of malaria in India, 2015–2016
- Ashwani Kumar 1 ,
- Himanshu K. Chaturvedi 2 ,
- Ajeet Kumar Mohanty 1 ,
- Surya Kant Sharma 3 ,
- Mantoshkumar S. Malhotra 3 &
- Arvind Pandey 2
Malaria Journal volume 19 , Article number: 156 ( 2020 ) Cite this article
6789 Accesses
11 Citations
1 Altmetric
Metrics details
India has launched the malaria elimination initiative in February 2016. Studies suggest that estimates of malaria are useful to rationalize interventions and track their impact. Hence, a national study was launched to estimate burden of malaria in India in 2015.
For sampling, all 624 districts of India were grouped in three Annual Parasite Incidence (cases per thousand population) categories, < two (low); two-five (moderate) and > five (high) API. Using probability proportional to size (PPS) method, two districts from each stratum were selected covering randomly 200,000 persons per district. Active surveillance was strengthened with 40 trained workers per study district. Data on malaria cases and deaths was collated from all health care providers i.e. pathological laboratories, private practitioners and hospitals in private and public health sectors and was used for analysis and burden estimation.
Out of 1215,114 population under surveillance, 198,612 (16.3%) tests were performed and 19,386 (9.7%) malaria cases were detected. The malaria cases estimated in India were 3875,078 (95% confidence interval 3792,018–3958,137) with API of 3.05 (2.99–3.12) including 2789,483 (2740,577–2838,389) Plasmodium falciparum with Annual Falciparum Incidence of 2.2 (2.16–2.24). Out of 8025 deaths investigated, 102 (1.27%) were attributed to malaria. The estimated deaths in India were 29,341 (23,354–35,327) including 19,067 (13,665–24,470) confirmed and 10,274 (7694–12,853) suspected deaths in 2015–2016.
Conclusions
Estimated malaria incidence was about four folds greater than one million reported by the national programme, but three folds lesser than thirteen million estimated by the World Health Organization (WHO). However, the estimated deaths were 93 folds more than average 313 deaths reported by the national malaria programme in 2015–2016. The 29,341 deaths were comparable with 24,000 deaths in 2015 and 22,786 deaths in 2016 estimated by the WHO for India. These malaria estimates can serve as a benchmark for tracking the success of malaria elimination campaign in India.
The World Health Organization (WHO) has reported 22% decline in malaria from the estimated 271 (177–382) million cases in the year 2000 to 212 (144–294) million in 2015 [ 1 ]. The reduction in estimated malaria attributable mortality is even more impressive from 856,728 (594,760–1204,220) deaths down to 426,791 (218,780–630,698). With these trends, the WHO has advocated elimination of malaria in at least 35 countries by the year 2030 [ 2 ]. Following the WHO path, India has launched the malaria elimination initiative in 2016.
The first set of global disease burden modelling studies was carried out a couple of decades ago for estimation of communicable and non-communicable diseases, injuries and deaths [ 3 , 4 ]. Many studies have also been conducted using country data and subjecting it to different methodologies, assumptions and epidemiological models to generate estimates of malaria burden [ 1 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ]. However, wide gaps between the estimates and the reported incidence have been the subject of intense debate calling to question not only deficiencies in surveillance and reporting systems, but also methodologies adopted to arrive at such estimates.
Outside of Africa, India is the main contributor to malaria related morbidity and mortality in the South-East Asia. Hence, several attempts have been made to estimate malaria burden in India from time to time using secondary data [ 1 , 8 , 12 ]. Mortality estimates for the year 2002 were provided by Dhingra et al. based on cause of death by verbal autopsy (COD VA) data of the Million Death Study from 2001 to 2003 [ 13 , 14 ]. It was estimated that below the age of 70 years, there were 205,000 deaths attributable to malaria/annum; < 5 years of age-55,000, 5–14 years of age-30,000 and 15–69 years of age-120,000 deaths. As the death estimates were about 300 times greater than the deaths reported by the Indian national programme, this publication triggered intense debate on the methodology adopted. It was surmised that besides issues related to the time gap between the death and the verbal autopsy, the overlapping of symptoms of other diseases with malaria could have influenced responses of the respondents [ 15 , 16 ]. Further, based on Vital Registration System and Medical Certification of Cause of Death, Kumar et al. estimated about 146,000 and 141,000 deaths due to malaria in India respectively in 1997 and 1998 [ 17 ]. A committee constituted by the Government of India arrived at an estimate of 9.751 million cases and 40,297 deaths due to malaria (30,014–48,660) in the year 2010 [ 18 ].
More recent global malaria mortality trends published suggest 46,970 (14,757–94,945) deaths for individuals of all ages in India in 2010 [ 5 ]. These included 4826 deaths (781–14,437) in children less than 5 years of age and 42,145 (11,340–88,615) deaths for individual of 5 years and older. Curiously, malaria ranked 7th among 291 causes of death and injuries in both 1990 and 2010 [ 9 ].
Malaria burden estimates at national and sub-national levels are vital not only as benchmark for priority setting and resource allocation but also to gauge programmatic achievements during disease elimination process. As malaria burden estimates based on a nationally representative sample of primary morbidity and mortality data are lacking, the present study is first such attempt globally which was carried out in three different malaria-endemic zones representing India in 2015–2016.
Sampling frame and sample size
A national sampling frame was prepared based on data provided by National Vector Borne Diseases Control Programme (NVBDCP) for stratification and selection of the clusters as a basic requirement of a sampling design. A list of all 624 districts of India with annual parasite incidence (API) of last 3 years (2011–2013), which was obtained from NVBDCP, served as the sampling frame. Based on the maximum API of last 3 years, all the districts were divided into three strata (S1, S2 and S3) of endemicity, i.e., high (S1: API ≥ 5), moderate (S2:2 ≥ API < 5) and low (S3: API < 2).
Selection of study districts
The sample size was worked out to provide the reliable estimate of API for each region and death rate due to malaria at the national level. It was based on the median API of the malaria endemic strata (7/1000 in S1, 3/1000 in S2 and 0.5/1000 in S3) with 10% margin of error in S1 and S2 and 20% margin of error in S3, 95% confidence interval, 10% non-response and design effect-2. As the median API of low endemic region (districts with API < 2) was low, the computed required sample size worked out to 400,000 persons. The same sample size was uniformly applied to the other two regions to maximize the possibility of capturing both malaria and death cases. The total sample size was thus 1200,000 from all the three regions. In this manner, two representative districts each from low, moderate and high burden districts and overall 6 study districts were selected in the country (Fig. 1 ). Further, three Primary Health Centres (PHCs) were selected randomly from the list of all PHCs of each selected district so that study population size within each selected PHC was approximately 70,000. In case the selected PHC was smaller (i.e. population was < 60,000), some population of the adjacent PHC or a sub-Centre was included in the study area to obtain the desired sample size. Similarly, the larger PHC (i.e. with a population > 80 000) was divided to select a contiguous segment of required population size. Overall study population of surveillance area was about 0.2 million/district. For concurrent death enumeration, an adjacent PHC of similar size and epidemiological features matching the surveillance PHC area was also selected.
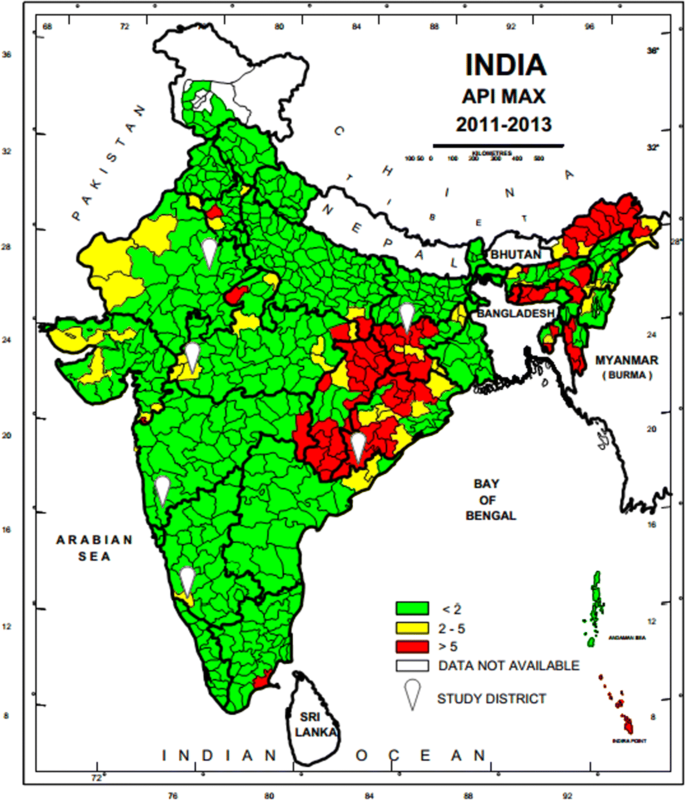
Map of India showing geographical location of six study districts (white balloons) for capturing malaria morbidity and mortality. Firstly district level stratification of India was done on the basis of three Annual Parasite Incidence (API) classes, < 2, 2–5 and > 5 taking into consideration APImax of malaria from 2011–2013 and then two study districts from each of the three strata were randomly selected as per PPS sampling method to conduct malaria burden estimation study
Study personnel
Six Technical Assistants (one in each district), 18 Field Workers (three in each district) and 240 (40 in each district) Voluntary Surveillance Monitors (VSMs), a statistical Assistant, an Epidemiologist, Consultant Biostatistician and 6 Data Entry Operators along with Co-Is and PIs were engaged for managing the project activities. The VSMs were chosen from the study or neighbouring villages/wards where they were assigned surveillance work. Field staff was trained in performing Rapid Diagnostic Test (RDT), preparing blood smears, filling up of study forms and record keeping. The VSMs worked in close collaboration with village Accredited Social Health Activists (ASHAs) and Multi-Purpose Workers (MPWs) and were instrumental in finding fever cases and testing their blood for malaria followed by treatment of malaria cases.
Surveillance
Active surveillance.
The community level active surveillance was carried out in each selected villages of PHC areas in rural and municipal areas or ward in urban area. House listing and complete enumeration of the study population was carried out in each study area (PHCs/Municipal wards) before initiation of surveillance by the 12 to 15 VSMs with help of ASHAs. One VSM was assigned a population of about 5000 or 1000 houses (one or more villages of PHCs or section of municipal areas) fortnightly to carry out the active surveillance of fever cases for the period of 12 months. During the surveillance, all the fever cases were recorded by the VSMs and their blood test for malaria was performed by the surveillance team. They were also referred to the PHCs or Sub-centres for confirmation of malaria and treatment. All the fever cases identified during the active surveillance were recorded with the result of blood test in the active surveillance format (A) and compiled at the end of every month. Field Workers (FW) were supervising VSMs, solving day to day problems, cross-checking all the cases reported by the VSMs every month, and maintaining the supplies of study related material and carrying out of the verbal autopsy of each death case using prescribed formats (D). Technical Assistants (TA) were responsible for overall field activities, logistics, and coordination with state health officials, solving of local problems, supervision, data collation and reporting to the project co-investigators.
Passive surveillance
The information related to passive cases detection was collated to ascertain morbidity and mortality due to malaria in the study PHC areas in rural areas and municipal wards in the urban areas (Figs. 2 and 3 ). All government and private health facilities in the study area and the vicinity were listed and empanelled to capture malaria cases coming from the study surveillance area and accessing these facilities. All records of fever and malaria cases were cross checked in both active and passive lists to avoid duplication. Blood tests were performed by bivalent RDTs (for both Plasmodium vivax and Plasmodium falciparum ) and by making thin and thick blood smear of fever cases encountered in the study population. Treatment of confirmed malaria cases was done by study personnel following the current national anti-malarial drug policy [ 19 ]. Data of all fever cases and their blood tests results collected through active and passive surveillance and also the VA of death cases were finally checked with name and address for confirmation of cases belonging to the surveillance study population by the project team (VSMs, FW, TA) during the district level monthly meeting and finally confirmed by the project co-investigator before sending the data to the central team for analysis.
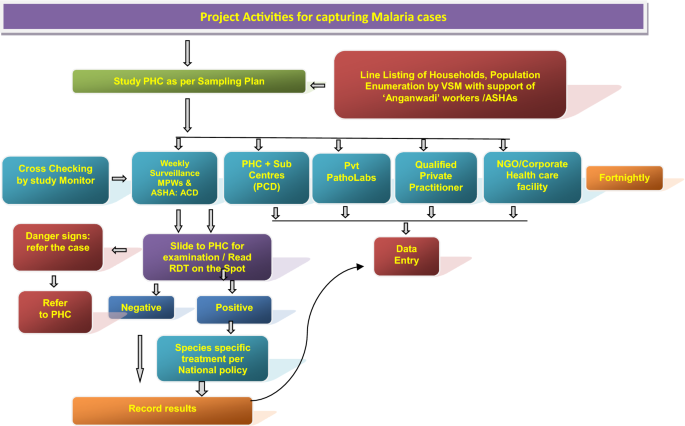
Flow diagram of activities carried out in the surveillance areas of the study districts to capture malaria cases by instituting surveillance and from enlisted health facilities, diagnostic laboratories, private practitioners and institutions
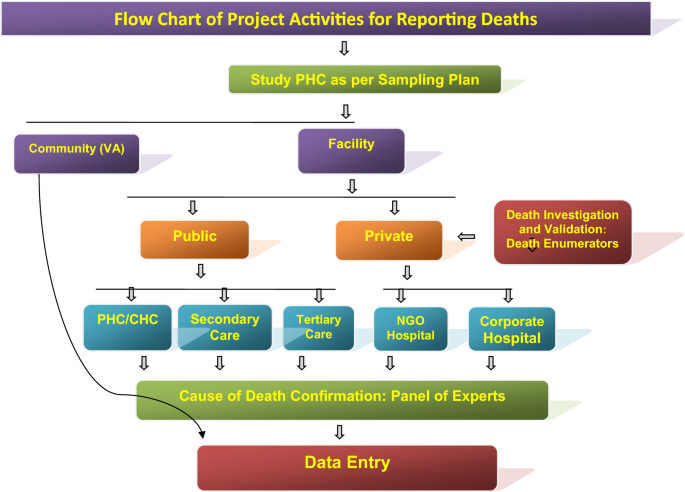
Flow diagram of the surveillance activities carried out in the study areas of the study districts to capture death cases by eliciting information from ‘Panchayats’ (Local self-Government bodies in the study villages), Municipal Councils/corporations, burial/cremation ground, public, community leaders, schools, shops, ASHAs, hospitals, etc. Verbal Autopsy of death cases was done after a fortnight of death occurrence by visiting residence of the deceased and information was captured on standard VA instrument in local language. Each VA report was examined by two medical experts independently for labeling the cause of death viz., probably due to malaria, confirmed due to malaria, cause other than malaria and unclassified death (cause cannot be discerned)
Deaths and cause of death assignment
Information on deaths captured through different sources (including hospital, death registry, cremation/burial records) was recorded. A pre-designed and tested verbal autopsy (VA) tool was filled up for all the death cases by visiting household of the deceased on day 15 post death. Two independent physicians after auditing all VA forms assigned the cause of death. In case of disagreement between the two physicians, a third physician was consulted and final cause of death based on agreement between any two physicians was assigned. If available, the medical records related to the death cases were also taken into consideration for cause of death assignment.
Data analysis
Data collected during the surveillance period of 1 year was analysed to obtain the crude and weighted estimates of annual incidence rates, death rates. The weights were calculated according to the study design adjusting the differences in sample coverage in each study area of district and overall estimates were obtained using the population weight of three endemicity strata (S1–S3).
Malaria morbidity
In Koraput district with high malaria endemicity, 15,563 cases with test positivity rate (TPR) of 19.69% and in Chatra district 916 cases (TPR 3.06%) were detected from study population. In the moderate endemic districts, malaria cases were 1947 with TPR of 10.01% in Jhabua, but in Dakshin Kannada 791 cases (TPR: 2.45%) were captured. In the low endemic areas, 36 and 133 malaria cases were captured in Jaipur and Kolhapur districts respectively with < 1 TPR (Fig. 4 , Table 1 ). Koraput and Jhabua districts showed predominance of P. falciparum (55–88%), while remaining 4 districts viz., Chatra, Dakshin Kannada, Jaipur and Kolhapur, showed predominance of P. vivax (44–95%). Mixed infections were reported in all the districts except Jaipur and Kolhapur which had extremely low incidence (Fig. 5 ). The observed Annual Parasite Incidence (API) which denotes malaria cases per 1000 population in Koraput being 74.5, was two-four folds higher than reported API in the previous 3 years, but observed API was within the range of reported API in Chatra, Dakshin Kannada and Jaipur districts. However, it was higher (9.3) in Jhabua compared to 3.6–8.0 reported in the previous years. In Kolhapur, though API of 0.7 was low in general, yet it was 17–35 folds higher when compared with reported API (0.02–0.04) in the years 2012–2014 (Table 1 ).
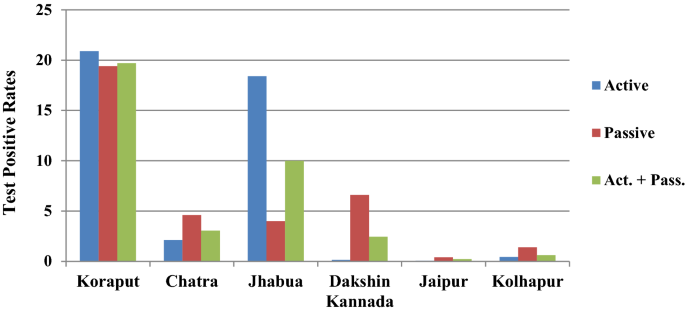
Test Positive Rates for malaria showed high variaility in active and passive collections in six study districts
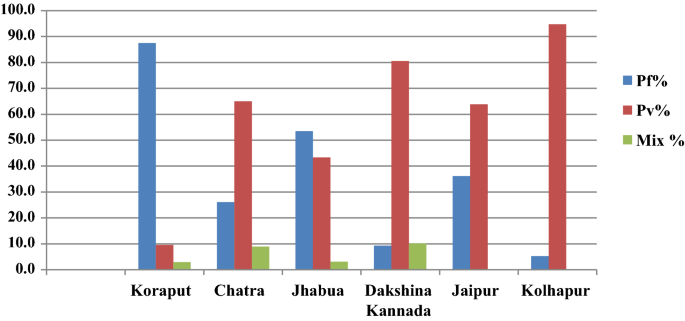
The proportion of P. falciparum and P. vivax varied in the 6 study districts
Malaria attributable deaths
From the six study districts, 8025 deaths were investigated (Table 2 ). Ten physicians assigned cause of death based on verbal autopsy (VA) narratives and available medical records. In high-malaria endemic Koraput district, out of 946 verbal autopsies performed, 60 and 35 deaths were labelled as attributed to malaria by the medical experts as confirmed and suspected deaths, respectively. In Chatra district only two deaths, one confirmed and one suspected were caused by malaria. In moderately malaria-endemic region, one suspected death due to P. falciparum malaria in Jhabua district and three malaria deaths (all due to P. vivax ) in Dakshin Kannada district were captured from the hospital records. In low malaria-endemic region, only one confirmed death due to complicated P. falciparum was reported in Kolhapur district (Table 2 ). Of these 102 total malaria deaths, 65 (63.7%) were among males and the rest 37 (36.3%) were among females in a male: female ratio of 1.75:1. The number of deaths was greater among males as compared to females in all the age groups except in children 1–14 years of age (Fig. 6 ). The number of deaths (62) was greatest in the broad age group of 15–70 years. In this age group, deaths were twice greater in males than in females. However, 18 deaths occurred among persons over 70 years of age involving both sexes almost equally.
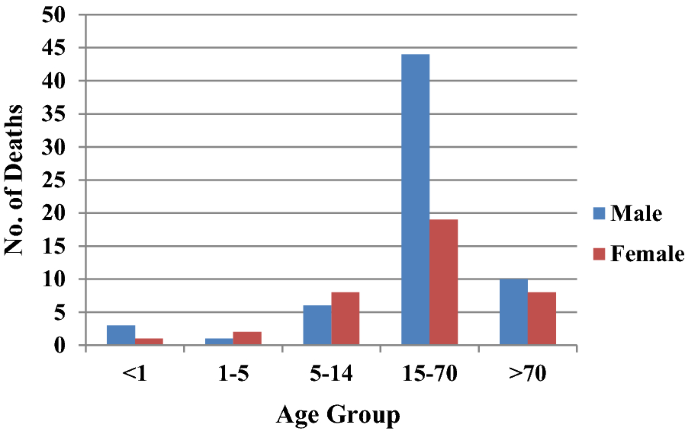
Age and sex distribution of deaths in the study districts
Plasmodium falciparum prevalence and mortality rates
When computed P. falciparum prevalence rate ( Pf PR) was highest (17.8%) in Koraput district followed by Jhabua (5.66%). In the remaining 4 districts, Pf PR was low from 0.03 to 1.0% (Table 3 ). Plasmodium falciparum specific mortality rates ( Pf MR) showed wide variation. In high malaria-endemic district, Pf MR was 0.67% and 0.62% respectively in Koraput and Chatra, while in Jhabua district Pf MR was low at 0.09% and in Dakshin Kannada district it was nil as all 3 deaths were due to P. vivax . Incidentally, in Kolhapur district with only 7 P. falciparum cases, one P. falciparum -attributable death was confirmed and hence and Pf MR rate stood abnormally high at 14.2%. The crude death rate was 47.5/100,000 persons in case of Koraput but < 1 in the remaining districts and the overall rate was 5.01/100,000 persons (Table 3 ).
Estimation of malaria morbidity and mortality burden
Based on the sample data of 6 districts, the number of malaria cases and deaths attributable to malaria were estimated for India by the weighted estimates of various rates such as annual fever rates and annual incidence of malaria and death rates due to malaria (Additional files 1 , 2 , 3 , 4 : Table S1–S4). As per Expert Group of Population Projection of India report, the projected population of India as on March 1, 2016 (mid of study period 2015–16) was worked out as 1.268 billion which was used to arrive at population-based malaria morbidity and mortality estimates. The population share of the three strata to the total population of India was 5.2% in case of high, 8.4% in moderate and 86.4% in case of low malaria endemic districts in strata S1, S2 and S3 respectively. This categorization was as per the initial sampling frame prepared for this study design.
The estimated Annual Parasite Incidence (API) was 41.66 for high, 6.53 for moderate and 0.39 for low malaria endemic areas (Table 4 ). Overall, the weighted estimate of API for the country worked out to 3.05 (2.99–3.12) per thousand population. Based on weighted estimates of API and standard error, the estimated number of malaria cases in the country ranged from 3792,018 to 3958,137 during the study period of one year with point estimate of 3875,078 malaria cases in India (Table 4 ).
The estimated AFI (Annual Falciparum Incidence) based on P . f malaria cases (both P. f & Mix P. f + P. v ) for the three regions was 36.57 for high, 3.0 for medium and 0.05 for low endemic area (Table 4 ). Overall, the weighted estimate of AFI for the country was 2.20 (2.16–2.24) per thousand populations. Based on weighted estimates of AFI and standard error of estimate, the estimated number of Pf malaria cases in the country was worked out between 2740,577 and 2838,389 with point estimate of 2789,483 P. falciparum cases (including mix infections).
Estimation of deaths due to malaria
All deaths attributed to malaria were categorized as confirmed and suspected deaths. In high malaria-endemic areas, the death rate due to confirmed malaria was estimated at 25.44/100,000 population and death rate due to suspected malaria as 15.0/100,000 population with overall death rate due to malaria as 40.44/100,000 population (Table 5 ). In high malaria prevalence area which embodies a population of 0.066 billion, the estimated deaths due to confirmed malaria were 16,789 and estimated deaths due to suspected malaria were 9901 hence 26,690 total deaths. In moderate malaria prevalence area, the death rate due to confirmed malaria was 0.5368/100,000 of population and death rate due to suspected malaria was 0.34/100,000. Hence in moderate prevalence area with 0.107 billion population, the estimated deaths were 945 of which 572 were due to confirmed malaria and 373 were due to suspected malaria. In low malaria burden area, the death rate due to confirmed malaria was 0.1556/100,000 and death rate due to suspected malaria was nil. In low malaria prevalence areas of India which had 1.096 billion populations, the estimated deaths due to confirmed malaria were 1706 and suspected malaria deaths were nil. Hence, the overall point-estimates of deaths due to confirmed malaria were 19,067 (13,665–24,470) and the point estimate of deaths due to suspected malaria was 10,274 (7694–12,853) with total deaths of 29,341 (23,354–35,327) due to malaria in a population of 1.269 billion in India (Table 5 ).
The foregoing effort on estimation of morbidity and mortality attributable to malaria has been made by conducting surveillance based prospective study for the first time in India. The findings of the study confirm that malaria scenario is highly diverse in the country. The observed malaria incidence in high endemic Koraput district situated in Odisha state of India was two-four folds greater than expected. On the other hand, Jhabua which represented districts of the country with moderate incidence showed much higher incidence of malaria than reported in the earlier years (Table 1 ). In the remaining districts, however, the observed incidence was quite as expected with over all indication that the sample districts could capture wide spectrum of variability of malaria normally observed in India. The same was also true for P. falciparum and P. vivax variability found between the study districts (Fig. 5 ). The overall contribution of P. falciparum and P. vivax was 72% and 28%, respectively in the present study as opposed to two-third and one-third ratio reported by the national programme, showing a differential of about 5% in both parasite species in 2015 [ 20 ].
The total number of estimated cases of malaria were about four folds more than about 1 million reported to the National Malaria Control Programme of India in the years 2015–2016 [ 20 ]. However, they were about one-third of 13 million (9.9–18 million) estimated by WHO for India for the year 2015 and 2016 [ 1 , 23 ]. Interestingly, 71% of the incident cases were contributed by only 4.7% of the total 1.268 billion population spread across eastern and north-eastern states of India. These states also contributed to 86.5% of the total estimated P. falciparum cases (Table 4 ).
During the present study, 93% of the reported deaths were in Koraput district of Odisha, a state which is highly malaria endemic with predominance of P. falciparum . It may be mentioned that Odisha state with only about 4% (43.7 million) population contributed 42% to the total reported malaria cases and 55.1% to the total P. falciparum cases in the country. Odisha also contributed to 31.8% of the total reported deaths due to malaria in India in 2015 [ 20 ]. The age-gender composition of deaths as seen in Fig. 6 confirms previously reported trends [ 5 , 12 ].
Malaria deaths in children in south and south-east Asia have been steadily decreasing since 1980 and accounted for a small proportion of the global deaths in this age group in 2010 [ 5 ]. Many studies have suggested that adult mortality due to malaria in India far exceeds in proportion than earlier known [ 5 , 12 , 13 ]. Even in Africa, adult absolute mortality is greater than child mortality than it was previously believed [ 23 ]. This has implications on the distribution of resources among affected populations both for surveillance as well as for vector control. Accordingly, the intervention focus needs to be widened covering both children as well as adults. This has epidemiological significance too. The re-enforced immunity after repeated infections in lower ages, which is expected to reduce adult malaria mortality, is short lived or not strong enough to prevent complications and deaths in adults in India [ 23 ].
India contributed 6% to the global estimated malaria cases and 49% to P. vivax cases in the year 2015 [ 1 ]. The country also contributed 6% to total deaths estimated and 51% to P. vivax mortality figures [ 1 ]. The time trends of malaria mortality estimated for India and endemic countries for 1980–2010 have also been recently published [ 5 ]. The WHO has reported a decline in the estimated number of malaria cases in India by 38% from 21 million in 2010 to 13 million in 2015 and by 55% (9.59 million) in 2017 and malaria deaths by 27%, i.e., from 33 000 in 2010 to 24 000 in 2015 and by 50% (16,733) in 2017 [ 1 , 24 ]. It is pertinent to mention that in the last decade, various programmatic changes have been introduced in India viz., improvement in the health infrastructure under National Health Mission and health care delivery over time through 600,000 village level health workers known as Accredited Social Health Activists (ASHAs). These workers have been providing better on-the-spot diagnosis (with RDT) of malaria at the doorsteps of the local people and enhanced ACT (artemisinin-based combination therapy) access for the treatment of P. falciparum malaria besides decentralized procurement and improving supply chain of LLINs to the communities in malaria high risk areas, etc. All these factors must have impacted trends of malaria morbidity and mortality in the country as observed in this study.
The estimated 29,341 (23,354–35,327) deaths from the primary data were comparable with WHO estimates of 24,000 (1500–47,000) for the year 2015; 22,786 (1580–45,300) for the year 2016 and 16,733 (1200–31,900) for the year 2017 [ 1 , 24 ]. However, these estimates were significantly lesser than 46,970 (14,757–94,945) estimated for India by Murray et al. and 205,000 by Dhingra et al. [ 5 , 13 ] while the deaths estimated in the present study were 76 folds greater than 384 deaths reported in India in 2015 [ 20 ].
The well cited limitations of the VA, notwithstanding, the observed crude P. falciparum mortality rate of 0.63% was on expected lines [ 21 , 22 ]. Najera and Hempel have reported that outside of Africa, malaria mortality has been estimated to be 1% of the estimated P. falciparum malaria incidence [ 23 ]. In the present study, malaria mortality rate was similarly 1.05% which was estimated taking 29,341 deaths as numerator and 2789,483 estimated P. falciparum cases as denominator in this study. Further, this agreement in P. falciparum mortality rates of the current study with that of earlier studies suggests that the methodology adopted in the current study for the burden estimation was quite appropriate. The recall period during VA was kept the shortest possible as 15 days during the study to elicit accurate information from the respondents. Another caveat of the study is that study population was enumerated just before initiation of surveillance and all the households and individuals were listed for follow up, but there is no information recorded about their movement or lost to follow up during the surveillance period.
Most importantly, this study has provided estimates of malaria cases and deaths in India at a time when they are most needed, i.e., at the inception of the malaria elimination campaign in the country. For such a vast and diverse country as India, the investigators recommend estimation of malaria burden at suitable intervals during the entire phase of malaria elimination till the target year 2027 and possibly beyond. This will offer distinct advantages as (1) the progress towards malaria elimination could be tracked when the current annual incidence is compared with the baseline numbers of malaria cases and deaths in different strata; (2) in the pre- and post-elimination phases, if any setbacks are observed in the targets, they could be timely addressed and (3) the regional priorities for resource allocation could be appropriately set to address residual transmission when the country is approaching malaria elimination targets to accelerate transmission control efforts and prevention of resumption of active transmission of malaria in areas of the country where malaria is eliminated.
The incidence of malaria in India were estimated at about 4 million in the year 2015–16. The estimates were four-fold improved over the number of malaria cases reported by the National Malaria Control Programme. Though they were about the three-fold lower than those which were estimated by the WHO for India for the same year, but the present estimates were based on an active surveillance sample survey design. The estimates depicted that over 70% of the total incident cases of malaria and 87% of the falciparum malaria cases were from about 5% of India’s population spread over eastern and north-eastern states of India.
The survey, during the year, estimated 29,341 (23,354–35,327) deaths due to malaria. They were the improved estimates over the earlier estimates and were comparable to the estimates provided by the WHO for the same period. The present estimates can serve as the benchmark for tracking the success of malaria elimination campaign in India.
Availability of data and materials
All the data and documents supporting the results of this study have been archived in ICMR-National Institute of Malaria Research, Dwarka, New Delhi. The raw and analysed data are available with ICMR-National Institute of Medical Statistics, Ansari Nagar, New Delhi.
Abbreviations
Annual parasite incidence
Annual falciparum incidence
Probability proportion to size
World Health Organization
Cause of death
Verbal autopsy
National Vector Borne Diseases Control Programme
Voluntary Surveillance Monitor
Rapid diagnostic test
Acredited Social Health Activist
Multipurpose health worker
Test positive rate
Plasmodium falciparum prevalence rate
Plasmodium falciparum mortality rate
WHO. World Malaria Report 2016: Geneva: World Health Organization; 2016. Accessed 18 July 2018.
WHO. Eliminating malaria. Geneva: World Health Organization; 2016. http://apps.who.int/iris/bitstream/10665/205565/1/WHO_HTM_GMP_2016.3_eng.pdf . Accessed 18 July 2018.
Murray CJ, Lopez AD. Evidence-based health policy-lessons from the Global Burden of Disease Study. Science. 1996;274:740–3.
Article CAS Google Scholar
Murray CJ, Lopez AD. The Global Burden of Disease 1990–2020: alternative projections of mortality and disability by cause for eight regions. Lancet. 1997;349:1498–504.
Murray CJ, Rossenfeld LC, Lim SS, Andrew KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31.
Article Google Scholar
Cibulskis RE, Aregawi M, Williams R, Otten M, Dye C. Worldwide incidence of malaria in 2009: estimates, time trends, and a critique of methods. PLoS Med. 2011;8:e1001142.
Hay SI, Okiro EA, Gething PW, Patil AP, Guerra CA, Snow RW. Estimating the Global Burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290.
Korenromp EL. Malaria incidence estimates at country level for the year 2004—proposed estimates and draft report. Geneva: World Health Organization, Roll Back Malaria, 2005. http://mosquito.who.int/docs/incidence_estimations2.pdf . Accessed 15 June, 2018.
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223.
Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106.
Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. Malaria risk: estimation of the malaria burden. Nature. 2005;434:214–7.
Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77(Suppl 6):69–78.
Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010;376:1768–74.
Registrar General India. Centre for Global Health Research. Causes of Death in India, 2001–2003: sample registration system. New Delhi: Government of India, (2009). Accessed 10 June 2018.
Shah NK, Dhariwal AC, Sonal GS, Gunasekara A, Dye C, Cibulskis R. Malaria—attributed death rates in India. Lancet. 2011;337:991.
Valecha N, Staedke S, Filler S, Mpimbaza A, Greenwood B, Chandarmohan D. Malaria –attributed death rates in India. Lancet. 2011;337:992–3.
Kumar A, Dua VK, Rathod P. Malaria–attributed death rates in India. Lancet. 2011;337:991–2.
Anonymous. Report of Expert Committee for estimating malaria mortality in the country. Ministry of Health and Family Welfare, Govt. of India; 2011, p. 1–53.
National Vector Borne Diseases Programme http://www.mrcindia.org/Diagnosis%20of%20Malaria%20pdf/Guidelines%202014.pdf . Accessed 15 June 2018.
National Vector Borne Diseases Programme. http://nvbdcp.gov.in/Doc/malaria-situation-March17.pdf . Accessed 15 June 2018.
Lozano R, Lopez AD, Atkinson C, Naghavi M, Flaxman AD, Murray CJ, Population Health Metrics Research Consortium (PHMRC). Performance of physician-certified verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9:32.
Snow R, Armstrong J, Forster D, Winstanley MT, Marsh VM, Newton CR, et al. Childhood deaths in Africa: uses and limitations of Verbal Autopsies. Lancet. 1991;340:351–5.
Najera JA, Hempel J. The burden of malaria. Geneva: World Health Organization 1996; CTD/MAL/96.10. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.597.5367&rep=rep1&type=pdf . Accessed 10 June 2019.
WHO. World Malaria Report 2018. Geneva: World Health Organization; 2018. Accessed 29 September 2019.
Download references
Acknowledgements
Authors are grateful to Secretary to the Govt. of India at the Department of Health Research & Director General, Indian Council of Medical Research; and the Directorate of National Vector Borne Diseases Control Programme for facilities and evincing keen interest in the study. They thank Director General of Health Services, Govt. of India for funding the study. They also thank Dr. Neena Valecha, the then Director ICMR-NIMR New Delhi, Dr. Manju Rahi, Scientist F, ECD, ICMR, New Delhi, Technical Review Group and the Chairman Dr. Shiv Lal and Dr. P.L. Joshi for technical, scientific and administrative advice. The logistic support from Dr. A. C. Dhariwal, Dr. G. S. Sonal and Dr. Sher Singh Kashyotia from National Vector Borne Diseases Control Programme was laudable. The advice of Dr. S. Sridhar is also sincerely acknowledged. Assistance received from the state and district Health officials, Drs. Mahesh Khalipe, Mahendra Jagtap, Arun Kumar, O. P. Thakan, P. Aswal, Narottam Sharma, Hari Shankar, S. Barve, D. Sisodia, P. K. Behra and S. N. Singh and NIMR colleagues Drs. S.K. Ghosh and Hemanth Kumar is thankfully acknowledged. The government and private health sectors, Municipal Corporations, Village bodies, burial/cremation staff, school teachers, Accredited Social Health Activists (ASHAs), community leaders provided information on cases and deaths which is gratefully acknowledged. The panel of doctors who assigned cause of death are also thanked. Finally, investigators thank NVBDCP, NIMS and NIMR staff for support and successful conclusion of the study. The manuscript is approved by the publication committee of NIMR and bears approval No. 019/2018.
The study was funded by the Ministry of Health and Family Welfare, Government of India.
Author information
Authors and affiliations.
Indian Council of Medical Research, National Institute of Malaria Research, Field Unit, Campal, Panaji, 403 001, Goa, India
Ashwani Kumar & Ajeet Kumar Mohanty
Indian Council of Medical Research, National Institute of Medical Statistics, Ansari Nagar, Medical Enclave, New Delhi, 110 029, India
Himanshu K. Chaturvedi & Arvind Pandey
Indian Council of Medical Research, National Institute of Malaria Research, Sector 8, Dwarka, 110 077, New Delhi, India
Surya Kant Sharma & Mantoshkumar S. Malhotra
You can also search for this author in PubMed Google Scholar
Contributions
AP planned and administered the study and arranged for its critical appraisal by Technical Review Group constituted by Ministry of Health and Family Welfare, Govt. of India. AK, AKM, SKS, MSM, HKC, prepared study formats, trained field teams, launched and supervised field study. MSM coordinated logistical support and provided administrative backing to all study teams, ensured timely supplies in all the six study districts and coordinated COD VA analysis and the cause of death assignment by medical experts. HKC and AP prepared sampling plan and performed analysis of data. HKC and AK interpreted data and prepared the manuscript and coordinated with all the authors. The opinion expressed and inferences drawn are exclusively of authors and not the organizations they belong to. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ashwani Kumar .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Institutional Ethics Committee of ICMR-National Institute of Malaria Research, New Delhi. However, during the study, no human subject was recruited for any drug trial. The blood test for malaria diagnosis by either microscopy or rapid diagnostic test was done as per standard procedures during routine fortnightly surveillance followed by treatment as per the national drug policy guidelines.
Consent for publication
Not applicable
Competing interests
The authors declare we have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ashwani Kumar and Himanshu K. Chaturvedi are equal first authors
Supplementary information
Additional file 1: table.
S1. Estimated Fever Rate in high, moderate and low malaria strata.
Additional file 2: Table
S2 . Estimated Fever Rate for all the study districts.
Additional file 3: Table
S3. Estimated Test Positive Rate Crude).
Additional file 4: Table
S4. Estimated Malaria Mortality Rate (weighted).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kumar, A., Chaturvedi, H.K., Mohanty, A.K. et al. Surveillance based estimation of burden of malaria in India, 2015–2016. Malar J 19 , 156 (2020). https://doi.org/10.1186/s12936-020-03223-7
Download citation
Received : 14 June 2019
Accepted : 06 April 2020
Published : 16 April 2020
DOI : https://doi.org/10.1186/s12936-020-03223-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Malaria burden
- Estimation of cases
- Test positivity rate
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
BRIEF RESEARCH REPORT article
Malaria elimination: situation analysis of cases in india, the state of madhya pradesh in central india, and district mandla of madhya pradesh.

