- Open access
- Published: 01 October 2021

Oral health care for the critically ill: a narrative review
- Lewis Winning 1 ,
- Fionnuala T. Lundy 2 ,
- Bronagh Blackwood 2 ,
- Daniel F. McAuley 2 &
- Ikhlas El Karim ORCID: orcid.org/0000-0002-5314-7378 2
Critical Care volume 25 , Article number: 353 ( 2021 ) Cite this article
21k Accesses
11 Citations
134 Altmetric
Metrics details
The link between oral bacteria and respiratory infections is well documented. Dental plaque has the potential to be colonized by respiratory pathogens and this, together with microaspiration of oral bacteria, can lead to pneumonia particularly in the elderly and critically ill. The provision of adequate oral care is therefore essential for the maintenance of good oral health and the prevention of respiratory complications.
Numerous oral care practices are utilised for intubated patients, with a clear lack of consensus on the best approach for oral care. This narrative review aims to explore the oral-lung connection and discuss in detail current oral care practices to identify shortcomings and offer suggestions for future research. The importance of adequate oral care has been recognised in guideline interventions for the prevention of pneumonia, but practices differ and controversy exists particularly regarding the use of chlorhexidine. The oral health assessment is also an important but often overlooked element of oral care that needs to be considered. Oral care plans should ideally be implemented on the basis of an individual oral health assessment. An oral health assessment prior to provision of oral care should identify patient needs and facilitate targeted oral care interventions.
Oral health is an important consideration in the management of the critically ill. Studies have suggested benefit in the reduction of respiratory complication such as Ventilator Associated Pneumonia associated with effective oral health care practices. However, at present there is no consensus as to the best way of providing optimal oral health care in the critically ill. Further research is needed to standardise oral health assessment and care practices to enable development of evidenced based personalised oral care for the critically ill.
Introduction
The oral cavity houses the second largest microbiota in the human body and includes bacteria, fungi, viruses, and archaea [ 1 ]. The majority of micro-organisms within the oral cavity are found within biofilms consisting of mostly commensal bacteria that are considered beneficial for the host. However, dysbiosis of the microbial biofilm can lead to dental diseases such as periodontitis and tooth decay [ 2 ]. Periodontitis is a chronic inflammatory disease affecting the supporting tissues of the teeth and is generally caused by oral anaerobic bacteria in a susceptible individual. The disease is highly prevalent, with severe forms affecting 10% of the population [ 3 ]. Tooth decay, on the other hand, is caused by acid produced by oral bacterial fermentation of dietary carbohydrates. Untreated dental caries is the 2nd most common chronic disease, with 2.4 billion individuals affected worldwide [ 4 ]. Untreated caries can ultimately lead to the death of the tooth and subsequent abscess formation in the underlying tissues.
Localised oral diseases, including periodontitis and caries-induced infections, have previously been shown to have systemic connections [ 5 ]. Oral bacteria commonly gain entrance to the circulation through ulcerated gingiva crevicular tissue that surrounds the teeth [ 6 ]. Invasion of the cariogenic Gram positive bacterium Streptococcus mutans into vascular endothelial cells is considered an exacerbating factor in infective endocarditis [ 7 ]. Additionally, oral bacteria including Staphylococcus aureus, Streptococcus sanguis, Enterococcus faecalis , and others have been implicated in the pathogenesis of infective endocarditis [ 8 ]. Poor oral hygiene in this regard, has been shown to be associated with an increased risk for infective endocarditis [ 9 ]. Gram negative oral bacteria and the local inflammatory response associated with periodontitis, can contribute to systemic inflammation and the initiation and progression of chronic inflammatory based diseases, including cardiovascular disease [ 10 ], diabetes [ 11 ] and respiratory disease [ 12 ].
This narrative review aims to provide an overview on the links between oral health and respiratory disease with particular consideration to the critically ill. We also consider the roles oral health assessment and oral care interventions have in the critically ill. A comprehensive search of the published English literature was conducted in PubMed, Medline, and Scopus until March 2021, using the following keywords: (“oral health” OR “oral disease” OR “periodontitis*” OR “caries” OR “oral health assessment” OR “oral health care” OR “oral prophylaxis”) AND (“critically ill” OR “critical care” OR “intensive care” OR “VAP”). Two of our investigators independently searched the databases (IEK and LW) and reviewed each of the retrieved articles.
Oral health and respiratory disease
The airway, including upper and lower segments, are a continuum of the oro-nasopharynx. Secretions of the upper airways are normally heavily contaminated with microorganisms originating from the oro-nasopharynx region. The lower airways, however, maintain a more sterile-like state supported by the cough reflex, the action of tracheobronchial secretions, mucociliary transport of inhaled microorganisms, and immune defence factors (cell-mediated immunity, humoral immunity, and neutrophils). In individuals with underlying chronic health problems, aspirated oral secretions containing potential pathogens are not always cleared effectively [ 13 ]. In these cases, pathogenic changes to the normal commensal microflora of the respiratory system, and more specifically potential infections that are derived from the oral cavity, represent a mechanistic pathway for an association with oral health.
The oral microbiome is comprised of over 600 prevalent taxa at the species level, with distinct subsets predominating in various oral habitats [ 1 ]. Dental caries and periodontitis are the most common oral diseases and are major causes of tooth loss [ 3 ]. Despite different aetiologies, caries and periodontal disease represent dysbiotic states of the oral microbiome [ 14 ]. In the absence of effective oral hygiene, initial dental plaque formation on a clean tooth surface will occur within 48 h. As the biofilm matures, its composition reflects the oral environment. If the pH in the oral cavity is low, then a cariogenic microbiota may predominate (Gram-positive bacteria and Candida albicans ), whereas if the gums are inflamed a periodontopathogenic microbiota is likely to predominate (anaerobic Gram-negative bacteria). Immunocompromised patients and individuals with low salivary flow rates will generally tend to be more susceptible to bacterial and fungal colonisation of the oral cavity. As well as leading to oral disease these pathogenic oral bacteria may be transported to the lungs where they have the potential to cause respiratory infections [ 15 ]. One cubic millimetre of dental plaque contains about 100 million bacteria [ 16 ], and may serve as a persistent reservoir for potential pathogens. Micro-aspiration of oral bacteria is common and frequently occurs during sleep. Studies have shown that typical aspirated volumes are of an amount likely to contain bacterial pathogens [ 17 ].
Amongst the associations between oral health and various respiratory diseases, the association with pneumonia has received much attention due to the strength of biological plausibility. Oral colonisation by respiratory pathogens, fostered by poor oral hygiene, has been associated with hospital-acquired pneumonia [ 12 , 18 ]. Hospital-acquired pneumonia is typically caused by bacteria that are not normally residents of the oropharynx but enter this milieu from the environment. These include Gram-negative bacilli, Pseudomonas aeruginosa , Staphylococcus aureus , and enteric species (such as Escherichia coli , Klebsiella pneumoniae, Serratia species, Enterobacter species ). In ventilator‐associated pneumonia (VAP), the placement of an endotracheal tube can transport oropharyngeal organisms into the lower airway [ 19 ]. The growth of a biofilm resistant to host defences and antibiotics, on the surface of the tube represents a further problem [ 20 ]. Recently, in an in vitro study, we showed that the opportunistic oral pathogen C. albicans enhanced bacterial numbers of the VAP pathogens; E . coli , S . aureus and MRSA in dual-species biofilms [ 21 ]. Studies have also linked community acquired pneumonia with poor oral hygiene [ 22 , 23 ].
There have been several systematic reviews that have aimed to investigate the association between oral health and pneumonia. Khadka et al. [ 24 ] performed a systematic review which included studies investigating pathogenic microorganisms in oral specimens of older people with aspiration pneumonia. Based on twelve studies (four cross-sectional, five cohort and three intervention) it was found that colonisation of the oral cavity by microorganisms commonly associated with respiratory infections. Furthermore, aspiration pneumonia occurred less in people who received professional oral care compared with no such care. In a systematic review focusing specifically on the association between periodontitis and nosocomial pneumonia, a meta-analysis was performed on 5 case–control studies that met the inclusion criteria [ 25 ]. A significant association was found between periodontitis and nosocomial pneumonia with an OR = 2.55, (95% CI 1.68–3.86). In a systematic review conducted by El-Rabbany et al. [ 26 ] focus was given to reviewing RCTs that evaluated the efficacy of prophylactic oral health procedures in reducing hospital-acquired pneumonia or ventilator-associated pneumonia. Twenty-eight trials were identified which found that good oral health care was associated with a reduction in the risk for hospital acquired and ventilator-associated pneumonia in high-risk patients.
Oral health in critically ill intubated patients
Critically ill patients in the ICU represent a uniquely vulnerable group. Patients that are unconscious or sedated in ICUs often require mechanical ventilation with an associated risk of VAP. VAP significantly increases mortality and complications, resulting in an increased period of ventilation, longer ICU stay and associated increased costs [ 27 ]. It has been shown that oral health deteriorates following admission to ICU [ 28 ]. Dental plaque accumulates rapidly in the mouths of critically ill patients with a significant shift in plaque microbial community observed in mechanically ventilated patients, including colonisation with potential VAP pathogens [ 29 , 30 ]. This confirmed previous findings that respiratory pathogens isolated from the lung are often genetically indistinguishable from strains of the same species isolated from the oral cavity in patients who receive mechanical ventilation [ 31 ]. Plaque accumulation is exacerbated in the absence of adequate oral care and by the drying of the oral cavity due to prolonged mouth opening, leading to severe inflammation of soft tissues. Pre-existing poor oral health on admission to ICU further complicates the picture and has been recognised as a specific risk factor in VAP development [ 32 ]. More recently, a case control study has demonstrated the impact of poor oral health in the form of periodontitis, and the associated higher risk of ICU admission, need for assisted ventilation and mortality during the COVID-19 pandemic [ 33 ].
Oral health assessment
The oral health of intubated patients deteriorates with time in ICU and this is particularly problematic for those with pre-existing dental disease. Several studies have verified that teeth and other oral surfaces of patients in ICU subjects serve as reservoirs for respiratory pathogen colonization, with the pathogens causing pneumonia appearing to first colonize the dental plaque on teeth or dentures, rather than soft tissues [ 34 ]. In intubated patients with poor baseline dental health, such as periodontal disease and tooth decay, the dysbiotic plaque is likely to be mature and its removal requires special considerations. Oral health assessment prior to provision of oral care is therefore important to identify oral disease and subsequently target specific oral care needs. Oral health assessment is a descriptive health measurement needed to establish the patient’s baseline oral health status, changes in oral health during the course of care, and response to interventions [ 35 ]. An oral health assessment should include a general observation and an intra-oral examination to detect changes in the oral cavity, including, teeth, soft tissues and saliva [ 36 ]. The oral assessment should be performed frequently as part of a systematic patient assessment and should be used to identify those at increased risk of oral complications.
Despite the obvious benefits, an oral health assessment is not routinely performed for critically ill patients [ 37 , 38 ], as the process is considered time-consuming and requires the training of nursing staff to identify oral disease. Furthermore, the tools that are available for oral assessment are variable, mostly not validated and are mostly developed for oral health assessment in different settings but adapted for use in ICU (Table 1 ). It is therefore not surprising that wide variability in oral care assessment practices exists [ 39 ]. In a recent consensus paper, the British Association of Critical Nurses (BACCN) emphasised the importance of oral assessment and identified the need for further research [ 36 ]. Oral care protocols that were based on an oral health assessment were previously found to be more cost-effective and resulted in a significant reduction of VAP [ 40 , 41 , 42 ]. As the provision of oral care for the critically ill and in particular those who are mechanically ventilated is complex and demanding, oral health assessment prior to provision of oral care to identify the oral disease and subsequent targeted oral care interventions could result in more clinically and cost-effective care [ 40 , 41 ] .
Oral care interventions for the critically ill
The importance of adequate oral care has been recognised in guideline interventions for the prevention of VAP [ 43 ]. Different oral practices have been adopted for intubated patients, including toothbrushing and the use of oral care solutions such as antiseptic mouthwash. However, the most effective way to achieve good oral care in the ICU is not known, and there is currently a lack of consensus [ 44 ].
Among oral care solutions, the oral antiseptic chlorhexidine digluconate was reported as the most widely used antiseptic for oral hygiene in European ICU patients [ 45 ]. Multiple systematic reviews including both randomised and non-randomised clinical trials have reported the effectiveness of chlorhexidine (CHX) in reducing VAP and mortality (Table 2 ). A recent Cochrane review performed a meta-analysis based on 18 RCTs and found that CHX reduced the risk of VAP compared to placebo or usual care from 24% to about 18% (RR 0.75, 95% confidence intervals (CI) 0.62–0.91, P = 0.004) [ 46 ]. Despite this, the use of CHX has been brought into question by the finding that a possible (non-significant) increase in mortality was reported [ 44 , 47 , 48 ]. It not clear, however how CHX increases the risk of mortality which has led to calls for further research to investigate its safety in critical care settings [ 49 , 50 ]. CHX exhibits broad-spectrum antimicrobial activity and is considered stable, safe and effective in reducing plaque formation [ 51 ]. However, it has some disadvantages including, tooth discolouration and mucosal ulcerations when used in high concentrations, as well as emerging evidence of microbial resistance [ 52 ]. Furthermore, CHX has limited antimicrobial activities on established biofilms and therefore mechanical plaque removal, such as tooth brushing, is required prior to supplemental use of CHX [ 53 , 54 ]. Future studies should be designed with these limitations in mind. Within the critical care context, the method of application of chlorhexidine is also worthy of consideration, as the use of gels may be safer than solutions, to reduce the risk of microaspiration.
Although the adjunct use of chemical plaque control may be useful, effective control of dental plaque biofilm requires physical disruption with mechanical devices such as toothbrushing. Control of dental plaque and oral disease using mechanical means alone is well documented in the general population [ 55 , 56 ]. In the critically ill, mechanical plaque control is widely used, but its efficacy in reducing the incidence of VAP is debatable. A systematic review of four RCT that included 828 patients showed toothbrushing did not significantly reduce the incidence of VAP (RR, 0.77; 95% CI 0.50–1.21) and mortality (RR, 0.88; 95% CI 0.70–1.10) [ 57 ]. On the other hand, Zhao et al., showed in a combined meta-analysis of five studies (910 participants), that toothbrushing reduced the incidence of VAP (RR 0.61, 95% CI 0.41–0.91, P = 0.01) [ 46 ]. In addition, toothbrushing compared to CHX was found to significantly reduce the duration of mechanical ventilation (MD − 1.46 days, 95% CI − 2.69 to − 0.23 days, P = 0.02) and ICU stay (MD − 1.89 days, 95% CI − 3.52 to − 0.27 days, P = 0.02), but had no effect on mortality (RR 0.86, 95% CI 0.70–1.05, P = 0.14). It is important to note here that the efficacy of toothbrushing in reducing plaque in these studies was reported in only one study [ 58 ] where the reduction in plaque scores was associated with a reduction in VAP.
