- DOI: 10.1038/ejhg.2009.109
- Corpus ID: 13716291

Triple X syndrome: a review of the literature
- M. Otter , C. Schrander‐Stumpel , L. Curfs
- Published in European Journal of Human… 1 March 2010
206 Citations
Triple x syndrome with a rare finding: cleft palate, triple x syndrome: characteristics of 42 italian girls and parental emotional response to prenatal diagnosis, the psychiatric phenotype in triple x syndrome: new hypotheses illustrated in two cases, reviewa review of trisomy x ( 47 , xxx ), a review of trisomy x (47,xxx), expanding the phenotype of triple x syndrome: a comparison of prenatal versus postnatal diagnosis, reviewof trisomy x (47,xxx), premature ovarian failure due to tetrasomy x in an adolescent girl, triple x syndrome and various abnormality of 3q in iraqi women: a case report, diminished ovarian reserve in girls and adolescents with trisomy x syndrome, 150 references, 47,xxx: what is the prognosis, psychosocial adaptation of 39 adolescents with sex chromosome abnormalities., long term outcome in children of sex chromosome abnormalities, antenatal diagnosis of an xxx female. a dilemma for genetic counseling., prevalence of the triple x syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders., verbal deficits in children with 47, xxy and 47, xxx karyotypes: a descriptive and experimental study, prognosis of prenatally diagnosed children with sex chromosome aneuploidy., speech and language development in 41 children with sex chromosome anomalies., head circumference and iq, of children with sex chromosome abnormalities, prenatal diagnosis of sex chromosome abnormalities: the 8-year experience of a single medical center, related papers.
Showing 1 through 3 of 0 Related Papers

Triple X syndrome: a review of the literature
Article Title: Triple X syndrome: a review of the literature
Authors: Otter, Schrander-Stumpel, and Curfs
Date of Publication: July 1, 2009
“Triple X syndrome is a syndrome with a high level of variety in the physical and behavioural phenotype. Triple X syndrome is not rare, but it is often undiagnosed. Notwithstanding the relatively high prevalence of triple X syndrome, there are many issues yet to be studied in physical and behavioural development up to old age.”
“Above all, further study is needed to establish evidence-based treatment and support protocols in physical treatments (endocrinological treatment, fertility issues and treatment in cases with EEG anomalies in relation to behaviour, etc.), educational support, psychiatric diagnosis and treatment, and psychological treatment, such as psychotherapy and family therapy.”
Share This Story, Choose Your Platform!
- Support & FAQ
Triple X syndrome: a review of the literature
- DA KG Polikliniek
- Genetica & Celbiologie
- CAPHRI School for Public Health and Primary Care
- GROW - School for Oncology and Reproduction
Research output : Contribution to journal › Article › Academic › peer-review
| Original language | English |
|---|---|
| Pages (from-to) | 265-271 |
| Journal | |
| Volume | 18 |
| Issue number | 3 |
| DOIs | |
| Publication status | Published - Mar 2010 |
- medical genetics
- sex-chromosome aberrations
- behavioural phenotypes of genetic syndromes
- development
- psychiatric disorders
Access to Document
- 10.1038/ejhg.2009.109
Fingerprint
- Triple X Syndrome Keyphrases 100%
- control INIS 100%
- brain INIS 100%
- reviews INIS 100%
- birth INIS 100%
- adults INIS 100%
- 47,XXX Syndrome Medicine and Dentistry 100%
- Triplex Keyphrases 75%
T1 - Triple X syndrome: a review of the literature
AU - Otter, Maarten
AU - Schrander-Stumpel, Constance T. R. M.
AU - Curfs, Leopold M. G.
PY - 2010/3
Y1 - 2010/3
N2 - The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47, XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased. Toddlers with triple X syndrome show delayed language development. The youngest girls show accelerated growth until puberty. EEG abnormalities seem to be rather common. Many girls show motor-coordination problems and auditory-processing disorders are not rare. Scoliosis is probably more common in adolescent cases. The IQ levels are 20 points below that of controls, and verbal IQ is lowest. The girls struggle with low self-esteem and they need psychological, behavioural and educational support. They perform best in stable families. After leaving school they seem to feel better. In adults, premature ovarian failure seems to be more prevalent than in controls. MRIs of the brain seem to show decreased brain volumes. The 47, XXX women most often find jobs that reflect their performance abilities. Psychotic illness seems to be more prevalent in triple X adult women than in controls. Psychotic disorders respond well to psychotropic drugs. Triple X adults suffer more frequently from cyclothymic and labile personality traits. Research on triple X syndrome may yield more insight into brain and behaviour relations, developmental psychopathology, auditory-processing disorders, EEG disorders, personality and psychotic disorders, etc.
AB - The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47, XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased. Toddlers with triple X syndrome show delayed language development. The youngest girls show accelerated growth until puberty. EEG abnormalities seem to be rather common. Many girls show motor-coordination problems and auditory-processing disorders are not rare. Scoliosis is probably more common in adolescent cases. The IQ levels are 20 points below that of controls, and verbal IQ is lowest. The girls struggle with low self-esteem and they need psychological, behavioural and educational support. They perform best in stable families. After leaving school they seem to feel better. In adults, premature ovarian failure seems to be more prevalent than in controls. MRIs of the brain seem to show decreased brain volumes. The 47, XXX women most often find jobs that reflect their performance abilities. Psychotic illness seems to be more prevalent in triple X adult women than in controls. Psychotic disorders respond well to psychotropic drugs. Triple X adults suffer more frequently from cyclothymic and labile personality traits. Research on triple X syndrome may yield more insight into brain and behaviour relations, developmental psychopathology, auditory-processing disorders, EEG disorders, personality and psychotic disorders, etc.
KW - review
KW - medical genetics
KW - sex-chromosome aberrations
KW - behavioural phenotypes of genetic syndromes
KW - development
KW - psychiatric disorders
U2 - 10.1038/ejhg.2009.109
DO - 10.1038/ejhg.2009.109
M3 - Article
C2 - 19568271
SN - 1018-4813
JO - European Journal of Human Genetics
JF - European Journal of Human Genetics
Last updated 27/06/24: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > Journals
- > European Psychiatry
- > Volume 66 Issue 1
- > Triple X syndrome: Psychiatric disorders and impaired...

Article contents
- Conclusions
Data Availability Statement
Author contributions, funding statement, conflict of interest, ethical standards, triple x syndrome: psychiatric disorders and impaired social functioning as a risk factor.
Published online by Cambridge University Press: 21 December 2022
- Supplementary materials
Women with triple X syndrome (TXS) have an extra X chromosome. TXS appeared to be associated with psychiatric disorders in biased or underpowered studies.
This study aims to describe the prevalence of psychiatric disorders in adults with TXS in a relatively large and less biased group of participants.
In this cross-sectional study, data were collected from 34 women with TXS (mean age = 32.9; s.d. = 13.1) and 31 controls (mean age = 34.9; s.d. = 13.7). Psychiatric disorders were assessed using the MINI International Neuropsychiatric Interview (MINI) and the adult behavior checklist (ABCL). Trait and state anxiety were assessed using the State–Trait Anxiety Inventory.
In the TXS group, MINI results showed a higher prevalence of major depressive episodes (43.3%), psychotic disorders (29.4%), and suicidality (23.5%). Only 50% of the TXS group earned a normal score for the total syndrome score using the ABCL. In addition, levels of trait anxiety were higher in the TXS group. Only three women in each group received psychotropic medication. Impaired social functioning appeared to represent a major risk factor in TXS as regards psychotic, affective disorders, trait anxiety, and low self-esteem.
Women with TXS are vulnerable to developing psychiatric disorders, and women with both TXS and impaired social functioning are even more vulnerable.
Knowledge with respect to the increased prevalence of psychiatric disorders in adult women diagnosed with triple X syndrome (TXS) is scarce [ Reference Freilinger, Kliegel, Hanig, Oehl-Jaschkowitz, Henn and Meyer 1 ]. Psychiatric disorders and psychological complaints, as well as the modifier impaired social functioning, are hardly studied in this group.
Modern psychiatrists are increasingly aware of the contribution of genetics to the etiology of psychiatric disorders. Genetics is a compulsory subject during the training to become a psychiatrist [ Reference Besterman, Moreno-De-Luca and Nurnberger 2 ]. Psychiatric disorders, like psychotic disorders and impaired social functioning, may be associated to copy number variants [ Reference Sullivan and Owen 3 ]. So today, psychiatrists may likely refer for genetic evaluation. Genetic evaluation of people with a psychotic disorder and impaired social functioning may reveal copy number variants but also a 47,XXY karyotype in men (Klinefelter syndrome) [ Reference Cederlof, Ohlsson Gotby, Larsson, Serlachius, Boman and Langstrom 4 ] or a 47,XXX karyotype in women [ Reference Otter, Crins, Campforts, Stumpel, van Amelsvoort and Vingerhoets 5 , Reference Otter, Schrander-Stumpel, Didden and Curfs 6 ]. These women with an extra X chromosome have so-called TXS, a genetic condition that occurs in 1 out of 1,000 women. TXS was first described in 1959 [ Reference Jacobs, Baikie, Brown, Macgregor, Maclean and Harnden 7 ]. Women with TXS who already know about their genetic condition—for example, after a prenatal diagnosis—may seek psychiatric expertise, for example, in specialist units for patients with genetic and neurodevelopmental disorders. However, there is almost no scientific literature on psychiatric problems in adult women with TXS. In 1973, Staffan Olanders, a Swedish psychiatrist, wrote his doctoral thesis on 39 women with an extra X chromosome and described different types of hallucinations, delusions, and affective disturbances, including suicidality, impaired social functioning, and behavioral disorders. The fact that he recruited the majority of his participants in mental hospitals created a highly biased group of participants [ Reference Olanders 8 ]. Nevertheless, there is evidence that women with psychotic disorders have a four times higher prevalence of TXS than the general population [ Reference Polani and Tanner 9 ]. In 2004, genetic evaluation was performed in 12 patients with mild learning disabilities and psychiatric disorders in our department, and TXS was diagnosed in three adult female patients and one adolescent girl. We subsequently performed a literature search on TXS [ Reference Otter, Schrander-Stumpel and Curfs 10 ] and found no systematic studies in unbiased groups of women with TXS and psychiatric disorders. In a case report, we described the psychopathology of two of these women [ Reference Otter, Schrander-Stumpel, Didden and Curfs 6 ]. They showed slightly decreased intelligence levels, psychotic disorders, impaired social functioning, suicidal ideations, traumatic experiences, affective disorders, and low self-esteem. More recently, Freilinger et al. [ Reference Freilinger, Kliegel, Hanig, Oehl-Jaschkowitz, Henn and Meyer 1 ] described symptoms of psychological distress in girls and women with TXS, and reported that half of them showed no behavioral or social deficits. Women without ( n = 20) and with ( n = 12) impaired social functioning were described in a previous report from our group [ Reference Otter, Crins, Campforts, Stumpel, van Amelsvoort and Vingerhoets 5 ]. A Danish nationwide study of hospital diagnoses and prescribed psychiatric medication in an unselected cohort of women with TXS ( n = 103) demonstrated that women with TXS have an increased risk to develop psychiatric conditions [ Reference Berglund, Stochholm and Gravholt 11 ]. In the current study on psychiatric disorders and psychological complaints, we analyzed the differences between these two groups.
