- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
News releases.
Media Advisory
Tuesday, November 22, 2022
NIH establishes website for self-reporting COVID-19 test results

Reporting a positive or negative test result just became easier through a new website from the National Institutes of Health. MakeMyTestCount.org, developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, allows users to anonymously report the results of any brand of at-home COVID-19 test.
COVID-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid COVID-19 test has become commonplace, test results are not often reported. COVID-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.
The National Institute of Biomedical Imaging and Bioengineering (NIBIB) supported development of MakeMyTestCount.org through the RADx Tech program.
NIBIB Director Bruce Tromberg, Ph.D., who leads the RADx Tech program, is available for comment.
About the Rapid Acceleration of Diagnostics (RADx ® ) initiative: The RADx initiative was launched on April 29, 2020, to speed innovation in the development, commercialization, and implementation of technologies for COVID-19 testing. The initiative has four programs: RADx Tech, RADx Advanced Technology Platforms, RADx Underserved Populations and RADx Radical. It leverages the existing NIH Point-of-Care Technology Research Network. The RADx initiative partners with federal agencies, including the Office of the Assistant Secretary of Health, Department of Defense, the Biomedical Advanced Research and Development Authority, and U.S. Food and Drug Administration. Learn more about the RADx initiative and its programs .
About the National Institute of Biomedical Imaging and Bioengineering (NIBIB): NIBIB’s mission is to improve health by leading the development and accelerating the application of biomedical technologies. The Institute is committed to integrating the physical and engineering sciences with the life sciences to advance basic research and medical care. NIBIB supports emerging technology research and development within its internal laboratories and through grants, collaborations, and training. More information is available at the NIBIB website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
Advertisement
Reporting Home Covid Test Results Can Be Confusing. Here’s How to Do It.
In many places, there is no system for sharing home test results with health officials, but the information may still be beneficial for public health.
- Share full article

By Knvul Sheikh
- June 4, 2022
So, you got a positive result on your home test for Covid-19. What do you do next? In addition to everything else on your plate — isolating and notifying close contacts, taking time off work and rescheduling appointments — it’s good practice to report your test results.
And while home tests have made it easier and faster to screen yourself and get treatment, it is not always easy to report an illness. This confusion has meant that many cases are left out of official counts.
If you test positive at a clinic or another community testing site, those results must be reported to public health departments under the CARES Act. Some home tests taken under the supervision of a trained telehealth provider are also reported to government health officials. But if your rapid test doesn’t fall into one of these categories, it can be unclear what to do.
The Centers for Disease Control and Prevention “ strongly encourages ” everyone who self-tests to report their positive results to a health care provider, who may order a P.C.R. test or otherwise report the data to state authorities. But only a few state health departments, including those in Colorado and Washington, collect data from home tests. Others, like in Massachusetts and New York, allow individual county health departments to decide whether they want to collect home test results.
The result is that official case counts are becoming an increasingly unreliable measure of the virus’s true toll. In New York City, for example, at the height of the Omicron wave, officials logged more than 538,000 new cases from January to mid-March. But a survey of New York City adults indicated that there could have been more than 1.3 million additional cases that were never detected or never reported during that time.
Health experts are concerned that public reporting of home testing is too sporadic and unpredictable.
“We do need a better sense of the amount of Covid in the community as people and organizations try to plan their behavior,” said Dr. Robert Wachter, the chair of the medicine department at the University of California, San Francisco. “When I’m deciding whether to eat indoors, for example, I don’t care about hospitalization numbers. I want to know the chance that my waiter or table-mate has Covid.”
But reporting relies on people being able to access home tests in the first place, which may put people in already underserved communities at a disadvantage. Data published by the C.D.C. in April suggested that home testing was most common among people who were young, white, highly educated and wealthy .
Even among people who have access to home tests, some may be nervous about volunteering personal information to local health authorities, said John Brownstein, an epidemiologist and chief innovation officer at Boston Children’s Hospital who led the recent C.D.C. research on home test use. Others may be too sick or overwhelmed to deal with the administrative burden of calling, emailing or otherwise figuring out how and when to report their test results.
Developing standardized, easy reporting systems for home tests could help solve part of the problem, Dr. Brownstein said. And information gleaned from home tests could allow researchers to calculate a better Covid index even if there isn’t uniform participation from the public.
“You don’t need every single person reporting their home test in order for the data to be valuable,” Dr. Brownstein said. “You have to understand how representative the sample is and make appropriate adjustments.” By combining test positivity data with other indicators, such as imputed case rates, wastewater information and community demographics, scientists can better understand how virus transmission is changing and assist with continued prevention efforts, he said.
Given how important it is to track case counts, here are four easy ways you can report a positive home test result.
Use a test’s mobile app.
Some rapid test kits, like the BinaxNOW, iHealth and Lucira kits, include a way to report your results through a mobile app, which usually also has instructional videos for using the tests.
Last year, a pilot program run by the C.D.C. and the National Institutes of Health distributed more than 1.4 million home tests to households in Tennessee and Michigan and found that, while overall test reporting was low and fewer than 10,000 test results were recorded in companion apps, those who used the apps were more likely to report their test results to public health authorities in both states. About 75 percent of the app users in Tennessee reported results and 84 percent in Michigan reported theirs.
Share results with your doctor.
If you test positive at home, another way to report your result is to contact your primary care provider, which is what the C.D.C. recommends you do.
When calling or emailing your doctor, make sure you’re ready to share a few key details: the kind of test you took, the time you took it, the date you started experiencing symptoms and your vaccination status.
Your doctor may recommend taking a P.C.R. test for confirmation, and can provide a medical report to help you take time off from work or school. Your doctor can also help you track new or concerning symptoms , give advice about antiviral treatments and clear you to return to work once you have fully recovered.
Contact your local health department.
Many local public health departments have ways for people to report their results online, though their methods for obtaining test data often vary from region to region. You can find your health department’s website and information through the National Association of County and City Health Officials directory .
Some reporting methods are straightforward, like the one in Marin County , Calif., which has a simple online form for reporting results. Residents of St. Louis County, Mo., can call in, email or submit their results online . In Washington, D.C., you can use an iPhone or Android app, in addition to the Department of Health’s self-reporting web portal .
Other health department websites are notoriously confusing to navigate or even understand. New York State’s Covid-19 resource page, for example, says that residents are not required to report their test results. A representative for the Department of Health said that this was because New York had used only results from laboratories or official testing providers “to analyze trends and report consistent data to the public” since the beginning of the pandemic. But some counties in New York, like Albany County and Tompkins County , allow reporting of home test results, which is separate from the data the state collects.
Participate in crowdsourcing.
Although a national home test surveillance website does not exist, researchers from Dr. Brownstein’s group have developed a platform for crowdsourcing home test results called OutbreaksNearMe.org that is fairly intuitive to use.
Originally designed to track flu outbreaks, the site has expanded to help create maps and analyze Covid-19 case data submitted by volunteers. The site shares information with the C.D.C. and local public health agencies and makes it available to the public, Dr. Brownstein said.
“Home tests represent a huge change in how quickly people can identify an infection, how quickly they can get care and access therapeutics,” Dr. Brownstein said. “They are going to be a core way that health care is delivered in the future, so we need to make sure that we in public health keep up with testing data.”
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Should You Report Your COVID-19 Home Test Results to Your Local Health Department?
simplehappyart / Getty Images
Key Takeaways
- Cases of COVID-19 may be undercounted in the U.S. because lab tests are the most likely to be reported to health departments.
- Long waits for tests and results are making many people opt for home COVID tests instead.
- At-home results can be reported to public health by consumers, but don’t have to be.
Data from the Centers for Disease Control and Prevention (CDC) this week shows that the average number of new COVID-19 cases has increased to more than 700,000 per day, over 200,000 more than a week ago.
But assume that’s an undercount.
That’s because CDC case counts are generally based on COVID-19 tests done at a testing site, clinic, or doctor’s office. These tests are analyzed by a laboratory, and laboratories are required to share results with public health departments to help track the virus regionally.
But current wait times for both scheduling clinic tests and receiving the results are taking days rather than hours. This is in part because of the recent holiday season, but also because as cases surge, people want to know if they have the virus. As a result, many people are opting for rapid home tests—many with 15-minutes results—instead of the clinic-based tests.
Why Unreported Test Results Are a Problem
Unreported test results jeopardizes the accuracy and utility of the case counts being published by health departments, academic institutions, and the CDC.
“The less information on positive [cases] and spread [of the virus], the less we can advise the public,” Lori Freeman, CEO of the National Association of County and City Health Officials, told Verywell.
Consumers can but don’t have to report home tests, according to a spokesperson from the Food and Drug Administration (FDA). And even when they do, the at-home results are not regularly added into health department case counts, Marci Layton, MD, chief medical officer of the Council of State and Territorial Epidemiologists , told Verywell.
“The challenge [reporting your results] from a public health perspective is that tracking cases through test results is usually done by results that are verifiable,” Layton said. “Home tests have always been challenging because there is no way for public health officials to verify that it was conducted correctly."
Public health experts understand that with the increase in home tests, they are likely undercounting COVID-19 cases by relying solely on laboratory-analyzed tests. In many cases, they are shifting to other measures to track the virus.
“We’re moving toward following trends, such as hospital and emergency room admissions and intensive care unit (ICU) and ventilator use," Layton said. “We are missing numbers on asymptomatic and mild cases by not having much data on home tests, but the data on severe cases is needed more.”
How to Report Your At-Home Test Results
In spite of the hazy accuracy, most public health personnel encourage you to submit your at-home test results to your local and/or state government.
“Public health, of course, would like to have the home test data,” Michael Fraser, PhD , CEO of the Association of State and Territorial Health Officers, told Verywell. “It would help us understand how quickly and where the virus is spreading. For now, we’re making assumptions.”
Some health departments are asking consumers for home test results. Summit County, Ohio, for example, offers an online form for reporting positive home tests. The same goes for Marin County in California. In Washington, D.C. , you can report results through an app. Still, other health departments ask test-takers to call in with their results.
You can find out how to contact your local health department by calling 311 and your state health department by calling 211.
Freeman suggests asking if your specific health department wants your home test results, “so you don’t overwhelm already busy health departments.”
Depending on the home test you take, your results may automatically be sent to local health authorities, especially if the test notifies you of your COVID status through a website or app.
According to the FDA, all home COVID-19 antigen tests must create a mechanism for consumers to report their results to the company, whether that's via an app, website, or phone call.
While the manufacturers must report any results they receive to health departments, consumers are not required to report their results to the manufacturers. But there may be advantages to doing so, Layton says. Many companies reply to positive results with up-to-date guidance on steps to take if you test positive and precautions if you test negative.
“Whether or not you do contact your health department [or test manufacturer] with the news from your test, the most important thing you can do with your test result is to follow CDC guidance,” Layton said.
The CDC has shortened its isolation guidance for people with COVID-19 from 10 days to five days without requiring an additional test.
Expect more information on what to do with a home test result if you test positive, especially since the FDA authorized two brand new home tests at the end of 2021, and the White House plans to send out at least half a million home test kits later this month.
"It's our hope that as we move forward, data collection and sharing will become much more consistent," Freeman said.
What This Means For You
If your home test sends you test results through a phone app or website, they should also send the results to public health departments, in which case you don't need to do anything further. Otherwise, you can find the number of your local health department by calling 311 and asking how to submit the information.
The information in this article is current as of the date listed, which means newer information may be available when you read this. For the most recent updates on COVID-19, visit our coronavirus news page .
By Fran Kritz Kritz is a healthcare reporter with a focus on health policy. She is a former staff writer for Forbes Magazine and U.S. News and World Report.

