- Chemistry Concept Questions and Answers

Inorganic Chemistry Questions
Inorganic Chemistry is a major branch of chemistry that deals with compounds not containing carbon. Such compounds are called inorganic compounds. The synthesis, properties and uses of inorganic compounds are studied in inorganic chemistry. The most common example of an inorganic compound is sodium chloride (NaCl). The most essential molecule, water (H 2 O), that governs life is inorganic. Inorganic chemistry helps us to understand the chemistry of elements other than carbon.
Inorganic Chemistry Questions with Solutions
Q1. What is the formula of blue vitriol?
- CuSO 4 .4H 2 O
- CuSO 4 .5H 2 O
- CuSO 4 .6H 2 O
- CuSO 4 .7H 2 O
Answer: (b)
Copper (II) Sulfate is also known as blue vitriol. It is used as an insecticide in agriculture.
Q2. Name few essential inorganic elements for our human body.
Answer: The essential inorganic elements for our human body are Magnesium (Mg), Sodium (Na), Potassium (K), Calcium (Ca).
Q3. Explain Isomerism.
Answer: Compounds which have the same molecular formula but differ in their arrangement of atoms in space are called isomers and the property is called isomerism. For example, cisplatin. It has two isomeric forms, the cis form and the trans form. The figure below shows cis and trans platin (left- cis platin, right- trans platin). The cis form is used in cancer therapy.

Q4. According to Werner’s theory, how many types of valencies do metal complexes have?
Answer: Metal complexes have two types of valencies, a primary valency and a secondary valency. Primary valency corresponds to the oxidation state of the metal ion and secondary valency corresponds to the coordination number of the metal ion.
Q5. List the different types of isomerism in coordination complexes.
Answer: The different types of isomerism in coordination complexes are:
- Ionization Isomerism
- Coordination Isomerism
- Linkage isomerism
- Geometrical isomerism
- Optical isomerism
Q6. Give two examples of ambidentate ligands.
Answer: Thiocyanate (-SCN) is an ambidentate ligand that can bind to the metal center (M) either by sulfur atom or by nitrogen atom. Nitrito (NO 2 ) is another example of ambidentate ligand as it can bind to the metal center (M) either by nitrogen atom or by oxygen atom.

Q7. Match the following items of column 1 with column 2 and choose the correct answer :
Q8. Match the following items of column 1 with column 2 and choose the correct answer:
Q9. Calculate the pH of HCl acid when the concentration of [H] + ions is 0.1 M.
Answer: pH is calculated by the formula:
pH = – log [H] +
pH = – log (0.1)
Therefore the pH of HCl acid is 1.
Q10. Alkali and Alkaline earth metals belong to which group of periodic table?
Answer: Alkali and Alkaline Earth metals belong to group 1 and group 2 of the periodic table respectively. They are collectively called s-block elements.
Q11. Which compound is called as inorganic benzene?
- B 3 H 6 N 3
- B 3 H 3 N 3
- B 6 H 3 N 3
Answer: (a)
B 3 H 6 N 3 is called as inorganic benzene or Borazine.

Q12. Calculate the pOH of base NaOH having a concentration of 1 ✕ 10 -10 M.
pOH = – log [OH] –
pOH = – log (1 ✕ 10 -10 )
pOH = -(-10 log 10)
pOH = 10 log 10
pOH = 10 ✕ 1
The pOH of NaOH is 10.
Q13. What are Arrhenius acids and Bases? Give examples.
Answer: The acids which give H + ions in water are called Arrhenius acids. The bases which give OH – ions in water are called Arrhenius bases. For example, CO 2 is an Arrhenius acid and CaO is an Arrhenius base.
CO 2 + H 2 O → H 2 CO 3 → 2H + + CO 3 2-
CaO + H 2 O → Ca(OH) 2 → Ca 2+ + 2OH –
Q14. What are Bronsted Lowry acids and bases?
Answer: Proton (H + ) donors are called Bronsted Lowry acids and proton acceptors are called Bronsted Lowry bases.
Q15. Give an example of Lewis acid.
Answer: BF 3 is an example of lewis acid. It is an electron pair acceptor.
Practice Questions on Inorganic Chemistry
Q1. What is the chemical formula of diborane? What is its structure?
Q2. How many banana bonds are there in diborane?
Q3. What is the HSAB principle?
Q4 . What is the geometry and shape of the SF 4 molecule?
Q5. What is the hybridization of methane?
Click the PDF to check the answers for Practice Questions. Download PDF
Recommended Videos
Chemical bonding introduction.

