
Snapsolve any problem by taking a picture. Try it in the Numerade app?


Solutions for Holt Chemistry 1st
R.thomas myers, keith oldham,savatore tocci, get access to all of the answers and step-by-step video explanations to this book and 5,000+ more. try numerade free., the science of chemistry.

Matter and Energy

Atoms and Moles

The Periodic Table

lons and lonic Compounds
Covalent compounds, the mole and chemical composition, chemical equations and reactions, stoichiometry, causes of change, states of matter and intermolecular forces, chemical equilibrium, acids and bases, reaction rates, oxidation, reduction, and electrochemistry, nuclear chemistry, carbon and organic compounds, biological chemistry.

Create an account to get free access
A free unlock just for you
Watch the video solution with this free unlock.

Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Use chemistry problems as a tool for mastering chemistry concepts. Some of these examples show using formulas while others include lists of examples.
Acids, Bases, and pH Chemistry Problems
Learn about acids and bases. See how to calculate pH, pOH, K a , K b , pK a , and pK b .
- Practice calculating pH.
- Get example pH, pK a , pK b , K a , and K b calculations.
- Get examples of amphoterism.
Atomic Structure Problems
Learn about atomic mass, the Bohr model, and the part of the atom.
- Practice identifying atomic number, mass number, and atomic mass.
- Get examples showing ways to find atomic mass.
- Use Avogadro’s number and find the mass of a single atom .
- Review the Bohr model of the atom.
- Find the number of valence electrons of an element’s atom.
Chemical Bonds
Learn how to use electronegativity to determine whether atoms form ionic or covalent bonds. See chemistry problems drawing Lewis structures.
- Identify ionic and covalent bonds.
- Learn about ionic compounds and get examples.
- Practice identifying ionic compounds.
- Get examples of binary compounds.
- Learn about covalent compounds and their properties.
- See how to assign oxidation numbers.
- Practice drawing Lewis structures.
- Practice calculating bond energy.
Chemical Equations
Practice writing and balancing chemical equations.
- Learn the steps of balancing equations.
- Practice balancing chemical equations (practice quiz).
- Get examples finding theoretical yield.
- Practice calculating percent yield.
- Learn to recognize decomposition reactions.
- Practice recognizing synthesis reactions.
- Practice recognizing single replacement reactions.
- Recognize double replacement reactions.
- Find the mole ratio between chemical species in an equation.
Concentration and Solutions
Learn how to calculate concentration and explore chemistry problems that affect chemical concentration, including freezing point depression, boiling point elevation, and vapor pressure elevation.
- Get example concentration calculations in several units.
- Practice calculating normality (N).
- Practice calculating molality (m).
- Explore example molarity (M) calculations.
- Get examples of colligative properties of solutions.
- See the definition and examples of saturated solutions.
- See the definition and examples of unsaturated solutions.
- Get examples of miscible and immiscible liquids.
Error Calculations
Learn about the types of error and see worked chemistry example problems.
- See how to calculate percent.
- Practice absolute and relative error calculations.
- See how to calculate percent error.
- See how to find standard deviation.
- Calculate mean, median, and mode.
- Review the difference between accuracy and precision.
Equilibrium Chemistry Problems
Learn about Le Chatelier’s principle, reaction rates, and equilibrium.
- Solve activation energy chemistry problems.
- Review factors that affect reaction rate.
- Practice calculating the van’t Hoff factor.
Practice chemistry problems using the gas laws, including Raoult’s law, Graham’s law, Boyle’s law, Charles’ law, and Dalton’s law of partial pressures.
- Calculate vapor pressure.
- Solve Avogadro’s law problems.
- Practice Boyle’s law problems.
- See Charles’ law example problems.
- Solve combined gas law problems.
- Solve Gay-Lussac’s law problems.
Some chemistry problems ask you identify examples of states of matter and types of mixtures. While there are any chemical formulas to know, it’s still nice to have lists of examples.
- Practice density calculations.
- Identify intensive and extensive properties of matter.
- See examples of intrinsic and extrinsic properties of matter.
- Get the definition and examples of solids.
- Get the definition and examples of gases.
- See the definition and examples of liquids.
- Learn what melting point is and get a list of values for different substances.
- Get the azeotrope definition and see examples.
- See how to calculate specific volume of a gas.
- Get examples of physical properties of matter.
- Get examples of chemical properties of matter.
- Review the states of matter.

