
Powerpoint Templates
Icon Bundle
Kpi Dashboard
Professional
Business Plans
Swot Analysis
Gantt Chart
Business Proposal
Marketing Plan
Project Management
Business Case
Business Model
Cyber Security
Business PPT
Digital Marketing
Digital Transformation
Human Resources
Product Management
Artificial Intelligence
Company Profile
Acknowledgement PPT
PPT Presentation
Reports Brochures
One Page Pitch
Interview PPT
All Categories


Quality Management System Powerpoint Presentation Slides
A Quality Management System (QMS) is a framework that helps organizations to manage their quality assurance and quality control processes. Quality management systems are designed to help organizations to improve their performance and meet their customers' expectations. SlideTeam has put together a comprehensive package of quality assurance ppt templates to help you get up to speed on the basics of quality management. Our templates are easy to use and can be customized to fit your specific needs. Plus, they’re free to download so you can start using them right away! So get started today and see how our quality assurance ppt templates can help you improve your business processes.

- Add a user to your subscription for free
You must be logged in to download this presentation.
Do you want to remove this product from your favourites?
PowerPoint presentation slides
Presenting this set of slides with name - Quality Management System Powerpoint Presentation Slides.The stages in this process are Quality Management System, Quality Assurance System, Qms.

People who downloaded this PowerPoint presentation also viewed the following :
- Business Slides , IT , Flat Designs , Strategic Planning Analysis , Visuals and Illustrations , Complete Decks , All Decks , Testing and Validation , Operations , Technology Quality Assurance , Management , Quality Management
- Quality Management System ,
- Quality Assurance System ,
Content of this Powerpoint Presentation
Quality management system powerpoint presentation slides with all 61 slides:.
Build belief in being able to achieve with our Quality Management System Powerpoint Presentation Slides. They are good confidence boosters.

Ratings and Reviews
by Donovan Cunningham
July 19, 2021
by Chester Kim
July 18, 2021
by Oumaima Cherqui
March 24, 2020
by Khanh Le
March 15, 2020
by Sawsun Awan
January 4, 2020

- Quality management systems: An introduction
How do successful businesses thrive in our ever-more competitive world? Some are driven by a charismatic leader; others rely on the power of the collective. But there is one ingredient which, from corner store to corporate powerhouse, is essential for healthy long-term success. Quality.
That is why effective quality management is an imperative for any successful business today. In our age of innovation and rapidly shifting expectations, keeping pace with the times means committing to a journey of continuous improvement. And achieving this goal requires a foundation of sound quality management systems .
An effective quality management system (QMS) provides the means to consistently meet consumer expectations and deliver products and services with minimal waste. In today’s highly competitive global economy, having a QMS in place is the prerequisite for sustainable success.
Table of contents
What is a quality management system .
In the most simple terms, a quality management system is a clearly defined set of processes and responsibilities that makes your business run how it’s supposed to. Each organization tailors its own QMS, comprising a formal set of policies, processes and procedures established to elevate consumer satisfaction. A QMS guides organizations as they standardize and enhance quality controls across manufacturing, service delivery and other key business processes.
The core benefits of a QMS include:
- Elevated consistency and standardization of processes and outputs
- Reduced errors and increased operational efficiency
- Improved customer satisfaction through the delivery of quality products and services
- Continuous evaluation and improvement of organizational operations
What is a digital QMS?
A QMS can be delivered digitally rather than using paper checklists and forms. This saves organizations time, mitigates risk and minimizes the chance of human error. Implementing a digital QMS requires meticulous planning and execution, and needs to be designed to comply with relevant regulations and industry standards, incorporating robust digital security measures to protect data.
All of these approaches call for expert guidance.
Types of quality management systems
A QMS may be based on either domestic or international standards. Different QMSs respond to different needs and scenarios, and organizations can choose to implement just one, or integrate a blend of different approaches. Among the most common are:
- Standardized systems : These set the bar for established standards and agreed-upon codes and practices, such as certifications against ISO standards. ISO 9001 outlines requirements for a comprehensive QMS and provides guidance for organizations looking to implement or improve their quality management strategy.
- Total quality management (TQM) : TQM is a management philosophy centred on customer satisfaction through the active participation of every employee. Its goal is to support the continuous improvement of quality across all levels and business functions.
- Lean management : Inefficiencies can result in unnecessary waste. Lean management strives to maximize customer value while minimizing waste using tools like value stream mapping, which helps fine-tune an organization’s processes for optimum efficiency.
- Six Sigma : Although perfection is almost impossible to reach, the pursuit of it is still worthwhile. Six Sigma uses data-driven techniques in the pursuit of producing near-perfect products and services, with a defect rate of 3.4 per one million opportunities. While that’s not perfect, it is pretty close.
Sign up for email updates
Register to receive resources and updates on quality management and related standards.
Almost done! You are only one step away from joining the ISO subscriber list. Please confirm your subscription by clicking on the email we've just sent to you. You will not be registered until you confirm your subscription. If you can't find the email, kindly check your spam folder and/or the promotions tab (if you use Gmail).
To learn how your data will be used, please see our privacy notice .
Benefits of using a quality management system
There are numerous reasons to establish a QMS. Standardized processes improve efficiency and enhance productivity through the reduction, or even elimination, of redundancies and waste. Defect prevention reduces costs associated with reworking or scrapping.
QMS audits excel at recognizing potential problems before they occur, thereby significantly reducing risk. What’s more, a QMS streamlines the record-keeping process, with improved documentation facilitating traceability and accountability – and aiding in regulatory compliance . A QMS also functions as a troubleshooting process, providing performance metrics and built-in audits to uncover weaknesses, establishing a solid foundation for improvement.
Consistent quality leads to happy, satisfied customers who become informal brand ambassadors within their communities. So they create further business opportunities and the potential for increased market share. Any real-world example of a QMS will aptly demonstrate this: Companies who have built a successful quality system are more likely to achieve their business goals, driving higher-loyalty, frictionless customer journeys.
Why is a quality management system important?
Every organization wants to strive for excellence. Because, ultimately, the quality of a product or service is what the customer gets out of it and is willing to pay for. Quality management plays a crucial role in delivering a superior experience, which in turn influences a company’s growth and performance.
Here are six good reasons to consider investing in a quality management system:
- Brand reputation : This is priceless, of course. A brand is more likely to gain international recognition when an organization surpasses established quality benchmarks.
- Customer retention : Consistently meeting, or exceeding, customer needs and expectations fosters loyalty. When high standards are met or surpassed, why would customers go anywhere else?
- Business sustainability : Consistently delivering excellence ensures and maintains a steady supply of customers. Doing business sustainably, and producing minimal waste, is the best way to grow and future-proof an organization.
- Compliance : Meeting regulatory, safety and quality standards is a must and a QMS seamlessly facilitates this process.
- Competitive edge : Higher-quality products and services give businesses a competitive advantage in complex times.
- Staff engagement : Employees who feel they are involved in quality improvements tend to experience higher engagement and productivity.
Journey to excellence
Developing an effective quality management system doesn’t happen overnight, but requires careful planning and execution. So, what are some of the key steps to success for an organization starting out on its QMS journey?
- Secure leadership commitment : Building a QMS requires alignment at the executive level.
- Document processes : Identify and thoroughly document procedures associated with existing quality processes.
- Define metrics : Performance-tracking metrics should be determined to ensure they meet QMS requirements.
- Training : All employees will need initial and ongoing training in order to build understanding and engagement with the QMS.
- Audits : Regular self-audits on processes and procedures will ensure compliance and effective implementation.
- Review system performance : Regularly assess system performance in order to make improvements as needed.
It’s important to note that while the steps outlined above provide a high-level overview, building and sustaining an impactful QMS takes considerable effort and commitment across multiple areas of an organization.
ISO 9001 Quality management systems
The bottom line
In today’s competitive marketplace, maintaining high-quality standards is more crucial than ever. As a business owner, you’re aware that customers will continue coming if they know that you will deliver them the product or service they need. This calls for company processes that are reliable, effective, trustworthy and streamlined – aligning business objectives and bottom lines with consistency and excellence. While this may sound like a no-brainer, how do you ensure a formalized process that documents each step, the desired outcomes, ways to improve, and the end results?
A quality management system may be just the solution you’re looking for.
Add to cart