P Block Elements Questions

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Free Download Problems and Solutions in Inorganic Chemistry for JEE 4th Edition by Vishal Joshi
for JEE (Main & Advanced)
Vishal Joshi
This book (Problems and Solutions in Inorganic Chemistry for JEE 4th Edition by Vishal Joshi) is designed to help aspiring engineers focus on the subject of Inorganic Chemistry from two standpoints: To develop their caliber, aptitude, and attitude for the engineering field and profession.
it’s a matter of great pleasure for me to present this edition of problems and solutions in inorganic chemistry for JEE before joint entrance examination aspirants. During teaching hours, I felt that the facts may be made more and more clear to the students through a problematic approach. although an ocean of material inorganic is available to the students, the approach to designing the problems has changed in recent years and if one tries in this ocean, it will be a very difficult task to make the students more familiar with the trends and tricks to solve problems. The present problem book has been presented in the current scenario of stiff competition and is well-equipped with efficiency. The book includes problems based on the latest patterns being followed by JEE.
Table of Contents
- Basic Concepts
- Periodic Properties
- Chemical Bonding
- Coordination Compounds
- Qualitative Analysis
- S-Block Elements
- P-Block Elements
- D-Block Elements
- F-Block Elements
- Hydrogen and Its Compounds
- Environmental Chemistry
File Size: 10.6 MB. Pages: 471. Please read Disclaimer .
Free Download Problems and Solutions in Inorganic Chemistry for JEE 4th Edition by Vishal Joshi pdf from the following links.
Download Link 1
You may also like to download “ Objective Chemistry for JEE MAIN ”.
Free download hundreds of chemistry books in pdf from HERE .
Please Subscribe to our Email list and get notified of our latest uploads ( Books, documents) and new updates. Email Subscription Box is provided on the sidebar (for PC) and on the bottom of this post (for Android Devices).
Kindly Like, Follow and Share our social media pages so that maximum people can benefit from this public service!
Facebook Instagram LinkedIn Twitter Pinterest
P.S: If the download link(s) is/are not working, kindly drop a comment below, so we’ll update the download link for you.
Happy reading!
Share this:
Inorganic Chemistry Problems and Solutions Vishal Joshi
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Subscribe via Email
Enter your email address to subscribe to this blog and receive notifications of new posts by email.
Email Address
Recent Posts
- Concept Development Studies in Chemistry by Dr. John S. Hutchinson (informative book of chemistry)
- Introductory Chemistry by Dr. David W. Ball (informative)
- Chemistry Atoms First 2e by Paul Flowers (informative)
- General Chemistry Principles Patterns and Applications by Bruce A. Averill (informative)
- Chemistry 2e by Paul Flowers (informative)
- Business Opportunities
- Chemistry Jobs
- Chemistry Notes
- Chemistry Test Preparation
- Chemistry Topics
- Uncategorized
Who we are?
“ The Prime Chemistry Porta l “. We provide a comprehensive platform for Chemistry Students, Researchers, Professionals of Academia, Industry, Entrepreneurs and businesspersons with Chemistry as the main interest.
- PRIVACY POLICY
ChemistryDocs.Com
© 2024 ChemistryDocs.Com. Built using WordPress and EmpowerWP Theme .

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Follow the author

Image Unavailable
![inorganic chemistry problem solving Problems and Solutions in Inorganic Chemistry for JEE (Main & Advanced) [Paperback] Vishal Joshi](https://m.media-amazon.com/images/I/51lwoe9aUzL._SX342_SY445_.jpg)
- To view this video download Flash Player
Problems and Solutions in Inorganic Chemistry for JEE (Main & Advanced) [Paperback] Vishal Joshi Paperback – July 1, 2019
Purchase options and add-ons.
- Language English
- Publisher CENGAGE ( TP)
- Publication date July 1, 2019
- Dimensions 10.63 x 8.27 x 0.75 inches
- ISBN-10 9353501091
- ISBN-13 978-9353501099
- See all details