- 1 Malaria Elimination Demonstration Project, Mandla, Madhya Pradesh, India
- 2 Indian Council of Medical Research, National Institute of Malaria Research, New Delhi, India
- 3 Department of Health Services, Government of Madhya Pradesh, Mandla, Madhya Pradesh, India
- 4 Directorate General of Health Services, Government of Madhya Pradesh, Bhopal, Madhya Pradesh, India
- 5 Foundation for Disease Elimination and Control of India (FDEC India), Mumbai, Maharashtra, India
- 6 Sun Pharmaceutical Industries Ltd., Mumbai, India
India contributed approximately 66% of the malaria cases in the WHO South-East Asia region in 2022. In India, approximately 44% of cases have been reported to be disproportionately contributed by approximately 27 districts. 1 A comparative analysis of reported malaria cases between January 2017 and December 2022 was performed in Mandla district, which is the site of a model malaria elimination demonstration project (MEDP) in Madhya Pradesh (MP), India. Compared to 2017, the decrease in malaria cases in Mandla from 2018 to 2022 was higher than MP and the rest of the country. The reduction of cases was significant in 2018, 2019, and 2021 ( p < 0.01) (Mandla vs. MP) and was highly significant during 2018–2022 ( p < 0.001) (Mandla vs. India). Robust surveillance and real-time data-based decisions accompanied by appropriate management, operational controls, and independent reviews, all designed for resource optimisation, were the reasons for eliminating indigenous malaria in Mandla district. The increase in infection rates during the months immediately following rains suggests that surveillance, vector control, and case management efforts should be specifically intensified for eliminating imported and indigenous cases in the near-elimination districts to work towards achieving the national elimination goal of 2030.
Introduction
Malaria remains one of the most important public health problems globally, with an estimated 249 million cases and 608,000 malaria-attributable deaths reported in 2022. Approximately 94% of global malaria cases are contributed by African countries, while 2% of cases are contributed by countries in the World Health Organization (WHO) South-East Asia (SEA) region. India accounted for approximately 66% of the malaria cases in the WHO SEA region in 2022 ( 1 ).
During the COVID-19 pandemic between 2019 and 2020, the global burden of malaria increased by 6%, primarily due to the disruption of anti-malarial activities. In comparison, India was the only High Burden High Impact (HBHI) country that reported a 46% decrease in malaria cases between 2019 and 2020. However, there was a 50% decrease in the distribution of insecticide-treated bed nets in India in 2020 ( 2 ).
In India, approximately 44% of the reported malaria cases and 43% deaths are disproportionately contributed by approximately 27 tribal-dominated districts that comprise 5% of the country's population. Among these cases, 57.3% are identified as Plasmodium falciparum infections ( 3 ).
India had set the target to achieve zero indigenous malaria cases in 26 low-to-moderate endemic malaria states/Union Territories (UTs) by 2022 and eliminate malaria throughout the country by 2027. The proposed strategies included strengthening malaria surveillance, establishing the mechanism for early case detection and prompt treatment, distribution and promotion of the use of long-lasting insecticidal nets (LLIN), effective indoor residual sprays (IRS), capacity-building of community healthcare service providers, and inter-sectorial coordination. The 2022 target of zero indigenous cases was achieved by only two states/UTs of Puducherry and Lakshadweep, with Chandigarh and newly formed UT of Ladakh reporting only two cases each in 2022 ( 3 , 4 ).
Inaccessible terrains, dense forest covers, perennial streams, poor socioeconomic indicators, poor health-seeking behavior, and inadequate health infrastructure are the significant challenges for malaria elimination in tribal-dominated areas of India. People living in these malaria-endemic areas have poor access to formal health facilities. Unqualified healthcare providers and traditional faith healers are often the first points of contact in the rural tribal areas, which is the primary cause of delay in prompt diagnosis and radical treatment ( 5 ).
In 2017, the MEDP was launched as a public–private partnership project between the Government of Madhya Pradesh (MP), Indian Council of Medical Research (ICMR), and Foundation for Disease Elimination and Control (FDEC) of India—a corporate social responsibility (CSR) subsidiary of Sun Pharmaceutical Industries Ltd.—to demonstrate that malaria elimination is possible in hard-to-reach, hilly, forested, and tribal-dominated areas. The MEDP's malaria operational elimination plan used the T4 (Track fever, Test fever, Treat malaria, and Track treatment) strategy, monitoring of vector control interventions, Mass Screening and Treatment (MSaT), needs-assessment followed by capacity-building, regular monitoring, and supervision for data-driven decision-making to ensure best outcomes of the resources deployed for the project ( 6 – 9 ).
The MEDP also estimated the burden of sub-microscopic malaria infection and the importation of cases into the district. From September 2017 to March 2021, for a total of 43 months of field operations, the MEDP achieved a 91% reduction in indigenous malaria cases with 10 consecutive months of zero transmission of indigenous malaria cases ( 10 ).
In the MEDP, the key interventions added to complement the interventions of the national programme included: (1) robust active surveillance using the T4 strategy, (2) periodic mass survey and treatment adopting the stratified clustered random sampling method, (3) molecular diagnosis of a subset of samples to estimate the burden of low-density malaria infection and asymptomatic cases, (4) supervised and quality-assured IRS and LLIN distribution for vector control efforts, (5) regular capacity-building of healthcare providers, (6) innovative information education communication/behavior change communication (IEC BCC) campaigns, and (7) robust reviews and accountability frameworks.
The present situation analysis was conducted to track malaria elimination progress in Mandla district post-MEDP and to highlight how the lessons learned could help achieve the national malaria elimination goal.
This study presents the situation analysis of the reported malaria cases between January 2017 and December 2022 in Mandla district. It compares them with the reported cases in MP and India during the same period. In Mandla, the monthly malaria prevalence data were collected from the MEDP data repository, which included the active and passive cases detected using rapid diagnostic tests (RDTs), microscopic examination of blood smears, and data provided by the District Malaria Office. During the study period, the total reported malaria cases of Mandla district were classified into two groups, namely indigenous and imported cases. Annual malaria prevalence data from the state of MP and India were obtained from the official website of the National Center for Vector Borne Disease Control (NCVBDC). The annual per cent decline in malaria cases, along with a 95% confidence interval, was estimated from 2017 as a reference year. The Chi-squared test was used to compare percentage change over multiple time points between Mandla vs. MP and India. The statistical analysis was performed using R for Windows version 4.3.2. The monthly trend of malaria cases during the MEDP (January 2017–March 2021) and the post-MEDP (April 2021–December 2022) period in Mandla district is presented in Figure 1 . The comparison of the annual per cent decline in malaria cases over multiple time points between Mandla district, the state of MP, and the country is shown in Table 1 .
Figure 1 . Monthly trend of Plasmodium falciparum , malaria cases and parasite incidence in district Mandla during 2017–2022.
Table 1 . Situation analysis of malaria cases during 2017 and 2022 in district Mandla, Madhya Pradesh and India.
Mandla district is located at the geo-coordinates of 22° 38' 25.476” N latitude and 80° 30' 48.384 E longitude. This district is among the tribal dominant and hilly forested districts in the state of MP. Approximately 58% of the population belonged to the ethnic tribal groups, mainly “ Gond” and “ Baiga” (Particularly Vulnerable Tribal Group). The transmission dynamics of malaria in the district is seasonal, and Anopheles culicifacies is the main malaria vector that breeds in perennial streams ( 11 ).
In reference to 2017 as the base year, the per cent decrease in malaria cases in Mandla district was 67.08%, 83.78%, 85.99%, 88.94%, and 93.37% from 2018 to 2022. At the state level, in MP, the percent decrease in malaria cases during this period was 51.75%, 69.36%, 85.36%, 93.11%, and 91.71%, respectively. Similarly, during the same period in India, the per cent decrease in malaria cases was 48.92%, 59.78%, 77.84%, 80.78%, and 79.03%, respectively ( Table 1 ).
Further analysis revealed that the per cent decrease in malaria cases in Mandla from 2018 to 2022, in reference to the year 2017, was significantly higher than MP during 2018 and 2019. The decrease was significantly higher in district Mandla than in the entire country from 2018 to 2022.
It should be noted that the data on malaria cases from district Mandla were obtained using active surveillance, passive surveillance, data from health camps, primary health centers, community health centers, district hospitals, and the sentinel surveillance network, which included registered public and private practitioners in the district. In comparison, the state and country data come solely from public (government) sources.
The monthly trend of P. falciparum malaria cases and parasite incidence/1,000 population showed a seasonal variation in the distribution of Plasmodium species in Mandla district. Most of the P. vivax cases were reported from March to August, showing peaks between June and August and then dominated by P. falciparum cases from September with peaks from October to December ( Figure 1 ).
Mandla district reported malaria cases throughout 2017 and 2018. Zero indigenous malaria cases were reported for 3 months each in 2019, 2020, and 2021, and there were zero indigenous malaria cases for seven months in 2022. Compared to the years 2017–2020, where there were two distinct peaks of malaria in February–March and July–September, there was only one peak with much lower intensity in the July–September months of 2021 and 2022. The month-wise trend at the state and country level could not be analyzed due to the non-availability of seasonal data in the public domain.
The comprehensive surveillance strategy provided a robust estimate of malaria cases in Mandla district as compared to the rest of the state and the country. The MEDP also implemented MSaT to diagnose and treat the asymptomatic malaria cases during 2018–2020 and adopted the stratified clustered sampling method based on the malaria endemicity ( 12 ). Therefore, the Mandla malaria estimates were a true “total” burden of malaria as compared to the state of MP and the rest of the country. Furthermore, a higher rate of decline in malaria cases was observed during the year 2022. Most of the malaria cases were reported during the monsoon season (June–September), along with zero reported indigenous malaria cases in the consecutive 8 months of the post-intervention period of the MEDP between 2021 and 2022.
Based on the findings from the malaria elimination project in Mandla district, the significant reduction in malaria cases over 4 years is attributable to robust (active and passive) surveillance using digital tools, the T4 strategy, active monitoring of vector control interventions, periodic capacity-building of the healthcare providers, regular community mobilization, MSaT, and molecular diagnosis of a subset of samples to estimate the burden of low-density malaria infection and asymptomatic cases. The institution of appropriate management and operational controls, along with frequent internal and external reviews, contributed to prompt actions and responses based on the real-time data ( 10 , 11 , 13 , 14 ). These protocols ensured the best outcomes of human, commodity, and financial resources used for the elimination project.
The MEDP regularly monitored and supervised vector control interventions in the district. The regular use of LLINs in the community increased from 34% (95% CI: 33.74–34.26) in 2017 to 47% (95% CI: 46.80–47.19) in 2019, and this difference was significant statistically ( p < 0.0001). The spraying quality of the IRS improved from 47.8% in 2017 to 88.6% in 2019, and the improvement in satisfaction with the IRS by the community increased from 66.8% to 90.5% within the same period ( 15 – 17 ).
Alphacypermethrin was used in LLIN and IRS in Mandla. As part of the MEDP, the insecticide susceptibility tests were conducted periodically during 2017, 2018, and 2019 to regularly monitor insecticide resistance to the vector species. The results showed that alphacypermethrin was possibly resistant to the Anopheles culicifacies in 2017 and further developed resistance in the year 2019 ( 15 ).
The strategies used in the MEDP can serve as a guide to develop and/or refine the district-specific malaria elimination operational plans by treating the district as an operational unit as has been already proposed by NCVBDC. In addition, linking data from each district through digital systems for robust surveillance, effective supply chain management, and real-time data analysis and reporting would be highly useful for programme managers and policy makers ( 18 ).
Two additional noteworthy observations from the MEDP study that are critical to malaria elimination are: (1) the finding of 1.51% of sub-microscopic infections in the community, detected through the diagnostic PCR method, and (2) the prevalence of asymptomatic malaria that was found to be 0.98% during the mass survey conducted by the MEDP from 2018 to 2020. Based on this information, the national programme should consider testing a subset of cases using sensitive PCR methods to determine sub-microscopic infection and conduct periodic mass surveys to identify and treat asymptomatic cases during the elimination phase ( 19 ).
The incidence data for Mandla, where indigenous transmission of malaria was interrupted several times during the conduct of the project, indicates that intensified surveillance, case management, and vector control efforts immediately after rains would lead to the elimination of infections, whether they are imported or are indigenous. Additionally, the gradual increase in the malaria-free months from 2019 to 2022 indicates that the gains achieved throughout the MEDP were progressive and sustained in the district. We firmly believe that the sustained gains were attributed to robust real-time internal and external reviews as part of the programme's management and operational controls.
Robust surveillance would provide information on individuals requiring treatment, including imported cases. Molecular diagnostic tests would help determine the burden of sub-microscopic/sub-RDT and asymptomatic infections. IRS and LLINs, together with minor engineering, should be deployed simultaneously, followed by an independent assessment of the households for the use of LLINs and IRS. Internal and external data quality reviews should be conducted for accountability at operational, human resource, supply chain, and scientific levels required for elimination goals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MS: Conceptualization, Data curation, Formal analysis, Writing—original draft. PB: Writing—review & editing. HR: Conceptualization, Writing—original draft, Writing—review & editing. RS: Writing—review & editing. HJ: Writing—review & editing. AA: Writing—review & editing. AL: Conceptualization, Supervision, Writing—original draft, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Foundation for Disease Elimination and Control (FDEC) of India.
Conflict of interest
AL was employed by Sun Pharmaceutical Industries Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ 27 high-burden districts - Lawngtlai, Lunglei, Mamit and Saiha (Mizoram); Dhalai, Gomati, Khowai, North Tripura, South Tripura (Tripura); South Garo Hills (Meghalaya); Gadchiroli (Maharashtra); Kolkata (West Bengal); Kalahandi, Kandhamal, Korapur, Malkangiri, Rayadgada (Odisha); Bastar, Bijapur, Dantewada Kanker, Kondagaon Narayanpur, Sukma (Chattisgarh); Khunti, West Singhbhum (Jharkhand); Nicobars (Andman and Nicobar).
1. WHO World Malaria Report . (2023). Available online at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023~ (accessed January 22, 2024).
Google Scholar
2. WHO World Malaria Report. Geneva: WHO (2022). p. 293.
3. NVBDCP Malaria Situation in India . (2023). Available online at: https://nvbdcp.gov.in/index4.php?lang=1andlevel=0andlinkid=564andlid=3867 (accessed June 20, 2023).
4. NVBDCP National Framework for Elimination of Malaria in India 2016-30 . (2016). Available online at: http://nvbdcp.gov.in/Doc/National-framework-for-malaria-elimination-in-India-2016%E2%80%932030.pdf (accessed January 22, 2024).
5. Sharma RK, Thakor HG, Saha KB, Sonal GS, Dhariwal AC, Singh N. Malaria situation in India with special reference to tribal areas. Indian J Med Res. (2015) 141:537–45. doi: 10.4103/0971-5916.159510
PubMed Abstract | Crossref Full Text | Google Scholar
6. Rajvanshi H, Bharti PK, Nisar S, Jain Y, Jayswar H, Mishra AK, et al. Study design and operational framework for a community-based Malaria Elimination Demonstration Project (MEDP) in 1233 villages of district Mandla, Madhya Pradesh. Malar J. (2020) 19:1–12. doi: 10.1186/s12936-020-03458-4
7. Rajvanshi H, Saha KB, Shukla MM, Nisar S, Jayswar H, Mishra AK, et al. Assessment of ASHA for knowledge, diagnosis and treatment on malaria in Mandla district of Madhya Pradesh as part of the malaria elimination demonstration project. Malar J. (2021) 20:1–8. doi: 10.1186/s12936-021-03610-8
8. Rajvanshi H, Nisar S, Bharti PK, Jayswar H, Mishra AK, Sharma RK, et al. Significance of training, monitoring and assessment of malaria workers in achieving malaria elimination goal of Malaria Elimination Demonstration Project. Malar J. (2021) 20:1–12. doi: 10.1186/s12936-020-03534-9
9. Singh MP, Rajvanshi H, Nisar S, Singh A, Jayswar H, Singh S, et al. A comparative assessment of the community frontline health workers for their knowledge and practices of malaria diagnosis and treatment in three contiguous districts Mandla, Balaghat, and Dindori of Madhya Pradesh, India. Malar J. (2023) 22:62. doi: 10.1186/s12936-023-04492-8
10. Bharti PK, Rajvanshi H, Nisar S, Jayswar H, Saha KB, Shukla MM, et al. Demonstration of indigenous malaria elimination through Track-Test-Treat-Track (T4) strategy in a malaria elimination demonstration project in Mandla, Madhya Pradesh. Malar J. (2020) 19:1–12. doi: 10.1186/s12936-020-03402-6
11. Singh MP, Rajvanshi H, Bharti PK, Jayswar H, Singh S, Mehra RK, et al. Evaluation of the model malaria elimination strategy in Mandla district along with its neighbouring districts: a time series analysis from 2008 to 2020. Malar J. (2023) 22:45. doi: 10.1186/s12936-023-04477-7
12. Singh A, Rajvanshi H, Singh MP, Bhandari S, Nisar S, Poriya R, et al. Mass screening and treatment (MSaT) for identifying and treating asymptomatic cases of malaria-malaria elimination demonstration project (MEDP), Mandla, Madhya Pradesh. Malar J. (2022) 21:395. doi: 10.1186/s12936-022-04423-z
13. Pradhan S, Pradhan MM, Dutta A, Shah NK, Joshi PL, Pradhan K, et al. Improved access to early diagnosis and complete treatment of malaria in Odisha, India. PLoS ONE. (2019) 14:e0208943. doi: 10.1371/journal.pone.0208943
14. Rajvanshi H, Singh MP, Bharti PK, Sahu RS, Anvikar A. Science of malaria elimination: using knowledge of bottlenecks and enablers from the Malaria Elimination Demonstration Project in Central India for eliminating malaria in the Asia Pacific region. Front Public Health. (2024) 11:1303095. doi: 10.3389/fpubh.2023.1303095
15. Mishra AK, Bharti PK, Vishwakarma A, Nisar S, Rajvanshi H, Sharma RK, et al. A study of malaria vector surveillance as part of the Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh. Malar J. (2020) 19:1–13. doi: 10.1186/s12936-020-03517-w
16. Rajvanshi H, Mishra K, Bharti PK, Sandhibigraha D, Nisar S, Jayswar H, et al. Learnings from two independent malaria elimination demonstration projects in India. Trans R Soc Trop Med Hyg. (2021) 115:1229–33. doi: 10.1093/trstmh/trab148
17. Mishra AK, Nisar S, Rajvanshi H, Bharti PK, Saha KB, Shukla MM, et al. Improvement of Indoor Residual Spraying and Long-Lasting Insecticidal Net services through structured monitoring and supervision as part of the Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh. Malar J. (2021) 20:1–12. doi: 10.1186/s12936-021-03639-9
18. Rajvanshi H, Bharti PK, Nisar S, Jayswar H, Mishra AK, Sharma RK, et al. A model for malaria elimination based on learnings from the Malaria Elimination Demonstration Project, Mandla district, Madhya Pradesh. Malar J. (2021) 20:1–15. doi: 10.1186/s12936-021-03607-3
19. Singh A, Singh MP, Bhandari S, Rajvanshi H, Nisar S, Telasey V, et al. Significance of nested PCR testing for the detection of low-density malaria infection amongst febrile patients from the Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh, India. Malar J. (2022) 21:341. doi: 10.1186/s12936-022-04355-8
Keywords: malaria elimination, MEDP, monitoring and accountability frameworks, robust surveillance, situation analysis, tribal malaria
Citation: Singh MP, Bharti PK, Rajvanshi H, Sahu RS, Jayswar H, Anvikar AR and Lal AA (2024) Malaria elimination: situation analysis of cases in India, the state of Madhya Pradesh in central India, and district Mandla of Madhya Pradesh. Front. Public Health 12:1363736. doi: 10.3389/fpubh.2024.1363736
Received: 31 December 2023; Accepted: 11 March 2024; Published: 09 April 2024.
Reviewed by:
Copyright © 2024 Singh, Bharti, Rajvanshi, Sahu, Jayswar, Anvikar and Lal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Altaf A. Lal, altaf.lal@gmail.com ; altaf.lal@sunpharma.com
† Present address: Harsh Rajvanshi, Asia Pacific Leaders Malaria Alliance (APLMA), Singapore, Singapore
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- International
- Today’s Paper
- Premium Stories
- Express Shorts
- Health & Wellness
- Brand Solutions
What the World Malaria Report says about India
Bucking global trends, malaria cases and deaths have continued to decline in india. what is behind india’s success story how does climate change play a part why is there a need for improved surveillance and what are the challenges ahead.
The recently released World Malaria Report shows that the number of cases and deaths due to the mosquito-borne infection India have continued to decline. With an estimated 33.8 lakh cases and 5,511 deaths, India saw a decline of 30 per cent in malaria incidence and 34 per cent in mortality in 2022, compared to the previous year.
India’s downward trend was reflected in the larger WHO South East Asian region that remained on track to achieving the 2030 target of reducing cases and deaths by 90 per cent, the report said.

