Search the world's largest collection of clinical case reports
Browse case reports by:

Publish in BMJ Case Reports
Global health case reports.
These are case reports that focus on the causes of ill health, the social determinants of health and access to healthcare services, prevailing local and national issues that affect health and wellbeing, and the challenges in providing care to vulnerable populations or with limited resources.
Read the full collection now
Images in… :
31 July 2023
Unusual association of diseases/symptoms :
24 January 2024
14 July 2023
Obstetrics and gynaecology :
5 March 2024
Case report :
18 October 2023
Case Reports: Rare disease :
Case Reports: Unusual presentation of more common disease/injury :
17 April 2024
Case Reports by specialty
- Anaesthesia
- Dentistry and oral medicine
- Dermatology
- Emergency medicine
- Endocrinology
- General practice and family medicine
- Geriatric medicine
- Haematology
- Infectious diseases
- Obstetrics and gynaecology
- Ophthalmology
- Orthopaedics
- Paediatrics
- Respiratory medicine
- Rheumatology

Global Health Competition
Every year BMJ Case Reports selects authors of global health case reports to join our editorial team as a global health associate editor.
This is an opportunity to gain some editorial experience or join our team on research and educational projects. Students and graduates may apply.
Simply select Global Health Competition when you submit.
Latest Articles
Case Reports: Unusual association of diseases/symptoms :
Case Reports: Novel treatment (new drug/interventions; established drug/procedure in new situation) :
22 May 2024
27 May 2024
Journal of Medical Case Reports
In the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge. Prof Michael Kidd AO, Editor-in- Chief

Join the Editorial Board
We are recruiting Associate Editors to join our Editorial Board. Learn more about the role and how to apply here !

- Meet the Editors
Get to know the Editors behind Journal of Medical Case Reports !

megaflopp / Getty Images / iStock
Requirements for case reports submitted to JMCR
• Patient ethnicity must be included in the Abstract under the Case Presentation section.
• Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Report of the Month
Superior mesenteric vein thrombosis due to covid-19 vaccination.
Vaccines have made a significant contribute to sowing the spread of the COVID-19 infection. However, side effects of the vaccination are beginning to appear, and one of which, thrombosis, is a particular problem as it can cuase serious complications. While cases of splanchnic venous thrombosis (SVT) after ChAdOx1 nCoV-19 vaccinations have been reported, cases of SVT mRNA-1273 vaccines are rare.
In this case report, clinicians describe a patient presenting with superior mesentric vein thrombosis following a COVID-19 vaccination, and examine the relationship between the mRNA-1273 vaccines and intestinal ischemia.
- Most accessed
Sinonasal immunoglobulin G4-related disease: a case report of an atypical and rare entity
Authors: Faiq I. Gorial, Nabaa Ihsan Awadh, Shahlaa B. Ali, Sazan Abdulwahab Mirza and Murtadha Hussein Abbas
Case analysis of hepatotoxicity caused by vancomycin
Authors: Jiayao Wu and Yulu Zhou
Endovascular stenting using a sagittal sinus approach for sigmoid sinus wall dehiscence related to intractable pulsatile tinnitus: a case series
Authors: Luis Alberto Ortega-Porcayo, Guillermo Gonzalez-Garibay, Ángel Lee, Juan A. Ponce-Gómez, Victor Alcocer-Barradas, Samuel Romano-Feinholz and Marco Antonio Zenteno Castellanos
Surgical intervention of Lemierre’s syndrome: a case report and review of the literature
Authors: Yiqi Pan, Zhihong Shi, Bin Ye, Qian Da, Chaofu Wang, Yilin Shen and Mingliang Xiang
Severe hypotension and postoperative cardiac arrest caused by 5-aminolevulinic acid: a case report
Authors: Taishi Miyazaki, Shinya Taguchi, Norihiko Obata and Satoshi Mizobuchi
Most recent articles RSS
View all articles
An itchy erythematous papular skin rash as a possible early sign of COVID-19: a case report
Authors: Alice Serafini, Peter Konstantin Kurotschka, Mariabeatrice Bertolani and Silvia Riccomi
Red ear syndrome precipitated by a dietary trigger: a case report
Authors: Chung Chi Chan and Susmita Ghosh
How to choose the best journal for your case report
Authors: Richard A. Rison, Jennifer Kelly Shepphird and Michael R. Kidd
The Erratum to this article has been published in Journal of Medical Case Reports 2017 11 :287
COVID-19 with repeated positive test results for SARS-CoV-2 by PCR and then negative test results twice during intensive care: a case report
Authors: Masafumi Kanamoto, Masaru Tobe, Tomonori Takazawa and Shigeru Saito
Recurrent knee arthritis diagnosed as juvenile idiopathic arthritis with a 10-year asymptomatic period after arthroscopic synovectomy: a case report
Authors: Atsushi Teramoto, Kota Watanabe, Yuichiro Kii, Miki Kudo, Hidenori Otsubo, Takuro Wada and Toshihiko Yamashita
Most accessed articles RSS
A Guide to Writing and Using Case Reports
This thematic series, published in 2016, provides a valuable resource for clinicians who are considered producing a case report. It comprises of a special editorial series of guides on writing, reviewing and using case reports.

Aims and scope
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports.
Case reports should show one of the following:
- Unreported or unusual side effects or adverse interactions involving medications
- Unexpected or unusual presentations of a disease
- New associations or variations in disease processes
- Presentations, diagnoses and/or management of new and emerging diseases
- An unexpected association between diseases or symptoms
- An unexpected event in the course of observing or treating a patient
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients.
Article accesses
Throughout 2022, articles were accessed from the journal website more than 4.17 million times; an average of over 11 ,400 accesses per day.
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Peer Review Mentoring Scheme
The Editors at Journal of Medical Case Reports endorse peer review mentoring of early career researchers.
If you are a senior researcher or professor and supervise an early career researcher with the appropriate expertise, we invite you to co-write and mentor them through the peer review process. Find out how to express your interest in the scheme here .
Call for Papers
The Journal of Medical Case Reports is calling for submissions to our Collection on COVID-19 – a look at the past, present and future of the pandemic . Guest Edited by Dr. Jean Karl Soler, The Family Practice Malta, Malta

About the Editor-in-Chief
Professor Michael Kidd AO FAHMS is foundation Director of the Centre for Future Health Systems at the University of New South Wales in Sydney, Australia, and Professor of Global Primary Care and Future Health Systems with the Nuffield Department of Primary Care Health Sciences at the University of Oxford. During the COVID-19 pandemic, Prof Kidd was the Deputy Chief Medical Officer and Principal Medical Advisor with the Australian Government Department of Health and Aged Care, and Professor of Primary Care Reform at the Australian National University. He holds honorary appointments with the University of Toronto, the University of Melbourne, Flinders University, and the Murdoch Children's Research Institute, and is the Emeritus Director of the World Health Organization Collaborating Centre on Family Medicine and Primary Care. He is an elected Fellow of the Australian Academy of Health and Medical Sciences (FAHMS). In the 2023 King's Birthday Honours List he was made an Officer of the Order of Australia. Prof Kidd served as president of the World Organization of Family Doctors (WONCA) from 2013-2016, and as president of the Royal Australian College of General Practitioners from 2002-2006. He is the founder and Editor-in-Chief of the Journal of Medical Case Reports, the world's first PubMed-listed journal devoted to publishing case reports from all medical disciplines.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
Explore 496,958 research studies in all 50 states and in 222 countries..
ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine.
IMPORTANT : Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits .
- Studies by Topic
- Studies on Map
Patients and Families
Researchers, study record managers.
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 5, May 2024 (In Progress)
- Volume 2024, Issue 4, April 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Editor-in-Chief
Richard Watts
Executive Editors
Tamim Alsuliman
Aloysious Aravinthan
Amanda Goodwin
Eleana Ntatsaki
Vassilis Vassiliou
About Oxford Medical Case Reports
Oxford Medical Case Reports (OMCR) is an open access, peer-reviewed online journal publishing original and educationally valuable case reports that expand the field of medicine. The journal deposits all articles in PubMed Central (PMC) and is indexed in the Web of Science Core Collection.

Browse by specialty
Oxford Medical Case Reports publish insightful cases across all medical specialties.
Browse collections including nephrology , palliative care , and geriatric medicine .
Explore more

- Submit your case
Oxford Medical Case Reports publishes original and educationally valuable case reports across all medical specialties.
- Author guidelines

Case Reports From Humanitarian And Resource Limited Settings
Médecins Sans Frontières (MSF) is working with OMCR to encourage clinicians in low-income and/or emergency contexts to submit interesting case reports and series from the field.
Browse the case reports from humanitarian and low resource settings

Enhanced discoverability
Oxford Medical Case Reports deposits all cases in PubMed Central . Physicians and researchers can find your work through PubMed , helping you reach the widest possible audience.
The journal is also indexed in the Web of Science Core Collection .
Latest articles

Email alerts
Register to receive table of contents email alerts as soon as new issues of Oxford Medical Case Reports are published online.
Join us on Facebook and Twitter
Be the first to read the latest news and cases by joining the Oxford Medical Case Reports community on Facebook , or by following us on Twitter .
Publish with OMCR
Editor-in-Chief Dr Richard Watts explains the benefits of publishing with Oxford Medical Case Reports.

Test your knowledge
A 57 year-old man has chest pain, but what is the diagnosis? Answer multiple choice questions to find out.
Take the test
Related Titles
Affiliations
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Department of Health and Human Services
- National Institutes of Health

COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient, about clinical research.
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.


Clinical Cases
Litfl clinical cases database.
The LITFL Clinical Case Collection includes over 250 Q&A style clinical cases to assist ‘ Just-in-Time Learning ‘ and ‘ Life-Long Learning ‘. Cases are categorized by specialty and can be interrogated by keyword from the Clinical Case searchable database.
Search by keywords; disease process; condition; eponym or clinical features…
Compendium of Clinical Cases
LITFL Top 100 Self Assessment Quizzes
Medical Case Reports

- Journal h-index : 7
- Journal cite score : 0.77
- Journal impact factor : 0.50
- Average acceptance to publication time (5-7 days)
- Average article processing time (30-45 days) Less than 5 volumes 30 days 8 - 9 volumes 40 days 10 and more volumes 45 days
Animal research and nutrition
About medical case reports.
The aim of the Journal is to provide a platform for the researchers and academicians throughout the world to speedily publish (at affordable fee), share and discuss rare and new findings in the areas of Medical Sciences.Medical Case Reports is an open access, peer-review journal which follows single-blind review system, it often follows Editorial Managing System for review process of an article.
Medical Case Reports delivers a focused valuable collection of cases in all disciplines so that healthcare professionals, researchers and others can easily find clinically important information on common and rare conditions. The journal mainly focuses on symptoms, signs, diagnosis, treatment, and follow-up of patient disease in different areas.
The journal covers all aspects from medical sciences including Diabetes Case Reports , Cancer Case Reports, HIV Case Reports , Obesity Case Reports, Abortion Case Reports, Mental Case Reports , Depression Case Reports, Cardiology , Clinical Biology, Developmental Biology, Dentistry, Genetics , Medical Biotechnology, Nephrology, Neurology, Obstetrics and Gynecology, Orthopedics, Otorhinolaryngology, Pharmacology, Reproductive Biology, Rheumatology, Surgery, Urology etc.
Submit your manuscript at : https://www.imedpub.com/submissions/medical-case-reports.html
or you can mail us at [email protected]
Diabetes Case Reports
Diabetes is a chronic metabolic disease that occurs when the human body is not able to produce enough of the hormone insulin or because cells do not respond to the insulin that is produced. High blood sugar produces symptoms of frequent urination, increased thirst and hunger. Globally in 2013, it is estimated that almost 382 million people suffer from diabetes for a prevalence of 8.3%. North America and the Caribbean is the region with the higher prevalence of 11% having 37 million people with diabetes followed by the Middle East and North Africa with a prevalence of 9.2% having 35 million people with diabetes. Western Pacific is the region with higher number of people living with diabetes (138 million), however its prevalence is 8.6%, close to the prevalence of the World.
Related Journals of Diabetes Case Repors
Journal of Diabetes Medication & Care , Medical Case Reports , Diabetes Medication & Care , Diabetes & Metabolism , Diabetic Complications and Medicine , Endocrinology & Diabetes Research , Clinical Diabetes , Current Opinion in Endocrinology, Diabetes and Obesity, Current Diabetes Reports, Current Diabetes Reviews Diabetes, Diabetes and Metabolism, Diabetes and Vascular Disease Research, Diabetes Care, Diabetes Educator, Diabetes Research and Clinical Practice, Diabetes Reviews, Diabetes Technology and Therapeutics, Diabetes, Nutrition and Metabolism - Clinical and Experimental Case Reports
Cancer Case Reports
In 2012, an estimated 14.1 million new cases of cancer occurred worldwide more than 4 in ten cancers occurring worldwide are in countries at a low or medium level of Human Development Index (HDI). The four most common cancers occurring worldwide are lung, female breast, bowel and prostate cancer . These four account for around 4 in 10 of all cancers diagnosed worldwide. Lung cancer is the most common cancer in men worldwide. More than 1 in 10 of all cancers diagnosed in men are lung cancers. Worldwide, almost 32.5 million people diagnosed with cancer within the five years previously were alive at the end of 2012 (estimated). An estimated 169.3 million years of healthy live were lost globally because of cancer in 2008.
Related Journals of Cancer Case Repors
Archives in Cancer Research , Colorectal Cancer: Open Access , Head and Neck Cancer Research , Journal of Adenocarcinoma , Journal of Neurooncology: Open Access , Biochimica et Biophysica Acta-Reviews on Cancer , Breast Cancer Research and Treatment , British Journal of Cancer , Ca-A Cancer Journal for Clinicians Cancer , Cancer and Metastasis Reviews , Cancer Causes and Control , Cancer Cell, Cancer Epidemiology Biomarkers and Prevention , Cancer Letters , Cancer Research, Clinical Cancer Research, European Journal of Cancer, International Journal of Cancer, Journal of the National Cancer Institute, Molecular Cancer Research, Molecular Cancer Therapeutics, Nature Reviews Cancer, Seminars in Cancer Biology
HIV Case Reports
HIV is the world’s leading infectious killer. According to WHO, Exit Disclaimer an estimated 39 million people have died since the first cases were reported in 1981 and 1.5 million people died of AIDS -related causes in 2013. HIV, the virus that causes AIDS, is one of the world’s most serious health and development challenges, According to the World Health Organization (WHO), Exit Disclaimer there were approximately 35 million people worldwide living with HIV/AIDS in 2013. Of these, 3.2 million were children (<15 years old). 2.1 million individuals worldwide became newly infected with HIV in 2013. This includes over 240,000 children (<15 years). Most of these children live in sub-Saharan Africa and were infected by their HIV-positive mothers during pregnancy, childbirth or breastfeeding. A UNAIDS report Exit Disclaimer shows that 19 million of the 35 million people living with HIV today do not know that they have the virus. The vast majority of people living with HIV are in low- and middle-income countries. According to WHO, Exit Disclaimer sub-Saharan Africa is the most affected region, with 24.7 million people living with HIV in 2013. Seventy-one percent of all people who are living with HIV in the world live in this region.
Related Journals of HIV Case Reports
Journal of HIV & Retro Virus , Advances in Influenza Research , Journal of Infectious Diseases and Treatment , HIV Medicine , Current HIV Research, HIV Clinical Trials , Current Opinion in HIV and AIDS , Current HIV/AIDS Reports , Journal of HIV Therapy , Journal of HIV/AIDS Prevention and Education for Adolescents and Children, Southern African Journal of HIV Medicine, Journal of HIV/AIDS and Social Services, HIV/AIDS policy law review / Canadian HIV/AIDS Legal Network, HIV Therapy, Journal of HIV/AIDS Prevention in Children and Youth, Future HIV Therapy, HIV/AIDS - Research and Palliative Care, Pediatric AIDS and HIV Infection, Canadian HIV/AIDS policy & law review / Canadian HIV/AIDS Legal Network, Archives of STD/hiv Research, HIV and AIDS Review, HIV clinician / Delta Region AIDS Education & Training Cente, Hopkins HIV report : a bimonthly newsletter for healthcare providers / Johns Hopkins University AIDS Service, Neurobehavioral HIV Medicine
Obesity Case Reports
In 2014, more than 1.9 billion adults, 18 years and older, were overweight. Of these over 600 million were obese. Overall, about 13% of the world’s adult population (11% of men and 15% of women) were obese in 2014, 39% of adults aged 18 years and over (38% of men and 40% of women) were overweight. The worldwide prevalence of obesity more than doubled between 1980 and 2014. In 2013, 42 million children under the age of 5 were overweight or obese. Once considered a high-income country problem, overweight and obesity are now on the rise in low- and middle-income countries, particularly in urban settings. In developing countries with emerging economies (classified by the World Bank as lower- and middle-income countries) the rate of increase of childhood overweight and obesity has been more than 30% higher than that of developed countries.
Related Journals of Obesity Case Reports
Medical case reports , Journal of Childhood Obesity , Journal of Obesity & Eating Disorders , Childhood Obesity , Current Opinion in Endocrinology , Diabetes and Obesity , Diabetes , Metabolic Syndrome and Obesity : Targets and Therapy Diabetes , Obesity and Metabolism Diabetes , Obesity and Metabolism , Supplement , International Journal of Obesity, Journal of Obesity, Obesity, Obesity and Metabolism (Italy), Obesity Facts, Obesity Research and Clinical Practice, Obesity Reviews, Obesity Surgery, Open Obesity Journal, Pediatric obesity,Surgery for Obesity and Related Diseases
Depression Case Reports
Depression is a common illness worldwide, with an estimated 350 million people affected. Depression is different from usual mood fluctuations and short-lived emotional responses to challenges in everyday life. Especially when long-lasting and with moderate or severe intensity, depression may become a serious health condition. It can cause the affected person to suffer greatly and function poorly at work, at school and in the family. At its worst, depression can lead to suicide. Suicide results in an estimated 1 million deaths every year.Even in some high-income countries, people who are depressed are not always correctly diagnosed, and others who do not have the disorder are occasionally misdiagnosed and prescribed antidepressants.The burden of depression and other mental health conditions is on the rise globally.
Related Journals of Depression Case Reports
Medical case reports , Acta Psychopathologica , Journal of Neuropsychiatry , Journal of Dementia & Mental health , Mental Health in Family Medicine , Clinical Depression , Depression and Anxiety , Depression Research and Treatment, Depression
Cardiology Case Reports
Heart disease s is responsible for the most deaths worldwide for both men and women of all races. Coronary artery disease, a blockage of the arteries that supply blood to the heart, is the most common type of heart disease. About 600,000 people in the United States die from heart disease every year—that’s one in four deaths. Every year, 715,000 Americans have a heart attack. Fifteen percent of people who have a heart attack will die from it. Heart disease affects whites and African Americans the most, accounting for 24.3 and 24.1 percent of deaths, respectively. Asians and Pacific Islanders are at third-highest risk for a heart disease-related death, at 22.5 percent. It accounts for 20.8 percent of deaths in the Hispanic community, and 17.9 percent in American Indians and Alaska Natives.
Related Journals of Heart Case Reports
Insights in Pediatric Cardiology , Invasive Cardiology: Future Medicine , Interventional Cardiology Journal , Medical case reports , American Journal of Physiology-Heart and Circulatory Physiology , American Heart Journal , Circulation : Heart Failure, Congestive Heart Failure , European Journal of Heart Failure , European Heart Journal , European Heart Journal , Cardiovascular Imaging , European Heart Journal, Supplement Heart, Heart and Lung: Journal of Acute and Critical Care, Heart and Vessels, Heart Disease, Heart Failure Reviews, Heart Rhythm, Heart Surgery Forum, Indian Heart Journal, International Heart Journal, Journal of Heart and Lung Transplantation, Journal of Heart Valve Disease, Texas Heart Institute Journal
Autism Case Reports
About 1 in 68 children has been identified with autism spectrum disorder (ASD) according to estimates from CDC's Autism and Developmental Disabilities Monitoring (ADDM) Network. ASD is reported to occur in all racial, ethnic, and socioeconomic groups. ASD is almost 5 times more common among boys (1 in 42) than among girls (1 in 189). Studies in Asia, Europe, and North America have identified individuals with ASD with an average prevalence of about 1%. A study in South Korea reported a prevalence of 2.6%. About 1 in 6 children in the United States had a developmental disability in 2006-2008, ranging from mild disabilities such as speech and language impairments to serious developmental disabilities, such as intellectual disabilities, cerebral palsy, and autism
Related Journals of Autism Case Reports
Journal of Neurology and Neuroscience , Interventional Neurology , Medical case reports , Autism-Open Access , Journal of Autism and Developmental Disorders , Autism , Autism Research , Research in Autism Spectrum Disorders , Focus on Autism and Other Developmental Disabilitie, Molecular Autism, Education and Training in Autism and Developmental Disab ilities
Drug Addiction Case Report
According to World Drugs report for 2012, 230 million people around the world - 1 in 20 in US - took illicit drugs in the last year. The report also says that problem drug users, mainly heroin - and cocaine -dependent people number about 27 million, roughly 0.6% of the world adult population. That′s 1 in every 200 people. The harmful use of alcohol results in 3.3 million deaths each year. On average every person in the world aged 15 years or older drinks 6.2 litres of pure alcohol per year. Less than half the population (38.3%) actually drinks alcohol, this means that those who do drink consume on average 17 litres of pure alcohol annually. At least 15.3 million persons have drug use disorders. Injecting drug use reported in 148 countries, of which 120 report HIV infection among this population.
Related Journals of Drug Addiction Case Report
Medical case reports , Journal of Drug Abuse , Acta Psychopathologica , Journal of Neuropsychiatry , Addiction , Addiction Biology , Addiction Research and Theory , Addiction science & clinical practice , American Journal on AddictionsCJAM Canadian Journal of Addiction Medicine , European Addiction Research , Heroin Addiction and Related Clinical Problems , International Journal of Mental Health and Addiction, Journal of Groups in Addiction and Recovery, Journal of Maintenance in the Addictions, Journal of Social Work Practice in the Addictions, Journal of Teaching in the Addictions, Journal of Addiction Medicine, Journal of Addictions and Offender Counseling, Journal of Addictions Nursing, Sexual Addiction and Compulsivity
Spinal Cord Injury Case Reports
The term spinal cord injury refers to damage to the spinal cord resulting from trauma (e.g. a car crash) or from disease or degeneration (e.g. cancer). There is no reliable estimate of global prevalence, but estimated annual global incidence is 40 to 80 cases per million population. Up to 90% of these cases are due to traumatic causes, though the proportion of non-traumatic spinal cord injury appears to be growing. Every year, around the world, between 250 000 and 500 000 people suffer a spinal cord injury (SCI). The majority of spinal cord injuries are due to preventable causes such as road traffic crashes, falls or violence. People with a spinal cord injury are two to five times more likely to die prematurely than people without a spinal cord injury, with worse survival rates in low- and middle-income countries. Spinal cord injury is associated with lower rates of school enrollment and economic participation, and it carries substantial individual and societal costs.
Related Journals of Spinal Cord Injury Case Reports
Medical case reports , Spine Research , Journal of Neurology and Neuroscience , Insights in Clinical Neurology , Spinal Cord , Journal of Spinal Disorders and Techniques , Journal of Spinal Cord Medicine , Topics in Spinal Cord Injury Rehabilitation , SCI nursing : a publication of the American Association of Spinal Cord Injury Nurses
Malnutrition Case Report
The United Nations Food and Agriculture Organization estimates that about 805 million people of the 7.3 billion people in the world, or one in nine, were suffering from chronic undernourishment in 2012-2014. Nearly half of all deaths in children under 5 are attributable to undernutrition. This translates into the unnecessary loss of about 3 million young lives a year. Malnutrition puts children at greater risk of dying from common infections, increases the frequency and severity of such infections, and contributes to delayed recovery. In addition, the interaction between undernutrition and infection can create a potentially lethal cycle of worsening illness and deteriorating nutritional status. Poor nutrition in the first 1,000 days of a child’s life can also lead to stunted growth, which is irreversible and associated with impaired cognitive ability and reduced school and work performance.
Related Journals of Malnutrition Case Report
Medical case reports , Journal of Animal Nutrition , Journal of Clinical Nutrition & Dietetics , Pediatric Infectious Diseases: Open Access , Clinical Pediatrics & Dermatology , Journal of Pediatric Care , American Journal of Clinical Nutrition , Annual Review of Nutrition , British Journal of Nutrition Clinical Nutrition , Critical Reviews in Food Science and Nutrition , Current Opinion in Clinical Nutrition and Metabolic Care , European Journal of Clinical Nutrition , European Journal of Nutrition , Journal of Parenteral and Enteral Nutrition, Journal of Pediatric Gastroenterology and Nutrition, Journal of the Academy of Nutrition and Dietetics, Journal of the American College of Nutrition, Journal of Nutrition, Journal of Nutritional Biochemistry, Molecular Nutrition and Food Research, Nutrition, Nutrition and Cancer, Nutrition Reviews, Proceedings of the Nutrition Society, Public Health Nutrition
Osteoporosis Case Report
Worldwide, osteoporosis causes more than 8.9 million fractures annually, resulting in an osteoporotic fracture every 3 seconds.Osteoporosis is estimated to affect 200 million women worldwide - approximately one-tenth of women aged 60, one-fifth of women aged 70, two-fifths of women aged 80 and two-thirds of women aged 90. Osteoporosis affects an estimated 75 million people in Europe, USA and Japan, for the year 2000, there were an estimated 9 million new osteoporotic fractures , of which 1.6 million were at the hip, 1.7 million were at the forearm and 1.4 million were clinical vertebral fractures. Europe and the Americas accounted for 51% of all these fractures, while most of the remainder occurred in the Western Pacific region and Southeast Asia. Worldwide, 1 in 3 women over age 50 will experience osteoporotic fractures, as will 1 in 5 men aged over 50. 80%, 75%, 70% and 58% of forearm, humerus, hip and spine fractures, respectively, occur in women. Overall, 61% of osteoporotic fractures occur in women, with a female-to-male ratio of 1.6. Nearly 75% of hip, spine and distal forearm fractures occur among patients 65 years old or over. A 10% loss of bone mass in the vertebrae can double the risk of vertebral fractures, and similarly, a 10% loss of bone mass in the hip can result in a 2.5 times greater risk of hip fracture
Related Journals of Osteoporosis Case Report
Medical Case Reports , Translational Biomedicine , Journal of Infectious Diseases and Treatment , Journal of Childhood & Developmental Disorders , Osteoporosis International , Current Osteoporosis Reports , Archives of Osteoporosis , Journal of Osteoporosis , International Journal of Osteoporosis and Metabolic Disorders
Medical Reporting Database
Each year, the FDA receives several hundred thousand medical device reports of suspected device-associated deaths, serious injuries and malfunctions. Medical Device Reporting (MDR) is one of the postmarket surveillance tools the FDA uses to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments of these products. Mandatory reporters (i.e., manufacturers, device user facilities, and importers) are required to submit certain types of reports for adverse events and product problems to the FDA about medical devices. In addition, the FDA also encourages health care professionals, patients, caregivers and consumers to submit voluntary reports about serious adverse events that may be associated with a medical device, as well as use errors, product quality issues, and therapeutic failures. These reports, along with data from other sources, can provide critical information that helps improve patient safety.
Related Journals of Medical Reporting Database
Medical Case Reports , Journal of Informatics and Data Mining , Translational Biomedicine , Invasive Cardiology: Future Medicine , Medical Mycology: Open Access , Distributed and Parallel Databases , Database : the journal of biological databases and curation, Foundations and Trends in Databases
Medical Transcription Reports
Medical transcription is part of the healthcare industry that renders and edits doctor dictated reports, procedures, and notes in an electronic format in order to create files representing the treatment history of patients. Health practitioners dictate what they have done after performing procedures on patients and MT’s transcribe the oral dictation and/or edit reports that have gone through speech recognition software. Pertinent up-to-date, confidential patient information is converted to a written text document by a medical transcriptionist (MT) which are called as the medical transcription reports. This text may be printed and placed in the patient's record and/or retained only in its electronic format. Medical transcription can be performed by MTs who are employees in a hospital or who work at home as telecommuting employees for the hospital; by MTs working as telecommuting employees or independent contractors for an outsourced service that performs the work offsite under contract to a hospital, clinic, physician group or other healthcare provider; or by MTs working directly for the providers of service (doctors or their group practices) either onsite or telecommuting as employees or contractors
Related Journals of Medical Transcription Reports
Medical Case Reports , Journal of Informatics and Data Mining , Translational Biomedicine , Invasive Cardiology: Future Medicine , Medical Mycology: Open Access , Journal of the American Association for Medical Transcription , Journal (American Association for Medical Transcription), Transcription
Veterinary Case Report
Veterinary Case Reports journals aims to publish cases in all disciplines so that veterinary professionals, researchers and others can easily find important information on both common and rare conditions pertaining to veternary. All articles are peer reviewed before publication.
Related Journals of Veterinary Case Report
Arabidopsis C. Elegans and Zebrafish , FisheriesSciences , Journal of Animal Nutrition , Medical case reports , American Journal of Veterinary Research , Canadian Veterinary Journal , Equine Veterinary Journal , Journal of the American Veterinary Medical Association , Journal of Veterinary Diagnostic Investigation , Journal of Veterinary Internal Medicine , Journal of Veterinary Pharmacology and Therapeutics , Medical and Veterinary Entomology , Preventive Veterinary Medicine, Research in Veterinary Science, Veterinary Clinics of North America - Food Animal Practice, Veterinary Clinics of North America - Small Animal Practice, Veterinary Immunology and Immunopathology, Veterinary Journal, Veterinary Microbiology, Veterinary Parasitology, Veterinary Pathology, Veterinary Record, Veterinary Research, Veterinary Surgery
Articles published in Medical Case Reports have been cited by esteemed scholars and scientists all around the world. Medical Case Reports has got h-index 7, which means every article in Medical Case Reports has got 7 average citations.
*2016 Journal Impact Factor was established by dividing the number of articles published in 2014 and 2015 with the number of times they are cited in 2016 based on Google Scholar Citation Index database. If 'X' is the total number of articles published in2014 and 2015, and 'Y'is the number of times these articles were cited in indexed journals during 2016 then, impact factor = Y/X
Recent Articles