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
Quality Management System
Published by Ansgar Iversen Modified over 5 years ago
Similar presentations
Presentation on theme: "Quality Management System"— Presentation transcript:

Understanding the Requirements Qimpro Standards Organization

ISO 9001 : 2000.

EPSON STAMPING ISO REV 1 2/10/2000.

1 Quality Management Standards. 2 THE ISO 9000 FAMILY ISO 9000: 2005 Identifies the fundamentals and vocabulary for Quality Management Systems (QMS) ISO.

The ISO 9002 Quality Assurance Management System

Quality Management Dr. S.W. Poon. Quality Management Introduction Meaning of quality Quality Control (QC) Quality Assurance (QA) Differences between QC.

Quality Management.

Quality Management Systems

ISO 9000:2000 Quality system standards adopted in 1987 by International Organization for Standardization; revised in 1994 and 2000 Technical specifications.

ISO 9001:2000 Intro Presented By: Brad D. Agenda Overview of QMS Fundamentals ISO 9001:2000 Overview & Requirements.

ISO 9000 Certification ISO 9001 and ISO
4. Quality Management System (QMS)

ISO 9000 Implementation Imran Hussain.
ISO 9000 Introduction Imran Hussain.

Welcome ISO9001:2000 Foundation Workshop.

JENN SHAFNER BRIAN KROUSE CLINT KEHRES. Pre ISO 9000 The BS 5750 standard required factories to document manufacturing procedures. BS 5750 was known.

Fundamentals of ISO.

Eng R. L. Nkumbwa-2010 Copperbelt University 1 ISO Quality Management Systems.

An Overview of the International Quality Management Standard
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
Home PowerPoint Templates Quality Management System
Quality Management System Templates for PowerPoint & Google Slides
Download our 100% editable Quality Management System Template to make analysis more accessible, faster, and accurate. The QMS assists businesses in documenting their quality procedures. This template makes it easier to communicate the idea to your audience or employees by displaying the entire process at once.
These Quality Management System Presentations are professionally and creatively designed, utilizing all necessary PowerPoint elements such as icons, shapes, charts, graphs, colors, clip-arts, etc. Download one of these templates to enjoy creativity at its peak.
Taguchi’s Quality Loss Function Curve PowerPoint Template

PDCA PowerPoint Template
Muda 7 Types Of Waste PowerPoint Template
9 Steps Circular Diagram With Service Icons
Flat DMAIC PowerPoint Template
Capability Maturity Model PowerPoint Template

Six Sigma Slide Designs for PowerPoint
Quality Management System Circular Diagram for PowerPoint
A Quality Management System (QMS) is a system deployed by organizations to document the internal strategies, methods, and management. It helps develop and produce high-quality products or services for customers. We have several-made templates that can assist you in analyzing your company’s quality. The Muda 7 Types Of Waste PowerPoint Template is one of these visually appealing templates that can help you identify loops that waste company resources and provide solutions.
In addition, QMS assists a company in reaching the regulatory and consumer standards and constantly modifying its processes. Furthermore, you must prioritize quality as it is critical in any business. It represents the company’s integrity, ensuring buyers always buy your products. Quality contributes to increased brand loyalty. It even promotes word-of-mouth marketing, lowering the cost of other forms of marketing. These templates include everything you need to make your Quality Management System Presentation flawless and communicate effectively with your audience.
What Are the Advantages of Having a Quality Management System Template?
The following are some of the reasons why you should use the QMS template:
- It assists businesses in maintaining consistency in the production and delivery of their products and services.
- It aids in the identification and analysis of compliance issues.
- Internal audits benefit from QMS data.
- It educates employees about an organization’s integrity and increases collaboration to meet customer expectations.
- It reduces waste and lowers business costs.
- It assists the company in identifying errors and risks and provides a better way to prevent them.
- Our pre-made templates simplify the analysis of QMS data.
How Can You Tell if a Quality Management System Is Working?
There are two primary methods for determining a quality management system’s effectiveness.
Data Comparison means comparing data obtained before QMS implementation to data obtained following implementation. The comparison will reveal whether the procedure is effective or not. This analysis will also highlight areas that require further investigation.
In Qualitative Feedback , we recognize: that the primary goal of QMS is to assist an organization in meeting the needs of its customers. Create a channel for your customers to provide honest feedback; this will reveal how effective the QMS you implemented was. You can collect data by conducting surveys.
Download Unlimited Content
Our annual unlimited plan let you download unlimited content from slidemodel. save hours of manual work and use awesome slide designs in your next presentation..

