Cochrane Switzerland
Pharmacological treatments for psoriasis - living systematic review with network meta analysis.

Psoriasis is a frequent inflammatory skin disease affecting between 2% and 8% of the general population. Psoriasis affects deeply quality of life. There is currently no cure for psoriasis, but various treatments can help to control the symptoms; thus, long-term treatment is usually needed.

In an interview authors Dr Laurence Le Cleach and Emilie Sbidian explain their Cochrane Living Systematic Review .
What is a « Living Systematic Review » ?
A systematic review, which is continually updated, incorporating relevant new evidence as it becomes available.
Read the full Review

Cochrane Skin
Systemic treatments for chronic plaque psoriasis: a living nma.

Our first living systematic review has been published. Read the interview with the authors from our French Satellite https://www.cochrane.org/news/living-systematic-review-pharmacological-treatments-psoriasis-network-meta-analysis and the review itself. Monthly searches are ongoing and the team are working on an update for later this year.
Associations of novel complete blood count-derived inflammatory markers with psoriasis: a systematic review and meta-analysis
- Published: 24 May 2024
- Volume 316 , article number 228 , ( 2024 )
Cite this article

- Yu-Cheng Liu 1 ,
- Shu-Han Chuang 2 ,
- Yu-Pin Chen 3 , 4 &
- Yi-Hsien Shih ORCID: orcid.org/0000-0003-2095-7064 5 , 6
63 Accesses
Explore all metrics
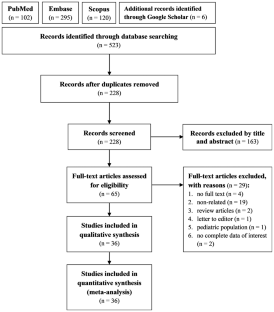
Psoriasis is an immune-mediated disorder which primarily affects skin and has systemic inflammatory involvement. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and monocyte-to-lymphocyte ratio (MLR) are novel complete blood count (CBC)-derived markers which can reflect systemic inflammation. This study aimed to systematically investigate the associations of NLR, PLR, SII, and MLR with psoriasis. This study was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. A comprehensive search of Pubmed, Embase, Scopus, and Google Scholar was conducted for relevant studies. Observational studies evaluating the correlations of NLR, PLR, SII, or MLR with psoriasis were included. The primary outcomes were the associations of these inflammatory markers with the presence and severity of psoriasis. The random-effect model was applied for meta-analysis. 36 studies comprising 4794 psoriasis patients and 55,121 individuals in total were included in the meta-analysis. All inflammatory markers were significantly increased in psoriasis groups compared to healthy controls (NLR: MD = 0.59, 95% CI: 0.47–0.7; PLR: MD = 15.53, 95% CI: 8.48–22.58; SII: MD = 111.58, 95% CI: 61.49-161.68; MLR: MD = 0.034, 95% CI: 0.021–0.048; all p < 0.001). Between-group mean differences in NLR and PLR were positively correlated with the mean scores of Psoriasis Area Severity Index (NLR: p = 0.041; PLR: p = 0.021). NLR, PLR, SII, and MLR are associated with the presence of psoriasis. NLR and PLR serve as significant indicators of psoriasis severity. These novel CBC-derived markers constitute potential targets in the screening and monitoring of psoriasis.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis
Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in psoriasis: a systematic review and meta-analysis

Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and other hematological parameters in psoriasis patients
Data availability.
No datasets were generated or analysed during the current study.
Armstrong AW, Read C, Pathophysiology (2020) Clinical presentation, and treatment of psoriasis: a review. JAMA 323(19):1945–1960. https://doi.org/10.1001/jama.2020.4006
Article CAS PubMed Google Scholar
Christophers E (2001) Psoriasis – epidemiology and clinical spectrum. Clin Exp Dermatol 26(4):314–320. https://doi.org/10.1046/j.1365-2230.2001.00832.x
Nestle FO, Kaplan DH, Barker J, Psoriasis (2009) N Engl J Med 361(5):496–509. https://doi.org/10.1056/NEJMra0804595
Arican O, Aral M, Sasmaz S, Ciragil P (2005) Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005(5):273–279. https://doi.org/10.1155/mi.2005.273
Article PubMed PubMed Central Google Scholar
Korman NJ (2020) Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol 182(4):840–848. https://doi.org/10.1111/bjd.18245
Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L (2017) Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis. Br J Dermatol 176(3):732–740. https://doi.org/10.1111/bjd.15149
Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A et al (2011) Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-Fluorodeoxyglucose Positron Emission tomography–computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol 147(9):1031–1039. https://doi.org/10.1001/archdermatol.2011.119
Article CAS PubMed PubMed Central Google Scholar
Youn SW, Kang SY, Kim SA, Park GY, Lee WW (2015) Subclinical systemic and vascular inflammation detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with mild psoriasis. J Dermatol 42(6):559–566. https://doi.org/10.1111/1346-8138.12859
Article PubMed Google Scholar
Beygi S, Lajevardi V, Abedini R (2014) C-reactive protein in psoriasis: a review of the literature. J Eur Acad Dermatol Venereol 28(6):700–711. https://doi.org/10.1111/jdv.12257
Chuang SH, Chang CH (2024) Platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in glaucoma: a meta-analysis. Biomark Med 18(1):39–49. https://doi.org/10.2217/bmm-2023-0651
Erre GL, Paliogiannis P, Castagna F, Mangoni AA, Carru C, Passiu G et al (2019) Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest 49(1):e13037. https://doi.org/10.1111/eci.13037
Fu W, Fu H, Ye W, Han Y, Liu X, Zhu S et al (2021) Peripheral blood neutrophil-to-lymphocyte ratio in inflammatory bowel disease and disease activity: a meta-analysis. Int Immunopharmacol 101(Pt B):108235. https://doi.org/10.1016/j.intimp.2021.108235
Liu Y-C, Yang T-I, Huang S-W, Kuo Y-J, Chen Y-P (2022) Associations of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with osteoporosis: a Meta-analysis. Diagnostics (Basel) 12(12):2968. https://doi.org/10.3390/diagnostics12122968
Wu Y, Chen Y, Yang X, Chen L, Yang Y (2016) Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 36:94–99. https://doi.org/10.1016/j.intimp.2016.04.006
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ (2020) Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 18(1):360. https://doi.org/10.1186/s12916-020-01817-1
Kou J, Huang J, Li J, Wu Z, Ni L (2023) Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and meta–analysis. Clin Exp Med 23(7):3895–3905. https://doi.org/10.1007/s10238-023-01035-y
Ng WW-S, Lam S-M, Yan W-W, Shum H-P, NLR (2022) MLR, PLR and RDW to predict outcome and differentiate between viral and bacterial pneumonia in the intensive care unit. Sci Rep 12(1):15974. https://doi.org/10.1038/s41598-022-20385-3
Ni J, Wang H, Li Y, Shu Y, Liu Y (2019) Neutrophil to lymphocyte ratio (NLR) as a prognostic marker for in-hospital mortality of patients with sepsis: a secondary analysis based on a single-center, retrospective, cohort study. Med (Baltim) 98(46):e18029. https://doi.org/10.1097/md.0000000000018029
Article Google Scholar
Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J et al (2014) Prognostic value of PLR in various cancers: a meta-analysis. PLoS ONE 9(6):e101119. https://doi.org/10.1371/journal.pone.0101119
Paliogiannis P, Satta R, Deligia G, Farina G, Bassu S, Mangoni AA et al (2019) Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med 19(1):37–45. https://doi.org/10.1007/s10238-018-0538-x
Ye JH, Zhang Y, Naidoo K, Ye S (2024) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in psoriasis: a systematic review and meta-analysis. Arch Dermatol Res 316(3):85. https://doi.org/10.1007/s00403-024-02823-6
Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM et al (2016) Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol 43(3):305–310. https://doi.org/10.1111/1346-8138.13061
Şener G, İnan Yuksel E, Gökdeniz O, Karaman K, Canat HD (2023) The relationship of hematological parameters and C-reactive protein (CRP) with Disease Presence, Severity, and response to systemic therapy in patients with psoriasis. Cureus 15(8):e43790. https://doi.org/10.7759/cureus.43790
Sugimoto E, Matsuda H, Shibata S, Mizuno Y, Koyama A, Li L et al (2023) Impact of pretreatment systemic inflammatory markers on treatment persistence with Biologics and Conventional systemic therapy: a retrospective study of patients with Psoriasis Vulgaris and Psoriatic Arthritis. J Clin Med 12(8). https://doi.org/10.3390/jcm12083046
Yorulmaz A, Hayran Y, Akpinar U, Yalcin B (2020) Systemic Immune-inflammation index (SII) predicts increased severity in Psoriasis and Psoriatic Arthritis. Curr Health Sci J 46(4):352–357. https://doi.org/10.12865/chsj.46.04.05
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27(6):1785–1805. https://doi.org/10.1177/0962280216669183
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Aktaş Karabay E, Demir D, Aksu Çerman A (2020) Evaluation of monocyte to high-density lipoprotein ratio, lymphocytes, monocytes, and platelets in psoriasis. Bras Dermatol 95(1):40–45. https://doi.org/10.1016/j.abd.2019.05.002
Amiri R, Iranmanesh B, Yazdizadeh K, Mohammadi S, Khalili M, Aflatoonian M (2023) The role of hematological markers in prediction of severity of psoriasis and associated arthritis. J Pak Assoc Dermatol 33(2):489–494
Google Scholar
Arısoy A, Karman K, Karayakalı M, Demirelli S, Seçkin HY, Çelik A et al (2017) Evaluation of ventricular repolarization features with novel electrocardiographic parameters (Tp-e, Tp-e/QT) in patients with psoriasis. Anatol J Cardiol 18(6):397–401. https://doi.org/10.14744/AnatolJCardiol.2017.7901
Arunadevi D, Raghavan V, Nott A (2022) Comparative and Correlative Study of Hematologic Parameters and selective inflammatory biomarkers in Psoriasis. Int J Nutr Pharmacol Neurol Dis 12(1):34–38. https://doi.org/10.4103/ijnpnd.ijnpnd_68_21
Ataseven A, Bilgin AU, Kurtipek GS (2014) The importance of neutrophil lymphocyte ratio in patients with psoriasis. Mater Sociomed 26(4):231–233
Çerman AA, Karabay EA, Altunay İK (2016) Evaluation of neutrophil-lymphocyte ratio and mean platelet volume in patients with psoriasis. Med Bull Sisli Etfal Hosp 50(2):137–141
COŞANSU NC, DİKİCİER BS (2020) Is there any association between the monocyte/lymphocyte ratio and the presence and severity of the disease in patients with psoriasis? A cross-sectional study. Sakarya Tıp Dergisi 10(3):430–436
Dincer Rota D, Tanacan E (2021) The utility of systemic-immune inflammation index for predicting the disease activation in patients with psoriasis. Int J Clin Pract 75(6):e14101. https://doi.org/10.1111/ijcp.14101
Guo H-h, Chen R-x (2024) Association of systemic inflammation index with psoriasis risk and psoriasis severity: a retrospective cohort study of NHANES 2009 to 2014. Med (Baltim) 103(8):e37236. https://doi.org/10.1097/md.0000000000037236
Article CAS Google Scholar
Hammad R, Hamdino M, El-Nasser AM (2020) Role of Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, Mean platelet volume in Egyptian patients with Psoriasis Vulgaris. Egypt J Immunol 27(1):157–168
PubMed Google Scholar
Hoffmann JHO, Enk AH (2022) Evaluation of Psoriasis Area and Severity Index Thresholds as proxies for systemic inflammation on an individual patient level. Dermatology 238(4):609–614. https://doi.org/10.1159/000520163
Hong J, Lian N, Li M (2023) Association between the neutrophil-to-lymphocyte ratio and psoriasis: a cross-sectional study of the National Health and Nutrition Examination Survey 2011–2014. BMJ Open 13(12):e077596. https://doi.org/10.1136/bmjopen-2023-077596
Kemeriz F, Tuǧrul B, Tuncer SC (2020) C-reactive protein to albumin ratio: is a new parameter for the disease severity in patients with psoriasis vulgaris? Dermatologica Sinica 38(4):199–204. https://doi.org/10.4103/ds.ds_42_20
Ma R, Cui L, Cai J, Yang N, Wang Y, Chen Q et al (2024) Association between systemic immune inflammation index, systemic inflammation response index and adult psoriasis: evidence from NHANES. Front Immunol 15. https://doi.org/10.3389/fimmu.2024.1323174
Melikoğlu M, Pala E (2023) Systemic Immune-inflammation index as a biomarker of Psoriasis Severity. Arch Basic Clin Res 5(2):291–295
Mishra S, Kadnur M, Jartarkar SR, Keloji H, Agarwal R, Babu S (2022) Correlation between serum uric acid, C-reactive protein, and neutrophil-to-lymphocyte ratio in patients with psoriasis: a case-control study. Turkderm - Turk Arch Dermatol Venereol 56(1):28–33. https://doi.org/10.4274/turkderm.galenos.2021.69797
Mondal S, Guha S, Saha A, Ghoshal L, Bandyopadhyay D (2022) Evaluation of Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic plaque psoriasis. Indian J Dermatol 67(4):477. https://doi.org/10.4103/ijd.ijd_935_21
Nguyen HT, Hoang Vo LD, Pham NN (2023) Neutrophil-To-lymphocyte and platelet-To-lymphocyte ratios as inflammatory markers in psoriasis: a case-control study. Dermatol Rep 15(1). https://doi.org/10.4081/dr.2022.9516
Pektas SD, Tugbaalatas E, Yilmaz N (2016) Plateletcrit is potential biomarker for presence and severity of psoriasis vulgaris. Acta Med Mediterranea 32(6):1785–1790. https://doi.org/10.19193/0393-6384_2016_6_164
Polat M, Bugdayci G, Kaya H, Oğuzman H (2017) Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol Alp Pannonica Adriat 26(4):97–100. https://doi.org/10.15570/actaapa.2017.28
Pujani M, Agarwal C, Chauhan V, Agarwal S, Passi S, Singh K et al (2022) Platelet parameters, neutrophil-lymphocyte ratio, platelet lymphocyte ratio, red cell distribution width: can they serve as biomarkers in evaluation of severity of psoriasis? J Appl Hematol 13(2):95–102. https://doi.org/10.4103/joah.joah_195_20
Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T, Sen N (2014) Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 33(3):223–227. https://doi.org/10.3109/15569527.2013.834498
Sikora M, Stec A, Chrabaszcz M, Waskiel-Burnat A, Zaremba M, Olszewska M et al (2019) Intestinal fatty acid binding protein, a Biomarker of Intestinal Barrier, is Associated with Severity of Psoriasis. J Clin Med 8(7). https://doi.org/10.3390/jcm8071021
Sirin MC, Korkmaz S, Erturan I, Filiz B, Aridogan BC, Cetin ES et al (2020) Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. Bras Dermatol 95(5):575–582. https://doi.org/10.1016/j.abd.2020.02.008
Solak B, Dikicier BS, Erdem T (2017) Impact of elevated serum uric acid levels on systemic inflammation in patients with psoriasis. Angiology 68(3):266–270. https://doi.org/10.1177/0003319716657980
Sunbul M, Seckin D, Durmus E, Ozgen Z, Bozbay M, Bozbay A et al (2015) Assessment of arterial stiffness and cardiovascular hemodynamics by oscillometric method in psoriasis patients with normal cardiac functions. Heart Vessels 30(3):347–354. https://doi.org/10.1007/s00380-014-0490-y
Toprak AE, Ozlu E, Ustunbas TK, Yalcınkaya E, Sogut S, Karadag AS (2016) Neutrophil/lymphocyte ratio, serum endocan, and nesfatin-1 levels in patients with psoriasis vulgaris undergoing phototherapy treatment. Med Sci Monit 22:1232–1237. https://doi.org/10.12659/MSM.898240
Article CAS PubMed Central Google Scholar
Wang WM, Wu C, Gao YM, Li F, Yu XL, Jin HZ (2021) Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and other hematological parameters in psoriasis patients. BMC Immunol 22(1):64. https://doi.org/10.1186/s12865-021-00454-4
Yavuz GÖ, Yavuz İH (2019) Novel inflammatory markers in patients with psoriasis. East J Med 24(1):63–68. https://doi.org/10.5505/ejm.2019.19327
Yildiz A, Ucmak D, Oylumlu M, Akkurt MZ, Yuksel M, Akil MA et al (2014) Assessment of atrial electromechanical delay and P-wave dispersion in patients with psoriasis. Echocardiography 31(9):1071–1076. https://doi.org/10.1111/echo.12530
Yurtdaş M, Yaylali YT, Kaya Y, Ozdemir M, Ozkan I, Aladağ N (2014) Neutrophil-to-lymphocyte ratio may predict subclinical atherosclerosis in patients with psoriasis. Echocardiography 31(9):1095–1104. https://doi.org/10.1111/echo.12511
Zhao Y, Yang XT, Bai YP, Li LF (2023) Association of Complete Blood Cell Count-Derived inflammatory biomarkers with psoriasis and mortality. Clin Cosmet Investig Dermatol 16:3267–3278. https://doi.org/10.2147/CCID.S437936
Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL (2019) Neutrophils in Psoriasis. Front Immunol 10:2376. https://doi.org/10.3389/fimmu.2019.02376
Golden JB, Groft SG, Squeri MV, Debanne SM, Ward NL, McCormick TS et al (2015) Chronic psoriatic skin inflammation leads to increased monocyte adhesion and aggregation. J Immunol 195(5):2006–2018. https://doi.org/10.4049/jimmunol.1402307
Ingersoll MA, Platt AM, Potteaux S, Randolph GJ (2011) Monocyte trafficking in acute and chronic inflammation. Trends Immunol 32(10):470–477. https://doi.org/10.1016/j.it.2011.05.001
Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A et al (2019) TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood 134(9):727–740. https://doi.org/10.1182/blood.2019000200
Herster F, Karbach S, Chatterjee M, Weber ANR, Platelets (2021) Underestimated regulators of Autoinflammation in Psoriasis. J Invest Dermatol 141(6):1395–1403. https://doi.org/10.1016/j.jid.2020.12.025
Langewouters AM, van Erp PE, de Jong EM, van de Kerkhof PC (2008) Lymphocyte subsets in peripheral blood of patients with moderate-to-severe versus mild plaque psoriasis. Arch Dermatol Res 300(3):107–113. https://doi.org/10.1007/s00403-007-0819-9
Núñez J, Miñana G, Bodí V, Núñez E, Sanchis J, Husser O et al (2011) Low lymphocyte count and cardiovascular diseases. Curr Med Chem 18(21):3226–3233. https://doi.org/10.2174/092986711796391633
Schulze-Koops H (2004) Lymphopenia and autoimmune diseases. Arthritis Res Ther 6(4):178. https://doi.org/10.1186/ar1208
Albayrak H (2023) Neutrophil-to-lymphocyte ratio, neutrophil-to-monocyte ratio, platelet-to-lymphocyte ratio, and systemic Immune-inflammation index in Psoriasis patients: response to treatment with Biological drugs. J Clin Med 12(17):5452. https://doi.org/10.3390/jcm12175452
Najar Nobari N, Shahidi Dadras M, Nasiri S, Abdollahimajd F, Gheisari M (2020) Neutrophil/platelet to lymphocyte ratio in monitoring of response to TNF-α inhibitors in psoriatic patients. Dermatol Ther 33(4):e13457. https://doi.org/10.1111/dth.13457
Download references
Acknowledgements
The authors have nothing to acknowledge.
Not applicable.
Author information
Authors and affiliations.
Department of General Medicine, Taipei Medical University Shuang Ho Hospital, New Taipei, 23561, Taiwan
Yu-Cheng Liu
Division of General Practice, Department of Medical Education, Changhua Christian Hospital, Changhua, 50006, Taiwan
Shu-Han Chuang
Department of Orthopedics, Taipei Municipal Wan Fang Hospital, Taipei, 11696, Taiwan
Yu-Pin Chen
Department of Orthopedics, School of Medicine, College of Medicine, Taipei Medical University, Taipei, 11031, Taiwan
Department of Dermatology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, 11031, Taiwan
Yi-Hsien Shih
Department of Dermatology, Taipei Medical University Shuang Ho Hospital, New Taipei City, 23561, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
Yu-Cheng Liu and Yi-Hsien Shih contributed to the study conception and design. Outcome data synthesis, analysis, and interpretation was performed by Yu-Cheng Liu. Yu-Cheng Liu and Shu-Han Chuang were the main contributors in drafting the manuscript. Yu-Pin Chen and Yi-Hsien Shih revised the original manuscript and provided expert opinions on the relevance of the results to clinical practice. Yi-Hsien Shih was the corresponding author. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Yi-Hsien Shih .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Liu, YC., Chuang, SH., Chen, YP. et al. Associations of novel complete blood count-derived inflammatory markers with psoriasis: a systematic review and meta-analysis. Arch Dermatol Res 316 , 228 (2024). https://doi.org/10.1007/s00403-024-02994-2
Download citation
Received : 15 April 2024
Revised : 15 April 2024
Accepted : 26 April 2024
Published : 24 May 2024
DOI : https://doi.org/10.1007/s00403-024-02994-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neutrophil-to-lymphocyte ratio
- Platelet-to-lymphocyte ratio
- Systemic immune-inflammation index
- Monocyte-to-lymphocyte ratio
- Find a journal
- Publish with us
- Track your research
Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review
Affiliations.
- 1 Center for Health Economics, University of York, York, United Kingdom. Electronic address: [email protected].
- 2 School of Medicine, Pharmacy, and Health, Durham University, Stockton-on-Tees, United Kingdom.
- 3 Academic Unit of Dermatology Research, University of Sheffield Medical School, Sheffield, United Kingdom.
- 4 Metaxis Ltd, Curbridge, United Kingdom.
- PMID: 24124809
- DOI: 10.1016/j.jaad.2013.06.027
Background: Chronic plaque psoriasis is the most common type of psoriasis and is characterized by redness, thickness, and scaling. First-line management is with topical treatments.
Objective: We sought to undertake a Cochrane review of topical treatments for chronic plaque psoriasis.
Methods: We systematically searched major databases for randomized controlled trials. Trials reported improvement using a range of related measures; standardized, pooled findings were translated onto a 6-point improvement scale.
Results: The review included 177 randomized controlled trials with 34,808 participants, including 26 trials of scalp psoriasis and 6 trials of inverse and/or facial psoriasis. Typical trial duration was 3 to 8 weeks. When compared with placebo (emollient base), the average improvement for vitamin-D analogues and potent corticosteroids was approximately 1 point, dithranol 1.2 points, very potent corticosteroids 1.8 points, and combined vitamin-D analogue plus steroid 1.4 points once daily and 2.2 points twice daily. However, these are indicative benefits drawn from heterogeneous trial findings. Corticosteroids were more effective than vitamin D for treating psoriasis of the scalp. For both body and scalp psoriasis, potent corticosteroids were less likely than vitamin D to cause skin irritation.
Limitations: Reporting of benefits, adverse effects, and safety assessment methods was often inadequate. In many comparisons, heterogeneity made the size of treatment benefit uncertain.
Conclusions: Corticosteroids are as effective as vitamin-D analogues and cause less skin irritation. However, further research is needed to inform long-term maintenance treatment and provide appropriate safety data.