A Rare Case of Inguinal Hernia with Complete Appendix Herniation to the Scrotum
Author(s) : Farshad Banouei and Mehdi Komaki
Hemoglobinosis D-Punjab in a Moroccan Family
Author(s) :
Arthritis and Renal Artery Stent Apoplexy in Takayasu
Author(s) : Eugene Mohan
Liver Transplant Immunosuppression in Sepsis Patients
Author(s) : Mariana Curceli
Hemodynamic Assessment of Renal Conduit Inclusion
Author(s) : Ding Yuan
Relevant Topics
Google scholar citation report, citations : 238.
Medical Case Reports received 238 citations as per google scholar report
Medical Case Reports peer review process verified at publons
Abstracted/Indexed in
- Google Scholar
- China National Knowledge Infrastructure (CNKI)
- Directory of Research Journal Indexing (DRJI)
- Secret Search Engine Labs
View More »

30+ Million Website Visitors
660806+ journal visitors.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Accelerating Biomedical Discovery and Data-Powered Health
Citations for biomedical literature
MedlinePlus
Reliable, up-to-date health information for you
An experimental multimedia search engine
Medical Subject Headings
ClinicalTrials.gov
A database of clinical studies, worldwide
Basic Local Alignment Search Tool
News and Highlights

NLM Announcements

Musings from the Mezzanine

NCBI Insights

Circulating Now

NIH MedlinePlus Magazine

Technical Bulletin

NIH Virtual Tour: National Library of Medicine
NLM is the world's largest biomedical library and a national resource for health professionals, scientists, and the public.
News Spotlight
Towards a smart bionic eye.
AI-Powered Artificial Vision for the Treatment of Incurable Blindness
These NLM-funded researchers bring together artificial intelligence, virtual reality, and visual prostheses – retinal and brain implants referred to as “bionic eyes” – in hopes of one day providing better assistive technology for incurable blindness that affects about 40 million people worldwide.
Despite recent advances in gene and stem cell therapies, which are showing great promise, there are no effective treatments for many people blinded by severe degeneration or damage to the retina, the optic nerve, or cortex. In such cases, an electronic visual prosthesis (“bionic eye”) may be the only option. The goal is thus to address fundamental questions at the intersection of neuroscience, computer science, and human-computer interaction that will enable the development of a Smart Bionic Eye.
Find more information in the NIH RePORTER or PubMed Central .
Research at NLM
Nlm intramural research program.
Intramural research at NLM consists of the development and application of computational approaches to a broad range of problems in biomedicine, molecular biology, and health. READ RESEARCH HIGHLIGHTS | MEET OUR PRINCIPAL INVESTIGATORS | EXPLORE TRAINING OPPORTUNITIES
Historical Collections at NLM
Biomedical and clinical informatics at nlm, health it and health data standards.

Efficient health care information exchange in the US and worldwide is made possible by NLM’s work with IT Data Standards.
Learn about NLM’s contributions to Health IT
Unified Medical Language System (UMLS) Terminology Services
This set of tooling services brings together many health and biomedical vocabularies and standards to enable interoperability between computer systems.
Explore UMLS
Biomedical Informatics Training Program

This training program provides biomedical and clinical informatics training and research opportunities for individuals at various stages in their career.
Investigate training opportunities

The Library started as a shelf of books in the Surgeon General’s office in 1836 but has grown to a collection of millions of print and electronic resources.
Explore our past
Organization
The diverse centers, divisions, advisory bodies and other organizational units that make up NLM contribute in myriad ways to the Library’s mission.
Explore the Library
Strategic Plan

This ten year plan outlines NLM's role in a future where data and information transform and accelerate biomedical discovery and improve health and health care.
VIEW OUR STRATEGIC PLAN
- Publications
- Conferences & Events
- Professional Learning
- Science Standards
- Awards & Competitions
- Instructional Materials
- Free Resources
- American Rescue Plan
- For Preservice Teachers
NCCSTS Case Collection
- Science and STEM Education Jobs
- Interactive eBooks+
- Digital Catalog
- Regional Product Representatives
- e-Newsletters
- Bestselling Books
- Latest Books
- Popular Book Series
- Prospective Authors
- Web Seminars
- Exhibits & Sponsorship
- Conference Reviewers
- National Conference • Denver 24
- Leaders Institute 2024
- National Conference • New Orleans 24
- Submit a Proposal
- Latest Resources
- Professional Learning Units & Courses
- For Districts
- Online Course Providers
- Schools & Districts
- College Professors & Students
- The Standards
- Teachers and Admin
- eCYBERMISSION
- Toshiba/NSTA ExploraVision
- Junior Science & Humanities Symposium
- Teaching Awards
- Climate Change
- Earth & Space Science
- New Science Teachers
- Early Childhood
- Middle School
- High School
- Postsecondary
- Informal Education
- Journal Articles
- Lesson Plans
- e-newsletters
- Science & Children
- Science Scope
- The Science Teacher
- Journal of College Sci. Teaching
- Connected Science Learning
- NSTA Reports
- Next-Gen Navigator
- Science Update
- Teacher Tip Tuesday
- Trans. Sci. Learning
MyNSTA Community
- My Collections
Case Study Listserv
Permissions & Guidelines
Submit a Case Study
Resources & Publications
Enrich your students’ educational experience with case-based teaching
The NCCSTS Case Collection, created and curated by the National Center for Case Study Teaching in Science, on behalf of the University at Buffalo, contains over a thousand peer-reviewed case studies on a variety of topics in all areas of science.
Cases (only) are freely accessible; subscription is required for access to teaching notes and answer keys.
Subscribe Today
Browse Case Studies
Latest Case Studies

Development of the NCCSTS Case Collection was originally funded by major grants to the University at Buffalo from the National Science Foundation , The Pew Charitable Trusts , and the U.S. Department of Education .
- Intranet Login
- Clinical Services
- Patient Care
- Department Videos
- Job Opportunities
- Diversity and Inclusion
- Administration
- Student Fellows
- Recent Graduates
- Affiliated Hospitals
- Pepper Talk
- Residency Matters
- Roth Report
- Application
- AP/Neuropathology
- Physician-Scientist Pathway
- Conferences
- Teaching Opportunities
- Salary & Benefits
- Living in Philly
- Breast Pathology
- Cellular Therapy
- Clinical Chemistry
- Clinical Informatics
- Clinical Microbiology
- Cytopathology
- GI/Hepatic Pathology
- Hematopathology
- Molecular Genetic Pathology
- Neuropathology
- Soft Tissue/Bone Pathology
- Surgical Pathology
- Transfusion Medicine
- Student Fellowship
- Graduate Studies
- Medical School
- Case Studies
- PennLab Summer Internship
- Cancer and Immunobiology
- Neuropathology and Neurodegeneration
- Diagnostic Innovation
- Info for Investigators
- Research Labs
- Centers and Institutes
- Clinical Cell and Vaccine Production Facility
- CRISPR/Cas9 Mouse Targeting Core
- Penn Cytomics and Cell Sorting Resource Laboratory
- Human Immunology
- Tumor Tissue/ Biospecimen Bank
- Publications
- Electron Microscopy
- Immunohistochemistry
- Medical Pathology
- Clinical Chemistry Core Lab
- Endocrinology
- Coagulation
- Immunology and HLA
- Microbiology
- Point-of-Care Testing
- Reference Testing
- Apheresis Unit
- Flow Cytometry
- Center for Personalized Diagnostics
- Clinical Cancer Cytogenetics Laboratory
- Molecular Pathology
- Rittenhouse Molecular Laboratory
- Test, Biospecimen, & Research Requests
- Clinical Assay Development
- Molecular Genetic Pathology Fellowship
- AP Consults
- Immunotherapy
- Meet Your Pathologist
- Making an Appointment
- Clinical Trials
- Patient Resources
CASE STUDIES
- All case studies
Filter by: Clear Filters
This page offers a collection of interesting cases from the Penn Department of Pathology and Laboratory Medicine that are available to download as PDFs. To view specific case studies by organ system or subspecialty, use the filter checkboxes in the left sidebar.
56-year-old woman with 3.5 cm large right nasal mass, resected after 2 nondiagnostic biopsies
33-year-old man with complex ethmoid sinus mass and imaging concerning for a sinonasal malignancy, 34-year-old man with aml with sudden onset of headache and fever, 36-year-old woman presenting with hemoptysis, 65-year-old man with 2.3 cm right lower thyroid nodule, 62-year-old man with a right posterior nasal mass, 56-year-old female presenting with a 3-month history of abdominal pain, 55-year-old male presenting with back pain, 65-year-old female with a mass involving the maxillary sinus, 74-year-old female with an extradural tumor compressing the right frontal lobe, 35-year-old man with chronic rhinosinusitis and nasal septal perforation, 54-year-old man with a 3.6 cm right neck mass, 21-year-old man with asthma, chronic sinusitis, polyps, headache and proptosis, 57-year-old woman with a renal mass, 63-year-old man with history of iv drug use, 72-year-old man with polypoid esophageal mass, 20-year-old woman with 3 cm mass in the tail of pancreas, 40-year-old man with increasing frequency of hypoglycemic spells, 52-year-old woman with transient symptomatic hyperthyroidism, stay connected.
- Therapeutic Pathology
- Anatomic Pathology
- Lab Medicine Advances
- Science Breakthroughs
Sign up for the Department Newsletter:
Thank you for subscribing!
Department of Pathology and Laboratory Medicine
Perelman School of Medicine at the University of Pennsylvania 3400 Spruce St. Philadelphia, PA 19104-4238
© 2024 Trustees of the University of Pennsylvania
- About This Site

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 June 2024
Risk factors for secondary thyroid cancer in patients with breast cancer: a propensity‑matched SEER analysis
- Yizhuo Diao 1 ,
- Ruiqi Wang 1 na1 ,
- Jiaxue Cui 1 na1 ,
- Chenxin Jin 1 ,
- Yongxing Chen 1 &
- Xiaofeng Li 1
Scientific Reports volume 14 , Article number: 12679 ( 2024 ) Cite this article
Metrics details
- Cancer epidemiology
- Cancer models
With the rapid development of imaging technology and comprehensive treatment in modern medicine, the early diagnosis rate of breast cancer is constantly improving, and the prognosis is also improving; As breast cancer patients survive longer, the risk of developing second primary cancers increases. Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously. This study extracted clinical data and survival outcomes of breast cancer patients registered in the Surveillance, Epidemiology and End Results (SEER) database between 2010 and 2019. After matching the case and controls with propensity scores, the selected patients were randomly split into training and test datasets at a ratio of 7:3. Univariate and multivariate COX proportional regression analysis is used to determine independent risk factors for secondary thyroid cancer and construct a column chart prediction model. Age, ethnicity, whether radiotherapy, tumor primary location, N stage, M stage were identified by Cox regression as independent factors affecting secondary thyroid cancer in patients with breast cancer patients, and a risk factor nomogram was established to predict patients’ 3 and 5 year survival probabilities. The AUC values for 3 and 5 years in the training set were 0.713, 0.707, and the c-index was 0.693 (95% CI 0.67144, 0.71456), and the AUC values for 3 and 5 years in the validation set were 0.681, 0.681, and the c-index was 0.673 (95% CI 0.64164, 0.70436), respectively.
Similar content being viewed by others
Risk of second primary thyroid cancer in cancer survivors

Breast cancer risk among thyroid cancer survivors and the role of I-131 treatment