This is, even as since the beginning of the pandemic, a trend of increasing incidence has been noted globally, after nearly a decade of the numbers remaining almost the same.
The number of malaria cases had dropped from 243 million to 233 million globally between 2000 and 2019. However, there was an increase of 11 million cases in 2020. They remained stable in 2021, before seeing another increase of 5 million in 2022 to 249 million. The number of malaria deaths also remained higher than the pre-pandemic levels. There were 608,000 deaths reported in 2022 as compared to 576,000 cases in 2019.
Behind India ’s success story
A focus on providing primary healthcare to the remotest areas, surveillance that is now being backed by digital data, and better handling of extreme weather events such as cyclones have been key to India’s success as per experts.

Good preventive practices, use of effective tools to keep the mosquito population in check, use of point of care tests for quick diagnosis, and good management of the malaria cases have been key to reducing cases and deaths due to malaria over the years, said Dr Neena Valecha, former director of National Institute of Malaria Research, who also served as the advisor on malaria to the WHO-Southeast Asia region previously.
He added that states such as Odisha that regularly see extreme weather events such as cyclones are now well prepared to handle it, thereby reducing incidences of malaria associated with such events.
Climate change and malaria
The malaria parasite and mosquito are both extremely sensitive to temperature, humidity, and rainfall, leaving experts worried about expanding reach of the disease. The report says that climate change can not only directly increase geographies for malaria spread, but also indirectly affect the impact of the disease by reducing access to healthcare facilities and timely treatment.
Dr Sarkar said: “Climate change is likely to lead to an increase in temperatures, with newer areas especially in the Himalayan belt suitable for the spread of the disease. High risk zones will also emerge in states that face very high rainfall periodically.” He said that planning for the disease should also take into account such extreme weather events as better planning for them can reduce incidence of malaria.
Almost half of the five million additional malaria cases reported globally in 2022 — 2.1 million — were from Pakistan that witnessed an extreme flood . The report said that the standing water after the floods became ideal breeding ground for mosquitoes and led to a five-fold increase in malaria cases in the country. “The floods destroyed infrastructure and isolated millions, hindering medical access and increasing disease risk,” it said.
With increasing frequency of such extreme weather events, the annual report for the first time focused on climate change and malaria.
Need for improved surveillance
With fewer cases being reported from the country, there has to be intensified efforts to find and treat the scattered cases. “When the burden of disease is higher, any intervention in areas reporting most of the cases results in drastic reduction in numbers. However, when the numbers go down, the cases are scattered and difficult to find. This is where the role of surveillance comes in,” Dr Sarkar said.
He added that it was important to have real-time digital data of these cases to help local administrations better plan the interventions.
Challenges ahead
While India is doing well when it comes to malaria, issues such as resistance may derail it from its target of elimination by 2030. “The biological threats include drug resistance, insecticide resistance, gene deletions in parasites which make diagnosis difficult,” said Dr Valecha.
Another challenge is vivax malaria, which accounts for over 40 per cent of malaria cases in India. The vivax plasmodium is known to hide in the liver and cause recurrent infections. To treat, a 14-day course of therapy has to be taken. Experts say the challenge with that is many do not complete the treatment and stop taking the drug once they feel better.
Dr Valecha added: “The last mile is always the most difficult. To achieve the malaria elimination target of 2030, there has to be emphasis on strengthening of surveillance as well as tailoring of malaria interventions at sub-national level which should be data driven. In addition updating policies and adopting new tools as per national and subnational need in line with WHO guidance is critical.”

Sexual harassment complaint against WB Guv & Article 361 Subscriber Only

History of religion-based reservations; question of Muslims’ inclusion Subscriber Only

In South Korea, remembering Rabindranath Tagore Subscriber Only

Laapataa Ladies: Fantasy by those who never lived in village Subscriber Only

Artificial General Intelligence (AGI): Why are people worried ? Subscriber Only

Modi appears to be throwing away his own lead in Subscriber Only

The clean energy transition has become messy Subscriber Only

UPSC Key |EC on deepfakes, Cooperative Federalism and more Subscriber Only

Message from Rae Bareli, Amethi: Rahul Gandhi de-risks, Priyanka waits Subscriber Only

Not native to India, gulmohar trees became signature of early Subscriber Only

Real Madrid's shift from dizzying days of Galacticos to current Subscriber Only

Heeramandi: Sanjay Leela Bhansali directs most offensive scene of his

Anonna Dutt is a Principal Correspondent who writes primarily on health at the Indian Express. She reports on myriad topics ranging from the growing burden of non-communicable diseases such as diabetes and hypertension to the problems with pervasive infectious conditions. She reported on the government’s management of the Covid-19 pandemic and closely followed the vaccination programme. Her stories have resulted in the city government investing in high-end tests for the poor and acknowledging errors in their official reports. Dutt also takes a keen interest in the country’s space programme and has written on key missions like Chandrayaan 2 and 3, Aditya L1, and Gaganyaan. She was among the first batch of eleven media fellows with RBM Partnership to End Malaria. She was also selected to participate in the short-term programme on early childhood reporting at Columbia University’s Dart Centre. Dutt has a Bachelor’s Degree from the Symbiosis Institute of Media and Communication, Pune and a PG Diploma from the Asian College of Journalism, Chennai. She started her reporting career with the Hindustan Times. When not at work, she tries to appease the Duolingo owl with her French skills and sometimes takes to the dance floor. ... Read More
- Explained Health
- Express Explained

Amid row over several controversial remarks, Sam Pitroda on Wednesday stepped down as the Chairman of the Indian Overseas Congress "of his own accord." His resignation was accepted by party President Mallikarjun Kharge, Congress General Secretary in-charge (Communications) Jairam Ramesh said.
UPSC Magazine

Read UPSC Magazine

More Explained

Best of Express

EXPRESS OPINION


May 08: Latest News
- 01 Israeli tanks have rolled into Rafah. What does this mean for the Palestinians sheltering there?
- 02 IPL 2024: Watch Sanju Samson’s dismissal by TV umpire sparks controversy in DC vs RR match
- 03 Punjab government refuses VRS to IAS officer who is BJP’s Lok Sabha candidate in Bathinda
- 04 Bombay HC seeks state govt reply to PIL alleging illegalities in ‘Dial 108’ ambulance project contract
- 05 Total 116 candidates from six seats in fray in Mumbai
- Elections 2024
- Political Pulse
- Entertainment
- Movie Review
- Newsletters
- Web Stories
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 December 2023
The effectiveness of malaria camps as part of the malaria control program in Odisha, India
- Danielle C. Ompad 1 ,
- Timir K. Padhan 2 na1 ,
- Anne Kessler 3 na1 ,
- Yesim Tozan 1 ,
- Abbey M. Jones 1 ,
- Anna Maria van Eijk 3 , 4 ,
- Steven A. Sullivan 3 ,
- Mohammed A. Haque 2 ,
- Madan Mohan Pradhan 5 na2 ,
- Sanjib Mohanty 2 na2 ,
- Jane M. Carlton 1 , 3 , 6 na2 &
- Praveen K. Sahu 2 na2
Scientific Reports volume 13 , Article number: 22998 ( 2023 ) Cite this article
1337 Accesses
6 Altmetric
Metrics details
- Epidemiology
Durgama Anchalare Malaria Nirakaran (DAMaN) is a multi-component malaria intervention for hard-to-reach villages in Odisha, India. The main component, malaria camps (MCs), consists of mass screening, treatment, education, and intensified vector control. We evaluated MC effectiveness using a quasi-experimental cluster-assigned stepped-wedge study with a pretest–posttest control group in 15 villages: six immediate (Arm A), six delayed (Arm B), and three previous interventions (Arm C). The primary outcome was PCR + Plasmodium infection prevalence. The time (i.e., baseline vs. follow-up 3) x study arm interaction term shows that there were statistically significant lower odds of PCR + Plasmodium infection in Arm A (AOR = 0.36, 95% CI = 0.17, 0.74) but not Arm C as compared to Arm B at the third follow-up. The cost per person ranged between US$3–8, the cost per tested US$4–9, and the cost per treated US$82–1,614, per camp round. These results suggest that the DAMaN intervention is a promising and financially feasible approach for malaria control.
Similar content being viewed by others

