
< Go back to Login
Forgot Password
Please enter your registered email ID. You will receive an email message with instructions on how to reset your password.


COVID(Coronavirus)-19 Impact PowerPoint Template

Product Description:
Download the Free COVID-19 Impact PowerPoint presentation to share with your senior management and customers. COVID-19 has impacted businesses massively. Many of us are at a point in reviewing what it all means in the short and long run. To help you build business reviews and have key discussions, we have created a PowerPoint presentation for you to download and use. This is draft material and will be updated frequently. We are making it available free of cost as a small gesture from our side.
You can also view a COVID-19 Business Impact Presentation to get ideas to showcase its impact on the business.
Check out more Change Management templates and COVID-19 Templates .
You May Also Like
- Covid 19 Timeline 02 - 4x3 – $4.99
- Covid 19 Timeline 02 - 16x9 – $4.99

Covid 19 Timeline 02 PowerPoint Template
Covid 19 Timeline 02 Presentation Template Use this Covid 19 Timeline 02 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Covid 19 Timeline 02 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
- COVID 19 Timeline 01 - 4x3 – $4.99
- COVID 19 Timeline 01 - 16x9 – $4.99
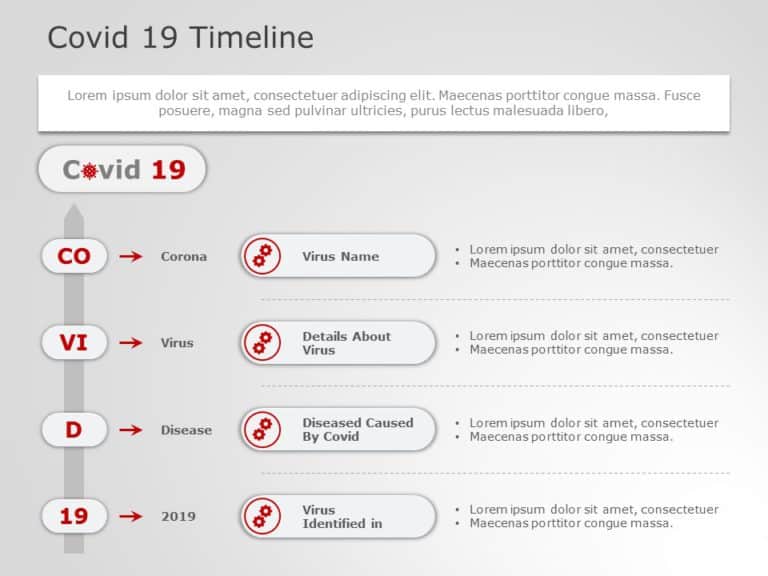
COVID 19 Timeline 01 PowerPoint Template
COVID 19 Timeline 01 Presentation Template Use this COVID 19 Timeline 01 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The COVID 19 Timeline 01 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
- COVID-19 Dashboard Global - 4x3 – $4.99
- COVID-19 Dashboard Global - 16x9 – $4.99

COVID-19 Dashboard Global PowerPoint Template
COVID-19 Dashboard Global Presentation Template Use this COVID-19 Dashboard Global PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The COVID-19 Dashboard Global PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly with our unique... read more
- COVID 19 Business Impact Presentation - 4x3 – $19.99
- COVID 19 Business Impact Presentation - 16x9 – $19.99

COVID-19 Business Impact Presentation PowerPoint Template
About COVID-19 Business Impact Presentation PowerPoint Template The recent coronavirus pandemic has forced many businesses and industries to stop their activities until society can resume a normal life. As a result, most nations are seeing an economic impact. Businesses are the ones who have suffered a lot due to this outbreak. Our Covid-19 Business Impact Presentation PowerPoint template can help... read more
- Change Impact Matrix Template - 4x3 – $4.99
- Change Impact Matrix Template - 16x9 – $4.99
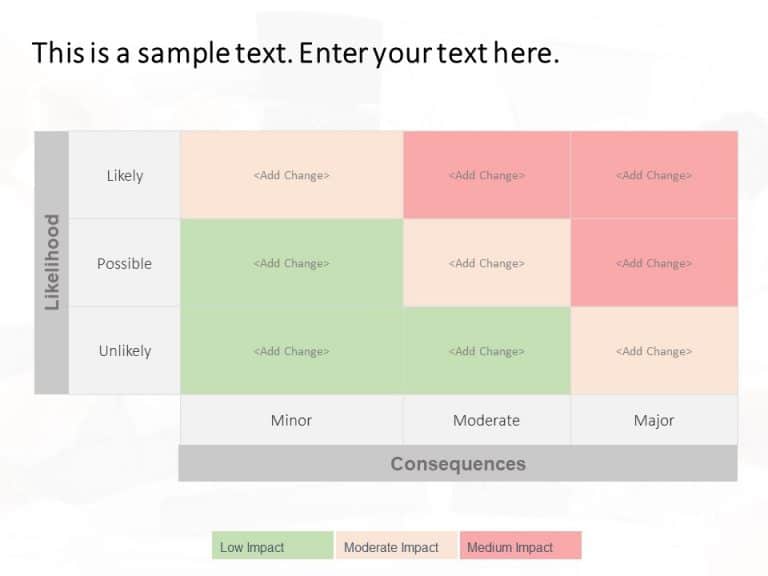
Change Impact Matrix PowerPoint Template
Change Impact Matrix Presentation Template Use this Change Impact Matrix PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Change Impact Matrix PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly with our unique... read more
- Covid-19-Timeline-05 - 4x3 – $4.99
- Covid-19-Timeline-05 - 16x9 – $4.99

COVID-19 Timeline Template for PowerPoint and Google Slides 05
COVID-19 Timeline Template for PowerPoint and Google Slides 05 The Covid-19 Timeline Template shows the timeline of Covid-19 symptoms and treatments over 22 days. The timeline shows the days from day 01 to day 22, making it clear how symptoms and treatments change over time. On the left side of the slide, there is a column where different symptoms are... read more
- Change Impact Assemment Matrix - 4x3 – $6.99
- Change Impact Assemment Matrix - 16x9 – $6.99
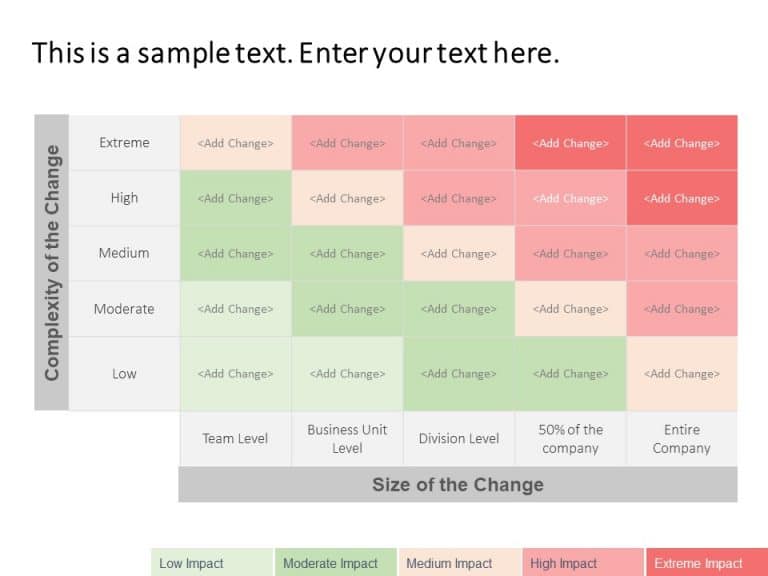
Change Impact Assemment Matrix PowerPoint Template
Change Impact Assessment Matrix Presentation Template Use this Change Impact Assessment Matrix PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Change Impact Assessment Matrix PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
- Covid 19 Timeline 04 - 4x3 – $6.99
- Covid 19 Timeline 04 - 16x9 – $6.99
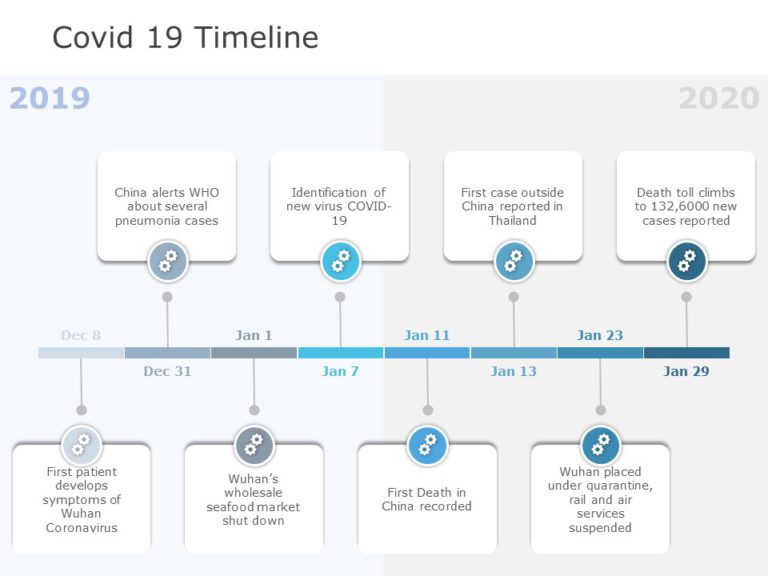
COVID-19 Timeline Template for PowerPoint and Google Slides 04
COVID-19 Timeline Template for PowerPoint and Google Slides 04 The COVID-19 Timeline Template is meant to track and present a timeline of important events related to the COVID-19 pandemic. It includes a timeline for key dates, milestones, and actions taken during the early stages of the outbreak. This template can be really helpful for educational, informational, or reporting purposes. The... read more
Recommended for you
- Covid 19 Timeline 03 - 4x3 – $4.99
- Covid 19 Timeline 03 - 16x9 – $4.99
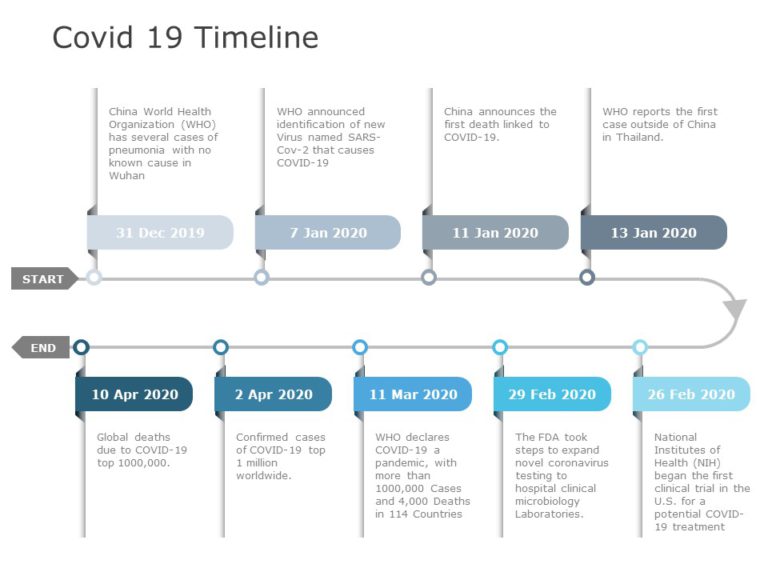
Covid 19 Timeline 03 PowerPoint Template
Covid 19 Timeline 03 Presentation Template Use this Covid 19 Timeline 03 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Covid 19 Timeline 03 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
- COVID Business Impact Grid Matrix - 4x3 – $4.99
- COVID Business Impact Grid Matrix - 16x9 – $4.99
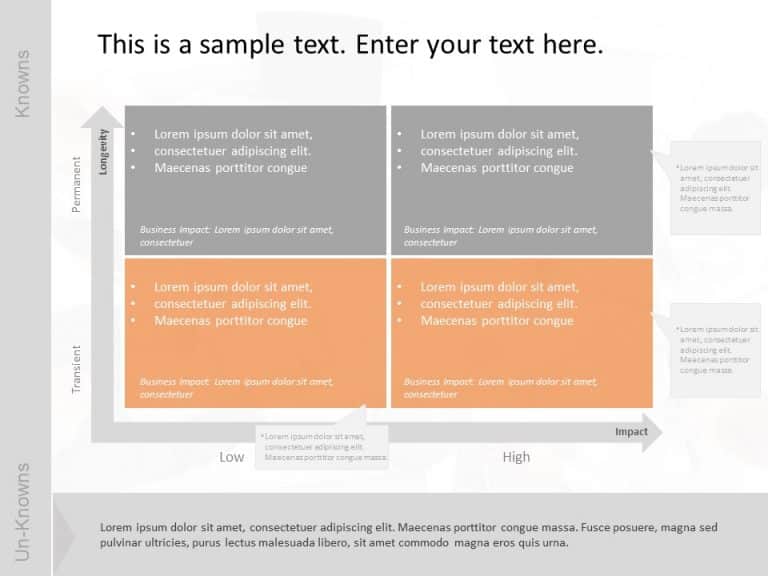
COVID Business Impact Grid Matrix PowerPoint Template
COVID Business Impact Grid Matrix Presentation Template Use this COVID Business Impact Grid Matrix PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The COVID Business Impact Grid Matrix PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey... read more
2 Steps Business 1 PowerPoint Template
Free 2 Steps Business 1 Presentation Template Use this Free 2 Steps Business 1 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Free 2 Steps Business 1 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey... read more
Business Steps 8 PowerPoint Template
Free Business Steps 8 Presentation Template Use this Free Business Steps 8 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Free Business Steps 8 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
2 Steps Roadmap PowerPoint Template
Free 2 Steps Roadmap Presentation Template Use this Free 2 Steps Roadmap PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Free 2 Steps Roadmap PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
- Change Management Strategy 02 PowerPoint Template - 4x3 – $4.99
- Change Management Strategy 02 PowerPoint Template - 16x9 – $4.99
Change Management Strategy 02 PowerPoint Template
Change Management Strategy 02 Presentation Template Use this Change Management Strategy 02 PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Change Management Strategy 02 PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly... read more
Timeline PowerPoint Template
Free Timeline Presentation Template Use this Free Timeline PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Free Timeline PPT template is professionally designed with the principles of vision sciences to capture your audience’s attention. Convey your message clearly with our unique set of editable... read more
- Coronavirus (COVID 19) Impact Implications & Immediate Actions - 4x3 – $19.99
- Coronavirus (COVID 19) Impact Implications & Immediate Actions - 16x9 – $19.99
Coronavirus (COVID-19) Impact Implications & Immediate Actions PowerPoint Template
Coronavirus (COVID-19) Impact Implications & Immediate Actions Presentation Template Use this Coronavirus (COVID-19) Impact Implications & Immediate Actions PowerPoint template to create visually appealing presentations in any professional setting. Its minimalistic design and ready-to-use features enhance your presentation slides ten folds. The Coronavirus (COVID-19) Impact Implications & Immediate Actions PPT template is professionally designed with the principles of vision sciences... read more
Forgot Password?
Join the SlideUpLift Discount Club- A Lifetime Value

Benefits never expire and apply to the whole SlideUplift library including future additions.
Upon paying a one time fee, you will remain a Discount Clubber for a lifetime and enjoy 20% discounts on all products that you purchase à la carte from SlideUpLift.com
Privacy Overview
Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.