Report Covid-19 Home Test Results Through New NIH Website

Reporting a positive or negative test result just became easier through a new website from NIH, MakeMyTestCount.org . Developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, the new website allows users to anonymously report the results of any brand of at-home Covid-19 test.
Covid-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid Covid-19 test has become commonplace, test results are not often reported. Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
How to Report COVID-19 Laboratory Data
Summary of recent changes.
- Effective May 11, 2023, HHS announced the Public Health Emergency declaration for COVID-19 has ended. In addition, HHS and CLIA regulatory requirements under the CARES Act Authority requiring reporting of laboratory test data to the federal government has also ended. State-specific reporting requirements may still apply. Laboratories and testing sites should review state-specific reporting requirements for COVID-19 testing. They may be required to continue reporting for COVID-19 testing to their State Health Departments.
Who can report
What to report.
- Using Standard Terminology
- Assistance with Electronic Reporting
All COVID-19 testing sites must
- have a Clinical Laboratory Improvement Amendments (CLIA) certificate,
- meet all requirements to perform testing, including only using FDA-authorized test systems according to their instructions for use, and
- follow all state specific-reporting requirements for COVID-19 test results.
COVID-19 testing sites are defined as
- laboratories that perform clinical diagnostic or screening testing under CLIA,
- non-laboratory COVID-19 diagnostic or screening testing locations, and
- other facilities or locations offering COVID-19 point-of-care diagnostic or screening tests, or in-home diagnostic or screening tests.
CMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN). This CDC- and CMS-preferred pathway to submit data to CDC’s NHSN applies only to CMS-certified long-term care facilities. Test data submitted to NHSN will be reported to appropriate state and local health departments using standard electronic laboratory messages. Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any.
Testing sites that perform COVID-19 surveillance testing on de-identified samples, regardless of their CLIA status, should not report the results of their surveillance testing to state, tribal, local, and territorial public health departments. If at any time a facility intends to report a patient-specific test result, it must first obtain a CLIA certificate and meet all requirements to perform testing. For more information, see the Center for Medicare and Medicaid Service’s (CMS) Research Testing and Clinical Laboratory Improvement Amendments of 1988 (CLIA) Regulations .
NOTE regarding self-test results : While there are no current mechanisms that require reporting of self-test results to public health authorities, CDC encourages everyone who uses a self-test to report any positive results to their healthcare provider. Healthcare providers can ensure that those who have tested positive for COVID-19 receive the most appropriate medical care, including specific treatments if necessary. COVID-19 home tests can be safely and privately reported at MakeMyTestCount.org .
Association of Public Health Laboratories (APHL), in collaboration with the Council of State and Territorial Epidemiologists (CSTE), CDC, and other public and private partners, has developed these CSTE tools to assist laboratories with reporting.
How to report
Laboratory data elements may be reported in the following ways:
- Submit laboratory testing data directly to state or local public health departments according to state/or local law or policy. Data must be sent using existing reporting channels to ensure rapid initiation of case investigations, and concurrent reporting of results must be shared with the ordering provider or patient, as applicable.
- Submit laboratory testing data to state and local public health departments through a centralized platform, where the data will then be routed to the appropriate state and local authorities and may be routed to CDC after removal of personally identifiable information according to applicable rules and regulations.
- Submit laboratory testing data through a state or regional Health Information Exchange (HIE) to the appropriate state or local public health department.
- CMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN). This CDC- and CMS-preferred pathway to submit data to CDC’s NHSN applies only to CMS-certified long-term care facilities. Test data submitted to NHSN will be reported to appropriate state and local health departments using standard electronic laboratory messages. Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any.
Laboratories should make every reasonable effort to provide the following data elements when reporting to state and jurisdictional health departments.
- Test ordered – use harmonized LOINC codes provided by CDC
- Device Identifier
- Test result–use appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC
- Test Result date (date format)
- Accession # / Specimen ID
- Patient age
- Patient race
- Patient ethnicity
- Patient sex
- Patient residence zip code
- Patient residence county
- Ordering provider name and nonpharmaceutical interventions (as applicable)
- Ordering provider zip code
- Performing facility name and CLIA number
- Performing facility zip code
- Specimen Source – use appropriate LOINC, SNOMED-CT, or SPM4 codes, or equivalently detailed alternative codes
- Date test ordered (date format)
- Date specimen collected (date format)
The following additional demographic data elements should also be collected and reported to state or local public health departments.
- Patient name (Last name, First name, Middle Initial)
- Patient street address
- Patient phone number with area code
- Patient date of birth
- Ordering provider address
- Ordering provider phone number
To protect patient privacy, any data that state and jurisdictional health departments send to CDC will be deidentified and will not include some patient-level information. The deidentified data shared with CDC will contribute to understanding COVID-19’s impact, case rate positivity trends, testing coverage, and will help identify supply chain issues for reagents and other materials.
How to report using standard terminology
CDC has posted a LOINC In-Vitro Diagnostic (LIVD) Test Code Mapping Guide for COVID-19 test results for tests with emergency use authorization from the U.S. Food and Drug Administration (FDA) that can be used by clinical laboratories and instrument manufacturers. This specification supports the use of standardized LOINC and SNOMED Clinical Terms (CT) codes to improve the accuracy of reporting tests for the SARS-CoV-2 virus. Using these harmonized LOINC and SNOMED-CT codes helps ensure that the same type of test is represented uniformly across the United States.
For those COVID-19 tests that have not yet received FDA emergency use authorization, CDC encourages test developers and laboratories that use COVID-19 tests to work together to obtain appropriate and interoperable LOINC and SNOMED-CT codes for reporting purposes.
LOINC codes must be used to represent the “question” a test asks of a specimen (e.g., does this specimen have SARS-CoV-2 RNA?), and SNOMED-CT codes must be used to represent the diagnostic “answer” (e.g., what was detected?). More background on these terminology standards can be found here:
Whenever possible, laboratories must use standard codes that already exist. Before requesting a new code, search the list of currently available LOINC codes for COVID-19 tests. If a LOINC test code cannot be identified whose attributes appropriately match the test for which coding is needed, new terms can be submitted, and a new code can be requested through LOINC .
Technical assistance for electronic reporting
Electronic reporting options are available to reduce the burden on providers reporting test results. Laboratories that are not currently reporting electronically to their state or local health department and want assistance in establishing electronic reporting can contact [email protected].
Below is a list of COVID-19 resources for laboratories:
HHS Guidance
- HHS Laboratory Reporting Guidance
CDC Resources
- COVID-19 Information for Laboratories
- CDC’s Laboratory Outreach Communication System (LOCS)
- Clinical Laboratory COVID-19 Response Calls
Technical Implementation Resources
- Guidance for Encoding School Information for COVID-19 Public Health Reporting
- LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests
- COVID-19 Response | CSTE EMERGENCY PREPAREDNESS & RESPONSE
- Interoperability Standards Advisory for COVID-19 Pandemic
CMS Resources
- Interim Final Rule with Comments (IFC)
Frequently Asked Questions on Laboratory Data Reporting Guidance for COVID-19 Testing
Data reporting information.
No, facilities that conduct tests for individuals from multiple states must report results to the appropriate state or local health department based on the patient’s residence. If the patient’s address isn’t available, results should be reported based on the provider’s location.
Facilities that conduct tests for individuals who are temporarily living away from their permanent residence, such as students in college or active duty military personnel, should report to the state health department based on the individual’s temporary address near their college campus or military installation.
COVID-19 home tests can be safely and privately reported at MakeMyTestCount.org , but there is currently limited use for collecting self-test result data to inform public health surveillance. There are no current mechanisms that require reporting of self-test results to public health authorities. Voluntary reporting of self-test results will often be anonymous or lack data necessary for public health analysis or action. Therefore, the self-test results are unlikely to enhance understanding of trends in disease transmission or severity and often do not provide sufficient information to support case investigations. The public health community, including CDC, is confident that situational awareness remains strong without receiving self-test results. Find more information: About CDC COVID-19 Data.
Clinicians and laboratories should contact their state or local public health department directly for more information on reporting requirements and the method for reporting.
Yes, all data related to the AOE questions should be collected and reported to state and local public health departments in the electronic laboratory report messages.
Technical aspects of reporting
Yes, information about LOINC codes and the specific harmonized LOINC codes for COVID-19 tests can be found on CDC’s website: LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests .
CDC’s LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests website has a mapping catalogue coded for the data elements associated with COVID-19 tests, including the LOINC test order, LOINC test result, SNOMED-CT test description and SNOMED-CT specimen source. Test developers and manufacturers of new tests should contact [email protected] for information about obtaining new codes.
The Association of Public Health Laboratories (APHL) , in collaboration with the Council of State and Territorial Epidemiologists (CSTE) , CDC, and other public and private partners , ha ve developed the National ELR Flat File and HL7 Generator Tool to assist laboratories with reporting.
The DI for some tests can be found in the National Institute of Health’s (NIH) Access GUDID Database . For a specific DI not located in the Access GUDID Database, contact the device manufacturer to obtain the DI. If the manufacturer does not yet have the DI for the device you are using, contact [email protected] for assistance.
Clinical research trial and study reporting
In general, no. Laboratories are not responsible for reporting these data. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
The reporting requirements differ for laboratories and clinicians:
Laboratories
Laboratories are not responsible for reporting these data since they do not have the patient-identifying information required to comply with reporting requirements. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
In clinical trials or other clinical studies, clinicians who are responsible for clinical care of trial or study participants are responsible for linking de-identified specimen test results to participant demographic information and may be required to report the results to the appropriate local, tribal, or state public health department based on the patient’s residence.
If a clinician receives test results related to COVID-19 from duplicate specimens that were collected in the same manner and tested with different test methods (e.g., different platforms) or in different CLIA-certified laboratories, the clinician should not report both results. In the case of two positive test results, the clinician should report the result that is provided first. In the case of discrepant test results, the clinician should report the positive result. However, local, tribal, or state health department rules and regulations apply and may differ from this general guidance.
If the clinician requests testing related to COVID-19 for study participants independent of research activities or for clinical management, results should be reported to the appropriate local, tribal, or state public health department.
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Medical Device Safety
- Emergency Situations (Medical Devices)
- Coronavirus (COVID-19) and Medical Devices
Understanding At-Home OTC COVID-19 Antigen Diagnostic Test Results