Keywords: BD; CI; IAGI; Investigator Assessment of Global Improvement; OD; SD; SMD; confidence interval; drug administration; drug safety; once daily; psoriasis; review; standard deviation; standardized mean difference; topical; treatment outcome; twice daily.
Copyright © 2013 American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Administration, Topical
- Adrenal Cortex Hormones / therapeutic use
- Chronic Disease
- Psoriasis / drug therapy*
- Randomized Controlled Trials as Topic
- Vitamin D / analogs & derivatives
- Vitamin D / therapeutic use
- Adrenal Cortex Hormones
- Systematic Review
- Open access
- Published: 23 May 2024
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
- Veronique Lambert ORCID: orcid.org/0000-0002-6984-0038 1 ,
- Sarah Kane ORCID: orcid.org/0009-0006-9341-4836 2 na1 ,
- Belal Howidi ORCID: orcid.org/0000-0002-1166-7631 2 na1 ,
- Bao-Ngoc Nguyen ORCID: orcid.org/0000-0001-6026-2270 2 na1 ,
- David Chandiwana ORCID: orcid.org/0009-0002-3499-2565 3 ,
- Yan Wu ORCID: orcid.org/0009-0008-3348-9232 1 ,
- Michelle Edwards ORCID: orcid.org/0009-0001-4292-3140 3 &
- Imtiaz A. Samjoo ORCID: orcid.org/0000-0003-1415-8055 2 na1
BMC Cancer volume 24 , Article number: 631 ( 2024 ) Cite this article
320 Accesses
1 Altmetric
Metrics details
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally advanced/metastatic breast cancer (HR+/HER2- LABC/mBC). Although there are many treatment options, there is no clear standard of care for patients following 1 L CDK4/6i. Understanding the real-world effectiveness of subsequent therapies may help to identify an unmet need in this patient population. This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC.
MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. Grey literature was searched to identify relevant conference abstracts published from 2019 to 2022. The review was conducted in accordance with PRISMA guidelines (PROSPERO registration: CRD42023383914). Data were qualitatively synthesized and weighted average median real-world progression-free survival (rwPFS) was calculated for NCCN/ESMO-recommended post-1 L CDK4/6i treatment regimens.
Twenty records (9 full-text articles and 11 conference abstracts) encompassing 18 unique studies met the eligibility criteria and reported outcomes for second-line (2 L) treatments after 1 L CDK4/6i; no studies reported disaggregated outcomes in the third-line setting or beyond. Sixteen studies included NCCN/ESMO guideline-recommended treatments with the majority evaluating endocrine-based therapy; five studies on single-agent ET, six studies on mammalian target of rapamycin inhibitors (mTORi) ± ET, and three studies with a mix of ET and/or mTORi. Chemotherapy outcomes were reported in 11 studies. The most assessed outcome was median rwPFS; the weighted average median rwPFS was calculated as 3.9 months (3.3-6.0 months) for single-agent ET, 3.6 months (2.5–4.9 months) for mTORi ± ET, 3.7 months for a mix of ET and/or mTORi (3.0–4.0 months), and 6.1 months (3.7–9.7 months) for chemotherapy. Very few studies reported other effectiveness outcomes and only two studies reported safety outcomes. Most studies had heterogeneity in patient- and disease-related characteristics.
Conclusions
The real-world effectiveness of current 2 L treatments post-1 L CDK4/6i are suboptimal, highlighting an unmet need for this patient population.
Peer Review reports
Introduction
Breast cancer (BC) is the most diagnosed form of cancer in women with an estimated 2.3 million new cases diagnosed worldwide each year [ 1 ]. BC is the second leading cause of cancer death, accounting for 685,000 deaths worldwide per year [ 2 ]. By 2040, the global burden associated with BC is expected to surpass three million new cases and one million deaths annually (due to population growth and aging) [ 3 ]. Numerous factors contribute to global disparities in BC-related mortality rates, including delayed diagnosis, resulting in a high number of BC cases that have progressed to locally advanced BC (LABC) or metastatic BC (mBC) [ 4 , 5 , 6 ]. In the United States (US), the five-year survival rate for patients who progress to mBC is three times lower (31%) than the overall five-year survival rate for all stages (91%) [ 6 , 7 ].
Hormone receptor (HR) positive (i.e., estrogen receptor and/or progesterone receptor positive) coupled with negative human epidermal growth factor 2 (HER2) expression is the most common subtype of BC, accounting for ∼ 60–70% of all BC cases [ 8 , 9 ]. Historically, endocrine therapy (ET) through estrogen receptor modulation and/or estrogen deprivation has been the standard of care for first-line (1 L) treatment of HR-positive/HER2-negative (HR+/HER2-) mBC [ 10 ]. However, with the approval of the cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) palbociclib in combination with the aromatase inhibitor (AI) letrozole in 2015 by the US Food and Drug Administration (FDA), 1 L treatment practice patterns have evolved such that CDK4/6i (either in combination with AIs or with fulvestrant) are currently considered the standard of care [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Other CDK4/6i (ribociclib and abemaciclib) in combination with ET are approved for the treatment of HR+/HER2- LABC/mBC; 1 L use of ribociclib in combination with an AI was granted FDA approval in March 2017 for postmenopausal women (with expanded approval in July 2018 for pre/perimenopausal women and for use in 1 L with fulvestrant for patients with disease progression on ET as well as for postmenopausal women), and abemaciclib in combination with fulvestrant was granted FDA approval in September 2017 for patients with disease progression following ET and as monotherapy in cases where disease progression occurs following ET and prior chemotherapy in mBC (with expanded approval in February 2018 for use in 1 L in combination with an AI for postmenopausal women) [ 18 , 19 , 20 , 21 ].
Clinical trials investigating the addition of CDK4/6i to ET have demonstrated significant improvement in progression-free survival (PFS) and significant (ribociclib) or numerical (palbociclib and abemaciclib) improvement in overall survival (OS) compared to ET alone in patients with HR+/HER2- advanced or mBC, making this combination treatment the recommended option in the 1 L setting [ 22 , 23 , 24 , 25 , 26 , 27 ]. However, disease progression occurs in a significant portion of patients after 1 L CDK4/6i treatment [ 28 ] and the optimal treatment sequence after progression on CDK4/6i remains unclear [ 29 ]. At the time of this review (literature search conducted December 14, 2022), guidelines by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend various options for the treatment of HR+/HER2- advanced BC in the second-line (2 L) setting, including fulvestrant monotherapy, mammalian target of rapamycin inhibitors (mTORi; e.g., everolimus) ± ET, alpelisib + fulvestrant (if phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation positive [PIK3CA-m+]), poly-ADP ribose polymerase inhibitors (PARPi) including olaparib or talazoparib (if breast cancer gene/partner and localizer of BRCA2 positive [BRCA/PALB2m+]), and chemotherapy (in cases when a visceral crisis is present) [ 15 , 16 ]. CDK4/6i can also be used in 2 L [ 16 , 30 ]; however, limited data are available to support CDK4/6i rechallenge after its use in the 1 L setting [ 15 ]. Depending on treatments used in the 1 L and 2 L settings, treatment in the third-line setting is individualized based on the patient’s response to prior treatments, tumor load, duration of response, and patient preference [ 9 , 15 ]. Understanding subsequent treatments after 1 L CDK4/6i, and their associated effectiveness, is an important focus in BC research.
Treatment options for HR+/HER2- LABC/mBC continue to evolve, with ongoing research in both clinical trials and in the real-world setting. Real-world evidence (RWE) offers important insights into novel therapeutic regimens and the effectiveness of treatments for HR+/HER2- LABC/mBC. The effectiveness of the current treatment options following 1 L CDK4/6i therapy in the real-world setting highlights the unmet need in this patient population and may help to drive further research and drug development. In this study, we conducted a systematic literature review (SLR) to qualitatively summarize the effectiveness and safety of treatment regimens in the real-world setting after 1 L treatment with CDK4/6i in patients with HR+/HER2- LABC/mBC.
Literature search
An SLR was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [ 31 ] and reported in alignment with the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) statement [ 32 ] to identify all RWE studies assessing the effectiveness and safety of treatments used for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy and received subsequent treatment in 2 L and beyond (2 L+). The Ovid® platform was used to search MEDLINE® (including Epub Ahead of Print and In-Process, In-Data-Review & Other Non-Indexed Citations), Ovid MEDLINE® Daily, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews by an experienced medical information specialist. The MEDLINE® search strategy was peer-reviewed independently by a senior medical information specialist before execution using the Peer Review of Electronic Search Strategies (PRESS) checklist [ 33 ]. Searches were conducted on December 14, 2022. The review protocol was developed a priori and registered with the International Prospective Register of Systematic Review (PROSPERO; CRD42023383914) which outlined the population, intervention, comparator, outcome, and study design (PICOS) criteria and methodology used to conduct the review (Table 1 ).
Search strategies utilized a combination of controlled vocabulary (e.g., “HER2 Breast Cancer” or “HR Breast Cancer”) and keywords (e.g., “Retrospective studies”). Vocabulary and syntax were adjusted across databases. Published and validated filters were used to select for study design and were supplemented using additional medical subject headings (MeSH) terms and keywords to select for RWE and nonrandomized studies [ 34 ]. No language restrictions were included in the search strategy. Animal-only and opinion pieces were removed from the results. The search was limited to studies published between January 2015 and December 2022 to reflect the time at which FDA approval was granted for the first CDK4/6i agent (palbociclib) in combination with AI for the treatment of LABC/mBC [ 35 ]. Further search details are presented in Supplementary Material 1 .
Grey literature sources were also searched to identify relevant abstracts and posters published from January 2019 to December 2022 for prespecified relevant conferences including ESMO, San Antonio Breast Cancer Symposium (SABCS), American Society of Clinical Oncology (ASCO), the International Society for Pharmacoeconomics and Outcomes Research (ISPOR US), and the American Association for Cancer Research (AACR). A search of ClinicalTrials.gov was conducted to validate the findings from the database and grey literature searches.
Study selection, data extraction & weighted average calculation
Studies were screened for inclusion using DistillerSR Version 2.35 and 2.41 (DistillerSR Inc. 2021, Ottawa, Canada) by two independent reviewers based on the prespecified PICOS criteria (Table 1 ). A third reviewer was consulted to resolve any discrepancies during the screening process. Studies were included if they reported RWE on patients aged ≥ 18 years with HR+/HER2- LABC/mBC who received 1 L CDK4/6i treatment and received subsequent treatment in 2 L+. Studies were excluded if they reported the results of clinical trials (i.e., non-RWE), were published in any language other than English, and/or were published prior to 2015 (or prior to 2019 for conference abstracts and posters). For studies that met the eligibility criteria, data relating to study design and methodology, details of interventions, patient eligibility criteria and baseline characteristics, and outcome measures such as efficacy, safety, tolerability, and patient-reported outcomes (PROs), were extracted (as available) using a Microsoft Excel®-based data extraction form (Microsoft Corporation, WA, USA). Data extraction was performed by a single reviewer and was confirmed by a second reviewer. Multiple publications identified for the same RWE study, patient population, and setting that reported data for the same intervention were linked and extracted as a single publication. Weighted average median real-world progression-free survival (rwPFS) values were calculated by considering the contribution to the median rwPFS of each study proportional to its respective sample size. These weighted values were then used to compute the overall median rwPFS estimate.
Quality assessment
The Newcastle-Ottawa scale (NOS) for nonrandomized (cohort) studies was used to assess the risk of bias for published, full-text studies [ 36 ]. The NOS allocates a maximum of nine points for the least risk of bias across three domains: (1) Formation of study groups (four points), (2) Comparability between study groups (two points), (3) Outcome ascertainment (three points). NOS scores can be categorized in three groups: very high risk of bias (0 to 3 points), high risk of bias (4 to 6), and low risk of bias (7 to 9) [ 37 ]. Risk of bias assessment was performed by one reviewer and validated by a second independent reviewer to verify accuracy. Due to limited methodological data by which to assess study quality, risk of bias assessment was not performed on conference abstracts or posters. An amendment to the PROSPERO record (CRD42023383914) for this study was submitted in relation to the quality assessment method (specifying usage of the NOS).
The database search identified 3,377 records; after removal of duplicates, 2,759 were screened at the title and abstract stage of which 2,553 were excluded. Out of the 206 reports retrieved and assessed for eligibility, an additional 187 records were excluded after full-text review; most of these studies were excluded for having patients with mixed lines of CDK4/6i treatment (i.e., did not receive CDK4/6i exclusively in 1 L) (Fig. 1 and Table S1 ). The grey literature search identified 753 records which were assessed for eligibility; of which 752 were excluded mainly due to the population not meeting the eligibility criteria (Fig. 1 ). In total, the literature searches identified 20 records (9 published full-text articles and 11 conference abstracts/posters) representing 18 unique RWE studies that met the inclusion criteria. The NOS quality scores for the included full-text articles are provided in Table S2 . The scores ranged from four to six points (out of a total score of nine) and the median score was five, indicating that all the studies suffered from a high risk of bias [ 37 ].
Most studies were retrospective analyses of chart reviews or medical registries, and all studies were published between 2017 and 2022 (Table S3 ). Nearly half of the RWE studies (8 out of 18 studies) were conducted in the US [ 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ], while the remaining studies included sites in Canada, China, Germany, Italy, Japan, and the United Kingdom [ 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ]. Sample sizes ranged from as few as 4 to as many as 839 patients across included studies, with patient age ranging from 26 to 86 years old.
Although treatment characteristics in the 1 L setting were not the focus of the present review, these details are captured in Table S3 . Briefly, several RWE studies reported 1 L CDK4/6i use in combination with ET (8 out of 18 studies) or as monotherapy (2 out of 18 studies) (Table S3 ). Treatments used in combination with 1 L CDK4/6i included letrozole, fulvestrant, exemestane, and anastrozole. Where reported (4 out of 18 studies), palbociclib was the most common 1 L CDK4/6i treatment. Many studies (8 out of 18 studies) did not report which specific CDK4/6i treatment(s) were used in 1 L or if its administration was in combination or monotherapy.
Characteristics of treatments after 1 L CDK4/6i therapy
Across all studies included in this review, effectiveness and safety data were only available for treatments administered in the 2 L setting after 1 L CDK4/6i treatment. No studies were identified that reported outcomes for patients treated in the third-line setting or beyond after 1 L CDK4/6i treatment. All 18 studies reported effectiveness outcomes in 2 L, with only two of these studies also describing 2 L safety outcomes. The distribution of outcomes reported in these studies is provided in Table S4 . Studies varied in their reporting of outcomes for 2 L treatments; some studies reported outcomes for a group of 2 L treatments while others described independent outcomes for specific 2 L treatments (i.e., everolimus, fulvestrant, or chemotherapy agents such as eribulin mesylate) [ 42 , 45 , 50 , 54 , 55 ]. Due to the heterogeneity in treatment classes reported in these studies, this data was categorized (as described below) to align with the guidelines provided by NCCN and ESMO [ 15 , 16 ]. The treatment class categorizations for the purpose of this review are: single-agent ET (patients who exclusively received a single-agent ET after 1 L CDK4/6i treatment), mTORi ± ET (patients who exclusively received an mTORi with or without ET after 1 L CDK4/6i treatment), mix of ET and/or mTORi (patients who may have received only ET, only mTORi, and/or both treatments but the studies in this group lacked sufficient information to categorize these patients in the “single-agent ET” or “mTOR ± ET” categories), and chemotherapy (patients who exclusively received chemotherapy after 1 L CDK4/6i treatment). Despite ESMO and NCCN guidelines indicating that limited evidence exists to support rechallenge with CDK4/6i after 1 L CDK4/6i treatment [ 15 , 16 ], two studies reported outcomes for this treatment approach. Data for such patients were categorized as “ CDK4/6i ± ET ” as it was unclear how many patients receiving CDK4/6i rechallenge received concurrent ET. All other patient groups that lacked sufficient information or did not report outcome/safety data independently (i.e., grouped patients with mixed treatments) to categorize as one of the treatment classes described above were grouped as “ other ”.
The majority of studies reported effectiveness outcomes for endocrine-based therapy after 1 L CDK4/6i treatment; five studies for single-agent ET, six studies for mTORi ± ET, and three studies for a mix of ET and/or mTORi (Fig. 2 ). Eleven studies reported effectiveness outcomes for chemotherapy after 1 L CDK4/6i treatment, and only two studies reported effectiveness outcomes for CDK4/6i rechallenge ± ET. Eight studies that described effectiveness outcomes were grouped into the “other” category. Safety data was only reported in two studies: one study evaluating the chemotherapy agent eribulin mesylate and one evaluating the mTORi everolimus.
Effectiveness outcomes
Real-world progression-free survival
Median rwPFS was described in 13 studies (Tables 2 and Table S5 ). Across the 13 studies, the median rwPFS ranged from 2.5 months [ 49 ] to 17.3 months [ 39 ]. Out of the 13 studies reporting median rwPFS, 10 studies reported median rwPFS for a 2 L treatment recommended by ESMO and NCCN guidelines, which ranged from 2.5 months [ 49 ] to 9.7 months [ 45 ].
Weighted average median rwPFS was calculated for 2 L treatments recommended by both ESMO and NCCN guidelines (Fig. 3 ). The weighted average median rwPFS for single-agent ET was 3.9 months ( n = 92 total patients) and was derived using data from two studies reporting median rwPFS values of 3.3 months ( n = 70) [ 38 ] and 6.0 months ( n = 22) [ 40 ]. For one study ( n = 7) that reported outcomes for single agent ET, median rwPFS was not reached during the follow-up period; as such, this study was excluded from the weighted average median rwPFS calculation [ 49 ].
The weighted average median rwPFS for mTORi ± ET was 3.6 months ( n = 128 total patients) and was derived based on data from 3 studies with median rwPFS ranging from 2.5 months ( n = 4) [ 49 ] to 4.9 months ( n = 25) [ 54 ] (Fig. 3 ). For patients who received a mix of ET and/or mTORi but could not be classified into the single-agent ET or mTORi ± ET treatment classes, the weighted average median rwPFS was calculated to be 3.7 months ( n = 17 total patients). This was calculated based on data from two studies reporting median rwPFS values of 3.0 months ( n = 5) [ 46 ] and 4.0 months ( n = 12) [ 49 ]. Notably, one study of patients receiving ET and/or everolimus reported a median rwPFS duration of 3.0 months; however, this study was excluded from the weighted average median rwPFS calculation for the ET and/or mTORi class as the sample size was not reported [ 53 ].
The weighted average median rwPFS for chemotherapy was 6.1 months ( n = 499 total patients), calculated using data from 7 studies reporting median rwPFS values ranging from 3.7 months ( n = 249) [ 38 ] to 9.7 months ( n = 121) [ 45 ] (Fig. 3 ). One study with a median rwPFS duration of 5.6 months was not included in the weighted average median rwPFS calculation as the study did not report the sample size [ 53 ]. A second study was excluded from the calculation since the reported median rwPFS was not reached during the study period ( n = 7) [ 41 ].
Although 2 L CDK4/6i ± ET rechallenge lacks sufficient information to support recommendation by ESMO and NCCN guidelines, the limited data currently available for this treatment have shown promising results. Briefly, two studies reported median rwPFS for CDK4/6i ± ET with values of 8.3 months ( n = 302) [ 38 ] and 17.3 months ( n = 165) (Table 2 ) [ 39 ]. The remaining median rwPFS studies reported data for patients classified as “Other” (Table S5 ). The “Other” category included median rwPFS outcomes from seven studies, and included a myriad of treatments (e.g., ET, mTOR + ET, chemotherapy, CDK4/6i + ET, alpelisib + fulvestrant, chidamide + ET) for which disaggregated median rwPFS values were not reported.
Overall survival
Median OS for 2 L treatment was reported in only three studies (Table 2 ) [ 38 , 42 , 43 ]. Across the three studies, the 2 L median OS ranged from 5.2 months ( n = 3) [ 43 ] to 35.7 months ( n = 302) [ 38 ]. Due to the lack of OS data in most of the studies, weighted averages could not be calculated. No median OS data was reported for the single-agent ET treatment class whereas two studies reported median OS for the mTORi ± ET treatment class, ranging from 5.2 months ( n = 3) [ 43 ] to 21.8 months ( n = 54) [ 42 ]. One study reported 2 L median OS of 24.8 months for a single patient treated with chemotherapy [ 43 ]. The median OS data in the CDK4/6i ± ET rechallenge group was 35.7 months ( n = 302) [ 38 ].
Patient mortality was reported in three studies [ 43 , 44 , 45 ]. No studies reported mortality for the single-agent ET treatment class and only one study reported this outcome for the mTORi ± ET treatment class, where 100% of patients died ( n = 3) as a result of rapid disease progression [ 43 ]. For the chemotherapy class, one study reported mortality for one patient receiving 2 L capecitabine [ 43 ]. An additional study reported eight deaths (21.7%) following 1 L CDK4/6i treatment; however, this study did not disclose the 2 L treatments administered to these patients [ 44 ].
Other clinical endpoints
The studies included limited information on additional clinical endpoints; two studies reported on time-to-discontinuation (TTD), two reported on duration of response (DOR), and one each on time-to-next-treatment (TTNT), time-to-progression (TTP), objective response rate (ORR), clinical benefit rate (CBR), and stable disease (Tables 2 and Table S5 ).
Safety, tolerability, and patient-reported outcomes
Safety and tolerability data were reported in two studies [ 40 , 45 ]. One study investigating 2 L administration of the chemotherapy agent eribulin mesylate reported 27 patients (22.3%) with neutropenia, 3 patients (2.5%) with febrile neutropenia, 10 patients (8.3%) with peripheral neuropathy, and 14 patients (11.6%) with diarrhea [ 45 ]. Of these, neutropenia of grade 3–4 severity occurred in 9 patients (33.3%) [ 45 ]. A total of 55 patients (45.5%) discontinued eribulin mesylate treatment; 1 patient (0.83%) discontinued treatment due to adverse events [ 45 ]. Another study reported that 5 out of the 22 patients receiving the mTORi everolimus combined with ET in 2 L (22.7%) discontinued treatment due to toxicity [ 40 ]. PROs were not reported in any of the studies included in the SLR.
The objective of this study was to summarize the existing RWE on the effectiveness and safety of therapies for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. We identified 18 unique studies reporting specifically on 2 L treatment regimens after 1 L CDK4/6i treatment. The weighted average median rwPFS for NCCN- and ESMO- guideline recommended 2 L treatments ranged from 3.6 to 3.9 months for ET-based treatments and was 6.1 months when including chemotherapy-based regimens. Treatment selection following 1 L CDK4/6i therapy remains challenging primarily due to the suboptimal effectiveness or significant toxicities (e.g., chemotherapy) associated with currently available options [ 56 ]. These results highlight that currently available 2 L treatments for patients with HR+/HER2- LABC/mBC who have received 1 L CDK4/6i are suboptimal, as evidenced by the brief median rwPFS duration associated with ET-based treatments, or notable side effects and toxicity linked to chemotherapy. This conclusion is aligned with a recent review highlighting the limited effectiveness of treatment options for HR+/HER2- LABC/mBC patients post-CDK4/6i treatment [ 56 , 57 ]. Registrational trials which have also shed light on the short median PFS of 2–3 months achieved by ET (i.e., fulvestrant) after 1 L CDK4/6i therapy emphasize the need to develop improved treatment strategies aimed at prolonging the duration of effective ET-based treatment [ 56 ].
