Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.

Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
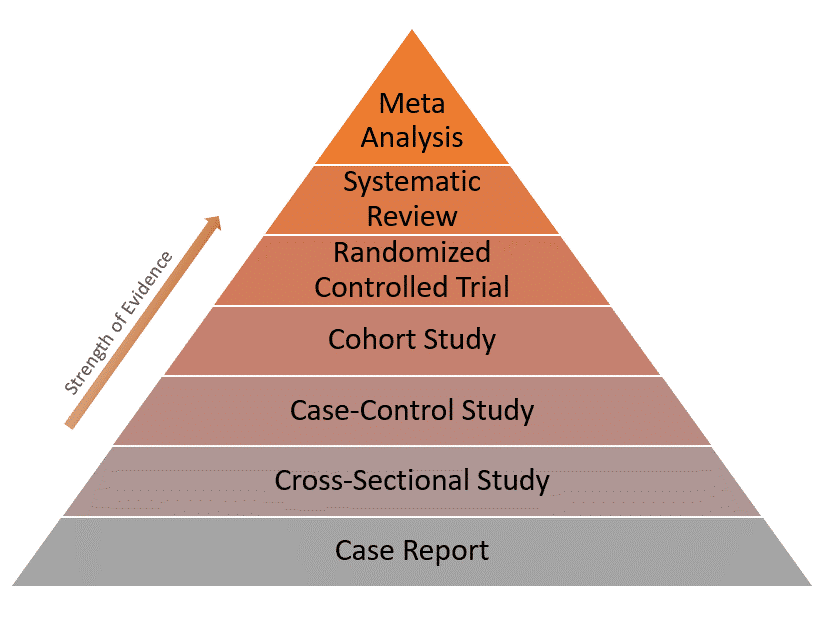
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to write a medical...
How to write a medical case report
- Related content
- Peer review
- Seema Biswas , editor-in-chief, BMJ Case Reports, London, UK ,
- Oliver Jones , student editor, BMJ Case Reports, London, UK
Two BMJ Case Reports journal editors take you through the process
This article contains...
- Choosing the right patient
- Choosing the right message
- Before you begin - patient consent
- How to write your case report
- How to get published
During medical school, students often come across patients with a unique presentation, an unfamiliar response to treatment, or even an obscure disease. Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care.
For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your curriculum vitae, particularly if it is on a topic of your chosen specialty. Publication will be an advantage when applying for foundation year posts and specialty training, and many job applications have points allocated exclusively for publications in peer reviewed journals, including case reports.
The writing of a case report rests on skills that medical students acquire in their medical training, which they use throughout their postgraduate careers: these include history taking, interpretation of clinical signs and symptoms, interpretation of laboratory and imaging results, researching disease aetiology, reviewing medical evidence, and writing in a manner that clearly and effectively communicates with the reader.
If you are considering writing a case report, try to find a senior doctor who can be a supervising coauthor and help you decide whether you have a message worth writing about, that you have chosen the correct journal to submit to (considering the format that the journal requires), that the process is transparent and ethical at all times, and that your patient is not compromised in your writing. Indeed, try to include your patient in the process from the …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article

Case Reports: How to Write a Case Report
- How to Write a Case Report
- Case Report Resources
- YouTube Resources
Consensus-Based Clinical Case Reporting Guidelines

