
- Previous Article
- Next Article

Research Design and Methods
Article information, literature review of type 2 diabetes management and health literacy.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Rulla Alsaedi , Kimberly McKeirnan; Literature Review of Type 2 Diabetes Management and Health Literacy. Diabetes Spectr 1 November 2021; 34 (4): 399–406. https://doi.org/10.2337/ds21-0014
Download citation file:
- Ris (Zotero)
- Reference Manager
The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges can be even more profound among minority populations and non-English speakers in the United States.
A literature search and standard data extraction were performed using PubMed, Medline, and EMBASE databases. A total of 1,914 articles were identified, of which 1,858 were excluded based on the inclusion criteria, and 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 articles were reviewed in detail.
Patients, including ethnic minorities and non-English speakers, who are engaged in diabetes education and health literacy improvement initiatives and ongoing follow-up showed significant improvement in A1C, medication adherence, medication knowledge, and treatment satisfaction. Clinicians considering implementing new interventions to address diabetes care for patients with low health literacy can use culturally tailored approaches, consider ways to create materials for different learning styles and in different languages, engage community health workers and pharmacists to help with patient education, use patient-centered medication labels, and engage instructors who share cultural and linguistic similarities with patients to provide educational sessions.
This literature review identified a variety of interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy.
Diabetes is the seventh leading cause of death in the United States, and 30.3 million Americans, or 9.4% of the U.S. population, are living with diabetes ( 1 , 2 ). For successful management of a complicated condition such as diabetes, health literacy may play an important role. Low health literacy is a well-documented barrier to diabetes management and can lead to poor management of medical conditions, low engagement with health care providers (HCPs), increased hospitalizations, and, consequently, higher health care costs ( 3 – 5 ).
The Healthy People 2010 report ( 6 ) defined health literacy as the “degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” Diabetes health literacy also encompasses a wide range of skills, including basic knowledge of the disease state, self-efficacy, glycemic control, and self-care behaviors, which are all important components of diabetes management ( 3 – 5 , 7 ). According to the Institute of Medicine’s Committee on Health Literacy, patients with poor health literacy are twice as likely to have poor glycemic control and were found to be twice as likely to be hospitalized as those with adequate health literacy ( 8 ). Associations between health literacy and health outcomes have been reported in many studies, the first of which was conducted in 1995 in two public hospitals and found that many patients had inadequate health literacy and could not perform the basic reading tasks necessary to understand their treatments and diagnoses ( 9 ).
Evaluation of health literacy is vital to the management and understanding of diabetes. Several tools for assessing health literacy have been evaluated, and the choice of which to use depends on the length of the patient encounter and the desired depth of the assessment. One widely used literacy assessment tool, the Test of Functional Health Literacy in Adults (TOFHLA), consists of 36 comprehension questions and four numeric calculations ( 10 ). Additional tools that assess patients’ reading ability include the Rapid Estimate of Adult Literacy in Medicine (REALM) and the Literacy Assessment for Diabetes. Tests that assess diabetes numeracy skills include the Diabetes Numeracy Test, the Newest Vital Sign (NVS), and the Single-Item Literacy Screener (SILS) ( 11 ).
Rates of both diabetes and low health literacy are higher in populations from low socioeconomic backgrounds ( 5 , 7 , 12 ). People living in disadvantaged communities face many barriers when seeking health care, including inconsistent housing, lack of transportation, financial difficulties, differing cultural beliefs about health care, and mistrust of the medical professions ( 13 , 14 ). People with high rates of medical mistrust tend to be less engaged in their care and to have poor communication with HCPs, which is another factor HCPs need to address when working with their patients with diabetes ( 15 ).
The cost of medical care for people with diabetes was $327 billion in 2017, a 26% increase since 2012 ( 1 , 16 ). Many of these medical expenditures are related to hospitalization and inpatient care, which accounts for 30% of total medical costs for people with diabetes ( 16 ).
People with diabetes also may neglect self-management tasks for various reasons, including low health literacy, lack of diabetes knowledge, and mistrust between patients and HCPs ( 7 , 15 ).
These challenges can be even more pronounced in vulnerable populations because of language barriers and patient-provider mistrust ( 17 – 19 ). Rates of diabetes are higher among racial and ethnic minority groups; 15.1% of American Indians and Alaskan Natives, 12.7% of Non-Hispanic Blacks, 12.1% of Hispanics, and 8% of Asian Americans have diagnosed diabetes, compared with 7.4% of non-Hispanic Whites ( 1 ). Additionally, patient-provider relationship deficits can be attributed to challenges with communication, including HCPs’ lack of attention to speaking slowly and clearly and checking for patients’ understanding when providing education or gathering information from people who speak English as a second language ( 15 ). White et al. ( 15 ) demonstrated that patients with higher provider mistrust felt that their provider’s communication style was less interpersonal and did not feel welcome as part of the decision-making process.
To the authors’ knowledge, there is no current literature review evaluating interventions focused on health literacy and diabetes management. There is a pressing need for such a comprehensive review to provide a framework for future intervention design. The objective of this literature review was to gather and summarize studies of health literacy–based diabetes management interventions and their effects on overall diabetes management. Medication adherence and glycemic control were considered secondary outcomes.
Search Strategy
A literature review was conducted using the PubMed, Medline, and EMBASE databases. Search criteria included articles published between 2015 and 2020 to identify the most recent studies on this topic. The search included the phrases “diabetes” and “health literacy” to specifically focus on health literacy and diabetes management interventions and was limited to original research conducted in humans and published in English within the defined 5-year period. Search results were exported to Microsoft Excel for evaluation.
Study Selection
Initial screening of the articles’ abstracts was conducted using the selection criteria to determine which articles to include or exclude ( Figure 1 ). The initial search results were reviewed for the following inclusion criteria: original research (clinical trials, cohort studies, and cross-sectional studies) conducted in human subjects with type 2 diabetes in the United States, and published in English between 2015 and 2020. Articles were considered to be relevant if diabetes was included as a medical condition in the study and an intervention was made to assess or improve health literacy. Studies involving type 1 diabetes or gestational diabetes and articles that were viewpoints, population surveys, commentaries, case reports, reviews, or reports of interventions conducted outside of the United States were excluded from further review. The criteria requiring articles to be from the past 5 years and from the United States were used because of the unique and quickly evolving nature of the U.S. health care system. Articles published more than 5 years ago or from other health care systems may have contributed information that was not applicable to or no longer relevant for HCPs in the United States. Articles were screened and reviewed independently by both authors. Disagreements were resolved through discussion to create the final list of articles for inclusion.

PRISMA diagram of the article selection process.
Data Extraction
A standard data extraction was performed for each included article to obtain information including author names, year of publication, journal, study design, type of intervention, primary outcome, tools used to assess health literacy or type 2 diabetes knowledge, and effects of intervention on overall diabetes management, glycemic control, and medication adherence.
A total of 1,914 articles were collected from a search of the PubMed, MEDLINE, and EMBASE databases, of which 1,858 were excluded based on the inclusion and exclusion criteria. Of the 56 articles that met criteria for abstract review, 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 studies identified various diabetes management interventions, including diabetes education tools such as electronic medication instructions and text message–based interventions, technology-based education videos, enhanced prescription labels, learner-based education materials, and culturally tailored interventions ( 15 , 20 – 28 ). Figure 1 shows the PRISMA diagram of the article selection process, and Table 1 summarizes the findings of the article reviews ( 15 , 20 – 28 ).
Findings of the Article Reviews (15,20–28)
SAHLSA, Short Assessment of Health Literacy for Spanish Adults.
Medical mistrust and poor communication are challenging variables in diabetes education. White et al. ( 15 ) examined the association between communication quality and medical mistrust in patients with type 2 diabetes. HCPs at five health department clinics received training in effective health communication and use of the PRIDE (Partnership to Improve Diabetes Education) toolkit in both English and Spanish, whereas control sites were only exposed to National Diabetes Education Program materials without training in effective communication. The study evaluated participant communication using several tools, including the Communication Assessment Tool (CAT), Interpersonal Processes of Care (IPC-18), and the Short Test of Functional Health Literacy in Adults (s-TOFHLA). The authors found that higher levels of mistrust were associated with lower CAT and IPC-18 scores.
Patients with type 2 diabetes are also likely to benefit from personalized education delivery tools such as patient-centered labeling (PCL) of prescription drugs, learning style–based education materials, and tailored text messages ( 24 , 25 , 27 ). Wolf et al. ( 27 ) investigated the use of PCL in patients with type 2 diabetes and found that patients with low health literacy who take medication two or more times per day have higher rates of proper medication use when using PCL (85.9 vs. 77.4%, P = 0.03). The objective of the PCL intervention was to make medication instructions and other information on the labels easier to read to improve medication use and adherence rates. The labels incorporated best-practice strategies introduced by the Institute of Medicine for the Universal Medication Schedule. These strategies prioritize medication information, use of larger font sizes, and increased white space. Of note, the benefits of PCL were largely seen with English speakers. Spanish speakers did not have substantial improvement in medication use or adherence, which could be attributed to language barriers ( 27 ).
Nelson et al. ( 25 ) analyzed patients’ engagement with an automated text message approach to supporting diabetes self-care activities in a 12-month randomized controlled trial (RCT) called REACH (Rapid Education/Encouragement and Communications for Health) ( 25 ). Messages were tailored based on patients’ medication adherence, the Information-Motivation-Behavioral Skills model of health behavior change, and self-care behaviors such as diet, exercise, and self-monitoring of blood glucose. Patients in this trial were native English speakers, so further research to evaluate the impact of the text message intervention in patients with limited English language skills is still needed. However, participants in the intervention group reported higher engagement with the text messages over the 12-month period ( 25 ).
Patients who receive educational materials based on their learning style also show significant improvement in their diabetes knowledge and health literacy. Koonce et al. ( 24 ) developed and evaluated educational materials based on patients’ learning style to improve health literacy in both English and Spanish languages. The materials were made available in multiple formats to target four different learning styles, including materials for visual learners, read/write learners, auditory learners, and kinesthetic learners. Spanish-language versions were also available. Researchers were primarily interested in measuring patients’ health literacy and knowledge of diabetes. The intervention group received materials in their preferred learning style and language, whereas the control group received standard of care education materials. The intervention group showed significant improvement in diabetes knowledge and health literacy, as indicated by Diabetes Knowledge Test (DKT) scores. More participants in the intervention group reported looking up information about their condition during week 2 of the intervention and showed an overall improvement in understanding symptoms of nerve damage and types of food used to treat hypoglycemic events. However, the study had limited enrollment of Spanish speakers, making the applicability of the results to Spanish-speaking patients highly variable.
Additionally, findings by Hofer et al. ( 22 ) suggest that patients with high A1C levels may benefit from interventions led by community health workers (CHWs) to bridge gaps in health literacy and equip patients with the tools to make health decisions. In this study, Hispanic and African American patients with low health literacy and diabetes not controlled by oral therapy benefited from education sessions led by CHWs. The CHWs led culturally tailored support groups to compare the effects of educational materials provided in an electronic format (via iDecide) and printed format on medication adherence and self-efficacy. The study found increased adherence with both formats, and women, specifically, had a significant increase in medication adherence and self-efficacy. One of the important aspects of this study was that the CHWs shared cultural and linguistic characteristics with the patients and HCPs, leading to increased trust and satisfaction with the information presented ( 22 ).
Kim et al. ( 23 ) found that Korean-American participants benefited greatly from group education sessions that provided integrated counseling led by a team of nurses and CHW educators. The intervention also had a health literacy component that focused on enhancing skills such as reading food package labels, understanding medical terminology, and accessing health care services. This intervention led to a significant reduction of 1–1.3% in A1C levels in the intervention group. The intervention established the value of collaboration between CHW educators and nurses to improve health information delivery and disease management.
A collaboration between CHW educators and pharmacists was also shown to reinforce diabetes knowledge and improve health literacy. Sharp et al. ( 26 ) conducted a cross-over study in four primary care ambulatory clinics that provided care for low-income patients. The study found that patients with low health literacy had more visits with pharmacists and CHWs than those with high health literacy. The CHWs provided individualized support to reinforce diabetes self-management education and referrals to resources such as food, shelter, and translation services. The translation services in this study were especially important for building trust with non-English speakers and helping patients understand their therapy. Similar to other studies, the CHWs shared cultural and linguistic characteristics with their populations, which helped to overcome communication-related and cultural barriers ( 23 , 26 ).
The use of electronic tools or educational videos yielded inconclusive results with regard to medication adherence. Graumlich et al. ( 20 ) implemented a new medication planning tool called Medtable within an electronic medical record system in several outpatient clinics serving patients with type 2 diabetes. The tool was designed to organize medication review and patient education. Providers can use this tool to search for medication instructions and actionable language that are appropriate for each patient’s health literacy level. The authors found no changes in medication knowledge or adherence, but the intervention group reported higher satisfaction. On the other hand, Yeung et al. ( 28 ) showed that pharmacist-led online education videos accessed using QR codes affixed to the patients’ medication bottles and health literacy flashcards increased patients’ medication adherence in an academic medical hospital.
Goessl et al. ( 21 ) found that patients with low health literacy had significantly higher retention of information when receiving evidence-based diabetes education through a DVD recording than through an in-person group class. This 18-month RCT randomized participants to either the DVD or in-person group education and assessed their information retention through a teach-back strategy. The curriculum consisted of diabetes prevention topics such as physical exercise, food portions, and food choices. Participants in the DVD group had significantly higher retention of information than those in the control (in-person) group. The authors suggested this may have been because participants in the DVD group have multiple opportunities to review the education material.
Management of type 2 diabetes remains a challenge for HCPs and patients, in part because of the challenges discussed in this review, including communication barriers between patients and HCPs and knowledge deficits about medications and disease states ( 29 ). HCPs can have a positive impact on the health outcomes of their patients with diabetes by improving patients’ disease state and medication knowledge.
One of the common themes identified in this literature review was the prevalence of culturally tailored diabetes education interventions. This is an important strategy that could improve diabetes outcomes and provide an alternative approach to diabetes self-management education when working with patients from culturally diverse backgrounds. HCPs might benefit from using culturally tailored educational approaches to improve communication with patients and overcome the medical mistrust many patients feel. Although such mistrust was not directly correlated with diabetes management, it was noted that patients who feel mistrustful tend to have poor communication with HCPs ( 20 ). Additionally, Latino/Hispanic patients who have language barriers tend to have poor glycemic control ( 19 ). Having CHWs work with HCPs might mitigate some patient-provider communication barriers. As noted earlier, CHWs who share cultural and linguistic characteristics with their patient populations have ongoing interactions and more frequent one-on-one encounters ( 12 ).
Medication adherence and glycemic control are important components of diabetes self-management, and we noted that the integration of CHWs into the diabetes health care team and the use of simplified medication label interventions were both successful in improving medication adherence ( 23 , 24 ). The use of culturally tailored education sessions and the integration of pharmacists and CHWs into the management of diabetes appear to be successful in reducing A1C levels ( 12 , 26 ). Electronic education tools and educational videos alone did not have an impact on medication knowledge or information retention in patients with low health literacy, but a combination of education tools and individualized sessions has the potential to improve diabetes medication knowledge and overall self-management ( 20 , 22 , 30 ).
There were several limitations to our literature review. We restricted our search criteria to articles published in English and studies conducted within the United States to ensure that the results would be relevant to U.S. HCPs. However, these limitations may have excluded important work on this topic. Additional research expanding this search beyond the United States and including articles published in other languages may demonstrate different outcomes. Additionally, this literature review did not focus on A1C as the primary outcome, although A1C is an important indicator of diabetes self-management. A1C was chosen as the method of evaluating the impact of health literacy interventions in patients with diabetes, but other considerations such as medication adherence, impact on comorbid conditions, and quality of life are also important factors.
The results of this work show that implementing health literacy interventions to help patients manage type 2 diabetes can have beneficial results. However, such interventions can have significant time and monetary costs. The potential financial and time costs of diabetes education interventions were not evaluated in this review and should be taken into account when designing interventions. The American Diabetes Association estimated the cost of medical care for people with diabetes to be $327 billion in 2017, with the majority of the expenditure related to hospitalizations and nursing home facilities ( 16 ). Another substantial cost of diabetes that can be difficult to measure is treatment for comorbid conditions and complications such as cardiovascular and renal diseases.
Interventions designed to address low health literacy and provide education about type 2 diabetes could be a valuable asset in preventing complications and reducing medical expenditures. Results of this work show that clinicians who are considering implementing new interventions may benefit from the following strategies: using culturally tailored approaches, creating materials for different learning styles and in patients’ languages, engaging CHWs and pharmacists to help with patient education, using PCLs for medications, and engaging education session instructors who share patients’ cultural and linguistic characteristics.
Diabetes self-management is crucial to improving health outcomes and reducing medical costs. This literature review identified interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy. Clinicians seeking to implement diabetes care and education interventions for patients with low health literacy may want to consider drawing on the strategies described in this article. Providing culturally sensitive education that is tailored to patients’ individual learning styles, spoken language, and individual needs can improve patient outcomes and build patients’ trust.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors conceptualized the literature review, developed the methodology, analyzed the data, and wrote, reviewed, and edited the manuscript. R.A. collected the data. K.M. supervised the review. K.M. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Portions of this research were presented at the Washington State University College of Pharmacy and Pharmaceutical Sciences Honors Research Day in April 2019.
Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 08 November 2019
Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000–2018
- Rami H. Al-Rifai ORCID: orcid.org/0000-0001-6102-0353 1 ,
- Maria Majeed 1 ,
- Maryam A. Qambar 2 ,
- Ayesha Ibrahim 2 ,
- Khawla M. AlYammahi 2 &
- Faisal Aziz 1
Systematic Reviews volume 8 , Article number: 268 ( 2019 ) Cite this article
11k Accesses
18 Citations
Metrics details
Investing in women’s health is an inevitable investment in our future. We systematically reviewed the available evidence and summarized the weighted prevalence of type 2 diabetes (T2DM) and pre-diabetes mellitus (pre-DM) in women of childbearing age (15–49 years) in the Middle East and North African (MENA) region.
We comprehensively searched six electronic databases to retrieve published literature and prevalence studies on T2DM and pre-DM in women of childbearing age in the MENA. Retrieved citations were screened and data were extracted by at least two independent reviewers. Weighted T2DM and pre-DM prevalence was estimated using the random-effects model.
Of the 10,010 screened citations, 48 research reports were eligible. Respectively, 46 and 24 research reports on T2DM and pre-DM prevalence estimates, from 14 and 10 countries, were included. Overall, the weighted T2DM and pre-DM prevalence in 14 and 10 MENA countries, respectively, were 7.5% (95% confidence interval [CI], 6.1–9.0) and 7.6% (95% CI, 5.2–10.4). In women sampled from general populations, T2DM prevalence ranged from 0.0 to 35.2% (pooled, 7.7%; 95% CI, 6.1–9.4%) and pre-DM prevalence ranged from 0.0 to 40.0% (pooled, 7.9%; 95% CI, 5.3–11.0%). T2DM was more common in the Fertile Crescent countries (10.7%, 95% CI, 5.2–17.7%), followed by the Arab Peninsula countries (7.6%, 95% CI, 5.9–9.5%) and North African countries and Iran (6.5%, 95% CI, 4.3–9.1%). Pre-DM prevalence was highest in the Fertile Crescent countries (22.7%, 95% CI, 14.2–32.4%), followed by the Arab Peninsula countries (8.6%, 95% CI, 5.5–12.1%) and North Africa and Iran (3.3%, 95% CI, 1.0–6.7%).
Conclusions
T2DM and pre-DM are common in women of childbearing age in MENA countries. The high DM burden in this vital population group could lead to adverse pregnancy outcomes and acceleration of the intergenerational risk of DM. Our review presented data and highlighted gaps in the evidence of the DM burden in women of childbearing age, to inform policy-makers and researchers.
Systematic review registration
PROSPERO CRD42017069231
Peer Review reports
The global burden of type 2 diabetes mellitus (T2DM) is rapidly increasing, affecting individuals of all ages. The global T2DM prevalence nearly doubled in the adult population over the past decade from 4.7% in 1980 to 8.5% in 2014 [ 1 ]. The global burden of T2DM in people 20–79 years is further projected to increase to 629 million in 2045 compared to 425 million in 2017 [ 1 ]. Low- and middle-income countries will be the most affected with the rise in the burden of T2DM. For the period between 2017 and 2045, the projected increase in the prevalence of T2DM in the Middle East and North Africa (MENA) region is 110% compared to 16% in Europe, 35% in North Africa and the Caribbean, and 62% in South and Central America [ 1 ]. Pre-diabetes (pre-DM) or intermediate hyperglycaemia is defined as blood glucose levels above the normal range, but lower than DM thresholds [ 1 ]. The burden of pre-DM is increasing worldwide. By 2045, the number of people aged between 20 and 79 years old with pre-DM is projected to increase to 587 million (8.3% of the adult population) compared to 352.1 million people worldwide in 2017 (i.e., 7.3% of the adult population of adults aged 20 to 79 years) [ 1 ]. About three quarters (72.3%) of people with pre-DM live in low- and middle-income countries [ 1 ].
Pre-DM or T2DM are associated with various unfavorable health outcomes. People with pre-DM are at high risk of developing T2DM [ 1 ]. Annually, it is estimated that 5–10% of people with pre-DM will develop T2DM [ 2 , 3 ]. Pre-DM and T2DM are also associated with early onset of nephropathy and chronic kidney disease [ 4 , 5 , 6 , 7 ], diabetic retinopathy [ 6 , 8 , 9 ], and increased risk of macrovascular disease [ 10 , 11 ]. T2DM is also reported to increase the risk of developing active [ 12 ] and latent tuberculosis [ 13 ]. The rising levels of different modifiable key risk factors, mainly body overweight and obesity, driven by key changes in lifestyle, are the attributes behind the continued burgeoning epidemics of pre-DM and T2DM [ 14 , 15 , 16 ]. Women of childbearing age (15–49 years) [ 17 ] are also affected by the global rise in pre-DM and T2DM epidemics. Rising blood glucose levels in women of childbearing age has pre-gestational, gestational, and postpartum consequences, including increased intergenerational risk of DM [ 18 ].
The total population in 20 countries (Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Malta, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Syria, Tunisia, the United Arab Emirates, and Yemen) in the Middle East and North Africa region comprises almost 6.7% (~ 421 million people) of the world’s population, with about 200 million females as of July 1, 2015 [ 19 ]. In adults ≥ 18 years, T2DM prevalence rose sharply by 2.3 times in each of the Eastern Mediterranean regions and the African region, between 1980 and 2014 [ 20 ]. This sharp increase in these two regions is higher than that reported in the region of the Americas (1.7 times), the European region (1.4 times), and the Western Pacific Region (1.9 times) [ 20 ].
Key pre-DM and T2DM risk factors, body overweight and obesity, are highly prevalent in people in the MENA countries. In 2013, the age-standardized prevalence of overweight and obesity among women ≥ 20 years was 65.5% (obese 33.9%) [ 21 ]. The high burden of overweight and obesity in several MENA countries attributed to the interrelated economic, dietary, lifestyle behavioral factors. The nutrition transitions and changes in the food consumption habits were supported by the witnessed economic development in most of the MENA countries. For instance, in the past five decades, the economic development in the Arab Gulf countries linked to the discovery of oil and gas reserves led to changes in eating habits towards the consumption of foods rich in fat and calories as well as increasing behavioral habits towards a sedentary lifestyle [ 22 , 23 ]. This is particularly true with the significant shift from the consumption of traditional low-fat food to fat-rich foods, as well as with a major change from an agricultural lifestyle to an urbanized lifestyle that is often accompanied by decreased levels of physical activity. The urbanized lifestyle increases exposure to fast foods through the high penetration of fast food restaurants serving fat-rich foods, the reliance on automobiles for transport, and the increasing penetration of cell phones, all of which facilitate low levels of physical activity. Globally, physical inactivity is estimated to cause around 27% of diabetes cases [ 24 ]. In eight Arab countries, based on national samples, low levels of physical activity in adults ranged from 32.1% of the population in Egypt in 2011–2012 to as high as 67% of the population in Saudi Arabia in 2005 [ 25 ]. Furthermore, fruit and vegetable consumption is inversely associated with weight gain [ 26 ]. Studies indicated a low intake of fruit and vegetables in some of the MENA countries [ 27 , 28 ]. The growing burden of the possible risk factors of body overweight and obesity in women may further affect and exacerbate the burden of DM and its associated complications in the MENA countries.
To develop effective prevention and control interventions, there is a need for understanding the actual burden of pre-DM and T2DM epidemics in vital population groups, such as women of childbearing age (15–49 years), in the MENA region. Thus, individual studies need to be compiled and summarized. According to our previously published protocol (with a slight deviation) [ 29 ], here, we present the results of the systematically reviewed published quantitative literature (systematic review “1”), to assess the burden (prevalence) of T2DM and pre-DM in women of childbearing age in the MENA region, from 2000 to 2018.
Investing in women’s health paves the way for healthier families and stronger economies. Societies that prioritize women’s health are likely to have better population health overall and to remain more productive for generations to come [ 30 ]. Against this background, our review was aimed at characterizing the epidemiology of T2DM and pre-DM in population groups of women of childbearing age in the MENA through (1) systematically reviewing and synthesizing all available published records of T2DM and pre-DM and (2) estimating the mean T2DM and pre-DM prevalence at national, sub-regional, and regional levels, from January 2000 to July 2018. The findings of the review fill an evidence gap to inform policy-makers on the epidemiologic burden of T2DM and pre-DM in women of childbearing age.
Following our published protocol [ 29 ] that is registered with the International Prospective Registry of Systematic Reviews (PROSPERO registration number “CRD42017069231” dated 12/06/2017), we reported here systematic review “1”. This review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 guidelines [ 31 , 32 , 33 ]. The PRISMA checklist is provided in the Additional file 1 .
Data source and search strategy
To identify eligible studies on T2DM and pre-DM prevalence measures in MENA countries, we implemented a comprehensive computerized search of six electronic databases (MEDLINE, EMBASE, Web of Science, SCOPUS, Cochrane library, and Academic Search Complete) from January 1, 2000, to July 12, 2018, using variant Medical Subject Headings (MeSH) and free-text (Text) terms. The detailed search strategy is presented in an additional box file (see Additional file 2 ). We also hand-searched the reference lists of eligible studies for further studies that might have been missed.
We defined the participants, exposure, comparator, outcome(s), and type of study “PECO(T)”. The PECO(T) statement provides the framework for the identification and selection of studies for inclusion [ 34 ]. As we were looking for prevalence studies, we only considered participants and the outcomes.
Inclusion and exclusion criteria
Participants : Women of childbearing age were defined according to the World Health Organization (WHO) as women aged between 15 and 49 years (thereafter, women of childbearing age) [ 35 ]. Pregnant women were also considered in this review as long as they were tested for T2DM and/or pre-DM according to what was reported in the individual studies.
Outcomes : T2DM and pre-DM. The included studies should have reported quantitative or calculable pre-DM or T2DM prevalence estimate(s) in women of childbearing age regardless of the sample size, pregnancy status, or pre-DM/T2DM ascertainment methodology, in any of the 20 MENA region countries [ 36 ]. We excluded studies of self-reported pre-DM/T2DM not supported with either anti-DM medications or a documented diagnosis. We also excluded studies on metabolic syndrome as long as there was no clear information on the proportion of women of childbearing age with pre-DM or T2DM. Studies were also excluded if they pooled women of childbearing age with pre-DM/T2DM with other non-communicable diseases in the same category, or together with males, or for each gender separately but without age stratification. We excluded studies with incalculable pre-DM/T2DM prevalence after attempting to contact the authors at least twice with no response.
Types of studies : We included observational studies if they were cross-sectional, comparative cross-sectional, case-control (not comparing T2DM/pre-DM vs. no T2DM/pre-DM), or cohort study designs. We excluded observational studies of other study designs.
Detailed eligibility criteria are available in the published protocol [ 29 ]. The PRISMA flow chart for the selection of studies is shown in Fig. 1 .
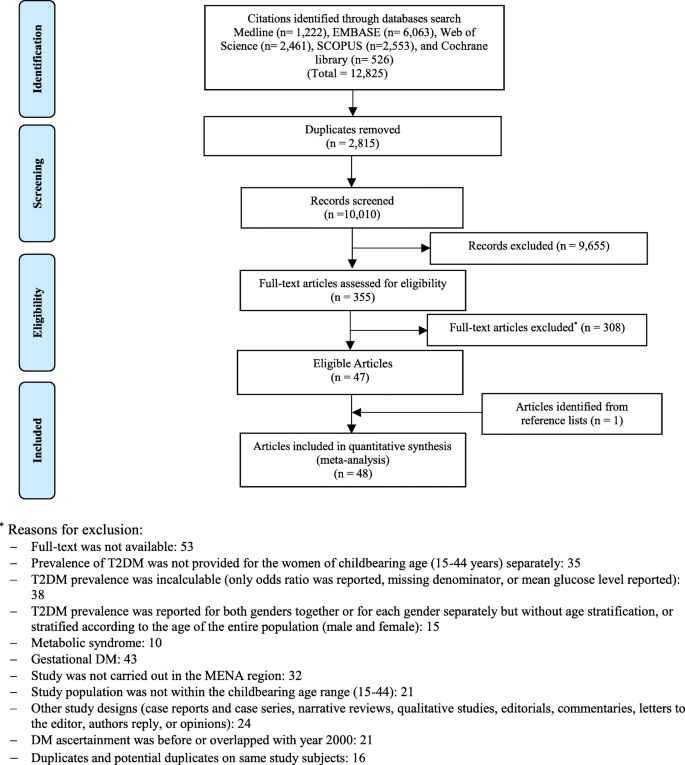
PRISMA flow chart
Identifying eligible studies
Titles and abstracts of the remaining citations were screened independently by four reviewers (AI, KA, MM, and MQ) for any potential study on pre-DM/T2DM in childbearing age women. Full-texts of the identified potentially eligible studies were thoroughly screened and independently assessed by the four reviewers. The qualities of the extracted studies were independently assessed by two other reviewers (RHA and FA). Discrepancies in data extraction were discussed and resolved.
Data extraction
Data from fully eligible studies were extracted into a pre-defined data extraction excel file using a pre-defined list of variables [ 29 ]. Our outcome of interest was the national/regional weighted pooled prevalence of T2DM and pre-DM in women of childbearing age in the MENA. We extracted the following data on the baseline characteristics of the eligible research reports (author names, year of publication, country, city, and study setting), study methodology (design, time period, sampling strategy, and T2DM/pre-DM ascertainment methodology), and study population (age, pregnancy status, co-morbidity, and number of women with the outcomes of interest).
In research reports which provided stratified T2DM/pre-DM prevalence estimates, the prevalence of the total sample was replaced with the stratified estimates keeping the rule of having at least 10 tested subjects per strata, otherwise we extracted information on the whole tested sample. We followed a pre-defined sequential order when extracting stratified prevalence estimates. Outcome measures stratified according to body mass index (BMI) were prioritized, followed by age and year. This prioritization scheme was used to identify the strata with more information on the tested women. When the strata were not prioritized, the overall outcome prevalence measured was extracted. For a research report that stratified the prevalence of the outcome of interest at these different levels (i.e., age and BMI), one stratum per research report was considered and included to avoid double counting. If the outcome measure was ascertained by more than one ascertainment guideline, we extracted relevant information based on the most sensitive and reliable ascertainment assay (i.e., prioritizing fasting blood glucose “FBG” over self-reported DM status), or the most recent and updated criteria (i.e., prioritizing WHO 2006 over WHO 1999 criteria).
We generated a funnel plot to explore the small-study effect on the pooled prevalence estimates. The funnel plot was created by plotting each prevalence measure against its standard error. The asymmetry of the funnel plot was tested using the Egger’s test [ 37 ] (see Additional files 3 and 4 ).
Quality appraisal and risk of bias
We assessed the methodological quality and risk of bias (ROB) of the studies on T2DM or pre-DM prevalence measures using six-quality items adapted from the National Heart, Lung, and Blood Institute (NIH) tool [ 38 ]. Of the 14 items proposed for observational studies on the NIH tool, eight items were not used as they are relevant only for cohort studies assessing the relationship between an exposure and an outcome [ 38 ]. We also assessed the robustness of the implemented sampling methodology and the ascertainment methodology of the measured outcome(s) using three additional quality criteria (sampling methodology, ascertainment methodology, and precision of the estimate). Studies were considered as having “high” precision if at least 100 women tested for T2DM/pre-DM; a reasonable precision, given a pooled prevalence of 7.2% for T2DM or 7.6% for pre-DM estimated in this study, was obtained. We computed the overall proportion of research reports with potentially low risk of bias across each of the nine quality criteria. We also computed the proportion (out of nine) of quality items with potentially a low risk of bias for each of the included research reports.
Quantitative synthesis: meta-analysis
Meta-analyses of the extracted data to estimate the weighted pooled prevalence of T2DM and pre-DM and the corresponding 95% confidence interval (CI) were executed. The variances of prevalence measures were stabilized by the Freeman-Tukey double arcsine transformation method [ 39 , 40 ]. The estimated pooled prevalence measures were weighted using the inverse variance method [ 40 ], and an overall pooled prevalence estimate was generated using a Dersimonian–Laird random-effects model [ 41 ]. Heterogeneity measures were also calculated using the Cochran’s Q statistic and the inconsistency index; I –squared ( I 2 ) [ 42 ]. In addition to the pooled estimates, the prevalence measures were summarized using ranges and medians. The prediction interval, which estimated the 95% interval in which the true effect size in a new prevalence study will lie, was also reported [ 42 , 43 ].
Country-level pooled estimates were generated according to the population group of tested women (general population, pregnant, non-pregnant with history of gestational DM (GDM), and patients with co-morbidity), and the overall country-level pooled prevalence, regardless of the tested population and study period. To assess if the prevalence of T2DM and pre-DM is changing over time, we stratified studies into two time periods: 2000–2009 and 2010–2018. In order not to miss any important data when estimating country-level, sub-regional, and regional prevalence, the period for studies that overlapped these two periods was defined as “overlapping”. In studies with an unclear data collection period, we used the median (~ 2 years) that was obtained from subtracting the year of publication from the year of data collection to estimate the year of data collection in those studies. The “patients with co-morbidity” included women of childbearing age with organ transplant, kidney dialysis, cancer, HIV, chronic obstructive pulmonary disease, polycystic ovarian syndrome (PCOS), or schizophrenia. Categorization of the study period was arbitrary with an aim to estimate the change in T2DM and pre-DM at the country-level and overall, over time.
We also estimated the weighted pooled prevalence, regardless of country, according to the tested women’s population group, study period, T2DM/pre-DM ascertainment guidelines (WHO guidelines, American DM Association (ADA) guidelines, International DM Association (IDF) guidelines, or medical records/anti-DM medications/self-reported), and sample size (< 100 or ≥ 100). The overall weighted pooled prevalence of T2DM and pre-DM regardless of the country, tested population, study period, ascertainment guidelines, and sample size was also generated. Providing pooled estimates regardless of the ascertainment guidelines was justified by the fact that the subject women were defined and treated as T2DM or pre-DM patients following each specific ascertainment guidelines.
To provide prevalence estimates at a more sub-regional level, countries in the MENA region were re-grouped into three sub-regions, namely, “Arab Peninsula, Fertile crescent, and North Africa and Iran.” The pooled prevalence in these three sub-regions was estimated according to the tested population group, study period, ascertainment guidelines, and sample size, as well as overall for each sub-region.
We also estimated the weighted pooled prevalence of T2DM and pre-DM according to age group. We categorized women of childbearing age into three age groups (15–29 years, 30–49 years) and not specified/overlapping. The “not specified/overlapping” category covers women who did fell in the other two age groups. For example, women with an age range of 25–34 years or 18–40 years. The age group weighted pooled prevalence produced regardless of the country, sub-region, and tested population as well as study period.
All meta-analyses were performed using the metaprop package [ 33 ] in Stata/SE v15 [ 44 ].
Sources of heterogeneity: meta-regression
Random-effects univariate and multivariable meta-regression models were implemented to identify sources of between-study heterogeneity and to quantify their contribution to variability in the T2DM and pre-DM prevalence. In univariate meta-regression models, analysis was performed by country, tested population, study period, ascertainment guidelines, and sample size. All variables with a p < 0.1, in the univariate models, were included in the multivariable model. In the final multivariable model, a p value ≤ 0.05 was considered statistically significant, contributing to heterogeneity in prevalence estimates.
All meta-regression analyses were performed using the metareg package in Stata/SE v15 [ 44 ].
Search and eligible research reports
Of the 12,825 citations retrieved from the six databases, 48 research reports were found eligible (Fig. 1 ); 46 reported T2DM prevalence [ 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 ] while 24 reported pre-DM prevalence [ 48 , 49 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 60 , 62 , 63 , 66 , 67 , 70 , 73 , 75 , 81 , 85 , 88 , 89 , 90 ].
Scope of reviewed T2DM reports
The 46 research reports on T2DM prevalence yielded 102 T2DM prevalence studies. The 46 reports were from 14 countries (Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Qatar, Saudi Arabia, Tunisia, the United Arab Emirates [UAE], and Yemen); ranging by year between 2000 in Saudi Arabia [ 79 ] and 2018 in UAE [ 81 ]. Sixteen (34.9%) research reports were reported in Saudi Arabia [ 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ], followed by 19.6% in the UAE [ 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ], and 15.2% in Iran [ 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. Over one third (37.3%) of the yielded 102 T2DM prevalence studies were in Saudi Arabia. Of the 102 T2DM prevalence studies, 79.4% were in women sampled from general populations and 11.8% in pregnant women. Over two thirds (69.6%) of the T2DM prevalence studies were in or before 2009 and 82.4% tested ≥ 100 women (Table 1 ).
Pooled T2DM prevalence
In the 14 countries, the weighted T2DM prevalence in women of childbearing age estimated at 7.5% (95% CI, 6.1–9.0%, I 2 , 98.2%) (Table 2 , Fig. 2 ). The weighted T2DM prevalence was not significantly different ( p = 0.4) in studies reported between 2000 and 2009 (7.9%, 95% CI, 6.2–9.7%, I 2 , 97.9%) and studies reported between 2010 and 2018 (5.8%, 95% CI, 3.4–8.7%, I 2 , 95.4%) (Table 2 ). The weighted T2DM prevalence was higher in women with an age range of 15–19 years (10.9%, 95% CI, 8.8–13.3%, I 2 , 97.9%) than women with an age range of 30–49 years (2.5%, 95% CI, 1.8–3.2%, I 2 , 83.6%) (see Additional file 5 ).