At-home over-the-counter (OTC) COVID-19 antigen tests, often referred to as rapid tests, detect proteins called antigens from SARS-CoV-2, the virus that causes COVID-19. At-home COVID-19 antigen tests are less likely to detect the SARS-CoV-2 virus than molecular tests, such as polymerase chain reaction (PCR) tests and other nucleic acid amplification tests (NAATs), which detect genetic material called RNA from the virus. This is especially true early in an infection or in people who do not have COVID-19 symptoms. Currently, all at-home COVID-19 antigen tests that are FDA-authorized are authorized for repeat testing, also called serial testing. This means people who receive a negative test result should use multiple tests over a certain period, testing at least twice over three days if they have symptoms and at least three times over five days if they do not have symptoms.
On this page:
When you should test for covid-19, what your at-home otc covid-19 test result means, what at-home covid-19 antigen tests do not tell you, reporting your test result, step-by-step guide: when to test and what your at-home covid-19 antigen test results mean, understanding covid-19 infection and the risk of spreading the virus, related information.
- If you have symptoms , test immediately and then test again per the instructions if your first result is negative.
- If you were exposed to someone who has COVID-19 and you do not have symptoms, wait at least 5 full days after your exposure before testing. If you test too early, you may have an inaccurate result.
- If you are in certain high-risk settings , you may need to test as part of a screening testing program.
- Consider testing before coming into contact with someone who has a high risk for severe COVID-19 ; people who are older adults or immunocompromised, or have other medical conditions, especially if you are in an area with a medium or high COVID-19 Hospital Admission Level .
Most FDA-authorized at-home OTC COVID-19 tests are antigen tests. While not perfect, they provide a fast and convenient COVID-19 testing option to detect the virus, so you may know if you are infected and should stay at home and away from people to help reduce the spread of the virus.
- A positive result using an at-home COVID-19 antigen test means you likely have COVID-19. Anyone who tests positive for COVID-19, or who likely has COVID-19, should contact their health care provider and follow the CDC's guidelines for staying at home and away from people.
- A negative result using at-home COVID-19 antigen test means the test did not detect the virus that causes COVID-19, but it does not rule out COVID-19 because some tests may not detect the virus early in an infection. Always do a repeat test at minimum after 48 hours following a negative test result when using an antigen test. Use the following guidance to help interpret your negative test result and determine what steps to take next.
In addition to COVID-19 test results, and when determining the likelihood of having the virus, consider:
- Recent symptoms
- Close contact with someone who has COVID-19
- The level of COVID-19 in your community
See more information about negative test results from at-home COVID-19 antigen test and repeat testing below.
- If you have an infection immediately after you are exposed to COVID-19 because it may take 2 to 5 days, and sometimes longer, for the virus to be detected by a COVID-19 antigen test. How long it takes before a test can detect the virus may vary between different COVID-19 variants and different tests.
- How contagious you are or if you can spread the virus to someone else. There are no tests that can tell you that. You could pass COVID-19 to others even before you get a positive result on a test.
- If you have another type of respiratory illness, such as flu or respiratory syncytial virus ( RSV ), unless it is a test specifically authorized for detection of these viruses.
Watch: CDC | How To Interpret Self-Test Results
The FDA encourages you to voluntarily and anonymously report your positive or negative test results every time you use an at-home COVID-19 test. You can send your test result to MakeMyTestCount.org or use an app or other digital option for self-reporting that may be included with your test. Report each test result one time.
The data from MakeMyTestCount.org can help public health departments know how fast the virus is spreading. This valuable test data help public health departments assess and modify their response to COVID-19 in their local communities, states, or across the country. The MakeMyTestCount website is developed through the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx) Tech program and allows consumers to anonymously report their test results from OTC at-home COVID-19 test.
When you are exposed to the virus that causes COVID-19, it may get into your nose, throat, and lungs (which make up your respiratory tract) and cause an infection. You may never develop symptoms of an infection or you may not have symptoms for several days after you are exposed to the virus, despite being infected. You could still pass the virus to others, even if you do not feel sick.
The amount of virus in an infected person's body may vary in different people, as well as at different times during the infection. There may also be differences in whether, or how easily, the virus can spread to another person throughout the course of the infection. Generally, the amount of virus in a person will start low, increase, then decrease again as a result of the body's immune response. The pattern of this increase and decrease, as well as the level of virus, varies from person to person, and there is no known level above which you can spread the virus and below which you cannot.
To prevent spreading COVID-19 to others, always follow the CDC's recommendations .
- At-Home OTC COVID-19 Diagnostic Tests
- List of Authorized At-Home OTC COVID-19 Diagnostic Tests
- At-Home COVID-19 Diagnostic Tests: Frequently Asked Questions
- COVID-19 Test Basics : Includes details on COVID-19 tests, types of samples, and other information.
- At-Home COVID-19 Antigen Tests-Take Steps to Reduce Your Risk of False Negative: FDA Safety Communication
- Counterfeit At-Home OTC COVID-19 Diagnostic Tests
- Find COVID-19 Tests at COVID.gov/tests
- Video: How to Interpret Self-Test Results | CDC
- COVID-19 Testing: What You Need to Know | CDC
- COVID-19 Self-Testing At-Home or Anywhere | CDC
- What to Do If You Were Exposed to COVID-19 | CDC
- Isolation and Precautions for People with COVID-19 | CDC
- Report your test result at MakeMyTestCount.org
- Diagram: Interpreting Your Negative Test Results
- Video: What Is an EUA? Describes how the FDA can issue an emergency use authorization (EUA) to provide more timely access to diagnostic tests that may help during the public health emergency when there is no adequate, approved, and available alternative.
Subscribe to Consumer Information for Medical Devices
Receive notifications for consumers about medical device information, recently approved devices, and alerts that may be of interest to the general public.
Should I report my at-home COVID-19 test results?
- Search Search

With hundreds of millions of free rapid-result COVID-19 tests being mailed out to homes, a common question people are asking is: Am I required to report a positive result to local health authorities?
No, says the Centers for Disease Control and Prevention , but the federal agency recommends that people isolate and inform their health-care providers. Some states, such as Washington , have set up a reporting hotline, while local health departments such as Marin County , California, and Tompkins County , New York, have online self-reporting forms.
Washington, D.C.’s health department also has an iPhone feature and Android app.
But a newly released national survey of nearly 11,000 people finds that 31% of those who tested positive at home for the virus that causes COVID-19 did not follow up with a more accurate polymerase chain reaction (PCR) test at their doctor’s office or a testing facility, and thus are likely not captured in official data.

