- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Trends in incidence of...
Trends in incidence of total or type 2 diabetes: systematic review
Visual summary available
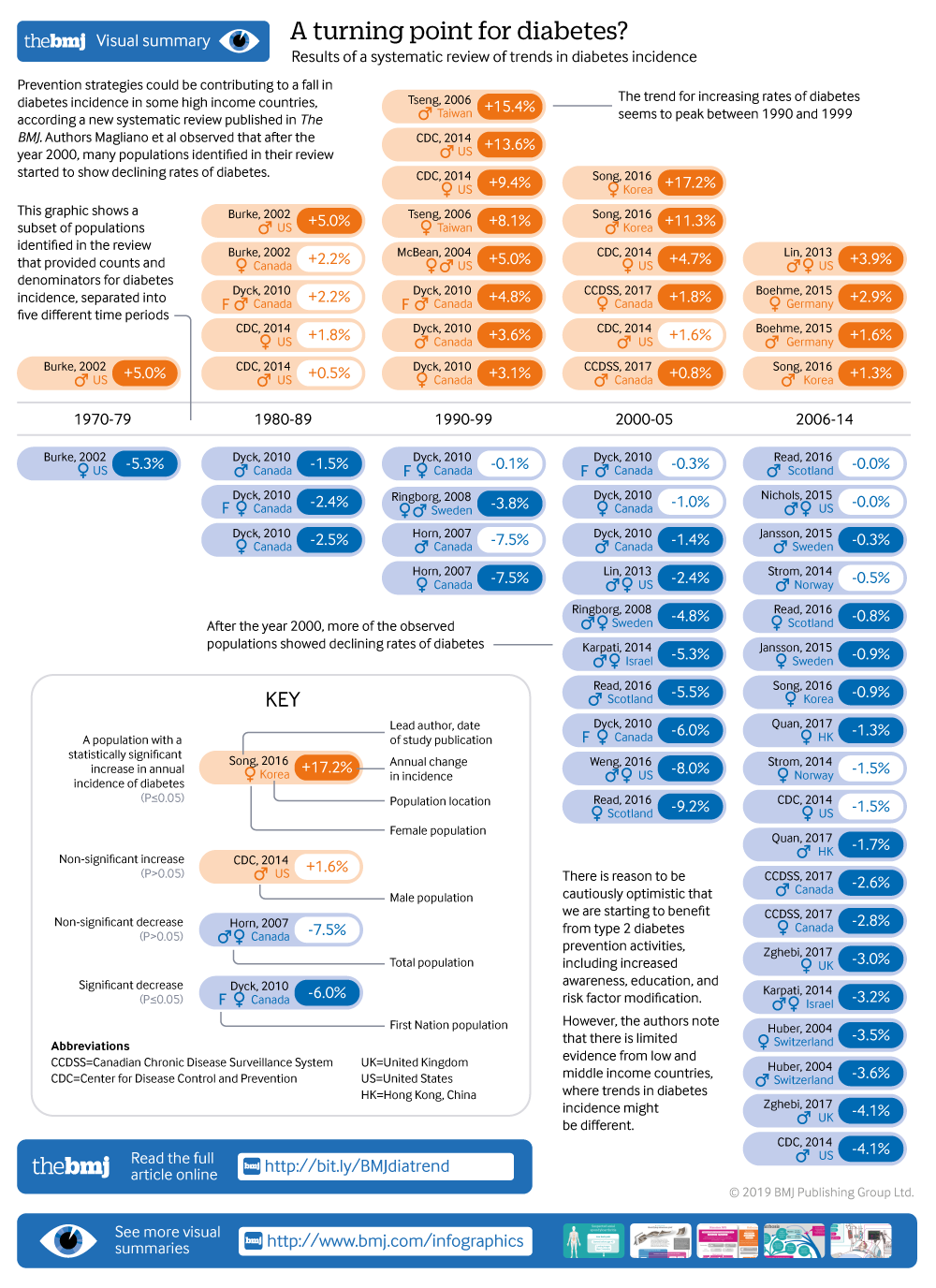
Showing the turning point in diabetes incidence in 61 populations
Linked editorial
Trends in type 2 diabetes
- Related content
- Peer review
- Dianna J Magliano , laboratory head of diabetes and population health 1 2 ,
- Rakibul M Islam , postdoctoral research fellow 1 2 ,
- Elizabeth L M Barr , postdoctoral research fellow 1 ,
- Edward W Gregg , chair in diabetes and cardiovascular disease epidemiology 3 4 ,
- Meda E Pavkov , physician scientist 3 ,
- Jessica L Harding , research fellow 3 ,
- Maryam Tabesh , research study coordinator 1 2 ,
- Digsu N Koye , postdoctoral research fellow 1 2 ,
- Jonathan E Shaw , deputy director of Baker Heart and Diabetes Institute 1 2
- 1 Baker Heart and Diabetes Institute, Melbourne, VIC 3004, Australia
- 2 School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC 3004, Australia
- 3 Centres for Diseases Control and Prevention, Division of Diabetes Translation, Atlanta, GA, USA
- 4 School of Public Health, Epidemiology and Biostatistics, Imperial College London, London, UK
- Correspondence to: D J Magliano dianna.magliano{at}baker.edu.au
- Accepted 16 July 2019
Objective To assess what proportions of studies reported increasing, stable, or declining trends in the incidence of diagnosed diabetes.
Design Systematic review of studies reporting trends of diabetes incidence in adults from 1980 to 2017 according to PRISMA guidelines.
Data sources Medline, Embase, CINAHL, and reference lists of relevant publications.
Eligibility criteria Studies of open population based cohorts, diabetes registries, and administrative and health insurance databases on secular trends in the incidence of total diabetes or type 2 diabetes in adults were included. Poisson regression was used to model data by age group and year.
Results Among the 22 833 screened abstracts, 47 studies were included, providing data on 121 separate sex specific or ethnicity specific populations; 42 (89%) of the included studies reported on diagnosed diabetes. In 1960-89, 36% (8/22) of the populations studied had increasing trends in incidence of diabetes, 55% (12/22) had stable trends, and 9% (2/22) had decreasing trends. In 1990-2005, diabetes incidence increased in 66% (33/50) of populations, was stable in 32% (16/50), and decreased in 2% (1/50). In 2006-14, increasing trends were reported in only 33% (11/33) of populations, whereas 30% (10/33) and 36% (12/33) had stable or declining incidence, respectively.
Conclusions The incidence of clinically diagnosed diabetes has continued to rise in only a minority of populations studied since 2006, with over a third of populations having a fall in incidence in this time period. Preventive strategies could have contributed to the fall in diabetes incidence in recent years. Data are limited in low and middle income countries, where trends in diabetes incidence could be different.
Systematic review registration Prospero CRD42018092287.
Introduction
Over the past few decades, the prevalence of diabetes in developed and developing countries has risen substantially, making diabetes a key health priority globally. 1 Examination of trends in total burden of diabetes is an essential part of the monitoring of this health priority area, but, to date, it has consisted primarily of studies looking at diabetes prevalence. 1 2 3 4 5 Prevalence estimates suggest that the diabetes burden is still rising in most countries, and this is often interpreted as evidence of increasing risk in the population. However, selective incidence studies 6 7 and some accompanying risk factor data 8 suggest otherwise. Prevalence can be a crude and misleading metric of the trajectory of an epidemic, because increasing prevalence of a disease might be due to either increasing incidence or to improved survival. Furthermore, prevalence cannot be reliably used to study the effects of changes in population risk factors, because their effects are detected earlier with incidence trends than with prevalence trends, and incidence is not affected by changes in survival.
Incidence measures the proportion of people who develop diabetes over a period of time among the population at risk. It is the appropriate measure of population risk, and a valuable way of assessing whether public health campaigns for diabetes prevention are succeeding. While prevalence can rise simply because mortality falls, incidence of diagnosed diabetes is affected only by the risk of the population and the amount of screening undertaken. Changes in prevalence might be an inadequate guide to the effects of prevention activities, and could lead to the inappropriate rejection of effective interventions. It is only by measuring both incidence and prevalence that a better understanding of the extent of diabetes can be achieved.
Among existing diabetes incidence data, a few studies suggest that diabetes incidence could be falling despite rising or stable prevalence, 6 7 9 but not all data are consistently showing the same trends. For example, studies from England and Wales (1994-98), 10 Portugal (1992-2015), 11 and Canada (1995-2007) 12 are reporting increases in diabetes incidence. To understand what is happening at a global level over time, a systematic approach to review all incidence trend data should be undertaken to study patterns and distributions of incidence trends by time, age, and sex. So far, no systematic reviews have reported on trends in the incidence of diabetes. Therefore, we conducted a systematic review of the literature reporting diabetes incidence trends.
Data sources and searches
We conducted a systematic review in accordance with PRISMA guidelines. 13 We searched Medline, Embase, and CINAHL from January 1980 to December 2017 without language restrictions. The full search strategy is available in supplementary table 1.
Study selection
Inclusion and exclusion criteria.
Eligible studies needed to report diabetes incidence in two or more time periods. Study populations derived from open, population based cohort studies (that is, with ongoing recruitment over time), diabetes registries, or administrative or health insurance databases based mainly or wholly in primary care (electronic medical records, health insurance databases, or health maintenance organisations). We also included serial, cross sectional, population based studies where incidence was defined as a person reporting the development of diabetes in the 12 months before the survey. Studies were required to report on the incidence of either total diabetes or type 2 diabetes. We excluded studies reporting incidence restricted to select groups (eg, people with heart failure) and studies reporting only on children or youth.
Each title and abstract was screened by at least two authors (DJM, JES, DNK, JLH, and MT) and discrepancies were resolved by discussion. We aimed to avoid overlap of populations between studies. Therefore, if national data and regional data were available from the same country over the same time period, we only included the national data. If multiple publications used the same data source, over the same time period, we chose the publication that covered the longest time period.
Outcome measure
Our outcome was diabetes incidence using various methods of diabetes ascertainment including: blood glucose, glycated haemoglobin (HbA1c), linkage to drug treatment or reimbursement registries, clinical diagnosis by physicians, administrative data (ICD codes (international classification of diseases)), or self report. Several studies developed algorithms based on several of these elements to define diabetes. We categorised the definition of diabetes into one of five groups: clinical diagnosis, diabetes treatment, algorithm derived, glycaemia defined (blood glucose or HbA1c, with or without treatment), and self report.
Data extraction and quality of studies
We extracted crude and standardised incidence by year (including counts and denominators) and the reported pattern of the trends (increasing, decreasing, or stable, (that is, no statistically significant change)) in each time period as well as study and population characteristics. Age specific data were also extracted if available. Data reported only in graphs were extracted by DigitizeIt software (European Organisation for Nuclear Research, Germany). We assessed study quality using a modified Newcastle-Ottawa scale for assessing the risk of bias of cohort studies 14 (supplementary material).
Statistical methods
Data were reported as incidence density (per person year) or yearly rates (percentage per year). From every study, we extracted data from every subpopulation reported, such that a study reporting incidence in men and women separately contributed two populations to this analysis. If studies reported two different trends over different time periods, we considered these as two populations. Further, if the study was over 10 years in duration, we treated these as two separate time periods. To avoid double counting, when the data were reported in the total population as well as by sex and ethnic groups, we only included data once and prioritised ethnicity specific data over sex specific data.
We extracted the age specific incidence data reported for every individual calendar year. These data were then categorised into four age bands (<40, 40-54, 55-69, and ≥70), and were plotted against calendar year. In studies where counts and denominators were reported by smaller age groups than we used, we recalculated incidence across our specified larger age groups. If we found multiple age groups within any of our broader age groups, but with insufficient information to combine the data into a new category, only data from one age group were used. To limit overcrowding on plots, if data were available for men, women, and the total population, only total population data were plotted. Data from populations with high diabetes incidence such as Mauritians 15 and First Nation populations from Canada 16 were plotted separately to allow the examination of most of the data more easily on a common scale (supplementary material). Furthermore, studies reporting data before 1991 or populations with fewer than three data points were not plotted. We also categorised studies into European and non-European populations on the basis of the predominant ethnicity of the population in which they were conducted. Studies conducted in Israel, Canada, and the United States were assigned to the European category.
We took two approaches to analyse trends of diabetes incidence over time. Firstly, we allocated the reported trend (increasing, decreasing, or stable (that is, no statistically significant change)) of each population to the mid-point of each study’s observational period, and then assigned this trend into one of five time periods (1960-79, 1980-89, 1990-99, 2000-05, and 2006-14). Where a test of significance of trends was not reported or when a time period was longer than 10 years, we performed Joinpoint trend analyses 17 18 to observe any significant trends in the data (assuming a constant standard deviation). Joinpoint Trend Analysis Software (version 4.5.0.1) uses permutation tests to identify points where linear trends change significantly in direction or in magnitude, and calculates an annual percentage change for each time period identified. In sensitivity analyses we also tested different cut points in the last two time periods.
The second approach was used to more accurately allocate trends to the prespecified time periods. Among the studies that reported raw counts of diabetes cases and denominators, we examined the association between calendar year and incidence, using Poisson models with the log person years as offset. The midpoints of age and calendar period were used as continuous covariates, and the effects of these were taken as linear functions. We analysed each study separately by prespecified time periods, and reported annual percentage change when the number of data points in the time period was at least four. For studies that did not provide raw data but did report a sufficient number of points, we analysed the relation between year and incidence using Joinpoint regression across the time periods specified above and reported annual percentage change. Analyses were conducted with Stata software version 14.0 (Stata Corporation, College Station, TX, USA), and Joinpoint (Joinpoint Desktop Software Version 4.5.0.1). 17 18
Patient and public involvement
No patients or members of the public were involved in setting the research question or the outcome measures for this study. No patients were asked to advise on interpretation or writing up of results. We intend to disseminate this research through press releases and at research meetings.
We found 22 833 unique abstracts from 1 January 1980 to the end of 2017. Among these, 80 described trends of diabetes incidence, of which 47 met all inclusion criteria. Articles describing trends were excluded for the following reasons: duplicated data (n=21), closed cohorts (n=5), populations included youth only (n=1), occupational cohorts (n=2), or no usable data presented (n=4; fig 1 ).

Flowchart of study selection
- Download figure
- Open in new tab
- Download powerpoint
Table 1 and supplementary material table 2 describe the characteristics of the included studies. Only 19% (9/47) of studies were from predominantly non-Europid populations and 4% (2/47) of studies were from low or middle income countries (China 25 and Mauritius 15 ). Administrative datasets, health insurance data, registry data, survey data, and cohort studies accounted for 38% (n=18), 21% (n=10), 19% (n=9), 11% (n=5), and 11% (n=5) of the 47 data sources, respectively. Among the 47 studies, diabetes was defined by a clinical diagnosis, diabetes treatment (via linkage to drug treatment registers), an algorithm, blood glucose, and self report in 28% (n=13), 9% (n=4), 47% (n=22), 11% (n=5), and 6% (n=3) of studies, respectively. Sample sizes of the populations were greater than 10 000 in every year in 85% (n=40) of the studies, and greater than 130 000 per year in 70% (n=33) of the studies. A total of 62% (n=29) of the 47 included studies exclusively reported on type 2 diabetes, and 38% (n=18) reported on total diabetes.
Characteristics of 47 included studies reporting on diabetes incidence trends, by country
- View inline
Summary of patterns of diabetes incidence trends based on analyses reported in publications in 1960-99
Trends of diabetes incidence
Among the 47 studies, 16 provided information on incidence by age group. Of these 16 studies, 14 were plotted in figure 2 , with those from high incidence countries plotted in supplementary figure 1. In these figures, incidence in most studies increased progressively until the mid-2000s in all age groups. Thereafter, most studies showed a stable or decreasing trend, apart from studies in Denmark 26 27 and Germany 31 and in a US health insurance population 9 where the incidence inflected upwards in the later years for some age groups.

Incidence of diabetes over time for populations aged under 40, 40-54, 55-69, and 70 or more, among studies reporting age specific data. Only populations with at least three points were plotted. NHIS=National Health Interview Survey
Using the first approach to analyse trends of diabetes incidence over time, we separated the data into populations based on sex and ethnicity, and allocated a time period to each population, generating 105 populations for analysis. Seventy four and 31 populations were predominantly Europid and non-Europid, respectively. Table 2 and table 3 show the reported trend for each population. Table 4 summarises the findings in table 2 and table 3 , and shows that the proportion of populations reporting increasing trends peaked in 1990-99 and fell progressively in the two later time periods. Between 1960 and 1989, 36% (8/22) of the populations studied had increasing trends in incidence of diabetes, 55% (12/22) had stable trends, and 9% (2/22) had decreasing trends. In 1990-2005, diabetes incidence increased in 66% (33/50) of populations, was stable in 32% (16/50), and decreased in 2% (1/50). In 2006-14, increasing trends were reported in 33% (11/33) of populations, whereas 30% (10/33) and 36% (12/33) had stable or declining incidence, respectively.
Summary of patterns of diabetes incidence trends based on analyses reported in publications in 2000-14
Summary of incidence trends over time of total or type 2 diabetes
Populations that reported a decrease in incidence after 2005 came from the US, 6 9 Israel, 34 Switzerland, 46 Hong Kong, 32 Sweden, 43 and Korea. 36 Populations reporting increasing incidence after 2005 included Portugal, 11 Denmark, 26 27 and Germany, 31 while populations from Canada, 19 Italy, 35 Scotland, 40 Norway, 39 US (non-Hispanic white), 56 and the United Kingdom 50 showed stable incidence. For two studies (16 populations), 16 29 we could not determine a direction of a trend (increasing, decreasing, or stable), because they showed three phases of change with the trend of the middle phase differing from the trend of the first and last phase. Across the total time period, we observed a higher proportion of populations reporting stable or decreasing trends in predominantly Europid than in non-Europid populations (52% v 41%).
Using the second approach to analyse trends of diabetes incidence over time, we modelled 21 studies (62 populations) that reported diabetes counts and denominators specifically within each time period ( table 5 ). The percentage of populations with a decreased or stable incidence was highest in 1980-89 (88%; 7/8), but this proportion was based on only eight populations in three studies. From 1990 onwards, the percentage with decreasing or stable incidence increased progressively, reaching 83% (19/23) of populations in 2006-14. Eight studies (21 populations) that were analysed by Joinpoint had no data on counts or denominators (supplementary table 3). When these data were considered with the data in table 5 , the percentage of populations in 2006-14 with decreasing or stable incidence fell to 70% (19/27), but this proportion was still the highest of all the time periods, whereas the percentage for 1990-99 remained the lowest at 31% (5/16).
Annual percentage change in diabetes incidence in men (M), women (W), or total population (T) among studies that provided counts and denominators, by time period
In a sensitivity analysis, we tested whether our selection of time periods was driving our results. When we defined the final time periods to be 2000-07 and 2008-14, our results were not altered, with 66% (21/32) of the populations in the last time period showing decreasing or stable trends. We also repeated the analysis in table 4 and excluded cohort studies and surveys, and found that the results were not materially altered, with 65% (20/31) of populations in the last time period (from 2006 onwards) showing decreasing or stable incidence of diabetes.
Quality of studies
The median score for study quality was 10 (interquartile range 8-11; supplementary table 4). We repeated the analyses reported in table 4 after excluding studies that had quality scores in the lowest quarter, and observed similar results to the main findings. For example, in 1960-89, 67% (10/15) of populations reported stable or decreasing incidence, while in the final time period, 67% (18/27) of populations reported stable or decreasing incidence of diagnosed diabetes.
Principal findings
In this systematic review of population based studies on diabetes incidence, we show evidence that the incidence of diagnosed diabetes increased in most populations from the 1960s to the early 2000s, after which a pattern emerged of levelling trends in 30% and declining trends in 36% of the reported populations. Although the lack of data for non-Europid populations leaves global trends in incidence unclear, these findings suggest that trends in the diabetes epidemic in some high income countries have turned in a more encouraging direction compared with previous decades. It is important to note that these results apply predominantly to type 2 diabetes, as even though many studies did not accurately define diabetes type, the incidence of type 2 diabetes in adults is an order of magnitude greater than that of type 1 diabetes.
The countries that showed stable or decreasing trends in the last time period were from Europe and east Asia, with no obvious clustering or commonalities. For the countries showing decreasing or stable diabetes trends, if the prevalence data were used to understand the diabetes epidemic in that country, a different message would be obtained. For example, national data from Korea showed that the prevalence of diabetes increased from 2000 to 2010. 59 Similarly in Sweden, the prevalence of pharmacologically treated diabetes increased moderately from 2006 to 2014. 43 In the US, the prevalence of diabetes reached a plateau when incidence began to decrease. However, we lacked incidence data from many areas of the world where the most steady and substantial increases in prevalence have been reported, including the Pacific Islands, Middle East, and south Asia. Large increases in incidence could still be occurring in these areas. The lack of incidence data for much of the world, combined with the common observation of discordance between incidence and prevalence rates where such data exist, both underscore the importance of using incidence data to understand the direction of the diabetes epidemic.
Incidence could be starting to fall for several reasons. Firstly, we might be starting to benefit from prevention activities of type 2 diabetes, including increased awareness, education, and risk factor modification. These activities have involved both targeted prevention among high risk individuals, similar to that conducted in the Diabetes Prevention study 60 and Diabetes Prevention Programme 61 62 in many countries, 63 and less intensive interventions with broader reach such as telephone counselling in the general community. 64 65 67 Secondly, health awareness and education programmes have also been implemented in schools and work places, and many changes to the physical environment, such as the introduction of bike tracks and exercise parks, have occurred. 68 Thirdly, favourable trends in selected risk factors of type 2 diabetes in some countries provide indirect evidence of positive changes to reduce diabetes incidence. Finally, in the US, there is some evidence in recent years of improved diets and related behaviours, which include reductions in intake of sugar sweetened beverages 69 and fat, 70 small declines in overall energy intake, and declines in some food purchases. 8 71
Similar reduction in consumptions of sugar sweetened beverages have occurred in Norway 72 and Australia 73 and fast food intake has decreased in Korea. 74 Some of these changes could be linked to a fall in diabetes incidence. Some places such as Scotland 75 have also had a plateauing of obesity prevalence, but this is not universal. In the US, despite earlier studies suggesting that the rate of increase in obesity might be slowing down, 76 77 more recent data show a small increase. 78 79 While some evidence supports the hypothesis that these prevention activities for type 2 diabetes and an improved environment could trigger sufficient behaviour change to have an effect on diabetes incidence, other data, such as the continuing rising obesity prevalence in the US, 79 casts some doubt over the explanations underpinning our findings on diabetes incidence trends.
Other factors might have also influenced reported diabetes incidence. Only 11% (n=5) of the studies reported here screened for undiagnosed diabetes, and therefore trends could have been influenced by secular changes in diagnostic behaviour. In 1997, the threshold for fasting plasma glucose for diagnosis of diabetes was reduced from 7.8 to 7.0 mmol/L, which could increase diagnosis of new cases of type 2 diabetes. In 2009-10, HbA1c was then introduced as an alternative way to diagnose diabetes. 80 Evidence from some studies suggests that the HbA1c diagnostic threshold detects fewer people with diabetes than do the thresholds for fasting plasma blood glucose, 80 81 potentially leading to a lowering of incidence estimates. However, across multiple studies, prevalence estimates based on fasting plasma glucose only versus HbA1c definitions are similar. 82 Furthermore, because HbA1c can be measured in the non-fasting state (unlike the fasting blood glucose or oral glucose tolerance test), the number of people who actually undergo diagnostic testing could be higher with HbA1c. Nichols and colleagues 56 reported that among seven million insured US adults, despite a shift towards HbA1c as the diagnostic test in 2010, the incidence of diabetes did not change from 2010 to 2011.
Another potential explanation for declining or stable diabetes incidence after the mid-2000s is a reduction in the pool of undiagnosed diabetes 83 through the intensification of diagnostic and screening activities 83 84 and changing diagnostic criteria during the previous decade. 80 Data from Read and colleagues provide some evidence to support this notion. 41
Among the included studies, two studies specifically examined clinical screening patterns in parallel with incidence trends. These studies reported that the proportion of the population screened for diabetes increased over time, and the incidence of diabetes remained stable 56 or fell. 34 While the Karpati study 34 combined data for glucose testing with HbA1c testing, the study by Nichols and colleagues 56 separated the two, and showed that both glucose testing and HbA1c testing increased over time. A third study, in Korea, 36 also noted that the incidence of diabetes decreased in the setting of an increase in the uptake of the national health screening programme. Despite the introduction of HbA1c for diagnosis of diabetes by the World Health Organization, this practice has not been adopted everywhere. For example, neither Scotland nor Hong Kong have introduced the use of HbA1c for screening or diagnosis of diabetes, and studies in these areas showed a levelling of diabetes incidence trends and decreasing trends, respectively.
Our findings appear to contrast with data showing increasing global prevalence of diabetes. 1 3 However, increasing prevalence could be influenced by improved survival of people with diabetes, because this increases the length of time that each individual remains within the diabetes population. As is shown in several studies in this review, 23 41 mortality from diabetes and incidence of diabetes might both be falling but as long as mortality is lower than incidence, prevalence will rise. Therefore, we argue that prevalence alone is an insufficient measure to track the epidemic of diabetes and other non-communicable diseases.
Strengths and weaknesses of this study
A key strength of this work was the systematic approach and robust methodology to describe trends in diagnosed diabetes incidence. We also presented the reported trends allocated to approximate time periods, as well as conducting our own regression within exact time periods. The following limitations should also be considered. Firstly, we did not formally search the grey literature, because a preliminary grey literature search revealed only low quality studies, with inadequate methodological detail to provide confidence in any observed incidence trends, and thus review could be subject to publication bias. Secondly, we were not able to source age or sex specific data on all populations. Thirdly, it was not possible to adjust for different methods of diabetes diagnosis or ascertain trends by different definitions of diabetes. Fourthly, most data sources reported only on clinically diagnosed diabetes and so were subject to influence from diagnostic behaviour and coding practices. Fifthly, study type changed over time, with large administrative datasets becoming more common and cohort studies becoming less common over time. Nevertheless, the size and absence of volunteer bias in administrative datasets likely make them less biased. Finally, data were limited in low and middle income countries.
Conclusions and unanswered questions
This systematic review shows that in most countries for which data are available, the incidence of diagnosed diabetes was rising from the 1990s to the mid-2000s, but has been stable or falling since. Preventive strategies and public health education and awareness campaigns could have contributed to this recent trend. Data are limited in low and middle income countries where trends in diabetes incidence might be different. Improvement of the collection, availability, and analysis of incidence data will be important to effectively monitor the epidemic and guide prevention efforts into the future.
What is already known on this topic
Monitoring of the diabetes epidemic has mainly focused on reporting diabetes prevalence, which continues to rise; however, increasing prevalence is partly driven by improved medical treatment and declining mortality
Studies on diabetes incidence are scarce, but among those that exist, some report a fall or stabilisation of diabetes incidence;
Whether the proportion of studies reporting falling incidence has changed over time is not known
What this study adds
This systematic review of published data reporting diabetes incidence trends over time shows that in most countries with available data, incidence of diabetes (mainly diagnosed diabetes) increased from the 1990s to the mid-2000s, and has been stable or falling since
Preventive strategies and public health education and awareness campaigns could have contributed to this flattening of rates, suggesting that worldwide efforts to curb the diabetes epidemic over the past decade might have been effective
Published data were very limited in low and middle income countries, where trends in diabetes incidence might be different
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
Contributors: MT, DNK, JLH, and RMI are postdoctoral fellows who screened abstracts for selection into the systematic review. JES and DJM also screened abstracts. ELMB applied the quality criteria to the selected articles. RMI extracted data, applied quality criteria to selected articles, and contributed to preparing the manuscript. DJM conceived the project, screened abstracts, extracted the data, analysed the data, and wrote the manuscript. JES, MEP, and EWG conceived the project, edited the manuscript, and provided intellectual input throughout the process. The funder of the study (CDC) was part of the study group and contributed to data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. DJM is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Funded by the CDC. The researchers were independent from the funders.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the CDC for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required because this work was a systematic review.
Data sharing: Data are available from the corresponding author ([email protected]).
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Finucane MM ,
- Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose)
- Whiting DR ,
- Guariguata L ,
- International Diabetes Federation
- NCD Risk Factor Collaboration (NCD-RisC)
- Karuranga S ,
- Abraham TM ,
- Pencina KM ,
- Pencina MJ ,
- Slining MM ,
- Kimball ES ,
- Newnham A ,
- de Sousa-Uva M ,
- Antunes L ,
- Johnson JA ,
- Hemmelgarn BR ,
- Liberati A ,
- Tetzlaff J ,
- Altman DG ,
- PRISMA Group
- ↵ Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute, 2014. www.ohri.ca/programs/clinical_epidemiology/oxford.asp Last accessed 14 December 2018.
- Söderberg S ,
- Tuomilehto J ,
- ↵ Joinpoint Regression Program. 4.6.0.0 version. Statistical Methodology and Applications Branch, Surveillance Research Program: National Cancer Institute, 2018. https://surveillance.cancer.gov/joinpoint/ .
- Midthune DN
- ↵ Canadian Chronic Disease Surveillance System. Canadian Chronic Disease Surveillance System 2017. https://www.canada.ca/en/public-health.html
- Blanchard JF ,
- Lipscombe LL ,
- Jacobs-Whyte H ,
- Paradis G ,
- Macaulay AC
- Carstensen B ,
- Kristensen JK ,
- Ottosen P ,
- Borch-Johnsen K ,
- Steering Group of the National Diabetes Register
- Jensen PB ,
- Abouzeid M ,
- Wikström K ,
- Peltonen M ,
- Reunanen A ,
- Klaukka T ,
- Maatela J ,
- Michaelis D ,
- Boehme MW ,
- Buechele G ,
- Frankenhauser-Mannuss J ,
- Vilbergsson S ,
- Sigurdsson G ,
- Sigvaldason H ,
- Hreidarsson AB ,
- Sigfusson N
- Karpati T ,
- Cohen-Stavi CJ ,
- Leibowitz M ,
- Feldman BS ,
- Baviera M ,
- Marzona I ,
- Zimmet PZ ,
- Ruwaard D ,
- Bartelds AI ,
- Hirasing RA ,
- Verkleij H ,
- Birkeland KI ,
- Barnett KN ,
- Ogston SA ,
- Kerssens JJ ,
- McAllister DA ,
- Scottish Diabetes Research Network Epidemiology Group
- Stenström G ,
- Sundkvist G
- Jansson SP ,
- Andersson DK ,
- Svärdsudd K
- Ringborg A ,
- Lindgren P ,
- Martinell M ,
- Stålhammar J
- Schwenkglenks M ,
- Holden SH ,
- Barnett AH ,
- Peters JR ,
- Zghebi SS ,
- Steinke DT ,
- Rutter MK ,
- Emsley RA ,
- Ashcroft DM
- Akushevich I ,
- Kravchenko J ,
- Ukraintseva S ,
- O’Brien P ,
- Centers for Disease Control and Prevention (CDC)
- McBean AM ,
- Gilbertson DT ,
- Narayanan ML ,
- Schraer CD ,
- Bulkow LR ,
- Nichols GA ,
- Schroeder EB ,
- Karter AJ ,
- SUPREME-DM Study Group
- Tabaei BP ,
- Chamany S ,
- Driver CR ,
- Pavkov ME ,
- Hanson RL ,
- Knowler WC ,
- Bennett PH ,
- Krakoff J ,
- Lindström J ,
- Eriksson JG ,
- Finnish Diabetes Prevention Study Group
- Barrett-Connor E ,
- Fowler SE ,
- Diabetes Prevention Program Research Group
- Saaristo T ,
- Moilanen L ,
- Korpi-Hyövälti E ,
- Troughton J ,
- Chatterjee S ,
- Schmittdiel JA ,
- Neugebauer R ,
- Solomon LS ,
- Giles-Corti B ,
- Vernez-Moudon A ,
- Bolt-Evensen K ,
- Brand-Miller JC ,
- ↵ Bromley C, Dowling S, L G. The Scottish Health Survey. Scotland: A National Statistics Publication for Scotland, 2013.
- Carroll MD ,
- Flegal KM ,
- Kruszon-Moran D ,
- Freedman DS ,
- American Diabetes Association
- Lorenzo C ,
- Rasmussen SS ,
- Johansen NB ,