Toothbrushing combined with antiseptics is a commonly used oral hygiene practice and showed efficacy in controlling plaque and periodontal disease [ 59 ]. In their meta-analysis Zhao et al. combined two studies (649 participants), investigating toothbrushing with chlorhexidine compared to chlorhexidine alone and no difference in the incidence of VAP (RR 0.74, 95% CI 0.50–1.09, P = 0.13), or mortality (RR 0.87, 95% CI 0.68–1.12, P = 0.28) was found [ 46 ]. Another systematic review compared CHX alone to oral hygiene protocols involving mechanical removal of biofilm (toothbrushing, scrapping) together with chlorhexidine [ 60 ]. Their meta-analysis of six studies (1276 patients) showed a reduction in the incidence of VAP in oral care protocols that combined mechanical plaque removal and CHX (risk difference: − 0.06 (95% CI − 0.11 to − 0.02; P = 0.007). CHX is known to be deactivated if used immediately following toothbrushing with toothpaste containing anionic surfactants [ 61 ] and it is not clear from these studies whether such considerations were taken into account.
Other oral care interventions
Several other oral care solutions are used in ICU in addition to CHX. These include antiseptics such as povidone iodine, Listerine and triclosan as well as non-antiseptics such as saline and bicarbonate. In their systematic review, Zhao et al. compared povidone iodine rinse with a saline rinse or placebo in a meta-analysis of three studies (356 participants). They showed evidence of a reduction in VAP in the povidone iodine group (RR 0.69, 95% CI 0.50–0.95, P = 0.02). On the contrary, their meta-analysis of 4 studies, which compared a saline rinse with a saline-soaked swab, found that saline rinse may reduce the incidence of VAP (RR 0.47, 95% CI 0.37–0.62, P < 0.001) [ 46 ]. A recent systematic review investigating the effectiveness of novel herbal oral care products in the prevention of VAP reported comparable affects to CHX [ 62 ]. However, with only a limited number of studies investigating these products, further studies are required.
It is apparent from the discussion above that there is no clear consensus on the most clinically relevant and cost-effective oral care intervention. In an attempt to define the most effective oral care intervention for the prevention of VAP, Sankaran and Sonis [ 64 ] exploited the existing meta-analysis data of a Cochrane systematic review [ 63 ], and performed a network meta-analysis (NMA) to compare different oral care interventions across different studies and rank the efficacy of each in the context of all of the interventions studied. The NMA included 25 studies (4473 subjects), 16 treatments, 29 pairwise comparisons, and 15 designs. The results based on the NMA most frequent ranking probability scores (P) showed that tooth brushing (P fixed-0.94, P random-0.89), tooth brushing with povidone-iodine (P fixed-0.90, Prandom-0.88), and furacillin (P fixed-0.88, P random-0.84) were the best three interventions for preventing VAP. CHX of 0.2% concentration (P score fixed of 0.65, P score random of 0.65) ranked as the second-best intervention in the network along with Biotene (P score fixed of 0.59, P score random 0.54) and potassium permanganate (P score fixed of 0.53, P score random 0.54). The NMA demonstrated the superiority of toothbrushing or mechanical cleaning and when combined with a mouthwash, NMA showed that tooth brushing is superior to a mouthwash alone and toothbrushing with povidone iodine is superior to any other mouthwash. The results of this NMA are however based on a mix of low risk and high risk of bias studies and are not recommended for clinical treatment needs. High quality clinical trials are needed taking into account the outcome of this NMA to determine the best intervention taking into account patient-specific oral care needs. A further consideration, relates to potential barriers in the implementation of oral care protocols. An ethnographic investigation found that the complexity of performing oral care in ICU setting is underestimated and undervalued [ 65 ]. Technical barriers included oral crowding with tubes and aversive responses by patients such as biting. Contextual impediments to oral care included time constraints, lack of training, and limited opportunities for interprofessional collaboration.
The contribution of poor oral hygiene and oral bacteria to the development of pneumonia is well established. Within the context of critical care, however, controversy exists as to the best practice to achieve optimal oral health care and whether this is reflected in better overall outcomes for ICU patients. Further research is needed to standardise oral care practices and personalise individuals’ oral health needs within the ICU.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
British Association of Critical Nurses
Beck Oral Assessment Score
Bedside oral exam
- Chlorhexidine
Cardiac surgery
Intensive care units
Mucosal Plaque Score
Non cardiac surgery
Network meta-analysis
Oral Assessment Guide
Randomised control trials
Ventilator associated pneumonia
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192(19):5002–17.
Article CAS PubMed PubMed Central Google Scholar
Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44(Suppl 18):S12-s22.
Article PubMed Google Scholar
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–53.
Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis: a comprehensive review. J Clin Periodontol. 2017;44(Suppl 18):S94-s105.
Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92(6):485–91.
Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118–25.
Abranches J, Zeng L, Bélanger M, Rodrigues PH, Simpson-Haidaris PJ, Akin D, Dunn WA Jr, Progulske-Fox A, Burne RA. Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol. 2009;24(2):141–5.
Del Giudice C, Vaia E, Liccardo D, Marzano F, Valletta A, Spagnuolo G, Ferrara N, Rengo C, Cannavo A, Rengo G. Infective endocarditis: a focus on oral microbiota. Microorganisms. 2021;9(6):1218.
Article PubMed PubMed Central Google Scholar
Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, Sasser HC. Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. J Am Dent Assoc. 2009;140(10):1238–44.
Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268–88.
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138–49.
Article CAS PubMed Google Scholar
Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol. 2013;40(Suppl 14):S8–19.
PubMed Google Scholar
Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465–82.
Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 2017;44(S18):S23–38.
Manger D, Walshaw M, Fitzgerald R, Doughty J, Wanyonyi KL, White S, Gallagher JE. Evidence summary: the relationship between oral health and pulmonary disease. Br Dent J. 2017;222(7):527–33.
MThoden van Velzenanger SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. J Clin Periodontol. 1984;11(4):209–20.
Article Google Scholar
Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111(5):1266–72.
Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8(1):54–69.
Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50(6):725–39 (; discussion 739–741 ).
Feldman C, Kassel M, Cantrell J, Kaka S, Morar R, Goolam Mahomed A, Philips JI. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J. 1999;13(3):546–51.
Luo Y, McAuley DF, Fulton CR, Sá Pessoa J, McMullan R, Lundy FT. Targeting Candida albicans in dual-species biofilms with antifungal treatment reduces Staphylococcus aureus and MRSA in vitro. PLoS ONE. 2021;16(4):e0249547.
van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3–9.
Kaneoka A, Pisegna JM, Miloro KV, Lo M, Saito H, Riquelme LF, LaValley MP, Langmore SE. Prevention of healthcare-associated pneumonia with oral care in individuals without mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Infect Control Hosp Epidemiol. 2015;36(8):899–906.
Khadka S, Khan S, King A, Goldberg LR, Crocombe L, Bettiol S. Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: a systematic review. Age Ageing. 2021;50(1):81–7.
Jeronimo LS, Abreu LG, Cunha FA, Lima RPE. Association between periodontitis and nosocomial pneumonia: a systematic review and meta-analysis of observational studies. Oral Health Prev Dent. 2020;18(1):11–7.
El-Rabbany M, Zaghlol N, Bhandari M, Azarpazhooh A. Prophylactic oral health procedures to prevent hospital-acquired and ventilator-associated pneumonia: a systematic review. Int J Nurs Stud. 2015;52(1):452–64.
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–46.
Terezakis E, Needleman I, Kumar N, Moles D, Agudo E. The impact of hospitalization on oral health: a systematic review. J Clin Periodontol. 2011;38(7):628–36.
Sands KM, Twigg JA, Lewis MAO, Wise MP, Marchesi JR, Smith A, Wilson MJ, Williams DW. Microbial profiling of dental plaque from mechanically ventilated patients. J Med Microbiol. 2016;65(2):147–59.
Sands KM, Wilson MJ, Lewis MAO, Wise MP, Palmer N, Hayes AJ, Barnes RA, Williams DW. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J Crit Care. 2017;37:30–7.
Heo SM, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 2008;47(12):1562–70.
Takahama A Jr, de Sousa VI, Tanaka EE, Ono E, Ito FAN, Costa PP, Pedriali MBBP, de Lima HG, Fornazieri MA, Correia LS, et al. Analysis of oral risk factors for ventilator-associated pneumonia in critically ill patients. Clin Oral Invest. 2021;25(3):1217–22.
Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, Hssain AA, Nicolau B, Sanz M, Tamimi F. Association between periodontitis and severity of COVID-19 infection: a case–control study. J Clin Periodontol. 2021;48(4):483–91.
Terpenning M, Bretz W, Lopatin D, Langmore S, Dominguez B, Loesche W. Bacterial colonization of saliva and plaque in the elderly. Clin Infect Dis. 1993;16(Suppl 4):S314-316.
Abidia RF. Oral care in the intensive care unit: a review. J Contemp Dent Pract. 2007;8(1):76–82.
Collins T, Plowright C, Gibson V, Stayt L, Clarke S, Caisley J, Watkins CH, Hodges E, Leaver G, Leyland S, et al. British association of critical care nurses: evidence-based consensus paper for oral care within adult critical care units. Nurs Crit Care. 2021;26(4):224–33.
Grap MJ, Munro CL. Preventing ventilator-associated pneumonia: evidence-based care. Crit Care Nurs Clin North Am. 2004;16(3):349–58.
Berry AM, Davidson PM. Beyond comfort: oral hygiene as a critical nursing activity in the intensive care unit. Intensive Crit Care Nurs. 2006;22(6):318–28.
Labeau S, Vandijck D, Rello J, Adam S, Rosa A, Wenisch C, Bäckman C, Agbaht K, Csomos A, Seha M, et al. Evidence-based guidelines for the prevention of ventilator-associated pneumonia: results of a knowledge test among European intensive care nurses. J Hosp Infect. 2008;70(2):180–5.
Ames NJ, Sulima P, Yates JM, McCullagh L, Gollins SL, Soeken K, Wallen GR. Effects of systematic oral care in critically ill patients: a multicenter study. Am J Crit Care. 2011;20(5):e103-114.
Labeau SO, Conoscenti E, Blot SI. Less daily oral hygiene is more in the ICU: not sure. Intensive Care Med. 2021;47(3):334–6.
Prendergast V, Kleiman C, King M. The bedside oral exam and the barrow oral care protocol: translating evidence-based oral care into practice. Intensive Crit Care Nurs. 2013;29(5):282–90.
Hellyer TP, Ewan V, Wilson P, Simpson AJ. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc. 2016;17(3):238–43.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50(3):1700582.
Article PubMed CAS Google Scholar
Rello J, Koulenti D, Blot S, Sierra R, Diaz E, De Waele JJ, Macor A, Agbaht K, Rodriguez A. Oral care practices in intensive care units: a survey of 59 European ICUs. Intensive Care Med. 2007;33(6):1066–70.
Zhao T, Wu X, Zhang Q, Li C, Worthington HV, Hua F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2020;12(12):008367.
Google Scholar
Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174(5):751–61.
Price R, MacLennan G, Glen J. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ Br Med J. 2014;348:g2197.
Cuthbertson BH, Dale CM. Less daily oral hygiene is more in the ICU: yes. Intensive Care Med. 2021;47(3):328–30.
Wittekamp BH, Plantinga NL. Less daily oral hygiene is more in the ICU: no. Intensive Care Med. 2021;47(3):331–3.
Bonez PC, dos Santos Alves CF, Dalmolin TV, Agertt VA, Mizdal CR, et al. Chlorhexidine activity against bacterial biofilms. Am J Infect Control. 2013;41(12):e119–22.
Saleem HG, Seers CA, Sabri AN, Reynolds EC. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016;16:214.
Article PubMed PubMed Central CAS Google Scholar
ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94(1):1–9.
deLacerdaVidal CF, Vidal AKdL, Monteiro JGDM, Cavalcanti A, Henriques APDC, Oliveira M, Godoy M, Coutinho M, Sobral PD, Vilela CÂ, et al. Impact of oral hygiene involving toothbrushing versus chlorhexidine in the prevention of ventilator-associated pneumonia: a randomized study. BMC Infect Dis. 2017;17(1):112–112.
van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55(1):104–23.
Slot DE, Valkenburg C, Van der Weijden GAF. Mechanical plaque removal of periodontal maintenance patients: a systematic review and network meta-analysis. J Clin Periodontol. 2020;47(Suppl 22):107–24.
Gu WJ, Gong YZ, Pan L, Ni YX, Liu JC. Impact of oral care with versus without toothbrushing on the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2012;16(5):R190.
Yao LY, Chang CK, Maa SH, Wang C, Chen CC. Brushing teeth with purified water to reduce ventilator-associated pneumonia. J Nurs Res. 2011;19(4):289–97.
Figuero E, Roldán S, Serrano J, Escribano M, Martín C, Preshaw PM. Efficacy of adjunctive therapies in patients with gingival inflammation: a systematic review and meta-analysis. J Clin Periodontol. 2020;47(Suppl 22):125–43.
Pinto A, Silva BMD, Santiago-Junior JF, Sales-Peres SHC. Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J Bras Pneumol. 2021;47(1):e20190286.
PubMed PubMed Central Google Scholar
Balagopal S, Arjunkumar R. Chlorhexidine: the gold standard antiplaque agent. J Pharm Sci Res. 2013;5:270–4.
Mojtahedzadeh M, et al. Systematic review: effectiveness of herbal oral care products on ventilator-associated pneumonia. Phytother Res. 2021;35(7):3665–72.
Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C: Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database Syst Rev 2016(10).
Sankaran SP, Sonis S. Network meta-analysis from a pairwise meta-analysis design: to assess the comparative effectiveness of oral care interventions in preventing ventilator-associated pneumonia in critically ill patients. Clin Oral Invest. 2021;25(5):2439–47.
Dale CM, Angus JE, Sinuff T, Rose L. Ethnographic investigation of oral care in the intensive care unit. Am J Crit Care. 2016;25(3):249–56.
Beck S. Impact of a systematic oral care protocol on stomatitis after chemotherapy. Cancer Nurs. 1979;2(3):185–99.
Henriksen BM, Ambjørnsen E, Axéll TE. Evaluation of a mucosal-plaque index (MPS) designed to assess oral care in groups of elderly. Spec Care Dentist. 1999;19(4):154–7.
Hayes J, Jones C. A collaborative approach to oral care during critical illness. Dent Health. 1995;34(3):6–10.
Silvestri L, Weir WI, Gregori D, Taylor N, Zandstra DF, van Saene JJM, van Saene HKF. Impact of oral chlorhexidine on bloodstream infection in critically Ill patients: systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2017;31(6):2236–44.
Villar CC, Pannuti CM, Nery DM, Morillo CM, Carmona MJ, Romito GA. Effectiveness of intraoral chlorhexidine protocols in the prevention of ventilator-associated pneumonia: meta-analysis and systematic review. Respir Care. 2016;61(9):1245–59.
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Dublin Dental University Hospital, Trinity College Dublin, Dublin, Ireland
Lewis Winning
Centre for Experimental Medicine, School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast, The Wellcome-Wolfson Building, 97 Lisburn Road, Belfast, BT9 7AE, Northern Ireland, UK
Fionnuala T. Lundy, Bronagh Blackwood, Daniel F. McAuley & Ikhlas El Karim
You can also search for this author in PubMed Google Scholar
Contributions
IEK, LW: conceptualization, draft preparation, FL, DMcA and BO’N: writing—original draft preparation, reviewing and editing. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Ikhlas El Karim .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Winning, L., Lundy, F.T., Blackwood, B. et al. Oral health care for the critically ill: a narrative review. Crit Care 25 , 353 (2021). https://doi.org/10.1186/s13054-021-03765-5
Download citation
Received : 17 June 2021
Accepted : 11 September 2021
Published : 01 October 2021
DOI : https://doi.org/10.1186/s13054-021-03765-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Oral health
- Oral bacteria
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 07 April 2017
Evidence summary: the relationship between oral health and pulmonary disease
- D. Manger 1 ,
- M. Walshaw 2 ,
- R. Fitzgerald 3 ,
- J. Doughty 4 ,
- K. L. Wanyonyi 5 ,
- S. White 6 &
- J. E. Gallagher 7
British Dental Journal volume 222 , pages 527–533 ( 2017 ) Cite this article
10k Accesses
65 Citations
40 Altmetric
Metrics details
- Respiratory tract diseases
- Tooth brushing
Presents moderate evidence of an association between oral health and two pulmonary conditions: chronic obstructive pulmonary disease (COPD) and pneumonia.
