

Quality Control Chemist Cover Letter Examples (Template & 20+ Tips)
Create a standout quality control chemist cover letter with our online platform. browse professional templates for all levels and specialties. land your dream role today.

As a quality control chemist, your role is essential in ensuring that products meet necessary standards and regulations. Crafting a compelling cover letter is your first opportunity to showcase your expertise and passion for the field. In this comprehensive guide, we'll provide you with helpful tips and a sample cover letter to help you land your next role as a quality control chemist.
We will cover:
- How to write a cover letter, no matter your industry or job title.
- What to put on a cover letter to stand out.
- The top skills employers from every industry want to see.
- How to build a cover letter fast with our professional Cover Letter Builder .
- Why you should use a cover letter template
Related Cover Letter Examples
- Welding Inspector Cover Letter Sample
- Patent Engineer Cover Letter Sample
- Chemical Process Engineer Cover Letter Sample
- Wind Technician Cover Letter Sample
- Fire Protection Engineer Cover Letter Sample
- Meteorologist Cover Letter Sample
- QA Director Cover Letter Sample
- Process Improvement Engineer Cover Letter Sample
- Electrical Drafter Cover Letter Sample
- Backend Developer Cover Letter Sample
- Voice Engineer Cover Letter Sample
- Environmental Planner Cover Letter Sample
- Sqa Engineer Cover Letter Sample
- Service Engineer Cover Letter Sample
- Entry Level Mechanical Engineer Cover Letter Sample
- Hydrogeologist Cover Letter Sample
- Embedded Engineer Cover Letter Sample
- Research Specialist Cover Letter Sample
- Water Resource Engineer Cover Letter Sample
- Proposal Engineer Cover Letter Sample
Quality Control Chemist Cover Letter Sample
Dear Hiring Manager,
I am writing to express my strong interest in the Quality Control Chemist position at your company. With a bachelor's degree in Chemistry and two years of experience in a similar role, I am confident in my ability to contribute to the success of your team. My attention to detail and dedication to ensuring quality standards are met make me the ideal candidate for this position.
During my previous role at XYZ Pharmaceuticals, I was responsible for conducting quality control tests on raw materials and finished products to ensure compliance with industry regulations and company standards. I utilized a variety of analytical techniques such as HPLC, GC, and FT-IR to analyze and interpret data, and effectively communicate any deviations or non-conformances to the relevant departments. My strong understanding of Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) allowed me to maintain a high level of accuracy and precision in my work.
In addition to my technical skills, I have a proven track record of working effectively in a team environment and collaborating with cross-functional teams to resolve quality issues. I am also adept at writing and updating standard operating procedures (SOPs) to reflect changes in production processes and quality control methods. My ability to stay organized and prioritize tasks has allowed me to meet tight deadlines and ensure that quality control activities are completed on time.
I am particularly drawn to the opportunity at your company because of the emphasis placed on continuous improvement and innovation. I am eager to contribute my ideas and expertise to help drive quality initiatives and contribute to the overall success of your team. Furthermore, I am confident that my strong work ethic and positive attitude will make me a valuable asset to your organization.
I am excited about the opportunity to bring my unique skills and experiences to the Quality Control Chemist position at your company. I am looking forward to the possibility of contributing to your team and I am available at your earliest convenience for an interview. Thank you for considering my application. I am looking forward to the possibility of being a part of your esteemed organization.
[Your Name]
Why Do you Need a Quality Control Chemist Cover Letter?
- Highlight your skills and experience: A quality control chemist cover letter allows you to showcase your relevant skills and experience in the field. This can help you stand out from other candidates and demonstrate to potential employers why you are the best fit for the position.
- Personalize your application: A cover letter provides you with the opportunity to personalize your application and explain why you are specifically interested in the role and the company. This can help you make a stronger connection with the hiring manager and show that you have done your research.
- Show your enthusiasm: A well-written cover letter can convey your enthusiasm and passion for the field of quality control chemistry. It allows you to explain why you are drawn to the work and how your skills and experience make you a great fit for the role.
- Address any potential concerns: If there are any potential concerns or gaps in your resume, a cover letter gives you the chance to address them head-on and provide context or explanations. This can help you mitigate any potential red flags and reassure the employer of your suitability for the position.
- Make a strong first impression: Your cover letter serves as your first introduction to a potential employer, and it can set the tone for the rest of your application. A well-crafted cover letter can make a strong first impression and pique the employer's interest in learning more about you.
A Few Important Rules To Keep In Mind
Writing rules for a quality control chemist cover letter.
- Address the letter to the specific hiring manager or department head
- Use a professional and formal tone throughout the letter
- Show your enthusiasm for the position and company
- Highlight your relevant experience and skills
- Customize the letter for each application, avoiding generic templates
- Keep the letter concise and focused on your qualifications
- Proofread the letter for grammar and spelling errors
- End the letter with a strong closing statement and a call to action
- Include your contact information and availability for an interview
What's The Best Structure For Quality Control Chemist Cover Letters?
After creating an impressive Quality Control Chemist resume , the next step is crafting a compelling cover letter to accompany your job applications. It's essential to remember that your cover letter should maintain a formal tone and follow a recommended structure. But what exactly does this structure entail, and what key elements should be included in a Quality Control Chemist cover letter? Let's explore the guidelines and components that will make your cover letter stand out.
Key Components For Quality Control Chemist Cover Letters:
- Your contact information, including the date of writing
- The recipient's details, such as the company's name and the name of the addressee
- A professional greeting or salutation, like "Dear Mr. Levi,"
- An attention-grabbing opening statement to captivate the reader's interest
- A concise paragraph explaining why you are an excellent fit for the role
- Another paragraph highlighting why the position aligns with your career goals and aspirations
- A closing statement that reinforces your enthusiasm and suitability for the role
- A complimentary closing, such as "Regards" or "Sincerely," followed by your name
- An optional postscript (P.S.) to add a brief, impactful note or mention any additional relevant information.
Cover Letter Header
A header in a cover letter should typically include the following information:
- Your Full Name: Begin with your first and last name, written in a clear and legible format.
- Contact Information: Include your phone number, email address, and optionally, your mailing address. Providing multiple methods of contact ensures that the hiring manager can reach you easily.
- Date: Add the date on which you are writing the cover letter. This helps establish the timeline of your application.
It's important to place the header at the top of the cover letter, aligning it to the left or center of the page. This ensures that the reader can quickly identify your contact details and know when the cover letter was written.
Cover Letter Greeting / Salutation
A greeting in a cover letter should contain the following elements:
- Personalized Salutation: Address the hiring manager or the specific recipient of the cover letter by their name. If the name is not mentioned in the job posting or you are unsure about the recipient's name, it's acceptable to use a general salutation such as "Dear Hiring Manager" or "Dear [Company Name] Recruiting Team."
- Professional Tone: Maintain a formal and respectful tone throughout the greeting. Avoid using overly casual language or informal expressions.
- Correct Spelling and Title: Double-check the spelling of the recipient's name and ensure that you use the appropriate title (e.g., Mr., Ms., Dr., or Professor) if applicable. This shows attention to detail and professionalism.
For example, a suitable greeting could be "Dear Ms. Johnson," or "Dear Hiring Manager," depending on the information available. It's important to tailor the greeting to the specific recipient to create a personalized and professional tone for your cover letter.
Cover Letter Introduction
An introduction for a cover letter should capture the reader's attention and provide a brief overview of your background and interest in the position. Here's how an effective introduction should look:
- Opening Statement: Start with a strong opening sentence that immediately grabs the reader's attention. Consider mentioning your enthusiasm for the job opportunity or any specific aspect of the company or organization that sparked your interest.
- Brief Introduction: Provide a concise introduction of yourself and mention the specific position you are applying for. Include any relevant background information, such as your current role, educational background, or notable achievements that are directly related to the position.
- Connection to the Company: Demonstrate your knowledge of the company or organization and establish a connection between your skills and experiences with their mission, values, or industry. Showcasing your understanding and alignment with their goals helps to emphasize your fit for the role.
- Engaging Hook: Consider including a compelling sentence or two that highlights your unique selling points or key qualifications that make you stand out from other candidates. This can be a specific accomplishment, a relevant skill, or an experience that demonstrates your value as a potential employee.
- Transition to the Body: Conclude the introduction by smoothly transitioning to the main body of the cover letter, where you will provide more detailed information about your qualifications, experiences, and how they align with the requirements of the position.
By following these guidelines, your cover letter introduction will make a strong first impression and set the stage for the rest of your application.
Cover Letter Body
I am writing to express my strong interest in the Quality Control Chemist position at your company. With a background in chemistry and extensive experience in quality control, I am confident in my ability to contribute to your team and make a positive impact on your organization.
- Education: I hold a Bachelor's degree in Chemistry from [University Name], where I gained a solid foundation in analytical chemistry, instrumental analysis, and quality control practices. My academic background has equipped me with the knowledge and skills necessary to excel in a quality control role.
- Experience: Over the past [number of years] years, I have worked as a Quality Control Chemist at [Previous Company Name], where I was responsible for performing routine and non-routine analysis of raw materials, in-process samples, and finished products. I have experience using a variety of analytical instruments, such as HPLC, GC, and FTIR, to ensure the quality and compliance of products.
- Attention to Detail: I have a keen eye for detail and a meticulous approach to my work. I am well-versed in conducting method validation, stability testing, and troubleshooting analytical methods to ensure accurate and reliable results. My commitment to precision and accuracy aligns with the high standards of quality control that your company upholds.
- Regulatory Compliance: I have a thorough understanding of regulatory guidelines, including FDA and cGMP requirements. I am adept at maintaining quality documentation, preparing regulatory submissions, and participating in audits to ensure compliance with industry standards.
- Team Collaboration: I am a collaborative team player who can effectively communicate with cross-functional teams, including research and development, manufacturing, and quality assurance, to drive continuous improvement and resolve quality issues.
Thank you for considering my application. I am excited about the opportunity to contribute to your team and apply my skills and expertise to support your company's quality control initiatives. I look forward to the possibility of discussing how my background, skills, and qualifications align with the needs of your organization.
Complimentary Close
The conclusion and signature of a cover letter provide a final opportunity to leave a positive impression and invite further action. Here's how the conclusion and signature of a cover letter should look:
- Summary of Interest: In the conclusion paragraph, summarize your interest in the position and reiterate your enthusiasm for the opportunity to contribute to the organization or school. Emphasize the value you can bring to the role and briefly mention your key qualifications or unique selling points.
- Appreciation and Gratitude: Express appreciation for the reader's time and consideration in reviewing your application. Thank them for the opportunity to be considered for the position and acknowledge any additional materials or documents you have included, such as references or a portfolio.
- Call to Action: Conclude the cover letter with a clear call to action. Indicate your availability for an interview or express your interest in discussing the opportunity further. Encourage the reader to contact you to schedule a meeting or provide any additional information they may require.
- Complimentary Closing: Choose a professional and appropriate complimentary closing to end your cover letter, such as "Sincerely," "Best Regards," or "Thank you." Ensure the closing reflects the overall tone and formality of the letter.
- Signature: Below the complimentary closing, leave space for your handwritten signature. Sign your name in ink using a legible and professional style. If you are submitting a digital or typed cover letter, you can simply type your full name.
- Typed Name: Beneath your signature, type your full name in a clear and readable font. This allows for easy identification and ensures clarity in case the handwritten signature is not clear.
Common Mistakes to Avoid When Writing a Quality Control Chemist Cover Letter
When crafting a cover letter, it's essential to present yourself in the best possible light to potential employers. However, there are common mistakes that can hinder your chances of making a strong impression. By being aware of these pitfalls and avoiding them, you can ensure that your cover letter effectively highlights your qualifications and stands out from the competition. In this article, we will explore some of the most common mistakes to avoid when writing a cover letter, providing you with valuable insights and practical tips to help you create a compelling and impactful introduction that captures the attention of hiring managers. Whether you're a seasoned professional or just starting your career journey, understanding these mistakes will greatly enhance your chances of success in the job application process. So, let's dive in and discover how to steer clear of these common missteps and create a standout cover letter that gets you noticed by potential employers.
- Not addressing the hiring manager or using a generic greeting
- Providing too much general information and not focusing on specific skills and experiences
- Using a generic template without customizing it for the specific job and company
- Failing to demonstrate knowledge of the company and its industry
- Overusing technical jargon that may not be relevant to the position
- Not showcasing accomplishments and instead listing job duties
- Ignoring the importance of proofreading for grammar and spelling errors
Key Takeaways For a Quality Control Chemist Cover Letter
- Proven track record in conducting quality control tests on raw materials, intermediates, and finished products
- Skilled in operating and maintaining analytical instruments such as HPLC, GC, UV-Vis, and FTIR
- Experience in developing and validating analytical methods
- Ability to troubleshoot and resolve instrument and method-related issues
- Understanding of regulatory requirements and compliance with cGMP and GLP standards
- Strong attention to detail and accuracy in documenting test results and maintaining records
- Excellent communication and collaboration skills to work effectively with cross-functional teams
- Committed to continuous improvement and quality enhancement processes
- Adherence to safety protocols and best practices in the laboratory

QC Chemist Cover Letter Example
The role of a QC Chemist is to ensure the quality and compliance of raw materials, intermediates, and finished products within a manufacturing or production environment. This position requires a strong understanding of analytical techniques, attention to detail, and the ability to collaboratively with other departments to ensure adherence to quality standards and regulatory requirements.
Enclosing an intriguing and compelling cover letter with your resume can enhance the prospects of landing your dream job. A cover letter displays your dedication and commitment to the work and how you responsibly share the accountabilities. However, writing a detailed and persuasive cover letter is cardinal to turn the potential hiring officer’s attention toward your cover letter from the vast pile. You can create a credible Cover Letter through our QC Chemist Cover Letter Sample below.

- Cover Letters
- Manufacturing
The QC Chemist plays a crucial role in the quality control process by conducting routine tests and analyses on chemical substances. The job role includes conducting various analytical tests, interpreting results, and providing critical input to maintain and improve product quality standards. The job duties include – performing analysis with a practical understanding of the test procedure and instrument operation, working under the direction provided by the supervisor, and performing laboratory analysis right first time.
What to Include in a QC Chemist Cover Letter?
Roles and responsibilities.
- Conducting routine and non-routine analysis of raw materials, in-process samples, and finished products using various analytical techniques.
- Monitoring and assessing the quality of products at different stages of the manufacturing process.
- Identifying deviations from quality standards and working collaboratively with production teams to address and resolve issues .
- Maintaining accurate and detailed records of all testing procedures, results, and any deviations from established quality standards.
- Preparing and updating standard operating procedures as necessary.
- Ensuring that laboratory instruments and equipment are calibrated and maintained in accordance with established procedures and regulatory requirements.
- Ensuring compliance with regulatory requirements, safety standards, and industry best practices.
- Analyzing and interpreting test results, preparing reports that summarize findings, and recommending corrective actions when needed.
Education & Skills
Qc chemist skills:.
- Proficiency in using various analytical instruments and techniques.
- The ability to troubleshoot and interpret complex data.
- Meticulous attention to detail in conducting tests, recording data, and maintaining accurate documentation.
- Familiarity with regulatory requirements and quality standards applicable to the industry – FDA and cGMP.
- Effective verbal and written communication skills to convey test results and collaborate with cross-functional teams.
- Strong problem-solving skills to identify and address deviations from quality standards.
- The ability to manage time efficiently to meet testing deadlines and prioritize tasks effectively.
QC Chemist Education Requirements:
- Bachelor’s degree in Chemistry, Analytical Chemistry, or a related field.
- Previous experience in a quality control or analytical chemistry role within a manufacturing environment is preferred.
QC Chemist Cover Letter Example (Text Version)
Dear Mr./ Ms.,
I’m writing to express my interest in the QC Chemist post at [Company Name], as advertised. With a solid background in chemistry, laboratory analysis, and quality control methods, I am delighted about the opportunity to help your team ensure product quality and compliance.
In my previous position as a Chemist at [Previous Company], I obtained vast expertise doing numerous analytical tests and investigations to determine the quality and purity of chemical compounds. I exhibited expertise in operating analytical instruments, interpreting test findings, and keeping accurate records of regulatory requirements. In addition, I worked directly with cross-functional teams to analyze and address quality-related concerns, taking corrective steps to prevent recurrence.
I am particularly drawn to [Company Name]’s demanding and dynamic workplace, where my talents and knowledge can applied to maintain the highest quality and safety standards. I am excited to use my chemistry education and love for scientific research to help your organization succeed.
My major accomplishments-
- Develops analytical techniques for estimating raw materials, intermediates, and final products to ensure regulatory compliance with industry standards.
- Implement quality control systems and procedures that enhance the test result accuracy and dependability and reduce product variability.
- Examine samples routinely and non-routinely utilizing various analytical techniques, including HPLC, GC, UV-Vis spectroscopy, titration, and wet chemistry.
- Participates in research and root cause analysis of deviations, complaints, and deviations, resulting in quick resolution and remedial action.
- Junior chemists and laboratory staff received training and mentorship, sharing skills in analytical methods, laboratory processes, and quality control systems.
I am excited about the chance to apply my knowledge to the QC Chemist position at [Company Name]. I can assure you that my experience and talents match the criteria of the role, and I am ready to contribute to the success of your quality control operations.
I’m grateful for this opportunity to apply. I am looking forward to discussing how my expertise and qualifications might assist [Company Name]. Please see my resume attached for evaluation.
Sincerely, [Your Name]
While writing an entry-level cover letter, you can include a brief written summary of your academic qualifications, industry-specific skillset, and relevant internship to supplement your job application.
While crafting a persuasive resume, you should always pay attention to the economy of words as the hiring manager is inundated with hundreds of job applications. We have a QC Chemist Resume Sample that shares a glimpse of your academic background and skill set meeting with the job description to help you create your own.

Customize QC Chemist Cover Letter
Get hired faster with our free cover letter template designed to land you the perfect position.
Related Manufacturing Cover Letters


Quality Control Chemist Cover Letter Examples & Writing Tips
Use these Quality Control Chemist cover letter examples and writing tips to help you write a powerful cover letter that will separate you from the competition.

Table Of Contents
- Quality Control Chemist Example 1
- Quality Control Chemist Example 2
- Quality Control Chemist Example 3
- Cover Letter Writing Tips
Quality control chemists are responsible for ensuring the quality of products by testing and analyzing samples. They work in a variety of industries, including food, pharmaceuticals, and manufacturing.
In order to be a successful quality control chemist, you need to be detail-oriented and have a strong understanding of the principles of chemistry.
Use these examples and tips to write a quality control chemist cover letter that stands out from the competition.
Quality Control Chemist Cover Letter Example 1
I am excited to be applying for the Quality Control Chemist position at ABC Corporation. I have a degree in Chemistry from a top university and two years of experience working in a quality control laboratory. I am confident that I have the skills and experience you are looking for in a candidate and I am eager to use my knowledge and abilities to help ABC Corporation reach its goals.
In my previous role at DEF Corporation, I was responsible for conducting daily quality control tests on products and raw materials. I also analyzed data to identify trends and potential problems. I was able to successfully improve the quality of the products by recommending changes to the manufacturing process.
I have experience with a variety of analytical techniques, including HPLC, GC, and UV-Vis spectroscopy. I am also proficient in using Microsoft Excel and other data analysis software. I am confident that I can quickly adapt to the methods and procedures used at ABC Corporation.
I am excited to be applying for this position and I believe that my qualifications make me the perfect candidate for the job. I look forward to meeting with you to discuss the Quality Control Chemist position in more detail. Thank you for your time and consideration.
Quality Control Chemist Cover Letter Example 2
I am writing to apply for the Quality Control Chemist position that was recently advertised on the company website. I am confident that I have the skills and qualifications that you are looking for, and I am eager to put my experience to work in this role.
As a Quality Control Chemist with three years of experience in the industry, I have the knowledge and skills necessary to ensure that all products meet the company’s high standards. I am experienced in using a variety of analytical instruments, and I have a deep understanding of the principles of quality control. I am also familiar with the latest industry trends and regulations.
I am a highly motivated individual who is always looking for ways to improve the quality of the products I am responsible for. I am confident that I can be a valuable asset to your team, and I look forward to the opportunity to discuss this position further with you.
Thank you for your time and consideration.
Quality Control Chemist Cover Letter Example 3
I am writing to express my interest in the Quality Control Chemist position that you have posted. I believe that my experience and education make me a strong candidate for this position.
I have been working as a chemist for the past five years, and I have gained valuable experience in the field of quality control. My most recent position was with a pharmaceutical company where I worked as a senior chemist. My duties included testing raw materials, finished products, and packaging materials for impurities and contamination. I also performed routine tests on equipment to ensure that it was functioning properly.
My previous positions have allowed me to develop strong analytical skills. I am able to identify impurities and contaminants using a variety of methods including gas chromatography, liquid chromatography, and mass spectrometry. I am also skilled at troubleshooting problems with instruments and equipment.
I believe that my background makes me an ideal candidate for your position. I am confident that I can perform all duties required of a quality control chemist. I am also familiar with the regulations governing the food and pharmaceutical industries. I would welcome the opportunity to discuss my qualifications with you in person.
Quality Control Chemist Cover Letter Writing Tips
1. highlight your experience.
When writing a cover letter for a quality control chemist position, it’s important to highlight your experience in the field. This can be done by providing specific examples of how you’ve ensured the quality of products in the past. For instance, you might discuss how you identified and corrected any errors in production, or how you developed and implemented new quality control procedures.
2. Showcase your problem-solving skills
As a quality control chemist, you’ll be responsible for solving problems related to product quality. To show that you have the skills necessary for the job, describe a time when you had to identify and solve a difficult problem. Explain how you gathered information, came up with a solution, and then implemented it.
3. Demonstrate your attention to detail
Hiring managers are looking for quality control chemists who have a keen eye for detail. To show that you have the attention to detail they’re looking for, provide an example of a time when you had to identify and correct an error in a product. Talk about how you identified the issue, determined the cause, and then fixed it.
4. Proofread your cover letter
It’s important to proofread your cover letter for any spelling or grammar mistakes before submitting it. Hiring managers will often disqualify candidates if their cover letter contains errors, so it’s important to take the time to review it carefully.
Facilities Supervisor Cover Letter Examples & Writing Tips
Client relations specialist cover letter examples & writing tips, you may also be interested in..., leasing manager cover letter examples & writing tips, senior caregiver cover letter examples & writing tips, patient access specialist cover letter examples & writing tips, game writer cover letter examples & writing tips.
Quality Control Chemist Cover Letter Examples
A great quality control chemist cover letter can help you stand out from the competition when applying for a job. Be sure to tailor your letter to the specific requirements listed in the job description, and highlight your most relevant or exceptional qualifications. The following quality control chemist cover letter example can give you some ideas on how to write your own letter.

