- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- When and how to update...
When and how to update systematic reviews: consensus and checklist
- Related content
Peer review
This article has a correction. please see:.
- Errata - September 06, 2016
- Paul Garner , professor 1 ,
- Sally Hopewell , associate professor 2 ,
- Jackie Chandler , methods coordinator 3 ,
- Harriet MacLehose , senior editor 3 ,
- Elie A Akl , professor 5 6 ,
- Joseph Beyene , associate professor 7 ,
- Stephanie Chang , director 8 ,
- Rachel Churchill , professor 9 ,
- Karin Dearness , managing editor 10 ,
- Gordon Guyatt , professor 4 ,
- Carol Lefebvre , information consultant 11 ,
- Beth Liles , methodologist 12 ,
- Rachel Marshall , editor 3 ,
- Laura Martínez García , researcher 13 ,
- Chris Mavergames , head 14 ,
- Mona Nasser , clinical lecturer in evidence based dentistry 15 ,
- Amir Qaseem , vice president and chair 16 17 ,
- Margaret Sampson , librarian 18 ,
- Karla Soares-Weiser , deputy editor in chief 3 ,
- Yemisi Takwoingi , senior research fellow in medical statistics 19 ,
- Lehana Thabane , director and professor 4 20 ,
- Marialena Trivella , statistician 21 ,
- Peter Tugwell , professor of medicine, epidemiology, and community medicine 22 ,
- Emma Welsh , managing editor 23 ,
- Ed C Wilson , senior research associate in health economics 24 ,
- Holger J Schünemann , professor 4 5
- 1 Cochrane Infectious Diseases Group, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK
- 2 Oxford Clinical Trials Research Unit, University of Oxford, Oxford, UK
- 3 Cochrane Editorial Unit, Cochrane Central Executive, London, UK
- 4 Department of Clinical Epidemiology and Biostatistics and Department of Medicine, McMaster University, Hamilton, ON, Canada
- 5 Cochrane GRADEing Methods Group, Ottawa, ON, Canada
- 6 Department of Internal Medicine, American University of Beirut, Beirut, Lebanon
- 7 Department of Mathematics and Statistics, McMaster University
- 8 Evidence-based Practice Center Program, Agency for Healthcare and Research Quality, Rockville, MD, USA
- 9 Centre for Reviews and Dissemination, University of York, York, UK
- 10 Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, Hamilton, ON, Canada
- 11 Lefebvre Associates, Oxford, UK
- 12 Kaiser Permanente National Guideline Program, Portland, OR, USA
- 13 Iberoamerican Cochrane Centre, Barcelona, Spain
- 14 Cochrane Informatics and Knowledge Management, Cochrane Central Executive, Freiburg, Germany
- 15 Plymouth University Peninsula School of Dentistry, Plymouth, UK
- 16 Department of Clinical Policy, American College of Physicians, Philadelphia, PA, USA
- 17 Guidelines International Network, Pitlochry, UK
- 18 Children’s Hospital of Eastern Ontario, Ottawa, ON, Canada
- 19 Institute of Applied Health Research, University of Birmingham, Birmingham, UK
- 20 Biostatistics Unit, Centre for Evaluation, McMaster University, Hamilton, ON, Canada
- 21 Centre for Statistics in Medicine, University of Oxford, Oxford, UK
- 22 University of Ottawa, Ottawa, ON, Canada
- 23 Cochrane Airways Group, Population Health Research Institute, St George’s, University of London, London, UK
- 24 Cambridge Centre for Health Services Research, University of Cambridge, Cambridge, UK
- Correspondence to: P Garner Paul.Garner{at}lstmed.ac.uk
- Accepted 26 May 2016
Updating of systematic reviews is generally more efficient than starting all over again when new evidence emerges, but to date there has been no clear guidance on how to do this. This guidance helps authors of systematic reviews, commissioners, and editors decide when to update a systematic review, and then how to go about updating the review.
Systematic reviews synthesise relevant research around a particular question. Preparing a systematic review is time and resource consuming, and provides a snapshot of knowledge at the time of incorporation of data from studies identified during the latest search. Newly identified studies can change the conclusion of a review. If they have not been included, this threatens the validity of the review, and, at worst, means the review could mislead. For patients and other healthcare consumers, this means that care and policy development might not be fully informed by the latest research; furthermore, researchers could be misled and carry out research in areas where no further research is actually needed. 1 Thus, there are clear benefits to updating reviews, rather than duplicating the entire process as new evidence emerges or new methods develop. Indeed, there is probably added value to updating a review, because this will include taking into account comments and criticisms, and adoption of new methods in an iterative process. 2 3 4 5 6
Cochrane has over 20 years of experience with preparing and updating systematic reviews, with the publication of over 6000 systematic reviews. However, Cochrane’s principle of keeping all reviews up to date has not been possible, and the organisation has had to adapt: from updating when new evidence becomes available, 7 to updating every two years, 8 to updating based on need and priority. 9 This experience has shown that it is not possible, sensible, or feasible to continually update all reviews all the time. Other groups, including guideline developers and journal editors, adopt updating principles (as applied, for example, by the Systematic Reviews journal; https://systematicreviewsjournal.biomedcentral.com/ ).
The panel for updating guidance for systematic reviews (PUGs) group met to draw together experiences and identify a common approach. The PUGs guidance can help individuals or academic teams working outside of a commissioning agency or Cochrane, who are considering writing a systematic review for a journal or to prepare for a research project. The guidance could also help these groups decide whether their effort is worthwhile.
Summary points
Updating systematic reviews is, in general, more efficient than starting afresh when new evidence emerges. The panel for updating guidance for systematic reviews (PUGs; comprising review authors, editors, statisticians, information specialists, related methodologists, and guideline developers) met to develop guidance for people considering updating systematic reviews. The panel proposed the following:
Decisions about whether and when to update a systematic review are judgments made for individual reviews at a particular time. These decisions can be made by agencies responsible for systematic review portfolios, journal editors with systematic review update services, or author teams considering embarking on an update of a review.
The decision needs to take into account whether the review addresses a current question, uses valid methods, and is well conducted; and whether there are new relevant methods, new studies, or new information on existing included studies. Given this information, the agency, editors, or authors need to judge whether the update will influence the review findings or credibility sufficiently to justify the effort in updating it.
Review authors and commissioners can use a decision framework and checklist to navigate and report these decisions with “update status” and rationale for this status. The panel noted that the incorporation of new synthesis methods (such as Grading of Recommendations Assessment, Development and Evaluation (GRADE)) is also often likely to improve the quality of the analysis and the clarity of the findings.
Given a decision to update, the process needs to start with an appraisal and revision of the background, question, inclusion criteria, and methods of the existing review.
Search strategies should be refined, taking into account changes in the question or inclusion criteria. An analysis of yield from the previous edition, in relation to databases searched, terms, and languages can make searches more specific and efficient.
In many instances, an update represents a new edition of the review, and authorship of the new version needs to follow criteria of the International Committee of Medical Journal Editors (ICMJE). New approaches to publishing licences could help new authors build on and re-use the previous edition while giving appropriate credit to the previous authors.
The panel also reflected on this guidance in the context of emerging technological advances in software, information retrieval, and electronic linkage and mining. With good synthesis and technology partnerships, these advances could revolutionise the efficiency of updating in the coming years.
Panel selection and procedures
An international panel of authors, editors, clinicians, statisticians, information specialists, other methodologists, and guideline developers was invited to a two day workshop at McMaster University, Hamilton, Canada, on 26-27 June 2014, organised by Cochrane. The organising committee selected the panel (web appendix 1). The organising committee invited participants, put forward the agenda, collected background materials and literature, and drafted the structure of the report.
The purpose of the workshop was to develop a common approach to updating systematic reviews, drawing on existing strategies, research, and experience of people working in this area. The selection of participants aimed on broad representation of different groups involved in producing systematic reviews (including authors, editors, statisticians, information specialists, and other methodologists), and those using the reviews (guideline developers and clinicians). Participants within these groups were selected on their expertise and experience in updating, in previous work developing methods to assess reviews, and because some were recognised for developing approaches within organisations to manage updating strategically. We sought to identify general approaches in this area, and not be specific to Cochrane; although inevitably most of the panel were somehow engaged in Cochrane.
The workshop structure followed a series of short presentations addressing key questions on whether, when, and how to update systematic reviews. The proceedings included the management of authorship and editorial decisions, and innovative and technological approaches. A series of small group discussions followed each question, deliberating content, and forming recommendations, as well as recognising uncertainties. Large group, round table discussions deliberated further these small group developments. Recommendations were presented to an invited forum of individuals with varying levels of expertise in systematic reviews from McMaster University (of over 40 people), widely known for its contributions to the field of research evidence synthesis. Their comments helped inform the emerging guidance.
The organising committee became the writing committee after the meeting. They developed the guidance arising from the meeting, developed the checklist and diagrams, added examples, and finalised the manuscript. The guidance was circulated to the larger group three times, with the PUGs panel providing extensive feedback. This feedback was all considered and carefully addressed by the writing committee. The writing committee provided the panel with the option of expressing any additional comments from the general or specific guidance in the report, and the option for registering their own view that might differ to the guidance formed and their view would be recorded in an annex. In the event, consensus was reached, and the annex was not required.
Definition of update
The PUGs panel defined an update of a systematic review as a new edition of a published systematic review with changes that can include new data, new methods, or new analyses to the previous edition. This expands on a previous definition of a systematic review update. 10 An update asks a similar question with regard to the participants, intervention, comparisons, and outcomes (PICO) and has similar objectives; thus it has similar inclusion criteria. These inclusion criteria can be modified in the light of developments within the topic area with new interventions, new standards, and new approaches. Updates will include a new search for potentially relevant studies and incorporate any eligible studies or data; and adjust the findings and conclusions as appropriate. Box 1 provides some examples.
Box 1: Examples of what factors might change in an updated systematic review
A systematic review of steroid treatment in tuberculosis meningitis used GRADE methods and split the composite outcome in the original review of death plus disability into its two components. This improved the clarity of the reviews findings in relation to the effects and the importance of the effects of steroids on death and on disability. 11
A systematic review of dihydroartemisinin-piperaquine (DHAP) for treating malaria was updated with much more detailed analysis of the adverse effect data from the existing trials as a result of questions raised by the European Medicines Agency. Because the original review included other comparisons, the update required extracting only the DHAP comparisons from the original review, and a modification of the title and the PICO. 12
A systematic review of atorvastatin was updated with simple uncontrolled studies. 13 This update allowed comparisons with trials and strengthened the review findings. 14
Which systematic reviews should be updated and when?
Any group maintaining a portfolio of systematic reviews as part of their normative work, such as guidelines panels or Cochrane review groups, will need to prioritise which reviews to update. Box 2 presents the approaches used by the Agency for HealthCare Research and Quality (AHRQ) and Cochrane to prioritise which systematic reviews to update and when. Clearly, the responsibility for deciding which systematic reviews should be updated and when they will be updated will vary: it may be centrally organised and resourced, as with the AHRQ scientific resource centre (box 2). In Cochrane, the decision making process is decentralised to the Cochrane Review Group editorial team, with different approaches applied, often informally.
Box 2: Examples of how different organisations decide on updating systematic reviews
Agency for healthcare research and quality (us).
The AHRQ uses a needs based approach; updating systematic reviews depends on an assessment of several criteria:
Stakeholder impact
Interest from stakeholder partners (such as consumers, funders, guideline developers, clinical societies, James Lind Alliance)
Use and uptake (for example, frequency of citations and downloads)
Citation in scientific literature including clinical practice guidelines
Currency and need for update
New research is available
Review conclusions are probably dated
Update decision
Based on the above criteria, the decision is made to either update, archive, or continue surveillance.
Of over 50 Cochrane editorial teams, most but not all have some systems for updating, although this process can be informal and loosely applied. Most editorial teams draw on some or all of the following criteria:
Strategic importance
Is the topic a priority area (for example, in current debates or considered by guidelines groups)?
Is there important new information available?
Practicalities in organising the update that many groups take into account
Size of the task (size and quality of the review, and how many new studies or analyses are needed)
Availability and willingness of the author team
Impact of update
New research impact on findings and credibility
Consider whether new methods will improve review quality
Priority to update, postpone update, class review as no longer requiring an update
The PUGs panel recommended an individualised approach to updating, which used the procedures summarised in figure 1 ⇓ . The figure provides a status category, and some options for classifying reviews into each of these categories, and builds on a previous decision tool and earlier work developing an updating classification system. 15 16 We provide a narrative for each step.
Fig 1 Decision framework to assess systematic reviews for updating, with standard terms to report such decisions
- Download figure
- Open in new tab
- Download powerpoint
Step 1: assess currency
Does the published review still address a current question.
An update is only worthwhile if the question is topical for decision making for practice, policy, or research priorities (fig 1 ⇑ ). For agencies, people responsible for managing a portfolio of systematic reviews, there is a need to use both formal and informal horizon scanning. This type of scanning helps identify questions with currency, and can help identify those reviews that should be updated. The process could include monitoring policy debates around the review, media outlets, scientific (and professional) publications, and linking with guideline developers.
Has the review had good access or use?
Metrics for citations, article access and downloads, and sharing via social or traditional media can be used as proxy or indicators for currency and relevance of the review. Reviews that are widely cited and used could be important to update should the need arise. Comparable reviews that are never cited or rarely downloaded, for example, could indicate that they are not addressing a question that is valued, and might not be worth updating.
In most cases, updated reviews are most useful to stakeholders when there is new information or methods that result in a change in findings. However, there are some circumstances in which an up to date search for information is important for retaining the credibility of the review, regardless of whether the main findings would change or not. For example, key stakeholders would dismiss a review if a study is carried out in a relevant geographical setting but is not included; if a large, high profile study that might not change the findings is not included; or if an up to date search is required for a guideline to achieve credibility. Box 3 provides such examples. If the review does not answer a current question, the intervention has been superseded, then a decision can be made not to update and no further intelligence gathering is required (fig 1 ⇑ ).
Box 3: Examples of a systematic review’s currency
The public is interested in vitamin C for preventing the common cold: the Cochrane review includes over 29 trials with either no or small effects, concluding good evidence of no important effects. 17 Assessment: still a current question for the public.
Low osmolarity oral rehydration salt (ORS) solution versus standard solution for acute diarrhoea in children: the 2001 Cochrane review 18 led the World Health Organization to recommend ORS solution formula worldwide to follow the new ORS solution formula 19 and this has now been accepted globally. Assessment: no longer a current question.
Routine prophylactic antibiotics with caesarean section: the Cochrane review reports clear evidence of maternal benefit from placebo controlled trials but no information on the effects on the baby. 20 Assessment: this is a current question.
A systematic review published in the Lancet examined the effects of artemisinin based combination treatments compared with monotherapy for treating malaria and showed clear benefit. 21 Assessment: this established the treatment globally and is no longer a current question and no update is required.
A Cochrane review of amalgam restorations for dental caries 22 is unlikely to be updated because the use of dental amalgam is declining, and the question is not seen as being important by many dental specialists. Assessment: no longer a current question.
Did the review use valid methods and was it well conducted?
If the question is current and clearly defined, the systematic review needs to have used valid methods and be well conducted. If the review has vague inclusion criteria, poorly articulated outcomes, or inappropriate methods, then updating should not proceed. If the question is current, and the review has been cited or used, then it might be appropriate to simply start with a new protocol. The appraisal should take into account the methods in use when the review was done.
Step 2: identify relevant new methods, studies, and other information
Are there any new relevant methods.
If the question is current, but the review was done some years ago, the quality of the review might not meet current day standards. Methods have advanced quickly, and data extraction and understanding of the review process have become more sophisticated. For example:
Methods for assessing risk of bias of randomised trials, 23 diagnostic test accuracy (QUADAS-2), 24 and observational studies (ROBINS-1). 25
Application of summary of findings, evidence profiles, and related GRADE methods has meant the characteristics of the intervention, characteristics of the participants, and risk of bias are more thoroughly and systematically documented. 26 27
Integration of other study designs containing evidence, such economic evaluation and qualitative research. 28
There are other incremental improvements in a wide range of statistical and methodological areas, for example, in describing and taking into account cluster randomised trials. 29 AMSTAR can assess the overall quality of a systematic review, 30 and the ROBIS tool can provide a more detailed assessment of the potential for bias. 31
Are there any new studies or other information?
If an authoring or commissioning team wants to ensure that a particular review is up to date, there is a need for routine surveillance for new studies that are potentially relevant to the review, by searching and trial register inspection at regular intervals. This process has several approaches, including:
Formal surveillance searching 32
Updating the full search strategies in the original review and running the searches
Tracking studies in clinical trial and other registers
Using literature appraisal services 33
Using a defined abbreviated search strategy for the update 34
Checking studies included in related systematic reviews. 35
How often this surveillance is done, and which approaches to use, depend on the circumstances and the topic. Some topics move quickly, and the definition of “regular intervals” will vary according to the field and according to the state of evidence in the field. For example, early in the life of a new intervention, there might be a plethora of studies, and surveillance would be needed more frequently.
Step 3: assess the effect of updating the review
Will the adoption of new methods change the findings or credibility.
Editors, referees, or experts in the topic area or methodologists can provide an informed view of whether a review can be substantially improved by application of current methodological expectations and new methods (fig 1 ⇑ ). For example, a Cochrane review of iron supplementation in malaria concluded that there was “no significant difference between iron and placebo detected.” 36 An update of the review included a GRADE assessment of the certainty of the evidence, and was able to conclude with a high degree of certainty that iron does not cause an excess of clinical malaria because the upper relative risk confidence intervals of harm was 1.0 with high certainty of evidence. 37
Will the new studies, information, or data change the findings or credibility?
The assessment of new data contained in new studies and how these data might change the review is often used to determine whether an update should go ahead, and the speed with which the update should be conducted. The appraisal of these new data can be carried out in different ways. Initially, methods focused on statistical approaches to predict an overturning of the current review findings in terms of the primary or desired outcome (table 1 ⇓ ). Although this aspect is important, additional studies can add important information to a review, which is more than just changing the primary outcome to a more accurate and reliable estimate. Box 4 gives examples.
Formal prediction tools: how potentially relevant new studies can affect review conclusions
- View inline
Box 4: Examples of new information other than new trials being important
The iconic Cochrane review of steroids in preterm labour was thought to provide evidence of benefit in infants, and this question no longer required new trials. However, a new large trial published in the Lancet in 2015 showed that in low and middle income countries, strategies to promote the uptake of neonatal steroids increased neonatal mortality and suspected maternal infection. 49 This information needs to somehow be incorporated into the review to maintain its credibility.
A Cochrane review of community deworming in developing countries indicates that in recent studies, there is little or no effect. 50 The inclusion of a large trial of two million children confirmed that there was no effect on mortality. Although the incorporation of the trial in the review did not change the review’s conclusions, the trial’s absence would have affected the credibility of the review, so it was therefore updated.
A new paper reporting long term follow-up data on anthracycline chemotherapy as part of cancer treatment was published. Although the effects from the outcomes remained essentially unchanged, apart from this longer follow-up, the paper also included information about the performance bias in the original trial, shifting the risk of bias for several outcomes from “unknown” to “high” in the Cochrane review. 51
Reviews with a high level of certainty in the results (that is, when the GRADE assessment for the body of evidence is high) are less likely to change even with the addition of new studies, information, or data, by definition. GRADE can help guide priorities in whether to update, but it is still important to assess new studies that might meet the inclusion criteria. New studies can show unexpected effects (eg, attenuation of efficacy) or provide new information about the effects seen in different circumstances (eg, groups of patients or locations).
Other tools are specifically designed to help decision making in updating. For example, the Ottawa 39 and RAND 45 methods focus on identification of new evidence, the statistical predication tool 15 calculates the probability of new evidence changing the review conclusion, and the value of information analysis approach 52 calculates the expected health gain (table 1 ⇑ ). As yet, there has been limited external validation of these tools to determine which approach would be most effective and when.
If potentially relevant studies are identified that have not previously been assessed for inclusion, authors or those managing the updating process need to assess whether including them might affect the conclusions of the review. They need to examine the weight and certainty of the new evidence to help determine whether an update is needed and how urgent that update is. The updating team can assess this informally by judging whether new studies or data are likely to substantively affect the review, for example, by altering the certainty in an existing comparison, or by generating new comparisons and analyses in the existing review.
New information can also include fresh follow-up data on existing included studies, or information on how the studies were carried out. These should be assessed in terms of whether they might change the review findings or improve its credibility (fig 1 ⇑ ). Indeed, if any study has been retracted, it is important the authors assess the reasons for its retraction. In the case of data fabrication, the study needs to be removed from the analysis and this recorded. A decision needs to be made as to whether other studies by the same author should be removed from the review and other related reviews. An investigation should also be initiated following guidelines from the Committee on Publication Ethics (COPE). Additional published and unpublished data can become available from a wide range of sources—including study investigators, regulatory agencies and industry—and are important to consider.
Preparing for an update
Refresh background, objectives, inclusion criteria, and methods
Before including new studies in the review, authors need to revisit the background, objectives, inclusion criteria, and methods of the current review. In Cochrane, this is referred to as the protocol, and editors are part of this process. The update could range from simply endorsing the current question and inclusion criteria, through to full rewriting of the question, inclusion criteria and methods, and republishing the protocol. As a field progresses with larger and better quality trials rigorously testing the questions posed, it may be appropriate to exclude weaker study designs (such as quasi-randomised comparisons or very small trials) from the update (table 2 ⇓ ). The PUGs panel recommended that a protocol refresh will require the authors to use the latest accepted methods of synthesis, even if this means repeating data extraction for all studies.
New authors and authorship
Updated systematic reviews are new publications with new citations. An authorship team publishing an update in a scientific or medical journal is likely to manage the new edition of a review in the same way as with any other publication, and follow the ICMJE authorship criteria. 56 If the previous author or author team steps down, then they should be acknowledged in the new version. However, some might perceive that their efforts in the first version warrant continued authorship, which may be valid. The management of authorship between versions can sometimes be complicated. At worst, it delays new authors completing an update and leads to long authorship lists of people from previous versions who probably do not meet ICMJE authorship criteria. One approach with updates including new authors is to have an opt-in policy for the existing authors: they can opt in to the new edition, provided that they make clear their contribution, and this is then agreed with the entire author team.
Although they are new publications, updates will generally include content from the published version. Changing licensing rights around systematic reviews to allow new authors of future updates to remix, tweak, or build on the contributions of the original authors of the published version (similar to the rights available via a Creative Commons licence; https://creativecommons.org ) could be a more sustainable and simpler approach. This approach would allow systematic reviews to continue to evolve and build on the work of a range of authors over time, and for contributors to be given credit for contributions to this previous work.
Efficient searching
In performing an update, a search based on the search conducted for the original review is required. The updated search strategy will need to take into account changes in the review question or inclusion criteria, for example, and might be further adjusted based on knowledge of running the original search strategy. The search strategy for an update need not replicate the original search strategy, but could be refined, for example, based on an analysis of the yield of the original search. These new search approaches are currently undergoing formal empirical evaluation, but they may well provide much more efficient search strategies in the future. Some examples of these possible new methods for review updates are described in web appendix 2.
In reporting the search process for the update, investigators must ensure transparency for any previous versions and the current update, and use an adapted flow diagram based on PRISMA reporting (preferred reporting items for systematic reviews and meta-analyses). 57 The search processes and strategies for the update must be adequately reported such that they could be replicated.
Systematic reviews published for the first time in peer reviewed journals are by definition peer reviewed, but practice for updates remains variable, because an update might have few changes (such as an updated search but no new studies found and therefore included) or many changes (such as revise methods and inclusion of several new studies leading to revised conclusions). Therefore, and to use peer reviewers’ time most effectively, editors need to consider when to peer review an update and the type of peer reviewer most useful for a particular update (for example, topic specialist, methodologist). The decision to use peer review, and the number and expertise of the peer reviewers could depend on the nature of the update and the extent of any changes to the systematic review as part of an editor assessment. A change in the date of the search only (where no new studies were identified) would not require peer review (except, arguably, peer review of the search), but the addition of studies that lead to a change in conclusions or significant changes to the methods would require peer review. The nature of the peer review could be described within the published article.
Reporting changes
Authors should provide a clear description of the changes in approach or methods between different editions of a review. Also, authors need to report the differences in findings between the original and updated edition to help users decide how to use the new edition. The approach or format used to present the differences in findings might vary with the target user group. 58 Publishers need to ensure that all previous versions of the review remain publically accessible.
Updates can range from small adjustments to reviews being completely rewritten, and the PUGs panel spent some time debating whether the term “new edition” would be a better description than “update.” However, the word “update” is now in common parlance and changing the term, the panel judged, could cause confusion. However, the debate does illustrate that an update could represent a review that asks a similar question but has been completely revised.
Technology and innovation
The updating of systematic review is generally done manually and is time consuming. There are opportunities to make better use of technology to streamline the updating process and improve efficiency (table 3 ⇓ ). Some of these tools already exist and are in development or in early use, and some are commercially available or freely available. The AHRQ’s evidence based practice centre team has recently published tools for searching and screening, and will provide an assessment of the use, reliability, and availability of these tools. 63
Technological innovations to improve the efficiency of updating systematic reviews
Other developments, such as targeted updates that are performed rapidly and focus on updating only key components of a review, could provide different approaches to updating in the future and are being piloted and evaluated. 64 With implementation of these various innovations, the longer term goal is for “living” systematic reviews, which identify and incorporate information rapidly as it evolves over time. 60
Concluding remarks
Updating systematic reviews, rather than addressing the same question with a fresh protocol, is generally more efficient and allows incremental improvement over time. Mechanical rules appear unworkable, but there is no clear unified approach on when to update, and how implement this. This PUGs panel of authors, editors, statisticians, information specialists, other methodologists, and guideline developers brought together current thinking and experience in this area to provide guidance.
Decisions about whether and when to update a systematic review are judgments made at a point in time. They depend on the currency of the question asked, the need for updating to maintain credibility, the availability of new evidence, and whether new research or new methods will affect the findings.
Whether the review uses current methodological standards is important in deciding if the update will influence the review findings, quality, reliability, or credibility sufficiently to justify the effort in updating it. Those updating systematic reviews to author clinical practice guidelines might consider the influence of new study results in potentially overturning the conclusions of an existing review. Yet, even in cases where new study findings do not change the primary outcome measure, new studies can carry important information about subgroup effects, duration of treatment effects, and other relevant clinical information, enhancing the currency and breadth of review results.
An update requires appraisal and revision of the background, question, inclusion criteria, and methods of the existing review and the existing certainty in the evidence. In particular, methods might need to be updated, and search strategies reconsidered. Authors of updates need to consider inputs to the current edition, and follow ICMJE criteria regarding authorship. 56
The PUGs panel proposed a decision framework (fig 1 ⇑ ), with terms and categories for reporting the decisions made for updating procedures for adoption by Cochrane and other stakeholders. This framework includes journals publishing systematic review updates and independent authors considering updates of existing published reviews. The panel developed a checklist to help judgements about when and how to update.
The current emphasis of authors, guideline developers, Cochrane, and consequently this guidance has been on effects reviews. The checklists and guidance here still applies to other types of systematic reviews, such as those on diagnostic test accuracy, and this guidance will need adapting. Accumulative experience and methods development in reviews other than those of effects are likely to help refine guidance in the future.
This guidance could help groups identify and prioritise reviews for updating and hence use their finite resources to greatest effect. Software innovation and new management systems are being developed and in early use to help streamline review updates in the coming years.
Contributors: HJS initiated the workshop. JC, SH, PG, HM, and HJS organised the materials and the agenda. SH wrote up the proceedings. PG wrote the paper from the proceedings and coordinated the development of the final guidance; JC, SH, HM, and HJS were active in the finalising of the guidance. All PUGs authors contributed to three rounds of manuscript revision.
Funding: Attendance at this meeting, for those attendees not directly employed by Cochrane, was not funded by Cochrane beyond the reimbursement of out of pocket expenses for those attendees for whom this was appropriate. Expenses were not reimbursed for US federal government attendees, in line with US government policy. Statements in the manuscript should not be construed as endorsement by the US Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Competing interests: All participants have a direct or indirect interest in systematic reviews and updating as part of their job or academic career. Most participants contribute to Cochrane, whose mission includes a commitment to the updating of its systematic review portfolio. JC, HM, RM, CM, KS-W, and MT are, or were at that time, employed by the Cochrane Central Executive.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/ .
- ↵ Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA 2001 ; 286 : 1461 - 7 . doi:10.1001/jama.286.12.1461 pmid:11572738 . OpenUrl CrossRef PubMed Web of Science
- ↵ Claxton K, Cohen JT, Neumann PJ. When is evidence sufficient? Health Aff (Millwood) 2005 ; 24 : 93 - 101 . doi:10.1377/hlthaff.24.1.93 pmid:15647219 . OpenUrl Abstract / FREE Full Text
- ↵ Fenwick E, Claxton K, Sculpher M, et al. Improving the efficiency and relevance of health technology assessment: the role of decision analytic modelling. Paper 179. Centre for Health Economics, University of York, 2000 .
- ↵ Sculpher M, Claxton K. Establishing the cost-effectiveness of new pharmaceuticals under conditions of uncertainty—when is there sufficient evidence? Value Health 2005 ; 8 : 433 - 46 . doi:10.1111/j.1524-4733.2005.00033.x pmid:16091019 . OpenUrl CrossRef PubMed Web of Science
- ↵ Sculpher M, Drummond M, Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res Policy 1997 ; 2 : 26 - 30 . pmid:10180650 . OpenUrl Abstract / FREE Full Text
- ↵ Wilson E, Abrams K. From evidence based economics to economics based evidence: using systematic review to inform the design of future research. In: Shemilt I, Mugford M, Vale L, et al, eds. Evidence based economics. Blackwell Publishing, 2010 doi:10.1002/9781444320398.ch12 .
- ↵ Chalmers I, Enkin M, Keirse MJ. Preparing and updating systematic reviews of randomized controlled trials of health care. Milbank Q 1993 ; 71 : 411 - 37 . doi:10.2307/3350409 pmid:8413069 . OpenUrl CrossRef PubMed Web of Science
- ↵ Higgins J, Green S, Scholten R. Chapter 3. Maintaining reviews: updates, amendments and feedback: Version 5.1.0 (updated March 2011). Cochrane Collaboration, 2011 .
- ↵ Cochrane. Editorial and publishing policy resource. http://community.cochrane.org/editorial-and-publishing-policy-resource . 2016.
- ↵ Moher D, Tsertsvadze A. Systematic reviews: when is an update an update? Lancet 2006 ; 367 : 881 - 3 . doi:10.1016/S0140-6736(06)68358-X pmid:16546523 . OpenUrl CrossRef PubMed Web of Science
- ↵ Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2016 ; 4 : CD002244 . pmid:27121755 . OpenUrl PubMed
- ↵ Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev 2014 ; 1 : CD010927 . pmid:24443033 . OpenUrl CrossRef PubMed
- ↵ Adams SP, Tsang M, Wright JM. Lipid lowering efficacy of atorvastatin. Cochrane Database Syst Rev 2012 ; 12 : CD008226 . pmid:23235655 . OpenUrl PubMed
- ↵ Higgins J. Convincing evidence from controlled and uncontrolled studies on the lipid-lowering effect of a statin. Cochrane Database Syst Rev 2012 ; 12 : ED000049 . pmid:23361645 . OpenUrl PubMed
- ↵ Takwoingi Y, Hopewell S, Tovey D, Sutton AJ. A multicomponent decision tool for prioritising the updating of systematic reviews. BMJ 2013 ; 347 : f7191 . doi:10.1136/bmj.f7191 pmid:24336453 . OpenUrl FREE Full Text
- ↵ MacLehose H, Hilton J, Tovey D, et al. The Cochrane Library: revolution or evolution? Shaping the future of Cochrane content. Background paper for The Cochrane Collaboration’s Strategic Session Paris, France, 18 April 2012. http://editorial-unit.cochrane.org/sites/editorial-unit.cochrane.org/files/uploads/2012-CC-strategic-session_full-report.pdf .
- ↵ Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2013 ;( 1 ): CD000980 . pmid:23440782 .
- ↵ Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. Cochrane Database Syst Rev 2002 ;( 1 ): CD002847 . pmid:11869639 .
- ↵ World Health Organization (WHO). Reduced osmolarity oral rehydration salts (ORS) formulation. A report from a meeting of Experts jointly organized by UNICEF and WHO. New York: Child and Adolescent Health and Development, 18 July 2001 http://apps.who.int/iris/bitstream/10665/67322/1/WHO_FCH_CAH_01.22.pdf .
- ↵ Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2014 ;( 10 ): CD007482 . pmid:25350672 .
- ↵ Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 2004 ; 363 : 9 - 17 . doi:10.1016/S0140-6736(03)15162-8 pmid:14723987 . OpenUrl CrossRef PubMed Web of Science
- ↵ Agnihotry A, Fedorowicz Z, Nasser M. Adhesively bonded versus non-bonded amalgam restorations for dental caries. Cochrane Database Syst Rev 2016 ; 3 : CD007517 . pmid:26954446 . OpenUrl PubMed
- ↵ Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011 ; 343 : d5928 . doi:10.1136/bmj.d5928 pmid:22008217 . OpenUrl FREE Full Text
- ↵ Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011 ; 155 : 529 - 36 . doi:10.7326/0003-4819-155-8-201110180-00009 pmid:22007046 . OpenUrl CrossRef PubMed Web of Science
- ↵ Sterne JAC, Higgins JPT, Reeves BC; on behalf of the development group for ROBINS-I. A tool for assessing risk of bias in non-randomized studies of interventions, version 7. March 2016. www.riskofbias.info .
- ↵ Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008 ; 336 : 924 - 6 . doi:10.1136/bmj.39489.470347.AD pmid:18436948 . OpenUrl FREE Full Text
- ↵ Schünemann HJ. Interpreting GRADE’s levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? J Clin Epidemiol 2016 ; 75 : 6 - 15 . doi:10.1016/j.jclinepi.2016.03.018 pmid:27063205 . OpenUrl CrossRef PubMed
- ↵ Gough D. Qualitative and mixed methods in systematic reviews. Syst Rev 2015 ; 4 : 181 . doi:10.1186/s13643-015-0151-y pmid:26670769 . OpenUrl CrossRef PubMed
- ↵ Richardson M, Garner P, Donegan S. Cluster randomised trials in Cochrane reviews: evaluation of methodological and reporting practice. PLoS One 2016 ; 11 : e0151818 . doi:10.1371/journal.pone.0151818 pmid:26982697 . OpenUrl CrossRef PubMed
- ↵ Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007 ; 7 : 10 . doi:10.1186/1471-2288-7-10 pmid:17302989 . OpenUrl CrossRef PubMed
- ↵ Whiting P, Savović J, Higgins JP, et al. ROBIS group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016 ; 69 : 225 - 34 . doi:10.1016/j.jclinepi.2015.06.005 pmid:26092286 . OpenUrl CrossRef PubMed
- ↵ Sampson M, Shojania KG, McGowan J, et al. Surveillance search techniques identified the need to update systematic reviews. J Clin Epidemiol 2008 ; 61 : 755 - 62 . doi:10.1016/j.jclinepi.2007.10.003 pmid:18586179 . OpenUrl CrossRef PubMed Web of Science
- ↵ Hemens BJ, Haynes RB. McMaster Premium LiteratUre Service (PLUS) performed well for identifying new studies for updated Cochrane reviews. J Clin Epidemiol 2012 ; 65 : 62 - 72.e1 . doi:10.1016/j.jclinepi.2011.02.010 pmid:21856121 . OpenUrl CrossRef PubMed
- ↵ Sagliocca L, De Masi S, Ferrigno L, Mele A, Traversa G. A pragmatic strategy for the review of clinical evidence. J Eval Clin Pract 2013 ; 19 : 689 - 96 . doi:10.1111/jep.12020 pmid:23317014 . OpenUrl CrossRef PubMed
- ↵ Rada G, Peña J, Capurro D, et al. How to create a matrix of evidence in Epistemonikos. Abstracts of the 22nd Cochrane Colloquium; Evidence-informed public health: opportunities and challenges; Hyderabad, India. Cochrane Database Syst Rev 2014 ; suppl 1 : 132 .
- ↵ Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev 2011 ;( 10 ): CD006589 . pmid:21975754 .
- ↵ Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev 2016 ; 2 : CD006589 . pmid:26921618 . OpenUrl PubMed
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011 ; 64 : 401 - 6 . doi:10.1016/j.jclinepi.2010.07.015 pmid:21208779 . OpenUrl CrossRef PubMed Web of Science
- ↵ Chung M, Newberry SJ, Ansari MT, et al. Two methods provide similar signals for the need to update systematic reviews. J Clin Epidemiol 2012 ; 65 : 660 - 8 . doi:10.1016/j.jclinepi.2011.12.004 pmid:22464414 . OpenUrl CrossRef PubMed
- Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med 2007 ; 147 : 224 - 33 . doi:10.7326/0003-4819-147-4-200708210-00179 pmid:17638714 . OpenUrl CrossRef PubMed Web of Science
- Shojania K, Sampson M, Ansari M, et al. Updating systematic reviews; AHRQ technical reviews; report no 07-0087. Agency for Healthcare Research and Quality, 2007 .
- Pattanittum P, Laopaiboon M, Moher D, Lumbiganon P, Ngamjarus C. A comparison of statistical methods for identifying out-of-date systematic reviews. PLoS One 2012 ; 7 : e48894 . doi:10.1371/journal.pone.0048894 pmid:23185281 . OpenUrl CrossRef PubMed
- Shekelle PG, Motala A, Johnsen B, Newberry SJ. Assessment of a method to detect signals for updating systematic reviews. Syst Rev 2014 ; 3 : 13 . doi:10.1186/2046-4053-3-13 pmid:24529068 . OpenUrl CrossRef PubMed
- Shekelle PG, Newberry SJ, Wu H, et al. Identifying signals for updating systematic reviews: a comparison of two methods; report no 11-EHC042-EF. Agency for Healthcare Research and Quality, 2011 .
- ↵ Shekelle P, Newberry S, Maglione M, et al. Assessment of the need to update comparative effectiveness reviews: report of an initial rapid program assessment (2005-2009). Agency for Healthcare Research and Quality, 2009 .
- Tovey D, Marshall R, Bazian, Hopewell S, Rader T. Fit for purpose: centralised updating support for high-priority Cochrane Reviews; National Institute for Health Research Cochrane-National Health Service Engagement Award Scheme, July 2011. https://editorial-unit.cochrane.org/sites/editorial-unit.cochrane.org/files/uploads/10_4000_01%20Fit%20for%20purpose%20-%20centralised%20updating%20support%20for%20high%20priority%20Cochrane%20Reviews%20FINAL%20REPORT.pdf .
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999 ; 18 : 341 - 64 . doi:10.1016/S0167-6296(98)00039-3 pmid:10537899 . OpenUrl CrossRef PubMed Web of Science
- Wilson EC. A practical guide to value of information analysis. Pharmacoeconomics 2015 ; 33 : 105 - 21 . doi:10.1007/s40273-014-0219-x pmid:25336432 . OpenUrl CrossRef PubMed
- ↵ Althabe F, Belizán JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet 2015 ; 385 : 629 - 39 . doi:10.1016/S0140-6736(14)61651-2 pmid:25458726 . OpenUrl CrossRef PubMed
- ↵ Taylor-Robinson D, Maayan N, Soares-Weiser K, et al. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev 2012 ;( 11 ): CD000371 .
- ↵ van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2016 ; 3 : CD005008 . pmid:26938118 . OpenUrl PubMed
- ↵ Wilson E. on behalf of the Cochrane Priority Setting and Campbell & Cochrane Economics Methods Groups. Which study when? Proof of concept of a proposed automated tool to help decision which reviews to update first. Cochrane Database Syst Rev 2014 ; suppl 2 : 29 - 31 .
- Rosenbaum SE, Glenton C, Nylund HK, Oxman AD. User testing and stakeholder feedback contributed to the development of understandable and useful Summary of Findings tables for Cochrane reviews. J Clin Epidemiol 2010 ; 63 : 607 - 19 . doi:10.1016/j.jclinepi.2009.12.013 pmid:20434023 . OpenUrl CrossRef PubMed
- Rosenbaum SE, Glenton C, Oxman AD. Summary-of-findings tables in Cochrane reviews improved understanding and rapid retrieval of key information. J Clin Epidemiol 2010 ; 63 : 620 - 6 . doi:10.1016/j.jclinepi.2009.12.014 pmid:20434024 . OpenUrl CrossRef PubMed Web of Science
- Vandvik PO, Santesso N, Akl EA, et al. Formatting modifications in GRADE evidence profiles improved guideline panelists comprehension and accessibility to information. A randomized trial. J Clin Epidemiol 2012 ; 65 : 748 - 55 . doi:10.1016/j.jclinepi.2011.11.013 pmid:22564503 . OpenUrl CrossRef PubMed
- ↵ International Committee of Medical Journal Editors (ICMJE). Defining the role of authors and contributors. 2016. www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html .
- ↵ Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev 2014 ; 3 : 54 . doi:10.1186/2046-4053-3-54 pmid:24886533 . OpenUrl CrossRef PubMed
- ↵ Newberry SJ, Shekelle PG, Vaiana M, et al. Reporting the findings of updated systematic reviews of comparative effectiveness: how do users want to view new information? report no 13-EHC093-EF. Agency for Healthcare Research and Quality, 2013 .
- Marshall IJ, Kuiper J, Wallace BC. Automating risk of bias assessment for clinical trials BCB’14. Proceedings of the 5th ACM conference on Bioinformatics, computational biology, and health informatics. 2014:88-95. http://thirdworld.nl/automating-risk-of-bias-assessment-for-clinical-trials .
- ↵ Elliott JH, Turner T, Clavisi O, et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med 2014 ; 11 : e1001603 . doi:10.1371/journal.pmed.1001603 pmid:24558353 . OpenUrl CrossRef PubMed
- Elliott J, Sim I, Thomas J, et al. #CochraneTech: technology and the future of systematic reviews. Cochrane Database Syst Rev 2014 ;( 9 ): ED000091 . pmid:25288182 .
- Cochrane. Project transform: the Cochrane Collaboration. 2016. http://community.cochrane.org/tools/project-coordination-and-support/transform .
- ↵ Paynter R, Bañez L, Berlinerm E, et al. EPC methods: an exploration of the use of text-mining software in systematic reviews. Research white paper. AHRQ publication 16-EHC023-EF. Agency for Healthcare Research and Quality, April 2016. https://www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productID=2214 .
- ↵ Soares-Weiser K, Marshall R, Bergman H, et al. Updating Cochrane Reviews: results of the first pilot of a focused update. Cochrane Database Syst Rev 2014 ; suppl 1 : 31 - 3 .
- A t tachments (0)
- Page History
- Page Information
- Resolved comments
- View in Hierarchy
- View Source
- Export to PDF
- Export to Word
Reporting search dates in Cochrane Reviews
- Created by Harriet MacLehose , last modified on Jul 15, 2019
Version history
This policy covers the reporting of search dates in Cochrane Reviews. It is informed by guidance on re-running searches covered i n MECIR conduct standard C37 . This standard requires that searches for all relevant databases be run (or re-run) within 12 months before publication of the review or review update, and that the results are screened for potentially eligible studies.
For definitions of search types (full, top-up, scoping) see Table below.
- Updates vs. amendments: a review is considered updated and receives a new citation in Cochrane Database of Systematic Reviews ( CDSR ) when a new search is conducted and the results of the search are fully incorporated. If a scoping search is conducted to determine if an update is required, then the date of this search will not change the 'Date of search' in the review or lead to a new citation version being created. This should be published as an amendment if necessary; see Assigning ‘What’s New’ events to Cochrane Reviews .
- If top-up searches are performed and the results incorporated then that top-up search date becomes the date of the full search (i.e. the date that appears in the ‘Date of Search’ field).
- The ‘Date of search’ remains the date of the search for which results were fully incorporated.
- Studies not yet fully incorporated into the review are added to ‘Studies awaiting classification’.
- The ' Search methods' in the abstract should focus on reporting the search dates related to the last fully incorporated studies. Brief mention of a top-up search may be made only if it was conducted for a completed update or new review. Do not refer to scoping searches for updating in the abstract.
- The 'Search methods for identification of studies' in the main text of the review should be used primarily to describe the details of the search for which the results have been fully incorporated, i.e. the dates of individual database searching and the hits retrieved should be based on the search date where results are fully incorporated. If a top-up search has been performed, but the results not yet fully incorporated, the search section may briefly describe this (see example below) and state how many studies have been placed in 'Studies awaiting classification'.
- In the 'Results of the search' section the authors should specify the number of studies yet to be fully incorporated into the review. This should also be reflected in the conclusions (both of the main review and the abstract).
- The PRISMA flow of studies diagram should also reflect the number of studies in the 'Studies awaiting classification' section.
- The 'What’s New' events must describe the number of studies that have been put into 'Studies awaiting classification' if the top-up search is mentioned in the search methods section.
- The search appendix should present only database strategies for searches conducted for which results were fully incorporated.
- If different databases were searched on different dates , the most recent date of the search for each database should be given within the text of the review and the earliest of these dates should be entered as the ‘Date of Search’. In the case of review updates or 'top-up' searches, if there is clear rationale for not searching one or more of the previously searched databases (e.g. because no unique relevant records were identified in the original/previous search, or the database is no longer being updated), the rationale should be stated within the text of the review. In this case, the 'Date of search' should be the earliest date of the searches performed for this smaller set of databases.
Definitions of search types (full, top-up, scoping)
|
|
|---|---|
Full search – results fully incorporated | Electronic search strategies run in full in all relevant databases AND all search results are assessed for eligibility as included, excluded, or ongoing studies. Only if all reasonable efforts to classify search results have failed should they be placed in ‘Studies awaiting classification.’* |
Top-up search – results not fully incorporated | Electronic search strategies run in full in all relevant databases BUT search results are not all assessed for eligibility, instead they are placed in 'Studies awaiting classification'. |
Scoping search for updating | Electronic search strategies run in selected databases to determine if an update is required. |
| *See R6 and R34 in ; and . | |
Examples of reporting top-up searches
The number of instances where a top-up search is performed and potential new studies are identified but not fully incorporated before publication should remain low. The following examples show how such searches should be described in various sections of a systematic review:
Do not change the 'Date of search' or th e 'Assessed as up-to-date' (see Note for editorial base staff ) in the Cochrane Review 'Information’ section. Also, if less than 10 trial reports then list here in parentheses and link. For example:
"The search was updated in month/year and n trial reports added to ‘Studies awaiting classification’ (e.g. Bertini 2005; Crowther 2005; Gillen 2004)."
Search methods
The focus should remain on the text about previous searches (fully incorporated) but the top-up search may be mentioned. For example:
"We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, and CINAHL (June 2013). We updated this search in September 2014, but these results have not yet been incorporated."
Search methods for identification of studies
The search should be reported as per MECIR reporting standards R34 to 39, including the dates for each source. At the end of the search methods section, it is appropriate to add the following text:
"We performed a further search in [month/year]. Those results have been added to 'Studies awaiting classification' and will be incorporated into the review at the next update."
Do not list all databases and the dates. If a top-up search in reported in this section, only a single month (or range of months) and year should be shown.
Results: Description of studies
This section will differ depending on the review, so add text where it is most appropriate); for example:
"[insert number] study reports from an updated search in [month/year] have been added to 'Studies awaiting classification'."
Discussion: Potential biases in the review process
Acknowledge the potential impact of un-incorporated studies as a source of potential bias, especially if studies concerned are potentially important in terms of sample size or direction of effect; for example:
"We attempted to conduct a comprehensive search for studies, but the fact that [insert number] studies have not yet been incorporated may be a source of potential bias."
Authors’ conclusions (Implications for practice)
This is not an implication for practice as such, but users should be alerted to the issue of un-incorporated studies, particularly if the studies concerned are potentially important in terms of sample size or direction of effect; for example:
"The [insert number] studies in 'Studies awaiting classification' may alter the conclusions of the review once assessed."
Note for editorial base staff
One date should be used to reflect the search and full incorporation of all search results into the review; this date is the ‘Date of search’. Standard practice has been to publish the 'Assessed as up-to-date' field and not the 'Date of search'. Until the 'Assessed as up-to-date' field is removed from RevMan these two dates must be the same. If these fields have been completed by a member of the author team, editorial base staff must check that there is agreement between dates.
Powered by a free Atlassian Confluence Community License granted to Cochrane. Evaluate Confluence today .
- Powered by Atlassian Confluence 8.5.4
- Printed by Atlassian Confluence 8.5.4
- Report a bug
- Atlassian News
Our site uses cookies to improve your experience. You can find out more about our use of cookies in About Cookies, including instructions on how to turn off cookies if you wish to do so. By continuing to browse this site you agree to us using cookies as described in About Cookies .
The Cochrane Library
Trusted evidence. Informed decisions. Better health.
Scolaris Search Portlet Scolaris Search Portlet
Scolaris language selector scolaris language selector.
| Select your preferred language for Cochrane Reviews and other content. Sections without translation will be in English. | ||
| Select your preferred language for the Cochrane Library website. | ||
Scolaris Content Language Banner Portlet Scolaris Content Language Banner Portlet
Web content display web content display.