Presents strong evidence that frail populations (such as ventilated, or community-living and hospital-based patients) would have a lower incidence of pneumonia after regular oral hygiene interventions which include use of chlorhexidine or povidone iodine, with stronger evidence supporting chlorhexidine in mouthwash, gel, or other forms.
Highlights that although evidence suggests that chlorhexidine reduces the incidence of ventilator-associated pneumonia, other outcomes such as mortality are not affected.
Introduction This paper is the second of four reviews exploring the relationships between oral health and general medical conditions, in order to support teams within Public Health England, health practitioners and policymakers.
Aim This review aimed to explore the most contemporary evidence on whether poor oral health and pulmonary disease occurs in the same individuals or populations, to outline the nature of the relationship between these two health outcomes, and discuss the implication of any findings for health services and future research.
Methods The work was undertaken by a group comprising consultant clinicians from medicine and dentistry, trainees, public health, and academics. The methodology involved a streamlined rapid review process and synthesis of the data.
Results The results identified a number of systematic reviews of medium to high quality which provide evidence that oral health and oral hygiene habits have an impact on incidence and outcomes of lung diseases, such as pneumonia and chronic obstructive pulmonary disease in people living in the community and in long-term care facilities. The findings are discussed in relation to the implications for service and future research.
Conclusion The cumulative evidence of this review suggests an association between oral and pulmonary disease, specifically COPD and pneumonia, and incidence of the latter can be reduced by oral hygiene measures such as chlorhexidine and povidone iodine in all patients, while toothbrushing reduces the incidence, duration, and mortality from pneumonia in community and hospital patients.
Similar content being viewed by others
Enhanced oral hygiene interventions as a risk mitigation strategy for the prevention of non-ventilator-associated pneumonia: a systematic review and meta-analysis
Perspectives of community-dwelling older adults with dementia and their carers regarding their oral health practices and care: rapid review

Knowledge, attitudes and practices of patients and healthcare professionals regarding oral health and COPD in São Paulo, Brazil: a qualitative study
Pulmonary diseases can be broadly divided into lung infections, lung cancer, and those which obstruct airflow (chronic obstructive pulmonary disease and asthma). Lung cancer, chronic obstructive pulmonary disease (COPD), and lower respiratory tract infections were three of the top six causes of years of life lost in England in 2013. 1 COPD and lung cancer are major causes of morbidity and mortality throughout the world. Pneumonia occurs in 1–2 individuals per 1,000, 2 is the cause of over 5% of all deaths for all ages in 2014, 3 and, together with influenza, accounted for the second-highest hospital bed days in the UK in 2014–2015. 4
Pneumonia is an inflammation of the lung, usually caused by infection. 5 Three common causes are bacteria, viruses and fungi, which may colonise the oral cavity and upper airway. 6 It is also possible to contract pneumonia by accidentally inhaling a liquid or chemical. People most at risk are aged over 65 or below two years, or have existing health problems; for example, mechanically ventilated patients who have an endotracheal tube placed from the oral cavity to the trachea to ensure a patent airway.
Ventilator-associated pneumonia (VAP) is a known complication of mechanical ventilation and defined as 'serious inflammation of the lung in patients who required the use of pulmonary ventilator'. 5 A patient may be ventilated for several reasons, primarily when they require critical care in intensive care units (ICUs) such as post-cardiac surgery, trauma, neurological or respiratory conditions, and for varying time periods.
Chronic obstructive pulmonary disease is a type of obstructive lung disease characterised by chronically poor airflow. The main symptoms include shortness of breath, cough, and sputum. Tobacco smoking is the most common cause of COPD, with a number of other factors such as air pollution and genetics playing a smaller role. 5 It is diagnosed by a combination of clinical judgement, patient factors, and spirometry.
The two most common diseases affecting oral health are dental caries and periodontitis. Dental caries (caries) is the localised destruction of susceptible dental hard tissues by acidic by-products from bacterial fermentation of dietary carbohydrates. 7 Periodontitis is a chronic inflammatory disease caused by bacterial infection of the supporting tissues around the teeth. 8 Approximately half of all adults in the UK are affected by some level of irreversible periodontitis, which increases with age, and almost one third have obvious dental decay. 9
It is suggested that there is biological plausibility for a causal link between pulmonary disease and oral health related to oral disease pathogens aspirated into the pulmonary tissues. In the absence of effective oral care, initial plaque formation will occur within forty-eight hours; the composition of the oropharyngeal flora becomes more heavily colonised by virulent gram-negative pathogens that, as well as leading to oral disease, may be transported to the lungs where they have the potential to cause respiratory infections. 10 The aim of good mouth care is to maintain oral cleanliness, remove plaque and thereby prevent infection. 11 Twice daily brushing is recommended to control both periodontal diseases and caries; 12 however, the extent to which this may impact on pulmonary disease is unclear. In view of the serious outcomes and high prevalence related to both pulmonary and oral diseases, the aim of this review is to collate the most contemporary evidence on any links between the two.
A rapid review methodology was employed to synthesise the evidence from articles published between 2005 and 2015 that explored the relationship between pulmonary and oral health. A rapid review is a synthesis of the most current and best evidence to inform decision-makers. 13 It combines elements of systematic reviews with a streamlined approach to summarise available evidence in a timely manner.
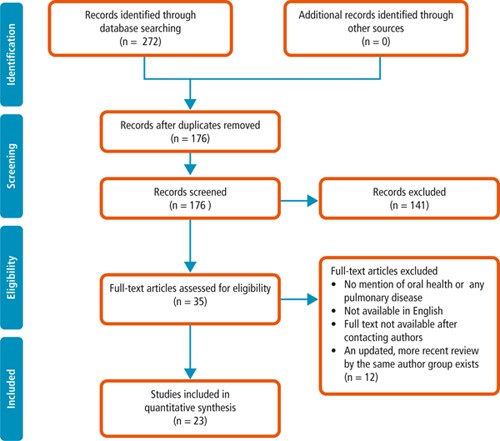
Search syntax was developed based on subject knowledge, MeSH terms, and task group agreements ( Box. 1 ), followed by duplicate systematic title and abstract searches of three electronic databases: Cochrane, PubMed, OVID (Embase, MEDLINE (R), and PsycINFO). Two independent searches were carried out: screening papers by abstract, and title, for relevance and duplication.
Studies were included if they were either a systematic review and/or meta-analysis, and explored a link between pulmonary and oral health. Disagreements between the reviewers and the wider research group were resolved by discussion. Papers were excluded for the following reasons: did not mention any term related to oral health or pulmonary health; were not available in English or in full text after contacting primary authors; or if a more up-to-date review covering the same topics by the same authors was found.
The following information was extracted from each paper: author, year, population studied, oral disease/intervention, definitions used, methods, comparison/intervention and controls, outcomes, results, authors' conclusions, quality and quality justification, as shown in data extraction Supplementary Table 1 .
From a total of 272 papers initially identified based on title and abstract, 35 remained after removal of duplicates, title screening and reviewing abstracts for relevance. These papers were examined in full and 23 papers were identified as relevant for the rapid review and synthesis of findings. A flow diagram of the process is provided in Figure 1 .
PRISMA 2009 Flow Diagram
Papers were reviewed and the following themes identified: association between oral health and pulmonary diseases; association of oral health interventions with the onset and outcomes of pneumonia in both (i) community-living and non-ventilated hospital-based patients (henceforth referred to simply as 'community' and 'hospital' patients respectively), and (ii) ventilated patients. The majority of evidence relates to patients who had difficulty in managing, or were unable to manage, their own oral hygiene measures; this included children, older people, patients with dementia, mechanically ventilated patients, and patients with functional disabilities and/or critical illness. Quality assessment was undertaken for each systematic review. An AMSTAR assessment was carried out on all papers with the methodological quality of the review being rated as 'High' with a score between eleven and eight, 'Moderate' between seven and four, and 'Low' between four and zero. The quality of all papers was also assessed by group discussion to reinforce the conclusion reached by the quality score.
The quality of the selected studies varied. Of the 23 systematic reviews, 13 were deemed to be high quality in line with the AMSTAR scoring system, following group discussion. Nine papers were found to be of moderate and one of low quality. Common AMSTAR missing points were the inclusion of grey literature, the listing of excluded papers with reasons for their exclusion, and the quality assessment of the included studies. Quality scores, as well as rationale for these scores, are presented for each paper included in this review in the data extraction table ( Supplementary Table 1 ).
Within the themes identified by this review, the papers examining oral hygiene interventions in ventilated patients were of particularly strong quality, 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 with all but five systematic reviews, 21 , 23 , 24 , 26 , 27 of high quality, while the systematic reviews examining community and hospital patients were more mixed with three of high, 28 , 29 , 30 and three of moderate quality. 31 , 32 , 33 Finally, the papers examining a direct association between oral health and pulmonary diseases were all of moderate quality. 33 , 34 , 35
Box 1 Search terms
1. (pulmonary or respiratory or lung) and (disease$ or infection$ or condition$) (all fields)
2. (pneumonia or respiratory tract infection or RTI) (all fields)
3. (chronic obstructive pulmonary dis$ or COPD) (all fields)
4. (dysphagia or aspirat$ or ventil$) (all fields)
5. (pulmonary or lung or respiratory) and (cancer or neoplasm) (all fields)
6. asthma or tuberculosis (all fields)
7. (oral or dental) and (health or hygiene or disease$ or care or infection) (all fields)
8. (periodon$ or gum) and disease (all fields)
9. (caries or tooth decay or DMFT) (all fields)
10. (plaque or oral bacteria or respiratory pathogen) (all fields)
11. (toothbrush$ or tooth brush $ or chlorhexidine) (all fields)
12. (systematic review) (all fields)
13. (meta ana$ or meta-ana$) (all fields)
Cochrane, PubMed, OVID (Embase, MEDLINE (R), PsycINFO)
Results: evidence synthesis
The findings are reported in two main sections. First, the nature of association between oral and pulmonary disease, including whether or not the latter is more likely in patients with oral disease. Second, the evidence from studies that have tested the impact of oral hygiene measures on pulmonary disease incidence and outcomes.
A] Association between oral and pulmonary disease
Overall the literature suggests associations of varying strength between oral health (periodontitis, caries, and plaque) and pulmonary disease (COPD and pneumonia). This was demonstrated by the increased presence of oral disease, or oral pathogens, in those participants who developed pulmonary disease when compared with those who did not. No evidence was discovered regarding any association between oral health and the presence of other conditions, notably lung cancer or tuberculosis. In the next sections, evidence of the associations between individual oral diseases and COPD and pneumonia are presented.
I] Periodontitis and COPD
In the case of periodontitis and COPD, three reviews of moderate methodological quality highlight an association between COPD and periodontal disease. The first, by Azarpazhooh and Leake, 35 provided weak evidence of an association between COPD and periodontal disease, suggesting study participants with significantly higher alveolar bone loss (ABL) and loss of clinical attachment had a higher risk of COPD than their counterparts. The second review by Sjogren et al . 33 also highlighted a weak association between ABL and dental plaque with COPD. And a third by Zeng et al . 34 reviewed fourteen observational studies assessing the relationship between COPD and periodontal disease and included pooled data stratified to control for smoking and other risk factors associated with the two diseases; the stratified results showed an attenuated, but significant, association between COPD and periodontal disease (P <0.001).
II] Periodontitis and pneumonia
Azarpazhooh and Leake (2006) 35 reviewed five studies that explored the relationship between pneumonia and oral health, suggesting that periodontal pathogens in saliva are a potentially important risk factor for pneumonia. No evidence was found linking periodontal disease itself with pneumonia.
III] Caries and pneumonia
The presence of caries was linked to the development of pneumonia in one moderate quality review, 35 which reported evidence from a nine-year cohort study indicating that decayed teeth (that is, dental caries) ([OR] ∼ 1.2 per decayed tooth) and cariogenic bacteria in saliva and plaque ([OR] 4 to 9.6) were associated with a higher risk of pneumonia. 35
IV] Plaque and pneumonia
Plaque, and its association with pulmonary disease, was examined by one moderate quality review. The evidence to support this was mixed with two prospective cohort studies suggesting that higher plaque scores were associated with a previous history of respiratory tract infection, whilst a third found no such significant association between pneumonia and plaque scores. 35
In summary, there is moderate evidence to suggest that patients with caries and plaque have a higher likelihood of developing pneumonia, and weak evidence suggesting an increased likelihood of people with more alveolar bone loss developing COPD than comparable counterparts.
B] Effect of oral hygiene interventions on incidence and outcomes of pulmonary disease
In this section the impact of oral hygiene interventions is reported in two sub-sections: first in relation to community or hospital patients; and, second, in relation to ventilated patients.
I] Effect of oral hygiene interventions on incidence and outcomes of pulmonary disease in community or hospital patients
Several reviews described oral hygiene interventions and their impact on incidence, or outcomes, of pneumonia in non-ventilated patients in community or hospital environments, while no evidence was found regarding any other pulmonary disease (including COPD). Therefore, this section will solely deal with oral hygiene inventions and their effects on pneumonia. These interventions include the use of chlorhexidine with concentrations between 0.12–2.0%, povidone iodine, the cleaning of prostheses, and mechanical interventions such as toothbrushing or professional care involving scaling and polishing.
a) Incidence of pneumonia in community and hospital patients
Seven systematic reviews investigated the relationship between oral hygiene interventions and incidence of pneumonia in these patients, and all suggest there is good evidence that oral hygiene interventions (chlorhexidine, toothbrushing, professional oral care, povidone iodine) reduce the risk of pneumonia. 28 , 29 , 30 , 31 , 32 , 33 , 35 The review quality ranged from high, 16 , 26 , 28 which included a meta-analysis, to moderate. 31 , 32 , 33 , 35 Two reviews suggest that there is a reduced risk of pneumonia with combined effect of mechanical and professional care, 28 , 33 and a third by Van der Maarel-Wierink et al . 32 suggests that manual toothbrushing, with or without povidone iodine, reduced the risk of pneumonia in frail older people by 67%. Of note, while mechanical plaque removal was shown to reduce pneumonia incidence in non-ventilated patients, this result was not repeated for ventilated patients.
In summary, there is good evidence that oral hygiene interventions reduce the risk of pneumonia in community and hospital patients.
b) Outcomes of pneumonia
Three high to moderate quality reviews found that mortality was reduced by mechanical plaque removal in community and hospital patients. 19 , 28 , 32 One high quality review by Silvestri et al . suggested no significant impact of chlorhexidine on pneumonia-associated mortality, although this paper included both ventilated and non-ventilated hospital patients. 29 Kaneoka et al . 28 in a high quality review, suggest that there is moderate evidence from two randomised, controlled trials, that mechanical oral care can lead to a risk reduction in fatal pneumonia but highlight a need for caution due to a risk of possible bias in the included studies. 19 Similarly, two studies included in the systematic review by Van der Maarel-Wierink et al . 32 found that toothbrushing without povidone iodine reduced pneumonia mortality (RR = 2.40 and 95% CI = 1.54–3.74 and OR = 3.57; 95% CI = 1.13–13.70).