Quality Management System: Implementation
Sep 18, 2012
2.18k likes | 5.7k Views
Quality Management System: Implementation. Objectives. To provide information on implementation of a quality management system including: An implementation plan Quality manager Document and records Policies, processes and procedures Quality system essentials Quality policy statement
Share Presentation
- incident accident
- appropriate document control procedures
- quality management system
- quality control
- analytical processes

Presentation Transcript
Objectives To provide information on implementation of a quality management system including: • An implementation plan • Quality manager • Document and records • Policies, processes and procedures • Quality system essentials • Quality policy statement • Quality manual • Continual quality improvement
Implementing a Quality Management System: Some Tips • QMP-LS does not dictate how a facility must approach the task of implementing processes or their QMS • Our program requirements lay out the basic elements of a quality management system • How a facility develops its QMS is up to facility management • OLA assesses the end result, not the manner in which your system was implemented • CLSI (NCCLS) outlines one approach in HS1-A and GP26-A • The following slides lay out a simplified version
Implementation of a Quality Management System 1. Get commitment from top management 2. Communicate your intent to all staff 3. Appoint a responsible individual (“quality manager”) 4. Review OLA requirements 5. Prepare an implementation plan 6. Prepare quality policy statement 7. Prepare quality manual: a compendium of policies* 8. Map processes as they are now * 9. Determine the gaps in processes and associated documentation * Note: Steps 7 and 8 can be reversed
Implementation of a QMS (continued) 10. Prioritize the redesign of processes 11. Plan and document the new processes 12. Verify the new processes 13. Plan and document the associated procedures 14. Communicate and train staff 15. Implement the processes 16. Measure the effectiveness of the processes 17. If problems are identified, modify the processes and go back to step 11
Quality Manager • An individual responsible for ensuring compliance with the quality management system; director retains ultimate responsibility • Can be either a full-time or part-time activity • Ensures the quality manual is kept up to date • Plans internal audits • Reports directly to the level of management where decisions are made on laboratory policy and resources
Define and Document What is Done • Take a systematic approach to address all operations in the path of workflow: pre-analytical, analytical and post-analytical • This is an enormous task, and will not take less than a year • Define what you currently do, then move toward improvement • Laboratory management should set priorities based on which processes most directly affect outcomes to patient results, and improve those first • Input from the people actually doing the work is essential • Input from users of the service is essential • Process mapping is essential
Documents and Records: Definitions • Documents: any information that provides direction • Policies, processes, procedures • Specifications • Plans • Regulations and standards • Records: any information that produces evidence • Requisitions • Examination results and reports • Quality Control and EQA records • Incident/Accident reports
Documenting the System • There are three types of documents used to describe laboratory protocols: • Policies • Processes • Procedures • The means of documentation can be electronic or paper • The next ten slides describe policies, processes, and procedures in detail
Policies Processes Procedures Documenting the System
Policies • Statements describing what is done and why • Define goals, briefly state the intent and direction • If your laboratory chooses to follow the CLSI model, typically, there is one policy for each Quality System Essential as listed on slide #24 • The twelve QSE policies form the basis for the Quality Manual • There may be additional “operational” policies that require documentation • Refer to supporting processes, procedures or other documentation, i.e. a list of related documents should be included
Processes • A series of inter-related steps involved in an activity or examination that uses resources and is managed to transform inputs into outputs • Usually displayed as flowcharts and illustrate the path of workflow and who is responsible • They do not include step-by-step instructions for individuals wanting to know exactly how to do something • Example: Process for Specimen Reception and Accessioning (a flow chart to show the steps involved in specimen reception and accessioning, involves more than one individual, but does not give detailed work instructions)
Processes • CLSI (NCCLS) documents HS1-A and GP26-A have some excellent examples of processes in both table and flow chart formats. • Typical processes for a laboratory service include those that follow a test from the time a health-care professional decides the test is needed through to the receipt of the test report: test ordering, specimen collection, specimen transport, specimen receipt, specimen referral, testing, interpretation, reporting. That is, pre-analytical, analytical and post-analytical processes. • Note that half of these processes do not occur within the laboratory itself, but that the laboratory still has a responsibility to document them.
Processes • Beyond these obvious processes all of the 12 Quality System Essentials (QSE) listed on slide #24 require process mapping • Examples of all of these can be found in CLSI (NCCLS) HS1-A and GP26-A • Each of the quality system essentials requires one or more processes, most of which will be linked • The output of one process is often the input to the next • Rarely is there a process that is not linked to another
Mapping Processes: Step One Map the BASIC process: • Identify and map the primary process in your facility • Begin at the highest and simplest level possible: a simple flow chart that traces a laboratory examination from physician order to the reported result • Identify the input (physician order) and the output (reported result) and all of the major steps that transform the physician order into a reported reported result • Begin with the “AS IS” process, i.e. what you are doing right now. This will allow you to identify problems and revise the process • You need to know where you are at, before you can determine where you are going!
Mapping Processes: Step Two Map the processes down to greater detail • Map processes for each of the essential elements of a quality management system: these are discussed in greater detail in unit 5 • Determine the sequence and interaction of the processes • Usually the output from one process will form the input to the next, but the relationship may be far more complex in some cases • Clarify the start and stop of each process to avoid creeping beyond the boundaries of the process being defined • Identify the personnel position responsible for each step of a process • Be sure to involve these individuals when mapping • Remember to map the processes AS THEY ARE NOW to allow you to identify problems and improve the processes
Implementation of Processes Define the criteria for determining that the process is effective. • This involves setting goals for quality performance, for example: • The Westgard rules for the quality control of quantitative results • The criteria for an acceptable and an unacceptable specimen • Target turnaround times Ensure that there are adequate resources available to implement the process • A primary function of laboratory management is to ensure that there is adequate staff and that the staff have the materials they need to do the job right Train staff and implement the process. • Effective communication and training is the key to success
Procedures • A procedure is the written work instructions that specify a way to carry out an activity, examination or step in a process. It is a set of step-by-step instructions that each individual follows • Form the classical procedure manual • Example: Procedure for performing an ABO group (the step-by-step instructions for a technologist to follow when performing the test)
Procedures • These should be written so that any individual who has a role in a process has detailed instructions to follow. • There must be step-by-step instructions available for a good number of tasks beyond technical procedures, such as responding to complaints from users of the laboratory service, validating equipment before use, accessioning specimens, entry of results into a laboratory information system. • The process flowcharts will prove to be invaluable tools to help pinpoint activities for which written procedures aremissing. • CLSI document GP2—A: Laboratory Documents: Development and Control is an excellent resource for procedure templates.
What is required for formatting? • Laboratories have the latitude to write their manuals in keeping with their own unique situation and scope of practice. • Laboratories are NOT required to revise manuals to conform to a specific template. • Assessors do not need to look for any particular format. • We do require that that appropriate document control procedures for review, identification, revision, etc. as described in section II.F (summarized on slide 21) are followed. • All analytical procedures must have the elements as described in program requirement VI.3. • The templates provided in CLSI GP26-A, and GP2-A meet our expectations and are an excellent resource.