Impact of thyroid hormone replacement on the risk of second cancer after thyroidectomy: a Korean National Cohort Study
Introduction.
Breast cancer is a malignant tumor that occurs in the Epithelium of the breast gland 1 . It is the “No. 1 public enemy” that threatens the physical and mental health of women around the world today 2 . It ranks first in the incidence and death of cancer among women in most countries around the world 3 . According to the data of the 2023 American Cancer Statistical Report, breast cancer is still one of the three most common cancers among women 4 ,occupying the first place in expected new cases among women; With the rapid development of imaging technology and comprehensive treatment in modern medicine 5 , the early diagnosis rate of breast cancer continues to improve, and the prognosis also improves 6 .The disease-free survival rate and overall survival rate of breast cancer patients have significantly improved; With the prolongation of the survival period of breast cancer patients, the risk of second primary cancer (SPC) may increase 7 ; Thyroid cancer is a malignant tumor originating from thyroid follicular epithelium 8 . It is considered to be an inert tumor 9 and the most common malignant tumor in the Endocrine system and head and neck tumors 10 ; Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously.
Materials and methods
Data sources.
The SEER database containing 13 registry centers prepared by the National Cancer Institute was selected for the data of this study, and a total of 692,555 patients diagnosed with breast cancer from 2010 to 2019 were extracted from the database using the SEER*Stat software program, and a total of 393,722 patients were screened according to the inclusion and exclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria.
(1) Patients diagnosed with breast cancer as the first tumor from 2010 to 2019 (2) Patients with no more than 2 tumors and the second type of tumor is thyroid cancer (3) Female patients (4) Patients with complete clinical data(Includes time of diagnosis, age at diagnosis, marital status at diagnosis, site of origin, mode of diagnostic confirmation, mode of case report, chemotherapy or not, radiotherapy or not, molecular typing, Progesterone Receptor, Estrogen Receptor, T,N, and M typing, type of secondary second tumor, interval between secondary second tumors, survival time, survival status)
Exclusion criteria
(1) Those with benign tumors (2) Proven only at autopsy or death (3) Patients with thyroid cancer occurring within 3 months of breast cancer diagnosis
Independent variables
The study data were converted to categorical variables in order to make the study more intuitive and standardized. Race was categorized as black, white, and other; marital status was categorized as married, unmarried; radiation and chemotherapy status was categorized as yes, no; primary tumor location was categorized as mid-breast, quadrant I, quadrant II, quadrant III, quadrant IV, and other; estrogen receptor (ER), and progesterone receptor (PR) status was categorized as estrogen receptor (ER) and progesterone receptor (PR) status positive and negative; and molecular staging included LuminalA, LuminalB, HER2 overexpression and triple-negative; T staging as T0T1, T2, T3 and T4; N staging as N0, N1, N2 and N3; M staging as M0 and M1; and secondary thyroid cancer status as yes or no.
Statistical methods
R4.2.3 statistical software was used for analysis. Patient standardized incidence rates (SIR) were calculated using the Multiple Principal Standardized Incidence Rates (MP-SIR) module of SEER Stat 8.4.1 software. Due to the large difference in sample sizes between the case and control groups in this study, in order to equalize the distribution of covariates between the groups, equalize confounders, and reduce selection bias, we introduced the propensity score matching (PSM) method 11 , in which the two groups of patients were matched in a ratio of 1:4 12 . Using the R software package MatchIt , the PSM method was used to match the case and control groups according to the year of diagnosis in a 1:4 ratio, and a total of 392,803 patients with unilateral breast cancer were included as controls, and a 1:4 matching of the case and control groups was accomplished based on the year of diagnosis of breast cancer in the 919 case group of thyroid cancer secondary to breast cancer. Patients served as controls. To ensure that the 919 cases were matched to the 3676 controls, caliper distances were chosen to be as small as possible. Propensity scores were calculated using a logistic regression model, and for better matching, the final matching caliper distance was 0.1. The standardized mean difference (SMD) was used to assess the balance of baseline information between the case and control groups after PSM. We considered SMD less than or equal to 0.1 to indicate a good match 11 .
Further univariate and multivariate analyses were performed using the COX proportional risk model to determine the risk factors for secondary thyroid cancer in breast cancer patients. R-studio software was used to randomly divide all the data into training and validation sets in the ratio of 7:3, and χ 2 test and t-test were performed on different variables in the training and validation sets, and then univariate and multivariate Cox regression analyses were carried out on the data in the training set in order to train the model, and the validation set was used to validate the model 13 . A nomogram was created using the R packages rms , foreign and survival for the final filtered variables and the ROC curves were used to create the nomograms under the ROC curves. The area under the ROC curve (AUC value) and C-index were used to evaluate the accuracy of the model, the AUC and C-index range from 0 to 1, the closer to 1 indicates that the model is more accurate, and it is usually considered that the model has a better predictive ability when the AUC reaches more than 0.7; the calibration curve was used to evaluate the degree of calibration of the model, and the closer the calibration curve is to the standard curve, the stronger the predictive ability of the model. Variables with a univariate COX regression of P < 0.1 were included in the multifactor analysis, and the multifactor analysis was included in the final model with the criterion that the difference was considered statistically significant at P < 0.05 14 .
Ethical approval and consent to participate
Not applicable. Data is available in a public database, ethics approval is not applicable.
Standardized incidence rate
The SEER Stat 8.4.1 analysis yielded a SIR result of 14.89 with a 95% CI of 14.02–15.79 for the period 2010–2019, a predicted number of people of 74.16, and a 10 year actual number of people of 1104, with an incidence rate of 159.41/100,000, which leads to the conclusion that patients who already have breast cancer are more likely to develop thyroid cancer than healthy people.
Propensity score matching
919 patients with thyroid cancer secondary to breast cancer after screening as case group and 392,803 patients with solitary breast cancer as control group were included in the propensity score matching, according to the year of diagnosis according to the PSM method to achieve 1:4 matching, and finally a total of 919 cases in the case group and 3676 cases in the control group were obtained, and the results are shown in Table 1 . The R language tableone package automatically uses analysis of variance for continuous variables, which is equivalent to t-test since this data is only divided into two groups. The result before matching was P < 0.001 and the result after matching was P = 1, SMD < 0.001, which can be considered as well balanced between the matched case group and the control group.
Baseline information
The 919 patients with thyroid cancer secondary to breast cancer and the 3676 patients only with breast cancer obtained after propensity score matching, for a total of 4595 patients, were included in subsequent model analyses. The baseline data of the 4595 patients are shown in Table 2 .
Comparison of baseline characteristics
The 4594 patients obtained after propensity score matching were randomly divided into training set and validation set according to the ratio of 7:3, 3219 patients in the training set and 1376 patients in the validation set. t-test was used for continuous variables, and chi-square test was used for categorical variables to characterize the intergroup differences between the training set and the validation set, and the p-values were all greater than 0.05, which proved that there were no intergroup differences in the results of the randomized splitting, and the results are shown in Table 3 .
Results of univariate and multivariate cox regressions
The training set patient data were included in univariate Cox regression analysis for each of the 12 variables. To avoid omission of important variables, 11 variables with P < 0.1 in the univariate Cox regression were included in the multivariate Cox regression. We included the variables with p < 0.1 in the univariate Cox regression analysis to the multivariate Cox model to examine the independent risk factors for second thyroid cancer; when P < 0.05 in multivariate Cox regression analysis, the factor was an independent risk factor affecting patients’ secondary thyroid cancer. The results of this univariate Cox regression showed that age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether radiotherapy, whether chemotherapy, T stage, N stage, M stage were the factors affecting the secondary thyroid cancer in breast cancer patients; while the results of multivariate Cox regression showed that age, race, whether radiotherapy, primary tumor location, N-stage, and M-stage were independent risk factors affecting the development of thyroid cancer in patients with breast cancer, and the results are shown in Table 4 .
Creation of nomogram
Variables screened in the multifactorial Cox regression analysis (P < 0.05) were included in the cox proportional risk model, and nomograms were created using R-studio software. Each variable was projected upward for breast cancer patients, and then each score on the scale was summed to obtain a total score, based on which the risk of secondary thyroid cancer in breast cancer patients at 3 and 5 years could be predicted, and the higher the total score the higher the risk, the prediction results are shown in Fig. 1 . Patients with breast cancer who were younger, received radiotherapy, had N1 staging, M1 staging, were white and other race, and had a primary in the inner lower quadrant were at greater risk of secondary thyroid cancer.
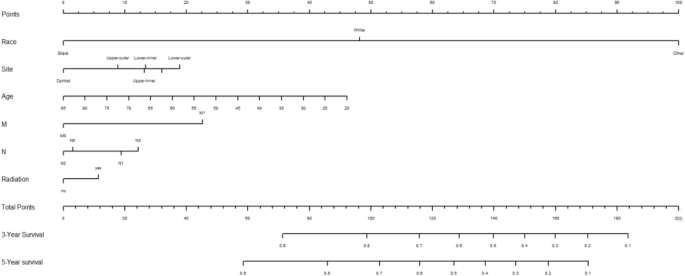
The nomogram of the COX proportional risk model.
Validation of nomogram
Using the ROC curve AUC value and c-index to evaluate the differentiation of the model, we found that, in the Cox proportional risk regression model, the AUC values of three and five years for the patients in the training set (Fig. 2 ) were 0.713, 0.707, and the c -index was 0.693 (95% CI 0.67144, 0.71456), respectively, and the AUC of three and five years for the patients in the validation set (Fig. 3 ) values were 0.681, 0.681,c-index was 0.673 (95% CI 0.64164, 0.70436 respectively), we can consider the model as having moderate predictive power. The calibration plots were plotted using the data from the validation set to evaluate the fit of the established model, and the results showed that in the Cox proportional risk regression model, the calibration curves of 3 and 5 years were close to the dotted line with a 45 ° inclination in the middle, which indicated that the model fit was better.The calibration curves of 3 and 5 years are shown in Fig. 4 .
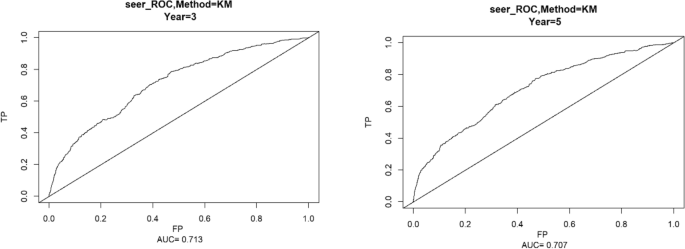
Three and Five year ROC curves for patients in the training center.
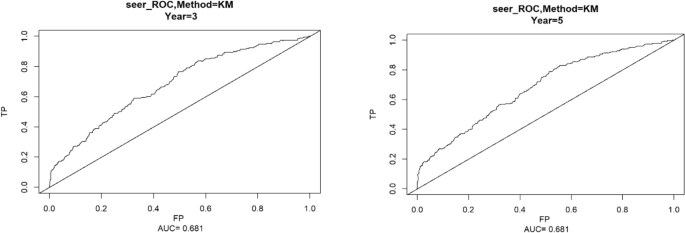
Three and Five year ROC curves for patients in the validation set.
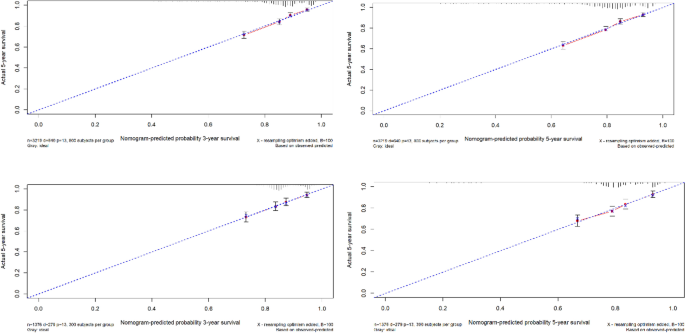
The calibration curves of 3 and 5 years.
Utility and discussion
Breast cancer is known as the world’s number one “red face killer”, according to the latest cancer data released by the World Health Organization, the number of new cases of breast cancer (2.26 million) has exceeded that of lung cancer (2.2 million), and has become the most prevalent cancer in the world 4 . In recent years, more and more general hospitals have set up thyroid breast surgery departments, mainly because it is generally recognized that both the thyroid and the breast are target organs for hormone regulation by the hypothalamic-pituitary endocrine axis, and there are some common pathogenic factors in the two. Therefore, the use of a combination of clinical diagnostics and intelligent means to determine the risk factors for secondary thyroid cancer in breast cancer patients is important for both doctors and patients, and it can guide breast cancer patients for follow-up screening and help clinicians develop treatment plans for the general population and breast cancer survivors.
Although the number of breast cancer patients has been increasing year by year, the low incidence of dual primary cancers, the fact that patients with second primary cancer may be seen in different hospitals, the difficulty of data collection and the long time required for follow-up make the collection of data for the study of dual primary cancers of the breast still difficult. Therefore, this study chooses the U.S. National Cancer Database (SEER), which covers about 30% of the U.S. population, updates patient data in March to April every year, and has a large number of patients with a long follow-up period, making it a more ideal source of data for the study of dual primary cancers.
A total of 692,555 patients diagnosed with breast cancer in the SEER database from 2010 to 2019 were included in this study, and the SEERSTATA software was used to calculate the SIR > 1 for thyroid cancer secondary to breast cancer, and it can be assumed that patients with breast cancer are more likely to develop thyroid cancer compared to cancer-free populations, which is consistent with the findings of previous literature. Due to the large difference in sample size between the case and control groups, in order to minimize bias, we matched 919 patients with thyroid cancer secondary to breast cancer as the case group and 393,722 patients with solitary breast cancer as the control group using propensity score matching. Because the diagnostic criteria, examination instruments, and staging criteria may differ between years, we performed 1:4 propensity score matching according to the year of diagnosis to minimize confounding bias and to include as many control cases as possible to ensure representativeness 12 . The matched 4595 patients were randomly divided into training set and validation set in the ratio of 7:3, and the obtained training set was used to train the cox regression model, and the validation set was used to verify the stability of the model 15 , 16 . The results of univariate cox regression showed that the 11 factors included except ER were independent factors for secondary thyroid cancer in breast cancer patients, and the results of multivariate cox regression showed that age, ethnicity, location of primary tumor, whether or not to have radiotherapy, N-stage, and M-stage were independent factors for secondary thyroid cancer in breast cancer patients.
Contrary to the general impression that age, as a continuous variable with an OR value of less than 1, is considered a protective factor against secondary thyroid cancer from breast cancer, the younger the age, the more likely it is to be secondary thyroid cancer, and previous studies by other scholars have also demonstrated that patients with secondary thyroid cancer after breast cancer were younger compared with those who had breast cancer only 17 , which we believe may be related to the recent years of rejuvenation of the incidence of breast cancer, the early diagnosis and early treatment that makes the breast cancer patient. We believe that this may be related to the recent rejuvenation of breast cancer incidence, prolonged survival due to early diagnosis and early treatment, and the modern lifestyle, where a good prognosis increases the likelihood of a secondary second tumor. However, in some scholars’ studies, age is a risk factor for survival in patients with thyroid cancer secondary to breast cancer relative to patients with solitary breast cancer, and older patients are more likely to have lower survival rates 18 . In conclusion, age is an extremely important factor affecting breast cancer patients with secondary thyroid cancer and warrants further study.
In terms of race, the SEER database has more white profiles due to region, and multivariate cox results show that whiteness and other ethnicities are independent risk factors for secondary thyroid cancers in patients with breast cancer. This may be related to the level of medical care, conditions, and other factors that were co-incorporated into the study, with genetic susceptibility playing a different role in different factors. Mariotto A.B. et al. suggest that the higher incidence of second primary cancers among white women is due to the higher overall survival and screening rates among white women compared to black female populations, and that more comprehensive medical coverage and higher levels of medical care make it easier to diagnose the disease, which would inevitably lead to an increase in the number of diagnoses if these cancers were diagnosed at an earlier stage, which would be consistent with the results of the present study 19 . If these cancers were diagnosed at an early stage, this would inevitably lead to an increase in the number of diagnoses. However the study by Shuting Li et al. suggests that black women with breast cancer should be given attention 13 . However, in a study by Karan Seegobin et al., it was found that there was no significant difference in the incidence of secondary breast and gynecologic cancers between Caucasians and Blacks 20 ,which may be due to the different target second primary cancer disease types in the study, and further research is needed in the future.
Breast cancer patients who receive radiation therapy are more likely to develop secondary thyroid cancers compared to those who do not, a finding that is generally consistent with current clinical opinion that radiation therapy can affect thyroid hormone secretion and thyroid function 21 . The relationship between radiation therapy and the risk of second primary cancers has long been recognized, and it has been demonstrated that radiation therapy is associated with an increased risk of second primary malignancies after exposure 21 . In several observational studies of breast cancer follow-up, the incidence of subsequent secondary acute myeloid leukemia was increased in patients with breast cancer, which may be related to the dose intensity of chemotherapy, the use of adjuvant radiotherapy, and the use of granulocyte colony-stimulating factor (GCSF) 22 . Data from the DBCG (The Danish Breast Cancer Cooperative Group) registry estimate that the proportion of second primary cancers after breast cancer associated with radiation therapy is about 9%. This is consistent with the results from the US SEER database registry 23 . However Grantzau’s analysis found that breast cancer radiotherapy was associated with a small but significant increase in the risk of second cancers for lung, esophageal, and soft tissue cancers, but was not significantly associated with second cancers for thyroid cancer 24 . Another study on the health of the population in Taiwan compared the risk of TC in BC patients who received radiotherapy and those who did not, and the risk of TC in women who received radiotherapy was not significantly higher than that in women who did not receive radiotherapy. This may be related to the selection of data from different ethnic groups in different regions.
In addition, some researchers have suggested that the ER/PR signaling pathway may be a common etiology of breast and thyroid carcinogenesis, and studies of its mechanisms, including the ER pathway and CHEK2 gene mutations in thyroid tissues. In our study, only PR receptor status was an independent factor for secondary thyroid cancer in breast cancer patients. Some researchers have suggested that patients with secondary thyroid cancer have a higher rate of both ER and PR positivity than patients with breast cancer only 25 , 26 .ER has two isoforms, Erα and Erβ, and overexpression of ERα in thyroid cancer tissues and lack of expression of ERβ in peripheral tissues was reported in 2011 27 , and under-expression or deletion of ER can be considered a hallmark of thyroid cancer 28 . Undifferentiated thyroid stem and progenitor cells express lower levels of ERβ compared to differentiated human thyroid cells 29 . Therefore, low levels of ER expression may suggest dedifferentiation of thyroid cancer 27 , 30 . The fact that this paper did not conclude that ER has an effect on secondary thyroid cancer in breast cancer patients may be related to the fact that gene-related variables were not included in this study, and that the SEER database contains only baseline and treatment information and no genetic data, which is a limitation that exists in this study 31 , 32 , 33 .
In AJCC staging, T stage represents the size and extent of the tumor, N stage represents lymph node metastasis, and M stage represents distant spread, with more stages representing more seriousness and worse prognosis. In this study, it was concluded that in N staging, breast cancer patients with N1 stage were more likely to develop secondary thyroid cancer compared to other stages, which may be related to the survival period, and we believe that only if the survival period is long enough, there is a possibility of developing a second primary tumor, while patients with N1 stage had lymph node metastasis, but it was not as serious as N2, N3, and N4 stage, so they were more likely to have a longer survival period; while patients with N0 stage had not No lymph node metastasis has occurred, and from the perspective of disease development, the possibility of secondary cancer is less than that of stage N1.In M stage, M0 stage represents no distant metastasis, and M1 stage represents distant metastasis, and we generally know that tumors with metastases are more likely to cause other cancers.
To summarize, in this study, univariate and multivariate cox analyses were used to screen the influencing factors of secondary thyroid cancer in breast cancer patients, respectively, and a column-line diagram was successfully established, and the calibration curves of the training and validation sets were well fitted, and the AUC and c-index reached significance. It is suggested that the column-line diagram has good predictive ability for the risk of secondary thyroid cancer in breast cancer patients 3 and 5 years after the onset of the disease. However, since the data were obtained from the United States, more studies are needed to verify whether the results obtained by applying this data can be applied to the Chinese population, and the results of this study can provide some references for clinicians 14 .
This study also has some limitations; first, this is a retrospective study, and selection bias due to incomplete data is inevitable. Although we used PSM to avoid selection bias, potential confounders cannot be excluded. Secondly, the Cox model fits well, but the AUC of the validation set is less than 0.7, which represents that the predictive ability of the model is still lacking, which may be related to the fact that breast cancer patients have a good prognosis and a long survival period, while only ten years of data have been observed in the present study, so in the future, we need more follow-up data to improve the model. Because the SEER database itself provides a limited amount of information and the database does not provide any information about genes, we were unable to study the genetic correlation of breast cancer secondary to thyroid cancer at the genetic level. The database has not been updated with data on tumor grading since 2017, so it is not possible to analyze the grading situation, which deserves further study in the future.

Conclusions
In summary, our study suggests that breast cancer patients are more likely to develop thyroid cancer compared to the general population. Among them, age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether or not radiotherapy, whether or not chemotherapy, T stage, N stage, and M stage are the factors affecting the secondary thyroid cancer in breast cancer patients; age, ethnicity, whether or not radiotherapy was performed, primary location of the tumor, N stage, and M stage are the independent factors affecting the secondary thyroid cancer in patients with breast cancer patients: the younger the age, the whiter the ethnicity, the whites and the other ethnicities, undergoing radiotherapy, internal lower quadrant and other locations, N1 stage, and M1 stage are more likely to develop thyroid cancer in breast cancer patients.
Data availability
The datasets analyzed during the current study are available in the SEER*Stat software (version 8.4.2, download from https://seer.cancer.gov/data/options.html ). A registration form needs to be completed before using and filtercriteria need to be added. The datasets are also available from the corresponding author on reasonable request.
Huang, J. et al. Effect of breast cancer as the first or second primary cancer on the prognosis of women with thyroid cancer: A SEER database analysis. Transl. Cancer Res. 911 , 6955–6962 (2020).
Article Google Scholar
Bolf, E. L., Sprague, B. L. & Carr, F. E. A linkage between thyroid and breast cancer: A common etiology?. Cancer Epidemiol. Biomark. Prev. 284 , 643–649 (2019).
Bakos, B. et al. Co-occurrence of thyroid and breast cancer is associated with an increased oncogenic SNP burden. BMC Cancer 21 , 1–11 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. A Cancer statistics, 2023.. CA Cancer J. Clin. 73 (1), 17–48 (2023).
Article PubMed Google Scholar
Zhang, W. et al. Metastasis patterns and prognosis in young breast cancer patients: A SEER database analysis[j]. Front. Oncol. 12 , 872862 (2022).
Article CAS PubMed PubMed Central Google Scholar
基于SEER数据库对乳腺癌...原发癌危险因素的回顾性分析_齐欣.pdf>.
Fu, J. et al. The clinical and genetic features in patients coexisting primary breast and thyroid cancers. Front. Endocrinol. 14 , 1136120 (2023).
Dong, L., Lu, J., Zhao, B., Wang, W. & Zhao, Y. Review of the possible association between thyroid and breast carcinoma. World J. Surg. Oncol. 16 , 1–7 (2018).
Ahn, J.-H. et al. The association between vitamin D supplementation and the long-term prognosis of differentiated thyroid cancer patients: A retrospective observational cohort study with propensity score matching. Front. Endocrinol. 14 , 1163671 (2023).
Lim, H., Devesa, S. S., Sosa, J. A., Check, D. & Kitahara, C. M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA https://doi.org/10.1177/10732748221135447 (2017).
Article PubMed PubMed Central Google Scholar
Cui, J. et al. Association of dietary pattern and Tibetan featured foods with high-altitude polycythemia in Naqu, Tibet: A 1:2 individual-matched case-control study. Front. Nutr. https://doi.org/10.3389/fnut.2022.946259 (2022).
Wang, J., Ning, Y., Du, Y. & Kang, Y. Lymphadenectomy benefits small cell carcinoma of ovary: A population-based analysis. Curr. Oncol. 2910 , 7802–7815 (2022).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 19 , 1 (2019).
Wang, R. et al. Risk factor analysis and nomogram establishment and verification of brain astrocytoma patients based on SEER database. Sci. Rep. 13 , 1 (2023).
Google Scholar
Qiu, X. et al. Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: A retrospective analysis of the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2023.1098686 (2023).
Zhou, Y. et al. The prognostic significance of further axillary dissection for sentinel lymph node micrometastases in female breast cancer: A competing risk analysis using the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2022.1012646 (2022).
Pu, C.-C., Yin, L. & Yan, J.-M. Risk factors and survival prediction of young breast cancer patients with liver metastases: A population-based study. Front. Endocrinol. https://doi.org/10.3389/fendo.2023.1158759 (2023).
Donin, N. et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 12219 , 3075–3086 (2016).
Mariotto, A. B., Rowland, J. H., Ries, L. A. G., Scoppa, S. & Feuer, E. J. Multiple cancer prevalence: A growing challenge in long-term survivorship. Cancer Epidemiol. Biomark. Prev. 163 , 566–571 (2007).
Seegobin, K. et al. Pilot study on the occurrence of multiple cancers following cancer-related therapy at the University of Florida, Jacksonville (2011–2016). J Investig. Med. 667 , 1050–1054 (2018).
Praga, C. et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: Correlation with doses of epirubicin and cyclophosphamide. J. Clin. Oncol. 2318 , 4179–91 (2005).
Smith, R. E., Bryant, J., DeCillis, A. & Anderson, S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The national surgical adjuvant breast and bowel project experience. J. Clin. Oncol. 217 , 1195–204 (2003).
Sokołowski, M., Mazur, G. & Butrym, A. Breast cancer and synchronous multiple myeloma as a diagnostic challenge: Case report and review of literature. Curr. Probl. Cancer 422 , 231–234 (2018).
Grantzau, T. & Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 1213 , 402–413 (2016).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 191 , 1592 (2019).
Ji, J., Zhang, X., Yuan, S., Liu, H. & Yang, L. Survival impact of gastrectomy and chemotherapy for gastric signet ring cell carcinoma with different metastatic lesions: A population-based study. Asian J. Surg. 47 (4), 1769–1775 (2024).
Di Vito, M. et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser-capture microdissection and molecular biology. Cancer Sci. 10210 , 1921–7 (2011).
Božović, A., Mandušić, V., Todorović, L. & Krajnović, M. Estrogen receptor beta: The promising biomarker and potential target in metastases. Int. J. Mol. Sci. 22 (4), 1656 (2021).
Xu, S., Chen, G., Peng, W., Renko, K. & Derwahl, M. Oestrogen action on thyroid progenitor cells: Relevant for the pathogenesis of thyroid nodules?. J. Endocrinol. 2181 , 125–33 (2013).
Thomas, C. & Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 118 , 597–608 (2011).
Lal, G. et al. Risk of subsequent primary thyroid cancer after another malignancy: Latency trends in a population-based study. Ann. Surg. Oncol. 196 , 1887–96 (2012).
Sun, L. M., Lin, C. L., Liang, J. A., Huang, W. S. & Kao, C. H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int. J. Cancer 13712 , 2896–903 (2015).
An, J. H. et al. A possible association between thyroid cancer and breast cancer. Thyroid 2512 , 1330–8 (2015).
Download references
Acknowledgements
We would like to thank Editage for English language editing.
Author information
These authors contributed equally: Ruiqi Wang and Jiaxue Cui.
Authors and Affiliations
Department of Epidemiology and Health Statistics, Dalian Medical University, 9 Lvshun South Road, Dalian, 116044, Liaoning, China
Yizhuo Diao, Ruiqi Wang, Jiaxue Cui, Chenxin Jin, Yongxing Chen & Xiaofeng Li
You can also search for this author in PubMed Google Scholar
Contributions
Y.D. (first author): design, original draft preparation, software analysis, data analysis and interpretation, manuscript writing, final approval of manuscript. R.W.: data interpretation, final approval of manuscript. J.C: collection data, final approval of manuscript. C.J.: methodology, final approval of manuscript. Y.C.: methodology, final approval of manuscript. X.L.: corresponding author, guide the revision of the article, final approval of manuscript.
Corresponding author
Correspondence to Xiaofeng Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Diao, Y., Wang, R., Cui, J. et al. Risk factors for secondary thyroid cancer in patients with breast cancer: a propensity‑matched SEER analysis. Sci Rep 14 , 12679 (2024). https://doi.org/10.1038/s41598-024-59209-x
Download citation
Received : 07 October 2023
Accepted : 08 April 2024
Published : 03 June 2024
DOI : https://doi.org/10.1038/s41598-024-59209-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Thyroid cancer
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Business of Medicine | Understanding the Medical Malpractice Litigation Process
May 31, 2024
Cardiology Magazine
Few things in a clinician's life generates more stress and disruption than an allegation of professional malpractice. The litigation process and the uncertainty it creates may cause a clinician to experience feelings of anger, anxiety and depression, or even physical reactions such as insomnia or stress-related headaches. The first step in alleviating the uncertainty is to understand the litigation process and the defendant's role during each step. The following is an overview of the various phases of the medical malpractice litigation process a defendant may be involved in and recommended strategies to maximize a successful outcome.
Early Indicators of Possible Litigation
Records Request The earliest indication of a potential lawsuit may be a request for medical records from a patient or a plaintiff's attorney. Such a request, however, may simply be part of an attorney's investigation of an accident or workers' compensation claim, in which case the request is usually made in a letter with a signed authorization enclosed, or by subpoena or other court order.
If you suspect the request for medical records is related to a potential medical malpractice action, notify your professional liability carrier. Ask for assistance in determining the validity of the records request and how to manage it promptly. Importantly, be sure to obtain valid signed authorization before releasing any medical records.
Notice of a Claim If a patient pursues a claim, the patient's attorney may notify the clinician by letter. In some states, notice may be triggered by statutory requirements, such as a notice of intent to sue or pre-suit notice. Additionally, a number of jurisdictions currently require a medical liability or malpractice case be heard by a screening panel before trial. 1
Statute of Limitations
The statute of limitations defines the time frame within which a plaintiff must file a lawsuit, often one to three years from the date of the alleged injury. The terms of the statutes vary by state and may be different for adults, minors and adults who are not mentally competent. If your attorney believes the statute of limitations has expired, your attorney will file a motion with the court to dismiss the lawsuit.
For the statute of limitations in your state, contact your local medical society or professional liability insurance carrier.
Never Alter a Medical Record
Read More, Learn More
Learn more about the litigation process with these articles from The Doctors Company.
Click here to read about tips for health care professionals to cope with legislation.
Click here to read about key factors in a deposition.
Click here to read about strategies to help assist your defense.
Upon receiving notice that a malpractice suit is about to commence or has been filed, clinicians must ensure the safety and integrity of the patient's medical record. Any changes made to the record after learning of a lawsuit raise questions about the clinician's truthfulness, motives and the quality of the care. Many clinicians and defense counsel have been embarrassed during discovery proceedings to learn that an earlier copy of the record differs materially from the record provided after litigation commenced.
Forensic document experts are frequently called to testify that a paper record has been augmented or altered. In situations in which a clinician has an electronic health record (EHR), counsel will retain information technology experts to conduct a metadata audit. The audit provides a complete analysis of every keystroke (including additions, deletions and changes) and when the entries were made, by whom, and how long a document was open for review and revision. If experts discover that the record has been altered, it can also expose the practitioner to punitive damages and result in a licensing board investigation.
You've Been Served. Now What?
Don't panic! If you receive a summons and complaint (a lawsuit), notify your professional liability carrier. This type of legal document requires a formal response within a prescribed time limit. Failure to respond appropriately may jeopardize your defense or even possibly result in a default judgment against you. Your professional liability carrier will assign a defense attorney who specializes in medical malpractice litigation and will handle the case through resolution.
Pre-Trial Discovery
Attorneys for both parties engage in written and oral discovery to understand the nature and extent of the care provided, as well as the merits of the patient's allegations. During discovery, attorneys for the plaintiff and defense review all medical records and other relevant documents related to the case to fully evaluate the claim. Interrogatories and depositions are two important parts of the discovery process.
Interrogatories (Written Discovery) Interrogatories are written questions directed by one party to another party designed to further develop the facts or the legal and clinical foundation of a case. Interrogatories directed to health care professionals usually seek background information concerning the individual's education, training, professional experience and credentials.
Interrogatory responses are legally admissible in court, therefore it is imperative that you review your answers carefully with your defense attorney. Your attorney will assist you in preparing accurate and appropriate responses.
Depositions (Oral Discovery) A deposition is a discovery tool used in virtually all forms of civil, administrative and criminal litigation. It provides an opportunity for both parties to obtain material information, assist in developing strategies for trial, and formally preserve testimony for use later. Testimony obtained in a deposition frequently proves to be the single most important event of the pretrial process. It is almost always crucial to the outcome of a case.
Depositions are conducted under oath in a verbal question-and-answer format. They are always recorded, traditionally by a certified shorthand reporter, who then transcribes the exchanges into a verbatim document that the deponent is required to sign. With increasing regularity over the past decade, the testimony is also preserved by separate audio and video technologies.
Deposition Testimony In preparation for a deposition, your attorney will meet with you to explain the process, offer recommendations on demeanor and dress, provide valuable suggestions on pitfalls to avoid, and identify probable areas of questioning by the attorneys who will attend the deposition.
Your attorney will also advise you of the best approach to use in answering questions. Your responses should be brief, concise, and delivered in a calm and thoughtful manner. Avoid guessing when you are uncertain of the answer. It is preferable to respond, "I do not know" or "I do not recall."
During your deposition, your attorney may perceive that a question is ambiguous or subject to a legal objection. Allow your attorney to state the objection and consider the objection when formulating your answer. The objection may alert you to an ambiguity or hidden meaning that is not otherwise apparent. Your attorney can also instruct you to refrain from answering a question that the attorney believes is an effort to elicit information that is not legally discoverable.
Key factors to keep in mind:
- Tell the truth. Deponents must promise to "tell the truth, the whole truth, and nothing but the truth." Failure to comply with the oath may be considered perjury (often a felony) that is punishable and may result in fines, sanctions and even imprisonment.
- Answer only the question asked. Deponents should listen carefully, answer only the question asked and then stop talking. Volunteering extraneous information prolongs the proceeding and identifies potential new areas of inquiry that opposing counsel may not have previously considered. One classic tactic is for the attorney to pause, leaving dead air that tends to be uncomfortable and can lead the deponent to resume talking.
- Maintain respect. When providing deposition testimony, be well prepared in advance, appear on time and appropriately attired, always act professionally and courteously, stay focused, and respond to the questions directly and with respect.
Expert Witnesses The use of expert witnesses is critical to professional liability cases. Expert witnesses help to define the standard of care and determine if any deviations have occurred. Both the plaintiff and defendant retain experts – sometimes more than one – to provide opinions on issues of causation and damages. Considerations when retaining an expert witness include the expert's education, training and experience. It is also important to have an expert who is articulate and likely to be well received by a jury.
Dismissal, Settlement or Trial
The litigation process can be lengthy, typically lasting two to five years, and even longer in some jurisdictions. Be prepared for extended periods of inactivity. The legal process is inefficient and impossible to control. Flurries of activity are often followed by prolonged periods of inactivity. Depositions are often scheduled, canceled and rescheduled.
At some point, enough information will have been gathered during the pre-trial discovery process for an assessment to be made about whether the case is defensible through trial or settlement should be considered. These decisions will be made between you, your attorney and your insurance carrier.
It's possible, however, that the case may be dismissed during the discovery process if the plaintiff's attorney determines the case lacks the elements needed to recover damages. According to the National Practitioner Data Bank, between 80% and 90% of defensible claims are dismissed with no settlement. 2 Additionally, 96.9% of successful medical malpractice claims are settled out of court. 3 Thus, only a very small percentage of medical malpractice cases ever proceed to trial.
Take Care of Yourself
Participating in a lawsuit can be challenging, difficult and stressful. Remember you are not alone. Emotional reactions to litigation are normal and there are people and resources available to help.
- Continue to maintain a healthy lifestyle with proper diet and exercise.
- Share your feelings to help maintain positive psychological health.
- Seek professional assistance when feelings of anxiety and distress interfere with daily work and relationships.
- Stay engaged with the litigation process. Your attorney needs your expertise and partnership. Staying engaged also minimizes uncertainty and allows you to feel more in control.
- Know that you will get through this stressful time.
This article was authored by Richard F. Cahill, JD , vice president and associate general counsel, Debra Davidson, MJ, CPHRM, CPPS , senior patient safety risk manager, and Douglas McCullough, JD , vice president of claims, all with The Doctors Company, part of TDC Group, and Sunny Jhamnani, MD, FACC , partner at Tri-City Cardiology in Chandler, AZ.
The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each healthcare provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
- Morton H. Medical Liability/Malpractice ADR and Screening Panels Statutes. National Conference of State Legislatures. Updated Aug. 10, 2021. Available here .
- U. S. Department of Health & Human Services. National Practitioner Data Bank. Data Analysis Tool. Available here .
- Rubin JB, Bishop TF. Characteristics of paid malpractice claims settled in and out of court in the USA: a retrospective analysis. BMJ Open 2013;3:e002985. doi:10.1136/bmjopen-2013-002985
Keywords: Cardiology Magazine, ACC Publications, Malpractice, Liability, Legal, Medical Records, Health Personnel, Lawyers
You must be logged in to save to your library.

Editors’ Corner | Refining Our Practice, Improving Patient Outcomes
Cover Story | Acute Coronary Syndromes: New Perspectives, New Data
Feature | JACC Family Series: Effect of Biological vs. Chronological Aging on CV Diseases
New in Clinical Documents | New ACC/AHA Guideline Focuses on Evaluation and Management of People With HCM
New in Clinical Documents | Management of Lower Extremity PAD Focus of New ACC/AHA Guideline
Focus on Heart Failure | Heart Failure Highlights From ACC.24
On the Move | Health Care Innovation Section Looks Ahead
Innovating Health Care | ACC.24 Innovation Pitch Challenge
Quality Improvement For Institutions | Advancing Quality CV Care Around the Globe
Quality Improvement For Institutions | ACC Accreditation Services: Leading Quality Improvement
Prioritizing Health | Lowering BP Without Drugs
Heart of Health Policy | CV Management Deep Dive: Navigating Ongoing Population and Workforce Trends
Heart of Health Policy | FTC Finalizes Ban on Noncompetes
The Pulse of ACC | JACC , The Lancet Align to Advance CV Health; Reset Your ACC Password; Dyad Leadership; More
Number Check | ACC Anywhere: Personalized Lifelong Learning
JACC in a Flash | RALES, EMPHASIS-HF; 6MWT Identifies ATTR-CA in High-Risk Patients; More
Just One More | Advocating For Women’s Health on Capitol Hill
JACC Journals on ACC.org
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Diversity and Inclusion
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
Latest in Cardiology
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
Education and Meetings
- Online Learning Catalog
- Understanding MOC
- Products and Resources
- Image and Slide Gallery
- Certificates and Certifications
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- CardioSmart
- Accreditation Services
- Clinical Solutions
- Clinician Well-Being Portal
- Mobile and Web Apps
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
The association between blood eosinophils and clinical outcome of acute exacerbations of chronic obstructive pulmonary disease: A systematic review and meta-analysis
Affiliations.
- 1 Department of Nursing, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China.
- 2 Department of Emergency and Critical Care Medicine, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China.
- 3 Department of Emergency Medicine, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China.
- 4 Department of Pharmacy, Lianyungang Clinical College of Nanjing Medical University, China.
- 5 Department of Humanities and Management, Kangda College of Nanjing Medical University, Lianyungang, China.
- 6 Department of Pharmacy, Lianyungang Clinical College of Nanjing Medical University, China. Electronic address: [email protected].
- 7 Department of Emergency and Critical Care Medicine, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China. Electronic address: [email protected].
- PMID: 38104787
- DOI: 10.1016/j.rmed.2023.107501
Objectives: Studies have shown an association between eosinophilia and clinical outcomes in acute exacerbation of chronic obstructive pulmonary disease (AECOPD). However, contradictory findings exist. Our study aims to systematically evaluate the association between elevated peripheral blood eosinophils and clinical outcome of patients with AECOPD.
Methods: An electronic search was conducted for relevant studies published from database inception to February 28, 2023, on PubMed, EMBASE, Cochrane Library, and Web of Science. The analysis covered studies on the correlation between EOS AECOPD and mortality, hospital stay duration, readmission and hospitalization rates, and invasive mechanical ventilation. Where applicable, relative risk (RR) and weighted mean difference (WMD) were extracted, pooled, and assessed using meta-analysis. Sensitivity analysis was performed to explore the source of heterogeneity.
Results: Fifteen high-quality studies including 14 cohort studies and one case-control study were included in the meta-analysis. Compared with non-eosinophilic AECOPD patients, those with eosinophilic AECOPD had a lower risk of mortality (RR = 0.65, 95 % confidence interval [CI] 0.54, 0.77, P < 0.001), shorter length of hospital stay (WMD = -1.56, 95%CI -2.16, -0.96, P < 0.001), and higher readmission rate (RR = 1.07, 95%CI 1.01,1.13, P = 0.029). No difference was found concerning the rate of hospitalization and invasive mechanical ventilation between the two groups.
Conclusion: Individuals diagnosed with eosinophilic AECOPD had a reduced mortality rate, a truncated period of hospitalization, and an insubstantial increase in the probability of readmission relative to their non-eosinophilic AECOPD counterparts. The level of eosinophils in blood has been shown to serve as a potential predictive biomarker for AECOPD patients.
Keywords: Blood eosinophils; Chronic obstructive pulmonary disease; Observational studies.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Publication types
- Meta-Analysis
- Systematic Review
- Case-Control Studies
- Disease Progression
- Eosinophils*
- Hospitalization
- Pulmonary Disease, Chronic Obstructive* / diagnosis
- Systematic Review
- Open access
- Published: 29 May 2024
Risk factors of chronic postoperative pain after total knee arthroplasty: a systematic review
- Junfei Li 1 ,
- Tingyu Guan 1 ,
- Yue Zhai 1 &
- Yuxia Zhang 2
Journal of Orthopaedic Surgery and Research volume 19 , Article number: 320 ( 2024 ) Cite this article
213 Accesses
Metrics details
There is a lack of relevant studies to grade the evidence on the risk factors of chronic pain after total knee arthroplasty (TKA), and only quantitative methods are used for systematic evaluation. The review aimed to systematically identify risk factors of chronic postoperative pain following TKA and to evaluate the strength of the evidence underlying these correlations.
PubMed, Web of Science, Cochrane Library, Embase, and CINAHL databases were searched from initiation to September 2023. Cohort studies, case-control studies, and cross-sectional studies involving patients undergoing total knee replacement were included. A semi-quantitative approach was used to grade the strength of the evidence-based on the number of investigations, the quality of the studies, and the consistency of the associations reported by the studies.
Thirty-two articles involving 18,792 patients were included in the final systematic review. Ten variables were found to be strongly associated with postoperative pain, including Age, body mass index (BMI), comorbidities condition, preoperative pain, chronic widespread pain, preoperative adverse health beliefs, preoperative sleep disorders, central sensitization, preoperative anxiety, and preoperative function. Sixteen factors were identified as inconclusive evidence.
Conclusions
This systematic review clarifies which risk factors could be involved in future research on TKA pain management for surgeons and patients. It highlights those factors that have been controversial or weakly correlated, emphasizing the need for further high-quality studies to validate them. Most crucially, it can furnish clinicians with vital information regarding high-risk patients and their clinical attributes, thereby aiding in the development of preventive strategies to mitigate postoperative pain following TKA.
Trial registration
This systematic review has been registered on the PROSPERO platform (CRD42023444097).
Introduction
Total knee arthroplasty (TKA) is the most common surgical intervention for patients with end-stage osteoarthritis [ 1 ].Despite a positive outcome for most patients, a sizeable portion of individuals experience significant pain following TKA [ 2 ]. Previous studies showed that the percentage of patients with unfavorable long-term pain outcomes ranged 10% ∼ to 34% following knee replacement [ 3 ]. The International Association for the Study of Pain (IASP) defines chronic postoperative pain (CPSP) as pain that persists for more than 3 months after surgery, excluding other causes (e.g., infection, surgical failure, recurrence of malignancy, etc.) [ 4 ]. In addition to disruption of daily activities brought on by the pain itself, adverse or chronic pain outcomes following joint replacement are of great concern to orthopedic surgeons and their patients. Chronic postoperative pain is also associated with deterioration in physical, functional, and mental domains, which implies significant personal, social, and healthcare costs with the rising prevalence of knee replacement surgeries [ 5 ].
Understanding the risk factors affecting chronic postsurgical pain can help increase the clinical staff’s understanding of the field, which can help clinicians make better decisions and help patients reduce the risk of developing chronic pain. Previous pain guidelines have only recommended perioperative interventions without doing an integration of risk factors [ 6 ]. Earlier systematic reviews that applied quantitative measures to identify predictors of persistent pain after TKA, without considering the grading of evidence, may result in limited quality outcome [ 7 ].
Therefore, this study will conduct a systematic review and critical appraisal of the risk factors affecting chronic pain after TKA, and use the Newcastle-Ottawa Scale (NOS) and the Agency for Healthcare Research and Quality (AHRQ) checklist to quality rate the level of evidence in the included literature.
This article used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement to guide implementation and reporting [ 8 ].
Data sources and search strategy
Five databases were searched (PubMed, Web of Science, Cochrane Library, Embase, CINAHL) from the time of the foundation of the database to July 2023. All pertinent keyword variations were used, including both the Medical Subject Headings (Mesh) of various databases as well as the free-text versions of these terms. Reference lists of selected studies and reviews were searched to find additional publications on the subject. Detailed information about the search strategy is shown in Appendix 1 .
Study selection and eligibility criteria
Studies meeting the following criteria were included: (1) cohort studies or case-control or cross-sectional studies; (2) patients undergoing total knee arthroplasty who are aged above 18 years old; (3) the outcome was defined as postoperative pain following total knee arthroplasty and follow-up had to be at least three months; (4) outcomes were predicted using preoperative, intraoperative or postoperative conditions. If total hip arthroplasty (THA) and total knee arthroplasty (TKA) patients were both included in the study, only TKA data were extracted. The exclusion criteria were as follows: (1) publications written in languages other than English and Chinese, (2) studies with incomplete methodology and full text not available. In addition, given the large number of possible confounding variables, cohort studies that failed to use a multivariate approach to assess risk factors were excluded.
Screening and data extraction
The titles and abstracts of all preliminary identified studies were screened by two investigators (JL and TG) independently following the selection criteria. Any differences of opinion were settled by consensus or discussion with a third independent reviewer. If there were multiple publications available, the most recent data were taken. To gather pertinent data, a predesigned electronic data extraction form was used. If there were multiple publications available, the most recent data were taken. The following information was extracted: participant characteristics, risk factors, pain outcome measures, follow-up period, and study design.
Assessing the risk of bias
The risk of bias assessment was independently assessed by two authors (JL and TG) in each included study by using the Newcastle Ottawa Quality Assessment Scale (NOS) and the checklist recommended by the Agency for Healthcare Research and Quality (AHRQ) [ 9 ].
The Newcastle-Ottawa Scale (NOS) is an important tool that evaluates case-control and cohort studies. It is composed of three main sections, which include a total of eight items. These sections cover various aspects of the study, including the selection of the study population, comparability, and exposure/outcome evaluation. The NOS uses a semi-quantitative star system to rate the study’s quality, with a maximum score of nine stars. Studies were categorized as high quality (7–9 points), moderate quality (4–6 points), and low quality (0–3 points) [ 10 ]. To evaluate the quality of the cross-sectional studies, we utilized the checklist recommended by the Agency for Healthcare Research and Quality (AHRQ). The AHRQ Risk of Bias Evaluation Tool assesses the risk of bias in five domains, including selection bias, implementation bias, follow-up bias, detection bias, and reporting bias. If the answer was “no” or “unclear”, the score was 0. If the answer is “yes”, the score is 1. Articles are rated as low (0–3), moderate (4–7), or high quality (8–11) [ 11 ].
Data synthesis and analysis
Semi-quantitative methods were used to summarize the strength of evidence supporting the association between risk factors and chronic postoperative pain. The best evidence synthesis included variables that were examined using a multivariate approach in at least two studies and demonstrated a statistically significant association. Three criteria were used to quantitatively evaluate the evidence of risk factors for chronic pain following total knee replacement: (1) the number of studies evaluating the variables; (2) the standard of the scores for each variable under assessment; (3) the consistency of the relationship between the factors and chronic postoperative pain. When 75% of the studies evaluating the variable reported the same direction of association, associations were deemed consistent [ 12 ]. Variables analyzed using multivariate methods that yielded no association were also taken into account. The level of evidence on risk factors for postoperative chronic pain was categorized into the following four categories: (1) strong: consistent findings were found in ≥ 2 high-quality articles; (2) moderate: with consistent results between 1 high-quality article and ≥ 1 moderate quality article or ≥ 3 moderate or low-quality articles; (3) inconclusive: When observed associations are inconsistent or assessed in 1 high-quality, < 3 moderate-quality studies or only in low-quality studies; (4) no association: no significant association was found in the high-quality multivariate analysis, or at least 3 high-quality studies found no association in the univariate analysis.
Study identification
Database search returned 18,792 articles, and 7 relevant articles were obtained through supplements from other resources. A total of 17,526 articles were obtained after eliminating duplicates. 17,239 references were excluded from the initial screening by reading titles and abstracts, leaving 287 references for full-text review. Among the remaining articles, 105 did not cover the outcome of concern, 66 did not match the target population, the full text was not available for one study, and 61 were excluded for other reasons. Therefore, a total of 32 studies were included in the systematic evaluation including five cross-sectional studies, one case-control study, and 26 cohort studies. The flowchart and reasons for exclusion are delineated in Fig. 1 .

Flowchart of study selection
Study characteristics
A total of 32,645 patients who underwent primary total knee arthroplasty were enrolled in this study (see Table 1 ). The sample size ranged from 71 to 11,373. The commonly used outcome measurement instruments in the studies were the visual analog scale (VAS) (10 studies), Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain scale (8 studies), and the Numerical Rating Scale (NRS) (7 studies). Five studies included total knee arthroplasty and total hip arthroplasty from which we extracted data for TKA. Study follow-up lasted a minimum of 3 months and a maximum of 10 years. Furthermore, 29 predictive factors associated with the development of postoperative chronic pain after TKA were identified.
Methodologic quality of included reviews
The research primarily focused on high or medium-quality literature, with no low-quality literature included in the analysis. The quality of cohort studies was evaluated using the NOS scale, with ratings ranging from moderate (four) to high (nine). The case-control study received a score of six out of nine on the NOS scale, indicating a moderate level of evidence. Five cross-sectional studies were assessed for quality using AHRQ, with three receiving a high-quality rating and two receiving a moderate rating. The scores for these studies ranged from 6 to 11. In studies rated as moderate quality, the most frequent reasons were attributed to the presence of confounding and measurement bias. Nine cohort studies have not reported or controlled for confounders, which may have led to an elevated risk of confounding bias. Furthermore, four cross-sectional studies exhibited indications of measurement bias, and the handling of missing data were not disclosed in the publication. The quality evaluation of the included studies according to the NOS and AHRQ checklist are shown in Appendix 2 .
The level of evidence for risk factors
Twenty-nine risk factors associated with the incidence of postoperative chronic pain were identified. The results of the best evidence analysis are presented in Table 2 . Upon conducting the study, it was found that ten variables exhibited a significant association with the onset of chronic pain following total knee arthroplasty (TKA). Age, body mass index (BMI), and comorbidities condition were discovered to possess strong evidence among demographic variables. As for preoperative factors, strong evidence was observed for preoperative pain, chronic widespread pain, preoperative adverse health beliefs, preoperative sleep disorders, central sensitization, preoperative anxiety, and preoperative function. No risk factors were strongly associated with the development of chronic pain among intraoperative and postoperative factors. Additionally, three factors were found to have a moderate association with outcome variables, namely gender, preoperative depression, and pain trajectory. At length, sixteen risk factors were identified as inconclusive, with the majority of them being statistically linked to chronic pain after TKA in just one study.
A total of 32 studies were included in our review, with a focus on case-control, cohort, and cross-sectional studies, and the grade of evidence in the literature was evaluated using the NOS scale, a quality assessment tool for cohort/case-control studies, and the AHRQ, a quality assessment tool for cross-sectional studies. Overall, the quality level of literature included in this study was high, and the reason for articles with a moderate level of evidence rating was the presence of potential confounding bias or measurement bias in the study. Twenty-nine risk factors connected with the development of chronic postoperative pain were identified, among which ten exhibited a strong correlation, three showed a moderate correlation, and sixteen factors yielded inconclusive results.
We employed a semi-quantitative approach to evaluate the level of evidence for risk factors and, in contrast to previous studies, identified two novel factors that exhibit a strong association with chronic pain following knee replacement surgery: preoperative sleep disturbances and preoperative poor health beliefs.
According to recent research that utilized machine learning and a large sample size, it has been determined that sleep problems can have a significant impact on chronic pain [ 13 ]. When we sleep, our body’s natural pain relief system is activated, and any disruptions to this system due to sleep deprivation or disturbances can negatively affect it [ 14 ]. A study was conducted to delve deeper into the relationship between sleep quality before total knee arthroplasty surgery and postoperative chronic pain syndrome (CPSP) [ 15 ]. The findings revealed that individuals who experienced sleep problems before the surgery were more likely to report higher pain scores three months after the procedure. This highlights the importance of addressing any pre-existing sleep issues before undergoing surgery to minimize the risk of postoperative chronic pain.
Health beliefs are thoughts, attitudes, or expectations that influence the experience of health and illness and related behaviors. Predictors such as illness perception, pain catastrophizing, preoperative expectations, and coping attitudes were grouped into the category of preoperative health beliefs in our article. Seven high-quality articles and one moderately quality article have demonstrated a statistically significant correlation between preoperative negative health beliefs and chronic postoperative pain [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ]. Research has shown that patients who experience greater levels of preoperative pain catastrophizing are more likely to suffer from moderate to severe pain after surgery. A study conducted by Giusti et al. has revealed that behavioral outcomes can forecast pain and functional outcomes up to 12 months after surgery [ 24 ]. Additionally, the study suggests that these outcomes partially mediate the relationship between catastrophizing and subsequent pain and function. Furthermore, a cohort study has identified the existence of psychological risk factors that may hinder the implementation of proper pain coping strategies and lead to the development of chronic postoperative pain.
Our review identified sixteen factors with insufficient evidence, as they were only statistically associated with CPSP in one study upon critical appraisal and lacked support from other literature. This highlights the necessity for further validation of these under-evidenced factors in future studies, specifically investigating their association with chronic pain. Moreover, it is crucial to prioritize factors backed by robust evidence and develop interventional clinical protocols based on these high-risk factors to provide comprehensive guidance to clinicians and nurses.
Limitations
This study has several limitations. In this systematic review, we only included patients with primary TKA and excluded those undergoing revision surgery and uni-compartmental arthroplasty; therefore, our findings may not extrapolate to other types of patients.
One of the major challenges in our study was the heterogeneity in the design of the included studies. We also found variations in the outcome indicators and measurement techniques used, which might account for the discrepancies in the results and hinder the integration of these findings.
Furthermore, we observed that some of the studies analyzed in this review did not adjust for potential confounders in their analyses. Confounding could have contributed to bias in our findings to some extent. Therefore, we recommend that future studies should put these factors into consideration when analyzing their results.
Clinical implications
This systematic review can inspire future personalized pain prevention and management measures. Enhanced monitoring of patient-reported pain before and early after surgery may lead to early detection and potential early intervention of patients at risk for CPSP. Early identification and targeted treatment of pain may reduce pain and prevent long-term disability. Improving awareness of the importance of biological, sociocultural, psychological, physical, and clinical factors will help to implement the role of interventions better.
This systematic review aims to assess the risk factors that contribute to the emergence of chronic pain after total knee arthroplasty. It further endeavors to appraise the evidence supporting these factors quantitatively. This analysis strives to enlighten surgeons and patients alike on potential risk factors that deserve exploration in future TKA pain management research, particularly those that have generated controversy or displayed weak correlations. Importantly, it underscores the necessity for additional high-quality studies to confirm these factors, thereby equipping clinicians with crucial knowledge regarding high-risk patients and their clinical characteristics. In turn, this knowledge contributes to the formulation of effective preventive measures aimed at reducing postoperative pain following TKA.
Data availability
No datasets were generated or analysed during the current study.
Hamilton D, Henderson GR, Gaston P, MacDonald D, Howie C, Simpson AH. Comparative outcomes of total hip and knee arthroplasty: a prospective cohort study. Postgrad Med J. 2012;88(1045):627–31.
Article PubMed Google Scholar
Fuzier R, Rousset J, Bataille B, Salces-y-Nédéo A, Maguès JP. One half of patients reports persistent pain three months after orthopaedic surgery. Anaesth Crit Care Pain Med. 2015;34(3):159–64.
Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435.
Article PubMed PubMed Central Google Scholar
Schug SA, Lavand’homme P, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52.
Kim DH, Pearson-Chauhan KM, McCarthy RJ, Buvanendran A. Predictive factors for developing chronic Pain after total knee arthroplasty. J Arthroplasty. 2018;33(11):3372–8.
Wainwright TW, Gill M, McDonald DA, Middleton RG, Reed M, Sahota O, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS(®)) Society recommendations. Acta Orthop. 2020;91(1):3–19.
Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114(4):551–61.
Article CAS PubMed Google Scholar
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Martínez-González L, Fernández-Villa T, Molina AJ, Delgado-Rodríguez M, Martín V. Incidence of Anorexia Nervosa in women: a systematic review and Meta-analysis. Int J Environ Res Public Health. 2020;17(11).
Liu L, Cai XC, Sun XY, Zhou YQ, Jin MZ, Wang J, et al. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: current evidence. J Eur Acad Dermatol Venereol. 2022;36(11):1969–79.
Gosselt AN, Slooter AJ, Boere PR, Zaal IJ. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. 2015;19(1):346.
Miettinen T, Mäntyselkä P, Hagelberg N, Mustola S, Kalso E, Lötsch J. Machine learning suggests sleep as a core factor in chronic pain. Pain. 2021;162(1):109–23.
Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–16.
Luo ZY, Li LL, Wang D, Wang HY, Pei FX, Zhou ZK. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res. 2019;14(1):378.
Yan Z, Liu M, Wang X, Wang J, Wang Z, Liu J, et al. Construction and Validation of Machine Learning Algorithms to Predict Chronic Post-surgical Pain among patients undergoing total knee arthroplasty. Pain Manag Nurs; 2023.
Lindberg MF, Miaskowski C, Rustøen T, Cooper BA, Aamodt A, Lerdal A. Preoperative risk factors associated with chronic pain profiles following total knee arthroplasty. Eur J Pain. 2021;25(3):680–92.
Shim J, McLernon DJ, Hamilton D, Simpson HA, Beasley M, Macfarlane GJ. Development of a clinical risk score for pain and function following total knee arthroplasty: results from the TRIO study. Rheumatol Adv Pract. 2018;2(2):rky021.
Rice DA, Kluger MT, McNair PJ, Lewis GN, Somogyi AA, Borotkanics R, et al. Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors. Br J Anaesth. 2018;121(4):804–12.
Yakobov E, Scott W, Stanish W, Dunbar M, Richardson G, Sullivan M. The role of perceived injustice in the prediction of pain and function after total knee arthroplasty. Pain. 2014;155(10):2040–6.
Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain. 2011;152(10):2287–93.
Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468(3):798–806.
Larsen DB, Laursen M, Edwards RR, Simonsen O, Arendt-Nielsen L, Petersen KK. The combination of Preoperative Pain, conditioned Pain Modulation, and Pain Catastrophizing predicts Postoperative Pain 12 months after total knee arthroplasty. Pain Med. 2021;22(7):1583–90.
Giusti EM, Manna C, Varallo G, Cattivelli R, Manzoni GM, Gabrielli S et al. The predictive role of executive functions and psychological factors on Chronic Pain after Orthopaedic surgery: a longitudinal cohort study. Brain Sci. 2020;10(10).
Download references
This study was supported by the National Key R&D Programmes (NKPs) subproject of China, Grant Numbered: No.2020YFC2008404-3.
Author information
Authors and affiliations.
Fudan University, Shanghai, China
Junfei Li, Tingyu Guan & Yue Zhai
Zhongshan Hospital, Shanghai, China
Yuxia Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: Junfei Li, Yuxia Zhang. Data acquisition analysis, or interpretation: Junfei Li, Tingyu Guan. Quality assessment: Junfei Li, Tingyu Guan. Manuscript preparation: Junfei Li. Critical revision of the manuscript: Yue Zhai, Yuxia Zhang. Study supervision and obtained funding: Yuxia Zhang. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Yuxia Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, J., Guan, T., Zhai, Y. et al. Risk factors of chronic postoperative pain after total knee arthroplasty: a systematic review. J Orthop Surg Res 19 , 320 (2024). https://doi.org/10.1186/s13018-024-04778-w
Download citation
Received : 03 April 2024
Accepted : 02 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s13018-024-04778-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic pain
- Pain, postoperative
- Arthroplasty, replacement, knee
- Risk factor
Journal of Orthopaedic Surgery and Research
ISSN: 1749-799X
- Submission enquiries: [email protected]
- Open access
- Published: 30 May 2024
Exploring the tradeoff between data privacy and utility with a clinical data analysis use case
- Eunyoung Im 1 , 2 ,
- Hyeoneui Kim 1 , 2 , 3 ,
- Hyungbok Lee 1 , 5 ,
- Xiaoqian Jiang 4 &
- Ju Han Kim 5 , 6
BMC Medical Informatics and Decision Making volume 24 , Article number: 147 ( 2024 ) Cite this article
83 Accesses
Metrics details
Securing adequate data privacy is critical for the productive utilization of data. De-identification, involving masking or replacing specific values in a dataset, could damage the dataset’s utility. However, finding a reasonable balance between data privacy and utility is not straightforward. Nonetheless, few studies investigated how data de-identification efforts affect data analysis results. This study aimed to demonstrate the effect of different de-identification methods on a dataset’s utility with a clinical analytic use case and assess the feasibility of finding a workable tradeoff between data privacy and utility.
Predictive modeling of emergency department length of stay was used as a data analysis use case. A logistic regression model was developed with 1155 patient cases extracted from a clinical data warehouse of an academic medical center located in Seoul, South Korea. Nineteen de-identified datasets were generated based on various de-identification configurations using ARX, an open-source software for anonymizing sensitive personal data. The variable distributions and prediction results were compared between the de-identified datasets and the original dataset. We examined the association between data privacy and utility to determine whether it is feasible to identify a viable tradeoff between the two.
All 19 de-identification scenarios significantly decreased re-identification risk. Nevertheless, the de-identification processes resulted in record suppression and complete masking of variables used as predictors, thereby compromising dataset utility. A significant correlation was observed only between the re-identification reduction rates and the ARX utility scores.
Conclusions
As the importance of health data analysis increases, so does the need for effective privacy protection methods. While existing guidelines provide a basis for de-identifying datasets, achieving a balance between high privacy and utility is a complex task that requires understanding the data’s intended use and involving input from data users. This approach could help find a suitable compromise between data privacy and utility.
Peer Review reports
Clinical data gathered through Electronic Health Records (EHR) is an invaluable asset for producing meaningful insights into patient care and healthcare service management. However, as this data includes sensitive personal information, there is a heightened risk of financial or social damage to individuals if their health data is improperly disclosed [ 1 , 2 ]. To address these concerns, many countries have implemented stringent regulations to safeguard patient privacy while still enabling the efficient use of data for health advancements [ 3 ]. In the United States, for example, the Health Insurance Portability and Accountability Act (HIPAA) sets forth provisions for data protection and usage [ 4 ]. Similarly, the General Data Protection Regulation (GDPR) offers a comprehensive data privacy framework within the European Union [ 5 ]. Additionally, South Korea’s Personal Information Protection Act delineates the guidelines for secure and permissible data handling [ 6 ].
The growing imperative for data privacy has spurred significant progress in privacy-preserving technologies. Differential Privacy (DP) safeguards data by integrating controlled random noise, thus ensuring individual data points remain confidential while aggregate analysis remains accurate [ 7 ]. In the biomedical field, DP is extensively employed in data query systems; the noise integrated into query responses helps protect sensitive inquiries pertaining to uncommon cases [ 8 , 9 ]. Current research in DP focuses on solving complex problems such as determining optimal privacy budgets and noise levels to balance confidentiality with data utility [ 8 , 10 , 11 ].
Homomorphic Encryption (HE) represents a breakthrough in cryptography for preserving privacy, enabling computations on encrypted data without altering the original values [ 12 ]. Recent research has validated the practicality of performing data analysis using HE [ 13 , 14 , 15 ]. Nonetheless, HE has not become mainstream in healthcare applications, primarily due to its substantial computational demands, intricate implementation, and the limited range of analytics that can be performed on data in its encrypted form [ 12 , 16 ].
Blockchain technology, recognized for its immutable, decentralized, and transparent nature [ 17 ], is gaining attention as an innovative approach for data privacy [ 18 , 19 , 20 ]. Despite this interest, the real-world application of blockchain is contingent upon enhancements in its capacity to process substantial data volumes, simplification of its implementation, and resolution of related regulatory challenges [ 21 , 22 , 23 , 24 ].
When preparing datasets with personal health information for secondary analysis, the prevailing practice is to mitigate the risk of re-identification of the subjects in the dataset by employing stringent de-identification procedures [ 25 , 26 ]. This involves the removal of direct identifiers that can uniquely pinpoint individual subjects within the dataset and altering quasi-identifiers, which alone do not identify subjects but could do so when merged with other data sources. Furthermore, the process considers sensitive information that, despite not directly identifying subjects, could have detrimental effects if disclosed, ensuring such data is also considered during the de-identification process.
The leading method for data de-identification employs strategies like K-anonymity, L-diversity, and T-closeness to modify data. K-anonymity safeguards against linkage attacks by ensuring that there are at least K identical records for any set of quasi-identifiers within a dataset, making it impossible to distinguish one individual from K-1 others [ 27 ]. In line with this, South Korea’s data publishing guidelines recommend adhering to a minimum of ‘K = 3’ for K-anonymity [ 28 , 29 ]. Additionally, L-diversity mandates a sensitive variable must have at least L distinct values, thereby offering protection against homogeneity attacks [ 30 ]. T-closeness, on the other hand, ensures that the distribution of a sensitive variable within any subset of the dataset closely approximates the distribution of that variable of the entire dataset, adhering to a specified threshold [ 31 ]. T-closeness prevents the likelihood that knowledge of the variable’s distribution could be exploited to reveal an individual’s identity [ 31 ]. The process of de-identification, which often involves masking or altering certain data values, can result in information loss and potentially reduce the utility of the dataset [ 32 ].
Determining the optimal threshold between data privacy and utility remains a complex challenge. Several studies have investigated how various de-identification strategies, specifically K-anonymity, L-diversity, and T-closeness, influence data utility. This is typically assessed by comparing the analytical results of de-identified datasets with those derived from the original dataset. Some researchers advocate that the privacy enhancements are overshadowed by a substantial reduction in data utility [ 33 , 34 ], while others argue that such utility loss might not be as severe as some studies imply [ 35 ]. However, these studies evaluated each de-identification technique in isolation, often resorting to simplified models that fail to fully capture the complexities of real-world data use, and led to mixed conclusions [ 34 , 35 ].
Moreover, the insights offered by such research into the tangible effects of data de-identification on actual data analysis tasks are somewhat restricted. This is because the analyses were either performed using overly simplistic examples [ 28 , 34 ] or on public datasets that have already undergone some form of de-identification [ 35 , 36 ], or focusing on theoretical aspects [ 37 ]. Therefore, there is a need for more intricate research that closely mirrors the complexities of real-life data analytics tasks and considers the multifaceted nature of data utility and privacy in actual applications.
This study explores the effects of different de-identification strategies on clinical datasets prepared for secondary analysis, with a focus on their implications for practical data analysis tasks. The aims of this study are twofold: firstly, to assess the effects of de-identification on both the dataset’s integrity and the outcomes of data analyses; and secondly, to ascertain if discernible trends emerge from the application of various de-identification techniques that could guide the establishment of a feasible balance between data privacy and data utility.
Data analysis use case
This study explores the impact of various de-identification techniques on datasets and their subsequent analysis results using a data analytic use case. The analytic use case involved predicting the Length of Stay (LOS) of high-acuity patients transferred to the emergency department (ED) of an academic medical center located in Seoul, South Korea. LOS in the ED serves as a crucial quality metric for ED services [ 38 , 39 , 40 ]. In Korea, an ED LOS under six hours is considered optimal [ 41 ]. Nonetheless, the overcrowding issues prevalent in tertiary hospital EDs elevate the risk of prolonged ED stays for patients transferred from other facilities for specialized care [ 42 , 43 ]. Understanding the factors affecting the ED LOS of transferred high-acuity patients is essential to providing timely care. The authors, HK and HL, previously developed a model to predict ED LOS using logistic regression, Random Forest, and Naïve Bayes techniques [ 44 ]. Building on insights from this earlier research, the current use case was crafted to develop a logistic regression model to predict ED LOS based on variables including the patient’s sex, age, medical conditions, the type and location of the transferring hospital, and the treatment outcomes.
The prediction model for ED LOS was developed using data from 1,155 patients who were transferred to the study site’s ED between January 2019 and December 2019. Patient demographics, clinical details, and transfer-related information were extracted from the study site’s Clinical Data Warehouse (CDW). The variables collected for this study are listed in Table 1 .
De-identification of the datasets
Developing de-identification scenarios.
Identifiers such as patient names and medical record numbers were removed. Quasi-identifiers play a critical role in de-identification as they form the foundation for assessing the adequacy of de-identification efforts and undergo most data transformations. To select the variables to test as quasi-identifiers, we first examined the extent to which each variable could uniquely link to individual subjects within the dataset, potentially identifying them. Table 2 displays the percentage of subjects in the dataset uniquely linked to either a single variable or a combination of variables. For instance, the sending hospital and primary diagnosis were uniquely linked to 27.71% and 17.75% of the subjects, respectively, and their combination linked up to 94% of the subjects. Consequently, information regarding the sending hospital and the primary diagnosis , coded using the International Classification of Disease (ICD) [ 45 ], were utilized as quasi-identifiers, along with sex and age , which are commonly considered quasi-identifiers in various de-identification efforts [ 4 , 46 ]. Treatment outcomes were identified as sensitive information. We developed 19 de-identification scenarios by varying the quasi-identifiers and sensitive information, and applying diverse configurations of privacy-preserving techniques such as K-anonymity, L-diversity, and T-closeness to each scenario.
Data transformation for de-identification
De-identification was performed using ARX, a publicly accessible and well-validated data anonymization tool that supports various de-identification methods [ 47 , 48 , 49 ]. We employed generalization and micro-aggregation techniques to modify the quasi-identifiers, both aimed at reducing the risk of re-identification by transforming original data into more general values. Generalization involves building a hierarchy for the given values by specifying minimum and maximum generalization levels. Generalization involves creating a hierarchy of values by specifying minimum and maximum levels, which can be adjusted based on criteria such as the number of digits masked in zip codes, size of intervals for age , condensation of 5-point Likert scores to 3-point scales, and generalization of full dates to broader time units such as week, month, or year [ 50 ]. Micro-aggregation, on the other hand, assigns representative values for alphanumeric data, such as using the mode for sex and the mean for age [ 50 ].
In our de-identification process, quasi-identifiers such as the sending hospital and primary diagnosis were transformed using generalization, while sex was modified through micro-aggregation. Age was subjected to both generalization and micro-aggregation. The generalization hierarchy for age included three levels with intervals of 5, 10, and 30 years respectively. For micro-aggregation, mean age values were used. The primary diagnosis was generalized into two levels based on higher-level ICD codes. For instance, a primary diagnosis with the ICD code I20.0, representing unstable angina , was generalized to I20 (i.e., angina pectoris ) at level 1, and further to I20-I25 (i.e., ischemic heart diseases ) at level 2. Generalization of the sending hospital also included two levels, where a specific facility such as “Hanmaeum Clinic in Jongno-gu, Seoul city” was generalized to the county level as “facility in Jongno-gu” at level 1 and then to the city level as “facility in Seoul” at level 2. For sex , micro-aggregation was employed, setting the mode as the representative value.
K-anonymity, L-diversity, and T-closeness were employed concurrently with specific parameters set for each: K and L were both set at 3, and T was set at 0.5. K-anonymity was specifically applied to quasi-identifiers to ensure that each individual is indistinguishable from at least two others. L-diversity and T-closeness, on the other hand, were applied to the variable designated as sensitive, ensuring that sensitive information is both sufficiently diverse and closely aligned with the overall distribution of the dataset. Table 3 details these 19 de-identification scenarios.
Data transformation was carried out in ARX according to the de-identification scenarios outlined in Table 3 . ARX provides options to adjust additional transformation parameters: the suppression limit , which sets the maximum proportion of records that can be omitted from the original dataset; approximation , which prioritizes solutions with shorter execution times; and precomputation , which determines the threshold for the fraction of unique data values in the dataset [ 50 ]. For this study, we utilized the default settings in ARX, where the suppression limit was set to 100%, and both approximation and precomputation features were disabled.
During execution, ARX evaluated various combinations of generalization and micro-aggregation levels to meet the requirements for K-anonymity, L-diversity, and T-closeness, ultimately recommending an optimal solution based on the balance between minimizing re-identification risk and preserving data utility. Figure 1 displays a screenshot of the data transformation solutions for the scenario where age , primary diagnosis , and sending hospital were designated as quasi-identifiers. Ultimately, we produced 19 versions of de-identified datasets, each based on the transformation solution that ARX identified as optimal.
The data transformation solutions suggested by ARX
Examination of the de-identified datasets
We reviewed the reduction in re-identification risk and the data utility scores that ARX estimated for the 19 de-identified datasets. To assess the similarity between each de-identified dataset and the original dataset, we employed Earth Mover’s Distance (EMD) [ 51 ]. Additionally, we calculated the dataset retention ratio. This metric is derived by dividing the number of data points in the transformed dataset by the number of data points in the original dataset. EMD and dataset retention ratio quantitatively evaluate the dissimilarity between the original dataset and the de-identified datasets, offering insights into how much the data has been altered through de-identification.
Testing the effects of de-identification on ED LOS prediction
Variable creation for predictive modeling.
To construct a logistic regression model for predicting ED LOS, we defined outcome and predictor variables. ED LOS, the outcome variable, was dichotomized into two categories: 6 h or less, and more than 6 h. We identified 13 predictors, including patient sex, age, medical conditions, treatment outcome, and the sending hospital type. Age , sending hospital location , and treatment outcome were dichotomized. Five dummy variables were created from primary diagnosis to represent high priority disease , neoplastic disease , circulatory disease , respiratory disease , and injury-related visits . The sending hospital type was derived from the sending hospital information . These variables, detailed in Table 4 , were consistently defined across all 19 de-identified datasets as well as the original dataset to facilitate comparative analyses.
Data analysis
After defining the outcome and predictor variables for logistic regression, we examined their distributions across the 19 de-identified datasets and the original dataset. To assess the differences in variable distributions, we utilized the proportion test [ 52 ]. Subsequently, logistic regression analysis was conducted using both the de-identified and original dataset. The predictive performance of these models was evaluated using the Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curve. We compared the AUC scores (AUROC) of the logistic regression models derived from the 19 de-identified datasets to that from the original dataset, employing the DeLong test [ 53 ]. Additionally, we analyzed the differences in the odds ratios of the predictors and their statistical significance to assess any impact the de-identification process might have had on the predictive capability of the models. All analyses were performed using R (version 4.0.4) [ 54 ].
Data transformation configurations applied for the de-identification of the datasets
Table 5 displays the optimal configurations for data transformation used in the 19 de-identified datasets. Variables subjected to generalization or micro-aggregation were designated as quasi-identifiers. Sensitive information is identified as ‘SI’ within the table. It is important to note that empty cells signify that the corresponding variable was treated as non-sensitive information in the specific dataset.
The de-identified datasets
Table 6 displays the re-identification reduction rates, ARX utility scores, EMD scores, and dataset retention ratios for the 19 transformed datasets. Additionally, the table presents the number of records retained post-transformation and the number of predictor variables generated. The ARX utility score reflects the extent of information loss, with a higher score indicating lower utility. It is important to note that the baseline re-identification risk varied among the datasets due to differences in the configuration of quasi-identifiers.
Overall, all 19 de-identification scenarios significantly reduced re-identification risk. However, the data transformation processes involved in de-identification led to record suppression and complete masking of variables used as predictors, thereby compromising dataset utility. Notably, except for three datasets (13, 15, 16), which used only sex and age as quasi-identifiers, there was a loss of one or more predictor variables. Datasets 13, 15, and 16 demonstrated the highest retention ratios and the lowest ARX utility and EMD scores, indicating minimal information loss and the highest similarity to the original dataset, thus reflecting superior dataset utility. They also exhibited the lowest baseline and post-transformation re-identification risks.
Datasets 7 and 8 underwent a transformation under the most complex de-identification scenarios, employing three quasi-identifiers and applying both L-diversity and T-closeness to two sensitive variables. Although these datasets achieved complete re-identification risk reduction, the extensive data transformation allowed only seven predictor variables to be generated. The de-identification scenarios 1 and 3, 2 and 4, and 13, 15, and 16 shared identical configurations of quasi-identifiers but varied in the L-diversity and T-closeness conditions applied to sensitive information, resulting in identical de-identified datasets (see Table 3 ).
Table 7 details the differences in variable distribution between each transformed dataset and the original dataset. As expected, variables designated as quasi-identifiers underwent the most transformation, leading to significant changes. Variables derived from these quasi-identifiers, such as sending hospital type, circulatory disease , and high priority disease , also exhibited notable distributional changes.
The prediction results
Logistic regression models were developed using both the original dataset and 19 de-identified datasets. The complete masking of variables classified as quasi-identifiers in some de-identified datasets resulted in differences in the number and types of predictors available for constructing the logistic regression models. Additionally, the number of records included in the regression analysis varied due to record suppression associated with the de-identification process. Figure 2 illustrates the ROC curves and the AUC values for all 20 datasets. The AUC values ranged from 0.695 to 0.787. The models generated from datasets 7 and 8, which only retained seven predictors due to extensive data masking, exhibited a statistically significant difference in AUC when compared to the original dataset, with a p-value of 0.002. For the models derived from the other datasets, no significant differences in AUC values were observed.
The number of records and predictors included in each model and the model performance
Figure 3 displays the Odds Ratios (OR) for predictors from selected datasets. Datasets 13, 15, and 16 were chosen because they retained all 13 predictor variables (Fig. 3 (a)). Dataset 9 was selected for having the next highest number of predictors ( N = 12) and for utilizing three quasi-identifiers: the sending hospital , which is identified as the most revealing variable in Table 2 , along with sex and age , which are commonly used as quasi-identifiers (Fig. 3 (b)). Dataset 19 was also included because it was configured using only the sending hospital and primary diagnosis as quasi-identifiers (Fig. 3 (c)). The ORs for all 19 datasets are detailed in Additional file 1: Figure S1 .
As depicted in Fig. 3 (a), the original dataset and de-identified datasets 13, 15, and 16 showed comparable prediction outcomes, with sex being the only predictor that displayed an OR notably different from the original dataset; however, it was not statistically significant in either model. Figure 3 (b) indicates that the ORs of the 12 predictors in dataset 9 were similar to those in the original dataset, although the OR for injury-related visits became insignificant. In contrast, dataset 19, which excluded two predictors, showed more pronounced differences in the ORs of the 11 remaining predictors (Fig. 3 (c)). Additionally, neoplastic disease and respiratory disease , significant predictors in the original dataset, became insignificant in dataset 9, while injury-related visits , previously insignificant, became significant (Fig. 3 (c)).
The Odds-Ratios of the predictors from the original dataset and the selected de-identified datasets
Data utility vs. data privacy
Figure 4 presents the correlations between re-identification risk reduction rates, ARX utility scores, EMD, and dataset retention ratios. There is a significant correlation between the re-identification reduction rate and the ARX utility score, indicating that greater reductions in re-identification risk are typically accompanied by larger losses of information. Conversely, the re-identification reduction rate exhibits a slight negative correlation with both EMD and dataset retention ratio; however, these correlations are not statistically significant.
The correlations between re-identification risk reduction and features of the de-identified datasets
This study tested various de-identification strategies on a clinical dataset, adjusting the number and types of quasi-identifiers and sensitive information, and configuring K-anonymity, L-diversity, and T-closeness in diverse ways. It aimed to address gaps left by earlier studies that utilized simplistic data use cases and de-identification configurations [ 28 , 34 , 35 ].
The results indicated that de-identification led to the suppression of records and variables, precluding the replication of analyses performed on the original dataset. Consequently, logistic regression models for predicting ED LOS yielded differing conclusions based on the de-identification approach, as illustrated in Fig. 3 . This highlights the need for the evolution of privacy technologies that maintain data integrity. Additionally, it cautions data users about potential biases introduced when working with de-identified datasets.
The study found optimal data utility when only sex and age were classified as quasi-identifiers, maintaining all variables and losing only six records. This configuration also significantly reduced the baseline re-identification risk, albeit sex and age by themselves did not strongly individualize records. However, this configuration did not account for the additional re-identification risk posed by the sending hospital and primary diagnosis , both of which were considered the most identifying variables in the dataset (Table 2 ). To eliminate any alterations to sex and age —key variables for clinical research—we examined the impact of designating only the sending hospital and primary diagnosis as quasi-identifiers (dataset 19). This strategy greatly reduced the chance of re-identification but at a considerable cost to data utility, resulting in the loss of over half the dataset and two predictor variables: the sending hospital type and high priority disease .
Seeking a compromise, datasets 5–12 incorporated sex , age , and either sending hospital or primary diagnosis as quasi-identifiers. In this series, datasets 7 and 8 achieved zero re-identification risk post-de-identification but sacrificed nearly half of the predictor variables. Datasets 11 and 12, while managing to retain all records, were considered less favorable due to the loss of four predictor variables. Datasets 5 and 6 struck a more acceptable balance, offering substantial re-identification risk reduction, retaining over 78% of records, and sacrificing only one predictor variable. Although dataset 5 had marginally better scores for risk reduction and data utility, dataset 6 was preferred because it retained information on high priority disease , a key predictor of ED LOS.
In this study, three different data utility metrics were examined, but only the ARX utility score exhibited a statistically significant correlation with the re-identification risk reduction rate. The EMD and dataset retention ratio both showed minor negative correlations with re-identification risk reduction; however, these were not statistically significant. This could suggest that the structural aspects of a dataset may not alone be adequate for assessing its utility, although further studies with a broader array of datasets would be required to substantiate this preliminary indication.
The scope of this research was limited to a single use case, analyzing data obtained from one hospital. Moreover, the range of de-identification scenarios tested did not encompass the full spectrum of complex configurations that could be employed. Despite these constraints, the research offers valuable insights into the nuanced interplay between data de-identification processes and data utility. It contributes to the ongoing conversation about how to approach data privacy in a way that still enables effective data usage.
As health data analysis grows more critical, so does the imperative to devise effective methods for ensuring data privacy. While established guidelines [ 47 ] offer a foundation for the de-identification of datasets, crafting a dataset that maintains a high level of privacy without unduly compromising its utility remains a nuanced challenge. It demands a thorough grasp of the data’s intended application. Incorporating input from data users during the de-identification process and considering the variety of potential data use cases could prove beneficial in finding a workable tradeoff between data privacy and utility.
Data availability
The clinical dataset used in this study is not made available due to the sensitive nature of clinical data. However, de-identified analytic datasets are available upon reasonable request from the corresponding author and with permission of Seoul National University Hospital.
Abbreviations
Area Under the receiver operating characteristic (ROC) Curve
Differential Privacy
Emergency Department
Earth Mover’s Distance
Homomorphic Encryption
International Classification of Disease
Length Of Stay
Receiver Operating Characteristic
Price WN, Cohen IG. Privacy in the age of medical big data. Nat Med. 2019;25(1):37–43.
Article CAS PubMed PubMed Central Google Scholar
Gostin LO, Halabi SF, Wilson K. Health data and privacy in the digital era. JAMA. 2018;320(3):233–4.
Article PubMed Google Scholar
Data Protection and Privacy Legislation Worldwide | UNCTAD. https://unctad.org/page/data-protection-and-privacy-legislation-worldwide . Accessed 6 Oct 2022.
Health and Human Services. Guidance Regarding Methods for De-identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html#coveredentities (2022). Accessed 28 Mar 2024.
General Data Protection Regulation (GDPR). Article 32 GDPR( https://gdprhub.eu/index.php?title=Article_32_GDPR (2023). Accessed 4 Apr 2024.
Personal Information Protection Commission. Pseudonymization Guidelines. Korea;2024.
Thapa C, Camtepe S. Precision health data: requirements, challenges and existing techniques for data security and privacy. Comput Biol Med. 2021;129:104130.
Cho H, Simmons S, Kim R, Berger B. Privacy-preserving biomedical database queries with optimal privacy-utility trade-offs. Cell Syst. 2020;10(5):408–16. e9.
Article CAS PubMed Google Scholar
Deldar F, Abadi M. Differentially private count queries over personalized-location trajectory databases. Data Brief. 2018;20:1510–4.
Article PubMed PubMed Central Google Scholar
Venkatesaramani R, Wan Z, Malin BA, Vorobeychik Y. Enabling tradeoffs in privacy and utility in genomic data beacons and summary statistics. Genome Res. 2023;33(7):1113–23.
PubMed PubMed Central Google Scholar
Xiong L, Post A, Jiang X, Ohno-Mochado L. New Methods to Protect Privacy When Using Patient Health Data to Compare Treatments. 2021.
Scheibner J, Raisaro JL, Troncoso-Pastoriza JR, Ienca M, Fellay J, Vayena E, et al. Revolutionizing medical data sharing using advanced privacy-enhancing technologies: technical, legal, and ethical synthesis. J Med Internet Res. 2021;23(2):e25120.
Bataa M, Song S, Park K, Kim M, Cheon JH, Kim S. Finding highly similar regions of genomic sequences through homomorphic encryption. J Comput Biol. 2024;31(3):197–212.
Kim D, Son Y, Kim D, Kim A, Hong S, Cheon JH. Privacy-preserving approximate GWAS computation based on homomorphic encryption. BMC Med Genom. 2020;13:1–12.
Article Google Scholar
Rovida L, Leporati A. Encrypted image classification with low memory footprint using fully homomorphic encryption. Cryptology ePrint Archive; 2024.
Acar A, Aksu H, Uluagac AS, Conti M. A survey on homomorphic encryption schemes: theory and implementation. ACM Comput Surv (Csur). 2018;51(4):1–35.
Kuo T-T, Kim H-E, Ohno-Machado L. Blockchain distributed ledger technologies for biomedical and health care applications. J Am Med Inform Assoc. 2017;24(6):1211–20.
Zhang F, Zhang Y, Ji S, Han Z. Secure and decentralized Federated Learning Framework with Non-IID Data based on Blockchain. Heliyon. 2024.
Wu C, Tang YM, Kuo WT, Yip HT, Chau KY. Healthcare 5.0: a secure and distributed network for system informatics in medical surgery. Int J Med Informatics. 2024:105415.
Ali A, Al-Rimy BAS, Tin TT, Altamimi SN, Qasem SN, Saeed F. Empowering Precision Medicine: Unlocking Revolutionary insights through Blockchain-enabled Federated Learning and Electronic Medical Records. Sensors. 2023;23(17):7476.
Chukwu E, Garg L. A systematic review of blockchain in healthcare: frameworks, prototypes, and implementations. Ieee Access. 2020;8:21196–214.
Fan C, Ghaemi S, Khazaei H, Musilek P. Performance evaluation of blockchain systems: a systematic survey. IEEE Access. 2020;8:126927–50.
Thantilage RD, Le-Khac N-A, Kechadi M-T. Healthcare data security and privacy in Data Warehouse architectures. Inf Med Unlocked. 2023:101270.
Tandon A, Dhir A, Islam AN, Mäntymäki M. Blockchain in healthcare: a systematic literature review, synthesizing framework and future research agenda. Comput Ind. 2020;122:103290.
Ahmed T, Aziz MMA, Mohammed N. De-identification of electronic health record using neural network. Sci Rep. 2020;10(1):18600.
Ahmed T, Aziz MMA, Mohammed N, Jiang X, editors. Privacy preserving neural networks for electronic health records de-identification. Proceedings of the 12th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics; 2021.
Sweeney L. Achieving k-anonymity privacy protection using generalization and suppression. Int J Uncertain Fuzziness Knowledge-Based Syst. 2002;10(05):571–88.
Jeon S, Seo J, Kim S, Lee J, Kim J-H, Sohn JW, et al. Proposal and assessment of a de-identification strategy to enhance anonymity of the observational medical outcomes partnership common data model (OMOP-CDM) in a public cloud-computing environment: anonymization of medical data using privacy models. J Med Internet Res. 2020;22(11):e19597.
Personal Information Protection Commission. uidelines for Personal Information De-identification Measures. 2016.
Machanavajjhala A, Kifer D, Gehrke J, Venkitasubramaniam M. l-diversity: privacy beyond k-anonymity. Acm Trans Knowl Discovery data (tkdd). 2007;1(1):3–es.
Li N, Li T, Venkatasubramanian S, editors. t-closeness: Privacy beyond k-anonymity and l-diversity. 2007 IEEE 23rd international conference on data engineering; 2006: IEEE.
Tomashchuk O, Van Landuyt D, Pletea D, Wuyts K, Joosen W, editors. A data utility-driven benchmark for de-identification methods. Trust, Privacy and Security in Digital Business: 16th International Conference, TrustBus 2019, Linz, Austria, August 26–29, 2019, Proceedings 16; 2019: Springer.
Brickell J, Shmatikov V, editors. The cost of privacy: destruction of data-mining utility in anonymized data publishing. Proceedings of the 14th ACM SIGKDD international conference on Knowledge discovery and data mining; 2008.
Wu L, He H, Zaïane OR, editors. Utility of privacy preservation for health data publishing. Proceedings of the 26th IEEE International Symposium on Computer-Based Medical Systems; 2013: IEEE.
Li T, Li N, editors. On the tradeoff between privacy and utility in data publishing. Proceedings of the 15th ACM SIGKDD international conference on Knowledge discovery and data mining; 2009.
Karagiannis S, Ntantogian C, Magkos E, Tsohou A, Ribeiro LL. Mastering data privacy: leveraging K-anonymity for robust health data sharing. Int J Inf Secur. 2024:1–13.
Zamani A, Oechtering TJ, Skoglund M. On the privacy-utility trade-off with and without direct access to the private data. IEEE Trans Inf Theory. 2023.
Baek S-M, Seo D-W, Kim Y-J, Jeong J, Kang H, Han KS, et al. Analysis of emergency department length of stay in patient with severe illness code. J Korean Soc Emerg Med. 2020;31(5):518–25.
Google Scholar
Laam LA, Wary AA, Strony RS, Fitzpatrick MH, Kraus CK. Quantifying the impact of patient boarding on emergency department length of stay: all admitted patients are negatively affected by boarding. J Am Coll Emerg Physicians Open. 2021;2(2):e12401.
Otto R, Blaschke S, Schirrmeister W, Drynda S, Walcher F, Greiner F. Length of stay as quality indicator in emergency departments: analysis of determinants in the German Emergency Department Data Registry (AKTIN registry). Intern Emerg Med. 2022;17(4):1199–209.
National Emergency Medical Center: Statistical yearbook of National Emergency Department Information System. https://www.e-gen.or.kr/nemc/statistics_annual_report.do?%20brdclscd=02 (2022). Accessed 7 Oct 2022.
Chang Y-H, Shih H-M, Chen C-Y, Chen W-K, Huang F-W, Muo C-H. Association of sudden in-hospital cardiac arrest with emergency department crowding. Resuscitation. 2019;138:106–9.
Kim J-s, Bae H-J, Sohn CH, Cho S-E, Hwang J, Kim WY, et al. Maximum emergency department overcrowding is correlated with occurrence of unexpected cardiac arrest. Crit Care. 2020;24:1–8.
Lee H, Lee S, Kim H. Factors affecting the length of stay in the emergency department for critically ill patients transferred to regional emergency medical center. Nurs Open. 2023;10(5):3220–31.
World Health Organization(WHO). International Statistical Classification of Diseases and Related Health Problems(ICD). https://www.who.int/standards/classifications/classification-of-diseases/1 (2019). Accessed 11 Oct, 2022.
Eicher J, Kuhn KA, Prasser F. An experimental comparison of quality models for health data de-identification. MEDINFO 2017: Precision Healthcare through Informatics: IOS; 2017. p. 704–8.
Jakob CE, Kohlmayer F, Meurers T, Vehreschild JJ, Prasser F. Design and evaluation of a data anonymization pipeline to promote Open Science on COVID-19. Sci data. 2020;7(1):435.
Meurers T, Bild R, Do K-M, Prasser F. A scalable software solution for anonymizing high-dimensional biomedical data. GigaScience. 2021;10(10):giab068.
Prasser F, Kohlmayer F, Lautenschläger R, Kuhn KA, editors. Arx-a comprehensive tool for anonymizing biomedical data. AMIA Annual Symposium Proceedings; 2014: American Medical Informatics Association.
ARX Configuration. n.d. https://arx.deidentifier.org/anonymization-tool/configuration/ . Accessed 4 Apr 2024.
Pele O, Werman M, editors. Fast and robust earth mover’s distances. 2009 IEEE 12th international conference on computer vision; 2009: IEEE.
Gart JJ. The comparison of proportions: a review of significance tests, confidence intervals and adjustments for stratification. Revue de l’Institut International de Statistique; 1971. pp. 148–69.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988:837–45.
R Core Team. R: a language and environment for statistical. Version 4.0.4. Vienna. Austria: R Foundation for Statistical Computing; 2021.
Download references
Acknowledgements
EI received a scholarship from the BK21 education program (Center for World-leading Human-care Nurse Leaders for the Future).
This study was supported in part by a research grant from the Korean Healthcare Bigdata showcase Project by the Korea Disease Control and Prevention Agency in the Republic of Korea (no.4800-4848-501). The funding body played no role in the design of the study and collection, analysis, interpretation of data, and writing the manuscript.
Author information
Authors and affiliations.
College of Nursing, Seoul National University, Seoul, South Korea
Eunyoung Im, Hyeoneui Kim & Hyungbok Lee
Center for World-leading Human-care Nurse Leaders for the Future by Brain Korea 21 (BK 21) four project, College of Nursing, Seoul National University, Seoul, South Korea
Eunyoung Im & Hyeoneui Kim
The Research Institute of Nursing Science, Seoul National University, Seoul, South Korea
Hyeoneui Kim
School of Biomedical Informatics, UTHealth, Houston, TX, USA
Xiaoqian Jiang
Seoul National University Hospital, Seoul, South Korea
Hyungbok Lee & Ju Han Kim
College of Medicine, Seoul National University, Seoul, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
EI conducted data de-identification and data analysis. HK conceived the initial project idea and interpreted the results. EI and HK designed the study and wrote the manuscript. HL prepared the clinical data and analyzed the utility of the de-identified dataset. XJ and JK interpreted the analysis results and provided critical insights into the data de-identification approaches. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Hyeoneui Kim .
Ethics declarations
Ethics approval and consent to participate.
This study utilized retrospective EHR data and was approved by the Institutional Review Board of the Seoul National University Hospital Biomedical Research Institute (IRB approval No: H-2009-156-1159). In accordance with Article 16 of the Korean Bioethics Law, informed consent was waived by the IRB. All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Im, E., Kim, H., Lee, H. et al. Exploring the tradeoff between data privacy and utility with a clinical data analysis use case. BMC Med Inform Decis Mak 24 , 147 (2024). https://doi.org/10.1186/s12911-024-02545-9
Download citation
Received : 01 June 2023
Accepted : 21 May 2024
Published : 30 May 2024
DOI : https://doi.org/10.1186/s12911-024-02545-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Data privacy
- Data utility
- Data de-identification
- Clinical data analysis
BMC Medical Informatics and Decision Making
ISSN: 1472-6947
- General enquiries: [email protected]
CrystEngComm
Acoustic shock wave-induced dynamic recrystallization of amino acids: a case study on l -serine.

* Corresponding authors
a Key Laboratory of High-Temperature and High-Pressure Study of the Earth's Interior, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, Guizhou 550081, China E-mail: [email protected]
b Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, Tamil Nadu, India
c Department of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh, Saudi Arabia
Amino acids are among the earliest organic molecules to exist on Earth, and the origin of such bio-molecules on Earth is still a topic of intense discussion in the associated scientific area. In this regard, one of the crucial methods to investigate this subject and to better comprehend the emergence of biomolecules on the planet is to carry out indoor laboratory-size shock wave recovery experiments. In this instance, we have investigated the structural and morphological properties of L -serine powder samples processed using acoustic shock waves. The effects of shock waves on the title sample have been studied using conventional diffraction and microscopy methods. According to the diffraction results, under shocked conditions, there has been no crystallographic phase change observed, whereas the entire test samples have undergone dynamic recrystallization with the same P 2 1 2 1 2 1 space group. Shock wave-driven dynamic recrystallization theories can explain the observed surface changes on the test samples, which underwent considerable surface modifications under shocked conditions.

Article information
Download citation, permissions.
Acoustic shock wave-induced dynamic recrystallization of amino acids: a case study on L -serine
S. Aswathappa, L. Dai, S. S. J. Dhas and R. S. Kumar, CrystEngComm , 2024, Advance Article , DOI: 10.1039/D4CE00384E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Res Notes

The clinical case report: a review of its merits and limitations
Trygve nissen.
1 Department of Clinical Medicine, University of Tromsø, N-9038 Tromsø, Norway
2 Division of General Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
3 Division of Addictions and Specialized Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. Some scholars point to its limited value for medical progress, while others assert that the genre is undervalued. We aimed to present the various points of view regarding the merits and limitations of the case report genre. We searched Google Scholar, PubMed and select textbooks on epidemiology and medical research for articles and book-chapters discussing the merits and limitations of clinical case reports and case series.
The major merits of case reporting were these: Detecting novelties, generating hypotheses, pharmacovigilance, high applicability when other research designs are not possible to carry out, allowing emphasis on the narrative aspect (in-depth understanding), and educational value. The major limitations were: Lack of ability to generalize, no possibility to establish cause-effect relationship, danger of over-interpretation, publication bias, retrospective design, and distraction of reader when focusing on the unusual.
Conclusions
Despite having lost its central role in medical literature in the 20th century, the genre still appears popular. It is a valuable part of the various research methods, especially since it complements other approaches. Furthermore, it also contributes in areas of medicine that are not specifically research-related, e.g. as an educational tool. Revision of the case report genre has been attempted in order to integrate the biomedical model with the narrative approach, but without significant success. The future prospects of the case report could possibly be in new applications of the genre, i.e. exclusive case report databases available online, and open access for clinicians and researchers.
Throughout history the clinical case report and case report series have been integral components of medical literature [ 1 ]. The case report genre held a strong position until it was sidelined in the second half of the 20 th century [ 2 , 3 ]. New methodologies for research articles paved the way for evidence-based medicine. Editors had to make space for these research articles and at the same time signaled less enthusiasm for publishing case reports [ 4 ]. This spurred some heated debates in medical journals as readers were worried that the traditional case report was in jeopardy [ 5 , 6 ]. Those who welcomed the new trend with fewer case reports being published pointed mainly to their low quality and inclination to emphasize mere curiosa [ 7 - 9 ]. Some of the proponents of the genre claimed that the case report had been and still was indispensible for furthering medical knowledge and that it was unique in taking care of the detailed study of the individual patient as opposed to the new research methods with their “…nomothetic approach [taking] precedence…” [ 5 ]. Still, the case report got a low ranking on the evidence hierarchy. After a decline in popularity a new interest for the case report emerged, probably beginning in the late 1990s [ 2 ]. A peer-reviewed ‘Case reports’ section was introduced in the Lancet in 1995 [ 10 ]. In 2007, the first international, Pubmed-listed medical journal publishing only case reports was established [ 11 , 12 ]. In the following years, several similar journals, for the most part online and open-access, have been launched.
The present debate is not so much focused on whether case reporting is obsolete or not. Some of the discussions after the turn of the century have been about adapting the case report genre to new challenges. One example is the suggestion of incorporating the narrative, i.e. “… stressing the patient’s story”, in the case report [ 13 ]. The authors termed their initiative “The storied case report”. Their endeavor was not met with success. In analyzing the causes for this, they wondered if “… junior trainees find it too hard to determine what is relevant and senior trainees find it too hard to change their habits” [ 13 ]. A similar attempt was done when the editors of the Journal of Medical Case Reports in 2012 encouraged authors to include the patients’ perspectives by letting patients describe their own experiences [ 14 ].
Notwithstanding, we feel there is much to be gained from having an ongoing discussion highlighting the indications and contraindications for producing case reports. This can to some degree be facilitated by getting an understanding of the merits and limitations of the genre. The objective of this article is to present the merits and limitations of case reports and case series reports.
We adopted Taber’s Cyclopedic Medical Dictionary’s definition of the case report : “A formal summary of a unique patient and his or her illness, including the presenting signs and symptoms, diagnostic studies, treatment course and outcome” [ 15 ]. A case report consists of one or two cases, most often only one. The case series or case series report usually consists of three to ten cases [ 16 ]. (In the following we use the term case report to denote both case reports and case series report). Case reports are most often naturalistic and descriptive. Sometimes, however, they can be prospective and experimental.
As literature specifically dealing with the case report genre seemed harder to elicit from the databases than the vast amount of particular case reports, we performed iterative searches. We searched Google Scholar and PubMed using the search terms ‘case report(s)’, ‘case series’, ‘case series report(s)’, ‘case reporting’ in various combinations with ‘clinical’, ‘medical’, ‘anecdotal’, ‘methodology’, ‘review’, ‘overview’, ‘strengths’, ‘weaknesses’, ‘merits’, and ‘limitations’. Further references were identified by examining the literature found in the electronic searches. We also consulted major textbooks on epidemiology [ 17 , 18 ], some scholars of medical genres [ 19 , 20 ] and a monograph on case reporting by the epidemiologist M. Jenicek [ 16 ]. We delimited our review to the retrospective, naturalistic, and descriptive case report, also labeled the “traditional” or “classic” case report, and case series including such reports. Thus we excluded other types, such as the planned, qualitative case study approach [ 21 ] and simulated cases [ 22 - 24 ]. Finally, we extracted the relevant data and grouped the merits and limitations items in rank order with the items we judged to be the most important first.
New observations
The major advantage of case reporting is probably its ability to detect novelties [ 16 ]. It is the only way to present unusual, uncontrolled observations regarding symptoms, clinical findings, course of illness, complications of interventions, associations of diseases, side effects of drugs, etc. In short, anything that is rare or has never been observed previously might be important for the medical community and ought to be published. A case report might sensitize readers and thus facilitate detection of similar or identical cases.
Generating hypotheses
From a single, or preferably several single case reports or a case series, new hypotheses could be formulated. These could then be tested with formal research methods that are designed to refute or confirm the hypotheses, i.e. comparative (observational and experimental) studies.
There are numerous examples of new discoveries or major advancements in medicine that started with a case report or, in some cases, as humbly as a letter to the editor. The first concern from the medical community about the devastating side effect of thalidomide, i.e. the congenital abnormalities, appeared as a letter to the editor in the Lancet in 1961 [ 25 ]. Soon thereafter, several case reports and case series reports were published in various journals. Case reporting is thus indispensable in drug safety surveillance (pharmacovigilance) [ 26 ].
Sometimes significant advancements in knowledge have come not from what researchers were pursuing, but from “accidental discoveries”, i.e. by serendipity. The story of Alexander Fleming’s discovery of penicillin in 1928 is well known in the medical field [ 27 ]. Psychiatry has profited to a large degree from this mode of advancing medical science as many of the drugs used for mental disorders have been discovered serendipitously [ 27 ]. One notable example is the discovery of the effect of lithium on manic episodes in patients with manic-depressive disorder [ 28 ]. A more recent discovery is the successful treatment of infantile hemangiomas with systemic propranolol. This discovery was published, as a case series report, in the correspondence section in New England Journal of Medicine [ 29 ]. However, the evidence for the effect of this treatment is still preliminary, and several randomized trials are under way [ 30 , 31 ].
Clear and operational entities are prerequisites for doing medical research. Descriptions must come before understanding. Clinical observations that lead to new disorders being described are well suited for case reporting. The medical literature is replete with case-based articles describing new diseases and syndromes. One notable example is the first description of neurasthenia by G. Beard in Boston Medical and Surgical Journal in 1869 [ 32 ].
Researching rare disorders
For rare disorders randomized controlled trials (RCTs) can be impossible to run due to lack of patients to be enrolled. Research on drug treatment and other kinds of interventions must therefore be based on less rigorous methodologies, among them case series and case reports. This would be in accordance with the European Commission’s recommendation to its members to improve health care for those with rare disorders [ 33 ].
Solving ethical constraints
Case reporting can be valuable when ethical constraints prohibit experimental research. Take as an example the challenge of how to manage the side effects of accidental extravasation of cytotoxic drugs. As RCTs on humans seem unethical in this clinical situation the current guidelines rest on small observational studies, case reports and animal studies [ 34 ]. Or another example: Physical restraint is sometimes associated with sudden, unexpected death. The cause or causes for this are to some degree enigmatic, and it is hard to conceive of a controlled study that could be ethical [ 35 , 36 ]. Case reports and case series being “natural experiments” might be the only evidence available for guiding clinical practice.
In-depth narrative case studies
Case reporting can be a way of presenting research with an idiographic emphasis. As contrasted to nomothetic research, an idiographic approach aims at in-depth understanding of human phenomena, especially in the field of psychology and psychiatry. The objective is not generalizable knowledge, but an understanding of meaning and intentionality for an individual or individuals. Sigmund Freud’s case studies are relevant examples. This usage of case reports borders on qualitative research. Qualitative studies, although developed in the social sciences, have become a welcome contribution within health sciences in the last two decades.
Educational value
Clinical medical learning is to a large degree case-based. Typical case histories and vignettes are often presented in textbooks, in lectures, etc. Unusual observations presented as published case reports are important as part of doctors’ continuing medical education, especially as they demonstrate the diversity of manifestations both within and between medical diseases and syndromes [ 37 , 38 ]. Among the various medical texts, the case report is the only one that presents day-to-day clinical practice, clinicians’ diagnostic reasoning, disease management, and follow-up. We believe that some case reports that are written with the aim of contributing to medical knowledge turn out to be of most value educationally because the phenomena have already been described elsewhere. Other case reports are clearly primarily written for educational value [ 37 ]. Some journals have regular sections dedicated to educational case reports, e.g. The Case Records of the Massachusetts General Hospital in the New England Journal of Medicine and the Clinical Case Conference found in the American Journal of Psychiatry.
The cost of doing a case report is low compared to planned, formal studies. Most often the necessary work is probably done in the clinical setting without specific funding. Larger studies, for instance RCTs, will usually need an academic setting.
Fast publication
The time span from observation to publication can be much shorter than for other kinds of studies. This is obviously a great advantage as a case report can be an important alert to the medical community about a serious event. The unexpected side effects of the sedative-antinauseant thalidomide on newborn babies is a telling story. The drug had been prescribed during pregnancy to the babies’ mothers. After the first published observation of severe abnormalities in babies appeared as a letter to the editor of the Lancet in December 16 th , 1961 [ 25 ], several case reports and series followed [ 39 , 40 ]. It should be mentioned though that the drug company had announced on December 2 nd , 1961, i.e. two weeks before the letter from McBride [ 25 ], that it would withdraw the drug form the market immediately [ 41 ].
Flexible structure
Riaz Agha, editor of the International Journal of Surgery Case Reports suggests that the case report, with its less rigid structure is useful as it “… allows the surgeon(s) to discuss their diagnostic approach, the context, background, decision-making, reasoning and outcomes” [ 42 ]. Although the editor is commenting on the surgical case report, the argument can be applied for the whole field of clinical medicine. It should be mentioned though, that other commentators have argued for a more standardized, in effect more rigid, structure [ 43 ].
Clinical practice can be changed
Case reporting can lead to or contribute to a change in clinical practice. A drug might be withdrawn from the market. Or a relabeling might change the attitude to and treatment of a condition. During Word War I the shell shock syndrome was labeled and described thoroughly in several articles in the Lancet , the first of them appearing in February 1915 [ 44 ]. The author was the British captain and military doctor Charles S. Myers. Before his efforts to bring good care and treatment to afflicted soldiers there had been a common misconception that many of these dysfunctional soldiers were malingerers or cowards.
Exercise for novice researchers
The case report format is well suited for young doctors not yet trained as researchers. It can be an opportunity for a first exercise in authoring an article and a preparation for a scientific career [ 37 , 45 , 46 ].
Communication between the clinical and academic fields
Articles authored by clinicians can promote communication between practicing clinicians and academic researchers. Observations published can generate ideas and be a trigger for further studies. For instance, a case series consisting of several similar cases in a short period can make up the case-group for a case–control study [ 47 ]. Clinicians could do the observation and publish the case series while the case–control study could be left to the academics.
Entertainment
Some commentators find reading case reports fun. Although a rather weak argument in favor of case reporting, the value of being entertained should not be dismissed altogether. It might inspire physicians to spend more time browsing and reading scientific literature [ 48 ].
Studying the history of medicine
Finally, we present a note on a different and unintended aspect of the genre. The accumulated case reports from past eras are a rich resource for researching and understanding medical history [ 49 , 50 ]. A close study of old case reports can provide valuable information about how medicine has been practiced through the centuries [ 50 , 51 ].
Limitations
No epidemiological quantities.
As case reports are not chosen from representative population samples they cannot generate information on rates, ratios, incidences or prevalences. The case or cases being the numerator in the equation, has no denominator. However, if a case series report consists of a cluster of cases, it can signal an important and possibly causal association, e.g. an epidemic or a side effect of a newly marketed drug.
Causal inference not possible
Causality cannot be inferred from an uncontrolled observation. An association does not imply a cause-effect relationship. The observation or event in question could be a mere coincidence. This is a limitation shared by all the descriptive studies [ 47 ]. Take the thalidomide tragedy already mentioned as an example; Unusual events such as congenital malformations in some of the children born to mothers having taken a specific drug during pregnancy does not prove that the drug is the culprit. It is a mere hypothesis until further studies have either rejected or confirmed it. Cause-effect relationships require planned studies including control groups that to the extent possible control for chance, bias and confounders [ 52 ].
Generalization not possible
From the argument above, it follows that findings from case reports cannot be generalized. In order to generalize we need both a cause-effect relationship and a representative population for which the findings are valid. A single case report has neither. A case series, on the other hand, e.g. many “thalidomide babies” in a short time period, could strengthen the suspicion of a causal relationship, demanding further surveillance and research.
Publication bias could be a limiting factor. Journals in general favor positive-outcome findings [ 53 ]. One group of investigators studying case reports published in the Lancet found that only 5% of case reports and 10% of case series reported treatment failures [ 54 ]. A study of 435 case reports from the field of dentistry found that in 99.1%, the reports “…clearly [had] a positive outcome and the intervention was considered and described as successful by the authors” [ 55 ].
Overinterpretation
Overinterpretation or misinterpretation is the tendency or temptation to generalize when there is no justification for it. It has also been labeled “the anecdotal fallacy” [ 56 ]. This is not a shortcoming intrinsic to the method itself. Overinterpretation may be due to the phenomenon of case reports often having an emotional appeal on readers. The story implicitly makes a claim to truth. The reader might conclude prematurely that there is a causal connection. The phenomenon might be more clearly illustrated by the impact of the clinician’s load of personal cases on his or her practice. Here exemplified by a young doctor’s confession: “I often tell residents and medical students, ‘The only thing that actually changes practice is adverse anecdote.’” [ 57 ].
Emphasis on the rare
As case reporting often deals with the rare and atypical, it might divert the readers’ attention from common diseases and problems [ 58 ].
Confidentiality
Journals today require written informed consent from patients before publishing case reports. Both authors and publishers are responsible for securing confidentiality. A guarantee for full confidentiality is not always possible. Despite all possible measures taken to preserve confidentiality, sometimes the patient will be recognized by someone. This information should be given to the patient. An adequately informed patient might not consent to publication. In 1995 in an Editorial in the British Journal of Psychiatry one commentator, Isaac Marks, feared that written consent would discourage case reports being written [ 59 ]. Fortunately, judged form the large number of reports being published today, it seems unlikely that the demand for consent has impeded their publication.
Other methodological limitations
Case reports and series are written after the relevant event, i.e. the observation. Thus, the reports are produced retrospectively. The medical record might not contain all relevant data. Recall bias might prevent us from getting the necessary information from the patient or other informants such as family members and health professionals.
It has also been held against case reporting that it is subjective. The observer’s subjectivity might bias the quality and interpretation of the observation (i.e. information bias).
Finally, the falsification criterion within science, which is tested by repeating an experiment, cannot be applied for case reports. We cannot design another identical and uncontrolled observation. However, unplanned similar “experiments” of nature can be repeated. Several such observations can constitute a case series that represents stronger indicative evidence than the single case report.
The major advantages of case reporting are the ability to make new observations, generate hypotheses, accumulate scientific data about rare disorders, do in-depth narrative studies, and serve as a major educational tool. The method is deficient mainly in being unable to deliver quantitative data. Nor can it prove cause-effect relationship or allow generalizations. Furthermore, there is a risk of overinterpretation and publication bias.
The traditional case report does not fit easily into the qualitative-quantitative dichotomy of research methods. It certainly shares some characteristics with qualitative research [ 16 ], especially with regard to the idiographic, narrative perspective – the patient’s “interior world” [ 60 ] – that sometimes is attended to. Apart from “The storied case report” mentioned in the Background-section, other innovative modifications of the traditional case report have been tried: the “evidence-based case report” [ 61 ], the “interactive case report” [ 62 ] and the “integrated narrative and evidence based case report” [ 63 ]. These modifications of the format have not made a lasting impact on the way case reports in general are written today.
The method of case reporting is briefly dealt with in some textbooks on epidemiology [ 17 , 18 ]. Journals that welcome case reports often put more emphasis on style and design than on content in their ‘instruction to authors’ section [ 64 ]. As a consequence, Sorinola and coworkers argue for more consensus and more consistent guidance on writing case reports [ 64 ]. We feel that a satisfactory amount of guidance concerning both style and content now exists [ 12 , 16 , 65 , 66 ]. The latest contribution, “The CARE guidelines”, is an ambitious endeavor to improve completeness and transparency of reports [ 66 ]. These guidelines have included the “Patient perspective” as an item, apparently a bit half-heartedly as this item is placed after the Discussion section, thus not allowing this perspective to influence the Discussion and/or Conclusion section. We assume this is symptomatic of medicine’s problem with integrating the biomedical model with “narrative-based medicine”.
In recent years the medical community has taken an increased interest in case reports [ 2 ], especially after the surge of online, exclusive case report journals started in 2007 with the Journal of Medical Case Reports (which was the first international, Pubmed-listed medical journal publishing only case reports) as the first of this new brand. The climate of skepticism has been replaced by enthusiasm and demand for more case reports. A registry for case reports, Cases Database, was founded in 2012 [ 67 ]. On the condition that it succeeds in becoming a large, international database it could serve as a register being useful for clinicians at work as well as for medical research on various clinical issues. Assuming Pamela P. Powell’s assertion that “[a]lmost all practicing physicians eventually will encounter a case worthy of being reported” [ 60 ] is valid, there should be no shortage of potential cases waiting to be reported and filed in various databases, preferably online and open access.
Limitations of this review
There are several limitations to this study. It is a weakness that we have not been able to review all the relevant literature. The number of publications in some way related to case reports and case report series is enormous, and although we have attempted to identify those publications relevant for our purpose (i.e. those that describe the merits and limitations of the case report genre), we might have missed some. It was difficult to find good search terms for our objective. Still, after repeated electronic searches supplemented with manual searches in reference lists, we had a corpus of literature where essentially no new merits or limitations emerged.
As we point out above, the ranking of merits and limitations represents our subjective opinion and we acknowledge that others might rank the importance of the items differently.
The perspective on merits and limitations of case reporting has been strictly medical. As a consequence we have not analyzed or discussed the various non-medical factors affecting the publication of case reports in different medical journals [ 2 ]. For instance, case reports are cited less often than other kinds of medical research articles [ 68 ]. Thus they can lower a journal’s impact factor, potentially making the journal less attractive. This might lead some high-impact journals to publish few or no case reports, while other journals have chosen to specialize in this genre.
Before deciding on producing a case report or case series based on a particular patient or patients at hand, the observant clinician has to determine if the case report method is the appropriate article type. This review could hopefully assist in that judgment and perhaps be a stimulus to the continuing debate in the medical community on the value of case reporting.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
TN contributed to the conception, drafting, and revision of the article. RW contributed to the conception, drafting, and revision of the article. Both authors approved the final manuscript.
Acknowledgements
There was no specific funding for this study.
- Cabán-Martinez AJ, Beltrán WF. Advancing medicine one note at a time: the educational value in clinical case reports. BMC Res Notes. 2012; 5 :293. doi: 10.1186/1756-0500-5-293. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The recent history of the clinical case report: a narrative review. JRSM Sh Rep. 2012; 3 :87. doi: 10.1258/shorts.2012.012046. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The history of the case report: a selective review. JRSM Open. 2014; 5 :4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G. Fare the well – the Editor’s last words. Br J Psychiatry. 2003; 182 :465–466. doi: 10.1192/bjp.182.6.465. [ CrossRef ] [ Google Scholar ]
- Williams DDR. In defence of case of the case report (Letter) Br J Psychiatry. 2004; 184 :84. doi: 10.1192/bjp.184.1.84. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enoch MD. Case reports (Letter) Br J Psychiatry. 2005; 185 :169. [ PubMed ] [ Google Scholar ]
- Nahum AM. The clinical case report: “Pot boiler” or scientific literature? Head Neck Surg. 1979; 1 :291–292. doi: 10.1002/hed.2890010402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doherty M. What value case reports? (Editorial) Ann Rheum Dis. 1994; 53 :1–2. doi: 10.1136/ard.53.1.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Procopio M. Publication of case reports. Br J Psychiatry. 2005; 187 :91. [ PubMed ] [ Google Scholar ]
- Bignall J, Horton R. Learning from stories – Lancet’s case reports. Lancet. 1995; 346 :1256. [ PubMed ] [ Google Scholar ]
- Kidd M, Hubbard C. Introducing Journal of Medical Case Reports. J Med Case Rep. 2007; 1 :1. doi: 10.1186/1752-1947-1-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rison RA. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes. J Med Case Reports. 2013; 7 :239. doi: 10.1186/1752-1947-7-239. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayoumi AM, Kopplin PA. The storied case report. CMAJ. 2004; 171 :569–570. doi: 10.1503/cmaj.1031503. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kidd MR, Saltman D. Case reports at the vanguard of the 21 st century medicine (Editorial) J Med Case Rep. 2012; 6 :156. doi: 10.1186/1752-1947-6-156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venes D. Taber’s Cyclopedic Medical Dictionary. 21. Philadelphia: F.A. Davis Company; 2009. [ Google Scholar ]
- Jenicek M. Clinical case reporting in evidence-based medicine. Oxford: Butterworth Heinemann; 1999. [ Google Scholar ]
- Fletcher RH, Fletcher SW. Clinical epidemiology: The essentials. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [ Google Scholar ]
- Sackett DL, Brian Haynes R, Tugwell P. Clinical epidemiology. Boston: Little, Brown and Company; 1985. [ Google Scholar ]
- Berkencotter C. Patient tales. Case histories and the uses of narrative in psychiatry. Columbia: University of South Carolina Press; 2008. [ Google Scholar ]
- Hunter KM. Doctors’ stories. The narrative structure of medical knowledge. Princeton: Princeton University Press; 1991. [ Google Scholar ]
- Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011; 11 :100. doi: 10.1186/1471-2288-11-100. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Kvalvik AM, Hynnekleiv T. Attitudes to coercion at two Norwegian psychiatric units. Nord J Psychiatry. 2011; 65 :133–137. doi: 10.3109/08039488.2010.513068. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Myklebust LH, Bratlid T. Psychologists and coercion: decisions regarding involuntary psychiatric admission and treatment in a group of Norwegian psychologists. Nord J Psychiatry. 2007; 61 :433–437. doi: 10.1080/08039480701773139. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaker E, Knudsen A, Wynn R, Lund A. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001; 19 :103–106. doi: 10.1080/028134301750235330. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McBride WG. Thalidomide and congenital abnormalities. (Letter) Lancet. 1961; 278 :1358. [ Google Scholar ]
- Chakra CAN, Pariente A, Pinet M, Nkeng L, Moore N, Moride Y. Case series in drug safety. A review to determine characteristics and quality. Drug Saf. 2010; 33 :1081–1088. doi: 10.2165/11539300-000000000-00000. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006; 8 :335–344. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949; 2 :349–352. [ PubMed ] [ Google Scholar ]
- Léauté-Labrèze C, de la Roque Dumas E, Hubiche T, Boralevi F. Propranolol for severe hemangiomas of infancy. (Letter to the editor) N Engl J Med. 2008; 358 :2649–2651. doi: 10.1056/NEJMc0708819. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A. Godt nok dokumentert? (Sufficiently documented?) (Editorial) Tidsskr Nor Legeforen. 2010; 130 :1806. [ PubMed ] [ Google Scholar ]
- U.S. National Institutes of Health. Studies found for the search 'propanolol and hemangiomas' in the ClinicalTrials.gov database. Accessed on 22 April, 2014 at: http://www.clinicaltrials.gov/ct2/results?term=propanolol+and+hemangiomas&Search=Search .
- Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869; 80 :217–221. doi: 10.1056/NEJM186904290801301. [ CrossRef ] [ Google Scholar ]
- Taylor DM. Developing a strategy for the management of rare disorders. BMJ. 2012; 344 :e2417. doi: 10.1136/bmj.e2417. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bjånes TK. Lokale bivirkninger ved parenteral administrering av legemidler. (Localized side effects caused by parenteral administration of drugs) Tidsskr Nor Legeforen. 2011; 131 :472–474. [ PubMed ] [ Google Scholar ]
- Nissen T, Rørvik P, Haugslett L, Wynn R. Physical restraint and near death of a psychiatric patient. J Forensic Sci. 2013; 58 :259–262. doi: 10.1111/j.1556-4029.2012.02290.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R. Coercion in psychiatric care: clinical, legal, and ethical controversies. Int J Psychiatry Clin Pract. 2006; 10 :247–251. doi: 10.1080/13651500600650026. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lundh A, Christensen M, Jørgensen AW. International or national publication of case reports. Dan Med Bul. 2011; 58 :A4242. [ PubMed ] [ Google Scholar ]
- Grønli O, Wynn R. Normocalcemic hyperparathyroidism and treatment resistant depression. Psychosomatics. 2013; 54 :493–497. doi: 10.1016/j.psym.2012.10.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward SP. Thalidomide and congenital abnormalities. BMJ. 1962; 2 (5305):646–647. doi: 10.1136/bmj.2.5305.646. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coodin FJ, Uchida CM, Murphy CH. Phocomelia: report of three cases. CMAJ. 1962; 87 :735–739. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heyman DJ. Managing director of the Destillers Company Ltd. (Letter) Lancet. 1961; 278 :1262. [ Google Scholar ]
- Agha R, Rosin RD. Time for a new approach to case reports. (Editorial) Int J Surg Case Rep. 2010; 1 :1–3. doi: 10.1016/j.ijscr.2010.04.001. doi:10.1016/j.ijscr.2010.04.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aronson JA. Anecdotes as evidence. (Editorial) BMJ. 2003; 326 :1346. doi: 10.1136/bmj.326.7403.1346. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Myers CS. A contribution to the study of the shell shock. Being an account of three cases of loss of memory, vision, smell and taste, admitted into the Duchess of Westminster’s War Hospital, Le Touquet. Lancet. 1915; 185 :316–320. doi: 10.1016/S0140-6736(00)52916-X. [ CrossRef ] [ Google Scholar ]
- Morris BA. The importance of case reports. (Letter to the Editor) CMAJ. 1989; 141 :875–876. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rison RA. Neurology case reports: a call for all. J Med Case Rep. 2011; 5 :113. doi: 10.1186/1752-1947-5-113. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002; 359 :145–149. doi: 10.1016/S0140-6736(02)07373-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S. Anecdotes in medicine - 15 years of Lancet case reports. Lancet. 2010; 376 :1448–1449. doi: 10.1016/S0140-6736(10)60624-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rose JC, Corn M. Dr. E. and other patients: new lessons from old case reports. J Hist Med Allied Sci. 1984; 39 :3–32. doi: 10.1093/jhmas/39.1.3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. Practice versus theory: tenth-century case histories from Islamic Middle East. Soc Hist Med. 2000; 13 :293–306. doi: 10.1093/shm/13.2.293. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. The case history in Medieval Islamic medical literature: Tajarib and Mujarrabat as source. Med Hist. 2010; 54 :195–214. doi: 10.1017/S0025727300006712. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Google Scholar ]
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991; 337 :867–872. doi: 10.1016/0140-6736(91)90201-Y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albrecht J, Meves A, Bigby M. Case reports and case series from the Lancet had significant impact on medical literature. J Clin Epidemiol. 2005; 58 :1227–1232. doi: 10.1016/j.jclinepi.2005.04.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oliveira GJ, Leles CR. Critical appraisal and positive outcome bias in case reports published in Brazilian dental journals. J Dent Educ. 2006; 70 :869–874. [ PubMed ] [ Google Scholar ]
- Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice. 1998; 4 :147–155. doi: 10.1111/j.1365-2753.1998.tb00081.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stuebe AM. Level IV evidence – adverse anecdote and clinical practice. N Engl J Med. 2011; 365 :8–9. doi: 10.1056/NEJMp1102632. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffman JR. Rethinking case reports. West J Med. 1999; 170 :253–254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G, Fahy T, Russell G, Healy D, Marks I, Tantam D, Dimond B. Case reports and confidentiality. (Editorial) Br J Psychiatry. 1995; 166 :555–558. doi: 10.1192/bjp.166.5.555. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Powell PP. The single-case report in medical literature: the ‘Elephant man’ serves as an exellent example. J Am Med Writers Assoc. 1990; 5 :9–12. [ Google Scholar ]
- Glasziou P. Evidence based case report: twenty year cough in a non-smoker. BMJ. 1998; 316 :1660–1661. doi: 10.1136/bmj.316.7145.1660. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harker N, Montgomery A, Fahey T. Interactive case report. Treating nausea and vomiting during pregnancy: case outcome. BMJ. 2004; 328 :276. doi: 10.1136/bmj.328.7434.276. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reis S, Hermoni D, Livingstone P, Borkan J. Integrated narrative and evidence based case report: case report of paroxysmal atrial fibrillation and anticoagulation. BMJ. 2002; 325 :1018–1020. doi: 10.1136/bmj.325.7371.1018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorinola O, Olufowobi O, Coomarasamy A, Khan KS. Instructions to authors for case reporting are limited: a review of a core journal list. BMC Med Educ. 2004; 4 :4. doi: 10.1186/1472-6920-4-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rossor MN. In: How to write a paper. 4. Hall GM, editor. London: Blackwell Publishing; 2008. How to write a case report; pp. 71–75. [ Google Scholar ]
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. CARE group. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013; 7 :223. doi: 10.1186/1752-1947-7-223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BioMed Central. Cases Database. Accessed on 22 April, 2014 at: http://www.casesdatabase.com .
- Mason RA. The case report – an endangered species? (Editorial) Anesthesia. 2001; 56 :99–102. doi: 10.1046/j.1365-2044.2001.01919.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Introduction
- Conclusions
- Article Information
Error bars show 95% CIs.
eTable 1. ICD codes, CPT codes, and generic drugs names
eFigure 1. Sample selection flowchart.
eFigure 2. Study design.
eTable 2. Balance after matching, MarketScan Research Databases 2008-2017.
eTable 3. Exposures to metformin, other diabetes medications, and statins over 2 years, MarketScan Research Databases 2008-2017.
eTable 4. Exposures to metformin, other diabetes medications, and statins over 2 years among subgroup of diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2008-2017.
eTable 5. Models for diabetes patients (N=81,262 cases, 79,497 controls), MarketScan Research Databases 2006-2017.*