Two high quality reviews suggest that the number of febrile days may be reduced by implementing oral health interventions. 17 , 29 One review found that toothbrushing with 1% iodine, or scaling combined with electric toothbrushing led to a reduction in febrile days. 30 These reviews do not include meta-analysis and should therefore be considered with caution.
Use of topical antiseptics and professional oral health care both appear to reduce microbial colonisation of the oral cavity. In a high quality review, Silvestri et al ., 29 report that chlorhexidine controls both gram-positive and gram-negative bacteria-related pneumonia as well as most (but not all) specific pneumonia-causing bacteria such as Streptococcus pneumoniae or Haemophilus influenza . However, when micro-organisms are classified into 'normal' and 'abnormal', chlorhexidine significantly reduces pneumonia due to 'normal' flora only. 29 One study in the review by Van der Maarel-Wierink et al . 32 suggests a reduction in levels of potential respiratory pathogens ( Streptococci, Staphylococci, Candida, Pseudomonas , and Black-pigmented Bacteroides species) after weekly professional oral healthcare. Professional oral care being defined as mechanical cleaning by a dentist/hygienist which varied in frequency from one to three times weekly.
A moderate quality review by Van der Maarel-Wierink et al ., which examined known risk factors for aspiration pneumonia reported an improvement in four out of five risk factors (swallowing latency time, activities of daily living scale, swallowing reflex, cough reflex sensitivity; but not salivary substance P) associated with regular oral hygiene. 32
In summary, good to moderate evidence suggests that oral hygiene interventions reduce many of the outcomes of pneumonia including febrile days, microbial colonisation, and mortality with the latter primarily being reduced by mechanical plaque removal.
II] The effect of oral hygiene interventions on incidence and outcomes of pulmonary disease in ventilated patients
There is a significant body of evidence relating to the effect of oral hygiene interventions on VAP, although no evidence regarding any other pulmonary disease. Again, this section focused on pneumonia and examines their impact on incidence and outcome, as well as cost-effectiveness and the role of different agents.
a) Incidence of VAP
In mechanically ventilated patients there is strong evidence from 13 systematic reviews that use of chlorhexidine (gel or mouthwash), when used in concentrations varying from 0.12–2.0%, reduces the risk of incidence of VAP. 14 , 16 , 17 , 18 , 19 , 20 , 21 , 24 , 26 , 27 , 29 , 30 , 31 Only one moderate-quality study, 25 the oldest included, did not find a significant reduction. The pooled relative risk of acquiring VAP reduced by approximately 40% when chlorhexidine-based oral decontamination was provided to ventilated patients in comparison to control groups (specifics of control groups varied among studies and included toothbrushing, 'standard oral care', placebo, other oral decontaminants, sterile water. Five reviews (two high, two moderate and one low quality) suggest the number needed to treat (NNT) as between 8 and 21 (with the high quality reviews finding a NNT of 14 and 15); meaning that between 8 and 21 ventilated patients in intensive care need to receive chlorhexidine oral decontamination for one case of VAP to be prevented. 20 , 22 , 26 , 27 , 33 Mechanical toothbrushing in addition to the use of chlorhexidine was not found to reduce the incidence of VAP by three high quality, and one moderate quality reviews. 14 , 15 , 20 , 23
In summary, there is strong evidence that regular chlorhexidine use in ventilated patients reduces the risk of VAP; with no evidence to show that mechanical plaque removal in addition to chlorhexidine provides further benefit.
b) Outcomes of VAP
No significant effect on mortality, duration of mechanical ventilation or duration of hospital stay was demonstrated, 14 , 17 , 18 , 19 , 20 , 22 , 24 , 25 , 26 and no evidence was found of a difference between chlorhexidine and placebo for the outcomes of VAP and mortality in children. 20 Other notable outcomes were that the use of chlorhexidine had a greater treatment effect in cardio-surgical patients, 24 , 29 , 36 and authors postulated that this was related to the planned nature of the intubation and the physical status of the patient at the time.
In relation to the impact of oral interventions on the use of systemic antibiotic therapy, Shi et al ., 20 a high quality review based on two randomised clinical trials, reported no significant difference in duration of antibiotic therapy, for the management of VAP, between intervention and control groups. One high quality systematic review, including four randomised-controlled trials, found no significant difference in antibiotic-free days between patients who received oral care and the control group. 15
Four reviews, 20 , 23 , 24 , 30 of high to medium quality, include evidence regarding oral health indices, in particular plaque scores. El-Rabbany et al ., 30 in a high quality review suggest that toothbrushing does improve oral health and has a positive effect on plaque scores when used on ventilated patients. It is suggested that this will reduce VAP, although as mentioned above, four reviews found toothbrushing had no effect. They do clarify that the studies reviewed were of moderate to high risk of bias. Two reviews, 23 , 24 report lower plaque levels in chlorhexidine groups versus controls in five trials, while one trial showed no such difference.
Shi et al . 20 reported the effect on plaque scores for toothbrushing versus no brushing and the use of chlorhexidine plus brushing versus a control group with chlorhexidine alone. The studies were of moderate to high risk of bias and presented ambivalent conclusions, when compared. One study indicated that plaque scores were improved, whereas the other three showed no difference.
In relation to microbial colonisation, Shi et al . found insufficient reliable and consistent evidence to confirm whether microbial colonisation of dental plaque varied between intervention and control groups for VAP. 20 On adverse effects of the interventions, two high and one moderate quality review 18 , 20 , 24 considered adverse effects in the evidence from the studies they included. One study reported that three patients receiving chlorhexidine complained of a transient, unpleasant taste and this compared to five patients in the control arm of the study. 20 In a further study, 9.8% of patients receiving chlorhexidine complained of mucosal irritation compared with 1% of the control group. 20 Snyder et al . 18 concurred with the comments from this study but added that further instruction to staff to be more gentle reduced the reports of irritation. Chlebicki et al . 24 reported no adverse effects.
Adverse effects/side effects reported were transient in nature and were reported in relation to both the chlorhexidine intervention and the control groups. The adverse effects of chlorhexidine were not unexpected and are those described within the drug proprietary literature. There was no reported evidence on the effect of oral hygiene interventions on the number of febrile days for ventilated patients.
In summary, there is moderate to low quality evidence that chlorhexidine does not have an effect on the following outcomes of VAP: mortality; duration of hospital stay; duration of ventilation; antibiotic use; plaque scores; microbial colonisation; or VAP in children. No unexpected side-effects of chlorhexidine were found.
c) Cost-effectiveness
Three systematic reviews reported on the cost-effectiveness of chlorhexidine as an oral care intervention. 16 , 18 , 24 Where chlorhexidine reduced the incidence of VAP by 43%, the comparative cost of a ye ar's supply of chlorhexidine (Peridex) was less than 10% of the cost associated with a single case of VAP. 16 The cost of chlorhexidine therapy for fourteen patients was suggested to be less than 10% of the cost of antibiotic therapy alone for one case of VAP. 16
Snyders et al . 18 also included two trials that considered the cost-effectiveness of chlorhexidine. Both suggested that chlorhexidine was cost-effective, and one suggested that the cost-effectiveness may be as much as ten times less per patient than the cost of antibiotics to treat VAP. 18 Chlebicki et al . 24 quotes studies examining costs of chlorhexidine, but notes no formal cost-effective analysis.
In summary, good evidence suggests that chlorhexidine is cost-effective when used to reduce pneumonia incidence.
d) Other antimicrobial agents
The effectiveness of topical application of povidone iodine for oral disinfection was considered in five systematic reviews of which four were high quality. 16 , 19 , 27 , 29 There is weak evidence that povidone iodine reduces the incidence of pneumonia, but this mode of oral disinfection was less effective than the use of chlorhexidine. 17 , 20 , 28 , 30 , 32
In summary, moderate evidence suggests both mechanical and chemical interventions have an impact on the incidence and outcomes of pneumonia in community and hospital patients. In regards to VAP, there is strong evidence that chemical interventions in general reduce incidence but do not affect other patient outcomes.
The cumulative evidence of this review suggests an association between oral and pulmonary disease, specifically COPD and pneumonia, and incidence of the latter can be reduced by oral hygiene measures such as chlorhexidine and povidone iodine in all patients, while toothbrushing reduces the incidence, duration, and mortality from pneumonia in community and hospital patients.
This review has a number of strengths and limitations which should be recognised. First, the review process conducted by a multidisciplinary team containing medical, dental, and public health professionals allowed for broad input and feedback and was thus considered a strength. Second, this is a 'rapid review', and so was intended to summarise existing evidence, rather than undertake quantitative synthesis of evidence. Third, there was large heterogeneity in the methodology of the studies in the literature reviewed including: variations in oral care interventions; varying measures of the chemical interventions such as chlorhexidine; and varying definitions/diagnoses of oral and pulmonary diseases; nonetheless there is important learning to inform future research.
The evidence has significant implications for research and services. First, the findings that highlight a reduction in the incidence of pneumonia in community and hospital patients after the implementation of oral hygiene measures (namely: toothbrushing, chlorhexidine, professional oral cleaning, and povidone iodine), provide useful data in planning for the oral health components of care pathways for patients with pneumonia. Second, a number of reviews demonstrated a reduction in the incidence of pneumonia after both chlorhexidine use and toothbrushing in community and hospital patients; and some studies, with a high risk of bias, additionally suggested that toothbrushing reduced the duration (days of fever) and mortality of pneumonia. Overall, this evidence supports the implementation of oral health protocols for pneumonia patients.
There was a greater volume of evidence on the role of oral hygiene interventions in reducing the incidence of VAP. Chlorhexidine was shown to be effective in reducing the incidence of VAP which has implications for patient well-being; it is also cost-effective, and without unexpected or severe adverse effects. In contrast to non-ventilated patients, toothbrushing alone had no effect on VAP incidence.
There is a clear need for further research, particularly around the cost-effectiveness and feasibility of implementation of oral hygiene interventions and their outcomes, as part of the care pathway for community-living and hospitalised frail patients in particular ( Table 1 ).
Although chlorhexidine was found to reduce the incidence of pneumonia as outlined in the paragraph above; other outcomes related to VAP, such as mortality or duration of ventilation/hospital-stay, were not affected by either chlorhexidine or toothbrushing. This seems contradictory and certainly warrants further investigation, especially as a low sample size and low attributable mortality of VAP may be the explanation. 37
So what can, and should, clinicians caring for community, hospital and ventilated patients do while waiting for this research? Numerous guidelines 38 , 39 , 40 recommend regular oral care, at least twice daily, to prevent oral disease and maintain oral health; this review highlights the additional importance of good oral hygiene for general health. Therefore, alongside oral health benefits, patients, carers, and relatives should be informed that improved oral hygiene may prevent episodes of pneumonia, and has been shown in some studies to reduce the incidence of mortality. In order to maintain optimal oral health, mechanical plaque removal by twice-daily toothbrushing is recommended. 12 Furthermore, the preventative effects of oral hygiene for the reduction of pneumonia can be further augmented by the oral application of chlorhexidine mouthwash, gels or other forms of delivery.
Where possible, this regimen can be carried out by the patient, with assistance from carers as required. An oral care plan should be created, implemented and reviewed at regular intervals; either by, or in consultation with, a dental professional. This is particularly important for patients who are unable to care for themselves. To prevent and improve the outcomes of pneumonia, commissioners and managers of services are advised to provide oral hygiene training for carers. Improving patients', relatives' and carers' knowledge of the effects of poor oral health has the potential to support health maintenance in vulnerable patients, deliver cost-effective care, and improve patient quality of life.
Newton J N, Briggs A D, Murray C J et al. Changes in health in England, with analysis by English regions and areas of deprivation, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386 : 2257–2274.
Article Google Scholar
Torres A, Peetermans W E, Viegi G, Blasi F . Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68 : 1057–1065.
Office for National Statistics. Mortality Statistics: Deaths Registered in England and Wales (Series DR). Available online at https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2015-07-15 (accessed March 2017).
Health and Social Care Information Centre. Hospital Episode Statistics. Admitted Patient Care – England, 2014–2015. 2015.
World Health Organisation. International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2016.
Scannapieco F A, Shay K . Oral health disparities in older adults: oral bacteria, inflammation, and aspiration pneumonia. Dent Clin North Am 2014; 58 : 771–782.
Fejerskov O, Nyvad B, Kidd E . Dental caries: the disease and its clinical management . Third edition. Wiley-Blackwell, 2015.
Google Scholar
Eke P I, Page R C, Wei L, Thornton-Evans G, Genco R J . Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 2012; 83 : 1449–1454.
Steele J, O'Sullivan I . Adult Dental Health Survey 2009. 2010. Available online at http://www.hscic.gov.uk/catalogue/PUB01061/adul-dent-heal-surv-firs-rele-2009-rep.pdf (accessed March 2017).
Eley B, Manson J . Periodontics . Fifth edition. Wright, 2004.
Mallett J D, Lisa. The Royal Marsden Hospital Manual of Clinical Nursing Procedures. 2000.
NHS Public Health England. Delivering better oral health: an evidence-based toolkit for prevention. 2014.
Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D . Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012; 1 : 10.
Alhazzani W, Smith O, Muscedere J, Medd J, Cook D . Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta-analysis of randomized trials evaluating ventilator-associated pneumonia. Crit Care Med 2013; 41 : 646–655.
Gu W J, Gong Y Z, Pan L, Ni Y X, Liu J C . Impact of oral care with versus without toothbrushing on the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2012; 16 : R190.
Zhang T T, Tang S S, Fu L J . The effectiveness of different concentrations of chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. J Clin Nurs 2014; 23 : 1461–1475.
Li L, Ai Z, Li L, Zheng X, Jie L . Can routine oral care with antiseptics prevent ventilator-associated pneumonia in patients receiving mechanical ventilation? An update meta-analysis from 17 randomized controlled trials. Int J Clin Exp Med 2015; 8 : 1645–1657.
PubMed PubMed Central Google Scholar
Snyders O, Khondowe O, Bell J . Oral chlorhexidine in the prevention of ventilator-associated pneumonia in critically ill adults in the ICU: A systematic review. S Afr J Crit Care 2011; 27.
Klompas M, Speck K, Howell M D, Greene L R, Berenholtz S M . Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med 2014; 174 : 751–761.
Shi Z, Xie H, Wang P et al. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev 2013; 8 : CD008367.
Kola A, Gastmeier P . Efficacy of oral chlorhexidine in preventing lower respiratory tract infections. Meta-analysis of randomized controlled trials. J Hosp Infect 2007; 66 : 207–216.
Chan E Y, Ruest A, Meade M O, Cook D J . Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 2007; 334 : 889.
Berry A M, Davidson P M, Masters J, Rolls K . Systematic literature review of oral hygiene practices for intensive care patients receiving mechanical ventilation. Am J Crit Care 2007; 16 : 552–562; quiz 63.
PubMed Google Scholar
Chlebicki M P, Safdar N . Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med 2007; 35 : 595–602.
Pineda L A, Saliba R G, El Solh AA . Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care 2006; 10 : R35.
Balamurugan E, Kanimoxhi A, Kumari G . Effectiveness of chlorhexidine oral decontamination in reducing the incidence of ventilator associated pneumonia: a meta-analysis. Br J Med Pract 2012; 5.
Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V . Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect Control Hosp Epidemiol 2008; 29 : 131–136.
Kaneoka A, Pisegna J M, Miloro K V et al. Prevention of Healthcare-Associated Pneumonia with Oral Care in Individuals Without Mechanical Ventilation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Infect Control Hosp Epidemiol 2015; 36 : 899–906.
Silvestri L, Weir I, Gregori D et al. Effectiveness of oral chlorhexidine on nosocomial pneumonia, causative micro-organisms and mortality in critically ill patients: a systematic review and meta-analysis. Minerva Anestesiol 2014; 80 : 805–820.
El-Rabbany M, Zaghlol N, Bhandari M, Azarpazhooh A . Prophylactic oral health procedures to prevent hospital-acquired and ventilator-associated pneumonia: a systematic review. Int J Nurs Stud 2015; 52 : 452–464.
Vilela M C, Ferreira G Z, Santos P S, Rezende N P . Oral care and nosocomial pneumonia: a systematic review. Einstein (Sao Paulo) 2015; 13 : 290–296.
van der Maarel-Wierink C D, Vanobbergen J N, Bronkhorst E M, Schols JM, de Baat C . Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology 2013; 30 : 3–9.
Sjogren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J . A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc 2008; 56 : 2124–2130.
Zeng X T, Tu M L, Liu D Y, Zheng D, Zhang J, Leng W . Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PloS One 2012; 7 : e46508.
Azarpazhooh A, Leake J L . Systematic review of the association between respiratory diseases and oral health. J Periodontol 2006; 77 : 1465–1482.
Labeau S O, Van de Vyver K, Brusselaers N, Vogelaers D, Blot S I . Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 2011; 11 : 845–854.
Damjanovic V, Silvestri L, Taylor N, van Saene H K, Piacente N . Oropharyngeal without intestinal decontamination does not make sense. J Hosp Infect 2014; 86 : 277–278.
Guidelines and Audit Implementation Network. Guidelines for the Oral Healthcare of Older People Living in Nursing and Residential Homes in Northern Ireland. Available online at https://rqia.org.uk/RQIA/files/12/12a65998-23a4-4610-a1f6-afe6ce2fa059.pdf (accessed March 2017).
Fiske J, Frenkel H, Griffiths J et al. Guidelines for the development of local standards of oral health care for people with dementia. Gerodontology 2006; 23 Suppl 1: 25–32.
NICE. NICE – Clinical Knowledge Summaries: Palliative care – oral. Available online at http://cks.nice.org.uk/palliative-care-oral#!annual_know_upd (accessed March 2017).
Download references
Acknowledgements
We would like to acknowledge the support of Public Health England, the Royal College of Surgeons, and the British Dental Association.
Author information
Authors and affiliations.
Deputy Medical Director, Specialist in Special Care Dentistry, Northamptonshire Healthcare NHS Foundation Trust, Salaried Primary Care Dental Service, Willowbrook Health Centre, Cottingham Road, Corby, NN17 2UR,
Department of Infection Microbiology and Immunology, Honorary Professor of Medicine, Liverpool University and Consultant Chest Physician, Liverpool Heart and Chest Hospital, Thomas Drive, Liverpool, L14 3PE,
Formerly Dental Core Trainee in Community Special Care Dentistry/Dental Public Health/Honorary Research Assistant,
R. Fitzgerald
Formerly Clinical Fellow in Special Care Dentistry, Northampton Healthcare NHS Foundation Trust, Academic Clinical Fellow in Special Care Dentistry, Eastman Dental Hospital, University College London, 256 Gray's Inn Road, London, WC1X 8LD,
Senior Lecturer in Dental Public Health, University of Portsmouth Dental Academy, William Beatty Building, Hampshire Terrace, Portsmouth PO1 2QG,
K. L. Wanyonyi
Population Health & Care Division, Director of Dental Public Health, Health and Wellbeing Directorate, Public Health England, Skipton House, 80 London Road, London, SE1 6LH,
Head of Population and Patient Health, Newland Pedley Professor of Oral Health Strategy, Honorary Consultant in Dental Public Health, King's College London Dental Institute, Denmark Hill Campus, Bessemer Road, London, SE5 9RS,
J. E. Gallagher
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to J. E. Gallagher .
Additional information
Refereed Paper
Supplementary information
Supplementary table 1.
Evidence from included papers (PDF 204 kb)
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Manger, D., Walshaw, M., Fitzgerald, R. et al. Evidence summary: the relationship between oral health and pulmonary disease. Br Dent J 222 , 527–533 (2017). https://doi.org/10.1038/sj.bdj.2017.315
Download citation
Accepted : 14 February 2017
Published : 07 April 2017
Issue Date : 07 April 2017
DOI : https://doi.org/10.1038/sj.bdj.2017.315
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Characterization of oral bacterial and fungal microbiome in recovered covid-19 patients.
- Guangqi Zhu
BMC Microbiology (2023)
Prevalence of oral complications in the course of severe SARS-CoV-2 infection under mechanical non-invasive ventilation
- Elzbieta Paszynska
- Maria Gawriolek
- Szczepan Cofta
European Journal of Medical Research (2023)
Opportunistic health screening for cardiovascular and diabetes risk factors in primary care dental practices: experiences from a service evaluation and a call to action
- Janine Doughty
- Simon M. Gallier
- Amanda J. Daley
British Dental Journal (2023)
Pulmonary disease and periodontal health: a meta-analysis
- ZhiDong Guo
Sleep and Breathing (2022)
Oral health care for the critically ill: a narrative review
- Lewis Winning
- Fionnuala T. Lundy
- Ikhlas El Karim
Critical Care (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Research article
- Open access
- Published: 14 June 2021
Oral status of older people in medium to long-stay health and social care setting: a systematic review
- Juan Antonio Ruiz-Roca 1 ,
- Dora Martín Fuentes 2 ,
- Francisco J. Gómez García 3 &
- Yolanda Martínez-Beneyto ORCID: orcid.org/0000-0002-1523-9415 4
BMC Geriatrics volume 21 , Article number: 363 ( 2021 ) Cite this article
2887 Accesses
13 Citations
8 Altmetric
Metrics details
Older patients who spend long periods hospitalized or those who are in a situation of institutionalization represent a risk group in this regard, as many of them suffer a degree of dependence and need help to perform the basic tasks of personal care. It is therefore important to learn more of the oral health status of this group of patients in order to make a proper assessment of the situation and to develop protocols for its management. The purpose of the study was to conduct a systematic review to ascertain the oral health status of older people patients admitted to institutions or hospitalized for a long period of time.
a systematic review of the literature published in two different databases (PubMed, Embase and Cochrane Library) was carried out, with 12 different combinations of keywords based on the following selection criteria: studies published in the last 5 years, in English and/or Spanish and/or Portuguese, with samples of ≥30 patients, performed in patients older than 65 years, admitted to any type of institution and/or hospital center for at least 7 days and in which the state of hard and/or soft tissues of the oral cavity were evaluated in some way. The selected articles were subjected to a thorough analysis.
The search strategy covered 1.014 articles: 689 from Pubmed and 325 from Cochrane Library. After applying the eligibility criteria, five articles were selected for our review. The level of evidence of the articles was, a sample of 773 patients most of them were women with an average age older than 70 years old.

Conclusions
The oral health of patients aged more than 65 is worse than that of the rest population. Long hospital stays or being institutionalized in a residence makes this group susceptible to a worsening of their oral health status. It is necessary to develop protocols for the oral health care of these patients, accompanied by training programs for the personnel responsible.
Peer Review reports
In light of the increase in life expectancy, aging is “on the verge of becoming one of the most significant social transformations of the twenty-first century” [ 1 ]. In Spain, people over 65 represent 19.2% of the total population [ 2 ], a figure that will reach 25.2% in 2033 [ 3 ].
This makes it necessary to reconsider the way in which we attend and treat older patients in society [ 4 ], not just those who have sufficient personal autonomy but also those, estimated to represent around 3% of the elderly [ 5 , 6 ], who live in institutions and need some kind of specific care. Despite this need, there are insufficient studies that describe the situation in which this population group find themselves and which might contribute to improving the attention given to them and therefore increase their quality of life. For example, in Spain there are no studies published in which the physical, medical and psychological conditions of the institutionalized older population are evaluated [ 5 ].
The progress and improvements that have been made in dentistry, as well as new patterns of care and prevention, have meant that it is increasingly possible to reach older people with a large number of teeth and in a better state of dentition than ever before [ 7 , 8 ] although there is still a tendency for the older to be vulnerable to caries and periodontitis [ 8 ]. Oral pathologies can significantly affect health and general welfare of the population, and lead to alterations in speech, the poor pronunciation of certain words, or deficient food intake, raising the risk of malnutrition [ 9 ] due to problems with chewing or swallowing. Moreover, oral health can have a negative effect on facial aesthetics, lowering self-esteem and harming the psychosocial well-being of the individual [ 10 , 11 , 12 ]. Numerous studies have described the relationship between poor oral health and the emergence of systemic diseases, ranging from heart disease or Diabetes Mellitus to respiratory diseases, such as pneumonia [ 8 , 10 , 13 , 14 ].
Diseases such as Parkinson’s or Alzheimer’s, or neuromuscular disorders, are some of the reasons that many are no longer able to carry out oral care tasks, due to a loss of manual dexterity, basically because of a loss of motor and cognitive skill, or because they do not remember how to brush their teeth or are not able to follow the instructions on how to do so themselves [ 11 ].
In the case of geriatric patients, the frequent coexistence of several diseases and disorders in the same patient must also be taken into account. Comorbidity in this population makes them especially susceptible to oral pathologies, often as a result of the medication they are taking, which increase the risk of tooth decay through hyposalivation [ 10 ]. In addition, some disorders may give rise to physical, cognitive or even motivational limitations that interfere with the development and habit of practicing good oral hygiene [ 11 , 15 , 16 ].
Added to the vulnerability of geriatric patients in this respect, other factors may limit their access to oral attention, such as an inability to assume the costs of treatments reduced physical mobility, the lack of transport or the absence of caregivers or family members who can accompany them. In addition, the work they used to do, their social environment or their own idiosyncrasies may mean the person lacks the ability to recognize the need for an a dental examination or treatment [ 10 ].
Despite the high prevalence of oral health problems in this group of patients, little or no importance is given to this problem [ 10 ], leading the World Health Organization (WHO) to advise on the need to increase awareness, on a social, cultural and medical level, of oral health as a major component of overall health and quality of life. The organization strongly recommends that countries develop programmes to meet the needs of their older citizens in this respect and to research the problem of oral care in the older people, due to an increase in the overall incidence of non-transmissible diseases [ 17 ]. A survey of the oral health of older patients carried out by the WHO revealed that oral health programmes targeting this population group are very rare [ 17 ], and that dental intervention tends to be therapeutic rather than (ideally) preventive. That is why hospitalization or long stays in care centers present a good opportunity for providing dental assistance that would otherwise not be offered to the general older population [ 10 ].
The removal of bacterial plaque at least twice a day (morning and evening) is essential for maintaining oral health, especially in dependent older people. However, despite the important role that staff in hospitals and other long stay centers such as nursing homes, could play in maintaining and influencing oral health, they do not know what care and oral hygiene protocols should be followed with the older people, except those patients who are at risk of pneumonia associated with mechanical ventilation [ 11 ].
Although oral pathologies are among the most common chronic diseases and represent an important public health problem due to their prevalence and the expense of treatment [ 15 ], there is a general but erroneous belief that oral hygiene and care are unimportant [ 11 ]. When patients, for different reasons, reject oral care, staff simply accept their refusal. However, refusing treatment would not be tolerated in other interventions - for example, measuring the level of glucose in the blood or the blood pressure of a patient. This situation is doubly severe in older patients with dementia who are reluctant to be cared for by third parties, Moreover, care providers may not be in a position to offer proper care, either because the patient refuses or because they are overworked and decide not to assist them. For all of the above, these patients can be considered extremely vulnerable and are at higher risk than the general older population [ 11 ].
Bilder et al. [ 15 ] describes how poor oral health and limited access to oral care for adults in long-term care centers, as well as the lack of detailed guidelines, are a reflection of insufficient scientific evidence concerning the dental care support techniques that can be offered [ 11 ]. This clearly does not help when attempts are made to reverse this situation. However, problems of oral health, ranging from dental caries to chewing problems or pain, constitute the most frequent treatment needs and are among the least successfully resolved health problems in the population group consisting of older people and the disabled [ 15 ].
For all these reasons, we think that the lack of information, documentation and prevention concerning the oral health of older patients can have an advese impact on health, i.e., on the state of complete physical, mental and social well-being.
Our main objetive, then, was to conduct a systematic review to ascertain the state of oral health of older patients in an institution for a long period of time, analysing those parameters that could reveal their current oral situation. Secondary objectives were: to see whether any deterioration of oral health detected in these patients is affected by their being in a hospital or residence; to ascertain whether a standard protocol exists concerning the oral health care of these patients; to compare the information obtained with published scientific literature, and, if no relevant information exists, to propose a line of research to establish a prevention-based protocol for oral care in the older population, especially those in long stay facilities.
The literature search strategy followed in making this systematic review was in accordance with the PICOS framework [ 18 ]. The focus question was: What is the state of oral health of institutionalized older patients?.
Study design
A systematic review of the literature was managed by two reviewers (JARR and DMF) independently and conducted an exhaustive search of each database.
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyzes Statement Check-list) 2009 statements (http: www.prismastatement.org ) throughout the selection process and the last manual updated by the Cochrane Collaboration, for the preparation of systematic reviews of the literature of the year 2009″ [ 19 ]. Institutional review board approval was not required for this review.
In the first round only titles and abstracts of retrieved articles were analyzed. Then in a second round all considered eligible studies were fully examined and final decisions about inclusions were made. In case of disagreement a third reviewer (YMB) participated in order to reach consensus. Cohen’s kappa coefficient was used to evaluate the disagreement between the researchers.
Following the methodology of evidence-based medicine, the PICO strategy was used, in order to prepare the research question to which we will try to answer in this work; Population (P): older patients, aged 65 and over admitted to hospital or geriatric center for periods of more 7 days; Intervention (I): To analyze the following parameters: Oral health indexes such as DMFT (Decayed, Missing, Filled Index) and treatment needs index and oral hygiene protocols Comparison (C): Oral health status among patients who are institutionalized versus non- older subjects; Outcome (O): Poorer results in patients institutionalized in the periodontal index score.
Search strategy and databases
An intensive search was performed in three of the main scientific databases such as the Cochrane Library, Medline via Pubmed and Embase. Only articles published in English, Spanish or Portuguese within the 5 year period 1 January 2014 to 1 January 2019 were consulted. The search strategy used terms from the controlled vocabulary MeSH (Medical Subject Headings) and the Boolean operators “AND”, “OR” and “NOT”, as well as terms related to the study population ( elderly inpatients, elderly hospitalized patients, long term hospitalization, long term inpatients, oral health oral status and oral pathology) .
Selection criteria
The following inclusion and exclusion criteria were followed in this systematic review (Table 1 ). The sample was ≥50 individuals, because that amount of sample is statistically representative, usually. In Spain, an older patient is considered to be ≥65 years old.