The present study aims to address the research gap in the description of psychiatric disorders in adult women with TXS. In this study, we examined the same group of 34 women with TXS and 31 controls, as described previously [ Reference Otter, Crins, Campforts, Stumpel, van Amelsvoort and Vingerhoets 5 , Reference Serrarens, Otter, Campforts, Stumpel, Jansma and van Amelsvoort 12 , Reference Otter, Campforts, Stumpel, Van Amelsvoort, Vingerhoets and Drukker 13 ]. First, we compared psychopathology between the TXS-group and the control group. Second, we assessed the contribution of Full-Scale IQ (FSIQ) to the levels of psychopathology. Third, we compared the risk of psychopathology in TXS women with impaired social functioning to those without it, because people with impaired social functioning are more at risk to develop psychiatric disorders, for example, psychotic disorders [ Reference Schalbroeck, Termorshuizen, Visser, van Amelsvoort and Selten 14 , Reference van der Linden, Simons, Viechtbauer, Ottenheijm, van Amelsvoort and Marcelis 15 ].
Participants
Sixty-five adult (≥18 years) women participated in the study, 34 with TXS (47,XXX karyotype) and 31 controls. In order to be eligible to participate in this study, subjects had to be capable of giving informed consent and had to be sufficiently proficient in the Dutch language.
Recruitment
Participants with TXS were recruited through flyers, digital newsletters, social media, the Dutch TXS support group, advertising, and the Department of Clinical Genetics of Maastricht University Medical Centre (MUMC+). The control group was recruited through families and friends of women with TXS and advertising. To lower the barrier to entry into the study, we encouraged the women with TXS to be accompanied by a friend or relative who participated in the study in several cases. This study was part of a larger research project on neuroimaging, neuropsychology, and neuropsychiatry in adults with TXS. When possible, all assessments were performed on the same day to make it as easy as possible for the participants. The data were collected between 2015 and 2018. Two women with TXS under legal guardianship, did not meet the inclusion criteria.
Study design and setting
This study was a cross-sectional study comparing a group of adults with TXS with a control group.
FSIQ was assessed using a shortened version of the Dutch Wechsler Adult Intelligence Scale, third edition (WAIS-III) [ Reference Velthorst, Levine, Henquet, de Haan, van Os and Myin-Germeys 16 ]. Psychopathology was assessed using the clinician rated MINI International Neuropsychiatric Interview (Dutch version; DSM-IV) (MINI) [ Reference Overbeek, Schruers and Griez 17 , Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller 18 ] and the Dutch authorized and tested version of the adult behavior checklist (ABCL) [ Reference Tenneij and Koot 19 ], the adult version of the child behavior checklist. The MINI was used to interview the participants, and the ABCL was completed by peer informants, like family members or friends. The MINI [ Reference Overbeek, Schruers and Griez 17 ] has been developed as a short structured interview for the most relevant psychiatric disorders (axis I) in the diagnostic and statistical manual of mental disorders—fourth edition (DSM-IV) [ 20 ]. The structured MINI interview allows administration by nonspecialized interviewers. The assessments were done by psychologists and medical students after their theoretical and practical training in psychiatry and training on the job. The students worked under the supervision of experienced research assistants. According to the DSM-IV, criteria for post-traumatic stress disorder requires past traumatic experiences and current re-experiencing. As traumatic experiences were described in TXS [ Reference Otter, Schrander-Stumpel, Didden and Curfs 6 ], results of traumatic experiences and current re-experiencing will be presented. The MINI interview does not provide details concerning traumatic experiences. However, some participants disclosed details about these experiences spontaneously.
The ABCL is an instrument to assess psychopathology in the general population and has been developed to be completed by proxy respondents. The ABCL is suitable for the 18- to 59-year age group. The ABCL includes 132 behavioral problems items which were evaluated for the preceding 6 months. Behavioral problem statements were scored by a peer informant on a three-level rating scale (“not true,” “somewhat or sometimes true,” and “very true”). Six DSM-oriented scales and eight syndrome scales were identified. The “internalizing syndrome scale” was derived from a summary score from the withdrawn, somatic complaints, and anxious/depressed syndrome scales. Similarly, the “externalizing syndrome scale” was derived from the rule-breaking behavior and aggressive behavior syndrome scales. The “total problem score” was derived from the sum of all syndrome scales. Item 79 on speech problems and item 91 on suicide talk were assessed separately.
Self-esteem was assessed using the ABCL items 33 (feels unloved), 35 (feels worthless), 47 (lacks self-confidence), and 107 (can’t succeed). We combined these items to assess self-esteem.
Trait and state anxiety were assessed using the State–Trait Anxiety Inventory-Dutch version (STAI) [ Reference Van der Ploeg 21 , Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs 22 ]. The STAI has 20 items for assessing trait anxiety and 20 items for state anxiety. All items were rated on a four-point Likert scale; higher scores indicated increased anxiety levels. The STAI has been developed in nonclinical samples and thus provides scores on anxiety levels that do not necessarily reach the strict cut-off levels from the DSM [ Reference Spielberger 23 ], but give essential information on mental health.
Social functioning was assessed using the Social Responsiveness Scale-Adults version (SRS-A) as described in a previous report [ Reference Otter, Crins, Campforts, Stumpel, van Amelsvoort and Vingerhoets 5 ]. This report described the results of the SRS-A in the TXS group in four classes: high functioning ( n = 1), normal functioning ( n = 19), mild-to-moderate deficits ( n = 7), and severe deficits ( n = 5). Because of the small numbers in some of the categories, we dichotomized social functioning into two groups, one with and one without impairments in social functioning.
Data on the use of medical compounds were collected.
Statistical analyses
To assess differences in psychopathology between the TXS and the control group, normally distributed continuous variables data were compared between the TXS group and the control group using the Student’s t -test (T-scores for the ABCL internalizing, externalizing, and total syndrome scales). Differences in categorical variables (MINI, raw scores of the ABCL) were analyzed using Fisher’s exact test.
In order to assess the contribution of FSIQ to the levels of psychopathology, the association between TXS and the ABCL T-scores were analyzed using linear regression analysis adjusting for FSIQ. In order to assess impaired social functioning as a risk factor for the development of psychiatric disorders, MINI scores, ABCL raw scores, and T-scores of the internalizing, externalizing, and total syndrome scores were analyzed in the TXS women with impaired social functioning in comparison to those without impaired social functioning.
All statistical analyses were performed using STATA/MP for Mac, version 13.1 (StataCorp, College Station, TX). All analyses were two-tailed, and alpha was set at 0.05. This study has an exploratory nature. Therefore, correction for multiple testing was not performed [ Reference Bender and Lange 24 ]. Bonferroni correction would set alpha at 0.0007.
Participant characteristics
The age of the participants (18–63 years of age) was relatively similar between the TXS group ( n = 34) and the control group ( n = 31), with mean age of 32.9 and 34.9 years (standard deviation [s.d.] = 13.1 and 13.7), respectively ( t (63) = −0.59, p = 0.56). Mean FSIQ was significantly lower in TXS subjects than in controls (mean [ M ] = 86.1, s.d. = 10.5, 95% CI 82.3–89.9 vs M = 96.8, s.d. = 12.7, 95% CI 92.1–101.4, p = 0.0005). Among the 34 women with TXS, 10 were diagnosed prenatally (mean age = 26.1 years, s.d. = 9.1), while the remaining 24 were diagnosed postnatally. The indications for postnatal testing included infertility/recurrent abortions ( n = 9; mean age = 44.3, s.d. = 9.4), atypical development ( n = 6; mean age = 28.5, s.d. = 11.5), history of a family member with a genetic condition ( n = 4; mean age = 45.8, s.d. = 11.7), small head ( n = 2), intestinal malformation ( n = 1), nuchal edema ( n = 1), and epicanthal folds ( n = 1). A total of 73.5 and 80% of the participants in the TXS and control groups were premenopausal at the time of the data collection. The number of the participants that used psychotropic medication was three in the TXS and three in the control group.
Psychiatric disorders in the TXS group compared to the control group
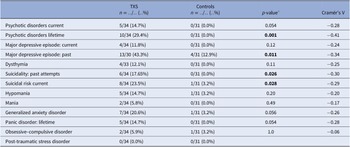
The frequency of lifetime psychotic disorders and major depressive episodes (MDEs) was higher in the TXS group than in the control group (Cramér’s V = −0.41; p = 0.001 and Cramér’s V = −0.34; p = 0.011, respectively; Table 1 ). Concerning suicidality, 17.65% of the TXS group reported past attempts and 23.5% of the TXS group reported a current suicidal risk. There was no difference between the groups in relation to substance abuse related disorders. In the TXS group and the control group, 60.6 and 29.3%, respectively, reported traumatic experiences (Cramér’s V = −0.32; p = 0.014). However, current re-experiencing showed only minor differences (in the TXS group 35% vs 22.2% in the control group, data not shown). Some participants disclosed that the traumatic experiences concerned sexual abuse ( n = 3) or bullying ( n = 3).
Table 1. MINI neuropsychiatric interview in comparison to the TXS group and the control group.
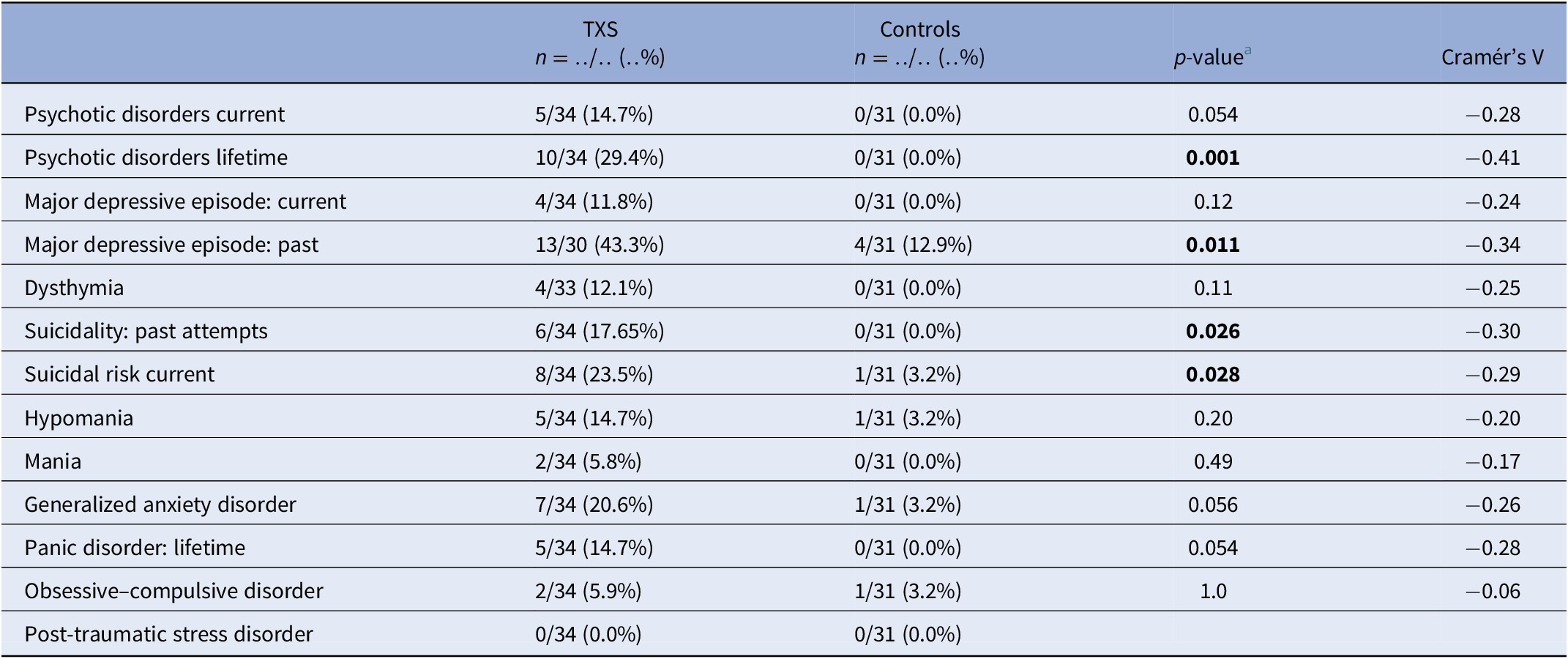
a Fisher’s exact test.
