- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
News releases.
Media Advisory
Tuesday, November 22, 2022
NIH establishes website for self-reporting COVID-19 test results

Reporting a positive or negative test result just became easier through a new website from the National Institutes of Health. MakeMyTestCount.org, developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, allows users to anonymously report the results of any brand of at-home COVID-19 test.
COVID-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid COVID-19 test has become commonplace, test results are not often reported. COVID-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.
The National Institute of Biomedical Imaging and Bioengineering (NIBIB) supported development of MakeMyTestCount.org through the RADx Tech program.
NIBIB Director Bruce Tromberg, Ph.D., who leads the RADx Tech program, is available for comment.
About the Rapid Acceleration of Diagnostics (RADx ® ) initiative: The RADx initiative was launched on April 29, 2020, to speed innovation in the development, commercialization, and implementation of technologies for COVID-19 testing. The initiative has four programs: RADx Tech, RADx Advanced Technology Platforms, RADx Underserved Populations and RADx Radical. It leverages the existing NIH Point-of-Care Technology Research Network. The RADx initiative partners with federal agencies, including the Office of the Assistant Secretary of Health, Department of Defense, the Biomedical Advanced Research and Development Authority, and U.S. Food and Drug Administration. Learn more about the RADx initiative and its programs .
About the National Institute of Biomedical Imaging and Bioengineering (NIBIB): NIBIB’s mission is to improve health by leading the development and accelerating the application of biomedical technologies. The Institute is committed to integrating the physical and engineering sciences with the life sciences to advance basic research and medical care. NIBIB supports emerging technology research and development within its internal laboratories and through grants, collaborations, and training. More information is available at the NIBIB website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Medical Device Safety
- Emergency Situations (Medical Devices)
- Coronavirus (COVID-19) and Medical Devices
Understanding At-Home OTC COVID-19 Antigen Diagnostic Test Results