Assessment of Bias in studies
From each of the articles, information was extracted, such as sample size, study design, any intervention and the measures of the results, how the results were measured/analysed/presented?. Articles are classified by reference to their level of scientific evidence according to the criteria described by the Scottish Intercollegiate Guidelines Network (SIGN), which provides checklists to assess the quality of: systematic reviews & meta-analyses, randomized clinical trials, cohort studies, case-control studies, diagnostic studies, and economic studies. Each checklist is accompanied by notes to aid completion, and written responses to the individual questions are used, with users then assigning studies an overall rating according to specified criteria. The full set of checklists and detailed notes on their use are available from SIGN [ 19 ].
The search culminated in five studies that fulfilled both the inclusion and exclusion criteria and which were conducted from 1 January 2014 to 1 January 2019 (Fig. 1 ).
Flow diagram of the search processes and results
Quality assessment
According to these criteria, the articles selected for our systematic review had the following levels of evidence and degrees of recommendation and the Risk of bias for non-randomized studies assessed with ROBINS-I Cochrane tool. (Tables 2 and 3 ).
Basic results
Of the five studies selected for this systematic review (Fig. 1 ), two were carried out in Europe [ 9 , 20 ], two in Asia [ 21 , 22 ], and one in Australia [ 23 ].
All the works were based on with a sample size that could offer extrapolated data (≥ 50 older patients): Poisson et al. [ 9 ] 159 patients, Gerritsen et al. [ 20 ] 355, Chen et al. [ 21 ] 120, and that smallest, Murray et al. [ 23 ] and Nakayama et al. [ 22 ] with 89 and 50 patients, respectively, making a total of 773 patients.
Although only two studies [ 20 , 21 ] specified the age range of the patients, the average age of all participants in the studies was over 70 years.
In three studies [ 9 , 20 , 21 ] the proportion of women in the sample was higher than that of men. As regards the total number of participants in the papers included in the review, the proportion of women who participated in the studies was higher (1:1.6 male to female ratio), which can be explained by the greater life expectancy of women.
Gerritsen and co-workers [ 20 ] took as their sample a group of older subjects from three retirement homes, while Chen et al. [ 21 ] studied subjects from a geriatric medical center and Murray et al. [ 23 ] patients from three rehabilitation centers for patients who had suffered a cerebrovascular accident (CVA). Poisson et al. and Nakayama et al. [ 9 , 22 ] developed their studies in hospitals, and Poisson et al . [ 9 ] worked in the geriatrics area of a hospital. Nakayama et al. [ 22 ] focused on patients suffering ALS (Amyotrophic Lateral Sclerosis) with nasogastric and artificial respiration. None of the selected studies specified whether they were in public or private centers.
Causes of admission of patients
Except for Murray et al. [ 23 ] and Nakayama et al. [ 22 ], who worked with very specific types of patient (patients in rehabilitation after CVA and patients with ALS, respectively), none of the studies specified the reason for admission to the centres, although Gerritsen et al. [ 20 ] and Chen et al. [ 21 ] gave a general outline. In particular, Gerritsen et al. [ 20 ] specified that 47% were in the residence for somatic reasons and 53% for psychogeriatric reasons, while Chen et al. [ 21 ] pointed out that the main diagnoses of their sample at admission were pneumonia, sepsis, idiopathic fever and infection of the urinary tract.
Two of the studies [ 22 , 23 ] did not specify the length of the stay in the institution, but, from the information provided in the articles, we understand that all the studied patients had been in institution for at least 7 days [ 21 , 23 ], while the longest times were those mentioned by Gerritsen et al. [ 20 ] (more than 2 years). Therefore, the subjects who had been the longest time in care were those mentioned in the only study carried out in retirement homes.
Three of the five studies [ 9 , 21 , 23 ] specify at least part of the systemic pathology that participants were suffering. The remaining two [ 20 , 22 ] did not mention whether the patients described in their studies suffered any other pathologies beyond those specified as the time of admission: somatic or psychogeriatric reasons in the case of Gerritsen et al. [ 20 ], and ALS in the case of Nakayama et al. [ 22 ]. In the study of Poisson et al. [ 9 ], 74.2% of the patients had some sort of cognitive problem. Murray et al. [ 23 ] mentioned only comorbidities derived from the CVA suffered by their patients (aphasia, apraxia, dependency, among others) and Chen et al. [ 21 ] describes the degree of dependence of their patients (total 45%; severe 35% and slight 20%), along with the more common pathologies such as Diabetes Mellitus (58.3% of patients) and high blood pressure (77.5%). However, the most striking thing in all the studies was the number of patients who had some sort of cognitive problem or degree of dependence that made them vulnerable if they did not receive good oral care (Table 3 ).
None of the studies evaluated the medication that the participants were taking despite the fact that medication could be associated with the state of their oral health. Nakayama et al. [ 22 ], who measured the salivation index of their participants, only mentioned that none of the patients in the study were following any treatment that would have affected their salivary flow (radiotherapy or botulinum toxin treatment).
Oral health and hygiene
Regarding the oral health of the participants in the studies, we conclude that the authors used different methods of assessment, and only Poisson et al. [ 9 ] and Nakayama et al. [ 22 ] used the DMFT index (Decayed, Missing, Filled Teeth). However, the vast majority of patients in all the studies had poor oral health and, we understand that they were also in great need of treatment, although only Gerritsen et al. [ 20 ] specified so.
As regards oral care measures, only one study [ 9 ] did not mention that subjects follow any kind of oral hygiene protocol. Gerritsen et al. [ 20 ] mentioned that patients in the caring homes had access to 16 h of dental care a week and 8 h of oral hygiene. This is probably why new patients had greater need of treatment than long-standing residents, although this relationship was only clear in the group of edentulous patients. This fact is possible due to they had no teeth, and this made it easier to offer care and because their mental condition meant they have received special attention. Nakayama et al. [ 22 ] described the protocol followed by nurses twice a day, in which they paid attention to both the hard and soft tissues. However, it must be borne in mind that the patients who participated in the study by these authors suffered from ALS, suggesting that they followed a special protocol (even though, in our opinion, such care should be considered normal). Chen et al. [ 21 ] suggested that the oral hygiene of patients is the responsibility of the nursing staff, but did not specify any guidelines or the frequency concerning the same. However, the authors do mention the improvements shown following the intervention (brushing and rinsing twice a day) with regard to halitosis, plaque and the state of mucous membranes. No significant differences were observed between the three types of rinses used for the different groups (Chlorhexidine, saline solution and boiled water) during the examinations carried out on the 7th day of the intervention, except for cases of halitosis, for which the best result was seen in the.
In the case of Murray et al. [ 23 ] it seems that patients only had their teeth brushed in the morning but that, due to the hygiene guidelines provided during the study (brushing with toothpaste after breakfast and dinner, and rinsing with water after the main meal, with the assistance of the staff when necessary), the oral situation of most of the patients with dysphagia improved; patients without dysphagia also improved, but not significantly so. In addition, the authors established a relationship between patient autonomy and their oral status. Improvement in the oral health of patients were recorded in the only two studies that provided oral hygiene guidelines during the studies and reassessed the oral situation of patients later [ 23 ]. It should be noted that only the studies of Poisson et al. and Gerritsen et al. [ 9 , 20 ] were supervised by dentists (Table 4 ).
In general, the studies included in our systematic review [ 9 , 20 , 3 ] found that the attention that should be given to the hygiene and oral care of patients is simply not given, and that staff, by implementing measures that are considered basic for maintaining good oral health, could improve the oral health of many people in this population.
Relationship between oral and general health
There is no doubt that a good oral health status is crucial for maintaining good general health [ 8 , 10 , 13 ]. In the older this relationship is much clearer, since many tend to suffer from conditions that make them susceptible to poorer oral health [ 10 , 11 , 15 ].
In a study carried out in 2001, Shimazaki et al. [ 24 ] showed that older edentulous subjects not using dentures were significantly ( p < 0.05) associated with hight risk of physical disability and mortality, independent of age and other variables (OR = 1.8, 95% CI). The decline in occlusal function resulting from tooth loss causes problems with chewing, swallowing, an food selection, and the nutritional status of edentulous people deteriorates. Therefore, Shimazaki et al. [ 24 ] concluded that those older inpatients with 20 or more teeth, leads to think that the conservation of teeth as the years pass exerts a protective role in the general state of health. These same authors studied the influence of oral health on febrile states in older inpatients during long hospital stays, and found that poor dental and oral health was linked to episodes of fever in both dentate and edentulous patients. In addition, many authors have described the relationship between poor oral health and the development of pneumonia as a result of aspiration and respiratory infections in patients with assisted ventilation [ 16 ]. This suggests that, while dental conservation work can favour the maintenance of a good general state of health in old age, the same does not apply if little attention has been paid to maintaining oral health previously.
Impact of hospitalization
Hospitalization changes the routines of people, and may cause stress or anxiety because of the pain and discomfort that they may experience during an illness [ 25 ]. For this reason, being hospitalized is an added risk when it comes to good oral health [ 16 ], as it usually results in a decline in self-esteem, leading patients to neglect personal care and hygiene at that same time that they feel worried about their disease [ 16 , 25 ]. This circumstance particularly affects patients with physical or cognitive limitations [ 12 , 25 , 26 , 27 ], who are the most vulnerable in terms of developing problems or deterioration in terms of oral health, especially during a long hospital stay or a situation of institutionalization.
Lack of data on longer term institutional care settings
Studies that have attempted to look for a relation between hospitalization and oral health were developed in Intensive Care Units (ICU), and so provide insufficient evidence since the vast majority of hospitalized patients attend other departments [ 25 ]. In addition, Sousa et al. [ 25 ] and Gibney et al. [ 16 ] found that short hospital stays in units that did not involve intensive care had a negative effect on the oral health of patients, corroborating the evidence of studies conducted in these units, and underlining the importance of studying the situation in other hospital services.
During the article selection process we were faced with the problem of the paucity of studies on elderly institutionalized or long-stay hospital patients and their oral health, although many authors [ 10 , 16 , 28 ] studied emergency and short-stay patients, who were found to have previous oral health as well as systemic problems. Other studies focused on dysphagia which elderly patients frequently suffer, its risk factors and relationship with malnutrition, but without analyzing the state of their oral health [ 29 , 30 , 31 , 32 ], despite its importance in this disorder. In our review [ 9 , 20 , 21 , 22 ], we observed that the vast majority of participants had poor oral health. For example, Gerritsen et al. [ 20 ] established the need for treatment in 70% of the patients in their sample, even though dental care was provided by their institution, which makes the lack of studies assessing the oral health of the older in this situation or during long hospital stays even more incomprehensible.
The need for standardize protocols
As we have seen in two of the studies included in our review [ 21 , 23 ], compliance with the protocols that involve the basic oral hygiene measures recommended for any patient these days leads to unquestionable improvements in the oral health of patients. This has also been found in other studies carried out in chronic care facilities and in areas of geriatric rehabilitation where oral hygiene measure were under the supervision of dental professionals and/or nursing staff following a standardized protocol [ 28 ].
Health care personnel recognise the importance of hygiene and oral care [ 33 ]. However, the lack of such care in long stay institutions and hospitals [ 34 ] is frequently attributed to a lack of training and time and the little cooperation of geriatric patients themselves [ 33 ]. Many studies have pointed to the difficulty posed by applying protocols of oral hygiene in institutions such as old people’s homes [ 20 ] due to the little training received by care workers concerning protocols of oral hygiene, the oral needs of older patients, and the risks and negative consequences of poor oral health [ 15 , 16 ], as well as on the availability of and access to material to carry out related tasks [ 11 , 35 ]. In addition, it has been described how a theoretical training programme is not sufficient to improve the oral care of these patients. In this context, Gammack et al. [ 36 ] found that when hygienists, auxiliary staff and nurses were given oral hygiene training on a theoretical basis using audiovisual aids and dummies rather than “real” patients, the oral health of dependent patients did not improve, perhaps because, among other reasons, staff had not received adequate training or information on the correct way to deal with the reactions of patients opposed to receiving much care [ 28 ]. However, some studies suggest that the attitude of the staff themselves towards providing oral care makes the difference between a patient accepting, asking for or neglecting oral care [ 11 ]. It is clear that oral health is not a priority in situations of lengthy hospitalization or institutionalization [ 11 , 37 ]. As we have seen in the results of the review, only one study [ 22 ] presented a detailed oral hygiene protocol to be applied twice a day, although, being a protocol used in patients with ALS, we understand that this is a special feature because of the medical condition in question.
The situation in Spain
In Spain, according to National Oral Health Surveys, carried out in 2015, the 20% of the population over 65 years old, worry less about their oral health, and visit the dentist less frequently [ 38 ]. Perhaps, for this reason, in the group of 65–74 years, the SIC (Significant Caries Index) of Bratthall, represents the highest value, a 25.27 ± 2.80, compared to the adult population (34–44 years) or adolescent population of 15 years old, whose values are 14.29 ± 3.86 and 3.73 ± 2.11, respectively [ 39 ].
Despite this situation, and the greater risk of developing oral pathologies as mentioned above, the number of complications and problems that can occur in this population group due to deficient oral health, there are no specific programmes dedicated to the prevention or promotion of oral health in the older population in Spain. In the published scientific literature, we only found one study dedicated to the development of a geriatric dental care programme (PADGE, in its Spanish acronym) [ 40 ] developed in the Public University of Navarra (Spain).
However, as in many cases the proposed programme remained just a proposal, and to this day remains to be implemented even at a regional level, and this in a Autonomous Community regarded as being a leader in preventive oral health programs. It seems that neither governmental nor local authorities consider worthwhile the logistic and economic effort that such a programme would involve. Population aging and the poor dental state of many people over 65 years of age, accompanied by a lack of specific studies and the quality of those that exist (the studies included in our systematic review had a level of evidence of 3 and grade of recommendation D, according to the SIGN criteria) on long term stays in hospitals and other institutions, together with the lack of protocols for promoting good oral hygiene and health care in nursing homes and hospitals, underline the importance of this line of study in the future. For this reason, and due to the lack of time available to develop the present overview, we intend to expand the study by developing a universal protocol for dental care in institutionalized patients.
The oral health of older patients aged over 65 years, whether hospitalized for long periods of time or living in institutions, is deficient, and a homogeneity in methodology of studies are needed. Furthermore, theoretical and practical continuous training courses would be necessary with the aim of training caregivers in oral health techniques.
Availability of data and materials
Data and materials are available ordering to the corresponding author.
Abbreviations
World Health Organization
Decayed, Missing, filled teeth
Juan Antonio Ruiz Roca
Dora Martin Fuentes
Yolanda Martínez Beneyto
Francisco Jose Gomez Garcia
Scottish Intercollegiate Guidelines Network
Ramdomized Controlled Trials
Cerebral vascular accident
Amyotrophic Lateral Sclerosis
Intensive Care Unit
Significant Caries Index
Geriatric Dental Care Programme
Confidence Interval
Decayed, Missing, Filled Index
UN. United Nations. Global affairs. Aging..2018 http://www.un.org/en/sections/issues-depth/ageing/ . Accessed 15 January 2019.
INE a: Statistics National Institute. Demography and population. Population figures and demographic censuses 2018 http://www.ine.es/prensa/cp_e2018_p.pdf Accessed 20 March 2019.
INE b: Statistics National Institute. Demography and population. Population figures and demographic censuses. Population projections. Latest data.2018 https://www.ine.es/prensa/pp_2018_2068.pdf . Accessed 25 April 2019.
WHO. World Health Organization. World report on aging and health. 2015. https://www.who.int/ageing/publications/world-report-2015/es/ . Accessed 2 February 2019.