Related Resources

Sonya’s Story (COVID-19)
A daughter prays that her mother’s death helps others understand the dangers of COVID-19

Contagious Chronicles: Don’t Be A Dreaded Spreader
In this episode, NFID experts offer insights on updated recommendations from the Centers for Disease Control and Prevention (CDC) to help prevent the spread of respiratory viruses, including COVID-19, influenza (flu), and respiratory syncytial virus (RSV)

Contagious Chronicles: Updates on COVID-19, RSV, and More
In the inaugural episode, NFID experts discuss new COVID-19 vaccine recommendations for older adults and other updates from the February 2024 meeting of the Advisory Committee on Immunization Practices (ACIP) …
- Create mode – the default mode when you create a requisition and PunchOut to Bio-Rad. You can create and edit multiple shopping carts
- Edit mode – allows you to edit or modify an existing requisition (prior to submitting). You will be able to modify only the cart that you have PunchedOut to, and will not have access to any other carts
- Inspect mode – when you PunchOut to Bio-Rad from a previously created requisition but without initiating an Edit session, you will be in this mode. You cannot modify any Cart contents
- Order Status
- Quick Order
- Bioprocessing
- Clinical Research
- Drug Discovery & Development
- Translational Research
- Wastewater Surveillance
- Diabetes / Hemoglobinopathies
- Hospital / Clinical Core Lab
- Infectious Disease
- Newborn Screening
- Transfusion Medicine
- Quality Control
- Food & Beverage Testing
- Classroom Education
- Bioprocess Analytics
- Bioprocess Chromatography
- Cell Line Development / Characterization
- Cell Research
- Gene Expression Analysis
- Mutation Detection
- Pathogen Detection
- Protein Expression / Characterization / Quantitation
- Viral / Vector Characterization
- Bacteriology
- Blood Typing, Screening & Antibody Identification
- Hemoglobinopathies
- Infectious Disease Testing
- Molecular Diagnostics
- Data Management Systems
- Proficiency Testing & EQAS
- Verification & Validation
- Food & Beverage Safety Testing
- Cannabis Testing
- Veterinary Diagnostics
- Water Quality Testing
- Biotechnology Textbook & Program
- DNA, PCR & Agarose Gel Electrophoresis
- Genetic Engineering, Microbiology & Model Organisms
- Proteins, Enzymes & ELISA
- COVID-19 Assay & Research
- Cell Isolation & Analysis
- Chromatography
- Digital PCR
- Electrophoresis & Blotting
- Flow Cytometers
- Immunoassays
- PCR & qPCR
- Sample Preparation & Quantitation
- Transfection
- Autoimmune Testing
- Blood Typing & Antibody Detection
- Diabetes Testing
- Hemoglobinopathy Testing
- Microbiology Testing
- Quality Controls
- Software & Data Analysis
- Molecular Testing
- B2B Commerce Solutions
- Custom PCR Plastics & Reagents
- Expert Care Service
- New Labs & New Grants
- Remote Diagnostic Services
- Supply Center Program
- Instrument Service Support Plans
- Trade-Up Program
- Certificate of Analysis
- Literature Library
- Electronic IFUs
- Product Safety Data Sheets
- Quality Management Systems Certificates
- Quality Control Inserts
- Life Science
- Clinical Testing Solutions
- Bioprocess Chromatography Resources
- Classroom Resources
- Product News
- Corporate News
COVID-19 Teaching Resources

Your students may have a lot of questions about COVID-19, from how it spreads to how it is detected and how it can be treated. This presents a rich opportunity to teach key concepts in biology through the lens of an ongoing real-world context. Bio-Rad offers a flexible array of hands-on kits, free resources, and lessons to help you teach the biology and detection of the SARS-CoV-2, the virus that causes COVID-19.
The Biology of SARS-CoV-2 and Detection Methods

What Is the SARS-CoV-2 Coronavirus?
Help your students understand the biology of SARS-CoV-2 by reviewing its origin, structure, and ways to prevent the spread of infection. This PowerPoint presentation walks you and your students through key biology concepts of the SARS-CoV-2 coronavirus.
Download PPT (PPT 16.3 MB)

How Do We Detect COVID-19?
Every day brings new developments in the race for effective and accurate COVID-19 testing, but most strategies are based on a few key fundamental technologies. This PowerPoint presentation explains some fundamental techniques and emerging strategies in COVID-19 detection.
Download PPT (PPT 23.3 MB)
Hands-On Laboratory Activities for Your Students
Teach your students the science behind SARS-CoV-2 detection using these hands-on laboratory activities. Use these three Bio-Rad Explorer Classroom Kits to teach relevant life science concepts in the context of COVID-19.
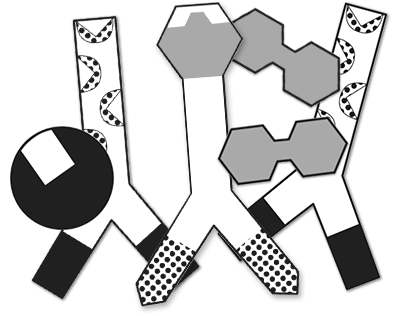
ELISA Antibody Detection
Several existing and emerging SARS-CoV-2 detection methods rely on the specificity of antibodies. In this activity, use real antibodies to determine whether simulated patients are or were infected with SARS-CoV-2.
This activity uses the reagents and antibodies from the ELISA Immuno Explorer Kit .
Download the instructions and presentation (PPT 23.3 MB)

PCR Detection
Investigate the real life spread of SARS-CoV-2 that occurred in a restaurant. In this activity, students use agarose gel electrophoresis to analyze pre-amplified DNA samples from simulated patients and propose ways the virus may have spread.
This activity uses the Virus Detection and Transmission Kit .
Download the instructions and presentation (PPT 13.4 MB)
Real-Time PCR Detection
Real-time PCR is currently the gold standard for COVID-19 diagnosis. In this activity, use real-time PCR to detect SARS-CoV-2 in simulated patient samples. Students analyze amplification and melt curves to determine which patients are positive and then quantify viral RNA.
This activity uses the reagents and DNA samples from the Crime Scene Investigator PCR Basics Real-Time PCR Starter Kit .
Download the instructions and presentation (PPT 27.4 MB)
Additional Resources
ELISA Paper Model Activity
Your students can use this paper model activity to get a solid grasp of the components of an ELISA and how they work together in antibody/antigen detection.
Download PDF (PDF 2.6 MB) Download PPT (PPT 65.9 MB)
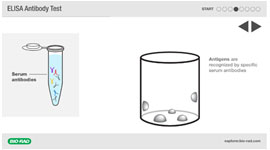
Animation: ELISA Antibody Test Animation: ELISA Antigen Test
Visualize two types of ELISA in these step-by-step animations.
Animation: Polymerase Chain Reaction
The steps of PCR are best visualized through animation.
These pages list our product offerings in these areas. Some products have limited regional availability. If you have a specific question about products available in your area, please contact your local sales office or representative .
- Bio-rad LinkedIn Bio-rad Antibodies LinkedIn
- Bio-rad YouTube Bio-rad Antibodies YouTube
- Bio-rad Twitter Bio-rad Antibodies Twitter
- Bio-rad Facebook Bio-rad Antibodies Facebook
- Bio-rad Instagram
- Bio-rad Pinterest
About Bio-Rad
Bioradiations, sustainability, investor relations.
Clinical Presentation
Clinical considerations for care of children and adults with confirmed COVID-19
‹ View Table of Contents
- The clinical presentation of COVID-19 ranges from asymptomatic to critical illness.
- An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity.
- Uncommon presentations of COVID-19 can occur, might vary by the age of the patient, and are a challenge to recognize.
- In adults, age is the strongest risk factor for severe COVID-19. The risk of severe COVID-19 increases with increasing age especially for persons over 65 years and with increasing number of certain underlying medical conditions .
Incubation Period
Data suggest that incubation periods may differ by SARS-CoV-2 variant. Meta-analyses of studies published in 2020 identified a pooled mean incubation period of 6.5 days from exposure to symptom onset. (1) A study conducted during high levels of Delta variant transmission reported an incubation period of 4.3 days, (2) and studies performed during high levels of Omicron variant transmission reported a median incubation period of 3–4 days. (3,4)
Presentation
People with COVID-19 may be asymptomatic or may commonly experience one or more of the following symptoms (not a comprehensive list) (5) :
- Fever or chills
- Shortness of breath or difficulty breathing
- Myalgia (Muscle or body aches)
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
The clinical presentation of COVID-19 ranges from asymptomatic to severe illness, and COVID-19 symptoms may change over the course of illness. COVID-19 symptoms can be difficult to differentiate from and can overlap with other viral respiratory illnesses such as influenza(flu) and respiratory syncytial virus (RSV) . Because symptoms may progress quickly, close follow-up is needed, especially for:
- older adults
- people with disabilities
- people with immunocompromising conditions, and
- people with medical conditions that place them at greater risk for severe illness or death.
The NIH COVID-19 Treatment Guidelines group SARS-CoV-2 infection into five categories based on severity of illness:
- Asymptomatic or pre-symptomatic infection : people who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test [NAAT] or an antigen test) but who have no symptoms that are consistent with COVID-19.
- Mild illness : people who may have any of the various signs and symptoms of COVID-19 but who do not have shortness of breath, dyspnea, or abnormal chest imaging.
- Moderate illness : people who have evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO 2 ) ≥94% on room air at sea level.
- Severe illness : people who have oxygen saturation <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO 2 /FiO 2 ) <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%
- Critical illness : people who have respiratory failure, septic shock, or multiple organ dysfunction.
Asymptomatic and presymptomatic presentation
Studies have documented SARS-CoV-2 infection in people who never develop symptoms (asymptomatic presentation) and in people who are asymptomatic when tested but develop symptoms later (presymptomatic presentation). ( 6,7 ) It is unclear what percentage of people who initially appear asymptomatic progress to clinical disease. Multiple publications have reported cases of people with abnormalities on chest imaging that are consistent with COVID-19 very early in the course of illness, even before the onset of symptoms or a positive COVID-19 test. (9)
Radiographic Considerations and Findings
Chest radiographs of patients with severe COVID-19 may demonstrate bilateral air-space consolidation. (23) Chest computed tomography (CT) images from patients with COVID-19 may demonstrate bilateral, peripheral ground glass opacities and consolidation. (24,25) Less common CT findings can include intra- or interlobular septal thickening with ground glass opacities (hazy opacity) or focal and rounded areas of ground glass opacity surrounded by a ring or arc of denser consolidation (reverse halo sign). (24)
Multiple studies suggest that abnormalities on CT or chest radiograph may be present in people who are asymptomatic, pre-symptomatic, or before RT-PCR detection of SARS-CoV-2 RNA in nasopharyngeal specimens. (25)

Common COVID-19 symptoms
Fever, cough, shortness of breath, fatigue, headache, and myalgia are among the most commonly reported symptoms in people with COVID-19. (5) Some people with COVID-19 have gastrointestinal symptoms such as nausea, vomiting, or diarrhea, sometimes prior to having fever or lower respiratory tract signs and symptoms. (10) Loss of smell and taste can occur, although these symptoms are reported to be less common since Omicron began circulating, as compared to earlier during the COVID-19 pandemic. (11,19-21) People can experience SARS-CoV-2 infection (asymptomatic or symptomatic), even if they are up to date with their COVID-19 vaccines or were previously infected. (8)
Several studies have reported ocular symptoms associated with SARS-CoV-2 infection, including redness, tearing, dry eye or foreign body sensation, discharge or increased secretions, and eye itching or pain. (13)
A wide range of dermatologic manifestations have been associated with COVID-19; timing of skin manifestations in relation to other COVID-19 symptoms and signs is variable. (14) Some skin manifestations may be associated with increased disease severity. (15) Images of cutaneous findings in COVID-19 are available from the American Academy of Dermatology .
Uncommon COVID-19 symptoms
Less common presentations of COVID-19 can occur. Older adults may present with different symptoms than children and younger adults. Some older adults can experience SARS-CoV-2 infection accompanied by delirium, falls, reduced mobility or generalized weakness, and glycemic changes. ( 12)
Transmission
People infected with SARS-CoV-2 can transmit the virus even if they are asymptomatic or presymptomatic. ( 16) Peak transmissibility appears to occur early during the infectious period (prior to symptom onset until a few days after), but infected persons can shed infectious virus up to 10 days following infection. (22 ) Both vaccinated and unvaccinated people can transmit SARS-CoV-2. ( 17,18) Clinicians should consider encouraging all people to take the following prevention actions to limit SARS-CoV-2 transmission:
- stay up to date with COVID-19 vaccines,
- test for COVID-19 when symptomatic or exposed to someone with COVID-19, as recommended by CDC,
- wear a high-quality mask when recommended,
- avoiding contact with individuals who have suspected or confirmed COVID-19,
- improving ventilation when possible,
- and follow basic health and hand hygiene guidance .
Clinicians should also recommend that people who are infected with SARS-CoV-2, follow CDC guidelines for isolation.
Table of Contents
- › Clinical Presentation
- Clinical Progression, Management, and Treatment
- Special Clinical Considerations
- Bhaskaran K, Bacon S, Evans SJ, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. Jul 2021;6:100109. doi:10.1016/j.lanepe.2021.100109
- Kim L, Garg S, O'Halloran A, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. Jul 16 2020;doi:10.1093/cid/ciaa1012
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Preventing chronic disease. Jul 1 2021;18:E66. doi:10.5888/pcd18.210123
- Ko JY, Danielson ML, Town M, et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. Sep 18 2020;doi:10.1093/cid/ciaa1419
- Wortham JM, Lee JT, Althomsons S, et al. Characteristics of Persons Who Died with COVID-19 - United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. Jul 17 2020;69(28):923-929. doi:10.15585/mmwr.mm6928e1
- Yang X, Zhang J, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. Open Forum Infect Dis. Sep 2021;8(9):ofab428. doi:10.1093/ofid/ofab428
- Rader B.; Gertz AL, D.; Gilmer, M.; Wronski, L.; Astley, C.; Sewalk, K.; Varrelman, T.; Cohen, J.; Parikh, R.; Reese, H.; Reed, C.; Brownstein J. Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep. April 1, 2022;71(13):489–494. doi:http://dx.doi.org/10.15585/mmwr.mm7113e1
- Pingali C, Meghani M, Razzaghi H, et al. COVID-19 Vaccination Coverage Among Insured Persons Aged >/=16 Years, by Race/Ethnicity and Other Selected Characteristics - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021. MMWR Morb Mortal Wkly Rep. Jul 16 2021;70(28):985-990. doi:10.15585/mmwr.mm7028a1
- Wiltz JL, Feehan AK, Molinari NM, et al. Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. Jan 21 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
- Murthy NC, Zell E, Fast HE, et al. Disparities in First Dose COVID-19 Vaccination Coverage among Children 5-11 Years of Age, United States. Emerg Infect Dis. May 2022;28(5):986-989. doi:10.3201/eid2805.220166
- Saelee R, Zell E, Murthy BP, et al. Disparities in COVID-19 Vaccination Coverage Between Urban and Rural Counties - United States, December 14, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. Mar 4 2022;71(9):335-340. doi:10.15585/mmwr.mm7109a2
- Burki TK. The role of antiviral treatment in the COVID-19 pandemic. Lancet Respir Med. Feb 2022;10(2):e18. doi:10.1016/S2213-2600(22)00011-X
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. Feb 10 2022;386(6):509-520. doi:10.1056/NEJMoa2116044
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med. Dec 17 2020;383(25):2477-2478. doi:10.1056/NEJMc2029240
- Jordan TB, Meyers CL, Schrading WA, Donnelly JP. The utility of iPhone oximetry apps: A comparison with standard pulse oximetry measurement in the emergency department. Am J Emerg Med. May 2020;38(5):925-928. doi:10.1016/j.ajem.2019.07.020
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):146-152. doi:10.15585/mmwr.mm7104e4
- Taylor CA, Whitaker M, Anglin O, et al. COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Delta and Omicron Variant Predominance, by Race/Ethnicity and Vaccination Status - COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. Mar 25 2022;71(12):466-473. doi:10.15585/mmwr.mm7112e2
- Johnson AG, Amin AB, Ali AR, et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):132-138. doi:10.15585/mmwr.mm7104e2
- Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 Infection and Hospitalization Among Adults Aged >/=18 Years, by Vaccination Status, Before and During SARS-CoV-2 B.1.1.529 (Omicron) Variant Predominance - Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep. Feb 4 2022;71(5):177-181. doi:10.15585/mmwr.mm7105e1
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Respiratory Syncytial Virus Infection in Older Adults: An Update
- Review Article
- Open access
- Published: 07 May 2024
Cite this article
You have full access to this open access article

- Franco Alfano 1 ,
- Tommaso Bigoni 1 ,
- Francesco Paolo Caggiano 1 &
- Alberto Papi ORCID: orcid.org/0000-0002-6924-4500 1
253 Accesses
1 Altmetric
Explore all metrics
Respiratory syncytial virus (RSV) infection represents one of the most common infections during childhood, with significant morbidity and mortality in newborns and in the early years of life. RSV is a common infection throughout all age groups, largely undetected and underestimated in adults, with a disproportionately high impact in older individuals. RSV infection has a wide range of clinical presentations, from asymptomatic conditions to acute pneumonia and severe life-threatening respiratory distress, including exacerbations of underlying chronic conditions. Overall, the incidence of RSV infections requiring medical attention increases with age, and it is highest among persons ≥ 70 years of age. As a consequence of a combination of an aging population, immunosenescence, and the related increased burden of comorbidities, high-income countries are at risk of developing RSV epidemics. The standard of care for RSV-infected patients remains supportive, including fluids, antipyretics, and oxygen support when needed. There is an urgent need for antivirals and preventive strategies in this population, particularly in individuals at higher risk of severe outcomes following RSV infection. In this review, we describe prevention and treatment strategies for RSV illnesses, with a deep focus on the novel data on vaccination that has become available (Arexvy, GSK, and Abrysvo, Pfizer) for older adults.
Similar content being viewed by others
Respiratory syncytial virus infection in older adults: an under-recognized problem.