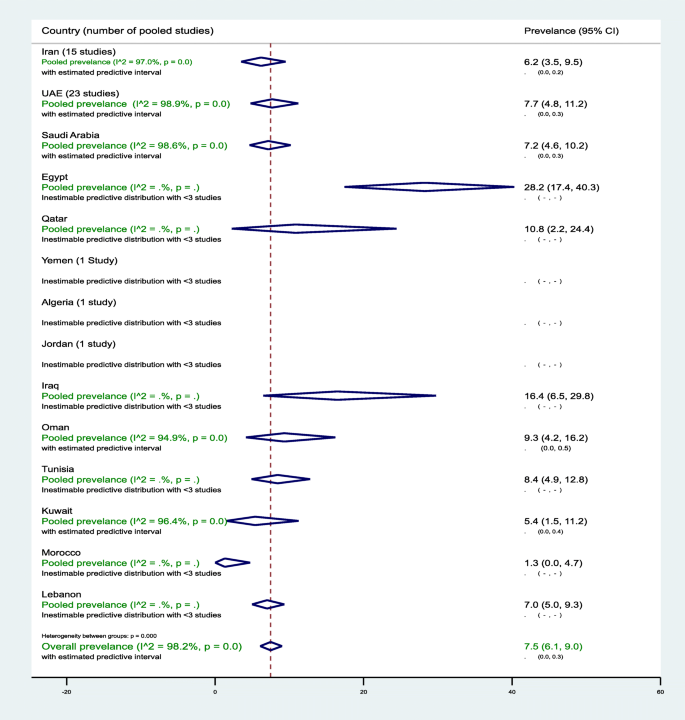
Forest plot of the meta-analyses for the 14 MENA countries’ studies on T2DM
Pooled findings of 102 T2DM prevalence estimates reported in 14 countries in the MENA region. The individual 102 estimates and their 95% confidence interval (CI) omitted to fit the plot. The diamond is centered on the summary effect estimate, and the width indicates the corresponding 95% CI. UAE, United Arab Emirates; T2DM, type 2 diabetes mellitus; MENA, Middle East and Northern Africa
The highest two weighted T2DM estimates were observed in infertile women of childbearing age in Egypt (28.2%, 95% CI, 17.4–40.3%) and in non-pregnant women with a history of GDM in Iran (24.7%, 95% CI, 18.5–31.5%) (Table 2 ). In general populations, the weighted T2DM prevalence ranged between 1.3% (95% CI, 0.0–4.7%) in 2001–2002 in Morocco [ 60 ] and 16.4% (95% CI, 6.5–29.8%, I 2 , 96.5%) in Iraq in 2007 [ 55 ] and in 2011–2012 [ 54 ]. In Saudi Arabia, in women of childbearing age sampled from general populations, the pooled T2DM prevalence estimated at 8.0% (95% CI, 5.3–11.3%, I 2 , 96.5%) (Table 1 ). In Saudi Arabia, the weighted T2DM prevalence in women of childbearing age, regardless of source of population and timeline, estimated at 7.2% (95% CI, 4.6–10.2%, I 2 , 98.6%) (Table 2 ). In Oman, the weighted T2DM prevalence in women of childbearing age sampled from general populations estimated at 8.0% (95% CI, 2.9–15.4%, I 2 , 95.9%) in 2000. In Qatar, the weighted T2DM was prevalence in women of childbearing age sampled from general populations 10.7% (95% CI, 2.2–24.4%, I 2 , 93.7%) between 2007 and 2008. In the UAE, in women of childbearing age sampled from general populations, the pooled T2DM prevalence estimated at 8.0% (95% CI, 4.8–11.9%, I 2 , 98.9%) that declined from 9.4% (95% CI, 5.6–14.1%, I 2 , 95.1%) between 2000 and 2009 to 6.0% (95% CI, 3.3–6.5%, I 2 , 90.5%) between 2010 and 2018 (Table 2 ).
Sub-regional pooled T2DM prevalence
The pooled T2DM prevalence measures estimated at 6.5% (95% CI, 4.3–9.1%, I 2 , 96.0%) in North African countries including Iran, 10.7% (95% CI 5.2–17.7%, I 2 , 90.7%) in the Fertile Crescent countries, and 7.6% (95% CI, 5.9–9.5%, I 2 , 98.5%) in the Arabian Peninsula countries (see Additional file 6 ).
Additional file 7 shows figures presenting the sub-regional-weighted prevalence of T2DM (Fig. 1 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Additional file 8 shows figures presenting timeline view of the weighted prevalence of T2DM (Fig. 1 ) by publication year.
Meta-bias in T2DM prevalence
The asymmetry in the funnel plot examining the small-study effects on the pooled T2DM prevalence among women of childbearing age indicates evidence for the presence of a small-study effect (Egger’s test p < 0.0001). The funnel plot is presented in an additional figure file (see Additional file 3 ).
Predictors of heterogeneity in T2DM prevalence
In the univariate meta-regression models, all variables except study period, T2DM ascertainment criteria, and sample size were associated with T2DM prevalence at p value < 0.1. In the adjusted meta-regression model, none of the included variables was significantly associated with T2DM prevalence at p value < 0.05. In two studies in infertile women of childbearing age in Egypt, the T2DM prevalence was higher (adjusted odds ratio (aOR), 5.26, 95% CI, 0.87–32.1) compared to women of childbearing age in Saudi Arabia. Overall, compared to women of childbearing age sampled from general populations, T2DM prevalence in non-pregnant women of childbearing age with a history of GDM was 234% higher (aOR, 3.34%, 95% CI, 0.90–12.41) (see Additional file 9 ).
Scope of reviewed pre-DM reports
The 24 research reports on pre-DM prevalence yielded 52 pre-DM prevalence studies and were from 10 countries (Iran, Iraq, Jordan, Kuwait, Morocco, Oman, Qatar, Saudi Arabia, UAE, and Yemen); ranging by year between 2002 in Oman [ 62 ] and 2018 in Saudi Arabia [ 81 ]. Thirteen (25.0%), 11 (21.2%), and 11 (21.2%) of the pre-DM prevalence studies were from Iran, Saudi Arabia, and UAE, respectively. Approximately 87.0% of the pre-DM prevalence studies tested women of childbearing age sampled from general populations. The pre-DM prevalence estimates ranged from 0.0% in various age groups in multiple countries [ 51 , 60 , 70 ] to 40.0% in Iraq in women aged 20–39 years, recruited from the general population [ 55 ] (Table 1 ).
Pooled pre-DM prevalence
In the 10 countries, the weighted pre-DM prevalence in women of childbearing age was estimated at 7.6% (95% CI, 5.2–10.4%, I 2 , 99.0%) (Table 3 , Fig. 3 ). The weighted pre-DM prevalence in studies reported between 2000 and 2009 (4.8%, 95% CI 4.0–7.8%, I 2 , 97.1%) was significantly lower ( p < 0.001) than the weighted prevalence estimated in studies reported between 2010 and 2018 (9.3%, 95%, 4.7–15.2%, I 2 , 93.9%) (Table 3 ). Weighted pre-DM prevalence was 1.70 times higher in women with an age range of 15–19 years (9.0%, 95% CI, 4.9–14.1%, I 2 , 99.2%) than women with an age range of 30–49 years (5.3%, 95% CI, 1.8–10.3%, I 2 , 99.0%) (see Additional file 5 ).
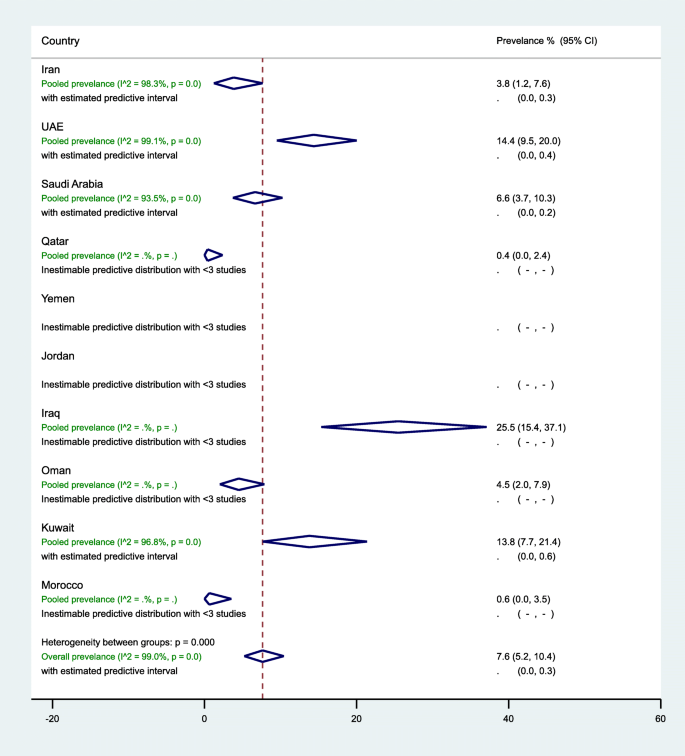
Forest plot of the meta-analyses for the 10 MENA countries’ studies on pre-DM pooled findings of 52 pre-DM prevalence estimates reported in 10 countries in the MENA region. The individual 52 estimates and their 95% confidence interval (CI) omitted to fit the plot. The diamond is centered on the summary effect estimate, and the width indicates the corresponding 95% CI. UAE, United Arab Emirates; pre-DM, pre-diabetes mellitus; MENA, Middle East and Northern Africa
In general populations, the highest three weighted pre-DM prevalence estimates were observed in women of childbearing age in Iraq (25.5%, 95% CI, 15.4–37.1%, I 2 , 92.2%), followed by UAE (15.5%, 95% CI, 10.5–21.2%, I 2 , 99.0%), and Kuwait (13.8%, 95% CI, 7.7–21.4%, I 2 , 96.8%) (Table 3 ). In 13 studies in Iran (7 from the general population), the prevalence of pre-DM ranged from 0.0 to 21.4% with an overall weighted prevalence of 3.8% (95% CI, 1.2–7.6%, I 2 , 98.3%). The 11 pre-DM studies in Saudi Arabia were in women of childbearing age sampled from the general population, with an overall weighted pre-DM prevalence of 6.6% (95% CI, 3.7–10.3%, I 2 , 93.5%) (2000–2009: 9.4% vs. 2010–2018: 4.4%). Regardless of the tested population in UAE, the weighted pre-DM prevalence was 6.6% (95% CI, 5.1–8.3%, I 2 , 65.6%) in studies reported between 2000 and 2009, and 12.0% (95% CI, 8.9–15.5%) in studies reported between 2010 and 2018 with an overall pre-DM prevalence of 14.4% (95% CI, 9.5–20.0%, I 2 , 99.1%) (Table 3 ).
Sub-regional pooled pre-DM prevalence
The pooled pre-DM prevalence estimated at 3.3% (95% CI, 1.0–6.7%, I 2 , 98.1%) in North African countries including Iran, 22.7% (95% CI, 14.2–32.4%, I 2 , 90.0%) in the Fertile crescent countries, and 8.6% (95% CI, 5.5–12.1%, I 2 , 99.1%) in the Arabian Peninsula countries (see Additional files 10 ). Additional file 7 shows figures presenting the sub-regional weighted prevalence of pre-DM (Fig. 2 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Additional file 8 shows figures presenting timeline view of the weighted prevalence of pre-DM (Fig. 2 ) by publication year.
Meta-bias in pre-DM prevalence measures
The asymmetry in the funnel plot examining the small-study effects on the pooled pre-DM prevalence among women of childbearing age indicates evidence for the presence of a small-study effect (Egger’s test p < 0.0001). The funnel plot is presented in an additional figure file (Additional file 4 ).
Predictors of heterogeneity in pre-DM prevalence
Country, study period, and pre-DM ascertainment criteria were associated with a difference in the pre-DM prevalence in the univariate meta-regression models at p value < 0.1. In the univariate meta-regression models, pre-DM prevalence in women of childbearing age in Iraq was 424% higher compared to such women in Saudi Arabia (OR, 5.24, 95% CI, 1.45–18.94%). This significant association turned insignificant in the multivariable model (aOR, 2.20, 95% CI, 0.52–10.82%). In the multivariable model, compared to Saudi Arabia, pre-DM prevalence in women of childbearing age was 70% lower in Iran (aOR, 0.30, 95% CI, 0.11–0.79%) and 88% lower in Morocco (aOR, 0.12, 95% CI, 0.01–0.91%) (see Additional file 11 ).
Quality assessment of the T2DM/pre-DM research reports
Findings of our summarized and research report-specific quality assessments for relevant DM prevalence studies can be found in Additional file 12 . Briefly, all the 48 research reports clearly stated their research questions or objectives, clearly specified and defined their study populations, and selected or recruited the study subjects from the same or similar populations. There was a clear gap in the reporting or justifying of the sample size calculation in 79.2% of the research reports. The majority (87.5%) of the research reports tested ≥ 100 women of childbearing age, and they were classified as having high precision.
Overall, the 48 research reports were of reasonable quality with potentially low ROB in an average of 7.2 items (range, 6–9). Four (8.3%) of the 48 reports had potentially low ROB in all the measured nine quality items [ 66 , 82 , 83 , 86 ] (see Additional file 12 ).
We provided, to our knowledge, the first regional study that comprehensively reviewed and estimated the regional, sub-regional, and country-level burden of T2DM and pre-DM in various populations of women of childbearing age in the MENA. Based on the available data from 14 and 10 studies in MENA countries, the present findings document the comparable burden of T2DM (7.5%, 95% CI 6.9–9.0%) and pre-DM (7.6%, 95% CI 5.2–10.4%) in women of childbearing age. The estimated prevalence of T2DM and pre-DM in 14 countries in the MENA is similar to the estimated worldwide crude diabetes prevalence of 8.2% (95% credible interval (CI) 6.6–9.9%) in adult women in 2014 (age-standardized 7.9%, 95% CI 6.4–9.7%) [ 91 ]. The T2DM and pre-DM prevalence in women of childbearing age varied across the three sub-regions in the MENA, by population group, time period, DM ascertainment criteria, and sample size. The obvious common prevalence of T2DM and pre-DM in women of childbearing age in the MENA countries reflects the highest prevalence of adult diabetes estimated for the MENA [ 91 ]. In this region, the crude diabetes prevalence in adult women increased from 5.0% in 1980 to 9.0% in 2014 [ 91 ]. This increase in diabetes prevalence among adult populations in the MENA over time is higher than many other regions including Europe and Central and West Africa [ 91 ]. The highest national adult diabetes prevalence estimates documented in the MENA is 5–10 times greater than the lowest national prevalence estimates documented in Western European countries [ 91 ].
T2DM is a significant public health problem in both developed and developing countries that can lead to various health complications including increased overall risk of dying prematurely [ 20 ]. The common burden of T2DM and pre-DM in women of childbearing age, which is reflected in the high burden of adult diabetes in this region [ 91 ], might be mainly driven by the sociodemographic changes in this region. In recent decades, there was an increase in median age, sedentary lifestyle, and physical inactivity in the MENA [ 92 ]. These lifestyle changes are linked to an increase in the burden of body overweight and obesity that are shared predisposing factors for pre-DM and T2DM [ 20 ]. At the population level, physical inactivity was very common in many MENA countries (Saudi Arabia 67.6% in 2005; Kuwait 62.6% in 2014; Qatar 45.9% in 2012; Egypt 32.1% in 2011–2012; Iraq 47.0% in 2015) [ 25 ]. The burden of body overweight and obesity is higher in many low-income and middle-income countries in the MENA than in Europe and Asia Pacific countries [ 93 ]. Obesity in women in several Middle Eastern countries was 40–50% [ 93 ]. The age-standardized prevalence of obesity was 32.0% in Egypt, 35.5% in Jordan, 30.4% in Iraq, 32.5% in Libya, and 35.4% in Saudi Arabia [ 94 ]. In Tunisia, 43.7% and 24.1% of 35–70-year-old females in urban and rural areas, respectively, were obese [ 95 ]. In 2016, in almost all of the countries in MENA, the mean BMI for people aged ≥ 18 years was ≥ 25.0 [ 96 ].
To curb the burden of DM and its associated complications in women of childbearing age in the MENA countries, our results suggest three main implications for care. First, based on the estimated 5–10% progression rate from pre-DM to T2DM [ 3 , 10 ], out of the 47,958 tested women of childbearing age for pre-DM (Table 3 ), we estimate that 2398 to 4796 women are expected to progress to T2DM. This risk of progression to T2DM could be reduced through lifestyle and drug-based interventions as it was reported elsewhere [ 97 , 98 , 99 ]. In England, 55–80% of participants with hyperglycemia at baseline had normal glycaemia at 10 year follow-up [ 3 ]. The high burden of DM along with pre-DM in women of childbearing age could accelerate maternal complications including GDM leading to increased intergenerational risk of DM. Programs to halt the growing epidemic of DM among different population groups could start by addressing the key risk factors including sedentary lifestyle and increased body weight. Addressing this problem would require social and public policies and efforts to reduce the national and regional burden of increased body weight and obesity through enhancing healthy eating behaviors and physical activity. Second, there is a critical need for strengthened surveillance systems that match the scale and nature of the DM epidemic in women of childbearing age in the MENA. Enhancing early detection and management of high-risk individuals requires accessible and affordable health care systems, outreach campaigns to raise public awareness, and social and medical support to induce and maintain a healthy lifestyle. Adult people at increased risk of T2DM and pre-DM can be predicted based on good screening tools from the Centers for Disease Control and Prevention (CDC) [ 100 ] and the American Diabetes Association (T2DM Risk Test) [ 101 ]. Early screening and detection will require government-funded prevention programs. Third, controlling the burden of T2DM and pre-DM in MENA countries requires strong and successful partnerships between public health and clinical departments. Physicians have a fundamental role in the care of individual patients to screen, diagnose, and treat both pre-DM and T2DM in clinical settings. In addition, physicians have a fundamental role in working to raise awareness and participating in developing prevention programs and engaging communities. Concerted efforts and partnership between physicians, health departments, and community agencies are needed to strengthen health care services, encouraging and facilitating early screening and detection, and promoting healthy diets and physical activity.
Providing summary estimates and up-to-date mapping gaps-in-evidence of T2DM and pre-DM prevalence in women of childbearing age in different MENA countries provides the opportunities for future public health interventions and research to better characterize the T2DM and pre-DM epidemiology nationally and regionally. Nevertheless, present review findings suggest that the DM burden in women of childbearing age in MENA countries is capturing only the tip of the iceberg. Identifying gaps-in-evidence through systematically reviewing and summarizing the literature has public health research implications. Our review shows that in many countries, the estimation of the burden of T2DM or pre-DM in women of childbearing age in general populations occurred more than a decade ago (Table 1 ). Additionally, the review shows that there was no data on the burden of T2DM and pre-DM in women of childbearing age in several countries in the MENA region. This lack of evidence on a key public heath outcome requires a strongly resourced research capacity and research funding schemes. There is evidence that federally funded research can impact important health issues that affect a large segment of the population [ 102 ].
This robust approach to the literature search and review as well as in retrieving and extracting relevant data from the published literature allowed us to provide summary estimates on the burden of T2DM and pre-DM in women of childbearing age from the 14 and 10 countries in the MENA, respectively. Once the diagnosis was established, regardless of the ascertainment criteria, patients were treated as having diabetes or pre-diabetes. Thus, generating pooled estimates, regardless of the DM ascertainment criteria, stratified according to various population groups, provided more insights into the actual burden of T2DM and pre-DM in various populations of women of childbearing age. The meta-regression analysis identified sources of variations in T2DM and pre-DM prevalence and sources of between-study heterogeneity in prevalence estimates. (Additional files 9 and 11 show these in more detail). The country-stratified and population-stratified T2DM and pre-DM prevalence reports revealed gaps in evidence that can help strengthen research and DM control programs in the most affected countries and populations. The use of probability sampling was very common in the studies included, which may provide broader insights on the representation of our findings to the general or specific group of women of childbearing age at the national, but not at the regional, level.
Limitations
There are important but unavoidable limitations when interpreting the results of our review. Despite the estimated DM prevalence, the actual DM burden could have been underestimated, at country, sub-regional, or regional level, due to several reasons. The inaccessibility of data on pre-DM or T2DM in women of childbearing age from several countries in the MENA may not necessarily mean an actual lack of data. To meet the aim of our review of estimating the burden of pre-DM and T2DM in women of childbearing age, in several published studies reviewed, women of childbearing age were found to have been combined with those of other age groups or with men. The presented overall pooled estimates, regardless of the tested population group, should not be interpreted as the total burden of the outcome at the population level. Utilizing data on T2DM and pre-DM from only 14 and 10 countries may limit the findings from being generalizable to the entire MENA region. Although we followed a thorough and well-defined search strategy, there is a potential of publication bias as shown in funnel plots (Additional files 3 and 4 ). The estimated T2DM and pre-DM prevalence suggest that only the tip of the iceberg was captured. The presented estimates may not be representative of the true prevalence for each population. This underestimation may be particularly true in low-resource settings where necessary resources and capacity in investigating pre-DM at the community level are lacking. The wide array of blood glucose cut-off points and criteria used for T2DM and pre-DM ascertainment also suggests that overestimation and underestimation bias cannot be excluded. Unless estimated from individual population-based studies only, the presented weighted pooled estimates at the country, sub-regional, or regional level should not be interpreted as the burden of the measured outcomes at the population level. Also, the presented pooled estimates according to the two time periods, from 2000 to 2009 and from 2010 to 2018, should not be interpreted as an over-time change in the burden of the measured outcomes. While our meta-analyses revealed substantial heterogeneity across studies, the meta-regression analyses identified the potential sources of between-study heterogeneity within the framework of the present study and the level of detail that can be used in describing these sources (Tables 1 and 2 ). Thus, much of the variability in T2DM and pre-DM prevalence across studies might remain unexplained.
Despite these potential limitations, our study provided a characterization of the scale of T2DM and pre-DM among women of childbearing age in several MENA countries based on the best available evidence. Data presented in this review can be used to (a) understand the burden of T2DM and pre-DM among a vital population group and to identify at high-risk populations within this specific population group; (b) guide the planning, implementation, and evaluation of programs to prevent and control DM; (c) implement immediate public health actions to prioritize the allocation of public health resources; and (d) formulate research hypotheses and provide a basis for epidemiologic studies. Future research opportunities should prioritize large country-level and multicenter comparable studies, to determine the prevalence of T2DM and pre-DM in various population groups of women of childbearing age. A definitive characterization of the burden of DM in women of childbearing age at the regional and sub-regional level would require comparable and empirical studies using standardized methodology and comparable DM ascertainment assays.
In conclusion, women of childbearing age in the MENA region bear an appreciable burden of T2DM and pre-DM. The estimated burden of T2DM and pre-DM was higher in the Arabian Peninsula and Fertile Crescent countries compared to the rest of the MENA countries identified with prevalence estimates in this review. Although both T2DM (7.5%) and pre-DM (7.6%) had similar overall estimated prevalence, there is need for a more focused attention on early detection and control by public health authorities to avoid DM-associated pre-gestational, gestational, and post-gestational complications. Country-level early DM detection and control programs should consider the key risk factors of DM, mainly the growing burden of body overweight and obesity. Furthermore, facilitating high-quality research and surveillance programs in countries with limited data on DM prevalence and reporting of DM prevalence estimates in women of childbearing age warrant focus.
Availability of data and materials
The datasets used and/or analyzed during the current study and its supplementary information files are available from the corresponding author on reasonable request.
Abbreviations
American DM association
Adjusted odds ratio
Confidence interval
Diabetes mellitus
Gestational diabetes mellitus
International Diabetes Mellitus Association
Middle East and North Africa
Medical Subject Headings
National Heart, Lung, and Blood Institute
Participants, exposure, comparator, and outcome
- Pre-diabetes mellitus
Preferred Reporting Items for Systematic Review and Meta-Analysis
Risk of bias
- Type 2 diabetes
United Arab Emirates
World Health Organization
International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels: International Diabetes Federation, 2017. https://diabetesatlas.org/resources/2017-atlas.html Accessed 5 Nov 2018.
Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–9.
Article CAS PubMed Google Scholar
Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med. 2007;24(2):200–7.
Metcalf PA, Baker JR, Scragg RK, Dryson E, Scott AJ, Wild CJ. Microalbuminuria in a middle-aged workforce. Effect of hyperglycemia and ethnicity. Diabetes Care. 1993;16(11):1485–93.
Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The inter-tribal Heart project. J Am Soc Nephrol. 2002;13(6):1626–34.
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23(8):1113–8.
Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673–82.
Article PubMed PubMed Central Google Scholar
Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care. 2007;30(10):2708–15.
Article PubMed Google Scholar
Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287(19):2528–33.
Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, obesity, and lifestyle study (AusDiab). Circulation. 2007;116(2):151–7.
Brunner EJ, Shipley MJ, Witte DR, Fuller JH, Marmot MG. Relation between blood glucose and coronary mortality over 33 years in the Whitehall study. Diabetes Care. 2006;29(1):26–31.
Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. https://doi.org/10.1371/journal.pone.0187967 .
Article CAS PubMed PubMed Central Google Scholar
Lee MR, Huang YP, Kuo YT, Luo CH, Shih YJ, Shu CC, et al. Diabetes mellitus and latent tuberculosis infection: a systematic review and metaanalysis. Clin Infect Dis. 2017;64(6):719–27.
PubMed Google Scholar
Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. 2014;176:149–52.
InterAct C, Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56:1520–30.
Article CAS Google Scholar
World Health Organisation. Sexual and reproductive health. 2015. http://www.who.int/reproductivehealth/topics/infertility/definitions/en/ . Accessed 5 Feb 2019.
Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6.
United Nations. Total Population - Both Sexes. World Population Prospects: The; 2015. pp. Revision 2016. https://esa.un.org/unpd/wpp/Download/Standard/Population/ . .
World Health Organization. Global report on diabetes. 2016. https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=B27DA6B4FB2DCD29CA71FB7C373A17FA?sequence=1 . Accessed 30 Jan 2019.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–81.
Galal O. Nutrition-related health patterns in the Middle East. Asia Pac J Clin Nutr. 2003;12(3):337–43 PubMed PMID: 14505998 .
Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12(1):1–13.
World Health Organization. Health education and promotion. Physical activity. Available at: http://www.emro.who.int/health-education/physical-activity/background.html . Accessed 15 Jan 2019.
Sharara E, Akik C, Ghattas H, Makhlouf OC. Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health. 2018;18(1):639.
Bes-Rastrollo M, Martinez-Gonzalez MA, Sanchez-Villegas A, de la Fuente AC, Martinez JA. Association of fiber intake and fruit/vegetable consumption with weight gain in a Mediterranean population. Nutrition. 2006;22(5):504–11.
Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85(1):19–26.
Nasreddine L, Mehio-Sibai A, Mrayati M, Adra N, Hwalla N. Adolescent obesity in Syria: prevalence and associated factors. Child Care Health Dev. 2010;36(3):404–13.
Al-Rifai RH, Aziz F. Prevalence of type 2 diabetes, prediabetes, and gestational diabetes mellitus in women of childbearing age in Middle East and North Africa, 2000-2017: protocol for two systematic reviews and meta-analyses. Syst Rev. 2018;1:96.
Article Google Scholar
Onarheim KH, Iversen JH, Bloom DE. Economic benefits of investing in women's health: a systematic review. PLoS One. 2016;11(3):e0150120.
Article PubMed PubMed Central CAS Google Scholar
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39.
Woodruff TJ, Sutton P. The navigation guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 2014;122(10):1007–14.
http://www.who.int/reproductivehealth/topics/infertility/definitions/en/ . .
The World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lendinggroups . Accessed 10 Nov 2017.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55.
National Heart, Lung, and blood institute. Quality assessment tool for observational cohort and cross-sectional studies https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . .
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607–11.
Miller JJ. The inverse of the freeman –Tukey double arcsine transformation. The American Statistician. 1978;32(4):138
Google Scholar
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Borenstein MHL, Higgins JP, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
Book Google Scholar
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
StataCorp. Stata statistical software: release 15. College Station: StataCorp LLC; 2017.
Salima T, Mounira K, Nadjia D. Assessment of nutritional status of pregnant women attending the City Tebessa PMI (Algeria). Natl J Physiol Pharm Pharmacol. 2011;1(2):97–105.
Eldesoky A-EE, Gad YZ, Ahmed N. Nonalcoholic fatty liver disease in young adult Egyptian women with polycystic ovary syndrome. Egyptian Liver J. 2013;3:15–9.
Ebrahimi H, Emamian MH, Hashemi H, Fotouhi A. High incidence of diabetes mellitus among a middle-aged population in Iran: a longitudinal study. Can J Diabetes. 2016;40(6):570–5.
Valizadeh M, Alavi N, Mazloomzadeh S, Piri Z, Amirmoghadami H. The risk factors and incidence of type 2 diabetes mellitus and metabolic syndrome in women with previous gestational diabetes. Int J Endocrinol Metab. 2015;13(2):e21696.
Hossein-Nezhad A, Mirzaei K, Maghbooli Z, Larijani B. Maternal glycemic status in GDM patients after delivery. Iran J Diabetes Lipid Disorders. 2009;8(1):95–104.
Azimi-Nezhad M, Ghayour-Mobarhan M, Safarian M, Esmailee H, Parizadeh SM, Rajabi-Moghadam M, et al. Anthropometric indices of obesity and the prediction of cardiovascular risk factors in an Iranian population. ScientificWorld J. 2009;9:424–30.
Azimi-Nezhad M, Ghayour-Mobarhan M, Parizadeh MR, Safarian M, Esmaeili H, Parizadeh SM, et al. Prevalence of type 2 diabetes mellitus in Iran and its relationship with gender, urbanisation, education, marital status and occupation. Singap Med J. 2008;49(7):571–6.
CAS Google Scholar
Hadaegh F, Bozorgmanesh MR, Ghasemi A, Harati H, Saadat N, Azizi F. High prevalence of undiagnosed diabetes and abnormal glucose tolerance in the Iranian urban population: Tehran Lipid and Glucose Study. BMC Public Health. 2008;8:176.
Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005;69(3):279–86.
Mansour AA, Al-Maliky AA, Kasem B, Jabar A, Mosbeh KA. Prevalence of diagnosed and undiagnosed diabetes mellitus in adults aged 19 years and older in Basrah. Iraq Diabetes Metab Syndr Obes. 2014;7:139–44.
Mansour AA, Wanoose HL, Hani I, Abed-Alzahrea A, Wanoose HL. Diabetes screening in Basrah, Iraq: a population-based cross-sectional study. Diabetes Res Clin Pract. 2008;79(1):147–50.
Abu-Zaiton A, Al-Fawwaz A. Prevalence of diabetes, obesity, hypertension and associated factors among students of Al-albayt University, Jordan. World J Med Sci. 2013;9(1):49–54.
Ahmed F, Waslien C, Al-Sumaie MA, Prakash P, Allafi A. Trends and risk factors of hyperglycemia and diabetes among Kuwaiti adults: National Nutrition Surveillance Data from 2002 to 2009. BMC Public Health. 2013;13:103.
Diejomaoh M, Jirous J, Al-Azemi M, Gupta M, Al-Jaber M, Farhat R, et al. Insulin resistance in women with recurrent spontaneous miscarriage of unknown aetiology. Med Princ Pract. 2007;16(2):114–8.
Tohme RA, Jurjus AR, Estephan A. The prevalence of hypertension and its association with other cardiovascular disease risk factors in a representative sample of the Lebanese population. J Hum Hypertens. 2005;19(11):861–8.
Rguibi M, Belahsen R. Prevalence and associated risk factors of undiagnosed diabetes among adult Moroccan Sahraoui women. Public Health Nutr. 2006;9(6):722–7.
Gowri V, Mathew M, Gravell D, AlFalahi K, Zakwani I, Ganguly SS, et al. Protein Z levels in pregnant Omani women: correlation with pregnancy outcome. J Thromb Thrombolysis. 2011;32(4):453–8.
Al-Lawati JA, Al Riyami AM, Mohammed AJ, Jousilahti P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002;19(11):954–7.
Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84(1):99–106.
Al-Nazhan SA, Alsaeed SA, Al-Attas HA, Dohaithem AJ, Al-Serhan MS, Al-Maflehi NS. Prevalence of apical periodontitis and quality of root canal treatment in an adult Saudi population. Saudi Med J. 2017;38(4):413–21.
Saeed AAW. Combined systolic diastolic hypertension among adults in Saudi Arabia: prevalence, risk factors and predictors: results of a national survey. Int J Med Res Health Sci. 2017;6(6):171–6.
Bahijri SM, Jambi HA, Al Raddadi RM, Ferns G, Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia--A Community-Based Survey. PLoS One. 2016;11(4):e0152559. https://doi.org/10.1371/journal.pone.0152559 .
Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7(5):622–32.
Serehi AA, Ahmed AM, Shakeel F, Alkhatani K, El-Bakri NK, Buhari BA, et al. A comparison on the prevalence and outcomes of gestational versus type 2 diabetes mellitus in 1718 Saudi pregnancies. Int J Clin Exp Med. 2015;8(7):11502–7.
PubMed PubMed Central Google Scholar
Al-Rubeaan K, Al-Manaa HA, Khoja TA, Youssef AM, Al-Sharqawi AH, Siddiqui K, et al. A community-based survey for different abnormal glucose metabolism among pregnant women in a random household study (SAUDI-DM). BMJ Open. 2014;4(8):e005906.
Amin TT, Al Sultan AI, Mostafa OA, Darwish AA, Al-Naboli MR. Profile of non-communicable disease risk factors among employees at a Saudi university. Asian Pac J Cancer Prev. 2014;15(18):7897–907.
Wahabi HA, Esmaeil SA, Fayed A, Al-Shaikh G, Alzeidan RA. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res Notes. 2012;5:496.
Saeed AA. Association of tobacco products use and diabetes mellitus-results of a national survey among adults in Saudi Arabia. Balkan Med J. 2012;29(3):247–51. https://doi.org/10.5152/balkanmedj.2012.035 .
Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9:76.
Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31(1):19–23.
Al-Baghli NA, Al-Ghamdi AJ, Al-Turki KA, Al Elq AH, El-Zubaier AG, Bahnassy A. Prevalence of diabetes mellitus and impaired fasting glucose levels in the Eastern Province of Saudi Arabia: results of a screening campaign. Singap Med J. 2010;51(12):923–30.
Al-Qahtani DA, Imtiaz ML, Saad OS, Hussein NM. A comparison of the prevalence of metabolic syndrome in Saudi adult females using two definitions. Metab Syndr Relat Disord. 2006;4(3):204–14.
Shaaban LA, Al-Saleh RA, Alwafi BM, Al-Raddadi RM. Associated risk factors with ante-partum intra-uterine fetal death. Saudi Med J. 2006;27(1):76–9 PubMed PMID: 16432598.
Habib FA. Incidence of post cesarean section wound infection in a tertiary hospital, Riyadh, Saudi Arabia. Saudi Med J. 2002;23(9):1059–63.
Karim A, Ogbeide DO, Siddiqui S, Al-Khalifa IM. Prevalence of diabetes mellitus in a Saudi community. Saudi Med J. 2000;21(5):438–42.
CAS PubMed Google Scholar
Ben Romdhane H, Ben Ali S, Aissi W, Traissac P, Aounallah-Skhiri H, Bougatef S, et al. Prevalence of diabetes in Northern African countries: the case of Tunisia. BMC Public Health. 2014;14:86.
Sulaiman N, Albadawi S, Abusnana S, Mairghani M, Hussein A, Al Awadi F, et al. High prevalence of diabetes among migrants in the United Arab Emirates using a cross-sectional survey. Sci Rep. 2018;8(1):6862.
Shah SM, Ali R, Loney T, Aziz F, ElBarazi I, Al Dhaheri S, et al. Prevalence of Diabetes among migrant women and duration of residence in the United Arab Emirates: a cross sectional study. PLoS One. 2017;12(1):e0169949.
Al Dhaheri AS, Mohamad MN, Jarrar AH, Ohuma EO, Ismail LC, Al Meqbaali FT, et al. A cross-sectional study of the prevalence of metabolic syndrome among young female Emirati adults. PLoS One. 2016;11(7):e0159378.
Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes mellitus prevalence: effect of the laboratory analytical variation. Diabetes Res Clin Pract. 2015;109(3):493–9.
Hajat C, Harrison O, Al SZ. Weqaya: a population-wide cardiovascular screening program in Abu Dhabi, United Arab Emirates. Am J Public Health. 2012;102(5):909–14.
Baynouna LM, Revel AD, Nagelkerke NJ, Jaber TM, Omar AO, Ahmed NM, et al. High prevalence of the cardiovascular risk factors in Al-Ain, United Arab Emirates. An emerging health care priority. Saudi Med J. 2008;29(8):1173–8.
Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract. 2007;78(3):369–77.
Malik M, Bakir A, Saab BA, King H. Glucose intolerance and associated factors in the multi-ethnic population of the United Arab Emirates: results of a national survey. Diabetes Res Clin Pract. 2005;69(2):188–95.
Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: implications of variation in post-partum follow-up criteria. Eur J Obstet Gynecol Reprod Biol. 2004;113(2):149–53.
Gunaid AA, Assabri AM. Prevalence of type 2 diabetes and other cardiovascular risk factors in a semirural area in Yemen. East Mediterr Health J. 2008;14(1):42–56.
Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30.
Engelhardt H, Schulz F, Büyükkeçeci Z. Demographic and Human Development in the Middle East and North Africa. https://doi.org/10.20378/irbo-50993 . Bamberg: University of Bamberg Press, Universitätsbibliothek Bamberg; 2018. 88 Seiten : Illustrationen, Diagramme p.
Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
World health Organization. Global Health Observatory (GHO) data. Overweight and obesity. Noncommunicable diseases. Obesity among adults. 2016. Available at: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/.1-8 Accessed 10 Dec 2018.
El Ati J, Traissac P, Delpeuch F, Aounallah-Skhiri H, Beji C, Eymard-Duvernay S, et al. Gender obesity inequities are huge but differ greatly according to environment and socio-economics in a North African setting: a national cross-sectional study in Tunisia. PLoS One. 2012;7(10):e48153.
World health Organization. Global Health Observatory (GHO) data. Overweight and obesity. Noncommunicable diseases. Mean body mass index (BMI) trends among adults. Available at https://www.who.int/gho/ncd/risk_factors/overweight_obesity/bmi_trends_adults/en/ Accessed 10 Dec 2018.
Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97.
Centers for Disease Control and Prevention. National Diabetes Prevention Program. https://www.cdc.gov/diabetes/prevention/index.html Accessed 10 Mar 2019.
American Diabetes Association. Type 2 Diabetes Risk Test. http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/?loc=atrisk-slabnav Accessed 2 Mar 2019.
Drees BM, Yun S. Reducing the burden of diabetes mellitus in the state of Missouri: a call to action. Mo Med. 2016;113(5):352–7.
Download references
Acknowledgments
Authors are grateful to the Institute of Public Health, College of Medicine and Health Sciences at the United Arab Emirates University for the infrastructure provided.
This systematic review was funded by the Summer Undergraduate Research Experience (SURE) PLUS-Grant of the United Arab Emirates University, 2017 (Research grant: 31M348). The funder had no role in the study design, collection, analysis, or interpretation of the data, nor in writing and the decision to submit this article for publication.
Author information
Authors and affiliations.
Institute of Public Health, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 15551, Al Ain, United Arab Emirates
Rami H. Al-Rifai, Maria Majeed & Faisal Aziz
Department of Biology, College of Sciences, United Arab Emirates University, P.O. Box 15551, Al Ain, United Arab Emirates
Maryam A. Qambar, Ayesha Ibrahim & Khawla M. AlYammahi
You can also search for this author in PubMed Google Scholar
Contributions
RHA conceptualized and designed the study. AI, MM, MQ, KA, and FA assessed the eligibility of the retrieved citations in the titles/abstracts and full-text screening phases. RHA, MM, and FA critically assessed the eligible studies and extracted data. RHA analyzed and interpreted the data. RHA drafted the manuscript. All authors critically reviewed the manuscript. RHA read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rami H. Al-Rifai .
Ethics declarations
Ethics approval and consent to participate.
There are no primary data used in this review. There is no need for any ethical approval or an exemption letter according to the United Arab Emirates University-Human Research Ethics Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA checklist.
Additional file 2.
Search strategies for the six databases, from January 1, 2000 to July 12, 2018.
Additional file 3
Funnel plots examining small-study effects on the pooled T2DM prevalence among women of childbearing age. Egger’s test p <0.0001.
Additional file 4
Funnel plots examining small-study effects on the pooled pre-DM prevalence among women of childbearing age. Egger’s test p <0.0001.
Additional file 5.
Weighted prevalence of T2DM and pre-DM in childbearing age women in MENA countries according to age group.
Additional file 6.
Sub-regional weighted prevalence of T2DM in women of childbearing age according to the tested population, data collection period, T2DM ascertainment, sample size, and overall, in 14 MENA countries.
Additional file 7.
Sub-regional weighted prevalence of T2DM (Figure 1 ) and pre-DM (Figure 2 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Square represents the estimated prevalence and lines around the square represent the upper and lower limit of the 95% confidence interval of the prevalence.
Additional file 8.
Timeline view of the weighted prevalence of T2DM (Figure 1 ) and pre-DM (Figure 2 ) in women of childbearing age, by publication year.
Additional file 9.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on T2DM prevalence in women of childbearing age by the different measured characteristics.
Additional file 10.
Sub-regional weighted prevalence of pre-DM in childbearing age women according to the tested population, data collection period, Pre-DM ascertainment, sample size, and overall, in the four sub regions of the 10 MENA countries.
Additional file 11.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on pre-DM prevalence in women of childbearing age by the different measured characteristics.
Additional file 12.
