Staff and Board
Funders and Supporters
Job Opportunities
News Releases
Media Coverage
Education and Training
Capacity Building Assistance
Research and Evaluation
Alive Maryland
- ASO/CBO National Directory
ASO/CBO Leadership Training

Black Women’s Health Collaborative Learning Series
Board Leadership Training
Effi Barry Training Institute
E-Learning Center
Fiscal Health Training
Harm Reduction Learning Institute
HealthLGBTQ
HIV Prevention Certified Provider™ Certification Program

Partnering and Communicating Together (PACT)
Pozitively Aging
REINFORCE Center for Retention in HIV Care
Remaining Relevant in the New Reality
"State of" National Surveys
SYNChronicity
TeleHealthHIV
HIV Treatment Innovation Certificate Program
HIV PrEP Navigation Certification Program
LGBTQ Health Training and Certificate Program™
Newsletter Sign-Up
Request ASO/CBO CBA
Request Fiscal Health Professional Services
Join the National Coalition for LGBTQ Health
- HealthHIV Resources
- External Resources
- HIV Prevention Certified Provider Program
- Mpox Resource Center
- HIV Testing and Care Services Locator


How I Knew I Had HIV: Stories by Real People Living with HIV
Everyone’s experience is different, and that’s important because NO ONE symptom just shows up to indicate that you might have HIV. In fact, most people feel no symptoms at all prior to finding out. That is why getting tested is so important. In the beginning, it can be frightening, but these individuals overcame their fears and have triumphed.
- February 1, 2022
Subject Matter
Related programs, related resources.
Aging and PrEP: Considerations for PrEP in People 50 Years of Age and Older
Integrating Frailty and Functional Outcomes into Clinical Trials
HIV Clinical, Comorbid, and Social Determinants of Health are Linked with Brain Aging
The Science of Aging: Lessons for HIV at the Interface of Commonality and Heterogeneity
HIV and Aging: Double Stigma
National HIV Curriculum: HIV in Older Adults
- 1630 Connecticut Ave, NW, Suite 500, Washington D.C. 20009
- 202.232.6749
- 202.232.6750
- [email protected]
© Copyright 2024 HealthHIV. All rights reserved.
- Open access
- Published: 07 December 2021
The lived experience of HIV-infected patients in the face of a positive diagnosis of the disease: a phenomenological study
- Behzad Imani ORCID: orcid.org/0000-0002-1544-8196 1 ,
- Shirdel Zandi 2 ,
- Salman khazaei 3 &
- Mohamad Mirzaei 4
AIDS Research and Therapy volume 18 , Article number: 95 ( 2021 ) Cite this article
7543 Accesses
3 Citations
3 Altmetric
Metrics details
AIDS as a human crisis may lead to devastating psychological trauma and stress for patients. Therefore, it is necessary to study different aspects of their lives for better support and care. Accordingly, this study aimed to explain the lived experience of HIV-infected patients in the face of a positive diagnosis of the disease.
This qualitative study is a descriptive phenomenological study. Sampling was done purposefully and participants were selected based on the inclusion and exclusion criteria. Data collection was conducted, using semi-structured interviews. Data analysis was performed using Colaizzi’s method.
12 AIDS patients participated in this study. As a result of data analysis, 5 main themes and 12 sub-themes were identified, which include : emotional shock (loathing, motivation of social isolation), the fear of the consequences (fear of the death, fear of loneliness, fear of disgrace), the feeling of the guilt (feeling of regret, feeling guilty, feeling of conscience-stricken), the discouragement (suicidal ideation, disappointment), and the escape from reality (denial, trying to hide).
The results of this study showed that patients will experience unpleasant phenomenon in the face of the positive diagnosis of the disease and will be subjected to severe psychological pressures that require attention and support of medical and laboratory centers.
Patients will experience severe psychological stress in the face of a positive diagnosis of HIV.
Patients who are diagnosed with HIV are prone to make a blunder and dreadful decisions.
AIDS patients need emotional and informational support when they receive a positive diagnosis.
As a piece of bad news, presenting the positive diagnosis of HIV required the psychic preparation of the patient
Introduction
HIV/AIDS pandemic is one of the most important economic, social, and human health problems in many countries of the world, whose, extent and dimensions are unfortunately ever-increasing [ 1 ]. In such circumstances, this phenomenon should be considered as a crisis, which seriously affects all aspects of the existence and life of patients and even the health of society [ 2 ]. Diagnosing and contracting HIV/AIDS puts a person in a vague and difficult situation. Patients suffer not only from the physical effects of the disease, but also from the disgraceful consequences of the disease. HIV/AIDS is usually associated with avoidable behaviors that are not socially acceptable, such as unhealthy sexual, relations and drug abuse: So the patients are usually held guilty for their illness [ 3 ]. On the other hand, the issue of disease stigma in the community is the cause of rejection and isolation of these patients, and in health care centers is a major obstacle to providing services to these patients [ 4 ]. Studies show that HIV/AIDS stigma has a completely negative effect on the quality of life of these patients [ 5 ]. Criminal attitudes towards these patients and disappointing behavior by family, community, and medical staff cause blame and discrimination in patients [ 6 ]. HIV/AIDS stigma is prevalent among diseases, making concealment a major problem in this behavioral disease. The stigma comes in two forms: a negative inner feeling and a negative feeling that other people in the community have towards the patient [ 7 ]. The findings of a study that conducted in Iran indicated that increasing HIV/AIDS-related stigma decreases quality of life of people living with HIV/AIDS [ 8 ]. Robert Beckman has defined bad news as “any news that seriously and unpleasantly affects persons’ attitudes toward their future”. He considers the impact of counseling on moderating a person’s feeling of being important [ 9 ]. Therefore, being infected by HIV / AIDS due to the stigma can be bad news, which will lead to unpleasant emotional reactions [ 10 ]. Studies that have examined the lives of these patients have shown that these patients will experience mental and living problems throughout their lives. These studies highlight the need for age-specific programming to increase HIV knowledge and coping, increase screening, and improve long-term planning [ 11 , 12 ].
A prerequisite for any successful planning and intervention for people living with HIV/AIDS is approaching them and conducting in-depth interviews in order to discover their feelings, attitudes; their views on themselves, their illness, and others; and finally, their motivation to follow up and the participation in interventions [ 13 ]. Accordingly, the present study aimed to explain the lived experience of HIV-infected patients in the face of a positive diagnosis of the disease, since the better understanding of the phenomena leads to the smoother ways to help and care for these patients.
Study setting
In this study, a qualitative method of descriptive phenomenology was used to discover and interpret the lived experience of HIV-positive patients, when they face a positive diagnosis of the disease. The philosophical strengths underlying descriptive phenomenology afford a deeper understanding of the phenomenon being studied [ 14 ]. Husserl’s four steps of descriptive phenomenology were employed: bracketing, intuiting, analyzing and interpreting [ 15 ].
Participants and sampling
Sampling was done purposefully and participants were selected based on inclusion criteria. In this purposeful sampling, participants were selected among those patients who had sufficient knowledge about this phenomenon. The sample size was not determined at the beginning of the study, instead, it continued until no new idea emerged and data-saturated. Participants were selected from patients who were admitted to the Shohala Behavioral Diseases Counseling Center in Hamadan-Iran. The center has been set up to conduct tests, consultations, medical and dental services, and to distribute medicines among the patients. Additional inclusion criteria for selecting a participant are: having a positive diagnosis experience at the center, Ability to recall events and mental thoughts in the face of the first positive diagnosis of the disease, having psychological and mental stability, having a favorable clinical condition, willingness to work with the research team, and the possibility of re-access for the second interview if needed. Exclusion criteria were unwillingness to participate in the study and inability of verbal communication in Persian language.
Data collection
The interviews began with a non-structured question (tell us about your experience with a positive diagnosis) and continued with semi-structured questions. Each interview lasted 35–70 min and was conducted in two sessions if necessary. All interviews were conducted by the main investigator (ShZ) that who has experience in qualitative research and interviewing. The interview was recorded and then written down with permission of the participant.
Data analysis
The descriptive Colaizzi method was used to analyses the collected data [ 16 ]. This method consists of seven steps: (1) collecting the participants’ descriptions, (2) understanding the meanings in depth, (3) extracting important sentences, (4) conceptualizing important themes, (5) categorizing the concepts and topics, (6) constructing comprehensive descriptions of the issues examined, and (7) validating the data following the four criteria set out by Lincoln and Guba.
Trustworthiness criteria were used to validate the research, due to the fact that importance of data and findings validity in qualitative research [ 17 ]. This study was based on four criteria of Lincoln and Guba: credibility, transferability, dependability, and conformability [ 18 ]. For data credibility, prolong engagement and follow-up observations, as well as samplings with maximum variability were used. For dependability of the data, the researchers were divided into two groups and the research was conducted as two separate studies. At the same time, another researcher with the most familiarity and ability in conducting qualitative research, supervised the study as an external observer. Concerning the conformability, the researchers tried not to influence their own opinions in the coding process. Moreover, the codes were readout by the participants as well as two researcher colleagues with the help of an independent researcher and expert familiar with qualitative research. Transferability of data was confirmed by offering a comprehensive description of the subject, participants, data collection, and data analysis.
Ethical considerations (ethical approval)
The present study was registered with the ethics code IR.UMSHA.REC.1398.1000 in Hamadan University of Medical Sciences. The purpose of the study was explained and all participants’ consents were obtained at first step. All participants were assured that the information obtained would remain confidential and no personal information would be disclosed. Participants were also told that there was no need to provide any personal information to the interviewer, including name, surname, phone number and address. To gain more trust, interviews were conducted by a person who was not resident of Hamadan and was not a native of the region, this case was also reported to the participants.
Twelve HIV-infected participated in this study. The mean age of the participants was 36.41 ± 4.12 years. 58.33% of the participants were male and 41.66% were married. Of these, 2 were illiterate, 2 had elementary diploma, 6 had high school diploma and 2 had academic education. Six of them were unemployed, 5 were self-employed and 1 was an official employee. These people had been infected by this disease for 6.08 ± 2.71 years, in average (Table 1 ).
Analysis of the HIV-infected patients’ experiences of facing the positive diagnosis of the disease by descriptive phenomenology revealed five main themes: emotional shock, the fear of the consequences, the feeling of the guilt, the discouragement, and the escape from reality (Table 2 ).
Emotional shock
Emotional shock is one of the unpleasant events that these patients have experienced after facing a positive diagnosis of the disease. This experience has manifested in loathing and motivation of social isolation.
These patients stated that after facing a positive diagnosis of the disease, they developed a strong inner feeling of hatred towards the source of infection. The patients feel hatred, since they hold the carrier as responsible for their infection. “…After realizing I was affected, I felt very upset with my husband, I did not want to see him again, because it made me miserable, I even decided to divorce ….”(P3).
Motivation of social isolation
The experiences of these patients showed that after facing the incident, they have suffered an internal failure that has caused them to try to distance from other people. These patients have become isolated, withdrawing from the community and sometimes even from their families. “…After this incident, I decided to live alone forever and stay away from all my family members. I made a good excuse and broke up our engagement…” (P7).
Fear of the consequences
Fear of the consequences is one of the unpleasant experiences that these patients will face, as soon as they receive a positive diagnosis of the disease. Based on experiences, these patients feel fear of loneliness, death, and disgrace as soon as they hear the positive diagnosis.
Fear of the death
The patients said that as soon as they got the positive test results, they thought that the disease was incurable and would end their lives soon. “…When I found I had AIDS, I was very upset and moved like a dead man because I was really afraid that at any moment this disease might kill me and I would die …” (P1).
Fear of loneliness
The participants stated that one of the feelings that they experienced as soon as they received a positive diagnosis of the disease was the fear of being alone. They stated that at that moment, the thought of being excluded from society and losing their intimacy with them was very disturbing. “…The thought that I could no longer have a family and had to stay single forever bothered me a lot, it was terrifying to me when I thought that society could no longer accept me as a normal person …” (P10).
Fear of disgrace
One of the feelings that these patients experienced when faced the positive diagnosis of the disease was the fear of disgrace. They suffer from the perception that the spread of news of the illness hurts the attitudes of those around them and causes them to be discredited. “…It was very annoying for me when I thought I would no longer be seen as a member of my family, I felt I would no longer have a reputation and everyone would think badly of me …” (P2).
Feeling of the guilt
From other experiences of these patients in facing the positive diagnosis of the disease is feeling guilty. This feeling appears in patients as feeling of regret, guilty and remorse.
Feeling of regret
These patients stated that they felt remorse for their lifestyle and actions as soon as they heard the positive diagnosis of the disease, because they thought that if they had lived healthier, they would not have been infected. “…After realizing this disease, I was very sorry for my past, because I really did not have a healthy life. I made a series of mistakes that caused me to get caught. At that moment, I just regretted why I had this disaster …” (P11).
Feeling guilty
The experience of these patients has shown that after receiving a positive diagnosis of the disease, they consider themselves guilty and complain about themselves. These patients condemn their lifestyle and sometimes even consider themselves deserving of the disease and think that it is a ransom that they have paid back. “…after getting the disease, I realized that I was paying the ransom because I was hundred percent guilty, I was the one who caused this situation with a series of bad deeds, and now I have to be punished …” (P5).
Feeling of conscience-stricken
One of the experiences that these patients reported is the pangs of conscience. These patients stated that after receiving a positive diagnosis of the disease, the thought that as a carrier they might have contaminated those around them was very unpleasant and greatly affected their psyche. “…after getting the disease. It was shocked and I was just crazy about the fact that if my wife and children had taken this disease from me, what would I do, I made them hapless … and this as very annoying for me …” (P8).
Discouragement
Discouragement is an unpleasant experience that patients experienced after receiving a positive HIV test results. Discouragement in these patients appears in the suicidal ideation and disappointment.
Suicidal ideation
The patients stated that they were so upset with the positive diagnosis of the illness and they immediately thought they could not live with the fact and the best thing to do was to end their own lives. “…The news was so bad for me that I immediately thought that if the test result was correct and I had AIDS, I would have to kill myself and end this wretch life, oh, I had a lot of problem and the thought of having to wait for a gradual death was horrible to me …” (P12).
Disappointment
The experience of these patients shows that a positive diagnosis of the disease for these patients leads to a destructive feeling of disappointment. So that they are completely discouraged from their lives. These patients think that their dreams and goals are vanished and that they have reached the end and everything is over. “…It was a horrible experience, so at that moment I felt my life was over, I had to prepare myself for a gradual death, I was at marriage ages when I thought I could no longer get married, I saw life as meaningless …” (P7).
Escape from reality
The lived experience of these patients shows that after receiving a positive diagnosis of the disease, they found that this fact was difficult to accept and somehow tried to escape from the reality. This experience has been in the form of denial and trying to hide from others.
One of the experiences of these patients in dealing with the positive test result of this disease has been to deny it. In this way, patients believed that the test result was wrong or that the result belonged to someone else. For this reason, the patients referred to other laboratories after receiving the first positive diagnosis of the disease. “…After the lab told me this and found out what the disease really was, I was really shocked and said it was impossible, it was definitely wrong and it is not true … I could not believe it at all, because I was a professional athlete and this could not happen to me. So I immediately went to a bigger city and there I went to a few laboratories for further tests …” (P6).
Trying to hide
These patients stated that after receiving the first positive diagnosis of the disease, they thought that no one should notice their disease and should remain anonymous as much as possible. “…I immediately decided that no one in my city should know that I got this disease and the news should not be spread anywhere, so I discard my phone number through which our city laboratory communicated with me and I came here to do a re-examination and go to the doctor, and after all these years, I always come here again for an examination …” (P4).
In this qualitative study, we attempted to discover lived experience of HIV-infected patients in the face of a positive diagnosis of the disease. Therefore, a descriptive phenomenological method was applied. As a result of this study, based on the experiences of the HIV-infected patients, the five main themes of emotional shock, fear of the consequences, feelings of guilt, discouragement and, escape from reality were obtained.
In this study, it was shown that the confrontation of these patients with the positive diagnosis of the disease causes them to experience a severe emotional shock. In this regard, Yangyang Qiu et al. [ 19 ] argued that anxiety and depression are very common among HIV-infected patients who have recently been diagnosed with the disease. The experience of the participants has shown that this emotional shock appears in the form of loathing and the motivation of social isolation. In fact, in these patients, the feeling of the loathing is an emotional response to the primary carrier that has infected them. The study of Imani et al. [ 20 ] have shown that decrease emotional intelligence in an environment where there is an HIV carrier, other people hate him/her, because they see him/her as a risk factor for their infection. The experience of the participants has also shown that receiving a positive diagnosis will motivate social isolation in these patients. Various studies have revealed that one of the consequences of AIDS/HIV that patients will suffer from, is social isolation [ 21 , 22 ].
Another experience of the participants, according to this study is fear of the consequences. This phenomenon appears in these patients as fear of the death, fear of loneliness, and fear of disgrace. Due to the nature of the disease, these patients feel an inner fear of premature death, as soon as they receive a positive diagnosis. In this regard, the study of Audrey K Miller et al. [ 23 ] showed that death anxiety in AIDS patients is a psychological complication. the participants have stated that they are very afraid of being alone after receiving a positive diagnosis, which is a natural feeling according to Keith Cherry and David H. Smith [ 24 ]; because these patients will mainly experience some degree of loneliness. HIV-infected patients also experienced a fear of disgrace, which will go back to the nature of the disease and people’s insight; but they should be aware that, as Newman Amy states, AIDS/ HIV is a disease, not a scandal [ 25 ].
Another experience of the participants in dealing with the positive diagnosis of the disease is guilt feeling. The patients will experience feelings of regret, the feeling guilty and feeling of the conscience-stricken. The experience of the participants shows that they regret their past. Earlier studies have also revealed that regret for the past is a common phenomenon among the patients living with HIV [ 26 , 27 , 28 ]. HIV-infected feel guilty while facing the positive diagnosis of the disease and consider themselves the main culprit of the situation. They often play a direct role in their infection, and their past lifestyle for sure [ 29 ]. Our study also found that these patients feel the conscience-stricken after a positive diagnosis, because they suspect that they may have infected people around them. This disease can be easily transmitted from the carrier to others if the health protocols are not followed [ 30 , 31 , 32 ].
Another experience of HIV-infected in dealing with the receiving a positive diagnosis of the disease is discouragement. These patients are disappointed and sometimes decide to suicide. Based on the lived experience of HIV-infected, it was found that receiving a positive diagnosis of the disease, will discourage them from life and patients will be disappointed in many aspects of life. Studies have shown that AIDS/HIV, as a crisis, will greatly reduce the patients' life expectancy and that they will continue to live in despair [ 33 ]. Studies also stated that they considered suicide as a solution to relieve stress when receiving a positive diagnosis. In this regard, various studies have emphasized that among the AIDS/HIV patients, loss of self-esteem and severe stress have led to high suicide rates [ 34 , 35 , 36 ].
According to the patients, trying to escape from reality is another phenomenon that they will experience. This phenomenon will occur in patients as denial and trying to hide the disease from others. Based on the lived experience of these patients, it was found that after facing a positive diagnosis, HIV-infected tend to deny that they are infected. In this regard, various studies have shown that AIDS/HIV patients in different stages of the disease and their lives try to deny it in different ways [ 37 , 38 , 39 ]. The HIV-infected also stated that at the beginning of the positive diagnosis of the disease, did not want others to know, so they wanted to hide themselves from others in any way possible. In this regard, Emilie Henry et al. [ 40 ] have shown that a high percentage of the patients living with AIDS/HIV have tried that others do not notice that they are ill.
One of the strengths of this study is the methodology of the study, because in this study, an attempt has been made to use descriptive phenomenology to explain the lived experience of HIV-infected patients when faced with a positive diagnosis of this disease. In fact, in this study, patients' experience of this particular situation was identified, and with careful analysis, the experiences of these people became codes and concepts, each of which can be a bridge that keeps the path of modern knowledge open to help these patients. One of the limitations of this study is the generalizability of the findings because patients’ experiences in different societies that have cultural, religious, subsistence, and economic differences can be different.
The results of this study showed that patients will experience unpleasant experiences in the face of receiving a positive diagnosis of the HIV. Patients’ unpleasant experiences at that moment include emotional shock, fear of the consequences, feeling guilty, discouragement and escape from reality. Therefore, medical and laboratory centers must pay attention to the patients' lived experience, and try to support the patients through education, counseling and other support programs to minimize the psychological trauma caused by the disease.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors through reasonable request.
Acknowledgements
The authors would like to express their gratitude to the Hamadan Health Network, the Hamadan Shohada Behavioral Diseases Counseling Center, and the participants who helped us in this study.
The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9812209934).
Author information
Authors and affiliations.
Department of Operating Room, School of Paramedicine, Hamadan University of Medical Sciences, Hamadan, Iran
Behzad Imani
Department of Operating Room, Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
Shirdel Zandi
Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
Salman khazaei
Department of Epidemiology, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
Mohamad Mirzaei
You can also search for this author in PubMed Google Scholar
Contributions
BI designed the study, collected the data, and provide the first draft of manuscript. ShZ designed the study and revised the manuscript. SKh participated in design of the study, the data collection, and revised the manuscript. MM participated in design of the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Shirdel Zandi .
Ethics declarations
Ethics approval and consent to participate.
This study is the result of a student project that has been registered in Hamadan University of Medical Sciences of Iran with the ethical code IR.UMSHA.REC.1398.1000.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Imani, B., Zandi, S., khazaei, S. et al. The lived experience of HIV-infected patients in the face of a positive diagnosis of the disease: a phenomenological study. AIDS Res Ther 18 , 95 (2021). https://doi.org/10.1186/s12981-021-00421-4
Download citation
Received : 15 July 2021
Accepted : 26 November 2021
Published : 07 December 2021
DOI : https://doi.org/10.1186/s12981-021-00421-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lived experience
- Phenomenological study
AIDS Research and Therapy
ISSN: 1742-6405
- Submission enquiries: [email protected]
- Patient Care & Health Information
- Diseases & Conditions
Acquired immunodeficiency syndrome (AIDS), is an ongoing, also called chronic, condition. It's caused by the human immunodeficiency virus, also called HIV. HIV damages the immune system so that the body is less able to fight infection and disease. If HIV isn't treated, it can take years before it weakens the immune system enough to become AIDS . Thanks to treatment, most people in the U.S. don't get AIDS .
HIV is spread through contact with genitals, such as during sex without a condom. This type of infection is called a sexually transmitted infection, also called an STI. HIV also is spread through contact with blood, such as when people share needles or syringes. It is also possible for a person with untreated HIV to spread the virus to a child during pregnancy, childbirth or breastfeeding.
There's no cure for HIV / AIDS . But medicines can control the infection and keep the disease from getting worse. Antiviral treatments for HIV have reduced AIDS deaths around the world. There's an ongoing effort to make ways to prevent and treat HIV / AIDS more available in resource-poor countries.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Pill Aids from Mayo Clinic Store
The symptoms of HIV and AIDS vary depending on the person and the phase of infection.
Primary infection, also called acute HIV
Some people infected by HIV get a flu-like illness within 2 to 4 weeks after the virus enters the body. This stage may last a few days to several weeks. Some people have no symptoms during this stage.
Possible symptoms include:
- Muscle aches and joint pain.
- Sore throat and painful mouth sores.
- Swollen lymph glands, also called nodes, mainly on the neck.
- Weight loss.
- Night sweats.
These symptoms can be so mild that you might not notice them. However, the amount of virus in your bloodstream, called viral load, is high at this time. As a result, the infection spreads to others more easily during primary infection than during the next stage.
Clinical latent infection, also called chronic HIV
In this stage of infection, HIV is still in the body and cells of the immune system, called white blood cells. But during this time, many people don't have symptoms or the infections that HIV can cause.
This stage can last for many years for people who aren't getting antiretroviral therapy, also called ART. Some people get more-severe disease much sooner.
Symptomatic HIV infection
As the virus continues to multiply and destroy immune cells, you may get mild infections or long-term symptoms such as:
- Swollen lymph glands, which are often one of the first symptoms of HIV infection.
- Oral yeast infection, also called thrush.
- Shingles, also called herpes zoster.
Progression to AIDS
Better antiviral treatments have greatly decreased deaths from AIDS worldwide. Thanks to these lifesaving treatments, most people with HIV in the U.S. today don't get AIDS . Untreated, HIV most often turns into AIDS in about 8 to 10 years.
Having AIDS means your immune system is very damaged. People with AIDS are more likely to develop diseases they wouldn't get if they had healthy immune systems. These are called opportunistic infections or opportunistic cancers. Some people get opportunistic infections during the acute stage of the disease.
The symptoms of some of these infections may include:
- Fever that keeps coming back.
- Ongoing diarrhea.
- Swollen lymph glands.
- Constant white spots or lesions on the tongue or in the mouth.
- Constant fatigue.
- Rapid weight loss.
- Skin rashes or bumps.
When to see a doctor
If you think you may have been infected with HIV or are at risk of contracting the virus, see a healthcare professional as soon as you can.
More Information
- Early HIV symptoms: What are they?
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
HIV is caused by a virus. It can spread through sexual contact, shooting of illicit drugs or use of shared needles, and contact with infected blood. It also can spread from parent to child during pregnancy, childbirth or breastfeeding.
HIV destroys white blood cells called CD4 T cells. These cells play a large role in helping the body fight disease. The fewer CD4 T cells you have, the weaker your immune system becomes.
How does HIV become AIDS?
You can have an HIV infection with few or no symptoms for years before it turns into AIDS . AIDS is diagnosed when the CD4 T cell count falls below 200 or you have a complication you get only if you have AIDS , such as a serious infection or cancer.
How HIV spreads
You can get infected with HIV if infected blood, semen or fluids from a vagina enter your body. This can happen when you:
- Have sex. You may become infected if you have vaginal or anal sex with an infected partner. Oral sex carries less risk. The virus can enter your body through mouth sores or small tears that can happen in the rectum or vagina during sex.
- Share needles to inject illicit drugs. Sharing needles and syringes that have been infected puts you at high risk of HIV and other infectious diseases, such as hepatitis.
- Have a blood transfusion. Sometimes the virus may be transmitted through blood from a donor. Hospitals and blood banks screen the blood supply for HIV . So this risk is small in places where these precautions are taken. The risk may be higher in resource-poor countries that are not able to screen all donated blood.
- Have a pregnancy, give birth or breastfeed. Pregnant people who have HIV can pass the virus to their babies. People who are HIV positive and get treatment for the infection during pregnancy can greatly lower the risk to their babies.
How HIV doesn't spread
You can't become infected with HIV through casual contact. That means you can't catch HIV or get AIDS by hugging, kissing, dancing or shaking hands with someone who has the infection.
HIV isn't spread through air, water or insect bites. You can't get HIV by donating blood.
Risk factors
Anyone of any age, race, sex or sexual orientation can have HIV / AIDS . However, you're at greatest risk of HIV / AIDS if you:
- Have unprotected sex. Use a new latex or polyurethane condom every time you have sex. Anal sex is riskier than is vaginal sex. Your risk of HIV increases if you have more than one sexual partner.
- Have an STI . Many STIs cause open sores on the genitals. These sores allow HIV to enter the body.
- Inject illicit drugs. If you share needles and syringes, you can be exposed to infected blood.
Complications
HIV infection weakens your immune system. The infection makes you much more likely to get many infections and certain types of cancers.
Infections common to HIV/AIDS
- Pneumocystis pneumonia, also called PCP. This fungal infection can cause severe illness. It doesn't happen as often in the U.S. because of treatments for HIV / AIDS . But PCP is still the most common cause of pneumonia in people infected with HIV .
- Candidiasis, also called thrush. Candidiasis is a common HIV -related infection. It causes a thick, white coating on the mouth, tongue, esophagus or vagina.
- Tuberculosis, also called TB. TB is a common opportunistic infection linked to HIV . Worldwide, TB is a leading cause of death among people with AIDS . It's less common in the U.S. thanks to the wide use of HIV medicines.
- Cytomegalovirus. This common herpes virus is passed in body fluids such as saliva, blood, urine, semen and breast milk. A healthy immune system makes the virus inactive, but it stays in the body. If the immune system weakens, the virus becomes active, causing damage to the eyes, digestive system, lungs or other organs.
- Cryptococcal meningitis. Meningitis is swelling and irritation, called inflammation, of the membranes and fluid around the brain and spinal cord, called meninges. Cryptococcal meningitis is a common central nervous system infection linked to HIV . A fungus found in soil causes it.
Toxoplasmosis. This infection is caused by Toxoplasma gondii, a parasite spread primarily by cats. Infected cats pass the parasites in their stools. The parasites then can spread to other animals and humans.
Toxoplasmosis can cause heart disease. Seizures happen when it spreads to the brain. And it can be fatal.
Cancers common to HIV/AIDS
- Lymphoma. This cancer starts in the white blood cells. The most common early sign is painless swelling of the lymph nodes most often in the neck, armpit or groin.
- Kaposi sarcoma. This is a tumor of the blood vessel walls. Kaposi sarcoma most often appears as pink, red or purple sores called lesions on the skin and in the mouth in people with white skin. In people with Black or brown skin, the lesions may look dark brown or black. Kaposi sarcoma also can affect the internal organs, including the lungs and organs in the digestive system.
- Human papillomavirus (HPV)-related cancers. These are cancers caused by HPV infection. They include anal, oral and cervical cancers.
Other complications
- Wasting syndrome. Untreated HIV / AIDS can cause a great deal of weight loss. Diarrhea, weakness and fever often happen with the weight loss.
- Brain and nervous system, called neurological, complications. HIV can cause neurological symptoms such as confusion, forgetfulness, depression, anxiety and difficulty walking. HIV -associated neurological conditions can range from mild symptoms of behavior changes and reduced mental functioning to severe dementia causing weakness and not being able to function.
- Kidney disease. HIV -associated nephropathy (HIVAN) is swelling and irritation, called inflammation, of the tiny filters in the kidneys. These filters remove excess fluid and waste from the blood and pass them to the urine. Kidney disease most often affects Black and Hispanic people.
- Liver disease. Liver disease also is a major complication, mainly in people who also have hepatitis B or hepatitis C.
There's no vaccine to prevent HIV infection and no cure for HIV / AIDS . But you can protect yourself and others from infection.
To help prevent the spread of HIV :
Consider preexposure prophylaxis, also called PrEP. There are two PrEP medicines taken by mouth, also called oral, and one PrEP medicine given in the form of a shot, called injectable. The oral medicines are emtricitabine-tenofovir disoproxil fumarate (Truvada) and emtricitabine-tenofovir alafenamide fumarate (Descovy). The injectable medicine is called cabotegravir (Apretude). PrEP can reduce the risk of sexually transmitted HIV infection in people at very high risk.
PrEP can reduce the risk of getting HIV from sex by about 99% and from injecting drugs by at least 74%, according to the Centers for Disease Control and Prevention. Descovy hasn't been studied in people who have sex by having a penis put into their vaginas, called receptive vaginal sex.
Cabotegravir (Apretude) is the first U.S. Food and Drug Administration-approved PrEP that can be given as a shot to reduce the risk of sexually transmitted HIV infection in people at very high risk. A healthcare professional gives the shot. After two once-monthly shots, Apretude is given every two months. The shot is an option in place of a daily PrEP pill.
Your healthcare professional prescribes these medicines to prevent HIV only to people who don't already have HIV infection. You need an HIV test before you start taking any PrEP . You need to take the test every three months for the pills or before each shot for as long as you take PrEP .
You need to take the pills every day or closely follow the shot schedule. You still need to practice safe sex to protect against other STIs . If you have hepatitis B, you should see an infectious disease or liver specialist before beginning PrEP therapy.
Use treatment as prevention, also called TasP. If you have HIV , taking HIV medicines can keep your partner from getting infected with the virus. If your blood tests show no virus, that means your viral load can't be detected. Then you won't transmit the virus to anyone else through sex.
If you use TasP , you must take your medicines exactly as prescribed and get regular checkups.
- Use post-exposure prophylaxis, also called PEP, if you've been exposed to HIV . If you think you've been exposed through sex, through needles or in the workplace, contact your healthcare professional or go to an emergency room. Taking PEP as soon as you can within the first 72 hours can greatly reduce your risk of getting HIV . You need to take the medicine for 28 days.
Use a new condom every time you have anal or vaginal sex. Both male and female condoms are available. If you use a lubricant, make sure it's water based. Oil-based lubricants can weaken condoms and cause them to break.
During oral sex, use a cut-open condom or a piece of medical-grade latex called a dental dam without a lubricant.
- Tell your sexual partners you have HIV . It's important to tell all your current and past sexual partners that you're HIV positive. They need to be tested.
- Use clean needles. If you use needles to inject illicit drugs, make sure the needles are sterile. Don't share them. Use needle-exchange programs in your community. Seek help for your drug use.
- If you're pregnant, get medical care right away. You can pass HIV to your baby. But if you get treatment during pregnancy, you can lessen your baby's risk greatly.
- Consider male circumcision. Studies show that removing the foreskin from the penis, called circumcision, can help reduce the risk of getting HIV infection.
- About HIV and AIDS . HIV.gov. https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/what-are-hiv-and-aids. Accessed Oct. 18, 2023.
- Sax PE. Acute and early HIV infection: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/search. Accessed Oct. 18, 2023.
- Ferri FF. Human immunodeficiency virus. In: Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV . HIV.gov. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations. Accessed Oct. 18, 2023.
- AskMayoExpert. Human immunodeficiency virus (HIV) infection. Mayo Clinic; 2023.
- Elsevier Point of Care. Clinical Overview: HIV infection and AIDS in adults. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Male circumcision for HIV prevention fact sheet. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/male-circumcision-HIV-prevention-factsheet.html. Accessed Oct. 19, 2023.
- Acetyl-L-carnitine. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Whey protein. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Saccharomyces boulardii. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Vitamin A. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Red yeast rice. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
Associated Procedures
- Liver function tests
News from Mayo Clinic
- Unlocking the mechanisms of HIV in preclinical research Jan. 10, 2024, 03:30 p.m. CDT
- Mayo Clinic expert on future of HIV on World AIDS Day Dec. 01, 2023, 05:15 p.m. CDT
- Mayo Clinic Minute: Know your status -- the importance of HIV testing June 27, 2023, 03:00 p.m. CDT
- Mayo Clinic Q&A podcast: The importance of HIV testing June 24, 2022, 12:18 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email