or download as PDF
Cover Letter Example (Text)
Ashiya Landrio
(446) 755-0402
Dear Emileigh Luthi,
I am writing to express my interest in the Quality Control Chemist position at Merck & Co. With a solid foundation of five years of experience in the pharmaceutical industry, particularly at Pfizer Inc., I bring a comprehensive skill set and hands-on understanding of quality control processes and analytical chemistry that I am eager to leverage to contribute to Merck's commitment to excellence in healthcare.
During my tenure at Pfizer Inc., I honed my ability to meticulously conduct assays and tests to ensure the quality and consistency of pharmaceutical products. My role required a keen attention to detail and a strict adherence to regulatory standards, including FDA and cGMP guidelines. I have developed a robust knowledge of analytical techniques such as HPLC, GC, and spectroscopy, and I have been recognized for my ability to troubleshoot and optimize methods to improve efficiency and accuracy.
I am particularly proud of my contributions to a cross-functional team that was tasked with reducing product testing times without compromising on quality. Through collaborative efforts and innovative thinking, we successfully streamlined several processes which resulted in a 15% reduction in testing times, thereby enhancing productivity and accelerating product release schedules.
My commitment to quality and continuous improvement is matched by my ability to work effectively in team settings. I am confident in my capacity to bridge interdepartmental communications and work alongside R&D, manufacturing, and regulatory affairs teams to ensure that quality standards are not just met, but exceeded.
I am excited about the opportunity to bring my expertise to Merck & Co., a company renowned for its innovative approach to healthcare and unwavering dedication to patient safety. I am looking forward to the possibility of discussing how my background, skills, and enthusiasms can be aligned with the dynamic team at Merck.
Thank you for considering my application. I am eager to further discuss how I can contribute to the success of your quality control department and am available at your convenience for an interview.
Warm regards,
Related Cover Letter Examples
- Quality Control Lab Technician
- Quality Control Analyst
- Quality Control Assistant
- Quality Control Associate
- Quality Control Auditor
- Quality Control Coordinator
- Resume Builder
- Resume Templates
- Resume Formats
- Resume Examples
- Cover Letter Builder
- Cover Letter Templates
- Cover Letter Formats
- Cover Letter Examples
- Career Advice
- Interview Questions
- Resume Skills
- Resume Objectives
- Job Description
- Job Responsibilities
- FAQ’s
Qc Chemist Cover Letter Example
Writing a cover letter for a position as a QC Chemist can seem overwhelming and time consuming. However, with the right approach and guidance, it can be a straightforward process. In this guide, you will find practical tips and advice for creating a convincing cover letter that will help you stand out from the competition. Included is an example to provide further guidance. With the help of this guide, you can make sure your cover letter makes it through the application process.

Download the Cover Letter Sample in Word Document – Click Below
If you didn’t find what you were looking for, be sure to check out our complete library of cover letter examples .

Start building your dream career today!
Create your professional cover letter in just 5 minutes with our easy-to-use cover letter builder!
Qc Chemist Cover Letter Sample
Dear [Name],
I am writing to apply for the position of QC Chemist. With my Bachelor’s in Chemistry and 4+ years of experience in quality assurance and control, I am confident I would be an asset to your team.
Having worked in a wide range of industries, I am well- versed in the principles and practice of quality assurance and control. I am a meticulous and detail- oriented professional, who thrives on creating and implementing process improvements. My ability to troubleshoot and problem solve, combined with my excellent communication and organizational skills, enables me to work effectively with stakeholders across the board.
I am proficient in utilizing a range of software programs, including Microsoft Office, LIMS and SAP. I am also comfortable working with laboratory equipment, and am highly adept at developing testing and validation protocols. I am familiar with a variety of laboratory techniques, including spectrometry and chromatography, and I have a strong understanding of GMP, GLP and ISO standards.
I am confident that my knowledge and experience make me an ideal candidate for the role of QC Chemist. I am eager to discuss the position and how I can add value to your organization.
Thank you for your time and consideration.
[Your Name]
Create My Cover Letter
Build a profession cover letter in just minutes for free.
Looking to improve your resume? Our resume examples with writing guide and tips offers extensive assistance.
What should a Qc Chemist cover letter include?
A Quality Control Chemist cover letter is an important element of any job application. It should clearly and concisely explain why you are the best candidate for the position and how your skills and experience match the job requirements.
Your Quality Control Chemist cover letter should include:
- Introduction: A brief explanation of your interest in the position and why you are the best candidate for the job.
- Summarize your professional qualifications and accomplishments, highlighting your expertise in the type of work the position requires.
- Work Experience: List your relevant work experience and share any successes you have had in the field.
- Education: Describe your educational background and relevant qualifications.
- Skills: Highlight your technical skills, such as understanding laboratory equipment and techniques, as well as any other talents that make you the ideal candidate.
- Referees: End the letter by providing contact details of two professional referees who can confirm your suitability for the role.
By including these components in your Quality Control Chemist cover letter, you can make a positive impression on the hiring manager and improve your chances of securing an interview.
Qc Chemist Cover Letter Writing Tips
Writing an effective cover letter for a QC chemist position is an important step in your job search. This is your chance to tell potential employers why you’re the right candidate for the position, and what you can bring to the company. Here are some tips to help you write the perfect cover letter:
- Start off with a strong introduction: Start your cover letter with a powerful introduction that will grab the reader’s attention and make them want to read more. Introduce yourself, your qualifications and experience, and why you’re the ideal candidate for the position.
- Highlight your skills and experience: Your cover letter should emphasize your relevant skills and experience that make you the best fit for the position. Focus on skills specifically related to the job such as laboratory analysis, report writing, and problem solving.
- Include relevant examples: Provide specific examples from your past experience that demonstrate your knowledge and proficiency. For example, if you have experience with a certain type of equipment, describe the techniques you used to operate it efficiently.
- Demonstrate your knowledge of the industry: Showing that you are knowledgeable about the industry will demonstrate to potential employers that you have the right qualifications for the position. Make sure to mention your familiarity with laboratory safety protocols and quality assurance procedures.
- Show your enthusiasm: Make sure that your cover letter conveys your enthusiasm and motivation. You want the reader to see that you are excited about the opportunity to work in the field and are committed to doing the job well.
- Keep it concise: Your cover letter should be clear and concise, and no more than a page in length. Focus on the important points and avoid rambling.
By following these tips, you can craft a compelling cover letter that will help you stand out from other applicants and get the job!
Common mistakes to avoid when writing Qc Chemist Cover letter
Writing a cover letter can be a daunting task, especially when applying for a position as a QC Chemist. It’s important to take your time and craft a well- written cover letter that will help you stand out from other candidates and make a positive impression on the hiring manager. To help you create an effective cover letter, here are some common mistakes to avoid:
- Not Tailoring Your Cover Letter: A generic, one- size- fits- all cover letter won’t make you stand out from the competition. Make sure to tailor your letter to the specific job requirements, noting relevant qualifications and experience.
- Not Including Specific Examples: Include specific examples of your experience and accomplishments, such as any projects you’ve managed or achievements you’ve made in previous roles. This will help to show the hiring manager what you can bring to the role.
- Not Following Directions: If the job posting asks for specific information to be included in the cover letter, make sure you comply. Failing to follow directions shows a lack of attention to detail.
- Not Checking Your Work: Before sending off your cover letter, make sure to double- check it for any typos or errors. Even a single typo could be enough to make the hiring manager think that you’re not serious about the role.
- Not Asking Questions: Asking thoughtful questions at the end of your letter can help to demonstrate your interest in the position. It also shows that you’ve taken the time to consider the role and how you can make a difference in the role.
By avoiding these common mistakes, you can ensure that your cover letter for the position of QC Chemist is as effective as possible. Taking the time to craft a well- written and tailored cover letter will help you stand out from the competition and make a positive impression on the hiring manager.
Key takeaways
Writing an impressive cover letter for a QC Chemist position is an important part of the job application process. A cover letter serves to introduce yourself to potential employers, highlight your qualifications, and give an overview of what you can bring to the job. To ensure that your cover letter is as effective as possible, here are some key takeaways to keep in mind when crafting your cover letter:
- Demonstrate your knowledge of the company and its mission. Show that you understand the company’s needs and how you can help meet them.
- Focus on the qualifications that make you the best fit for the job. Highlight your relevant skills, experience, and education.
- Keep your cover letter concise and to the point. Use clear, concise language that gets the point across in a few sentences.
- Use clear and professional formatting. Make sure to use a professional font and font size, and use white space to break up the text.
- Proofread and edit your cover letter thoroughly. Check for any spelling and grammar errors.
Following these key takeaways will help ensure that your cover letter for a QC Chemist position is as impressive as possible and will make you stand out from other applicants.
Frequently Asked Questions
1. how do i write a cover letter for an qc chemist job with no experience.
When writing a cover letter for a QC Chemist job with no experience, you should focus on your related educational background and transferable skills. Highlight your lab and research experience, as well as any relevant internships or volunteer work. Emphasize your commitment to quality assurance and your passion for chemistry. Explain why you are interested in the job and why you would be a great fit for the role. Provide concrete examples to back up your qualifications and be sure to proofread your cover letter for errors before sending it.
2. How do I write a cover letter for an Qc Chemist job experience?
When writing a cover letter for a QC Chemist job with experience, you should focus on your specific experience and accomplishments within the field. Provide concrete examples of projects you have worked on and the results you achieved. Demonstrate your technical competency and commitment to quality assurance, and explain why you are the best candidate for the role. Be sure to proofread your cover letter for errors before submitting it.
3. How can I highlight my accomplishments in Qc Chemist cover letter?
When highlighting your accomplishments in your cover letter, focus on specific achievements and results. Provide concrete examples of successful projects or initiatives you have worked on, as well as any awards or recognition you have received. Emphasize your commitment to quality assurance, and explain why you are the ideal candidate for the role.
4. What is a good cover letter for an Qc Chemist job?
A good cover letter for a QC Chemist job should be tailored to the specific role and company. Emphasize your transferable skills, lab and research experience, and commitment to quality assurance. Explain why you are the ideal candidate for the role and provide concrete examples to back up your qualifications. Be sure to proofread your cover letter for errors before submitting it.
In addition to this, be sure to check out our cover letter templates , cover letter formats , cover letter examples , job description , and career advice pages for more helpful tips and advice.
Let us help you build your Cover Letter!
Make your cover letter more organized and attractive with our Cover Letter Builder

Quality Control Chemist Cover Letter Example: 4 Templates
We are providing you with a few templates on quality control chemist cover letters and you can choose any of them as per your preference. A cover letter is basically a job application which is sent by the applying candidate which contains all of the important information like the skills qualifications, education background of the applying candidate and is sent to the hiring company.
Template : 1
Table of Contents
Quality Control Chemist Cover Letter
James Brown
238 Broadway
United States
The HR Manager
XYZ Company
354 F 63rd Street
Subject- Quality control chemist cover letter
Respected sir/ma’am,
I am [mention the name of the applaying candidate] and I am writing this letter as a form of job application to apply for the job of [mention the name of the job position] in [mention the name of the hiring company]. I got the details about this job from [mention the name of the source from where the sender got the details].
Now let me start by informing you that I have completed my bachelor’s degree in chemistry from [mention the name of a college] and after that I did an internship in [mention the name of another company] for over [mention the duration].
After completing my internship I started working with [mention the name of another company] as a [mention the name of a job related to this job] where I was responsible for assisting with the production activities when required, helping the senior staff with advanced chemical analysis techniques, work with computer softwares and hardwares et cetera.
I would like to thank you for accepting my cover letter as a farmers job application. I would be grateful to you if you go through my resume which is endless with this letter. Thank you so much.
[handwritten signature]
[Mention the contact details]
[Mention here, if there is any post note to be given]
Template : 2
My name is [mention the name of the applying candidate] and I am an employee of [mention the name of another company] where I used to work as a [mention the name of a job related to this job]. I am writing this letter to apply for the job of [mention the name of the job position] in [mention the name of the hiring company].
As I have worked in [mention the name of the company mentioned above] for a long period of time I think that now I should extend my boundaries by working in [mention the name of the hiring company]. I have all the proper skills and qualifications within me and I will be a perfect fit for this job.
Let me get you started by informing you that I have a bachelor’s degree along with a master’s degree in [mention the name of the course related to this job] from [mention the name of two different colleges] and I have excellent knowledge of Microsoft office along with proper communication skills within me.
In my previous job at [mention the name of the company mentioned above] I used to carry out the instructions which were given by the senior staff, maintain a clean and assigned area, have excellent understanding of analytical chemistry et cetera.
Lastly, I would like to bring to your notice that my resume is enclosed with this letter and it would be great if you can go through it once. Thank you so much for your precious time and consideration. I hope that you will get back to me as soon as possible.
Similar Posts:
- Chemist Cover Letter Examples: 4 Templates
- Analytical Chemist Cover Letter Example: 4 Templates
- Chemist Letter Of Farewell Templates
- How To Write a Cover Letter With No Experience: 82 Templates
- Quality Auditor Cover Letter Examples: 6 Templates
- Stem Teacher Cover Letter Example: 4 Templates
- Sound Engineer Cover Letter Example: 12 Templates
- Lab Technician Cover Letter: 8 Types Templates
- Chemist Welcome Letter Template
- Warehouse Supervisor Cover Letter: 10 Templates
“Business, marketing, and blogging – these three words describe me the best. I am the founder of Burban Branding and Media, and a self-taught marketer with 10 years of experience. My passion lies in helping startups enhance their business through marketing, HR, leadership, and finance. I am on a mission to assist businesses in achieving their goals.”
Leave a Comment

- Resume Samples
- Resume Examples
- Resume Templates
- Cover Letters
- Writing Objectives
- Interview Tips
- Career Options
QC Chemist Cover Letter
Contact Us : Privacy Policy
Resume Worded | Proven Resume Examples
- Resume Examples
- Research & Science Resumes
- Quality Control Resume Guide & Examples
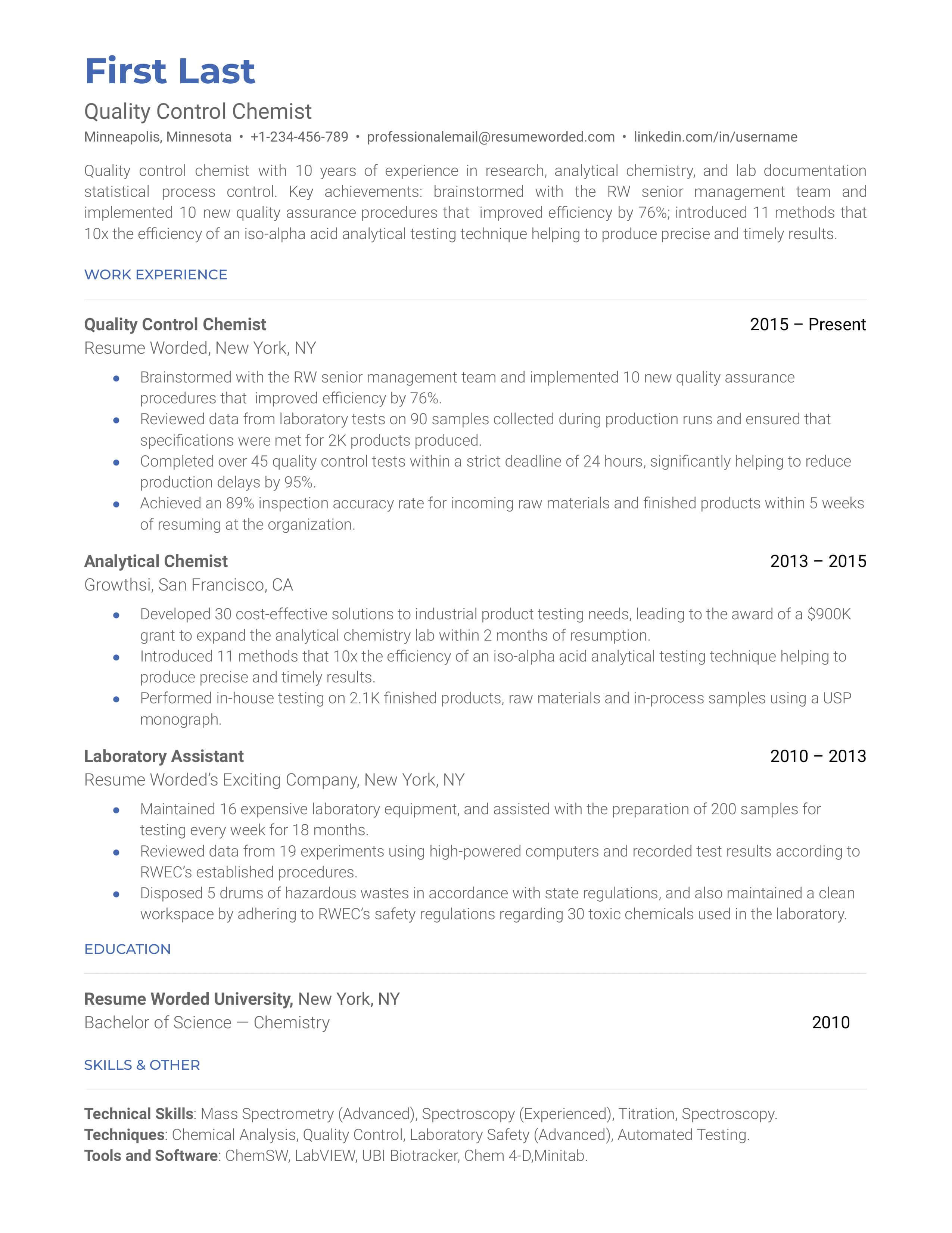
Quality Control Chemist Resume Examples: Proven To Get You Hired In 2024

Jump to a template:
- Quality Control Chemist
- Quality Control Analyst
- Analytical Chemist
Get advice on each section of your resume:
Jump to a resource:
- Quality Control Chemist Resume Tips
Quality Control Chemist Resume Template
Download in google doc, word or pdf for free. designed to pass resume screening software in 2022., quality control chemist resume sample.
A quality control chemist is responsible for ensuring safety at the workplace. They’re also in charge of conducting tests in the drug/food/chemicals manufacturing process. They should evaluate materials, production conditions, and product safety to ensure they meet quality goals. A quality control chemist must also develop documentation and reports regarding the product’s quality and performance.
We're just getting the template ready for you, just a second left.
Recruiter Insight: Why this resume works in 2022
Tips to help you write your quality control chemist resume in 2024, explain how you’ve ensured safety in the work environment..
A quality control chemist must perform certain tasks to ensure workplace safety. This includes performing laboratory reviews, checking materials, and developing lab reports. In your quality control resume, you should mention your strategies to maintain safety procedures in the work environment.
Tailor your resume to the chemistry field.
When writing your resume, make sure you focus on chemistry-related keywords and quality control terms. This will help you get past ATSs and get the recruiters’ attention. Remember that job posts also include keywords, so you should try to spot them to get some inspiration.