The results of this review reveal a paucity of additional real-world effectiveness and safety evidence after 1 L CDK4/6i treatment in HR+/HER2- LABC/mBC. OS and DOR were only reported in two studies while other clinical endpoints (i.e., TTD, TTNT, TTP, ORR, CBR, and stable disease) were only reported in one study each. Similarly, safety and tolerability data were only reported in two studies each, and PROs were not reported in any study. This hindered our ability to provide a comprehensive assessment of real-world treatment effectiveness and safety following 1 L CDK4/6i treatment. The limited evidence may be due to the relatively short period of time that has elapsed since CDK4/6i first received US FDA approval for 1 L treatment of HR+/HER2- LABC/mBC (2015) [ 35 ]. As such, almost half of our evidence was informed by conference abstracts. Similarly, no real-world studies were identified in our review that reported outcomes for treatments in the third- or later-lines of therapy after 1 L CDK4/6i treatment. The lack of data in this patient population highlights a significant gap which limits our understanding of the effectiveness and safety for patients receiving later lines of therapy. As more patients receive CDK4/6i therapy in the 1 L setting, the number of patients requiring subsequent lines of therapy will continue to grow. Addressing this data gap over time will be critical to improve outcomes for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy.
There are several strengths of this study, including adherence to the guidelines outlined in the Cochrane Handbook to ensure a standardized and reliable approach to the SLR [ 58 ] and reporting of the SLR following PRISMA guidelines to ensure transparency and reproducibility [ 59 ]. Furthermore, the inclusion of only RWE studies allowed us to assess the effectiveness of current standard of care treatments outside of a controlled environment and enabled us to identify an unmet need in this patient population.
This study had some notable limitations, including the lack of safety and additional effectiveness outcomes reported. In addition, the dearth of studies reporting PROs is a limitation, as PROs provide valuable insight into the patient experience and are an important aspect of assessing the impact of 2 L treatments on patients’ quality of life. The studies included in this review also lacked consistent reporting of clinical characteristics (e.g., menopausal status, sites of metastasis, prior surgery) making it challenging to draw comprehensive conclusions or comparisons based on these factors across the studies. Taken together, there exists an important gap in our understanding of the long-term management of patients with HR+/HER2- LABC/mBC. Additionally, the effectiveness results reported in our evidence base were informed by small sample sizes; many of the included studies reported median rwPFS based on less than 30 patients [ 39 , 40 , 41 , 46 , 49 , 51 , 60 ], with two studies not reporting the sample size at all [ 47 , 53 ]. This may impact the generalizability and robustness of the results. Relatedly, the SLR database search was conducted in December 2022; as such, novel agents (e.g., elacestrant and capivasertib + fulvestrant) that have since received FDA approval for the treatment of HR+/HER2- LABC/mBC may impact current 2 L rwPFS outcomes [ 61 , 62 ]. Finally, relative to the number of peer-reviewed full-text articles, this SLR identified eight abstracts and one poster presentation, comprising half (50%) of the included unique studies. As conference abstracts are inherently limited by how much content that can be described due to word limit constraints, this likely had implications on the present synthesis whereby we identified a dearth of real-world effectiveness outcomes in patients with HR+/HER2- LABC/mBC treated with 1 L CDK4/6i therapy.
Future research in this area should aim to address the limitations of the current literature and provide a more comprehensive understanding of optimal sequencing of effective and safe treatment for patients following 1 L CDK4/6i therapy. Specifically, future studies should strive to report robust data related to effectiveness, safety, and PROs for patients receiving 2 L treatment after 1 L CDK4/6i therapy. Future studies should also aim to understand the mechanism underlying CDK4/6i resistance. Addressing these gaps in knowledge may improve the long-term real-world management of patients with HR+/HER2- LABC/mBC. A future update of this synthesis may serve to capture a wider breadth of full-text, peer-reviewed articles to gain a more robust understanding of the safety, effectiveness, and real-world treatment patterns for patients with HR+/HER2- LABC/mBC. This SLR underscores the necessity for ongoing investigation and the development of innovative therapeutic approaches to address these gaps and improve patient outcomes.
This SLR qualitatively summarized the existing real-world effectiveness data for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. Results of this study highlight the limited available data and the suboptimal effectiveness of treatments employed in the 2 L setting and underscore the unmet need in this patient population. Additional studies reporting effectiveness and safety outcomes, in addition to PROs, for this patient population are necessary and should be the focus of future research.
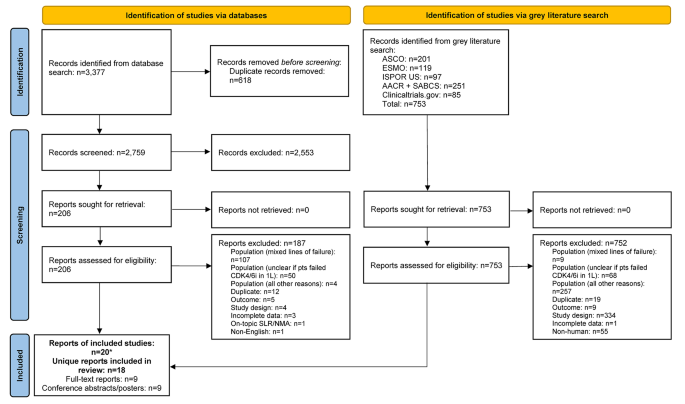
PRISMA flow diagram. *Two included conference abstracts reported the same information as already included full-text reports, hence both conference abstracts were not identified as unique. Abbreviations: 1 L = first-line; AACR = American Association of Cancer Research; ASCO = American Society of Clinical Oncology; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ISPOR = Professional Society for Health Economics and Outcomes Research; n = number of studies; NMA = network meta-analysis; pts = participants; SABCS = San Antonio Breast Cancer Symposium; SLR = systematic literature review.
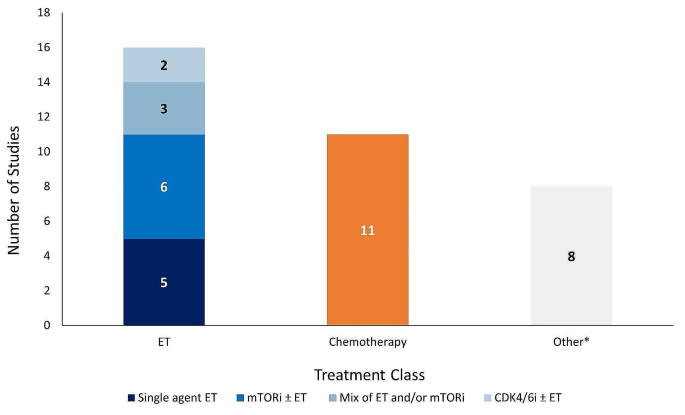
Number of studies reporting effectiveness outcomes exclusively for each treatment class. *Studies that lack sufficient information on effectiveness outcomes to classify based on the treatment classes outlined in the legend above. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ET = endocrine therapy; mTORi = mammalian target of rapamycin inhibitor.
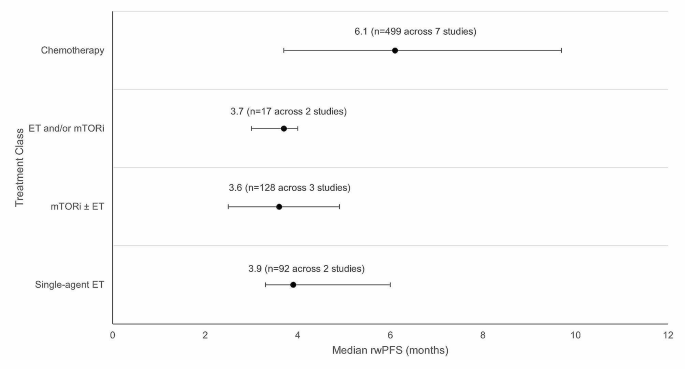
Weighted average median rwPFS for 2 L treatments (recommended in ESMO/NCCN guidelines) after 1 L CDK4/6i treatment. Circular dot represents weighted average median across studies. Horizontal bars represent the range of values reported in these studies. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ET = endocrine therapy, mTORi = mammalian target of rapamycin inhibitor; n = number of patients; NCCN = National Comprehensive Cancer Network; rwPFS = real-world progression-free survival.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. This study is registered with PROSPERO (CRD42023383914).
Abbreviations
Second-line
Second-line treatment setting and beyond
American Association of Cancer Research
Aromatase inhibitor
American Society of Clinical Oncology
- Breast cancer
breast cancer gene/partner and localizer of BRCA2 positive
Clinical benefit rate
Cyclin-dependent kinase 4/6 inhibitor
Complete response
Duration of response
European Society for Medical Oncology
Food and Drug Administration
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 negative
Hormone receptor
Hormone receptor positive
Professional Society for Health Economics and Outcomes Research
Locally advanced breast cancer
Metastatic breast cancer
Medical Literature Analysis and Retrieval System Online
Medical subject headings
Mammalian target of rapamycin inhibitor
National Comprehensive Cancer Network
Newcastle Ottawa Scale
Objective response rate
Poly-ADP ribose polymerase inhibitor
Progression-free survival
Population, Intervention, Comparator, Outcome, Study Design
Partial response
Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses
Patient-reported outcomes
- Real-world evidence
San Antonio Breast Cancer Symposium
- Systematic literature review
Time-to-discontinuation
Time-to-next-treatment
Time-to-progression
United States
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A, Breast, Cancer—Epidemiology. Risk factors, classification, prognostic markers, and current treatment Strategies—An. Updated Rev Cancers. 2021;13(17):4287.
Google Scholar
World Health Organization (WHO). Breast Cancer Facts Sheet [updated July 12 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95(1130):20211033.
Article PubMed Google Scholar
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524– 41.
National Cancer Institute (NIH). Cancer Stat Facts: Female Breast Cancer [updated 2020. https://seer.cancer.gov/statfacts/html/breast.html .
American Cancer Society. Key Statistics for Breast Cancer [ https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html .
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. npj Breast Cancer. 2022;8(1):95.
Article CAS PubMed PubMed Central Google Scholar
Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25(Suppl 1):S131–41.
Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:17588359221113694.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus guidelines for advanced breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–57.
Article CAS PubMed Google Scholar
US Food Drug Administration. Palbociclib (Ibrance) 2017 [updated March 31, 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer 2018 [updated July 18. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib for HR positive, HER2-negative breast cancer 2017 [updated Sept 28. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer .
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer 2022 [ https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: Palbociclib for the Treatment of Postmenopausal Patients with estrogen Receptor-Positive, HER2-Negative metastatic breast Cancer. Clin Cancer Res. 2015;21(21):4760–6.
US Food Drug Administration. Ribociclib (Kisqali) [ https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali#:~:text=On%20March%2013%2C%202017%2C%20the,hormone%20receptor%20(HR)%2Dpositive%2C .
US Food Drug Administration. FDA approves new treatment for certain advanced or metastatic breast cancers [ https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. 2018 [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer .
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall survival with Palbociclib and fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–36.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. J Clin Oncol. 2017;35(32):3638–46.
Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, Hou W, et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget. 2018;9(100):37352–66.
Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17.
Goetz M. MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. SABCS; 2023.
Munzone E, Pagan E, Bagnardi V, Montagna E, Cancello G, Dellapasqua S, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2– metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6):100332.
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Annals of Oncology. 2021;32(12):1475-95.
European Society for Medical Oncology (ESMO). ESMO Metastatic Breast Cancer Living Guideline: ER-positive HER2-negative Breast Cancer [updated May 2023. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Welch PM VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). www.training.cochrane.org/handbook : Cochrane; 2021.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Fraser C, Murray A, Burr J. Identifying observational studies of surgical interventions in MEDLINE and EMBASE. BMC Med Res Methodol. 2006;6(1):41.
US Food Drug Administration. Palbociclib (Ibrance). Silver Spring, MD: US Food and Drug Administration; 2017.
Book Google Scholar
GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos PT. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [ https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–6.
Kalinsky KM, Kruse M, Smyth EN, Guimaraes CM, Gautam S, Nisbett AR et al. Abstract P1-18-37: Treatment patterns and outcomes associated with sequential and non-sequential use of CDK4 and 6i for HR+, HER2- MBC in the real world. Cancer Research. 2022;82(4_Supplement):P1-18-37-P1-18-37.
Choong GM, Liddell S, Ferre RAL, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res Treat. 2022;196(1):229–37.
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective Analysis of Treatment Patterns and effectiveness of Palbociclib and subsequent regimens in metastatic breast Cancer. J Natl Compr Canc Netw. 2019;17(2):141–7.
Rozenblit M, Mun S, Soulos P, Adelson K, Pusztai L, Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14.
Bashour SI, Doostan I, Keyomarsi K, Valero V, Ueno NT, Brown PH, et al. Rapid breast Cancer Disease Progression following cyclin dependent kinase 4 and 6 inhibitor discontinuation. J Cancer. 2017;8(11):2004–9.
Giridhar KV, Choong GM, Leon-Ferre R, O’Sullivan CC, Ruddy K, Haddad T, et al. Abstract P6-18-09: clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Cancer Res. 2019;79:P6–18.
Article Google Scholar
Mougalian SS, Feinberg BA, Wang E, Alexis K, Chatterjee D, Knoth RL, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–44.
Moscetti LML, Riggi L, Sperduti I, Piacentini FOC, Toss A, Barbieri E, Cortesi L, Canino FMA, Zoppoli G, Frassoldati A, Schirone A, Dominici MECF. SEQUENCE OF TREATMENTS AFTER CDK4/6 THERAPY IN ADVANCED BREAST CANCER (ABC), A GOIRC MULTICENTER RETRO/ PROSPECTIVE STUDY. PRELIMINARY RESULTS IN THE RETROSPECTIVE SERIES OF 116 PATIENTS. Tumori. 2022;108(4S):80.
Menichetti AZE, Giorgi CA, Bottosso M, Leporati R, Giarratano T, Barbieri C, Ligorio F, Mioranza E, Miglietta F, Lobefaro R, Faggioni G, Falci C, Vernaci G, Di Liso E, Girardi F, Griguolo G, Vernieri C, Guarneri V, Dieci MV. CDK 4/6 INHIBITORS FOR METASTATIC BREAST CANCER: A MULTICENTER REALWORLD STUDY. Tumori. 2022;108(4S):70.
Marschner NW, Harbeck N, Thill M, Stickeler E, Zaiss M, Nusch A, et al. 232P Second-line therapies of patients with early progression under CDK4/6-inhibitor in first-line– data from the registry platform OPAL. Annals of Oncology. 2022;33:S643-S4
Gousis C, Lowe KMH, Kapiris M. V. Angelis. Beyond First Line CDK4/6 Inhibitors (CDK4/6i) and Aromatase Inhibitors (AI) in Patients with Oestrogen Receptor Positive Metastatic Breast Cancer (ERD MBC): The Guy’s Cancer Centre Experience. Clinical Oncology2022. p. e178.
Endo Y, Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, et al. Time to chemotherapy for patients with estrogen receptor-positive breast Cancer and cyclin-dependent kinase 4 and 6 inhibitor use. J Breast Cancer. 2022;25(4):296–306.
Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Amaro CP, Batra A, Lupichuk S. First-line treatment with a cyclin-dependent kinase 4/6 inhibitor plus an aromatase inhibitor for metastatic breast Cancer in Alberta. Curr Oncol. 2021;28(3):2270–80.
Crocetti SPM, Tassone L, Marcantognini G, Bastianelli L, Della Mora A, Merloni F, Cantini L, Scortichini L, Agostinelli V, Ballatore Z, Savini A, Maccaroni E. Berardi R. What is the best therapeutic sequence for ER-Positive/HER2- Negative metastatic breast cancer in the era of CDK4/6 inhibitors? A single center experience. Tumori. 2020;106(2S).
Nichetti F, Marra A, Giorgi CA, Randon G, Scagnoli S, De Angelis C, et al. 337P Efficacy of everolimus plus exemestane in CDK 4/6 inhibitors-pretreated or naïve HR-positive/HER2-negative breast cancer patients: A secondary analysis of the EVERMET study. Annals of Oncology. 2020;31:S382
Luhn P, O’Hear C, Ton T, Sanglier T, Hsieh A, Oliveri D, et al. Abstract P4-13-08: time to treatment discontinuation of second-line fulvestrant monotherapy for HR+/HER2– metastatic breast cancer in the real-world setting. Cancer Res. 2019;79(4Supplement):P4–13.
Mittal A, Molto Valiente C, Tamimi F, Schlam I, Sammons S, Tolaney SM et al. Filling the gap after CDK4/6 inhibitors: Novel Endocrine and Biologic Treatment options for metastatic hormone receptor positive breast Cancer. Cancers (Basel). 2023;15(7).
Ashai N, Swain SM. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel). 2023;15(6).
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). www.training.cochrane.org/handbook : Cochrane; 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502.
US Food Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [updated January 27 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves capivasertib with fulvestrant for breast cancer [updated November 16 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer .
Download references
Acknowledgements
The authors would like to acknowledge Joanna Bielecki who developed, conducted, and documented the database searches.
This study was funded by Pfizer Inc. (New York, NY, USA) and Arvinas (New Haven, CT, USA).
Author information
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen and Imtiaz A. Samjoo contributed equally to this work.
Authors and Affiliations
Pfizer, 10017, New York, NY, USA
Veronique Lambert & Yan Wu
EVERSANA, Burlington, ON, Canada
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen & Imtiaz A. Samjoo
Arvinas, 06511, New Haven, CT, USA
David Chandiwana & Michelle Edwards
You can also search for this author in PubMed Google Scholar
Contributions
VL, IAS, SK, BH, BN, DC, YW, and ME participated in the conception and design of the study. IAS, SK, BH and BN contributed to the literature review, data collection, analysis, and interpretation of the data. VL, IAS, SK, BH, BN, DC, YW, and ME contributed to the interpretation of the data and critically reviewed for the importance of intellectual content for the work. VL, IAS, SK, BH, BN, DC, YW, and ME were responsible for drafting or reviewing the manuscript and for providing final approval. VL, IAS, SK, BH, BN, DC, YW, and ME meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Corresponding author
Correspondence to Imtiaz A. Samjoo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors of this manuscript declare that the research presented was funded by Pfizer Inc. and Arvinas. While the support from Pfizer Inc. and Arvinas was instrumental in facilitating this research, the authors affirm that their interpretation of the data and the content of this manuscript were conducted independently and without bias to maintain the transparency and integrity of the research. IAS, SK, BH, and BN are employees of EVERSANA, Canada, which was a paid consultant to Pfizer in connection with the development of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lambert, V., Kane, S., Howidi, B. et al. Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i. BMC Cancer 24 , 631 (2024). https://doi.org/10.1186/s12885-024-12269-8
Download citation
Received : 26 January 2024
Accepted : 16 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12885-024-12269-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- First-line CDK4/6i
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Systematic review
- Open access
- Published: 28 May 2024
Exploring the content and delivery of feedback facilitation co-interventions: a systematic review
- Michael Sykes ORCID: orcid.org/0000-0002-2039-7397 1 ,
- Zahava R. S. Rosenberg-Yunger 2 ,
- Matthew Quigley 3 ,
- Lavanya Gupta 3 ,
- Owen Thomas 4 ,
- Lisa Robinson 5 ,
- Karen Caulfield 5 ,
- Noah Ivers 6 &
- Sarah Alderson 4
Implementation Science volume 19 , Article number: 37 ( 2024 ) Cite this article
3 Altmetric
Metrics details
Policymakers and researchers recommend supporting the capabilities of feedback recipients to increase the quality of care. There are different ways to support capabilities. We aimed to describe the content and delivery of feedback facilitation interventions delivered alongside audit and feedback within randomised controlled trials.
We included papers describing feedback facilitation identified by the latest Cochrane review of audit and feedback. The piloted extraction proforma was based upon a framework to describe intervention content, with additional prompts relating to the identification of influences, selection of improvement actions and consideration of priorities and implications. We describe the content and delivery graphically, statistically and narratively.
We reviewed 146 papers describing 104 feedback facilitation interventions. Across included studies, feedback facilitation contained 26 different implementation strategies. There was a median of three implementation strategies per intervention and evidence that the number of strategies per intervention is increasing. Theory was used in 35 trials, although the precise role of theory was poorly described. Ten studies provided a logic model and six of these described their mechanisms of action. Both the exploration of influences and the selection of improvement actions were described in 46 of the feedback facilitation interventions; we describe who undertook this tailoring work. Exploring dose, there was large variation in duration (15–1800 min), frequency (1 to 42 times) and number of recipients per site (1 to 135). There were important gaps in reporting, but some evidence that reporting is improving over time.
Conclusions
Heterogeneity in the design of feedback facilitation needs to be considered when assessing the intervention’s effectiveness. We describe explicit feedback facilitation choices for future intervention developers based upon choices made to date. We found the Expert Recommendations for Implementing Change to be valuable when describing intervention components, with the potential for some minor clarifications in terms and for greater specificity by intervention providers. Reporting demonstrated extensive gaps which hinder both replication and learning. Feedback facilitation providers are recommended to close reporting gaps that hinder replication. Future work should seek to address the ‘opportunity’ for improvement activity, defined as factors that lie outside the individual that make care or improvement behaviour possible.
Review registration
The study protocol was published at: https://www.protocols.io/private/4DA5DE33B68E11ED9EF70A58A9FEAC02 .
Peer Review reports
Contribution to the literature
Feedback facilitation delivered alongside audit and feedback is recommended to increase improvement in care.
We describe the content and delivery of feedback facilitation within randomized controlled trials and identify design choices.
Feedback facilitation is a heterogenous intervention that uses different implementation strategies. The number of strategies being used is increasing. The target of these strategies varies from direct impact upon care to seeking to implement improvement activities. We describe the use of theory logic models and the enactment of tailoring.
The ability to replicate a study underpins implementation science and impact. Few studies provide sufficient detail to enable replication.
Audit and feedback is a complex intervention that involves the delivery of feedback on performance over a specific period [ 1 ]. Health professionals may not have the knowledge and skills to engage and respond to feedback, and this may create variation in the effectiveness of audit and feedback [ 2 , 3 ]. Health systems are investing in quality improvement support to feedback recipients [ 4 , 5 ]
Brown and colleagues [ 2 ] describe quality improvement co-interventions as supporting feedback recipients, “to identify the reasons for and develop solutions to sub-optimal performance” (p16). Quality improvement support is a form of feedback facilitation that might help recipients to identify, “barriers and enablers for making change” [ 6 ; p3]. The identification of influences and selection of actions to address these is known as ‘tailoring’ [ 7 ]. In addition to tailoring, authors have described the need to develop commitment, as the shared resolve to implement a change [ 8 ]; for example, through describing implications of current audit performance [ 9 ].