How Can You Tell If You Have HIV?
The only way to know for sure if you have HIV is to get tested. You can’t rely on symptoms to tell whether you have HIV.
Knowing your HIV status gives you powerful information so you can take steps to keep yourself and your partner(s) healthy:
- If you test positive , you can take medicine to treat HIV. People with HIV who take HIV medicine (called antiretroviral therapy or ART) as prescribed and get and keep an undetectable viral load can live long and healthy lives and will not transmit HIV to their HIV-negative partners through sex . An undetectable viral load is a level of HIV in the blood so low that it can’t be detected in a standard lab test.
- If you test negative , you have more HIV prevention tools available today than ever before, like pre-exposure prophylaxis (PrEP) , medicine people at risk for HIV take to prevent getting HIV from sex or injection drug use, and post-exposure prophylaxis (PEP ), HIV medicine taken within 72 hours after a possible exposure to prevent the virus from taking hold.
- If you are pregnant , you should be tested for HIV so that you can begin treatment if you're HIV-positive. If you have HIV and take HIV medicine as prescribed throughout your pregnancy and childbirth and give HIV medicine to your baby for 4 to 6 weeks after giving birth, your risk of transmitting HIV to your baby can be less than 1%. HIV medicine will protect your own health as well.
Use the HIV Services Locator to find an HIV testing site near you.
HIV self-testing is also an option. Self-testing allows people to take an HIV test and find out their result in their own home or other private location. You can buy a self-test kit at a pharmacy or online, or your health care provider may be able to order one for you. Some health departments or community-based organizations also provide self-test kits for a reduced cost or for free. Learn more about HIV self-testing and which test might be right for you .
What Are the Symptoms of HIV?
There are several symptoms of HIV. Not everyone will have the same symptoms. It depends on the person and what stage of the disease they are in.
Below are the three stages of HIV and some of the symptoms people may experience.
Stage 1: Acute HIV Infection
Within 2 to 4 weeks after infection with HIV, about two-thirds of people will have a flu-like illness. This is the body’s natural response to HIV infection.
Flu-like symptoms can include:
- Night sweats
- Muscle aches
- Sore throat
- Swollen lymph nodes
- Mouth ulcers
These symptoms can last anywhere from a few days to several weeks. But some people do not have any symptoms at all during this early stage of HIV.