- Previous Article
- Next Article
Lifestyle Modification
Bariatric surgery, pharmacological agents, thiazolidinediones, vascular outcomes, translation and cost-effectiveness of diabetes prevention, who should be targeted for diabetes prevention, conclusions, type 2 diabetes prevention: a review.
Leena A. Ahmad, MD, is a fellow in the Department of Medicine, Division of Endocrinology, and Jill P. Crandall, MD, is an associate professor of clinical medicine and director of the Diabetes Clinical Trials Unit at Albert Einstein College of Medicine in Bronx, N.Y.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Leena A. Ahmad , Jill P. Crandall; Type 2 Diabetes Prevention: A Review. Clin Diabetes 1 January 2010; 28 (2): 53–59. https://doi.org/10.2337/diaclin.28.2.53
Download citation file:
- Ris (Zotero)
- Reference Manager
This review offers a discussion of various strategies for the prevention of type 2 diabetes. It includes results from recent clinical trials targeting patients who are at highest risk for the development of diabetes, with a particular emphasis on lifestyle modification strategies and the implementation of such programs in community-based settings.
T ype 2 diabetes has increased dramatically in the past two decades, with 1.6 million cases diagnosed each year in the United States. 1 Diabetes prevalence is highest among the elderly and in certain ethnic groups, especially African Americans, Hispanic Americans, and Native Americans. People with diabetes have a two- to fourfold increased risk of developing cardiovascular disease, peripheral vascular disease, and stroke. These complications account for 65% of mortality from diabetes and, as of 2006, have made diabetes the seventh leading cause of death in the United States. 1 , 2
Unfortunately, diabetes is often diagnosed relatively late in the course of the disease, at a point when many patients have already developed complications. In addition, management efforts are labor intensive and challenging for both patients and physicians. Furthermore, the economic burden associated with diabetes is substantial, with U.S. costs estimated at $174 billion in 2007 and one of every five health care dollars spent on caring for someone diagnosed with diabetes. 2 The impact of diabetes on individuals' health and its economic burden to society have made its prevention a major goal of the current era.
In the past decade, major advances have been made in our understanding of the prevention of type 2 diabetes. Interventions that can reverse impaired glucose regulation early in its course may be the key to primary prevention of the long-term complications of diabetes.
Type 2 diabetes is a heterogeneous disorder characterized by two interrelated metabolic defects: insulin resistance coupled with impaired insulin secretion by β-cells in the pancreas. 3 Therefore, strategies that target these two mechanisms by improving insulin sensitivity and protecting β-cell function have become the focus of prevention efforts. Weight loss and physical activity, as well as some medications, are thought to improve both insulin sensitivity and secretion. The results of major clinical diabetes prevention trials will be reviewed here.
In the past decade, several randomized, controlled clinical trials have examined the role of diet and exercise in the prevention of type 2 diabetes. 4 One of the earliest studies was conducted in a Chinese community among 577 men and women with impaired glucose tolerance who were randomized to a program of diet, exercise, or both. 5 Dietary intervention focused on increased amounts of vegetables and reduced consumption of alcohol and simple sugars; overweight individuals (those with a BMI > 25 kg/m 2 ) were encouraged to lose weight. The exercise group was instructed to increase their daily activity by the equivalent of 20 minutes of moderate activity, such as brisk walking, and the diet-plus-exercise group was asked to do both exercise and dietary modification.
After 6 years of follow-up, all three interventions were similarly effective, with risk reductions of 31–46% compared to an untreated control group. During long-term follow-up of this cohort, most participants had progressed to diabetes, although diabetes prevalence was still lower in the former intervention groups (80% compared to 93% in the placebo group). 6
More recently, the Finnish Diabetes Prevention Study (DPS) 7 randomized 522 overweight (average BMI 31 kg/m 2 ) middle-aged individuals to either intensive lifestyle modification or a control group. The former entailed both specific dietary recommendations and exercise guidelines, including a weight-loss goal of 5% of total body weight and at least 30 minutes per day of combined aerobic activity and resistance training.
This study demonstrated a clinically significant impact of intensive lifestyle changes in the reduction of diabetes. At the 3-year follow-up, the group reduced their cumulative risk by 58% compared to the control subjects. During the first year, the intervention group lost an average of 4.2 kg, which appeared to be the primary mediator of diabetes risk reduction. Further analysis demonstrated the impact of exercise on the risk reduction of diabetes: moderate to vigorous activity of at least 2.5 hours per week reduced the incidence of diabetes by 63–69%. In the extended follow-up (3 years after the active intervention was completed), the intensive lifestyle group maintained a 36% relative reduction in diabetes incidence, suggesting that these benefits could be maintained outside of a structured clinical trial setting. 8
The largest clinical trial to date to study lifestyle intervention for the prevention of diabetes was the Diabetes Prevention Program (DPP). 9 The DPP randomized 3,234 overweight participants with impaired glucose tolerance and elevated fasting glucose from 22 sites in the United States to one of three interventions: intensive lifestyle intervention (ILS), metformin, or placebo. The participants were mostly middle aged and had an average BMI of 34 kg/m 2 . Forty-five percent were from ethnic and racial minority groups known to be at high risk for diabetes. The ILS group was instructed to follow a low-calorie, low-fat diet, with a weight-loss target of 7% of baseline body weight and an exercise goal of at least 150 minutes per week of moderate-intensity physical activity. The ILS group participated in a 16-week core curriculum focused on behavior modification, diet, and exercise education during the first 24 weeks, followed by at least monthly reinforcement.
After an average follow-up of 2.8 years, the ILS group achieved a mean weight loss of 7%, and three-fourths of the participants met the exercise targets during the first 6 months of the study. The ILS group had a 58% reduction in the development of diabetes compared to the placebo group. Weight loss was the predominant predictor of reduced diabetes incidence, with a 16% reduction of developing diabetes for each kilogram of weight lost. However, participants who did not achieve their weight-loss targets but were able to achieve the exercise goal also benefited (44% risk reduction compared to placebo). The effectiveness of the ILS intervention was similar in men and women and among racial and ethnic groups. The greatest risk reduction was in participants older than 60 years of age, most likely because they achieved the biggest weight loss and the greatest increase in physical activity. 10
After completion of the initial masked phase of the DPP, all participants were offered the ILS program in a group session format and then were enrolled in the DPP Outcome Study (DPPOS), which aimed to examine whether the diabetes prevention was sustainable over time. During DPPOS, all participants were provided with quarterly lifestyle sessions, and the original ILS subjects received additional group classes.
Results from an additional 6.8 years of follow-up in DPPOS were recently published. 11 After a median total follow-up of 10 years, the ILS group, which had initially lost ~7 kg in the first year of the DPP, weighed 2 kg less on average than at DPP randomization. During DPPOS, diabetes incidence rates in the metformin and former placebo groups fell to equal those in the former ILS group, but the cumulative incidence remained lowest in the ILS group (34% risk reduction compared with placebo).
These results demonstrate that prevention or delay of diabetes achieved through lifestyle change can persist for at least 10 years. Furthermore, the decrease in diabetes incidence rates among former metformin and placebo groups suggests that lifestyle intervention provided in a group format is an effective approach.
Studies conducted in Japanese and Indian populations have also demonstrated the effectiveness of lifestyle modification in the prevention of diabetes. 12 , 13
Bariatric surgery as a means of achieving weight loss has proven to be successful in diabetes prevention. In one prospective trial of > 2,000 patients who underwent a variety of surgical procedures (most commonly, vertical banded gastroplasty) and a matched standard-care control group, the risk of diabetes in the surgical group was reduced by 86% at 2 years and 75% at 10 years of follow-up. None of those who lost at least 12% of their baseline weight developed diabetes, compared to 7% of those with 2% weight loss and 9% of those who gained weight. 14 , 15
Bariatric surgery has also been reported to induce remission of existing diabetes. In a randomized, controlled trial of gastric banding versus conventional diet therapy, 73% of surgical patients achieved a remission compared to 13% of control subjects. 16 Gastric banding procedures improve glycemic control in patients with established diabetes, further supporting the potential benefit in diabetes prevention for appropriately selected patients. 17
Although moderate-intensity exercise and weight loss clearly have been shown to be effective in reducing diabetes risk, not all patients are able to achieve these lifestyle goals. For these patients and those who progress despite successful weight loss, additional therapeutic options are needed. Several pharmacological agents have been studied in clinical diabetes prevention trials.
Metformin is the most widely studied drug for diabetes prevention. In the DPP, participants randomized to metformin (850 mg, twice daily) achieved a 31% reduction in diabetes compared to placebo. 9 Metformin was most effective in more obese participants (baseline BMI > 35 kg/m 2 ), who experienced a 53% reduction of diabetes incidence, and in participants < 45 years of age, who saw a 44% reduction. Metformin had little benefit for older individuals who were 60–85 years of age at baseline. The effectiveness of metformin was attributed in part to weight loss, which averaged 1.7 kg and accounted for 64% of the beneficial effect of metformin. 9 Importantly, after an average of 10 years of follow-up, the metformin group had maintained an average weight loss of 2.5 kg, and diabetes risk was reduced by 18% compared to the former placebo group. 11 Smaller studies conducted in India and China reported similar reductions in diabetes risk. 13 , 18
In general, metformin is widely available, inexpensive, and relatively well tolerated. These studies suggest that this medication is an appropriate treatment approach in appropriately selected patients, especially those who are younger and overweight.
The α-glucosidase inhibitor acarbose was studied in the Study to Prevent Non-Insulin-Dependent Diabetes (STOP-NIDDM) trial, which randomized 1,429 participants with impaired glucose tolerance to either acarbose, 100 mg, or placebo three times daily for a mean of 3.3 years. 19 In this study, subjects in the acarbose treatment arm had a 25% reduction in the incidence of diabetes. However, almost one-third of the acarbose group was unable to complete the study because of gastrointestinal side effects, which makes the results of the study difficult to interpret and the applicability to clinical care unclear.
The thiazolidinediones (TZDs) have also been studied as potential agents for diabetes prevention. In the first year of the DPP, diabetes incidence was reduced by 75% in the troglitazone arm before it was discontinued because of evidence of hepatotoxicity. 20 Troglitazone was also studied in a cohort of women with recent gestational diabetes and reduced diabetes by ~50% compared to untreated controls. 21 Rosiglitazone was studied in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial, 22 a large, international study that randomized high-risk patients (impaired fasting glucose, impaired glucose tolerance, or both) to rosiglitazone, 8 mg daily, or placebo. After an average follow-up of 3 years, the incidence of diabetes in the rosiglitazone group was reduced by 62% compared to placebo. Glucose intolerance was normalized in 50% of the rosiglitazone group compared to only 30% in the placebo group.
However, rosiglitazone does have well-known side effects, such as weight gain and peripheral edema; in the DREAM trial, the TZD group gained 2.2 kg more weight than the placebo group. Additional concerns include the controversy surrounding the potential cardiotoxicity of rosiglitazone and a report of increased fractures in women taking this medication, both of which have diminished enthusiasm for its routine use in diabetes prevention. 23 , 24
Although delay of the diagnosis of diabetes is the primary outcome in all diabetes prevention studies, the critical clinical issue is the prevention of the micro- and macrovascular complications of diabetes. Indeed, these complications account for the morbidity and mortality of the disease, and the ultimate goal of diabetes prevention is to avoid these devastating outcomes.
Investigators from the STOP-NIDDM trial reported a 49% reduction in cardiovascular events in the acarbose-treated group during the 3.3 years of follow-up, but the number of events was small, and this finding remains to be confirmed. 25 Cardiovascular disease risk markers were improved in the ILS group in the DPP, including lipoproteins, C-reactive protein, and fibrinogen. 26 During long-term follow-up, this group continued to show improvements in both lipids and blood pressure measurements, despite the fact that they were receiving less drug treatment for these conditions. 11 Longer-term follow-up of the DPP cohort may provide more definitive data on cardiovascular and microvascular outcomes.
The protocols employed in most lifestyle intervention trials are labor intensive and require dedicated staff and resources, raising issues about the economics of implementing these programs. Analyses of the costs of various strategies are conflicting, and two fundamental questions have emerged. First, if we elect to treat prediabetes, which of the strategies is the most cost-effective? Second, is it more economically prudent to start such a program in patients who are at high risk for diabetes, or should treatment be initiated only after diabetes has developed?
The DPP investigators analyzed the cost per quality-adjusted life year (QALY), comparing the lifestyle and metformin interventions to placebo. 27 The cost per QALY for the ILS intervention was ~ $1,100 compared to $31,300 for the metformin intervention. This led investigators to conclude that, compared to placebo, the ILS intervention was not only the most effective treatment for diabetes prevention, but also the most cost-efficient. Furthermore, when compared to other well-accepted interventions, they concluded that both DPP interventions would be cost-effective from societal and health system perspectives.
However, another analysis concluded that such programs are too expensive for widespread implementation and suggested that it may be preferable to delay intervention until diabetes is diagnosed. 28 Much of the discrepancy between these analyses derives from varying assumptions about rates of progression to diabetes and its complications and differences in analytic approach. However, cost-benefit analyses have been reported from other diabetes prevention trials with generally favorable results. 29 , 30
Resources for Implementing Lifestyle Modification
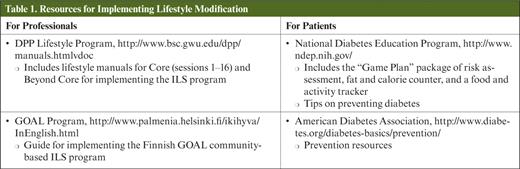
Lifestyle intervention has been conclusively proven effective in reducing diabetes risk, but for such an approach to be broadly implemented, it must be translated into community-based settings that are both accessible and affordable. Although such translation efforts are in their infancy, a number of significant efforts have been initiated ( Table 1 ).
Finnish investigators have developed a community-based model for intensive lifestyle intervention called Good Ageing in Lahti (GOAL). 31 This program identified high-risk participants from Finnish primary care settings and enrolled them in six 2-hour group counseling sessions that were based on a social-cognitive health behavior model and led by public health nurses. 32 , 33 Although the results of the GOAL trial were not as robust as the DPS in terms of meeting weight-loss and physical-activity targets (12 versus 43% and 65 versus 86%, respectively), this primary care–based program demonstrated a significant reduction in weight and BMI in high-risk individuals. Of the participants who had impaired glucose tolerance at baseline, 12% went on to develop type 2 diabetes at 3 years, and 43% returned to normal glucose tolerance.
Marrero and Ackermann developed a community-based program closely modeled after the DPP ILS for implementation at local YMCAs. 34 This program included a three-step approach: a 16-week core curriculum, a 4-week “training and refinement” phase, and a long-term maintenance phase. The core curriculum included weekly small-group sessions focused on mapping out explicit exercise plans and building problem-solving skills. In the second phase, participants met twice weekly with either a training partner or as a group to exercise. In the maintenance phase, monthly meetings included participants and their family members and addressed common barriers to weight loss and exercise (e.g., holidays and restaurant meals) and used many of the same tools as the original DPP.
High-risk individuals randomized to the group lifestyle program achieved a mean weight loss of 6% compared to only a 2% weight loss in a control group, which was sustained at 12 months. 35 Furthermore, the intervention group had a significantly reduced estimated 10-year risk of coronary heart disease (based on blood pressure, lipid levels, and A1C), supporting the potential for this community-based program to delay or prevent not only the onset of diabetes, but also the associated cardiovascular complications. 36 The cost per person to implement this type of community lifestyle intervention program was estimated at between $275 and $325 annually compared to the original DPP ILS intervention cost of $1,400 per participant for the first year. 37 , 38 This provides strong evidence that dissemination of the DPP lifestyle intervention in a well-established community organization is feasible and can be cost-effective.
There are similar group-based lifestyle intervention programs underway in communities throughout the United States. A recent review examined several such programs that were implemented in a wide variety of environments, including a rural Southern church community and an inner-city urban population in the Northeast. 39 Although the programs varied in length and target population, all reported significant weight loss and increased physical activity.
One of the larger translation efforts was reported by the Montana Diabetes Control Program, which collaborated with four health care facilities (urban and rural) to implement a group-based lifestyle program based on the DPP. This effort produced weight-loss results comparable to the DPP (mean weight loss 6.7 kg at 6 months), and most participants also achieved physical-activity goals. 40
Such results reinforce the feasibility of effective community-based lifestyle intervention strategies for diabetes prevention in diverse populations and in varied settings. However, much remains to be done to gain commitment from insurers and health care systems to ensure broad implementation for high-risk populations.
Recommendations for Screening for Pre-Diabetes and Diabetes 41

The first step in diabetes prevention is identifying patients who are at highest risk. This group includes individuals of any age who are overweight and obese (BMI > 25 kg/m 2 ) with at least one risk factor (such as high-risk ethnic group, first-degree relative with diabetes, personal history of gestational diabetes, or sedentary lifestyle). The American Diabetes Association (ADA) recommends that these patients should be screened every 3 years ( Table 2 ). All other patients should begin screening at the age of 45 years. 41
The laboratory diagnosis of “at risk” has traditionally been determined by the presence of impaired fasting glucose or impaired glucose tolerance. However, the current ADA clinical practice recommendations recommend that A1C measurement may be used as a screening tool, with levels between 5.7 and 6.4% defining those at highest risk for diabetes. 41 This simple blood test is readily available in most primary care settings, can be performed regardless of fasting status, and has the potential to more easily identify patients who would benefit from diabetes prevention measures. Validation of this approach remains to be completed, however.
Recent clinical trials have convincingly shown that lifestyle modification is the most effective tool in the prevention or delay of type 2 diabetes. For overweight and obese patients, a modest weight-loss goal of 5–10% (often < 20 lb) can substantially reduce the risk of diabetes. Moderate-intensity physical activity such as brisk walking for at least 150 minutes per week also plays an important role in reducing diabetes risk, even in the absence of weight loss ( Table 3 ).
Recommendations and Resources for Lifestyle Modification for Diabetes Prevention

For patients who are unable to achieve these lifestyle goals or those who progress despite exercising and losing weight, metformin has also been proven effective, especially in younger obese patients. Acarbose, when tolerated at the maximum effective dose, may also confer a moderate risk reduction. Data regarding thiazolidinediones are conflicting, and the reports of cardiovascular and fracture risk make this option less attractive as a prevention strategy. However, none of these medications are as robust in diabetes prevention as the lifestyle intervention strategies, and cost-effectiveness analyses suggest that pharmacotherapy may have greater financial costs.
Perhaps the most pressing clinical question remaining is whether these prevention strategies will reduce the vascular complications of diabetes that are the cause of the greatest financial burden and personal suffering in patients with diabetes. Prevention of diabetes is our most powerful intervention, and successful implementation of these proven strategies should be the focus of our efforts.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,160,796 articles, preprints and more)
- Free full text
- Citations & impact
The Effectiveness of Clinician-Led Community-Based Group Exercise Interventions on Health Outcomes in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis.
Author information, affiliations.
- Christie V 2
ORCIDs linked to this article
- Kirwan M | 0000-0002-0319-5670
- Gwynne K | 0000-0002-6897-4528
- Christie V | 0000-0003-3887-8305
International Journal of Environmental Research and Public Health , 07 May 2024 , 21(5): 601 https://doi.org/10.3390/ijerph21050601 PMID: 38791815 PMCID: PMC11120654
Abstract
Free full text .

The Effectiveness of Clinician-Led Community-Based Group Exercise Interventions on Health Outcomes in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
1 Faculty of Medicine, Health and Human Sciences, Macquarie University, Talavera Road, North Ryde, NSW 2109, Australia; moc.liamg@8etihwtmail (L.W.); [email protected] (M.K.); [email protected] (L.H.)
Morwenna Kirwan
Vita christie.
2 Djurali Centre for Aboriginal and Torres Strait Islander Health Research, Heart Research Institute, Eliza Street, Newtown, NSW 2042, Australia; [email protected]
3 DVC Indigenous Office, University of New South Wales, High Street, Sydney, NSW 2052, Australia
Lauren Hurst
Kylie gwynne.
- Associated Data
Data can be made available upon request.
This systematic review and meta-analysis evaluated the combined effects of clinician-led and community-based group exercise interventions on a range of health outcomes in adults with type 2 diabetes mellitus. Our literature search spanned Medline, Scopus, PubMed, Embase, and CINAHL databases, focusing on peer-reviewed studies published between January 2003 and January 2023. We included studies involving participants aged 18 years and older and articles published in English, resulting in a dataset of eight studies with 938 participants. Spanning eight peer-reviewed studies with 938 participants, the analysis focused on the interventions’ impact on glycemic control, physical fitness, and anthropometric and hematological measurements. Outcomes related to physical fitness, assessed through the six-minute walk test, the 30 s sit-to-stand test, and the chair sit-and-reach test, were extracted from five studies, all of which reported improvements. Anthropometric outcomes from seven studies highlighted positive changes in waist circumference and diastolic blood pressure; however, measures such as body mass index, systolic blood pressure, weight, and resting heart rate did not exhibit significant changes. Hematological outcomes, reviewed in four studies, showed significant improvements in fasting blood glucose, triglycerides, and total cholesterol, with glycemic control evidenced by reductions in HbA1c levels, yet LDL and HDL cholesterol levels remained unaffected. Ten of the fifteen outcome measures assessed showed significant enhancement, indicating that the intervention strategies implemented may offer substantial health benefits for managing key type 2 diabetes mellitus-related health parameters. These findings in combination with further research, could inform the refinement of physical activity guidelines for individuals with type 2 diabetes mellitus, advocating for supervised group exercise in community settings.
- 1. Introduction
Diabetes affects more than half a billion individuals globally, with type 2 diabetes mellitus comprising 90% to 95% of these cases [ 1 ]. This prevalence, representing nearly one in ten adults, is increasing and poses a significant threat to the health and well-being of people, impacting individuals, families, and societies [ 1 ].
Physical activity, alongside nutritional and medical therapies, is critical for managing type 2 diabetes mellitus [ 2 ]. Exercise, a specific category of physical activity, involves activities that improve strength, endurance, agility, balance, and flexibility, all of which are beneficial for type 2 diabetes mellitus patients. These benefits extend beyond physical health, positively affecting the psychological and cognitive aspects of health [ 2 ]. Current guidelines advise adults aged 18–64 with type 2 diabetes mellitus to undertake at least 150 min of moderate-to-vigorous intensity exercise weekly and to participate in resistance training sessions at least twice a week [ 2 ]. Despite such guidelines, over 1.4 billion adults globally fall short of meeting these physical activity recommendations, regardless of their type 2 diabetes mellitus status [ 3 ].
Researchers have investigated several physical activity intervention techniques to support people with type 2 diabetes mellitus to be more active. Clinician-led facility-based fitness training is one such tactic, and it has the potential to enhance glycemic management and other cardiovascular risk factors of type 2 diabetes mellitus [ 4 , 5 , 6 ]. These interventions are frequently resource-intensive, only accessible in large cities, and their long-term viability is uncertain [ 7 ]. Other methods to encourage physical activity in type 2 diabetes mellitus adults include individual-based treatments, medication use, and behavior modification. It can be difficult to persuade people with type 2 diabetes mellitus to embrace behavior change with only short visits to their GP [ 7 ]. These self-management techniques are also only moderately effective in the near term, and long-term evaluations are frequently relatively few [ 8 ]. Furthermore, those with low incomes, low levels of education, limited access to healthcare, and linguistic and cultural hurdles may find these types of treatments to be inaccessible [ 8 ].
The burgeoning prevalence of type 2 diabetes mellitus necessitates an expansion of existing intervention strategies to manage the disease effectively. The synthesis of clinician-led and community-based exercise interventions presents a promising hybrid model, leveraging the structured guidance of healthcare professionals with the accessibility of community settings. Recognizing the potential of this integrated approach could be instrumental in shaping future health policies and guidelines that seek to amplify the reach and impact of type 2 diabetes mellitus management strategies. Community-based exercise interventions might overcome the limitations of facility-based and individual approaches by providing culturally relevant health education. Facility-based interventions are administered in controlled, institutional environments such as hospitals or clinics, while community-based interventions take place within local settings, utilizing area resources and engaging community members, potentially increasing adherence to self-management practices [ 9 , 10 ]. The World Health Organization advocates for such interventions to promote physical activity among people with type 2 diabetes mellitus [ 11 ]. Updates to physical activity guidelines now recommend clinician-led exercise as a beneficial strategy [ 12 ]. A systematic review in 2018 indicated that supervised aerobic and resistance training yields better health outcomes than unsupervised activities [ 13 ]. Studies combining community-based and clinician-led exercises have shown health benefits [ 14 , 15 ], with some effects persisting for up to twelve months post-intervention [ 16 ].
This study evaluated the combination of clinician-led and community-based exercise interventions of adults with type 2 diabetes mellitus through a systematic review and meta-analysis. In this context, a “clinician” is a qualified health worker who delivers services in community settings. The study design anticipated sufficient quantitative data to include a meta-analysis. Unlike previous studies conducted in a workplace or traditional clinical settings, this research investigates the effectiveness of group exercise interventions for adults with type 2 diabetes mellitus implemented in community-based settings such as recreation centers, local facilities, and community centers. To our knowledge, this is the first systematic review targeting the efficacy of supervised group exercise interventions in community settings for enhancing health outcomes in type 2 diabetes mellitus adults.
- 2. Materials and Methods
2.1. Study Design
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 17 ] and was registered with the International Prospective Register of Systematic Reviews (PROSPERO: ID no. CRD42023363265).
In adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 17 ], the systematic search and selection process for relevant studies was conducted across multiple databases. A comprehensive search yielded a total of 693 studies from CINAHL, Scopus, Embase, Medline, and PubMed. Following the removal of 264 duplicates, 429 abstracts were screened. Of these, 415 studies were excluded based on exclusion criteria, leaving 14 full-text articles to be assessed for eligibility. Reasons for exclusion at this stage included the absence of outcome data reported as mean and standard deviation and the lack of integration of both clinician-led and community-based approaches in the interventions. Ultimately, 8 studies met all criteria and were included in the systematic review and meta-analysis.
2.2. Search Strategy and Data Sources
The search strategy was developed in consultation with two senior health researchers (MK, KG) and a health research librarian. A comprehensive literature search across Medline, Scopus, Pubmed, Embase, and CINAHL databases for peer-reviewed studies published from January 2003 until January 2023 was conducted. We employed the following search strings to gather relevant data: “(Type 2 Diabetes OR Diabetes) AND (Clinician Led OR Supervised) AND (Community-based OR Community) AND (Exercise OR Physical Activity OR Fitness OR Outcome Measures) AND (Management)”. These search parameters were refined to include subjects aged 18 years and older and articles published in the English language ( Supplementary File: Table S1. MeSH Terms and Database Searches ).
Initial identification of titles and abstracts was independently performed by two authors (LW and MK), with disparities resolved through discussion or consultation with a third reviewer (KG) if required. Full texts of potentially relevant studies were then retrieved and assessed for eligibility. The final inclusion of articles was determined by checking the references of selected studies for additional relevant literature. All search results were systematically organized using Microsoft Excel (Version 16.84).
For this review, we adopted a PICO framework focusing on:
Participants/Population: Adults (18 years or older) involved in clinician-led, community-based exercise programs for managing type 2 diabetes mellitus.
Intervention(s)/Exposure(s): Eligible studies were those conducted in high-income countries—specified regions (Australia, New Zealand, Canada, UK, and Europe), with interventions predominantly based on PA (over 50%), targeting adults with pre-existing type 2 diabetes mellitus, using community-based settings, and overseen by qualified clinicians, presenting quantitative studies of original data in peer-reviewed journals.
Comparator(s)/Control: Participants receiving standard care without the specified clinician-led, community-based exercise.
Main Outcome(s): Measurable pre- and post-health outcomes related to PA, including weight loss, BMI changes, waist-to-hip ratios, HbA1c levels, and six-minute walk test (6MWT) improvements.
Additional Outcome(s): Compliance with PA programs, clinician experience, and details on the interventions’ setting and delivery.
2.3. Inclusion and Exclusion Criteria
This review included full-text, published, peer-reviewed literature with original outcome data that reported on the effectiveness of clinician-led, community-based, group exercise interventions for the management of type 2 diabetes mellitus. The inclusion criteria included lifestyle interventions where exercise was a purposeful, structured, and required part of the intervention and conducted in high-income countries (Australia, New Zealand, Canada, UK, and Europe). Studies published 2003–2023 were included to obtain contemporary evidence.
Studies were omitted if they were not peer-reviewed, published prior to 2003, were not published in English, and did not feature interventions primarily based in the community. Exclusions also applied to research not primarily focused on physical activity, those that involved minors, or adults with diabetes types other than type 2 diabetes mellitus where specific data were not differentiated. Additional exclusion criteria encompassed studies conducted exclusively in clinical environments, those without the oversight of a professionally trained clinician, and research relying solely on qualitative data sources.
2.4. Data Extraction and Management
Following the study selection, data were extracted into a Microsoft Excel spreadsheet. This data included the title, authors, publication year, study design, location, clinician type, inclusion and exclusion criteria, participant details, type of physical activity intervention, outcome measures, and results.
2.5. Meta-Analysis
For the synthesis of results, we conducted a meta-analysis of comparable outcome measures, including glycemic control, physical fitness outcomes, and anthropometric measurements, employing the mean difference pooled using a random effects model [ 18 ] in IBM SPSS Statistics (Version 28). The effect size was calculated for each relevant outcome measure at a 95% confidence interval. Heterogeneity was evaluated using the I-squared statistic. The included studies’ effect estimations were represented graphically by forest plots. A p -value of <0.05 was considered statistically significant. Revman Web was used to display figures represented within this systematic review [ 19 ].
2.6. Risk of Bias Assessment
The risk of bias in included studies was appraised using the EPHPP tool [ 20 ] by two authors (LW and LH) independently. The EPHPP tool was selected as a suitable tool for assessment of quantitative public health research. Any conflicting evaluations were discussed until a consensus was reached. The final inclusion of studies in the review depended on achieving at least a medium-quality rating.
3.1. Study Selection Process
This systematic review and meta-analysis was conducted in strict accordance with the PRISMA guidelines [ 17 ], which dictate standards for reporting such research. The review started with a detailed search across several databases, namely CINAHL, Scopus, Embase, Medline, and PubMed. This initial search yielded 693 studies. From these, 264 duplicates were identified and removed, leaving 429 for abstract screening. After careful consideration, 415 studies were excluded because they did not meet the specific criteria set out for this research.
The eligibility of the remaining 14 full-text articles was closely examined. Five of these articles were excluded for reasons including not reporting outcomes in the form of mean and standard deviation, or because they did not feature both clinician-led and community-based intervention approaches. Consequently, eight studies were selected for inclusion in the final review and analysis. This work was also officially registered with the International Prospective Register of Systematic Reviews (PROSPERO) [ 21 ] under the ID CRD42023363265, ensuring that the process was systematically planned and recorded. This has been displayed in our PRISMA flowchart ( Figure 1 ).

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram illustrating the selection process for studies included in the systematic review and meta-analysis.
3.2. Study Characteristics
The eight studies encompassed a total of 938 participants and explored 15 health outcomes to ascertain the impact of clinician-led and community-based group exercise programs on adults with type 2 diabetes mellitus. We have detailed the characteristics of these studies in Table 1 , which includes participant demographics, intervention types, durations, and settings. Requests for additional information were made to the authors of two studies [ 22 , 23 ].
Individual study characteristics.
Abbreviations: N = Number; % Female = Percentage of Female Participants; y = Years; SD = Standard Deviation; NR = Not Reported.
3.3. Quality of Included Studies
Quality assessment using the EPHPP framework indicated that five studies were rated as “strong” overall, with the remaining three rated as “medium” ( Table 2 ).
Risk of bias assessment—EPHPP scores of included studies.
Most of the studies recruited participants through diabetes management clinics or patient databases. Among them, one study was conducted as a randomized controlled trial [ 23 ], while the other seven were pre-post studies. Confounding variables were identified in four of the studies [ 14 , 23 , 25 , 27 ]. It is noteworthy that all studies employed data collection methods recognized for their validity and reliability. However, there was a lack of clarity in the reporting of participant withdrawals and dropouts in two of the studies [ 14 , 15 ].
3.4. Cardiometabolic Health Indicators
Anthropometric measurements, as well as heart rate and blood pressure, were focal outcomes in seven studies, as depicted in Figure 2 [ 14 , 15 , 23 , 24 , 25 , 27 , 28 ]. A notable and statistically significant reduction emerged in both waist–hip circumference (CI: −4.27 to −1.70) and diastolic blood pressure (CI: −6.53 to −2.38). Trends suggesting improvement were seen in BMI (CI: −22.79 to 3.46) and systolic blood pressure (CI: 13.99 to 2.19), though these changes did not reach statistical significance. No associations were noted for either weight (CI: −2.17 to 1.54) or resting heart rate (CI: −1.20 to 1.46).

Forest plots depicting the effects of interventions on cardiometabolic parameters: body mass index (BMI, kg/m 2 where kg = kilograms, m 2 = square meters), waist circumference (cm, where cm = centimeters), weight (kg), resting heart rate (bpm, where bpm = beats per minute), systolic blood pressure and diastolic blood pressure, both measured in mmHg (millimeters of mercury). Each plot displays mean values, standard deviations (SD), total participant counts, and study-specific weights. Mean differences are shown with 95% confidence intervals (CI), calculated using a random effects model. Heterogeneity is quantified by I 2 and tau-squared (τ 2 ) values. Studies referenced: Kirwan et al., 2022 [ 14 ], Kirwan et al., 2021 [ 15 ], Akinci et al., 2018 [ 22 ], Mendes et al., 2017 [ 23 ], Higgs et al., 2016 [ 24 ], Cugusi et al., 2015 [ 26 ], Negri et al., 2010 [ 27 ].
3.5. Physical Fitness and Functional Capacity
A suite of tests designed to evaluate physical and functional fitness, presented in Figure 3 [ 14 , 15 , 23 , 26 , 28 ], indicated significant improvements. These were quantified in the 6 min walk test (6MWT) (CI: 42.38 to 88.42), the 30 s sit-to-stand test (STS 30) (CI: 2.92 to 4.67), and the chair sit-and-reach test (CI: 2.68 to 5.52).

Forest plots of intervention effects on physical and functional fitness measures. Top panel: Six-Minute Walk Test (6MWT) measured in meters (m), evaluating walking distance. Middle panel: Sit-to-Stand in 30 Seconds Test (STS 30), counting the number of repetitions. Bottom panel: Chair Sit-and-Reach Test measured in centimeters (cm), assessing reach and flexibility. Each plot includes data on mean and standard deviation (SD) for intervention and baseline, total study weight, and the mean difference with 95% confidence intervals (CI). Heterogeneity across studies is quantified by I 2 statistics and Tau 2 values. Notably, significant improvements in physical performance measures are observed following the intervention, as indicated by the mean differences and confidence intervals. Studies referenced: Kirwan et al., 2022 [ 14 ], Kirwan et al., 2021 [ 15 ], Akinci et al., 2018 [ 22 ], Higgs et al., 2016 [ 24 ], Mendes et al., 2016 [ 25 ], Negri et al., 2010 [ 27 ].
3.6. Glycemic and Lipid Profiles
The efficacy of the interventions on glycemic control and lipid metabolism was captured through hematological measures in four studies, as shown in Figure 4 [ 23 , 24 , 27 , 28 ]. There were statistically significant improvements in HbA1c (CI: −0.94 to −0.30), fasting blood glucose (CI: −26.37 to −9.73), triglycerides (CI: −39.95 to −18.48), and total cholesterol (CI: −26.41 to −2.89). LDL cholesterol presented a favorable trend (CI: −22.79 to 3.46), although not statistically significant. HDL cholesterol did not exhibit significant change post-intervention (CI: −3.33 to 4.17).