Identification of Documents All quality management system documents must have an identification in the form of a header or footer that contains the following information: • Date of issue • Edition and/or current revision date and/or revision number • The number of pages within • Authority for issue • An electronic identification, if applicable
Document and Record Control • All documents must be approved prior to use • Changes to documents must be approved by authorized personnel • May be on any appropriate medium • Available at all locations • Reviewed periodically • For obsolete documents • Remove promptly or assure against inadvertent use • Retain for a defined length of time • Identify the document as obsolete
Documentation Hint • Since a major goal of implementing a QMS is CONTINUAL IMPROVEMENT, be sure that the system documenting policies, processes, and procedures can be easily modified by authorized personnel. • The expectation is that laboratory management will respond to opportunities for improvements, possibly by modifying protocols.
Organization Personnel Equipment Purchasing and inventory Process control Documents and records Information management Investigation of non-conformities Assessment Process improvement Service and satisfaction Facilities and safety The Quality System Essentials (QSE) • The QMS must encompass all management activities and processes relating to quality assurance. • These can be categorized into twelve essentials:
Quality Policy Statement • A document that defines the overall intentions and direction of the quality management system • Demonstrates the facility’s commitment to quality with clear leadership by top management • Leaders shape the culture of the lab: their commitment is the key to success
QMP-LS Quality Policy Statement • QMP-LS commits to maintain a quality management system to support high quality laboratory accreditation, external quality assessment and education services that: • Meet all clients’ needs; applicable regulatory, statutory and contractual requirements; and relevant national/international standards. • Evaluates and continually improves the effectiveness of the services provided. • Ensures this policy is communicated to and understood by all employees. • Provides a process for establishment, review and modification of quality objectives. • Reviews and modifies this policy for continued suitability.
Quality Manual • A compendium of policies to describe the laboratory’s quality management system • Format can vary, usually will be contained in a single volume • Can be electronic or paper • Must be easy to update • All personnel given instructions on its use • Maintained and reviewed by the quality manager
Quality Manual • Includes a description of the laboratory • Includes quality policy statement • Defines policies of the laboratory • Makes references to supporting processes and procedures, but does not include them • If a policy is documented and included in the quality manual, it must reflect actual laboratory practice • Validation of policies will be sought during the assessment visit
Quality Manual Review • Prior to each assessment visit, laboratories are asked to submit the results of a self-assessment of their quality manual. • Self-assessment form includes an evaluation of the physical aspects of the manual (i.e. table of contents, introduction, glossary, identification). • In addition, the form provides a pre-assessment of the facility’s policies as defined by the requirements and serves as a guide for assessors to locate laboratory documents when on-site
Continual Quality Improvement • Once your Quality Management system is fully documented, do not expect it to stay static for long. • The next phase is continual improvement through the use of quality indicators, internal auditing, investigation of non-conformities, and management review. • This is a primary objective of the QMS. • Continual improvement is a never-ending loop wherein a facility : • plans for a policy/process/ procedure (PLAN); • communicates it and implements it (DO); • monitors it to see if it works (CHECK) and • if a problem is identified, formulates a new or revised plan (ACT).
Continual Quality Improvement
Analysis of Indicators • Term used to describe ways of measuring how well the quality system is working. • The analysis of daily quality control charts is the indicator most familiar to laboratory personnel, but there are measures of the pre- and post-analytical phases as well. • OLA does not dictate the use of any specific indicators. • Facilities are expected to choose indicators to reflect critical issues to outcome, or problematic issues. • Facilities should include all aspects of the service, pre-analytical, analytical and post-analytical, inpatients and outpatients, referral labs, point-of-care tests
Analysis of Indicators • Analysis is conducted by comparing against a “benchmark” where available • Lab’s own experience • Practice guidelines • Published references • Trend analysis • Remember that something must be done with the data gathered: identify opportunities for improvement and formulate an action plan.
Analysis of Indicators • Examples of pre-analytic indicators: • Specimen transport times • Wrist band checking • Quality of phlebotomy • Specimen acceptability rates • Examples of analytic indicators: • QC and EQA results • Computer downtimes • Correlation of tests e.g. POCT vs lab based results • Examples of post-analytic indicators: • Critical value reporting • Reflexive testing • Turnaround times • Reporting errors
Non-conformities • Non-fulfillment of any requirement in the performance of a laboratory examination or test. Identified from many sources, including audits, quality control, staff comments and clinician complaints. • Instances where something has gone wrong in a process. • Laboratories are expected to keep records of non-conformities and corrective actions taken. • Non-conformance reports are the biggest and best source of information for continual improvement.
Internal Audits • Recommend conducting once per year • Planned by the quality manager • Personnel should not audit their own work • Reviewed by management • Corrective or preventive actions identified, documented, and measured
Management Review • Recommend that the quality management system documentation be reviewed and signed periodically by laboratory management. • Review: reports from managerial/supervisory personnel, internal audits, external assessments, non-conformity reports, indicator analyses, feedback, etc. • Recommend that action plans for improvement are appropriately identified, developed, implemented and monitored.
Final Comments • Everyone is responsible for ensuring that the protocols of the QMS are followed. • This includes top management, directors, managers, MLTs, laboratory assistants and clerical staff. • All personnel need to be aware of what is expected of them as individuals. • It is NOT ENOUGH just to write down a series of protocols—they must be followed and they must be proven to WORK. • Each laboratory should be able to provide evidence that its processes are effective in meeting the goals defined in their policies. • It is up to the laboratory to define what records and documentation are necessary to provide this supporting evidence.
Self-Assessment
Question 1 Which of the following statements is correct? • A process states the goals of the facility. • Processes do not include step-by-step instructions. • A process only involves one individual. • Processes should always be discrete and never linked.
Question 1 The correct answer to question 1 is b) Processes do not include step-by-step instructions, these are PROCEDURES.
Question 2 Which of the statements regarding procedure manuals is correct? • Laboratories have the latitude to write their manuals in keeping with their own unique situation and scope of practice. • Laboratories are required to revise manuals to conform to the templates in CLSI GP2-A • Assessors must ensure that all manuals conform to the QMP-LS format. • Procedure manuals do not fall under the rules for document control.
Question 2 The answer to question 2 is a) Laboratories have the latitude to write their manuals in keeping with their own unique situation and scope of practice. While GP2-A is an excellent resource for those looking for a template, we do not require every facility to revise their manuals to conform to it.
Question 3 A suitable plan for continual improvement can be summarized as: • CHECK PLAN DO ACT • DO ACT CHECK PLAN • PLAN DO CHECK ACT • PLAN ACT CHECK DO
Question 3 The answer to question 3 is c)
Question 4 To be in compliance with OLA program requirements, each facility must designate a full-time “Quality Manager” • TRUE • FALSE
Question 4 The correct answer to question 4 is b) FALSE The responsibility for quality management must be assigned to an individual (quality manager or otherwise titled), but this can be either a full-time or part-time activity given in addition to other responsibilities.
Question 5 Which of the following statements is incorrect? • OLA assesses the manner in which each facility implemented their QMS. • Policies define goals and briefly state the intent and direction of the facility. • There must be step-by-step instructions for tasks other than technical procedures in the analytical phase. • The quality manual makes references to supporting processes and procedures, but does not include them.
Question 5 The answer to question 5 is a). OLA assesses the end result, not the manner in which a facility’s QMS was implemented.
Additional Optional Resources • ISO 9000:2005. Quality management systems—Fundamentals and vocabulary. • ISO 9001:2000. Quality management systems—Requirements. • ISO 9004-2000. Quality management systems—Guidelines for performance improvements • ISO 17025. General Requirements for the Competence of Testing and Calibration Laboratories.
- More by User