Acevedo Alcaraz E, Alcaraz Baños M, Benito Martínez J, Robert Muir B, Navalon VC. Situation of our institutionalized elderly in residences and needs for their social integration. Azarbe Revista Internacional de Trabajo Social y Bienestar. 2014;3:279–82.
Google Scholar
INE c: Statistics National Institute. Demography and population. Population figures and demographic censuses. Population and housing censuses. Results. Population characteristics in groups. 2018. https://www.ine.es/prensa/np777.pdf . Accessed 24 April 2019.
Peltola P, Vehkalahti MM, Wuolijoki-Saaristo K. Oral health and treatment needs of the long-term hospitalised elderly. Gerodontology. 2004;21:93–9.
Article Google Scholar
Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol. 2016;72:153–75.
Poisson P, Laffond T, Campos S, Dupuis V, Bourdel-Marchasson I. Relationships between oral health, dysphagia and undernutrition in hospitalised elderly patients. Gerodontology. 2016;33:161–8.
Chróinín D, Montalto A, Jahromi S, Ingham N, Beveridge A. Foltyn POral Health Status Is Associated with Common Medical Comorbidities in Older Hospital Inpatients. J Am Geriatr Soc. 2016;64(8):1696–700.
Coker E, Ploeg J, Kaasalainen S, Carter N. Nurses' oral hygiene care practices with hospitalised older adults in postacute settings. Int J Older People Nurs. 2017;12:1–12.
Ngo DYJ, Thomson WM, Subramaniam M, Abdin E, Ang KY. The oral health of long-term psychiatric inpatients in Singapore. Psychiatry Res. 2018;266:206–11.
Liao YM, Tsai JR, Chou FH. The effectiveness of an oral health care program for preventing ventilator-associated pneumonia. Nurs Crit Care. 2015;20(2):89–97.
Górska R, Dembowska E, Konopka TP, Wysokinska-Miszczuk J, Pietruska M, Ganowicz E. Correlation between the state of periodontal tissues and selected risk factors for periodontitis and myocardial infarction. Adv Clin Exp Med. 2017;26(3):505–14.
Bilder L, Yavnai N, Zini A. Oral health status among long-term hospitalized adults: a cross sectional study. Peer J. 2014;2:E423.
Gibney JM, Wright C, Sharma A, D'Souza M, Naganathan V. The oral health status of older patients in acute care on admission and Day 7 in two Australian hospitals. Age Ageging. 2017;46:852–6.
Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Commun Dentistry Oral Epidemiol. 2005;33(2):81.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA GROUP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–6.
Article CAS Google Scholar
Gerritsen P, Cune M, van der Bilt A, Abbink J, de Putter C. Effects of integrated dental care on oral treatment needs in residents of nursing homes older than 70 years. Spec Care Dentist. 2015;35:132–7.
Chen SC, Weng LC, Tsai SC, Wang SM, Han HM. Effectiveness of Oral Rinsing Solutions on Mucus, Odor, and Plaque in the Hospitalized Elderly in Taiwan. Clin Nurs Res. 2017;1:1054773817744151.
Nakayama R, Nishiyama A, Matsuda C, Nakayama Y, Hakuta C, Shimada M. Oral health status of hospitalized amyotrophic lateral sclerosis patients: a single-centre observational study. Acta Odontol Scand. 2018;76:294–8.
Murray J, Scholten I. An oral hygiene protocol improves oral health for patients in inpatient stroke rehabilitation. Gerodontology. 2018;35:18–24.
Shimazaki Y, Soh I, Saito T, Yamashita Y, Koga T, Miyazaki H, et al. Influence of dentition status on physical disability, mental impairment and mortality in institutionalized elderly people. J Dent Res. 2001;80:340–5.
Sousa LLA. Silva Filho WLSe, Mendes RF, Moita Neto JM, Prado Junior RR. Oral health of patients under short hospitalization period: observational study. J Clin Periodontol. 2014;41:558–63.
Chu KY, Yang NP, Chou P, Chiu HJ, Chi LY. Comparison of oral health between inpatients with schizophrenia and disabled people or the general population. J Formos Med Assoc. 2012;111:214–9.
Kossioni AE, Kossionis GE, Polychronopoulou A. Oral health status of elderly hospitalised psychiatric patients. Gerodontology. 2012;29(4):272–83.
Gibney JM, Wright FA, D'Souza M, Naganathan V. Improving the oral health of older people in hospital. Australas J Ageing. 2019;38:33–8.
Kuroda Y. Factors associated with the level of oral intake in hospitalized older adults with dysphagia: The importance of mental activity. Clin Nutr ESPEN. 2016;13:e52–4.
Leder SB, Suiter DM, Agogo GO, Cooney LM Jr. An Epidemiologic Study on Ageing and Dysphagia in the Acute Care Geriatric-Hospitalized Population: A Replication and Continuation Study. Dysphagia. 2016;31(5):619–25.
Maeda K, Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30(1):80–7.
Martín A, Ortega O, Roca M, Arús M, Clavé P. Effect of A Minimal-Massive Intervention in Hospitalized Older Patients with Oropharyngeal Dysphagia: A Proof of Concept Study. J Nutr Health Aging. 2018;22:739–47.
Frenkel H, Harveys I, Needs K. Oral health care education and its effect on caregivers’ knowledge and attitudes: a randomized controlled trial. Community Dent Oral Epidemiol. 2002;30:91–100.
Shay K. Oral neglect in the institutionalized elderly part 2: The role of the dentist and the standard of care. Spec Care Dentist. 1990;10:200–3.
Zimmerman S, Austin S, Cohen L, Reed D, Poole P, Ward K, et al. Readily Identifiable Risk Factors of Nursing Home Residents’ Oral Hygiene: Dementia, Hospice, and Length of Stay. J AM Geriatr Soc. 2017;65:2516–21.
Gammack J, Pulisetty S. Nursing education and improvement in oral care delivery in long term care. JAMDA. 2009;10:658–61.
PubMed Google Scholar
Fitzpatrick J. Oral health care needs of dependent older people: Responsibilities of nurses and care staff. J Adv Nurs. 2000;32:1325–32.
White book. Populaton survey. Oral Health in Spain 2015. Consejo General de Colegios de Dentistas de España. Procter & Gamble España, SA. 2016. https://www.consejodentistas.es/comunicacion/actualidad-del-consejo/publicaciones-del-consejo/libros-del-consejo/item/1204-libro-blanco-2015.html . Accessed 2 April 2019.
Bravo Pérez M, Almerich Silla JM, Ausina Márquez V, Avilés Gutiérrez P, Blanco González JM, Canorea Díaz E, et al. Oral Health survey in Spain 2015. RCOE. 2016;21:8–48.
Fernández Pan A. Gerodontological dental assistance planning-PADGE [TFM en Internet]. [Navarra]: University of Navarra. 2014. http://academicae.unavarra.es/bitstream/handle/2454/14023/Adriana%20Fernández%20Pan.pdf?sequence=1 . Accessed 19 April 2019.
Download references
Acknowledgements
“not applicable”.
Authors´ contributions
J.A.R.R. and D.M.F. conducted the systematic literature search and carried out study selection. In cases of disagreement, the two authors consulted Y.M.B., who helped them reach a consensus. J.A.R.R. and F.J.G.G. extracted data from the articles included in the review and assessed the quality of the studies. J.A.R.R., Y.M.B. and F.J.G.G wrote the main text. J.A.R.R.,and D.M.F. prepared the figures and tables. All authors have reviewed the manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Gerodontology, Faculty of Medicine-Dentistry, University of Murcia, Avda Marqués de los Velez s/n. Clínica Odontológica Universitaria, Murcia, 30008, Spain
Juan Antonio Ruiz-Roca
Department of Gerodontology, Research Investigations External, Faculty of Medicine-Dentistry, University of Murcia, Murcia, Spain
Dora Martín Fuentes
Department of Oral Medecine, Faculty of Medicine-Dentistry, University of Murcia, Murcia, Spain
Francisco J. Gómez García
Department of Preventive and Community Dentistry, Faculty of Medicine-Dentistry, University of Murcia, Murcia, Spain
Yolanda Martínez-Beneyto
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yolanda Martínez-Beneyto .
Ethics declarations
Ethics approval and consent to participate.
“not applicable, there is not data from patients”.
Consent for publication
Not applicable.
Competing interests
We declare that we have no proprietary, financial, professional or other personal interest of any nature or kind, in any product, service and/or company that could be construed as influencing the position presented in the manuscript entitled “Oral Status of older people in medium to long-stay health and social care setting: a systematic review”,
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ruiz-Roca, J.A., Fuentes, D.M., Gómez García, F.J. et al. Oral status of older people in medium to long-stay health and social care setting: a systematic review. BMC Geriatr 21 , 363 (2021). https://doi.org/10.1186/s12877-021-02302-x
Download citation
Received : 24 November 2020
Accepted : 26 May 2021
Published : 14 June 2021
DOI : https://doi.org/10.1186/s12877-021-02302-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- “Older inpatients”
- “older hospitalized patients”
- “long term hospitalization”
- “long term inpatients”
- “Oral health”
- “Oral status”
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Countering the poor oral health of people with intellectual and developmental disability: a scoping literature review
Affiliations.
- 1 School of Nursing and Midwifery, Centre for Oral Health Outcomes & Research Translation (COHORT), Western Sydney University, Hawkesbury Campus, Locked Bag 3, Richmond, NSW, 2753, Australia. [email protected].
- 2 School of Nursing and Midwifery, Western Sydney University, Hawkesbury Campus, Locked Bag 3, Richmond, NSW, 2753, Australia.
- 3 COHORT, Western Sydney University, South Western Sydney Local Health District, Ingham Institute Applied Medical Research, Penrith, Australia.
- 4 Bangalore Baptist Hospital, Bellary Road, Hebbal, Bangalore, Karnataka State, 560024, India.
- 5 School of Dentistry, Faculty of Medicine and Health, University of Sydney, Sydney, Australia.
- 6 Translational Health Research Institute, Campbelltown, NSW, 2560, Australia.
- PMID: 31729967
- PMCID: PMC6858643
- DOI: 10.1186/s12889-019-7863-1
Background: People with intellectual and developmental disability (IDD) have poor oral health and need support to maintain optimal oral health outcomes. Little is known about how, when and where to intervene for this population. Thus the aim of this review was to summarise the existing evidence surrounding improving oral health outcomes for people with IDD.
Methods: A scoping literature review was conducted focusing on 'oral health' and 'intellectual disability'. Systematic searches of five electronic databases were conducted in line with the study aims and two authors independently examined all records for relevance, with consensus achieved by a third author.
Results: A small number of approaches and interventions were identified to support people with IDD to independently maintain optimal oral hygiene. Identified studies highlighted that caregivers play a vital role in the provision of oral health support, emphasising the effectiveness of educational interventions for caregivers. However, there was uncertainty regarding the efficacy of specific tooth brushing interventions for people with IDD. In cases of more severe IDD and/or dental-related behavioural problems, dental treatment under general anaesthesia was often both a necessary and effective method of oral health care provision. The findings also identified outreach and exclusive oral health services as successful strategies for increasing the limited access of people with IDD to oral care services.
Conclusions: A uniform approach to supporting oral health for people with IDD is unlikely to succeed. A system-based approach is needed to address the diverse needs of the population of people with IDD, their caregivers and service context. Further high quality evidence is required to confirm these findings.
Keywords: Dental interventions; Gender; Health disparities; Intellectual disability; Nursing; Oral health.
Publication types
- Developmental Disabilities*
- Health Status*
- Intellectual Disability*
- Oral Health*
- Oral Hygiene
- Toothbrushing
Advertisement
Aspiration Pneumonia and Oral Health
- Published: 02 May 2023
- Volume 11 , pages 161–165, ( 2023 )
Cite this article

- Maryana Hamad 1 &
- Nogah Nativ-Zeltzer 1
342 Accesses
Explore all metrics
Purpose of Review
The goal of this review is to summarize the literature regarding the association between poor oral health and aspiration pneumonia as well as the effect of oral care interventions on the incidence of aspiration pneumonia in older adults.
Recent Findings
Accumulating evidence suggests that poor oral hygiene and dental status are primary risk factors for the development of aspiration pneumonia. Although some evidence supports the potential of oral care intervention to prevent pneumonia and reduce its associated mortality, the limited number of high-quality studies precludes reaching robust conclusions regarding its efficacy and establishing clinical guidelines.
There is a strong association between oral hygiene and aspiration pneumonia risk. The review highlights the need for further randomized controlled trials examining the efficacy of individual oral care interventions for aspiration pneumonia prevention.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Evidence summary: the relationship between oral health and pulmonary disease

Effect of poor oral health status at hospital admission on in-hospital outcomes of older patients with aspiration pneumonia

Impact of oral health status on oral intake ability prognosis after pneumonia in older patients: a retrospective cohort study
Papers of particular interest, published recently, have been highlighted as: • of importance •• of major importance.
Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primer. 2021;7(1):1–28. https://doi.org/10.1038/s41572-021-00259-0 .
Article Google Scholar
DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30(1):40–8. https://doi.org/10.1016/j.jcrc.2014.07.011 .
Article PubMed Google Scholar
Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296–302. https://doi.org/10.1111/j.1532-5415.2005.00608.x .
Article CAS PubMed Google Scholar
Regunath H, Oba Y. Community-acquired pneumonia. In: StatPearls. StatPearls Publishing; 2022. Accessed January 7, 2023. http://www.ncbi.nlm.nih.gov/books/NBK430749/ .
Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71. https://doi.org/10.1056/nejm200103013440908 .
Neill S, Dean N. Aspiration pneumonia and pneumonitis: a spectrum of infectious/noninfectious diseases affecting the lung. Curr Opin Infect Dis. 2019;32(2):152–7. https://doi.org/10.1097/QCO.0000000000000524 .
Kosutova P, Mikolka P. Aspiration syndromes and associated lung injury: incidence, pathophysiology and management. Physiol Res. 2021;70(Suppl 4):S567–83. https://doi.org/10.33549/physiolres.934767 .
Article CAS PubMed PubMed Central Google Scholar
Mason CM. Bacterial translocation in the pathogenesis of pneumonia. Clin Pulm Med. 1994;1(4):215.
Quinton LJ, Walkey AJ, Mizgerd JP. Integrative physiology of pneumonia. Physiol Rev. 2018;98(3):1417–64. https://doi.org/10.1152/physrev.00032.2017 .
Grousd JA, Rich HE, Alcorn JF. Host-pathogen interactions in gram-positive bacterial pneumonia. Clin Microbiol Rev. 2019;32(3):e00107–e118. https://doi.org/10.1128/CMR.00107-18 .
Niederman MS, Cilloniz C. Aspiration pneumonia. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2022;35 Suppl 1(Suppl 1):73–77. https://doi.org/10.37201/req/s01.17.2022 .
van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344–54. https://doi.org/10.1016/j.jamda.2010.12.099 .
Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13(2):69–81. https://doi.org/10.1007/pl00009559 .
Nativ-Zeltzer N, Nachalon Y, Kaufman MW, et al. Predictors of aspiration pneumonia and mortality in patients with dysphagia. The Laryngoscope. n/a(n/a). https://doi.org/10.1002/lary.29770 .
Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20(6):740–5. https://doi.org/10.1097/00003246-199206000-00007 .