Forest plots displaying the effects of interventions on glycemic and lipid profiles measured in milligrams per deciliter (mg/dL). The plots are organized by biomarker: hemoglobin A1c (HbA1c), Fasting blood glucose, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL). Each plot provides the mean and standard deviation (SD) at baseline and post-intervention, total sample size, mean difference with 95% confidence intervals (CI), and the weight of each study in the meta-analysis. Heterogeneity is quantified using I 2 statistics, indicating the percentage of total variation across studies due to heterogeneity rather than chance. The intervention effect is assessed with a random effects model, showcasing significant changes in each biomarker following the intervention. Studies referenced: Akinci et al., 2018 [ 22 ], Mendes et al., 2017 [ 23 ], Cugusi et al., 2015 [ 26 ], Negri et al., 2010 [ 27 ].
- 4. Discussion
This systematic review supports the assertion that a dual strategy encompassing clinician-led and community-based group exercise interventions can improve important health outcomes in adults with type 2 diabetes mellitus. Out of fifteen health outcomes assessed through meta-analysis, nine demonstrated statistically significant improvements associated with the intervention. This resonates with findings from prior systematic reviews, which similarly concluded that exercise interventions delivered in community or clinical settings are efficacious for type 2 diabetes mellitus management [ 7 , 13 ].
Cardiometabolic Health Indicators: Our analysis identified significant enhancements in waist circumference and diastolic blood pressure among the intervention group. The implications of these improvements are noteworthy considering the pivotal role these indicators play in forecasting glycemic control within the type 2 diabetes mellitus population [ 28 , 29 ]. Although no substantial association was found between the interventions and BMI, body weight, resting heart rate, or systolic blood pressure, these parameters have been reported to improve in earlier interventions as per previous reviews [ 7 , 13 ].
Physical Fitness and Functional Capacity: The interventions included in our meta-analysis also significantly improved cardiovascular fitness, lower body strength, and flexibility. These findings hold particular importance because individuals with type 2 diabetes mellitus are prone to a gradual decline in physical function due to aging, which often leads to muscle atrophy and an increase in fat mass. These changes can substantially restrict mobility and physical function [ 30 ]. Our review indicates that intervention periods as brief as 2 to 3 months are capable of significantly enhancing the functional fitness of adults with type 2 diabetes mellitus.
Glycemic and Lipid Profiles: Hematological measures, including HbA1c, fasting blood glucose, triglycerides, and total cholesterol, also showed significant improvements post-intervention. Notably, despite a shorter intervention span of 8 weeks, the study by Akinci et al. [ 23 ] reported changes in mean values comparable to those from studies with longer durations, each extending beyond 3 months [ 24 , 27 , 28 ]. This observation is particularly striking as HbA1c levels reflect an individual’s average blood glucose control over a three-month period [ 31 ], suggesting that even brief interventions can be beneficial. However, due to the nature of HbA1c, long-term follow-up in future studies may be required to understand the full impact of these interventions [ 24 ].
Research that evaluates the health benefits of physical activity for older adults with type 2 diabetes mellitus yields findings of considerable importance. A concerning trend highlighted by current research is the lower fitness levels observed in individuals with type 2 diabetes mellitus as compared to non-diabetic individuals, a disparity that is worsened with aging [ 32 ]. Improvements in physical and functional fitness are crucial for this group, as they are intimately linked with reductions in cardiovascular risks and improvements in insulin sensitivity [ 33 ]. Nonetheless, the task of evaluating the clinical importance of such physical and functional fitness improvements is complex. Quantifying the clinical impact of the physical and functional fitness improvements noted in our systematic review is difficult due to limited data on what constitutes a clinically significant change for this population [ 34 ].
To address this, validated criterion standards, though not specific to type 2 diabetes mellitus, offer valuable benchmarks for the fitness levels necessary for older adults to remain independent [ 35 ]. Our systematic review incorporated two studies [ 14 , 15 ] that used these benchmarks to assess participants’ baseline fitness and subsequent improvements. These benchmarks were critical for quantifying participants’ fitness levels in relation to the standards needed for independence with aging. The findings from Kirwan et al. [ 14 , 15 ] were encouraging, showing a considerable number of participants reaching or surpassing the target fitness levels after the intervention. These findings bolster the case for future research to utilize these benchmarks in community-based, clinician-led group exercise programs, which would allow for a more nuanced interpretation of changes in the physical and functional fitness among individuals with type 2 diabetes mellitus.
In the current discourse on geriatric health, the significance of functional fitness emerges as a critical factor, particularly within the demographic contending with type 2 diabetes mellitus. Maintaining an adequate level of functional fitness is instrumental in diminishing fall risks and fostering the capacity for independent living, which in turn exerts a substantial influence on the quality of life [ 36 ]. For individuals navigating the complexities of type 2 diabetes mellitus management, preserving their independence and the ability to conduct daily living activities constitutes a fundamental health objective [ 32 ]. A deficiency in effective, targeted interventions may result in a trajectory that culminates in dependency, precipitating substantial long-term economic burdens on healthcare systems. Complicating this issue is the prevalence of physical inactivity among individuals with type 2 diabetes mellitus, which further exacerbates the risk of functional decline [ 37 , 38 ].
The studies included in this review showed favorable health outcomes for participants; however, these studies varied in duration from 8 weeks to 9 months. It remains to be seen whether the short-term benefits translate into long-term health improvements over the years. Longitudinal studies are needed to determine if the observed benefits persist and to investigate methods to encourage sustained engagement with these exercise programs.
The cost-effectiveness of combined clinician-led and community-based interventions also warrants further exploration. Community-based interventions are often perceived as more economical compared to their clinic-based counterparts. Nevertheless, additional financial considerations such as staffing, program development, and the upkeep of facilities must not be overlooked. A recent systematic review has summarized the existing economic evaluations of physical activity interventions specifically in the context of type 2 diabetes mellitus management [ 39 ]. The findings are encouraging, indicating that such interventions are generally a sound investment—four out of ten interventions were deemed cost-saving, while six were considered cost-effective, and two displayed favorable cost-utility characteristics. Future research should, therefore, extend to a thorough cost-effectiveness and cost-utility assessment of combined community-based and clinician-led group exercise interventions. Such inquiry is essential to assess the practicality and potential for broader application within diverse healthcare systems.
This research acknowledges several limitations that warrant caution in interpreting the findings. Primarily, the recruitment of participants from referred sources such as clinics may introduce bias, as opposed to random selection from a representative target population which could offer a more balanced perspective. Furthermore, the reporting of participant adherence was often absent or noted to be moderate at best. For instance, Higgs et al. observed a dropout rate of approximately 40 percent before the follow-up measures could be taken [ 24 ]. Additionally, the participant gender ratio in many included studies was skewed, potentially affecting the extrapolation of results to the broader type 2 diabetes mellitus community, there are no molecular data or experimentally derived data and it is not clear whether these interventions can be applied to different geographical locations and different people. Despite these constraints, efforts were made to design this review to maximize the translational potential of the findings.
In terms of strengths, this review’s robust sample size of 938 participants enhances the reliability of the conclusions drawn. The deliberate inclusion of anthropometric, functional, and hematological measures offers a comprehensive view of the multifaceted impacts that clinician-led and community-based exercise interventions may have on adults with type 2 diabetes mellitus. Additionally, the selection of studies from countries with analogous cultures—such as Australia, New Zealand, Canada, the UK, and Europe—was intended to ensure participant homogeneity.
The insights garnered from this systematic review could inform refinements to physical activity guidelines tailored for individuals with type 2 diabetes mellitus, advocating for community-based, clinician-led exercise modalities. Notably, current Australian exercise recommendations for type 2 diabetes mellitus management mirror those for the general population, with specific guidance on blood glucose management during physical activity [ 2 , 40 ]. However, our review does not conclusively favor a particular community setting or clinician type, which may stem from the diverse community structures and clinician roles across the various countries of the included studies. This area might benefit from further investigative efforts.
- 5. Conclusions
In conclusion, our analysis of eight studies presenting quantitative data corroborates the expanding evidence that both community-based and clinician-led group exercise interventions can positively influence health outcomes in individuals with type 2 diabetes mellitus. Yet, the need for additional research is evident, especially regarding the interventions’ effectiveness within culturally and linguistically diverse groups. By addressing this gap, we can move towards resolving persisting uncertainties and creating customized interventions that address the distinct needs of these communities.
- Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph21050601/s1 , Table S1: MeSH Terms and Database Searches.
- Funding Statement
This research received no external funding.
- Author Contributions
Conceptualization, M.K. and K.G.; methodology, M.K., K.G. and L.W.; validation, L.W. and L.H.; formal analysis, L.W. and M.K.; resources, M.K.; writing—original draft preparation, L.W. and M.K.; writing—review and editing, V.C., K.G., L.W. and M.K.; visualization, L.W. and M.K.; supervision, K.G. and M.K.; project administration, L.W. and V.C. All authors have read and agreed to the published version of the manuscript.
- Informed Consent Statement
Not applicable.
- Data Availability Statement
- Conflicts of Interest
The authors declare that there are no conflicts of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Full text links
Read article at publisher's site: https://doi.org/10.3390/ijerph21050601
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics.

Europe PMC is part of the ELIXIR infrastructure
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 28 May 2024
Prediabetes remission for type 2 diabetes mellitus prevention
- Andreas L. Birkenfeld ORCID: orcid.org/0000-0003-1407-9023 1 , 2 , 3 , 4 &
- Viswanathan Mohan ORCID: orcid.org/0000-0001-5038-6210 5 , 6
Nature Reviews Endocrinology ( 2024 ) Cite this article
171 Accesses
9 Altmetric
Metrics details
- Medical research
- Pre-diabetes
Current guidelines for the delay and prevention of type 2 diabetes mellitus recommend for people with prediabetes to lose at least 7% of their body weight. Here, we advocate to use glycaemic remission as a goal of prevention in people with prediabetes and those who are at high risk for type 2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Nathan, D. M. et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 62 , 1319–1328 (2019).
Article PubMed PubMed Central Google Scholar
American Diabetes Association Professional Practice Committee. prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2024. Diabetes Care 47 (Suppl 1), S43–S51 (2024).
Article Google Scholar
Sandforth, A. et al. Mechanisms of weight loss-induced remission in people with prediabetes: a post-hoc analysis of the randomised, controlled, multicentre Prediabetes Lifestyle Intervention Study (PLIS). Lancet Diabetes Endocrinol. 11 , 798–810 (2023).
Article PubMed Google Scholar
Fritsche, A. et al. Different effects of lifestyle intervention in high- and low-risk prediabetes: results of the randomized controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 70 , 2785–2795 (2021).
Article CAS PubMed Google Scholar
Zhyzhneuskaya, S. V. et al. Time course of normalization of functional β-cell capacity in the diabetes remission clinical trial after weight loss in type 2 diabetes. Diabetes Care 43 , 813–820 (2020).
Tabák, A. G. et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373 , 2215–2221 (2009).
Jumpertz von Schwartzenberg, R., Vazquez Arreola, E., Sandforth, A. et al. Role of weight loss-induced prediabetes remission in the prevention of type 2 diabetes: time to improve diabetes prevention. Diabetologia https://doi.org/10.1007/s00125-024-06178-5 (2024).
Taylor, R. Type 2 diabetes and remission: practical management guided by pathophysiology. J. Intern. Med. 289 , 754–770 (2021).
Sathish, T. et al. Effect of conventional lifestyle interventions on type 2 diabetes incidence by glucose-defined prediabetes phenotype: an individual participant data meta-analysis of randomized controlled trials. Diabetes Care 46 , 1903–1907 (2023).
Wagner, R. et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 27 , 49–57 (2021).
Download references
Acknowledgements
The authors acknowledge the members of ‘The Prediabetes Group’, who are listed in the Supplementary Information document, for their assistance with reviewing this article. A.L.B. acknowledges the support of funding from the German Federal Ministry for Education and Research (01GI0925) via the German Center for Diabetes Research (DZD e.V.).
Author information
Authors and affiliations.
German Center for Diabetes Research (DZD), Neuherberg, Germany
Andreas L. Birkenfeld
Department of Internal Medicine IV, Diabetology, Endocrinology and Nephrology, Eberhard-Karls University Tübingen, Tübingen, Germany
Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen, Tübingen, Germany
Department of Diabetes, Life Sciences & Medicine Cardiovascular Medicine & Sciences, King’s College London, London, UK
Madras Diabetes Research Foundation, Chennai, India
Viswanathan Mohan
Dr. Mohan’s Diabetes Specialities Centre, Chennai, India
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andreas L. Birkenfeld .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Related links.
Diabetes mellitus: https://www.who.int/news-room/fact-sheets/detail/diabetes
World Health Organization’s global targets for noncommunicable diseases: https://www.who.int/publications/i/item/9789241506236
Supplementary information
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Birkenfeld, A.L., Mohan, V. Prediabetes remission for type 2 diabetes mellitus prevention. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00996-8
Download citation
Published : 28 May 2024
DOI : https://doi.org/10.1038/s41574-024-00996-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, a systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms.

- 1 Baycrest Centre, Rotman Research Institute, Toronto, ON, Canada
- 2 Sunnybrook Research Institute, Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, Toronto, ON, Canada
- 3 Department of Medical Biophysics, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 4 Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 5 Departments of Psychology and Psychiatry, University of Toronto, Toronto, ON, Canada
The rising prevalence of type 2 diabetes (T2DM) and hypertension in older adults, and the deleterious effect of these conditions on cerebrovascular and brain health, is creating a growing discrepancy between the “typical” cognitive aging trajectory and a “healthy” cognitive aging trajectory. These changing health demographics make T2DM and hypertension important topics of study in their own right, and warrant attention from the perspective of cognitive aging neuroimaging research. Specifically, interpretation of individual or group differences in blood oxygenation level dependent magnetic resonance imaging (BOLD MRI) or positron emission tomography (PET H 2 O 15 ) signals as reflective of differences in neural activation underlying a cognitive operation of interest requires assumptions of intact vascular health amongst the study participants. Without adequate screening, inclusion of individuals with T2DM or hypertension in “healthy” samples may introduce unwanted variability and bias to brain and/or cognitive measures, and increase potential for error. We conducted a systematic review of the cognitive aging neuroimaging literature to document the extent to which researchers account for these conditions. Of the 232 studies selected for review, few explicitly excluded individuals with T2DM (9%) or hypertension (13%). A large portion had exclusion criteria that made it difficult to determine whether T2DM or hypertension were excluded (44 and 37%), and many did not mention any selection criteria related to T2DM or hypertension (34 and 22%). Of all the surveyed studies, only 29% acknowledged or addressed the potential influence of intersubject vascular variability on the measured BOLD or PET signals. To reinforce the notion that individuals with T2DM and hypertension should not be overlooked as a potential source of bias, we also provide an overview of metabolic and vascular changes associated with T2DM and hypertension, as they relate to cerebrovascular and brain health.