Chapter Three Quality Management
Chapter Three Quality Management “Implementation of the quality principles in your organization requires time; however, the end result will bring customer loyalty, satisfaction, increased market share, and financial contribution.”
1.02k views • 46 slides

Developing a Quality Management System for the Latvian Health Inspectorate
Developing a Quality Management System for the Latvian Health Inspectorate. Health inspectorate of Latvia Head of Quality Division Evija Jekabsone [email protected]. Agenda. Introduction Organization Profile Situation before the solution The implementation project Results
823 views • 30 slides

EFFORTS IN THE HUNGARIAN VET SYSTEM FOR THE IMPLEMENTATION OF QUALITY ASSURANCE
EFFORTS IN THE HUNGARIAN VET SYSTEM FOR THE IMPLEMENTATION OF QUALITY ASSURANCE. Dr. Magdolna Benke National Institute for Vocational and Adult Education INAP Conference, September 17-18, 2009 Turin. I. Actual situation: parallel employment of different systems .
352 views • 18 slides

Quality Management Principles Become CEO Management Practices
2. A Quality System versus a Management System. The management system of a company should be determined strategically and must meet the company's business objectives. The ISO standards and guides can be used to accomplish strategic business objectives, to create a quality management system that fit
645 views • 34 slides

Chapter 12: File System Implementation
Chapter 12: File System Implementation. File System Structure File System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery Log-Structured File Systems NFS. File-System Structure. File structure Logical storage unit
720 views • 47 slides

Chapter 7. Operations Quality Management. World- Class Service. Overview. Nature of Quality Traditional Quality Management Modern Quality Management Quality Management Recognition Total Quality Management (TQM) Programs Quality Management in Services
689 views • 42 slides

Catalog Management Implementation
West Virginia University’s. Catalog Management Implementation. Contents. Introduction Implementation Workflow Access Training DegreeWorks Lessons Learned. Introduction. Moved from PDF catalog on Web to online Catalog Management System during 2011-2012
322 views • 12 slides


10 :File-System Implementation
10 :File-System Implementation. File-System Structure Allocation Methods Free-Space Management Directory Implementation Efficiency and Performance Recovery. File-System Structure. File structure Logical storage unit Collection of related information
324 views • 20 slides

Implementation of a Risk Management System by Cameroon Customs
Implementation of a Risk Management System by Cameroon Customs. Prepared by: Gasper KONNEH NEBA Inspector of Customs 10 July 2012. Outline . Background Implementation issues Benefits Some challenges Some lessons learned. Background: motivation for the reform .
300 views • 8 slides

Essentials of Quality and Quality Management
Essentials of Quality and Quality Management. Jeroen van Esch Deputy Area Quality Manager Cap Gemini SBA 4. Contents. Generic. Part I : Quality in general Quality, Quality system, Quality management Part II : Quality and business needs Part III : Principles for a quality system
805 views • 33 slides

Chapter 12: File System Implementation. Chapter 12: File System Implementation. File-System Structure File-System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery NFS Example: WAFL File System. Objectives.
809 views • 65 slides

Chapter 12: File System Implementation. File System Structure File System Implementation Directory Implementation Allocation Methods Free-Space Management Unix File System, Inodes, etc. Moving-Head Disk Mechanism. Disk Addressing.
381 views • 21 slides
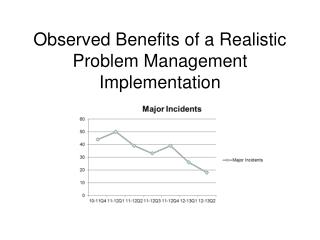
Observed Benefits of a Realistic Problem Management Implementation
Observed Benefits of a Realistic Problem Management Implementation. Presenter – Harry Sachs. 29 years IT industry experience: Software Development System Management Network Management System Performance, Capacity and Modeling System Architecture Problem Management. G o a l.
294 views • 16 slides

How to Measure Data Quality
How to Measure Data Quality. Data Quality Management System (DQMS) Self Assessment Product Inspection & KPIs Continuous Improvement. “Quality is a measurable, manageable business issue” John Guaspari. How to Measure Data Quality Key Elements of DQF. Data Quality Management System
1.05k views • 34 slides

Lecture 19: File System Implementation (Ch 11)
Lecture 19: File System Implementation (Ch 11). Chapter 11: File System Implementation. File-System Structure File-System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery NFS Example: WAFL File System.
812 views • 58 slides