Product details
- Publisher : CENGAGE ( TP) (July 1, 2019)
- Language : English
- ISBN-10 : 9353501091
- ISBN-13 : 978-9353501099
- Item Weight : 1.43 pounds
- Dimensions : 10.63 x 8.27 x 0.75 inches
- Best Sellers Rank: #6,667,158 in Books ( See Top 100 in Books )
About the author
Vishal joshi.
Discover more of the author’s books, see similar authors, read author blogs and more
Customer reviews
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
- Sort reviews by Top reviews Most recent Top reviews
Top reviews from the United States
Top reviews from other countries.
- Amazon Newsletter
- About Amazon
- Accessibility
- Sustainability
- Press Center
- Investor Relations
- Amazon Devices
- Amazon Science
- Sell on Amazon
- Sell apps on Amazon
- Supply to Amazon
- Protect & Build Your Brand
- Become an Affiliate
- Become a Delivery Driver
- Start a Package Delivery Business
- Advertise Your Products
- Self-Publish with Us
- Become an Amazon Hub Partner
- › See More Ways to Make Money
- Amazon Visa
- Amazon Store Card
- Amazon Secured Card
- Amazon Business Card
- Shop with Points
- Credit Card Marketplace
- Reload Your Balance
- Amazon Currency Converter
- Your Account
- Your Orders
- Shipping Rates & Policies
- Amazon Prime
- Returns & Replacements
- Manage Your Content and Devices
- Recalls and Product Safety Alerts
- Conditions of Use
- Privacy Notice
- Consumer Health Data Privacy Disclosure
- Your Ads Privacy Choices
Simplifying Organic Chemistry
Orgosolver provides study tools to help students with their organic chemistry homework and preparation for quizzes, exams, or even the MCAT. Our tools, quizzes, and study guides are designed to help students test every reaction or mechanism with any molecule they draw!
Need help studying for your next exam?
Check out our study guides and our dynamic reaction quizzes, 📋 orgo 1 final exam study guide, 📋 orgo 2 final exam study guide, 📋 reaction quizzes, who we are..
Two former organic chemistry students from the University of Maryland.
While taking Organic Chemistry back in 2009, we often discussed the lack of readily available resources on the web for this course. With the challenges presented by 2020 and the pandemic, we found ourselves again thinking about the ideas that we had discussed in our undergraduate days. Putting our professional skills together, we developed OrgoSolver; the first dynamic Organic Chemistry reaction solver.
Testimonials
Stockton university.
"It helped me with the mechanisms I needed. It allowed me to visualize what reactions I was working with!"
San Francisco State University
"The reaction solver is a great help for both learning reactions and solving retrosynthesis problems."
UC Berkeley
"Orgosolver helped me when nothing else could!"
Questions, Comments, Feedback? Email us at [email protected]


Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
In-cite ai reference generator, legal text refiner, job search ai assistant.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.5: Practice problems
- Last updated
- Save as PDF
- Page ID 151356

- Kathryn Haas
- Duke University
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
These questions are designed to check your understanding of the reading in this chapter.
- What is the historic definition of inorganic chemistry ? What are some problems that arise from this definition?
- Name three sub-fields of inorganic chemistry.
- What is the class of inorganic compounds that have bonds between metals and carbon?
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Glimpse of next-generation internet

Science is making anti-aging progress. But do we want to live forever?
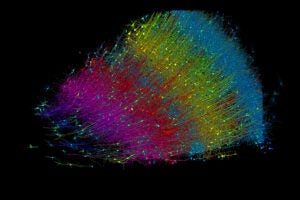
Epic science inside a cubic millimeter of brain