At-home over-the-counter (OTC) COVID-19 antigen tests, often referred to as rapid tests, detect proteins called antigens from SARS-CoV-2, the virus that causes COVID-19. At-home COVID-19 antigen tests are less likely to detect the SARS-CoV-2 virus than molecular tests, such as polymerase chain reaction (PCR) tests and other nucleic acid amplification tests (NAATs), which detect genetic material called RNA from the virus. This is especially true early in an infection or in people who do not have COVID-19 symptoms. Currently, all at-home COVID-19 antigen tests that are FDA-authorized are authorized for repeat testing, also called serial testing. This means people who receive a negative test result should use multiple tests over a certain period, testing at least twice over three days if they have symptoms and at least three times over five days if they do not have symptoms.
On this page:
When you should test for covid-19, what your at-home otc covid-19 test result means, what at-home covid-19 antigen tests do not tell you, reporting your test result, step-by-step guide: when to test and what your at-home covid-19 antigen test results mean, understanding covid-19 infection and the risk of spreading the virus, related information.
- If you have symptoms , test immediately and then test again per the instructions if your first result is negative.
- If you were exposed to someone who has COVID-19 and you do not have symptoms, wait at least 5 full days after your exposure before testing. If you test too early, you may have an inaccurate result.
- If you are in certain high-risk settings , you may need to test as part of a screening testing program.
- Consider testing before coming into contact with someone who has a high risk for severe COVID-19 ; people who are older adults or immunocompromised, or have other medical conditions, especially if you are in an area with a medium or high COVID-19 Hospital Admission Level .
Most FDA-authorized at-home OTC COVID-19 tests are antigen tests. While not perfect, they provide a fast and convenient COVID-19 testing option to detect the virus, so you may know if you are infected and should stay at home and away from people to help reduce the spread of the virus.
- A positive result using an at-home COVID-19 antigen test means you likely have COVID-19. Anyone who tests positive for COVID-19, or who likely has COVID-19, should contact their health care provider and follow the CDC's guidelines for staying at home and away from people.
- A negative result using at-home COVID-19 antigen test means the test did not detect the virus that causes COVID-19, but it does not rule out COVID-19 because some tests may not detect the virus early in an infection. Always do a repeat test at minimum after 48 hours following a negative test result when using an antigen test. Use the following guidance to help interpret your negative test result and determine what steps to take next.
In addition to COVID-19 test results, and when determining the likelihood of having the virus, consider:
- Recent symptoms
- Close contact with someone who has COVID-19
- The level of COVID-19 in your community
See more information about negative test results from at-home COVID-19 antigen test and repeat testing below.
- If you have an infection immediately after you are exposed to COVID-19 because it may take 2 to 5 days, and sometimes longer, for the virus to be detected by a COVID-19 antigen test. How long it takes before a test can detect the virus may vary between different COVID-19 variants and different tests.
- How contagious you are or if you can spread the virus to someone else. There are no tests that can tell you that. You could pass COVID-19 to others even before you get a positive result on a test.
- If you have another type of respiratory illness, such as flu or respiratory syncytial virus ( RSV ), unless it is a test specifically authorized for detection of these viruses.
Watch: CDC | How To Interpret Self-Test Results
The FDA encourages you to voluntarily and anonymously report your positive or negative test results every time you use an at-home COVID-19 test. You can send your test result to MakeMyTestCount.org or use an app or other digital option for self-reporting that may be included with your test. Report each test result one time.
The data from MakeMyTestCount.org can help public health departments know how fast the virus is spreading. This valuable test data help public health departments assess and modify their response to COVID-19 in their local communities, states, or across the country. The MakeMyTestCount website is developed through the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx) Tech program and allows consumers to anonymously report their test results from OTC at-home COVID-19 test.
When you are exposed to the virus that causes COVID-19, it may get into your nose, throat, and lungs (which make up your respiratory tract) and cause an infection. You may never develop symptoms of an infection or you may not have symptoms for several days after you are exposed to the virus, despite being infected. You could still pass the virus to others, even if you do not feel sick.
The amount of virus in an infected person's body may vary in different people, as well as at different times during the infection. There may also be differences in whether, or how easily, the virus can spread to another person throughout the course of the infection. Generally, the amount of virus in a person will start low, increase, then decrease again as a result of the body's immune response. The pattern of this increase and decrease, as well as the level of virus, varies from person to person, and there is no known level above which you can spread the virus and below which you cannot.
To prevent spreading COVID-19 to others, always follow the CDC's recommendations .
- At-Home OTC COVID-19 Diagnostic Tests
- List of Authorized At-Home OTC COVID-19 Diagnostic Tests
- At-Home COVID-19 Diagnostic Tests: Frequently Asked Questions
- COVID-19 Test Basics : Includes details on COVID-19 tests, types of samples, and other information.
- At-Home COVID-19 Antigen Tests-Take Steps to Reduce Your Risk of False Negative: FDA Safety Communication
- Counterfeit At-Home OTC COVID-19 Diagnostic Tests
- Find COVID-19 Tests at COVID.gov/tests
- Video: How to Interpret Self-Test Results | CDC
- COVID-19 Testing: What You Need to Know | CDC
- COVID-19 Self-Testing At-Home or Anywhere | CDC
- What to Do If You Were Exposed to COVID-19 | CDC
- Isolation and Precautions for People with COVID-19 | CDC
- Report your test result at MakeMyTestCount.org
- Diagram: Interpreting Your Negative Test Results
- Video: What Is an EUA? Describes how the FDA can issue an emergency use authorization (EUA) to provide more timely access to diagnostic tests that may help during the public health emergency when there is no adequate, approved, and available alternative.
Subscribe to Consumer Information for Medical Devices
Receive notifications for consumers about medical device information, recently approved devices, and alerts that may be of interest to the general public.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
How to Report COVID-19 Laboratory Data
Summary of recent changes.
- Effective May 11, 2023, HHS announced the Public Health Emergency declaration for COVID-19 has ended. In addition, HHS and CLIA regulatory requirements under the CARES Act Authority requiring reporting of laboratory test data to the federal government has also ended. State-specific reporting requirements may still apply. Laboratories and testing sites should review state-specific reporting requirements for COVID-19 testing. They may be required to continue reporting for COVID-19 testing to their State Health Departments.
Who can report
What to report.
- Using Standard Terminology
- Assistance with Electronic Reporting
All COVID-19 testing sites must
- have a Clinical Laboratory Improvement Amendments (CLIA) certificate,
- meet all requirements to perform testing, including only using FDA-authorized test systems according to their instructions for use, and
- follow all state specific-reporting requirements for COVID-19 test results.
COVID-19 testing sites are defined as
- laboratories that perform clinical diagnostic or screening testing under CLIA,
- non-laboratory COVID-19 diagnostic or screening testing locations, and
- other facilities or locations offering COVID-19 point-of-care diagnostic or screening tests, or in-home diagnostic or screening tests.
CMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN). This CDC- and CMS-preferred pathway to submit data to CDC’s NHSN applies only to CMS-certified long-term care facilities. Test data submitted to NHSN will be reported to appropriate state and local health departments using standard electronic laboratory messages. Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any.
Testing sites that perform COVID-19 surveillance testing on de-identified samples, regardless of their CLIA status, should not report the results of their surveillance testing to state, tribal, local, and territorial public health departments. If at any time a facility intends to report a patient-specific test result, it must first obtain a CLIA certificate and meet all requirements to perform testing. For more information, see the Center for Medicare and Medicaid Service’s (CMS) Research Testing and Clinical Laboratory Improvement Amendments of 1988 (CLIA) Regulations .
NOTE regarding self-test results : While there are no current mechanisms that require reporting of self-test results to public health authorities, CDC encourages everyone who uses a self-test to report any positive results to their healthcare provider. Healthcare providers can ensure that those who have tested positive for COVID-19 receive the most appropriate medical care, including specific treatments if necessary. COVID-19 home tests can be safely and privately reported at MakeMyTestCount.org .
Association of Public Health Laboratories (APHL), in collaboration with the Council of State and Territorial Epidemiologists (CSTE), CDC, and other public and private partners, has developed these CSTE tools to assist laboratories with reporting.
How to report
Laboratory data elements may be reported in the following ways:
- Submit laboratory testing data directly to state or local public health departments according to state/or local law or policy. Data must be sent using existing reporting channels to ensure rapid initiation of case investigations, and concurrent reporting of results must be shared with the ordering provider or patient, as applicable.
- Submit laboratory testing data to state and local public health departments through a centralized platform, where the data will then be routed to the appropriate state and local authorities and may be routed to CDC after removal of personally identifiable information according to applicable rules and regulations.
- Submit laboratory testing data through a state or regional Health Information Exchange (HIE) to the appropriate state or local public health department.
- CMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN). This CDC- and CMS-preferred pathway to submit data to CDC’s NHSN applies only to CMS-certified long-term care facilities. Test data submitted to NHSN will be reported to appropriate state and local health departments using standard electronic laboratory messages. Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any.
Laboratories should make every reasonable effort to provide the following data elements when reporting to state and jurisdictional health departments.
- Test ordered – use harmonized LOINC codes provided by CDC
- Device Identifier
- Test result–use appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC
- Test Result date (date format)
- Accession # / Specimen ID
- Patient age
- Patient race
- Patient ethnicity
- Patient sex
- Patient residence zip code
- Patient residence county
- Ordering provider name and nonpharmaceutical interventions (as applicable)
- Ordering provider zip code
- Performing facility name and CLIA number
- Performing facility zip code
- Specimen Source – use appropriate LOINC, SNOMED-CT, or SPM4 codes, or equivalently detailed alternative codes
- Date test ordered (date format)
- Date specimen collected (date format)
The following additional demographic data elements should also be collected and reported to state or local public health departments.
- Patient name (Last name, First name, Middle Initial)
- Patient street address
- Patient phone number with area code
- Patient date of birth
- Ordering provider address
- Ordering provider phone number
To protect patient privacy, any data that state and jurisdictional health departments send to CDC will be deidentified and will not include some patient-level information. The deidentified data shared with CDC will contribute to understanding COVID-19’s impact, case rate positivity trends, testing coverage, and will help identify supply chain issues for reagents and other materials.
How to report using standard terminology
CDC has posted a LOINC In-Vitro Diagnostic (LIVD) Test Code Mapping Guide for COVID-19 test results for tests with emergency use authorization from the U.S. Food and Drug Administration (FDA) that can be used by clinical laboratories and instrument manufacturers. This specification supports the use of standardized LOINC and SNOMED Clinical Terms (CT) codes to improve the accuracy of reporting tests for the SARS-CoV-2 virus. Using these harmonized LOINC and SNOMED-CT codes helps ensure that the same type of test is represented uniformly across the United States.
For those COVID-19 tests that have not yet received FDA emergency use authorization, CDC encourages test developers and laboratories that use COVID-19 tests to work together to obtain appropriate and interoperable LOINC and SNOMED-CT codes for reporting purposes.
LOINC codes must be used to represent the “question” a test asks of a specimen (e.g., does this specimen have SARS-CoV-2 RNA?), and SNOMED-CT codes must be used to represent the diagnostic “answer” (e.g., what was detected?). More background on these terminology standards can be found here:
Whenever possible, laboratories must use standard codes that already exist. Before requesting a new code, search the list of currently available LOINC codes for COVID-19 tests. If a LOINC test code cannot be identified whose attributes appropriately match the test for which coding is needed, new terms can be submitted, and a new code can be requested through LOINC .
Technical assistance for electronic reporting
Electronic reporting options are available to reduce the burden on providers reporting test results. Laboratories that are not currently reporting electronically to their state or local health department and want assistance in establishing electronic reporting can contact [email protected].
Below is a list of COVID-19 resources for laboratories:
HHS Guidance
- HHS Laboratory Reporting Guidance
CDC Resources
- COVID-19 Information for Laboratories
- CDC’s Laboratory Outreach Communication System (LOCS)
- Clinical Laboratory COVID-19 Response Calls
Technical Implementation Resources
- Guidance for Encoding School Information for COVID-19 Public Health Reporting
- LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests
- COVID-19 Response | CSTE EMERGENCY PREPAREDNESS & RESPONSE
- Interoperability Standards Advisory for COVID-19 Pandemic
CMS Resources
- Interim Final Rule with Comments (IFC)
Frequently Asked Questions on Laboratory Data Reporting Guidance for COVID-19 Testing
Data reporting information.
No, facilities that conduct tests for individuals from multiple states must report results to the appropriate state or local health department based on the patient’s residence. If the patient’s address isn’t available, results should be reported based on the provider’s location.
Facilities that conduct tests for individuals who are temporarily living away from their permanent residence, such as students in college or active duty military personnel, should report to the state health department based on the individual’s temporary address near their college campus or military installation.
COVID-19 home tests can be safely and privately reported at MakeMyTestCount.org , but there is currently limited use for collecting self-test result data to inform public health surveillance. There are no current mechanisms that require reporting of self-test results to public health authorities. Voluntary reporting of self-test results will often be anonymous or lack data necessary for public health analysis or action. Therefore, the self-test results are unlikely to enhance understanding of trends in disease transmission or severity and often do not provide sufficient information to support case investigations. The public health community, including CDC, is confident that situational awareness remains strong without receiving self-test results. Find more information: About CDC COVID-19 Data.
Clinicians and laboratories should contact their state or local public health department directly for more information on reporting requirements and the method for reporting.
Yes, all data related to the AOE questions should be collected and reported to state and local public health departments in the electronic laboratory report messages.
Technical aspects of reporting
Yes, information about LOINC codes and the specific harmonized LOINC codes for COVID-19 tests can be found on CDC’s website: LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests .
CDC’s LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests website has a mapping catalogue coded for the data elements associated with COVID-19 tests, including the LOINC test order, LOINC test result, SNOMED-CT test description and SNOMED-CT specimen source. Test developers and manufacturers of new tests should contact [email protected] for information about obtaining new codes.
The Association of Public Health Laboratories (APHL) , in collaboration with the Council of State and Territorial Epidemiologists (CSTE) , CDC, and other public and private partners , ha ve developed the National ELR Flat File and HL7 Generator Tool to assist laboratories with reporting.
The DI for some tests can be found in the National Institute of Health’s (NIH) Access GUDID Database . For a specific DI not located in the Access GUDID Database, contact the device manufacturer to obtain the DI. If the manufacturer does not yet have the DI for the device you are using, contact [email protected] for assistance.
Clinical research trial and study reporting
In general, no. Laboratories are not responsible for reporting these data. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
The reporting requirements differ for laboratories and clinicians:
Laboratories
Laboratories are not responsible for reporting these data since they do not have the patient-identifying information required to comply with reporting requirements. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
In clinical trials or other clinical studies, clinicians who are responsible for clinical care of trial or study participants are responsible for linking de-identified specimen test results to participant demographic information and may be required to report the results to the appropriate local, tribal, or state public health department based on the patient’s residence.
If a clinician receives test results related to COVID-19 from duplicate specimens that were collected in the same manner and tested with different test methods (e.g., different platforms) or in different CLIA-certified laboratories, the clinician should not report both results. In the case of two positive test results, the clinician should report the result that is provided first. In the case of discrepant test results, the clinician should report the positive result. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
If the clinician requests testing related to COVID-19 for study participants independent of research activities or for clinical management, results should be reported to the appropriate local, tribal, or state public health department.
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Report Covid-19 Home Test Results Through New NIH Website

Reporting a positive or negative test result just became easier through a new website from NIH, MakeMyTestCount.org . Developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, the new website allows users to anonymously report the results of any brand of at-home Covid-19 test.
Covid-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid Covid-19 test has become commonplace, test results are not often reported. Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.