Abbreviations: MINI, MINI International Neuropsychiatric Interview; TXS, triple X syndrome.
The results of the ABCL ( Table 2 ) showed statistically significant differences in relation to internalizing problems and total problems, but not externalizing problems. Thought problems in the ABCL syndrome scale were indicative of psychotic disorders (Cramér’s V = −0.39; p = 0.004). The DSM oriented scores on depressive problems showed statistically significant differences, but anxious problems did not ( Table 2 ). The assessment of self-esteem in the ABCL revealed differences between the two groups, with higher scores on low self-esteem in the TXS group (Cramér’s V = −0.59; p = 0.001). Speech problems (ABCL item 79) were significantly more often reported in the TXS group (Cramér’s V = −0.41; p = 0.002; data not shown), but “suicide talks” (ABCL item 91) were not (Cramér’s V = −0.24; p = 0.147; data not shown). This might indicate that women with TXS avoid talking about their suicidal feelings and thoughts.
Table 2. Summary of group differences of ABCL results in the triple X syndrome (TXS) and control groups.
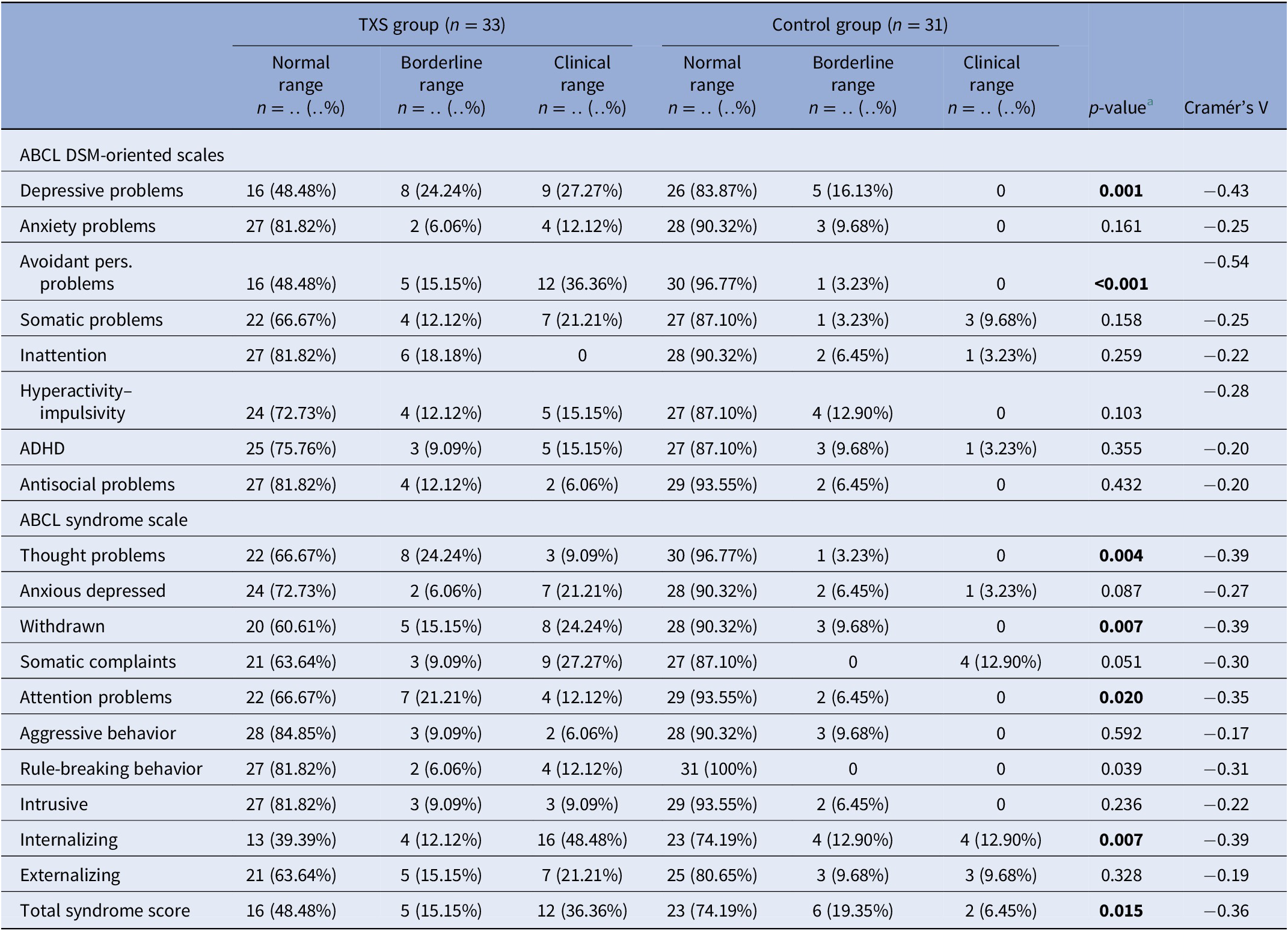
Abbreviations: ABCL, adult behavior checklist; ADHD, attention deficit hyperactivity disorder; DSM, diagnostic and statistical manual of mental disorders.
The TXS group reported higher levels of anxiety as assessed with the STAI in comparison to the control group. This was at the time of the interview (TXS STAI: M = 34.3; s.d. = 10.0; 95% CI = 30.7, 37.8 vs control group: M = 30.2; s.d. = 4.6; 95% CI = 28.5, 31.9; t (62) = 2.07; p = 0.042) as well as in the weeks before the interview (TXS STAI: M = 42.6; s.d. = 11.9; 95% CI = 38.4, 46.8; vs control group: M = 33.3; s.d. = 8.9; 95% CI = 30.1, 36.6; t (62) = 3.51; p = 0.0008).
When we controlled for FSIQ as a potential confounder, partial eta-squared (η p 2 ) values of the internalizing problems (η p 2 = 0.18), externalizing (η p 2 = 0.04), and total problems (η p 2 = 0.13) appeared to be low. This means that the differences between the TXS and the control groups were only partially explained by differences in FSIQ.
Psychiatric disorders in women with TXS without and with impaired social functioning
MINI results ( Table 3 ) in relation to psychotic disorders showed the strongest association (Cramér’s V = 0.45), so women with TXS as well as impaired social functioning more often suffer from psychotic disorders than women with TXS without impaired social functioning. ABCL DSM oriented results ( Table 4 ) showed the strongest association in relation to anxiety problems (Cramér’s V = 0.62), inattention (Cramér’s V = 0.62), and antisocial problems (Cramér’s V = 0.62), so women with TXS and impaired social functioning more often suffer from anxiety, inattention, and antisocial problems. The ABCL syndrome scales ( Table 4 ) showed strong associations between the prevalence of anxiety, attention deficit hyperactivity disorder (ADHD) related behavior, somatic complaints, and behavioral problems and impaired social functioning. The TXS group without impaired social functioning showed higher levels of self-esteem ( M = 2.9; s.d. = 1.5; 95% CI = 2.2, 3.6; data not shown) in comparison with the TXS group with impaired social functioning ( M = 4.7; s.d. = 2.1; 95% CI = 3.4, 6.1; t (30) = −2.86; p = 0.0076; data not shown). Speech problems showed small differences (Cramér’s V = 0.36; p = 0.12; data not shown). In contrast, the results in relation to “suicide talks” (item 91) showed major differences (Cramér’s V = 0.56; p = 0.004; data not shown).
Table 3. MINI neuropsychiatric interview: comparison between the TXS without and with social impairments.
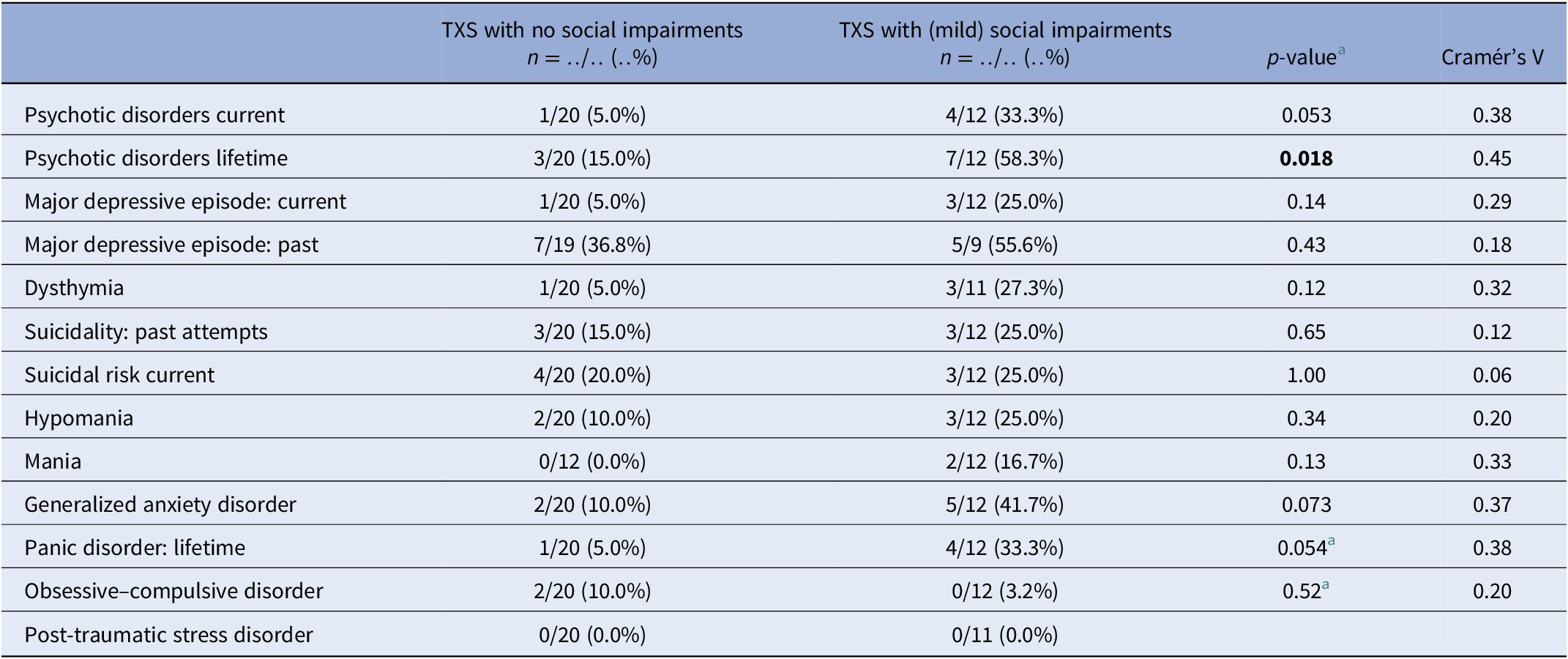
Table 4. Summary of group differences of ABCL results in the TXS without and with social impairments.