There is a lack a clarity about the content and delivery of feedback facilitation. Facilitation is associated with enabling and making a target behaviour easier. In the context of audit and feedback, facilitation might include how to use feedback, undertake quality improvement or set goals and plans [ 10 ]. Beyond feedback-specific facilitation, Richie and colleagues describe 22 implementation facilitation skills, including engaging stakeholders, problem-identification/solving and education skills. The template for intervention description and replication (TIDieR) [ 11 ] provides a guide to describe the content of interventions. TIDieR highlights the importance of describing what is delivered and why, who delivers the intervention, how, where, when and how much, whether there is tailoring, modifications and if fidelity is assessed and delivered. In relation to what is delivered, the Expert Recommendations for Implementing Change (ERIC) [ 12 ] describes 73 different types of implementation intervention. In relation to why a particular intervention is delivered, Colquhoun and colleagues [ 13 ] described gaps in the use of theory within audit and feedback interventions. Feedback facilitation could be considered an implementation strategy. Within the current manuscript we will refer to feedback facilitation as an intervention (Table 1 ), to be consistent with the description of multi-faceted interventions [ 1 , 2 ], co-interventions [ 2 ] and complex interventions [ 14 ], and to reflect that feedback facilitation may be composed of multiple implementation strategies.
Multiple authors (e.g. [ 18 , 19 , 20 ]) recommend using logic models to describe the programme theory for an intervention. Describing the intervention components enables replication across contexts with fidelity of identified core components [ 14 ]. Lewis and colleagues [ 17 ] describe potential components in the causal pathway of interventions: intervention mechanism(s); context; pre-conditions and/or moderators; proximal and distal outcomes. Such frameworks provide a further lens through which to describe the content and delivery of feedback facilitation interventions.
The effectiveness of audit and feedback with or without a feedback facilitation co-intervention is being explored during the updated Cochrane review of randomised controlled trials. Understanding the content and delivery, as well as the effectiveness, of an intervention is extremely valuable and supports the interpretation and use of the findings. The aim of the current study is to describe the content and delivery of feedback facilitation co-interventions used in trials of audit and feedback.
We explored the content of feedback facilitation co-interventions reported in randomised controlled trials of audit and feedback (A&F). Feedback facilitation trials were identified from the latest update of the Cochrane review of audit and feedback. Within the Cochrane review, co-interventions were described as a form of feedback facilitation, which “could be training about how to use feedback, or to do quality improvement in the practice, or set goals and plans, etc.” [ 10 ].
The search criteria and identification of studies is reported by the Cochrane review [ 21 ] team, who provided the papers identified as containing feedback facilitation. We reviewed these papers and their citations for further details describing the intervention content.
Inclusion criteria: Papers describing interventions delivered in randomised controlled trials of audit and feedback with additional feedback facilitation co-intervention delivered to health care workers. There were no exclusion criteria.
Participants
Audit and feedback and/or feedback facilitation developers and/or deliverers.
Intervention
Feedback facilitation co-interventions delivered alongside audit and feedback.
Quality assessment
Quality was assessed as part of the Cochrane review.
Data collection and management
We extracted data from papers describing the trial, from publicly available protocols and from companion papers. Eight reviewers extracted data from the included studies using a specifically designed and piloted proforma adapted from the TIDieR framework [ 11 ]. The adapted proforma extended TIDieR to capture the form of implementation strategy [ 12 ], theory and logic model, the identification of influences upon performance and work to align improvement actions to influences, whether information to describe the implications of performance was reported and the level of change sought.
The adapted proforma also enabled us to explore whether and how the feedback facilitation co-interventions supported teams to tailor their response to feedback. In identifying whether the facilitation explored influences upon performance, we looked for whether influences or causes were given, sought by data recipients or not recorded. The extraction guide (Appendix A) provided the example of using a framework to identify determinants or other potential list of influences from which recipients selected. The data extractors described the procedure to explore influences using language similar to that in the text and categorised this as ‘sought by data recipients’, ‘given by study team’, ‘co-produced’ or ‘not recorded’. The extractors then described the presence or absence of a process by which implementation strategies were determined; for example, whether they were given by the study team or selected by data recipients.
Data was recorded and managed in Excel
Duration of feedback facilitation was calculated in minutes; where specified in days, duration was converted to 450 min per day. Maximum duration was used unless an average time was given. The deliverer of facilitation was classified into expert, peers or improvement specialists [ 22 ].
We developed and piloted reviewer guidance notes to accompany the proforma (Appendix A). Each paper was reviewed by 2 reviewers. The reviewers were health service researchers, five of whom were also clinicians. Six reviewers were involved in the development of the codebook through iterative discussion, design and testing. Two further reviewers received training and supervision in use of the code book. The reviewers extracted separately, and disagreements were resolved through consensus between the two reviewers.
Data analysis and synthesis
Two members of the team (MS and SA, both experienced implementation scientists) cleaned the data set and used the extracted data to codify the ERIC strategies, referring to source papers where necessary. MS and SA analysed the data narratively, graphically and statistically using Excel and StataMP 17. Our analysis drew upon the full data set, with the exception of the narrative analysis of the use of theory, which focussed on the period since a review of the use of theory in audit and feedback [ 13 ]. Statistical analysis involved a linear regression to determine if the number of TIDieR framework items not reported changed with publication year. We examined plots of residuals from the regression analyses and performed a Breusch-Pagan test for heteroskedasticity. The synthesis was presented to the research team for challenge. We summarised the content of feedback facilitation interventions and drew upon guidance and wider literature to consider implications for research and practice. To support feedback facilitation providers, we made the different forms of content and delivery that we identified explicit as a series of design choices.
The protocol for this review has been published separately [ 23 ]. We report upon variations from the protocol in the discussion.
The Cochrane review identified 104 randomised controlled trials that delivered feedback facilitation alongside audit and feedback. We included 146 papers describing these trials, as detailed in the below flowchart (Fig. 1 ).
The PRISMA flowchart
Table 2 summarises the content and delivery of feedback facilitation in 104 trials. Additional data is provided in the Supplementary Materials. Table 3 presents a cumulative summary of the content across included studies.
Date and setting
Included trials dated from 1982 to 2020 (Fig. 2 ). The included studies took place in primary care ( n = 54; 52%), secondary care ( n = 43; 41%), two in both primary and secondary care, 3 in nursing homes, 1 in an antenatal clinic (unclear whether primary or secondary care) and 1 in dental practice.
A graph describing the date and frequency of included studies of feedback facilitation and the mean number of strategies used per feedback facilitation intervention
Expert Recommendations for Implementing Change (ERIC) strategies
We identified 26 different implementation strategies within feedback facilitation (Fig. 3 ). The median number of strategies per trial was 3 (IQR 2–4 strategies). Figure 2 shows that the number of strategies used within feedback facilitation interventions has increased over time. There were no apparent differences in the number of strategies used depending on whether the feedback facilitation intervention was undertaken in primary or secondary care (Supplementary materials 6 & 7).
A graph of the frequency of implementation strategy use within included studies [*Added to ERIC coding]

Use of theory and logic models
We found 35 studies (34%) that described using theory. A total of 31 theories were referenced within the included papers. The most frequent were adult learning theory ( n = 5; 5%) [e.g. 171 ], Rogers’ diffusion of innovation theory ( n = 4; 4%) [ 172 ], Bandura’s self-efficacy theory ( n = 4; 4%) [ 173 ], and Bandura’s social learning theory ( n = 4; 4%) [ 174 ]. We found that theory was most frequently used in intervention design. Data from papers published since Colquhoun and colleagues’ exploration of the use of theory in studies of audit and feedback [ 13 ] are presented in the Supplementary Materials 2. As illustrated by the quotes, we found that it was often difficult to understand how the authors’ applied theory; for example, “(we) combined strategies shown to change providers’ behaviour with those based on the diffusion of innovation theory” [ 24 ] and “technology-assisted learning resources were also developed using motivational systems and instructional design theory” [ 45 ].
We found 10 studies provided a logic model to describe the intervention. Table 4 summarises the content of these logic models.
Materials used in feedback facilitation
Feedback facilitation interventions used a range of materials (Supplementary Materials 1). We grouped these into the following categories:
Materials to support clinician behaviour change by addressing capability, for example, evidence-based guidelines (e.g. [ 28 , 34 , 99 ]), reminder stickers and cards ([e.g. 100 , 116 , 143 ]), written educational materials (e.g. [ 111 , 118 , 149 ]). We identified a subset of these materials that was administrative equipment such as patient care record [ 84 ], x-ray ordering stamps [ 93 ] and ordering sets [ 99 ].
Materials to address clinician motivation; for example, information about reimbursement [ 132 ]. It is possible that some of the other materials described above as addressing capability may have addressed motivation (e.g. relating to patient outcome), although this was not clear from the description.
Materials to support patient behaviour change by addressing capability; for example, patient information leaflets (e.g. [ 155 ]) and self-help materials [e.g. 121 ].
Clinical equipment to support clinician behaviour change by addressing ‘opportunity’; for example, testing kits (e.g. [ 24 ]) and clinical assessment tools (e.g. [ 114 ]).
Materials to support the improvement work: Help to analyse influences (e.g. critical event analysis form [ 58 ]; a description of ways to use the audit results, including discussions with colleagues, detailed follow up surveys among patients, and establishment of a patient panel [ 159 ]); Help both to select strategies (e.g. written recommendations [ 27 ]) and to enact strategies (e.g. action plan [ 58 ], amendable template to give information to stakeholders [ 164 ]).
Identification of priorities
We explored whether and by whom priorities were identified from within the performance feedback during feedback facilitation. In 43 studies (43%), priorities for improvement were identified by the feedback facilitators; for example, Hendryx and colleagues’ educational outreach included that “the (study) team member reviewed the findings, and offered concrete, practical suggestions for improvement” ([ 82 ] p420). In 19 studies (18%), priorities were identified by feedback recipients; for example, Ivers and colleagues provided a worksheet “to facilitate goal-setting” ([ 91 ] p3). In 4 studies (4%), there was evidence that priorities were co-designed between the study team and the feedback recipients [ 60 , 61 , 84 , 85 ]. For example, Frijling and colleagues provided feedback facilitation where “the facilitator and the GPs discussed the content of the feedback reports, prioritized specific aspects of decision making to be improved and made change plans” ([ 60 ] p837). In 39 studies (38%), it was not possible to determine whether or by whom priorities were identified within the performance feedback.
Exploration of influences upon performance
We explored whether and how influences upon performance were investigated within feedback facilitation: In 12 studies (12%), influences upon performance were given by the feedback facilitators; for example, “data presented included hospital-specific baseline performance data and information on knowledge and organizational barriers to stroke care identified by the surveys... (including) organisational barriers such as lack of order sets and pathways” ([ 99 ] p1635). In 32 studies (31%), influences upon performance were explored by feedback recipients; for example, “a 90-min standardized small group quality improvement meeting, supervised by the medical coordinator of the diagnostic center… (including) a thorough discussion of the difficulties of achieving changes at the individual primary care physician level, the practice level, or at the patient level” ([ 157 ] p2408). In 9 studies (9%), we identified that a description of influences upon performance was co-produced by the study team and the feedback recipients. However, there were blurred boundaries between co-produced identification of influences and where influences were sought by feedback recipients; for example, where a focus group might have a facilitator, it was unclear the extent to which they provided a structure or were more directional. In Kennedy et al.’s study, co-ordinators facilitated interdisciplinary care teams to identify “barriers and facilitators to implementing evidence-based strategies, particularly changes that could be made at an organizational level” (p4). In 51 studies, it was not possible to determine whether/how influences upon performance were explored.
Where influences upon performance were given, these may be based upon previous research, including as part of intervention development. Influences upon performance were sought by recipients both in discussion and using proformas. Some focussed on specific barriers (e.g. confidence [ 78 ]) whilst others used a broader lens; for example, Chaillet et al. [ 43 ] described that, "the training program also sensitized participants to social, economic, organizational, cultural and legal factors”. Proformas were used to support recipients to explore influences (e.g. [ 58 ]). The depth of exploration varied (e.g. a 3-h training session [ 155 ] or 20-min exercise [ 111 ]) and may be a collective (e.g. focus group [ 27 ]) or individual exercise (e.g. [ 116 ]). Co-production included national analysis followed by local tailoring, information gathering from patients followed by healthcare worker selection and the sharing of learning between sites.
There were no apparent differences in whether the influences were sought by recipients, given or co-produced depending on whether the feedback facilitation intervention was undertaken in primary or secondary care (Supplementary materials 7).
Determining implementation strategies
We explored how strategies were selected: In 33 studies (32%), improvement strategies were given by the feedback facilitators, in 27 studies (26%) they were determined by the feedback recipients. Improvement strategies were co-designed in 20 studies (19%). In 24 studies (23%), it was not reported who determined the improvement strategies.
The suggested strategies given by the study team were sometimes generic suggestions to all teams (e.g. [ 52 ]), and sometimes site specific (e.g. [ 34 ]). Where the strategies were determined by recipients, this included doing so with the support of learning from other sites [ 126 ] and using a plan-do-study-act template [ 93 ]. Co-produced strategy selection included selection from a list of strategies provided by the study team and adaptation of proposed strategies (e.g. [ 68 ]). Proposed strategies could be in a list presented by peers (e.g. [ 47 ]), and/or described in meeting, webinars or calls (e.g. [ 146 ]).
The Sankey chart (Supplementary Materials 3) illustrates the lack of relationship between who identified influences and who identified strategies: In 10 (10%) trials, both the identification of influences and identification of strategies was undertaken by recipients; in 4 (4%) trials, both were given by the study team.
There were no apparent differences in whether the actions were determined by recipients, given or co-produced depending on whether the feedback facilitation intervention was undertaken in primary or secondary care (Supplementary materials 7).
Identification of implications of performance
We explored whether feedback facilitation involved identifying implications of performance. We found that implications were given as part of feedback facilitation in 36 studies and identified by feedback recipients in 7 studies (7%). In 61 studies (59%), consideration of implications was not reported. There were no apparent differences in whether the feedback facilitation intervention was undertaken in primary or secondary care (Supplementary materials 7).
Other intervention components
We looked for additional components to the intervention, not described above. We found additional components that sought to address capability and motivation: Components to address capability targeted both capability to improve (e.g. [ 68 ]) and capability to deliver care (e.g. [ 89 ]). Interventions to increase motivation included motivational text messages (e.g. [ 47 ]), celebrating good practice (e.g. [ 82 ]), and positional leader prioritisation [e.g. [ 82 ]. These may have had some impact upon the social opportunity by changing the social environment (e.g. giving permission). We did not identify additional components that specifically targeted ‘opportunity’ for the target behaviours, defined as factors that lie outside the individual that make the care or improvement behaviour possible.
Delivery of feedback facilitation
A variety of modes were used to deliver facilitation, with the most common being face-to-face ( n = 86; 83%) and educational materials ( n = 52; 50%). Virtual delivery by telephone ( n = 16;15%) and online ( n = 12; 12%) was less commonly used, which is likely to be due in-part to the age of the literature. Most studies used one ( n = 45; 43%) or two ( n = 50; 48%) methods of delivery, with fewer using three ( n = 7; 7%; [ 38 , 45 , 47 , 52 , 82 , 84 , 136 ]).
Frequency of feedback facilitation
Most studies delivered feedback facilitation between 1 and 3 times (median = 3, interquartile range 1–5). Six studies (6%) delivered facilitation 15 times or more [ 24 , 60 , 61 , 100 , 114 , 115 ]). The maximum times feedback facilitation was delivered was 42 times [ 115 ]. Data was not available for 25 studies (24%).
Duration of feedback facilitation
Feedback facilitation delivery took between 15–1800 min, with a median of 120 min and IQR of 75–420 min. For studies with over 420 min of facilitation, this was delivered over several consecutive days and/or as follow up calls following initial delivery. 45 studies (43%) did not record the delivery time.
Timing of feedback facilitation
Most facilitation was delivered with ( n = 37; 36%) or after ( n = 32; 31%) feedback delivery, so that the feedback could be reviewed with the participants. There were some studies that delivered before ( n = 14; 13%), although ten of these studies (10%) also included facilitation during and/or after feedback. The five studies (5%) that only delivered facilitation pre-feedback all included educational materials. Three of these studies (3%) reported local identification of priorities [ 39 , 42 , 58 ], whilst it was not reported in the other two [ 43 , 76 ].
Who delivered feedback facilitation
Most facilitation was delivered by experts (e.g.specialist physicians with expertise in osteoporosis or geriatrics [ 93 ]) ( n = 50; 48%), followed by peers (e.g. local co-ordinators [ 24 ] ( n = 31; 30%) and then quality improvement specialists ( n = 21; 20%)) (Supplementary Materials 4). Facilitation was delivered virtually through a computer programme in two studies (2%) [ 39 , 151 ] (Supplementary Materials 1). We discuss challenges with coding this data below.
Who received feedback facilitation
The majority of facilitation was delivered to clinicians ( n = 86; 83%), with a smaller number including both clinicians and non-clinical/managerial ( n = 10; 10%). There were no instances of facilitation being delivered to managers only. In eight studies (8%), it was unclear who were the recipients.
Number of recipients receiving feedback facilitation per site
It was difficult to determine the number of recipients of facilitation per site, with 68 studies (65%) either not reporting or providing unclear descriptions. The number of recipients per site ranged from 1 to 135. Studies variably described both minimum and maximum recipients per site, with others giving averages but no range. Of the 36 studies (35%) reporting recipients per site, most sites had small groups of 10 or fewer recipients ( n = 28; 28%).
Number of intervention sites receiving feedback facilitation
The number of intervention sites ranged from 1 to 811, with a median of 19 and IQR of 12–38. Data was skewed to the right by 18 studies with large intervention site numbers over 50. Two studies (2%) did not report the number of intervention sites [ 123 , 127 ].
Comparison of recipients per site with number of intervention arm sites
Where both number of recipients per site and number of intervention sites were recorded ( n = 33; 32%), the trend was for the number of recipients per site to decrease as the number of intervention sites increased, however this was not statistically significant on linear regression ( p = 0.86, Confidence intervals –0.55 to 0.21) (Supplementary Materials 5).
Number of people receiving the intervention at one time
Most studies ( N = 71; 70%) did not record the number of people receiving the intervention at each time; Of the 33 studies (32%) that did, the intervention was delivered most frequently to an individual ( n = 9; 9%) and most were delivered to 10 or fewer individuals ( n = 28; 27%). Two studies (2%) [ 98 , 102 ] delivered to 11–20 people and three (3%) to 21 or more [ 70 , 147 , 162 ]. The maximum number of people the intervention was delivered to at one time was 45 [ 70 ].
Comparison of number of people receiving the intervention at one time by setting
Interventions delivered in secondary care were often delivered to a larger number of people than those delivered in primary care (secondary care median = 8, IQR = 2.5–16; primary care median = 3.5, IQR = 1–7.5), however this was not a statistically significant finding on a Mann–Whitney U test ( p = 0.16) (Supplementary materials 6). This is likely due to primary care studies involving feedback to individual practitioners and smaller team sizes compared to secondary care. The lack of studies describing how many people received the intervention at one time makes drawing conclusions difficult.
Level of change sought
Most facilitation sought a level of change at the team level ( n = 74, 71%), with fewer studies seeking level of change at multi-team organisation levels ( n = 23, 22%), at the wider system ( n = 5, 5%), 2 studies directly targeted patient-level change [ 45 , 150 ]. For example, Clarke and colleagues provided evidence-based education for women through two antenatal classes as part of an intervention to increase the rates of vaginal birth after caesarean section.
Tailoring of feedback facilitation
Only 19 studies (19%) reported tailoring of the intervention delivery. Types of tailoring included tailoring of the content to identified needs and barriers and local context (e.g. [ 34 , 73 , 122 , 161 , 166 ]) and additional episodes of facilitation in response to need (40,131). For example, Quinley and colleagues focussed facilitation on physicians with poorer performance where a practice contained multiple physicians [ 132 ]. Brown and colleagues [ 42 ] tailored content to existing level of knowledge and delivery through, “the use of a variety of media including individualised tuition and feedback” (p443).
Assessment of fidelity
Fidelity of facilitation was reported and described as assessed in 41 studies (39%). Where assessed, 27 out of the 41 studies reported fidelity achievement, given either as a range or mean adherence. Fidelity ranged from 29 to 100%.
Modification
Most studies ( n = 60, 58%) did not report whether any modifications to the intervention took place. Of those that did, 18% ( n = 8) reported making modifications whereas 82% ( n = 36) did not. Examples of modifications included additional re-training sessions [ 24 ], modifications due to online system malfunctions [ 38 ], changes to number of facilitation sessions offered [ 74 ] and delivery mode, for example, where the source was unable to continue to deliver feedback facilitation in-person, so later delivery changed to teleconference [ 93 ]. Reporting of the presence or not of modifications to facilitation interventions is improving over time, with 53% of studies reporting modifications published in 2010 or later and 88% since 2000.
Reporting of TIDieR intervention content items
The number of TIDieR items not reported within each study was determined to give a score out of 18. The results are presented in Supplementary Table 1b. The non-reporting of items ranged from 2 to 14, with a median of six content items not recorded (IQR 4.75–8). The number of items not reported reduced over time ( p < 0.05) on linear regression, however this only explained 5% of the variation. Heteroskedasticity was not present on testing ( p = 0.72).
We describe the content and delivery of the feedback facilitation to support designers of future feedback facilitation interventions. Our systematic review of 146 papers describes feedback facilitation delivered alongside audit and feedback in 104 randomised controlled trials. The papers were identified during the Cochrane review of audit and feedback. [ 21 ] The Cochrane review includes an assessment of the effectiveness of feedback facilitation.
We found feedback facilitation is a heterogeneous intervention containing at least one of 26 different implementation strategies and drawing upon each of the 9 implementation strategy groupings [ 175 ]. We found evidence that the number of strategies used per intervention is increasing over time (Fig. 2 ). To support future delivery of feedback facilitation we have used this heterogeneity to illuminate previous intervention design choices (Table 5 ). This is not intended to represent an exhaustive list of choices. In making these choices, guidance [e.g. 14 ] recommends intervention developers draw upon evidence, theory and stakeholder views about patient outcomes, proximal outcomes, mechanisms, context, pre-conditions and/or moderators and the intervention content. Articulating these may both support consideration of the coherence of the intervention and evaluation of whether it was provided as planned. Detailed description of planned and actual content also supports learning and replication of delivery. We propose both future work with stakeholders to evolve the design options, and further studies evaluating the impact of these choices upon effectiveness and implementation outcomes such as feasibility, appropriateness and acceptability [ 176 ].
These design choices have important implications, including those related to tailoring and dose
In relation to tailoring, the source of both the influences and the selection of improvement actions may impact upon the effectiveness of the intervention; for example, strategies selected by the study team may have a more explicit link to theory and evidence, and may include external stakeholders able to challenge existing mental models. Conversely, the study team’s interpretation of the influences upon performance and the alignment between influences and strategies may differ from those involved in change-making, which might undermine buy-in and create barriers to specification of the change. Future research that investigates the impact of different sources and of co-produced tailoring would support providers of feedback facilitation.
We measured the ‘dose’ of the facilitation and found wide variation, including the duration (15 to 1800 min), frequency of facilitation (1 to 42 episodes) and the number of recipients per site (1 to 135). There was also wide variation in the number of people receiving the intervention at once (1 to 45) and different modes used (e.g. through materials, face-to-face or virtual approaches). Future work could investigate the most (cost-) effective way to deliver feedback facilitation; for example, through the use of SMART optimisation designs [ 177 ] with economic evaluations. Such studies should assess both cost of delivery and of receipt. Consideration of real-life scalability would be valuable, given only one study delivered to more than 150 sites. All studies delivered facilitation to an intervention group. Questions remain about whether an adaptive intervention delivering sequences of feedback facilitation strategies as a co-intervention to audit and feedback, where the type, intensity or modality of the co-intervention evolve according to changing recipient responsiveness to feedback, might be more (cost-) effective.