| This was developed by to correspond with key components of a case report and capture useful clinical information (including 'meaningful use' information mandated by some insurance plans). The narrative: A case report tells a story in a narrative format that includes the presenting concerns, clinical findings, diagnoses, interventions, outcomes (including adverse events), and follow-up. The narrative should include a discussion of the rationale for any conclusions and any take-away messages. |
| Title | 1 | The words "case report: should be in the title, along with area of focus |
| Keywords | 2 | Four to seven keywords - include "case report" as one of the keywords |
| Abstract | 3a | Background: What does this case report add to the medical literature? |
| 3b | Case summary: chief complaint, diagnoses, interventions, and outcomes | |
| 3c | Conclusion: What is the main "take-away" lesson from this case? | |
| Introduction | 4 | The current standard of care and contributions of this case - with references (1-2 paragraphs) |
| Timeline | 5 | Information from this case report organized into a timelines (table or figure) |
| Patient Information | 6a | De-identified demographic and other patient or client specific information |
| 6b | Chief complaint - what prompted the visit? | |
| 6c | Relevant history including past interventions and outcomes | |
| Physical Exam | 7 | Relevant physical examination findings |
| Diagnostic Assessment | 8a | Evaluations such as surveys, laboratory testing, imaging, etc. |
| 8b | Diagnostic reasoning including other diagnoses considered and challenges | |
| 8c | Consider tables or figures linking assessment, diagnoses, and interventions | |
| 8d | Prognostic characteristics where applicable | |
| Interventions | 9a | Types such as lifestyle recommendations, treatments, medications, surgery |
| 9b | Intervention administration such as dosage, frequency, and duration | |
| 9c | Note changes in intervention with explanation | |
| 9d | Other concurrent interventions | |
| Follow-up and Outcomes | 10a | Clinician assessment (and patient or client assessed outcomes when appropriate) |
| 10b | Important follow-up diagnostic evaluations | |
| 10c | Assessment of intervention adherence and tolerability, including adverse events | |
| Discussion | 11a | Strengths and limitations in your approach to the case |
| 11b | Specify how this case report informs practice or Clinical Practice Guidelines (CPG) | |
| 11c | How does this case report suggest a testable hypothesis? | |
| 11d | Conclusions and rationale | |
| Patient Perspective | 12 | When appropriate, include the assessment of the patient or client on this episode of care |
| Informed Consent | 13 | Informed consent from the person who is the subject of this case report, required by most journals |
| Additional Information | 14 | Acknowledgement section; Competing Interests; IRB approval when required |
Gagnier JJ, Riley D, Altman DG, Moher D, Sox H, Kienle GS, for the CARE group: The CARE guidelines: Consensus-based clinical case reporting guideline development. Dtsch Arztebl Int 2013; 110(37): 603-8.
Select Journals Accepting Case Reports
| The following journals are indexed in Medline and currently accepting case reports (as of 12/2/2016) either regularly or under specific circumstances. Click on the links below to view the author instructions for each journal and determine if your case meets the journal's criteria. |
Case Report Templates
The CAse REporting (CARE) team created templates in nine languages to assist clinicians, researchers, and educators with the ultimate goal of improving the completeness, transparency, and usefulness of case reports.
English , Spanish , German , Chinese , Dutch , French , Japanese , Korean , Portuguese
- Case Report Journals
A list of case report journals can be found in the pdf below. It provides information on the year launched, open-access status, reported questionable publishing practices, and whether the journal is indexed in Medline. The majority of these journals are open-access and will require a submission fee.
BMJ Case Reports
- BMJ Consent Form
The Library has an institutional fellowship with BMJ Case Reports which allows faculty, staff, and students at Weill Cornell Medicine to submit case reports without paying an individual fellowship fee. Use our fellowship code when you are ready to publish.
Please note: BMJ Case Reports, like most journals, requires a signed consent form in order for a case report to be considered for publication.
- << Previous: Home
- Next: Case Report Resources >>
- Last Updated: Aug 15, 2022 12:15 PM
- URL: https://med.cornell.libguides.com/casereports
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023
A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
26 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Search Menu
Sign in through your institution
- Advance articles
- AHFS First Release
- AJHP Voices
- AJHP Residents Edition
- Top Twenty-Five Articles
- ASHP National Surveys of Pharmacy Practice in Hospital Settings
- Medication Safety
- Pharmacy Technicians
- Specialty Pharmacy
- Emergency Preparedness and Clinician Well-being
- Author Guidelines
- Submission Site
- Open Access
- Information for Reviewers
- Self-Archiving Policy
- Author Instructions for Residents Edition
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Editorial Board
- Permissions
- Journals on Oxford Academic
- Books on Oxford Academic
- < Previous
How to write a patient case report
- Article contents
- Figures & tables
- Supplementary Data
Henry Cohen, How to write a patient case report, American Journal of Health-System Pharmacy , Volume 63, Issue 19, 1 October 2006, Pages 1888–1892, https://doi.org/10.2146/ajhp060182
- Permissions Icon Permissions
Purpose. Guidelines for writing patient case reports, with a focus on medication-related reports, are provided.
Summary. The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion. The abstract of a patient case report should succinctly include the four sections of the main text of the report. The introduction section should provide the subject, purpose, and merit of the case report. It must explain why the case report is novel or merits review, and it should include a comprehensive literature review that corroborates the author’s claims. The case presentation section should describe the case in chronological order and in enough detail for the reader to establish his or her own conclusions about the case’s validity. The discussion section is the most important section of the case report. It ought to evaluate the patient case for accuracy, validity, and uniqueness; compare and contrast the case report with the published literature; derive new knowledge; summarize the essential features of the report; and draw recommendations. The conclusion section should be brief and provide a conclusion with evidence-based recommendations and applicability to practice.
Conclusion. Patient case reports are valuable resources of new and unusual information that may lead to vital research.
American Society of Health-System Pharmacists members
Personal account.
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Short-term Access
To purchase short-term access, please sign in to your personal account above.
Don't already have a personal account? Register
| Month: | Total Views: |
|---|---|
| January 2019 | 253 |
| February 2019 | 915 |
| March 2019 | 810 |
| April 2019 | 784 |
| May 2019 | 759 |
| June 2019 | 735 |
| July 2019 | 687 |
| August 2019 | 511 |
| September 2019 | 377 |
| October 2019 | 265 |
| November 2019 | 237 |
| December 2019 | 97 |
| January 2020 | 205 |
| February 2020 | 151 |
| March 2020 | 143 |
| April 2020 | 103 |
| May 2020 | 115 |
| June 2020 | 133 |
| July 2020 | 129 |
| August 2020 | 154 |
| September 2020 | 133 |
| October 2020 | 147 |
| November 2020 | 85 |
| December 2020 | 84 |
| January 2021 | 104 |
| February 2021 | 105 |
| March 2021 | 108 |
| April 2021 | 153 |
| May 2021 | 109 |
| June 2021 | 87 |
| July 2021 | 124 |
| August 2021 | 113 |
| September 2021 | 126 |
| October 2021 | 126 |
| November 2021 | 101 |
| December 2021 | 137 |
| January 2022 | 105 |
| February 2022 | 105 |
| March 2022 | 149 |
| April 2022 | 186 |
| May 2022 | 116 |
| June 2022 | 155 |
| July 2022 | 115 |
| August 2022 | 111 |
| September 2022 | 176 |
| October 2022 | 123 |
| November 2022 | 126 |
| December 2022 | 101 |
| January 2023 | 151 |
| February 2023 | 127 |
| March 2023 | 168 |
| April 2023 | 147 |
| May 2023 | 103 |
| June 2023 | 50 |
| July 2023 | 76 |
| August 2023 | 117 |
| September 2023 | 94 |
| October 2023 | 138 |
| November 2023 | 115 |
| December 2023 | 76 |
| January 2024 | 103 |
| February 2024 | 108 |
| March 2024 | 108 |
| April 2024 | 85 |
| May 2024 | 63 |
| June 2024 | 15 |
Email alerts
Citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1535-2900
- Print ISSN 1079-2082
- Copyright © 2024 American Society of Health-System Pharmacists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Get new issue alerts Get alerts
- Submit a Manuscript
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
How to Write Case Reports and Case Series
Ganesan, Prasanth
Department of Medical Oncology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Address for correspondence: Dr. Prasanth Ganesan, Medical Oncology, 3 rd Floor, SSB, Jawaharlal Institute of Postgraduate Medical Education and Research, Dhanvantari Nagar, Puducherry - 605006, India. E-mail: [email protected]
Received March 13, 2022
Received in revised form April 10, 2022
Accepted April 10, 2022
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Case reports are considered the smallest units of descriptive studies. They serve an important function in bringing out information regarding presentation, management, and/or outcomes of rare diseases. They can also be a starting point in understanding unique associations in clinical medicine and can introduce very effective treatment paradigms. Preparing the manuscript for a case report may be the first exposure to scientific writing for a budding clinician/researcher. This manuscript describes the steps of writing a case report and essential considerations when publishing these articles. Individual components of a case report and the “dos and don'ts” while preparing these components are detailed.
INTRODUCTION
A case report describes several aspects of an individual patient's presentation, investigations, management decisions, and/or outcomes. This is a type of observational study and has been described as the smallest publishable unit in medical literature.[ 1 ] A case series involves a group of patients with similar presentations or treatments. In modern medicine [ Figure 1 ], these publications are categorized as the “lowest level of evidence”.[ 2 ] However, they serve several essential functions. For example, there are rare diseases where large, randomized trials, or even observational studies may not be possible. Medical practice, in these conditions, is often guided by well-presented case reports or series. There are situations where a single case report has heralded an important therapy change.[ 3 ] Further, case reports are often a student's first exposure to manuscript writing. Hence, these serve as training for budding scholars to understand scientific writing, learn the process of manuscript submission, and receive and respond to reviewer comments. This article explains the reasons why case reports are published and provides guidance for writing such type of articles.

WHY ARE CASE REPORTS PUBLISHED?
A case report is often published to highlight the rarity of a particular presentation. However, it may be of much more value if it also informs some aspects of management. This could be in the form of rare expressions of a common disease so that clinicians who read will be aware and can consider additional possibilities and differential diagnoses when encountering similar situations. A new form of evaluation of a patient, either to facilitate the diagnostics or to improve understanding of the disease condition, may stimulate a case report. Novel treatments may be tried, and the results might be necessary to disseminate. This may be encountered either in rare diseases or conditions where treatment options are exhausted. Moreover, randomized trials report outcomes of a group and often do not inform about the individual patient. [ Table 1 ] describes a few examples of case reports/case series which have had a remarkable impact on medical practice.