‘Younger people are engaging in more interactive behaviors, and they are at lower risk of dying if they get sick, but they are at higher risk of getting sick,’ says David Lazer, distinguished professor of political science and computer science. Photo by Adam Glanzman/Northeastern University
Researchers discovered that hours-long waits for testing coupled with the fact that some health-care facilities actually discouraged people from coming in are some of the causes for people not following through.
Still, wouldn’t it be better if the government had more accurate data on positive case counts? Yes, says David Lazer , university distinguished professor of political science and computer science at Northeastern, and one of the study’s authors, but the data was already messed up to begin with.
“Early in the pandemic, people who were sick couldn’t get tested at all, and there were a lot of asymptomatic people,” he says. “Better information is better for sure, but our data was already so screwed up.”
The fact that nearly 3 in 10 people didn’t report their positive results didn’t faze Lazer. “I was surprised that it wasn’t higher, honestly,” he says. For one thing, antigen tests tend to lean toward a false negative—meaning someone has the virus but the test didn’t pick it up. For peace of mind, some people may choose to visit a testing facility or a doctor.
“They end up testing positive at the doctor’s office and then they enter into the official system that way,” he says.
The U.S. study was conducted nationally by the Covid States Project, a collaborative effort by researchers from Northeastern, Harvard, Northwestern, and Rutgers universities The online poll lasted about a month, starting shortly after Christmas during the omicron surge.
Home antigen tests flew off pharmacy shelves as an alternative to standing in hours-long lines at local testing sites for PCR tests, results of which can come back from a lab in a day or two—sometimes longer.
Among those who did get a follow-up test, 88% received confirmation of a positive result while 12% got a negative result. Researchers noted in the study that they did not distinguish between antigen or PCR testing in the subsequent test, so they couldn’t estimate what proportion of the 12% might have indicated that the original test was a false positive.
Thus, it is possible that both results could be correct.
With the prevalence of skewed data, the study calculated that official counts may underestimate positive COVID-19 cases by about 6%, partially alleviating concerns that official case counts are only capturing the tip of the iceberg because of the growing prevalence of home testing.
“I expected that figure to be higher,” Lazer says. He thought that rapid tests would be more common than going to the doctor or a testing facility. In fact, antigen tests have been used less as a proportion of all tests because they’re expensive, he adds.
Hundreds of millions of free tests have begun heading out to people’s homes under a plan announced by President Joe Biden. The U.S. went from 24 million home rapid tests on the market in August to 375 million in January, according to the White House.
Their use is particularly frequent with people 18 to 24 years old, city dwellers, Democrats, and the well-educated, the Northeastern study found.
Researchers surmise that younger people just don’t go to the doctor as much and are more inclined to favor home tests. Higher usage among young adults also could be traced to the possibility that the coronavirus has been spreading more among younger people, or that they’re more likely to be exposed to it.
“In general younger people are engaging in more interactive behaviors, and they are at lower risk of dying if they get sick, but they are at higher risk of getting sick,” Lazer says.
On the flip side, people 65 years and older were least likely to use home tests, as were Republicans, and rural residents.
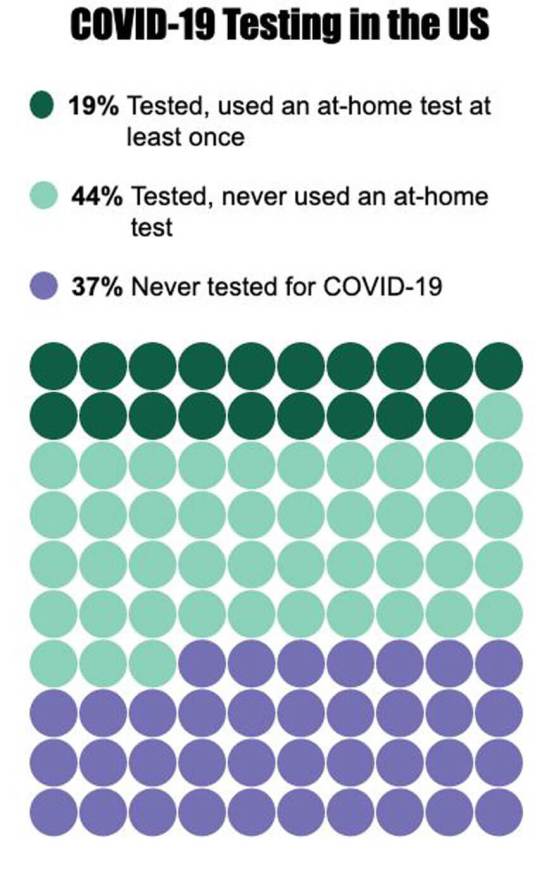
In all, 19% of survey-takers used a home test at least once, but that is expected to ramp up sharply in the weeks ahead, Lazer predicts.
Nearly two years into the pandemic, researchers hit upon a key finding—37% of people said they had never taken a COVID-19 test of any sort, antigen or PCR. This was most prevalent among men, Asian Americans and white people, rural residents, and people 45 years and older, the study found.
“I was really surprised that so many older people haven’t been tested given they’re at higher risk,” says Lazer. It raises questions about access and overall attitudes about getting tested, he adds. “There’s a good chunk of the country in general that doesn’t go to the doctor unless absolutely necessary because it’s a big sociological and psychological barrier.”
For media inquiries , please contact [email protected].
Editor's Picks
‘republic day’ event at northeastern celebrates the 78th anniversary of democracy in italy, a guide to pride month events and activities across northeastern’s global campuses, what is bladed beef mechanically tenderized steaks shouldn’t be eaten rare, food policy expert explains, how can you stay safe during a music festival, northeastern computer scientist receives prestigious early-career award for game design research, featured stories, meet the northeastern co-op helping to upgrade the world’s largest particle accelerator at the european organization for nuclear research, .ngn-magazine__shapes {fill: var(--wp--custom--color--emphasize, #000) } .ngn-magazine__arrow {fill: var(--wp--custom--color--accent, #cf2b28) } ngn magazine dominique biron turned weaknesses into strengths on her way to the ncaa track and field championships, how did us prisoner blood infect thousands in the uk northeastern experts unpack the scandal, donald trump was convicted without cameras in the new york courtroom. will his other court cases be televised.

Recent Stories

Why the Rise of Rapid Tests Makes COVID-19 Case Counts Hard to Trust
B y official counts, fewer people are being diagnosed with COVID-19 right now than at almost any other point during the pandemic. There were an average of 40,000 new cases per day as of April 19, compared to more than 800,000 per day at the height of the U.S. Omicron wave.
But official counts are increasingly misleading. More Americans than ever are testing positive on at-home tests —the results of which are rarely reported to public-health authorities, and are thus missing from official tallies. Public-health experts worry that case numbers are now an unreliable way to judge the state of the pandemic, and that there are countless more infections than statistics show.
Under the CARES Act , COVID-19 testing sites are required to report results to public-health departments. The results of proctored remote tests—which are sometimes required for activities including travel and involve a health professional supervising the test over video—are usually reported, too. But individuals aren’t required to report the results of their standard home tests. Some state health departments, like those in Colorado and Washington , collect self-reported data. Others, like Massachusetts , defer to local health departments. But in many places, there’s no established system.
The CDC recommends that people share their positive results with their health care provider, who may in turn recommend a laboratory test to confirm the result and add it to official tallies. But many people don’t tell their doctors they’ve had a positive rapid test—25% of American adults don’t even have a primary care doctor, according to one study —and some doctors don’t bother recommending a secondary test. About 30% of people who tested positive for COVID-19 via a DIY diagnostic did not get a confirmatory test and thus probably weren’t counted, according to a January survey from the COVID States Project .
That may help explain why overall laboratory testing volume declined from more than 2 million tests per day in January to around half a million per day in mid-April—along with the closure of some mass testing sites , the end of free testing programs for people who are uninsured , and the nationwide relaxation of pandemic precautions.
In some respects, it’s surprising that so many people do get another test after getting a positive result at home. David Lazer, co-author of the COVID States Project survey and a professor of political and computer sciences at Northeastern University, says he was surprised by his group’s findings; he expected more than 30% of people to skip the secondary test. At this point in the pandemic, he suspects that the real number is higher, since people are increasingly comfortable with at-home tests and it’s growing harder to find free testing sites.
“There’s every reason to believe that the missingness is much, much larger now than it was in January,” Lazer says.
That’s a problem, health experts agree. Along with wastewater surveillance and hospitalization rates, testing data is one of the major ways public health officials track the virus’ spread and look for potential surges and hotspots. Agencies including the CDC have said measures like mask mandates can be applied fluidly depending on current transmission patterns in a given area. But if health officials don’t have an accurate picture of where the virus is spreading, they won’t be able to use appropriate mitigation strategies.
A national reporting system for home-test data could help solve that problem—but the question is how to make one work, and whether it’s the best use of increasingly strained public-health resources.
Read More : How Wearing a Mask Can Help Protect You Even If No One Else Wears One
The mixed blessing of home tests
Dr. Michael Mina, chief science officer at the remote testing company eMed, has long argued that rapid tests are crucial to controlling the pandemic. Quickly swabbing before travel or social events, for example, can prevent people from unknowingly infecting others. It’s great that people are finally using self-tests regularly, Mina says, but it’s time to better track the resulting data.
“Two years ago, I was pushing for at-home tests regardless of reporting, out of this massive urgency and need” for better prevention tools, he says. “Now, we’ve had two years to catch up.”
The need for better tracking is clear. During the Omicron wave, about 20% of people in the U.S. who had COVID-like symptoms used an at-home test, according to CDC data . Now, people are testing at home more than ever. For the first time during the pandemic, more people tested positive on at-home tests than other types of tests during the week ending April 16, according to new data from researchers at Boston Children’s Hospital and survey company Momentive (which has not yet been published in a peer-reviewed journal). About 58% of the positive cases reported by the 474,000 people surveyed were picked up by an at-home test.
That’s better for individuals because it’s convenient, says John Brownstein, chief innovation officer at Boston Children’s Hospital. “But it’s not better for public health, because public-health data relies on detailed reporting.”
Many at-home test kits include a way to voluntarily report results to the manufacturer, often by downloading an app; the company may then choose to share the results with public-health officials. But few people use that option. Through a pilot program run by the CDC and the U.S. National Institutes of Health, more than 1.4 million DIY tests were distributed to households in Tennessee and Michigan in 2021—but fewer than 10,000 test results were later logged in a companion app , according to an article in Health Affairs.
Read More : The First COVID-19 Breathalyzer Test Is Coming to the U.S.
Similarly, only about 5,700 people have reported a positive result through Washington State’s hotline since August 2021, a health department representative told TIME. That, too, represents a tiny fraction of the tests taken during that time frame; during the peak of the Omicron surge, the state was recording thousands of cases every day.
The search for a better system
It would be technologically easy for the CDC or another U.S. government agency to build a website where users could quickly log their at-home diagnoses. Brownstein’s research group already runs such a website to “put the ‘public’ back in public health,” he says. Crowdsourcing data benefits individuals as well as researchers, because “you get a disease weather map, where you can understand what’s going on and make decisions for yourself and your family.”
But using that approach to inform federal statistics is risky, Lazer says, because a couple of “bad apples” could choose to falsely report many cases and skew the data. And without knowing how many total tests have been taken, it’s hard to know the significance of the few results that are reported, Mina says. (Brownstein, however, thinks there’s value in a national surveillance site, even without 100% participation. “Not many people [write Amazon reviews], but there are enough people who are willing to give you a sense of the value of a product,” he says.)
For more people to opt in to a reporting system, they would need a reason beyond being a “Good Samaritan,” Mina says. His company, eMed, is trying to incentivize self-reporting. After someone uses an eMed-compatible home test, the company generates a lab report that is shared with public-health departments. That also benefits the individual, Mina says, because they can use the report to be cleared for travel , work, or school if they’re negative. If they’re positive, they have proof of that result and will be connected by telemedicine to a doctor who can prescribe treatment. Those may be better motivators for the average person than simply contributing to statistics, Mina says.
Public-health officials should also take advantage of existing tools by working with diagnostics companies to make their self-reporting systems easier and more accessible, Brownstein says. Instead of downloading an app, for example, people could send in their results via text message.
Another option, Lazer says, would be to conduct repeated, large surveys of American households, asking if anyone in the home recently tested positive for COVID-19 and, if so, on which type of test.
A problem bigger than self tests
To Beth Blauer, executive director of the Centers for Civic Impact at Johns Hopkins University and an expert on government data systems, the data problem in the U.S. involves more than at-home tests. Two years into the pandemic, states still don’t have a standardized way of collecting and assessing the test results they get from testing sites, which means federal case and testing data is flawed even before considering the missing data from unlogged rapid tests, she says.
The situation is especially bad now that some public testing sites are shutting down and uninsured people can no longer get tested for free, Blauer adds. Some people might test at home instead, but many won’t. Data show that home testing is most common among those who are fairly young, white, highly educated, and wealthy —perhaps unsurprising, given that each test costs about $10. Many people, especially those from underserved communities, simply won’t get tested if they can’t get a free diagnostic through work, school, or a convenient public test site, Blauer says, which means many cases will never be detected.
“If COVID has taught us anything, it’s that we have to be much more agile in the way that we dial up and dial down public-health interventions,” Blauer says. “As we dilute that data, it becomes harder and harder to be agile.”
Finding ways to include at-home test data in official case counts could make a dent in that problem. But that will only work if everyone has access to at-home tests and knows what to do with the information they reveal, says Benjamin Rader, a graduate research fellow at Boston Children’s Hospital.
“When we try to create a comprehensive surveillance system, it’s imperative that we make sure we’re reaching everyone in society,” Rader says. “We need to make sure we’re doing things to target everyone and not miss pockets of the U.S.”
More Must-Reads from TIME
- How Selena Gomez Is Revolutionizing the Celebrity Beauty Business
- TIME100 Most Influential Companies 2024
- Javier Milei’s Radical Plan to Transform Argentina
- How Private Donors Shape Birth-Control Choices
- The Deadly Digital Frontiers at the Border
- What's the Best Measure of Fitness?
- The 31 Most Anticipated Movies of Summer 2024
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Write to Jamie Ducharme at [email protected]
How to Report T-Test Results (With Examples)
We can use the following general format to report the results of a one sample t-test :
A one sample t-test was performed to compare [variable of interest] against the population mean. The mean value of [variable of interest] (M = [Mean], SD = [standard deviation]) was significantly [higher, lower, or different] than the population mean; t(df) = [t-value], p = [p-value].
We can use the following format to report the results of an independent two samples t-test :
A two sample t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
We can use the following format to report the results of a paired samples t-test :
A paired samples t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
Note: The “M” in the results stands for sample mean, the “SD” stands for sample standard deviation, and “df” stands for degrees of freedom associated with the t-test statistic.
The following examples show how to report the results of each type of t-test in practice.
Example: Reporting Results of a One Sample T-Test
A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test.
The following screenshot shows the results of the test:

Here’s how to report the results of the test:
A one sample t-test was performed to compare the mean height of a certain species of plant against the population mean. The mean value of height (M = 14.33, SD = 1.37) was not significantly different than the population mean; t(11) = -1.685, p = .120.
Example: Reporting Results of an Independent Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average miles per gallon of a certain car. To test this, they conduct an experiment in which 12 cars receive the new fuel treatment and 12 cars do not.
The following screenshot shows the results of the independent samples t-test:

A two sample t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was not a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(22) = -1.428, p = .167.
Example: Reporting Results of a Paired Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average mpg of a certain car. To test this, they conduct an experiment in which they measure the mpg of 12 cars with and without the fuel treatment.
The following screenshot shows the results of the paired samples t-test:

A paired samples t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(11) = -2.244, p = .046.
Additional Resources
Use the following calculators to automatically perform various t-tests:
One Sample t-test Calculator Two Sample t-test Calculator Paired Samples t-test Calculator
How to Calculate the Sum by Group in Excel
The complete guide: how to report anova results, related posts, how to normalize data between -1 and 1, vba: how to check if string contains another..., how to interpret f-values in a two-way anova, how to create a vector of ones in..., how to determine if a probability distribution is..., what is a symmetric histogram (definition & examples), how to find the mode of a histogram..., how to find quartiles in even and odd..., how to calculate sxy in statistics (with example), how to calculate expected value of x^3.

- Calculators
- Descriptive Statistics
- Merchandise
- Which Statistics Test?
How to Report a T -Test Result in APA Style
The APA style guide details precise requirements for citing the results of statistical tests, which means as well as getting the basic format right, you've got watch out for punctuation, the placing of brackets, italicisation, and the like.
There are a number of different t -tests, the most common being single sample t -test, independent t -test and dependent t -test. The basic format for reporting the result of a t -test is the same in each case (the color red means you substitute in the appropriate value from your study):
t ( degress of freedom ) = the t statistic , p = p value .
It's the context you provide when reporting the result that tells the reader which type of t -test was used. Here are some examples.
Single Sample T -Test
United fans reported higher levels of stress ( M = 83, SD = 5) than found in the population as a whole, t (48) = 2.3, p = .026.
Coffee drinkers spent more time awake ( M = 17.8, SD = 1.4) than the population norm, t (28) = 2.6, p < .05.
Independent T -Test
The 25 participants who received the drug intervention ( M = 480, SD = 34.5) compared to the 28 participants in the control group ( M = 425, SD = 31) demonstrated significantly better peak flow scores, t (51) = 2.1, p = .04.
There was no significant effect for sex, t (38) = 1.7, p = .097, despite women ( M = 55, SD = 8) attaining higher scores than men (M = 53, SD = 7.8).
Dependent T -Test
The results from the pre-test ( M = 13.5, SD = 2.4) and post-test ( M = 16.2, SD = 2.7) memory task indicate that the presence of caffeine in the bloodstream resulted in an improvement in memory recall, t (19) = 3.1, p = .006.
There was a significant increase in the volume of alcohol consumed in the week after the end of semester ( M = 8.7, SD = 3.1) compared to the week before the end of semester ( M = 3.2, SD = 1.5), t (52) = 4.8, p < .001.
1. The abbreviations M and SD stand for mean and standard deviation respectively.
2. If your t -test is one-tailed, you need to say so.
3. There are two ways to report p values. The first way is to cite the alpha value, as in the second of the single sample t -test examples above. The second way, very much the preferred way in the age of computer aided calculations (and the way recommended by the APA), is to report the exact p value (as in our first example). If you report the exact p value, then you need to state your alpha level early in your results section. The other thing to note here is that if your p value is less than .001, it's conventional simply to state p < .001, rather than give the exact value.
4. Remember to drop the leading 0 from the p value.
5. No need to provide a formula for t .
6. Degrees of freedom are N - 1 for the single sample and dependent measures t -tests; and ( N 1 - 1) + ( N 2 - 1) for the independent t -test.
7. If you're hypothesis testing, then remember to restate your hypothesis.


Statistics Made Easy

How to Report T-Test Results (With Examples)
We can use the following general format to report the results of a one sample t-test :
A one sample t-test was performed to compare [variable of interest] against the population mean. The mean value of [variable of interest] (M = [Mean], SD = [standard deviation]) was significantly [higher, lower, or different] than the population mean; t(df) = [t-value], p = [p-value].
We can use the following format to report the results of an independent two samples t-test :
A two sample t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
We can use the following format to report the results of a paired samples t-test :
A paired samples t-test was performed to compare [response variable of interest] in [group 1] and [group 2]. There [was or was not] a significant difference in [response variable of interest] between [group1] (M = [Mean], SD = [standard deviation]) and [group2] (M = [Mean], SD = [standard deviation]); t(df) = [t-value], p = [p-value].
Note: The “M” in the results stands for sample mean, the “SD” stands for sample standard deviation, and “df” stands for degrees of freedom associated with the t-test statistic.
The following examples show how to report the results of each type of t-test in practice.
Example: Reporting Results of a One Sample T-Test
A botanist wants to know if the mean height of a certain species of plant is equal to 15 inches. She collects a random sample of 12 plants and performs a one sample-test.
The following screenshot shows the results of the test:

Here’s how to report the results of the test:
A one sample t-test was performed to compare the mean height of a certain species of plant against the population mean. The mean value of height (M = 14.33, SD = 1.37) was not significantly different than the population mean; t(11) = -1.685, p = .120.
Example: Reporting Results of an Independent Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average miles per gallon of a certain car. To test this, they conduct an experiment in which 12 cars receive the new fuel treatment and 12 cars do not.
The following screenshot shows the results of the independent samples t-test:

A two sample t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was not a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(22) = -1.428, p = .167.
Example: Reporting Results of a Paired Samples T-Test
Researchers want to know if a new fuel treatment leads to a change in the average mpg of a certain car. To test this, they conduct an experiment in which they measure the mpg of 12 cars with and without the fuel treatment.
The following screenshot shows the results of the paired samples t-test:

A paired samples t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment. There was a significant difference in miles per gallon between fuel treatment (M = 22.75, SD = 3.25) and no fuel treatment (M = 21, SD = 2.73); t(11) = -2.244, p = .046.
Additional Resources
Use the following calculators to automatically perform various t-tests:
One Sample t-test Calculator Two Sample t-test Calculator Paired Samples t-test Calculator
Featured Posts