Introduction
Amongst middle-aged and older adults, the rising prevalence of T2DM, hypertension, and other conditions that comprise the metabolic syndrome is a global health epidemic, attributed largely to sedentary lifestyles, poor diet, and lack of exercise. In 2008, it was estimated that 347 million adults worldwide had T2DM, up from 153 million in 1980 ( Danaei et al., 2011 ). Over the next two decades, it is expected that these numbers will continue to rise, by as much as 38% by 2030 ( Shaw et al., 2010 ). Prevalence rates of hypertension are even higher. In 2000, the global prevalence of hypertension was 26.4%, affecting an estimated 972 million people worldwide. Again, these numbers are expected to increase by approximately 60% by 2025, to a total of 1.56 billion people ( Kearney et al., 2005 ). Critically, hypertension is present in up to 75% of individuals with T2DM ( Colosia et al., 2013 ). The growing number of middle-aged and older adults living with T2DM and/or hypertension makes these conditions important topics of study in their own right.
Better long-term health care and disease management allow middle-aged and older adults to live with T2DM and hypertension for many years; however, both of these conditions have long-term deleterious effects on cerebrovascular and brain health, and contribute to cognitive impairment and decline ( Gorelick et al., 2011 ). T2DM and midlife hypertension confer a high risk for development of mild cognitive impairment (MCI) and dementia ( Launer et al., 2000 ; Kloppenborg et al., 2008 ; Creavin et al., 2012 ; Crane et al., 2013 ; Roberts et al., 2014 ), and older individuals with T2DM progress to dementia at faster rates ( Xu et al., 2010 ; Morris et al., 2014 ). These changing health demographics have created a discrepancy: what we define as “normal” or “typical” cognitive aging is becoming farther and farther removed from what would be considered optimal, or “healthy” cognitive aging.
This trend warrants attention from the perspective of cognitive aging research. Without adequate screening procedures in place, inclusion of individuals with T2DM and hypertension in otherwise healthy study samples may introduce unwanted variability and bias to brain and/or cognitive measures, and increase the potential for type 1 and type 2 errors. Functional neuroimaging studies may be particularly vulnerable in this regard. Blood oxygenation level dependent magnetic resonance imaging (BOLD MRI) and positron emission tomography (PET H 2 O 15 ) measure hemodynamic changes associated with neural activity, and thus provide an indirect measure of neural function ( Logothetis et al., 2001 ). To interpret individual or group differences in BOLD or PET signaling as reflective of individual or group differences in neural activation underlying a cognitive operation of interest, we rely on assumptions of intact neurovascular signaling, cerebrovascular reactivity, and vascular health amongst the study participants. These assumptions may be true in young and healthy individuals, but do not hold in older adults with conditions that affect vascular health ( D'Esposito et al., 2003 ). Even normal, age-related changes in the integrity of the cerebrovascular system can undermine these assumptions ( D'Esposito et al., 1999 ).
Yet, it was our impression that relatively few studies in the cognitive aging neuroimaging literature consider T2DM or hypertension during recruitment, or control for potential confounds associated with these conditions during analysis. To clarify the extent to which current research practices consider T2DM and hypertension in study design, we present the results of a systematic review of the cognitive aging neuroimaging literature, looking at study inclusion/exclusion criteria and methodology related to T2DM and hypertension. Then, to reinforce the notion that individuals with T2DM and hypertension should not be overlooked as a potential source of bias, we provide an overview of metabolic and vascular changes associated with T2DM and hypertension, as they relate to vascular health, structural brain atrophy, and functional integrity. The final section discusses best practices moving forward.
Systematic Review
This review focuses on the cognitive aging neuroimaging literature, however the issues associated with inclusion of individuals with T2DM and hypertension in study samples are by no means limited to this area of research. Any research study whose population of interest has high prevalence rates of T2DM or hypertension should be cognizant of these issues. For example, psychiatric populations have a higher incidence of metabolic disruption and T2DM that is mediated, at least partially, by the use of mood stabilizers, anticonvulsants, and antipsychotic medications ( Regenold et al., 2002 ; Newcomer and Haupt, 2006 ).
It should also be noted that the purpose of this review is not to quantitatively compare the results of studies that have excluded T2DM and/or hypertension with those that have not. This type of comparison is not feasible for numerous reasons, the primary one being that the extent to which individuals with T2DM or hypertension were present in study samples that did not screen for either condition is unknown. Rather, the aim of this review is to highlight the proportion of studies in the cognitive aging neuroimaging literature that consider T2DM and/or hypertension in their inclusion/exclusion criteria, or attempt to account for the potential bias introduced by inclusion of these individuals in their study groups.
We searched PsychInfo, MedLine, and PubMed between 1995 and February, 2013 using the search terms [“functional magnetic resonance imaging” or “positron emission tomography”], [“geriatrics” or “aging” or “age differences”], and [“cognit*” or “neuropsych*” or “memory” or “attention”]. Across the three databases, these search terms produced 704 unique empirical studies. From these results, we excluded studies that did not include a “healthy” or “normal” older adult sample ( n = 125), included a clinical sample other than MCI or Alzheimer disease (AD)/dementia (e.g., psychiatric; n = 46), did not use BOLD or PET H 2 O 15 imaging ( n = 227), and did not scan during a cognitive or resting state task ( n = 74; Figure 1 ).
Figure 1. Literature search terms and exclusion criteria . Based on these criteria, 232 studies were selected for review.
Based on these criteria, 232 studies were selected for review. These studies are identified with an asterisk (*) in the reference section. Two hundred and nineteen of these used BOLD imaging, one used both BOLD and PET H 2 O 15 , and 12 used PET H 2 O 15 only. One hundred and sixty five of these studies compared a “healthy” older group with a group of young participants, 34 studies compared a “healthy” older sample to an MCI and/or AD group (two of which also included a young adult comparison group), and the remaining 33 studies looked only at a “healthy” older sample. The majority of surveyed studies employed a memory paradigm during imaging (e.g., encoding/recognition of words, pictures, scenes, faces, autobiographical memory, spatial memory, associative memory, implicit learning). Working memory and executive processes were also well-studied (e.g., cognitive control, inhibition, decision making, mental rotation, task-switching, attention, judgment, processing speed, naming, imagery, verb generation, fluency). We also included resting-state studies in the sample.
Our primary concern was how sample selection was reported to have occurred. In particular, we were interested to learn how many studies specifically screened for T2DM and/or hypertension in their healthy older adult samples. For each of the 232 identified studies, the inclusion/exclusion criteria were examined according to the following criteria: (i) explicit exclusion of T2DM and/or hypertension, or exclusion of medical disorders/physical illnesses/systemic illnesses (implying that all medical conditions, including T2DM and hypertension, were excluded); (ii) exclusion of “significant,” “major,” or “severe” medical/physical/systemic disorders; or (iii) no screening criteria related to T2DM and/or hypertension provided. We also surveyed each of the 232 studies to determine how subjects were screened (e.g., self-report questionnaire, clinical assessment with a medical doctor, laboratory testing), and how—if at all—the potential influence of intersubject vascular variability on the measured BOLD or PET signals was addressed.
In each section below, superscript numbers, letters, and symbols are used to represent the extent to which studies screened for T2DM and hypertension, the screening method, and the degree to which studies attempted to account for intersubject vascular variability, respectively. The identified studies are denoted in the reference section according to these superscript classifiers.
Inclusion/exclusion of T2DM and hypertension
Of the 232 studies surveyed, only 22 (9.5%) explicitly excluded individuals with T2DM( 1 ), and only 29 (12.5%) explicitly excluded individuals with hypertension( 2 ). Thirteen studies—approximately 6%—excluded both T2DM and hypertension. Fourteen studies (6.0%) excluded individuals on antihypertensive medication( 3 ), however few of these studies also clarified whether individuals were assessed for untreated hypertension and excluded, if present. Nineteen studies (8.2%) excluded medical illnesses, systemic illnesses, medical disorders or physical illnesses( 4 ). This criterion implies that all medical conditions, including T2DM and hypertension, were excluded.
In contrast, almost half of the included studies (102; 44.0%) had exclusion criteria that made it difficult to determine whether T2DM was excluded( 5 ), and 85 studies (36.6%) had exclusion criteria that made it difficult to determine whether hypertension and/or antihypertensive medications were excluded( 6 ). These studies listed “major medical illnesses,” “significant medical conditions,” “serious systemic illnesses,” “conditions/medications interfering with cognitive and/or brain function,” “vascular disease,” “cardiovascular disease,” and/or “conditions/medications interfering with the fMRI signal” as exclusion criteria, or simply described their sample as “healthy.” There were also many studies that did not mention any selection criteria related to T2DM (80; 34.5%)( 7 ) or hypertension (51; 22.0%)( 8 ).
In addition, 26 studies (11.2%) included individuals with controlled hypertension( 9 ), 8 studies (3.5%) included controlled and uncontrolled hypertension( 10 ), 3 studies (1.3%) included individuals with controlled T2DM( 11 ), and 6 studies (2.5%) included individuals with controlled and uncontrolled T2DM in their healthy cohort( 12 ). Figure 2 provides a visual depiction of these results.
Figure 2. The extent to which T2DM and hypertension were accounted for in the inclusion/exclusion criteria of the healthy samples that were surveyed .
Screening method
The majority of studies (173; 75%) did not report how they conducted their medical screening( a ). Only 28 studies (12%) reported having screened subjects with physician-conducted medical examinations and/or laboratory testing( b ). Sixteen studies (7%) screened participants with telephone interviews, in-person clinical interviews, medical history, chart reviews, or a combination of these methods( c ). The remaining 15 studies (6%) used a self-report questionnaire to assess medical status( d ).
Accounting for intersubject vascular variability
A survey of the 232 included studies found that just under one third (29%) acknowledged and/or addressed the potential influence of intersubject vascular variability on the reported results. Many excluded subjects with a high vascular burden by screening for white matter hyperintensities in the imaging data( ■ ). Others compared groups on vascular risk factors( + ), compared outcome measures on hypertension status or antihypertensive treatment status( ♦ ), or attempted to control for health, blood pressure, and/or white matter hyperintensities in the reported associations ( ❖ ). Several studies noted in their discussion the possibility that the reported results were influenced by vascular factors, or explained why they did not think this was an issue( • ). A few studies used the measured BOLD or PET signals to examine and account for individual differences in vascular health( □ ); for example, by ensuring that groups were equated on BOLD signal variability, by comparing the temporal characteristics of the hemodynamic response curve across groups, with proportional scaling of the BOLD or PET signal, or by focusing on group by task interactions (instead of group main effects) or comparing within-subject task contrasts across individuals or groups to minimize any individual or group differences in vascular integrity.
There are rigorous ways to account for intersubject vascular variability, such as additional task data or an additional imaging contrast. Several studies included in the present review used arterial spin labeling (ASL) MRI ( ▴ ) or PET ( ▾ ) to measure resting cerebral blood flow and control for individual differences in perfusion. Three studies used a breath-hold task to index individual differences in cerebrovascular reactivity ( ❍ ), and two studies included a low-level motor or baseline task to ensure that participants demonstrated an adequate hemodynamic response ( × ).
Our results found that fewer than 10% of the selected functional imaging studies on cognitive aging explicitly excluded individuals with T2DM from their normative samples, and fewer than 15% explicitly excluded individuals with hypertension. A number of studies reported selection criteria that were insufficient to determine whether T2DM or hypertension were screened. Critically, one third of included studies had no reported inclusion or exclusion criteria related to T2DM, while almost a quarter had no reported inclusion or exclusion criteria related to hypertension. Only 67 of the 232 selected studies (29%) acknowledged or addressed the potential influence of intersubject vascular variability on the measured BOLD or PET signals.
Moreover, the large majority of studies did not include information about the medical screening process itself (e.g., laboratory testing vs. clinical interview vs. self-report questionnaire). This is not ideal when established tests for T2DM and hypertension are available (for example, 24-h ambulatory blood pressure monitoring would be the gold-standard for determining hypertension status, and an oral glucose tolerance test for determining T2DM status). Furthermore, we posit that participants may be less likely to volunteer T2DM or hypertension status as a “significant” medical illness without specific probing (i.e., compared to cancer, HIV, multiple sclerosis, or heart disease), because when these conditions are well-controlled they can have a minimal impact on day-to-day functioning, and, in the case of T2DM, can be controlled by diet alone. Collectively, these observations point to a lack of awareness that T2DM and hypertension are major medical illnesses that interfere significantly with cognitive and brain function in older adults.
Overview: Metabolic and Vascular Complications of Type 2 Diabetes Mellitus and Hypertension
To reinforce the position that T2DM and hypertension are conditions that can have a major effect on brain health and cognitive aging, this next section reviews evidence on the cognitive deficits, structural changes, and functional consequences associated with T2DM and hypertension, and describes some of the mechanisms that mediate these changes.
Type 2 Diabetes Mellitus
T2DM is the result of peripheral insulin resistance, which leads to insulin dysregulation and hyperglycemia. These metabolic changes affect cerebrovascular health, structural integrity, and brain function, and underlie the associations between T2DM, cognitive decline, and dementia risk.
Insulin dysregulation
Insulin is a peptide hormone that is critical for regulation of blood glucose levels. Binding of insulin to its receptors, found on nearly all cells throughout the body, facilitates the cellular uptake of glucose from the blood. When bound, insulin and insulin-like growth factor also activate complex intracellular signaling pathways that promote cell growth and survival, regulate glucose metabolism, and inhibit oxidative stress and apoptosis (for a review, see Nakae et al., 2001 ).
The defining characteristic of T2DM is peripheral insulin resistance, which occurs when cells in the body decrease their response to insulin stimulation. In the developing stage of this disease, the pancreas is able to produce enough insulin to overcome this resistance. This results in peripheral hyperinsulinemia, and blood glucose levels remain within the normal range. As the disease progresses, however, the pancreas can no longer keep up, and blood glucose levels begin to rise. When blood glucose levels are high even in the fasting state, T2DM is diagnosed.
Peripheral insulin resistance and hyperinsulinemia have a counterintuitive impact on insulin levels within the central nervous system. In the face of peripheral hyperinsulinemia, insulin transport across the blood brain barrier is effectively reduced, resulting in a brain hypo -insulinemic state (e.g., Heni et al., 2013 ). Low brain insulin levels and disrupted insulin signaling contribute to cognitive impairments directly, particularly in medial temporal lobe regions where insulin receptors are abundant ( Convit, 2005 ; Craft, 2006 ). Indirectly, low brain insulin levels exacerbate amyloid beta (Aβ) and tau pathology, hallmarks of Alzheimer disease (AD). It is here that we see the link between T2DM and Alzheimer disease pathology: brain insulin deficiency results in the down-regulation of insulin degrading enzyme (IDE; Luchsinger, 2008 ), which also has a role in degrading Aβ ( Carlsson, 2010 ). As a result, Aβ degradation is effectively reduced, contributing to its aggregation and amyloid plaque formation. Decreased brain insulin levels also suppress the enzymes involved in tau phosphorylation, contributing to the formation of neurofibrillary tangles ( Akter et al., 2011 ). While the downstream impact of T2DM-mediated brain insulin deficiency and insulin resistance is more moderate than that associated with AD, the underlying pathogenic mechanisms are similar ( Steen et al., 2005 ), and it has been proposed that AD is a form of diabetes mellitus that selectively affects the brain (T3DM; for discussion, see de la Monte and Wands, 2008 ). Given this, is not surprising that individuals with T2DM show a pattern of memory impairment, medial temporal lobe atrophy, and reduced hippocampal connectivity that is similar to the classic pattern of memory deficits, neurodegeneration, and network disruption in AD (e.g., Gold et al., 2007 ; Zhou et al., 2010 ; Baker et al., 2011 ; Cui et al., 2014 ).
Hyperglycemia
When cells in the body become resistant to the effects of insulin, blood glucose levels rise, resulting in hyperglycemia. Endothelial cells are particularly vulnerable to the effects of hyperglycemia, because they are less efficient at reducing glucose uptake in the face of high blood glucose levels ( Kaiser et al., 1993 ). Under such conditions, the resultant intracellular hyperglycemia induces an overproduction of reactive oxygen species in the mitochondria, which increases oxidative stress within the cell. This initiates a cascade of biochemical events that mediate much of the microvascular and macrovascular damage associated with T2DM including, but not limited to, increased intracellular formation of advanced glycation end-products (AGEs) and protein kinase C activation ( Du et al., 2000 ; Nishikawa et al., 2000 ; Brownlee, 2005 ; Giacco and Brownlee, 2010 ; Johnson, 2012 ).
AGEs are formed during normal metabolism on proteins with slower rates of turnover, in almost all cells throughout the body. AGE accumulation over time is a major factor in normal aging; however, under hyperglycemic conditions, AGE production is exacerbated beyond normal levels. AGEs cause intracellular damage and induce apoptosis through a process called cross-linking ( Shaikh and Nicholson, 2008 ). AGEs also contribute to oxidative stress, and themselves activate inflammatory signaling cascades (for a review, see Yan et al., 2008 ). Critically, under hyperglycemic conditions, the Aβ protein itself can act as an AGE ( Granic et al., 2009 ), which enhances its own aggregation and further increases amyloid plaque formation.
Protein kinase C activation, on the other hand, affects a variety of changes in gene expression that culminate in vascular dysfunction. Production of nitric oxide (NO), a vasodilator, is decreased, and production of endothelin-1, a vasoconstrictor, is increased. As a result, blood vessels are less able to dilate to accommodate increased blood flow demand. Over time, chronic exposure to high concentrations of endothelin-1 and decreased concentrations of NO contribute to diminished vessel elasticity, and structural changes in the vessel wall that result in atherosclerotic plaque formation ( Kalani, 2008 ).
In the brain, hyperglycemia-mediated macro- and microvascular damage reduces the delivery of nutrients and oxygen required to meet metabolic demands. Altered cerebral autoregulation has been observed in middle-aged adults with T2DM ( Brown et al., 2008 ), and may be an early manifestation of microvascular disease ( Kim et al., 2008 ). Older adults with T2DM show decreased blood flow velocity, increased cerebrovascular resistance, and impaired vasoreactivity ( Novak et al., 2006 ). Over time, declines in cerebrovascular health and reduced perfusion of brain tissue lead to structural atrophy and altered brain function.
Cognitive effects
The cognitive profile of individuals with T2DM includes deficits in attention, processing speed, learning and memory, and executive function (e.g., Reaven et al., 1990 ; Brands et al., 2007 ; Yeung et al., 2009 ; Whitehead et al., 2011 ). Moreover, these individuals, and individuals with pre-diabetes (impaired glucose tolerance), show an accelerated trajectory of cognitive decline relative to that associated with healthy aging ( Gregg et al., 2000 ; Fontbonne et al., 2001 ; Arvanitakis et al., 2004 ; Yaffe et al., 2004 ; Fischer et al., 2009 ; Nooyens et al., 2010 ; Espeland et al., 2011 ; for conflicting results, see van den Berg et al., 2010 ).
Cognitive deficits in T2DM have been linked to multiple disease-related processes, including: (i) poor glucose control (i.e., hemoglobin A1c [HbA1c]; Ryan and Geckle, 2000 ; Kanaya et al., 2004 ; Cukierman-Yaffe et al., 2009 ; Maggi et al., 2009 ; Luchsinger et al., 2011 ; Tuligenga et al., 2014 ; for conflicting results, see Christman et al., 2011 ), (ii) glucose intolerance ( Rizzo et al., 2010 ; Zhong et al., 2012b ), (iii) high peripheral AGE levels ( Yaffe et al., 2011 ), (iv) high levels of inflammatory cytokines ( Marioni et al., 2010 ), and (v) peripheral hyperinsulinemia and insulin resistance ( Bruehl et al., 2010 ; Zhong et al., 2012a ). Even in non-diabetic adults, poorer glucoregulation has been associated with deficits and/or declines in verbal memory, working memory, processing speed, and executive function ( Dahle et al., 2009 ; Bruehl et al., 2010 ; Messier et al., 2010 , 2011 ; Ravona-Springer et al., 2012 ).
The link between cognitive impairment and poor metabolic control may be largely mediated by the structural and functional brain changes that occur in the presence of chronic insulin dysregulation and hyperglycemia. Associations between glucoregulation, hypoperfusion in temporal regions, hippocampal atrophy, and memory impairment have been observed in T2DM ( Gold et al., 2007 ; Last et al., 2007 ), and in non-diabetic adults with decreased peripheral glucose regulation ( Convit et al., 2003 ), or high fasting plasma glucose levels within the normal range ( Cherbuin et al., 2012 ; Kerti et al., 2013 ). In other studies of T2DM, cognitive deficits and structural brain atrophy were linked to cerebral hypoperfusion and altered vascular reactivity ( Last et al., 2007 ; Brundel et al., 2012 ), and disrupted default-mode network connectivity was associated with peripheral hyperinsulinemia, insulin resistance, and white matter integrity ( Musen et al., 2012 ; Hoogenboom et al., 2014 ). Regardless of the underlying cause, brain atrophy in T2DM is associated with poor cognition ( Moran et al., 2013 ), and cognitive declines have been associated with progression of brain atrophy over time ( van Elderen et al., 2010 ; Reijmer et al., 2011 ). Some studies suggest that structural changes may occur early in the course of T2DM; enlarged lateral ventricles, particularly within the frontal horns, have been observed less than a year after diagnosis ( Lee et al., 2013 ), and middle-aged, as well as older adults with T2DM, show reduced prefrontal volumes ( Bruehl et al., 2009 ) and generalized global atrophy ( de Bresser et al., 2010 ; Kamiyama et al., 2010 ; Espeland et al., 2013 ).
Hypertension
The brain is one of the most highly perfused organs. The cerebral hemispheres are supplied by capillary beds connected to the pial vasculature by penetrating arterioles, and the pial vasculature stems from a system of arteries branching off the anterior, middle, and posterior cerebral arteries. Maintenance of brain function depends on a constant blood supply through this network. Hypertension causes changes to the structure and function of these blood vessels, which impacts perfusion in affected areas. Hypoperfusion, for example, can interfere with the delivery of oxygen and nutrients required to meet metabolic demands, and makes hypertension a major risk factor for vascular cognitive impairment, stroke, and dementia.
Cerebrovascular changes
Hypertension places enormous stress on the cerebral circulation (for a comprehensive review, see Pires et al., 2013 ). A hallmark of chronic hypertension is increased vascular resistance, particularly in the small blood vessels that perfuse the brain. Vascular resistance increases as vessel walls thicken. This remodeling is an adaptive response required to maintain chronically increased blood pressure, but it decreases the interior space of the blood vessels (the lumen). Vascular resistance also increases as the number of blood vessels decrease. Rat models of hypertension have shown both of these effects: reductions in lumen diameter and in the number of capillaries making up capillary beds in the cerebral vasculature ( Sokolova et al., 1985 ).
Blood flow is reduced when vascular resistance is high, and chronic hypertension-mediated hypoperfusion has been linked to white matter degradation, gray matter atrophy, and cognitive deficits. Studies of older adults with hypertension show reduced blood flow, particularly in occipito-temporal, prefrontal, and medial temporal lobe regions ( Beason-Held et al., 2007 ), positive correlations between blood pressure and white matter burden ( White et al., 2011 ; Raji et al., 2012 ), and negative correlations between blood pressure and total brain volume ( Nagai et al., 2008 ). Blood vessel function is also impacted by hypertension. Cerebral autoregulation (i.e., the ability to maintain a constant perfusion rate over a range of arterial pressures) is impaired, as is cerebrovascular reactivity, the ability of blood vessels to dilate to accommodate increased blood flow demand ( Last et al., 2007 ; Hajjar et al., 2010 ).
The cognitive profile of older adults with hypertension includes poorer performance on tests of executive function, including verbal fluency, Trails B-A switching score, Stroop interference scores ( Bucur and Madden, 2010 ), slowed processing speed ( Dahle et al., 2009 ), and deficits in attention and memory (see Gifford et al., 2013 for a meta-analysis). Prospective cohort studies show that midlife cardiovascular risk factors like hypertension predict cognitive impairment in later life (e.g., Virta et al., 2013 ), and, similarly, cross-sectional studies show a relation between higher systolic blood pressure and poorer cognitive performance, even within the normotensive range, a relation that is particularly strong in midlife (e.g., Knecht et al., 2008 , 2009 ). Hypertension is associated with decreases in cognitive reserve ( Giordano et al., 2012 ), and older adults with MCI and cardiovascular risk factors like hypertension are twice as likely to develop dementia compared to those without such risk factors ( Johnson et al., 2010 ; Ettorre et al., 2012 ). Moreover, cognitive declines may be faster in those with MCI and hypertension, compared to those without hypertension ( Li et al., 2011 ; Goldstein et al., 2013 ).
The association between hypertension and cognitive decline appears to be strongest in executive and processing speed domains, and weakest in memory and language domains. Hypertension increased the risk of non-amnestic MCI, but not amnestic MCI, regardless of APOEε 4 genotype or hypertensive medication status ( Reitz et al., 2007 ), and predicted progression to dementia in non-amnestic MCI, but not amnestic or multi-domain MCI ( Oveisgharan and Hachinski, 2010 ). The impact of hypertension on executive and processing speed domains is consistent with studies that show a positive relation between hypertension and white matter changes ( Kennedy and Raz, 2009 ; Raz et al., 2012 ), and between white matter changes and deficits in processing speed, executive function, and attention, but not memory (e.g., Debette et al., 2011 ).
Cognitive deficits in hypertensive adults are linked to various indicators of vascular and brain health. There are correlations between white matter integrity and performance on tests of executive function and attention ( Hannesdottir et al., 2009 ), and between decreased flow-mediated dilation and poorer executive function ( Smith et al., 2011 ). Deficits in attention and psychomotor speed in late middle-aged adults with hypertension are associated with reductions in global brain perfusion, reductions that were not fully ameliorated following 6-months of antihypertensive treatment ( Efimova et al., 2008 ). Global cognitive decline has been linked to reduced cerebral blood flow in the face of white matter lesions and lacunar infarcts ( Kitagawa et al., 2009 ), to higher pulse pressure and arterial stiffness ( Scuteri et al., 2007 ; Waldstein et al., 2008 ; Triantafyllidi et al., 2009 ), and to hypertension-mediated deep-brain vascular pathology ( Yakushiji et al., 2012 ). In another large study of patients with MCI, those with hypertension and deep white matter lesions were at higher risk of dementia ( Clerici et al., 2012 ).
Conclusions
Taken together, these studies provide abundant evidence that middle-aged and older adults with T2DM and hypertension, relative to healthy older adults, are more likely to show signs of cognitive dysfunction, widespread structural atrophy, vascular damage, and functional changes. In light of their rising prevalence amongst older adults, there is an increasing likelihood that, without adequate screening at recruitment, individuals with T2DM and/or hypertension will be included in healthy older adult samples. This may introduce unwanted variability and bias to brain and/or cognitive measures, and increase the potential for type 1 and type 2 errors. Given the state of the neuroimaging literature on this topic and the need to advance our understanding, we view T2DM and hypertension as important new frontiers in cognitive neuroscience.
Moving forward, there is an opportunity to develop best practices when it comes to cognitive neuroscience research in older adult populations. Reconciling the vascular risk component in T2DM and hypertension may be the most tractable option since there are myriad approaches one can take to do this. The most rigorous approach in this respect may be inclusion of a breath-hold task, or a measure of cerebral blood flow (e.g., ASL) in the functional imaging protocol, as this allows for a direct estimate of each subject's vascular health. Breath-hold tasks can be used to index cerebrovascular reactivity in response to non-neuronal signals. The breath-hold period induces hypercapnia, which stimulates vasodilation and increases blood flow and blood volume in the brain, a signal change that occurs independently of neuronal activation. ASL or resting-state PET scans provide a direct measure of blood flow, and can be used to account for individual differences in perfusion. As noted above, these methods have already been used in some studies of cognitive aging to account for individual differences in cerebrovascular health. Whether other means of equating vascular risk across participants or across groups (e.g., screening participants for excessive white matter hyperintensities, post-hoc comparison of outcome measures or study groups on vascular risk factors, or statistical analyses aimed at controlling for the effects of vascular variability in the reported results) are similarly effective requires further study.
It may also be important for investigators to acknowledge a distinction between “healthy” and “typical” brain aging. Studies characterizing healthy aging should adopt T2DM and hypertension as exclusion criteria. Conversely, given the high prevalence of T2DM and hypertension in older adults, community- or population-based studies characterizing the typical trajectory of cognitive aging would benefit by including these participants to maximize the generalizability of results, and reconciling the heterogeneity through study design groups (e.g., stratifying based on diagnosis of T2DM and hypertension) or covariates in their analysis.
As the proportion of older adults living with T2DM and hypertension increase, it is imperative that functional imaging studies are designed to account for these population trends. The current state of the cognitive aging neuroimaging literature suggests that there is limited appreciation and/or awareness that T2DM and hypertension are significant medical illnesses that disrupt brain vasculature, brain structure, and brain function. By adopting best practices that take T2DM and hypertension into account, we can advance our understanding of these conditions, and of cognitive aging in general.
Author Contributions
Liesel-Ann C. Meusel selecting, indexing, and reviewing articles, writing of drafts; Nisha Kansal selecting articles, editing of drafts; Ekaterina Tchistiakova contributing to the first draft, editing of drafts; William Yuen selecting articles, contributing to the first draft, editing of drafts; Bradley J. MacIntosh provided conceptual foundation for paper, editing of drafts; Carol E. Greenwood provided conceptual foundation for paper, editing of drafts; Nicole D. Anderson provided conceptual foundation for paper, editing of drafts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported in part by postdoctoral fellowships from the Centre for Stroke Recovery and the Alzheimer Society of Canada awarded to Liesel-Ann C. Meusel, and grant funds from CIHR (MOP111244).
Akter, K., Lanza, E. A., Martin, S. A., Myronyuk, N., Rua, M., and Raffa, R. B. (2011). Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br. J. Clin. Pharmacol . 71, 365–376. doi: 10.1111/j.1365-2125.2010.03830.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
* 5,6,a Anderson, K. E., Lynch, K., Zarahn, E., Scarmeas, N., Van Heertum, R., Sackeim, H. et al. (2005). H215O PET study of impairment of nonverbal recognition with normal aging. J. Neuropsychiatry Clin. Neurosci . 17, 192–200. doi: 10.1176/appi.neuropsych.17.2.192
* 7,8,d Anguera, J. A., Reuter-Lorenz, P. A., Willingham, D. T., and Seidler, R. D. (2011). Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J. Cogn. Neurosci . 23, 11–25. doi: 10.1162/jocn.2010.21451
* 5,6,a Ansado, J., Monchi, O., Ennabil, N., Faure, S., and Joanette, Y. (2012). Load-dependent posterior-anterior shift in aging in complex visual selective attention situations. Brain Res . 1454, 14–22. doi: 10.1016/j.brainres.2012.02.061
* 5,6,a,□ Antonova, E., Parslow, D., Brammer, M., Dawson, G. R., Jackson, S. H. D., and Morris, R. G. (2009). Age-related neural activity during allocentric spatial memory. Memory 17, 125–143. doi: 10.1080/09658210802077348
Arvanitakis, Z., Wilson, R. S., Bienias, J. L., Evans, D. A., and Bennett, D. A. (2004). Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol . 61, 661–666. doi: 10.1001/archneur.61.5.661
* 5,6,b Bäckman, L., Karlsson, S., Fischer, H., Karlsson, P., Brehmer, Y., Rieckmann, A. et al. (2011). Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol. Aging 32, 1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018
* 5,6,a Bagurdes, L. A., Mesulam, M. M., Gitelman, D. R., Weintraub, S., and Small, D. M. (2008). Modulation of the spatial attention network by incentives in healthy aging and mild cognitive impairment. Neuropsychologia 46, 2943–2948. doi: 10.1016/j.neuropsychologia.2008.06.005
* 5,6,c Bai, F., Liao, W., Watson, D. R., Shi, Y., Wang, Y., Yue, C. et al. (2011). Abnormal whole-brain functional connection in amnestic mild cognitive impairment patients. Behav. Brain Res . 216, 666–672. doi: 10.1016/j.bbr.2010.09.010
* 5,6,a Bai, F., Zhang, Z., Yu, H., Shi, Y., Yuan, Y., Zhu, W. et al. (2008). Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci. Lett . 438, 111–115. doi: 10.1016/j.neulet.2008.04.021
Baker, L. D., Cross, D. J., Minoshima, S., Belongia, D., Watson, G. S., and Craft, S. (2011). Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol . 68, 51–57. doi: 10.1001/archneurol.2010.225
* 5,6,c,▴ Bangen, K. J., Kaup, A. R., Mirzakhanian, H., Wierenga, C. E., Jeste, D. V., and Eyler, L. T. (2012). Compensatory brain activity during encoding among older adults with better recognition memory for face-name pairs: an integrative functional, structural, and perfusion imaging study. J. Int. Neuropsychol. Soc . 18, 402–413. doi: 10.1017/S1355617712000197