ETHICAL ISSUES
If there is a possibility of patient identification from the report, it is mandatory to obtain informed consent from the patient while approval from the institutional ethics committee (IEC) may also be needed depending on institutional policies.[ 7 ] If identifying information is absent (or if suitable steps are taken to remove identifying information or hide the identity, (such as by covering the eyes), it may still be required by some journals to obtain ethics committee approval for certain types of case reports. If a case series involves retrospective chart review, “waiver-of-consent” may be sought from the ethics committee. Indian Ethical Guidelines do not separately address this issue in case reports.[ 8 ] The Committee on Publication Ethics has described best practices for journals when publishing case reports which also gives links to model consent forms.[ 9 ]
HOW TO START?
If you are a beginner and you have identified an interesting case which you want to report, the first step would be to sit with your team and discuss the aspects of the case you want to highlight in your publication.[ 10 ] Do a literature search and try to summarize available information before writing the draft. It would also be a good idea to understand which journal you are targeting; this will assist in determining the number of figures, the word limits, and ethical requirements (such as informed consent). Discussions with senior faculty about the authors and their order should also be done at this point to avoid issues later. For a beginner, it would be a good practice to present the case in the department or in an institutional scientific forum before writing up the manuscript.
COMPONENTS OF a CASE REPORT
A case report usually has the following sections: an abstract, a brief introduction, the actual description of the case, and finally, the discussion which highlights the uniqueness of the case and includes a conclusion statement. Many journals these days publish case reports only as a letter to editor; in such cases, an abstract is not usually required.
The title must be informative about the problem being reported. It may refer to the particular issue being highlighted in the report, or it may refer to the educational aspect of that particular report. Catchy titles are often used by authors to trigger interest among the readers and make them want to read the article. Authors may remember to use titles which will help people locate the article when searching the literature.
When writing a title, it may be best to avoid terms such as “case report,” “review of literature,” “unique,” “rare,” “first-report”; these do not add value to the presentation.
Introduction
This must introduce the condition and clearly state why the case report is worth reading. It may also contain a brief mention of the current status of the problem being described with supporting references.
Describing the case
The case must be presented succinctly, in a chronological order, clearly highlighting the salient aspects of the case being reported. Relevant negative findings may be provided. For example, if a case is being reported for elaborating a new type of treatment, then more attention must be given to treatment aspects (e.g., name of the drug, dosage, schedule, dose modifications, or the type of surgery, duration, and type of anesthesia) after briefly describing the presentation and diagnostics. The idea is that the reader must be able to apply the treatment in his/her practice if required.
However, if the case is being presented for diagnostic rarity/unusual clinical features/pathological aspects, then more attention must be given to these aspects. For example, if the emphasis is on tissue pathology, then the description must include details about tissue processing, types of stains, and immunohistochemistry details.
Figures and tables
Figures, as in any publication, should be self-explanatory. A properly constructed figure legend can be used for describing certain aspects of the case much better than long-winded text in the main manuscript. This will also help to reduce the word count in the main manuscript. If there are multiple figures (e.g., follow-up radiology series and response to treatment images), these can be combined as [ Figure 1 ]a, [ Figure 1 ]b, [ Figure 1c ] or [ Figure 1 ]a, [ Figure 1 ]b, [ Figure 1 ]c, [ Figure 1d ]. This will help conform to the figure number limits prescribed by the journal. While preparing the figures, one must ensure that the quality of the art/photograph is not compromised. Further, patient identifying features must be masked, unless necessary to show.
Tables are usually not part of case reports but may be used. One example is presenting the baseline investigations in a tabular format which can facilitate assimilation as well as reduce the word count. Tables are more often used in case series. The most common is a type of table where the features of all the cases included are summarized with each row referring to an individual patient. This usually works for a series of up to ten patients; beyond that, the table may become crowded and difficult to understand. Tables may also be used in the discussion section to summarize related, published reports to date.
Discussion including review
A case report may help to alter the approach to patient management in the clinic or it may even stimulate original research evaluating a new treatment. Thus, the discussion must summarize the unique aspects of the case (why is the case different?) and the essential learning points/implications (how will it change management?/What further research needs to be done?). In addition to stating the differences from existing literature, the discussion should also attempt to explain these differences.
If the condition or treatment approach being focused on is sufficiently rare, reviewing all available cases published until that point is critical. This review may be presented in a table with each case described briefly. A more nuanced study might attempt to summarize the relevant demographics and clinical details of the various cases published to date in the form of a table (e.g., median age, gender distribution, and survival outcomes).
CASE SERIES - WHAT IS DIFFERENT?
There is no formal definition as to what is case series and what would be considered a retrospective cohort study. In general, a case series comprises <10 cases; beyond that, it may be feasible to apply formal statistics and may be considered a cohort study.
Both case reports and case series are descriptive studies. Case series must have similar cases and hence the inclusion must be clearly defined. The interventions must be documented in a way that is reproducible and follow-up of each individual in the report must be available. Although formal statistical analyses are usually not a part of case series, authors may attempt to summarize baseline demographic parameters using descriptive statistics.
ABSTRACT OF a CASE REPORT
As explained earlier, a few journals do not require abstracts for case report submissions. When required, one should try to highlight the salient aspects of the case presented and the reason for the publication within the abstract word limit, which may be as short as 100–200 words. Spend time and effort in writing a good abstract as this is a portion which is usually read by the editor during manuscript screening and may have implications for whether the article progresses to the next stage of editorial processing.
REFERENCES IN a CASE REPORT
One may only cite key references in a case report or series as there is limited scope for elaborate literature search. Most journals have a limit of 10–15 references for case reports; when publishing as a letter to editor (or correspondence), the allowed reference limit may be even lower (five or less for some journals).
CHOOSING THE RIGHT JOURNAL
Many journals have recently stopped publishing case reports and series. This is often an attempt by journals to optimize their resources (space and reviewer time) to attain the highest possible impact. Although this is unfortunate, it is a reality which must be acknowledged. Nonetheless, the advent of online-only journals has led to more options for aspiring authors. Some journals accept case series, whereas others have “sister” journals created to accept case reports and other, less definitive, contributions to the literature.[ 11 ] It is an important exercise to study all available journals accepting case reports of the type being written. The case report must be tailored to the journal's requirements. Many journals may charge an article processing fee; author(s) must consider whether they are willing to pay and publish. Some of these may be predatory journals; authors must be wary of them and scrupulously avoid publishing in such journals as they can permanently stain the publication records of a researcher.
PUBLISHING THE CASE REPORT/SERIES AS a LETTER TO EDITOR/IMAGE SERIES
When the matter to be conveyed is very minimal or is being published mainly for its rarity, letters to editor may be an alternate route to publish case report data. Interesting images may be published in the form of “images” series which is now a part of many journals. The flexibility of web-based publishing also allows interesting videos to be published online.
GUIDELINES FOR CASE REPORTS
There are guidelines which help authors in the preparation and submission of case reports. The CAse REports (CARE) checklist is one such popular guideline. It provides a “checklist” and other resources for authors that can help navigate the process of writing a case report, especially when a person is doing it for the first time.[ 12 ]
AUTHORSHIP IN CASE REPORTS
Although there are no separate guidelines for authorship in “case reports,” general authorship rules follow that for any manuscript. “Gift” authorship must be avoided. All authors must have contributed to the creation of the manuscript in addition to being involved in some aspect of care of the patient being reported. Authorship order should be ideally predecided based on mutual consensus.
CONCLUSIONS
A case report is a useful starting point for one's scientific writing career. There are useful online resources which describe the steps for a newbie writer.[ 13 14 ] [ Table 2 ] summarizes the important components to follow and understand when writing case reports. Although many frontline journals have reduced their acceptance of case reports, these publications continue to serve an essential scientific and academic role.

Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Case reports; manuscript writing; case series; references
- + Favorites
- View in Gallery
Readers Of this Article Also Read
How to revise and resubmit the manuscript after a favorable peer review, glossopharyngeal neuralgia following coronavirus disease 2019 infection, polymorphous adenocarcinoma of the parotid – an uncommon site of occurrence, primary vulval mucinous adenocarcinoma of intestinal type masquerading as..., detection of rare blood group ax phenotype in blood donor.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
How to write a patient case report
Affiliation.
- 1 Arnold & Marie Schwartz College of Pharmacy & Health Sciences, Long Island University, Brooklyn, NY 11203, USA. [email protected]
- PMID: 16990637
- DOI: 10.2146/ajhp060182
Purpose: Guidelines for writing patient case reports, with a focus on medication-related reports, are provided.
Summary: The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion. The abstract of a patient case report should succinctly include the four sections of the main text of the report. The introduction section should provide the subject, purpose, and merit of the case report. It must explain why the case report is novel or merits review, and it should include a comprehensive literature review that corroborates the author's claims. The case presentation section should describe the case in chronological order and in enough detail for the reader to establish his or her own conclusions about the case's validity. The discussion section is the most important section of the case report. It ought to evaluate the patient case for accuracy, validity, and uniqueness; compare and contrast the case report with the published literature; derive new knowledge; summarize the essential features of the report; and draw recommendations. The conclusion section should be brief and provide a conclusion with evidence-based recommendations and applicability to practice.
Conclusion: Patient case reports are valuable resources of new and unusual information that may lead to vital research.
PubMed Disclaimer
Similar articles
- Instructions for Radiological Case Reports. Bannas P. Bannas P. Rofo. 2017 Apr;189(4):333-338. doi: 10.1055/s-0043-101525. Epub 2017 Mar 23. Rofo. 2017. PMID: 28335058 English.
- Writing patient case reports for publication. Juyal D, Thaledi S, Thawani V. Juyal D, et al. Educ Health (Abingdon). 2013 May-Aug;26(2):126-9. doi: 10.4103/1357-6283.120707. Educ Health (Abingdon). 2013. PMID: 24200736
- Rules to be adopted for publishing a scientific paper. Picardi N. Picardi N. Ann Ital Chir. 2016;87:1-3. Ann Ital Chir. 2016. PMID: 28474609
- Writing a qualitative research report. Burnard P. Burnard P. Accid Emerg Nurs. 2004 Jul;12(3):176-81. doi: 10.1016/j.aaen.2003.11.006. Accid Emerg Nurs. 2004. PMID: 15234716 Review.
- [Writing and publication of a clinical case report]. Târcoveanu E, Roca M, Mihăescu T. Târcoveanu E, et al. Chirurgia (Bucur). 2011 Sep-Oct;106(5):581-4. Chirurgia (Bucur). 2011. PMID: 22165055 Review. Romanian.
- Academic Footprint of Journal of Orthopedic Case Reports in the Past 5 Years - A Scientometric Analysis. Muthu S, Annamalai S. Muthu S, et al. J Orthop Case Rep. 2024 Mar;14(3):1-4. doi: 10.13107/jocr.2024.v14.i03.4264. J Orthop Case Rep. 2024. PMID: 38560307 Free PMC article. No abstract available.
- Writing Case Reports Can Improve Seven Components in Clinical Reasoning. Nishizawa T, Ishizuka K, Otsuka Y, Nakanishi T, Kawashima A, Miyagami T, Yamashita S. Nishizawa T, et al. Int Med Case Rep J. 2024 Mar 21;17:195-200. doi: 10.2147/IMCRJ.S449310. eCollection 2024. Int Med Case Rep J. 2024. PMID: 38533427 Free PMC article.
- Twelve Tips for Creating an All-Day Writing Retreat for Health Profession Educators: An Immersive, Product-Oriented Learning Experience. Ligon BL, Elizondo R, Thammasitboon S. Ligon BL, et al. MedEdPublish (2016). 2019 Oct 8;8:188. doi: 10.15694/mep.2019.000188.1. eCollection 2019. MedEdPublish (2016). 2019. PMID: 38089256 Free PMC article.
- Journals accepting case reports. Gotschall T, Spencer A, Hoogland MA, Cortez E, Irish E. Gotschall T, et al. J Med Libr Assoc. 2023 Oct 2;111(4):819-822. doi: 10.5195/jmla.2023.1747. J Med Libr Assoc. 2023. PMID: 37928130 Free PMC article.
- Case Report-Driven Medical Education in Rural Family Medicine Education: A Thematic Analysis. Ohta R, Sano C. Ohta R, et al. Healthcare (Basel). 2023 Aug 11;11(16):2270. doi: 10.3390/healthcare11162270. Healthcare (Basel). 2023. PMID: 37628468 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Case report
Journal of Medical Case Reports welcomes well-described reports of cases that include the following:
- Unreported or unusual side effects or adverse interactions involving medications.
- Unexpected or unusual presentations of a disease.
- New associations or variations in disease processes.
- Presentations, diagnoses and/or management of new and emerging diseases.
- An unexpected association between diseases or symptoms.
- An unexpected event in the course of observing or treating a patient.
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect.
Case reports submitted to Journal of Medical Case Reports should make a contribution to medical knowledge and must have educational value or highlight the need for a change in clinical practice or diagnostic/prognostic approaches. The journal will not consider case reports describing preventive or therapeutic interventions, as these generally require stronger evidence.
Authors are encouraged to describe how the case report is rare or unusual as well as its educational and/or scientific merits in the covering letter that accompanies the submission of the manuscript.
Any images should protect the patient’s anonymity as far as possible. Any photos or medical imaging should not show the patient's name, medical record number, or date of birth. Images should be cropped only to show the key feature. As per journal policy, JMCR does not consider images with patient faces or patient facial features. If an image of a face must be published, this should be cropped so that only the affected area is shown.
Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Patient ethnicity must be included in the Abstract and in the Main Body under the Case Presentation section.
Reporting standards
For case reports, Journal of Medical Case Reports requires authors to follow the CARE guidelines . The CARE checklist should be provided as an additional files. Submissions received without these elements will be returned to the authors as incomplete.
The checklist will not be used as a tool for judging the suitability of manuscripts for publication in Journal of Medical Case Reports , but is intended as an aid to authors to clearly, completely, and transparently let reviewers and readers know what authors did and found. Using the CARE guideline to write the case report and completing the CARE checklist are likely to optimize the quality of reporting and make the peer review process more efficient.

Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
Title page
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review, A case report etc."
- or, for non-clinical or non-research studies: a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. The abstract must include the following separate sections:
- Background: why the case should be reported and its novelty
- Case presentation: a brief description of the patient’s clinical and demographic details, the diagnosis, any interventions and the outcomes
- Conclusions: a brief summary of the clinical impact or potential implications of the case report
Keywords
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the case report or study, its aims, a summary of the existing literature.
Case presentation
This section should include a description of the patient’s relevant demographic details, medical history, symptoms and signs, treatment or intervention, outcomes and any other significant details.
Discussion and Conclusions
This should discuss the relevant existing literature and should state clearly the main conclusions, including an explanation of their relevance or importance to the field.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
- Editorial Board
- Manuscript editing services
- Meet the Editors
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
For authors
What we publish, how to submit.
- BMJ Case Reports Author Guide
Case report templates
Author statements, reviewer guidance, editorial policy, patient consent and confidentiality, peer review, competing interests, what will it cost, open access, rapid responses.
- flowcharts that show clinical course time lines
- illustrative diagrams that facilitate the interpretation of clinical images
- graphs of results
- management algorithms
- referenced guidelines
- assess the efficacy or effectiveness of new interventions, new drugs, unlicensed substances, or lifestyle changes
- describe drug efficacy, drug interactions or adverse drug effects in patients enrolled in ongoing clinical trials
- describe single-instance, off-label or experimental use of an existing drug or a combination of drugs used for a new clinical indication or the results of phase 2 clinical trials
- have been previously submitted to a preprint server as there are patient confidentiality concerns
- have more than one case (case series). If we feel that an article is strengthened by the inclusion of more than one case, we may consider the article provided it includes no more than three patients. Please contact the editor-in-chief before submitting a case series
- the learning outcomes should be important and novel
- there should be a detailed and balanced review of relevant up-to-date literature
- include diagrams, flowcharts and algorithms that you have drawn so that each case may be used as a textbook case
- there should be comprehensive and critical appraisal of relevant global health literature
- include published public health and epidemiological data
- include an in-depth understanding of the anthropological background of the case
- Videos are published under the same copyright terms as the associated article
- The content and focus of the video must relate directly to the case report
- If audio narration is used, please, ensure that this is clear
- Annotate and label essential structures in videos
- Do not add background music or colourful animation
- Use the compression parameters that video sharing sites use. Often these are standard options in your editing software. A comprehensive guide is available from Vimeo
- Do not show any identifiable features of living patients and/or identifiable personal details in the foreground or background
- Clinical Case Report reviewer guidance >>
- Global Health Case Report reviewer guidance >>
- Images in/Videos reviewer guidance >>
- be involved in the clinical care of the patient
- give final approval of the manuscript
- be responsible for drafting of the text, sourcing and editing of clinical images, results of clinical investigations, drawing of original diagrams and management algorithms, and critical revision for important intellectual content
- agree to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
- authors are required to declare in the author statement that they are the patient in the case report, this statement will appear in the case report
- we do not publish case reports where the patient is the sole author or the patient is a relative of the author
- the report will require careful anonymisation - patients with concerns about anonymity are advised not to co-author manuscripts but to add their patient perspective instead
- we require consent from everyone whose medical information is disclosed in the manuscript (e.g., parents, siblings, etc)
- signature of the consent form should be after the patient has seen and approved the manuscript
- if the manuscript is substantially changed as a result of revisions, the authors should confirm that the patient has seen and approved the final manuscript
- patients should be made aware that published online content may be picked up by non-BMJ or non-medical media
- after publication of a case report, should authors wish to submit a second manuscript describing the progress of the same patient, up to date informed consent will be needed with a new consent form signed by the patient
- anonymise all details of patients in the text, tables, figures, figure legends and within the patient perspective section
- unless clinically relevant, ethnicity and occupation should not be included
- when describing family history in the case report use “first degree relative” or ”second degree relative” for parents or siblings or grandparents or cousins
- exclude specific ages, instead use “early”/”mid”/”late” “20s”, “30s”, “40s”….
- childhood age ranges include preterm neonatal, term neonatal (birth – 27 days), infancy (28 days – 12 months), toddler (13 months – 2 years), early childhood (2 – 5 years), middle childhood (6 – 11 years), early adolescence (12 – 18 years) and late adolescence (19 – 21 years)
Case Study Research Method in Psychology
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews).
The case study research method originated in clinical medicine (the case history, i.e., the patient’s personal history). In psychology, case studies are often confined to the study of a particular individual.
The information is mainly biographical and relates to events in the individual’s past (i.e., retrospective), as well as to significant events that are currently occurring in his or her everyday life.
The case study is not a research method, but researchers select methods of data collection and analysis that will generate material suitable for case studies.
Freud (1909a, 1909b) conducted very detailed investigations into the private lives of his patients in an attempt to both understand and help them overcome their illnesses.
This makes it clear that the case study is a method that should only be used by a psychologist, therapist, or psychiatrist, i.e., someone with a professional qualification.
There is an ethical issue of competence. Only someone qualified to diagnose and treat a person can conduct a formal case study relating to atypical (i.e., abnormal) behavior or atypical development.
Famous Case Studies
- Anna O – One of the most famous case studies, documenting psychoanalyst Josef Breuer’s treatment of “Anna O” (real name Bertha Pappenheim) for hysteria in the late 1800s using early psychoanalytic theory.
- Little Hans – A child psychoanalysis case study published by Sigmund Freud in 1909 analyzing his five-year-old patient Herbert Graf’s house phobia as related to the Oedipus complex.
- Bruce/Brenda – Gender identity case of the boy (Bruce) whose botched circumcision led psychologist John Money to advise gender reassignment and raise him as a girl (Brenda) in the 1960s.
- Genie Wiley – Linguistics/psychological development case of the victim of extreme isolation abuse who was studied in 1970s California for effects of early language deprivation on acquiring speech later in life.
- Phineas Gage – One of the most famous neuropsychology case studies analyzes personality changes in railroad worker Phineas Gage after an 1848 brain injury involving a tamping iron piercing his skull.
Clinical Case Studies
- Studying the effectiveness of psychotherapy approaches with an individual patient
- Assessing and treating mental illnesses like depression, anxiety disorders, PTSD
- Neuropsychological cases investigating brain injuries or disorders
Child Psychology Case Studies
- Studying psychological development from birth through adolescence
- Cases of learning disabilities, autism spectrum disorders, ADHD
- Effects of trauma, abuse, deprivation on development
Types of Case Studies
- Explanatory case studies : Used to explore causation in order to find underlying principles. Helpful for doing qualitative analysis to explain presumed causal links.
- Exploratory case studies : Used to explore situations where an intervention being evaluated has no clear set of outcomes. It helps define questions and hypotheses for future research.
- Descriptive case studies : Describe an intervention or phenomenon and the real-life context in which it occurred. It is helpful for illustrating certain topics within an evaluation.
- Multiple-case studies : Used to explore differences between cases and replicate findings across cases. Helpful for comparing and contrasting specific cases.
- Intrinsic : Used to gain a better understanding of a particular case. Helpful for capturing the complexity of a single case.
- Collective : Used to explore a general phenomenon using multiple case studies. Helpful for jointly studying a group of cases in order to inquire into the phenomenon.
Where Do You Find Data for a Case Study?