Abe S, Ishihara K, Okuda K. Prevalence of potential respiratory pathogens in the mouths of elderly patients and effects of professional oral care. Arch Gerontol Geriatr. 2001;32(1):45–55. https://doi.org/10.1016/S0167-4943(00)00091-1 .
Bartlett JG, Finegold SM. Anaerobic infections of the lung and pleural space. Am Rev Respir Dis. 1974;110(1):56–77. https://doi.org/10.1164/arrd.1974.110.1.56 .
Imsand M, Janssens JP, Auckenthaler R, Mojon P, Budtz-Jørgensen E. Bronchopneumonia and oral health in hospitalized older patients. A pilot study Gerodontology. 2002;19(2):66–72. https://doi.org/10.1111/j.1741-2358.2002.00066.x .
Quagliarello V, Ginter S, Han L, Van Ness P, Allore H, Tinetti M. Modifiable risk factors for nursing home-acquired pneumonia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40(1):1–6. https://doi.org/10.1086/426023 .
Abe S, Ishihara K, Adachi M, Okuda K. Tongue-coating as risk indicator for aspiration pneumonia in edentate elderly. Arch Gerontol Geriatr. 2008;47(2):267–75. https://doi.org/10.1016/j.archger.2007.08.005 .
Pace CC, McCullough GH. The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–22. https://doi.org/10.1007/s00455-010-9298-9 .
Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–63. https://doi.org/10.1046/j.1532-5415.2001.49113.x .
Awano S, Ansai T, Takata Y, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87(4):334–9. https://doi.org/10.1177/154405910808700418 .
Bassim CW, Gibson G, Ward T, Paphides BM, Denucci DJ. Modification of the risk of mortality from pneumonia with oral hygiene care. J Am Geriatr Soc. 2008;56(9):1601–7. https://doi.org/10.1111/j.1532-5415.2008.01825.x .
Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465–82. https://doi.org/10.1902/jop.2006.060010 .
• Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50(3):430–433. https://doi.org/10.1046/j.1532-5415.2002.50106.x . Yoneyama et al. conducted a randomized controlled trial of 417 nursing home patients. Results of the study indicated that pneumonia, fever days, and death from pneumonia decreased significantly in patients who received oral care compared to those that did not receive any oral care intervention .
Adachi M, Ishihara K, Abe S, Okuda K, Ishikawa T. Effect of professional oral health care on the elderly living in nursing homes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(2):191–5. https://doi.org/10.1067/moe.2002.123493 .
Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc. 2008;56(11):2124–30. https://doi.org/10.1111/j.1532-5415.2008.01926.x .
Hollaar VRY, van der Putten GJ, van der Maarel-Wierink CD, Bronkhorst EM, de Swart BJM, Creugers NHJ. The effect of a daily application of a 0.05% chlorhexidine oral rinse solution on the incidence of aspiration pneumonia in nursing home residents: a multicenter study. BMC Geriatr. 2017;17:128. https://doi.org/10.1186/s12877-017-0519-z .
Sørensen RT, Rasmussen RS, Overgaard K, Lerche A, Johansen AM, Lindhardt T. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. J Neurosci Nurs J Am Assoc Neurosci Nurses. 2013;45(3):139–46. https://doi.org/10.1097/JNN.0b013e31828a412c .
Higashiguchi T, Ohara H, Kamakura Y, et al. Efficacy of a new post-mouthwash intervention (wiping plus oral nutritional supplements) for preventing aspiration pneumonia in elderly people: a multicenter, randomized, comparative trial. Ann Nutr Metab. 2017;71(3–4):253–60. https://doi.org/10.1159/000485044 .
Maeda K, Akagi J. Oral care may reduce pneumonia in the tube-fed elderly: a preliminary study. Dysphagia. 2014;29(5):616–21. https://doi.org/10.1007/s00455-014-9553-6 .
•• Kaneoka A, Pisegna JM, Miloro KV, et al. Prevention of healthcare-associated pneumonia with oral care in individuals without mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Infect Control Hosp Epidemiol. 2015;36(8):899–906. https://doi.org/10.1017/ice.2015.77 . This meta-analysis aimed to assess the effect of oral care on the incidence of healthcare-associated pneumonia in nonventilated patients. While the results of the review suggest a prophylactic effect of oral care, the authors note the high risk of bias in the reviewed studies .
Liu C, Cao Y, Lin J, et al. Oral care measures for preventing nursing home-acquired pneumonia. Cochrane Database Syst Rev. 2018;9:Cd012416. https://doi.org/10.1002/14651858.CD012416.pub2 .
• Khadka S, Khan S, King A, Goldberg LR, Crocombe L, Bettiol S. Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: a systematic review. Age Ageing. 2021;50(1):81–87. https://doi.org/10.1093/ageing/afaa102 . This systematic review identified pathogenic microorganisms which were associated with aspiration pneumonia in adults in residential aged care and concluded that professional oral hygiene was useful in reducing the risk of aspiration pneumonia .
El-Solh AA. Association between pneumonia and oral care in nursing home residents. Lung. 2011;189(3):173–80. https://doi.org/10.1007/s00408-011-9297-0 .
Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94(3 Suppl):14S–16S. https://doi.org/10.1177/0022034514552494 .
Article PubMed PubMed Central Google Scholar
Koeman M, van der Ven AJAM, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173(12):1348–55. https://doi.org/10.1164/rccm.200505-820OC .
Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2007;35(2):595–602. https://doi.org/10.1097/01.CCM.0000253395.70708.AC .
Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care Lond Engl. 2006;10(1):R35. https://doi.org/10.1186/cc4837 .
Hellyer TP, Ewan V, Wilson P, Simpson AJ. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc. 2016;17(3):238–43. https://doi.org/10.1177/1751143716644461 .
Download references
Author information
Authors and affiliations.
Department of Communication Disorders, Sackler Faculty of Medicine, Tel Aviv University, P.O. Box 39040, 6997801, Tel Aviv, Israel
Maryana Hamad & Nogah Nativ-Zeltzer
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nogah Nativ-Zeltzer .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Hamad, M., Nativ-Zeltzer, N. Aspiration Pneumonia and Oral Health. Curr Otorhinolaryngol Rep 11 , 161–165 (2023). https://doi.org/10.1007/s40136-023-00455-4
Download citation
Accepted : 17 April 2023
Published : 02 May 2023
Issue Date : June 2023
DOI : https://doi.org/10.1007/s40136-023-00455-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aspiration pneumonia
- Oral hygiene
- Pneumonia prevention
- Find a journal
- Publish with us
- Track your research

5 serious side effects of poor oral hygiene on your health
D o you not brush your teeth regularly or twice a day? Brushing your teeth twice a day can help avoid buildup of plaque and tartar. When you don't do the basics, you put your oral health in jeopardy. Did you know oral hygiene not only helps clean your mouth and teeth but keeps your overall health in check? From Alzheimer's to diabetes to liver disease and heart conditions, the implications of neglecting oral care can be far-reaching. However, these risks can be mitigated by taking simple everyday steps to prioritize one’s oral hygiene. Know the side effects of poor oral hygiene and what you can do to avoid them.
How does poor oral hygiene affect your health?
If you have been neglecting your oral health, you should know how it affects your health, as explained by Dentist Dr Rajiv Verma .
1. Alzheimer’s disease
Alzheimer's disease is a progressive brain disorder that affects your memory, behaviour, and thinking. It is the most common type of dementia that can get worse with age. A study published in the journal Microorganisms found a link between oral diseases and Alzheimer’s disease, specifically through a protein called ‘amyloid-beta’. "This protein is produced by one’s body when there is an infection. People who suffer from Alzheimer’s have this protein present in high quantities in their brain. Since oral diseases are also driven by infections, the ‘amyloid-beta’ protein is often found around the outside of infected teeth and gums. The protein may then filter into one’s blood stream, where it can potentially be transported to the brain, hence the potential link between poor oral health and Alzheimer’s," explains Dr Verma.
2. Diabetes
People with diabetes are at a higher risk of developing gum disease? However, the link works both ways. Gum disease and infection can increase your blood sugar levels, suggesting that if one has developed, there’s an increased risk of the other developing too. So, make sure you are following a proper oral health routine to avoid complications.
3. Liver cancer and liver disease
Poor oral health is linked to a 75 percent increase in liver cancer risk, found a study published in the journal SAGE The liver contributes to the removal of bacteria, so when it is affected by diseases, its function will decline, and bacteria will survive for longer and potentially cause more harm. Some bacteria have been found to originate in the oral cavity, explains the expert.
4. Lung conditions
Your mouth contains a lot of bacterial plaque, and when you do not follow a proper oral hygiene, it gets inhaled and spread the bacteria to the lungs. This can cause infection, which can aggravate existing lung conditions. People with aspiration pneumonia, a condition which occurs when food or liquid is breathed into the lungs, are at a higher risk. It is difficult for a dentist to diagnose whether a patient has a lung problem, so it is best to look out for symptoms of any lung disease , advises the dentist.
5. Heart disease and strokes
People who suffer from periodontal disease are more likely to be diagnosed with a heart disease, found a study published in the Journal of Indian Society of Periodontology . Several other studies have also found a link between inflammatory markers (signs of body-wide inflammation) found in the bloodstream of those with chronic gum disease and those who have suffered from strokes and heart disease.
How to maintain better oral hygiene?
Maintaining a healthy and balanced oral microbiome is essential for good oral health. Here are some simple tips by the expert that you can follow:
1. Eat a diverse range of foods, particularly legumes, beans, and fruit.
2. Consume a diet rich in fibre and foods that promote the growth of good bacteria.
3. Include fermented foods such as yogurt and kefir in your diet, which contain healthy bacteria and can reduce the prevalence of pathogenic bacteria.
4. Consume more prebiotic foods like fibre-rich foods that stimulate the growth of healthy bacteria. These include artichokes, bananas, asparagus, oats, and apples.
5. A high-sugar diet can encourage the growth of acid-loving bacteria like Streptococcus Mutans, which contributes to caries and periodontal (gum) disease and inhibits good bacteria. So, avoid eating sugary foods.
4. Avoid fizzy and diet drinks, as these can contribute to dental erosion and cavities by affecting the balance of microorganisms, and upset the good bacteria.
5. Choose a microbiome-boosting toothpaste for good oral hygiene.
6. Brush your teeth twice daily using a sonic toothbrush and floss daily.
7. Saltwater is a good natural remedy, as it kills bad bacteria and promotes good bacteria.
By understanding the hidden risks associated with poor oral hygiene and implementing the steps outlined above, individuals can significantly improve their oral health and reduce the risk of serious health conditions.


COMMENTS
Oral hygiene is related to every aspect of our lives but is often taken for granted. Our mouth is the window to the health of our bodies. It can show signs of nutritional deficiencies or general infection. Whether you are 90 or 9 years of age, oral hygiene is important. Oral hygiene is a vital aspect of everyone including university students ...
There is a century-old conflict on whether dental caries is caused by poor oral hygiene or poorly formed teeth (ie, teeth with dental defects). ... The primary conclusion of this systematic review, that oral hygiene has no impact on dental caries rates, is strengthened by other independent lines of evidence. ... Citing Literature. Volume 35 ...
Poor oral hygiene in this regard, has been shown to be associated with an increased risk for infective endocarditis . Gram ... Schols JMGA, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9.
3. Poor oral hygiene and alcohol: Alcohol is a known carcinogen involved in causation of oral cancer [51, 52]. A Chinese case-control study found that subjects who consumed alcohol and had POH (represented by inadequate dentition) had 5 times more risk of having oral cancer (OR = 9.1, 95% CI = 4.419) than those. -.
Request PDF | Role of Poor Oral Hygiene in Causation of Oral Cancer—a Review of Literature | Oral squamous cell carcinomas (OSCC) are among the commonest cancers in South East Asia and more so ...
Within the themes identified by this review, the papers examining oral hygiene interventions in ventilated patients were of particularly strong quality, 14,15,16,17,18,19,20,21,22,23,24,25 with ...
This systematic review aims to outline the 4-dimensional (4-D) impact of OHRQoL within patient populations routinely treated by dental hygiene and/or dental therapy providers, as there is limited literature present for these oral health care professionals.
Oral squamous cell carcinomas (OSCC) are among the commonest cancers in South East Asia and more so in the Indian subcontinent. The role of tobacco and alcohol in the causation of these cancers is well-documented. Poor oral hygiene (POH) is often seen to co-exist in patients with OSCC. However, the role of poor oral hygiene in the etio-pathogenesis of these cancers is controversial. We decided ...
Poor oral hygiene (POH) is often seen to co-exist in patients with OSCC. However, the role of poor oral hygiene in the etio-pathogenesis of these cancers is controversial. ... During our review of literature, we found that the majority of studies, instead of using these indices, utilized various other parameters as a measure of POH, like tooth ...
The evidence for oral hygiene care practices, outcomes of nurse-administered oral care and nursing's role in influencing the oral health literacy of patients require further study. References Adams R. ( 1996 ) Qualified nurses lack adequate knowledge related to oral health, resulting in inadequate oral care of patients on medical wards .
This review will include the effects of poor oral hygiene on the esophagus, stomach, small and large bowel, liver and pancreas. Methods A systemic review of the literature was performed using the Medline database. The search was performed using the terms " oral hygiene" or "periodontal" or "periodontitis" or "gingivitis" and ...
Many studies have pointed to the difficulty posed by applying protocols of oral hygiene in institutions such as old people's homes due to the little training received by care workers concerning protocols of oral hygiene, the oral needs of older patients, and the risks and negative consequences of poor oral health [15, 16], as well as on the ...
Background: People with intellectual and developmental disability (IDD) have poor oral health and need support to maintain optimal oral health outcomes. Little is known about how, when and where to intervene for this population. Thus the aim of this review was to summarise the existing evidence surrounding improving oral health outcomes for people with IDD.
The practice of personal hygiene of the mouth. It includes the maintenance of oral cleanliness, tissue tone, and general preservation of oral health. | Explore the latest full-text research PDFs ...
A LITERATURE REVIEW ON ORAL HEALTH IN PRESCHOOLERS. ISBN: 978-1-927303-54-2 HPA Report No. - RSC0210 Prepared for the Health Promotion Agency by: Sarah Dallas Dr Judy Li Kerri Kruse Dr Karen McBride-Henry HEALTH PROMOTION AGENCY PO Box 2142 Wellington 6140 New Zealand www.hpa.org.nz May 2015. 1. ACKNOWLEDGEMENT.
This study aims at examining the relationship between depression and oral health problems such as oral conditions, access to dental care, and oral hygiene measures. Methods: A cross-sectional study using a secondary data analysis of 9,693 participants from the 2017 to March 2020 prepandemic National Health and Nutrition Examination Survey (NHANES).
Purpose of Review The goal of this review is to summarize the literature regarding the association between poor oral health and aspiration pneumonia as well as the effect of oral care interventions on the incidence of aspiration pneumonia in older adults. Recent Findings Accumulating evidence suggests that poor oral hygiene and dental status are primary risk factors for the development of ...
4. Lung conditions. Your mouth contains a lot of bacterial plaque, and when you do not follow a proper oral hygiene, it gets inhaled and spread the bacteria to the lungs. This can cause infection ...
Current conceptualizations of dental caries etiology center primarily on the local role of sugar, starch, or other fermentable carbohydrates on tooth enamel demineralization—a well-established and empirically supported mechanism. However, in addition to this mechanism, studies dating back to the early 1900s point to an important systemic role of diet and nutrition, particularly from pasture ...