Undernutrition as a risk factor for TB

Anti-VEGF biosimilars for nAMD

Magnesium for preterm babies
- Highlighted Reviews
- Special Collections
Scolaris Content List Scolaris Content List
Peritoneal dialysis versus haemodialysis for people commencing dialysis
Isabelle Ethier, Ashik Hayat, Juan Pei, Carmel M Hawley, David W Johnson, Ross S Francis, Germaine Wong, Jonathan C Craig, Andrea K Viecelli, Yeoungjee Cho, Htay Htay, Samantha Ng, Saskia Leibowitz
Treatments for intractable constipation in childhood
Morris Gordon, Ciaran Grafton-Clarke, Shaman Rajindrajith, MA Benninga, Vassiliki Sinopoulou, Anthony K Akobeng
Factors that influence participation in physical activity for people with bipolar disorder: a synthesis of qualitative evidence
Claire J McCartan, Jade Yap, Paul Best, Josefien Breedvelt, Gavin Breslin, Joseph Firth, Mark A Tully, Paul Webb, Chris White, Simon Gilbody, Rachel Churchill, Gavin Davidson
Metformin for preventing the progression of chronic kidney disease
Ragada El-Damanawi a , Isabelle Kitty Stanley a , Christine Staatz, Elaine M Pascoe, Jonathan C Craig, David W Johnson, Andrew J Mallett, Carmel M Hawley, Elasma Milanzi, Thomas F Hiemstra, Andrea K Viecelli
External electrical and pharmacological cardioversion for atrial fibrillation, atrial flutter or atrial tachycardias: a network meta‐analysis
Kishore Kukendrarajah, Mahmood Ahmad, Mafalda Carrington, Adam Ioannou, Julie Taylor, Yousuf Razvi, Nikolaos Papageorgiou, Gillian E Mead, Immaculate F Nevis, Fabrizio D'Ascenzo, Stephen B Wilton, Pier D Lambiase, Carlos A Morillo, Joey SW Kwong, Rui Providencia
View Current Issue
Editorial List Editorial List

Zoe Jordan, Vivian Welch, Karla Soares-Weiser

Yi Feng Lai, Stephanie Q Ko

Rebecca E Ryan, Sophie Hill

Patrick M Bossuyt, Jonathan J Deeks, Mariska M Leeflang, Yemisi Takwoingi, Ella Flemyng

Sharon R Lewis, Xavier L Griffin
Scolaris Special Collection Scolaris Special Collection

- Browse by PICOs
Browse by Topic
Browse by picos .
The PICO model is widely used and taught in evidence-based health care as a strategy for formulating questions and search strategies and for characterizing clinical studies or meta-analyses . PICO stands for four different potential components of a clinical question: Patient, Population or Problem; Intervention; Comparison; Outcome.
See more on using PICO in the Cochrane Handbook .
| Population | Intervention & Comparison | Outcome |
|---|---|---|
Browse Cochrane Reviews, Protocols and Clinical Answers.
- Allergy & intolerance
- Blood disorders
- Child health
- Complementary & alternative medicine
- Consumer & communication strategies
- Dentistry & oral health
- Developmental, psychosocial & learning problems
- Ear, nose & throat
- Effective practice & health systems
- Endocrine & metabolic
- Eyes & vision
- Gastroenterology & hepatology
- Genetic disorders
- Gynaecology
- Health & safety at work
- Health professional education
- Heart & circulation
- Infectious disease
- Insurance medicine
- Kidney disease
- Lungs & airways
- Mental health
- Methodology
- Neonatal care
- Orthopaedics & trauma
- Pain & anaesthesia
- Pregnancy & childbirth
- Public health
- Reproductive & sexual health
- Rheumatology
- Skin disorders
- Tobacco, drugs & alcohol

Scolaris Language Sponsor Footer Scolaris Language Sponsor Footer
Sign in sign in, scolaris content display scolaris content display.
Search for your institution's name below to login via Shibboleth
Previously accessed institutions
If you have a Wiley Online Library institutional username and password, enter them here.
Institutional users
Other access options.
- Individual access - via Wiley Online Library

- McGill Library
Systematic Reviews, Scoping Reviews, and other Knowledge Syntheses
- Updating the database searches
- Types of knowledge syntheses
- Identifying the research question
- Developing the protocol
- Database-specific operators and fields
- Search filters and tools
- Exporting and documenting search results
- Deduplicating
- Grey literature and other supplementary search methods
- Documenting the search methods
Updating the database searches using Covidence
Alternative: updating the database searches using indexing dates, bibliography.
- Resources for screening, appraisal, and synthesis
- Writing the review
- Additional training resources
To update your searches, we recommend rerunning your saved searches, exporting all the records to RIS files, and then importing those RIS files into Covidence for deduplication. This is our recommended approach to updating searches to retrieve new records only, as well as for removing duplicate records.
If you do this, be sure to follow the instructions provided by Covidence (e.g., download your PRISMA flow diagram before adding the new RIS files to a previous review).
How to update a review with new studies
There are different methods that you can use to update your searches, and they are usually database-specific. What you are trying to do is avoid screening the same records all over again.
In Covidence, you can simply import all the records generated by the new/updated searches, and Covidence will remove records that have already been uploaded . If you do this, be sure to follow the instructions provided by Covidence (e.g., download your PRISMA flow diagram before adding the new RIS files to a previous review).
Another method is to restrict records to those entered into the database on or after the date you last ran the search (note: this is NOT the publication date, this is the date the record was added to the database, and this date field is not available in all databases).
This can be done in PubMed, MEDLINE (Ovid), Embase (Ovid), Scopus, CINAHL (EBSCOhost), APA PsycInfo (Ovid), CENTRAL (Cochrane Library/Wiley), and Web of Science Core Collection (as well as others), but the entry date is not available as a search field in all databases and it may miss records .
Please note: The field codes are subject to change and we strongly suggest checking the database information, particularly at the beginning of a new year, when indexing changes are more likely to occur. Please also let us know if you identify issues with these codes. This table was last updated April 2, 2024 (Correction: da refers to MeSH Date in Ovid MEDLINE).
Many thanks to those who reach out to let us know when there are issues or corrections needed!
| Database | Platform | Suggested Search Field(s) | Example of a search last run on July 1, 2019 and updated on May 1, 2023 |
|---|---|---|---|
| CENTRAL/Trials | Cochrane Library/Wiley | Use "Date" filter | View search results / Select Trials tab > Under "Filter your results" (left column), enter date range ("Date added to CENTRAL trials database") as custom range 01/07/2019 to 01/05/2023
|
| PubMed ( ) | - | CRDT OR EDAT OR MHDA | #x AND ("2019/07/01"[CRDT] : "3000"[CRDT] OR "2019/07/01"[EDAT] : "3000"[EDAT] OR "2019/07/01"[MHDA] : "3000"[MHDA]) (MHDA refers to the date that MeSH are added and may increase duplicates but can identify indexed records previously missed by textword searching) |
| MEDLINE ( ) | OvidSP | .dt,ez,da. (Create Date, Entrez date; MeSH date) | (201907* OR 201908* OR 201909* OR 20191* OR 202*).dt,ez,da. (da refers to the date that MeSH are added and may increase duplicates but can identify indexed records previously missed by textword searching) |
| Embase ( ) | OvidSP | .dc. | limit x to dc=20190701-20230501 |
| PsycInfo ( ) | OvidSP | .up. | limit x to up=20190701-20230501 |
| CINAHL ( ) | EBSCOhost | (EM yyyymmdd- OR (ZD "in process" AND RD yyyymmdd-)) | (EM 20190701- OR (ZD "in process" AND RD 20190701-)) |
| Scopus | ORIG-LOAD-DATE AFT | ORIG-LOAD-DATE AFT 20190630 | |
| Web of Science Core Collection (new Web of Science platform) | Web of Science | LD | Select Index Date: 2019-07-01 to 2023-05-01 OR From Advanced Search Query Builder > Query Preview: LD=(2019-07-01/2023-05-01)
|
See also: Garner P, Hopewell S, Chandler J, MacLehose H, Akl EA, Beyene J, Chang S, Churchill R, Dearness K, Guyatt G, Lefebvre C, Liles B, Marshall R, Martínez García L, Mavergames C, Nasser M, Qaseem A, Sampson M, Soares-Weiser K, Takwoingi Y, Thabane L, Trivella M, Tugwell P, Welsh E, Wilson EC, Schünemann HJ. When and How to Update Systematic Reviews: Consensus and Checklist . BMJ. 2016;354. 10.1136/bmj.i3507
Due to a large influx of requests, there may be an extended wait time for librarian support on knowledge syntheses.
Find a librarian in your subject area to help you with your knowledge synthesis project.
Or contact the librarians at the Schulich Library of Physical Sciences, Life Sciences, and Engineering s [email protected]
Need help? Ask us!
- << Previous: Documenting the search methods
- Next: Resources for screening, appraisal, and synthesis >>
- Last Updated: Jun 27, 2024 12:05 PM
- URL: https://libraryguides.mcgill.ca/knowledge-syntheses
McGill Library • Questions? Ask us! Privacy notice
- Interlibrary Loan
Ask an Expert
Ask an expert about access to resources, publishing, grants, and more.
MD Anderson faculty and staff can also request a one-on-one consultation with a librarian or scientific editor.
- Library Calendar
Log in to the Library's remote access system using your MyID account.

- Library Home
- Research Guides
- Systematic Reviews
Updating a Systematic Review
Systematic reviews: updating a systematic review.
- Introduction
- Step-by-Step
- Standards and Guidelines
- Designing the Protocol
- Selecting Studies
- Appraising Studies
- Data Extraction
- Writing a Systematic Review
- Meta-analyses
- Evaluating a Systematic Review
- Cochrane Systematic Reviews
- Get more help
- Related Review Types
Systematic reviews need to be evaluated on a periodic basis to determine if they need to be updated. If it's likely that new studies have come out and the topic is still of interest to clinicians and decision makers then it should be updated.
Cochrane Handbook Ch IV: Updating a Review
Cochrane MECIR Manual: Reporting Standards Specific to Updates
Cochrane Handbook Ch 22.2 Maintaining the currency of systematic reviews
PRISMA Flow Diagrams for SR Updates
Living Systematic Reviews
"Living" systematic reviews are updated on a periodic basis, often monthly. Any new evidence is immediately incorporated into the published review. Needless to say, this is very time-consuming so this type of updating is usually only used for topics that are of high importance to decision makers, on which new evidence is frequently published. More information and examples are at Cochrane's LSRs and LSR Protocols .
Searching for New Studies
It can be challenging to search the databases and retrieve only the new studies that have been added since the last time you searched. Each database uses different date fields and some databases are not able to restrict a search to a specific month and year. Another challenge is that sometimes older articles are added to the databases so if you only search the new time period you might miss articles that were retrospectively added.
There are two solutions.
1. Run new searches on the entire time period, even the years you've already looked at. De-duplicate your new search results against the previous search results which you have hopefully saved. This is relatively easy to do in EndNote but can be cumbersome and slow if you have thousands of results.
2 Run new searches not by publication date but by the date they were entered into the databases. This will retrieve articles that weren't in the databases the last time you searched no matter what their publication date is. Unfortunately, this isn't possible in all databases but it is in most. Here are the methods to do it:
- PubMed: XYZSearchString AND ("2018/12/25"[EDAT] : "3000"[EDAT])
- Ovid Medline: limit LastLineOfSearch to dt="20181225-20201225"
- Ovid Embase: limit LastLineOfSearch to dc=20181225-20201225
- Cochrane Library: use the Custom Date Range box [publication date, not entry date]
- Ovid PsychINFO: limit LastLineOfSearch to up=20181225-20201225
- Scopus: Search Within Results: ORIG-LOAD-DATE AFT 20181225
- CINAHL (Ebsco): AND new line: EM 20181225-20201225
- Web of Science : AND PY=(2018-2020) [can only search by publication date and year]
- ClinicalTrials.gov : Use the "First Posted" date field
- << Previous: Evaluating a Systematic Review
- Next: Cochrane Systematic Reviews >>
- Last Updated: May 2, 2024 2:38 PM
- URL: https://mdanderson.libguides.com/systematicreviews
Improving your search strategy: date limit filters (2/2)
May 6, 2021

This blog post follows on from ‘ Improving your search strategy: Randomised controlled trial filters ’.
Electronic database searches can be run on multiple platforms. This article focusses primarily on applying date limits in Ovid.
When to use date limit filters
Study selection criteria for systematic literature reviews (SLRs) may specify publication date limits, for example:
- Only studies published after a drug or device approval date are relevant in an SLR searching for real-world evidence of clinical efficacy and safety
- Date limits may be needed in SLRs of rapidly evolving fields where previous interventions have become obsolete
- Health technology assessment agencies often require SLRs to be conducted within 6 months prior to submission – an SLR using date limit filters may be performed to update a previous SLR.
Various date limit filters can be used to search different electronic databases. Like other filters applied to search strings, these influence the sensitivity (number of relevant records retrieved) and precision (number of irrelevant records retrieved).
Limiting searches by year
In Ovid, a built-in ‘Publication Year’ filter can be used to limit a search from a specific year, for example ‘2015 to current’ (Figure 1).
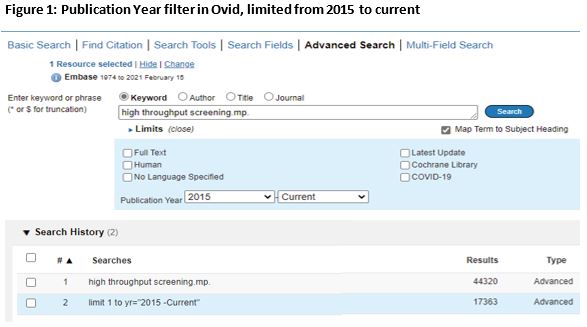
Limiting searches using specific dates
Limiting searches by a specific date, rather than just publication year, is more complex. One option is to use the ‘Date of Publication’ field. However, citation formatting is inconsistent in Embase (some publication dates are structured DD MMM YYYY [e.g. 18 JAN 2018]; others are unstructured [e.g. Jan-Feb 2018, or Winter 2018]) (1, 2). In Medline, the ‘Date of Publication’ is consistently formatted (YYYY MM DD); however, the month and/or day are not present in every record (3). This variability makes ‘Date of Publication’ unsuitable for limiting a search by specific dates (1). Therefore, other date limiting fields must be selected and these vary across databases.
Date limit filters available in Embase
Several date limits are available when searching Embase in Ovid, all formatted YYYYMMDD (2):
- Date Delivered (.dd.): the date a citation XML file was produced for distribution to Ovid with the state=”new”. This includes only new records; The Date Delivered is removed when a record is revised (1)
- Revised Date (.rd.): the date the citation XML file was produced for distribution to Ovid with the state=”update”. This date can change if an updated record is delivered to Ovid
- Date Created (.dc.): the date of final activity on a citation before the XML file is produced for distribution to Ovid. This date is always present in an Embase record. The Date Created is not removed when a record is revised but will change as a new record is added. There may be a short lag between the Date Created and Date Delivered while the record is processed and added to the database (1)
- Entry Week (.em.): the year and week (01–52) when records were added to Embase on Ovid, since the yearly database reload. The Entry Week includes all records but can only be used within the current year (1).
Date limit filters available in MEDLINE
Several date limits are available when searching MEDLINE in Ovid, all formatted YYYYMMDD (3):
- Create Date (.dt.): the date a record is added to PubMed before processing begins (adding Medical Subject Headings [MeSH] terms, completing quality validations, and distributing the record to PubMed). This is not the same as the publication date. This date remains the same throughout any revisions
- Revised Date (.rd.): the date a record is revised or corrected in Ovid MEDLINE
- Entry Date (.ed.) : the NLM internal Date Completed, when processing of the record ends. The entry date may change when a record is revised, and some records do not have an entry date
- Update Date (.up.): the date a record was added to MEDLINE since the yearly reload completion. This date changes with each reload reflect the date Ovid starts processing the reload data, so can be used as a limit only within the current year
- Electronic Date of Publication (.ep.): the date a record was sent to NLM for inclusion in MEDLINE
- Entrez Date (.ez.): the date a citation was added to PubMed
- MeSH Date (.da.): the date MeSH terms were added to a citation. The MeSH date is set equal to the Entrez date until MeSH terms are added.
Date limit filters available in Cochrane databases
When searching Cochrane Central Register of Controlled Trials in Ovid, the only available date limit is Ovid’s built-in ‘Publication Year’ filter. When searching Cochrane Database of Systematic Reviews in Ovid, three date limit fields are available (4):
- Date of Publication (.dp.) : the month, year, and possibly day that the journal was published. This can only be limited by year
- Date of Most Recent Amendment (.dr.): the month, year, and possibly day that the review or protocol was last updated. Searches can be limited by a range of years, or searches can be limited to more specific dates, including day and month, only for a single date, not a date range (format, MM-DD-YYYY)
- Date of Most Recent Substantive Amendment (.ds.) : the month, year, and possibly day that the review or protocol was revised in a substantial way. Searches can be limited by a range of years, or searches can be limited to more specific dates, including day and month, only for a single date, not a date range (format, MM-DD-YYYY).
Working example
In a recent project, SLR search strings were limited to identify evidence published after the 1 st January 2010. As part of the search string development process, a set of relevant records were identified to validate the search strategy. In the first iteration, Date Delivered (.dd) was used when searching Embase in Ovid, missing 13 out of 37 relevant records. To improve the sensitivity of the search string, we assessed two alternative date limit filters:
- Publication year
- Date Created.
Both filters identified all 37 relevant records. As there was no requirement to limit the search to a specific date or month within a year, the best approach was to use the publication year filter. However, if there had been a requirement to limit the search to a specific date within 2010, the Date Created filter would have been the most appropriate to identify all relevant evidence.
Conclusions
Each date limit filter varies in sensitivity and precision. Although the Date Delivered field in Embase is commonly used to limit searches, our experience is it can reduce sensitivity. We have identified alternative methods that use other fields, increasing the search sensitivity. Combining the Date Delivered and Revised Date limits in Embase, or the Create Date and Revised Date limits in MEDLINE, retrieves the most studies. This increased sensitivity reduces the risk of missing relevant studies. However, the precision is likely to be reduced, resulting in retrieval of more irrelevant studies, as records revised within the date limits but published earlier may be included. Therefore, the date limit filter, or combination of filters, should be chosen on a project-by-project basis.
If you would like to learn more about systematic literature reviews, please contact Source Health Economics , an independent consultancy specialising in evidence generation, health economics, and communication.
- Shete S. How can I limit search results by a date range in Ovid? : Wolters Kluwer Health; 2020 [Available from: https://wkhealth.force.com/ovidsupport/s/article/Limit-by-date-range-in-Ovid .
- Wolters Kluwer Health. Embase: Excerpta Medica Database Guide 2021 [Available from: https://ospguides.ovid.com/OSPguides/embase.htm#em .
- Wolters Kluwer Health. MEDLINE® 2020 Database Guide 2020 [Available from: https://ospguides.ovid.com/OSPguides/medline.htm#ED .
- Wolters Kluwer Health. Evidence Based Medicine Reviews: Cochrane Database of Systematic Reviews 2014 [Available from: http://ospguides.ovid.com/OSPguides/cochdb.htm .
More Insights

National Numeracy Day 2024: making maths work for women and girls
Written by Abby Paine, Holly Pilkington, and Dom Partridge Two years ago we wrote a blog for National Numeracy Day 2022, and enjoyed the process so much that we thought we would write another one! Last time, we wrote about network ... Read more

Quality assessment in single-arm trials: insights for systematic reviews
Written by Hannah Shapiro Quality assessment in single-arm trials Quality assessment is a vital aspect of conducting a thorough systematic literature review (SLR), as the validity of the conclusions of the review depends on the ... Read more

A ‘NICE’ Innovation: The Proportionate Approach
Written by Kiera Lander, Assistant Project Manager The National Institute for Health and Care Excellence (NICE) has long been at the forefront of health technology assessment (HTA), ensuring that patients have access to innovative, ... Read more