There are several places to find data for a case study. The key is to gather data from multiple sources to get a complete picture of the case and corroborate facts or findings through triangulation of evidence. Most of this information is likely qualitative (i.e., verbal description rather than measurement), but the psychologist might also collect numerical data.
1. Primary sources
- Interviews – Interviewing key people related to the case to get their perspectives and insights. The interview is an extremely effective procedure for obtaining information about an individual, and it may be used to collect comments from the person’s friends, parents, employer, workmates, and others who have a good knowledge of the person, as well as to obtain facts from the person him or herself.
- Observations – Observing behaviors, interactions, processes, etc., related to the case as they unfold in real-time.
- Documents & Records – Reviewing private documents, diaries, public records, correspondence, meeting minutes, etc., relevant to the case.
2. Secondary sources
- News/Media – News coverage of events related to the case study.
- Academic articles – Journal articles, dissertations etc. that discuss the case.
- Government reports – Official data and records related to the case context.
- Books/films – Books, documentaries or films discussing the case.
3. Archival records
Searching historical archives, museum collections and databases to find relevant documents, visual/audio records related to the case history and context.
Public archives like newspapers, organizational records, photographic collections could all include potentially relevant pieces of information to shed light on attitudes, cultural perspectives, common practices and historical contexts related to psychology.
4. Organizational records
Organizational records offer the advantage of often having large datasets collected over time that can reveal or confirm psychological insights.
Of course, privacy and ethical concerns regarding confidential data must be navigated carefully.
However, with proper protocols, organizational records can provide invaluable context and empirical depth to qualitative case studies exploring the intersection of psychology and organizations.
- Organizational/industrial psychology research : Organizational records like employee surveys, turnover/retention data, policies, incident reports etc. may provide insight into topics like job satisfaction, workplace culture and dynamics, leadership issues, employee behaviors etc.
- Clinical psychology : Therapists/hospitals may grant access to anonymized medical records to study aspects like assessments, diagnoses, treatment plans etc. This could shed light on clinical practices.
- School psychology : Studies could utilize anonymized student records like test scores, grades, disciplinary issues, and counseling referrals to study child development, learning barriers, effectiveness of support programs, and more.
How do I Write a Case Study in Psychology?
Follow specified case study guidelines provided by a journal or your psychology tutor. General components of clinical case studies include: background, symptoms, assessments, diagnosis, treatment, and outcomes. Interpreting the information means the researcher decides what to include or leave out. A good case study should always clarify which information is the factual description and which is an inference or the researcher’s opinion.
1. Introduction
- Provide background on the case context and why it is of interest, presenting background information like demographics, relevant history, and presenting problem.
- Compare briefly to similar published cases if applicable. Clearly state the focus/importance of the case.
2. Case Presentation
- Describe the presenting problem in detail, including symptoms, duration,and impact on daily life.
- Include client demographics like age and gender, information about social relationships, and mental health history.
- Describe all physical, emotional, and/or sensory symptoms reported by the client.
- Use patient quotes to describe the initial complaint verbatim. Follow with full-sentence summaries of relevant history details gathered, including key components that led to a working diagnosis.
- Summarize clinical exam results, namely orthopedic/neurological tests, imaging, lab tests, etc. Note actual results rather than subjective conclusions. Provide images if clearly reproducible/anonymized.
- Clearly state the working diagnosis or clinical impression before transitioning to management.
3. Management and Outcome
- Indicate the total duration of care and number of treatments given over what timeframe. Use specific names/descriptions for any therapies/interventions applied.
- Present the results of the intervention,including any quantitative or qualitative data collected.
- For outcomes, utilize visual analog scales for pain, medication usage logs, etc., if possible. Include patient self-reports of improvement/worsening of symptoms. Note the reason for discharge/end of care.
4. Discussion
- Analyze the case, exploring contributing factors, limitations of the study, and connections to existing research.
- Analyze the effectiveness of the intervention,considering factors like participant adherence, limitations of the study, and potential alternative explanations for the results.
- Identify any questions raised in the case analysis and relate insights to established theories and current research if applicable. Avoid definitive claims about physiological explanations.
- Offer clinical implications, and suggest future research directions.
5. Additional Items
- Thank specific assistants for writing support only. No patient acknowledgments.
- References should directly support any key claims or quotes included.
- Use tables/figures/images only if substantially informative. Include permissions and legends/explanatory notes.
- Provides detailed (rich qualitative) information.
- Provides insight for further research.
- Permitting investigation of otherwise impractical (or unethical) situations.
Case studies allow a researcher to investigate a topic in far more detail than might be possible if they were trying to deal with a large number of research participants (nomothetic approach) with the aim of ‘averaging’.
Because of their in-depth, multi-sided approach, case studies often shed light on aspects of human thinking and behavior that would be unethical or impractical to study in other ways.
Research that only looks into the measurable aspects of human behavior is not likely to give us insights into the subjective dimension of experience, which is important to psychoanalytic and humanistic psychologists.
Case studies are often used in exploratory research. They can help us generate new ideas (that might be tested by other methods). They are an important way of illustrating theories and can help show how different aspects of a person’s life are related to each other.
The method is, therefore, important for psychologists who adopt a holistic point of view (i.e., humanistic psychologists ).
Limitations
- Lacking scientific rigor and providing little basis for generalization of results to the wider population.
- Researchers’ own subjective feelings may influence the case study (researcher bias).
- Difficult to replicate.
- Time-consuming and expensive.
- The volume of data, together with the time restrictions in place, impacted the depth of analysis that was possible within the available resources.
Because a case study deals with only one person/event/group, we can never be sure if the case study investigated is representative of the wider body of “similar” instances. This means the conclusions drawn from a particular case may not be transferable to other settings.
Because case studies are based on the analysis of qualitative (i.e., descriptive) data , a lot depends on the psychologist’s interpretation of the information she has acquired.
This means that there is a lot of scope for Anna O , and it could be that the subjective opinions of the psychologist intrude in the assessment of what the data means.
For example, Freud has been criticized for producing case studies in which the information was sometimes distorted to fit particular behavioral theories (e.g., Little Hans ).
This is also true of Money’s interpretation of the Bruce/Brenda case study (Diamond, 1997) when he ignored evidence that went against his theory.
Breuer, J., & Freud, S. (1895). Studies on hysteria . Standard Edition 2: London.
Curtiss, S. (1981). Genie: The case of a modern wild child .
Diamond, M., & Sigmundson, K. (1997). Sex Reassignment at Birth: Long-term Review and Clinical Implications. Archives of Pediatrics & Adolescent Medicine , 151(3), 298-304
Freud, S. (1909a). Analysis of a phobia of a five year old boy. In The Pelican Freud Library (1977), Vol 8, Case Histories 1, pages 169-306
Freud, S. (1909b). Bemerkungen über einen Fall von Zwangsneurose (Der “Rattenmann”). Jb. psychoanal. psychopathol. Forsch ., I, p. 357-421; GW, VII, p. 379-463; Notes upon a case of obsessional neurosis, SE , 10: 151-318.
Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Money, J., & Ehrhardt, A. A. (1972). Man & Woman, Boy & Girl : The Differentiation and Dimorphism of Gender Identity from Conception to Maturity. Baltimore, Maryland: Johns Hopkins University Press.
Money, J., & Tucker, P. (1975). Sexual signatures: On being a man or a woman.
Further Information
- Case Study Approach
- Case Study Method
- Enhancing the Quality of Case Studies in Health Services Research
- “We do things together” A case study of “couplehood” in dementia
- Using mixed methods for evaluating an integrative approach to cancer care: a case study
Related Articles
Research Methodology
Qualitative Data Coding

What Is a Focus Group?
Cross-Cultural Research Methodology In Psychology

What Is Internal Validity In Research?

Research Methodology , Statistics
What Is Face Validity In Research? Importance & How To Measure

Criterion Validity: Definition & Examples
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
APA Sample Paper

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Note: This page reflects the latest version of the APA Publication Manual (i.e., APA 7), which released in October 2019. The equivalent resource for the older APA 6 style can be found here .
Media Files: APA Sample Student Paper , APA Sample Professional Paper
This resource is enhanced by Acrobat PDF files. Download the free Acrobat Reader
Note: The APA Publication Manual, 7 th Edition specifies different formatting conventions for student and professional papers (i.e., papers written for credit in a course and papers intended for scholarly publication). These differences mostly extend to the title page and running head. Crucially, citation practices do not differ between the two styles of paper.
However, for your convenience, we have provided two versions of our APA 7 sample paper below: one in student style and one in professional style.
Note: For accessibility purposes, we have used "Track Changes" to make comments along the margins of these samples. Those authored by [AF] denote explanations of formatting and [AWC] denote directions for writing and citing in APA 7.
APA 7 Student Paper:
Apa 7 professional paper:.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian Dermatol Online J
- v.12(5); Sep-Oct 2021
How to Write a Case Report?
Department of Dermatology, KPC Medical College and Hospital, Kolkata, West Bengal, India
Inderpal Singh
1 Department of Dermatology, Maharishi Markandeshwar Medical College, Sultanpur Road, Himachal Pradesh, India
Case report is regarded as one of the first line of evidence in medical science. There have been numerous circumstances, where the initial dissemination (and breakthrough) of scientific knowledge had been done, with the help of case reports. Case report is a particular variety of manuscript that showcases the unusual features and management of a patient. The words of William Osler (Father of modern medicine) “Always note and record the unusual…publish it. Place it on permanent record as a short, concise note. Such communications are always of value,” still hold relevance in today's era. In this article, we shall discuss the keys to draft a case report worthy of publication.
Case report is a distinct way of conveying a piece of information.[ 1 ] In addition, writing a case report provides a good opportunity to young physicians to get acquainted with the nitty gritties of writing a scientific article.[ 2 ] Case reports have more chances of acceptance if they provide a message which has the ability to alter clinical practice. They are also accepted if they add to the existing literature and/or raise a novel research question, which has the potential of generating a large-scale research.[ 3 , 4 ]
A case report is expected to be authentic and thought provoking. The use of propranolol for the management of infantile hemangiomas is an example; which came into limelight with the publication of case reports; and thereafter, large-scale experimental studies were designed.[ 5 , 6 , 7 ] Therefore, case reports aid in designing tenable protocol for performing good quality scientific research.[ 8 ] The CARE (CAse REport) guidelines for writing a case report are universally followed.[ 9 ]
Brevity and clarity, are the two pre-requisites for any case report.[ 9 ] The various components of a case report and the preferable formatting of the same have been summarized below:
- Title : Authors should attempt to provide a short and crisp title, and ensure that the title attracts the reviewers, editors, and the readers, to go through the entire article. The title should be well-thought of and comprehensive. It should always give a fair idea of what lies within the article, and should not be vague and non-specific. The new message of the article should preferably be reflected in the title. Most importantly, the title should be scientifically sound. Abbreviations in the first place should be avoided, and authors should avoid the use of “superfluous words”.
- Abstract : The abstract must clearly state the new information and the key takeaway messages.[ 10 ] There should be adequate patient data in terms of relevant history, clinical findings, tests, and interventions. Both physician and patient assessed outcomes should be mentioned.[ 11 ] Abstract is one of the most crucial components of an article, because, the reviewers and the editors would be having a fair perception of the entire manuscript after reading the abstract, and many times, the articles get rejected due to a poorly written abstract (lack of flow and message). Abstract should be revised every time the manuscript is revised or changed.
- Keywords : Choosing a proper keyword is very important, while writing a case report. Keywords aid the indexers and search engines find relevant papers. Keywords should represent the content of the manuscript, and should be specific to the entity in question
- Introduction : Introduction of a case report must initially address the magnitude and importance of the disease in question. Authors should highlight as to what is unknown about the entity and why they are reporting the case.
- Case report : This section should describe all the necessary details of the patient including de-identified patient-specific information, concerns, and symptoms, relevant history (medical, surgical, and family), significant clinical examination findings (both positive and negative), diagnostic tests, differentials, provisional diagnosis, and prognosis. Authors should always mention how they ruled out the clinical and histpathological differentials (preferably in a tabular format) and how they arrived at the final diagnosis. If applicable, authors should also describe the details of therapeutic intervention (pharmacological name of drug, dose, frequency and duration). The response to treatment (objective and subjective parameters) must be mentioned clearly, without any ambiguity. When the authors are reporting a drug reaction, it is advisable to mention the details of Naranjo's adverse drug reaction probability scale, Hartwig's severity assessment scale and modified Schumock and Thornton scale. Besides, it is prudent to register the drug reaction under Pharmacovigilance Program of India (PvPI) and mention the necessary details about the same, in the case report.
- Discussion : The discussion should include the relevant medical literature and the rationale for the conclusion. It is always advisable to add the perspective of the patients, whenever possible.[ 12 ] A thorough literature search must be done and the relevant references must be cited. Some widely used reference management and formatting software applications are BibTeX, Papers, Zotero, EndNote, RefWords, ReadCube, and Mendeley. Authors should always avoid the claims the first publication, because this is not possible to be verified by the reviewers and editors, and such claims are not desirable while publishing any article. Authors should summarize the key findings and unique points about their case, highlighting the need for publishing the case report (as to how this will add to the literature). The primary take-away messages of the case report should be given at the end of the article, in the form of a one-line conclusion.
- Images and figures : The requirements are always mentioned in the website of the journal. Some of the vital considerations include a non-identifiable patient, high-quality JPEG or TIFF-centered clear and sharply focused images, and not exceeding 3-4 MB. Authors should never crop the original image, and ensure that the background is clean. If the figure has been published before, authors should acknowledge the original source and submit written permission from the copyright holder to reproduce the material. A credit line should appear in the legend for such figures. Low-resolution images, grainy images due to high ISO and pixelated images should never be submitted. The photographs must be clicked in a manner that makes it clear to the readers, as to which region is involved. If the authors are describing a case whose uniqueness lies in the unilateral distribution of lesions on the lower limb, the photograph must be clicked in a fashion which displays both the lower limbs. When it comes to therapeutic interventions, the pre-treatment and post-treatment photographs should have the same resolution, exposure, brightness and background. The figure legends must precisely mention about the stain and magnification for dermatopathology images; and the type of dermoscope used (and the magnification).[ 13 ]
Apart from the aforementioned things, some of the important issues which need to be taken care of, include informed consent by the patient, potential conflicts of interest, de-identification of patient-related data, and ethics committee approval, if obtained or necessary.[ 14 , 15 ] We propose the format as shown in Apppendix 1 for case report. This worksheet can streamline the process of writing, by providing a structure. Once completed, it can be quickly formatted into a manuscript for submission. We believe that this worksheet will serve as a handy tool for penning down case reports. The greatest barrier is paucity of time, and a certain anxiety about the process of publication. Following a structured standard approach to organizing and presenting clinical observations, may remove these barriers for busy physicians who are genuinely interested in writing case reports.
Most authors, reviewers and editors are of the opinion, that authors should strictly follow the instructions pertaining to preparation of manuscript so as to increase the probability of acceptance of manuscript. Authors may either follow the instructions which are available on the specific website of the journal, or they can also follow the guidelines which are uniformly followed by all biomedical journals ( http://www.icmje.org/index.html ). Manuscripts are often rejected outright by the journals, when the instructions are not followed properly.[ 10 ]
An accurate, thoroughly worked-up and well-written case report strengthens the medical literature. Therefore, authors must strictly follow the instructions while preparing the draft of the case report – comprehensive, crisp, and apt, and most importantly, the ability to add to clinical practice.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Appendix 1: Case report check sheet
| Authorship: |
| ICMJE (International Committee of Medical Journal Editors) criteria |
| Names should be listed in the sequence of their contribution to the manuscript |
| Patient Privacy: |
| Identifying information should be deleted |
| Informed consent from the patient to publish the case |
| Written approval from the Institutional Review Board/privacy officer has been obtained |
| Title: |
| Crisp description of the case in discussion |
| Abstract: |
| ≤250 words |
| Written in a properly organized design |
| Clearly stated objective |
| Details of management discussed in the case report subsection |
| Discussion summarizes the contribution of the case to the literature |
| Indexing terms from Pub Med are provided |
| Introduction: |
| Clearly stated purpose |
| Significance of the entity (in discussion) is mentioned |
| Definitions of the relevant terminologies are mentioned |
| It is mentioned as to how the case adds to the existing literature |
| Case Report: |
| The case is outlined in a comprehensive style |
| The course of events is presented chronologically |
| Patient characteristics are described accurately |
| Positive and noteworthy negative results are mentioned |
| Newer investigational procedures are described properly, with properly cited references |
| All unusual terms are defined precisely |
| A diagnosis is provided, with a clear presentation of the treatment |
| Discussion: |
| The case is weighed up against the existing knowledge, with a brief discussion of differential diagnoses |
| Scientific basis for the management is provided |
| The authors hypothesize a mechanism behind the findings |
| Limitations are mentioned |
| Authors suggest avenues for future research |
| Conclusion: |
| The conclusion is relatable to the reason behind reporting the case. |
| New information is well-summarized |
| Acknowledgements: |
| Those who have contributed to the case, but they have not been included as authors in the manuscript, should be acknowledged |
| References: |
| The authors provide relevant and suitable references |
| References must be strictly written in accordance with the journal instructions |
| Figures and Clinical Images: |
| Figures conform to the specifications of the journal (mentioned in the instructions to authors) |
| Authors must obtain permission from the publisher, if the figures have been published elsewhere |
TechRepublic
Artificial intelligence.

The 10 Best AI Courses in 2024
Today’s options for best AI courses offer a wide variety of hands-on experience with generative AI, machine learning and AI algorithms.

ChatGPT Cheat Sheet: A Complete Guide for 2024
Get up and running with ChatGPT with this comprehensive cheat sheet. Learn everything from how to sign up for free to enterprise use cases, and start using ChatGPT quickly and effectively.

Microsoft Copilot Cheat Sheet: Price, Versions & Benefits
The Surface Laptop 7 and Surface Pro 11, which ship in June, will be able to run Copilot features on-device.

Llama 3 Cheat Sheet: A Complete Guide for 2024
Learn how to access Meta’s new AI model Llama 3, which sets itself apart by being open to use under a license agreement.

OpenAI’s GPT-4 Can Autonomously Exploit 87% of One-Day Vulnerabilities, Study Finds
Researchers from the University of Illinois Urbana-Champaign found that OpenAI’s GPT-4 is able to exploit 87% of a list of vulnerabilities when provided with their NIST descriptions.
Latest Articles

OpenAI, Anthropic Research Reveals More About How LLMs Affect Security and Bias
Anthropic opened a window into the ‘black box’ where ‘features’ steer a large language model’s output. OpenAI dug into the same concept two weeks later with a deep dive into sparse autoencoders.
AWS Custom Silicon Chips Range a Sign of What’s Coming to APAC Cloud Computing
Rampant demand for AI computing is driving cloud providers like Microsoft, Google and AWS to build custom silicon chips. Soon, APAC customers will have more choice based on use case and cost.

Get an Introduction to AI Services Like ChatGPT for Just $50
This three-course bundle teaches you how to use ChatGPT and Midjourney to full effect to help your business. It's on sale for $100 off now.

Raspberry Pi Embraces AI With Hailo Collaboration
Plus, Raspberry Pi will list as an IPO on the London Stock Exchange, a boon for the market.

Some Generative AI Company Employees Pen Letter Wanting ‘Right to Warn’ About Risks
Both the promise and the risk of "human-level" AI has always been part of OpenAI’s makeup. What should business leaders take away from this letter?

IBM’s Think 2024 News That Should Help Skills & Productivity Issues in Australia
TechRepublic interviewed IBM’s managing director for Australia about how announcements from the recent Think event could impact the tech industry in particular.

