C x H y + O 2 ---> CO 2 + H 2 O
CH 4 + O 2 ---> CO 2 + H 2 O C 2 H 6 + O 2 ---> CO 2 + H 2 O C 6 H 12 O 6 + O 2 ---> CO 2 + H 2 O C 2 H 5 OH + O 2 ---> CO 2 + H 2 O
C 21 H 24 N 2 O 4 + O 2 ---> CO 2 + H 2 O + NO 2 C 2 H 5 SH + O 2 ---> CO 2 + H 2 O + SO 2
1) C 7 H 6 O + O 2 ---> 2) CH 3 COCH 3 + O 2 ---> 3) H 2 C 2 O 4 + O 2 --->
A carbon-hydrogen compound reacting with oxygen
are always CO 2 and H 2 O
C 7 H 6 O + O 2 ---> CO 2 + H 2 O
CH 3 COCH 3 + O 2 ---> CO 2 + H 2 O
H 2 C 2 O 4 + O 2 ---> CO 2 + H 2 O


Combustion Reactions: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
\[CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O\]
\[CH_4 + \frac{3}{2} O_2 \rightarrow CO + 2H_2O\]
- Basis of 100 moles methane
- 25% excess air
- Complete conversion of methane
- No partial combustion
- Moles of air fed to reactor
- Moles of each component in stack gas
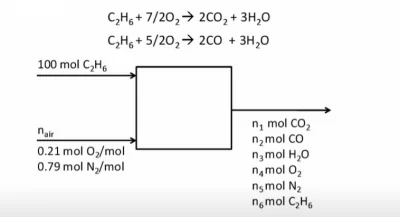
Example Problem 2
- Basis of 100 moles ethane
- 50% excess air
- Ratio of water/dry gas in stack gas
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
High school chemistry
Course: high school chemistry > unit 3.
- Balancing chemical equations
- Balancing more complex chemical equations
- Visually understanding balancing chemical equations
Balancing another combustion reaction
- Apply: balancing equations
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page


Video transcript

Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
Resume improvement assistant, global branding and cultural considerations tutor, brand extensions and line extensions tutor.
Let your curiosity lead the way:
Apply Today
- Arts & Sciences
- Graduate Studies in A&S
Computational Problem Solving in the Chemical Sciences
Chemistry 4050.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.2: Calorimetry (Problems)
- Last updated
- Save as PDF
- Page ID 98797
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
PROBLEM \(\PageIndex{1}\)
A 500-mL bottle of water at room temperature and a 2-L bottle of water at the same temperature were placed in a refrigerator. After 30 minutes, the 500-mL bottle of water had cooled to the temperature of the refrigerator. An hour later, the 2-L of water had cooled to the same temperature. When asked which sample of water lost the most heat, Student A replied that both bottles lost the same amount of heat because they started at the same temperature and finished at the same temperature. Student B thought that the 2-L bottle of water lost more heat because there was more water. A third student believed that the 500-mL bottle of water lost more heat because it cooled more quickly. A fourth student thought that it was not possible to tell because we do not know the initial temperature and the final temperature of the water. Indicate which of these answers is correct and describe the error in each of the other answers.
Student A is incorrect because the mass of water in both containers is not the same.
Student C is incorrect because the bottle cooled quicker due to less mass of water.
Student D is incorrect because it doesn't matter what the change in temperature is as long as it is the same for both bottles.
Student B is correct: if the change in temperature is the same, the one with the more mass (the 2L bottle) had more heat loss. We could prove this using \(q=c×m×ΔT=c×m×(T_\ce{final}−T_\ce{initial})\) from Section 8.1 .
PROBLEM \(\PageIndex{2}\)
How many milliliters of water at 23 °C with a density of 1.00 g/mL must be mixed with 180 mL (about 6 oz) of coffee at 95 °C so that the resulting combination will have a temperature of 60 °C? Assume that coffee and water have the same density and the same specific heat (4.184 J/g °C).
*The section number changed after this video was made*
PROBLEM \(\PageIndex{3}\)
How much will the temperature of a cup (180 g) of coffee at 95 °C be reduced when a 45 g silver spoon (specific heat 0.24 J/g °C) at 25 °C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.
The temperature of the coffee will drop 1 degree.
PROBLEM \(\PageIndex{4}\)
A 45-g aluminum spoon (specific heat 0.88 J/g °C) at 24 °C is placed in 180 mL (180 g) of coffee at 85 °C and the temperature of the two become equal.
- What is the final temperature when the two become equal? Assume that coffee has the same specific heat as water.
- The first time a student solved this problem she got an answer of 88 °C. Explain why this is clearly an incorrect answer.