Out of the frying pan and into the fire: Moving to a market access consultancy company
Written by Brian O'Toole, HTA Writing Lead Last year I changed jobs. It was a major life decision that took time to make as it meant leaving the comfort and familiarity of academia for the hustle and bustle of a market access ... Read more
Cookies Policy
Our Website uses cookies to improve your experience. Please visit our Cookies page for more information about cookies and how we use them.
- Open access
- Published: 28 May 2013
Are systematic reviews up-to-date at the time of publication?
- Elaine M Beller 1 ,
- Joyce Kee-Hsin Chen 2 , 3 , 4 , 5 ,
- Una Li-Hsiang Wang 3 , 6 &
- Paul P Glasziou 1
Systematic Reviews volume 2 , Article number: 36 ( 2013 ) Cite this article
12k Accesses
122 Citations
27 Altmetric
Metrics details
Systematic reviews provide a synthesis of evidence for practitioners, for clinical practice guideline developers, and for those designing and justifying primary research. Having an up-to-date and comprehensive review is therefore important. Our main objective was to determine the recency of systematic reviews at the time of their publication, as measured by the time from last search date to publication. We also wanted to study the time from search date to acceptance, and from acceptance to publication, and measure the proportion of systematic reviews with recorded information on search dates and information sources in the abstract and full text of the review.
A descriptive analysis of published systematic reviews indexed in Medline in 2009, 2010 and 2011 by three reviewers, independently extracting data.
Of the 300 systematic reviews included, 271 (90%) provided the date of search in the full-text article, but only 141 (47%) stated this in the abstract. The median (standard error; minimum to maximum) survival time from last search to acceptance was 5.1 (0.58; 0 to 43.8) months (95% confidence interval = 3.9 to 6.2) and from last search to first publication time was 8.0 (0.35; 0 to 46.7) months (95% confidence interval = 7.3 to 8.7), respectively. Of the 300 reviews, 295 (98%) stated which databases had been searched, but only 181 (60%) stated the databases in the abstract. Most researchers searched three (35%) or four (21%) databases. The top-three most used databases were MEDLINE (79%), Cochrane library (76%), and EMBASE (64%).
Conclusions
Being able to identify comprehensive, up-to-date reviews is important to clinicians, guideline groups, and those designing clinical trials. This study demonstrates that some reviews have a considerable delay between search and publication, but only 47% of systematic review abstracts stated the last search date and 60% stated the databases that had been searched. Improvements in the quality of abstracts of systematic reviews and ways to shorten the review and revision processes to make review publication more rapid are needed.
Peer Review reports
Systematic reviews provide a synthesis of evidence for practitioners, for clinical practice guideline developers, and for those designing and justifying new primary research [ 1 , 2 ]. Because systematic reviews help to set new trials in the context of previous similar research, some healthcare journals have made this a requirement for reporting new research [ 3 ]. An up-to-date systematic review should also be considered before future trials on the same topic are conducted [ 4 ]. Being able to readily identify up-to-date and comprehensive systemic reviews is therefore important to several groups. Hence the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guideline requires describing the information sources (item 7) and the search method (item 8) of systematic reviews [ 5 , 6 ]. These items suggest that review authors describe all information sources searched (for example, databases with dates of coverage, contact with study authors to identify additional studies, and the date they were last searched) [ 6 ]. The PRISMA Statement also suggests including the first of these (information sources) in the abstract of the systematic review report [ 5 ].
Many readers of systematic reviews scan only the abstract in order to determine the relevance of the review to their needs [ 7 ]. Part of this scan should assess the comprehensiveness and recency of the review. For users of reviews the crucial date for assessing recency is the date of last search, rather than the date of publication. Whilst more complex algorithms for assessing whether a review is up-to-date exist [ 8 ], the length of delay from last search is a simple way of assessing the recency of a review when scanning abstracts for relevant papers on a topic.
Although the search dates are usually reported in the main text of reviews, the reporting of these in abstracts is less well documented. We believe that the dates should be reported in the abstract, as this is often where readers assess whether to obtain the full text of articles [ 7 ].
Delays in publication are well documented for some types of research. For clinical trials, one study showed that the median time from completion to first submission of the main results was 10 months, and the time to publication was 23 months [ 9 ]. An analysis of 100 systematic reviews suggested that new research published between the conduct and publication of the review meant that 7% of the reviews were out of date on the day of publication, but did not analyze the length of delay between search and publication [ 8 ]. A study in 2008, prior to the release of the PRISMA Statement, found that the median time from search to publication was 61 weeks [ 10 ]. To document the extent of delay between search and publication in more recent systematic reviews, we decided to sample reviews published between 2009 and 2011 to determine the dates of search completion in relation to the date of publication, and how well this was reported in the abstract and full text of the review.
The primary study objective was to evaluate how up-to-date systematic reviews are at the time of first publication, as measured by the time lag from last search date to publication. Secondary objectives were to ascertain how much of the time from search date to publication was caused by delays in submission and revision of manuscripts, as compared with delays in the publishing lead time, and to determine whether authors provided information on search dates and database sources in the abstract of the review, as this is often the only part of a systematic review that is read by someone screening for relevant papers.
Data search, study selection and data extraction
We collected all systematic review articles indexed in Medline each year from 2009 to 2011 from the National Library of Medicine’s Core Clinical Journals (CCJ) subset of journals [ 11 ]. The CCJ subset was chosen because we wanted a broader selection of journals than the major general medical ones, but needed to limit the search due to the large number of citations to screen to determine which of these were systematic reviews. The CCJ journals are those ‘recommended for individual practitioners and libraries of small hospitals and clinics’ [ 11 ]. We used the broad definition of a systematic review previously used by Moher and colleagues in their study of the epidemiology of systematic reviews: ‘… the authors’ stated objective was to summarize evidence from multiple studies, and the article described explicit methods, regardless of the details provided’ [ 12 ]. The eligible reviews were found using the same search strategy as was used in their study. One reviewer screened titles and abstracts initially, and then full texts, to determine whether the article was a systematic review using only two criteria: that a search strategy was described, and it appeared that all eligible papers were used in the review (for example, table of included studies or similar). A second reviewer independently assessed any reports where the classification was deemed unclear. All systematic reviews about interventions ( n = 860) formed the population from which to sample. Using the random number generator in Excel, we randomly selected 100 intervention reviews from each year.
Data were collected from abstracts and full texts by one reviewer (EMB), with a 10% sample also independently extracted by two reviewers (JK-HC and UL-HW) for quality checking. The data extraction items included the following descriptive information: name of the journal, first author, and year of publication. From each study we extracted details on date of search, date of first publication (for example, online publication if ahead of print), and date of acceptance (where available). If the exact search date was not presented, the end of the month was used (for example, 31 October). Additionally, we checked the date of publication from the journal website if it was not printed on the article. Finally, the databases that had been searched in each systematic review were recorded.
Outcome measures
First, the primary outcome was measured using the time from the last search date to the first date of publication. Second, delays in submission and publication were measured using the time from the last search date to the date of acceptance (where available), and the time from acceptance to first publication. Finally, the proportion of articles reporting the last search date and data sources in the full text and abstract was calculated.
Data management and statistical analysis
All data extraction was managed by Microsoft Office InfoPath. Statistical analysis was conducted with SPSS, v.17 (Chicago, IL, USA). Descriptive statistics were used to summarize the data, using the number and proportion (%) to describe categorical variables and the mean, median, minimum, maximum and standard deviation for continuous variables. A survival analysis was conducted to determine the median time from search to acceptance and publication in published systematic reviews. A Kaplan–Meier curve was used to represent graphically the results of the survival analysis.
Publication location of systematic reviews
Systematic reviews of interventions appeared in 85 of the 118 CCJ journals during 2009 to 2011. Six journals had more than 10 reviews of interventions published in that period ( BMJ , Annals of Internal Medicine , British Journal of Surgery , Annals of Surgery , Pediatrics , Lancet ). Thirty-five of the journals published only one systematic review of interventions during that time.
Time from search to publication
We included 300 systematic reviews. The median (minimum to maximum) time from last search to acceptance was 5.1 (0 to 43.8) months (95% confidence interval = 3.9 to 6.2) and from last search to first publication time was 8.0 (0 to 46.7) months (95% confidence interval =7.3 to 8.7), respectively. The times are shown with the Kaplan–Meier curve in Figure 1 .
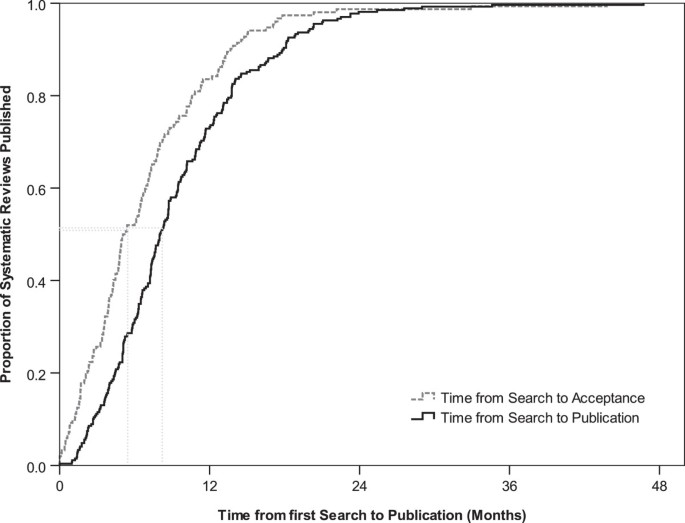
Kaplan–Meier curve demonstrating the time to publication of 300 systematic reviews. The median (minimum to maximum) time from last search to acceptance was 5.1 (0 to 43.8) months (95% confidence interval =3.9 to 6.2) and from last search to first publication time was 8.0 (0 to 46.7) months (95% confidence interval =7.3 to 8.7).
Search date and databases stated in the abstract and full text
In the full text of articles, 90.3% (271/300) stated the search date and 98.3% (295/300) stated the databases that were searched. However, only 47.0% (141/300) of articles stated the search dates and 60.3% (181/300) stated the databases that were searched in the abstract. Interestingly, there were respectively 29 (9.7%) and five (1.7%) articles that did not provide the search date and databases they used even in the full text, as shown in Figure 2 .
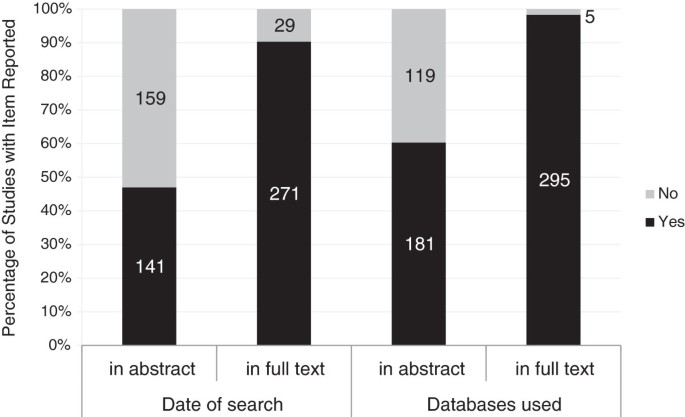
Date of last search and databases searched stated in full text and abstract. Percentage of systematic reviews with date of last search and databases searched stated in the full text and abstract.
Characteristics of information sources in systematic reviews
In 300 included systematic reviews, the mean (standard deviation) number of databases searched was 3.2 (1.6), with the range of databases being one to nine. Most researchers searched three (34.7%, 104/300) or four (21.0%, 63/300) databases in their systematic review. Thirty-four (11.3%) searches were conducted on only one database. Another six (2.0%) articles did not mention how many databases had been searched, as shown in Figure 3 . Overall, the top three most used databases were MEDLINE (78.9% of reviews), Cochrane library (76.0%), and EMBASE (63.5%), as shown in Table 1 .
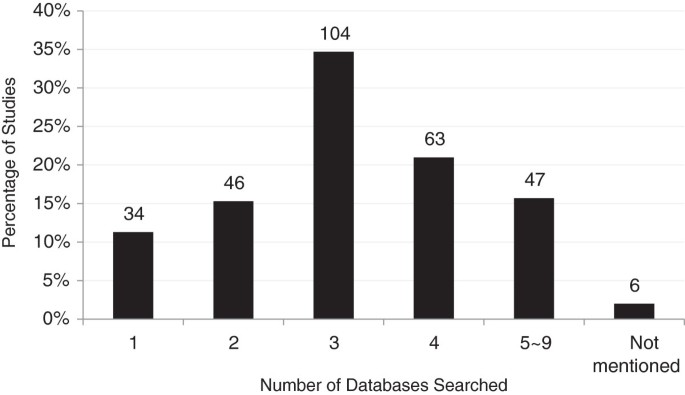
Number of databases searched in 300 published systematic reviews.
Of the 90% of our 300 systematic reviews that provided a date of search, the median time from last search to publication was 8.0 months. This is an improvement over the results reported in 2008 where the median time was around 14 months [ 10 ]. However, the distribution in our study was skewed, with around 10% of reviews having a last search date to publication time of more than 18 months. Since reviews can date rapidly [ 8 ], this delay is important to users of reviewers.
For a reader searching for an up-to-date review, the relevant date is that of the last search not the date of publication, but this was provided in only 47% of abstracts. Hence readers would need to check, and possibly purchase, the full text to determine recency. Similarly, readers may wish to know the list of databases searched to assess completeness of the review, but this was missing from 40% of abstracts.
The time from search to publication can be usefully compared with the half-life of a review’s conclusions. One analysis of 100 systematic reviews found the half-life was 5.5 years until there was a change in the clinical conclusions of a review [ 8 ]. That analysis also found that 7% of reviews were out of date on the day of publication. That is, new research that changed the clinical conclusions was published between the date of search and the date of publication. This is consistent with our finding of a median time from last search to publication delay of 8.0 months.
We found no previous studies on the reporting of dates in abstracts, but several studies have examined the search dates and other items in the full text of reviews. An analysis of 65 Cochrane reviews found that 91% reported the years searched, but only 11% gave the date of last search [ 13 ]. Similarly, a study of 297 systematic reviews found that 70% reported the dates covered by the search, and 77% gave the end date of search, but these were better reported in Cochrane reviews (83% and 91%, respectively) than in non-Cochrane reviews (60% and 67%, respectively) [ 14 ].
Our analysis has some limitations. First, we only selected systematic reviews from MEDLINE's CCJ. If the noncore journals have longer delays then our results are likely to be an underestimate for all reviews. Second, we could only analyze the time from acceptance to publication, and only in some of the reviews. It would be helpful to obtain data on other components such as time for review, revision, re-review, and how often authors did search updates during the revision process. Third when authors did not present an exact date of search we rounded up to the end of the month (for example, October was coded as 31 October).
Given clinicians’ and other decision-makers’ needs for up-to-date reviews, the current length of delay and lack of dates in abstracts needs improvement. Journal publishers need to work with authors to find ways to shorten the time between search and publication. This could be through more rapid review and revision processes, or by providing means to do an additional prepublication search, as some Cochrane review groups do. Authors and editors should both ensure that the date of last search is included in the abstract, in keeping with the PRISMA Statement guidance. Editors and peer reviewers should expect authors to demonstrate compliance with the PRISMA Statement guidance on submission of their article. Publication of the search date in the abstract would make future monitoring of publication delays more feasible.
Being able to identify comprehensive, up-to-date reviews is important to clinicians, guideline groups, and those designing clinical trials. This study demonstrates that some reviews have a considerable delay between search and publication; only 47% of systematic reviews abstracts stated the last search date; and 60% stated the databases that had been searched. To aid readers in rapidly determining the recency of a systematic review, we believe that the date of search should be present in its abstract. Improvements in the quality of abstracts of systematic reviews and ways to shorten the review and revision processes to make review publication more rapid are needed.
Abbreviations
Core Clinical Journals
Preferred Reporting Items for Systematic Reviews and meta-analyses.
Clarke M, Hopewell S, Chalmers I: Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J R Soc Med. 2007, 100: 187-190. 10.1258/jrsm.100.4.187.
Article PubMed PubMed Central Google Scholar
Chung M, Newberry SJ, Ansari MT, Yu WW, Wu H, Lee J, Suttorp M, Gaylor JM, Motala A, Moher D, Balk EM, Shekelle PG: Two methods provide similar signals for the need to update systematic reviews. J Clin Epidemiol. 2012, 65: 660-668. 10.1016/j.jclinepi.2011.12.004.
Young C, Horton R: Putting clinical trials into context. Lancet. 2005, 366: 107-108. 10.1016/S0140-6736(05)66846-8.
Article PubMed Google Scholar
Sutton A, Cooper N, Jones D: Evidence synthesis as the key to more coherent and efficient research. BMC Med Res Methodol. 2009, 9: 29-10.1186/1471-2288-9-29.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009, 6: e1000100-10.1371/journal.pmed.1000100.
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6: e1000097-10.1371/journal.pmed.1000097.
Dogan RI, Murray GC, Neveol A, Lu Z: Understanding PubMed® user search behaviour through log analysis. Database. 2009, 2009: bap018-10.1093/database/bap018.
Google Scholar
Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D: How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007, 147: 224-233. 10.7326/0003-4819-147-4-200708210-00179.
Unalp A, Tonascia S, Meinert C: Presentation in relation to publication of results from clinical trials. Contemp Clin Trials. 2007, 28: 358-369. 10.1016/j.cct.2006.10.005.
Sampson M, Shojania KG, Garritty C, Horsley T, Ocampo M, Moher D: Systematic reviews can be produced and published faster. J Clin Epidemiol. 2008, 61: 531-536. 10.1016/j.jclinepi.2008.02.004.
Abridged Index Medicus (AIM or ‘Core Clinical’) Journal Titles: http://www.nlm.nih.gov/bsd/aim.html ,
Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG: Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007, 4: e78-10.1371/journal.pmed.0040078.
Yoshii A, Plaut DA, McGraw KA, Anderson MJ, Wellik KE: Analysis of the reporting of search strategies in Cochrane systematic reviews. J Med Libr Assoc. 2009, 97: 21-29. 10.3163/1536-5050.97.1.004.
Sampson M, McGowan J, Tetzlaff J, Cogo E, Moher D: No consensus exists on search reporting methods for systematic reviews. J Clin Epidemiol. 2008, 61: 748-754. 10.1016/j.jclinepi.2007.10.009.
Download references
Acknowledgements
This work was in part supported by an NHMRC Australia Fellowship grant number 0527500.
Author information
Authors and affiliations.
Centre for Research in Evidence-Based Practice, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, QLD, 4229, Australia
Elaine M Beller & Paul P Glasziou
Department of Nursing, Taipei Medical University, Wan Fang Hospital, 111, Section 3, Hsing-Long Road, Taipei, 116, Taiwan
Joyce Kee-Hsin Chen
Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan, Taoyuan, 333, Taiwan
Joyce Kee-Hsin Chen & Una Li-Hsiang Wang
School of Nursing, College of Nursing, Taipei Medical University, 250 Wu-Hsing Street, Taipei, 110, Taiwan
Center for Evidence-Based Medicine, Taipei Medical University, 250 Wu-Hsing Street, Taipei, 110, Taiwan
College of Nursing, Chang Gung University of Science and Technology, 261 Wen-Hwa 1st Road, Kwei-Shan, Taoyuan, 333, Taiwan
Una Li-Hsiang Wang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul P Glasziou .
Additional information
Competing interests.
All authors declare that they have no competing interests.
Authors’ contributions
PPG and EMB conceived the idea for the study and design the research protocol. EMB devised the search strategy, searched for studies, and extracted data from abstracts and full texts into Microsoft Office InfoPath. JK-HC and UL-HW extracted 10% data in duplicate for quality checking. JK-HC, UL-HW and EMB conducted the statistical analysis. All authors contributed to data interpretation. JK-HC and UL-HW drafted the manuscript. All authors contributed to revision of the manuscript and approved the final version. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Beller, E.M., Chen, J.KH., Wang, U.LH. et al. Are systematic reviews up-to-date at the time of publication?. Syst Rev 2 , 36 (2013). https://doi.org/10.1186/2046-4053-2-36
Download citation
Received : 07 December 2012
Accepted : 16 May 2013
Published : 28 May 2013
DOI : https://doi.org/10.1186/2046-4053-2-36
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic reviews
- Reporting guidance
- Quality of reporting
- Information retrieval
- Dissemination of results
- Presentation and publication policy
- Time factors
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 August 2022
Transparency of reporting search strategies in systematic reviews
- K. M. Saif-Ur-Rahman 1 , 2 , 3 , 4
Hypertension Research volume 45 , pages 1838–1839 ( 2022 ) Cite this article
1755 Accesses
1 Citations
7 Altmetric
Metrics details
The Original Article was published on 08 August 2022
Systematic reviews are considered the highest level of evidence, as they provide precise information that is essential for decision making at different levels. Searching databases for the literature is one of the fundamental components of a systematic review [ 1 ]. A poor search strategy may lead to low-quality evidence. The recently published systematic review by Zhang et al. [ 2 ] highlighted the association between altitude and the prevalence of hypertension among permanent highlanders. The authors demonstrated methodological robustness in all aspects of the systematic review except for the search strategy. The authors provided two search strategies as supplementary material. Search 1 yielded 1035 citations from MEDLINE (through PubMed) after applying the search date restrictions mentioned by the authors (from inception to April 30, 2021). Search 2 yielded 1204 citations in MEDLINE (through PubMed) after applying the same filter for the date. Interestingly, the authors mentioned in the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) flow diagram that the total number of retrieved articles is 1273, and 694 of them were retrieved from PubMed. This mismatch certainly calls into question the transparency of the review. It seems that the authors failed to report the search strategy transparently to make it replicable. Another issue is that the authors did not provide a comprehensive search strategy for all the databases that they searched (MEDLINE/PubMed, Embase, and Web of Science).
The PRISMA statement [ 3 ] provides the standard norms for reporting a systematic review and meta-analysis. The PRISMA 2020 [ 4 ] statement is the latest updated guideline that specifically recommends that authors should provide the full search strategies for all databases, registries, and websites searched. Systematic reviews should be reported in a way so that they can be replicated and updated to add to the evidence base. For transparent reporting, the presentation of the comprehensive search strategy with all filters applied is essential. The Peer Review of Electronic Search Strategies (PRESS) guidelines have provided a comprehensive direction for developing search strategies for systematic reviews and health technology assessment reports [ 1 ]. The checklist for the PRESS guidelines included the translation of the research question into population, intervention, comparison, and outcome elements; the use of Boolean and proximity operators; the use of database-specific subject headings, text word or free text searching, considerations of spelling, syntax, and line numbers; and limitations and filters. The PRESS guidelines involve the peer review process of the search strategy. However, even if the review is not peer-reviewed, developing search strategies in accordance with the PRESS guidelines is very useful.
The use of artificial intelligence (AI) enhances the robustness of the systematic review process and overcomes resource constraints [ 5 ]. The systematic review accelerator program provides support in developing and refining search strategies with the help of AI. The “search refiner” is a tool that supports understanding the formulation of search strategy, visualizing, and use of Boolean queries. The tool also helps to refine the search queries in more effective ways [ 6 ]. Another interesting tool of the systematic review accelerator is the Polyglot Search Translator [ 7 ], which supports the translation of the search strings across different databases. The use of AI can thus help to develop search strategies efficiently and effectively. This will also support the authors in reporting the search strategies transparently and increase the reliability of the process undertaken. Systematic reviews should be conducted following standard methods to avoid bias in the review process. Standard systematic reviews may provide strong evidence for clinicians, policymakers, researchers, and end users. Systematic reviews conducted without following standard methods and reporting guidelines may lead to waste of research and are simply considered “garbage in and garbage out”. Journals should be strict in requiring that authors perform systematic reviews in accordance with established robust methods.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021
Article PubMed Google Scholar
Zhang Y, Yang Y, Wu X, Han B, Mao A, Gu D, et al. The association between altitudes and the prevalence of hypertension among permanent highlanders. Hypertens Res. 2022. https://doi.org/10.1038/s41440-022-00985-2
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Article PubMed PubMed Central Google Scholar
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 https://doi.org/10.1136/bmj.n71
Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90. https://doi.org/10.1016/j.jclinepi.2020.01.008
Scells H, Zuccon G. searchrefiner: a query visualisation and understanding tool for systematic reviews. Proceedings of the 27th ACM International Conference on Information and Knowledge Management. 2018;1939–42.
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108:195–207. https://doi.org/10.5195/jmla.2020.834
Download references
Acknowledgements
The author acknowledges the different systematic review method groups.
Author information
Authors and affiliations.
Evidence Synthesis Ireland and Cochrane Ireland, University of Galway, Galway, Ireland
K. M. Saif-Ur-Rahman
College of Medicine, Nursing and Health Sciences, University of Galway, Galway, Ireland
Health Systems and Population Studies Division, icddr,b, Dhaka, Bangladesh
Department of Public Health and Health Systems, Nagoya University Graduate School of Medicine, Nagoya, Japan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to K. M. Saif-Ur-Rahman .
Ethics declarations
Conflict of interest.
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Saif-Ur-Rahman, K.M. Transparency of reporting search strategies in systematic reviews. Hypertens Res 45 , 1838–1839 (2022). https://doi.org/10.1038/s41440-022-01003-1
Download citation
Received : 05 July 2022
Revised : 18 July 2022
Accepted : 21 July 2022
Published : 22 August 2022
Issue Date : November 2022
DOI : https://doi.org/10.1038/s41440-022-01003-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic review
- Search strategy
- Transparency
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Research Guides
- Health Sciences Library
Systematic Reviews & Library Assistance
- Systematic Reviews
What is a Systematic Review?
Sample timeline, standards & guidelines, parts of a systematic review.
- Steps & Helpful Tools
- Preparing for your Systematic Review
- Finding the Search Terms
- Running the Search
- Screening & Selecting Studies
- Synthesizing Date & Writing Review
- Other Types of Reviews
- HSL Systematic Review Policy
Systematic Review Self-Paced Module
Prefer a module over a guide? Want to learn a bit more? Check out our new module:
- So you want to conduct a systematic review...
Get Library Help
Need help? Use the buttons to access information on frequently asked questions.
Ask a Question
Library Workshops
Request Articles/Books
How To Tutorials
Review eBooks

A systematic review is a type of literature review that follows certain standards and guidelines. The review involves a rigorous, well documented, transparent, and reproducible search and selection process, where researchers are attempting to gather and synthesize all evidence that answers a specific clinical question.
What is required?
- A team: A systematic review cannot be completed by 1 person.
- Time: Systematic reviews typically take 12 to 18 months to complete (see sample timeline below). Use PredicTER to estimate the amount of time needed.
- A clear question: Systematic reviews are geared to answer clearly defined clinical questions.
- Comprehensive Literature Searches : With a systematic review you are attempting to find and synthesize all relevant information with a reproducible search.
Where do I start?
- Explore the various standards & guidelines of systematic reviews .
- Examine other review types to determine the best option for your project.

Project Management Tools to assist with the systematic review process:
- Systematic Review Timeline Roles & Responsibilities Checklist
There a variety of standards and guidelines geared towards conducting and writing systematic reviews. Some publishers may require that certain guidelines and/or standards are followed.
- PRISMA Guidelines
- Cochrane Handbook for Systematic Reviews of Interventions
- National Academies Standards for Systematic Reviews
Systematic Reviews contain 5 major parts with many steps in each of those sections. Use the table below to learn more about these specific steps and tools that can help you through the review process.
Prefer a visual flowchart? Check out: A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research .
|
| ||
|
|
|
|
| Formulate your question using PICO | ||
| Determine if there is already a review on this topic | , , or | If a systematic review already exists or is in process, then conducting an additional review may result in publication challenges. |
| Create and register a protocol | Checklist: |
|
| Define your inclusion and exclusion criteria |
|
|
| Identify 3 to 10 “gold standard” articles (GSAs) | These are ideal articles that you want for your review. | |
| Identify databases for search |
|
|
|
| ||
| Subject heading frequency analysis of gold standard articles |
| Use subject explosion when fitting. |
| Term Harvesting | or | Include synonyms and acronyms. |
| Create search string 1 |
| Combine terms with Boolean operators and add in advanced searching features: truncation, proximity searching, field codes, and wild cards.
|
| Test search string 1 against gold standard articles, if GSAs are not found modify the search string |
| |
| Translate search string to remaining databases | , , or | Remember controlled vocabulary will and advanced search features may vary between databases. |
|
| ||
| Run all database searches on the same day | Note date ran and the number of results from each database. | |
| Search for grey literature, hand searching, etc. (if applicable) |
|
|
|
| ||
| Deduplicate Results | , or | have the ability to detect duplicates. Note number of duplicates removed! |
| Title & Abstract Screening | , , or | Use a minimum of two independent reviewers. |
| Full text Screening | (if necessary) | Use a minimum of two independent reviewers. |
| Quality & Risk of Bias Assessment | , , , or |
|
| Document the Process | & |
|
|
| ||
| Extract data | or | Have two reviewers complete the data extraction and develop a plan for resolving conflicts. Determine the data you need to collect, ideal method to collect this data, and a tool to organize data. Finally run a pilot and adjust if necessary. |
| Synthesize findings (Qualitative or Quantitative) |
|
|
| Write & Publish Review | & |
|
- Next: Preparing for your Systematic Review >>
- Last Updated: May 29, 2024 2:32 PM
- URL: https://guides.libraries.uc.edu/ReviewAssistance
University of Cincinnati Libraries
PO Box 210033 Cincinnati, Ohio 45221-0033
Phone: 513-556-1424
Contact Us | Staff Directory
University of Cincinnati
Alerts | Clery and HEOA Notice | Notice of Non-Discrimination | eAccessibility Concern | Privacy Statement | Copyright Information
© 2021 University of Cincinnati
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Systematic Reviews
Describes what is involved with conducting a systematic review of the literature for evidence-based public health and how the librarian is a partner in the process.
Several CDC librarians have special training in conducting literature searches for systematic reviews. Literature searches for systematic reviews can take a few weeks to several months from planning to delivery.
Fill out a search request form here or contact the Stephen B. Thacker CDC Library by email [email protected] or telephone 404-639-1717.
Campbell Collaboration
Cochrane Collaboration
Eppi Centre
Joanna Briggs Institute
McMaster University
PRISMA Statement
Systematic Reviews – CRD’s Guide
Systematic Reviews of Health Promotion and Public Health Interventions
The Guide to Community Preventive Services
Look for systematic reviews that have already been published.
- To ensure that the work has not already been done.
- To provides examples of search strategies for your topic
Look in PROSPERO for registered systematic reviews.
Search Cochrane and CRD-York for systematic reviews.
Search filter for finding systematic reviews in PubMed
Other search filters to locate systematic reviews
A systematic review attempts to collect and analyze all evidence that answers a specific question. The question must be clearly defined and have inclusion and exclusion criteria. A broad and thorough search of the literature is performed and a critical analysis of the search results is reported and ultimately provides a current evidence-based answer to the specific question.
Time: According to Cochrane , it takes 18 months on average to complete a Systematic Review.
The average systematic review from beginning to end requires 18 months of work. “…to find out about a healthcare intervention it is worth searching research literature thoroughly to see if the answer is already known. This may require considerable work over many months…” ( Cochrane Collaboration )
Review Team: Team Members at minimum…
- Content expert
- 2 reviewers
- 1 tie breaker
- 1 statistician (meta-analysis)
- 1 economist if conducting an economic analysis
- *1 librarian (expert searcher) trained in systematic reviews
“Expert searchers are an important part of the systematic review team, crucial throughout the review process-from the development of the proposal and research question to publication.” ( McGowan & Sampson, 2005 )
*Ask your librarian to write a methods section regarding the search methods and to give them co-authorship. You may also want to consider providing a copy of one or all of the search strategies used in an appendix.
The Question to Be Answered: A clearly defined and specific question or questions with inclusion and exclusion criteria.
Written Protocol: Outline the study method, rationale, key questions, inclusion and exclusion criteria, literature searches, data abstraction and data management, analysis of quality of the individual studies, synthesis of data, and grading of the evidience for each key question.
Literature Searches: Search for any systematic reviews that may already answer the key question(s). Next, choose appropriate databases and conduct very broad, comprehensive searches. Search strategies must be documented so that they can be duplicated. The librarian is integral to this step of the process. Before your librarian creates a search strategy and starts searching in earnest you should write a detailed PICO question , determine the inclusion and exclusion criteria for your study, run a preliminary search, and have 2-4 articles that already fit the criteria for your review.
What is searched depends on the topic of the review but should include…
- At least 3 standard medical databases like PubMed/Medline, CINAHL, Embase, etc..
- At least 2 grey literature resources like Clinicaltrials.gov, COS Conference Papers Index, Grey Literature Report, etc…
Citation Management: EndNote is a bibliographic management tools that assist researchers in managing citations. The Stephen B. Thacker CDC Library oversees the site license for EndNote.
To request installation: The library provides EndNote to CDC staff under a site-wide license. Please use the ITSO Software Request Tool (SRT) and submit a request for the latest version (or upgraded version) of EndNote. Please be sure to include the computer name for the workstation where you would like to have the software installed.
EndNote Training: CDC Library offers training on EndNote on a regular basis – both a basic and advanced course. To view the course descriptions and upcoming training dates, please visit the CDC Library training page .
For assistance with EndNote software, please contact [email protected]
Vendor Support and Services: EndNote – Support and Services (Thomson Reuters) EndNote – Tutorials and Live Online Classes (Thomson Reuters)
Getting Articles:
Articles can be obtained using DocExpress or by searching the electronic journals at the Stephen B. Thacker CDC Library.
IOM Standards for Systematic Reviews: Standard 3.1: Conduct a comprehensive systematic search for evidence
The goal of a systematic review search is to maximize recall and precision while keeping results manageable. Recall (sensitivity) is defined as the number of relevant reports identified divided by the total number of relevant reports in existence. Precision (specificity) is defined as the number of relevant reports identified divided by the total number of reports identified.
Issues to consider when creating a systematic review search:
- All concepts are included in the strategy
- All appropriate subject headings are used
- Appropriate use of explosion
- Appropriate use of subheadings and floating subheadings
- Use of natural language (text words) in addition to controlled vocabulary terms
- Use of appropriate synonyms, acronyms, etc.
- Truncation and spelling variation as appropriate
- Appropriate use of limits such as language, years, etc.
- Field searching, publication type, author, etc.
- Boolean operators used appropriately
- Line errors: when searches are combined using line numbers, be sure the numbers refer to the searches intended
- Check indexing of relevant articles
- Search strategy adapted as needed for multiple databases
- Cochrane Handbook: Searching for Studies See Part 2, Chapter 6
A step-by-step guide to systematically identify all relevant animal studies
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- UNC Libraries
- HSL Academic Process
- Systematic Reviews
Systematic Reviews: Home
Created by health science librarians.

- Systematic review resources
What is a Systematic Review?
A simplified process map, how can the library help, publications by hsl librarians, systematic reviews in non-health disciplines, resources for performing systematic reviews.
- Step 1: Complete Pre-Review Tasks
- Step 2: Develop a Protocol
- Step 3: Conduct Literature Searches
- Step 4: Manage Citations
- Step 5: Screen Citations
- Step 6: Assess Quality of Included Studies
- Step 7: Extract Data from Included Studies
- Step 8: Write the Review
Check our FAQ's
Email us
Call (919) 962-0800
Make an appointment with a librarian
Request a systematic or scoping review consultation

A systematic review is a literature review that gathers all of the available evidence matching pre-specified eligibility criteria to answer a specific research question. It uses explicit, systematic methods, documented in a protocol, to minimize bias , provide reliable findings , and inform decision-making. ¹
There are many types of literature reviews.
Before beginning a systematic review, consider whether it is the best type of review for your question, goals, and resources. The table below compares a few different types of reviews to help you decide which is best for you.
| Systematic Review | Scoping Review | Systematized Review |
|---|---|---|
| Conducted for Publication | Conducted for Publication | Conducted for Assignment, Thesis, or (Possibly) Publication |
| Protocol Required | Protocol Required | No Protocol Required |
| Focused Research Question | Broad Research Question | Either |
| Focused Inclusion & Exclusion Criteria | Broad Inclusion & Exclusion Criteria | Either |
| Requires Large Team | Requires Small Team | Usually 1-2 People |
- Scoping Review Guide For more information about scoping reviews, refer to the UNC HSL Scoping Review Guide.

- UNC HSL's Simplified, Step-by-Step Process Map A PDF file of the HSL's Systematic Review Process Map.
- Text-Only: UNC HSL's Systematic Reviews - A Simplified, Step-by-Step Process A text-only PDF file of HSL's Systematic Review Process Map.

The average systematic review takes 1,168 hours to complete. ¹ A librarian can help you speed up the process.
Systematic reviews follow established guidelines and best practices to produce high-quality research. Librarian involvement in systematic reviews is based on two levels. In Tier 1, your research team can consult with the librarian as needed. The librarian will answer questions and give you recommendations for tools to use. In Tier 2, the librarian will be an active member of your research team and co-author on your review. Roles and expectations of librarians vary based on the level of involvement desired. Examples of these differences are outlined in the table below.
| Tasks | Tier 1: Consultative | Tier 2: Research Partner / Co-author |
|---|---|---|
| Guidance on process and steps | Yes | Yes |
| Background searching for past and upcoming reviews | Yes | Yes |
| Development and/or refinement of review topic | Yes | Yes |
| Assistance with refinement of PICO (population, intervention(s), comparator(s), and key questions | Yes | Yes |
| Guidance on study types to include | Yes | Yes |
| Guidance on protocol registration | Yes | Yes |
| Identification of databases for searches | Yes | Yes |
| Instruction in search techniques and methods | Yes | Yes |
| Training in citation management software use for managing and sharing results | Yes | Yes |
| Development and execution of searches | No | Yes |
| Downloading search results to citation management software and removing duplicates | No | Yes |
| Documentation of search strategies | No | Yes |
| Management of search results | No | Yes |
| Guidance on methods | Yes | Yes |
| Guidance on data extraction, and management techniques and software | Yes | Yes |
| Suggestions of journals to target for publication | Yes | Yes |
| Drafting of literature search description in "Methods" section | No | Yes |
| Creation of PRISMA diagram | No | Yes |
| Drafting of literature search appendix | No | Yes |
| Review other manuscript sections and final draft | No | Yes |
| Librarian contributions warrant co-authorship | No | Yes |
- Request a systematic or scoping review consultation
The following are systematic and scoping reviews co-authored by HSL librarians.
Only the most recent 15 results are listed. Click the website link at the bottom of the list to see all reviews co-authored by HSL librarians in PubMed
Researchers conduct systematic reviews in a variety of disciplines. If your focus is on a topic outside of the health sciences, you may want to also consult the resources below to learn how systematic reviews may vary in your field. You can also contact a librarian for your discipline with questions.
- EPPI-Centre methods for conducting systematic reviews The EPPI-Centre develops methods and tools for conducting systematic reviews, including reviews for education, public and social policy.
Environmental Topics
- Collaboration for Environmental Evidence (CEE) CEE seeks to promote and deliver evidence syntheses on issues of greatest concern to environmental policy and practice as a public service
Social Sciences
- Siddaway AP, Wood AM, Hedges LV. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu Rev Psychol. 2019 Jan 4;70:747-770. doi: 10.1146/annurev-psych-010418-102803. A resource for psychology systematic reviews, which also covers qualitative meta-syntheses or meta-ethnographies
- The Campbell Collaboration
Social Work
Software engineering
- Guidelines for Performing Systematic Literature Reviews in Software Engineering The objective of this report is to propose comprehensive guidelines for systematic literature reviews appropriate for software engineering researchers, including PhD students.
Sport, Exercise, & Nutrition
- Application of systematic review methodology to the field of nutrition by Tufts Evidence-based Practice Center Publication Date: 2009
- Systematic Reviews and Meta-Analysis — Open & Free (Open Learning Initiative) The course follows guidelines and standards developed by the Campbell Collaboration, based on empirical evidence about how to produce the most comprehensive and accurate reviews of research
- Systematic Reviews by David Gough, Sandy Oliver & James Thomas Publication Date: 2020
Updating reviews
- Updating systematic reviews by University of Ottawa Evidence-based Practice Center Publication Date: 2007
- Next: Step 1: Complete Pre-Review Tasks >>
- Last Updated: May 16, 2024 3:24 PM
- URL: https://guides.lib.unc.edu/systematic-reviews
- University of Michigan Library
- Research Guides
Systematic Reviews
- Search Strategy
- Work with a Search Expert
- Covidence Review Software
- Types of Reviews
- Evidence in a Systematic Review
- Information Sources
Developing an Answerable Question
Creating a search strategy, identifying synonyms & related terms, keywords vs. index terms, combining search terms using boolean operators, a sr search strategy, search limits.
- Managing Records
- Selection Process
- Data Collection Process
- Study Risk of Bias Assessment
- Reporting Results
- For Search Professionals
Validated Search Filters
Depending on your topic, you may be able to save time in constructing your search by using specific search filters (also called "hedges") developed & validated by researchers in the Health Information Research Unit (HiRU) of McMaster University, under contract from the National Library of Medicine. These filters can be found on
- PubMed’s Clinical Queries & Health Services Research Queries pages
- Ovid Medline’s Clinical Queries filters or here
- Embase & PsycINFO
- EBSCOhost’s main search page for CINAHL (Clinical Queries category)
- HiRU’s Nephrology Filters page
- American U of Beirut, esp. for " humans" filters .
- Countway Library of Medicine methodology filters
- InterTASC Information Specialists' Sub-Group Search Filter Resource
- SIGN (Scottish Intercollegiate Guidelines Network) filters page
Why Create a Sensitive Search?
In many literature reviews, you try to balance the sensitivity of the search (how many potentially relevant articles you find) & specificit y (how many definitely relevant articles you find ), realizing that you will miss some. In a systematic review, you want a very sensitive search: you are trying to find any potentially relevant article. A systematic review search will:
- contain many synonyms & variants of search terms
- use care in adding search filters
- search multiple resources, databases & grey literature, such as reports & clinical trials
PICO is a good framework to help clarify your systematic review question.
P - Patient, Population or Problem: What are the important characteristics of the patients &/or problem?
I - Intervention: What you plan to do for the patient or problem?
C - Comparison: What, if anything, is the alternative to the intervention?
O - Outcome: What is the outcome that you would like to measure?
Beyond PICO: the SPIDER tool for qualitative evidence synthesis.
5-SPICE: the application of an original framework for community health worker program design, quality improvement and research agenda setting.
A well constructed search strategy is the core of your systematic review and will be reported on in the methods section of your paper. The search strategy retrieves the majority of the studies you will assess for eligibility & inclusion. The quality of the search strategy also affects what items may have been missed. Informationists can be partners in this process.
For a systematic review, it is important to broaden your search to maximize the retrieval of relevant results.
Use keywords: How other people might describe a topic?
Identify the appropriate index terms (subject headings) for your topic.
- Index terms differ by database (MeSH, or Medical Subject Headings , Emtree terms , Subject headings) are assigned by experts based on the article's content.
- Check the indexing of sentinel articles (3-6 articles that are fundamental to your topic). Sentinel articles can also be used to test your search results.
Include spelling variations (e.g., behavior, behaviour ).
Both types of search terms are useful & both should be used in your search.
Keywords help to broaden your results. They will be searched for at least in journal titles, author names, article titles, & article abstracts. They can also be tagged to search all text.
Index/subject terms help to focus your search appropriately, looking for items that have had a specific term applied by an indexer.
Boolean operators let you combine search terms in specific ways to broaden or narrow your results.

An example of a search string for one concept in a systematic review.