The potential public health consequences of COVID-19 on malaria in Africa
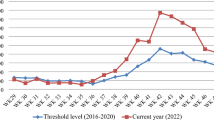
Malaria surveillance, outbreak investigation, response and its determinant factors in Waghemra Zone, Northeast Ethiopia: unmatched case–control study
Factors associated with the decline of malaria in Myanmar’s Ayeyarwady Region between 2013 and 2017
Introduction.
India has made noteworthy progress towards malaria elimination, with cases decreasing from 20 million in 2000 to approximately 4.1 million cases in 2020 1 . Despite this decline over the past two decades, it remains an important public health problem. India was one of eleven countries accounting for 70% of the global burden of malaria in 2020 1 , and it continues to account for 79% of all malaria cases and 83% of all malaria deaths in the South-East Asia region 2 . Within India, the state of Odisha has the highest burden of malaria, accounting for 22.4% of all cases in the country in 2020, of which 91.4% were Plasmodium falciparum infections 3 . The malaria burden in Odisha has been persistently high in the remote, forested areas of the state. In response to this, the Government of Odisha implemented the Durgama Anchalare Malaria Nirakaran (DAMaN; ‘malaria control in inaccessible areas’) program in 2017. DAMaN was designed to supplement existing and routine malaria control programs that serve approximately 5000 inaccessible villages and hamlets in the rural parts of the state 4 .
A key activity of the DAMaN program is the implementation of ‘malaria camps’ (MCs). In the MC model, teams of health workers visit villages and hamlets to provide the intervention which includes seven key activities in each cycle: (1) one round of mass screen and treat (MSAT) before the monsoon season conducted with point-of-care rapid diagnostic tests (RDTs), (2) one or two rounds of fever screen and treat (FSAT) during or after the monsoon season, (3) IRS (indoor residual spraying), (4) other vector control methods, (5) LLIN (long-lasting insecticidal net) distribution (not on an annual basis), (6) educational programming, and (7) maternal and child health visits and screenings. The intervention has been described in detail 4 , 5 , 6 , 7 . The control condition, as a part of the National Vector Control Strategy, is standard of care (SOC) whereby Accredited Social Health Activists (ASHAs) conduct door-to-door fever surveillance weekly. Fever cases are tested with RDTs and treated; severe cases are referred to hospitals 8 . IRS is conducted twice in a year during the transmission season in selected high risk areas having an annual parasite index (API) > 5, and LLINs are distributed in high risk areas having API > 2 9 . Thus key distinguishing features of MCs versus SOC include MSAT (everyone is offered malaria screening regardless of fever status in the MCs), educational programming, and maternal and child health visits and screenings.
Odisha state has seen a > 80% decline in malaria cases since 2017, attributed to the large-scale distribution of LLINs and the DAMaN program 10 . The aim of our project was to evaluate the effectiveness of the DAMaN MCs through a quasi-experimental cluster-assigned (i.e., non-randomized) stepped-wedge study with a pretest–posttest control group design.
We enrolled 2463 participants into three study arms: six immediate interventions (Arm A), six delayed interventions (Arm B), and three previous interventions (Arm C), and sampled them at baseline, with three follow-ups from August 2019 to December 2020. The primary outcome was PCR + Plasmodium infection prevalence. A time (i.e., visit) \(\times\) study arm interaction revealed statistically significant lower odds of PCR + malaria in Arm A versus B at the third follow-up. Our results suggest that the DAMaN program’s malaria camps were associated with lower malaria incidence relative to standard-of-care.
Study population and study design
Fifteen villages were selected in the northern districts of Keonjhar and Jharsuguda in Odisha state, India, in consultation with the Odisha Malaria Control Program (Fig. 1 ). The villages were distributed between three study arms: six villages in Arm A ‘new-MC’ (communities receiving MCs for the first time in year one), six villages in Arm B ‘delayed-MC’ (communities undergoing routine malaria control in year one and receiving MCs for the first time in year two), and three villages in Arm C ‘old-MC’ villages, where MCs had already been implemented prior to the study period. A flow chart of the study is shown in Fig. 2 .
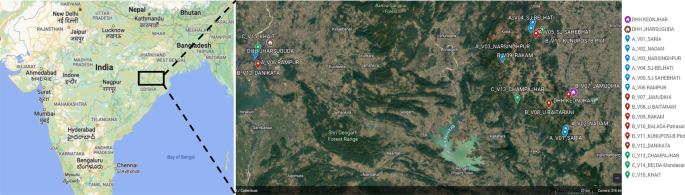
Location of 15 hard-to-reach villages in Odisha, India. The two DHH (district headquarter hospitals) are indicated, and each village is named with its study arm letter (A, B, C) and village number (V1–15) provided. Maps created with Google©2023, INEGI.
Cluster-assigned quasi-experimental study of malaria camps as part of the DAMaN malaria elimination program in Odisha, India. Flow chart providing a summary of the 15 clusters (villages) distributed between three arms and sampled at baseline and three follow-ups. A timeline of the activities is also given, and whether COVID-19 shutdowns are known to have occurred.
We enrolled 2463 participants in the study and Table 1 presents their baseline demographic characteristics. A total of 14.1% of the participants were aged 5 years or younger, 29.4% were aged 6 to 17, 23.6% were 18–34, and 32.8% were aged 35 or older, with females comprising 56.1% of the participants. The participants generally had low educational attainment: almost half (49.3%) had no schooling/less than primary school whereas 5.7% had higher secondary school or more. With respect to occupation, 28.8% were housewives, 34.9% were students, 5.6% were farmers/agricultural laborers, 18.6% were employed in another trade, 9.6% were children that were not in school, and 2.6% did not have an occupation.
Across the study arms there were statistically significant differences in age, educational attainment, and occupation, but not sex. As evidenced by the age category distribution and the mean age for each arm, Arm A was older (mean age [M age ] = 27.2, standard deviation [SD] = 19.0) and Arm C was younger (M age = 22.2, SD = 18.8) than Arm B (M age = 25.7, SD = 19.6, p ANOVA < 0.001). A higher proportion of participants in Arm A had higher secondary education or higher while a higher proportion in Arm B had middle, secondary, or matric and in Arm C a high proportion had primary education. There were a higher proportion of farmers/agricultural laborers and people with other employment/trades in Arm A, a higher proportion of housewives in Arm B, and higher proportions of students and children not in school in Arm C. Given the differences across arms, adjusted odds ratios (aORs) control for demographics.
Intent-to-treat analysis for primary and secondary malaria outcomes
The MC intervention was revised from our initial protocol paper 7 with two major and consistent differences. First, all MCs conducted MSAT rather than FSAT in all rounds of the intervention. Second, LLINs were not distributed in our study villages because their 3-yearly replenishment distribution scheduled for 2020 was delayed due to the Covid-19 pandemic.
Table 1 presents the malaria outcomes at baseline and the third follow-up (FU3) visit; associations are considered statistically significant if p < 0.013, based on a Bonferroni correction. At baseline, 9.8% of the sample had PCR + Plasmodium infection, with statistically significant differences across study arms. Arm A had the highest prevalence of Plasmodium infection (14.2%), followed by Arm B (8.1%) and Arm C (3.9%, p < 0.001) (Fig. 3 , panel A). Prevalence of RDT + Plasmodium infection was 1.9% and there were no significant differences across arms; Arm A again had the highest prevalence of Plasmodium infection (2.7%), followed by Arm B (1.8%) and Arm C (0.5%, p = 0.014). When malaria was defined by any positive PCR or RDT test, prevalence overall was 9.7% at baseline. With respect to species, 236 (98.3%) were P. falciparum cases and 9 (3.8%) were P. vivax ; 5 (2.1%) were co-infected with P. falciparum and P. vivax [data not shown]. Approximately 48.8% of Plasmodium infections were asymptomatic (defined as RDT + or PCR + and no fever) at baseline with Arm C having the highest proportion (94.1%) of asymptomatic cases as compared to Arms A and B (Fig. 3 , panel C: 40.4 and 53.7%, respectively; p < 0.001). A substantial majority (80.3%) of Plasmodium cases were subpatent (i.e., RDT- and PCR +), with no significant differences across arms (Fig. 3 , panel D).
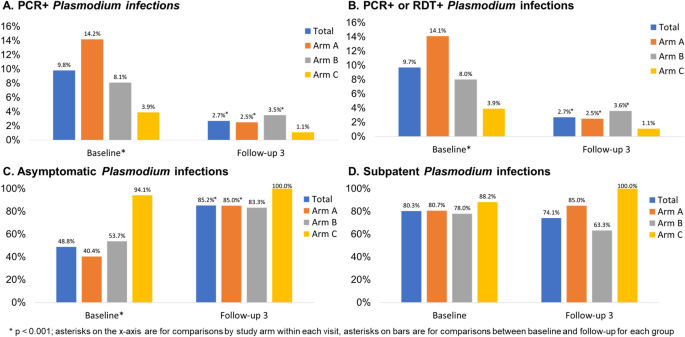
Plasmodium infection outcomes at baseline and follow-up 3 by study arm, Odisha, India. Four panels of data showing: (A) PCR + and (B) PCR + or RDT + Plasmodium infection prevalence at baseline (n = 2463) and follow-up 3 (n = 1999); (C) asymptomatic Plasmodium infection prevalence at baseline (among n = 240 total infections) and follow-up 3 (among n = 54 total infections); (D) subpatent Plasmodium infection prevalence at baseline (among n = 240 total infections) and follow-up 3 (among n = 54 total infections).
At FU3, 2.7% of the sample had PCR + Plasmodium infection, with no significant differences across arms (Fig. 3 , panel A). Prevalence of RDT + Plasmodium infection was 0.7% overall at follow-up, with 0.4% prevalence in Arm A, 1.3% prevalence in Arm B, and no observed cases in Arm C Table 1 , p = 0.017). When malaria was defined by any positive PCR or RDT test, prevalence overall was 2.7% at FU3 (Fig. 3 , panel B). When we compared malaria outcomes from baseline to follow-up for the entire sample as well as each arm, we found that there was a statistically significant reduction in both RDT + and PCR + Plasmodium infection in the sample overall and for Arm A. We observed a statistically significant reduction in PCR + but not RDT + Plasmodium infection in Arm B and no significant differences in Arm C. With respect to species, 53 (98.1%) were P. falciparum cases and 1 (1.9%) was P. vivax ; none were co-infected with P. falciparum and P. vivax at FU3. Approximately 85.2% of Plasmodium infections were asymptomatic at FU3 with no significant differences across arms but statistically significant increases in the proportion of cases that were asymptomatic from baseline to FU3 for the entire sample (Fig. 3 , panel C, p < 0.001), Arm A (p = < 0.001), and Arm B (p = 0.004) but not Arm C. A substantial majority (74.1%) of Plasmodium cases were subpatent at FU3, with no significant change from baseline to FU3 (Fig. 3 , panel D).
The overall follow-up rate for FU3 was 81.2% with a range of 80.6 to 83.4% across arms, with no significant differences across arms (Table 1 ). The sociodemographic and malaria outcome correlates of participants lost-to-follow-up (LTFU) is shown in Supplemental Table 1. There were no significant differences in LTFU by study arm or sex. Younger people were more likely to be LTFU as were those with lower educational attainment, students, and children not in school. Those with malaria, regardless of detection method, were statistically significantly more likely to be LTFU but there were no significant differences by asymptomatic or subpatent cases.
Table 2 presents the crude and adjusted multilevel mixed effects logistic regression models for the primary and secondary malaria outcomes. The adjusted models controlled for baseline Plasmodium infection, age, and gender given baseline differences in Plasmodium infection prevalence and demographics across study arms. Education and occupation were not included in the model, although they were statistically significant in the bivariate analyses, due to multicollinearity. The time (i.e., baseline vs. FU3) x study arm interaction term shows that there were statistically significant lower odds of PCR + Plasmodium infection in Arm A (AOR = 0.36, 95% CI = 0.17, 0.74) but not Arm C as compared to Arm B at follow-up. The adjusted model for PCR + or RDT + Plasmodium infection showed similar results. For RDT + Plasmodium infection, odds ratios could not be calculated due to small numbers of RDT + Plasmodium infections at FU3. Village-specific Plasmodium infection detected by PCR at baseline and FU3 are provided in Supplemental Table 2.
Intent-to-treat analysis for secondary outcomes
The anthropometric and clinical outcomes at baseline and the third follow-up visit are shown in Table 3 ; associations are considered statistically significant if p < 0.013. At baseline, 2.4% were severely underweight, with no differences across study arms. At follow-up, the prevalence of severe underweight decreased to 1.5%; there were still no differences across arms in underweight at follow-up. Few (2.2%) were severely anemic, with significantly more anemia in Arm A (5.1%) as compared to Arms B and C (0.1 and 0.7%, respectively; p < 0.001). At follow-up there were no significant differences in anemia across arms, but there were statistically significant decreases from baseline to follow-up for the total sample and Arm A.
At baseline, prevalence of fever was 24.2%. Arm B had the highest prevalence (38.0%) as compared to Arm A (14.6%) and Arm C (13.9%, p < 0.001). There were no significant differences in fever across arms at follow-up, although there were statistically significant reductions from baseline to follow-up overall and for each arm (p < 0.001). Prevalence of malnutrition measured by mid-upper arm circumference (MUAC) was 19.3% at baseline, with higher prevalence in Arm C (26.4%) as compared to Arms A (18.2%) and B (17.2%, p < 0.001). At follow-up there were statistically significant differences in malnutrition across arms but no significant differences from baseline to follow-up. Crude and adjusted multilevel mixed effects logistic regression models were not estimated for these secondary outcomes due to small numbers (i.e., severe underweight, severe anemia, fever) and/or few significant differences in the bivariate analyses comparing baseline to follow-up (i.e., severe anemia and MUAC malnutrition).
Malaria exposure and serology in a subset of study participants
We assayed 285 baseline samples (238 PCR + and 47 PCR-) for anti- Plasmodium antibodies to 13 P. falciparum -specific antigens and four P. vivax -specific antigens using the Luminex MAGPIX platform and xPONENT software to estimate mean fluorescence intensity (MFI). Data were missing for six markers (n = 174 missing for PvMSP10; n = 20 missing for PfEBA140 R3-5, PfETRAMP5_ag1, PfMSP2CH150, PfRh2_2030, and PvMSP8). There were no significant differences by gender for P. falciparum and P. vivax antibodies, but the proportion positive increased as age increased (data not shown).
Table 4 summarizes the antibody results by study arm and infection status. There were no significant differences between Arms A and B in terms of P. falciparum antibodies at baseline; a higher proportion of Arm B participants had P. vivax antibodies at baseline compared to Arm A participants. There were statistically significant differences in antibodies across all three arms, with a lower proportion of Arm C participants having Plasmodium antibodies. With respect to baseline infection status, a higher proportion of symptomatic participants had P. falciparum antibodies and P. vivax antibodies as compared to uninfected and asymptomatic participants.
Village-level costs of MCs
We conducted an analysis of the implementation costs of MCs in the study villages from the service provider perspective. The results of the cost analysis are presented in Table 5 . In the study villages, the cost per person participated ranged between US$3–8, the cost per tested between US$4–9, and the cost per treated between US$82–1614 per camp round.
In this quasi-experimental study conducted in rural villages in Odisha, intent-to-treat analyses suggest that the DAMaN program’s malaria camps were associated with lower malaria incidence relative to standard-of-care. This is suggested by the reductions in Plasmodium infection prevalence at baseline versus FU3. We note that all villages received MCs at some point, with Arm A receiving MCs four times, Arm B receiving it twice, and Arm C receiving it prior to the study’s inception and three times during the study. Intervention activities were impacted by the COVID-19 pandemic-related shutdowns and, as a result, 9 villages (2 of 6 in Arm A, 6 of 6 in Arm B, and 2 of 3 in Arm C) did not receive any malaria camp activities during the first follow-up. There were no serious adverse events.
Our findings are in contrast to a recent meta-analysis that noted a marginal and non-significant pooled effect size for MSAT interventions on Plasmodium infection prevalence and incidence 11 . One major limitation of previous evaluations of MSAT is low sensitivity of the diagnostic methods (i.e., RDTs and light microscopy) 11 . The Odisha DAMaN MCs use RDTs for MSAT while this evaluation used both an RDT and PCR, overcoming some previous evaluations’ limitations.
There were no significant differences in the subset of Arm A and B participants tested for Plasmodium antibodies, suggesting that Arms A and B had similar malaria exposure prior to the intervention. There were statistically significant differences in Plasmodium antibodies across all 3 arms, with a lower proportion of Arm C participants having antibodies; this suggests lower transmission of Plasmodium in these villages and/or impact of the previous exposure to the MC intervention.
At baseline, more symptomatic (i.e., febrile) participants had P. falciparum and P. vivax antibodies as compared to uninfected and asymptomatic participants. These results suggest possible cross-reactivity of P. falciparum and P. vivax antibodies and antigens in the assay as well as potential previous exposure to P. vivax . The lower proportions of participants with P. vivax antibodies relative to P. falciparum antibodies is consistent with RDT and PCR data demonstrating lower prevalence of P. vivax in this study sample, as well as data from the National Center for Vector Borne Diseases Control showing that 91.4% of cases in 2021 were P. falciparum infections 3 .
The Malaria Control Program implemented MSAT across all malaria camp rounds rather than FSAT in the subsequent rounds. This likely contributed to the impact of the intervention, as 49 of the 69 RDT + cases (71.0%) detected in the study were afebrile [data not shown]. In other words, they treated more Plasmodium infections than they otherwise would have if they had only tested and treated febrile cases each time.
The cost per person for the MCs ranged between US$3–8, the cost per tested person between US$4–9, and the cost per treated person between US$82–1,614 per MC round. According to our systematic literature review on the effectiveness and cost-effectiveness of intermittent mass MSAT interventions for malaria 11 , these results are comparable to the costs reported in the published literature, ranging between US$3.5–14.3 per person tested per round, and corresponds roughly to 20–35% of the per-capita domestic general health expenditure in India (US$19.6 in 2018) 12 .
We note several important study limitations. First, as discussed in our study protocol 7 , the villages were not randomized to study arms, which may result in potential selection bias. Second, COVID-19 pandemic shutdowns resulted in disruption in delivery of the MCs at FU1 which meant that several villages may not have received all or any of the activities in that MC round. Third, while the follow-up was over 80%, people with Plasmodium infection at baseline were less likely to be followed-up but there were no significant differences in follow-up among asymptomatic or subpatent cases.
Collectively, our quasi-experimental cluster-assigned stepped-wedge study results suggest that the DAMaN malaria camp intervention is associated with reductions in malaria in rural villages and thus is a promising, financially feasible approach for malaria control in rural settings. However, given the quasi-experimental design and COVID-related study challenges, we cannot infer causality. This is timely considering that Odisha State government extended the DAMaN initiative in October 2022 for five more years in 21 districts in a bid to achieve malaria elimination in Odisha by 2030 13 .
Ethics statement
Ethical approval for the trial was obtained from the Odisha State Research and Ethics Committee (Odisha, India, dated 24 June 2019), institutional ethics committee at Community Welfare Society Hospital (Rourkela, Odisha, India) and the institutional review board at New York University (New York, NY, USA). All research was performed in accordance with relevant guidelines/regulations and the Declaration of Helsinki.
Study design and enrollment procedures
The quasi-experimental cluster-assigned stepped-wedge study design, power analysis, and recruitment/enrollment procedures are described in a protocol paper 7 and the study was registered at ClinicalTrials.gov (NCT03963869; first posted 28 May 2019). Briefly, 12 intervention villages were selected in two districts of Odisha state, Keonjhar and Jharsuguda, and distributed in equal numbers between two study arms: Arm A ‘new-MC’ (communities receiving MCs for the first time in year one) and Arm B ‘delayed-MC’ (communities undergoing routine malaria control in year one and receiving MCs for the first time in year two); control Arm C contained three ‘old-MC’ villages, where MCs had already been implemented prior to the study period. A cohort was planned with target enrollment of 2,700 individuals across all 15 villages (i.e., 1100 in Arms A and B; 500 in Arm C).
Study participants in each village were recruited and enrolled at baseline before the initial administration of the intervention by the Government of Odisha DAMaN team and subsequently surveyed prior to each round of their screen and treat program. RDT diagnosis and treatment of the study participants followed the Government of Odisha Department of Health and Family Welfare Standard Treatment Guidelines ( https://health.odisha.gov.in/sites/default/files/2020-02/STG-2018.pdf ). Study participants in the three villages not receiving the intervention were recruited and enrolled in parallel temporally. Residents of the study villages aged 1–69 were eligible for the study. Written informed consent was obtained in the local language from all adult study participants aged 18–69 years with apparent full comprehension of the study procedures. Assent was obtained in addition to parental or legal guardian informed consent for participants aged 7–17 years. Parental informed consent was obtained for children aged 1–6 years. During each survey round, all subjects completed a health questionnaire that captured demographic, malaria knowledge and prevention, and Plasmodium infection and treatment history data and provided a blood sample for Plasmodium parasite detection.
Intervention adaptations due to COVID-19 pandemic
Arm A (new MC) and C (old MC) villages were to receive two cycles of MCs, each with two rounds of MSAT. The first MC cycle was from August to November 2019 (Round 1) and January to March 2020 (Round 2). We note that the Round 2 MSAT was interrupted by COVID-19 pandemic shutdowns. The second MC cycle was from June to September 2020 (Round 1) and October to December 2020 (Round 2). We note that Round 1 MSAT was also interrupted by COVID-19 pandemic shutdowns. Arm B (delayed MC) villages were to receive SOC in the first cycle (August 2019–March 2020) and one MC cycle with two rounds of MSAT from June to September 2020 (Round 1) and October to December 2020 (Round 2). Again, Round 1 was interrupted by the pandemic; see Supplemental Fig. 1 for details.
Primary outcomes
The primary outcome measures were any Plasmodium as detected by PCR and Plasmodium species (i.e., P. falciparum and/or Plasmodium vivax ) as detected by PCR. Secondary malaria outcome measures included Plasmodium infection as detected by RDT and Plasmodium -specific serology. Plasmodium infection by RDT is defined as RDT negative (RDT−) or RDT positive (RDT+). We further classified infections as ‘asymptomatic’, defined as PCR+ and/or RDT+ for any Plasmodium species with absence of documented fever or self-reported fever in the last 48 h, and/or ‘subpatent’, defined as RDT− and PCR+. Plasmodium -specific serology was analyzed as a continuous antibody titer variable as well as a categorical (seropositive vs. seronegative) variable.
Blood sample collection and processing
Blood samples for blood smears, blood spots (Whatman FTA cards), a small volume microvette, and micro volumes required for point of care hemoglobin and RDTs (FalciVax), were collected from consenting study participants by sterile lancet finger prick as previously described 7 . Vacutainers (5 ml; BD Vacutainer glass blood collection tubes with acid citrate dextrose) of venous blood were requested from study participants found to be positive by RDT. Microvette and vacutainer samples were refrigerated until separated into plasma and infected red blood cell (iRBC) components by centrifugation. Plasma was stored at −80 °C until assayed for Plasmodium immunoglobulin G (IgG), and total DNA was extracted from the iRBC pellet using QIamp DNA mini kit (QIAGEN) with a final elution volume of 50 μl.
Molecular detection of Plasmodium parasites by species-specific PCR
DNA samples were tested for Plasmodium species by a PCR assay targeting multi-copy loci Pfr364 and Pvr47 14 as previously described 7 . Five μl of DNA was used in a total reaction volume of 30 μl, and amplification products were visually confirmed by ethidium bromide-stained gel electrophoresis using a Gel Doc EZ documentation system (BioRad Laboratories, Inc.).
Detection of anti-Plasmodium IgG by Luminex MAGPIX cytometric bead array
Plasma isolated from a subset of microvette samples of finger prick blood was assayed for 13 anti- P. falciparum and four anti- P. vivax antibodies as previously described. 7 The panel included antigens whose corresponding antibodies have been suggested as indicators of protection from clinical disease 15 , 16 , long-term or cumulative exposure 17 , 18 , 19 , 20 , and recent infection 17 , 18 , 19 , 20 . Briefly, n = 300 plasma samples were diluted 1/200 and assayed in duplicate according to standard procedures 21 , 22 . xPONENT software was used for data acquisition (Luminex Corp., Austin, TX). Specifically, the net Mean Fluorescence Intensity (MFI; net MFI Ag = raw MFI Ag − background MFI Ag where background MFI Ag is the mean MFI of a given antigen in the blank wells) was calculated for each antigen in each sample. The presence of antibody to each antigen (e.g., seropositivity) was defined as the mean net MFI naive pool plus three standard deviations. Composite variables for any P. falciparum , any P. vivax , and any P. falciparum or P. vivax antibodies were created.
Secondary anthropometric and clinical outcomes
We report on several anthropometric measurements, including severe underweight, severe anemia, fever, and malnutrition as measured by mid-upper arm circumference (MUAC). For adults aged 18 and older, severe underweight was defined as a BMI < 16. For children, severe underweight was defined as three standard deviations below the median based on the WHO growth charts used for children < 5 years and the revised Indian Academy of Pediatrics (IAP) growth charts for Indian children and adolescents aged 5–17 23 . Severe anemia was defined as hemoglobin < 5 g/dl for persons aged < 12 and 7 g/dl among those aged 12 and older 24 . Fever was defined as a temperature of 99.5°F or higher. MUAC malnutrition was defined as < 23 cm for adults and adolescents aged 15 and older 25 , 26 , < 16 cm for 10–14 year olds 27 , < 12.9 cm for 5–9 year olds 27 , and < 11.5 cm for children 6 to 60 months 28 .
Costing study design, data collection, and analyses
Using an activity-based costing method, we conducted an analysis of the implementation costs of malaria camps (MCs) in the study villages from the service provider perspective 29 . Interviews were conducted with the malaria camp supervisors who led the MCs in the study villages, and programmatic documentation from the Odisha State malaria control program were reviewed to identify the activities related to the planning and implementation of DAMaN at the village-level. These included: (1) Training activities (training of public health supervisors and workers as well as village volunteers, including the village specific ASHAs; (2) Micro-plan development for MCs; (3) Planning and sensitization activities (identification of village volunteers, selection of camp venue and time); (4) Community sensitization activities; (5) Community mobilization activities; (6) Health activities (malaria testing and treatment, child health, maternal health); (7) Monitoring and supervision activities; and (8) Follow-up activities. Resource use was linked to specific activities associated with an intervention. During interviews, the MC supervisors identified the time dedicated by MC staff to different activities for each camp round. Time costs of MC staff were calculated based on average annual gross wage rates, apportioned according to time devoted to DAMaN activities per camp round, and summed across all MC staff. Unit costs of rapid diagnostic kits and antimalarial drugs were extracted from the DAMaN Operational Guidelines 30 . The costs of these commodities were calculated by multiplying the quantities used during a camp round by their unit costs based on participation in each camp round in each village. Personnel and implementation costs per round were summed and divided by the population that participated in the MC, the population tested for malaria, and the population treated for malaria, in each study village to calculate cost per person participated, cost per person tested, and cost per person treated per camp round. All costs were collected in local currency and converted to and presented in 2020 US dollars (US$), using the average exchange rate for 2020 (1 US$ = 74.27 Indian Rupees) 31 .
Statistical analyses
The primary outcome was Plasmodium presence and species as detected by PCR at the third follow-up visit. Secondary outcomes included Plasmodium presence and species as detected by RDT and anthropometric and clinical outcomes including underweight, anemia, fever, and malnutrition as measured by mid-upper arm circumference (MUAC) at the third follow-up visit. Missing data was minimal among the analytic sample and ranged from 0 to 1.5% depending on the variable.
Bivariate analyses compared outcomes and key covariates across study arms with Pearson’s χ 2 or Fishers exact tests (if cell sizes were < 5) at baseline and outcomes at follow-up 3. We use a Bonferroni adjusted α of 0.013 to account for multiple comparisons in the bivariate analyses comparing baseline to follow-up outcomes. Follow-up rates were calculated as the proportion of baseline participants that were followed up at the third time point.
Multilevel mixed-effects logistic regression was used to estimate the intervention effects, which enabled us to account for the repeated measure and nesting of individuals within the 15 villages. Mixed models are recommended when there are at least 10 clusters and fewer than 40 clusters 32 , 33 . The equation is:
where \({y}_{ij}^{*}\) is the expected logit of plasmodium infection, \(i\) is the individual, \(j\) is the cluster (i.e., village), \(\beta\) s are the fixed effects, \({u}_{j}\) is the random intercept for the villages, and \({\epsilon }_{ij}\) is the error term. Study arm, age, sex, education, and occupation are time-invariant covariates. Study arm is intent-to-treat, so even if a participant moved to another village, they remain assigned to the village where they were initially enrolled. Visit number in the only time-varying covariate. An interaction term (study arm x visit [baseline vs. FU3]) was used to estimate time effects for each arm and village was included as a random effect. We first estimated the crude associations for each outcome by study arm. We then adjusted these estimates for age, sex, education, and occupation. Odds ratios with 95% confidence intervals are reported. All analyses were conducted with Stata 17.0 (StataCorp, College Station, TX, USA) and were intent-to-treat. The study was registered at ClinicalTrials.gov (#NCT03963869).
Data availability
Epidemiology study data are available in the Clinical Epidemiology Database 34 ( https://clinepidb.org ) under the ‘India ICEMR DAMaN Quasi-experimental Stepped-wedge’ study title.
World malaria report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. ( Geneva, 2021).
World malaria report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. (2022).
National Vector Borne Disease Control Program, D. G. o. H. S., Ministry of Health and Family Welfare, Government of India. (2020).
Rajvanshi, H. et al. Learnings from two independent malaria elimination demonstration projects in India. Trans. R. Soc. Trop. Med. Hyg. (2021)
Pradhan, M. M. et al. Comprehensive case management of malaria: Operational research informing policy. J. Vect. Borne Dis. 56 , 56–59 (2019).
Article CAS Google Scholar
Bal, M. et al. Assessment of effectiveness of DAMaN: A malaria intervention program initiated by Government of Odisha, India. PLoS ONE 15 , e0238323 (2020).
Article CAS PubMed PubMed Central Google Scholar
Ompad, D. C. et al. The effectiveness of malaria camps as part of the Durgama Anchalare Malaria Nirakaran (DAMaN) program in Odisha, India: Study protocol for a cluster-assigned quasi-experimental study. Glob. Health Action 14 , 1886458 (2021).
Article PubMed PubMed Central Google Scholar
National Vector Borne Disease Control Programme. (2007).
Department of Health & Family Welfare (India). National Vector Borne Disease Control Programme (NVBDCP). https://health.odisha.gov.in/NVBDCP.asp . (Accessed 16 Dec 2021).
Pradhan, M. M. & Meherda, P. K. Malaria elimination drive in Odisha: Hope for halting the transmission. J. Vect. Borne Dis. 56 , 53–55 (2019).
Article Google Scholar
Kim, S., Luande, V. N., Rocklov, J., Carlton, J. M. & Tozan, Y. A Systematic Review of the Evidence on the Effectiveness and Cost-Effectiveness of Mass Screen-and-Treat Interventions for Malaria Control. Am. J. Trop. Med. Hyg. 105 , 1722–1731 (2021).
World Health Organization. Global Health Expenditure database , https://apps.who.int/nha/database (2021).
Service, E. N. in The New Indian Express (2022).
Demas, A. et al. Applied genomics: data mining reveals species-specific malaria diagnostic targets more sensitive than 18S rRNA. J. Clin. Microbiol. 49 , 2411–2418 (2011).
Osier, F. H. et al. Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol. 29 , 387–394 (2007).
Osier, F. H. et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med. 12 , 108 (2014).
Elliott, S. R. et al. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep. 6 , 100 (2014).
Achan, J. et al. Serologic markers of previous malaria exposure and functional antibodies inhibiting parasite growth are associated with parasite kinetics following a plasmodium falciparum controlled human infection. Clin Infect Dis 70 , 2544–2552 (2020).
Article CAS PubMed Google Scholar
Wu, L. et al. Sero-epidemiological evaluation of malaria transmission in The Gambia before and after mass drug administration. BMC Med. 18 , 331 (2020).
Wu, L. et al. Antibody responses to a suite of novel serological markers for malaria surveillance demonstrate strong correlation with clinical and parasitological infection across seasons and transmission settings in The Gambia. BMC Med. 18 , 304 (2020).
Wu, L. et al. Optimisation and standardisation of a multiplex immunoassay of diverse Plasmodium falciparum antigens to assess changes in malaria transmission using sero-epidemiology. Wellcome Open Res. 4 , 26 (2019).
Article PubMed Google Scholar
Helb, D. A. et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc. Natl. Acad. Sci. USA 112 , E4438-4447 (2015).
Indian Academy of Pediatrics Growth Charts, C. et al. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr 52 , 47–55 (2015)
Malaria, S. Trop Med Int Health 19 , 7–131 (2014).
Tang, A. M. et al. Determining a global mid-upper arm circumference cut-off to assess underweight in adults (men and non-pregnant women). Public Health Nutr 23 , 3104–3113 (2020).
Van Tonder, E. et al. Mid-upper arm circumference (MUAC) as a feasible tool in detecting adult malnutrition. South Afr. J. Clin. Nutrit. 32 , 93–98 (2019).
WHO. Guidelines for an Integrated Approach to the Nutritional Care of HIV-Infected Children (6 Months-14 Years) . (2009).
Roberfroid, D. et al. Utilization of mid-upper arm circumference versus weight-for-height in nutritional rehabilitation programmes: A systematic review of evidence. WHO commissioned white paper (2013). https://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren_review1.pdf .
Drummond, M. F., Sculpher, M. J., Torrance, G. W., O’Brien, B. J. & Stoddart, G. L. Methods for the Economic Evaluation of Health Care Programmes . (Oxford University Press, 2005).
Secretary, P. (ed Health &Family Welfare Department) (National Vector Borne Disease Control Programme, 2016–2020).
Fund, I. M. in Exchange Rates (2020).
Snijders, T. A. B. & Bosker, R. J. Multilevel analysis : an introduction to basic and advanced multilevel modeling . 2nd edn, (Sage, 2012).
McNeish, D. & Stapleton, L. M. Modeling clustered data with very few clusters. Multivar. Behav. Res. 51 , 495–518 (2016).
Ruhamyankaka, E. et al. ClinEpiDB: An open-access clinical epidemiology database resource encouraging online exploration of complex studies. Gates Open Res. 3 , 1661 (2019).
Download references
Acknowledgements
We thank study participants and the DAMaN teams in the study villages of Keonjhar and Jharsuguda districts for their cooperation, and Dr Deben Das, Mr. Baladev Nanda, and Mr. Birat R Padhan of the Odisha State Malaria Control Programme for their support. We thank field team members Asit Samal, Anna Bage, Pratibha Ekka, Satyanarayana Panigrahi, Sahil Ekka, John P Lakra, Sobharani Toppo, and Madhusmita Parichha and lab technicians Swagatika Dash and Satyaranjan Chhatria at Community Welfare Society Hospital for their efforts, and Dr. Kevin Tetteh, Ms. Elin Dumont, and Ms. Catriona Patterson of the London School of Hygiene and Tropical Medicine (England, UK) for providing serology assay support. We also thank Dr. James Beeson (Burnet Institute, Australia) and Dr. Simon Draper (University of Oxford, UK) for providing serology assay antigens. This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number U19AI089676 as part of the International Centers of Excellence for Malaria Research. The content does not necessarily represent the official views of the US NIH or NIAID.
Author information
These authors contributed equally: Timir K. Padhan and Anne Kessler.
These authors jointly supervised this work: Madan Mohan Pradhan, Sanjib Mohanty, Jane M. Carlton and Praveen K. Sahu.
Authors and Affiliations
School of Global Public Health, New York University, New York, NY, 10003, USA
Danielle C. Ompad, Yesim Tozan, Abbey M. Jones & Jane M. Carlton
Department of Molecular Biology and Infectious Diseases, Community Welfare Society Hospital, Rourkela, Odisha, 769042, India
Timir K. Padhan, Mohammed A. Haque, Sanjib Mohanty & Praveen K. Sahu
Center for Genomics and Systems Biology, Department of Biology, New York University, New York, NY, 10003, USA
Anne Kessler, Anna Maria van Eijk, Steven A. Sullivan & Jane M. Carlton
Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Anna Maria van Eijk
Department of Health and Family Welfare, State Vector Borne Disease Control Programme, Bhubaneswar, Odisha, 751001, India
Madan Mohan Pradhan
Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Global Public Health, Baltimore, MD, 21205, USA
Jane M. Carlton
You can also search for this author in PubMed Google Scholar
Contributions
D.O., A.K., A.V.E., Y.T., S.A.S., P.K.S., S.M., M.M.P., and J.M.C. conceptualized and developed the study design and methodology with input from all authors. P.K.S. led the field and laboratory work in Odisha, India with support from T.K.P., M.A.H., S.M., and M.M.P. A.K. undertook the serology assay. D.O. and S.A.S. managed the data. D.O., A.J., and Y.T. completed the data analyses. J.M.C. acquired the funding. D.O., A.K., Y.T., J.M.C., and P.K.S. wrote the manuscript with input from all authors.
Corresponding author
Correspondence to Danielle C. Ompad .
Ethics declarations
Competing interests.
Dr. Madan M. Pradhan is employed by the Odisha State Vector Borne Disease Control Programme, which is the organization that is implementing the intervention. He has a potentially self-serving stake in the research results via potential promotion or career advancement based on outcomes. The other authors have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ompad, D.C., Padhan, T.K., Kessler, A. et al. The effectiveness of malaria camps as part of the malaria control program in Odisha, India. Sci Rep 13 , 22998 (2023). https://doi.org/10.1038/s41598-023-46220-x
Download citation
Received : 10 January 2023
Accepted : 30 October 2023
Published : 28 December 2023
DOI : https://doi.org/10.1038/s41598-023-46220-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- International
- International aid and development
- Health in developing countries
Battling malaria in India
How a DFID supported health programme in rural India is helping to raise awareness of malaria prevention and treatment among vulnerable tribal communities