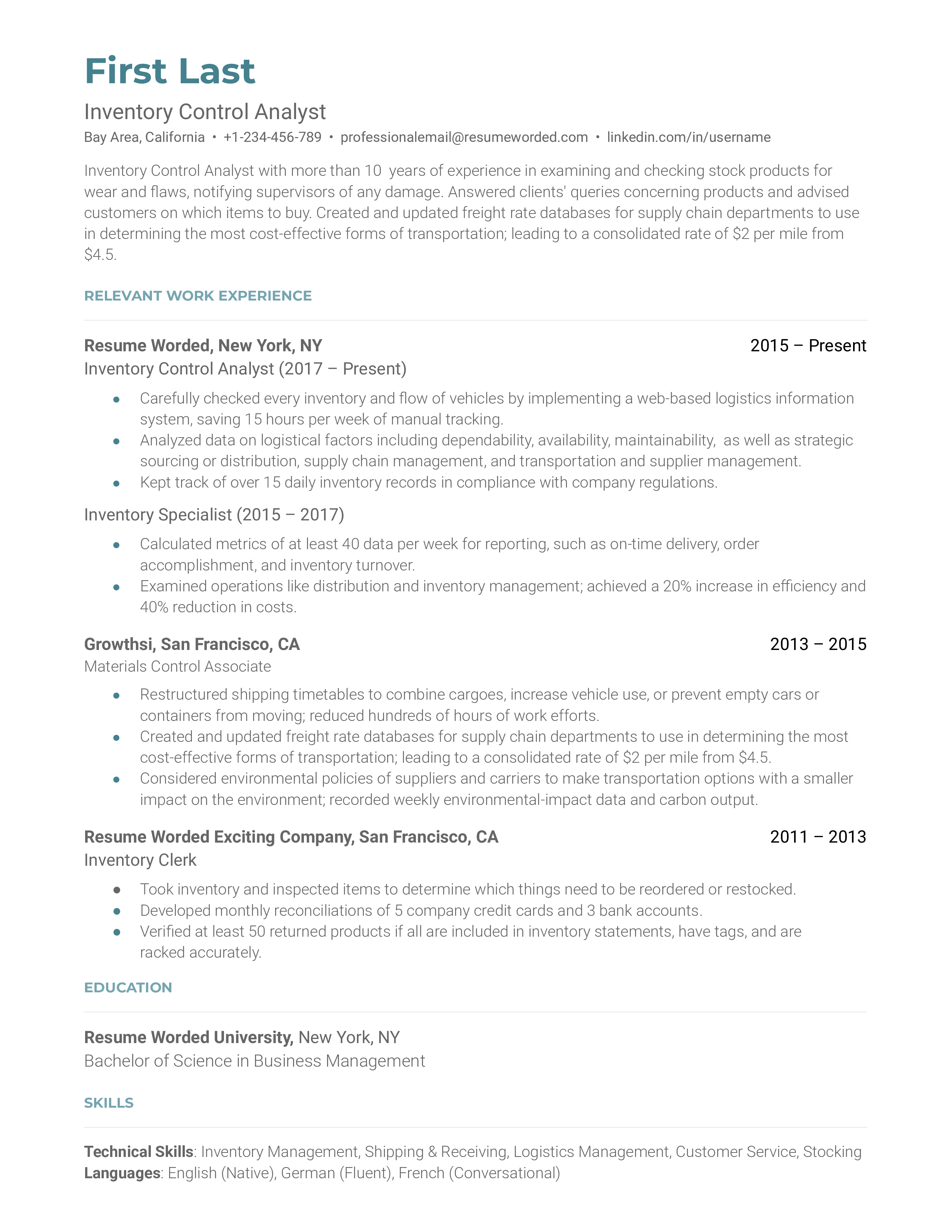
Quality Control Analyst Resume Sample
Analytical chemist resume sample.
As a hiring manager who has recruited quality control chemists at companies like Pfizer, Merck, and Johnson & Johnson, I've seen countless resumes over the years. The best ones always stand out by effectively highlighting the candidate's relevant skills, experience, and accomplishments. In this article, we'll share some tips to help you create a strong resume that will catch the attention of hiring managers in the quality control chemistry field.
Showcase your technical skills and certifications
Hiring managers want to see that you have the necessary technical skills and certifications for the job. Make sure to:
- Highlight your proficiency in analytical techniques like HPLC, GC, and UV/Vis spectroscopy
- Mention any relevant certifications, such as ASQ Certified Quality Engineer or Six Sigma Green Belt
- Describe your experience with quality control software and statistical analysis tools
For example, instead of simply listing 'HPLC' as a skill, you could say:
Conducted over 500 HPLC analyses to ensure product quality and compliance with USP standards

Quantify your achievements and impact
When describing your work experience, use specific numbers and metrics to quantify your achievements and show the impact you made. Compare the following:
- Responsible for testing raw materials and finished products
Instead, try something like:
- Analyzed 50+ raw material batches per week, resulting in a 15% reduction in material waste and $200K annual cost savings
- Implemented a new QC testing protocol that increased throughput by 25% and reduced turnaround time from 7 to 5 days
By using specific numbers, you give the hiring manager a clear picture of your capabilities and the value you can bring to their team.
Highlight your problem-solving skills with examples
Quality control chemists often face challenges and need to troubleshoot issues in the lab. Show the hiring manager that you have strong problem-solving skills by providing concrete examples:
- Investigated and resolved a recurring contamination issue, leading to a 20% reduction in batch failures
- Developed a new method for analyzing a difficult-to-test product, improving accuracy by 30%
These examples demonstrate your ability to think critically, analyze problems, and find effective solutions – valuable skills for any quality control role.
Tailor your resume to the job description
One common mistake job seekers make is using the same generic resume for every application. Instead, take the time to tailor your resume to each specific job you apply for:
- Read the job description carefully and identify the key skills and requirements
- Make sure your resume highlights your relevant experience and accomplishments that match those requirements
- Use similar language and terminology as the job posting to show you're a good fit
For example, if the job emphasizes experience with GMP regulations, make sure to include any GMP-related work you've done:
Conducted weekly GMP audits of the manufacturing facility, ensuring 100% compliance with FDA regulations
Include relevant projects and publications
In addition to your work experience, consider including any relevant projects, research, or publications that showcase your expertise in quality control chemistry:
- Developed and validated a new HPLC method for analyzing a novel drug compound, resulting in a successful IND filing
- Published a research paper in the Journal of Chromatography on a new approach for impurity testing
- Presented findings on QC best practices at the American Chemical Society annual conference
These examples help demonstrate your knowledge, initiative, and contributions to the field, making you a more competitive candidate.
Use industry-specific keywords
Many companies use applicant tracking systems (ATS) to screen resumes for relevant keywords before a human even looks at them. To ensure your resume makes it past this initial screening, use industry-specific keywords throughout your resume:
- Quality control, QA/QC, GMP, GLP, FDA regulations, USP, EP, JP
- Analytical techniques: HPLC, GC, UV/Vis, IR, MS, NMR, titration, Karl Fischer
- Statistical analysis, SPC, DOE, ANOVA, regression analysis
Incorporate these keywords naturally into your job descriptions, skills section, and other areas of your resume to increase your chances of being noticed by both the ATS and the hiring manager.
Writing Your Quality Control Chemist Resume: Section By Section
summary.
A summary on your resume is optional. It's a chance to provide context about your career, especially if you're changing fields or have a lot of experience. Avoid using an objective statement, as it's outdated. Instead, focus on highlighting your relevant skills and experience for the Quality Control Chemist role you're targeting.
When writing your summary, avoid repeating information that's already in other sections of your resume. Keep it concise, ideally no more than a paragraph. Use it as an opportunity to showcase your most relevant qualifications and mention your target job title and keywords to help with applicant tracking systems (ATS).

To learn how to write an effective resume summary for your Quality Control Chemist resume, or figure out if you need one, please read Quality Control Chemist Resume Summary Examples , or Quality Control Chemist Resume Objective Examples .
1. Tailor your summary to the quality control chemist job
When applying for a Quality Control Chemist position, it's crucial to customize your summary to the specific role and company. Many job seekers make the mistake of using a generic summary:
Experienced chemist with a proven track record in quality control. Skilled in analytical techniques and problem-solving. Seeking a challenging position to utilize my expertise.
Instead, highlight your most relevant skills and experience that match the job description:
Meticulous Quality Control Chemist with 5+ years of experience ensuring product quality and compliance in the pharmaceutical industry. Expertise in analytical method development, validation, and troubleshooting. Seeking to leverage my skills in HPLC, GC, and UV/Vis spectroscopy to contribute to [Company]'s commitment to delivering safe and effective products.
2. Quantify your quality control impact and achievements
When possible, use numbers and metrics to quantify your achievements and impact in quality control. This helps hiring managers understand the scope of your responsibilities and the value you can bring. Compare these two examples:
- Conducted quality control testing on raw materials and finished products
- Investigated and resolved quality issues
Now see how much stronger they become with quantification:
- Performed quality control testing on 50+ raw materials and product batches per week, ensuring 100% adherence to specifications
- Investigated and resolved 30+ quality deviations, reducing rework by 25% and saving $50K annually
Experience
Your work experience section is the heart of your quality control chemist resume. It's where you show hiring managers how you've applied your skills in real-world settings to drive results. To make this section compelling, focus on your achievements and the value you delivered in each role, rather than listing responsibilities.
Let's break down the key steps to write an effective work experience section for a quality control chemist resume, with examples:
1. Highlight key QC chemist skills
Analyze the job description for the quality control chemist role you're targeting. Identify the core skills and requirements, and mirror that language in your resume. Highlight key skills like:
- Analytical chemistry techniques like HPLC, GC, or spectroscopy that you've used to assess product quality
- Experience with cGMP, FDA, or other relevant regulations for your industry
- Proficiency in statistical analysis tools to analyze quality data
- Collaboration with cross-functional teams like R&D or manufacturing
Focusing on the skills from the job description will help your resume pass applicant tracking systems and grab the hiring manager's attention.
2. Use strong action verbs
When describing your work experience, start each bullet point with a strong action verb that showcases your contributions. Avoid overused, generic verbs like "managed" or "led." Instead, use industry-specific verbs that paint a vivid picture of your work, like:
- Validated HPLC methods for assessing product purity
- Investigated and resolved out-of-specification results
- Collaborated with R&D to develop stability testing protocols
- Spearheaded initiatives to streamline quality control processes
Responsible for conducting quality tests on incoming raw materials
While this bullet isn't terrible, it's a bit generic. Instead, try quantifying your impact like:
Conducted quality tests on 50+ raw materials per week, ensuring 100% adherence to specifications

3. Quantify your impact with metrics
Numbers are your best friend when writing your work experience section. They give hiring managers a concrete sense of your contributions and help your resume stand out. Look for opportunities to quantify your achievements, like:
- Reduced out-of-specification results by 30% through implementing Six Sigma quality control processes
- Collaborated with manufacturing to increase yield by 12% while maintaining product quality
- Completed 100% of assigned quality investigations within 48 hours
- Revised SOPs to standardize QC testing, saving 10 hours per week
Not every bullet point needs a number, but aim to include at least 1-2 per role. If you don't have exact numbers, estimates are okay as long as they're realistic.
4. Showcase promotions and career growth
Hiring managers love to see candidates who have progressed and taken on more responsibility in their careers. If you've been promoted or grown your role, make that clear in your work experience section. For example:
- Quality Control Chemist II, ABC Pharma (2018-Present)
- Promoted from Quality Control Chemist I in 2018 after demonstrating exceptional analytical skills and leadership
- Oversee a team of 5 QC chemists, coordinating testing schedules and providing technical guidance
- Developed and validated a new HPLC method that reduced testing time by 20%
- Quality Control Chemist I, ABC Pharma (2016-2018)
- Conducted routine QC testing on raw materials, in-process samples, and finished products
- Assisted in investigating out-of-specification results and implementing corrective actions
By showing your progression, you demonstrate your ability to learn, grow, and take on new challenges - all qualities hiring managers seek.
Education
As a quality control chemist, your education section needs to show you have the right knowledge for the job. But the details you include depend on how much work experience you have. Here are tips for writing a strong education section on your quality control chemist resume:

1. Put your highest degree first
List your degrees in reverse chronological order, with the most recent one at the top. For each degree, include:
- Name of institution
- Degree earned
- Graduation year
- Relevant coursework, thesis, or projects
For example:
Bachelor of Science in Chemistry, ABC University, 2018 Relevant Coursework: Analytical Chemistry, Organic Chemistry, Instrumental Analysis
2. Highlight chemistry coursework
If you're a recent grad applying for entry-level quality control chemist jobs, listing relevant courses can show you have the right skills, even without much work experience. Include classes like:
- Analytical chemistry
- Organic chemistry
- Instrumental analysis
- Quality assurance
But don't do this:
- Intro to Chemistry
- General Biology
- College Algebra
3. Add certifications to education
Industry certifications show specialized knowledge and can boost your resume. You can list them in your education section or a separate certifications section. For example:
Certified Quality Engineer (CQE), American Society for Quality (ASQ), 2020
Other relevant certifications for quality control chemists could include:
- Certified Quality Auditor (CQA)
- Certified Quality Improvement Associate (CQIA)
- Six Sigma Green Belt or Black Belt
4. Keep it short for senior roles
If you're a senior-level quality control chemist with many years of experience, your work history is more important than education. Keep your education section to just 1-2 lines with your degree, major, and university name.
MS in Chemistry, XYZ University
Master of Science in Chemistry, XYZ University, 1995 Bachelor of Science in Chemistry, ABC University, 1993 Relevant Coursework: Analytical Chemistry, Physical Chemistry, Biochemistry Senior Thesis: Analysis of Heavy Metal Contaminants in River Sediments
Skills
The skills section is a key part of your resume as a quality control chemist. It's where you highlight your technical abilities, tools you're proficient in, and techniques you've mastered. Hiring managers and recruiters look at your skills section to quickly assess if you have the right background for the job.
Let's break down what to include step-by-step, with examples of good and bad skills sections so you can make yours stand out:

1. Categorize your skills by specialization area
As a specialized role, quality control chemists should group their skills into categories. This quickly shows hiring managers what your areas of expertise are. Include categories like:
- Analytical Techniques : HPLC, GC, Mass Spectrometry, UV/Vis Spectroscopy
- Testing Methods : Dissolution Testing, Stability Testing, Raw Materials Testing
- Lab Skills : Instrument Calibration, Lab Equipment Maintenance, GMP
Grouping your skills like this, instead of just listing them all out, makes it easy for busy hiring managers to skim your resume and understand the breadth of your abilities.
2. Focus on hard skills and tools used in quality control
When detailing your skills, emphasize hard skills related directly to quality control in the pharmaceutical or chemical industry. Avoid listing soft skills like 'strong communication' or 'attention to detail'. Instead, focus on specific tools, techniques, and technical skills.
Skills: Teamwork, Strong Work Ethic, Organized, GMP, GLP, Detail-Oriented, Dissolution Testing, Critical Thinking, Troubleshooting, Leadership
While this skills section includes some relevant hard skills, it's mixed in with too many soft skills that don't provide concrete evidence of your abilities. Instead, focus on tools and techniques like this:
Skills: Analytical Techniques : HPLC, GC, UV/Vis, Dissolution Testing, Karl Fischer Titration Quality Management : GMP, GLP, FDA Guidelines, USP Instrumentation : Agilent 1100, Waters Alliance, Perkin Elmer Lambda
3. Include your proficiency level for technical skills
Many quality control roles require expertise in specific techniques or instrumentation. Conveying your level of proficiency can help you stand out from other applicants. You can add proficiency in parentheses after each skill, like this:
Skills: HPLC (Expert), GC (Advanced), Mass Spectrometry (Intermediate), Karl Fischer Titration (Expert), Dissolution Testing (Advanced)
This quickly shows the hiring manager which techniques you're most skilled in. They can compare your abilities to what's needed for the role. Just make sure your proficiency levels are honest and can be backed up by your work experience.
4. Avoid outdated or generic skills
In the fast-paced pharmaceutical industry, it's important to show that your skills are up-to-date. Avoid listing skills that are outdated or too generic, like:
Skills: Microsoft Office, HPLC, Wet Chemistry, GMP, Detail-Oriented, Teamwork
'Microsoft Office' is too broad to be meaningful, and 'Wet Chemistry' is an outdated term. The soft skills listed also don't add much value. Instead, focus on more specific, modern techniques and tools:
Skills: Chromatography : HPLC, UPLC, GC Spectroscopy : Mass Spectrometry, NMR, FTIR Regulatory : GMP, FDA 21 CFR Part 11, USP Data Analysis : JMP, VirtualLab, ChromQuest
This skills section demonstrates your knowledge of modern industry tools and techniques, making you a more competitive applicant.
Skills For Quality Control Chemist Resumes
Here are examples of popular skills from Quality Control Chemist job descriptions that you can include on your resume.
- High-Performance Liquid Chromatography (HPLC)
- Analytical Chemistry
- Wet Chemistry
- UV/Vis Spectroscopy
- Karl Fischer Titration
- Chromatography
- U.S. Food and Drug Administration (FDA)
- Gas Chromatography
Skills Word Cloud For Quality Control Chemist Resumes
This word cloud highlights the important keywords that appear on Quality Control Chemist job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more likely you should include it in your resume.

How to use these skills?
Similar resume templates.

Inventory Manager
- Clinical Research Resume Guide
- Environmental Scientist Resume Guide
- Health and Safety Resume Guide
- Quality Control Resume Guide
- Chemistry Resume Guide
Resume Guide: Detailed Insights From Recruiters
- Quality Control Resume Guide & Examples for 2022
Improve your Quality Control Chemist resume, instantly.
Use our free resume checker to get expert feedback on your resume. You will:
• Get a resume score compared to other Quality Control Chemist resumes in your industry.
• Fix all your resume's mistakes.
• Find the Quality Control Chemist skills your resume is missing.
• Get rid of hidden red flags the hiring managers and resume screeners look for.
It's instant, free and trusted by 1+ million job seekers globally. Get a better resume, guaranteed .
Quality Control Chemist Resumes
- Template #1: Quality Control Chemist
- Template #2: Quality Control Chemist
- Template #3: Quality Control Analyst
- Template #4: Analytical Chemist
- Skills for Quality Control Chemist Resumes
- Free Quality Control Chemist Resume Review
- Other Research & Science Resumes
- Quality Control Chemist Interview Guide
- Quality Control Chemist Sample Cover Letters
- Alternative Careers to a Quality Control Chemist
- All Resumes
- Resume Action Verbs
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 4 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 4 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

Privacy preference center
We care about your privacy
When you visit our website, we will use cookies to make sure you enjoy your stay. We respect your privacy and we’ll never share your resumes and cover letters with recruiters or job sites. On the other hand, we’re using several third party tools to help us run our website with all its functionality.
But what exactly are cookies? Cookies are small bits of information which get stored on your computer. This information usually isn’t enough to directly identify you, but it allows us to deliver a page tailored to your particular needs and preferences.
Because we really care about your right to privacy, we give you a lot of control over which cookies we use in your sessions. Click on the different category headings on the left to find out more, and change our default settings.
However, remember that blocking some types of cookies may impact your experience of our website. Finally, note that we’ll need to use a cookie to remember your cookie preferences.
Without these cookies our website wouldn’t function and they cannot be switched off. We need them to provide services that you’ve asked for.
Want an example? We use these cookies when you sign in to Kickresume. We also use them to remember things you’ve already done, like text you’ve entered into a registration form so it’ll be there when you go back to the page in the same session.
Thanks to these cookies, we can count visits and traffic sources to our pages. This allows us to measure and improve the performance of our website and provide you with content you’ll find interesting.
Performance cookies let us see which pages are the most and least popular, and how you and other visitors move around the site.
All information these cookies collect is aggregated (it’s a statistic) and therefore completely anonymous. If you don’t let us use these cookies, you’ll leave us in the dark a bit, as we won’t be able to give you the content you may like.
We use these cookies to uniquely identify your browser and internet device. Thanks to them, we and our partners can build a profile of your interests, and target you with discounts to our service and specialized content.
On the other hand, these cookies allow some companies target you with advertising on other sites. This is to provide you with advertising that you might find interesting, rather than with a series of irrelevant ads you don’t care about.
Chemist Cover Letter Samples & Examples That Worked in 2024

Creating a stand-out chemist cover letter is the catalyst that can speed up your job search. Behind every great chemist is a cover letter that highlights their skills in researching new substances and mastering complex chemical processes.
By adding our expert tips, illustrative examples, and adaptable templates to your beaker, you can craft a cover letter that persuades employers you've got the perfect formula they need.

