- Harvard Business School →
- Faculty & Research →
- November 2013
- HBS Case Collection

GlaxoSmithKline in China (A)
- Format: Print
- | Language: English
About The Author
John A. Quelch
Related work.
- November 2013 (Revised September 2015)
- Faculty Research
GlaxoSmithKline in China (B)
- November 2013 (Revised January 2014)
GlaxoSmithKline In China (A), (B) and (C)
- January 2014 (Revised January 2014)
GlaxoSmithKline in China (C)
- GlaxoSmithKline in China (B) By: John A. Quelch and Margaret L. Rodriguez
- GlaxoSmithKline In China (A), (B) and (C) By: John A. Quelch
- GlaxoSmithKline in China (C) By: John Quelch and Margaret L. Rodriguez
- GlaxoSmithKline in China (A) By: John A. Quelch and Margaret L. Rodriguez
Brought to you by:

GlaxoSmithKline in China (A)
By: John A. Quelch, Margaret Rodriguez
Four GlaxoSmithKline employees were accused of bribing Chinese health care workers to prescribe the company's drugs. The accusations brought to light the questionable incentive structures of the…
- Length: 15 page(s)
- Publication Date: Nov 7, 2013
- Discipline: Marketing
- Product #: 514049-PDF-ENG
What's included:
- Teaching Note
- Educator Copy
$4.95 per student
degree granting course
$8.95 per student
non-degree granting course
Get access to this material, plus much more with a free Educator Account:
- Access to world-famous HBS cases
- Up to 60% off materials for your students
- Resources for teaching online
- Tips and reviews from other Educators
Already registered? Sign in
- Student Registration
- Non-Academic Registration
- Included Materials
Four GlaxoSmithKline employees were accused of bribing Chinese health care workers to prescribe the company's drugs. The accusations brought to light the questionable incentive structures of the Chinese health care system and the pressure on companies to adhere to local customs while still observing local laws.
Learning Objectives
To explore why executives engage in bribery. To assess the crisis management capacity of a multinational company in an important, emerging economy.
Nov 7, 2013 (Revised: Sep 8, 2015)
Discipline:
Geographies:
China, United Kingdom, United States
Industries:
Apparel accessories, Healthcare sector, Healthcare service industry, Insurance industry, Pharmaceutical industry, Public administration
Harvard Business School
514049-PDF-ENG
We use cookies to understand how you use our site and to improve your experience, including personalizing content. Learn More . By continuing to use our site, you accept our use of cookies and revised Privacy Policy .
China’s pharma scandal and the ethics of the global drug market
Postdoctoral Research Fellow, Bioethics, UNSW Sydney
Associate Professor in Bioethics & Director, Centre for Values and Ethics and the Law in Medicine, University of Sydney
Disclosure statement
Wendy Lipworth receives funding from the National Health & Medical Research Council.
Ian Kerridge has previously received NHMRC receives funding from the NHMRC to investigate the ethics of DTCA of prescription medicines and the interaction between the medical profession and the pharmaceuticl industry. He is also a member of the Ethics Committee of the Royal Australasian College of Physicians and has made submissions to Medicines Australia regarding their Code of Conduct.
University of Sydney and UNSW Sydney provide funding as members of The Conversation AU.
View all partners

China is in the midst of conducting a series of corruption investigations of pharmaceutical companies that have been operating in the country.
It all started with the investigation of officials from pharmaceutical company GlaxoSmithKline, who were reportedly engaged in “ bribery and corruption ” in China. The officials apparently used travel agencies to funnel illegal payments to doctors and government officials .
That was in July. In August, Associated Press reported French drug company Sanofi was being investigated for bribing Chinese doctors in 2007.
And late last week, South China Morning Post reported that German pharmaceutical conglomerate Bayer had joined the ranks of companies being investigated by the Chinese. That report mentioned pharmaceutical companies Eli Lilly, Novo Nordisk, H Lundbeck, AstraZeneca and UCB had also been contacted by Chinese investigators.
This wide-ranging, ongoing scandal highlights the many regulatory and ethical challenges of globalised drug development.
Globalised drug development
The development of prescription pharmaceuticals has long been an international endeavour, and in recent times the locus of activity has shifted from North America and Europe to Asia (particularly India and China), Eastern Europe, and Latin and South America.
Until recently, these regions participated primarily in the manufacture and testing of prescription pharmaceuticals, while North America and Europe remained the major sites for their sale.
Ethicists became concerned about the exploitation of research participants, who might be coerced into participating in research and would not have access to the medicines tested on them.
At the same time, scientists, clinicians and regulators worried about the validity and generalisability of research results derived from populations with different genetic profiles, diets, co-morbidities, life expectancies, and so on.
They also worried about the quality and safety of medicines, particularly the possibility of tainted or counterfeit medicines making their way to the West.
More recently, the pharmaceutical industry has become alert to the rapid economic progress of developing countries. Their enormous populations are increasingly seen as major “emerging markets” for the sale of patented as well as generic prescription pharmaceuticals.
Chronic, lifestyle (non-communicable) diseases such as diabetes, heart disease and respiratory illness are affecting people in these countries at rates comparable to those in the West. This makes them prime targets for many “blockbusters” drugs, which make the most money for pharmaceutical companies.
Marketing medicines
While access to medicines that are effective in the prevention and treatment of non-communicable diseases is undoubtedly a good thing, the sale of medicines in developing countries raises a new suite of regulatory and ethical issues.
Many of these issues have been extensively debated in the developed world, and resulted in an incremental increase of regulations controlling the activities of pharmaceutical companies.
It’s now broadly accepted in most developed countries that advertising medicines to the public needs to be tightly controlled (currently only the United States and New Zealand allow direct-to-consumer advertising of prescription pharmaceuticals).

It’s also widely accepted that professional and regulatory controls should limit promotion and marketing activities by the pharmaceutical industry so that they don’t inappropriately influence research, medical education, policymaking and prescribing.
The code of conduct of Australia’s pharmaceutical industry body, Medicines Australia, for example, now allows pharmaceutical companies to provide doctors with medical and educational items that enhance patient care, but these cannot be branded with drug names.
While an anatomical model might still be allowed as a gift, the ubiquitous branded pens and post-it notes will soon be a relic of the past.
Companies also must disclose their interactions with doctors. And pharmaceutical company representatives need to undertake formal training to ensure that they understand relevant legislation and guidelines.
Physicians have developed similarly detailed guidelines governing their interactions with the pharmaceutical industry, such as those of the Royal Australasian College of Physicians (currently being revised).
The debates surrounding these intricate guidelines stand in stark contrast to the blatant corruption being investigated in China.
A global issue
But it would be a mistake to see this issue simply as evidence of high-level industrial and professional corruption in a less regulated emerging economy.
Inappropriate corporate behaviour and professional practice still flourish in developed countries despite regulations. Indeed, these are frequently more extensive than those recently reported in China.
GlaxoSmithKline, for instance, agreed to pay a fine of US$3 billion in 2012 to settle charges of inappropriate promotion of antidepressants and failure to report safety data about the diabetes drug Avandia.
Physicians, likewise, have been complicit. In 2007, for example, orthopaedic device manufacturers paid 939 orthopaedic surgeons in the United States US$198 million to use their devices. In 2008, following a lawsuit by the US Department of Justice that was settled, this figure increased to US$228 million!
This makes the estimated US$3.34 million that fraudulently exchanged hands in China in the GlaxoSmithKline case look like peanuts.
As well as being a reminder of the need to concentrate on local as well as global corporate practices, the scandal highlights the need to think through the responsibility of corporations for their employees’ actions in a globalised world.
GlaxoSmithKline’s chief executive officer, for instance, denied any knowledge of the corruption in China. The denial may be plausible given the company has more than 100,000 employees worldwide, but it’s nearly impossible to promise corporate integrity in the face of large-scale global expansion.
This raises questions about the power and purpose of “corporate integrity agreements”, such as the one signed by GlaxoSmithKline , in the wake of its fraud settlement with the US government. That included the provision that the company would change the way its sales force was compensated.
Scandals implicating pharmaceutical companies, such as the one unfolding in China, show that we need better strategies if “corporate integrity” is to mean anything in the globalised medicine market.
- Drug regulation

Case Management Specialist

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)

China and Corruption: The Case of GlaxoSmithKline

By: Conner Lee
GlaxoSmithKline (GSK) is Britain’s biggest drug maker. Chinese authorities found GSK guilty of bribing both hospitals and doctors to help promote their products in China, using a network of nearly seven hundred travel agencies to pay medical professionals, health-related organizations, and government officials. According to Chinese authorities, GSK funneled about 3 billion yuan, or US$482 million, through this network to recipients. Receipts were forged for purchases and transactions that never took place, including fake conferences. At first, GSK denied any involvement in the bribes. Then, after an internal investigation, GSK admitted that certain executives acted independently in ways that broke Chinese law. (Rajagopalan) Chinese television even went so far as to air an alleged confession of one of the four senior GSK executives under investigation of how the scheme relied on “fake conferences and travel agencies to create receipts for services that were never performed.” (Thompson) GSK denies the sums of money are as high as Chinese officials suggest.
The Chinese officials also seemed to emphasize how the cost of the bribes was passed directly to Chinese consumers. In other words, doctors and other medical staff were bribed to sell their products and the cost of those bribes was added to the price of the products that consumers paid for. In some cases, the final price of the product was several times the cost in other countries. (BBC News) Chinese officials also claim GSK bribed officials to obstruct Chinese investigations, according to a security ministry official. (Bloomberg)
Five senior executives of GSK were arrested and subsequently found guilty of bribery. Mark Reilly, the chief executive of GlaxoSmithKline operations in China, received a suspended prison sentence. Four other GSK managers in China received similar suspended sentences. GSK’s local subsidiary in China was found guilty of bribery and fined nearly US$500 million, the largest corporate fine in China, according to the official Chinese news agency Xinhua. While the total fine is large, it is dwarfed by GSK’s annual free cash flow of about £4 billion. The company is also being investigated in other countries, and faces allegations that it bribed doctors in “Poland, Iraq, Jordan, and Lebanon.” (Rajagopalan) GSK wants to be the “The first company in the drugs industry to stop paying outside doctors to promote its products.” (Rajagopalan) It also claims to want to stop company policies that incentivize sales representatives to bribe doctors, and end payments for medical professionals to attend conferences. (Rajagopalan) In addition, since GSK was accused of bribery, its sales in China have taken a hit as well, and may be down permanently. In 2013, GSK’s sales dropped thirty percent after it was accused of corruption. (Financial Times) Once one of GSK’s fastest-growing markets, GSK’s medicine and vaccine sales, dropped 61% in the country, and sales of its consumer health products dropped by 29%. (Jack)
Corruption in China
China in the middle of a growing anti-corruption program, a program initiated by Chinese President Xi Jinping (Shobert). Cases of corruption used to be rare; however, due to public discontent with corruption in the Chinese Communist Party, the government was forced to respond. Announcements of investigations of party officials and businesses are now constantly in China’s headlines. (First Source from Hatton)
Corruption is a serious problem in China; even low-level officials can easily make small fortunes. Party officials can make millions of dollars a year in bribes and blackmail. The family of the official who launched the anti-corruption campaign, according to Bloomberg, holds an estimated $376 million. (Second Source from Hatton) In 2013, China was ranked 80 th out of 178 countries in the Transparency International’s Corruption Perceptions Index . Corruption is widely believed to be one of the major barriers to China’s social and economic development; some analysts warn that corruption threatens the country’s future and the popularity (and power) of the Communist Party. The Chinese public views corruption as a major problem. Many citizens in surveys say it is the biggest problem the country faces. Citizens describe corruption as unrestricted and rapidly growing. According to some estimates, approximately 10 percent of Chinese government funding is used as bribes, kickbacks, or is stolen. Even when investigations take place, the likelihood of corrupt officials going to jail is less than three percent. (Pei)
Anticorruption attempts have largely been failures in China. It is hard to enforce laws and regulations when everyone is breaking them. However, it is also possible the Western media has a false perception of how bad corruption is in China. As one source notes, “Between 1979 and 2000, over 700,000 cases for investigation were filed against officials by investigators.” Of these, approximately 56 percent of such cases were embezzlement, 28 percent bribery, and misuse of public funds 16 percent. If this is true, then it is possible the Chinese government may be doing more than it seems to confront corruption. (Manion, 87)
Corruption in China increased dramatically after 1978, and has grown hand-in-hand with the economy. As one source notes, “The Chinese economy has, it would seem, flourished even as corruption worsened.” (Wedeman, 4) Some nations are able to succeed despite high levels of corruption, and China seems to be one such country. (Wedeman, 4) Many authors seem to agree that while corruption and rapid growth may coexist together in the short-term, they are essentially contradictory. While the steps the Chinese government has taken to curtail corruption may have been ineffective, it is possible these efforts have prevented corruption from spiraling out of control. (Wedeman, 8)
China’s Healthcare System
China is in the midst of reforming its health care system, which is being expanded at a rapid pace. This includes a new national health insurance plan, which covers basic health needs. Unfortunately, these additions are “Being built on a top of a very weak foundation.” “Doctors are chronically over-worked and under-paid” (Shobert), working long hours and earning far too little. In addition, hospitals are stuck between fund shortages and rising health-care costs. Consequently, alternative means of revenue have been found. In this case, doctors seem to be making up for low salaries with bribes, as seen in cases such as the GSK scandal. In addition, Chinese citizens often bribe health care professionals to ensure that they receive good treatment when needed. (Shobert)
There are several major forms of corruption in the Chinese health-care system. The first is the pricing system. The government has laws on how much hospitals can charge for various products and services, but hospitals often simply ignore these laws, setting their own prices or simply overbilling patients. (Tam, 267) There have been several cases in which hospitals have charged patients for care they did not receive. Hospital staff at all levels were found to be accepting bribes, from patients that expect better medical services to medical equipment and pharmaceutical firms hoping to sell their products. Doctors, especially, accept bribes often. (Tam, 268) Hospitals have been documented to sell patients cheaper fake and substandard medications, illegally charging them the cost of the real medication and then pocketing the difference. Hospitals reap vast profits this way, especially in medical departments that treat serious illness, such as oncology. (Tam, 269)
The medical industry is stuck between rising expenses and declining budgets. China has a desperately underfunded public health care system. Between 1985 and 2005, government spending as a percentage of total health-care spending dropped from an estimated 38.6% to 17.9%. Health expenditure by private organizations declined as well. Meanwhile, personal spending on health care increased, from 20.4% in 1978 to 52.2% in 2005. Hospitals are forced to make up for the difference using illicit income to cover their expenses. (Tam, 270, 272)
Many health-care professionals use corruption as a means for personal gains. Loopholes and lax enforcement of law worsen the problem. One way in which physicians gain personal profit is by prescribing drugs and medical procedures that patients do not need. Sometimes part of the money paid for the medicine is kicked back to the physician. (Tam, 273) Doctors who are found accepting bribes are rarely penalized (Tam, 274), authorities who are aware of the budgetary problems the health care system faces are often unwilling to confront corruption.
Competition in the Chinese health-care system is intense, and many pharmaceutical firms resort to bribery and corruption as a means of selling their products. Chinese firms typically spend twenty to thirty percent of the price of their products on bribing doctors and hospitals, and customers are forced to pay the costs of these bribes. (Tam, 274)
The problems in the health industry have diminished public trust in the hospital systems. Various surveys indicate that many patients feel they must bribe doctors to ensure they receive good care. Otherwise, they fear poor treatment within the health care system. This sentiment cannot be understated. (Tam, 277)
Many foreign companies complain that, “China is broadly becoming a less hospitable place for multinational companies to operate.” (Shobert) Many foreign firms are convinced they are being scrutinized by regulatory oversight, and they are being held to higher regulatory standards than their domestic counterparts. If these suspicions are in fact true, then domestic firms in China are likely behaving far worse than their foreign competitors. (Shobert)
Public pressure for reform in the health-care industry is growing. A backlash threatens to undermine the Chinese government, and public resentment regarding corrupt officials in the industry is growing. While past attempts to deal with corruption in the industry have fallen short, it is possible the government may eventually be forced to deal with the problem. For instance, in 2006, 2,000 people rioted outside a hospital after a three-year old boy died of ingesting pesticide. The doctors there had refused to treat the boy because the parents did not have cash on hand when they arrived at the hospital. According to some estimates, there were about 17,000 such incidents in 2010. Officials have good reason to fear a public backlash. It is also possible that reform attempts will fall apart as in the past.
Since the scandal, China has passed several bills aimed at dealing with corruption in the health-care industry. The National Health and Family Planning Commission issued two bills with “anti-corruption compliance requirements” (Ross and Zhou), as well as a “blacklist” of certain medical-device firms and pharmaceutical companies that have violated the law. Chapter 49 establishes “Nine prohibitions” aimed at preventing bribery in the health-care sector. These prohibitions are aimed at preventing institutions and individuals from accepting various types of bribes and cutbacks, including preventing medical personnel from accepting commissions or kickbacks of any kind from medical institutions other than the one they work at. Chapter 50, however, establishes a blacklist system that shuts firms and individuals who have participated in bribery out of the health-care system. The last attempt at doing this was a 2007 law that was ineffective, and this new law aims to be much more effective. These bills are now law. (Ross and Zhou)
The GlaxoSmithKline Case
The case that GlaxoKlineSmith bribed Chinese officials does have merit. The company itself has acknowledged that there seems to have been some misconduct by certain executives, so it is almost undeniable the bribery took place. There is another side to this case, however. Bribery and other forms of corruption are not just common in China, companies are actually expected to bribe simply as a way of business. In many hospitals, all of the staff can be expected to be involved in the bribery system. It is so common there are literally systems in some hospitals to divide the profits from the bribes.
GSK was wrong in participating in the bribery schemes. It also was wrong in participating in corruption even if it was following expected industry norms in the country. It is never ethical to do a wrong act (in this case bribery) even if everyone else is doing the same act.
Given that large numbers of foreign companies complain of being unfairly scrutinized by Chinese authorities, it is likely the Chinese government unfairly investigated GSK because it was a large, foreign company with rapid market share growth that threatened domestic industry. Because bribery is so common, and because everyone does it, the Chinese government has the power to pick winners and losers. The government can arrest and press charges against any company it does not like, and rightfully claim the company was in violation of the law. GSK, in particular, was targeted for being a big, foreign firm with high market share growth in China.
Ultimately the consequences of the bribery scandal had a net negative impact on many people especially patients who carried most of the cost of corruption. The bribes caused many doctors to prescribe certain medications when they should have prescribed others, and it’s caused certain patients to receive better treatment than others. Corruption also vastly undermined public trust in the Chinese health care system, with most of the public deeply skeptical of hospitals and physicians. Unfortunately, bribery and corruption are the only ways that the Chinese health care system gets the funding it needs. Without bribery and other such forms of illegal funding, the Chinese health care system would either go bankrupt or go into debt. But bribery doesn’t just affect the people who have direct association with the act. It affects the behavior of a large segment of society. Even when everyone else is doing something bad, doing the action yourself only makes the problem worse. In the case of GlaxoSmithKline, the consequences of bribery actually contribute in undermining society, making the act unethical.
Works Cited:
Jack, Andrew, Patrick Jenkins, and David Oakley. “GlaxoSmithKline China Sales Face Growing Pressure – FT.com.” Financial Times . Financial Times LTD, 23 Sept. 2013. Web. 18 July 2014.
Shobert, Benjamin. “Three Ways To Understand GSK’s China Scandal.” Forbes . Forbes Magazine, 04 Sept. 2013. Web. 25 June 2014.
Rajagopalan, Megha, and Kazunori Takada. “Chinese Police Charge British Former Head of GSK in China with Bribery.” Reuters . Thomson Reuters, 14 May 2014. Web. 24 June 2014.
“UK Executive Accused in GlaxoSmithKline China Probe.” BBC News . BBC, 14 May 2014. Web. 25 June 2014.
“Glaxo’s Former China Head Accused of Ordering Bribes.” Bloomberg.com . Bloomberg, 14 May 2014. Web. 25 June 2014.
Insider, The. “The Glaxo-China Bribery Scandal: A New Policeman Walks The Beat.” Forbes . Forbes Magazine, 25 July 2013. Web. 25 June 2014.
Thompson, Mark. “Bribery Scandal Will Hit Glaxo’s China Growth.” CNNMoney . Cable News Network, 24 July 2013. Web. 25 June 2014.
(First Source from) Hatton, Celia. “How Real Is China’s Anti-corruption Campaign?” BBC News . BBC News, 4 Sept. 2013. Web. 25 June 2014.
(Second Source from) Hatton, Celia. “How Serious Is China on Corruption?” BBC News . BBC News, 28 Jan. 2013. Web. 25 June 2014.
Pei, Minxin. “Corruption Threatens China’s Future.” Carnegie Endowment for International Peace . Carnegie Endowment, 9 Oct. 2007. Web. 25 June 2014.
Ross, Lester, and Kenneth Zhou. “China’s New Anti-Corruption Policies in the Health Care Industry.” Wilmerhale . Wilmer Cutler Pickering Hale and Dorr LLP, 9 Jan. 2014. Web. 25 June 2014.
Tam, W. “Organizational Corruption By Public Hospitals In China.” Crime Law And Social Change 56.3 (n.d.): 265-282. Social Sciences Citation Index . Web. 25 June 2014.
Jack, Andrew. “GSK China Sales Plummet 60% since Scandal – FT.com.” Financial Times . The Financial Times LTD, 23 Oct. 2013. Web. 25 June 2014.
Manion, Melanie. Corruption by Design: Building Clean Government in Mainland China and Hong Kong . Cambridge, MA: Harvard UP, 2004. Print.
Wedeman, Andrew Hall. Double Paradox: Rapid Growth and Rising Corruption in China . Ithaca: Cornell UP, 2012. Print.
- Trust/trustworthiness
- Name First Last
- Your Message
- Share full article
Advertisement
Supported by
China Fines GlaxoSmithKline Nearly $500 Million in Bribery Case
By Keith Bradsher and Chris Buckley
- Sept. 19, 2014

HONG KONG — Global multinationals have invested billions of dollars in China over the last decade, with the prospect of selling to 1.4 billion people. But the promise of China’s growth is increasingly offset by the dangers of being caught up in the country’s anticorruption campaigns and rising economic nationalism.
In the strongest signal yet, a Chinese court on Friday imposed a fine of nearly $500 million on the British pharmaceutical giant GlaxoSmithKline for bribery, dwarfing the penalties in earlier criminal cases.
Multinational companies broadly have been under pressure in China, with technology companies, automakers and food manufacturers under investigation. As new cases and penalties have emerged, companies have been nervously preparing for their own potential fallout.
Last week, Chinese authorities fined the Audi unit of Volkswagen $40.5 million for violations of antitrust laws. In a similar case, a dozen Japanese auto parts and bearing manufacturers were assessed $200 million in penalties last month.
Beijing officials have gone out of their way in the last two weeks to deny complaints by foreign business groups and governments that China’s continuing legal crackdown represents an effort to discriminate against multinational companies and help Chinese companies compete. The Glaxo case showed that “an open China is not a lawless one,” Xinhua, the official news agency, said in a commentary.
But the Glaxo case underlines the dangers for multinationals as they continue to do business in a country where corruption has been widespread and where the legal and regulatory system has shown a greater willingness to prosecute foreign companies.
Two antitrust lawyers involved in other cases said in separate interviews that Chinese officials had rushed investigations along, sometimes in a few weeks, with little chance for multinationals to present their side. In some antimonopoly cases this summer, multinational company executives have not even been allowed to bring their lawyers to meetings with regulators, the lawyers said, both of whom insisted on anonymity because they were representing clients in litigation.
In many cases, regulators demanded that multinationals sharply reduce prices for products. Glaxo and a growing list of automakers have already done so.
Few companies have faced the level of scrutiny that Glaxo has. But no other multinational has acknowleged that its senior managers oversaw such a spree of bribe-giving and illicit sales tactics.
Chinese authorities accused Glaxo of bribing hospitals and doctors, channeling illicit kickbacks through travel agencies and pharmaceutical industry associations — a scheme that brought the company higher drug prices and illegal revenue of more than $150 million. In a rare move, authorities also prosecuted the foreign-born executive who ran Glaxo’s Chinese unit.
After a one-day trial held in secrecy, the court sentenced Glaxo’s British former country manager, Mark Reilly, and four other company managers to potential prison terms of up to four years. The sentences were suspended, allowing the defendants to avoid incarceration if they stay out of trouble, according to Xinhua. The verdict indicated that Mr. Reilly could be promptly deported. The report said they had pleaded guilty and would not appeal.
Glaxo said in a statement that it “fully accepts the facts and evidence of the investigation, and the verdict of the Chinese judicial authorities.” “GSK P.L.C. sincerely apologizes to the Chinese patients, doctors and hospitals, and to the Chinese government and the Chinese people.”
The British Embassy in Beijing said that it had no information on the possible deportation of Mr. Reilly and that while an appeal remained possible, it would have no comment on the trial.
“We note the verdict in this case,” an embassy spokesman said. “We have continually called for a just conclusion in the case in accordance with Chinese law. It would be wrong to comment while the case remains open to appeal.”
The court said that in deciding how to punish Mr. Reilly, it had taken into account that he had returned from Britain to face the investigators, and that he had “truthfully recounted the crimes of his employer,” meriting a relatively lenient punishment, the Xinhua report said. The other defendants also confessed and also earned relatively light sentences, according to the report.
The Glaxo case — and sizable fine — represents a setback for the company.
When the accusations first emerged last year, the company said that employees were “outside of our systems of controls.” It said the scandal involved a few rogue Chinese-born employees.
But the case escalated in May, when Chinese police accused Mr. Reilly, a Briton, of orchestrating a “massive bribery network.” Mr. Reilly and two Chinese-born executives, Zhang Guowei and Zhao Hongyan, had even bribed government officials in Beijing and Shanghai, they said. The names of the other defendants are Liang Hong and Huang Hong.
In its statement, Glaxo said that the court, the Changsha Intermediate People’s Court, had found the company guilty only of bribing nongovernmental personnel. The statement made no mention of any conviction for bribing government officials, a more politically delicate issue as President Xi Jinping of China pursues a broad campaign to root out corruption.
The accusations sent a chill through the industry when they came out last year. Many global drug makers used the same Shanghai travel agency that the authorities in the Glaxo case said had altered corporate travel expenses to pay cash bribes.
“It’s very hard to do business in the Chinese health care and pharmaceutical sectors without doing payoffs,” said David Zweig, the director of the Center on China’s Transnational Relations at the Hong Kong University of Science and Technology. “Everyone else pays bribes. Glaxo just got caught.”
Glaxo has also loomed large over another case.
In August, business partners in the investigative firm ChinaWhys were sentenced by a Chinese court after they were hired by Glaxo to look into whether a former employee was passing information about suspicions of fraud at the company to Chinese authorities.
Glaxo hired the couple in spring 2013 to look into whether a former employee had sent the company emails and a sex video of Mr. Reilly recorded without his knowledge or consent, according to people who were briefed on the situation and spoke on the condition of anonymity. The video was recorded with a camera inside his Shanghai apartment bedroom.
ChinaWhys, which specialized in due diligence work , completed an inconclusive preliminary report on the sex video of Mr. Reilly by June 2013 and suggested continuing the inquiry. In July 2013, the couple was detained, and they were formally arrested a month later, accused of illegally obtaining private information for their company.
The couple’s family has said the arrests were almost certainly linked to the Glaxo investigation, adding that Glaxo had not told Peter Humphrey, one of the investigators, the full details of the person suspected of being a whistle-blower.
Mr. Humphrey was sentenced to two and a half years in prison. The other, his wife, Yu Yingzeng, who is a Chinese-born American citizen, was sentenced to two years. The court said Mr. Humphrey would be deported after he served his term.
Glaxo appeared to distance itself from ChinaWhys in its statement Friday evening, saying that, “GSK P.L.C. also apologizes for the harm caused to individuals who were illegally investigated by” one of its subsidiaries in China.
Jane Perlez contributed reporting from Beijing.
GlaxoSmithKline in China: Challenges Faced
China is undoubtedly the largest source of income for multinational pharmaceutical enterprises, and it is also the market they attach the most importance to. This article mainly analyzes the annual report of GSK to study its development in China and the challenges it faces.

22 Years in China
The pharmaceutical baron, GlaxoSmithKline plc, known as GSK, was formed in 2000 after the merger of Glaxo Wellcome and SmithKline Beecham. At that time, GSK already had three subsidiary companies and five manufacturing sites in China.
In 2007, the Group set up an R&D center in Shanghai, focusing on research into neurodegeneration. However, CFDA's protection policy for local pharmaceutical enterprises at that time makes it difficult for MNCs to obtain market entrance approval.
As the most populous developing country in the world, China has become one of the world's biggest and fastest-growing drug markets. In 2012, its China Pharmaceuticals and Vaccines turnover surged to GBP 759 million by 17%.
GSK grabbed the world headlines in June 2013 when the police visited its Chinese offices for bribery allegations. One month later, the Chinese authority confirmed an ongoing investigation into alleged economic crimes by GSK China. In the same year, the Group's Pharmaceuticals and Vaccines sales slumped by 18% and its total wellness sales, part of the Consumer Healthcare business, tumbled by 40%.
Its Chinese affiliate was eventually found guilty of bribing doctors to prescribe its medicines, for which it was fined GBP 301 million in 2014. In that year's annual report, GSK said that its Pharmaceuticals and Vaccines sales in China fell 1% due to the effects of the government investigation. Its global turnover also saw a nosedive from GBP 26.5 billion in 2013 to GBP 23 billion in 2014.
Following the scandal, the Group claimed it would adopt a new way of rewarding medical representatives, strengthen expense monitoring and compliance efforts and hire external investigators to review its Chinese operations.
In the aftermath of the scandal, GSK was struggling with a tarnished reputation and its pharmaceuticals turnover in China was down 18% in 2015 mainly due to, it said, "increased pricing pressures and the ongoing reshaping of the business." In the same year, they removed the R&D center in Shanghai.
It seems that they did not take this step wisely. The CFDA changed the review policy and announced data verification on July 22, 2015, which marked an opening up – the overseas innovative drugs could now compete with the local ones on the same platform.
In 2016, GSK had a mixed result concerning its sales in China. Its pharmaceutical sales in the country declined by 12%, while its sales in the sector of Consumer Healthcare delivered a strong growth performance.
The reason behind this is that digitalization and e-commerce swept China. GSK went with the tide by distributing its products through an e-commerce platform and developing a patient support app. In 2016, its products like Sensodyne and Voltaren benefited particularly from e-commerce and retail distribution expansion. GSK also created an app where patients could order medication directly through Alibaba, China's online retail giant.
This year, two products of GSK have been approved by NMPA. One is Cervarix, a two-dose vaccine for girls aged between 9 to 14 years to prevent cervical cancer caused by HPV types 16 and 18. Another is Benlysta, a drug treating adult patients with active lupus nephritis.
Even though the turnover of GSK continues to increase these years, its profit performance has been poor. In order to solve this problem, GSK has chosen to split its consumer healthcare business to generate more cash.

A Spin-off of consumer healthcare business
In 2020, GSK announced a two-year split plan, and a week ago, on July 18, 2022, it announced the official split of its consumer health business. After the spin-off, Haleon was listed on the London Stock Exchange.

It is worth noting that GSK and Pfizer jointly established the split company in the consumer business in August 2019. Less than half a year after its establishment, GSK announced the divestiture of the joint venture. From a commercial perspective, the re-listing of the stripped Haleon can broaden financing channels, obtain cash flow, and improve the company's operational efficiency, so that GSK focuses on vaccines and specialty medicines while Haleon focuses on consumer business.
However, the reality is not as optimistic as expected. According to GSK's 2021 annual report, its turnover in 2021 was GBP 34.1 billion (USD 40.71 billion), the same as that in 2020, of which the pharmaceutical business was GBP 17.7 billion, an increase of 4%. The vaccine business was GBP 6.8 billion, continued negative growth, and the consumer healthcare business was GBP 9.6 billion, a decrease of 4%. In other words, GSK spun off a business accounting for nearly 36% of its turnover to relieve the pressure on cash flow, use the cash obtained to carry out research and development, expand existing pipelines, or seek new growth points through mergers and acquisitions.
GSK consumer healthcare possesses many familiar brands in oral health, pain relief, respiratory and nutrition/gastro-intestinal. However, the business was still split, and it had to be suspected that the move was to weaken the power of the current CEO, Dame Emma Walmsley. As a woman from a non-pharmaceutical background, Emma, who joined GSK's European consumer healthcare team in 2010, faces more pressure than this.
Vaccines – A major focus for the so-called ‘new GSK’
Qi Xin, vice president of GSK and general manager of China prescription drugs and vaccines, once said that GSK pays great attention to vaccines because prevention is better than treatment. Vaccines are of great help to the health of China's 1.4 billion people. However, when COVID-19 came, GSK did not give a satisfactory result on vaccine research and development but provided adjuvants to enhance immune response and production capacity. Although it is currently working with Sanofi, CureVac and other companies to develop vaccines, it is ultimately a late-moving enterprise compared with Pfizer. Pfizer has returned to the first place, but GSK's revenue growth has not improved.
In addition to the missed COVID-19 opportunity, the development of its 'crown jewel' Shingrix has also been bumpy.
The Centers for Disease Control (CDC) has advised people not to vaccinate another vaccine within two weeks after Covid-19 vaccination, which has seriously affected the sales of vaccines in the United States. The 2021 annual report showed that Shingrix decreased 13% to GBP 1,721 million. The UK is also regarded as a critical market by the company. But its sales strategy has been criticized by some critics for going too far.
The same situation also occurs in China, which is another cornerstone of the growth strategy of GSK's shingles vaccine. However, its approach of using online influencers to publicize attracted criticism from netizens, making it a hot topic on Weibo for the wrong reasons. Although the aging problem in China seems to be a huge market, as a class II vaccine (self-paid, not covered by national health insurance), the price of up to CNY 3200 (USD 437) also makes most people take more of a wait-and-see attitude. A staff member of a community service center said, "Last month, we reported ten shingles vaccines with CDC, the same with last few months. There were many consultations, but the price persuaded most of them to retreat." Search the major e-commerce platforms in China. The store with the highest monthly sales in Alibaba sold only 38 shots, and the total sales volume of JD was 200 shots plus up to July 2022. GSK still needs more time to recover from public sentiment. However, there is not much time left for him. The application for NMPA approval of the shingles vaccine of BCHT (Chinese: 百克生物) was accepted in April 2022.

Although GSK underperforms in covid-19 vaccine and Shingrix, it still has a respiratory syncytial virus (RSV) vaccine under development. Its meningitis sales decreased 7% globally. In order to realize its double revenue ambitions, the enterprise is communicating with NMPA, hoping to introduce meningitis vaccines into China in appropriate ways.
Vaccines are expected to be one of the most significant growth drivers for GSK, with high single-digit percentage sales growth (CAGR) anticipated over the 2021-2026 period. GSK aims to double the revenues of Shingrix in those five years and to double both meningitis and flu vaccine sales in the next decade. According to the answer paper handed in in 2021, the prospect is not optimistic.
GSK seems to have not been blessed by fortune along the way. We expected GSK could fulfill commitments to shareholders and realize a GBP 10 billion growth in the next ten years.

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- The Attorney General
- Organizational Chart
- Budget & Performance
- Privacy Program
- Press Releases
- Photo Galleries
- Guidance Documents
- Publications
- Information for Victims in Large Cases
- Justice Manual
- Business and Contracts
- Why Justice ?
- DOJ Vacancies
- Legal Careers at DOJ
- Our Offices
Archived Press Releases
Archived News
Para Notícias en Español
GlaxoSmithKline to Plead Guilty and Pay $3 Billion to Resolve Fraud Allegations and Failure to Report Safety Data
Global health care giant GlaxoSmithKline LLC (GSK) agreed to plead guilty and to pay $3 billion to resolve its criminal and civil liability arising from the company’s unlawful promotion of certain prescription drugs, its failure to report certain safety data, and its civil liability for alleged false price reporting practices, the Justice Department announced today. The resolution is the largest health care fraud settlement in U.S. history and the largest payment ever by a drug company.
GSK agreed to plead guilty to a three-count criminal information, including two counts of introducing misbranded drugs, Paxil and Wellbutrin, into interstate commerce and one count of failing to report safety data about the drug Avandia to the Food and Drug Administration (FDA). Under the terms of the plea agreement, GSK will pay a total of $1 billion, including a criminal fine of $956,814,400 and forfeiture in the amount of $43,185,600. The criminal plea agreement also includes certain non-monetary compliance commitments and certifications by GSK’s U.S. president and board of directors. GSK’s guilty plea and sentence is not final until accepted by the U.S. District Court.
GSK will also pay $2 billion to resolve its civil liabilities with the federal government under the False Claims Act, as well as the states. The civil settlement resolves claims relating to Paxil, Wellbutrin and Avandia, as well as additional drugs, and also resolves pricing fraud allegations.
“Today’s multi-billion dollar settlement is unprecedented in both size and scope. It underscores the Administration’s firm commitment to protecting the American people and holding accountable those who commit health care fraud,” said James M. Cole, Deputy Attorney General. “At every level, we are determined to stop practices that jeopardize patients’ health, harm taxpayers, and violate the public trust – and this historic action is a clear warning to any company that chooses to break the law.”
“Today’s historic settlement is a major milestone in our efforts to stamp out health care fraud,” said Bill Corr, Deputy Secretary of the Department of Health and Human Services (HHS). “For a long time, our health care system had been a target for cheaters who thought they could make an easy profit at the expense of public safety, taxpayers, and the millions of Americans who depend on programs like Medicare and Medicaid. But thanks to strong enforcement actions like those we have announced today, that equation is rapidly changing.”
This resolution marks the culmination of an extensive investigation by special agents from HHS-OIG, FDA and FBI, along with law enforcement partners across the federal government. Moving forward, GSK will be subject to stringent requirements under its corporate integrity agreement with HHS-OIG; this agreement is designed to increase accountability and transparency and prevent future fraud and abuse. Effective law enforcement partnerships and fraud prevention are hallmarks of the Health Care Fraud Prevention and Enforcement Action Team (HEAT) initiative, which fosters government collaboration to fight fraud.
Criminal Plea Agreement
Under the provisions of the Food, Drug and Cosmetic Act, a company in its application to the FDA must specify each intended use of a drug. After the FDA approves the product as safe and effective for a specified use, a company’s promotional activities must be limited to the intended uses that FDA approved. In fact, promotion by the manufacturer for other uses – known as “off-label uses” – renders the product “misbranded.”
Paxil: In the criminal information, the government alleges that, from April 1998 to August 2003, GSK unlawfully promoted Paxil for treating depression in patients under age 18, even though the FDA has never approved it for pediatric use. The United States alleges that, among other things, GSK participated in preparing, publishing and distributing a misleading medical journal article that misreported that a clinical trial of Paxil demonstrated efficacy in the treatment of depression in patients under age 18, when the study failed to demonstrate efficacy. At the same time, the United States alleges, GSK did not make available data from two other studies in which Paxil also failed to demonstrate efficacy in treating depression in patients under 18. The United States further alleges that GSK sponsored dinner programs, lunch programs, spa programs and similar activities to promote the use of Paxil in children and adolescents. GSK paid a speaker to talk to an audience of doctors and paid for the meal or spa treatment for the doctors who attended. Since 2004, Paxil, like other antidepressants, included on its label a “black box warning” stating that antidepressants may increase the risk of suicidal thinking and behavior in short-term studies in patients under age 18. GSK agreed to plead guilty to misbranding Paxil in that its labeling was false and misleading regarding the use of Paxil for patients under 18.
Wellbutrin: The United States also alleges that, from January 1999 to December 2003, GSK promoted Wellbutrin, approved at that time only for Major Depressive Disorder, for weight loss, the treatment of sexual dysfunction, substance addictions and Attention Deficit Hyperactivity Disorder, among other off-label uses. The United States contends that GSK paid millions of dollars to doctors to speak at and attend meetings, sometimes at lavish resorts, at which the off-label uses of Wellbutrin were routinely promoted and also used sales representatives, sham advisory boards, and supposedly independent Continuing Medical Education (CME) programs to promote Wllbutrin for these unapproved uses. GSK has agreed to plead guilty to misbranding Wellbutrin in that its labeling did not bear adequate directions for these off-label uses. For the Paxil and Wellbutrin misbranding offenses, GSK has agreed to pay a criminal fine and forfeiture of $757,387,200.
Avandia: The United States alleges that, between 2001 and 2007, GSK failed to include certain safety data about Avandia, a diabetes drug, in reports to the FDA that are meant to allow the FDA to determine if a drug continues to be safe for its approved indications and to spot drug safety trends. The missing information included data regarding certain post-marketing studies, as well as data regarding two studies undertaken in response to European regulators’ concerns about the cardiovascular safety of Avandia. Since 2007, the FDA has added two black box warnings to the Avandia label to alert physicians about the potential increased risk of (1) congestive heart failure, and (2) myocardial infarction (heart attack). GSK has agreed to plead guilty to failing to report data to the FDA and has agreed to pay a criminal fine in the amount of $242,612,800 for its unlawful conduct concerning Avandia.
“This case demonstrates our continuing commitment to ensuring that the messages provided by drug manufacturers to physicians and patients are true and accurate and that decisions as to what drugs are prescribed to sick patients are based on best medical judgments, not false and misleading claims or improper financial inducements,” said Carmen Ortiz, U.S. Attorney for the District of Massachusetts.
“Patients rely on their physicians to prescribe the drugs they need,” said John Walsh, U.S. Attorney for Colorado. “The pharmaceutical industries’ drive for profits can distort the information provided to physicians concerning drugs. This case will help to ensure that your physician will make prescribing decisions based on good science and not on misinformation, money or favors provided by the pharmaceutical industry.”
Civil Settlement Agreement
As part of this global resolution, GSK has agreed to resolve its civil liability for the following alleged conduct: (1) promoting the drugs Paxil, Wellbutrin, Advair, Lamictal and Zofran for off-label, non-covered uses and paying kickbacks to physicians to prescribe those drugs as well as the drugs Imitrex, Lotronex, Flovent and Valtrex; (2) making false and misleading statements concerning the safety of Avandia; and (3) reporting false best prices and underpaying rebates owed under the Medicaid Drug Rebate Program.
Off-Label Promotion and Kickbacks: The civil settlement resolves claims set forth in a complaint filed by the United States alleging that, in addition to promoting the drugs Paxil and Wellbutrin for unapproved, non-covered uses, GSK also promoted its asthma drug, Advair, for first-line therapy for mild asthma patients even though it was not approvedor medically appropriate under these circumstances. GSK also promoted Advair for chronic obstructive pulmonary disease with misleading claims as to the relevant treatment guidelines. The civil settlement also resolves allegations that GSK promoted Lamictal, an anti-epileptic medication, for off-label, non-covered psychiatric uses, neuropathic pain and pain management. It further resolves allegations that GSK promoted certain forms of Zofran, approved only for post-operative nausea, for the treatment of morning sickness in pregnant women. It also includes allegations that GSK paid kickbacks to health care professionals to induce them to promote and prescribe these drugs as well as the drugs Imitrex, Lotronex, Flovent and Valtrex. The United States alleges that this conduct caused false claims to be submitted to federal health care programs.
GSK has agreed to pay $1.043 billion relating to false claims arising from this alleged conduct. The federal share of this settlement is $832 million and the state share is $210 million.
This off-label civil settlement resolves four lawsuits pending in federal court in the District of Massachusetts under the qui tam , or whistleblower, provisions of the False Claims Act, which allow private citizens to bring civil actions on behalf of the United States and share in any recovery.
Avandia: In its civil settlement agreement, the United States alleges that GSK promoted Avandia to physicians and other health care providers with false and misleading representations about Avandia’s safety profile, causing false claims to be submitted to federal health care programs. Specifically, the United States alleges that GSK stated that Avandia had a positive cholesterol profile despite having no well-controlled studies to support that message. The United States also alleges that the company sponsored programs suggesting cardiovascular benefits from Avandia therapy despite warnings on the FDA-approved label regarding cardiovascular risks. GSK has agreed to pay $657 million relating to false claims arising from misrepresentations about Avandia. The federal share of this settlement is $508 million and the state share is $149 million.
Price Reporting: GSK is also resolving allegations that, between 1994 and 2003, GSK and its corporate predecessors reported false drug prices, which resulted in GSK’s underpaying rebates owed under theMedicaid Drug Rebate Program. By law, GSK was required to report the lowest, or “best” price that it charged its customers and to pay quarterly rebates to the states based on those reported prices. When drugs are sold to purchasers in contingent arrangements known as “bundles,” the discounts offered for the bundled drugs must be reallocated across all products in the bundle proportionate to the dollar value of the units sold. The United States alleges that GSK had bundled sales arrangements that included steep discounts known as “nominal” pricing and yet failed to take such contingent arrangements into account when calculating and reporting its best prices to the Department of Health and Human Services. Had it done so, the effective prices on certain drugs would have been different, and, in some instances, triggered a new, lower best price than what GSK reported. As a result, GSK underpaid rebates due to Medicaid and overcharged certain Public Health Service entities for its drugs, the United States contends. GSK has agreed to pay $300 million to resolve these allegations, including $160,972,069 to the federal government, $118,792,931 to the states, and $20,235,000 to certain Public Health Service entities who paid inflated prices for the drugs at issue.
Except to the extent that GSK has agreed to plead guilty to the three-count criminal information, the claims settled by these agreements are allegations only, and there has been no determination of liability.
“This landmark settlement demonstrates the Department’s commitment to protecting the American public against illegal conduct and fraud by pharmaceutical companies,” said Stuart F. Delery, Acting Assistant Attorney General for the Justice Department’s Civil Division. “Doctors need truthful, fair, balanced information when deciding whether the benefits of a drug outweigh its safety risks. By the same token, the FDA needs all necessary safety-related information to identify safety trends and to determine whether a drug is safe and effective. Unlawful promotion of drugs for unapproved uses and failing to report adverse drug experiences to the FDA can tip the balance of those important decisions, and the Justice Department will not tolerate attempts by those who seek to corrupt our health care system in this way.”
Non-monetary Provisions and Corporate Integrity Agreement
In addition to the criminal and civil resolutions, GSK has executed a five-year Corporate Integrity Agreement (CIA) with the Department of Health and Human Services, Office of Inspector General (HHS-OIG). The plea agreement and CIA include novel provisions that require that GSK implement and/or maintain major changes to the way it does business, including changing the way its sales force is compensated to remove compensation based on sales goals for territories, one of the driving forces behind much of the conduct at issue in this matter. Under the CIA, GSK is required to change its executive compensation program to permit the company to recoup annual bonuses and long-term incentives from covered executives if they, or their subordinates, engage in significant misconduct. GSK may recoup monies from executives who are current employees and those who have left the company. Among other things, the CIA also requires GSK to implement and maintain transparency in its research practices and publication policies and to follow specified policies in its contracts with various health care payors.
“Our five-year integrity agreement with GlaxoSmithKline requires individual accountability of its board and executives,” said Daniel R. Levinson, Inspector General of the U.S. Department of Health and Human Services. “For example, company executives may have to forfeit annual bonuses if they or their subordinates engage in significant misconduct, and sales agents are now being paid based on quality of service rather than sales targets.”
“The FDA Office of Criminal Investigations will aggressively pursue pharmaceutical companies that choose to put profits before the public’s health,” said Deborah M. Autor, Esq., Deputy Commissioner for Global Regulatory Operations and Policy, U.S. Food and Drug Administration. “We will continue to work with the Justice Department and our law enforcement counterparts to target companies that disregard the protections of the drug approval process by promoting drugs for uses when they have not been proven to be safe and effective for those uses, and that fail to report required drug safety information to the FDA.”
“The record settlement obtained by the multi-agency investigative team shows not only the importance of working with our partners, but also the importance of the public providing their knowledge of suspect schemes to the government,” said Kevin Perkins, Acting Executive Assistant Director of the FBI’s Criminal, Cyber, Response and Services Branch. “Together, we will continue to bring to justice those engaged in illegal schemes that threaten the safety of prescription drugs and other critical elements of our nation’s healthcare system.”
“ Federal employees deserve health care providers and suppliers, including drug manufacturers, that meet the highest standards of ethical and professional behavior,” said Patrick E. McFarland, Inspector General of the U.S. Office of Personnel Management. “Today’s settlement reminds the pharmaceutical industry that they must observe those standards and reflects the commitment of Federal law enforcement organizations to pursue improper and illegal conduct that places health care consumers at risk.”
“Today’s announcement illustrates the efforts of VA OIG and its law enforcement partners in ensuring the integrity of the medical care provided our nation’s veterans by the Department of Veterans Affairs,” said George J. Opfer, Inspector General of the Department of Veterans Affairs. “The monetary recoveries realized by VA in this settlement will directly benefit VA healthcare programs that provide for veterans’ continued care.”
“This settlement sends a clear message that taking advantage of federal health care programs has substantial consequences for those who try,” said Rafael A. Medina, Special Agent in Charge of the Northeast Area Office of Inspector General for the U.S. Postal Service. “The U.S. Postal Service pays more than one billion dollars a year in workers' compensation benefits and our office is committed to pursuing those individuals or entities whose fraudulent acts continue to unfairly add to that cost.”
A Multilateral Effort
The criminal case is being prosecuted by the U.S. Attorney’s Office for the District of Massachusetts and the Civil Division’s Consumer Protection Branch. The civil settlement was reached by the U.S. Attorney’s Office for the District of Massachusetts, the U.S. Attorney’s Office for the District of Colorado and the Civil Division’s Commercial Litigation Branch. Assistance was provided by the HHS Office of Counsel to the Inspector General, Office of the General Counsel-CMS Division and FDA’s Office of Chief Counsel as well as the National Association of Medicaid Fraud Control Units.
This matter was investigated by agents from the HHS-OIG; the FDA’s Office of Criminal Investigations; the Defense Criminal Investigative Service of the Department of Defense; the Office of the Inspector General for the Office of Personnel Management; the Department of Veterans Affairs; the Department of Labor; TRICARE Program Integrity; the Office of Inspector General for the U.S. Postal Service and the FBI.
This resolution is part of the government’s emphasis on combating health care fraud and another step for the Health Care Fraud Prevention and Enforcement Action Team (HEAT) initiative, which was announced in May 2009 by Attorney General Eric Holder and Kathleen Sebelius, Secretary of HHS. The partnership between the two departments has focused efforts to reduce and prevent Medicare and Medicaid financial fraud through enhanced cooperation. Over the last three years, the department has recovered a total of more than $10.2 billion in settlements, judgments, fines, restitution, and forfeiture in health care fraud matters pursued under the False Claims Act and the Food, Drug and Cosmetic Act.
Court documents related to today’s settlement can be viewed online at www.justice.gov/opa/gsk-docs.html .
Related Materials:
Remarks by the Deputy Attorney General James M. Cole at the GSK Press Conference Remarks by Acting Assistant Attorney General for the Civil Division Stuart F. Delery at the GSK Press Conference
Related Content
Endo Health Solutions Inc. (EHSI) was ordered today to pay $1.086 billion in criminal fines and an additional $450 million in criminal forfeiture — the second-largest set of criminal financial...
A sixth Nigerian national pleaded guilty to operating a transnational inheritance fraud scheme that defrauded elderly and vulnerable consumers across the United States.
Sixteen individuals were charged in connection with a sprawling “grandparent scam” to defraud elderly Americans out of millions of dollars, the Justice Department announced today during a virtual announcement .

- Predictive Analytics Workshops
- Corporate Strategy Workshops
- Advanced Excel for MBA
- Powerpoint Workshops
- Digital Transformation
- Competing on Business Analytics
- Aligning Analytics with Strategy
- Building & Sustaining Competitive Advantages
- Corporate Strategy
- Aligning Strategy & Sales
- Digital Marketing
- Hypothesis Testing
- Time Series Analysis
- Regression Analysis
- Machine Learning
- Marketing Strategy
- Branding & Advertising
- Risk Management
- Hedging Strategies
- Network Plotting
- Bar Charts & Time Series
- Technical Analysis of Stocks MACD
- NPV Worksheet
- ABC Analysis Worksheet
- WACC Worksheet
- Porter 5 Forces
- Porter Value Chain
- Amazing Charts
- Garnett Chart
- HBR Case Solution
- 4P Analysis
- 5C Analysis
- NPV Analysis
- SWOT Analysis
- PESTEL Analysis
- Cost Optimization
GlaxoSmithKline in China (B)
- Sales & Marketing / MBA EMBA Resources
Next Case Study Solutions
- Planned Parenthood Federation of America (B) Case Study Solution
- Thrive or Revive? The Kaiser Permanente "Thrive" Marketing Programs Case Study Solution
- New York Against AIDS (A): The Saatchi & Saatchi Compton Advertising Campaign Case Study Solution
- Promoting Healthcare Tourism in India Case Study Solution
- Medtronic: Patient Management Initiative (A) Case Study Solution
Previous Case Solutions
- Tengion: Bringing Regenerative Medicine to Life Case Study Solution
- LifeSpan Inc.: Abbott Northwestern Hospital Case Study Solution
- Amicon Corp. (B) Case Study Solution
- Vicks Health Care Division: Project Scorpio (C), Spanish Version Case Study Solution
- Amicon Corp. (C) Case Study Solution

Predictive Analytics
May 20, 2024

Popular Tags
Case study solutions.

Case Study Solution | Assignment Help | Case Help
Glaxosmithkline in china (b) description.
In 2013, Chinese investigators detained four GSK employees for allegedly bribing health care staff to sell GSK pharmaceuticals. A month later, GSK's Asia Pacific regional president, Abbas Hussain, said the company would help identify corrupt practices. Two days later, GSK's CEO, Andrew Witty, called the allegations "shameful" and said the company would use the opportunity to "make changes."
Case Description GlaxoSmithKline in China (B)
Strategic managment tools used in case study analysis of glaxosmithkline in china (b), step 1. problem identification in glaxosmithkline in china (b) case study, step 2. external environment analysis - pestel / pest / step analysis of glaxosmithkline in china (b) case study, step 3. industry specific / porter five forces analysis of glaxosmithkline in china (b) case study, step 4. evaluating alternatives / swot analysis of glaxosmithkline in china (b) case study, step 5. porter value chain analysis / vrio / vrin analysis glaxosmithkline in china (b) case study, step 6. recommendations glaxosmithkline in china (b) case study, step 7. basis of recommendations for glaxosmithkline in china (b) case study, quality & on time delivery.
100% money back guarantee if the quality doesn't match the promise
100% Plagiarism Free
If the work we produce contain plagiarism then we payback 1000 USD
Paypal Secure
All your payments are secure with Paypal security.
300 Words per Page
We provide 300 words per page unlike competitors' 250 or 275
Free Title Page, Citation Page, References, Exhibits, Revision, Charts
Case study solutions are career defining. Order your custom solution now.
Case Analysis of GlaxoSmithKline in China (B)
GlaxoSmithKline in China (B) is a Harvard Business (HBR) Case Study on Sales & Marketing , Texas Business School provides HBR case study assignment help for just $9. Texas Business School(TBS) case study solution is based on HBR Case Study Method framework, TBS expertise & global insights. GlaxoSmithKline in China (B) is designed and drafted in a manner to allow the HBR case study reader to analyze a real-world problem by putting reader into the position of the decision maker. GlaxoSmithKline in China (B) case study will help professionals, MBA, EMBA, and leaders to develop a broad and clear understanding of casecategory challenges. GlaxoSmithKline in China (B) will also provide insight into areas such as – wordlist , strategy, leadership, sales and marketing, and negotiations.
Case Study Solutions Background Work
GlaxoSmithKline in China (B) case study solution is focused on solving the strategic and operational challenges the protagonist of the case is facing. The challenges involve – evaluation of strategic options, key role of Sales & Marketing, leadership qualities of the protagonist, and dynamics of the external environment. The challenge in front of the protagonist, of GlaxoSmithKline in China (B), is to not only build a competitive position of the organization but also to sustain it over a period of time.
Strategic Management Tools Used in Case Study Solution
The GlaxoSmithKline in China (B) case study solution requires the MBA, EMBA, executive, professional to have a deep understanding of various strategic management tools such as SWOT Analysis, PESTEL Analysis / PEST Analysis / STEP Analysis, Porter Five Forces Analysis, Go To Market Strategy, BCG Matrix Analysis, Porter Value Chain Analysis, Ansoff Matrix Analysis, VRIO / VRIN and Marketing Mix Analysis.
Texas Business School Approach to Sales & Marketing Solutions
In the Texas Business School, GlaxoSmithKline in China (B) case study solution – following strategic tools are used - SWOT Analysis, PESTEL Analysis / PEST Analysis / STEP Analysis, Porter Five Forces Analysis, Go To Market Strategy, BCG Matrix Analysis, Porter Value Chain Analysis, Ansoff Matrix Analysis, VRIO / VRIN and Marketing Mix Analysis. We have additionally used the concept of supply chain management and leadership framework to build a comprehensive case study solution for the case – GlaxoSmithKline in China (B)
Step 1 – Problem Identification of GlaxoSmithKline in China (B) - Harvard Business School Case Study
The first step to solve HBR GlaxoSmithKline in China (B) case study solution is to identify the problem present in the case. The problem statement of the case is provided in the beginning of the case where the protagonist is contemplating various options in the face of numerous challenges that Gsk's Gsk is facing right now. Even though the problem statement is essentially – “Sales & Marketing” challenge but it has impacted by others factors such as communication in the organization, uncertainty in the external environment, leadership in Gsk's Gsk, style of leadership and organization structure, marketing and sales, organizational behavior, strategy, internal politics, stakeholders priorities and more.
Step 2 – External Environment Analysis
Texas Business School approach of case study analysis – Conclusion, Reasons, Evidences - provides a framework to analyze every HBR case study. It requires conducting robust external environmental analysis to decipher evidences for the reasons presented in the GlaxoSmithKline in China (B). The external environment analysis of GlaxoSmithKline in China (B) will ensure that we are keeping a tab on the macro-environment factors that are directly and indirectly impacting the business of the firm.
What is PESTEL Analysis? Briefly Explained
PESTEL stands for political, economic, social, technological, environmental and legal factors that impact the external environment of firm in GlaxoSmithKline in China (B) case study. PESTEL analysis of " GlaxoSmithKline in China (B)" can help us understand why the organization is performing badly, what are the factors in the external environment that are impacting the performance of the organization, and how the organization can either manage or mitigate the impact of these external factors.
How to do PESTEL / PEST / STEP Analysis? What are the components of PESTEL Analysis?
As mentioned above PESTEL Analysis has six elements – political, economic, social, technological, environmental, and legal. All the six elements are explained in context with GlaxoSmithKline in China (B) macro-environment and how it impacts the businesses of the firm.
How to do PESTEL Analysis for GlaxoSmithKline in China (B)
To do comprehensive PESTEL analysis of case study – GlaxoSmithKline in China (B) , we have researched numerous components under the six factors of PESTEL analysis.
Political Factors that Impact GlaxoSmithKline in China (B)
Political factors impact seven key decision making areas – economic environment, socio-cultural environment, rate of innovation & investment in research & development, environmental laws, legal requirements, and acceptance of new technologies.
Government policies have significant impact on the business environment of any country. The firm in “ GlaxoSmithKline in China (B) ” needs to navigate these policy decisions to create either an edge for itself or reduce the negative impact of the policy as far as possible.
Data safety laws – The countries in which Gsk's Gsk is operating, firms are required to store customer data within the premises of the country. Gsk's Gsk needs to restructure its IT policies to accommodate these changes. In the EU countries, firms are required to make special provision for privacy issues and other laws.
Competition Regulations – Numerous countries have strong competition laws both regarding the monopoly conditions and day to day fair business practices. GlaxoSmithKline in China (B) has numerous instances where the competition regulations aspects can be scrutinized.
Import restrictions on products – Before entering the new market, Gsk's Gsk in case study GlaxoSmithKline in China (B)" should look into the import restrictions that may be present in the prospective market.
Export restrictions on products – Apart from direct product export restrictions in field of technology and agriculture, a number of countries also have capital controls. Gsk's Gsk in case study “ GlaxoSmithKline in China (B) ” should look into these export restrictions policies.
Foreign Direct Investment Policies – Government policies favors local companies over international policies, Gsk's Gsk in case study “ GlaxoSmithKline in China (B) ” should understand in minute details regarding the Foreign Direct Investment policies of the prospective market.
Corporate Taxes – The rate of taxes is often used by governments to lure foreign direct investments or increase domestic investment in a certain sector. Corporate taxation can be divided into two categories – taxes on profits and taxes on operations. Taxes on profits number is important for companies that already have a sustainable business model, while taxes on operations is far more significant for companies that are looking to set up new plants or operations.
Tariffs – Chekout how much tariffs the firm needs to pay in the “ GlaxoSmithKline in China (B) ” case study. The level of tariffs will determine the viability of the business model that the firm is contemplating. If the tariffs are high then it will be extremely difficult to compete with the local competitors. But if the tariffs are between 5-10% then Gsk's Gsk can compete against other competitors.
Research and Development Subsidies and Policies – Governments often provide tax breaks and other incentives for companies to innovate in various sectors of priority. Managers at GlaxoSmithKline in China (B) case study have to assess whether their business can benefit from such government assistance and subsidies.
Consumer protection – Different countries have different consumer protection laws. Managers need to clarify not only the consumer protection laws in advance but also legal implications if the firm fails to meet any of them.
Political System and Its Implications – Different political systems have different approach to free market and entrepreneurship. Managers need to assess these factors even before entering the market.
Freedom of Press is critical for fair trade and transparency. Countries where freedom of press is not prevalent there are high chances of both political and commercial corruption.
Corruption level – Gsk's Gsk needs to assess the level of corruptions both at the official level and at the market level, even before entering a new market. To tackle the menace of corruption – a firm should have a clear SOP that provides managers at each level what to do when they encounter instances of either systematic corruption or bureaucrats looking to take bribes from the firm.
Independence of judiciary – It is critical for fair business practices. If a country doesn’t have independent judiciary then there is no point entry into such a country for business.
Government attitude towards trade unions – Different political systems and government have different attitude towards trade unions and collective bargaining. The firm needs to assess – its comfort dealing with the unions and regulations regarding unions in a given market or industry. If both are on the same page then it makes sense to enter, otherwise it doesn’t.
Economic Factors that Impact GlaxoSmithKline in China (B)
Social factors that impact glaxosmithkline in china (b), technological factors that impact glaxosmithkline in china (b), environmental factors that impact glaxosmithkline in china (b), legal factors that impact glaxosmithkline in china (b), step 3 – industry specific analysis, what is porter five forces analysis, step 4 – swot analysis / internal environment analysis, step 5 – porter value chain / vrio / vrin analysis, step 6 – evaluating alternatives & recommendations, step 7 – basis for recommendations, references :: glaxosmithkline in china (b) case study solution.
- sales & marketing ,
- leadership ,
- corporate governance ,
- Advertising & Branding ,
- Corporate Social Responsibility (CSR) ,
Amanda Watson
Leave your thought here

© 2019 Texas Business School. All Rights Reserved
USEFUL LINKS
Follow us on.
Subscribe to our newsletter to receive news on update.

Dark Brown Leather Watch
$200.00 $180.00


Dining Chair
$300.00 $220.00

Creative Wooden Stand
$100.00 $80.00
2 x $180.00
2 x $220.00
Subtotal: $200.00
Free Shipping on All Orders Over $100!

Wooden round table
$360.00 $300.00
Hurley Dry-Fit Chino Short. Men's chino short. Outseam Length: 19 Dri-FIT Technology helps keep you dry and comfortable. Made with sweat-wicking fabric. Fitted waist with belt loops. Button waist with zip fly provides a classic look and feel .

MBA Case Pro

GlaxoSmithKline in China (B) PESTEL Analysis
MBA Case Pro Case Study Solution | Term Papers | Essays | Assignment
M BA case pro PESTEL Analysis of case study "GlaxoSmithKline in China (B)" includes 6 parts - Political Factors, Economic Factors, Social Factors, Technological Factors, Environmental Factors, and Legal Factors. Case Study "GlaxoSmithKline in China (B)" is written - John A. Quelch, Margaret Rodriguez.
Sales & Marketing Case Study Solution
Change management, entrepreneurship, ethics, government, health, marketing, social responsibility, strategy execution , case study solution, term papers , gsk's gsk, political factors that impact glaxosmithkline in china (b), russia ukraine war, intellectual and property rights, stability of the political system, data privacy laws, government policies, government regulations, political intervention, political activism and protests, taxation policies, antitrust and other competitive issues, economic factors that impact glaxosmithkline in china (b), fiscal and monetary policies of various governments post covid, economic inequality and income distribution, currency risks, rising energy costs because of russia ukraine war, rising real estate prices, global slowdown, rising inflation, natural disasters and climate emergency, rising unemployment rates, increasing wages in emerging economies, social factors that impact glaxosmithkline in china (b), customer loyalty and recommendations, diversity and inclusivity at workplace, health and services products boom, labor practices and unions, digital technology and market expansion, data privacy and security, consumer behavior, cultural differences, demographic changes, increasing urbanization, technological factors that impact glaxosmithkline in china (b), machine learning and artificial intelligence, investments in automation and robotics, 5g and other mobile innovations, augmented reality and virtual reality, cloud computing and saas services, foray into adjacent markets, personalization of product offerings, internet penetration, expansion of internet of things (iot), ai and other voice input technologies, environmental factors that impact glaxosmithkline in china (b), renewable energy strategy, corporate social responsibility, customer awareness, packaging and logistics emissions, sustainability efforts across the value chain, carbon footprint of warehouses and distribution centers, heating and water resources, reduction of paper based packaging, conservation and sustainable practices, e-waste and other wastes, legal factors that impact glaxosmithkline in china (b), labor laws compliance, cyber security safeguards, different taxes and laws in various countries, anti trust laws, different regulations for digital content, protection of intellectual property rights, copyright infringement and counterfeit problem, business law and other practices, consumer activism, environmental laws compliance.
The New Leadership Imperative: Embracing Digital Transformation PESTEL Analysis
Hotel Perennial PESTEL Analysis
Medtronic: Patient Management Initiative (B) PESTEL Analysis
Icebreaker: The China Entry Decision PESTEL Analysis
Komatsu in China, Chinese Version PESTEL Analysis
Caffebene: Master Brewer of Growth and Global Ambition PESTEL Analysis
Egghead to Egghead.com (B), Portuguese Version PESTEL Analysis
Cause-Related Marketing: 3M as a Corporate Sponsor of the Canadian Breast Cancer Foundation PESTEL Analysis
Tax Impropriety: Judicial Sanctions and Professional Repercussions PESTEL Analysis
Camel's Milk and Lamb's Liver (C) PESTEL Analysis
George McClelland at KSR (B) PESTEL Analysis
Mrs. Ebtissam Algosaibi: An Entrepreneur in the Kingdom of Saudi Arabia PESTEL Analysis
ZS Associates: Sales Force Sizing PESTEL Analysis
XM Satellite Radio (A) PESTEL Analysis
Endius Inc.: Alternatives for Developing a New Medical Device PESTEL Analysis
SUNDAY Communications Ltd.: A Marketing Strategy for a Wireless Future PESTEL Analysis
Narayana Hrudayalaya: From Heart Care to Human Care PESTEL Analysis
EdGE Networks: Making HR Intelligent PESTEL Analysis
Airbus vs. Boeing (B): The Storm Intensifies PESTEL Analysis
Managing Religion in the Workplace: Abercrombie & Fitch and Masterpiece Cakeshop PESTEL Analysis
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 May 2024
Dynamics of measles immunity from birth and following vaccination
- Wei Wang ORCID: orcid.org/0000-0003-4056-3732 1 ,
- Megan O’Driscoll ORCID: orcid.org/0000-0002-7972-5703 2 ,
- Qianli Wang 3 ,
- Sihong Zhao 1 ,
- Henrik Salje ORCID: orcid.org/0000-0003-3626-4254 2 na1 &
- Hongjie Yu ORCID: orcid.org/0000-0002-6335-5648 1 , 3 , 4 na1
Nature Microbiology ( 2024 ) Cite this article
948 Accesses
1662 Altmetric
Metrics details
- Antimicrobial responses
- Epidemiology
- Risk factors
Measles remains a major threat to human health despite widespread vaccination. While we know that maternal antibodies can impair vaccine-induced immunity, the relative contributions of pre-existing immunity levels, maternal and infant characteristics on vaccine responses remain unclear, hampering evidence-based vaccination policy development. Here we combine serological data from 1,505 individuals (aged 0–12 years) in a mother–infant cohort and in a child cohort with empirical models to reconstruct antibody trajectories from birth. We show that while highly heterogeneous across a population, measles antibody evolution is strongly predictive from birth at the individual level, including following vaccination. Further, we find that caesarean section births were linked with 2.56 (95% confidence interval: 1.06–6.37) increased odds of primary vaccine failure, highlighting the long-term immunological consequences of birth route. Finally, we use our new understanding of antibody evolution to critically assess the population-level consequences of different vaccination schedules, the results of which will allow country-level evaluations of vaccine policy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
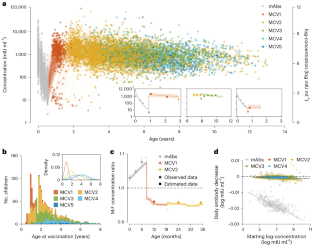
Similar content being viewed by others
Long-term measles antibody profiles following different vaccine schedules in China, a longitudinal study
Longitudinal antibody dynamics after COVID-19 vaccine boosters based on prior infection status and booster doses

Predicted effectiveness of vaccines and extended half-life monoclonal antibodies against RSV hospitalizations in children
Data availability.
Because of privacy and ethical reasons, individual epidemiological and serological data cannot be made public, but they are available from the corresponding author (H.Y.) on reasonable request. The time frame for a response to requests is 1–2 weeks. We have provided the synthetic dataset, and aggregated de-identified data generated in this study. These datasets have been deposited in Zenodo at https://doi.org/10.5281/zenodo.11018560 (ref. 35 ). Source data are provided with this paper.
Code Availability
The codes to reproduce the main results and Extended data in this study have been deposited in Zenodo at https://doi.org/10.5281/zenodo.11018560 (ref. 35 ).
Minta, A. A. et al. Progress toward regional measles elimination—worldwide, 2000–2021. MMWR Morb. Mortal. Wkly Rep. 71 , 1489–1495 (2022).
Article PubMed PubMed Central Google Scholar
Ma, C. et al. Progress toward measles elimination—China, January 2013–June 2019. MMWR Morb. Mortal. Wkly Rep. 68 , 1112–1116 (2019).
Patel, M. et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb. Mortal. Wkly Rep. 68 , 402–404 (2019).
Article PubMed Google Scholar
Wiedermann, U., Garner-Spitzer, E. & Wagner, A. Primary vaccine failure to routine vaccines: why and what to do? Hum. Vaccin. Immunother. 12 , 239–243 (2016).
Failure to vaccinate and vaccine failure. Nat. Microbiol. 4 , 725 (2019).
Guerra, F. M. et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect. Dis. 17 , e420–e428 (2017).
Nic Lochlainn, L. M. et al. Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: a systematic review and meta-analysis. Lancet Infect. Dis. 19 , 1246–1254 (2019).
Article CAS PubMed PubMed Central Google Scholar
Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32 , e00084–18 (2019).
Cáceres, V. M., Strebel, P. M. & Sutter, R. W. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin. Infect. Dis. 31 , 110–119 (2000).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16 , 626–638 (2016).
Article CAS PubMed Google Scholar
Carazo, S., Billard, M.-N., Boutin, A. & De Serres, G. Effect of age at vaccination on the measles vaccine effectiveness and immunogenicity: systematic review and meta-analysis. BMC Infect. Dis. 20 , 251 (2020).
Leuridan, E. et al. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ 340 , c1626 (2010).
Wang, Q. et al. Long-term measles antibody profiles following different vaccine schedules in China, a longitudinal study. Nat. Commun. 14 , 1746 (2023).
Wei, X. et al. The transfer and decay of maternal antibodies against enterovirus A71, and dynamics of antibodies due to later natural infections in Chinese infants: a longitudinal, paired mother-neonate cohort study. Lancet Infect. Dis. 21 , 418–426 (2021).
Steinhoff, M. C. et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N. Engl. J. Med. 362 , 1644–1646 (2010).
Perret, C. et al. Dengue infection during pregnancy and transplacental antibody transfer in Thai mothers. J. Infect. 51 , 287–293 (2005).
Metcalf, C. J. E. et al. Comparing the age and sex trajectories of SARS-CoV-2 morbidity and mortality with other respiratory pathogens. R. Soc. Open Sci. 9 , 211498 (2022).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. USA 111 , 869–874 (2014).
van Panhuis, W. G. et al. Decay and persistence of maternal dengue antibodies among infants in Bangkok. Am. J. Trop. Med. Hyg. 85 , 355–362 (2011).
Waaijenborg, S. et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J. Infect. Dis. 208 , 10–16 (2013).
Teunis, P. F. M., van Eijkeren, J. C. H., de Graaf, W. F., Marinović, A. B. & Kretzschmar, M. E. E. Linking the seroresponse to infection to within-host heterogeneity in antibody production. Epidemics 16 , 33–39 (2016).
Li, H.-T., Hellerstein, S., Zhou, Y.-B., Liu, J.-M. & Blustein, J. Trends in Cesarean delivery rates in China, 2008–2018. JAMA 323 , 89–91 (2020).
de Koff, E. M. et al. Mode of delivery modulates the intestinal microbiota and impacts the response to vaccination. Nat. Commun. 13 , 6638 (2022).
Liu, H. et al. Vaginal delivery and breastfeeding benefit infant immune response to hepatitis B vaccine: a prospective cohort study. J. Clin. Transl. Hepatol. 11 , 899–907 (2023).
PubMed PubMed Central Google Scholar
Li, H. et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 584 , 274–278 (2020).
Strömbeck, A. et al. Earlier infantile immune maturation is related to higher DTP vaccine responses in children. Clin. Transl. Immunol. 5 , e65 (2016).
Article Google Scholar
Rosenthal, S. R. & Clements, C. J. Two-dose measles vaccination schedules. Bull. World Health Organ. 71 , 421–428 (1993).
CAS PubMed PubMed Central Google Scholar
Chen, R. T. et al. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162 , 1036–1042 (1990).
Launay, O. et al. Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 / TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study. EBioMedicine 75 , 103810 (2022).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357 , 1903–1915 (2007).
Amanna, I. J. & Slifka, M. K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 411 , 206–215 (2011).
Yang, J. et al. Seroepidemiology of enterovirus A71 infection in prospective cohort studies of children in southern China, 2013–2018. Nat. Commun. 13 , 7280 (2022).
He, Y. et al. Assessing vaccination coverage, timeliness, and its temporal variations among children in a rural area in China. Hum. Vaccin. Immunother. 17 , 592–600 (2021).
Argüelles, M. H. et al. Measles virus-specific antibody levels in individuals in Argentina who received a one-dose vaccine. J. Clin. Microbiol. 44 , 2733–2738 (2006).
Yu Group. Analysis data and code for the measles_Ab_dynamics. Zenodo https://doi.org/10.5281/zenodo.11018560 (2024).
Download references
Acknowledgements
We thank all contributors and study participants for their participation and trust; Fudan University for providing the computational resources to perform this study and data management support. H.Y. acknowledges financial support from the Key Program of the National Natural Science Foundation of China (82130093). W.W. acknowledges financial support from the Young Scientists Fund of the National Natural Science Foundation of China (82304205), the National Postdoctoral Program for Innovative Talent (BX2021072) and the China Postdoctoral Science Foundation (2022M720753). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We also acknowledge B. T. Grenfell and C. J. E. Metcalf of Princeton University, and A. T. Huang of the University of Cambridge for helpful comments on the paper.
Author information
These authors jointly supervised this work: Henrik Salje, Hongjie Yu.
Authors and Affiliations
School of Public Health, Fudan University, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China
Wei Wang, Sihong Zhao & Hongjie Yu
Department of Genetics, University of Cambridge, Cambridge, UK
Megan O’Driscoll & Henrik Salje
Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, Shanghai, China
Qianli Wang & Hongjie Yu
Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, China
You can also search for this author in PubMed Google Scholar
Contributions
H.S. and H.Y. conceived the study. Q.W. and S.Z. performed the laboratory tests. W.W. collated data and performed the analysis. M.O. reviewed the code. W.W., M.O. and H.S. wrote the first draft of the paper. W.W., M.O. H.S. and H.Y. discussed the results and contributed to revisions of the paper.
Corresponding authors
Correspondence to Henrik Salje or Hongjie Yu .
Ethics declarations
Competing interests.
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, Shanghai Roche Pharmaceutical Company, and SINOVAC Biotech Ltd. None of these research fundings is related to measles. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 age- and sex-specific concentration distributions..
( a ) Number of observations and ( b ) measured concentrations (mIU/ml) by age and sex. In ( b ), the points with error bars represent the measured mean concentrations and the 95% confidence intervals for 555 adult women participants, as well as for 554, 328, 774, 1004, 820 and 478 participants aged 0 months (301 boys and 253 girls), 6 months (175 boys and 153 girls), 1–2 years (390 boys and 384 girls), 3–4 years (509 boys and 495 girls), 5–6 years (408 boys and 412 girls) and 7–11 years (235 boys and 243 girls), respectively. The abbreviation ‘M’ denotes the mothers in the mother-infant cohort.
Source data
Extended data fig. 2 comparison of measured log-concentrations and log-concentration estimates from model a..
( a – g ) The consistency between measured and model estimated log-concentrations (log mIU/ml) among 350, 350, 224, 276, 350, 235 and 226 participants who were followed at 0, 2, 4, 6, 12, 24 and 36 months of age. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model A, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions ( n = 3,000).
Extended Data Fig. 3 Differences in vaccine responses between MCV1 responders and non-responders.
( a ) Probability density distribution of MCV1-induced peak log-concentration increase (log mIU/ml); ( b ) pre-MCV1, ( c ) MCV1-induced and ( d ) post-MCV1 log-concentrations (log mIU/ml) in MCV1 responders ( n = 324) and non-responders ( n = 26). Note that the dashed line in ( a ) is used to differentiate the peak log-concentration rise from MCV1 in MCV1 non-responders (left curve) and responders (right curve); in ( b – d ), each box plot shows minimum, first quartile, median, third quartile, and maximum values, while each point represents individual prediction.
Extended Data Fig. 4 The blunting of primary vaccine response due to maternal antibodies.
Note that the solid lines and shaded areas show the mean of posterior median estimated concentrations (mIU/ml) and the 95% credible intervals in each quartile group.
Extended Data Fig. 5 Comparison of measured and estimated log-concentrations before and after MCV1 vaccination.
( a – g ) The consistency between measured and estimated log-concentrations (log mIU/ml) among 350 cohort women and 350, 350, 224, 276, 350, and 9 child participants who were followed at 0, 2, 4, 6, 12 and 24 months of age. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model C, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions ( n = 3,000).
Extended Data Fig. 6 Comparison of measured log-concentrations and log-concentration estimates from model B in the mother-infant cohort.
( a – g ) The consistency between measured and estimated log-concentrations (log mIU/ml) from 350, 350, 224, 276, 350, 235 and 226 participants aged 0, 2, 4, 6, 12, 24 and 36 months. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model B, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions ( n = 3,000).
Extended Data Fig. 7 Comparison of measured log-concentrations and log-concentration estimates from model B in the child cohort.
( a – g ) The consistency between measured and estimated log-concentrations (log mIU/ml) from 1148, 325, 1033, 303, 1031, 306 and 1061 participants at baseline and six subsequent follow-up visits. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model B, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions ( n = 3,000).
Extended Data Fig. 8 The validation of the expanded model framework.
Note that the filled points with error bars show the measured mean concentrations (mIU/ml) and the 95% confidence intervals for 350, 350, 268, 298, 102, 237, 78, 262, 41, 91, 22 participants who were followed at 0 (350 in the infant cohort), 2–5 (350 in the infant cohort), 6–11 (268 in the infant cohort), 12–17 (86 in the infant cohort, 212 in the child cohort), 18–23 (35 in the infant cohort, 67 in the child cohort), 24–29 (85 in the infant cohort, 152 in the child cohort), 30–35 (37 in the infant cohort, 41 in the child cohort), 36–41 (90 in the infant cohort, 172 in the child cohort), 42–47 (41 in the child cohort), 48–53 (91 in the child cohort), and 54–60 (22 in the child cohort) months. The open points with error bars show the predicted mean concentrations and the 95% credible intervals ( n = 10,000). The points in grey, red and yellow areas indicate the mean estimates of maternal, post-MCV1 and post-MCV2 antibody concentrations, respectively.
Extended Data Fig. 9 Antibody responses and population immunity dynamics following different measles vaccination schedules.
In ( a ) and ( b ), the lines and shaded area are based on 10,000 simulated datasets. ( a ) Antibody responses (mIU/ml) and ( b ) the proportion of children with above-threshold (120 mIU/ml) concentration, stratified by the age at dose 1. The solid line represents the median of the individual mean concentrations ( a ) or the proportion of protected individuals ( b ) based on the simulated observations from children receiving a two-dose schedule (that is, dose 1 at 6/8/12 months and dose 2 at 15/24 months), while the dotted line denotes those from children receiving a single-dose schedule (that is, dose 1 at 6/8/12 months). The colour of shaded area in ( a ) from light to darkness reflects the 2.5 and 97.5 percentiles and the interquartile range of the individual mean concentration from the simulated individuals’ antibody responses, whereas it represents 95% confidence intervals in ( b ).
Extended Data Fig. 10 Estimated population immunity dynamics across different measles vaccination schedules, using a protective threshold of 200 mIU/ml.
The lines and shaded areas are based on 10,000 simulated datasets. The solid and dotted line represents the proportion of protected individuals based on the simulated observations from children receiving a two-dose schedule (that is, dose 1 at 6/8/12 months and dose 2 at 18 months) or a single-dose schedule. The colour of the shaded area represents 95% confidence intervals.
Supplementary information
Supplementary information.
Supplementary Tables 1–9 and Figs. 1–4.
Reporting Summary
Source data fig. 1.
Statistical source data.
Source Data Fig. 2
Source data fig. 3, source data extended data fig. 1, source data extended data fig. 2, source data extended data fig. 3, source data extended data fig. 4, source data extended data fig. 5, source data extended data fig. 6, source data extended data fig. 7, source data extended data fig. 8, source data extended data fig. 9, source data extended data fig. 10, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Wang, W., O’Driscoll, M., Wang, Q. et al. Dynamics of measles immunity from birth and following vaccination. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01694-x
Download citation
Received : 21 December 2023
Accepted : 03 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41564-024-01694-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Fern Fort University
Glaxosmithkline in china (a) case study analysis & solution, harvard business case studies solutions - assignment help.
GlaxoSmithKline in China (A) is a Harvard Business (HBR) Case Study on Sales & Marketing , Fern Fort University provides HBR case study assignment help for just $11. Our case solution is based on Case Study Method expertise & our global insights.
Sales & Marketing Case Study | Authors :: John A. Quelch, Margaret Rodriguez
Case study description.
Four GlaxoSmithKline employees were accused of bribing Chinese health care workers to prescribe the company's drugs. The accusations brought to light the questionable incentive structures of the Chinese health care system and the pressure on companies to adhere to local customs while still observing local laws.
Entrepreneurship, Ethics, Government, Health, Marketing, Social responsibility, Strategy execution
Order a Sales & Marketing case study solution now
To Search More HBR Case Studies Solution Go to Fern Fort University Search Page
[10 Steps] Case Study Analysis & Solution
Step 1 - reading up harvard business review fundamentals on the sales & marketing.
Even before you start reading a business case study just make sure that you have brushed up the Harvard Business Review (HBR) fundamentals on the Sales & Marketing. Brushing up HBR fundamentals will provide a strong base for investigative reading. Often readers scan through the business case study without having a clear map in mind. This leads to unstructured learning process resulting in missed details and at worse wrong conclusions. Reading up the HBR fundamentals helps in sketching out business case study analysis and solution roadmap even before you start reading the case study. It also provides starting ideas as fundamentals often provide insight into some of the aspects that may not be covered in the business case study itself.
Step 2 - Reading the GlaxoSmithKline in China (A) HBR Case Study
To write an emphatic case study analysis and provide pragmatic and actionable solutions, you must have a strong grasps of the facts and the central problem of the HBR case study. Begin slowly - underline the details and sketch out the business case study description map. In some cases you will able to find the central problem in the beginning itself while in others it may be in the end in form of questions. Business case study paragraph by paragraph mapping will help you in organizing the information correctly and provide a clear guide to go back to the case study if you need further information. My case study strategy involves -
- Marking out the protagonist and key players in the case study from the very start.
- Drawing a motivation chart of the key players and their priorities from the case study description.
- Refine the central problem the protagonist is facing in the case and how it relates to the HBR fundamentals on the topic.
- Evaluate each detail in the case study in light of the HBR case study analysis core ideas.
Step 3 - GlaxoSmithKline in China (A) Case Study Analysis
Once you are comfortable with the details and objective of the business case study proceed forward to put some details into the analysis template. You can do business case study analysis by following Fern Fort University step by step instructions -
- Company history is provided in the first half of the case. You can use this history to draw a growth path and illustrate vision, mission and strategic objectives of the organization. Often history is provided in the case not only to provide a background to the problem but also provide the scope of the solution that you can write for the case study.
- HBR case studies provide anecdotal instances from managers and employees in the organization to give a feel of real situation on the ground. Use these instances and opinions to mark out the organization's culture, its people priorities & inhibitions.
- Make a time line of the events and issues in the case study. Time line can provide the clue for the next step in organization's journey. Time line also provides an insight into the progressive challenges the company is facing in the case study.
Step 4 - SWOT Analysis of GlaxoSmithKline in China (A)
Once you finished the case analysis, time line of the events and other critical details. Focus on the following -
- Zero down on the central problem and two to five related problems in the case study.
- Do the SWOT analysis of the GlaxoSmithKline in China (A) . SWOT analysis is a strategic tool to map out the strengths, weakness, opportunities and threats that a firm is facing.
- SWOT analysis and SWOT Matrix will help you to clearly mark out - Strengths Weakness Opportunities & Threats that the organization or manager is facing in the GlaxoSmithKline in China (A)
- SWOT analysis will also provide a priority list of problem to be solved.
- You can also do a weighted SWOT analysis of GlaxoSmithKline in China (A) HBR case study.
Step 5 - Porter 5 Forces / Strategic Analysis of Industry Analysis GlaxoSmithKline in China (A)
In our live classes we often come across business managers who pinpoint one problem in the case and build a case study analysis and solution around that singular point. Business environments are often complex and require holistic solutions. You should try to understand not only the organization but also the industry which the business operates in. Porter Five Forces is a strategic analysis tool that will help you in understanding the relative powers of the key players in the business case study and what sort of pragmatic and actionable case study solution is viable in the light of given facts.
Step 6 - PESTEL, PEST / STEP Analysis of GlaxoSmithKline in China (A)
Another way of understanding the external environment of the firm in GlaxoSmithKline in China (A) is to do a PESTEL - Political, Economic, Social, Technological, Environmental & Legal analysis of the environment the firm operates in. You should make a list of factors that have significant impact on the organization and factors that drive growth in the industry. You can even identify the source of firm's competitive advantage based on PESTEL analysis and Organization's Core Competencies.
Step 7 - Organizing & Prioritizing the Analysis into GlaxoSmithKline in China (A) Case Study Solution
Once you have developed multipronged approach and work out various suggestions based on the strategic tools. The next step is organizing the solution based on the requirement of the case. You can use the following strategy to organize the findings and suggestions.
- Build a corporate level strategy - organizing your findings and recommendations in a way to answer the larger strategic objective of the firm. It include using the analysis to answer the company's vision, mission and key objectives , and how your suggestions will take the company to next level in achieving those goals.
- Business Unit Level Solution - The case study may put you in a position of a marketing manager of a small brand. So instead of providing recommendations for overall company you need to specify the marketing objectives of that particular brand. You have to recommend business unit level recommendations. The scope of the recommendations will be limited to the particular unit but you have to take care of the fact that your recommendations are don't directly contradict the company's overall strategy. For example you can recommend a low cost strategy but the company core competency is design differentiation.
- Case study solutions can also provide recommendation for the business manager or leader described in the business case study.
Step 8 -Implementation Framework
The goal of the business case study is not only to identify problems and recommend solutions but also to provide a framework to implement those case study solutions. Implementation framework differentiates good case study solutions from great case study solutions. If you able to provide a detailed implementation framework then you have successfully achieved the following objectives -
- Detailed understanding of the case,
- Clarity of HBR case study fundamentals,
- Analyzed case details based on those fundamentals and
- Developed an ability to prioritize recommendations based on probability of their successful implementation.
Implementation framework helps in weeding out non actionable recommendations, resulting in awesome GlaxoSmithKline in China (A) case study solution.
Step 9 - Take a Break
Once you finished the case study implementation framework. Take a small break, grab a cup of coffee or whatever you like, go for a walk or just shoot some hoops.
Step 10 - Critically Examine GlaxoSmithKline in China (A) case study solution
After refreshing your mind, read your case study solution critically. When we are writing case study solution we often have details on our screen as well as in our head. This leads to either missing details or poor sentence structures. Once refreshed go through the case solution again - improve sentence structures and grammar, double check the numbers provided in your analysis and question your recommendations. Be very slow with this process as rushing through it leads to missing key details. Once done it is time to hit the attach button.
Previous 5 HBR Case Study Solution
- EILEEN FISHER: Repositioning the Brand Case Study Solution
- UnME Jeans: Branding in Web 2.0 Case Study Solution
- SCI Ontario: Achieving, Measuring and Communicating Strategic Success Case Study Solution
- Amazon in 2016, Spanish Version Case Study Solution
- TaKaDu Case Study Solution
Next 5 HBR Case Study Solution
- Fabindia: Branding India's Artisanal Crafts for Mass Retail Case Study Solution
- Reinventing Retail: ShopRunner's Network Bet Case Study Solution
- Retail Credit Scoring for Auto Finance Limited Case Study Solution
- Retail Relay (C) Case Study Solution
- Spencer's Retail Limited: Repositioning in a Changing Retail Environment Case Study Solution
Special Offers
Order custom Harvard Business Case Study Analysis & Solution. Starting just $19
Amazing Business Data Maps. Send your data or let us do the research. We make the greatest data maps.
We make beautiful, dynamic charts, heatmaps, co-relation plots, 3D plots & more.
Buy Professional PPT templates to impress your boss
Nobody get fired for buying our Business Reports Templates. They are just awesome.
- More Services
Feel free to drop us an email
- fernfortuniversity[@]gmail.com
- (000) 000-0000

IMAGES
VIDEO
COMMENTS
The authors present the GSK corruption scandal in the People's Republic of China (PRC) as a revelatory case study which constitutes one of the best opportunities in recent years to explore the dynamics of the relationship between China and the West, and multinational corporations-Chinese party-state (MNC-PS) engagement in particular ...
China Engagement: A Revelatory Case Study Martin Thorley and Andreas Fulda Abstract This article critically examines multinational corporation (MNC)-host government rela-tions in the People's Republic of China (PRC) through the prism of the GlaxoSmithKline (GSK) corruption scandal. The article takes the episode as a revelatory case study and
The GSK China scandal unfolded when the China division of the global drugmaker GlaxoSmithKline (GSK) admitted to engaging in bribery to promote its products within the Chinese market. The scandal originated with the discovery of sex tapes featuring Mark Reilly, the head of GSK's operations in China, and his Chinese girlfriend, circulated among senior company executives.
Ethical Insight. Corruption is the abuse of power or position for personal gain. It often involves bribery, as in the case of British pharmaceutical company GlaxoSmithKline (GSK) in China. GSK bribed medical professionals to push their drugs into the market. The company also bribed government officials to ease up on regulation.
Case Study - Curbing Corruption: GlaxoSmithKline in China - Page 1 of 3 Curbing Corruption: GlaxoSmithKline in China Multinational companies have often turned to China with the prospect of marketing to a large population that has seen major economic growth in recent decades. As China has become an
Andreas Fulda is assistant professor at the School of Politics and International Relations, University of Nottingham, and senior fellow at the University of Nottingham Asia Research Institute. Dr Fulda has specialised in the fields of democratisation studies; philanthropy and civil society; citizen diplomacy; and EU-China relations.
Abstract. Four GlaxoSmithKline employees were accused of bribing Chinese health care workers to prescribe the company's drugs. The accusations brought to light the questionable incentive structures of the Chinese health care system and the pressure on companies to adhere to local customs while still observing local laws.
GlaxoSmithKline in China (A) By: John A. Quelch, Margaret Rodriguez. Four GlaxoSmithKline employees were accused of bribing Chinese health care workers to prescribe the company's drugs. The accusations brought to light the questionable incentive structures of the…. Length: 15 page (s)
In September 2014, the British pharmaceutical company GlaxoSmithKline (GSK) was. found guilty of bribing Chinese doctors and hospitals to prescribe its medical products. and was levied a record ...
This article critically examines multinational corporation (MNC)-host government relations in the People's Republic of China (PRC) through the prism of the GlaxoSmithKline (GSK) corruption scandal. The article takes the episode as a revelatory case study and analyses it with a view to uncovering further data on the imperatives that govern interactions between the PRC and MNCs.
A case study GlaxoSmithKline is facing serious charges of misconduct as enforcement grows fierce. The entwining Chinese anti-bribery laws, the US FCPA and UK Bribery Act call for ... executives, the bribery case in China has also been followed by investigations by the US and UK regulators. The US Foreign Corrupt Practices Act (FCPA) of 1977, as
Indeed, these are frequently more extensive than those recently reported in China. GlaxoSmithKline, for instance, agreed to pay a fine of US$3 billion in 2012 to settle charges of inappropriate ...
The case in China has been followed by investigations in the US and UK, which further compounds the consequences and liabilities facing GSK. The facts. June 28 2013: the police in Changsha City announced investigations into certain GlaxoSmithKline (China) Investment (GSK China) executives for potential economic crimes.
GSK's local subsidiary in China was found guilty of bribery and fined nearly US$500 million, the largest corporate fine in China, according to the official Chinese news agency Xinhua. While the total fine is large, it is dwarfed by GSK's annual free cash flow of about £4 billion. The company is also being investigated in other countries ...
In the strongest signal yet, a Chinese court on Friday imposed a fine of nearly $500 million on the British pharmaceutical giant GlaxoSmithKline for bribery, dwarfing the penalties in earlier ...
This article mainly analyzes the annual report of GSK to study its development in China and the challenges it faces. 22 Years in China. The pharmaceutical baron, GlaxoSmithKline plc, known as GSK, was formed in 2000 after the merger of Glaxo Wellcome and SmithKline Beecham. At that time, GSK already had three subsidiary companies and five ...
Global health care giant GlaxoSmithKline LLC (GSK) agreed to plead guilty and to pay $3 billion to resolve its criminal and civil liability arising from the company's unlawful promotion of certain prescription drugs, its failure to report certain safety data, and its civil liability for alleged false price reporting practices.
GlaxoSmithKline in China (B) case study will help professionals, MBA, EMBA, and leaders to develop a broad and clear understanding of casecategory challenges. GlaxoSmithKline in China (B) will also provide insight into areas such as - wordlist , strategy, leadership, sales and marketing, and negotiations. ...
Implementation framework helps in weeding out non actionable recommendations, resulting in awesome GlaxoSmithKline in China (A) case study solution. Step 9 - Take a Break . Once you finished the case study implementation framework. Take a small break, grab a cup of coffee or whatever you like, go for a walk or just shoot some hoops. ...
MBA case pro PESTEL Analysis of case study "GlaxoSmithKline in China (B)" includes 6 parts - Political Factors, Economic Factors, Social Factors, Technological Factors, Environmental Factors, and Legal Factors. Case Study "GlaxoSmithKline in China (B)" is written - John A. Quelch, Margaret Rodriguez. In 2013, Chinese investigators detained four ...
DOI: 10.1007/s11069-024-06653-7 Corpus ID: 269654878; Spatial and temporal changes in social vulnerability to natural hazards: a case study for China counties @article{Li2024SpatialAT, title={Spatial and temporal changes in social vulnerability to natural hazards: a case study for China counties}, author={Xueting Li and Leiwen Jiang}, journal={Natural Hazards}, year={2024}, url={https://api ...
Taking China as a case study, we estimated the number of susceptible children under the age of 5 between the two schedules, which is defined as the mean susceptible probability multiplied by the ...
Beijing, China, 19 April 2024 - UN-Habitat, in cooperation with Chaoyang Environmental Group Co., Ltd. (Chaoyang Environment), organized an inception and expert group meeting in Beijing. The meeting brought together a diverse group of experts from China and abroad to exchange insights on climate change and municipal solid waste management.
AMA Style. Zhang Y, Zhang B, Hou J. Simulation Study on Student Residential Energy Use Behaviors: A Case Study of University Dormitories in Sichuan, China.
Nanling Mountain region is a typical southern hilly region, which plays an important ecological and environmental protection role in China's overall land protection pattern. Based on the remote sensing image data of Longnan City in Nanling Mountain region in 2013, 2018 and 2023, this paper interpreted the land use type and analyzed the land use transfer situation by using land use transfer ...
Implementation framework helps in weeding out non actionable recommendations, resulting in awesome GlaxoSmithKline in China (A) case study solution. Step 9 - Take a Break . Once you finished the case study implementation framework. Take a small break, grab a cup of coffee or whatever you like, go for a walk or just shoot some hoops. ...
Investigating how land use patterns impact on carbon emissions is crucial, as land use change is a major cause of increased carbon emission. High Efficiency Eco-economic Zone of Yellow River Delta (HEEZ-YRD) are typical of land use patterns affecting carbon emissions due to the fact they have greater dramatic land use changes. Here, we used carbon emission model and the decoupling analysis to ...
We conducted a case study of Xining, China, which has the highest population on the Tibetan Plateau (Pan et al., 2021). The aims of this study were to explore the spatiotemporal characteristics of ESs from 2000 to 2020 in the context of urbanization, establish a GI network by identifying ecological sources and corridors using the reconstructed ...