Access your test results

About your test results
Knowing your results helps you and your doctor understand what’s best for your health.
2 ways to get your lab results
1. directly through myquest ®.
Receive easy-to-understand Quest lab results and more, directly on your smartphone, tablet, or desktop:
- Schedule and manage your Quest appointments
- Manage test results from your family or those in your care with My Circle
- Share health information with your healthcare providers
- Organize your health information in one convenient place
2. From your doctor's office
- You can ask your physician to send you a copy of your previous test results
- During your next visit, you can ask your physician to indicate on your requisition that Quest should also send you a copy of your results
Most test results can be shared by phone, while others are best shared during a follow-up doctor's visit and discussion. Work with your doctor on how best to share your test results. Access Test Results, Manage Appointments & More | MyQuest (questdiagnostics.com)
Discuss with your doctor
While MyQuest will guide you through your lab results, it's important to discuss them with your doctor. Together, you two can decide what's best for your health.
Download the MyQuest ® app
MyQuest makes it easy to get your test results, schedule appointments, track your health history, and more, all in one place.
We do not charge a fee to use this app. However, your carrier’s data and usage charges apply.
For Apple ® iPhone, iPod Touch, iPad, Android™ Available on iPhones running IOS 11.3 or later.
Download on the App Store Get It On Google Play
Frequently asked questions about test results
Unless otherwise noted, the answers to the following questions apply to test results by healthcare professionals only.
How long does it take to receive test results?
COVID-19 RESULTS: Test results are typically available by the end of the next day. As testing conditions continue to evolve, stay up-to-date on estimated turnaround times .
If you're looking for your results from tests you've purchased directly from Quest, go here for more information. If your testing was ordered by your healthcare professional, your lab test results (performed by a Quest Diagnostics laboratory) will be delivered to MyQuest as soon as they are available. You can go to the Results page and look at the Pending Results section to determine the current status of your lab work. If it has been more than 5 days since you came in for testing, you can go to the Results page and click Request Test Results (or the plus [+] icon > Request Test Results on your mobile device) and then follow the instructions for requesting lab test results.
After requesting your lab test results, a new item appears on your Activity and Results pages. The item on your Activity page indicates you requested a test result and the item on your Results page provides information about the status of that request.
Note: If you are waiting for lab results in CA, PA, OR, or MD, your lab results may be held for a period of time before they are released. This hold time is in place to comply with state-specific laws.
Can I track the status of my test results?
You can check the current status of your test results in MyQuest on the Results page under Pending Results . Test tracking cards appear on the Results page under Pending Results , and indicate where the test is in the process. Testing can be in one of the following states:
- Test Ordered. Quest has received an order for your testing. After your sample has been collected, it will be sent to a Quest laboratory for processing.
- In Process. Quest has received your sample. Once testing is complete, your results will be ready for review.
- Physician Notification. Processing has been completed and your test results are being prepared. You'll receive an email when your test results are available for review in MyQuest. (In some cases a delivery date may be provided.)
- Canceled. This order was canceled on <date>. Please determine if testing is still needed.
Note: If no status is available, you should see the following message At this time, we are unable to provide the status of pending lab results. As soon as we have status information it will be updated here. Please check back later .
Where do I go for help with MyQuest?
If you don't find the answers you are looking for in the FAQs, you can:
- Access Quest Chatbot. The Quest Chatbot can answer many frequently asked questions about purchases, MyQuest, and Appointment Scheduling. Quest Chatbot is accessible from the bottom of each page or by clicking the Contact Quest > Message Quest menu option. Once Quest Chat opens, just type your question.
- Access the Message Quest form. You can fill out a form that sends a message directly to our customer support representatives. While filling out the form, please specify a Reason for Contact, as it helps us get the message to the right support team.
What other health apps can be used to get results?
We offer integrated results through Apple Health, CLEAR, and TrustAssure. If you do not see your health app listed, please click on the link below to share your request with us.
Request a FHIR API
Access family test results
MyQuest ® helps you monitor your family's health with My Circle
Billing and insurance
Check your health plan coverage and pay your bill.
Browse within Quest
Testing options • Preparing for a test • Preparing for child visits • MyQuest • Purchase tests • Financial assistance • FAQs

Our company
- How we operate
- Corporate responsibility
- Inclusion and diversity
- Actions and insights
- Specialty labs

- Site map •
- Privacy Notices •
- Terms •
- Contact us •
- Language assistance / non-discrimination •
- Asistencia de idiomas / Aviso de no discriminación •
- 語言協助 / 不歧視通知 •
- Accessibility •
- Your Privacy Choices •
Quest® is the brand name used for services offered by Quest Diagnostics Incorporated and its affiliated companies. Quest Diagnostics Incorporated and certain affiliates are CLIA certified laboratories that provide HIPAA covered services. Other affiliates operated under the Quest® brand, such as Quest Consumer Inc., do not provide HIPAA covered services.
Quest®, Quest Diagnostics®, any associated logos, and all associated Quest Diagnostics registered or unregistered trademarks are the property of Quest Diagnostics. All third-party marks—® and ™—are the property of their respective owners. © 2024 Quest Diagnostics Incorporated. All rights reserved. Image content features models and is intended for illustrative purposes only.
www.QuestDiagnostics.com says
You are now leaving the Quest Diagnostics web site. Quest Diagnostics does not control the site you are about to enter and accepts no responsibility for its content.
We'll redirect you to MyDocBill.com, the website of Quest’s billing services provider. Here, Quest patients have secure access to pay their bill, update insurance, edit their profile and view their account history.
Employer Health and Wellness
To schedule an appointment for your employer health and wellness screening (biometric screening), we need to redirect you to the Health & Wellness website.
- Your doctor must have ordered your COVID-19 antibody testing or you must have purchased a test through QuestDirect. COVID-19 is highly contagious. If you are currently experiencing COVID-19 symptoms, you must first contact your doctor for next steps..
- We can only accept patients in our Patient Service Centers who have been confirmed to be COVID-19 symptoms-free for at least 10 days.
Purchased Tests
Please visit our appointment scheduling page to make your appointment.

Test Summary Reports Tutorial: Learn with Example & Template
Test Report
Test Report is a document which contains a summary of all test activities and final test results of a testing project. Test report is an assessment of how well the Testing is performed. Based on the test report, stakeholders can evaluate the quality of the tested product and make a decision on the software release.
For example, if the test report informs that there are many defects remaining in the product, stakeholders can delay the release until all the defects are fixed.
Test Report Example
Why Test Report?
The following scenario will show you why we do need the Test Report
Do you know the root cause of this problem? Why does the website still has defects even when your Team has already tested it?
The problem is you ignored the reporting & evaluation phase in Test Management. The boss has no information to evaluate the quality of this website. They just trusted what you said and released the website without knowing its testing performance.
The typical benefits of a test report include:

How to make a good Test Report?
To answer this, you must know –
What does a test report contain?

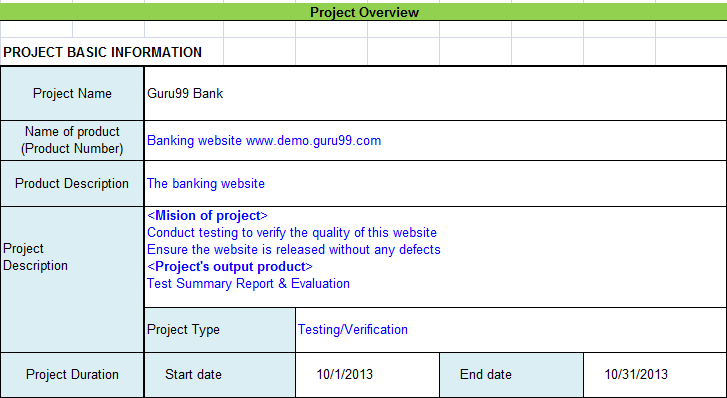
Project Information
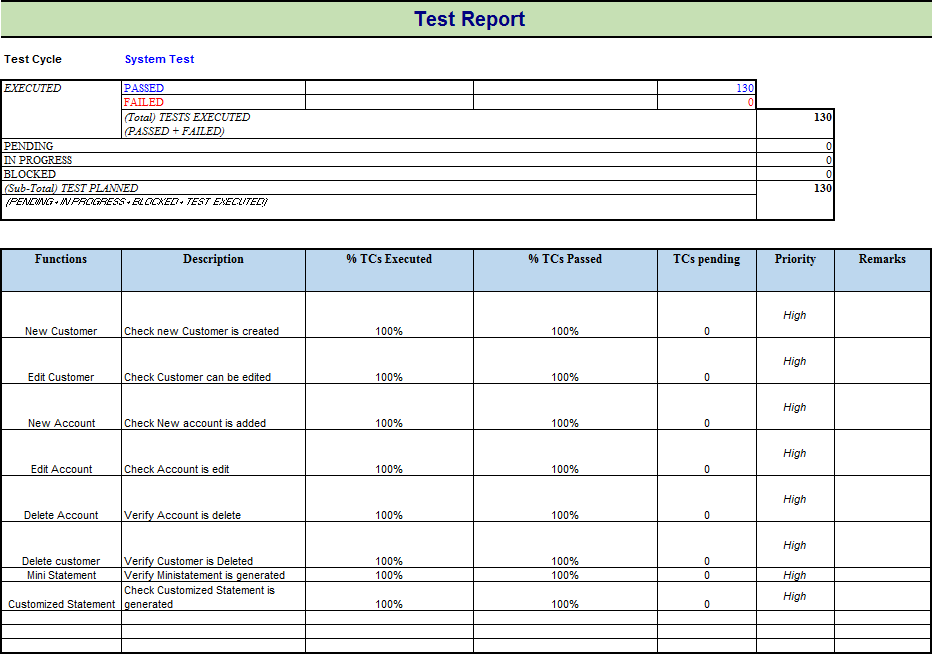
All information of the project such as the project name, product name, and version should be described in the test report. For example, the information of Guru99Bank project will be as follows
Test Objective
As mentioned in Test Planning tutorial, Test Report should include the objective of each round of testing, such as Unit Test, Performance Test, System Test …Etc.
Test Summary
This section includes the summary of testing activity in general. Information detailed here includes
- The number of test cases executed
- The numbers of test cases pass
- The numbers of test cases fail
- Pass percentage
- Fail percentage
This information should be displayed visually by using color indicator , graph, and highlighted table .
Take a look at Test Report of the website Guru99 Bank to know more detail about Test report
One of the most important information in Test Report is defect. The report should contain following information
- Total number of bugs
- Status of bugs (open, closed, responding)
- Number of bugs open, resolved, closed
- Breakdown by severity and priority
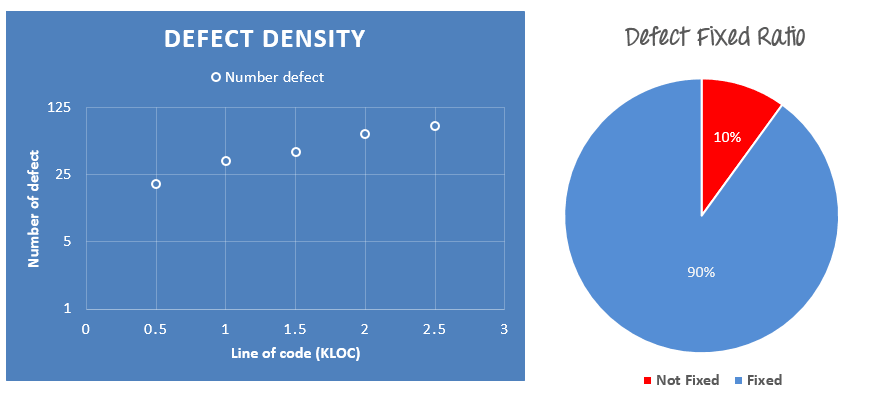
Like test summary, you can include some simple metrics like Defect density, % of fixed defects.
The project team sent you the Defect information as following
- Defect density is 20 defects/1000 lines of code average
- 90% defects fixed in total
- The detail of the bugs are described in this Defect tracker here
You can represent the data as following graph
Tips to write a good test report
Test report is a communication tool between the Test Manager and the stakeholder. Through the test report, the stakeholder can understand the project situation, the quality of product and other things.
The following scenario shows you why we need a good Test Report
You co-operate with outsourcing company, its tester after having performed Performance Testing of the website Guru99 Bank, sends you a test report like this
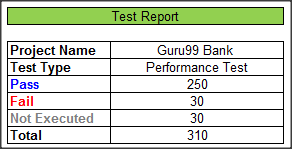
The information of that report is too abstract . It does not have any detailed information. The stakeholder who will read it might be slightly puzzled when they get it. They might ask or have following sets of questions: –
- Why did they not execute 30 TCs that remains
- What are these failed Test Cases
- Doesn’t have any bugs description
To solve that problem, a good Test Report should be:

- Detail : You should provide a detailed description of the testing activity, show which testing you have performed. Do not put the abstract information into the report, because the reader will not understand what you said.
- Clear: All information in the test report should be short and clearly understandable.
- Standard: The Test Report should follow the standard template. It is easy for stakeholder to review and ensure the consistency between test reports in many projects.
- Specific: Do not write an essay about the project activity. Describe and summarize the test result specification and focus on the main point.
For example, to correct the above Test Report, the tester should provide more information such as:
- Project information
- Test cycle: (System Test, Integration Test…etc.)
- Which functions have already tested (% TCs executed, % TCs passed or fail…)
- Defect report (Defect description, Priority or status…)
- Defect Management Process in Software Testing
- 5 Steps to Master Team Management Skills
- TEST Management Tutorials: Complete Training Course
- TestLink Tutorial: A Complete Guide
- 16 BEST Test Management Tools (2024)
- Top 20 QA Manager / Test Lead Interview Questions (2024)
- Test Management in Software Testing PDF for Beginners
- 10 Best Test Management Tools For Jira (2024)
- Extensive end-to-end automation of QA process
- Comparative analysis of app performance against peers
- Continuous monitoring of app performance using synthetic data for higher availability of apps
- Easy-to-use developer friendly platform
ADD-ON PRODUCTS
Enterprise addon products, resource center, resource center.