Cisco Live 2024: New Unified Observability Experience Packages Cisco & Splunk Insight Tools
The observability suite is the first major overhaul for Splunk products since the Cisco acquisition. Plus, Mistral AI makes a deal with Cisco’s incubator.

Intel Lunar Lake NPU Brings 48 TOPS of AI Acceleration
Competition for AI speed heats up. Plus, the first of the two new Xeon 6 processors is now available, and Gaudi 3 deals have been cinched with manufacturers.

Cisco Live 2024: Cisco Unveils AI Deployment Solution With NVIDIA
A $1 billion commitment will send Cisco money to Cohere, Mistral AI and Scale AI.

AMD Reveals Ryzen 9000 CPUs and AI PC Architecture at Computex 2024
The next generation of Ryzen processors for desktops is built on AMD’s Zen 5 microarchitecture.

The 5 Best Udemy Courses That Are Worth Taking in 2024
Udemy is an online platform for learning at your own pace. Boost your career with our picks for the best Udemy courses for learning tech skills online in 2024.

TechRepublic Premium Editorial Calendar: Policies, Checklists, Hiring Kits and Glossaries for Download
TechRepublic Premium content helps you solve your toughest IT issues and jump-start your career or next project.

What is the EU’s AI Office? New Body Formed to Oversee the Rollout of General Purpose Models and AI Act
The AI Office will be responsible for enforcing the rules of the AI Act, ensuring its implementation across Member States, funding AI and robotics innovation and more.

Google, Microsoft, Meta and More to Develop Open Standard for AI Chip Components in UALink Promoter Group
Notably absent from the group is NVIDIA, which has its own equivalent technology that it may not wish to share with its closest rivals.

Gartner Predicts Worldwide AI Chip Revenue Will Gain 33% in 2024
AI chip revenue for 2024 is expected to hit $71.3 billion. Plus, AI device makers are increasingly bringing chips in-house.
Create a TechRepublic Account
Get the web's best business technology news, tutorials, reviews, trends, and analysis—in your inbox. Let's start with the basics.
* - indicates required fields
Sign in to TechRepublic
Lost your password? Request a new password
Reset Password
Please enter your email adress. You will receive an email message with instructions on how to reset your password.
Check your email for a password reset link. If you didn't receive an email don't forgot to check your spam folder, otherwise contact support .
Welcome. Tell us a little bit about you.
This will help us provide you with customized content.
Want to receive more TechRepublic news?
You're all set.
Thanks for signing up! Keep an eye out for a confirmation email from our team. To ensure any newsletters you subscribed to hit your inbox, make sure to add [email protected] to your contacts list.

IMAGES
VIDEO
COMMENTS
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Follow the same steps as for a Case Report. Template for writing a Case Series Title: Choose an informative title and, if need be, sub-title; always include "Case Series" so that the. reader knows immediately what study type you have chosen. Authors: Check the Instructions to Authors of the journal you are submitting to for formatting ...
Case report can be classified as a single case report, two case reports or case series, which aggregate more than two cases in a report. Case reports are usually retrospective by nature, however, it can be prospectively designed, for example, applying a new diagnostic or management approach or guideline of a particular health condition to ...
Below is the general format adopted for most case reports. Summarise your case report in a sentence. Mention how rare this condition is, and why your case report is important eg as a differential to consider in a patient presenting with X, Y and Z. Narrate your case in a way that is easy and enjoyable to follow.
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care. For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your ...
This checklist was developed by CARE to correspond with key components of a case report and capture useful clinical information (including 'meaningful use' information mandated by some insurance plans).. The narrative: A case report tells a story in a narrative format that includes the presenting concerns, clinical findings, diagnoses, interventions, outcomes (including adverse events), and ...
writing a clinical case report either for ... For example - Patient X is a 10-year-old girl who presented to the emergency department of the School of Dentistry. Short, simple, and to the point. ...
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow the diagnostic pathway. For example - Patient X is a 10 ...
According to the University of Texas Health Science Center, most journals publish case reports that deal with one or more of the following: 1. Unusual observations. 2. Adverse response to therapies. 3. Unusual combination of conditions leading to confusion. 4. Illustration of a new theory.
Abstract. Purpose. Guidelines for writing patient case reports, with a focus on medication-related reports, are provided. Summary. The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a ...
Do this after writing the case report. Information should include: (1) Background, (2) Key points from the case; and (3) Main lessons to be learned from this case report. Keywords. Provide 2 to 5 keywords that will identify important topics covered by this case report. References.
can introduce very effective treatment paradigms. Preparing the manuscript for a case report may be the first exposure to scientific writing for a budding clinician/researcher. This manuscript describes the steps of writing a case report and essential considerations when publishing these articles. Individual components of a case report and the "dos and don'ts" while preparing these ...
Select the Journal of Submission and Look at the Literature. Before you submit your manuscript, carefully peruse the potential journals of submission and read the author instructions for those journals. It is important to ensure that the journal of submission is the right one for your work. Carefully considering journal selection will allow you ...
The abstract of a patient case report should succinctly include the four sections of the main text of the report. The introduction section should provide the subject, purpose, and merit of the case report. It must explain why the case report is novel or merits review, and it should include a comprehensive literature review that corroborates the ...
the case report 4he discussion shouldevaluate thepatient case for accuracyvalidity and uniqueness compareand thecase re portwith the published literature andderive new knowledge andap plicabilitytopractice 4heauthor datapresented andby establishing atemporal andcausal relationship &ordrug
Submit Your Case: The Sky Is the Limit. As a last message, to all of the young authors who are trying to get their clinical cases published, remember that the "sky is the limit"—be creative, find a good mentor, and write up an interesting case. Once your manuscript is accepted, it is just the beginning of your academic career.
Case Report abstract s that do not provide meaningful teaching points will not be accepted. Title . The abstract title should emphasize the clinical condition and main teaching point. Format . Case Report abstracts must be submitted in the following structured format: • Introduction or Background • Clinical Case (including diagnostic ...
10a-P_Cohen.indd. case report should be descriptive, ac-curate, and succinct. Abstract. Case reports should in-clude an abstract of 100-250 words. The availability of an abstract will allow for easier retrieval from elec-tronic databases and help research-ers discern their levels of interest in the case report.
Presentations, diagnoses and/or management of new and emerging diseases. An unexpected association between diseases or symptoms. An unexpected event in the course of observing or treating a patient. Findings that shed new light on the possible pathogenesis of a disease or an adverse effect. Case reports submitted to Journal of Medical Case ...
All submissions to BMJ Case Reports require the completion of author statements. Case reports should have a maximum of four authors. One of the co-authors should be the lead clinician responsible for the patient's care and responsible for the integrity of the content of the case report, signed informed consent and author statements accompanying each manuscript.
Introduction. For many doctors and other healthcare professionals, writing a case report represents the first effort at getting articles published in medical journals and it is considered a useful exercise in learning how to write scientifically due to similarity of the basic methodology.1 Case reports aim to convey a clinical message.2,3 Despite different types of case reports, they all aim ...
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews). The case study research method originated in clinical medicine (the case history, i.e., the patient's personal history). In psychology, case studies are ...
Crucially, citation practices do not differ between the two styles of paper. However, for your convenience, we have provided two versions of our APA 7 sample paper below: one in student style and one in professional style. Note: For accessibility purposes, we have used "Track Changes" to make comments along the margins of these samples.
EPTV NA ESCOLA 2024 | Tema deste ano é: "Inteligência artificial, aliada ou inimiga do nosso futuro?". #eptv #eptvsuldeminas #eptv1 #eptvnaescola
The various components of a case report and the preferable formatting of the same have been summarized below: Title: Authors should attempt to provide a short and crisp title, and ensure that the title attracts the reviewers, editors, and the readers, to go through the entire article. The title should be well-thought of and comprehensive.
Gartner Predicts Worldwide AI Chip Revenue Will Gain 33% in 2024. AI chip revenue for 2024 is expected to hit $71.3 billion. Plus, AI device makers are increasingly bringing chips in-house. By ...