And so, continue reading to learn all about:
- Creating a chemist cover letter header and headline
- Personalizing your chemist cover letter
- Writing a compelling chemist cover letter introduction
- Describing your skills and accomplishments as a chemist
- Persuasively concluding your chemist cover letter
- Accessing the best job search resources for chemists
1. How to create an effective chemist cover letter header and headline
Before you begin writing the paragraphs of your cover letter that inform the employer of all your key qualifications, your first key step is to create your cover letter header and cover letter headline .
A header should always come first and be placed at the top of the page, typically aligned to the left. In this header, you will include your name and contact information, as well as include a letter address to the employer and their company.
Here is an example of a well-formatted chemist cover letter header
Jack Jones , Chemist (123) 456-7890 | [email protected] | linkedin.com/in/jackjones
To: Harold Cleaning Supply Development Co. Chemistry Department 1234 Street Address Chattanooga, TN 37405
Next, you will write your cover letter headline. Headlines serve as title statements that help to hook the employer’s attention and compel them to continue reading.
You should always use a keyword related to the position, an eye-catching number or trigger word , and a powerful adjective or verb when writing a cover letter headline.
Here is an example of a great headline from a chemist's cover letter
My 4-Step Approach to Safe Lab Practices as a Chemist & How I Will Use It to Improve Your Lab
Create your cover letter fast with artificial intelligence.
2. how to personalize your chemist cover letter.
Writing a cover letter can be daunting, especially when you are debating how to make it unique and stand out to employers.
Where most job applicants fail in the cover letter writing process is by making their letters too generalized. To truly impress an employer, your letter should be personalized to them and their exact wants and needs.
This requires you to do some research about the company before applying. Think of it like you are developing a new product for the company — what information do you need to include that will make your letter highly relevant and specific to them?
Oftentimes, this will include references to the company’s values, goals, and current projects.
Additionally, you should take the time to identify which staff member at the company is responsible for hiring and onboarding new employees. Once you discover this, include a personalized greeting on your cover letter that addresses this specific person by name.
Here are 3 examples of personalized chemist cover letter greetings
Dear Head Chemist Mike Miller,
- Dear Mr. Mike Miller,
- Dear Lab Hiring Manager Paula Jean,
3. How to make your chemist cover letter introduction compelling
With your header and headline out of the way, you can now write your cover letter introduction.
You should always strive to make your cover letter introduction as concise and compelling as possible . Important information to include in your introduction can include:
- A summary of your professional history in chemistry
- Any key specializations you possess, such as chemical engineering or product development
- A statement on why you are a great fit for the role
Here is an example of a compelling introduction from a chemist's cover letter
I am with 8+ years of specialized experience in cosmetics development and designing improved production processes. As an expert in developing hypoallergenic products safe for 99% of people, I know I can provide your company with the expertise needed to expand your product range and attract a wider variety of customers.
Find out your resume score!
4. How to properly describe your skills and accomplishments as a chemist
Once you have introduced yourself and your top qualifications in your introductory paragraph, you will then dive into the specifics of your best skills and achievements as a chemist.
To describe your best skills and achievements, it is essential to include information that is highly relevant to the position you are applying for , provides contextual details that give the employer a sense of the real-life value you offer, and quantifies achievements through specific data and statistics.
Here are 6 skills to describe in a chemist cover letter
- Lab & substance safety protocols
- Handling dangerous substances
- Research and experimentation methodologies
- Collecting and analyzing research findings
- Documenting and writing reports on experiments
- Communicating clearly with other research departments
Here is an example of how to describe an accomplishment in a cover letter
In my time as a chemist at [Former Employer], I developed 9 new formulas for lipstick products that could be worn for an average of 45% more time compared to competing lipstick formulas. Additionally, I focused on safe product development which led the company to win three awards for hypoallergenic products.
5. How to persuasively conclude your chemist cover letter
Finally, the last step to writing a chemist cover letter is to create a persuasive conclusion .
To do so, your conclusion must reiterate your excitement for the role, as well as how the employer can get in contact with you. You want to encourage the employer to contact you promptly by listing a time and date when you plan to follow up as well.
Don’t forget to include a professional and formal sign-off before signing your name at the end!
Here is an example of a persuasive conclusion from a chemist's cover letter
I am grateful for your time in considering me for this role and hope to hear from you in the next week regarding my application. You may contact me any day of the week between the hours of 8 a.m. to 4 p.m. at (123) 456-7890. As I am incredibly excited about this opportunity, I plan to follow up next Monday afternoon about the position if I have yet to hear back.
Many Thanks,
[Applicant Name]
If you have ever wondered how a cover letter differs from a resume, this article will tell you everything about the key differences between the two.
6. The best job search resources for chemists
If you're a chemist in the job market, it's crucial to know the right places to look. Here are some valuable resources designed specifically for your scientific pursuits:
- American Chemical Society (ACS): ACS offers a comprehensive job board loaded with opportunities for chemists. They also provide career advice and guidance.
- Chemistry World Jobs : This dedicated job site lists positions specifically for chemists and biochemists, ranging from academic to industrial roles.
- Chemistry World : This magazine of the Royal Society of Chemistry, provides a job board packed with opportunities worldwide.
- Indeed : As one of the largest job boards, Indeed offers plentiful listings under their 'chemist' category, covering a wide range of roles and locations.
- LinkedIn : This professional networking site also offers an extensive job search function. Use the right keywords like 'chemist,' or 'chemical research' to find suitable positions.
- Science Careers : This site offers job postings in a range of science research fields, including chemistry.
Each of these platforms provides plentiful opportunities for chemists, making your next career step just a search away.
Chemist Cover Letter FAQ
In essence, the key is tailoring. You should align your specialized skills closely with the job requirements and express your genuine passion for the chemistry field.
Your cover letter should ideally not exceed a single page. Aim for three to four succinct yet detailed paragraphs.
Not necessary. This isn't an exhaustive biography. Highlight pertinent roles and responsibilities that strongly relate to the job you're applying for.
Just as in a chemical formula, every element matters. A clean, professional, and logistic format enhances readability and shows an attention to detail.
Without doubt. Showcase your unique qualities and experiences just like showcasing rare elements in chemistry. Embrace individuality but remember to stay relevant and professional.


Julia Gergelova
Julia is a professional writer, translator and graphic designer. She holds degrees in translation and interpretation, and has international work experience from a number of different countries in Europe as well as China and Panama. Julia formerly taught academic writing and as a graphic designer contributed to outlets such as The Business of Business . She has a passion for lifelong learning and good coffee.
All science cover letter examples
- Agricultural Scientist
- Formal Scientist
- Humanities Scientist
- Medical Researcher / Scientist
- Natural Scientist
- Nature Scientist
- Social Scientist
All chemist cover letter examples

Related chemist resume examples

Let your resume do the work.
Join 5,000,000 job seekers worldwide and get hired faster with your best resume yet.

6 Professional Quality Control Cover Letter Examples for 2024
Your quality control cover letter must immediately highlight your eye for detail. It should reflect your ability to detect and address errors efficiently. Showcase in your cover letter specific instances where your intervention improved product quality. Make it clear that your skills are key to maintaining the highest quality standards.
All cover letter examples in this guide

Quality Control Inspector

Quality Control Manager

Quality Control Specialist

Quality Control Technician

Cover Letter Guide
Quality Control Cover Letter Sample
Cover Letter Format
Cover Letter Salutation
Cover Letter Introduction
Cover Letter Body
Cover Letter Closing
No Experience Quality Control Cover Letter
Key Takeaways

Embarking on your job search, you've likely discovered that a compelling quality control cover letter is your gateway to catching a recruiter's eye. Don't simply echo your resume; instead, sharpen your focus on a professional triumph that makes you proud. Aim for a blend of formality with a fresh, cliché-free narrative to stand out. Remember, brevity is key – keep your spotlight story to a single page to ensure a captivating read.
- Writing the essential quality control cover letter sections: balancing your professionalism and personality;
- Mixing storytelling, your unique skill set, and your greatest achievement;
- Providing relevant (and interesting) information with your quality control cover letter, despite your lack of professional experience;
- Finding the perfect format for your[ quality control cover letter, using templates from industry experts.
Leverage the power of Enhancv's AI: upload your resume and our platform will map out how your quality control cover letter should look, in mere moments.
If the quality control isn't exactly the one you're looking for we have a plethora of cover letter examples for jobs like this one:
- Quality Control resume guide and example
- Quality Technician cover letter example
- Quality Control Manager cover letter example
- Quality Assurance Associate cover letter example
- Quality Specialist cover letter example
- Quality Manager cover letter example
- Quality Auditor cover letter example
- Junior QA Tester cover letter example
- Quality Assurance Engineer cover letter example
- Quality Control Engineer cover letter example
- Quality Assurance Technician cover letter example
Quality Control cover letter example
ALEX JOHNSON
San Francisco, CA
+1-(234)-555-1234
- Highlighting Quantifiable Achievements: Mentioning the 20% boost in inspection efficiency demonstrates the candidate's ability to deliver measurable results, which is compelling evidence of their expertise.
- Relevant Experience: Emphasizing experience in process enhancement and meticulous product inspection directly relates to the demands of a Quality Control Specialist role, indicating a strong fit.
- Problem-Solving Abilities: The candidate showcases analytical and proactive problem-solving skills, which are crucial for identifying and implementing process improvements in quality control.
- Call to Action: The candidate ends the cover letter with an invitation to arrange an interview, which is a strong call to action that can prompt the hiring manager to move forward with the application process.
What about your quality control cover letter format: organizing and structuring your information
Here is one secret you should know about your quality control cover letter assessment. The Applicant Tracker System (or ATS) won't analyze your cover letter.
You should thus focus on making an excellent impression on recruiters by writing consistent:
- Introduction
- Body paragraphs (and explanation)
- Promise or Call to action
- Signature (that's optional)
Now, let's talk about the design of your quality control cover letter.
Ensure all of your paragraphs are single-spaced and have a one-inch margins on all sides (like in our cover letter templates ).
Also, our cover letter builder automatically takes care of the format and comes along with some of the most popular (and modern) fonts like Volkhov, Chivo, and Bitter.
Speaking of fonts, professionals advise you to keep your quality control cover letter and resume in the same typography and avoid the over-used Arial or Times New Roman.
When wondering whether you should submit your quality control cover letter in Doc or PDF, select the second, as PDF keeps all of your information and design consistent.
The top sections on a quality control cover letter
- Header: This includes your contact information, the date, and the employer's contact information, and sets a professional tone for your cover letter.
- Greeting: Address the hiring manager by name if possible to personalize your cover letter and show attention to detail, which is critical in a quality control role.
- Introduction: Briefly mention your relevant experience in quality control and express your enthusiasm for the role, showcasing your commitment to maintaining high standards.
- Body: Highlight your key achievements and specific examples of how you've implemented quality control processes or improvements, demonstrating your expertise and value-add to the potential employer.
- Closing: Summarize your qualifications, restate your interest in the position, and include a call to action, like requesting an interview, showing proactivity which is essential in a quality control position.
Key qualities recruiters search for in a candidate’s cover letter
Attention to Detail : Because quality control requires identifying even the smallest deviations from standards, precision and meticulousness are paramount for ensuring product consistency and safety.
Analytical Skills : Proficiency in analyzing data from quality tests to identify trends, pinpoint issues, and determine the root cause of quality problems is essential for continuous improvement.
Knowledge of Quality Standards : Familiarity with relevant quality standards such as ISO 9001, Six Sigma principles, or industry-specific regulations ensures compliance and guides the quality assurance processes.
Experience with Quality Control Tools : Practical experience with tools and equipment used in quality inspections, such as calipers, micrometers, and CMM (Coordinate Measuring Machines), provides the technical ability to perform accurate assessments.
Problem-Solving Abilities : The capability to troubleshoot issues, provide solutions, and implement corrective actions is crucial to maintaining product quality and preventing future defects.
Communication Skills : Clear communication is needed to convey quality issues and recommendations to team members, management, and sometimes suppliers or customers to facilitate understanding and prompt action.
How to personalize your quality control cover letter greeting
Before you start writing your quality control cover letter, take the time to find out who is recruiting for the role.
Search for the recruiter's name on LinkedIn or the corporate website to address them personally in your quality control cover letter salutation .
What if you can't find out who's recruiting for the role?
Always aim to avoid the very impersonal "Dear Sir/Madam" - instead, opt out for "Dear HR Team" or "Dear Hiring Manager" to make a better first impression.
List of salutations you can use
- Dear Hiring Manager,
- Dear [Company Name] Team,
- Dear [Department Name] Hiring Committee,
- Dear [Mr./Ms./Dr. Last Name],
- Dear [Job Title] Hiring Team,
- Dear [Job Title] Search Committee,
Using your quality control cover letter intro to show your dedication
We know just how difficult it is to start writing your quality control cover letter introduction .
There are so many great qualities you have as a professional, which one should you choose?
How about writing up to two sentences about your passion and commitment to the work you do or are set to do?
Try to describe exactly what you enjoy about the potential role.
A positive attitude from the get-go will help you stand out as a motivated quality control professional.
How to write an achievement-focused quality control cover letter body
We've got the intro and greeting covered. Now, comes the most definitive part of your quality control cover letter - the body .
In the next three to six paragraphs, you'd have to answer why should recruiters hire you.
What better way to do this than by storytelling?
And, no, you don't need a "Once upon a time" or "I started from the bottom and made it to the top" career-climbing format to tell a compelling narrative.
Instead, select up to three most relevant skills for the job and look back on your resume.
Find an achievement, that you're proud of, which has taught you these three job-crucial skills.
Quantify your accomplishment, using metrics, and be succinct in the way you describe it.
The ultimate aim would be to show recruiters how this particular success has built up your experience to become an invaluable candidate.
Ending your quality control cover letter: a closing paragraph with a promise
If you're thinking of finishing your quality control cover letter with a "Sincerely yours" or "Thanks for the consideration," you need to read on.
End the final paragraph of your quality control cover letter with a twist:
- a promise - of how you'd grow as a professional, part of the company, or improve organizational metrics;
- a call to action - prompt interviewers with some follow-up actions if they are interested in your profile.
A personalized ending would surely help you to stand out by being a memorable candidate.
What to write on your quality control cover letter, when you have zero experience
The best advice for candidates, writing their quality control cover letters with no experience , is this - be honest.
If you have no past professional roles in your portfolio, focus recruiters' attention on your strengths - like your unique, transferrable skill set (gained as a result of your whole life), backed up by one key achievement.
Or, maybe you dream big and have huge motivation to join the company. Use your quality control cover letter to describe your career ambition - that one that keeps you up at night, dreaming about your future.
Finally, always ensure you've answered why employers should hire precisely you and how your skills would benefit their organization.
Key takeaways
Your quality control cover letter is your best shot at standing out by showing your motivation and the unique skills you'd bring to the job:
- Chose no more than one achievement, which you'd be talking about in the body of your quality control cover letter, by focusing on skills and outcomes;
- Address recruiters with their first or last name, or "Dear Hiring Manager" in your quality control cover letter greeting;
- Introduce in no more than two sentences what makes your profile unique (perhaps it's your motivation, enthusiasm, or appreciation of the company you're applying for);
- Select the same font you have used in your resume (avoid Times New Roman and Arial, as most candidates tend to invest in them);
- Close your quality control cover letter with a promise of how you see yourself growing in the company and the benefits you'd bring about.
Quality Control cover letter examples
Explore additional quality control cover letter samples and guides and see what works for your level of experience or role.

Cover letter examples by industry
AI cover letter writer, powered by ChatGPT
Enhancv harnesses the capabilities of ChatGPT to provide a streamlined interface designed specifically focused on composing a compelling cover letter without the hassle of thinking about formatting and wording.
- Content tailored to the job posting you're applying for
- ChatGPT model specifically trained by Enhancv
- Lightning-fast responses