Photos by Stephanie Mitchell/Harvard Staff Photographer
‘The scientist is not in the business of following instructions.’
Alvin Powell
Harvard Staff Writer
George Whitesides became a giant of chemistry by keeping it simple
Part of the experience series.
Leaders at Harvard in and out of the classroom tell their stories in the Experience series.
When George Whitesides started as a teenage technician in his father’s Kentucky lab in the early 1950s, the bond was immediate — and lasting.
Today one of the world’s most influential chemists , Whitesides, the Woodford L. and Ann A. Flowers University Research Professor, has worked on a wide array of scientific problems, shifting focus periodically to uncharted territory. Over the course of his long career — Harvard College, Cal Tech, MIT, and more than four decades as a researcher and teacher back at Harvard — he’s explored nuclear magnetic spectroscopy, organometallic chemistry, molecular self-assembly, soft robotics, unconventional data storage, microfabrication, nanotechnology, and the origin of life. He has published more than 1,200 scientific articles and holds more than 100 patents, and his many honors include the National Medal of Science. He’s also known for his ability to spin discoveries into new companies, including biotech giant Genzyme, purchased in 2011 by Sanofi.
In a conversation with the Gazette, Whitesides looked back on his life and career. The interview has been edited for clarity and length.
Your interest in chemistry starts in your father’s lab?
I don’t know where it came from. It was always interesting to me that the world was made of atoms and how those atoms combined, and I was a good chemist from the very beginning, so studying chemistry seemed like a sensible thing to do. I assumed I would end up working in the chemical industry.
It must have been a cut above the typical teenage summer job.
It was much more boring than that. I measured the pour-point viscosity of coal tar. You heat it up and put it in a cup. The cup has a hole of calibrated size in it and this very thick liquid dribbles out of the hole. You measure how long it takes for a given amount of liquid to dribble out and record that, then you can calculate from those data pour-point viscosity. It was part of the process of producing a standardized product and was boring to do, but it was also satisfying and something a high school student could manage.
You were at Phillips Andover before enrolling at Harvard. How was that experience?
I enjoyed Andover. I had a couple of teachers who were very good. I learned how to study and I certainly learned some chemistry. It was pretty standard for students who’d done well in prep schools to get early admission to Harvard, which I got. But I was not a star student. I got advanced placement in one course, maybe analytical chemistry. I took the first hour exam and got an F. I took the second hour exam and got an F. That same thing happened for the third hour exam. I don’t remember exactly how long the string went on, but I went to the teacher and said, “What do I do to salvage this? It’s going really badly.” And he looked at me very briefly and said, “Learn the material.” That was a very useful lesson. So I went away and learned the material.
What was Harvard like in the late 1950s?
It was the usual undergraduate experience. I knew it mostly from the collection of courses that I took. I had a good time while I was here but most of the good time came from courses I took and people who took the same courses. The one woman I met would eventually become my wife, Barbara. Her brother was my roommate, arbitrarily assigned at some point.

After Harvard, you headed to Cal Tech.
I ended up in the lab of a guy named Jack Roberts, who turned out to be a perfect fit for me. He was in physical organic chemistry, which made sense. You looked at previous reactions and you learned how they went. Then, if you had a new reaction or a new process, you asked “What is it analogous to?” And you predicted that if all the pieces were the same, the reaction would probably go roughly the same way. And it often did, which made it a pretty logical discipline.
The nice thing about Roberts was that, unlike many research directors, he never told me what to do. I would come up with an idea and do the research. Then I would write a draft of a paper and send him the draft, and he would look at it, correct it — largely the grammar but sometimes the chemistry — and give it back to me. After I’d done the necessary work, I’d give it back to him. This would usually go on for a couple of cycles and then we’d send it off to the journal.
It’s easy to look back at a career and imagine that one step led to another. But when you’re living it, the next step is often not clear. Were there times when you wondered whether you should be doing something other than chemistry?
No. I thought chemistry was pretty straightforward, very general, very interesting, and a good thing to do. I was enjoying it and making some progress.
“I prefer to think that, to the extent that we’ve been successful, it’s because we do stuff that’s simple and useful and solves problems.”
Your focus has shifted periodically from one major area to another. Were those shifts intentional, evolutionary, or accidental?
It was intentional. My central point of instruction to students is this: If somebody else is working on something, don’t work on it. There’s an old saying in chemistry that if somebody else has developed something and you work on it, you are working for them. If you produce an idea and someone else works on it, they’re working for you.
An example of where this has succeeded is in something called self-assembled monolayers. There is a very highly developed chemistry focused on making and observing the smallest causal structures: nanostructures. This fits in a peripheral way with the general importance of nanoscience in making electronic components. But if there are billions of dollars being spent by industry making electronic components, why should a little university research group do that?
So we worked on an alternative way to do this without expensive equipment. We worked out a technique in which you basically take a gold film and dip it in a solution of appropriate chemical. You reliably get a monolayer film one molecule thick. That can then be manipulated using the tools of physical organic chemistry to give you very, very small structures. We’ve made structures that are a couple of angstroms wide and connected in various ways. The reason that’s important is it makes it possible for organic chemists, inorganic chemists, and biochemists to use this technique to enter nanoscience. It’s a technique that everybody can use. I’m a believer in problems and a believer in easy.
You gave a TED talk on the importance of simplicity. With so much science focused on complex problems, how did you come to that view?
Something that’s simple is easier to work with than something that is complicated, and you’re going to make more rapid progress with a simple technique than a complicated one. I don’t like competition just for the sake of competition, but in a sense it’s obvious that if you work on something somebody else is working on, then it’s a competition and you want to be making more rapid progress. But I don’t choose to compete. I choose to work on problems because I think they’re interesting and important.
Is there a philosophy there?
Yes. Do things that are easy to do rather than things that are complicated. You’ll find our laboratory is just like ordinary chemistry laboratories, while a physical chemistry laboratory that works on nanostructures has elaborate equipment that sometimes takes years to build. I don’t want to build elaborate equipment — that’s not my skill.
Is this approach part of the reason you’ve been successful?
Success is in the eye of the beholder. I prefer to think that, to the extent that we’ve been successful, it’s because we do stuff that’s simple and useful and solves problems.
You also do it frugally. You’ve talked in the past about the importance of frugal science in an era when the price tag for science is rising.
You don’t need much more than an evaporator and a beaker. You buy the chemicals you need or you make them yourself because they’re easy to make. And then, the underlying principles are the principles of physical organic chemistry, which makes it relatively easy to predict outcomes and which contributes to the simplicity. What we do is apply physical organic chemistry through techniques that we develop to solve complicated problems, problems that in other hands require complicated equipment or complicated ideas.

