- A-Z Publications
- Annual Review of Clinical Psychology

Annual Review of Clinical Psychology - Current Issue
- Navigate this Journal
- Early Publication
Previous Volumes
- Editorial Committee
Volume 19, 2023
A clinical psychologist who studies alcohol.
In this article, I describe why I believe the study of alcohol use and its consequences is a rich and rewarding area of scholarly activity that touches on multiple disciplines in the life sciences, the behavioral sciences, and the humanities. I then detail the circuitous path I took to become an alcohol researcher and the various challenges I encountered when starting up my research program at the University of Missouri. A major theme of my journey has been my good fortune encountering generous, brilliant scholars who took an interest in me and my career and who helped guide and assist me over the course of my career. I also highlight selected, other professional activities I've been involved in, focusing on editorial work, quality assurance, and governance of professional societies. While the focus is on my training and work as a psychologist, the overarching theme is the interpersonal context that nurtures careers.
- Add to my favorites Favourites
Community Mental Health Services for American Indians and Alaska Natives: Reconciling Evidence-Based Practice and Alter-Native Psy-ence
This review updates and extends Gone & Trimble's (2012) prior review of American Indian (AI) and Alaska Native (AN) mental health. First, it defines AI/AN populations in the USA, with an explanation of the importance of political citizenship in semisovereign Tribal Nations as primary for categorizing this population. Second, it presents an updated summary of what is known about AI/AN mental health, with careful notation of recurrent findings concerning community inequities in addiction, trauma, and suicide. Third, this article reviews key literature about AI/AN community mental health services appearing since 2010, including six randomized controlled trials of recognizable mental health treatments. Finally, it reimagines the AI/AN mental health enterprise in response to an “alter-Native psy-ence,” which recasts prevalent mental health conditions as postcolonial pathologies and harnesses postcolonial meaning-making through Indigenized therapeutic interventions. Ultimately, AI/AN Tribal Nations must determine for themselves how to adopt, adapt, integrate, or refuse specific mental health treatments and services for wider community benefit.
Culturally Responsive Cognitive Behavioral Therapy for Ethnically Diverse Populations
Cognitive behavioral therapy (CBT) is often referred to as the “gold standard” treatment for mental health problems, given the large body of evidence supporting its efficacy. However, there are persistent questions about the generalizability of CBTs to culturally diverse populations and whether culturally sensitive approaches are warranted. In this review, we synthesize the literature on CBT for ethnic minorities, with an emphasis on randomized trials that address cultural sensitivity within the context of CBT. In general, we find that CBT is effective for ethnic minorities with diverse mental health problems, although nonsignificant trends suggest that CBT effects may be somewhat weaker for ethnic minorities compared to Whites. We find mixed support for the cultural adaptation of CBTs, but evidence for cultural sensitivity training of CBT clinicians is lacking, given a dearth of relevant trials. Based on the limited evidence thus far, we summarize three broad models for addressing cultural issues when providing CBT to diverse populations.
What Four Decades of Meta-Analysis Have Taught Us About Youth Psychotherapy and the Science of Research Synthesis
Intervention scientists have published more than 600 randomized controlled trials (RCTs) of youth psychotherapies. Four decades of meta-analyses have been used to synthesize the RCT findings and identify scientifically and clinically significant patterns. These meta-analyses have limitations, noted herein, but they have advanced our understanding of youth psychotherapy, revealing ( a ) mental health problems for which our interventions are more and less successful (e.g., anxiety and depression, respectively); ( b ) the beneficial effects of single-session interventions, interventions delivered remotely, and interventions tested in low- and middle-income countries; ( c ) the association of societal sexism and racism with reduced treatment benefit in majority-girl and majority-Black groups; and, importantly, ( d ) the finding that average youth treatment benefit has not increased across five decades of research, suggesting that new strategies may be needed. Opportunities for the future include boosting relevance to policy and practice and using meta-analysis to identify mechanisms of change and guide personalizing of treatment.
Evaluation of Pressing Issues in Ecological Momentary Assessment
The use of repeated, momentary, real-world assessment methods known as the Experience Sampling Method and Ecological Momentary Assessment (EMA) has been broadly embraced over the last few decades. These methods have extended our assessment reach beyond lengthy retrospective self-reports as they can capture everyday experiences in their immediate context, including affect, behavior, symptoms, and cognitions. In this review we evaluate nine conceptual, methodological, and psychometric issues about EMA with the goal of stimulating conversation and guiding future research on these matters: the extent to which participants are actually reporting momentary experiences, respondents’ interpretation of momentary questions, the use of comparison standards in responding, efforts to increase the EMA reporting period beyond the moment to longer periods within a day, training of EMA study participants, concerns about selection bias of respondents, the impact of missing EMA assessments, the reliability of momentary data, and for which purposes EMA might be considered a gold standard for assessment. Resolution of these issues should have far-reaching implications for advancing the field.
Machine Learning and the Digital Measurement of Psychological Health
Since its inception, the discipline of psychology has utilized empirical epistemology and mathematical methodologies to infer psychological functioning from direct observation. As new challenges and technological opportunities emerge, scientists are once again challenged to define measurement paradigms for psychological health and illness that solve novel problems and capitalize on new technological opportunities. In this review, we discuss the theoretical foundations of and scientific advances in remote sensor technology and machine learning models as they are applied to quantify psychological functioning, draw clinical inferences, and chart new directions in treatment.
The Questionable Practice of Partialing to Refine Scores on and Inferences About Measures of Psychological Constructs
Partialing is a statistical approach researchers use with the goal of removing extraneous variance from a variable before examining its association with other variables. Controlling for confounds through analysis of covariance or multiple regression analysis and residualizing variables for use in subsequent analyses are common approaches to partialing in clinical research. Despite its intuitive appeal, partialing is fraught with undesirable consequences when predictors are correlated. After describing effects of partialing on variables, we review analytic approaches commonly used in clinical research to make inferences about the nature and effects of partialed variables. We then use two simulations to show how partialing can distort variables and their relations with other variables. Having concluded that, with rare exception, partialing is ill-advised, we offer recommendations for reducing or eliminating problematic uses of partialing. We conclude that the best alternative to partialing is to define and measure constructs so that it is not needed.
Eating Disorders in Boys and Men
While boys and men have historically been underrepresented in eating disorder research, increasing interest and research during the twenty-first century have contributed important knowledge to the field. In this article, we review the epidemiology of eating disorders and muscle dysmorphia (the pathological pursuit of muscularity) in boys and men; specific groups of men at increased risk for eating disorders; sociocultural, psychological, and biological vulnerability factors; and male-specific assessment measures. We also provide an overview of current research on eating disorder and muscle dysmorphia prevention efforts, treatment outcomes, and mortality risk in samples of boys and men. Priorities for future research are including boys and men in epidemiological studies to track changes in incidence, identifying (neuro)biological factors contributing to risk, eliminating barriers to treatment access and utilization, and refining male-specific prevention and treatment efforts.
Mental Health of Transgender and Gender Diverse Youth
Transgender and gender diverse (TGD) children and adolescents are an increasingly visible yet highly stigmatized group. These youth experience more psychological distress than not only their cisgender, heterosexual peers but also their cisgender, sexual minority peers. In this review, we document these mental health disparities and discuss potential explanations for them using a minority stress framework. We also discuss factors that may increase and decrease TGD youth's vulnerability to psychological distress. Further, we review interventions, including gender-affirming medical care, that may improve mental health in TGD youth. We conclude by discussing limitations of current research and suggestions for the future.
Behavioral Interventions for Children and Adults with Tic Disorder
Over the past decade, behavioral interventions have become increasingly recognized and recommended as effective first-line therapies for treating individuals with tic disorders. In this article, we describe a basic theoretical and conceptual framework through which the reader can understand the application of these interventions for treating tics. The three primary behavioral interventions for tics with the strongest empirical support (habit reversal, Comprehensive Behavioral Intervention for Tics, and exposure and response prevention) are described. Research on the efficacy and effectiveness of these treatments is summarized along with a discussion of the research evaluating the delivery of these treatments in different formats and modalities. The article closes with a review of the possible mechanisms of change underlying behavioral interventions for tics and areas for future research.
The Garrett Lee Smith Memorial Act: A Description and Review of the Suicide Prevention Initiative
The Garrett Lee Smith (GLS) Memorial Act, continuously funded since 2004, has supported comprehensive, community-based youth suicide prevention efforts throughout the United States. Compared to matched communities, communities implementing GLS suicide prevention activities have lower population rates of suicide attempts and lower mortality among young people. Positive outcomes have been more pronounced with continuous years of implementation and in less densely populated communities. Cost analyses indicate that implementation of GLS suicide prevention activities more than pays for itself in reduced health care costs associated with fewer emergency department visits and hospitalizations. Although findings are encouraging, the heterogeneity of community suicide prevention programs and the lack of randomized trials preclude definitive determination of causal effects associated with GLS. The GLS initiative has never been brought fully to scale (e.g., simultaneously impacting all communities in the United States), so beneficial effects on nationwide suicide rates have not been realized.
Racism and Social Determinants of Psychosis
The Centers for Disease Control and Prevention has identified racism as a serious threat to public health. Structural racism is a fundamental cause of inequity within interconnected institutions and the social environments in which we live and develop. This review illustrates how these ethnoracial inequities impact risk for the extended psychosis phenotype. Black and Latinx populations are more likely than White populations to report psychotic experiences in the United States due to social determining factors such as racial discrimination, food insecurity, and police violence. Unless we dismantle these discriminatory structures, the chronic stress and biological consequences of this race-based stress and trauma will impact the next generation's risk for psychosis directly, and indirectly through Black and Latina pregnant mothers. Multidisciplinary early psychosis interventions show promise in improving prognosis, but coordinated care and other treatments still need to be more accessible and address the racism-specific adversities many Black and Latinx people face in their neighborhoods and social environments.
Developmental Consequences of Intimate Partner Violence on Children
Numerous studies associate childhood exposure to intimate partner violence (IPV) with adverse adjustment in the domains of mental health, social, and academic functioning. This review synthesizes this literature and highlights the critical role of child self-regulation in mediating children's adjustment outcomes. We discuss major methodological problems of the field, including failure to consider the effects of prenatal IPV exposure and the limitations of variable-oriented and cross-sectional approaches. Finally, we present a comprehensive theoretical model of the effects of IPV on children's development. This model includes three mechanistic pathways—one that is unique to IPV (maternal representations) and two that are consistent with the effects of other stressors (maternal mental health and physiological functioning). In our model, the effects of these three pathways on child adjustment outcomes are mediated through parenting and child self-regulation. Future research directions and clinical implications are discussed in the context of the model.
Psychoneuroimmunology: An Introduction to Immune-to-Brain Communication and Its Implications for Clinical Psychology
Research conducted over the past several decades has revolutionized our understanding of the role of the immune system in neural and psychological development and function across the life span. Our goal in this review is to introduce this dynamic area of research to a psychological audience and highlight its relevance for clinical psychology. We begin by introducing the basic physiology of immune-to-brain signaling and the neuroimmune network, focusing on inflammation. Drawing from preclinical and clinical research, we then examine effects of immune activation on key psychological domains, including positive and negative valence systems, social processes, cognition, and arousal (fatigue, sleep), as well as links with psychological disorders (depression, posttraumatic stress disorder, anxiety, schizophrenia). We also consider psychosocial stress as a critical modulator of neuroimmune activity and focus on early life adversity. Finally, we highlight psychosocial and mind–body interventions that influence the immune system and may promote neuroimmune resilience.
Racial, Ethnic, and Cultural Resilience Factors in African American Youth Mental Health
Racism constitutes a significant risk to the mental health of African American children, adolescents, and emerging adults. This review evaluates recent literature examining ethnic and racial identity, ethnic-racial socialization, religiosity and spirituality, and family and parenting as racial, ethnic, and cultural resilience factors that shape the impact of racism on youth mental health. Representative studies, purported mechanisms, and critiques of prior research are presented for each factor. Recent studies of racism and resilience revisit foundational resilience factors from prior research while reflecting new and important advances (e.g., consideration of gender, cultural context, structural racism), providing important insights for the development of prevention and intervention efforts and policy that can alleviate mental health suffering and promote health and mental health equity for African American youth.
Acculturation and Psychopathology
Acculturation and psychopathology are linked in integrated, interactional, intersectional, and dynamic ways that span different types of intercultural contact, levels of analysis, timescales, and contexts. A developmental psychopathology approach can be useful to explain why, how, and what about psychological acculturation results in later adaptation or maladaptation for acculturating youth and adults. This review applies a conceptual model of acculturation and developmental psychopathology to a widely used framework of acculturation variables producing an Integrated Process Framework of Acculturation Variables (IP-FAV). This new comprehensive framework depicts major predisposing acculturation conditions (why) as well as acculturation orientations and processes (how) that result in adaptation and maladaptation across the life span (what). The IP-FAV is unique in that it integrates both proximal and remote acculturation variables and explicates key acculturation processes to inform research, practice, and policy.
Posttraumatic Stress Disorder in Refugees
The number of refugees and internally displaced people in 2022 is the largest since World War II, and meta-analyses demonstrate that these people experience elevated rates of mental health problems. This review focuses on the role of posttraumatic stress disorder (PTSD) in refugee mental health and includes current knowledge of the prevalence of PTSD, risk factors, and apparent differences that exist between PTSD in refugee populations and PTSD in other populations. An emerging literature on understanding mechanisms of PTSD encompasses neural, cognitive, and social processes, which indicate that these factors may not function exactly as they have functioned previously in other PTSD populations. This review recognizes the numerous debates in the literature on PTSD in refugees, including those on such issues as the conceptualization of mental health and the applicability of the PTSD diagnosis across cultures, as well as the challenge of treating PTSD in low- and middle-income countries that lack mental health resources to offer standard PTSD treatments.
Risk and Resilience Among Children with Incarcerated Parents: A Review and Critical Reframing
Parental incarceration is a significant, inequitably distributed form of adversity that affects millions of US children and increases their risk for emotional and behavioral problems. An emerging body of research also indicates, however, that children exhibit resilience in the context of parental incarceration. In this article, we review evidence regarding the adverse implications of parental incarceration for children's adjustment and consider factors that account for these consequences with special attention to naturally occurring processes and interventions that may mitigate risk and contribute to positive youth development. We also offer a critical reframing of resilience research and argue that ( a ) scholars should adopt more contextualized approaches to the study of resilience that are sensitive to intersecting inequalities and ( b ) resilience research and practice should be conceptualized as important complements to, rather than substitutes for, social and institutional change. We conclude by offering social justice–informed recommendations for future research and practice.
Supernatural Attributions: Seeing God, the Devil, Demons, Spirits, Fate, and Karma as Causes of Events
For many people worldwide, supernatural beliefs and attributions—those focused on God, the devil, demons, spirits, an afterlife, karma, or fate—are part of everyday life. Although not widely studied in clinical psychology, these beliefs and attributions are a key part of human diversity. This article provides a broad overview of research on supernatural beliefs and attributions with special attention to their psychological relevance: They can serve as coping resources, sources of distress, psychopathology signals, moral guides, and decision-making tools. Although supernatural attributions sometimes involve dramatic experiences seen to violate natural laws, people more commonly think of supernatural entities working indirectly through natural events. A whole host of factors can lead people to make supernatural attributions, including contextual factors, specific beliefs, psychopathology, cognitive styles and personality, and social and cultural influences. Our aim is to provide clinical psychologists with an entry point into this rich, fascinating, and often overlooked literature.
Volume 19 (2023)
Volume 18 (2022), volume 17 (2021), volume 16 (2020), volume 15 (2019), volume 14 (2018), volume 13 (2017), volume 12 (2016), volume 11 (2015), volume 10 (2014), volume 9 (2013), volume 8 (2012), volume 7 (2011), volume 6 (2010), volume 5 (2009), volume 4 (2008), volume 3 (2007), volume 2 (2006), volume 1 (2005), volume 0 (1932).
Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident Due to planned maintenance there will be periods of time where the website may be unavailable. We apologise for any inconvenience.
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > The Psychologist's Companion
- > Writing a Literature Review

Book contents
- Frontmatter
- Acknowledgments
- Introduction
- Part I Macro-Challenges in Writing Papers: Planning and Formulating Papers
- 1 Eight Common Misconceptions about Psychology Papers
- 2 How to Generate, Evaluate, and Sell Your Ideas for Research and Papers
- 3 Literature Research
- 4 Writing a Literature Review
- 5 Planning and Writing the Experimental Research Paper
- 6 Ethics in Research and Writing
- Part II Micro-Challenges in Writing Papers: Presenting Your Ideas in Writing
- Part III Writing and Preparing Articles for Journal Submission
- Part IV Presenting Yourself to Others
4 - Writing a Literature Review
from Part I - Macro-Challenges in Writing Papers: Planning and Formulating Papers
Published online by Cambridge University Press: 24 November 2016
Most undergraduate research papers, and many graduate and professional research papers as well, are based on literature reviews. The aims of a literature review are different from those of an empirical research paper, and hence the skills required differ somewhat as well. The goals of literature reviews are the following (American Psychological Association, 2009):
1. To define and clarify problems
2. To inform the reader about a subject by summarizing and evaluating studies
3. To identify inconsistencies, gaps, contradictions, and relationships in the literature
4. To suggest future steps and approaches to solve the issues identified
There are five kinds of literature reviews that can be distinguished on the basis of the aim of the review. Reviews can strive to (a) generate new knowledge, (b) test theories, (c) integrate theories, (d) develop a new theory, or (e) integrate existing knowledge.
If you plan to submit your literature review to a journal and have to decide where to submit it, you may want to read some literature reviews that have been published in the journals you are considering to find out whether your paper is a good fit to the journal. Generally, the probability of an article being accepted is highest when you develop new knowledge, a new theory, or integrate several theories (instead of just reviewing and summarizing the literature on a particular topic) (Eisenberg, 2000). In general, the best literature reviews do not merely summarize literature; they also create new knowledge by placing the literature into a new framework or at least seeing the literature in a new way.
The literature review can proceed smoothly if you follow a sequence of simple steps:
1. Decide on a topic for a paper.
2. Organize and search the literature.
3. Prepare an outline.
4. Write the paper.
5. Evaluate the paper yourself and seek others’ feedback on it.
DECIDING ON A TOPIC FOR A PAPER
Your first task is to decide on a topic for a paper. This is, in a sense, the most important task because the paper can be no better than the topic. We have found five mistakes that repeatedly turn up in writers’ choices of topics:
1. The topic doesn't interest the writer.
2. The topic is too easy or too safe for the writer.
3. The topic is too difficult for the writer.
4. There is inadequate literature on the topic.
5. The topic is too broad.
Access options
Save book to kindle.
To save this book to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service .
- Writing a Literature Review
- Robert J. Sternberg , Cornell University, New York , Karin Sternberg , Cornell University, New York
- Book: The Psychologist's Companion
- Online publication: 24 November 2016
- Chapter DOI: https://doi.org/10.1017/CBO9781316488935.006
Save book to Dropbox
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Dropbox .
Save book to Google Drive
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Google Drive .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 February 2022
Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics
- Christoph U. Correll ORCID: orcid.org/0000-0002-7254-5646 1 , 2 , 3 ,
- Amber Martin 4 ,
- Charmi Patel 5 ,
- Carmela Benson 5 ,
- Rebecca Goulding 6 ,
- Jennifer Kern-Sliwa 5 ,
- Kruti Joshi 5 ,
- Emma Schiller 4 &
- Edward Kim ORCID: orcid.org/0000-0001-8247-6675 7
Schizophrenia volume 8 , Article number: 5 ( 2022 ) Cite this article
15k Accesses
53 Citations
14 Altmetric
Metrics details
- Schizophrenia
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature review of English-language CPGs and synthesized current recommendations for the acute and maintenance management with antipsychotics. Searches for schizophrenia CPGs were conducted in MEDLINE/Embase from 1/1/2004–12/19/2019 and in guideline websites until 06/01/2020. Of 19 CPGs, 17 (89.5%) commented on first-episode schizophrenia (FES), with all recommending antipsychotic monotherapy, but without agreement on preferred antipsychotic. Of 18 CPGs commenting on maintenance therapy, 10 (55.6%) made no recommendations on the appropriate maximum duration of maintenance therapy, noting instead individualization of care. Eighteen (94.7%) CPGs commented on long-acting injectable antipsychotics (LAIs), mainly in cases of nonadherence (77.8%), maintenance care (72.2%), or patient preference (66.7%), with 5 (27.8%) CPGs recommending LAIs for FES. For treatment-resistant schizophrenia, 15/15 CPGs recommended clozapine. Only 7/19 (38.8%) CPGs included a treatment algorithm.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