Implementation strategies may contain different behaviour change techniques and act upon different mechanisms [ 17 , 178 ]. The heterogeneity of feedback facilitation undermines the ability to draw conclusions about its effectiveness. We found that the ERIC compilation provided a valuable tool for identifying component strategies. However, given more recent work describing potential behaviour change techniques within strategies [ 178 ], it would support replication and learning if future papers describe the active ingredients (such as, instruction on how to perform behaviour, information about health consequences or social support) within strategies. We identified overlap in the content of ERIC strategies; for example, learning collaboratives often contained educational meetings, re-examining implementation, small tests of change, whilst other studies that also delivered these elements to multiple sites at once may not be described as a learning collaborative. Where an intervention was in the overlap between ERIC definitions, we used the terms used by the authors to categorise the intervention components. We were unable to code motivational text messages [ 47 ] using ERIC and included them as an additional strategy. Similarly, we determined that ‘clinical decision support systems’ incorporated both ‘change the clinical record system’ and ‘remind clinicians’ as the closest match. We found that 47 ERIC strategies were not incorporated into feedback facilitation (Supplementary materials 8) and may provide alternative content to future feedback facilitation providers; for example, to promote adaptability.
We explored whether reporting was improving over time. We found that later reported studies had fewer non-reports of TIDieR items as expected with changes in publishing requirements, but this only explains 5% of the variance. Further action to improve reporting may be needed to support interpretation of results, replication of interventions and the advancement of implementation science. We draw particular attention to the omission of the rationale and proximal target of the intended change.
As recommended in TIDieR [ 11 ], we sought the underlying rationale for the use of feedback facilitation: 35 studies referenced the use of theory and 10 studies provided a logic model describing their programme theory. Understanding the underlying rationale for an intervention supports replication, as adaptation around core components increases fit to the new context [ 14 ]. Describing the programme theory of an intervention also supports interpretation of results; for example, consideration of the coherence of the intervention, the proposed mechanism of effect, the context, the work being done by the intervention recipient and the assessed outcomes. Detailing causal pathways helps advance implementation science [ 17 ]. We found that within the 10 trials that had a logic model, there were gaps in the reporting of mechanisms (reported in 6 studies) and of contextual, predisposing or moderating factors (reported in 5 studies); Studies reporting this detail dated from 2011.
We found that feedback facilitation interventions sought to address motivation and capability. This was evidenced within: the proximal and distal outcomes where logic models were provided; the intervention materials (e.g. providing guidelines, detailing impacts upon outcomes and information about reimbursement and providing patient information and self-help materials); and the additional components. In relation to capabilities, the interventions sought to target both capabilities to improve (e.g. support to analyse influences upon care using a critical event analysis form or action plan template) and capabilities to deliver care (e.g. reminder cards or guideline documents). However, the target of the intended proximal change was often unclear; for example, whether education targeted improvement capabilities or knowledge about clinical care. Behaviour change literature (e.g. [ 179 ]) recommends specifying the target behaviour prior to the development of interventions. Omitting this information again hampers replication and the advancement of knowledge about what influences different behaviours. We found few examples of interventions addressing opportunity. Interventions may be enhanced by supporting the opportunity to undertake the improvement work; for example, by explicitly bringing that work into a workshop [ 9 ].
Strengths and limitations
There were minor variations from the protocol: We had planned to exclude papers that provided training in the target care practice, rather than the use of feedback, but found that it was not possible to identify the target behaviour of such training. We also planned to explore the extent to which the co-intervention was solely feedback facilitation but heterogeneity within feedback facilitation undermined our ability to assess this.
We sought the presence of a logic model, as recommended by guidance [ 19 ]. More recent guidance [ 14 ] recommends that a logic model is accompanied by a more detailed description of the programme theory; some studies (e.g. [ 123 ]) provided a narrative summary of the programme theory without a logic model. We included 146 papers describing 104 trials, however as with all reviews, there is a risk that we missed papers. We focussed on feedback facilitation within trials, which may differ from feedback facilitation undertaken outside of clinical trials. We included one paper [ 156 ] which described feedback facilitation alongside audit, but that was subsequently excluded from the Cochrane review due to the nature of the outcomes measured. Our data extraction template was adapted from the TIDieR framework, with the addition of prompts to seek strategy type, whether/how priorities for improvement were identified, whether/how influences upon performance were sought, whether/how strategies were selected and whether/how implications from performance were identified. Whilst we also sought other components to the intervention, it is possible that different prompts may have identified alternative factors important to the design and delivery of feedback facilitation. It is possible that increased granularity by categorising at the level of behaviour change technique (BCT) rather than ERIC strategy may have been useful, however gaps in recording would have been amplified at the active ingredient level. It is also possible that future feedback facilitation reviewers are seeking information about the mode of delivery found in ERIC strategies but missing from BCTs. We resolved disagreements through discussion but did not keep a record of the content of the discussion. The intervention deliverer (e.g. expert, peer) was difficult to assess from the information provided. It is possible that what is key is whether they are perceived as 'experts' or 'peers' (for example, if they are viewed as ‘credible source’ [ 180 ]), an assessment which might be made by each participant, rather than on the basis of a job title. In piloting, the reviewers found it difficult to agree on whether strategies addressed capability, opportunity or motivation. As a result, this was assessed by two reviewers (MS and SA) with training and experience of using COM-B [ 15 ] as part of a focussed assessment of the target of specific strategies. We focussed on the content and delivery and characteristics of the feedback recipients, the setting and the level of change sought. We did not collect information about the target behaviours upon which feedback is being given. Further work to explore the relationship between characteristics of the target behaviour(s) and the content and delivery of feedback facilitation may identify additional design choices.
Feedback facilitation is a much-used intervention delivered alongside large-scale audit and feedback to increase effectiveness. Health system policy and theory-informed hypotheses advocate for the delivery of feedback facilitation, often referred to as support for quality improvement. We describe heterogeneity in the design of feedback facilitation, highlighting some of the design choices for future providers (Table 5 ). We were able to describe the components with feedback facilitation using ERIC, but there was the opportunity for some minor clarifications in terms and for intervention providers to provide greater specificity. Whilst reporting demonstrated extensive gaps, hindering replication and learning, there was some evidence that reporting is improving over time. We recommend future work to consider the role of ‘opportunity’ within intervention designs and the use of evaluation techniques to maximise intervention efficiency.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary materials].
Abbreviations
Audit and facilitation
Behaviour change techniques
Expert Recommendations for Implementing Change
Feedback facilitation
Health Quality Improvement Partnership
Inter-quartile range
Preferred Reporting Items for Systematic Reviews & Meta-Analyses (Appendix B)
Quality Improvement Collaborative
Sequential Multiple Assignment Randomized Trial
Theoretical domains framework
Template for Intervention Description and Replication
Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, O’Brien MA, Johansen M, Grimshaw J, Oxman AD. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6:CD000259.
Google Scholar
Brown B, Gude WT, Blakeman T, van der Veer SN, Ivers N, Francis JJ, Lorencatto F, Presseau J, Peek N, Daker-White G. Clinical performance feedback intervention theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research. Implement Sci. 2019;14(1):40.
Article PubMed PubMed Central Google Scholar
Colquhoun HL, Carroll K, Eva KW, Grimshaw JM, Ivers N, Michie S, Brehaut JC. Informing the research agenda for optimizing audit and feedback interventions: results of a prioritization exercise. BMC Med Res Methodol. 2021;21(1):1–8.
Article Google Scholar
Health Service Executive. National Review of Clinical Audit. Health Service Executive: Dublin, Ireland. 2019. Available from: national-review-of-clinical-audit-report-2019.pdf. Accessed 26 Sept 2023.
Healthcare Quality Improvement Partnership. Maximising the Quality Improvement potential of the National Clinical Audit and Patient Outcomes Programme. HQIP: London, UK; 2021. Available at: https://www.hqip.org.uk . Accessed 26 Sept 2023.
Cooke LJ, Duncan D, Rivera L, Dowling SK, Symonds C, Armson H. How do physicians behave when they participate in audit and feedback activities in a group with their peers? Implement Sci. 2018;13(1):104.
McHugh SM, Riordan F, Curran GM, Lewis CC, Wolfenden L, Presseau J, Lengnick-Hall R, Powell BJ. Conceptual tensions and practical trade-offs in tailoring implementation interventions. Front Health Serv. 2022;17(2):974095.
Weiner BJ. A theory of organizational readiness for change. Implement Sci. 2009;4(1):1–9.
Sykes M, O’Halloran E, Mahon L, McSharry J, Allan L, Thomson R, Finch T, Kolehmainen N. Enhancing national audit through addressing the quality improvement capabilities of feedback recipients: a multi-phase intervention development study. Pilot Feasibility Stud. 2022;8(1):1–8.
Ivers N. Audit and Feedback Systematic Review: Data Abstraction Guide. 2019. Unpublished.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014;348. https://doi.org/10.1136/bmj.g1687 .
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21.
Colquhoun HL, Brehaut JC, Sales A, Ivers N, Grimshaw J, Michie S, Carroll K, Chalifoux M, Eva KW. A systematic review of the use of theory in randomized controlled trials of audit and feedback. Implement Sci. 2013;8(1):1–8.
Skivington K, Matthews L, Craig P, Simpson S, Moore L. Developing and evaluating complex interventions: updating Medical Research Council guidance to take account of new methodological and theoretical approaches. Lancet. 2018;1(392):S2.
Michie S, Van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(1):1–2.
Rychetnik L, Hawe P, Waters E, Barratt A, Frommer M. A glossary for evidence based public health. J Epidemiol Comm Health. 2004;58:538–45.
Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, Walsh-Bailey C, Weiner B. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health. 2018;7(6):136.
Smith JD, Li DH, Rafferty MR. The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. 2020;15:1–2.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. https://doi.org/10.1136/bmj.a1655 .
Mills T, Lawton R, Sheard L. Advancing complexity science in healthcare research: the logic of logic models. BMC Med Res Methodol. 2019;19(1):1–1.
Ivers N, Antony J, Konnyu, K, O'Connor D, Presseau, J,Grimshaw J. Audit and feedback: effects on professional practice [protocol for a Cochrane review update]. 2022. Available from: https://zenodo.org/record/6354035 Accessed 26 Sept 2023.
Cooke LJ, Duncan D, Rivera L, Dowling SK, Symonds C, Armson H. The Calgary Audit and Feedback Framework: a practical, evidence-informed approach for the design and implementation of socially constructed learning interventions using audit and group feedback. Implement Sci. 2018;13(1):1–8.
Sykes M, Alderson S, Thomas O, Caulfield K, Robinson L, Quigley M, Gupta L, Rosenberg-Yunger Z. Exploring the components of feedback facilitation: a systematic review. Available from: https://www.protocols.io/view/exploring-the-components-of-feedback-facilitation-8epv5jm8dl1b/v1 . Accessed 26 Sept 2023.
Althabe F, Chomba E, Tshefu AK, Banda E, Belizán M, Bergel E, Berrueta M, Bertrand J, Bose C, Cafferata ML, Carlo WA. A multifaceted intervention to improve syphilis screening and treatment in pregnant women in Kinshasa, Democratic Republic of the Congo and in Lusaka, Zambia: a cluster randomised controlled trial. Lancet Glob Health. 2019;7(5):e655–63.
Nkamba D, Mwenechanya M, Kilonga AM, et al. Barriers and facilitators to the implementation of antenatal syphilis screening and treatment for the prevention of congenital syphilis in the Democratic Republic of Congo and Zambia: results of qualitative formative research. BMC Health Serv Res. 2017;17:556.
Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Boyd M, Cresswell K, Eden M, Elliott R, Franklin M, Hippisley-Cox J, Howard R. PINCER trial: a cluster randomised trial comparing the effectiveness and cost-effectiveness of a pharmacist-led IT-based intervention with simple feedback in reducing rates of clinically important errors in medicines management in 113, general practices. Available at: https://web.archive.org/web/20170809093609id_/http://www.birmingham.ac.uk/Documents/college-mds/haps/projects/cfhep/psrp/finalreports/PS024PINCERFinalReportOctober2010.pdf . Accessed 29 Aug 2023.
Awad AI, Eltayeb IB, Baraka OZ. Changing antibiotics prescribing practices in health centers of Khartoum State, Sudan. Eur J Clin Pharmacol. 2006;62:135–42.
Article CAS PubMed Google Scholar
Ayieko P, Ntoburi S, Wagai J, Opondo C, Opiyo N, Migiro S, Wamae A, Mogoa W, Were F, Wasunna A, Fegan G. A multifaceted intervention to implement guidelines and improve admission paediatric care in Kenyan district hospitals: a cluster randomised trial. PLoS Med. 2011;8(4):e1001018.
Wagai J, Mbindyo P, Mbaabu L, et al. Implementation experience during an eighteen month intervention to improve paediatric and newborn care in Kenyan district hospitals. Implement Sci. 2009;4:45.
Ayieko P, Irimu G, Ogero M, Mwaniki P, Malla L, Julius T, Chepkirui M, Mbevi G, Oliwa J, Agweyu A, Akech S. Effect of enhancing audit and feedback on uptake of childhood pneumonia treatment policy in hospitals that are part of a clinical network: a cluster randomized trial. Implement Sci. 2019;14(1):1–4.
English M, Ayieko P, Nyamai R, Were F, Githanga D, Irimu G. What do we think we are doing? How might a clinical information network be promoting implementation of recommended paediatric care practices in Kenyan hospitals? Heal Res Policy Syst. 2017;15:4. https://doi.org/10.1186/s12961-017-0172-1 .
Baker R, Farooqi A, Tait C, Walsh S. Randomised controlled trial of reminders to enhance the impact of audit in general practice on management of patients who use benzodiazepines. BMJ Qual Saf. 1997;6(1):14–8.
Article CAS Google Scholar
Baldwin NS, Gilpin DF, Tunney MM, Kearney MP, Crymble L, Cardwell C, Hughes CM. Cluster randomised controlled trial of an infection control education and training intervention programme focusing on meticillin-resistant Staphylococcus aureus in nursing homes for older people. J Hosp Infect. 2010;76(1):36–41.
Barkun AN, Bhat M, Armstrong D, Dawes M, Donner A, Enns R, Martin J, Moayyedi P, Romagnuolo J, Stitt L. Effectiveness of disseminating consensus management recommendations for ulcer bleeding: a cluster randomized trial. CMAJ. 2013;185(3):E156–66.
Bertoni AG, Bonds DE, Chen H, Hogan P, Crago L, Rosenberger E, Barham AH, Clinch CR, Goff DC. Impact of a multifaceted intervention on cholesterol management in primary care practices: guideline adherence for heart health randomized trial. Arch Intern Med. 2009;169(7):678–86.
Bloos F, Rueddel H, Thomas-Rueddel D, Schwarzkopf D, Pausch C, Harbarth S, Schreiber T, Gründling M, Marshall J, Simon P, Levy MM. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med. 2017;43:1602–12.
Article PubMed Google Scholar
Bloos F, Thomas-Rüddel D, Rüddel H, Engel C, Schwarzkopf D, Marshall JC, Harbarth S, Simon P, Riessen R, Keh D, Dey K. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18(2):1.
Bond TC, Patel PR, Krisher J, Sauls L, Deane J, Strott K, McClellan W. A group-randomized evaluation of a quality improvement intervention to improve influenza vaccination rates in dialysis centers. Am J Kidney Dis. 2011;57(2):283–90.
Bonevski B, Sanson-Fisher RW, Campbell E, Carruthers A, Reid AL, Ireland M. Randomized controlled trial of a computer strategy to increase general practitioner preventive care. Prev Med. 1999;29(6):478–86.
Borgiel AE, Williams JI, Davis DA, Dunn EV, Hobbs N, Hutchison B, Wilson CR, Jensen J, O’Neil JJ, Bass MJ. Evaluating the effectiveness of 2 educational interventions in family practice. CMAJ. 1999;161(8):965–70.
CAS PubMed PubMed Central Google Scholar
Bregnhøj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol. 2009;65:199–207.
Brown LF, Keily PA, Spencer AJ. Evaluation of a continuing education intervention “Periodontics in General Practice.” Commun Dent Oral Epidemiol. 1994;22(6):441–7.
Chaillet N, Dumont A, Abrahamowicz M, Pasquier JC, Audibert F, Monnier P, Abenhaim HA, Dubé E, Dugas M, Burne R, Fraser WD. A cluster-randomized trial to reduce cesarean delivery rates in Quebec. N Engl J Med. 2015;372(18):1710–21.
Charrier L, Allochis MC, Cavallo MR, Gregori D, Cavallo F, Zotti CM. Integrated audit as a means to implement unit protocols: a randomized and controlled study. J Eval Clin Pract. 2008;14(5):847–53.
Clarke M, Devane D, Gross MM, Morano S, Lundgren I, Sinclair M, Putman K, Beech B, Vehviläinen-Julkunen K, Nieuwenhuijze M, Wiseman H. OptiBIRTH: a cluster randomised trial of a complex intervention to increase vaginal birth after caesarean section. BMC Pregnancy Childbirth. 2020;20:1–7.
Grylka-Baeschlin S, Nicoletti J, Sinclair M, Maguire R, Carroll M, Begley C. Process evaluation for OptiBIRTH, a randomised controlled trial of a complex intervention designed to increase rates of vaginal birth after caesarean section. Trials. 2018;19(1):9. https://doi.org/10.1186/s13063-017-2401-x .
Cundill B, Mbakilwa H, Chandler CI, Mtove G, Mtei F, Willetts A, Foster E, Muro F, Mwinyishehe R, Mandike R, Olomi R. Prescriber and patient-oriented behavioural interventions to improve use of malaria rapid diagnostic tests in Tanzania: facility-based cluster randomised trial. BMC Med. 2015;13:1–6.
Chandler CI, Meta J, Ponzo C, Nasuwa F, Kessy J, Mbakilwa H, et al. The development of effective behaviour change interventions to support the use of malaria rapid diagnostic tests by Tanzanian clinicians. Implement Sci. 2014;9:83.
Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, Back AL, Rubenfeld GD, Engelberg RA. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183(3):348–55.
Curtis JR, Treece PD, Nielsen EL, Downey L, Shannon SE, Braungardt T, Owens D, Steinberg KP, Engelberg RA. Integrating palliative and critical care: evaluation of a quality-improvement intervention. Am J Respir Crit Care Med. 2008;178:269–75.
Treece PD, Engelberg RA, Shannon SE, Nielsen EL, Braungardt T, Rubenfeld GD, Steinberg KP, Curtis JR. Integrating palliative and critical care: description of an intervention. Crit Care Med. 2006;34:S380–7.
DeVore AD, Cox M, Heidenreich PA, Fonarow GC, Yancy CW, Eapen ZJ, Peterson ED, Hernandez AF. Cluster-randomized trial of personalized site performance feedback in get with the guidelines-heart failure. Circ Cardiovasc Qual Outcomes. 2015;8(4):421–7.
Everett GD, deBlois CS, Chang PF. Effect of cost education, cost audits, and faculty chart review on the use of laboratory services. Arch Intern Med. 1983;143(5):942–4.
Fabbri C, Dutt V, Shukla V, Singh K, Shah N, Powell-Jackson T. The effect of report cards on the coverage of maternal and neonatal health care: a factorial, cluster-randomised controlled trial in Uttar Pradesh, India. Lancet Glob Health. 2019;7(8):e1097–108.
Filardo G, Nicewander D, Herrin J, Edwards J, Galimbertti P, Tietze M, Mcbride S, Gunderson J, Collinsworth A, Haydar Z, Williams J. A hospital-randomized controlled trial of a formal quality improvement educational program in rural and small community Texas hospitals: one year results. Int J Qual Health Care. 2009;21(4):225–32.
Filardo G, Nicewander D, Herrin J, et al. A hospital-randomized controlled trial of an educational quality improvement intervention in rural and small community hospitals in texas following implementation of information technology. Am J Med Qual. 2007;22:418–27.
Filardo G, Nicewander D, Herrin J, et al. Challenges in conducting a hospital-randomized trial of an educational quality improvement intervention in rural and small community hospitals. Am J Med Qual. 2008;23:440–7.
Foster JM, Hoskins G, Smith B, Lee AJ, Price D, Pinnock H. Practice development plans to improve the primary care management of acute asthma: randomised controlled trial. BMC Fam Pract. 2007;8(1):1–3.
Foy R, Penney GC, Grimshaw JM, Ramsay CR, Walker AE, MacLennan G, Stearns SC, McKenzie L, Glasier A. A randomised controlled trial of a tailored multifaceted strategy to promote implementation of a clinical guideline on induced abortion care. BJOG. 2004;111(7):726–33.
Frijling BD, Lobo CM, Hulscher ME, Akkermans RP, Braspenning JC, Prins A, Van Der Wouden JC, Grol RP. Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice. Diabet Med. 2002;19(10):836–42.
Frijling BD, Lobo CM, Hulscher ME, Akkermans RP, van Drenth BB, Prins A, van der Wouden JC, Grol RP. Intensive support to improve clinical decision making in cardiovascular care: a randomised controlled trial in general practice. BMJ Qual Saf. 2003;12(3):181–7.
Lobo CM, Frijling BD, Hulscher ME, Bernsen RM, Braspenning JC, Grol RP, Prins A, van der Wouden JC. Organisational determinants of cardiovascular prevention in general practice. Scand J Prim Health Care. 2003;21(2):99–105.
Frijling BD, Lobo CM, Hulscher ME, van Drenth BB, Braspenning JC, Prins A, van der Wouden JC, Grol RP. Provision of information and advice in cardiovascular care: clinical performance of general practitioners. Patient Educ Couns. 2002;48(2):131–7.
Frijling BD, Spies TH, Lobo CM, Hulscher ME, van Drenth BB, Braspenning JC, Prins A, van der Wouden JC, Grol RP. Blood pressure control in treated hypertensive patients: clinical performance of general practitioners. Br J Gen Pract. 2001;51(462):9–14.
Frijling BD, Lobo CM, Keus IM, Jenks KM, Akkermans RP, Hulscher ME, Prins A, van der Wouden JC, Grol RP. Perceptions of cardiovascular risk among patients with hypertension or diabetes. Patient Educ Couns. 2004;52(1):47–53.
Lobo CM, Frijling BD, Hulscher ME, Bernsen RM, Braspenning JC, Grol RP, Prins A, van der Wouden JC. Improving quality of organizing cardiovascular preventive care in general practice by outreach visitors: a randomized controlled trial. Prev Med. 2002;35(5):422–9.
Lobo CM, Frijling BD, Hulscher ME, Braspenning JC, Grol RP, Prins A, van der Wouden JC. Organizing cardiovascular preventive care in general practice: determinants of a successful intervention. Prev Med. 2002;35(5):430–6.
Gilkey MB, Dayton AM, Moss JL, Sparks AC, Grimshaw AH, Bowling JM, Brewer NT. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics. 2014;134(2):e346–53.
Gilkey MB, Moss JL, Roberts AJ, Dayton AM, Grimshaw AH, Brewer NT. Comparing in-person and webinar delivery of an immunization quality improvement program: a process evaluation of the adolescent AFIX trial. Implement Sci. 2014;9(1):21.
Gilkey MB, Parks MJ, Margolis MA, McRee AL, Terk JV. Implementing evidence-based strategies to improve HPV vaccine delivery. Pediatrics. 2019;144(1):e20182500.