* 9,12,b,▴ Bangen, K. J., Restom, K., Liu, T. T., Jak, A. J., Wierenga, C. E., Salmon, D. P. et al. (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol. Aging 30, 1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012
* 7,8,a,▾ Beason-Held, L. L., Kraut, M. A., and Resnick, S. M. (2008). I. Longitudinal changes in aging brain function. Neurobiol. Aging 29, 483–496. doi: 10.1016/j.neurobiolaging.2006.10.031
Beason-Held, L. L., Moghekar, A., Zonderman, A. B., Kraut, M. A., and Resnick, S. M. (2007). Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 38, 1766–1773. doi: 10.1161/STROKEAHA.106.477109
* 9,12,c,+ Beeri, M. S., Lee, H., Cheng, H., Wollman, D., Silverman, J. M., and Prohovnik, I. (2011). Memory activation in healthy nonagenarians. Neurobiol. Aging 32, 515–523. doi: 10.1016/j.neurobiolaging.2009.02.022
* 1,2,a Berlingeri, M., Bottini, G., Danelli, L., Ferri, F., Traficante, D., Sacheli, L. et al. (2010). With time on our side? Task-dependent compensatory processes in graceful aging. Exp. Brain Res . 205, 307–324. doi: 10.1007/s00221-010-2363-7
* 4,a Bernard, F. A., Desgranges, B., Eustache, F., and Baron, J.-C. (2007). Neural correlates of age-related verbal episodic memory decline: a PET study with combined subtraction/correlation analysis. Neurobiol. Aging 28, 1568–1576. doi: 10.1016/j.neurobiolaging.2006.07.004
* 5,6,a Bollinger, J., Rubens, M. T., Masangkay, E., Kalkstein, J., and Gazzaley, A. (2011). An expectation-based memory deficit in aging. Neuropsychologia 49, 1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021
Brands, A. M. A., Van den Berg, E., Manschot, S. M., Biessels, G. J., Kappelle, L. J., De Haan, E. H. F. et al. (2007). A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. J. Int. Neuropsychol. Soc . 13, 288–297. doi: 10.1017/S1355617707070312
* 2,5,a Braskie, M. N., Landau, S. M., Wilcox, C. E., Taylor, S. D., O'Neil, J. P., Baker, S. L. et al. (2011). Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum. Brain Mapp . 32, 947–961. doi: 10.1002/hbm.21081
* 5,6,a Braskie, M. N., Small, G. W., and Bookheimer, S. Y. (2009). Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Hum. Brain Mapp . 30, 3981–3992. doi: 10.1002/hbm.20823
* 1,10,b,❖ Braskie, M. N., Small, G. W., and Bookheimer, S. Y. (2010). Vascular health risks and fMRI activation during a memory task in older adults. Neurobiol. Aging 31, 1532–1542. doi: 10.1016/j.neurobiolaging.2008.08.016
Brown, C. M., Marthol, H., Zikeli, U., Ziegler, D., and Hilz, M. J. (2008). A simple deep breathing test reveals altered cerebral autoregulation in type 2 diabetic patients. Diabetologia 51, 756–761. doi: 10.1007/s00125-008-0958-3
Brownlee, M. (2005). The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625. doi: 10.2337/diabetes.54.6.1615
Bruehl, H., Sweat, V., Hassenstab, J., Polyakov, V., and Convit, A. (2010). Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J. Clin. Exp. Neuropsychol . 32, 487–493. doi: 10.1080/13803390903224928
Bruehl, H., Wolf, O. T., Sweat, V., Tirsi, A., Richardson, S., and Convit, A. (2009). Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res . 1280, 186–194. doi: 10.1016/j.brainres.2009.05.032
Brundel, M., van den Berg, E., Reijmer, Y. D., de Bresser, J., Kappelle, L. J., and Biessels, G. J. (2012). Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J. Diabetes Complicat . 26, 205–209. doi: 10.1016/j.jdiacomp.2012.03.021
Bucur, B., and Madden, D. J. (2010). Effects of adult age and blood pressure on executive function and speed of processing. Exp. Aging Res . 36, 153–168. doi: 10.1080/03610731003613482
* 7,9,a Burgmans, S., van Boxtel, M. P. J., Vuurman, E. F. P. M., Evers, E. A. T., and Jolles, J. (2010). Increased neural activation during picture encoding and retrieval in 60-year-olds compared to 20-year-olds. Neuropsychologia 48, 2188–2197. doi: 10.1016/j.neuropsychologia.2010.04.011
* 2,5,a Cabeza, R., Anderson, N. D., Locantore, J. K., and McIntosh, A. R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402. doi: 10.1006/nimg.2002.1280
* 2,5,d Cabeza, R., Daselaar, S. M., Dolcos, F., Prince, S. E., Budde, M., and Nyberg, L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex 14, 364–375. doi: 10.1093/cercor/bhg133
* 2,5,a Cabeza, R., Grady, C. L., Nyberg, L., McIntosh, A. R., Tulving, E., Kapur, S. et al. (1997). Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J. Neurosci . 17, 391–400.
Pubmed Abstract | Pubmed Full Text
* 7,8,a Campbell, K. L., Grady, C. L., Ng, C., and Hasher, L. (2012). Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia 50, 2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025
* 7,8,a Cappell, K. A., Gmeindl, L., and Reuter-Lorenz, P. A. (2010). Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473. doi: 10.1016/j.cortex.2009.11.009
* 7,8,a Carlson, M. C., Erickson, K. I., Kramer, A. F., Voss, M. W., Bolea, N., Mielke, M. et al. (2009). Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J. Gerontol. A Biol. Sci. Med. Sci . 64, 1275–1282. doi: 10.1093/gerona/glp117
Carlsson, C. M. (2010). Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J. Alzheimers. Dis . 20, 711–722. doi: 10.3233/JAD-2010-100012
* 7,8,a Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L. et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci . 26, 10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006
* 2,5,a Chen, N., Chou, Y., Song, A. W., and Madden, D. J. (2009). Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct. Funct . 213, 571–585. doi: 10.1007/s00429-009-0218-4
Cherbuin, N., Sachdev, P., and Anstey, K. J. (2012). Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH Study. Neurology 79, 1019–1026. doi: 10.1212/WNL.0b013e31826846de
Christman, A. L., Matsushita, K., Gottesman, R. F., Mosley, T., Alonso, A., Coresh, J. et al. (2011). Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 54, 1645–1652. doi: 10.1007/s00125-011-2095-7
* 5,6,a Chua, E. F., Schacter, D. L., and Sperling, R. A. (2009). Neural basis for recognition confidence in younger and older adults. Psychol. Aging 24, 139–153. doi: 10.1037/a0014029
* 5,6,b Clément, F., and Belleville, S. (2009). Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum. Brain Mapp . 30, 4033–4047. doi: 10.1002/hbm.20827
Clerici, F., Caracciolo, B., Cova, I., Fusari Imperatori, S., Maggiore, L., Galimberti, D. et al. (2012). Does vascular burden contribute to the progression of mild cognitive impairment to dementia? Dement. Geriatr. Cogn. Disord . 34, 235–243. doi: 10.1159/000343776
Colosia, A. D., Palencia, R., and Khan, S. (2013). Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab. Syndr. Obes . 6, 327–338. doi: 10.2147/DMSO.S51325
Convit, A. (2005). Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol. Aging 26(Suppl. 1), 31–35. doi: 10.1016/j.neurobiolaging.2005.09.018
Convit, A., Wolf, O. T., Tarshish, C., and de Leon, M. J. (2003). Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. U.S.A . 100, 2019–2022. doi: 10.1073/pnas.0336073100
* 7,8,b Cook, I. A., Bookheimer, S. Y., Mickes, L., Leuchter, A. F., and Kumar, A. (2007). Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int. J. Geriatr. Psychiatry 22, 332–342. doi: 10.1002/gps.1678
Craft, S. (2006). Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord . 20, 298–301. doi: 10.1097/01.wad.0000213866.86934.7e
Crane, P. K., Walker, R., Hubbard, R. A., Li, G., Nathan, D. M., Zheng, H. et al. (2013). Glucose levels and risk of dementia. N. Engl. J. Med . 369, 540–548. doi: 10.1056/NEJMoa1215740
Creavin, S. T., Gallacher, J., Bayer, A., Fish, M., Ebrahim, S., and Ben-Shlomo, Y. (2012). Metabolic syndrome, diabetes, poor cognition, and dementia in the Caerphilly prospective study. J. Alzheimers Dis . 28, 931–939. doi: 10.3233/JAD-2011-111550
Cui, Y., Jiao, Y., Chen, Y.-C., Wang, K., Gao, B., Wen, S. et al. (2014). Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63, 749–760. doi: 10.2337/db13-0519
Cukierman-Yaffe, T., Gerstein, H. C., Williamson, J. D., Lazar, R. M., Lovato, L., Miller, M. E. et al. (2009). Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32, 221–226. doi: 10.2337/dc08-1153
Dahle, C. L., Jacobs, B. S., and Raz, N. (2009). Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol. Aging 24, 154–162. doi: 10.1037/a0014283
Danaei, G., Finucane, M. M., Lu, Y., Singh, G. M., Cowan, M. J., Paciorek, C. J. et al. (2011). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378, 31–40. doi: 10.1016/S0140-6736(11)60679-X
* 2,5,d Daselaar, S. M., Fleck, M. S., Dobbins, I. G., Madden, D. J., and Cabeza, R. (2006). Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex 16, 1771–1782. doi: 10.1093/cercor/bhj112
* 7,8,d Daselaar, S. M., Veltman, D. J., Rombouts, S. A. R. B., Raaijmakers, J. G. W., and Jonker, C. (2003). Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126, 43–56. doi: 10.1093/brain/awg005
* 2,5,a Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., and Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. doi: 10.1093/cercor/bhm155
Debette, S., Seshadri, S., Beiser, A., Au, R., Himali, J. J., Palumbo, C. et al. (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. doi: 10.1212/WNL.0b013e318227b227
de Bresser, J., Tiehuis, A. M., van den Berg, E., Reijmer, Y. D., Jongen, C., Kappelle, L. J. et al. (2010). Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 33, 1309–1314. doi: 10.2337/dc09-1923
* 7,9,a de Chastelaine, M., Wang, T. H., Minton, B., Muftuler, L. T., and Rugg, M. D. (2011). The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb. Cortex 21, 2166–2176. doi: 10.1093/cercor/bhq294
de la Monte, S. M., and Wands, J. R. (2008). Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol . 2, 1101–1113. doi: 10.1177/193229680800200619
* 5,6,a Dennis, N. A., and Cabeza, R. (2011). Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol. Aging 32, 2318.e17–30. doi: 10.1016/j.neurobiolaging.2010.04.004
* 5,6,a Dennis, N. A., Daselaar, S., and Cabeza, R. (2007a). Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging 28, 1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006
* 5,6,a Dennis, N. A., Hayes, S. M., Prince, S. E., Madden, D. J., Huettel, S. A., and Cabeza, R. (2008a). Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn . 34, 791–808. doi: 10.1037/0278-7393.34.4.791
* 5,6,a Dennis, N. A., Kim, H., and Cabeza, R. (2008b). Age-related differences in brain activity during true and false memory retrieval. J. Cogn. Neurosci . 20, 1390–1402. doi: 10.1162/jocn.2008.20096
* 5,6,a Dennis, N. A., Kim, H., and Cabeza, R. (2007b). Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia 45, 3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003.
D'Esposito, M., Deouell, L. Y., and Gazzaley, A. (2003). Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci . 4, 863–872. doi: 10.1038/nrn1246
D'Esposito, M., Zarahn, E., Aguirre, G. K., and Rypma, B. (1999). The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10, 6–14. doi: 10.1006/nimg.1999.0444
* 5,6,b,• Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M. et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi: 10.1212/01.wnl.0000171450.97464.49
* 5,6,a,× DiGirolamo, G. J., Kramer, A. F., Barad, V., Cepeda, N. J., Weissman, D. H., Milham, M. P. et al. (2001). General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport 12, 2065–2071. doi: 10.1097/00001756-200107030-00054
* 1,6,b,■ Donix, M., Poettrich, K., Weiss, P. H., Werner, A., von Kummer, R., Fink, G. R. et al. (2010). Age-dependent differences in the neural mechanisms supporting long-term declarative memories. Arch. Clin. Neuropsychol . 25, 383–395. doi: 10.1093/arclin/acq037
* 5,6,a Drobyshevsky, A., Baumann, S. B., and Schneider, W. (2006). A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 31, 732–744. doi: 10.1016/j.neuroimage.2005.12.016
Du, X. L., Edelstein, D., Rossetti, L., Fantus, I. G., Goldberg, H., Ziyadeh, F. et al. (2000). Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U.S.A . 97, 12222–12226. doi: 10.1073/pnas.97.22.12222
* 1,2,a,■ Duarte, A., Graham, K. S., and Henson, R. N. (2010). Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol. Aging 31, 1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014
* 1,2,a,■ Duarte, A., Henson, R. N., and Graham, K. S. (2008). The effects of aging on the neural correlates of subjective and objective recollection. Cereb. Cortex 18, 2169–2180. doi: 10.1093/cercor/bhm243
* 3,5,a Dulas, M. R., and Duarte, A. (2011). The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage 57, 1192–1204. doi: 10.1016/j.neuroimage.2011.05.036
* 3,5,a Dulas, M. R., and Duarte, A. (2012). The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cereb. Cortex 22, 37–50. doi: 10.1093/cercor/bhr056
* 7,9,a Duverne, S., Motamedinia, S., and Rugg, M. D. (2009). The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb. Cortex 19, 733–744. doi: 10.1093/cercor/bhn122
Efimova, I. Y., Efimova, N. Y., Triss, S. V., and Lishmanov, Y. B. (2008). Brain perfusion and cognitive function changes in hypertensive patients. Hypertens. Res . 31, 673–678. doi: 10.1291/hypres.31.673
* 1,2,a Emery, L., Heaven, T. J., Paxton, J. L., and Braver, T. S. (2008). Age-related changes in neural activity during performance matched working memory manipulation. Neuroimage 42, 1577–1586. doi: 10.1016/j.neuroimage.2008.06.021
* 7,8,a Erickson, K. I., Colcombe, S. J., Wadhwa, R., Bherer, L., Peterson, M. S., Scalf, P. E. et al. (2007). Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol. Aging 28, 272–283. doi: 10.1016/j.neurobiolaging.2005.12.012
Espeland, M. A., Bryan, R. N., Goveas, J. S., Robinson, J. G., Siddiqui, M. S., Liu, S. et al. (2013). Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women's Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 36, 90–97. doi: 10.2337/dc12-0555
Espeland, M. A., Miller, M. E., Goveas, J. S., Hogan, P. E., Coker, L. H., Williamson, J. et al. (2011). Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women's health initiative study of cognitive aging. J. Womens. Health (Larchmt) . 20, 1435–1443. doi: 10.1089/jwh.2011.2812
Ettorre, E., Cerra, E., Marigliano, B., Vigliotta, M., Vulcano, A., Fossati, C. et al. (2012). Role of cardiovascular risk factors (CRF) in the patients with mild cognitive impairment (MCI). Arch. Gerontol. Geriatr . 54, 330–332. doi: 10.1016/j.archger.2011.04.025
* 5,6,a Fakhri, M., Sikaroodi, H., Maleki, F., Ali Oghabian, M., and Ghanaati, H. (2012). Age-related frontal hyperactivation observed across different working memory tasks: an fMRI study. Behav. Neurol . 25, 351–361. doi: 10.3233/BEN-2012-120280
* 4,a,• Fera, F., Weickert, T. W., Goldberg, T. E., Tessitore, A., Hariri, A., Das, S. et al. (2005). Neural mechanisms underlying probabilistic category learning in normal aging. J. Neurosci . 25, 11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005
* 7,9,a Fernandes, M. A., Pacurar, A., Moscovitch, M., and Grady, C. (2006). Neural correlates of auditory recognition under full and divided attention in younger and older adults. Neuropsychologia 44, 2452–2464. doi: 10.1016/j.neuropsychologia.2006.04.020
* 2,11,a,■,▴ Filippini, N., Ebmeier, K. P., MacIntosh, B. J., Trachtenberg, A. J., Frisoni, G. B., Wilcock, G. K. et al. (2011). Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 54, 602–610. doi: 10.1016/j.neuroimage.2010.08.009
* 2,12,a,■,• Filippini, N., Nickerson, L. D., Beckmann, C. F., Ebmeier, K. P., Frisoni, G. B., Matthews, P. M. et al. (2012). Age-related adaptations of brain function during a memory task are also present at rest. Neuroimage 59, 3821–3828. doi: 10.1016/j.neuroimage.2011.11.063
Fischer, A. L., de Frias, C. M., Yeung, S. E., and Dixon, R. A. (2009). Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. J. Clin. Exp. Neuropsychol . 31, 809–822. doi: 10.1080/13803390802537636
Fontbonne, A., Berr, C., Ducimetière, P., and Alpérovitch, A. (2001). Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 24, 366–370. doi: 10.2337/diacare.24.2.366
* 3,5,a Gandini, D., Lemaire, P., Anton, J.-L., and Nazarian, B. (2008). Neural correlates of approximate quantification strategies in young and older adults: an fMRI study. Brain Res . 1246, 144–157. doi: 10.1016/j.brainres.2008.09.096
* 5,6,d,■ Garrett, D. D., Kovacevic, N., McIntosh, A. R., and Grady, C. L. (2011). The importance of being variable. J. Neurosci . 31, 4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011
* 3,5,a,□ Gazzaley, A., Cooney, J. W., Rissman, J., and D'Esposito, M. (2005). Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci . 8, 1298–1300. doi: 10.1038/nn1543
Giacco, F., and Brownlee, M. (2010). Oxidative stress and diabetic complications. Circ. Res . 107, 1058–1070. doi: 10.1161/CIRCRESAHA.110.223545
Gifford, K. A., Badaracco, M., Liu, D., Tripodis, Y., Gentile, A., Lu, Z. et al. (2013). Blood pressure and cognition among older adults: a meta-analysis. Arch. Clin. Neuropsychol . 28, 649–664. doi: 10.1093/arclin/act046
* 7,8,a,■ Gigi, A., Babai, R., Penker, A., Hendler, T., and Korczyn, A. D. (2010). Prefrontal compensatory mechanism may enable normal semantic memory performance in mild cognitive impairment (MCI). J. Neuroimaging 20, 163–168. doi: 10.1111/j.1552-6569.2009.00386.x
Giordano, N., Tikhonoff, V., Palatini, P., Bascelli, A., Boschetti, G., De Lazzari, F. et al. (2012). Cognitive functions and cognitive reserve in relation to blood pressure components in a population-based cohort aged 53 to 94 years. Int. J. Hypertens . 2012:274851. doi: 10.1155/2012/274851
* 5,6,a,+ Giovanello, K. S., De Brigard, F., Hennessey Ford, J., Kaufer, D. I., Burke, J. R., Browndyke, J. N. et al. (2012). Event-related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc . 18, 886–897. doi: 10.1017/S1355617712000689
* 5,6,a Giovanello, K. S., Kensinger, E. A., Wong, A. T., and Schacter, D. L. (2010). Age-related neural changes during memory conjunction errors. J. Cogn. Neurosci . 22, 1348–1361. doi: 10.1162/jocn.2009.21274
Gold, S. M., Dziobek, I., Sweat, V., Tirsi, A., Rogers, K., Bruehl, H. et al. (2007). Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50, 711–719. doi: 10.1007/s00125-007-0602-7
* 5,6,b Gold, B. T., Jiang, Y., Jicha, G. A., and Smith, C. D. (2010a). Functional response in ventral temporal cortex differentiates mild cognitive impairment from normal aging. Hum. Brain Mapp . 31, 1249–1259. doi: 10.1002/hbm.20932
* 2,7,d,■ Gold, B. T., Powell, D. K., Xuan, L., Jicha, G. A., and Smith, C. D. (2010b). Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol. Aging 31, 512–522. doi: 10.1016/j.neurobiolaging.2008.04.005
Goldstein, F. C., Levey, A. I., and Steenland, N. K. (2013). High blood pressure and cognitive decline in mild cognitive impairment. J. Am. Geriatr. Soc . 61, 67–73. doi: 10.1111/jgs.12067
Gorelick, P. B., Scuteri, A., Black, S. E., DeCarli, C., Greenberg, S. M., Iadecola, C. et al. (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. doi: 10.1161/STR.0b013e3182299496
* 4, a,• Gould, R. L., Brown, R. G., Owen, A. M., Ffytche, D. H., and Howard, R. J. (2003). fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 20, 1006–1019. doi: 10.1016/S1053-8119(03)00365-3
* 1,9,a Grady, C. L., Grigg, O., and Ng, C. (2012). Age differences in default and reward networks during processing of personally relevant information. Neuropsychologia 50, 1682–1697. doi: 10.1016/j.neuropsychologia.2012.03.024
* 5,6,d,■,• Grady, C. L., Protzner, A. B., Kovacevic, N., Strother, S. C., Afshin-Pour, B., Wojtowicz, M. et al. (2010). A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex 20, 1432–1447. doi: 10.1093/cercor/bhp207
* 7,9,a,■,• Grady, C. L., Springer, M. V., Hongwanishkul, D., McIntosh, A. R., and Winocur, G. (2006). Age-related changes in brain activity across the adult lifespan. J. Cogn. Neurosci . 18, 227–241. doi: 10.1162/089892906775783705
Granic, I., Dolga, A. M., Nijholt, I. M., van Dijk, G., and Eisel, U. L. M. (2009). Inflammation and NF-kappaB in Alzheimer's disease and diabetes. J. Alzheimers Dis . 16, 809–821. doi: 10.3233/JAD-2009-0976
Gregg, E. W., Yaffe, K., Cauley, J. A., Rolka, D. B., Blackwell, T. L., Narayan, K. M. et al. (2000). Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch. Intern. Med . 160, 174–180. doi: 10.1001/archinte.160.2.174
* 5,6,c Grön, G., Bittner, D., Schmitz, B., Wunderlich, A. P., Tomczak, R., and Riepe, M. W. (2003). Variability in memory performance in aged healthy individuals: an fMRI study. Neurobiol. Aging 24, 453–462. doi: 10.1016/S0197-4580(02)00128-8
* 6,7,a,□ Grossman, M., Cooke, A., DeVita, C., Alsop, D., Detre, J., Chen, W. et al. (2002). Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage 15, 302–317. doi: 10.1006/nimg.2001.0971
Hajjar, I., Zhao, P., Alsop, D., and Novak, V. (2010). Hypertension and cerebral vasoreactivity: a continuous arterial spin labeling magnetic resonance imaging study. Hypertension 56, 859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002
* 5,6,a Hampstead, B. M., Stringer, A. Y., Stilla, R. F., Amaraneni, A., and Sathian, K. (2011). Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 49, 2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008
* 5,6,a Hampstead, B. M., Stringer, A. Y., Stilla, R. F., Giddens, M., and Sathian, K. (2012). Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus 22, 1652–1658. doi: 10.1002/hipo.22006
* 7,8,a Han, S. D., Arfanakis, K., Fleischman, D. A., Leurgans, S. E., Tuminello, E. R., Edmonds, E. C. et al. (2012a). Functional connectivity variations in mild cognitive impairment: associations with cognitive function. J. Int. Neuropsychol. Soc . 18, 39–48. doi: 10.1017/S1355617711001299
* 4, b Han, Y., Lui, S., Kuang, W., Lang, Q., Zou, L., and Jia, J. (2012b). Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS ONE 7:e28664. doi: 10.1371/journal.pone.0028664
Hannesdottir, K., Nitkunan, A., Charlton, R. A., Barrick, T. R., MacGregor, G. A., and Markus, H. S. (2009). Cognitive impairment and white matter damage in hypertension: a pilot study. Acta Neurol. Scand . 119, 261–268. doi: 10.1111/j.1600-0404.2008.01098.x
* 7,8,a Hartley, A. A., Jonides, J., and Sylvester, C.-Y. C. (2011). Dual-task processing in younger and older adults: similarities and differences revealed by fMRI. Brain Cogn . 75, 281–291. doi: 10.1016/j.bandc.2011.01.004
* 3,7,a,• Hedden, T., Van Dijk, K. R. A., Shire, E. H., Sperling, R. A., Johnson, K. A., and Buckner, R. L. (2012). Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb. Cortex 22, 1038–1051. doi: 10.1093/cercor/bhr172
Heni, M., Schöpfer, P., Peter, A., Sartorius, T., Fritsche, A., Synofzik, M. et al. (2013). Evidence for altered transport of insulin across the blood–brain barrier in insulin-resistant humans. Acta Diabetol . doi: 10.1007/s00592-013-0546-y. [Epub ahead of print].
* 5,9,b Holtzer, R., Rakitin, B. C., Steffener, J., Flynn, J., Kumar, A., and Stern, Y. (2009). Age effects on load-dependent brain activations in working memory for novel material. Brain Res . 1249, 148–161. doi: 10.1016/j.brainres.2008.10.009
Hoogenboom, W. S., Marder, T. J., Flores, V. L., Huisman, S., Eaton, H. P., Schneiderman, J. S. et al. (2014). Cerebral white matter integrity and resting-state functional connectivity in middle-aged patients with type 2 diabetes. Diabetes 63, 728–738. doi: 10.2337/db13-1219
* 5,6,a Hosseini, S. M. H., Rostami, M., Yomogida, Y., Takahashi, M., Tsukiura, T., and Kawashima, R. (2010). Aging and decision making under uncertainty: behavioral and neural evidence for the preservation of decision making in the absence of learning in old age. Neuroimage 52, 1514–1520. doi: 10.1016/j.neuroimage.2010.05.008
* 5,6,a Huang, C.-M., Polk, T. A., Goh, J. O., and Park, D. C. (2012). Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia 50, 55–66. doi: 10.1016/j.neuropsychologia.2011.10.022
* 5,6,a Hubert, V., Beaunieux, H., Chételat, G., Platel, H., Landeau, B., Viader, F. et al. (2009). Age-related changes in the cerebral substrates of cognitive procedural learning. Hum. Brain Mapp . 30, 1374–1386. doi: 10.1002/hbm.20605
* 4, c, • Iidaka, T., Sadato, N., Yamada, H., Murata, T., Omori, M., and Yonekura, Y. (2001). An fMRI study of the functional neuroanatomy of picture encoding in younger and older adults. Brain Res. Cogn. Brain Res . 11, 1–11. doi: 10.1016/S0926-6410(00)00058-6
* 1,2,b,♦ Jennings, J. R., van der Veen, F. M., and Meltzer, C. C. (2006). Verbal and spatial working memory in older individuals: a positron emission tomography study. Brain Res . 1092, 177–189. doi: 10.1016/j.brainres.2006.03.077
* 1,2,a Jimura, K., and Braver, T. S. (2010). Age-related shifts in brain activity dynamics during task switching. Cereb. Cortex 20, 1420–1431. doi: 10.1093/cercor/bhp206
* 5,6,a Jin, M., Pelak, V. S., and Cordes, D. (2012). Aberrant default mode network in subjects with amnestic mild cognitive impairment using resting-state functional MRI. Magn. Reson. Imaging 30, 48–61. doi: 10.1016/j.mri.2011.07.007
Johnson, E. L. (2012). Glycemic variability in type 2 diabetes mellitus: oxidative stress and macrovascular complications. Adv. Exp. Med. Biol . 771, 139–154.
* 5,6,a Johnson, M. K., Mitchell, K. J., Raye, C. L., and Greene, E. J. (2004). An age-related deficit in prefrontal cortical function associated with refreshing information. Psychol. Sci . 15, 127–132. doi: 10.1111/j.0963-7214.2004.01502009.x
Johnson, J. K., Pa, J., Boxer, A. L., Kramer, J. H., Freeman, K., and Yaffe, K. (2010). Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dement. Geriatr. Cogn. Disord . 30, 344–351. doi: 10.1159/000318836
* 9,11,a,■ Johnson, S. C., Schmitz, T. W., Asthana, S., Gluck, M. A., and Myers, C. (2008). Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn . 15, 129–145. doi: 10.1080/13825580601139444
* 10,12,c Jones, D. T., Machulda, M. M., Vemuri, P., McDade, E. M., Zeng, G., Senjem, M. L. et al. (2011). Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–1531. doi: 10.1212/WNL.0b013e318233b33d
Kaiser, N., Sasson, S., Feener, E. P., Boukobza-Vardi, N., Higashi, S., Moller, D. E. et al. (1993). Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42, 80–89. doi: 10.2337/diab.42.1.80
Kalani, M. (2008). The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc. Health Risk Manag . 4, 1061–1068. doi: 10.2147/VHRM.S3920
* 5,6,a Kalkstein, J., Checksfield, K., Bollinger, J., and Gazzaley, A. (2011). Diminished top-down control underlies a visual imagery deficit in normal aging. J. Neurosci . 31, 15768–15774. doi: 10.1523/JNEUROSCI.3209-11.2011
Kamiyama, K., Wada, A., Sugihara, M., Kurioka, S., Hayashi, K., Hayashi, T. et al. (2010). Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel-based morphometry VSRAD study. Jpn. J. Radiol . 28, 266–272. doi: 10.1007/s11604-009-0416-2
Kanaya, A. M., Barrett-Connor, E., Gildengorin, G., and Yaffe, K. (2004). Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch. Intern. Med . 164, 1327–1333. doi: 10.1001/archinte.164.12.1327
* 5,6,a,° Kannurpatti, S. S., Motes, M. A., Rypma, B., and Biswal, B. B. (2010). Neural and vascular variability and the fMRI-BOLD response in normal aging. Magn. Reson. Imaging 28, 466–476. doi: 10.1016/j.mri.2009.12.007
* 5,6,a,° Kannurpatti, S. S., Motes, M. A., Rypma, B., and Biswal, B. B. (2011). Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum. Brain Mapp . 32, 1125–1140. doi: 10.1002/hbm.21097
* 5,6,a Kaufmann, L., Ischebeck, A., Weiss, E., Koppelstaetter, F., Siedentopf, C., Vogel, S. E. et al. (2008). An fMRI study of the numerical Stroop task in individuals with and without minimal cognitive impairment. Cortex 44, 1248–1255. doi: 10.1016/j.cortex.2007.11.009
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., and He, J. (2005). Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223. doi: 10.1016/S0140-6736(05)17741-1
Kennedy, K. M., and Raz, N. (2009). Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res . 1297, 41–56. doi: 10.1016/j.brainres.2009.08.058
* 7,8,a Kennedy, K. M., Rodrigue, K. M., Devous, M. D. Sr., Hebrank, A. C., Bischof, G. N., and Park, D. C. (2012). Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage 62, 1–8. doi: 10.1016/j.neuroimage.2012.03.077
Kerti, L., Witte, A. V., Winkler, A., Grittner, U., Rujescu, D., and Floel, A. (2013). Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81, 1746–1752. doi: 10.1212/01.wnl.0000435561.00234.ee
* 9,11,a,■,× Kikuchi, M., Hirosawa, T., Yokokura, M., Yagi, S., Mori, N., Yoshikawa, E. et al. (2011). Effects of brain amyloid deposition and reduced glucose metabolism on the default mode of brain function in normal aging. J. Neurosci . 31, 11193–11199. doi: 10.1523/JNEUROSCI.2535-11.2011
Kim, E., Cho, M. H., Cha, K. R., Park, J. S., Ahn, C.-W., Oh, B. H. et al. (2008). Interactive effect of central obesity and hypertension on cognitive function in older out-patients with Type 2 diabetes. Diabet. Med . 25, 1440–1446. doi: 10.1111/j.1464-5491.2008.02612.x
* 6,7,a Kim, S.-Y., and Giovanello, K. S. (2011). The effects of attention on age-related relational memory deficits: fMRI evidence from a novel attentional manipulation. J. Cogn. Neurosci . 23, 3637–3656. doi: 10.1162/jocn_a_00058
* 4,b Kircher, T., Weis, S., Leube, D., Freymann, K., Erb, M., Jessen, F. et al. (2008). Anterior hippocampus orchestrates successful encoding and retrieval of non-relational memory: an event-related fMRI study. Eur. Arch. Psychiatry Clin. Neurosci . 258, 363–372. doi: 10.1007/s00406-008-0805-z
* 1,9,a Kirchhoff, B. A., Anderson, B. A., Barch, D. M., and Jacoby, L. L. (2012). Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb. Cortex 22, 788–799. doi: 10.1093/cercor/bhr129
Kitagawa, K., Oku, N., Kimura, Y., Yagita, Y., Sakaguchi, M., Hatazawa, J. et al. (2009). Relationship between cerebral blood flow and later cognitive decline in hypertensive patients with cerebral small vessel disease. Hypertens. Res . 32, 816–820. doi: 10.1038/hr.2009.100
Kloppenborg, R. P., van den Berg, E., Kappelle, L. J., and Biessels, G. J. (2008). Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur. J. Pharmacol . 585, 97–108. doi: 10.1016/j.ejphar.2008.02.049
* 2,5,a Klostermann, E. C., Braskie, M. N., Landau, S. M., O'Neil, J. P., and Jagust, W. J. (2012). Dopamine and frontostriatal networks in cognitive aging. Neurobiol. Aging 33, 623.e15–24. doi: 10.1016/j.neurobiolaging.2011.03.002
Knecht, S., Wersching, H., Lohmann, H., Berger, K., and Ringelstein, E. B. (2009). How much does hypertension affect cognition? Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J. Neurol. Sci . 283, 149–152. doi: 10.1016/j.jns.2009.02.362
Knecht, S., Wersching, H., Lohmann, H., Bruchmann, M., Duning, T., Dziewas, R. et al. (2008). High-normal blood pressure is associated with poor cognitive performance. Hypertension 51, 663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577
* 7,8,b,• Koch, W., Teipel, S., Mueller, S., Buerger, K., Bokde, A. L. W., Hampel, H. et al. (2010). Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage 51, 280–287. doi: 10.1016/j.neuroimage.2009.12.008
* 7,8,a Krause, J. B., Taylor, J. G., Schmidt, D., Hautzel, H., Mottaghy, F. M., and Müller-Gärtner, H. W. (2000). Imaging and neural modelling in episodic and working memory processes. Neural Netw . 13, 847–859
* 1,9,a Kühn, S., Schmiedek, F., Schott, B., Ratcliff, R., Heinze, H.-J., Düzel, E. et al. (2011). Brain areas consistently linked to individual differences in perceptual decision-making in younger as well as older adults before and after training. J. Cogn. Neurosci . 23, 2147–2158. doi: 10.1162/jocn.2010.21564
* 7,8,a Kukolja, J., Thiel, C. M., Wilms, M., Mirzazade, S., and Fink, G. R. (2009). Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiol. Aging 30, 630–645. doi: 10.1016/j.neurobiolaging.2007.08.015
* 6,7,a Kukolja, J., Thiel, C. M., Wolf, O. T., and Fink, G. R. (2008). Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology (Berl.) 201, 293–304. doi: 10.1007/s00213-008-1275-8
* 1,2,a,• Lamar, M., Yousem, D. M., and Resnick, S. M. (2004). Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage 21, 1368–1376. doi: 10.1016/j.neuroimage.2003.11.018
* 7,8,a Langenecker, S. A., Briceno, E. M., Hamid, N. M., and Nielson, K. A. (2007). An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Res . 1135, 58–68. doi: 10.1016/j.brainres.2006.11.068
* 5,6,a Langenecker, S. A., and Nielson, K. A. (2003). Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage 20, 1384–1392. doi: 10.1016/S1053-8119(03)00372-0
* 5,6,a Langenecker, S. A., Nielson, K. A., and Rao, S. M. (2004). fMRI of healthy older adults during Stroop interference. Neuroimage 21, 192–200. doi: 10.1016/j.neuroimage.2003.08.027
Last, D., Alsop, D. C., Abduljalil, A. M., Marquis, R. P., de Bazelaire, C., Hu, K. et al. (2007). Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 30, 1193–1199. doi: 10.2337/dc06-2052
Launer, L. J., Ross, G. W., Petrovitch, H., Masaki, K., Foley, D., White, L. R. et al. (2000). Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol. Aging 21, 49–55. doi: 10.1016/S0197-4580(00)00096-8
Lee, J. H., Yoon, S., Renshaw, P. F., Kim, T.-S., Jung, J. J., Choi, Y. et al. (2013). Morphometric changes in lateral ventricles of patients with recent-onset type 2 diabetes mellitus. PLoS ONE 8:e60515. doi: 10.1371/journal.pone.0060515
* 5,6,a Leshikar, E. D., Gutchess, A. H., Hebrank, A. C., Sutton, B. P., and Park, D. C. (2010). The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex 46, 507–521. doi: 10.1016/j.cortex.2009.07.011
* 5,6,a Li, C., Zheng, J., Wang, J., Gui, L., and Li, C. (2009a). An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer's disease. Curr. Alzheimer Res . 6, 525–530. doi: 10.2174/156720509790147142
Li, J., Wang, Y. J., Zhang, M., Xu, Z. Q., Gao, C. Y., Fang, C. Q. et al. (2011). Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76, 1485–1491. doi: 10.1212/WNL.0b013e318217e7a4
* 7,8,a Li, Z., Moore, A. B., Tyner, C., and Hu, X. (2009b). Asymmetric connectivity reduction and its relationship to “HAROLD” in aging brain. Brain Res . 1295, 149–158. doi: 10.1016/j.brainres.2009.08.004
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. doi: 10.1038/35084005
Luchsinger, J. A. (2008). Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur. J. Pharmacol . 585, 119–129. doi: 10.1016/j.ejphar.2008.02.048
Luchsinger, J. A., Palmas, W., Teresi, J. A., Silver, S., Kong, J., Eimicke, J. P. et al. (2011). Improved diabetes control in the elderly delays global cognitive decline. J. Nutr. Health Aging 15, 445–449. doi: 10.1007/s12603-011-0057-x
* 3,5,a MacDonald, S. W. S., Nyberg, L., Sandblom, J., Fischer, H., and Bäckman, L. (2008). Increased response-time variability is associated with reduced inferior parietal activation during episodic recognition in aging. J. Cogn. Neurosci . 20, 779–786. doi: 10.1162/jocn.2008.20502
* 2,5,a,■ Madden, D. J., Costello, M. C., Dennis, N. A., Davis, S. W., Shepler, A. M., Spaniol, J. et al. (2010). Adult age differences in functional connectivity during executive control. Neuroimage 52, 643–657. doi: 10.1016/j.neuroimage.2010.04.249
* 1,2,d,■ Madden, D. J., Langley, L. K., Denny, L. L., Turkington, T. G., Provenzale, J. M., Hawk, T. C. et al. (2002a). Adult age differences in visual word identification: functional neuroanatomy by positron emission tomography. Brain Cogn . 49, 297–321. doi: 10.1006/brcg.2001.1502
* 2,5,d,■ Madden, D. J., Spaniol, J., Whiting, W. L., Bucur, B., Provenzale, J. M., Cabeza, R. et al. (2007). Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol. Aging 28, 459–476. doi: 10.1016/j.neurobiolaging.2006.01.005
* 1,2,d,■ Madden, D. J., Turkington, T. G., Provenzale, J. M., Denny, L. L., Langley, L. K., Hawk, T. C. et al. (2002b). Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol. Aging 17, 24–43. doi: 10.1037/0882-7974.17.1.24
Maggi, S., Limongi, F., Noale, M., Romanato, G., Tonin, P., Rozzini, R. et al. (2009). Diabetes as a risk factor for cognitive decline in older patients. Dement. Geriatr. Cogn. Disord . 27, 24–33. doi: 10.1159/000183842
* 1,9,a Maillet, D., and Rajah, M. N. (2011). Age-related changes in the three-way correlation between anterior hippocampus volume, whole-brain patterns of encoding activity and subsequent context retrieval. Brain Res . 1420, 68–79. doi: 10.1016/j.brainres.2011.08.071
Marioni, R. E., Strachan, M. W. J., Reynolds, R. M., Lowe, G. D. O., Mitchell, R. J., Fowkes, F. G. R. et al. (2010). Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59, 710–713. doi: 10.2337/db09-1163
* 3,5,b Mathis, A., Schunck, T., Erb, G., Namer, I. J., and Luthringer, R. (2009). The effect of aging on the inhibitory function in middle-aged subjects: a functional MRI study coupled with a color-matched Stroop task. Int. J. Geriatr. Psychiatry 24, 1062–1071. doi: 10.1002/gps.2222
* 4,a Mattay, V. S., Fera, F., Tessitore, A., Hariri, A. R., Berman, K. F., Das, S. et al. (2006). Neurophysiological correlates of age-related changes in working memory capacity. Neurosci. Lett . 392, 32–37. doi: 10.1016/j.neulet.2005.09.025
* 1,2,a,• Matthäus, F., Schmidt, J.-P., Banerjee, A., Schulze, T. G., Demirakca, T., and Diener, C. (2012). Effects of age on the structure of functional connectivity networks during episodic and working memory demand. Brain Connect . 2, 113–124. doi: 10.1089/brain.2012.0077
* 7,8,a,° Mayhew, S. D., Li, S., Storrar, J. K., Tsvetanov, K. A., and Kourtzi, Z. (2010). Learning shapes the representation of visual categories in the aging human brain. J. Cogn. Neurosci . 22, 2899–2912. doi: 10.1162/jocn.2010.21415
* 7,8,a,□ McGeown, W. J., Shanks, M. F., Forbes-McKay, K. E., and Venneri, A. (2009). Patterns of brain activity during a semantic task differentiate normal aging from early Alzheimer's disease. Psychiatry Res . 173, 218–227. doi: 10.1016/j.pscychresns.2008.10.005