Receiving the National Medal of Science from President Bill Clinton in 1998.
Courtesy of George Whitesides
How does idea-generation work in your lab?
We make a list of the 10 most important things we can think of. I’ll suggest specific problems and the students will come up with ways of attacking them. The initial ideas may be mostly mine, but the important ideas are often mostly the students’. The scientist is not in the business of following instructions. Students should experience coming up with an idea and pursuing it themselves.
What do we work on now? We work on the origin of life. We work on “what is magnetism” and what can you do with it? We work on a series of problems related to small structures. And we work on soft robots. Those are all areas that are important, for one or another reason, to some community in the technical world.
You’ve said that the real product of your lab is the students. Your team has generated 1,200 papers, more than 100 patents, and several commercial enterprises. Why is teaching more important than the generation of knowledge?
They’re both important, but the students go out themselves and teach, so there’s an amplification there. Students come to the group and learn a particular style — or develop their own variant of that style. Then they go off and many get academic jobs. They have students, who they teach in their own way, and it goes on from there.
“One of the wonderful things about science is it gives you an enormous scope in not only what you do, but also how you want to do it.”
You’ve had a hand in starting a number of companies and have clearly put an emphasis on making sure things get commercialized. Do you help launch a company and then step back or do you stay involved?
It’s not straightforward. You have to have an idea, you have to have a market, and you have to have people who can deal with the exigencies of a small company.
One thing that’s never been quite clear is how you take bright young people and teach them to be entrepreneurs. You may have a technology but you won’t know whether it has an application until people take your technology and pay you — or the company — to use that product. That’s not what universities do particularly well, but it is what CTOs, CFOs, and CEOs — the people who run the company — do well. So there’s an entirely different part of the story that’s important, which concerns the identification and recruitment of people who can make a small company prosper. And it can take a long time for that to happen.
There are many variables in that process. Is one more important than the others?
People ultimately run the company, but it’s as complicated a problem as doing the research. Often at a small company that is succeeding you find a good application, a good product identification, and a good CEO. And the CEO may often be the one who comes up with a product identification and all the rest. Money is also critical.
And in the end it’s important — with respect to guiding principles — that since funding for your work comes from your neighbors, a benefit goes out to them in some way?
In jobs, which provide income, or in some other way. People generally don’t like just giving away money if they’re going to get nothing in return. We have a system of taxation — and we could argue about its fairness or lack of fairness — but the fact of the matter is people prefer to see something come from their money.
Lecturing at Harvard in 2005.
File photo by Stephanie Mitchell/Harvard Staff Photographer
Students are sometimes told that failure is good for learning. Do you agree?
Certainly, a failure is good for instruction. If you look at the companies we’ve started that have done well, you would find an equal number that have not prospered. Those failures often come from a bad understanding of how the market works.
We recently developed a method for storing information that doesn’t involve electronics but instead uses dyes. That was based on my sense that information storage is an extremely important area but consumes a lot of energy and is subject to hacking. I thought that if you could provide an alternative which didn’t use energy and was not subject to hacking, people would be very interested in finding applications for it. I have so far been wrong. Nobody has shown an appropriate level of interest.
Might that take time to find its application, or is it just a miss?
A lot of small companies don’t go anywhere for quite a while after they get started. When they finally do, what changed is never entirely clear.
A big question you’re working on that has resisted explanation is the origin of life. Why is this problem so difficult?
For starters, you’re not going to make bugs in a test tube, so how do you tell whether you’ve succeeded or not?
The “RNA world” is a leading hypothesis for a plausible way of going from random chemicals — basically generated in outer space and then raining on the Earth — to the components of a living organism. A prime proponent, John Sutherland , thinks that RNA came first, then the RNA somehow propagated the DNA, and you go from there. It makes perfectly respectable sense on paper but you don’t know that it actually happened that way.
We do know, though, that you can make an RNA that way. If you have pools that are acidic and have sulfur in them — because they’re near volcanic fumaroles — and then it rains on them, the rain forms other chemicals. If these pools sit on hot rocks so that there’s heat to do chemistry with, then chemistry will occur and some of it may well produce RNA. But does that mean that that’s the origin of life? Does that mean that that’s the right hypothesis? No, it doesn’t.
These are very legitimate questions and they’re good scientific questions, but there’s a difference between something that could plausibly happen and something that probably did happen.
Do you have a favorite theory?
We’re working on an approach where our preferred source of energy is lightning and we’re learning all sorts of things about chemistry going on in lightning. Instead of making lightning, we make sparks that are energetic, very hot, and have curious things associated with them. A lot of lightning strikes occur over oceans and all around the ocean there are cavities in rocks, which if they get hot, are good places to think about chemistry happening. Now, whether that is the solution to the origin of life is another question.
“My central point of instruction to students is this: If somebody else is working on something, don’t work on it.”
Have your teaching methods changed over the course of your career?
No. We do our own approach and the students, whether they’re graduate students or undergraduate students, are free to say, “I think that is an interesting way of doing things” or “That’s not for me, that’s not the kind of problem I want to solve.” One of the wonderful things about science is it gives you an enormous scope in not only what you do, but also how you want to do it.
Let’s close with the areas of science you find most interesting right now.
You can make a list of maybe 10 or 15 problems and you’ll find that each requires separate ideas to solve. I won’t make any broad generalization about what’s more interesting and what’s less interesting, but I don’t think I want to leave to my grandchildren a world which is significantly hotter than it is now. I do think that it’s a good thing to think about whether the countless stars with planets around them also have countless intelligences on them. There are a wide variety of problems that can make the list. They’re all interesting but they’re all different and it’s not obvious how to solve or even contribute to many of them.
Also in this series:

Studying ‘why women are interesting, and men are boring’
Nobel laureate Claudia Goldin recounts pioneering career spent tracing major part of U.S. workforce, economy hidden in plain sight

‘I realized that I couldn’t say no — not because of personal ambition, but given the moment.’
Harvard’s 29th president shares memories and lessons from his early life and career.

‘If you stay the same in everything you do as things around you are changing, eventually you’re going to hit a wall. You just have to adapt and evolve and change.’
Head football coach Tim Murphy has led the Crimson to nine Ivy League championships, three unbeaten seasons, and a 186-83 record.

‘I’ve never done work that I was not interested in. That is a very good reason to go on.’
Indian economist and philosopher, Amartya Sen, the 1998 Nobel laureate in economics, talks about his life as the son of distinguished Hindu academics and how the inequities all around him in colonial India of the 1930s would shape his intellectual destiny.
‘I wanted to warn future social movements that listening only to one’s own side can generate dangerous amounts of unrealism’
Jane Mansbridge, one of the world’s leading scholars of democratic theory talks about her “jagged trajectory” toward success.

‘I developed a sense of the enormous, great luck in managing to survive, giving me a strong feeling that I had an obligation to pay it forward’
As he prepares to retire after 52 years, Harvard Law School’s Laurence H. Tribe retraces his journey from awkward immigrant math whiz to leading constitutional law scholar and admired professor.

‘When you see death all the time, you go into this mode of increased energy and sharper focus’
Pioneering AIDS researcher Myron “Max” Essex was one of the first to propose that a retrovirus was the cause of AIDS.

‘Integrating oral health and primary care can really help the health of this nation and of the world’
Harvard School of Dental Medicine’s dean of 28 years, Bruce Donoff, steps down in January. He discusses his years in leadership and life lessons learned along the way.

‘To be horrified by inequality and early death and not have any kind of plan for responding — that would not work for me’
In the Experience series, Paul Farmer talks Partners In Health, “Harvard-Haiti,” and making the lives of the poor the fight of his life.

‘I was confused and inspired. I wanted to do everything’
The first woman to earn tenure at the GSD and the first to chair the department of architecture has made a career of making statements.

‘The greatest gift you can have is a good education, one that isn’t strictly professional’
The professor who put forward the idea of multiple intelligences talks about his adventures in learning for the Experience series.

‘What the hell — why don’t I just go to Harvard and turn my life upside down?’
Family, history, and the 1960s all helped to shape the higher ed leader, but it was illness that urged her forward.
Share this article
You might like.
Physicists demo first metro-area quantum computer network in Boston

Nobel laureate details new book, which surveys research, touches on larger philosophical questions

Researchers publish largest-ever dataset of neural connections
Finding right mix on campus speech policies
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier

IMAGES
VIDEO
COMMENTS
Q2. Name few essential inorganic elements for our human body. Answer: The essential inorganic elements for our human body are Magnesium (Mg), Sodium (Na), Potassium (K), Calcium (Ca). Q3. Explain Isomerism. Answer: Compounds which have the same molecular formula but differ in their arrangement of atoms in space are called isomers and the ...
Name three sub-fields of inorganic chemistry. What is the class of inorganic compounds that have bonds between metals and carbon? 1.5: Practice problems is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.
Problems. Exercise 1. Exercise 2. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Inorganic Chemistry 4th Edition, you'll learn how to solve your toughest homework problems. Our resource for Inorganic ...
Vibrational Modes of Small Molecules. 1 H NMR Spectra of Small Molecules. Nomenclature of Inorganic Compounds. (Drill & Practice) Binary Ionic Compounds Containing a Metal Ion With a Fixed Charge. (50 questions) Hydrated Ionic Compounds. (20 questions) Binary Covalent Compounds.
The theory that each elements is composed of tiny indestructible particles called atoms, that all atoms of a given element simple, whole-number rations to form compounds. Matter. Anything that occupies space and has mass. State. A classification of the form of matter as a solid, liquid, or gas. Composition.
Inorganic Chemistry. Practice. Assessments. Want the full Albert experience for your school or class? Learn more! Tags. Study bonding, synthesis, and behavior of inorganic compounds and materials.
Now, with expert-verified solutions from Inorganic Chemistry 3rd Edition, you'll learn how to solve your toughest homework problems. Our resource for Inorganic Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems ...
Description. Dear student, Welcome to this online chemistry course. This 14h course will introduce you to the main and fundamentals topics in general chemistry using hands on demos and playing english crystal clear explanation. This course is primarily designed for students at high school, college or university.
Inorganic chemistry is a beautiful, rich, and exciting discipline, but it also has its challenges. This solutions manual for Inorganic Chemistry, Seventh Edition will prove invaluable in meeting those challenges. As you master each chapter in Inorganic Chemistry, having detailed solutions handy allows you to confirm your answers and develop your ability to think through the problem-solving ...
Unlike static PDF Inorganic Chemistry 5th Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. No need to wait for office hours or assignments to be graded to find out where you took a wrong turn. You can check your reasoning as you tackle a problem using our interactive solutions viewer.
Jeanne Tan. Understanding Advanced Chemistry Through Problem Solving : The Learner's Approach Volume 1: Physical Chemistry and Inorganic Chemistry. Understanding Advanced Chemistry Through Problem Solving : The Learner's Approach Volume 2. Tools. Share. Recommend to Library. Purchase Save for later. ISBN: 978-981-4578-90-5 (softcover) USD 48.00.
Leading the reader from the fundamental principles of inorganic chemistry, right through to cutting-edge research at the forefront of the subject, Inorganic Chemistry, Seventh Edition is the ideal course companion for the duration of a student's degree. The authors have drawn upon their extensive teaching and research experience to update this text; the seventh edition retains the much-praised ...
I can solve problems in inorganic chemistry research. 8: I can think critically about inorganic chemistry research. 9: Whether the science content is difficult or easy, I am sure that I can understand it. Science identity: 10: I have come to think of myself as a "scientist". 11: My interest in science is an important reflection of who I am.