Gjelstad S, Høye S, Straand J, Brekke M, Dalen I, Lindbæk M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ. 2013;26:347.
Gjelstad S, Fetveit A, Straand J, Dalen I, Rognstad S, Lindbaek M. Can antibiotic prescriptions in respiratory tract infections be improved? A cluster-randomized educational intervention in general practice–the Prescription Peer Academic Detailing (Rx-PAD) Study [NCT00272155]. BMC Health Serv Res. 2006;6:1–2.
Guadagnoli E, Soumerai SB, Gurwitz JH, Borbas C, Shapiro CL, Weeks JC, Morris N. Improving discussion of surgical treatment options for patients with breast cancer: local medical opinion leaders versus audit and performance feedback. Breast Cancer Res Treat. 2000;61:171–5.
Gude WT, van Engen-Verheul MM, van der Veer SN, Kemps HM, Jaspers MW, de Keizer NF, Peek N. Effect of a web-based audit and feedback intervention with outreach visits on the clinical performance of multidisciplinary teams: a cluster-randomized trial in cardiac rehabilitation. Implement Sci. 2016;11:1–6.
van Engen-Verheul MM, de Keizer NF, van der Veer SN, Kemps H, Reimer WJ, Jaspers MW, Peek N. Evaluating the effect of a web-based quality improvement system with feedback and outreach visits on guideline concordance in the field of cardiac rehabilitation: rationale and study protocol. Implement Sci. 2014;9(1):1–5.
Gulliford MC, Juszczyk D, Prevost AT, Soames J, McDermott L, Sultana K, Wright M, Fox R, Hay AD, Little P, Moore M. Electronically delivered interventions to reduce antibiotic prescribing for respiratory infections in primary care: cluster RCT using electronic health records and cohort study. Health Technol Assess. 2019;23(11):1.
Gullion DS, Tschann JM, Adamson TE, Coates TJ. Management of hypertension in private practice: a randomized controlled trial in continuing medical education. J Contin Educ Heal Prof. 1988;8(4):239–55.
Harris MF, Parker SM, Litt J, Van Driel M, Russell G, Mazza D, Jayasinghe UW, Del Mar C, Lloyd J, Smith J, Zwar N. Implementing guidelines to routinely prevent chronic vascular disease in primary care: the Preventive Evidence into Practice cluster randomised controlled trial. BMJ Open. 2015;5(12):e009397.
Hayes R, Armour B, Bratzler D, Moore L, Murray C, Stevens BR, Radford M, Fitzgerald D, Elward K, Ballard DJ. Comparison of an enhanced versus a written feedback model on the management of Medicare inpatients with venous thrombosis. Jt Comm J Qual Improv. 2001;27(3):155–68.
CAS PubMed Google Scholar
Hayes RP, Baker DW, Luthi JC, Baggett RL, McClellan W, Fitzgerald D, Abrams FR, Bratzler D, Ballard DJ. The effect of external feedback on the management of medicare inpatients with congestive heart failure. Am J Med Qual. 2002;17(6):225–35.
Hayes RP, Ballard DJ. Feedbck about practice patterns for measurable iprovements in quality of care: a challenge for PROs under the Health Care Quality Improvement Initiative. Clin Perform Qual Health Care. 1995;3:15–22.
Hendryx MS, Fieselmann JF, Jeanne Bock M, Wakefield DS, Helms CM, Bentler SE. Outreach education to improve quality of rural ICU care: results of a randomized trial. Am J Respir Crit Care Med. 1998;158(2):418–23.
Herbert CP, Wright JM, Maclure M, Wakefield J, Dormuth C, Brett-MacLean P, Legare J, Premi J. Better Prescribing Project: a randomized controlled trial of the impact of case-based educational modules and personal prescribing feedback on prescribing for hypertension in primary care. Fam Pract. 2004;21(5):575–81.
Hogg W, Lemelin J, Graham ID, Grimshaw J, Martin C, Moore L, Soto E, O’Rourke K. Improving prevention in primary care: evaluating the effectiveness of outreach facilitation. Fam Pract. 2008;25(1):40–8.
Lemelin J, Hogg W, Baskerville N. Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care. CMAJ. 2001;164(757–763):11.
Houston TK, Sadasivam RS, Allison JJ, Ash AS, Ray MN, English TM, Hogan TP, Ford DE. Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implement Sci. 2015;10(1):1–6.
Houston TK, Sadasivam RS, Ford DE, Richman J, Ray MN, Allison JJ. The QUIT-PRIMO provider-patient Internet-delivered smoking cessation referral intervention: a cluster-randomized comparative effectiveness trial: study protocol. Implement Sci. 2010;5(1):1–9.
Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, Viswanathan S. Effect of a quality improvement intervention on clinical outcomes in patients in India with acute myocardial infarction: the ACS QUIK randomized clinical trial. JAMA. 2018;319(6):567–78.
Huis A, Schoonhoven L, Grol R, Donders R, Hulscher M, van Achterberg T. Impact of a team and leaders-directed strategy to improve nurses’ adherence to hand hygiene guidelines: a cluster randomised trial. Int J Nurs Stud. 2013;50(4):464–74.
Ivers NM, Tu K, Young J, Francis JJ, Barnsley J, Shah BR, Upshur RE, Moineddin R, Grimshaw JM, Zwarenstein M. Feedback GAP: pragmatic, cluster-randomized trial of goal setting and action plans to increase the effectiveness of audit and feedback interventions in primary care. Implement Sci. 2013;8:1–1.
Ivers NM, Tu K, Francis J, Bransley J, Shah B, Upshur R, Kiss A, Grimshaw JM, Zwarenstein M. Trial of Goal-setting and Action-Plans to increase the effectiveness of audit and feedback interventions in primary care. Study protocol. Implement Sci. 2010;5.
Kaufmann-Kolle P, Szecsenyi J, Broge B, Haefeli WE, Schneider A. Führt die Implementierung von offenem Benchmarking in datengestützten Qualitätszirkeln zur Verbesserung der hausärztlichen Versorgung bei Arzneimittelinteraktion und Asthma bronchiale? Z Evid Fortbild Qual Gesundhwes. 2011;105(5):389–95.
Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, Morin SN, Crilly RG, Josse RG, Lohfeld L, Pickard LE. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials. 2015;16(1):1–1.
Kennedy CC, Ioannidis G, Giangregorio LM, Adachi JD, Thabane L, Morin SN, Crilly RG, Marr S, Josse RG, Lohfeld L, Pickard LE. An interdisciplinary knowledge translation intervention in long-term care: study protocol for the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Implement Sci. 2012;7(1):1–2.
Kennedy CC, Thabane L, Ioannidis G, Adachi JD, Papaioannou A. Implementing a knowledge translation intervention in long-term care: feasibility results from the Vitamin D and Osteoporosis Study (ViDOS). J Am Med Dir Assoc. 2014;15:943–5.
Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285(22):2871–9.
Kritchevsky SB, Braun BI, Bush AJ, Bozikis MR, Kusek L, Burke JP, Wong ES, Jernigan J, Davis CC, Simmons B, TRAPE Study Group*. The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients: a randomized trial. Ann Intern Med. 2008;149(7):472–80.
Kritchevsky SB, Simmons BP, Braun BI. The project to monitor indicators: a collaborative effort between the Joint Commission on Accreditation of Healthcare Organizations and the Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 1995;16:33–5.
Lakshminarayan K, Borbas C, McLaughlin B, Morris NE, Vazquez G, Luepker RV, Anderson DC. A cluster-randomized trial to improve stroke care in hospitals. Neurology. 2010;74(20):1634–42.
Article CAS PubMed PubMed Central Google Scholar
Lemelin J, Hogg W, Baskerville N. Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care. CMAJ. 2001;164(6):757–63.
Baskerville NB, Hogg W, Lemelin J. Process evaluation of a tailored multifaceted approach to changing family physician practice patterns improving preventive care. J Fam Pract. 2001;50(3):W242–9.
Lesuis N, van Vollenhoven RF, Akkermans RP, Verhoef LM, Hulscher ME, den Broeder AA. Rheumatologists’ guideline adherence in rheumatoid arthritis: a randomised controlled study on electronic decision support, education and feedback. Clin Exp Rheumatol. 2018;36(1):21–8.
PubMed Google Scholar
Levi CR, Attia JA, D’Este C, Ryan AE, Henskens F, Kerr E, Parsons MW, Sanson-Fisher RW, Bladin CF, Lindley RI, Middleton S. Cluster-Randomized trial of thrombolysis implementation support in metropolitan and regional Australian stroke centers: lessons for individual and systems behavior change. J Am Heart Assoc. 2020;9(3):e012732.
Paul CL, Levi CR, D’Este CA, Parsons MW, Bladin CF, Lindley RI, Attia JR, Henskens F, Lalor E, Longworth M, Middleton S. Thrombolysis ImPlementation in Stroke (TIPS): evaluating the effectiveness of a strategy to increase the adoption of best evidence practice–protocol for a cluster randomised controlled trial in acute stroke care. Implement Sci. 2014;9(1):1–3.
Lopez-Picazo JJ, Ruiz JC, Sanchez JF, Ariza A, Aguilera B. A randomized trial of the effectiveness and efficiency of interventions to reduce potential drug interactions in primary care. Am J Med Qual. 2011;26(2):145–53.
Lynch EA, Cadilhac DA, Luker JA, Hillier SL. Education-only versus a multifaceted intervention for improving assessment of rehabilitation needs after stroke; a cluster randomised trial. Implement Sci. 2015;11(1):1–3.
Lynch EA, Luker JA, Cadilhac DA, Hillier SL. Rehabilitation assessments for patients with stroke in Australian hospitals do not always reflect the patients’ rehabilitation requirements. Arch Phys Med Rehabil. 2015;96:782–9.
Lynch EA, Luker JA, Cadilhac DA, Fryer CE, Hillier S. A qualitative study using the Theoretical Domains Framework to investigate why patients were or were not assessed for rehabilitation after stroke. Clin Rehabil. 2017;31(7):966–77.
McCartney P, Macdowall W, Thorogood M. A randomised controlled trial of feedback to general practitioners of their prophylactic aspirin prescribing. BMJ. 1997;315(7099):35–6.
McClellan WM, Hodgin E, Pastan S, McAdams L, Soucie M. A Randomized Evaluation of Two Health Care Quality Improvement Program (HCQIP) Interventions to Improve the Adequacy of Hemodialysis Care of ESRD Patients: Feedback Alone: versus: Intensive Intervention. J Am Soc Nephrol. 2004;15(3):754–60.
McCluskey A, Ada L, Kelly PJ, Middleton S, Goodall S, Grimshaw JM, Logan P, Longworth M, Karageorge A. A behavior change program to increase outings delivered during therapy to stroke survivors by community rehabilitation teams: the Out-and-About trial. Int J Stroke. 2016;11(4):425–37.
Mertens JR, Chi FW, Weisner CM, Satre DD, Ross TB, Allen S, Pating D, Campbell CI, Lu YW, Sterling SA. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial. Addict Sci Clin Pract. 2015;10(1):1–7.
Moher M, Yudkin P, Wright L, Turner R, Fuller A, Schofield T, Mant D. Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ. 2001;322(7298):1338.
Mold JW, Fox C, Wisniewski A, Lipman PD, Krauss MR, Harris DR, Aspy C, Cohen RA, Elward K, Frame P, Yawn BP. Implementing asthma guidelines using practice facilitation and local learning collaboratives: a randomized controlled trial. Ann Fam Med. 2014;12(3):233–40.
Mold JW, Aspy CA, Nagykaldi Z. Implementation of evidence-based preventive services delivery processes in primary care: an Oklahoma Physicians Resource/Research Network (OKPRN) study. J Am Board Fam Med. 2008;21(4):334–44.
Myers RE, Turner B, Weinberg D, Hyslop T, Hauck WW, Brigham T, Rothermel T, Grana J, Schlackman N. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38(4):375–81.
Myers RE, Turner B, Weinberg D, Hauck WW, Hyslop T, Brigham T, Rothermel T, Grana J, Schlackman N. Complete diagnostic evaluation in colorectal cancer screening: research design and baseline findings. Prev Med. 2001;33(4):249–60.
Nilsson G, Hjemdahl P, Hässler A, Vitols S, Wallen NH, Krakau I. Feedback on prescribing rate combined with problem-oriented pharmacotherapy education as a model to improve prescribing behaviour among general practitioners. Eur J Clin Pharmacol. 2001;56:843–8.
Palmer RH, Louis TA, Hsu LN, Peterson HF, Rothrock JK, Strain R, Thompson MS, Wright EA. A randomized controlled trial of quality assurance in sixteen ambulatory care practices. Med Care. 1985;1:751–70.
Palmer RH, Strain R, Maurer JV, Thompson MS. A method for evaluating performance of ambulatory pediatric tasks. Pediatrics. 1984;73(3):269–77.
Papadakis S, Cole AG, Reid RD, Assi R, Gharib M, Tulloch HE, Mullen KA, Wells G, Pipe AL. From good to great: the role of performance coaching in enhancing tobacco-dependence treatment rates. Ann Fam Med. 2018;16(6):498–506.
Papadakis S, Pipe AL, Reid RD, Tulloch H, Mullen KA, Assi R, Cole AG, Wells G. Effectiveness of performance coaching for enhancing rates of smoking cessation treatment delivery by primary care providers: study protocol for a cluster randomized controlled trial. Contemp Clin Trials. 2015;1(45):184–90.
Patel MS, Kurtzman GW, Kannan S, Small DS, Morris A, Honeywell S, Leri D, Rareshide CA, Day SC, Mahoney KB, Volpp KG. Effect of an automated patient dashboard using active choice and peer comparison performance feedback to physicians on statin prescribing: the PRESCRIBE cluster randomized clinical trial. JAMA Netw Open. 2018;1(3):e180818.
Peiris D, Usherwood T, Panaretto K, Harris M, Hunt J, Redfern J, Zwar N, Colagiuri S, Hayman N, Lo S, Patel B. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8(1):87–95.
Peiris D, Usherwood T, Panaretto K, Harris M, Hunt J, Patel B, Zwar N, Redfern J, MacMahon S, Colagiuri S, Hayman N. The Treatment of cardiovascular Risk in Primary care using Electronic Decision suppOrt (TORPEDO) study: intervention development and protocol for a cluster randomised, controlled trial of an electronic decision support and quality improvement intervention in Australian primary healthcare. BMJ Open. 2012;2(6):e002177.
Pettersson E, Vernby Å, Mölstad S, Lundborg CS. Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? A cluster randomized controlled trial. J Antimicrob Chemother. 2011;66(11):2659–66.
Price-Haywood EG, Harden-Barrios J, Cooper LA. Comparative effectiveness of audit-feedback versus additional physician communication training to improve cancer screening for patients with limited health literacy. J Gen Intern Med. 2014;29:1113–21.
Price-Haywood EG, Roth KG, Shelby K, Cooper LA. Cancer risk communication with low health literacy patients: a continuing medical education program. J Gen Intern Med. 2010;25:126–9.
Article PubMed Central Google Scholar
Quanbeck A, Brown RT, Zgierska AE, Jacobson N, Robinson JM, Johnson RA, Deyo BM, Madden L, Tuan WJ, Alagoz E. A randomized matched-pairs study of feasibility, acceptability, and effectiveness of systems consultation: a novel implementation strategy for adopting clinical guidelines for opioid prescribing in primary care. Implement Sci. 2018;13:1–3.
Quanbeck A, Brown R, Zgierska A, Jacobson N, Robinson J, Alagoz E, Deyo B, Madden L, Johnson R, Tuan WJ. Systems consultation: a novel implementation strategy for adopting clinical guidelines for opioid prescribing in primary care. Annual Research Meeting. 2017. Academy Health: Wisconsin, US. Available from: https://academyhealth.confex.com/academyhealth/2017arm/mediafile/Presentation/Paper16946/Andrew%20Quanbeck_%20academyhealth%20oral%20present%206-23-17.pdf . Accessed 26 Sept 2023.
Quanbeck A, Almirall D, Jacobson N, Brown RT, Landeck JK, Madden L, Cohen A, Deyo BM, Robinson J, Johnson RA, Schumacher N. The Balanced Opioid Initiative: protocol for a clustered, sequential, multiple-assignment randomized trial to construct an adaptive implementation strategy to improve guideline-concordant opioid prescribing in primary care. Implement Sci. 2020;15:1–3.
Quinley JC, Shih A. Improving physician coverage of pneumococcal vaccine: a randomized trial of a telephone intervention. J Community Health. 2004;29:103–15.
Raasch BA, Hays R, Buettner PG. An educational intervention to improve diagnosis and management of suspicious skin lesions. J Contin Educ Heal Prof. 2000;20(1):39–51.
Raasch B. The epidemiology, diagnosis and management of nonmelanoma skin cancer in general practice in Townsville. PhD thesis, James Cook University, Australia. 1999.
Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of performance feedback reports on adherence to evidence-based guidelines in use of CT for evaluation of pulmonary embolism in the emergency department: a randomized trial. Am J Roentgenol. 2015;205(5):936–40.
Rantz MJ, Popejoy L, Petroski GF, Madsen RW, Mehr DR, Zwygart-Stauffacher M, Hicks LL, Grando V, Wipke-Tevis DD, Bostick J, Porter R. Randomized clinical trial of a quality improvement intervention in nursing homes. Gerontologist. 2001;41(4):525–38.
Rask K, Kohler SA, Wells KI, Williams JA, Diamond CC. Performance improvement interventions to improve delivery of screening services in diabetes care. JCOM-WAYNE PA-. 2001;8(11):23–30.
Ruangkanchanasetr S. Laboratory investigation utilization in pediatric out-patient department Ramathibodi Hospital. J Med Assoc Thai. 1993;1(76):194–208.
Rubin GL, Schofield WN, Dean MG, Shakeshaft AP. Appropriateness of red blood cell transfusions in major urban hospitals and effectiveness of an intervention. Med J Aust. 2001;175(7):354–8.
Sauaia A, Ralston D, Schluter WW, Marciniak TA, Havranek EP, Dunn TR. Influencing care in acute myocardial infarction: a randomized trial comparing 2 types of intervention. Am J Med Qual. 2000;15(5):197–206.
Schectman JM, Schroth WS, Verme D, Voss JD. Randomized controlled trial of education and feedback for implementation of guidelines for acute low back pain. J Gen Intern Med. 2003;18(10):773–80.
Schneider A, Wensing M, Biessecker K, Quinzler R, Kaufmann-Kolle P, Szecsenyi J. Impact of quality circles for improvement of asthma care: results of a randomized controlled trial. J Eval Clin Pract. 2008;14(2):185–90.
Scholes D, Grothaus L, McClure J, Reid R, Fishman P, Sisk C, Lindenbaum JE, Green B, Grafton J, Thompson RS. A randomized trial of strategies to increase chlamydia screening in young women. Prev Med. 2006;43(4):343–50.
Sinclair C, Frankel M. The effect of quality assurance activities on the quality of mental health services. QRB Qual Rev Bull. 1982;8(7):7–15.
Siriwardena AN, Rashid A, Johnson MR, Dewey ME. Cluster randomised controlled trial of an educational outreach visit to improve influenza and pneumococcal immunisation rates in primary care. Br J Gen Pract. 2002;52(482):735–40.
PubMed PubMed Central Google Scholar
Smith-Bindman R, Chu P, Wang Y, Chung R, Lopez-Solano N, Einstein AJ, Solberg L, Cervantes LF, Nelson TR, Boswell W, Delman BN. Comparison of the effectiveness of single-component and multicomponent interventions for reducing radiation doses in patients undergoing computed tomography: a randomized clinical trial. JAMA Intern Med. 2020;180(5):666–75.
Solomon DH, Katz JN, La Tourette AM, Coblyn JS. Multifaceted intervention to improve rheumatologists’ management of glucocorticoid-induced osteoporosis: a randomized controlled trial. Arthritis Care Res. 2004;51(3):383–7.
Søndergaard J, Hansen DG, Aarslev P, Munck AP. A multifaceted intervention according to the Audit Project Odense method improved secondary prevention of ischemic heart disease: a randomised controlled trial. Fam Pract. 2006;23(2):198–202.
Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, Morris N, McLaughlin B, Gao X, Willison DJ, Asinger R. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279(17):1358–63.
Stewardson AJ, Sax H, Gayet-Ageron A, Touveneau S, Longtin Y, Zingg W, Pittet D. Enhanced performance feedback and patient participation to improve hand hygiene compliance of health-care workers in the setting of established multimodal promotion: a single-centre, cluster randomised controlled trial. Lancet Infect Dis. 2016;16(12):1345–55.
Tierney WM, Hui SL, McDonald CJ. Delayed feedback of physician performance versus immediate reminders to perform preventive care: effects on physician compliance. Med Care. 1986;1:659–66.
Trietsch J, van Steenkiste B, Grol R, Winkens B, Ulenkate H, Metsemakers J, van der Weijden T. Effect of audit and feedback with peer review on general practitioners’ prescribing and test ordering performance: a cluster-randomized controlled trial. BMC Fam Pract. 2017;18(1):1–3.
Trietsch J, van der Weijden T, Verstappen W, Janknegt R, Muijrers P, Winkens R, van Steenkiste B, Grol R, Metsemakers J. A cluster randomized controlled trial aimed at implementation of local quality improvement collaboratives to improve prescribing and test ordering performance of general practitioners: study Protocol. Implement Sci. 2009;4(1):1–4.
van der Velden AW, Kuyvenhoven MM, Verheij TJ. Improving antibiotic prescribing quality by an intervention embedded in the primary care practice accreditation: the ARTI4 randomized trial. J Antimicrob Chemother. 2016;71(1):257–63.
van der Weijden TR, Grol RP, Knottnerus JA. Feasibility of a national cholesterol guideline in daily practice. A randomized controlled trial in 20 general practices. Int J Qual Health Care. 1999;11(2):131–7.
Veninga CC, Øv PL, Wahlstrom RO, Muskova M, DenigG P, Berkhof J, Kochen MM, Haaijer-Ruskamp FM, Drug Education Project Group. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Am J Respir Crit Care Med. 1999;160(4):1254–62.
Verstappen WH, van der Weijden T, Sijbrandij J, Smeele I, Hermsen J, Grimshaw J, Grol RP. Effect of a practice-based strategy on test ordering performance of primary care physicians: a randomized trial. JAMA. 2003;289(18):2407–12.
Verstappen WH, van der Weijden T, Dubois WI, Smeele I, Hermsen J, Tan FE, Grol RP. Improving test ordering in primary care: the added value of a small-group quality improvement strategy compared with classic feedback only. Ann Fam Med. 2004;2(6):569–75.
Vingerhoets E, Wensing M, Grol RP. Feedback of patients’ evaluations of general practice care: a randomised trial. BMJ Qual Saf. 2001;10(4):224–8.
Wahlström R, Kounnavong S, Sisounthone B, Phanyanouvong A, Southammavong T, Eriksson B, Tomson G. Effectiveness of feedback for improving case management of malaria, diarrhoea and pneumonia–a randomized controlled trial at provincial hospitals in Lao PDR. Tropical Med Int Health. 2003;8(10):901–9.
Walsh M, Laptook A, Kazzi SN, Engle WA, Yao Q, Rasmussen M, Buchter S, Heldt G, Rhine W, Higgins R, Poole K. A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics. 2007;119(5):876–90.