How Many Bullet Points Should I Have Per Job on a Resume
How to follow up on a job application, what are red flags on a resume, how to list temporary work on a resume, how to use freelance work to add value to your resume, creating a professional development plan – a guide based on experience.
- Create Resume
- Terms of Service
- Privacy Policy
- Cookie Preferences
- Resume Examples
- Resume Templates
- AI Resume Builder
- Resume Summary Generator
- Resume Formats
- Resume Checker
- Resume Skills
- How to Write a Resume
- Modern Resume Templates
- Simple Resume Templates
- Cover Letter Builder
- Cover Letter Examples
- Cover Letter Templates
- Cover Letter Formats
- How to Write a Cover Letter
- Resume Guides
- Cover Letter Guides
- Job Interview Guides
- Job Interview Questions
- Career Resources
- Meet our customers
- Career resources
- English (UK)
- French (FR)
- German (DE)
- Spanish (ES)
- Swedish (SE)
© 2024 . All rights reserved.
Made with love by people who care.
Quality Control Chemist Resume Sample
The resume builder.
Create a Resume in Minutes with Professional Resume Templates
Work Experience
- Maintain a clear understanding and working knowledge of GMP/GLP requirements, Safety requirements, test methods and SOP’s. Adhere to and enforces those policies within the team
- Assist with environmental monitoring
- Support sampling and testing for cleaning validation, process validation, and method validation as required
- Maintain analytical testing compliance with national and international testing standard for pharmaceuticals
- Data analysis and evaluation of identifiable factors
- Write stability protocols, maintain stability chambers/programs, review/manage stability reports, interact with customers
- Develop testing methods to support cleaning verification program; validate or verify methods as necessary
- Perform cleaning verification by executing existing methods
- Perform preliminary review of analytical data for accuracy and quality
- Help train new or less experienced personnel in SOP content and analytical techniques and methodologies
- Execute method transfers into QC and write summary reports
- Perform method validations and write technical reports
- Perform instrument calibration (IQ/OQ/PQ/PM) and routine equipment maintenance/repair
- Analytically test in-process, final products, intermediates and raw materials
- Uncover problems with analytical test results or method performance and take appropriate steps to address them
- Participate in OOS investigations via testing
- Thoroughly investigate deviations, identify root causes, and propose appropriate action
- Perform requalification of reference standards maintaining inventory/documentation
- Document testing, including laboratory notebooks
- B.S. in Chemistry or related biological sciences required
- Familiarity with method validation, method development, technical writing, & analytical chemistry (HPLC,GC, IC, KF, Compendium)
- In a GLP or cGMP analytical laboratory environment
- Work in analytical testing labs with hazardous and toxic chemicals
- Knowledge and understanding of organic analytical chemistry
- Sample preparation for 2 test methods to be run on HPLC
- Scheduling runs of the 2 separate test methods on the HPLC
Professional Skills
- Perform analysis of a variety of drug substances and drug products with emphasis upon purity, content uniformity, and dissolution in a GMP-compliant manner using analytical instruments such as HPLC and GC required
- Demonstrated excellent problem solving and troubleshooting skills
- Demonstrated teamwork, communication, planning/organizing, leadership skills, and strong commitment to safety
- Proficient in Microsoft Word and Microsoft Excel and possess strong technical writing skills
- Excellent scientific writing skills with the ability to troubleshoot analytical issues and author deviation reports suitable for review by regulatory agencies
- Strong interpersonal skills and ability to work as a team player
- Strong communication skills, both written and verbal; attention to detail
How to write Quality Control Chemist Resume
Quality Control Chemist role is responsible for organizational, microsoft, instrumentation, analytical, chemistry, research, training, database, purchasing, shipping. To write great resume for quality control chemist job, your resume must include:
- Your contact information
- Work experience
- Skill listing
Contact Information For Quality Control Chemist Resume
The section contact information is important in your quality control chemist resume. The recruiter has to be able to contact you ASAP if they like to offer you the job. This is why you need to provide your:
- First and last name
- Telephone number
Work Experience in Your Quality Control Chemist Resume
The section work experience is an essential part of your quality control chemist resume. It’s the one thing the recruiter really cares about and pays the most attention to. This section, however, is not just a list of your previous quality control chemist responsibilities. It's meant to present you as a wholesome candidate by showcasing your relevant accomplishments and should be tailored specifically to the particular quality control chemist position you're applying to. The work experience section should be the detailed summary of your latest 3 or 4 positions.
Representative Quality Control Chemist resume experience can include:
- Demonstrates math and computer skills (Microsoft Office)
- Strong laboratory skills desired with ability to follow written procedures
- Assist in validation of manufacturing and production lines and equipment pertinent to chemistry (e.g. equipment and room cleaning validation) if requested
- Communicate effectively with company employees and all levels of management
- Experience in a chemistry laboratory; or equivalent combination of education and experience
- Well-developed interpersonal skills. Ability to get along with diverse personalities
Education on a Quality Control Chemist Resume
Make sure to make education a priority on your quality control chemist resume. If you’ve been working for a few years and have a few solid positions to show, put your education after your quality control chemist experience. For example, if you have a Ph.D in Neuroscience and a Master's in the same sphere, just list your Ph.D. Besides the doctorate, Master’s degrees go next, followed by Bachelor’s and finally, Associate’s degree.
Additional details to include:
- School you graduated from
- Major/ minor
- Year of graduation
- Location of school
These are the four additional pieces of information you should mention when listing your education on your resume.
Professional Skills in Quality Control Chemist Resume
When listing skills on your quality control chemist resume, remember always to be honest about your level of ability. Include the Skills section after experience.
Present the most important skills in your resume, there's a list of typical quality control chemist skills:
- Strong laboratory skills required including both scientific practices andhousekeeping
- Good interpersonal skills are also required
- Encourage understanding of the tasks being described in procedures by using written communication skills
- Understand the Validation and Technology Transfer process and have experience with writing procedures/ reports and execution of laboratory test
- Solid computer experience including lab software, Microsoft Word and Excel
- Experience in a chemist role (GMP/GLP, cosmetics, FMCG or pharma) with color matching experience required
List of Typical Experience For a Quality Control Chemist Resume
Experience for chemist, quality control resume.
- Exhibit organizational skills and attention to detail
- Work effectively as part of a team and independently with minimal supervision
- Working knowledge of USP6. Experience in a FDA regulated QC environment
- Proficiency in MS Word and MS Excel and demonstrated application of problem solving/trouble shooting tools
- Freely can accept, good housekeeping, orderliness and safety in a lab are a requisite
- Able to demonstrate accuracy and thoroughness while completing work in a timely manner
- Experience with wet chemical methods
- Analytical test method validation
- Experience with analytical chemistry in a pharmaceutical/Nutraceutical industry
Experience For Quality Control Data Review Chemist Resume
- Quality Control laboratory experience in a cGMP facility
- Maintain and troubleshoot laboratory equipment and analytical instrumentation within level of experience
- 6 mos. experience with analytical instrumentation, such as HPLC, GC, FTIR, UV-VIS, and dissolution apparatus
- Able to organize, prioritize and perform concurrent tasks with minimal supervision
- Operate basic laboratory equipment. Knowledge of Quality Systems Regulations (QSR) is highly desirable
- Experience with Chemstation software
- Working knowledge of statistical methods and computer data/information handling systems
- Testing on fuels and chemicals to include: GC, IC, Xray, ICP, etc
Experience For Chemist, / II, Quality Control Resume
- Working knowledge of computer systems such as Microsoft Office programs
- Create and implement a system recording and reporting group metrics including testing time, reporting time, review time, and release time
- Perform microbiological testing of all collected samples according to standard operating procedures (SOP’s)
- Supports a Drug Substance manufacturing team by providing in-process analytical testing for critical process parameters
- Hear at normal speaking levels, talking and ability to exchange ideas
- Occasional lifting and/or moving up to 20 pounds of boxes
- Facilitate the resolution of manufacturing and/or QC processing problems
- Perform all work according to cGMP following regulatory and GSK standards
- Assist the Team Leader and Scientists in determining the root cause of laboratory deviations providing clear and concise documentation of the event
Experience For Chemist, Quality Control Operations Resume
- Compile and enter data into computer database (i.e. LIMS - Laboratory Information Management System) to be used for reporting and trending purposes
- Occasional lifting and/or moving up to 20 pound of boxes
- Maintenance and troubleshooting of the HPLC
- Operating an HPLC
- Work in a team or independent setting and collaborate with employees from various departments
- Lift 20 – 30 lbs involving the movement of raw materials within the quarantine cage
Experience For Quality Control Chemist, Microbiology Resume
- Normal laboratory and manufacturing situation
- Assist in quality control testing of samples provided to the QC laboratory
- Operation and understanding of GC instrumentation
- Read and understand Standard Operating Procedures (SOP) related to job function
- Performs a variety of analytical EIA (ELISAs and HEIA) testing
- Perform qualitative and quantitative analyses using analytical instrumentation such as HPLC, GC, UV/Vis, KF and FTIR
- Analyze data against approved specifications, notifying management of any OOS and aberrant results
- Perform peer review of laboratory testing, as required
- Provide assistance and make recommendations in the design of incoming inspection and/or chemical analysis of raw materials or finished products
Experience For Review Chemist, Quality Control Resume
- Participate in the qualification/certification of new and/or existing suppliers
- Aid in transferring these new methods into a Quality Control function for routine use
- Characterize polymer size by GPC/SEC chromatography using established standard processes
- Characterization of polymers by HPLC/FPLC using established standard processes
- Optical characterization of the polymer dyes including measurement of absorbance and fluorescence emission spectra
- Other responsibilities or projects as assigned by reporting manager
- Assist with the setting up and implementation of the PPCP QC laboratory
Experience For Senior Chemist, Quality Control Operations Resume
- Perform and/or assist with analytical testing of raw materials, in-process samples, and finished products
- Comply with cGMP regulations and follow all standard operating procedures
- Adherence to follow standard operating procedures (cGMP’s, SOP’s, and DOP’s)
- Assists with the testing of surveys
- Maintains personal training files for work performed
Experience For Associate Chemist, Quality Control Resume
- Adhere to the pertinent aspects of Corporate and plant safety programs and adhering to GMP's, ISO standards or other regulations
- Operation and understanding of GC/MS instrumentation
- Theoretical and working knowledge of instrumentation such as HPLC, GC, UV/Vis and analytical methods
- Maintain and follow all laboratory systems, GMPS, safety and housekeeping requirements
- Performs USP, EP and ACS testing on raw materials
- Analyze using wet, physical, and instrumental methods to determine product composition and characteristics (qualitative and quantitative analyses)
- Autoclave polymerization testing on finished product
- Maintain the laboratory in according to 5S principles
Experience For Quality Control Chemist, QC Resume
- Tabulate and present quality data to the internal customers including sales, R & D and production
- Assist with training of the analysts in areas of expertise and knowledge and in new methods, SOP’s and updates
- Detailed knowledge of quality management systems, pharmaceutical manufacturing operations, requirements for cGLP, pharmacopoeial methods and stability
- Basic Understanding of GMP/GLP
- Familiar with Raw Material testing, Global compendia and ICH
- Knowledge and understanding of refinery processes
- Working in an analytical laboratory
Experience For Quality Control Bench Chemist Resume
- Interpretation of raw data from HPLC
- Input results into SAP for official records
- Knowledge and understanding of acceptance activity requirements for incoming, in-process and final inspection requirements (as associated with job type/position) to ensure that a device/drug conforms to its specification and out of specification items are properly handled
- Knowledge and understanding of Stop Orders, Concessions, ESD, Calibration, Preventative Maintenance, Material Identification & Segregation, documentation configuration control practices; documentation & disposition of non-conforming material and Good Documentation Practices requirements, as associated with this job type/position
- Aware of and comply with the GEHC Quality Manual, Quality Management System, Quality Management Policy, Quality Goals and applicable laws and regulations as they apply to this job type/position
- Assist with raw material release
- Helps prepare monthly metrics for raw material release as needed
- Assist with sample and release of materials as needed
- Assist with wet chemical analysis of material as needed
List of Typical Skills For a Quality Control Chemist Resume
Skills for chemist, quality control resume.
- Testing experience within the pharmaceutical industry
- Perform testing within the Quality Control department involving analytical Instrumentation experience including HPLC, GC, and FTIR
- Strong understanding of NMR spectroscopy and NMR data processing
- Experience working in an industrial manufacturing environment - Chemical Industrial / Adhesives /Sealants / Paint
- Familiarity with guidelines such as good manufacturing processes and ISO standards
- Assists with the validation of processes and equipment according to established procedures
Skills For Quality Control Data Review Chemist Resume
- Experienced in leading technical lab investigations
- Hands on experience performing refinery test methods (e.g. ASTM methods for: GC, AA, IC, XRF, elemental analysis, and wet chemistry)
- Demonstrated success delivering business value through laboratory innovation
- Demonstrated ability to consult on analytical instrumentation and test methods, including method development and equipment/instrumentation repair and purchase
- Experience working with data analytics tools (Spotfire, MS Power BI)
- Experience with chemometric modeling and analysis
- Experience with the proper operation of laboratory instrumentation, troubleshooting, and the generation and revision of SOPs and test methods
- Participate in validation and measurement system analysis of various testing methods
- Previous pipetting experience
Skills For Chemist, / II, Quality Control Resume
- Industry experience using HPLC
- At least one-year experience in the production of reference material manufacturing
- Lab experience in QC manufacturing environment
- Good with multi-tasking
- Performance of routine HPLC method development and validation of HPLC protocols of bulk APIs and new drug products required
- Experience in a GMP or GLP Quality Contol (QC) lab
- Industrial lab experience
- Complete visual inspection of product labeling prior to the issuance of the prepared labels to manufacturing
Skills For Chemist, Quality Control Operations Resume
- Performs raw material, in-process material and finished goods testing
- Perform routine chemical testing of commercial, validation and stability samples in accordance to written methodology
- Documentation experience is desirable
- Assure conformance to Good Laboratory Practice within the QC Group
- Assist with the validation of analytical methods
- Responsible for the quality release of goods in SAP QM system as well as product CoA creation
Skills For Quality Control Chemist, Microbiology Resume
- B.A. or B.S. in Chemistry with 1+ year(s) experience within a Quality Control or Analytical Chemistry Department
- Demonstrated ability to partner with co-workers and build rapport with other departments
- Meeting multiple testing cycle time requirements through scheduling, testing and documentation review
- Identifying, planning and completing continuous improvement projects within the laboratory and Northridge facility
- Navigating Ambiguity- Respond positively to changing situations
- Preparing and routing ingredient usage sheets
- Maintaining laboratory cleanliness and housekeeping
Skills For Review Chemist, Quality Control Resume
- Analyzing Packaging Materials, Raw Materials and Finished Products per USP/NF/EP Compendia and approved Test methods and Specifications
- Testing using HPLC, FTIR, ICP-OES, UV/VIS, Liquid Scintillation and Gamma Spectrometer
- Understanding of Adage and Chemstation software
- Preparing test chemical solutions
- Ensuring world-class safety within the quality control laboratory and Northridge Facility
Skills For Senior Chemist, Quality Control Operations Resume
- Supporting resolution of non-conformances and investigations in the quality control laboratory
- Supporting introduction of new analytical techniques and equipment to the quality control laboratory
- Understanding of polymer chemistry and structure property relationships
- Working closely with other employees in Quality Control and R&D to investigate out of specification results
- Working knowledge of gas chromatography, NDIR, GC/MS analytical methods
- Working closely with others in the group to achieve business objectives
- Testing and inspection of incoming raw materials and chemicals
- Assisting with internal audits of the facility
- Handling of air sensitive materials in an inert environment (glovebox)
Skills For Associate Chemist, Quality Control Resume
- Testing is In support of Manufacturing and sample receipt functions
- Performing Sample Receipt functions
- Intermittently sitting, standing, stooping. Moderate lifting or carrying 25-50 pounds. Climbing ladders or scaffolds. Typically standing and/or walking
- Assists with the maintenance of real time, accelerated and shipping stability testing according to established Standard Operating Procedures (SOPs)
- Assist in evaluating new testing applications and instrumentation, either developing methods or adopting compendial methods
- Activities include drafting Analytical Investigation Report text, performing action steps, and drawing justifiable conclusions based on data
- Support lab operations by completing other tasks associated with testing
- Regularly perform wet chemistry, dissolution testing, titrations and pH testing
- Perform Quality Control (QC) testing of raw materials, components, and finished kits according to established specifications
Skills For Quality Control Chemist, QC Resume
- Performs return authorization product testing according to established procedures
- Assists technical service with customer complaint testing when needed according to QC specifications
- Ensure there are no other interferences coming from the product formulations causing the OOS result
- Perform small scale dye labeling reactions according to established standard processes
- Conduct QC testing of samples using various techniques such as HPLC, GC, and wet chemistry techniques
- Assists in the stocking and supplying of the Quality Control lab
Skills For Quality Control Bench Chemist Resume
- Support qualification of new QC equipment and coordinating preventative maintenance of existing lab equipment
- Maintain adequate inventory of supplies needed for all raw material sampling including but not limited to pipets, containers, and consumables
- Perform testing of raw material, in-process, finished product and stability samples
- Report all identified problems to designated senior personnel and assist them with troubleshooting problems
- Thoroughly document justification for all changes being made
- Participate in housekeeping performance and audits
- Opportunity for progression in a fast paced diverse working environment
- Use critical thinking to solve complex problems with multiple variables
- Assists with the evaluation of in-process materials according to established procedures
List of Typical Responsibilities For a Quality Control Chemist Resume
Responsibilities for chemist, quality control resume.
- Operating at least two of the following analytical instruments or systems: GC, HPLC, UV-Vis, FTNIR, IR, Titrations, USP Monograph Testing, Viscometer, Particle Size Analyzer, GPC
- Demonstrated strong mechanical skills and ability to use tools to maintain instrumentation
- Good working knowledge of basic biological and chemical laboratory procedures
- Perform accurate and timely analyses on finished products and stability of different dose forms (solid dose, liquids, topicals)
- BS in Chemistry or related discipline with 2-5 years of pharmaceutical industry or laboratory experience
- Knowledge of cGMPs and good documentation practices
- Strong background in analytical chemistry and quanititative analysis
- Demonstrated ability to work on multiple projects and meet timelines
- BS in Chemistry with 0-3 years of pharmaceutical industry or laboratory experience
Responsibilities For Quality Control Data Review Chemist Resume
- Experience using statistical quality control tools and practices
- Demonstrated ability to communicate chemistry/technical information to audiences of varying technical background
- Modify and validate analytical procedures to meet business needs
- Experience within a QC laboratory is required
- Polyurethane foam formulation experience is required
- Experience in data collection, manipulation and analysis
- Experience with Chromatography Data Acquisition systems and Laboratory Information Systems
- Experience in the maintenance and calibration of analytical equipment is an asset
- Experience in a GMP/GxP or a comparable federally regulated environment
Responsibilities For Chemist, / II, Quality Control Resume
- Analytical laboratory experience in a GMP/GxP or a comparable federally regulated environment
- Experience as Analytical or QC Chemist, or as a Research Associate/Assistant or Wet Assay Lab Technician required
- Opportunity to gain experience with a diverse range of analytical equipment
- Experience with analytical technique and instrumentation
- Perform routine HPLC method development and validation of HPLC protocols of bulk APIs and new drug products
- Work in accordance with approved methods, SOPs, and cGMP’s/GLPs, while performing tests
- Assists with the maintenance of QC equipment according to SOPs as requested by supervisor
Responsibilities For Chemist, Quality Control Operations Resume
- Perform and follow standard operating procedures
- Perform troubleshooting of instrument and method
- Assist the QC Manager in training others in this area of expertise if needed
- Development and implementation of new and existing test methods
- Assists in training of QC staff on use and operation of new laboratory equipment
- Test and approve incoming raw materials
Responsibilities For Quality Control Chemist, Microbiology Resume
- Support Manufacturing, Quality, Sales and Product Management functions
- Operation of analytical, mechanical property and chemical processing equipment
- Be on call and available for nights and weekends. (At least twice a month/Every other weekend rotate with team members)
- One (1) to three (3) years of experience with the following analytical equipment: HPLC, UV/Vis, balances, pH meters, Karl Fisher, disintegration baths and dissolution apparatus
- Experienced in performing raw material, in-process, and finished product analysis by wet-chemistry, TLC, titration (manual and automated), FTIR, UV-Vis, Refractometers, HPLC, ICP, AA, and other routine QC analytical instruments
- Experienced with ingredient characteristics such as, bulk and tap density, particle size, purity, moisture content, form, composition, etc
- Without assistance can professionally organize and prep test samples, reagents, and solutions to perform analytical work
Responsibilities For Review Chemist, Quality Control Resume
- Can accurately record data, perform calculations, and report results in a GMP compliant environment
- Authoritative ability to perform and coordinate analytical testing and related activities, conducting chemical tests as defined by our test methods and protocols
- Review the work of laboratory technicians, third party test results, lab notebooks, and data generated by other lab members
- Familiar with analytical quality control tests for vitamins, minerals, botanical compounds, and enzymes
- Analyzes and maintain data per applicable SOPs
- Documents activities in a cGMP manner
- Recognize problems and inform the lab manager to perform corrective actions
Responsibilities For Senior Chemist, Quality Control Operations Resume
- Performs investigations and resolution of out-of-specification events
- Prepares reagents and solutions as necessary to conduct analyses
- Maintain instrumentation logs for laboratory equipment
- Perform daily/weekly/monthly 5S activates as needed
- Understand instrumentation logbooks/logs for laboratory equipment
- Wear PPE such as nitrile gloves, dust mask or respirator, when needed
- Development, implementation, and completion of the incoming inspection and/or chemical analysis of raw materials or finished products distributed in accordance with good laboratory practice
- Complete batch record review and release approval of products in accordance with finished product inspection procedures
- Create, receive, and approve product certifications
Responsibilities For Associate Chemist, Quality Control Resume
- Supports the lab manager as required to coordinate the activities of the QC group
- Customer complaint and/or process improvement report investigation and resolution
- Development, implementation, and completion of calibration procedures for all laboratory instruments and equipment
- Promote continuous process improvement through participation in team projects
- Performs HPLC, GC and wet chemical analyses in quick turnaround and high throughput quality control laboratory
- Is responsible for minor instrument maintenance and document review
- Adheres to all applicable cGMP regulations
Responsibilities For Quality Control Chemist, QC Resume
- Quality test and release of all materials received and produced on site
- Standard tests include Karl Fischer titration, NMR spectroscopic analysis, ICPOES and ICPMS
- Ability and eagerness to collaborate with both junior and senior scientists in order to expedite project completion
- Competency in MS Office required
- Review, revise, and write SOPs
- Work flexible hours as needed to support production demands
- Work well in both team and individual environments
- Ensure at the least, these methods are accurate, precise, and robust for daily use on specific samples
Responsibilities For Quality Control Bench Chemist Resume
- Maintain equipment logs, calibration logs, and solvent/mobile phase preparation records
- Test products for long-term stability under various conditions
- Provide timely and high-quality data reports that document test results
- Interface, maintain, and populate data and results in specified database(s)
- Serves as the technical resource of the PPCP QC group
- Utilize analytical laboratory instrumentation with supervision
Related to Quality Control Chemist Resume Samples
Associate chemist resume sample, quality chemist resume sample, scientist, quality control resume sample, science resume sample, research science resume sample, life science resume sample, resume builder.
- ResumeBuild
5 Amazing qc chemist Resume Examples (Updated 2023) + Skills & Job Descriptions
Build your resume in 15 minutes, qc chemist: resume samples & writing guide, george wilson, employment history.
- Maintain laboratory equipment and ensure accuracy of results
- Review and approve production documentation
- Work with production and quality assurance to ensure compliance with applicable regulations and standards
- Ensure compliance with Good Laboratory Practices (GLP)
- Monitor and review product quality data
- Train and mentor junior chemists
- Collaborate with other departments on process improvement initiatives
Do you already have a resume? Use our PDF converter and edit your resume.
Virginia Ingram
Professional summary.
- Prepare reports, protocols, and documents related to testing activities
- Troubleshoot and resolve laboratory issues
- Analyze raw materials, in-process samples, and finished products using analytical techniques
- Monitor and review laboratory safety practices
- Provide technical support to other departments
- Perform other duties as assigned
- Participate in investigations, root cause analysis, and corrective and preventive actions
- Develop and validate analytical test methods
Umberto Foster
Not in love with this template? Browse our full library of resume templates

Table of Content
- Introduction
- Resume Samples & Writing Guide
- Resume Example 1
- Resume Example 2
- Resume Example 3
- Resume Example 4
- Resume Example 5
- Jobs Description
- Jobs Skills
- Technical Skills
- Soft Skills
- How to Improve Your Resume
- How to Optimize Your Resume
- Cover Letter Example
qc chemist Job Descriptions; Explained
If you're applying for an qc chemist position, it's important to tailor your resume to the specific job requirements in order to differentiate yourself from other candidates. Including accurate and relevant information that directly aligns with the job description can greatly increase your chances of securing an interview with potential employers. When crafting your resume, be sure to use action verbs and a clear, concise format to highlight your relevant skills and experience. Remember, the job description is your first opportunity to make an impression on recruiters, so pay close attention to the details and make sure you're presenting yourself in the best possible light.
- We Use the Quality control skills for Effulent managment of the Unit.
- Quality Analysis of Raw materials & Finished products.
- Clinical microbiology and biochemical testing.
- Maintain databases of experiment characteristics or results.
- Microbial contamination of Finished product.
- Quantitative analysis Of Chemical Auxiliaries and Dyes testing Reactive dyes (Remazol , Levafix)
- Solubility test, ASS, PLS, Viscosity, Filter test etc.
- Standardization of plant Batch liquid.
- Standardization of plant Blender
- Standardization & Preparation of solution used in analysis
- Calibration of instrument
- Dyeing like exhaust, Silicate Pad Batch
- Calibration Of Equipment Such as Practical Size Analyzer, Surface Tension,
- Viscometer, Tensiometer
- Knowledge of SAP
- Complete Knowledge of Color Computer Matching
- Person in charge for schedule waste collection, arrangement of chemical stock in and out from chemical room.
- Details documentation for chemical room.
- Backup side for documentation in quality control for assembly department.
- Documentation for new product, proton part.
- Quantitative analysis Of Chemical Auxiliaries and Dyes testing Reactive dyes (Remazol , Levafix ).
- Solubility test, ASS, PLS, Viscosity, Filter test etc.
- l Standardization of plant Batch liquid.l Standardization of plant Blender
- l Standardization & Preparation of solution used in analysis
- l Calibration of instrument
- l Knowledge of Color Computer Matching
qc chemist Job Skills
For an qc chemist position, your job skills are a key factor in demonstrating your value to the company and showing recruiters that you're the ight fit for the role. It's important to be specific when highlighting your skills and ensure that they are directly aligned with the job requirements, as this can greatly improve your chances of being hired. By showcasing your relevant skills and experience, you can make a compelling case for why you're the best candidate for the job.
How to include technical skills in your resume:
Technical skills are a set of specialized abilities and knowledge required to perform a particular job effectively. Some examples of technical skills are data analysis, project management, software proficiency, and programming languages, to name a few. Add the technical skills that will get hired in your career field with our simple-to-use resume builder. Select your desired resume template, once you reach the skills section of the builder, manually write in the skill or simply click on "Add more skills". This will automatically generate the best skills for your career field, choose your skill level, and hit "Save & Next."
- Chromatography
- Spectroscopy
- Quality Control
- Data Interpretation
- GMP Compliance
- Laboratory Safety
- Hazardous Waste Management
- Calibration
- Laboratory Techniques
- Sample Preparation
- Data Validation
- Data Analysis
- Microbiology
- Calculation
- Instrumentation
- Aseptic Techniques
- Statistical Analysis
- Troubleshooting
- Quality Assurance
How to include soft skills in your resume:
Soft skills are non-technical skills that relate to how you work and that can be used in any job. Including soft skills such as time management, creative thinking, teamwork, and conflict resolution demonstrate your problem-solving abilities and show that you navigate challenges and changes in the workplace efficiently. Add competitive soft skills to make your resume stand-out to recruiters! Simply select your preferred resume template in the skills section, enter the skills manually or use the "Add more skills" option. Our resume builder will generate the most relevant soft skills for your career path. Choose your proficiency level for each skill, and then click "Save & Next" to proceed to the next section.
- Communication
- Interpersonal
- Time Management
- Problem Solving
- Decision Making
- Critical Thinking
- Adaptability
- Organization
- Public Speaking
- Negotiation
- Conflict Resolution
- Attention to Detail
- Self-Motivation
- Stress Management
- Collaboration
- Strategic Thinking
- Emotional Intelligence
- Flexibility
- Reliability
- Professionalism
- Computer Literacy
- Project Management
- Customer Service
- Presentation
- Written Communication
- Social Media
- Supervisory
- Risk Management
- Database Management
- Documentation
- Financial Management
- Visualization
- Business Acumen
- Process Improvement
- Relationship Management.
How to Improve Your qc chemist Resume
Navigating resume pitfalls can mean the difference between landing an interview or not. Missing job descriptions or unexplained work history gaps can cause recruiters to hesitate. Let's not even talk about the impact of bad grammar, and forgetting your contact info could leave your potential employer hanging. Aim to be comprehensive, concise, and accurate.
Vincent White
Unexplained year gaps and missing job experiences are a no-no, gaps in your resume can prevent recruiters from hiring you if you don't explain them..
- It's okay to have gaps in your work experience but always offer a valid explanation instead of just hiding it.
- Use the gap to talk about positive attributes or additional skills you've learned.
- Be honest and straightforward about the gap and explain it using a professional summary.
How to Optimize Your qc chemist Resume
Keep an eye out for these resume traps. Neglecting to detail your job roles or explain gaps in your career can lead to unnecessary doubts. Grammar blunders can reflect negatively on you, and without contact information, how can employers reach you? Be meticulous and complete.
Francis Quinn
- Collaborate with other departements on process improvement initiativs
- Monitor and reviw product quality data
- Develop and validte analytical test methods
- Monitering and reviews product quality data
- Work with production and quality assurance too ensure compliance with applicable regulations and standards.
- Maintain laboratory equipement, and ensuare accuracy of results
- Work with production and quality assurance, to ensuare compliance with applicable regulations and standards
- Participate in investigations, root cause anaylsis, and corrective and preventative actions.
Avoid Spelling Mistakes and Include your Contact Information
Missing contact information prevents recruiters from understanding you're the best fit for the position..
- Make sure you're not missing contact information on your resume. That should include your full name, telephone number and email address.
- Make sure to use a professional email address as part of your contact information.
- Highlight your contact information and double check that everything is accurate to help recruiters get in touch with you.
qc chemist Cover Letter Example
A cover letter can be a valuable addition to your job application when applying for an qc chemist position. Cover letters provide a concise summary of your qualifications, skills, and experience, also it also gives you an opportunity to explain why you're the best fit for the job. Crafting a cover letter that showcases your relevant experience and enthusiasm for the Accounts Payable role can significantly improve your chances of securing an interview.
To the respected Celgene Corporation Recruitment Team
I am writing to express my interest in the Senior Qc Chemist role at Celgene Corporation. As a Qc Chemist with 5 years of experience, I am confident that I possess the necessary skills and qualifications to excel in this position.
As someone who has faced challenges in various areas of my life and has overcome them, I am confident in my ability to adapt and thrive in any environment. I have developed a reputation for being a collaborative team player and an effective problem solver, which has been instrumental in my career's success. With my experience and passion for Science & Biotech, I am excited to apply my skills to this role and contribute to your organization's growth and success.
I appreciate the opportunity to apply for the Senior Qc Chemist position. I am confident that I can make a valuable contribution to your organization and that together there is no challenge that we cannot overcome. I will be waiting, hopeful for what the future will bring.
Showcase your most significant accomplishments and qualifications with this cover letter. Personalize this cover letter in just few minutes with our user-friendly tool!
Related Resumes & Cover Letters

Contemporary

Professional

Looking to explore other career options within the Science & Biotech field?
Check out our other resume of resume examples.
- Health Technician Resume
FIND EVERYTHING YOU NEED HERE.
IF YOU HAVE QUESTIONS, WE HAVE ANSWERS.

4 Ways a Career Test Can Jump-Start Your Future (and Help Your Resume)
If you’re looking for a fresh path or a new passion, a career test could help you find it. You can take these tests online, in the comfort of your...

Avoid These 3 Resume Mistakes at All Costs
Your resume is your first impression for a prospective employer. The way you present yourself in that little document can make or break you – it can clinch you an...

Resume Design Tips and Tricks
Creating a resume that stands out from the rest doesn’t have to be rocket science. With just a few tips and tricks, you can make your professional resume a shining...
Build your Resume in 15 minutes
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
- Resume Samples
- Science and Biotech
Qc Chemist Resume Samples
The guide to resume tailoring.
Guide the recruiter to the conclusion that you are the best candidate for the qc chemist job. It’s actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get hired.
Craft your perfect resume by picking job responsibilities written by professional recruiters
Pick from the thousands of curated job responsibilities used by the leading companies, tailor your resume & cover letter with wording that best fits for each job you apply.
Create a Resume in Minutes with Professional Resume Templates
- Perform elementary method analysis and development for troubleshooting purposes. Make recommendations to technical support on analytical methods
- Perform other duties as assigned or deemed necessary to assist the QC Lab Manager in achieving key objectives
- Manage daily tasks with in the lab to ensure work queue is managed
- Perform all miscellaneous analytical testing as directed by West Chester’s QC Supervisor, Methods Development Chemist, and Analytical Services Manager
- Perform and assist with additional duties as may be directed by the QC supervisor
- Generate, review and maintain detailed records/documents and assist with QC data management and control charting
- Work in accordance with approved methods, SOPs, and cGMPs/GLPs, while performing tests
- Support in establishing and controlling of raw material’s supplier qualification program
- Ensure raw materials have been manufactured, tested or inspected according to specifications and current testing
- Assist in optimizing test procedures to improve efficiency whenever possible
- Preparation and reviewing of equipment qualification protocols and reports
- Establish and maintain QC Raw Materials laboratory in full cGMP-compliance
- Participate in the validation and transfer of internal test methods, as well as method verification of compendial methods
- Perform raw material testing following United States Pharmacopoeia (USP), European Pharmacopoeia (EP), American Chemical Society (ACS) and/or Internal Validated Methods
- Set up, troubleshoot, and make adjustments to laboratory equipment
- Serves as a technical lead on projects and provides direction to various levels of chemist
- Applies cGMP concepts in association with department specific responsibilities
- Performs routine to complex laboratory analyses and experiments with no supervision
- Performs testing for the qualification of reference standards
- Transfer, modify, and improve analytical methods
- Provide technical advice to team members, and other Johnson Matthey Pharma Services (JMPS) departments
- Strong knowledge of analytical chemistry principles and methods with the ability to interpret results
- Strong knowledge of wet chemistry and GLP’s
- Ability to perform basic math functions
- Proficient in SAP and Microsoft Office with excellent written and verbal communication skills
- Excellent interpersonal, verbal, and written communication skills with the ability to communicate with all levels of the organization
- Strong command of Quality Control processes/systems
- Good understanding of ISO/AS 9001 Quality Management Systems
- Ability to communicate in a professional manner, both written and orally, with individuals at all levels of the organization
- Ability to use personal judgment and knowledge to disseminate information
- Highly proficient in usage of IT tools – MS Office Suite, SPC, SAP, ETQ, Access, and other computer systems
15 Qc Chemist resume templates