767 views • 63 slides

Chapter 11: File System Implementation
Chapter 11: File System Implementation. Chapter 11: File System Implementation. File-System Structure File-System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery NFS Example: WAFL File System. Objectives.
810 views • 63 slides

665 views • 51 slides

File System Implementation
File System Implementation. File System Implementation. File System Structure File System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery Log-Structured File Systems NFS. File-System Structure. File structure
575 views • 41 slides

Implementing a Quality Management System
Implementing a Quality Management System. A Financial Perspective By Cecil D. White. World Quality Day Symposium – Nov. 10, 2011. Presentation Topics. 1. Defining the QMS?. 2. The benefits?. 3. The Costs?. 4. Is it worth it?. What is a Quality Management System?. P olicies,
892 views • 54 slides

Chapter 12 File-System Implementation
Chapter 12 File-System Implementation. Outline. File System Structure File System Implementation Directory Implementation Allocation Methods Free-Space Management Efficiency and Performance Recovery Log-Structured File Systems NFS. File-System Structure. File structure
633 views • 48 slides
Create an account
Create a free IEA account to download our reports or subcribe to a paid service.
News and commentaries
- Toggle filter China (16)
- Toggle filter India (16)
- Toggle filter United States (11)
- Toggle filter Brazil (4)
- Toggle filter Ukraine (3)
- Toggle filter Democratic Republic of the Congo (2)
- Toggle filter Georgia (2)
- Toggle filter Russia (2)
- Toggle filter Bangladesh (1)
- Toggle filter Cambodia (1)
- Toggle filter Africa (1)
- Toggle filter Middle East (1)
- Toggle filter Oil (18)
- Toggle filter Electric vehicles (12)
- Toggle filter Wind (11)
- Toggle filter Hydrogen (10)
- Toggle filter Coal (6)
- Toggle filter Demand response (6)
- Toggle filter Bioenergy (5)
- Toggle filter Heat pumps (3)
- Toggle filter Heating (3)
- Toggle filter Methane abatement (3)
- Toggle filter Electricity (6)
- Toggle filter Carbon Capture, Utilisation and Storage (5)
- Toggle filter Fossil Fuels (5)
- Toggle filter Buildings (3)
- Toggle filter Industry (2)
- Toggle filter Energy Efficiency and Demand (1)
- Toggle filter Renewables (1)
- Toggle filter Transport (1)
- Toggle filter Climate Change (44)
- Toggle filter Investment (35)
- Toggle filter Energy Security (25)
- Toggle filter Covid-19 (23)
- Toggle filter Energy Efficiency (21)
- Toggle filter Net Zero Emissions (20)
- Toggle filter Energy Access (18)
- Toggle filter Russia's War on Ukraine (13)
- Toggle filter Energy and Water (11)
- Toggle filter Renewable Integration (10)
- Toggle filter Clean Energy Transitions Programme (40)
- Toggle filter Digital Demand-Driven Electricity Networks Initiative (9)
- Toggle filter People-Centred Clean Energy Transitions (5)
- Toggle filter Clean Energy Transitions in Emerging Economies (3)
- Toggle filter Electric Vehicles Initiative (2)
- Toggle filter Energy Efficiency in Emerging Economies (2)
- Toggle filter Global Commission for Urgent Action on Energy Efficiency (2)
- Toggle filter Technology Collaboration Programme (2)
- Toggle filter CEM Hydrogen Initiative (1)
- Toggle filter Clean Energy Ministerial (1)
- Toggle filter News (936)
- Toggle filter Commentary (274)
- Toggle filter Press release (162)
- Toggle filter 2024 (15)
- Toggle filter 2023 (49)
- Toggle filter 2022 (15)
- Toggle filter 2021 (43)
- Toggle filter 2020 (50)
- Toggle filter 2019 (43)
- Toggle filter 2018 (33)
- Toggle filter 2017 (25)
- Toggle filter 2016 (1)
Regional cooperation key to tap into the North Sea’s hydrogen potential
Clean energy is boosting economic growth
Europe has taken its energy destiny back into its own hands
Oil demand growing at a slower pace as post-Covid rebound runs its course
More efficient and flexible buildings are key to clean energy transitions
Africa's electricity access planners turn to geospatial mapping
Energy is vital to a well-functioning water sector
It is time for CCUS to deliver
A strong focus on oil security will be critical throughout the clean energy transition
How access to clean cooking empowers women
Innovation in all its forms in spotlight at first Energy Innovation Forum at IEA Ministerial
India could triple its biofuel use and accelerate global deployment.
The relationship between growth in GDP and CO2 has loosened; it needs to be cut completely
The clean energy economy demands massive integration investments now
Russia’s attacks on Ukraine’s energy sector have escalated again as winter sets in
China’s petrochemical surge is driving global oil demand growth
Financial headwinds for renewables investors: what’s the way forward.
Natural gas is now stronger than ever in the United States power sector
What does COP28 need to do to keep 1.5 °C within reach? These are the IEA's five criteria for success
Cost of capital survey shows investments in solar pv can be less risky than gas power in emerging and developing economies, though values remain high.
Now is the time to climate-proof Europe’s economy
Data tool gives fine-grained view of climate vulnerabilities in the energy system and beyond
Setting the standard: How Central America is harmonising energy efficiency for appliances
Reaching net zero emissions demands faster innovation, but we’ve already come a long way
Subscription successful
Thank you for subscribing. You can unsubscribe at any time by clicking the link at the bottom of any IEA newsletter.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About Adverse Childhood Experiences
- Risk and Protective Factors
- Program: Essentials for Childhood: Preventing Adverse Childhood Experiences through Data to Action
- Adverse childhood experiences can have long-term impacts on health, opportunity and well-being.
- Adverse childhood experiences are common and some groups experience them more than others.