Quality assessment of the 48 research reports included in the analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Al-Rifai, R.H., Majeed, M., Qambar, M. et al. Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000–2018. Syst Rev 8 , 268 (2019). https://doi.org/10.1186/s13643-019-1187-1
Download citation
Received : 17 March 2019
Accepted : 07 October 2019
Published : 08 November 2019
DOI : https://doi.org/10.1186/s13643-019-1187-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Women of childbearing age
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Registered Report Protocol
Registered Report Protocols describe a study’s rationale and methods for which the planned work was peer-reviewed prior to data collection.
See all article types »
Type 1 and type 2 diabetes mellitus: Clinical outcomes due to COVID-19. Protocol of a systematic literature review
Contributed equally to this work with: Juan Pablo Pérez Bedoya, Alejandro Mejía Muñoz
Roles Conceptualization, Investigation, Methodology, Project administration, Writing – original draft
* E-mail: [email protected]
Current address: National Faculty of Public Health, University of Antioquia, Medellin, Antioquia, Colombia
Affiliation Epidemiology Group, National Faculty of Public Health, University of Antioquia, Medellín, Colombia
Affiliation Biology and Control of Infectious Diseases Group, Faculty of Exact and Natural Sciences, University of Antioquia, Medellín, Colombia
Roles Supervision, Validation, Writing – review & editing
¶ ‡ NCB and PADV also contributed equally to this work.
Affiliation Department of Translational Medicine, Herbert Wertheim College of Medicine & Department of Global Health, Robert Stempel College of Public Health and Social Work, Florida International University, Miami, FL, United States of America
- Juan Pablo Pérez Bedoya,
- Alejandro Mejía Muñoz,
- Noël Christopher Barengo,
- Paula Andrea Diaz Valencia

- Published: September 9, 2022
- https://doi.org/10.1371/journal.pone.0271851
- See the preprint
- Peer Review
- Reader Comments
Introduction
Diabetes has been associated with an increased risk of complications in patients with COVID-19. Most studies do not differentiate between patients with type 1 and type 2 diabetes, which correspond to two pathophysiological distinct diseases that could represent different degrees of clinical compromise.
To identify if there are differences in the clinical outcomes of patients with COVID-19 and diabetes (type 1 and type 2) compared to patients with COVID-19 without diabetes.
Observational studies of patients with COVID-19 and diabetes (both type 1 and type 2) will be included without restriction of geographic region, gender or age, whose outcome is hospitalization, admission to intensive care unit or mortality compared to patients without diabetes. Two authors will independently perform selection, data extraction, and quality assessment, and a third reviewer will resolve discrepancies. The data will be synthesized regarding the sociodemographic and clinical characteristics of patients with diabetes and without diabetes accompanied by the measure of association for the outcomes. The data will be synthesized regarding the sociodemographic and clinical characteristics of patients with diabetes and without diabetes accompanied by the measure of association for the outcomes.
Expected results
Update the evidence regarding the risk of complications in diabetic patients with COVID-19 and in turn synthesize the information available regarding type 1 and type 2 diabetes mellitus, to provide keys to a better understanding of the pathophysiology of diabetics.
Systematic review registry
This study was registered at the International Prospective Registry for Systematic Reviews (PROSPERO)— CRD42021231942 .
Citation: Pérez Bedoya JP, Mejía Muñoz A, Barengo NC, Diaz Valencia PA (2022) Type 1 and type 2 diabetes mellitus: Clinical outcomes due to COVID-19. Protocol of a systematic literature review. PLoS ONE 17(9): e0271851. https://doi.org/10.1371/journal.pone.0271851
Editor: Alok Raghav, GSVM Medical College, INDIA
Received: July 7, 2022; Accepted: August 23, 2022; Published: September 9, 2022
Copyright: © 2022 Pérez Bedoya et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding: This research was developed within the framework of the project "Repository for the surveillance of risk factors for chronic diseases in Colombia, the Caribbean and the Americas" and has the financial support of the Ministry of Science, Technology and Innovation of Colombia—Minciencias 844 (grant number 111584467754). The opinions expressed are those of the authors and not necessarily of Minciencias.
Competing interests: The authors have declared that no competing interests exist.
The Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2), the causal viral agent of coronavirus disease 2019 (COVID-19), currently has the world in one of the greatest public health crises of recent times since its appearance at the end of 2019 in the city of Wuhan, China [ 1 ]. The infection has a mild or even asymptomatic course in most cases, but in elderly patients (over 60 years-of-age) and in those with pre-existing chronic comorbidities, it can progress severe complications such as pneumonia, acute respiratory distress (ARDS) with hyperinflammatory involvement and multi-organ failure, leading in some cases to death [ 2 ].
Different studies have reported that patients diagnosed with diabetes who suffer from COVID-19 disease have higher morbidity and mortality compared with people without diabetes [ 3 ]. An analysis by Gude Sampedro et al. using prognostic models found that diabetic patients had greater odds of being hospitalized (OR 1.43; 95% CI: 1.18 to 1.73), admitted to the intensive care unit (OR 1.61; 95% CI: 1.12 to 2.31) and dying from COVID-19 (OR 1.79; 95% CI %: 1.38 to 2.32) compared with patients without diabetes [ 4 ]. However, it is difficult to establish whether diabetes alone directly contributed to the increase likelihood of complications.
Several studies using secondary data have emerged during the course of the pandemic that seek to determine the association of diabetes with mortality and other clinical outcomes in patients with COVID-19, such as, for example, a meta-analysis carried out by Shang et al. of severe infection and mortality from COVID-19 in diabetic patients compared with those without diabetes. They reported that patients with COVID-19 and diabetes had higher odds of serious infection (OR = 2.38, 95% CI: 2.05 to 2.78) and mortality (OR = 2, 21, 95% CI: 1.83 to 2.66) than patients without diabetes [ 5 ]. Despite the fact that there are several primary studies that attempt to explain the association between diabetes and COVID-19, most studies lack epidemiological rigor in the design and methodology used [ 6 ]. In addition, many of them did not distinguish between type 1 and type 2 diabetes, which are two very different conditions with different clinical development and pathophysiological mechanisms [ 7 ]. This may lead to different degrees of clinical complications from COVID-19. Currently, there is a gap in knowledge about the complications in patients with COVID-19 according to the type of diabetes. Moreover, only limited information exist how COVID-19 affects type 1 patients [ 8 , 9 ].
The objective of this systematic literature review will be to identify whether there are differences in the clinical outcomes of both type 1 and type 2 diabetes patients diagnosed with COVID-19 compared with patients with COVID-19 without a diagnosis of diabetes. This study will provide scientific evidence regarding the risk of complications in diabetic patients with COVID-19 and, in turn, synthesize the available information regarding to type 1 and type 2 diabetes.
Study design
This systematic literature review protocol was prepared according to the Preferred Reporting Elements for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [ 10 ] ( S1 Appendix ). The results of the final systematic review will be reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) [ 11 , 12 ]. In the event of significant deviations from this protocol, they will be reported and published with the results of the review.
Eligibility criteria
Participants (population)..
Patients with a confirmed diagnosis of COVID-19 without restriction of geographic region, sex, or age. For the diagnosis of COVID-19, the operational definition of confirmed case of the World Health Organization in its latest update will be used as a reference. Confirmed case of SARS-CoV-2 infection: a person with a positive Nucleic Acid Amplification Test (NAAT), regardless of clinical criteria OR epidemiological criteria or a person meeting clinical criteria AND/OR epidemiological criteria (suspect case A) with a positive professional- use or self-test SARS-CoV-2 Antigen RDT [ 13 ].
Patients with COVID-19 and concomitant diagnosis of unspecified diabetes mellitus, differentiated into type 1 diabetes mellitus or type 2 diabetes mellitus, without restriction of geographic region, gender, or age of the patients, who present definition of clinical criteria and /or paraclinical tests used by researchers to classify patients according to their diabetes status.
The operational definition of a confirmed case of diabetes mellitus provided by the American Diabetes Association will be used as a guide. The reference diagnostic criteria for diabetes are fasting plasma glucose ≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 h or 2-h plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during OGTT or hemoglobin A1C ≥6.5% (48 mmol/mol) or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, at random plasma glucose ≥200 mg/dL [ 14 ].
In selected primary studies, identification of diabetes status may be based on medical history and International Classification of Diseases codes for type 1 or type 2 diabetes, use of antidiabetic medications, or previously defined diagnostic criteria.
Comparator.
Patients with COVID-19 who do not have a concomitant diagnosis of diabetes mellitus.
The main endpoint is all-cause mortality (according to the definitions of each primary study) and the secondary outcomes are hospitalization and admission to the ICU, where the authors specify a clear definition based on clinical practice guidelines and provide a well-defined criteria for patient outcomes.
Type of study.
Primary observational original research studies (prospective or retrospective cohort, case-control design, and cross-sectional studies) will be included in this systematic review.
Exclusion criteria
Clinical trials, editorials, letters to the editor, reviews, case reports, case series, narrative reviews or systematic reviews and meta-analyses, as well as research in the field of basic sciences based on experimental laboratory models, will be excluded. Original research articles that only include other types of diabetes, such as monogenic diabetes, gestational diabetes, latent autoimmune diabetes in adults, ketosis-prone diabetes, among others, or articles with publication status prior to publication will not be considered. In addition, articles whose main hypothesis is not diabetes and do not have the established outcomes will be excluded.
Information sources and search strategy
Electronic bibliographic databases..
For the preparation of the search strategy, the recommendations of the PRISMA-S guide [ 15 ] will be adopted. Relevant articles will be identified by electronic search applying the equation previously developed by the researchers and validated by an expert librarian ( S2 Appendix ). The following electronic bibliographic databases will be used: MEDLINE, EMBASE, LILACS, OVID MEDLINE, WHO (COVID-19 Global literature on coronavirus disease) and SCOPUS with a publication date from December 2019 to August 15, 2022, without language restriction.
The search for potential primary studies published in gray literature will be performed through the World Health Organization database for COVID-19 (WHO COVID-19 Global literature on coronavirus disease). This database contains different electronic bibliographic databases incorporated into its browser, including Web of Science, EuropePMC and Gray literature, among others.
Unlike electronic bibliographic databases.
To identify other potentially eligible studies, the references of relevant publications will be reviewed to perform a snowball manual search. This technique consists of searching for new articles from the primary studies already selected in order to guarantee exhaustiveness in the search.
Study selection process
Two researchers will independently evaluate all the titles and abstracts of the retrieved articles, using the free access Rayyan® software [ 16 ] with previously established selection criteria. Disagreements will be resolved in first instance through discussion and in the second instance through a third reviewer. Subsequently, the full text of the articles selected in the eligibility phase will be read independently by two researchers, both using the same instrument previously validated in Excel according to predefined criteria. Discrepancies will be resolved by discussion or a third reviewer. The process of identification, selection and inclusion of primary studies will be described and presented using the flowchart recommended by the PRISMA statement in its latest version 2020 [ 11 , 12 ].
Data collection and extraction
Standardized and validated forms will be used to collect the data extracted from the primary studies, accompanied by a detailed instruction manual to specify the guiding questions, and avoid the introduction of bias. Data will be extracted from those articles in full text format. If the full text is not available, contact the author or search for the manuscript with the help of the library system. This process will be carried out by two researchers independently. A third investigator will verify the extracted data to ensure the accuracy of the records. The authors of the primary studies will be contacted to resolve any questions that may arise. The reviewers will resolve the disagreements through discussion and one of the two referees will adjudicate the discrepancies presented through discussion and consensus.
In specific terms, the following data will be collected both for the primary studies that report diabetes and COVID-19 and for those that differentiate between DMT1 and DMT2: author, year and country where the study was carried out; study design; general characteristics of the population, sample size, demographic data of the participants (sex, age, ethnicity), percentage of patients with diabetes, percentage of patients with type 1 and/or type 2 diabetes, percentage of patients without diabetes, frequency of comorbidities in diabetics and non-diabetics, percentage of diabetic and non-diabetic patients who presented the outcomes (hospitalization, ICU admission and mortality) and association measures reported for the outcomes. Data extraction will be done using a Microsoft Excel 365 ® spreadsheets.
Quality evaluation
The study quality assessment tool provided by the National Institutes of Health (NIH) [ 17 ] will be used for observational studies such as cohort, case-control, and cross-sectional. Two tools will be sued: one for cohort and cross-sectional studies (14 questions/domains) and one for case-control studies (12 questions/domains). These tools are aimed at detecting elements that allow evaluation of possible methodological problems, including sources of bias (for example, patient selection, performance, attrition and detection), confounding, study power, the strength of causality in the association between interventions and outcomes, among other factors. The different tools that will be used reflect a score of "1" or "0" depending on the answer "yes" or "no", respectively for each question or domain evaluated, or failing that, the indeterminate criterion option. For observational cohort studies, which consist of 14 risk of bias assessment domains, the studies will be classified as having good quality if they obtain ≥10 points, of fair quality if they obtain 8 to 9 points, and of poor quality if they obtain less than 8 points. On the other hand, in the case of case-control studies that consist of 12 bias risk assessment domains, the studies will be classified as good quality if they obtained ≥8 points, regular quality if they obtained 6 to 7 points and of poor quality if they obtained less than 6 points. However, the internal discussion between the research team will always be considered as the primary quality criterion.
Data synthesis
A narrative synthesis with summary tables will be carried out according to the recommendations adapted from the Synthesis Without Meta-analysis (SWiM) guide to describe in a structured way the methods used, and the findings found in the primary studies, as well as the criteria for grouping of the studies [ 18 ]. A narrative synthesis will be presented in two sections, one for patients with COVID-19 and diabetes and another for patients with COVID-19 and type 1 or type 2 diabetes.
Assessment of clinical and methodological heterogeneity will determine the feasibility of the meta-analysis. Possible sources of heterogeneity identified are the clinical characteristics of the study population, the criteria used to define the outcomes in the groups of patients, the time period of the pandemic in which the study was carried out, and the availability of measurement and control for potential confounding factors. For this reason, it is established a priori that this diversity of findings will make it difficult to carry out an adequate meta-analysis [ 19 ]. However, if meta-analysis is considered feasible, the random effects model will be used due to the high probability of heterogeneity between studies. Statistical heterogeneity will be assessed using the X 2 test and the I 2 statistic, and publication bias assessed using funnel plots if there are sufficient (>10) studies [ 20 ].
Exploratory ecological analysis
An exploratory ecological analysis of the association between the frequency of clinical outcomes of diabetic patients with COVID-19 and the indicators related to the health care dimension, reported for the different countries analyzed by means of the correlation coefficient, will be carried out. The open public databases of the World Bank (WB) [ 21 ], the World Health Organization (WHO) [ 22 ] and Our World In Data [ 23 ] will be used to extract population indicators related to health care, among those prioritized, universal health coverage, hospital beds per 1,000 people, doctors per 1,000 people, current health spending as a percentage of gross domestic product (GDP), percentage of complete vaccination coverage for COVID-19.
Since the first epidemiological and clinical reports were released from the city of Wuhan regarding the clinical characteristics of patients with COVID-19, a high incidence of chronic non-communicable diseases has been observed in Covid-19 patients. Current scientific evidence has shown that certain comorbidities increase the risk for hospitalization, severity of illness or death from COVID-19, such as hypertension, cardiovascular disease, chronic kidney disease, chronic respiratory disease, diabetes, among others [ 24 ].
One of the main chronic comorbidities affected by the COVID-19 pandemic is diabetes. Multivariate analysis of several observational epidemiological studies have revealed that COVID-19 patients with diabetes were at increased risk of hospitalization, ICU admission, and mortality compared with patients without diabetes [ 4 ].
For this reason, it is expected that this systematic literature review will provide scientific support regarding the outcomes and complications that patients diagnosed with COVID-19 with type 1 or type 2 diabetes present compared with patients without diabetes. This information will be useful for healthcare personnel, public health professionals and epidemiologists involved in patient care or decision making, generating epidemiological evidence. Thus, highlighting the decisive role of epidemiological research in the context of the pandemic, especially in the field of diabetes epidemiology may improve comprehensive management and care of diabetic patients. This study may also provide important information that can be used to update of clinical practice guidelines.
Limitations
There are some potential limitations to the proposed systematic review. Firstly, both type 1 and type 2 diabetes may have different key search terms and some studies may be missed. To minimize this limitation, different search equations have been designed for each database in an exhaustive and sensitive manner. In addition to reading references and level ball as an additional strategy. Another limitation is that observational studies evaluating the effect of an intervention may be susceptible to significant confounding bias and may present high heterogeneity in the findings. To report these possible biases, an adequate quality assessment will be carried out, with highly sensitive and previously validated tools, exclusive for each type of observational design. The review is intended for publication in a peer-reviewed journal.
The status of the study
The study is in the selection phase of the records by applying the eligibility criteria to the titles and abstracts. Completion of the project is expected in September 2022 with the publication of the results.
Conclusions
This report describes the systematic review protocol that will be utilized to update the evidence regarding the risk of complications in diabetic patients with COVID-19 and in turn synthesize the information available regarding DM1 and DM2, to provide keys to a better understanding of the pathophysiology of diabetics.
Supporting information
S1 appendix. prisma-p (preferred reporting items for systematic review and meta-analysis protocols) 2015 checklist: recommended items to address in a systematic review protocol..
https://doi.org/10.1371/journal.pone.0271851.s001

S2 Appendix. Search string details for each database.
https://doi.org/10.1371/journal.pone.0271851.s002
- View Article
- PubMed/NCBI
- Google Scholar
- 13. World Health Organization. WHO COVID-19 Case definition Updated in Public health surveillance for COVID-19. 2022 July 22. [cited 18 August 2022]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1
- 17. National Institutes of Health (NIH) [Internet]. Study Quality Assessment Tools; 2021. [cited 16 June 2022]. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 19. Cochrane Handbook for Systematic Reviews of Interventions; 2022. [cited 16 June 2022]. Available at: https://training.cochrane.org/handbook/current
- 21. World Bank Group [Internet]. World Bank Indicators; 2022. [cited 16 June 2022]. Available at: https://datos.bancomundial.org/indicador
- 22. World Health Organization (WHO) [Internet]. Global Health Observatory Data Repository; 2021. [cited 16 June 2022]. Available at: https://apps.who.int/gho/data/node.home
- 23. Our World In Data [Internet]. Statistics and Research Coronavirus Pandemic (COVID-19); 2022. [cited 16 June 2022]. Available at: https://ourworldindata.org/coronavirus
- 24. Centers for Disease Control and Prevention [Internet]. COVID-19. People with Certain Medical Conditions; 2021. [cited 16 June 2022]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=ht
6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024
Collaborators.
- American Diabetes Association Professional Practice Committee : Nuha A ElSayed , Grazia Aleppo , Raveendhara R Bannuru , Dennis Bruemmer , Billy S Collins , Laya Ekhlaspour , Marisa E Hilliard , Eric L Johnson , Kamlesh Khunti , Ildiko Lingvay , Glenn Matfin , Rozalina G McCoy , Mary Lou Perry , Scott J Pilla , Sarit Polsky , Priya Prahalad , Richard E Pratley , Alissa R Segal , Jane Jeffrie Seley , Elizabeth Selvin , Robert C Stanton , Robert A Gabbay
- PMID: 38078586
- PMCID: PMC10725808 (available on 2025-01-01 )
- DOI: 10.2337/dc24-S006
The American Diabetes Association (ADA) "Standards of Care in Diabetes" includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, an interprofessional expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
© 2024 by the American Diabetes Association.
Publication types
- Diabetes Mellitus* / therapy
- Endocrinology*
- Hypoglycemia* / diagnosis
- Hypoglycemia* / prevention & control
- Reference Standards
- Societies, Medical
- Standard of Care
Grants and funding
- K23 DK121942/DK/NIDDK NIH HHS/United States
- Associations
- Working Groups
- Escardio.org
- ESC eLearning

Show navigation Hide navigation
- European Society of Cardiology
- Diabetes and CVD Programme
- Recommended Reading
Literature review: Diabetes as risk factor for cardiovascular disease
Comment by elisa dal canto and joline wj beulens, eapc diabetes and cvd educational programme amsterdam university medical centers – location vu.
Diabetes mellitus (DM) is an important risk factor for cardiovascular disease (CVD), which in turn represents the principal cause of disability and mortality in individuals with DM. The pathophysiological basis of this link is atherosclerosis, which accounts for the majority of CVD and it is accelerated by DM. However, conventional vascular risk factors (e.g., obesity, physical inactivity, hypertension and high cholesterol level) cannot fully explain the excess CVD risk associated with DM, although HbA1c appears to be an important mediator (1).
Furthermore, while myocardial infarction (MI) and stroke are the most commonly investigated complications of DM, the associations of DM with other vascular disorders are for a large part unknown. Two recent large scale studies that thoroughly investigated the relationship between DM and the risk of CVD shed some light on this topic. A meta-analysis of 102 studies from the Emerging Risk Factors Collaboration including data of 698,782 people without initial CVD evaluated the associations between DM and fasting plasma glucose (FPG) concentrations and a wide range of fatal and non-fatal cardiovascular outcomes (2). According to their results, DM accounted for 11% of vascular deaths which corresponds to an estimated 325,000 death/year in high income countries alone. Subjects with DM compared to those without it, had about a two-fold higher risk for (CHD), ischaemic stroke, unclassified stroke, and deaths for other vascular diseases, independently of other risk factors (age, sex, smoking status, BMI, and systolic blood pressure) (2). Interestingly, greater HRs for CHD with DM were found in groups at lower absolute cardiovascular risk, such as younger individuals, women, non-smokers, those with below average BMI and with below average systolic blood pressure. In subjects with DM, FPG concentrations were non-linearly related to risk of CHD or ischaemic stroke (2), but information about haemoglobin A1c (HbA1c), which is known to be more strongly associated with CVD as compared with FPG (3), were not provided. A large prospective cohort using health records from the CALIBER programme implemented the knowledge on the link between DM and CVD evaluating the association between diabetes status and 12 different initial cardiovascular presentations, including angina, MI, peripheral arterial disease, heart failure (HF), abdominal aortic aneurysm (4). The cohort consisted of 1,921,260 individuals, of whom 34,198 (1.8%) had type 2 DM. 113,638 cardiovascular events occurred during a median follow-up time of 5.5 years; among these, peripheral arterial disease was the first presentation in 992 (16.2%) of the 6137 subjects with type 2 DM who had cardiovascular events whereas HF was the first presentation in 866 (14.1%) of them (5). This result is consistent with the previous finding of a high prevalence of HF among diabetic patients (30.6%); interestingly, the proportion of patients with preserved (HFpEF) vs reduced ejection fraction (HFrEF) was considerably higher (24.8% vs 5.8%) (5). This suggests that other pathophysiological mechanisms, in addition to atherosclerosis, affect the myocardium of subjects with DM, such as cardiomyocyte hypertrophy, caused by hyperinsulinemia and endothelial dysfunction caused by hyperglycaemia (6). While hyperglycaemia is one of the principal causes of macrovasculopathy In DM, it also represents the driver of microvascular complications (retinopathy, nephropathy, and neuropathy). In this regard, macro- and microvascular complications can be seen as part of the same spectrum, as the two disorders are strongly interconnected, and thus require a common preventive strategy. Taken together these data not only confirm that DM represents a strong and independent risk factor for CVD, but they also point out that the association is not restricted to CHD or stroke goes but concerns multiple acute and chronic cardiovascular outcomes.
Moreover, DM confers an increased risk of CHD even in those categories such as women and young individuals which are traditionally regarded as low risk groups. These findings have several important implications; the surveillance and prevention of DM complications should be directed towards multiple diseases and multiple subgroups of patients, and DM clinical trials should start considering other CV events, in addition to MI and stroke, as possible outcomes.
Note : The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology
Elisa Dal Canto, Joline WJ Beulens were commenting on these articles:
Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-22.
Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105-13
1. Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ. 2001 Jan 6;322(7277):15-8. 2. Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-22. 3. Selvin E, Steffes MW, Zhu H, et al. Glycated haemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800-11. 4. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105-13. 5. Boonman-de Winter LJ, Rutten FH, Cramer MJ, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55(8):2154-62. 6. Petar M. Seferović, Walter J. Paulus; Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes, Eur Heart J. 2015;36(27):1718-27.

- ESC Board and Committees
- ESC Policies
- Statutes & Reports
- ESC Press Office
- Press Releases
- ESC Congress
- ESC Cardio Talk
- Our Offices
- Conference Facilities
- Jobs in Cardiology
- Terms & Conditions
- Update your cookie settings
Need help?
Help centre Contact us
© 2024 European Society of Cardiology. All rights reserved.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 July 2015
Type 2 diabetes mellitus
- Ralph A. DeFronzo 1 ,
- Ele Ferrannini 2 ,
- Leif Groop 3 ,
- Robert R. Henry 4 ,
- William H. Herman 5 ,
- Jens Juul Holst 6 ,
- Frank B. Hu 7 ,
- C. Ronald Kahn 8 ,
- Itamar Raz 9 ,
- Gerald I. Shulman 10 ,
- Donald C. Simonson 11 ,
- Marcia A. Testa 12 &
- Ram Weiss 13
Nature Reviews Disease Primers volume 1 , Article number: 15019 ( 2015 ) Cite this article
47k Accesses
1084 Citations
125 Altmetric
Metrics details
- Diabetes complications
- Type 2 diabetes
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both microvascular complications (including retinopathy, nephropathy and neuropathy) and macrovascular complications (such as cardiovascular comorbidities), owing to hyperglycaemia and individual components of the insulin resistance (metabolic) syndrome. Environmental factors (for example, obesity, an unhealthy diet and physical inactivity) and genetic factors contribute to the multiple pathophysiological disturbances that are responsible for impaired glucose homeostasis in T2DM. Insulin resistance and impaired insulin secretion remain the core defects in T2DM, but at least six other pathophysiological abnormalities contribute to the dysregulation of glucose metabolism. The multiple pathogenetic disturbances present in T2DM dictate that multiple antidiabetic agents, used in combination, will be required to maintain normoglycaemia. The treatment must not only be effective and safe but also improve the quality of life. Several novel medications are in development, but the greatest need is for agents that enhance insulin sensitivity, halt the progressive pancreatic β-cell failure that is characteristic of T2DM and prevent or reverse the microvascular complications. For an illustrated summary of this Primer, visit: http://go.nature.com/V2eGfN
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
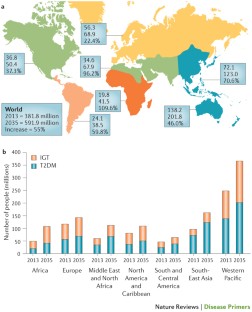
Similar content being viewed by others
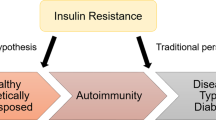
Double or hybrid diabetes: A systematic review on disease prevalence, characteristics and risk factors
Novel therapies with precision mechanisms for type 2 diabetes mellitus
Heterogeneity and endotypes in type 1 diabetes mellitus
DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58 , 773–795 (2009). A classic review of the aetiology of T2DM, with a therapeutic approach based on its pathophysiology.
Article CAS PubMed PubMed Central Google Scholar
Abdul-Ghani, M. A., Tripathy, D. & DeFronzo, R. A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29 , 1130–1139 (2006).
Article CAS PubMed Google Scholar
Gerstein, H. C. et al . Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 78 , 305–312 (2007).
Article PubMed Google Scholar
Hawa, M. I. et al . Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care 36 , 908–913 (2013).
Article PubMed PubMed Central Google Scholar
Gardner, D. S. & Tai, E. S. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes. Metab. Syndr. Obes. 5 , 101–108 (2012).
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 37 , S14–S80 (2014). A comprehensive overview of the standards of medical care published by the ADA.
Article Google Scholar
DeFronzo, R. A. & Abdul-Ghani, M. A. Preservation of β-cell function: the key to diabetes prevention. J. Clin. Endocrinol. Metab. 96 , 2354–2366 (2011).
Ferrannini, E., Gastaldelli, A. & Iozzo, P. Pathophysiology of prediabetes. Med. Clin. North Am. 95 , 327–339 (2011).
Garvey, W. T. et al . Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 37 , 912–921 (2014).
Nathan, D. M. et al . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30 , 753–759 (2007).
DeFronzo, R. A. et al . Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364 , 1104–1115 (2011). A large prospective study demonstrating the efficacy of thiazolidinediones in preventing the progression of IGT to T2DM.
Zinman, B. et al . Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 376 , 103–111 (2010).
Dansinger, M. L., Tatsioni, A., Wong, J. B., Chung, M. & Balk, E. M. Meta-analysis: the effect of dietary counseling for weight loss. Ann. Intern. Med. 147 , 41–50 (2007).
Purcell, K. et al . The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2 , 954–962 (2014).
Ali, M. K., Echouffo-Tcheugui, J. & Williamson, D. F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. (Millwood) 31 , 67–75 (2012).
Tuomilehto, J. et al . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344 , 1343–1350 (2001).
Inzucchi, S. E. et al . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35 , 1364–1379 (2012). ADA position statement on the treatment of T2DM, advocating a stepped care approach starting with metformin.
American Association of Clinical Endocrinologists. AACE Comprehensive Diabetes Algorithm 2013 Consensus Statement. Endocr. Pract. Suppl. 1 , 1–87 (2015). AACE position statement on the treatment of T2DM, advocating initial monotherapy or combination therapy based upon the starting HbA1c, and recommending various antidiabetic medications as initial therapy.
Google Scholar
Pozzilli, P. et al . The A1C and ABCD of glycaemia management in type 2 diabetes: a physician's personalized approach. Diabetes Metab. Res. Rev. 26 , 239–244 (2010). The first published report by key opinion leaders recommending individualized therapy based on the age and body weight of patients, the presence or absence of complications, and duration and aetiology of disease.
International Diabetes Federation. IDF Diabetes Atlas 6th Edition. IDF [online] , (2013).
Hu, F. B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34 , 1249–1257 (2011). An important study emphasizing the role of diet, physical activity and genes — beyond obesity — in the diabetes epidemic that is engulfing Asian countries as they are exposed to westernization.
Chan, J. C. et al . Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301 , 2129–2140 (2009).
Ley, S. H., Hamdy, O., Mohan, V. & Hu, F. B. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383 , 1999–2007 (2014).
Grøntved, A., Rimm, E. B., Willett, W. C., Andersen, L. B. & Hu, F. B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 172 , 1306–1312 (2012).
Grøntved, A. & Hu, F. B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 305 , 2448–2455 (2011).
Cappuccio, F. P., D'Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33 , 414–420 (2009).
Pan, A., Schernhammer, E. S., Sun, Q. & Hu, F. B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 8 , e1001141 (2011).
Barnett, A. H., Eff, C., Leslie, R. D. & Pyke, D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia 20 , 87–93 (1981).
Wang, Y. C., McPherson, K., Marsh, T., Gortmaker, S. L. & Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378 , 815–825 (2011).
Wang, X. et al . Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36 , 166–175 (2013).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302 , 179–188 (2009).
Ding, E. L. et al . Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361 , 1152–1163 (2009).
Wang, T. J. et al . Metabolite profiles and the risk of developing diabetes. Nat. Med. 17 , 448–453 (2011).
Esteve, E., Ricart, W. & Fernández-Real, J.-M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care 14 , 483–490 (2011).
Hu, F. B. et al . Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Schellenberg, E. S., Dryden, D. M., Vandermeer, B., Ha, C. & Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes. Ann. Intern. Med. 159 , 543–551 (2013). A comprehensive review of the effectiveness of lifestyle intervention in the treatment of T2DM, emphasizing that, although initially successful, most subjects with diabetes regain the majority of lost weight over the subsequent 3–5 years.
DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53 , 1270–1287 (2010). A comprehensive review describing the role of excess tissue lipid deposition in the development of insulin resistance, β-cell failure and atherosclerotic cardiovascular disease: that is, lipotoxicity.
Hemminki, K., Li, X., Sundquist, K. & Sundquist, J. Familial risks for type 2 diabetes in Sweden. Diabetes Care 33 , 293–297 (2010).
Groop, L. et al . Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 45 , 1585–1593 (1996).
Lyssenko, V. et al . Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54 , 166–174 (2005).
Grant, S. F. et al . Variant of transcription factor 7-like 2 ( TCF7L2 ) gene confers risk of type 2 diabetes. Nat. Genet. 38 , 320–323 (2006).
Lyssenko, V. et al . Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Invest. 117 , 2155–2163 (2007).
Sladek, R. et al . A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445 , 881–885 (2007).
Saxena, R. et al . Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 , 1331–1336 (2007).
Morris, A. P. et al . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44 , 981–990 (2012).
Flannick, J. et al . Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46 , 357–363 (2014).
Lyssenko, V. et al . Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41 , 82–88 (2009).
Rosengren, A. H. et al . Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327 , 217–220 (2010).
Tang, Y. et al . Genotype-based treatment of type 2 diabetes with an α2A-adrenergic receptor antagonist. Sci. Transl Med. 6 , 257ra139 (2014). These paper provides an example in which a genetic finding in an animal model of diabetes has been translated into a drug target in humans, the ADRA2A gene.
De Jesus, D. F. & Kulkarni, R. N. Epigenetic modifiers of islet function and mass. Trends Endocrinol. Metab. 25 , 628–636 (2014).
Ozcan, S. Minireview: microRNA function in pancreatic β cells. Mol. Endocrinol. 28 , 1922–1933 (2014).
Lyssenko, V. et al . Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 359 , 2220–2232 (2008). This paper presents a genetic explanation for the development of T2DM.
Travers, M. E. et al . Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 62 , 987–992 (2013).
Gulli, G., Ferrannini, E., Stern, M., Haffner, S. & DeFronzo, R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 41 , 1575–1586 (1992).
Martin, B. C. et al . Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340 , 925–929 (1992).
Ferrannini, E. & Mari, A. β-cell function in type 2 diabetes. Metabolism 63 , 1217–1227 (2014).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383 , 1068–1083 (2014).
Muller, D. C., Elahi, D., Tobin, J. D. & Andres, R. Insulin response during the oral glucose tolerance test: the role of age, sex, body fat and the pattern of fat distribution. Aging (Milano) 8 , 13–21 (1996).
CAS Google Scholar
Nauck, M. A., Vardarli, I., Deacon, C. F., Holst, J. J. & Meier, J. J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54 , 10–18 (2011).
Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 16 , 9–21 (2014).
Bays, H., Mandarino, L. & DeFronzo, R. A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89 , 463–478 (2004).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510 , 84–91 (2014). An excellent review of the specific lipid varieties and the molecular events through which they cause insulin resistance in the liver.
Bensellam, M., Laybutt, D. R. & Jonas, J.-C. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol. Cell. Endocrinol. 364 , 1–27 (2012).
Ritzel, R. A., Meier, J. J., Lin, C.-Y., Veldhuis, J. D. & Butler, P. C. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 56 , 65–71 (2007).
Collins, S., Pi, J. & Yehuda-Shnaidman, E. Uncoupling and reactive oxygen species (ROS) — a double-edged sword for β-cell function? “Moderation in all things”. Best Pract. Res. Clin. Endocrinol. Metab. 26 , 753–758 (2012).
Cabrera, O. et al . The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103 , 2334–2339 (2006).
Hodson, D. J. et al . Lipotoxicity disrupts incretin-regulated human β cell connectivity. J. Clin. Invest. 123 , 4182–4194 (2013).
Brandhorst, H., Brandhorst, D., Brendel, M. D., Hering, B. J. & Bretzel, R. G. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 7 , 489–495 (1998).
Rahier, J., Guiot, Y., Goebbels, R. M., Sempoux, C. & Henquin, J. C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10 (Suppl. 4), 32–42 (2008). A post-mortem study demonstrating a decline in β-cell mass with preservation of α-cell mass in individuals with T2DM.
Marselli, L. et al . Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 57 , 362–365 (2014).
Marchetti, P. et al . The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50 , 2486–2494 (2007).
Marchetti, P. & Masini, M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 5 , 1055–1056 (2009).
Gupta, D. & Leahy, J. L. Islet amyloid and type 2 diabetes: overproduction or inadequate clearance and detoxification? J. Clin. Invest. 124 , 3292–3294 (2014).
Mezza, T. et al . Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 63 , 994–1007 (2014). This work in human islets describes the impact of insulin resistance on the relative proportion of α-cells and β-cells, and the functional consequences — in terms of insulin and glucagon secretion — of this chronic adaptation.
Deng, S. et al . Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53 , 624–632 (2004).
Igoillo-Esteve, M. et al . Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 53 , 1395–1405 (2010).
Giacca, A., Xiao, C., Oprescu, A. I., Carpentier, A. C. & Lewis, G. F. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am. J. Physiol. Endocrinol. Metab. 300 , E255–E262 (2010).
Halban, P. A. et al . β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 99 , 1983–1992 (2014).
Ferrannini, E. et al . Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 54 , 1507–1516 (2011). This longitudinal study of non-diabetic subjects identifies baseline insulin resistance and β-cell dysfunction as predictors of future dysglycaemia.
Michaliszyn, S. F. et al . β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 63 , 3846–3855 (2014).
Mari, A. et al . Mechanisms of the incretin effect in subjects with normal glucose tolerance and patients with type 2 diabetes. PLoS ONE 8 , e73154 (2013).
Holst, J. J., Knop, F. K., Vilsbøll, T., Krarup, T. & Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 34 , S251–S257 (2011).
Camastra, S. et al . Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes 62 , 3709–3717 (2013).
Ferrannini, E. The stunned β cell: a brief history. Cell Metab. 11 , 349–352 (2010).
Shulman, G. I. et al . Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322 , 223–228 (1990). This study demonstrated that defects in insulin-stimulated muscle glycogen synthesis was the major factor responsible for whole-body insulin resistance in patients with T2DM.
Groop, L. C. et al . Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84 , 205–213 (1989).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9 , 367–377 (2008).
Gerich, J. E., Meyer, C., Woerle, H. J. & Stumvoll, M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24 , 382–391 (2001).
Honka, H. et al . Validation of [ 18 F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia 56 , 893–900 (2013).
Meijer, R. I. et al . Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19 , 494–500 (2012).
Blázquez, E., Velázquez, E., Hurtado-Carneiro, V. & Ruiz-Albusac, J. M. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front. Endocrinol. (Lausanne) 5 , 161 (2014).
Kleinridders, A., Ferris, H. A., Cai, W. & Kahn, C. R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63 , 2232–2243 (2014).
Kulkarni, R. N. et al . Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96 , 329–339 (1999). An insightful study documenting that β-cell-specific insulin receptor knockout results in markedly impaired insulin secretion and overt diabetes, thereby providing a unifying mechanism whereby insulin resistance explains both the defects in insulin-stimulated tissue glucose uptake and decreased insulin secretion.
Oliveira, J. M., Rebuffat, S. A., Gasa, R. & Gomis, R. Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can. J. Physiol. Pharmacol. 92 , 613–620 (2014).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148 , 852–871 (2012). An excellent review of the molecular mechanism responsible for insulin resistance in T2DM and obesity.
Magnusson, I., Rothman, D. L., Katz, L. D., Shulman, R. G. & Shulman, G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Invest. 90 , 1323–1327 (1992). This study demonstrated that increased rates of hepatic glucose production in patients with poorly controlled T2DM could entirely be attributed to increased rates of gluconeogenesis.
Matsuda, M. et al . Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 51 , 1111–1119 (2002).
Samuel, V. T. et al . Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl Acad. Sci. USA 106 , 12121–12126 (2009).
Baron, A. D., Schaeffer, L., Shragg, P. & Kolterman, O. G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36 , 274–283 (1987).
DeFronzo, R. A., Ferrannini, E., Hendler, R., Wahren, J. & Felig, P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc. Natl Acad. Sci. USA 75 , 5173–5177 (1978).
Ferrannini, E. et al . The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 37 , 79–85 (1988).
DeFronzo, R. A. et al . Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36 , 3169–3176 (2013).
Barrett, E. J., Wang, H., Upchurch, C. T. & Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 301 , E252–E263 (2011).
Baron, A. D. Hemodynamic actions of insulin. Am. J. Physiol. 267 , E187–E202 (1994).
CAS PubMed Google Scholar
Krüger, M. et al . Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl Acad. Sci. USA 105 , 2451–2456 (2008).