Respiratory Syncytial Virus Infection: An Update
The Future of Respiratory Syncytial Virus Disease Prevention and Treatment
Avoid common mistakes on your manuscript.
1 Epidemiology, Virology, and Immunopathology
1.1 introduction.
Respiratory syncytial virus (RSV) is a ubiquitous respiratory virus belonging to the Pneumoviridae family, genus Orthopneumoviridae . There are two subgroups (A and B), differing from each other in their molecular structure. Like other respiratory viruses, RSV infection results in annual recurring events (seasonal epidemics). RSV infection represents one of the most common infections during childhood, with significant morbidity and mortality in newborns and in the early years of life [ 1 ]. In recent decades, epidemiological data have reinforced the evidence of the many faces of RSV infection throughout all age groups, with a disproportionately high impact in elderly individuals. RSV infection has a wide range of clinical presentations, from asymptomatic infections to severe lower respiratory tract infections (LTRIs), including exacerbations of underlying chronic conditions. Overall, RSV-related acute respiratory infections (RSV-ARIs) are largely undetected in adults and substantially underestimated. Currently, two monoclonal antibodies (palivizumab and nirsevimab) are approved and available for preventing RSV infection in the high-risk infant population, whereas no such option is available for adults. Recently, active immunization has been approved in adults based on the efficacy of candidate vaccines in phase III studies. In this article, maintaining a deep focus on elderly individuals and people with comorbidities, we discuss the latest epidemiological data about RSV, its virology and immunopathology, and the clinical characteristics and therapeutic and preventative perspectives of RSV infection.
1.2 Source and Selection Criteria
Our search sites included references in PubMed and Google Scholar between 1957 and 2023. The main search terms included “RSV” and “respiratory infections”, in conjunction with “therapeutics”, “vaccine”, “epidemiology”, “adults”, “elderly”, “frail”, and “comorbidities”. We searched for recent guidelines and consensus statements. We included observational and animal studies if clinically relevant. Finally, we searched ClinicalTrials.gov for updated clinical trial information.
1.3 Epidemiology
In 2015, the Global Burden of Disease (GBD) study estimated 1.7 million deaths caused by LTRIs, with RSV representing one of the important viral pathogens [ 2 ]. RSV has a seasonal pattern of infectivity, commonly seen in respiratory viruses, including influenza, with annual recurrence. In temperate climate countries, it spreads throughout the winter season, with a peak between December and January, whereas in tropical countries, it circulates in the summer season [ 3 ]. Either a single subgroup or both (A and B) remain each season, determining reinfection and seasonal outbreaks [ 3 ]. Reinfections are common throughout life due to a short-term, or incomplete, immunity response. Antigenic variations are considered less important, at variance with other respiratory viruses such as influenza [ 4 ]. Before the coronavirus disease 2019 (COVID-19) pandemic, RSV followed a predictable seasonal pattern of infection. During the pandemic, due to the reduced spread of infections related to generic hygiene procedures such as social distancing, wearing face masks, and hand washing/disinfecting, all respiratory viral infection rates dramatically decreased, especially the clinical manifestations of RSV and influenza. Notably, there was a rebound of viral infections following the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic season [ 5 ]. The concept of “immunity debt” has been proposed for this resurgence of cases, which caused a heavy burden on healthcare services defined as a “tridemic”, a triple threat from the increasing spread of SARS-CoV-2, flu, and RSV at risk of overwhelming the healthcare systems [ 5 ]. After the SARS-CoV-2 pandemic period and the lifting of public health and social measures, a greater number of RSV cases were observed in different countries, with out-of-season epidemics and with a delayed surge of RSV, compared with the usual seasonality [ 6 ].
The highest burden of RSV infections occurs in children < 5 years old (with a global incidence of 17 per 1000 people) and older adults aged > 70 years (incidence of 6.3 per 1000 people) [ 5 ]. A global systematic analysis recently conducted in children aged < 5 years old estimated that 33.0 million RSV-LTRIs occurred in 2019, of which, 6.6 million occurred in infants 0–6 months old, with more than 95% of RSV-LRTIs occurring in low- and middle-income countries [ 7 ]. In children aged < 5 years, 3.6 million RSV hospital admissions were estimated, with 26,300 in-hospital deaths globally [ 7 ]. RSV accounts for as much as 70% of all childhood respiratory infections, with almost all children infected with RSV by the age of 3 [ 8 , 9 ]. Known risk factors for severe RSV infection in younger people include prematurity, age < 2 months, underlying chronic lung or heart diseases, serious neurological or metabolic disorders, Down’s syndrome, immune deficiency, crowded living conditions, and indoor smoke pollution.
Data on RSV infection in elderly individuals and in people with comorbidities are some decades behind the robustness of the paediatric evidence, although they have recently had exponential growth. The RSV burden in three European countries in both healthy older adults and adults with comorbidities is comparable to that caused by influenza [ 10 ]. RSV-ARI is the third most commonly identified viral cause of hospitalization, mostly in adults aged > 65 years old, with less than 1% of adults with RSV infection requiring hospitalization [ 3 ]. Data on adults from developing countries are scarce. No significant difference in sex or economic status has been reported in the incidence of RSV infection, even when tropical and subtropical regions were considered [ 11 , 12 ].
Prospectively, in high-income countries, it is expected in 2025 that the number of RSV-ARIs in older people > 65 years could reach 10 million cases, 800,000 hospitalizations, and 74,000 in-hospital deaths [ 10 ]. A recent meta-analysis conducted by Savic et al. analysed 21 different studies regarding RSV infections in people aged 60 years and older in high-income countries, highlighting that, in 2019, the RSV-ARI attack rate was 1.62% [ 10 ]. The RSV-ARI hospitalization rate was estimated to be 0.15%, while the RSV-ARI in-hospital case fatality rate (hCFR) was 7.13% [ 10 ]. For comparison, each year influenza causes between 3 and 5 million severe cases, with 290,000–650,000 deaths globally, most of them occurring in older patients [ 13 ]. The influenza mortality rate in people aged > 70 years old is 16.4 per 100,000 people, compared to 1.9 in the general population [ 14 ]. Similarly to RSV, the population at higher risk of severe disease or complications due to influenza infection are children under 5 years of age, older people, individuals with chronic medical conditions (i.e. cardiorespiratory, renal, or immunosuppression conditions), and pregnant women [ 15 ].
RSV infection has been increasingly identified as a cause of respiratory viral diseases in adults with comorbidities, including cardiopulmonary diseases. A meta-analysis by Shi et al. considered 20 studies on RSV-ARIs in adults with comorbidities, 18 from developed countries and two from developing countries, and ten in adults aged > 18 years and ten in older groups of > 50 years [ 2 ]. The analysis reported an incidence rate for RSV-ARI in adults with comorbidities (e.g. cystic fibrosis, chronic heart failure [CHF], chronic obstructive pulmonary disease [COPD] immunocompromised status) of 30.3 per 1000 persons per year/season [ 2 ]. The hospitalization rate in CHF or COPD adults aged > 65 years was 13.2 per 1000 persons per year, with an hCFR of 11% [ 16 ].
Indeed, it has been estimated that as a consequence of a combination of an aging population, immunosenescence, and the related increased burden of comorbidities, high-income countries are at risk of developing RSV epidemics [ 17 ]. Notably, clinicians for a long time have not been considering RSV infection as a potential cause of hospitalization and relevant morbidity in adults. This, combined with the lack of specific antiviral treatment, has discouraged physicians from performing RSV diagnostic testing and contributed to the under recognition/estimation of the real impact of RSV (particularity in at-risk populations) [ 10 ]. Indeed, to date, few ad hoc RSV surveillance systems are in place to assess the real impact of RSV infection and to raise awareness of the risks of RSV infections in sensitive populations [ 17 ]. In recent years, RSV testing has substantially increased in relation to more efficient and affordable detection methods, such as the molecular polymerase chain reaction (PCR) technique [ 3 ].
In a recent European study, almost 40% of all RSV-associated hospitalizations occurred in patients aged 65 years and older [ 17 ]. Of the 158,000 RSV-associated hospital admissions among adults older than 18 years old [ 17 ], 92% (145,000) occurred in patients older than 65 years of age (Fig. 1 ). Adults aged 75–84 years showed the highest annual frequency of RSV hospitalization, accounting for 75,000 cases/year, at a rate of 2.24 per 1000 adults annually, with the highest rate among elderly individuals > 85 years of age [ 17 ]. Comparable data have been reported in the United States (US), with a burden of 159,000 RSV hospitalizations in adults > 65 years of age [ 18 ]. The overall mortality for patients admitted for RSV is 6–8%, accounting for 25% of the excess winter mortality [ 3 ]. Data from Southern and Eastern Europe are still scarce. Moreover, even if data available for RSV hospital admission are likely to reflect the overall burden of the infection, to date, the estimated burden of the disease in the general population is still uncertain.
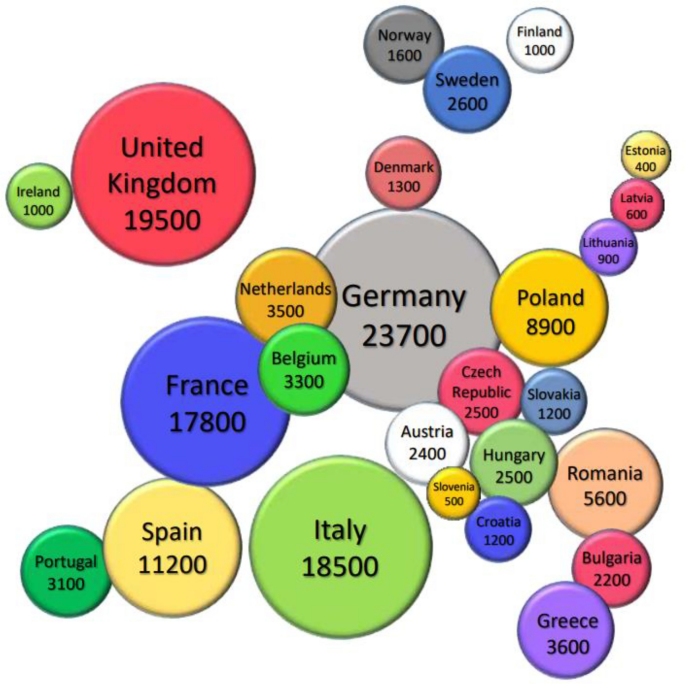
(data from [ 17 ]).
Geographical distribution of the estimated annual respiratory syncytial virus-associated hospitalizations in European Union countries (including the United Kingdom) and Norway in patients aged > 65 years old
Between 10% and 31% of adults hospitalized for RSV infection require intensive care support, while 3–17% of them require mechanical ventilation. Of all high-risk patients (with cardiopulmonary disease or immunodeficiency) infected by RSV, 32% will require hospitalization and 26% will need intensive care support [ 19 ]. Data indicate that mortality rates range from 11 to 18% in hospitalized adults 65 years of age and older with RSV infection [ 2 , 20 ], with an estimated 13% RSV-related case fatality for patients with cardiopulmonary disease among high-risk European adults [ 19 ].
In general, RSV infections occur more frequently in patients with underlying chronic lung diseases. In patients with obstructive lung diseases, RSV is one of the major triggers of acute exacerbations of asthma and COPD [ 3 ]. RSV is the second most common respiratory virus detected in acute exacerbations of COPD, accounting for up to 15% of exacerbations. In COPD patients, the clinical presentation, radiological findings, length of stay, hospitalization, intensive care unit (ICU) admission, and mortality rates of RSV infection are similar to those of influenza [ 21 ]. RSV is also a major risk factor for developing asthma exacerbations, particularly in patients hospitalized for RSV-ARIs in infancy [ 4 ]. Moreover, RSV can induce bronchial hyperresponsiveness, contributing to the pathogenesis of asthma in infancy [ 22 ].
1.4 Virology and Immunopathology
RSV is a respiratory virus belonging to the Pneumoviridae family, Orthopneumoviridae genus. RSV is a single-stranded negative-sense RNA virus containing an approximately 15,200-nt genome and encoding 11 proteins (Fig. 2 ) [ 23 ]. Proteins G and F are the two most relevant surface antigenic proteins, accounting for attachment and fusion, respectively, to host cells. RSV is categorized into two antigenic subtypes, A and B, based on the second hypervariable region of the G gene [ 4 , 24 ]. RSV-A and RSV-B subtypes may cocirculate during an outbreak; RSV-A represents the predominant strain in most years [ 4 ]. Reinfection is frequent and can occur throughout life, while the severity of RSV-ARI tends to diminish after subsequent exposure [ 4 ].
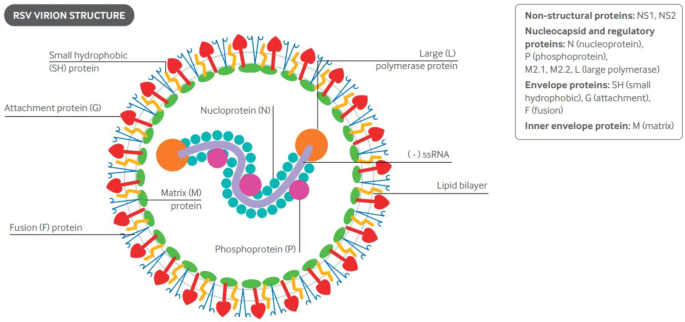
(Reproduced with permission from [ 3 ]). ssRNA single-stranded ribonucleic acid
Structure and genomic RNA of respiratory syncytial virus
RSV spreads through respiratory droplets or fomites. RSV primarily infects polarized ciliated human airway epithelial cells (hAECs) [ 25 ], reaching the upper (URT) and lower respiratory tracts (LRT). hAEC infection determines morphological alterations of infected cells, cilia loss, mucus hypersecretion, and syncytia formation (thus, the name of syncytial virus) [ 4 ]. The G protein (RSV G) is responsible for virus attachment, binding to receptors such as nucleolin, CX3C motif chemokine receptor 1 (CX3CR1), Heparan Sulfate ProteoGlycans (HSPGs), and Intercellular Adhesion Molecule 1 (ICAM-1). After RSV G connects RSV to epithelial cells, RSV F protein merges with the surface membrane and enters through pores into the cytoplasm. Viral entry and infection are catalysed by the prefusion F protein after a conformational change into a post-fusion conformation, fusing the virion to host cells. Herein, viral RNA initiates transcription and viral replication [ 23 ].
During the first phase of infection, host cell pattern recognition receptors (PRRs) recognize RSV and initiate a signalling cascade resulting in the expression of interferon (IFN) molecules, upregulating major histocompatibility complex I (MHC-I), NLR family CARD domain containing 5 (NLRC5), and IFN-β [ 4 ]. During the later stage of RSV infection, Toll-like receptor 3 (TLR3) binds to viral RNA, activating the transcription of Nuclear factor-κB (NF-kB) and type I IFNs. The upregulation of type I IFN predisposes cells to an “antiviral state”, which restricts viral replication and warns and activates uninfected cells [ 4 ]. In mammalian models and humans, RSV proteins such as NS1, NS2, G RSV, and F RSV reduce type I responses, while several RSV proteins can interfere with type III IFNs located on hAECs, facilitating viral replication [ 23 ]. hAEC-infected cells release chemo-attractants and adhesion molecules such as tumour necrosis factor (TNFα), C-X-C motif chemokine ligand 6 (CXCL6), interleukin (IL)-1β, IL-6, IL-8, chemokine ligand 2 (CCL2), granulocyte-monocyte colony stimulating factor (GM-CSF), Macrophage inflammatory protein-1 alpha (MIP-1α), ICAM-1, and MHC-I/II, thus fully activating innate and adaptive immune responses [ 4 ]. Each different RSV protein plays a role in infection and in eliciting an immune response. NS1 and NS2 are involved in immune modulation by interacting with different immune signalling pathways, including the JAK/STAT suppressing pathway, and with the host’s gene expression and activation pathways, including NF-kB. In vitro and in vivo, NS1 and NS2 induce necroptosis of hAECs, an inflammatory programmed cell death, and subsequently inhibiting the classic apoptosis pathway [ 26 ]. RSV proteins in infected hAECs not only interfere with intrinsic and classical immune pathways but also impair the activation of several genes involved in the production of cytokines and chemokines, which are critical for the recruitment of innate and adaptive immune cells [ 26 ].
Indeed, both innate and adaptive immune responses are elicited by RSV infection; they both contribute to the pathogenetic mechanisms related to the infection [ 23 ], as summarized in Fig. 3 [ 21 ]. RSV innate immune responses involve polymorphonuclear leukocytes (PMNs, such as neutrophils and eosinophils), alveolar macrophages (AMs), natural killer (NK) cells, and dendritic cells (DCs).
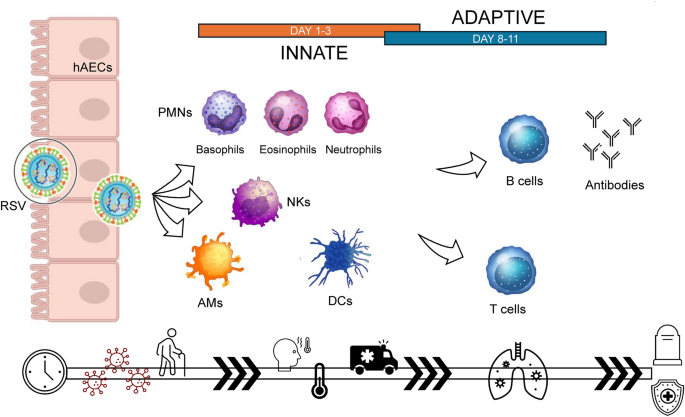
Pathogenesis of RSV infections, involving the innate and adaptive immune response. AMs alveolar macrophages, DCs dendritic cells, hAECs human airway epithelial cells, NKs natural killers, PMNs polymorphonuclear cells, RSV respiratory syncytial virus
Neutrophils can migrate into tissues during acute viral infections and are involved in virus identification and elimination, antigen presentation, recruitment of proinflammatory cells, and cytokine production. Neutrophil activity during RSV infection consists of phagocytosis, degranulation, and NETosis, i.e. a neutrophil-death inflammation signalling pathway. Polymorphonuclear cells exhibit a bivalent role during RSV infection and could lead to more severe and symptomatic infections [ 4 ].
Eosinophils have phagocytic and antigen-presenting activity. RSV-infected in vitro hAECs secrete chemoattractant mediators such as CCL5 or MIP-1α, leading to eosinophil recruitment to the site of infection [ 4 ]. Eosinophils activate signalling cascades, resulting in increased expression of type I IFNs such as IFN-β. Eosinophils contribute to viral elimination by degranulation and secretion of RNA-degrading enzymes, such as eosinophilic cationic protein (ECP) [ 4 ]. Excessive recruitment of eosinophils may result in tissue damage and an unbalanced T cell response towards a T2-high phenotype [ 4 , 22 ]. Indeed, infants with severe RSV disease have a high Th2/Th1 (T helper) ratio profile and an increased risk of developing asthma [ 22 , 23 ].
NK cells are crucial to restrict viral spread in the early stages of infections, given their cytotoxic and immunological activities. In the later stages of infection, NK cells switch to a regulatory phenotype to limit immune cell-mediated lung inflammation [ 4 ]. Functional monocytes migrate into the lungs during viral infections, and their role is pivotal for protection, immune regulation, and supply to the macrophage population during the acute phases of viral infection [ 4 ]. Lung DCs are professional antigen-presenting cells that are chief producers of type I IFNs and bridge innate and adaptive immune responses during viral infection. DC depletion may result in increased viral load [ 4 , 23 ].
Adaptive immune responses to RSV occur via B cell and T cell activation, and B cells prime the production of virus-specific antibodies, neutralizing the virus and thus preventing RSV cell entry. B cells produce antibodies against RSV F (to both the prefusion and post-fusion structures) that are highly neutralizing and cross-react with both RSV-A and RSV-B subtypes [ 27 ]. Unfortunately, RSV-specific B cells rapidly decrease once the infection is over, usually within months after exposure, due to an immunosuppressive effect of RSV on the generation of memory T cells [ 4 ]. CD8+ cytotoxic T cells can directly kill RSV-infected cells, while CD4+ T cells enhance B cell activation and antibody production [ 23 ]. Older adults have comparable RSV antibody responses to younger people, even if they are more susceptible to severe RSV infections, indicating that other immunological parameters, such as dysfunctional T cells, should be considered to account for the immunological deficiencies associated with immunosenescence [ 4 ].