The Role of Test Reporting in Software Testing: A Comprehensive Overview
Introduction
Continuous testing plays a pivotal role in ensuring the quality and reliability of software products. Test reporting, an essential aspect of the continuous testing process, provides valuable insights into the test execution and helps stakeholders make informed decisions.
This blog will explore why test reporting in continuous testing is so important and the benefits, challenges, and best practices associated with it. By understanding these key elements of test reporting in continuous testing , teams can ensure success on any project.
Understanding Test Reporting in Software Testing
Test reporting is a crucial process in software testing that revolves around gathering, analyzing, and presenting essential test data and results to stakeholders. At its core, it serves as a vital communication channel, providing insights into a software application's progress, quality, and readiness throughout the testing lifecycle. By consolidating test outcomes, test reporting empowers teams to identify patterns, make informed decisions based on data, and proactively tackle potential issues.
The fundamental purpose of test reporting is to keep all project stakeholders informed and aligned. This includes developers, testers, product owners, project managers, and key team members. By sharing a comprehensive view of the software's testing status, test management reporting fosters collaboration and makes sure everyone involved is on the same page. This level of transparency is essential in complex development environments, as it promotes a shared understanding of the software's health and assists in streamlining the development process.
Read: 4 Essential Tips for Software Test Management
When diving into the details of test reporting, it is essential to recognize its three primary functions: collection, analysis, and presentation of test data.
- Data Collection: A vast amount of data is generated during the testing process. This includes test case execution results, defect reports, test coverage metrics, and other relevant information. Test reporting involves meticulously collecting and organizing this data to ensure it is accurate and up-to-date. Data collection not only encompasses the outcomes of individual test cases but also includes information about the test environment, such as the hardware and software configurations used for testing. This context helps stakeholders understand the conditions under which testing occurred and provides insights into potential environmental factors that may have influenced the test results.
- Data Analysis: Once the test data is collected, it undergoes rigorous analysis to derive meaningful insights. The goal is to identify trends, patterns, and correlations within the data. This analysis provides a detailed understanding of the software's performance and helps uncover potential problem areas. For instance, data analysis might reveal an increasing number of defects in a particular software functional area. This insight would prompt the team to investigate further and take corrective measures to improve the quality of that specific component.
- Data Presentation: The final step of test reporting is presenting the analyzed data in a clear and comprehensible format. Stakeholders need information that is easily digestible and allows them to make informed decisions quickly. To achieve this, test reporting utilizes various visualization techniques, such as charts, graphs, and dashboards. These visual aids provide a bird's-eye view of the test results, enabling stakeholders to grasp the software's testing status at a glance. Additionally, test reports often include textual summaries that provide context and explain the significance of the presented data.
Also read: How Does Continuous Testing Accelerate DevOps?
The Advantages of a Test Summary Report in Software Testing
Amidst various types of test reports, the test summary report stands out for its concise nature and a high-level overview of the testing process. This specialized report brings several distinct benefits, making it a valuable tool for decision-makers and project stakeholders.
1. Management Visibility: Test summary reports are specifically tailored for management and higher-level stakeholders who seek an executive-level understanding of the testing progress and results. These reports provide a snapshot of the overall testing status without overwhelming readers with intricate technical details. For busy executives and project managers, the test summary report becomes an indispensable source of information to assess the software's testing health quickly and effectively.
The test summary report streamlines communication between testing teams and management by offering a concise yet informative view of the software's quality status. This enhanced visibility ensures that decision-makers stay informed, remain in the loop, and have the necessary insights to make well-informed strategic decisions.
Check: Integration of Extent Reports
2. Facilitating Quick Decision-Making: Quick decision-making is essential for meeting tight deadlines and maintaining a competitive edge in the fast-paced world of software development. Test summary reports excel, presenting crucial information clearly and straightforwardly. Decision-makers can swiftly grasp the software's quality status, including the number of tests executed, the overall test outcomes, and key metrics.
The concise format of the test summary report enables stakeholders to identify critical areas that require immediate attention or additional resources. With this information, project leaders can make timely decisions about prioritizing tasks, allocating resources, and adjusting development strategies. The ability to act promptly based on insights from the test summary report ensures that the software development process stays on track and aligned with project goals.
3. Optimizing Resource Allocation: Efficient resource allocation is a cornerstone of successful project management. Test summary reports play a vital role in helping organizations allocate resources effectively. By providing a high-level view of testing outcomes and areas that require attention, these reports enable teams to optimize their resource distribution.
For example, if the test summary report indicates that certain test cases consistently fail in a specific functional area, the team can allocate additional resources and focus on improving the testing effort in that domain. On the other hand, areas with a high success rate may require fewer resources, allowing them to be reallocated to other critical parts of the project. As a result, the test summary report becomes an invaluable tool for ensuring that resources are utilized optimally, promoting cost-effectiveness, and enhancing overall project efficiency.
Also check: How ReportPortal Helps Continuous Integration and Testing Processes
4. Empowering Proactive Risk Management: Software development projects are not without risks, and identifying potential roadblocks early on is crucial for successful project delivery. Test summary reports are vital in risk assessment by highlighting key risk areas and potential vulnerabilities.
By providing a clear overview of test outcomes and defects, the test summary report enables teams to manage risks proactively. Project stakeholders can focus on addressing critical issues before they escalate, minimizing the impact of potential setbacks. This proactive approach to risk management enhances project resilience and contributes to a smoother development process.
What Are the Challenges Associated with Test Reporting in Continuous Testing?
Test reporting in continuous testing can present challenges, especially when multiple test cases have different outcomes. To accurately and effectively report on results, teams must ensure that they collect the right data and utilize an appropriate reporting system. Manual test reporting in software testing can be time-consuming as well as labor-intensive, making it difficult to keep up with the speed of development cycles. Compiling data into meaningful metrics and reports that stakeholders can easily understand is also challenging.
Read: What is Continuous Monitoring in DevOps?
Some common challenges include:
- Data Overload : In large-scale projects, generating comprehensive test reports may lead to an overwhelming amount of data, making it difficult for stakeholders to extract relevant insights.
- Report Accuracy : Accurate test reporting relies on consistent and reliable test data. If the test data is erroneous or incomplete, it can compromise the credibility of the entire reporting process.
- Report Interpretation : Understanding and interpreting test reports can be challenging, especially for non-technical stakeholders. Testers must ensure that the reports are presented in a clear and understandable format.
- Time Constraints : Preparing detailed test reports can be time-consuming, especially when testing cycles are short and frequent.
Also read: Exploring the top CI/CD tools for DevOps
What are the Key Components of a Test Report?
Creating an effective test report involves structuring it into several key sections. Each section serves a specific purpose and contributes to the overall comprehensiveness of the report. The different sections of a test report include:
1. Introduction: The introduction section of a test report serves as the gateway to the entire document. Its purpose is to provide a clear and concise overview of the test report, giving readers a preview of what to expect. Key components of the introduction include:
- Purpose: Clearly state the objective of the test report. This could be to present the results of a specific testing phase, provide an update on the software's testing progress, or assess the software's readiness for release.
- Scope: Define the scope of the testing effort covered in the report. Specify the aspects of the tested software, the testing types conducted (e.g., functional testing, performance testing, security testing), and any limitations or exclusions that might impact the report's findings.
- Software Being Tested: Identify the specific software application or module under test. Include version information and other relevant details that help readers understand the testing context.
A well-crafted introduction sets the stage for the test report, offering readers a clear understanding of its purpose, scope, and the software's context within the testing process.
2. Test Environment: The test environment section provides essential details about the setup where the testing occurred. These details are crucial for understanding the context and the factors that may have influenced the test results. Key components of the test environment section include:
- Hardware: List the hardware components used for testing, such as servers, workstations, devices, and network equipment. Include specifications like CPU, RAM, storage capacity, and any special configurations relevant to the testing process.
- Software: Enumerate the software components involved in the testing, such as operating systems, databases, web browsers, and other dependencies. Specify their versions and any specific settings or configurations applied during testing.
- Configurations: Detail any configurations or setups used during testing, such as network settings, user accounts, and permissions.
- Versions: Clearly state the software versions being tested, including any patches or updates applied during the testing process.
The test environment section ensures transparency and replicability of the testing process, enabling others to reproduce the tests and verify the results under similar conditions.
Check: Writing an Appium Test in Kotlin
3. Test Execution Summary: This section provides a high-level overview of the test execution, offering stakeholders a quick glimpse of the testing outcomes. Key components of the test execution summary include:
- Total Test Cases: Mention the total number of test cases planned for execution.
- Test Cases Executed: Indicate the number of test cases executed during the testing phase.
- Test Cases Passed: Specify the count of test cases that successfully passed without encountering any defects.
- Test Cases Failed: Provide the number of test cases that resulted in failures and a brief explanation of the failure reasons.
The test execution summary is crucial for decision-makers and management, as it provides an at-a-glance understanding of the overall testing progress and outcomes.
4. Detailed Test Results: In this section, testers provide a comprehensive breakdown of the test results, diving into the specifics of each test case executed. Key components of the detailed test results include:
- Test Case ID: Assign a unique identifier to each test case for easy reference.
- Test Case Description: A concise description of each test case, outlining its objective and expected behavior.
- Test Case Status: Indicate the status of each test case (passed, failed, blocked, etc.).
- Defects: If a test case fails, include details about the defects encountered, including their severity, priority, and steps to reproduce.
- Test Data: Specify any specific test data used for each test case to ensure reproducibility.
- Screenshots/Attachments: Include relevant screenshots or attachments to support the test results and provide additional context.
The detailed test results section forms the core of the test report, presenting a granular view of the testing outcomes and facilitating in-depth analysis.
Check out: Decoding codeless Appium test automation with HeadSpin
5. Defect Summary: The defect summary section consolidates all the defects found during testing and provides a concise overview of their impact. Key components of the defect summary include:
- Total Defects: State the total number of defects identified during testing.
- Defect Categories: Categorize the defects based on severity levels (e.g., critical, major, minor) and priority (e.g., high, medium, low).
- Defect Status: Specify the current status of each defect (open, closed, retested, etc.).
- Defect Resolution: Include information about how to address and resolve each defect.
The defect summary section helps stakeholders understand the software's overall quality by highlighting defects' presence and status, enabling effective issue management and resolution.
6. Test Coverage: The test coverage section provides insights into the extent to which the software has been tested and which areas remain untested. Key components of the test coverage section include:
- Functional Areas: Enumerate the software's functional areas or modules covered in the testing process.
- Percentage of Code Covered: Provide the percentage of code exercised during testing.
- Test Types: Specify the types of testing performed for each functional area (e.g., unit testing, integration testing, system testing).
- Uncovered Areas: Identify any functional areas or aspects of the software that were not tested and the reasons for the omission.
Also check: A Detailed Guide to Code Coverage and Test Coverage
Test coverage ensures that all critical aspects of the software have been thoroughly tested, minimizing the risk of undiscovered defects.
7. Conclusion and Recommendations: The conclusion section summarizes the key findings and outcomes of the testing effort and presents actionable recommendations for improvement. Key components of the conclusion and recommendations section include:
- Summary of Testing Outcomes: Recapitulate the main results and trends observed during testing.
- Testing Objectives Met: Evaluate whether the testing objectives set at the beginning of the phase have been achieved.
- Improvement Areas: Highlight areas where the software can be further improved based on the testing findings.
- Recommendations: Provide actionable recommendations to address the identified issues and enhance the software's quality.
The conclusion and recommendations section forms the basis for future improvements and actions to ensure a successful software development process.
Different Types of Test Reports for Comprehensive Insights
Test reporting is a multifaceted process, and various test reports cater to other stakeholders and objectives. Each type offers unique insights into specific aspects of the testing effort, contributing to a well-rounded understanding of the software's quality and development progress. Let's delve into the details of some common types of test reports:
- Test Summary Report: The test summary report is significant in test reporting due to its concise nature and a high-level overview of the testing process. This report is specifically designed for management and higher-level stakeholders who need an executive view of the testing progress and results without getting entangled in granular details.
- Defect Report: The defect report focuses on the defects encountered during testing. It provides detailed information about each defect, including its status, priority, and associated steps to reproduce.
- Test Execution Report: The test execution report provides insights into the test execution process, outlining the number of test cases executed, passed, and failed. It offers a detailed view of test results for each implemented test case.
- Test Coverage Report: The test coverage report assesses the extent to which the software has been tested and the untested areas. It helps stakeholders understand the completeness of the testing effort and identify gaps in test coverage.
Read: Navigating Testing Success - The Ultimate Guide to Test Strategy Document Mastery