What are adverse childhood experiences?
Adverse childhood experiences, or ACEs, are potentially traumatic events that occur in childhood (0-17 years). Examples include: 1
- Experiencing violence, abuse, or neglect.
- Witnessing violence in the home or community.
- Having a family member attempt or die by suicide.
Also included are aspects of the child’s environment that can undermine their sense of safety, stability, and bonding. Examples can include growing up in a household with: 1
- Substance use problems.
- Mental health problems.
- Instability due to parental separation.
- Instability due to household members being in jail or prison.
The examples above are not a complete list of adverse experiences. Many other traumatic experiences could impact health and well-being. This can include not having enough food to eat, experiencing homelessness or unstable housing, or experiencing discrimination. 2 3 4 5 6
Quick facts and stats
ACEs are common. About 64% of adults in the United States reported they had experienced at least one type of ACE before age 18. Nearly one in six (17.3%) adults reported they had experienced four or more types of ACEs. 7
Preventing ACEs could potentially reduce many health conditions. Estimates show up to 1.9 million heart disease cases and 21 million depression cases potentially could have been avoided by preventing ACEs. 1
Some people are at greater risk of experiencing one or more ACEs than others. While all children are at risk of ACEs, numerous studies show inequities in such experiences. These inequalities are linked to the historical, social, and economic environments in which some families live. 5 6 ACEs were highest among females, non-Hispanic American Indian or Alaska Native adults, and adults who are unemployed or unable to work. 7
ACEs are costly. ACEs-related health consequences cost an estimated economic burden of $748 billion annually in Bermuda, Canada, and the United States. 8
ACEs can have lasting effects on health and well-being in childhood and life opportunities well into adulthood. 9 Life opportunities include things like education and job potential. These experiences can increase the risks of injury, sexually transmitted infections, and involvement in sex trafficking. They can also increase risks for maternal and child health problems including teen pregnancy, pregnancy complications, and fetal death. Also included are a range of chronic diseases and leading causes of death, such as cancer, diabetes, heart disease, and suicide. 1 10 11 12 13 14 15 16 17
ACEs and associated social determinants of health, such as living in under-resourced or racially segregated neighborhoods, can cause toxic stress. Toxic stress, or extended or prolonged stress, from ACEs can negatively affect children’s brain development, immune systems, and stress-response systems. These changes can affect children’s attention, decision-making, and learning. 18
Children growing up with toxic stress may have difficulty forming healthy and stable relationships. They may also have unstable work histories as adults and struggle with finances, jobs, and depression throughout life. 18 These effects can also be passed on to their own children. 19 20 21 Some children may face further exposure to toxic stress from historical and ongoing traumas. These historical and ongoing traumas refer to experiences of racial discrimination or the impacts of poverty resulting from limited educational and economic opportunities. 1 6
Adverse childhood experiences can be prevented. Certain factors may increase or decrease the risk of experiencing adverse childhood experiences.
Preventing adverse childhood experiences requires understanding and addressing the factors that put people at risk for or protect them from violence.
Creating safe, stable, nurturing relationships and environments for all children can prevent ACEs and help all children reach their full potential. We all have a role to play.
- Merrick MT, Ford DC, Ports KA, et al. Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention — 25 States, 2015–2017. MMWR Morb Mortal Wkly Rep 2019;68:999-1005. DOI: http://dx.doi.org/10.15585/mmwr.mm6844e1 .
- Cain KS, Meyer SC, Cummer E, Patel KK, Casacchia NJ, Montez K, Palakshappa D, Brown CL. Association of Food Insecurity with Mental Health Outcomes in Parents and Children. Science Direct. 2022; 22:7; 1105-1114. DOI: https://doi.org/10.1016/j.acap.2022.04.010 .
- Smith-Grant J, Kilmer G, Brener N, Robin L, Underwood M. Risk Behaviors and Experiences Among Youth Experiencing Homelessness—Youth Risk Behavior Survey, 23 U.S. States and 11 Local School Districts. Journal of Community Health. 2022; 47: 324-333.
- Experiencing discrimination: Early Childhood Adversity, Toxic Stress, and the Impacts of Racism on the Foundations of Health | Annual Review of Public Health https://doi.org/10.1146/annurev-publhealth-090419-101940 .
- Sedlak A, Mettenburg J, Basena M, et al. Fourth national incidence study of child abuse and neglect (NIS-4): Report to Congress. Executive Summary. Washington, DC: U.S. Department of Health an Human Services, Administration for Children and Families.; 2010.
- Font S, Maguire-Jack K. Pathways from childhood abuse and other adversities to adult health risks: The role of adult socioeconomic conditions. Child Abuse Negl. 2016;51:390-399.
- Swedo EA, Aslam MV, Dahlberg LL, et al. Prevalence of Adverse Childhood Experiences Among U.S. Adults — Behavioral Risk Factor Surveillance System, 2011–2020. MMWR Morb Mortal Wkly Rep 2023;72:707–715. DOI: http://dx.doi.org/10.15585/mmwr.mm7226a2 .
- Bellis, MA, et al. Life Course Health Consequences and Associated Annual Costs of Adverse Childhood Experiences Across Europe and North America: A Systematic Review and Meta-Analysis. Lancet Public Health 2019.
- Adverse Childhood Experiences During the COVID-19 Pandemic and Associations with Poor Mental Health and Suicidal Behaviors Among High School Students — Adolescent Behaviors and Experiences Survey, United States, January–June 2021 | MMWR
- Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics. 2004 Feb;113(2):320-7.
- Miller ES, Fleming O, Ekpe EE, Grobman WA, Heard-Garris N. Association Between Adverse Childhood Experiences and Adverse Pregnancy Outcomes. Obstetrics & Gynecology . 2021;138(5):770-776. https://doi.org/10.1097/AOG.0000000000004570 .
- Sulaiman S, Premji SS, Tavangar F, et al. Total Adverse Childhood Experiences and Preterm Birth: A Systematic Review. Matern Child Health J . 2021;25(10):1581-1594. https://doi.org/10.1007/s10995-021-03176-6 .
- Ciciolla L, Shreffler KM, Tiemeyer S. Maternal Childhood Adversity as a Risk for Perinatal Complications and NICU Hospitalization. Journal of Pediatric Psychology . 2021;46(7):801-813. https://doi.org/10.1093/jpepsy/jsab027 .
- Mersky JP, Lee CP. Adverse childhood experiences and poor birth outcomes in a diverse, low-income sample. BMC pregnancy and childbirth. 2019;19(1). https://doi.org/10.1186/s12884-019-2560-8 .
- Reid JA, Baglivio MT, Piquero AR, Greenwald MA, Epps N. No youth left behind to human trafficking: Exploring profiles of risk. American journal of orthopsychiatry. 2019;89(6):704.
- Diamond-Welch B, Kosloski AE. Adverse childhood experiences and propensity to participate in the commercialized sex market. Child Abuse & Neglect. 2020 Jun 1;104:104468.
- Shonkoff, J. P., Garner, A. S., Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, & Section on Developmental and Behavioral Pediatrics (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. https://doi.org/10.1542/peds.2011-2663
- Narayan AJ, Kalstabakken AW, Labella MH, Nerenberg LS, Monn AR, Masten AS. Intergenerational continuity of adverse childhood experiences in homeless families: unpacking exposure to maltreatment versus family dysfunction. Am J Orthopsych. 2017;87(1):3. https://doi.org/10.1037/ort0000133 .
- Schofield TJ, Donnellan MB, Merrick MT, Ports KA, Klevens J, Leeb R. Intergenerational continuity in adverse childhood experiences and rural community environments. Am J Public Health. 2018;108(9):1148-1152. https://doi.org/10.2105/AJPH.2018.304598 .
- Schofield TJ, Lee RD, Merrick MT. Safe, stable, nurturing relationships as a moderator of intergenerational continuity of child maltreatment: a meta-analysis. J Adolesc Health. 2013;53(4 Suppl):S32-38. https://doi.org/10.1016/j.jadohealth.2013.05.004 .
Adverse Childhood Experiences (ACEs)
ACEs can have a tremendous impact on lifelong health and opportunity. CDC works to understand ACEs and prevent them.