In childhood, the mechanisms of RSV-associated diseases are considered related to the immaturity of the defensive system; in analogy, in elderly people, immunosenescence plays a relevant role in increasing susceptibility to infection and increasing the risk of severe output of the infective event. Immunosenescence refers to the diminished efficiency of the innate and adaptive immune systems due to biological aging [ 28 ]. It is a complex condition accounting for a series of alterations in immune homeostasis and cell regulation, resulting, among others, in increased susceptibility to severe infections, poor vaccine responses, and a higher likelihood of long-term sequelae [ 29 ]. Older adults show reduced viral clearance and mucus production, which favour the occurrence of airway infections [ 4 ]. In the elderly population, B cell and antibody responses are lower in quantity and less efficient than in younger individuals. Similarly, the T cell repertoire is poorer and has low functioning, with dysregulation of cytokine production and fibrosis of lymph nodes [ 29 ]. These factors contribute to increasing susceptibility to infectious diseases. Indeed, influenza-related deaths occur in 70–90% of adults older than 65 years of age, while pneumococcal disease mortality in older adults (15–30%) is higher than that in the younger population [ 29 ]. In addition, as a consequence of the dysregulation of immune responses due to immune senescence, elderly individuals express a lower rate of seroconversion after vaccination than younger individuals, as documented for influenza vaccines [ 30 ].
2 Clinical Presentation, Risk Factors, and Comorbidities in Adult Patients
In general, RSV-ARIs are not clinically distinguishable from those related to other respiratory viruses. There is a wide range of clinical presentations (Table 1 ), from asymptomatic conditions to acute pneumonia and severe life-threatening respiratory distress [ 31 ]. In adults, asymptomatic infections are less frequent than in younger patients (< 5%). The majority of adults develop signs of URT infection, such as rhinorrhoea, nasal congestion (22–78%), or pharyngodynia (16–64%), within 5 days of infection [ 3 ]. LRTIs can cause cough (85–95% of cases), wheezing (33–90%), and dyspnoea (51–93%), reflecting RSV direct/indirect damage to the lower tract [ 3 ]. Nonspecific systemic symptoms are fever, asthenia, and anorexia (48–56%), which could vary in intensity and severity [ 3 ], although they occur less commonly than in influenza infection. Unlike influenza virus infections, whose clinical expression peaks at 2–3 days after the onset of the first symptom, RSV infection typically develops symptoms from 4 to 7 days after exposure, peaking between 7 and 13 days [ 32 ].
RSV LRTIs can cause pneumonia and exacerbations of chronic airway diseases such as asthma or COPD [ 33 ]. Respiratory failure has been reported in 8–13% of older adults hospitalized for RSV, with a mortality of 2–5% [ 34 ]. RSV infections can be complicated by concomitant bacterial or viral respiratory coinfection in up to 21–23% of cases, with the former pathogen coinfection being associated with an enhanced risk of death and severe RSV disease [ 3 ]. During the recent COVID-19 pandemic, significant RSV coinfection was demonstrated in patients with SARS-CoV-2 infections. The most common coinfections were rhinovirus/enterovirus (6.9%), RSV (5.2%), and non–SARS-CoV-2 Coronaviridae (4.3%) [ 35 ].
Clinical manifestations of RSV infection in the elderly are hardly distinguishable from those of influenza or other respiratory viruses, but some symptoms may be more suggestive for one of the pathogens. High fever is more frequently associated with influenza. Similarly, malaise, asthenia, and myalgia, as well as gastrointestinal symptoms are less common in RSV infection than during influenza episodes [ 34 ].
Compared to influenza adults, adults hospitalized with RSV present a longer length of stay (6.0 days vs 3.6 days) [ 17 ] and increased morbidity. In a study conducted on adults in patients hospitalized for influenza-like illness during three consecutive seasons, 15% of RSV-positive patients were admitted to the ICU, and 8% died. The percentages were similar in the influenza-infected individuals. RSV patients were significantly more likely than patients with influenza or without RSV to develop pneumonia (44% vs 28% and 26%, respectively) [ 36 ].
RSV infection usually occurs more than once in life. Although our knowledge of the burden of RSV in the elderly has increased [ 1 , 5 ], it is still lacking compared to the vast literature available for RSV in children [ 2 ]. Indeed, RSV was not considered a potentially serious problem in older adults until the 1970s, when outbreaks of RSV infection occurred in long-term care facilities [ 12 , 37 ]. Overall, the incidence of RSV infections requiring medical attention increases with age, and it is highest (199 episodes per 10,000) among persons ≥ 70 years of age [ 38 ].
2.1 RSV Infections in Older Patients with Chronic Diseases
Many hospitalized RSV infections involve adults with chronic medical conditions. In these cases, even when the RSV infection is clinically mild, it can result in hospital admission, serious complications, and death [ 10 ]. Advanced age, pneumonia, ventilator support, and secondary bacterial infection are all associated with an increased risk of death among hospitalized patients with RSV [ 17 ].
Viral infections, particularly rhinovirus and RSV, are commonly associated with acute exacerbations of asthma [ 39 ] and have been claimed to contribute to the development of asthma in preschool children [ 40 , 41 ]. A prospective study with a cohort of 206 children demonstrated that severe RSV bronchiolitis in the first year of life is followed by a diagnosis of childhood asthma by the seventh birthday in nearly half of children. High asthma rates (48% by age 7 years) have been reported after RSV bronchiolitis in childhood [ 42 ].
RSV infection is an important cause of COPD exacerbation; it has been identified with variable frequencies ranging from 0.8 to 22% of acute cases according to the different diagnostic tools [ 31 ].
A multicentre study was conducted in 28 hospitals between January 2015 and December 2018 in the Republic of South Korea in a cohort of 1177 patients hospitalized with a diagnosis of acute exacerbation of COPD. The most commonly detected viruses were rhinovirus (11%) and influenza virus A (11%), followed by RSV (4.3%) [ 43 ].
In a post hoc analysis of two longitudinal studies that examined RSV infection in high risk adults for ≤ 2 RSV seasons, an increased risk of illness with RSV among patients with COPD was associated with exposure to children and the presence of CHF [ 44 ]. The association between exposure in children and the risk of acquiring RSV illness is not surprising because respiratory secretions of infants with primary infections contain high viral titres of RSV for relatively long periods, and infectious fomites may contaminate environmental surfaces [ 45 ].
In a secondary analysis of a multicentre prospective study in hospitalized patients with acute respiratory diseases in Canada, hospitalized patients with COPD who were RSV positive had a significant morbidity compared to individuals with influenza infection. In hospitalized patients with COPD and RSV infection, 24% needed non-invasive ventilation (vs 11% with influenza) and 18% needed ICU admission. Once in the ICU, half of the patients with RSV required mechanical ventilation. A lower mortality rate (2.8%) was observed among RSV-positive versus influenza-positive individuals. However, a significantly higher proportion of patients with RSV received non-invasive ventilation (23.6% with RSV vs 11.2% with influenza), which may have affected the likelihood of survival in the context of underlying COPD [ 46 ].
The association between cardiopulmonary diseases and the severity of RSV-associated respiratory disease is likely multifactorial and includes changes in immune function, hypoxia, and fever stress and possible prothrombotic changes induced by the infection and the related inflammatory conditions. With disruption of endothelial function, the inflammatory response can lead to plaque destabilization and rupture and thus contribute to acute coronary syndrome, especially in patients already at risk. In a large multicentre review of 607 patients hospitalized with RSV in Hong Kong, 14% of patients had cardiovascular complications; likewise, 13.3% of 547 influenza virus-positive patients had cardiovascular complications. Cardiovascular complications (heart failure, atrial fibrillation, acute coronary event) occurred in 19.4% of adults (18–65 years of age) with RSV infection, as well as 21.3% of immunocompetent elderly (> 65 years of age) and 25.6% of patients with COPD [ 47 ]. In a Canadian study with 22% of patients hospitalized for RSV infection experiencing cardiovascular complications (14% CHF exacerbation, 8% new arrhythmia, 2% stroke, and 1% myocardial infarction), 52% of patients had a past medical history of cardiac disease [ 48 ]. Acute cardiovascular events were the direct cause of mortality in 16.7% of the 72 patients with RSV who died within 60 days of hospital admission [ 33 ].
A number of high-risk factors for RSV infection have been identified in haematopoietic stem cell transplantation, such as male sex, type of transplant (i.e. allogeneic), cytomegalovirus seropositivity, and engraftment status [ 4 , 37 ]. Infection with RSV is also of special concern in lung transplant recipients [ 26 ]. The estimated incidence of community-acquired respiratory virus infections in lung transplant recipients is 15–50 cases per 100 patient-years, and RSV accounts for 19% of these infections (i.e. 2–10 per 100 patient-years) [ 49 ].
3 Diagnostic Tools and Innovation
Four principal methods of diagnosing RSV acute infection are currently available: viral culture; antigen detection by immunofluorescence assay (IFA) or enzyme immunoassay (EIA); RNA detection by reverse transcription-PCR (RT-PCR); and serological assessment of RSV-specific Immunoglobulin M (IgM) antibodies or a significant rise in RSV-specific IgG antibodies between acute- and convalescent-phase sera. The latter method provides only a retrospective diagnosis [ 50 ]. Molecular techniques, such as nucleic acid amplification tests, which can detect very low viral titres, have high diagnostic accuracy in adults and provide rapid results. Reverse transcriptase real-time PCR (RT-PCR) has become the reference diagnostic method for RSV detection [ 51 , 52 ].
Nearly all recent RSV incidence estimates have been obtained by RT-PCR testing of nasal or nasopharyngeal (NP) swabs [ 53 ]. The advantages of adding multiple tools for detection are questionable [ 54 ]. NP swabs have become a common self-administered tool for detecting viral infection during the COVID-19 pandemic; thus, currently, obtaining accurate epidemiological/diagnostic data has become simpler.
In the case of respiratory symptoms appearing in an elderly individual, early detection of RSV would allow a more effective containment of virus spread and prevention of outbreaks, as we learned from the recent COVID-19 pandemic. Additionally, the opportunity to use swabs to simultaneously identify multiple respiratory pathogens would enable a faster and more targeted management approach. Therefore, considering the simplicity of (and familiarity with) NP swab collection in the general population (particularly among healthcare professionals and caregivers), the use of tools such as nasal and NP swabs should be encouraged, particularly in nursing homes and long-term care facilities, where the most vulnerable individuals reside and are at higher risk of severe clinical manifestations.
Radiological findings associated with RSV infection are usually nonspecific and nondiagnostic, particularly on chest X-ray.
In an observational and retrospective study, hospitalized adults with laboratory-confirmed RSV infection underwent chest radiography at admission. The chest radiography was abnormal in 50% of patients, with consolidation (48%) and ground glass opacity (GGO) in a single unilateral lower zone (40%) being the most frequent findings. [ 55 ].
Computed tomography may reveal pulmonary nodules and GGOs [ 56 ]. In general, imaging studies should be considered in most severe cases for differential diagnosis. In children with RSV infection (1 day to 10 years [median 7 months]), chest X-ray demonstrates radiological findings of central pneumonia and peribronchitis or the absence of abnormalities in equal proportions [ 57 ]. In an observational study in adults, lung bilateral involvement was less frequently reported in RSV cases than in other non-RSV viral infections [ 58 ].
The diagnosis of RSV infection in immunocompromised individuals is also associated with findings such as GGOs, nodular lesions, and signs of organizing pneumonia. In contrast to RSV, tree-in-bud opacities are frequently reported in influenza infections [ 59 ].
4 Management and Prevention
The standard of care for RSV-infected patients remains supportive, including fluids, antipyretics, and oxygen support when needed [ 60 ]. In children, only short-term clinical benefits are achieved with the use of available drugs and antivirals, with little or no effect from pharmacotherapy-based bronchodilators and glucocorticoids. There is no recommendation for the routine use of 3% nebulized hypertonic solution in children experiencing acute bronchiolitis [ 61 ]. Two antibody drugs, palivizumab and nirsevimab, have been approved for preventing RSV infection in infants. No such option is currently available for the treatment of RSV infection in adults. Therefore, there is an urgent need for antivirals and preventive strategies in this population, particularly in individuals at higher risk of severe outcomes following RSV infection. In the next sections, we will analyse some of the molecules currently under investigation for the treatment of RSV infection, as well as novel data for the prevention of RSV infection in adults/elderly people.
4.1 Pharmacological Treatment
A number of treatments (mainly antivirals) have been developed and tested in RSV acute infections. They have been recently reviewed in detail [ 62 ]. Here, we summarized only some of the relevant data and antiviral drugs currently in clinical development.
4.1.1 Ribavirin
The only antiviral treatment for RSV approved by the Food and Drug Administration (FDA) in infants is ribavirin. It inhibits replication of both RNA and DNA viruses. Ribavirin can be administered intravenously, orally, and by aerosolization and has often been given in combination with intravenous immunoglobulin.
Compared to the original inhaled formulation, the oral formulation is equally effective and cheaper; thus, it is the preferred route of administration. The efficacy data are controversial, and use is restricted to life-threatening RSV LRTIs and lung transplant recipients [ 63 ].
4.1.2 Presatovir (GS-5806)
GS-5806 is an oral small molecule that inhibits RSV entry at low nanomolar concentrations by blocking viral-envelope fusion with the host cell membrane. A phase I study in humans showed no adverse events. The study was conducted in healthy adults experimentally infected with a clinical strain of RSV and showed a significant reduction in the viral load, along with a reduction in mucus production and the total symptom score [ 64 ]. A phase IIb study conducted in patients undergoing lung transplantation found no efficacy in reducing the viral load, symptom improvement, or prevention of lung function deterioration in lung transplant recipients infected with RSV [ 49 ].
4.1.3 Sisunatovir (RV521)
RV521 is an orally bioavailable inhibitor of RSV fusion to host cells. Oral bioavailability in preclinical species ranged from 42 to > 100%, with evidence of highly efficient penetration into lung tissue. In healthy adult human volunteers experimentally infected with RSV, a potent antiviral effect was observed with a significant reduction in both viral load and symptoms [ 65 ].
4.1.4 Ziresovir (AK0529)
Ziresovir is an RSV fusion inhibitor that reduces cytopathic effects in HEp-2 cell cultures [ 66 ] infected with both RSV A and B strains [ 67 ]. In a phase II study, AK0529 was well tolerated in hospitalized RSV-infected infant patients, and treatment with AK0529 2 mg/kg bid reduced viral load [ 68 ].
4.2 Prevention
4.2.1 hygiene measures.
As for other respiratory viruses, RSV is classically spread through inhalation of aerosolized droplets and, especially in children, by contact with fomites. Good hygiene practices therefore have a fundamental role in limiting the transmission of this infection. Multiple strategies could be applied, such as careful and periodic hand washing, social distancing, especially outside the house and with non-family members, home isolation of people presenting respiratory symptoms, covering coughs and sneezes with a single-use tissue or the elbow, using easily cleanable toys if working with children, cleaning frequently touched surfaces with bleach solution or disinfectants, and wearing a facial mask in public situations when social distancing is not feasible. Furthermore, structural and procedural measures should be implemented for the frailest population, such as those living in long-term care facilities. During the COVID-19 pandemic, the broad application of these rules deeply impacted the circulation of all respiratory viruses, including RSV, as reported by the Centers for Disease Control and Prevention (CDC). In the 4 years before the COVID-19 pandemic, the weekly percentage of positivity of RSV detection tests in the US usually rose from 3% to 12.5–16.7% between October and December. In contrast, from January to April 2020, it fell from 15.3% to 1.4% and persisted < 1% weekly during the next year. From October 2020 to April 2021, the cumulative incidence of hospitalization related to RSV disease decreased to 0.3 cases per 100,000 people, starting from 27.1 and 33.4 in the preceding two seasons (Fig. 4 ) [ 69 ]. In addition, COVID-19 spread in nursing homes and long-term care facilities was deeply reduced by procedural and structural measures, such as protected zones for family visits, entry restrictions, and COVID-19 isolation areas [ 70 ].
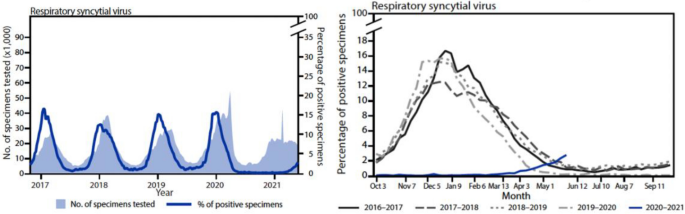
Evolution of RSV epidemiology during the COVID-19 pandemic. Reproduced from Ref. [ 69 ] (Content source: Centers for Disease Control and Prevention). COVID-19 coronavirus disease 2019, RSV respiratory syncytial virus
Preventive hygiene measures should therefore be performed, especially in older people, those with social contact with children and frail patients.
4.2.2 Passive Immunization
The main purpose of passive immunization consists of reducing the spread of RSV from the URT to the LRT using anti-RSV antibodies, limiting the severity of the infection to an URT infection. Currently, passive immunization is an option exclusively for high-risk infants [ 71 ]. Studies are needed to evaluate the efficacy and cost-effectiveness of these drugs in adult patients to provide more preventive opportunities, especially for those with a contraindication to vaccination.
4.2.2.1 Palivizumab
Palivizumab, an RSV-specific monoclonal antibody approved for the prevention of RSV infection, is effective in reducing RSV hospitalization rates by approximately 60%. The use is indicated in infants at risk (overall in 3% of the total infant population), e.g. those who are born prematurely or have underlying conditions (chronic lung disease, congenital heart disease, immunodeficiencies, or other severe chronic illnesses). This antibody protects against severe disease; it is expensive and therefore, it is only used in high-income countries of high-risk preterm infants. Palivizumab exhibits no efficacy in the treatment of RSV infection [ 72 ].
Some evidence has shown a safe and well-tolerated profile of this drug in immunocompromised adults [ 73 , 74 , 75 , 76 ], although it has been tested in small studies and there is no approved indication.
4.2.2.2 Nirsevimab
Nirsevimab is a derivative antibody from a precursor of the prefusion F protein; it has been engineered to increase efficacy and prolong the antibody half-life. In a recent efficacy trial in infants, nirsevimab achieved the primary endpoint of reduction of medically attended LRTIs and the secondary endpoint of reduction of hospitalizations and was granted prime eligibility status by the European Medicines Agency (EMA) and breakthrough status by the US FDA. This monoclonal antibody was 100 times more potent than palivizumab. Thanks to the increased antibody efficacy and the extended half-life via engineering technology, a single dose administration at birth provides protection for the entire RSV first season [ 77 ]. Nirsevimab is currently recommended only in infants [ 78 ].