Cusi, K. et al . Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105 , 311–320 (2000). The first study in humans with T2DM to demonstrate impaired insulin signal transduction through the IRS1–PI3K pathway in muscle, with normal to increased insulin signalling through the MAPK pathway.
Krook, A. et al . Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49 , 284–292 (2000).
Copps, K. D. & White, M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55 , 2565–2582 (2012).
Bouzakri, K. et al . IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55 , 785–791 (2006).
Hiratani, K. et al . Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem. Biophys. Res. Commun. 335 , 836–842 (2005).
Krssak, M. et al . Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42 , 113–116 (1999).
Petersen, K. F. et al . Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109 , 1345–1350 (2002).
Petersen, K. F. et al . Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54 , 603–608 (2005).
Lara-Castro, C. & Garvey, W. T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. North Am. 37 , 841–856 (2008).
Yu, C. et al . Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277 , 50230–50236 (2002).
Bezy, O. et al . PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J. Clin. Invest. 121 , 2504–2517 (2011).
Samuel, V. T. et al . Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279 , 32345–32353 (2004).
Samuel, V. T. et al . Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117 , 739–745 (2007).
Choi, C. S. et al . Suppression of diacylglycerol acyltransferase-2 ( DGAT2 ), but not DGAT1 , with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282 , 22678–22688 (2007).
Morino, K. et al . Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 115 , 3587–3593 (2005).
Szendroedi, J. et al . Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111 , 9597–9602 (2014).
Larsen, P. J. & Tennagels, N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 3 , 252–260 (2014).
Turpin, S. M. et al . Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20 , 678–686 (2014).
Cantley, J. L. et al . CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl Acad. Sci. USA 110 , 1869–1874 (2013).
Patti, M.-E. & Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31 , 364–395 (2010). Mitochondrial dysfunction as a causative factor in the development of insulin resistance in T2DM is reviewed.
Ritov, V. B. et al . Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54 , 8–14 (2005).
Petersen, K. F. et al . Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300 , 1140–1142 (2003).
Petersen, K. F., Dufour, S., Befroy, D., Garcia, R. & Shulman, G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350 , 664–671 (2004).
Mogensen, M. et al . Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56 , 1592–1599 (2007).
Petersen, K. F., Dufour, S. & Shulman, G. I. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2 , e233 (2005).
Wang, C.-H., Wang, C.-C., Huang, H.-C. & Wei, Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 280 , 1039–1050 (2013).
Rains, J. L. & Jain, S. K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 50 , 567–575 (2011).
Morino, K. et al . Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes 61 , 877–887 (2012).
Romeo, G. R., Lee, J. & Shoelson, S. E. Metabolic syndrome, insulin resistance, and roles of inflammation — mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 32 , 1771–1776 (2012).
Arkan, M. C. et al . IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11 , 191–198 (2005).
De Alvaro, C., Teruel, T., Hernandez, R. & Lorenzo, M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279 , 17070–17078 (2004).
Howard, J. K. & Flier, J. S. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 17 , 365–371 (2006).
Lebrun, P. & Van Obberghen, E. SOCS proteins causing trouble in insulin action. Acta Physiol. (Oxf.) 192 , 29–36 (2008).
Article CAS Google Scholar
Uysal, K. T., Wiesbrock, S. M. & Hotamisligil, G. S. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology 139 , 4832–4838 (1998).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45 , 881–885 (1996).
Kim, J. K. et al . Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest. 108 , 437–446 (2001).
Yuan, M. et al . Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKK β. Science 293 , 1673–1677 (2001).
Goldfine, A. B. et al . The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152 , 346–357 (2010).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121 , 2111–2117 (2011).
Nishimura, S. et al . CD8 + effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15 , 914–920 (2009).
Feuerer, M. et al . Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15 , 930–939 (2009).
Bertola, A. et al . Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61 , 2238–2247 (2012).
Cai, D. et al . Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11 , 183–190 (2005).
Perry, R. J. et al . Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160 , 745–758 (2015).
Mori, M. A. et al . A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes 59 , 2960–2971 (2010).
Mauer, J. et al . Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 6 , e1000938 (2010).
Shi, H. et al . TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116 , 3015–3025 (2006).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8 , 519–529 (2007).
Boden, G. et al . Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57 , 2438–2444 (2008).
Eizirik, D. L., Cardozo, A. K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29 , 42–61 (2008). A comprehensive review of ER stress and the UPR in the development of insulin resistance and obesity.
Gregor, M. F. et al . Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58 , 693–700 (2009).
Ozawa, K. et al . The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54 , 657–663 (2005).
Herschkovitz, A. et al . Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282 , 18018–18027 (2007).
Boden, G. Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes 58 , 518–519 (2009).
Sengupta, S., Peterson, T. R. & Sabatini, D. M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40 , 310–322 (2010).
Shah, O. J., Wang, Z. & Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14 , 1650–1656 (2004).
Ozcan, U. et al . Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29 , 541–551 (2008).
Park, S. W. et al . The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16 , 429–437 (2010).
Stratton, I. M. et al . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321 , 405–412 (2000). A seminal UK Prospective Diabetes Study study unequivocally demonstrating that improved glycaemic control reduced the incidence of microvascular, and to a lesser extent, macrovascular complications in patients with T2DM.
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359 , 1577–1589 (2008). A long-term follow-up of the UK Prospective Diabetes Study showing that early intensive glycaemic control has a persistent impact on preventing both microvascular and macrovascular complications long after initiation of the intensified antidiabetic regimen has been discontinued: that is, the ‘legacy effect’.
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54 , 1615–1625 (2005). A lucid discussion of the molecular pathways involved in the development of diabetic microvascular complications.
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107 , 1058–1070 (2010).
Coutinho, M., Gerstein, H. C., Wang, Y. & Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22 , 233–240 (1999).
Taskinen, M.-R. & Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 239 , 483–495 (2015). An up-to-date review of the pathogenesis of diabetic dyslipidaemia and its treatment.
Isomaa, B. et al . Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24 , 683–689 (2001).
Adler, A. I. et al . Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321 , 412–419 (2000).
Williams, B. Treating hypertension in patients with diabetes: when to start and how low to go? JAMA 313 , 573–574 (2015). The optimal blood pressure goal in hypertensive patients with T2DM is discussed in light of the controversial results observed in the blood pressure arm of the ACCORD trial.
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C. & Sowers, J. R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. North Am. 43 , 103–122 (2014).
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32 , 1327–1334 (2009).
[No authors listed.] Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20 , 1183–1197 (1997). A reference publication by the ADA on the diagnosis and classification of diabetes mellitus.
Herman, W. H. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award Lecture, 2006. Diabetes Care 30 , 1912–1919 (2007). A landmark lecture providing a comprehensive overview of the epidemiology of T2DM and the public health implications for diabetes prevention.
DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 26 , 688–696 (2003).
Engelgau, M. M., Narayan, K. M. & Herman, W. H. Screening for type 2 diabetes. Diabetes Care 23 , 1563–1580 (2000).
LeFevre, M. L. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 161 , 587–593 (2014).
Lindström, J. & Tuomilehto, J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26 , 725–731 (2003).
Tabaei, B. P. & Herman, W. H. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care 25 , 1999–2003 (2002).
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus (WHO, 1999).
Pan, X. R. et al . Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT Diabetes Study. Diabetes Care 20 , 537–544 (1997).
Knowler, W. C. et al . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 , 393–403 (2002).
Ramachandran, A. et al . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49 , 289–297 (2006).
Chiasson, J.-L. et al . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359 , 2072–2077 (2002).
Kawamori, R. et al . Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 373 , 1607–1614 (2009).
Knowler, W. C. et al . Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54 , 1150–1156 (2005).
Gerstein, H. C. et al . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368 , 1096–1105 (2006).
Li, G. et al . The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371 , 1783–1789 (2008).
Lindström, J. et al . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368 , 1673–1679 (2006).
Knowler, W. C. et al . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374 , 1677–1686 (2009). Long-term follow-up of body weight regain and diabetes incidence in patients with IGT in the Diabetes Prevention Program treated with lifestyle heavy, lifestyle light and metformin, showing that gradual weight regain is the norm and that 40–50% of patients with IGT develop diabetes despite successful weight loss.
DeFronzo, R. A., Eldor, R. & Abdul-Ghani, M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 36 , S127–S138 (2013). A rational approach to the treatment of T2DM is presented based on its pathophysiology.
Raz, I. et al . Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 36 , 1779–1788 (2013).
Nakagami, T., Kawahara, R., Hori, S. & Omori, Y. Glycemic control and prevention of retinopathy in Japanese NIDDM patients. A 10-year follow-up study. Diabetes Care 20 , 621–622 (1997).
Lim, E. L. et al . Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54 , 2506–2514 (2011).
Jazet, I. M. et al . Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia 51 , 309–319 (2008).
Abdul-Ghani, M. A. et al . Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes. Metab. 17 , 268–275 (2015). This prospective randomized trial using a combination of antidiabetic agents proven to reverse known pathophysiological abnormalities in T2DM demonstrated superiority of glycaemic control compared with the stepped approach of metformin followed by a sulfonylurea and then basal insulin recommended by most national diabetes organizations.
Harrison, L. B., Adams-Huet, B., Raskin, P. & Lingvay, I. β-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 35 , 1406–1412 (2012).
Gram, J. et al . Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish Diabetes Study. Diabetes Care 34 , 27–33 (2011).
DeFronzo, R. A. et al . Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38 , 384–393 (2015).
Weng, J. et al . Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371 , 1753–1760 (2008).
Hu, Y. et al . Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care 34 , 1848–1853 (2011). One of several recent studies demonstrating that intensive insulin therapy to correct the decompensated metabolic state in newly diagnosed patients with T2DM can lead to durable glycaemic control without or with a marked reduction in antidiabetic medications.
Xiang, A. H. et al . Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 55 , 517–522 (2006).
Astrup, A. et al . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond.) 36 , 843–854 (2012).
Cusi, K., Consoli, A. & DeFronzo, R. A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 81 , 4059–4067 (1996).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281 , 2005–2012 (1999). A landmark UK Prospective Diabetes Study documenting the need for progressive add-on therapies in newly diagnosed patients with T2DM receiving initial therapy with metformin or with a sulfonylurea.
Brown, J. B., Conner, C. & Nichols, G. A. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 33 , 501–506 (2010).
Kahn, S. E. et al . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355 , 2427–2443 (2006). A 5-year ADOPT study demonstrating long-term durable HbA1c reduction with rosiglitazone compared with a progressive rise in HbA1c observed with metformin and sulfonylureas, and a more rapid deterioration of glycaemic control with sulfonylureas compared with metformin.
Madiraju, A. K. et al . Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510 , 542–546 (2014).
Ferrannini, E. The target of metformin in type 2 diabetes. N. Engl. J. Med. 371 , 1547–1548 (2014).
[No authors listed.] Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352 , 854–865 (1998).
Maedler, K. et al . Sulfonylurea induced β-cell apoptosis in cultured human islets. J. Clin. Endocrinol. Metab. 90 , 501–506 (2005).
Roumie, C. L. et al . Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann. Intern. Med. 157 , 601–610 (2012).
Simpson, S. H., Majumdar, S. R., Tsuyuki, R. T., Eurich, D. T. & Johnson, J. A. Dose–response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174 , 169–174 (2006).
Simpson, S. H. et al . Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 3 , 43–51 (2015). A review of the published literature that examines the relationship between sulfonylurea therapy and the development of adverse cardiovascular events.
Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 351 , 1106–1118 (2004).
Eldor, R., DeFronzo, R. A. & Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 36 , S162–S174 (2013). An exhaustive review of the mechanism of action, efficacy and side-effect profile of the thiazolidinedione class of antidiabetic medications.
Miyazaki, Y., He, H., Mandarino, L. J. & DeFronzo, R. A. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 52 , 1943–1950 (2003).
Gastaldelli, A. et al . Thiazolidinediones improve β-cell function in type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 292 , E871–E883 (2007).
DeFronzo, R. A. et al . Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 62 , 3920–3926 (2013).
Kahn, S. E. et al . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 60 , 1552–1560 (2011).
Dormandy, J. A. et al . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366 , 1279–1289 (2005). A large prospective study (PROactive) demonstrating that pioglitazone significantly reduced the second principal end point of myocardial infarction, stroke and cardiovascular death; the primary end point did not reach statistical significance because of the inclusion of peripheral arterial disease and leg revascularization, which is known to be refractory to medical intervention, including statin therapy.
Aronoff, S. et al . Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 23 , 1605–1611 (2000).
Erdmann, E., Song, E., Spanheimer, R., van Troostenburg de Bruyn, A.-R. & Perez, A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes. Metab. 16 , 63–74 (2014).
[No authors listed.] Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. Takeda [online] , (2014).
Levin, D. et al . Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia 58 , 493–504 (2015).
Kjems, L. L., Holst, J. J., Vølund, A. & Madsbad, S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52 , 380–386 (2003).
Vilsbøll, T., Krarup, T., Madsbad, S. & Holst, J. J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 45 , 1111–1119 (2002).
Aroda, V. R. et al . Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin. Ther. 34 , 1247–1258.e22 (2012).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13 , 7–18 (2011).
Balas, B. et al . The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 92 , 1249–1255 (2007).
Drucker, D. J. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62 , 3316–3323 (2013). A comprehensive review of the effect of incretin hormones on pancreatic hormone secretion and pathology by one of the world's leading authorities.
White, W. B. et al . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369 , 1327–1335 (2013).
Scirica, B. M. et al . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369 , 1317–1326 (2013).
Cervera, A. et al . Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 294 , E846–E852 (2008).
Bunck, M. C. et al . Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 34 , 2041–2047 (2011). A landmark 3-year prospective study demonstrating the marked and durable improvement in β-cell function using the combined hyperglycaemic and euglycaemic insulin clamp techniques following exenatide treatment in patients with T2DM.
Klonoff, D. C. et al . Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24 , 275–286 (2008).
Schwartz, S. & Kohl, B. A. Type 2 diabetes mellitus and the cardiometabolic syndrome: impact of incretin-based therapies. Diabetes Metab. Syndr. Obes. 3 , 227–242 (2010).
Eng, C., Kramer, C. K., Zinman, B. & Retnakaran, R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384 , 2228–2234 (2014).
Egan, A. G. et al . Pancreatic safety of incretin-based drugs — FDA and EMA assessment. N. Engl. J. Med. 370 , 794–797 (2014).
Van de Laar, F. A. et al . Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2 , CD003639 (2005).
Esposito, K. et al . Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 13 , 594–603 (2011).
Richter, B., Bandeira-Echtler, E., Bergerhoff, K. & Lerch, C. L. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2 , CD006739 (2008).
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 32 , 515–531 (2011). An excellent review of the mechanism of action, efficacy and safety of the recently approved SGLT2 inhibitor class of antidiabetic medications.
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91 , 733–794 (2011).
Merovci, A. et al . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124 , 509–514 (2014).
Ferrannini, E. et al . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124 , 499–508 (2014).
Abdul-Ghani, M. A., DeFronzo, R. A. & Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62 , 3324–3328 (2013).
Cherney, D. Z. I. et al . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129 , 587–597 (2014).
Holman, R. R. et al . Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361 , 1736–1747 (2009). A comparison of the efficacy and side-effect profile of commonly used complex insulin regimens for the treatment of patients with T2DM.
Gough, S. C. L. et al . Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2 , 885–893 (2014).
Wilding, J. P. et al . Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann. Intern. Med. 156 , 405–415 (2012).
Anderson, M., Powell, J., Campbell, K. M. & Taylor, J. R. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab. Syndr. Obes. 7 , 85–94 (2014).
PubMed PubMed Central Google Scholar
Schopman, J. E. et al . The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab. Res. Rev. 30 , 11–22 (2014).
Desouza, C., Salazar, H., Cheong, B., Murgo, J. & Fonseca, V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 26 , 1485–1489 (2003).
Gerstein, H. C. et al . Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358 , 2545–2559 (2008). The ORIGIN trial demonstrated that physiological insulin replacement doses (30–40 units per day) in newly diagnosed patients with T2DM could control HbA1c without an increased risk of cardiovascular events; however, the risk of hypoglycaemia was significantly increased, and the study did not examine the effect of higher doses of insulin, which are usually required to normalize glycaemia in more long-standing diabetes, on cardiovascular risk or other potential side effects of insulin therapy.
Cushman, W. C. et al . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362 , 1575–1585 (2010).
James, P. A. et al . 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311 , 507–520 (2014).
Emdin, C. et al . Association of cardiovascular trial registration with positive study findings: Epidemiological Study of Randomized Trials (ESORT). JAMA Intern. Med. 175 , 304–307 (2015).
Testa, M. A. & Simonson, D. C. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA 280 , 1490–1496 (1998). This was the first randomized trial to demonstrate that better glucose control improves QOL, cognitive function and general perceived health, and reduces symptom distress and absenteeism from work.
Testa, M. A. & Simonson, D. C. Assesment of quality-of-life outcomes. N. Engl. J. Med. 334 , 835–840 (1996).
Testa, M. A., Simonson, D. C. & Turner, R. R. Valuing quality of life and improvements in glycemic control in people with type 2 diabetes. Diabetes Care 21 , C44–C52 (1998).
Bode, B. W. et al . Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes. Metab. 12 , 604–612 (2010).
Testa, M. A. et al . Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J. Clin. Endocrinol. Metab. 97 , 3504–3514 (2012). This randomized trial demonstrated that patient satisfaction with treatment was more positively affected by improved QOL, reduced glucose variability and better glycaemic control with a basal-bolus regimen than negatively affected by the burden of additional injections.
Cotter, A. P., Durant, N., Agne, A. A. & Cherrington, A. L. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J. Diabetes Complications 28 , 243–251 (2014).
Rose, M. et al . The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J. Clin. Epidemiol. 67 , 516–526 (2014).
DeFronzo, R. A. & Triplitt, C. Novel agents for T2DM. Diabetes Spectr. 27 , 100–112 (2014). This article presents a more detailed review of novel antidiabetic agents that currently are being investigated in animals and humans for the treatment of T2DM.
Wong, A. K., Howie, J., Petrie, J. R. & Lang, C. C. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond.) 116 , 607–620 (2009).
Agrawal, N. K. & Kant, S. Targeting inflammation in diabetes: newer therapeutic options. World J. Diabetes 5 , 697–710 (2014). Inflammation in insulin target tissues and β-cells is a now well-established pathogenetic abnormality T2DM. This article reviews the mechanism by which inflammation contributes to glucose intolerance in T2DM and potential interventions to suppress inflammation and improve insulin sensitivity and β-cell function.
Poy, M. N. et al . miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl Acad. Sci. USA 106 , 5813–5818 (2009).
Burant, C. F. et al . TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379 , 1403–1411 (2012).
Assmann, A., Hinault, C. & Kulkarni, R. N. Growth factor control of pancreatic islet regeneration and function. Pediatr. Diabetes 10 , 14–32 (2009).
Vasavada, R. C. et al . Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes 56 , 2732–2743 (2007).
Wiederkehr, A. & Wollheim, C. B. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol. Cell. Endocrinol. 353 , 128–137 (2012).
Wang, C. et al . Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia 56 , 1999–2009 (2013).
Sivitz, W. I. & Yorek, M. A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 12 , 537–577 (2010).
Li, N., Stojanovski, S. & Maechler, P. Mitochondrial hormesis in pancreatic β cells: does uncoupling protein 2 play a role? Oxid. Med. Cell. Longev. 2012 , 740849 (2012).
Aquilano, K., Baldelli, S., Pagliei, B. & Ciriolo, M. R. Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. 13 , 140–154 (2013).
Matschinsky, F. M. et al . Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care 34 , S236–S243 (2011).
Engel, S. S. Glycemic and lipid effects of the short-acting glucagon receptor antagonist MK-3577 in patients with type 2 diabetes. Diabetes Abstr. 61 , A266 (2012).
Gumbiner, B. Pronounced glucose (G) reduction in poorly controlled T2DM with MB07803, a novel fructose-1, 6-biphosphatase inhibitor (FBPasel) with reduced potential for acid-base disturbance versus the 1st generation FBPasel CS-917. Diabetes Abstr. 58 , LB4 (2009).
Kumashiro, N. et al . Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62 , 2183–2194 (2013).
Stark, R. et al . A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J. Biol. Chem. 289 , 7257–7263 (2014).
Harlan, D. M., Kenyon, N. S., Korsgren, O. & Roep, B. O. Current advances and travails in islet transplantation. Diabetes 58 , 2175–2184 (2009).
Motté, E. et al . Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab. 307 , E838–E846 (2014).
Pagliuca, F. W. et al . Generation of functional human pancreatic β cells in vitro . Cell 159 , 428–439 (2014).
Blum, B. et al . Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 3 , e02809 (2014).
Pickup, J. C. Banting Memorial Lecture 2014* Technology and diabetes care: appropriate and personalized. Diabet. Med. 32 , 3–13 (2015).
Peyser, T., Dassau, E., Breton, M. & Skyler, J. S. The artificial pancreas: current status and future prospects in the management of diabetes. Ann. NY Acad. Sci. 1311 , 102–123 (2014). This article presents an up-to-to-date status report on progress with the artificial pancreas (closed-loop system).
Klonoff, D. C. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. J. Diabetes Sci. Technol. 8 , 1071–1073 (2014).
Eldor, R., Arbit, E., Corcos, A. & Kidron, M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS ONE 8 , e59524 (2013).
DeFronzo, R. A. Dissociation between metformin plasma exposure and its glucose-lowering effect: a novel gut-mediated mechanism of action. Diabetes 62 , a281 (2013).
DePaoli, A. M., Higgins, L. S., Henry, R. R., Mantzoros, C. & Dunn, F. L. Can a selective PPARγ modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care 37 , 1918–1923 (2014).
Colca, J. R., Tanis, S. P., McDonald, W. G. & Kletzien, R. F. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin. Investig. Drugs 23 , 1–7 (2014).
Suh, J. M. et al . Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513 , 436–439 (2014).
Gaich, G. et al . The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18 , 333–340 (2013).
Jeoung, N. H. & Harris, R. A. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J. 34 , 274–283 (2010).
Povel, C. M. et al . Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care 36 , 362–368 (2013).
Pacini, G., Mari, A., Fouqueray, P., Bolze, S. & Roden, M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes. Metab. 17 , 541–545 (2015).
Birch, A. M., Buckett, L. K. & Turnbull, A. V. DGAT1 inhibitors as anti-obesity and anti-diabetic agents. Curr. Opin. Drug Discov. Devel. 13 , 489–496 (2010).
Liu, L. et al . Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117 , 1679–1689 (2007).
Harrima, G., Greenwood, J. & Bhar, S. Acetyl-CoA carboxylase inhibition by NDI-630 inhibits fatty acid synthesis stimulates fatty acid oxidative, reduces body weight, improvise insulin sensitivity, and modulates dyslipidemia in rats. Diabetes Abstr. 62 , A161 (2013).
Tao, H., Zhang, Y., Zeng, X., Shulman, G. I. & Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20 , 1263–1269 (2014).
Perry, R. J. et al . Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18 , 740–748 (2013).
Garvey, W. T. et al . Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95 , 297–308 (2012).
Carlsson, L. M. et al . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 367 , 695–704 (2012). The effectiveness and safety of bariatric surgery in the treatment of obesity and T2DM is reviewed in this longest ongoing study on surgical intervention.
Neuschwander-Tetri, B. A. et al . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385 , 956–965 (2014).
Out, C., Groen, A. K. & Brufau, G. Bile acid sequestrants: more than simple resins. Curr. Opin. Lipidol. 23 , 43–55 (2012).
Cellitti, S. A novel GLP-1-FGF21 fusion protein for the treatment of diabetes and obesity. Keystone Symp. Obes. (2014).
Thareja, S., Aggarwal, S., Bhardwaj, T. R. & Kumar, M. Protein tyrosine phosphatase 1B inhibitors: a molecular level legitimate approach for the management of diabetes mellitus. Med. Res. Rev. 32 , 459–517 (2012).
Chakraborty, C., Doss, C. G., Bandyopadhyay, S. & Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 5 , 697–712 (2014).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. Gut 63 , 1513–1521 (2014).
Patel, S. R., Hakim, D., Mason, J. & Hakim, N. The duodenal–jejunal bypass sleeve (EndoBarrier Gastrointestinal Liner) for weight loss and treatment of type 2 diabetes. Surg. Obes. Relat. Dis. 9 , 482–484 (2013).
Bhatt, M. P., Lim, Y.-C. & Ha, K.-S. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc. Res. 104 , 234–244 (2014).
Bhat, M., Pouliot, M., Couture, R. & Vaucher, E. The kallikrein–kinin system in diabetic retinopathy. Prog. Drug Res. 69 , 111–143 (2014).
PubMed Google Scholar
Hajhosseiny, R. et al . Have we reached the limits for the treatment of diabetic nephropathy? Expert Opin. Investig. Drugs 23 , 511–522 (2014).
Williams, M. E. et al . Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 27 , 605–614 (2007).
De Zeeuw, D. et al . The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25 , 1083–1093 (2014).
Boussageon, R. et al . Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343 , d4169 (2011).
Colditz, G. A., Willett, W. C., Rotnitzky, A. & Manson, J. E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 122 , 481–486 (1995).
Chan, J. M., Rimm, E. B., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17 , 961–969 (1994).
Download references
Acknowledgements
The authors acknowledge grants from: the South Texas Veterans Healthcare System to R.A.D.; the National Institutes of Health (grants R01DK24092 to R.A.D.; DK58845 and P30 DK46200 to F.B.H.; R01 DK-040936, R01 DK-049230, R24 DK-085836, UL1 RR-045935, R01 DK-082659 and R24 DK085610 to G.I.S.; P30 DK036836 to C.R.K. Novo Nordisk Foundation for Basic Metabolic Research and the University of Copenhagen to G.I.S. and C.R.K.; DVA-Merit Review grant and VA San Diego Healthcare System to R.H.; National Institute for Diabetes and Digestive and Kidney Disease (grant P30DK092926) to W.H.; the Swedish Research Council (grants 2010–3490 and 2008–6589) and European Council (grants GA269045) to L.G.; Italian Ministry of University & Research (MIUR 2010329EKE) to E.F.; the Patient-Centered Outcomes Research Institute (PCORI) Program Award (CE1304-6756) to D.C.S. and M.A.T.; NovoNordisk Foundation to the NNF Center for Basic Metabolic Research to J.H. W.H. acknowledges the Michigan Center for Diabetes Translational Research and I.R. thanks R. Sprung for editorial assistance.
Author information
Authors and affiliations.
Diabetes Division, Department of Medicine, University of Texas Health Science Center, South Texas Veterans Health Care System and Texas Diabetes Institute, 701 S. Zarzamoro, San Antonio, 78207, Texas, USA
Ralph A. DeFronzo
CNR Institute of Clinical Physiology, Pisa, Italy
Ele Ferrannini
Department of Clinical Science Malmoe, Diabetes & Endocrinology, Lund University Diabetes Centre, Lund, Sweden
University of California, San Diego, Section of Diabetes, Endocrinology & Metabolism, Center for Metabolic Research, VA San Diego Healthcare System, San Diego, California, USA
Robert R. Henry
University of Michigan, Ann Arbor, Michigan, USA
William H. Herman
University of Copenhagen, Kobenhavn, Denmark
Jens Juul Holst
Department of Nutrition, Harvard T.H. Chan School of Public Health and Department of Epidemiology, Harvard T.H. Chan School of Public Health and Channing Division of Network Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA
Frank B. Hu
Harvard Medical School and Joslin Diabetes Center, Boston, Massachusetts, USA
C. Ronald Kahn
Division of Internal Medicine, Diabetes Unit, Hadassah Hebrew University Hospital, Jerusalem, Israel
Howard Hughes Medical Institute and the Departments of Internal Medicine and Cellular & Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut, USA
Gerald I. Shulman
Division of Endocrinology, Diabetes and Hypertension, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA
Donald C. Simonson
Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA
Marcia A. Testa
Department of Human Metabolism and Nutrition, Braun School of Public Health, Hebrew University, Jerusalem, Israel
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (R.R.H.); Epidemiology (F.B.H.); Mechanisms/pathophysiology (L.C.G., C.R.K., E.F., G.I.S. and R.A.D.); Diagnosis, screening and prevention (W.H.H.); Management (R.A.D.); Quality of life (D.C.S. and M.A.T.); Outlook (I.R., J.J.H. and R.W.); overview of Primer (R.A.D.).
Corresponding author
Correspondence to Ralph A. DeFronzo .
Ethics declarations
Competing interests.
The authors declare the following potential COI: (1) R.A.D.: Research Grant Support - AstraZeneca, Bristol Myers Squibb, Janssen; Speaker's Bureau - AstraZeneca, Novo Nordisk, Advisory Board/Consultant - AstraZeneca, Janssen, Novo Nordisk, Boehringer Ingelheim, Lexicon, Intarcia; (2) E.F.: Research Grant Support - Boehringer Ingelheim, Eli Lilly; Consultant/Speaker Bureau-Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Takeda, Medtronic, Intarcia; (3) C.R.K. serves as a consultant for Medimmune, Merck, Five Prime Therapeutics, CohBar, Antriabio, and Catabasis; (4) L.G. has no conflict of interest; (5) R.H. has received grant support from Hitachi, Janssen, Eli Lilly, Sanofi-Aventis and Viacyte and is a consultant/advisory board member for Alere, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Clin Met, Eisai, Elcelyx, Gilead, Intarcia, Isis, Janssen, Merck, Novo Nordisk, Sanofi-Aventis, and Vivus; (6) W.H.H. has no conflict of interest; (7) J.J.H. has received grant support from Novartis and Merck and is a consultant/advisory board member for Glaxo, Smith, Kline, Novo Nordisk, and Zealand Pharmaceuticals; (8) M.A.T. has no conflict of interest; (9) R.W. serves as a consultant for Medtronics and Kamada and is on the speaker's bureau for Medtronics and Novo Nordisk; (10) F.H. has received research support from California Walnut Commission and Metegenics; (11) G.I.S. serves on scientific advisory boards for Merck and Novartis and he has received research grant support from Gilead Pharmaceuticals; (12) D.C.S. has no conflict of interest; (13) I.R. – Advisory Board: Novo Nordisk, Astra Zeneca/BMS, MSD, Eli Lilly, Sanofi, Medscape Cardiology; Consultant: Astra Zeneca/BMS, Insuline; Speaker's Bureau: Eli Lilly, Novo Nordisk, Astra Zeneca/BMS, J&J, Sanofi, MSD, Novartis, Teva; Shareholder: Insuline, Labstyle.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, rights and permissions.
Reprints and permissions
About this article
Cite this article.
DeFronzo, R., Ferrannini, E., Groop, L. et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 1 , 15019 (2015). https://doi.org/10.1038/nrdp.2015.19
Download citation
Published : 23 July 2015
DOI : https://doi.org/10.1038/nrdp.2015.19
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
SYSTEMATIC REVIEW article
Meta-analysis shows that mesenchymal stem cell therapy can be a possible treatment for diabetes.

- 1 Research and Development (R&D) Department, R3 Medical Research LLC, Scottsdale, AZ, United States
- 2 Research and Development (R&D) Department, Pak-American Hospital Pvt. Ltd., Islamabad, Pakistan
- 3 Research and Development (R&D) Department, R3 Stem Cell LLC, Scottsdale, AZ, United States
- 4 Research and Development (R&D) Department, Bello Bio Labs and Therapeutics Pvt. Ltd., Islamabad, Pakistan
- 5 Department of Statistics, Quaid-i-Azam University, Islamabad, Pakistan
Objective: This meta-analysis includes the systematic literature review and meta-analysis involving clinical trials to assess the efficacy and safety of mesenchymal stem cell (MSC) transplantation for treating T1DM and T2DM.
Methods: We searched PubMed, ScienceDirect, Web of Science, clinicaltrials.gov, and Cochrane Library for “published” research from their inception until November 2023. Two researchers independently reviewed the studies’ inclusion and exclusion criteria. Our meta-analysis included 13 studies on MSC treatment for diabetes.
Results: The MSC-treated group had a significantly lower HbA1c at the last follow-up compared to the baseline (MD: 0.95, 95% CI: 0.33 to 1.57, P -value: 0.003< 0.05), their insulin requirement was significantly lower (MD: 0.19, 95% CI: 0.07 to 0.31, P -value: 0.002< 0.05), the level of FBG with MSC transplantation significantly dropped compared to baseline (MD: 1.78, 95% CI: -1.02 to 4.58, P -value: 0.212), the FPG level of the MSC-treated group was significantly lower (MD: -0.77, 95% CI: -2.36 to 0.81, P -value: 0.339 > 0.05), and the fasting C-peptide level of the MSC-treated group was slightly high (MD: -0.02, 95% CI: -0.07 to 0.02, P- value: 0.231 > 0.05).
Conclusion: The transplantation of MSCs has been found to positively impact both types of diabetes mellitus without signs of apparent adverse effects.
Introduction
Diabetes is a serious and growing health problem worldwide. This is a persistent health condition that arises when the body is incapable of adequately controlling the levels of glucose in the bloodstream. Each year, the prevalence of diabetes mellitus (DM) rises. According to the International Diabetes Federation, approximately 4.51 million adults across the globe were diagnosed with diabetes in 2017. Furthermore, it is expected that this figure will escalate to 6.93 million by the year 2045 ( 1 ). Diabetes holds two types which vary in their mode of action on the human body. Type 1 diabetes mellitus (T1DM) is an immune system infection, and insusceptible assaults result in the obliteration of islet cells, causing islet aggravation related to outright insulin lack. Eventually, several related complications arise, compromising the patient’s quality of life and reducing their durability ( 2 ). Around 90% of adults with diabetes are diagnosed with the most common form of diabetes, known as type 2 diabetes mellitus (T2DM) ( 3 ). The leading causes of diabetes are the malfunctioning of islet cells and the body’s reduced sensitivity to insulin ( 4 ).
High blood glucose levels in individuals with diabetes are managed through a combination of insulin injections, daily oral hypoglycemic agents, exercise, and diet. However, while these conventional therapeutic approaches aim to regulate insulin levels, they may not always be effective in doing so, which can result in severe hypoglycemia and poor adherence to treatment plans. In fact, only 14% of patients with diabetes in the United States meet the glucose, lipid, and blood pressure control and quitting smoking targets. Despite significant research efforts devoted to understanding the disease process and the experimental therapeutics of diabetes, there is still an urgent need for more effective treatments to prevent or manage this severe metabolic illness ( 5 ).
Stem cell-based transplantation has emerged as a promising strategy for treating diabetes in recent years, which offers numerous benefits. Mesenchymal stem cells (MSCs), unlike embryonic stem cells, are not associated with tumorigenic risks or ethical concerns when treating diabetes ( 6 – 8 ). Due to its ease of access and wide availability, MSC transplantation is an appealing option ( 9 ); it has low immunogenicity, the ability to self-renew, the potential for multi-differentiation, the secretion of various cytokines, and other biological characteristics. It does not raise any ethical issues ( 10 – 12 ).
In the past decade, MSCs have demonstrated their therapeutic potential in both clinical and preclinical studies for the treatment of diabetes. In vitro studies have proposed that MSCs are capable to self-renew and differentiate into multiple mesenchymal lineages such as adipogenic, chondrogenic, and osteogenic lineages. Furthermore, they have low immunogenicity due to the interstitial expression of major histocompatibility complex (MHC) class I and the lack of MHC class II. MSCs release cytokines, growth factors, and exosomes, which modulate insulin sensitivity and β-cell dysfunction. Earlier studies have recommended that MSCs have the capability to exert antidiabetic effects because several dose administrations of MSCs may help improve hyperglycemia in DM patients.
Recent experimental explorations shed light on the complex mechanisms that highlight the therapeutic effects of MSCs in diabetes management. In STZ diabetic animal models, β-cell dysfunction can be caused by pancreatic microenvironment inflammation. The MSC treatment has been validated to facilitate the proliferation of regulatory T cells (Tregs) and incorporate long-term immunomodulatory effects. The secretion of cytokines such as Th2 secreted by Tregs and interleukins (IL-10 and IL-3) pose an anti-inflammatory profile which supports pancreatic β-cell regeneration and function ( 13 ).
In addition, MSCs exhibit a response toward inflammatory stimuli by shifting macrophages from pro-inflammatory (M1) to anti-inflammatory (M2) phenotypes. This is promoted by an overexpression of IL-6 and monocyte chemoattractant protein (MCP-1). MSCs may alleviate the systemic inflammation by downregulating the inflammatory cytokines, reducing insulin receptor action, and secreting IL-1Ra in response to IL-1β and tumor necrosis factor (TNF-α) signals from diabetic islets. This reaction reduces the synthesis of NLRP3 production in adipose tissue and liver. These findings highlight MSCs’ complex immunomodulatory characteristics and potential as a therapeutic method for controlling type 2 diabetes and its consequences. Conclusively, MSC infusion has been utilized to treat diabetes by reconstructing β cells, enhancing and regulating glucose homeostasis, alleviation of insulin resistance, and lowering/regulating systemic inflammation ( 14 ).
The therapeutic potential of bone marrow-obtained mesenchymal stem cell (BM-MSC) transplantation in treating T2DM was demonstrated for the first time in a 2009 study conducted by Bhansali et al. ( 15 ). The study involved 10 T2DM patients and showed that BM-MSC transplantation developed a significant decline in insulin requirement and improvement in stimulated C-peptide levels. Several subsequent studies have been carried out to validate the safety and effectiveness of using BM-MSCs and placenta-derived mesenchymal stem cells (PD-MSCs) in treating T2DM. These investigations have confirmed the initial results reported in ( 15 ) and have provided further evidence of the therapeutic potential of BM-MSCs and PD-MSCs for treating T2DM ( 16 ).
A recent investigation demonstrated that patients with T2DM experienced a decrease in HbA1c levels and insulin dose at the 6-month mark following treatment and after receiving a combination of intravenous and intrapancreatic endovascular injection of umbilical cord-derived mesenchymal stem cells (UC-MSCs) with a 5-day interval. The study also discovered that 41% of the patients became insulin independent, and 29% had 50% or greater reduction in insulin requirement. Nonetheless, these positive outcomes were not sustained over the next 3–6 months as the HbA1c levels and insulin dose reverted to their pre-treatment levels ( 17 ).
Multipotent stem cells have been utilized in the treatment of different autoimmune-related disorders, with some commercial products resulting from these treatments ( 18 – 21 ). However, the assessment of the safety and effectiveness of stem cell transplantation for DM is still in its preliminary stages. Clinical trials involving MSCs and hematopoietic stem cells (HSCs) in patients with T1DM have been carried out since 2000 ( 22 – 25 ), yet there is still no strong consensus on their efficacy. To date, no research has been carried out to compare the effectiveness of mesenchymal stem cells (MSCs) in treating type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) despite earlier studies demonstrating the efficacy of MSCs in both forms of diabetes. Furthermore, a number of studies have been published in the context of systematic reviews and meta-analyses. They examined the impact of stem cell therapy (SCT) on diabetes mellitus, but the absence of several critical components in each of these investigations has led to differences in their findings and has resulted in them being inadequate in providing a complete understanding of previous interventional studies.
Recently, Madani et al. ( 26 ) compared the efficacy of MSCs and HSCs in a meta-analysis of SCT research in T1DM. The search period for this paper was limited to September 2019, and it does not include both types of DM to compare the effectiveness of MSCs and HSCs simultaneously. Similarly, a meta-analysis ( 27 ) was carried out in 2021 to predict the safety and efficacy profile of transplanting mesenchymal stem cells for treating T1DM and T2DM. However, this meta-analysis did not include four papers ( 28 – 31 ) as their search window was limited to November 2011 to November 2020. The reason of not including these studies is not mentioned. Our meta-analysis includes the recent clinical trial conducted by Zang et al. ( 32 ), which involved testing UC-MSCs on Chinese adults with T2DM. The primary objective of this study was to evaluate the distinct therapeutic effects of MSCs on diabetes mellitus and its subtypes as well as their safety to lay a speculative foundation for medical assessment and diabetes therapeutic interventions based on trials conducted until November 2023. The outcomes of this research could have the potential to guide in the design of future clinical trials investigating the effectiveness and safety of MSC therapy for DM as well as provide evidence to support the development of clinical guidelines for the use of this therapy.
Materials and methods
Selection criteria.