Hey there. My name is Zach Bobbitt. I have a Masters of Science degree in Applied Statistics and I’ve worked on machine learning algorithms for professional businesses in both healthcare and retail. I’m passionate about statistics, machine learning, and data visualization and I created Statology to be a resource for both students and teachers alike. My goal with this site is to help you learn statistics through using simple terms, plenty of real-world examples, and helpful illustrations.
One Reply to “How to Report T-Test Results (With Examples)”
I really liked you explanation and examples. You solved my problem of mixing the concepts of independent sample t-test & paired sample t-test
Thank you very much
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Join the Statology Community
Sign up to receive Statology's exclusive study resource: 100 practice problems with step-by-step solutions. Plus, get our latest insights, tutorials, and data analysis tips straight to your inbox!
By subscribing you accept Statology's Privacy Policy.
- Español – América Latina
- Português – Brasil
- Tiếng Việt
- Android Studio
- Android Developers
Compose Preview Screenshot Testing
Screenshot testing is an effective way to verify how your UI looks to users. The Compose Preview Screenshot Testing tool combines the simplicity and features of composable previews with the productivity gains of running host-side screenshot tests. Compose Preview Screenshot Testing is designed to be as easy to use as composable previews.
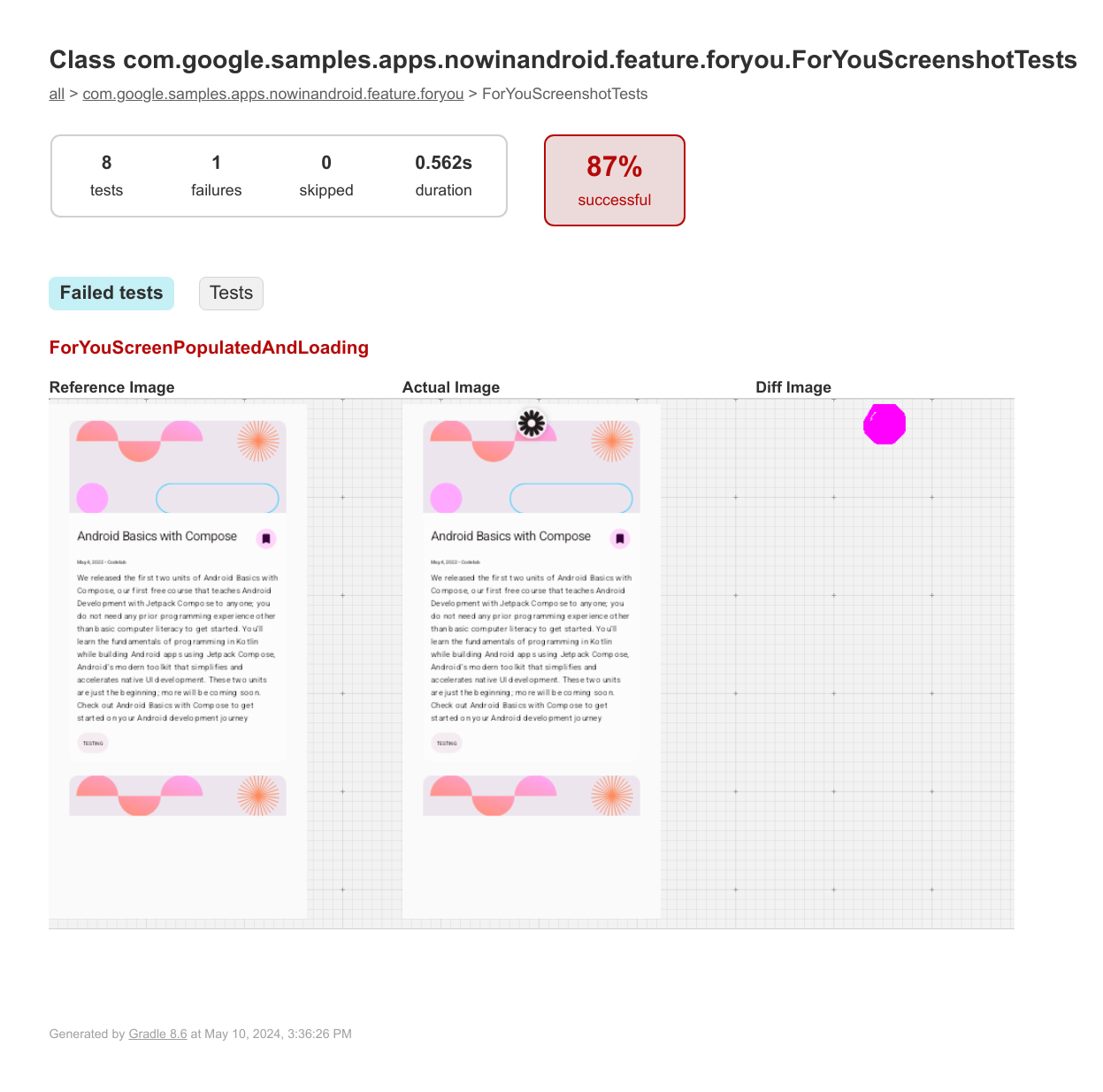
A screenshot test is an automated test that takes a screenshot of a piece of UI and then compares it against a previously approved reference image. If the images don't match, the test fails and produces an HTML report to help you compare and find the differences.
With the Compose Preview Screenshot Testing tool, you can:
- Identify a number of existing or new composable previews you want to use for screenshot tests.
- Generate reference images from those composable previews.
- Generate an HTML report that identifies changes to those previews after you make code changes.
- Use @Preview parameters, such as uiMode or fontScale , and multi-previews to help you scale your tests.
- Modularize your tests with the new screenshotTest source set.
Requirements
To use Compose Preview Screenshot Testing, you need the following:
- Android Gradle 8.5.0-beta01 or higher.
- Kotlin 1.9.20 or higher.
To enable the tool, follow these steps:
- Add the com.android.compose.screenshot plugin, version 0.0.1-alpha01 to your project.
- Add the plugin to your version catalogs file: [versions] agp = "8.5.0-beta01" kotlin = "1.9.20" ... screenshot = "0.0.1-alpha01" [plugins] ... screenshot = { id = "com.android.compose.screenshot", version.ref = "screenshot"}
- In your module-level build.gradle.kts file, add the plugin in the plugins {} block: plugins { ... alias(libs.plugins.screenshot) }
- Enable the experimental property in your project's gradle.properties file. android.experimental.enableScreenshotTest=true
- Add it to your version catalogs: [libraries] androidx-compose-ui-tooling = { group = "androidx.compose.ui", name = "ui-tooling"}
- Add it to your module-level build.gradle.kts file: dependencies { screenshotTestImplementation(libs.androidx.compose.ui.tooling) }
Designate composable previews to use for screenshot tests
To designate the composable previews you want to use for screenshot tests, place the previews in a test class. The test class file must be located in the new screenshotTest source set, for example app/src/screenshotTest/kotlin/com/google/yourapp/ExamplePreviewScreenshots.kt ( {module}/src/screenshotTest/{kotlin|java}/com/your/package ).
You can add more composables and/or previews, including multi-previews, in this file or other files created in the same sourceset.
Generate reference images
After you set up a test class, you need to generate reference images for each preview. These reference images are used to identify changes later, after you make code changes. To generate reference images for your composable preview screenshot tests, run the following Gradle task:
- Linux and macOS: ./gradlew updateDebugScreenshotTest ( ./gradlew {:module:}update{Variant}ScreenshotTest )
- Windows: gradlew updateDebugScreenshotTest ( gradlew {:module:}update{Variant}ScreenshotTest )
After the task completes, find the reference images in app/src/debug/screenshotTest/reference ( {module}/src/{variant}/screenshotTest/reference ).
Generate a test report
Once the reference images exist, run the validate task to take a new screenshot and compare it with the reference image:
- Linux and macOS: ./gradlew validateDebugScreenshotTest ( ./gradlew {:module:}validate{Variant}ScreenshotTest )
- Windows: gradlew validateDebugScreenshotTest ( gradlew {:module:}validate{Variant}ScreenshotTest )
The verification task creates an HTML report at {module}/build/reports/screenshotTest/preview/{variant}/index.html .
Known issues
You can find the current list of known issues in the tool's Issue Tracker component . Report any other feedback and issues through the issue tracker .
Content and code samples on this page are subject to the licenses described in the Content License . Java and OpenJDK are trademarks or registered trademarks of Oracle and/or its affiliates.
Last updated 2024-05-17 UTC.
- My View My View
- Following Following
- Saved Saved
Japan auto safety scandal widens, Toyota halts some shipments
- Medium Text

- Company Toyota Motor Corp Follow
- Company Honda Motor Co Ltd Follow
- Company Mazda Motor Corp Follow
SHARES FALL
Sign up here.
Reporting by Daniel Leussink and Rocky Swift; Additional reporting by Kaori Kaneko and Satoshi Sugiyama; Editing by Edwina Gibbs, David Dolan and Muralikumar Anantharaman
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Thomson Reuters
Daniel Leussink is a correspondent in Japan. Most recently, he has been covering Japan’s automotive industry, chronicling how some of the world's biggest automakers navigate a transition to electric vehicles and unprecedented supply chain disruptions. Since joining Reuters in 2018, Leussink has also covered Japan’s economy, the Tokyo 2020 Olympics, COVID-19 and the Bank of Japan’s ultra-easy monetary policy experiment.

Business Chevron

Mexican peso, stocks tumble on fears of ruling coalition super-majority in Congress
Mexican stocks fell over 6% on Monday and the peso closed at its weakest to the dollar since November after the country's ruling party scored a surprisingly strong election result and looked poised for a super-majority in Congress that markets fear might bring constitutional change and diminish checks and balances.

Complex PTSD Test: How To Identify And Diagnose C-PTSD
Complex post-traumatic stress disorder (also referred to as complex PTSD or C-PTSD) usually results from chronic traumatic experiences. This disorder can involve traditional PTSD symptoms like re-experiencing, avoidance, and hyperarousal, as well as emotional regulation difficulties, negative self-beliefs, and relationship challenges. You can take C-PTSD self-report questionnaires to evaluate your symptoms, but for an official diagnosis, a licensed mental health professional must administer official screening tools. Many types of therapy can be effective in treating C-PTSD, including cognitive processing therapy and prolonged exposure.
Identifying complex PTSD (C-PTSD)
Complex PTSD or complex post-traumatic stress disorder can develop in response to prolonged or repeated trauma.
While post-traumatic stress disorder (PTSD) may occur in the aftermath of a single traumatic experience, C-PTSD is typically seen in those who have endured traumatic life circumstances, particularly those in which a person was unable to escape. These can include the following:
- Childhood abuse or neglect
- Domestic violence
- Repeated physical or sexual abuse
- Torture or slavery
- War and genocide campaigns
Diagnostic criteria for complex PTSD
C-PTSD usually differs from PTSD in several key ways due to the nature of the trauma and the wide-ranging effects it can have on an individual's mental, emotional, and social function. Here are the criteria for a diagnosis of complex PTSD :
1. Exposure to extreme traumatic events
C-PTSD typically involves uniquely threatening or disturbing circumstances, such as those involving prolonged or repeated violence or abuse.
While each person may have a different level of tolerance for distressing situations, the types of trauma that trigger C-PTSD are generally more extreme and sustained. These traumatic experiences may involve not only direct threats to safety or integrity, but also prolonged or repeated exposure, which can result in a deep-seated impact on an individual's psychological and emotional well-being.
2. Core symptoms of PTSD
C-PTSD normally involves the core symptoms of PTSD:
- Re-experiencing: Intrusive thoughts, nightmares, or flashbacks
- Avoidance: Avoiding thoughts, conversations, people, places, or activities that may trigger traumatic memories
- Hyperarousal: Irritability, difficulty sleeping, difficulty concentrating, feeling jumpy
3. Additional symptoms specific to C-PTSD
Beyond the core symptoms of PTSD, C-PTSD usually involves additional symptoms that strain the way a person relates to themselves and others.
Sometimes, these symptoms may be described as changes in personality, and they can significantly alter the way an individual perceives the world around them.
Symptoms specific to C-PTSD can include the following:
- Severe and pervasive emotional regulation issues: Anger, frustration, persistent sadness, or suicidal thoughts
- Persistent negative self-beliefs: Helplessness, shame, guilt, stigma, or worthlessness
- Persistent interpersonal difficulties: Social withdrawal, trust issues, or problems sustaining healthy relationships
In general, to be diagnosed with C-PTSD, a person must meet all of these diagnostic criteria. In situations where an individual exhibits only some of these symptoms, but not enough to meet the full diagnostic criteria, they may be considered to have PTSD or subthreshold PTSD.
Common C-PTSD tests
Researchers and clinical psychologists have developed several screening tools and questionnaires to help individuals and their clinicians assess and identify symptoms associated with C-PTSD.
Self-report questionnaires for C-PTSD
Some C-PTSD tests are designed to be taken by the individual. These self-report measures can provide a convenient way to assess your symptoms so you can make an informed decision about how to approach treatment.
- The International Trauma Questionnaire (ITQ): This questionnaire is based on the 11th edition of the World Health Organization’s International Classification of Diseases (ICD-11). The ITQ is usually considered a reliable and valid measure for assessing and diagnosing both PTSD and C-PTSD.
- Complex Trauma Questionnaire (ComplexTQ): Specifically designed to assess complex trauma, the ComplexTQ evaluates symptoms across emotional regulation, interpersonal difficulties, and identity disturbances. Preliminary research indicates that the Complex TQ test is valid and reliable .
- Developmental Trauma Inventory (DTI): This inventory is primarily geared toward identifying exposure to trauma during childhood and its long-term effects. It can pinpoint developmental disruptions linked to trauma, which tend to be crucial in diagnosing C-PTSD. While not as well-studied as other tests, preliminary research indicates that the DTI may be promising for assessing PTSD and C-PTSD .
Note that these tools are generally meant to help you understand how closely your symptoms may align with a C-PTSD diagnosis. However, they are not intended to diagnose the disorder. For an official diagnosis, you will typically need to undergo a clinical evaluation with a licensed mental health professional.