The serotonin theory of depression: a systematic umbrella review of the evidence

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Introduction.
Schizophrenia is an often debilitating, chronic, and relapsing mental disorder with complex symptomology that manifests as a combination of positive, negative, and/or cognitive features 1 , 2 , 3 . Standard management of schizophrenia includes the use of antipsychotic medications to help control acute psychotic episodes 4 and prevent relapses 5 , 6 , whereas maintenance therapy is used in the long term after patients have been stabilized 7 , 8 , 9 . Two main classes of drugs—first- and second-generation antipsychotics (FGA and SGA)—are used to treat schizophrenia 10 . SGAs are favored due to the lower rates of adverse effects, such as extrapyramidal effects, tardive dyskinesia, and relapse 11 . However, pharmacologic treatment for schizophrenia is complicated because nonadherence is prevalent, and is a major risk factor for relapse 9 and poor overall outcomes 12 . The use of long-acting injectable (LAI) versions of antipsychotics aims to limit nonadherence-related relapses and poor outcomes 13 .
Patient treatment pathways and treatment choices are determined based on illness acuity/severity, past treatment response and tolerability, as well as balancing medication efficacy and adverse effect profiles in the context of patient preferences and adherence patterns 14 , 15 . Clinical practice guidelines (CPG) serve to inform clinicians with recommendations that reflect current evidence from meta-analyses of randomized controlled trials (RCTs), individual RCTs and, less so, epidemiologic studies, as well as clinical experience, with the goal of providing a framework and road-map for treatment decisions that will improve quality of care and achieve better patients outcomes. The use of clinical algorithms or other decision trees in CPGs may improve the ease of implementation of the evidence in clinical practice 16 . While CPGs are an important tool for mental health professionals, they have not been updated on a regular basis like they have been in other areas of medicine, such as in oncology. In the absence of current information, other governing bodies, healthcare systems, and hospitals have developed their own CPGs regarding the treatment of schizophrenia, and many of these have been recently updated 17 , 18 , 19 . As such, it is important to assess the latest guidelines to be aware of the changes resulting from consideration of updated evidence that informed the treatment recommendations. Since CPGs are comprehensive and include the diagnosis as well as the pharmacological and non-pharmacological management of individuals with schizophrenia, a detailed comparative review of all aspects of CPGs for schizophrenia would have been too broad a review topic. Further, despite ongoing efforts to broaden the pharmacologic tools for the treatment of schizophrenia 20 , antipsychotics remain the cornerstone of schizophrenia management 8 , 21 . Therefore, a focused review of guideline recommendations for the management of schizophrenia with antipsychotics would serve to provide clinicians with relevant information for treatment decisions.
To provide an updated overview of United States (US) national and English language international guidelines for the management of schizophrenia, we conducted a systematic literature review (SLR) to identify CPGs and synthesize current recommendations for pharmacological management with antipsychotics in the acute and maintenance phases of schizophrenia.
Systematic searches for the SLR yielded 1253 hits from the electronic literature databases. After removal of duplicate references, 1127 individual articles were screened at the title and abstract level. Of these, 58 publications were deemed eligible for screening at the full-text level, from which 19 were ultimately included in the SLR. Website searches of relevant organizations yielded 10 additional records, and an additional three records were identified by the state-by-state searches. Altogether, this process resulted in 32 records identified for inclusion in the SLR. Of the 32 sources, 19 primary CPGs, published/issued between 2004 and 2020, were selected for extraction, as illustrated in the PRISMA diagram (Fig. 1 ). While the most recent APA guideline was identified and available for download in 2020, the reference to cite in the document indicates a publication date of 2021.
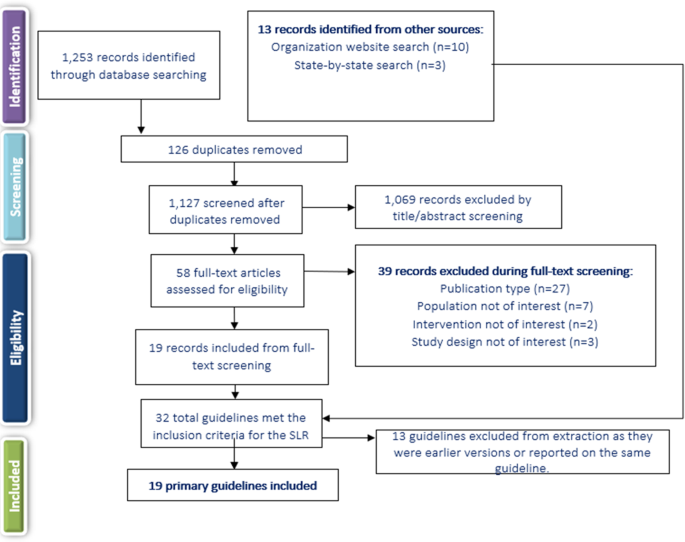
SLR systematic literature review.
Of the 19 included CPGs (Table 1 ), three had an international focus (from the following organizations: International College of Neuropsychopharmacology [CINP] 22 , United Nations High Commissioner for Refugees [UNHCR] 23 , and World Federation of Societies of Biological Psychiatry [WFSBP] 24 , 25 , 26 ); seven originated from the US; 17 , 18 , 19 , 27 , 28 , 29 , 30 , 31 , 32 three were from the United Kingdom (British Association for Psychopharmacology [BAP] 33 , the National Institute for Health and Care Excellence [NICE] 34 , and the Scottish Intercollegiate Guidelines Network [SIGN] 35 ); and one guideline each was from Singapore 36 , the Polish Psychiatric Association (PPA) 37 , 38 , the Canadian Psychiatric Association (CPA) 14 , the Royal Australia/New Zealand College of Psychiatrists (RANZCP) 39 , the Association Française de Psychiatrie Biologique et de Neuropsychopharmacologie (AFPBN) from France 40 , and Italy 41 . Fourteen CPGs (74%) recommended treatment with specific antipsychotics and 18 (95%) included recommendations for the use of LAIs, while just seven included a treatment algorithm Table 2 ). The AGREE II assessment resulted in the highest score across the CPGs domains for NICE 34 followed by the American Psychiatric Association (APA) guidelines 17 . The CPA 14 , BAP 33 , and SIGN 35 CPGs also scored well across domains.
Acute therapy
Seventeen CPGs (89.5%) provided treatment recommendations for patients experiencing a first schizophrenia episode 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 41 , but the depth and focus of the information varied greatly (Supplementary Table 1 ). In some CPGs, information on treatment of a first schizophrenia episode was limited or grouped with information on treating any acute episode, such as in the CPGs from CINP 22 , AFPBN 40 , New Jersey Division of Mental Health Services (NJDMHS) 32 , the APA 17 , and the PPA 37 , 38 , while the others provided more detailed information specific to patients experiencing a first schizophrenia episode 14 , 18 , 19 , 23 , 24 , 28 , 33 , 34 , 35 , 36 , 39 , 41 . The American Association of Community Psychiatrists (AACP) Clinical Tips did not provide any information on the treatment of schizophrenia patients with a first episode 29 .
There was little agreement among CPGs regarding the preferred antipsychotic for a first schizophrenia episode. However, there was strong consensus on antipsychotic monotherapy and that lower doses are generally recommended due to better treatment response and greater adverse effect sensitivity. Some guidelines recommended SGAs over FGAs when treating a first-episode schizophrenia patient (RANZCP 39 , Texas Medication Algorithm Project [TMAP] 28 , Oregon Health Authority 19 ), one recommended starting patients on an FGA (UNHCR 23 ), and others stated specifically that there was no evidence of any difference in efficacy between FGAs and SGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , Singapore guidelines 36 ), or did not make any recommendation (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , Schizophrenia Patient Outcomes Research Team [PORT] 30 , 31 ). The BAP 33 and WFBSP 24 noted that while there was probably no difference between FGAs and SGAs in efficacy, some SGAs (olanzapine, amisulpride, and risperidone) may perform better than some FGAs. The Schizophrenia PORT recommendations noted that while there seemed to be no differences between SGAs and FGAs in short-term studies (≤12 weeks), longer studies (one to two years) suggested that SGAs may provide benefits in terms of longer times to relapse and discontinuation rates 30 , 31 . The AFPBN guidelines 40 and Florida Medicaid Program guidelines 18 , which both focus on use of LAI antipsychotics, both recommended an SGA-LAI for patients experiencing a first schizophrenia episode. A caveat in most CPGs was that physicians and their patients should discuss decisions about the choice of antipsychotic and that the choice should consider individual patient factors/preferences, risk of adverse and metabolic effects, and symptom patterns 17 , 18 , 19 , 22 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 41 .
Most CPGs recommended switching to a different monotherapy if the initial antipsychotic was not effective or not well tolerated after an adequate antipsychotic trial at an appropriate dose 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 32 , 33 , 35 , 36 , 39 . For patients initially treated with an FGA, the UNHCR recommended switching to an SGA (olanzapine or risperidone) 23 . Guidance on response to treatment varied in the measures used but typically required at least a 20% improvement in symptoms (i.e. reduction in Positive and Negative Syndrome Scale or Brief Psychiatric Rating Scale scores) from pre-treatment levels.
Several CPGs contained recommendations on the duration of antipsychotic therapy after a first schizophrenia episode. The NJDMHS guidelines 32 recommended nine to 12 months; CINP 22 recommended at least one year; CPA 14 recommended at least 18 months; WFSBP 25 , the Italian guidelines 41 , and NICE 34 recommended 1 to 2 years; and the RANZCP 39 , BAP 33 , and SIGN 35 recommended at least 2 years. The APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy.
Twelve guidelines 14 , 18 , 22 , 24 , 28 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 (63.2%) discussed the treatment of subsequent/multiple episodes of schizophrenia (i.e., following relapse). These CPGs noted that the considerations guiding the choice of antipsychotic for subsequent/multiple episodes were similar to those for a first episode, factoring in prior patient treatment response, adverse effect patterns and adherence. The CPGs also noted that response to treatment may be lower and require higher doses to achieve a response than for first-episode schizophrenia, that a different antipsychotic than used to treat the first episode may be needed, and that a switch to an LAI is an option.
Several CPGs provided recommendations for patients with specific clinical features (Supplementary Table 1 ). The most frequently discussed group of clinical features was negative symptoms, with recommendations provided in the CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , APA 17 , and NJDMHS guidelines; 32 negative symptoms were the sole focus of the guidelines from the PPA 37 , 38 . The guidelines noted that due to limited evidence in patients with predominantly negative symptoms, there was no clear benefit for any strategy, but that options included SGAs (especially amisulpride) rather than FGAs (WFSBP 24 , CINP 22 , AFPBN 40 , SIGN 35 , NJDMHS 32 , PPA 37 , 38 ), and addition of an antidepressant (WFSBP 24 , UNHCR 23 , SIGN 35 , NJDMHS 32 ) or lamotrigine (SIGN 35 ), or switching to another SGA (NJDMHS 32 ) or clozapine (NJDMHS 32 ). The PPA guidelines 37 , 38 stated that the use of clozapine or adding an antidepressant or other medication class was not supported by evidence, but recommended the SGA cariprazine for patients with predominant and persistent negative symptoms, and other SGAs for those with full-spectrum negative symptoms. However, the BAP 33 stated that no recommendations can be made for any of these strategies because of the quality and paucity of the available evidence.
Some of the CPGs also discussed treatment of other clinical features to a limited degree, including depressive symptoms (CINP 22 , UNHCR 23 , CPA 14 , APA 17 , and NJDMHS 32 ), cognitive dysfunction (CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , and NJDMHS 32 ), persistent aggression (CINP 22 , WFSBP 24 , CPA 14 , AFPBN 40 , NICE 34 , SIGN 35 , BAP 33 , and NJDMHS 32 ), and comorbid psychiatric diagnoses (CINP 22 , RANZCP 39 , BAP 33 , APA 17 , and NJDMHS 32 ).
Fifteen CPGs (78.9%) discussed treatment-resistant schizophrenia (TRS); all defined it as persistent, predominantly positive symptoms after two adequate antipsychotic trials; clozapine was the unanimous first choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 . However, the UNHCR guidelines 23 , which included recommendations for treatment of refugees, noted that clozapine is only a reasonable choice in regions where white blood cell monitoring and specialist supervision are available, otherwise, risperidone or olanzapine are alternatives if they had not been used in the previous treatment regimen.
There were few options for patients who are resistant to clozapine therapy, and evidence supporting these options was limited. The CPA guidelines 14 therefore stated that no recommendation can be given due to inadequate evidence. Other CPGs discussed options (but noted there was limited supporting evidence), such as switching to olanzapine or risperidone (WFSBP 24 , TMAP 28 ), adding a second antipsychotic to clozapine (CINP 22 , NICE 34 , TMAP 28 , BAP 33 , Florida Medicaid Program 18 , Oregon Health Authority 19 , RANZCP 39 ), adding lamotrigine or topiramate to clozapine (CINP 22 , Florida Medicaid Program 18 ), combination therapy with two non-clozapine antipsychotics (Florida Medicaid Program 18 , NJDMHS 32 ), and high-dose non-clozapine antipsychotic therapy (BAP 33 , SIGN 35 ). Electroconvulsive therapy was noted as a last resort for patients who did not respond to any pharmacologic therapy, including clozapine, by 10 CPGs 17 , 18 , 19 , 22 , 24 , 28 , 32 , 35 , 36 , 39 .
Maintenance therapy
Fifteen CPGs (78.9%) discussed maintenance therapy to various degrees via dedicated sections or statements, while three others referred only to maintenance doses by antipsychotic agent 18 , 23 , 29 without accompanying recommendations (Supplementary Table 2 ). Only the Italian guideline provided no reference or comments on maintenance treatment. The CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 recommended keeping patients on the same antipsychotic and at the same dose on which they had achieved remission. Several CPGs recommended maintenance therapy at the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , and TMAP 28 ). The CPA 14 and SIGN 35 defined the lower dose as 300–400 mg chlorpromazine equivalents or 4–6 mg risperidone equivalents, and the Singapore guidelines 36 stated that the lower dose should not be less than half the original dose. TMAP 28 stated that given the relapsing nature of schizophrenia, the maintenance dose should often be close to the original dose. While SIGN 35 recommended that patients remain on the same antipsychotic that provided remission, these guidelines also stated that maintenance with amisulpride, olanzapine, or risperidone was preferred, and that chlorpromazine and other low-potency FGAs were also suitable. The BAP 33 recommended that the current regimen be optimized before any dose reduction or switch to another antipsychotic occurs. Several CPGs recommended LAIs as an option for maintenance therapy (see next section).
Altogether, 10/18 (55.5%) CPGs made no recommendations on the appropriate duration of maintenance therapy, noting instead that each patient should be considered individually. Other CPGs made specific recommendations: Both the Both BAP 33 and SIGN 35 guidelines suggested a minimum of 2 years, the NJDMHS guidelines 32 recommended 2–3 years; the WFSBP 25 recommended 2–5 years for patients who have had one relapse and more than 5 years for those who have had multiple relapses; the RANZCP 39 and the CPA 14 recommended 2–5 years; and the CINP 22 recommended that maintenance therapy last at least 6 years for patients who have had multiple episodes. The TMAP was the only CPG to recommend that maintenance therapy be continued indefinitely 28 .
Recommendations on the use of LAIs
All CPGs except the one from Italy (94.7%) discussed the use of LAIs for patients with schizophrenia to some extent. As shown in Table 3 , among the 18 CPGs, LAIs were primarily recommended in 14 CPGs (77.8%) for patients who are non-adherent to other antipsychotic administration routes (CINP 22 , UNHCR 23 , RANZCP 39 , PPA 37 , 38 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , TMAP 28 , NJDMHS 32 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ). Twelve CPGs (66.7%) also noted that LAIs should be prescribed based on patient preference (RANZCP 39 , CPA 14 , AFPBN 40 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , Schizophrenia PORT 30 , 31 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ).
Thirteen CPGs (72.2%) recommended LAIs as maintenance therapy 18 , 19 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 . While five CPGs (27.8%), i.e., AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and the Florida Medicaid Program 18 recommended LAIs specifically for patients experiencing a first episode. While the CPA 14 did not make any recommendations regarding when LAIs should be used, they discussed recent evidence supporting their use earlier in treatment. Five guidelines (27.8%, i.e., Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ) noted that evidence around LAIs was not sufficient to support recommending their use for first-episode patients. The AFPBN guidelines 40 also stated that LAIs (SGAs as first-line and FGAs as second-line treatment) should be more frequently considered for maintenance treatment of schizophrenia. Four CPGs (22.2%, i.e., CINP 22 , UNHCR 23 , Italian guidelines 41 , PPA guidelines 37 , 38 ) did not specify when LAIs should be used. The AACP guidelines 29 , which evaluated only LAIs, recommended expanding their use beyond treatment for nonadherence, suggesting that LAIs may offer a more convenient mode of administration or potentially address other clinical and social challenges, as well as provide more consistent plasma levels.
Treatment algorithms
Only Seven CPGs (36.8%) included an algorithm as part of the treatment recommendations. These included decision trees or flow diagrams that map out initial therapy, durations for assessing response, and treatment options in cases of non-response. However, none of these guidelines defined how to measure response, a theme that also extended to guidelines that did not include treatment algorithms. Four of the seven guidelines with algorithms recommended specific antipsychotic agents, while the remaining three referred only to the antipsychotic class.
LAIs were not consistently incorporated in treatment algorithms and in six CPGs were treated as a separate category of medicine reserved for patients with adherence issues or a preference for the route of administration. The only exception was the Florida Medicaid Program 18 , which recommended offering LAIs after oral antipsychotic stabilization even to patients who are at that point adherent to oral antipsychotics.
Benefits and harms
The need to balance the efficacy and safety of antipsychotics was mentioned by all CPGs as a basic treatment paradigm.
Ten CPGs provided conclusions on benefits of antipsychotic therapy. The APA 17 and the BAP 33 guidelines stated that antipsychotic treatment can improve the positive and negative symptoms of psychosis and leads to remission of symptoms. These CPGs 17 , 33 as well as those from NICE 34 and CPA 14 stated that these treatment effects can also lead to improvements in quality of life (including quality-adjusted life years), improved functioning, and reduction in disability. The CPA 14 and APA 17 guidelines noted decreases in hospitalizations with antipsychotic therapy, and the APA guidelines 17 stated that long-term antipsychotic treatment can also reduce mortality. The UNHCR 23 and the Italian 41 guidelines noted that early intervention increased positive outcomes. The WFSBP 24 , AFPBN 40 , CPA 14 , BAP 33 , APA 17 , and NJDMHS 32 affirmed that relapse prevention is a benefit of continued/maintenance treatment.
Some CPGs (WFSBP 24 , Italian 41 , CPA 14 , and SIGN 35 ) noted that reduced risk for extrapyramidal adverse effects and treatment discontinuation were potential benefits of SGAs vs. FGAs.
The risk of adverse effects (e.g., extrapyramidal, metabolic, cardiovascular, and hormonal adverse effects, sedation, and neuroleptic malignant syndrome) was noted by all CPGs as the major potential harm of antipsychotic therapy 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 . These adverse effects are known to limit long-term treatment and adherence 24 .
This SLR of CPGs for the treatment of schizophrenia yielded 19 most updated versions of individual CPGs, published/issued between 2004 and 2020. Structuring our comparative review according to illness phase, antipsychotic type and formulation, response to antipsychotic treatment as well as benefits and harms, several areas of consistent recommendations emerged from this review (e.g., balancing risk and benefits of antipsychotics, preferring antipsychotic monotherapy; using clozapine for treatment-resistant schizophrenia). On the other hand, other recommendations regarding other areas of antipsychotic treatment were mostly consistent (e.g., maintenance antipsychotic treatment for some time), somewhat inconsistent (e.g., differences in the management of first- vs multi-episode patients, type of antipsychotic, dose of antipsychotic maintenance treatment), or even contradictory (e.g., role of LAIs in first-episode schizophrenia patients).
Consistent with RCT evidence 43 , 44 , antipsychotic monotherapy was the treatment of choice for patients with first-episode schizophrenia in all CPGs, and all guidelines stated that a different single antipsychotic should be tried if the first is ineffective or intolerable. Recommendations were similar for multi-episode patients, but factored in prior patient treatment response, adverse effect patterns, and adherence. There was also broad consensus that the side-effect profile of antipsychotics is the most important consideration when making a decision on pharmacologic treatment, also reflecting meta-analytic evidence 4 , 5 , 10 . The risk of extrapyramidal symptoms (especially with FGAs) and metabolic effects (especially with SGAs) were noted as key considerations, which are also reflected in the literature as relevant concerns 4 , 45 , 46 , including for quality of life and treatment nonadherence 47 , 48 , 49 , 50 .
Largely consistent with the comparative meta-analytic evidence regarding the acute 4 , 51 , 52 and maintenance antipsychotic treatment 5 effects of schizophrenia, the majority of CPGs stated there was no difference in efficacy between SGAs and FGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , and Singapore guidelines 36 ), or did not make any recommendations (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , and Schizophrenia PORT 30 , 31 ); three CPGs (BAP 33 , WFBSP 24 , and Schizophrenia PORT 30 , 31 ) noted that SGAs may perform better than FGAs over the long term, consistent with a meta-analysis on this topic 53 .
The 12 CPGs that discussed treatment of subsequent/multiple episodes generally agreed on the factors guiding the choices of an antipsychotic, including that the decision may be more complicated and response may be lower than with a first episode, as described before 7 , 54 , 55 , 56 .
There was little consensus regarding maintenance therapy. Some CPGs recommended the same antipsychotic and dose that achieved remission (CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 ) and others recommended the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , TMAP 28 , CPA 14 , and SIGN 35 ). This inconsistency is likely based on insufficient data as well as conflicting results in existing meta-analyses on this topic 57 , 58 , 59 .
The 15 CPGs that discussed TRS all used the same definition for this condition, consistent with recent commendations 60 , and agreed that clozapine is the primary evidence-based treatment choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , reflecting the evidence base 61 , 62 , 63 . These CPGs also agreed that there are few options well supported by evidence for patients who do not respond to clozapine, with a recent meta-analysis of RCTs showing that electroconvulsive therapy augmentation may be the most evidence-based treatment option 64 .
One key gap in the treatment recommendations was how long patients should remain on antipsychotic therapy after a first episode or during maintenance therapy. While nine of the 17 CPGs discussing treatment of a first episode provided a recommended timeframe (varying from 1 to 2 years) 14 , 22 , 24 , 32 , 33 , 34 , 35 , 39 , 41 , the APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy. Similarly, six of the 18 CPGs discussing maintenance treatment recommended a specific duration of therapy (ranging from two to six years) 14 , 22 , 25 , 32 , 39 , while as many as 10 CPGs did not point to a firm end of the maintenance treatment, instead recommending individualized decisions. The CPGs not stating a definite endpoint or period of maintenance treatment after repeated schizophrenia episodes or even after a first episode of schizophrenia, reflects the different evidence types on which the recommendation is based. The RCT evidence ends after several years of maintenance treatment vs. discontinuation supporting ongoing antipsychotic treatment; however, naturalistic database studies do not indicate any time period after which one can safely discontinue maintenance antipsychotic care, even after a first schizophrenia episode 8 , 65 . In fact, stopping antipsychotics is associated not only with a substantially increased risk of hospitalization but also mortality 65 , 66 , 67 . In this sense, not stating an endpoint for antipsychotic maintenance therapy should not be taken as an implicit statement that antipsychotics should be discontinued at any time; data suggest the contrary.
A further gap exists regarding the most appropriate treatment of negative symptoms, such as anhedonia, amotivation, asociality, affective flattening, and alogia 1 , a long-standing challenge in the management of patients with schizophrenia. Negative symptoms often persist in patients after positive symptoms have resolved, or are the presenting feature in a substantial minority of patients 22 , 35 . Negative symptoms can also be secondary to pharmacotherapy 22 , 68 . Antipsychotics have been most successful in treating positive symptoms, and while eight of the CPGs provided some information on treatment of negative symptoms, the recommendations were generally limited 17 , 22 , 23 , 24 , 32 , 33 , 35 , 40 . Negative symptom management was a focus of the PPA guidelines, but the guidelines acknowledged that supporting evidence was limited, often due to the low number of patients with predominantly negative symptoms in clinical trials 37 , 38 . The Polish guidelines are also one of the more recently developed and included the newer antipsychotic cariprazine as a first-line option, which although being a point of differentiation from the other guidelines, this recommendation was based on RCT data 69 .
Another area in which more direction is needed is on the use of LAIs. While all but one of the 19 CPGs discussed this topic, the extent of information and recommendations for LAI use varied considerably. All CPGs categorized LAIs as an option to improve adherence to therapy or based on patient preference. However, 5/18 CPGs (27.8%) recommended the use of LAI early in treatment (at first episode: AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and Florida Medicaid Program 18 ) or across the entire illness course, while five others stated there was not sufficient evidence to recommend LAIs for these patients (Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ). The role of LAIs in first-episode schizophrenia was the only point where opposing recommendations were found across CPGs. This contradictory stance was not due to the incorporation of newer data suggesting benefits of LAIs in first episode and early-phase patients with schizophrenia 70 , 71 , 72 , 73 , 74 in the CPGs recommending LAI use in first-episode patients, as CPGs recommending LAI use were published between 2005 and 2020, while those opposing LAI use were published between 2011 and 2020. Only the Florida Medicaid CPG recommended LAIs as a first step equivalent to oral antipsychotics (OAP) after initial OAP response and tolerability, independent of nonadherence or other clinical variables. This guideline was also the only CPG to fully integrate LAI use in their clinical algorithm. The remaining six CPGs that included decision tress or treatment algorithms regarded LAIs as a separate paradigm of treatment reserved for nonadherence or patients preference rather than a routine treatment option to consider. While some CPGs provided fairly detailed information on the use of LAIs (AFPBN 40 , AACP 29 , Oregon Health Authority 19 , and Florida Medicaid Program 18 ), others mentioned them only in the context of adherence issues or patient preference. Notably, definitions of and means to determine nonadherence were not reported. One reason for this wide range of recommendations regarding the placement of LAIs in the treatment algorithm and clinical situations that prompt LAI use might be due to the fact that CPGs generally favor RCT evidence over evidence from other study designs. In the case of LAIs, there was a notable dissociation between consistent meta-analytic evidence of statistically significant superiority of LAIs vs OAPs in mirror-image 75 and cohort study designs 76 and non-significant advantages in RCTs 77 . Although patients in RCTs comparing LAIs vs OAPs were less severely ill and more adherent to OAPs 77 than in clinical care and although mirror-image and cohort studies arguably have greater external validity than RCTs 78 , CPGs generally disregard evidence from other study designs when RCT evidence exits. This narrow focus can lead to disregarding important additional data. Nevertheless, a most updated meta-analysis of all 3 study designs comparing LAIs with OAPs demonstrated consistent superiority of LAIs vs OAPs for hospitalization or relapse across all 3 designs 79 , which should lead to more uniform recommendations across CPGs in the future.
Only seven CPGs included treatment algorithms or flow charts to guide LAI treatment selection for patients with schizophrenia 17 , 18 , 19 , 24 , 29 , 35 , 40 . However, there was little commonality across algorithms beyond the guidance on LAIs mentioned above, as some listed specific treatments and conditions for antipsychotic switches, while others indicated that medication choice should be based on a patient’s preferences and responses, side effects, and in some cases, cost effectiveness. Since algorithms and flow charts facilitate the reception, adoption and implementation of guidelines, future CPGs should include them as dissemination tools, but they need to reflect the data and detailed text and be sufficiently specific to be actionable.
The systematic nature in the identification, summarization, and assessment of the CPGs is a strength of this review. This process removed any potential bias associated with subjective selection of evidence, which is not reproducible. However, only CPGs published in English were included and regardless of their quality and differing timeframes of development and publication, complicating a direct comparison of consensus and disagreement. Finally, based on the focus of this SLR, we only reviewed pharmacologic management with antipsychotics. Clearly, the assessment, other pharmacologic and, especially, psychosocial interventions are important in the management of individuals with schizophrenia, but these topics that were covered to varying degrees by the evaluated CPGs were outside of the scope of this review.
Numerous guidelines have recently updated their recommendations on the pharmacological treatment of patients with schizophrenia, which we have summarized in this review. Consistent recommendations were observed across CPGs in the areas of balancing risk and benefits of antipsychotics when selecting treatment, a preference for antipsychotic monotherapy, especially for patients with a first episode of schizophrenia, and the use of clozapine for treatment-resistant schizophrenia. By contrast, there were inconsistencies with regards to recommendations on maintenance antipsychotic treatment, with differences existing on type and dose of antipsychotic, as well as the duration of therapy. However, LAIs were consistently recommended, but mainly suggested in cases of nonadherence or patient preference, despite their established efficacy in broader patient populations and clinical scenarios in clinical trials. Guidelines were sometimes contradictory, with some recommending LAI use earlier in the disease course (e.g., first episode) and others suggesting they only be reserved for later in the disease. This inconsistency was not due to lack of evidence on the efficacy of LAIs in first-episode schizophrenia or the timing of the CPG, so that other reasons might be responsible, including possibly bias and stigma associated with this route of treatment administration. Lastly, gaps existed in the guidelines for recommendations on the duration of maintenance treatment, treatment of negative symptoms, and the development/use of treatment algorithms whenever evidence is sufficient to provide a simplified summary of the data and indicate their relevance for clinical decision making, all of which should be considered in future guideline development/revisions.
The SLR followed established best methods used in systematic review research to identify and assess the available CPGs for pharmacologic treatment of schizophrenia with antipsychotics in the acute and maintenance phases 80 , 81 . The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including use of a prespecified protocol to outline methods for conducting the review. The protocol for this review was approved by all authors prior to implementation but was not submitted to an external registry.
Data sources and search algorithms
Searches were conducted by two independent investigators in the MEDLINE and Embase databases via OvidSP to identify CPGs published in English. Articles were identified using search algorithms that paired terms for schizophrenia with keywords for CPGs. Articles indexed as case reports, reviews, letters, or news were excluded from the searches. The database search was limited to CPGs published from January 1, 2004, through December 19, 2019, without limit to geographic location. In addition to the database sources, guideline body websites and state-level health departments from the US were also searched for relevant CPGs published through June 2020. A manual check of the references of recent (i.e., published in the past three years), relevant SLRs and relevant practice CPGs was conducted to supplement the above searches and ensure and the most complete CPG retrieval.
This study did not involve human subjects as only published evidence was included in the review; ethical approval from an institution was therefore not required.
Selection of CPGs for inclusion
Each title and abstract identified from the database searches was screened and selected for inclusion or exclusion in the SLR by two independent investigators based on the populations, interventions/comparators, outcomes, study design, time period, language, and geographic criteria shown in Table 4 . During both rounds of the screening process, discrepancies between the two independent reviewers were resolved through discussion, and a third investigator resolved any disagreement. Articles/documents identified by the manual search of organizational websites were screened using the same criteria. All accepted studies were required to meet all inclusion criteria and none of the exclusion criteria. Only the most recent version of organizational CPGs was included for data extraction.
Data extraction and synthesis
Information on the recommendations regarding the antipsychotic management in the acute and maintenance phases of schizophrenia and related benefits and harms was captured from the included CPGs. Each guideline was reviewed and extracted by a single researcher and the data were validated by a senior team member to ensure accuracy and completeness. Additionally, each included CPG was assessed using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool. Following extraction and validation, results were qualitatively summarized across CPGs.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of the SLR are available from the corresponding author upon request.
Correll, C. U. & Schooler, N. R. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 16 , 519–534 (2020).
Article PubMed PubMed Central Google Scholar
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1 , 15067 (2015).
Article PubMed Google Scholar
Millan, M. J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15 , 485–515 (2016).
Article CAS PubMed Google Scholar
Huhn, M. et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 , 939–951 (2019).
Article CAS PubMed PubMed Central Google Scholar
Kishimoto, T., Hagi, K., Nitta, M., Kane, J. M. & Correll, C. U. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18 , 208–224 (2019).
Leucht, S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379 , 2063–2071 (2012).
Carbon, M. & Correll, C. U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 16 , 505–524 (2014).
Correll, C. U., Rubio, J. M. & Kane, J. M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17 , 149–160 (2018).
Emsley, R., Chiliza, B., Asmal, L. & Harvey, B. H. The nature of relapse in schizophrenia. BMC Psychiatry 13 , 50 (2013).
Correll, C. U. & Kane, J. M. Ranking antipsychotics for efficacy and safety in schizophrenia. JAMA Psychiatry 77 , 225–226 (2020).
Kane, J. M. & Correll, C. U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 12 , 345–357 (2010).
Kane, J. M., Kishimoto, T. & Correll, C. U. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12 , 216–226 (2013).
Correll, C. U. et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J. Clin. Psychiatry 77 , 1–24 (2016).
Remington, G. et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 62 , 604–616 (2017).
American Psychiatric Association. Treatment recommendations for patients with schizophrenia. Am. J. Psychiatry 161 , 1–56 (2004).
Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines, Field, M. J., & Lohr, K. N. (Eds.). Clinical Practice Guidelines: Directions for a New Program. (National Academies Press (US), 1990).
American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia , 3rd edn. (2021). https://doi.org/10.1176/appi.books.9780890424841 .
Florida Medicaid Drug Therapy Management Program. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults . (2020). Available at: https://floridabhcenter.org/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf .
Mental Health Clinical Advisory Group, Oregon Health Authority. Mental Health Care Guide for Licensed Practitioners and Mental Health Professionals. (2019). Available at: https://sharedsystems.dhsoha.state.or.us/DHSForms/Served/le7548.pdf .
Krogmann, A. et al. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 24 , 38–69 (2019).
Fountoulakis, K. N. et al. The report of the joint WPA/CINP workgroup on the use and usefulness of antipsychotic medication in the treatment of schizophrenia. CNS Spectr . https://doi.org/10.1017/S1092852920001546 (2020).
Leucht, S. et al. CINP Schizophrenia Guidelines . (CINP, 2013). Available at: https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf .
Ostuzzi, G. et al. Mapping the evidence on pharmacological interventions for non-affective psychosis in humanitarian non-specialised settings: A UNHCR clinical guidance. BMC Med. 15 , 197 (2017).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 13 , 318–378 (2012).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry 14 , 2–44 (2013).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int. J. Psychiatry Clin. Pract. 21 , 82–90 (2017).
Moore, T. A. et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J. Clin. Psychiatry 68 , 1751–1762 (2007).
Texas Medication Algorithm Project (TMAP). Texas Medication Algorithm Project (TMAP) Procedural Manual . (2008). Available at: https://jpshealthnet.org/sites/default/files/inline-files/tmapalgorithmforschizophrenia.pdf .
American Association of Community Psychiatrists (AACP). Clinical Tips Series, Long Acting Antipsychotic Medications. (2017). Accessed at: https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view .
Buchanan, R. W. et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36 , 71–93 (2010).
Kreyenbuhl, J. et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr. Bull. 36 , 94–103 (2010).
New Jersey Division of Mental Health Services. Pharmacological Practice Guidelines for the Treatment of Schizophrenia . (2005). Available at: https://www.state.nj.us/humanservices/dmhs_delete/consumer/NJDMHS_Pharmacological_Practice_Guidelines762005.pdf .
Barnes, T. R. et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 34 , 3–78 (2020).
National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. (2014). Available at: http://www.nice.org.uk/guidance/cg178 .
Scottish Intercollegiate Guidelines Network (SIGN). Management of Schizophrenia: A National Clinical Guideline . (2013). Available at: https://www.sign.ac.uk/assets/sign131.pdf .
Verma, S. et al. Ministry of Health Clinical Practice Guidelines: Schizophrenia. Singap. Med. J. 52 , 521–526 (2011).
CAS Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 1. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 1. 53 , 497–524 (2019).
Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 2. 53 , 525–540 (2019).
Galletly, C. et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust. N. Z. J. Psychiatry 50 , 410–472 (2016).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13 , 340 (2013).
De Masi, S. et al. The Italian guidelines for early intervention in schizophrenia: development and conclusions. Early Intervention Psychiatry 2 , 291–302 (2008).
Article Google Scholar
Barnes, T. R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 25 , 567–620 (2011).
Correll, C. U. et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74 , 675–684 (2017).
Galling, B. et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16 , 77–89 (2017).
Pillinger, T. et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 , 64–77 (2020).
Rummel-Kluge, C. et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 38 , 167–177 (2012).
Angermeyer, M. C. & Matschinger, H. Attitude of family to neuroleptics. Psychiatr. Prax. 26 , 171–174 (1999).
CAS PubMed Google Scholar
Dibonaventura, M., Gabriel, S., Dupclay, L., Gupta, S. & Kim, E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12 , 20 (2012).
McIntyre, R. S. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J. Clin. Psychiatry 70 (Suppl 3), 5–11 (2009).
Tandon, R. et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: a worldwide, cross-sectional, web-based survey. Ann. Gen. Psychiatry 19 , 42 (2020).
Zhang, J. P. et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 16 , 1205–1218 (2013).
Zhu, Y. et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 4 , 694–705 (2017).
Kishimoto, T. et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol. Psychiatry 18 , 53–66 (2013).
Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174 , 927–942 (2017).
Samara, M. T., Nikolakopoulou, A., Salanti, G. & Leucht, S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr. Bull. 45 , 639–646 (2019).
Zhu, Y. et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 27 , 835–844 (2017).
Bogers, J. P. A. M., Hambarian, G., Michiels, M., Vermeulen, J. & de Haan, L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. A systematic review and meta-analysis. Schizophr. Bull. Open 46 , Suppl 1 S326 (2020).
Tani, H. et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology 45 , 887–901 (2020).
Uchida, H., Suzuki, T., Takeuchi, H., Arenovich, T. & Mamo, D. C. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr. Bull. 37 , 788–799 (2011).
Howes, O. D. et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174 , 216–229 (2017).
Masuda, T., Misawa, F., Takase, M., Kane, J. M. & Correll, C. U. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry 76 , 1052–1062 (2019).
Siskind, D., McCartney, L., Goldschlager, R. & Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 209 , 385–392 (2016).
Siskind, D., Siskind, V. & Kisely, S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can. J. Psychiatry 62 , 772–777 (2017).
Wang, G. et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J. Psychiatr. Res. 105 , 23–32 (2018).
Tiihonen, J., Tanskanen, A. & Taipale, H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am. J. Psychiatry 175 , 765–773 (2018).
Taipale, H. et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res . 197 , 274–280 (2018).
Taipale, H. et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 19 , 61–68 (2020).
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19 (Suppl 1), 38–52 (2014).
PubMed Google Scholar
Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 , 625–639 (2018).
Kane, J. M. et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry 77 , 1217–1224 (2020).
Schreiner, A. et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr. Res. 169 , 393–399 (2015).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72 , 822–829 (2015).
Tiihonen, J. et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 168 , 603–609 (2011).
Zhang, F. et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr. Dis. Treat. 11 , 657–668 (2015).
Kishimoto, T., Nitta, M., Borenstein, M., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J. Clin. Psychiatry 74 , 957–965 (2013).
Kishimoto, T. et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr. Bull. 44 , 603–619 (2018).
Kishimoto, T. et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr. Bull. 40 , 192–213 (2014).
Kane, J. M., Kishimoto, T. & Correll, C. U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 66 , S37–41 (2013).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8 , 387–404 (2021).
Cook, D. J., Mulrow, C. D. & Haynes, R. B. Systematic reviews: synthesis of best evidence for clinical decisions. Ann. Intern. Med. 126 , 376–380 (1997).
Higgins, J. P. T. & Green, S. Cochrane Collaboration Handbook for Systematic Reviews of Interventions . (The Cochrane Collaboration and John Wiley & Sons, Ltd, 2008).
Download references
Acknowledgements
This study received financial funding from Janssen Scientific Affairs.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
The Zucker Hillside Hospital, Department of Psychiatry, Northwell Health, Glen Oaks, NY, USA
- Christoph U. Correll
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, NY, USA
Charité Universitätsmedizin Berlin, Department of Child and Adolescent Psychiatry, Berlin, Germany
Evidera, Waltham, MA, USA
Amber Martin & Emma Schiller
Janssen Scientific Affairs, LLC, Titusville, NJ, USA
Charmi Patel, Carmela Benson, Jennifer Kern-Sliwa & Kruti Joshi
Goulding HEOR Consulting Inc., Vancouver, BC, Canada
Rebecca Goulding
Biohaven Pharmaceuticals, New Haven, CT, USA
You can also search for this author in PubMed Google Scholar
Contributions
C.C., A.M., R.G., C.P., C.B., K.J., J.K.S., E.S. and E.K. contributed to the conception and the design of the study. A.M., R.G. and E.S. conducted the literature review, including screening, and extraction of the included guidelines. All authors contributed to the interpretations of the results for the review; A.M. and C.C. drafted the manuscript and all authors revised it critically for intellectual content. All authors gave their final approval of the completed manuscript.
Corresponding author
Correspondence to Christoph U. Correll .
Ethics declarations
Competing interests.
C.C. has received personal fees from Alkermes plc, Allergan plc, Angelini Pharma, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Inc, Janssen Pharmaceutica/Johnson & Johnson, LB Pharma International BV, H Lundbeck A/S, MedAvante-ProPhase, Medscape, Neurocrine Biosciences, Noven Pharmaceuticals, Inc, Otsuka Pharmaceutical Co, Inc, Pfizer, Inc, Recordati, Rovi, Sumitomo Dainippon Pharma, Sunovion Pharmaceuticals, Inc, Supernus Pharmaceuticals, Inc, Takeda Pharmaceutical Company Limited, Teva Pharmaceuticals, Acadia Pharmaceuticals, Inc, Axsome Therapeutics, Inc, Indivior, Merck & Co, Mylan NV, MedInCell, and Karuna Therapeutics and grants from Janssen Pharmaceutica, Takeda Pharmaceutical Company Limited, Berlin Institute of Health, the National Institute of Mental Health, Patient Centered Outcomes Research Institute, and the Thrasher Foundation outside the submitted work; receiving royalties from UpToDate; and holding stock options in LB Pharma. A.M., R.G., and E.S. were all employees of Evidera at the time the study was conducted on which the manuscript was based. C.P., C.B., K.J., J.K.S., and E.K. were all employees of Janssen Scientific Affairs, who hold stock/shares, at the time the study was conducted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Correll, C.U., Martin, A., Patel, C. et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophr 8 , 5 (2022). https://doi.org/10.1038/s41537-021-00192-x
Download citation
Received : 26 February 2021
Accepted : 02 November 2021
Published : 24 February 2022
DOI : https://doi.org/10.1038/s41537-021-00192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Psychosis superspectrum ii: neurobiology, treatment, and implications.
- Roman Kotov
- William T. Carpenter
- Katherine G. Jonas
Molecular Psychiatry (2024)
Sex Differences Between Female and Male Individuals in Antipsychotic Efficacy and Adverse Effects in the Treatment of Schizophrenia
- Megan Galbally
- Karen Wynter
- Susanna Every-Palmer
CNS Drugs (2024)
Antipsychotic Discontinuation through the Lens of Epistemic Injustice
- Helene Speyer
- Lene Falgaard Eplov
Community Mental Health Journal (2024)
Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery
- Celso Arango
- Andrea Fagiolini
BMC Psychiatry (2023)
Machine learning methods to predict outcomes of pharmacological treatment in psychosis
- Lorenzo Del Fabro
- Elena Bondi
- Paolo Brambilla
Translational Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Maps & Floorplans
- Libraries A-Z

- Ellis Library (main)
- Engineering Library
- Geological Sciences
- Journalism Library
- Law Library
- Mathematical Sciences
- MU Digital Collections
- Veterinary Medical
- More Libraries...
- Instructional Services
- Course Reserves
- Course Guides
- Schedule a Library Class
- Class Assessment Forms
- Recordings & Tutorials
- Research & Writing Help
- More class resources
- Places to Study
- Borrow, Request & Renew
- Call Numbers
- Computers, Printers, Scanners & Software
- Digital Media Lab
- Equipment Lending: Laptops, cameras, etc.
- Subject Librarians
- Writing Tutors
- More In the Library...
- Undergraduate Students
- Graduate Students
- Faculty & Staff
- Researcher Support
- Distance Learners
- International Students
- More Services for...
- View my MU Libraries Account (login & click on My Library Account)
- View my MOBIUS Checkouts
- Renew my Books (login & click on My Loans)
- Place a Hold on a Book
- Request Books from Depository
- View my ILL@MU Account
- Set Up Alerts in Databases
- More Account Information...
Clinical Psychology Capstone: Literature Review & Peer Review
- Articles & Guidelines
- Facts & Figures
- Laws & Policies
- Managing Citations
- Literature Review & Peer Review
Literature Review
What are the differences between literature reviews?
- Literature Review - A general summary, or overview of the topic that is typically qualitative and subjective
- Systematic Review - A type of literature review that answers a focused clinical question
- Meta-Analysis - A type of systematic review using statistical methods to combine data from systematic reviews
What is the best way to find literature reviews?
- PsycINFO - A psychology database with the capacity to limit by Methodology
How do I know a journal is peer reviewed?
- If searching in a database (eg: Social Work Abstracts,GenderWatch), select Peer Review from the Refine/Limit Results options.
- Check the journal's website: look for the 'about' or 'about this journal' section.
- Check Ulrich's Periodicals Directory ; Search by journal name and look for the little black referee's jersey icon.
- Ask us : Call, text, email, or chat
What does Peer Review mean anyway?
When you submit an article to a journal, someone has to determine if it's worth printing. Peer review was developed as a way to screen articles and determine the quality of your article.
At a peer reviewed journal, the editor sends your article out to several reviewers (usually three) who are in the same field, or 'peers'. Generally, your name will be taken off of the article so personalities don't interfer with the process. The reviewers read through your article looking to see if: the topic is unique or novel, if the data or research is sound, and if it's well written. The reviewers can: reject the article; accept it with revisions; accept it as is.
Benefits of peer review is that multiple people decide vs just the editor and the review process weeds out poorly written or researched articles.
Drawbacks of peer review is that it's only as good as the reviewers so poorly written or researched articles have gotten published. Also, peer review was established as a way to check quality not catch fraud.
For more on peer review (I know that someone is interested...), check out Nature Peer Review Debate
- << Previous: Managing Citations
- Last Updated: Apr 24, 2024 1:00 PM
- URL: https://libraryguides.missouri.edu/clinicalpsych

- Open access
- Published: 01 December 2022
The psychological impact, risk factors and coping strategies to COVID-19 pandemic on healthcare workers in the sub-Saharan Africa: a narrative review of existing literature
- Freddy Wathum Drinkwater Oyat 1 ,
- Johnson Nyeko Oloya 1 , 2 ,
- Pamela Atim 1 , 3 ,
- Eric Nzirakaindi Ikoona 4 ,
- Judith Aloyo 1 , 5 &
- David Lagoro Kitara ORCID: orcid.org/0000-0001-7282-5026 1 , 6 , 7
BMC Psychology volume 10 , Article number: 284 ( 2022 ) Cite this article
6028 Accesses
9 Citations
1 Altmetric
Metrics details
The ongoing COVID-19 pandemic has significantly impacted the physical and mental health of the general population worldwide, with healthcare workers at particular risk. The pandemic's effect on healthcare workers' mental well-being has been characterized by depression, anxiety, work-related stress, sleep disturbances, and post-traumatic stress disorder. Hence, protecting the mental well-being of healthcare workers (HCWs) is a considerable priority. This review aimed to determine risk factors for adverse mental health outcomes and protective or coping measures to mitigate the harmful effects of the COVID-19 crisis among HCWs in sub-Saharan Africa.
We performed a literature search using PubMed, Google Scholar, Cochrane Library, and Embase for relevant materials. We obtained all articles published between March 2020 and April 2022 relevant to the subject of review and met pre-defined eligibility criteria. We selected 23 articles for initial screening and included 12 in the final review.
A total of 5,323 participants in twelve studies, predominantly from Ethiopia (eight studies), one from Uganda, Cameroon, Mali, and Togo, fulfilled the eligibility criteria. Investigators found 16.3–71.9% of HCWs with depressive symptoms, 21.9–73.5% with anxiety symptoms, 15.5–63.7% experienced work-related stress symptoms, 12.4–77% experienced sleep disturbances, and 51.6–56.8% reported PTSD symptoms. Healthcare workers, working in emergency, intensive care units, pharmacies, and laboratories were at higher risk of adverse mental health impacts. HCWs had deep fear, anxious and stressed with the high transmission rate of the virus, high death rates, and lived in fear of infecting themselves and families. Other sources of fear and work-related stress were the lack of PPEs, availability of treatment and vaccines to protect themselves against the virus. HCWs faced stigma, abuse, financial problems, and lack of support from employers and communities.
The prevalence of depression, anxiety, insomnia, and PTSD in HCWs in sub-Saharan Africa during the COVID-19 pandemic has been high. Several organizational, community, and work-related challenges and interventions were identified, including improvement of workplace infrastructures, adoption of correct and shared infection control measures, provision of PPEs, social support, and implementation of resilience training programs. Setting up permanent multidisciplinary mental health teams at regional and national levels to deal with mental health and providing psychological support to HCWs, supported with long-term surveillance, are recommended.
Peer Review reports
Introduction
When coronavirus disease 2019 (COVID-19) was declared a pandemic in March 2020, healthcare workers (HCWs) globally and in sub-Saharan Africa (SSA) were unprepared for the scale of the physical and mental health devastation that was to follow [ 1 ]. The impact of the COVID-19 pandemic on healthcare workers has been profound, characterized by death, disability, and untenable burden on mental health and well-being [ 2 ]. Factors impacting their mental health include high risks of exposure and infection, financial insecurity, separation from loved ones, stigma, difficult triage decisions, stressful work environment, scarcity of supplies including personal protective equipment (PPEs), exhaustion, traumatic experiences due to regular witnessing of deaths among patients and colleagues [ 2 , 3 ]. Greenberg et al. [ 4 ] observed that the COVID-19 pandemic put healthcare professionals worldwide in an unprecedented situation, making difficult decisions to provide care for many severely ill patients with constrained or inadequate resources.
In almost all WHO regions, data indicates that infection rates among healthcare workers are higher than in the general population [ 5 ]. Scholars suggest that the end of the COVID-19 pandemic is not yet in sight. Neither are they sure about the virulence of the following variant when it appears as caseloads are still rising, with more than 621 million infections and 6.5 million deaths reported worldwide by 19th October 2022 [ 6 ]; mainly driven by the newer omicron variants. However, recently in October 2022, we received with gratitude a reassuring message from US President Biden declaring the end of the COVID-19 pandemic in the United States of America.
Meanwhile, previous studies found high levels of depression, anxiety, and PTSD in survivors among the general population and healthcare workers (HCWs) one-to-three years after the control of the SARS epidemic [ 7 ] and the 2014–2016 Ebola epidemic in West Africa [ 8 ]. In addition, recent surveys [ 9 , 10 , 11 , 12 , 13 , 14 ], reviews, and meta-analyses [ 15 , 16 , 17 , 18 ] are pointing to early evidence that a considerable proportion of healthcare workers have experienced stress, anxiety, depression, and sleep disturbances during the COVID-19 pandemic, raising concerns about risks to their long-term mental health.
Studies from the global north countries [ 19 , 20 ], UK [ 21 ], USA [ 22 ], and in India [ 23 ], and China [ 24 , 25 ] have shed light on the vulnerability that characterizes frontline healthcare workers during this pandemic, especially regarding their mental health and well-being. However, evidence in sub-Saharan Africa is scanty, and the pattern and prevalence of psychological disorders are not well understood.
Evidence from a systematic review by Pappa S et al. on 33,062 Chinese HCWs in April 2020 found a pooled prevalence rate of mental health problems among respondents; anxiety 23.2%, depression 22.8%, and insomnia 38.9% [ 26 ]. Similarly, Singapore study, Tan et al . [ 27 ], Li et al . [ 28 ], BMA [ 29 ] and in China [ 31 ] found high levels of psychological disorders among health workers.
Since the beginning of the pandemic, we found one systematic review involving 919 frontline HCWs, 3928 general HCWs, and 2979 medical students conducted in Africa from December 2019 to April 2020 [ 31 ]. The study by Chen J et al . reported a high prevalence of depression, anxiety, and insomnia among frontline HCWs in sub-Saharan Africa (SSA) at 45%, 51%, and 28%, respectively. In comparison, the prevalence of depression, anxiety, and insomnia among the general population was much lower at 30%, 31%, and 24%, respectively [ 31 ]. Furthermore, we found that only a few studies investigated protective and coping measures, given the many uncertainties surrounding the evolution of the COVID-19 pandemic [ 32 ]. Adequate data are needed to equip frontline HCWs and healthcare managers in sub-Saharan Africa to mitigate the medium and long-term adverse effects of the COVID-19 pandemic [ 33 ].
This review aimed to answer three questions (1) What is the psychological impact of the COVID-19 pandemic on HCWs in Sub-Saharan Africa?
(2) What are the associated risk factors during the COVID-19 pandemic?
(3) What interventions (mitigating and coping strategies) protect and support the mental health and well-being of HCWs during the ongoing crises and after the pandemic?
Methodology
Search methodology and article selection.
This current article is a mixed-method narrative review of existing literature on mental health disorders, risk factors, and interventions relevant to the COVID-19 pandemic on HCWs in sub-Saharan. A search on the PubMed electronic database was undertaken using the search terms "novel coronavirus", "COVID-19", "nCoV", "mental health", "psychiatry", "psychology", "anxiety", "depression" and "stress" in various permutations and combinations.
Search processes
We conducted a comprehensive literature search on original articles published from March 2020 to 30 April 2022 in electronic databases of Embase, PubMed, Google Scholar, and the daily updated WHO COVID-19 database. Our search terms included but were not limited to ('COVID-19'/exp OR COVID-19 OR 'coronavirus'/exp OR coronavirus) AND ('psychological'/exp OR psychological OR 'mental'/exp OR mental OR 'stress'/exp OR stress OR 'anxiety' OR anxiety OR 'depression' OR depression OR 'post-traumatic' OR 'post-traumatic'/exp OR 'trauma' OR 'trauma'/exp) OR Health care workers, medical workers of health care professionals, sub-Saharan Africa, for Embase. ("COVID-19" [All Fields] OR "coronavirus" [All Fields]) AND ("Stress, Psychological" [Mesh] OR "mental" OR "anxiety" OR "depression" OR "stress" OR "post-traumatic" OR "trauma") for PubMed, for the WHO COVID-19 database, and ("COVID-19" OR "coronavirus") AND ("Psychological" OR "mental" OR "anxiety" OR "depression" OR "stress" OR "post-traumatic" OR "trauma") for Google Scholar. On reviewing the above citations, twelve articles met the inclusion criteria relevant for this review and are in Table 1 . All twelve articles were cross-sectional, with one qualitative and the others quantitative observational studies.
Eligibility criteria
We included original qualitative and quantitative studies examining the risk factors, psychological impact of COVID-19 and coping strategies of healthcare workers (HCWs) in sub-Saharan Africa during the COVID-19 pandemic. We excluded studies if they were.
1. Not reported in the English language 2. Studies which were not primary research 3. Studies that had not been published in a peer-reviewed journal 4. Studies that did not include data on HCWs’ mental health or psychological well-being 5. Duplicate studies 6. not using validated instruments to measure the risks and psychological impact.
FWDO performed the search of articles. DLK reviewed the articles involving screening of titles, followed by examination of abstracts. The potential articles identified were further reviewed in full text to examine their eligibility. In addition, four of the authors independently reviewed the full articles to abstract the relevant data required for the review. Thereafter, a meeting to harmonise findings were done and presented in a report.
Data extraction and appraisal of the study
We extracted information from each study, including author, study population, year of publication, country, socio-demographic characteristics, sample size, response rate, gender proportion, age, and study time, areas assessed, the validated instrument used and the prevalence. The appraisal involved assessing the research design, recruitment of respondents, inclusion and exclusion criteria, reliability of outcome determination, statistical analyses, ethical compliance, strengths, limitations, and clinical implications of the articles.
Our review protocol was not registered on PROSPERO because of the significant variation in the methodologies of the articles used in the review. The results precluded using a meta-analytic approach and made a narrative review the most suitable for this work. In addition, we did not use the Cochrane Collaboration GRADE method to assess the quality of evidence of outcomes included in this narrative review. Instead, we used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) 22 items checklist to gauge the quality of the twelve articles included in this review. We qualitatively validated the articles based on additional considerations namely study design, sample sizes, sampling procedures, response rates, statistical methods used, measures taken by the authors to deal with bias and confounding factors and ethical consideration.
Definition of healthcare worker (HCW)
For this narrative review, we adhered to the Centres for Disease Control and Prevention (CDC) definition of HCWs, which includes physicians, nurses, emergency medical personnel, dental professionals and students, medical and nursing students, laboratory technicians, pharmacists, hospital volunteers, and administrative staff [ 34 ].
Search results
The search found twenty-three studies of interest. Full texts of potentially relevant studies underwent eligibility assessment, and twelve articles met the inclusion criteria for this narrative review.
Study characteristics
The twelve articles comprised eleven quantitative and one qualitative study. The common mental health conditions assessed were depression, anxiety, perceived stress, and post-traumatic stress disorder (PTSD). The coping strategy, perceived health status, health distress (including burnout), insomnia, and perceived stigma were also assessed [ 35 , 36 ]. The total number of respondents in these studies was 5,323. The qualitative study had fifty respondents [ 35 ], while the most significant number of participants, 420 was recorded in one of the quantitative studies from Ethiopia [ 37 ]. The questionnaire response rates varied between 90%-100%, with most studies dominated by male respondents at 51.9%-69.2% [ 38 ]. Nurses were the commonest study population, followed by doctors, pharmacists, and laboratory technicians, and no study involved non-HCWs of facilities. Most papers utilized probability sampling procedures, and four quantitative studies used non-random sampling procedures limiting generalizability of their findings and increasing the risk of selection bias. Eight studies were from Ethiopia, and one was from Cameroon, Uganda, Mali, and Togo, respectively (Table 1 ). Most studies were conducted in urban tertiary public hospitals, university teaching hospitals, and rural and urban general hospitals, including primary care facilities operated by Non-Governmental Organizations (NGOs) for example in Mali [ 39 ]. Several validated tools assessed depression, anxiety, insomnia, stress, and PTSD (Table 1 ).
Table 1 provides an overview of the studies selected and validated instruments used to measure psychological disorders.
Table 2 provides comparisons with studies conducted outside of sub-Saharan Africa.
Table 3 provides information on studies showing the classification of psychological outcomes.
Table 4 are studies showing risk factors associated with psychological disorders.
Table 5 are studies that identified protective factors for psychological disorders.
Risks of bias and confounding factors
Most articles selected were cross-sectional studies that employed probability sampling procedures (Table 1 ). Cross-sectional study design minimized selection biases, but many used structured questionnaires, including online self-administered questionnaires, which increased bias due to social desirability. It was not clear how confounding variables were controlled in five papers reviewed [ 38 , 39 , 40 , 43 , 45 ] leading to excessive and perhaps inappropriate determination of associations.
Socio-demographic factors
In this review, the mean age of the respondents ranged between 23 and 35 years, and predominantly males. Age was associated with anxiety, and stress symptoms in 6(50%) of all the studies reviewed [ 35 , 37 , 40 , 41 , 42 , 44 ]. An age of over 40 years was associated with moderate to severe symptoms of PTSD. Two studies concluded that respondents aged over 40 years were more likely to develop PTSD symptoms than their younger counterparts [ 37 , 41 ].
Female gender was significantly associated with depression, anxiety, and stress symptoms among HCWs in seven studies reviewed [ 36 , 37 , 38 , 41 , 42 , 43 ]. Many studies found that being female, married, and a nurse were independent predictors of stress symptoms. Moreover, sex, age, marital status, type of profession, and working environment were significant factors for PTSD symptoms [ 37 , 41 ]. However, one study in Ethiopia found that the odds of depression were twice higher among male healthcare providers than among female healthcare providers [ 35 ].
Psychological impact on healthcare workers
Most studies reviewed directly assessed the prevalence of depression, anxiety, stress, insomnia, and PTSD in HCWs. Common causes of anxiety, fear, or psychological distress that health professionals reported were: lack of access to PPEs and other equipment, being exposed to COVID-19 at work and taking the infection home to their families, uncertainties that their organization will support/take care of their personal and family needs if they got infection, long working hours, death of colleagues, lack of social support, stigmatization, high rates of transmission and poor income [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. However, the prevalence of mental health symptoms exhibited great variations for example depressive symptoms were examined in nine studies [ 35 , 36 , 37 , 39 , 43 , 44 , 45 , 46 ], and varied between 16.3% and 71.9% among HCWs [ 38 , 39 ].
In addition, nine other studies reported high prevalence of anxiety symptoms among HCWs [ 35 , 36 , 37 , 40 , 43 , 44 , 45 , 46 , 47 ] which varied between 21.9% and 73.5% [ 36 , 39 ]. Five studies investigated HCWs' perceived stress during the pandemic; 15.5%-63.7% of HCWs reported high levels of work-related stress [ 35 , 36 , 37 , 43 , 45 ]. Three studies reported 12.4–77% of HCWs experienced sleep disturbances during the COVID-19 pandemic [ 37 , 39 , 40 ].
Post-traumatic stress disorder (PTSD) was in three studies [ 38 , 41 , 42 ], and the prevalence of PTSD-like symptoms varied between 51.6 and 56.8% in HCWs [ 38 , 41 ]. A qualitative study from Uganda reported high symptoms of depression, anxiety, and PTSD among HCWs [ 35 ]. Additionally, factors that increased the risk of PTSD symptoms were for example, working in emergency units and being frontline workers. Furthermore, many studies found that frontline HCWs had increased symptoms of mental disorders and being a frontline worker was an independent risk factor for depression, anxiety, and PTSD [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ].
Risk factors associated with adverse mental health outcomes
The qualitative study from Uganda reported the factors associated with mental disorder symptoms among HCWs. These were long working hours, lack of equipment (PPEs, testing kits), lack of sleep, exhaustion, high death rates, death of colleagues, and a high COVID-19 transmission rate among HCWs [ 35 ]. Lack of equipment (PPEs, ventilators, and testing kits), overworking, and lack of logistic support were in Ethiopian studies [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 45 ]. Most studies identified several risk factors for adverse mental health outcomes among respondents for example those with medical and mental illnesses, contacts with confirmed COVID-19 patients, and poor social support which were significantly associated with depression [ 42 , 43 ]. Other factors were females, nurses, married, frontline workers, ICU, emergency units, living alone, and lack of social support [ 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Too, participants’ families with chronic illnesses, had contacts with confirmed COVID-19 cases, and poor social support were significantly associated with anxiety. Other risk factors associated with anxiety include exhaustion, long working hours, frontline workers, emergencies, nurses, pharmacists, laboratory technicians, married, older, younger, living alone, being female, working at general and referral hospitals, and perceived stigma. In addition, participants’ families with chronic illnesses, those who had contacts with confirmed COVID-19 cases, and those with poor social support were predictors of stress during the COVID-19 pandemic [ 37 , 38 , 40 , 41 , 42 , 43 , 45 ]. Other stress symptoms include having a medical illness, a mental illness, being a frontline worker, married, nurse, female, pharmacist, laboratory technician, physician, older age, lack of standardized PPE supply, low incomes, and living with a family [ 36 , 37 , 40 , 41 , 42 , 43 , 44 , 45 ]. Healthcare providers with low monthly incomes were significantly more likely to develop stress than those with high monthly incomes [ 38 ]. In addition, participants living alone, living with a family, and being married were associated with symptoms of psychological disorders among HCWs [ 36 , 37 , 38 , 45 ]. Overall, the risk factors for adverse psychological impacts are categorized in three thematic areas (i) occupational, (ii) psychosocial, and (iii) environmental aspects.
Occupational factors
Most studies showed that frontline HCWs, nurses, doctors, pharmacists, and laboratory technicians had significantly higher levels of mental health risks compared to non-frontline HCWs [ 35 , 36 , 37 , 38 , 40 , 42 , 43 , 45 ]. They experienced higher frequency of insomnia, anxiety, depression, and somatization than non-frontline medical HCWs. In contrast, Mali [ 39 ] and Cameroon [ 46 ] studies found a higher prevalence of depression, anxiety, and PTSD in non-frontline HCWs [ 39 , 46 ]. However, among HCWs, physicians were 20% less likely to develop mental health disorders than nurses, pharmacists, and laboratory technicians [ 39 ]. In addition, healthcare workers with low monthly incomes had higher symptoms of depression, anxiety, stress, and insomnia [ 37 ].
Healthcare groups
Five studies found that being a nurse was associated with worse mental disorders than doctors [ 36 , 37 , 40 , 44 , 45 ].
Frontline staff with direct contact with COVID-19
Most papers in the review found that being in a “frontline” position or having direct contact with COVID-19 patients was associated with higher level of psychological distress [ 35 , 36 , 37 , 38 , 40 , 42 , 43 , 45 ]. In addition, studies found that contact with COVID-19 patients was independently associated with an increased risk of sleep disturbances [ 40 , 46 ]. Moreover, HCWs who had contact with confirmed COVID-19 cases were more likely to develop depression, anxiety, and stress symptoms than those who had no contact with COVID-19 patients [ 36 , 37 , 38 , 43 , 45 ].
Lack of personal protective equipment (PPEs)
Most studies reported that the lack of PPEs was associated with higher symptoms of depression, anxiety, stress, and insomnia, while its availability was associated with fewer mental disorder symptoms [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. In Mali, workers from centres that provided facemasks were 51% less likely to suffer from depression, 62% less likely to develop anxiety, and 45% less likely to develop insomnia [ 39 ]. In Ethiopia, the odds of developing post-traumatic stress disorder were much higher among HCWs who did not receive standardized PPEs supplies than those who had [ 38 , 41 , 42 ]. In Uganda, the lack of PPEs was associated with depression, anxiety, and PTSD [ 35 ].
Heavy workload
Longer working hours, increased work intensity, increased patient load, and exhaustion were risk factors in Ugandan [ 35 ] and Ethiopian studies [ 36 ].
Psychosocial factors: perceived stigma and fear of infection
The fear of infection was in the qualitative study from Uganda [ 35 ], one quantitative study from Cameroon [ 47 ] and seven cross-sectional studies from Ethiopia [ 36 , 37 , 38 , 41 , 42 , 43 , 44 ]. Poor social support was associated with PTSD symptoms, depression, anxiety, and stress [ 35 , 36 , 37 , 38 , 42 , 43 ]. Two studies reported that HCWs with perceived stigmatization were more likely to suffer from depression, anxiety, stress, and PTSD [ 37 , 42 ].
family concerns
This came up as one of the main risk factors of stress in almost all studies, especially among those HCWs in direct contact with confirmed COVID-19 cases [ 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 ]. A family member suffering from COVID-19 was associated with poor mental health outcomes in HCWs [ 36 , 37 ].
Protective psychosocial factors
Two studies suggest a reduction of perceived stigma can be achieved by sensitization of communities about COVID-19 [ 37 , 42 ], and four studies recommend solid social support [ 36 , 37 , 42 , 43 ].
Safety of family
Family safety had the most significant impact in reducing stress. Safety from COVID-19 infection and financial protection of families were essential coping strategies for HCWs [ 35 , 36 ].
Underlying illnesses
We found three studies that reported an underlying medical and mental illness as an independent risk factor for poor psychological outcomes [ 42 , 43 , 45 ].
Protective factors against adverse mental health outcomes
The review identified protective factors to adverse mental health outcomes during COVID-19. The qualitative study from Uganda and four quantitative cross-sectional studies from Ethiopia identified some protective factors [ 35 , 38 , 41 , 42 , 45 ]. The protective factors are grouped under three thematic areas (i) occupational, (ii) psychosocial, and (iii) environmental aspects.
The qualitative study identified many social coping strategies among respondents, including family networks, community networks, help from family, responsibility to society, assistance from community members, availability of assistance from strangers, and the symbiotic nature of assistance in the community [ 35 ].

Protective occupational factors
Studies suggest that physicians suffered fewer mental health disorders partly because of their experience with previous epidemics [ 37 , 42 , 45 ].
Some necessary coping measures include good hospital guidance and ongoing training of frontline HCWs [ 37 , 42 , 45 ].
Adequate supply of PPEs
As mentioned above, PPE was a protective factor when adequate and a risk factor for poor mental health outcomes when deemed inadequate [ 35 , 36 , 37 , 42 , 43 ].
The COVID-19 pandemic has been an ongoing global public health emergency that has burdened healthcare workers' physical and mental well-being (HCWs) [ 1 , 5 ]. Our review confirms the enormous magnitude of mental health impact of COVID-19 on healthcare workers in sub-Saharan Africa, and it is widespread, with significant levels of depression, anxiety, distress, and insomnia; especially those working directly with COVID-19 patients at particular risk [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Out of the twelve articles reviewed, eight studies (66%) came from Ethiopia, and this has implications on the results (Table 1 ). This finding indicates few research published to date on the psychological impact of the pandemic on the mental health of HCWs in sub-Saharan Africa; a subregion that the COVID-19 pandemic has severely impacted.
Overview of the study sites
Studies in this review were conducted predominantly in hospital settings. We found only one study relating to primary healthcare workers or facilities [ 38 ]. This finding is of concern, as there is increasing evidence that many non-frontline HCWs continue to suffer psychological symptoms long after the conclusion of infectious disease epidemics [ 7 , 8 ]. In addition, a significant mortality due to COVID-19 was due to excess morbidity, some of which were from primary care facilities. Given that this study is the first narrative review in sub-Saharan Africa, it would be helpful to briefly compare our findings with some published reviews and surveys from other regions (Table 2 ).
High prevalence of psychological disorders among participants
Investigators in this review found 16.3–71.9% HCWs with depressive symptoms, 21.9–73.5% had anxiety symptoms, 15.5–63.7% experienced work-related stress symptoms, 12.4–77% experienced sleep disturbances, and 51.6–56.8% PTSD symptoms [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. This high prevalence of mental health symptoms among HCWs in our review is consistent with previous reviews conducted early in the pandemic in sub-Saharan Africa [ 31 ], Asia [ 17 , 18 , 26 , 28 ], USA & Europe [ 15 , 16 ], and supported by a batch of cross-sectional studies globally [ 11 , 12 , 13 , 14 , 19 , 27 , 30 ]. We found mixed results with significant variations within and among regions and countries, as depicted in Tables 1 and 2 .
Risk factors of psychological disorders among participants
Studies established that HCWs responding to the COVID-19 pandemic in sub-Saharan Africa were exposed to long working hours, overworking, exhaustion, high risk of infection, and shortage of personal protective equipment (Tables 3 and 4 ). In addition, HCWs had deep fear, were anxious and stressed with the high transmission rate of the virus among themselves, high death rates among themselves and their patients, and lived under constant fear of infecting themselves and their families with obvious consequences [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Some HCWs were deeply worried about the lack of standardized PPEs, known treatments and vaccines to protect against the virus. Many health workers had financial problems, lacked support from families and employers if they contracted the virus [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 44 ]. An additional source of fear and anxiety was the perceived stigma attached to being infected with COVID-19 by the public [ 36 , 41 ]. Studies found that HCWs, especially those working in emergency, intensive care units, infectious disease wards, pharmacies, and laboratories, were at higher risk of developing adverse mental health impacts compared to others [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 ]. This is supported by previous reviews [ 15 , 16 , 17 , 18 , 26 , 28 ] and cross-sectional studies [ 10 , 11 , 12 , 13 , 14 , 20 , 21 , 23 , 25 , 30 ]. However, findings were inconsistent on the impact of COVID-19 on frontline health workers, with ten studies [ 35 , 36 , 37 , 39 , 40 , 41 , 42 , 44 , 45 ] suggesting they are at higher risk than peers and two studies showing no significant difference in psychological disorders relating to the departments [ 38 , 43 ].
The Mali’s study was conducted exclusively in primary care facilities among HCWs not involved in treating COVID-19 cases but still registered a very high prevalence of depression 71.9%, anxiety 73.6%, and insomnia 77.0% [ 39 ]. In contrast, two studies conducted among HCWs at COVID-19 treatment facilities in Ethiopia [ 36 , 38 ] registered much lower prevalence of depression 20.2%, anxiety 21.0%, and insomnia12.4% [ 36 ], and 16.3%, 30.7% and 15.9% respectively, in the second study [ 38 ]. These findings show that not only frontline HCWs experienced mental health disorders during this pandemic but highlight the need for direct interventions for all HCWs regardless of occupation or workstation during this and future pandemics. The significant disparity in the studies could be due to structural, occupational, and environmental issues for example challenges faced by Mali's healthcare systems, characterized by acute equipment shortages, lack of PPEs, human resources, lack of trained and experienced HCWs, ongoing nationwide insecurity, and terrorism compared to Ethiopia. Therefore, local context needs to be considered as contributing factor to mental health disorders among HCWs.
Regional variations of psychological disorders
Tan et al . found a higher prevalence of anxiety among non-medical HCWs in Singapore [ 27 ]. As previously noted, the prevalence of poor psychological outcomes varied between countries. Compared to sub-Saharan Africa and China, data from India [ 23 ] and Singapore [ 27 ] revealed an overall lower prevalence of anxiety and depression than similar cross-sectional data from sub-Saharan Africa [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ] and China [ 9 , 25 , 30 ]. This finding suggests that different contexts and cultures may reveal different psychological findings and that, it is possible that being at different countries’ outbreak curve may play a part, as there is evidence that it is influential.
Tan et al . suggests that medical HCWs in Singapore had experienced a SARS outbreak and thus were well prepared for COVID-19 psychologically and infection control measures [ 27 ]. What can be deduced is that context and cultural factors play a role, not just the cadre or role of healthcare workers [ 16 ]. It also highlights the importance of reviewing evidence regularly as more data emerge from other countries.
One hospital in Ethiopia found that the thought of resignation was associated with higher chances of mental health disorders and that pharmacists and laboratory technicians who did not receive prior training exhibited higher symptoms of mental health disorders compared to others [ 36 ]. Work shift arrangement, considering a dangerous atmosphere presented by working in COVID-19 wards, was one which exacerbated or relieved mental health symptoms among HCWs, with shorter exposure periods being most beneficial [ 36 ]. Meanwhile, studies found that financial worries caused by severe lockdowns and erratic payment of salaries and allowances were also major stressors [ 35 ]. This finding is like studies in Pakistan [ 13 ] and China [ 30 , 32 ].
In this review, HCWs who had contact with confirmed COVID-19 patients were more affected by depression, anxiety, and stress than their counterparts who had not [ 35 , 36 , 37 , 40 , 41 , 43 , 45 ]. This finding is like previous reviews [ 15 , 16 , 17 , 18 , 26 , 28 , 31 ] and cross-sectional studies [ 9 , 10 , 11 , 12 , 13 , 14 , 21 , 23 , 24 , 25 , 27 , 30 ], which reported higher depression, anxiety, and psychological symptoms of distress in HCWs who were in direct contact with confirmed or suspected COVID-19 patients.
A study in Pakistan showed that 80% of participants expected the provision of PPE from authority [ 13 ], and 86% were anxious. Some respondents alluded to forced deployment, while in Mali, 73.3% were anxious, with the majority worrying about the shortage of nurses [ 39 ]. Therefore, prospects of being deployed at a workstation where one had not been trained or oriented contributed to fear among health workers. In the sub-Saharan African context, this scenario can best be represented in HCWs involved in internship who must endure hard work during their training. Tan et al . found that junior doctors were more stressed than nurses in Singapore [ 27 ].
Socio-demographic characteristics
Nearly all studies in our review suggest that socio-demographic variables for example age, gender, marital status, and living alone or with families contribute to the high mental disorder symptoms [ 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 ]. We, the authors suggest that these observations are handled cautiously as several investigators of these reviewed articles did not entirely control the influence of confounding variables. An alternative explanation for this study's findings may be the more significant risks of frontline exposure amongst women and junior HCWs, predominantly employed in lower-status roles, many of whom lacked experience and appropriate training within healthcare system globally. It is also important to note that respondents to all studies, when disaggregated by gender, and age, were predominantly younger or female, which may have impacted the outcomes of these findings [ 16 ]. In addition, the consistently higher mortality rates, and risk of severe COVID-19 disease amongst men would suggest that the complete picture regarding gender and mental health during this pandemic is still incomplete [ 16 ]. Moreover, in several studies, both younger and older age groups were equally affected by mental health symptoms but for different reasons. Cai et al . [ 32 ] in a Chinese study on HCWs for example observed that irrespective of age, colleagues' safety, self and families' safety, the lack of treatment for COVID-19 was a factor that induced stress in HCWs. Similarly, in our review, the lack of PPEs, high infection transmission rates, high death rates among HCWs, and the fear of infecting their families were the factors that induced stress in all HCWs [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ].
We, the authors propose that paying close attention to concerns of HCWs by employers would greatly relieve some stressors and contribute to increased mental well-being of participants. Compared with physicians, our review showed that nurses were more likely to suffer from depression, anxiety, insomnia, PTSD, and stress [ 35 , 37 , 39 , 40 , 41 , 44 , 45 ]. Workloads and night shifts in healthcare facilities, as well as contacts with risky patients, enhanced nurses' mental distress risks [ 15 , 16 , 17 , 18 , 26 , 27 , 28 ]. In addition, nursing staff have more extended physical contacts and closer interactions with patients than other professionals, providing round-the-clock care required by patients with COVID-19 and thus the increased risk [ 15 ]. On the one hand, we posit that most senior physicians are experienced and always keep well-informed with emerging medical emergencies. The majority become aware of emerging epidemic early and actively protect themselves from infections through regular scientific literature updates compared to their junior counterparts. Senior physicians also spend less time in emergency wards unless there is a need to conduct specific procedures which cannot be undertaken by senior housemen or general medical officers. Cai et al . [ 32 ] concluded that it is essential to have a high level of training and professional experience for healthcare workers engaging in public health emergencies, especially for the new staff. As a result, these findings highlight the importance of focusing on all the frontline HCWs sacrificing to contain the COVID-19 pandemic.
Regular monitoring of high-risk groups
There is a need to continue monitoring the high-at-risk groups, including nursing staff, interns, support staff, and all deployed in emergency wards. These high-at-risk groups should be encouraged to undertake screening, treatment, and vaccination to avoid the medium and long-term consequences of such epidemics [ 15 , 16 , 35 , 37 , 40 , 44 ].
Social support and coping mechanisms
The effect of social support and coping measures is in the qualitative study [ 34 ] and three other quantitative studies [ 36 , 41 , 42 ] which concluded that respondents with good social support were less likely to suffer from severe depression, anxiety, work-related stress, and PTSD. The qualitative study identified several coping measures, including community and organizational support, family, and community networks, help from family, responsibility to society, and assistance from community members and strangers, including the symbiotic nature of assistance in the community [ 35 ]. Other measures include providing accommodation and food to employees [ 35 ].
Interestingly, no study examined the association of resilience and self-efficacy with sleep quality, degrees of anxiety, depression, PTSD, and stress. However, a Chinese study by Cai et al. [ 32 ] suggests that the social support given to HCWs causes a reduction in anxiety and stress levels and increases their self-efficacy. In divergence, Xiao et al . [ 46 ] found no relationship between social support and sleep quality.
Only two studies in our review examined the effects of stigma on the mental health of HCWs [ 36 , 41 ] and found that HCWs with perceived stigma were more likely to be depressed, anxious, stressed, and prone to poor sleep quality [ 36 , 41 ]. We, the authors suggest that better community sensitization by creating public awareness involving appropriate local community structures and networks are essential. The broader community in sub-Saharan Africa may have suffered severely from infodemics with severe consequences on their mental health, especially during the difficult lockdowns. In addition, removing discrimination/inequalities at the workplace based on race and other social standings have a powerful influence on the mental health outcomes of HCWs. Also, because emotional exhaustion is long associated with depression, anxiety, and sleep disturbances, none of the studies in our review examined burnout as an essential component of mental health disorders in HCWs in sub-Saharan Africa.
Protective and coping measures
In this review we have provided evidence about personal, occupational, and environmental factors that were important protective and coping measures against psychological disorders. Based on these factors we suggest some protective and coping measures which can help to reduce the negative effects of the pandemic on mental health of HCWs in sub-Saharan Africa. Organizations and healthcare managers need to be aware that primary prevention is key to any successful interventions to contain and control any epidemic. This should take the form of planned regular training, orientation and continuing medical education grounded on proven infection control measures. These measures need to be backed up by timely provision of protective equipment, drugs, testing facilities, vaccines, isolation facilities, clinical and mental health support, and personal welfare of HCWs [ 35 , 36 , 37 , 42 , 45 ]. The effect of community and organizational support and coping measures was shown by the qualitative study [ 35 ] and five other quantitative studies [ 36 , 37 , 41 , 42 , 43 ] indicating that respondents who had good social and organizational support were less likely to suffer from severe depression, anxiety, work related stress and PTSD. Prior experience with comparable pandemics and training are suggested as beneficial coping strategies for healthcare workers during this pandemic but also local social structural and geopolitical conditions appear to determine the pattern and evolution of mental health symptoms among HCWs [ 14 , 15 , 31 , 32 , 47 ]. In our case the high prevalence of all mental health symptoms in non-frontline primary health care facilities in Mali [ 39 ] which was already plagued with instability and weak healthcare systems prior to the pandemic is a case in point. Results are particularly consistent in showing that provision of PPEs, testing kits, orientation training of workers, work shift arrangements, provision of online counselling, provision of food and accommodation and prompt payment of allowances by employers were important protective measures [ 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ]. The feeling of being protected is associated with higher work motivation with implication for staff turnover [ 35 , 38 , 43 , 45 ]. Hence, physical protective materials [ 14 ], together with frequent provision of information, should be the cornerstone of any interventions to prevent deterioration in mental health of HCWs (Table 5 ). Finally, provision of rest rooms, online consultation with psychologists/psychiatrists, protection from financial hardships, access to social amenities and religious activities are some important coping measures [ 35 , 36 , 38 , 42 , 45 ]. In this era of digital health care with plentiful internet and smartphones, organization can conduct online trainings, online mental health education, online psychological counselling services, and online psychological self-help intervention tailored to the needs of their HCWs [ 35 , 37 , 42 ]. In addition, it is essential to understand and address the sources of anxiety among healthcare professionals during this COVID-19 pandemic, as this has been one of the most experienced mental health symptoms [ 48 ]. Adequate protective equipment provided by health facilities is one of the most important motivational factors for encouraging continuation of work in future outbreaks. Furthermore, availability of strict infection control guidelines, specialized equipment, recognition of their efforts by facility management, government, and reduction in reported cases of COVID-19 provide psychological benefits [ 15 , 32 ]. Finally, we call upon Governments (the largest employers of HCWs) in sub-Saharan Africa to do what it takes to improve investments in the mental health of HCWs and plan proactively in anticipation of managing infectious disease epidemics, including other expected and unexpected disasters.
Future research direction
There was no study that examined the association of resilience and self-efficacy with sleep quality, degrees of anxiety, depression, PTSD, and stress. Although emotional exhaustion has long been associated with depression, anxiety, and sleep disturbances, no study in our review examined burnout as an important component of mental health disorders in HCWs in sub-Saharan Africa. The impacts of infodemics, stringent lockdown measures, discrimination/inequalities at workplaces based on race, and other social standings on mental health outcomes of HCWs need to be investigated.
Future studies are needed on the above including other critical areas like suicidality, suicidal ideations, and substance abuse during the COVID-19 pandemic. In addition, there is a significant variation of related literature calling for more rigorous research in future. More systematic studies will be required to clarify the full impact of the pandemic so that meaningful interventions can be planned and executed at institutional and national levels in the Sub-Saharan Africa.
Limitations of this study
There are some limitations to this study. First, most of the studies are from one country, limiting the generalizability of the results to the whole African continent. Second, all the studies were cross-sectional and only looked at associations and correlations. There is a need for prospective or retrospective cohort or case–control studies on this subject matter. Longitudinal research studies on the prevalence of mental disorders in the COVID-19 pandemic in the sub-Saharan Africa are urgently required. Third, most studies reviewed did not adequately examine protective factors or coping measures of the health workers in their settings. In addition, most studies did not pay strict attention to confounding variables which could have led to inappropriate results and conclusions. Fourth, most sample sizes were small and unlikely representative of the population and yet larger sample sizes would better identify the extent of mental health problems among health workers in the region. Fifth, depression, anxiety, and stress were assessed solely through self-administered questionnaires rather than face-to-face psychiatric interviews. Sixth, these studies employed various instruments and different cut-off thresholds to assess severity. Notably, the magnitude and severity of reported mental health outcomes may vary based on the validity and sensitivity of the measurement tools. Seventh, there was no mention of mental baseline information among the studied population and therefore it was unknown if the studied population had pre-existing mental health illnesses that decompensated during the pandemic crisis. Eight, investigators did not give much attention to stigma, burnout, resilience, and self-efficacy among study participants.
Furthermore, our review did not employ systematic reviews or meta-analyses methods for the information generated. This narrative review paper precluded deeper insight into the quality of reviewed articles for this paper. Still, our observation was that investigators did not consider the strict lockdown measures, quarantine, and isolation imposed by many countries in sub-Saharan Africa as possible risk factors for mental health disorders among HCWs.
Based on the articles reviewed, the prevalence of depression, anxiety, insomnia, and PTSD in HCWs in the sub-Saharan Africa during the COVID-19 pandemic is high. We implore health authorities to consider setting up permanent multidisciplinary mental health teams at regional and national levels to deal with mental health issues and provide psychological support to patients and HCWs, always supported with sufficient budgetary allocations.
Long-term surveillance is essential to keep track of insidiously rising mental health crises among community members. There is a significant variation of related literature thus calling for more rigorous research in the future. More systematic studies will be needed to clarify the full impact of the pandemic so that meaningful interventions can be planned better and executed at institutional and national levels in sub-Saharan Africa.
Availability of data and materials
Datasets analysed in the current study are available from the corresponding author at a reasonable request.
Abbreviations
Coronavirus disease 2019
Healthcare workers.
Mental health
Public health emergency
Personal protective equipment
World Health Organisation
World Health Organization. Coronavirus disease (COVID-19) outbreak. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 1 March 2020).
Veenema TG, Closser S, Thrul J. Mental Health, and Social Support for Healthcare and Hospital Workers During the COVID-19 Pandemic. Baltimore, MD: Johns Hopkins Center for Health Security; 2021.
Chersich MF, Gray G, Fairlie L. COVID-19 in Africa: care and protection for frontline healthcare workers. Global Health. 2020;16:46. https://doi.org/10.1186/s12992-020-00574-3Interventions .
Article PubMed PubMed Central Google Scholar
Greenberg N, Docherty M, Gnanapragasam S, Wessely S. Managing mental health challenges faced by healthcare workers during a COVID-19 pandemic. BMJ. 2020;368: m1211. https://doi.org/10.1136/bmj.m1211 .
Article PubMed Google Scholar
WHO. Keep health workers safe to keep patients safe: WHO. 2020. https:// www.who.int/news/item/17-09-2020-keep-health-workers-safe-to-keep - patients-safe-who. Accessed 21 October 2020.
WHO. Weekly epidemiological update on COVID-19- 19 th October 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-october-2022 .
Maunder R, Lancee W, Balderson K, Bennett J, Borgundvaag B, Evans S, et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerging Infect Dis. 2006;12(12):1924–32. https://doi.org/10.3201/eid1212.060584 .
Article Google Scholar
Jalloh MF, Li W, Bunnell RE. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Glob Health. 2018;3: e000471. https://doi.org/10.1136/bmjgh-2017-000471 .
Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7(3): e14.
Huang JZ, Han MF, Luo TD, Ren AK, Zhou XP. Mental health survey of 230 medical staff in a tertiary infectious disease hospital for COVID-19. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi. Chinese J Indus Hygiene Occup Dis. 2020;38:E001.
Google Scholar
Cai W, Lian B, Song X, Hou T, Deng G, Li H. A cross-sectional study on mental health among health care workers during the outbreak of coronavirus disease. Asian J Psychiatr. 2020. https://doi.org/10.1016/j.ajp.2020.102111 .
Elhadi M, Msherghi A, Elgzairi M, Alhashimi A, Bouhuwaish A, Biala M, Abuelmeda S, et al. Psychological status of healthcare workers during the civil war and COVID-19 pandemic: a cross-sectional study. J Psychosom Res. 2020;137: 110221.
Urooj U, Ansari A, Siraj A, Khan S, Tariq H. Expectations, fears, and perceptions of doctors during COVID-19 pandemic. Pak J Med Sci. 2020;36:S37–42. https://doi.org/10.12669/pjms.36.COVID19-S4.2643 .
Khanal P, Devkota N, Dahal M, Paudel K, Joshi D. Mental health impacts among health workers during COVID-19 in a low resource setting: a cross-sectional survey from Nepal. Global Health. 2020;16(1):89. https://doi.org/10.1186/s12992-020-00621-z .
Preti E, Di Mattei V, Perego G. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43. https://doi.org/10.1007/s11920-020-01166-z .
De Kock JH, Latham HA, Leslie SJ, Grindle M, Munoz SA, Ellis L, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. https://doi.org/10.1186/s12889-020-10070-3 .
Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and the general public. A systematic review and meta-analysis. Psychiatry Res. 2020;291:113190. https://doi.org/10.1016/j.psychres.2020.113190 .
Bareeqa SB, Ahmed SI, Samar SS, Yasin W, Zehra S, Monese GM, et al. Prevalence of depression, anxiety, and stress in china during COVID-19 pandemic: a systematic review with meta-analysis. Int J Psychiatry Med. 2021;56:210–27.
Søvold LE, Naslund JA, Kousoulis AA. Prioritizing the mental health and well-being of healthcare workers: an urgent global public health priority. Front Public Health. 2021;9: 679397. https://doi.org/10.3389/fpubh.2021.679397 .
Consolo U, Bellini P, Bencivenni D, Iani C, Checchi V. Epidemiological aspects, and psychological reactions to COVID-19 of dental practitioners in the Northern Italy districts of Modena and Reggio Emilia. Int J Environ Res Public Health. 2020;17(10):3459. https://doi.org/10.3390/ijerph17103459 .
Gilleen J, Santaolalla A, Valdearenas L, Salice C, Fusté M. Impact of the COVID-19 pandemic on the mental health and well-being of UK healthcare workers. BJPsych Open. 2021;7(3): e88. https://doi.org/10.1192/bjo.2021.42 .
Shechter A, Diaz F, Moise N, Anstey DE, Ye S, Agarwal S, et al. psychological distress, coping behaviours, and preferences for support among New York healthcare workers during the COVID-19 pandemic. Gen Hosp Psychiatry. 2020;66:1–8. https://doi.org/10.1016/j.genhosppsych.2020.06.007 .
Wilson W, Raj JP, Rao S, Ghiya M, Nedungalaparambil NM, Mundra H, et al. Prevalence and predictors of stress, anxiety, and depression among healthcare workers managing COVID-19 pandemic in India: a nationwide observational study. Indian J Psychol Med. 2020;42(4):353–8. https://doi.org/10.1177/0253717620933992 .
Wang S, Xie L, Xu Y, Yu S, Yao B, Xiang D. Sleep disturbances among medical workers during the outbreak of COVID-2019. Occup Med. 2020. https://doi.org/10.1093/occmed/kqaa074 .
Kang L, Ma S, Chen M, Yang J, Wang Y, Li R. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav Immun. 2020. https://doi.org/10.1016/j.bbi.2020.03.028 .
Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behavi Immun. 2020. https://doi.org/10.1016/j.bbi.2020.05.026 .
Tan BYQ, Chew NWS, Lee GKH, Jing M, Goh Y, Yeo LLL. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1083 .
Li Y, Scherer N, Felix L, Kuper H. Prevalence of depression, anxiety, and post-traumatic stress disorder in health care workers during the COVID-19 pandemic: a systematic review and meta-analysis. PLoS One. 2021;16(3): e0246454. https://doi.org/10.1371/journal.pone.0246454 .
British Medical Association. The mental health and well-being of the medical workforce – now and beyond COVID-19. 2020. Available from URL: https://www.bma.org.uk/media/2475/bma-covid-19-and-nhs-staff-mental-healthwellbeing-report-may-2020.pdf .
Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3): e203976. https://doi.org/10.1001/jamanetworkopen.2020.3976 .
Chen J, Farah N, Dong RK, Chen RZ, Xu W, Yin J, et al. Mental health during the COVID-19 crisis in Africa: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:10604. https://doi.org/10.3390/ijerph182010604 .
Gupta S, Sahoo S. Pandemic, and mental health of the frontline healthcare workers: a review and implications in the Indian context amidst COVID-19. Gen Psychiatr. 2020;33(5): e100284. https://doi.org/10.1136/gpsych-2020-100284 .
Cai H, Tu B, Ma J, Chen L, Fu L, Jiang Y, et al. psychological impact, and coping strategies of frontline medical staff in Hunan between January and March 2020 during the outbreak of coronavirus disease 2019 (COVID-19) in Hubei, China. Med Sci Monit. 2020;26:924171. https://doi.org/10.12659/MSM.924171 .
CDC. National Center for Immunization and Respiratory Diseases. CDC (2020) Available online at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html .
Muzyamba C, Makova O, Mushibi GS. Exploring health workers’ experiences of mental health challenges during care of patients with COVID-19 in Uganda: a qualitative study. BMC Res Notes. 2021;14(1):286. https://doi.org/10.1186/s13104-021-05707-4 .
GebreEyesus FA, Tarekegn TT, Amlak BT, Shiferaw BZ, Emeria MS, Geleta OT, et al. Levels and predictors of anxiety, depression, and stress during COVID-19 pandemic among frontline healthcare providers in Gurage zonal public hospitals, Southwest Ethiopia, 2020: a multicentre cross-sectional study. PLoS One. 2021;16(11): e0259906. https://doi.org/10.1371/journal.pone.0259906 .
Mulatu HA, Tesfaye M, Woldeyes E. The prevalence of common mental disorders among health care professionals during the COVID-19 pandemic at a tertiary Hospital in East Africa. Med Rxiv. 2020. https://doi.org/10.1101/2020.10.29.20222430 .
Ayalew M, Deribe B, Abraham Y, Reta Y, Tadesse F, Defar S. Post-traumatic stress disorder symptoms and its predictors among healthcare workers following COVID-19 pandemic in Southern Ethiopia: a cross-sectional study. Front Psychiatry. 2021. https://doi.org/10.3389/fpsyt.2021.818910 .
Sagaon-Teyssier L, Kamissoko A, Yattassaye A, Diallo F, Castro DR, Delabre R, et al. Assessment of mental health outcomes and associated factors among workers in community-based HIV care centers in the early stage of the COVID-19 outbreak in Mali. Health Policy Open. 2020. https://doi.org/10.1016/j.hpopen.2020.100017 .
Kemal J. Psychological distress, early behavioral response, and perception toward the COVID-19 pandemic among health care workers in North Shoa Zone, Oromiya region. Front Psychiatry. 2020. https://doi.org/10.3389/fpsyt.2021.628898 .
Chekole YA, Minaye SY, Abate SM, Mekuriaw B. Perceived stress, and its associated factors during COVID-19 among healthcare providers in Ethiopia: a cross-sectional study. Adv Public Heal. 2020. https://doi.org/10.1155/2020/5036861 .
Asnakew S. Prevalence of post-traumatic stress disorder on health professionals in the era of COVID-19 pandemic, Northwest Ethiopia 2020: a multi-centred cross-sectional study. PLoS One. 2021;16(9):e0255340.
Asnakew S, Amha H, Kassew T. Mental health adverse effects of COVID-19 pandemic on health care workers in Northwest Ethiopia: a multicenter cross-sectional study. Neuropsychiatr Dis Treat. 2021;17:1375–84. https://doi.org/10.2147/NDT.S306300 .
Kounou KB, Guédénon KM, Foli AAD, Gnassounou-Akpa E. Mental health of medical professionals during the COVID-19 pandemic in Togo. Psychiatry Clin Neurosci. 2020;74:559–60.
Ayalew MB. Prevalence and determinant factors of mental health problems among healthcare professionals during COVID-19 pandemic in southern Ethiopia: a multicentre cross-sectional study. BMJ Open. 2021. https://doi.org/10.1136/bmjopen-2021-057708 .
Keubo FRN, Mboua PC, Tadongfack TD, Tchoffo EF, Tatang CT, Zeuna JI, et al. psychological distress among health care professionals of the three COVID-19 most affected Regions in Cameroon: Prevalence and associated factors. In: Annales Médico-Psychologiques, Revue Psychiatrique; Elsevier Masson: Paris, France. 2021;179:141–146.
Xiao H, Zhang Y, Kong D, Li S, Yang N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Med Sci Monit. 2020;26:e923549. https://doi.org/10.12659/MSM.923549 .
Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among healthcare professionals during the COVID-19 pandemic. JAMA. 2020. https://doi.org/10.1001/jama.2020.5893 .
Download references
Acknowledgements
We thank Uganda Medical Association Acholi-branch members for the financial assistance which enabled the team to conduct this study successfully.
Author information
Authors and affiliations.
Uganda Medical Association (UMA), UMA-Acholi Branch, Gulu City, Uganda
Freddy Wathum Drinkwater Oyat, Johnson Nyeko Oloya, Pamela Atim, Judith Aloyo & David Lagoro Kitara
Moroto Regional Referral Hospital, Moroto, Uganda
Johnson Nyeko Oloya
St. Joseph’s Hospital, Kitgum District, Uganda
Pamela Atim
ICAP at Columbia University, Freetown, Sierra Leone
Eric Nzirakaindi Ikoona
Rhites-N, Acholi, Gulu City, Uganda
Judith Aloyo
Faculty of Medicine, Department of Surgery, Gulu University, P.O. Box 166, Gulu City, Uganda
David Lagoro Kitara
Harvard University, Cambridge, USA
You can also search for this author in PubMed Google Scholar
Contributions
FWDO, JA, JNO, ENI and DLK searched and screened studies and extracted data from selected articles. FWDO wrote the first draft of the manuscript, and all authors reviewed and edited the final draft. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to David Lagoro Kitara .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
All authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Oyat, F.W.D., Oloya, J.N., Atim, P. et al. The psychological impact, risk factors and coping strategies to COVID-19 pandemic on healthcare workers in the sub-Saharan Africa: a narrative review of existing literature. BMC Psychol 10 , 284 (2022). https://doi.org/10.1186/s40359-022-00998-z
Download citation
Received : 03 September 2022
Accepted : 22 November 2022
Published : 01 December 2022
DOI : https://doi.org/10.1186/s40359-022-00998-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- COVID-19 pandemic
- Social support
- Occupational health and safety
- Mental health surveillance
- Workplace organization
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]

American Psychological Association

The impact of transgender legislation
Psychological science points to an increased risk of suicide and poor mental health amid a record number of bills aimed at restricting the rights of the LGBTQ+ population
APA policy supporting transgender, gender diverse, nonbinary individuals
Membership in APA

APA Community
A new exclusive destination tailored for APA members

Membership benefits
Unlock the tools, discounts, and services included with your membership

Renew your membership
Keep your benefits and access to leading psychological information
Psychology topics spotlight

Misinformation and disinformation

Resources to navigate trauma

Tips to foster a healthy workplace
Science and practice of psychology

Ethics Code

Continuing Education

Grants, Awards, and Funding

Standards and Guidelines
Networks and communities

Network with peers, enhance your professional development, expand your personal growth, and more

APA Divisions

High school teachers

Undergraduate educators

Graduate students

Early career psychologists

Managing your career
Resources to help you throughout your career in psychology, including finding a job, salary data, finances and money management, mentoring and supervision, and training and professional development

Explore career paths

Psychologist profiles

How did you get that job?

Events and training
Featured jobs
Apa publications and products.

Write with clarity, precision, and inclusion
Children’s books
Monitor on Psychology
Newsletters
Reports and surveys
Continuing education
Merchandise

Real Siblings

Jacob's Missing Book

Harper Becomes a Big Sister
Attachment-Based Family Therapy for Sexual and Gender Minority Young Adults and Their Non-Accepting Parents
Dismantling Everyday Discrimination
APA Services

Learn how you can help APA advocate for psychology-informed federal policy and legislation, and support psychological research

APA Services, Inc.
A companion professional organization to APA, serving all members and advocating for psychology
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Heart rate recovery: up to date in heart failure—a literature review.

1. Introduction
2. autonomic influence in exercise and the recovery period, 3. main hrr parameters, 3.1. difference between peak hr and hr at a certain moment of recovery.
- HRR1 —HRR at 1 minute of recovery subtracted from the peak HR [ 24 , 25 , 26 , 27 , 28 , 29 , 30 ].
- HRR2 —HRR at 2 min of recovery subtracted from the peak HR [ 25 , 28 , 29 , 31 , 32 ].
- HRR150 sec —measured as the difference between the maximum HR and the HR after 150 seconds of recovery [ 33 ].
- HRR3 —measured as the difference between the maximum HR and the HR after three minutes of recovery [ 34 ].
3.2. A Ratio between Different Phases of the Effort
- Heart rate recovery index —measured as the ratio between heart rate acceleration time (AT) and heart rate deceleration time (DT) [ 35 ].
3.3. A Delay in the Maximum Heart Rate
- Delay of peak HR : HRR assessment 6 months after heart transplantation reflects the cardiac denervation and the loss of vagal tone, which would normally induce HR drop after exercise [ 32 ].
4. Clinical Applications of HRR
5. methodology, 6. hrr in heart failure: bedside studies.
| Study (Year) | Patients Enrolled (n) | HF Population | Purpose of the Study | Exercise Test Methodology | Beta-Blocker Treatment | HRR Evaluation Method and Cut-Off | Conclusions |
|---|---|---|---|---|---|---|---|
| Hossri et al. (2024) [ ] | 106 | HFpEF and HFmrEF with concomitant CAD | Benefits of CPMR in HF patients with CAD | Treadmill CPET using an incremental maximal protocol, followed later by a submaximal constant load protocol at 80% of the initial test; recovery period of 6 min | 87% of the patients. Dosage N/A. | HRR1 was evaluated at the first recovery min; Cut-off N/A | 12 weeks of CPMR were associated with improved NYHA class, significant exercise test performance, an increased HRR, and an enhanced QOL. |
| Irfanullah et al. (2023) [ ] | 39 | HFpEF, HFmrEF, HFrEF | The effects of cycle ergometer training on heart rate recovery and mindfulness in patients with NYHA Class I and II heart failure | 6 MWT on a 400–700 m distance. | All patients received either beta-blockers or CCB. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut-off N/A | HRR1 and HRR2 improved after 6 weeks of cycle-ergometer training, as well as the MAAS. |
| Carneiro et al. (2021) [ ] | 2066 | Participants without HF | Incidence of HF and its type (HFpEF and HFrEF) during the follow-up period (16.8 years) | Submaximal treadmill exercise test using the Bruce protocol; recovery in a supine position. | N/A | HRR3 = HRmax-HR at 3 min of recovery; Cut-off N/A | Slower HRR3 is associated with a higher risk of developing heart failure, particularly HFrEF. |
| Hajdusek et al. (2017) [ ] | 103 | 78 advanced HFrEF and 25 healthy controls, assessed for device implantation or transplant eligibility | Evaluation of HRR and MCR as outcome determinants in HF, during a follow-up of ~3.4 years | Symptom limited using a bicycle CPET, with a 25 W increase/3 min. | 97% of HF patients. Daily low-middle doses (12.5–50 mg Carvedilol; 2.5–200 mg Metoprolol and 2.5–10 mg Bisoprolol) | The difference between HRmax and HR at 150 s of recovery; Cut-off N/A | MCR slope correlates with distinct clinical variables compared to HRR. In heart failure patients, the MCR slope offers significant prognostic value beyond HRR. |
| Yaylalı et al. (2015) [ ] | 41 | HFrEF and HFmrEF | Correlation between exercise training and HRR improvement, before and after entering a training program | Symptom limited bicycle CPET with 10 W/min followed by 3 min active cool-down | 51.2% of patients. Dosage N/A | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; N.V. Preestablished HRR1 > 12 bpm; HRR2 > 22 bpm | The training enhanced only HRR2, with IT showing a greater impact on the HRR2 improvement. Both HRR (1 and 2) in those with an abnormal HRR baseline have improved after exercise. |
| Imamura et al. (2015) [ ] | 21 | Heart transplant patients | Impact of post-heart transplantation parasympathetic reinnervation | Symptom limited Cycle-ergometer CPET using a 10 W/min incremental protocol, followed by a 5 min passive recovery in a seated position. | 19% of patients. Dosage N/A. | HRR2-the difference between peak HR and HR after 2 min of recovery (measured 2 years after transplant) Delay of peak HR-the delay from the peak HR after the recovery time initiation (measured 6 months after transplant). Cut-off N/A | Parasympathetic reinnervation coincides with enhanced post-exercise recovery and heart failure-specific quality of life during the 2 years following heart transplantation. |
| Lindemberg et al. (2014) [ ] | 161. 154 completed the test | 126 patients with HFrEF and 35 healthy individuals. | Correlations between HRR1 and 6 MWD | 6 MWT followed by passive (seated) recovery. | 70.58% of HF patients. Carvedilol mean dose 30 ± 29 mg. | HRR1 = HRmax-HR at 1 min of recovery; N.V. Preestablished HRR1 > 12 bpm | HF patients who received beta-blockers had better exercise tolerance than those without receiving beta-blocker medication, even though they had altered HRR. |
| Cahalin et al. (2014) [ ] | 240 | 200 patients with HFrEF and 40 patients with HFpEF | Correlations between HRR measured post 6 MWT and CPET and predictors of abnormal HRR. | 6 MWT followed by a passive recovery. A symptom-limited bicycle CPET lasting 8–10 min. 1 min active cool-down period. | 60% of the entire study group. Dosage N/A | HRR1 = HRmax-HR at 1 min of recovery; N.V. Preestablished HRR1 > 12 bpm | The 6 MWT and CPET were correlated concerning HRR, HR Reserve, and peak HR. Predictors of abnormal HRR were found to be Peak HR, EOV and E/e’ ratio. |
7. Echocardiographic Correlations
8. discussion, 9. further perspectives, 10. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Dewar, A.; Kass, L.; Stephens, R.C.M.; Tetlow, N.; Desai, T. Heart Rate Recovery Assessed by Cardiopulmonary Exercise Testing in Patients with Cardiovascular Disease: Relationship with Prognosis. Int. J. Environ. Res. Public Health 2023 , 20 , 4678. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yu, Y.; Liu, T.; Wu, J.; Zhu, P.; Zhang, M.; Zheng, W.; Gu, Y. Heart rate recovery in hypertensive patients: Relationship with blood pressure control. J. Hum. Hypertens. 2017 , 31 , 354–360. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023 , 24 , 15472. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fecchio, R.Y.; Brito, L.; Leicht, A.S.; Forjaz, C.L.M.; Peçanha, T. Reproducibility of post-exercise heart rate recovery indices: A systematic review. Auton. Neurosci. 2019 , 221 , 102582. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U.; Yin, J.; Jin, X.; Shan, Z.; et al. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017 , 6 , e005505. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L.D.M. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014 , 34 , 327–339. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Okutucu, S.; Karakulak, U.N.; Aytemir, K.; Oto, A. Heart rate recovery: A practical clinical indicator of abnormal cardiac autonomic function. Expert Rev. Cardiovasc. Ther. 2011 , 9 , 1417–1430. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Imai, K.; Sato, H.; Hori, M.; Kusuoka, H.; Ozaki, H.; Yokoyama, H.; Takeda, H.; Inoue, M.; Kamada, T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994 , 24 , 1529–1535. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023 , 44 , 3627–3639. [ Google Scholar ] [ CrossRef ]
- Severino, P.; D’amato, A.; Prosperi, S.; Cas, A.D.; Mattioli, A.V.; Cevese, A.; Novo, G.; Prat, M.; Pedrinelli, R.; Raddino, R.; et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? J. Clin. Med. 2022 , 11 , 857. [ Google Scholar ] [ CrossRef ]
- Gronda, E.; Dusi, V.; D’elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 2022 , 24 , E4–E11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020 , 12 , 373–408. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, D.Y.; Anderson, A.S. The Sympathetic Nervous System and Heart Failure. Cardiol. Clin. 2014 , 32 , 33–45. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumar, H.U.; Nearing, B.D.; Mittal, S.; Premchand, R.K.; Libbus, I.; DiCarlo, L.A.; Amurthur, B.; KenKnight, B.H.; Anand, I.S.; Verrier, R.L. Autonomic regulation therapy in chronic heart failure with preserved/mildly reduced ejection fraction: ANTHEM-HFpEF study results. Int. J. Cardiol. 2023 , 381 , 37–44. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peçanha, T.; de Brito, L.C.; Fecchio, R.Y.; de Sousa, P.N.; Junior, N.D.d.S.; de Abreu, A.P.; da Silva, G.V.; Mion-Junior, D.; Forjaz, C.L.d.M. Metaboreflex activation delays heart rate recovery after aerobic exercise in never-treated hypertensive men. J. Physiol. 2016 , 594 , 6211–6223. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Roberto, S.; Mulliri, G.; Milia, R.; Solinas, R.; Pinna, V.; Sainas, G.; Piepoli, M.F.; Crisafulli, A. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J. Appl. Physiol. 2017 , 122 , 376–385. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. In Comprehensive Physiology ; Wiley: Hoboken, NY, USA, 2015; pp. 1–32. [ Google Scholar ] [ CrossRef ]
- Gourine, A.V.; Ackland, G.L. Cardiac vagus and exercise. Physiology 2019 , 34 , 71–80. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic Effects on Heart Rate Recovery after Exercise. J. Investig. Med. 2004 , 52 , 394–401. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Facioli, T.P.; Philbois, S.V.; Gastaldi, A.C.; Almeida, D.S.; Maida, K.D.; Rodrigues, J.A.L.; Sanchez-Delgado, J.C.; Souza, H.C.D. Study of heart rate recovery and cardiovascular autonomic modulation in healthy participants after submaximal exercise. Sci. Rep. 2021 , 11 , 3620. [ Google Scholar ] [ CrossRef ]
- Roberto, S.; Milia, R.; Doneddu, A.; Pinna, V.; Palazzolo, G.; Serra, S.; Orrù, A.; Kakhak, S.A.H.; Ghiani, G.; Mulliri, G.; et al. Hemodynamic abnormalities during muscle metaboreflex activation in patients with type 2 diabetes mellitus. J. Appl. Physiol. 2019 , 126 , 444–453. [ Google Scholar ] [ CrossRef ]
- Smarz, K.; Tysarowski, M.; Zaborska, B.; Pilichowska-Paszkiet, E.; Sikora-Frac, M.; Budaj, A.; Jaxa-Chamiec, T. Chronotropic incompetence limits aerobic exercise capacity in patients taking beta-blockers: Real-life observation of consecutive patients. Healthcare 2021 , 9 , 212. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart Rate Recovery after Submaximal Exercise Testing as a Predictor of Mortality in a Cardiovascularly Healthy Cohort. Ann. Intern. Med. 2000 , 132 , 552–555. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hossri, C.A.C.; Araujo, F.; Baldi, B.; Otterstetter, R.; Uemoto, V.; Carvalho, C.; Mastrocola, L.; Albuquerque, A. Association among cardiopulmonary and metabolic rehabilitation, arrhythmias, and myocardial ischemia responses of patients with HFpEF or HFmrEF. Braz. J. Med. Biol. Res. 2024 , 57 , e13174. [ Google Scholar ] [ CrossRef ]
- Andrade, G.N.; Rodrigues, T.; Takada, J.; Braga, L.; Umeda, I.; Nascimento, J.; Pereira-Filho, H.; Grupi, C.; Salemi, V.; Jacob-Filho, W.; et al. Prolonged heart rate recovery time after 6-minute walk test is an independent risk factor for cardiac events in heart failure: A prospective cohort study. Physiotherapy 2022 , 114 , 77–84. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lindemberg, S.; Chermont, S.; Quintão, M.; Derossi, M.; Guilhon, S.; Bernardez, S.; Marchese, L.; Martins, W.; Nóbrega, A.C.L.; Mesquita, E.T. Heart Rate Recovery in the First Minute at the Six-Minute Walk Test in Patients with Heart Failure. Arq. Bras. Cardiol. 2014 , 102 , 279–287. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tanaka, S.; Miyamoto, T.; Mori, Y.; Harada, T.; Tasaki, H. Heart rate recovery is useful for evaluating the recovery of exercise tolerance in patients with heart failure and atrial fibrillation. Heart Vessels 2021 , 36 , 1551–1557. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- da Fonseca, G.W.P.; dos Santos, M.R.; de Souza, F.R.; da Costa, M.J.A.; von Haehling, S.; Takayama, L.; Pereira, R.M.R.; Negrão, C.E.; Anker, S.D.; Alves, M.J.d.N.N. Sympatho-Vagal Imbalance is Associated with Sarcopenia in Male Patients with Heart Failure. Arq. Bras. Cardiol. 2019 , 112 , 739–746. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Youn, J.C.; Lee, H.S.; Choi, S.-W.; Han, S.-W.; Ryu, K.-H.; Shin, E.-C.; Kang, S.-M. Post-exercise heart rate recovery independently predicts clinical outcome in patients with acute decompensated heart failure. PLoS ONE 2016 , 11 , e0154534. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cahalin, L.P.; Arena, R.; Labate, V.; Bandera, F.; Guazzi, M. Predictors of abnormal heart rate recovery in patients with heart failure reduced and preserved ejection fraction. Eur. J. Prev. Cardiol. 2014 , 21 , 906–914. [ Google Scholar ] [ CrossRef ]
- Yaylali, Y.T.; Fındıkoglu, G.; Yurtdas, M.; Konukcu, S.; Senol, H. The effects of baseline heart rate recovery normality and exercise training protocol on heart rate recovery in patients with heart failure. Anatol. J. Cardiol. 2015 , 15 , 727–734. [ Google Scholar ] [ CrossRef ]
- Imamura, T.; Kinugawa, K.; Okada, I.; Kato, N.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Kinoshita, O.; Nawata, K.; et al. Parasympathetic Reinnervation Accompanied by Improved Post-Exercise Heart Rate Recovery and Quality of Life in Heart Transplant Recipients. Int. Heart J. 2015 , 56 , 180–185. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hajdusek, P.; Kotrc, M.; Kautzner, J.; Melenovsky, V.; Benesova, E.; Jarolim, P.; Benes, J. Heart rate response to exercise in heart failure patients: The prognostic role of metabolic–chronotropic relation and heart rate recovery. Int. J. Cardiol. 2017 , 228 , 588–593. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carneiro, H.A.; Song, R.J.; Lee, J.; Schwartz, B.; Vasan, R.S.; Xanthakis, V. Association of Blood Pressure and Heart Rate Responses to Submaximal Exercise with Incident Heart Failure: The Framingham Heart Study. J. Am. Heart Assoc. 2021 , 10 , e019460. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cozlac, A.-R.; Petrescu, L.; Crisan, S.; Luca, C.T.; Vacarescu, C.; Streian, C.G.; Lazar, M.-A.; Gurgu, A.; Dragomir, A.; Goanta, E.V.; et al. A Novel and Simple Exercise Test Parameter to Assess Responsiveness to Cardiac Resynchronization Therapy. Diagnostics 2020 , 10 , 920. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peçanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017 , 227 , 795–802. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sydó, N.; Sydó, T.; Merkely, B.; Carta, K.G.; Murphy, J.G.; Lopez-Jimenez, F.; Allison, T.G. Impaired Heart Rate Response to Exercise in Diabetes and Its Long-term Significance. Mayo Clin. Proc. 2016 , 91 , 157–165. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alihanoglu, Y.I.; Yildiz, B.S.; Kilic, I.D.; Uludag, B.; Demirci, E.E.; Zungur, M.; Evrengul, H.; Kaftan, A.H. Impaired systolic blood pressure recovery and heart rate recovery after graded exercise in patients with metabolic syndrome. Medicine 2015 , 94 , e428. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maeder, M.T.; Ammann, P.; Schoch, O.D.; Rickli, H.; Korte, W.; Hürny, C.; Myers, J.; Münzer, T. Determinants of postexercise heart rate recovery in patients with the obstructive sleep apnea syndrome. Chest 2010 , 137 , 310–317. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shim, C.Y. Stress Testing in Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2021 , 17 , 435–445. [ Google Scholar ] [ CrossRef ]
- Vukomanovic, V.; Suzic-Lazic, J.; Celic, V.; Cuspidi, C.; Grassi, G.; Galderisi, M.; Djukic, V.; Tadic, M. Is there association between left atrial function and functional capacity in patients with uncomplicated type 2 diabetes? Int. J. Cardiovasc. Imaging 2020 , 36 , 15–22. [ Google Scholar ] [ CrossRef ]
- Tadic, M.; Suzic-Lazic, J.; Vukomanovic, V.; Cuspidi, C.; Ilic, S.; Celic, V. Functional capacity and left ventricular diastolic function in patients with type 2 diabetes. Acta Diabetol. 2021 , 58 , 107–113. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lin, Y.T.; Lin, L.Y.; Chuang, K.J. N terminal prohormone of brain natriuretic peptide is associated with improved heart rate recovery after treadmill exercise test. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023 , 18 , 200203. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- van de Vegte, Y.J.; van der Harst, P.; Verweij, N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J. Am. Heart Assoc. 2018 , 7 , e008341. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019 , 13 , 1–10. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ren, C.; Zhu, J.; Shen, T.; Song, Y.; Tao, L.; Xu, S.; Zhao, W.; Gao, W. Comparison Between Treadmill and Bicycle Ergometer Exercises in Terms of Safety of Cardiopulmonary Exercise Testing in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2022 , 9 , 864637. [ Google Scholar ] [ CrossRef ]
- Irfanullah, M.W.; Yaqoob, N.; Shah, S.; Tariq, I.; Chaudhary, F.; Ibrahim, U.; Huma, Z.E.; Khan, N. Effects of Cycle Ergometer Training on Heart Rate Recovery and Mind Fullness in NYHA class I, II Heart Failure Patients. Pak. J. Med. Health Sci. 2023 , 17 , 10–13. [ Google Scholar ] [ CrossRef ]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021 , 23 , 352–380. [ Google Scholar ] [ CrossRef ]
- Maldonado-Martín, S.; Brubaker, P.H.; Ozemek, C.; Jayo-Montoya, J.A.; Becton, J.T.; Kitzman, D.W. Impact of β-Blockers on Heart Rate and Oxygen Uptake During Exercise and Recovery in Older Patients with Heart Failure With Preserved Ejection Fraction. J. Cardiopulm. Rehabil. Prev. 2020 , 40 , 174. [ Google Scholar ] [ CrossRef ]
- Skaluba, S.J.; Litwin, S.E. Doppler-derived left ventricular filling pressures and the regulation of heart rate recovery after exercise in patients with suspected coronary artery disease. Am. J. Cardiol. 2005 , 95 , 832–837. [ Google Scholar ] [ CrossRef ]
- Gharacholou, S.M.; Scott, C.G.; Borlaug, B.A.; Kane, G.C.; McCully, R.B.; Oh, J.K.; Pellikka, P.A. Relationship between diastolic function and heart rate recovery after symptom-limited exercise. J. Card. Fail. 2012 , 18 , 34–40. [ Google Scholar ] [ CrossRef ]
- Mashayekhi, B.; Mohseni-Badalabadi, R.; Hosseinsabet, A.; Ahmadian, T. Correlation between Heart rate recovery and Left Atrial phasic functions evaluated by 2D speckle-tracking Echocardiography after Acute Myocardial infarction. BMC Cardiovasc. Disord. 2023 , 23 , 164. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arshi, B.; Geurts, S.; Tilly, M.J.; Berg, M.v.D.; Kors, J.A.; Rizopoulos, D.; Ikram, M.A.; Kavousi, M. Heart rate variability is associated with left ventricular systolic, diastolic function and incident heart failure in the general population. BMC Med. 2022 , 20 , 91. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tsai, C.H.; Ma, H.-P.; Hung, C.-S.; Huang, S.-H.; Chuang, B.-L.; Lin, C.; Lo, M.-T.; Peng, C.-K.; Lin, Y.-H. Usefulness of heart rhythm complexity in heart failure detection and diagnosis. Sci. Rep. 2020 , 10 , 14916. [ Google Scholar ] [ CrossRef ]
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of Autonomic Function in Cardiovascular Disease. Physiological Basis and Prognostic Implications. J. Am. Coll. Cardiol. 2008 , 51 , 1725–1733. [ Google Scholar ] [ CrossRef ]
- Julario, R.; Mulia, E.P.B.; Rachmi, D.A.; A’yun, M.Q.; Septianda, I.; Dewi, I.P.; Juwita, R.R.; Dharmadjati, B.B. Evaluation of heart rate variability using 24-hour Holter electrocardiography in hypertensive patients. J. Arrhythm. 2021 , 37 , 157–164. [ Google Scholar ] [ CrossRef ]
- Bechke, E.; Kliszczewicz, B.; McLester, C.; Tillman, M.; Esco, M.; Lopez, R. An examination of single day vs. multi-day heart rate variability and its relationship to heart rate recovery following maximal aerobic exercise in females. Sci. Rep. 2020 , 10 , 14760. [ Google Scholar ] [ CrossRef ]
- Vacarescu, C.; Luca, C.-T.; Feier, H.; Gaiță, D.; Crișan, S.; Negru, A.-G.; Iurciuc, S.; Goanță, E.-V.; Mornos, C.; Lazăr, M.-A.; et al. Betablockers and Ivabradine Titration According to Exercise Test in LV Only Fusion CRT Pacing. Diagnostics 2022 , 12 , 1096. [ Google Scholar ] [ CrossRef ]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018 , 15 , 731–743. [ Google Scholar ] [ CrossRef ]
- Sardu, C.; Massetti, M.M.; Rambaldi, P.; Gatta, G.; Cappabianca, S.; Sasso, F.C.; Santamaria, M.; Volpicelli, M.; Ducceschi, V.; Signoriello, G.; et al. SGLT2-inhibitors reduce the cardiac autonomic neuropathy dysfunction and vaso-vagal syncope recurrence in patients with type 2 diabetes mellitus: The SCAN study. Metabolism 2022 , 137 , 155243. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- van Bilsen, M.; Patel, H.C.; Bauersachs, J.; Böhm, M.; Borggrefe, M.; Brutsaert, D.; Coats, A.J.; de Boer, R.A.; de Keulenaer, G.W.; Filippatos, G.S.; et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2017 , 19 , 1361–1378. [ Google Scholar ] [ CrossRef ]
- Franssen, W.M.A.; Beyens, M.; Al Hatawe, T.; Frederix, I.; Verboven, K.; Dendale, P.; Eijnde, B.O.; Massa, G.; Hansen, D. Cardiac function in adolescents with obesity: Cardiometabolic risk factors and impact on physical fitness. Int. J. Obes. 2019 , 43 , 1400–1410. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ackland, G.L.; Minto, G.; Clark, M.; Whittle, J.; Stephens, R.C.; Owen, T.; Prabhu, P.; del Arroyo, A.G. Autonomic regulation of systemic inflammation in humans: A multi-center, blinded observational cohort study. Brain Behav. Immun. 2018 , 67 , 47–53. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teodoru, M.; Negrea, M.O.; Cozgarea, A.; Cozma, D.; Boicean, A. Enhancing Pulmonary Embolism Mortality Risk Stratification Using Machine Learning: The Role of the Neutrophil-to-Lymphocyte Ratio. J. Clin. Med. 2024 , 13 , 1191. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020 , 17 , 269–285. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rachieru, C.; Luca, C.T.; Văcărescu, C.; Petrescu, L.; Cirin, L.; Cozma, D. Future Perspectives to Improve CHA2 DS2 VASc Score: The Role of Left Atrium Remodelling, Inflammation and Genetics in Anticoagulation of Atrial Fibrillation. Clin. Interv. Aging 2023 , 18 , 1737–1748. [ Google Scholar ] [ CrossRef ]
- da Fonseca, R.X.; da Cruz, C.J.G.; Soares, E.M.K.V.K.; Garcia, G.L.; Porto, L.G.G.; Molina, G.E. Post-exercise heart rate recovery and its speed are associated with resting-reactivity cardiovagal modulation in healthy women. Sci. Rep. 2024 , 14 , 5526. [ Google Scholar ] [ CrossRef ]
| Study (Year) | Patients Enrolled (n) | HF Population | Purpose of the Study | Exercise Test Methodology | Beta-Blocker Treatment | HRR Evaluation Method and Cut-Off | Conclusions |
|---|---|---|---|---|---|---|---|
| Andrade et al. (2022) [ ] | 76 | HFrEF | Correlation of all-cause mortality and HRR1 and HRR2 during a 2-year follow-up | 6 MWT followed by passive supine recovery. | 97% of patients. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Preestablished N.V. HRR1 > 12 bpm; HRR2 > 22 bpm | Decreased HRR1 and HRR2 are associated with increased mortality. |
| Tanaka et al. (2021) [ ] | 84 | HFrEF with AF | Correlation of HRR and exercise capacity of HFrEF and AF patients before and after the rehabilitation program | Cycle ergometer CPET using a ramp protocol of 10 W/min until exhaustion, with an active 1 min recovery, followed by a 4 min passive recovery. | 90% of patients. Dosage N/A | HRR1 = HRmax-HR at 1 min of recovery. For AF patients, HR was determined by averaging the last ten beats at each point; Cut-off N/A | Improved HRR is associated with improved exercise capacity in patients with HFrEF and AF after completing the cardiac rehabilitation program. |
| Cozlac et al. (2020) [ ] | 109 | HFrEF patients following CRT implantation | Correlation of HRRI and CRT responsiveness | Cycle ergometer using the Bruce Protocol with a 25 W increase/2 min. | 82.8% of the patients. Dosage N/A. | HRRI = The ratio between HR AT and DT. The cut-off for CRT response predictability was 1.51. | HRRI was significantly higher in CRT responders vs. non-responders. |
| Fonseca et al. (2019) [ ] | 116 | HFrEF | Association of sarcopenia and autonomic regulation | Symptom limited cycle ergometer CPET using a ramp protocol 5–10 W/min. Active recovery for 2 min, followed by 4 min of passive recovery. | 100% of sarcopenic and 94% of non-sarcopenic patients. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut-off N/A | Sarcopenia is associated with decreased HRR1 and HRR2 in HF patients. |
| Youn et al. (2016) [ ] | 107 | Recovered acute decompensated HFrEF (Eligible for discharge) | Correlation between HRR and pro-inflammatory states with clinical outcomes | Treadmill CPET using a modified Bruce Protocol. Passive recovery in seated position. | Total of 33.3% in the CV-events group and 68.8% in the no-CV-events group. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut off HRR1 < 13, HRR2 < 27 | Impaired HRR is associated with an exaggerated pro-inflammatory response and independently predicts clinical outcomes. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Cozgarea, A.; Cozma, D.; Teodoru, M.; Lazăr-Höcher, A.-I.; Cirin, L.; Faur-Grigori, A.-A.; Lazăr, M.-A.; Crișan, S.; Gaiță, D.; Luca, C.-T.; et al. Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review. J. Clin. Med. 2024 , 13 , 3328. https://doi.org/10.3390/jcm13113328
Cozgarea A, Cozma D, Teodoru M, Lazăr-Höcher A-I, Cirin L, Faur-Grigori A-A, Lazăr M-A, Crișan S, Gaiță D, Luca C-T, et al. Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review. Journal of Clinical Medicine . 2024; 13(11):3328. https://doi.org/10.3390/jcm13113328
Cozgarea, Andreea, Dragoș Cozma, Minodora Teodoru, Alexandra-Iulia Lazăr-Höcher, Liviu Cirin, Adelina-Andreea Faur-Grigori, Mihai-Andrei Lazăr, Simina Crișan, Dan Gaiță, Constantin-Tudor Luca, and et al. 2024. "Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review" Journal of Clinical Medicine 13, no. 11: 3328. https://doi.org/10.3390/jcm13113328
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts
- PMC10082633

Discontinuing Methadone and Buprenorphine: A Review & Clinical Challenges
Joan e. zweben.
Clinical Professor of Psychiatry, University of California, San Francisco, Staff Psychologist, San Francisco VA Medical Center
James L. Sorensen
Department of Psychiatry and Behavioral Sciences, Zuckerberg San Francisco General Hospital, University of California, San Francisco
Mallory Shingle
Christopher k. blazes.
Vista Taos Drug and Alcohol Rehabilitation Center, Taos, New Mexico
This paper offers a review and recommendations for clinicians working with patients interested in discontinuing their opioid medication-assisted treatment. As buprenorphine has gained widespread acceptance for opioid addiction, many treatment providers and patients have a range of hopes and expectations about its optimal use. A surprising number assume buprenorphine is primarily useful as a medication to transition off illicit opioid use, and success is partially defined by discontinuing the medication. Despite accumulating evidence that a majority of patients will need to remain on medication to preserve their gains, clinicians often have to address a patient’s fervent desire to taper off. Using the concept of “recovery capital”, our review addresses 1) the appropriate duration of medication for opioid use disorders (MOUD), 2) risks associated with discontinuing, 3) a checklist that guides the patient through self-assessment of the wisdom of discontinuing MOUD, and 4) shared decision making about how to proceed.
1. Introduction
An opioid crisis of unprecedented proportions 1 has led to a reconsideration of medications to treat opioid use disorder. Desperation about overdose deaths plus education campaigns for the public and professionals have made the use of opioid medication for opioid use disorder (MOUD) more understandable and acceptable. 2 . Use of buprenorphine/naloxone (a prescription medication that combines buprenorphine and naloxone) has increased greatly, and methadone has received renewed attention. Since 2002, when buprenorphine/naloxone was approved to be dispensed from physicians’ offices, 3 many patients have gained access to the medication who would not consider going to a structured methadone program. Buprenorphine/naloxone has proven more acceptable to private sector programs than methadone, and widespread efforts have also made it available in prisons, jails, emergency departments, primary care facilities and other settings. However, both patients and treatment professionals possess widely varying knowledge and expectations for what medication can accomplish, and that variation creates challenges.
Despite growing public acceptance, old misunderstandings and biases persist in more subtle forms. Even though rehabilitation, not abstinence, has long been the goal of opioid replacement therapy 4 one of the most prominent misunderstandings is the view that successful treatment requires the patient to discontinue the medication at some point and still maintain stable recovery. This view, often implicit, makes itself known in discussions with patients and staff. Even some recent clinical guidance acknowledging the high level of risk inherent to tapering offers prescriptive ways to taper from buprenorphine, acknowledging that clinicians continue to face this obstacle to providing effective treatment. 5 The narrative promoting discontinuation often goes unchallenged, even by those best situated to promote examination of the issues. A long and growing list of research studies suggests that some or perhaps most patients, will be unable to discontinue the medication and preserve their gains. This is documented in the literature on methpatients, 6 , 7 , 8 and the American Society of Addiction Medicine (ASAM) concluded two decades ago that methadone is best considered as a long-term treatment. 9 At least a subset of buprenorphine/naloxone patients appear to achieve lasting rehabilitation when used as a long-term treatment as well. 10 , 11 , 12 , 13 , 14 While research into effective methods for encouraging patients to continue treatment must continue, physicians, counselors, and other frontline staff with varied levels of medical training are being asked to meet the current needs of their patients expressing the urge to taper from their Medication for Opioid Use Disorder.
As defined by the Chair of the UK Recovery Academy David Best and U.S. psychologist Alexandre Laudet, recovery is a lived experience with principles focused on “the central ideas of hope, choice, freedom and aspiration that are experienced rather than diagnosed and occur in real life settings rather than in the rarefied atmosphere of clinical settings.” 15 When patients choose to self-define recovery as discontinuation of buprenorphine/naloxone, despite physician encouragement to continue MOUD, it is the view of the present authors that a patient’s desire to discontinue should be fully respected as they grow in self-empowerment. The desire should also be addressed in a context in which the patient is fully informed and prepared for the challenges. 16 In this article, we suggest ways to discuss issues with patients and treatment providers, adapting the framework of “recovery capital” and using The Recovery Capital and Physician Risk Factor Checklists. We understand that addressing recovery issues is unlikely to be sufficient for patients to discontinue MOUD, but addressing these recovery tasks will greatly improve the quality of life for the patient whether medication is continued or not.
Best and Laudet’s understanding of recovery capital, originally defined by Granfield and Cloud, 17 builds upon the idea of recovery as a lived experience: “Recovery capital refers to the sum of resources that may facilitate the process. 18 It thus encompasses a range of elements shown to contribute to quality of life. 19 , 20 These elements include social support, life meaning, spirituality in some form, and a community that supports the recovery process—all serving to reduce stress and increase stability of recovery, which in some cases patients choose to define by discontinuing MOUD. While some contributing elements of recovery capital rest outside of the clinical setting, there is also immense variability in the type of support offered in the clinical environment. White and Cloud 21 assert that the appropriate interventions depend in part on the balance of recovery capital and problem severity/complexity. Since we know Opioid Use Disorder to often have high levels of problem severity and complexity, physicians, counselors, and other frontline staff must be prepared to respond to the acuteness of a patient taper with a high level of recovery capital. Although most experienced clinicians working with patients in long term treatment address these issues over time, the checklist facilitates a more systematic approach. Most recently Vilsaint and colleagues 22 published a 10-item Brief Assessment of Recovery Capital (BARC-10) for alcohol and drug use disorder that psychological, physical, social, and environmental resources indicative of recovery capital.
2. Historical Background
Methadone was the first opioid medication to be used widely in outpatient addiction treatment, and it was controversial from the beginning. 23 As a result, an extensive research base developed and continues to this day. During the 1960s and 1970s, it was assumed that methadone could stabilize patients, who were given up to two years to steady their lives before they were required to taper off. Over time, research indicated these hopes were unrealistic. Studies conducted over the last 50 years confirm that it is extremely difficult for individuals to discontinue from methadone without returning to heroin. Research suggests that most patients will be unable to discontinue the medication and preserve the gains they made during treatment with methadone. 24
Buprenorphine/naloxone:
Since the FDA approved buprenorphine/naloxone as an OUD treatment, clinicians and researchers discovered similarly discouraging results associated with discontinuing the medication. 25 Meanwhile, clinicians observed high levels of success with continuation of buprenorphine/naloxone. 26 Compared with methadone, buprenorphine/naloxone offers some advantages, as a partial opioid agonist with less toxicity, 27 , 28 and it is available through prescription services, though limited, 29 rather than enrollment at a licensed clinic, which may be more convenient for patients and viewed as less stigmatizing. 30
Methadone to Buprenorphine/naloxone:
Breen et al. 31 explored transferring patients’ MOUD from methadone to buprenorphine and then tapering off buprenorphine, but without much success. While 38 of the 51 (75%) of patients reached zero dosage, only 31% were not using heroin or methadone at the one-month follow-up. Four patients (13%) switched to buprenorphine/naloxone, one of whom tapered off buprenorphine/naloxone. Twenty patients (67%) stopped their tapers due to feeling unstable/withdrawal symptoms, drug use/positive urinalysis results, psychiatric in these life, and pain management problems.
Discontinuing buprenorphine/naloxone:
Many studies have explored and compared methods for discontinuing buprenorphine. Several review articles summarized this literature, finding that the majority of people who attempt withdrawal from buprenorphine do not succeed. Dunn, Sigmon, Strain, Heil, & Higgins 32 compared 27 studies of the duration used to taper from buprenorphine. The review included 8 studies that conducted post-taper follow-up (with lengths of follow-up varying widely from 8–365 days after last buprenorphine dose). Collapsing across the 8 studies, a median of 23% of participants provided opioid-negative samples collected at the first post-taper follow-up visit (e.g. samples gathered in closest proximity to the final taper day). Retention in buprenorphine treatment also appears to be a problem when patients enter directly from illegal opioid use. In a retrospective longitudinal cohort analysis of 17,329 Medicaid patients (2013–2017), Samples et al. 33 found over 25% of the sample discontinued in the first month of treatment and most discontinued before 180 days. Risk factors for early discontinuation include younger adults, minorities, those with a history of non-opioid SUDS, and a low initial dose. These authors did not find that psychiatric comorbidities were a significant risk factor. They recommended focusing on the treatment barriers for those at high risk for discontinuation.
A systematic review by Bentzley, Barth, Bak, & Book 34 found that most patients who discontinued buprenorphine maintenance therapy did it involuntarily, because they had been failing to meet strict program requirements. Rates of relapse to illicit opioid use 1 month after discontinuation were over 50% in every study; collapsing across studies, 18% of patients were abstinent from opioids in the first month following discontinuance of buprenorphine. Sordo and colleagues 35 conducted a meta-analysis that synthesized evidence from cohort studies published until 2016 on risk of mortality in MOUD patients during and after treatment. They examined cohorts on buprenorphine and methadone separately and reported that retention in either treatment is associated with substantial reductions in the risk for all cause and overdose mortality. Adverse events are common even among patients who discontinued after 6 months of continuous buprenorphine treatment. In the large-scale study of patients with Medicaid, Williams et al. 36 found risks of acute care service use and opioid overdose were high. Almost half the patients were seen in emergency departments at least once, although adverse events diminished with longer time in treatment.
General conclusion--Discontinuing not recommended:
While discontinuing therapy with methadone or buprenorphine/naloxone may be a personal goal for many patients, family members, and addiction treatment staff, there are discouraging odds of completing a taper and remaining abstinent from illicit opioids. Weinstein et al. 37 conclude that, though many patients want to discontinue, few are successful, and the “medical community must work to address any barriers to long-term maintenance.” Robert Newman went further, challenging the significance of attempting to build programs to make patients medication-free when they are already doing well on a maintenance medication. Newman asked, “to what end?” (p.1429). 38
3. With all the problems with tapering, why do people still want to discontinue MOUD?
The vignette below illustrates the factors that fuel the patient’s desire to stop taking buprenorphine/naloxone:
Sam was entering his third inpatient detoxification program since he began using prescription opioids and then heroin 16 years ago. He expressed shame over his relapse and doubts about his ability to remain abstinent. His longest period of abstinence occurred during the two years he was on buprenorphine/naloxone. When asked to describe his life then, he reported being comfortable and stable in his ability to meet work obligations. Under perceived pressure, he discontinued the medication and relapsed to heroin. When he entered private-sector detoxification program, buprenorphine treatment was instituted for detoxification from heroin, and he expressed the desire to taper off buprenorphine by the end of this treatment episode. Asked why, he stated that he had concealed his addiction from his live-in partner, who would strongly disapprove of his being on medication. Treatment interventions during his 30 day stay included education about opioid addiction, buprenorphine/naloxone, and exploration of how his life would change if he could communicate honestly with his partner about this and other issues .
Several misconceptions drive the desire to discontinue opioid medications, which can be addressed in counseling patients who are considering tapering off methadone or buprenorphine-naloxone.
Misconception: Discontinuing medications is necessary:
Stigma 39 , 40 is a powerful force in perpetuating negative attitudes toward opioid medication. It is at its most ferocious with respect to methadone, but similar issues influence attitudes about buprenorphine. Many patients and treatment professionals implicitly or openly view discontinuation as a desirable or necessary goal. As a counter to that idea, treatment staff can point out that both methadone and buprenorphine are dependence-producing medications, a property they share with synthetic thyroid, antidepressants, antipsychotics, antihistamines, blood pressure medications, antiepileptic drugs and others less influenced by stigma.
Misconception: People are not really “clean” if they are on methadone or buprenorphine/naloxone.
A long-held attitude that needs to change is that receiving maintenance opioids reflects an illness, a defect, or moral weakness. 41 With methadone, this stigma is common among patients, e.g. that the medication “takes your heart,” 42 along with numerous misconceptions and myths about methadone. 43 Family members and peers were influenced by the persistent stigma and devalued the patients’ accomplishments if they remained on medications. Patients fear the medication would be detected in employee drug testing and their jobs would be in jeopardy. As a counter to that idea, a counselor can express disagreement with the patient’s initial statement at face value and can probe further to clarify the issues. It is important to encourage them to elaborate, examine their reactions, and reconsider what is in their best interest. A counseling framework of shared decision-making is most likely to increase patient receptivity to cautions from the treatment provider.
Misconception: “If I tried harder I could get off opioid medications”:
This ignores the research suggesting that genetic factors influence vulnerability to opioid addiction 44 and that long-term opioid use alters neurobiological factors in ways that may mean that most of these patients are unlikely to be able to discontinue for extended periods of time.
Misconception: Medications that are easier to taper are better.
Buprenorphine is seen as preferable in this regard. There is no consistent relationship between ease of discontinuation and long-term abstinence. Amato et al. 45 conducted a Cochrane review of 23 studies comparing the use of methadone with other medications aiming to manage opioid withdrawal symptoms. The medications compared with methadone in the 23 reviewed studies included: 1. Other opioid agonists (LAAM (levo-acetyl-methadol), Buprenorphine, propoxyphene, etc); 2. Adrenergic agonists (clonidine, lofexidine, guanfacine); 3. Opioid antagonists (naltrexone, naloxone); and 4. Placebo. They found that, while some methods are superior to others in reducing withdrawal symptoms, research comparing withdrawal methods has not identified any that are associated with long-term abstinence.
4. Optimal Clinical Stance
Despite clinician recommendations based on research, some patients still express a strong desire to taper. It is the authors’ experience doing training and consultation in a variety of treatment settings that counselors and medical providers often do not know how to have the conversation with the patient. It is desirable for treatment providers to maintain a balance between respect for a patient’s choice and realistic feedback on what it will take to succeed no matter what the patient chooses. It is useful for the patient who wishes to remain on medication to have a plan that includes identifying a prescribing medical provider and a counselor to explore how to handle charged situations like peer and family pressure to discontinue. Patients who choose to taper can be asked to use the Recovery Capital Checklist to identify challenges and formulate specific plans for addressing them. Ideally, this includes minimizing stress in other aspects of their lives. This plan should also include signals that tapering is not working and resuming maintenance medication should be considered, preferably with a counselor knowledgeable about opioid addiction. Clinicians should address any sense of failure in patients who have done the recovery work but find themselves unable to taper. They should be encouraged to focus on their achievements in recovery and to focus on the goals that medication can make possible.
5. Recovery Capital and Physician Checklists for Patients and Counselors, and Medical Staff
We introduce the Recovery Capital Checklist as an updated and expanded tool for patients, counselors, and medical providers to identify and address issues related to stable recovery. This guides patients to make their own assessment of whether they have made enough changes to tackle a high risk effort. In the process of discussing the elements on this checklist, some conclude they are not prepared to discontinue their medication. Whatever they decide, the Checklist provides guidance in optimizing the recovery effort, whether or not the patient remains on medication.
The Recovery Capital Checklist is based on an earlier tool The Tapering Readiness Inventory, developed by Sorensen et al in 1987 46 as part of a research study designed to investigate whether enriched psychosocial services could improve outcomes when patients discontinued their medication. This study and many subsequent studies did not find psychosocial interventions predicted long-term success in tapering. However, clinicians have downloaded and shared the original tool many times since 1987. Counselors report it provides a helpful framework for discussion, allowing for a dialogue without endorsing the goal of tapering. The patient and counselor’s section of the checklist specifies elements of emotional growth and life-style changes that increases the potential for stability. It is important for counselors to be clear that no research to date has identified predictive factors for a successful taper, but other goals are more attainable.
The newly created medical provider’s section, the Physician Risk Factor Checklist, indicates warning signs that the patient is likely not stable enough to consider a taper. The goal is to provide a framework for informed consent about the high risk of tapering, recommendations to maximize chances of a good outcome whether the patient remains on medication or not, and to identify areas for future research.
The Recovery Capital Checklist, the section for patients and counselors, is based on updated literature for tapering from either methadone or buprenorphine. We use the term “checklist” to signal that it has points of consideration or reminders in planning, rather than a comprehensive formal catalogue. The Checklist highlights factors that have been associated with a readiness to discontinue methadone or buprenorphine/naloxone. It does not predict success; rather, it helps assess whether the patient is prepared for a high risk endeavor. Having many factors working in one’s favor may suggest that a person has a better chance of attempting a highly stressful endeavor without returning to illegal drugs. Having very few of the factors on one’s side may indicate greater danger of serious relapse. The item on spirituality was added based on many patients’ reports of its importance in their recovery. For example, in her large study of how people in recovery define the key elements, Kaskutas et al. 47 reported strong support for “spirituality of recovery.” It may be a reflection of their participation in 12-Step groups but not necessarily so. Others report drawing strength from meditation, church attendance, and private prayer. Patients without a strong spiritual connection can do well, but it appears that this element is a key part of the support system for many who are struggling to make and sustain progress.
The Physician Risk Factor Checklist, the section intended for medical providers, is new and contains items that should be explored, as they represent factors associated with higher risk of relapse. It contains several indicators of possible drug-seeking behavior, or other signs of instability. It gives the physician a framework to discuss unrealistic expectations in the light of clear warning signs. Specific responses need to be evaluated by the clinician familiar with the individual patient’s history. For example, it is quite possible that prescriptions for anxiolytics or stimulant medications are appropriate, but they need to be closely monitored to identify possible abuse before the patient becomes unstable. We stress that the checklist is based on the authors’ experience and, like the Tapering Readiness Checklist, has not been psychometrically evaluated. There are a variety of published physician-administered screening instruments available to assess risk of opioid diversion. 48
In general, the items in the two sections of the Checklists can promote a fruitful conversation with the patient, potentially offering specific areas that need to be addressed if a taper is under serious consideration.
6. Indications that Maintenance Medications are Needed for Best Results
The Checklists’ sections for patients and their counselors and for medical providers, provide criteria to guide clinicians and may promote future research. Many patients who wish to taper may not be ready to do so. Tapering is highly stressful, and a supportive social network, including family, helps to weather the storms. Although patients do succeed despite unfavorable social conditions, it is certainly desirable to do the recovery work prior to attempting a taper. 49 Coping skills need to be strengthened, and psychological issues such as anxiety or depression need to be addressed.
Clinicians report that patients using alcohol and other drugs such as stimulants have less likelihood of success. Relapse may not occur immediately but can happen weeks or months after using another intoxicant. There is surprisingly little research on the role of non-opioid substances in precipitating relapse to the primary drug of abuse. Many patients who use opioids report extended periods of sobriety, followed by relapse when they use alcohol or stimulants, thinking they will not have problems because it is not their drug of choice. Some state that drugs like marijuana “help” them abstain from opioids, but this claim needs systematic examination. The Recovery Capital and Physician Risk Factor Checklists also call attention to alcohol and other drug use that needs to be discussed.
It is also important that patients be familiar with the triggers and stressors, and have acquired skills to manage them. Various manualized treatments, such as cognitive behavioral therapy, help to develop and consolidate those skills. 50 , 51 It is also important that co-occurring psychiatric disorders, mild are appropriately addressed. Medication alone can be helpful, but it is preferable to also explore how the patient has adapted in the past and what coping strategies might need to change.
7. Limitations
Many gaps remain in the evidence base. Studies use different terminology, interventions, inclusion criteria, follow-up methods, and indices of success. There is a need for greater standardization of methodology when possible, so results can be compared. As noted in the review articles, most studies used very short follow-up windows; most were essentially open single-group follow-up studies with no blinding of participants or staff to dosage or intervention group. Studies with such blinding often had strict admission criteria, lessening their generalizability to the general patient population.
The National Institute of Health’s Helping to End Addiction Long-term (HEAL) initiative 52 includes addressing the question of the optimal time that patients should be on opioid replacement therapy (ORT), and suggest adding to this research initiative what are the predictors of completing tapering from ORT and remaining abstinent from illicit opiates. Connery and Weiss 52 offer a recent summary of some of the outstanding questions in their Editorial in the February 2020 issue The American Journal of Psychiatry , which features studies on this topic.
Little is known about how well patients on buprenorphine do without the relatively firm requirements of the methadone treatment system. What percent have discontinued on their own, with what result? Most importantly, can we identify factors that predict long-term abstinence from opioids and other substances for both methadone and buprenorphine/naloxone?
Studies of the workforce, particularly counselors, would be timely. How well do they understand opioid medications? Are physicians clear on how to train the counselors to discuss meds with their patients? Do either of groups have skills beyond instruction (e.g., “it is too risky to go off medication”) to help patients work through their resistance? These aforementioned areas are implementation issues, and far too little is spent understanding these barriers to effective care.
8. Conclusions
Individuals who use opioids are at historically high risk for overdose or other negative consequences, likely related to the emergence of the synthetic fentanyl analogues. Thus, it is important for medication decisions to be made carefully, with full knowledge of the high risks. Many patients on methadone or buprenorphine grow comfortable and put less than optimal effort into improving their psychosocial functioning and making life-style changes. Even for those who do make such changes, success without medication is unpredictable. We offer the Recovery Capital and Physician Risk Factor Checklists as frameworks to examine and address these issues within a process of shared decision-making. The goal is for patients to focus on what they have achieved and what remains to be done, independent of whether they remain on medication.
The Recovery Capital Checklist (Patients and Counselors Section)
| 1. | Have you been abstaining from illegal drugs, such as heroin, cocaine, and speed? | Yes | No |
| 2. | Do you think you are able to cope with difficult situations without using drugs? | Yes | No |
| 3. | Are you employed or in school? | Yes | No |
| 4. | Are you staying away from contact with users and illegal activities? | Yes | No |
| 5. | Have you gotten rid of your drug paraphernalia? | Yes | No |
| 6. | Are you living in a neighborhood that doesn’t have a lot of drug use? | Yes | No |
| 7. | And are you comfortable there? | Yes | No |
| 8. | Do you have nonuser friends that you spend time with? | Yes | No |
| 9. | Are you living in a stable household or family? | Yes | No |
| 10. | Do you have friends or family who would be helpful to you during a taper? | Yes | No |
| 11. | Do you have a spiritual practice? | Yes | No |
| 12. | Have you been participating in counseling that has been helpful? | Yes | No |
| 13. | Does your counselor think you are ready to taper? | Yes | No |
| 14. | Do you think you would ask for help when you are feeling bad during a taper? | Yes | No |
| 15. | Are you in good mental and physical health? | Yes | No |
| 16. | Do you want to get off methadone or buprenorphine? | Yes | No |
The purpose of this section of the Checklist is to help patients and counselors to decide if the patient is ready to taper or discontinue from MOUD at this time, Each item represents an important part of the process of being ready to discontinue MOUD.
The more questions that can honestly be answered “yes,” the greater the likelihood that the patient is ready to taper from opioid medication. Consider that each “no” response represents an area that the patient and counselor probably need to work on to increase the odds of a successful taper and recovery. Circle the appropriate response.
Physician Risk Factor Checklist (Medical Providers Section)
| 1. | Any unexpected findings on POMP | Yes | No |
| 2. | Frequent emergency department visits/minor injuries/MVCs | Yes | No |
| 3. | Recently appeared intoxicated/impaired | Yes | No |
| 4. | Increased dose without authorization | Yes | No |
| 5. | Needed to take medications belonging to someone else | Yes | No |
| 6. | Patient or others worried about how patient is handling medications | Yes | No |
| 7. | Had to make an emergency phone call or go to the clinic without an appointment | Yes | No |
| 8. | Used pain medication for symptoms other than pain—sleep, mood, stress relief | Yes | No |
| 9. | Changed route of administration | Yes | No |
| 10. | Serious co-morbid mental illness | Yes | No |
| 11. | Recent requests for early refills | Yes | No |
| 12. | Recent reports of lost or stolen prescriptions | Yes | No |
| 13. | Hoarding or stockpiling of medications | Yes | No |
| 14. | Increasingly unkempt | Yes | No |
| 15. | Attempted to obtain prescriptions from other doctors | Yes | No |
| 16. | Concurrent benzodiazepine prescriptions | Yes | No |
| 17. | Concurrent stimulant prescription | Yes | No |
| 18. | Maintenance dose greater than 8 mg or buprenorphine or 80 mg methadone | Yes | No |
| 19. | Current reports of disturbances in sleep | Yes | No |
| 20. | Current reports of problems or lability in mood or energy | Yes | No |
The purpose of this section of the Checklist is to help medical providers to assess potential signs or barriers that may lessen the patient’s likelihood of being able to succeed with a taper or discontinuation of MOUD.
Acknowledgements:
The authors are grateful for editorial comments and suggestions from colleagues David Kan, MD, Laurel Koepernik, MPA, Jack McCarthy, MD, Lisa Najavits, PhD, and Laurie Wermuth, PhD.
Sources of Support
This paper was supported in part by the second author’s Fulbright-Canada Fellowship while at the University of Calgary, Calgary, Alberta, Canada. Additional support through NIH U10DA15815, R25DA028567, and R25DA035163.
Contributor Information
Joan E. Zweben, Clinical Professor of Psychiatry, University of California, San Francisco, Staff Psychologist, San Francisco VA Medical Center.
James L. Sorensen, Department of Psychiatry and Behavioral Sciences, Zuckerberg San Francisco General Hospital, University of California, San Francisco.
Mallory Shingle, Department of Psychiatry and Behavioral Sciences, Zuckerberg San Francisco General Hospital, University of California, San Francisco.
Christopher K. Blazes, Vista Taos Drug and Alcohol Rehabilitation Center, Taos, New Mexico.
Loading metrics
Open Access
Peer-reviewed
Research Article
Clinical outcomes of chikungunya: A systematic literature review and meta-analysis
Roles Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft
* E-mail: [email protected]
Affiliation Asc Academics B.V., Groningen, Netherlands
Roles Conceptualization, Writing – original draft
Affiliations Valneva Austria GmbH, Vienna, Austria, Department of Health Sciences, University Medical Center Groningen, Groningen, Netherlands
Roles Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing
Affiliations Asc Academics B.V., Groningen, Netherlands, Department of Health Sciences, University Medical Center Groningen, Groningen, Netherlands
Roles Investigation, Project administration
Roles Data curation, Investigation
Roles Conceptualization, Supervision
Affiliation Valneva Austria GmbH, Vienna, Austria
Roles Supervision
Affiliations Department of Health Sciences, University Medical Center Groningen, Groningen, Netherlands, Department of Economics, Econometrics & Finance, University of Groningen, Faculty of Economics & Business, Groningen, The Netherlands, Center of Excellence for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia, Division of Pharmacology and Therapy, Faculty of Medicine Universitas Airlangga, Surabaya, Indonesia
Affiliations Asc Academics B.V., Groningen, Netherlands, Department of Health Sciences, University Medical Center Groningen, Groningen, Netherlands, Department of Health Technology and Services Research, University of Twente, Enschede, The Netherlands
- Kris Rama,
- Adrianne M. de Roo,
- Timon Louwsma,
- Hinko S. Hofstra,
- Gabriel S. Gurgel do Amaral,
- Gerard T. Vondeling,
- Maarten J. Postma,
- Roel D. Freriks

- Published: June 7, 2024
- https://doi.org/10.1371/journal.pntd.0012254
- Peer Review
- Reader Comments
This is an uncorrected proof.
Chikungunya is a viral disease caused by a mosquito-borne alphavirus. The acute phase of the disease includes symptoms such as fever and arthralgia and lasts 7–10 days. However, debilitating symptoms can persist for months or years. Despite the substantial impact of this disease, a comprehensive assessment of its clinical picture is currently lacking.
We conducted a systematic literature review on the clinical manifestations of chikungunya, their prevalence and duration, and related hospitalization. Embase and MEDLINE were searched with no time restrictions. Subsequently, meta-analyses were conducted to quantify pooled estimates on clinical outcomes, the symptomatic rate, the mortality rate, and the hospitalization rate. The pooling of effects was conducted using the inverse-variance weighting methods and generalized linear mixed effects models, with measures of heterogeneity reported.
The systematic literature review identified 316 articles. Out of the 28 outcomes of interest, we were able to conduct 11 meta-analyses. The most prevalent symptoms during the acute phase included arthralgia in 90% of cases (95% CI: 83–94%), and fever in 88% of cases (95% CI: 85–90%). Upon employing broader inclusion criteria, the overall symptomatic rate was 75% (95% CI: 63–84%), the chronicity rate was 44% (95% CI: 31–57%), and the mortality rate was 0.3% (95% CI: 0.1–0.7%). The heterogeneity between subpopulations was more than 92% for most outcomes. We were not able to estimate all predefined outcomes, highlighting the existing data gap.
Chikungunya is an emerging public health concern. Consequently, a thorough understanding of the clinical burden of this disease is necessary. Our study highlighted the substantial clinical burden of chikungunya in the acute phase and a potentially long-lasting chronic phase. Understanding this enables health authorities and healthcare professionals to effectively recognize and address the associated symptoms and raise awareness in society.
Author summary
Chikungunya disease is an emerging public health concern. The disease is transmitted by mosquitoes and characterized by arthralgia and fever in the acute phase, lasting 7–10 days. Additionally, some individuals experience chronic symptoms such as arthralgia and tiredness that can last from months to years. Chikungunya is mainly present in the Americas and Asian countries, but the mosquitoes transmitting the disease are spreading to other regions due to climate change, amongst others. This increased disease threat highlights the importance of understanding chikungunya symptoms. However, there are currently no precise estimates on the prevalence of chikungunya symptoms. Therefore, we analysed the available literature on the clinical manifestations of chikungunya. We found that 75% of infected people develop symptoms, primarily characterized by arthralgia in 90% and fever in 88% of cases. Chronic symptoms affect 44% of symptomatic people, and 0.3% of patients with chikungunya die. Unfortunately, we were not able to estimate all predefined outcomes of interest because we did not find enough studies publishing on some of these, demonstrating that there is still much unknown around the clinical manifestations of chikungunya. However, the results can help healthcare workers early identifying chikungunya and raise awareness of this debilitating disease.
Citation: Rama K, de Roo AM, Louwsma T, Hofstra HS, S. Gurgel do Amaral G, Vondeling GT, et al. (2024) Clinical outcomes of chikungunya: A systematic literature review and meta-analysis. PLoS Negl Trop Dis 18(6): e0012254. https://doi.org/10.1371/journal.pntd.0012254
Editor: Richard A. Bowen, Colorado State University, UNITED STATES
Received: February 26, 2024; Accepted: May 28, 2024; Published: June 7, 2024
Copyright: © 2024 Rama et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting information files.
Funding: This paper was funded by Valneva Austria GmbH. AMR and GTV are Valneva employees. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: I have read the journal’s policy and the authors of this manuscript have the following competing interests: KR, TL, HSH, and GSG are employees of Asc Academics. Asc Academics has received consultancy fees for this project from Valneva Austria GmbH. AMR and GTV are Valneva employees and own stock options of Valneva. MJP reports grants and honoraria from various pharmaceutical companies, including those developing, producing, and marketing vaccines. He holds stocks in Health-Ecore (Zeist, Netherlands) and PAG BV (Groningen, Netherlands), and advises ASC Academics (Groningen, Netherlands). These competing interest will not alter adherence to PLOS policies on sharing data and materials.
Introduction
Chikungunya is a viral disease caused by a mosquito-borne alphavirus, the chikungunya virus (CHIKV) [ 1 ]. The infection is characterized by an acute phase with symptoms including fever, arthralgia, and myalgia. While most infected individuals fully recover after the acute phase of the disease, between 30–40% of patients develop persistent symptoms, such as chronic arthritis, fatigue, stiffness, depression, and sleep and neurological disorders, which can last from months to several years [ 2 , 3 ]. Long-term effects lead to significant limitations in daily activities and reduce the patients’ overall quality of life [ 4 – 6 ]. Nevertheless, despite the negative impact of the disease on the quality of life, the awareness and societal understanding of chikungunya remain limited, even among the afflicted populations and healthcare workers [ 7 ]. Chikungunya has been identified as a public health threat based on several records of CHIKV outbreaks worldwide, with a risk of exacerbation in the future due to the global spread of CHIKV [ 8 ]. The distribution of the CHIKV vectors ( Aedes aegypti and Aedes albopictus ) is one of the main factors contributing to the disease’s dissemination. This expansion is attributed to globalization and climate change, allowing the Aedes mosquitos to survive in areas previously considered unsuitable [ 9 , 10 ]. Prevention against the disease consists predominantly of mosquito population control [ 11 ]. Recently, the FDA approved the first chikungunya vaccine, presenting a new tool to fight the disease and potentially alleviate the associated economic and health burdens [ 12 ]. Despite the increasing interest in CHIKV and the recent announcement of a vaccine, uncertainties persist regarding the clinical burden of chikungunya. Although multiple studies have explored one or more health outcomes associated with chikungunya [ 3 , 13 , 14 ], to the best of our knowledge, no extensive meta-analysis was performed to quantify pooled estimates on the clinical presentation of chikungunya. To address this gap, we conducted a comprehensive systematic literature review (SLR) on the clinical manifestations of chikungunya, and proceeded with a robust yet flexible meta-analysis. This approach allowed us to provide estimates on a broad spectrum of endpoints on the health outcomes of chikungunya. We paid particular attention to the symptomatic, mortality, and chronicity rates for a comprehensive understanding of the disease in both acute and chronic phases. Our study aims to contribute valuable insights into the overall clinical outcomes of chikungunya. This, in turn, can inform public health intervention strategies and enhance global surveillance by enabling earlier detection of outbreaks.
Literature search and study selection
The SLR adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) guidelines, with searches conducted on MEDLINE In-Process via PubMed.com, and Embase via Embase.com without time limits. Grey literature searches were performed for the years 2019–2023 to capture data that may not have yet been included in the databases. The search string included terms related to chikungunya and study design. Eligibility criteria were developed using a Population, Intervention, Comparator, Outcomes, Study (PICOS) framework. The inclusion criteria focused on the clinical manifestations of chikungunya, their prevalence and duration, and related hospitalization, and excluded in vitro/preclinical studies, reviews, and non-English articles. Specifics can be found in S1 Text .
Screening and data extraction
All retrieved articles were deduplicated and titles and abstracts were screened against the PICOS criteria using Rayyan. From the selected articles, full texts were examined for eligibility, followed by detailed data extraction organized by study design, patient characteristics, and outcomes of interest. The whole screening process was conducted by two independent reviewers (GG, HH), resolving conflicts through consensus. An exhaustive feasibility assessment ensured the inclusion of studies with explicit criteria and comparable reporting methods, reducing heterogeneity and potential outlier influence. The risk of bias was determined using a modified Downs and Black checklist [ 15 ] and NIH quality assessment tool for observational studies [ 16 ], see S2 Text . Discrepancies were resolved by consensus. No protocol for this systematic review and meta-analysis was registered.
Population and data analysis
The meta-analysis was performed using the meta package of the R statistical software to create a pooled estimate of the most important clinical outcomes of chikungunya. The outcomes of interest were the overall symptomatic, chronicity, and mortality rates, the underreporting factor, the duration of the acute and chronic phase, the hospitalization and outpatient rate (acute and chronic), the mortality rate per region, and the rate and duration of the following symptoms: arthralgia, arthritis, fatigue, fever, headache, joint swelling, myalgia, nausea, rash, and vomiting. The distinction between arthralgia and arthritis was made based on the definition used in the original study.
Both fixed-effects and random-effects models with logit transformation were estimated, where a random-effects model was chosen in case of high heterogeneity. Fixed-effects meta-analyses employed inverse-variance weighting, while random-effects accounted for between-study heterogeneity and are better suited to account for the larger variations in outcomes reported. Heterogeneity was assessed using Cochran’s Q , I 2 , H 2 statistics, and τ 2 estimation. Outlier analyses employed the leave-one-out method, Baujat plots, and statistical distance measures. All results were visually represented using forest plots, providing a clear and concise graphical representation of the individual study findings and the overall meta-analysis result.
Our study utilized subpopulations—subsets of the original populations defined by particular demographic and clinical features. These features correspond to the data reported in the studies we analyzed and the segmentation into subpopulations was based on the inclusion or exclusion criteria set forth in the original research papers. This approach allowed us to perform a more granular analysis. The clinical outcomes of interest were analyzed for a target population to ensure comparability among included studies, which excluded children under 15, individuals with comorbidities or concurrent infections, and pregnant women. Additionally, we excluded unconfirmed CHIKV cases and studies involving chronic patients reporting on the acute phase due to recall bias. Lastly, retrospective studies focusing on mortality were excluded as they exhibited evidence of selection bias. Meta-analyses were performed when an endpoint was reported at least five times for a given subpopulation.
A preliminary search indicated that data on chronicity, mortality, and symptomatic cases was predominantly reported for a more general population, including individuals under the age of 15 and chronic patients. Therefore, we decided to apply less strict criteria on the studies reporting these outcomes, allowing us to estimate these endpoints. Additionally, to detail the development of chronic symptoms, we estimated the chronic rate at various points from disease onset by dividing studies reporting on chronicity rates following a CHIKV infection into subgroups based on time intervals (three, six, and 12 months). The inclusion criteria for each subgroup were to fall within the time windows created by consecutive intervals (e.g., 90–180 days for three months). We excluded studies extending beyond 24 months to avoid a selection bias, as these already focused on patients with pre-existing chronic conditions.
For the mortality rates, we separated the groups that reported outcomes for high-risk populations from those dealing with the general population with lower risk. This stratification allowed us to account for potential confounding variables. Older age and comorbidities have been identified to increase the risk for mortality [ 2 , 17 ]. Therefore, we classify as high-risk of mortality the groups with a minimum age over 65 (or median above 70 when missing), and previous conditions that induced prior intensive care exceeding 24 hours.
To estimate the overall symptomatic rate, we included studies that explicitly reported symptomatic rates based on one or more of the symptoms commonly associated with the disease. Symptomatic patients were often an implicit inclusion criteria, or a precondition for laboratory testing, making most of the studies reporting on the symptomatic rate unusable. We excluded the studies that had a 100% symptomatic rate to prevent selection bias, as including those would lead to a skewed perspective due to symptoms being part of their inclusion criteria.
Literature search
The SLR was conducted on 4 July 2023 and yielded 16,308 hits. After removing 6,285 duplicates, 10,023 studies were screened by titles and abstracts. From these, a total of 316 articles were deemed suitable for inclusion. The process of the SLR is detailed in Fig 1 , which illustrates the PRISMA diagram of the included studies. The complete PRISMA checklist is provided in S3 Text . The quality assessment of included studies can be found in S1 Table .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pntd.0012254.g001
Study characteristics
The categorization of study designs in the included articles was made with careful consideration, taking into account the diversity in how these studies defined their methodologies. The judgment used in categorizing these studies was guided by the definitions provided within the paper itself. When a study described its design in a way that matched more than one predefined category, the predominant one was chosen. This approach aimed to respect the original terminology used by the study authors while also creating a coherent framework for analysis.
Of the 316 articles included, 231 studies were observational, 11 were experimental, and for 74 studies this was not reported. Of the observational studies, 106 were cross-sectional, 35 were cohort, 29 were longitudinal, 25 were retrospective, 23 were prospective, and 13 were case-control or case-series studies. Of the experimental studies, there were 6 trials from phase I to III with double and single-blind designs. Goals ranged from assessing treatments like chloroquine and vaccines’ effectiveness to exploring seroprevalence and chronic CHIKV effects. Two trials investigated new mRNA treatment mechanisms. The focus was solely on CHIKV, not on coinfections.
The study location varied: Southern Asia was the most represented with 78 articles, followed by South America, with 67. There were 41 articles from The Caribbean region, 41 from Eastern Africa, 28 from South-Eastern Asia, 14 from Central America. Eight, six, five, four, and three articles were from Western Europe, Northern America, Southern Europe, Middle Africa, and Western Africa, respectively. Two or one articles were from Eastern Asia, Micronesia, Northern Europe, or Southern Africa. A total of 193 studies reported mean or median age. Data on co-infection with Zika and/or dengue were reported in 11 studies. An overview of the study characteristics, including details on the experimental studies, can be found in S2 Table .
The most commonly reported symptom was fever, reported in 57.9% of the studies (N = 183), followed by rash in 54.1% (N = 171), headache in 51.3% (N = 162), and arthralgia in 47.8% (N = 151). Most studies reported high rates (70% to 100%) of fever. Among the 151 studies reporting arthralgia rates, the symptom prevalence ranged from 1% to 100%, as studies presented heterogeneous settings, including, for example, recovered patients, patients in the acute phase, or chronic patients. Duration of symptoms was reported in 22 studies. Taking all symptoms into account, the mean duration of symptoms ranged from two days (fever) to 342 days (arthralgia). It is important to note that the studies presented heterogeneous groups of patients when reporting on the duration of symptoms, which could explain the wide range reported in literature. The hospitalization rate was reported by 53 studies. The hospitalization rate varied between 0%, reported by five different studies [ 18 – 22 ], and 93% in a study by Reller and colleagues [ 23 ].
The development of chronic disease after CHIKV infection was reported in 68 studies. Most studies defined chronic CHIKV infection as the presence of symptoms three months after the infection. Arthralgia was reported as a chronic symptom in 67 studies, joint swelling was reported in 11 studies, myalgia was reported in eight studies, stiffness, especially in the morning, was reported in six studies, and arthritis was reported in four studies. The percentage of patients developing chronic disease ranged from 16% in a study conducted during an outbreak in Chennai, India [ 24 ] to 100% in two other studies [ 25 , 26 ]. Fifty of the included studies reported data on mortality, of which 22 reported no deaths in the study population. The highest reported mortality rate was 36.67%, or 36,670 per 100,000 population, reported by Gupta and colleagues. This study population consisted of chikungunya patients who had been admitted to the intensive care [ 27 ].
Meta-analyses feasibility and results
From the 316 articles retrieved from the SLR, we extracted 756 distinct subpopulations. Each subpopulation corresponds to a group defined by a unique set of inclusion and exclusion criteria as per the definitions provided in each original study. Out of the 756 subpopulations, 335 were used for the analysis of the general population, while 52 where used for the target population. From the 28 selected clinical outcomes, we were able to conduct 11 meta-analyses for the target population, see Fig 2 . The number of studies and subpopulations available for each endpoint is shown in Table 1 . The forest plots from the individual meta-analyses can be found in S1 Fig , and the outlier analysis for each endpoint with the Baujat plot is presented in S2 Fig . No studies or subpopulations were excluded based on outlier analyses. Below, we present the 11 estimates from the meta-analyses on the target population, followed by the results of the analysis on mortality, chronicity, and overall symptomatic rates in the general population.
https://doi.org/10.1371/journal.pntd.0012254.g002
Presented are the number of studies and number of subpopulations reporting on the specific outcomes, the pooled estimates, confidence intervals and I 2 of the estimated endpoints. CI: confidence interval. I 2 : I-squared statistic of heterogeneity.
https://doi.org/10.1371/journal.pntd.0012254.t001
Chikungunya symptoms estimates in the target population.
The prevalence of arthralgia in symptomatic adults with confirmed chikungunya was estimated at 89.7%, while arthritis was less frequent at 17.6%. Fatigue was observed in 56% of patients, fever in 87.8%, and headache affected 49.5% of the population. Joint swelling was reported in 50% of patients, myalgia in 62.9%, nausea in 34.7%, rash in 44.3%, and vomiting in 17.1%. The hospitalization rate during the acute phase of chikungunya was reported by nine subpopulations and estimated at 17%. All results are presented in Table 1 , showing the pooled effect estimate for each symptom, reflecting the average rate of occurrence in the studied populations within specified confidence intervals. Each symptom analysis showed substantial heterogeneity between subpopulations, indicated by high I 2 statistics.
Chronicity, mortality, and overall symptomatic rate.
The meta-analysis for chronicity rate showed declining rates over time: 43.89% at three months, 34.39% at six months, and 31.87% at twelve months, see Fig 3 . Notably, persistent high heterogeneity was observed across subgroups ( I 2 between 96–97%). Mortality rates were estimated at 0.32% (320 per 100,000 population), for normal-risk populations and 15.34% (15,340 per 100,000 population) for high-risk populations, see Fig 4 . The latter displayed higher heterogeneity ( I 2 = 97%) compared to the normal risk ( I 2 = 87%). The meta-analysis estimates that 74.9% of the general population with CHIKV infection were symptomatic, with a 95% confidence interval from 63% to 84%, see Fig 5 . A total of eight studies with corresponding eight subgroups were included in this analysis. I 2 statistics showed a heterogeneity of 91%. Results of the outlier and influential cases analysis can be found in S2 Fig .
https://doi.org/10.1371/journal.pntd.0012254.g003
https://doi.org/10.1371/journal.pntd.0012254.g004
https://doi.org/10.1371/journal.pntd.0012254.g005
Chikungunya poses an emerging global health threat; however, uncertainties around the health burden of this infectious disease persist. This SLR and meta-analyses aim to consolidate existing research on the clinical manifestations of chikungunya. The objective of this study was to provide accurate estimates on the symptomatology of this disease, with a specific focus on the chronicity, mortality, and overall symptomatic rates. Overall, our findings emphasize the substantial disease burden associated with a CHIKV infection.
Arthralgia, fever, and myalgia were the most prevalent symptoms, affecting 89.7%, 87.8%, and 62.9% of symptomatic adults, respectively. These symptoms are also described in previous literature as most common for chikungunya [ 17 , 28 ]. It’s important to note that these symptoms were often implicitly used when initially detecting suspected cases. Although we removed all explicit inclusion criteria, these estimates are likely affected by selection bias. The hospitalization rate of 17% underscores the challenges for healthcare systems during outbreaks. The disease burden related to these symptoms makes chikungunya a significant burden for local healthcare systems, highly influencing the quality of life of infected individuals [ 6 ].
The number of studies that provided data on mortality, chronicity, and overall symptomatic rate was limited for the target adult population. Thus, we decided to use less restrictive population criteria for these specific outcomes. Within this broader general population, we found a 0.32% (320 per 100,000 population) mortality rate in the low-risk group. This is slightly higher than the common reported case-fatality rate of 0.1% (100 per 100,000 population), although reports on mortality rated may vary [ 2 , 6 ]. To our knowledge, no previous meta-analysis on mortality rates has been performed. Therefore, we argue that 0.32% (320 per 100,000 population) is a realistic estimate for the general population. While this percentage is still relatively low compared to other arboviruses [ 29 ], mortality rates can be drastically higher in high-risk groups. Our meta-analysis revealed a mortality rate of 15.34% (15,340 per 100,000 population) in elderly and individuals with previous emergency department or intensive care admissions.
In defining the high-risk group for mortality, we included hospitalized patients who are typically older. As a result, the average age within this group was higher and advanced age is a recognized risk factor for increased mortality from CHIKV infection [ 30 ]. The task of separating the effects of comorbid conditions from the direct impact of CHIKV on mortality rates is complex. These factors often interact with each other, complicating the attribution of cause of death to CHIKV alone—particularly when our analysis could not conclusively establish the causes listed on death certificates. Furthermore, we recognize the possibility of publication bias in existing research on severe CHIKV cases. There may be an overrepresentation of studies focusing on severe outcomes and elevated mortality rates among individuals with underlying health complications or atypical presentations of CHIKV. Such a bias could lead to an overestimation of the mortality risk associated with the virus. Nonetheless, our SLR showed mortality rates up to 36.67% (36,670 per 100,000 population) in specific populations, demonstrating that despite its low rates in the general population, the impact of mortality should not be overlooked [ 27 ].
The chronic phase of chikungunya can be debilitating and long-lasting, leading to a significant health burden for individuals affected. Results from our meta-analysis showed a chronicity rate of 43.89% at three months, 34.39% at six months, and 31.87% at 12 months post-infection, indicating the lasting health burden. A meta-analysis conducted by Paixao and colleagues on the chronicity rate of chikungunya showed similar outcomes, with an overall no-recovery rate of 43% after three months [ 3 ]. One notable difference, possibly due to variations in methodologies, is that Paixao and colleagues reported slightly lower rates over time. Both studies indicate a stabilization over time, but more research is needed to comprehensively map the progression of the chronic phase. In conclusion, long-term chronic illness majorly impacts the quality of life of chikungunya patients [ 4 , 6 ], making these results alarming, especially in light of the potential growing spread of the disease [ 9 , 10 ].
The significant disease burden related to chikungunya was further underlined by an overall symptomatic rate of 74.9% in the general population. The symptomatic rate of chikungunya was estimated between 72% and 97% by the CDC Yellow Book, showing that our estimate could be on the low end [ 17 ]. A reason for this could be the various definitions of symptomatic manifestations across studies, which posed a challenge in deriving a precise estimate for this outcome. Additionally, estimates in the literature are mainly based on patients showing healthcare-seeking behaviour, leaving out asymptomatic patients. Therefore, these estimates are likely to be overestimated. Because we created our estimate based on the total general population, we expect them to provide a better reflection of reality.
Two studies identified in the SLR were designed to investigate treatment options for Chikungunya and therefore included control groups. However, we excluded control populations without confirmed CHIKV from our analysis because our focus was on populations with confirmed CHIKV. In instances where multiple treatment options were assessed among confirmed CHIKV populations, these groups were included in the analysis as we aimed to understand the symptomatology of the disease at presentation in its acute phase. It should be noted that the inclusion of these populations did not significantly influence the outcomes of our study since the primary interest was in the manifestation of symptoms rather than treatment efficacy.
Although we obtained estimates for 11 of the 28 predefined endpoints, estimation for several endpoints proved infeasible due to their infrequent reporting as identified in the SLR. We did not obtain estimates for the underreporting factor, the length of the acute and the chronic phase, the duration of the different symptoms, and the frequencies of hospitalization and outpatient care. Even considering the subpopulation analysis method used, we could not estimate more endpoints. The limited number of studies reflects the uncertainty and novelty associated with chikungunya and the need for more research in this field.
In cases where meta-analyses were feasible for the endpoints, we encountered challenges due to poor data quality or absent data. This is attributable to two main reasons: firstly, the reporting of several endpoints varied inconsistently across studies, preventing their combination in a meta-analysis; and secondly, some studies that reported the desired endpoint did not meet the inclusion criteria, resulting in sparse data that hindered meaningful analysis. As a result, significant knowledge gaps persist regarding various aspects of chikungunya. Further research is necessary to fill these gaps and enhance our understanding of this disease. Additionally, consistent and strict reporting criteria on the clinical picture of chikungunya are needed to help create more comprehensive estimates. Enhanced quality and quantity of data could facilitate the possibility to study potential differences in symptomatology for the different CHIKV subtypes. Furthermore, it could enable investigations into the pathogenicity of CHIKV over the years by comparing data from previous outbreaks.
A strength of our study is the use of subpopulation analyses. We discovered that extracting subpopulations from individual studies allows more endpoints to be estimated, offering comparable populations that limit heterogeneity. The use of subgroups could be useful for future research and mitigate some of the data discrepancies detected in the SLR.
The main limitation of our study is the significant presence of heterogeneity indicated by an average I 2 statistic of 92%. This reflects substantial differences in the inclusion criteria among the studies, a tendency inherent in the disease area of CHIKV as shown by other meta-analyses reporting similar, or even higher, levels of heterogeneity [ 3 ]. There are several reasons for this high heterogeneity. First, data collection on chikungunya is conducted mostly during the outbreaks which limits the possibility of establishing strict scientific protocols as researchers must adapt to the dynamic nature of the event. Secondly, a standardized methodology for reporting endpoints is lacking, making it challenging to compare studies in a meta-analysis. Thirdly, we noticed that including older individuals affected our results, by showing lower symptomatic rates but higher mortality and hospitalization rates. Future studies might exclude this demographic for more precise age-related outcomes. Additionally, other, less known, symptoms might have influenced the disease estimates. An example of this is depressive symptoms related to chikungunya. A study included in our analysis has potentially skewed our meta-analysis results with inflated estimates for fatigue, headache, and rash because they investigated depressive symptoms during the CHIKV infection [ 31 ]. This highlights how undisclosed factors that increase the population’s vulnerability to chikungunya symptoms can potentially impact the research. Another limitation is the potential for confounding factors contributing to symptom prevalence, which we were unable control for in our study. There’s an implicit assumption that the symptoms described have a causal association with Chikungunya; however, some symptoms such as myalgia and fatigue are commonly prevalent in the population and may not be causally related to CHIKV infection. The difficulty in establishing a direct causal relationship between these symptoms and CHIKV should be taken into consideration when interpreting the results. We acknowledge that this could affect the precision of the associations drawn in our analysis and suggest that future research should aim to discern the specific attributable risk of CHIKV for these symptoms. Lastly, outbreaks often occur in locations with limited surveillance systems, leading to lacking or less accurate data from these areas. The high heterogeneity shows the need for additional research in the fields, as well as standardized methodologies in studying chikungunya. Additionally, it emphasizes the importance of meta-analyses like these to come to accurate estimates.
Chikungunya is recognized as a global public health threat, and the disease is expected to spread further due to globalization and climate change. At the same time, vector control and surveillance systems remain limited. Consequently, a thorough understanding of the clinical burden of chikungunya is important to inform public health intervention strategies and improve global surveillance. Our study showed that chikungunya poses a significant health burden, with 74.9% of infected individuals experiencing symptomatic disease. Chronic symptoms are found in 43.4% of patients and can be debilitating and long-lasting. We were not able to create pooled estimates on all endpoints, highlighting the still existing data gap in literature here. Nevertheless, the outcomes determined add to the growing body of evidence underlining the debilitating consequences of chikungunya. With the growing threat of chikungunya, health authorities and healthcare professionals must be prepared to adequately diagnose patients affected by the disease and consider public health interventions to limit its burden. Our findings contribute to the comprehension of chikungunya’s clinical outcomes, essential for improving global surveillance and detecting potential outbreaks.
Supporting information
S1 text. literature search and study selection..
Containing the search strategy and PICOs of the studies included in the SLR.
https://doi.org/10.1371/journal.pntd.0012254.s001
S2 Text. Quality assessment tools.
Modified Downs & Black checklist and the NIH quality assessment tool.
https://doi.org/10.1371/journal.pntd.0012254.s002
S3 Text. PRISMA 2020 checklist.
https://doi.org/10.1371/journal.pntd.0012254.s003
S1 Table. Quality assessment of included studies.
https://doi.org/10.1371/journal.pntd.0012254.s004
S2 Table. Summary of study characteristics.
https://doi.org/10.1371/journal.pntd.0012254.s005
S1 Fig. Forest plots of the clinical outcomes of chikungunya.
https://doi.org/10.1371/journal.pntd.0012254.s006
S2 Fig. Influential case and outlier analysis with Baujat plots.
https://doi.org/10.1371/journal.pntd.0012254.s007
Acknowledgments
We would like to thank the internal teams of Asc Academics who helped during the data extraction phase of the SLR, as well as Roma Kwiatkiewicz from Asc Academics for providing medical writing support.
- View Article
- PubMed/NCBI
- Google Scholar
- 2. European Centre for Disease Prevention and Control [Internet]. Factsheet about chikungunya. [cited 2024 February 20]. Available from: https://www.ecdc.europa.eu/en/chikungunya/facts/factsheet .
- 8. Zavala-Colon M, Gonzalez-Sanchez JA. 2. In: Engohang-Ndong J, editor. History and Geographic Distribution of Chikungunya Virus. Rijeka: IntechOpen; 2022. p. 15–24. Available from: https://doi.org/10.5772/intechopen.98662 .
- 12. Food and Drug Administration [Internet]. FDA Approves First Vaccine to Prevent Disease Caused by Chikungunya Virus. [cited 2024 February 20]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-prevent-disease-caused-chikungunya-virus .
- 16. National Heart, Lung, and Blood Institute [Internet]. Study Quality Assessment Tools. [cited 2024 February 20]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools .
- 17. Centers for Disease Control and Prevention [Internet]. CDC Yellow Book 2024. [cited 2024 February 20]. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/chikungunya .

IMAGES
VIDEO
COMMENTS
Clinical Psychology Review publishes substantive reviews of topics germane to clinical psychology. Papers cover diverse issues including: psychopathology, psychotherapy, behavior therapy, cognition and cognitive therapies, behavioral medicine, community mental health, assessment, and child …. View full aims & scope. $4670.
The purpose of this guide is to provide information and resources for clinical psychology students engaged in writing a literature review.
This systematic review examines the empirical evidence on how clinical psychologists make ethical decisions and identifies the factors that influence their choices.
A literature review differs from a systematic review, which addresses a specific clinical question by combining the results of multiple clinical trials (an article on this topic will follow as part of this series of publications).
Clinical Psychology: Science and Practice ( CPSP) publishes cutting-edge reviews and developments in the science and practice of clinical psychology and related mental health fields. This is accomplished by publishing scholarly articles, primarily involving narrative and systematic reviews, as well as meta-analyses, related to assessment ...
Searching the scientific literature: Implications for quantitative and qualitative reviews. Yelena P. Wu 1, Brandon S. Aylward 1, Michael C. Roberts ⁎, Spencer C. Evans. Clinical Child Psychology Program, University of Kansas, 2010 Dole Human Development Center, 1000 Sunnyside Avenue, Lawrence, KS 66045, USA. H I G H L I G H T S.
LITERATURE REVIEW. no. Reflective practice in clinical psychology: Reflections from basic psychological science. Scott O. Lilienfeld, ... Published on behalf of the Society of Clinical Psychology, Division 12 of the American Psychological Association. More from this journal. News; CPSP Ethics Statement; Wiley Job Network;
AIMS AND SCOPE OF JOURNAL: The Annual Review of Clinical Psychology provides comprehensive reviews of significant developments in the field of clinical psychology and psychiatry. The journal covers research, theory, and the application of psychological principles to address recognized disorders, including schizophrenia, mood, anxiety, childhood, substance use, cognitive, and personality disorders.
Although clinical supervision is considered to be a major component of the development and maintenance of psychotherapeutic competencies, and despite an increase in supervision research, the empirical evidence on the topic remains sparse. Because most previous reviews lack methodological rigor, we aimed to review the status and quality of the empirical literature on clinical supervision, and ...
The aims of a literature review are different from those of an empirical research paper, and hence the skills required differ somewhat as well. The goals of literature reviews are the following (American Psychological Association, 2009): 1. To define and clarify problems. 2. To inform the reader about a subject by summarizing and evaluating ...
The purpose of this guide is to provide information and resources for clinical psychology students engaged in writing a literature review.
The objective of this article is to provide guidance on how to conduct systematic literature review. By surveying publications on the methodology of literature review, we summarize the typology of literature review, describe the procedures for conducting the review, and provide tips to planning scholars.
Writing a Literature Review in Psychology What is a literature review? How is a literature review different from a research article? The two purposes: describe/compare and evaluate Getting started Select a topic and gather articles Choose a current, well-studied, specific topic Search the research literature Read the articles Write the ...
Reflective practice has gained traction in clinical psychology largely to address the fact that practitioners must frequently "use their heads" when scientific data are not readily available. Despite...
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature ...
The purpose of this guide is to provide information and resources for clinical psychology students engaged in writing a literature review.
The first section of your thesis is the literature review. You should choose one of the following formats when writing your literature review: 1. Stand-Alone Paper (SAP) In this type of literature review, a question that is related to the topic of the empirical paper is identified, and then addressed via a systematic search of the literature. 2.
Systematic Review - A type of literature review that answers a focused clinical question; Meta-Analysis - A type of systematic review using statistical methods to combine data from systematic reviews; What is the best way to find literature reviews? PsycINFO - A psychology database with the capacity to limit by Methodology
Within the past few decades, there has been a surge of interest in the investigation of mindfulness as a psychological construct and as a form of clinical intervention. This article reviews the empirical literature on the effects of mindfulness on psychological ...
DOCTORATE IN CLINICAL PSYCHOLOGY Literature Review The Association between Maternal Sensitivity and Child Social and Emotional Development: Trainee Name:Lara Best Supervisors: Laura Miller, Research Associate, University of Bristol. Dr. Rebecca Pearson, Post-doctoral Research Assistant, University of
It was a requirement of the funding team that the research design comprised a literature review, and that the involved and worked collaboratively with young people with lived experience of anxiety and/or depression throughout the course of the project. Members of the funding team provided feedback on an early draft of this manuscript.
Search methodology and article selection. This current article is a mixed-method narrative review of existing literature on mental health disorders, risk factors, and interventions relevant to the COVID-19 pandemic on HCWs in sub-Saharan.
The American Psychological Association (APA) is a scientific and professional organization that represents psychologists in the United States. APA educates the public about psychology, behavioral science and mental health; promotes psychological science and practice; fosters the education and training of psychological scientists, practitioners and educators; advocates for psychological ...
This review addresses the significance of HRR in HF assessment, analyzing recently conducted studies, and providing a foundation for further research and clinical application.
Considering a career in clinical psychology? We outline the latest in master's program requirements, licensure details, and professional opportunities.
Discontinuing Methadone and Buprenorphine: A Review & Clinical Challenges. This paper offers a review and recommendations for clinicians working with patients interested in discontinuing their opioid medication-assisted treatment. As buprenorphine has gained widespread acceptance for opioid addiction, many treatment providers and patients have ...
Methods. We conducted a systematic literature review on the clinical manifestations of chikungunya, their prevalence and duration, and related hospitalization.