Wang Y, Li Z, Zhao X, Wang C, Wang X, Wang D, Liang L, Liu L, Wang C, Li H, Shen H. Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. 2018;320(3):245–54.
Wang Y, Li Z, Xian Y, Zhao X, Li H, Shen H, Wang C, Liu L, Wang C, Pan Y, Wang D. Rationale and design of a cluster-randomized multifaceted intervention trial to improve stroke care quality in China: the GOLDEN BRIDGE–Acute Ischemic Stroke. Am Heart J. 2015;169(6):767–74.
Wathne JS, Kleppe LK, Harthug S, Blix HS, Nilsen RM, Charani E, Smith I. The effect of antibiotic stewardship interventions with stakeholder involvement in hospital settings: a multicentre, cluster randomized controlled intervention study. Antimicrob Resist Infect Control. 2018;7(1):1–2.
Whidden C, Kayentao K, Liu JX, Lee S, Keita Y, Diakité D, Keita A, Diarra S, Edwards J, Yembrick A, Holeman I. Improving community health worker performance by using a personalised feedback dashboard for supervision: a randomised controlled trial. J Glob Health. 2018;8(2):020418.
Willis TA, Collinson M, Glidewell L, Farrin AJ, Holland M, Meads D, Hulme C, Petty D, Alderson S, Hartley S, Vargas-Palacios A. An adaptable implementation package targeting evidence-based indicators in primary care: a pragmatic cluster-randomised evaluation. PLoS Med. 2020;17(2):e1003045.
Willis TA, Hartley S, Glidewell L, Farrin AJ, Lawton R, McEachan RRC, et al. Action to Support Practices Implement Research Evidence (ASPIRE): protocol for a cluster-randomised evaluation of adaptable implementation packages targeting ‘high impact’ clinical practice recommendations in general practice. Implement Sci. 2016;11(1):1–11.
CAS Google Scholar
Glidewell L, Willis TA, Petty D, Lawton R, McEachan RRC, Ingleson E, et al. To what extent can behaviour change techniques be identified within an adaptable implementation package for primary care? A prospective directed content analysis. Implement Sci. 2018;13(32):32.
Wu Y, Li S, Patel A, Li X, Du X, Wu T, Zhao Y, Feng L, Billot L, Peterson ED, Woodward M. Effect of a quality of care improvement initiative in patients with acute coronary syndrome in resource-constrained hospitals in China: a randomized clinical trial. JAMA cardiology. 2019;4(5):418–27.
Li S, Wu Y, Du X, Li X, Patel A, Peterson ED, Turnbull F, Lo S, Billot L, Laba T, Gao R. Rational and design of a stepped-wedge cluster randomized trial evaluating quality improvement initiative for reducing cardiovascular events among patients with acute coronary syndromes in resource-constrained hospitals in China. Am Heart J. 2015;169(3):349–55.
Fox RD, Mazmanian PE, Putnam RW. Changing and learning in the lives of physicians. New York: Praeger; 1989.
Rogers EM. Diffusion of innovations. 3rd ed. New York: Free Press; 1983.
Bandura A. Self-efficacy: toward a unifying theory of behavior change. Psychol Rev. 1977;84:191–215.
Bandura A. Social foundation and thought of action: a social cognitive theory. New York: Prentice Hall; 1986.
Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, Proctor EK, Kirchner JE. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10:1–8.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Griffey R, Hensley M. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76.
Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5):S112–8.
McHugh S, Presseau J, Luecking CT, Powell BJ. Examining the complementarity between the ERIC compilation of implementation strategies and the behaviour change technique taxonomy: a qualitative analysis. Implement Sci. 2022;17(1):1–23.
Presseau J, McCleary N, Lorencatto F, Patey AM, Grimshaw JM, Francis JJ. Action, actor, context, target, time (AACTT): a framework for specifying behaviour. Implement Sci. 2019;14:1–3.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, Foy R, Duncan EM, Colquhoun H, Grimshaw JM, Lawton R. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12(1):1–8.
Download references
Not applicable.
Author information
Authors and affiliations.
Northumbria University, Newcastle Upon Tyne, UK
Michael Sykes
Toronto Metropolitan University, Toronto, Canada
Zahava R. S. Rosenberg-Yunger
Monash University, Melbourne, Australia
Matthew Quigley & Lavanya Gupta
University of Leeds, Leeds, UK
Owen Thomas & Sarah Alderson
Newcastle Upon Tyne NHS Foundation Trust, Newcastle Upon Tyne, UK
Lisa Robinson & Karen Caulfield
University of Toronto, Toronto, Canada
You can also search for this author in PubMed Google Scholar
Contributions
MS conceived the study and worked with SA, ZRY, MQ, LG, NI to develop the protocol. OT, LR, KC, MS, SA, ZRY, MQ, LG were involved in data extraction. MS and SA undertook the analysis, MQ produced the Sankey chart. All authors, contributed to the interpretation of the findings and production of the manuscript.
Corresponding author
Correspondence to Michael Sykes .
Ethics declarations
Ethics approval and consent to participate.
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Competing interests.
MS, MQ and SA have previously developed feedback facilitation interventions, although not ones included within the current review.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sykes, M., Rosenberg-Yunger, Z.R.S., Quigley, M. et al. Exploring the content and delivery of feedback facilitation co-interventions: a systematic review. Implementation Sci 19 , 37 (2024). https://doi.org/10.1186/s13012-024-01365-9
Download citation
Received : 04 October 2023
Accepted : 13 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1186/s13012-024-01365-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Implementation Science
ISSN: 1748-5908
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Jump to navigation
- Bahasa Malaysia

Lifestyle changes for treating psoriasis
Review question
We wanted to see whether lifestyle changes (e.g. changing diet, exercising, and avoiding smoking and drinking alcohol), alone or combined, were useful in treating psoriasis when compared to no such changes or another psoriasis treatment.
Psoriasis is a long-lasting, inflammatory skin disease; it causes thick, red, itching, and scaling patches. Obesity, drinking, smoking, and an inactive lifestyle can worsen psoriasis. We intended to find out if lifestyle changes can improve psoriasis severity and quality of life, and reduce comorbidities (other conditions occurring alongside a primary condition).
Trial characteristics
We included 10 trials, with 1163 participants, which assessed the effects of low-calorie diet alone; low-calorie diet combined with an exercise programme; a combination of walking exercise and continuous health education; and educational instructions to promote a healthy lifestyle (diet, smoking, and alcohol abstinence). We examined the research evidence up to July 2018.
Non-profit organisations funded four trials, one trial received funding for the education programme from pharmaceutical companies, and the other five trials had no funding or did not report the funding source. All participants were aged at least 18 years (mean age: 43 to 61 years). Where reported, the trials included 656 men and 478 women; all were set in a hospital. In four trials, the participants were limited to people with moderate-to-severe psoriasis. One trial included participants who had initially been treated with oral medicines for moderate-to-severe psoriasis but whose psoriasis had not cleared after four weeks. In four trials, all severities of psoriasis were eligible, but these trials either did not report the participants’ psoriasis severity or only provided average severity scores. One trial included participants with mild psoriasis. Trials compared lifestyle change interventions with usual care (including to continue healthy eating), information only, no treatment, or medical treatment alone. Treatment was given for between 12 weeks to three years.
Key results
The following results are based on obese participants and compare lifestyle change interventions (low-calorie diet) to usual care. A low-calorie diet may reduce the severity of psoriasis (when assessed as the proportion of participants achieving at least 75% improvement from the start of treatment in the Psoriasis Area and Severity Index (PASI 75), a widely used tool for the measurement of psoriasis severity) (low-quality evidence) and probably improves quality of life (moderate-quality evidence). Participants on a low-calorie diet may be more likely to stick to treatment (treatment adherence), but treatment effects vary so it is possible that it may make little or no difference (low-quality evidence). A low-calorie diet probably improves BMI (body mass index: a healthy weight calculator) (moderate-quality evidence). The trials did not say how long they treated participants before they stopped dieting (time to relapse).
The following results are based on obese participants and compare lifestyle change interventions (low-calorie diet plus an exercise programme) to weight-loss information aimed at improving psoriasis. A low-calorie diet plus an exercise programme probably results in a greater reduction in the severity of psoriasis (based on PASI 75), but the effects of this treatment vary, so it is possible that it may make little or no difference. There is probably no difference in treatment adherence between the two groups; however, a low-calorie diet plus exercise programme probably improves BMI reduction (all outcomes based on moderate-quality evidence). Trials did not report quality of life or time to relapse.
Only two trials in this review assessed side effects. In one trial side effects were caused by an additional therapy given to both groups of participants (they were not caused by the dietary treatment). The other trial, which compared two dietary treatments to no treatment, did not observe any side effects. Generally, participants complied with the assessed lifestyle changes successfully.
We found no trials on alcohol abstinence or smoking cessation.
Quality of the evidence
The quality of evidence was moderate to low for the outcomes 'Severity of psoriasis' and 'Adherence to the intervention' and moderate for 'Reduction in comorbidities: change in Body Mass Index (BMI)'. Quality of life, measured in only one of the two key comparisons, was based on moderate-quality evidence. Trial limitations included participants knowing which treatment they were receiving and large number of participants withdrawing from trials.
Dietary intervention may reduce the severity of psoriasis (low-quality evidence) and probably improves quality of life and reduces BMI (moderate-quality evidence) in obese people when compared with usual care, while combined dietary intervention and exercise programme probably improves psoriasis severity and BMI when compared with information only (moderate-quality evidence). None of the trials measured quality of life.
We did not detect a clear difference in treatment adherence between those in the combined dietary intervention and exercise programme group and those given information only (moderate-quality evidence). Adherence may be improved through dietary intervention compared with usual care (low-quality evidence). Participants generally adhered well to the lifestyle interventions assessed in the review.
No trials assessed the time to relapse. Trial limitations included unblinded participants and high dropout rate.
Future trials should reduce dropouts and include comprehensive outcome measures; they should examine whether dietary intervention with or without an exercise programme is effective in non-obese people with psoriasis, whether an additional exercise programme is more effective than dietary intervention alone, whether the time to relapse prolongs in people who receive dietary intervention with or without exercise programme, and whether smoking cessation and alcohol abstinence are effective in treating psoriasis.
Psoriasis is an inflammatory skin disease that presents with itching, red, scaling plaques; its worsening has been associated with obesity, drinking, smoking, lack of sleep, and a sedentary lifestyle. Lifestyle changes may improve psoriasis.
To assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions.
We searched the following databases up to July 2018: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched the China National Knowledge Infrastructure, the Airiti Library, and five trials registers up to July 2018. We checked the references of included trials for further relevant trials, and we asked the authors of the included trials if they were aware of any relevant unpublished data.
We included randomised controlled trials (RCTs) of lifestyle changes (either alone or in combination) for treating psoriasis in people diagnosed by a healthcare professional. Treatment had to be given for at least 12 weeks. Eligible comparisons were no lifestyle changes or another active intervention.
We used standard methodological procedures expected by Cochrane. The primary outcome measures were 'Severity of psoriasis' and 'Adherence to the intervention'. Secondary outcomes were 'Quality of life', 'Time to relapse', and 'Reduction in comorbidities'. We used GRADE to assess the quality of the evidence for each outcome.
We included 10 RCTs with 1163 participants (mean age: 43 to 61 years; 656 men and 478 women were reported). Six trials examined the effects of dietary intervention (low-calorie diet) in 499 obese participants (mean age: 44.3 to 61 years; where reported, 395 had moderate-to-severe psoriasis). One trial assessed a combined dietary intervention and exercise programme in 303 obese participants with moderate-to-severe psoriasis who had started a systemic therapy for psoriasis and had not achieved clearance after four weeks of continuous treatment (median age: 53 years). Another trial assessed a walking exercise and continuous health education in 200 participants (mean age: 43.1 years, severity not reported). Finally, two trials included education programmes promoting a healthy lifestyle in 161 participants (aged 18 to 78 years), with one trial on mild psoriasis and the other trial not reporting severity.
Comparisons included information only; no intervention; medical therapy alone; and usual care (such as continuing healthy eating).
All trials were conducted in hospitals and treated participants for between 12 weeks and three years. One trial did not report the treatment period. Seven trials measured the outcomes at the end of treatment and there was no additional follow-up. In two trials, there was follow-up after the treatment ended. Five trials had a high risk of performance bias, and four trials had a high risk of attrition bias.
We found no trials assessing interventions for alcohol abstinence or smoking cessation. No trials assessed time to relapse. Only two trials assessed adverse events; in one trial these were caused by the add-on therapy ciclosporin (given in both groups). The trial comparing two dietary interventions to a no-treatment group observed no adverse events.
The results presented in this abstract are based on trials of obese participants.
Outcomes for dietary interventions versus usual care were measured 24 weeks to six months from baseline. Compared to usual care, dietary intervention (strict caloric restriction) may lead to 75% or greater improvement from baseline in the Psoriasis Area and Severity Index (PASI 75) (risk ratio (RR) 1.66, 95% confidence interval (CI) 1.07 to 2.58; 2 trials, 323 participants; low-quality evidence). Adherence to the intervention may be greater with the dietary intervention than usual care, but the 95% CI indicates that the dietary intervention might also make little or no difference (RR 1.26, 95% CI 0.76 to 2.09; 2 trials, 105 participants; low-quality evidence). Dietary intervention probably achieves a greater improvement in dermatology quality-of-life index (DLQI) score compared to usual care (MD −12.20, 95% CI −13.92 to −10.48; 1 trial, 36 participants; moderate-quality evidence), and probably reduces the BMI compared to usual care (MD −4.65, 95% CI −5.93 to −3.36; 2 trials, 78 participants; moderate-quality evidence).
Outcomes for dietary interventions plus exercise programme were measured 16 weeks from baseline and are based on one trial (303 participants). Compared to information only (on reducing weight to improve psoriasis), combined dietary intervention and exercise programme (dietetic plan and physical activities) probably improves psoriasis severity, but the 95% CI indicates that the intervention might make little or no difference (PASI 75: RR 1.28, 95% CI 0.83 to 1.98). This combined intervention probably results in a greater reduction in BMI (median change −1.10 kg/m², P = 0.002), but there is probably no difference in adherence (RR 0.95, 95% CI 0.89 to 1.01; 137/151 and 145/152 participants adhered in the treatment and control group, respectively). There were no data on quality of life. These outcomes are based on moderate-quality evidence.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Pilot study on large language models for risk-of-bias assessments in systematic reviews: A(I) new type of bias?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-7989-6994 Joseph Barsby 1 , 2 ,
- http://orcid.org/0000-0003-4417-0370 Samuel Hume 1 , 3 ,
- http://orcid.org/0000-0002-7679-9125 Hamish AL Lemmey 1 , 4 ,
- http://orcid.org/0000-0002-2867-7393 Joseph Cutteridge 5 , 6 ,
- http://orcid.org/0000-0001-8621-5165 Regent Lee 7 ,
- http://orcid.org/0000-0003-3795-6762 Katarzyna D Bera 3 , 7
- 1 Oxford Medical School , Oxford , UK
- 2 Newcastle Upon Tyne Hospitals NHS Foundation Trust , Newcastle Upon Tyne , UK
- 3 University of Oxford St Anne's College , Oxford , UK
- 4 University of Oxford Magdalen College , Oxford , UK
- 5 York and Scarborough Teaching Hospitals NHS Foundation Trust , York , UK
- 6 Hull University Teaching Hospitals NHS Trust , Hull , UK
- 7 Nuffield Department of Surgical Sciences , University of Oxford , Oxford , UK
- Correspondence to Dr Katarzyna D Bera, University of Oxford Nuffield Department of Surgical Sciences, Oxford, UK; katarzyna.bera{at}st-annes.ox.ac.uk
https://doi.org/10.1136/bmjebm-2024-112990
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Systematic Reviews as Topic
Risk-of-bias (RoB) assessment is used to assess randomised control trials for systematic errors. Developed by Cochrane, it is considered the gold standard of assessing RoB for studies included within systematic reviews, representing a key part of Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 1 The RoB tool comprises six domains that may signify bias: random sequence generation, allocation concealment, blinding of participants and personnel, attrition bias, reporting bias and other potential biases. 2 This assessment is an integral component of evaluating the quality of evidence; however, it is a time-consuming and labour-intensive process.
Large language models (LLMs) are a form of generative artificial intelligence (AI) trained on large volumes of data. ChatGPT is an LLM developed by OpenAI, capable of generating a wide variety of responses in response to user prompts. Concerns exist around the application of such AI tools in research, including ethical, copyright, plagiarism and cybersecurity risks. 3 However, LLMs are increasingly popular with investigators seeking to streamline analyses. Studies have begun investigating the potential role of LLMs in the RoB assessment process. 4 5 Given the flexibility and rapidly evolving nature of LLMs, our goal was to explore whether ChatGPT could be used to automate the RoB assessment process without sacrificing accuracy. This study offers an assessment of the applicability of LLMs in SRs as of December 2023.
This study sits within an SR (PROSPERO CRD420212479050). Two reviewers (SH and HALL) implemented RoB across n=15 full-length papers in portable document format (PDF) format ( table 1 ). Six domains were assessed independently alongside an added ‘overall assessment’ domain ranking each as high, low or unclear RoB. Alongside RoB assessment, reviewers recorded author name, DOI and publication year using a Microsoft Office form. Any conflicts were resolved by discussion, with a third reviewer (KDB) available for arbitration, although this was not required.
- View inline
List of included papers (n=15), numbered 1–15
In parallel, a fourth reviewer (JB) uploaded the same PDF files to ChatPDF.com, a plug-in powered by ChatGPT3.5 that facilitates the upload of PDF files for analysis by ChatGPT3.5. ChatGPT3.5 was prompted to assess the RoB within each paper through prompts pertaining to each of the six domains. Responses were recorded in a separate, but identical, Microsoft Office form. Reviewer 4 (JB) was blinded to the assessment of reviewers 1 and 2 throughout. JB then repeated this process using ChatGPT4.
Responses from decisions across all domains were compared considering percentage of concurrent decisions, opposite decisions and indeterminable decisions. All data were analysed and stored in Microsoft Excel. Gemini was trialled but was unable to perform a RoB assessment in its current form.
In total, n=105 decisions were undertaken by GPT3.5, GPT4 and human reviewers. ChatGPT3.5 was concurrent with human (gold standard) assessment in 41/105 (39.0%) decisions and disagreed on 10/105 (9.5%). ChatGPT4 agreed with human assessment on 34/105 (34.3%) decisions and disagreed on 15/105 (14.3%). Both ChatGPT3.5 and ChatGPT4 delivered an indeterminate response on 54/105 (51.4%) decisions. ChatGPT3.5 outperformed or matched the performance of ChatGPT4 in 6/7 (85.6%) domains (aside from selective reporting), with ChatGPT3.5 performing best in assessing sequence generation and completeness of data, with 8/15 (53.3%) concurrent with human assessment. ChatGPT4 performed best in assessing selective reporting, with 14/15 (93.3%) correct decisions. Results by domain are summarised in table 2 . Notably, GPT4 performed superiorly in one domain (selective reporting), returning a correct decision in 14/15 (93.3%) cases, while GPT3.5 was correct in 10/15 (66.7%) decisions.
Summary of risk-of-bias assessment outcome per domain assessed by ChatGPT3.5 and ChatGPT4 compared with human assessment
When assessing Karanikolas (2011) and Mann (1983), ChatGPT3.5 returned an assessment as ‘moderate’, and ‘low to moderate’, in the overall domain. Both were recorded as unclear as substitute. On one occasion, ChatGPT identified an author as ‘P. C. Weaver’, who is not an author on any included papers. When discussing Lastoria (2006), ChatGPT responded initially in Portuguese.
We explored the potential for ChatGPT as a tool for assessing RoB. Overall, ChatGPT demonstrated moderate agreement, and minor disagreement with gold standard (human) assessment. While encouraging, this suboptimal performance precludes us from recommending ChatGPT be used in real-world RoB assessment.
To emulate end-users, prompts were not standardised, and some questions were repeated to ensure accuracy. When responses were ‘moderate’, ChatGPT was prompted to reassess. Similarly, ChatGPT often declined to perform assessments, which required further prompting with alternate question structure or wording. When prompted to assess allocation concealment for Choksy (2006), ChatGPT summarised the process as follows: ‘randomisation was performed using a random number table and randomisation details were placed in sealed envelopes which were opened in the operating theatre’. Human assessment ranked this as low RoB, whereas ChatGPT ranked this as unclear stating ‘it [was] unclear whether these envelopes were opaque’, demonstrating a literal interpretation of the literature not seen in human assessment.
In this analysis, ChatGPT4 did not offer improvement on ChatGPT3.5 in any domain, aside from selective reporting. For selective reporting, ChatGPT4 returned a correct decision in 14/15 (93.3%) cases, and ChatGPT3.5 in 10/15 (66.7%). However, human assessment returned a decision of unclear on n=14/15 of these assessments. ChatGPT4’s inability to give a definitive assessment is perhaps best outlined by ChatGPT4’s response: ‘To make a definitive assessment, one would ideally compare the outcomes reported in the paper with those specified in the study’s protocol registration’. This could represent an improvement through appreciation of the wider context of a given paper.
Our findings have limitations: the sample size of included papers in this study was small and fairly homogenous. Additionally, we chose not to use standardised prompts. Prompt variability has been demonstrated to introduce output variability. 5 Future research could benefit from a larger and more diverse dataset, with standardised prompts to assess and improve consistency of responses.
Supplemental material
Ethics statements, patient consent for publication.
Not applicable.
Ethics approval
- McKenzie JE ,
- Bossuyt PM , et al
- Higgins JPT ,
- Altman DG ,
- Gøtzsche PC , et al
- Arshad HB ,
- Khan SU , et al
- Talukdar JR
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
X @scullingmonkey
Contributors Conception of study: JB and KDB. Data collection: JB, JC, KDB, SH, HALL. Data analysis: JB, JC and KDB. Data interpretation: JB, KDB and LR. Drafting of paper: JB, JC and KDB. Approval of final version: all authors. ChatGPT is the subject of this study and was only used as described in methods and results. ChatGPT was not used in analysing, drafting or rewriting of the paper.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dermatol Pract Concept
- v.14(2); 2024 Apr
- PMC11135956

Efficacy of Intralesional Methotrexate Injection versus Triamcinolone Acetonide in Nail Psoriasis: A Systematic Review and Meta-Analysis
Stephanie nathania.
1 Department of Dermatovenereology, Faculty of Medicine, Diponegoro University/Dr. Kariadi General Hospital Medical Center, Semarang, Indonesia
Diah Adriani Malik
2 Department of Physiology, Faculty of Medicine, Diponegoro University/Dr. Kariadi General Hospital Medical Center, Semarang, Indonesia
Introduction
Psoriasis is a chronic inflammatory skin disease that can affect many parts of the body. Psoriatic involvement of the nail bed or nail matrix results in nail psoriasis, which is common. Patients with psoriatic nails have impaired quality of life due to the appearance of nails, and significant morbidity and functional impairments may arise in large cases. The management of nail psoriasis is challenging because it is usually time-consuming, with uncertain outcomes. The existing evidence suggests that intralesional injections are particularly effective for nail psoriasis. Current studies provide recommendations on the intralesional injection technique, recommending an optimal concentration of methotrexate (MTX), triamcinolone acetonide (TA), and cyclosporine, but the comparison of these treatment is still limited.