Clinician-administered screening tools
Other C-PTSD tests are meant to be used in clinical interviews with licensed mental health professionals. These may be used to gain a deeper understanding of an individual’s trauma history and symptomatology, as well as to provide a comprehensive diagnostic assessment.
To give you an official diagnosis, your mental health provider may use one of the following tools:
- Symptoms of Trauma Scale (SOTS): The SOTS can measure the frequency and intensity of trauma-related symptoms across various domains, including emotional, psychological, and physical responses to trauma.
- Cameron Complex Trauma Interview (CCTI): The Cameron Complex Trauma Interview (CCTI) is a specialized diagnostic tool normally used to assess complex trauma in children ages five to 11.
- Complex PTSD Item Set additional to the Clinician Administered PTSD Scale (COPISAC): This is an extension of a common PTSD screening tool used by mental health professionals. It is designed specifically to screen for complex PTSD.
How to get tested for complex PTSD
If you are concerned that you may be experiencing C-PTSD, you may benefit from taking self-report measures and formal assessments. Here’s how the testing process might look:
1. Take a self-report measure
You can start by completing a recognized self-report tool like the ones mentioned above. These tests can provide clarity and awareness surrounding your symptoms, and your results can help guide the conversation during your initial consultation with a mental health professional.
2. Seek professional help
If your results indicate that you may be experiencing PTSD or C-PTSD, it may be advisable to connect with a mental health professional for further evaluation. A licensed provider can assess your symptoms and work with you to develop a treatment plan.
Note that, for an official diagnosis, state licensing boards typically require that providers meet with clients face-to-face for a clinical interview. This may be necessary in situations where you need prescription medication, disability accommodations, or insurance coverage for treatment.
3. Undergo a formal assessment
If you need an official diagnosis, your mental health provider will likely ask you to take a formal C-PTSD test. This generally involves a clinical interview in which they will ask you detailed questions about your personal history, symptoms, and experiences.
The goal is usually to establish a comprehensive understanding of your trauma history and its impact on your life. The interview may also assess how your symptoms meet the diagnostic criteria for C-PTSD and differ from other common concerns, such as PTSD or borderline personality disorder .
4. Develop a treatment plan
With a detailed understanding of your condition, you and your mental health provider can work together to develop a comprehensive treatment plan. Treatment for C-PTSD may include psychotherapy, lifestyle adjustments, and, in some cases, medication.
Understanding therapy options for complex PTSD
Experts recommend a few types of trauma therapy for the treatment of complex PTSD. Though the treatment process may differ slightly depending on the type, each tends to target the parts of the brain responsible for memory and emotional regulation.
These therapies usually aim to help individuals process and integrate traumatic memories, reduce the intensity of emotional responses to trauma cues, and improve overall emotional and behavioral control. Here are the treatments most commonly recommended for trauma disorders:
- Eye movement desensitization and reprocessing (EMDR): EMDR is primarily designed to help individuals process and integrate traumatic memories in a way that reduces their lingering effects. This therapy typically uses guided eye movements to help the brain work through traumatic memories, aiming to alter the emotional response associated with those memories.
- Cognitive processing therapy: CPT usually focuses on helping individuals understand and modify their distressing thoughts related to the trauma, aiming to change how they think about the trauma and reduce its ongoing negative effects on their emotions and behavior.
- Prolonged exposure: This therapy normally involves repeated, detailed imagining of the trauma or progressive exposures to trauma reminders. The goal is typically to reduce the distress and avoidance behavior that can come with traumatic memories.

You can find clinicians who specialize in trauma-informed care through your local mental health clinic or online. If you require an official diagnosis, you may need to attend an in-person appointment with a licensed mental health professional. If you have taken a self-report assessment and are ready to begin treatment without a formal diagnosis, online therapy may be an option worth exploring.
Online trauma therapy for complex PTSD
Online therapy platforms like BetterHelp generally allow you to attend weekly therapy sessions with a licensed therapist who specializes in treating complex trauma. Through BetterHelp, you can find a mental health provider offering the type of treatment you are seeking, plus attend weekly sessions by phone, video call, or live chat.
While research is still in its early stages, preliminary studies suggest that online therapy sessions can be an effective way to deliver trauma-focused cognitive therapy, EMDR, and prolonged exposure. In traditional face-to-face settings, these therapies are among the most well-founded trauma interventions, having been backed by decades of research.
If you are concerned that you or a loved one may be living with C-PTSD, you can use a self-report tool such as the ITQ, ComplexTQ, or DTI to help you understand your symptoms. For a formal diagnosis, you will need to be clinically assessed by a mental health provider. They may use a C-PTSD test like the SOTS or COPISAC to evaluate and diagnose you. With a thorough understanding of how your trauma has affected you, you may be ready to begin treatment in your local area or online.
- What To Know About Soldiers And PTSD Medically reviewed by Melissa Guarnaccia , LCSW
- Understanding The VA PTSD Rating Scale Medically reviewed by Nikki Ciletti , M.Ed, LPC
- Relationships and Relations
Ad-free. Influence-free. Powered by consumers.
The payment for your account couldn't be processed or you've canceled your account with us.
We don’t recognize that sign in. Your username maybe be your email address. Passwords are 6-20 characters with at least one number and letter.
We still don’t recognize that sign in. Retrieve your username. Reset your password.
Forgot your username or password ?
Don’t have an account?
- Account Settings
- My Benefits
- My Products
- Donate Donate
Save products you love, products you own and much more!
Other Membership Benefits:
Suggested Searches
- Become a Member
Car Ratings & Reviews
2024 Top Picks
Car Buying & Pricing
Which Car Brands Make the Best Vehicles?
Tires, Maintenance & Repair
Car Reliability Guide
Key Topics & News
Listen to the Talking Cars Podcast
Home & Garden
Bed & Bath
Top Picks From CR
Best Mattresses
Lawn & Garden
TOP PICKS FROM CR
Best Lawn Mowers and Tractors
Home Improvement
Home Improvement Essential
Best Wood Stains
Home Safety & Security
HOME SAFETY
Best DIY Home Security Systems
REPAIR OR REPLACE?
What to Do With a Broken Appliance
Small Appliances
Best Small Kitchen Appliances
Laundry & Cleaning
Best Washing Machines
Heating, Cooling & Air
Most Reliable Central Air-Conditioning Systems
Electronics
Home Entertainment
FIND YOUR NEW TV
Home Office
Cheapest Printers for Ink Costs
Smartphones & Wearables
BEST SMARTPHONES
Find the Right Phone for You
Digital Security & Privacy
MEMBER BENEFIT
CR Security Planner
Take Action
How Effective Are Portable Air Conditioners?
Consumer Reports' tests have found that these heavy units are iffy at cooling—and hardly portable
When you shop through retailer links on our site, we may earn affiliate commissions. 100% of the fees we collect are used to support our nonprofit mission. Learn more .