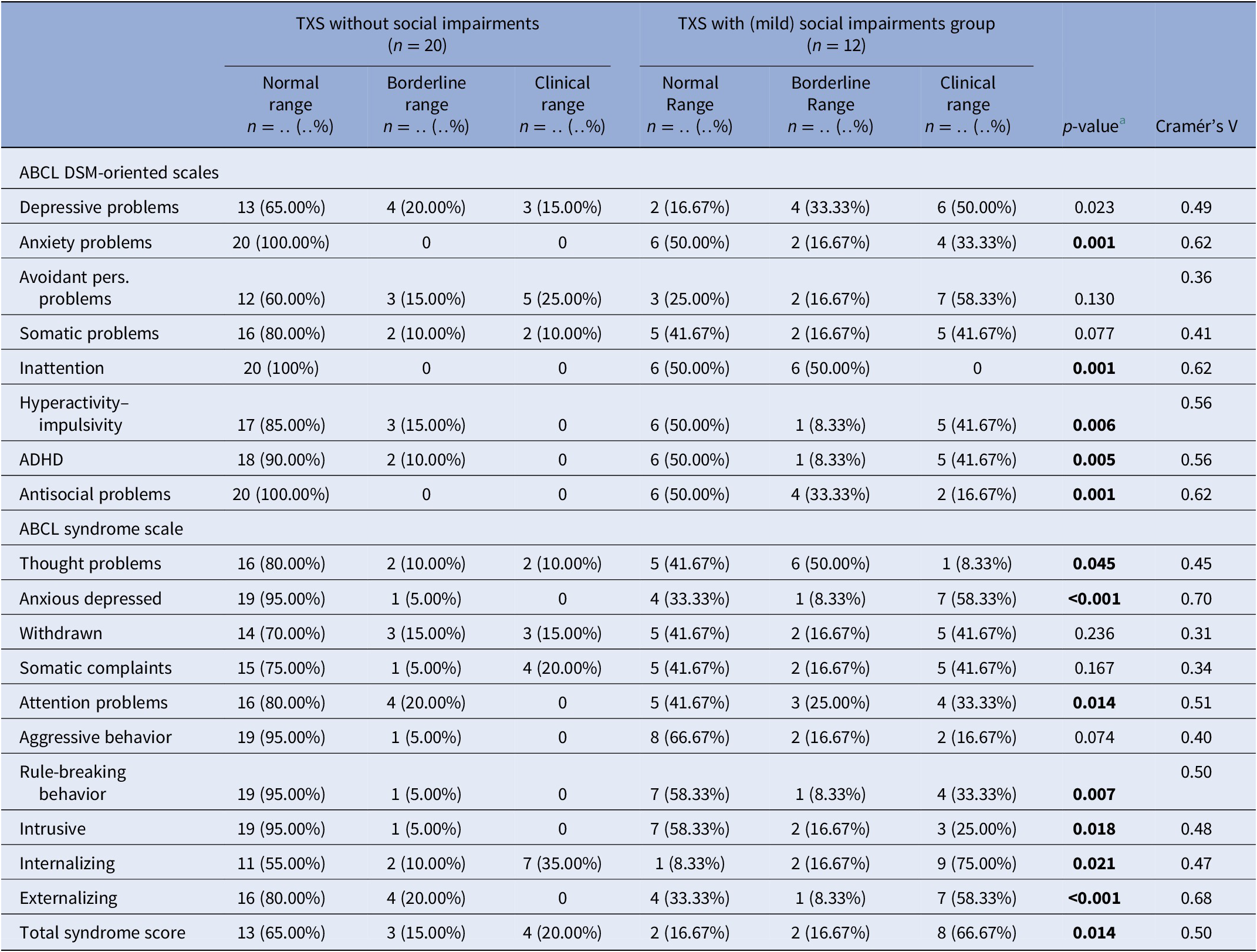
Abbreviations: ABCL, adult behavior checklist; ADHD, attention deficit hyperactivity disorder; DSM, diagnostic and statistical manual of mental disorders; MINI, MINI International Neuropsychiatric Interview; TXS, triple X syndrome.
The TXS group without impaired social functioning reported lower levels of anxiety as assessed with the STAI ( M = 31.6; s.d. = 9.3; CI = 27.3, 36.0) in comparison to the TXS group with social impairments ( M = 39.4; s.d. = 10.3; CI = 32.5, 46.4; t (29) = −2.15; p = 0.04) at the time of the interview and also lower levels of anxiety in the weeks before the interview (the TXS group without social impairments: M = 39.0; s.d. = 12.4; CI = 33.3, 44.8; vs the TXS group with social impairments: M = 49.5; s.d. = 9.1; CI = 43.4, 55.7; t (29) = −2.46; p = 0.02). In summary, women with TXS and impaired social functioning more often have psychiatric disorders and psychological complaints than women with TXS without social impairments.
Main findings and comparison with findings from other studies
Only 50% of the TXS group earned a normal score in the ABCL total syndrome score, and even a smaller part of the TXS group scored normal in the internalizing syndrome score (39.4%; Table 2 ). The results of the MINI interview revealed a much higher prevalence of past MDEs (43.3%), lifetime psychotic disorders (29.4%), and current suicidality (23.5%) ( Table 1 ). As opposed to a more extended version of the MINI (the MINI Plus), the MINI cannot differentiate between the subtypes of psychotic disorders, like delusional or schizophrenic disorders. The results of the current psychotic disorders and current melancholic features ( Table 1 ) may not be representative, as it was not expected that subjects with current melancholic features would join this study as a participant. The STAI revealed higher levels of anxious complaints in the TXS group ( t (62) = 3.51; p = 0.0008).
In cases with lifetime psychotic disorders, impaired social functioning (Cramér’s V = 0.45; p = 0.018) appeared to be a risk factor to the women with TXS ( Table 3 ). The ABCL internalizing (Cramér’s V = 0.47; p = 0.021), like the anxious/depressed syndrome scores (Cramér’s V = 0.70; p ≤ 0.001) and externalizing syndrome scores including antisocial and rule-breaking behavior (Cramér’s V = 0.68; p ≤ 0.001), revealed higher levels of psychopathology in the group of women with TXS and impaired social functioning.
Previous studies recruited smaller groups of participants [ Reference Freilinger, Kliegel, Hanig, Oehl-Jaschkowitz, Henn and Meyer 1 ] or suffered from higher levels of recruitment bias as in Olanders [ Reference Olanders 8 ]. Freilinger (2018) showed that 50% of a small group of adult women with TXS function without psychiatric disorders, which is in accordance with our results. Our previous report on two cases with TXS demonstrated slightly decreased intelligence levels, psychotic disorders, impaired social functioning, suicidal ideations, traumatic experiences, affective disorders, and low self-esteem [ Reference Otter, Schrander-Stumpel, Didden and Curfs 6 ]. The current study adds to the knowledge of TXS syndrome that impaired social functioning appear to represent a risk factor in TXS as regards psychotic, affective disorders, attentional problems, and low self-esteem, but not in relation to traumatic experiences and suicidality. Attentional problems has been discussed in more detail in another report from our group on neuropsychological findings in the same group of participants [ Reference Otter, Campforts, Stumpel, Van Amelsvoort, Vingerhoets and Drukker 13 ]. Decreased levels of FSIQ appear not to represent a significant risk factor. Importantly, we observed severe medical undertreatment, as only three women in the TXS group ( n = 34) and three in the control group ( n = 31) received psychotropic medication Table 5 . This is in contrast with the findings of the Danish nationwide study that described increased prescriptions in the TXS group of psycholeptic drugs (26.2% in the TXS group and 20.7% in the control group), antipsychotics (11.7% in the TXS group and 5.5% in the control group), psychoanaleptic drugs (29.1% in the TXS group and 20.7% in the control group), antidepressant drugs (26.2% in the TXS group and 17.3% in the control group), and ADHD medication and nootropics (3.9% in the TXS group and 1.5% in the control group) [ Reference Berglund, Stochholm and Gravholt 11 ]. This can be explained by the fact that the Danish study collected data from medical settings. In the Netherlands, the care for people with genetic disorders like TXS is mainly provided by psychologists as the first tier of healthcare providers. Psychologists—of course—prefer nonmedical treatments and are not allowed to prescribe medication in the Netherlands. Furthermore, it is our experience that women with TXS are reluctant to use psychiatric medication.
Table 5. Current medications.