How to Create Effective Test Reports: A Step-by-Step Guide
Creating good test reports requires a structured approach that ensures clarity, relevance, and accuracy. An effective test report is a critical communication tool, providing stakeholders valuable insights into the software's testing progress and quality. To craft comprehensive and impactful test reports, follow this step-by-step guide:
Step 1: Define the Report's Purpose
Before creating the test report, clearly define its purpose and identify the target audience. Understand what specific information stakeholders need to make informed decisions. For instance, management may require a high-level overview of progress, while testers may need granular details of test results.
Step 2: Gather Comprehensive Data
Accurate and comprehensive data is the backbone of any test report. Ensure that the testing process captures all relevant information, including test case execution results, defects, test environment details, and any other pertinent metrics. This may involve integrating testing tools and test management systems to collect data seamlessly.
Step 3: Choose the Right Metrics
Selecting appropriate metrics that align with the report's purpose is crucial for delivering meaningful insights. Common testing metrics include test pass rate, defect density, defect trend, test coverage, and test execution progress. Tailor the choice of metrics to meet the stakeholders' needs and measure the software's quality effectively.
Step 4: Use Clear and Concise Language
Present information in straightforward, easy-to-understand language. Avoid using technical jargon that might confuse non-technical stakeholders. Use simple and concise sentences to convey complex information efficiently.
Step 5: Visualize Data
Visual aids make data more accessible and understandable. Utilize charts, graphs, and tables to present test results and metrics in a visually appealing format. For example, use pie charts to show the distribution of test case status (passed, failed, blocked), line graphs to depict defect trends over time, and bar charts to compare test pass rates across different test cycles.
Step 6: Add Context and Analysis
Mere data representation is insufficient; providing context and analysis is equally important. Accompany the visualized data with brief explanations and interpretations. Describe the significance of certain trends, anomalies, or critical defects found during testing. The context helps stakeholders understand the implications of the presented data and supports informed decision-making.
Step 7: Proofreading and Review
Review the test report for errors, inconsistencies, and coherence. A well-polished report enhances credibility and professionalism. Check for accurate data representation and ensure the conclusions drawn align with the presented data.
Step 8: Automate Reporting
Consider leveraging test reporting tools to automate the generation of reports. These tools can integrate with testing frameworks and test management systems, automatically collecting data and producing standardized reports. Automation saves time, reduces human errors, and ensures consistency in report generation.
Learn more: How Enterprises Conduct Automated Continuous Testing at Scale with Jenkins
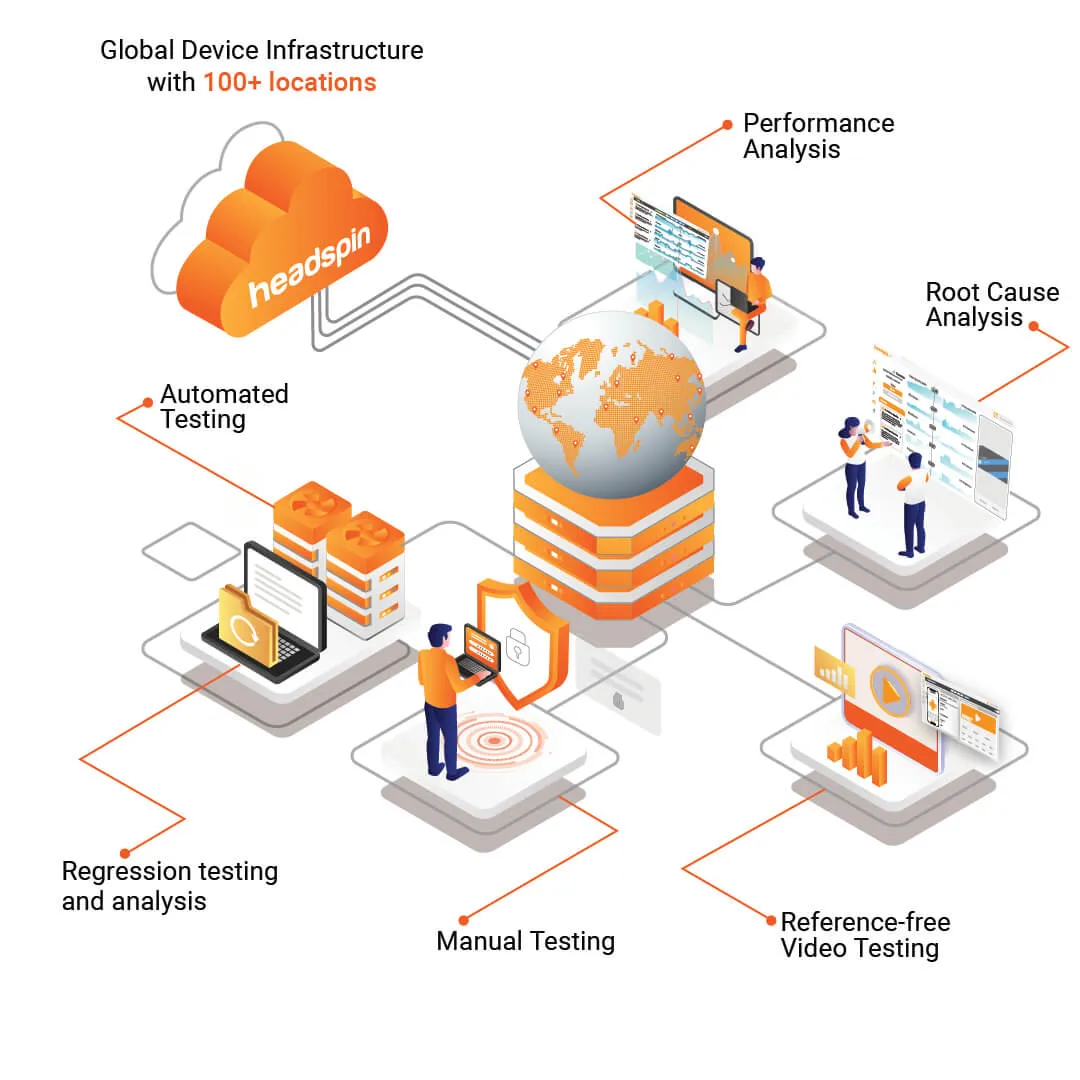
How HeadSpin's Cutting-Edge Solutions Empower Businesses with Seamless Test Reporting
HeadSpin offers a comprehensive and cutting-edge solution that covers no-code and low-code automation, setting new standards in continuous testing. Through strategic partnerships with industry-leading test reporting tools like Tricentis’ Tosca and ACCELQ, HeadSpin empowers organizations to integrate robust test reporting capabilities into their testing processes seamlessly.
Let's explore a few unique capabilities and advantages of HeadSpin's solution:
- Ease of CI/CD Workflow Integration: HeadSpin’s partnership with Tricentis and other tools ensures seamless integration of the testing process into Continuous Integration/Continuous Deployment (CI/CD) workflows. This integration streamlines the testing effort, allowing automated tests to be executed automatically as part of the development pipeline. As a result, teams can identify defects early in the development cycle and accelerate the release process with greater confidence in software quality.
- Quality of Experience Issue Identification: By connecting to thousands of mobile and browser testing devices in global locations, the partnership enables thorough testing of various distributed edge nodes or "edges." This approach helps identify Quality of Experience (QoE) issues, such as performance bottlenecks, latency problems, and user experience glitches, that may arise from different network conditions and geographical locations. Detecting and addressing these issues enhances the overall quality and reliability of the software.
- Higher Test Automation Rates: The collaboration between HeadSpin and other test reporting tools leads to higher test automation rates of up to 90%. Test automation significantly reduces manual testing efforts, freeing resources for more valuable tasks and accelerating the software development lifecycle. Increased automation rates also drive innovation in enterprise packaged applications ecosystems, as teams can focus on implementing new features and improvements rather than manual testing.
- Test with HeadSpin's Real Device Cloud: The partnership enables users to leverage HeadSpin's global device infrastructure to implement test cases with Tosca's codeless power. As a leading test automation tool, Tosca complements HeadSpin's device cloud capabilities. Using codeless automation features, testers can create test cases without writing extensive code, making the testing process more accessible to a broader range of team members.
What’s Next?
Test reporting in software testing is an indispensable aspect of continuous testing that provides vital information to stakeholders, ensuring software quality and reliability. Effective test reports offer numerous benefits, including insights into test progress, early defect detection, data-driven decision-making, regulatory compliance, and efficient project communication.
Leveraging HeadSpin's comprehensive solution with no-code automation capabilities, in collaboration with top-notch test reporting tools, amplifies the effectiveness of your testing efforts.
If you want to enhance your test reporting capabilities and streamline your continuous testing process, take the next step with HeadSpin.
Connect Now
Q1. how can test reporting tools automate the generation of test reports.
Ans: Test reporting tools automate report generation by integrating with testing frameworks and test management systems, automatically collecting comprehensive data, and presenting it in a visually appealing format, reducing human errors and saving time.
Q2. What are the benefits of using visualization techniques in test reporting?
Ans: Visualizing techniques in test reporting simplify complex data, enabling stakeholders to grasp testing insights quickly, make informed decisions, and enhance communication and collaboration for better quality assurance and efficient problem-solving.
Related blogs

The Key to Digital Success: A Comprehensive Guide to Continuous Testing Integration

How Usability Testing Impacts Mobile Game Testing

A Comparative Analysis of Functional and Non-Functional Testing
-1280X720-Final-2.jpg)
Regression Intelligence practical guide for advanced users (Part 1)
Regression Intelligence practical guide for advanced users (Part 2)
Regression intelligence practical guide for advanced users (part 3).
-1280X720-Final-2.jpg)
Regression Intelligence practical guide for advanced users (Part 4)

Discover how HeadSpin can empower your business with superior testing capabilities

Book Free Trial
Perfect Digital Experiences with Data Science Capabilities
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Testing for COVID-19
Report a free NHS COVID-19 rapid lateral flow test result
You no longer need to report the result from a free NHS rapid lateral flow test if you live in England.
If you have an old free NHS rapid lateral flow test, you can take it but you do not need to report the results.
Find out more about COVID-19 symptoms, testing and vaccination and how to avoid catching and spreading COVID-19 .
If you live in Northern Ireland, Scotland or Wales
Find out about:
- COVID-19 testing in Northern Ireland
- COVID-19 testing in Scotland
- COVID-19 testing in Wales
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .

- Calculators
- Descriptive Statistics
- Merchandise
- Which Statistics Test?
How to Report a T -Test Result in APA Style
The APA style guide details precise requirements for citing the results of statistical tests, which means as well as getting the basic format right, you've got watch out for punctuation, the placing of brackets, italicisation, and the like.
There are a number of different t -tests, the most common being single sample t -test, independent t -test and dependent t -test. The basic format for reporting the result of a t -test is the same in each case (the color red means you substitute in the appropriate value from your study):
t ( degress of freedom ) = the t statistic , p = p value .
It's the context you provide when reporting the result that tells the reader which type of t -test was used. Here are some examples.
Single Sample T -Test
United fans reported higher levels of stress ( M = 83, SD = 5) than found in the population as a whole, t (48) = 2.3, p = .026.
Coffee drinkers spent more time awake ( M = 17.8, SD = 1.4) than the population norm, t (28) = 2.6, p < .05.
Independent T -Test
The 25 participants who received the drug intervention ( M = 480, SD = 34.5) compared to the 28 participants in the control group ( M = 425, SD = 31) demonstrated significantly better peak flow scores, t (51) = 2.1, p = .04.
There was no significant effect for sex, t (38) = 1.7, p = .097, despite women ( M = 55, SD = 8) attaining higher scores than men (M = 53, SD = 7.8).
Dependent T -Test
The results from the pre-test ( M = 13.5, SD = 2.4) and post-test ( M = 16.2, SD = 2.7) memory task indicate that the presence of caffeine in the bloodstream resulted in an improvement in memory recall, t (19) = 3.1, p = .006.
There was a significant increase in the volume of alcohol consumed in the week after the end of semester ( M = 8.7, SD = 3.1) compared to the week before the end of semester ( M = 3.2, SD = 1.5), t (52) = 4.8, p < .001.
1. The abbreviations M and SD stand for mean and standard deviation respectively.
2. If your t -test is one-tailed, you need to say so.
3. There are two ways to report p values. The first way is to cite the alpha value, as in the second of the single sample t -test examples above. The second way, very much the preferred way in the age of computer aided calculations (and the way recommended by the APA), is to report the exact p value (as in our first example). If you report the exact p value, then you need to state your alpha level early in your results section. The other thing to note here is that if your p value is less than .001, it's conventional simply to state p < .001, rather than give the exact value.
4. Remember to drop the leading 0 from the p value.
5. No need to provide a formula for t .
6. Degrees of freedom are N - 1 for the single sample and dependent measures t -tests; and ( N 1 - 1) + ( N 2 - 1) for the independent t -test.
7. If you're hypothesis testing, then remember to restate your hypothesis.