This study aimed to evaluate the efficacy of intralesional injections of MTX compared with TA in treating nail psoriasis using the Nail Psoriasis Severity Index (NAPSI) score.
A systematic literature search was performed on EBSCOhost, Scopus, ProQuest, ScienceDirect, SpringerLink, Elsevier Clinical Key, Cochrane library, and ClinicalTrials.gov using subgroups terms: “ intralesional methotrexate injections for nail psoriasis ,” “ intralesional triamcinolone acetonide injections for nail psoriasis ,” and “ NAPSI Score. ” Three studies were included in the qualitative synthesis and meta-analysis.
The overall standardized mean difference in NAPSI scores after administration of intralesional injection of MTX and TA was −0.213±0.232 (95% CI: −0.667–0.241). The Q statistic value was −0.921 (p=0.357), indicating the insignificant difference in the effectiveness of both therapies.
Both MTX and TA were effective in treating nail psoriasis based on the reduction of NAPSI score.
Psoriasis is a chronic inflammatory skin disease with predominantly skin and joint involvement. Psoriatic involvement of the nail bed or nail matrix results in nail psoriasis. Nail psoriasis is more common in adults, with a prevalence of up to 10–78% [ 1 ]. It can manifest clinically as a wide variety of nail changes, like discoloration, subungual hyperkeratosis, pitting, onycholysis, and splinter hemorrhaging of the nail bed, depending on the part of the nail unit affected. The most observed forms are psoriasis of the nail matrix, nail bed, and nail fold. Patients with psoriatic nails have impaired quality of life due to the appearance of nails, and significant morbidity and functional impairments may arise in large cases [ 2 , 3 ].
The management of nail psoriasis is challenging because it is usually time-consuming, with uncertain outcomes. The treatment options mainly depend upon the severity and extent of disease. Patients with nail psoriasis are treated with either topical, intralesional, or systemic therapies [ 4 ]. Methotrexate (MTX), acitretin, and leflunomide are the options for systemic therapy of psoriasis but, as they result in mild improvement of nail psoriasis, many experts consider systemic treatment inadequate for treating nail psoriasis. Topical therapy represents one of the oldest and most well-studied treatment methods for nail psoriasis. Multiple medications have been studied, including corticosteroids, calcipotriol, tazarotene, 5-fluorouracil, cyclosporin, psoralene, and topical calcineurin inhibitors. However, achieving optimal therapeutic concentrations of topical medications is challenging with nail psoriasis given the presence of the nail plate, which can serve as an impermeable physical barrier. The existing evidence suggests that intralesional injections into the nail bed and matrix are particularly effective for alleviating lesions caused by psoriasis of the nail matrix and also have moderate effects on nail bed signs [ 5 ]. Current studies provide recommendations on the intralesional injection technique, recommending an optimal concentration of triamcinolone acetonide (TA), methotrexate (MTX), and cyclosporine [ 6 ]. Mittal et al. compared MTX with other active treatments such as TA and cyclosporine (CsA); good outcomes were reported for TA and MTX, which both appeared to be better than cyclosporine. Both TA and MTX acted as anti-inflammatory, anti-proliferation, and immunosuppressive agents [ 7 ].
We conducted a systematic review and meta-analysis for the evaluation of treatments for nail psoriasis. This study was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009.
Literature Search
A computer-based literature search was performed to identify relevant articles publish on MEDLINE PubMed, EBSCOhost, Scopus, ProQuest, ScienceDirect, SpringerLink, Elsevier Clinical Key, Cochrane library, and ClinicalTrials.gov as well as hand searching from Indonesia libraries. The main search terms using medical subject headings (MeSH) to create subgroups terms were: “ intralesional methotrexate injections for nail psoriasis ,” “ intralesional triamcinolone acetonide injections for nail psoriasis ,” and “ NAPSI Score. ”
Study Selection
The inclusion and exclusion criteria were determined before the search. The included studies fulfilled the following inclusion criteria: (1) the study discussed intralesional MTX and TA injections from 2018 until 2022; (2) study design was clinical trials with/without randomization; (3) the study participants should not have received systemic or topical therapy within 3 months; (4) the evaluated interventions included 3–4 intralesional MTX injections or intralesional MTX injections for 4–6-week interval; (5) the outcome of the studies was reduction in NAPSI score.
Data Abstraction
Three independent reviewers abstracted data using a predefined data extraction form. The following information was extracted from each study: author, year of publication, design of study, blind time period, patient type, details of the interventions (intralesional MTX and TA injections), and the improvement after the treatment using NAPSI score.
Data Analysis
We performed statistical analyses using the Cochrane systematic review software (Review Manager (RevMan) [Computer program] Version 5.4.1., 2020). Categorical data are displayed as percentages, and numerical data are displayed as mean and standard deviation (SD). The meta-analysis assessed the weighted mean between changes in the mean and SD from baseline of the treatment groups and control groups.
Systematic Review
We identified 51 articles matching the search criteria. We extracted 42 articles after reading the title and removing duplicate publications. After reading the abstract, 18 articles were excluded. Furthermore, we retained 33 articles after a full-text review. Three complete articles were assessed for eligibility in order to evaluate the efficacy of MTX intralesional injection as the treatment of nail psoriasis in qualitative and quantitative synthesis. The literature search was conducted based on the PRISMA flow diagram ( Figure 1 ) [ 7 ].

Flow diagram of search strategy and study selection.
These three studies included 38 patients and were conducted in India (n=1), Egypt (n=1), and Italy (n=1). All of the included trials used the MTX intralesional injection as the intervention and TA intralesional injection for the control group. Two of the trials used De Beker injection technique in four injection sites. One of the trials used V-shaped injection technique in two injection sites. The duration of the intervention in all of the trials was 24 weeks.
Results of Qualitative Data Analysis
A study by Starace et al (2022) assessed a pilot study that compared intralesional methotrexate injections versus triamcinolone acetonide in patients affected by nail matrix psoriasis. The study participants were enrolled in Italy between January 2019 and September 2020. Participants included a total of 12 patients with 20 nails affected with psoriasis who had not received any treatment in three months. Patients were divided into two groups of six patients each: Group 1 was treated with MTX 25 mg/mL and Group 2 with TA 10 mg/mL. Each group had a baseline NAPSI score of 5.3. Depending on the group, either MTX or TA was injected into each affected nail, following the De Berker technique and without digital anesthesia. The patients were asked to take folic acid 5 mg once weekly (not on the day of injection) to reduce MTX-associated adverse events (AEs) and toxicities. Patients were treated every six weeks for 24 weeks (total of four treatment sessions) and followed up for an additional six months.
Assessment by NAPSI was performed during each treatment session and at each follow-up visit. At the end of the four sessions, all patients showed improvement in their nail psoriasis, and no new nail disease was noted: mean NAPSI at one month after the last treatment session had a mean value of 0.3 for the MTX group and 1.8 for the TA group. These data were confirmed at the 6-month follow-up for MTX. All patients were satisfied with the procedure. Side effects included procedural pain, which was tolerable. Subungual hematoma occurred in one patient treated with MTX and in one patient treated with TA. Hypopigmentation of the proximal nail fold was instead reported in two of the six patients treated with TA. No major AE was reported in this study.
The study by Mittal et al. (2018) assessed an open-label comparative study of triamcinolone, methotrexate, and cyclosporine. The study participants were enrolled in India in 2018. Patients having at least three affected fingernails with or without concomitant skin lesions who had not been on any systemic and topical antipsoriatic medications for at least the previous three months were enrolled. Ninety fingernails in 17 patients were assigned to three groups of thirty nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/ml), methotrexate (25 mg/ml), or cyclosporine (50 mg/ml), respectively. Digital nerve blocks with plain lignocaine (2%) were administered before intervention. A volume of 0.05 ml was injected from each lateral angle, forming a V . Two injections were administered into each treated nail, with an interval of six weeks.
The severity of nail psoriasis was evaluated using NAPSI score after 12 and 24 weeks. The NAPSI score was graded as: G0 = No improvement; G1 = 25%–50% improvement; G2 = 51%–75% improvement; G3 = 76%–99% improvement; G4 = Complete recovery. At the end of the study, 15 patients (50%) from the TA group, 17 patients (56.7%) from MTX group, and 10 patients (33.3%) from the cyclosporine group showed G3 and G4 improvement. In the cyclosporine group, 11 patients (36.7%) showed only G2 improvement at 24 weeks. This study showed that intramatricial injection therapy was a safe, economical, simple, and effective modality in the management of nail psoriasis. Pain, the most common side effect, was transient with injections of MTX and TA in this study, but with CsA injections, pain was severe and lasted for a few hours in about 50% of nails and for 2–3 days in eight nails injected with cyclosporine. In this study, though the differences in the efficacies of intramatricial TA, MTX, and CsA were not statistically significant, MTX yielded the best results, with the maximum number of nails showing complete recovery (G4 improvement).
A comparative study by Abdelmeniem et al. (2022) evaluated the efficacy of topical calcipotriol combined with urea 20% versus intralesional injection of triamcinolone acetonide, 5-fluorouracil, and methotrexate in the treatment of nail psoriasis. The study participants were enrolled in Egypt in 2022. This study included 60 patients with nail psoriasis who were randomly assigned to four groups, each containing 15 patients. The first three groups received intralesional injection of 0.1 ml of 5-FU (group A), MTX (group B), and TA (group C) into the nail matrix and bed monthly for three months. Group D received a topical combination of calcipotriol/urea 20% twice daily for three months. Patients that received intralesional injections were anesthetized with a combination of topical lidocaine and prilocaine cream 30 minutes before injection. Four injections were administered: two injections at the nail matrix and two injections in the nail bed. The injections were administered twice a day for three months and followed up after six months. Therapeutic response was assessed every month for three months using the NAPSI score.
At the end of the study, the mean percentage of improvement was significantly higher in topical calcipotriol/urea combination (57.1 ± 26.4) than intralesional TA (44.2 ± 32.7), intralesional MTX (37.7 ± 14.2), and intralesional 5-FU (29.6 ± 14). Adverse effects were mild and insignificant in the studied groups. In this study, topical calcipotriol/urea combination seemed to be more effective and safer than intralesional injections of 5-FU, MTX, and TA.
Results of Quantitative Data Analysis (Meta-Analysis)
The difference in mean NAPSI scores after MTX and TA intralesional injections is shown in inTable Table 2 . All of the studies reported a reduction in NAPSI score in both the MTX group and the TA group.
Characteristics of included studies.
The difference in mean NAPSI score after MTX intralesional injections (n=38) and TA intralesional injections (n=38).
The results of the meta-analysis of the effect of MTX intralesional injection therapy compared to TA intralesional injection therapy are shown in Figure 2 . The heterogeneity test obtained Q value = 2.066; df=2; p<0.356, I 2 =3.201. This indicated that the data were homogenous, and the analysis was assessed in fixed effects model.

Forest plot showing the efficacy of MTX intralesional injection compared with placebo from all studies that were evaluated in the meta-analysis. 95% CI = 95% Confidence Interval;
The results of the meta-analysis showed an overall difference in NAPSI scores after administration of intralesional injection of MTX and TA that was −0.213±0.232 (95% CI: −0.667–0.241). This showed that the reduction in NAPSI score after MTX intralesional injection was greater than that after TA intralesional injection. The Q statistic value was z value= −0.921 (p=0.357), showing an insignificant difference in the effectiveness of intralesional MTX injection compared to TA in the management of nail psoriasis.
Risk of Bias in Included Studies
The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system. The studies by Starace et al. and by Mitral et al. showed the possibility of risk of bias, while the study by Adelmeniem et al. showed a low risk of bias. Overall, the result of the meta-analysis evaluating the efficacy of MTX versus TA injection in nail psoriasis treatment was considered to be of moderate-quality (⊕⊕⊕⊖).
Moderate-quality evidence indicated that there was a moderate level of confidence in the estimated effect size from the meta-analysis, where the actual effect size was likely to be close to the estimated value, although there was a possibility that the actual effect size was substantially different. Further studies are likely to have an important impact on the estimated effect size and the level of confidence in the estimated effect size.
This was a systematic review and meta-analysis evaluating the efficacy of MTX versus TA intralesional injection in treating nail psoriasis. Three studies were included in the qualitative review (systematic review), and all three could be reviewed quantitatively (meta-analysis) to determine the efficacy of MTX and TA intralesional injection in nail psoriasis treatment based on the changes in the NAPSI score.
Methotrexate (MTX) has been shown to improve NAPSI score in several studies. MTX is a folic acid analog that irreversibly binds to dihydrofolate reductase and blocks deoxyribonucleic acid synthesis. MTX acts as the anti-inflammatory, anti-proliferative, and immunosuppressant agent [ 11 ]. It is usually taken orally or administered by injection (intramuscular, intravenous, subcutaneous) and has several indications, including nail psoriasis. Current studies suggest injections of MTX (10–25 mg/ml) be administered every 4–8 weeks [ 11 , 12 ].
Triamcinolone acetonide (TA) is a synthetic corticosteroid [ 13 ]. The existing evidence suggests that TA intralesional injections into the nail bed and matrix are particularly effective for alleviating lesions caused by psoriasis of the nail matrix, and they also have moderate effects on nail bed signs [ 14 , 15 ]. TA acts as the anti-inflammatory, anti-proliferative, and immunosuppressant agent. Current studies suggest injections of triamcinolone acetonide (5–10 mg/ml) be administered every 4–8 weeks. Side effects after these procedures are well known [ 16 ]. Local side effects include telangiectasia, skin atrophy, subungual drug deposition, subungual or subcutis hematoma, pigmentation change, necrosis, and ulceration of the skin. Systemic side effect includes Cushing syndrome [ 12 , 13 ].
Patients in the MTX group were expected to have lower NAPSI scores after the intralesional injection of MTX. All of the studies showed a reduction in NAPSI scores after the injection of MTX. Starace et al. reported reduction in NAPSI score in MTX group (−5±1.62), and there was no recurrence during the six months of follow-up [ 10 ]. Mittal et al. also reported a reduction in NAPSI score in MTX group after four weeks of follow-up. The reduction in NAPSI score was 3.04±1.19 and was statistically significant [ 7 ]. Lastly, Abdelmeniem et al. also reported a reduction in NAPSI score in MTX group (−2.4±1.28) [ 9 ]. These findings were consistent with previous studies, which reported significant improvement in nail psoriasis receiving MTX intralesional injections [ 11 – 15 ].
Patients in the TA group were also expected to have lower NAPSI scores after intralesional injections of TA. All the studies showed a reduction in NAPSI scores after the injection of TA. Starace et al. reported a reduction in NAPSI score in the TA group (−3.5±1.48) during the six months of follow-up [ 10 ]. Mittal et al. also reported a reduction in NAPSI score in the TA group after 24 weeks of follow-up (−2.77±1.35) [ 7 ]. Lastly, Abdelmeniem et al. also reported a reduction in NAPSI score in the TA group (−2.5 ± 2.21) [ 9 ]. These findings were consistent with previous studies, which reported significant improvement in nail psoriasis receiving TA intralesional injections [ 11 – 15 ]. Various side effects of intralesional injection of steroids lead us to suggest that MTX intralesional injection is currently preferable in the treatment of nail psoriasis.
We initially suggested that the reduction in NAPSI score in the MTX group was greater than TA group. Starace et al. and Mittal et al. reported significantly greater reduction in NAPSI score after intralesional injection of MTX (Starace et al.: −5±1.62; Mittal et al.: −3.5±1.48) [ 7 , 10 ]. Different outcomes were reported by Abdelmeniem et al.: the reduction in TA group (−2.5±2.21) was greater than in the MTX group (−2.4±1.28). However, Abdelmeniem et al. reported no significance in their findings [ 9 ].
The meta-analysis of the effect of MTX intralesional injection therapy on nail psoriasis treatment was statistically insignificant compared to TA intralesional injection. ( P =0.357 ). The statistical analysis and meta-analysis in this study were limited because of the limited number of patients. Heterogeneity of the dosage, technique procedure, and frequency of the therapy that had not been standardized also affected the results of this study.
However, this study has some limitations, including the limited number of RCTs evaluating the administration of MTX and PRP injection in nail psoriasis, thus affecting the limited number of participants in this study, and that some studies only presented their results in the form of boxplot graph without specifying the numerical value or assessed the treatment response using different scores, thus disqualifying that study from being included in the meta-analysis.
Both methotrexate and triamcinolone acetonide are effective in treating nail psoriasis based on the reduction in NAPSI score. However, larger studies with more participants are necessary to establish the optimal dosage, number and frequency of injections, and technique of injection. A standardized assessment score is also needed to obtain more accurate results.
Abbreviations
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.

IMAGES
VIDEO
COMMENTS
We interpreted the I 2 statistic according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2017): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable ...
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
The objectives of our review were to compare the efficacy and safety of conventional systemic agents for patients with moderate to severe psoriasis and to provide a ranking of these treatments according to their efficacy and safety by the mean of a network meta-analysis. Our review included 140 studies (31 new studies for the update) and 51,749 ...
This is the baseline update of a Cochrane Review first published in 2017, in preparation for this Cochrane Review becoming a living systematic review. Objectives: To compare the efficacy and safety of conventional systemic agents, small molecules, and biologics for people with moderate-to-severe psoriasis, and to provide a ranking of these ...
However, the Cochrane Review on FAEs, published in 2015, included people with all types of psoriasis and not only plaque‐type psoriasis . Adaptive criteria for considering studies for this review As a living systematic review , we are continually identifying new evidence for interventions already in the network of trials but also for novel ...
Psoriasis is an immune‐mediated disease for which some people have a genetic predisposition. The condition manifests in inflammatory effects on either the skin or joints, or both, and it has a major impact on quality of life. ... Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. Unlock the full ...
Psoriasis is an immune‐mediated disease with either skin or joints manifestations, or both, and it has a major impact on quality of life. ... incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. Unlock the full review .
RESULTS: We included six Cochrane systematic reviews assessing interventions for treating psoriasis. The findings from high-quality evidence were that (a) etanercept reduced the psoriasis severity index, compared with placebo and (b) steroids plus vitamin D, compared with vitamin D alone, improved the skin clearance rate, as assessed by investigators, but was associated with a higher ...
Psoriasis is a frequent inflammatory skin disease affecting between 2% and 8% of the general population. Psoriasis affects deeply quality of life. There is currently no cure for psoriasis, but various treatments can help to control the symptoms; thus, long-term treatment is usually needed. In an interview authors Dr Laurence Le Cleach and Emilie Sbidian explain their Cochrane Living Systematic ...
A Cochrane Review on FAEs for psoriasis was published in 2015 . Small molecules ... For each of the following domains and according to the general principles in section 8.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we graded the following 'Risk of bias' domains as 'low', 'high', or 'unclear'.
Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. ... Naldi L, Kinberger M, Afach S, Le Cleach L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews 2023, Issue 7. Art. No.: CD011535. DOI: 10.1002/14651858.CD011535.pub6.
The results from all the Cochrane systematic reviews that were included were summarized and presented in a narrative synthesis. Results: We included six Cochrane systematic reviews assessing interventions for treating psoriasis. The findings from high-quality evidence were that (a) etanercept reduced the psoriasis severity index, compared with ...
This is a protocol for a Cochrane Review (intervention). ... Sbidian E, Le Cleach L. Serious adverse events associated with systemic treatments for psoriasis: a network meta-analysis of observational studies and randomized controlled trials (Protocol). Cochrane Database of Systematic Reviews 2024, Issue 4. Art. No.: CD015263. DOI: 10.1002 ...
The results are presented as a systematic review, and the risk of bias was assessed by the corresponding author using the Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 ...
Our first living systematic review has been published. Read the interview with the authors from our French Satellite https://www.cochrane.org/news/living-systematic ...
Cochrane Database of Systematic Reviews Review - Intervention. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Emilie Sbidian; Anna Chaimani; Ignacio Garcia-Doval; Liz Doney; Corinna Dressler; ... Select your preferred language for Cochrane Reviews. You will see translated Review sections in your ...
Cochrane reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Library should be consulted for the most recent version of the review. Our objective was to compare the effectiveness, tolerability, and safety of topical treatments for chronic plaque psoriasis, relative to placebo; and to compare ...
The 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) served as a guideline for this systematic review and meta-analysis . Database searches were conducted using Ovid MEDLINE, Embase, Cochrane CENTRAL, and Web of Science for all publications until February 27th, 2021.
Chronic plaque psoriasis is the most common type of psoriasis. Although any part of the body may be affected, the most commonly affected sites are the elbows, knees, and scalp. 'Topical' treatments (i.e. treatments applied to the skin) are usually tried first. These include vitamin D products, topical corticosteroids, tar-based preparations ...
From treatment to trigger: a systematic review of imiquimod-induced psoriasis. Nirmay Shah, Corresponding Author. Nirmay Shah [email protected] ... Dermatrials Research & Venderm Innovations in Psoriasis, Hamilton, ON, Canada. Search for more papers by this author. First published: 28 May 2024.
Psoriasis is an immune-mediated disorder which primarily affects skin and has systemic inflammatory involvement. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and monocyte-to-lymphocyte ratio (MLR) are novel complete blood count (CBC)-derived markers which can reflect systemic inflammation. This study aimed to systematically ...
Background: Chronic plaque psoriasis is the most common type of psoriasis and is characterized by redness, thickness, and scaling. First-line management is with topical treatments. Objective: We sought to undertake a Cochrane review of topical treatments for chronic plaque psoriasis. Methods: We systematically searched major databases for randomized controlled trials.
Background/Aim: Nutrition is a key element of the prehabilitation process prior to surgery. The aim of this study was to identify the clinical pathways of nutritional prehabilitation before cystectomy. Methods: A systematic literature review was conducted in PubMed, the Cochrane Library, CINAHL, Scopus and the Web of Science databases. Quality and risk of bias assessment was conducted adhering ...
Other non‐Cochrane systematic reviews have also reported the superiority of FAEs over placebo in the treatment of psoriasis,24, 25, 26 and similar efficacy to methotrexate.24, 26 However, GRADEpro assessment of the level of quality of evidence in our review demonstrated that the latter conclusion is unreliable due to the very low quality of ...
This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC. MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022.
Our systematic review of 146 papers describes feedback facilitation delivered alongside audit and feedback in 104 randomised controlled trials. The papers were identified during the Cochrane review of audit and feedback. The Cochrane review includes an assessment of the effectiveness of feedback facilitation.
We wanted to see whether lifestyle changes (e.g. changing diet, exercising, and avoiding smoking and drinking alcohol), alone or combined, were useful in treating psoriasis when compared to no such changes or another psoriasis treatment. Background. Psoriasis is a long-lasting, inflammatory skin disease; it causes thick, red, itching, and ...
Risk-of-bias (RoB) assessment is used to assess randomised control trials for systematic errors. Developed by Cochrane, it is considered the gold standard of assessing RoB for studies included within systematic reviews, representing a key part of Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.1 The RoB tool comprises six domains that may signify bias: random ...
This was a systematic review and meta-analysis evaluating the efficacy of MTX versus TA intralesional injection in treating nail psoriasis. Three studies were included in the qualitative review (systematic review), and all three could be reviewed quantitatively (meta-analysis) to determine the efficacy of MTX and TA intralesional injection in ...