The studies were included on the following criteria: (1) research studies published in Chinese and English, (2) clinical studies/trials involving MSCs as a treatment regimen for DM, (3) MSCs were used to treat diabetes in all patients, regardless of age, race, sex, extent of disease, or geographical location, and (4) all findings evaluated the treatment of diabetes with MSCs. There were no restrictions on the time, duration, or dosing frequency of MSCs used in the treatments. The treatment’s control group is either placebo or absolutely nothing. The treatment duration and dosage for the placebo was the same as for the MSC group. Finally, these are (5) studies with multiple follow-up timeframes, ranging from 3-, 6-, 9-, and 12 months, which was consistent with the majority of the studies analyzed.
The exclusion criteria were (1) research studies/trials in languages apart from Chinese and English, (2) studies with lacking reports or data (such as conference abstracts with missing sections), and (3) repeat publications. The meta-analysis incorporated the most up-to-date and comprehensive studies available, including clinical trials.
The study looked at two types of outcomes:
1. Primary outcomes: These were variations in insulin requirements, HbA1c, fasting blood glucose (FBG), fasting plasma glucose (FPG), and C-peptide between baseline and after therapy (3-, 6-, 9-, and 12-month follow-up).
2. Secondary outcomes: These included hypoglycemia episodes, self-limiting upper respiratory tract infections, mild fever, nausea, and vomiting.
Search strategy
We started searching numerous directories for eligible studies, including the Cochrane Library, PubMed, ScienceDirect, Web of Science, and clinicaltrials.gov while following the PRISMA 2020 guidelines. We used a combination of keywords, including (“mesenchymal stem/stromal cell, Wharton’s jelly cells, progenitor cells, bone marrow” or “MSCs”) AND (“diabetic, diabetes mellitus or hyperglycemia”) AND (“type 1 diabetes” or “type 2 diabetes”) AND “clinical trial” AND (“English language” OR “Chinese language”). In addition to database searches, we also performed manual searches of reference lists and descriptive reviews from applicable trials. The exploration was strictly limited to human subjects, published studies, case studies, and English and Chinese papers, with unpublished studies being excluded. The search period covered all publications up to February 2023. A detailed description of the search strategies is provided in Appendix 1.
Data extraction and basic characteristics
Two researchers (DLG and SS) worked independently on the comprehensive literature screening and data retrieval. In cases where discrepancies arose during the study selection process, a third reviewer was consulted. We collected pertinent information for all selected studies, such as the first author’s name, year of publication, sample size, study type, mean patient age in years, mean dose of injected cells, treatment route, number of patients who achieved insulin-free status, and timeframe of follow-up duration in months ( Table 1 ).
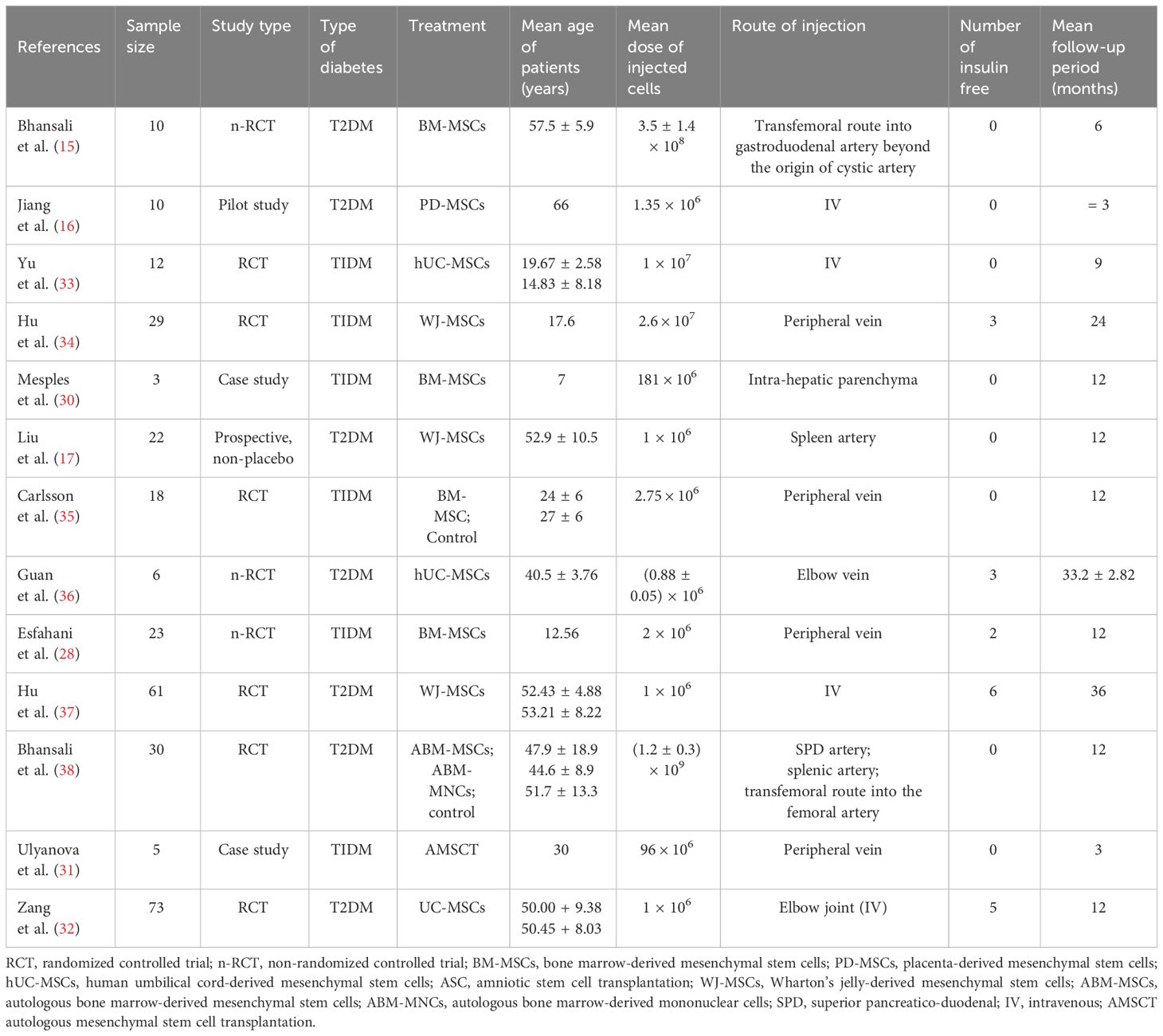
Table 1 Baseline characteristics of 13 eligible papers included in this meta-analysis.
Statistical data analysis
This analysis utilized mean difference (MD) to compare continuous variables between baseline and follow-ups. MD was selected to compare continuous variables because of its simplicity, interpretability, and compatibility with meta-analysis techniques, representing the absolute difference in means between treatment groups, quantifying the magnitude of difference, and providing a straightforward measure of treatment effect. It is suitable for synthesizing data from diverse studies, focusing on comparing means rather than specific statistical assumptions, and allows to estimate the overall effect. We considered P -value less than 0.05 with a 95% confidence interval (CI) as statistically significant. We calculated the heterogeneity of the included studies using the I 2 statistic, where values of 25%, 50%, and 75%–100% indicated low, medium, and high heterogeneity, respectively. In instances where significant heterogeneity was detected ( I 2 > 50% and P < 0.10), we used a random-effects model for the meta-analysis ( 39 ). Otherwise, the data were evaluated using a fixed-effects model.
These models offer the flexibility to incorporate prior information and estimate heterogeneity more comprehensively. The decision to utilize fixed-effects or random-effects models in our meta-analysis was indeed based on the observed level of heterogeneity among the included studies. While fixed-effects models assume a common treatment effect across all studies, random-effects models account for both within-study and between-study variability, acknowledging potential differences in treatment effects. Moreover, in case of higher heterogeneity, the Knapp and Hartung adjustment was also applied. This adjustment accounts for potential variability in effect sizes across studies and provides more conservative estimates of the overall effect. We also performed sensitivity analysis to evaluate the robustness of results by testing the impact of different assumptions, models, or inclusion criteria, thus ensuring the reliability and validity of conclusions amidst varying methodological choices and potential biases. The externally standardized residuals, DFFITS values, Cook’s distances, covariances ratios, leave-one-out Tau estimates, Hat values, and weights were plotted. This allows for a comprehensive assessment of the data and helps identify influential data points or outliers. This approach enhances the transparency and reliability of the analysis. In this meta-analysis, we compared the treatment group, i.e., MSCs and the control group (if any) from the selected studies using Jamovi version 2.3 ( 40 ), and the results were depicted by forest plots ( Tables 2 – 6 ). We assessed the heterogeneity and publication bias using several methods, including the Q Cochrane test and I 2 statistic, the Cochrane ROB tool, meta-regression analysis, and examination of publication bias using funnel plots, and Begg’s and Egger’s regression tests.
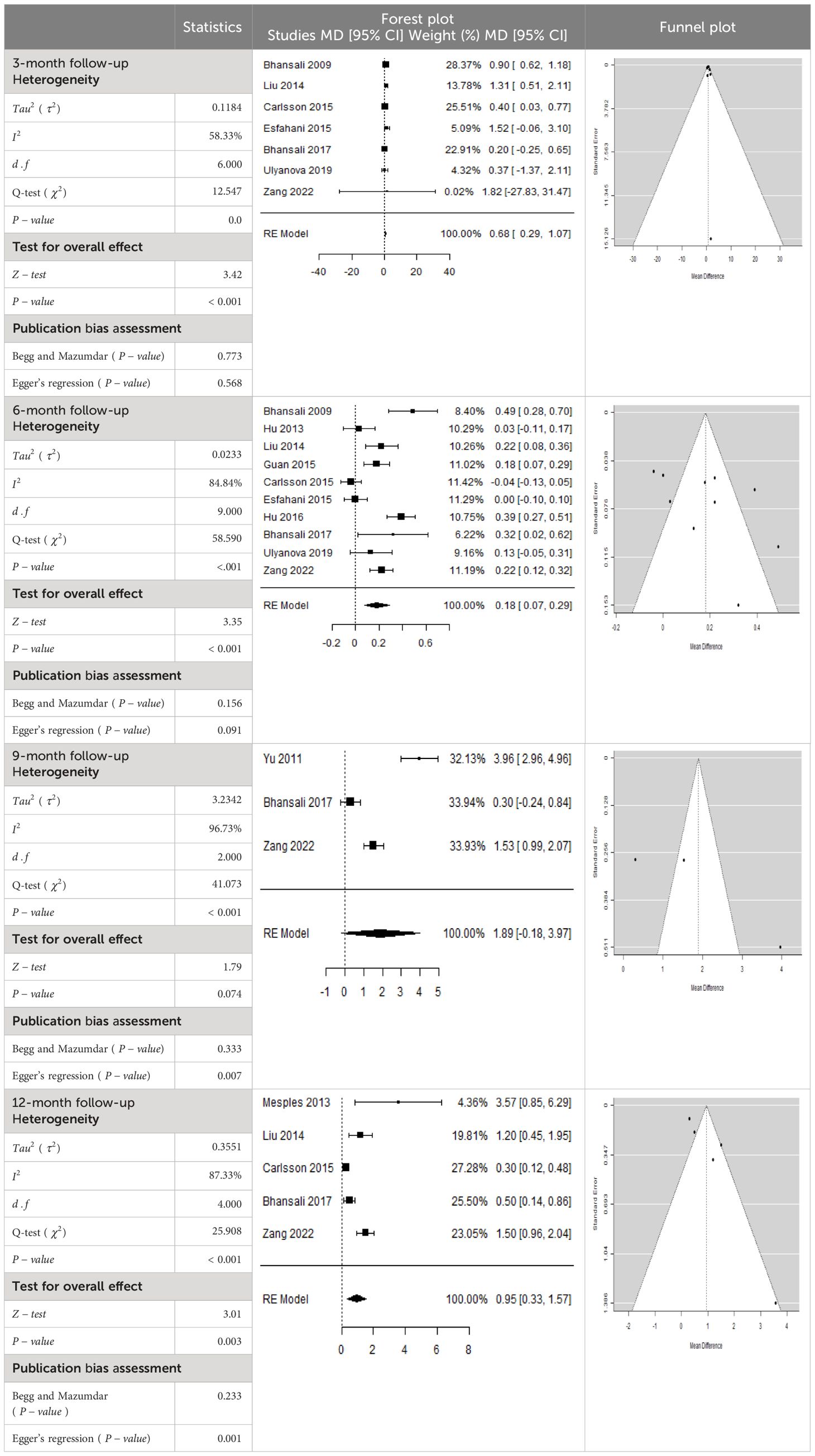
Table 2 Forest plots with the corresponding 95% CIs for the mean difference (MD) of HbA1c.
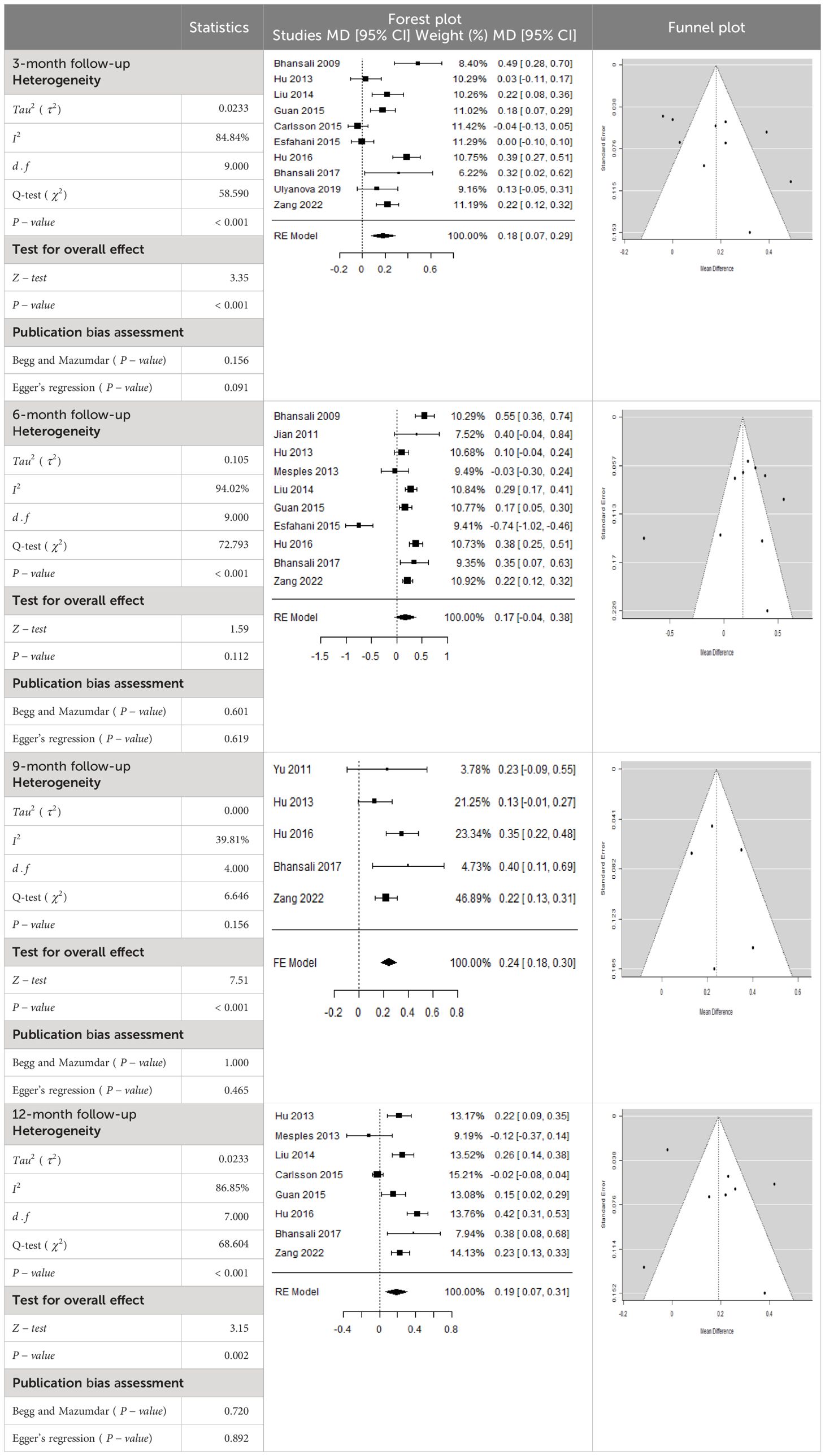
Table 3 Forest plots with the corresponding 95% CIs for the mean difference (MD) of insulin requirement.
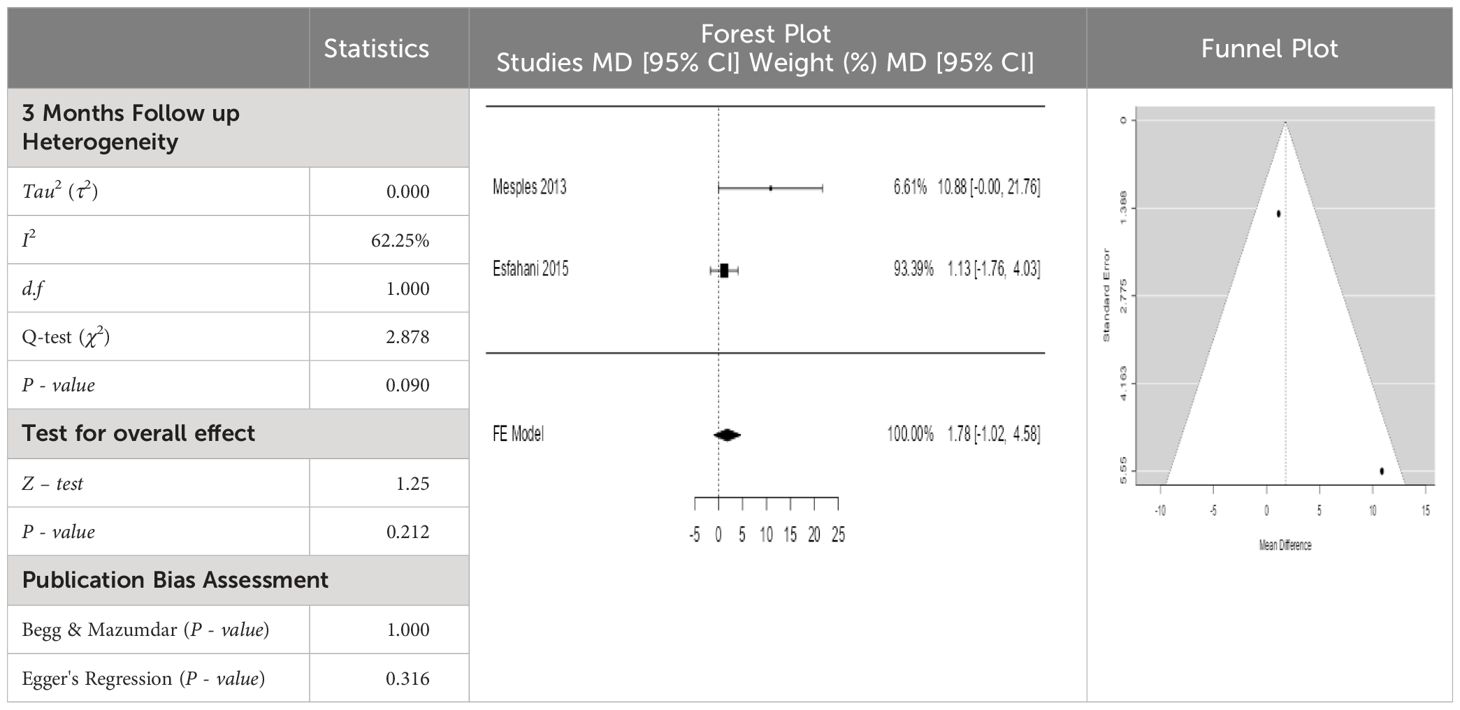
Table 4 Forest plots with the corresponding 95% CIs for the mean difference (MD) of fasting blood glucose.
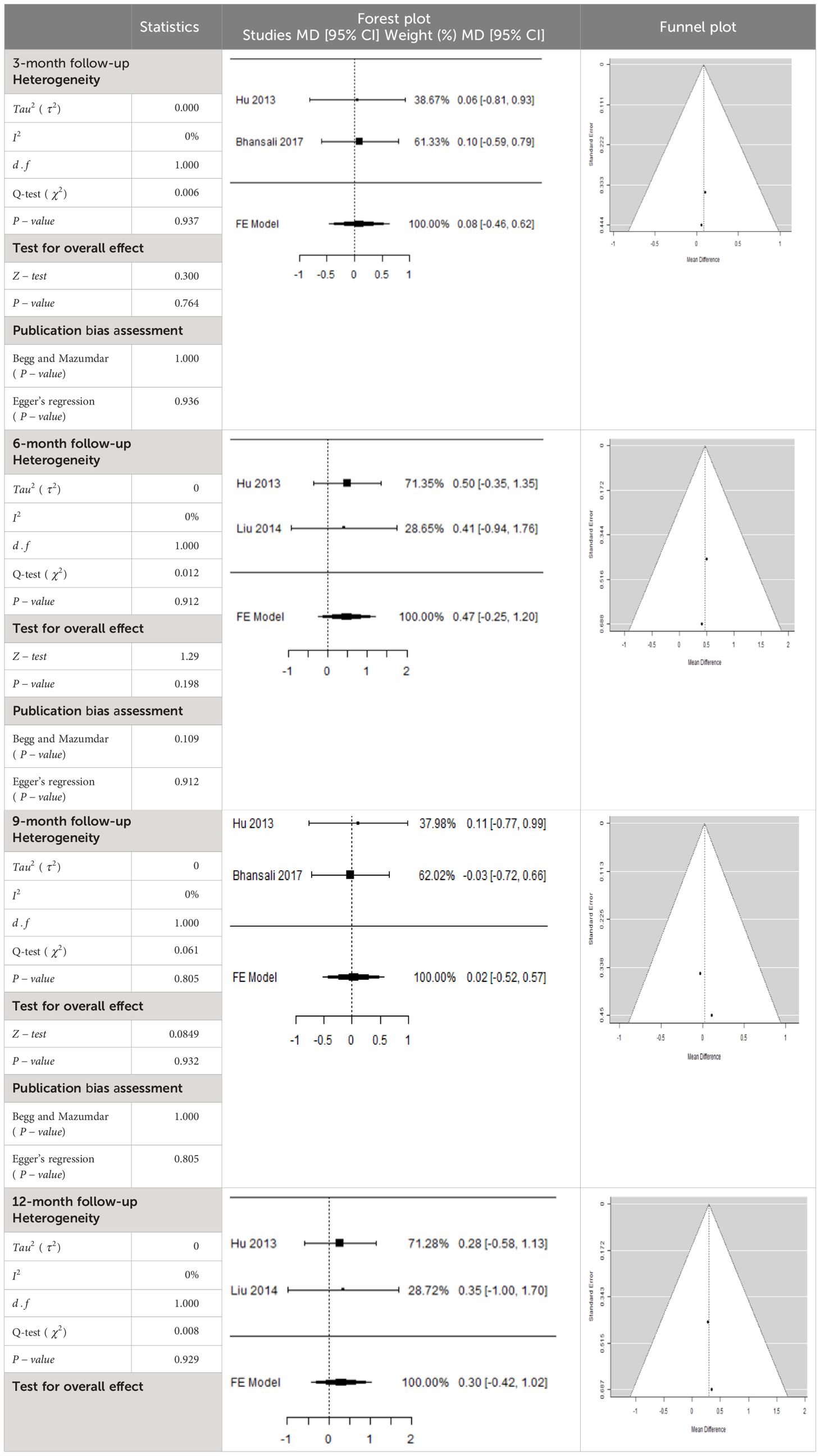
Table 5 Forest plots with the corresponding 95% CIs for the mean difference (MD) of fasting plasma glucose.
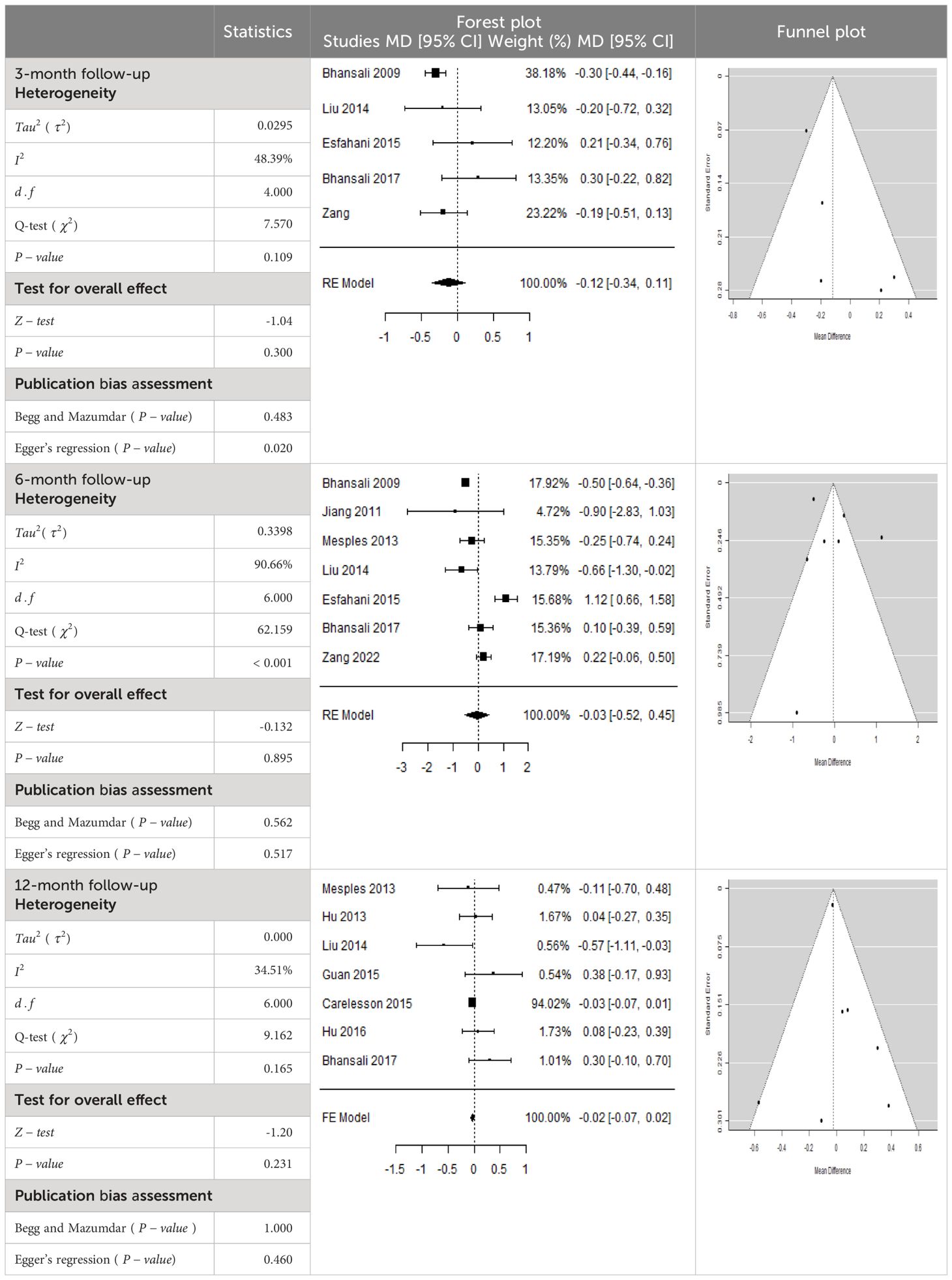
Table 6 Forest plots with the corresponding 95% CIs for the mean difference (MD) of C-peptide.
Search results
The search strategy identified a total of 2,280 articles from selected databases and prior bibliographies. Following a review of the titles and abstracts, 2,231 studies were eliminated due to their lack of relevance in terms of purpose, goal, intervention, and/or measures. After a thorough evaluation of the remaining 49 papers, 34 were excluded. In total, 15 clinical studies met the inclusion criteria and were embraced in the quantitative data analysis for selected outcome measures. However, two of the studies ( 41 , 42 ) were unable to be retrieved. We searched them on different resources, but they were inaccessible. Finally, 13 clinical studies ( 15 – 17 , 28 – 38 ), consisting of 302 subjects, were embraced in the meta-analysis. The selection process of studies is often presented in a flow diagram, which may be visualized in Figure 1 . This diagram provides an overview of the steps taken to identify, screen, and include studies in a systematic review or meta-analysis.
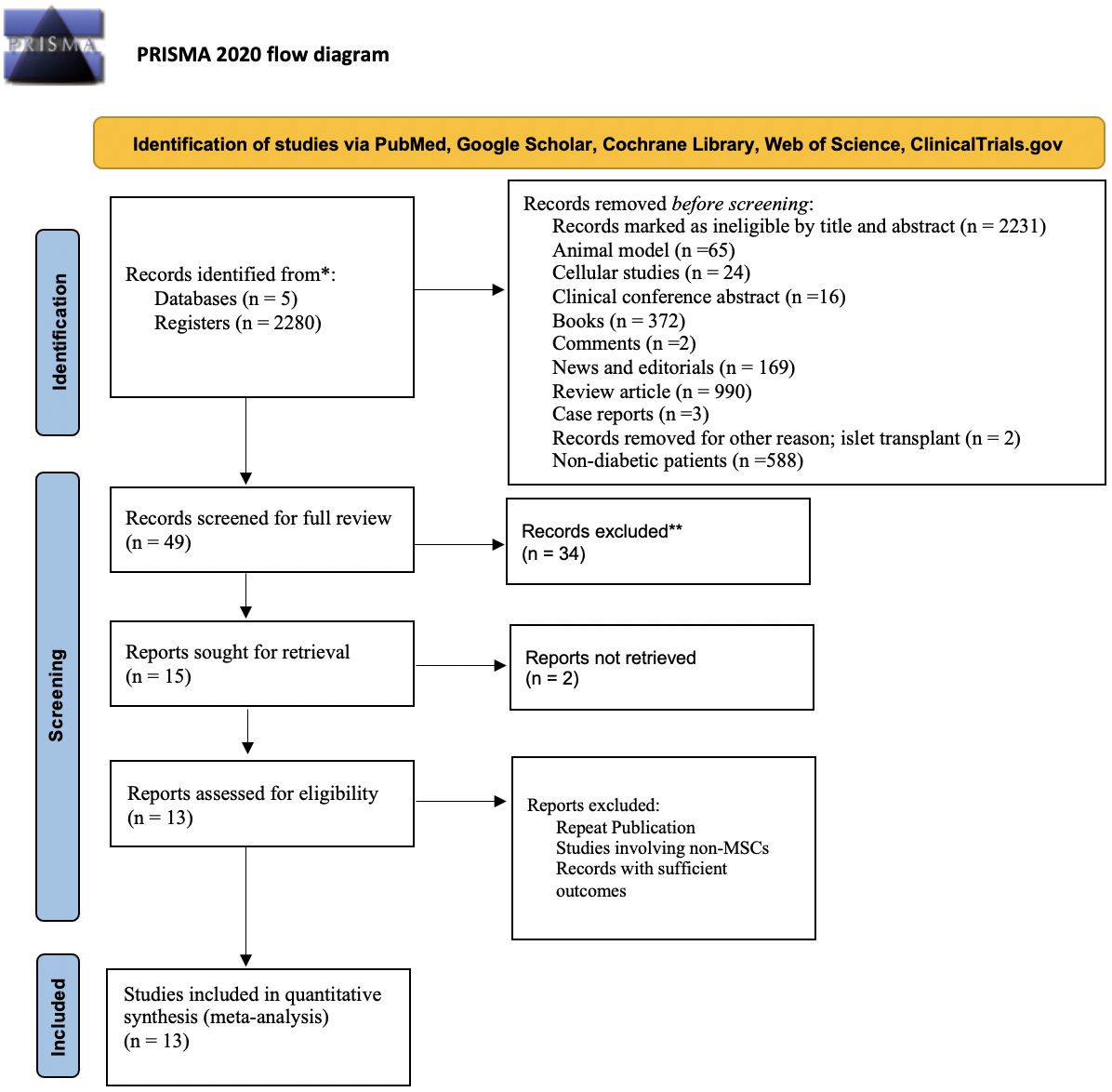
Figure 1 Flow diagram illustrating the identification, screening, and selection of the eligible clinical trials/studies for meta-analysis.
Attributes of the included studies
Table 1 presents clinical data from studies that were included in the analysis. The studies were published between 2000 and November 2023 and included sample sizes ranging from 1 to 73. The subjects’ mean age varied from 17.6 to 57.6 years, with a predominance of male participants. Some trials, however, were unable to collect enough clinical data, such as body mass, blood pressure, liver and renal function tests, and fasting plasma insulin. The eligible studies involved four types of MSCs: including BM-MSCs, Wharton’s jelly-derived (WJ-MSCs), and UC-MSCs, with various cell doses used. The intervention regimen involved administering MSCs via intravenous or intra-arterial delivery, with doses ranging from (0.88 ± 0.05) × 10 6 to (1.2 ± 0.3) × 10 9 . The follow-up period varied from =3 to 12 months. Of the eligible studies, six reported data on MSCs and T1DM ( 28 – 31 , 33 – 35 ), and seven reported data on MSCs and T2DM ( 15 – 17 , 32 , 36 – 38 ).
Effects of stem cell transplantation on HbA1c (%)
In Table 2 , the results of the meta-analysis for the parameter HbA1c with 3-, 6-, 9-, and 12-month follow-up demonstrated that the MSC transplantation is associated with a reduction of HbA1c. It was also found that the MSCs had a significant effect on both T1DM and T2DM with 3-, 6-, 9-, and 12-month follow-up. According to the forest plots, the overall effect size measured with mean difference (MD) revealed comparing the administration of the MSCs and baseline which had shown the significant reduction in HbA1c at 5% level of significance in 3-, 6-, 9-, and 12-month follow-up as (MD: 0.68, 95% CI: 0.29 to 1.07, P -value:< 0.001, I 2 : 58.33%), (MD: 0.18, 95% CI: 0.07 to 0.29, P -value:< 0.001, I2:84.84%), (MD: 1.89, 95% CI: -0.18 to 3.97, P -value: 0.074 > 0.05, I2: 96.73%), and (MD: 0.95, 95% CI: 0.33 to 1.57, P -value: 0.003< 0.05, I2: 87.33%), respectively.
In RCT, we observed that the HbA1c level was lower in the MSC-treated group than in the control group after 3, 6, and 12 months. Furthermore, the difference was statistically significant with 3-, 6-, and 12-month follow-up as (MD = 0.32, 95% CI 0.03 to 0.61, P -value = 0.028), (MD = 0.17, 95% CI 0.01 to 0.34, P -value = 0.043), and (MD = 0.95, 95% CI 0.12 to 1.77, P -value = 0.025), respectively, while in n-RCT, the HbA1c in the MSC-treated group showed a significant decrease from its baseline level to those at the 3- and 6-month follow-up period (MD = 0.96, 95% CI 0.70 to 1.22, P -value< 0.001), and (MD = 0.19, 95% CI 0.04 to 0.34, P -value = 0.012), respectively. The observed MDs in all included studies (100%) along with 3-, 6-, 9-, and 12-month follow-up were being positive (100%), which had indicated a decrease in HbA1c due to MSC transplantation ( Table 7 ). Moreover, from graph/ Figure 2 , the pooled mean of HbA1c computed from all studies with 3-, 6-, 9-, and 12-month follow-up showed a reduction in the levels of HbA1c due to stem cell transplantation when compared with the control group.
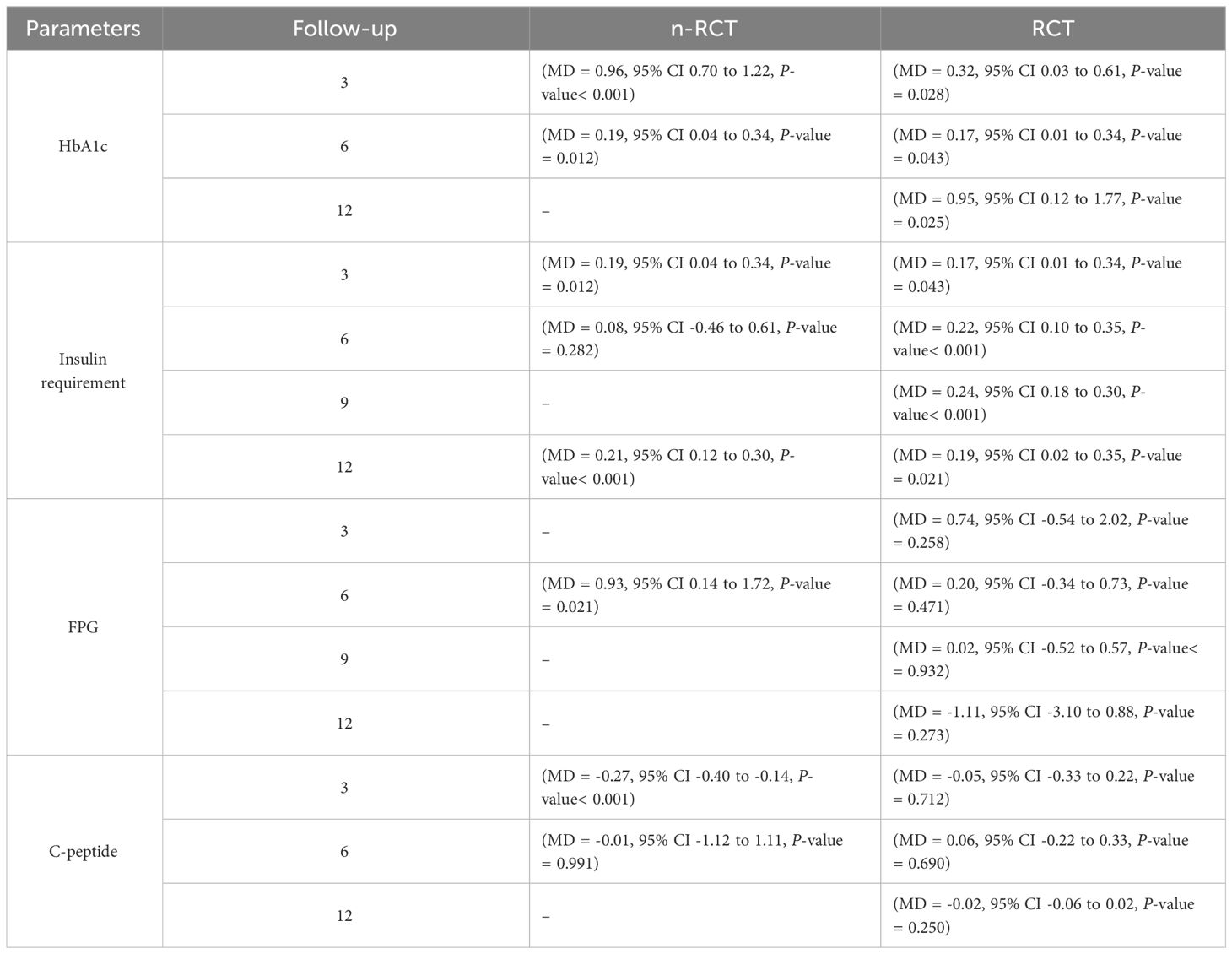
Table 7 Summarized results about HbA1c, insulin requirement, fasting blood glucose, fasting plasma glucose, and C-peptide for n-RCT and RCT.