Molecular Structure Chemistry Problems
See chemistry problems writing chemical formulas. See examples of monatomic and diatomic elements.
- Practice empirical and molecular formula problems.
- Practice simplest formula problems.
- See how to calculate molecular mass.
- Get examples of the monatomic elements.
- See examples of binary compounds.
- Calculate the number of atoms and molecules in a drop of water.
Nomenclature
Practice chemistry problems naming ionic compounds, hydrocarbons, and covalent compounds.
- Practice naming covalent compounds.
- Learn hydrocarbon prefixes in organic chemistry.
Nuclear Chemistry
These chemistry problems involve isotopes, nuclear symbols, half-life, radioactive decay, fission, fusion.
- Review the types of radioactive decay.
Periodic Table
Learn how to use a periodic table and explore periodic table trends.
- Know the trends in the periodic table.
- Review how to use a periodic table.
- Explore the difference between atomic and ionic radius and see their trends on the periodic table.
Physical Chemistry
Explore thermochemistry and physical chemistry, including enthalpy, entropy, heat of fusion, and heat of vaporization.
- Practice heat of vaporization chemistry problems.
- Practice heat of fusion chemistry problems.
- Calculate heat required to turn ice into steam.
- Practice calculating specific heat.
- Get examples of potential energy.
- Get examples of kinetic energy.
- See example activation energy calculations.
Spectroscopy and Quantum Chemistry Problems
See chemistry problems involving the interaction between light and matter.
- Calculate wavelength from frequency or frequency from wavelength.
Stoichiometry Chemistry Problems
Practice chemistry problems balancing formulas for mass and charge. Learn about reactants and products.
- Get example mole ratio problems.
- Calculate percent yield.
- Learn how to assign oxidation numbers.
- Get the definition and examples of reactants in chemistry.
- Get the definition and examples of products in chemical reactions.
Unit Conversions
There are some many examples of unit conversions that they have their own separate page!
- Physical Chemistry
Ch. 9 Practice Test - Doral Academy Preparatory

Related documents

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
Balancing, Identifying & Predicting Chemical Equations Quiz
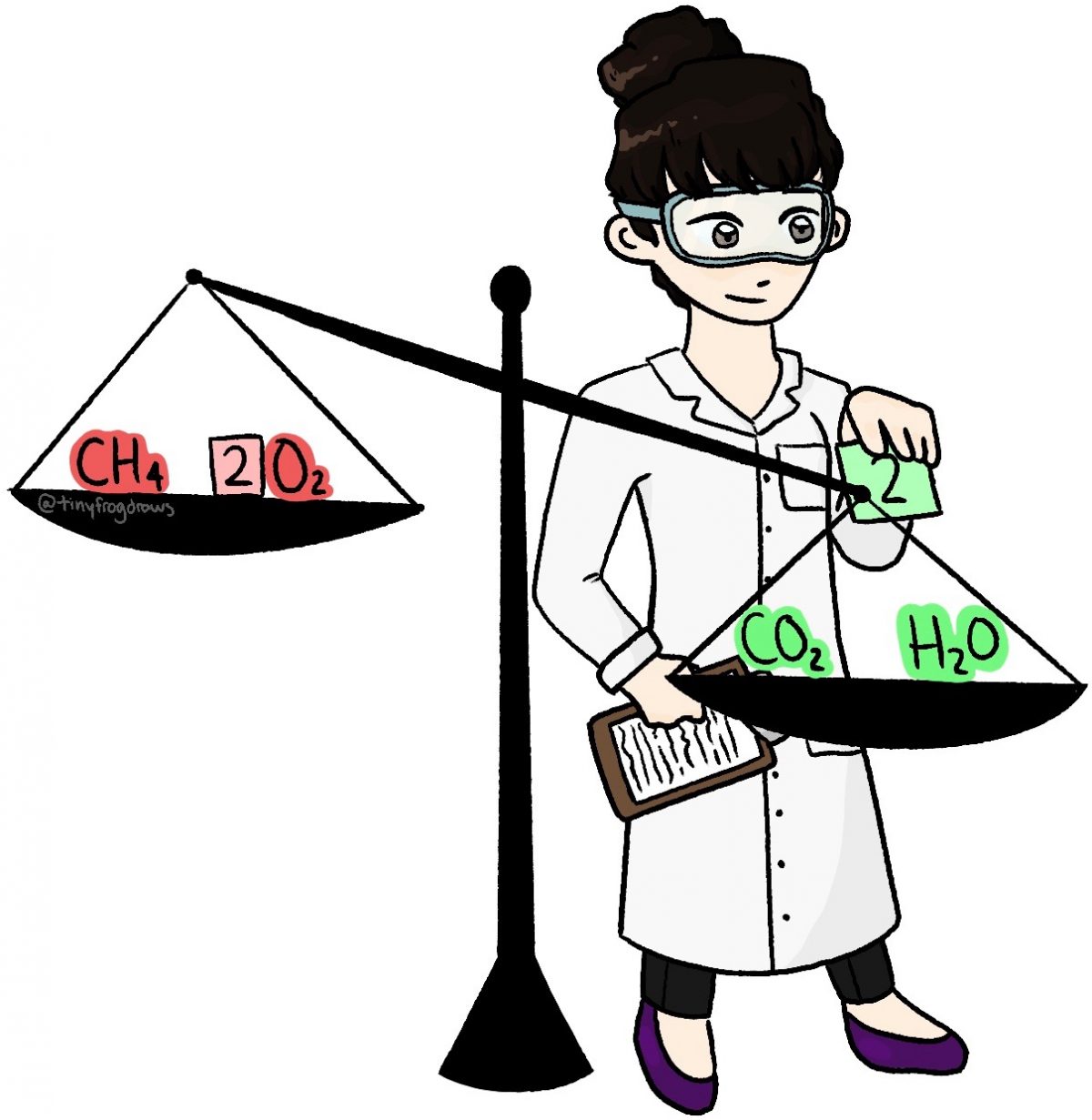
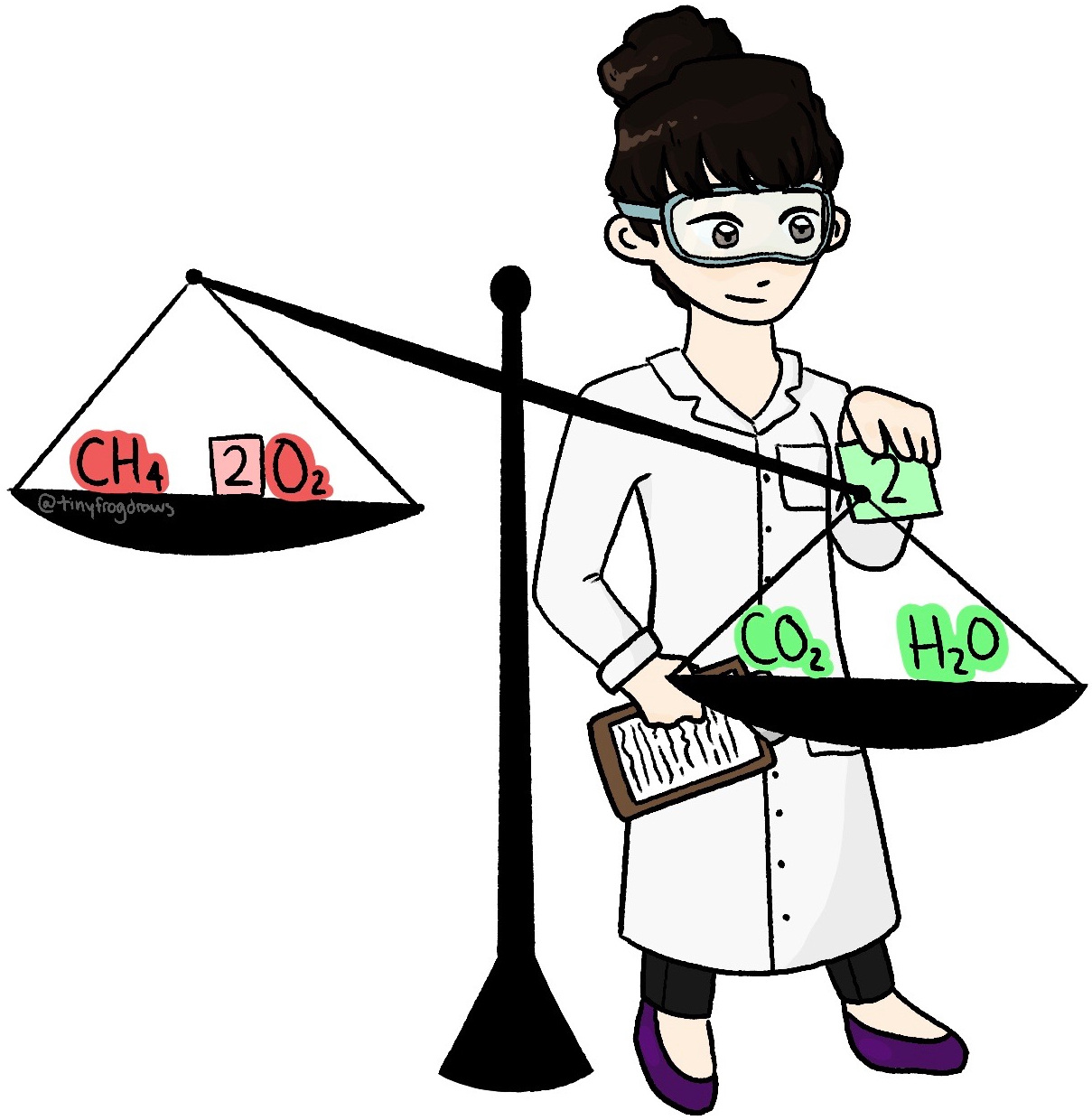
This online quiz is intended to give you extra practice in balancing, identifying and predicting a random selection of over 150 chemical equations. This quiz aligns with the following NGSS standard(s): HS-PS1-2 , HS-PS1-7
Select your preferences below and click 'Start' to give it a try!

IMAGES
VIDEO
COMMENTS
Exercise 7. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Holt Chemistry 6th Edition, you'll learn how to solve your toughest homework problems. Our resource for Holt Chemistry includes answers to chapter ...
Unlike static PDF Holt Chemistry 6th Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. No need to wait for office hours or assignments to be graded to find out where you took a wrong turn. You can check your reasoning as you tackle a problem using our interactive solutions viewer.
Fortunately, you do have a way to relate mass and numbers of atoms. One iron atom has a mass of 55.847 amu, and 55.847 g of iron contains 6.022 137 1023 atoms of iron. Likewise, 32.066 g of sulfur contains 6.022 137 1023 atoms of sul-fur. Knowing this, you can measure out 55.847 g of iron and 32.066 g of sulfur and be pretty certain that you ...
So far in your chemistry course, you have learned that chemists count quantities ... Yes; the answer is 2/3 of 4.0. Practice 1. ... Holt ChemFile: Problem-Solving Workbook 102 Stoichiometry Name Class Date Problem Solving continued Sample Problem 2 Potassium chlorate is sometimes decomposed in the laboratory to gener-ate oxygen. The reaction is ...
Find step-by-step solutions and answers to Modern Chemistry - 9780030367779, as well as thousands of textbooks so you can move forward with confidence. ... Chemistry; Modern Chemistry. 6th Edition. Rinehart, Winston and Holt. ISBN: 9780030367779. Rinehart, Winston and Holt. More textbook info. Rinehart, Winston and Holt. ISBN: 9780030367779 ...
Snapsolve any problem by taking a picture. Try it in the Numerade app? No Try it. ... Textbooks; Holt Chemistry; Solutions for Holt Chemistry 1st R.Thomas Myers, Keith Oldham,Savatore Tocci Get access to all of the answers and step-by-step video explanations to this book and 5,000+ more. Try Numerade free.
So far in your chemistry course, you have learned that chemists count quantities ... Yes; the answer is 2/3 of 4.0. Practice 1. ... Holt ChemFile: Problem-Solving Workbook 102 Stoichiometry Name Class Date Problem Solving continued Sample Problem 2 Potassium chlorate is sometimes decomposed in the laboratory to gener-ate oxygen. The reaction is ...
Class. CHAPTER 3 TEST. Atoms: The Building Blocks of Matter. MULTIPLE CHOICE On the line at the left of each statement, write the letter of the. choice that best completes the statement or answers the question. 1. The behavior of cathode rays in a glass tube containing gas at low pressure led scientists to. conclude that the rays were composed of.
It's easier to figure out tough problems faster using Chegg Study. Unlike static PDF Holt Mcdougal Modern Chemistry 1st Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. No need to wait for office hours or assignments to be graded to find out where you took a wrong turn.
Holt ChemFile: Problem-Solving Workbook 122 Percentage Yield ... Is the answer reasonable? Yes; 83% is about 5/6, which appears to be close to the ratio 0.71/0.86. ... Problem Solving continued Practice 1. In the commercial production of the element arsenic, arsenic(III) oxide is
AP Chemistry Practice Questions Sterling Test Prep,2021-07-25 Recommended by teachers. Trusted by students. High-yield AP Chemistry practice questions and detailed explanations to achieve a high score. The Publishers' Trade List Annual ,1979
Diman Regional
17.0 g/mol NH3. 3H2(g) → 2NH3(g) a. Determine to one decimal place the molar mass of each substance and express each mass in grams per mole. b. There are six different mole ratios in this system. Write out each one. 3 mol H2:1 mol N2; 2 mol NH3:1 mol N2; 2 mol NH3:3 mol H2; or their reciprocals. 7.
mass/person = 85 kg Note that the numerical answer, 11.8 people, must be rounded down to 11 people. 11 people 1.08 × 109 km 1 examiner 1 nanogoat 1 microphone 2 kilomockingbirds 1 kmockingbirds 1 × 103 mockingbirds 1 dekaration 9.7 m/s 4.62 × 10−2 cm 6.75 × 10−4 g 7.5 × 104 cm 1.6 × 107 µg 7.8 × 103 s 2 × 102 mm I Ch. 1-2 Holt ...
Exercise 4. Exercise 5. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Modern Chemistry 1st Edition, you'll learn how to solve your toughest homework problems. Our resource for Modern Chemistry includes ...
Books. Holt Chemistry File: Mini-Guide to Problem Solving. Houghton Mifflin, 1998 - Education - 297 pages. This reference is a must for students who need extra help, reteaching, or extra practice. The guide moves students through the same concepts as the text, but at a slower pace. More descriptive detail, along with visual algorithms, provides ...
Some chemistry problems ask you identify examples of states of matter and types of mixtures. While there are any chemical formulas to know, it's still nice to have lists of examples. Practice density calculations. Identify intensive and extensive properties of matter. See examples of intrinsic and extrinsic properties of matter.
Assessment Ch. 9 Practice Test Chapter: Stoichiometry In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. _____ 1. A balanced chemical equation allows one to determine the a. mole ratio of any two substances in the reaction. b. energy released in the reaction. c.
This online quiz is intended to give you extra practice in balancing, identifying and predicting a random selection of over 150 chemical equations. This quiz aligns with the following NGSS standard (s): HS-PS1-2, HS-PS1-7. Select your preferences below and click 'Start' to give it a try! Number of problems: 1. 5.
Practice Problems with Answers. (Organized mostly as in Zumdahl Chemistry) All Practice Problems provided include Answers. Chemical Foundations. measurement, significant figures, precision & accuracy, conversion factors, matter. Atoms, Molecules and Ions. atomic theory, intro to Periodic Table, formulas & names of compounds.
Exercise 512. Exercise 513. Exercise 514. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Holt Physics 6th Edition, you'll learn how to solve your toughest homework problems.