How to Report T-Test Results (With Examples)
We can use the following general format to report the results of a one sample t-test :
A one sample t-test was performed to compare [variable of interest] against the population mean. The mean value of [variable of interest] (M = [Mean], SD = [standard deviation]) was significantly [higher, lower, or different] than the population mean; t(df) = [t-value], p = [p-value].
We can use the following format to report the results of an independent two samples t-test :
A two sample t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
We can use the following format to report the results of a paired samples t-test :
A paired samples t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
Note: The “M” in the results stands for sample mean, the “SD” stands for sample standard deviation, and “df” stands for degrees of freedom associated with the t-test statistic.
The following examples show how to report the results of each type of t-test in practice.
Example: Reporting Results of a One Sample T-Test
A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test.
The following screenshot shows the results of the test:

Here’s how to report the results of the test:
A one sample t-test was performed to compare the mean height of a certain species of plant against the population mean. The mean value of height (M = 14.33, SD = 1.37) was not significantly different than the population mean; t(11) = -1.685, p = .120.
Example: Reporting Results of an Independent Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average miles per gallon of a certain car. To test this, they conduct an experiment in which 12 cars receive the new fuel treatment and 12 cars do not.
The following screenshot shows the results of the independent samples t-test:

A two sample t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was not a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(22) = -1.428, p = .167.
Example: Reporting Results of a Paired Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average mpg of a certain car. To test this, they conduct an experiment in which they measure the mpg of 12 cars with and without the fuel treatment.
The following screenshot shows the results of the paired samples t-test:

A paired samples t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(11) = -2.244, p = .046.
Additional Resources
Use the following calculators to automatically perform various t-tests:
One Sample t-test Calculator Two Sample t-test Calculator Paired Samples t-test Calculator
How to Calculate the Sum by Group in Excel
The complete guide: how to report anova results, related posts, how to normalize data between -1 and 1, vba: how to check if string contains another..., how to interpret f-values in a two-way anova, how to create a vector of ones in..., how to determine if a probability distribution is..., what is a symmetric histogram (definition & examples), how to find the mode of a histogram..., how to find quartiles in even and odd..., how to calculate sxy in statistics (with example), how to calculate expected value of x^3.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
How Long Does Blood Work Take?
- What Is a Blood Draw?
- Factors Affecting Timing
- Common Blood Tests
- Inpatient vs. Outpatient Testing
- Getting Faster Results
A blood draw is a medical procedure used to screen for health conditions, evaluate how certain organ systems work, and monitor treatments. There are many types of blood tests available. The turnaround time to receive test results will be based on the type of test and where the test was taken.
This article will discuss common blood tests and how long it takes to get their results.
Boy_Anupong / Getty Images
How Does a Blood Draw Work?
A blood draw is a procedure that is medically known as a venipuncture. Phlebotomists are medical technicians who perform blood draws. A blood test takes only a few minutes to complete.
To perform a venipuncture a phlebotomist will:
- Wash their hands and wear gloves
- Wrap a tourniquet around the upper portion of the arm
- Examine your arm for a vein to use for the venipuncture
- Clean the insertion site
- Insert the needle into the vein
- Collect the blood in vials
- Remove the needle and apply a bandage
After the blood has been drawn the phlebotomist will send the vials to the laboratory for testing.
What Factors Affect Blood Test Result Timings?
The timing of blood test results will vary based on several factors. These factors can include:
- Where the test was performed
- If the test can be run in the facility it was drawn or needs to be sent to a different location
- The type of blood test
Another factor that affects result timing is if the healthcare provider has received the result but has not released it for the patient to see. This might be done when the healthcare provider wants to talk on the phone with the patient or would like to order additional testing.
Common Blood Tests and How Long Each Takes
Below is a description of common blood tests and the turnaround time for each test.
Complete Blood Count (CBC)
CBCs are a very common test that looks at the red blood cells , white blood cells, and platelets in the blood. It is used to see how the immune system is working, diagnose blood diseases, and other medical conditions. The results are typically reported to the healthcare provider's office the next day.
Basic Metabolic Panel
A basic metabolic panel looks at eight different components of the blood. These components show fluid balances, electrolyte levels, and kidney functions. You may have to stop drinking and eating 8–12 hours before this type of test. Specific tests within a basic metabolic panel are:
- Carbon dioxide
- Blood urea nitrogen (BUN)
The basic metabolic panel results are usually available within a day.
Complete Metabolic Panel
A complete metabolic panel includes the same tests as a basic metabolic panel and six additional components. The additional tests look closer at liver function. Just like a basic metabolic panel, a person may be asked to fast for a complete metabolic panel.
The extra six tests in a complete metabolic panel are:
- Total protein
- Alkaline phosphatase (ALP)
- Alanine transaminase (ALT)
- Aspartate aminotransferase (AST)
The turnaround time for a CMP is a few days.
Lipid Panel
A lipid panel looks at several factors that measure the different fats found in the blood. It helps a healthcare provider determine if someone is at risk for various conditions like heart disease. Most people will need to fast before their lipid panel, but ask your healthcare provider for specific instructions ahead of your scheduled blood draw. The tests within a lipid panel are:
- Total cholesterol (a calculation of the cholesterol circulating in your body)
- High-density lipoprotein (HDL) cholesterol (considered “good” cholesterol)
- Low-density lipoprotein (LDL) cholesterol (considered “bad” cholesterol)
- Triglycerides (a type of fat in the blood)
The results should be back in about one day.
Pregnancy Test
A pregnancy blood test looks at the hormone human chorionic gonadotropin (HCG ) that is produced during pregnancy. The results can be either qualitative or quantitative.
A qualitative pregnancy test provides either a negative or positive result, depending on the presence of HCG in the blood. This is usually done to confirm pregnancy. Quantitative HCG blood tests provide a HCG level. This test is used to confirm pregnancy or to look for abnormalities in the pregnancy.
Pregnancy blood tests can take anywhere from a few hours to a day to receive the results.
Blood Test for Cancer
A healthcare provider can use certain blood tests to detect cancer. The type of test will determine how long it takes to get results which can be one to a few days.
Thyroid Test
A thyroid blood test is used to see how the thyroid is performing. The thyroid is a small butterfly-shaped gland at the front of the neck. It produces hormones that control how the body uses energy.
The test can determine a diagnosis and monitor the treatment of thyroid-related health conditions. Results are usually ready a few days after the blood draw.
Test for Anemia
Anemia is a medical condition where the body does not carry enough oxygen in the red blood cells to the body. The blood test most commonly used to diagnose anemia is a CBC. The CBC is used to diagnose anemia; other specialized tests can be used to diagnose what kind of anemia. This test looks at the levels of hemoglobin, which carries oxygen. A CBC result is typically back the next day.
Tests for Sexually Transmitted Infections (STIs)
There are several types of STIs and not every type can be detected on a blood test. STIs are tested through urine samples, genital swabs, or oral swabs. The below STIs are tested with blood tests and have a turnaround result time listed:
- Syphilis: A few hours to a few days
- Human immunodeficiency virus (HIV): 30 minutes or less for a rapid antibody test or several days for a nuclear acid test (NAT)
- Hepatitis B: Three to six days
Inpatient vs. Outpatient Blood Testing
Inpatient is a term referring to people who have been admitted to the hospital, where they will stay while receiving medical care. For example, they may be ill, are preparing for or recovering from surgery, or are receiving other treatment, such as giving birth. Inpatients who receive blood tests tend to get their results quicker than outpatients. This may be because most hospitals have a laboratory on campus and are able to process blood results when urgently needed.
Outpatient refers to people who have not been admitted to a hospital. They may come to a lab clinic or medical office to get their blood drawn for testing. Outpatient blood results tend to take longer than those for inpatients because blood samples sometimes need to be processed at another location.
Tips to Get Faster Blood Test Results
The turnaround time for blood test results vary based on the type of test and where it was performed. It is important to have a conversation with your healthcare provider to determine when to expect results and how they will be provided to you. Results can be given through your electronic health record (EHR), phone calls, or in person during an appointment.
Blood tests are a medical procedure that can be taken in a healthcare provider's office, at a clinic, or in the hospital. They are used to evaluate health conditions, find illnesses, or monitor treatments. The blood draw itself is a fast procedure that takes only minutes, while results can take minutes to days to receive. A blood test's turnaround time will vary from test to test.
MedlinePlus. What you need to know about blood testing .
Johns Hopkins Medicine. Blood test .
MedlinePlus. Complete blood count (CBC) .
LabCorp. Complete blood count - CBC .
MedlinePlus. Basic metabolic panel (BMP) .
Children's Minnesota. Blood test: basic metabolic panel (BMP) .
MedlinePlus. Basic metabolic panel .
American Association for Clinical Chemistry. Comprehensive metabolic panel .
Children's Minnesota. Blood test: Lipid panel .
University of California San Francisco Health. HCG blood test - quantitative .
National Cancer Institute. How cancer is diagnosed .
MedlinePlus. Thyroid tests .
National Institute of Health. Anemia diagnosis .
Mount Sinai. VDRL test .
Centers for Disease Control and Prevention. Types of HIV tests .
Public Health Ontario. Hepatitis B - serology .
Centers for Medicare and Medicaid Services. Advanced copy- revisions to state operations manual (SOM) hospital appendix A .
Centers for Medicare and Medicaid Services. Hospital outpatient quality reporting program .
Kaiser Permenante. What happens to your blood?
By Patty Weasler, RN, BSN Weasler is a Wisconsin-based registered nurse with over a decade of experience in pediatric critical care.