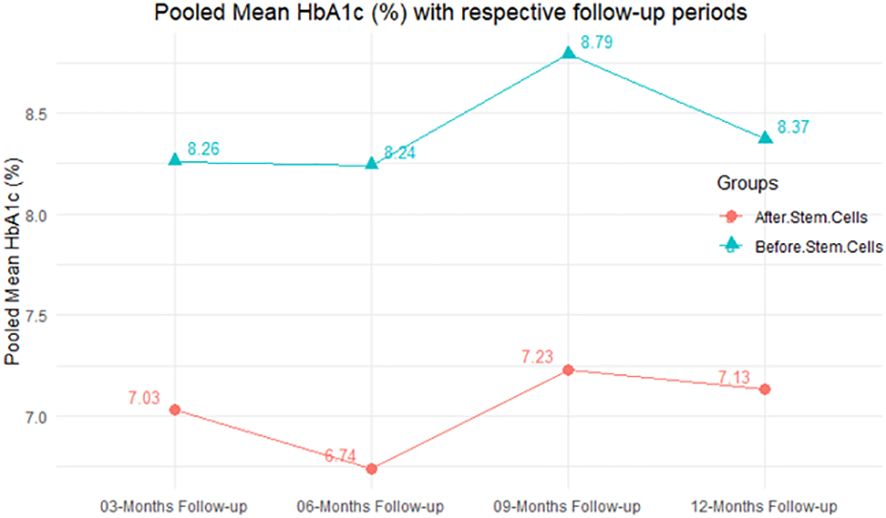
Figure 2 Pooled mean HbA1c (%) with respective follow-up periods.
Effects of stem cell transplantation on insulin (IU/kg/day) requirement
According to the forest plots presented in Table 3 , the overall effect size evaluated using difference (MD) demonstrated a substantial decrease in insulin requirement between the administration of MSCs and the control group at 5% level of significance in 3-, 6-, 9-, and 12-month follow-up as (MD: 0.18, 95% CI: 0.07 to 0.29, P -value:< 0.001, I 2 : 84.84%), (MD: 0.17, 95% CI: -0.04 to 0.38, P -value: 0.112 > 0.05, I 2 : 88.49%), (MD: 0.24, 95% CI: 0.18 to 0.30, P -value:< 0.001, I 2 : 39.81%), and (MD: 0.19, 95% CI: 0.07 to 0.31, P -value: 0.002< 0.05, I 2 : 86.85%), respectively.
In RCT, it was shown that the insulin requirement level was lower in the MSC-treated group than in the control group after 3, 6, 9, and 12 months of follow-up. Moreover, the difference was statistically significant with 3-, 6-, 9-, and 12-month follow-ups as (MD = 0.17, 95% CI 0.01 to 0.34, P -value = 0.043), (MD = 0.22, 95% CI 0.10 to 0.35, P -value< 0.001), (MD = 0.24, 95% CI 0.18 to 0.30, P -value< 0.001), and (MD = 0.19, 95% CI 0.02 to 0.35, P -value = 0.021), respectively, while in n-RCT the insulin requirement in the MSC-treated group showed a significant decrease from its baseline level to those at the 3-, 6-, and 12-month follow-up period (MD = 0.19, 95% CI 0.04 to 0.34, P -value = 0.012) and (MD = 0.21, 95% CI 0.12 to 0.30, P -value< 0.001), respectively. However, the difference was not statistically significant at 6 months (MD = 0.08, 95% CI -0.46 to 0.61, P -value = 0.282) ( Table 7 ). The observed MDs in all included studies (100%) along with 3-, 6-, 9-, and 12-month follow-up were being positive (100%), which had indicated a reduction in insulin due to MSCs therapy. Furthermore, from graph/ Figure 3 , the pooled mean of insulin requirement computed from all studies with 3-, 6-, 9-, and 12-month follow-up showed a reduction in the levels of insulin requirement due to stem cell transplantation.
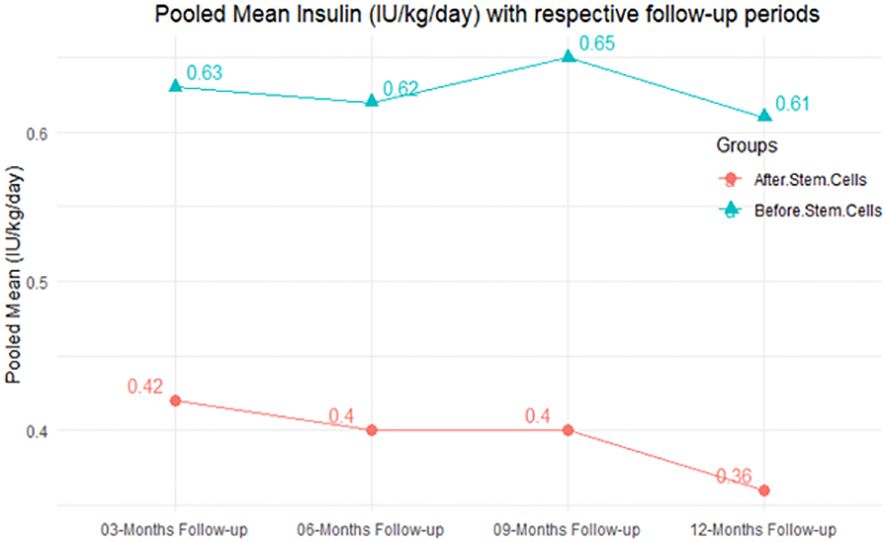
Figure 3 Pooled mean insulin requirement (IU/kg/day) with respective follow-up periods.
Effects of stem cell transplantation on FBG (mmol/L)
From Table 4 , according to the forest plots, the overall effect size measured with difference (MD) showed that when the MSCs were given to the control group, the fasting blood glucose level dropped by a fair number at 5% level of significance in 6-month follow-up as (MD: 1.78, 95% CI: -1.02 to 4.58, P -value: 0.212, I 2 : 62.25%). The observed MDs in all included studies (100%) along with 6-month follow-up were being positive (100%), which indicated a decline in fasting blood glucose due to MSC transplantation. In addition, from graph/ Figure 4 , the pooled mean of FBG was computed from all studies with 3-month follow-up.
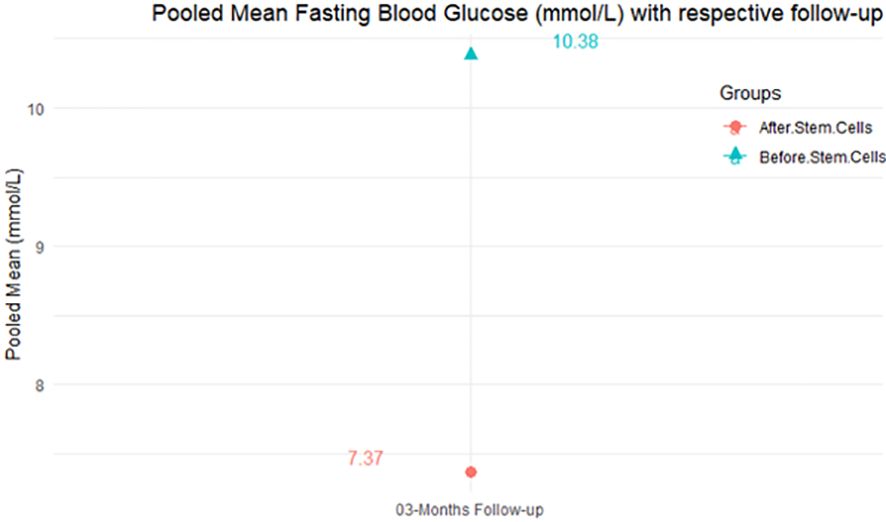
Figure 4 Pooled mean fasting blood glucose (mmol/L) with respective follow-up periods.
Effects of stem cell transplantation on FPG (mmol/L)
In Table 5 , the total effect size evaluated by mean difference (MD) revealed by comparing the MSC administration and the baseline group showed a considerably lower fasting plasma glucose, as represented by the forest plots at 5% level of significance in 3-, 6-, 9-, and 12-month follow-up as (MD: 0.08, 95% CI: -0.46 to 0.62, P -value: 0.764 > 0.05, I 2 : 0%), (MD: 0.47, 95% CI: -0.25 to 1.20, P -value: 0.198 > 0.05, I 2 : 0%), (MD: 0.02, 95% CI: -0.52 to 0.57, P -value: 0.061 > 0.05, I 2 : 0%), and (MD: 0.30, 95% CI: -0.42 to 1.02, P -value: 0.417 > 0.05, I 2 : 0%).
In RCT, we observed that the FPG level was lower in the MSC-treated group than in the control group after 3, 6, and 9 months, but the FPG level was higher in the MSC-treated group than in the control group after a 12-month follow-up period. Moreover, the difference was not statistically significant with 3-, 6-, 9-, and 12-month follow-up as (MD = 0.74, 95% CI -0.54 to 2.02, P -value = 0.258), (MD = 0.20, 95% CI -0.34 to 0.73, P -value = 0.471), (MD = 0.02, 95% CI -0.52 to 0.57, P -value< = 0.932), and (MD = -1.11, 95% CI -3.10 to 0.88, P -value = 0.273), respectively, while in n-RCT the FPG in the MSC-treated group showed a significant decrease from its baseline level to that at the 6-month follow-up period (MD = 0.93, 95% CI 0.14 to 1.72, P -value = 0.021) ( Table 7 ).
The observed mean differences (MDs) in all included studies along with 3-, 6-, and 12-month follow-up were positive, which had indicated a decrease in fasting plasma glucose due to stem cell therapy, while the observed mean differences (MDs) in one study with 9-month follow-up were negative, which had indicated an increase in fasting plasma glucose due to stem cell therapy. The overall effect size for 3, 6, and 12 months was non-significant at P -value > 0.05. The results are shown in Table 5 . The pooled mean of fasting plasma glucose computed from all studies with 3-, 6-, and 9-month follow-up showed a decrease in the levels of FPG due to stem cell transplantation, but at 12-month follow-up the levels of fasting plasma glucose showed an increase (see the graph/ Figure 5 ).
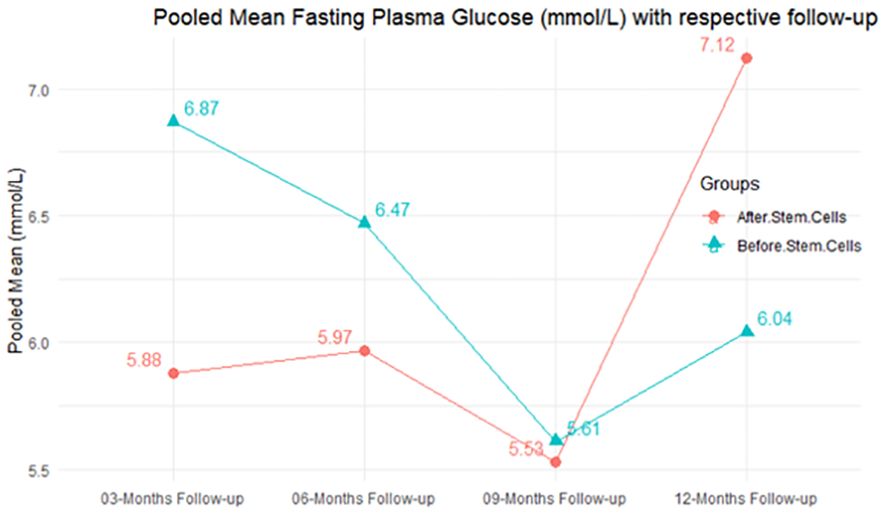
Figure 5 Pooled mean fasting plasma glucose (mmol/L) with respective follow-up periods.
Effects of stem cell transplantation on C-peptide (ng/mL)
According to the forest plots shown in Table 6 , the overall effect size evaluated with difference (MD) was disclosed when comparing the administration of the MSCs and the control group, which had shown a considerably reduced level of C-peptide at 5% level of significance in 3-, 6-, and 12-month follow-up as (MD: -0.12, 95% CI: -0.34 to 0.11, P -value: 0.0.300 > 0.05, I 2 : 48.39%), (MD: -0.03, 95% CI: -0.52 to 0.45, P- value: 0.895 > 0.05, I 2 : 90.66%), and (MD: -0.02, 95% CI: -0.07 to 0.02, P- value: 0.231 > 0.05, I 2 : 34.51%), respectively.
In RCT, we found that the level of C-peptide was increased in the MSC-treated group than in the control group after 3 and 12 months, but the C-peptide level was lower in the MSC-treated group than in the control group after a 6-month follow-up period. The difference was statistically non-significant with 3-, 6-, 9-, and 12-month follow-up as (MD = -1.11, 95% CI -3.10 to 0.88, P -value = 0.273), (MD = -0.05, 95% CI -0.33 to 0.22, P -value = 0.712), (MD = 0.06, 95% CI -0.22 to 0.33, P -value = 0.690), and (MD = -0.02, 95% CI -0.06 to 0.02, P -value = 0.250), respectively, while in n-RCT the C-peptide in the MSC-treated group showed an increase from its baseline level and at 3- and 6-month follow-up period (MD = -0.27, 95% CI -0.40 to -0.14, P -value< 0.001 and (MD = -0.01, 95% CI: -1.12 to 1.11, P -value = 0.991) ( Table 7 ).
The observed mean differences (MDs) in all included studies along with 3-, 6-, and 12-month follow-up were negative (52.63%), which had indicated a minor increase in C-peptide due to MSC transplantation, while 47.37% showed a positive response. From the graph/ Figure 6 , the pattern of the levels of C-peptide was random as observed at 3-, 6-, and 12-month follow-up. As all results of the comparison between stem cell therapy and control group for C-peptide were non-significant, there is a need, therefore, to conduct more studies with a long follow-up.
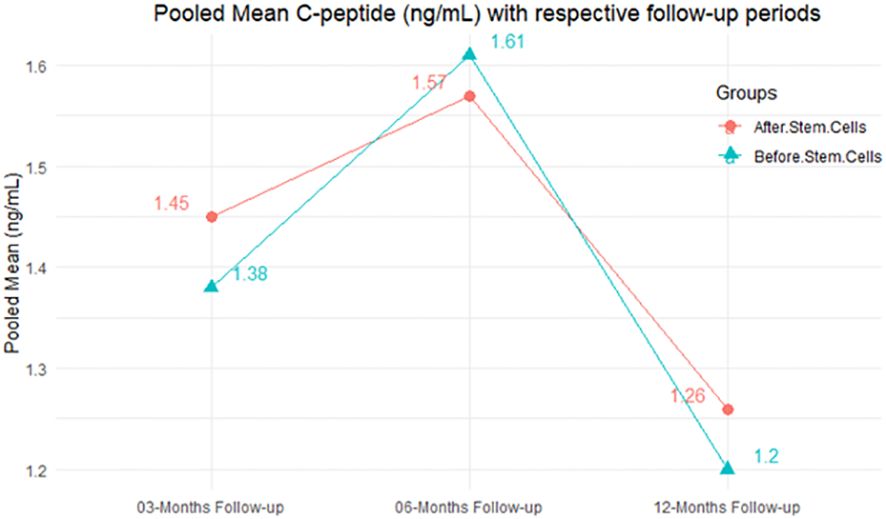
Figure 6 Pooled mean C-peptide (ng/mL) with respective follow-up periods.
Heterogeneity
Cochran’s Q -test and I 2 statistic were applied to measure the heterogeneity of the true outcome of the following parameters: HbA1c, insulin requirement, fasting blood glucose, fasting plasma glucose, and C-peptide with 3-, 6-, 9-, and 12-month follow-ups. According to the Q-test, the true outcomes had appeared to be heterogeneous significantly for HbA1c with 6-, 9-, and 12-month follow-up as ( Q -test: 12.547, P -value: 0.049< 0.05, tau-square: 0.1184, I 2 : 58.33%), ( Q -test: 58.590, P -value< 0.001, tau-square: 0.0233, I 2 : 84.84%), ( Q -test: 41.073, P -value< 0.001, tau-square: 3.2342, I 2 : 96.73%), and ( Q -test: 25.908, P -value< 0.001, tau-square: 0.3551, I 2 : 87.33%), respectively (shown in Table 2 ). Similar results for other parameters can be found in Tables 2 – 6 . The random-effect model was implemented for significance of heterogeneous true outcomes.
Publication bias assessment
The publication bias is estimated through funnel plots and Begg’s and Egger’s regression tests for each forest plot of the following parameters: HbA1c, insulin, fasting blood glucose, fasting plasma glucose, and C-peptide with 3-, 6-, 9-, and 12-month follow-up. The empirical estimation of publication bias was indicated as non-significant bias at 5% level of significance for HbA1c in all 3-, 6-, 9-, and 12-month follow-up as (Begg and Mazumdar test, P -value: 0.733 > 0.05 and Egger’s regression P -value: 0.568 > 0.05), (Begg and Mazumdar test, P- value: 0.156 > 0.05 and Egger’s regression P -value: 0.091 > 0.05), (Begg and Mazumdar test, P -value: 0.333 > 0.05 and Egger’s regression P -value: 0.007< 0.05), and (Begg and Mazumdar test, P -value: 0.233 > 0.05 and Egger’s regression P- value: 0.001< 0.05), respectively. Similar results for publication bias about the parameters insulin requirement, FPG, FBG, and C-peptide can be found in Tables 3 – 6 .
Sensitivity analysis
In the present meta-analysis, sensitivity analysis was conducted to evaluate the impact of key methodological decisions on the synthesized effect estimates and associated uncertainty measures. Specifically, we explored the effects of alternative statistical models (e.g., fixed-effects vs. random-effects models), inclusion/exclusion of studies based on specific criteria (e.g., sample size, study quality), and variations in data synthesis techniques. Through this rigorous examination, we aimed to ascertain the robustness of our findings against potential sources of bias and heterogeneity inherent in meta-analytic research. By identifying influential studies, assessing the sensitivity of results to methodological assumptions, and exploring the consistency of conclusions across different analytical approaches, the sensitivity analysis provides valuable insights into the reliability and generalizability of our study findings.
Multiple methods including the externally standardized residuals, DFFITS values, Cook’s distances, covariances ratios, leave-one-out tau estimates, Hat values, and weights were applied and examined in instances that their residuals of fasting plasma glucose fall out of the control limits ( Figure 7 ) due to a previously conducted study ( 37 ). After excluding this study, a similar approach was iteratively repeated and excluded ( 15 ) to minimize the potential risk of bias. The revised results for FPG are shown in Table 5 . The plots of these sensitivity analyses for HbA1c, insulin requirement, FPG, FBG, and C-peptide are presented in Figures 7 – 11 .
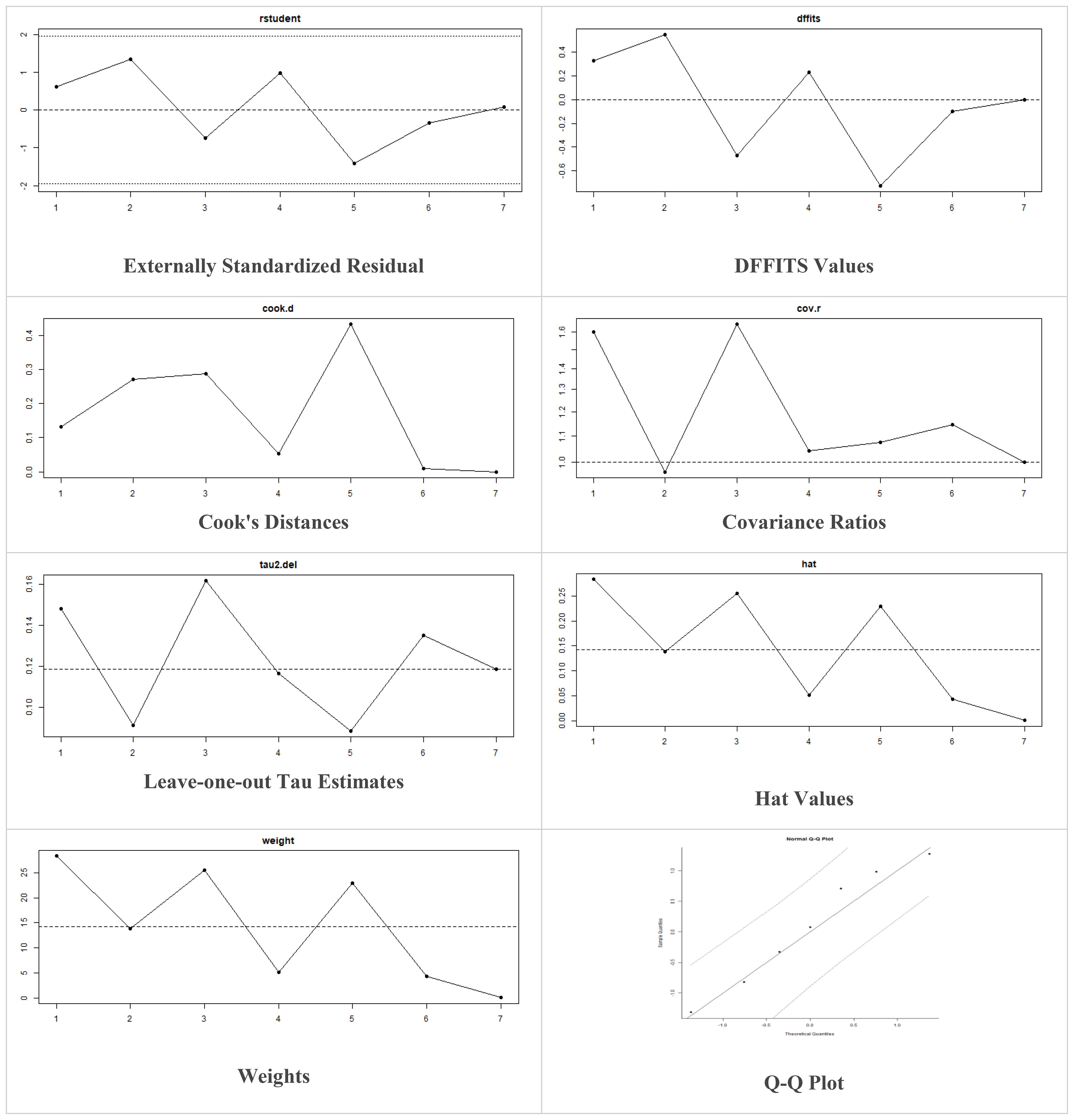
Figure 7 Plots of Sensitivity Analysis for HbA1c (%).
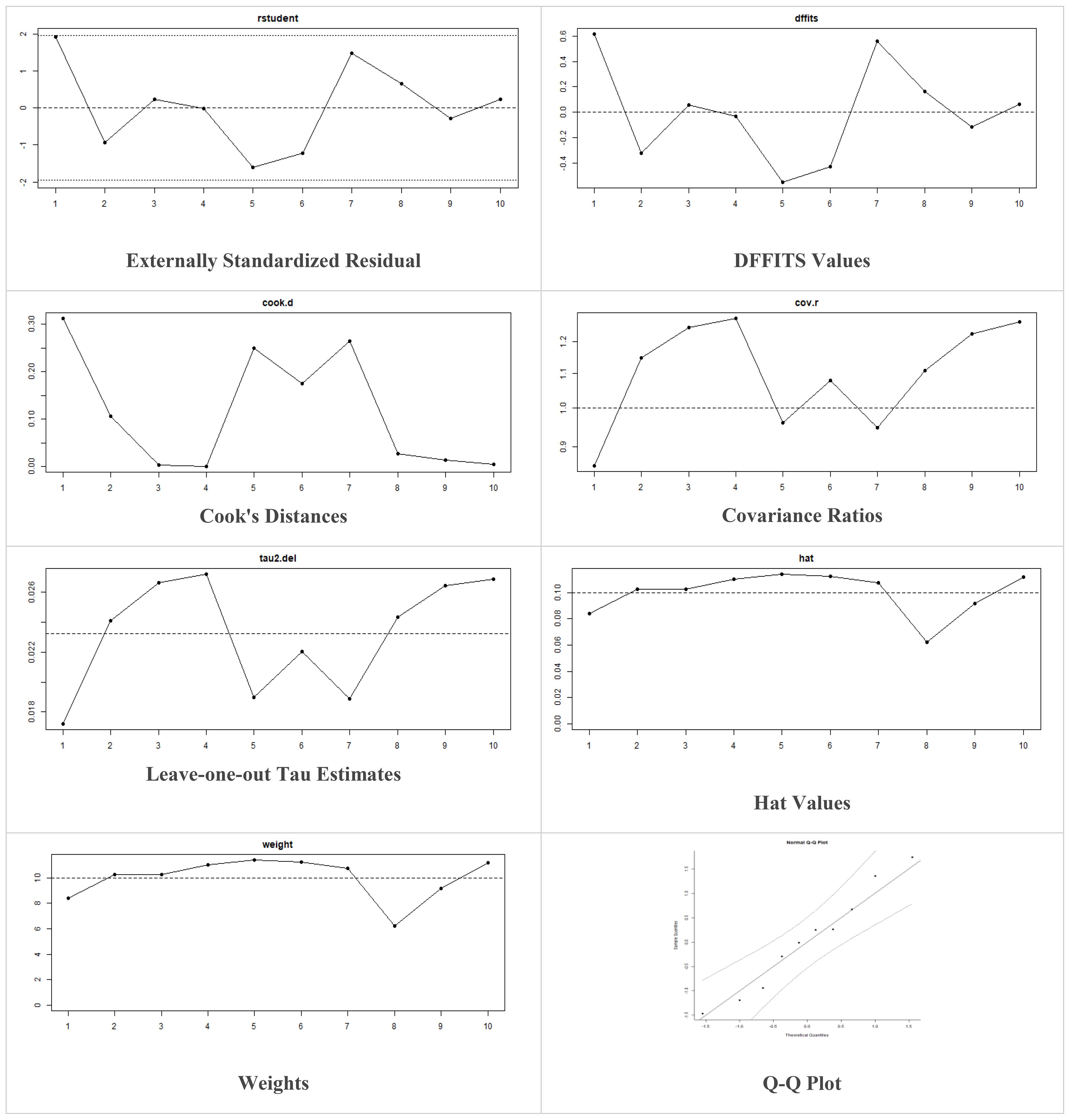
Figure 8 Plots of Sensitivity Analysis Tests for Insulin requirement (IU/kg/day).
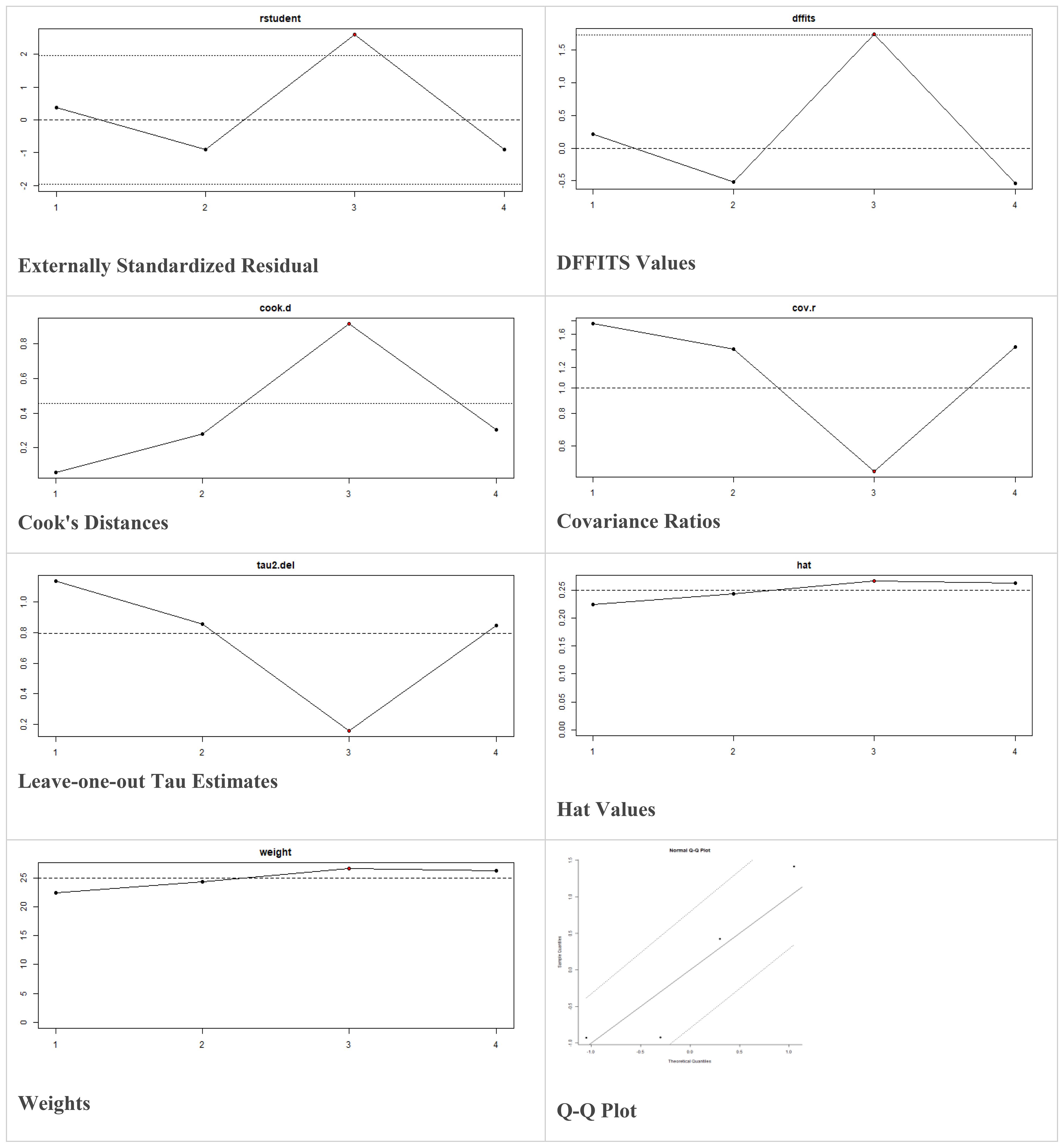
Figure 9 Plots of Sensitivity Analysis Tests for FPG (mmol/L).
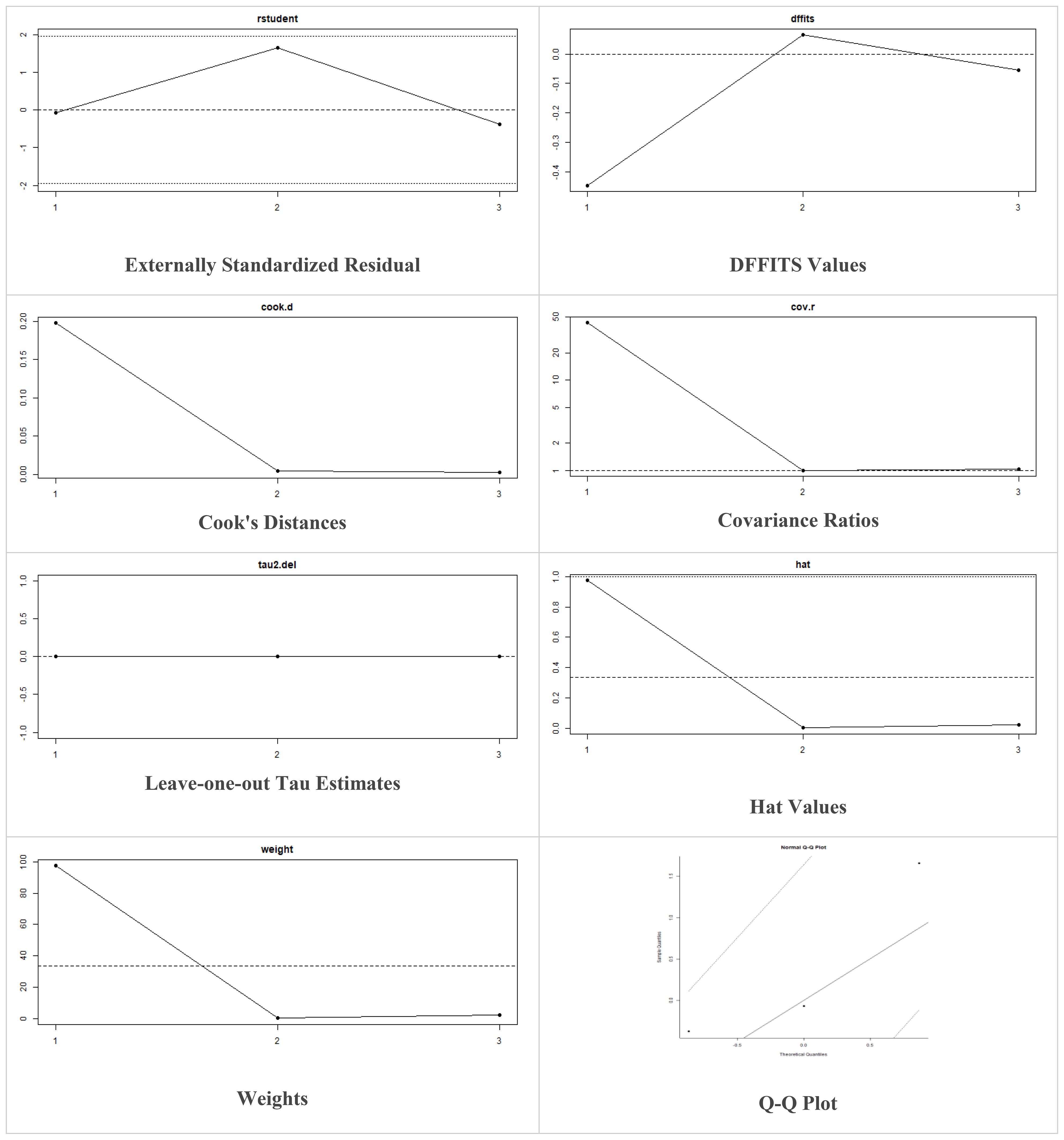
Figure 10 Plots of Sensitivity Analysis Tests for FBG (mmol/L).
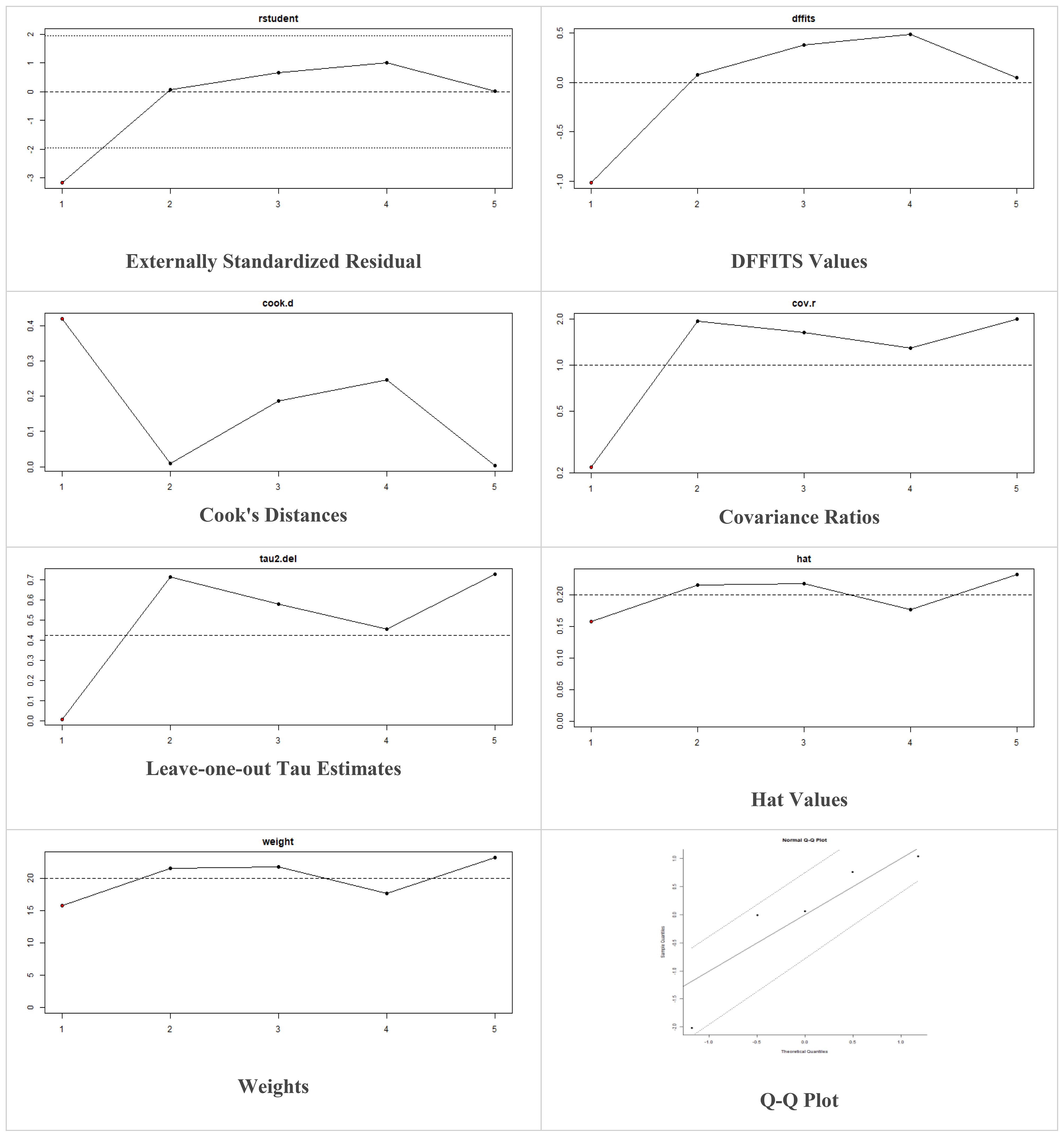
Figure 11 Plots of Sensitivity Analysis Tests for C-peptide (ng/mL).
MSC transplantation safety and adverse events
To clearly differentiate between potential complications caused by the intervention, the fundamental complications of T1DM and insulin therapy must be identified. Compliance to Good Clinical Practice (GCP) guidelines, including randomization and the inclusion of a control group, is recommended to facilitate this. However, the majority of clinical trials investigating stem cell transplantation for the treatment of T1DM have been of poor quality, with many lacking a control group or randomization to allow for comparisons of outcomes and adverse events. Consequently, there have been conflicting judgments regarding the side effects of stem cell therapy in these trials. Hypoglycemia was excluded from consideration as an adverse event, as it can happen due to insulin therapy and autoimmune disorders of the thyroid in individuals with T1DM without any intervention.
Minor hypoglycemic episodes were mentioned in three studies ( 15 , 37 , 38 ), but these episodes were not classified as severe. Nausea and vomiting were mentioned in three studies, with ( 38 ) not specifying the number of patients affected, while ( 15 ) and ( 30 ) reported one patient each. Bhansali et al. ( 15 ) also reported hemorrhage at the injection puncture site in one patient, a drop in hemoglobin level in two patients, and a self-limiting upper respiratory tract infection in one patient. Mild fever was reported in three out of 22 T2DM patients by Liu et al. ( 17 ). There were no serious or persistent adverse reactions or legacy effects observed during the follow-up timespan, indicating that MSCs are reasonably safe in the treatment of DM.
This meta-analysis provides quick insights about MSC transplantation along with their statistical significance ( P -value< 0.05) and was associated with improvements in both T1DM and T2DM. The absence of observed adverse effects in the patients suggests that MSC transplantation may be a safe and promising approach to improve glucose metabolism in individuals with T1DM and T2DM. The data confirms the use of MSC transplantation as an effectual diabetes treatment. The significant reduction in fasting blood glucose, plasma blood glucose, and HbA1c levels at baseline suggests that MSC therapy can improve blood glucose regulation in diabetic patients. The use of FBG and PBG as diabetes diagnostic criteria, as well as HbA1c levels as a measure of diabetes control, supports the conclusion that MSC transplantation has a therapeutic effect on blood glucose regulation in DM patients.
The meta-analysis results indicate a slight but non-significant rise in fasting C-peptide levels in the group that received MSC transplantation ( P -value > 0.05). The escalation in F-CP level decreased as the duration of follow-up increased. This increase in F-CP indicates an improvement in insulin secretion by the pancreatic islet cells, implying that MSC transplantation has a beneficial effect on insulin secretion. The elevated insulin secretion could be due to either a rise in the number of insulin-secreting cells or an improved performance in the function of the remaining β cells. These findings suggest that MSC transplantation has potential as a treatment for diabetes, but further research with longer follow-up periods is necessary to fully comprehend the underlying mechanisms and ensure its long-term safety and effectiveness.