81.95 °C
This temperature is higher than the starting temperature of the coffee, which is impossible.
PROBLEM \(\PageIndex{5}\)
The temperature of the cooling water as it leaves the hot engine of an automobile is 240 °F. After it passes through the radiator it has a temperature of 175 °F. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g °C.
\(5.7 \times 10^2\; kJ\)
PROBLEM \(\PageIndex{6}\)
When 50.0 g of 0.200 M NaCl( aq ) at 24.1 °C is added to 100.0 g of 0.100 M AgNO 3 ( aq ) at 24.1 °C in a calorimeter, the temperature increases to 25.2 °C as AgCl( s ) forms. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat in joules produced.
PROBLEM \(\PageIndex{7}\)
The addition of 3.15 g of Ba(OH) 2 •8H 2 O to a solution of 1.52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the following equation:
\[Ba(OH)_2 \cdot 8H_2O_{(s)} + 2NH_4SCN_{(aq)} \rightarrow Ba(SCN)_{2(aq)} + 2NH_{3(aq)} + 10H_2O_{(l)}\]
PROBLEM \(\PageIndex{8}\)
When 1.0 g of fructose, C 6 H 12 O 6 ( s ), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °C. If the heat capacity of the calorimeter and its contents is 9.90 kJ/°C, what is q for this combustion?
PROBLEM \(\PageIndex{9}\)
One method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. To determine the rate at which coal is to be fed into the burner in this type of plant, the heat of combustion per ton of coal must be determined using a bomb calorimeter. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °C. If the heat capacity of the calorimeter is 21.6 kJ/°C, determine the heat produced by combustion of a ton of coal (2000 pounds). Remember 1 kg = 2.2 pounds
2.91 x 10 7 kJ
PROBLEM \(\PageIndex{10}\)
A teaspoon of the carbohydrate sucrose (common sugar) contains 16 Calories (16 kcal). What is the mass of one teaspoon of sucrose if the average number of Calories for carbohydrates is 4.1 Calories/g?
*This problem was renumbered after the video was made*
PROBLEM \(\PageIndex{11}\)
What is the maximum mass of carbohydrate in a 6-oz serving of diet soda that contains less than 1 Calorie per can if the average number of Calories for carbohydrates is 4.1 Calories/g?
PROBLEM \(\PageIndex{12}\)
A pint of premium ice cream can contain 1100 Calories. What mass of fat, in grams and pounds, must be produced in the body to store an extra 1.1 × 10 3 Calories if the average number of Calories for fat is 9.1 Calories/g? Remember 1 kg = 2.2 pounds
120.87 g or 1.2 x 10 2 g with 2 significant figures
0.266 lbs or 0.27 lbs with 2 significant figures
PROBLEM \(\PageIndex{13}\)
A serving of a breakfast cereal contains 3 g of protein, 18 g of carbohydrates, and 6 g of fat. What is the Calorie content of a serving of this cereal if the average number of Calories for fat is 9.1 Calories/g, for carbohydrates is 4.1 Calories/g, and for protein is 4.1 Calories/g?
1.4 × 10 2 Calories
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology

IMAGES
VIDEO
COMMENTS
The molecular formula is C 2 H 6. Problem #10: The osmotic pressure of a solution containing 2.04 g of an unknown molecular compound dissolved in 175.0 mL of solution at 25.0 °C is 2.13 atm. The combustion of 22.08 g of the unknown compound produced 36.26 g CO 2 and 14.85 g H 2 O.
Solution. Step 1: Plan the problem. Ethanol and oxygen are the reactants. As with a hydrocarbon, the products of the combustion of an alcohol are carbon dioxide and water. Step 2: Solve. Write the skeleton equation: C2H5OH(l) +O2(g) → CO2(g) +H2O(g) C 2 H 5 OH ( l) + O 2 ( g) → CO 2 ( g) + H 2 O ( g) Balance the equation.
After combustion with excess oxygen, a 12.501 g of a petroleum compound produced 38.196 g of carbon dioxide and 18.752 of water. A previous analysis determined that the compound does not contain oxygen. Establish the empirical formula of the compound. × mol CO2 1 mol C. 38.196 g CO2 = 0.86787 mol C ÷ 0.86787 = 1 mol C 44.011 g CO21 mol CO2. mol.
Combustion is a reaction in which a substance reacts with oxygen gas. C 2 H 5 OH ( l) + O 2 ( g ) →. Since C 2 H 5 OH contains carbon, CO 2 will be one of the products. Since it also contains hydrogen, H 2 O will be another product. H 2 O is produced in the gaseous phase due to the high temperatures that accompany combustion reactions.
Problem. In the presence of a small amount of oxygen a combustion reaction will not only produce carbon dioxide, but also carbon monoxide. The incomplete combustion of naphthalene, a hydrocarbon used in many dyes, produced 2.80 g CO, 4.40 g CO 2 and 1.44 g H 2 O. Determine its empirical formula. A.
Combustion of 2.78 mg of ethyl butyrate produces 6.32 mg of CO2 C O 2 and 2.58 mg of H2O H 2 O. What is the empirical formula is the compound is composed of C, H, and O? Q4. Calculate. the number of grams of solute in 0.250 L of 0.150 M KBr K B r. Molar concentration of a solution containing 4.75 g of Ca(NO3)2 C a ( N O 3) 2 in 0.200L.
This chemistry video tutorial explains how to find the empirical formula and molecular formula using combustion analysis. It explains how to calculate the n...
In this video, Mr. Krug shows students how to solve combustion analysis problems, determining an empirical formula and molecular formula.
4 PRACTICE PROBLEM. Only carbon, hydrogen, nitrogen, and chlorine are present in compound X. When excess silver nitrate (AgNO3) is added to 1.52 g of X after it has been dissolved in water, all of the chlorine in X reacts, forming 3.01 g of solid AgCl. Complete combustion of 1.52 g of X results in the formation of 1.39 g of CO2 and 0.95 g of H2O.
Combustion Analysis Practice Problems. 1.) Researchers used a combustion method to analyze a compound used as an antiknock additive in gasoline. A 9.394 mg sample of the compound yielded 31.154 mg of carbon dioxide and 7.977 mg of water in the combustion. Calculate the percent composition of the compound.
Combustion analysis of 0.1500 g of methyl tert-butyl ether, an octane booster used in gasoline, gave 0.3744 g of CO2 and 0.1838 g of H2O. When a flask having a volume of 1.00 L was evacuated and then filled with methyl tertbutyl ether vapor at a pressure of 100.0 kPa and a temperature of 54.8 °C, the mass of the flask increased by 3.233 g.
Practice Problems. Note that none of the example problems above are balanced. Your teacher may require this, but the ChemTeam will only provide some of the following answers balanced. The rest are up to you!! Write correct formulas for the products in these combustion reactions. 1) C 6 H 6 + O 2---> 2) C 12 H 22 O 11 + O 2---> 3) C 25 H 52 + O ...
This video describes how to solve a combustion analysis problem where there are two quantities of unknown compounds, a more difficult variation of the stand...
Try to solve these problems before watching the solutions in the screencasts.
Solving Combustion Analysis Problems. Term. 1 / 21. Combustion Reaction reactants. Click the card to flip 👆. Definition. 1 / 21. 1. Some type of hydrocarbon - can contain another element (usually O)
Balancing chemical equations 1. Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O. Note: All reactants and products require a coefficient of at least one. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with ...
Determine the empirical formula of a compound using combustion analysis. When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon is converted to CO 2 and the hydrogen to H 2 O (Figure 3.8.1 3.8. 1 ). The amount of carbon produced can be determined by measuring the ...
- All right now we have another combustion reaction. Instead of ethylene, we now have ethane, C2H6, has two carbons and six hydrogen atoms in each molecule of ethane, and it is reacting. It's ethane gas, it is reacting with molecular oxygen in gaseous form and they combust to form carbon dioxide gas and liquid water, and like we've seen in ...
The focus of this problem is the combustion of a 0.5000 g... This video walks through how to do the first problem in the Combustion Practice Problems Worksheet. AP Chemistry
This AI tool helps answer chemistry questions and solve chemistry problems. HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.
By the end of this course, you will be proficient in using computational tools, understanding atomic interactions, and approaching chemical problems with a structured and strategic thought process. Join us to unlock the secrets of the molecular world and transform the way you see chemistry! Prerequisites: Chem 106/112A, Math 132, Physics 191 ...
PROBLEM 8.2.7 8.2. 7. The addition of 3.15 g of Ba (OH) 2 •8H 2 O to a solution of 1.52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented ...
DOI: 10.1007/s10910-024-01619-3 Corpus ID: 269548592; Family of phase fitted 3-step second-order BDF methods for solving periodic and orbital quantum chemistry problems @article{Saadat2024FamilyOP, title={Family of phase fitted 3-step second-order BDF methods for solving periodic and orbital quantum chemistry problems}, author={Hosein Saadat and Sanaz Hami Hassan Kiyadeh and Ramin Goudarzi ...
This is a video tutorial on the ALEKS topic regarding thermochemistry and combustion reactions. It is only the hot air balloon subtopic, but the same subtopi...