* 5,6,a Meier, T. B., Desphande, A. S., Vergun, S., Nair, V. A., Song, J., Biswal, B. B. et al. (2012). Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage 60, 601–613. doi: 10.1016/j.neuroimage.2011.12.052
* 7,9,c Meinzer, M., Flaisch, T., Seeds, L., Harnish, S., Antonenko, D., Witte, V. et al. (2012a). Same modulation but different starting points: performance modulates age differences in inferior frontal cortex activity during word-retrieval. PLoS ONE 7:e33631. doi: 10.1371/journal.pone.0033631
* 5,6,d Meinzer, M., Flaisch, T., Wilser, L., Eulitz, C., Rockstroh, B., Conway, T. et al. (2009). Neural signatures of semantic and phonemic fluency in young and old adults. J. Cogn. Neurosci . 21, 2007–2018. doi: 10.1162/jocn.2009.21219
* 7,9,c Meinzer, M., Seeds, L., Flaisch, T., Harnish, S., Cohen, M. L., McGregor, K. et al. (2012b). Impact of changed positive and negative task-related brain activity on word-retrieval in aging. Neurobiol. Aging 33, 656–669. doi: 10.1016/j.neurobiolaging.2010.06.020
Messier, C., Awad-Shimoon, N., Gagnon, M., Desrochers, A., and Tsiakas, M. (2011). Glucose regulation is associated with cognitive performance in young nondiabetic adults. Behav. Brain Res . 222, 81–88. doi: 10.1016/j.bbr.2011.03.023
Messier, C., Tsiakas, M., Gagnon, M., and Desrochers, A. (2010). Effect of age and glucoregulation on cognitive performance. J. Clin. Exp. Neuropsychol . 32, 809–821. doi: 10.1080/13803390903540323
* 7,9,a Meulenbroek, O., Kessels, R. P. C., de Rover, M., Petersson, K. M., Rikkert, M. G. M. O., Rijpkema, M. et al. (2010a). Age-effects on associative object-location memory. Brain Res . 1315, 100–110. doi: 10.1016/j.brainres.2009.12.011
* 1,2,a Meulenbroek, O., Petersson, K. M., Voermans, N., Weber, B., and Fernández, G. (2004). Age differences in neural correlates of route encoding and route recognition. Neuroimage 22, 1503–1514. doi: 10.1016/j.neuroimage.2004.04.007
* 7,8,a Meulenbroek, O., Rijpkema, M., Kessels, R. P. C., Rikkert, M. G. M. O., and Fernández, G. (2010b). Autobiographical memory retrieval in patients with Alzheimer's disease. Neuroimage 53, 331–340. doi: 10.1016/j.neuroimage.2010.05.082
* 7,8,b,■ Miettinen, P. S., Pihlajamäki, M., Jauhiainen, A. M., Niskanen, E., Hänninen, T., Vanninen, R. et al. (2011). Structure and function of medial temporal and posteromedial cortices in early Alzheimer's disease. Eur. J. Neurosci . 34, 320–330. doi: 10.1111/j.1460-9568.2011.07745.x
* 3,7,a,• Milham, M. P., Erickson, K. I., Banich, M. T., Kramer, A. F., Webb, A., Wszalek, T. et al. (2002). Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn . 49, 277–296. doi: 10.1006/brcg.2001.1501
* 5,6,a Mitchell, K. J., Johnson, M. K., Raye, C. L., and D'Esposito, M. (2000). fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res. Cogn. Brain Res . 10, 197–206. doi: 10.1016/S0926-6410(00)00029-X
* 7,8,a Mitchell, K. J., Johnson, M. R., Higgins, J. A., and Johnson, M. K. (2010). Age differences in brain activity during perceptual versus reflective attention. Neuroreport 21, 293–297. doi: 10.1097/WNR.0b013e32833730d6
* 7,8,a Mitchell, K. J., Raye, C. L., Johnson, M. K., and Greene, E. J. (2006). An fMRI investigation of short-term source memory in young and older adults. Neuroimage 30, 627–633. doi: 10.1016/j.neuroimage.2005.09.039
* 1,2,a,▴ Mohtasib, R. S., Lumley, G., Goodwin, J. A., Emsley, H. C. A., Sluming, V., and Parkes, L. M. (2012). Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. Neuroimage 59, 1143–1151. doi: 10.1016/j.neuroimage.2011.07.092
Moran, C., Phan, T. G., Chen, J., Blizzard, L., Beare, R., Venn, A. et al. (2013). Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36, 4036–4042. doi: 10.2337/dc13-0143
* 3,5,a Morcom, A. M., and Friston, K. J. (2012). Decoding episodic memory in ageing: a Bayesian analysis of activity patterns predicting memory. Neuroimage 59, 1772–1782. doi: 10.1016/j.neuroimage.2011.08.071
* 3,5,a,□ Morcom, A. M., Good, C. D., Frackowiak, R. S. J., and Rugg, M. D. (2003). Age effects on the neural correlates of successful memory encoding. Brain 126, 213–229. doi: 10.1093/brain/awg020
* 5,6,a Mormino, E. C., Brandel, M. G., Madison, C. M., Marks, S., Baker, S. L., and Jagust, W. J. (2012). Aβ Deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb. Cortex 22, 1813–1823. doi: 10.1093/cercor/bhr255
* 5,6,a Mormino, E. C., Smiljic, A., Hayenga, A. O., Onami, S. H., Greicius, M. D., Rabinovici, G. D. et al. (2011). Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex 21, 2399–2407. doi: 10.1093/cercor/bhr025
Morris, J. K., Vidoni, E. D., Honea, R. A., and Burns, J. M. (2014). Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol. Aging 35, 585–589. doi: 10.1016/j.neurobiolaging.2013.09.033
* 5,6,a Mowinckel, A. M., Espeseth, T., and Westlye, L. T. (2012). Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. Neuroimage 63, 1364–1373. doi: 10.1016/j.neuroimage.2012.08.004
* 5,6,a Murphy, K., and Garavan, H. (2004). Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage 21, 219–228. doi: 10.1016/j.neuroimage.2003.09.016
* 3,7,b Murty, V. P., Sambataro, F., Das, S., Tan, H.-Y., Callicott, J. H., Goldberg, T. E. et al. (2009). Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J. Cogn. Neurosci . 21, 1920–1933. doi: 10.1162/jocn.2009.21130
Musen, G., Jacobson, A. M., Bolo, N. R., Simonson, D. C., Shenton, M. E., McCartney, R. L. et al. (2012). Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61, 2375–2379. doi: 10.2337/db11-1669
Nagai, M., Hoshide, S., Ishikawa, J., Shimada, K., and Kario, K. (2008). Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J. Hypertens . 26, 1636–1641. doi: 10.1097/HJH.0b013e3283018333
* 7,8,a Nagel, I. E., Preuschhof, C., Li, S.-C., Nyberg, L., Bäckman, L., Lindenberger, U. et al. (2009). Performance level modulates adult age differences in brain activation during spatial working memory. Proc. Natl. Acad. Sci. U.S.A . 106, 22552–22557. doi: 10.1073/pnas.0908238106
* 10,12,a Nagel, I. E., Preuschhof, C., Li, S.-C., Nyberg, L., Bäckman, L., Lindenberger, U. et al. (2011). Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J. Cogn. Neurosci . 23, 2030–2045. doi: 10.1162/jocn.2010.21560
Nakae, J., Kido, Y., and Accili, D. (2001). Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev . 22, 818–835. doi: 10.1210/edrv.22.6.0452
Newcomer, J. W., and Haupt, D. W. (2006). The metabolic effects of antipsychotic medications. Can. J. Psychiatry 51, 480–491.
* 4, b, ■ Nichols, L. M., Masdeu, J. C., Mattay, V. S., Kohn, P., Emery, M., Sambataro, F. et al. (2012). Interactive effect of apolipoprotein e genotype and age on hippocampal activation during memory processing in healthy adults. Arch. Gen. Psychiatry 69, 804–813. doi: 10.1001/archgenpsychiatry.2011.1893
* 7,8,a,• Nielson, K. A., Douville, K. L., Seidenberg, M., Woodard, J. L., Miller, S. K., Franczak, M. et al. (2006). Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol. Aging 27, 1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022
* 5,6,a Nielson, K. A., Langenecker, S. A., and Garavan, H. (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol. Aging 17, 56–71. doi: 10.1037/0882-7974.17.1.56
* 3,5,a,□ Nielson, K. A., Langenecker, S. A., Ross, T. J., Garavan, H., Rao, S. M., and Stein, E. A. (2004). Comparability of functional MRI response in young and old during inhibition. Neuroreport 15, 129–133. doi: 10.1097/00001756-200401190-00025
Nishikawa, T., Edelstein, D., Du, X. L., Yamagishi, S., Matsumura, T., Kaneda, Y. et al. (2000). Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790. doi: 10.1038/35008121
Nooyens, A. C. J., Baan, C. A., Spijkerman, A. M. W., and Verschuren, W. M. M. (2010). Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care 33, 1964–1969. doi: 10.2337/dc09-2038
* 7,10,c,❖ Nordahl, C. W., Ranganath, C., Yonelinas, A. P., Decarli, C., Fletcher, E., and Jagust, W. J. (2006). White matter changes compromise prefrontal cortex function in healthy elderly individuals. J. Cogn. Neurosci . 18, 418–429. doi: 10.1162/089892906775990552
Novak, V., Last, D., Alsop, D. C., Abduljalil, A. M., Hu, K., Lepicovsky, L. et al. (2006). Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 29, 1529–1534. doi: 10.2337/dc06-0261
* 5,6,a Nyberg, L., Dahlin, E., Stigsdotter Neely, A., and Bäckman, L. (2009). Neural correlates of variable working memory load across adult age and skill: dissociative patterns within the fronto-parietal network. Scand. J. Psychol . 50, 41–46. doi: 10.1111/j.1467-9450.2008.00678.x
* 7,8,a,• O'Brien, J. L., O'Keefe, K. M., LaViolette, P. S., DeLuca, A. N., Blacker, D., Dickerson, B. C. et al. (2010). Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976. doi: 10.1212/WNL.0b013e3181e3966e
* 7,8,a Osaka, M., Otsuka, Y., and Osaka, N. (2012a). Verbal to visual code switching improves working memory in older adults: an fMRI study. Front. Hum. Neurosci . 6:24. doi: 10.3389/fnhum.2012.00024
* 7,8,a Osaka, M., Yaoi, K., Otsuka, Y., Katsuhara, M., and Osaka, N. (2012b). Practice on conflict tasks promotes executive function of working memory in the elderly. Behav. Brain Res . 233, 90–98. doi: 10.1016/j.bbr.2012.04.044
* 7,8,a Otsuka, Y., Osaka, N., Morishita, M., Kondo, H., and Osaka, M. (2006). Decreased activation of anterior cingulate cortex in the working memory of the elderly. Neuroreport 17, 1479–1482. doi: 10.1097/01.wnr.0000236852.63092.9f
Oveisgharan, S., and Hachinski, V. (2010). Hypertension, executive dysfunction, and progression to dementia: the canadian study of health and aging. Arch. Neurol . 67, 187–192. doi: 10.1001/archneurol.2009.312
* 6,7,a Pacheco, J., Beevers, C. G., McGeary, J. E., and Schnyer, D. M. (2012). Memory monitoring performance and PFC activity are associated with 5-HTTLPR genotype in older adults. Neuropsychologia 50, 2257–2270. doi: 10.1016/j.neuropsychologia.2012.05.030
* 7,8,a Park, D. C., Welsh, R. C., Marshuetz, C., Gutchess, A. H., Mikels, J., Polk, T. A. et al. (2003). Working memory for complex scenes: age differences in frontal and hippocampal activations. J. Cogn. Neurosci . 15, 1122–1134. doi: 10.1162/089892903322598094
* 4, a, □ Park, J., Carp, J., Hebrank, A., Park, D. C., and Polk, T. A. (2010). Neural specificity predicts fluid processing ability in older adults. J. Neurosci . 30, 9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010
* 1,2,a Paxton, J. L., Barch, D. M., Racine, C. A., and Braver, T. S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb. Cortex 18, 1010–1028. doi: 10.1093/cercor/bhm135
* 1,9,b Persson, J., Kalpouzos, G., Nilsson, L.-G., Ryberg, M., and Nyberg, L. (2011). Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus 21, 753–766. doi: 10.1002/hipo.20794
* 7,10,a,+ Persson, J., Nyberg, L., Lind, J., Larsson, A., Nilsson, L.-G., Ingvar, M. et al. (2006). Structure-function correlates of cognitive decline in aging. Cereb. Cortex 16, 907–915. doi: 10.1093/cercor/bhj036
* 7,10,c,♦ Persson, J., Pudas, S., Lind, J., Kauppi, K., Nilsson, L.-G., and Nyberg, L. (2012). Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb. Cortex 22, 2297–2304. doi: 10.1093/cercor/bhr306
* 2,5,a Persson, J., Sylvester, C.-Y. C., Nelson, J. K., Welsh, K. M., Jonides, J., and Reuter-Lorenz, P. A. (2004). Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage 23, 1382–1390. doi: 10.1016/j.neuroimage.2004.08.004
* 7,8,a Petrella, J. R., Townsend, B. A., Jha, A. P., Ziajko, L. A., Slavin, M. J., Lustig, C. et al. (2005). Increasing memory load modulates regional brain activity in older adults as measured by fMRI. J. Neuropsychiatry Clin. Neurosci . 17, 75–83. doi: 10.1176/appi.neuropsych.17.1.75
* 5,6,a Piefke, M., Onur, Ö. A., and Fink, G. R. (2012). Aging-related changes of neural mechanisms underlying visual-spatial working memory. Neurobiol. Aging 33, 1284–1297. doi: 10.1016/j.neurobiolaging.2010.10.014
* 7,8,a Pihlajamäki, M., and Sperling, R. A. (2009). Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer's disease and at-risk older individuals. Behav. Neurol . 21, 77–91. doi: 10.3233/BEN-2009-0231
Pires, P. W., Dams Ramos, C. M., Matin, N., and Dorrance, A. M. (2013). The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol . 304, H1598–H1614. doi: 10.1152/ajpheart.00490.2012
* 4, c Podell, J. E., Sambataro, F., Murty, V. P., Emery, M. R., Tong, Y., Das, S. et al. (2012). Neurophysiological correlates of age-related changes in working memory updating. Neuroimage 62, 2151–2160. doi: 10.1016/j.neuroimage.2012.05.066
* 7,8,a Prakash, R. S., Erickson, K. I., Colcombe, S. J., Kim, J. S., Voss, M. W., and Kramer, A. F. (2009). Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn . 71, 328–335. doi: 10.1016/j.bandc.2009.07.005
* 7,8,a Protzner, A. B., Mandzia, J. L., Black, S. E., and McAndrews, M. P. (2011). Network interactions explain effective encoding in the context of medial temporal damage in MCI. Hum. Brain Mapp . 32, 1277–1289. doi: 10.1002/hbm.21107
* 7,8,a,• Putcha, D., O'Keefe, K., LaViolette, P., O'Brien, J., Greve, D., Rentz, D. M. et al. (2011). Reliability of functional magnetic resonance imaging associative encoding memory paradigms in non-demented elderly adults. Hum. Brain Mapp . 32, 2027–2044. doi: 10.1002/hbm.21166
* 1,9,d Rajah, M. N., Languay, R., and Grady, C. L. (2011). Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci . 31, 17941–17954. doi: 10.1523/JNEUROSCI.1690-11.2011
* 7,8,a,• Rajah, M. N., Languay, R., and Valiquette, L. (2010). Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex 46, 535–549. doi: 10.1016/j.cortex.2009.07.006
* 5,6,a,□ Rajah, M. N., and McIntosh, A. R. (2008). Age-related differences in brain activity during verbal recency memory. Brain Res . 1199, 111–125. doi: 10.1016/j.brainres.2007.12.051
Raji, C. A., Lopez, O. L., Kuller, L. H., Carmichael, O. T., Longstreth, W. T. Jr., Gach, H. M. et al. (2012). White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol. Aging 33, 834.e7–16. doi: 10.1016/j.neurobiolaging.2011.08.010
* 2,7,d,▴ Ramsøy, T. Z., Liptrot, M. G., Skimminge, A., Lund, T. E., Sidaros, K., Christensen, M. S. et al. (2012). Healthy aging attenuates task-related specialization in the human medial temporal lobe. Neurobiol. Aging 33, 1874–1889. doi: 10.1016/j.neurobiolaging.2011.09.032
* 6,7,a Rand-Giovannetti, E., Chua, E. F., Driscoll, A. E., Schacter, D. L., Albert, M. S., and Sperling, R. A. (2006). Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol. Aging 27, 173–182. doi: 10.1016/j.neurobiolaging.2004.12.013
Ravona-Springer, R., Moshier, E., Schmeidler, J., Godbold, J., Akrivos, J., Rapp, M. et al. (2012). Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J. Alzheimers Dis . 30, 299–309. doi: 10.3233/JAD-2012-120106
* 5,6,a Raye, C. L., Mitchell, K. J., Reeder, J. A., Greene, E. J., and Johnson, M. K. (2008). Refreshing one of several active representations: behavioral and functional magnetic resonance imaging differences between young and older adults. J. Cogn. Neurosci . 20, 852–862. doi: 10.1162/jocn.2008.20508
Raz, N., Yang, Y., Dahle, C. L., and Land, S. (2012). Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta 1822, 361–369. doi: 10.1016/j.bbadis.2011.08.007
Reaven, G. M., Thompson, L. W., Nahum, D., and Haskins, E. (1990). Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care 13, 16–21. doi: 10.2337/diacare.13.1.16
Regenold, W. T., Thapar, R. K., Marano, C., Gavirneni, S., and Kondapavuluru, P. V. (2002). Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J. Affect. Disord . 70, 19–26. doi: 10.1016/S0165-0327(01)00456-6
Reijmer, Y. D., van den Berg, E., de Bresser, J., Kessels, R. P. C., Kappelle, L. J., Algra, A. et al. (2011). Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab. Res. Rev . 27, 195–202. doi: 10.1002/dmrr.1163
Reitz, C., Tang, M.-X., Manly, J., Mayeux, R., and Luchsinger, J. A. (2007). Hypertension and the risk of mild cognitive impairment. Arch. Neurol . 64, 1734–1740. doi: 10.1001/archneur.64.12.1734
* 5,6,a,▴ Restom, K., Bangen, K. J., Bondi, M. W., Perthen, J. E., and Liu, T. T. (2007). Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage 37, 430–439. doi: 10.1016/j.neuroimage.2007.05.024
* 7,8,a Rieckmann, A., Fischer, H., and Bäckman, L. (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage 50, 1303–1312. doi: 10.1016/j.neuroimage.2010.01.015
* 7,8,a Rieckmann, A., Karlsson, S., Fischer, H., and Bäckman, L. (2011). Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J. Neurosci . 31, 14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011
Rizzo, M. R., Marfella, R., Barbieri, M., Boccardi, V., Vestini, F., Lettieri, B. et al. (2010). Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 33, 2169–2174. doi: 10.2337/dc10-0389
Roberts, R. O., Knopman, D. S., Geda, Y. E., Cha, R. H., Pankratz, V. S., Baertlein, L. et al. (2014). Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers. Dement . 10, 18–26. doi: 10.1016/j.jalz.2013.01.001
* 5,6,a Rombouts, S. A. R. B., Barkhof, F., Goekoop, R., Stam, C. J., and Scheltens, P. (2005a). Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp . 26, 231–239. doi: 10.1002/hbm.20160
* 5,6,a,• Rombouts, S. A. R. B., Goekoop, R., Stam, C. J., Barkhof, F., and Scheltens, P. (2005b). Delayed rather than decreased BOLD response as a marker for early Alzheimer's disease. Neuroimage 26, 1078–1085. doi: 10.1016/j.neuroimage.2005.03.022
* 7,10,b,❖ Rosano, C., Venkatraman, V. K., Guralnik, J., Newman, A. B., Glynn, N. W., Launer, L. et al. (2010). Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J. Gerontol. A Biol. Sci. Med. Sci . 65, 639–647. doi: 10.1093/gerona/glq038
* 3,5,a Rosen, A. C., Gabrieli, J. D. E., Stoub, T., Prull, M. W., O'Hara, R., Yesavage, J. et al. (2005). Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex 41, 595–602. doi: 10.1016/S0010-9452(08)70199-0
Ryan, C. M., and Geckle, M. O. (2000). Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care 23, 1486–1493. doi: 10.2337/diacare.23.10.1486
* 4, b Rypma, B., Berger, J. S., Genova, H. M., Rebbechi, D., and D'Esposito, M. (2005). Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex 41, 582–594. doi: 10.1016/S0010-9452(08)70198-9
* 4, b Rypma, B., Eldreth, D. A., and Rebbechi, D. (2007). Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex 43, 65–76. doi: 10.1016/S0010-9452(08)70446-5
* 5,6,a Sala-Llonch, R., Arenaza-Urquijo, E. M., Valls-Pedret, C., Vidal-Piñeiro, D., Bargall,ó, N., Junqu,é, C. et al. (2012). Dynamic functional reorganizations and relationship with working memory performance in healthy aging. Front. Hum. Neurosci . 6, 152. doi: 10.3389/fnhum.2012.00152
* 5,6,b Salami, A., Eriksson, J., and Nyberg, L. (2012). Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J. Neurosci . 32, 10749–10757. doi: 10.1523/JNEUROSCI.0278-12.2012
* 4, a, • Sambataro, F., Murty, V. P., Callicott, J. H., Tan, H.-Y., Das, S., Weinberger, D. R. et al. (2010). Age-related alterations in default mode network: impact on working memory performance. Neurobiol. Aging 31, 839–852. doi: 10.1016/j.neurobiolaging.2008.05.022
* 3,5,a Schneider-Garces, N. J., Gordon, B. A., Brumback-Peltz, C. R., Shin, E., Lee, Y., Sutton, B. P. et al. (2010). Span, CRUNCH, and beyond: working memory capacity and the aging brain. J. Cogn. Neurosci . 22, 655–669. doi: 10.1162/jocn.2009.21230
* 7,9,c Schulte, T., Müller-Oehring, E. M., Chanraud, S., Rosenbloom, M. J., Pfefferbaum, A., and Sullivan, E. V. (2011). Age-related reorganization of functional networks for successful conflict resolution: a combined functional and structural MRI study. Neurobiol. Aging 32, 2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002
Scuteri, A., Tesauro, M., Appolloni, S., Preziosi, F., Brancati, A. M., and Volpe, M. (2007). Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J. Hypertens . 25, 1035–1040. doi: 10.1097/01.hjh.0000170384.38708.b7
* 7,8,a Shafto, M. A., Stamatakis, E. A., Tam, P. P., and Tyler, L. K. (2010). Word retrieval failures in old age: the relationship between structure and function. J. Cogn. Neurosci . 22, 1530–1540. doi: 10.1162/jocn.2009.21321
Shaikh, S., and Nicholson, L. F. B. (2008). Advanced glycation end products induce in vitro cross-linking of alpha-synuclein and accelerate the process of intracellular inclusion body formation. J. Neurosci. Res . 86, 2071–2082. doi: 10.1002/jnr.21644
Shaw, J. E., Sicree, R. A., and Zimmet, P. Z. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract . 87, 4–14. doi: 10.1016/j.diabres.2009.10.007
* 5,6,b,• Siedlecki, K. L., Habeck, C. G., Brickman, A. M., Gazes, Y., and Stern, Y. (2009). Examining the multifactorial nature of cognitive aging with covariance analysis of positron emission tomography data. J. Int. Neuropsychol. Soc . 15, 973–981. doi: 10.1017/S1355617709990592
* 7,8,a Simon, J. R., Vaidya, C. J., Howard, J. H., and Howard, D. V. (2012). The effects of aging on the neural basis of implicit associative learning in a probabilistic triplets learning task. J. Cogn. Neurosci . 24, 451–463. doi: 10.1162/jocn_a_00116
Smith, P. J., Blumenthal, J. A., Babyak, M. A., Hinderliter, A., and Sherwood, A. (2011). Association of vascular health and neurocognitive performance in overweight adults with high blood pressure. J. Clin. Exp. Neuropsychol . 33, 559–566. doi: 10.1080/13803395.2010.537648
Sokolova, I. A., Manukhina, E. B., Blinkov, S. M., Koshelev, V. B., Pinelis, V. G., and Rodionov, I. M. (1985). Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc. Res . 30, 1–9. doi: 10.1016/0026-2862(85)90032-9
* 2,5,d Solbakk, A.-K., Fuhrmann Alpert, G., Furst, A. J., Hale, L. A., Oga, T., Chetty, S. et al. (2008). Altered prefrontal function with aging: insights into age-associated performance decline. Brain Res . 1232, 30–47. doi: 10.1016/j.brainres.2008.07.060
* 5,6,a Solé-Padullés, C., Bartrés-Faz, D., Junqué, C., Vendrell, P., Rami, L., Clemente, I. C. et al. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 30, 1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008
* 5,6,d,■ Spaniol, J., and Grady, C. (2012). Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol. Aging 33, 425.e3–18. doi: 10.1016/j.neurobiolaging.2010.10.005
* 6,7,a Sperling, R. A., Bates, J. F., Chua, E. F., Cocchiarella, A. J., Rentz, D. M., Rosen, B. R. et al. (2003). fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr . 74, 44–50. doi: 10.1136/jnnp.74.1.44
* 10,12,b Stebbins, G. T., Carrillo, M. C., Dorfman, J., Dirksen, C., Desmond, J. E., Turner, D. A. et al. (2002). Aging effects on memory encoding in the frontal lobes. Psychol. Aging 17, 44–55. doi: 10.1037/0882-7974.17.1.44
Steen, E., Terry, B. M., Rivera, E. J., Cannon, J. L., Neely, T. R., Tavares, R. et al. (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J. Alzheimers Dis . 7, 63–80.
* 5,6,b Steffener, J., Brickman, A. M., Rakitin, B. C., Gazes, Y., and Stern, Y. (2009). The impact of age-related changes on working memory functional activity. Brain Imaging Behav . 3, 142–153. doi: 10.1007/s11682-008-9056-x
* 5,6,a Stern, Y., Habeck, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J. et al. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 15, 394–402. doi: 10.1093/cercor/bhh142
* 4, a Stern, Y., Rakitin, B. C., Habeck, C., Gazes, Y., Steffener, J., Kumar, A. et al. (2012). Task difficulty modulates young-old differences in network expression. Brain Res . 1435, 130–145. doi: 10.1016/j.brainres.2011.11.061
* 7,8,a Stern, Y., Zarahn, E., Habeck, C., Holtzer, R., Rakitin, B. C., Kumar, A. et al. (2008). A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb. Cortex 18, 959–967. doi: 10.1093/cercor/bhm134
* 4, a Stevens, W. D., Hasher, L., Chiew, K. S., and Grady, C. L. (2008). A neural mechanism underlying memory failure in older adults. J. Neurosci . 28, 12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008
* 7,9,a St Jacques, P. L., Rubin, D. C., and Cabeza, R. (2012). Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol. Aging 33, 1298–1310. doi: 10.1016/j.neurobiolaging.2010.11.007
* 5,6,a Thiyagesh, S. N., Farrow, T. F. D., Parks, R. W., Accosta-Mesa, H., Young, C., Wilkinson, I. D. et al. (2009). The neural basis of visuospatial perception in Alzheimer's disease and healthy elderly comparison subjects: an fMRI study. Psychiatry Res . 172, 109–116. doi: 10.1016/j.pscychresns.2008.11.002
* 7,8,a Thomsen, T., Specht, K., Rimol, L. M., Hammar, A., Nyttingnes, J., Ersland, L. et al. (2004). Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage 22, 912–919. doi: 10.1016/j.neuroimage.2004.02.015
* 5,9,a Townsend, J., Adamo, M., and Haist, F. (2006). Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage 31, 1682–1692. doi: 10.1016/j.neuroimage.2006.01.045
Triantafyllidi, H., Arvaniti, C., Lekakis, J., Ikonomidis, I., Siafakas, N., Tzortzis, S. et al. (2009). Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am. J. Hypertens . 22, 525–530. doi: 10.1038/ajh.2009.35
* 5,6,b Trivedi, M. A., Murphy, C. M., Goetz, C., Shah, R. C., Gabrieli, J. D. E., Whitfield-Gabrieli, S. et al. (2008a). fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement. Geriatr. Cogn. Disord . 26, 123–137. doi: 10.1159/000148190
* 1,6,a Trivedi, M. A., Schmitz, T. W., Ries, M. L., Hess, T. M., Fitzgerald, M. E., Atwood, C. S. et al. (2008b). fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer's disease. Neuropsychologia 46, 1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035
* 4, b Trivedi, M. A., Stoub, T. R., Murphy, C. M., George, S., deToledo-Morrell, L., Shah, R. C. et al. (2011). Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav . 5, 126–136. doi: 10.1007/s11682-011-9117-4
* 5,6,a Tsukiura, T., Sekiguchi, A., Yomogida, Y., Nakagawa, S., Shigemune, Y., Kambara, T. et al. (2011). Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. J. Cogn. Neurosci . 23, 200–213. doi: 10.1162/jocn.2010.21476
Tuligenga, R. H., Dugravot, A., Tabák, A. G., Elbaz, A., Brunner, E. J., Kivimäki, M. et al. (2014). Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol . 2, 228–235. doi: 10.1016/S2213-8587(13)70192-X.
* 7,8,a Tyler, L. K., Shafto, M. A., Randall, B., Wright, P., Marslen-Wilson, W. D., and Stamatakis, E. A. (2010). Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex 20, 352–364. doi: 10.1093/cercor/bhp105
* 7,8,a Vallesi, A., McIntosh, A. R., and Stuss, D. T. (2009). Temporal preparation in aging: a functional MRI study. Neuropsychologia 47, 2876–2881. doi: 10.1016/j.neuropsychologia.2009.06.013
* 5,6,a,■ Vallesi, A., McIntosh, A. R., and Stuss, D. T. (2011). Overrecruitment in the aging brain as a function of task demands: evidence for a compensatory view. J. Cogn. Neurosci . 23, 801–815. doi: 10.1162/jocn.2010.21490
van den Berg, E., Reijmer, Y. D., de Bresser, J., Kessels, R. P. C., Kappelle, L. J., and Biessels, G. J. (2010). A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 53, 58–65. doi: 10.1007/s00125-009-1571-9
* 4, a Vandenbroucke, M. W. G., Goekoop, R., Duschek, E. J. J., Netelenbos, J. C., Kuijer, J. P. A., Barkhof, F. et al. (2004). Interindividual differences of medial temporal lobe activation during encoding in an elderly population studied by fMRI. Neuroimage 21, 173–180. doi: 10.1016/j.neuroimage.2003.09.043
* 4, c van der Veen, F. M., Nijhuis, F. A. P., Tisserand, D. J., Backes, W. H., and Jolles, J. (2006). Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia 44, 2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023
van Elderen, S. G. C., de Roos, A., de Craen, A. J. M., Westendorp, R. G. J., Blauw, G. J., Jukema, J. W. et al. (2010). Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 75, 997–1002. doi: 10.1212/WNL.0b013e3181f25f06
* 7,8,a van Impe, A., Coxon, J. P., Goble, D. J., Wenderoth, N., and Swinnen, S. P. (2011). Age-related changes in brain activation underlying single- and dual-task performance: visuomanual drawing and mental arithmetic. Neuropsychologia 49, 2400–2409. doi: 10.1016/j.neuropsychologia.2011.04.016
* 5,6,a,• Velanova, K., Lustig, C., Jacoby, L. L., and Buckner, R. L. (2007). Evidence for frontally mediated controlled processing differences in older adults. Cereb. Cortex 17, 1033–1046. doi: 10.1093/cercor/bhl013
* 7,8,a,❖ Venkatraman, V. K., Aizenstein, H., Guralnik, J., Newman, A. B., Glynn, N. W., Taylor, C. et al. (2010). Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage 49, 3436–3442. doi: 10.1016/j.neuroimage.2009.11.019
Virta, J. J., Heikkilä, K., Perola, M., Koskenvuo, M., Räihä, I., Rinne, J. O. et al. (2013). Midlife cardiovascular risk factors and late cognitive impairment. Eur. J. Epidemiol . 28, 405–416. doi: 10.1007/s10654-013-9794-y
* 7,8,a Voelcker-Rehage, C., Godde, B., and Staudinger, U. M. (2010). Physical and motor fitness are both related to cognition in old age. Eur. J. Neurosci . 31, 167–176. doi: 10.1111/j.1460-9568.2009.07014.x
* 7,8,a Waiter, G. D., Fox, H. C., Murray, A. D., Starr, J. M., Staff, R. T., Bourne, V. J. et al. (2008). Is retaining the youthful functional anatomy underlying speed of information processing a signature of successful cognitive ageing? An event-related fMRI study of inspection time performance. Neuroimage 41, 581–595. doi: 10.1016/j.neuroimage.2008.02.045
Waldstein, S. R., Carrington, S., Thayer, J. F., Najjar, S. S., Scuteri, A., and Zonderman, A. B. (2008). Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51, 99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674
* 5,6,a Wang, L., Laviolette, P., O'Keefe, K., Putcha, D., Bakkour, A., Van Dijk, K. R. A. et al. (2010a). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 51, 910–917. doi: 10.1016/j.neuroimage.2010.02.046
* 7,9,a Wang, L., Li, Y., Metzak, P., He, Y., and Woodward, T. S. (2010b). Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage 50, 862–872. doi: 10.1016/j.neuroimage.2010.01.044
* 7,9,a Wang, T. H., Kruggel, F., and Rugg, M. D. (2009). Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia 47, 1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030
Weis, S., Leube, D., Erb, M., Heun, R., Grodd, W., and Kircher, T. (2011). Functional neuroanatomy of sustained memory encoding performance in healthy aging and in Alzheimer's disease. Int. J. Neurosci . 121, 384–392. doi: 10.3109/00207454.2011.565892
White, W. B., Wolfson, L., Wakefield, D. B., Hall, C. B., Campbell, P., Moscufo, N. et al. (2011). Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 124, 2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036
Whitehead, B. P., Dixon, R. A., Hultsch, D. F., and MacDonald, S. W. S. (2011). Are neurocognitive speed and inconsistency similarly affected in type 2 diabetes? J. Clin. Exp. Neuropsychol . 33, 647–657. doi: 10.1080/13803395.2010.547845
* 7,9,a,× Wierenga, C. E., Benjamin, M., Gopinath, K., Perlstein, W. M., Leonard, C. M., Rothi, L. J. G. et al. (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol. Aging 29, 436–451. doi: 10.1016/j.neurobiolaging.2006.10.024
* 7,9,b,■,□ Wierenga, C. E., Stricker, N. H., McCauley, A., Simmons, A., Jak, A. J., Chang, Y.-L. et al. (2010). Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer's disease. Neuroimage 51, 1222–1233. doi: 10.1016/j.neuroimage.2010.03.021
* 5,6,a Wood, G., Ischebeck, A., Koppelstaetter, F., Gotwald, T., and Kaufmann, L. (2009). Developmental trajectories of magnitude processing and interference control: an FMRI study. Cereb. Cortex 19, 2755–2765. doi: 10.1093/cercor/bhp056
* 7,8,a Woodard, J. L., Seidenberg, M., Nielson, K. A., Antuono, P., Guidotti, L., Durgerian, S. et al. (2009). Semantic memory activation in amnestic mild cognitive impairment. Brain 132, 2068–2078. doi: 10.1093/brain/awp157
* 5,6,a Woodard, J. L., Seidenberg, M., Nielson, K. A., Miller, S. K., Franczak, M., Antuono, P. et al. (2007). Temporally graded activation of neocortical regions in response to memories of different ages. J. Cogn. Neurosci . 19, 1113–1124. doi: 10.1162/jocn.2007.19.7.1113
* 5,6,c Woodard, J. L., Seidenberg, M., Nielson, K. A., Smith, J. C., Antuono, P., Durgerian, S. et al. (2010). Prediction of cognitive decline in healthy older adults using fMRI. J. Alzheimers Dis . 21, 871–885. doi: 10.3233/JAD-2010-091693
* 5,6,c Woodard, J. L., Sugarman, M. A., Nielson, K. A., Smith, J. C., Seidenberg, M., Durgerian, S. et al. (2012). Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Curr. Alzheimer Res . 9, 436–446. doi: 10.2174/156720512800492477
* 5,6,a,• Wu, J.-T., Wu, H.-Z., Yan, C.-G., Chen, W.-X., Zhang, H.-Y., He, Y. et al. (2011). Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci. Lett . 504, 62–67. doi: 10.1016/j.neulet.2011.08.059
Xu, W., Caracciolo, B., Wang, H.-X., Winblad, B., Bäckman, L., Qiu, C. et al. (2010). Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 59, 2928–2935. doi: 10.2337/db10-0539
Yaffe, K., Blackwell, T., Kanaya, A. M., Davidowitz, N., Barrett-Connor, E., and Krueger, K. (2004). Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63, 658–663. doi: 10.1212/01.WNL.0000134666.64593.BA
Yaffe, K., Lindquist, K., Schwartz, A. V., Vitartas, C., Vittinghoff, E., Satterfield, S. et al. (2011). Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 77, 1351–1356. doi: 10.1212/WNL.0b013e3182315a56
Yakushiji, Y., Noguchi, T., Hara, M., Nishihara, M., Eriguchi, M., Nanri, Y. et al. (2012). Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke 43, 1800–1805. doi: 10.1161/STROKEAHA.111.647065
Yan, S. F., Ramasamy, R., and Schmidt, A. M. (2008). Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab . 4, 285–293. doi: 10.1038/ncpendmet0786
Yeung, S. E., Fischer, A. L., and Dixon, R. A. (2009). Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology 23, 1–9. doi: 10.1037/a0013849
* 5,6,c Ystad, M., Eichele, T., Lundervold, A. J., and Lundervold, A. (2010). Subcortical functional connectivity and verbal episodic memory in healthy elderly—a resting state fMRI study. Neuroimage 52, 379–388. doi: 10.1016/j.neuroimage.2010.03.062
* 5,6,c Ystad, M., Hodneland, E., Adolfsdottir, S., Haász, J., Lundervold, A. J., Eichele, T. et al. (2011). Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage 55, 24–31. doi: 10.1016/j.neuroimage.2010.11.016
Zhong, Y., Miao, Y., Jia, W. P., Yan, H., Wang, B. Y., and Jin, J. (2012a). Hyperinsulinemia, insulin resistance and cognitive decline in older cohort. Biomed. Environ. Sci . 25, 8–14. doi: 10.3967/0895-3988.2012.01.002
Zhong, Y., Zhang, X. Y., Miao, Y., Zhu, J. H., Yan, H., Wang, B. Y. et al. (2012b). The relationship between glucose excursion and cognitive function in aged type 2 diabetes patients. Biomed. Environ. Sci . 25, 1–7. doi: 10.3967/0895-3988.2012.01.001
Zhou, H., Lu, W., Shi, Y., Bai, F., Chang, J., Yuan, Y. et al. (2010). Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci. Lett . 473, 5–10. doi: 10.1016/j.neulet.2009.12.057
* 7,8,a,• Zhu, D. C., Zacks, R. T., and Slade, J. M. (2010). Brain activation during interference resolution in young and older adults: an fMRI study. Neuroimage 50, 810–817. doi: 10.1016/j.neuroimage.2009.12.087
Keywords: type 2 diabetes mellitus, hypertension, cognition, aging, imaging
Citation: Meusel L-AC, Kansal N, Tchistiakova E, Yuen W, MacIntosh BJ, Greenwood CE and Anderson ND (2014) A systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms. Front. Aging Neurosci . 6 :148. doi: 10.3389/fnagi.2014.00148
Received: 25 January 2014; Accepted: 17 June 2014; Published online: 08 July 2014.
Reviewed by:
Copyright © 2014 Meusel, Kansal, Tchistiakova, Yuen, MacIntosh, Greenwood and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole D. Anderson, Rotman Research Institute, Baycrest, 3560 Bathurst Street, Toronto, ON M6A 2E1, Canada e-mail: [email protected]
A systematic literature review: prescribing indicators related to type 2 diabetes mellitus and cardiovascular risk management
Affiliation.
- 1 Department of Clinical Pharmacology, University Medical Centre Groningen, University of Groningen, the Netherlands. [email protected]
- PMID: 19960483
- DOI: 10.1002/pds.1894
Purpose: Valid prescribing indicators (PI) are needed for reliable assessment of prescribing quality. The purpose of this study is to describe the validity of existing PI for type 2 diabetes mellitus and cardiovascular risk management.
Methods: We conducted a systematic literature search for studies describing the development and assessment of relevant PIs between January 1990 and January 2009. We grouped identified PI as drug- or disease-oriented, and according to the aspects of prescribing addressed and the additional clinical information included. We reviewed the clinimetric characteristics of the different types of PI.
Results: We identified 59 documents describing the clinimetrics of 16 types of PI covering relevant prescribing aspects, including first-choice treatment, safety issues, dosing, costs, sufficient and timely treatment. We identified three types of drug-oriented, and five types of disease-oriented PI with proven face and content validity as well as operational feasibility in different settings. PI focusing on treatment modifications were the only indicators that showed concurrent validity. Several solutions were proposed for dealing with case-mix and sample size problems, but their actual effect on PI scores was insufficiently assessed. Predictive validity of individual PI is not yet known.
Conclusion: We identified a range of existing PI that are valid for internal quality assessment as they are evidence-based, accepted by professionals, and reliable. For external use, problems of patient case-mix and sample size per PI should be better addressed. Further research is needed for selecting indicators that predict clinical outcomes.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Cardiovascular Diseases / etiology
- Cardiovascular Diseases / prevention & control*
- Diabetes Mellitus, Type 2 / complications
- Diabetes Mellitus, Type 2 / drug therapy*
- Drug Prescriptions / standards*
- Practice Patterns, Physicians' / standards*
GLP1-GIP receptor co-agonists: a promising evolution in the treatment of type 2 diabetes
- Review Article
- Open access
- Published: 03 June 2024
Cite this article
You have full access to this open access article