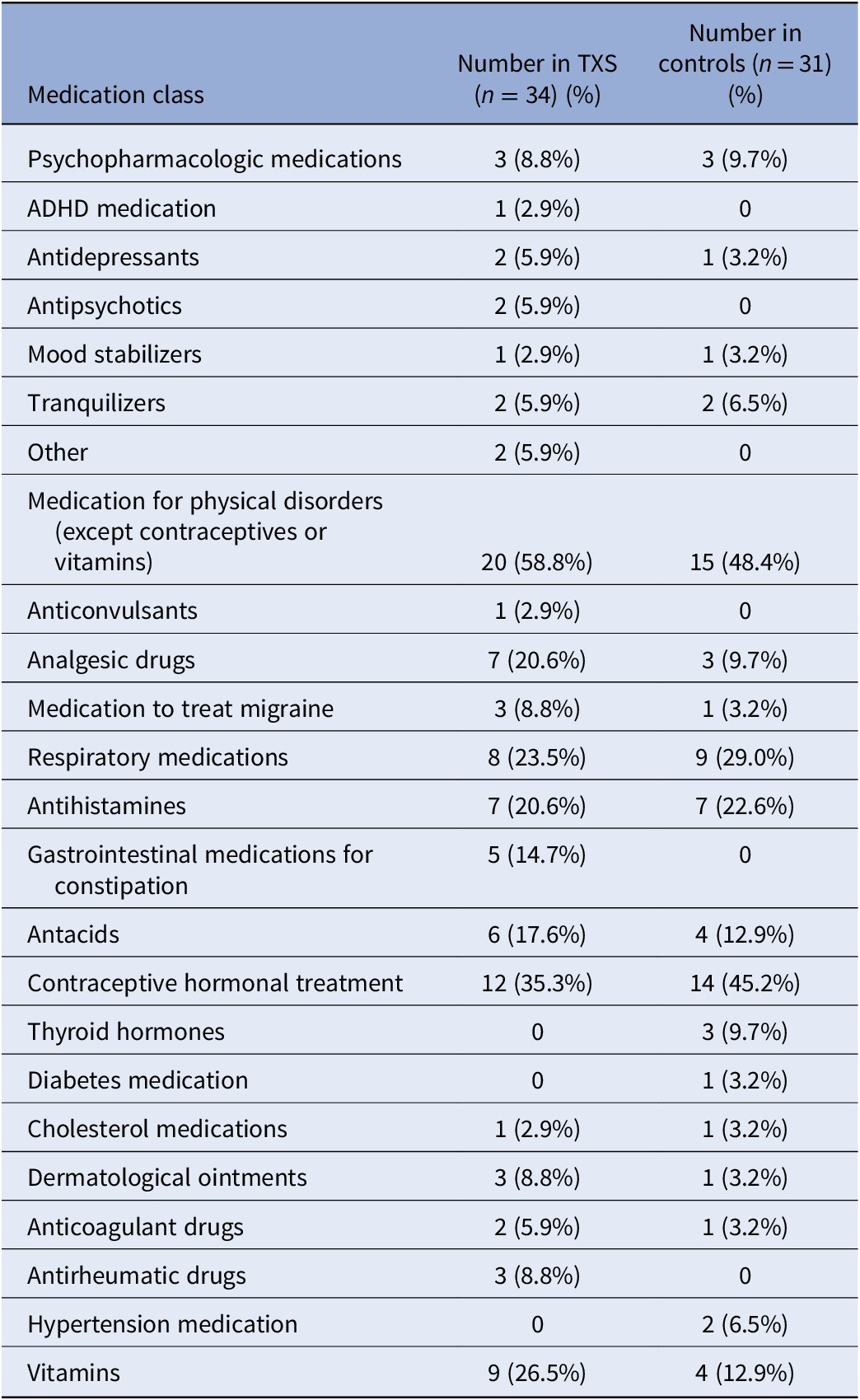
Abbreviations: ADHD, attention deficit hyperactivity disorder; TXS, triple X syndrome.
Implications
Clinicians who work with women with TXS should be aware of the risk of psychiatric disorders, including psychotic disorders, affective disorders and suicidality. Clinicians should be aware of the possibility of a not yet recognized TXS diagnosis, especially those who work with women with psychotic disorders and impaired social functioning. It is essential to consider that women with TXS may suffer from expressive language disorders. A structured clinical interview like MINI can help uncover all complaints, including somewhat embarrassing ones such as traumatic experiences or suicidal thoughts, that were not already shared with the clinician. Improving the psychological and psychiatric diagnostic procedures will therefore have to lead to improvement of psychotherapeutic treatments and treatment with psychiatric medication in accordance with the guidelines.
Strengths, limitations, and how to design future studies
Our group focuses on research on adult women with TXS. Several groups have been investigating sex chromosomal disorders without enough attention to the features of the distinct disorders [ Reference Mankiw, Park, Reardon, Fish, Clasen and Greenstein 25 – Reference Wilson, King and Bishop 27 ]. This way, the special needs of women with TXS are underestimated. Moreover, previous studies mainly focus on children with sex chromosomal disorders [ Reference Mankiw, Park, Reardon, Fish, Clasen and Greenstein 25 – Reference Wilson, King and Bishop 27 ]. The study of TXS is far behind the study of other sex chromosomal disorders. Even in 2019, a review of sex chromosomal disorders and psychiatric disorders mentions that women with TXS are not at risk of developing impaired social functioning [ Reference Green, Flash and Reiss 28 ]. These differences may be explained by the lack of physical features in TXS. Clinicians seldom suspect a TXS diagnosis based on external or endocrinological characteristics, contrasting with Turner and Klinefelter syndrome [ Reference Skuse, Printzlau and Wolstencroft 29 ]. Therefore, this study is important in filling the gap in our knowledge of psychiatric disorders in TXS.
This descriptive and explorative study has limitations. Our group of adults with TXS was more extensive and less biased than any other sample, but the numbers were relatively small. As TXS is not rare, it should be possible to establish larger groups of participants and investigate them in a longitudinal design. The number of participants in the current study was too small to search for differences in various age groups and for differences between premenopausal and postmenopausal women with TXS. A longitudinal design also offers the opportunity to identify early markers of “at risk” development and assess early interventions’ effectiveness [ Reference van Rijn 26 ]. As soon as early interventions appear to be effective, early recognition of TXS is the next step, probably by noninvasive prenatal testing [ Reference Gadsboll, Petersen, Gatinois, Strange, Jacobsson and Wapner 30 ] or screening of every newborn and subsequent counseling of the parents [ Reference Nielsen and Wohlert 31 ] and potentially preventing psychological problems. Another limitation of this study is the assessment of self-esteem with an unvalidated tool.
Despite the small numbers, this study may suggest new themes to focus on in future studies. The present paper dichotomized social functioning, but this is not a dichotomous construct. Studies in larger groups are necessary to investigate differences in the prevalence of psychopathology between groups with various levels of social functioning. The study of self-esteem, until now seldom recognized as a biologically based personality feature [ Reference Rosenberg 32 ], deserves further study. Furthermore, the previously described variability in self-esteem in people with psychotic disorders deserves further scientific attention in the study of TXS, preferably in an ecological study design [ Reference van der Linden, Simons, Viechtbauer, Ottenheijm, van Amelsvoort and Marcelis 15 , Reference Daemen, van Amelsvoort, Group and Reininghaus 33 ]. Comparably, suicidality in TXS deserves further scientific attention, which could be helpful to women with TXS, but also could extend the knowledge about genetic and other factors that contribute to suicidal thoughts and behavior [ Reference Turecki and Brent 34 ].
Klinefelter syndrome has been cited as a genetic model of psychotic disorders in men [ Reference DeLisi, Maurizio, Svetina, Ardekani, Szulc and Nierenberg 35 ]. TXS may provide a unique model to study psychotic disorders associated with impaired social functioning, which is important as both may interact and may be associated with poor daily life outcomes in women [ Reference Isvoranu, Ziermans, Schirmbeck, Borsboom, Geurts and de Haan 36 ]. The relation between the extra X chromosome and psychiatric disorders in TXS remains to be elucidated. Several pathogenetic mechanisms have been hypothesized. The extra X chromosome might cause decreased cell-division rates [ Reference Otter, Schrander-Stumpel and Curfs 10 ], which might explain the smaller head circumference and the decreased total brain volumes in TXS [ Reference Leibovitz, Lerman-Sagie and Haddad 37 – Reference Reardon, Clasen, Giedd, Blumenthal, Lerch and Chakravarty 39 ]. Gene dosage imbalances in X chromosomal genes that escape X chromosome inactivation [ Reference Carrel and Willard 40 – Reference Nielsen, Trolle, Vang, Hornshoj, Skakkebaek and Hedegaard 43 ] and autosomal genes [ Reference Raznahan, Parikshak, Chandran, Blumenthal, Clasen and Alexander-Bloch 42 – Reference Penrose 45 ] might also play a role in the pathophysiological process from the extra X chromosome to neurobiological disturbances and the subsequent psychiatric disorders. Several X-linked genes have been mentioned as candidate genes [ Reference Zhang, Yang, Li, Ma and Li 46 ]. These studies addressed fundamental scientific issues concerning the biology of sex chromosomes. They yielded several new candidate genes to be explored in future studies because some are linked to mental retardation and brain development [ Reference Raznahan, Parikshak, Chandran, Blumenthal, Clasen and Alexander-Bloch 42 , Reference Nielsen, Trolle, Vang, Hornshoj, Skakkebaek and Hedegaard 43 ]. However, these interesting studies did not find an explanation for the variability of the psychiatric phenotype in women with TXS. Nielsen et al. suggested [ Reference Nielsen, Trolle, Vang, Hornshoj, Skakkebaek and Hedegaard 43 ] that future studies should use brain tissue to explore the pathogenetic mechanisms behind TXS, but brain tissue is unavailable from living humans. We suggest using brains from animals like nonhuman primates or infertile cattle with an extra X chromosome [ Reference Otter, Schrander-Stumpel and Curfs 10 ] or neuronal tissue generated using human induced pluripotent stem cells, preferably from women with TXS and differences in the psychiatric phenotype. The use of human induced pluripotent stem cells harbors the promise of discovering new treatment options for neuropsychiatric disorders [ Reference Bardy, Greenberg, Perry, Licinio and Baune 47 ].
In summary, women with TXS are vulnerable to developing psychiatric disorders, and women with TXS and impaired social functioning are even more vulnerable. Psychotic disorders, major depression, anxiety disorders, suicidality, and low self-esteem, should be considered in the clinical examination of women with TXS. Clinicians who work with women with impaired social functioning and psychiatric disorders should consider referral to a clinical geneticist. Future research should use a longitudinal design, larger groups of participants and preferably an ecological design. We know that the participants of this study gave their time and efforts to find medical and psychological treatments. Future studies also should develop and evaluate treatments for psychiatric disorders in women with TXS.
The data that support the findings of this study are available from the corresponding author (M.O.), upon reasonable request.
Acknowledgements
We would like to express our thanks to the participants for their participation in this study. We thank Ida Bakker and Thea van der Velde from the Dutch TXS support group (Contactgroep Triple-X Syndroom) for their contribution. Ida and Thea supported the writing of the flyers and were very supportive during the recruitment of participants. And thanks in advance for their contribution to the presentation of the results during a meeting of the TXS support group.
M.O., C.T.R.M.S., and T.A.M.J.v.A. had the idea for the study and contributed to the study design. T.A.M.J.v.A. secured funding for the study. M.O. and B.C.M.C. collected the data. M.O. and M.D. led the statistical analyses and drafting of the manuscript. All authors contributed to the drafting and approved the final manuscript for submission.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors declare none.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects were approved by the medical ethics committee of MUMC+ and Maastricht University (NL46871.068.14/METC143051). Written informed consent was obtained from all subjects before starting the data collection after explaining the study procedures.
Otter et al. supplementary material

No CrossRef data available.
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Volume 66, Issue 1
- Maarten Otter (a1) (a2) (a3) , Bea C. M. Campforts (a1) , Constance T. R. M. Stumpel (a4) , Thérèse A. M. J. van Amelsvoort (a1) and Marjan Drukker (a1)
- DOI: https://doi.org/10.1192/j.eurpsy.2022.2355
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Triple X syndrome: a review of the literature

2010, European Journal of Human Genetics
Related Papers
European Psychiatry
Bea Campforts
Background Women with triple X syndrome (TXS) have an extra X chromosome. TXS appeared to be associated with psychiatric disorders in biased or underpowered studies. Aim This study aims to describe the prevalence of psychiatric disorders in adults with TXS in a relatively large and less biased group of participants. Method In this cross-sectional study, data were collected from 34 women with TXS (mean age = 32.9; s.d. = 13.1) and 31 controls (mean age = 34.9; s.d. = 13.7). Psychiatric disorders were assessed using the MINI International Neuropsychiatric Interview (MINI) and the adult behavior checklist (ABCL). Trait and state anxiety were assessed using the State–Trait Anxiety Inventory. Results In the TXS group, MINI results showed a higher prevalence of major depressive episodes (43.3%), psychotic disorders (29.4%), and suicidality (23.5%). Only 50% of the TXS group earned a normal score for the total syndrome score using the ABCL. In addition, levels of trait anxiety were higher ...