4.2.2.3 Clesrovimab (MK-1654)
Clesrovimab is a monoclonal antibody similar to nirsevimab, and it targets site IV of the RSV prefusion F protein and the same YTE mutation. Studies in children are ongoing to evaluate whether clesrovimab reduces the incidence of RSV-associated medically attended LTRI from day 1 through day 150 post-dose and to assess its safety profile [ 79 ].
4.2.3 Active Immunization
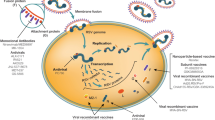
In the last decade, there have been multiple efforts to develop RSV vaccines, with variable results. Several approaches have been developed, such as live attenuated vaccines (LAVs) for infants, stabilized pre-F subunit vaccines for pregnant women, and subunit, vector-based and nucleic acid vaccines for older adults (Fig. 5 ) [ 80 ].
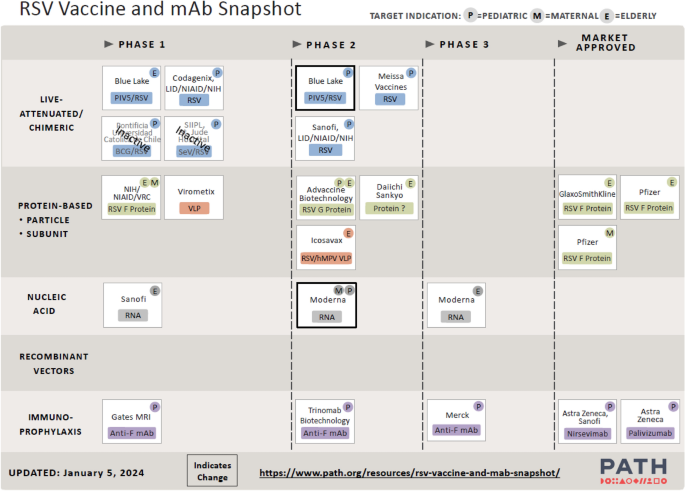
Picture of the effort to track the development of RSV vaccine and mAb candidates worldwide. (Reproduced from the PATH website at www.path.org , April 2024). mAb monoclonal antibody, RSV respiratory syncytial virus, BCG Bacillus Calmette-Guérin, hMPV human metapneumovirus, NIAID National Institute of Allergy and Infectious Diseases, NIH National Institutes of Health, PIV5 Parainfluenza virus 5, SeV Sendai virus, VLP virus-like particle, VRC Vaccine Research Center
LAVs, attenuated through various techniques to reduce virulence [ 81 ], mimic natural RSV infection and are able to induce both a potent humoral mucosal and cellular immune response. Several LAV candidates have been developed and are currently being tested in phase I or II trials, with promising results. Among the others, MV-012-968 was reported to be safe and able to induce a mucosal IgA response in seropositive patients [ 82 ].
Chimeric live virus vaccines consist of related attenuated viral particles expressing RSV-specific proteins. They are reported to have more effective antigen presentation than vectored vaccines, leading to the activation of an intense specific adaptive immune response with a good safety profile [ 83 , 84 ]. Among the two chimeric vaccines that are being studied, the rBCG-N-hRSV vaccine, an attenuated recombinant BCG (Bacillus Calmette-Guérin) vaccine producing RSV nucleoprotein, was proven to be well tolerated in a phase I trial [ 85 ].
Subunit and inactivated virus vaccine development has been abandoned since a formalin-inactivated RSV vaccine was reported to have enhanced the severity of the RSV disease of the first natural RSV infection subsequent to vaccination in RSV-naive children [ 86 ].
In adults, the Adult Respiratory Syncytial Virus (AReSVi-006) study was the first positive trial with a subunit RSV vaccine. It is an ongoing, randomized, multicentric, placebo-controlled trial evaluating the efficacy of the RSVPreF3 OA vaccine (Arexvy, GSK) in adults aged 60 years old or older. This vaccine includes a stabilized RSV F protein, which stimulates the development of specific neutralizing antibodies, and the AS01E adjuvant system. The primary outcome consisted of evaluating the efficacy of this vaccine in preventing RSV-lower respiratory tract disease (LRTD) in older patients during the first season after vaccination. The definition of RSV-LRTD relied on the presence of at least three LRT symptoms or two LRT symptoms or signs (with at least one sign) for at least 1 day. Approximately 25,000 patients were included in this study and randomized 1:1 to receive either placebo or a single dose of vaccine (treatment arm) before the commencement of the RSV season. This vaccination reduced the number of patients who had an RSV-LRTD, with vaccine efficacy detected: 82.6% (96.95% confidence interval [CI] 57.9–94.1) for a median follow-up of 6.7 months (Fig. 6 ). A similar result was achieved in terms of severe RSV-LRTD, with a vaccine efficacy of 94.1% (95% CI 62.4–99.9). The numbers of patients who reported at least one acute respiratory infection (ARI) due to RSV, defined as at least one systemic plus one respiratory sign or symptom or no less than two respiratory signs or symptoms for at least 1 day, were 95 and 27 in the two groups, respectively (vaccine efficacy of 71.7% [95% CI 56.2–82.3]). An equivalent efficacy was reported according to viral subtype (A or B), and it remained high in the two major age groups (60–69 and 70–79) and in frail adults. The responses were specifically evaluated in subgroups according to the presence of specific comorbidities known to be associated with a higher risk of severe disease (described as “conditions of interest” [COI]), such as cardiopulmonary, endocrine, and metabolic comorbidities, where vaccine efficacy was similarly obtained. In addition, the investigational vaccine had a good safety profile [ 87 ].
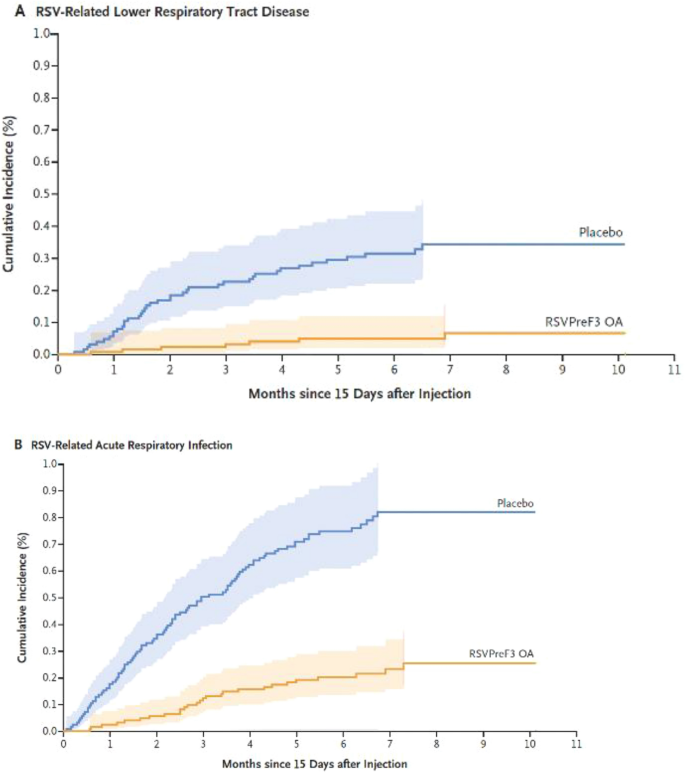
RSVPreF3 OA vaccine efficacy in reducing incidence of RSV-LRTD and acute respiratory infections. (Reproduced with permission from [ 87 ]). LRTD lower respiratory tract disease, RSV respiratory syncytial virus
For the second season, the initial cohort of patients was divided into three subgroups (placebo, annual vaccination, and single dose) evaluating the vaccine efficacies over two seasons of annual revaccination and single dose. The annual revaccination group showed a vaccine efficacy over two seasons of 67.1% (97.5% CI 48.1–80.0), similar to that of the single dose of vaccine (67.2%, 97.5% CI 48.2–80.0). A sustained vaccine efficacy over two seasons was also reported against RSV-ARI, severe RSV-LRTD, and RSV-LRTD in the groups 60–69 years old and 70–79 years old, patients with at least one COI, and in the prefrail subgroup [ 88 ].
The preventive efficacy on RSV-LRTD and ARI was elevated in patients with at least one COI (94.6% and 81.0%, respectively), at least one cardiopulmonary comorbidity (92.1% and 88.1%, respectively), at least one endocrine/metabolic comorbidity (100% and 79.4%, respectively), and at least two COI (92.0% and 88.0%, respectively). Similarly, the antibody responses to the vaccine were not influenced by the presence of at least one COI [ 89 ].
The RSV Vaccine Efficacy Study in Older Adults Immunized against RSV Disease (RENOIR) trial was a randomized 1:1, multicentre, placebo-control, phase III study evaluating a bivalent vaccine containing an RSV prefusion F protein (RSVpreF vaccine [Abrysvo, Pfizer]) from the two major viral subgroups (RSV-A and B) in older adults 60 years old or older. This formulation did not include an adjuvant system. At the time of this publication, this study was ongoing [ 90 ]. The two primary outcomes of the prespecified interim analysis were the evaluation of the efficacy of the investigational vaccine in the prevention of RSV-LRTD with either two or three symptoms or signs with a duration of at least 24 h in older patients with an RSV infection confirmed by an RT-PCR assay or equivalent test. Approximately 34,000 patients were randomized to receive either the RSVpreF vaccine (treatment arm) or placebo before the beginning of the RSV season, and the mean follow-up was 7 months. The reported RSV-LRTDs with at least two signs/symptoms were 1.19 per 1000 person-years of follow-up in the treatment arm and 3.58 per 1000 person-years of follow-up in the placebo arm (the vaccine efficacy was 66.7%, 96.66% CI 28.8–85.8). RSV-LRTDs with at least three signs/symptoms were 0.22 per 1000 person-years of follow-up in the treatment group and 1.52 per 1000 person-years of follow-up in the other one (the vaccine efficacy was 85.7%, 96.66% CI 32.0–98.7). In addition, the vaccine efficacy did not significantly differ according to viral subtype (A or B), age group (60–69, 70–79, or ≥ 80), and presence of high-risk conditions for adverse outcomes. The cases of RSV-ARI with at least one sign or symptom and a confirmed infection (RT-PCR assay) were 2.38 per 1000 person-years and 6.30 per 1000 person-years in the two study arms, respectively (vaccine efficacy was 62.1%, 95% CI 37.1–77.9). Interestingly, the vaccine efficacy increased with increasing severity of RSV-LRTD, in concordance with the available evidence for other vaccines (e.g. SARS-CoV-2 or influenza virus) [ 90 , 91 , 92 , 93 ].
Of note, the efficacy of the RSVpreF vaccine evaluated in the RENOIR study (adults) was tested in pregnancy for the reduction of RSV-LRTD in infants in the Maternal Immunization Study for Safety and Efficacy (MATISSE) study. The trial showed a significant reduction in medical attended severe RSV-LRTD within 90 and 180 days after delivery and no significant difference for the total cases of RSV-LRTD analysis [ 94 ].
mRNA vaccines consist of messenger RNA coding for specific viral antigens that are able to induce a specific immune response (this vaccination platform was used with COVID-19). The mRNA-1345 vaccine (Moderna) consists of an mRNA encoding a stabilized RSV pre-F protein and a lipid formulation that is able to induce and augment the humoral and cellular immune response to this protein. The ConquerRSV study was a randomized 1:1, multicentre, placebo-controlled, phase III trial evaluating the mRNA-1345 vaccine in a population of approximately 37,000 adults of at least 60 years of age. The two primary outcomes were the prevention of RSV-LRTD with at least two or three symptoms. An interim analysis showed a vaccine efficacy of 83.7% (95.88% CI 66.0–92.2%, p < 0.0001) in reducing RSV-LRTD defined as at least two signs or symptoms, with 55 and nine cases reported in the placebo arm and vaccine arm, respectively. In addition, RSV-LRTDs with at least three signs or symptoms were reduced from 17 in the placebo arm to three in the vaccine arm, corresponding to a vaccine efficacy of 82.4% (96.36% CI 34.8–95.3%, p = 0.0078). The investigational vaccine was reported to have a good safety profile and was well tolerated [ 95 ]. The company announced that marketing authorization applications for this investigational vaccine had been submitted to EMA, Swissmedic, and the Therapeutic Goods Administration (TGA) in Australia, along with a rolling submission of a Biologics License Application to the FDA [ 96 ].
Other different vaccine strategies of RSV vaccination already tested and/or currently in development for adults have been recently reviewed in detail [ 62 ]. Here, we summarized only some of the relevant data.
The particle-based vaccines are based on the presentation of multiple antigens via assembled particles inducing a highly effective immune response. These investigational vaccines are currently in early development but with promising results. Icosavax’s IVX-121 consists of a synthetic virus-like particle that delivers multiple copies of stabilized trimeric pre-F proteins and was reported to induce a persistent humoral response at 12 months after the first administration in the interim analysis of the phase Ib extension trial [ 97 ].
The IVX-A12, a combination of the RSV vaccine IVX-121 and the human metapneumovirus (hMPV) vaccine IVX-241, is currently being evaluated in adults in an ongoing phase II clinical study. [ 98 ]
Recombinant vector vaccines consist of modified replication-defective viruses that contain RSV genes codifying for specific antigens stimulating a specific cellular and antibody response. In this field, the MVA-BN-RSV vaccine (Modified vaccinia Ankara-Bavarian Nordic) consists of a poxvirus acting as a vector to express RSV intracellular proteins, such as N and M2, and surface proteins F and G [ 99 ]. MVA-BN-RSV vaccination was associated with a depletion of viral load, a 79% reduction in RSV disease, and a high title humoral response lasting at least 6 months after administration [ 100 , 101 ]. The phase III VANIR study evaluated the efficacy of this vaccine in more than 20,000 participants aged over 60 years. A recent press release reported the preliminary results with a 59% vaccine efficacy in the prevention of LRTD with at least two symptoms. A 42.9% efficacy was reported in the prevention of LRTD with at least three symptoms, which failed to meet one of the co-primary outcomes of the trial, resulting in the discontinuation of Bavarian Nordic’s RSV vaccine development programme [ 102 ].
The Ad26-RSV-pre-F vaccine (Janssen) consists of an adenoviral vector that leads to the expression of the RSV pre-F protein in combination with a recombinant pre-F protein. Ad26-RSV-pre-F was evaluated in the CYPRESS study, a phase IIb, proof-of-concept trial. The primary endpoint was the first occurrence of RSV-LRTD, showing an efficacy of 80% for the most severe disease, defined as three or more LRTD symptoms (94.2% CI 52.2–92.9, p < 0.001) and 69.8% for all RSV-ARIs (94.2% CI 43.7–84.7, p < 0.001). The vaccine reduced the severity of symptoms with a faster recovery compared to placebo [ 103 ]. The EVERGREEN study, a phase III trial evaluating this vaccine in older adults, was finally discontinued following a company’s assessment of the RSV vaccine landscape [ 104 , 105 ].
Based on evidence and decisions of regulatory agencies, Arexvy and Abrysvo have been approved and are currently available in the US and Europe. To date, both the FDA and EMA, together with the 2024 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, recommend RSV vaccination in patients older than 60 years old and/or with chronic heart and lung comorbidities. The CDC suggests a single dose of the RSV vaccine for those older than 60 years old, preferably administered a few months before the viral seasonal peak, such as in late summer or early fall. The CDC suggests a shared clinical decision-making (SDM) approach for RSV vaccination. In other words, the decision to proceed with vaccination should be based on an informed patient’s preference reached through an active discussion with the healthcare provider (HCP). This discussion should cover various topics, including available evidence, the patient's health status, risk factors for severe RSV disease, HCP’s suggestions, and the safety profile of the vaccine, among others [ 106 , 107 , 108 , 109 , 110 , 111 ].
In the UK, the Joint Committee on Vaccination and Immunisation (JCVI) suggests RSV vaccination for adults aged at least 75 years old [ 112 ].
In Spain, the Spanish NeumoExperts Prevention Group (NEP) recommends this vaccination among adults over 60 years old, prioritizing those with chronic comorbidities deemed at high risk of severe RSV illness, as well as healthcare workers, who are at elevated risk of both acquiring and transmitting RSV [ 113 ].
Both vaccines should be administered via intramuscular route into the deltoid muscle, and Abrysvo is the only vaccine approved in pregnancy [ 114 , 115 , 116 ].
5 Discussion
Not considered a disease of the youngest anymore, RSV burden remains to date an underestimated cause of morbidity and hospitalization globally in the elderly and immunocompromised population. RSV is an insidious respiratory infection, partly due to nonspecific symptoms, often similar to other viral respiratory tract infections, along with a seasonal pattern of transmission during the wintertime in epidemic outbreaks that recurs annually [ 3 ]. Early diagnosis is challenging, particularly in elderly or fragile patients, where the virus can lead to more severe clinical presentations such as pneumonia, respiratory failure, and Acute Respiratory Distress Syndrome (ARDS). Among the elderly older than 65 years, the European Union is facing 145,000 RSV-associated hospitalizations each year, and the US, 159,000. The mortality rate in elderly hospitalized RSV patients is significant, up to 18% [ 1 , 20 ]. The documented coinfection with other respiratory pathogens, which further complicates the clinical presentation, should not be overlooked. Several studies have demonstrated an increased risk of exacerbation of chronic respiratory diseases (such as asthma and COPD) and an elevated cardiovascular risk in both immunocompetent elderly individuals and those with multiple comorbidities, adding to the overall burden on public health costs. Currently, the most accurate and rapid diagnostic investigation for detecting RSV is RT-PCR amplification on nasal or NP swabs. During the recent COVID-19 pandemic, these tests have become readily available and easy to perform, even by the patients' caregivers themselves. At present, instrumental radiological examinations do not allow the specific detection of pleuro-parenchymal alterations caused by this pathogen. Due to the absence of effective therapies against RSV disease, prophylaxis has rapidly become the most efficient measure to reduce RSV diffusion and clinical impact in the population, especially among the most vulnerable patients such as older adults, polymorbid patients, or those living in nursing facilities. Hygiene measures, such as procedural or structural interventions, have significantly lowered respiratory virus transmission [ 70 ], but active prophylaxis remains essential and should be pursued, targeting those at higher risk of severe disease. Several clinical trials have been conducted and published that show a significant reduction in the incidence of RSV disease, especially in its most severe form. In addition, vaccination was proven to be highly effective regardless of the presence of specific comorbidities and persisted as effective across different seasons [ 87 , 88 , 89 , 90 ]. As a result, regulatory agencies such as the EMA and the FDA have now included RSVPreF3 (Arexvy, GSK) and RSVpreF (Abrysvo, Pfizer) in the suggested vaccinations for individuals over 60 years of age. While Arexvy contains the AS01E adjuvant system, the latter does not contain any adjuvant. Both vaccines protect against the two major RSV subtypes (A and B); Abrysvo is bivalent, containing an RSV prefusion F protein from both RSV-A and B, while Arexvy, even though it is not technically bivalent, demonstrated an optimal efficacy for both subtypes in clinical trials [ 87 , 90 ]. Both Arexvy and Abrysvo can be administered on the same occasion of the influenza vaccination, and national health authorities may approve the simultaneous administration of RSV vaccines with the others [ 111 , 117 ].
In the last decades, significant economic resources have been invested in influenza vaccination. Now it is the time to invest also in RSV vaccination, leading to wider protection against seasonal respiratory viruses in frail and older patients.
6 Conclusions
RSV is a ubiquitous respiratory virus with a seasonal pattern of infection. While RSV represents one of the most common infections during childhood, epidemiological data indicate a substantial impact throughout all ages, with a disproportionately high impact in elderly individuals. RSV carries a serious burden affecting older adults with comorbidities, i.e. the frailest population. No therapeutic option is available for adults. Active immunisation has been recently approved in older adults aged > 60 years old based on the results of multiple phase III studies, such as the RENOIR and AReSVi-006 studies, showing a substantial reduction of RSV-related LTRD and severe LTRD [ 87 , 90 ]. In addition, a single dose of Arexvy has been shown to be as effective as annual revaccination, simplifying the vaccination schedule [ 88 ]. To date, vaccinations represent the best protection from developing severe disease and serious adverse events related to RSV infection, especially for the elderly population with multimorbidities (in particular, cardiopulmonary and metabolic) and immunocompromised individuals.
Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health. 2015;5(1): 010408.
Article PubMed PubMed Central Google Scholar
Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H, Investigators R. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis. 2022;226(Suppl 1):S17–21.
Article PubMed Google Scholar
Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;10(366): l5021.
Article Google Scholar
Agac A, Kolbe SM, Ludlow M, Osterhaus A, Meineke R, Rimmelzwaan GF. Host responses to respiratory syncytial virus infection. Viruses. 2023;15(10):1999.