- Methodological Considerations for the Case-Control Study of Metformin and Age-Related Macular Degeneration—Reply JAMA Ophthalmology Comment & Response August 1, 2021 Andrea L. Blitzer, MD; Sandra A. Ham, MS; Dimitra Skondra, MD, PhD
- Methodological Considerations for the Case-Control Study of Metformin and Age-Related Macular Degeneration JAMA Ophthalmology Comment & Response August 1, 2021 Mahyar Etminan, PharmD, MSc(Epi); Mohit Sodhi, MSc; Lingyi Li, MSc
- Is There a Case for Case-Control Studies in the Exploration of Retrospective Data Sets? JAMA Ophthalmology Invited Commentary March 1, 2021 Myra B. McGuinness, MBiostat, PhD; Jessica Kasza, BSc(Hons), PhD; Robyn H. Guymer, MBBS, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Blitzer AL , Ham SA , Colby KA , Skondra D. Association of Metformin Use With Age-Related Macular Degeneration : A Case-Control Study . JAMA Ophthalmol. 2021;139(3):302–309. doi:10.1001/jamaophthalmol.2020.6331
Manage citations:
© 2024
- Permissions
Association of Metformin Use With Age-Related Macular Degeneration : A Case-Control Study
- 1 Department of Ophthalmology and Visual Science, University of Chicago Medical Center, Chicago, Illinois
- 2 Center for Health and the Social Sciences, The University of Chicago, Chicago, Illinois
- 3 Department of Ophthalmology, New York University, New York
- Invited Commentary Is There a Case for Case-Control Studies in the Exploration of Retrospective Data Sets? Myra B. McGuinness, MBiostat, PhD; Jessica Kasza, BSc(Hons), PhD; Robyn H. Guymer, MBBS, PhD JAMA Ophthalmology
- Comment & Response Methodological Considerations for the Case-Control Study of Metformin and Age-Related Macular Degeneration—Reply Andrea L. Blitzer, MD; Sandra A. Ham, MS; Dimitra Skondra, MD, PhD JAMA Ophthalmology
- Comment & Response Methodological Considerations for the Case-Control Study of Metformin and Age-Related Macular Degeneration Mahyar Etminan, PharmD, MSc(Epi); Mohit Sodhi, MSc; Lingyi Li, MSc JAMA Ophthalmology
Question Is there an association between metformin use and the development of age-related macular degeneration (AMD)?
Findings In this large case-control study using a national database of patients, we found that metformin use was associated with decreased odds of developing AMD in a dose-dependent manner, with the greatest benefit at low to moderate dosages.
Meaning The use of metformin may protect against the development of AMD and lead to a novel therapeutic strategy for the prevention of this disease.
Importance Age-related macular degeneration (AMD), the leading cause of irreversible blindness in older adults, appears to have no effective preventive measures. The common antidiabetic drug metformin has been shown to have protective outcomes in multiple age-associated diseases and may have the potential to protect against the development of AMD.
Objective To determine whether metformin use is associated with reduced odds of developing AMD.
Design, Setting, and Participants This case-control study of patients from a nationwide health insurance claims database included a population-based sample of patients. Those aged 55 years and older with newly diagnosed AMD from January 2008 to December 2017 were defined as cases and matched with control participants. Data analyses were completed from June 2019 to February 2020.
Exposures Dosage of metformin and exposure to other prescribed medications, as identified from outpatient drug claims.
Main Outcomes and Measures Risk of developing AMD.
Results A total of 312 404 affected individuals were included (181 817 women [58.2%]). After matching, 312 376 control participants were included (172 459 women [55.2%]; age range, 55 to 107 years). The case group had a slightly higher percentage of participants with diabetes (81 262 participants [26.0%]) compared with the control group (79 497 participants [25.5%]). Metformin use was associated with reduced odds of developing AMD (odds ratio [OR], 0.94 [95% CI, 0.92-0.96]). This association was dose dependent, with low to moderate doses of metformin showing the greatest potential benefit (dosages over 2 years: 1-270 g, OR, 0.91 [95% CI, 0.88-0.94]; 271-600 g, OR, 0.90 [95% CI, 0.87-0.93]; 601-1080 g, OR, 0.95 [95% CI, 0.92-0.98]). Doses of more than 1080 g of metformin over 2 years did not have reduced odds of developing AMD. Both the reduction in odds ratio and the dose-dependent response were preserved in a cohort consisting only of patients with diabetes. Metformin use was associated with a decreased OR of AMD in patients with diabetes without coexisting diabetic retinopathy (OR, 0.93 [95% CI, 0.91-0.95]) but was a risk factor in patients with diabetic retinopathy (OR, 1.07 [95% CI, 1.01-1.15]).
Conclusion and Relevance In this study, metformin use was associated with reduced odds of developing AMD. This association was dose dependent, with the greatest benefit at low to moderate doses. When looking only at patients with diabetes, we saw a preservation of the dose-dependent decrease in the odds of patients developing AMD. Metformin does not appear to be protective in patients with diabetes and coexisting diabetic retinopathy. This study suggests that metformin may be useful as a preventive therapy for AMD and provides the basis for potential prospective clinical trials.
In the US, age-related macular degeneration (AMD) is the leading cause of irreversible blindness in adults older than 50 years. As the older adult population increases worldwide, there is a growing economic burden of AMD. 1 Currently, there are no efficacious preventive measures for AMD development or available treatments for the nonexudative form, which accounts for 90% of cases.
Metformin, the most commonly prescribed oral antihyperglycemic drug for diabetes, has been shown to have antiaging and protective effects against many age-associated diseases. 2 In epidemiology studies, metformin lowers the risk of developing cardiovascular disease, stroke, cancer, dementia, and primary open-angle glaucoma. 3 - 10 Metformin also reduces inflammatory markers and increases life span in murine models. 11 , 12 These exciting findings have led to the Targeting Aging With Metformin (TAME) trial, a prospective randomized clinical trial to assess metformin’s antiaging effects. 13
Given the known antiaging effects of metformin and its relatively safe adverse-effect profile, we sought to determine if the use of metformin is associated with reduced odds of developing AMD in a large, nationwide sample. We replicated a case-control study that was conducted in a small health care system. 14 To do this, we conducted a case-control study using a nationwide health insurance claims database. We also investigated a potential dose-dependent association in the study population, as well as in a subgroup of only patients with diabetes. Additionally, we evaluated whether the findings were dependent on the presence or absence of coexisting diabetic retinopathy (DR).
Quiz Ref ID Data were derived from the IBM MarketScan Commercial and Medicare Supplemental Databases from January 2006 to December 2017. These databases represent the annual health services of approximately 30 to 50 million employees, dependents, and retirees annually in the US with primary or Medicare supplemental coverage through privately insured health plans. The databases include enrollment records and inpatient, outpatient, and outpatient prescription drug claims. The University of Chicago institutional review board approved this study and granted an exemption to requiring consent from study participants because identifiable private information had been removed from the data. Using the MarketScan databases, we identified cases as adults aged 55 years and older who were newly diagnosed with AMD from January 2008 to December 2017 and had at least 2 eye examinations during the previous 12 months. We identified AMD using International Classification of Disease , Ninth Revision and Tenth Revision ( ICD-9 and ICD-10 ) codes, and eye examinations were identified using Current Procedural Terminology ( CPT ) codes (eTable 1 in the Supplement ). We excluded patients who did not have continuous health insurance plan enrollment that included prescription drug coverage for 24 months prior to the diagnosis (eFigure 1 in the Supplement ). Since we had the option of using more or fewer years of data, based on preliminary analyses, we chose to power the study to detect odds ratios (ORs) of 0.95 with 90% power in a subgroup of 76 000 individuals with diabetes, which required 10 years of cases. Odds ratios were calculated via logistic regression.
Age on the index date was classified into 10-year age groups (55-64, 65-74, 75-84, 85-94, and >95 years); there were very few participants older than 95 years, so they were combined with the subgroup aged 84 to 95 years for data analysis. Sex information was classified as men and women. Comorbidities associated with AMD were identified using ICD-9 and ICD-10 codes from inpatient and outpatient claims during the 12 months prior to and including the index date (eFigure 2 in the Supplement ). Known risk factors for AMD and comorbidities were anemia, hypertension, diabetes, smoking, hyperlipidemia, nonproliferative DR, and proliferative DR (eTable 1 in the Supplement ). 15 - 21 Patients were considered to have a comorbidity if at least 1 ICD code was observed. We also computed modified Charlson Comorbidity Index (CCI) scores using inpatient and outpatient data for 12 months, ending on the index date. Charlson comorbidities were myocardial infarction, congestive heart failure, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease/rheumatic disease, peptic ulcer disease, mild liver disease, paraplegia and hemiplegia, kidney disease, cancer, moderate or severe liver disease, metastatic carcinoma, and HIV/AIDS. Patients were classified as having a weighted CCI score of 0, 1, 2 or 3 or more. Patients were classified into 4 regions (Northeast, South, North Central, and West) using the location of residence during the year of the index event.
We selected a control group from the general population represented in MarketScan data, matched 1:1 to the cohort of affected patients based on age, anemia, hypertension, region, and CCI score. The selected matching variables were based on methods used in similar studies. 8 , 10 , 14 , 20 To identify a pool of candidates from all patients in the MarketScan databases, each year, each enrolled adult 55 years or older was screened for eye examinations. Eligible candidates had 2 or more eye examinations during the prior 12 months. An index date was randomly selected from all dates of eye examinations. Candidates also had continuous health insurance plan coverage that included prescription drug coverage during the 24 months prior to the index date. Potential control participants could be included in the pool in multiple years but were excluded from the control pool once they had an incident or previous AMD diagnosis. Such patients would initially be counted as a control participant and then become a participant with a case in later years. Since the control pool was quite large, for each year’s individuals with cases, control participants were randomly selected from among those with exact matches based on 5 criteria: age on index date, anemia, hypertension, region, and CCI score (0, 1, 2, or ≥3). We chose not to match by diabetes status because, by design, matching criteria do not vary between groups; however, we wanted to test the independent effect of diabetes in adjusted models. After selection, the remaining comorbidities were operationalized for control participants using the same criteria as for participants with cases.
To operationalize exposures, outpatient prescription drug claims for participants with cases and control participants were examined for the 24 months preceding the index date. We identified claims by National Drug Code for drugs with generic names that included names of diabetes medications (metformin, insulin, sulfonureas, glitazones, meglitinides, and others) and statins, which are listed in eTable 1 in the Supplement . Patients with exposures had to have at least 1 outpatient prescription drug claim for a medication within 2 years preceding the index date. We calculated total exposure to metformin and classified the dosage by approximate quartiles as 0, 1 to 270, 271 to 600, 601 to 1080, and more than 1080 g over 2 years. We excluded participants with cases involving ambiguous or incomplete claims data.
Descriptive statistics for participants with cases, the control pool, and control participants were frequencies and percentages for categorical variables or means and SDs for continuous variables. To examine significant differences in categorical sample characteristics and exposures between participants with cases and control participants, we used χ 2 tests. We report standard differences between the 2 groups to assess the balance of the matched samples. Univariable logistic regressions tested the bivariate associations between incident AMD and sex, diabetes, smoking, hyperlipidemia, nonproliferative and proliferative DR, metformin, insulin, sulfonureas, glitazones, meglitinides, other diabetes medications, and statins. Multivariable logistic regression models were constructed to determine whether the medications were risk factors for AMD by estimating the influence of diabetes medication and various comorbidities on incident AMD. One model tested any medication use, and a second model tested categories of 2-year cumulative dosage of metformin. We repeated these regressions using the subgroup of participants with cases and control participants with diabetes. All regressions were stratified by year, 10-year age group, anemia, hypertension, region, and CCI score. The comorbidities included in our multivariable analyses were age, sex, region, hypertension, hyperlipidemia, obesity, CCI score, diabetes, smoking, diabetic medications, statins, and DR. Two-sided tests were considered significant with an α of .05. We used SAS version 9.4 (SAS Institute) for all analyses. Analyses took place from June 2019 to February 2020.
We identified 312 404 patients who met the case inclusion criteria for AMD (eFigure 2 in the Supplement ). The total control pool was 31 343 467 eligible patients ( Table 1 ). Compared with control participants, those with cases were older (eg, 55-64 years: control participants, 20 477 450 [65.3%] vs participants with cases, 65 891 [21.1%]; 75-84 years: 3 233 025 [10.3%] vs 108 734 [34.8%]; ≥85 years: 774 606 [2.5%] vs 51 955 [16.6%]), more often resided in the US Northeast region (control participants, 4 829 594 [15.4%] vs participants with cases, 60 734 [19.4%]) and the US North Central region (control participants, 8 686 649 [27.7%] vs participants with cases, 96 413 [30.9%]), were more likely to have hypertension (control participants, 16 689 204 [53.3%] vs participants with cases, 203 463 [65.1%]) and anemia (control participants, 1 209 904 [3.9%] vs participants with cases, 20 197 [6.5%]), and had a higher CCI score (eg, CCI score of 1: control participants, 5 305 055 [16.9%] vs participants with cases, 63 305 [20.3%]; CCI score of 2: 3 377 497 [10.8%] vs 47 842 [15.3%]; CCI score of ≥3: 2 327 571 [7.4%] vs 46 525 [14.9%]). After matching, 312 376 control participants were included in the analysis ( Table 2 ). The case group included 181 817 women (58.2%), compared with 172 459 women (55.2%) in the control group ( P < .001); the overall age range was 55 to 107 years. Those with cases were slightly more likely to have diabetes than control participants (participants with cases, 81 262 [26.0%] vs control participants, 79 497 [25.5%]; P < .001) and also more likely to smoke (17 841 [5.7%] vs 12 920 [4.1%]) and have hyperlipidemia (155 080 [49.6%] vs 149 627 [47.9%]), nonproliferative DR (11 400 [3.7%] vs 5281 [1.7%]), and proliferative DR (2241 [0.7%] vs 1229 [0.4%]). As expected from exact matching, the samples were balanced on age, region, year, CCI score, hypertension, and anemia (eTable 2 in the Supplement ).
The exposure rate to metformin for individuals with cases was 12.8% (40 081 of 312 404), and for control participants, it was 13.0% (40 730 of 312 376; P = .01; eTable 3 in the Supplement ), with very small differences in the dosages of each group. The case group had slightly more patients with no exposure (participants with cases, 272 323 [87.2%] vs control participants, 271 646 [87.0%]) and with the highest doses (9833 [3.2%] vs 9121 [2.9%]) and slightly smaller percentages of those with low to moderate doses (271-600 g/2 years: 9097 [2.9%] vs 9780 [3.1%]; 601-1080 g/2 years: 11 010 [3.5%] vs 11 075 [3.6%]: P < .001). Mean total metformin dose over 2 years was 116 g in both groups ( P = .06).
Quiz Ref ID Univariable analysis identified several associations in patients with AMD, including known risk factors such as smoking (OR, 1.43 [95% CI, 1.39-1.46]) and diabetes (OR, 1.03 [95% CI, 1.02-1.04]) ( Table 3 ). This analysis also showed an association with metformin use. The use of any metformin over 2 years reduced the odds of developing AMD (OR, 0.98 [95% CI, 0.97-1.00]; P = .01). In the multivariable model adjusting for known risk factors and other medications, the reduced odds associated with metformin persisted (OR, 0.94 [95% CI, 0.92-0.96]; P < .001), and a dose-dependency was revealed, with low to moderate doses being the most protective. Metformin doses of 1 to 270 g over 2 years had reduced odds of AMD (OR, 0.91 [95% CI, 0.89-0.94]; P < .001), with a similar finding for 271 to 600 g over 2 years. Taking 601 to 1080 g of metformin over 2 years had an OR of 0.95 (95% CI, 0.93-0.98; P = .002). The highest dose of more than 1080 g over 2 years had no difference in AMD incidence.
Quiz Ref ID Since metformin is most commonly used as an antidiabetic drug, subgroup analyses were performed to examine associations of exposures with AMD among patients with diabetes. As seen in the full cohort of patients, patients with diabetes had a reduced odds of developing AMD when using metformin in the 2 years prior (OR, 0.95 [95% CI, 0.93-0.97]; P < .001) ( Figure 1 ). A dose-dependent outcome was redemonstrated, with the largest benefit seen at small to moderate doses of metformin. The greatest reduction in risk was seen at metformin doses of 271 to 600 g over 2 years with an OR of 0.91 (95% CI, 0.88-0.94). Doses of 1 to 270 g over 2 years and 600 to 1080 g over 2 years were also associated with reduced odds (ORs, 0.93 [95% CI, 0.90-0.96] and 0.95 [95% CI, 0.92-0.98], respectively). As in the full cohort, the highest metformin dose of more than 1080 g over 2 years had no association with AMD incidence. Furthermore, metformin use was only reduced the odds of AMD in the absence of DR (OR, 0.93 [95% CI, 0.91-0.95]; P < .0001). In contrast, the presence of DR was associated with an increased odds of AMD in patients using metformin (OR, 1.07 [95% CI, 1.01-1.15]; P = .03) ( Figure 2 ).
Although we did not include diabetes as a matching variable by design, the matched samples had similar percentages with diabetes (81 262 [26.0%] in the case group and 79 497 [25.5%] in the control group). When looking at exposure to the subgroup of only patients with diabetes, there was an expected increase in the proportion of patients taking metformin (participants with cases, 38 580 [47.5%] vs those without cases, 38 388 [48.3%]; P = .001), as well as insulin (18 277 [22.5%] vs 17 175 [21.6%]; P < .001), sulfonureas (25 509 [31.4%] vs 25 919 [32.6%]; P < .001), glitazones (9863 [12.1%] vs 9958 [12.5%]; P = .02), and other antidiabetic medications (11 969 [14.7%] vs 10 987 [13.8%]; P < .001) (eTable 4 in the Supplement ). Although exposure to medications was greater, the differences in exposure between participants with cases and control participants were the same as in the full sample. In bivariate analyses, the unadjusted risk of incident AMD was similar to the full sample, as were the adjusted models, suggesting that diabetes is not among the most important risk factors for AMD (eTable 5 in the Supplement ).
In evaluation of other medications, univariable and multivariable analyses showed a small decrease in the odds of developing AMD with insulin (univariable OR, 1.06 [95% CI, 1.03-1.08]; P < .001; multivariable OR, 0.91 [95% CI, 0.89-0.93]; P < .001), sulfonureas (univariable OR, 0.96 [95% CI, 0.95-0.98]; P < .001; multivariable OR, 0.92 [95% CI, 0.90-0.94]; P < .001), glitazones (univariable OR, 0.97 [95% CI, 0.94-0.99]; P < .001; multivariable OR, 0.96 [95% CI, 0.93-0.98]; P < .001), and statins (univariable OR, 0.98 [95% CI, 0.97-0.99]; P < .001; multivariable OR, 0.95 [95% CI, 0.94-0.96]; P < .001). In the full sample, patients with AMD had higher rates of insulin use (18 257 [5.8%] in the case group and 17 383 [5.6%] in the control group; P < .001) and lower rates of metformin (40 081 [12.8%] vs 40 730 [13.0%]; P = .01), sulfonurea (25 775 [8.3%] vs 26 702 [8.6%]; P < .001), and statin use (statin (162 856 [52.1%] vs 165 393 [53.0%]; P < .001) (eTable 3 in the Supplement ). Although statistically different in this large database, exposure to all medications differed by less than 1.0% between groups.
This case-control study using a large, national sample suggests that metformin use over 2 years in adults aged 55 years and older is associated with 5% to 10% reduced odds ratio of developing AMD. Moreover, there is a dose-dependent association of this potential protective effect, with low to moderate doses of metformin being associated with the lowest odds ratio for the development of AMD, suggestive of a J-shaped or U-shaped nonlinear dose-response curve. The association of high doses of metformin was similar to no exposure. It is possible that high doses of metformin may be more commonly used in patients with poorly controlled diabetes and such patients may benefit less from metformin use. In a subgroup of only patients with diabetes, similar ORs were seen when compared with the study population as a whole. Metformin was found to decrease the odds of new AMD among patients with diabetes and without DR, but this same outcome was not seen in patients with coexisting DR. To our knowledge, this is the largest study investigating the association between metformin and the development of AMD.
Metformin has been shown to have beneficial effects on multiple age-associated diseases. The UK Prospective Diabetes Study (UKPDS), a randomized intervention trial on patients with overweight and newly diagnosed diabetes, suggested a cardioprotective outcome of metformin use. 3 This was substantiated in the Hyperinsulinemia: the Outcome of its Metabolic Effects (HOME) trial, a placebo-controlled randomized clinical trial that showed that addition of metformin to the drug regimens of patients already receiving insulin treatment was associated with the prevention of macrovascular events. 4 Metformin has also been associated with a decreased rate of several cancers, particularly breast, lung, and colorectal cancers. 2 , 5 - 8 The Singapore Longitudinal Aging Study revealed a significant protective outcome of metformin use in cognitive impairment. 9 Metformin use has also been shown to reduce the risk of ocular age-associated disease, such as primary open-angle glaucoma. 10
Work in in vitro studies and animal models has demonstrated that metformin affects multiple pathways that may modulate the biology of aging. Metformin acts directly and indirectly on several targets, including 5′ adenosine monophosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), and sirtuin-1 (SIRT1) to affect important cell functions, such as survival, stress defense, autophagy, oxidative stress, protein synthesis, and inflammatory response. 11 , 22 - 29 The scope of this study does not determine the molecular pathways involved in the potential protective outcome of metformin use in the development of AMD. Further studies are needed to investigate the role of metformin on these pathways specifically as it pertains to the pathogenesis of AMD.
Brown et al 14 recently reported the protective outcomes of metformin use in AMD development in patients at the University of Florida. They found a 42% reduction in the odds of developing AMD with metformin use, in contrast with the 5% to 10% in this study. 22 There are several key differences between the 2 studies, some of which are highlighted by the characteristics of the study populations. Importantly, the patients at the University of Florida have an exceptionally high mean CCI score (4.2, compared with 1.1 in this study), suggesting a substantially sicker population. Additionally, the frequency of diabetes in the University of Florida case and control groups was dissimilar (43% in the case group and 70% in the control group, compared with 26.0% and 25.5%, respectively, in this study). Although Brown et al 14 included other potential drugs in their analysis, the omission of insulin, which was also shown to reduce the odds of AMD development in this study, may have inflated the outcome of metformin use. They also did not collect data on smoking, a known risk factor for the development of AMD. It is likely that our large population of patients drawn from a nationwide database is more representative of the US as a whole.
An additional finding of interest from our study is the possible association between DR and AMD. Diabetes appears to be a risk factor for AMD, 17 but the presence of DR has shown an inconsistent role in the development of AMD. Two longitudinal studies have suggested that DR may increase the risk of AMD, 20 , 21 while other studies have revealed a possible protective outcome of DR. 30 , 31 Our study provides additional evidence that DR may be a risk factor for the development of AMD. In this large cohort of patients, those with AMD were more likely to have DR. We also found that the potential protective outcome of metformin use is seen in patients with diabetes in the absence of DR.
There are strengths of using a large cohort of patients for a case-control study. The use of diabetic medications may have small but meaningful effects on the risk of developing incident AMD; yet, by using MarketScan data, we are able to capture these outcome with a high degree of precision.
Quiz Ref ID A limitation of our study is that we are unable to determine the probability of developing AMD; rather, we are reporting the ORs of the association of metformin use with having AMD. Another limitation of this study is that it relies on diagnosis codes from billing records. It is possible that billing records underreport diagnoses or include incorrect diagnoses. For example, mild AMD may be coded as drusen, which, in the absence of an additional AMD code, was not included in our analysis, because it may often refer to peripheral retinal drusen. Using ICD coding for smoking has an excellent specificity but poor sensitivity and likely does not capture all of the individuals who smoked within our study cohort. 32 The large population in this database would be expected to outweigh small numbers of misdiagnoses or incorrect coding. Additionally, the MarketScan database includes information on patients from private insurance only, which is not fully representative of the older population. Because we do not have access to medical records, we are unable to see patient characteristics, including demographic information, such as ethnicity, as well as medical information, such as the level of hemoglobin A 1c . Because of this limitation, it is possible that there are ethnic differences in the patients who are taking metformin compared with the patients who do not take metformin. Knowledge of hemoglobin A 1c levels may be useful in separating the antihyperglycemic effect of metformin from its anti-inflammatory or other effects. These data are insufficient to determine if metformin is acting as a director mediator of AMD or if the outcome of metformin use is, in part, through a reduction in blood glucose levels. Future studies can be aimed at identifying the pathways responsible for the potential beneficial effect of metformin, to further uncover the potential role of metformin as a novel strategy to prevent or slow AMD.
Knowing the beneficial associations that metformin has been shown to have with multiple diseases of aging, we asked if it could also play a role in reducing the odds of AMD. We found that not only does metformin reduce the odds of developing AMD, but that this outcome is strongest at low to moderate doses and is only seen in the absence of coexisting DR. This study highlights metformin as a possible therapeutic intervention to prevent or slow the progression of AMD. Future studies will be important to further validate and confirm this finding, in addition to determining the molecular mechanisms involved and which pathogenic pathways of AMD are affected by metformin. If a protective effect of metformin is confirmed in clinical trials, this may lead to a novel therapeutic strategy for this disease, which is the leading cause of blindness in older adults and has no previously established preventive measures.
Accepted for Publication: November 11, 2020.
Published Online: January 21, 2021. doi:10.1001/jamaophthalmol.2020.6331
Corresponding Author: Dimitra Skondra, MD, PhD, Department of Ophthalmology and Visual Science, University of Chicago Medical Center, 5841 S Maryland Ave, Chicago, IL 60637 ( [email protected] ).
Author Contributions: Dr Skondra and Ms Ham had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: Blitzer, Ham, Skondra.
Drafting of the manuscript: Blitzer, Ham.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ham.
Administrative, technical, or material support: Colby.
Supervision: Colby, Skondra.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by a grant from The University of Chicago Institute for Translational Medicine (Dr Skondra).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
The largest online collection of medical case reports. Validation period: 6/4/2024, 3:01:30 AM - 6/4/2024, 9:01:30 AM. Subscribe Login. Latest content Archive For authors About Help. Email alerts. Search the world's largest collection of clinical case reports ... Every year BMJ Case Reports selects authors of global health case reports to join ...
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports. Case reports should show one of the following: Unreported or unusual side effects or adverse interactions involving medications. Unexpected or unusual presentations of a disease.
ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Explore 496,616 research studies in all 50 states and in 222 countries. See listed clinical studies related to the coronavirus disease (COVID-19)
PubMed is a comprehensive database of biomedical literature from various sources, including MEDLINE, life science journals, and online books. You can search for citations, access full text content, and explore topics related to health, medicine, and biology. PubMed also provides advanced search options and tools for researchers and clinicians.
About Oxford Medical Case Reports. Oxford Medical Case Reports (OMCR) is an open access, peer-reviewed online journal publishing original and educationally valuable case reports that expand the field of medicine.The journal deposits all articles in PubMed Central (PMC) and is indexed in the Web of Science Core Collection.. Click here to find out more about the journal.
The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953.
Some of the new journals cover general medicine and others cover specific therapeutic areas. Most case report journals (94%) are open access and approximately 40% are indexed in PubMed. Clinical issues covered by case report journals include previously unreported adverse effects of drugs or other treatments, unexpected events that occur in the ...
SAGE Open Medical Case Reports is a peer-reviewed, open access journal, which focusses on providing a publication home for short case reports and case series, which often do not find a place in traditional primary research journals, but provide key insights into real medical cases that are essential for physicians, and may ultimately help to improve patient outcomes.
To continue please click the button below. 120. LOGOUT. CONTINUE. Connect with NLM. National Library of Medicine. 8600 Rockville Pike. Bethesda, MD 20894. Web Policies.
Clinical Case Reports is inviting applications for the role of Associate Editor (Veterinary Science). We are excited to be seeking an Associate Editor to build on the journal's strong foundations and continue to develop Clinical Case Reports as a core community resource. The deadline for applications is June 15 th 2024.Please click here for further details about the role and how to apply.
The LITFL Clinical Case Collection includes over 250 Q&A style clinical cases to assist ' Just-in-Time Learning ' and ' Life-Long Learning '. Cases are categorized by specialty and can be interrogated by keyword from the Clinical Case searchable database. Search by keywords; disease process; condition; eponym or clinical features….
Cases Database is limited by the fact that only 252 titles are currently indexed, but the developers have a standing call for other publishers to allow indexing of more journals. Until then, it would be difficult to recommend this database to other user groups, as a limit to case studies in MEDLINE sources would provide a more authoritative search.
Search all biomedical databases provided by the National Center for Biotechnology Information (NCBI), an agency of the U.S. National Library of Medicine at the NIH ... Privately and publicly funded clinical studies conducted around the world. ClinVar. Human variations of clinical significance. dbGaP. Genotype/phenotype interaction studies. dbSNP.
Clinical Case Studies (CCS), peer-reviewed & published bi-monthly electronic only, is the only journal devoted entirely to innovative psychotherapy case studies & presents cases involving individual, couples, & family therapy.The easy-to-follow case presentation format allows you to learn how interesting & challenging cases were assessed & conceptualized, & how treatment followed such ...
The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as ...
Identification and description of patients with multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection using the Premier Healthcare Database, Epidemiology and Infection ...
James B. Cutrell, MD and James M. Sanders, PhD, PharmD. This commentary on a case describes need for clinician collaboration to optimize therapeutic use of antimicrobials in clinical settings. AMA J Ethics. 2024;26 (6):E441-447. doi: 10.1001/amajethics.2024.441. Case and Commentary. May 2024.
Cosmos IF: 3.634. Medical Case Reports is an open access, peer-reviewed online journal publishing original and educationally valuable case reports that expand the field of medicine. The journal covers all medical specialties comprising a comprehensive resource for physicians in all fields and at all stages of training.
These NLM-funded researchers bring together artificial intelligence, virtual reality, and visual prostheses - retinal and brain implants referred to as "bionic eyes" - in hopes of one day providing better assistive technology for incurable blindness that affects about 40 million people worldwide. Despite recent advances in gene and stem ...
The NCCSTS Case Collection, created and curated by the National Center for Case Study Teaching in Science, on behalf of the University at Buffalo, contains over a thousand peer-reviewed case studies on a variety of topics in all areas of science. Cases (only) are freely accessible; subscription is required for access to teaching notes and ...
CASE STUDIES. This page offers a collection of interesting cases from the Penn Department of Pathology and Laboratory Medicine that are available to download as PDFs. To view specific case studies by organ system or subspecialty, use the filter checkboxes in the left sidebar.
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...
Data sources. The SEER database containing 13 registry centers prepared by the National Cancer Institute was selected for the data of this study, and a total of 692,555 patients diagnosed with ...
Additionally, a number of jurisdictions currently require a medical liability or malpractice case be heard by a screening panel before trial. 1. Statute of Limitations. The statute of limitations defines the time frame within which a plaintiff must file a lawsuit, often one to three years from the date of the alleged injury. ...
Our study aims to systematically evaluate the association between elevated peripheral blood eosinophils and clinical outcome of patients with AECOPD. Methods: An electronic search was conducted for relevant studies published from database inception to February 28, 2023, on PubMed, EMBASE, Cochrane Library, and Web of Science.
Study identification. Database search returned 18,792 articles, and 7 relevant articles were obtained through supplements from other resources. A total of 17,526 articles were obtained after eliminating duplicates. 17,239 references were excluded from the initial screening by reading titles and abstracts, leaving 287 references for full-text review.
Nonetheless, few studies investigated how data de-identification efforts affect data analysis results. This study aimed to demonstrate the effect of different de-identification methods on a dataset's utility with a clinical analytic use case and assess the feasibility of finding a workable tradeoff between data privacy and utility.
Amino acids are among the earliest organic molecules to exist on Earth, and the origin of such bio-molecules on Earth is still a topic of intense discussion in the associated scientific area. In this regard, one of the crucial methods to investigate this subject and to better comprehend the emergence of biomolecule
The climate of skepticism has been replaced by enthusiasm and demand for more case reports. A registry for case reports, Cases Database, ... Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice. 1998; 4:147-155. doi: 10.1111/j.1365-2753.1998.tb00081.x. [Google Scholar] Stuebe AM. ...
We replicated a case-control study that was conducted in a small health care system. 14 To do this, we conducted a case-control study using a nationwide health insurance claims database. We also investigated a potential dose-dependent association in the study population, as well as in a subgroup of only patients with diabetes.