European Journal of Pediatrics
Florinda Ceriani
Indian Journal of Pediatrics
sujatha jagadeesh
Triple X syndrome is a rare numerical chromosomal anomaly, occurring as a result of non dysjunction in meiosis I. Most cases have neurodevelopmental defects and functional problems. We report two cases diagnosed in our centre. The first was a fetus with cleft lip and palate, 47, XXX was identified by Fetal Blood Sampling. The second was a child with multisystem anomaly including cleft lip and palate, whose karyotype also revealed 47, XXX. Though isolated cases of associated abnormalities have been reported there have not been consistent phenotypic changes reported with this condition.
Developmental Neurorehabilitation
Caroline Junge
European Child & Adolescent Psychiatry
Nollaig Byrne
Orphanet Journal of Rare Diseases
Lennie Wilson
The Journal of Pediatric Research
Journal of Intellectual Disability Research
International Journal of Reproduction, Contraception, Obstetrics and Gynecology
Dr. Purnima Nadkarni
… Medicine & Child …
Gaia Scerif
AimTo review systematically the neurodevelopmental characteristics of individuals with sex chromosome trisomies (SCTs).MethodA bibliographic search identified English-language articles on SCTs. The focus was on studies unbiased by clinical referral, with power of at least 0.69 to detect an effect size of 1.0.ResultsWe identified 35 articles on five neonatally identified samples that had adequate power for our review. An additional 11 studies were included where cases had been identified for reasons other than neurodevelopmental concerns. Individuals with an additional X chromosome had mean IQs that were within broadly normal limits but lower than the respective comparison groups, with verbal IQ most affected. Cognitive outcomes were poorest for females with XXX. Males with XYY had normal-range IQs, but all three SCT groups (XXX, XXY, and XYY) had marked difficulties in speech and language, motor skills, and educational achievement. Nevertheless, most adults with SCTs lived independently. Less evidence was available for brain structure and for attention, social, and psychiatric outcomes. Within each group there was much variation.InterpretationIndividuals with SCTs are at risk of cognitive and behavioural difficulties. However, the evidence base is slender, and further research is needed to ascertain the nature, severity, and causes of these difficulties in unselected samples.
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
American Journal of Medical Genetics
William Brown
Case Reports in Pediatrics
Marco Armando
International Journal of Human Genetics
Jhumur Pani
American Journal of Medical Genetics Part A
Robin Hansen
Journal of genetics
Tahir Malla
Indian Journal of Human Genetics
Sujatha Madireddi , Tella Sunitha
Shivanand Patil
Clinical Science
Martyn Bell
Kjeld Rasmussen
Journal of Pediatrics
Sebastian Vasquez
Acta Medica Philippina
Mary Chiong
American Journal of Medical Genetics Part C: Seminars in Medical Genetics
Talia Thompson
Natascha Lantschner
Neurosciences (Riyadh, Saudi Arabia)
Adel Mahmoud
Journal of Neurodevelopmental Disorders
Marcelo Cordoba
Yitzchak Frank
Claus Gravholt
Osamu Samura
NeuroImage: Clinical
Jonathan Blumenthal
Journal of Genetics
daniela mendez
Clinical Genetics
Maija Wilska
Andrea Gropman
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Triple x syndrome with short stature: case report and literature review
Affiliation.
- 1 Department of Child Health Care, Children's Hospital of Zhejiang University School of Medicine and Zhejiang Key Laboratory for Diagnosis and Therapy of Neonatal Disease, Hangzhou, China.
- PMID: 23056899
- PMCID: PMC3446055
Background: Triple X syndrome is a sex chromosomal aneuploidy condition characterized by tall stature, microcephaly, hypertelorism, congenital abnormalities, and motor and language delays. It is mainly derived from maternal nondisjunctional errors during meiosis. To highlight the clinical features and diagnosis of triple X syndrome, we present a rare phenotype of the syndrome.
Case presentation: A 5.9 year-old girl was admitted to our hospital because of short stature. Both her height and weight were below the 3(rd) percentile compared to the normal peers. She was found with mild motor and speech delay. Laboratory investigation showed low level of IGF-1 and zinc, elevated estradiol level and normal result of arginine provocation test.
Conclusion: Our data suggest that triple X syndrome should also be suspected in patients with short stature, elevated estradiol and low level of IGF-1, even with normal result of arginine provocation test.
Keywords: 47, XXX; Insulin-Like Growth Factor-1; Sex Chromosome Aneuploidy; Short Stature; Triple X Syndrome.
PubMed Disclaimer
The growth of height (A)…
The growth of height (A) and weight (B) from birth to 5.9 years.…
Similar articles
- Growth hormone therapy for children with KBG syndrome: A case report and review of literature. Ge XY, Ge L, Hu WW, Li XL, Hu YY. Ge XY, et al. World J Clin Cases. 2020 Mar 26;8(6):1172-1179. doi: 10.12998/wjcc.v8.i6.1172. World J Clin Cases. 2020. PMID: 32258089 Free PMC article.
- Homozygous loss of function BRCA1 variant causing a Fanconi-anemia-like phenotype, a clinical report and review of previous patients. Freire BL, Homma TK, Funari MFA, Lerario AM, Leal AM, Velloso EDRP, Malaquias AC, Jorge AAL. Freire BL, et al. Eur J Med Genet. 2018 Mar;61(3):130-133. doi: 10.1016/j.ejmg.2017.11.003. Epub 2017 Nov 10. Eur J Med Genet. 2018. PMID: 29133208 Review.
- Tall stature, insulin resistance, and disturbed behavior in a girl with the triple X syndrome harboring three SHOX genes: offspring of a father with mosaic Klinefelter syndrome but with two maternal X chromosomes. Kanaka-Gantenbein C, Kitsiou S, Mavrou A, Stamoyannou L, Kolialexi A, Kekou K, Liakopoulou M, Chrousos G. Kanaka-Gantenbein C, et al. Horm Res. 2004;61(5):205-10. doi: 10.1159/000076532. Epub 2004 Jan 29. Horm Res. 2004. PMID: 14752208
- De novo dup(X)(q22.3q26) in a girl with evidence that functional disomy of X material is the cause of her abnormal phenotype. Armstrong L, McGowan-Jordan J, Brierley K, Allanson JE. Armstrong L, et al. Am J Med Genet A. 2003 Jan 1;116A(1):71-6. doi: 10.1002/ajmg.a.10727. Am J Med Genet A. 2003. PMID: 12476455 Review.
- Changes in serum IGF-I and IGFBP-3 concentrations during the IGF-I generation test performed prospectively in children with short stature. Cotterill AM, Camacho-Hübner C, Duquesnoy P, Savage MO. Cotterill AM, et al. Clin Endocrinol (Oxf). 1998 Jun;48(6):719-24. doi: 10.1046/j.1365-2265.1998.00407.x. Clin Endocrinol (Oxf). 1998. PMID: 9713560
- 47, XXX syndrome with infertility, premature ovarian insufficiency, and streak ovaries. Rafique M, AlObaid S, Al-Jaroudi D. Rafique M, et al. Clin Case Rep. 2019 May 14;7(6):1238-1241. doi: 10.1002/ccr3.2207. eCollection 2019 Jun. Clin Case Rep. 2019. PMID: 31183102 Free PMC article.
- Jacobs PA, Baikie AG, Brown WM, et al. Evidence for the existence of the human “super female”. Lancet. 1959;2(7100):423–5. - PubMed
- Stochholm K, Juul S, Gravholt CH. Mortality and incidence in women with 47,XXX and variants. Am J Med Genet A. 2010;152A(2):367–72. - PubMed
- Tartaglia NR, HoweL S, Sutherland A, et al. A review of trisomy X (47,XXX) Orphanet J Rare Dis. 2010;5:8. - PMC - PubMed
- Lenroot RK, Lee NR, Giedd JN. Effects of sex chromosome aneuploidies on brain development: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15(4):318–27. - PMC - PubMed
- Tennes K, Puck M, Bryant K, et al. A develop-mental study of girls with trisomy X. Am J Hum Genet. 1975;27(1):71–80. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Iran J Pediatr
- v.22(2); 2012 Jun

Triple X Syndrome with Short Stature: Case Report and Literature Review
Triple X syndrome is a sex chromosomal aneuploidy condition characterized by tall stature, microcephaly, hypertelorism, congenital abnormalities, and motor and language delays. It is mainly derived from maternal nondisjunctional errors during meiosis. To highlight the clinical features and diagnosis of triple X syndrome, we present a rare phenotype of the syndrome.
Case Presentation
A 5.9 year-old girl was admitted to our hospital because of short stature. Both her height and weight were below the 3 rd percentile compared to the normal peers. She was found with mild motor and speech delay. Laboratory investigation showed low level of IGF-1 and zinc, elevated estradiol level and normal result of arginine provocation test.
Our data suggest that triple X syndrome should also be suspected in patients with short stature, elevated estradiol and low level of IGF-1, even with normal result of arginine provocation test.
Introduction
Triple X syndrome (trisomy X, 47,XXX), first described by Jacobs 1959, is a sex chromosomal aneuploidy condition with female phenotype[ 1 ]. The incidence of the syndrome is estimated at 10.7 per 100000 live born girls[ 2 ]. Its symptoms vary very widely, including tall stature, hypertelorism, epicanthal folds, clinodactyly, congenital heart disease, genitourinary and some other anomalies,[ 3 ]. Neuroimaging studies showed significantly smaller brain volume in these patients[ 4 ], which might associate with poor learning and language skills at school age[ 5 ]. These could eventually lead to shyness, stress, and disturbance in their interpersonal relationships. Some cases even may experience delayed menarche and premature ovarian function. Mortality of the syndrome is significantly higher than in age-matched females[ 2 ].
Herein, we report a case of 47,XXX who was discovered to be slow growing. To our knowledge, this is the first case report of a triple-X karyotype with short stature. The patient's parents provided written informed consent for karyotype sampling and taking pictures.
A 5.9-year-old girl presented to our unit due to slow growing for 4 years. She was the only child of unrelated parents. She was born at term by uterine-incision delivery following an uneventful pregnancy, weighing 2700 g and measuring 47 cm. No medication history was reported. She started walking independently and speaking at the 17th month. Her mother was 27 years and father was 32 years at conception, without any history of disease or use of drugs before or during pregnancy. Her mother and father had a height of 154 cm and 164 cm, respectively. Both of them are healthy and have normal karyotypes. Her family history revealed that no clinical features were noted in other family members, and they had not examined their karyotype.
The girl was 105 cm (<3 rd percentile for normal population, height SD score -1.9) with a weight of 14.6 kg (<3 rd percentile for normal population, weight SD score <-2SD). Her body mass index (BMI) was 13.2 (<15 th percentile for normal population, BMI SD score <-1SD) and head circumference 49.5 cm (percentile for normal population, BMI SD score). She grew 4-5 cm every year in the past 4 years, as shown in Fig. 1 .

The growth of height (A) and weight (B) from birth to 5.9 years. The patient was below the 3 rd percentile according to the WHO Child Growth Standards.
She had a higher interpupillary distance with Tanner 1 stage of breasts ( Fig. 2a - -b). b ). The genitalia were of normal female phenotype. No other abnormal features were noted.

A: Normal features in appearance. B: widened papillae distance
The score on the Peabody Picture Vocabulary Test and the social viability measuring list on infants-junior middle school students revised by Zuo Qihua[ 6 ] were within the average range. The outcome of Gesell Developmental Schedules indicated mild development delay in gross motor, fine motor and language ( Table 1 ).
Development quotient (DQ) scores and developmental age (DA) of Gesell
| DQ | DA (month) | |
|---|---|---|
| 78 | 56.1 | |
| 64 | 46 | |
| 75 | 54 | |
| 73 | 52.7 | |
| 82 | 58.8 |
Normal ≥ 85; borderline development 75 < DQ < 85; mild delay 55 ≤ DQ ≤ 75; moderate delay 40 ≤ DQ ≤ 54; severe delay 25 ≤ DQ ≤ 39; extremely severe delay 25 < DQ
Laboratory tests showed decreased serum insulin-like growth factor-1 (IGF-1) from 46.4 mg/L (normal range 42-114 mg/L) measured 2 years ago to 31.1 mg/L (normal range 60-158 mg/L). The peak value of growth hormone (GH) in arginine provocation test was 11.4 mg/L, while 36.6 mg/L in insulin provocation test. Both of them were in normal range. Her blood zinc level was normal two years ago, but this time, it was 69.4 µg/L (normal range 72-180 µg/L). Basal hormone test 2 years ago indicated follicle stimulating hormone (FSH 18.6 IU/L; normal range 0.5-3.7 IU/L), luteinizing hormone (LH 2.3 IU/L; normal range 0.6∼1.7 mIU/mL) and prolactin (PRL 29.00 IU/L; normal range 2.34-26.7 IU/L) were elevated, while this time the basal level of LH was <0.10 IU/L and estradiol was 25.8 ng/L (normal range <20 ng/L). Other parameters including liver and kidney function, blood routine test, blood glucose, cortisol and adrenocorticotropic hormone, thyroid function, insulin, and hepatitis virus were all within normal limits.
Bone radiographic imaging examination of the left carpal demonstrated that there was 7/10 wrist ossification center, the difference between bone age and chronological age was in normal range ( Fig. 3A ). Chromosome analysis using G-banding technique demonstrated a 47,XXX karyotype ( Fig. 3B ). The ultrasound examination of heart, uterus, ovary and urinary system, as well as cranial magnetic resonance imaging (MRI) were normal.

A , Radiological findings of bone age. B , 47, XXX Karyotype of the patient
Triple X syndrome is usually of sporadic origin. X chromosomes in these patients fail to separate during cell division, in a process called nondisjunction. It mostly derives from maternal nondisjunctional errors during meiosisⅠ(63%) or Ⅱ(17.4%). Only one of three X chromosomes is activated and the other two are inactivated to Barr bodies. The variable phenotypic abnormalities mentioned above are thought to be related to the over expression of the genes situated on the extra X chromosomes that escape X-inactivation [ 3 ]. Advanced maternal age and aberrant recombination are risk factors of the syndrome [ 7 ].
It is reported that only 10% of the patients were diagnosed [ 3 ], because the identification of a fetus with 47,XXX on ultrasound is difficult and prenatal diagnosis via amniocentesis, chorionic villi sampling or postnatal karyotype analysis are not routine in the clinic. Variable symptoms will also contribute to the high rate of misdiagnosis. In this case, other family members were phenotypically normal and the girl would not have been suspected from physical examination. Thus, the karyotype of 47,XXX was an unexpected finding.
Triple X females are tending to display moderately tall stature [ 8 ], their final height ranges from -1 to 3 SDS of the normal population. It was supposed to be related to the short-stature-homeobox-containing gene (SHOX gene) in the pseudoautosomal region of X and Y chromosomes [ 9 ]. The excessive copies of the gene will prolong the period of growth, while haploinsufficiecy of it may lead to short stature. Also, the alteration of non-inactivated region and hormone factors might contribute to the height increase [ 10 ]. In this report, the girl was short. To our knowledge, this is the first case of a triple X patient with short stature. Her body length ranged between the 15 th to 50 th percentile in the first year, and later on, it was below the 3 rd percentile, but she grew about 4∼5cm annually in recent years. So, in our case, the height of the patient was opposite to previous reports in the literature. There was no evidence showing that her short stature was related to growth hormone deficiency, because the result of the arginine provocation test was normal. The low level of blood zinc and IGF-1 might have an effect on her stature in some extent, since low blood zinc level will reduce the level of IGF-1 and lead to slow growing. However, the blood zinc and IGF-1 level were in normal ranges 2 years ago, but they were significantly decreased this time, especially the IGF-1 level, which was below -2SD for age. Meanwhile, we found that the girl's growth rate did not change a lot in the past two years. Thus, it may indicate that the circulating levels of IGF-1 and blood zinc level are not the main causes of the abnormal growth pattern in our case. This was the same as Lise Aksglaede's opinion that growth pattern was not reflected in circulating levels of IFG-1 in trisomy[ 11 ]. Some studies proposed that neither SHOX overdosage nor estrogen deficiency alone were sufficient to lead to a tall stature, since their combination permitted a prolonged growth period and a higher final height[ 9 ]. In this study, the girl showed elevated estrogen level. Thus, the SHOX over dosage per se may not lead to over growth and we hypothesized that a mutation in the SHOX gene may result in a short height in our case. But we did not do the FISH analysis of SHOX, so we cannot make any conclusions.
Previous literature showed that 47,XXX females usuaLy had high levels of estrogen and progesterone, causing menstrual disorder and sexual precocity [ 12 ]. However, most of them will have normal reproductive functions. In our case, when the girl was 4.2 years old, her hormone test showed that the level of FSH, LH and PRL were all above the normal range, especially FSH. This does not happen before puberty in normal populations. Thus, it indicates that in our case the hypothalamus-adenohypophysis-gonadal (HPG) axis was activated in advance. The level of estradiol increased two years later, while the level of FSH and LH decreased to normal and below the lower limit, respectively. It prevented the estradiol from increasing too much, and no physical changes occurred. The abnormal level of gonadal hormone in our case was probably due to the existence of the extra X chromosome and the expression of genes which had escaped X-inactivation. However, continued monitoring into adolescence will be required for evaluation of hormone levels and pubertal development.
Some studies reported that 50% of 47,XXX females have delayed motor development and poor language skills[ 5 ]. In our case, 17 months of age was the time first words were pronounced and first steps taken, showing a slight delay from the normal population. The result of Gesell developmental schedule demonstrated minor development delay on motor and language skills. These characteristics were consistent with the clinical features in previous reports.
Our case provides a rare example of 47,XXX associated with short stature, elevated level of estradiol and decreased IGF-1 level. Therefore, clinicians should be aware of possible association between triple X and short stature. Further study on the correlation and mechanism between 47,XXX and abnormal growth is required.
Acknowledgments
We thank parents of the patient for permitting to publish her data. This work was supported by grants from the Zhejiang Health Bureau Fund (2006QN017).
- Open access
- Published: 25 June 2024
Association of the stress hyperglycemia ratio with coronary artery disease complexity as assessed by the SYNTAX score in patients with acute coronary syndrome
- Sheng Zhao 1 na1 ,
- Zuoxiang Wang 1 na1 ,
- Ping Qing 1 na1 ,
- Minghui Li 1 ,
- Qingrong Liu 2 ,
- Keke Wang 3 ,
- Xiaojin Gao 1 , 4 ,
- Jie Zhao 1 , 4 &
- Yongjian Wu 1 , 4
Diabetology & Metabolic Syndrome volume 16 , Article number: 139 ( 2024 ) Cite this article
104 Accesses
Metrics details
Mounting evidence supports a significant correlation between the stress hyperglycemia ratio (SHR) and both short- and long-term prognoses in patients with acute coronary syndrome (ACS). Nevertheless, research examining the association between the SHR and the complexity of coronary artery disease (CAD) is scarce. Therefore, this study aimed to explore the association between the SHR and CAD complexity, as assessed by the SYNTAX score, in patients with ACS.
A total of 4715 patients diagnosed with ACS were enrolled and divided into five groups according to the quintiles of the SHR. CAD complexity was assessed using the SYNTAX score and categorized as low (≤ 22) or mid/high (> 22) levels. Logistic regression was utilized to examine the association between the SHR and CAD severity (mid-/high SYNTAX score). Restricted cubic spline (RCS) curves were generated to assess the association between the SHR and CAD severity. Subgroup analyses were conducted to stratify outcomes based on age, sex, diabetes mellitus (DM) status, and clinical presentation.
Among the total ACS population, 503 (10.7%) patients had mid/high SYNTAX scores. Logistic regression analysis revealed that the SHR was an independent risk factor for mid/high SYNTAX scores in a U-shaped pattern. After adjusting for confounding variables, Q1 and Q5 demonstrated elevated odds ratios (ORs) relative to the reference category Q3, with ORs of 1.61 (95% CI: 1.19 ∼ 2.19) and 1.68 (95% CI: 1.24 ∼ 2.29), respectively. Moreover, the ORs for Q2 (1.02, 95% CI: 0.73 ∼ 1.42) and Q4 (1.18, 95% CI: 0.85 ∼ 1.63) resembled that of Q3. Compared with the merged Q2-4 group, the ORs were 1.52 (95% CI: 1.21 ∼ 1.92) for Q1 group and 1.58 (95% CI: 1.25 ∼ 2) for the Q5 group. Subgroup analysis revealed that the U-shaped association between the SHR and mid/high SYNTAX score was attenuated in DM patients (P for interaction = 0.045).
Conclusions
There were U-shaped associations between the SHR and CAD complexity in ACS patients, with an SHR ranging from 0.68 to 0.875 indicating a relatively lower OR for mid/high SYNTAX scores. Further studies are necessary to both evaluate the predictive value of the SHR in ACS patients and explore the underlying mechanisms of the observed U-shaped associations.
The complexity of coronary artery disease (CAD) directly correlates with its severity and is linked to adverse clinical outcomes [ 1 , 2 ]. The Synergy between the PCI with TAXUS™ and Cardiac Surgery (SYNTAX) score is a widely used angiographic tool for grading the complexity of CAD and helps in decision-making between coronary artery bypass grafting surgery (CABG) and percutaneous coronary intervention (PCI) for patients with complex CAD [ 1 , 3 , 4 , 5 , 6 ]. Patients with intermediate to high SYNTAX scores (> 22) face a heightened risk of major adverse cardiovascular events (MACEs) and are better candidates for CABG [ 1 , 4 , 5 , 7 , 8 ]. Diabetes and blood glucose levels are closely linked to CAD development as traditional risk factors. However, the direct correlation between acute and chronic blood glucose markers and the SYNTAX score remains debating [ 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 ].