Our findings revealed a substantial reduction in insulin demands following MSC therapy in patients with diabetes, which was consistent across all included studies with follow-up periods of 3, 6, 9, and 12 months. This decrease in insulin requirements was found to be statistically significant ( P -value< 0.05). The observed efficacy of MSC therapy in reducing insulin requirements was retained at the end of most follow-up intervals. However, further studies with prolonged follow-up time points and complete data must confirm these findings. The cessation of insulin treatment is a crucial component in enhancing the overall quality of life of individuals with diabetes. In some studies, it was regarded as the primary outcome. A total of three patients in ( 34 ), three in ( 36 ), two in ( 28 ), six in ( 37 ), and five in ( 32 ) experienced an insulin-free period.
The findings of the meta-analysis indicate a potential improvement in the efficacy of stem cell transplantation for diabetes treatment from 3 to 12 months after transplantation. However, some of the trends were not statistically significant. The results suggest that the MSC transplantation group experienced improvement from 3-, 6-, 9-, and 12-month follow-up periods. However, to ensure the safety and efficacy profiles of SCT for diabetes treatment, long-term follow-up studies are necessary. Thus, there is a need to conduct more studies with extended follow-up periods to obtain a better understanding of the effects of SCT on diabetes. Moreover, further studies that will emphasize on clarifying the different follow-up phases or describing the primary outcomes related to the impact of SCT on diabetes morbidity and mortality are recommended.
According to the analysis, MSCs have been demonstrated as a secure option for stem cell transplantation in diabetes mellitus. The short-term findings indicated that MSCs could help enhance blood glucose regulation; however, additional research is necessary to assess their long-term impacts. Across the 13 studies, no significant adverse reactions or occurrences of hypoglycemic events were detected in subjects who received MSCs treatment. This suggests that MSC transplantation can be regarded as a safe treatment option for DM.
Strengths and limitations
The performance of MSC transplantation in the treatment of diabetes mellitus was analyzed in this systematic review and meta-analysis. The study searched numerous databases and trial registries from their establishment until February 2023. The study utilized a consensus approach to settle disputes, neutral supervision for data extraction, inclusion and exclusion criteria, top-notch impact illustration of original research studies findings on meta-analysis results, and confidence intervals for cumulative facts. Regardless of these advantages, the study had flaws, such as insufficient well-designed clinical trials with control groups, randomization, and blinding. Most of the SCT clinical trials in DM were single arm, leading to inconclusive results. Therefore, standardization and uniformity in the production, culture, and administration of MSCs in clinical trials are needed. In addition, the long-term safety and efficacy of MSC-based therapies have yet to be established, and larger sample sizes, more extended follow-up periods, and well-designed randomized controlled trials are needed to provide a comprehensive assessment of the benefits and risks of these treatments. It is also suggested that expressing daily insulin levels in units/kg/day instead of just customary units can provide a more standardized and comparable measure of the treatment’s effect. Furthermore, presenting findings in numeric form, rather than just figures, can increase the clarity and comprehensiveness of the results. In conclusion, while MSC transplantation shows promise in treating T1DM and T2DM, further research is necessary to fully understand its safety and efficacy and establish best practices for its use in clinical trials. Furthermore, limitations exist due to the lack of individual-level data required for subgroup analysis, such as age, gender, sickness status, and duration of disease history. The decision of selecting confounding factors was contingent upon data availability. The selection of confounding factors for inclusion in the study was determined subjectively, acknowledging inherent limitations. Complete elimination of potential interference from other factors was not feasible. Therefore, future research endeavors should encompass a broader scope, incorporating additional articles to further elucidate the collective impact of multiple factors on diabetes mellitus. Additionally, constraints in implementing alternative statistical methods such as t -tests, ANOVA, or regression analysis due to the lack of original research data and distribution details of variables pose challenges. However, the inability to conduct detailed subgroup analyses and the reliance on summarized data may limit the depth of insights and generalizability of findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
UH: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. NK: Data curation, Formal analysis, Project administration, Supervision, Validation, Writing – review & editing. DG: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review & editing. KA: Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. SS: Data curation, Resources, Writing – review & editing. AU: Data curation, Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to Pak-American Hospital Pvt. Ltd. (Jahangir Multiplex, Peshawar Road, Sector H-13 Islamabad 44000, Pakistan) for providing the platform and support in conducting this study.
Conflict of interest
Authors UH, SS, NK, DG, and AU were employed by the companies R3 Medical Research LLC, Pak-American Hospital Pvt. Ltd., and R3 Stem Cell LLC. Authors NK and DG were employed by the company Bello Bio Labs and Therapeutics Pvt. Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1380443/full#supplementary-material
1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract . (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet . (2001) 358:221–9. doi: 10.1016/S0140-6736(01)05415-0
3. IDF. IDF Diabetes Atlas (2018). Available online at: http://www.diabetesatlas.org/ .
Google Scholar
4. Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab . (2014) 16:467–77. doi: 10.1111/dom.12273
5. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care . (2013) 36:S127–38. doi: 10.2337/dcS13-2011
6. Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Trans Med . (2012) 1:59–63. doi: 10.5966/sctm.2011-0017
CrossRef Full Text | Google Scholar
7. Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant . (2010) 19:667–79. doi: 10.3727/096368910X508762
8. Nanette J, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative diseases. Regener Med . (2010) 5:933–46. doi: 10.2217/rme.10.72
9. Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion . (2014) 54:1418–37. doi: 10.1111/trf.12421
10. Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironmentIDO-expressing murine MSCs and tumor immunity. Cancer Res . (2014) 74:1576–87. doi: 10.1158/0008-5472.CAN-13-1656
11. Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell Immunol . (2015) 293:113–21. doi: 10.1016/j.cellimm.2015.01.002
12. Zanone MM, Favaro E, Miceli I, Grassi G, Camussi E, Caorsi C, et al. Human mesenchymal stem cells modulate cellular immune response to islet antigen glutamic acid decarboxylase in type 1 diabetes. J Clin Endocrinol Metab . (2010) 95:3788–97. doi: 10.1210/jc.2009-2350
13. Gao S, Zhang Y, Liang K, Bi R, Du Y. Mesenchymal stem cells (MSCs): a novel therapy for type 2 diabetes. Stem Cells Int . (2022) 2022:1–17. doi: 10.1155/2022/8637493
14. Sun X, Hao H, Han Q, Song X, Liu J, Dong L, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther . (2017) 8:1–14. doi: 10.1186/s13287-017-0668-1
15. Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, et al. Efficacy of autologous bone marrow–derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev . (2009) 18:1407–16. doi: 10.1089/scd.2009.0164
16. Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med . (2011) 5:94–100. doi: 10.1007/s11684-011-0116-z
17. Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, et al. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther . (2014) 5:1–9. doi: 10.1186/scrt446
18. Kreisel W, Potthoff K, Bertz H, Schmitt-Graeff A, Ruf G, Rasenack J, et al. Complete remission of Crohn's disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone marrow Transplant . (2003) 32:337–40. doi: 10.1038/sj.bmt.1704134
19. Mannon PJ. Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn's disease. Expert Opin Biol Ther . (2011) 11:1249–56. doi: 10.1517/14712598.2011.602967
20. Martin P, Uberti JP, Soiffer RJ, Klingemann H, Waller EK, Daly AS, et al. Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD. Biol Blood Marrow Transplant . (2010) 16:S169–70. doi: 10.1016/j.bbmt.2009.12.057
21. Matar AA, Chong JJ. Stem cell therapy for cardiac dysfunction. Springerplus . (2014) 3:1–14. doi: 10.1186/2193-1801-3-440
22. Lock LT, Tzanakakis ES. Stem/progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng . (2007) 13:1399–412. doi: 10.1089/ten.2007.0047
23. Davey GC, Patil SB, O’Loughlin A, O’Brien T. Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol . (2014) 5:86. doi: 10.3389/fendo.2014.00086
24. D’Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes . (2014) 63:3041–6. doi: 10.2337/db14-0295
25. Mabed M, Shahin M. Mesenchymal stem cell-based therapy for the treatment of type 1 diabetes mellitus. Curr Stem Cell Res Ther . (2012) 7:179–90. doi: 10.2174/157488812799859829
26. Madani S, Amanzadi M, Aghayan HR, Setudeh A, Rezaei N, Rouhifard M, et al. Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: a systematic review and meta-analysis. Systematic Rev . (2022) 11:1–18. doi: 10.1186/s13643-022-01950-3
27. Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, et al. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther . (2021) 12:273. doi: 10.1186/s13287-021-02342-5
28. Esfahani EN, Ghavamzadeh A, Mojahedyazdi N, Hashemian E, Alimoghadam K, Aghel N, et al. Administration of autologous mesenchymal stem cell transplantation for treatment of type 1 diabetes mellitus. Iranian J Public Health . (2015) 44:55–68.
29. Liu Y, Cao D-L, Guo L-B, Guo S-N, Xu J-K, Zhuang H-F, et al. Amniotic stem cell transplantation therapy for type 1 diabetes: a case report. J Int Med Res . (2013) 41:1370–7. doi: 10.1177/0300060513487640
30. Mesples A, Majeed N, Zhang Y, Hu X. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: preliminary results. Med Sci monitor . (2013) 19:852. doi: 10.12659/MSM.889525
31. Ulyanova O, Askarov M, Kozina L, Karibekov T, Shaimardanova G, Zhakupova A, et al. Autologous mesenchymal stem cell transplant in patients with type 1 diabetes mellitus. Exp Clin Transplant . (2019) 17:236–8. doi: 10.6002/ect
32. Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B, et al. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther . (2022) 13:1–10. doi: 10.1186/s13287-022-02848-6
33. Yu W-l, Hong G, Xiao-long Y, Li W, Sheng-li Y, Yan-gang W, et al. Umbilical cord mesenchymal stem cells transplantation for newly-onset type 1 diabetes. Chin J Tissue Eng Res . (2011) 15:4363. doi: 10.3969/j.issn.1673-8225.2011.23.042
34. Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine J . (2013) 60:347–57. doi: 10.1507/endocrj.EJ12-0343
35. Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes . (2015) 64:587–92. doi: 10.2337/db14-0656
36. Guan LX, Guan H, Li H-B, Ren C-A, Liu L, Chu J-J, et al. Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. Exp Ther Med . (2015) 9:1623–30. doi: 10.3892/etm.2015.2339
37. Hu J, Wang Y, Gong H, Yu C, Guo C, Wang F, et al. Long term effect and safety of Wharton's jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med . (2016) 12:1857–66. doi: 10.3892/etm.2016.3544
38. Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, et al. Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: a randomized, placebo-controlled comparative study. Stem Cells Dev . (2017) 26:471–81. doi: 10.1089/scd.2016.0275
39. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin trials . (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
40. Şahin M, Aybek E. Jamovi: an easy to use statistical software for the social scientists. Int J Assess Tools Educ . (2019) 6:670–92. doi: 10.21449/ijate.661803
41. Zhang X, Xu L, Zhou Y. Safety and efficacy of allogeneic amniotic mesenchymal stem cells transplantation in the treatment of newly-onset type 1 diabetes mellitus. Shandong Med J . (2016) 56:44–6.
42. Kong D, Zhuang X, Wang D, Qu H, Jiang Y, Li X, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab . (2014) 60:1969–76. doi: 10.7754/Clin.Lab.2014.140305
Keywords: diabetes mellitus, mesenchymal stem cell, stem cell therapy, regenerative medicine, clinical trials
Citation: Habiba UE, Khan N, Greene DL, Ahmad K, Shamim S and Umer A (2024) Meta-analysis shows that mesenchymal stem cell therapy can be a possible treatment for diabetes. Front. Endocrinol. 15:1380443. doi: 10.3389/fendo.2024.1380443
Received: 02 February 2024; Accepted: 09 April 2024; Published: 10 May 2024.
Reviewed by:
Copyright © 2024 Habiba, Khan, Greene, Ahmad, Shamim and Umer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nasar Khan, [email protected] ; [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The effect of incretin-based drugs on the riks of acute pancreatitis: a review
- Review article
- Published: 05 May 2024
Cite this article

- Agata Czaplicka ORCID: orcid.org/0000-0002-8933-6357 1 &
- Beata Kaleta ORCID: orcid.org/0000-0002-0697-2594 2
Explore all metrics
In recent years, new hypoglycaemic drugs that affect the incretin system have become increasingly popular in the treatment of type 2 diabetes mellitus (T2DM): glucagon-like receptor 1 agonists (GLP1RAs), dipeptidyl peptidase 4 inhibitors (DPP4is) and the recently developed dual glucagon-like receptor 1 agonist and glucose-dependent insulinotropic polypeptide (tirzepatide). Their main role of these drugs is to normalise blood glucose levels. In addition, GLP1RAs are approved for the treatment of excessive body weight. The efficacy of drugs affecting the incretin system is well described in the literature, however, there are still only few reports about their safety. This review aims to summarize the results of current research and meta-analyses on risk of acute pancreatitis (AP) during incretin-affecting drugs treatment.
A narrative review was performed using present literature in an attempt to identify the relationship between AP and incretin-affecting drugs. The following keywords were used: acute pancreatitis, glucagon-like receptor 1 agonists, dipeptidyl peptidase 4 inhibitors and tirzepatide.
It was demonstrated that the use of DPP4is is safe for the majority of patients with T2DM, whereas a risk of AP should be noted in case of GLP1RAs therapy. To date, most studies found no significant association between tirzepatide therapy and the increased risk of AP.
The majority of studies have shown that DPP4is, GLP1RAs and tirzepatide are effective and safe in most T2DM patients. However, the follow-up time for patients treated with tirzepatide is short, therefore more studies are required to confirm the safety of this drug.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Incretin-based therapies and acute pancreatitis risk: a systematic review and meta-analysis of observational studies.
Association of the gallbladder or biliary diseases with dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes: a meta-analysis of randomized controlled trials

Balancing Benefits and Risks in Patients Receiving Incretin-Based Therapies: Focus on Cardiovascular and Pancreatic Side Effects
Wang YW, Lin JH, Yang CS. Meta-analysis of the association between new hypoglycemic agents and digestive diseases. Med (Baltim). 2022;101(34):e30072. https://doi.org/10.1097/MD.0000000000030072 .
Article CAS Google Scholar
Vázquez LA, Romera I, Rubio-de Santos M, Escalada J. Glycaemic Control and Weight reduction: a narrative review of New therapies for type 2 diabetes. Diabetes Ther. 2023;14(11):1771–84. https://doi.org/10.1007/s13300-023-01467-5 .
Article PubMed PubMed Central Google Scholar
Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P. Obesity and diabetes-not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018;34(7):e3042. https://doi.org/10.1002/dmrr.3042 .
American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–42. https://doi.org/10.2337/dc24-S002 .
Article Google Scholar
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to Glycemic Treatment: standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S158–78. https://doi.org/10.2337/dc24-S009 .
Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(13):1506–31. https://doi.org/10.1016/j.jacc.2018.08.1037 .
Article PubMed Google Scholar
Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010. https://doi.org/10.1155/2015/697010 .
Liu Y, Ruan B, Jiang H, Le S, Liu Y, Ao X, et al. The weight-loss effect of GLP-1RAs glucagon-like Peptide-1 receptor agonists in non-diabetic individuals with overweight or obesity: a systematic review with Meta-Analysis and Trial Sequential Analysis of Randomized controlled trials. Am J Clin Nutr. 2023;118(3):614–26. https://doi.org/10.1016/j.ajcnut.2023.04.017 .
Article CAS PubMed Google Scholar
Roussey B, Calame P, Revel L, Zver T, Konan A, Piton G, et al. Liver spontaneous hypoattenuation on CT is an imaging biomarker of the severity of acute pancreatitis. Diagn Interv Imaging. 2022;103(9):401–7. https://doi.org/10.1016/j.diii.2022.03.008 .
Kinoshita H, Zhang J, Ponthisarn A, Sharma M, Binh N, Siam A, et al. Clinical practice guidelines in the diagnosis and management of acute pancreatitis. Med Studies/Studia Medyczne. 2019;35(4):304–11. https://doi.org/10.5114/ms.2019.91248 .
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. https://doi.org/10.1136/gutjnl-2012-302779 .
Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C. et al Acute Pancreatitis: Diagnosis Treat Drugs. 2022;82(12):1251–76. https://doi.org/10.1007/s40265-022-01766-4 .
Goodarzi MO, Petrov MS. Diabetes of the exocrine pancreas: implications for Pharmacological Management. Drugs. 2023;83(12):1077–90. https://doi.org/10.1007/s40265-023-01913-5 .
Article CAS PubMed PubMed Central Google Scholar
Boer GA, Holst JJ. Incretin Hormones and type 2 diabetes-mechanistic insights and therapeutic approaches. Biology (Basel). 2020;9(12):473. https://doi.org/10.3390/biology9120473 .
Gumieniczek A, Berecka-Rycerz A. Metabolism and chemical degradation of New Antidiabetic drugs: a review of Analytical approaches for Analysis of Glutides and Gliflozins. Biomedicines. 2023;11(8):2127. https://doi.org/10.3390/biomedicines11082127 .
Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1–2):8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x .
Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Horm (Athens). 2018;17(3):333–50. https://doi.org/10.1007/s42000-018-0038-0 .
Popoviciu MS, Păduraru L, Yahya G, Metwally K, Cavalu S. Emerging role of GLP-1 agonists in obesity: a Comprehensive Review of Randomised controlled trials. Int J Mol Sci. 2023;24(13):10449. https://doi.org/10.3390/ijms241310449 .
Cesaro A, De Michele G, Fimiani F, Acerbo V, Scherillo G, Signore G, et al. Visceral adipose tissue and residual cardiovascular risk: a pathological link and new therapeutic options. Front Cardiovasc Med. 2023;10:1187735. https://doi.org/10.3389/fcvm.2023.1187735 .
Shi FH, Li H, Cui M, Zhang ZL, Gu ZC, Liu XY. Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes: a systematic review and Meta-analysis of Randomized controlled trials. Front Pharmacol. 2018;9:576. https://doi.org/10.3389/fphar.2018.00576 .
Liao C, Liang X, Zhang X, Li Y. The effects of GLP-1 receptor agonists on visceral fat and liver ectopic fat in an adult population with or without diabetes and nonalcoholic fatty liver disease: a systematic review and meta-analysis. PLoS ONE. 2023;18(8):e0289616. https://doi.org/10.1371/journal.pone.0289616 .
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. https://doi.org/10.1016/j.molmet.2020.101102 .
Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: a real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol (Lausanne). 2022;13:1043789. https://doi.org/10.3389/fendo.2022.1043789 .
El-Arabey AA, Zhang H, Abdalla M, Al-Shouli ST, Alkhalil SS, Liu Y. Metformin as a promising target for DPP4 expression: computational modeling and experimental validation. Med Oncol. 2023;40(10):277. https://doi.org/10.1007/s12032-023-02140-4 .
Chen SY, Kong XQ, Zhang KF, Luo S, Wang F, Zhang JJ. DPP4 as a potential candidate in Cardiovascular Disease. J Inflamm Res. 2022;15:5457–69. https://doi.org/10.2147/JIR.S380285 .
Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(11):642–53. https://doi.org/10.1038/s41574-020-0399-8 .
Röhrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front Immunol. 2015;6:386. https://doi.org/10.3389/fimmu.2015.0038 .
Bernardini F, Nusca A, Coletti F, La Porta Y, Piscione M, Vespasiano F, et al. Incretins-based therapies and their Cardiovascular effects: New Game-Changers for the management of patients with diabetes and Cardiovascular Disease. Pharmaceutics. 2023;15(7):1858. https://doi.org/10.3390/pharmaceutics15071858 .
Scirica BM, Im K, Murphy SA, Kuder JF, Rodriguez DA, Lopes RD, et al. Re-adjudication of the Trial evaluating Cardiovascular outcomes with Sitagliptin (TECOS) with study-level meta-analysis of hospitalization for heart failure from cardiovascular outcomes trials with dipeptidyl peptidase-4 (DPP-4) inhibitors. Clin Cardiol. 2022;45(7):794–801. https://doi.org/10.1002/clc.23844 .
Seino Y, Kaku K, Kadowaki T, Okamoto T, Sato A, Shirakawa M, et al. A randomized, placebo-controlled trial to assess the efficacy and safety of sitagliptin in Japanese patients with type 2 diabetes and inadequate glycaemic control on ipragliflozin. Diabetes Obes Metab. 2021;23(6):1342–50. https://doi.org/10.1111/dom.14346 .
Li J, He K, Ge J, Li C, Jing Z. Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108656. https://doi.org/10.1016/j.diabres.2021.108656 .
Tseng CM, Liao WC, Chang CY, Lee CT, Tseng CH, Hsu YC, et al. Incretin-based pharmacotherapy and risk of adverse pancreatic events in the ethnic Chinese with diabetes mellitus: a population-based study in Taiwan. Pancreatology. 2017;17(1):76–82. https://doi.org/10.1016/j.pan.2016.10.003 .
Storgaard H, Cold F, Gluud LL, Vilsbøll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906–8. https://doi.org/10.1111/dom.12885 .
Singh AK, Gangopadhyay KK, Singh R. Risk of acute pancreatitis with incretin-based therapy: a systematic review and updated meta-analysis of cardiovascular outcomes trials. Expert Rev Clin Pharmacol. 2020;13(4):461–8. https://doi.org/10.1080/17512433.2020.1736041 .
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183 .
Shu Y, He X, Wu P, Liu Y, Ding Y, Zhang Q. Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA adverse event reporting system. Front Public Health. 2022;10:996179. https://doi.org/10.3389/fpubh.2022.996179 .
Aroda VR, Erhan U, Jelnes P, Meier JJ, Abildlund MT, Pratley R, et al. Safety and tolerability of semaglutide across the SUSTAIN and PIONEER phase IIIa clinical trial programmes. Diabetes Obes Metab. 2023;25(5):1385–97. https://doi.org/10.1111/dom.14990 .
Javed H, Kogilathota Jagirdhar GS, Kashyap R, Vekaria PH. Liraglutide-Induced Pancreatitis: a Case Report and Literature Review. Cureus. 2023;15(4):e38263. https://doi.org/10.7759/cureus.38263 .
Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38(6):1058–66. https://doi.org/10.2337/dc13-1210 .
Caparrotta TM, Templeton JB, Clay TA, Wild SH, Reynolds RM, Webb DJ, et al. Glucagon-like peptide 1 receptor agonist (GLP1RA) exposure and outcomes in type 2 diabetes: a systematic review of Population-based Observational studies. Diabetes Ther. 2021;12(4):969–89. https://doi.org/10.1007/s13300-021-01021-1 .
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58. https://doi.org/10.2337/dc13-2761 .
Chin R, Nagaoka S, Nakasawa H, Tanaka Y, Inagaki N. Safety and effectiveness of dulaglutide 0.75 mg in Japanese patients with type 2 diabetes in real-world clinical practice: 36 month post-marketing observational study. J Diabetes Investig. 2023;14(2):247–58. https://doi.org/10.1111/jdi.13932 .
Al-Kawas F, Anderson MA, Enns R, Wilson TH, Johnson S, Mallory JM, PANCREATIC SAFETY IN STUDIES OF THE GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST ALBIGLUTIDE. Endocr Pract. 2019;25(7):698–716. https://doi.org/10.4158/EP-2018-0507 .
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. https://doi.org/10.1016/S0140-6736(18)32261-X .
Shahbazi M, Qudsiya Z, Fahel A, Amini A, Tanoli T. First reported case of Dulaglutide-Induced Acute Pancreatitis with normal serum lipase level. Cureus. 2023;15(6):e40576. https://doi.org/10.7759/cureus.40576 .
Khan AB, Shah A, Ahmad S, Khan MI, Amir A. Dulaglutide (Trulicity)-Induced Acute Pancreatitis: a Case Report. Cureus. 2023;15(5):e38630. https://doi.org/10.7759/cureus.38630 .
AlSaadoun AR, AlSaadoun TR, Al Ghumlas AK. Liraglutide Overdose-Induced Acute Pancreatitis. Cureus. 2022;14(1):e21616. https://doi.org/10.7759/cureus.21616 .
Dolan RD, Bazarbashi AN, Lo A, Smith BN. Liraglutide-Induced Hemorrhagic Pancreatitis in a nondiabetic patient. ACG Case Rep J. 2020;7(5):e00380. https://doi.org/10.14309/crj.0000000000000380 .
Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE, et al. 2 DIABETES MANAGEMENT ALGORITHM– 2020 EXECUTIVE SUMMARY. Endocr Pract. 2020;26(1):107–39. https://doi.org/10.4158/CS-2019-0472 .
Yabe D, Kuwata H, Kaneko M, Ito C, Nishikino R, Murorani K, et al. Use of the Japanese health insurance claims database to assess the risk of acute pancreatitis in patients with diabetes: comparison of DPP-4 inhibitors with other oral antidiabetic drugs. Diabetes Obes Metab. 2015;17(4):430–4. https://doi.org/10.1111/dom.12381 .
Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. Association between Incretin-based drugs and the risk of Acute Pancreatitis. JAMA Intern Med. 2016;176(10):1464–73. https://doi.org/10.1001/jamainternmed.2016.1522 .
Ueki K, Tanizawa Y, Nakamura J, Yamada Y, Inagaki N, Watada H, et al. Long-term safety and efficacy of alogliptin, a DPP-4 inhibitor, in patients with type 2 diabetes: a 3-year prospective, controlled, observational study (J-BRAND Registry). BMJ Open Diabetes Res Care. 2021;9(1):e001787. https://doi.org/10.1136/bmjdrc-2020-001787 .
Lee M, Sun J, Han M, Cho Y, Lee JY, Nam CM, et al. Nationwide trends in Pancreatitis and Pancreatic Cancer Risk among patients with newly diagnosed type 2 diabetes receiving Dipeptidyl Peptidase 4 inhibitors. Diabetes Care. 2019;42(11):2057–64. https://doi.org/10.2337/dc18-2195 .
Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of Acute Pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40(2):284–6. https://doi.org/10.2337/dc15-1707 .
Sayiner ZA, Inan Demiroğlu G, Akarsu E, Araz M. The relationship between Dipeptidyl Peptidase-4 inhibitor usage and Asymptomatic Amylase Lipase Increment in type 2 diabetes Mellitus patients. Turk J Pharm Sci. 2020;17(1):68–73. https://doi.org/10.4274/tjps.galenos.2018.83788 .
Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of Subcutaneous Tirzepatide vs Placebo added to titrated insulin glargine on Glycemic Control in patients with type 2 diabetes: the SURPASS-5 Randomized Clinical Trial. JAMA. 2022;327(6):534–45. https://doi.org/10.1001/jama.2022.0078 .
Karagiannis T, Avgerinos I, Liakos A, Del Prato S, Matthews DR, Tsapas A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251–61. https://doi.org/10.1007/s00125-022-05715-4 .
Xie Z, Hu J, Gu H, Li M, Chen J. Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: a systematic review. Front Endocrinol (Lausanne). 2023;14:1244432. https://doi.org/10.3389/fendo.2023.1244432 .
Mishra R, Raj R, Elshimy G, Zapata I, Kannan L, Majety P, et al. Adverse events related to Tirzepatide. J Endocr Soc. 2023;7(4):bvad016. https://doi.org/10.1210/jendso/bvad016 .
Tang Y, Zhang L, Zeng Y, Wang X, Zhang M. Efficacy and safety of tirzepatide in patients with type 2 diabetes: a systematic review and meta-analysis. Front Pharmacol. 2022;13:1016639. https://doi.org/10.3389/fphar.2022.1016639 .
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus Semaglutide once Weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15. https://doi.org/10.1056/NEJMoa2107519 .
Download references
Author information
Authors and affiliations.
Department of Internal Medicine and Gastroenterology, Brodnowski Hospital of the Mazovian, Kondratowicza 8, 03-242, Warsaw, Poland
Agata Czaplicka
Department of Clinical Immunology, Medical University of Warsaw, 02-006, Warsaw, Poland
Beata Kaleta
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to conceptualization, review and editing of the manuscript. All authors approved the final manuscript for submission.
Ethics declarations
Conflict of interest, financial interests.
The authors declare they have no financial interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Czaplicka, A., Kaleta, B. The effect of incretin-based drugs on the riks of acute pancreatitis: a review. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01430-6
Download citation
Received : 28 November 2023
Accepted : 27 March 2024
Published : 05 May 2024
DOI : https://doi.org/10.1007/s40200-024-01430-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute pancreatitis
- Dipeptidyl peptidase 4
- Inhibitors glucagon-like receptor 1 agonists
- Tirzepatide
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 09 May 2024
Diabetes, life course and childhood socioeconomic conditions: an empirical assessment for Mexico
- Marina Gonzalez-Samano 1 &
- Hector J. Villarreal 1
BMC Public Health volume 24 , Article number: 1274 ( 2024 ) Cite this article
158 Accesses
9 Altmetric
Metrics details
Demographic and epidemiological dynamics characterized by lower fertility rates and longer life expectancy, as well as higher prevalence of non-communicable diseases such as diabetes, represent important challenges for policy makers around the World. We investigate the risk factors that influence the diagnosis of diabetes in the Mexican population aged 50 years and over, including childhood poverty.
This work employs a probabilistic regression model with information from the Mexican Health and Aging Study (MHAS) of 2012 and 2018. Our results are consistent with the existing literature and should raise strong concerns. The findings suggest that risk factors that favor the diagnosis of diabetes in adulthood are: age, family antecedents of diabetes, obesity, and socioeconomic conditions during both adulthood and childhood.
Conclusions
Poverty conditions before the age 10, with inter-temporal poverty implications, are associated with a higher probability of being diagnosed with diabetes when older and pose extraordinary policy challenges.
Peer Review reports
One of the major public health concerns worldwide is the negative consequences that the demographic (with its epidemiological) transition could bring. This demographic transition is driven by increasing levels of life expectancy (caused by technological innovation and scientific breakthroughs in many cases) and decreasing fertility rates. While during the 20th century, the main health concerns were related to infectious and parasitic diseases, at the present time, non-communicable diseases (NCDs), such as diabetes, constitute a harsh burden in terms of economic and social impact. NCDs most commonly affect the health of adults and the elderly. The economic and social costs associated with NCDs increase sharply with age. These patterns have implications for economic growth, poverty-reduction efforts and social welfare [ 1 ].
Mexico’s demographic trends are reflecting a significant shift over the past decades, much like those observed globally. In 1950, the fertility rate stood at 6.7 children per woman, and the proportion of the population aged 60 or over was about 2%. Since the 1970s, there has been a considerable decrease in fertility rates; by 2017, it had dropped to 2.2 children per woman [ 2 ]. Even more pressing, according to CONAPO Mexico had a total fertility rate of 1.91 during 2023 [ 3 ]. Alongside the declining fertility, the aging population is becoming a more prominent feature in Mexico’s demographic profile. In 2017, individuals aged 60 and over constituted around 10% of the population. Forecasts for 2050 project that this figure will more than double, with those 60 and over representing 25% of the total population. These trends suggest substantial changes in Mexico’s population structure, with implications for policy-making in areas such as healthcare, pensions, and workforce development [ 2 ].
Regarding NCDs, in 2017 13% of the Mexican adult population suffered from diabetes, which is twice the Organisation for Economic Cooperation and Development (OECD) average and it is also the highest rate among its members. Some of the risk factors associated with this disease are being overweight or obese, unhealthy diets and sedentary lifestyles. In 2017 72.5% of the Mexican population was overweight or obese [ 4 ] and the country had the highest OECD rate of hospital admissions for diabetes. During the period of 2012 to 2017, the number of hospital admissions for amputations related to this condition, increased by more than 10%, which suggests a deterioration in quality and control of diabetes treatments [ 4 ]. Moreover, it is estimated that diabetes prevalence will continue with its upward trend; forecasts anticipate that in 2030 there will be around 17.2 million people in Mexico with this condition [ 5 ].
Despite the increasing proportion of older people, most of the research regarding the effects of socioeconomic conditions on health focuses on economically active populations. Those which do consider older people, do not investigate length factors such as childhood conditions [ 6 , 7 ]. In this sense, the Social Determinants of Health (SDH) throughout the Life Course approach provide a framework to ponder and direct the design of public policies on population aging and health [ 8 , 9 ]. They focus on well-being and the quality of life of populations from a multi-factorial perspective [ 10 , 11 , 12 ].
In this study, we explore the impact of childhood and adulthood conditions and other demographic and health aspects on diabetes among older people. The literature has proposed several mechanisms through which the mentioned drivers could operate. In general, these approaches imply that satisfactory socioeconomic outcomes for adults may relatively atone for poor socioeconomic conditions in early childhood [ 13 , 14 , 15 , 16 ].
Poverty conditions during the first years of life have critical implications, and yet children are twice as likely to live in poverty as adults [ 16 , 17 ]. On the other hand, poverty is known to be closely linked to NCDs such as diabetes. According to [ 13 ], NCDs are expected to obstruct poverty reduction efforts in low and middle-income countries (LMICs) by increasing costs associated with health care. Moreover, the costs resulting from NCDs such as diabetes could deplete household incomes rapidly and impulse millions of people into poverty [ 16 ].
The United Nations Children’s Fund (UNICEF) has highlighted the consequences of what it describes as the “invisible epidemic”: non-communicable diseases. NCDs are the leading cause of death worldwide, accounting for 71% or 41 million of the annual deaths globally. The majority (85%) of NCD deaths among people under 70 years of age occur in low and middle-income countries [ 17 ].
According the World Health Organization (WHO), SDH are non-medical factors that influence health outcomes, such as the circumstances in which people are born, grow, work, live, and age, and the broader set of forces and systems that shape the conditions of daily life Footnote 1 .
These forces include economic policies and systems, development agendas, social norms and policies, and political systems [ 11 , 18 ]. In this regard, SDH have an important influence on health inequities in countries of all income levels. Health and disease follow a social gradient, that is, the lower the socioeconomic status, a lesser health is expected [ 11 , 18 ].
On the other hand, the Life Course perspective distinguishes the opportunity to inhibit and control illnesses at key phases of life from preconception to pregnancy, infancy, childhood, adolescence, and through adulthood. This does not follow the health model where an individual is healthy until disease occurs, the trajectory is determined earlier in life. Evidence suggests that age related mortality and morbidity can be anticipated in early life with factors such as maternal diet [ 19 ] and body composition, low childhood intelligence, and negative childhood experiences acting as antecedents of late-life diseases [ 13 ].
The consequential diversity in the capacities and health needs of older people is not accidental. They are rooted in events throughout the life course and SDH that can often be modified, hence opening intervention opportunities. This framework is central in the proposed “Healthy Aging”. According to WHO [ 20 ], Healthy Aging is “the process of developing and maintaining the functional ability that enables well-being in older age”.
In this way, the Life Course and SDH approaches allow to better distinguish how social differences in health are perpetuated and propagated, and how they can be diminished or assuaged through generations. Several research efforts suggest that age related mortality and morbidity can be predicted in early life with aspects such as maternal nutrition, low childhood intelligence, difficult childhood experiences acting as antecedents of late-life diseases [ 13 ]. The Life Course acknowledges the contribution of earlier life conditions on adult health outcomes [ 15 , 21 ]. In addition, SDH have an important influence on inequality and, therefore, on people’s well-being and quality of life [ 22 ]. Trends in health literacy across life are also influenced by various SDH such as income, educational level, gender and ethnicity [ 23 ].
Finally, though the research that links early life conditions and health outcomes in adulthood is scarce in low and middle-income countries, our study aims to address the gaps in knowledge regarding the impact of childhood socioeconomic conditions on long-term health outcomes, including the prevalence of non-communicable diseases in LMICs. We specifically focus on the incidence of diabetes in Mexico. Advocating for early-life targeted interventions, we highlight the critical need to address the root causes of NCDs to reduce their impact on the most vulnerable groups. Utilizing data from the Mexican Health and Aging Study (MHAS), which provides comprehensive health, demographic, and socioeconomic information on individuals aged 50 and older, as well as details on their childhoods (before the age of 10) and family health backgrounds [ 24 ], our research emphasizes the importance of developing targeted interventions on early life course stages.
Health, childhood and adulthood conditions
Multiple studies highlight that childhood experiences can influence patterns of disease, aging, and mortality later in life [ 10 , 11 , 16 , 20 , 25 ]. The conditions in health and its social determinants accumulate over the life course. This process initiates with pregnancy and early childhood, continues throughout school years and the transition to working life and later in retirement. The main priority should be for countries to ensure a good start in life during childhood. This requires at least adequate social and health protection for women, plus affordable good early childhood education and care systems for infants [ 11 ].
However, demonstrating links between childhood health conditions and adult development and health is complex. Frequently, researchers do not have the data necessary to distinguish the health effects of changes in living standards or environmental conditions with respect to childhood illnesses [ 26 ]. A study conducted in Sweden, concluded that reduced early exposure to diseases is related to increases in life expectancy. Additionally, research with data from two surveys of Latin America countries found associations between early life conditions and disabilities later in life. In this sense, the study suggests that older people who were born and raised in times of poor nutrition and a higher risk of exposure to infectious diseases, were more likely to have some disability. In a survey in Puerto Rico, it was observed that the probability of being disabled was greater than 64% for people who grew up in poor conditions than for those who grew up in good conditions. Another survey that considered seven urban centers in Latin America found that the probability of disability was 43% higher for those with disadvantaged backgrounds, than for those with favorable ones [ 26 ].
Recent studies have focused on childhood circumstances to explain later life outcomes [ 12 , 27 , 28 , 29 , 30 , 31 ]. These research findings have shown the importance of considering socioeconomic aspects during childhood, including child poverty from a multidimensional perspective [ 12 ], as a determinant of health status of adults and health disparities. When disadvantaged as children, irreversible effects on health show-up frequently. One clear example is the association of socioeconomic aspects during childhood with type 2 diabetes and obesity in adulthood [ 32 , 33 ].
The future development of children is linked to present socioeconomic levels and social mobility in adulthood [ 27 ]. Some studies [ 28 , 34 , 35 ] indicate that the effects of childhood exposure to lower socioeconomic status or conditions of poverty on health in old age may persist independently of upward social mobility in adulthood. Hence, children who grow up in poverty are more likely to present health problems during adulthood, while those who did not grow up in poverty have a higher probability of remaining healthy.
Another important consideration regards developmental mismatches [ 36 ]. Their article emphasizes how developmental and evolutionary mismatches impact the risk of diseases like diabetes. There could be a disparity between the early life environment and the one encountered in adulthood, turning adaptations that were once beneficial into risk factors for non-communicable diseases. High-calorie diets and sedentary lifestyles could trigger diabetes prevalence.
If these connections between early life and health in old age can be established firmly, it is expected that aging people in low and middle-income countries have another disadvantage regarding elders in developed countries, including a higher risk of developing health problems in old age and frequently multiple NCDs [ 26 ]. Under this context, the effective management of NCDs such as diabetes is crucial, and childhood living standards would be a variable to ponder [ 26 , 37 ]. Work related to the Life Course approach has emphasized the importance of considering socioeconomic aspects during childhood, including poverty [ 12 ] as a determinant of adult health status and its disparities [ 28 , 29 , 30 , 31 ].
Data and methods
Data source.
The Mexican Health and Aging Study (MHAS) is a national longitudinal survey of adults aged 50 years and over in Mexico. The baseline survey has national, urban, and rural representation of adults born in 1951 or earlier. It was conducted in 2001 with follow-up interviews in 2003, 2012, 2015, 2018 and 2021 [ 38 ]. New samples of adults were added in 2012 and 2018 to refresh the panel. The survey includes information on health measures (self-reports of conditions and functional status), background (education and childhood living conditions), family demographics, and economic measures. The MHAS (Mexican Health and Aging Study) is partly sponsored by the National Institutes of Health/National Institute on Aging (grant number NIH R01AG018016) in the United States and the Instituto Nacional de Estadística y Geografía (INEGI) in Mexico. Data files and documentation are public use and available at www.MHASweb.org .
In this research, the analysis was based on data from the survey conducted in 2018 (it was the most recent when the project started, later the 2021 survey became available). The study focused exclusively on participants who were aged 50 or older at the time of the 2018 survey. To minimize response bias, the study included only observations from direct interviewees, excluding proxy respondents, and particularly those who completed the section of the questionnaire pertaining to “Childhood Characteristics before the age of 10 years” Footnote 2 . Furthermore, to expand the sample size, individuals who first joined the survey during the 2012 cycle were identified, utilizing data from both the 2012 and 2018 surveys [ 39 ]. After locating the same individuals in both datasets, responses related to childhood conditions from the 2012 survey were extracted and integrated into the 2018 dataset. Biases in the samples were not found. This approach resulted in a total sample size of 8,082 observations.