In this example from a PubMed search, [mh] = MeSH & [tiab] = Title/Abstract, a more focused version of a keyword search.
A typical database search limit allows you to narrow results so that you retrieve articles that are most relevant to your research question. Limit types vary by database & include:
- Article/publication type
- Publication dates
In a systematic review search, you should use care when applying limits, as you may lose articles inadvertently. For more information, see, particularly regarding language & format limits. Cochrane 2008 6.4.9
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Methods and guidance on conducting, reporting, publishing and appraising living systematic reviews: a scoping review protocol
Affiliations.
- 1 Evidence-based Oncology, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, 50937, Germany.
- 2 Department of Medicine, American University of Beirut, Beirut, Lebanon.
- 3 Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Canada.
- 4 Editorial and Methods Department, Cochrane Central Executive, Cochrane, London, SW1Y 4QX, UK.
- 5 Rafic Hariri School of Nursing, American University of Beirut, Beirut, Lebanon.
- 6 F1000 Research Ltd, London, SE1 8BU, UK.
- 7 School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
- 8 Stockholm Environment Institute, Stockholm, Sweden.
- 9 Mercator Research Institute on Global Commons and Climate Change, Berlin, Germany.
- 10 Africa Centre for Evidence, University of Johannesburg, Johannesburg, South Africa.
- PMID: 35186269
- PMCID: PMC8822136
- DOI: 10.12688/f1000research.55108.1
Background: The living systematic review (LSR) approach is based on an ongoing surveillance of the literature and continual updating. A few guidance documents address the conduct, reporting, publishing and appraisal of systematic reviews (SRs), but the methodology described is either not up-to date or not suitable for LSRs and misses additional LSR-specific considerations. The objective of this scoping review is to systematically collate methodological literature and guidance on how to conduct, report, publish and appraise the quality of LSRs. The scoping review will allow the mapping of the existing evidence on the topic to support LSRs authors seeking guidance and identify related gaps. Methods: To achieve our objectives, we will conduct a scoping review to survey and evaluate existing evidence, using the standard scoping review methodology. We will search MEDLINE, EMBASE, and Cochrane using the OVID interface. The search strategy was developed by a researcher experienced in developing literature search strategies with the help of an information specialist. As for searching grey literature, we will seek existing guidelines and handbooks on LSRs from organizations that conduct evidence syntheses using the Lens.org website. Two review authors will extract and catalogue the study data on LSR methodological aspects into a standardized and pilot-tested data extraction form. The main categories will reflect proposed methods for (i) conducting LSRs, (ii) reporting of LSRs, (iii) publishing and (iv) appraising the quality of LSRs. Data synthesis and conclusion: By collecting these data from methodological surveys and papers, as well as existing guidance documents and handbooks on LSRs, we might identify specific issues and components lacking within current LSR methodology. Thus, the systematically obtained findings of the scoping review could be used as basis for the revision of existing methods tools on LSR, for instance a PRISMA statement extension for LSRs.
Keywords: Living systematic reviews; appraisal; conducting LSRs; methods and guidance; reporting; scoping review.
Copyright: © 2021 Iannizzi C et al.
PubMed Disclaimer
Conflict of interest statement
Competing interests: JMB is a Publishing Executive for F1000 Research Ltd. JMB was involved in editing and drafting of the article, but had no involvement following submission of the final version for publication, nor in the post-publication peer review of the article.
Figure 1.. Framework on the methodological plan,…
Figure 1.. Framework on the methodological plan, from search to data synthesis, for this scoping…
Similar articles
- Methods and guidance on conducting, reporting, publishing, and appraising living systematic reviews: a scoping review. Iannizzi C, Akl EA, Anslinger E, Weibel S, Kahale LA, Aminat AM, Piechotta V, Skoetz N. Iannizzi C, et al. Syst Rev. 2023 Dec 14;12(1):238. doi: 10.1186/s13643-023-02396-x. Syst Rev. 2023. PMID: 38098023 Free PMC article.
- Methodological quality and reporting quality of COVID-19 living systematic review: a cross-sectional study. Luo J, Chen Z, Liu D, Li H, He S, Zeng L, Yang M, Liu Z, Xiao X, Zhang L. Luo J, et al. BMC Med Res Methodol. 2023 Jul 31;23(1):175. doi: 10.1186/s12874-023-01980-y. BMC Med Res Methodol. 2023. PMID: 37525117 Free PMC article.
- Conceptualizing the reporting of living systematic reviews. Khabsa J, Chang S, McKenzie JE, Barker JM, Boutron I, Kahale LA, Page MJ, Skoetz N, Akl EA. Khabsa J, et al. J Clin Epidemiol. 2023 Apr;156:113-118. doi: 10.1016/j.jclinepi.2023.01.008. Epub 2023 Feb 1. J Clin Epidemiol. 2023. PMID: 36736707 Review.
- Methods of conduct and reporting of living systematic reviews: a protocol for a living methodological survey. Khamis AM, Kahale LA, Pardo-Hernandez H, Schünemann HJ, Akl EA. Khamis AM, et al. F1000Res. 2019 Feb 26;8:221. doi: 10.12688/f1000research.18005.2. eCollection 2019. F1000Res. 2019. PMID: 31231512 Free PMC article.
- What guidance is available for researchers conducting overviews of reviews of healthcare interventions? A scoping review and qualitative metasummary. Pollock M, Fernandes RM, Becker LA, Featherstone R, Hartling L. Pollock M, et al. Syst Rev. 2016 Nov 14;5(1):190. doi: 10.1186/s13643-016-0367-5. Syst Rev. 2016. PMID: 27842604 Free PMC article. Review.
- Extension of the PRISMA 2020 statement for living systematic reviews (LSRs): protocol. Kahale LA, Piechotta V, McKenzie JE, Dorando E, Iannizzi C, Barker JM, Page MJ, Skoetz N, Akl EA. Kahale LA, et al. F1000Res. 2022 Jun 10;11:109. doi: 10.12688/f1000research.75449.2. eCollection 2022. F1000Res. 2022. PMID: 38813137 Free PMC article.
- Barriers to and facilitators of living guidelines use in low-income and middle-income countries: a scoping review. Meteku BT, Quigley M, Turner T, Green SE. Meteku BT, et al. BMJ Open. 2024 Jan 17;14(1):e074311. doi: 10.1136/bmjopen-2023-074311. BMJ Open. 2024. PMID: 38233061 Free PMC article. Review.
- Involving Health Care Professionals in the Development of Electronic Health Records: Scoping Review. Busse TS, Jux C, Laser J, Rasche P, Vollmar HC, Ehlers JP, Kernebeck S. Busse TS, et al. JMIR Hum Factors. 2023 Jul 10;10:e45598. doi: 10.2196/45598. JMIR Hum Factors. 2023. PMID: 37428524 Free PMC article. Review.
- Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Emran TB, Islam F, Mitra S, Paul S, Nath N, Khan Z, Das R, Chandran D, Sharma R, Lima CMG, Awadh AAA, Almazni IA, Alhasaniah AH, Guiné RPF. Emran TB, et al. Molecules. 2022 Oct 31;27(21):7405. doi: 10.3390/molecules27217405. Molecules. 2022. PMID: 36364232 Free PMC article. Review.
- Elliott JH, Turner T, Clavisi O, et al. : Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap. PLoS Med. 2014;11(2):e1001603. 10.1371/journal.pmed.1001603 - DOI - PMC - PubMed
- Elliott J, Synnot A, Turner T, et al. : Living systematic review 1: Introduction - the Why, What, When and How. J Clin Epidemiol. 2017;91. 10.1016/j.jclinepi.2017.08.010 - DOI - PubMed
- Cochrane: Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. 2019.
- Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). 2021;n71:372. 10.1136/bmj.n71 - DOI - PMC - PubMed
- Shea BJ, Reeves BC, Wells G, et al. : AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. 2017;358:j4008. 10.1136/bmj.j4008 - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- F1000 Research Ltd
- PubMed Central
Research Materials
- NCI CPTC Antibody Characterization Program
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- About PROSPERO |
- How to register |
- Service information
- My PROSPERO |
- Log in | Join Logout:

PROSPERO is fast-tracking registration of protocols related to COVID-19
PROSPERO accepts registrations for systematic reviews, rapid reviews and umbrella reviews. PROSPERO does not accept scoping reviews or literature scans . Sibling PROSPERO sites registers systematic reviews of human studies and systematic reviews of animal studies .
Before registering a new systematic review, check PROSPERO and the resources on COVID-END to see whether a similar review already exists. If so, please do not duplicate without good reason . Your efforts may be much more useful if switched to a different topic. This will avoid research waste and contribute more effectively to tackling the pandemic.
Shortcut for already registered reviews of human studies relevant to Covid-19
Shortcut for already registered reviews of animal studies relevant to Covid-19
Shortcut for already registered reviews of human and animal studies relevant to Covid-19, tagged by research area
If you do not already have a PROSPERO account, you will need to create one to register a review
Register a review
- Accessing and completing the registration form
Search PROSPERO
Important notice.
To allow the PROSPERO team to focus on COVID-19 and to avoid further delay, during the pandemic all submissions that have been waiting for registration for more than 30 days and which pass a basic automated check will be published automatically . The PROSPERO team will not check these submissions; this will be stated clearly on the published record. Records will be published exactly as submitted; therefore extra care should be taken to ensure that submitted information is accurate. Submissions which do not pass the basic automated check will be automatically rejected.
Due to technical issues, you will not receive an email notification if your record is automatically published. Please check your account after 10 days to confirm registration.
For records that are within 10 days of submission, registrations from the UK will continue to be prioritised because PROSPERO is funded by the National Institute for Health Research (NIHR).
We are receiving a large volume of emails enquiring about progress. As answering these takes time away from processing records, please only email should if it is absolutely necessary. If your enquiry is related to a review on COVID-19 registration please add #COVID-19 to your subject line. For other reviews please allow at least 14 days from submission before enquiring about progress.
Students: We do not (have the resources to) register reviews done as part of training courses, modules or other 'mini' reviews. Feel free to use the system in your learning and to help you develop a full protocol, but do not press the button.
What you will find in PROSPERO
PROSPERO includes protocol details for systematic reviews relevant to health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome.
Systematic review protocols on PROSPERO can include any type of any study design. Reviews of reviews and reviews of methodological issues that contain at least one outcome of direct patient or clinical relevance are also accepted.
From October 2019 PROSPERO will require earlier registration.
Previously, we accepted registrations provided the reviewers had not completed their data extraction but, from 1st October 2019, we will only accept reviews provided that data extraction has not yet started .
We hope that this will reduce the potential for bias by reducing the opportunity for data extraction to lead to conscious or subconscious effects on the review, for instance by influencing decisions to include or exclude certain items in order to shape a review so that it reaches a desired conclusion.
PROSPERO will continue to accept registrations if formal screening of search results against the review’s eligibility criteria is complete, because we understand that the steps of a review up to that point do not always follow a strictly sequential manner. We also recognise that registration before then may be challenging for reviews being done to a short timeline or strict deadline.
We continue to encourage authors to have written a full protocol before they register their review and to provide full and specific details on their methods in the PROSPERO registration record. Generic ‘cut and paste’ statements are discouraged.
Latest new registrations
- Digital health applications (DiGA) for treating depression and generalized anxiety disorder: protocol for a systematic health app review and systematic review of published evidence
- Experiences of healthcare professionals and women aged 70 years and older in the primary care management of osteoporosis
- Online Interventions for carers of people with dementia that focus on support strategies for daily living: a mixed methods systematic review
- Why are cervical screening rates lower in women with learning disabilities? A systematic review
- Suicide in individuals with depression: an updated systematic review and meta-analysis
Accessibility
Cookies and Privacy
Centre for Reviews and Dissemination University of York York, UK YO10 5DD
- Integrity of data and prospective registration
When to register your review
Do not register too early. Your systematic review protocol should be complete before you submit your registration request.
Registering reviews that are never performed is unhelpful to the research community and may discredit the research team. You should therefore have the necessary resource in place to complete the review before you register your protocol (notification of award of research funding or firm commitment that author time is available for unfunded projects).
PROSPERO relies on the integrity of researchers for the accuracy of the data supplied, and the named contacts are accountable for the content of their records. We routinely monitor the time frame given in submissions and seek clarification where this appears overly ambitious prior to confirming registration and providing a PROSPERO registration number. Amendments and updates to the record are made transparent in the audit trail within each record.
On rare occasions, peer reviewers and editors using PROSPERO to compare what was planned with what is reported in the final manuscript, have identified that the initial registration date in PROSPERO post-dates the manuscript submission date to the journal. In these cases the logical explanation is that the stage of review was inaccurately completed in the PROSPERO registration form; otherwise the submission would have been rejected. In such cases, the named contact will be alerted to the issue and given the opportunity to respond within two weeks. If it is confirmed that incorrect information was provided, or no response is received, the content of the PROSPERO record will be removed, leaving the title and the details of the named contact and the following statement:
Since publication of this record it has been established that information provided initially about the stage of the review and anticipated completion date was inaccurate. The review had actually progressed beyond the stage of eligibility for PROSPERO.
Prospective registration aims to facilitate the comparison of reported review findings with what was planned a priori in the protocol. Reviews should ideally be registered before screening against eligibility criteria commences. However, reviews are currently accepted provided they have not progressed beyond the completion of data extraction.
Protocol details for this review should be sought from the named contact.
Please tell us why this record has been updated.
Please provide a brief description of any major changes made to your record e.g. to inclusion criteria or outcomes and why these have been made. This is important information for those reading your record. It helps safeguard against readers assuming that changes have been done to manipulate results and supports the credibility of your review.
PLEASE NOTE: the text you enter here will appear in the published review under the Revision Notes section
- Help with registration
- The registration data set
What happens after submitting a form
Making changes, amendments and updating a published record, what to do after completing a review and after publishing the findings, registering an update of a completed review, inclusion criteria.
- About PROSPERO
- What is registration?
- Cochrane protocols on PROSPERO
- How to cite a PROSPERO record
- Promotion of PROSPERO
- Support for PROSPERO
- References and resources
PROSPERO Covid-19 filters
Click any of the keywords below to search PROSPERO for Covid-19 registrations or click here to see all Covid-19 human studies or here to see all Covid-19 animal studies.
Click to hide the Covid-19 filters and go back to standard PROSPERO searching
| Tag | Count |
|---|---|
| Chinese medicine | 80 |
| Diagnosis | 64 |
| Epidemiological | 247 |
| Genetics | 7 |
| Health impacts | 217 |
| Mental health | 116 |
| Other | 45 |
| PPE | 20 |
| Prognosis | 58 |
| Public health | 19 |
| Transmission | 42 |
| Treatments | 246 |
| Vaccines | 5 |
Click to hide your search history and show search results show your search history and hide search results . Open the Filters panel to find records with specific characteristics (e.g. all reviews about cancer or all diagnostic reviews etc). See our Guide to Searching for more details.
Click to hide the standard search and use the Covid-19 filters.
Health area of review
Review authors may add as many Health Area tags as they want. These tags are not currently applied by Cochrane authors so Cochrane Protocols will not be retrieved with this filter.
- Any health area
- Alcohol/substance misuse/abuse
- Blood and immune system
- Cardiovascular
- Care of the elderly
- Child health
- Complementary therapies
- Crime and justice
- Digestive system
- Ear, nose and throat
- Endocrine and metabolic disorders
- Eye disorders
- General interest
- Health inequalities/health equity
- Infections and infestations
- International development
- Mental health and behavioural conditions
- Musculoskeletal
- Neurological
- Obstetrics and gynaecology
- Oral health
- Palliative care
- Perioperative care
- Physiotherapy
- Pregnancy and childbirth
- Public health (including social determinants of health)
- Rehabilitation
- Respiratory disorders
- Service delivery
- Skin disorders
- Social care
- Tropical Medicine
- Wounds, injuries and accidents
- Violence and abuse
Type and method of the review
- Any review type or method
- Epidemiologic
- Intervention
- Service Delivery
- Systematic review
- Meta-analysis
- Individual patient data (IPD) meta-analysis
- Prospective meta-analysis (PMA)
- Pre-clinical review
- Methodology
- Network meta-analysis
- Review of reviews
- Qualitative synthesis
- Cost effectiveness
Source of the review
- All protocols
- Exclude Cochrane protocols
- Exclude reviews of animal studies for human health protocols
- Cochrane protocols only
- Reviews of animal studies for human health protocols only
| All protocols | ||
| Exclude Cochrane protocols | ||
| Exclude Pre-clinical protocols | ||
| Cochrane protocols only | ||
| Pre-clinical protocols only | ||
Status of the review
- Any review status
- Ongoing update
- Discontinued
Restrict search to specific fields
- Common choices
- PROSPERO Registration number [AN]
- Intervention [IV]
- Funding sources [FU]
- Other fields
- Assessment of bias [BA]
- Collaborators [CL]
- Comparator [CM]
- Completion date [CD]
- Condition studied [CS]
- Conflict of interest [CF]
- Contact address [AD]
- Context [CT]
- Country [CO]
- Data extraction [DE]
- Data synthesis [DY]
- Dissemination [DS]
- Existing review details [ER]
- Final report [FR]
- Health area [HA]
- Keywords [KW]
- Language [LA]
- Named contact [NC]
- Named contact email [EM]
- Organisational affiliation [OA]
- Original language title [LT]
- Other registrations [RE]
- Participants [PA]
- Primary outcomes [OP]
- Published protocol [PR]
- Review question [RQ]
- Review team [TE]
- Searches [SS]
- Secondary outcomes [OS]
- Start date [SD]
- Status of the review [RS]
- Study type [ST]
- Subgroup analysis [SG]
- Summary [SM]
- Telephone [TL]
- Type of review [RT]
- URL for search [UR]

Date added to PROSPERO
| Select all | Unselect all | Clear history | AND | OR| NOT --> |
| Line | Search for | Hits |
|---|
Export the results of your search to a tagged text file. The file will be prepared and downloaded to your device when you click "Export now" below
An Endnote import filter is available for these records here .
Registering a review on PROSPERO
PROSPERO is an international database of prospectively registered systematic reviews in health and social care. Key features from the review protocol are recorded and maintained as a permanent record in PROSPERO. The aim is to provide a comprehensive listing of systematic reviews registered at inception, to help avoid unplanned duplication. By promoting transparency in the process and enabling comparison of reported review findings with what was planned in the protocol PROSPERO also aims to minimise the risk of bias in systematic review.
PROSPERO has been developed and is managed by the Centre for Reviews and Dissemination (CRD) at the University of York and is funded by the UK’s National Institute for Health Research (NIHR).
What does registration on PROSPERO involve?
Registration in PROSPERO involves the prospective submission and publication of key information about the design and conduct of a systematic review.
Registration on PROSPERO is free of charge. In return, registrants are accountable for the accuracy and updating of information submitted.
PROSPERO includes details of any planned or on-going systematic review that has a health related outcome.
PROSPERO accepts:
Systematic review protocols assessing:
- Interventions (Including qualitative and individual participant data reviews )
- Diagnostic accuracy
- Prognostic factors
- Epidemiological reviews relevant to health and social care
- Public health
- Service delivery in health and social care
- Methodological
Further information on protocol development specific to review types is accessible below.

Requirements for registration
- A full protocol should be ready before registering with PROSPERO
- Submissions must be made before data extraction commences (from October 2019)
- Registration forms must be complete.
- Submissions must be in English (search strategies and protocols attached to a record may be in any language).
PROSPERO does not accept:
- Systematic reviews without an outcome of clear relevance to the health of humans
- Scoping reviews
- Literature reviews that use a systematic search
- Systematic reviews assessing sports performance as an outcome
- Methodological reviews that assess ONLY the quality of reporting
- Systematic critical appraisals
Other considerations
- Cochrane protocols are automatically uploaded- To avoid duplication of records, Cochrane protocols should not be registered separately with PROSPERO.
- Systematic reviews of animal studies only are not eligible for registration in the section of PROSPERO dedicated to reviews of human studies. These should be registered in the section of PROSPERO for animal studies.
- Systematic reviews of in-vitro studies only are not eligible for registration on PROSPERO. We recommend registering such protocols elsewhere, for instance on Open Science Framework.
If you are in any doubt about the eligibility of your review, including the stage of progress please contact us by email using the details on the contact page for advice.
Do not register too early. Your systematic review protocol should be complete before you submit your registration request
Registering reviews that are never performed is unhelpful to the research community and may discredit the research team. You should therefore have the necessary resource in place to complete the review before you register your protocol (notification of award of research funding or firm commitment that author time is available for unfunded projects).
Accessing and navigating the registration form
Obtain a username and password by following the ‘Join’ link in the top right hand corner of the PROSPERO website.
Once you have joined, you can ‘Sign in’ and then you will be able to select ‘Register a review’ in the left hand column. This opens a page that encourages you to check that your review will meet the inclusion criteria and that the review is not already registered, if you are happy to continue, click on Open the registration form. The Open the registration form option opens a page where you are asked to confirm your review is eligible for inclusion and sufficiently different from any other review registered. If you are happy to confirm the information you may proceed to the registration form by clicking on the Register a review box. This will take you to the electronic registration form which has 22 required fields and 18 optional fields. ‘Required’ fields, marked with a red asterisk, must be completed before the Submit button can be accessed. You may save and exit the form at any time, and return at a later date changes are also automatically saved when a field is exited. You are able to add or edit information at a later time by signing in at the main page and going to ‘My PROSPERO records’.
Providing access to a protocol is not a substitute for entering data into the required fields.
Most registrants complete the form in 60 minutes or less.

Access to your record to make further changes or updates is suspended during the administrative process. Receipt of submission is acknowledged in an automated email sent to the named contact.
Registration forms are checked against the eligibility criteria for PROSPERO and if they meet the inclusion criteria are checked for clarity of content before being approved and published on the register, returned for clarification or rejected. We endeavor to provide an update within five working days. However if you wish to enquire on the progress of your submission please contact the administration team by email using the details on the contact page .
Once published on the register, the record will again become accessible for future editing. However the original document submitted will remain in the register to provide a permanent record for the audit trail and for reference. The form can also be saved as a pdf or word processing document if you want to share with others working on the review before submitting.
Should your submission be rejected for registration the record will then be locked and no further edits can be made. Further access to the record for editing is not then possible without contacting us by email using the details on the contact page .
Changes, amendments and updates can be made to a published record by signing in, going to My PROSPERO records and opening the saved record. Once the changes have been made you must click the Submit button. You will then be asked to give brief details of the changes made. The information entered here will appear in the public record and should inform users of the database of the nature of the changes made (e.g. removed one of the outcome measures; changed the anticipated completion date).
Full guidance notes are available here .
All submitted edits and changes to a PROSPERO record will be recorded, dated, and made available within the public record audit trail. The most recent version will appear with previous versions accessible from dated links on the right-hand side of the screen, with the revision notes.
Records remain permanently on PROSPERO. Once the review is completed this information should be updated in the record together with the anticipated publication date. The bibliographic reference and electronic links to publications should be added to the record by the authors. In the absence of a publication, details of availability of the review’s unpublished results, or reasons for the discontinuation of the review, should be documented. Reminder emails with detailed instructions on what to do, are sent on the anticipated completion and publication dates.
If you decide to update the registration of a review you have already completed, you can access the record by signing in and going to My PROSPERO records. You can make changes to the protocol and submit it as an update and it will be processed as for a new review. It is important to decide if you are updating a review, or in fact because of changes to the protocol, are doing a new review. The following definitions have been provided to help you decide.
What is an update of a review?
Updating a systematic review is a discrete event during which efforts are made to identify and incorporate new evidence into a previously completed systematic review An ‘update’ may be any modified version of a review that includes the findings of a more recent search than the previously completed version of the review. It can still be considered an update even if the new search reveals no additional studies. Any newly identified studies should be assessed and, if appropriate, incorporated into the updated review. An update might also be an opportunity to conduct new analyses or add additional information to the review.
What constitutes a new review rather than an update?
It can be difficult to decide whether an update to a review is in fact a new review. There is little published guidance on this. PROSPERO adopts a pragmatic approach. If changes to the review questions or methods are so substantial that they require major changes to the original protocol, this should be regarded as a new review rather than an update. Examples that would constitute a new review:
- addition of new treatment comparisons e.g. direct comparison of different drugs, when the old review included only comparisons of drug with placebo;
- substantial changes to the population being studied e.g. adding adults to a review that was previously restricted to children;
- exclusion criteria in the old review become inclusion criteria in the new review;
- introduction of new analysis techniques e.g. a switch from aggregate data meta-analyses to individual participant meta-analyses.
If in doubt, a new record for a new review should be created. This will minimise the complexity of the editing to the original record in PROSPERO and make it easier for users to distinguish between the original review and the later version. Links between the new and original review can be added in field #37 in the registration form.
Guide to completing the registration fields
The following guidance notes follow the format of the registration form. The guidance includes a description and example of what is required for each of the fields within each section.
SPECIAL NOTES: We accept information in good faith and rely upon the integrity of researchers to ensure the validity of all the data presented in PROSPERO records. Action will be taken if inaccuracies in data, particularly stage of review and anticipated completion date , are identified at any time.
PROSPERO records need to be fully searchable so the information requested needs to be in the fields, even if access to a protocol is given in Field 34. The records are permanent but links are not. We therefore do not accept submissions that refer the reader to the protocol without providing the basic information in the fields.
Registering a review of animal studies on PROSPERO
PROSPERO for animal studies is an international database of prospectively registered systematic reviews of animal studies relevant to human health. Key features from the review protocol are recorded and maintained as a permanent record in PROSPERO. The aim is to provide a comprehensive listing of systematic reviews registered at inception, to help avoid unplanned duplication. By promoting transparency in the process and enabling comparison of reported review findings with what was planned in the protocol PROSPERO also aims to minimise the risk of bias in systematic review.
PROSPERO for animal studies has been developed and is managed by the SYRCLE-CAMARADES team and the Centre for Reviews and Dissemination (CRD) at the University of York. It is funded by the UK’s National Institute for Health Research (NIHR).
- Systematic reviews of human studies only are not eligible for registration in the section of PROSPERO dedicated to reviews of animal studies. These should be registered in the section of PROSPERO for human studies.
Registering reviews that are never performed is unhelpful to the research community and may discredit the research team. You should therefore have the necessary resources in place to complete the review before you register your protocol (notification of award of research funding or firm commitment that author time is available for unfunded projects).
Once you have joined, you can ‘Sign in’ and then you will be able to select ‘Register a review’ in the left hand column. This opens a page that encourages you to check that your review will meet the inclusion criteria and that the review is not already registered. If you are happy to continue, open the registration form by clicking on the "Register a systematic review of animal research studies (study subjects are animals) that is of direct relevance to human health" link. This opens a page where you are asked to confirm your review is eligible for inclusion and sufficiently different from any other review registered. If you are happy to confirm the information you may proceed to the registration form by clicking on the "Register a review" link. This will take you to the electronic registration form which has 26 required fields and 14 optional fields. All subfields of required fields, marked with a red asterisk, must be completed before the protocol can be submitted. You may save and exit the form at any time, and return at a later date changes are also automatically saved when a field is exited. You are able to add or edit information at a later time by signing in at the main page and going to ‘My PROSPERO records’.
Registration forms are checked against the eligibility criteria for PROSPERO. If they meet the inclusion criteria, they are checked for clarity of content before either being approved and published on the register, returned for clarification, or rejected. We endeavour to provide an update within five working days. If you wish to enquire on the progress of your submission please contact the administration team by email using the details on the contact page .
Once published on the register, the record will again become accessible for future editing. However, the original document submitted will remain in the register to provide a permanent record for the audit trail and for reference.
Should your submission be rejected for registration, the record will be locked and no further edits can be made. Further access to the record for editing is not possible without contacting us by email using the details on the contact page .
Changes, amendments and updates can be made to a published record by signing in, going to “My PROSPERO records” and opening the saved record. Once the changes have been made, click the “Submit” button. You will then be asked to give brief details of the changes made. The information entered here will appear in the public record and should inform users of the database of the nature of the changes made (e.g. removed one of the outcome measures; changed the anticipated completion date).
If you decide to update the registration of a review you have already completed, you can access the record by signing in and going to “My PROSPERO records”. You can make changes to the protocol and submit it as an update and it will be processed as for a new review. It is important to decide if you are updating a review, or in fact are doing a new review because of changes to the protocol. The following definitions have been provided to help you decide.
- substantial changes to the population being studied e.g. adding non-rodents to a review that was previously restricted to rodents;
PROSPERO records need to be fully searchable. We can therefore not accept submissions in which only a link to a protocol registered elsewhere is provided, without providing the basic information in the fields.
These screening questions check whether your review is eligible for inclusion in PROSPERO and avoid wasting your time if it is not eligible.
Will your registration record be in English?
Is this a scoping, literature or mapping review.
PROSPERO does not accept scoping reviews, literature reviews or mapping reviews. This should not stop you from submitting your full protocol or completed review for publication in a journal.
To copy this explanation to the clipboard to include with a journal submission click here .
Resources describing scoping/mapping reviews can be found here .
Does your review include a health outcome with direct relevance to human health?
(e.g. reviews of educational interventions to improve maths skills are not eligible, reviews of educational interventions to promote breastfeeding are eligible)
Is your review of methodological studies that have a clear link to human health?
(e.g. relates to systematic review or clinical study methods)
Is this a Cochrane review?
Cochrane reviews are usually uploaded to PROSPERO from the Cochrane Library and do not need to be registered via the PROSPERO website. However, this is temporarily suspended because of technical issues that we are working to resolve.
If you have a special case review please talk to your Cochrane Review Group and/or contact the PROSPERO team.
Is this a mini or partial review done for a training course or classwork or are you using the system to learn how to register?
PROSPERO does not have resources to process applications for reviews done only for training purposes. This includes mini reviews restricted to a subset of eligible studies, demonstrator reviews where a whole class does the same review, or any other projects that are less than full systematic reviews.
For learning purposes you may download and complete the PROSPERO form as a PDF document . If you do complete the form online, please save this in your own space and do not SUBMIT it for publication.
Please check PROSPERO for similar systematic reviews before proceeding
Checking whether a similar review already exists should be one of the first steps taken in a systematic review.
Knowingly repeating an existing systematic review is not necessarily wrong, but to avoid research waste there should be a reason for doing this - for example if the new review will incorporate additional studies, use new or alternative methods of analysis, or have a different focus.
PROSPERO does not prevent registration of similar reviews. However, registrations are dated in PROSPERO and a journal could decide not to publish a review that has repeated an already registered review without justification.
If you find a similar review in PROSPERO, but are unsure if it is the same or if it will be completed and published, we suggest that you contact the author to find out before proceeding. Contact details can be found within the PROSPERO record.
I have searched PROSPERO and...
Have you written a protocol.
PROSPERO registration captures key information about the design and conduct of a planned systematic review. It is not a full protocol. We strongly encourage you to write a full protocol before completing the PROSPERO registration form (although you may proceed without doing this).
Will more than one person be involved in the systematic review?
We strongly recommend that you follow best practice and include more than one person in the review team. PROSPERO will not accept registrations unless there is more than one person conducting the review. You must include details of the other author(s) in the registration form.
Do you intend to publish the results of your systematic review and/or make them publicly available when completed?
PROSPERO aims to increase transparency and help prevent unintended duplication of effort. This requires that the results of systematic reviews should be made publicly available e.g. by publication in an academic journal, posting in a research repository or being made available on a permanent website. We therefore do not accept registrations from systematic reviews that will not be made available to others e.g. projects that are internal to an organization or company, or masters dissertations if it is known that these will not be shared.
Have you started your review?
Stage of review.
What work have you already done on your systematic review?
Reviews that have started data extraction (at the time of initial submission) are not eligible for inclusion in PROSPERO. If we find that incorrect status and/or start or completion dates have been supplied, the published PROSPERO record will be withdrawn.
Preliminary searches
Searches conducted prior to a systematic review to assess the extent of the existing literature.
Piloting the study selection process
A process to determine whether the study selection criteria will identify studies eligible for inclusion in the project.
Full searches
Exhaustive searches to identify all publications eligible for inclusion in the review.
Full screening of search results against eligibility criteria
Data extraction
Extracting or obtaining data from included studies or other sources.
Risk of bias or quality assessment
Assessment of the risk of bias or quality of included studies.
Data synthesis
The main project analyses to combine data from eligible studies using formal data synthesis methods.
Please now go ahead and register your review .
Please go back and search PROSPERO before continuing
We look forward to receiving future submissions from you.
DEMO VERSION ONLY
Fields that have an asterisk (*) next to them means that they must be answered. Word limits provide guidance but do not actually limit the number of words that can be entered in each section. You are encouraged to follow maximum length. Registrant means the person filling out the form.
Fields that have an asterisk (*) next to them means that they must be answered. Word limits are provided for each section. You will be unable to submit the form if the word limits are exceeded for any section. Registrant means the person filling out the form .
Please select one of the options below to edit your record. Either option will create a new version of the record - the existing version will remain unchanged.
A list of fields that can be edited in an update can be found here
- 1. * Review title.
- 2. Original language title.
- 3. * Anticipated or actual start date.
- 4. * Anticipated completion date.
- 5. * Stage of review at time of this submission.
- Section 2 Review team details
- 6. * Named contact. Email salutation (e.g. "Dr Smith" or "Joanne") for correspondence:
- 7. * Named contact email.
- 8. Named contact address PLEASE NOTE this information will be published in the PROSPERO record so please do not enter private information, i.e. personal home address
- 9. Named contact phone number.
- 10. * Organisational affiliation of the review. Organisation web address:
- 11. * Review team members and their organisational affiliations.
- 12. * Funding sources/sponsors.
- 12. * Funding sources/sponsors. Grant number(s) State the funder, grant or award number and the date of award
- 13. * Conflicts of interest.
- 14. Collaborators.
- Section 3 Review method
- 15. * Review question.
- 16. * Searches.
- 17. URL to search strategy.
- 18. * Condition or domain being studied.
- 19. * Participants/population.
- 20. * Intervention(s), exposure(s).
- 21. * Comparator(s)/control.
- 22. * Types of study to be included.
- 23. Context.
- 24. * Main outcome(s). Measures of effect
- 25. * Additional outcome(s). * Timing and effect measures --> Measures of effect
- 26. * Data extraction (selection and coding).
- 27. * Risk of bias (quality) assessment.
- 28. * Strategy for data synthesis.
- 29. * Analysis of subgroups or subsets.
- Section 4 General information
- 30. * Type and method of review.
- 31. Language.
- 32. * Country.
- 33. Other registration details.
- 34. Reference and/or URL for published protocol.
- 35. Dissemination plans.
- 36. Keywords.
- 37. Details of any existing review of the same topic by the same authors.
- 38. * Current review status.
- 39. Any additional information.
- 40. Details of final report/publication(s) or preprints if available.
- * MeSH headings.
- 8. * Named contact address. PLEASE NOTE this information will be published in the PROSPERO record so please do not enter private information
- 9. Named contact phone number
- 12. * Funding sources/sponsors. Grant number(s)
- 15. * Review question. Context and rationale
- 18. * Human disease modelled.
- 19. * Animals/population. Inclusion criteria: Exclusion criteria:
- 20. * Intervention(s), exposure(s). Inclusion criteria: Exclusion criteria:
- 21. * Comparator(s)/control. Inclusion criteria: Exclusion criteria:
- 22. * Study designs to be included. Inclusion criteria: Exclusion criteria:
- 23. Other selection criteria or limitations applied.
- 24. * Outcome measure(s). Inclusion criteria: Exclusion criteria:
- 26. * Study selection and data extraction. Example: Screening will be performed in two phases, namely initial screening based on title and abstract, followed by full-text screening of the eligible articles for final inclusion. In each phase, 2 observers will independently assess each article. Discrepancies will be resolved through discussion, or by consulting a third investigator. ">Procedure for study selection Example : Title-abstract screening: 1. Not an original full research paper (e.g. review, editorial) 2. Not an in vivo animal study 3. No metastases/ only primary tumor 4. No control group 5. Combination therapy or contamination 6. Not about analgesics used in the clinic Full text-screening: As above, with the addition of: 7. No relevant outcome measure reported ">Prioritise the exclusion criteria Example: Two reviewers will independently extract data from each article. We first try to extract numerical data from tables, text or figures. If these are not reported, we will extract data from graphs using digital ruler software. In case data are not reported or unclear, we will attempt to contact authors by e-mail (max. 2 attempts). In case an outcome is measured at multiple time points, data from the time point where efficacy is highest will be included. ">Methods for data extraction Example: Experimental groups, control group(s) and number of animals per group. ">Data to be extracted: study design Example: Species, sex, weight, age, co‐morbidity, anaesthetic agent used, method of induction of cardiac ischemia, duration of ischemia and duration of reperfusion (if applicable). ">Data to be extracted: animal model Example: Dose, timing of administration, frequency of administration, route of administration, vehicle. ">Data to be extracted: intervention of interest Example: Serum creatinine; continuous; umol/L (may be recalculated from mg/dL). ">Data to be extracted: primary outcome(s) Example: Blood urea nitrogen; continuous; mmol/L (may be recalculated from mg/dL); Renal histological damage as assessed by Jablonski scale; continuous; Jablonski score. ">Data to be extracted: secondary outcome(s) Example: 1st author, year of publication, language, journal. ">Data to be extracted: other
- 27. * Risk of bias and/or quality assessment.
- 28. * Strategy for data synthesis. An introduction as well as a practical guide to meta-analysis of pre-clinical studies are available. Example: A meta‐analysis will be performed for all outcome measures reported in 10 or more articles. For subgroup analysis a minimum of 8 studies per subgroup is required. If meta‐analysis is not possible, data will be reported through a descriptive summary. ">Planned approach If a meta-analysis is planned , please specify the following:"> Example: number of metastases: standardized mean difference; incidence of metastasis: risk ratio. ">Effect measure The random-effects model is the typical model of choice for pre-clinical meta-analyses. This is because in the fixed-effect model, it is assumed that the differences in observed effect between studies is solely due to sampling error (i.e. differences in sample size), and that the true effect is the same (fixed) across all studies. However, this assumption is unlikely to hold true for data from animal studies, which generally include various species, strains and treatment regimes, for which different true effects are likely to exist. The random-effects model takes into account both the within-study (sampling error) and between-study (differences in the true effect size) variance. Should the excessive between-study variance be very low or zero, the random-effects model will yield the same results as the fixed-effect model. For further details, see the introduction and practical guide to pre-clinical meta-analysis. Example: Because of the exploratory nature of animal studies, a random effects model will be used to account for anticipated heterogeneity. ">Effect models introduction and practical guide to pre-clinical meta-analysis. Example: Heterogeneity will be assessed using the (residual) I2 and adjusted R2 statistics. ">Heterogeneity For further guidance please refer to the introduction and practical guide to pre-clinical meta-analysis. Example: Whenever a control group serves more than one experimental group, we will correct the total number of control animals in the meta-analysis by dividing the number of animals in the control group by the number of treatment groups served. Where applicable, Holm-Bonferroni correction for testing multiple subgroup analyses will be performed. If one or more subgroup analyses cannot be performed due to insufficient data, the p-value will be adjusted accordingly. ">Other
- 29. * Analysis of subgroups or subsets. Subgroup analysis or meta-regression are used to explore between-study heterogeneity and can provide insight into the relationship between study characteristics (e.g. species, sex or drug class or dose) and effect size. They should be considered hypothesis-generating. Ideally, a threshold describing the number of studies per subgroup required for analysis should be specified. For further guidance please refer to the introduction and practical guide to pre-clinical meta-analysis. Example: The following study characteristics will be examined as potential source of heterogeneity: species (stratified per species); sex (stratified per sex); duration of index ischemia (linear); stem cell dose (linear); blinding of outcome assessment reported (stratified yes vs no). For stratified analyses, a minimum number of 8 studies per subgroup is required. ">Subgroup analyses A sensitivity analysis is conducted to assess the impact of decisions taken in the review process on the meta-analysis outcome. These decisions may have been made in various stages of the review, e.g. the decision to exclude certain disease models, the decision to pool certain units of measurement for an outcome, the choice of effect measure, how subgroup variables are stratified etc. In order to assess the robustness of the findings of the meta-analysis, the analyses are re-run using the alternative options for each decision. If the results of both meta-analyses are similar, the results seem robust. When the conclusions of a meta-analysis significantly change, this should be discussed. Example: To test the robustness of our findings when selecting the time point of greatest efficacy we will re-run the analysis with data from the latest possible time point (in studies reporting an outcome at multiple time points). We will test the robustness of linear regression of time-to-treatment by performing stratified analysis (treatment pre-ischemia vs during vs post-ischemia). We will assess the effect of our decision to pool all reported scales for histological damage by re-running the analyses using only data from studies using the Jablonski scale. ">Sensitivity Example: For meta-analyses using the mean difference or risk ration as effect measure and containing at least 20 studies, we will produce funnel plots and assess publication bias using Egger’s regression test. ">Publication bias
- 30. * Review type.
- 36. * Keywords.
Log in to PROSPERO
Your password request has been confirmed. Please log in with your new password.
Please enter your email address and password.
| --> | |
See our privacy notice here
Reset/change password
Please note that the reset password function is currently not available. We apologise for any inconvenience and expect a fix to be in place later today.
Please enter your email address and hit the Reset button. If your account exists an email will be sent to you with a link to change your password. The link will expire in one hour
PROSPERO - My login details
Please note, your email address, postal address and name will be used to populate automatically any records you create and will therefore be published with the records. BY COMPLETING THIS FORM YOU AGREE TO THIS INFORMATION BEING PUBLISHED (see our privacy notice here)
| * Denotes required field. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Service information pagePROSPERO is currently prioritising registration of COVID-19 protocols and continues to receive a vast and increasing number of registrations. To allow the PROSPERO team to focus on COVID-19 and to avoid further delay, during the pandemic all submissions that have been waiting for registration for more than 30 days and which pass a basic automated check will be published automatically . The PROSPERO team will not check these submissions; this will be stated clearly on the published record. Records will be published exactly as submitted; therefore extra care should be taken to ensure that submitted information is accurate. Submissions which do not pass the basic automated check will be automatically rejected. Due to technical issues, you will not receive an email notification if your record is automatically published. Please check your account after 30 days to confirm registration For records that are within 30 days of submission, registrations from the UK will continue to be prioritised because PROSPERO is funded by the National Institute of Health Research (NIHR). We are receiving a huge volume of emails enquiring about progress. Answering these takes time away from processing records, so we ask that you only email should it be absolutely necessary. If your enquiry is related to a review on COVID-19 registration please add #COVID-19 to your subject line. For other reviews please allow at least 34 days from submission before enquiring about progress. We thank you for your understanding in advance. Previous changes With effect From 1st October 2019, PROSPERO only accepts reviews provided that data extraction has not yet started. This is intended to reduce potential for bias by reducing the opportunity for (conscious or subconscious) selection or manipulation of data during extraction to shape a review so that it reaches a desired conclusion. PROSPERO will continue to accept registrations if formal screening of search results against the review’s eligibility criteria is complete, because we understand that the steps of a review up to that point do not always follow a strictly sequential manner. We also recognise that registration before then may be challenging for reviews being done to a short timeline or strict deadline. Records are now date stamped to show initial submission date and date of receipt of revised records (for those returned to authors for amendment), as well as registration date. Authors should have written a full protocol before they register and provide full and specific details on their methods in the PROSPERO registration record. Generic ‘cut and paste’ statements should not be used. STUDENTS doing mini-reviews or other training exercises should NOT register PROSPERO has limited resource and is unable to process student work done as part of their training these (handling them takes time away from full projects intended for publication). Students may use the system to create and store a record by saving but please do not submit. Substantial reviews done for dissertations or theses may be registered but will require email confirmation by supervisors. PROSPERO has temporarily suspended the automatic uploading of Cochrane protocols because of technical issues. We are working to resolve these and hope to resume the automatic registration of Cochrane protocols over the coming months. NIHR SurveyWe have been asked by NIHR to investigate if and how PROSPERO is helping to reduce unintended duplication of systematic reviews. This information will be used in support of our forthcoming application for renewed funding for PROSPERO. Please help us by agreeing to participate in a short on-line survey that should take no more than 15 minutes to complete. At this stage we just need your permission to send you a link to the survey in June. Yes please, send me the link No thanks PRIVACY STATEMENT - Information about you: how we use it and with whom we share it We will add your email address to a list that we will use to send you a link to the above survey. By agreeing to participate in the survey, you give your consent for us to do so. We will not hold or use this information for any other purpose and we will not share with any third party. We do not use profiling or automated decision-making processes. Thank you for helping us to secure future funding for PROSPERO. Your code has expired. Please contact us by email using the details on the contact page to get a new code Thank you. We will send you a link to the survey when it is launched in June Thank you. We have recorded your preference not to be sent a link to the survey If you have an issue you can contact us... Systematic reviews of humans Email: [email protected] Tel: +44 (0)1904 321049 Systematic reviews of animals Email: [email protected] This field uses answers to initial screening questions. It cannot be edited until after registration. Tick the boxes to show which review tasks have been started and which have been completed. Update this field each time any amendments are made to a published record. Give the working title of the review, for example the one used for obtaining funding. Ideally the title should state succinctly the interventions or exposures being reviewed and the associated health or social problems. Where appropriate, the title should use the PI(E)COS structure to contain information on the Participants, Intervention (or Exposure) and Comparison groups, the Outcomes to be measured and Study designs to be included. Acronyms may be included in titles, but should not be used alone without expansion unless they are regarded as more usual than the expansion (e.g. HIV). The title in this field must be in English. If the original title is in a different language the English version must be entered here, with the non-English version entered into the field labelled “Original Language Title”. If the final title of the review differs, this can be displayed in the Publication of Final Report Field. Example: Systematic review and meta-analysis of recurrence and survival following pre- versus post-operative radiation in localized, resectable soft-tissue sarcoma. For reviews in languages other than English, this field should be used to enter the title in the language of the review. This will be displayed together with the English language title. Example: Revisión sistemática y meta-análisis de la recurrencia y la supervivencia tras la fase de radiación en comparación con post-operatorio en el sarcoma localizados resecables de tejido blando. Give the date when the systematic review commenced, or is expected to commence. For the purposes of PROSPERO, the date of commencement for the systematic review can be defined as any point after completion of a protocol but before formal screening of the identified studies against the eligibility criteria begins. A protocol can be deemed complete when it is approved by a funder or the person commissioning the review; when peer review is complete; when the protocol is published or when the authors decide that it is complete and they do not anticipate any major revisions to the design of the systematic review. This field may be edited at any time. All edits to published records will appear in the record audit trail. A brief explanation of the reason for changes should be given in the Revision Notes facility. Example: 01 June 2011 Give the date by which the review is expected to be completed. In the absence of an agreed contractual date, a realistic anticipated date for completion should be set. It can be modified should the schedule change. When this date is reached, the named contact will receive an automated email to ask them to provide an update on progress. This field may be edited at any time. All edits will appear in the record audit trail. A brief explanation of the reason for changes should be given in the Revision Notes facility. Indicate the stage of progress of the review by ticking the relevant Started and Completed boxes. Additional information may be added in the free text box provided. Please note: Reviews that have progressed beyond the point of completing data extraction at the time of initial registration are not eligible for inclusion in PROSPERO. Should evidence of incorrect status and/or completion date being supplied at the time of submission come to light, the content of the PROSPERO record will be removed leaving only the title and named contact details and a statement that inaccuracies in the stage of the review date had been identified. This field should be updated when any amendments are made to a published record and on completion and publication of the review. Example: Preliminary searches ticked as completed, pilot of the study selection process ticked as started. The named contact acts as the guarantor for the accuracy of the information presented in the register record. This should be the lead reviewer or a representative of the review team. This person is also responsible for submitting details of any amendments while the review is ongoing and publication details after the review is completed. The named contact is the person to whom users of PROSPERO would send questions or comments. This field is automatically populated from the named contact’s signing in details. The named contact’s name will be displayed in the public record. Example: Dr Joseph Bloggs N.B. To change the named contact for a published record, send details of the existing and new contact to [email protected] Give the electronic mail address of the named contact. This may be a generic email address to which the named contact has access. This field is automatically populated from the named contact’s joining details, but can be changed if required. The email address supplied here will be displayed in the public record. Examples: [email protected] or [email protected] Give the full postal address for the named contact. (N.B. This field is automatically populated from the named contact’s joining details.) This address will be displayed in the public record. If you do not wish it to appear in the public record delete the content of this field. Example: Alcuin B Block,University of York, York, YO10 5DD, UK Give the telephone number for the named contact, including international dialling code. (N.B. This field is automatically populated from the named contact’s joining details.) This number will be displayed in the public record. If you do not wish it to appear in the public record delete the content of this field. Example: +44 (0)10904 321040 Full title of the organisational affiliations for this review and website address if available. This field may be completed as ‘None’ if the review is not affiliated to any organisation. Example: Andalusian Agency for Health Technology Assessment (AETSA) Give the personal details and the organisational affiliations of each member of the review team. Affiliation refers to groups or organisations to which review team members belong. NOTE: email and country are now mandatory fields for each person. . Affiliation refers to groups or organisations to which review team members belong. Review team members will be listed ‘manuscript’ style in the order entered in this list. The named contact will be automatically added to this field, but can be deleted if not a member of the review team. To place the named contact somewhere other than first in order, delete the automatic entry and enter members’ details in the required order. Membership of the review team and details of affiliations can be updated at any time. All edits will appear in the record audit trail. Example: Mr Joseph Bloggs, Centre for Reviews and Dissemination, University of York, UK. Dr Jane Smith, Department of Health Sciences, University of York, UK. Prof. Steven Jones, Centre for Health Statistics, Medical Research Centre, Canada. Give details of the individuals, organizations, groups or other legal entities who take responsibility for initiating, managing, sponsoring and/or financing the review. Include any unique identification numbers assigned to the review by the individuals or bodies listed. Examples: NIHR HTA Programme (Project ref 09/13/02). The Terry Fox New Frontiers Program in Cancer (Ref 201006TFL). Funding provided by Amgen, Merck, Roche, and Sanofi-aventis. List any conditions that could lead to actual or perceived undue influence on judgements concerning the main topic investigated in the review. The conflicts of interest listed should cover the review team as a whole, as well as individuals in the team. Conflicts of interest arise when a team member or the team as a whole (e.g. because of the team’s institution) has financial or personal relationships that may inappropriately influence (bias) their actions (such relationships are also known as dual commitments, competing interests, or competing loyalties).These relationships vary from being negligible to having great potential for influencing judgement. Not all relationships represent true conflict of interest. On the other hand, the potential for conflict of interest can exist regardless of whether a person believes that the relationship affects his or her scientific judgement. Financial relationships (such as employment, consultancies, stock ownership, honoraria, and paid expert testimony) are the most easily identifiable conflicts of interest and the most likely to undermine the credibility of the review. However, conflicts can occur for other reasons, such as personal relationships, academic competition, and intellectual passion. For the purposes of disclosure, the term “competing interest” should be considered synonymous with conflict of interest. 1 Example: The lead reviewer (JB) has given talks on this topic at workshops, seminars, and conferences for which travel and accommodation has been paid for by the organisers. The other authors declare that they have no known conflicts of interest. Give the name and affiliation of any individuals or organisations who are working on the review but who are not listed as review team members. NOTE: email and country are now mandatory fields for each person. Example: Dr Eric Porter, Oncologist, University Hospital, Brighton, UK. Clinical advisor. State the question(s) to be addressed by the review, clearly and precisely. Review questions may be specific or broad. It may be appropriate to break very broad questions down into a series of related more specific questions. Questions may be framed or refined using PI(E)COS where relevant. Example: How does pre-operative chemotherapy impact on survival of early stage non-small cell lung cancer compared to surgery alone? State the sources that will be searched. Give the search dates, and any restrictions (e.g. language or publication period). Do NOT enter the full search strategy (it may be provided as a link or attachment.) The search strategy reported in systematic review protocols should:
Sources include (but are not limited to) bibliographic databases, reference lists of eligible studies and review articles, key journals, conference proceedings, trials registers, Internet resources and contact with study investigators, experts and manufacturers. Systematic reviews typically use more than one database. Examples of electronic bibliographic databases include MEDLINE, EMBASE, PsycINFO. Other database sources include The Cochrane Library, Health Technology Assessment Database, and Web of Science.
It is considered good practice for searches to be re-run just before the final analyses and any further studies identified, retrieved for inclusion.
Give a link to the search strategy or an example of a search strategy for a specific database if available (including the keywords that will be used in the search strategies). Alternatively, an electronic file could be supplied which will be linked to from the Register record. This will be made publicly available from the published record immediately, or it can be held in confidence until the review has been completed, at which time it will be made publicly available. Example: http://www.biomedcentral.com/1756-0500/3/250 Give a short description of the disease, condition or healthcare domain being studied. This could include health and wellbeing outcomes. Examples: Type 2 diabetes. Physical activity in children. Give summary criteria for the participants or populations being studied by the review. The preferred format includes details of both inclusion and exclusion criteria. Example: Inclusion: Adults with schizophrenia (as diagnosed using any recognised diagnostic criteria). Exclusion: Adolescents (under 18 years of age) and elderly people (over 70). Give full and clear descriptions or definitions of the nature of the interventions or the exposures to be reviewed. This is particularly important for reviews of complex interventions (interventions involving the interaction of several elements). If appropriate, an operational definition describing the content and delivery of the intervention should be given. Ideally, an intervention should be reported in enough detail that others could reproduce it or assess its applicability to their own setting. The preferred format includes details of both inclusion and exclusion criteria. For reviews of qualitative studies give details of the focus of the review. Example: Population-level tobacco control interventions are defined as those applied to populations, groups, areas, jurisdictions or institutions with the aim of changing the social, physical, economic or legislative environment to make them less conducive to smoking. These are approaches that mainly rely on state or institutional control, either of a link in the supply chain or of smokers' behaviour in the presence of others. Examples include tobacco crop substitution or diversification, removing subsidies on tobacco production, restricting trade in tobacco products, measures to prevent smuggling, measures to reduce illicit cross-border shopping, restricting advertising of tobacco products, restrictions on selling tobacco products to minors, mandatory health warning labels on tobacco products, increasing the price of tobacco products, restricting access to cigarette vending machines, restricting smoking in the workplace, and restricting smoking in public places. Such approaches could also form part of wider, multifaceted interventions in schools, workplaces or communities. 3 Where relevant, give details of the alternatives against which the main subject/topic of the review will be compared (e.g. another intervention or a non-exposed control group). The preferred format includes details of both inclusion and exclusion criteria. Control or comparison interventions should be described in as much detail as the intervention being reviewed. If the comparator is ‘treatment as usual’ or ‘standard care’, this should be described, with attention being paid to whether it is ‘standard care’ at the time that an eligible study was done, or at the time the review is done. Systematic reviews of qualitative studies rarely have a comparator or control; stating ‘Not applicable’ is therefore acceptable. Examples: Placebo. A group of hospital in-patients who were not exposed to the infectious agent. Give details of the types of study (study designs) eligible for inclusion in the review. If there are no restrictions on the types of study design eligible for inclusion, or certain study types are excluded, this should be stated. The preferred format includes details of both inclusion and exclusion criteria. If different study designs are needed for different parts of the review, this should be made clear. Where qualitative evidence will be incorporated in or alongside a review of quantitative data, this should be stated. Example: We will include randomised trials to assess the beneficial effects of the treatments, and will supplement these with observational studies (including cohort and case–control studies) for the assessment of harms. Give summary details of the setting and other relevant characteristics which help define the inclusion or exclusion criteria. Include relevant details if these form part of the review’s eligibility criteria but are not reported elsewhere in the PROSPERO record. Examples: Studies in hospital accident and emergency departments. Research in low- and middle-income countries only will be included. Give the pre-specified primary (most important) outcomes of the review, including details of how the outcome is defined and measured and when these measurement are made, if these are part of the review inclusion criteria. For systematic reviews of qualitative studies give details of what the review aims to achieve. Examples: Change in depression score from baseline to the last available follow-up, measured using the Beck Depression Inventory. Five year progression-free survival (measured from randomisation). Establishing the barriers and facilitators to smoking cessation in pregnancy. List the pre-specified secondary (additional) outcomes of the review, with a similar level of detail to that required for primary outcomes. Where there are no secondary outcomes please state ‘None’ or ‘Not applicable’ as appropriate to the review Example: Apgar scores for the baby at 1 and 5 minutes after birth. Describe how studies will be selected for inclusion. State what data will be extracted or obtained. State how this will be done and recorded. Data extraction methods reported in systematic review protocols should include: Study selection
Describe the method of assessing risk of bias or quality assessment. State which characteristics of the studies will be assessed and any formal risk of bias tools that will be used. Methods for assessing risk of bias reported in systematic review protocols should include:
Provide details of the planned synthesis including a rationale for the methods selected. This must not be generic text but should be specific to your review and describe how the proposed analysis will be applied to your data. Data synthesis methods reported in systematic review protocols should be specific about how they apply to the review and data in question and include:
State any planned investigation of ‘subgroups’. Be clear and specific about which type of study or participant will be included in each group or covariate investigated. State the planned analytic approach. Planned ‘subgroup’ analysis or investigation of potential effect modifiers in reported in systematic review protocols should include:
Select the type of review and the review method from the lists below. Select the health area(s) of interest for your review. N.B. The information required here relates to the topic and outcome of the systematic review rather than the methods to be used. It is used to facilitate accurate searching of the database. Select each country individually to add it to the list below, use the bin icon to remove any added in error. The entry will default to English if no other selection is made. For languages other than English, registrants are asked to indicate whether a summary or abstract will be made available in English. Example: English, French. Select the country in which the review is being carried out from the drop down list. For multi-national collaborations select all the countries involved. Example: England, Canada. Give the name of any organisation where the systematic review title or protocol is registered (such as with The Campbell Collaboration, or The Joanna Briggs Institute) together with any unique identification number assigned. (N.B. Registration details for Cochrane protocols will be automatically entered). If extracted data will be stored and made available through a repository such as the Systematic Review Data Repository (SRDR), details and a link should be included here. If none, leave blank. Example: The title for this review and the review protocol are recorded in the Campbell Library as Project 27 Give the citation and link for the published protocol, if there is one. This may be to an external site such as a journal or organisational website. Alternatively an unpublished protocol may be deposited with CRD in pdf format. A link to this will be automatically added. Example: Free C, Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Research Notes 2010, 3:250 doi:10.1186/1756-0500-3-250 http://www.biomedcentral.com/1756-0500/3/250 . Give brief details of plans for communicating essential messages from the review to the appropriate audiences. Any knowledge transfer or implementation activities beyond publication of the final report that are planned should be included. Example: In addition to producing a report for the funders of this review, which will be made available free of charge on their website, a paper will be submitted to a leading journal in this field. Furthermore, should the findings of the review warrant a change in practice, a one page summary report will be prepared and sent to lead clinicians and healthcare professionals in the National Health Service. Give words or phrases that best describe the review. Keywords will help users find the review in the Register (the words do not appear in the public record but are included in searches). Be as specific and precise as possible. Avoid acronyms and abbreviations unless these are in wide use. The addition of keywords is particularly important for non-effectiveness reviews. These records are likely to contain fewer relevant terms in other fields such as comparators and outcomes. Information specialists at the Centre for Reviews and Dissemination (CRD) will assign MeSH terms, which will appear in the public record. Example: Systematic review; meta-analysis; recurrence; survival; radiation; resectable; soft-tissue; sarcoma. Give details of earlier versions of the systematic review if an update of an existing review is being registered, including full bibliographic reference if possible. Example: This review is an update of our earlier systematic review and economic model and is being undertaken in the light of the publication of significant new research which will assist in developing our model. The citation for the existing review is Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, Glasziou P, Bland M, Stirk L, Westwood M. A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions. Health Technol Assess . 2007;11(22):1-87. Select from drop down list to indicate the current status of the review: Review status should be updated when the review is completed and when it is published. Select from the list below to indicate the current status of the review. Use the free text box to provide an explanation of the status of the review. Example: Discontinued: This review has been abandoned as we have been unable to secure adequate funding to proceed. Provide any other information the review team feel is relevant to the registration of the review. Example: This review is being undertaken as part of the planning for a randomised trial to compare all different types of radiotherapy for localised, resectable soft-tissue sarcoma. This field should be left empty until details of the completed review are available OR you have a link to a preprint. Give the full citation for the preprint or final report or publication of the systematic review, including the URL where available. This field may also be used to record the availability of an un-published final report, summary results etc. Example: Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, Papadimas I. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol. 2010 Apr;162(4):643- 52. Epub 2009 Dec 2. http://eje-online.org/cgi/content/full/162/4/643 Give the working title of the review. This must be in English. The title should have the interventions or exposures being reviewed and the associated health or social problems. Where appropriate, the title should use the PI(E)CO structure to contain information on the Population, Intervention (or Exposure) and Comparison groups, and the Outcomes to be measured. Acronyms may be included in titles, but should not be used alone without expansion unless they are regarded as more usual than the expansion (e.g. HIV). If the original title is in a different language, the English version must be entered here, with the non-English version entered into Field #2 (Original language title). If the final title of the (published) review differs from the one entered here, this can be recorded in Field #40 (Details of the final report/publication(s)). Example: Efficacy of ischemic postconditioning against renal ischemia-reperfusion injury in animal models, a systematic review and meta-analysis. For the purposes of PROSPERO, the date of commencement for the systematic review can be defined as any point after completion of a protocol, but before formal screening of the identified studies against the eligibility criteria begins. A protocol can be deemed complete when it is approved by a funder or the person commissioning the review, when peer review is complete, when the protocol is published, or when the authors decide that it is complete and they do not anticipate any major revisions to the design of the systematic review. Give the date by which the review is expected to be completed. In the absence of an agreed contractual date, a realistic anticipated date for completion should be set. It can be modified should the schedule change. When this date is reached, the named contact will receive an automated email to ask them to provide an update on progress. This field should be updated when any amendments are made to a published record and on completion and publication of the review. Example: “ Preliminary searches” ticked as completed, “Piloting of the study selection process” ticked as started. The named contact acts as the guarantor for the accuracy of the information presented in the register record. This should be the lead reviewer or a representative of the review team. This person is also responsible for submitting details of any amendments while the review is ongoing and publication details after the review is completed. The named contact is the person to whom users of PROSPERO would send questions or comments. This field is automatically populated from the named contact’s signing in details. The named contact will be displayed in the public record. Example: Dr Joseph Bloggs Enter the electronic mail address of the named contact. This may be a generic email address to which the named contact has access. This field is automatically populated from the named contact’s signing in details, but can be changed if required. The email address supplied here will be displayed in the public record. Examples: [email protected]; [email protected] Enter the full postal address for the named contact. This field is automatically populated from the named contact’s signing in details. This address will be displayed in the public record. If you do not wish it to appear in the public record delete the content of this field. Example: Alcuin B Block, University of York, York, YO10 5DD, UK Enter the telephone number for the named contact, including international dialling code. This field is automatically populated from the named contact’s signing in details. This number will be displayed in the public record. If you do not wish it to appear in the public record delete the content of this field. Example: +44 (0)10904 32104 Full title of the organisational affiliations for this review and website address if available. This field may be completed as ‘none’ if the review is not affiliated to any organisation. Example: Radboud university medical center (Radboudumc) Give the personal details and the organisational affiliations of each member of the review team. NOTE: email and country are now mandatory fields for each person. . Affiliation refers to groups or organisations to which review team members belong. The named contact will be automatically added to this field, but can be deleted if not a member of the review team. To place the named contact somewhere other than first in order, delete the automatic entry and enter members’ details in the required order. Membership of the review team and details of affiliations can be updated at any time. All edits will appear in the record audit trail. Examples: Mr Joseph Bloggs, Centre for Reviews and Dissemination, University of York, UK. Dr Jane Smith, Department of Health Sciences, University of York, UK. Prof. Steven Jones, Centre for Health Statistics, Medical Research Centre, Canada. Give details of the individuals, organisations, groups or other legal entities who take responsibility for initiating, managing, sponsoring and/or financing the review. Any unique identification numbers assigned to the review by the individuals or bodies listed should be included. List any conditions that could lead to actual or perceived undue influence on judgements concerning the main topic investigated in the review. The conflicts of interest listed should cover the review team as a whole, as well as individuals in the team. Conflicts of interest arise when a team member or the team as a whole (e.g. because of the team’s institution) has financial or personal relationships that may inappropriately influence (bias) their actions (such relationships are also known as dual commitments, competing interests, or competing loyalties). These relationships vary from being negligible to having great potential for influencing judgement. Not all relationships represent true conflict of interest. On the other hand, the potential for conflict of interest can exist regardless of whether a person believes that the relationship affects his or her scientific judgement. Financial relationships (such as employment, consultancies, stock ownership, honoraria, and paid expert testimony) are the most easily identifiable conflicts of interest and the most likely to undermine the credibility of the review. However, conflicts can occur for other reasons, such as personal relationships, academic competition, and intellectual passion. For the purposes of disclosure, the term “competing interest” should be considered synonymous with conflict of interest. Example: The lead reviewer (JB) has given talks on this topic at workshops, seminars, and conferences for which travel and accommodation has been paid for by the organisers. The other authors declare that they have no known conflicts of interest. Give the name, affiliation and role of any individuals or organisations who are working on the review but who are not listed as review team members. Give details of the question to be addressed by the review, clearly and precisely. This should be clearly and precisely defined, but may be specific or broad. The question may be framed or refined using PI(E)COS where relevant. Further guidance is available in e.g. the step by step search guide. Example: Does analgesic treatment reduce the number or incidence of metastasis in animal cancer models? Give details of the sources to be searched, and any restrictions (e.g. language or publication period). The full search strategy is not required, but may be supplied as a link or attachment. A step by step search guide , as well as animal search filters for Pubmed and EMBASE , are available to facilitate the search process. List all sources that will be used to identify studies for the review. Sources include (but are not limited to) bibliographic databases, reference lists of eligible studies and review articles, key journals, trials registers, conference proceedings, internet resources and contact with experts and manufacturers. Example: We will search the following electronic bibliographic databases: MEDLINE, EMBASE, and Web of Science. The full search strategy (see pdf) is based on the search components “animal” (using Pubmed and EMBASE search filters [ref, ref]), “laparoscopic surgery” and “renal function”. No publication date or language restrictions will be applied. We will screen the reference lists of included studies for additional eligible studies not retrieved by our search. The searches will be re-run just before the final analyses to retrieve the most recent studies eligible for inclusion. Give a link to the search strategy or an example of a search strategy for a specific database if available (including the keywords that will be used in the search strategies). Alternatively, an electronic file could be supplied which will be linked to from the Register record. This will be made publicly available from the published record immediately, or it can be held in confidence until the review has been completed, at which time it will be made publicly available. Example: http://www.biomedcentral.com/1756-0500/3/250 Give a short description of the disease, condition or healthcare domain being modelled. This could include health and wellbeing outcomes. Example: Type 2 diabetes; Myocardial infarction; Physical activity in the elderly. Give summary criteria for the animals being studied by the review, e.g. species, sex, details of disease model. Please include details of both inclusion and exclusion criteria. Example: Inclusion criteria: all animal models with experimental cancer in which metastasis can develop (all species, all sexes). Exclusion criteria: animals with co-morbidities; ex vivo, in vitro and in silico models; experimental cancer without metastasis Give full and clear descriptions of the nature of the interventions or the exposures to be reviewed (e.g. dosage, timing, frequency). Please include details of both inclusion and exclusion criteria. For reviews of pre-clinical animal studies, the intervention would be e.g. treatment with a drug, or a therapeutic intervention such as exercise. For reviews of animal exposure studies, e.g. in toxicology, the intervention would be exposure to a certain compound. For reviews aiming to provide an overview of animal models for a certain health problem or disease, this would be the intervention(s) used to induce the disease model (e.g. high-fat diet to induce obesity, or transverse aortic constriction to induce heart failure). See also Field #30 for additional information on review types. Example: Inclusion criteria: analgesic treatment with compounds registered for use in clinical practice, including pre-treatment of tumor cells with analgesics before injection. All timings, frequencies and dosages of treatment are eligible for inclusion. Exclusion criteria: treatment with analgesics not registered for use in clinical practice. Where relevant, give details of the type(s) of control interventions against which the experimental condition(s) will be compared (e.g. another intervention or a non-exposed control group). Please include details of both inclusion and exclusion criteria. Control or comparison interventions should be described in as much detail as the intervention being reviewed. A “control group” may refer to vehicle-treated animals, sham-treated animals, animals undergoing no treatment at all, baseline measurements, etc. Indicate which of these control conditions are eligible for inclusion. If the review aims to provide an overview of available animal models for a certain health problem / disease (animal model review, see Field #30), the comparator would generally be a healthy, naive animal”. Example: Inclusion criteria: vehicle-treated control animals. Exclusion criteria: all other control conditions (e.g. no treatment, saline-treated if vehicle is not saline). Give details of the types of study (study designs) eligible for inclusion in the review. If there are no restrictions on the types of study design eligible for inclusion, or certain study types are excluded, this should be stated. Please include details of both inclusion and exclusion criteria. Example: Inclusion criteria: controlled studies with a separate control group. Exclusion criteria: case studies, cross-over studies, studies without a separate control group. Give details of any other inclusion and exclusion criteria (e.g. publication date or language restrictions). Examples: Inclusion criteria: all languages, all publication dates. Exclusion criteria: none. Give details of the outcome measures to be considered for inclusion in the review. Please include details of both inclusion and exclusion criteria. Example: Inclusion criteria: tumor number and/or tumor incidence reported. Exclusion criteria: no relevant outcomes reported (e.g. tumor weight only). This question does not apply to systematic reviews of animal studies for human health submissions Give the procedure for selecting studies for the review and extracting data, including the number of researchers involved and how discrepancies will be resolved. List the data to be extracted. Other relevant details could include whether study selection and/or data extraction will be blinded (researchers unaware of author/journal details) and whether and how authors of eligible studies will be contacted to provide missing or additional data. For reviews of individual participant data, this field should include the data to be sought and how this will be collected. A description of any other manipulation or transformation of the extracted data that is planned may be included. Example: Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources will be screened independently by two review authors to identify studies that potentially meet the inclusion criteria outlined above. The full text of these potentially eligible studies will be retrieved and independently assessed for eligibility by two review team members. Any disagreement between them over the eligibility of particular studies will be resolved through discussion with a third reviewer. A standardised, pre-piloted form will be used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted information will include: study setting; study population and participant demographics and baseline characteristics; details of the intervention and control conditions; study methodology; recruitment and study completion rates; outcomes and times of measurement; indicators of acceptability to users; suggested mechanisms of intervention action; information for assessment of the risk of bias. Two review authors will extract data independently, discrepancies will be identified and resolved through discussion (with a third author where necessary). Missing data will be requested from study authors. Example for IPD: Those responsible for the included studies will be asked to supply line by line individual participant data comprising: de-identified patient reference; allocated treatment, date of randomisation; date of birth, gender, tumour stage, tumour histology, survival status, date of last follow up or death. State whether and how risk of bias and/or study quality will be assessed. Assessment tools specific for pre-clinical animal studies include SYRCLE’s risk of bias tool and the CAMARADES checklist for study quality. SYRCLE’s risk of bias tool is used to perform an assessment of internal validity, addressing selection, performance, detection, attrition, and other types of bias. The CAMARADES checklist is used to perform a combined assessment of the reporting of a number of measures to reduce bias, and several indicators of external validity and study quality. Both tools may be adapted by adding or removing items. If this is planned, specify which adaptations have been made. “No risk of bias and/or quality assessment planned” is acceptable only if the aim of the review is limited to providing an overview of available animal models, without presenting any outcome data. Give the planned general approach to synthesis, e.g. whether aggregate or individual participant data will be used and whether a quantitative or narrative (descriptive) synthesis is planned. It is acceptable to state that a quantitative synthesis will be used if the included studies are sufficiently homogenous. Where appropriate, the planned analytical approaches (e.g. Bayesian or frequentist (classical), fixed or random effects; categorising studies within a narrative synthesis) should be outlined. Whether and how statistical heterogeneity will be explored and how any observed heterogeneity will impact on or modify the planned approach to analysis should be stated, along with any planned sensitivity analyses. Example: We will provide a narrative synthesis of the findings from the included studies, structured around the type of intervention, target population characteristics, type of outcome and intervention content. We will provide summaries of intervention effects for each study by calculating risk ratios (for dichotomous outcomes) or standardised mean differences (for continuous outcomes). We anticipate that there will be limited scope for meta-analysis because of the range of different outcomes measured across the small number of existing trials. However, where studies have used the same type of intervention and comparator, with the same outcome measure, we will pool the results using a random-effects meta-analysis, with standardised mean differences for continuous outcomes and risk ratios for binary outcomes, and calculate 95% confidence intervals and two sided P values for each outcome. In studies where the effects of clustering have not been taken into account, we will adjust the standard deviations for the design effect. Heterogeneity between the studies in effect measures will be assessed using both the χ2 test and the I 2 statistic. We will consider an I 2 value greater than 50% indicative of substantial heterogeneity. We will conduct sensitivity analyses based on study quality. We will use stratified meta-analyses to explore heterogeneity in effect estimates according to: study quality; study populations; the logistics of intervention provision; and intervention content. We will also assess evidence of publication bias. Example for IPD: Individual data from all randomised participants will be included in the analyses, which will be performed on an intention to treat basis. A two-stage approach to synthesis will be used. For time to event outcomes, the individual times to event will be used in the stratified (by trial) logrank test to produce hazard ratio estimates of the effect of treatment for individual trials. These hazard ratios will then be combined across studies using a fixed effect model to give combined hazard ratios. For dichotomous outcomes, the number of events and the number of patients will be used to calculate Peto odds ratio estimates of treatment effect. These will be generated for individual trials and then combined across trials using a fixed effect model. For all outcomes, trial results will also be combined using a random effects model to test robustness to model choice. Give details of any plans for the separate presentation, exploration or analysis of different types of participants (e.g. by age, disease status, ethnicity, socioeconomic status, presence or absence or co-morbidities); different types of intervention (e.g. drug dose, presence or absence of particular components of intervention); different settings (e.g. country, acute or primary care sector, professional or family care); or different types of study (e.g. randomised or non-randomised). The approach to be taken should be stated, e.g. whether subgroup analyses, meta-regression or modelling of covariates is planned and, where appropriate, details of categorisation (e.g. BMI <25, 25-30,>30) should be given. Where it is not possible or appropriate to specify subgroups or subsets in advance, for example in a qualitative synthesis, please make a statement to this effect. Example: If the necessary data are available, subgroup analyses will be done for people with stage I and stage II disease separately. Within each stage, and overall, we also plan to do a subgroup analysis by age (<20, 20-30, 30-40, >40 years). This is a qualitative synthesis and while subgroup analyses may be undertaken it is not possible to specify the groups in advance Pre-clinical animal intervention reviews have a review question in the PICO format and concern pre-clinical testing of intervention(s) in animal models of disease. Animal model reviews aim to provide an overview of animal models for a certain health problem or disease (area) and do not investigate the efficacy of treatment. They may still have a PICO review question where the intervention is the induction of a disease model. Experimental animal exposure reviews have a review question in PECO format and concern the effect of exposure to certain compounds in experimental animals, e.g. safety studies in toxicology. Select each country individually to add it to the list below, use the bin icon to remove any added in error. The entry will default to English if no other selection is made. For languages other than English, registrants are asked to indicate whether a summary or abstract will be made available in English. List other places where the systematic review protocol is registered. The name of the organisation and any unique identification number assigned to the review by that organisation should be included. Example: All Wales Systematic Review Register (AWSRR2 Sciaticax) Example: G.P.J. van Hout, S.J. Jansen of Lorkeers, K.E. Wever, E.S. Sena, W.W. van Solinge, P.A. Doevendans, G. Pasterkamp, S.A.J. Chamuleau and I.E. Hoefer. Anti-inflammatory compounds to reduce infarct size in large-animal models of myocardial infarction: A meta-analysis (pages 4–10). Version of Record online: 20 JAN 2015 | DOI: 10.1002/ebm2.4 Give words or phrases that best describe the review. Keywords will help users find the review in the Register. Be as specific and precise as possible. Avoid acronyms and abbreviations unless these are in wide use. Information specialists at the Centre for Reviews and Dissemination (CRD) will assign MeSH terms. When the review is published, NLM assigned MeSH terms will be added where these are available. Example: This is an update of our earlier systematic review and is being undertaken in the light of the publication of significant new research. The citation for the existing review is Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Brain. 2007 Dec;130(Pt 12):3063-74. Review status should be updated when the review is completed and when it is published
Loading metrics Open Access Peer-reviewed Research Article Magnitude and clinical characteristics of cerebral palsy among children in Africa: A systematic review and meta-analysisRoles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing * E-mail: [email protected] Affiliation Assistant Professor in Pediatrics and Child Health Nursing, College of Health Science, Woldia University, Weldiya, Ethiopia Affiliation MSc in Psychiatry, College of Health Science, Woldia University, Weldiya, Ethiopia Affiliation MSc in Pediatrics and Child Health Nursing, College of Health Science, Woldia University, Weldiya, Ethiopia Affiliation MSc in Emergency Medicine and Critical Care Nursing, College of Health Science, Woldia University, Weldiya, Ethiopia Roles Conceptualization, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing Roles Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing Affiliation MSc in Adult Health Nursing, College of Health Science, Woldia University, Weldiya, Ethiopia

Cerebral palsy (CP) is the most common motor disability in childhood which causes a child’s behavioral, feeding, and sleep difficulties. It remains a poorly studied health problem in Africa. The main aim of this study was assessing the pooled prevalence of Cerebral Palsy (CP) and its clinical characteristics in Africa context. Systematic review and meta-analysis were conducted using Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines to search articles from electronic databases (Cochrane library, Ovid platform) (Medline, Embase, and Emcare), Google Scholar, CINAHL, PubMed, Maternity and Infant Care Database (MIDIRS). The last search date was on 12/05/ 2023 G. C. A weighted inverse variance random-effects model was used to estimate the pooled estimates of cerebral palsy and its types. The subgroup analysis, publication bias and sensitivity analysis were done. Studies on prevalence and clinical characteristics of cerebral palsy were included. The primary and secondary outcomes were prevalence and clinical characteristics of cerebral palsy respectively. A total of 15 articles with (n = 498406 patients) were included for the final analysis. The pooled prevalence of cerebral palsy in Africa was found to be 3·34 (2·70, 3·98). The most common type is spastic cerebral palsy accounting 69·30% (66·76, 71·83) of all cases. The second one is quadriplegic cerebral palsy which was found to be 41·49% (33·16, 49·81). Ataxic cerebral palsy accounted 5·36% (3·22, 7·50). On the other hand, dyskinetic cerebral palsy was found to be 10.88% (6·26, 15·49). About 32·10% (19·25, 44.95) of cases were bilateral while 25·17% (16·84, 33·50) were unilateral. The incidence of cerebral palsy in Africa surpasses the reported rates in developed nations. Spastic and quadriplegic subtypes emerge as the most frequently observed. It is recommended to channel initiatives toward the strategic focus on preventive measures, early detection strategies, and comprehensive management protocols. Citation: Abate BB, Tegegne KM, Zemariam AB, Wondmagegn Alamaw A, Kassa MA, Kitaw TA, et al. (2024) Magnitude and clinical characteristics of cerebral palsy among children in Africa: A systematic review and meta-analysis. PLOS Glob Public Health 4(6): e0003003. https://doi.org/10.1371/journal.pgph.0003003 Editor: Julia Robinson, PLOS: Public Library of Science, UNITED STATES Received: November 15, 2023; Accepted: May 22, 2024; Published: June 21, 2024 Copyright: © 2024 Abate et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Data Availability: All data are available in the manuscript and supporting files . Funding: The author(s) received no specific funding for this work. Competing interests: The authors have declared that no competing interests exist. Cerebral palsy (CP) is a group of permanent disorders of development that affect different body parts and result in activity limitations with the manner of walking, muscle tone, posture, and coordination of movement. This is attributed to a non-progressive disturbance that occurs in the development of the fetal or infant brain [ 1 ]. There are various forms of cerebral palsy, each distinguished by the area of the brain that is injured. This occurs when signals are not correctly sent from the brain to the muscle. Despite the fact that numerous studies show that CP can result from a wide range of conditions, such as early birth, illnesses, injuries, and medical issues, the pathophysiology of this issue is unclear [ 2 ]. Cerebral palsy movement abnormalities are frequently accompanied with sensory, perception, cognition, communication, and behavior issues, seizures, and subsequent musculoskeletal problems [ 3 ]. Children with CP are present with certain characteristics/types which include spasticity, hypotonia, diplegia, hemiplegia, and dystonia [ 4 ]. Usually, motor disorders are more common and accompanied by perception cognition, sensation, communication and behavior due to epilepsy and secondary musculoskeletal problems [ 1 ]. CP greatly affects the childhood characteristics such as behavioral difficulties, feeding difficulties, social skills, and sleep [ 5 ]. CP is the most common neurologic problem causing motor disability in children [ 6 , 7 ]. According to the World Health Organization(WHO) report globally, there were 10% of children (approximately 200 million) suffer from physical disability, mental deficiencies or developmental delay, and impaired learning abilities [ 8 ]. Furthermore, more than three fourth of the world’s disabled population live in low-income countries, many of these in Africa [ 9 ]. Recent population-based studies from around the world reported that the prevalence of CP was ranging from 1 to nearly 4 per 1,000 live births [ 4 ]. The prevalence of CP is variably reported across different countries which are 1.89 per 1,000 live births in Norway [ 10 ], and 2.2 per 1,000 live births in Denmark [ 11 ]. Furthermore, a population-based study in Bangladesh reported that 3.4 per 1,000 children [ 12 ]. In Africa, the prevalence of CP is also compared to developed nations. Evidently, studies on hospital clinical samples suggest prevalence ranging from 2 to 10 cases per 1000 live births from Egypt, Uganda, South Africa, and South Egypt, respectively [ 13 – 18 ]. Moreover, a population-based study in Uganda revealed that the prevalence of CP was 2.9 per 1,000 children in 2017 [ 19 ]. In line with the global trend, CP places a heavy burden of disease on children, families, and society in both developed and developing countries [ 4 , 11 ]. The previous studies done in different countries showed that several factors were associated with CP. Among these factors, asphyxia at birth, low birth weight, intrauterine infections and multiple gestations were the most important determinants for CP [ 20 – 22 ]. Cases of CP varies in presentation, etiology, evolution, severity, medical and rehabilitation needs, comorbidities, and outcomes [ 23 ]. Furthermore, compared to younger ages, older children with cerebral palsy (CP) were substantially less prevalent, and this tendency was reflected in the decline in prevalence. These results implied that a substantial death rate existed among children with cerebral palsy, especially in the most severely impacted children. The paucity of information on the epidemiology of cerebral palsy (CP) in low- and middle-income countries (LMICs) highlights the maltreatment of and shortage of resources for children with CP in these countries, which lowers their survival rate for consecutive birth dates [ 19 ]. It might be appreciated at early or later age. Cerebral palsy was diagnosed in 43.4% of children after the first year of life, 32.4% after the second half of life, and 24.1% before the age of six months [ 24 ]. This figure shows there is time delay in the diagnosis of CP. Globally, several strategies and interventions have been tried to reduce the prevalence and adverse sequels of CP among children [ 7 , 9 ]. It was anticipated that advancements in these areas would lead to lower rates of cerebral palsy because prenatal events are thought to account for about 75% of all cases of cerebral palsy. These include electronic fetal monitoring, cesarean sections, and generally improving obstetric and neonatal care [ 4 , 8 , 25 ]. Beyond these efforts, the problem has still a public concern, particularly in developing nations [ 26 ], because their obstetric and neonatal advanced medical care is limited [ 27 ]. Few rigorous population-based studies have recently been published from Uganda [ 19 ], and Bangladesh [ 12 ] revealing large discrepancy in prevalence between them and studies from high income countries. Large differences in the prevalence of CP were found between studies conducted in high-income countries and those conducted in African nations. These investigations unequivocally showed that the data from these disparate pieces of evidence or from research conducted in wealthy nations, and they suggested the necessity of a thorough evaluation of CP from low- and middle-income nations [ 28 ]. There is a lack of structured and consistent screening policy for developmental disabilities amongst children in Africa [ 18 ]. Due to this circumstance and delayed presentation, many children with disabilities have gone undiagnosed and consequently have not received the necessary assistance. Consequently, the severity and impact of CP in Africa are understated. Because of stigma, families with disabled children in African nations often find themselves shut out of society. Because they are frequently denied access to the necessities of recognition, education, health care, and socialization, the majority of these children thereafter face numerous social, economic, and political obstacles [ 29 ]. In Africa different studies have been conducted regarding the magnitude and clinical characteristics of cerebral palsy, however findings from these small studies lack consistency and results are variable making it challenging to recommend actions. To date, a rigorous systematic review and meta-analysis of the overall prevalence of CP are lacking in resource-poor settings despite the fact that large burden of the disease. Hence, this study is intended to assess the pooled prevalence of CP and its clinical characteristics among children in Africa. The result of this systematic review and meta-analysis will provide a pooled data on the burden of cerebral palsy which can be used as a baseline in designing strategies for prevention and control of cerebral palsy. Search strategyThis systematic review and meta-analysis review assessed studies that provide data on the magnitude and clinical characteristics of cerebral palsy in the Africa context. We searched these articles from the following databases: Cochrane library, Ovid platform (Medline, Embase, and Emcare), Google Scholar, CINAHL, PubMed, Maternity and Infant Care Database (MIDIRS), and institutional repositories in Africa countries on 12/05/ 2023 G. C. The search in all database included keywords that are the combinations of population, condition/outcome, and context. A snowball searching for the references of relevant papers for linked articles was also performed. The following search map was applied: (prevalence OR magnitude) AND (Children [MeSH Terms] OR infant OR child OR childhood) AND (cerebral palsy [MeSH Terms] OR “developmental disabilities”, OR “neurological impairment,” OR “childhood disability”) AND (ataxia OR dyskinesia OR spastic) AND (“clinical characteristics [MeSH Terms]” OR type) AND (Africa OR “developing countries”) on PubMed database ( S1 Table ). These search terms were further paired with the names of African countries. Thus, the key searching terms were considering Africa countries that compose of Ethiopia, Djibouti, Somalia, Egypt, Eritrea, Sudan, Tanzania, Kenya, Nigeria, Uganda etc. Using those key terms, we used the Boolean operator "OR" (to connect key terms/phrases within the same concept), "AND" (to connect key terms /phrases between two concepts), and "NOR" to filter out. In addition, we used truncation (*), adjacency searching ( ADJn) , and wildcard symbols to find variations in spelling and variant word endings on the Ovid databases. Moreover, we applied relevant limits (filters) such as a limit to human studies only. The sample search strategy for Medline is provided in S1 Table . Study selection and screeningThe retrieved studies were exported to Endnote version 8 reference managers to remove duplicate studies. Two investigators (BB and KM) independently screened the selected studies using article’s title and abstracts before retrieval of full-text papers. We used pre-specified inclusion criteria to further screen the full-text articles. Disagreements were discussed during a consensus meeting with other reviewers (AB and AW) for the fin selection of studies to be included in the systematic review and meta-analysis. The retrieved studies were imported in covidence platform to remove duplicate studies and to do the whole screening process. Inclusion and exclusion criteriaStudies that assess the magnitude and clinical characteristics of cerebral palsy among children under the age of 18 were considered. Citations without abstract and/or full text, anonymous reports, editorials, and qualitative studies were excluded from the analysis. The Prevalence of cerebral palsy was considered as the proportion of children with cerebral palsy among 1000 risk population. Studies conducted among children under the age of 18 were considered. InterventionNot applicable. Studies conducted in Africa context. Study designAll observational studies. Prevalence of cerebral palsy and its clinical characteristics/type. Quality assessmentThe authors appraised the quality of the studies by using the Joanna Briggs Institute (JBI) quality appraisal checklist [ 17 , 30 ]. There was a team of four reviewers and the papers were split amongst the team. Each paper was then assessed by two reviewers and any disagreements were discussed with the third and the fourth reviewers. Studies were considered as low risk or good quality when it scored 4 and above for all designs (cross-sectional, and cohort) [ 19 ], whereas the studies scored 3 and below were considered as high risk or poor quality and excluded ( S2 Table ). Furthermore, we thoroughly extract adjusted confounders and main findings from all included studies ( Table 1 ). Similar methodology has been used in previously published works [ 17 , 18 , 31 – 37 ].
https://doi.org/10.1371/journal.pgph.0003003.t001 Data extractionThe authors developed a data extraction form on the excel sheet and the following data were extracted for eligible studies: year of publication, country, and study design, the definition of cerebral palsy, and clinical characteristics / type of cerebral palsy. The data extraction sheet was piloted using 4 papers randomly, and it was adjusted after piloted the template. Two of the authors (BB and KM) extracted the data using the extraction form in collaboration. The third and fourth (MA and AB) authors checked the correctness of the data independently. Any disagreements between reviewers were resolved through discussions with third and fourth reviewers when required. The mistyping of data was resolved through crosschecking with the included papers. Synthesis of resultsThe authors transformed the data to STATA 17 for analysis after it was extracted in an excel sheet considering prevalence, and type/characteristics reported. We pooled the overall prevalence estimates of cerebral palsy by a random effect meta-analysis model. We examined the heterogeneity of effect size using the Q statistic and the I 2 statistics. In this study, the I 2 statistic value of zero indicates true homogeneity, whereas the value 25%, 50%, and 75% represented low, moderate and high heterogeneity, respectively [ 38 – 41 ]. Subgroup analysis was done by the study country, study design, and year of publication. Sensitivity analysis was employed to examine the effect of a single study on the overall estimation. Publication bias was checked by the funnel plot and more objectively through Egger’s regression test. A total of 5394 studies were identified; 5380 from different databases and 14 from other sources. After duplication removed, a total of 2,431 articles remained (2963 removed by duplication). Finally, 206 studies were screened for full-text review, and 15 articles with (n = 498406 patients) were included for the final analysis ( Fig 1 , and S2 Table ). https://doi.org/10.1371/journal.pgph.0003003.g001 Characteristics of included studiesFifteen studies were included in this systematic review and meta-analysis [ 14 , 16 , 19 , 42 – 52 ]. Two studies were from Ethiopia [ 42 , 47 ], two from Uganda [ 19 , 43 ], two from Tanzania [ 44 , 45 ], three from Egypt [ 16 , 46 , 50 ], one from Cameroon [ 51 ], one from Nigeria [ 52 ], two from South Africa [ 14 , 48 ], and one from Sudan [ 49 ] ( Table 1 ). Prevalence of cerebral palsy in AfricaMost of the included studies (n = 11) have reported the prevalence of cerebral palsy per 1000 live births [ 14 , 16 , 19 , 45 , 46 , 50 – 52 ]. The prevalence of cerebral palsy was ranged from 1(95% CI: 0·96, 1·04) to 17·77 (95% CI: 10·93, 24·61). The random-effects model analysis from those studies revealed that, the pooled prevalence of cerebral palsy in Africa was found to be 3·34 (95% CI: 2·70, 3·98) (95% CI: I 2 = 99·4%; p < 0·001) ( Fig 2 ). https://doi.org/10.1371/journal.pgph.0003003.g002 Subgroup analysisS ubgroup analysis was done through stratified by country, and sample size. Based on this, the prevalence of cerebral palsy was found to be 2·90(2·72–3·08) in Uganda, 13·68(6·08–21·28) in Tanzania, 2·12 (1·37, 2·88) in Egypt, 4·86 (4·20, 5·52), 2·30 (2·23, 2·37) in Nigeria and 10·00 (8·70,11·30) in South Africa ( Fig 3 ). https://doi.org/10.1371/journal.pgph.0003003.g003 Publication biasA funnel plot showed asymmetrical distribution. The Egger’s regression test-value was 0·009, which indicated that, the presence of publication bias. Due to the presence of publication bias ( S1 Fig ), we employed a trim and fill analysis and one study was added and the prevalence of cerebral palsy becomes 3·193 ( S2 Fig ). Sensitivity analysisWe also employed a leave-one-out sensitivity analysis to identify the potential source of heterogeneity in the analysis of the prevalence of cerebral palsy in Africa. The results of this sensitivity analysis showed that the findings were not dependent on a single study. Our pooled estimated prevalence of cerebral palsy varied from 2·76 (2·12–3·39) to 3·55 (2·84–4·25) after the deletion of a single study ( S3 Fig ). Clinical characteristics of cerebral palsy in AfricaSpastic cerebral palsy.. Pooled prevalence . Spastic cerebral palsy is the most common type of cerebral palsy in Africa. It is characterized by jerky movements, muscle tightness and joint stiffness. Six of the included studies have reported the magnitude of spastic cerebral palsy in percentage [ 16 , 19 , 42 , 43 , 46 , 52 ]. The prevalence of spastic cerebral palsy was ranged from 23·00%(95% CI: 15·90, 30·10) to 88·90 (95% CI: 85·83, 91·97). The random-effects model analysis from those studies revealed that, the pooled prevalence of spastic cerebral palsy in Africa was found to be 69·30% (95% CI: 66·76, 71·83) (95% CI: I 2 = 99·5%; p < 0·001) ( Fig 4 ). https://doi.org/10.1371/journal.pgph.0003003.g004 Subgroup analysis . The subgroup analysis was done through stratified by country, and sample size. Based on this, the prevalence of spastic cerebral palsy was found to be 23·00%(95% CI: 15·90, 30·10) in Uganda, 70·13% (65·54,74·72) in Egypt, 69·90%(69·68, 70·12) in Nigeria, and 88·9%(85·83, 91·97) in Ethiopia ( S4 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·468, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S5 Fig ). Quadriplegic cerebral palsy in Africa.Pooled prevalence . Dyskinetic CP is characterized by involuntary, uncontrolled, and recurring movements with fluctuating muscle tone [ 3 , 28 ]. Most of the included studies (n = 5) have reported the magnitude of quadriplegic cerebral palsy in percentage from total cerebral palsy cases [ 16 , 19 , 42 , 46 , 48 , 49 ]. The prevalence of unilateral cerebral palsy was ranged from 27.60% (95% CI: 18.56, 36.64) to 62·50 (95% CI: 57·77, 67·23). The random-effects model analysis from those studies revealed that, the pooled prevalence of quadriplegic cerebral palsy in Africa was found to be 41·49% (95% CI: 33·16, 49·81) (95% CI: I 2 = 99·7%; p < 0·001) ( Fig 5 ). https://doi.org/10.1371/journal.pgph.0003003.g005 Subgroup analysis . The subgroup analysis was done through stratified by country. Based on this, the prevalence of quadriplegic unilateral cerebral palsy was found to be 36·40% (24·44–48·35) in Egypt, 62·5% (57·77, 67·23) in Ethiopia, 27·60% (18·56, 36·64) in South Africa, and 43·50% (34·15, 52·85) in Sudan ( S6 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·360, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S7 Fig ). Ataxic cerebral palsy in Africa.Pooled prevalence . Ataxic cerebral palsy characterized by trouble with balance and coordination [ 53 ]. Most of the included studies (n = 11) have reported the prevalence of ataxic cerebral palsy per 1000 [ 16 , 19 , 42 , 43 , 45 – 49 , 52 ]. The prevalence of cerebral palsy was ranged from 1·4(95% CI: 0·25, 2·55) to 9·80 (95% CI: 9·66, 9·94). The random-effects model analysis from those studies revealed that, the pooled prevalence of cerebral palsy in Africa was found to be 5·36 (95% CI: 3·22, 7·50) (95% CI: I 2 = 99·8%; p < 0·001) ( Fig 6 ). https://doi.org/10.1371/journal.pgph.0003003.g006 Subgroup analysis . The subgroup analysis was done through stratsified by country, and sample size. Based on this, the prevalence of ataxic cerebral palsy was found to be 5·38(2·02–12·78) in Uganda, 9·00(3·39–14·61) in Tanzania, 4·90 (2·46, 7·35) in Egypt, 2·09 (0·23, 3·96) in Ethiopia, 9·60 (3·64, 15·56) in South Africa and 10·00 (8·70,11·30) in Sudan ( S8 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·633, which indicated that, the absence of publication bias. Due to the absence of publication bias, we did not employ a trim and fill analysis ( Fig 7 ). https://doi.org/10.1371/journal.pgph.0003003.g007 Dyskinestic cerebral palsy in Africa.Pooled prevalence . Dyskinetic cerebral palsy is characterized by dystonia, athetosis, and chorea [ 54 ]. Most of the included studies (n = 8) have reported the magnitude of ataxic cerebral palsy [ 16 , 19 , 42 , 43 , 46 – 48 , 52 ]. The prevalence of ataxic cerebral palsy was ranged from 3·80% (95% CI: 3·57, 4·03) to 29·80 (95% CI: 20·55, 39·05). The random-effects model analysis from those studies revealed that, the pooled prevalence of unilateral cerebral palsy in Africa was found to be 10·88% (95% CI: 6·26, 15·49) (95% CI: I 2 = 100%; p < 0.001) ( Fig 8 ). https://doi.org/10.1371/journal.pgph.0003003.g008 Subgroup analysis . The subgroup analysis was done through stratified by country, and sample size. Based on this, the prevalence of unilateral cerebral palsy was found to be 9·67%(6·92–12·42) in Uganda, 9·90% (2·06, 21·86) in Egypt, 4·60%(4·50, 4·70) in Nigeria, 8·51%(5·94, 11·06) in Ethiopia, and 29·80% (20·55, 39·05) in South Africa ( S10 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·728, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S11 Fig ). Bilateral cerebral palsy in Africa.Pooled prevalence . Bilateral cerebral palsy is a problem with movement, co-ordination and development which affects both sides of the body [ 55 ]. Most of the included studies (n = 10) have reported the magnitude of bilateral cerebral palsy in percentage from total cerebral palsy cases [ 16 , 19 , 42 , 43 , 46 – 48 , 52 ]. The prevalence of unilateral cerebral palsy was ranged from 9·60% (95% CI: 9·24, 9·96) to 60·40 (95% CI: 53·74, 67·06). The random-effects model analysis from those studies revealed that, the pooled prevalence of bilateral cerebral palsy in Africa was found to be 32·10% (95% CI: 19·25, 44·95) (95% CI: I 2 = 100%; p < 0·001) ( Fig 9 ). https://doi.org/10.1371/journal.pgph.0003003.g009 Subgroup analysis . The subgroup analysis was done through stratified by country, and sample size. Based on this, the prevalence of unilateral cerebral palsy was found to be 41·40%(36·48–46·31) in Uganda, 20·00% (12·16–27·84) in Tanzania, 28·94% (8·96, 66·83) in Egypt, 60·20%(59·97, 60·43) in Nigeria, 34·96 (14·73, 84·4) in Ethiopia, 16·00% (8·59, 23·41) in South Africa, and 13·9% (7·38, 20·42) in Sudan ( S12 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·529, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S13 Fig ). Unilateral cerebral palsy in Africa.Pooled prevalence . Unilateral Cerebral Palsy is characterized by hemiplegia and hemiparesis, is a condition that affects muscle control and function on one side of the body [ 56 ]. Most of the included studies (n = 10) have reported the magnitude of unilateral cerebral palsy in percentage from total cerebral palsy cases [ 16 , 19 , 42 , 43 , 45 – 49 , 52 ]. The prevalence of unilateral cerebral palsy was ranged from 13·50% (95% CI: 13·08, 13·92) to 23·70 (95% CI: 16·53, 30·87). The random-effects model analysis from those studies revealed that, the pooled prevalence of unilateral cerebral palsy in Africa was found to be 25·17% (95% CI: 16·84, 33·50) (95% CI: I 2 = 100%; p < 0·001) ( Fig 4 ). Subgroup analysis . The subgroup analysis was done through stratified by country. Based on this, the prevalence of unilateral cerebral palsy was found to be 35·15%(13·30–56·99) in Uganda, 15%(8·00–22·00) in Tanzania, 17·45% (9·71, 25·19) in Egypt, 39·8%(39·57, 40·03) in Nigeria and 10·00 (8·70,11·30), 24·76%(19·61, 29· 92) in Ethiopia, 16·00% (8·59,23·41) in South Africa, and 25·9% (17·64, 34·16) in Sudan ( S14 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·903, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S15 Fig ). Mixed type cerebral palsy in Africa.Pooled prevalence . Mixed cerebral palsy occurs when a child exhibits symptoms of more than one type of cerebral palsy [ 57 ]. Most of the included studies (n = 10) have reported the magnitude of mixed cerebral palsy in percentage [ 19 , 42 , 43 , 45 – 49 , 52 ]. The prevalence of mixed cerebral palsy in Africa was ranged from to 2·00%(1·85, 2·15) to 26·9% (95% CI: 26·36, 27·44). The random-effects model analysis from those studies revealed that, the pooled prevalence of mixed cerebral palsy in Africa was found to be 8·58% (95% CI: 4·06, 13·11) (95% CI: I 2 = 99·9%; p < 0·001) ( Fig 10 ). https://doi.org/10.1371/journal.pgph.0003003.g010 Subgroup analysis . The subgroup analysis was done through stratified by country. Based on this, the prevalence of unilateral cerebral palsy was found to be 4·60%(1·31–10·51) in Uganda, 5·0%(0·73–9·27) in Tanzania, 25·21% (21·88, 28·54) in Egypt, 8·3%(8·17, 8·43) in Nigeria and 2·70 (0·97, 4·42) in Ethiopia, 2·10% (0·80, 5·00) in South Africa, and 2·80% (0·31, 6·59) in Sudan ( S16 Fig ). Publication bias . A funnel plot showed symmetrical distribution. The Egger’s regression test-value was 0·468, which indicated that, the absence of publication bias. As a result, we didn’t conduct trim and fill analysis ( S17 Fig ). The most prevalent form of motor disability that affects children is cerebral palsy (CP). The brain may malfunction during fetal or prenatal development due to insufficient oxygenation, or it may result from damage to the developing infant’s brain during the postpartum period. In this systematic review and meta-analysis, we aimed to assess the magnitude and clinical characteristics of CP among children in Africa. Accordingly, the pooled prevalence of cerebral palsy was found to be 3·3 per 1000 live births (2·69,3·92). This finding is in line with the study done in USA [ 58 ]. This might be because African descendants were surveyed in that study. However, this finding is higher than CP prevalence in most other countries in United State and Europe which is estimated to be 2–2·5 of 1000 [ 59 – 61 ]. Compared to some studies conducted in Arabic-speaking countries, 1·8 [ 7 ], Europe, 2·25 [ 62 ], India, 2·27 [ 63 ], Canada, 2·57 [ 64 ], and Japan 1·88 [ 65 ], the pooled prevalence of cerebral palsy is greater in Africa. This discrepancy might be because of the high magnitude of perinatal complications such as birth asphyxia and neonatal infections in Africa [ 66 ]. In addition, this might be due to the fact that in Africa, there are challenges to manage cerebral palsy due to: inaccessibility of basic care, limited availability of diagnostic facilities, limited number of trained and expertise personal in managing cerebral palsy and exacerbated by lack of appropriate intervention, medication, surgical procedure and regular physical care [ 67 ]. In fact, these improper managements might have contributed to the prevalence of cerebral palsy. Furthermore, prematurity, low birthweight, kernicterus, asphyxia and neonatal infections are among the main causes of cerebral palsy, and they are more common in Africa than in western nations [ 67 ]. This implies there may be a greater proportion of children with more severe disability in resource-poor countries because of delayed presentation of a range of disorders and absence of early intervention services. Moreover, the clinical spectrum of CP differs from that of affluent countries in resource-poor, developing nations. The discrepancy probably explained by the multifactorial causes of CP and difference in quality of health care delivery between the two regions, low- and middle-income countries and developed countries [ 19 , 68 ]. The burden difference might also be due to absence or inadequacy of inter-disciplinary team approach in developing countries [ 24 ]. On the other hand, most studies done in developing countries were institution based and at referral hospitals. Since referral hospitals receive CP patients from different district hospitals, health center and regional hospitals where there are few experts and facilities hence the number of CP patients’ data might be aggregated to the referral hospitals [ 44 , 69 ]. In addition, the high prevalence may be due to improvement in health seeking behavior of the community in developing countries where previously people in Africa believed that having CP patient at home is a curse and tried to hide them at their homes. Tanzania had a higher prevalence of cerebral palsy, at 13·68/1000 (6·08–21·28) compared to studies from other African countries. This might be due to the small sample size included in studies from Tanzania. It is also higher than that of other countries study, Norway 2·1 [ 70 ], Thailand 1·0 [ 71 ]. This difference might be advance technology used, utilization of advance treatment modalities, regular follow up of antenatal care and sample size difference in other countries like Norway and Thailand. The results of the current review revealed that the spastic type of cerebral palsy affected the majority of children in Africa which accounts 63·4% of all form cerebral palsy. This finding is in line with different studies done in other regions such as north east Italy, Bangladesh and Nepal [ 58 , 72 , 73 ]. During assessment of perinatal factors, studies identified that those children with spastic subtype of CP had higher rate of fetal distress and PROM during the perinatal period, higher rate of language and speech difficulty and worse functional impairment while those with Dyskinetic and Ataxic CP were found to have higher rate of precipitated labor and deep jaundice during the neonatal period [ 74 ]. These findings may indicate that spastic subtype of CP might be related with the high rate of perinatal hypoxic insult in Africa as in cases of fetal distress, while dyskinetic and ataxic forms may be associated with bleeding and injuries to the deep grey matters of the brain that can happen in cases of precipitated labor. Children with severe forms of CP were shown to have lower levels of communication function, according to a study from Sweden that demonstrated the relationship between communication function and gross and fine motor and cognitive function [ 75 ]. Numerous severe (quadriplegic) bilateral spastic type of CP arises from injuries to the full-term brain due to complications during the birth process such as birth asphyxia or acquired central nervous system infections like meningitis [ 76 ]. Study done in Misurata Hospital -LIBYA around 50% of cases had malnutrition and anemia [ 77 ]. In Greece study, nearly one-third of patients suffered from iron deficiency anemia [ 78 ]. Children with disabilities and their families in African countries are frequently excluded from society because of stigmatization. Most of these children consequently confront many challenges socially, economically, and politically because they are often denied the basics of health care, education, socialization, and recognition. This systematic review and meta-analysis have limitations. There is variation between African countries in terms of culture, the economic profile, political stability, research funding and infrastructure, and health care systems. This makes it very difficult to generalize the results of this review across all countries. Besides, although we have tried to extensively search articles from all countries in Africa, we able to found 15 articles with from few countries; this may also affect the generalizability of the pooled findings. Furthermore, differences in methodology, including hospital versus community sittings, in the included studies also contributed to high levels of heterogeneity. Despite these limitations, these meta-analyses provide a picture of CP and its types among children in Africa. Conclusions and recommendationsThe overall prevalence of cerebral palsy in Africa found to be high (3·34 /1000) (95% CI: 2·70, 3·98). Compared to findings from developed countries spastic, and quadriplegic cerebral palsy were found to be the most common with pooled prevalence of 69·30% (95% CI: 66·76, 71·83) and 41·49% (95% CI: 33·16, 49·81) respectively. Governmental and non-governmental organizations should target their effort on the prevention, early detection and management of cerebral palsy in Africa. Large multicenter studies, qualitative or mixed-methods studies assessing patient and family understanding of CP, access to resources, and barriers to care are virtually absent in Africa to address recent evidence gap. In addition, there is a need for longitudinal studies that would assess outcomes over time for patients with CP as well as for randomized controlled trials of community-based treatment strategies that would be appropriate in an African setting. Supporting informationS1 table. search strategy used for one of the databases.. https://doi.org/10.1371/journal.pgph.0003003.s001 S2 Table. Quality appraisal result of included studies in Africa, using Joanna Briggs Institute (JBI) quality appraisal checklist [ 16 ].https://doi.org/10.1371/journal.pgph.0003003.s002 S1 Fig. Shows test of publication bias for prevalence of cerebral palsy among children in by sample size in Africa.https://doi.org/10.1371/journal.pgph.0003003.s003 S2 Fig. Trim and fill analysis for prevalence of cerebral palsy among children in by sample size in Africa.https://doi.org/10.1371/journal.pgph.0003003.s004 S3 Fig. Sensitivity analysis for prevalence of cerebral palsy among children in by sample size in Africa.https://doi.org/10.1371/journal.pgph.0003003.s005 S4 Fig. Subgroup analysis for prevalence of spastic cerebral palsy among children by countries in Africa.https://doi.org/10.1371/journal.pgph.0003003.s006 S5 Fig. Publication bias for prevalence of spastic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s007 S6 Fig. Subgroup analysis for prevalence of quadriplegic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s008 S7 Fig. Publication bias for prevalence of quadriplegic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s009 S8 Fig. Subgroup analysis for prevalence of ataxic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s010 S9 Fig. Publication bias for prevalence of ataxic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s011 S10 Fig. Subgroup analysis for prevalence of dyskinetic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s012 S11 Fig. Publication bias for prevalence of dyskinetic cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s013 S12 Fig. Subgroup analysis for prevalence of bilateral cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s014 S13 Fig. Publication bias for prevalence of bilateral cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s015 S14 Fig. Subgroup analysis for prevalence of unilateral cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s016 S15 Fig. Publication bias assessment for prevalence of unilateral cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s017 S16 Fig. Subgroup analysis for prevalence of mixed type cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s018 S17 Fig. Publication bias for prevalence of mixed type cerebral palsy among children in Africa.https://doi.org/10.1371/journal.pgph.0003003.s019 https://doi.org/10.1371/journal.pgph.0003003.s020
An official website of the United States government The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site. The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
A systematic approach to searching: an efficient and complete method to develop literature searchesAssociated data. Creating search strategies for systematic reviews, finding the best balance between sensitivity and specificity, and translating search strategies between databases is challenging. Several methods describe standards for systematic search strategies, but a consistent approach for creating an exhaustive search strategy has not yet been fully described in enough detail to be fully replicable. The authors have established a method that describes step by step the process of developing a systematic search strategy as needed in the systematic review. This method describes how single-line search strategies can be prepared in a text document by typing search syntax (such as field codes, parentheses, and Boolean operators) before copying and pasting search terms (keywords and free-text synonyms) that are found in the thesaurus. To help ensure term completeness, we developed a novel optimization technique that is mainly based on comparing the results retrieved by thesaurus terms with those retrieved by the free-text search words to identify potentially relevant candidate search terms. Macros in Microsoft Word have been developed to convert syntaxes between databases and interfaces almost automatically. This method helps information specialists in developing librarian-mediated searches for systematic reviews as well as medical and health care practitioners who are searching for evidence to answer clinical questions. The described method can be used to create complex and comprehensive search strategies for different databases and interfaces, such as those that are needed when searching for relevant references for systematic reviews, and will assist both information specialists and practitioners when they are searching the biomedical literature. INTRODUCTIONLibrarians and information specialists are often involved in the process of preparing and completing systematic reviews (SRs), where one of their main tasks is to identify relevant references to include in the review [ 1 ]. Although several recommendations for the process of searching have been published [ 2 – 6 ], none describe the development of a systematic search strategy from start to finish. Traditional methods of SR search strategy development and execution are highly time consuming, reportedly requiring up to 100 hours or more [ 7 , 8 ]. The authors wanted to develop systematic and exhaustive search strategies more efficiently, while preserving the high sensitivity that SR search strategies necessitate. In this article, we describe the method developed at Erasmus University Medical Center (MC) and demonstrate its use through an example search. The efficiency of the search method and outcome of 73 searches that have resulted in published reviews are described in a separate article [ 9 ]. As we aimed to describe the creation of systematic searches in full detail, the method starts at a basic level with the analysis of the research question and the creation of search terms. Readers who are new to SR searching are advised to follow all steps described. More experienced searchers can consider the basic steps to be existing knowledge that will already be part of their normal workflow, although step 4 probably differs from general practice. Experienced searchers will gain the most from reading about the novelties in the method as described in steps 10–13 and comparing the examples given in the supplementary appendix to their own practice. CREATING A SYSTEMATIC SEARCH STRATEGYOur methodology for planning and creating a multi-database search strategy consists of the following steps:
Each step in the process is reflected by an example search described in the supplementary appendix . 1. Determine a clear and focused questionA systematic search can best be applied to a well-defined and precise research or clinical question. Questions that are too broad or too vague cannot be answered easily in a systematic way and will generally result in an overwhelming number of search results. On the other hand, a question that is too specific will result into too few or even zero search results. Various papers describe this process in more detail [ 10 – 12 ]. 2. Describe the articles that can answer the questionAlthough not all clinical or research questions can be answered in the literature, the next step is to presume that the answer can indeed be found in published studies. A good starting point for a search is hypothesizing what the research that can answer the question would look like. These hypothetical (when possible, combined with known) articles can be used as guidance for constructing the search strategy. 3. Decide which key concepts address the different elements of the questionKey concepts are the topics or components that the desired articles should address, such as diseases or conditions, actions, substances, settings, domains (e.g., therapy, diagnosis, etiology), or study types. Key concepts from the research question can be grouped to create elements in the search strategy. Elements in a search strategy do not necessarily follow the patient, intervention, comparison, outcome (PICO) structure or any other related structure. Using the PICO or another similar framework as guidance can be helpful to consider, especially in the inclusion and exclusion review stage of the SR, but this is not necessary for good search strategy development [ 13 – 15 ]. Sometimes concepts from different parts of the PICO structure can be grouped together into one search element, such as when the desired outcome is frequently described in a certain study type. 4. Decide which elements should be used for the best resultsNot all elements of a research question should necessarily be used in the search strategy. Some elements are less important than others or may unnecessarily complicate or restrict a search strategy. Adding an element to a search strategy increases the chance of missing relevant references. Therefore, the number of elements in a search strategy should remain as low as possible to optimize recall. Using the schema in Figure 1 , elements can be ordered by their specificity and importance to determine the best search approach. Whether an element is more specific or more general can be measured objectively by the number of hits retrieved in a database when searching for a key term representing that element. Depending on the research question, certain elements are more important than others. If articles (hypothetically or known) exist that can answer the question but lack a certain element in their titles, abstracts, or keywords, that element is unimportant to the question. An element can also be unimportant because of expected bias or an overlap with another element.  Schema for determining the optimal order of elements Bias in elementsThe choice of elements in a search strategy can introduce bias through use of overly specific terminology or terms often associated with positive outcomes. For the question “does prolonged breastfeeding improve intelligence outcomes in children?,” searching specifically for the element of duration will introduce bias, as articles that find a positive effect of prolonged breastfeeding will be much more likely to mention time factors in their titles or abstracts. Overlapping elementsElements in a question sometimes overlap in their meaning. Sometimes certain therapies are interventions for one specific disease. The Lichtenstein technique, for example, is a repair method for inguinal hernias. There is no need to include an element of “inguinal hernias” to a search for the effectiveness of the Lichtenstein therapy. Likewise, sometimes certain diseases are only found in certain populations. Adding such an overlapping element could lead to missing relevant references. The elements to use in a search strategy can be found in the plot of elements in Figure 1 , by following the top row from left to right. For this method, we recommend starting with the most important and specific elements. Then, continue with more general and important elements until the number of results is acceptable for screening. Determining how many results are acceptable for screening is often a matter of negotiation with the SR team. 5. Choose an appropriate database and interface to start withImportant factors for choosing databases to use are the coverage and the presence of a thesaurus. For medically oriented searches, the coverage and recall of Embase, which includes the MEDLINE database, are superior to those of MEDLINE [ 16 ]. Each of these two databases has its own thesaurus with its own unique definitions and structure. Because of the complexity of the Embase thesaurus, Emtree, which contains much more specific thesaurus terms than the MEDLINE Medical Subject Headings (MeSH) thesaurus, translation from Emtree to MeSH is easier than the other way around. Therefore, we recommend starting in Embase. MEDLINE and Embase are available through many different vendors and interfaces. The choice of an interface and primary database is often determined by the searcher’s accessibility. For our method, an interface that allows searching with proximity operators is desirable, and full functionality of the thesaurus, including explosion of narrower terms, is crucial. We recommend developing a personal workflow that always starts with one specific database and interface. 6. Document the search process in a text documentWe advise designing and creating the complete search strategies in a log document, instead of directly in the database itself, to register the steps taken and to make searches accountable and reproducible. The developed search strategies can be copied and pasted into the desired databases from the log document. This way, the searcher is in control of the whole process. Any change to the search strategy should be done in the log document, assuring that the search strategy in the log is always the most recent. 7. Identify appropriate index terms in the thesaurus of the first databaseSearches should start by identifying appropriate thesaurus terms for the desired elements. The thesaurus of the database is searched for matching index terms for each key concept. We advise restricting the initial terms to the most important and most relevant terms. Later in the process, more general terms can be added in the optimization process, in which the effect on the number of hits, and thus the desirability of adding these terms, can be evaluated more easily. Several factors can complicate the identification of thesaurus terms. Sometimes, one thesaurus term is found that exactly describes a specific element. In contrast, especially in more general elements, multiple thesaurus terms can be found to describe one element. If no relevant thesaurus terms have been found for an element, free-text terms can be used, and possible thesaurus terms found in the resulting references can be added later (step 11). Sometimes, no distinct thesaurus term is available for a specific key concept that describes the concept in enough detail. In Emtree, one thesaurus term often combines two or more elements. The easiest solution for combining these terms for a sensitive search is to use such a thesaurus term in all elements where it is relevant. Examples are given in the supplementary appendix . 8. Identify synonyms in the thesaurusMost thesauri offer a list of synonyms on their term details page (named Synonyms in Emtree and Entry Terms in MeSH). To create a sensitive search strategy for SRs, these terms need to be searched as free-text keywords in the title and abstract fields, in addition to searching their associated thesaurus terms. The Emtree thesaurus contains more synonyms (300,000) than MeSH does (220,000) [ 17 ]. The difference in number of terms is even higher considering that many synonyms in MeSH are permuted terms (i.e., inversions of phrases using commas). Thesaurus terms are ordered in a tree structure. When searching for a more general thesaurus term, the more specific (narrower) terms in the branches below that term will also be searched (this is frequently referred to as “exploding” a thesaurus term). However, to perform a sensitive search, all relevant variations of the narrower terms must be searched as free-text keywords in the title or abstract, in addition to relying on the exploded thesaurus term. Thus, all articles that describe a certain narrower topic in their titles and abstracts will already be retrieved before MeSH terms are added. 9. Add variations in search terms (e.g., truncation, spelling differences, abbreviations, opposites)Truncation allows a searcher to search for words beginning with the same word stem. A search for therap* will, thus, retrieve therapy, therapies, therapeutic, and all other words starting with “therap.” Do not truncate a word stem that is too short. Also, limitations of interfaces should be taken into account, especially in PubMed, where the number of search term variations that can be found by truncation is limited to 600. Databases contain references to articles using both standard British and American English spellings. Both need to be searched as free-text terms in the title and abstract. Alternatively, many interfaces offer a certain code to replace zero or one characters, allowing a search for “pediatric” or “paediatric” as “p?ediatric.” Table 1 provides a detailed description of the syntax for different interfaces. Field codes in five most used interfaces for biomedical literature searching
Searching for abbreviations can identify extra, relevant references and retrieve more irrelevant ones. The search can be more focused by combining the abbreviation with an important word that is relevant to its meaning or by using the Boolean “NOT” to exclude frequently observed, clearly irrelevant results. We advise that searchers do not exclude all possible irrelevant meanings, as it is very time consuming to identify all the variations, it will result in unnecessarily complicated search strategies, and it may lead to erroneously narrowing the search and, thereby, reduce recall. Searching partial abbreviations can be useful for retrieving relevant references. For example, it is very likely that an article would mention osteoarthritis (OA) early in the abstract, replacing all further occurrences of osteoarthritis with OA . Therefore, it may not contain the phrase “hip osteoarthritis” but only “hip oa.” It is also important to search for the opposites of search terms to avoid bias. When searching for “disease recurrence,” articles about “disease free” may be relevant as well. When the desired outcome is survival , articles about mortality may be relevant. 10. Use database-appropriate syntax, with parentheses, Boolean operators, and field codesDifferent interfaces require different syntaxes, the special set of rules and symbols unique to each database that define how a correctly constructed search operates. Common syntax components include the use of parentheses and Boolean operators such as “AND,” “OR,” and “NOT,” which are available in all major interfaces. An overview of different syntaxes for four major interfaces for bibliographic medical databases (PubMed, Ovid, EBSCOhost, Embase.com, and ProQuest) is shown in Table 1 . Creating the appropriate syntax for each database, in combination with the selected terms as described in steps 7–9, can be challenging. Following the method outlined below simplifies the process:
The supplementary appendix explains the method of building a query in more detail, step by step for different interfaces: PubMed, Ovid, EBSCOhost, Embase.com, and ProQuest. This method results in a basic search strategy designed to retrieve some relevant references upon which a more thorough search strategy can be built with optimization such as described in step 11. 11. Optimize the searchThe most important question when performing a systematic search is whether all (or most) potentially relevant articles have been retrieved by the search strategy. This is also the most difficult question to answer, since it is unknown which and how many articles are relevant. It is, therefore, wise first to broaden the initial search strategy, making the search more sensitive, and then check if new relevant articles are found by comparing the set results (i.e., search for Strategy #2 NOT Strategy #1 to see the unique results). A search strategy should be tested for completeness. Therefore, it is necessary to identify extra, possibly relevant search terms and add them to the test search in an OR relationship with the already used search terms. A good place to start, and a well-known strategy, is scanning the top retrieved articles when sorted by relevance, looking for additional relevant synonyms that could be added to the search strategy. We have developed a unique optimization method that has not been described before in the literature. This method often adds valuable extra terms to our search strategy and, therefore, extra, relevant references to our search results. Extra synonyms can be found in articles that have been assigned a certain set of thesaurus terms but that lack synonyms in the title and/or abstract that are already present in the current search strategy. Searching for thesaurus terms NOT free-text terms will help identify missed free-text terms in the title or abstract. Searching for free-text terms NOT thesaurus terms will help identify missed thesaurus terms. If this is done repeatedly for each element, leaving the rest of the query unchanged, this method will help add numerous relevant terms to the query. These steps are explained in detail for five different search platforms in the supplementary appendix . 12. Evaluate the initial resultsThe results should now contain relevant references. If the interface allows relevance ranking, use that in the evaluation. If you know some relevant references that should be included in the research, search for those references specifically; for example, combine a specific (first) author name with a page number and the publication year. Check whether those references are retrieved by the search. If the known relevant references are not retrieved by the search, adapt the search so that they are. If it is unclear which element should be adapted to retrieve a certain article, combine that article with each element separately. Different outcomes are desired for different types of research questions. For instance, in the case of clinical question answering, the researcher will not be satisfied with many references that contain a lot of irrelevant references. A clinical search should be rather specific and is allowed to miss a relevant reference. In the case of an SR, the researchers do not want to miss any relevant reference and are willing to handle many irrelevant references to do so. The search for references to include in an SR should be very sensitive: no included reference should be missed. A search that is too specific or too sensitive for the intended goal can be adapted to become more sensitive or specific. Steps to increase sensitivity or specificity of a search strategy can be found in the supplementary appendix . 13. Check for errorsErrors might not be easily detected. Sometimes clues can be found in the number of results, either when the number of results is much higher or lower than expected or when many retrieved references are not relevant. However, the number expected is often unknown, and very sensitive search strategies will always retrieve many irrelevant articles. Each query should, therefore, be checked for errors. One of the most frequently occurring errors is missing the Boolean operator “OR.” When no “OR” is added between two search terms, many interfaces automatically add an “AND,” which unintentionally reduces the number of results and likely misses relevant references. One good strategy to identify missing “OR”s is to go to the web page containing the full search strategy, as translated by the database, and using Ctrl-F search for “AND.” Check whether the occurrences of the “AND” operator are deliberate. Ideally, search strategies should be checked by other information specialists [ 18 ]. The Peer Review of Electronic Search Strategies (PRESS) checklist offers good guidance for this process [ 4 ]. Apart from the syntax (especially Boolean operators and field codes) of the search strategy, it is wise to have the search terms checked by the clinician or researcher familiar with the topic. At Erasmus MC, researchers and clinicians are involved during the complete process of structuring and optimizing the search strategy. Each word is added after the combined decision of the searcher and the researcher, with the possibility of directly comparing results with and without the new term. 14. Translate to other databasesTo retrieve as many relevant references as possible, one has to search multiple databases. Translation of complex and exhaustive queries between different databases can be very time consuming and cumbersome. The single-line search strategy approach detailed above allows quick translations using the find and replace method in Microsoft Word (<Ctrl-H>). At Erasmus MC, macros based on the find-and-replace method in Microsoft Word have been developed for easy and fast translation between the most used databases for biomedical and health sciences questions. The schema that is followed for the translation between databases is shown in Figure 2 . Most databases simply follow the structure set by the Embase.com search strategy. The translation from Emtree terms to MeSH terms for MEDLINE in Ovid often identifies new terms that need to be added to the Embase.com search strategy before the translation to other databases.  Schematic representation of translation between databases used at Erasmus University Medical Center Dotted lines represent databases that are used in less than 80% of the searches. Using five different macros, a thoroughly optimized query in Embase.com can be relatively quickly translated into eight major databases. Basic search strategies will be created to use in many, mostly smaller, databases, because such niche databases often do not have extensive thesauri or advanced syntax options. Also, there is not much need to use extensive syntax because the number of hits and, therefore, the amount of noise in these databases is generally low. In MEDLINE (Ovid), PsycINFO (Ovid), and CINAHL (EBSCOhost), the thesaurus terms must be adapted manually, as each database has its own custom thesaurus. These macros and instructions for their installation, use, and adaptation are available at bit.ly/databasemacros. 15. Test and reiterateIdeally, exhaustive search strategies should retrieve all references that are covered in a specific database. For SR search strategies, checking searches for their recall is advised. This can be done after included references have been determined by the authors of the systematic review. If additional papers have been identified through other non-database methods (i.e., checking references in included studies), results that were not identified by the database searches should be examined. If these results were available in the databases but not located by the search strategy, the search strategy should be adapted to try to retrieve these results, as they may contain terms that were omitted in the original search strategies. This may enable the identification of additional relevant results. A methodology for creating exhaustive search strategies has been created that describes all steps of the search process, starting with a question and resulting in thorough search strategies in multiple databases. Many of the steps described are not new, but together, they form a strong method creating high-quality, robust searches in a relatively short time frame. Our methodology is intended to create thoroughness for literature searches. The optimization method, as described in step 11, will identify missed synonyms or thesaurus terms, unlike any other method that largely depends on predetermined keywords and synonyms. Using this method results in a much quicker search process, compared to traditional methods, especially because of the easier translation between databases and interfaces (step 13). The method is not a guarantee for speed, since speed depends on many factors, including experience. However, by following the steps and using the tools as described above, searchers can gain confidence first and increase speed through practice. What is new?This method encourages searchers to start their search development process using empty syntax first and later adding the thesaurus terms and free-text synonyms. We feel this helps the searcher to focus on the search terms, instead of on the structure of the search query. The optimization method in which new terms are found in the already retrieved articles is used in some other institutes as well but has to our knowledge not been described in the literature. The macros to translate search strategies between interfaces are unique in this method. What is different compared to common practice?Traditionally, librarians and information specialists have focused on creating complex, multi-line (also called line-by-line) search strategies, consisting of multiple record sets, and this method is frequently advised in the literature and handbooks [ 2 , 19 – 21 ]. Our method, instead, uses single-line searches, which is critical to its success. Single-line search strategies can be easily adapted by adding or dropping a term without having to recode numbers of record sets, which would be necessary in multi-line searches. They can easily be saved in a text document and repeated by copying and pasting for search updates. Single-line search strategies also allow easy translation to other syntaxes using find-and-replace technology to update field codes and other syntax elements or using macros (step 13). When constructing a search strategy, the searcher might experience that certain parentheses in the syntax are unnecessary, such as parentheses around all search terms in the title/abstract portion, if there is only one such term, there are double parentheses in the proximity statement, or one of the word groups exists for only one word. One might be tempted to omit those parentheses for ease of reading and management. However, during the optimization process, the searcher is likely to find extra synonyms that might consist of one word. To add those terms to the first query (with reduced parentheses) requires adding extra parentheses (meticulously placing and counting them), whereas, in the latter search, it only requires proper placement of those terms. Many search methods highly depend on the PICO framework. Research states that often PICO or PICOS is not suitable for every question [ 22 , 23 ]. There are other acronyms than PICO—such as sample, phenomenon of interest, design, evaluation, research type (SPIDER) [ 24 ]—but each is just a variant. In our method, the most important and specific elements of a question are being analyzed for building the best search strategy. Though it is generally recommended that searchers search both MEDLINE and Embase, most use MEDLINE as the starting point. It is considered the gold standard for biomedical searching, partially due to historical reasons, since it was the first of its kind, and more so now that it is freely available via the PubMed interface. Our method can be used with any database as a starting point, but we use Embase instead of MEDLINE or another database for a number of reasons. First, Embase provides both unique content and the complete content of MEDLINE. Therefore, searching Embase will be, by definition, more complete than searching MEDLINE only. Second, the number of terms in Emtree (the Embase thesaurus) is three times as high as that of MeSH (the MEDLINE thesaurus). It is easier to find MeSH terms after all relevant Emtree terms have been identified than to start with MeSH and translate to Emtree. At Erasmus MC, the researchers sit next to the information specialist during most of the search strategy design process. This way, the researchers can deliver immediate feedback on the relevance of proposed search terms and retrieved references. The search team then combines knowledge about databases with knowledge about the research topic, which is an important condition to create the highest quality searches. Limitations of the methodOne disadvantage of single-line searches compared to multi-line search strategies is that errors are harder to recognize. However, with the methods for optimization as described (step 11), errors are recognized easily because missed synonyms and spelling errors will be identified during the process. Also problematic is that more parentheses are needed, making it more difficult for the searcher and others to assess the logic of the search strategy. However, as parentheses and field codes are typed before the search terms are added (step 10), errors in parentheses can be prevented. Our methodology works best if used in an interface that allows proximity searching. It is recommended that searchers with access to an interface with proximity searching capabilities select one of those as the initial database to develop and optimize the search strategy. Because the PubMed interface does not allow proximity searches, phrases or Boolean “AND” combinations are required. Phrase searching complicates the process and is more specific, with the higher risk of missing relevant articles, and using Boolean “AND” combinations increases sensitivity but at an often high loss of specificity. Due to some searchers’ lack of access to expensive databases or interfaces, the freely available PubMed interface may be necessary to use, though it should never be the sole database used for an SR [ 2 , 16 , 25 ]. A limitation of our method is that it works best with subscription-based and licensed resources. Another limitation is the customization of the macros to a specific institution’s resources. The macros for the translation between different database interfaces only work between the interfaces as described. To mitigate this, we recommend using the find-and-replace functionality of text editors like Microsoft Word to ease the translation of syntaxes between other databases. Depending on one’s institutional resources, custom macros can be developed using similar methods. Results of the methodWhether this method results in exhaustive searches where no important article is missed is difficult to determine, because the number of relevant articles is unknown for any topic. A comparison of several parameters of 73 published reviews that were based on a search developed with this method to 258 reviews that acknowledged information specialists from other Dutch academic hospitals shows that the performance of the searches following our method is comparable to those performed in other institutes but that the time needed to develop the search strategies was much shorter than the time reported for the other reviews [ 9 ]. CONCLUSIONSWith the described method, searchers can gain confidence in their search strategies by finding many relevant words and creating exhaustive search strategies quickly. The approach can be used when performing SR searches or for other purposes such as answering clinical questions, with different expectations of the search’s precision and recall. This method, with practice, provides a stepwise approach that facilitates the search strategy development process from question clarification to final iteration and beyond. SUPPLEMENTAL FILEAcknowledgments. We highly appreciate the work that was done by our former colleague Louis Volkers, who in his twenty years as an information specialist in Erasmus MC laid the basis for our method. We thank Professor Oscar Franco for reviewing earlier drafts of this article.
Poor glycemic control impairs oral health in children with type 1 diabetes mellitus - a systematic review and meta-analysis
BMC Oral Health volume 24 , Article number: 748 ( 2024 ) Cite this article 34 Accesses Metrics details There are more than one million children and adolescents living with type 1 diabetes mellitus, and their number is steadily increasing. Diabetes affects oral health through numerous channels, including hyposalivation, immune suppression, and the inflammatory effect of glycation end-products. However, patients with type 1 diabetes must follow a strict sugar free diet that is proven to be carioprotective. Therefore, the aim of this systematic review and meta-analysis is to investigate whether children with type 1 diabetes have a difference in Decayed, Missing, Filled Teeth index (DMFT), salivary function, and periodontal status than children without diabetes, with an emphasis on glycemic control. Materials and MethodsPubMed, Embase and Cochrane libraries were screened for articles, using predefined search keys without any language or date restrictions. Two independent authors performed the selection procedure, extracted data from the eligible articles, carried out a manual search of the reference lists, and assessed the risk of bias using the Newcastle-Ottawa scale. Meta-analysis was performed in R using the random-effects model. Effect sizes were mean differences; subgroup analysis was performed on glycemic control. 33 studies satisfied the eligibility criteria. 22 studies did not show a significant difference regarding the DMFT index between the diabetes and non-diabetes groups; six studies found that children living with diabetes had higher DMFT scores, compared to five studies that found significantly lower scores. Meta-analysis found no statistically significant differences in plaque, gingival, and calculus indexes, however it found significant differences in pooled DMFT indexes, and salivary flow rate. Subgroup analysis on glycemic control using DMFT values found significant differences in children with good and poor glycemic control with results of 0.26 (CI95%=-0.50; 1.03) and 1.46 (CI95%=0.57; 2.35), respectively. ConclusionsChildren with poor glycemic control face higher risk of developing caries compared to good control and non-diabetes children. Regular dental check-ups and strict control of glycemic levels are highly advised for children living with type 1 diabetes, further emphasizing the importance of cooperation between dentists and diabetologists. Peer Review reports IntroductionDiabetes Mellitus (DM) is a disorder that is caused by either the lack of insulin secretion or the insufficient effect of the hormone [ 1 ], that leads to a chronically increased blood glucose level, which harms human health in several ways [ 2 ]. DM has four main types: type 1 is caused by an autoimmune response against the beta-cells of the pancreas; type 2 can develop on a multifactorial basis, mainly by an unhealthy lifestyle with the addition of bad diet and obesity; gestational diabetes develops and usually recedes within the gestational period; and lastly secondary diabetes that is either caused by certain medications or other illnesses [ 3 ]. There is still some uncertainty on the exact reason behind the development of type 1 DM; numerous causes are mentioned in the current literature including genetic (HLA proteins) and nongenetic factors (viral infections such as Coxsackievirus B) [ 4 , 5 ]. It was estimated that the number of people affected by DM to be at 536,3 million in 2021, and projected to reach 783 million by 2045 [ 6 ]. A significant portion of the affected individuals consists of children and adolescents and approximately 1.2 million of them have type 1 DM [ 6 ]. According to Chobot et al., the incidence of type 1 DM increased from 5.36 to 22.74 per 100 000 capita in 24 years’ time [ 7 ]. Several studies showed that there is a consistent increase in the number of affected children, approximately 3%, per year [ 8 ]. Hyperglycemia is the main cause of the clinical symptoms: elevated blood sugar levels can cause polyuria, weight loss despite heightened appetite, blurred vision, excessive thirst, constant tiredness and diabetic ketoacidosis [ 9 ]. Diagnosis relies on symptoms alongside an oral glucose tolerance test (OGTT), although evaluating metabolic control can also be achieved through measuring the HbA1c level; furthermore, the presence of autoantibodies associated with diabetes can be examined [ 10 ]. Dental caries is widespread all around the world [ 11 ]. Facilitated by biofilms and various factors, leads to localized demineralization of teeth [ 12 ]. Additionally, there were studies that reported on the harmful effects of DM on oral health, namely higher caries rate in children with type 1 DM, significantly higher plaque accumulation, gingivitis and calculus deposition [ 13 , 14 , 15 ]. According to Nederfors, salivary dysfunctions can be classified into three main groups: xerostomia, hyposalivation and changes in the composition of saliva [ 16 ]. Xerostomia is known to be the subjective complaint of oral dryness [ 17 ], whereas hyposalivation means the decrease in salivary outflow, that can be objectively measured [ 18 ]. Hyposalivation can go together with xerostomia, but that’s not always the case – on the other hand, sometimes xerostomia is present without real salivary gland dysfunction [ 19 ]. DM is considered to cause lower salivary flow rate [ 2 ], which can also induce harmful complications such as caries [ 20 ] and oral candidiasis [ 21 ]. Hyposalivation, poor immune defense, and high blood sugar levels are the main risk factors of developing oral candidiasis [ 21 , 22 ]. A suppressed immune system does not only make the human body susceptible to infections [ 22 ], but it also has a negative effect on wound healing [ 23 ]. DM has a bidirectional relationship with periodontal health, namely because DM promotes periodontal inflammation through various pathophysiological pathways that influence immune cells, collagen and lipid metabolism [ 11 , 12 , 24 ], while periodontitis can have serious adverse effects on glycemic control [ 25 ]. High blood sugar levels can lead to the formation of advanced glycation end-products, which enhance the production of inflammatory cytokines. In this manner the speed of periodontal bone resorption increases rapidly [ 26 ]. There is still debate on the overall effect of type 1 DM on oral health; on one hand, lower salivary functions and higher salivary glucose levels shift the oral environment towards a more cariogenic milieu, on the other hand patients with DM should follow a strict sugar-free diet, that has a serious carioprotective effect [ 27 ]. The relationship between type 2 DM and oral health is more certain, however, the impact of type 1 DM is still contradictory. There is data in the literature that type 1 DM decreases [ 28 ], or has no significant effect on caries prevalence [ 29 ], and also that it increases calculus and gingival indices [ 30 ]. There is no previous analysis in the literature that investigates the effect of different glycemic controls on oral health in children with type 1 DM. Therefore, we decided to investigate the effect of type 1 DM and glycemic control on caries prevalence, salivary flow rate and periodontal indices. Materials and methodsThis review was created according to the standards of the PRISMA® (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement. The PICO (P, population/patient/problem; I, intervention; C, comparison; O, outcome) question we investigated in this review was formed according to the rules of PRISMA®: “Do children (P) living with Type 1 Diabetes Mellitus (I), compared to healthy children (C), have worse caries and periodontal indexes? (O) The protocol of the review was preregistered on PROSPERO (CRD42023449223). Inclusion and exclusion criteriaStudies were included, if they (1) were cross-sectional and case-control studies; (2) included patients under the age of 19; (3) included only type 1 DM. Studies were excluded if they (1) did not report on any of the predefined outcomes; (2) were about other fields of dentistry; (3) were animal studies; (4) were inadequate article types, such as notes, reviews, letters, conference abstracts or randomized controlled studies; (5) had high risk of bias. Information sources, search strategy and the selection processAn extensive search strategy was employed to identify eligible studies through the following electronic databases: Pubmed, Cochrane Library, and Embase. The complete search key used was the following: ((diabetes OR DM OR diabetes mellitus OR diabetic) AND (type 1 OR type-1 OR type one OR insulin dependent OR IDDM)) AND (children OR child) AND (caries OR decay OR oral health status OR DMF OR gingival index OR calculus index OR salivary flow rate OR plaque index). The keywords were linked with the help of Boolean operators. The databases were screened on May 30, 2024. The results were exported to Endnote [ 31 ]. After duplicate removal, which was done with the help of the automatic duplicate finder in Endnote, two calibrated independent authors searched for articles according to the predefined inclusion and exclusion criteria with the help of Rayyan.ai [ 32 ], where the title and abstract selection was conducted. Disagreements were solved by consensus. If no consensus was achieved a third reviewer helped with the decision. The final pool of included studies was decided upon completing the full-text selection procedure under similar conditions. Agreements between the reviewers were calculated by Cohen’s Kappa. A manual search of the included papers reference list was conducted using the online Citation chaser tool [ 33 ]. Quality assessment and data extractionThe quality assessment of the included studies was done by the same two independent reviewers based on the guidelines of the Newcastle-Ottawa scale for case-control and cross-sectional studies. Two authors have extracted the necessary data independently using Excel (Microsoft) forms. The following data were extracted: first, the year the article was published; second, the names of the authors; and third, the title of the study. The number and type of different case and control groups were recorded, the parameters they examined, the number of the examined children in their respective groups, ages, and sex distributions were recorded. Data on Decayed, Missing due to caries, and Filled Teeth (DMFT) index (categorical outcomes) and the parameters of the saliva, including salivary flow rate (continuous outcomes) and the quantity of the saliva (continuous outcomes were recorded). Some studies recorded the results of the Oral Hygiene Index-Simplified (OHI-S), the Plaque Index (PI) (Silness-Löe), the Calculus Index (CI) (Greene and Vermilion), and the Gingival Index (GI) (Löe-Silness) which were also extracted. The results and conclusions of each study were summarized to make the comparison more easily manageable and the results straightforwardly accessible. Publication bias and certainty of evidencePublication bias was assessed by funnel plots when at least 10 studies were available. Certainty of evidence was assessed by one reviewer with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool. Data Synthesis and AnalysisFor the analysis, a random-effects model was chosen based on the assumption of significant between-study heterogeneity. The predefined included outcomes were all continuous, therefore the effect size measure was the difference between the means (MD) with 95% CI. A result that didn’t contain the null value was considered statistically significant. Subgroup analysis was performed based on the glycemic control of the patients; differentiation was made between well-, and poorly controlled patients based on their HbA1c values; for standardization purposes patients with lower than 7,5–8% HbA1c were allocated to the well-controlled, and higher than 7,5–8% were allocated to the poorly controlled group. Furthermore, between-study heterogeneity was calculated with the I2 statistics. Descriptive statistics were used to show the results of the meta-analysis with forest plots. Subgroup analyses were performed using the glycemic control data of the patient groups. All statistical analyses were carried out with R (version 4.3.0) using the meta (version 6.2.1) package for basic meta-analysis calculations and plots. Result of the systematic search and quality assessmentFrom the systematic search 1723 articles were retrieved, after the duplication removal 1499 articles were assessed by title and abstract selection (κ = 0.81). Conducting the full text selection, 34 eligible articles were identified for further analysis (κ = 1). The databases were screened on May 30, 2024. No additional eligible studies were found at the manual searches of the reference lists. The detailed selection procedure can be found in Fig. 1 .  Prisma flowchart (2020), detailed explanation of the selection procedure For the included studies it was required to have transparent inclusion and exclusion criteria, measurements of outcomes, adequate statistical analysis and consistent reporting of outcomes. To increase the certainty of the evidence, studies with low to moderate risk of bias (above a score of five) were included, whereas studies with high risk (below a score of five) were excluded from further analysis. The risk of bias assessment of studies is shown in Table 1 . The study of Al-Mutari et al. has received high risk of bias due to the contradictions in the abstract and in the full text of the article. They had conflicting outcomes in the Results section compared to the conclusion in the main text [ 34 ]. General aspects of the included studiesAll in all, the included articles were from 14 countries. There were five studies from India [ 35 , 36 , 37 , 38 , 39 ], four from Iran [ 27 , 40 , 41 , 42 ],two from Saudi Arabia [ 43 , 44 ], two from Egypt [ 45 , 46 ], two from Greece [ 47 , 48 ], one from Kuwait [ 14 ], one from The United States [ 49 ] one from Poland [ 50 ], one from Portugal [ 51 ], one from Montenegro [ 52 ], one from Kosovo [ 53 ], one from Turkey [ 54 ], one from Brazil [ 55 ], one from Iraq [ 56 ], one from Libya [ 57 ], one from Sweden [ 58 ], one from Belgium [ 29 ], one from Romania [ 59 ], one from Italy [ 60 ], one from Hong Kong [ 61 ], one from Finland [ 62 ], one from Lithuania [ 63 ] and one from Hungary [ 64 ]. The youngest child in the cohort was two-year-olds, while the oldest one was eighteen years old. Altogether, 5048 children were examined: 2547 children living with type 1 DM and 2501 non-DM children. The included articles analyzed the oral health of children with DM in comparison with their sex and age-matched controls without DM. The parameters under investigation included the following: DMFT, DMFS (Decayed, Missing due to caries, and Filled Surface), dmft (decayed, missing, and filled primary teeth) indexes, ICDAS (International Caries Detection and Assessment System), stimulated or unstimulated salivary flow rate, buffer capacity, viscosity and glucose level of the saliva, CI, PI, GI (Table 2 ). Glycemic controlSeveral articles differentiated between the quality of glycemic control. Ten study divided the DM study group into further groups according to their metabolic control [ 27 , 29 , 40 , 46 , 47 , 48 , 50 , 59 , 60 , 63 ]; five articles defined good glycemic control (GGC) and poor glycemic control (PGC) [ 47 , 48 , 50 , 60 , 63 ]. Whereas five articles included a third group called intermediate glycemic control (IGC) [ 27 , 40 , 46 , 29 , 59 ]. The HbA1c values used to define the sub-groups are shown in Table 3 . Seven articles examined the buffer capacity in relation to the prevalence of caries [ 14 , 44 , 47 , 48 , 52 , 53 , 63 ], two reported significantly worse buffer capacity in children living with DM [ 43 , 53 ], and one of these two have reported significantly higher scores on DMFT index [ 53 ]. From the three article reporting no significant differences between the study and the control group with respect to buffer capacity, two did not find a significant difference concerning the DMFT index either [ 48 , 63 ] and one found significantly higher DMFT [ 14 ]. Two articles have reported higher buffer capacity, though not significantly higher values, while there was no significant difference between the DMFT indexes either [ 47 , 52 ] (Table 4 ). Caries indexesThe included studies exhibited a high degree of heterogeneity with respect to the analysis of DMFT index, which stands for the number of decayed, missing due to caries, and filled teeth [ 65 ]. Twenty-two studies did not find statistically significant differences between the study group and the control group [ 27 , 29 , 38 , 41 , 42 , 43 , 44 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 55 , 57 , 59 , 60 , 61 , 62 , 64 ]. There were six studies revealing higher DMFT values in the DM groups [ 14 , 35 , 40 , 45 , 53 , 56 ]; and five studies found that children living with type 1 DM had lower DMFT values, which means a better caries prevalence [ 36 , 37 , 39 , 58 , 63 ]. All those studies that found the DMFT index significantly worse revealed poorer results in many other aspects, such as higher PI and GI [ 45 ], lower buffer capacity and salivary flow rate [ 53 ]. Interestingly, the study conducted by Elheeny et al. reported higher DMFT index in the DM group, even though they brushed their teeth significantly more [ 45 ]. Babu et al. reported that the DMFT index was higher in children with DM, however their GI was comparable [ 35 ]. The study of Geetha et al. disclosed that the DMFT index in children with DM was significantly lower, while their CI were significantly higher [ 36 ]. One other study stated that the study group had better DMFS and PI indexes despite having a lower salivary flow rate and a higher salivary glucose level [ 37 ]. All the other studies revealed that there was no statistically significant difference between the study and control groups regarding the DMFT or DMFS indexes. From these 22 articles, twelve showed a higher DMFT value in DM groups, but these differences were not significant [ 27 , 38 , 44 , 46 , 29 , 42 , 49 , 51 , 55 , 57 , 62 , 64 ], and there were five studies in which children with DM had better DMFT values than healthy controls [ 41 , 43 , 52 , 54 , 61 ]. The remaining five articles did not report on the comparison of healthy and DM individuals, only comparing the groups divided by metabolic control [ 47 , 48 , 50 , 59 , 60 ]. There were 17 studies included in the meta-analysis [ 14 , 27 , 29 , 35 , 36 , 38 , 40 , 41 , 42 , 44 , 46 , 49 , 52 , 55 , 56 , 61 , 62 ]. Statistically significant differences were found between the groups, with a result of 0.41 (CI95%=0.03; 0.78). The between study heterogeneity was considered very high and significant I2=98% (Fig. 2 ).  Meta-analysis of the pooled DMFT values compared in children with and without DM After dividing children living with DM into groups according to their metabolic control, there were a few articles that did not find statistically significant differences between the groups [ 27 , 40 , 46 , 47 , 29 , 59 , 60 ]. Three articles found significant differences between different metabolic controls [ 48 , 50 , 63 ]. Pachonski et al. reported that there was a significant difference between children with PGC and GGC regarding the DMFT index, and children with GGC had the best DMFT values among the groups, including the healthy controls, while children with PGC had the worst values [ 50 ]. According to the study of Pappa et al., even though there was no significant difference between children with GGC and no DM in terms of DMFT, there was a significant difference between the GGC and PGC groups and a significant difference between the PGC and control group [ 48 ]. Babatzia reported that children with PGC had higher DMFS values, although not significant [ 47 ]. According to the study of Siudikiene, children living with DM had significantly lower DMFS score compared to non-DM children, patients with well-controlled DM had significantly less decayed surface, to poorly controlled individuals [ 63 ]. There were five studies included in the meta-analysis of DMFT with subgroup analysis based on their glycemic control [ 27 , 29 , 40 , 48 , 50 ]. There was a statistically significant difference between poorly controlled patients and non-DM patients with a result of 1.46 (CI95%=0.57; 2.35). The between study heterogeneity was considered very high and statistically significant I2=92%; there was no difference between the well-controlled and non-DM patients (Fig. 3 ).  Subgroup Meta-analysis of DMFT index in well- and poorly controlled children compared with children without DM Salivary parametersSeven articles investigated the buffer capacity of children with DM [ 14 , 44 , 47 , 48 , 52 , 53 , 62 ]. Two articles showed statistically significantly worse buffer capacity [ 44 , 53 ], three articles did not find significant differences between the study and control group [ 14 , 48 , 62 ], and two studies reported better results in the DM group, while the buffer capacity of these children was not significantly higher compared to children without DM [ 47 , 52 ]. Eleven study examined salivary flow rate, from which five studies examined stimulated salivary flow rate [ 44 , 47 , 52 , 53 , 62 ], four study examined the unstimulated flow rate [ 37 , 39 , 51 , 55 ], and two examining both the stimulated and the resting salivary flow rate [ 14 , 48 ]. Five of them have reported significantly worse results [ 37 , 39 , 52 , 53 , 55 ], five studies revealed no significant difference between the study and control groups [ 14 , 44 , 48 , 51 , 62 ], and lastly, one study reported comparable outcomes in children with DM to non-DM children [ 47 ]. Out of the three articles where they found the flow rate significantly worse in the study group than in the control group [ 37 , 39 , 52 , 53 , 55 ], there was one article that reported significantly higher DMFT scores [ 53 ], two with no significant difference [ 52 , 55 ], and two with significantly lower DMFT index [ 37 , 39 ]; whereas the five articles where they found no significant difference in the salivary flow rate, four of them also showed no significant difference in the DMFT scores [ 44 , 48 , 51 , 62 ], except for the study of Akpata, where the DMFT index was significantly higher in DM children [ 14 ] (Table 3 ). Seven articles examined the buffer capacity in relation to the prevalence of caries [ 14 , 44 , 47 , 48 , 52 , 53 , 62 ], two reported significantly worse buffer capacity in children living with DM [ 43 , 53 ], and one of these two have reported significantly higher scores on DMFT index [ 53 ]. From the three article reporting no significant differences between the study and the control group with respect to buffer capacity, two did not find a significant difference concerning the DMFT index either [ 48 , 62 ] and one found significantly higher DMFT [ 14 ]. Two articles have reported higher buffer capacity, though not significantly higher values, while there was no significant difference between the DMFT indexes either [ 47 , 52 ] (Table 4 ). There were seven studies included in the meta-analysis of salivary flow rate [ 14 , 44 , 52 , 53 , 62 , 63 ]. There were statistically significant differences between the groups with a result of -0.21 (CI95%=-0.36; -0,07). The between study heterogeneity was considered very high and significant I2=97% (Fig. 4 ).  Meta-analysis of stimulated salivary flow rate compared in children with and without DM Only three of the seven articles recorded data about metabolic control and salivary parameters. Pappa et al. reported that salivary flow rate and pH values were significantly lower in the PGC group than in the GGC group and controls [ 48 ], while others found that the flow rate of all children was normal with sufficient capacity [ 47 ]. Siudikiene et al. found that there were no significant differences between the groups in terms of salivary flow rate and buffering capacity [ 63 ]. Periodontal indexesConsidering periodontal indexes, GI, PI, and CI were examined. There were nine studies reporting on GI scores. Four articles showed higher GI scores in children living with DM [ 40 , 42 , 45 , 46 ], and five articles did not find significant differences [ 35 , 38 , 47 , 50 , 61 ]. There were no data about significantly better GI scores; however, in one study the gingival conditions of DM children were considered healthy [ 35 ]. Seven studies were included in the quantitative analysis of GI that was comparable and used the Löe and Silness index [ 35 , 39 , 40 , 46 , 49 , 50 , 54 , 61 ]. There were no statistically significant differences between the groups with a result of 0.05 (CI95%=-0.01; 0.11). The between study heterogeneity was considered low and statistically non-significant I2=44% (Fig. 5 ).  Meta-analysis of Löé & Silness gingival index values compared in children with and without DM Regarding CI, two out of five studies have reported significantly higher scores in children living with DM [ 36 , 51 ], and three did not find statistically significant differences between the groups [ 40 , 47 , 61 ]. Just as in the case of GI scores, there was not a significantly better CI score recorded in the DM group. Meta-analysis was conducted on three studies regarding CI that used Greene and Vermilion indexes [ 40 , 52 , 61 ]. There were no statistically significant differences between the groups with a result of 0,04 (CI95%=-0,00; 0,09). The between study heterogeneity was considered very low and non-significant I2=0% (Fig. 6 ).  Meta-analysis of Greene and Vermilion calculus index values compared in children with and without DM Nine articles reported on PI, from which five articles found significantly higher PI scores in the DM group [ 40 , 45 , 46 , 51 ]. Among these four articles, one applied this observation only to children with poor metabolic control [ 47 ]. There were two studies with non-significant differences between the groups [ 42 , 50 ], while two studies have reported lower PI scores in the DM group [ 37 , 54 ]. There were seven studies included in the meta-analysis of PI [ 37 , 40 , 46 , 50 , 52 , 54 , 61 ]. There were no statistically significant differences between the groups with a result of 0.17 (CI95%=-0.40; 0.74). The between study heterogeneity was considered very high and statistically significant I2=95% (Fig. 7 ).  Meta-analysis of Silness & Löé plaque index values compared in children with and without DM Three studies that examined DM children according to different metabolic controls did not find significant differences between the groups regarding the conditions of the periodontium and oral hygiene (PI, GI, and CI) [ 40 , 46 , 50 ]. Even though Babatzia et al. have reported that there was no significant difference between GI and CI scores, they found that children with PGC had significantly more dental plaque [ 47 ]. With analyses containing at least 10 studies, publication bias was assessed by generating funnel plots. DMFT outcomes have provided symmetrical funnel plots, hence the probability of the existence of publication bias is low (Fig. 8 ).  Funnel plotof publication bias in DMFT outcomes Outcomes DMFT, GI, and CI have received low certainty of evidence, whereas outcomes salivary flow rate and PI have received very low certainty of evidence (Fig. 9 ).  Assessment of the certainty of evidence with GRADE tool The results of our meta-analysis regarding the pooled values of DMFT differences between patients with and without DM are in line with current state of the literature, however we only found a small difference between the groups, that is even though statistically significant, also clinically irrelevant, therefore a more complex approach is necessary to identify the connections more accurately [ 66 ]. The measurement of metabolic values in children holds significant importance as it facilitates early diagnosis and timely intervention. This approach enables full understanding of the potential consequences of DM, especially the effects of elevated blood glucose levels. For instance, certain studies did not report statistically significant differences between the study and control groups. However, taking into account the differences in metabolic control, significant differences are found. For instance, Pachonski et al. reported no significant differences between DM and non-DM children concerning DMFT values. However, they observed statistically significant differences between PGC and non-DM children. [ 50 ]. Differences in metabolic control within the populations could give an explanation for some of the differences between the included studies, that may be responsible for some of the between study heterogeneity. The most recent meta-analysis in the topic have found similar results regarding the differences in pooled DMFT values, however it did not investigate the effect of different glycemic controls on DMFT values [ 20 ]. Therefore, this meta-analysis sought to fill this gap in the literature. The study of Elheeny et al. did not group the children with DM according to their quality of metabolic control, despite that, the study can be informative in this aspect. The frequency of children with PGC was higher in the age group between 8 and 10, than 11 and 14 with percentages of 93,6% and 76,3%, respectively – which means, especially for the early adolescent group, that they basically examined children with poorly controlled DM. They found significantly higher caries scores in both of these age groups [ 45 ]. However, in some cases, even when they examined more children with PGC, they did not observe significant differences between the study groups and the control groups. In the study of Lai, 70.6% of the children living with DM had PGC; in the study of Sadeghi, 40% of the DM children had PGC; and in the study of Mesaro S., 66.7% of the study group had high HbA1c values [ 40 , 59 , 60 ]. However, these percentages are significantly lower than those previously mentioned. Most of the articles showed no significant differences between the study groups and the control groups. Some even reported significantly better DMFT indexes in children living with DM type I [ 36 , 37 , 39 , 58 , 63 ]. There could be several factors behind these results. Lower caries prevalence corresponds with the lower plaque scores, which could mean that DM children have better oral health routines than healthy children [ 37 ]. We have found no significant difference in PI between children with and without DM, that is in line with other studies [ 67 ]. Dental plaque is the strongest risk factor of developing caries, and the fact that PI is similar in the two population elevates the evidence of the impact of DM on caries risk [ 68 ]. It is said that children living with type 1 DM represent a more health-conscious and motivated group of society, due to the fact that these children are diagnosed with a metabolic disease at a young age and their parents are willing to cooperate with doctors and dentists to provide better life circumstances for their children [ 64 ]. This is confirmed in few studies; children with GGC had the best results not only compared to children with PGC but also to healthy controls [ 48 , 50 ]. Lai et al. have reported that children with GGC are counted as patients with lower caries risk in contrast to children living with PGC. They did not observe a significant difference between the study and the control group, but there were significantly more caries-free children in the GGC group compared to the PGC group, and there was a statistically significant difference concerning many cariogenic bacteria [ 60 ]. Another reason for the outstanding DMFT values of DM patients are their strict, sucrose-restricted diet and frequent monitoring, which might answer the question of why children with GGC represent the lowest DMFT values [ 37 , 48 ]. Furthermore, an important factor that could influence the results is the selection of patients in each group. For example, in the study of Iscan et al. 2020, control patients were children who sought treatment at the faculty, which could be a reason for an elevated value of DMFT score among them [ 54 ]. In another case, data of children with DM were collected at events organized to promote health-conscious lifestyles. Therefore, it may not represent the average DM population, hence parents that bring their children to such events are usually more health-conscious [ 64 ]. There is already evidence in the literature, that poor glycemic control in patients with type 2 DM elevates the risk of caries, periodontitis and peri-implantitis, however there were no previous analysis in the matter that investigated children with type 1 DM [ 69 , 70 , 71 ]. In order to fill this gap, we conducted the necessary analyses and found statistically significant, and clinically relevant differences between GGC and PGC children. To have good glycemic control, it is essential to attend regular meetings with a diabetologist, who helps with motivation, cooperation, and education of health. Therefore, when examining the effects of DM, not only the presence of the illness is the most relevant factor, but the quality of metabolic control. In a few studies, the children living with type 1 DM had better parameters than the controls [ 36 , 37 , 39 , 58 , 63 ]. In other cases, only the children with GGC had better scores [ 60 ]. There was not a single case where children with PGC had better oral health parameters than controls or the GGC group. We have found significantly lower salivary flow rate in children with DM, that could also provide a possible explanation for higher caries indices, that is in line with other studies conducted in the topic [ 72 ]. There was no article showing significantly better salivary flow rate in the DM group compared to the control groups’ scores. Pappa et al. examined not only the measurable salivary flow rate but the subjective feeling of xerostomia as well. Although they did not find a significant difference between the healthy and the DM groups, they found statistically significantly more children living with PGC suffering from xerostomia and lower salivary flow rate [ 48 ]. Children with GGC did not have significantly lower flow rates than the control patients; however, they reported xerostomia more often. According to Pappa et al., that could be a consequence of the frequent changes in blood sugar levels [ 48 ]. Also, we have found similar results regarding GI and CI parameters, that are closely connected with dental plaque induced inflammation, that further strengthens the connections of DM and caries [ 73 , 74 ]. However, in the study of Babatzia et al., they found elevated amounts of plaque in the group of PGC children, there were no significantly higher GI index associated with it [ 47 ]. Additionally, some studies did not find significantly different values in CI either. However, it is important to note, that the formation of calculus and the induction of gingival inflammation could be affected by individual characteristics as well, not only the presence or absence of DM and dental plaque [ 40 ]. According to the results of our analysis, it is possible to conclude that PGC leads to higher prevalence of caries. There are many tools that enable dentists to measure their patients HbA1c levels without blood taking, pain, and with a relatively good cost- and time-efficient method, in the dental office [ 75 ]. Therefore, we suggest HbA1c measurements in the dental office for patients with DM, to check their quality of glycemic control, and to suggest diabetologist consultation when poor control is found. Due to the nature of our research question, we could only include observational studies. Therefore, our certainty in our evidence is limited. Some included studies have not used the same indexes to report on periodontal condition, so it was not possible to include them in the quantitative analysis. The results for the meta-analysis have shown very high heterogeneity, which affects the certainty of the evidence. The strength of our study is, that to the best of our knowledge, there is no up-to-date analysis in the available literature on the topic that also investigates the impact of glycemic control on caries and periodontal outcomes. Hence, we could provide important insight in the topic. According to our results, our implication for practice is that HbA1c measurements are highly advised among children with DM to screen for poor glycemic control and to prevent any possible further damage on oral and systemic health. The strive for good glycemic control, by improving patient compliance and encouraging good cooperation with diabetologists and dentists would benefit the oral and systemic health of children with type 1 DM. Furthermore, we highly suggest more studies with rigorous protocols to compare children with different qualities of glycemic control according to their HbA1c levels to non-DM children, with cohorts matched for oral hygiene values. Children living with poorly controlled type 1 DM have higher DMFT values, while well-controlled children have comparable or better DMFT values to children with no DM. Chairside HbA1c measurement is highly suggested at dental checkups in order to identify underlying DM and verify the quality of glycemic control with close cooperation with diabetologist specialists. Availability of data and materialsThe datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis. Reference listGuthrie RA, Guthrie DW. Pathophysiology of diabetes mellitus. Crit Care Nurs Q. 2004;27(2):113–25. Article PubMed Google Scholar Hoseini A, et al. Salivary flow rate and xerostomia in patients with type I and II diabetes mellitus. Electron Physician. 2017;9(9):5244–9. Article PubMed PubMed Central Google Scholar Committee ADAPP. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care. 2023;47(Supplement1):S20–42. Google Scholar Novotna M, Podzimek S, Broukal Z, Lencova E, Duskova J. Periodontal diseases and dental caries in children with type 1 diabetes mellitus. Mediators Inflamm. 2015;2015:379626. https://doi.org/10.1155/2015/379626 . Hober D, Alidjinou EK. Enteroviral pathogenesis of type 1 diabetes: queries and answers. Curr Opin Infect Dis. 2013;26(3):263–9. Article CAS PubMed Google Scholar Federation ID. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes Federation. 2021; https://www.diabetesatlas.org . Chobot A, et al. Updated 24-year trend of Type 1 diabetes incidence in children in Poland reveals a sinusoidal pattern and sustained increase. Diabet Med. 2017;34(9):1252–8. Patterson CC, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62:408–17. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62. Article Google Scholar Neu A, et al. Diagnosis, Therapy and Follow-Up of Diabetes Mellitus in Children and Adolescents. Exp Clin Endocrinol Diabetes. 2019;127:01. Dicembrini I, et al. Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020;57(12):1405–12. Kumar M, et al. Diabetes and gum disease: the diabolic duo. Diabetes Metab Syndr. 2014;8(4):255–8. Ismail AF, McGrath CP, Yiu CK. Oral health of children with type 1 diabetes mellitus: a systematic review. Diabetes Res Clin Pract. 2015;108(3):369–81. Akpata ES, et al. Caries experience among children with type 1 diabetes in Kuwait. Pediatr Dent. 2012;34(7):468–72. PubMed Google Scholar del López LM, Ocasio-López C. Comparing the oral health status of diabetic and non-diabetic children from Puerto Rico: a case-control pilot study. P R Health Sci J. 2011;30(3):123–7. Nederfors T. Xerostomia and hyposalivation. Adv Dent Res. 2000;14:48–56. Rayman S, Dincer E, Almas K. Xerostomia. Diagnosis and management in dental practice. N Y State Dent J. 2010;76(2):24–7. Thakkar JP, Lane CJ. Hyposalivation and Xerostomia and Burning Mouth Syndrome: Medical Management. Oral Maxillofac Surg Clin North Am. 2022;34(1):135–46. López-Pintor RM, et al. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. 2016;2016:4372852. Liu T, et al. Caries status and salivary alterations of type-1 diabetes mellitus in children and adolescents: a systematic review and meta-analysis. J Evid Based Dent Pract. 2021;21(1):101496. Patel PN, et al. Oral candidal speciation, virulence and antifungal susceptibility in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2017;125:10–9. Mauri-Obradors E, et al. Oral manifestations of diabetes mellitus. a systematic review. Med Oral Patol Oral Cir Bucal. 2017;22(5):e586–94. CAS PubMed PubMed Central Google Scholar Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31(8):817–36. Zheng M, et al. Prevalence of periodontitis in people clinically diagnosed with diabetes mellitus: a meta-analysis of epidemiologic studies. Acta Diabetol. 2021;58(10):1307–27. Baeza M, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. 2020;28:e20190248. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. 2020;82(1):214–24. Kamran S, Moradian H, Yazdan E, Bakhsh. Comparison of the mean DMF index in type i diabetic and healthy children. J Dent (Shiraz). 2019;20(1):61–5. Singh-Hüsgen P, et al. Investigation of the oral status and microorganisms in children with phenylketonuria and type 1 diabetes. Clin Oral Invest. 2016;20:841–7. Tagelsir A, et al. Dental caries and dental care level (restorative index) in children with diabetes mellitus type 1. Int J Pediatr Dent. 2011;21(1):13–22. Siudikiene J, et al. Oral hygiene in children with type I diabetes mellitus. Stomatologija. 2005;7(1):24–7. The EndNote Team. EndNote. Philadelpia, PA: Clarivate Analytics; 2013. Ouzzani M, et al. Rayyan—a web and mobile app for systematic reviews. Syst Reviews. 2016;5(1):210. Haddaway NR, Grainger MJ, Gray CT. citationchaser: an R package for forward and backward citations chasing in academic searching. 2021. AlMutairi FFJ, et al. Relationship between type-I diabetes mellitus and oral health status and oral health-related quality of life among children of Saudi Arabia. J Family Med Prim Care. 2020;9(2):647–51. Babu KLG, Subramaniam P, Kaje K. Assessment of dental caries and gingival status among a group of type 1 diabetes mellitus and healthy children of South India - a comparative study. J Pediatr Endocrinol Metab. 2018;31(12):1305–10. Geetha S, et al. Oral health status and knowledge among 10-15years old type 1 diabetes mellitus children and adolescents in Bengaluru. Indian J Dent Res: Off Publ Indian Soc Dent Res. 2019;30(1):80–6. CAS Google Scholar Manjushree R, et al. Evaluation of salivary components and dental plaque in relation to dental caries status in type 1 diabetes mellitus. Int J Clin Pediatr Dent. 2022;15(Suppl 2):S121–5. Govindaraju L, Gurunathan D. Comparison of the oral hygiene status in children with and without juvenile diabetes - a comparative study. Indian J Dent Res. 2023;34(4):410–2. Rai K, et al. Dental caries and salivary alterations in Type I Diabetes. J Clin Pediatr Dent. 2011;36(2):181–4. Sadeghi R, et al. The effect of diabetes mellitus type I on periodontal and dental status. J Clin Diagn Res. 2017;11(7):ZC14–7. PubMed PubMed Central Google Scholar Bassir L, et al. Relationship between dietary patterns and dental health in type I diabetic children compared with healthy controls. Iran Red Crescent Med J. 2014;16(1):e9684. Rafatjou R, et al. Dental health status and hygiene in children and adolescents with Type 1 diabetes mellitus. J Res Health Sci. 2016;16(3):122–6. Al-Badr AH, et al. Dental caries prevalence among Type 1 diabetes mellitus (T1DM) 6- to 12-year-old children in Riyadh, Kingdom of Saudi Arabia compared to non-diabetic children. Saudi Dent J. 2021;33(5):276–82. Assiri SA, et al. Assessment of dental caries and salivary characteristics among type 1 diabetic Saudi children. J Dent Sci. 2022;17(4):1634–9. Elheeny AAH. Oral health status and impact on the oral health-related quality of life of Egyptian children and early adolescents with type-1 diabetes: a case-control study. Clin Oral Invest. 2020;24(11):4033–42. El-Tekeya M, et al. Caries risk indicators in children with type 1 diabetes mellitus in relation to metabolic control. Pediatr Dent. 2012;34(7):510–6. Babatzia A, et al. Clinical and microbial oral health status in children and adolescents with type 1 diabetes mellitus. Int Dent J. 2020;70(2):136–44. Pappa E, Vastardis H, Rahiotis C. Chair-side saliva diagnostic tests: an evaluation tool for xerostomia and caries risk assessment in children with type 1 diabetes. J Dent. 2020;93:103224. Goteiner D, et al. Periodontal and caries experience in children with insulin-dependent diabetes mellitus. J Am Dent Assoc. 1986;113(2):277–9. Pachonski M, et al. Dental caries and periodontal status in children with type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metabolism. 2020;26(1):39–44. Coelho A, et al. Oral health of Portuguese children with Type 1 Diabetes: a multiparametric evaluation. J Clin Pediatr Dent. 2018;42(3):231–5. Djuričkovic M, Ivanovic M. Dental health status in children with type 1 diabetes mellitus in Montenegro. Vojnosanit Pregl. 2021;78(2):171–8. Ferizi L, et al. The influence of Type 1 diabetes mellitus on dental caries and salivary composition. Int J Dent. 2018;2018:p5780916. Iscan TA, Ozsin-Ozler C, Ileri-Keceli T, Guciz-Dogan B, Alikasifoglu A, Uzamis-Tekcicek M. Oral health and halitosis among type 1 diabetic and healthy children. J Breath Res. 2020;14(3):036008. https://doi.org/10.1088/1752-7163/ab8d8b . Alves C, Menezes R, Brandão M. Salivary flow and dental caries in Brazilian youth with type 1 diabetes mellitus. Indian J Dent Res. 2012;23(6):758–62. Amer Y, Saeed LM, Naser N. Biochemical evaluation of saliva in insulin dependent diabetes mellitus children in hilla city-iraq. J Pharm Sci Res. 2019;11(2):627–30. Arheiam A, Omar S. Dental caries experience and periodontal treatment needs of 10- to 15-year old children with type 1 diabetes mellitus. Int Dent J. 2014;64(3):150–4. Matsson L, Koch G. Caries frequency in children with controlled diabetes. Scand J Dent Res. 1975;83(6):327–32. CAS PubMed Google Scholar MesaroS AS, et al. Does Diabetes Influence Oral Health in Children?. Rom J Diabetes Nutr Metabolic Dis. 2019;26(1):65–71. Lai S, et al. Evaluation of the difference in caries experience in diabetic and non-diabetic children-a case control study. PLoS ONE. 2017;12(11):e0188451. Ismail AF, McGrath CP, Yiu CKY. Oral health status of children with type 1 diabetes: a comparative study. J Pediatr Endocrinol Metab. 2017;30(11):1155–9. Swanljung O, et al. Caries and saliva in 12-18-year-old diabetics and controls. Scand J Dent Res. 1992;100(6):310–3. Siudikiene J, et al. Dental caries and salivary status in children with type 1 diabetes mellitus, related to the metabolic control of the disease. Eur J Oral Sci. 2006;114(1):8–14. Banyai D, Vegh D, Vegh A, Ujpal M, Payer M, Biczo Z, et al. Oral health status of children living with type 1 diabetes mellitus. Int J Environ Res Public Health. 2022;19(1):545. https://doi.org/10.3390/ijerph19010545 . Article CAS PubMed PubMed Central Google Scholar Pitts NB, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. Taylor GD. Children with type 1 diabetes and caries - are they linked?. Evid Based Dent. 2020;21(3):94–5. Duda-Sobczak A, Zozulinska-Ziolkiewicz D, Wyganowska M. Better gingival status in patients with comorbidity of type 1 diabetes and thyroiditis in comparison with patients with type 1 diabetes and no thyroid disease-A preliminary study. Int J Environ Res Public Health. 2023;20(4):3008. https://doi.org/10.3390/ijerph20043008 . Figuero E, et al. Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: a systematic review. J Clin Periodontol. 2017;44:S116–34. de Lima AKA, et al. Diabetes mellitus and poor glycemic control increase the occurrence of coronal and root caries: a systematic review and meta-analysis. Clin Oral Investig. 2020;24(11):3801–12. Wu CZ, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. Bencze B, et al. Prediabetes and poorly controlled type-2 diabetes as risk indicators for peri-implant diseases:a systematic review and meta-analysis. J Dent. 2024;146:105094. Shimazaki Y, et al. Stimulated salivary flow rate and oral health status. J Oral Sci. 2017;59(1):55–62. Murakami S, et al. Dental plaque-induced gingival conditions. J Periodontol. 2018;89(Suppl 1):S17–27. Velsko IM, et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome. 2019;7(1):102. Masiero S, Alberti A, Corbella S, Francetti L. Chairside screening for undiagnosed diabetes and prediabetes in patients with periodontitis. Int J Dent. 2022;2022:9120115. https://doi.org/10.1155/2022/9120115 . Download references AcknowledgementsFinancial interest. The authors have no relevant financial or non-financial interests to disclose. Open access funding provided by Semmelweis University. The authors did not receive support from any organization for the submitted work Author informationZsuzsanna Triebl and Bulcsú Bencze should be considered joint first authors. Authors and AffiliationsDiabetes-Dental Workgroup, Semmelweis University, Szentkirályi 47, Budapest, 1088, Hungary Zsuzsanna Triebl, Bulcsú Bencze, Dorottya Bányai & Dániel Végh Department of Paediatric Dentistry and Orthodontics, Semmelweis University, Szentkirályi 47, Budapest, 1088, Hungary Zsuzsanna Triebl, Dorottya Bányai & Noémi Rózsa Department of Prosthodontics, Semmelweis University, Szentkirályi 47, Budapest, 1088, Hungary Bulcsú Bencze, Péter Hermann & Dániel Végh You can also search for this author in PubMed Google Scholar ContributionsZS.T.:, methodology, formal analysis, writing – original draft; writing - review & editing; visualization; data curation B.B.: methodology, formal analysis, writing – original draft; writing - review & editing; visualization; data curation; statistical analyses; P.H., N.R.: conceptualization, writing – review & editing; V.D., B.D.: methodology; formal analysis; conceptualization; supervision; writing – original draft.; writing review & editing All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Corresponding authorCorrespondence to Dániel Végh . Ethics declarationsEthics approval and consent to participate. Not applicable. Consent to publicationCompeting interests. The authors declare no competing interests. Additional informationPublisher’s note. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Rights and permissionsOpen Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data. Reprints and permissions About this articleCite this article. Triebl, Z., Bencze, B., Bányai, D. et al. Poor glycemic control impairs oral health in children with type 1 diabetes mellitus - a systematic review and meta-analysis. BMC Oral Health 24 , 748 (2024). https://doi.org/10.1186/s12903-024-04516-y Download citation Received : 29 March 2024 Accepted : 21 June 2024 Published : 28 June 2024 DOI : https://doi.org/10.1186/s12903-024-04516-y Share this articleAnyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative
BMC Oral HealthISSN: 1472-6831
An official website of the Department of Health & Human Services
 1. Use quotes to search for an exact match of a phrase. 2. Put a minus sign just before words you don't want. 3. Enter any important keywords in any order to find entries where all these terms appear.
Preventable or potentially inappropriate psychotropics and adverse health outcomes in older adults: systematic review and meta-analysis.Corvaisier M, Brangier A, Annweiler C, et al. Preventable or potentially inappropriate psychotropics and adverse health outcomes in older adults: systematic review and meta-analysis. J Nutr Health Aging. 2024;28(4):100187. doi:10.1016/j.jnha.2024.100187. Older adults who take potentially inappropriate medications (PIM) are at increased risk of adverse events such as falls. This review focuses specifically on potentially inappropriate psychotropic (PIP) use in older adults. These studies investigated the association with PIP use association of falls , mortality, negative impact on ability to participate in activities of daily living, and unplanned hospitalizations; the use of a PIP increased the risk of all four outcomes. Drug related problems and pharmacist interventions in a geriatric unit employing electronic prescribing. November 20, 2013 Overnight stay in the emergency department and mortality in older patients. November 29, 2023 Descriptive analysis of patient misidentification from incident report system data in a large academic hospital federation. October 20, 2021 Error in intensive care: psychological repercussions and defense mechanisms among health professionals. October 29, 2014 Feelings of trust and of safety are related facets of the patient's experience in surgery: a descriptive qualitative study in 80 patients. August 24, 2022 Guidelines on Human Factors in Critical Situations 2023. August 9, 2023 Overview of adverse events related to invasive procedures in the intensive care unit. May 30, 2012 Accuracy of emergency department clinical findings for diagnosis of coronavirus disease 2019. July 29, 2020 Effectiveness of a ‘do not interrupt’ vest intervention to reduce medication errors during medication administration: a multicenter cluster randomized controlled trial. October 13, 2021 Clinical reasoning in dire times- analysis of cognitive biases in clinical cases during the COVID-19 pandemic. February 23, 2022 Identification of warning signs during selection of surgical trainees. January 16, 2019 When do supervising physicians decide to entrust residents with unsupervised tasks? September 8, 2010 Agreement of expert judgment in causality assessment of adverse drug reactions. May 4, 2005 Error disclosure in neonatal intensive care: a multicentre, prospective, observational study. May 10, 2023 Preventable hospital admissions related to medication (HARM): cost analysis of the HARM study. March 1, 2011 Medication errors at hospital admission and discharge: risk factors and impact of medication reconciliation process to improve healthcare. October 20, 2021 Crossover of the patient satisfaction surveys, adverse events and patient complaints for continuous improvement in radiotherapy department. April 20, 2022 Anticoagulation-associated adverse drug events in hospitalized patients across two time periods. July 26, 2023 The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. December 2, 2009 Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. August 19, 2009 Epidemiology and patient outcome after medical emergency team calls triggered by atrial fibrillation. April 13, 2011 Influence of shift duration on cognitive performance of emergency physicians: a prospective cross-sectional study. August 29, 2018 Operating room organization and surgical performance: a systematic review. March 20, 2024 Exploring stakeholder perceptions around implementation of the Operating Room Black Box for patient safety research: a qualitative study using the theoretical domains framework. November 13, 2019 The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. April 15, 2009 Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. October 1, 2008 Reliability of the assessment of preventable adverse drug events in daily clinical practice. April 2, 2008 Why do hospital prescribers continue antibiotics when it is safe to stop? Results of a choice experiment survey. September 2, 2020 Patient handoffs and multi-specialty trainee perspectives across an institution: informing recommendations for health systems and an expanded conceptual framework for handoffs. August 23, 2023 Success in hospital-acquired pressure ulcer prevention: a tale in two data sets. December 12, 2018 Managing competing organizational priorities in clinical handover across organizational boundaries. January 21, 2015 Prospective risk analysis and incident reporting for better pharmaceutical care at paediatric hospital discharge. December 17, 2014 Impact of including readmissions for qualifying events in the Patient Safety Indicators. April 22, 2015 Use of maternal early warning trigger tool reduces maternal morbidity. April 6, 2016 Risk and pharmacoeconomic analyses of the injectable medication process in the paediatric and neonatal intensive care units. May 12, 2010 Identifying organizational cultures that promote patient safety. November 18, 2009 Inappropriate medications in elderly ICU survivors: where to intervene? June 29, 2011 Clinical care checklists: salvations or frustrations? June 1, 2011 Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. March 12, 2014 Effect of nonpayment for hospital-acquired, catheter–associated urinary tract infection: a statewide analysis. September 19, 2012 Patient safety culture and the association with safe resident care in nursing homes. April 18, 2012 Errors in electronic health record–based data query of statin prescriptions in patients with coronary artery disease in a large, academic, multispecialty clinic practice. May 2, 2018 Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. May 17, 2017 Simulation-based assessment of the management of critical events by board-certified anesthesiologists. September 13, 2017 The development and implementation of checklists in obstetrics. September 27, 2017 An innovative collaborative model of care for undiagnosed complex medical conditions. May 31, 2017 Relationship of safety climate and safety performance in hospitals. April 1, 2009 Patient safety climate in 92 US hospitals: differences by work area and discipline. February 4, 2009 Patient safety climate in US hospitals: variation by management level. November 12, 2008 Effects of work hour reduction on residents' lives: a systematic review. September 28, 2005 Quality improvement implementation and hospital performance on patient safety indicators. January 31, 2006 Workforce perceptions of hospital safety culture: development and validation of the patient safety climate in healthcare organizations survey. September 26, 2007 Will my patient fall? January 17, 2007 Use of a safety climate questionnaire in UK health care: factor structure, reliability and usability. November 22, 2006 Use of a prospective risk analysis method to improve the safety of the cancer chemotherapy process. November 23, 2005 Liability reform should make patients safer: "Avoidable classes of events" are a key improvement. October 19, 2005 Systematic review: effects of resident work hours on patient safety. September 28, 2005 Differences in the reporting of care-related patient injuries to existing reporting systems. March 6, 2005 Defining, identifying and addressing problematic polypharmacy within multimorbidity in primary care: a scoping review. June 12, 2024 Physician antipsychotic overprescribing letters and cognitive, behavioral, and physical health outcomes among people with dementia: a secondary analysis of a randomized clinical trial. May 29, 2024 Managers' perceptions of the factors affecting resident and patient safety work in residential settings and nursing homes: a qualitative systematic review. May 1, 2024 Potentially inappropriate prescribing in long-term care and its relationship with probable delirium. January 24, 2024 Patient safety in nursing homes from an ecological perspective: an integrated review. January 17, 2024 Informatics tools in deprescribing and medication optimization in older adults: development and dissemination of VIONE methodology in a high reliability organization. November 15, 2023 Intervention of pharmacist included in multidisciplinary team to reduce adverse drug event: a qualitative systematic review. November 1, 2023 A virtual breakthrough series collaborative to support deprescribing interventions across Veterans Affairs healthcare settings. October 4, 2023 STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. September 27, 2023 Do not PIMP my nursing home ride! The impact of Potentially Inappropriate Medications Prescribing on residents' emergency care use. September 20, 2023 Handling polypharmacy--a qualitative study using focus group interviews with older patients, their relatives, and healthcare professionals. September 13, 2023 Potentially inappropriate medication use is associated with increased risk of incident disability in healthy older adults. September 6, 2023 Exploring the impact of safety culture on incident reporting: lessons learned from machine learning analysis of NHS England staff survey and incident data. August 30, 2023 American Geriatrics Society 2023 updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. August 16, 2023 Value assessment of deprescribing interventions: suggestions for improvement. August 16, 2023 The spectrum of hospitalization-associated harm in the elderly. July 26, 2023 Association of polypharmacy and potential drug-drug interactions with adverse treatment outcomes in older adults with advanced cancer. July 19, 2023 Prevalence of potentially inappropriate medication prescribing in US nursing homes, 2013-2017. July 5, 2023 Drug-related problems among older people with dementia: a systematic review. July 5, 2023 Family conferences to facilitate deprescribing in older outpatients with frailty and with polypharmacy: the COFRAIL cluster randomized trial. May 10, 2023 Medicines related problems (MRPs) originating in primary care settings in older adults - a systematic review. May 3, 2023 A cluster randomized trial of two implementation strategies to deliver audit and feedback in the EQUIPPED medication safety program. April 26, 2023 Evaluation of effectiveness and safety of pharmacist independent prescribers in care homes: cluster randomised controlled trial. March 1, 2023 A scoping review of adverse incidents research in aged care homes: learnings, gaps, and challenges. February 8, 2023 Long-Term Trends of Psychotropic Drug Use in Nursing Homes. February 1, 2023 Exploring nursing-sensitive events in home healthcare: a national multicenter cohort study using a trigger tool. January 25, 2023 Interventions to increase patient safety in long-term care facilities-umbrella review. January 25, 2023 Deprescribing medicines in older people living with multimorbidity and polypharmacy: the TAILOR evidence synthesis. October 5, 2022 Potentially inappropriate prescribing for adults living with diabetes mellitus: a scoping review. October 5, 2022 Factors associated with potentially harmful medication prescribing in nursing homes: a scoping review. September 28, 2022  Connect With UsSign up for Email UpdatesTo sign up for updates or to access your subscriber preferences, please enter your email address below. Agency for Healthcare Research and Quality5600 Fishers Lane Rockville, MD 20857 Telephone: (301) 427-1364
Submit Your InnovationsPlease select your preferred way to submit an innovation. Continue as a GuestTrack and save your innovation in My Innovations Edit your innovation as a draft Continue Logged InPlease select your preferred way to submit an innovation. Note that even if you have an account, you can still choose to submit an innovation as a guest. Continue logged inNew users to the psnet site. Access to quizzes and start earning CME, CEU, or Trainee Certification. Get email alerts when new content matching your topics of interest in My Innovations.  |
IMAGES
VIDEO
COMMENTS
When you decide to update a systematic review search, there are two ways of identifying new articles: ... An entry date filter will find any articles added to the results since you last ran the search, unlike a publication date filter, which would only find more recent articles. Some examples of entry date filters for articles entered since ...
Systematic reviews synthesise relevant research around a particular question. Preparing a systematic review is time and resource consuming, and provides a snapshot of knowledge at the time of incorporation of data from studies identified during the latest search. ... or if an up to date search is required for a guideline to achieve credibility ...
Updating a search. Sometimes you may need to update database searches. This may be because: you have taken leave from working on your review. The Cochrane Handbook states that 'The search must be rerun close to publication, if the initial search date is more than 12 months (preferably six months) from the intended publication date, and the ...
The search strategy for systematic reviews is the main method of collecting the data which will underpin the review's findings. This means that the search must be sufficiently robust - both sensitive and specific - to capture all relevant articles. ... Date range of publication. Study design type. Whether a study focuses on the review's ...
Median time from the final search date to indexing 1.4 years (inter-quartile range; 0.96-2.0 years). Lags from search to publication were shortest for ... When considering the results of a particular systematic review, users should search for more recent reviews or trials to see if any exist and determine if the results are consistent with the ...
Updates vs. amendments: a review is considered updated and receives a new citation in Cochrane Database of Systematic Reviews (CDSR) when a new search is conducted and the results of the search are fully incorporated. If a scoping search is conducted to determine if an update is required, then the date of this search will not change the 'Date ...
Performing, writing, and publishing a systematic review take a long time. In a cohort of journal-published systematic reviews, Cochrane reviews, and health technology assessment reports, the median time lag between the stated last search date and publication was 61 weeks (interquartile range, 33-87 weeks) [].In the same cohort of reviews, 7% were out of date at the time of publication [].
The PICO model is widely used and taught in evidence-based health care as a strategy for formulating questions and search strategies and for characterizing clinical studies or meta-analyses . ... RevMan Web Use Cochrane's industry-leading software for all of your systematic reviews. Follow @cochranelibrary. Scolaris Language Sponsor Footer ...
Another method is to restrict records to those entered into the database on or after the date you last ran the search (note: this is NOT the publication date, this is the date the record was added to the database, and this date field is not available in all databases). ... When and How to Update Systematic Reviews: Consensus and Checklist. BMJ ...
"Living" systematic reviews are updated on a periodic basis, often monthly. Any new evidence is immediately incorporated into the published review. ... Scopus: Search Within Results: ORIG-LOAD-DATE AFT 20181225; CINAHL (Ebsco): AND new line: EM 20181225-20201225; Web of Science: AND PY=(2018-2020) [can only search by publication date and year]
Background. Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Systematic reviews are characterized by a methodical and replicable methodology and presentation. They involve a comprehensive search to locate all relevant published and unpublished work on a subject; a systematic integration of search results; and a critique of the extent, nature, and quality of evidence in relation to a particular research question.
When searching Cochrane Database of Systematic Reviews in Ovid, three date limit fields are available (4): Date of Publication (.dp.): the month, year, and possibly day that the journal was published. This can only be limited by year; Date of Most Recent Amendment (.dr.): the month, year, and possibly day that the review or protocol was last ...
Background Systematic reviews provide a synthesis of evidence for practitioners, for clinical practice guideline developers, and for those designing and justifying primary research. Having an up-to-date and comprehensive review is therefore important. Our main objective was to determine the recency of systematic reviews at the time of their publication, as measured by the time from last search ...
Transparency of reporting search strategies in systematic reviews. Hypertension Research 45 , 1838-1839 ( 2022) Cite this article. The Original Article was published on 08 August 2022 ...
Polyglot Search, Peer Review of Electronic Search Strategies, or Systematic Review Information Retrieval Checklist. Remember controlled vocabulary will and advanced search features may vary between databases. RUNNING THE SEARCH. Run all database searches on the same day : Note date ran and the number of results from each database.
Several CDC librarians have special training in conducting literature searches for systematic reviews. Literature searches for systematic reviews can take a few weeks to several months from planning to delivery. Fill out a search request form or contact the Stephen B. Thacker CDC Library by email [email protected] or telephone 404-639-1717.
a systematic search that attempts to identify all studies that would meet the eligibility criteria; ... Keeping the review up to date: Source: Green, S. and Higgins, J.P. (2008). Preparing a cochrane review. In J.P. Higgins and S. Green (eds.), Cochrane handbook for systematic reviews of interventions.
A systematic review is a literature review that gathers all of the available evidence matching pre-specified eligibility criteria to answer a specific research question. It uses explicit, systematic methods, documented in a protocol, to minimize bias, provide reliable findings, and inform decision-making.
Creating a Search Strategy. A well constructed search strategy is the core of your systematic review and will be reported on in the methods section of your paper. The search strategy retrieves the majority of the studies you will assess for eligibility & inclusion. The quality of the search strategy also affects what items may have been missed.
Conclusions: Being able to identify comprehensive, up-to-date reviews is important to clinicians, guideline groups, and those designing clinical trials. This study demonstrates that some reviews have a considerable delay between search and publication, but only 47% of systematic review abstracts stated the last search date and 60% stated the ...
Methods: The author performed a systematic search of the literature from 27 September 2022-22 November 2022 in PUBMED, in the first 6 pages of Google Scholar and in the online catalog, the ...
Background: The living systematic review (LSR) approach is based on an ongoing surveillance of the literature and continual updating. A few guidance documents address the conduct, reporting, publishing and appraisal of systematic reviews (SRs), but the methodology described is either not up-to date or not suitable for LSRs and misses additional LSR-specific considerations.
PROSPERO accepts registrations for systematic reviews, rapid reviews and umbrella reviews. PROSPERO does not accept scoping reviews or literature scans.Sibling PROSPERO sites registers systematic reviews of human studies and systematic reviews of animal studies.. Before registering a new systematic review, check PROSPERO and the resources on COVID-END to see whether a similar review already ...
Systematic reviews of interventions can support informed healthcare practices and decision-making. SoF tables are useful tools for distilling key information from these reviews into a succinct and accessible format. This tutorial has outlined guidance for presenting accurate and informative SoF tables. AUTHOR CONTRIBUTIONS
The last search date was on 12/05/ 2023 G. C. A weighted inverse variance random-effects model was used to estimate the pooled estimates of cerebral palsy and its types. The subgroup analysis, publication bias and sensitivity analysis were done. ... To date, a rigorous systematic review and meta-analysis of the overall prevalence of CP are ...
INTRODUCTION. Librarians and information specialists are often involved in the process of preparing and completing systematic reviews (SRs), where one of their main tasks is to identify relevant references to include in the review [].Although several recommendations for the process of searching have been published [2-6], none describe the development of a systematic search strategy from ...
Result of the systematic search and quality assessment. From the systematic search 1723 articles were retrieved, after the duplication removal 1499 articles were assessed by title and abstract selection (κ = 0.81). Conducting the full text selection, 34 eligible articles were identified for further analysis (κ = 1).
A systematic search of four academic databases, ProQuest Central, Taylor and Francis Journals, SAGE Journals and APA PsycInfo, web and citation search was conducted between April 2022 and October 2022. ... Date: 11 September 2001-11 September 2021 publication years ... This systematic review underscores the pressing necessity for gaining a ...
Older adults who take potentially inappropriate medications (PIM) are at increased risk of adverse events such as falls. This review focuses specifically on potentially inappropriate psychotropic (PIP) use in older adults. These studies investigated the association with PIP use association of falls, mortality, negative impact on ability to participate in activities of daily living, and ...