A health worker runs a malaria diagnosis test on an elderly woman. Picture: DFID
Update: This case study was updated in November 2012
Milu Jani lives in Labangi, a small village in a remote area of Western Odisha - one of the poorest states in India. The village is around 3 hours from the nearest district town of Angul and part of the journey needs to be done on foot. There is no health centre, no electricity and no school in the village. Milu works as a forest guard at the Satakosia wildlife reserve, earning a mere 90 rupees (£1.20) a day.
Like most villagers, Milu has lost a loved one to malaria. His eyes tear up as he recalls his father’s death last winter. Milu’s father fell ill with a high fever and was tragically diagnosed with malaria only a day before his death, leaving no time for proper treatment. Malaria can be treated effectively with drugs, but treatment is most effective when administered within 24 hours of the onset of fever. Odisha accounts for a quarter of India’s malaria cases amd 16% of its malaria deaths.
Milu knew mosquito bites can cause malaria but didn’t know the deadly mosquitos were breeding in the water pools around the village. After his father passed away, Milu met a local health worker who explained the risks of malaria and how to prevent his family from becoming ill with this deadly disease.
Getting healthcare to rural communities
The DFID supported state health programme in Odisha trains health workers to help raise awareness of malaria prevention and treatment among vulnerable tribal communities like Milu’s. Health workers like Suhasini Behera in Milu’s village are trained in the use of diagnostic tests and how to administer appropriate medicines. They also distribute bednets and promote the proper use of nets to prevent malaria.
“It was not easy to convince people to use bed nets,” Suhasini says. “People feared the nets were poisonous as there had been reports of rashes and itching from the insecticide” - misconceptions that she sought to dispel while visiting their homes.
By working with the community to raise awareness of malaria, Suhasini and her fellow health workers help to prevent people from contracting malaria and help the villagers to understand how to access treatment.
Bednets - a simple prevention tool
DFID’s support to the Odisha state health programme has helped Milu and his family purchase bednets that will prevent them from contracting malaria in the future. By subsidising the cost of the nets, DFID enabled poor families like Milu’s to purchase two bednets for only 20 rupees (25p). The nets are treated with insecticide and last up to five years.

Milu Jani and his family under their new bednet.
Milu’s mother is content with her new net. “I always had disturbed sleep due to the mosquitoes. This new net has brought me a lot of comfort and I also use it for my afternoon nap”.
Milu is equally satisfied. “Malaria was a huge problem in our area. Now everyone in the village is using a net. I have lost my father but now I can keep my family safe from malaria”.
The DFID supported Odisha state health programme distributed 4.3 million bed nets to villages between 2009 and 2012, integrated with education and awareness activities of health workers. The programme has trained nearly 39,000 health workers to use diagnostic tests, give medicines and distribute bed nets.
DFID support has helped provide an extra 240,000 bed nets to young expectant mothers and tribal children to prevent anaemia and malaria-related deaths between 2010 and 2012. The fact that most women sleep with their newborn under the treated bednet has helped reduce deaths in children due to Malaria. DFID’s technical support has also helped the state government establish evidence required for assessing and up-scaling malaria initiatives.
Odisha has recorded a 24% decline in malaria cases and 60% decline in reported deaths in 2011 compared to 2010. The total number of reported malaria deaths declined from 247 to 100 between 2010 and 2011.
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.
We have updated our terms and conditions and privacy policy Click "Continue" to accept and continue with ET HealthWorld
We use cookies to ensure best experience for you
We use cookies and other tracking technologies to improve your browsing experience on our site, show personalize content and targeted ads, analyze site traffic, and understand where our audience is coming from. You can also read our privacy policy , We use cookies to ensure the best experience for you on our website.
By choosing I accept, or by continuing being on the website, you consent to our use of Cookies and Terms & Conditions .
- Leaders Speak
- Brand Solutions
- Malaria linked with genetic changes associated with ageing: Study
According to the 2023 World Health Organisation (WHO) Malaria report, published in 'The Lancet Microbe' journal, around 70 per cent of the global burden of the mosquito-borne disease is concentrated in 11 countries, including India and 10 African countries.
- Updated On May 4, 2024 at 06:39 AM IST
- Published On May 4, 2024 at 06:36 AM IST
All Comments
By commenting, you agree to the Prohibited Content Policy
Find this Comment Offensive?
- Foul Language
- Inciting hatred against a certain community
- Out of Context / Spam
Join the community of 2M+ industry professionals
Subscribe to our newsletter to get latest insights & analysis., download ethealthworld app.
- Get Realtime updates
- Save your favourite articles
- Health news
- genetic changes
- telomere length
- white blood cells
- endemic regions
- DNA analysis
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.

Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene .
Burden of malaria in india: retrospective and prospective view.
Ashwani Kumar , Neena Valecha , Tanu Jain , and Aditya P. Dash .
Affiliations
In India, nine Anopheline vectors are involved in transmitting malaria in diverse geo-ecological paradigms. About 2 million confirmed malaria cases and 1,000 deaths are reported annually, although 15 million cases and 20,000 deaths are estimated by WHO South East Asia Regional Office. India contributes 77% of the total malaria in Southeast Asia. Multi-organ involvement/dysfunction is reported in both Plasmodium falciparum and P. vivax cases. Most of the malaria burden is borne by economically productive ages. The states inhabited by ethnic tribes are entrenched with stable malaria, particularly P. falciparum with growing drug resistance. The profound impact of complicated malaria in pregnancy includes anemia, abortions, low birth weight in neonates, still births, and maternal mortality. Retrospective analysis of burden of malaria showed that disability adjusted life years lost due to malaria were 1.86 million years. Cost–benefit analysis suggests that each Rupee invested by the National Malaria Control Program pays a rich dividend of 19.7 Rupees.
- Introduction
Malaria imposes great socio-economic burden on humanity, and with six other diseases (diarrhea, HIV/AIDS, tuberculosis, measles, hepatitis B, and pneumonia), accounts for 85% of global infectious disease burden. 1 , 2 Malaria afflicts ∼90 countries and territories in the tropical and subtropical regions, and almost one half of them are in Africa, South of Sahara. About 36% of the world population (i.e., 2020 million) is exposed to the risk of contracting malaria. The World Health Organization estimates 300-500 million malaria cases annually, with 90% of this burden being in Africa. In addition, the estimated annual mortality attributed to malaria ranges from 700,000 to 2.7 million globally and > 75% of them are African children and expectant mothers. Doubts have been expressed about reliability of these estimates because most of the hyper- and holoendemic countries, especially in Africa, lack credible diagnostic facilities and reporting systems. 3 , 4
In the Southeastern Asian Region of WHO, of ∼1.4 billion people living in 11 countries (land area, 8,466,600 km 2 ; i.e., 6% of global area), 1.2 billion are exposed to the risk of malaria, most of whom live in India. 5 However, Southeast Asia contributed to only 2.5 million cases to the global burden of malaria. Of this, India alone contributed 76% of the total cases. Taking into account clinical episodes, it has now been estimated with the help of epidemiologic models and geographical and demographic data that Plasmodium falciparum estimates outside Africa, especially in Southeast Asia, are 200% higher than reported by the World Health Organization (i.e., 118.94 million of global estimates of 515 million cases). 4 In addition to this, burden of P. vivax malaria in the world has been calculated at 71-80 million cases, of which Southeast Asia and Western pacific countries contributed 42 million cases. 6
Health Care and the Malaria Control Program in in-Dia
India has 29 states and 7 union territories. There are a number of well-structured National Disease Control/Elimination Programs that are implemented by the state governments following national policies. There are three tiers of government-funded health care system throughout India (primary health care system having network of primary health centers and subcenters in rural areas, urban health centers, and urban health posts or dispensaries in the towns functional under municipal councils and corporations; district hospitals for secondary care; and medical colleges and hospitals for tertiary care). Among private health care providers, there are general practitioners and quacks besides hospitals and poly-clinics that are professionally managed. Tertiary care hospitals are also operated by large public sector industrial units and a large private sector industry. An organized National Vector Borne Disease Control Program (NVBDCP) provides technical and operational guidelines to the state governments besides sharing one half the costs for the control of malaria, filariasis, Japanese encephalitis, leishmaniasis, and dengue/Dengue Hemorrhagic Fever control in India. NVBDCP is implemented through the primary health care system with the assistance of multi-purpose workers at the grass roots level. In inaccessible areas, drug distribution centers (DDCs) and fever treatment depots (FTDs) are provided at the community level. Early detection and complete treatment, selective vector control, and behavioral change communication are the key components of current malaria control strategy of the NVBDCP. World Bank assisted Intensified Malaria Control Project (IMCP) has been launched recently in India. In the 1990s, Enhanced Malaria Control Project (EMCP) in 181 selected districts of the country was operational with the assistance of the World Bank. Another project supported by Global Fund for AIDS, Tubercolosis and Malaria (GFATM) is on the anvil under NVBDCP and would be launched in the districts hitherto not covered either under EMCP or IMCP.
Malaria Incidence in India
In India, the epidemiology of malaria is complex because of geo-ecological diversity, multi-ethnicity, and wide distribution of nine anopheline vectors transmitting three Plasmodial species: P. falciparum, P. vivax, and P. malariae. Anopheles culicifacies is widely distributed and is the principal vector of rural malaria, An. stephensi is the primary urban vector, An. fluviatilis is a vector in the hills and foothills, and An. minimus , An. nivipes , An. philippinen-sis, and An. dirus are vectors in the northeast and An. sun-daicus is restricted to Andaman and Car Nicobar islands. An. annularis and An. varuna are secondary vectors with wide distribution.
In 1947, when India became independent, 75 million malaria cases in a population of 330 million were estimated. 7 During the eradication era in the late 1950s and early 1960s, a spectacular achievement was witnessed on the malaria eradication front because malaria cases significantly declined to just 100,000 in 1964. However, reversal was experienced, and malaria staged a comeback. By 1976, malaria cases had touched the 6.4 million mark. A continued rise in P. falci-parum was witnessed, and its proportion has gradually risen to nearly 50% in recent years ( Figure 1 ).
Trends of malaria incidence in India from 1960 to 2005. Nearing eradication in 1960s (< 100,000 cases) to resurgence in the mid-1970s (∼6.4 million cases) and stabilizing trend to ∼2 million cases in the 1990s. P. falciparum proportion (more...)
Distribution of Malaria in Different States of India
The annual parasite incidence (API) is a malariometric index to express malaria cases per thousand population. As per the NVBDCP incidence records, in most of India, the API was < 2, whereas 2–5 API was in scattered regions, and regions with > 5 API were scattered in the states of Rajasthan, Gujarat, Karnataka, Goa, Southern Madhya Pradesh, Chhattisgarh, Jharkhand, and Orissa and in northeastern states ( Figure 2 ).
Distribution of malaria incidence in India according to annual parasite incidence in 2004 (data source: NVBDCP). Majority of India had < 2 cases per 1,000 population, 2–5 cases in some scattered regions, and > 5 cases per 1,000 (more...)
The proportion of P. vivax and P. falciparum varies in different parts of India. Although mostly indo-gangatic plains and northern hilly states, northwestern India and southern Tamil Nadu state have < 10% P. falciparum, and the rest are P. vivax infections; in the forested areas inhabited by ethnic tribes, the situation is reversed, and the P. falciparum proportion is 30–90%, and in the remaining areas, it is between 10% and 30% ( Figure 3 ).
Plasmodium falciparum proportion distribution in In-dia. High proportion of P. falciparum up to 90% is seen in zones inhabited by ethnic tribes in forest ecosystems where stable malaria conditions occur.
In India, malaria is contributed the most by Orissa state ( Figure 4 ). Although Orissa has a population of 36.7 million (3.5%), it contributed 25% of a total of 1.5–2 million reported annual malaria cases, 39.5% of P. falciparum malaria, and 30% of deaths caused by malaria in India (Source NVBDCP, India). Similarly, in the other states inhabited by ethnic tribes mainly in the forest ecosystems, meso to hyperendemic conditions of malaria exist with the preponderance of P. falci-parum to the extent of 90% or even more.
Contribution of different states to malaria in India. Orissa, Chhattisgarh, West Bengal, Jharkhand, and Karnataka contributed the most.
Annual Blood Examination Rate
The National Vector Borne Disease Control Program prescribes that the annual blood examination rate (ABER) for malaria should be at least 10% on the presumption that 10% of the population in a year will have fever at one point in time. It is assumed that if all or most of the fever cases are examined for malaria, most of the incidence of malaria could be captured during fortnightly active surveillance. A look at the 2004 data ( Figure 5 ) shows that the average ABER was 9% in India. In 14 of 29 states, however, it ranged from 1% to 8%, and in the remaining 15 states and union territories, ABER ranged from 10% to 40%.
Annual blood examination rates (ABERs) for detection of malaria in different states of India in 2004. Ten percent is considered adequate to reflect a true picture of malaria, but there are some highly endemic states where ABER is much less than the norm. (more...)
Widespread Problem of Drug Resistance
In India, chloro-quine resistance in P. falciparum was first reported by Manjha in the Karbi Anglong District in 1973 8 and from Nowgaon in 1974 in the northeastern state of Assam. More cases were detected in the next 3-4 years in Assam, Arunachal Pradesh, Mizoram, and Nagaland. Although foci of resistance to chlo-roquine are present in the entire country, the problem is more pronounced in areas with intense P. falciparum transmission like the northeastern states and Orissa and in areas where there is intermixing of the population, such as project areas, including construction sites, in big metropolitan areas, and along international borders ( Figure 6 ). In most of the studies, only late treatment failure to chloroquine has been observed, probably because of the semi-immune nature of the population.
Areas shown in gray (triangles and patches) where chloroquine resistance in P. falciparum has been confirmed, qualifying for use of the second-line drug SP (source: Data from National Vector Borne Diseases Control Program, India).
The problem of drug resistance has also been studied using molecular markers. Molecular studies in 274 Indian Pf isolates detected K76T mutations in all patients who did not respond to chloroquine and 96% of cases who were cured with chloroquine, showing the lack of a correlation between the K76T mutation and clinical cure. 9 However, in this study, a significant association of the K76T mutation was observed with the in vitro response to chloroquine in P. falciparum . Alleles of the PfMDR 1 gene showed a strong association but incomplete correlation with chloroquine resistance. 10
Although the available data on sulfadoxine-pyrimethamine (SP) resistance is limited, it seems that efficacy for this drug is within acceptable limits, except in limited areas such as the Indo-Myanmar border in Arunachal Pradesh and some parts of Assam and West Bengal. 11 , 12 In one study, of 40 clinical isolates, 87.5% had dihydrofolate reductase and 15% had di-hydropteroate synthase mutations. 13 Parasites carrying double or single mutants also showed increased minimum inhibitory concentration values for both pyrimethamine and sulfadoxine.
Only limited reports of chloroquine resistance in P. vivax malaria are available from India. Two cases from Mumbai did not respond to a full dose of chloroquine (1,500 mg) and peripheral smear continued to be positive despite adequate blood concentration of drug. 14 Similarly, there is another case report from Mathura (U.P.) of nonresponse to standard dose of chloroquine as confirmed by repeated blood examination. 15 Recently 16% RI and 6.7%, RII resistance in P. vivax was reported in a study conducted in 75 patients in Bihar. 16 In addition, multi-drug resistance has also been reported. 17 Contrary to these reports, in a study in West Bengal and Orissa during 1998–2001, 100% cure rates by Day 7 in 480 P. vivax malaria patients were observed. 18 Incidentally, these areas, where P. vivax is still sensitive to chloroquine, have high drug pressure and chloroquine resistance in P. falciparum. Similar findings were confirmed in a therapeutic efficacy study with chloroquine in vivax malaria in Gautam Budh Nagar (Uttar Pradesh) in the north, Navi Mumbai (Maharashtra) in the west, and Chennai (Tamil Nadu) in south India in 287 patients in 2002. The curative efficacy of chloroquine was 100% in these patients with vivax malaria. Rapid parasite and fever clearance was observed in all cases, and the drug was well tolerated. 19 From the data available thus far, it is evident that the problem of drug resistance in P. vivax is not of major concern; however, one needs to be vigilant because P. vivax produces a relapsing type of infection and is a predominant species in India.
Based on the results of 28-day in vivo studies until 2001 and therapeutic studies from 2002 onward conducted by the NVB-DCP and research institutes including the National Institute of Malaria Research, the drug policy has been revised in 241 primary health centers (PHCs) of 71 districts in 20 states of India ( Figure 6 ).
The salient features of the national drug policy that has been modified and approved in January 2007 by the NVB-DCP are as follows:
- All efforts should be made to confirm the diagnosis of malaria. If it is not possible, chloroquine in full therapeutic dose of 25 mg/kg body weight over 3 days should be given to all cases of clinical malaria including confirmed cases at all levels, irrespective of high- or low-risk malaria status of district/block. In high-risk areas, in addition to chloro-quine, primaquine (single dose) should be given. Practice of presumptive treatment with 600 mg chloroquine will be discontinued.
- Change of drug from chloroquine (first line of treatment) to artesunate plus sulfadoxine-pyrimethamine (ACT) combination therapy (second line of treatment) will be done at the treatment failure of > 10%. The change will be done in PHCs with drug resistance and clusters of blocks around that PHC.
- Mefloquine is available in the country and is only to be provided to patients with the prescription of medical practitioners supported by a laboratory report showing asexual stages of P. falciparum in the peripheral smear.
- Artemisinin derivatives may only be used in injectable form for the treatment of severe and complicated P. falci-parum malaria in adults. Use of oral forms of these derivatives (for monotherapy) is not recommended.
- Chemoprophylaxis is recommended for 1) pregnant women in high-risk areas and 2) travelers, including service personnel, who temporarily go on duty to high malarious areas. In chloroquine-sensitive areas, chloroquine is given weekly, but in chloroquine-resistant areas, chloroquine should be supplemented by daily proguanil.
- For treatment of vivax malaria, treatment with 1500 mg chloroquine over 3 days and Primaquine 0.25 mg/kg for 14 days (under medical supervision) is recommended.
Malaria Prevalence According to Age and Sex in India
Most of the point prevalence studies in India have been carried out for outbreak/epidemic investigations. There is very limited information on age- and sex-specific seasonal prevalence of malaria in different paradigms in the country. In the available studies, age and sex classification used is arbitrary. 20–26 The burden is generally higher in men than women in all age groups. Children in the states of Assam, 20–22 Arunachal Pradesh, 23 and Rajasthan 24 had a higher incidence of malaria than adults, whereas in the indo-gangatic plains, the situation was reversed. 25 , 26
Incidence Gap
In 1990, it was estimated that, of a population of 843.7 million in India, 75, 240, and 500 million people were, respectively, at high, moderate, and low risk of contracting malaria. The situation has not changed much since then except for the population growth in each risk category. 27 It is now well accepted that the reported incidence of malaria at the national level on the basis of surveillance carried out in the primary health care system at best reflects a trend and not the true burden of malaria. In the 1990s, the reported malaria incidence in India was ∼1.5–2.6 million cases and 666–1000 deaths/yr whereas the estimated incidence by WHO was 15 million malaria cases with 19,500–20,000 deaths/yr (WHO SEARO website) ( Figure 7 ).
World Health Organization estimates that India has 15 million cases of malaria with 19,500–20,000 deaths annually vs. ∼2 million cases and 1,000 deaths reported (WHO SEARO website).
A comparison of malaria incidence reported during routine surveillance under the primary health care system with that of parallel longitudinal studies carried out in some states of In-dia showed that a huge gap of 68% to 98% existed between the reported and true incidence of malaria ( Table 1 ). 28–32 Reasons attributed to this gap are inadequacies in surveillance and examination and underreporting of malaria cases. Underreporting of malaria because of misdiagnosis has been observed in Gujarat, where re-examination of blood smears in nine PHCs revealed that 6.7% of them had been misdiag-nosed. As a result, 1,262 malaria cases went undetected and unreported. Consequently, the API of malaria should have been 9.0 instead of the 5.9 reported. 33 How reliable was the clinical diagnosis alone for the treatment of malaria was shown in a hospital-based study. Although there were 24% malaria cases on the basis of clinical judgment alone, the cases were actually 52% when microscopic diagnosis was done, showing a gap of 28%. 34 In a recent study conducted in Ahmedabad in Gujarat state, it was estimated that there were on average 25,465 malaria cases/yr versus 4,119 cases reported and at least 22 malaria deaths/million population versus 0.3/million reported. 35 Thus, there existed glaring gaps between the reported and true burden of malaria in India.
Malaria incidence gap between routine surveillance and parallel longitudinal surveys/point prevalence studies carried out in Uttar Pradesh (now Uttaranchal), Haryana and Orissa state of India
Burden of Complicated Malaria
In India, reports suggested that mortality in complicated P. falciparum malaria in Vellore in the southern state of Tamil Nadu was 7.9%, whereas in Jabalpur (Madhya Pradesh) and Rourkela (Orissa), it was 25.6% and 30%, respectively. 36 , 37 In Jabalpur Medical College, 1,783 patients were admitted with complicated P. falci-parum infection, of which 152 (8.5%) had cerebral malaria. 37 Of these, 39 (25.6%) died, and most of them were in the 16-to 40-year age group. Mortality was significantly higher in patients with hyperparasitemia and hypoglycemia. Delayed diagnosis and comatose condition were the main determinants of death. In a tertiary care industrial hospital at Rour-kela, a comparative analysis revealed that the total number of patients admitted with complicated malaria significantly increased from 14.4% (62/431) in 1995–1997 to 23.7% (236/996) in 2000–2002. Similarly, cases of acute renal failures doubled from 22.5% (83/369) to 44.2% (117/265), and deaths in patients without renal involvement also increased from 12.8% (47/369) to 16.3% (119/731) (Ispat General Hospital, Rour-kela, unpublished data). A general shift in the clinical profile in patients with complicated malaria has been observed, and multiple organ dysfunction/failure is becoming a common feature. For example, in a tertiary care hospital in Cuttack, only 10.9% (96/879) of cases admitted were without complications, whereas 382 (43.5%) had cerebral, renal, or hepatic involvement, 298 (33.9%) had cerebral malaria with either renal or hepatic involvement, 103 (11.7%) had multi-organ failure, and 138 of 783 (17.6%) died from malaria (BK Das, personal communication).
Complications caused by the hitherto considered benign species P. vivax have been reported from Bikaner, India 38 as from elsewhere in recent years. 39–41 It was observed that 72 of the 440 patients with microscopically and polymerase chain reaction–confirmed monoinfection of P. vivax had severe manifestations, which included jaundice (33; 45.8%), severe anemia (11; 15.3%), respiratory distress with acidosis (8; 11.1%), acute renal failure (7; 9.7%), cerebral dysfunction with multiple convulsions (6; 8.6%), abnormal bleeding (6; 8.3%), shock (hypotension; 5; 6.9%), pulmonary edema (3; 4.2%), and hemoglobinuria (3; 4.2%). 38 Many combinations of severe manifestations were observed in 35 of the 72 P. vivax cases followed. In 12 pregnant women with P. vivax infection, there were 2 abortions, 2 still births, and 4 preterm deliveries.
Burden of Malaria in Pregnancy in India
It is well known that pregnant women constitute an important risk group for malaria infection, particularly in hyper- and holoendemic situations. The well-known effects include effectiveness of placental barrier, parasite sequestration in placenta, suboptimal nutrition of the fetus, congenital malaria, intrauterine growth retardation, low birth weight, premature interruption of pregnancy, infant mortality, and maternal death. 42–44 It may be the cause of cerebral malaria and severe anemia. In low transmission areas, maternal mortality is ∼1%, whereas in Africa it could be between 84 and 2,000 per 100,000 live births (0.00084–2%). 45
In southeast Asia, malaria is a serious burden in pregnancy, with a spectrum of ill effects as shown by Slide Positive Rate (1.1–58%; N = 45–365), parasitemia (1–70%; N = 55,365), cerebral malaria (7–76%; N = 45–365), anemia (8.6–90%; N = 45–365), maternal mortality (7–66.6%; N = 45–365), placental malaria (18–29%; N = 256,365), abortions (2–11%; N = 45–365), intrauterine fetal development impairment (2–31%; N = 45–322), stillbirth (2–13%, N = 45–365), preterm (4.2–60%; N = 45–322), and low birth weight (5.4–89%; N = 55–365). 46
In northwestern India, in a hospital-based study in Bi-kaner, 43 it was found that the mortality rate in 45 pregnant women with P. falciparum infection was highly significant (37.8%) compared with non-pregnant women with P. falci-parum infection (14.81%) at P < 0.001. Similarly, the incidence of cerebral malaria (75.55%), severe anemia (< 5 g%; 20%), hepatic failure (13.3%), and renal failure (20%) was significantly higher in pregnant women than non-pregnant women at 32.92%, 4.11%, 9.05%, and 6.17%, respectively.
From central India, it has been reported that pregnant women ( N = 365) suffer significantly more from both P. vivax ( N = 121) and P. falciparum ( N = 244) malaria than non-pregnant women ( N = 150). 47 The weight of neonates born to infected mothers was 300–350 g less on average than neonates born to non-infected mothers ( N = 1762). The weights continued to be significantly lower until 6 months, affecting the growth of babies in infancy. It was found that rates of malaria infection reduced from the first to third pregnancy. The mean parasitemia in pregnant women suffering from P. vivax ( P < 0.05) or P. falciparum ( P < 0.0001) malaria was much higher than non-pregnant malaria-infected women. Similarly, women with P. falciparum infection were significantly more anemic than the non-infected pregnant women ( P < 0.0001) or infected non-pregnant women ( P < 0.001). The pregnant women with P. falciparum malaria were significantly more anemic than those suffering from P. vivax infection. Of the 244 pregnant women who had P. falciparum infection, 3(1.22%) died, whereas in another 3, abortions were recorded, and 2 others had still births. Only one still birth and abortion each were recorded in P. vivax –infected women who were primigravidae. Among non-infected women, however, one abortion (in a primagravida) and one still birth (in a multigravida) were recorded.
Mortality Attributable to Malaria and Gaps
In India, malaria is one of the most important causes of direct or indirect infant, child, and adult mortality. In pre-independent India, the death toll caused by malaria was estimated at 1 million during normal years and 2 million during epidemic years. 7 Malaria mortality steeply declined after the National Malaria Eradication Program was launched in 1958. The National Program reported 879, 666, 1,057, 946, and 938 deaths caused by complicated P. falciparum malaria from 1997 to 2001, showing a specific malaria mortality ratio (SMMR) of 0.30–0.48 in these years, which was one of the lowest in the world. However, as per the WHO SEARO, 19,500–20,000 deaths occurred annually in India. Other than these sources, there are scanty reports on deaths caused by malaria that are primarily based on outbreak or epidemic studies. 25 , 48–54 Age-, sex-, and cause-specific deaths are most extensively covered in the Government of India report on the basis of Medical Certification of Cause of Death (MCCD). 55 The most recent available report is for 1998, during which there were 4,481 certified malarial deaths reported from various categories of hospitals in rural and urban areas of India. Significantly, in this report, only 14.9% of the total registered deaths were medically certified and to which a specific cause of death was attributed. A simple conversion to 100% certification would mean that the deaths caused by malaria could be 49,796, assuming that the malarial deaths were uniformly distributed in the remaining 85.1% of the sample ( Table 2 ). During the same year, only 666 deaths were reported by the National Vector Borne Disease Control Program; hence, these estimates were incomparable. It may be further noted that the MCCD-1998 report 55 contained death statistics from only 15 states and union territories of a total of 29 states and 7 union territories. Certified death data from many malaria endemic states such as Uttar Pradesh, Bihar, Assam, West Bengal and Tamil Nadu were not available. Had there been reporting of deaths from these states, the malarial deaths would have been much more than the estimated 49,796. Hence, available data on deaths are incomplete, and there seems to be a large gap between reported and actual deaths caused by malaria in India.
Estimates of deaths caused by malaria in 15 states and Union Territories (UT) in India based on report on medically certified deaths in 1998
Age and Sex Distribution of Malaria Mortality
Age-sex distribution of malaria deaths shows that, in general, malaria mortality across all ages was comparatively higher in males than in females ( Figure 8 ). This mortality gap in sex widens after the age of 25 years. 55 Overall, the number of deaths in males was 2,827 (63.1%) compared with 1,654 (36.9%) in females, with a male: female ratio of 1:0.56. Unlike in Africa, where most of the deaths are reported in infants and children, it is seen that in India, malarial deaths increased up to the age of 44 years in both sexes and declined thereafter. Although the deaths in infants and children < 14 years of age accounted for 20.6%, in older ages (15–54 years), they accounted for 56.1%, and the rest 23.3%, were in those > 55 years of age. Hence, most of the burden of malarial mortality was borne by the economically productive ages.
Age and sex distribution of malaria mortality in India in 1998. The deaths are more in men than women across all ages, whereas middle productive ages in general have much higher mortality than children. (N.S. = age not specified).
Burden of Malaria in Terms of Disability-Adjusted Life Years Lost in India: A Preliminary Estimate
In 1993, the Harvard School of Public Health in collaboration with World Bank and WHO assessed the Global Burden of Disease (GBD). 2 The GBD study introduced a new metric—the (DALY)—to quantify the burden of disease. One DALY means 1 lost year of healthy life on account of disease and is a common currency for disease morbidity and mortality expressed in time. This concept has gained importance in the past decade. WHO undertook the GBD study of 135 major causes for 2002 and estimated DALYs for each cause in different regions and countries. 56
We computed DALYs lost because of malaria in India for 1997. Deaths caused by malaria were estimated at 71,396 based on MCCD-1997 report. 57 Deaths were proportionately distributed according to age and sex based on the MCCD data. From a population census of the India-1991 report, 58 the mid-year population was calculated and assigned to different ages of both sexes. The incidence of malaria was taken as per the WHO estimates of 15 million. Disability weights estimated in the GBD 2000 study for episodes (0.172–0.211), anemia (0.012–0.013), and neurologic sequelae (0.581) were used. Duration of an episode of malaria was taken as 7 days. DALYs were estimated using a GBD template with age weighting and discounting. The total DALYs lost because of malaria were 1.86 million years. Among females, DALYs lost were 0.786 million versus 1.074 million in males ( Figure 9 ). The maximum DALYs lost (53.25%) were in the middle productive ages from 15 to 44 years of age, followed by children < 14 years of age (27.68%), and 19% in those > 45 years of age ( Figure 10 ).
Years of life lost (YLL), years lost because of disability (YLD), and disability-adjusted life years lost (DALYs) caused by malaria in both sexes in 1997 in India.
DALYs lost according to age and sex in India in 1997.
Socio-Economic Burden of Malaria in India
Malaria obviously has a devastating socio-economic impact on affected countries, the majority of which are developing, poor, and located in the tropics. Such is the effect that the cost of control measures is worthwhile, considering the disability, mortality, economic loss and industrial inefficiency the afflicted population and the country faces. 59 A treatise written by Sinton 7 on “what malaria costs India” stated that the problem of the very existence in many parts of India was in fact the problem of malaria in the 1930s. In those days, it constituted one of the most important causes of economic misfortune, engendering poverty, lowering the physical and intellectual standards of the nation, and hampering prosperity and economic progress in every way. This was not only true for India but for all the malarious countries in the world.
Efforts were made to calculate the economic burden of malaria as early as 1933 using evidence that there were 100 million cases of malaria annually, and of these, one third were in adults (33 million). 60 The adults of productive age earned on an average of 7.5 Rupees per month at that time. Furthermore, it was worked out that actual working days lost per man per year were ∼15 days because of the disability during primary attacks, relapses, and re-infections. However, based on the financial loss in the community in terms of lost wages, Sinton 7 calculated a total loss of Rupees 1237 lacs (£10 million at the then prevailing exchange rates, and ∼US$27.49 million). Because of sublevel performance as a result of 10–25% loss of efficiency, there would be an additional loss of 29.7 crores (£22 million equivalent to US$62 million at current prices) when computed at a 10% loss. Together, the loss would be Rupess 42 crores at 1935 currency conversion level (£32 million equivalent to ∼US$93.3 million at current prices). This loss does not include cost of medical attendance, loss to agriculture, mining, industry, and other fields. Had estimates for these losses also been computed, the figure would have been much higher.
Similarly, Kondrachine and Trigg estimated that the annual direct and indirect cost of malaria in Africa was US$800 million in 1987 and US$1,800 million in 1995. 61 They opined that, outside Africa, the economic impact is equally high. In South America, the days of work lost because of malaria have been shown to vary from 1.5 to 14.3, with an average loss of income estimated to be 13.2% of the minimum salary, ranging between 7% and 75.8%. In Asia, studies in Nepal have shown that P. falciparum and P. vivax are responsible for 10 and 5 days total disability and 2.5 and 1 days of partial disability, respectively. Monitory loss because of malaria per case was between US$3.7 and US$35.8. For the control of disease alone, the WHO Action Plan for malaria control (1995–2000) estimated that ∼US$28 million/yr of external investment in malaria control was needed in Africa. Outside Africa, malaria control programs cost an estimated US$175–350 million a year.
The prevalence of malaria and economic loss was studied in the iron ore mines in the Sundargarh district of Orissa. 62 It was calculated that Rupees 11,04,841 (US$24,282 at current prices) were lost in three mines because of malaria in 1988. Individually average annual loss or expenditure per patient per episode was Rupees 178.38 ($3.9) in case of laborers, Rupees 77.93 (US$1.71) for regular mine employees, and Rupees 228.51 (US$5.02) for businessmen. In other words, poor casual labor was the worst hit by malaria in these mines. In a more recent study carried out in Gujarat state, 63 it was estimated that, per malaria episode, monitory loss in the urban area was Rupees 393.59 ($8.65) compared with Rupees 157.59 ($3.46) in rural areas, which was less than one half spent in the urban area.
In 1994, Shiv Lal and others estimated that, if there were no control activities and malaria was allowed to transmit from the 1947 level, there would have been an expenditure of Rupees 76,600 million (US$1,670 million) for medication, medical advise, hospitalization, and absenteeism. Even if the estimates of the Malaria Research Center, Delhi (now NIMR; 25 million cases/yr) 64 were taken into account for calculation of economic loss, the cost would have been Rupees 68,600 million (US$1,508 million) versus expenditures of Rupees 3,467.9 million (US$76.2 million) for control. Thus, the net savings due to malaria control was estimated at Rupees 65,132.1 million (US$1,431 million). These authors inferred that every Rupee invested in malaria control has produced a direct return of Rupees 19.70. The estimated man-days saved were 1,328.75 million per year.
In the 1950s and early 1960s, a major global initiative of WHO to eradicate malaria also brought malaria under firm control in India and almost on the verge of eradication, but a reverse followed in the mid-1960s until the mid-1970s; the disease staged a comeback with vengeance. In the 1980s, new malaria ecotypes developed from environmental and developmental impact and were followed by outbreaks and epidemics in the 1990s. 20–22 , 24–27 There are vast lands inhabited by ethnic tribes in Madhya Pradesh, Chattisgarh, Jharkhand, Orissa, and the entire northeastern region, where malaria has remained deeply entrenched, P. falciparum preponderance is persistent, and asymptomatic burden in these areas is not known. The emergence of resistance to chloroquine in P. fal-ciparum in many pockets of the country and reports of reducing sensitivity in P. vivax are major causes of concern. 14–17 In some areas in the northeastern region, even foci of multi-drug–resistant P. falciparum have been found. Alternate therapies such as mefloquine and artemisnin derivatives or combination therapies are expensive, and since they have been selectively introduced in the control program, would require constant monitoring for their judicious use and to observe emergence of resistance against them.
The changing clinical manifestations with multi-organ involvement in P. falciparum , emerging trends of complications in P. vivax malaria, and burden of malaria in pregnancy are other important issues that merit attention and formulation of suitable intervention strategies. Historically, P. vivax has been suspected to impose a significant burden of mortality, resulting from its interaction with other diseases and conditions. 6 The malaria-specific mortality gap needs to be bridged. While the reported number of deaths is ∼1,000, the actual mortality caused by malaria is many folds higher. Even the MCCD report, which covers both rural and urban areas, extensively suffers from serious limitations because of non-reporting of mortality by some key malaria-affected states and also because of the overall incomplete medical certification of deaths and attribution of specific cause of death. 55
Health planners and administrators need estimates of the true burden of malaria for allocation of much needed resources for interventions. The current reported incidence of ∼2 million/yr in India at best reflects a trend, and given the gaps identified in various studies, the actual incidence is definitely far more than presently known. The reasons attributed to such a gap are deficiencies in coverage, collection, and examination of blood smears and reporting systems. 27 , 29–31 , 33 , 34 Moreover, in India, the government health sector, which provides free or highly subsidized health care, caters to the needs of 20% of the population, mainly in rural areas, whereas the rest of the population seeks health care in the private sector as their first point of contact, where the bulk of malaria is generally treated empirically. 65 , 66 The clinically treated cases never or rarely find place in the official statistics. This gap needs to be bridged to build burden estimates. Coupled with this, there is the likelihood of a sizable population acting as asymptomatic carriers of plasmodial infection, particularly in malarious areas inhabited by ethnic tribes in India, where meso- to hyperendemic conditions exist. In such areas, inaccessibility and insurgency seem to be major causes of deficient routine surveillance services. In many such remote places, DDCs have been opened in India, where malaria is symptomatically treated by trained community volunteers without accounting for the treated cases. Similar doubts have been expressed about the validity of estimates available for Africa because of inadequate detection and reporting and general inadequacies in the surveillance in malarious countries of Af-rica. The known and missing incidence of malaria in affected countries has been compared with the ears of hippopotamus, which are visible above water, whereas the bulk lies unseen underneath. 3 This statement may also apply to many parts of India.
The true incidence of morbidity and mortality are of paramount importance in the estimation of DALYs lost. In the absence of true burden estimates, we computed DALYs lost for India using WHO projections and mortality estimations on the basis of MCCD data. Although our DALY estimates are conservative, they are much higher at 1.86 million years lost compared with WHO estimates of 0.844 million years for 2002. 56 India, therefore, must initiate burden estimation studies based on primary incidence and prevalence data to highlight the actual malaria burden in the country.
Malaria is well known for its debilitating, demoralizing, and impoverishing consequences, and therefore, estimation of its true burden and control is central to addressing these issues, with the final aim of lifting the human resource above the poverty line. The poor may find it hard to deal with persistent malaria problem, as coping with the disease is economically disastrous for the communities living on the edge. The estimated 20-fold returns on expenditure makes a strong case for adequate investment in malaria control in India. A good investment in malaria control not only makes public health sense but also economic sense in the present era of economic liberalization in India. Firm malaria control is imperative for human resource development, which in turn is imperative for equitable and sustained economic growth.
Acknowledgments: The authors thank Prof N. K. Ganguly, Director General, Indian Council of Medical Research, for encouragement; Dr Lalit Kant, Senior Deputy Director General, for initiating Disease Burden Estimation Studies in the Institutes of Indian Council of Medical Research and arranging training of the first author; and the World Health Organization, South Eastern Regional Office, New Delhi, and the USAID for providing funds for review of literature and expert group meetings on malaria burden in India
Reprint requests: Ashwani Kumar, National Institute of Malaria Research, Field Station, Campal, Panaji, Goa, Pin 403 001, India.
Authors’ addresses: Ashwani Kumar and Tanu Jain, National Institute of Malaria Research, Field Station, Campal, Panaji, Goa, Pin 403 001, India, Telephone: 91-832-2222444, Fax: 91-832/2421406, E-mail: moc.liamg@70inawhsa . Neena Valecha and Aditya P. Dash, National Institute of Malaria Research, 22 Sham Nath Marg, Delhi 110 054, India, Telephone: 91-11-23915658, Fax: 91-11-23946150, E-mails: gro.aidnicrm@rotcerid and moc.liamg@ahcelavneen .
- Cite this Page Kumar A, Valecha N, Jain T, et al. Burden of Malaria in India: Retrospective and Prospective View. In: Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.
- PDF version of this page (795K)
In this Page
Other titles in this collection.
- The Intolerable Burden of Malaria
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Burden of Malaria in India: Retrospective and Prospective View - Defining and De... Burden of Malaria in India: Retrospective and Prospective View - Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Approximately usd 10.8 million invested in new drug development for malaria and ntds in partners including japanese pharma shionogi, mitsubishi tanabe, and eisai and europe-based institutions.
TOKYO , May 9, 2024 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total investment of approximately JPY 1.64 billion ( USD 10.8 million 1 ) in four projects for the development of new drugs for malaria and neglected tropical diseases (NTDs). 2
The GHIT Fund invests in the development of new drugs, vaccines, and diagnostics for neglected infectious diseases such as malaria, tuberculosis, and NTDs, which predominantly affect the world's vulnerable and underserved populations. These R&D projects must involve partnerships between Japanese and non-Japanese organizations, and the four new projects consist of diverse partnerships including Japanese pharmaceutical companies and universities, as well as non-Japanese research institutions and universities.
The GHIT Fund will invest approximately JPY 444 million ( USD 2.9 million 1 ) in two malaria projects: the development of malaria chemoprevention drug through a partnership among Shionogi & Co., Ltd., Nagasaki University, National Institute of Infectious Diseases, and Medicines for Malaria Venture (MMV), and therapeutic drug development led by Eisai Co., Ltd., the Scripps Research Institute , and International Centre for Genetic Engineering and Biotechnology (ICGEB).
For NTDs, the GHIT Fund will also invest approximately JPY 1.2 billion ( USD 7.9 million 1 ) in two projects: drug development for onchocerciasis and lymphatic filariasis led by Eisai Co., Ltd., University Hospital Bonn, and Helmholtz Centre for Infection Research, and the development of a treatment for Chagas disease led by Mitsubishi Tanabe Pharma Corporation and Drugs for Neglected Diseases initiative (DNDi).
The GHIT Fund plays a catalytic role in matching Japanese and global partners to accelerate R&D resulting in the support of over 180 development partners since 2013. Through global partnerships, the GHIT Fund aims to promote open innovation in R&D and create an environment that enables the rapid delivery of products to patients in need.
Please refer to Appendix 1 for a detailed description of each project and its development stage.
As of March 31, 2024 , the GHIT Fund invested in 36 projects, including 12 discovery projects, 16 preclinical projects, and 8 clinical trials 3 in the portfolio. The total amount of investments since 2013 is JPY 33.2 billion ( USD 219 million ) (Appendix 2).
1 USD1 = JPY151.33 , the approximate exchange rate on March 31, 2024 . 2 These awarded projects were selected and approved as new investments from among proposals to RFP2023-001 for the Product Development Platform, which was open for applications from November 2022 to July 2023 . 3 This number includes projects in the registration phase.
The GHIT Fund is a Japan -based international public-private partnership (PPP) fund that was formed between the Government of Japan , multiple pharmaceutical companies, the Bill & Melinda Gates Foundation, Wellcome, and the United Nations Development Programme (UNDP). The GHIT Fund invests in and manages an R&D portfolio of development partnerships aimed at addressing neglected diseases, such as malaria, tuberculosis, and neglected tropical diseases, which afflict the world's vulnerable and underserved populations. In collaboration with global partners, the GHIT Fund mobilizes Japanese industry, academia, and research institutes to create new drugs, vaccines, and diagnostics for malaria, tuberculosis, and neglected tropical disease s. https://www.ghitfund.org/en
Appendix 1. Project Details
*All amounts are listed at an exchange rate of USD1 = JPY151.33 , the approximate exchange rate on March 31, 2024 .
Appendix 2. Investment Overview (as of March 31, 2024 )
Investments to date Total investments: 33.2 billion yen ( USD 219 million 1 ) Total invested projects: 125 (36 active projects and 89 completed projects)
To learn more about GHIT Fund's investments, please visit Investment Overview: https://www.ghitfund.org/investment/overview/en Portfolio: https://www.ghitfund.org/investment/portfolio/en Advancing Portfolio: https://www.ghitfund.org/investment/advancingportfolio/en Clinical Candidates: https://www.ghitfund.org/investment/clinicalcandidates/en
For more information, contact: Nancy Moss , 254-729-991-028, [email protected] Mina Ohata at +81-36441-2032 or [email protected]
SOURCE Global Health Innovative Technology Fund (GHIT Fund)
- Personal Finance
- Today's Paper
- Partner Content
- Entertainment
- Social Viral
- Pro Kabaddi League
FLiRT 2024: New group of Covid variants spreads in US; all you need to know
Flirt, a group of new covid variants in the omicron jn.1 lineage, is spreading in the us. kp.2 and kp 1.1 are more infectious than earlier omicron variants, which, however, have similar side effects.
)
FLiRT 2024. Photo: Freepik
Listen to This Article
What is flirt and what are its symptoms, do indians need to panic about the 'flirt', world malaria day 2024: 10 signs and symptoms of malaria one should know, 21 new cases of covid-19 sub-variant jn.1 found in 3 states: niti aayog, jn.1 covid variant: states issue advisories, mandaviya holds review meet, serum institute of india to apply for licence of jn.1 covid variant vaccine, will covid sub-variant jn.1 bring mask mandates here's what experts say, genetic defects, not lack of oxygen cause cerebral palsy in children: study, number of medical tourists in india to exceed pre-pandemic levels in cy24, wockhardt hospitals launches early recovery program for liver transplant, top yoga tips for hydration and preventing dehydration in summer season, india needs to expand universal health coverage for ageing population: adb, will indians get ‘flirt’ in future.
Don't miss the most important news and views of the day. Get them on our Telegram channel
First Published: May 06 2024 | 4:39 PM IST
Explore News
- Suzlon Energy Share Price Adani Enterprises Share Price Adani Power Share Price IRFC Share Price Tata Motors Share Price Tata Steel Share Price Yes Bank Share Price Infosys Share Price SBI Share Price Tata Power Share Price HDFC Bank Share Price
- Latest News Company News Market News India News Politics News Cricket News Personal Finance Technology News World News Industry News Education News Opinion Shows Economy News Lifestyle News Health News
- Today's Paper About Us T&C Privacy Policy Cookie Policy Disclaimer Investor Communication GST registration number List Compliance Contact Us Advertise with Us Sitemap Subscribe Careers BS Apps
- Budget 2024 Lok Sabha Election 2024 IPL 2024 Pro Kabaddi League IPL Points Table 2024

