Nested Study
- Reference work entry
- Cite this reference work entry

- J. Rick Turner 3
1575 Accesses
Nested case-control study
A nested case-control study is one that is “nested” within a cohort study.
In many cohort studies, all subjects provide a wide range of information at the time of recruitment, e.g., results from a physical examination, answers to multiple questionnaires, blood and urine samples, and results from imaging techniques. Because of the large numbers of subjects in these studies and the cost of analyzing some biological samples, some of these resources are often not analyzed in detail at the time of collection, but are stored for future use. The nested case-control study is performed using subjects who develop the disease of interest in due course, and control subjects who are selected from those who were disease-free at the time the case subjects (those who developed the disease) were diagnosed.
The appropriate data sets and samples are then retrieved and analyzed for these two subsets (cases and controls) of the original cohort recruited into...
This is a preview of subscription content, log in via an institution to check access.

Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
References and Readings
Webb, P., Bain, C., & Pirozzo, S. (2005). Essential epidemiology: An introduction for students and health professionals . Cambridge, UK: Cambridge University Press.
Google Scholar
Download references
Author information
Authors and affiliations.
Cardiovascular Safety, Quintiles, Durham, NC, USA
Dr. J. Rick Turner
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to J. Rick Turner .
Editor information
Editors and affiliations.
Behavioral Medicine Research Center, Department of Psychology, University of Miami, Miami, FL, USA
Marc D. Gellman
J. Rick Turner
Rights and permissions
Reprints and permissions
Copyright information
© 2013 Springer Science+Business Media, New York
About this entry
Cite this entry.
Turner, J.R. (2013). Nested Study. In: Gellman, M.D., Turner, J.R. (eds) Encyclopedia of Behavioral Medicine. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1005-9_1046
Download citation
DOI : https://doi.org/10.1007/978-1-4419-1005-9_1046
Publisher Name : Springer, New York, NY
Print ISBN : 978-1-4419-1004-2
Online ISBN : 978-1-4419-1005-9
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Research article
- Open access
- Published: 21 July 2008
Advantages of the nested case-control design in diagnostic research
- Cornelis J Biesheuvel 1 , 2 ,
- Yvonne Vergouwe 1 ,
- Ruud Oudega 1 ,
- Arno W Hoes 1 ,
- Diederick E Grobbee 1 &
- Karel GM Moons 1
BMC Medical Research Methodology volume 8 , Article number: 48 ( 2008 ) Cite this article
50k Accesses
88 Citations
2 Altmetric
Metrics details
Despite its benefits, it is uncommon to apply the nested case-control design in diagnostic research. We aim to show advantages of this design for diagnostic accuracy studies.
We used data from a full cross-sectional diagnostic study comprising a cohort of 1295 consecutive patients who were selected on their suspicion of having deep vein thrombosis (DVT). We draw nested case-control samples from the full study population with case:control ratios of 1:1, 1:2, 1:3 and 1:4 (per ratio 100 samples were taken). We calculated diagnostic accuracy estimates for two tests that are used to detect DVT in clinical practice.
Estimates of diagnostic accuracy in the nested case-control samples were very similar to those in the full study population. For example, for each case:control ratio, the positive predictive value of the D-dimer test was 0.30 in the full study population and 0.30 in the nested case-control samples (median of the 100 samples). As expected, variability of the estimates decreased with increasing sample size.
Our findings support the view that the nested case-control study is a valid and efficient design for diagnostic studies and should also be (re)appraised in current guidelines on diagnostic accuracy research.
Peer Review reports
In diagnostic research it is essential to determine the accuracy of a test to evaluate its value for medical practice [ 1 ]. Diagnostic test accuracy is assessed by comparing the results of the index test with the results of the reference standard in the same patients. Given the cross-sectional nature of a diagnostic accuracy question, the design may be referred to as a cross-sectional cohort design. The (cohort) characteristic by which the study subjects (cohort members) are selected is 'the suspicion of the target disease', defined by the presence of particular symptoms or signs [ 2 ]. The collected study data allow for calculation of all diagnostic accuracy parameters of the index test, such as sensitivity, specificity, odds ratio, receiver operating characteristic (ROC) curve and predictive values, i.e. the probabilities of presence and absence of the disease given the index test result(s).
Subjects are not always selected on their initial suspicion of having the disease but often on the true presence or absence of the disease among those who underwent the reference test in routine care practice, which merely reflects a cross-sectional case-control design [ 3 , 4 ]. Appraisal of such conventional case-control design in diagnostic accuracy research has been limited due to its problems related to the incorrect sampling of cases and controls [ 3 – 7 ]. These problems may be overcome by applying a nested (cross-sectional) case-control study design, which may be advantageous over a full (cross-sectional) cohort design. The rationale, strengths and limitations of a nested case-control approach in epidemiology studies have widely been discussed in the literature [ 8 – 11 ], but not so much in the context of diagnostic accuracy research [ 6 ].
We therefore aim to show advantages of the nested case-control design for addressing diagnostic accuracy questions and discuss its pros and cons in relation to a conventional case-control design and to the full (cross sectional) cohort design in this domain. We will illustrate this with data from a recently conducted diagnostic accuracy study.
Case-control versus nested case-control design
The essence of a case-control study is that cases with the condition under study arise in a source population and controls are a representative sample of this same source population. Not the entire population is studied, what would be a full cohort study or census approach, but rather a random sample from the source population [ 12 ]. A major flaw inherent to case-control studies, described as early as 1959 [ 13 ], is the difficulty to ensure that cases and controls are a representative sample of the same source population. In a nested case-control study the cases emerge from a well-defined source population and the controls are sampled from that same population. The main difference between a case-control and a nested case-control study is that in the former the cases and controls are sampled from a source population with unknown size, whereas the latter is 'nested' in an existing predefined source population with known sample size. This source population can be a group or cohort of subjects that is followed over time or not.
The term 'cohort' is commonly referred to a group of subjects followed over time in etiologic or prognostic research. But in essence, time is no prerequisite for the definition of a cohort. A cohort is a group of subjects that is defined by the same characteristic. This characteristic can be a particular birth year, a particular living area, and also the presence of a particular sign or symptom that makes them suspected of having a particular disease as in diagnostic research. Accordingly, a cross-sectional study can either be a cross-sectional case-control study or a cross-sectional cohort study.
Case-control and nested case-control design in diagnostic accuracy research
In diagnostic accuracy research the case-control design is incorrectly applied when subjects are selected from routine care databases. First, this design commonly leads to biased estimates of diagnostic accuracy of the index test due to referral or (partial) verification bias [ 4 , 14 – 18 ]. In routine care, physicians selectively refer patients for additional tests, including the reference test, based on previous test results. This is good clinical practice but a bad starting point for diagnostic research. As said, for diagnostic research purposes all subjects suspected of the target disease preferably undergo the index test(s) plus reference test irrespective of previous test results. Second, selection of patients with a negative reference test result as 'controls' may lead to inclusion of controls that correspond to a different clinical domain, i.e. patients who underwent the reference test but not necessarily because they were similarly suspected of the target condition [ 16 , 17 ]. A third disadvantage of such case-control design is that absolute probabilities of disease presence given the index test results, i.e. the predictive values or post-test probabilities, that are the desired parameters for patient care, cannot be obtained. Cases and controls are sampled from a source population of unknown size. The total number of patients that were initially suspected of the target disease based on the presence of symptoms or signs, i.e. the true source population, is commonly unknown as in routine care patients are hardly classified by their symptoms and signs at presentation [ 18 ]. Hence, the sampling fraction of cases and controls is unknown and valid estimates of the absolute probabilities of disease presence cannot be calculated [ 12 ].
A nested case-control study in diagnostic research includes the full population or cohort of patients suspected of the target disease. The 'true' disease status is obtained for all these patients with the reference standard. Hence, there is no referral or partial verification bias. The results of the index tests can then be obtained for all subjects with the target condition but only for a sample of the subjects without the target condition. Usually all patients with the target disease are included, but this could as well be a sample of the cases. Besides the absence of bias, all measures of diagnostic accuracy, including the positive and negative predictive values, can simply be obtained by weighing the controls with the case-control sampling fraction, as explained in Figure 1 .
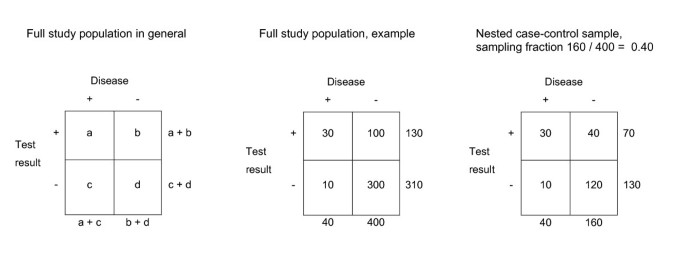
Theoretical example of a full study population and a nested case-control sample . The index test result and the outcome are obtained for all patients of the study population. The case-control ratio was 1:4 (sampling fraction (SF) = 160/400 = 0.40). Valid diagnostic accuracy measures can be obtained from the nested case-control sample, by multiplying the controls with 1/sampling fraction. For example, the positive predictive value (PPV) of a full study population can be calculated with a/(a + b), in this example 30/(30 + 100) = 0.23. In a nested case-control sample the PPV is calculated with a/(a + (1/SF)*b), in this example: 30/(30 + 2.5*40) = 0.23. In a case-control sample however, the controls are sampled from a source population with unknown size. Therefore, the sample fraction is unknown and valid estimate of the PPV cannot be calculated.
Potential advantages of a nested case-control design in diagnostic research
The nested case-control study design can be advantageous over a full cross-sectional cohort design when actual disease prevalence in subjects suspected of a target condition is low, the index test is costly to perform, or if the index test is invasive and may lead to side effects. Under these conditions, one limits patient burden and saves time and money as the index test is performed in only a sample of the control subjects.
Furthermore, the nested case-control design is of particular value when stored data (serum, images etc.) of an existing study population are re-analysed for diagnostic research purposes. Using a nested case-control design, only data of a sample of the full study population need to be retrieved and analysed without having to perform a new diagnostic study from the start. This may for example apply to evaluation of tumour markers to detect cancer, but also for imaging or electrophysiology tests.
Diagnostic accuracy estimates derived from a nested case-control study, should be virtually identical to a full cohort analysis. However, the variability of the accuracy estimates will increase with decreasing sample size. We illustrate this with data of a diagnostic study on a cohort of patients who were suspected of DVT.
A cross-sectional study was performed among a cohort of adult patients suspected of deep vein thrombosis (DVT) in primary care. This suspicion was primarily defined by the presence of a painful and swollen or red leg that existed no longer than 30 days. Details on the setting, data collection and main results have been described previously. [ 19 , 20 ] In brief, the full study population included 1295 consecutive patients who visited one of the participating primary care physicians with above symptoms and signs of DVT. Patients were excluded if pulmonary embolism was suspected. The general practitioner systematically documented information on patient history and physical examination. Patient history included information such as age, gender, history of malignancy, and recent surgery. Physical examination included swelling of the affected limb and difference in circumference of the calves calculated as the circumference (in centimetres) of affected limb minus circumference of unaffected limb, further referred to as calf difference test. Subsequently, all patients were referred to undergo D-dimer testing. In line with available guidelines and previous studies, the D-dimer test result was considered abnormal if the test yielded a D-dimer level ≥ 500 ng/ml. [ 21 , 22 ] Finally, they all underwent the reference test, i.e. repeated compression ultrasonography (CUS) of the lower extremities. In patients with a normal first CUS measurement, the CUS was repeated after seven days. DVT was considered present if one CUS measurement was abnormal. The echographist was blinded to the results of patient history, physical examination, and the D-dimer assay.
Nested case-control samples
Nested case-control samples were drawn from the full study population (n = 1295). In all samples, we included always all 289 cases with DVT. Controls were randomly sampled from the 1006 subjects without DVT. We applied four different and frequently used case-control ratios, i.e. one control for each case (1:1), two controls for each case (1:2), three controls for each case (1:3) and four controls for each case (1:4). For example, a sample with case-control ratio of 1:1 contained 289 cases and 289 random subjects out of 1006 controls (sampling fraction 289/1006 = 0.287). In the 1:4 approach, we sampled with replacement. For each case-control ratio, 100 nested case-control samples were drawn.

Statistical analysis
We focussed on two important diagnostic tests for DVT, i.e. the dichotomous D-dimer test and the continuous calf difference test. The latter was specifically chosen as it allowed for the estimation and thus comparison of the area under the ROC curve (ROC area). Diagnostic accuracy measures of both tests were estimated for the four case-control ratios and compared with those obtained from the full study population. Measures of diagnostic accuracy included sensitivity and specificity, positive and negative predictive values and the odds ratio (OR) for the D-dimer test, and the OR and the ROC area for the calf difference test.
In the analysis of the nested case-control samples, we multiplied control samples by [1/sample fraction] corresponding to the case-control ratio (1:1 = 3.48; 1:2 = 1.74; 1:3 = 1.16; 1:4 = 0.87). For each case-control ratio, the point estimates and variability were determined. The median estimate of the 100 samples was considered as the point estimate. Analyses were performed using SPSS version 12.0 and S-plus version 6.0.
In the full study population, the prevalence of DVT was 22% (n = 289), the D-dimer test was abnormal in 69% of the patients (n = 892) and the mean difference in calf circumference was 2.3 cm (Table 1 ). The prevalence of DVT was 50%, 33%, 25% and 20% in the nested case-control samples as a result of the sampling ratios (1:1, 1:2, 1:3 and 1:4, respectively). The distributions of the test characteristics in the control samples were similar as for the patients from the full study population without DVT (Table 1 ).
In the full study population the sensitivity and negative predictive value were high for the D-dimer test, 0.94 and 0.96, respectively (Table 2 ), whereas the specificity and positive predictive value were relatively low. The OR for the calf difference test was 1.44 and the ROC area was 0.69.
The average estimates of diagnostic accuracy for each of the four case-control ratios were similar to the corresponding estimates of the full study population (Figure 2 ). For example, the negative predictive value of the D-dimer test was 0.955 in both the full study population and for the four case-control ratios. The OR of the calf difference test was 1.44 in the full study population and the OR derived from the nested case-control samples were on average also 1.44.
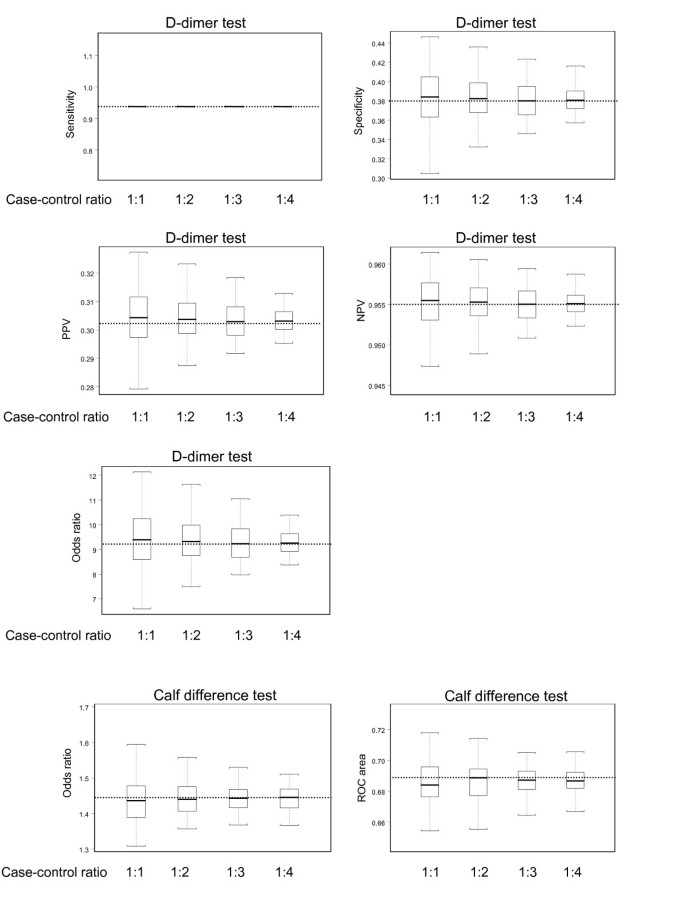
Estimates of diagnostic accuracy of the D-dimer test and calf difference test for the 100 nested case-control samples with case-control ratios ranging from 1:1 to 1:4 . The boxes indicate mean values and corresponding interquartile ranges (25 th and 75 th percentile). Whiskers indicate 2.5 th and 97.5 th percentiles. The dotted lines represent the values estimated in the full study population.
The use of (conventional) case-control studies in diagnostic research has often been associated with biased estimates of diagnostic accuracy, due to the incorrect sampling of subjects [ 3 – 6 , 18 ]. Moreover, this study design does not allow for the estimation of the desired absolute disease probabilities. We discussed and showed that a case-control study nested within a well defined cohort of subjects suspected of a particular target disease with known sample size can yield valid estimates of diagnostic accuracy of an index test, including the absolute probabilities of disease presence or absence. Diagnostic accuracy parameters derived from a full (cross-sectional) cohort of patients suspected of DVT were similar to the estimates derived from various nested case-control samples averaged over 100 simulations. Expectedly, the variability decreased with increasing number of controls, making the measures estimated in the larger case-control samples more precise.
As discussed, the number of subjects from which the index test results need to be retrieved can substantially be reduced with a nested case-control design. Hence, the nested case-control design is particularly advantageous when the prevalence of the target condition in the cohort of patients suspected of the target disease is rare, when the index test results are costly or difficult to collect and for re-analysing stored images or specimen. However, precision of the diagnostic accuracy measures will be hampered by increased variability when too little control patients are included.
Rutjes et al nicely discussed limitations of different study designs in diagnostic research [ 6 ]. They proposed the 'two-gate design with representative sampling' (which resembles the nested case-control design in this paper) as a valid design. We confirmed their proposition with a quantitative analysis of a diagnostic study. Rutjes et al suggested not to use the term 'nested case-control' to prevent confusion with etiologic studies where this design is commonly applied. Indeed, diagnostic and etiologic research differs fundamentally, first and foremost on the concept of time. Diagnostic accuracy studies are, in contrast to etiologic studies, typically cross-sectional in nature. Furthermore, diagnostic associations between index and reference tests are purely descriptive, whereas in etiologic studies causal associations and potential confounding are involved. Despite these major differences we believe there is no reason not to use the term nested case-control study in diagnostic research as well. The term inherently refers to the method of sampling of study subjects which can be the same in a diagnostic or etiologic setting, and has no direct bearing on the other issues typically related to etiologic case control studies.
Our findings support the view that the nested case-control study is a valid and efficient design for diagnostic studies. We believe that the nested case-control approach should be applied more often in diagnostic research, and also be (re)appraised in current guidelines on diagnostic methodology.
Knottnerus JA, van Weel C, Muris JW: Evaluation of diagnostic procedures. BMJ. 2002, 324 (7335): 477-480. 10.1136/bmj.324.7335.477.
Article PubMed PubMed Central Google Scholar
Knottnerus JA, Muris JW: Assessment of the accuracy of diagnostic tests: the cross-sectional study. J Clin Epidemiol. 2003, 56 (11): 1118-1128. 10.1016/S0895-4356(03)00206-3.
Article CAS PubMed Google Scholar
Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, Meulen van der JHP, Bossuyt PMM: Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999, 282: 1061-1066. 10.1001/jama.282.11.1061.
Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM: Evidence of bias and variation in diagnostic accuracy studies. CMAJ. 2006, 174 (4): 469-476.
Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J: Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004, 140 (3): 189-202.
Article PubMed Google Scholar
Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM: Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005, 51 (8): 1335-1341. 10.1373/clinchem.2005.048595.
Kraemer H: Evaluating Medical Tests. 1992, London, UK , Sage Publications
Google Scholar
Mantel N: Synthetic retrospective studies and related topics. Biometrics. 1973, 29 (3): 479-486. 10.2307/2529171.
Essebag V, Genest J, Suissa S, Pilote L: The nested case-control study in cardiology. Am Heart J. 2003, 146 (4): 581-590. 10.1016/S0002-8703(03)00512-X.
Ernster VL: Nested case-control studies. Prev Med. 1994, 23 (5): 587-590. 10.1006/pmed.1994.1093.
Langholz B: Case-Control Study, Nested. Encyclopedia of Biostatistics. Edited by: Armitage PCT. 2005, New York , John Wiley & Sons, 646-665. 2nd
Rothman KJ, Greenland S: Modern epidemiology. 1998, Philadelphia , Lincot-Raven Publishers, Second
Mantel N, Haenszel W: Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959, 22 (4): 719-748.
CAS PubMed Google Scholar
Ransohoff DF, Feinstein AR: Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978, 299 (17): 926-930.
Begg CB, Greenes RA: Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics. 1983, 39: 297-215. 10.2307/2530820.
Article Google Scholar
Knottnerus JA, Leffers JP: The influence of referral patterns on the characteristics of diagnostic tests. J Clin Epidemiol. 1992, 45: 1143-1154. 10.1016/0895-4356(92)90155-G.
van der Schouw YT, van Dijk R, Verbeek ALM: Problems in selecting the adequate patient population from existing data files for assessment studies of new diagnostic tests. J Clin Epidemiol. 1995, 48: 417-422. 10.1016/0895-4356(94)00144-F.
Oostenbrink R, Moons KG, Bleeker SE, Moll HA, Grobbee DE: Diagnostic research on routine care data: prospects and problems. J Clin Epidemiol. 2003, 56 (6): 501-506. 10.1016/S0895-4356(03)00080-5.
Oudega R, Hoes AW, Moons KG: The Wells rule does not adequately rule out deep venous thrombosis in primary care patients. Ann Intern Med. 2005, 143 (2): 100-107.
Oudega R, Moons KG, Hoes AW: Limited value of patient history and physical examination in diagnosing deep vein thrombosis in primary care. Fam Pract. 2005, 22 (1): 86-91. 10.1093/fampra/cmh718.
Perrier A, Desmarais S, Miron M, de Moerloose P, Lepage R, Slosman D, Didier D, Unger P, Patenaude J, Bounameaux H: Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999, 353: 190-195. 10.1016/S0140-6736(98)05248-9.
Schutgens RE, Ackermark P, Haas FJ, Nieuwenhuis HK, Peltenburg HG, Pijlman AH, Pruijm M, Oltmans R, Kelder JC, Biesma DH: Combination of a normal D-dimer concentration and a non-high pretest clinical probability score is a safe strategy to exclude deep venous thrombosis. Circulation. 2003, 107 (4): 593-597. 10.1161/01.CIR.0000045670.12988.1E.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/8/48/prepub
Download references
Acknowledgements
For this research project we received financial support from the Netherlands Organization for Scientific Research, grant number: ZON-MW904-66-112. The funding source had no influence on the design, data analysis and report of this study.
Author information
Authors and affiliations.
Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, The Netherlands
Cornelis J Biesheuvel, Yvonne Vergouwe, Ruud Oudega, Arno W Hoes, Diederick E Grobbee & Karel GM Moons
The Children's Hospital at Westmead, Sydney, Australia
Cornelis J Biesheuvel
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Karel GM Moons .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
All authors commented on the draft and the interpretation of the findings, read and approved the final manuscript. CJB was responsible for the design, statistical analysis and wrote the original manuscript. YV was responsible for the design and statistical analysis. RO was responsible for the data collection. AWH was responsible for expertise in case-control design. DEG and KGMM were responsible for conception and design of the study and coordination.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Biesheuvel, C.J., Vergouwe, Y., Oudega, R. et al. Advantages of the nested case-control design in diagnostic research. BMC Med Res Methodol 8 , 48 (2008). https://doi.org/10.1186/1471-2288-8-48
Download citation
Received : 07 March 2008
Accepted : 21 July 2008
Published : 21 July 2008
DOI : https://doi.org/10.1186/1471-2288-8-48
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diagnostic Accuracy
- Deep Vein Thrombosis
- Target Disease
- Diagnostic Accuracy Study
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
The nested case-control study in cardiology
Affiliation.
- 1 Division of Cardiology, McGill University Health Center, Montreal, Quebec, Canada.
- PMID: 14564310
- DOI: 10.1016/S0002-8703(03)00512-X
Background: The nested case-control study is an efficient epidemiological design whereby a case-control approach is employed within an established cohort. The large number of recent prospective studies and randomized trials conducted in cardiology provide cohorts within which the nested case-control approach is increasingly used.
Methods: This paper describes the design of the nested case-control study, and evaluates its role in cardiology by reviewing all such studies indexed in Medline from 1966 to 2000. The example of homocysteine is used to illustrate how discrepancies between results of nested case-control and case-control studies played an important role in the decisions and recommendations of national and international organizations.
Results: Seventy-seven nested case-control studies in cardiology were reviewed. The number of studies per year has been increasing since the first publication in 1987. The majority (96%) of studies evaluated potential risk factors for cardiovascular disease while the remainder evaluated drugs with cardiac adverse effects. In studies of homocysteine and coronary artery disease, nested case-control studies did not confirm the strong association suggested by early case-control studies that may have been influenced by bias (eg, selection, publication, or reverse causality). This led national and international organizations to advise against routine screening.
Conclusions: The nested case-control study is increasingly used to study causal relationships in cardiology. The large cohorts of cardiac patients created by prospective studies, clinical trials, and administrative databases should be exploited using this methodology to assess potential cardiac risk factors and other causal relationships that cannot be studied in randomized trials.
Publication types
- Research Support, Non-U.S. Gov't
- Biomarkers / blood
- Cardiology*
- Case-Control Studies*
- Coronary Disease / blood
- Coronary Disease / etiology*
- Homocysteine / blood*
- Meta-Analysis as Topic
- Prospective Studies
- Risk Factors
- Homocysteine

EP717 Module 5 - Epidemiologic Study Designs – Part 2:
Case-control studies.
- Page:
- 1
- | 2
- | 3
- | 4
- | 5
- | 6
- | 7

A Nested Case-Control Study
Interpretation of the odds ratio, test yourself, recap of case-control design.

Now consider a hypothetical prospective cohort study among 89,949 women in whom the investigators took blood samples and froze them at baseline for possible future use. After following the cohort for 12 years the investigators wanted to investigate a possible association between the pesticide DDT and breast cancer. Since they had frozen blood samples collected at baseline, they had the option of having the samples tested for DDT levels. If they had done this, the table below shows what they would have found.
If they had had this data, they could have calculated the risk ratio:
RR = (360/13,636) / (1,079/76,313) = 1.87
However, the cost of analyzing each sample for DDT was $20, and to analyze all of them would have cost close to $1.8 million. So, like the previous study, the exposure data was very costly.
Although this was a prospective cohort study, we could regard the cohort as a source population and conduct a case-control study drawing samples from the cohort . We could, for example, analyze the blood samples on all of the women who had developed breast cancer during the 12 year follow up and on 2,878 randomly selected samples from the women without breast cancer (i.e., twice as many controls as cases). This would be described as a nested case-control study , i.e., nested within a cohort study.
The results might have looked like this:
Odds Ratio = (a/c) / (b/d) = (360/1,079) / (432/2,446)
= 1.89 during the 12 year follow up study
So, they could achieve an odds ratio that is very close to what the risk ratio would have been at a much lower cost: (1,439+2,878) x $20 = $86,340.
The odds ratio is a legitimate measure of association, and, when the outcome of interest is uncommon, it provides a good estimate of what the risk ratio would have been if a cohort study had been possible. When looking at increasingly common outcomes, the odds ratio gives estimates that are more extreme than the risk ratio, i.e., further away from the null value.
Not surprisingly, the interpretation of an odds is therefore similar to the interpretation of a risk ratio.
- The null value (no difference) is 1.0.
- Odds ratios > 1 suggest an increase in risk
- Odds ratios < 1 suggest a decrease in risk
The odds ratio above would be interpreted as follows:
"Women with high DDT blood levels at baseline had 1.89 times the odds of developing breast cancer compared to women with low blood levels of DDT during the 12 year observation period."
Calculate the odds ratio for the association between playing video games and development of hypertension. Interpret the odds ratio you calculate in a sentence. See if you can do both of these correctly before looking at the answer.
return to top | previous page | next page
Content ©2021. All Rights Reserved. Date last modified: April 21, 2021. Wayne W. LaMorte, MD, PhD, MPH

IMAGES
VIDEO
COMMENTS
An example of a nested case-control study design (This is an example of a nested case-control design (m= 2) from a small cohort of ten subjects. For example, three subjects (i = 1, 2, 3) failed with no ties before the remaining seven subjects (i = 4, …, 10) were censored. The subjects 2 and 4 were selected as the controls from the risk set at ...
The analysis of nested case-control studies uses a proportional hazards model and a modification to the partial likelihood used in full-cohort studies, giving estimates of hazard ratios. Extensions to other survival models are possible. In the standard design, controls are selected randomly from the risk set for each case; however, more ...
The main advantages of a nested case-control study are as follows: (1) cost reduction and effort minimization, as only a fraction of the parent cohort requires the necessary outcome assessment; (2) reduced selection bias, as both case and control subjects are sampled from the same population; and (3) flexibility in analysis by allowing testing ...
A nested case-control (NCC) study is a variation of a case-control study in which cases and controls are drawn from the population in a fully enumerated cohort. [1] Usually, the exposure of interest is only measured among the cases and the selected controls. Thus the nested case-control study is more efficient than the full cohort design.
The nested case-control study (NCC) design within a prospective cohort study is used when outcome data are available for all subjects, but the exposure of interest has not been collected, and is difficult or prohibitively expensive to obtain for all subjects. A NCC analysis with good matching procedures yields estimates that are as efficient and unbiased as estimates from the full cohort study.
A major flaw inherent to case-control studies, described as early as 1959 , is the difficulty to ensure that cases and controls are a representative sample of the same source population. In a nested case-control study the cases emerge from a well-defined source population and the controls are sampled from that same population.
The case-control study can be subcategorized into four different subtypes based on how the control group is selected and when the cases develop the disease of interest as described in the following sections. Nested Case-Control Study When a case-control study is performed within a cohort study, it is called a nested case-control study. In a nested
A nested case-control study is an efficient design that can be embedded within an existing cohort study or randomised trial. It has a number of advantages compared to the conventional case-control design, and has the potential to answer important research questions using untapped prospectively collected data. We demonstrate the utility of the matched nested case-control design by applying it ...
3.1 |. Nested case-control studies: univariate case. In this article, we develop methods for performing estimation and inference for the joint frailty model for recurrent events and a terminal event in contexts where complete data is not available - for example, it may be expensive, time-consuming, or otherwise infeasible to collect certain exposure or covariate measures on the full cohort.
Abstract. The nested case-control study design (or the case-control in a cohort study) is described here and compared with other designs, including the classic case-control and cohort studies and the case-cohort study. In the nested case-control study, cases of a disease that occur in a defined cohort are identified and, for each, a specified ...
Abstract. The nested case-control study design (or the case-control in a cohort study) is described here and compared with other designs, including the classic case-control and cohort studies and the case-cohort study. In the nested case-control study, cases of a disease that occur in a defined cohort are identified and, for each, a specified ...
Definition. A nested case-control study is one that is "nested" within a cohort study. In many cohort studies, all subjects provide a wide range of information at the time of recruitment, e.g., results from a physical examination, answers to multiple questionnaires, blood and urine samples, and results from imaging techniques.
Nested case-control studies refer to a class of cohort sampling designs used in epidemiologic research. This article describes the general principles, analysis methods, statistical properties, and relationship to other approaches. Methods are illustrated by studies from the epidemiological literature.
The case-control study can be subcategorized into four different subtypes based on how the control group is selected and when the cases develop the disease of interest as described in the following sections. Nested Case-Control Study. When a case-control study is performed within a cohort study, it is called a nested case-control study.
Researchers investigated whether antipsychotic drugs were associated with venous thromboembolism. A population based nested case-control study design was used. Data were taken from the UK QResearch primary care database consisting of 7 267 673 patients. Cases were adult patients with a first ever record of venous thromboembolism between 1 January 1996 and 1 July 2007. For each case, up to four ...
A Nested Case-Control Study. Suppose a prospective cohort study were conducted among almost 90,000 women for the purpose of studying the determinants of cancer and cardiovascular disease. After enrollment, the women provide baseline information on a host of exposures, and they also provide baseline blood and urine samples that are frozen for ...
Background Despite its benefits, it is uncommon to apply the nested case-control design in diagnostic research. We aim to show advantages of this design for diagnostic accuracy studies. Methods We used data from a full cross-sectional diagnostic study comprising a cohort of 1295 consecutive patients who were selected on their suspicion of having deep vein thrombosis (DVT). We draw nested case ...
The nested case-control study design employs case-control methodology within an established prospective cohort study . It first emerged in the 1970-80s and was typically used when it was expensive or difficult to obtain data on a particular exposure for all members of the cohort; instead a subset of controls would be selected at random [ 2 ].
The nested case-control design is the most widely used method for sampling from epidemiologic cohorts when investigators need to collect additional data in a reduced sample. 1 Using incidence density sampling, the potential impact of exposures on disease occurrence can be studied by hazard ratios in a reduced data set. 1, 2 Furthermore, the cumulative incidence function can also be estimated ...
Nested case-control studies Risk sets di er depending on whether time since entry into the study or age is used as the underlying time scale Figure:Sampling a nested case-control study with one control per case, using time since study recruitment or age as the timescale. The solid lines represent the time period over which individuals are observed.
In summary, nested case-control and case-cohort designs are efficient in terms of cost and can be used to evaluate the relationship between the exposure and diseases. Compared to a nested case-control design, the case-cohort design is more efficient and allows an investigator to study several disease outcomes by using the same random sample.
Nested case-control studies refer to a class of cohort sampling designs used in epidemiologic research. This article describes the general principles, analysis methods, statistical properties, and relationship to other approaches. Methods are illustrated by studies from the epidemiological literature.
Abstract. Background: The nested case-control study is an efficient epidemiological design whereby a case-control approach is employed within an established cohort. The large number of recent prospective studies and randomized trials conducted in cardiology provide cohorts within which the nested case-control approach is increasingly used.
A Nested Case-Control Study. Now consider a hypothetical prospective cohort study among 89,949 women in whom the investigators took blood samples and froze them at baseline for possible future use. After following the cohort for 12 years the investigators wanted to investigate a possible association between the pesticide DDT and breast cancer.