Read our complete resume writing guides
How to tailor your resume, how to make a resume, how to mention achievements, work experience in resume, 50+ skills to put on a resume, how and why put hobbies, top 22 fonts for your resume, 50 best resume tips, 200+ action words to use, internship resume, killer resume summary, write a resume objective, what to put on a resume, how long should a resume be, the best resume format, how to list education, cv vs. resume: the difference, include contact information, resume format pdf vs word, how to write a student resume, qc chemist resume examples & samples.
- Ensure compliance with mandatory training requirements and ensure that your training records are up to date and compliant to cGMP
- Your responsibilities may include test method development and validation, raw material, in process, finished product and Stability testing
- Ensure all analytical testing is carried out as defined in the Quality System
- Provide training (induction/ on the job/ procedures and updates), technical guidance / trouble shooting to laboratory staff including review of associated training records
- Proficient in the use various software application utilized in the laboratory. As required the development configuration and validation of software in accordance with industry standards
- Management of laboratory spend and approval of vendor invoices
- Management of laboratory inventory and supplies and ordering of same
- Participate on LBP/VIP and Continuous project teams as required
- BSc or similar degree with minimum 3 years experience in a relevant area
- Proven ability to work well as part of a team & on own with minimum supervision
- Perform testing, utilizing analytical equipment and standard wet chemistry techniques, on raw materials, intermediate and bulk (including lacquer)
- Write/revise standard operating procedures and methods
- Assist training of other chemists on procedures and processes as needed
- Responsible for calibration, maintenance and troubleshooting of test instruments
- Maintains a clear understanding and working knowledge of GMP/GLP requirements, Safety requirements, test methods and SOP’s. Adheres to and enforces those policies within the team
- Working knowledge of a wide variety of laboratory equipment and processes
- Strong knowledge of wet chemistry and GLP’s
- Process and Method Validation
- Excellent application of Root Cause Problem Solving
- CGMP (Cosmetic Good Manufacturing Practices)
- Technical Writing skills relative to SOPs, Test Methods, and Work Instructions
- Team Oriented
- Effective influence and persuasion skills
- Strong time management and prioritization capability
- Excellence in Execution
- Constructive Networker
- To operate within the chemistry laboratory for the analysis of raw materials, in process and finish products
- Sampling of materials, utilities and environment
- Ensure all operations are conducted in accordance with GMP's and good scientific principles
- Manage documentation and equipment maintenance systems within the laboratory
- Review chemical analysis data of raw materials, in process and finish products
Bulk QC Chemist Resume Examples & Samples
- Adhere to H&S, Quality, GLP, GMP (ISO 22716) and Hygiene Policies
- Complete all daily activities required within the bulk QC laboratory
- Perform analysis of bulks according to quality and IC/R&D specifications
- Responsible for timely and effective quality testing
- Record, interpret and communicate results. Report results using defined documentation systems/computer systems
- Review and analyze quality results for quality improvements e.g. RFT
- Raise quality notifications and partake in root cause/problem solving investigations
- Maintain bulk retains and Reference Standards
- Create, edit, review and maintain quality control procedures
- Review and manage inventory, raising disposal notes for bulks to be destroyed when necessary
- Partake in internal and external quality/H&S audits
- Present and share information on processes or practices with visitors or external auditors
- Provide quality advice and guidance to central weighing, manufacturing, pressing and assembly departments
- Liaise with IC/R&D
- Provide information and training to new staff
- Contribute to and participate in continuous improvement activities if required (lean events)
- Act today, shape tomorrow, and deliver continuous improvement/lean improvements
- Represent department at key meetings, projects or working groups, delegate for Senior Bulk QC Chemist where necessary
- Testing all current finished products using assigned techniques, methods and procedures to agreed timescales
- Complete testing data in accordance with Company Policy and legislative compliance to agreed time scales
- Carry out peer checking of analytical test documentation as required
- Report all OOS and Atypical data and results to the QC Supervisor
- Participate in laboratory investigations as required,
- To prepare and maintain all chemicals and solutions according to current procedures
- Be conversant with the hazardous properties of chemicals and observe the safety rules
- Assist in the management of laboratory stock items
- Maintenance and calibration of instruments
- To give assistance in maintaining and updating laboratory procedures, reports, logbooks, certificates of analysis and other documentation
- To participate in training programmes
- To assist in GMP and safety audits of the laboratory and other areas as required
- Third level degree in science discipline
- Minimum 2-3 years laboratory experience with good hands-on knowledge of common laboratory techniques and instruments including HPLC, GC, Dissolution, KF, LOD, Raw material testing experience is preferred
- Maintain credibility and integrity
- Strong work ethics and goal focused
- Demonstrate enthusiasm and energy
- Software - Empower / Velquest / LIMS
QC Chemist Fresno Resume Examples & Samples
- Promptly performs reports and records all Quality Control procedures and test results in a precise and accurate manner
- Maintains inventory status of reagents, equipment, etc. in order to keep the lab at full operational capacity
- Prepares lab standard test reagents, and performs standardization on reagents and calibration of equipment on a regular basis. Keeps detailed records and maintains control and/or trend charts
- Maintains a clean, safe and orderly work environment and assures smooth operation of the QA Laboratory by communication, planning and preparation in support of other shifts. Understands and is active in the company 5S and Lean initiatives
- Associate’s degree in Chemistry or a related field
- Immigration sponsorship not offered for this role
- Bachelor’s degree in Chemistry or other Science preferred
- The QC Chemist shall conduct analytical and physical tests on raw materials, drug substances, in-process samples and drug products in accordance with approved procedures, with minimal supervision of the laboratory supervisor
- The incumbent will, on occasion, supervise the work of one or more Technicians, if deemed necessary by the Laboratory Supervisor
- The incumbent should be able to demonstrate a working knowledge of all required analytical techniques used in the Impax QC Laboratory. He/she should be able to perform all required duties independently or with minimal assistance of the lab supervisor
- The incumbent shall assure that products are tested and evaluated in accordance with laboratory SOPs and cGMP regulations and are dispositioned accordingly
- The incumbent should be fully aware of version and change control as they relate to all lab documentation
- He/she shall maintain laboratory instrumentation, logbooks, test results, databases and notebooks in compliance with laboratory SOPs and cGMPs as they relate to the QC Laboratory (i.e., 21 CFR 211.65)
- The incumbent should be fully aware of all USP/NF and other compendial requirements, ICH and FDA Guidances, Corporate laboratory requirements and other policies and procedures as they relate to QC Laboratory activities
- The incumbent will be responsible for providing and documenting training to less experienced laboratory personnel
- The incumbent shall be familiar with software such as Microsoft Word, Microsoft Excel, and other such relevant software packages, such as TotalChrom® and/or other Chromatographic Data and Control software
- He/she maintains a safe work environment for self and others in the laboratory. Assures that all safety information such as MSDSs are available for all lab personnel
- The incumbent may be called upon to perform other tasks to support the attainment of business objectives
- The incumbent is responsible for mentoring less experience lab personnel in good documentation practices (notebook, investigative and other technical report writing, annotating and archiving supporting data, maintaining procedural formats, etc.)
- The incumbent may perform such metrology functions as instrument/system calibration and qualifications
Lead / Coordinating QC Chemist Resume Examples & Samples
- Responsible for QC release of finished products and raw materials / analytical project management and delivery
- Responsible for scheduling of workload within the QC labs ensuring that QC metrics are met
- Provide analytical services and support to the site, ensuring compliance to GMP's and SOP's
- Subject matter expert on quality control activities
- Use technical skills and experience to troubleshoot and problem solve product testing issues. Lead lab investigations and deviations
- Ensure QC Laboratories meet current Good Laboratory Practice (cGLP) requirements and ensure always audit ready
- Compile, review and trend chemistry test results on a daily basis to ensure compliance with cGLP
- Stakeholder management with other departments / sites on matters related Quality Control
- Assist and/or lead projects for safety improvements/lean implementation/continuous improvement/compliance
- Assist in testing where required
- 3rd level degree in a science related discipline required (chemistry or biochemistry degree an advantage)
- 5 years + experience gained a cGMP manufacturing laboratory environment within the pharmaceutical sector
- Provide analytical services and support to the site, ensuring compliance to GMP's and SOP's. Assist in routine testing procedures in support of team targets
- Use technical skills and experience to troubleshoot and problem solve product testing issues
- Ensure QC Laboratories meet current Good Laboratory Practice (cGLP) requirements
- Maintain, update and issue chemical methods, specifications and SOP's in compliance with pharmacopeia and regulatory requirements
- Compile and review chemistry test results on a daily basis to ensure compliance with cGLP
- Training other team members as required
- Effective interaction with other departments on matters related to raw materials, in-process, packaging and finished batch release
- Assist in method transfer/development activities as required
- 2 years experience gained a cGMP manufacturing laboratory environment - ideally part within the pharmaceutical sector
- · Handle internal and external problems related to quality, dig out root cause and propose corrective action and preventive measurements, issue investigation report on time
- · Be an active participant in quality and efficiency improvement projects, design or propose action plan for these projects, implement action plan and monitor project progress as required
- · Recognize customer and statutory specific requirements on products, support supervisor to guarantee compliance
- · Maintain interaction with solution lab and technical service as well as counterparts at other locations
- · Take the coordinating role in work-off activities; make every effort to reduce COPQ
- · Train QC employee with standardized SOP and product information on the job training program
- · Take responsibility to daily maintain lab facilities and organize 5S activities
- · Ensure that all activities in the areas of responsibility comply with the SSHE policy of AkzoNobel
- · Other responsibilities as assigned by supervisor or line Manager
Entry Level QC Chemist Resume Examples & Samples
- Under the supervision perform analytical testing of raw materials, in-process materials, stability samples, finished products as per the test method
- Perform wet chemistry testing as per Test Method, SOP or USP compendia procedure
- Analytical testing will include Assay, preservative content, LOD, ROI, water content, identification test, dissolution, disintegration, heavy metals etc
- Operates general analytical instruments during routine testing including but not limited to HPLC, GC, KF /Automatic titrator, FT-IR, Polarimeter, UV/Vis spectrophotometer, melting point apparatus, dissolution and disintegration units, Malvern Particle size analyzer, and laboratory ovens
- Recognizes deviations from normal trend and inform group leader or laboratory management of any problems and/or deviations that may affect the integrity of the data and participates in corrective action of problems
- Complies with all current Good Manufacturing Practices (cGMP) current Good Laboratory Practices (cGLP) and Environmental Health & Safety (EHS) requirements, laboratory Standard Operating Procedures (SOPs) and company policies and procedures
- Perform standard qualitative and quantitative analysis on: purchased raw materials, in-process products, pure drug substances and finished pharmaceutical preparations employing accepted gravimetric and spectrophotometer procedures, in accordance with approved testing procedures
- Utilize standard "bench" chemistry technique as well as sophisticated electronic instrumentation, including spectrophotometer, viscometer, etc
- Maintain records of all analysis information of assigned products on data sheets for permanent file and proper entry of information on laboratory forms
- Perform routine analytical testing of raw materials purchased from prospective suppliers to determine that the materials meet compendia and/or company standards
- Assist with troubleshooting analytical methodology and instrumentation malfunctions
- Assist in validation of manufacturing and production lines and equipment pertinent to chemistry and microbiology (e.g. equipment and room cleaning validation)
- If assigned to the QA Receiving area, develop specifications for packaging components and prepare final specification sheets for new, revised, or updated components in conjunction with packaging departments, other facilities and component vendors
- Bachelors in Chemistry/related field or an Associates in Chemistry/related field
- Previous pharmaceutical (cGMP) lab experience preferred
- Experience using fundamental analytical instrumentation and techniques (such as HPLC,GC, viscometer , titration, UV-Vis, IR)
- Computer knowledge and use of Microsoft Office software
- Legible and thorough documentation skills
Electronic Chemicals Lab QC Chemist Resume Examples & Samples
- Perform analytical testing of finished products, in-process samples, stability samples, experimental samples, and raw materials in support of manufacturing and product development
- Perform analytical testing using titration, GC, FTIR, and other common chemistry analytical techniques
- Document testing results in accordance with quality procedures and maintain a well-documented laboratory notebook
- Execute method transfer protocols, method validation protocols, investigation protocols, and instrument qualification protocols
- Develop new analytical methods to support product development and technical service
- Maintain awareness of state-of-the-art analytical chemistry technology
- Work in a fast paced, results-oriented team environment to assist in developing new technologies to provide solutions to customers' needs
- Adhere to Dynaloy environmental, health, and safety program
- Perform common laboratory procedures such as weighing on analytical balances, glassware preparation, equipment calibrations, and data review
- Be an immediate contributor
- College degree in Chemistry, Biology, or related field. Graduate degree preferred
- 5 or more years experience in an analytical/QC laboratory setting is required
- Previous experience with GC method development is required
- Previous experience in a wet chemistry laboratory is required
- Previous experience with Quality Control and ISO 9001 is required
- Previous experience performing customer and ISO audits is required
- Strong collaboration skills
- Strong results focus
- The QC Chemist shall conduct analytical and physical tests on raw materials, drug substances, in-process samples and drug products in accordance with approved procedures with minimal supervision
- Development of analytical methods of new DP and API by using HPLC, GLC and UV-Vis NIR Spectrophotometer
- Experience maintaining laboratory instrumentation, logbooks, test results, databases and notebooks in compliance with laboratory SOPs and cGMP as they relate to the QC Laboratory
- Experience working with Oral Solids is a must
- Must have excellent interpersonal, communication, and written skill
- Data generated will be utilized to nominate dosage forms
- Bachelor of Science (B.S.) degree in Chemistry is required. A Master of Science (M.S.) degree in Chemistry is preferred
- Three to five years of experience in pharmaceutical quality control laboratory in stability department
- A minimum of 2 years’ experience working in generics environment
- Running tests on samples originating from the plant, the field, QC Management, vendors, toll manufacturers, or Global Technology personnel, including making usage decisions on batches of products produced at the plant or lots of raw materials brought into the plant
- Calculating material additions to batches based on test results, and assisting in batch troubleshooting activities
- Taking part in the QC Lab safety program and monthly QC Lab operational meetings
- Characterizing shelf stability of batch retains samples
- Calibrating and maintaining QC Lab equipment
- Administrative activities such as creating certificates of analysis; general laboratory housekeeping activities
- Previous laboratory experience required
- Bachelor’s degree (Chemistry or a related technical field) preferred
- Chemical manufacturing experience a plus
- Assists QC Manager with updates of quality systems for analytical testing of raw materials, bulk powder, and finished product
- Develops new methods/techniques, and improves existing test methods, as needed
- Reviews and approves SOPs, analytical data, and corrective action reports
- Assesses any non-conformance/out-of-specification (OOS) results, determines impact on product, and ensures timely resolution or corrective action
- Identifies and coordinates qualification of contract testing laboratories as needed
- Follows appropriate protocols, SOPs, compendial and analytical methods, and stability protocols for sampling and testing active pharmaceutical ingredient and finished product
- Maintains appropriate equipment, records, files, and logs according to SOPs and applicable regulatory requirements
- Performs basic diagnostic and troubleshooting procedures for test methods and instrumentation
- Assists in the maintenance of laboratory requirements, including reagents and supplies
- Reviews trending of data and helps to manage sample backlogs
- Bachelor's Degree in Biochemistry, Chemistry, or a related science degree
- Intimate familiarity with instrumental analysis of protein samples (i.e., HPLC, IVD, SDS-PAGE, viscosity, particle size)
- Computer skills (Excel, Word, SmartLab, Empower, LIMS).Method and/or equipment validation experience
- Experience in protein manufacture, highly recommended
- Support supervisor to guarantee released batch compliant with available laws and regulations, Understand TS16969 requirements on the position, comply with those requirements in daily works
- Collect data basing on daily work and keep test records for every batch
- Follow all the lab safety rules and AKZO regulations. Be responsible for daily housekeeping and equipment maintenance of working area
- Other responsibilities as assigned by supervisor or line Manager
- Perform required testing on raw materials, intermediates and finished products to ensure quality products and customer satisfaction
- Evaluate analytical data and recommend corrective action to production personnel
- Maintain lab assets in accordance with written procedures and schedules as required by GMP, GLP, and ISO 9001 when applicable
- Recommend improvements to existing analytical methods and develop new methods when appropriate
- Assists in maintaining appropriate inventory levels of lab supplies
- Responsible for the approval / rejection of finished product samples for incoming and outbound shipments
- In collaboration with lead operators, provide resolution for activities on the offshift
- Responsible for the maintenance of quality related SAP modules (QM and MM)
- Responsible for managing customer and Huntsman product specifications
- Manage non-conforming product system, including blocked inventory
- Transition existing hard copy QC batch data to an electronic system- LIMS, MS Excel, MS Access, etc
- Monitor batch product data with SQC techniques, including process capability studies, to alert production of potential off-spec and advise business and technical teams about specification change opportunities
- Interact with Customer Service and technical teams providing quality related feedback on customer inquires about materials and specification related issues
- Maintain lab instrumentation and calibrations and commission new equipment and instrumentation
- Identify opportunities to continually improve department equipment, techniques and work processes
- Work with Process Development and Technical Innovation (R&D) teams on new product specifications and testing protocols
- Coordinate work assignment and priorities with lab technicians and administrative assistant
- Follows instructions and performs other duties as may be assigned by supervisor
- A minimum of a Bachelor's Degree in Chemistry or related discipline
- 5 years of laboratory experience in a similar role, minimum 2 years of experience with quality management systems: ISO9001 and AS9100, the Aerospace Standard
- Must have a level of proficiency with Internet, Email, and Microsoft programs
- QC Chemist with experience in working in a manufacturing site quality control laboratory
- Must have excellent multitasking skills to be able to maintain real time communication between production, planning and customer service in quality related issues
- Must work closely with external and internal customers and be capable to coordinate laboratory schedule deadlines while adhering to company’s safety guidelines
- Must have a good understanding of mechanical and analytical test methods for materials used in different industrial and aerospace applications
- Conduct method validation and round-robin testing in site QC lab for products of manufactuer or formulation sample
- Develop new method for residual impurity or contaminant in product or cleaning medium
- Coordinate the daily analysis under concept of good laboratory practise, support supervisor on updating the lab SOPs to maintain good operation of QC lab,provide the training to lab technician
- Double check the test result records to ensure all of data are gengerated properly under SOPs
- Monitor the consumables inventory and chemical stock, make the request to purchase reagents, consumbles and reference standard etc
- Work as lab safety office to lead HIP topics, drill, lab housekeeping
- Report to supervisor or relevant department any unusual findings/abnomalities in lab with recommendation on corrective actions
- Maintain the SAP-QM-LIMS to ensure the system working properly
- Develop and implement maintenance plan of lab equipment to ensure the lab equipment working propely, and carry out troubleshooting as well
- Coordinate the lab auxiliary facility maintenance together with site maintenace
- Under the direction of supervision, the scientist manages change control for Analytical documents (including drafting of testing methods and specifications), Reviews methods and specifications to ensure alignment with Good Manufacturing Practices (GMPs)And other applicable worldwide regulations
- Review and approve production and analytical documentation accompanying the release of API, Safety Assessment or clinical supply lots (bulk and packaged) to ensure conformance to appropriate regulatory requirements
- In addition, the Scientist communicates and resolves comments with client areas
- B.S. and/or M.S. in an appropriate Science or Engineering discipline
- Minimum of 2 years of laboratory experience
- Experience should be in the pharmaceutical/chemical industry or government drug-regulatory agency with a working knowledge of cGMP regulations
- Working experience should include performing laboratory methods, assay development and document review
- Effective communications (oral/written) and interpersonal experience along with technical writing skills
- Follow company SOPs and current Good Manufacturing Practices (cGMP) in the performance of all responsibilities
- Analyze samples in support of: instrument qualification, method validations, the raw material testing program, process validation, in-process testing requirements, R&D, stability testing, API testing, and finished product test
- Carry out method development and method validation for specified projects, under supervision
- Prepare solvent mixture, buffers, and reagents as needed for testing
- Process and analyze analytical data
- Review analytical data and test results, and compare to test specifications. Enter test results in lab reports
- Learn to troubleshoot instrument problems and work with the supervisor, laboratory chemists, and equipment manufacturer’s technical services to resolve issues
- Perform instrument maintenance, calibration, and standardizations as required
- Write standard operating procedures (SOPs) for equipment operation, methods, and general laboratory procedures as needed
- Order and maintain laboratory supplies as needed
- Experience in small molecule drug analysis
- Hands-on instrumentation experience in HPLC and/or Gas chromatography (GC)
- Instrumentation experience and expertise in HPLC (waters, empower), UV/VIS and FTIR
- Proficient in Microsoft Office including Excel and Word
- Experience with chromatography application programs
Senior QC Chemist Resume Examples & Samples
- Independently conducts analyses, interprets results, and develops solutions
- Develops efficient and timely project objectives. Detect and evaluate problems with analytical processes and contribute in developing methods and techniques for solutions to those problems
- Detect problems in using standardized procedures
- Prepare scientific reports and documents
- Reviews other team member’s analytical data for simple tests
- Interact with internal and external clients
- Compose Standard Operating Procedures (SOPs) and GMP documents
- Develop technical proficiency, think independently, exercise sound judgments that correspond with experience, and adhere to safe work practices
- BS in Analytical Chemistry or equivalent with 5-7 years related experience in a pharmaceutical industry
- MS in Analytical Chemistry or equivalent with 3-5 years related experience in a pharmaceutical industry
- Ability to write clear and concise technical reports
- Good understanding of Microsoft Office and statistical software
- Experience with common analytical techniques such as: HPLC, GC, NMR, UV, LC-MS
- Experience communicating effectively to management
- BA or BS in chemistry. Minimum two years of experience in a the lab performing chemistry related duties
- Experience with High Pressure Liquid Chromatography, Infra-Red (IR), Gas Chromatograph (GC), Ultra Violet/Visible, Particle Size Analysis, KF Titration and wet chemistry skills are required. Familiarity with HPLC/GC analytical software
- Familiarity with GLP/GMP guidelines
- Good Communication and writing skills
- Familiarity with out-of-specification (OOS) and out-of-trend (OOT) investigation
- Responsible for the laboratory testing in accordance with company SOP’s as well as cGMP’s and GLP’s
- Maintains lab work area in a neat and ordered fashion
- Works in support of production may require weekend or off-shift work
- Has miniumal customer interaction
- Maintains data within a laboratory notebook in a neat (legible) and organized manner
- Records entries in laboratory logbooks as necessary
- Generally works in agreeable conditions. May require extended periods of standing or sitting. Requires the ability to wear a respirator or breathing apparatus
- Performs work in accordance with general and specific safety precautions
- Responsible for ensuring safety and integrity of product testing in the department
- Works in a fast paced, moderately stressful environment. Adherence to project deadlines is critical
- Consolidates all notebook pages and Chromatographic scans with the QC section of the batch record and forwards results to documentation
- Assists with the orientation of new employees
- Initiates DCR’s to correct or clarify SOP’s
QC Chemist / Technician Resume Examples & Samples
- Preparation of chemical test reports
- May have more responsibility in compiling, analysing and interpreting results to internal/external customers
- May perform moderately complex assignments which are non-routine which may include equipment, facilities or product design
- Conducts all business activities in accordance to the Company’s HSE policies, legal compliance requirements and Core Values
- Qualifications with a chemical/quality focus would be beneficial
- Experience in a chemical manufacturing facility
- Awareness of chemical hazards and general health, safety, environment and welfare considerations
- Ensure compliance to cGMP's in the QC laboratory
- Development of Analytical methods of new drug products and API using HPLC, GLC and UV-Vis, Dissolution, NIR Spectrophotometer
- Validation of Analytical Methods
- Calibration of laboratory equipment and instruments
- Analysis of stability samples and finished products
- 5 years of experience with a minimum of 2 years with Finished Products
- Prefers Chemists to have prior work experience with Generics manufacturing or CMO
- Applies Good Laboratory Practices to accurately record and file all raw test data, including pertinent paperwork –e.g., chromatograms, graphs, spectra and test results. Communicates results and dispositions to Production and the QA Supervisor as required. Calculates and recommends batch adjustments to Production
- Reports promptly all problems regarding procedures, equipment, products, raw materials, specifications, etc., to the QC Supervisor or designate, as well as other QC Chemists
- Associate's degree in Chemistry or a related field
- Up to 6 months of relevant work experience
- Ability to work 12 hour shifts including nights, weekends and holidays
- Bachelor's degree in Chemistry or other Science preferred
- Experience working in a quality control setting at a chemical manufacturing facility a plus
- Perform analytical testing per UT’s SOPs on raw material samples according to USP, EP and JP where applicable
- Perform analytical testing on in-process, finished product and stability samples per UT test methods and SOPs
- Prepare SOPs, set up calibration and PM programs for the applicable equipment identified for the analytical laboratory
- Plan, prepare and execute method transfer and method validation protocols as required
- Check quality control laboratory documentation to include laboratory notebooks, LIMS, SOPs, and Protocols
- Prepare appropriate reports (method transfer, method validation, and / or data summary)
- Lead investigations involving analytical out of specification results
- Troubleshoot laboratory equipment
- Proficient in Microsoft Office; Word, Excel, and Outlook
- Proficient in Waters Empower Software
- Proficient in LIMS
- Report and protocol experience preferred
- Must be detail oriented, self-motivated, organized and have the ability to prioritize work
- Must be able to work independently and as part of a multi-functional team, with the ability to handle high workloads, stressful situations and deadlines
Seasonal Ink Materials / QC Chemist Resume Examples & Samples
- BS degree in Chemistry, Engineering or related field with an emphasis in chemistry or materials engineering
- A 2 year degree or work experience in the ink or paint industry will be considered if candidate demonstrates a high level of technical knowledge
- Strong knowledge of chemistry, mathematics and statistics
- Knowledge in the following areas is helpful: paste ink water-based liquid inks (gravure, flexo or inkjet, etc.)
- The hours for this position are day shift Monday-Friday, with occasional work on the weekends
- Ensure compliance with all company policies and procedures and appropriate regulations, including FDA and ISO 9001, ISO 13485, CMDR and the Medical Device Directive
- Perform and document instrumental (i.e., UV-Vis, HPLC) and wet chemistry techniques/analyses
- Maintain GMP/GLP laboratory notebooks and documentation relevant to the duties and responsibilities assigned
- Perform laboratory testing, as well as general laboratory maintenance, including in process and final product release testing and stability testing
- Bachelors Degree in chemistry, or biological sciences, preferred
- Five to Seven years related experience
- Working knowledge of standard laboratory practices and safety
- Experience with UV-Vis and HPLC highly desired
- High school diploma or equivalent
- A minimum of 60 hours of college-level work from an accredited college including a minimum of 8 hours of Chemistry or related science course(s)
- Ability to read, write and verbally communicate in the English language
- Ability to reason through operational issues as they arise and resolve or communicate to others
- Must have comprehensive knowledge of plant operations as they affect quality of product
- Must have general knowledge of calculators and computers
- A high degree of manual dexterity and accuracy required
- Preferred knowledge of all types of equipment used in the manufacturing of cement
- Knowledge of hazards associated with areas of responsibility
- Must have a basic understanding of the plant operations
- Must have excellent work and safety record
- Must understand Lockout/Tagout procedure and safety requirements
QC Chemist Supervisor Resume Examples & Samples
- Supervise and assign work to QC chemist to support production. Work with Value Stream Lead to understand the priority and schedule work in lab to get testing completed on timely manner
- Review test result and ensure that all testing are performed accurately prior to approve them in LIMS system
- Review analytical methods, protocols, SOPs and other technical documents and train QC chemist on relevant procedures
- Assist with resolution of customer complaints and reviewing batch records. Investigate consumer complaints and communicate with manager and/or cross functional department as needed
- Support continuous improvement activities within group
- Conducts and writes laboratory investigations and identify Root cause for the failure
- Ensure laboratory equipment is properly calibrated and maintained
- Test samples in accordance with established procedures and using all instrumentation required in the procedures, including AA, IR, UV-VIS
- Prepare and calibrate reagent standards
- Bachelor’s degree in Chemistry, Biochemistry or Chemical Engineering
- A minimum 5 years of laboratory experience Wet chemistry
- Proficiency with most wet chemistry techniques and gravimetric assays
- Must be able to independently evaluate results from routine analysis and identify problems and potential problems as they occur
- Prior experience as Supervisor or a very strong team leader or experience coaching/mentoring junior chemists and addressing performance and employee development in regulated QC environment is required
- Proficient in Microsoft Office, LIMS (Lab Information Management System)
- Test samples in accordance with established procedures and using all instrumentation required in the procedures, including AA, LC, GC-FID, GC-EC, GC-MS, IR, ICAP, UV-VIS
- Repair, maintain and calibrate laboratory instruments
- Review biological test results received from outside testing lab
- Assist with resolution of customer complaints and reviewing batch records
- Knowledge of biological/Microbiological testing is plus
- Performs required analytical testing on in-process and final products
- Performs USP, EP, and ACS testing on raw materials
- Testing using HPLC, FTIR, ICP-OES, UV/VIS, Liquid Scintillation, and Gamma Spectrometer
- Tracking and trending of analytical data
- Participates in validation projects requiring analytical support
- Writing and developing SOPs
- Contributes to process improvement through Lean, 5S and six sigma tools
- Assists in the stocking and supplying of the QC lab
- Performs visual inspection of finished product
- Associate degree plus 2 years working experience in Manufacturing or Laboratory or Bachelor’s
- Proficient with software applications applicable to the job
- Must be available for nights and weekends as needed
- Must have the ability to distinguish color
- Ability to lift 25lbs
- Be able to work weekends/Rotation of shifts
- Equipment experience with HPLC, ICP, UV/Vis, FT-IR
- Experience working with radioactive material including the use of gamma spectrometers, Ion
- Apply basic scientific knowledge to perform analysis and to solve simple analytical problems
- Learn and follow all Standard Operating Procedures (SOPs) and Good Manufacturing Practices. (GMPs)
- Revise SOPs, test methods, and GMP documents with guidance
- Conduct analysis and interpret results
- Transfer, modify, and improve analytical methods with guidance from experienced team members
- Prepare scientific reports with guidance from experienced team members
- Review all data regarding raw materials, intermediates, and APIs
- Perform cleaning validations and verifications
- Calibrate instruments and contribute to laboratory organization and compliance
- Demonstrates technical proficiency and self-assuredness in applying cGMP standards
- Ensures all documentation produced is in compliance with cGMP standards
- Support the Company’s commitment for environmental health & safety by applying ISO 14001, OHSAS 18001, and Sustainability 2025 principals into daily activities
- Perform all analytical testing relating to incoming raw materials
- Perform all analytical testing relating to West Chester in-process testing
- Perform all analytical testing relating to finished goods testing
- Perform all analytical testing related to resale and toll production that is shipped from the West Chester facility
- Perform all analytical testing related to pilot plant production
- Perform all analytical testing related to reapprovals, and returned goods
- Perform all miscellaneous analytical testing as directed by West Chester’s QC Supervisor, Methods Development Chemist, and Analytical Services Manager
- Perform all necessary test method calibrations as defined in the West Chester Quality Control ISO procedures
- Perform all necessary instrument maintenance as defined in the West Chester Quality Control ISO procedures
- Perform all necessary instrument troubleshooting as defined in the West Chester Quality Control ISO procedures
- Maintain good laboratory practices in the areas of safety and cleanliness while performing the assigned analytical duties
- Reports directly to the West Chester QC Supervisor and indirectly to the Analytical Services Manager
- ¨This part time Quality Control position is designed to support West Chester production. The position includes working most, if not all weekends as well as covering shifts open due to vacation or sick. It is the duty of the part time Quality Control chemist to work safely and responsibly in all areas of the plant. It is also the responsibility of the part time Quality Control chemist to report accurate analytical results, so the West Chester facility will not ship any non-conforming product. Lastly, it is expected that the part time Quality Control chemist work with a good attitude and be a team player in decisions relating to the functioning of the laboratory
- A minimum of three years of study in a science curriculum
- Organizational and prioritization skills for efficient task completion
- Reliable decision-making skills
- Proficient in Word, Excel, Microsoft Office, Microsoft Access, and Microsoft Power Point
- Ability to use scientific logic to deal with different types of problems
- Ability to manage multiple tasks and projects concurrently and efficiently
- Ability to maintain confidences within the normal course of business
- Ability to be a self-starter, manage time and work without direct supervision
- Ability to communicate established procedures in the training of personnel
- Ability to work in a consulting, advisory, and coordinating role
- Ability to communicate and work as a member of a team
- Ability to organize and prioritize work in a timely manner and react well under pressure
- Ability to interpret documents such as safety and emergency response regulations
- Ability to add, subtracts, multiply, and divide in all units of measure, using whole numbers, common fractions, and decimals
- In a fast-paced environment, perform stability testing on solid dose pharmaceutical product using techniques such as HPLC, Dissolution, UV/VIS, and KF moisture
- Evaluate and documenting test results in accordance with company SOPs
- Troubleshoot lab equipment and perform any lab investigations in conjunction with supervisor
- Apply GMP principles throughout the process, ensuring compliance with established regulations
- Perform routine and complex sample analysis using HPLC, GC, Dissolution, USP/EP raw material testing, wet chemistry, spectrophotometric techniques, or microbiological techniques
- Performs routine and complex sample analysis to assess the conformance of raw materials, finished products, and/or stability samples per product specifications
- Performs testing and documents data, and reports results
- Demonstrates an advanced knowledge of SOP’s and GMP regulations as related to the pharmaceutical quality control laboratory
- Conduct tasks in compliance with cGXP requirements, current industry standards, compendia standards, FDA expectations and internal SOPs
- Ensure lab investigations are com[leted in a timely manner
- Schedule and/or perform necessary validation, qualification and calibration of laboratory equipment/instruments as needed
- Communicate with clients, contractors, and regulatory agents as required
- Provide leadership and guidance to junior staff
- Work independently and seek supervision when required
- Update various GMP documents including but not limited to SOPs, QATs, HOCs, change controls, safe practices, protocols, templates and reports demonstrating strong technical writing skills
- Perform specialized maintenance and calibration of laboratory equipment (Metrology) as needed
- Perform daily maintenance and/or calibration of laboratory equipment/instruments as needed
QC Chemist, Raw Material Resume Examples & Samples
- Assist in the installation of laboratory equipment and qualification of laboratory equipment for intended use in QC laboratory
- Perform raw material and consumables sampling in accordance to written testing SOP’s and in accordance with local/ international regulations
- Support in establishing and controlling of raw material’s supplier qualification program
- Monitoring and optimization of work flows and methods/procedures, in-process control, method controls and reference’s and performs trending of on-going in process control data to pursue an on-going quality assurance program
- Participate in laboratory investigations as per local SOP and facilitate root cause analysis
- Review QC documents to ensure completeness, accuracy, consistency, and clarity and raw materials have been manufactured, tested, or inspected according to specification, local SOP and cGMPs
- Prepare and participate to health authority’s inspections and internal audits in QC Raw Material area
QC Chemist, In-process Control Resume Examples & Samples
- Support lab equipment installation and qualify/validate lab equipment for QC laboratory for operations according to cGMP standards
- Responsible in providing technical support to run and validate necessary test methods on lab equipment and later perform product testing including maintenance of product reference and assay control programs
- Ensure that priorities for testing and review are set and followed in a timely manner and involve in laboratory investigations and root cause finding
- Optimizes test procedures to improve efficiency whenever possible
- Participate to health authorities inspections and internal audits in his or her area
- Monitoring and optimization of work flows and methods/procedures, in-process control, method controls and references and perform trending of on-going in process control dat
- Test and release raw materials, mediums, and finished products using laboratory equipment
- Clean tech-ware and calibrate instruments daily by following the procedures as required
- Enter testing data and close batch tickets and purchasing orders in database
- File batch tickets and store retains
- Maintain the micro samples receiving log and submit samples for testing
- Provide support for investigation of nonconforming batches
- Perform daily housekeeping in the laboratory to ensure a safe, clean work environment
- 1-2 years quality control laboratory experience (or equivalent) in a manufacturing environment
- Possess a technical understanding of quality control tests and their relationship to the manufacturing process and/or customer processes and applications
- Good organizational skills, verbal/written communication skills and attention to detail
- Strong MS Office products knowledge and skills
- Must be able to lift up to 25lbs
- Required to work in shift & Support the in-process, finished goods sampling
- Required to do testing & reporting the in process, finished good sample
- Follow the SOP for carrying out required analysis, operating & maintaining testing equipment
- Accountable to ensure the Operation and Calibration of analytical Instruments as per laid down procedure
- Prepare all instruments Calibration Schedule, Instrument History card and servicing follow up
- Managing all documentation & data relating to Quality Control & Quality Assurance
- Ensure sample retaining & Housekeeping of the QC lab
- Handle sophisticated instrument like IR, Spectrometer & X-ray Spectrometer,
- Experience in industry related to Batch operations, compounding –preferably solid / powder material
- Demonstrated success in GMP regulated analytical environments
- Familiarity with GE TOC analyzers and FTIR with ATR (Thermo Fisher)
- Excellent written and verbal communication skills and experience interacting with people from a wide range of skill levels and experience
- 15 %- Perform analytical tests, both automated and manual to include Wet chemistries, Flashpoint, Relative Density, Refractive Index, Color, Optical Rotation, particle size, moisture content, preparation of GC encapsulation samples and viscosity on all IFF technologies
- 25 %- Perform Data entry into various databases, reporting results and tracking (SAP, PLM, & IFFMAN). Ensure timely approval of materials in analytical and GC for all IFF technologies. Interfacing with chemists, evaluators, corporate QC on special testing and reporting results as required
- 50% - Load GC and GCMS, trouble shooting, adjustment, etc
- 10 %- Other activities as assigned by supervisor. customer complain investigation, etc
- BS Chemistry
- Strong working knowledge of Encapsulation analyses and GC analyses (trouble shooting)
- 1-2 years of work experience using QC Laboratory equipment for scientific testing
- Working experience with SAP System (or related production manufacturing software system)
- Working experience with Microsoft office - specifically Excel and Word
- Provides analytical support for production by performing GC, HPLC, and/or Wet Chemistry analysis on Raw Material, in-process and finished samples
- Supports analytical and chemical development by testing samples
- Performs wet chemistry testing: pH, LOD, MP, titrations, KF, ROI, extractions, IR, UV and physical characteristics. GC and HPLC testing will also be required
- Assists in writing of SOP’s, testing standards, protocols and reports
- Works closely with Production Shift supervisor to determine priorities, deadlines and requirements in production area. Responsibilities in production area may include: witness DEA transactions, swabbing, witness charging, documentation evaluation and any other Quality or Production functions deemed necessary
- Calibrates production pH meter
- Assists in training of lab personnel on instrumentation, methods, and cGMP
- An BS/BA Degree in chemistry or related science with 3-6 years direct QC lab experience
- 4-6 years’ operating HPLC, and GC in a cGMP regulated QC laboratory
- Strong working knowledge of operating and troubleshooting GC and HPLC, and utilizing Totalchrom software
- Knowledge of cGMP is a plus
- Knowledge and understanding of Stop Orders, Concessions, ESD, Calibration, Preventative Maintenance, Material Identification & Segregation, documentation configuration control practices; documentation & disposition of non-conforming material, and Good Documentation Practices requirements, as associated with this job type/position
- Complete all planned Quality & Compliance training within the defined deadlines
- Associate degree plus 2 years working experience in Manufacturing or Laboratory or Bachelor’s degree
- Ability to speak, understand, read, and write in local language
- Waters Empower Experience or similar software
- Experience working with radioactive material including the use of gamma spectrometers, Ion Chambers and liquid scintillation analyzers
- Visual inspection experience
- Working knowledge of USP
- Experience in a FDA regulated QC environment
- Background in Math or Chemistry
Chemist, QC Chemist Resume Examples & Samples
- 0-2 year experience in the application of laboratory techniques in a manufacturing QC environment
- Demonstrate proficiency in the use of basic laboratory techniques and equipment
- Able to follow detailed procedures and have good documentation skills
- Ability to work in a team environment, make decision of a limited scope which is subject to review
- Good organization, communication, multi-tasking and computer skills
- Basic math skills in a science environment
QC Chemist Intern Resume Examples & Samples
- Perform routine physical testing on clinical and stability samples such as pH, viscosity, and osmolality
- Prepare solvents and samples for HPLC analysis and operate the HPLC for analysis of clinical, stability, and raw material samples
- Run routine raw material testing as necessary
- All work and documentation will be performed per cGMP and InSite Vision’s procedures
- Help maintain the labs in a clean, organized, and safe condition
- Assist QA or QC Microbiology as trained and directed
- Requires coursework in Chemistry at the High School level, or equivalent
- Must possess excellent analytical skills and laboratory techniques and the ability to multi-task and work in time sensitive situations
- Must possess excellent interpersonal, communication (oral and written), and organizational skills
- Ability to anticipate and resolve issues and work effectively in a team environment
- Must possess ability to navigate computer systems with little assistance. Basic knowledge in the use and application of the Microsoft Office (Excel, PowerPoint, and Word) is required
Shift QC Chemist Mon-thur Resume Examples & Samples
- Perform physical and chemical testing of non-pesticide and pesticide products packaged for consumer use
- Maintain Technical Quality laboratory in compliance with cGMP and GLP regulations
- Calibrate, maintain, operate, and troubleshoot analytical and laboratory equipment (GC, HPLC, FTIR, and other general lab instruments)
- Prepare solutions for treating consumer packaged products for production, quality and shipment
- Work on project developments and stability studies
- Monitor incoming ingredient quality control
- Set up equipment used in the Research and Development Pilot Lab for experimental and process development studies
- Assist in the scale up and transfer of R&D products to commercial manufacturing sites
- Assist in the preparation and reconciliation of scientific paperwork
- Support (set-up, tear down, maintenance) for cGMP and Non-GMP Laboratory Equipment
- Support Scientists and Technicians in Batching Process Equipment Setup and Manufacture
- Support Engineering on New Equipment Qualifications
- 3+ yrs of laboratory experience required
- Perform analytical testing per United Therapeutics (UT) SOPs on raw material samples according to USP, EP and JP where applicable
- Perform analytical testing on in process, finished product and stability samples per UT test methods and SOPs
- Execute method transfer and method validation protocols
- Check quality control technicians’ documentation
- Proficient in Microsoft Office (Word, Excel, PowerPoint, Outlook)
- Working knowledge of HPLC, Dissolution, GC and other analytical techniques
- Must be detail oriented, self motivated, organized and have the ability to prioritize work
- Ability to work independently and as part of a multi-functional team
- Ability to handle high workloads, stressful situations and deadlines
QA / QC Chemist Resume Examples & Samples
- Support lab functions by maintaining instruments (calibrations, standards, troubleshooting)
- Interface with suppliers for instrument maintenance contracts, repairs, and calibrations
- Interface with external customers to assist with product issues
- New method development, data gathering, statistical evaluations, etc
- Plant support by troubleshooting and performing analyses and reporting results effectively
- Perform safety, quality and ISO audits
- Bachelor’s of Science in Chemistry or Bio-Chemistry required
- 5 or more years’ experience in analytical or polymer chemistry a plus
- Familiarity with LIMS, databases, Gas Chromatography, XRF, and titrations a plus
- Understanding Design of Experiments (DOE) preferred
- Six Sigma certified preferred
- Knowledge of ISO 9001 and IATF 16949 requirements preferred
- Must be able to demonstrate strong problem-solving skills
- Must possess strong interpersonal and teamwork skills – ability to work with customers in cross functional groups
QC Chemist / Documentation Specialist Fremont Resume Examples & Samples
- Writes and edits SOPs relating to Quality Control testing/process instructions, policies, and procedures
- Independently completes writing assignments according to set standards regarding order, clarity, style, and terminology
- Reviews published materials and recommend revisions for changes in scope, format, and content. Maintains records and files of work and revisions
- Work with QC Scientists and Supervisors on the design, layout, review editing and revision of procedures, and other testing documentation
- Organizes large amounts of technical information utilizing Advanced MS Word and Excel functions, as well as managing many technical documents simultaneously
- Participate in cross functional project teams, as required
- Bachelor’s degree or equivalent experience
- Must be computer literate and competent in PC based software such as Office 2010 Professional (Word, Excel, Access, and PowerPoint)
- Minimum of 2 years work related experience using a Document Management System
- Proficient with configuration change management priniciples and working knowledge of following and tracking record retention schedules
- Proficient with 55+ wpm minimum typing speed
- Knowledge of ISO 13485, FDA QSRs and GMP is preferred
- Ability to work in high-pressured deadline oriented environment
- Good skills at alpha and numeric sequencing & filing
- Proofreading and editing skills, and an aptitude for numbers and detail oriented work requiring a high degree of accuracy
- Must have excellent customer service and listening skills
- Basic arithmetic skills including the ability to calculate figures and amounts and to interpret the data
- Able to sit, stand and/or use keyboard for long periods of time
- Must have the skills to work as a strong contributor in a team effort
- Test raw materials using HPLC, GC, KF and various wet chemical methods meeting stringent time lines and schedules
- Enter data into LIMS (Laboratory Information Management System)
- Accurately document all tests/test materials/equipment used and all results obtained
- Science Degree in Chemistry or equivalent with at least 2 years of laboratory experience
- Manages the chemistry laboratory activities to assure timely availability and accuracy of results
- Reviews and approves all required chemistry testings for utilities, raw materials, manufacturing processes and products and ensures that the tests are analyzed in accordance with corporate, regulatory and external agency regulations
- Enforces all laboratory policies and practices, and ensure that all in-use procedures are current and compliant
- Maintains all safety and security policies and practices
- Supports equipment/software/method validation and performs (re)validation when necessary
- Monitor results and issues corrective actions when appropriate
- Provides support and technical expertise to ensure that manufactured products meet applicable regulatory standards and guidelines with respect to Quality control operations
- Ensures expenditures are within budget
- Keeps up-to-date on the regulations concerning biopharmaceutical QC activities
- Participate in investigation of non-conformities (deviations and exceptions)
- Problem solving
- Identifies and reports problems in QC. Solves problems and implements corrective actions
- Presents in a timely manner major issues to the management with strategies on their resolution
- Analyze in process samples of ETP plant
- Analyze treated Effluent samples
- Analyze water samples
- Analyze outlet sample for consent parameters
- Calibration of lab instruments
- Documentation of lab results
- Experience in NABL accredited lab
- Ensure EHS compliance
- Ensure best house keeping
- Responsible for calibrating and testing various analytical instruments to ensure that test results are reliable
- Prepares appropriate documentation for test results so that trends can be identified and analyzed
- Collaborates with S & T to perform and establish specific quality standards and specifications for chemically or biologically modified products
- Ensures compliance with federal, state, and local regulatory requirements
- May investigate customer complaints and provide management with test results information
- Calibration, operation and results interpretation of HPLC, GC, FTIR. Perform wet chemical analysis and Titration, Percent of Non-Volatile Materials, Refractive Index, Karl Fisher Moisture, Viscosity as well as other miscellaneous test methods
- Performs special assignments upon request
- Must be available to work all shifts and weekends as required
- Ensure that the Quality System of DIQCL is properly implemented and adhered to according to at all times
- Ensure that all quality and technical operations are in compliance with ISO 17025 DuPont standard and other compliance obligation
- Review the LQM, LPM, LWIM and all other associated documents every 3 years
- Ensure that internal quality audits conducted at least once a year
- Ensure that all staff are committed to quality and continuous improvement and maintain discipline among staff within the laboratory
- Ensure all staff are properly trained and able to perform their jobs professionally without any undue influence from any quarter
- Document and data control according to ISO 17025, DuPont lab standards and other compliance obligation
- Plan and implement all quality assurance programmes and activities such as ISO 9001, GMP and HACCP, EMS effectively
- Shall adhere to all company’s policies, rules and regulations and committed to company’s quality and continuous improvement programs such as MS ISO 9001, GMP, HACCP, RSPO, BRC, EMS
- Ensure implementation of Environmental Monitoring Program on monthly basis and escalate to QA Manager when deviation arise
- Handles customer requirement eg, lab test, customer complaint investigation responds, pre shipment sample, lab blend and other internal customer request
- Ensure smooth operation during the working hours of the laboratory
- Any other tasks or projects assigned by the superior
- Maintains current, complete, legible notebooks and laboratory records in compliance with good scientific and regulatory practices. Maintains adequate inventories of reagents, glassware, and other supplies
- Writes and executes laboratory investigations for known laboratory errors and OOS results using Trackwise and/or other QC procedures
- Utilizes Personal Protective Equipment (PPE) as necessary
- BS/BA degree preferred; ideally in a scientific or technical discipline and/or relevant laboratory experience within a Pharmaceutical Quality Control Laboratory
- Basic understanding of cGMPs and regulatory requirements as they relate to laboratory practices and manufacturing processes
- Ability to prioritize workload and manage multiple and varied tasks with enthusiasm
- Analyze and / or test under cGMP, raw materials, intermediates and finished products by applying validated or compendia methods
- Release testing of products per current compendia, regulatory and corporate quality systems, and principles
- Performs analysis in the laboratory with minimal supervision
- Prepares standards, mobile phases, and reagents
- Calibrates and monitors laboratory equipment (HPLC, GC, ICP, etc.)
- Analyzes raw materials, intermediates, stability samples and finished products for required tests by various analytical and instrumental methods
- Performs calculations, collects and prepares data for evaluation
- Keeps supervisor informed of work status
- Investigation of OOS results under guidance of senior staff
- Maintains good documentation practices
- Work according to SOP’s and Specification sheets
- Demonstrate the ability to learn and perform lab specific analytical methods
- Complete analysis on raw materials and finished products within an agreed upon turnaround time
- Ensures SOP’s and specifications are up to date and in compliance with current Compendia and updates them if required
- Performs and documents all work associated in adherence with cGMP guidelines
- AS/BS Chemistry
- 1-3 years lab experience
- Exp. w/ quality control techniques and methods. (raw material testing, HPLC, GC)
- Perform analytical and physical testing on in-process, finished product and stability samples
- Maintain analytical methods in the laboratory in a state of validation
- Assist in addressing laboratory investigations within specified time frame
- Perform accurate and timely testing of routine and some non-routine lab samples in accordance with appropriate GMP and safety guidelines
- Participates in laboratory root cause investigations and quality system improvement initiates by executing well defined protocols and procedures
- Shares technical information and best practice within the group
- Conduct studies to validate methods, develop methods, evaluate new technologies. Sample Analysis
- Coordination of method transfers and co-validations
- Qualifying, calibrating, and maintaining analytical instrumentation
Related Job Titles