Problems and Solutions in Inorganic Chemistry for JEE 4th Edition by Vishal Joshi . for JEE (Main & Advanced) Authors: ... it will be a very difficult task to make the students more familiar with the trends and tricks to solve problems. The present problem book has been presented in the current scenario of stiff competition and is well-equipped ...
Problem-based learning (PBL) is an acclaimed educational concept for laboratory teaching in chemistry, which significantly affects learner motivation. A central aim of PBL is to overcome educational problems with "cook-book" laboratories. For example, when students receive experimental instructions and apply the instructions similar to recipes, they do not necessarily understand what they ...
Problems and Solutions in Inorganic Chemistry for JEE (Main & Advanced) [Paperback] Vishal Joshi Paperback - July 1, 2019 . ... purchased many books related to jee problems and solutions but this thing is something different and after reading and solving problem from this book I realised that this is itself THE BOOK FROM HEAVEN . The best ...
Simplifying Organic Chemistry. Orgosolver provides study tools to help students with their organic chemistry homework and preparation for quizzes, exams, or even the MCAT. Our tools, quizzes, and study guides are designed to help students test every reaction or mechanism with any molecule they draw!
This AI tool helps answer chemistry questions and solve chemistry problems. HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.
Advanced Problem Solving Part - VI. Lesson 6 • May 2 • 1h 32m. + See all lessons. Enrol for IIT JEE Advance Problem Solving Course on Inorganic Chemistry for JEE 2024 conducted by Piyush Maheshwari on Unacademy. The course is taught in Hinglish.
The authors have also retained the popular discourse feature from their previous two books — Understanding Advanced Physical Inorganic Chemistry and Understanding Advanced Organic and Analytical Chemistry — to help the learners better understand and see for themselves, how the concepts should be applied during solving problems.
Name three sub-fields of inorganic chemistry. What is the class of inorganic compounds that have bonds between metals and carbon? This page titled 1.5: Practice problems is shared under a not declared license and was authored, remixed, and/or curated by Kathryn Haas.
Champs: Problem Solving Course on Inorganic Chemistry - JEE Main & Advance 2022 Piyush Maheshwari. In this course, Piyush Maheshwari will provide in-depth knowledge of Inorganic Chemistry. The course will be helpful for aspirants preparing for IIT JEE. Learners at any stage of their preparation will be benefi...
Advanced Problem Solving Part - VI. Lesson 6 • Mar 30 • 47m. Enrol for IIT JEE Advanced Problem Solving Course on Inorganic Chemistry for JEE 2024 conducted by Piyush Maheshwari on Unacademy. The course is taught in Hinglish.
What we do is apply physical organic chemistry through techniques that we develop to solve complicated problems, problems that in other hands require complicated equipment or complicated ideas. Receiving the National Medal of Science from President Bill Clinton in 1998. Courtesy of George Whitesides. How does idea-generation work in your lab?
Kyushu University. "Expanding on the fundamental principles of liquid movement." ScienceDaily. ScienceDaily, 20 May 2024. <www.sciencedaily.com / releases / 2024 / 05 / 240520122705.htm>. July 8 ...
Inorganic Chemistry: Amines Polymers Aromatic compounds: Physical Chemistry: Mole concept Chemical kinetics Chemical equilibrium: JEE Advanced Chemistry High weightage chapters. ... If a student notices a pattern of silly mistakes while solving a problem, they must make a note of it and practise more such questions in order to clarify their ...
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, No. 5625 Renmin Rd., Changchun 130022, China School of Applied Chemistry and Engineering, University of Science and Technology of China, No. 96 Jinzhai Rd., Hefei 230026, China ...