Article CAS PubMed PubMed Central Google Scholar
Piralla A, Chen Z, Zaraket H. An update on respiratory syncytial virus. BMC Infect Dis. 2023;23(1):734.
Chuang YC, Lin KP, Wang LA, Yeh TK, Liu PY. The impact of the COVID-19 pandemic on respiratory syncytial virus infection: a narrative review. Infect Drug Resist. 2023;16:661–75.
Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):11S-S15.
Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94(8):1101–8.
Savic M, Penders Y, Shi T, Branche A, Pircon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023;17(1): e13031.
Article CAS PubMed Google Scholar
Childs A, Zullo AR, Joyce NR, McConeghy KW, van Aalst R, Moyo P, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(1):210.
Vikerfors T, Grandien M, Olcen P. Respiratory syncytial virus infections in adults. Am Rev Respir Dis. 1987;136(3):561–4.
Thompson WW, Weintraub E, Dhankhar P, Cheng PY, Brammer L, Meltzer MI, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3(1):37–49.
Conrad A, Valour F, Vanhems P. Burden of influenza in the elderly: a narrative review. Curr Opin Infect Dis. 2023;36(4):296–302.
World Health Organization. Influenza (Seasonal). [cited 2024 8 April]. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) .
Falsey AR, Walsh EE, Esser MT, Shoemaker K, Yu L, Griffin MP. Respiratory syncytial virus-associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J Med Virol. 2019;91(1):65–71.
Osei-Yeboah R, Spreeuwenberg P, Del Riccio M, Fischer TK, Egeskov-Cavling AM, Boas H, et al. Estimation of the number of respiratory syncytial virus-associated hospitalizations in adults in the European Union. J Infect Dis. 2023;228(11):1539–48.
McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300.
Nguyen-Van-Tam JS, O’Leary M, Martin ET, Heijnen E, Callendret B, Fleischhackl R, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31(166): 220105.
Kwon YS, Park SH, Kim MA, Kim HJ, Park JS, Lee MY, et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect Dis. 2017;17(1):785.
Ramaswamy M, Groskreutz DJ, Look DC. Recognizing the importance of respiratory syncytial virus in chronic obstructive pulmonary disease. COPD. 2009;6(1):64–75.
Urbani F, Cometa M, Martelli C, Santoli F, Rana R, Ursitti A, et al. Update on virus-induced asthma exacerbations. Expert Rev Clin Immunol. 2023;19(10):1259–72.
Bergeron HC, Tripp RA. Immunopathology of RSV: an updated review. Viruses. 2021;13(12):2478.
Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987;84(16):5625–9.
Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(12):519–30.
Van Royen T, Rossey I, Sedeyn K, Schepens B, Saelens X. How RSV proteins join forces to overcome the host innate immune response. Viruses. 2022;14(2):419.
Andreano E, Paciello I, Bardelli M, Tavarini S, Sammicheli C, Frigimelica E, et al. The respiratory syncytial virus (RSV) prefusion F-protein functional antibody repertoire in adult healthy donors. EMBO Mol Med. 2021;13(6): e14035.
Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82(1):50–5.
Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25.
Nunez IA, Carlock MA, Allen JD, Owino SO, Moehling KK, Nowalk P, et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS ONE. 2017;12(11): e0185666.
Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(6):639–43.
Kaler J, Hussain A, Patel K, Hernandez T, Ray S. Respiratory syncytial virus: a comprehensive review of transmission, pathophysiology, and manifestation. Cureus. 2023;15(3): e36342.
PubMed PubMed Central Google Scholar
Lee N, Lui GC, Wong KT, Li TC, Tse EC, Chan JY, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–77.
Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–9.
Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–6.
Loubet P, Lenzi N, Valette M, Foulongne V, Krivine A, Houhou N, et al. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin Microbiol Infect. 2017;23(4):253–9.
Hart RJ. An outbreak of respiratory syncytial virus infection in an old people’s home. J Infect. 1984;8(3):259–61.
Belongia EA, King JP, Kieke BA, Pluta J, Al-Hilli A, Meece JK, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults >/=60 years old. Open Forum Infect Dis. 2018;5(12):ofy316.
Dulek DE, Peebles RS Jr. Viruses and asthma. Biochim Biophys Acta. 2011;1810(11):1080–90.
Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–10.
Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9(9):731–45.
Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130(1):91-100 e3.
Sim YS, Lee JH, Lee EG, Choi JY, Lee CH, An TJ, et al. COPD exacerbation-related pathogens and previous COPD treatment. J Clin Med. 2022;12(1):111.
Mehta J, Walsh EE, Mahadevia PJ, Falsey AR. Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD. 2013;10(3):293–9.
Hall CB, Douglas RG Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr. 1976;89(1):11–5.
Mulpuru S, Andrew MK, Ye L, Hatchette T, LeBlanc J, El-Sherif M, et al. Impact of respiratory viral infections on mortality and critical illness among hospitalized patients with chronic obstructive pulmonary disease. Influenza Other Respir Viruses. 2022;16(6):1172–82.
Anderson NW, Binnicker MJ, Harris DM, Chirila RM, Brumble L, Mandrekar J, et al. Morbidity and mortality among patients with respiratory syncytial virus infection: a 2-year retrospective review. Diagn Microbiol Infect Dis. 2016;85(3):367–71.
Volling C, Hassan K, Mazzulli T, Green K, Al-Den A, Hunter P, et al. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;13(14):665.
Gottlieb J, Torres F, Haddad T, Dhillon G, Dilling DF, Knoop C, et al. A randomized controlled trial of presatovir for respiratory syncytial virus after lung transplant. J Heart Lung Transplant. 2023;42(7):908–16.
Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–84.
Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53(12):3738–49.
Popow-Kraupp T, Aberle JH. Diagnosis of respiratory syncytial virus infection. Open Microbiol J. 2011;5:128–34.
Onwuchekwa C, Moreo LM, Menon S, Machado B, Curcio D, Kalina W, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023;228(2):173–84.
Onwuchekwa C, Atwell J, Moreo LM, Menon S, Machado B, Siapka M, et al. Pediatric respiratory syncytial virus diagnostic testing performance: a systematic review and meta-analysis. J Infect Dis. 2023;228(11):1516–27.
Wong SS, Yu JW, Wong KT, Lee N, Lui GC, Chan PK, et al. Initial radiographic features as outcome predictor of adult respiratory syncytial virus respiratory tract infection. AJR Am J Roentgenol. 2014;203(2):280–6.
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415–27.
Kern S, Uhl M, Berner R, Schwoerer T, Langer M. Respiratory syncytial virus infection of the lower respiratory tract: radiological findings in 108 children. Eur Radiol. 2001;11(12):2581–4.
Kim MC, Kim MY, Lee HJ, Lee SO, Choi SH, Kim YS, et al. CT findings in viral lower respiratory tract infections caused by parainfluenza virus, influenza virus and respiratory syncytial virus. Medicine (Baltimore). 2016;95(26): e4003.
Ricco M, Corrado S, Palmieri S, Marchesi F. Respiratory syncytial virus: a systematic review and meta-analysis of tomographic findings (2000–2022). Children (Basel). 2023;10(7):1169.
Empey KM, Peebles RS Jr, Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50(9):1258–67.
Manti S, Staiano A, Orfeo L, Midulla F, Marseglia GL, Ghizzi C, et al. UPDATE—2022 Italian guidelines on the management of bronchiolitis in infants. Ital J Pediatr. 2023;49(1):19.
Langedijk AC, Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol. 2023;21(11):734–49.
Turner TL, Kopp BT, Paul G, Landgrave LC, Hayes D Jr, Thompson R. Respiratory syncytial virus: current and emerging treatment options. Clinicoecon Outcomes Res. 2014;6:217–25.
DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–22.
Cockerill GS, Angell RM, Bedernjak A, Chuckowree I, Fraser I, Gascon-Simorte J, et al. Discovery of sisunatovir (RV521), an inhibitor of respiratory syncytial virus fusion. J Med Chem. 2021;64(7):3658–76.
Gao Y, Cao J, Xing P, Altmeyer R, Zhang Y. Evaluation of small molecule combinations against respiratory syncytial virus in vitro. Molecules. 2021;26(9):2607.
Zheng X, Gao L, Wang L, Liang C, Wang B, Liu Y, et al. Discovery of ziresovir as a potent, selective, and orally bioavailable respiratory syncytial virus fusion protein inhibitor. J Med Chem. 2019;62(13):6003–14.
Huang LM, Schibler A, Huang YC, Tai A, Chi H, Chieng CH, et al. Safety and efficacy of AK0529 in respiratory syncytial virus-infected infant patients: a phase 2 proof-of-concept trial. Influenza Other Respir Viruses. 2023;17(7): e13176.
Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1013–9.
Malara A, Noale M, Trevisan C, Abbatecola AM, Borselli G, Cafariello C, et al. Efficacy of COVID-19 control measures on post-vaccination outbreak in Italian Long Term Care Facilities: implications for policies. Front Public Health. 2023;11:1091974.
Samson L. Prevention of respiratory syncytial virus infection. Paediatr Child Health. 2009;14(8):521–32.
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7.
Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184(3):350–4.
Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transpl Infect Dis. 2010;12(1):38–44.
McCoy D, Wong E, Kuyumjian AG, Wynd MA, Sebti R, Munk GB. Treatment of respiratory syncytial virus infection in adult patients with hematologic malignancies based on an institution-specific guideline. Transpl Infect Dis. 2011;13(2):117–21.
Tsitsikas DA, Oakervee H, Cavenagh JD, Gribben J, Agrawal SG, Mattes FM. Treatment of respiratory syncytial virus infection in haemopoietic stem cell transplant recipients with aerosolized ribavirin and the humanized monoclonal antibody palivizumab: a single centre experience. Br J Haematol. 2009;146(5):574–6.
Ruckwardt TJ, Morabito KM, Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. 2019;51(3):429–42.
Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks O, Sanchez PJ, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(34):920–5.
Esposito S, Amirthalingam G, Bassetti M, Blasi F, De Rosa FG, Halasa NB, et al. Monoclonal antibodies for prophylaxis and therapy of respiratory syncytial virus, SARS-CoV-2, human immunodeficiency virus, rabies and bacterial infections: an update from the World Association of Infectious Diseases and Immunological Disorders and the Italian Society of Antinfective Therapy. Front Immunol. 2023;14:1162342.
Mazur NI, Terstappen J, Baral R, Bardaji A, Beutels P, Buchholz UJ, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23(1):e2–21.
Karron RA, Atwell JE, McFarland EJ, Cunningham CK, Muresan P, Perlowski C, et al. Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. Am J Respir Crit Care Med. 2021;203(5):594–603.
Meissa Vaccines I. Meissa vaccines provides a pipeline update on vaccine candidates for COVID-19 and RSV. June 30, 2020. https://www.meissavaccines.com/post/meissa-vaccines-provides-a-pipeline-update-on-vaccine-candidates-for-covid-19-and-rsv Accessed 12 Apr 2022.
Scaggs Huang F, Bernstein DI, Slobod KS, Portner A, Takimoto T, Russell CJ, et al. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Hum Vaccin Immunother. 2021;17(2):554–9.
Wiegand MA, Gori-Savellini G, Gandolfo C, Papa G, Kaufmann C, Felder E, et al. A respiratory syncytial virus vaccine vectored by a stable chimeric and replication-deficient Sendai virus protects mice without inducing enhanced disease. J Virol. 2017;91(10).
Abarca K, Rey-Jurado E, Munoz-Durango N, Vazquez Y, Soto JA, Galvez NMS, et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine. 2020;27: 100517.
Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209-15.
Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608.
Ison MG, Papi A, Langley JM. Efficacy of one dose of the respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in adults ≥ 60 years of age persists for 2 RSV seasons. Abstract presented at IDWeek; October 11–15, 2023; Boston, MA. https://www.patientcareonline.com/view/idweek-2023-late-breaker-efficacy-of-single-rsvpref3-dose-sustained-over-2-seasons-in-high-risk-older-adults . Accessed 14 Dec 2023.
Feldman RG, Antonelli-Incalzi R, Steenackers K, Lee DG, Papi A, Ison MG, et al. Respiratory syncytial virus prefusion f protein vaccine is efficacious in older adults with underlying medical conditions. Clin Infect Dis. 2023.
Walsh EE, Perez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–77.
Ferdinands JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine. 2021;39(28):3678–95.
Florentino PTV, Millington T, Cerqueira-Silva T, Robertson C, de Araujo OV, Junior JBS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22(11):1577–86.
He X, Su J, Ma Y, Zhang W, Tang S. A comprehensive analysis of the efficacy and effectiveness of COVID-19 vaccines. Front Immunol. 2022;13: 945930.
Kampmann B, Madhi SA, Munjal I, Simoes EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F Vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–64.
Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N Engl J Med. 2023;389(24):2233–44.
Moderna Announces Global Regulatory Submissions For Its Respiratory Syncytial Virus (RSV) Vaccine, MRNA-1345. In Investor Relations—Moderna. 2023. https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-Global-Regulatory-Submissions-For-Its-Respiratory-Syncytial-Virus-RSV-Vaccine-MRNA-1345/default.aspx . Accessed 02 Apr 2024.
Icosavax Announces Positive Topline Interim Phase 1/1b Results for VLP Vaccine Candidate IVX-121 Against RSV. In: Icosavax; 2022. https://ir.icosavax.com/news-releases/news-release-details/icosavax-announces-positive-topline-interim-phase-11b-results/ . Accessed 02 Apr 2024.
Icosavax Initiates Phase 2 Trial of IVX-A12 Against RSV and hMPV in Older Adults. In: Icosavax; 2023. https://ir.icosavax.com/news-releases/news-release-details/icosavax-initiates-phase-2-trial-ivx-a12-against-rsv-and-hmpv/ . Accessed 02 Apr 2024.
Samy N, Reichhardt D, Schmidt D, Chen LM, Silbernagl G, Vidojkovic S, et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: a randomized phase I clinical trial. Vaccine. 2020;38(11):2608–19.
Weidenthaler H, Schultz S, Sanos S, et al. VT&T-77: efficacy, safety and immunogenicity of the recombinant MVA-BN-RSV vaccine against respiratory syncytial virus (RSV) infection in a human challenge trial (HCT) in healthy adult participants. In: RSVVW’21 Abstract Booklet. Virtual: RSVVW’21: 6th ReSViNet Conference: November 10–12, 2021 (abstr VT&T-77).
Jordan E, Kabir G, Schultz S, Silbernagl G, Schmidt D, Jenkins VA, et al. Reduced respiratory syncytial virus load, symptoms, and infections: A human challenge trial of MVA-BN-RSV vaccine. J Infect Dis. 2023;228(8):999–1011.
Bavarian Nordic Provides Update On Rsv Vaccine Program. In: Bavarian Nordic. 2023. https://www.bavarian-nordic.com/Investor/News/News.aspx?News=6808 . Accessed 02 Apr 2024.
Falsey AR, Williams K, Gymnopoulou E, Bart S, Ervin J, Bastian AR, et al. Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N Engl J Med. 2023;388(7):609–20.
ClinicalTrails.gov. Identifier: NCT04908683, a study of an adenovirus serotype 26 pre-fusion conformation-stabilized F protein (Ad26. RSV. pre-F) based respiratory syncytial virus (RSV) vaccine in the prevention of lower respiratory tract disease in adults aged 60 years and O. June 1, 2021. https://clinicaltrials.gov/ct2/show/ NCT04908683. Accessed 14 Dec 2023.
Janssen provides portfolio update. News release. Johnson & Johnson. March 29, 2023. Accessed 14 Dec 2023. https://www.jnj.com/janssen-provides-portfolio-update .
Global Initiative for Chronic Obstructive Lung Disease—GOLD. Global strategy for prevention, diagnosis and management of COPD: 2024 Report. https://goldcopd.org/wp-content/uploads/2023/11/KEY-CHANGES-GOLD-2024.pdf . Accessed 14 Dec 2023.
Pfizer Inc. U.S. FDA Approves ABRYSVOTM, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults. In: Pfizer. 2023. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention . Accessed 02 Apr 2024.
U.S. Food and Drug Administration. FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine. In: U.S. Food and Drug Administration. 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine . Accessed 02 Apr 2024.
GlaxoSmithKline. European Commission authorises GSK’s Arexvy, the first respiratory syncytial virus (RSV) vaccine for older adults. In: GlaxoSmithKline. 2023. https://www.gsk.com/en-gb/media/press-releases/european-commission-authorises-gsk-s-arexvy-the-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/ . Accessed 02 Apr 2024.
Pfizer Inc. European Commission Approves Pfizer’s ABRYSVOTM to Help Protect Infants through Maternal Immunization and Older Adults from RSV. In: Pfizer. 2023. https://www.pfizer.com/news/press-release/press-release-detail/european-commission-approves-pfizers-abrysvotm-help-protect . Accessed 02 Apr 2024.
Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801.
Joint Committee on Vaccination and Immunisation (JCVI). Respiratory syncytialvirus (RSV) immunisation programme for infants and older adults: JCVI fullstatement. In: Department of Health & Social Care, UK. 2023. https://www.gov.uk/government/publications/rsv-immunisation-programme-jcvi-advice-7-june-2023/respiratory-syncytial-virus-rsv-immunisation-programme-for-infants-and-older-adults-jcvi-full-statement-11-september-2023#background . Accessed 8 Apr 2024.
Redondo E, Rivero-Calle I, Mascaros E, Ocana D, Jimeno I, Gil A, et al. Respiratory syncytial virus vaccination recommendations for adults aged 60 years and older: The NeumoExperts prevention group position paper. Arch Bronconeumol. 2024;60(3):161–70.
EMA-EPAR. Abrysvo Respiratory syncytial virus vaccine (bivalent, recombinant). 2023.
EMA-EPAR. Arexvy (Recombinant respiratory syncytial virus pre-fusion F pro-tein, adjuvanted with AS01E. 2023.
Centers for Disease Control and Prevention. Healthcare Providers: RSV Vaccination for Pregnant People. In: Centers for Disease Control and Prevention; 2023. https://www.cdc.gov/vaccines/vpd/rsv/hcp/pregnant-people.html . Accessed 02 Apr 2024.
Melgar M. Updated Evidence to Recommendation Framework: respiratory syncytial virus (RSV) in adults. Advisory Committee on Immunization Practices meeting; June 21. Atlanta, GA; 2023.
Download references
Acknowledgements
The authors thank Elisa Veratelli for editorial support. This editorial support was funded by Consorzio Futuro as authorized by the University of Ferrara.
Author information
Authors and affiliations.
Respiratory Unit, Department of Translational Medicine, University of Ferrara Medical School, University of Ferrara, Sant’Anna University Hospital, Via Aldo Moro, 8, 44124, Ferrara, Italy
Franco Alfano, Tommaso Bigoni, Francesco Paolo Caggiano & Alberto Papi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Alberto Papi .
Ethics declarations
Author contributions.
All authors contributed to literature review and data evaluation. AP prepared the first draft of manuscript. All authors critically revised and contributed to the finalization of the manuscript. All authors read and approved the final version of the manuscript.
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Data Availability
Not applicable.
Conflict of Interest
AP received grants for research from Chiesi, Astrazeneca, GSK, and Sanofi; consulting fees or advisory board fees from Chiesi, Astrazeneca, GSK, Mundipharma, Novartis, Edmond, Sanofi, Avillion, IQVIA, Elpen Pharmaceuticals, TEVA, and MSD, and lecture fees form Chiesi, Astrazeneca, GSK, Mundipharma, Sanofi, MSD, Novartis, and Zambon outside the submitted work. FA, TB, and FPC have nothing to disclose.
Ethics Approval
Not applicable
Informed Consent
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Alfano, F., Bigoni, T., Caggiano, F.P. et al. Respiratory Syncytial Virus Infection in Older Adults: An Update. Drugs Aging (2024). https://doi.org/10.1007/s40266-024-01118-9
Download citation
Accepted : 19 April 2024
Published : 07 May 2024
DOI : https://doi.org/10.1007/s40266-024-01118-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
News & Publications
Johns hopkins all children's hosts the 2024 central florida triangle metabolism meeting.

Researchers from four Central Florida medical institutions — Johns Hopkins All Children’s Hospital, the H. Lee Moffitt Cancer Center and Research Institute, the AdventHealth Translational Research Institute for Diabetes and Metabolism and the University of Florida — co-sponsored the second annual Central Florida Triangle Metabolism Meeting, held March 14-15 on the Johns Hopkins All Children’s Hospital campus in St. Petersburg, Florida.
The conference title highlights the collaborative partnership among these four major central Florida Research organizations, focused on metabolism and physiology and emphasizes that the regulatory principles of fundamental metabolism are deeply rooted in all physiologic processes. The symposium provided an opportunity for cross-disciplinary biomedical researchers to present their recent research and to continue to explore unifying theory of metabolism.
Close to 100 attendees pre-registered for the event and an additional 25-50 people logged onto the video conference link to participate in the two-day event. “We could not have handled this event without the skilled assistance of Lorenzo Thomas, who expertly handled all the logistics as our academic coordinator,” says Timothy Osborne, Ph.D. , conference co-organizer, associate dean for basic research, director of the Institute for Fundamental Biomedical Research at the Johns Hopkins All Children’s Hospital, and professor of medicine in the Division of Endocrinology, Diabetes and Metabolism of the Johns Hopkins University School of Medicine. “This collaboration among institutions has proven a tremendous success.
“This second annual Central Florida Triangle Metabolism Meeting was sub-themed around metabolic control through the central nervous system and cancer. This year, the conference featured two keynote lectures, each emphasizing this year’s focus on the brain, which also integrated our conference with two special lectureship programs at Johns Hopkins All Children’s.
“On the first day of the meeting, Joel Elmquist, D.V.M., Ph.D., from the University of Texas Southwestern Medical School, delivered the Vice Dean’s Lecture which is a semi-annual event hosted by Dr. George Jallo. On the second day of the meeting, Tamas Horvath, D.V.M., Ph.D., professor and chair of the Department of Comparative Medicine at Yale University, delivered the Pediatric Grand Rounds Lecture, which is a weekly event hosted by the Continuing Medical Education program.”
Keynote Address
Joel Elmquist, D.V.M., Ph.D. , director of the Center for Hypothalamic Research and vice chair of research in the department of internal medicine at the University of Texas Southwestern Medical School, offered a keynote address titled “SF-1 Targets in the Hypothalamus: A Molecular Link Between Energy Balance and Exercise.” His lecture described laboratory experiments using animal models and focusing on steroidogenic factor-1 (SF-1) transcription factor and its role in the ventromedial nucleus of the hypothalamus (VMH), a complex brain structure important for neuroendocrine functions, including glucose regulation and also social and sexual behaviors.
The researchers aimed at developing a better understanding of the functions of SF-1 in the VMH by looking at what happens when those genes are “knocked out” in one group of laboratory animals as compared to control groups that maintained those genes.
Their ongoing investigations into SF-1 and VMH, as well as other genes, are ultimately aimed at shedding light on metabolism in humans to be potentially informative with regard to human metabolism and metabolic diseases.
Other Selected Presentations
After the keynote address, the conference continued with 20 speakers from over a dozen research institutions and universities across the United States (see box), each presenting their research on metabolism and physiology, covering a wide range of topics, including cancer; glaucoma; metabolic diseases; sensory systems; pulmonary research; fatty liver disease and diabetes; neural metabolism in aging and neurodegeneration; and obesity.
Selected presentation highlights:
Padhu Pattabiraman, Ph.D. (Indiana University) spoke on a potential advancement in the treatment of glaucoma. In his presentation, titled "Novel Mechanistic Insights into the Role of Sterol Regulatory Element Binding Proteins in the Regulation of Intraocular Pressure,” Pattabiraman discussed a novel way to reduce high intraocular pressure (IOP) in the eyes, a cause of glaucoma.
According to Pattabiraman, glaucoma is a leading cause of blindness and the goal for slowing the progression of glaucoma rests in finding new ways to lower IOP by keeping open the internal pathway by which the IOP can be “drained.” Current treatments depend on eye drops or surgery to lower IOP.
He discussed the experimental use of a small molecule called “fatostatin” to target this pathway and reducing IOP by blocking activation of sterol regulatory element binding proteins (SREBPs), thereby changing the cellular biomechanics in the eye by affecting the lipids that modulate “cell stiffening.” Cell stiffening causes pathway blockage and fatostatin helps in restructuring the mechanical forces that cause cell stiffening and, in doing so, potentially lowers IOP.
Mioara Larion, Ph.D. , (NIH), who leads the Cancer Metabolism Research Program at the Neuro-Oncology Branch (NOB) at NIH, studies the metabolic needs of cancer cells and how tumors process nutrients for their growth. A goal is to develop targeted metabolic approaches that can delay tumor growth. In her presentation, titled “Brain Tumor Metabolism: An Underexplored Therapeutic Vulnerability,” Larion spoke on tumor metabolism and how a “lipid imbalance” can trigger defects in organelles, subcellular structures that perform a variety of functions in the cell. One aspect of her research is related to how targeting that imbalance may induce “apoptosis,” or programmed cell death, a process that can kill cancer cells.
She is interested in the metabolic needs of cancer cells and how they process nutrients, in order to develop targeted approaches that delay tumor growth. She and her colleagues are particularly interested in identifying metabolic vulnerabilities in glioblastomas, a fast-growing type of central nervous system tumor that forms from tissues of the brain and spinal cord.
Xiangbo Ruan, Ph.D. , (Johns Hopkins All Children’s Institute for Fundamental Biomedical Research) spoke on the “Mechanism of impaired amino acid catabolism (the break-down of complex molecules) in fatty liver diseases and type 2 diabetes.” For Ruan, impaired amino acid metabolism in the liver is a hallmark of both non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D). Despite the critical role of impaired AA catabolism linking NAFLD with T2D, a molecular mechanism explaining this dysregulation is unknown. However, Ruan and his colleagues have found a mechanism of action for a human-specific long non-coding RNA (lncRNA). Gene expression is regulated by lncRNAs at multiple levels and plays a role in suppressing amino acid catabolism. This finding may explain impaired amino acid catabolism, present in both NAFLD and T2D.
Aya G. Elmarsafawi, Ph.D., (The H. Lee Moffitt Cancer Center and Research Institute) offered a presentation titled “The polyamine-hypusine axis regulates CD8+ T-cell fate and functions” which was the topic of her Ph.D. studies in the laboratory of John Cleveland, Ph.D., who is the Director of the Moffitt Cancer Institute. Elmarsafawi discussed the role played by CD8+ immune cells in “adoptive cell therapy” (ACT), a recent advancement in cancer immunotherapy that sources the patient’s own CD8+ immune cells which are subsequently “reprogrammed” and expanded in the laboratory, then injected back into the patient to boost immunity.
According to Elmarsafawi, strategies that would enhance the function of CD8+ cells are “an urgent clinical need.” He explained how the “polyamine-hypusine axis,” shown to regulate T-cell function, is a metabolic “checkpoint” that can be exploited to augment to generation of CD8+ cells to improve the efficacy of T-cell based immunotherapies.
Tamas Horvath (Yale University)
Tamas Horvath, D.V.M., Ph.D., professor and chair of the Department of Comparative Medicine and also the director of the Yale “Program on Integrative Cell Signaling and Neurobiology of Metabolism,” offered the second keynote lecture titled “Hunger drives life.” Horvath explained recent research carried out in his lab that investigated specific neurons that potentially play a role in anorexia nervosa (AN), an eating disorder characterized by an abnormally low body weight that, among other specifics, often may result in addictive exercise to help cause and maintain low body weight. AN is a condition that is more likely to be fatal in women than in men.
The Yale researchers found that food-restricted mice all died within 72 hours of their compulsive running, but when elevated fats were added to their diet, deaths were prevented and the researchers have hypothesized that a specific neuron that is activated by food restriction, called the “hypothalamic agouti-related peptide” (AgRP), plays a role in determining when anorexia becomes fatal, as the exercise-addicted animals lacking a high fat diet were unable to properly mobilize fuel during food restricted exercise. The finding suggests that those in medical care for anorexia nervosa, and at-risk of dying, may benefit from a high fat diet.
“We were very happy with our attendance this year,” says co-conference organizer Gina De Nicola, Ph.D., from Moffitt Cancer Center’s Department of Metabolism and Physiology and leader of Moffitt’s Metabolism Program. “We had twice as many posters as last year, and the poster session was popular as many great discussions took place around them. Also, the presentations included interesting new themes and new technologies for studying metabolism and physiology.”
Universities and Research Organization Affiliations of Invited Speakers
Johns Hopkins All Children’s
The Johns Hopkins University
The H. Lee Moffitt Cancer Center and Research Institute
AdventHealth Translational Research Institute
University of Florida
University of Kansas
University of Illinois, Chicago
University of Massachusetts
The Ohio State University
University of Colorado
National Institutes of Health
Indiana University
Yale University