In addition, we selected a suite of predictor variables to provide a comprehensive examination of the demographic, socioeconomic, and health-related characteristics within our sample (Table 1 ). The cohort consists of 8,082 participants with males exhibiting a marginally higher mean age (58.3 years) compared to females (56.7 years). In terms of educational achievement, males attained a slightly higher level of schooling, averaging 8.3 years, as opposed to 7.6 years for females.
Regarding the spatial distribution of the study population reveals that 1,717 individuals reside in areas with 2,500 inhabitants or fewer, indicating a rural setting, while the majority, 6,365 individuals, are found in regions with more than 2,500 inhabitants, suggesting an urban setting. Among the subjects, a significant number of males (23%) are located in the former, rural settings, which is higher than their female counterparts (19.7%). The data on living arrangements indicate notable gender differences, with 86% of males cohabiting with partners against 68.8% of females. The state of being single-a term here encompassing a spectrum of prior marital experiences but currently not cohabiting-is observed in 31.2% of females and 14% of males. The socioeconomic dimension is gauged using “proxy variables” such as the absence of poverty in adulthood and presence of childhood poverty, both of which are evenly represented across genders. Health-related self-reporting data reveals that females have a higher incidence of diagnosed diabetes (24.4%) compared to males (20.1%), and a larger percentage of females (26.6%) manage their diabetes with insulin. The propensity for medication use to control diabetes is high among both sexes, though more pronounced in females (91.5%) relative to males (85.3%). Additionally, obesity rates, determined by a Body Mass Index Footnote 3 of 30 or greater, are substantially elevated in females (34.8%) versus males (24.6%). Furthermore, a familial history of diabetes is slightly more prevalent in females, affecting 32.6% with diabetic mothers and 20% with diabetic fathers.
There is a serious concern about self-reporting medical conditions, to what extent this information is reliable. For [ 40 , 41 ] the validity and high accuracy of self-reported diagnosis of diabetes mellitus has been confirmed by previous research, and previous studies using WHO data have also used this question to evaluate diabetes mellitus [ 42 , 43 ].
For the survey employed in this paper, [ 44 ] confirm a correspondence between self-reported and objective measures. Nonetheless, [ 45 ] warn about true prevalence and this kind of reporting. In addition, the implications of relying on diagnosed diabetes, rather than total diabetes prevalence, include the potential under-representation of the condition’s true prevalence due to undiagnosed cases. Since the study’s analysis is based on self-reported data from the Mexican Health and Aging Study, it might not capture those individuals who are unaware of their condition [ 45 ]. The existence of statistical biases could be a potential limitation in the analysis.
Equally or even more troublesome is the problem of recalling conditions during childhood. While some factors (depression among others) can produce limited recalling [ 46 ], specific conditions are well recalled, if not their details and timing [ 47 ].
Regarding the age distribution, the sample is mostly concentrated in three groups: 67.6% for individuals between 50 and 59 years of age, followed by 29.6% for those between 60 and 69 years of age, and 2.5% for those between 70 and 79 years of age. On average, the educational level for women is 7.6 years of schooling while for men it is 8.2 years, which suggests an incomplete level of secondary education for both. On the other hand, from the total number of women in the sample (4,368), 24% of them indicated the presence of diabetes, and 20% of men in the sample (3,714) reported this condition. In addition, around 68% of women with diabetes reported being overweight or obese, for men this percentage was 69%. Meanwhile, 71.4% women with diabetes reported parental history of diabetes, for men this percentage was 68%. The next subsections describe the construction and identification of the key dependent and independent variables.
Dependent variable
The dependent variable is binary, which refers to the individual’s diagnosis of diabetes. This variable was taken from section C of the basic questionnaire of the MHAS 2018. The question is as follows: Has a doctor or medical professional ever told you that you have diabetes? If the answer is “yes” it was assigned a value of 1 and if the answer was “no”, a 0. The absence of answers was left empty, non-imputed. Regarding the individuals who reported being diagnosed with diabetes, 94.2% were taking medication or using insulin injections or pumps, and / or following a special diet to manage diabetes, without statistical differences when interchanging the samples.
Independent variables
For the explanatory variables of the model, sociodemographic, socioeconomic (“proxy” Footnote 4 of poverty in childhood and non-poverty in old age) Footnote 5 , and geographical variables were considered, as well as other variables related the parents of the interviewees. Given the difficulty of constructing a robust variable that reflects respondents’ income, internet access was considered as a proxy variable that would allow to ascertain the poverty status of the individual in old age. Several tests were performed for robustness Footnote 6 .
Internet access in Mexico is more common among relative well-off Mexicans than it was among the poorest sector of the population. Thus, according to [ 49 , 50 ], 7 out of 10 individuals from the highest income segment were internet users, while for the lowest income deciles, this was only 2 out of 10. Furthermore, a low level of schooling was related to internet access opportunities. Therefore, people who only received primary education were 4 times less likely to use the internet in Mexico.
Additionally, for the variable of poverty during childhood, a proxy was considered which corresponds to the answer of the question “Before you were 10 years old, did your home have an indoor toilet?” Footnote 7 , United Nations Children’s Fund (UNICEF) collaborators [ 12 ], pointed out that the severe deprivation of sanitation facilities has critical long-term effects on various aspects of an infant. In this regard, UNICEF highlights the crucial importance of eradicating severe sanitation deprivation as a method to eradicate absolute child poverty, emphasizing that sanitation facilities should be a priority for children.
Statistical analysis
Linear Probability Models (LPM) define the probability:
They assume (require) that: i) \(Pr(Y=1 \mid X)\) is an increasing function in X for \(\beta _{0}>0\) , and ii) \(0 \le Pr(Y=1 \mid X) \le 1 \forall X\) .
This implies a cumulative distribution function that guarantees that for any value of the parameters of X , probabilities are well-defined, with values in the interval [0, 1].
The dependent variable to be explained is binary (diabetes diagnosis is 1 if the person has been diagnosed with diabetes and 0 for the person who has not been diagnosed with diabetes). Hence, a special class of regression models (with limited dependent variable), is considered. There are two probability models with these characteristics frequently used: the Logit model, and the Probit model. In relation to this, [ 48 ] points out that, theoretically, both models are very similar. A potential advantage of Probit models is they could feed other related inquiries. For example, when testing selection via Inverse Mill’s Ratios.
The Probit model is expressed as:
In the Probit model with multiple regressors, \(X_1,X_2,\ldots ,X_k\) , \(\phi (.)\) the cumulative standard normal distribution function is \(\phi (Z)=P(X\le z)\) , \(Z\sim N(0,1)\) .
Therefore, in ( 2 ) \(P(Y=1 \mid X_1,X_2,\ldots ,X_k )\) means the probability that an event occurs given the values of other explanatory variables, where Z is distributed as a standard normal \(Z\sim N(0,1)\) . While a series of tests could be performed in the model, two are critical for this investigation: the linearity between the independent variables and the underlying latent variable, and the normality of errors.
In ( 2 ), the coefficient \(\beta _{1}\) represents the change in z associated to a unit of change in \(X_1\) . It is then observed that, although the effect of z on a change is linear, the link between z and the dependent variable Y is not linear since \(\phi\) is a non-linear function of X . Therefore, the coefficients of X do not have a simple interpretation. In that sense, marginal effects must be calculated. Considering that in the linear regression model, the slope coefficient measures the change in the average value of the returned variable, due to a unit of change in the value of the regressor, maintaining the other variables constant. In these models, the slope coefficient directly measures the change in the probability of an event occurring, as a result of a unit change in the value of the regressor, holding all other variables constant, a discussion can be found at [ 51 ]. The \(\beta\) parameters are frequently estimated by maximum likelihood. The likelihood function is the joint probability distribution of the data treated as a function of the unknown coefficients Footnote 8 .
The maximum likelihood function is the conditional density of \(Y_1,\ldots ,Y_k\) given \(X_1,\ldots ,X_k\) as a function of the unknown parameters \(\beta\) . Thus, the Maximum Likelihood Estimation (MLE) is the value of the parameters \(\beta\) that maximizes the maximum likelihood function. Hence, the MLE is the value of \(\beta\) that best describes the distribution of the data. In this regard and in large samples, the MLE is consistent, normally distributed, and efficient (it has the lowest variance among all the estimators). The \(\beta\) is solved by numerical methods. The resulting \({\hat{\beta }}\) is consistent, normally distributed, and asymptotically efficient.
A Probit model is proposed as follows. The dependent variable is diagnosed diabetes in adulthood correlated to several independent variables: sex, age, marital status, locality size, a dummy variable (to identify observations sourced from the 2012 survey wave, which is focused on childhood-related questions), obesity condition (Body Mass Index \(\ge\) 30), family history of diabetes, childhood poverty, no poverty in adulthood and the interaction of childhood poverty and no poverty in adulthood.
The variables should have analogous probability distributions and behave mutually independent. If errors violate the assumptions, the estimated values would be biased and inconsistent. Therefore, estimated values will also be shown with the Linear Probability Model.
In this type of model, \(y_i\) is a latent dependent variable that takes values of 1 if the person has been diagnosed with diabetes, that is, if individual i has a certain characteristic or quality and 0 otherwise; X is a set of explanatory variables that are assumed to be strictly exogenous, which implies that \(Cov\left[ x_i,\varepsilon _j\right] =0\ \forall\) the i individuals. In addition, the error term \(\varepsilon\) is assumed to be i . i . d . In this way, the probability of an event occurring given a set of explanatory variables is obtained:
In ( 1 ) G is a function that strictly takes values between 0 and 1, \(0<G(z)<1\) , for all real numbers z . As noted at the beginning of this section, in the Probit model, G represents a standardized normal cumulative distribution function given by:
Finally, to know the effects of the changes in the explanatory variables on the probability of the event occurring, a partial derivative can show that:
The term \(g\left( z\right)\) corresponds to a probability density function. Since the Probit model \(G\left( .\right)\) is a strictly positive cumulative distribution function, \(g\left( z\right) >0\ \forall \ z\) , the sign of the partial effect is the same as that of \(\beta _j\) .
This section reviews the factors associated with the probability of being diagnosed with diabetes for men and women and discusses their significance. Table 2 summarizes the main results of the Probit model.
Sociodemographic
Marginal effects on the dependent variable show that the age of individuals is highly significant with a positive correlation. This suggests that age is a factor leading to a higher probability (1%) of obtaining a diagnosis of diabetes, which could imply that as the person ages, the likelihood of developing diabetes increases. This result is consistent with studies conducted on the age-related decline in mitochondrial function, which in turn contributes to insulin resistance in old age. These conditions may foster the development of glucose intolerance and type 2 diabetes [ 53 , 54 ].
In addition, the outcomes indicate that women have an associated probability increase of 4% of suffering from this disease compared to men Footnote 9 . Regarding the differences by marital status, women and men living in a couple have a higher probability of being diagnosed with diabetes. In a study for Mexico using MHAS 2012, [ 45 ] found that being a woman and being married are significantly associated with a higher likelihood of self-reported diabetes Footnote 10 .
On the other hand, the results by size of locality suggest that individuals residing in urban areas have a non-negligible higher probability of suffering from diabetes compared to people living in rural locations. This is in line with the phenomenon of “nutritional transition”, which initially occurred in high-income countries and later in low-income countries, first in urban areas and then in rural areas [ 56 , 57 ]. For Mexico, [ 58 ] despite the prevalence of diabetes presents heterogeneous patterns, this condition is strongly greater in urban areas compared with rural areas.
Health and lifestyle
The results suggest a significant positive effect on the probability of diagnosis of diabetes for the individuals in the sample when the father and/or mother have this condition. In the case of a mother with diabetes, the associated probability of diabetes is 13%, while for a father with diabetes, it is 12%. Additionally, obesity is an important risk factor in the diagnosis of diabetes, the linked marginal effect of this comorbidity in the diagnosis of diabetes is 4%. In this regard, no significant differences were found by sex or locality size Footnote 11 .
Socioeconomic
The findings indicate a lower probability that individuals are diagnosed with diabetes if during adulthood they are not poor (-5%). On the other hand, from the interaction of the variables poverty in childhood and non-poverty in old age, a considerable positive effect is observed. This suggests that when the individual was poor in childhood, despite no longer poor in adulthood, the probability associated with the diagnosis of diabetes is positive and significant. Thus, it is possible that conditions of poverty in childhood influence the development of this disease later in life Footnote 12 . While this is a correlation, the fact that an interaction of socioeconomic characteristics has bigger linear effect than a key biological characteristic (obesity) is non trivial, and reinforces the importance of life course analysis.
Social mobility, defined as the change in an individual’s socioeconomic status relative to their parents or over their lifetime, is a crucial metric for assessing equal opportunity-a measure of whether people have the same chances to achieve success regardless of their initial socioeconomic position. Our study aligns with the broader evidence [ 65 , 66 ], suggesting that those from disadvantaged backgrounds often face significant barriers to socioeconomic advancement Footnote 13 .
A compelling finding of this paper, refers how poverty conditions during childhood remain an important risk factor associated with the greater probability of being diagnosed with diabetes during adulthood in Mexico. Despite these circumstances do not determine the diagnosis of diabetes in older adults, they have a strong correlation with the ailment. On the other hand, even when individuals have not experienced poverty during childhood, but it occurs during adulthood, the probability associated with the diagnosis of diabetes increases. Not surprisingly, the probability of being diagnosed with diabetes scales when the person was poor in both stages. These effects are persistent for men and women, although for women the associated probability was higher than for men. Likewise, there is a positive and high correlation of the parents’ history of diabetes and the obesity condition on the probability of developing this disease. Biological aspects could be present, but also modifiable factors, with the generational transmission of elements related to lifestyle (eating habits and physical activity). Similarly, people who live with a partner have a higher associated probability of being diagnosed with diabetes. The literature suggests that this is due to the tendency of individuals to select spouses based on the preference for similar phenotype characteristics and the convergence of their behaviors and lifestyle. Moreover, these issues have been exacerbated by urbanization processes and by the “food transition” Footnote 14 that has made processed and ultra-processed products more and more accessible. Such products are characterized by being high in fat, salt, and sugar. Regarding the effect of the size of the locality on the probability of being diagnosed with diabetes, the results show differences for people residing in rural and urban areas. In urban localities, the associated probability is higher compared to rural ones. Likewise, aging is an important factor that affects the probability of suffering from diabetes: as the individual ages, the probability of developing this disease increases.
In terms of the analysis and empirical strategy used, the findings show valuable relationships. Aligned with efforts to improve the accuracy and reliability of health data by combining biomarkers and objective measurements with self-reported data [ 70 ], biomarkers in the survey were employed. These biomarkers were used for diabetes (the dependent variable) and obesity condition (as one of the independent variables) in the model of Results section. The results are consistent with the previous findings (See Appendix ).
There is ample space for additional work and get over the limitations of this work. For example, being MHAS a longitudinal survey, an econometric model can be developed in order to explore (test) causal relationships among the extensive set of variables. Also self-reporting could present different types of biases. While the use of biomarkers was an important robustness test, calculating bounds and checking selection biases would be valuable. Moreover, the survey also captures information related to social protection variables and social programs transfers, which could be useful for testing policies.
Given the interconnection of childhood conditions and the importance of these in the development of adult capacities and their success in their future life, they should be considered within the design and formulation of public policies and programs. The policies should focus and prioritize objectives of reducing the inequality gaps and pre-existing poverty in the country. Adopting measures to reduce inequalities in the social sphere is essential to protect future generations. In this sense, it is important to act on the Social Determinants of Health throughout the course of life in a broader social and economic context. Acting on the SDH would improve prospects for health and generate considerable social benefits that would allow people to achieve their capabilities and reduce the intergenerational perpetuation of inequalities. Thus, the SDH together with the Life Course approach, provide a sensible framework to identify risk clusters that can be broken in periods of effective interventions (e.g. childhood), as well as to improve the design of public policies on population aging and health, from a perspective focused on the well-being and quality of life of the Mexican population.
In this way, and to face the demographic transition and the diabetes epidemic in Mexico, comprehensive public policies that consider interventions from childhood will be required to reduce inequality and poverty. For some years now, the WHO has emphasized the importance and role of the inclusion of long-term care policies and programs focused on older adults. The forecasts in case of untimely acting indicate a significant negative effect on the social, economic and health structures for the coming years.
Finally, despite the increase of older population, much of the research on the effects of socioeconomic conditions on health is concentrated in economically active populations, and those ignore older people, and pay restricted attention to long term factors such as childhood conditions. The results presented in this document contribute to studies on population aging and public health. Evidence is found with respect to health determinants in a demographic group that is growing rapidly and not sufficiently considered.
Availability of data and materials
Data files and documentation are public use and available at www.MHASweb.org . Data and code used during the current study are available from the corresponding author on reasonable request.
Social Determinants of Health. Retrieved from https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 . Accessed on January 22, 2024.
Given the survey design, people responding the childhood questionnaire are new participants.
A Body Mass Index (BMI) was constructed considering the variables of height and weight reported in the MHAS 2018 survey (C6: “What is your current weight in kilograms?”, C67: “What is your height without shoes in meters?”). For adults, the World Health Organization (WHO) defines overweight as a BMI of 25 or higher, and obesity as a BMI of 30 or higher. BMI was calculated by dividing a person’s weight in kilograms by the square of their height in meters (kilograms/m 2 ).This information is available at: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight , accessed on January 10, 2024.
In this context, the term “proxy”, was employed to describe variables that serve as stand-ins for factors that are not directly observable within our dataset. As noted by [ 48 ].
Numerous variables that could reflect household income were tested, but since they were self-reported and not part of the survey’s core, there is a large number of missing values.
We thank one referee for her suggestions regarding education years.
This question is found in section J.18 of the basic questionnaire and corresponds to the question “Does this home have ... internet?” If the person answers “yes”, that means that they have internet service and were assigned a value of 1, and 0 if the person does not have this service.
There is an interesting possibility of comparing the linear marginal effects with direct estimations from a Logit model (risk differences), [ 52 ]. We thank a referee for pointing this out.
This is consistent with what was stated in Aging in Mexico: The Most Vulnerable Adults of the MHAS Newsletter: May 20-2, 2020, which indicates that women are more likely to report diabetes than men. Retrieved from http://www.enasem.org/images/ENASEM-20-2-Aging_In_Mexico_AdutosMasVulnerables_2020.pdf . Accessed on February 10, 2024.
Furthermore, Danish researchers found a connection between the Body Mass Index of one spouse and the other spouse’s risk of developing type 2 diabetes. According to this study, spouses tend to be similar in terms of body weight, as people often tend to marry someone similar to themselves and share dietary and exercise habits when living together [ 55 ].
It has long been known that type 2 diabetes is, in part, hereditary. Family studies have revealed that first-degree relatives of people with type 2 diabetes are approximately 3 times more likely to develop the disease than people without a positive family history of the disease [ 59 , 60 , 61 ]. Likewise, in a study for Mexico, [ 62 ] point out that obesity and a history of type 2 diabetes in parents and genes play an important role in the development of type 2 diabetes. Furthermore, [ 63 ], points out that the frequency of diabetes mellitus also varies between different races and ethnicities.
This is consistent with the research by [ 64 ] who find that the conditions in which the person lived at the age of 10 affect health in old age.
According to [ 67 ] in a regional analysis on the degree of social mobility in Mexico, it indicates that social mobility is higher than the national average in the North and Central North regions, similar to the national average in the Central region, and lower than the average in the South region. In particular, it notes that children of poor parents made above-average progress if they grew up in the northern region, and less than average progress if they grew up in the southern region.
The country’s food environment has been transformed; it is becoming easier to access unhealthy products. In this sense, for the last 40 years, important changes have been observed in the Mexican diet, mainly from fresh and unprocessed foods to processed and ultra-processed products with a high content of sugar, salt, and fat. Marrón-Ponce et al. [ 68 ], point out that in 2016 around 23.1% of the energy in the Mexican population’s diet came from ultra-processed products, even though the WHO recommendations suggest that at most, this percentage should present between 5 and 10% of total energy per day. In addition, Mexico is the worldwide largest consumer of sugary beverages; its consumption represents approximately 10% of the total daily energy intake in adults and children and constitutes 70% of the total added sugar in the diet [ 69 ].
The study incorporates biomarkers to evaluate health conditions related to diabetes and obesity. Glycosylated hemoglobin results are employed as an indicator of diabetes [ 71 ], with a value equal to or exceeding 6.5% signifying a positive diagnosis (coded as “1”), while values below this threshold are coded as “0”, indicating the absence of the condition. Concurrently, Body Mass Index (BMI) is calculated from weight and height measurements to determine obesity, with a BMI of 30 or more classified as obese. These biomarkers provide quantifiable and reliable means of assessing the presence of these two critical health issues within the study’s population.
Abbreviations
Mexican Health and Aging Study
non-communicable diseases
Social Determinants of Health
World Health Organization
Organisation for Economic Cooperation and Development
National Institute of Statistics and Geography
United Nations Children’s Fund
Low and middle-income countries
Linear Probability Models
Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–724.
Article Google Scholar
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017-Highlights. New York: United Nations; 2017.
Consejo Nacional de Población (CONAPO). Conciliación demográfica de México 1950-2019 y Proyecciones de la Población de México y las Entidades Federativas 2020-2070; n.d. Retrieved from Consejo Nacional de Población (CONAPO). 2023. https://conapo.segob.gob.mx/work/models/CONAPO/pry23/Mapa_Ind_Dem23/index_2.html .
Organisation for Economic cooperation and development. Health at a Glance 2019: OECD Indicators. Paris: OECD; 2019.
International Diabetes Federation. IDF Diabetes Atlas. Brussels: International Diabetes Federation; 2019.
Roy K, Chaudhuri A. Influence of socioeconomic status, wealth and financial empowerment on gender differences in health and healthcare utilization in later life: evidence from India. Soc Sci Med. 2008;66:1951–62.
Article PubMed Google Scholar
Mete C. Predictors of elderly mortality: health status, socioeconomic characteristics and social determinants of health. Health Econ. 2005;14:135–48.
Osler M. The life course perspective: A challenge for public health research and prevention. Eur J Public Health. 2006;16(3):230. https://doi.org/10.1093/eurpub/ckl030 .
Marmot M. Social determinants of health inequalities. Lancet (London, England). 2005;365(9464):1099–104. https://doi.org/10.1016/S0140-6736(05)71146-6 .
Wise PH. Child poverty and the promise of human capacity: childhood as a foundation for healthy aging. Acad Pediatr. 2016;16:S37–45.
Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P, et al. WHO European review of social determinants of health and the health divide. Lancet. 2012;380(9846):1011–29.
Gordon D, Nandy S, Pantazis C, Pemberton S, Townsend P. The distribution of child poverty in the developing world. Bristol: Centre for International Poverty Research; 2003.
Google Scholar
Jacob CM, Baird J, Barker M, Cooper C, Hanson M. The importance of a life-course approach to health: chronic disease risk from preconception through adolescence and adulthood: white paper. Geneva: World Health Organization; 2017.
Graham H. Building an inter-disciplinary science of health inequalities: the example of lifecourse research. Soc Sci Med. 2002;55:2005–16.
Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME. Mortality in adults aged 26–54 years related to socioeconomic conditions in childhood and adulthood: post war birth cohort study. BMJ. 2002;325:1076–80.
Article PubMed PubMed Central Google Scholar
United Nations International Children’s Emergency Fund (UNICEF) and International Labour Organization (ILO). Towards universal social protection children: Achieving SDG 1.3. Geneva; UNICEF-ILO; 2019.
UNICEF. Programme guidance for early life prevention of non-communicable diseases. New York: United Nations Children’s Fund; 2019. https://www.unicef.org/media/61431/file .
Commission on Social Determinants of Health, et al. Closing the gap in a generation: health equity through action on the social determinants of health: final report of the commission on social determinants of health. Geneva: World Health Organization; 2008.
Cusick SE, Georgieff MK. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days’’. J Pediatr. 2016;175:16–21. https://doi.org/10.1016/j.jpeds.2016.05.013 .
World Health Organization. World report on ageing and health. Geneva: World Health Organization; 2015.
Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Commun Health. 2003;57:778–83.
Article CAS Google Scholar
Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–9.
Maindal HT, Aagaard-Hansen J. Health literacy meets the life-course perspective: towards a conceptual framework. Global Health Action. 2020;13:1775063.
INEGI. Diseño conceptual. Encuesta Nacional sobre Salud y Envejecimiento en México (ENASEM) 2018. 2018. https://www.inegi.org.mx/contenidos/programas/enasem/2018/doc/enasem_2018_diseno_conceptual.pdf . Accessed 30 Oct 2023.
Pan American Health Organization. Building Health Throughout the Life Course. Concepts, Implications, and Application in Public Health. Washington, D.C.: Pan American Health Organization; 2020.
World Health Organization. Global Health and Aging. Geneva: National Institute on Aging and World Health Organization; 2011.
Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–47.
Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60:593–S101.
Haas SA. The long-term effects of poor childhood health: An assessment and application of retrospective reports. Demography. 2007;44:113–35.
Haas SA. Trajectories of functional health: The “long arm’’ of childhood health and socioeconomic factors. Soc Sci Med. 2008;66:849–61.
Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. J Gerontol Ser B Psychol Sci Soc Sci. 2012;67:238–48.
Tamayo T, Herder C, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010;10:1–15.
Kohler IV, Soldo BJ. Childhood predictors of late-life diabetes: the case of Mexico. Soc Biol. 2005;52:112–31.
Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–5.
Fass S, Dinan KA, Aratani Y . Child poverty and intergenerational mobility. New York: Mailman School of Public Health, Columbia University; 2009.
Gluckman PD, Hanson MA, Low FM. Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk. Philos Trans R Soc B Biol Sci. 2019;374(1770):20180109. https://doi.org/10.1098/rstb.2018.0109 .
World Health Organization. Global report on diabetes. Geneva: World Health Organization; 2016.
Wong R, Michaels-Obregon A, Palloni A. Cohort Profile: The Mexican Health and Aging Study (MHAS). Int J Epidemiol. 2017;46(2):e2. https://doi.org/10.1093/ije/dyu263 .
MHAS Mexican Health and Aging Study 2012 and 2018. Retrieved from www.MHASweb.org on [20 Feb, 2024]. Data Files and Documentation (public use): Mexican Health and Aging Study, [Core survey Data and Documentation].
Pastorino S, Richards M, Hardy R, Abington J, Wills A, Kuh D, et al. Validation of self-reported diagnosis of diabetes in the 1946 British birth cohort. Prim Care Diabetes. 2015;9(5):397–400. https://doi.org/10.1016/j.pcd.2014.05.003 .
Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2012;176(8):738–43. https://doi.org/10.1093/aje/kws156 .
Koyanagi A, Smith L, Shin JI, Oh H, Kostev K, Jacob L, et al. Multimorbidity and Subjective Cognitive Complaints: Findings from 48 Low- and Middle-Income Countries of the World Health Survey 2002–2004. J Alzheimers Dis. 2021;81(4):1737–47. https://doi.org/10.3233/JAD-201592 .
Ma R, Romano E, Vancampfort D, Firth J, Stubbs B, Koyanagi A. Physical Multimorbidity and Social Participation in Adult Aged 65 Years and Older From Six Low- and Middle-Income Countries. J Gerontol Ser B Psychol Sci Soc Sci. 2021;76(7):1452–62. https://doi.org/10.1093/geronb/gbab056 .
Palloni A, Beltrán-Sánchez H, Novak B, Pinto G, Wong R. Adult obesity, disease and longevity in Mexico. Salud Publica Mex. 2015;57(Suppl 1):S22–S30. https://doi.org/10.21149/spm.v57s1.7586 .
Kumar A, Wong R, Ottenbacher KJ, Al Snih S. Prediabetes, undiagnosed diabetes, and diabetes among Mexican adults: findings from the Mexican Health and Aging Study. Ann Epidemiol. 2016;26(3):163–70. https://doi.org/10.1016/j.annepidem.2015.12.006 .
Goltermann J, Meinert S, Hülsmann C, Dohm K, Grotegerd D, Redlich R, et al. Temporal stability and state-dependence of retrospective self-reports of childhood maltreatment in healthy and depressed adults. Psychol Assess. 2023;35(1):12–22. https://doi.org/10.1037/pas0001175 .
Tustin K, Hayne H. Defining the boundary: Age-related changes in childhood amnesia. Dev Psychol. 2010;46(5):1049–61. https://doi.org/10.1037/a0020105 .
Greene WH. Econometric analysis. New Jersey: Prentice Hall; 1993.
Mecinas-Montiel JM. The digital divide in Mexico: A mirror of poverty. Mex Law Rev. 2016;9:93–102.
García-Mora F, Mora-Rivera J. Exploring the impacts of Internet access on poverty: A regional analysis of rural Mexico. New Media Soc. 2023;25(1):26–49. https://doi.org/10.1177/14614448211000650 .
Adams CP. Learning Microeconometrics with R. 1st ed. New York: Chapman and Hall/CRC; 2020. https://doi.org/10.1201/9780429288333 .
Norton EC, Dowd BE, Maciejewski ML. Marginal Effects-Quantifying the Effect of Changes in Risk Factors in Logistic Regression Models. JAMA. 2019;321(13):1304–5. https://doi.org/10.1001/jama.2019.1954 .
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2.
Article CAS PubMed PubMed Central Google Scholar
Kmemmare Z. Sarcopenia and diabetes: pathogenesis and consequences. Br J Diabetes Vasc Dis. 2011;11:230–4.
University of Copenhagen The Faculty of Health and Medical Sciences. Married couples share risk of developing diabetes. 2018. www.sciencedaily.com/releases/2018/05/180522123324.htm . Accessed 27 Apr 2024.
Popkin BM. Global changes in diet and activity patterns as drivers of the nutrition transition. In: Kalhan SC, Prentice AM, Yajnik CS, editors. Emerging societies-coexistence of childhood malnutrition and obesity, vol. 63. Vevey: Karger Publishers; 2009. p. 1–14.
Chapter Google Scholar
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21.
Soto-Estrada G, Moreno Altamirano L, García-García JJ, Ochoa Moreno I, Silberman M. Trends in frequency of type 2 diabetes in Mexico and its relationship to dietary patterns and contextual factors. Gac Sanit. 2018;32:283–90.
Flores JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–91.
Hansen L. Candidate genes and late-onset type 2 diabetes mellitus. Susceptibility genes or common polymorphisms? Dan Med Bull. 2003;50:320–46.
CAS PubMed Google Scholar
Gloyn AL. The search for type 2 diabetes genes. Ageing Res Rev. 2003;2:111–27.
Article CAS PubMed Google Scholar
Berumen J, Orozco L, Betancourt-Cravioto M, Gallardo H, Zulueta M, Mendizabal L, et al. Influence of obesity, parental history of diabetes, and genes in type 2 diabetes: A case-control study. Sci Rep. 2019;9:1–15.
World Health Organization. Classification of diabetes mellitus. 2019. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus . Accessed 27 Apr 2024.
Grimard F, Laszlo S, Lim W. Health, aging and childhood socio-economic conditions in Mexico. J Health Econ. 2010;29:630–40.
OECD. Understanding social mobility; n.d. https://www.oecd.org/stories/social-mobility/ . Accessed 15 Mar 2024.
Clarke C, et al. The economic costs of childhood socio-economic disadvantage in European OECD countries. OECD Papers on Well-being and Inequalities. 2022;(9). https://doi.org/10.1787/8c0c66b9-en .
Delajara M, Graña D. Intergenerational social mobility in Mexico and its regions results from rank-rank regressions. Sobre Mex Temas Econ. 2018;1:22–37.
Marrón-Ponce JA, Tolentino-Mayo L, Hernández-F M, Batis C. Trends in ultra-processed food purchases from 1984 to 2016 in Mexican households. Nutrients. 2018;11:45.
Sánchez-Pimienta TG, Batis C, Lutter CK, Rivera JA. Sugar-sweetened beverages are the main sources of added sugar intake in the Mexican population. J Nutr. 2016;146:1888S–1896S.
Wong R, Michaels-Obregón A, Palloni A, Gutiérrez-Robledo LM, González-González C, López-Ortega M, et al. Progression of aging in Mexico: The Mexican Health and Aging Study (MHAS) 2012. Salud Publica Mex. 2015;57:s79–89.
Eyth E, Naik R. Hemoglobin A1C. StatPearls [Internet]; 2023. Last Update: March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK549816/ . Accessed 27 Apr 2024.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Tecnologico de Monterrey, School of Government and Public Transformation, EGyTP, Mexico City, Mexico
Marina Gonzalez-Samano & Hector J. Villarreal
You can also search for this author in PubMed Google Scholar
Contributions
MGS (Marina Gonzalez-Samano) contributed to the design of the study and the final document with guidance and conceptual insights from HJV (Hector J. Villarreal). MGS and HJV carried out the search, analysed the documents and wrote the first draft of the article. All authors were involved in the conception of the research, revisions and editing of the article. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Marina Gonzalez-Samano .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
For robustness testing a model specification was employed where self-reported diabetes and obesity measures are substituted with biomarkers obtained from the MHAS 2012. Table 3 summarizes the main results of the Probit model.
The analytical results from Table 2 (Model 1), and those derived from the utilization of biomarkers in Table 3 (Model 2) exhibit a considerable likeness, especially in the context of diabetes and obesity indicators. Notably, there is a significant reduction in the sample size when biomarkers Footnote 15 are introduced, which might account for the increased standard errors observed in Table 3. Consequently, certain variables such as: being “woman”, “living with a couple” and “residing in an urban locality”, have lost statistical significance in the biomarker analysis. Despite these differences, the general conclusions derived from this specification remain consistent with those presented in Model 1 (Table 2 ). Moreover, the linear effect of the interaction effect of poverty in childhood with no poverty in adulthood is bigger with the biomarker specification. Nonetheless, the larger confidence intervals need to be considered.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gonzalez-Samano, M., Villarreal, H. Diabetes, life course and childhood socioeconomic conditions: an empirical assessment for Mexico . BMC Public Health 24 , 1274 (2024). https://doi.org/10.1186/s12889-024-18767-5
Download citation
Received : 19 August 2023
Accepted : 03 May 2024
Published : 09 May 2024
DOI : https://doi.org/10.1186/s12889-024-18767-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiological transition
- Life course
- Childhood conditions
- Social determinants of health
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Additionally, this literature review did not focus on A1C as the primary outcome, although A1C is an important indicator of diabetes self-management. A1C was chosen as the method of evaluating the impact of health literacy interventions in patients with diabetes, but other considerations such as medication adherence, impact on comorbid ...
Abstract. Objective: The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges ...
In this paper, we present a systematic review of the literature on the association of these risk factors with the incidence/prevalence of type 2 diabetes. We give insights on the contribution of independent risk factors in the development of type 2 diabetes along with possible solutions towards a preventive approach.
In a systematic review, diabetes mellitus distress was ... Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence ...
Introduction. Diabetes mellitus (DM), as a growing epidemic of bipolar disorder, affects near 5.6% of the world's population ().Its global prevalence was about 8% in 2011 and is predicted to rise to 10% by 2030 ().Likewise, its prevalence in China also increased rapidly from 0.67% in 1980 to 10.4% in 2013 ().Therefore, DM is a contributing factor to morbidity and mortality.
Introduction. Diabetes mellitus (DM) is probably one of the oldest diseases known to man. It was first reported in Egyptian manuscript about 3000 years ago. 1 In 1936, the distinction between type 1 and type 2 DM was clearly made. 2 Type 2 DM was first described as a component of metabolic syndrome in 1988. 3 Type 2 DM (formerly known as non-insulin dependent DM) is the most common form of DM ...
The present review indicates that the rate of published qualitative research on lived experience of diabetes has increased dramatically over the last 25 years. We developed an innovative Next-Generation mixed-method approach to qualitative secondary analysis and used it to review this literature, derived from a systematic search of PubMed.
There is a growing awareness that type 1 diabetes mellitus (T1DM) is a heterogeneous disease that can be characterized into distinct endotypes. This Review discusses the evidence for endotypes in ...
This is a literature review aiming to overview, summarise and discuss the role and effect of patient empowerment, self-management education and lifestyle modification in the management of people with DM. ... Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: A systematic review with ...
The global burden of type 2 diabetes mellitus (T2DM) is rapidly increasing, affecting individuals of all ages. The global T2DM prevalence nearly doubled in the adult population over the past decade from 4.7% in 1980 to 8.5% in 2014 [].The global burden of T2DM in people 20-79 years is further projected to increase to 629 million in 2045 compared to 425 million in 2017 [].
Misra, Wagner et al. systematically review if strategies to subclassify type 2 diabetes (T2D) are associated with high quality evidence and patient outcomes. Cluster-based stratification yields ...
Diabetes mellitus is a group of physiological dysfunctions characterized by hyperglycemia resulting directly from insulin resistance, inadequate insulin secretion, or excessive glucagon secretion. ... The purpose of this article is to review the basic science of type 2 diabetes and its complications, and to discuss the most recent treatment ...
Introduction Diabetes has been associated with an increased risk of complications in patients with COVID-19. Most studies do not differentiate between patients with type 1 and type 2 diabetes, which correspond to two pathophysiological distinct diseases that could represent different degrees of clinical compromise. Objective To identify if there are differences in the clinical outcomes of ...
Abstract. Diabetes mellitus arises as a result of insulin resistance or a decrease in its production. This work consists of analyzing the various immunological and pathophysiological factors of ...
The American Diabetes Association (ADA) "Standards of Care in Diabetes" includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, an interprofessional ...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Risk Factors and Prevention. Diabetes mellitus (DM) is an important risk factor for cardiovascular disease (CVD), which in turn represents the principal cause of disability and mortality in individuals with DM. The pathophysiological basis of this link is atherosclerosis, which accounts for the majority of CVD and it is accelerated by DM.
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both microvascular complications ...
This systematic literature review aims to identify diabetes self-management education (DSME) features to improve diabetes education for Black African/Caribbean and Hispanic/Latin American women with Type 2 diabetes mellitus. ... Given the results from our systematic literature review, we propose that the balance between tailoring care and ...
Background: Dietary patterns play a critical role in diabetes management, while the best dietary pattern for Type 2 diabetes (T2DM) patients is still unclear. The aim of this network meta-analysis was to compare the impacts of various dietary approaches on the glycemic control of T2DM patients. Methods: Relevant studies were retrieved from PubMed, Embase, Web of Knowledge, Cochrane Central ...
Prediabetes & Diabetes Self-Management Literature Review 108 Cherry Street, Burlington, VT 05401 802-863-7200 www.healthvermont.gov Search Terms The terms in the table below contain the search terms used to identify records for consideration in PubMed. These terms were used as OR statements in their respective searches with medical
ObjectiveThis meta-analysis includes the systematic literature review and meta-analysis involving clinical trials to assess the efficacy and safety of mesenc... Skip to main content. ... Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Trans Med. (2012) 1:59-63. doi: 10.5966/sctm.2011-0017.
Diabetes is a constant, chronic condition with good days and bad days. 'The reality of diabetes is: it's 24 hours a day, every day'. P12 (woman and carer of husband with T1D) 'It is never going to be perfect. Diabetes is like a constant rollercoaster(…)Really, it's a marathon not a sprint'. P20 (woman, mother of a teen with T1D)
This review aims to summarize the results of current research and meta-analyses on risk of acute pancreatitis (AP) during incretin-affecting drugs treatment. A narrative review was performed using present literature in an attempt to identify the relationship between AP and incretin-affecting drugs.
Demographic and epidemiological dynamics characterized by lower fertility rates and longer life expectancy, as well as higher prevalence of non-communicable diseases such as diabetes, represent important challenges for policy makers around the World. We investigate the risk factors that influence the diagnosis of diabetes in the Mexican population aged 50 years and over, including childhood ...
This narrative literature review aims to highlight the literature available while examining the underlying physiology of exercise in diabetes, the benefits and risks of physical exercise, the strategies for minimizing complications, the protocols suggested, and the potential limitations. ... HbA1c in adults with type 2 diabetes mellitus and the ...
Read the latest articles of Endocrinología, Diabetes y Nutrición (English ed.) at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature