- Search Menu
- Advance Articles
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Advertising & Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Paediatrics & Child Health
- About The Canadian Paediatric Society
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Case 1 diagnosis: allergy bullying, clinical pearls.
- < Previous
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation
- Article contents
- Figures & tables
- Supplementary Data
Lopamudra Das, Michelle GK Ward, Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation, Paediatrics & Child Health , Volume 19, Issue 2, February 2014, Pages 69–70, https://doi.org/10.1093/pch/19.2.69
- Permissions Icon Permissions
A 12-year-old girl with a history of asthma presented to the emergency department with a three-day history of increased work of breathing, cough and wheezing. She reported no clear trigger for her respiratory symptoms, although she had noted some symptoms of a mild upper respiratory tract infection. With this episode, the patient had been using a short-acting bronchodilator more frequently than she had in the past, without the expected resolution of symptoms.
On the day of presentation, the patient awoke feeling ‘suffocated’ and her mother noted her lips to be blue. In the emergency department, her oxygen saturation was 85% and her respiratory rate was 40 breaths/min. She had significantly increased work of breathing and poor air entry bilaterally to both lung bases, with wheezing in the upper lung zones. She was treated with salbutamol/ipratropium and received intravenous steroids and magnesium sulfate. Her chest x-ray showed hyperinflation and no focal findings.
Her medical history revealed that she was followed by a respirologist for her asthma, had good medication adherence and had not experienced a significant exacerbation for six months. She also had a history of wheezing, dyspnea and pruritis with exposure to peanuts, chickpeas and lentils; she had been prescribed an injectible epinephrine device for this. However, her device had expired at the time of presentation. In the past, her wheezing episodes had been seasonal and related to exposure to grass and pollens; this presentation occurred during the winter. Further history revealed the probable cause of her presentation.
Although reluctant to disclose the information, our patient later revealed that she had been experiencing significant bullying at school, which was primarily related to her food allergies. Three days before her admission, classmates had smeared peanut butter on one of her schoolbooks. She developed pruritis immediately after opening the book and she started wheezing and coughing later that day. This event followed several months of being taunted with peanut products at school. The patient was experiencing low mood and reported new symptoms of anxiety related to school. The review of systems was otherwise negative, with no substance use.
The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. The trigger for her asthma exacerbation was likely multifactorial, related to exposure to the food allergen as well as the upper respiratory infection. A psychologist was consulted to assess the symptoms of anxiety and depression that had occurred as a result of the bullying. During the hospitalization, the medical team contacted the patient's school to provide education on allergy bullying, treatment of severe allergic reactions and its potential for life-threatening reactions with exposure to allergens. The medical team also recommended community resources for further education of students and staff about allergy bullying and its prevention.
Allergy bullying is a form of bullying with potentially severe medical outcomes. In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study [ 1 ]), this bullying is related directly to the food allergy. From a medical perspective, there are little published data regarding allergy bullying, and many health care providers may not be aware of the issue.
Allergy bullying can include teasing a child about their allergy, throwing food at a child, or even forcing them to touch or eat allergenic foods. Most episodes of allergy bullying occur at school, and can include episodes perpetrated by teachers and/or staff ( 2 ).
Allergy bullying can lead to allergic reactions, which may be mild or severe (eg, urticaria, wheezing, anaphylaxis), but may also lead to negative emotional consequences (sadness, depression) ( 2 ) and an overall decrease in quality of life measures ( 1 ). Adolescents commonly resist using medical devices, such as injectible epinephrine devices, and bullying may be a contributing factor for this ( 3 ). Attempting to conceal symptoms in a bullying situation may place children at risk for a worse outcome.
Physicians can play a key role in detecting allergy bullying and its health consequences. In many cases, children have not discussed this issue with their parents ( 1 ). Given the prevalence of bullying, its potential to lead to severe harm, including death, and the lack of awareness of this issue, clinicians should specifically ask about bullying in all children and teens with allergies. Physicians can also work with families and schools to support these children, educate their peers and school staff, and help prevent negative health outcomes from allergy bullying.
Online resources
www.anaphylaxis.ca − A national charity that aims to inform, support, educate and advocate for the needs of individuals and families living with anaphylaxis, and to support and participate in research. This website includes education modules for schools and links to local support groups throughout Canada.
www.whyriskit.ca/pages/en/live/bullying.php − A website for teenagers with food allergies; includes a segment that addresses food bullying.
www.foodallergy.org − Contains numerous resources for children and their families, including a significant discussion on bullying and ways to prevent it.
Allergy bullying is common but is often unrecognized as a factor in clinical presentations of allergic reactions.
Physicians should make a point of asking about bullying in patients with allergies and become familiar with resources for dealing with allergy bullying.
Physicians can play roles as advocates, educators and collaborators with the school system to help make the school environment safer for children with allergies who may be at risk for allergy bullying.
Google Scholar
Email alerts
Citing articles via, looking for your next opportunity.
- About Paediatrics & Child Health
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1918-1485
- Print ISSN 1205-7088
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Presentation
A 12 year old girl, Annie, enters the Pediatrician’s office with complaints of dyspnea, wheezing, and chest tightness.
The history of the illness from the family includes:
A recent upper respiratory infection with cough, congestion and runny nose with a low grade fever of 100.8 degrees Fahrenheit.
Mother also states this has happened the last few times Annie had cold symptoms and does seem to get relief from Albuterol that was prescribed previously. Annie is behind on her well checks and usually only comes in for sick visits.
Family/Social History
Family history revealed that mother has asthma and because of this uses a lot of hand sanitizer for herself and family to keep them from “catching colds”.
In the home lives Annie, her mother, father and little brother age 6. Annie’s father is a smoker but mother states he smokes outside.
The family is living in a home currently under renovations.
Upon assessment Annie has the following:

(Parakh, 2019)
- intermittent coughing
- expiratory wheezing
- subcostal retractions
- temperature of 100.8 PO
- heart rate of 100 bpm
- respiratory rate of 40
- pulse oximetry 92 %
- purulent mucous from bilateral nares
- catching her breath often while speaking
- Case report
- Open access
- Published: 21 February 2018
Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
- Virginia Mirra 1 ,
- Silvia Montella 1 &
- Francesca Santamaria 1
BMC Pediatrics volume 18 , Article number: 73 ( 2018 ) Cite this article
11k Accesses
11 Citations
12 Altmetric
Metrics details
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Peer Review reports
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 , 3 , 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 , 12 , 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 , 20 , 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 , 23 , 24 , 25 , 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].
A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 , 33 , 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.
Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 , 61 , 62 , 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 , 66 , 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 , 82 , 83 , 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 , 86 , 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 , 90 , 91 , 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Abbreviations
Anti-immunoglobulin E
Body mass index
IgE receptor
Forced expiratory flow between 25% and 75%
Forced expiratory volume in the first second
Gastroesophageal reflux
Inhaled corticosteroids
Intensive care unit
Interleukin
Long-acting β 2 -agonist
Oral leukotriene receptor antagonist
Quality of life
Randomized controlled trials
Short-acting β 2 -agonists
Sleep-disordered breathing
O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–3.
Article PubMed Google Scholar
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–8.
Article Google Scholar
Hedlin G. Management of severe asthma in childhood-state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25:111–21.
Konradsen JR, Nordlund B, Lidegran M, Pedroletti C, Grönlund H, van Hage M, et al. Problematic severe asthma: a proposed approach to identifying children who are severely resistant to therapy. Pediatr Allergy Immunol. 2011;22:9–18.
Global Initiative for Asthma Report. Global strategy for asthma management and prevention (updated 2016). https://www.ginasthma.org . Accessed 07 June 2017.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–53.
Article CAS PubMed Google Scholar
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–507.
Lødrup Carlsen KC, Hedlin G, Bush A, Wennergren G, de Benedictis FM, De Jongste JC, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37:432–40.
Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–5.
Article CAS PubMed PubMed Central Google Scholar
Lang A, Mowinckel P, Sachs-Olsen C, Riiser A, Lunde J, Carlsen KH, et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr Allergy Immunol. 2010;21:945–53.
Nordlund B, Konradsen JR, Pedroletti C, Kull I, Hedlin G. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr. 2011;100:1454–60.
Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tinkelman D. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes. 2010;8:6.
Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46.
British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, 2014. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline . Accessed 13 Apr 2016.
Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, et al. Severe asthma features in children: a case-control online survey. Ital J Pediatr. 2016;42:9.
Article PubMed PubMed Central Google Scholar
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34.
Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36:196–201.
Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's severe asthma research program. Pediatr Allergy Immunol Pulmonol. 2010;23:131–8.
Konradsen JR, Caffrey Osvald E, Hedlin G. Update on the current methods for the diagnosis and treatment of severe childhood asthma. Expert Rev Respir Med. 2015;9:769–77.
Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10.
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul WJ. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. Asthma. 2012;49:586–92.
Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–8.
Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: consider all of the possibilities. Can Respir J. 2000;7:415–8.
Wener RR, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–35.
Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–4.
De Groot EP, Kreggemeijer WJ, Brand PL. Getting the basics right resolves most cases of uncontrolled and problematic asthma. Acta Paediatr. 2015;104:916–21.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–9.
Article PubMed Central Google Scholar
Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373-381.
Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901.
CAS PubMed Google Scholar
De Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7.
Rotiroti G, Roberts G, Scadding GK. Rhinitis in children: common clinical presentations and differential diagnoses. Pediatr Allergy Immunol. 2015;26:103–10.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:S8–160.
Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–21.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855–64.
De Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41:1068–73.
Barker NJ, Jones M, O'Connell NE, Everard ML. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013;12:CD010376.
Google Scholar
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, Weaver DE, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50:1128–36.
Ross KR, Storfer-Isser A, Hart MA, Kibler AM, Rueschman M, Rosen CL, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42.
Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011;19:1623–8.
Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–5.
Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43.
Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012;13:822–33.
Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46.
Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116:1163–4.
Van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Katzmarzyk PT, Bouchard C. Where is the beef? Waist circumference is more highly correlated with BMI and total body fat than with abdominal visceral fat in children. Int J Obes. 2014;38:753–4.
Article CAS Google Scholar
De Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36:671–8.
Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016; https://doi.org/10.1136/thoraxjnl-2015-207630 .
Federal Drug Administration Advisory for Omalizumab. Available at: https://wayback.archive-it.org/7993/20170111075347/ . http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm . Accessed 4 Feb 2018.
European Medicines Agency: assessment report for Xolair. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124 . Accessed 7 June 2017.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96.
Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR. Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun. 2015;71:419–26.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674–7.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, Cao C, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. “real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2015; https://doi.org/10.1111/all.12815 .
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6.
Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–7.
Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–70.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–33.
Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9.
Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–6.
Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9.
Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–8.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–9.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–10.
Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6.
Busse WW, Trzaskoma B, Omachi TA, Canvin J, Rosen K, Chipps BE, et al. Evaluating Xolair persistency of response after long-term therapy (XPORT). Am J Respir Crit Care Med. 2014;189:A6576.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49.
National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma—full report 2007. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf . Accessed 4 Feb 2018.
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunolgy. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85.
Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30.
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13.
Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82.
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Hedlin G, van Hage M. The role of immunotherapy in the management of childhood asthma. Ther Adv Respir Dis. 2012;6:137–46.
Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25:829–32.
Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9.
Stelmach I, Kaczmarek-Woźniak J, Majak P, Olszowiec-Chlebna M, Jerzynska J. Efficacy and safety of high-doses sublingual immunotherapy in ultra-rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–8.
Download references
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
All relevant data and materials are published in the manuscript.
Author information
Authors and affiliations.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131, Naples, Italy
Virginia Mirra, Silvia Montella & Francesca Santamaria
You can also search for this author in PubMed Google Scholar
Contributions
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Francesca Santamaria .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mirra, V., Montella, S. & Santamaria, F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18 , 73 (2018). https://doi.org/10.1186/s12887-018-1019-9
Download citation
Received : 24 May 2016
Accepted : 29 January 2018
Published : 21 February 2018
DOI : https://doi.org/10.1186/s12887-018-1019-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Adolescents
- Asthma exacerbations
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Episode 79 – Management of Acute Pediatric Asthma Exacerbations

In this EM Cases episode on Pediatric Asthma we discuss risk stratification (including the PASS and PRAM scores), indications for CXR, the value of blood gases, MDIs with spacer vs nebulizers for salbutamol and ipatropium bromide, the best way to give corticosteroids, the value of inhaled steroids, the importance of early administration of magnesium sulphate in the sickest kids, and the controversies around the use of ketamine, heliox, high flow nasal cannuala oxygen, NIPPV, epinephrine and IV salbutamol in severe asthma exacerbations. So, with the multinational and extensive experience of Dr. Dennis Scolnik , the clinical fellowship Program Director at The Hospital for Sick Children in Toronto and Dr. Sanjay Mehta , multiple award winning educator who you might remember from his fantastic work on our Pediatric Orthopedics episode, we’ll help you become more comfortable the next time you are faced with a child with asthma who is crashing in your ED…
Podcast: Play in new window | Download (Duration: 1:09:19 — 63.5MB)
Subscribe: Apple Podcasts
Written Summary and blog post written by Anton Helman, April 2016
Cite this podcast as: Scolnik, D, Mehta, S, Helman, A. Management of Acute Pediatric Asthma Exacerbations. Emergency Medicine Cases. April, 2016. https://emergencymedicinecases.com/pediatric-asthma/. Accessed [date].
Pediatric Asthma Severity Indicators on History
- life-threatening exacerbations
- admissions to ICU
- deterioration while already on systemic steroids
- using more than 2 canisters of short acting B-agonist per month
- cardiopulmonary and psychiatric comorbidities
Its important to realize that a lack of risk factors does not necessarily confer a lack of risk, so even if a patient has none of these risk factors, they can still be at risk for deterioration from their asthma.
Reliable Validated Measures of Pediatric Asthma Severity
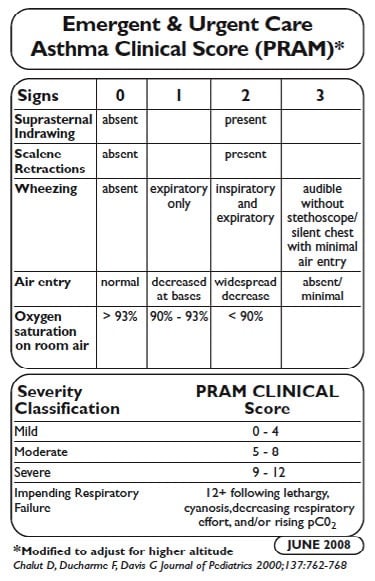
PASS Score for Pediatric Asthma Severity
VBG in Pediatric Asthma
A PaCO2 >42 is indicative but not diagnostic of a severe exacerbation A PaCO2 >50 is a risk factor for impending respiratory failure Metabolic Acidosis is an indicator of impending arrest!
A VBG is seldom indicated unless the child has no clinical improvement with maximal therapy. The timing of the VBG is important: It may be most useful as a baseline after ED treatment in a patient going to the ICU.
Don’t forget the classic teaching: A ‘normal’ Hg partial pressure of CO2 in a patient with extreme tachypnea and retractions could indicate impaired ventilation and impending respiratory failure.
Indications for CXR in presumed Pediatric Asthma
The rate of CXR use in kids with asthma increased significantly from the mid 90’s to around 2010. Although it’s not unreasonable for first time wheezers to get a baseline CXR, it’s important to realize that an unsuspected diagnosis made on the basis of a CXR in an acutely wheezing child is rare, even if the child has never wheezed before.
In fact there are no set of predictors in the literature that can accurately identify children likely to have abnormalities on CXR.
Nonetheless, some situations that might warrant a CXR in a child with a wheeze, are focal chest findings, fever, subcutaneous emphysema or a history of choking.
MDI vs Nebs vs IV B-agonists in Pediatric Asthma
Compared with nebulized treatments, metered-dose inhaler (MDI) with a spacer use has been shown to be equally effective for children of all ages with a wide range of illness severity and by multiple outcome measures. Among children 1 to 4 years old, using a MDI with a spacer was associated with a greater reduction in wheezing and a lower hospitalization rate in one study.
Furthermore, a recent cost analysis determined that the use of MDIs to treat children with mild to moderate asthma exacerbations in the ED could yield significant cost savings compared with nebulized treatments. MDIs with a spacer should not be used in patients with impending respiratory failure and it can be difficult to coordinate breathing with administration of the inhaler for patients less than 1 year old.
IV beta-agonists have not been shown to be superior to inhaled beta-agonists. IV beta-agonists should be considered in those who are unable to tolerate nebulized or MDI treatments.
For < 15 kg: Salbutamol MDI 4 puffs or 2.5mg nebulized in 2-3ml NS x3 back to back (continuously) For > 15kg: Salbutamol MDI 8 puffs or 5mg nebulized in 2-3ml NS x3 back to back (continuously)
A Cochrane review found that those treated with continuously nebulized bronchodilators had lower rates of hospitalization, greater improvements in pulmonary function test results, and similar rates of adverse events compared with those treated intermittently. Continuous treatment allows greater compliance with the goal of delivering the equivalent of three intermittent bronchodilator treatments in the first hour of care. In addition, this method will result in less respiratory therapy time and costs; it has been shown to be safe, and it may benefit the sickest patients the most.
B-agonists with Ipatropium Bromide are more effective than B-agonists alone in Pediatric Asthma
In a systematic review and meta-analysis comparing the use of beta-agonists plus anticholinergics with beta-agonists alone, combination therapy was associated with significantly lower hospitalization rates and improvements in asthma scores and pulmonary function test results.
So multiple doses of ipatropium bromide added to beta-agonists are indicated for kids with moderate to severe asthma exacerbations. However, there are no clinical trials supporting ipratropium use beyond the first hour or first 3 doses in children.
Ipatromium Bromide dosing: MDI 4 puffs (80mcg) or 250mcg nebulized
Single dose Dexamethasone is the preferred oral corticosteroid for Pediatric Asthma
A study out of the Annals of EM entitled “ A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department ”, showed that a single dose of dexamethasone dosed at 0.3mg/kg orally compared to prednisolone dosed at 1mg/kg for 3 days in 245 children with known asthma had equivalent PRAM scores at day 4. This is consistent with 3 previous RCTs, the largest of which dosed dexamethasone at 0.6mg/kg po.
So, it is reasonable to give one dose of dexamethasone at 0.3-0.6mg/kg po just prior to starting bronchodilators to all but the sickest of kids who present to the ED with an asthma exacerbation, obviating the need for an outpatient prescription.
It’s also worth noting that dexamethasone is associated with less vomiting compared to prednisolone as well.
Update 2022: A prospective, randomized, single center randomized clinical trial of 318 patients 2 to 20 years of age presenting to a pediatric ED with mild to moderate asthma exacerbation found no difference in the rate of return visits for continued or worsened symptoms, days to symptom resolution, missed school days, or vomiting between patient randomized to 1 or 2 doses of dexamethasone. Abstract
Inhaled corticosteroids
While there’s no evidence that the use of inhaled steroids in the ED are beneficial there is evidence that they decrease relapse rates in the outpatient setting.
The maximum dose of inhaled steroid is the equivalent of Fluticasone (Flovent in Canada) of 100 micrograms 2 puffs twice daily for a maximum of 200 micrograms per day. There is evidence based on observational data from the Canadian Paediatric Surveillance Program that higher doses may lead to adrenal suppression and in some cases adrenal shock.
Discharge Criteria for Pediatric Asthma
Discharge criteria from the ED include:
- Needing beta-agonists less often than q4 h after 4 to 8 h of conventional treatment
- A reading of SpO2 94% on room air
- Minimal or no signs of respiratory distress
- Improved air entry
Discharge Instructions for Pediatric Asthma
- Prepare a written asthma action plan with medications and signs to look out for that would necessitate a return to the ED
- Continue to use a short-acting beta-agonists such as salbutamol until exacerbation resolves and then as needed, with directions to see a health care professional if therapy is needed more often than every 4h.
- For all but the mildest of asthma patients seen in the ED, a prescription for 3 weeks of inhaled streroid such as fluticasone 50 micrograms, 2 puffs twice daily.
- Review techniques for using inhaled asthma medications as well as for cleaning/maintaining the inhaler device. Parents must understand that they need to use the MDI spacer and that the mask fits properly, to use the B-agonist BEFORE the inhanled steroid and to wash the mouth out after the steroid inhaler to prevent thrush.
- Encourage follow-up with the patient’s primary care physician or a local asthma clinic to review asthma control, environmental history and symptom recognition.
Peak expiratory flow should NOT be relied upon solely as a measure of severity or as a sole determinate for discharge.

IV Magnesium Sulphate in Pediatric Asthma
A meta-analyses suggests that use of magnesium sulphate results in improved outcomes for both adults and children, improving respiratory function and decreasing hospital admissions. IV magnesium sulphate may be considered in cases of moderate and severe asthma with incomplete response to conventional.
IV magmesium sulphate 40mg/kg should be given EARLY to patients with severe asthma who do not substantially improve after the first 6o minutes of bronchodilator and steroid therapy.
The most common adverse effect is hypotension; this may be avoided by infusion of the dose over 20 minutes and giving a fluid bolus prior to or during the magnesium infusion.
If there is a delay in obtaining an IV, magnesium sulphate can be given IO or inhaled via nebulizer.
What about Nebulized Magnesium Sulphate?
The RCT entitled MAGNETIC trial in 2013 of about 500 children showed that MgSO4 2.5mL of 250mmol/L solution q20mins x 3 added to the salbutamol and ipratropium bromide nebulizer in the first hour for kids with acute severe asthma, significantly improved asthma severity scores without any increase in adverse events.
Pediatric Asthma Therapy with Equivocal or Mixed Evidence that may be indicated when all else has failed
Up to 26% of children intubated due to asthma suffer complications including pneumothorax, impaired venous return, and cardiovascular collapse because of increased intrathoracic pressure. Mechanical ventilation during an asthma exacerbation is associated with an increased risk of death and should be considered as a last resort and in conjunction with the support of a paediatric ICU specialist.
IM Epinephrine – time tested but no good evidence
IM epinephrine at the same doses used in anaphylaxis (0.01mg/kg of 1:1000, max 0.3mg given in the anterolateral thigh) has been used for decades in children with severe asthma, however there have been no robust RCTs to support it’s use.
Heliox – reserve for the ICU
According to the Canadian Pediatric Society Guidelines for Managing the Patient with Acute Asthma Exacerbation , using a helium-oxygen gas mixture should be reserved for children in the ICU setting with severe asthma exacerbation who have failed to improve despite maximized therapy.
Ketamine to avoid intubation – 3 Mixed Studies
A limited case series has reported the effectiveness of a bolus (2 mg/kg) followed by a continuous infusion (2 to 3 mg/kg/h) of ketamine in children with severe asthma who were approaching respiratory failure. In this study, the use of ketamine resulted in prompt improvement and avoided the need for endotracheal intubation. This is an appealing use of ketamine, because it may allow one to avoid the hazards of endotracheal intubation and mechanical ventilation in the patient with asthma.
A randomized control trial showed no improvement in pulmonary index scores with the administration of ketamine to patients with moderate to severe asthma. Patients were randomized to 0.2mg/kg ketamine bolus followed by 0.5mg/kg/h for 2 hours vs placebo.Pulmonary index scores were measured throughout the 2 hours and no difference was found.
In a 2001 prospective, observational, single-arm pilot study in two pediatric EDs over three months, the effect of IV ketamine added to standard therapy in status asthmaticus wasevaluated. Initiation of ketamine in patients with severe asthma was associated with clinical improvement. Side effects were easily managed with treatment or discontinuation of ketamine.
The take home message is that more convincing evidence is required before ketamine can be recommended for routine treatment of severe pediatric asthma to avoid intubation.
Ketamine, however, is safe at dissociative dosages, and is a reasonable option when all others measures have failed.
BiPAP – the pediatric literature isn’t quite as impressive as the adult literature
A few case reports and observational studies of the use of BiPAP in pediatric asthma show some promise. The one RCT of only 20 patients does show a benefit in clinical asthma scores, respiratory rate, and supplemental oxygen need. While intuitively sensible, there is no evidence that NIPPV prevents the need for intubation in children with status asthmaticus.
Similar to other rescue measures, NIPPV can be considered when all others measures have failed in hopes of avoiding endotracheal intubation.
High Flow Nasal Cannula – gaining popularity
Another way of providing a bit of noninvasive positive pressure that seems to becoming popular among the pedatricians is high flow nasal cannula oxygen. The evidence is conflicting for this, and most studies were done in kids with bronchiolitis rather than asthma.
One study from Pediatric Emergency Care in 2012 showed that the use of high flow nasal oxygen reduced the need for intubation in pediatric acute respiratory failure, but there was no change in mortality or ICU length of stay.
However, a Cochrane review in 2014 based on 11 studies concluded that no evidence could be found to allow determination of the safety or effectiveness of HFNC therapy in children.
The latest study out of Emergency Medicine Journal concluded that HFNC may have a role, but about 1/3 of patients required BiPAP or intubation.
IV salbutamol – may improve recovery time and length of stay
Two RCTs showed a more rapid recovery time and earlier discharge from hospital when IV salbutamol was compared to nebulized ipratopium bromide in one of the studies, and compared to continuous salbutamol nebs in another. When using IV salbutamol, be on the lookout for excessive tachycardia, low DBP and rising lactate. Start at 1mcg/kg/min infusion and titrate to 5mcg/kg/min.
The decision to intubate should be based on clinical judgement as opposed to any single vital sign or blood gas result. Some variables to consider for intubation are worsening hypercapnea, patient exhaustion and changes in mental status.
Putting it all together for Severe Pediatric Asthma Exacerbation: A Step-wise Approach
*note that the blue indicates evidence-based treatment while the red indicates therapies that are reasonable to try when all else has failed but do not have strong evidence for benefit
Put the child on the cardiac monitor | Obtain IV access and draw blood work including electrolytes and a VBG (with particular attention to the K) | Call your RT and pediatric intensivist early | Continuous salbutamol nebulizers with the first 3 including ipratropium bromide | IV steroids: methylprednisolone 1mg/kg or hydrocortisone 5mg/kg (if dexamethasone 0.3mg/kg, max 10mg was not given prior to starting nebs) | IV NS 20mL/kg bolus (preferably before the MgSO4) | IV Magnesium Sulphate 40mg/kg to a maximum of 2g over 20 mins (in the first hour if possible) | Consider epinephrine 0.01mg/kg IM and nebulized MgSO4 (especially if you are having trouble obtaining IV access) | Consider BiPaP or high flow nasal oxygen | Consider IV salbutamol 1-5mcg/kg/min (beware tachycardia, low DBP, rising lactate) | Consider subdissociative dose ketamine | Consider Heliox

Quote of the Month
“Knowledge is not only power; it is happiness,
and being taught is the intellectual analog of being loved.”
– Isaac Asimov
For more on Paediatric Emergencies download our free interactice eBook EM Cases Digest Vol. 2 Pediatric Emergencies
Dr. Helman, Dr. Mehta and Dr. Scolnik have no conflicts of interest to declare
Key References
Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med 2004;11(1):10-8.
Belessis Y, Dixon S, Thomsen A, et al. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol 2004;37(3):201-9.
Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): A responsive index of acute asthma severity. J Pediatr 2000;137(6):762-8.
Birken CS, Parkin PC, Macarthur C. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity, and responsiveness. J Clin Epidemiol 2004;57(11):1177-81.
Gershel JC, et al: The usefulness of chest radiographs in first asthma attacks. Engl J Med 309: 336, 1983.
Castro-Rodriguez JA, Rodrigo GJ: Beta-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: A systematic review with meta-analysis. J Pediatr 2004; 145:172.
Camargo CA, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003;(4):CD001115.
Randolph C. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;134 Suppl 3:S178-9.
Cronin JJ, Mccoy S, Kennedy U, et al. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann Emerg Med. 2015; 134:432.
Rodrigo GJ, Castro-Rodriguez JA: Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax 2005; 60:740.)
Goldbloom E, Ahmet A. Adrenal suppression: An under-recognized complication of a common therapy. Paediatr Child Health. 2010;15(7):411-2. Full PDF
Rowe BH, et al: Intravenous magnesium sulfate treatment for acute asthma in the emergency department: A systematic review of the literature. Ann Emerg Med 2000; 36:181. Full PDF
Cheuk DK, Chau TC, Lee SL: A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child 2005; 90:74.
Powell CV, Kolamunnage-dona R, Lowe J, et al. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol Assess. 2013;17(45):v-vi, 1-216.
O Ortiz-Alvarez, A Mikrogianakis; Managing the Patient with an acute asthma exacerbation. Canadian Paediatric Society,Paediatr Child Health 2012;17(5):251-5
Denmark TK, Crane HA, Brown L: Ketamine to avoid mechanical ventilation in severe pediatric asthma. J Emerg Med 30: 163, 2006.
Allen JY, Macia CG. The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Ann Emerg Med. 2005;46(1):43-50.
Petrillo TM, Fortenberry JD, Linzer JF, Simon HK. Emergency department use of ketamine in pediatric status asthmaticus. J Asthma. 2001; 38(8):657-664.
Basnet S, Mander G, Andoh J, Klaska H, Verhulst S, Koirala J. Safety, efficacy, and tolerability of early initiation of noninvasive positive pressure ventilation in pediatric patients admitted with status asthmaticus: a pilot study. Pediatr Crit Care Med. 2012;13(4):393-8.
Wing R, James C, Maranda LS, Armsby CC. Use of high-flow nasal cannula support in the emergency department reduces the need for intubation in pediatric acute respiratory insufficiency. Pediatr Emerg Care. 2012;28(11):1117-23.
Mayfield S, Jauncey-cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014;3:CD009850.
Long E, Babl FE, Duke T. Is there a role for humidified heated high-flow nasal cannula therapy in paediatric emergency departments?. Emerg Med J. 2016.
Browne GJ, Trieu L, Van asperen P. Randomized, double-blind, placebo-controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Crit Care Med. 2002;30(2):448-53.
Browne GJ, Penna AS, Phung X, Soo M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;349(9048):301-5.
Additional FOAMed Resources for Pediatric Asthma
Evidence-based slide set on ED pediatric asthma management from CAEP 2015 Conference
Mechanical Ventilation in Severe Asthma on Pediatric EM Morsels
Asthma and The Vent on PEM ED podcast
Best Bets review on Ketamine in Severe Asthma
St. Emlyns on Why don’t we just use dexamethasone?
Ryan Radecki on Early Administration of Steroids in Pediatric Asthma
For more EM Cases content on Pediatric Emergencies download our free eBook,
EM Cases Digest Vol. 2 Pediatric Emergencies here .

About the Author: Anton Helman
Recent Posts

EM Quick Hits 56 – Nitroglycerin in SCAPE, REBOA, Diverticulitis Imaging, CRAO, Penicillin Allergy, Physician Personality

Ep 193 The Crashing Asthmatic – Recognition and Management of Life Threatening Asthma

ECG Cases 49 – ECG and POCUS for Dyspnea and Chest Pain
[…] Helman goes through an evidence based approach to management of asthma exacerbations in children. […]
Simple but covers all the basics, enjoyable read through Thank you
Leave A Comment Cancel reply
Nursing Case Study for Pediatric Asthma
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Anthony is a 6-yr-old male patient brought to the pediatric ER with a history of asthma since he came home from the NICU as an infant. He lives with his parents, Bob and Josh, who adopted him after fostering him from age 4 months. They have tried the usual nebulizer treatments but Anthony is not responding as usual, so they brought him for evaluation.
Initial assessment in triage reveals both inspiratory and expiratory wheezes, dyspnea, tachypnea, diaphoresis, and retractions.
BP 70/40 mmHg SpO2 93% on room air HR 131 bpm RR 32bpm at rest Temp 38.3°C
What physiologic issue is Anthony suffering from based on the assessment findings?
- Respiratory distress is evidenced by both vital signs and physical assessment findings. His RR and HR are high. He is also sweating and having retractions which may indicate he is working hard to try to establish oxygen exchange.
What signs and symptoms might occur that would show worsening of his condition?
- Skin color changes (i.e. blue or bluish around the mouth or even inside the mouth, blue nail beds, gray or pale compared to usual)
- Grunting on exhalation (this indicates the body is trying to keep air in the lungs)
- Stridor (this is heard in the upper airway and can be an ominous sign)
- Changes in the level of consciousness (becoming lethargic or drowsy)
Anthony is pale but not gray. His lips do indicate a very faint bluish tinge. He can speak but it appears difficult.
What medications might the nurse expect the provider to order?
- Short-acting beta-agonists (SABAs)
- Racemic albuterol – A racemic mixture of albuterol (salbutamol) is the primary SABA used for quick relief of acute asthma symptoms and exacerbations.
- Levalbuterol – Levalbuterol (Levosalbutamol), the R-enantiomer, is the active isomer of racemic albuterol that confers the bronchodilator effects. Levalbuterol is approved in the United States for the treatment of bronchospasm in children ≥4 years of age via hydrofluoroalkane (HFA) metered-dose inhaler (MDI) and ≥6 years of age via solution for nebulizer
What side effects might occur from the medications ordered?
- “The most common side effects are tremor, increased heart rate, and palpitations” Anthony may report feeling jittery due to the activation of beta receptors.
After administration of racemic albuterol, Anthony now has a RR of 22 and O2 saturation of 95% on room air. However, the provider decides to admit him to the inpatient pediatric observation unit. His parents ask if there are ways to keep him from continually being admitted to the hospital.
What are some education topics to bring up to Anthony’s parents?
- Controlling asthma triggers — The factors that set off or worsen asthma symptoms are called “triggers.” Identifying and avoiding asthma triggers is essential to keeping symptoms under control. Common asthma triggers generally fall into several categories:
- Allergens (including dust mites, pollen, mold, cockroaches, mice, cats, and dogs)
- Respiratory infections, such as the common cold or the flu
- Irritants (such as tobacco smoke, chemicals, and strong odors or fumes)
- Exercise or other physical activity
What does the nurse understand about this medication?
- Systemic corticosteroids are an essential treatment option for many disease states, especially asthma. These medications reduce the length and severity of asthma exacerbations and reduce the need for hospitalization or ED visits. It is important for asthma patients to receive prednisolone as soon as possible after the onset of symptoms that are bronchodilator-unresponsive to attain these benefits.
- Although usually prescribed for a 5- to 7-day period, oral corticosteroids are not without adverse effects. The most common adverse effects are the same for the majority of oral corticosteroids and include increased appetite, weight gain, flushed face.
- Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Thinning bones (osteoporosis) and fractures happen over time, be mindful this may cause problems in an energetic child. Suppressed adrenal gland hormone production may result in a variety of signs and symptoms, including severe fatigue, loss of appetite, nausea and muscle weakness.
Anthony sleeps during the night shift and the next day, his pediatrician makes rounds and discusses a change in the severity rating of Anthony’s asthma.
What does the nurse know about asthma severity and how it is determined?
- Asthma severity is the intrinsic intensity of the disease. Assessment of asthma severity is made on the basis of components of current impairment and future risk. The severity is determined by the most severe category measured
Bob and Josh are interested in meeting with respiratory therapy for assistance with inhalers. They say that Anthony has trouble using inhaler devices.
Inviting respiratory therapy to provide parent teaching is an example of what? How can this department help the family?
- Interdisciplinary team collaboration.
- Teaching about medications, proper inhaler (or other equipment) use, thorough explanation of peak expiratory flow (PEF) measuring, ways to help control RR.
After lunch, Anthony is ready to be discharged. His parents verbalize gratitude to the staff and thank the team for helping with education.
What can the nurse help Bob and Josh start to establish to try to help them with Anthony’s condition?
- Setting goals and planning. Preparing for an action plan (“Asthma ‘action plan’ is a form or document that your child’s provider can help you put together; it includes instructions about how to monitor symptoms and what to do when they happen. Asthma action plans are available for children up to five years old, for children five years and older and adults and for school. An action plan can tell you when to add or increase medications, when to call your child’s provider, and when to get immediate emergency help. This can help you know what to do in the event of an asthma attack. Different people can have different action plans, and your child’s action plan will likely change over time.”)
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
References:
View the full transcript, nursing case studies.

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
Sustainment of a Successful Pediatric Asthma Management Program Case Study

Executive Summary
In 2016, Children’s Hospital of Orange County (CHOC) was awarded the HIMSS Davies Award. One of the award-winning case studies focused on using embedded, evidence-based care guidelines to control pediatric asthma and an alert system that triggered when patients were about to be discharged before a home management plan was created. CHOC drove down the average length of stay for pediatric asthma patients from 2.14 days to 1.72 days. Pediatric asthma readmissions within 30 days also fell from an average of 1.7 per quarter to 0.7 per quarter. To sustain and expand on this work, in 2017 the organization decided to re-focus on the pediatric condition registries which had first been installed in 2015. It organized and stabilized the number of primary care patients who received an pediatric asthma action plan (AAP) and asthma control test (ACT) resulting in lower emergency room visits and inpatient hospital admissions. The process re-design was driven by analytical data which was reviewed on a monthly basis for transparency into how providers were performing against their value measurements. The utilization of a population health platform empowered the team to take disparate sources of data (different electronic health records, claims, labs, pharmacy data, etc., and transform the data in to meaningful, useful information and integrate the required documentation into the EHR. In total, CHOC built six ambulatory guidelines of care into their EMR, one of which was asthma, leading to the overall improvement in the ambulatory care of pediatric asthmatics. The results included:
- In December 2019, ~59% of primary care patients received the pediatric asthma action plan—up from 28.89% in April 2017.
- In December 2019, 57% of primary care patients received the asthma control test—up from 29.61% in April 2017.
- Patients being seen in the emergency department (ED) for asthma decreased, as well as the readmission rate within seven days of discharge per 100 discharges decreased from 1.84 to 0.3.
The key difference in this case study as compared to the one in 2016 is the work to improve the ambulatory care aspect, as well as continued work in the Breathmobile™ which is the only mobile asthma clinic serving preschool and school-aged kids in Orange County.
Lessons learned include:
The primary and most important take away is to first manage the care of the patients who show up for treatment—and then create an ambulatory plan of care that prevents them from requiring emergent care or hospital admission.
- When choosing registries for implementation, determine which diseases are most prominent in your population.
- Identify which of these diseases lead to increased morbidity, mortality or overall healthcare utilization.
- Understand which of these diseases have well established care guidelines.
- Target disease groups that have willing physicians to partner with on disease registry development.
- Patient and family education, access to care, overcoming social determinants of health issues, improving the use of the Asthma Control Test and the Asthma Action Plan are key to preventing pediatric asthma ED visits and readmissions.
- Engaging the community, schools, and resources like the Breathmobile help to prevent ED asthma visits.
- Care managers and school nurses also play a huge role in asthma management.
Defining the Clinical Problem and Pre-Implementation Performance
CHOC Medical Group is composed of 54 pediatricians and pediatric nurse practitioners who practice in five clinical sites in Orange County, California. Two sites are on the campus of the Children’s Hospital in the city of Orange, and three sites are in surrounding communities (Santa Ana and Garden Grove) where they support a multicultural population which is entirely Medicaid (MediCal) funded.
The population that CHOC Medical Group serves struggles with barriers such as language discordance, transportation, food and housing insecurity, and various cultural beliefs related to their healthcare. Given the proximity to the U.S./Mexico border, there is a large Latin American population who struggle with immigration issues and in some cases delay/avoid contact with the medical system.
Finally, given CHOCH’s affiliation with the children’s hospital, which is a free-standing tertiary/quaternary children’s hospital, CHOCH primary care clinics support a large volume of hospital graduates with a high degree of complex medical conditions. Preventing emergency room visits and hospital admissions is key to claiming “successful” management of this population. The medical group was struggling to maintain incremental increases in the percentage of pediatric asthmatic patients who had an updated annual AAP and ACT. This was an initiative that the organization and Practice Transformation Network (Southwest PTN—CHOC Children’s and Rady Children’s) had embraced as part of the chronic disease management goal of the Population Health Division’s engagement in the Transforming Clinical Practice Initiative Award CHOC received from the Centers for Medicare and Medicaid Services. CHOC’s subject matter experts identified a wide range of provider/panel completion rates and, as a collective, the group was averaging in the low 30% range. Difficulties with this stemmed from insufficient time for providers to address asthma during routine sick visits and well visits, ineffective capture of patients in the EMR Chronic Disease Registries, inconsistent standards of work for the care team, and variable approaches to compliance from provider to provider and clinic to clinic.
The numerators are respectively the patients who received the AAP and ACT, and patients seen in the ED with the diagnosis of asthma. The denominator is the number of patients in the asthma registry with the diagnosis of asthma.
The American Academy of Pediatrics, the Centers for Disease Control, and the National Institutes of Health (NIH) all support the utilization of an Asthma Action Plan and care guidelines specific to pediatric asthma management. Following these guidelines, the chief medical information officer (CMIO) led the asthma registry development with input from an expert panel consisting of asthma experts in pulmonary and allergy, primary care physicians, practice staff, and other pertinent providers. The common goal was to improve compliance and decrease the ED visits and hospital admissions.
The ultimate target to date is to meet and exceed a 54% compliance rate—the benchmark from the American Academy of Pediatrics and Mayo Clinic is 43%.
The organization is currently continuing these efforts and at the time of this writing is working toward The Joint Commission (TJC) Disease Specific Care Pediatric Asthma Certification. This certification will help ensure our program meets clinical performance standards and targeted metrics as well as other compliance standards from TJC.
Designing and Implementing Model Practices and Governance
The decision to purchase a population health platform to augment other sources of data such as claims data and the enterprise data warehouse was a turning point in improving our workflows and outcomes. The ability to join the platform supplier as a development partner for pediatric content provided an opportunity to focus on the specific needs of the pediatric population. The purchase of the population health platform was a collaborative decision between the information systems department leadership (the chief medical information officer and the chief information officer at the time), the head of CHOC Health Alliance (independent physician association or IPA) and director over the CHOC population health program, and the vice president of strategic planning, along with other ambulatory and senior leaders. The population health platform, HealtheIntent™ was chosen after a formal RFP process involving several vendors. None of the other population health platforms CHOC evaluated had pediatric content, and the HealtheIntent provider had a long history with our organization of working with CHOC to develop pediatric applications, leading to the choice of HealtheIntent. The multidisciplinary team driving pediatric asthma care is referenced below.
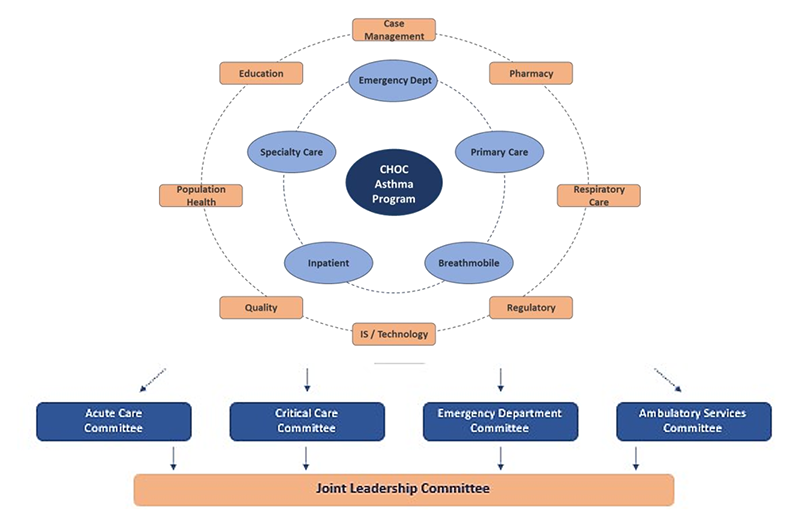
Care guidelines supported by the population health management tool which include measures from the asthma registry were embedded in the standard primary care workflow. Processes were developed to create a uniform and consistent standard of work regardless of provider or location and to identify patients with asthma requiring an asthma AAP and ACT.
Learning resources included:
- HealtheRegistries® Help Page
- User Guides for Providers, Office Staff, and Personnel
- Ex: Pamphlets—Ideal for scattered audiences or as a supplement educational piece
- Ex: Hospital Sessions—Ideal for large group trainings
- Ex: Individual Clinic Visits—Ideal for onboarding new physicians
Enabling Clinical Transformation Through Information and Technology
The foundational component of the CHOC Children’s Population Health program is the creation of a single patient-centered plan of care that can be used by the entire care team across the complete care continuum. Having the actionable data visible at the point of care helps to ensure compliance and outcomes are constantly at the forefront of patient care. The primary office staff prep the asthma charts prior to the visit, the care team medical assistants, licensed vocational nurses, mid-levels, physicians, care managers, school nurses, and Breathmobile staff all have access to the same clinical data.
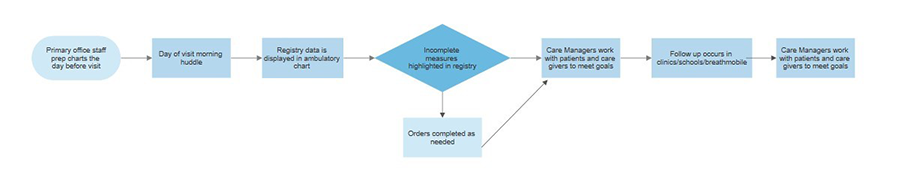
To develop the condition-specific pediatric registries, the team conducted research to determine which diseases were most prevalent in CHOC’s population and which of those diseases led to the most frequent ED visits and hospitalizations. The pediatric asthma registry was created because of the volume and impact this population of patients brings to CHOC. Approximately 13% of CHOC’s capitated, (value-based per member, per month payment system), population has a diagnosis of asthma, and it is the most frequent cause of visits to the ED as 30% of children with asthma visit the ED every year. Asthma is also one of CHOC’s most frequent causes of hospitalization and 5% of children with asthma are hospitalized every year, while 40% miss five or more school days every year from asthma exacerbations. Asthma severity is classified as mild intermittent, moderate, and severe persistent, per NIH guidelines. The team’s recommended care includes assessing an ACT at least once a year and annually updating an AAP specific for that patient. The asthma registry focusses on these two and other measures like appropriate controller meds being given as prescribed.
Operationalizing the registries is another key to our success. Prior to the patient visit, the primary office staff have already identified key elements which may need to be addressed and can intervene to raise awareness to the appropriate care team member, including the patient’s family. Running in the background of the EHR is technology that goes beyond just the claims data, and pulls in disease specific clinical data. This data platform allows specific care plan and treatment modalities to be pushed to the provider’s workflow at the point of care. This population health platform is a cloud-based, programmable platform that is vendor-agnostic, which means it can receive data from any EHR, existing health IT system and other data sources, but also pharmacy, eligibility, laboratory and other sources of relevant information. In building the disease specific registries, the first step was to consider the data needs, and then to prioritize the development of the tools. The steps include acquiring the data, normalizing and data transformation, creating the disease specific algorithms, and building specific tools to push the information into the workflow at the point of care as shown in the interface diagram.
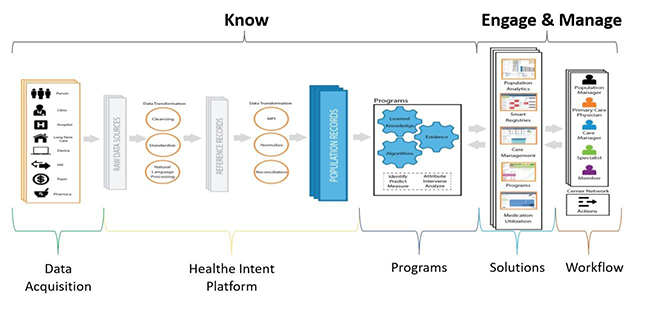
Based on the figures below, you can see a patient who has met all measures, compared to a patient who has some measures that have not been achieved.
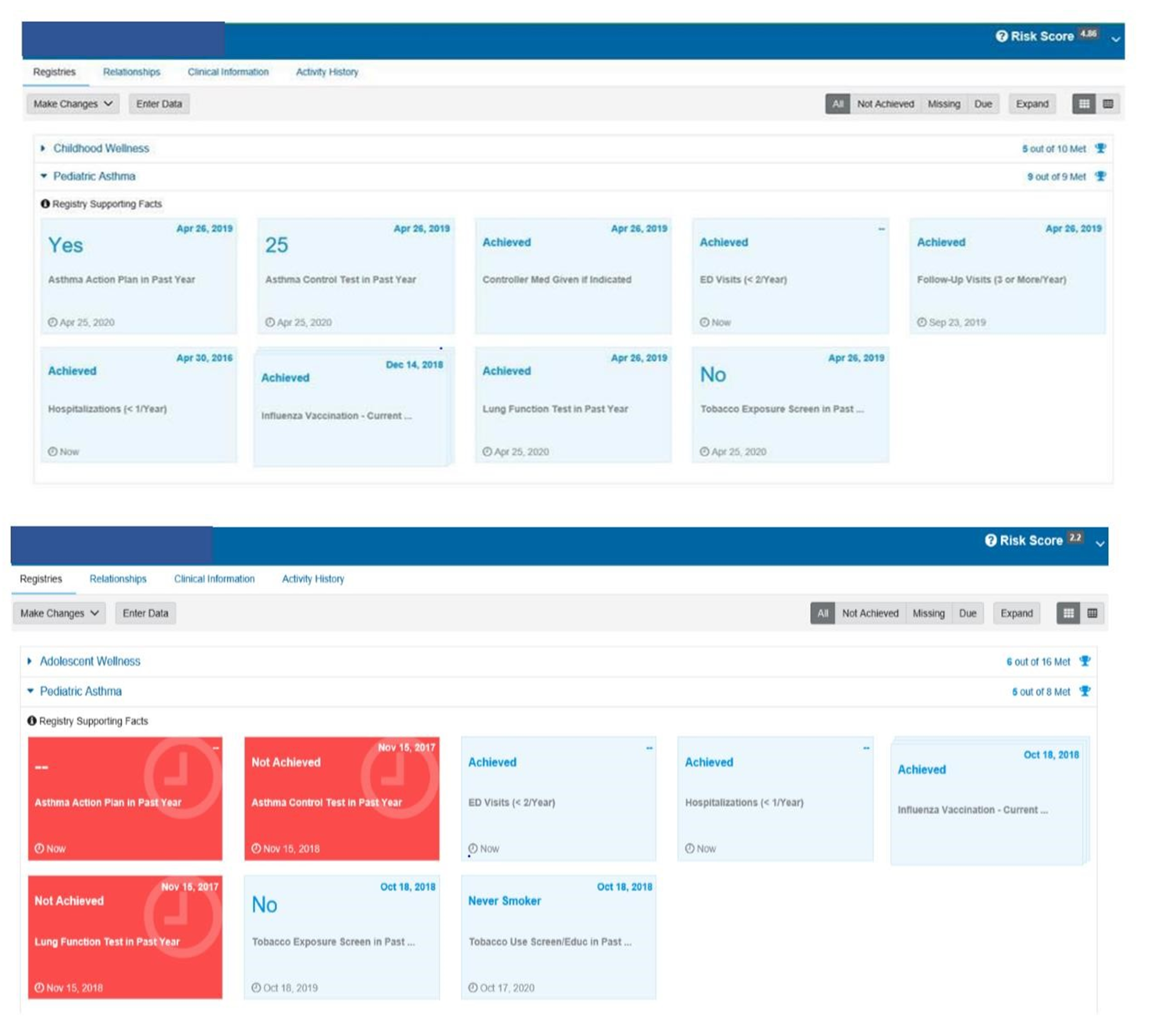
The provider’s view is a push of the registry requirements to the point of care within the workflow.
The primary care team participated in a LEAN Kaizen event to gain an understanding of and improve upon the current state of asthma management. Care team education was based on a train-the-trainer format and materials included job aids, classroom time, online videos, tutorials, and elbow to elbow support during the rollout.
Following the importance of the design and implementation of the asthma registry, is access to care managers who are front and center receiving alerts, providing availability for telehealth visits, and working with the care team to keep everyone updated on their asthma population.
The organization is dedicated to keeping the pediatric asthma patient healthy, managing the disease holistically, and preventing exacerbations. CHOC is ranked as one of the top pulmonology programs in the nation by U.S. News & World Report. CHOC offers patient and family-centered care and comprehensive inpatient and outpatient management for children with asthma. The organization offers comprehensive asthma education by a certified asthma educator for families to learn how to manage and control asthma symptoms. CHOC proudly offer the Breathmobile, the only mobile asthma clinic serving preschool and school-aged kids in Orange County. CHOC also provides the only high-risk asthma clinic in the region dedicated to treating life-threatening asthma. The Breathmobile is another key to the success in reducing the ED visits and hospitalization. In 2019 alone, this team helped to reduce ED visits, hospitalizations, and missed school days.

Improving Adherence to the Standard of Care
A foundational component of CHOC Children’s Population Health program is the creation of a single patient-centered plan of care that can be used by the entire care team across the complete care continuum, including schools and the Breathmobile. The foundation of this care sits upon the population health platform which also supplies the data for the system review. The utilization of the population health platform empowers the team to take disparate sources of data (different electronic health records, claims, labs, pharmacy data, etc.) and transform the data in to meaningful, useful information and integrate the required documentation into the EHR. The results included (at top performance):
- In December 2019, ~59% of primary care patients received the asthma action plan—up from 28.89% in April 2017.
- Patients being seen in the ED for asthma deceased as well as the readmission rate within seven days of discharge per 100 patients decreased from 1.84 to 0.3. The numerators are respectively the patients who received the AAP and ACT, patients seen in the ED with the diagnosis of asthma. The denominator is the number of patients in the asthma registry with the diagnosis of asthma. The adherence to the standard of care in the use of the registry, the ACT, and the AAP, along with clinically driven evidenced-based processes which are managed by real-time analytics and standing meetings to address compliance has proven effective in driving outcomes and enhancing utilization of the tools.
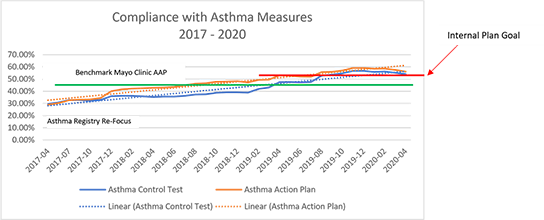
Improving Patient Outcomes
The organization implemented pediatric condition registries with an initial focus on pediatric asthma to be used by primary care providers. The organization increased and stabilized the number of primary care patients who received an AAP and ACT. This resulted in lower ED visits, seven-day readmissions, and overall readmissions for asthma. CHOC achieved these results by using analytical data on a monthly basis for transparency into how providers were performing against their value measures. The numerators are respectively the patients who received the AAP and ACT, patients seen in the ED with the diagnosis of asthma, and patients readmitted after seven days. The denominator is the number of patients in the asthma registry with the diagnosis of asthma.
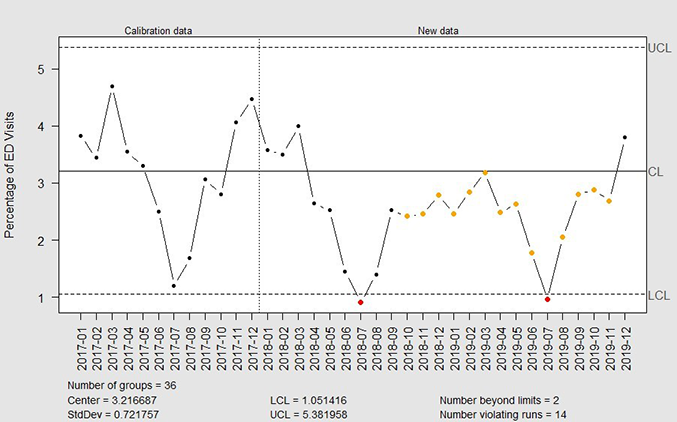
Accountability and Driving Resilient Care Redesign
For a pediatric healthcare system, effective management of the pediatric asthma population improves the well-being of the patients and their families, reduces emergency department visits and improves overall health outcomes. With this goal in mind, physicians, IT leaders, and care management teams at Children’s Hospital of Orange County leveraged a population health registry to improve the health of pediatric asthma patients. As part of this registry, the CHOC Children’s team worked together to research and define measures that were appropriate for the care of pediatric asthma patients. Ten measures were defined and built into the population health asthma registry. Of these, two were selected for a focus on improvement—patients with an asthma control test completed in the past year and patients with an asthma action plan completed in the past year. Key factors for effectively using the measures and the patient registry was making the status of those measures available in real time in the clinical workflow and incorporating the registry data into the standard work of every patient encounter. For example, if a child who has asthma visits with a physician at a primary care clinic and that patient has not had an asthma control test in the past year, that information is front facing to the providers and the care teams. In addition, to track improvements, baseline data was documented, and dashboards were built to provide information on the completion of measures in real time. Since the care team can identify gaps in the service and respond proactively to close those gaps, the organization can maximize the ability to see patients more efficiently.
Along with the push of information directly to the point of care, CHOC also utilizes analytics to measure the compliance of registry and care guideline utilization. Each physician group and each physician are held accountable for care compliance. The organization can focus on local variations in care to reduce frequent ED visits and hospital admissions. CHOC is able to track improvements in compliance with registry metrics over time in reports, dashboards, and scorecards. A re-focus on the asthma registries in 2017 led to improved compliance and improved outcomes.
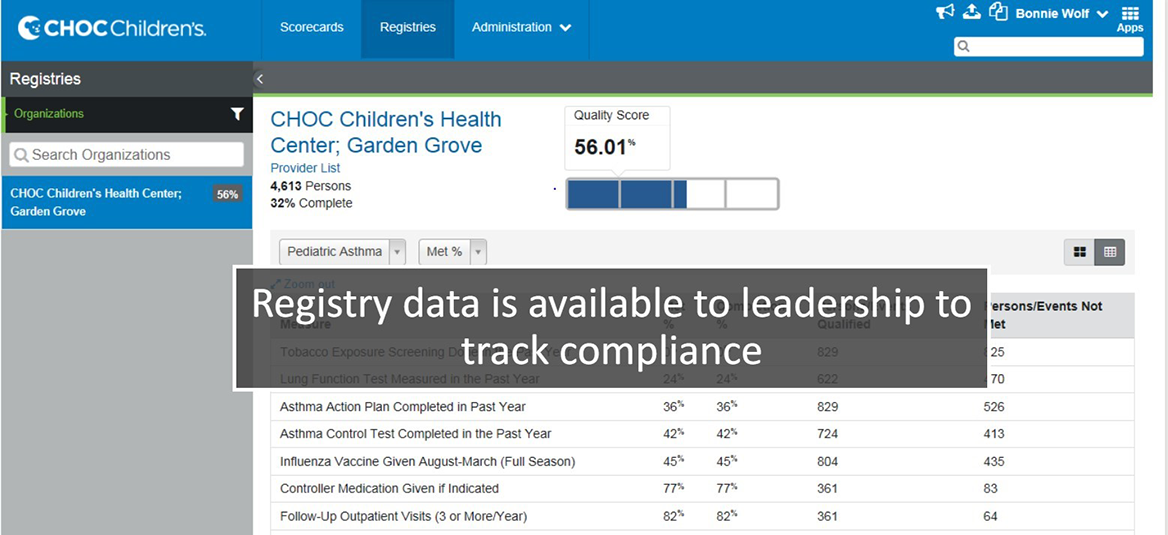
The views and opinions expressed in this content or by commenters are those of the author and do not necessarily reflect the official policy or position of HIMSS or its affiliates.
HIMSS Davies Awards
The HIMSS Davies Award showcases the thoughtful application of health information and technology to substantially improve clinical care delivery, patient outcomes and population health.
Begin Your Path to a Davies Award


IMAGES
VIDEO
COMMENTS
In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study ), this bullying is related directly to the food allergy. From a medical perspective, there are little published data ...
Patient Presentation. A 12 year old girl, Annie, enters the Pediatrician's office with complaints of dyspnea, wheezing, and chest tightness. The history of the illness from the family includes: A recent upper respiratory infection with cough, congestion and runny nose with a low grade fever of 100.8 degrees Fahrenheit.
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
Symptom pattern consistent with asthma and asthma symptoms not well-controlled, or µW Symptom pattern not consistent with asthma but wheezing episodes occur frequently, e.g. every 6 adherence, exposures 8 weeks. Give diagnostic trial for 3 months. Asthma diagnosis, and not well-controlled on low dose ICS
A 9-year-old boy was admitted to the hospital because of fever, cough, respiratory distress, and chest pain. Imaging revealed ill-defined nodular opacities throughout all lung lobes, areas of conso...
A few case reports and observational studies of the use of BiPAP in pediatric asthma show some promise. The one RCT of only 20 patients does show a benefit in clinical asthma scores, respiratory rate, and supplemental oxygen need. ... and most studies were done in kids with bronchiolitis rather than asthma. One study from Pediatric Emergency ...
Hey everyone. My name is Abby. We're going to cover a case study today about pediatric asthma. Let's get started. Anthony is a six year old male patient brought to the ER with a history of asthma. Since he came home from the NICU as an infant, he has lived with his parents, Bob and Josh, who adopted him after fostering him from the age of four ...
Introduction. This paper presents the findings from a case study which formed one of seven case studies designed to identify the nursing contribution to chronic disease management. 1 All of the case studies were selected using criteria which indicated innovation and nurse leadership. Findings from the other six case studies, reported elsewhere, 2 highlighted considerable fragmentation in the ...
In December 2019, 57% of primary care patients received the asthma control test—up from 29.61% in April 2017. Patients being seen in the emergency department (ED) for asthma decreased, as well as the readmission rate within seven days of discharge per 100 discharges decreased from 1.84 to 0.3. The key difference in this case study as compared ...
Summary. This chapter presents a case study of a 10-year-old girl with moderate intermittent asthma diagnosed at age 4 and was admitted to the pediatric intensive care unit with status asthmaticus. The case study includes details about history of present illness, past medical history, past surgical history, family history, and current status.
Pediatric Asthma Case Study . Breakout: Applying the Care Manager Process . Marcus is a 17 year-old high school junior. Marcus was well, with the "usual colds," until the age of six. He then began having a larger number of lower respiratory tract illnesses and was diagnosed with asthma. He had eczema as a child but that has since resolved.
Asthma is the most common chronic childhood disease and a common reason for pediatric emergency medical treatment. It affects approximately 10-15% of all children in the United States. [1] Risk ...
Three trials were included that involved children with mild to moderate asthma who received beclomethasone, 200 μg twice a day. The final conclusion was that the use of beclomethasone, 400 μg a day, caused a decrease in linear growth of -1.54 cm per year (95% CI, -1.15 to -1.94 cm).
Quick-Relief Medication for All Patients: SABA as needed for symptoms. Intensity of treatment depends on severity of symptoms: up to 3 treatments at 20-minute intervals as needed. Short course of systemic oral corticosteroids may be needed. Caution: Increasing of b-agonist or use >2x/week for symptoms control indicates.
Asthma is a complex disease that involves physiological, environmental, and psychosocial factors. This paper reviews childhood asthma case management by social service professionals, lay health workers, and nurses, and it presents a new randomized controlled study using nurse case management in a local community coalition. Evidence suggests the common factor for success involves case managers ...
WHY IT MATTERS. Acute asthma exacerbations in children are extremely common. Most asthmatic exacerbations respond quickly to basic treatment with beta-agonists, anticholinergics, and steroids. This case highlights the management of those patients who need treatment that goes beyond the basics. Critically ill pediatric patients are anxiety ...
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
UNFOLDING Reasoning Case Study: STUDENT Pediatric Asthma History of Present Problem: Jared Johnson is a 10 year-old African-American boy with a history of moderate persistent asthma. He is being admitted to the pediatric unit of the hospital from the walk-in clinic with an acute asthma exacerbation. Jared started complaining of increased chest ...
G.J is an African American, a five-year-old boy who is brought up in a Christian setting home by his parents. The mother is aged 32 years, the father is 35 years and has a brother who is 4 years. The primary language that is used at their home is English. G.J was taken for medical evaluation and admitted diagnosis was asthma.
A 4-month-old girl presents with a 1-week history of a temperature to 102°F (38.9°C), congestion, rhinorrhea, and cough. She has had fatigue and diaphoresis with feedings over the last week, although this did not occur before this time. She was born at term following normal findings on prenatal ultrasonography. Initially, she had difficulty gaining weight, but she is now growing along the ...
Pediatric Drowning. Posted on May 9, 2023May 9, 2023by Jared Baylis. A three-year-old child was swimming with their family, when they wandered into the deep end and submerged under water. The parents noticed the child was below the surface. When the child was brought to the surface, they were unconscious and coughing up foam.
Asthma is a chronic disease that is characterized by narrowing and inflammation of the airways. The obstruction of the airways occurs due to three processes that occur with asthma: inflammatory cell infiltration, mucus hypersecretion with mucus plug formation, and smooth muscle contraction (Hashmi et al., 2022).
What does your child's room once per week (Also wet mop the child's room and parent should also vacuum all carpets and furniture weekly using a HEPA filter. this will decrease the child's exposure to airborne irritants and allergens That can trigger the asthma attack) Study with Quizlet and memorize flashcards containing terms like A nurse is ...
We calculated pediatric asthma burden using a U.S. pediatric asthma incidence rate averaged between 2006 and 2008 (12.5 cases per 1000 children) multiplied by the current U.S. population under 18. We used state-by-state incidence of pediatric asthma from the U.S. Centers for Disease Control and Prevention from 2021 ( 73 ), the most recent date ...
A study published Friday suggests that around 50,000 current cases of pediatric asthma in the U.S. are linked to long-term exposure to nitrogen dioxide from gas and propane stoves.