Don’t assume you have HIV just because you have any of these symptoms—they can be similar to those caused by other illnesses. But if you think you may have been exposed to HIV, get an HIV test.
Here’s what to do:
- Find an HIV testing site near you —You can get an HIV test at your primary care provider’s office, your local health department, a health clinic, or many other places . Use the HIV Services Locator to find an HIV testing site near you.
- Request an HIV test for recent infection —Most HIV tests detect antibodies (proteins your body makes as a reaction to HIV), not HIV itself. But it can take a few weeks after you have HIV for your body to produce these antibodies. There are other types of tests that can detect HIV infection sooner. Tell your doctor or clinic if you think you were recently exposed to HIV and ask if their tests can detect early infection.
- Know your status —After you get tested, be sure to learn your test results. If you’re HIV-positive, see a health care provider as soon as possible so you can start treatment with HIV medicine. And be aware: when you are in the early stage of infection, you are at very high risk of transmitting HIV to others. It is important to take steps to reduce your risk of transmission. If you are HIV-negative, there are prevention tools like pre-exposure prophylaxis (PrEP) that can help you stay negative.
Stage 2: Clinical Latency
In this stage, the virus still multiplies, but at very low levels. People in this stage may not feel sick or have any symptoms. This stage is also called chronic HIV infection .
Without HIV treatment, people can stay in this stage for 10 or 15 years, but some move through this stage faster.
If you take HIV medicine exactly as prescribed and get and keep an undetectable viral load, you can live and long and healthy life and will not transmit HIV to your HIV-negative partners through sex.
But if your viral load is detectable, you can transmit HIV during this stage, even when you have no symptoms. It’s important to see your health care provider regularly to get your viral load checked.
Stage 3: AIDS
If you have HIV and you are not on HIV treatment, eventually the virus will weaken your body’s immune system and you will progress to AIDS ( acquired immunodeficiency syndrome) .
This is the late stage of HIV infection.
Symptoms of AIDS can include:
- Rapid weight loss
- Recurring fever or profuse night sweats
- Extreme and unexplained tiredness
- Prolonged swelling of the lymph glands in the armpits, groin, or neck
- Diarrhea that lasts for more than a week
- Sores of the mouth, anus, or genitals
- Red, brown, pink, or purplish blotches on or under the skin or inside the mouth, nose, or eyelids
- Memory loss, depression, and other neurologic disorders
Each of these symptoms can also be related to other illnesses. The only way to know for sure if you have HIV is to get tested. If you are HIV-positive, a health care provider will diagnose if your HIV has progressed to stage 3 (AIDS) based on certain medical criteria .
Many of the severe symptoms and illnesses of HIV disease come from the opportunistic infections that occur because your body’s immune system has been damaged. See your health care provider if you are experiencing any of these symptoms.
But be aware: Thanks to effective treatment, most people in the U.S. with HIV do not progress to AIDS. If you have HIV and remain in care, take HIV medicine as prescribed, and get and keep an undetectable viral load, you will stay healthy and will not progress to AIDS.
Read more about the difference between HIV and AIDS .
Related HIV.gov Blogs
- Testing HIV Testing
- Self-Testing HIV Self-Testing
- CDC – HIV Basics: How Do I Know If I Have HIV?
- NICHD – Symptoms of HIV/AIDS
- VA – HIV/AIDS Basics: Symptoms
- Research article
- Open access
- Published: 18 March 2022
Experiences of new diagnoses among HIV-positive persons: implications for public health
- Adobea Yaa Owusu ORCID: orcid.org/0000-0003-2223-7896 1
BMC Public Health volume 22 , Article number: 538 ( 2022 ) Cite this article
2876 Accesses
4 Citations
Metrics details
Ready acceptance of experiences of new diagnoses among HIV-positive persons is a known personal and public health safety-net. Its beneficial effects include prompt commencement and sustenance of HIV-positive treatment and care, better management of transmission risk, and disclosure of the HIV-positive status to significant others. Yet, no known study has explored this topic in Ghana; despite Ghana’s generalised HIV/AIDS infection rate. Existing studies have illuminated the effects of such reactions on affected significant others; not the infected.
This paper studied qualitatively the experiences of new diagnoses among 26 persons living with HIV/AIDS. Sample selection was random, from two hospitals in a district in Ghana heavily affected by HIV/AIDS. The paper applied the Hopelessness Theory of Depression.
As expected, the vast majority of respondents experienced the new diagnoses of their HIV-positive infection with a myriad of negative psychosocial reactions, including thoughts of committing suicide. Yet, few of them received the news with resignation. For the vast majority of respondents, having comorbidities from AIDS prior to the diagnosis primarily shaped their initial reactions to their diagnosis. The respondents’ transitioning to self-acceptance of their HIV-positive status was mostly facilitated by receiving counselling from healthcare workers.
Conclusions
Although the new HIV-positive diagnosis was immobilising to most respondents, the trauma faded, paving the way for beneficial public health actions. The results imply the critical need for continuous education on HIV/AIDS by public health advocates, using mass media, particularly, TV. Healthcare workers in VCTs should empathise with persons who experience new diagnoses of their HIV-positive status.
Peer Review reports
News of a newly-diagnosed HIV-positive status has the tendency to lead to negative psychosocial outcomes for persons living with HIV/AIDS (PLWHAs) [ 1 , 2 , 3 ]. In this paper, the concept of newly-diagnosed refers to the first time a respondent was informed of his/her HIV-positive status. Implications of this operational definition are discussed later on in the paper. Humans depict cognitive, emotional, and motivational deficits on hearing bad news and experiencing what is deemed uncontrollable events [ 4 ]. This is very likely to apply to PLWHAs particularly, when confronted with the news of being HIV-positive for the first time. Particularly, PLWHAs are known to mostly experience neurocognitive disorders associated with the infection [ 5 ], and often experience common mental disorders [ 6 , 7 ]. This is especially the case when PLWHAs fairly expect or self-perceive negative social reactions such as spousal abuse, dismissal from employment, stigma and discrimination, among others [ 8 ]. Peterson and Seligman ([ 4 ], p. 347) christened these series of responses on such occasions as “learned helplessness phenomenon.”
There are numerous news reports of persons who have committed suicide shortly after an HIV-positive diagnosis. This includes even the case of a female physician from the Eket local government area of Akwa Ibom, Nigeria [ 9 ]. Previous researchers note that after the initial diagnosis of a disease believed to be life-threatening, and particularly incurable, patients are known to experience an immediate descent into several distressing psychological and emotional states of mind [ 1 , 2 , 10 ]. These range from disbelief to denial to being utterly scared and shocked. Some even think that their life is not worth living anymore and imagine that indeed, the disease has already taken a final toll on them [ 2 , 3 ]. This brings to the fore the need to study the reactions of PLWHAs right after their initial diagnosis. Research and programme interventions on adaptation to a new HIV diagnosis provide personal and public health safety-nets and are thus needed [ 2 , 11 ]. Such research and interventions are helpful in educating newly-diagnosed PLWHAs on HIV [ 1 , 10 ] and promoting their health [ 1 , 8 , 12 ].
Acceptance of the news of an HIV-positive diagnosis is critical for several reasons [ 2 ]. Engagement in and sustenance of HIV treatment and care [ 1 , 2 ], viral load (VL), CD4 counts [ 2 , 13 ], and transmission risk [ 2 , 11 , 14 ] are affected by the reaction to the news of diagnosis. Reactions also affect perceptions of stigma and disclosure activity [ 2 , 11 ]. Conversely, difficulties with accepting diagnosed HIV-positive status has serious potential negative consequences for individuals and the general public [ 15 , 16 ]. From the angle of healthcare practitioners, better handling of new diagnosis of an HIV-positive status and related disclosure could greatly buffer the psychosocial and mental health of the newly-diagnosed PLWHAs, and help facilitate healthcare seeking and retention. These include the management of less traumatised disclosure, reduction of self-stigmatisation, and better management of romantic and family relationships [ 2 , 14 ]. Others are the reduction of viral transmission [ 14 , 17 ], and improvement in general public health and well-being of populations [ 1 , 14 ]. Knowing the experiences of new diagnoses among HIV-positive persons is, therefore, likely to facilitate successful linkage and retention of such persons in healthcare for HIV [ 1 , 2 ]. This is critical and is also known to be a significant gap in the HIV care continuum in some parts of the world, even the U.S. with all its known medical advancement [ 11 ].
Based on previous related literature, this paper primarily examines qualitatively the experiences of new diagnoses of 26 Ghanaian PLWHAs to the first news of their HIV-positive status. Second, it attempts to untangle the situations surrounding these experiences to determine what might have influenced such reactions. Third, the paper delineates the processes that particularly helped the PLWHAs transition from negative psychological reactions to showing positive reactions and seeking HIV treatment. Fourth, it adds to the core literature on managing experiences of new diagnoses of an HIV-positive status, and illuminates their importance to public health. It is hoped that this paper will facilitate a better acceptance of an HIV-positive sense of self which is known to aid PLWHAs to accept, adjust, and more effectively cope with their diagnosis. This will aid them to better manage the complexities of living with the infection [ 2 , 18 ]. This paper specifies the knowledge to Ghana and more importantly, Ghana’s most HIV/AIDS-affected district [ 19 , 20 ]. A thorough search on the experiences of PLWHAs’ new diagnoses in Ghana yielded no known results despite the fact that Ghana has a generalised HIV/AIDS epidemic: more than 1% of the residents have the infection [ 21 ]. This paper aims to fill that gap.
Immediate reactions to news of HIV positive status in Africa
The literature on the immediate reactions to the initial diagnosis of HIV-positive status is also sparse, and a majority of what research there is comes from South Africa. Such findings overwhelmingly corroborate each other: immediately after being diagnosed HIV-positive, PLWHAs studied depict deep negative emotions. Visser et al. [ 22 ] studied the phenomenon in South Africa, using a semi-structured interview of 293 pregnant women who were undergoing HIV test during antenatal care. On hearing the news, those who tested positive were shocked, and got frightened that they would be abandoned and discriminated against.
Fabianova [ 23 ] undertook a longitudinal study in Nairobi, Kenya, on the psychosocial aspects of being PLWHA. When the respondents who visited VCTs were first informed of their HIV-positive status, 89% of them felt sad due to their HIV-positive status, 60% had feelings of fear and anxiety, 30% felt angry, 25% felt distressed, and 15% cried. Additional psychosocial behaviours exhibited by the respondents included grief, guilt, hopelessness, helplessness, anger, disbelief, self-blame or blamed others, and aggression towards a counsellor. Their sadness was mostly in reference to close relatives who had died of AIDS. Their fears related mostly to the loss of their social position. Other fears surrounded loss of life, ambition, sexual relations, independence, physical performance, and financial stability [ 23 ]. While Fabianova [ 23 ] observed from the extant literature that suicide is a common reaction for persons who are first informed of their HIV/AIDS status, her study found that less than 1% of participants attempted suicide on hearing of their confirmed HIV-positive status. Most of Fabianova’s ([ 23 ], p. 201) respondents already considered themselves to be “walking corpses” and even visualised their funeral and grave.
Fabianova [ 23 ] enumerated several explanatory factors that unpacked her respondents’ reactions to their initial diagnoses of being HIV-positive. These included gender, level of preparedness of a client in the pre-testing session, and type of sexual relationship(s) they were in. Others included levels of general knowledge of HIV/AIDS, and HIV/AIDS-related stigma in their community. Fabianova ([ 23 ], p. 199) discovered that males responded to the initial HIV/AIDS diagnosis with anger, disbelief, and aggression. The females cried, got shocked, “swallowed big lumps of air, saliva subconsciously, shook both their hands in refusal and blame [sic] the others almost immediately.”
Other key explanatory factors for Fabianova’s ([ 23 ], p. 199) respondents’ immediate reactions included concerns about “lack of immediate elaborate support structures”, extent of level of awareness about HIV/AIDS, level of HIV/AIDS-related stigma, availability of antiretroviral therapy (ART), and support groups to enable them move on with their lives. Feelings of guilt for the infection were explained by whether the individual felt his/her lifestyle exposed him/her to it, and type of sexual involvement they were engaged in. They felt guilty that they would infect a spouse if they were married. If they were in an unstable/non-married relationship, they did not feel guilty, and shared the blame with the casual sexual partner. Sixty-two percent blamed their partners or the environment with the excuse that they stayed loyal to their partners. Fabianova’s ([ 23 ], p. 200) respondents who tested on their own volition did so based on their own or a partner’s “failure”, poor health or “accidental happening”, or work commitments.
The theoretical framework adopted for this paper, the Hopelessness Theory of Depression, is grounded on depression. Depression is currently one of the five leading causes of the disease burden internationally, except in sub-Saharan Africa (SSA) [ 24 ]. Researchers note that depressive disorders and other common mental health disorders (CMDs--depression, anxiety and somatization) were critically linked to the Millennium Development Goals, particularly gender equity, poverty, HIV/AIDS, and maternal and child health [ 24 , 25 ]. Importantly, depression is the most diagnosed psychiatric disorder among PLWHAs. Depression also serves as a risk factor for the progression of HIV/AIDS. In African settings, a growing appreciation of an important link between CMDs and HIV/AIDS has been established [ 7 , 23 ] as well. HIV has unleashed “a significant strain” on mental health in Africa ([ 24 ], p. 61; [ 26 ]). The huge burden of HIV/AIDS in SSA accounts for 16% of depression in the sub-region [ 24 ]. Alternatively, a neurobiological association exists between HIV and CMDs: “the HIV virus has quite specific detrimental effects on neuronal function” ([ 24 ], pp. 61 & 65). Evidence-based reports in African settings and other developing nations (studies conducted in Sao Paulo, Bangkok, Kinshasa, Nairobi, and Ethiopia), found both more symptoms and a higher prevalence of depression among symptomatic PLWHAs than among non-symptomatic or HIV-negative persons [ 24 ].
Theoretical framework: the hopelessness theory of depression
The Hopelessness Theory of Depression is applied to this paper. It is a diathesis-stress theory which posits that organisms express some form of cognitive and emotional deficits after experiencing a bad event. The theory argues that three depressogenic inferential styles serve as risk factors of depression [ 27 , 28 ]. These are the tendency to attribute a bad event to a global or stable cause; the tendency to perceive bad events as having many disastrous consequences; and the propensity to view oneself as flawed or inefficient [ 28 , 29 ]. Making negative inference upsurges the possibility of hopelessness while feeling hopeless makes depression inevitable. With this explanation, the theory assumes hopelessness as a critical underlying factor to depression. Adding to the causal explanation, Seligman [ 30 ] stated that the symptoms, cure and prevention of a bad event also model depression. In societies like Ghana, where HIV is associated with nonconformity to societal expectations and/or sexual promiscuity, PLWHAs may be more exposed to adverse emotional and cognitive symptoms after receiving an HIV-positive report.
The application of the thesis of the Hopelessness Theory of Depression to HIV-positive populations in SSA is not new. Govender and Schlebusch’s [ 31 ] study in Kwa-Zulu Natal, South Africa, applied Beck’s Hopelessness Scale and Beck’s Depression Inventory [ 32 ] to their assessment of the correlation between depression, hopelessness, and suicidal thoughts in PLWHAs. Schlebusch and Govender [ 33 ] used the same inventories to study PLWHAs in a University-affiliated hospital in South Africa. Primarily, they studied the prevalence of risk of suicidal ideation in PLWHAs immediately after their first diagnosis. Kylmä et al. [ 34 ] also studied the full gamut/dynamics of the concept of hope (hope, despair, hopelessness) among PLWHAs. Their study yielded information on how PLWHAs’ perceptions of hope could facilitate their clinical care. The Hopelessness Theory of Depression is applied to the discussion of the findings.
Study setting and cultural context of LMKM
The LMKM, the catchment area for the study, is situated in the Eastern Region of Ghana. The region, one of ten at the time of data collection, had 2,633,154 residents by Ghana’s last Population and Housing census of 2010, making it the third most-populated region. The Eastern Region is mostly semi-urban [ 35 ]. The LMKM, one of 26 administrative municipalities/districts in the Eastern Region by the time of data collection, covers 12.4% of the region, with total land mass of 304.4 km 2 . The 2010 Population and Housing Census recorded 89,246 residents of the Municipality comprising 46.5% males and 53.5% females. Christians were 92.8%; other religious groups include Muslims and traditionalists [ 36 ]. The indigenes are ethnic Dangmes and speak Krobo. They are a patrilineal descent group, which means they inherit property through their father’s lineage.
Sampling and data collection
This study used a “descriptive, multiple case study approach” ([ 2 ], p. 2; [ 37 ]). This method generates interviewees’ in-depth descriptions of their situations, views and realities regarding issues. This provides deep insights of their actions and choices [ 38 ]. In this study, the pool of cases of the individual respondents is considered a “multiple case study approach” ([ 2 , p. 2; 37 ]). Additionally, the findings from the study municipality forms a case study. As Kutnick et al. [ 2 ] note, case studies are exceptionally useful in eliciting contextual situations, when they are important to a particular study. Examples are cultural, social and structural impediments (such as stigma, and fear) to post-diagnosis HIV care [ 37 ].
This paper analysed data from 26 (13 each from two hospitals studied) out of 38 PLWHAs interviewed qualitatively through personal interviews from June to July 2015. The data used for this study formed part of a large data set from a project which primarily studied the linkages between housing conditions and the reported health status of PLWHAs (see [ 39 , 40 , 41 , 42 ]). The implications of recruiting individuals further away from their diagnosis for this paper are addressed in the section on limitations. The interviews were conducted with the aid of a pretested semi-structured question guide. Table 1 has the key questions asked. Some of these key questions in Table 1 were probing questions that emanated from the main/initial interview guide, because it is open ended, as is the norm with data collection tools for qualitative studies. The initial sample of 38 comprised both males and females who were selected using random sampling [ 2 ] as part of a research project which primarily studied the nexus between the health status of PLWHAs in the LMKM and their housing conditions.
The project comprised both qualitative and quantitative data collection. The qualitative interview guide has been submitted as Additional file 1 . First, the study district, LMKM, was conveniently selected based on its lead, by the time of developing the project proposal, in having persons with HIV/AIDS in Ghana, due to which the government had focused on strengthening healthcare institutions and personnel in the district for the fight against HIV/AIDS. Second, two out of three health facilities in the study district were also conveniently selected—a government and quasi-government hospitals. These had been specially equipped by the Government since 2002, to manage HIV/AIDS cases, thus conveniently leaving out the other government hospital [ 39 , 40 , 41 , 42 ]. Respondents had come to HIV/AIDS Voluntary Counseling and Testing (VCT) Centres in the two hospitals--St. Martins de Porres Hospital in Agormanya and Atua Government Hospital in Atua, near Agormanya, for care. Third, respondents were initially selected conveniently for having been medically diagnosed of being HIV-positive prior to the study, after which they had to verbally self-confirm their status to the PIs of the study.
Interviewing took place in these VCTs. The VCTs operated only on weekdays. For each day during the weekdays, based on prior reconnaissance survey, 40 respondents were targeted for both qualitative and quantitative interviews from the daily list of attendees in each hospital. One-third of these were selected randomly and interviewed. If there were less than 40 people in a day, half of them were randomly selected for interviews. For each day fewer than one-fourth of our initial sample were males, we increased the chances of having males in the sample, by including every fourth male from the initial list of persons who did not make it to the list from which final sampling was done [see [ 20 , 40 , 42 ]. This is justified based on a preponderance of female PLWHAs in the study district [ 20 , 40 , 42 ], Ghana, and also, sub-Sahara Africa generally [ 40 , 42 , 43 , 44 ]. Respondents were given unique codes to prevent being re-interviewed during the course of the study. Five male and female graduate student interviewers were assigned to each study hospital, including one qualitative interviewer.
Qualitative or otherwise, a respondent got randomly selected for an interview when a prior interview had ended, beginning with a random start from the list of assigned codes, till the selected list was exhausted in a day. Similar sampling arrangements were made for each VCT used. The random selection of the respondents was necessary to spread their selection over the designated one month of study, for purposes of having a variety of cases/stories over the study period, for richer analysis. Out of the estimated average of about 40 PLWHAs who would visit each VCT per day, it was necessary to randomly select some and leave others out, to facilitate spreading respondent selection over time as said already. Additionally, it was expedient to randomly distribute the respondents between the qualitative study and survey data collection approaches for the larger project, to avoid possible biases in the responses from these two approaches.
Respondents were males and females, 18 years or older, and confirmed their HIV-positive status to the principal investigators (PIs). The interviewing time for a respondent in the qualitative study ranged between 35 and 50 min. The language used for the interview was left to the respondent’s choice between Krobo, the indigenous language in the LMKM, Twi, the indigenous language spoken the most in Ghana, and English, Ghana’s official language due to its colonial past. With prior permission from the respondents, the interviews were audio recorded.
This paper uses the qualitative data from the project. The original sample of 38 for the initial qualitative data was primarily informed by previous literature on the need to reach saturation in qualitative interviews [ 20 , 42 , 45 , 46 ] which was achieved subjectively after interviewing about 10 respondents in each of the two study sites [ 20 ]. Although thematic saturation is usually achieved with 30 qualitative participants [ 20 , 45 , 46 ], 10 respondents gave us thematic saturation at each VCT, because they were quite homogenous [ 20 ]. Previous studies [ 20 , 38 , 42 , 45 , 47 ] have defined thematic saturation as recurrence or repetitiveness of responses from qualitative respondents. Despite reaching saturation with a combined 20 respondents from both VCT centres, we continued interviewing up to 38 of them to meet an initial objective of spreading respondent selection over a month [ 40 , 42 ], with the hope of having some variety of responses due to the case study nature. The sample of 26 for this paper were selected from the 38 because the rest did not respond to the primary question on how they experienced the new diagnoses of their HIV-positive status (see [ 2 ]).
Ethical clearance and data quality approaches
Ethical clearance was sought from the Ethical Committee for Humanities at the University of Ghana, Legon ( ECH 017/14–15 ) , and the Ethical Review Board of the Memorial University at St. Johns, Newfoundland, Canada. Permissions were also sought from the Ethical Review Committee of the Ghana Health Service (GHS-ERC: 02/11/14) for using institutions under its jurisdiction for the study, as well as from the Eastern Regional Directorate of the Ghana Health Service. The District Health Management Directorate of the LMKM and the administrators of the study hospitals responded to the written permissions with verbal permissions to undertake the study. The respondents were informed of the objectives of the study, assured of confidentiality, and their informed consent was sought. They were also informed that their participation was voluntary and they would not be penalised if they opted to turn down the interview at any time during the process.
One person declined to be interviewed, citing time constraints. No identifiable markers were used for the respondents (see [ 20 , 39 , 40 , 42 ]).
Data analysis
The data were transcribed verbatim, and reviewed by the author and team of transcribers to ensure accuracy. The adequacy of translation from the local languages to English was ascertained by holding several meetings between the author and the transcribers to interrogate the correct translations forth and back till a mutual agreement was reached on the correct translations.
Based on the deductive approach, the data analysis was done focusing on the objectives of the study [ 40 ]. Qualitative thematic content analysis, which enables interrogating narratives from all cases studied for a combined result [ 20 , 40 , 42 , 48 ], guided generating themes and subthemes for this paper. In this paper, the qualitative thematic content analysis used also qualifies to be called a discourse or conversation analysis [ 49 , 50 ]. It used a qualitative thematic coding [ 50 , 51 ] of the different interviews/conversations with the respondents and observed the three key features of qualitative content analysis described by Schreier [ 50 ]. These are reducing the data, systematically analysing them, and applying flexibility in the analysis. The systematic analysis meant carefully examining every single aspect of the transcribed data and systematically describing their meaning [ 50 , 52 ], as well as comparing and relating different parts of the data to one another [ 50 ]. NVivo version 11 professional software [ 53 ] was used to identify themes and sub-themes relevant to the objectives of the study and other relevant information. Again, back and forth meetings were held between the author and the team of two coding assistants, who coded the data independently, to mutually agree on their meaning and context.
These repeated processes of reviewing the data enhance intercoder reliability subjectively and improve the credibility/validity and reliability of qualitative data [ 40 , 42 , 54 ].
Based on previous research [ 2 , 11 , 34 ] respondents’ experiences of the new diagnoses of their HIV-positive status were adjudged as negative if the respondent either expressed shock, disbelief, worry, fear/panic, felt the HIV infection would kill him/her, or immediately contemplated suicide. Respondents’ experiences of their new diagnoses were classified as resigned acceptance if they readily accepted the new diagnoses and/or showed some optimism instantly that they could survive the infection. Finally, their experiences of the new diagnoses were considered resigned neutral when the respondent indicated he/she showed no emotion upon receiving the first news of his/her HIV-positive status (Fig. 1 & Table 2 ).
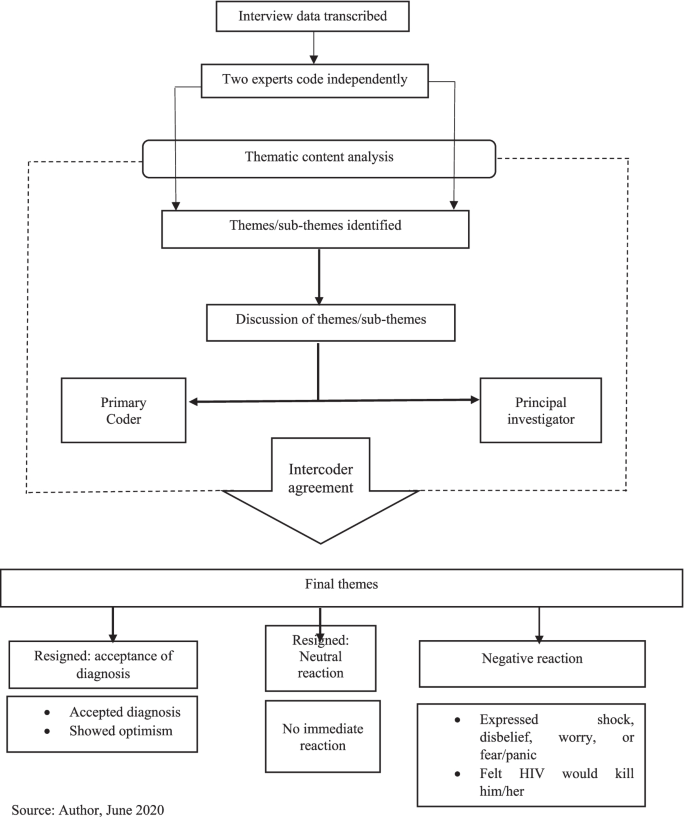
Data analysis workflow
Reflexivity
Reflexively, the author’s cultural and theoretical population health orientation admittedly had some influence on aspects of the research process, although these were minimal. These included the choice of the title. Being fully aware of the extent of stigmatisation culturally associated with HIV/AIDS in Ghana [ 20 , 40 , 42 ], the PIs of the project from which data for this paper emanates were curious about how respondents would experience the new diagnoses with the infection.
Nevertheless, given the author’s extensive training in the dogma and ethics of scientific research, particularly regarding the need for value-neutrality and objectivity [ 54 ], the author’s cultural and theoretical backgrounds did not otherwise influence the data analysis and interpretation. This was applicable to most of the research process as well.
Socio-demographic characteristics of respondents
The respondents’ ages ranged from 25 to 68 years old. They were mostly females (80.77%, n = 21), primarily indigenous Dangmes (84.62%, n = 22), and generally had low socio-economic status. Also, they were mostly single (separated/widowed after cohabiting/never married/widowed), and they had been officially diagnosed HIV-positive for a range of six months to 12 years (Table 3 ).
Thematic findings
Experiences of new diagnoses of hiv/aids.
Table 2 summarises the experiences of new diagnoses among the respondents, upon hearing of their HIV seropositive status. There were negative and resigned reactions. Verbatim responses are identified with the type of hospital the respondent used: government or quasi-government.
Negative reactions
The data showed that the vast majority of the respondents seemed to have been very hard hit and traumatised by the news of HIV-positive test result, and expressed very negative sentiments. For these, the experiences of new diagnoses ranged from disbelief, to being very disturbed. Few mentioned the thought of killing themselves, incessant crying, and feeling that they would die from AIDS. Other reactions included being very frustrated, very surprised, very frightened, deep shock, deep worry, being heartbroken/devastated, self-blame, and avoiding going to hospital till conversion to AIDS (Table 2 ):
“ It was very hard for me when I first heard it; in fact, I cried and cried.” ( Government hospital).
“In fact I was very frustrated. I even decided to take poison so that I will die before the sickness [HIV] becomes worse.” (Government hospital).
“I was very surprised and shocked when I was first told I had this disease. It was very heart-breaking.” (Government hospital).
Resigned reactions
On the contrary, few respondents showed resignation towards the bad news. These comprised three who readily accepted the diagnosis and two who seemed neutral to it. From their responses, respondents who accepted their diagnosis seemed to have readily and consciously accepted/embraced the diagnosis of HIV-positive and encoded it rather smoothly as part of their identity.
“When I was told I was not perturbed because I knew people who were also taking the drug” [ARVs]. (Quasi-government hospital).
The very few respondents who seemed to have been neutral to their new diagnosis of HIV/AIDS seemed to have continued with their lives seamlessly on the spare of the moment they received the news, and did not take a break to react to the news of being HIV-positive.
“I was ill for long time so when I was told about it I only continued to take medications.” (Government hospital) .
Further analysis indicated that these experiences of the new diagnoses were influenced by certain background situations. These are discussed in the next section.
Factors influencing experiences of the new diagnoses
For the very few respondents whose new diagnoses experiences were neutral, being ill for a long time before the HIV-positive diagnosis, and accepting that sickness is inevitable and could happen anytime, were what influenced their experiences of the new HIV-positive diagnoses:
“ … as for sickness, it’s sickness; you have less control over sickness, so I didn’t really do anything.” (Quasi-government hospital).
Two of the few respondents who readily accepted their new diagnosed HIV-positive status said they experienced persistent comorbidities from the HIV infection. Resultantly, they voluntarily went to the VCTs to test for their HIV status:
“I started falling sick and strange rashes kept appearing on my skin, so when I took it to the hospital, I was told it was the virus.” (Government hospital).
“I frequently fell sick … and it was not getting better so I came to the hospital myself … I came (willingly) to them to be tested when I realised I have changed. So they were even happy I walked to them myself to be tested.” (Quasi-government hospital).
Including two multiple responses, the vast majority who responded negatively to the experiences of new diagnoses of HIV-positive status, mentioned four main situations that informed their experiences of the new diagnoses. Mostly, they suffered comorbidities from their infection.
“For about seven months I was not feeling well. It even got to a point I lost consciousness. All forms of tests were ran on me … but they could not find exactly what was wrong...My boss advised me … so I went to the hospital for the test and the nurses said I was HIV-positive.” (Government hospital).
Next, nearly half of the respondents who reacted negatively said a spouse/partner was HIV-positive and thus knew they had contracted the infection from them, and/or due to that, health personnel tested them also for the infection. Importantly, half of these PLWHAs did not know their partners’ HIV status prior to respondent’s diagnosis.
“ I was staying with my husband by then but he died so it was after he died that the doctors realised that it was this disease he died of … afterwards I was tested and diagnosed with this disease.” (Government hospital).
Similar to those who readily embraced their diagnosis, a few of those whose new diagnoses experiences were negative were tested voluntarily based on their sexual partner’s infection. Likewise, several participants whose new diagnoses experiences were triggered by the onset of comorbidities said they voluntarily went for the testing. Additionally, for a slim minority of the latter, their conviction to go for the VCT was based on mass media (often television) announcements and discussions on the signs and symptoms of HIV/AIDS.
“Already I suspected that my husband had all the symptoms that are discussed on TV and radio about this disease. He fell sick often and also coughed most of the time.” (Government hospital).
“Even though I wasn’t falling sick, I came to be tested willingly after they announced everybody should get tested … .” (Government hospital).
“… I used to fall sick frequently and that was when a lot of noise and adverts were made about this disease on television and radio...I went to the St. Martin’s hospital and told the doctors about my situation and he asked me go for an HIV test and that was when I got to know I had this disease.” (Quasi-government hospital ).
Finally, few (including two who gave multiple responses—primarily that a spouse/partner was HIV-positive) mentioned that they got tested when they were pregnant.
“I was about to give birth to my second born … my husband is also HIV-positive.” (Government hospital ).
“I was diagnosed when I was pregnant when my second born and I visited the hospital … Yes, [my husband is HIV-positive].” (Government hospital).
Factors that facilitated transitioning to accept HIV-positive self
For the few respondents who showed resignation towards the experiences of their new HIV-positive diagnoses, there was no need for transitioning to accepting an HIV-positive self. Two of these respondents who readily embraced the new HIV-positive diagnoses said they already knew persons who were HIV-positive and were taking antiretroviral medications (ARVs):
“I realised I was not the only victim; many people are also victims …” (Quasi-government hospital ).
Two others said they already had comorbidities from AIDS and started treatment right after diagnoses:
“ I was ill for a long time so when I was told about it I only continued to take medications.”
(Government hospital ).
Another participant thought sickness is inevitable and thus did not need to worry about such diagnosis.
Nearly one-third of the respondents whose experiences of the new HIV-positive diagnoses were negative gave no response regarding what influenced their transitioning to accepting their new HIV-positive diagnoses. They mostly felt uncomfortable/reluctant talking about it. The rest mentioned what facilitated their transitioning more spontaneously and/or more readily after some amount of probing. The vast majority of the rest who experienced negative reactions said the most important help with their transitioning was counselling from health workers who encouraged them to initiate and continue treatment, with the assurance that if they did so, they would survive the infection.
“… After I came here [VCT] and was advised and encouraged to see something to live for, I have been okay. They [health personnel] have been very friendly and encouraging. In fact, they have helped me a lot .” (Government hospital).
“ They [health personnel] counsel us and tell us the fact that we have this virus does not mean our world has come to an end.” (Government hospital).
“… After being put on medication, going through counselling and tests, and being told what to do, I was hopeful that if you adhered to the medication you could live long.” ( Quasi-government hospital).
For one of these, her mother-in-law was the main person who empathised with her:
“I was really worried and disturbed but the encouragement and advice from the nurses and my mother-in-law … has helped me. After I told her [mother-in-law] I had been diagnosed … she brought me here [VCT] to introduce me [to a nurse] , so she has been helpful.” (Government hospital).
The second main issue that facilitated the transitioning for few of the respondents was their awareness of the current medical advancement in the treatment of HIV infection, due to which “you would live long” ( Quasi-government hospital) despite the infection:
“… because they have drugs to treat it, I was OK. Provided it will not cut short my life span I am happy.” ( Quasi-government hospital).
“In the olden days when there were no drugs you thought you would die … after being told, so you become afraid, but now we know there are drugs available so if you are able to take your drugs you don’t have any problem.” ( Quasi-government hospital).
Three respondents alluded to accepting medical diagnoses, facing reality, and living by Biblical principles as facilitating their acceptance of their new diagnoses.
“ I accepted it because you can’t deny what a doctor says” ( Quasi-government hospital).
“I was disturbed but I thought to myself that it had already happened ” ( Quasi-government hospital) .
“I didn’t kill myself because I am a Christian and the Bible speaks against that. I forgot about everything and decided to keep coming for the medications and now by the grace of God I have lived for over twelve years .” ( Government hospital).
There was, however, a lone-voice who said she had still not settled down to the reality of being HIV-positive after having been diagnosed three years prior and seeking treatment for one-year post diagnosis. Thus, she has not been consistent in getting treatment and is already feed up with seeking treatment:
“It seemed to be untrue. From time to time I stopped taking the medication … I am fed up with coming to seek treatment. I have been treating the sickness for over a year but the symptoms recur after I see the doctor.” ( Quasi-government hospital).
This lone-voice mentioned having severe comorbidities which were probably due to starting healthcare for HIV two years after diagnosis. She also mentioned experiencing extreme discrimination and ostracisation both at home and in public, which no doubt, are linked to her comorbidities.
This section discusses the findings of the study, with a bearing on the tenets of the Hopelessness Theory of Depression. Fear has been associated with HIV since its discovery in the 1980s. Fear is mostly fueled by misconceptions associated with the virus which is mostly linked to death and stigmatisation [ 55 ]. Grounded on fear of death and a feeling of helplessness, a wide range of reactions are exhibited upon diagnosis or disclosure of an individual’s HIV-positive status. Although these reactions are both from the individual in question and significant others of this individual, the literature has mostly focused on the reactions of the persons affiliated with these individuals after disclosure [ 56 , 57 ]. Indeed, very few studies explore the individual’s reactions upon diagnosis or testing positive for the virus [ 55 , 58 ]. Based on this drawback and the known public health significance of such immediate reactions to HIV-positive diagnosis, this paper examined the experiences of new diagnoses of Ghanaian PLWHAs after hearing the “bad news.”
Importantly, a critical examination of differences between participants from each of the two hospitals studied shows that 11 respondents from the government hospital had negative reactions to the new experience of their HIV/AIDS diagnosis while ten in the quasi-government hospital did so. On the other hand, the experiences of two respondents from the quasi-government hospital was adjudged as “Resigned: accepted diagnosis” while one participant from the government hospital showed a ‘Resigned: accepted diagnosis.” There were no other differences in the participants’ reactions between the two study sites (Table 2 ). Rather, the difference between the respondents basically stemmed from the influences on their experiences of the new HIV-positive diagnoses discussed earlier (Table 2 ), which did not have a relationship with the particular hospital they received healthcare from. While previous researchers have articulated the lack of major differences in the findings from the respondents used for the qualitative study in the project [ 39 , 41 ], they have further clarified that the respondents’ similar socio-bio-demographic background is mostly responsible for the lack of major differences in the findings [ 20 , 39 , 41 ].
The findings from this study have corroborated those of previous authors that receiving the news of newly-diagnosed HIV-positive status is often met with reactions that are “complex and multi-faceted” ([ 2 ], p. 12). Additionally, the findings that respondents had varied responses and a myriad of experiences to the new diagnoses of their HIV-positive status is in line with reports from several studies (example: [ 2 , 58 ]). Most of the initial reactions of respondents in this study were very traumatising and discouraging. These included a few who contemplated suicide upon hearing the news; supporting Fabianova’s [ 23 ] findings in Nairobi.
Similar to previous research [ 55 , 58 ], findings from this study indicate that though negative emotional and psychological reactions may occur upon learning of an HIV-positive status, respondents may also resign themselves to their fate—to accept or numb their feelings about the new disclosure of their HIV+ diagnoses. Likewise, this study found that respondents who were already exposed to HIV/AIDS needed no transitioning to self-acceptance of their HIV-positive status. This corroborates previous findings [ 55 ]. Nearly all the respondents settled down later, after experiencing the new diagnoses, initiated and continued with their healthcare for HIV/AIDS. This affirms previous assertions that such initial feelings regarding information on a newly-diagnosed HIV-positive status mostly fade away eventually [ 3 , 23 ].
Again, the paper corroborates previous findings and highlights the buffering role of healthcare providers in moderating the experiences of new diagnoses of HIV-positive persons [ 1 , 11 ]: the vast majority of the respondents who adapted to their HIV-positive status attributed it to counselling and support from healthcare personnel. This role of the healthcare workers in aiding respondents’ transition to accepting their HIV-positive selves affirms the documented importance of social support in ameliorating the otherwise negative effects of experiencing a health trauma [ 23 , 59 ]. Research has identified HIV counsellors’ or health providers’ choice of words and emotions as crucial in determining peoples’ reaction to the initial diagnosis of HIV infection [ 55 , 58 ]. When these emotions were hopeful and assuaging, patients were more likely to be calm and comforted, and vice versa [ 58 ].
The findings of this study underscore the fact that PLWHAs who experience new HIV-positive diagnosis will need to receive such interventions early [ 12 ]. These interventions are needed to give them hope in life to abate extreme psychosocial trauma that can be associated with experiences of new HIV-positive status [ 11 , 60 ]. Per the findings of this paper, such interventions should include educating PLWHAs experiencing new HIV-positive diagnosis that new medical advances for HIV-positive infection make it possible for PLWHAs experiencing new HIV-positive diagnosis to lead normal lives and live long, if they seek early treatment and adhere to prescribed healthcare.
A fair number of the respondents mentioned being infected by their spouses/partners who were alive or deceased (also see [ 40 ]), implying that they probably were not using condoms and other modes to prevent HIV transmission from their sexual partners. This finding is not unlikely considering that Owusu [ 42 ] found that the PLWHAs studied were hardly using any form of protection against HIV with their sexual partners, whether in stable or unstable relationships. Two respondents were exceptions--they used condoms, but inconsistently [ 40 ]. Furthermore, Owusu [ 40 ] found that the unmarried or non-co-habiting PLWHAs had not disclosed their HIV-positive status to their sexual partners, with the exception of one respondent. PLWHAs who fail to disclose their status to their partners may have been living in a state of denial and or may fear/have feared the ramifications of disclosing their status. They may thus refuse to disclose as a way of attenuating the anticipated effect of disclosing, as previous authors have attested to [ 12 , 15 , 23 ].
Additionally, findings from this paper illuminate the importance of voluntary counseling and testing. Majority of the respondents mentioned having had comorbidities before they tested for their HIV-positive status. Other researchers have indicated that anticipated reaction influences decisions on voluntary testing for HIV [ 55 , 58 ]. Previous research clearly notes potential barriers to voluntary testing for HIV in Ghana. These include the fear of stigma, discrimination and abuse, and possible dissolution of romantic relationships associated with being a PLWHA. Importantly, there is fear of the perception that an HIV-positive diagnosis is a death warrant [ 16 , 61 ]. Antenatal-linked VCT is a policy strategy in Ghana for HIV control through a nationwide integration of VCT and antenatal care [ 62 ]. However, clients may disagree to it.
The findings from this paper mostly tally with the Hopelessness Theory of Depression’s core proposition; the respondents overwhelmingly perceived the experiences of their new HIV-positive status as translating into unwelcome consequences and negative inferences, as did respondents in other SSA settings [ 12 , 23 ]. This plausibly led to depression and feelings of hopelessness among them. Also, this perspective may have underlain the self-blame by a few of them for being HIV-positive [ 28 , 29 ]. Furthermore, this paper confirms the Hopelessness Theory of Depression’s propositions that underlying perceived bad situations and experiences which are attributed to internal factors lead to depression. Conversely, those ascribed to presumed external factors/influences give comfort/are reassuring [ 4 ]. In this study, the experiences of new HIV-positive diagnoses were mostly negative for respondents with internal factors such as having comorbidities and having a spouse/partner with HIV/AIDS.
In consonance with Peterson’s and Seligman’s [ 4 ] Hopelessness Theory of Depression’s propositions, respondents of this study said that external factors motivated them to take commendable actions such as going for VCT. These included the influence of health education through mass media, counselling by healthcare practitioners, and information regarding modern medical advancements which can help PLWHAs live without comorbidities, and possibly survive the infection. Furthermore, these factors facilitated their adaptation to their HIV-positive selves.
Nevertheless, this paper does not substantiate Peterson and Seligman’s [ 4 ] Hopelessness Theory of Depression fully. Contrary to their proposition, in this study, fewer respondents mentioned that a series of external factors such as knowing someone who has HIV/AIDS, and being given a near-mandatory HIV test at an antenatal clinic were what influenced their negative reactions. Conversely, other internal factors such as having comorbidities and having an HIV-positive romantic partner influenced few of the respondents to positively adapt to their HIV-positive diagnoses. In this study, therefore, the clear diathesis of external factors giving psychosocial comfort and internal factors unleashing mental discomfort in response to hearing the often unwelcome first news of being HIV-positive was not fully supported. This was also true of Assen et al.’s [ 12 ] study in Ethiopia.
Hence, this paper corroborates Govender and Schlebusch’s [ 31 ] synthesis of the Hopelessness Theory of Depression. These authors emphasise that the numerous internal and external challenges that face PLWHAs such as discrimination, stigmatisation, abuse, financial, marital, and healthcare challenges, among others, may [also combine to] have connotations for the loss of control over one’s life, fear of the future, and feeling of helplessness. As well, they may underlie the negative inferences which exacerbate the feeling of hopelessness and increase the likelihood of depressive symptoms stemming from experiencing new diagnosis of HIV/AIDS.
This paper adds to the knowledge on the personal and public health effects of experiencing new HIV-positive diagnosis. Particularly, it highlights the commendable public health effects of receiving new diagnosis for HIV-positive status and illuminates the role of social support in seeking and continuing healthcare for the diagnosis. Also, it attests to the role of healthcare workers and behaviour change communication, using mass media, in fighting the menace of HIV/AIDS. Furthermore, as articulated above, this paper did not fully support the conclusions of Peterson’s and Seligman’s [ 4 ] Hopelessness Theory of Depression’s framework. Rather, the paper contributes additional information to it in the form of an anti-thesis. Additionally, unlike Fabianova’s [ 23 ] study in Nairobi, this study did not find that the respondents acted with aggression towards counsellors who first broke the news of their HIV-positive status to them.
More importantly, this paper has navigated new frontiers in the body of knowledge in its thematic area of study. First, contrary to Fabianova’s [ 23 ] findings that some of her respondents attempted suicide, non-of the respondents in this study mentioned having attempted to take their lives, although a few of them revealed they had suicidal ideation. This may be due to the fewer respondents this study engaged as well as its cross-sectional design, compared to Fabianova’s [ 23 ] respondents, and the longitudinal approach to her study. Second, this study has newly articulated outstanding information on factors which facilitate PLWHAs’ transitioning to accepting and settling down to their experiences of new HIV-positive status. Third, unlike previous literature, this study has uniquely found that having comorbidities from HIV/AIDS was the primary reason that influenced the respondents to voluntarily test for their HIV-positive status. The uniqueness of this finding may be linked to the awareness that VCT of HIV/AIDS status is very rare in Ghana [ 63 , 64 ].
Limitations
The qualitative nature of this study better facilitates unearthing the complexities associated with experiences of new diagnoses among HIV-positive persons and learning about the adaptation process [ 2 ]. Also, the repeated data analyses this paper employed strengthens its reliability and validity. However, when interpreting these findings, some limitations should be considered. With HIV-positive status being a very sensitive issue, and highly stigmatised in Ghana [ 16 , 65 , 66 ], social desirability of responses may have influenced the findings [ 2 ]. Being a retrospective study, recall bias may also affect the reliability of the responses [ 2 , 20 ]. This is with particular reference to the time lapse between the moment a respondent was newly-diagnosed with HIV/AIDS, as operationalised above, and the time of the study. Recruiting individuals further away from their diagnosis is a limitation; it could lead to recall bias. Furthermore, the study is cross-sectional and does not permit inference of causality [ 67 ]. Additionally, being a qualitative study with a fairly small sample size, the findings are not generalisable to non-respondents in the LMKM and also, Ghana as a whole [ 67 ].
Omona ([ 68 ], p. 181) posits that although random sampling is rare in qualitative studies, they are not out of place as they facilitate sampling the “desired number of individuals …” from a generated sampling frame, which he asserts improves the credibility of the sample (also see [ 69 ], p. 28). Yet, as hinted previously in this paper, random sampling in qualitative studies could pose some limitations. A careful search of the literature does not specify such limitations. However, the author guesstimates that random sampling in qualitative studies may rob the data of the quality of focusing on some key persons/texts of interest to the study which convenience sampling will provide. Lastly, based on the primary focus of the project, this paper did not undertake a diagnostic assessment for depression among the respondents. The paper is thus unable to ascertain if depression contributed to experiences of the new diagnoses of HIV-positive status among the PLWHAs studied.
Evidence elsewhere suggests that upon receiving the initial news of an HIV-positive diagnosis, most people have strong psychosocial reactions [ 23 , 55 , 70 ]. Such reactions are also known to have very critical public health implication [ 1 , 2 , 14 ]. Yet little research has focused on the initial reactions of newly-diagnosed PLWHAs in SSA, particularly Ghana. Neither has the implications of their reactions for personal and public health been extensively studied [ 2 , 11 ]]. This makes this study of the experiences of new HIV-positive diagnoses very timely. The personal and public health implications of one’s experience of new diagnosis of HIV-positive status is critically important for Ghana, which has a generalised HIV-positive infection [ 21 ].
Consistent with the literature, the vast majority of the respondents became extremely traumatised and immobilised when they experienced new diagnoses of their HIV-positive status. A few, however, more readily resigned to their HIV-positive identity. Regardless of their experiences of new diagnoses of HIV/AIDS, having comorbidities prior to diagnosis influenced their experiences the most. This was followed by having/having had a spouse/partner especially, and/or knowing someone who was HIV-positive, prior to their diagnoses. Next, respondents mentioned being influenced by health education through mass media, TV particularly, on signs and symptoms of HIV/AIDS. Importantly, health education, counselling, reassurance and empathy from healthcare workers provided hope. Furthermore, these facilitated their transitioning to settling down to self-acceptance of an HIV-positive status and continuing with healthcare. Finally, this paper concludes that to a large extent, the findings are applicable to the tenets of the Hopelessness Theory of Depression used as a framework for this study. Consequently, this paper found hopelessness as an important driving force to negative reactions towards one’s experience of his/her new HIV-positive diagnosis.
Conclusions from this paper have several public health significances. It highlights the continuous need for and strengthening of behaviour change communication on HIV/AIDS by the Ghana AIDS Commission and Ghana Health Service. This should emphasise its signs and symptoms, the need to seek early treatment, and adherence to prescribed ARVs. Strengthening the use of mass media, small groups, schools, churches/mosques and person-to-person channels in such endeavours is important. HIV/AIDS-related health promotion and education should also continue to emphasise prevention, but importantly, state that once infected, HIV/AIDS can be controlled; the infected person can live without comorbidities and need not succumb to the infection. Stakeholders should work harder towards educating residents in the LMKM, and for that matter, Ghanaians generally, about voluntary testing of HIV status. Most of the respondents in this study mentioned having had comorbidities from HIV/AIDS prior to their new diagnoses. Onset of comorbidities for HIV/AIDS prior to diagnosis and treatment can make the treatment expensive; it can also diminish the chances of surviving the infection severely [ 71 , 72 ].
Given the high rates of HIV/AIDS in the study district and Region, the health education should also emphasise the need for persons whose sexual partners are HIV-positive—particularly those who show signs and symptoms of the infection, and all who engage in at-risk sex, to practice safer sex. Finally, the paper recommends increased social support and empathy for PLWHAs in LMKM particularly and in Ghana generally, from family, friends, neighbors, community leaders, healthcare professionals, and organised groups such as members of their religious affiliation, if any [ 20 , 40 , 42 ]. This study has unearthed social support as a critical moderating element in the transitioning of PLWHAs to integrating their HIV-positive self-concept, initiating, and adhering to prescribed healthcare. Families, social groups, and healthcare professionals should empathise with PLWHAs.
Availability of data and materials
The datasets used and/or analysed during this study are available from the author on reasonable request. The key questions for the study have been provided in the paper, in Table 1 . The complete question guide used to collect the data has also been provided online as Additional file 1 .
Abbreviations
Acquired immune deficiency syndrome
Anti-retroviral therapy
Antiretroviral medications
Common mental health disorders
Ethics Committee on Humanities
Ethical Review Committee
Ghana AIDS Commission
Ghana Health Service
Ghana Statistical Service
Human immunodeficiency virus
Lower Manya Krobo Municipality
Principal Investigator
Persons living with HIV/AIDS
Research Assistants
Sub-Saharan Africa
University of California San Francisco
Voluntary counselling and testing
Chu C, Selwyn PA. Diagnosis and initial management of acute HIV infection. Am Fam Physician. 2010;81(10):1239–44.
PubMed Google Scholar
Kutnick AH, Gwadz MV, Cleland CM, Leonard NR, Freeman R, Ritchie AS, et al. It’s a process: reactions to HIV diagnosis and engagement in HIV care among high-risk heterosexuals. Front Public Health. 2017;10(5):100. https://doi.org/10.3389/fpubh.2017.00100 .
Article Google Scholar
University of California San Franscisco. Coping with HIV/AIDS: Mental Health. HIV in Site Project: the UCSF Center for HIV Information. 2018. http://hivinsite.ucsf.edu/insite?page=pb-daily-mental . Accessed 2 May 2018.
Google Scholar
Peterson C, Seligman ME. Causal explanations as a risk factor for depression: theory and evidence. Psychol Rev. 1984;91(3):347. https://doi.org/10.1037/0033-295X.91.3.347 .
Article CAS PubMed Google Scholar
Alford K, Banerjee S, Nixon E, O’Brien C, Pounds O, Butler A, et al. Assessment and management of HIV-associated cognitive impairment: experience from a multidisciplinary memory service for people living with HIV. Brain Sci. 2019;9(2):37. https://doi.org/10.3390/brainsci9020037 .
Article PubMed Central Google Scholar
Stein DJ, Seedat S, Emsley RA, Olley BO. HIV/AIDS in Africa—a role for the mental health practitioner? S Afr J Psychiatry. 2005;95(3):167–8.
Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. 2014;5(6). https://doi.org/10.4172/2155-9899.1000276 .
Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Sawires S, et al. Missed opportunities for HIV testing and late-stage diagnosis among HIV-infected patients in Uganda. PLoS One. 2011;6(7). https://doi.org/10.1371/journal.pone.0021794 .
Nairaland Forum. A female doctor commits suicide after testing positive to HIV. 2011. http://www.nairaland.com/720421/female-doctor-commits-suicide-after . Accessed 25 May 2018.
Hosek SG, Lemos D, Harper GW, Telander K. Evaluating the acceptability and feasibility of project ACCEPT: An intervention for youth newly diagnosed with HIV. AIDS Educ Prev. 2011;23(2):128–44. https://doi.org/10.1521/aeap.2011.23.2.128 .
Article PubMed PubMed Central Google Scholar
Moitra E, Chan PA, Stein MD. Open trial of an acceptance-based behavior therapy intervention to engage newly diagnosed HIV patients in care: rationale and evidence of feasibility and acceptability. Behav Modif. 2015;39(5):670–90. https://doi.org/10.1177/0145445515590977 .
Assen A, Molla F, Wondimu A, Abrha S, Melkam W, Tadesse E, et al. Late presentation for diagnosis of HIV infection among HIV positive patients in South Tigray zone, Ethiopia. BMC Public Health. 2016;16(1):558. https://doi.org/10.1186/s12889-016-3263-y .
Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyper-endemic settings. AIDS (London, England). 2010;24(5):729–35. https://doi.org/10.1097/QAD.0b013e32833433fe .
Pettifor A, MacPhail C, Corneli A, Sibeko J, Kamanga G, Rosenberg N, et al. NIAID center for HIV/AIDS vaccine immunology. Continued high risk sexual behavior following diagnosis with acute HIV infection in South Africa and Malawi: implications for prevention. AIDS Behav. 2011;15(6):1243–50. https://doi.org/10.1007/s10461-010-9839-0 .
Skinner D, Mfecane S. Stigma, discrimination and the implications for people living with HIV/AIDS in South Africa. SAHARA J. 2004;1(3):157–64.
Article CAS Google Scholar
Koka E, Ahorlu CK, Agyeman DK. Social death through HIV and AIDS stigmatization and discrimination in Ghana: a case study of the central regional hospital, Cape Coast, Ghana. Advanc Appl Sociol. 2013;3(06):231–6.
Miller WC, Rosenberg NE, Rutstein SE, Powers KA. The role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):277–82. https://doi.org/10.1097/COH.0b013e32833a0d3a .
Gwadz M, de Guzman R, Freeman R, Kutnick A, Silverman E, Leonard NR, et al. Exploring how substance use impedes engagement along the HIV care continuum: a qualitative study. Front Public Health. 2016;4:62. https://doi.org/10.3389/fpubh.2016.00062 .
Lund R, Agyei-Mensah S. Queens as mothers: the role of the traditional safety net of care and support for HIV/AIDS orphans and vulnerable children in Ghana. Geo J. 2008;71(2–3):93–106. https://doi.org/10.1007/s10708-008-9145-9 .
Owusu AY, Laar A. Managing HIV-positive sero-status in Ghana’s most HIV concentrated district: self-perceived explanations and theoretical discourse. Afr J AIDS Res. 2018;17(1):82–90. https://doi.org/10.2989/16085906.2017.1419268 .
Article PubMed Google Scholar
GAC. Summary of the 2016 sentinel survey report. Accra: GAC; 2017. http://www.ghanaids.gov.gh/gac1/aids_info.php . Accessed 10 Jan 2018
Visser MJ, Makin JD, Vandormael A, Sikkema KJ, Forsyth BW. HIV/AIDS stigma in a south African community. AIDS Care. 2009;21(2):197–206.
Fabianova L. Psychosocial aspects of people living with HIV/AIDS. In Social and psychological aspects of HIV/AIDS and their ramifications 2011. IntechOpen. doi: https://doi.org/10.5772/21148 . https://www.intechopen.com/books/social-and-psychological-aspects-of-hiv-aids-and-their-ramifications/psychosocial-aspects-of-people-living-with-hiv-aids . Accessed 14 Oct 2019.
Patel V, Stein DJ. Common mental disorders in sub-Saharan Africa: the triad of depression, anxiety and somatization. In: Akyeampong E, Hill A, Kleinman A, editors. The culture of mental illness and psychiatric practice in Africa. Bloomington: Indiana University Press; 2015. p. 50–72. http://www.jstor.org/stable/j.ctt16gz69f.6 . Accessed 26 Jun 2019.
Miranda JJ, Patel V. Achieving the Millennium Development Goals: does mental health play a role? PLoS Med. 2005;2(10):e291.
Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS (London, England). 2006;20(12):1571–82.
Abela JR. The hopelessness theory of depression: a test of the diathesis–stress and causal mediation components in third and seventh grade children. J Abnorm Child Psychol. 2001;29(3):241–54.
Abela JR, Payne AV. A test of the integration of the hopelessness and self-esteem theories of depression in schoolchildren. Cogn Ther Res. 2003;27(5):519–35. https://doi.org/10.1023/A:1026303020478 .
Waszczuk MA, Coulson AE, Gregory AM, Eley TC. A longitudinal twin and sibling study of the hopelessness theory of depression in adolescence and young adulthood. Psychol Med. 2016;46(9):1935–49. https://doi.org/10.1017/S0033291716000489 .
Seligman ME. Helplessness. On depression, development and death. San Francisco: Freeman; 1975.
Govender RD, Schlebusch L. Hopelessness, depression and suicidal ideation in HIV-positive persons. S Afr J Psychiatry. 2012;18(1):16–21. https://doi.org/10.4102/sajpsychiatry.v18i1.302 .
Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42(6):861 52. GSS. Population and Housing census 2010. Final results. Accra: GSS.2012.
Schlebusch L, Govender RD. Elevated risk of suicidal ideation in HIV-positive persons. Depress Res Treat. 2015;2015. https://doi.org/10.1155/2015/609172 .
Kylmä J, Vehviläinen-Julkunen K, Lähdevirta J. Hope, despair and hopelessness in living with HIV/AIDS: a grounded theory study. J Adv Nurs. 2001;33(6):764–75. https://doi.org/10.1046/j.1365-2648.2001.01712.x .
GSS. Population and Housing census 2010. Final results. Accra: GSS; 2012.
GSS. Population and Housing Report: District Analytical Report 2010, Lower Manya Krobo Municipal. 2014. http://www.statsghana.gov.gh/docfiles/2010_District_Report/Eastern/LOWER%20 MANYA%20KROBO.pdf. Accessed 14 Jul 2019.
Yin RK. Case study research: design and methods. Thousand Oaks, SAGE 2013;14(1):69–71.
Baxter P, Jack S. Qualitative case study methodology: study design and implementation for novice researchers. Qual Rep. 2008;13(4):544–59.
Tenkorang EY, Owusu AY, Laar AK. Housing and health outcomes of persons living with HIV/AIDS (PLWHAs) in the lower Manya Krobo district, Ghana. J Health Care Poor Underserved. 2017;28(1):191–215. https://doi.org/10.1353/hpu.2017.0017 .
Owusu AY. A gendered analysis of living with HIV/aids in the eastern region of Ghana. BMC Public Health. 2020;20:751. https://doi.org/10.1186/s12889-020-08702-9 .
Tenkorang EY, Owusu AY, Laar AK, Yeboah EH. Housing, psychosocial and adherence counseling among HIV+ persons in Ghana. Health Promot Int. 2019;34(2):204–14. https://doi.org/10.1093/heapro/dax072 .
Owusu AY. Social contexts of living with HIV/AIDS in the eastern region of Ghana. Istanbul Univ J Sociol. 2019;39(2):425–54. https://doi.org/10.26650/SJ.2019.39.2.0109 .
NACP. HIV sentinel survey report. Accra: Ghana Health Service/Ministry of Health; 2018. p. 2019.
Owusu, A. Y. Gender, Adolescents, and Achieving Sustainable Development Goals in Ghana. In A. Williams & I. Luginaah (Eds.). Gender Matters Globally: Geography, Health and Sustainability (Chapter 4). Routledge. Forthcoming. ISBNS: Hardback: 9780367743901; Ebook: 9780367743918; Future paperback: 9780367743925.
Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
Mason M. Sample size and saturation in PhD studies using qualitative interviews. In Forum qualitative Sozialforschung/Forum: qualitative social research 2010;11(3).
Sedziafa AP, Tenkorang EY, Owusu AY. “… he always slaps me on my ears”: the health consequences of intimate partner violence among a group of patrilineal women in Ghana. Culture Health Sexual. 2016;18(12):1379–92. https://doi.org/10.1080/13691058.2016.1187291 .
Lieblich A, Tuval-Mashiach R, Zilber T. Narrative research: reading, analysis, and interpretation, vol. 27: Thousand Oaks, Sage; 1998.
Book Google Scholar
Krippendorff K. Content analysis: an introduction to its methodology. Thousand Oaks: Sage; 2004.
Schreier M. Qualitative content analysis in practice. Thousand Oaks: Sage; 2012.
Boyatzis RE. Transforming qualitative information: thematic analysis and code development. Thousand Oaks: Sage; 1998.
Mayring P. Qualitative content analysis. Forum Qualitative Sozialforschung/ Forum. Qual Soc Res. 2000;1(2):20. https://doi.org/10.17169/fqs-1.2.1089 https://www.qualitative-research.net/index.php/fqs/article/view/1089 . Accessed 20 Apr 2021.
QSR International. NVivo professional version 11. Boston: QSR International; 2015.
Babbie ER. The practice of social research. 13th ed. Scarborough: Wadsworth/Cengage Learning; 2013.
Anderson M, Elam G, Gerver S, Solarin I, Fenton K, Easterbrook P. “It took a piece of me”: initial responses to a positive HIV diagnosis by Caribbean people in the UK. AIDS Care. 2010;22(12):1493–8. https://doi.org/10.1080/09540121.2010.482125 .
Greeff M, Phetlhu R, Makoae LN, Dlamini PS, Holzemer WL, Naidoo JR, et al. Disclosure of HIV status: experiences and perceptions of persons living with HIV/AIDS and nurses involved in their care in Africa. Qual Health Res. 2008;18(3):311–24. https://doi.org/10.1177/1049732307311118 .
Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2007;83(1):29–34.
Hult JR, Maurer SA, Moskowitz JT. “I'm sorry, you're positive”: a qualitative study of individual experiences of testing positive for HIV. AIDS Care. 2009;21(2):185–8. https://doi.org/10.1080/09540120802017602 .
de-Graft Aikins A. Mental illness and destitution in Ghana: A Social-psychological perspective. In: Akyeampong E, Hill AG, Kleinman AM, editors. The Culture of Mental Illness and Psychiatric Practice in Africa. Bloomington: Indiana university press; 2015. p. 112–43. http://www.jstor.org/stable/j.ctt16gz69f.6 Accessed 26 Jun 2019.
Baumgartner LM, David KN. Accepting being poz: the incorporation of the HIV identity into the self. Qual Health Res. 2009;19(12):1730–43. https://doi.org/10.1177/1049732309352907 .
NAP+, GAC and UNAIDS. Persons living with HIV Stigma Index Study, Ghana. Accra: NAP+, GAC and UNAIDS; 2014. http://www.stigmaindex.org/sites/default/files/reports/GHANA%20Stigma%20Index%20report%202014.pdf . Accessed 4 Apr 2016. Revised report
Baiden F, Baiden R, Williams J, Akweongo P, Clerk C, Debpuur C, et al. Review of antenatal-linked voluntary counseling and HIV testing in sub-Saharan Africa: lessons and options for Ghana. Ghana Med J. 2005;39(1):8–13. https://doi.org/10.4314/gmj.v39i1.35974 .
Article CAS PubMed PubMed Central Google Scholar
Gadegbeku C, Saka R, Mensah B. Attitude of the Youth towards Voluntary Counselling and Testing (VCT) of HIV/AIDS in Accra, Ghana. J Biol Agri Healthcare. 2013;3(11):133 www.iiste.org .
Oppong AK. HIV/AIDS knowledge and uptake of HIV counselling and testing among undergraduate private university students in Accra, Ghana. Reprod Health. 2013;10:17. https://doi.org/10.1186/1742-4755-10-17 .
Tenkorang EY, Owusu AY. Examining HIV-related stigma and discrimination in Ghana: what are the major contributors? Sex Health. 2013;10(3):253–62. https://doi.org/10.1071/SH12153 .
Dapaah JM, Spronk R. When the clinic becomes a home. Successful VCT and ART services in a stressful environment. J Soc Aspects HIV/AIDS. 2016;13(1):142–51. https://doi.org/10.1080/17290376 .
Badaru UM, Ogwumike OO, Adeniyi AF, Nelson EE. Determinants of caregiving burden and quality of life of informal caregivers of African stroke survivors: literature review. Int J Disabil Human Dev. 2017;16(3):249–58. https://doi.org/10.1515/ijdhd-2016-0041 .
Omona J. Sampling in qualitative research: improving the quality of research outcomes in higher education. Makerere J Higher Educ. 2013;4(2):169–85. https://doi.org/10.4314/majohe.v4i2.4 .
Miles M, Huberman AM. Qualitative data analysis: An expanded sourcebook. 2nd ed. Thousand oaks: Sage; 1994.
Peirce A. The emotional impact of an HIV diagnosis. 2017. https://www.everydayhealth.com/hiv-aids/hiv-diagnosis-emotional-impact.aspx . Accessed 2 May 2018.
GAC. National HIV & AIDS Strategic Plan 2016-2020. 2016. http://www.ghanaids.gov.gh/gac1/pubs/COMPREHENSIVE%20NSP%202016-2020.pdf . Accessed 17 May 2020.
Owusu AY, Asante KT. Current HIV/AIDS status, access to antiretroviral treatment, and HIV related stigma in Ghana. Policy Brief. 2020. Accra: ISSER.
Download references

Acknowledgements
Professor Eric Y. Tenkorang of the Department of Sociology, Memorial University of Newfoundland and Labrador in Canada, was the PI for the project and participated in the data collection. Professor Tenkorang also principally solicited for the funds for this study from the IDRC. The Author is grateful to him. Dr. Kofi Takyi Asante of ISSER, University of Ghana, Legon, is also acknowledged for proofreading an earlier version of this paper.
Funding for data collection for this research was provided by the International Development Research Centre (IDRC), through the Canadian-African Research Grant. Matching grants were provided by the Department of Sociology, Memorial University of Newfoundland and Labrador, Canada. IDRC and the Memorial University played no role in the design of the study, data collection, analysis, and interpretation, as well as the writing of the manuscript.
Author information
Authors and affiliations.
Institute of Statistical, Social and Economic Research (ISSER), University of Ghana, P. O. Box LG 74, Legon, Ghana
Adobea Yaa Owusu
You can also search for this author in PubMed Google Scholar
Contributions
AYO contributed to conceptualising the study and served as a co-Principal Investigator (co-PI). AYO mostly focused on the qualitative aspect of the project from which the data for this paper emanates. AYO also developed the in-depth interview guide, helped to liaise with technical staff of the Ghana Health Service and Ghana AIDS Commission for explanations to some HIV/AIDS technical and policy-related issues in Ghana. Furthermore, AYO led in the acquisition of ethical clearance from the University of Ghana. Additionally, AYO co-supervised field staff during the data collection, and handled the quality control, and analysis of the qualitative data. AYO wrote and finalised this paper single-handedly. Since this paper is sole-authored, the final version of the manuscript does not require the approval of any other person prior to being submitted and published.
Author’s information
AYO is an Associate Professor at the Institute of Statistical, Social and Economic Research, College of Humanities, University of Ghana, Legon, Ghana. AYO is a Medical Sociologist, Public Health, and behavioural health specialist.
Corresponding author
Correspondence to Adobea Yaa Owusu .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval were sought from the following: 1) Ethical Review Board of the Memorial University at St. Johns, Newfoundland, Canada, 2) the Ethics Committee for Humanities at the University of Ghana, Legon (ECH 017/14–15), and 3) the Ghana Health Service’s Ethical Review Board (GHS-ERC: 02/11/14). The Protocol Submission form which was filled to seek ethical permission from the Ghana-based ethical committees included five options for types of consent to be sought from the respondents, under the section on “consent process”, with instructions to “circle all that applies.” We chose all of "written”, “oral”, “English language”, and "local language” options. These were approved by the ethics committees, as part of the general approval we received. The need to approve non-written consent as well as the use of the local languages is informed by the fact that not everyone in the study’s catchment area, which is applicable to the rest of Ghana, can read and write. Respondents who consented to participate in the study by writing had to append their signature or initials to a written form provided by the Ethical Committee for Humanities at the University of Ghana. Field enumerators recorded verbal consent on the said form for respondents who could not read and write. Permission to conduct the study were also received from 1) the Ghana Health Service’s Eastern Regional Directorate, 2) The LMKM Directorate of the Ghana Health Service, and 3) the Administrators of the Atua Government, and St. Martins De Pores hospitals. Each participant gave a written or verbal informed consent to participate before they were interviewed.
Consent for publication
Not applicable.
Competing interests
The author declares that there are no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Qualitative/Indepth Interview Guide. Housing and Health Needs of Persons Living with HIV/AIDS Project. Open-ended qualitative indepth interview guide with sections A to G and ending with demographic/background data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Owusu, A.Y. Experiences of new diagnoses among HIV-positive persons: implications for public health. BMC Public Health 22 , 538 (2022). https://doi.org/10.1186/s12889-022-12809-6
Download citation
Received : 04 July 2020
Accepted : 21 February 2022
Published : 18 March 2022
DOI : https://doi.org/10.1186/s12889-022-12809-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Comorbidities
- Psychosocial outcomes
- Qualitative research
- Experiences of new HIV-positive diagnoses
- Hopelessness theory of depression
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Consensus Statement
- Published: 13 June 2023
Cognitive impairment in people living with HIV: consensus recommendations for a new approach
- Sam Nightingale ORCID: orcid.org/0000-0001-7870-5287 1 ,
- Beau Ances 2 ,
- Paola Cinque 3 ,
- Ameet Dravid 4 ,
- Anna J. Dreyer ORCID: orcid.org/0000-0003-1230-3431 1 ,
- Magnus Gisslén 5 , 6 ,
- John A. Joska 1 ,
- Judith Kwasa 7 ,
- Ana-Claire Meyer 8 ,
- Nombeko Mpongo 9 ,
- Noeline Nakasujja 10 ,
- Roger Pebody 11 ,
- Anton Pozniak 12 , 13 ,
- Richard W. Price ORCID: orcid.org/0000-0001-5429-6044 14 ,
- Christopher Sandford 15 ,
- Deanna Saylor 8 , 16 ,
- Kevin G. F. Thomas 17 ,
- Jonathan Underwood ORCID: orcid.org/0000-0001-6963-2821 18 , 19 ,
- Jaime H. Vera 20 &
- Alan Winston 21 , 22
Nature Reviews Neurology volume 19 , pages 424–433 ( 2023 ) Cite this article
7981 Accesses
16 Citations
17 Altmetric
Metrics details
- Central nervous system infections
- Infectious diseases
Current approaches to classifying cognitive impairment in people living with HIV can overestimate disease burden and lead to ambiguity around disease mechanisms. The 2007 criteria for HIV-associated neurocognitive disorders (HAND), sometimes called the Frascati criteria, can falsely classify over 20% of cognitively healthy individuals as having cognitive impairment. Minimum criteria for HAND are met on the basis of performance on cognitive tests alone, which might not be appropriate for populations with diverse educational and socioeconomic backgrounds. Imprecise phenotyping of cognitive impairment can limit mechanistic research, biomarker discovery and treatment trials. Importantly, overestimation of cognitive impairment carries the risk of creating fear among people living with HIV and worsening stigma and discrimination towards these individuals. To address this issue, we established the International HIV-Cognition Working Group, which is globally representative and involves the community of people living with HIV. We reached consensus on six recommendations towards a new approach for diagnosis and classification of cognitive impairment in people living with HIV, intended to focus discussion and debate going forward. We propose the conceptual separation of HIV-associated brain injury — including active or pretreatment legacy damage — from other causes of brain injury occurring in people living with HIV. We suggest moving away from a quantitative neuropsychological approach towards an emphasis on clinical context. Our recommendations are intended to better represent the changing profile of cognitive impairment in people living with HIV in diverse global settings and to provide a clearer framework of classification for clinical management and research studies.
Similar content being viewed by others
Global prevalence of mild cognitive impairment among older adults living in nursing homes: a meta-analysis and systematic review of epidemiological surveys

Comparing different approaches for operationalizing subjective cognitive decline: impact on syndromic and biomarker profiles
Long-term cognitive impairment after ICU treatment: a prospective longitudinal cohort study (Cog-I-CU)
Introduction.
The most frequently used criteria for cognitive impairment in people living with HIV are the HIV-associated neurocognitive disorders (HAND) criteria, developed in 2007 by a working group convened by the US National Institute of Mental Health 1 . The HAND criteria (sometimes referred to as the Frascati criteria) were intended to harmonize research methodology to allow comparisons across diverse study settings. Although originally intended for use in research, HAND terminology has become widely used to refer to the clinical burden of cognitive impairment 2 . The HAND criteria have been successful in providing a consistent system of classification in global research studies for 15 years. The spectrum of HIV disease has, however, changed dramatically in the past two decades: the majority of people living with HIV globally are now virally supressed by effective antiretroviral therapy (ART), and life expectancy approaches that of uninfected cohorts 3 , 4 . Minimum criteria for HAND are met on the basis of cognitive test performance compared with HIV-negative populations, without the need for a clinical assessment of cognitive status. Several authors have argued that this approach overestimates disease burden and that the HAND criteria are not appropriate for the modern era 5 , 6 , 7 , 8 , 9 , 10 , 11 .
Criticism of the HAND criteria centres on three main points, as outlined by authors from our group in 2021 (ref. 12 ). First, the statistical approach applied to cognitive data has the potential for a very high false-positive rate: the current approach defines over 20% of cognitively healthy HIV-negative control participants as having cognitive impairment 8 , 10 . Second, cognitive test performance is strongly influenced by complex educational, cultural and socioeconomic factors, which can interact with HIV risk such that low cognitive test performance might not correspond to a pathological state 13 , 14 . Last, in the modern era of effective ART and an ageing population of people living with HIV, cognitive impairment in these individuals is frequently multifactorial and, hence, is not synonymous with the direct effect of HIV on the brain and not best described as ‘HIV-associated’, which implies a degree of causation 15 , 16 .
The HAND criteria typically classify 20–60% (and sometimes up to 90%) of people living with HIV as cognitively impaired 2 , 14 , 17 . This figure does not seem to align with clinical observations that cognitive impairment in people living with HIV presents less frequently in the modern era, usually affecting individuals who are not on effective ART or have relevant comorbidities, or occurring as a result of damage caused by CNS HIV replication before effective ART 18 , 19 , 20 . Lack of diagnostic precision could hamper clinical studies of cognitive impairment in people living with HIV and reduce the power to identify biomarkers relating to neuropathogenesis 21 .
A label of cognitive impairment can affect self-esteem and confidence and raise fears for future health in people living with HIV 22 . Overestimation of cognitive impairment might also worsen stigma and discrimination towards these individuals 23 . For example, people living with HIV in the UK were denied the opportunity to become airline pilots owing to concerns over the development of cognitive impairment. Following a campaign by a pilot living with HIV, the UK Civil Aviation Authority removed this ban in 2022 to reflect the improved HIV outcomes in the modern ART era 24 . Conversely, underestimation or misclassification of cognitive impairment in people living with HIV carries the risk of missing cases and preventing access to care. Cognitive impairment is an important complication of HIV with far-reaching consequences for quality of life 22 . Approaches to the diagnosis and classification of cognitive impairment must reflect the modern spectrum of disease so that prognostic information is accurate and affected individuals can receive the help they need.
The original HAND publication of 2007 acknowledged several of these potential methodological issues and recommended strongly that the criteria should be field tested and further refined going forward 1 . In response to the issues described above, we established the International HIV-Cognition Working Group. The broad aim of the group was to propose improvements to the diagnostic approach to cognitive impairment in people living with HIV so as to reflect changes in the spectrum of HIV disease in the modern ART era. In this Consensus Statement, we provide specific recommendations around key issues to focus discussion and help the field move forward.
The International HIV-Cognition Working Group was initiated by the HIV Mental Health Research Unit at the University of Cape Town, South Africa, and follows our HAND critique published in 2021 (ref. 12 ). The group was intended to be globally representative, and hence, preference was given to experts based in low-income and middle-income countries with high HIV prevalence. In high-income countries, members were invited in approximately equal numbers from Europe and the USA. We aimed to include people with direct clinical experience of people living with HIV, as well as leading researchers in the field. Representatives were invited from the community of people living with HIV in both high-income and low-income settings. Of 25 representatives invited, three declined and two withdrew after initially accepting, citing time commitments. Of the resulting 20 members, nine (45%) were based in low-income and middle-income countries in the Global South. Members included academics and clinicians from the neurology, psychiatry, neuropsychology and infectious disease fields, as well as three HIV community representatives.
Working group meetings were held virtually. The framework laid out in the 2021 HAND critique was used as a starting point for discussion 12 , with the specific aim of outlining a diagnostic approach for cognitive impairment in people living with HIV that is applicable in the clinic as well as in research, is appropriate for diverse populations of people living with HIV globally, is applicable in both low-resource and high-resource settings, and reduces the risk of fear, stigma and discrimination for people living with HIV. Members participated in videoconference discussions and engaged further via the group e-mail chain. On the basis of this communication, a manuscript draft was prepared by S.N. and distributed to the group for comment and further input. Multiple iterations of the manuscript were reviewed, revised and redistributed. Additional smaller meetings were held virtually or in person at international conferences, and the outcomes of these meetings were subsequently shared for discussion with the wider group. This iterative process continued until a broad consensus was reached on all points by all working group members.
This process led to the six recommendations outlined below and summarized in Box 1 . These recommendations should be interpreted as representing the consensus opinion of a diverse group of experts rather than being a definitive new set of criteria. Further validation and a broader consensus within the field will be required to define and implement definitive new criteria for cognitive impairment in people living with HIV.
Box 1 Summary of recommendations from the International HIV-Cognition Working Group
Recommendation 1
HIV-associated brain injury (HABI) should be considered as one cause of cognitive impairment alongside other potential causes of brain injury occurring in people living with HIV.
Recommendation 2
HABI should be differentiated on the basis of HIV RNA suppression and the activity of pathology.
Recommendation 3
Low performance on cognitive tests should not be labelled as cognitive impairment without clinical context.
Recommendation 4
When interpreting cognitive data, the false-classification rate should be considered.
Recommendation 5
A research classification of cognitive impairment in people living with HIV should consider a combination of cognitive symptoms, low performance on cognitive testing, and abnormality on neurological investigations.
Recommendation 6
Cognitive symptoms should refer to any change in cognition that has been noticed by the individual or an observer, whether or not this change has an impact on daily functioning.
Recommendations
Classifying cause of brain injury in people living with hiv.
Recommendation 1: HIV-associated brain injury (HABI) should be considered as one cause of cognitive impairment alongside other potential causes of brain injury occurring in people living with HIV.
In the modern era, cognitive impairment is frequently multifactorial, with a direct effect of HIV on the brain representing only one cause 15 , 19 . To distinguish direct effects of HIV from other causes of brain injury in people living with HIV, we recommend that the term HABI should be used to refer to damage caused directly by HIV. Other causes of brain injury include various comorbidities and medication effects 19 , 25 , as summarized in Box 2 . We recommend that HABI is conceptually separated from other causes of brain injury, although we accept that this can be difficult in practice, given that several causes can coexist and clinical manifestations can lead to overlapping symptoms and signs. Nevertheless, we feel that separating the concept of HABI from all-cause cognitive impairment in people living with HIV reduces ambiguity in terminology and facilitates examination of brain injury mechanisms.
HAND is defined as being caused by HIV, at least in part 1 . The HAND criteria do acknowledge, however, that people living with HIV are potentially vulnerable to the cognitive effects of other conditions. Where present, such comorbid conditions are termed ‘contributing’, as they contribute to cognitive impairment alongside the effect of HIV. If an individual has an alternative cause of cognitive impairment, this is considered a ‘confounding’ condition and a classification of HAND is not made. As such, a label of HAND means that cognitive impairment is caused by a direct effect of HIV on the brain, with or without additional contributions from comorbidities. This terminology might no longer be appropriate owing to dramatic improvements in HIV clinical care, which have reduced the frequency of brain injury caused by HIV. We recommend that a classification of cognitive impairment in people living with HIV should encompass all potential causes of brain injury, regardless of whether HABI is the cause or even a contributing factor in any given case. Moving to a classification that considers multiple causes of cognitive impairment will more accurately represent changes to the clinical burden of disease and facilitate the study of more representative samples in research, compared with the current classification.
Parallels can be drawn between milder forms of cognitive impairment in people living with HIV and mild cognitive impairment (MCI) in relation to Alzheimer disease (AD). The underlying pathology associated with MCI is heterogeneous and the majority of individuals with MCI do not go on to develop AD 26 . Biomarkers have been identified that reliably differentiate MCI with underlying AD pathology from other causes of MCI, and can be used to predict progression to AD 27 . The comparable situation in people living with HIV is to distinguish between HABI and other underlying causes of cognitive impairment. This distinction is more difficult with HABI than with AD pathology as, in contrast to AD, HABI does not generally progress to a marked dementia syndrome in individuals who receive suppressive ART 28 .
Numerous potential pathological mechanisms could underlie HABI, including persistent immune activation, blood–brain barrier dysfunction and more direct virus-induced neurotoxicity. Neuronal damage can be mediated by both immune-active molecules and HIV products, and could involve several mechanisms including oxidative stress, metabolic changes, glutamate dysregulation and NMDA excitotoxity 29 , 30 , 31 . Of note, HABI differs slightly from the existing terms HIV encephalopathy and HIV encephalitis. HIV encephalopathy refers to a predominantly subcortical cognitive–motor syndrome, also known as HIV-associated dementia, which is an AIDS-defining condition. HIV encephalitis refers to the histopathological finding of multinucleated giant cells and microglial nodules 32 . Although these complications continue to occur, particularly in people with untreated or advanced HIV disease, they no longer represent the prominent neuropathology of HABI in the modern ART era. For example, in one study, of 20 people diagnosed with HIV-associated dementia, only one had histopathological evidence of HIV encephalitis post mortem 33 . Our proposed term HABI is intended to encompass any mechanism of brain injury caused directly by HIV, including those previously described by the terms HIV encephalopathy and HIV encephalitis.
Box 2 Potential causes of brain injury in people living with HIV
This is not an exhaustive list, as any neuropathological process can potentially affect people living with HIV.
HIV-associated brain injury (HABI) (Fig. 1 )
Legacy HABI: inactive brain injury from pretreatment damage
Active HABI: ongoing brain injury leading to clinical or radiological progression
Other causes of brain injury
Previous or ongoing CNS infections (for example, neurosyphilis, CNS tuberculosis, CNS toxoplasmosis, CNS cryptococcosis and progressive multifocal leukoencephalopathy)
Cerebrovascular disease
Traumatic brain injury
Neurodegenerative disorders such as Alzheimer disease
Other non-HIV-related neurological condition (for example, multiple sclerosis or uncontrolled epilepsy)
Developmental disability
Nutritional deficiencies (for example, vitamin B 12 or niacin deficiency)
Coinfections (for example, syphilis or hepatitis C)
Hazardous alcohol use
Substance misuse
Antiretroviral CNS neurotoxicity
Differentiating legacy and active HABI
Recommendation 2: HABI should be differentiated on the basis of HIV RNA suppression and the activity of pathology.
In the modern era, the majority of people living with HIV globally are virally supressed on effective ART and, as a result, are largely protected from progressive HIV disease 3 . Therefore, defining the risk of progressive disease caused by HABI in people with HIV RNA suppression is particularly important. Current evidence is conflicting, with regard to both how commonly HIV causes cognitive impairment in this group 2 , 28 and whether the brain injury is the result of a progressive or static process 34 , 35 , 36 , 37 , 38 . To reduce ambiguity in this area, we recommend that HABI is subdivided into legacy and active HABI on the basis of progression (Fig. 1 ). This differentiation is important as it has implications for treatment and prognosis 25 , 39 . Of note, legacy and active HABI can coexist, as active HABI can occur on a background of legacy HABI.
HIV-associated brain injury (HABI) should be differentiated from other causes of brain injury, such as comorbid factors and medication effects. In individuals with plasma HIV RNA suppression, HABI is characterized as either legacy (inactive brain injury from pretreatment damage) or active (ongoing brain injury leading to clinical and/or radiological progression). Whether active HABI occurs in people with sustained HIV RNA suppression in plasma and cerebrospinal fluid has not been definitively shown.
HABI can also occur in people living with HIV with untreated, or incompletely treated, HIV infection 40 , 41 . In such patients, the focus should be on systemic HIV viral control 25 , 39 , 42 , 43 .
Legacy HABI
Irreversible or only partially reversible CNS damage resulting from HABI can be sustained during periods of untreated HIV infection, particularly in the context of advanced immunosuppression 20 . This sustained damage has been referred to as the legacy effect and represents brain injury that occurs before an individual initiates ART. In adults with vertically acquired HIV (transmitted from mother to child in utero, intrapartum or postnatally through breast feeding), legacy HABI can include sequelae from the effects of HIV infection on the developing brain 44 . Legacy effects are inactive and permanent and, hence, not amenable to treatment. It is possible that subclinical legacy HABI could lower cognitive reserve, thereby increasing vulnerability to cognitive impairment from other causes, such as ageing 45 , 46 , 47 .
Active HABI
Active HABI refers to evidence of sustained clinical or radiological progression of CNS damage over time, beyond that expected from normal ageing or variability in cognitive performance testing, with careful exclusion of alternative causes. Progression of CNS damage in the context of HIV RNA suppression in plasma should prompt examination for cerebrospinal fluid (CSF) HIV RNA escape 39 , 43 . Definitions of CSF HIV RNA escape vary, but the consensus definition is the presence of HIV RNA in the CSF combined with absence or lower levels in the plasma 48 . CSF RNA escape can indicate compartmentalized HIV replication in the CNS resulting from low treatment potency in the intrathecal compartment, owing to ART resistance, less effective or older ART regimens, or low adherence to treatment. CSF HIV RNA escape can lead to varying presentations, including rapidly progressive neurological disease and diffuse white matter signal abnormality on MRI 49 , 50 , although it can also be transient and asymptomatic 49 . Evidence suggests that CSF RNA escape is becoming less common with modern ART 51 . Low levels of HIV RNA in the CSF might not necessarily be the cause of active neuropathology, and its presence should not be taken as definitive evidence of CNS-compartmentalized HIV. However, in the presence of clinically active disease, CSF HIV RNA should be investigated and, if present, ideally treated in the first instance with ART directed to CSF resistance profiles 52 .
Another cause of active HABI is CD8 encephalitis, a severe inflammatory disorder characterized by T cell infiltration into the brain, leading to swelling and raised intracranial pressure that can be fatal 31 . CD8 encephalitis typically occurs in people on ART and can be associated with a number of triggers, including CSF HIV RNA escape and immune reconstitution inflammatory syndrome (IRIS), suggesting some overlap among these conditions 31 , 53 . CD8 encephalitis is responsive to corticosteroids 54 .
IRIS can occur in the weeks to months following the initiation of ART and can affect the brain in the absence of opportunistic pathogens 31 , 55 . This condition is thought to be a result of an immune response directed at HIV viral reservoirs in the brain and has been associated with CSF HIV RNA escape 55 , 56 . Similar to CD8 encephalitis, IRIS can lead to a severe, potentially fatal T cell encephalitis with brain oedema, which can respond to immune modulation with corticosteroids 57 . IRIS directed at viral, fungal, bacterial or parasitic opportunistic pathogens in the brain is considered to be a secondary effect and not part of HABI.
Beyond the uncommon cases of CD8 encephalitis and CNS IRIS described above, HIV has not been definitively shown to cause a progressive cognitive syndrome in the context of sustained HIV RNA suppression in both plasma and CSF. Nevertheless, several mechanisms have been proposed for progressive cognitive decline in controlled HIV. These mechanisms include HIV protein-associated encephalopathy; ongoing CNS HIV replication below the threshold of detection; and a neuroinflammatory process established during legacy damage that persists after effective HIV control with ART 29 , 30 , 31 . The 2013 consensus report from the MIND Exchange program stated that “it is not possible from existing data to conclude whether patients with successful treatment (ie, plasma HIV RNA <50 copies/mL) are at risk of progression [of cognitive impairment]” 43 . Since this consensus report was published, several studies have examined this question, with some suggesting that active HABI can occur in the context of controlled HIV 34 , 58 , particularly in ageing cohorts 37 , 59 , 60 . By contrast, others have provided longitudinal cognitive outcome data with low rates of progression 20 , 35 , 38 , falling prevalence rates of cognitive impairment over time 28 , 61 , cognitive deterioration associated with comorbidities rather than HIV factors 19 , and ageing trajectories similar to those in lifestyle-matched control individuals 36 . Whether controlled HIV can cause active HABI remains an important question to answer so that people living with HIV can obtain accurate prognostic information, and those at risk of cognitive impairment can be identified for treatment and management.
Biomarker changes without brain injury
Some CSF, plasma and imaging biomarkers that are used in research have indicated an active and, hence, potentially injurious process in the brain despite viral suppression and stable cognition 34 , 58 , 62 , 63 , 64 , 65 . Examples include diffusion tensor imaging and functional MRI, and various CSF biomarkers of immune activation and neuronal damage 66 . Although such markers sometimes indicate ongoing inflammation in the CNS of people living with HIV, the extent to which this inflammation has a clinical correlate or represents an injurious process, as opposed to subclinical effects or a healing process in response to pre-existing damage, is not clear 25 . Furthermore, some biomarkers change with age and can be altered in HIV-negative control individuals matched with people living with HIV for comorbidities and lifestyle factors 67 . Therefore, we recommend that abnormalities indicated by such markers should not be considered definitive evidence of an active injurious process, unless they are demonstrated to correspond to clinical or radiological progression as described earlier in this section. This distinction is intended not to undermine the potential importance of biomarker abnormalities but to acknowledge the difference between a research finding of concern and definitive evidence of a clinical effect.
A biomarker that consistently corresponds to clinical or radiological progression of brain injury could be used as evidence of active HABI, similar to the use of CSF amyloid and tau proteins to predict progression of AD 27 . One biomarker that might be useful in this context is CSF neurofilament light chain (NfL), a robust and sensitive marker of neuronal injury 68 . Research is needed to investigate the use of NfL as a biomarker for active HABI in people with sustained HIV RNA suppression. It should be noted that NfL is not specific for HABI versus other causes of brain injury, so other causes should be carefully excluded.
Neuroinflammation, as indicated by raised biomarker levels, could cause an active process of neuronal dysfunction without progressive injury, which could lower cognition in a stable or fluctuant way without sustained progression, akin to a metabolic encephalopathy. Supporting this theory, some CSF and imaging biomarkers were reported to correlate with clinical outcomes in people living with HIV; however, the associations were inconsistent and generally weak 66 , 69 . Trials of anti-inflammatory and neuroprotective compounds aimed at improving cognition in this patient population have not shown clinical effects 70 , 71 , 72 , 73 . Further research is needed to determine whether a non-progressive yet active process of neuronal dysfunction that is potentially amenable to treatment can occur in people with sustained suppression of HIV RNA. If shown to occur, this condition would warrant a third HABI subtype in addition to legacy and active HABI.
Interpretation of cognitive tests
Recommendation 3: Low performance on cognitive tests should not be labelled as cognitive impairment without clinical context.
Cognitive testing is an important element of assessment in people living with HIV who are suspected of having cognitive impairment. Despite the appeal of cognitive scores as an objective measure of neuronal function, the results vary widely depending on non-biological factors such as educational background and socioeconomic status 13 , 14 . Indeed, this issue was stressed in the original HAND criteria publication 1 . For example, the average score on the Montreal Cognitive Assessment (MoCA) in a study of healthy HIV-negative individuals without cognitive impairment in a low-income area of Cape Town was 21.7 out of 30 (ref. 74 ), which falls below what is considered a normal score in North America (26–30), where the MoCA was developed. These differences do not imply impaired cognition, but rather that performance on these tests can be culture-bound and vary substantially between groups with different educational and sociodemographic backgrounds.
In research studies, cognitive scores are typically compared with those in normative control populations. These comparisons can be improved by the collection of normative data from populations with similar demographics to the measured population of people living with HIV, or by controlling for demographic factors in established normative datasets with regression-based techniques 75 . However, these approaches have several limitations. First, studies have demonstrated wide variation in normative data among and within countries 76 , and developing extensive normative data for each setting in which people living with HIV reside would be impractical. Second, matching for all lifestyle and comorbid factors associated with HIV status is difficult owing to the broad diversity of HIV demographics. Last, in some regions, HIV acquisition is associated with poverty and low education levels 77 , 78 , increasing the likelihood that people living with HIV will return lower test scores than the population average. In clinical practice, these factors are taken into account by neuropsychologists and clinicians with experience in cognitive testing, who use cut-offs appropriate for the population or interpret an individual’s performance subjectively on the basis of educational background and estimates of premorbid functioning. In research studies, these approaches are often impractical and might be considered too subjective, and hence normative control scores are used to make comparisons.
The HAND criteria define statistical methodology to determine cut-offs for cognitive performance in people living with HIV compared with normative controls 1 . Cognitive performance within a particular domain must fall more than 1 s.d. below the normative average for that domain to be considered impaired. This threshold must be crossed in two or more cognitive domains for a classification of HAND. Several other statistical approaches to define cognitive impairment have also been used as alternatives to the HAND criteria, including the Global Deficit Score, Multivariate Normative Comparison, Novel Multivariate Method and the Gisslén criteria 8 , 25 . Some of these methods can be applied in several different ways (discussed under Recommendation 4 below), which creates the potential for large variation in the statistical methodology used to define cognitive impairment in people living with HIV. The percentage of individuals classified as having cognitive impairment can vary widely with different methods. For example, when 20 different methods were applied to a clinical cohort in South Africa, the rate of cognitive impairment ranged from 20% to 97% 79 . Therefore, the same individual can be classified as having cognitive impairment by one statistical method but not by another.
Fluctuation of cognitive scores in an individual over time presents another challenge 80 . Minor variation around domain cut-offs can have large effects on binary classifications 81 , so an individual on the margin of impairment can be classified as having impairment at one time point but not at another. This issue is evident in longitudinal studies, where fluctuations in cognitive function are frequently observed 1 , 82 .
For the reasons above, we suggest that the use of cross-sectional quantitative neuropsychological approaches alone is limited as a method of determining impairment in diverse populations. No tool provides a perfect indication of neuronal function, and any statistical method of dichotomization on the basis of cognitive performance will be to some extent arbitrary. Perfectly matching a normative population to factors associated with HIV acquisition in all settings is extremely difficult. Although defining a group at the lower end of the cognitive spectrum can be useful, we recommend that they be classified as having low cognitive performance rather than being diagnosed with cognitive impairment, unless supporting information exists in other areas of assessment (see Recommendation 5 below).
Comparisons can be made with the diagnosis of MCI in relation to AD. Statistical cut-offs for low cognitive performance in MCI vary from 1 to 2 s.d., resulting in wide variation in MCI prevalence depending on the method used 83 . Less stringent definitions using 1 s.d. are generally not favoured as they have a high false-positive rate 84 , fail to show an association with medial temporal atrophy and apolipoprotein E genotype (an important genetic determinant of AD risk) 85 , and result in considerable diagnostic instability — that is, fluctuation between a classification of MCI and cognitively normal — over time 83 . The potential for false classification of MCI is mitigated by the requirement for symptomatology, in contrast to HAND, for which symptoms are not a requirement (see Recommendation 6).
Individuals with isolated low cognitive performance, though not meeting our proposed criteria for cognitive impairment, might represent an important group for future research. Subclinical impairment and/or reduced cognitive reserve in those with low cognitive performance could increase vulnerability to other brain injuries, which is particularly important as the population of people living with HIV ages 45 , 46 , 47 . It should be noted that individuals at the lower end of the spectrum of cognitive performance might be more likely to have lower education levels and socioeconomic status, as well as different comorbid and lifestyle factors, compared with those at the upper end 86 , 87 . Consequently, those with low cognitive performance might have worse general health-related outcomes, owing to the interaction with social determinants of health 88 .
False classifications in cognitive data
Recommendation 4: When interpreting cognitive data, the false-classification rate should be considered.
As stated in the previous section, HAND criteria define cognitive impairment as performance of at least 1 s.d. below the normative average in at least two cognitive domains 1 . If population performance is normally distributed, then approximately 16% of scores on each test will fall more than 1 s.d. below the mean, so a sizeable proportion of individuals without cognitive impairment will be falsely classified as impaired. This false-classification rate depends on the number of domains measured, the number of tests used per domain, and the relationships among different tests, but is typically in excess of 20% and can rise to over 70% in some cases 9 , 10 , 80 .
As mentioned in the previous section, several other statistical approaches can be used to determine cut-offs for cognitive performance in people living with HIV compared with normative controls 8 , 25 . Some methods are more stringent than others, with improved false-positive rates generally at the expense of decreased sensitivity. Some methods can be applied in several different ways; for example, when more than one test is used per cognitive domain to improve accuracy, that domain can be defined as impaired by one test being positive, both tests being positive, or by the average domain T -score. These variations can alter the false-classification rate quite markedly 80 , 81 .
The false-classification rate is an important consideration when interpreting study findings: a study reporting low performance on cognitive tests in 30% of a population has a different interpretation when the false-classification rate is known to be 25% compared with 2.5%, for example. Currently, the false-classification rate is rarely acknowledged or reported in studies reporting HAND prevalence 11 . The range of tools to help estimate the false-classification rate for different statistical methodologies should be expanded 8 .
To help avoid false classification of cognitive impairment, alternative approaches can be used to handle cognitive data in research studies. One approach is to longitudinally assess the trajectory of cognitive performance rather than applying dichotomization cross-sectionally. For longitudinal assessment, fluctuation in cognitive performance and practice effects must be taken into account 80 , 89 . Another approach is to use cognitive performance as a continuous variable rather than as a binary measure with a statistically determined cut-off. The use of continuous variables captures the full spectrum of cognition and provides greater statistical power than the comparison of proportions below a cut-off 81 .
Defining cognitive impairment in people living with HIV
Recommendation 5: A research classification of cognitive impairment in people with HIV should consider a combination of cognitive symptoms, low performance on cognitive testing and abnormality on neurological investigations.
Assessment for cognitive impairment falls broadly into three areas: clinical history, performance on cognitive testing and the results of neurological investigations. Each area has strengths and weaknesses if used alone to determine cognitive impairment (Table 1 ). The presence of cognitive symptoms, defined here as a change in cognition that has been noticed by the individual, is clinically important but is a subjective measure, and reporting of symptoms varies among settings (see Recommendation 6). The results of cognitive testing can be more objective than clinical history but are strongly influenced by complex educational and socioeconomic factors and must be interpreted in the context of the individual or population background (as discussed in Recommendation 3). Evidence of brain injury on neurological investigations, such as neuroimaging or CSF examination, is the most objective measure of pathology, but abnormalities can represent subclinical damage, and these tests are not universally available or accessible in low-resource settings. In addition, neurological investigations can be insensitive for some causes of brain injury, including HABI, and the absence of evident abnormalities on routine investigations does not exclude the presence of brain injury 50 .
Given the limitations discussed above, we propose that in research settings, a classification of cognitive impairment can be made when an individual shows abnormalities in at least two of the three assessment areas. Using this pragmatic approach, someone with low cognitive performance would be considered to have cognitive impairment if supporting evidence of cognitive symptoms and/or brain injury was available. Similarly, someone with cognitive symptoms and evidence of brain injury would be considered to have cognitive impairment, even if cognitive performance did not fall below a threshold and, hence, would not currently be classified as HAND — for example, individuals with high pre-morbid function and/or cognitive reserve.
The importance of an isolated abnormality in any one assessment area should not be undermined by altering the criteria for cognitive impairment as described here. Individuals in whom the criteria for cognitive impairment are not met could still represent a group with clinical and research importance, and our recommendation is simply to alter the terminology used to describe these groups. Individuals previously defined as having asymptomatic neurocognitive impairment (ANI) — a form of HAND and therefore considered a neurocognitive disorder — would instead be referred to as having ‘low performance on cognitive tests’. These individuals would not be considered to have cognitive impairment in the absence of supporting evidence of abnormality from another assessment area.
Defining cognitive symptoms
Recommendation 6: Cognitive symptoms should refer to any change in cognition that has been noticed by the individual or an observer, whether or not this change impacts daily functioning.
When assessing someone at risk of cognitive impairment, obtaining a history of cognitive symptoms is important. We recommend a subtly different use of the term ‘symptom’ from that applied in the HAND criteria. HAND criteria define symptomatic cognitive impairment as a change in activities of daily living (ADLs) resulting from cognitive issues 1 . As treatment for HIV has improved, cognitive symptoms in people living with HIV have generally become milder 28 . Symptoms such as forgetfulness or difficulty concentrating can considerably affect an individual’s quality of life and ability to work but might not be severe enough to limit ADLs 22 . This level of symptoms is similar to that required for the diagnosis of MCI in relation to AD, for which cognitive change should be noticeable but not yet have a substantial effect on ADLs 90 .
Cognitive symptoms are inherently subjective: some cultures might be reluctant to acknowledge cognitive issues and some languages have limited vocabulary for cognitive complaints 91 , 92 . Furthermore, cognitive dysfunction can impair insight, reducing the probability of an individual reporting their difficulties 93 . Where available, an observer account — for example, a collateral history from a partner, family member or carer — can improve accuracy of symptom reporting and already forms part of the criteria for MCI 94 . In these cases, gaining consent from the individual with HIV is important, as the observer account can include sensitive information. Difficulties with ADLs reported on functional scales should be confirmed as related to cognitive issues rather than physical disability, intercurrent illness, psychological factors or fatigue.
Cognitive symptoms can be transient and reactive to psychological stressors or life events 95 , 96 . Symptoms can be more common in people with depression, which could be related to the effects of mood on cognitive processes or to shared biological mechanisms of neuroinflammation 97 , 98 , 99 . Where uncertainty arises, repeated assessments over longer periods of time are useful. Rapidly evolving symptoms should trigger urgent investigation for CNS opportunistic infection, CD8 encephalitis, fulminant presentations of symptomatic CSF HIV RNA escape, or neurological disorders unrelated to HIV infection 49 , 55 .
Discussion and potential limitations
Our recommendations differ from the HAND criteria in two main ways: first, by distinguishing HABI as a separate entity from all-cause cognitive impairment, and second, by recommending a clinical assessment for a label of cognitive impairment to be applied. Although perhaps not as appealingly simple as the HAND classification, our approach reflects the complexity of assessing cognitive impairment in people living with HIV in the modern era. Application of this approach in clinical settings would require no additional measures beyond the current recommended standard of care. Assessment of a person suspected of having cognitive issues should, at a minimum, involve a clinical history (ideally with an observer account), backed up with a cognitive measure. Assessment for brain injury depends on locally available resources, and detailed neurological investigations are not essential for this classification.
Historically, not all research studies have collected the information necessary to diagnose cognitive impairment in this way. We feel that collecting a clinical history and objective markers of brain injury is important to conduct research with relevance to the outcomes and concerns of people living with HIV. Studies that consider individual assessment areas in isolation without a clinical history (for example, a study of cross-sectional cognitive performance or neuroimaging), could provide useful mechanistic information. However, we suggest that such studies avoid reporting rates of cognitive impairment and making assumptions about the cause of impairment. Even in advanced HIV disease, diagnostic measures interpreted without clinical context have been shown to have poor inter-rater agreement for assigning the aetiology of cognitive impairment, owing to a myriad of comorbidities 100 .
Although we highlight issues with the HAND criteria and their potential to overestimate prevalence, our approach should not be interpreted as implying that cognitive impairment is no longer an issue in people living with HIV. Cognitive impairment remains a vitally important complication of HIV with multiple causes and can have profound effects on many aspects of an individual’s functioning and quality of life 22 . Furthermore, cognitive issues could become even more important as the population of people living with HIV ages. A robust set of criteria is essential to focus research and ensure that those at risk are identified and receive the help they need.
Our proposed approach has several potential limitations. The recommendation to include a clinical assessment to determine clinically meaningful cognitive impairment can be difficult to transfer to the research environment. In many low-resource settings, standardized cognitive measures are applied by local-language-speaking research assistants who do not have sufficient medical or neuropsychology training to obtain a detailed history 17 . Despite this challenge, clinical assessments often form part of the inclusion criteria for studies of other diseases, including studies that involve people living with HIV in low-resource settings 101 . The 1991 American Academy of Neurology criteria for HIV-associated dementia and minor cognitive motor disorder required a clinical history. The criteria stated that mild cognitive deficits should be verified by a reliable history, if possible from an informant, to ensure that the timing and nature of impairment are consistent with HIV as the cause 102 . The 2007 HAND criteria moved away from this requirement by creating the category of ANI 1 . ANI was intended to represent a preclinical stage of impairment that might be amenable to treatment. However, as ANI is based on cognitive performance alone, without clinical correlates or other evidence of brain injury, the ability to reliably identify a pathological phenotype could be limited. To facilitate the collection of clinical assessments and observer accounts in research, tools are needed that can distinguish cognitive limitations from physical or mental health issues. Such tools exist for other diseases; for example, the Community Screening Instrument for Dementia includes a cognitive assessment, functional measures and an observer account, and has been used across diverse settings globally 103 .
Sensitivity to detect cognitive impairment on the basis of overlap of at least two areas of assessment (Recommendation 5) would differ depending on the method. For example, the percentage of individuals identified as having brain injury would vary depending on whether neuroimaging was available and, if so, whether MRI or CT was used. This limitation is not uncommon in clinical situations, where sensitivity to detect disease can be lower when access to investigative methods is limited. We propose Recommendation 5 as a pragmatic alternative to HAND that considers clinical context. As this recommendation is perhaps the most speculative of those proposed here, validation against clinical diagnosis compared with existing approaches will be important.
The term HABI has been coined to refer to a degree of brain injury as a direct result of HIV, but what constitutes sufficient injury in this context is to some extent subjective. HIV enters the brain during early stages of infection, making it difficult to exclude some level of HABI in all people living with HIV 29 . Furthermore, the separation between HABI and other causes of brain injury might not be completely distinct. For example, cerebrovascular disease can be exacerbated by HIV-associated endothelial dysfunction and/or ART effects 104 . As another example, some evidence indicates that HIV interacts with substance abuse to compound the injurious effects on the brain 105 . Nevertheless, we feel that distinguishing injury caused directly by HIV, classed as HABI, from indirect or combined effects, which would not be described as HABI, is useful.
Although a combination of investigations and clinical context can diagnose HABI, no single imaging or CSF biomarker has been identified for all stages and types of HABI that is robust enough for clinical use 69 . This lack could in part be attributable to variations in the underlying pathology in people categorized with HAND. As discussed in Recommendations 1 and 4, HAND can encompass a mixture of different pathologies and include individuals without true cognitive impairment. As a result, any genuine association between biomarkers and HABI-related cognitive impairment could be diluted by the inclusion of individuals without HABI. Conceptual separation of HABI from low cognitive performance is intended to improve clarity in this area. Notably, this issue is not unique to HABI: many other causes of brain injury have no definitive test and rely on clinical judgement of medical history, risk factors and investigative findings. Although not always straightforward, attempting to identify the relative contribution of HABI to cognitive impairment is important, particularly as treatment for HIV becomes more effective and widespread. This knowledge will allow people living with HIV to better understand the risk of developing cognitive impairment and enable treatments to be tailored to the cause of impairment.
Conclusions
In this Consensus Statement, we have outlined a series of recommendations for a new approach to cognitive impairment in people living with HIV. These recommendations reflect the consensus opinion of our diverse group of experts and are intended to drive discussion and debate towards the development of new criteria for cognitive impairment in people living with HIV. Our recommendations will require assessment, validation, refinement and a broader consensus within the field. More detail will be needed in several areas, including: which cognitive tools and functional scales to use in different settings; which statistical methods to apply to determine low cognitive performance; how best to obtain a history of cognitive symptoms; how to determine severity of cognitive impairment; and how to interpret investigations of HABI. Overall, our approach is intended to better represent the changing profile of cognitive impairment in people living with HIV in diverse global settings and to provide a clearer framework of classification for clinical management and research studies.
Antinori, A. et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69 , 1789–1799 (2007).
Article CAS PubMed Google Scholar
Wang, Y. et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95 , e2610–e2621 (2020).
Frescura, L. et al. Achieving the 95 95 95 targets for all: a pathway to ending AIDS. PLoS ONE 17 , e0272405 (2022).
Article CAS PubMed PubMed Central Google Scholar
van Sighem, A. I. et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24 , 1527–1535 (2010).
Article PubMed Google Scholar
Torti, C., Foca, E., Cesana, B. M. & Lescure, F. X. Asymptomatic neurocognitive disorders in patients infected by HIV: fact or fiction? BMC Med. 9 , 138 (2011).
Article PubMed PubMed Central Google Scholar
Ciccarelli, N. Considerations on nosology for HIV-associated neurocognitive disorders: it is time to update? Infection 48 , 37–42 (2020).
Nightingale, S. et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 13 , 1139–1151 (2014).
Underwood, J. et al. Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS ONE 13 , e0194760 (2018).
Meyer, A. C., Boscardin, W. J., Kwasa, J. K. & Price, R. W. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology 41 , 208–216 (2013).
Gisslen, M., Price, R. W. & Nilsson, S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect. Dis. 11 , 356 (2011).
Nightingale, S., Joska, J., Winston, A., Gisslén, M. & Barber, T. Reader response: global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95 , e2610–e2621 (2020).
Google Scholar
Nightingale, S. et al. Moving on from HAND: why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin. Infect. Dis. 73 , 1113–1118 (2021).
Guinosso, S. A., Johnson, S. B. & Riley, A. W. Multiple adverse experiences and child cognitive development. Pediatr. Res. 79 , 220–226 (2016).
Wei, J. et al. The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front. Neurol. 11 , 581346 (2020).
Clifford, D. B. HIV-associated neurocognitive disorder. Curr. Opin. Infect. Dis. 30 , 117–122 (2017).
Heaton, R. K. et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75 , 2087–2096 (2010).
Nyamayaro, P., Chibanda, D., Robbins, R. N., Hakim, J. & Gouse, H. Assessment of neurocognitive deficits in people living with HIV in Sub Saharan Africa: a systematic review. Clin. Neuropsychol. 33 , 1–26 (2019).
Ferretti, F. et al. Cognitive impairment in a clinical setting. J. Acquir. Immune Defic. Syndr. 77 , e10–e13 (2018).
Heaton, R. K. et al. Twelve-year neurocognitive decline in HIV is associated with comorbidities, not age: a CHARTER study. Brain 146 , 1121–1131 (2023).
Qu, Y. et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS 36 , 19–27 (2022).
Lu, J. et al. Effects of Glasgow Outcome Scale misclassification on traumatic brain injury clinical trials. J. Neurotrauma 25 , 641–651 (2008).
Alford, K. et al. “A fog that impacts everything”: a qualitative study of health-related quality of life in people living with HIV who have cognitive impairment. Qual. Life Res. 31 , 3019–3030 (2022).
Low, L. F. & Purwaningrum, F. Negative stereotypes, fear and social distance: a systematic review of depictions of dementia in popular culture in the context of stigma. BMC Geriatr. 20 , 477 (2020).
UK Civil Aviation Authority. Infectious Diseases Guidance Material https://www.caa.co.uk/aeromedical-examiners/medical-standards/pilots/conditions/infectious-disease/infectious-diseases-guidance-material-gm/ (2023).
Winston, A. & Spudich, S. Cognitive disorders in people living with HIV. Lancet HIV 7 , e504–e513 (2020).
Petersen, R. C. et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90 , 126–135 (2018).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397 , 1577–1590 (2021).
Mastrorosa, I. et al. Declining prevalence of HIV-associated neurocognitive disorders in more recent years and associated factors, in a large cohort of ART-treated HIV-infected individuals. Clin. Infect. Dis. 76 , e629–e637 (2023).
Saylor, D. et al. HIV-associated neurocognitive disorder – pathogenesis and prospects for treatment. Nat. Rev. Neurol. 12 , 309 (2016).
Irollo, E., Luchetta, J., Ho, C., Nash, B. & Meucci, O. Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders. Cell Mol. Life Sci. 78 , 4283–4303 (2021).
Johnson, T. P. & Nath, A. Biotypes of HIV-associated neurocognitive disorders based on viral and immune pathogenesis. Curr. Opin. Infect. Dis. 35 , 223–230 (2022).
Navia, B. A., Cho, E. S., Petito, C. K. & Price, R. W. The AIDS dementia complex: II. Neuropathology. Ann. Neurol. 19 , 525–535 (1986).
Levine, A. J. et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol. 22 , 431–441 (2016).
Rourke, S. B. et al. Asymptomatic neurocognitive impairment is a risk for symptomatic decline over a 3-year study period. AIDS 35 , 63–72 (2021).
Cole, M. A. et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology 69 , 2213–2220 (2007).
Cole, J. H. et al. No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin. Infect. Dis. 66 , 1899–1909 (2018).
Goodkin, K. et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV 4 , e411–e422 (2017).
Sanford, R. et al. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin. Infect. Dis. 67 , 1697–1704 (2018).
[No authors listed] IN BRIEF: BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022. HIV Med. 23 (Suppl. 3), 3–14 (2022).
Heaton, R. K. et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17 , 3–16 (2011).
Cysique, L. A. & Brew, B. J. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol. Rev. 19 , 169–185 (2009).
Liner, K. J. 2nd, Hall, C. D. & Robertson, K. R. Effects of antiretroviral therapy on cognitive impairment. Curr. HIV/AIDS Rep. 5 , 64–71 (2008).
Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the Mind Exchange Program. Clin. Infect. Dis. 56 , 1004–1017 (2013).
Article Google Scholar
Nichols, S. L. Central nervous system impact of perinatally acquired HIV in adolescents and adults: an update. Curr. HIV/AIDS Rep. 19 , 121–132 (2022).
Narsi, K., Tomita, A. & Ramlall, S. Neuropsychological functioning and cognitive reserve in newly HIV diagnosed antiretroviral-naive South African adults from peri-urban and informal settlements. PLoS ONE 16 , e0260260 (2021).
Narsi, K., Tomita, A. & Ramlall, S. Cognitive reserve and its determinants in newly HIV diagnosed antiretroviral-naive adults from periurban and informal settlements: evidence from an HIV hyperendemic South African setting. J. Acquir. Immune Defic. Syndr. 85 , 387–393 (2020).
Chan, P. & Valcour, V. Neurocognition and the aging brain in people with HIV: implications for screening. Top. Antivir. Med. 29 , 423–429 (2022).
PubMed Google Scholar
Winston, A. et al. Defining cerebrospinal fluid HIV RNA escape: editorial review AIDS. AIDS 33 , S107–S111 (2019).
Ferretti, F., Gisslen, M., Cinque, P. & Price, R. W. Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr. HIV/AIDS Rep. 12 , 280–288 (2015).
Kugathasan, R. et al. Diffuse white matter signal abnormalities on magnetic resonance imaging are associated with human immunodeficiency virus type 1 viral escape in the central nervous system among patients with neurological symptoms. Clin. Infect. Dis. 64 , 1059–1065 (2017).
Perez-Valero, I. et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33 , 475–481 (2019).
Underwood, J. & Winston, A. Guidelines for evaluation and management of cognitive disorders in HIV-positive individuals. Curr. HIV/AIDS Rep. 13 , 235–240 (2016).
Lucas, S. B., Wong, K. T., Nightingale, S. & Miller, R. F. HIV-associated CD8 encephalitis: a UK case series and review of histopathologically confirmed cases. Front. Neurol. 12 , 628296 (2021).
Lescure, F. X. et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin. Infect. Dis. 57 , 101–108 (2013).
Johnson, T. & Nath, A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr. Opin. Neurol. 24 , 284–290 (2011).
Venkataramana, A. et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67 , 383–388 (2006).
Ringelstein, A. et al. Severe aseptic leucoencephalopathy as immune reconstitution inflammatory syndrome in Caucasian and African patients. AIDS 23 , 1435–1437 (2009).
Chang, K. et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 34 , 203–213 (2020).
Clifford, K. M. et al. Progressive brain atrophy despite persistent viral suppression in HIV patients older than 60 years. J. Acquir. Immune Defic. Syndr. 76 , 289–297 (2017).
Sheppard, D. P. et al. Accelerated and accentuated neurocognitive aging in HIV infection. J. Neurovirol. 23 , 492–500 (2017).
Oliveira, N. L. et al. Longitudinal 5-year prediction of cognitive impairment among men with HIV disease. AIDS 35 , 889–898 (2021).
Vera, J. H. et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 86 , 1425–1432 (2016).
Eden, A. et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS ONE 11 , e0157160 (2016).
Hagberg, L. et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 7 , 15 (2010).
Milanini, B. et al. Longitudinal brain atrophy patterns and neuropsychological performance in older adults with HIV-associated neurocognitive disorder compared with early Alzheimer’s disease. Neurobiol. Aging 82 , 69–76 (2019).
McLaurin, K. A., Booze, R. M. & Mactutus, C. F. Diagnostic and prognostic biomarkers for HAND. J. Neurovirol. 25 , 686–701 (2019).
Robertson, J. et al. Increased immune activation and signs of neuronal injury in HIV-negative people on preexposure prophylaxis. AIDS 35 , 2129–2136 (2021).
Yilmaz, A. et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert. Rev. Mol. Diagn. 17 , 761–770 (2017).
Bandera, A. et al. HIV-associated neurocognitive impairment in the modern ART era: are we close to discovering reliable biomarkers in the setting of virological suppression? Front. Aging Neurosci. 11 , 187 (2019).
Ellis, R. J. et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin. Infect. Dis. 58 , 1015–1022 (2014).
Decloedt, E. H. et al. Moderate to severe HIV-associated neurocognitive impairment: a randomized placebo-controlled trial of lithium. Medicine 95 , e5401 (2016).
Sacktor, N. et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J. Neurovirol. 24 , 16–27 (2018).
Sacktor, N. et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77 , 1135–1142 (2011).
Robbins, R. N. et al. Exploring the utility of the Montreal Cognitive Assessment to detect HIV-associated neurocognitive disorder: the challenge and need for culturally valid screening tests in South Africa. Clin. Neuropsychol. 27 , 437–454 (2013).
Cysique, L. A. et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J. Clin. Exp. Neuropsychol. 33 , 505–522 (2011).
Robertson, K. et al. International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J. Neurovirol. 22 , 472–478 (2016).
Pellowski, J. A., Kalichman, S. C., Matthews, K. A. & Adler, N. A pandemic of the poor: social disadvantage and the US HIV epidemic. Am. Psychol. 68 , 197–209 (2013).
Ward-Peterson, M. et al. Using multilevel models to evaluate the influence of contextual factors on HIV/AIDS, sexually transmitted infections, and risky sexual behavior in sub-Saharan Africa: a systematic review. Ann. Epidemiol. 28 , 119–134 (2018).
Dreyer, A. J. et al. Rates of cognitive impairment in a South African cohort of people with HIV: variation by definitional criteria and lack of association with neuroimaging biomarkers. J. Neurovirol. 27 , 579–594 (2021).
Binder, L. M., Iverson, G. L. & Brooks, B. L. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch. Clin. Neuropsychol. 24 , 31–46 (2009).
MacCallum, R. C., Zhang, S., Preacher, K. J. & Rucker, D. D. On the practice of dichotomization of quantitative variables. Psychol. Methods 7 , 19–40 (2002).
Sacktor, N. et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86 , 334–340 (2016).
Jak, A. J. et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17 , 368–375 (2009).
Loewenstein, D. A. et al. Using different memory cutoffs to assess mild cognitive impairment. Am. J. Geriatr. Psychiatry 14 , 911–919 (2006).
Schinka, J. A. et al. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am. J. Geriatr. Psychiatry 18 , 684–691 (2010).
Maki, P. M. et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84 , 231–240 (2015).
Watson, C. W. et al. Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychol. 38 , 33–42 (2019).
Burch, L. S. et al. Socioeconomic status and treatment outcomes for individuals with HIV on antiretroviral treatment in the UK: cross-sectional and longitudinal analyses. Lancet Public Health 1 , e26–e36 (2016).
Bartels, C., Wegrzyn, M., Wiedl, A., Ackermann, V. & Ehrenreich, H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 11 , 118 (2010).
Jongsiriyanyong, S. & Limpawattana, P. Mild cognitive impairment in clinical practice: a review article. Am. J. Alzheimers Dis. Other Demen. 33 , 500–507 (2018).
Faure-Delage, A. et al. Socio-cultural perceptions and representations of dementia in Brazzaville, Republic of Congo: the EDAC survey. Dement. Geriatr. Cogn. Dis. Extra 2 , 84–96 (2012).
Mushi, D. et al. Social representation and practices related to dementia in Hai district of Tanzania. BMC Public Health 14 , 260 (2014).
Cacciamani, F. et al. Awareness of cognitive decline trajectories in asymptomatic individuals at risk for AD. Alzheimers Res. Ther. 12 , 129 (2020).
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256 , 183–194 (2004).
Lukasik, K. M., Waris, O., Soveri, A., Lehtonen, M. & Laine, M. The relationship of anxiety and stress with working memory performance in a large non-depressed sample. Front. Psychol. 10 , 4 (2019).
Storbeck, J. Performance costs when emotion tunes inappropriate cognitive abilities: implications for mental resources and behavior. J. Exp. Psychol. Gen. 141 , 411–416 (2012).
De Francesco, D. et al. Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med. 20 , 274–285 (2019).
Hammond, E. R. et al. Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms; a prospective cohort study. J. Neurovirol. 22 , 479–487 (2016).
Rubin, L. H. & Maki, P. M. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr. HIV/AIDS Rep. 16 , 82–95 (2019).
Woods, S. P. et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J. Clin. Exp. Neuropsychol. 26 , 759–778 (2004).
Maritz, J. et al. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve 41 , 599–606 (2010).
[No authors listed] Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 41 , 778–785 (1991).
Joska, J. A. et al. Prevalence of HIV-1 Infection in an elderly rural population and associations with neurocognitive impairment. AIDS 33 , 1765–1771 (2019).
Benjamin, L. A. et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 11 , 878–890 (2012).
Chilunda, V., Calderon, T. M., Martinez-Aguado, P. & Berman, J. W. The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era. Brain Res. 1724 , 146426 (2019).
Download references
Acknowledgements
The authors dedicate this manuscript to Christopher Sandford, a tireless advocate for the HIV community who died shortly before its publication. His insightful input and enthusiasm for the project were invaluable to this manuscript’s development.
Author information
Authors and affiliations.
HIV Mental Health Research Unit, Division of Neuropsychiatry, Department of Psychiatry and Mental Health, Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Sam Nightingale, Anna J. Dreyer & John A. Joska
Department of Neurology, Washington University School of Medicine, St Louis, MO, USA
Unit of Infectious Diseases, San Raffaele Institute, Milan, Italy
- Paola Cinque
Department of Medicine, Poona Hospital and Research Centre and Noble Hospital, Pune, India
Ameet Dravid
Institute of Biomedicine, Department of Infectious Diseases, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
Magnus Gisslén
Region Västra Götaland, Sahlgrenska University Hospital, Department of Infectious Diseases, Gothenburg, Sweden
Department of Clinical Medicine and Therapeutics, Faculty of Health Science, University of Nairobi, Nairobi, Kenya
Judith Kwasa
Department of Neurology, Johns Hopkins University, Baltimore, MD, USA
Ana-Claire Meyer & Deanna Saylor
Desmond Tutu HIV Centre, Cape Town, South Africa
Nombeko Mpongo
Department of Psychiatry, College of Health Sciences, Makerere University, Kampala, Uganda
Noeline Nakasujja
NAM, London, UK
Roger Pebody
Department of HIV Medicine, Chelsea and Westminster Hospital NHS Foundation Trust, London, UK
Anton Pozniak
Department of Clinical Research, London School of Hygiene & Tropical Medicine, London, UK
Department of Neurology, University of California San Francisco, San Francisco, CA, USA
Richard W. Price
UK Community Advisory Board (UK-CAB), London, UK
Christopher Sandford
University Teaching Hospital, Lusaka, Zambia
Deanna Saylor
Applied Cognitive Science and Experimental Neuropsychology Team (ACSENT), Department of Psychology, University of Cape Town, Cape Town, South Africa
Kevin G. F. Thomas
Division of Infection and Immunity, Cardiff University, Cardiff, UK
Jonathan Underwood
Department of Infectious Diseases, Cardiff and Vale University Health Board, Cardiff, UK
Department of Global Health and Infection, Brighton and Sussex Medical School, Brighton, UK
Jaime H. Vera
Department of Infectious Disease, Imperial College London, London, UK
- Alan Winston
HIV Clinical Trials, Winston Churchill Wing, St Mary’s Hospital, London, UK
You can also search for this author in PubMed Google Scholar
Contributions
S.N. wrote the article. All authors contributed substantially to discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Sam Nightingale .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neurology thanks J. McArthur, T. Barber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Nightingale, S., Ances, B., Cinque, P. et al. Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nat Rev Neurol 19 , 424–433 (2023). https://doi.org/10.1038/s41582-023-00813-2
Download citation
Accepted : 19 April 2023
Published : 13 June 2023
Issue Date : July 2023
DOI : https://doi.org/10.1038/s41582-023-00813-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Reply to ‘cognitive criteria in hiv: greater consensus is needed’.
- Sam Nightingale
Nature Reviews Neurology (2024)
Cognitive criteria in HIV: greater consensus is needed
- Lucette A. Cysique
- Bruce J. Brew
- Sean B. Rourke
Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management
- Ronald J. Ellis
- María J. Marquine
- Johannes C. M. Schlachetzki
Nature Reviews Neurology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
Health-Related Quality of Life in People with Advanced HIV Disease, from 1996 to 2021: Systematic Review and Meta-analysis
- Substantive Review
- Open access
- Published: 14 May 2024
Cite this article
You have full access to this open access article

- I. Portilla-Tamarit ORCID: orcid.org/0000-0002-2187-8731 1 , 2 , 3 , 4 ,
- M. Rubio-Aparicio ORCID: orcid.org/0000-0002-2599-4246 2 , 5 ,
- M. J. Fuster-RuizdeApodaca ORCID: orcid.org/0000-0003-4304-7194 6 , 7 ,
- J. Portilla-Tamarit ORCID: orcid.org/0000-0003-0881-9646 2 , 3 , 4 ,
- S. Reus ORCID: orcid.org/0000-0002-3320-9754 2 , 3 , 4 , 8 &
- J. Portilla ORCID: orcid.org/0000-0002-3676-9511 2 , 3 , 4 , 8
184 Accesses
1 Altmetric
Explore all metrics
The purpose of the study was to assess the effects of advanced HIV disease (AHD) on health-related quality of life (HRQoL) in PLHIV, the changes in HRQoL outcomes over the last 25 years, and the differences between countries according to level of economic development. We conducted a systematic review and meta-analysis. The search was conducted in PubMed and Web of Science using the terms: “health-related quality of life”, “HQRoL”, “HIV”, “AIDS”, “advanced HIV disease” and “low CD4 cells”. Studies inclusion criteria were: adult population; initiated after 1996 and published before July 2021; clinical trials, cross-sectional, cohort, and case–control studies; studies analyzing the relationship between AHD and HRQoL; English or Spanish language. Standardized mean differences ( d +) were calculated to estimate the effect size for the meta-analyses. Summary statistics were calculated using a random-effects model, and analyses of effect moderators, using mixed-effects models. The meta-analysis included 38 studies. The results indicated that HRQoL is worse in patients with AHD compared to those without. The main HRQoL domains affected were overall health perception and concern and physical and functional health and symptoms. We found a moderate impact for age and gender on some HRQoL domains. There were no differences in relation to socioeconomic inequities, country of residence, or time period analyzed. In conclusion, advanced HIV disease has a negative impact on health and well-being in PLHIV. Our results show that despite all the advances in antiretroviral treatments over the last 25 years, AHD persists as a source of extreme vulnerability, regardless of where PLHIV live.
El objetivo del estudio fue evaluar los efectos de la enfermedad avanzada de sida (EAS) en la calidad de vida relacionada con la salud (CVRS) en personas que viven con el VIH (PVVIH), los cambios experimentados en la CVRS en los últimos 25 años y las diferencias entre países. Realizamos una revisión sistemática y metaanálisis. La búsqueda se llevó a cabo en PubMed y Web of Science utilizando los términos: “calidad de vida relacionada con la salud”, “CVRS”, “VIH”, “SIDA”, “enfermedad avanzada por VIH” y “células CD4 bajas”. Los criterios de inclusión de los estudios fueron: población adulta; iniciado después de 1996 y publicado antes de julio de 2021; ensayos clínicos, estudios transversales, de cohorte y de casos y controles; estudios que analizan la relación entre EAS y CVRS; idioma inglés o español. Se calcularon diferencias de medias estandarizadas ( d +) para estimar el tamaño del efecto para los metaanálisis. Los efectos promedios se calcularon utilizando un modelo de efectos aleatorios, y el análisis de moderadores utilizando modelos de efectos mixtos. El metaanálisis incluyó 38 estudios. Los resultados indicaron que la CVRS es peor en pacientes con EAS en comparación con aquellos sin EAS. Los principales dominios de CVRS afectados son la percepción de salud general y su preocupación , y la función física y de salud y los síntomas asociados . Encontramos un impacto moderado por edad y género en algunos dominios de CVRS. No encontramos diferencias en cuanto a las desigualdades socioeconómicas, país de residencia o período de tiempo analizado. En conclusión, la enfermedad avanzada por VIH tiene un impacto negativo en la salud y el bienestar en las personas con VIH. Nuestros resultados muestran que, a pesar de todos los avances en los tratamientos antirretrovirales en los últimos 25 años, el EAS persiste como una fuente de extrema vulnerabilidad, independientemente de dónde vivan las personas con VIH.
Similar content being viewed by others

Health, Health-Related Quality of Life, and Quality of Life: What is the Difference?

Lecanemab Clarity AD: Quality-of-Life Results from a Randomized, Double-Blind Phase 3 Trial in Early Alzheimer’s Disease

A systematic review of quality of life research in medicine and health sciences
Avoid common mistakes on your manuscript.
Introduction
In 1996, the advent of combined active antiretroviral therapy (cART) dramatically transformed the face of AIDS [ 1 ]. Since then, the efficacy and safety of antiretroviral therapies have improved, while AIDS-related morbidity and mortality have continued to decrease [ 1 ]. In 2014, the World Health Organization (WHO) approved a new goal on HIV and AIDS: “ to end the AIDS epidemic as a public health threat by 2030 ”. To achieve this objective, UNAIDS established the “90-90-90” targets, defined as: “90% of people living with HIV (PLHIV) will know their HIV status, 90% of people who know their HIV-positive status will be accessing treatment, and 90% of PLHIV on treatment will have suppressed viral loads.” This strategy promoted healthy lives and well-being for all PLHIV, regardless of their age or country of residence [ 2 , 3 ]. Recently, these objectives have become more ambitious, raising the bar to ensure that 95% of PLHIV achieve these targets [ 4 ]. Since then, different authors have highlighted the importance of health-related quality of life (HRQoL) for attaining long-term health and well-being in PLHIV, calling on health systems, international societies, and others providing AIDS services to include it as an essential goal of HIV care [ 5 , 6 ].
As a multidimensional concept, HRQoL can be influenced by many factors in PLHIV, including ageing, gender, HIV-related symptoms, comorbidities, substance abuse, depression, socioeconomic inequalities, employment, and financial stress, among others [ 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. Understanding all the factors associated with HRQoL is critical for designing interventions to support PLHIV, especially vulnerable populations such as those accessing services with advanced HIV disease (AHD).
The WHO defines AHD as a CD4 + count under 200 cells/µL or a WHO clinical stage 3 or 4 [ 14 ]. Despite all the efforts to rapidly diagnose HIV infection, diagnosis of AHD is still frequent in PLHIV even in middle- and high-income countries. In 2021, of the 692,000 people in the European Union/European Economic Area who received a new HIV diagnosis, 35% were diagnosed with AHD [ 15 ]. Worldwide, about 650,000 people died from AIDS-related diseases in 2021, over half in the WHO Africa and Asia–Pacific regions [ 16 ].
AHD has a negative impact on the health of PLHIV [ 17 , 18 ], increasing the likelihood of developing AIDS- and non-AIDS-defining disease, especially cancer, cardiovascular disease, kidney disease, and neurocognitive impairment. All these risks predispose those with AHD to experience more physical symptoms, including chronic pain, extreme fatigue, and a significant decline in their ability to carry out activities of daily living [ 19 , 20 , 21 , 22 ]. Moreover, AHD is associated with an increased risk of mortality and reduced life expectancy [ 21 , 23 ]. Managing multiple medical conditions simultaneously can be overwhelming, negatively impacting emotional and psychological quality of life [ 24 ]. Furthermore, the stigma and discrimination associated with HIV may intensify in this advanced stage, leading to social isolation, self-esteem issues, and difficulties in accessing adequate medical care [ 25 ]. All these aspects suggest that AHD has a devastating impact on the physical, mental, and emotional health in PLHIV, ultimately affecting HRQoL.
However, there is no consensus on the impact of AHD on HRQoL. Different authors have reported that PLHIV with low CD4 counts or an AIDS-defining event have worse HRQoL than those with higher CD4 counts or without AIDS. However, there is no agreement about which HRQoL domains are most affected in people with AHD. Regarding CD4 + cells counts, studies by Fuster-RuizdeApodaca et al., Venturi et al., and Aden et al. have reported that lower CD4 counts correspond to lower overall HRQoL scores [ 26 , 27 , 28 ]. Emuren et al. and Liu et al. described that CD4 + cell counts below 200/µL were associated with worse physical but not mental health [ 29 , 30 ]. On the other hand, Fumaz et al., focusing on gender differences, observed that poorer mental health in women was associated with lower CD4 cell counts but not with physical health [ 31 ]. Préau et al. reported similar results in a mixed-gender sample, finding a negative association between low CD4 cells and mental HRQoL [ 32 ]. Regarding AIDS, Emuren et al. and Préau et al. found that an AIDS diagnosis was linked with worse physical health but not with the mental health dimension [ 29 , 33 ], while Fumaz et al. reported that AIDS-defining events were similarly associated with unacceptably low physical and mental HRQoL, regardless of gender [ 31 ]. Nevertheless, Badia et al. did not find any relationship between low CD4 counts or AIDS with lower HRQoL scoring [ 34 ]. The variability of these findings in different settings supports the need to synthesize all available evidence to clarify how AHD affects HRQoL.
Another aspect that warrants investigation is the effect of improvements in antiretroviral therapy (ART) over time. Since its introduction in 1996, ART has made gradual but steady advances in terms of better efficacy, decreased toxicity, and improved convenience. In the first years of the AIDS pandemic, surviving to an AHD diagnosis was the main challenge for PLHIV. In the mid-1990s, treatments with protease inhibitors (PIs) like ritonavir and indinavir plus zidovudine represented a significant breakthrough in the approach to HIV/AIDS, providing an opportunity for people with AHD [ 1 ]. The first PIs were associated with diarrhea, metabolic side effects, and lipodystrophy [ 35 ], while zidovudine, the first nucleoside retrotranscriptase inhibitor (NRTI), was associated with anemia and bone marrow suppression [ 36 ]. Other first-generation NRTIs, such as stavudine, zalcitabine, and didanosine were associated with severe mitochondrial toxicity, peripheral neuropathy, and pancreatitis [ 37 ]. Efavirenz, the first non-nucleoside retrotranscriptase inhibitor (NNRTI), and nevirapine were approved at the end of the twentieth century. Efavirenz was associated with neurotoxicity, while nevirapine could cause severe hypersensitivity hepatitis [ 38 , 39 ]. In the 2000s, the second-generation therapies appeared, including boosted PI, new NRTI, and NNRTI, with greater efficacy, tolerance, and convenience [ 40 ]. These new drugs permitted the development of fixed-dose combination (FDC) therapies, reducing the pill burden and improving adherence. In this second period, PIs were associated with a higher cardiovascular risk [ 41 ], and tenofovir disoproxil-fumarate (TDF) with renal and bone toxicity [ 42 ]. In the mid-2000s, raltegravir, the first integrase strand-transfer inhibitor (INSTI), was approved for the treatment of HIV infection, followed by elvitegravir, dolutegravir, and bictegravir. The introduction of the latest INSTIs marked the third period of antiretroviral treatment, characterized by the widespread use of FDC in therapies and the development of potent bi-therapies for treating HIV infection. INSTIs have been associated with neuropsychiatric toxicity and obesity, but these adverse effects rarely lead to treatment discontinuation [ 43 ]. The success of ART has permitted the establishment of new strategies, such as early treatment of HIV infection regardless of the number of CD4 cells and the “test and treat” strategy, initiating ART as soon as possible [ 44 , 45 ]. These extraordinary changes could support the hypothesis that improvements in ART from 1996 to 2021 have impacted HRQoL even in PLHIV with AHD. Unfortunately, access to remarkable advances in antiretroviral therapy has been disparate worldwide due to the varying availability and allocation of economic resources in different countries [ 46 ].
The aims of this meta-analysis are: (1) to assess the effects of AHD on HRQoL in PLHIV; (2) to analyze the probable changes on HRQoL outcomes over the last 25 years, from the advent of the first cART with PI to the new regimens of ART that use INSTI; (3) to assess heterogeneity among studies; and (4) to evaluate potential differences in HRQoL in PLHIV with AHD from different countries and levels of economic development: low-, middle- and high- income countries, and to examine the influence of other potential variables.
Protocol and Registration
The study protocol was pre-registered on the Open Science Framework (see https://doi.org/10.17605/OSF.IO/RZAMQ for further information). Furthermore, this systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA guidelines) [ 47 ]. Supplementary Table 1 presents the PRISMA checklist.
Study Eligibility Criteria
Our study population was adults with AHD defined as AIDS (CDC clinical stage C3 or WHO HIV-clinical stage 3/4), or CD4 < 200 cells/µL [ 14 ]. Inclusion criteria were: (1) studies in PLHIV aged 18 years or older; (2) studies initiated after the advent of cART in 1996, and published before 31 July 2021; (3) clinical trials, cross-sectional, cohort, and case–control studies that included any HRQoL intervention; (4) studies analyzing the relationship between AHD and HRQoL, comparing HRQoL with versus without AIDS and/or with CD4 + lymphocytes ≥ 200 cells/μL versus < 200 cells/μL; (5) studies written in English or Spanish. Exclusion criteria were: (1) meta-analyses, systematic reviews, and secondary studies; (2) abstracts, conference proceedings, books or book chapters, unpublished material, and reports that were not peer-reviewed; (3) articles reporting HRQoL measures obtained using a non-validated questionnaire; (4) articles reporting lymphocyte CD4 + counts, not categorized as having more or less than 200 CD4 + cells/μL.
Search Strategy
The search was conducted from 1 to 31 July 2021 in PubMed and the Web of Science. The following keywords were used: “health-related quality of life” [OR] “HQRoL” AND “HIV” [OR] “AIDS” [OR] “advanced HIV disease” [OR] “low CD4 cells”. Supplementary Table 2 presents the full search strategy. Additionally, the reference lists of the selected articles were handsearched to identify other potentially eligible studies.
Search results were de-duplicated, and four review authors (IPT, JP, JPT and SR) independently screened the titles and abstracts against the selection criteria. All relevant or potentially relevant records were retrieved for full-text review, which was also performed independently by IPT, JP, JPT, and SR. Disagreements were resolved by discussion between the four review authors. Doubts about HRQoL measures or meta-analysis methods were resolved by MJF, an expert in HRQoL, and MRA, an expert in meta-analyses.
Data Extraction and Quality Assessment
Study characteristics were independently extracted by the researchers according to a predefined protocol and categorized based on article characteristics. The following information was collected: first author, publication year, country, World Bank income level, sample size, demographic characteristics of the sample (i.e., age, sex), study design, HRQoL questionnaires, recruitment period, percentage of patients on ART, percentage of patients with undetectable viral load, percentage of patients with CD4 + < 200 cells/µL, and percentage of patients with AIDS (Table 1 ). Information was also extracted on study funding, authors’ conflict of interests, and other ethical issues.
The methodological quality of the studies included in the meta-analysis was assessed with the Newcastle–Ottawa Scale (NOS), adapted for cross-sectional studies [ 48 ]. This scale uses a “star system” to judge quality based on three dimensions: selection, comparability, and outcome. The NOS consists of 7 items, and the total maximum quality score is 9 stars. Study quality was classified as “high” (8–9 stars), “moderate” (5–7 stars) or “low” (≤ 4 stars).
To assess the reliability of the data extraction, all studies were independently coded by two review authors (IPT and MRA), and inconsistencies were resolved by consensus. For categorical variables, kappa coefficients ranged between 0.810 and 1.0 (M = 0.931), and for continuous variables all intra-class correlations were equal to 1.0.
Computation of Effect Sizes
Usually, HRQoL is measured as a multidimensional concept; however, not all HRQoL questionnaires have exactly the same dimensions. To make the comparison feasible, we categorized the most frequent dimensions according to similarity or by grouping the instruments containing summary indexes. Three review authors (PI, RM, MJF) carried out this process, discussing and resolving discrepancies by consensus. Six outcome domains were finally created: overall health perception and concern , physical and functional health and symptoms , psychological health , social relationships , mental health summary , and physical health summary . Supplementary Table 3 provides details on how dimensions of the questionnaires were categorized.
Once the six HQRoL domains were summarized, the effect sizes for each individual study were calculated. The effect size index was the standardized mean difference ( d ), defined as the difference between the mean of the treatment group and the mean of the control group, divided by a pooled standard deviation (SD): d = c(m)(yT − yC)∕SD, with c(m) representing a correction factor for small sample sizes [ 49 ]. In this meta-analysis, the d index was calculated to compare the differences in HRQoL between people diagnosed versus not diagnosed with AIDS, and between those with CD4 counts under versus over 200 cells/µL. In any case, the mean values of the non-AIDS and the CD4 ≥ 200 cells/µL groups (control groups) were subtracted from the means for the AIDS and the CD4 < 200 cells/µL groups (treatment groups), respectively, such that negative d values indicate poorer HRQoL in the AIDS and CD4 < 200 cells/µL groups. For standardization, absolute values of d of around 0.2, 0.5, and 0.8 were interpreted as small, moderate, and large magnitudes of effect, respectively [ 50 ]. For effect size calculations, the available means and SDs for each group were used. When this information was not reported, the corresponding authors were contacted to request the required values. In some studies, when the results were reported by means of odds ratio or correlation, conversion formulas were applied to obtain the corresponding d value [ 51 , 52 ].
Statistical Analysis
Separate meta-analyses were carried out with the effect sizes for each of the six outcome measures, for the comparison of the AIDS versus non-AIDS groups, and for the comparison of the CD4 < 200 cells/µL versus CD4 ≥ 200 cells/µL groups.
Random-effects models were used to account for the expected variability in effect sizes. This model involves weighting each effect size by its inverse variance, defined as the sum of the within-study variance and between-study variance, the latter being estimated by restricted maximum likelihood [ 53 ]. For each meta-analysis, the method proposed by Hartung was applied to compute the mean effect size along with its 95% confidence intervals (CI) [ 54 ]. To check the variability in effect sizes, Cochran’s heterogeneity Q statistic and the I 2 index (values of 0%, 25%, 50% and 75% representing no, low, moderate, and high heterogeneity, respectively) were calculated [ 55 ]. For each meta-analysis, a forest plot was also constructed.
Publication bias was assessed by constructing funnel plots using the trim-and-fill method, which consists of imputing missing effect sizes to achieve symmetry [ 56 ]. Furthermore, Egger’s regression test was also applied [ 57 ]. Evidence of publication bias was defined as a statistically significant result for Egger’s test, defined at p < 0.10 instead of the usual p < 0.05 because of the lower statistical power with a small number of studies [ 58 ].
Finally, in the presence of heterogeneity and at least 10 studies for the outcome, analyses of potential effect moderators were performed [ 59 ]. Meta-regressions and weighted ANOVAs for continuous and categorical moderators, respectively, were applied by assuming mixed-effect models by means of the F statistic, described by Knapp-Hartung [ 60 , 61 ]. The Q E statistic was calculated to assess the model misspecification of the moderator analyses, together with an estimate of the percentage of variance accounted for by the moderator, R 2 [ 62 ].
All statistical analyses were carried out with the metafor package in R version 3.2.3 [ 63 ].
Characteristics of Included Studies
The search yielded 1079 results in PubMed and 1487 in the Web of Science. After removing duplicates, a total of 1585 unique records were screened, and 531 full-text articles were assessed for eligibility (see Fig. 1 ). After exclusion criteria were applied, 38 studies were included in the meta-analysis. One of these used two samples, so a total of 39 samples were analyzed (Table 1 ; Fig. 1 ) [ 24 , 27 , 28 , 29 , 33 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 ]. According to the assessment with the NOS scale, 18 studies (46.2%) were deemed high quality and 21 (53.8%) moderate quality (Supplementary Table 4 ). All studies met criteria for the ascertainment of the exposure and the assessment of the outcome, as these were inclusion criteria for the study.
Flow chart of study selection process
Mean Effect Sizes and Heterogeneity
Table 2 presents the results of the meta-analysis for the comparison of the groups with CD4 < 200 cells/µL versus CD4 ≥ 200 cells/µL, for each of the six HRQoL outcome domains. Forest plots are displayed in Fig. 2 . The mean effect sizes for all six outcomes were negative, indicating worse HRQoL in the group with lower CD4 counts. All results were statistically significant except for psychological health ( d + − 0.133, 95% CI − 0.319, 0.054) and mental health summary ( d + − 0.260, 95% CI − 0.851, 0.332). The highest mean effect size was found for overall health perception and concern ( d + − 0.598, 95% CI − 0.949, − 0.248), followed by physical and functional health and symptoms ( d + − 0.395, 95% CI − 0.740, − 0.049) and physical health summary ( d + − 0.362, 95% CI − 0.557, − 0.167), with similar magnitudes. The meta-analysis showed heterogeneity (I 2 > 70% and statistically significant Q), except for physical health summary , where the I 2 value was 36.7% and Cochran’s Q was not significant (Table 2 ). This large variability is also reflected in the forest plots (Fig. 2 ).
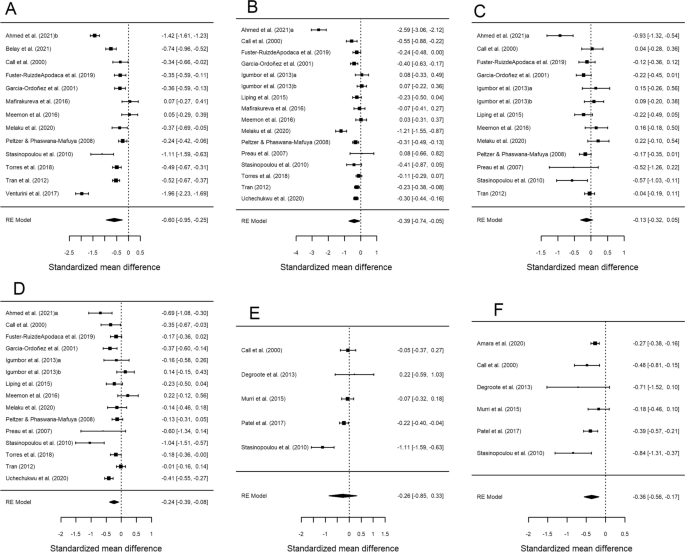
Forest plots displaying the standardized mean differences with 95% confidence intervals for the comparison of the CD4 < 200 cells/μL and CD4 > 200 cells/μL groups for each of the six outcome measures: overall health perception and concern ( A ), physical and functional health and symptoms ( B ), psychological health ( C ), social relationship ( D ), mental health summary ( E ) and physical health summary ( F ). RE (random-effects) model refers to the statistical model used in the calculations
Table 3 presents the results of the meta-analysis comparing AIDS versus non-AIDS groups for each of the six HRQoL outcome measures. Forest plots are displayed in Fig. 3 . As previously, the mean effect sizes were negative for all six outcomes, indicating poor HRQoL in the AIDS group. All analyses were statistically significant with the exception of psychological health ( d + − 0.136; 95% CI − 0.305, 0.032). In this case, the highest mean effect size was found for physical health summary ( d + − 0.670; 95% CI − 1.002, − 0.338), while the rest of the mean effect sizes showed a low to moderate magnitude. Regarding the variability among effect sizes, very high heterogeneity was found for all outcomes (I 2 > 80% and statistically significant Q) (Table 3 ; Fig. 3 ).
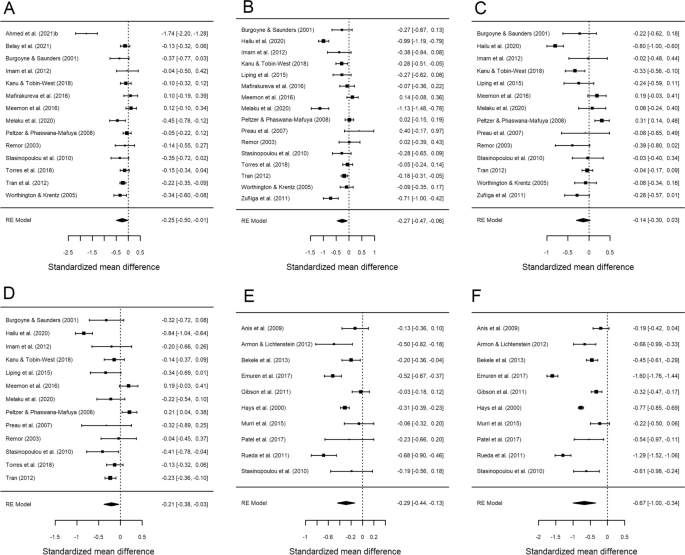
Forest plots displaying the standardized mean differences with 95% confidence intervals for the comparison of the AIDS with non-AIDS groups for each of the six outcome measures: overall health perception and concern ( A ), physical and functional health and symptoms ( B ), psychological health ( C ), social relationship ( D ), mental health summary ( E ) and physical health summary ( F ). RE (random-effects) model refers to the statistical model used in the calculations
Publication Bias
Publication bias was assessed through Egger’s tests and funnel plots, using the trim-and-fill method. Figure 4 presents the funnel plots for the comparison according to CD4 counts (< 200 cells/µL versus ≥ 200 cells/µL) for the HRQoL measures. For physical health summary , two additional effect sizes were imputed to the original set of estimates to achieve symmetry in the funnel plot (Fig. 4 F). When a mean effect (and its 95% CI) was calculated using the six effect sizes plus the two imputed values, the mean effect was d + − 0.317 (95% CI − 0.490, − 0.144). However, Egger’s test did not reach statistical significance ( p = 0.117).
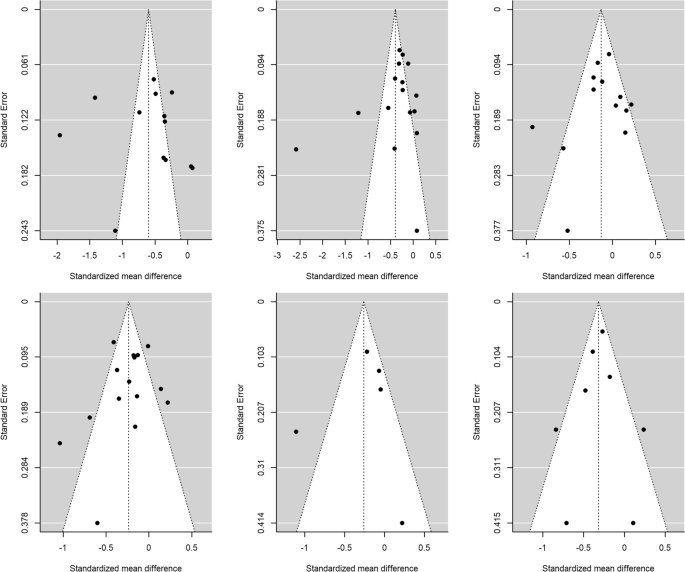
Funnel plots for the comparison of the CD4 < 200 cells/μL and CD4 > 200 cells/μL groups for overall health perception and concern ( A ), physical and functional health and symptoms ( B ), psychological health ( C ), social relationship ( D ), mental health summary ( E ) and physical health summary ( F ) measures. The white circles are the imputed standardized mean changes by means of the Duval and Tweedie's trim and-fill method
Figure 5 presents the funnel plots for the comparison of the AIDS versus non-AIDS groups. For social relationships , four additional effect sizes were imputed to achieve symmetry in the funnel plot (Fig. 5 D). When a mean effect (and its 95% CI) was calculated using the 13 effect sizes plus the four imputed values, the mean effect was d + − 0.083 (95% CI − 0.262, 0.095). Nevertheless, once again Egger’s test was not statistically significant ( p = 0.61).
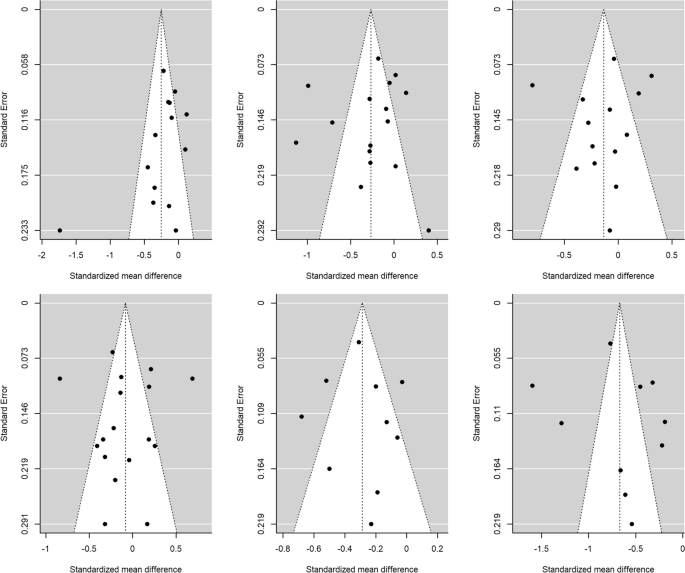
Funnel plots for the comparison of the AIDS with non-AIDS groups for overall health perception and concern ( A ), physical and functional health and symptoms ( B ), psychological health ( C ), social relationship ( D ), mental health summary ( E ) and physical health summary ( F ) measures. The white circles are the imputed standardized mean changes by means of the Duval and Tweedie's trim and-fill method
For the rest of meta-analyses, the trim-and-fill method did not require imputing new values to the funnel plots (Figs. 4 , 5 ), and the Egger’s tests were not statistically significant. Thus, the results of these meta-analyses are at low risk for publication bias.
Analysis of Moderator Variables
The heterogeneity observed in the standardized mean differences prompted an analysis of moderator variables for outcome measures with at least 10 studies. Tables 4 and 5 show the results of the meta-regressions, and Tables 6 and 7 of the weighted ANOVAs, comparing groups by CD4 counts and AIDS status, respectively.
Of the different potential moderator variables analyzed, only two continuous variables yielded a statistically significant result for the comparison of the CD4 groups (see Table 4 ): mean age exhibited a statistically significant relationship with the effect size for overall health perception and concern ( p = 0.038), accounting for 35.5% of the variance observed. The negative sign of the regression coefficient for this moderator indicated larger standardized mean differences, as the mean age of participants decreased for this outcome measure. On the other hand, the percentage of men showed a significant relationship with the effect sizes of the social relationships measure ( p = 0.042), accounting for 30.8% of the variance. Once again, a negative relationship was found, such that the larger the proportion of men, the lower the standardized mean differences for this outcome measure.
Finally, for the comparison of groups with AIDS versus without AIDS, country income level yielded a marginally significant result ( p = 0.059) as an effect modifier, explaining a notable percentage of the variance ( R 2 = 38.21%) for the physical and functional health and symptoms measure (see Table 7 ). The highest effect was found in the low-income category ( d + − 0.786).
We conducted a meta-analysis to assess the influence of AHD on HRQoL and its different domains. We included papers published over the last 25 years of the AIDS pandemic, analyzing possible differences in HRQoL and AHD in different periods of time as well as differences between countries of different income levels. Our main finding was that HRQoL is worse in patients with AHD compared to those without. People with CD4 counts of fewer than 200 cells/µL sustain negative impacts in all domains of HRQoL, especially in overall health perception and concern , followed by physical and functional health and symptoms , and physical health summary , with similar mean effect sizes but large inter-study variability. The outcomes related to psychological health and mental health summary were also negatively affected, though comparisons did not reach statistical significance. In patients with an AIDS diagnosis, the six HRQoL domains showed lower scores than in those without AIDS. The greatest difference was observed for physical health summary . As in patients with low CD4 cells, psychological health scores did not show a significant difference between people with versus without AIDS. This finding is probably related to the negative psychological impact of an HIV diagnosis on mental health in PLHIV, regadless of AIDS diagnosis or number of CD4 cells [ 96 , 97 ]. PLHIV often experience intersecting types of discrimination (marginalized identities, internalized HIV stigma, limited economic resources, etc.) or suffer from uncertainty about a non-curable and potentially lethal infection. Thus, PLHIV are highly vulnerable to mental health problems [ 98 , 99 ], independently of their immunovirological status.
In this meta-analysis, we found that overall health perception and concern and physical and functional health and symptoms are the main HRQoL domains affected in PLHIV with AHD. Physical and functional health and symptoms includes different dimensions, including physical functioning, energy, mobility, effects and severity of pain, and level of independence. The symptomatology of advanced stages of HIV infection, the associated comorbidities, and the loss of vitality caused by progression of HIV infection could explain the worse scores in these domains [ 19 , 20 , 21 , 22 ].
Our meta-analysis considers numerous effect modifiers (age, gender, treatment with ART, and HIV viral load) that were analyzed for their potential influence on the effect sizes of the HRQoL outcome. We found a moderate impact for age and gender on some HRQoL domains. Age showed an inverse relationship with the effect sizes for overall health perception and concern , such that older age was associated with worse scores, probably due to ageing, comorbidities, and disability associated with AHD [ 100 ]. The domain for overall health perception and concern comprises perceptions, distress, concerns, and worries related to general health. Thus, this result is consistent with previous studies showing that HRQoL indexes of physical health are negatively affected in older PLHIV [ 101 ]. Furthermore, male sex showed a significant negative relationship with the effect sizes for social relationships . In high-income countries, men with HIV are usually reported as having better scores in social relationships and other related dimensions or predictors than women with HIV [ 27 , 31 ]. Our results shows that this trend could differ between countries depending on income level, that AHD could affect men’s social relationships more than women’s, or that in low- and medium-income countries women use social support as coping strategy to reduce the stressors on health outcomes [ 8 , 102 ]. Another possibility is that men are overrepresented in this meta-analysis, especially in studies from low- and middle-income countries.
Regarding the relationship between HRQoL and country income level, we hypothesized that people with AHD from middle- or high-income countries would report higher HRQoL scores than in those living in low-income countries, due to the direct impact of socioeconomic status on HRQoL [ 8 ]. However, we did not find differences between the analyzed countries according to this parameter. When we compared the group with versus without AIDS, we found only a borderline significant result for physical and functional health and symptoms , in PLHIV with AIDS from low-income countries. These results could be related with worse conditions in housing, nutrition, health resources, employment difficulties, and other socioeconomic indicators in these settings [ 88 , 95 ]. In fact, there is evidence supporting the degenerative impact that material deprivation has on HRQoL [ 103 ].
Furthermore, given the continuous improvements in the efficacy and safety of ART from 1996 to the present, we hypothesized that HRQoL in PLHIV with AHD in the era of the new ART (2012–2019) would be better than when these drugs were first introduced (1996). We analyzed three 8-year periods: 1996–2003, 2004–2011, and 2012–2019. Surprisingly, we observed no differences between the time periods analyzed. AHD continues being the worst condition in the spectrum of HIV infection, occurring in high-income and low-income countries alike, despite the availability of new antiretroviral treatments, early diagnosis strategies, and easy access to ART. Ghiasvand et al.’s meta-analysis of studies from 2005 to 2016 in low-, middle- and high-income countries likewise found no impact on HRQoL from ART in PLHIV [ 103 ]. The present meta-analysis broadens this evidence by also including earlier periods with less effective and more toxic treatments in PLHIV with AHD. These results suggest that improving quality of life for PLHIV may require additional interventions beyond just the provision of ART.
To address the significant impact of AHD on HRQoL, the initial step should be the implementation of prevention programs, early diagnosis, and early treatment to decrease the prevalence of AHD worldwide [ 44 ]. Regarding strategies to enhance HRQoL in PLHIV, these should first focus on addressing basic needs such as nutrition, access to healthcare resources, and employment, which have been associated with low HRQoL [ 6 , 95 ]. This is particularly crucial in low-income countries, where inequalities are often more pronounced and require steadfast policy responses. Indeed, a systemic and comprehensive approach that considers the special individual needs of this population is essential everywhere. This should include bolstering resilience resources, economic empowerment, and self-stigma detection. Moreover, PLHIV with AHD need preventive interventions that focus on both AIDS and non-AIDS events, among other aspects [ 6 ]. Other strategies should provide health education along with comprehensive disease management information and training to ensure adherence to ART, thereby empowering patients to effectively participate in controlling their disease [ 76 ]. Emotional support services and counseling should be offered to manage stress and other psychological challenges [ 29 ]. Encouraging participation in support groups, social activities, and community programs is also crucial for reducing loneliness [ 74 ]. Additionally, efforts should be directed towards eradicating the stigma associated with AHD while promoting inclusivity and understanding for PLHIV in society [ 25 ]. To achieve all these goals, individuals with AHD should receive multidisciplinary, comprehensive, and personalized care from a team of physicians, nurses, social workers, and psychologists.
Our study has some limitations. First of all, we could only make use of data available from published studies. Second, we were unable to explore the influence of some factors associated with reduced HRQoL: intravenous drug use or substance misuse, socioeconomic inequalities, refugee or migrant status, lower educational level, social support, and internalized stigma [ 104 ]. Third, some countries and WHO regions are underrepresented. We did not find data from Latin America (except from Mexico and Guyana), eastern Europe countries, the Western Pacific and Eastern Mediterranean regions (except Pakistan), India, Brazil, or others. The languages used in this meta-analysis (Spanish or English), along with the databases chosen (PubMed and the Web of Science, both dominated by research from Western countries) probably generated some selection bias in the included studies.
On the other hand, the review also has some important strengths. First, we obtained enough data to demonstrate the highly negative impact of AHD on HRQoL. Second, the long study period enabled a comparison of HRQoL in different periods of the AIDS pandemic and in countries with different income levels. Its results show that despite all the advances in the treatment of HIV infection over the last 25 years, AHD persists as a source of extreme vulnerability for PLHIV.
In conclusion, this meta-analysis shows that AHD has a negative impact on the health and well-being of PLHIV, affecting all HRQoL domains, especially overall self-perceived health, physical health summary, and psychological health. These effects have not changed in the last 25 years and affect all PLHIV with AHD independently of country of residence. HIV clinicians and researchers should focus future studies on improving HRQoL and better understanding the special needs of this vulnerable population.
Data Availability
Dataset is available upon request.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60.
Article PubMed Google Scholar
World Health Organization. Draft global health sector strategies HIV, 2016–2021 [Internet]. 2016. https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_31-en.pdf?ua=1 .
UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014.
UNAIDS. Understanding fast-track targets. Accelerating action to end the AIDS epidemic by 2030. [Internet]. 2015. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf
Lazarus JV, Van Hout MC, Fuster-Ruizdeapodaca MJ, Brown G, Guaraldi G. A call for health systems to monitor the health-related quality of life of people living with HIV . HIV Med. 2023;24:107–10.
Lazarus JV, Safreed-Harmon K, Kamarulzaman A, Anderson J, Leite RB, Behrens G, et al. Consensus statement on the role of health systems in advancing the long-term well-being of people living with HIV. Nat Commun. 2021;12:4450.
Article CAS PubMed PubMed Central Google Scholar
Briongos Figuero L, Bachiller Luque P, Palacios Martín T, González Sagrado M, Eiros BJ. Assessment of factors influencing health-related quality of life in HIV-infected patients: assessment of factors influencing health-related quality of life. HIV Med. 2011;12:22–30.
Article CAS PubMed Google Scholar
Degroote S, Vogelaers D, Vandijck DM. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 2014;72:40.
Article PubMed PubMed Central Google Scholar
Douab T, Marcellin F, Vilotitch A, Protopopescu C, Préau M, Suzan-Monti M, et al. Health-related quality of life of people living with HIV followed up in hospitals in France: comparing trends and correlates between 2003 and 2011 (ANRS-VESPA and VESPA2 national surveys). AIDS Care. 2014;26:S29-40.
Millar BM, Parsons JT, Redline S, Duncan DT. What’s sleep got to do with it?: Sleep health and sexual risk-taking among men who have sex with men. AIDS Behav. 2019;23:572–9.
Nigusso FT, Mavhandu-Mudzusi AH. Health-related quality of life of people living with HIV/AIDS: the role of social inequalities and disease-related factors. Health Qual Life Outcomes. 2021;19:63.
Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16.
Turan B, Budhwani H, Fazeli PL, Browning WR, Raper JL, Mugavero MJ, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav. 2017;21:283–91.
Ford N, Meintjes G, Calmy A, Bygrave H, Migone C, Vitoria M, et al. Managing advanced HIV disease in a public health approach. Clin Infect Dis. 2018;66:S106-SS110.
ECDC. HIV/AIDS Surveillance in Europe 2021. [Internet]. 2022. https://www.ecdc.europa.eu/sites/default/files/documents/2022-Annual_HIV_Report_final.pdf
World Health Organization. Key facts HIV 2021 [Internet]. 2022. https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/key-facts-hiv-2021-26july2022.pdf?sfvrsn=8f4e7c93_5
Calmy A, Ford N, Meintjes G. The persistent challenge of advanced HIV disease and AIDS in the era of antiretroviral therapy. Clin Infect Dis. 2018;66:S103-SS105.
Levy I, Maor Y, Mahroum N, Olmer L, Wieder A, Litchevski V, et al. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: a retrospective cohort study. BMJ Open. 2016;6: e012721.
Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–97.
Pettit AC, Giganti MJ, Ingle SM, May MT, Shepherd BE, Gill MJ, et al. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J Intern AIDS Soc. 2018;21: e25031.
Article Google Scholar
Sobrino-Vegas P, Moreno S, Rubio R, Viciana P, Bernardino JI, Blanco JR, et al. Impact of late presentation of HIV infection on short-, mid- and long-term mortality and causes of death in a multicenter national cohort: 2004–2013. J Infect. 2016;72:587–96.
Zhang S, van Sighem A, Kesselring A, Gras L, Prins J, Hassink E, et al. Risk of non-AIDS-defining events among HIV-infected patients not yet on antiretroviral therapy: Non-AIDS-defining events in untreated patients. HIV Med. 2015;16:265–72.
May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–202.
Anis AH, Nosyk B, Sun H, Guh DP, Bansback N, Li X, et al. Quality of life of patients with advanced HIV/AIDS: measuring the impact of both AIDS-defining events and non-AIDS serious adverse events. JAIDS. 2009;51:631–9.
PubMed Google Scholar
Gesesew HA, Tesfay Gebremedhin A, Demissie TD, Kerie MW, Sudhakar M, Mwanri L. Significant association between perceived HIV related stigma and late presentation for HIV/AIDS care in low and middle-income countries: a systematic review and meta-analysis. PLoS ONE. 2017;12: e0173928.
Aden B, Nosyk B, Wittenberg E, Schackman BR. Health-related quality of life in HIV-infected and at-risk women: the impact of illicit drug use and hepatitis C on a community preference weighted measure. Med Decis Making. 2014;34:800–8.
Fuster-RuizdeApodaca MJ, Laguía A, Safreed-Harmon K, Lazarus JV, Cenoz S, del Amo J. Assessing quality of life in people with HIV in Spain: psychometric testing of the Spanish version of WHOQOL-HIV-BREF. Health Qual Life Outcomes. 2019;17:144.
Venturini A, Cenderello G, Di Biagio A, Giannini B, Ameri M, Giacomini M, et al. Quality of life in an Italian cohort of people living with HIV in the era of combined antiretroviral therapy (Evidence from I.A.N.U.A. study-investigation on antiretroviral therapy). AIDS Care. 2017;29:1373–7.
Emuren L, Welles S, Evans AA, Polansky M, Okulicz JF, Macalino G, et al. Health-related quality of life among military HIV patients on antiretroviral therapy. PLoS ONE. 2017;12: e0178953.
Liu C, Johnson L, Ostrow D, Silvestre A, Visscher B, Jacobson LP. Predictors for lower quality of life in the HAART era among HIV-infected men. JAIDS. 2006;42:470–7.
Fumaz CR, Larrañaga-Eguilegor M, Mayordomo-López S, Gómez-Martínez S, González-García M, Ornellas A, et al. Health-related quality of life of people living with HIV infection in Spain: a gender perspective. AIDS Care. 2019;31:1509–17.
Préau M, Marcellin F, Carrieri MP, Lert F, Obadia Y, Spire B. Health-related quality of life in French people living with HIV in 2003: results from the national ANRS-EN12-VESPA Study. AIDS. 2007;21:S19-27.
Preau M, Apostolidis T, Francois C, Raffi F, Spire B. Time perspective and quality of life among HIV-infected patients in the context of HAART. AIDS Care. 2007;19:449–58.
Badia X, Podzamczer D, Garcia M, López-Lavid CC, Consiglio E. A randomized study comparing instruments for measuring health-related quality of life in HIV-infected patients. Spanish MOS-HIV and MQOL-HIV validation group. Medical outcomes study HIV health survey. AIDS. 1999;13:1727–35.
Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050.
Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, et al. The Toxicity of Azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:192–7.
Walker UA, Bäuerle J, Laguno M, Murillas J, Mauss S, Schmutz G, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004;39:311–7.
Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5.
Vogel M, Rockstroh JK. Hepatotoxicity and liver disease in the context of HIV therapy. Curr Opin HIV AIDS. 2007;2:306–13.
Bartlett JA, Fath MJ, DeMasi R, Hermes A, Quinn J, Mondou E, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–64.
Ryom L, Lundgren JD, El-Sadr W, Reiss P, Kirk O, Law M, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A: D international prospective multicohort study. The Lancet HIV. 2018;5:e291-300.
Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–75.
Sax PE, Arribas JR, Orkin C, Lazzarin A, Pozniak A, DeJesus E, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. ClinicalMedicine. 2023;59: 101991.
US Public Health Service. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents [Internet]. 2011. www.aidsinfo.nih.gov .
US Public Health Service. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. [Internet]. 2006. www.aidsinfo.nih.gov/guidelines/default_db2.asp?id=50 .
Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS. 2018;32:1551–61.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Wells, G. A., Shea, B., O’Connell, D. A., Peterson, J., Welch, V., Losos, M., & Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2020. http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso/Perfezionamento/EBM_Faggiano/NOS_oxford.pdf
Hedges LV, Olkin I. Statistical methods for meta-analysis. Saint Louis: Academic Press; 1985.
Google Scholar
Cohen J. Statistical power analysis for the behavioral sciences. Oxfordshire: Routledge; 1988.
Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to meta-analysis. 2nd ed. Hoboken: Wiley; 2021.
Book Google Scholar
Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–67.
Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 3rd edition. New York: Russell Sage Foundation; 2019
Hartung J. An alternative method for meta-analysis. Biom J. 1999;41:901–16.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I 2 index? Psychol Methods. 2006;11:193–206.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Rothstein HR. Publication bias as a threat to the validity of meta-analytic results. J Exp Criminol. 2008;4:61–81.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Aguinis H, Gottfredson RK, Wright TA. Best-practice recommendations for estimating interaction effects using meta-analysis: interaction effects in meta-analysis. J Organiz Behav. 2011;32:1033–43.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710.
Rubio-Aparicio M, López-López JA, Viechtbauer W, Marín-Martínez F, Botella J, Sánchez-Meca J. Testing categorical moderators in mixed-effects meta-analysis in the presence of heteroscedasticity. J Exp Educ. 2020;88:288–310.
López-López JA, Marín-Martínez F, Sánchez-Meca J, Van den Noortgate W, Viechtbauer W. Estimation of the predictive power of the model in mixed-effects meta-regression: a simulation study. Br J Math Stat Psychol. 2014;67:30–48.
Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft. 2010. https://doi.org/10.18637/jss.v036.i03 .
Ahmed A, Saqlain M, Akhtar N, Hashmi F, Blebil A, Dujaili J, et al. Translation and cross-cultural adaptation of WHOQOL-HIV Bref among people living with HIV/AIDS in Pakistan. Health Qual Life Outcomes. 2021;19:48.
Ahmed A, Saqlain M, Bashir N, Dujaili J, Hashmi F, Mazhar F, et al. Health-related quality of life and its predictors among adults living with HIV/AIDS and receiving antiretroviral therapy in Pakistan. Qual Life Res. 2021;30:1653–64.
Amara PS, Naveed Z, Wichman CS, Fox HS, Baccaglini L. Neurocognitive impairment and health-related quality of life among people living with Human Immunodeficiency Virus (HIV). PLoS ONE. 2021;16: e0248802.
Armon C, Lichtenstein K. The associations among coping, nadir CD4+ T-cell count, and non-HIV-related variables with health-related quality of life among an ambulatory HIV-positive patient population. Qual Life Res. 2012;21:993–1003.
Bekele T, Rourke SB, Tucker R, Greene S, Sobota M, Koornstra J, et al. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care. 2013;25:337–46.
Belay YB, Ali EE, Sander B, Gebretekle GB. Health-related quality of life of patients with HIV/AIDS at a tertiary care teaching hospital in Ethiopia. Health Qual Life Outcomes. 2021;19:24.
Burgoyne RW, Saunders DS. Quality of life among urban Canadian HIV/AIDS clinic outpatients. Int J STD AIDS. 2001;12:505–12.
Call SA, Klapow JC, Stewart KE, Westfall AO, Mallinger AP, DeMasi RA, et al. Health-related Quality of Life and Virologic outcomes in an HIV clinic. Qual Life Res. 2000;9:977–85.
Degroote S, Vogelaers DP, Vermeir P, Mariman A, De Rick A, Van Der Gucht B, et al. Socio-economic, behavioural, (neuro)psychological and clinical determinants of HRQoL in people living with HIV in Belgium: a pilot study. J Int AIDS Soc. 2013;16:18643.
Garcia-Ordoñez MA, Mansilla Francisco JJ, Nieto Aragon E, Cereto MR, Salas Samper F, Vallejo Diaz M, et al. Quality of life associated with the health of patients with HIV infection measured with the Health Questionnaire SF-36. An Med Interna. 2001;18:74–9.
Gibson K, Rueda S, Rourke SB, Bekele T, Gardner S, Fenta H, et al. Mastery and coping moderate the negative effect of acute and chronic stressors on mental health-related quality of life in HIV. AIDS Patient Care STDS. 2011;25:371–81.
Hailu T, Yitayal M, Yazachew L. Health-related quality of life and associated factors among adult HIV mono-infected and TB/HIV co-infected patients in public health facilities in northeast Ethiopia: a comparative cross-sectional study. PPA. 2020;14:1873–87.
Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV cost and services utilization study. Am J Med. 2000;108:714–22.
Igumbor J, Stewart A, Holzemer W. Comparison of the health-related quality of life, CD4 count and viral load of AIDS patients and people with HIV who have been on treatment for 12 months in rural South Africa. SAHARA-J J Soc Aspects HIV/AIDS. 2013. https://doi.org/10.1080/17290376.2013.807070 .
Imam MH, Flora MS, Moni MA, Shameem RK, Haque MA, Mamun SA. Health related quality of life with HIV/AIDS in different stages of HIV infection. Mymensingh Med J. 2012;21:509–15.
CAS PubMed Google Scholar
Kanu NE, Tobin-West CI. Health-related quality of life of HIV patients with and without tuberculosis registered in a Tertiary Hospital in Port Harcourt. Nigeria Hivar. 2018;17:210–7.
Liping M, Peng X, Haijiang L, Lahong J, Fan L. Quality of life of people living with HIV/AIDS: a cross-sectional study in Zhejiang Province, China. PLoS ONE. 2015;10: e0135705.
Mafirakureva N, Dzingirai B, Postma MJ, van Hulst M, Khoza S. Health-related quality of life in HIV/AIDS patients on antiretroviral therapy at a tertiary care facility in Zimbabwe. AIDS Care. 2016;28:904–12.
Meemon N, Paek SC, Yenchai D, Wan TTH. Application of the WHOQOL-HIV-BREF questionnaire in HIV-infected Thai patients: reliability and validity of the instrument. J Assoc Nurses AIDS Care. 2016;27:698–708.
Melaku T, Mamo G, Chelkeba L, Chanie T. Health-related quality of life among people living with human immunodeficiency virus on highly active antiretroviral therapy in Ethiopia: PROQOL-HIV based survey. PROM. 2020;11:73–86.
Murri R, Fantoni M, Del Borgo C, Visona R, Barracco A, Zambelli A, et al. Determinants of health-related quality of life in HIV-infected patients. AIDS Care. 2003;15:581–90.
Patel AR, Lester RT, Marra CA, van der Kop ML, Ritvo P, Engel L, et al. The validity of the SF-12 and SF-6D instruments in people living with HIV/AIDS in Kenya. Health Qual Life Outcomes. 2017;15:143.
Peltzer K, Phaswana-Mafuya N. Health-related quality of life in a sample of HIV-infected South Africans. Afr J AIDS Res. 2008;7:209–18.
Remor E. Reliability and validity of the Spanish version of the MOS-SF-30 to assess the health related quality of life in people infected by HIV. Aten Primaria. 2003;32:15–22.
Rueda S, Raboud J, Mustard C, Bayoumi A, Lavis JN, Rourke SB. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care. 2011;23:435–43.
Schnall R, Liu J, Cho H, Hirshfield S, Siegel K, Olender S. A health-related quality-of-life measure for use in patients with HIV: a validation study. AIDS Patient Care STDS. 2017;31:43–8.
Stasinopoulou PG, Tzavara C, Dimitrakaki C, Georgiou O, Baraboutis IG, Skoutelis A, et al. Reliability and validity of the Greek translation of the MOS-HIV health survey in HIV-infected individuals. Qual Life Res. 2010;19:199–205.
Torres TS, Harrison LJ, La Rosa AM, Lavenberg JA, Zheng L, Safren SA, et al. Quality of life among HIV-infected individuals failing first-line antiretroviral therapy in resource-limited settings. AIDS Care. 2018;30:954–62.
Tran B, Ohinmaa A, Nguyen L. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual Life Outcomes. 2012;10:132.
Tran B. Quality of life outcomes of antiretroviral treatment for HIV/AIDS patients in Vietnam. PLoS ONE. 2012;7: e41062.
Uchechukwu D, Iorfa SK, Ugwu DI. Negative centralisation of HIV/AIDS trauma and health-related quality of life: do post-traumatic stress symptoms explain the link? Afr J AIDS Res. 2020;19:206–13.
Worthington C, Krentz HB. Socio-economic factors and health-related quality of life in adults living with HIV. Int J STD AIDS. 2005;16:608–14.
de Oliveira Gomes M, Castro R, Correa da Mota J, De Boni RB. Association of syndemic conditions and quality of life among people living with HIV/AIDS. AIDS Care. 2022. https://doi.org/10.1080/09540121.2022.2080801 .
Schönnesson LN. Psychological and existential issues and quality of life in people living with HIV infection. AIDS Care. 2002;14:399–404.
Collins PY, Velloza J, Concepcion T, Oseso L, Chwastiak L, Kemp CG, et al. Intervening for HIV prevention and mental health: a review of global literature. J Int AIDS Soc. 2021. https://doi.org/10.1002/jia2.25710 .
Rasoolinajad M, Abedinia N, Noorbala AA, Mohraz M, Badie BM, Hamad A, et al. Relationship among HIV-related stigma, mental health and quality of life for HIV-positive patients in Tehran. AIDS Behav. 2018;22:3773–82.
Cahill S, Valadéz R. Growing older with HIV/AIDS: new public health challenges. Am J Public Health. 2013;103:e7-15.
Hsieh E, Polo R, Qian H-Z, Fuster-RuizdeApodaca MJ, Del Amo J. Intersectionality of stigmas and health-related quality of life in people ageing with HIV in China, Europe, and Latin America. Lancet Healthy Longev. 2022;3:e206–15.
Cobb S. Social support as a moderator of life stress. Psychosom Med. 1976;38:300–14.
Ghiasvand H, Higgs P, Noroozi M, Ghaedamini Harouni G, Hemmat M, Ahounbar E, et al. Social and demographical determinants of quality of life in people who live with HIV/AIDS infection: evidence from a meta-analysis. Biodemography Soc Biol. 2020;65:57–72.
Portilla-Tamarit J, Reus S, Portilla I, Fuster Ruiz-de-Apodaca MJ, Portilla J. Impact of advanced HIV disease on quality of life and mortality in the era of combined antiretroviral treatment. JCM. 2021;10:716.
Download references
Acknowledgements
We acknowledge people living with HIV who have contributed to each study included.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research received no external funding.
Author information
Authors and affiliations.
Department of Health Psychology, University of Alicante, Alicante, Spain
I. Portilla-Tamarit
Alicante Institute for Health and Biomedical Research (ISABIAL), Alicante, Spain
I. Portilla-Tamarit, M. Rubio-Aparicio, J. Portilla-Tamarit, S. Reus & J. Portilla
Infectious Diseases Unit, Department of Internal Medicine, Alicante University General Hospital, Alicante, Spain
I. Portilla-Tamarit, J. Portilla-Tamarit, S. Reus & J. Portilla
Spanish AIDS Research Network, Carlos III Health Institute, Madrid, Spain
Department of Basic Psychology & Methodology, Faculty of Psychology and Speech Therapy, University of Murcia, Avda. Teniente Flomesta, 5, 30003, Murcia, Spain
M. Rubio-Aparicio
Faculty of Psychology, National Distance Learning University (UNED), Madrid, Spain
M. J. Fuster-RuizdeApodaca
Spanish Interdisciplinary AIDS Society (SEISIDA), 28036, Madrid, Spain
Department of Clinical Medicine, Miguel Hernandez University, Elche, Alicante, Spain
S. Reus & J. Portilla
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to M. Rubio-Aparicio .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 33 KB)
Supplementary file1 (docx 32 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Portilla-Tamarit, I., Rubio-Aparicio, M., Fuster-RuizdeApodaca, M.J. et al. Health-Related Quality of Life in People with Advanced HIV Disease, from 1996 to 2021: Systematic Review and Meta-analysis. AIDS Behav (2024). https://doi.org/10.1007/s10461-024-04298-y
Download citation
Accepted : 16 February 2024
Published : 14 May 2024
DOI : https://doi.org/10.1007/s10461-024-04298-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Advanced HIV disease
- Health related quality of life
- Systematic review
- Meta-analysis
Palabras Clave
- Enfermedad avanzada de sida
- Calidad de vida realacionada con la salud
- Revision sistemática
- Meta-analisis
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This publication is provided for historical reference only and the information may be out of date.

StatPearls [Internet].
Case study: 33-year-old female presents with chronic sob and cough (archive).
Sandeep Sharma ; Muhammad F. Hashmi ; Deepa Rawat .
Affiliations
Last Update: February 20, 2023 .
- Case Presentation
History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician’s office six months ago. At that time, she was diagnosed with acute bronchitis and treated with bronchodilators, empiric antibiotics, and a short course oral steroid taper. This management did not improve her symptoms, and she has gradually worsened over six months. She reports a 20-pound (9 kg) intentional weight loss over the past year. She denies camping, spelunking, or hunting activities. She denies any sick contacts. A brief review of systems is negative for fever, night sweats, palpitations, chest pain, nausea, vomiting, diarrhea, constipation, abdominal pain, neural sensation changes, muscular changes, and increased bruising or bleeding. She admits a cough, shortness of breath, and shortness of breath on exertion.
Social History: Her tobacco use is 33 pack-years; however, she quit smoking shortly prior to the onset of symptoms, six months ago. She denies alcohol and illicit drug use. She is in a married, monogamous relationship and has three children aged 15 months to 5 years. She is employed in a cookie bakery. She has two pet doves. She traveled to Mexico for a one-week vacation one year ago.
Allergies: No known medicine, food, or environmental allergies.
Past Medical History: Hypertension
Past Surgical History: Cholecystectomy
Medications: Lisinopril 10 mg by mouth every day
Physical Exam:
Vitals: Temperature, 97.8 F; heart rate 88; respiratory rate, 22; blood pressure 130/86; body mass index, 28
General: She is well appearing but anxious, a pleasant female lying on a hospital stretcher. She is conversing freely, with respiratory distress causing her to stop mid-sentence.
Respiratory: She has diffuse rales and mild wheezing; tachypneic.
Cardiovascular: She has a regular rate and rhythm with no murmurs, rubs, or gallops.
Gastrointestinal: Bowel sounds X4. No bruits or pulsatile mass.
- Initial Evaluation
Laboratory Studies: Initial work-up from the emergency department revealed pancytopenia with a platelet count of 74,000 per mm3; hemoglobin, 8.3 g per and mild transaminase elevation, AST 90 and ALT 112. Blood cultures were drawn and currently negative for bacterial growth or Gram staining.
Chest X-ray
Impression: Mild interstitial pneumonitis
- Differential Diagnosis
- Aspiration pneumonitis and pneumonia
- Bacterial pneumonia
- Immunodeficiency state and Pneumocystis jiroveci pneumonia
- Carcinoid lung tumors
- Tuberculosis
- Viral pneumonia
- Chlamydial pneumonia
- Coccidioidomycosis and valley fever
- Recurrent Legionella pneumonia
- Mediastinal cysts
- Mediastinal lymphoma
- Recurrent mycoplasma infection
- Pancoast syndrome
- Pneumococcal infection
- Sarcoidosis
- Small cell lung cancer
- Aspergillosis
- Blastomycosis
- Histoplasmosis
- Actinomycosis
- Confirmatory Evaluation
CT of the chest was performed to further the pulmonary diagnosis; it showed a diffuse centrilobular micronodular pattern without focal consolidation.
On finding pulmonary consolidation on the CT of the chest, a pulmonary consultation was obtained. Further history was taken, which revealed that she has two pet doves. As this was her third day of broad-spectrum antibiotics for a bacterial infection and she was not getting better, it was decided to perform diagnostic bronchoscopy of the lungs with bronchoalveolar lavage to look for any atypical or rare infections and to rule out malignancy (Image 1).
Bronchoalveolar lavage returned with a fluid that was cloudy and muddy in appearance. There was no bleeding. Cytology showed Histoplasma capsulatum .
Based on the bronchoscopic findings, a diagnosis of acute pulmonary histoplasmosis in an immunocompetent patient was made.
Pulmonary histoplasmosis in asymptomatic patients is self-resolving and requires no treatment. However, once symptoms develop, such as in our above patient, a decision to treat needs to be made. In mild, tolerable cases, no treatment other than close monitoring is necessary. However, once symptoms progress to moderate or severe, or if they are prolonged for greater than four weeks, treatment with itraconazole is indicated. The anticipated duration is 6 to 12 weeks total. The response should be monitored with a chest x-ray. Furthermore, observation for recurrence is necessary for several years following the diagnosis. If the illness is determined to be severe or does not respond to itraconazole, amphotericin B should be initiated for a minimum of 2 weeks, but up to 1 year. Cotreatment with methylprednisolone is indicated to improve pulmonary compliance and reduce inflammation, thus improving work of respiration. [1] [2] [3]
Histoplasmosis, also known as Darling disease, Ohio valley disease, reticuloendotheliosis, caver's disease, and spelunker's lung, is a disease caused by the dimorphic fungi Histoplasma capsulatum native to the Ohio, Missouri, and Mississippi River valleys of the United States. The two phases of Histoplasma are the mycelial phase and the yeast phase.
Etiology/Pathophysiology
Histoplasmosis is caused by inhaling the microconidia of Histoplasma spp. fungus into the lungs. The mycelial phase is present at ambient temperature in the environment, and upon exposure to 37 C, such as in a host’s lungs, it changes into budding yeast cells. This transition is an important determinant in the establishment of infection. Inhalation from soil is a major route of transmission leading to infection. Human-to-human transmission has not been reported. Infected individuals may harbor many yeast-forming colonies chronically, which remain viable for years after initial inoculation. The finding that individuals who have moved or traveled from endemic to non-endemic areas may exhibit a reactivated infection after many months to years supports this long-term viability. However, the precise mechanism of reactivation in chronic carriers remains unknown.
Infection ranges from an asymptomatic illness to a life-threatening disease, depending on the host’s immunological status, fungal inoculum size, and other factors. Histoplasma spp. have grown particularly well in organic matter enriched with bird or bat excrement, leading to the association that spelunking in bat-feces-rich caves increases the risk of infection. Likewise, ownership of pet birds increases the rate of inoculation. In our case, the patient did travel outside of Nebraska within the last year and owned two birds; these are her primary increased risk factors. [4]
Non-immunocompromised patients present with a self-limited respiratory infection. However, the infection in immunocompromised hosts disseminated histoplasmosis progresses very aggressively. Within a few days, histoplasmosis can reach a fatality rate of 100% if not treated aggressively and appropriately. Pulmonary histoplasmosis may progress to a systemic infection. Like its pulmonary counterpart, the disseminated infection is related to exposure to soil containing infectious yeast. The disseminated disease progresses more slowly in immunocompetent hosts compared to immunocompromised hosts. However, if the infection is not treated, fatality rates are similar. The pathophysiology for disseminated disease is that once inhaled, Histoplasma yeast are ingested by macrophages. The macrophages travel into the lymphatic system where the disease, if not contained, spreads to different organs in a linear fashion following the lymphatic system and ultimately into the systemic circulation. Once this occurs, a full spectrum of disease is possible. Inside the macrophage, this fungus is contained in a phagosome. It requires thiamine for continued development and growth and will consume systemic thiamine. In immunocompetent hosts, strong cellular immunity, including macrophages, epithelial, and lymphocytes, surround the yeast buds to keep infection localized. Eventually, it will become calcified as granulomatous tissue. In immunocompromised hosts, the organisms disseminate to the reticuloendothelial system, leading to progressive disseminated histoplasmosis. [5] [6]
Symptoms of infection typically begin to show within three to17 days. Immunocompetent individuals often have clinically silent manifestations with no apparent ill effects. The acute phase of infection presents as nonspecific respiratory symptoms, including cough and flu. A chest x-ray is read as normal in 40% to 70% of cases. Chronic infection can resemble tuberculosis with granulomatous changes or cavitation. The disseminated illness can lead to hepatosplenomegaly, adrenal enlargement, and lymphadenopathy. The infected sites usually calcify as they heal. Histoplasmosis is one of the most common causes of mediastinitis. Presentation of the disease may vary as any other organ in the body may be affected by the disseminated infection. [7]
The clinical presentation of the disease has a wide-spectrum presentation which makes diagnosis difficult. The mild pulmonary illness may appear as a flu-like illness. The severe form includes chronic pulmonary manifestation, which may occur in the presence of underlying lung disease. The disseminated form is characterized by the spread of the organism to extrapulmonary sites with proportional findings on imaging or laboratory studies. The Gold standard for establishing the diagnosis of histoplasmosis is through culturing the organism. However, diagnosis can be established by histological analysis of samples containing the organism taken from infected organs. It can be diagnosed by antigen detection in blood or urine, PCR, or enzyme-linked immunosorbent assay. The diagnosis also can be made by testing for antibodies again the fungus. [8]
Pulmonary histoplasmosis in asymptomatic patients is self-resolving and requires no treatment. However, once symptoms develop, such as in our above patient, a decision to treat needs to be made. In mild, tolerable cases, no treatment other than close monitoring is necessary. However, once symptoms progress to moderate or severe or if they are prolonged for greater than four weeks, treatment with itraconazole is indicated. The anticipated duration is 6 to 12 weeks. The patient's response should be monitored with a chest x-ray. Furthermore, observation for recurrence is necessary for several years following the diagnosis. If the illness is determined to be severe or does not respond to itraconazole, amphotericin B should be initiated for a minimum of 2 weeks, but up to 1 year. Cotreatment with methylprednisolone is indicated to improve pulmonary compliance and reduce inflammation, thus improving the work of respiration.
The disseminated disease requires similar systemic antifungal therapy to pulmonary infection. Additionally, procedural intervention may be necessary, depending on the site of dissemination, to include thoracentesis, pericardiocentesis, or abdominocentesis. Ocular involvement requires steroid treatment additions and necessitates ophthalmology consultation. In pericarditis patients, antifungals are contraindicated because the subsequent inflammatory reaction from therapy would worsen pericarditis.
Patients may necessitate intensive care unit placement dependent on their respiratory status, as they may pose a risk for rapid decompensation. Should this occur, respiratory support is necessary, including non-invasive BiPAP or invasive mechanical intubation. Surgical interventions are rarely warranted; however, bronchoscopy is useful as both a diagnostic measure to collect sputum samples from the lung and therapeutic to clear excess secretions from the alveoli. Patients are at risk for developing a coexistent bacterial infection, and appropriate antibiotics should be considered after 2 to 4 months of known infection if symptoms are still present. [9]
Prognosis
If not treated appropriately and in a timely fashion, the disease can be fatal, and complications will arise, such as recurrent pneumonia leading to respiratory failure, superior vena cava syndrome, fibrosing mediastinitis, pulmonary vessel obstruction leading to pulmonary hypertension and right-sided heart failure, and progressive fibrosis of lymph nodes. Acute pulmonary histoplasmosis usually has a good outcome on symptomatic therapy alone, with 90% of patients being asymptomatic. Disseminated histoplasmosis, if untreated, results in death within 2 to 24 months. Overall, there is a relapse rate of 50% in acute disseminated histoplasmosis. In chronic treatment, however, this relapse rate decreases to 10% to 20%. Death is imminent without treatment.
- Pearls of Wisdom
While illnesses such as pneumonia are more prevalent, it is important to keep in mind that more rare diseases are always possible. Keeping in mind that every infiltrates on a chest X-ray or chest CT is not guaranteed to be simple pneumonia. Key information to remember is that if the patient is not improving under optimal therapy for a condition, the working diagnosis is either wrong or the treatment modality chosen by the physician is wrong and should be adjusted. When this occurs, it is essential to collect a more detailed history and refer the patient for appropriate consultation with a pulmonologist or infectious disease specialist. Doing so, in this case, yielded workup with bronchoalveolar lavage and microscopic evaluation. Microscopy is invaluable for definitively diagnosing a pulmonary consolidation as exemplified here where the results showed small, budding, intracellular yeast in tissue sized 2 to 5 microns that were readily apparent on hematoxylin and eosin staining and minimal, normal flora bacterial growth.
- Enhancing Healthcare Team Outcomes
This case demonstrates how all interprofessional healthcare team members need to be involved in arriving at a correct diagnosis. Clinicians, specialists, nurses, pharmacists, laboratory technicians all bear responsibility for carrying out the duties pertaining to their particular discipline and sharing any findings with all team members. An incorrect diagnosis will almost inevitably lead to incorrect treatment, so coordinated activity, open communication, and empowerment to voice concerns are all part of the dynamic that needs to drive such cases so patients will attain the best possible outcomes.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Histoplasma Contributed by Sandeep Sharma, MD
Disclosure: Sandeep Sharma declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Deepa Rawat declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Sharma S, Hashmi MF, Rawat D. Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough (Archive) [Updated 2023 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Similar articles in PubMed
- Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. [J Adv Pract Oncol. 2017] Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. Doverspike L, Kurtz S, Selvaggi K. J Adv Pract Oncol. 2017 May-Jun; 8(4):382-386. Epub 2017 May 1.
- Review Breathlessness with pulmonary metastases: a multimodal approach. [J Adv Pract Oncol. 2013] Review Breathlessness with pulmonary metastases: a multimodal approach. Brant JM. J Adv Pract Oncol. 2013 Nov; 4(6):415-22.
- A 50-Year Old Woman With Recurrent Right-Sided Chest Pain. [Chest. 2022] A 50-Year Old Woman With Recurrent Right-Sided Chest Pain. Saha BK, Bonnier A, Chong WH, Chenna P. Chest. 2022 Feb; 161(2):e85-e89.
- Suicidal Ideation. [StatPearls. 2024] Suicidal Ideation. Harmer B, Lee S, Rizvi A, Saadabadi A. StatPearls. 2024 Jan
- [Clinical analysis of the first patient with imported Middle East respiratory syndrome in China]. [Zhonghua Wei Zhong Bing Ji Jiu...] [Clinical analysis of the first patient with imported Middle East respiratory syndrome in China]. Ling Y, Qu R, Luo Y. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015 Aug; 27(8):630-4.
Recent Activity
- Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough (Archive) - S... Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough (Archive) - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About Mild TBI and Concussion
- After a Mild TBI or Concussion
- Health Disparities in TBI
- Comparing Head Impacts
- Clinical Guidance
- Mild Traumatic Brain Injury Management Guideline
- Resources for Health Care Providers
Traumatic Brain Injury & Concussion
About Moderate and Severe TBI

Preventing TBI
Symptoms of Mild TBI and Concussion

Where to Get Help
Facts About TBI
For Medical Professionals

Clinical Guidance for Pediatric mTBI

Health Care Provider Resources
CDC Programs

HEADS UP Online Training Courses
National Concussion Surveillance System
Core State Injury Prevention Program (Core SIPP)
A traumatic brain injury, or TBI, is an injury that affects how the brain works. TBI is a major cause of death and disability in the United States.
For Everyone
Health care providers.
- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .

IMAGES
VIDEO
COMMENTS
I have participated in the care of this patient and am aware of the diagnosis in this case. ... unilateral nature of the symptoms, ... study. Lancet HIV 2017;4(11):e495-e504. Crossref. PubMed.
A 35-year-old HIV-positive man was admitted to our hospital with intermittent fever for several months (Figure 1) and chronic diarrhea. He was diagnosed HIV-positive 4 years earlier and had subsequently developed several AIDS-defining diseases such as Pneumocystis carinii pneumonia and CMV retinitis. Three months before admission the patient began complaining of watery diarrhea and recurrent ...
An estimated 1 in 2 Black men who have sex with men in the United States will become infected with HIV over a lifetime. 1 Among the nearly 50,000 adolescents and young adults (13 to 24 years of ...
Everyone's experience is different, and that's important because NO ONE symptom just shows up to indicate that you might have HIV. In fact, most people feel no symptoms at all prior to finding out. That is why getting tested is so important. In the beginning, it can be frightening, but these individuals overcame their fears and have triumphed.
This case study is about Anamika (name changed), a 35-year-old female who is an empowered HIV-positive and presently works as Community Co-ordinator at an anti-retroviral treatment (ART) centre. Her transformation from a victim of social stigma and discrimination due to her HIV-positive status to her present role has been possible due to her ...
Several studies have shown that in health care ... symptoms of acute seroconversion syndrome may manifest in the patient at approximately day 7 to day 12. ... in that case, an HIV-1 NAAT to detect ...
AIDS as a human crisis may lead to devastating psychological trauma and stress for patients. Therefore, it is necessary to study different aspects of their lives for better support and care. Accordingly, this study aimed to explain the lived experience of HIV-infected patients in the face of a positive diagnosis of the disease. This qualitative study is a descriptive phenomenological study.
HIV can cause neurological symptoms such as confusion, forgetfulness, depression, anxiety and difficulty walking. HIV -associated neurological conditions can range from mild symptoms of behavior changes and reduced mental functioning to severe dementia causing weakness and not being able to function. Kidney disease.
Case Studies. The Berlin Patient ... A good amount of moderate exercise can help fight HIV symptoms of nerve pain, loss of appetite and reduce the risks of other chronic diseases like heart disease, diabetes and osteoporosis. Taking the prescribed medication on time is known as adherence. This is vital to help reduce the risk of HIV becoming ...
Diarrhea that lasts for more than a week. Sores of the mouth, anus, or genitals. Pneumonia. Red, brown, pink, or purplish blotches on or under the skin or inside the mouth, nose, or eyelids. Memory loss, depression, and other neurologic disorders. Each of these symptoms can also be related to other illnesses.
Human immunodeficiency virus (HIV) is an infection that attacks the body's immune system. Acquired immunodeficiency syndrome (AIDS) is the most advanced stage of the disease. HIV targets the body's white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
A study of global trends in HIV among adolescents and young adults showed a decrease in HIV incidence from 34.5 per 100,000 population in 1990 to ... Confirmed HIV Case. For all adults, adolescents, and children 18 months and older: ... Patients have 1 or more symptoms that are associated with an acute HIV infection or a constellation of ...
In 2020, over 30,600 people received an HIV diagnosis in the United States, and 20,758 cases were due to male-to-male sexual contact; data for 2020 should be interpreted with caution due to the impact of the COVID-19 pandemic on access to HIV testing, care-related services, and case surveillance activities.
Ready acceptance of experiences of new diagnoses among HIV-positive persons is a known personal and public health safety-net. Its beneficial effects include prompt commencement and sustenance of HIV-positive treatment and care, better management of transmission risk, and disclosure of the HIV-positive status to significant others. Yet, no known study has explored this topic in Ghana; despite ...
Interview with Dr. Anthony Fauci on progress made during the past four decades of the HIV/AIDS pandemic and ongoing efforts to end this threat. 18m 44s Download. The dramatic saga of the acquired ...
As in other studies 18,39,41,42,43,44,45,46, education was noted as a significant driver of awareness of HIV among adolescents in this study. Higher education might be associated with increased ...
For example, in one study, of 20 people diagnosed with HIV-associated dementia, only one had histopathological evidence of HIV encephalitis post mortem 33. Our proposed term HABI is intended to ...
The purpose of the study was to assess the effects of advanced HIV disease (AHD) on health-related quality of life (HRQoL) in PLHIV, the changes in HRQoL outcomes over the last 25 years, and the differences between countries according to level of economic development. We conducted a systematic review and meta-analysis. The search was conducted in PubMed and Web of Science using the terms ...
CASE STUDY OF A PATIENT WITH HIV-AIDS AND VISCERAL LEISHMANIASIS CO-INFECTION IN MULTIPLE EPISODES ... In individuals with HIV/AIDS and presenting symptoms such as asthenia, anorexia, and weight loss, VL might be responsible for 7-23% of instances of fever of unknown origin 11. This patient presented classic clinical manifestations during the ...
Cost-effectiveness of HIV screening and testing. Initial studies reported voluntary HIV screening to be cost-effective in health care settings where undiagnosed HIV infection is less than ≥0.1% 1 2.It was also reported to be more cost-effective than many established screening programs for chronic disease (e.g., hypertension, colon cancer, and breast cancer). 2 3 Treatment costs are lowered ...
Additionally, the patient was advised to refrain from smoking, as it can worsen respiratory symptoms and hinder recovery. Investigation In the case of a 21-year-old male patient presenting with respiratory symptoms and a history of HIV, a thorough investigation is necessary to establish a comprehensive understanding of the underlying condition and guide appropriate management.
High-Impact Prevention High-Impact Prevention Case Studies Questions and Answers about High-Impact Prevention All April 23, 2024 ... HCV causes no symptoms or only a mild illness which goes away over a few weeks. For the majority of infected persons, untreated HCV can cause serious, chronic illness. ... National Center for HIV, Viral Hepatitis ...
Case Presentation. History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician's office six months ago.
Details on symptoms and danger signs. Nov. 17, 2023. Where to Get Help. Information about organizations that can help with care and recovery. Apr. 29, 2024. Facts About TBI. Learn about causes, groups at risk, and potential effects. Apr. 29, 2024. TBI Data. Get the latest TBI data.
A study suggests that genetics can be a cause of Alzheimer's, not just a risk, raising the prospect of diagnosis years before symptoms appear. Determining whether someone has Alzheimer's ...
We used a case-finding method to identify adults in the community with respiratory symptoms without diagnosed lung disease. Participants who were found to have undiagnosed COPD or asthma on ...