- Stefano Ciardullo ORCID: orcid.org/0000-0003-2422-3041 1 , 2 ,
- Mario Luca Morieri 3 ,
- Giuseppe Daniele 4 , 5 ,
- Teresa Vanessa Fiorentino 6 ,
- Teresa Mezza 7 , 8 ,
- Domenico Tricò 4 ,
- Agostino Consoli 9 , 10 ,
- Stefano Del Prato 11 ,
- Francesco Giorgino 12 ,
- Salvatore Piro 13 ,
- Anna Solini 14 &
- Angelo Avogaro 3
Explore all metrics
Type 2 diabetes represents a growing challenge for global public health. Its prevalence is increasing worldwide, and, like obesity, it affects progressively younger populations compared to the past, with potentially greater impact on chronic complications. Dual glucagon like peptide 1 (GLP1) and glucose-dependent insulinotropic peptide (GIP) receptor agonists are among the new pharmacological strategies recently developed to address this challenge. Tirzepatide, characterized by its ability to selectively bind and activate receptors for the intestinal hormones GIP and GLP-1, has been tested in numerous clinical studies and is already currently authorized in several countries for the treatment of type 2 diabetes and obesity. In this context, the aim of the present document is to summarize, in the form of a narrative literature review, the currently available data on the main mechanisms of action of GIP/GLP-1 co-agonists and the clinical effects of tirzepatide evaluated in various clinical trials.
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) and obesity pose a growing challenge to public health, with millions of people affected by these pathological conditions and socio-economic costs steadily increasing worldwide. In Italy, one in twenty adults aged 18–69 years has been diagnosed with diabetes, and four in ten adults are overweight [ 1 ]. The prevalence of these conditions rises with age and is higher in men than women and in individuals with lower incomes and education.
In parallel, the landscape of pharmacological therapies for T2DM and (to a lesser extent) obesity has expanded in recent decades with new classes of molecules coming into the market. These novel therapies have often exceeded expectations by demonstrating beneficial effects that extend to complications that were not the primary focus of their development. Among these new therapeutic options, tirzepatide has gathered significant interest in the scientific and medical community as the first member of a new class of drugs characterized by their ability to selectively bind and activate the receptors for the intestinal hormones GIP and GLP-1. Tirzepatide has received approval for use in patients with T2D following several phase 3 studies testing and demonstrating its marked anti-hyperglycemic efficacy and positive actions on multiple cardiovascular risk factors, associated with an excellent safety profile [ 2 , 3 ]. Approval for the treatment of obesity, irrespective of the presence of diabetes, has also been grated [ 4 ]. Additionally, further investigations are underway to explore potential additional uses of tirzepatide in the clinical management of other cardiometabolic conditions associated with obesity, including heart failure and non-alcoholic (or metabolic-dysfunction associated) steatohepatitis (NASH/MASH).
In this narrative literature review, we explore the pharmacological properties of tirzepatide and critically summarize and comment on the results of the clinical studies conducted thus far, as well as discuss the potential implications for the treatment of obesity, T2DM, and associated cardiometabolic complications.
New evidence on the actions of GLP-1
GLP-1 is secreted by L cells located in the distal ileum and colon in response to nutrient ingestion. The interaction of GLP-1 with its receptor leads to an increase in intracellular cAMP levels and activation of numerous cellular processes that vary depending on the organ or system involved [ 5 ]. Among the most well-known and studied effects of GLP-1 is its impact on the pancreatic islet, where the activation of GLP-1-associated signaling pathways results in increased insulin secretion and reduced glucagon production. This response promotes proper nutrient metabolism by increasing glucose utilization in insulin-dependent tissues such as muscle and adipose tissue, reducing endogenous glucose production, and enhancing glycogen synthesis.
GLP-1 is also responsible for a multitude of extra-pancreatic effects that made its pharmacological development even more interesting. This has led to the creation of GLP-1 agonists and analogues capable of effectively activating all receptor and cellular systems compatible with GLP-1. These agonists/analogues of GLP-1 slow down gastric emptying, modulate calorie intake by increasing the sense of satiety, modulate cardiovascular activity, and regulate natriuresis at the renal level [ 6 ]. Recently, several pieces of evidence have expanded our understanding of the effects of GLP-1, demonstrating innovative actions even in well-known targets such as the pancreatic islet. In particular, GLP-1 analogues have shown direct effects on the plasticity of the pancreatic islet in both normal glucose tolerance and throughout the whole spectrum of alterations leading to overt diabetes [ 7 ]. Evidence indicates that the pancreatic islet is dynamic, plastic, and characterized by processes of trans-differentiation and de-differentiation in a high percentage of T2DM subjects. GLP-1, in addition to its known effects on stimulating beta-cell proliferation and inhibiting apoptosis, is involved in the modulation of trans-differentiation and de-differentiation processes [ 7 ].
Through the analysis of numerous in vivo and in vitro studies aimed at determining the mechanisms by which GLP-1 enhances insulin secretion in individuals with normal glucose tolerance or T2DM, two main intracellular mechanisms underlying the incretin effect have emerged. The first mechanism involves the enhancement of the reloading of the insulin pool available for immediate release, occurring only in the presence of incretin and at a certain glucose threshold. The second mechanism is characterized by the modulation of intracellular calcium, which seems to function as a trigger for rapid insulin exocytosis and complements the amplification phenomena [ 8 ].
The functional and structural modulation of the pancreatic islet mediated by GLP-1 also has an impact on liver pathophysiology. The complex interaction of factors such as insulin resistance, glucotoxicity and lipotoxicity contribute to the pathogenesis and coexistence of diabetes and metabolic-dysfunction associated steatotic liver disease (MASLD) in a reciprocal process of exacerbating the underlying conditions [ 9 ]. Diabetes promotes the progression of MASLD to steatohepatitis, cirrhosis, and hepatocellular carcinoma. GLP-1 agonists/analogues show beneficial effects on most of the multiple alterations underlying MASLD and cirrhosis progression [ 10 ]. On the other hand, it is still not entirely clear whether the hepatic effects of GLP-1 are direct or indirect, as recent studies have not clearly demonstrated the presence of GLP-1 receptors in human hepatocytes. A recent in vivo study in healthy subjects showed that acute intravenous infusion of GLP-1 during a pancreatic clamp, in which insulin and glucagon levels were maintained at basal levels, caused a reduction in hepatic glucose production, suggesting a potential direct effect on gluconeogenesis [ 11 ]. Another study showed that acute administration of exenatide was associated with increased hepatic glucose uptake during an oral glucose tolerance test (OGTT) and a reduction in endogenous glucose production, reinforcing the hypothesis of a direct action of GLP-1 on the liver [ 12 ].
The gastrointestinal system is highly integrated with the central nervous system (CNS). Enteroendocrine cells (EECs), the microbiota, and metabolites produced by the microbiota constitute a complex signaling system [ 13 ] that involves the enteric plexus, CNS afferents and efferents, and the modulation of numerous brain nuclei like the nucleus tractus solitarius, hypothalamus, thalamus, and many others directly involved in the control of glucose and energy homeostasis [ 14 ]. GLP-1 has been shown to effectively contribute to the functional integration between the CNS and peripheral metabolism; modulation of calorie intake, satiety, and energy balance are just a few of the most evident effects caused by these actions [ 15 ]. In recent years, the effects of GLP-1 agonists/analogues on the CNS have gained relevance due to emerging evidence supporting the neuroprotective effects of these molecules. Diabetes is associated with an increased risk of developing dementia and neurodegenerative diseases, which is inversely related to the degree of glucose tolerance and directly to the disease duration [ 16 ]. On the other hand, strict glucose control does not limit or improve the damage caused by diabetes and its comorbidities to the CNS [ 17 , 18 ] as the impact of insulin resistance, obesity, and diabetes on the CNS begins very early in the natural history of the disease [ 19 ]. CNS plasticity and cerebral glucose uptake are directly modulated by GLP-1 receptor activation [ 20 , 21 ]. The potential clinical implications of these observations have been confirmed by a recent multicenter trial involving over 4000 patients, in which dulaglutide resulted in approximately a 14% reduction in the risk of cognitive decline after 5 years of therapy [ 22 ]. The neuroprotective effect of GLP-1 is also evident in intervention studies on neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Individuals with diabetes have an increased risk of developing neurodegenerative diseases, and the combination of cerebral and peripheral insulin resistance constitutes one of the main mechanisms underlying the initiation and progression of neurodegenerative diseases [ 23 ]. In a trial conducted on a small number of subjects with Alzheimer’s disease, treatment with liraglutide was able to prevent the progressive decline in cerebral glucose uptake that characterizes patients with the disease [ 24 ]. Similarly, patients with Parkinson’s disease showed an improvement in motor parameters according to the criteria of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) after treatment with exenatide [ 25 , 26 ]. Therefore, GLP-1 agonists/analogues represent a continuously evolving class of drugs that can modulate mechanisms aimed at achieving increasingly ambitious glycemic and body weight goals. Additionally, the pleiotropic actions of GLP-1 expand the therapeutic potential of GLP-1 agonists/analogues through combination with other drug classes, suggesting new scenarios in terms of enhancing cardio-renal protection and expanding clinical indications.
Biological effects of GIP
The Gastric Inhibitory Polypeptide (GIP), isolated for the first time from porcine small intestine in 1971 [ 27 ], was named as GIP on the basis of its ability to inhibit gastric hydrochloric acid secretion [ 28 ]. It is a 42-amino acid peptide secreted by the K-cells, present in high density in the duodenum and upper jejunum, following oral ingestion of nutrients such as glucose, amino acids, and long-chain fatty acids (Fig. 1 ). Incretin function of GIP has emerged some years after its identification [ 29 ]. Several pieces of evidence have demonstrated that GIP administration results in an improved glucose-dependent insulin secretion [ 30 , 31 ]. Similarly to GLP-1, GIP exerts several beneficial effects on β cells. Indeed, GIP administration reduces β cell apoptosis and enhances β cell mass in animal models [ 32 ]. Additionally, GIP modulates α cells function in a glucose-dependent manner. In hypoglycemic and normoglycemic conditions, GIP administration stimulates glucagon secretion in healthy subjects. On the other hand, during a hyperglycemic clamp, GIP has no effect on glucagon secretion [ 30 , 31 ]. Interestingly, several alterations in intra-pancreatic actions of GIP have been found in subjects affected by T2DM. Incretin action of GIP is dramatically reduced in the presence of diabetes, as demonstrated by the evidence that glucose-dependent insulin secretion is not potentiated by GIP administration in subjects with diabetes [ 33 , 34 ]. On the contrary, the positive effect of GIP on glucagon secretion is preserved [ 34 ]. The reduced incretin effect of GIP in diabetic subjects may be due to the down-regulation of GIP receptor expression, which has been found in pancreatic islets of diabetic Zucker rats and reverted following correction of hyperglycemia [ 35 ].
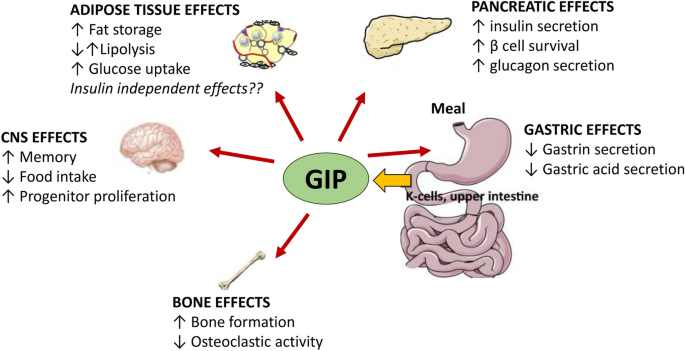
Gastro-intestinal and extra gastro-intestinal effects of glucose-dependent insulinotropic peptide
Notably, GIP exerts several metabolic actions on extra-pancreatic tissues including adipose tissue, brain and bone (Fig. 1 ). Studies exploring the effects of GIP on adipose tissue have provided controversial results. Deletion of GIP receptor has been found to counteract high-fat diet induced weight gain in mice, suggesting a causal role of GIP in development of obesity [ 36 ]. Moreover, GIP administration reduces lipolysis-related genes expression in adipose tissue of overweight subjects and consequently decreases circulating free fatty acid levels [ 37 ]. On the other hand, Timper et al. [ 38 ] described an augmented lipolysis in differentiated human preadipocyte-derived adipocytes upon GIP stimulation. This divergent evidence may be due to the different experimental conditions of the studies; moreover it is still unclear whether GIP has direct, insulin independent, metabolic effects on adipose tissue. GIP receptor has been found to be expressed in the central nervous system. In particular, mature neurons and progenitor cells in the adult rat hippocampus express GIP receptors, and treatment with GIP is able to promote neuronal cell proliferation [ 39 ]. Additionally, GIP contributes to the regulation of hunger sensation. Indeed, acute activation of GIP signaling in hypothalamic cells results in a decreased food intake in rodents [ 40 ]. Furthermore, several preclinical studies have demonstrated the beneficial effects of GIP on bone mass. GIP has been found to directly stimulate bone formation by osteoblastic cells and inhibit osteoclastic activity [ 41 , 42 ]. The protective effects of GIP on the bone have been also observed in humans with and without diabetes [ 43 , 44 ].
Effects of tirzepatide on body weight
GLP-1 receptor agonists, initially developed and marketed to exploit their relevant hypoglycemic effects, have shown positive but heterogeneous effects on body weight control. These effects depend on the type, route of administration, dose, and duration of action of each specific GLP-1 receptor agonist, as well as individual characteristics such as baseline body weight and degree of glucose tolerance. In the SCALE study, liraglutide, administered daily at a dose of 0.6–3.0 mg, was associated with a body weight loss 5.6 kg greater than placebo [ 45 ].
The efficacy of liraglutide on weight management was subsequently surpassed by semaglutide, which, when administered weekly at a dose of 2.4 mg in obese patients without diabetes, resulted in a weight reduction of 12.4% compared to placebo in the STEP 1 study [ 46 ], and a reduction of 9.4% compared to liraglutide 3.0 mg in the STEP-8 study [ 47 ]. With the development of the first GIP/GLP-1 co-agonist, tirzepatide, there has been an exponential increase in the effectiveness of incretin-mimetic pharmacological therapy for weight control in obese patients with and without diabetes. In subjects with T2DM, weekly administration of tirzepatide at doses of 5 mg, 10 mg, or 15 mg was associated with greater weight loss compared to placebo (− 6.3 kg, − 8.4 kg, and − 9.4 kg, respectively), and 1.7 kg, 4.8 kg, and 7.2 kg greater weight loss compared to selective GLP-1 receptor agonists, demonstrating a dose-dependent effect sustained for up to 2 years [ 48 ].
In the SURPASS-2 study, tirzepatide 15 mg resulted in a faster and greater decline in body weight (− 5.5 kg) compared to semaglutide 1.0 mg, more than doubling the proportion of patients achieving at least 10% weight loss (57% vs 24%) [ 3 ]. Greater weight loss efficacy of tirzepatide 10 mg (− 3.2 kg) and 15 mg (− 5.2 kg) compared to semaglutide was also observed in an indirect comparison with data from the SUSTAIN FORTE study, where semaglutide was administered at the maximum hypoglycemic dose of 2.0 mg [ 49 ].
The effects of tirzepatide on body weight control have also been confirmed in non-diabetic individuals in the SURMOUNT-1 study, where treatment with tirzepatide 10–15 mg was associated with an average weight loss of approximately 20% at 72 weeks, exceeding 25% in one third of the patients. It is interesting to note that the positive effect of tirzepatide on body weight is associated with a significant improvement in quality of life and physical performance [ 50 ] and is independent of sex [ 51 ], baseline body mass index (BMI) [ 52 ] and potential side effects, predominantly gastrointestinal [ 53 ]. Furthermore, the weight loss induced by tirzepatide appears to be primarily due to a greater reduction in fat mass compared to metabolically active lean mass, resulting in a favorable redistribution of abdominal ectopic adipose tissue and a marked reduction in waist circumference and intrahepatic fat [ 54 ]. The exact mechanisms underlying tirzepatide-induced weight loss remain to be clarified. The simultaneous activation of GIP and GLP-1 receptors may have central synergistic effects on appetite control, greater than selective GLP-1 receptor agonists [ 55 ], although preliminary studies do not support this hypothesis [ 56 ]. Alternative and non-mutually exclusive mechanisms may involve favorable effects on basal energy expenditure [ 57 ] or on the choice of less energy-rich foods consumed in free-living conditions [ 58 ], although these latter hypotheses remain to be validated in clinical studies.
Glucose control
The SURPASS studies enrolled populations that were similar in terms of baseline HbA1c levels, which ranged from 7.9 to 8.5% [ 2 , 3 , 59 , 60 , 61 ] and found a statistically significant reduction in HbA1c already at the 5 mg dose, demonstrating a greater efficacy of tirzepatide compared to placebo or active comparator [ 2 , 3 , 59 , 60 , 61 ]. Interestingly, the reduction in HbA1c was already evident after the first 4 weeks of treatment and remained statistically significant up to the end of the 52-week observation period, supporting the evidence of rapid efficacy of the drug and great durability, which was independent from weight loss.
In terms of achievement of HbA1c target, 81% and 97% of patients treated with tirzepatide reached an HbA1c less than 7%, while 71% and 95% of patients achieved a HbA1c of less than 6.5%. In addition, 23% and 61% of patients achieved HbA1c values below 5.7%, with no documented hypoglycemia episodes in SURPASS 1 (vs. add on to other therapeutic schemes). It is interesting to note that the efficacy of Tirzepatide evaluated on a 7-point daily glucose profile demonstrated better control at all points of the glycemic profile compared to semaglutide 1 mg [ 3 ]; on the other hand, the comparison with insulin degludec showed that although insulin, titrated during the study, obtained a better result on fasting blood glucose, treatment with tirzepatide 10 and 15 mg demonstrated better control on all other points of glucose profile [ 60 ].
In addition to the improvement in HbA1c and glucose profile, tirzepatide has also demonstrated to improve insulin resistance, as assessed by HOMA-IR. This effect was independent from the dose of tirzepatide used and was similar in case of placebo [ 59 ] or treatment with active comparator semaglutide 1 mg [ 3 ].
In order to evaluate the mechanisms of action of tirzipatide, in a recent study [ 62 ] subjects with T2DM performed a deep metabolic evaluation with measures of beta cell function and insulin sensitivity by hyperglycemic clamp, mixed meal test and euglycemic clamp before and 28 weeks after treatment with tirzepatide 15 mg or semaglutide 1 mg. This study confirmed that tirzepatide induces a great improvement in insulin sensitivity compared to semaglutide, as already observed with HOMA-IR. In addition, a significant improvement in the first and second phase of insulin secretion was observed with tirzepatide treatment, as well as an improvement in beta cell function, estimated with beta-cell glucose sensitivity. Compared with semaglitude 1 mg, treatment with tirzepatide demonstrated reduced glucagon secretion in response to mixed meal, suggesting an additive effect compared to GPL1 RA on glucagon suppression.
The efficacy of GIP/GLP1 receptor co-agonists on glucose control is surprising, and allows to reach glucose levels similar to diabetes remission in a greater percentage of patients compared to other treatments. The mechanisms of action of tirzepatide are multiple and need to be further investigated, but the results obtained so far suggest an effect both in improving insulin resistance and in enhancing beta cell function by restoring insulin secretion.
Prevention of cardiorenal complications and beyond
With the improvement in the management of cardiovascular risk factors, a progressive decline in the annual incidence rate of cardiovascular morbidity and mortality has been observed in both the general population and the diabetic population over the past decades. However, this reduction has progressed in parallel among groups of patients with and without diabetes, and the gap in CVD risk between individuals with and without diabetes is unchanged [ 63 ]. At the same time, the incidence of diabetic nephropathy, although reduced compared to 20 years ago, is stabilized in recent years, and the incidence of end-stage renal disease (due to reduced cardiovascular mortality and increased life expectancy) is increasing [ 64 ]. Furthermore, the secular trends of causes of mortality in patients with diabetes shows an increase for neurodegenerative causes (e.g., dementia) and liver diseases [ 65 ]. This leads to a scenario where new therapeutic strategies for diabetes must inevitably consider not only glycemic and weight control but also the reduction of both vascular and non-vascular complications. In this regard, the GIP and GLP-1 agonist, tirzepatide, currently shows promising data derived from phase 2 and 3 clinical trials.
Hepatic Steatosis : Recently, a sub-study of the phase 3 clinical trial SURPASS-3 showed that, among patients receiving metformin and/or SGLT2 inhibitors, the addition of tirzepatide (10 or 15 mg) as compared to insulin degludec was able to significantly reduce hepatic fat content (evaluated by magnetic resonance) after 52 weeks of treatment. The absolute difference in liver fat content [LFC] was − 4.7% (95% CI from − 6.7 to − 2.7), with a relative decrease in LFC from baseline of − 35.9% and − 28.4% for the two individual dosages ( p < 0.0005), and a significant reduction of − 18.6% even at the 5 mg dosage. This reduction in LFC was largely explained by weight loss and improved glycemic control induced by tirzepatide (50%), although it is possible to hypothesize that the remaining effect may be related to improvements in lipotoxicity, inflammation, and mitochondrial function caused by tirzepatide [ 54 ]. These data will need biopsy evaluation to assess the benefits of tirzepatide on the histological features of NAFLD/NASH or fibrosis, which are currently being collected in an ongoing clinical trial (SYNERGY-NASH-NCT04166773).
Diabetic Kidney Disease : The effect of tirzepatide on the progression of renal damage has been evaluated so far in the post-hoc analysis of the SURPASS-4 study (phase 3, open-label), which randomized patients receiving metformin, sulfonylurea, or SGLT2 inhibitors to tirzepatide (5 mg, 10 mg, or 15 mg weekly) versus insulin glargine (100 U/ml) for 104 weeks. After a median of 85 weeks, the decline in estimated glomerular filtration rate (eGFR) was 2.2 ml/min/1.73m 2 /year (95% CI 1.6 to 2.8) in favor of tirzepatide, accompanied by a difference in the urinary albumin/creatinine ratio (uACR) of − 31.9% (95% CI from − 37.7 to − 25.7%), also in favor of tirzepatide. The composite renal outcome (ESRD, eGFR decline > 40%, renal death, new onset of macroalbuminuria) strongly favored tirzepatide (HR 0.58, 95% CI from 0.43 to 0.80, p = 0.0008); such results were primarily driven by the reduced incidence of macroalbuminuria. The relatively small numbers (n = 1989) and the short duration of the study require caution, but the results appear highly promising while awaiting the results of ongoing studies (SURPASS-CVOT) [ 66 ].
Cardiovascular Safety : The cardiovascular safety of tirzepatide has been evaluated and confirmed to date through a pre-specified meta-analysis of phase 2 and 3 randomized trials comparing tirzepatide with other placebo, insulin glargine, degludec, semaglutide, dulaglutide for a minimum of 26 weeks (including the GPGB, SURPASS 1,2,3,4,5, and J-mono studies) [ 67 ]. The analysis of 4887 participants treated with tirzepatide and 2,328 participants in the control groups identified 142 participants who developed at least one MACE-4p (inclusive of cardiovascular mortality, myocardial infarction, stroke, and hospitalization for unstable angina) and confirmed the cardiovascular safety with an hazard ratio (HR) of 0.80 (95% CI 0.57–1.11) for the comparison between tirzepatide and controls. This finding was consistent for cardiovascular mortality (HR 0.90, 95% CI from 0.50 to 1.61) and all-cause mortality (HR 0.80, 95% CI from 0.51 to 1.25).
However, it is important to exercise caution in interpreting these hazard ratios. On one hand, the confidence intervals only allow for conclusions regarding non-inferiority, and on the other hand, the average duration of observation was only 1 year. It should also be noted that, from a methodological perspective, the model used (Cox with stratification by cardiovascular risk class of the trials, i.e., SURPASS-4: high risk, and all others: low risk) requires various assumptions, including the homogeneity of the relative effect of tirzepatide treatment on MACE regardless of the baseline cardiovascular risk level of the patients. This assumption is desirable but will need to be confirmed in subsequent studies. Therefore, for now, it is better to focus on the conclusions that tirzepatide is safe from a cardiovascular perspective. However, it is only a matter of time before we will have more definitive answers. Indeed, the SURPASS-CVOT study, which compares tirzepatide head-to-head with dulaglutide, will likely provide more comprehensive answers (expected by the end of 2024).
Perspectives on clinical impact and adherence
The World Health Organization defines adherence as “the extent to which a person’s behavior—taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider” [ 68 ]. Several factors influence the patient’s level of adherence and can be distinguished as social and economic factors, patient-related factors (health beliefs, health literacy), therapy-related factors (complexity of the treatment, adverse events), presence of comorbidities (neurological/psychiatric or of different origins), and factors related to the healthcare system (doctor-patient relationship, difficulty in obtaining follow-up visits). These barriers account to varying degrees for the reduced level of adherence described for multiple asymptomatic chronic conditions, including diabetes. A meta-analysis has indeed highlighted that only about 50–60% of patients with diabetes adhere to antidiabetic therapy [ 69 ], with a significant impact on quality of life and life expectancy. Several real-world studies based on administrative data and pharmacy reports have demonstrated that reduced adherence to antidiabetic therapy is associated not only with poorer glycemic control but also with a significant increase in overall mortality and hospitalizations for all causes [ 70 , 71 ].
There are several interventions capable of promoting adherence to pharmacological treatment. Education plays a critical role, as demonstrated by various randomized trials [ 72 ]. It is essential to verify the patient’s understanding of the prescribed therapeutic regimen, clarify the benefits that can result from the therapy, discuss possible adverse events, and provide useful advice to minimize them, as well as simplify the therapeutic regimen when appropriate [ 73 ]. While real-world data on the level of adherence to dual GLP1-RA/GIP agonists are not available to date, some considerations can be drawn from available clinical trials and experiences with other pharmacological classes. In the SURPASS program, gastrointestinal side effects associated with the use of tirzepatide were similar to those observed with semaglutide and GLP1-RA drugs in general, both in terms of frequency and duration [ 3 ]. Tirzepatide has also demonstrated superior efficacy compared to any other therapy used in the treatment of T2DM, comparable or superior even to regimes based on the use of multiple pharmacological classes. Since several studies document a decrease in adherence as therapeutic complexity increases, an improvement in therapeutic adherence can be expected with the use of this drug, especially considering the excellent results that patients can achieve in terms of weight reduction. Lastly, as demonstrated by a large online survey, patients with T2DM generally have a positive attitude towards the option of a weekly-administered antidiabetic therapy rather than a daily one [ 74 ], especially when it involves a user-friendly device [ 75 ]. These data instill optimism about the impact that this drug might have in the treatment of T2DM and its complications in every day clinical practice.
Conclusions
The currently available evidence on the mechanisms of action and clinical effects of GLP-1/GIP dual agonism supports very optimistic prospects for the future use of tirzepatide. This new molecule, by acting synergistically on combined systems known to be altered in T2DM [ 33 , 34 ], could indeed expand the therapeutic potential of GLP-1 agonists/analogues, suggesting new scenarios in terms of improving glycemic control, weight management, and hopefully providing protection against chronic micro-macrovascular complications and not only limited to hepatic complications.
The potential of this molecule should be understood within the context of the epidemiological evolution of T2DM, characterized by several key elements, including: 1. The progressive increase in prevalence both globally and nationally [ 1 ]. 2. The reduction in the average age of onset of diabetes (and obesity) [ 76 ] 3. The greater impact on morbidity and mortality of diabetes in patients who develop the condition at a younger age compared to those who develop it later [ 77 ]. Therefore, while efforts undoubtedly need to be intensified towards preventing the onset of the disease and making lifestyle modifications (a fundamental aspect of the therapy for every patient with T2DM), it is clear, as indicated by the most recent guidelines [ 78 ], that the pharmacological approach should focus on timely treatment that leaves no room for therapeutic inertia and employs medications that are as effective as possible in both metabolic control and the prevention of chronic complications.
In this context, the data currently available on tirzepatide, showing its efficacy in glycemic control to the extent that a considerable percentage of patients achieve glucose levels indicative of diabetes remission [ 3 , 59 ], as well as its high effectiveness in weight management, are promising regarding its future use in clinical practice. These clear effects (observed through comparison with various comparators, including current weekly GLP1-RAs on the market) are complemented by interesting data on cardiovascular safety and efficacy (from post-hoc studies) regarding the incidence and progression of nephropathy and hepatic steatosis. It is still too early to draw definitive conclusions, but the numerous ongoing clinical trials, including the cardiovascular safety trial versus an active comparator (dulaglutide), the first of its kind, will provide us shortly with important confirmations.
Istituto Superiore di Sanità (2021) Dati Sorveglianza PASSI 2020–2021. 2023
Ludvik B, Giorgino F, Jodar E et al (2021) Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398(10300):583–598. https://doi.org/10.1016/S0140-6736(21)01443-4
Article CAS PubMed Google Scholar
Frias JP, Davies MJ, Rosenstock J et al (2021) Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 385(6):503–515. https://doi.org/10.1056/NEJMoa2107519
Jastreboff AM, Aronne LJ, Ahmad NN et al (2022) Tirzepatide once weekly for the treatment of obesity. N Engl J Med 387(3):205–216. https://doi.org/10.1056/NEJMoa2206038
Deganutti G, Liang Y-L, Zhang X et al (2022) Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation. Nat Commun 13(1):92
Article CAS PubMed PubMed Central Google Scholar
Laurindo LF, Barbalho SM, Guiguer EL et al (2022) GLP-1a: going beyond traditional use. Int J Mol Sci 23(2):739
Mezza T, Cinti F, Cefalo CMA, Pontecorvi A, Kulkarni RN, Giaccari A (2019) β-cell fate in human insulin resistance and type 2 diabetes: a perspective on islet plasticity. Diabetes 68(6):1121–1129
Grespan E, Giorgino T, Natali A, Ferrannini E, Mari A (2021) Different mechanisms of GIP and GLP-1 action explain their different therapeutic efficacy in type 2 diabetes. Metabolism 114:154415
Budd J, Cusi K (2020) Role of agents for the treatment of diabetes in the management of nonalcoholic fatty liver disease. Curr DiabRep 20:1–9
Google Scholar
Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G (2021) Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites 11(2):73
Seghieri M, Rebelos E, Gastaldelli A et al (2013) Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 56:156–161
Gastaldelli A, Gaggini M, Daniele G et al (2016) Exenatide improves both hepatic and adipose tissue insulin resistance: a dynamic positron emission tomography study. Hepatology 64(6):2028–2037
Wachsmuth HR, Weninger SN, Duca FA (2022) Role of the gut–brain axis in energy and glucose metabolism. Exp Mol Med 54(4):377–392
Clemmensen C, Müller TD, Woods SC, Berthoud H-R, Seeley RJ, Tschöp MH (2017) Gut-brain cross-talk in metabolic control. Cell 168(5):758–774
Frias JP, Bonora E, Nevarez Ruiz L et al (2021) Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care 44(3):765–773
Article PubMed PubMed Central Google Scholar
Kim WJ, Lee SJ, Lee E, Lee EY, Han K (2022) Risk of incident dementia according to glycemic status and comorbidities of hyperglycemia: a nationwide population-based cohort study. Diabetes Care 45(1):134–141
Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ (2015) Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol 14(3):329–340
Article PubMed Google Scholar
Biessels GJ, Verhagen C, Janssen J et al (2019) Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care 42(10):1930–1938
Lunghi C, Daniele G, Binda P et al (2019) Altered visual plasticity in morbidly obese subjects. Iscience 22:206–213
Animali S, Steinwurzel C, Dardano A et al (2023) Effect of fasting on short-term visual plasticity in adult humans. Eur J Neurosci 57(1):148–162
Daniele G, Iozzo P, Molina-Carrion M et al (2015) Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes 64(10):3406–3412
Cukierman-Yaffe T, Gerstein HC, Colhoun HM et al (2020) Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol 19(7):582–590
Onaolapo AY, Ojo FO, Adeleye OO, Falade J, Onaolapo OJ (2023) Diabetes mellitus and energy dysmetabolism in Alzheimer’s disease: understanding the relationships and potential therapeutic targets. Curr Diabetes Rev 19:31–45
Article Google Scholar
Gejl M, Gjedde A, Egefjord L et al (2016) In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci 108:198350
Athauda D, Maclagan K, Skene SS et al (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390(10103):1664–1675
Athauda D, Gulyani S, Kumar Karnati H et al (2019) Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol 76(4):420–429
Brown JC, Dryburgh JR (1971) A gastric inhibitory polypeptide II: the complete amino acid sequence. Can J Biochem 49(8):867–872
Brown J, Dryburgh J, Ross S, Dupre J (1975) Identification and actions of gastric inhibitory polypeptide. In: Proceedings of the 1974 Laurentian hormone conference, Elsevier, pp 487–532
Dupre J, Ross S, Watson D, Brown J (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37(5):826–828
Elahi D, Andersen DK, Brown JC et al (1979) Pancreatic alpha-and beta-cell responses to GIP infusion in normal man. Am J Physiol 237(2):E185-191. https://doi.org/10.1152/ajpendo.1979.237.2.E185
Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK (2011) Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 60(12):3103–3109
Yanagimachi T, Fujita Y, Takeda Y et al (2016) Pancreatic glucose-dependent insulinotropic polypeptide (GIP) (1–30) expression is upregulated in diabetes and PEGylated GIP(1–30) can suppress the progression of low-dose-STZ-induced hyperglycaemia in mice. Diabetologia 59(3):533–541. https://doi.org/10.1007/s00125-015-3842-y
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W (1993) Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91(1):301–307. https://doi.org/10.1172/jci116186
Chia CW, Odetunde JO, Kim W, Carlson OD, Ferrucci L, Egan JM (2014) GIP contributes to islet trihormonal abnormalities in type 2 diabetes. J Clin Endocrinol Metab 99(7):2477–2485. https://doi.org/10.1210/jc.2013-3994
Piteau S, Olver A, Kim SJ et al (2007) Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat. Biochem Biophys Res Commun 362(4):1007–1012. https://doi.org/10.1016/j.bbrc.2007.08.115
Miyawaki K, Yamada Y, Ban N et al (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8(7):738–742
Gögebakan Ö, Andres J, Biedasek K et al (2012) Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes 61(2):292–300. https://doi.org/10.2337/db10-0902
Timper K, Grisouard J, Sauter NS et al (2013) Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am J Physiol Endocrinol Metab 304(1):E1-13. https://doi.org/10.1152/ajpendo.00100.2012
Nyberg J, Anderson MF, Meister B et al (2005) Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci 25(7):1816–1825. https://doi.org/10.1523/jneurosci.4920-04.2005
Adriaenssens AE, Biggs EK, Darwish T et al (2019) Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab 30(5):987-996.e986. https://doi.org/10.1016/j.cmet.2019.07.013
Bollag RJ, Zhong Q, Phillips P et al (2000) Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 141(3):1228–1235. https://doi.org/10.1210/endo.141.3.7366
Zhong Q, Itokawa T, Sridhar S et al (2007) Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab 292(2):E543-548. https://doi.org/10.1152/ajpendo.00364.2006
Christensen MB, Lund AB, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK (2020) Glucose-dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with type 2 diabetes. J Endocr Soc 4(9):bvaa097. https://doi.org/10.1210/jendso/bvaa097
Christensen MB, Lund A, Calanna S et al (2018) Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab 103(1):288–294. https://doi.org/10.1210/jc.2017-01949
Pi-Sunyer X, Astrup A, Fujioka K et al (2015) A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373(1):11–22. https://doi.org/10.1056/NEJMoa1411892
Wilding JPH, Batterham RL, Calanna S et al (2021) Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183
Rubino DM, Greenway FL, Khalid U et al (2022) Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA 327(2):138–150. https://doi.org/10.1001/jama.2021.23619
Karagiannis T, Avgerinos I, Liakos A et al (2022) Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia 65(8):1251–1261. https://doi.org/10.1007/s00125-022-05715-4
Vadher K, Patel H, Mody R et al (2022) Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: an adjusted indirect treatment comparison. Diabetes Obes Metab 24(9):1861–1868. https://doi.org/10.1111/dom.14775
Matza LS, Stewart KD, Landó LF, Patel H, Boye KS (2022) Exit interviews examining the patient experience in clinical trials of tirzepatide for treatment of type 2 diabetes. Patient Patient-Centered Outcomes Res 15(3):367–377. https://doi.org/10.1007/s40271-022-00578-8
Plat AW, Rasouli N, Peleshok J, Sapin H, Wilding J (2022) Change in body weight from baseline with tirzepatide: sex subgroup analysis of the SURPASS studies. Diabetes. https://doi.org/10.2337/db22-720-P
Wilding JPH, Kwan AYM, Maldonado JM, Wang H, Rasouli N (2022) Tirzepatide induces weight loss in patients with type 2 diabetes regardless of baseline BMI: a post hoc analysis of SURPASS-1 through SURPASS-5 studies. Diabetologia 65(Suppl 1):S289–S289
Patel H, Khunti K, Rodbard HW et al (2022) Tirzepatide-induced weight loss in type 2 diabetes is independent of nausea, vomiting, or diarrhoea. Diabetologia 65(Suppl 1):S290–S291
Gastaldelli A, Cusi K, Fernandez Lando L, Bray R, Brouwers B, Rodriguez A (2022) Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 10(6):393–406. https://doi.org/10.1016/S2213-8587(22)00070-5
Samms RJ, Coghlan MP, Sloop KW (2020) How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab 31(6):410–421. https://doi.org/10.1016/j.tem.2020.02.006
Coskun T, Heise T, DeVries J et al (2022) Tirzepatide reduces appetite, energy intake and fat mass in people with type 2 diabetes. Diabetologia 65(Suppl 1):S288–S288
Samms RJ, Zhang G, He W et al (2022) Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol Metab 64:101550. https://doi.org/10.1016/j.molmet.2022.101550
Geisler CE, Antonellis MP, Trumbauer W et al (2023) Tirzepatide suppresses palatable food intake by selectively reducing preference for fat in rodents. Diabetes Obes Metab 25(1):56–67
Rosenstock J, Wysham C, Frias JP et al (2021) Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398(10295):143–155. https://doi.org/10.1016/S0140-6736(21)01324-6
Del Prato S, Kahn SE, Pavo I et al (2021) Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398(10313):1811–1824. https://doi.org/10.1016/S0140-6736(21)02188-7
Dahl D, Onishi Y, Norwood P et al (2022) Effect of subcutaneous tirzepatide vs placebo added to titrated Insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 327(6):534–545. https://doi.org/10.1001/jama.2022.0078
Heise T, Mari A, DeVries JH et al (2022) Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol 10(6):418–429. https://doi.org/10.1016/S2213-8587(22)00085-7
Rawshani A, Rawshani A, Gudbjörnsdottir S (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 377(3):300–301. https://doi.org/10.1056/NEJMc1706292
Halminen J, Sattar N, Rawshani A et al (2022) Range of risk factor levels, risk control, and temporal trends for nephropathy and end-stage kidney disease in patients with type 1 and type 2 diabetes. Diabetes Care 45(10):2326–2335. https://doi.org/10.2337/dc22-0926
Pearson-Stuttard J, Cheng YJ, Bennett J et al (2022) Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 10(1):46–57. https://doi.org/10.1016/s2213-8587(21)00288-6
Heerspink HJL, Sattar N, Pavo I et al (2022) Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol 10(11):774–785. https://doi.org/10.1016/s2213-8587(22)00243-1
Sattar N, McGuire DK, Pavo I et al (2022) Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 28(3):591–598. https://doi.org/10.1038/s41591-022-01707-4
Sabaté E, Sabaté E (2003) Adherence to long-term therapies: evidence for action. World Health Organization, Geneva
Krass I, Schieback P, Dhippayom T (2015) Adherence to diabetes medication: a systematic review. Diabet Med 32(6):725–737
Khunti K, Seidu S, Kunutsor S, Davies M (2017) Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care 40(11):1588–1596
Lee DSU, Lee H (2022) Adherence and persistence rates of major antidiabetic medications: a review. Diabetol Metab Syndr 14(1):12
Bogner HR, Morales KH, de Vries HF, Cappola AR (2012) Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Family Med 10(1):15–22
Rubin RR (2005) Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 118(5):27–34
Polonsky W, Fisher L, Hessler D, Bruhn D, Best J (2011) Patient perspectives on once-weekly medications for diabetes. Diabetes Obes Metab 13(2):144–149
Matza LS, Boye KS, Stewart KD et al (2020) Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: a crossover study (PREFER). Diabetes Obes Metab 22(3):355–364
Ruiz PLD, Stene LC, Bakken IJ, Håberg SE, Birkeland KI, Gulseth HL (2018) Decreasing incidence of pharmacologically and non-pharmacologically treated type 2 diabetes in Norway: a nationwide study. Diabetologia 61(11):2310–2318. https://doi.org/10.1007/s00125-018-4681-4
Koye DN, Ling J, Dibato J, Khunti K, Montvida O, Paul SK (2020) Temporal trend in young-onset type 2 diabetes—macrovascular and mortality risk: study of UK primary care electronic medical records. Diabetes Care 43(9):2208–2216. https://doi.org/10.2337/dc20-0417
Davies MJ, Aroda VR, Collins BS et al (2022) Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 45(11):2753–2786. https://doi.org/10.2337/dci22-0034
Download references
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement. The study was funded by the Italian Diabetes Society and partly supported by a grant from Eli Lilly.
Author information
Authors and affiliations.
Department of Medicine and Surgery, Università degli Studi di Milano Bicocca, Milan, Italy
Stefano Ciardullo
Department of Medicine and Rehabilitation, Policlinico di Monza, Via Modigliani 10, 20900, Monza, Italy
Unit of Metabolic Disease, University Hospital of Padua, Padua, Italy
Mario Luca Morieri & Angelo Avogaro
Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Giuseppe Daniele & Domenico Tricò
CISUP, Center for Instrument Sharing, University of Pisa, 56124, Pisa, Italy
Giuseppe Daniele
Department of Medical and Surgical Sciences, University Magna Graecia of Catanzaro, 88100, Catanzaro, Italy
Teresa Vanessa Fiorentino
Department of Medicine and Translational Surgery, Università Cattolica del Sacro Cuore, Rome, Italy
Teresa Mezza
Digestive Disease Center, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
Department of Medicine and Aging Sciences, Center for Advanced Studies and Technology (CAST, Ex CeSIMet) G. d’Annunzio University Chieti-Pescara, Chieti, Italy
Agostino Consoli
Endocrinology and Metabolism Unit, Pescara Health Service, Pescara, Italy
Sant’Anna School of Advanced Studies, Pisa, Italy
Stefano Del Prato
Department of Precision and Regenerative Medicine and Ionian Area, Section of Internal Medicine, Endocrinology, Andrology and Metabolic Diseases, University of Bari Aldo Moro, 70124, Bari, Italy
Francesco Giorgino
Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Salvatore Piro
Department of Surgical, Medical, Molecular and Critical Area Pathology, University of Pisa, Pisa, Italy
Anna Solini
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stefano Ciardullo .
Ethics declarations
Conflict of interest.
SC received lecture or consultancy fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk; MLM received lecture or consultancy fees from Amarin, Amgen, AstraZeneca, Eli Lilly, MSD, Novo Nordisk, SlaPharma, and Servier; DT received lecture or consultancy fees from Amarin, AstraZeneca, Eli Lilly, and Novo Nordisk. SDP has received research funding from AstraZeneca, Boehringer Ingelheim, Novartis Pharmaceuticals Co., and Merck Sharpe & Dohme, and is a consultant for or has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, Merck Sharp & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi, and Takeda. AC has received consulting or speaker fees from Bristol Meyer Squibb, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo-Nordisk, Sanofi-Aventis, Sigma-Tau, Takeda. FG reports consultancies or paid advisory board memberships for Eli Lilly, Medtronic, Novo Nordisk, Roche Diabetes Care, and Sanofi; grants received from Eli Lilly, Lifescan, and Roche Diabetes Care; lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lifescan, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche Diabetes Care, and Sanofi; and patents pending from Roche Diabetes Care. AS reports consultant fees from, Bayer, Eli Lilly Novo Nordisk and Sankyo, and lecture fees from Bayer, Eli Lilly, Novo Nordisk, and Sanofi-Aventis. AA received research grants and lecture or advisory board fees from MSD, AstraZeneca, Novartis, Boehringer Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier and Takeda. No other potential conflicts of interest relevant to this article were reported.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ciardullo, S., Morieri, M.L., Daniele, G. et al. GLP1-GIP receptor co-agonists: a promising evolution in the treatment of type 2 diabetes. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02300-6
Download citation
Received : 13 February 2024
Accepted : 04 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1007/s00592-024-02300-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tirzepatide
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Healthcare (Basel)
- PMC10671220