IMAGES
VIDEO
COMMENTS
A Quality Management System (QMS) is a framework that helps organizations to manage their quality assurance and quality control processes. Quality management systems are designed to help organizations to improve their performance and meet their customers' expectations. SlideTeam has put together a comprehensive package of quality assurance ppt ...
ISO 9001: 2015 QUALITY MANAGEMENT SYSTEMS. May 23, 2018 •. 7 likes • 7,900 views. Subhendu Datta. This presentation gives a bried overview of the various parts & purpose of the ISO 9001:2015 QMS. It revolves around the PDCA Cycle and useful in Manufacture & construction Industry. Read more. Leadership & Management.
A quality management system (QMS) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. A QMS helps coordinate and direct an organization's activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous ...
Quality management systems (QMS) Jul 19, 2017 • Download as PPTX, PDF •. 10 likes • 10,887 views. salman-fuu. Follow. A typical approach. Education. Download now. Quality management systems (QMS) - Download as a PDF or view online for free.
And achieving this goal requires a foundation of sound quality management systems. An effective quality management system (QMS) provides the means to consistently meet consumer expectations and deliver products and services with minimal waste. In today's highly competitive global economy, having a QMS in place is the prerequisite for ...
Dec 12, 2018 •. 6 likes • 6,548 views. SlideTeam. Follow. Presenting this set of slides with name - Quality Management System PowerPoint Presentation Slides.The stages in this process are Quality Management System, Quality Assurance System, Qms. Read more. Design. 1 of 61. Download now.
This is a quality management system essentials ppt infographics. This is a four stage process. The stages in this process are continual improvement, ethics, quality policy and strategy, quality assurance, training, management support, quality control, standard operating procedures. Slide 1 of 7.
ISO 9001 is officially titled "Quality management systems - requirements." It's intent is provided in Section 1.0 "Scope" (this statement in unchanged in the past three editions of ISO 9001) "This International Standard specifies requirements for a quality management system when an organization needs to demonstrate
This template covers normative requirements in ISO 9001, requirements or checklist for quality audit and statements about QMS. Deliver and pitch your topic in the best possible manner with this international standard for quality management system deployment of statements about qms slides pdf. Use them to share invaluable insights on deployment ...
This is a quality management system essentials ppt infographics. This is a four stage process. The stages in this process are continual improvement, ethics, quality policy and strategy, quality assurance, training, management support, quality control, standard operating procedures. Slide 1 of 7.
Quality is the cornerstone of excellence, the bedrock upon which success is built. Our beautiful Quality Management System (QMS) presentation template for MS PowerPoint and Google Slides is perfect for describing the set of policies, processes, and procedures that help businesses ensure consistent product or service quality.
Presentation on theme: "Quality Management Systems (QMS)"— Presentation transcript: 6 Quality Policy The overall intentions and directions of an organization related to quality as formally expressed by top management (ISO 9000) Clear statement of intent for each activity. Must be: The appropriate Involve everybody Out line organizational ...
A management system which follows the model - or "conforms to the standard" - is built on a firm foundation of state-of-the-art practices. 11 Quality Management System Understanding our QMS for the achievement of organization and business success. 2 Objectives of the Orientation. To learn the benefits of implementing the requirements of the ...
62 Thankyou. Download ppt "Quality Management System". Session Brief Introduction Quality Management Systems - Fundamentals & Vocabulary ISO 9000 : 2000 Quality Management System - Requirements Quality Management Systems - Guidelines for performance improvements Case Study of Gammon India Interaction Conclusion.
This template makes it easier to communicate the idea to your audience or employees by displaying the entire process at once. These Quality Management System Presentations are professionally and creatively designed, utilizing all necessary PowerPoint elements such as icons, shapes, charts, graphs, colors, clip-arts, etc. Download one of these ...
The schematic illustrates this concept: Quality Management System Issues to be considered when setting up a QMS include its: • Design • Build • Control • Deployment • Measurement • Review • Improvement. Quality Management System Quality Management Principles ISO 9000 contains eight quality management principles, upon which to base ...
Presentation Transcript. Implementation of a Quality Management System 1. Get commitment from top management 2. Communicate your intent to all staff 3. Appoint a responsible individual ("quality manager") 4. Review OLA requirements 5. Prepare an implementation plan 6. Prepare quality policy statement 7.
Presenting this set of slides with name - Quality Management System Powerpoint Presentation Slides.The stages in this process are Quality Management System, Quality Assurance System, Qms. https://bit.ly/2W3wMac. Read more. Business. 1 of 61. Download now.
This is a quality management system ppt powerpoint presentation complete deck with slides. This is a one stage process. The stages in this process are quality, management,, business, marketing, opportunity. Slide 1 of 72. Slide 1 of 72.
Qms. Dec 6, 2016 • Download as PPTX, PDF •. 165 likes • 92,046 views. HANU DADHWAL. Quality management system. Education. Download now. Qms - Download as a PDF or view online for free.
Natural gas is now stronger than ever in the United States power sector. 04 December 2023. COP28. What does COP28 need to do to keep 1.5 °C within reach? These are the IEA's five criteria for success. 30 November 2023. Investment.
Toxic stress, or extended or prolonged stress, from ACEs can negatively affect children's brain development, immune systems, and stress-response systems. These changes can affect children's attention, decision-making, and learning. 18. Children growing up with toxic stress may have difficulty forming healthy and stable relationships.