Statistics Made Easy
How to Report T-Test Results (With Examples)
We can use the following general format to report the results of a one sample t-test :
A one sample t-test was performed to compare [variable of interest] against the population mean. The mean value of [variable of interest] (M = [Mean], SD = [standard deviation]) was significantly [higher, lower, or different] than the population mean; t(df) = [t-value], p = [p-value].
We can use the following format to report the results of an independent two samples t-test :
A two sample t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
We can use the following format to report the results of a paired samples t-test :
A paired samples t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
Note: The “M” in the results stands for sample mean, the “SD” stands for sample standard deviation, and “df” stands for degrees of freedom associated with the t-test statistic.
The following examples show how to report the results of each type of t-test in practice.
Example: Reporting Results of a One Sample T-Test
A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test.
The following screenshot shows the results of the test:

Here’s how to report the results of the test:
A one sample t-test was performed to compare the mean height of a certain species of plant against the population mean. The mean value of height (M = 14.33, SD = 1.37) was not significantly different than the population mean; t(11) = -1.685, p = .120.
Example: Reporting Results of an Independent Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average miles per gallon of a certain car. To test this, they conduct an experiment in which 12 cars receive the new fuel treatment and 12 cars do not.
The following screenshot shows the results of the independent samples t-test:

A two sample t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was not a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(22) = -1.428, p = .167.
Example: Reporting Results of a Paired Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average mpg of a certain car. To test this, they conduct an experiment in which they measure the mpg of 12 cars with and without the fuel treatment.
The following screenshot shows the results of the paired samples t-test:

A paired samples t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(11) = -2.244, p = .046.
Additional Resources
Use the following calculators to automatically perform various t-tests:
One Sample t-test Calculator Two Sample t-test Calculator Paired Samples t-test Calculator
Featured Posts

Hey there. My name is Zach Bobbitt. I have a Masters of Science degree in Applied Statistics and I’ve worked on machine learning algorithms for professional businesses in both healthcare and retail. I’m passionate about statistics, machine learning, and data visualization and I created Statology to be a resource for both students and teachers alike. My goal with this site is to help you learn statistics through using simple terms, plenty of real-world examples, and helpful illustrations.
One Reply to “How to Report T-Test Results (With Examples)”
I really liked you explanation and examples. You solved my problem of mixing the concepts of independent sample t-test & paired sample t-test
Thank you very much
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Join the Statology Community
Sign up to receive Statology's exclusive study resource: 100 practice problems with step-by-step solutions. Plus, get our latest insights, tutorials, and data analysis tips straight to your inbox!
By subscribing you accept Statology's Privacy Policy.
- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .

IMAGES
VIDEO
COMMENTS
What you need to know. NIH has developed a website that allows people to easily and securely report their at-home COVID-19 and flu test results. MakeMyTestCount.org doesn't ask for personal information when you report your positive or negative result. It just wants to know what your ZIP code is, when you took the test, and how old you are.
To report your test your result on MakeMyTestCount.org, start by clicking either "Positive" or "Negative" on the homepage. From there, you'll be prompted to answer a few more general questions, including which brand of test you took, when you took the test, your age, and your zip code. What to Do If You Get a Positive At-Home COVID-19 ...
COVID-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation. Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a ...
If you test positive at home, another way to report your result is to contact your primary care provider, which is what the C.D.C. recommends you do. When calling or emailing your doctor, make ...
Report your test result to MakeMyTestCount.org. The FDA encourages you to voluntarily and anonymously report your positive or negative test results every time you use an at-home COVID-19 test.
Cases of COVID-19 may be undercounted in the U.S. because lab tests are the most likely to be reported to health departments. Long waits for tests and results are making many people opt for home COVID tests instead. At-home results can be reported to public health by consumers, but don't have to be. Data from the Centers for Disease Control ...
In the case of two positive test results, the clinician should report the result that is provided first. In the case of discrepant test results, the clinician should report the positive result. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
A Test Analysis Report is a document that summarizes the results and findings from the testing phase of a software development project. It provides a comprehensive overview of the testing process, including details about what was tested, how it was tested, and the outcomes.
The COVID-19 test result is available through a Labcorp Patient™ account or from your healthcare provider. If your healthcare provider or a telemedicine program ordered a COVID-19 test from Labcorp, your result will be delivered directly to a Labcorp Patient™ account as soon as it is available.*. You can create an account anytime.
Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation. Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a ...
Here are five actions you can take to create an efficient test summary report: Describe the scope of testing. Document test environment details. Summarize the types of testing performed and test results. Capture any lessons learned throughout testing. Report on the status of exit criteria.
2 ways to get your lab results. 1. Directly through MyQuest ®. Receive easy-to-understand Quest lab results and more, directly on your smartphone, tablet, or desktop: 2. From your doctor's office. Most test results can be shared by phone, while others are best shared during a follow-up doctor's visit and discussion.
The pillars of a good test summary report are: Specification: You don't need to write a very long test summary report. It should have all the test result specifications concisely and to the point. Standard: The test summary report should follow a standard template as it is easy for stakeholders to review and understand.
In most cases, lab test results delivery times should not exceed two weeks. The most common reason for delay in receiving results is inaccurate or out-of-date personal information on record with your health care providers or in your Labcorp Patient™ portal personal profile. Please check and confirm the following:
Test Report is a document which contains a summary of all test activities and final test results of a testing project. Test report is an assessment of how well the Testing is performed. Based on the test report, stakeholders can evaluate the quality of the tested product and make a decision on the software release. For example, if the test ...
Also included a sample Test summary report template for download. This article explains simple 12 Steps Guide to writing an effective Test Summary Report. Also included a sample Test summary report template for download. ... After performing exhaustive testing, publishing the test results, metrics, best practices, lessons learned, conclusions ...
Test reporting is a crucial process in software testing that revolves around gathering, analyzing, and presenting essential test data and results to stakeholders. At its core, it serves as a vital communication channel, providing insights into a software application's progress, quality, and readiness throughout the testing lifecycle.
If you have an old free NHS rapid lateral flow test, you can take it but you do not need to report the results. Find out more about COVID-19 symptoms, testing and vaccination and how to avoid ...
The basic format for reporting the result of a t -test is the same in each case (the color red means you substitute in the appropriate value from your study): t ( degress of freedom) = the t statistic, p = p value. It's the context you provide when reporting the result that tells the reader which type of t -test was used. Here are some examples.
Here is the exact wording we can use: A Mann-Whitney U test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] and [group2]; z = [z-value], p = [p-value]. The following example shows how to report the results of ...
The following examples show how to report the results of each type of t-test in practice. Example: Reporting Results of a One Sample T-Test. A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test. The following screenshot shows the ...
The following screenshot shows the output of the regression model: Here is how to report the results of the model: Multiple linear regression was used to test if hours studied and prep exams taken significantly predicted exam score. The fitted regression model was: Exam Score = 67.67 + 5.56* (hours studied) - 0.60* (prep exams taken)
A blood test's turnaround time will vary from test to test. Weasler is a Wisconsin-based registered nurse with over a decade of experience in pediatric critical care. It typically takes less than three minutes to get your blood drawn, and the results can take anywhere from a few minutes to a few weeks to come back.
The following examples show how to report the results of each type of t-test in practice. Example: Reporting Results of a One Sample T-Test. A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test. The following screenshot shows the ...
Summary. The results of a cholesterol test show people the amounts of different types of cholesterol in their blood. This can indicate a person's risk of heart disease and stroke, among other ...
Email Content STAAR Report Card (Word Doc) Updated May 14, 2024. Email Content Single-Sign-On (Word Doc) Updated May 14, 2024. ... Head to TexasAssessment.gov to access scores and resources, including how your child answered test questions. STAAR results offer valuable insight into your child's progress in key subject areas. ...
This report shows your child's performance on the Virginia Standards of Learning test. This report, combined with teacher input, classroom work, report cards, and the Student Detail by Question reports, gives families and teachers a more complete picture of student progress. You can use these results to help identify where your child is doing ...
May 6, 2024. Scientists are proposing a new way of understanding the genetics of Alzheimer's that would mean that up to a fifth of patients would be considered to have a genetically caused form ...
The individual bank results from the stress test will factor directly into a bank's capital requirements, mandating each bank to hold enough capital to survive a severe recession. If a bank does not stay above its capital requirements, it is subject to automatic restrictions on capital distributions and discretionary bonus payments.
Flint Residential and Business Testing Report - results collected January 01, 2024 through May 13, 2024 2 Bottle Kit 1 Bottle Kit 2 Bottle Kit 1 Bottle Kit Sample Number Date Submitted Analysis (Lead) 250 ml Bottle (PPB) 750 ml Bottle (PPB) 1 Liter Calculated (PPB) 1 Liter (PPB) Analysis (Copper) 250 ml Bottle (PPB) 750 ml