A Narrative Literature Review on the Role of Exercise Training in Managing Type 1 and Type 2 Diabetes Mellitus
Alessandro piras.
1 Department of Life Quality Studies, University of Bologna, 40126 Bologna, Italy
Milena Raffi
2 Department of Biomedical and Neuromotor Sciences, University of Bologna, 40126 Bologna, Italy; [email protected]
Associated Data
Data are contained within the article.
Diabetes mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia associated with impaired carbohydrate, lipid, and protein metabolism, with concomitant absence of insulin secretion or reduced sensitivity to its metabolic effects. Patients with diabetes mellitus have a 30% more risk of developing heart failure and cardiovascular disease compared to healthy people. Heart and cardiovascular problems are the first cause of death worldwide and the main complications which lead to high healthcare costs. Such complications can be delayed or avoided by taking prescribed medications in conjunction with a healthy lifestyle (i.e., diet and physical activity). The American College of Sports Medicine and the American Diabetes Association recommend that diabetic people reduce total sedentary time by incorporating physical activity into their weekly routine. This narrative literature review aims to summarize and present the main guidelines, pre-exercise cardiovascular screening recommendations, and considerations for patients with diabetes and comorbidities who are planning to participate in physical activity programs.
1. Introduction
Physical activity (PA) is normally suggested in the management of type 1 (T1DM) and type 2 (T2DM) diabetes mellitus and can improve glucose uptake by increasing insulin sensitivity, glucose transportation into the cells, and lowering body adiposity. In T2DM, the practice of PA, both alone and when combined with diet and drug therapy (e.g., oral hypoglycemic agents), can result in improved glycemic control [ 1 ]. In addition, PA can also help to prevent the onset of T2DM and has an important role in reducing the significant worldwide problem of this pathology. Different studies have enhanced our understanding of the acute and long-term physiological benefits of PA, although the precise duration, intensity, and type have yet to be fully elucidated [ 1 , 2 , 3 ]. In T1DM, however, the expected improvements in glycemic control with PA have not been clearly established. Instead, significant physical and psychological benefits of physical exercise can be achieved while careful education, screening, and planning allow the metabolic, microvascular, and macrovascular risks to be predicted and diminished [ 4 ].
The probable hypoglycemic advantage associated with PA was initially observed by Aristotle and later promoted by Allen and Joslin in 1920 [ 2 ]. Even though PA is considered one of the main solutions for diabetes management, indications about the exercise protocols for diabetes are scarce. Notwithstanding, different results have highlighted that many characteristics of the pathology can be improved and probably avoided following regular PA [ 2 , 3 , 4 ]. At the moment, we do not have recommendations regarding screening, exercise protocols, or treatment programs for people with diabetes. It is the same situation for personal frustration and support regarding insulin dosage, nutrition, and the potential limits on performance and safety [ 5 ].
It has been estimated that more than 600 million individuals will suffer from T2DM by 2035 [ 6 ]. Increasing attention has been paid to people at increased risk of developing T2DM, such as those living with impaired fasting glucose (i.e., prediabetes) [ 7 ]. PA is beneficial for preventing the progression of impaired fasting glucose to diabetes [ 8 , 9 ], but people at high risk of developing diabetes often fail to meet the PA guidelines of 150 min of moderate-to-vigorous aerobic exercise per week [ 10 ]. Innovative trials, such as the Diabetes Prevention Program (DPP), have demonstrated that a lifestyle intervention that comprises 150 min of moderate-intensity PA per week can reduce the progression of impaired fasting glucose by up to 58% compared to a non-exercise group and is almost twice as effective as the leading pharmaceutical intervention [ 11 ]. This represents a valid procedure designed to assess the potency of lifestyle modification for the prevention of T2DM [ 7 ]. Nowadays, even though the benefits of PA as a therapeutic measure for diabetic patients are well known and accepted, it is difficult to put exercise recommendations into action for several reasons. Insufficient knowledge among diabetologists and exercise professionals and a lack of dedicated facilities are indicated as important limitations [ 2 ]. Moreover, to provide effective PA support to people with diabetes, research is required to develop clinically effective, cost-effective, scalable interventions that bridge the gap between supervised PA advice. Print-based materials [ 12 ] and technology-based support, such as websites [ 13 , 14 , 15 ], smartphone apps [ 16 , 17 ], and telephone helplines [ 12 , 18 ], have also been shown to be effective in supporting people with diabetes to increase their level of PA. However, such interventions would have better efficacy if grounded in health behavior change theory [ 15 ]. For this reason, wearable technologies, offering biometric data to patients and healthcare professionals, bridging the gap between supervised PA advice, and enabling patients to engage in regular, long-term, physically active lifestyles [ 19 , 20 ], seem to be very helpful in diabetes prevention and management. It has been shown that professional exergames, as well as mobile app-based programs [ 21 ], have the potential to be effective treatment options, especially because they seem to at least partly solve the adherence problem [ 22 ]. Prescribing physical exercise is not generally undertaken, either by the general practitioner or by the diabetologist. This may be because there is insufficient awareness of the benefits of physical exercise or because there is a lack of specific knowledge regarding current recommendations. Thus, prescriptions, when suggested, are generic and more oriented towards ‘physical activity’ rather than ‘exercise therapy’, without appropriate indication about type, intensity, frequency, timing, progression, and precautions [ 2 ]. Furthermore, barriers to, and discriminations in, PA and exercise adoption and maintenance must be addressed to maximize participation [ 3 ].
This narrative literature review aims to highlight the literature available while examining the underlying physiology of exercise in diabetes, the benefits and risks of physical exercise, the strategies for minimizing complications, the protocols suggested, and the potential limitations.
2. Acute Effect of Exercise on Sugar Metabolism
PA allows for improving the glucose-tolerance curve by ameliorating insulin sensitivity in any subject, either with T2DM or T1DM [ 23 ]. Traditionally, physical exercise is promoted in T2DM, where insulin action is scarce in the context of insulin resistance and/or inappropriate insulin secretion. Nevertheless, even in the dysregulation of immune system function found in T1DM, the β-cell toxicity is facilitated by a complex interaction between oxidative stress and inflammation, for which the chronic exercise effect could be protective. During physical exercise, large changes in energy utilization require fine adjustments of glucose and fatty acid concentrations present in the blood. During the first 5–10 min of moderate-intensity exercise, a mixture of fatty acid and glucose provides the major fuel source for skeletal muscle. As exercise duration is prolonged and maintained at a moderate intensity, the contribution of fatty acid becomes more significant, thanks to hormonal responses, such as increased levels of norepinephrine and glucagon that promote the release of fatty acids from adipose tissue (fat stores) into the bloodstream (lipolysis) [ 24 ]. The liberated fatty acids circulate in the blood and are available for uptake by muscle cells. There is also an upregulation of fatty acid transporters in muscle cells. This increased expression of transport proteins facilitates the uptake of fatty acids from the bloodstream into the muscle fibers, making them available for energy production. The oxidation of fatty acids produces a larger amount of ATP compared to glucose metabolism. By relying more on fatty acids for energy, the body can conserve its limited glycogen stores [ 25 ]. Preserving glycogen is crucial for sustaining exercise performance, especially during endurance activities or when exercise intensity increases. Glycogen is the primary fuel source for high-intensity exercise, and by sparing its usage, it helps delay the onset of fatigue. There is a direct relationship between exercise intensity and glucose utilization: when the exercise intensity increases, we observe an augmented glucose utilization via the glycogen deposit from muscles and liver [ 25 ]. A complex hormonal and autonomic response allows an intensification in hepatic glucose production and tissue uptake by the mobilization of non-esterified fatty acid from adipose tissue deposits [ 24 ]. This is produced both by a fall in circulating insulin concentrations and a wide variety of counterregulatory hormones (glucagon, adrenaline, cortisol, and growth hormone) that counteract the hypoglycemic action of insulin [ 26 ]. Elevations in the blood concentrations of these hormones promote both increased glucose production and mobilization of non-esterified fatty acids from adipose storage sites. In addition, the production of new glucose in the liver (gluconeogenesis) from substrates such as lactate is enhanced [ 27 ]. Direct sympathetic stimulation of the pancreas and liver after muscle contraction may also bypass initial hormonal control and additional fuel supplies are provided by ketone formation and mobilization of lactate from inactive muscle glycogen. Glucose transport into muscle is again provided by the transporter protein GLUT4, which is recruited to the membrane surface in large quantities in contracting muscle, independently of insulin. Altogether, these changes maintain the increased fuel supply for exercising muscles and prevent hypoglycemia from excessive utilization [ 27 ].
Higher-intensity exercise or shorter-duration activities primarily rely on glycogen as the predominant fuel source, while lower-intensity or longer-duration exercise shifts towards a greater reliance on fatty acids for energy [ 25 ]. When exercise is ended, the body goes into a fasted state in which glycogen stores in muscle and liver are low and hepatic glucose production is enhanced. The level of the counter-regulatory hormones could remain higher for a long time with an associated hyperglycemic and hyperinsulinaemic response [ 28 ]. Improved GLUT4 transport and insulin sensitivity produce augmented glycogen resynthesis at the muscle level. Thereafter, when glycogen, glucose, and hormone levels return to normal levels, and homoeostasis is reached, insulin can produce supplementary glucose uptake and glycogen resynthesis in muscle and liver. When insulin is scarce, or the body is in a resistant state, glucose storage could be reduced within muscle as a consequence of the inadequate transport with an associated decrement in glycogen synthase activity [ 5 , 26 ].
3. Exercise Benefits for People with Diabetes
PA, sports, and exercise should be encouraged in people with T1DM for the same reasons as it should be promoted in people with T2DM or in the general population. The prescription of physical exercise for diabetic control should be considered for a variety of associated and independent health benefits. The full scope of these benefits can be seen in a number of reviews and include weight loss, weight loss maintenance, lipid profiles, reduction in blood pressure, good psychological profile, and the regulation of symptoms implicated in the metabolic syndrome [ 1 , 29 ]. It would appear that the combined effect of PA and diet provides the first and possibly most effective intervention in improving cardiovascular risk [ 30 ].
It has become evident that the most important effect of physical exercise is the improvement of blood sugar control, weight loss, and weight loss maintenance. PA helps in lowering blood sugar levels by increasing the uptake of glucose into muscles, even without the need for insulin. It enhances insulin sensitivity, making the body more efficient at using insulin to regulate blood sugar. Regular exercise can contribute to better long-term glycemic control. Moreover, physical exercise plays a crucial role in weight management, which is particularly important for individuals with T2DM. Regular PA reduces body fat, increases muscle mass, and improves overall body composition. Maintaining a healthy weight can enhance insulin sensitivity, allowing for more efficient glucose utilization. This can lead to decreased insulin resistance, which is a key factor in T2DM. By improving insulin sensitivity, physical exercise can reduce the reliance on medication or insulin therapy in managing diabetes [ 5 , 31 , 32 ].
PA can also improve cardiovascular health by strengthening the heart, reducing blood pressure, lowering LDL cholesterol levels, increasing HDL cholesterol levels, and improving blood circulation, significantly reducing the risk of heart disease and stroke. Cardiovascular complications are also worsened by stress, and PA is an excellent stress reliever [ 33 ]. Managing stress is important for individuals with diabetes because stress hormones can raise blood sugar levels. Physical exercise reduces stress levels, improves mood, and promotes overall mental well-being. By improving blood sugar control, weight management, cardiovascular health, and overall well-being, regular exercise can lower the risk of diabetes-related complications such as heart disease, stroke, nerve damage, kidney disease, and eye problems [ 5 , 31 , 32 , 33 , 34 ].
4. Exercise and Diabetes: General Recommendations
All levels of PA, including leisure activities, recreational sports, and competitive professional performance, can be performed by people with DM who do not have complications and are in good blood glucose control. A recent systematic review and meta-analysis of eight randomized controlled trials (5190 participants) reported that effective exercise interventions have the potential to reduce the personal and economic costs associated with diabetes [ 12 , 35 ]. They questioned the efficacy and cost-effectiveness of exercise referral schemes, increasing the exercise level and recommending further trials, especially those that are theory-driven [ 36 ]. It should be noted that many of the referral schemes used leisure centers for group-based programs, which did not provide expert supervision. James et al. [ 18 ] demonstrated a significant increase in PA among insufficiently active primary care patients who were provided a referral to receive five sessions from an exercise specialist. It has been suggested that those who potentially have the most to gain from exercise are often the ones who find it most difficult to engage in healthy behavior [ 35 , 37 ]. The effectiveness of PA as a therapeutic intervention is also seriously hampered by uncertainty concerning how clinical care teams should support people with diabetes to meet and maintain exercise guidelines [ 38 ].
It has been largely confirmed that daily PA has different implications in improving people's lifestyles by improving insulin sensitivity, reducing exogenous insulin injection, managing body weight and lipid profiles, boosting self-confidence, improving psychological problems associated with the pathology, reducing systemic inflammation, and most importantly, enhancing long-term protection against cardiovascular disease. Chronic hyperglycemia is supposed to be the mechanism by which coronary artery disease, stroke, nephropathy, retinopathy, and neuropathy occur over decades after disease onset. Moreover, hyperglycemia is related to increased inflammation, as reflected by activation of immune cells and increased systemic concentrations of proinflammatory cytokines/chemokines [ 39 ]. This inflammatory status persists for several hours or days after hyperglycemia has been resolved [ 40 ]. Preventing hyperglycemia is the main aim of DM treatment and may eliminate the excess inflammation caused by physical exercise. When this occurs, the subject can (i) postpone or cancel the planned exercise activity until the pro-inflammatory effects are reduced, (ii) perform physical exercise in a pro-inflammatory status, or (iii) reduce physical exercise-associated inflammation via medical treatment. Given that prior hyperglycemia alone (without ketosis) is not currently included in the contraindications to physical exercise, the second option is good [ 41 ]. General guidelines that may prove helpful in regulating the glycemic response to PA are presented below [ 2 , 42 ].
5. Metabolic Control before Exercise
- I. Avoid physical exercise if the fasting glucose level is >250 mg/dL and ketosis is present, and take care if the glucose level is >300 mg/dL and no ketosis is present.
- II. Ingest additional carbohydrates if glucose levels are <100 mg/dL.
Blood glucose monitoring before, during, and after physical exercise
- I. Identify when changes in medication (insulin/hypoglycemic agent) or food intake are necessary.
- II. Learn the glycemic response to different PA conditions.
5.1. Food Intake the Physical Exercise Day
- I. Consume additional carbohydrates as required to avoid hypoglycemia.
- II. Carbohydrate-based foods should be readily available during and after PA.
5.2. Food Intake the Rest Day
- I. Reduce carbohydrate intake (<50 g/day).
- II. A low carbohydrate diet has been shown to be effective for weight loss due to its effect on decreasing appetite and calorie intake.
- III. Lowering dietary carbohydrate intake has demonstrated benefits for insulin resistance, the underlying cause of T2DM, by independently promoting both weight loss and a reduction in insulin levels.
6. Exercise in Managing T1DM
The ability to adjust the therapeutic regimen (insulin administration and timing, type and quantity of food ingestion before and after physical exercise) that allows safe participation and high performance has recently been recognized as an important management strategy in individuals with T1DM. In particular, the important role played by the patient in collecting self-monitored blood glucose data related to physical exercise responses to improve performance and enhance safety is now fully accepted [ 1 , 2 , 43 ].
When amateurs exercise at 50–60% of VO 2max , which is below their anaerobic threshold, glucose levels rise following the increased uptake of skeletal muscle. The level of glycemia is generally reduced 30/45 min after PA, and it is preserved within the physiological range by two processes: a rapid increase in endogenous glucose production and a reduction in systemic insulin levels [ 41 ]. Patients should reduce or stop insulin infusion before exercise. This is to avoid the hypoglycemic effect due to the increase in glucose uptake at the skeletal muscle and to suppress endogenous glucose production.
Different is the situation in which the subject is involved in physical exercise above the anaerobic threshold and close to the VO 2max . In this condition, the body produces a higher adrenergic activation that facilitates, beyond cardiovascular response, an endogenous increase in glucose level, exceeding the metabolic need at the peripheral level. This creates a state of moderate, transient hyperglycemia that does not exceed 140 mg/dL in healthy people. In T1DM, given that insulin cannot be secreted in response to this hyperglycemic response, the hyperglycemia value often continues to increase after exercise, sometimes becoming dangerous (>400 mg/dL) [ 41 ].
Hypoglycemia, which can occur during, immediately after, or many hours after PA, can be avoided. Indeed, during physical exercise, the body requires approximately 30–50% less insulin than in resting conditions to transport glucose across the cell membrane of myocytes. This is due to a considerable fraction of exercise-stimulated transmembrane glucose transport that occurs via noninsulin-dependent mechanisms [ 44 ]. In this case, it is important that the patient has both an adequate knowledge of the metabolic and hormonal responses to PA and well-tuned self-management skills. The increasing use of intensive insulin therapy has provided patients with the flexibility to make appropriate insulin dose adjustments for various activities. Moreover, in T1DM, after 2–4 years of disease onset, the ability to increase glucagon secretion (the main counterregulatory hormone) in response to hypoglycemia is permanently and completely abolished. However, in T1DM, hypoglycemia is caused by excessive exogenous insulin injection, involving low glycemia and high insulinemia that blocks glucagon release [ 41 ].
Aerobic performance is reduced in T1DM because of cardiovascular, muscular, and metabolic impairments. When compared to their nondiabetic counterparts, young patients with T1DM showed a reduction in VO 2max despite insulin therapy [ 45 ], a difference exacerbated in adults with neuropathic complications or sedentary lifestyles [ 46 ]. Different is the situation in which athletes with T1DM are compared to their nondiabetic counterparts, where VO 2peak was found to be similar [ 47 ]. Moreover, in T1DM, various cardiovascular parameters, such as end-diastolic volume and left ventricular ejection fraction, do not show a normal increment due to exercise, meaning that exercise can lead to normal aerobic and cardiovascular parameters [ 41 ].
The general recommendations for PA in adults with T1DM, free of complications, are the same for children, considering that kids are more subjected to a greater variability in glycemic level. For this reason, attention should be focused on glycemic fluctuation during exercise so that parents, teachers, and athletic coaches are properly trained. When dealing with adolescents, hormonal variations can increase the difficulty in managing glycemic levels. Notwithstanding these additional difficulties, it is out of the question that following the useful recommendations to avoid hypoglycemia, PA is a safe and satisfying practice for most children and adolescents with T1DM [ 42 ].
7. Exercise in Managing T2DM
A standard recommendation for people with and without diabetes is to start PA with a warm-up and end with a cool-down phase. The warm-up involves 5–10 min of aerobic exercises (walking, cycling, etc.) at low intensity. The aim is to prepare the skeletal muscles, heart, and lungs for a progressive increase in exercise intensity. Then, muscles can be gradually stretched for an additional 5–10 min to maintain a good range of motion in the joints [ 48 ]. After the main training session, a 5–10 min cool-down should be organized similarly to the warm-up. The aim of the cool-down is to gradually bring the heart rate back to its pre-exercise value. There are some recommendations that are particularly important and specific for people with T2DM. Moderate weight training exercises with light weights and high repetitions can be suggested for maintaining or enhancing upper body strength in people with diabetes. Different long-term studies have established long-lasting beneficial effects of regular PA on carbohydrate metabolism and insulin sensitivity, which can be maintained for at least 5 years. They suggested PA programs with an intensity in the range of 50–80% of VO 2max , three to four times a week, for 30–60 min a session. Aerobic exercise should be suggested, considering safety measures for PA involving the feet, which are essential for many patients with diabetes [ 1 , 2 , 48 , 49 , 50 , 51 ]. High-resistance exercise using weights may be acceptable for young individuals with diabetes but not for older individuals with long-standing diabetes.
Good hydration is also crucial, as dehydration can adversely affect glycemic levels and heart function. Through PA, fluid should be frequently consumed to compensate for losses in sweat reflected in body weight loss or the maximal amount of fluid tolerated (e.g., 0.5 L of fluid consumed 2 h before PA) [ 52 ]. Precautions should be taken when exercising in extremely hot or cold environments.
It has been shown that improvements in glycated hemoglobin (HbA1c) are generally 10–20% of the baseline and are most marked in patients with mild T2DM and in those who are likely to show insulin resistance [ 53 ]. It remains true, unfortunately, that most of these studies suffer from inadequate randomization and controls and are confounded by associated lifestyle changes. The general agreement is that regular exercise should not be expected to dramatically affect HbA1c values; other variables, such as increased food intake or reduced insulin dosages, compensate for any increases in glucose disposal. Nonetheless, epidemiological evidence confirmed that being physically active, rather than sedentary, can lower mortality and morbidity for any given level of HbA1c [ 54 ].
It seems that long-lasting programs of PA are good enough and demonstrate a higher rate of adherence in patients with prediabetes or uncomplicated T2DM. In this kind of study, researchers started their training programs with initial supervision, followed by home-training programs with follow-up to assess the level of adherence [ 4 ]. A lot of them have shown good results in the maximum oxygen consumption with few complications for patients [ 55 ].
8. Psychological Profile, Muscular, and Cardiovascular Evaluations
Other important aspects to consider when prescribing an exercise program are the evaluation of motor responses and the consideration of the psychological profile. Evaluations represent a fundamental moment for understanding the real capabilities of an individual, setting up protocols and assessing results over time. People with diabetes show both impaired exercise tolerance and an excessive risk of developing heart failure, which are not entirely explained by known cardiovascular risk factors or coronary artery disease [ 56 ]. The risk for cardiovascular disease and other diabetes-related complications, including neuropathy, retinopathy, and nephropathy in persons with long-standing disease, is high, and care should be taken to properly screen individuals before recommending a new exercise program. Caution is warranted for those with advanced disease complications and medical screening; before initiating any new vigorous exercise program, a graded exercise stress test with ECG and blood pressure monitoring should be performed. The main assessments that should be made before carrying out any type of physical exercise are, in order: (i) the Health Assessment Questionnaire; (ii) assessment of balance level and risk of falls; (iii) cardiopulmonary exercise testing; (iv) the assessment of muscle strength.
Health assessment questionnaires [ 57 ]. While several tools are available to measure health-related quality of life (HRQoL) for patients with diabetes, the design and, therefore, duration of these measurements may limit their feasibility in the daily routine of a sports facility. Furthermore, these measures do not distinguish items for diabetes-specific quality of life. Thus, a specific questionnaire on the diabetics’ quality of life (DMQoL) was developed with only 10 questions, sensitive to the change related to the progression of diabetes compared to the initial stages (e.g., glycemic changes). The combination of the DMQoL and the WHOQOL-BREF (the shortened version of the quality of life questionnaire designed by WHO) provides a comprehensive picture of overall health-related quality of life in patients with diabetes and improves the ability to detect changes clinically significant to the pathology.
Tests that determine the level of balance and the risk of falls. Patients with T2DM, particularly those ≥65 years old, exhibit an increased rate of falls. It is, therefore, important to assess the risk of falling before the prescription of an exercise intervention. The most used tests are the Timed Up and Go Test (TUG), the Functional Reach Test, (FRT), the Berg Balance Scale (BBS), and the Dynamic Gait Index (DGI). Among those tests, the TUG showed the greatest sensitivity (90%) and specificity (88%) to the phenomenon [ 58 ]. In this assessment, the patient gets up from a chair, walks 3 m, turns around, returns to the chair, and sits down again. This task must be completed within 10.6 s. Times between 11 and 20 s are within the normal range for frail elderly and disabled patients; times ≥20 s indicate that the person needs external assistance. A score ≥30 s predicts a higher risk of falling.
Cardiopulmonary exercise testing (CPET). Measurements of ventilation, gas exchange, and electrocardiography during an incremental exercise test are noninvasive protocols that provide an assessment of pulmonary, cardiovascular, and muscle function during exercise (see Table 1 ). The addition of echocardiographic monitoring (“imaging-CPET”), mainly used in patients with heart failure, may provide further insight into different aspects of cardiac function during exercise and their impact on exercise intolerance [ 59 ]. Even though guidelines recommend the application of objective exercise prescriptions using CPET data [ 59 ], it is common to find programs without CPET information or with limited resources to establish exercise intensity on the basis of resting heart rate (e.g., exercising heart rate threshold set 20 or 30 beats/min above the resting heart rate). This simple method has been criticized and demonstrated to be inadequate by several researchers. Moreover, it should be noted that performing this test routinely and its cost mean that specific criteria must be defined regarding patients for whom it would be imperative to perform the test.
Physiological variables measured during CPET.
Legend: VO 2 %, percentage of peak oxygen uptake; HR, heart rate; BP, blood pressure; ECG, electrocardiography; VE/VO 2 ; VE/VCO 2 , ventilator equivalents of oxygen and carbon dioxide; SpO 2 , arterial oxygen saturation; PETO 2 and PETCO 2 , end-expiratory pressure of oxygen and carbon dioxide; Δ(a − v)O 2 , arteriovenous difference in oxygen; RER, respiratory exchange ratio; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; E/A, transmittal flow velocity; DT, deceleration time.
Assessment of muscle strength. A significant percentage of T2DM patients (prevalence 16%, mean age 58 years old) are sarcopenic compared to age-matched healthy individuals [ 34 ]. Considering that 80% of insulin-mediated glucose uptake occurs in skeletal muscle (lean mass) [ 60 ], we should consider the importance of increasing lean mass for improving glycemic control in these individuals [ 61 ]. Furthermore, muscle strength is reduced by 30% to 50% in T2DM patients compared to their healthy counterparts. Dynamometry is considered the gold standard for examining muscle strength. However, considering the cost of this device and the technical skills required, this test is not feasible for those working in private and home care settings. There are different types of tests, such as handgrip strength and sit-to-stand tests. Handgrip strength shows moderate to high correlations with extremity muscle strength. The patient sits in a chair with their elbow flexed to 90 degrees and a force device in one hand. Subsequently, the patient grips the device as tightly as possible for 3 s. This test is performed three times, alternating hands. Another strength test that could be considered to assess muscle strecngth in T2DM patients is the sit-to-stand test, although the validity of this test is currently under intense debate. Briefly, in this test, the person, without the help of the hands and arms, but with only the work of the legs, must perform, in 1 min, as many reps as possible and sit with legs bent at 90 degrees. Another version foresees a 30 s duration (30s-STS) [ 62 ], and another comprises five reps executed as quickly as possible (FTSST) [ 63 ].
9. Exercise Prescription
Regular exercise enhances overall fitness, strength, and endurance [ 61 ]. It boosts energy levels and can alleviate symptoms of fatigue commonly experienced by individuals with diabetes. It is important for individuals with diabetes to consult with their healthcare team before starting an exercise program. They can provide guidance on the type, intensity, duration, and frequency of exercise that suits individual needs and medical conditions. Additionally, monitoring blood sugar levels before, during, and after exercise is crucial to prevent hypoglycemia or hyperglycemia episodes.
As soon as the patient has been inspected and the risk factors and exercise tolerance identified, regular exercise should be suggested. Most patients with T2DM are sedentary, overweight, and middle-aged or older. In this population, PA may well be beneficial but needs to be carefully applied. Guidelines published by the American Diabetes Association (ADA) and by the American College of Sports Medicine (ACSM) suggest a proper warm-up of 5–10 min, followed by stretching, then the main activity session, ended with 5–10 min of an active cool-down epoch to bring back the physiological variables to their pre-exercise values [ 49 ]. The intensity, duration, and frequency of exercise necessary for good health should be in the range of 60–80% of maximal oxygen consumption delineated in the ACSM guidelines in 1976 [ 32 ]. The aim of an adult should be to maintain continuous moderate exercise for 30 min, equivalent to brisk walking, for five or six days a week, with the possibility to do shorter bouts of more intense exercises. Exercises at vigorous intensity are widely suggested for their health benefits and can be safely recommended for people with diabetes if cardiovascular and hypertensive complications are considered [ 5 , 32 ]. To our knowledge, no studies have accurately defined the most suitable exercise programs for people with diabetes up until now. It is unsuitable to be too prescriptive, and instead, we should concentrate on adherence and compliance. When ACSM guidelines are suggested, there is a dropout rate of 40–70% after 12–18 months, even with an active intervention program [ 48 ]. Nevertheless, recent guidelines have gained wider acceptance, and much greater success has been shown in the Malmo intervention studies with mixed high and low-intensity exercises, although exercises below 30% VO 2max have demonstrated lower benefit [ 64 ].
PA guidelines for patients with DM are the same as for healthy people unless co-occurring health conditions or advanced age affect their physical ability. In particular, interventions that combine aerobic and resistance training appear to show greater efficacy than the two training modalities taken individually [ 65 ]. Combined exercise three times/week may be of greater benefit for glycemic control than aerobic or resistance exercise alone. Additionally, total exercise duration and calorie expenditure were greater with the combo workout than individual workouts [ 65 ]. Several types of exercise protocols enhance health and glycemic management in individuals with DM, although structured exercise training has been studied most frequently, with benefits resulting from enhanced insulin sensitivity, reduced postprandial hyperglycemia, and reduced cardiovascular risk.
9.1. High-Intensity Interval Training (HIIT)
There has been a growing interest in high-intensity interval training (HIIT) over the past 20 years. HIIT has received considerable attention due to its positive metabolic and cardiovascular adaptations that are similar, or even superior, to moderate-intensity continuous training in a variety of populations [ 7 , 66 , 67 ]. Specific to diabetes prevention, a recent meta-analysis demonstrated that HIIT leads to greater improvements in insulin resistance compared with training at moderate intensity [ 68 ]. Given the positive health adaptations, HIIT may represent a promising PA strategy for individuals with impaired fasting glucose [ 7 ]. HIIT involves alternating short sets of high-intensity exercise, typically achieving values ≥85% peak heart rate (HRmax), with passive recovery periods or light exercise typically performed at ≤70% HRmax. HIIT has been proposed as a time-efficient form of exercise. A typical HIIT session can be up to three times shorter than that of traditional moderate-intensity continuous training (MICT) and can lead to both cardiovascular and metabolic improvements in less time than MICT [ 69 , 70 ]. Several authors have studied the potential benefits of HIIT in multiple aspects, including cardiorespiratory fitness, anthropometric variables, mental health, and cardiovascular and cardiometabolic diseases in different populations, both healthy and with pathologies [ 7 , 69 , 71 ]. In individuals with T2DM and without complications, training adaptations induced by HIIT and MICT are equally capable of rapidly attenuating some local limiting factors governing the initially impaired VO 2 kinetics response during submaximal exercise [ 72 , 73 ]. For this reason, HIIT at low volume should also be considered a suitable and effective exercise modality to enhance oxidative metabolism in individuals living with T2DM [ 74 ]. However, there are limited data on people’s adherence to HIIT in the long term and outside of a supervised or laboratory environment. Preliminary evidence shows that individuals with impaired fasting glucose can independently adhere to HIIT training for more than 4 weeks, and they do so at a higher rate with respect to the adherence to moderate-intensity continuous training [ 69 ]. While these findings are encouraging, more research is required to determine if samples drawn from a pre- or diabetic population can adhere to long-term interval exercise [ 75 ].
9.2. Peripheral Heart Action Training
A particular form of HIIT is PHA (Peripheral Heart Action) training [ 76 ]. PHA is a circuit training exercise that involves several stations that involve different muscles or muscle groups, following a well-defined pattern: the alternation between the exercises that involve the muscles located “above and below” the heart in such a way as to avoid passive breaks between stations. The HIIT session is repeated five times in the first 2 days of training and gradually increased in subsequent sessions according to the subject’s heart rate, which is monitored with the heart rate monitor. In the PHA session the subjects perform 15 repetitions on each piece of equipment and then move on to the next station with active breaks until the completion of the circuit of training. Active breaks imply that the subjects train the lower limbs as soon as they finish the upper limbs and vice versa. This circuit training is performed four times, separated by 1 min of rest, and resistance is increased for the next exercise session if the subject is able to perform 15 full repetitions during the final set for each exercise. Subjects wear a heart rate monitor and maintain intensity at 55–60% of 1RM, which corresponds to approximately 60–80% of HRmax.
9.3. Water Exercise
Compared to land-based exercise, aquatic exercise displays different advantages, including its effects on cardiovascular regulation because the blood flow to the lower limbs decreases due to the existence of hydrostatic pressure. This increases the redistribution of blood flow and the cardiac preload, thus increasing the stroke volume. Moreover, due to the height of the water, the pressure on systemic circulation increases, further affecting respiratory effort. These changes are helpful in increasing the elasticity and strength of the respiratory muscles, improving oxygen uptake [ 77 ]. The resistance and heat dissipation effects of water are conducive to energy expenditure and improve the effects of exercise [ 77 ]. Therefore, exercise in water could be considered an alternative protocol to land-based exercise. Such protocol could be started with a 5 min warm-up consisting of articular mobilization, followed by 30 min of easy swimming (alternating the various swimming styles—front crawl, breaststroke, backstroke and butterfly), 5 min of leg-only swimming and 5 min of arm-only swimming, 5 min of aquatic skills, and 5 min of cool-down. An alternative protocol, with head-out water immersion, may be used successfully and induce positive metabolic adaptations in patients with diabetes [ 78 ].
Exercise prescription must also consider patients’ readiness to exercise, attitudes, and belief systems while positively encouraging decisions to exercise. Support can be provided through a team of doctors, nurses, physiotherapists, lifestyle counsellors, and exercise consultants and even through health policy decision-making at the government and local levels. Moreover, exercise should be prescribed based on the type of diabetes, distinguishing between type 1 and type 2.
10. Conclusions and Future Recommendations
The major challenge is to persuade diabetic people to practice PA and to follow dietary recommendations. Sedentary persons with disabilities are very resistant to changing their lifestyle; in particular, adult diabetic people are resistant to changing their habits, maybe because of this pathology, except for those who have complications. Successful interventions to promote long-term changes have used specific strategies to promote sustainable effects of interventions [ 79 ]. Considering that drug-resistant diseases could become the leading cause of death by 2050, an intervention aiming at sustainable lifestyle change must, therefore, include components that facilitate the maintenance of PA levels and dietary changes over time.
It is well known that men and women of all ages and abilities can improve their quality of life through regular PA associated with well-designed dietary recommendations and nutrition therapy. Being physically active is one of the most important actions that people of all ages can take to improve their health. The regular practice of physical exercise fosters normal growth and development, makes people feel better, function better, sleep better, and reduces the risk of a large number of chronic diseases. Health benefits start immediately after exercising, and even short episodes of PA are beneficial. Evidence regarding the health benefits of regular PA is well established, and research continues to provide insight into what works to get people moving, both at the individual and community levels. Achieving the benefits of PA depends on our personal efforts to increase activity in ourselves, family, friends, patients, and colleagues. Action is also required at the school, workplace, and community levels. Future recommendations for elderly interventions should emphasize the importance of naturalistic or personally meaningful environments and designs that should induce a mismatch of supply and demand; they should have high task variability, fulfilling basic individual senior needs, but also be engaging to maximize long-term adherence to physical exercise and an active lifestyle.
Funding Statement
The authors declare that the work was supported by the Erasmus+ Project BE-NEW 622371-EPP-1-2020-1-IT-SPO-SCP with European funding. The content of the publication is the sole responsibility of the publisher and that the European Commission is not liable for any use that may be made of the information. It was also funded by the Ministero Università e Ricerca PRIN2022 n. 2022KZ4KMY, the Fondazione Del Monte di Bologna e Ravenna Prot. n. 0001883 - 30/05/2022; the Fondazione Carisbo n. 2021.0101 - Bando Ricerca Medica e Alta Tecnologia 2021.
Author Contributions
All authors contributed to the conception of the article. The corresponding author drafted the article and performed the literature search and analysis. All authors contributed to the interpretation of the results and critically revised the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors have no relevant financial or non-financial interests to disclose.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs.
In this paper, we present a systematic review of the literature on the association of these risk factors with the incidence/prevalence of type 2 diabetes. We give insights on the contribution of independent risk factors in the development of type 2 diabetes along with possible solutions towards a preventive approach.
Abstract. Objective: The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges ...
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of ...
Type 2 diabetes mellitus (T2DM) accounts for >90% of the cases of diabetes in adults. Resistance to insulin action is the major cause that leads to chronic hyperglycemia in diabetic patients. ... Arora T, Taheri S. Sleep optimization and diabetes control: a review of the literature. Diabetes Ther. 2015 Dec; 6 ((4)):425-68. [PMC free article ...
PLoS ONE 8, e59524 (2013). Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both ...
Objective To evaluate current risk models and scores for type 2 diabetes and inform selection and implementation of these in practice. Design Systematic review using standard (quantitative) and realist (mainly qualitative) methodology. Inclusion criteria Papers in any language describing the development or external validation, or both, of models and scores to predict the risk of an adult ...
Introduction. Over the last 25 years, healthcare providers and researchers have come to recognize the importance of the patient-centred perspective for people with a medical condition 1, 2.This is especially relevant for diabetes because most diabetes care is self-care, not administered by healthcare providers.
Objective To assess what proportions of studies reported increasing, stable, or declining trends in the incidence of diagnosed diabetes. Design Systematic review of studies reporting trends of diabetes incidence in adults from 1980 to 2017 according to PRISMA guidelines. Data sources Medline, Embase, CINAHL, and reference lists of relevant publications. Eligibility criteria Studies of open ...
This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations ...
Background: Different methodologic approaches for constructing dietary patterns and differences in their composition limit conclusions on healthful patterns for diabetes prevention.Objective: We summarized evidence from prospective studies that examined associations of dietary patterns with type 2 diabetes by considering different methodologic approaches.
The body of literature that outlines higher risk of microvascular or macrovascular complications in early-onset type 2 diabetes has focussed on comparing people with type 2 diabetes to those ...
Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies 1 2. Author links open overlay panel Jannasch Franziska 4 5, Kröger Janine 4 5, ... Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of ...
In the past decade, several randomized, controlled clinical trials have examined the role of diet and exercise in the prevention of type 2 diabetes. 4 One of the earliest studies was conducted in a Chinese community among 577 men and women with impaired glucose tolerance who were randomized to a program of diet, exercise, or both. 5 Dietary intervention focused on increased amounts of ...
We randomly assigned patients with type 2 diabetes and chronic kidney disease (defined by an estimated glomerular filtration rate [eGFR] of 50 to 75 ml per minute per 1.73 m 2 of body-surface area ...
This review explores the current conventional drugs used in the treatment of type 2 DM, the associated limitations related to their usage and the cutting edge novel nanoformulations that are under continual research for circumventing the stated drawbacks of the conventional drug use. 2. Pathophysiology of diabetes.
Introduction. Diabetes mellitus (DM) is probably one of the oldest diseases known to man. It was first reported in Egyptian manuscript about 3000 years ago. 1 In 1936, the distinction between type 1 and type 2 DM was clearly made. 2 Type 2 DM was first described as a component of metabolic syndrome in 1988. 3 Type 2 DM (formerly known as non-insulin dependent DM) is the most common form of DM ...
This systematic review and meta-analysis evaluated the combined effects of clinician-led and community-based group exercise interventions on a range of health outcomes in adults with type 2 diabetes mellitus. Our literature search spanned Medline, Scopus, PubMed, Embase, and CINAHL databases, focusing on peer-reviewed studies published between ...
Although good glycemic control in patients with type 2 diabetes (T2D) can prevent cardiovascular complications, many diabetic patients still have poor optimal control. A new class of antidiabetic drugs (e.g., glucagon-like peptide-1-GLP-1 receptor agonists, sodium-glucose co-transporters-SGLT2 inhibitors), in addition to the low hypoglycemic effect, exert multiple beneficial effects at a ...
Aim To summarize, critically review, and interpret the evidence related to the systematic reviews on health literacy (HL) amongst type 2 diabetes mellitus (T2DM). Methods The methodology for this ...
Type 2 diabetes mellitus (T2DM) is a disease affecting mostly the adults but is being increasingly recognized in children and adolescents. However, the information about the glycemic profile and ...
A systematic literature review of diabetes self-management education features to improve diabetes education in women of Black African/Caribbean and Hispanic/Latin American ethnicity. ... Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care, 29 (2006), pp. 1851-1855.
Pre-diabetes. Current guidelines for the delay and prevention of type 2 diabetes mellitus recommend for people with prediabetes to lose at least 7% of their body weight. Here, we advocate to use ...
The rising prevalence of type 2 diabetes (T2DM) and hypertension in older adults, and the deleterious effect of these conditions on cerebrovascular and brain health, is creating a growing discrepancy between the "typical" cognitive aging trajectory and a "healthy" cognitive aging trajectory. These changing health demographics make T2DM and hypertension important topics of study in ...
The purpose of this study is to describe the validity of existing PI for type 2 diabetes mellitus and cardiovascular risk management. Methods: We conducted a systematic literature search for studies describing the development and assessment of relevant PIs between January 1990 and January 2009. We grouped identified PI as drug- or disease ...
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. It may be due to impaired insulin secretion, resistance to peripheral actions of insulin, or both. According to the International Diabetes Federation (IDF), approximately 415 million adults between the ages of 20 to 79 years had diabetes mellitus in 2015.[1] DM is proving to be a global public ...
Type 2 diabetes represents a growing challenge for global public health. Its prevalence is increasing worldwide, and, like obesity, it affects progressively younger populations compared to the past, with potentially greater impact on chronic complications. Dual glucagon like peptide 1 (GLP1) and glucose-dependent insulinotropic peptide (GIP) receptor agonists are among the new pharmacological ...
Introduction The rise of new technologies in the field of health is yielding promising results. In certain chronic conditions such as type 2 diabetes mellitus, which ranks among the top five causes of global mortality, it could be useful in supporting patient management. Materials and methods A systematic review will be conducted on scientific publications from the last 5 years (January 2019 ...
Methods and materials: A review of chosen literature from PubMed database, GoogleScholar database between the years 2000-2024 was carried out using the following keywords: "low-carbohydrate", low-carb", "low carbohydrate diet", "low-carb diet", "type 2 diabetes", "insulin", "insulin resistance", "obesity".
1. Introduction. Physical activity (PA) is normally suggested in the management of type 1 (T1DM) and type 2 (T2DM) diabetes mellitus and can improve glucose uptake by increasing insulin sensitivity, glucose transportation into the cells, and lowering body adiposity.