IMAGES
VIDEO
COMMENTS
14. Quality Control Chemist. Cover Letters. Approved by real hiring managers, these Quality Control Chemist cover letters have been proven to get people hired in 2024. A hiring manager explains why. Compiled by: Kimberley Tyler-Smith. Senior Hiring Manager. 20+ Years of Experience. Jump to a Cover Letter.
Quality Control Chemist Cover Letter Sample. Dear Hiring Manager, I am writing to express my strong interest in the Quality Control Chemist position at your company. With a bachelor's degree in Chemistry and two years of experience in a similar role, I am confident in my ability to contribute to the success of your team. My attention to detail ...
The top sections on a quality control chemist cover letter. Header: Include your contact information, the date, and the employer's contact information; this makes it easy for the recruiter to get in touch and demonstrates your attention to detail, which is crucial in a quality control chemist role.
QC Chemist Cover Letter Example. The role of a QC Chemist is to ensure the quality and compliance of raw materials, intermediates, and finished products within a manufacturing or production environment. This position requires a strong understanding of analytical techniques, attention to detail, and the ability to collaboratively with other ...
In order to be a successful quality control chemist, you need to be detail-oriented and have a strong understanding of the principles of chemistry. Use these examples and tips to write a quality control chemist cover letter that stands out from the competition. Quality Control Chemist Cover Letter Example 1
The following quality control chemist cover letter example can give you some ideas on how to write your own letter.Quality Control Chemist Cover Letter Example Use this template. or download as PDF. Cover Letter Example (Text) Ashiya Landrio (446) 755-0402. [email protected]. Dear Emileigh Luthi, I am writing to express my interest in ...
Qc Chemist Cover Letter Sample. Dear [Name], I am writing to apply for the position of QC Chemist. With my Bachelor's in Chemistry and 4+ years of experience in quality assurance and control, I am confident I would be an asset to your team. Having worked in a wide range of industries, I am well- versed in the principles and practice of ...
Our cover letter examples are written by certified cover letter writers and is a great representation of what hiring managers are looking for in a QC Chemist cover letter resume. Use this example for reference as you create your own cover letter or use this easy cover letter builder that will guide you through every step of your building your ...
These are some steps you can follow to write an effective chemist cover letter: 1. Format the letter. The first step to creating an effective chemist cover letter is formatting the document professionally. After creating the document, use 1-inch margins, a font like Times New Roman or Arial and a font size between 10 and 12 points.
How to Write the QC Chemist Cover Letter. 41592 Crist Plain. Emmerichside, OH 68555-4053. Dear Spencer Denesik, I submit this application to express my sincere interest in the QC chemist position. Previously, I was responsible for inspection of incoming chemicals and analysis of in-process product components prior to the release to ...
The HR Manager. XYZ Company. 354 F 63rd Street. New York. NY 10022. United States. Subject- Quality control chemist cover letter. Respected sir/ma'am, My name is [mention the name of the applying candidate] and I am an employee of [mention the name of another company] where I used to work as a [mention the name of a job related to this job].
This chemist cover letter example will give you a blueprint for writing your own cover letter: Adaptable cover letter sample. Dear Dr. Walkenden, I have worked as a chemist within the Bioscience industry for the past two years and am looking for a new role after my move to New York.
Sample QC Chemist Cover Letter. Sample One. From Eddie Watkins 1917, Palmer Lane, Brinton Square Oakland California 63810 United States 239-860-4715 [email protected] Date: November 25, 2011 To Judy Thompson Recruitment Executive Morris Pharmaceuticals Oakland California 63810 United States
Free Chemist cover letter example. Dear Mr. Adams: I am submitting my resume for the position of Chemist. As a skilled chemist with experience ensuring top-flight quality control while performing various testing and research projects, I am confident that I will make a significant contribution to your company. My professional experience includes ...
Pfizer Inc. - Seattle, USA January 2014 - April 2017. Quality Control Chemist. Performed chemical and physical testing of finished products, contributing to a 20% improvement in batch release times. Contributed to the implementation of FTC-IR to detect impurities, resulting in a 25% improvement in product purity metrics.
Here is an example of a persuasive conclusion from a chemist's cover letter. I am grateful for your time in considering me for this role and hope to hear from you in the next week regarding my application. You may contact me any day of the week between the hours of 8 a.m. to 4 p.m. at (123) 456-7890.
The following are six steps you can take to write an effective cover letter for a quality control job opportunity: 1. Address the cover letter personally. The first step you can take to get the attention of quality control hiring managers is to address your cover letter personally. This means avoiding using generic titles, such as Sir or Madam ...
5 Professional Chemist Cover Letter... Your chemist cover letter must quickly grab the hiring manager's attention. Highlight your most relevant experience in the first few lines. Ensure you convey your enthusiasm for the position. Tailor your skills to match the job description perfectly. Create a Cover Letter.
Cover Letter Examples >. 6 Professional Quality Control... Your quality control cover letter must immediately highlight your eye for detail. It should reflect your ability to detect and address errors efficiently. Showcase in your cover letter specific instances where your intervention improved product quality.
Quality Control Chemist. 05/2013 - 09/2015. Detroit, MI. Help train new or less experienced personnel in SOP content and analytical techniques and methodologies. Execute method transfers into QC and write summary reports. Perform method validations and write technical reports. Perform instrument calibration (IQ/OQ/PQ/PM) and routine equipment ...
Follow the steps below to write an impressive cover letter when you apply for a role in chemistry: 1. Start with a professional header. At the top of any cover letter, include a professional header. Include contact details that are up to date, including at least a phone number and an email address. Make sure you check your emails regularly ...
qc chemist Cover Letter Example. A cover letter can be a valuable addition to your job application when applying for an qc chemist position. Cover letters provide a concise summary of your qualifications, skills, and experience, also it also gives you an opportunity to explain why you're the best fit for the job. Crafting a cover letter that ...
Perform all miscellaneous analytical testing as directed by West Chester's QC Supervisor, Methods Development Chemist, and Analytical Services Manager. Perform and assist with additional duties as may be directed by the QC supervisor. Generate, review and maintain detailed records/documents and assist with QC data management and control charting.
Resume Builder offers free, HR-approved resume templates to help you create a professional resume in minutes. Start Building. 1. Write a brief summary of your chemistry qualifications. Grab the hiring manager's attention with an engaging profile that encapsulates your most relevant qualifications.
1. Use the correct cover letter formatting. A cover letter is a formal business document, and it should look like one, says Aylward. In the upper left corner of your page, put the name, email ...