IMAGES
COMMENTS
This study analysed historical malaria cases in India from 1990 to 2022 to assess the annual trends and the impact of key anti-malarial interventions on malaria incidence. ... and Holt's models were used to forecast malaria cases from 2023 to 2030. The reported annual malaria cases in India during 1990-2000 were 2.38 million, which dropped ...
The National Vector Borne Disease Control Program of India reported ~1.6 million cases and ~1100 malaria deaths in 2009. Some experts argue that this is a serious underestimation and that the actual number of malaria cases per year is likely between 9 and 50 times greater, with an approximate 13-fold underestimation of malaria-related mortality.
India is slated for malaria elimination by 2030. 4 It contributed 83% of the estimated malaria cases and 82% of malaria deaths in South-East Asia Region (SEAR) in 2020, according to the 2021 WMR. Plasmodium falciparum and P. vivax are the major prevalent parasites in India. The country contributed 51% of the global P. vivax cases in 2016, when the country launched the National Framework for ...
The WHO's World Malaria Report 2020 highlighted India's gains in the path to elimination. India recorded impressive 60% reduction in reported cases compared with 2017, and a 46% reduction in cases compared with 2018, which built momentum to reach the goal of zero indigenous malaria cases by 2027. India's National Strategic Plan for ...
Summary of World Malaria Report 2021. In 2020, 29 of the 85 countries that were malaria-endemic accounted for 96% of malaria cases. India contributed 1.7% of malaria cases and 1.2% deaths globally. Between 2019 and 2020, all high burden to high impact (HBHI) countries except India reported increases in cases and deaths (and in India, the rate ...
In India, in the last decade, malaria cases and deaths have declined significantly; ... Studies predict malaria transmission is bounded by the thermal optimum of 17 °C and 34 °C 51,52,53.
The World Malaria Report (WMR) 2020 released by WHO, which gives the estimated cases for malaria across the world, based on mathematical projections, indicates that India has made considerable progress in reducing its malaria burden. India is the only high endemic country which has reported a decline of 17.6% in 2019 as compared to 2018.
Malaria remains a major public health burden in the south-east Asia region, and approximately 80% of malaria cases of the nine countries in this region occur in India 1.As the seventh largest and ...
India is slated for malaria elimination by 2030.4 It contributed 83% of the estimated malaria cases and 82% of malaria deaths in South-East Asia Region (SEAR) in 2020, according to the 2021 WMR. Plasmodium falciparum and P. vivax are the major prevalent parasites in India. The country contributed 51% of the global P. vivax cases in 2016, when the country launched the National Framework for ...
In Odisha, the state with the greatest malaria burden in India, there were an estimated 295 000 reported cases from July through December 2016 - 56 of them fatal. During the same time period in 2017, the number of cases had fallen by nearly 50%, to approximately 156 000; fatalities fell by more than two thirds, to 16 deaths.
Purulia is a malaria-prone district in West Bengal, India, with approximately half of the blocks defined as malaria endemic. We analyzed the malaria case in each block of the Purulia district from ...
Background India has launched the malaria elimination initiative in February 2016. Studies suggest that estimates of malaria are useful to rationalize interventions and track their impact. Hence, a national study was launched to estimate burden of malaria in India in 2015. Methods For sampling, all 624 districts of India were grouped in three Annual Parasite Incidence (cases per thousand ...
In India, approximately 44% of cases have been reported to be disproportionately contributed by approximately 27 districts. 1 A comparative analysis of reported malaria cases between January 2017 and December 2022 was performed in Mandla district, which is the site of a model malaria elimination demonstration project (MEDP) in Madhya Pradesh ...
A lesson from this study is that lethal falciparum malaria can be transmitted in regions of India, believed to be non-endemic for the disease, resulting in fatal outcomes if diagnosis is missed or delayed. ... The locality of the malaria cases reported herein is a remote village called Shishila, situated in a forest area (12°54′2″N75°30 ...
The recently released World Malaria Report shows that the number of cases and deaths due to the mosquito-borne infection India have continued to decline. With an estimated 33.8 lakh cases and 5,511 deaths, India saw a decline of 30 per cent in malaria incidence and 34 per cent in mortality in 2022, compared to the previous year.
21. Malaria morbidity decreased by 43% in districts targeted by the Enhanced Malaria Control Project and nationwide by 38% with almost 1 million fewer cases diagnosed in 2004 than in 1997. Three states, Gujarat, Andra Pradesh, and Maharashtra reduced malaria morbidity by 65-70%.
The team undertook census studies at three field sites, finding common use of repellents (mats, coils, vaporizers, or creams) for mosquito control, but with inconsistent association with reduction in malaria cases, indicating that further clinical trial testing of repellents is required to consolidate their role in vector-borne disease control.
The surveillance information of Malaria of October, 2023 in India is enclosed in this Monthly Malaria Situation Information Report. The various indicators analyzed in this report are *BSE, *TPC, *TPR & *PF. *BSE (Blood Slide Examination), TPC (Total Positive Cases), PF (Plasmodium falciparum) and TPR (Total Positivity ... · Timely referral and ...
More than 90% of India's 161,516 reported malaria cases in 2021 were concentrated in just 8 states, all of which have significant tribal populations. The tribal districts that report malaria ...
India was one of eleven countries accounting for 70% of the global burden of malaria in 2020 1, and it continues to account for 79% of all malaria cases and 83% of all malaria deaths in the South ...
One study from East Timor in 2006 reported a co-infection with Plasmodium falciparum malaria and dengue in a 7-year-old girl who subsequently died. 6 Three further descriptive studies and one case control study were published from India 7 8 and French Guiana, 9 10 all patients in these studies being adult patients. Malaria and dengue co ...
DFID's technical support has also helped the state government establish evidence required for assessing and up-scaling malaria initiatives. Odisha has recorded a 24% decline in malaria cases and ...
New Delhi: Malaria infection is linked with genetic changes, known to be brought about by ageing, according to a new study.Researchers extracted genetic material from blood samples of more than ...
This paper draws on case studies of two large-scale national land and housing programs: in India, a national slum upgrading program called Basic Services for the Urban Poor (BSUP); and, in Tanzania, a national land delivery scheme called the 20,000 Plots Project (see Figure 1). In each country, these programs are compared with local-level ...
Consequently, the API of malaria should have been 9.0 instead of the 5.9 reported. 33 How reliable was the clinical diagnosis alone for the treatment of malaria was shown in a hospital-based study. Although there were 24% malaria cases on the basis of clinical judgment alone, the cases were actually 52% when microscopic diagnosis was done ...
The GHIT Fund will invest approximately JPY 444 million (USD 2.9 million 1) in two malaria projects: the development of malaria chemoprevention drug through a partnership among Shionogi & Co., Ltd ...
The GHIT Fund will invest approximately JPY 444 million (USD 2.9 million 1) in two malaria projects: the development of malaria chemoprevention drug through a partnership among Shionogi & Co., Ltd ...
The most prominent FLiRT variant is KP.2, which has become the dominant strain in the U.S and accounting for around 25% of new COVID-19 cases as of April 2024". "The FLiRT variants, especially KP.2, appear to have increased transmissibility compared to previous Omicron sub variants.