Based on Consumer Reports’ testing of portable air conditioners , they should be seen as a last resort for cooling a home when fans aren’t enough or other types of air conditioners aren’t an option. Despite manufacturer claims, these units barely get a room below sweltering, let alone to the 78° F that’s considered the upper threshold of indoor comfort .
- How Portable ACs Work
- How to Compare Btu Ratings
- Portable AC Tips
- Best Portable ACs
- How CR Tests Portable ACs
Portable air conditioners are intended for homes in which window configurations or building regulations prevent the installation of window units .
“A portable air conditioner is an alternative, but not an ideal one,” says Chris Regan, who oversees Consumer Reports’ air conditioner tests. They’re typically bigger, noisier, and more expensive than window units, and they use more energy. In fact, retailers report that many portable air conditioners are returned each season by dissatisfied customers.
How Portable Air Conditioners Work
Unlike a window air conditioner or a central heating, ventilation, and air conditioning system (HVAC) , all of the mechanical parts of a portable air conditioner sit inside the room you’re trying to cool. This contributes to the noise they can make.
It’s also a reason for less-than-capable cooling. While a window AC uses outside air to cool the coils on the outdoor part of the unit, a portable air conditioner uses conditioned air from the room it’s in to cool the mechanicals. That creates negative pressure that can cause warm, unconditioned air from nearby rooms or the outdoors to flow into the room you’re trying to keep cool.
Moreover, it’s debatable how portable they are. Once the hose is connected to the kit in the window (to vent it outdoors), you won’t want to move the unit. In addition, most portable AC units weigh 50 to 80 pounds, sometimes more, making them difficult to move from room to room. And, while they do have wheels, portable air conditioners can be difficult to roll on carpets and over raised thresholds between rooms as well as stairs and steps.
Portable AC units also need their space. The hose is 5 to 7 feet long, and the air conditioner needs to be at least 2 feet away from any walls or furniture that may block its airflow.
How to Compare Btu Ratings Between Different Air Conditioners
While window air conditioners have been subject to federal energy efficiency standards for more than 25 years, portable air conditioners have not. In 2016 the Department of Energy set new efficiency standards for them, but they don’t go into effect until 2025. Many manufacturers have already been producing units that meet those standards.
The result? When you’re shopping, you might see portable air conditioners that list a Btu rating according to the new standard—and some that list an inflated or misleading Btu rating. (Btu, or British thermal unit, is a measurement of its cooling capacity.) And during this transition, you might see two Btu ratings listed on the same box.
For example, a portable model that was formerly listed at 14,000 Btu (called the ASHRAE rating, from the American Society of Heating, Refrigerating and Air-Conditioning Engineers) may now carry a DOE rating of 10,000 Btu.
But even with this change, you still can’t compare a portable air conditioner with a window unit regarding Btu. “The DOE’s test conditions for window ACs are more demanding than those for portable ACs,” says Joanna Mauer, who tracks energy efficiency for the Appliance Standards Awareness Project, an advocacy group. “A window AC rated at 6,000 Btu will therefore deliver more cooling than a portable AC unit rated at 6,000 Btu.”
How to Get the Most Out of a Portable Air Conditioner
They aren’t for everyone, but some people may have no viable alternatives. If that’s you, keep the following tips in mind to make the most of its cooling capabilities.
Install it right. All portable air conditioners come with a kit that you install in a window. It consists of a plastic panel with connections for the exhaust hose and can be installed horizontally in a double-hung window or vertically in a sliding window. Make sure all the connections are tight, and seal any air gaps.
Get a ceiling fan. Create a breeze by running a ceiling fan . It will make the room feel cooler, but only if it’s rotating in the direction that’s designed to push cool air downward. Many ceiling fans allow you to switch their rotational direction, and most should be set for optimal airflow out of the box. Still, you should double-check whether your model allows this and which settings it’s on to ensure you’re getting the most out of it.
Block the sun. Close the curtains and shades to keep the sun from overheating the room. You might also consider getting darker shades, curtains, or blinds that can more effectively stave off the sun’s warmth.
Best Portable Air Conditioners
None of the portable air conditioners in our tests make our list of recommended air conditioners, but if you have no alternative, consider one of these five high-performing models.
How CR Tests Portable Air Conditioners
At Consumer Reports we test each air conditioner in a room appropriate for its claimed size. We’ve adjusted our testing according to the DOE’s new standard. “We now go by the DOE’s Btu rating,” Regan says. “That means we are testing each unit in a room more appropriate to its cooling capacity.”
In our portable air conditioner tests, we measure how long it takes the appliance to lower the temperature in a test chamber from 90° F to 85° F. We found that it takes at least 20 minutes—and often much longer. By comparison, the best window air conditioners can cool a room by 10° F in about 15 minutes or less.
To read more about the differences between portable, window, and central air conditioners, be sure to check out our air conditioner buying guide . You can also browse our database to see more details on all of the air conditioner models that CR tests .
Sharing is Nice
We respect your privacy . All email addresses you provide will be used just for sending this story.
Keystone KSTAW08INV-HC
Windmill "08w2wi ", commerical cool cwam12w6c, ge ahte06aa, costway n4-ah-10n24-a7u1-it, lg lw1224ivsm, friedrich ccv10a10a, friedrich ccv08a10a, koldfront wac12003wco, hisense aw1023tw1w (lowes), koldfront wac8003wco, vissani vwa10 (home depot).
See All Ratings
Trending in Portable air conditioners
We Tried It: The Chillwell 2.0 Portable Air Cooler
Best Portable Air Conditioners of 2024
Most and Least Reliable Room Air Conditioner Brands
Best Portable Air Conditioners for a Home Office

IMAGES
VIDEO
COMMENTS
What you need to know. NIH has developed a website that allows people to easily and securely report their at-home COVID-19 and flu test results. MakeMyTestCount.org doesn't ask for personal information when you report your positive or negative result. It just wants to know what your ZIP code is, when you took the test, and how old you are.
What. Reporting a positive or negative test result just became easier through a new website from the National Institutes of Health. MakeMyTestCount.org, developed through NIH's Rapid Acceleration of Diagnostics (RADx®) Tech program, allows users to anonymously report the results of any brand of at-home COVID-19 test.
Clinics and doctors' offices are required by law to report Covid test results to state and local public health officials, but people who take at-home tests aren't — and many of those test ...
If you test positive at home, another way to report your result is to contact your primary care provider, which is what the C.D.C. recommends you do. When calling or emailing your doctor, make ...
To report your test your result on MakeMyTestCount.org, start by clicking either "Positive" or "Negative" on the homepage. From there, you'll be prompted to answer a few more general questions, including which brand of test you took, when you took the test, your age, and your zip code. What to Do If You Get a Positive At-Home COVID-19 ...
Cases of COVID-19 may be undercounted in the U.S. because lab tests are the most likely to be reported to health departments. Long waits for tests and results are making many people opt for home COVID tests instead. At-home results can be reported to public health by consumers, but don't have to be. Data from the Centers for Disease Control ...
An Easton, New Hampshire, resident prepares to take a Covid-19 self-test on December 7, 2021. John Tully/Bloomberg/Getty Images. Ideally, you should report positive results to both your provider ...
While taking a rapid Covid-19 test has become commonplace, test results are not often reported. Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation. Lab tests have a well-established technology system for sharing test results.
NIH Establishes Website for Self-Reporting COVID-19 Test Results . Published: November 22, 2022. Reporting a positive or negative test result just became easier through a new website from the National Institutes of Health. Learn More For NIH Staff NIH Strategic Response to COVID-19.
Residential households in the U.S. can order free at-home tests from the U.S. Postal Service. Visit covidtests.gov to place an order. NIH has created MakeMyTestCount, a site that allows you to report your at-home COVID-19 test results safely and privately. Public health officials can use this anonymous data to track COVID-19 levels in our ...
Summary of Recent Changes. Effective May 11, 2023, HHS announced the Public Health Emergency declaration for COVID-19 has ended. In addition, HHS and CLIA regulatory requirements under the CARES Act Authority requiring reporting of laboratory test data to the federal government has also ended. State-specific reporting requirements may still apply.
The FDA encourages you to voluntarily and anonymously report your positive or negative test results every time you use an at-home COVID-19 test. You can report your test result by going to ...
Keeping tests at room temperature is fine. A study in the Journal of Clinical Virology found that storing kits above 86° F or below about 37° F decreased the accuracy of the tests, but most home ...
The U.S. went from 24 million home rapid tests on the market in August to 375 million in January, according to the White House. Their use is particularly frequent with people 18 to 24 years old, city dwellers, Democrats, and the well-educated, the Northeastern study found. Researchers surmise that younger people just don't go to the doctor as ...
About 58% of the positive cases reported by the 474,000 people surveyed were picked up by an at-home test. That's better for individuals because it's convenient, says John Brownstein, chief ...
Test reports are documents containing a summary of all the test details of software projects, like the environments where QA teams validate the test code, who performs the test, and when and how the test was performed. During testing, it acts as a physical log that records what code was tested, in what configuration, and what bugs were found.
To report the results of a t test, include the following: the degrees of freedom (df) in parentheses; the t value (also referred to as the t statistic) the p value; Example: Reporting t test results. Older adults experienced significantly more loneliness than younger adults, t(32) = 2.94, p = .006.
Here are five actions you can take to create an efficient test summary report: Describe the scope of testing. Document test environment details. Summarize the types of testing performed and test results. Capture any lessons learned throughout testing. Report on the status of exit criteria.
We can use the following general format to report the results of a one sample t-test: A one sample t-test was performed to compare [variable of interest] against the population mean. The mean value of [variable of interest] (M = [Mean], SD = [standard deviation]) was significantly [higher, lower, or different] than the population mean; t (df ...
The basic format for reporting the result of a t -test is the same in each case (the color red means you substitute in the appropriate value from your study): t ( degress of freedom) = the t statistic, p = p value. It's the context you provide when reporting the result that tells the reader which type of t -test was used. Here are some examples.
To test this, they conduct an experiment in which 12 cars receive the new fuel treatment and 12 cars do not. The following screenshot shows the results of the independent samples t-test: Here's how to report the results of the test: A two sample t-test was performed to compare miles per gallon between fuel treatment and no fuel treatment.
Speedtest by Ookla offers free desktop and mobile apps to test your internet speed and diagnose connectivity issues.
From the Settings screen, tap Hardware . From the Hardware screen, tap Test connectivity . Once run, the report will provide a status report that can be shared with your IT department or Square Support teams for further assistance if needed. The Hardware Hub can also be accessed from the My devices option in the menu bar at the top of the screen.
A screenshot test is an automated test that takes a screenshot of a piece of UI and then compares it against a previously approved reference image. If the images don't match, the test fails and produces an HTML report to help you compare and find the differences. With the Compose Preview Screenshot Testing tool, you can:
Report Assistance Fraud. Program Resources & Information. DHS Executive Leadership. DHS Offices. Our offices offer essential services and benefits catering to the diverse needs of Pennsylvanians and are dedicated to helping individuals and families thrive. OCDEL. Office of Child Development & Early Learning.
TOKYO, June 3 (Reuters) - A safety test scandal at Japanese automakers widened on Monday, with Toyota Motor (7203.T) , opens new tab both halting shipments of some vehicles after Japan's transport ...
Speedtest can help you test the speed and overall performance of your internet for free from any device. Click here to open a new page and take a Speedtest. You can then compare your results with what you've learned about internet performance near you. If you aren't getting the results you expect, you can either use this guide to use your ...
Today Purina Animal Nutrition launched Purina® Systemiq™ Probiotic Supplement, a live and active probiotic to support gut health in horses, and the Purina® MQ™ Equine Microbiome Testing Kit ...
Diagnostic criteria for complex PTSD. C-PTSD usually differs from PTSD in several key ways due to the nature of the trauma and the wide-ranging effects it can have on an individual's mental, emotional, and social function. Here are the criteria for a diagnosis of complex PTSD: 1. Exposure to extreme traumatic events.
In our portable air conditioner tests, we measure how long it takes the appliance to lower the temperature in a test chamber from 90° F to 85° F. We found that it takes at least 20 minutes—and ...