IMAGES
COMMENTS
Premium Google Slides theme and PowerPoint template. The coronavirus outbreak has become one of the most notorious events of the decade, if not the current century. Every bit of information helps a lot, so let us help you create useful and informative presentations about this virus with our latest template. We've got a serious matter at hand ...
COVID-19 is an infectious disease of the human respiratory system caused by the virus SARS-CoV-2. The disease is almost always mild and causes fever, dry cough, shortness of breath, and fatigue. Older people and other at-risk populations may develop life-threatening symptoms. There is no vaccine or treatment.
Covid-19 Virus - Medical PowerPoint Template. The Covid-19 is a medical PowerPoint template with more than 70 unique and modern slides. This Covid presentation template has a gorgeous duotone color scheme, but you can easily customize it to match your brand. You'll also find unique mockup devices, image placeholders, infographic elements, and ...
The COVID 19 PowerPoint templates go beyond traditional static slides to make your professional presentations stand out. Given the sleek design and customized features, they can be used as PowerPoint as well as Google Slides templates. Inculcated with visually appealing unique and creative designs, the templates will double your presentation ...
Here you can find 195 PowerPoint templates and themes. Take a look at the entire library. Make your presentations look the best! Covid19 - Covid Powerpoint Template. By wearepixoo. Covirus - Covid Medical Powerpoint Template. By Yumnacreative. COVID - Clean Presentation Powerpoint.
Œ° Š (Lúšh9zk…8¿ë«VÿÎÕÇý 5 µ; »"åyšzœ …Uó~h¬Ë¶µåµÐ¶àÞë· Áî ÿÿ PK !®·:ÒÌ - ppt/slides/slide13.xmlìX[oÛ6 ~ °ÿÀéyŠnÔͨSH²U h× N±gš¢-.º ¢ xEÿû )ɱ"´M»‡ ÛbÀ¢ÈCòœï|ç ¿xyWWhÏDÏÛfn8 ¶ XCÛ‚7Û¹ñá:7# õ'4 ©Ú†Í ë ——?ÿô¢›õU `wÓÏÈÜ(¥ìf ...
These covid19 ppt templates are 100% editable, totally customized, easily downloadable, and ready to use. ... Download the Free COVID-19 Impact PowerPoint presentation to share with your senior management and customers. COVID-19 has impacted businesses massively. Many of us are at a point in reviewing what it all means in the short and long run.
Six coronaviruses (CoVs) are known to infect humans: 229E, OC43, SARS-CoV, NL63, HKU1, and MERS-CoV. Many CoVs are simultaneously maintained in nature, allowing for genetic recombination, resulting in novel viruses. SARS-CoV-2 is the third pathogenic novel coronavirus to emerge over the past two decades.
COVID-19 presentation for educators - Google Slides. Parts of this slide didn't load.
To help with the current coronavirus emergency, we have put together these free coronavirus tips Powerpoint slide templates for you to use. The slides include coronavirus prevention tips, symptoms and instructions on what to do if you have symptoms. Feel free to adjust the templates based on your local health authority instructions and contact ...
Presentation template - 28 April 2021. 28 April 2021. | COVID-19: Critical preparedness, readiness and response. Download (3.8 MB)
If a patient tests negative by Rapid Antigen Test (RAT) FDA recommends. If symptomatic, test at least twice 48 hours apart. A third test might be needed if the patient is concerned they have COVID-19. If asymptomatic, but believe they have been exposed, test with RAT at least 3 times, each 48 hours apart to be considered truly negative.
COVID-19 Communications Framework Presentation Slides (Template) DATE. April 30, 2021. Download File. Real Stories, Real People.
This PowerPoint presentation walks you and your students through key biology concepts of the SARS-CoV-2 coronavirus. Download PPT (PPT 16.3 MB) How Do We Detect COVID-19? Every day brings new developments in the race for effective and accurate COVID-19 testing, but most strategies are based on a few key fundamental technologies.
The version of the browser you are using is no longer supported. Please upgrade to a supported browser. Dismiss
The clinical presentation of COVID-19 ranges from asymptomatic to critical illness. An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity. Uncommon presentations of COVID-19 can occur, might vary by the age of the ...
Below are the PowerPoints that were included in the presentation. Coronavirus Disease 2019 (COVID-19) David Calfee, M.D., M.S. SARS-CoV-2 Molecular Diagnostics Testing Implementation. Hanna Rennart, Ph.D. COVID-19 Clinical Management and Treatment. Kristen Marks, M.D. Find a Physician Giving Contact Us Events.
Post-COVID-19 condition presents an important challenge to health care decision-makers in Canada and across the world due to the high volume of potential patients, diverse presentations of symptoms, few clinical guidelines and treatments, and unknown pathophysiology.
The impact of COVID-19 on small business outcomes and expectations. Proceedings of the national academy of sciences, 117(30), pp.17656-17666. Fairlie, R., 2020. The impact of COVID‐19 on small business owners: Evidence from the first three months after widespread social‐distancing restrictions. Journal of economics & management strategy, 29 ...
Summer 2024 CHPC Presentation Schedule. All CHPC presentations are conducted exclusively via Zoom. There are no in-person presentations at this time. Presentations are typically one hour, from 1-2pm, while hands on-presentations, marked with * in the schedule below, run two hours from 1-3pm. The ACCESS (formerly XSEDE) HPC workshops will take ...
RSV infection has a wide range of clinical presentations, from asymptomatic conditions to acute pneumonia and severe life-threatening respiratory distress, including exacerbations of underlying chronic conditions. ... such as those living in long-term care facilities. During the COVID-19 pandemic, the broad application of these rules deeply ...
United Airlines - Airline Tickets, Travel Deals and Flights If you're seeing this message, that means JavaScript has been disabled on your browser, please enable JS ...
Covid-19. Masks Strongly Recommended but Not Required in Maryland, Starting Immediately ... Other Selected Presentations. ... offered a presentation titled "The polyamine-hypusine axis regulates CD8+ T-cell fate and functions" which was the topic of her Ph.D. studies in the laboratory of John Cleveland, Ph.D., who is the Director of the ...