The stress hyperglycemia ratio (SHR) is a novel marker intended to reflect genuine glucose status and estimate relative hyperglycemia [ 16 , 17 ]. Increasing evidence supports a significant association between the SHR and short- and long-term adverse cardiovascular outcomes in patients with acute coronary syndrome (ACS) [ 18 , 19 ]. A recent study investigated the association between this ratio and CAD severity, revealing a noteworthy correlation between the SHR and the incidence of multivessel CAD (MVD) [ 20 ]. However, there is currently no research examining the relationship between the SHR and CAD complexity in ACS patients using the more comprehensive SYNTAX score as an indicator of CAD complexity.
Therefore, the aim of this study was to investigate the complexity of CAD across the SHR continuum and assess the association between the SHR and the SYNTAX score in patients with ACS.
Study design and population
This study was a prospective, observational cohort study conducted at Fuwai Hospital, National Center for Cardiovascular Diseases. The study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Fuwai Hospital Ethics Review Committee. All participants provided informed consent prior to enrollment. From January 1, 2013, to December 31, 2013, a total of 10,724 patients who underwent percutaneous coronary intervention (PCI) at Fuwai Hospital were consecutively screened. The inclusion criterion was patients who presented with acute coronary syndrome (ACS). The following exclusion criteria were used: (1) had an invalid SYNTAX score, (2) had a previous PCI or CABG history, (3) lacked crucial laboratory data (admission glucose or glycated hemoglobin A1c [HbA1c]), (4) were < 18 years old or > 80 years old, (5) had an eGFR < 30 ml/min/1.73 m2 , and (6) had other cooccurring diseases in the acute phase. Ultimately, 4715 ACS patients were successfully enrolled and divided into five groups based on quintiles. The detailed process of population enrollment is depicted in Fig. 1 .

Restricted cubic splines for the odds ratio of mid/high SYNTAX score. Adjusted for age, sex, body mass index, current smoker, DM, hypertension, dyslipidemia, previous MI, previous stroke, PVD, eGFR, TG, HDL-C, LDL-C, Lp (a), hs-CRP, uric acid, LVEF < 40% and clinical presentation. Abbreviations as shown in Table 1
Data collection and definitions
We collected baseline demographic and clinical data prospectively for all patients. Demographic information included age, sex, BMI, comorbidities, smoking status, and prior history of myocardial infarction (MI). Clinical data included clinical presentation, laboratory test results, auxiliary examination findings and angiographic characteristics. Glycemic status upon admission was assessed using the LABOSPECT 008 system (Hitachi, Tokyo, Japan), and the glycated hemoglobin (HbA1c) level was determined using high-performance liquid chromatography (G8; TOSOH, Tokyo, Japan). Body mass index (BMI) was determined by dividing weight (in kilograms) by the square of height (in meters). The estimated glomerular filtration rate (eGFR) was assessed using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [ 21 ]. Following the completion of coronary angiography (CAG), the characteristics of coronary disease, including the number of stenotic vessels, unusual types of coronary stenosis, and SYNTAX score, were assessed by two coronary intervention specialists [ 3 ].
The SHR was calculated using the following formula: ABG (mmol/l)/[1.59×HbA1c (%) − 2.59] [ 16 , 17 ]. Acute coronary syndrome (ACS) was defined as unstable angina (UA), non-ST segment elevation myocardial infarction (NSTEMI), or ST segment elevation myocardial infarction (STEMI) [ 22 ]. Diabetes status was documented for patients with a prior diagnosis of diabetes, current or previous use of oral hypoglycemic drugs or insulin, or HbA1c levels > 6.5%. CAD was defined as the presence of at least one major coronary artery with ≥ 50% stenosis confirmed by CAG, including the left anterior descending, left circumflex, and right coronary arteries. The severity of CAD was assessed using the SYNTAX score, categorizing patients into two groups based on their scores: the low SYNTAX score group with scores ≤ 22 and the mid/high SYNTAX score group with scores > 22. Participants with one major coronary artery with ≥ 50% stenosis were categorized as having single-vessel CAD, while those with stenosis in more than two coronary arteries were classified as having multivessel CAD. Additionally, left main (LM) disease was defined as > 50% stenosis in the left main coronary artery, which also qualifies as multivessel CAD.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD) or as the median and interquartile range (IQR), while categorical variables are presented as numbers and percentages. Statistical analyses included the χ2 test for comparing categorical variables and the t test, analysis of variance, Mann–Whitney U test, or Kruskal–Wallis H test for continuous variables. Logistic regression was utilized to examine the association between the SHR and CAD severity (mid/high SYNTAX score), with odds ratios (ORs) and corresponding 95% confidence intervals (CIs) calculated. Restricted cubic spline (RCS) curves were generated to assess the association between the SHR and CAD severity. Furthermore, subgroup analyses were conducted to stratify outcomes based on age, sex, diabetes mellitus (DM), and clinical presentation. These analyses were performed using comprehensive regression models adjusted for potential confounders. All the statistical analyses were performed using R version 4.3.0 software (R Foundation for Statistical Computing, Vienna, Austria), with statistical significance set at a P value < 0.05.
Baseline characteristics
A total of 4715 patients with ACS were included in our study. The mean age was 57.88 ± 10.26 years, with 3542 (75.1%) being male. The mean body mass index (BMI) was 25.83 ± 3.28 kg/m 2 . Among the enrolled patients, 3267 (69.3%) presented with unstable angina (UA), while 1418 (30.7%) were diagnosed with acute myocardial infarction (AMI). Hypertension was prevalent in 2997 (63.6%) patients, and hyperlipidemia was diagnosed in 3017 (64.0%) patients. Additionally, 1909 (40.5%) patients had diabetes, and 57.7% were current smokers. The mean SYNTAX score was 11.81 ± 7.73, with 10.7% of patients having a mid/high SYNTAX score (> 22). Patients were stratified into five groups based on the SHR: the Q1 group (SHR ≤ 0.68), n = 942; the Q2 group (0.68 < SHR ≤ 0.737), n = 944; the Q3 group (0.737 < SHR ≤ 0.795), n = 942; the Q4 group (0.795 < SHR ≤ 0.875), n = 944; and the Q5 group (SHR > 0.875), n = 943.
Significant differences were observed across different SHR groups concerning several demographic and clinical parameters. These included age, sex, BMI, systolic blood pressure (SBP), incidence of diabetes mellitus (DM), and history of previous myocardial infarction (MI) and stroke. Furthermore, noteworthy variations were evident in laboratory parameters, including triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein (a) [Lp(a)], high-sensitivity C-reactive protein (Hs-CRP), albumin (ALB), admission blood glucose (ABG), glycated hemoglobin (HbA1c), hemoglobin levels, and uric acid levels. Additionally, angiographic characteristics such as left main (LM) disease, triple-vessel disease (TVD), chronic total occlusion (CTO) disease, and SYNTAX score were significantly different across SHRs. Across Q1 to Q5, both age and the proportion of females decreased gradually. Q5 demonstrated a notably greater incidence of previous MI than did the other quintiles. Conversely, the prevalence of DM in Q2, Q3, and Q4 was lower than that in Q1 and Q5. The incidence of AMI increased progressively from Q1 to Q5, while that of UA decreased. Laboratory parameters such as TG, TC, HDL-C, LDL-C, hs-CRP, ALB, and hemoglobin steadily increased from Q1 to Q5. ABG and HBA1c showed contrasting trends, with ABG increasing and HBA1c decreasing. For most angiographic characteristics, the proportion of complex lesions in Q2, Q3, and Q4 was lower than that in Q1 and Q5, resulting in similar distribution trends observed for the SYNTAX scores. The detailed baseline characteristics are presented in Table 1 .
Associations between the SHR and CAD complexity assessed by the SYNTAX score
Among the total ACS population, 503 (10.7%) patients had mid/high SYNTAX scores (> 22). Logistic regression analyses revealed a significant U-shaped association between the SHR and mid/high SYNTAX score in ACS patients, as shown in Table 2 . According to the unadjusted model, Q1 and Q5 exhibited greater odds ratios (ORs) than Q3 (reference), with ORs of 1.76 (95% CI: 1.3 ∼ 2.38) and 1.86 (95% CI: 1.4 ∼ 2.54), respectively. Moreover, the ORs of Q2 (1.06, 95% CI: 0.76 ∼ 1.46) and Q4 (1.16, 95% CI: 0.84 ∼ 1.6) were similar to those of Q3, with no significant differences observed. This U-shaped association persists consistently in both Model 1 (the basic model) and Model 2 (the comprehensive model). Patients with intermediate SHR levels (Q2, Q3 and Q4) exhibited the lowest odds of risk, with the risk increasing progressively in other groups. After adjusting for all confounding demographic and clinical variables, the ORs for Q1 and Q5 compared to Q3 were 1.61 (95% CI: 1.19 ∼ 2.19) and 1.68 (95% CI: 1.24 ∼ 2.29), respectively. Additionally, no significant differences were detected among Q2, Q3, and Q4. Given the relatively consistent low ORs for mid/high SYNTAX scores in the Q2, Q3, and Q4 groups, we merged them into one group (Q2-4) and reanalyzed them compared to Q1 and Q5. Similar risk associations were identified. Compared to Q2-4, the ORs were 1.52 (95% CI: 1.2 ∼ 1.92) for the Q1 group and 1.58 (95% CI: 1.25 ∼ 2) for the Q5 group.
To further explore the association between the SHR and CAD complexity, we conducted an RCS analysis, as depicted in Fig. 2 . In line with the aforementioned results, the RCS analysis validated a U-shaped pattern in the relationship between the SHR and mid/high SYNTAX scores. The lowest odds ratio (OR) was identified at a nadir SHR of 0.74, corresponding to the 0.737 ∼ 0.795 interval (Q3, reference group).
Subgroup analysis of the association between the SHR and CAD complexity
Table 3 displays the findings of a stratified analysis examining the association between the SHR and CAD complexity, considering factors such as age, sex, BMI, DM, and clinical presentation. This U-shaped association was consistently observed in various subgroups. This distinct pattern underscores the critical importance of the nadir interval at 0.68 ∼ 0.875, wherein any deviation from this optimal SHR value range (whether increased or decreased) is consistently correlated with an elevated risk of a mid/high SYNTAX score. Significant interactions between SHR and DM (P for interaction = 0.045) were identified. Among patients without DM, the U-shaped association between the SHR and mid/high SYNTAX score was stronger than that between the SHR and DM. This difference was primarily evident in the lowest SHR (Q1), where the OR for Q1 in the non-DM group significantly increased to 2.07 (95% CI: 1.51–2.84) compared to that in the Q2-4 group, while the OR in the DM group showed only an increasing trend to 1.17 (95% CI: 0.84–1.64). At the highest SHR (Q5), there were consistently elevated ORs compared to those in Q2-4, both in the non-DM group and in the DM group, with values of 1.69 (95% CI: 1.21–2.35) and 1.59 (95% CI: 1.15–2.18), respectively.
We investigated the correlation between the SHR and CAD complexity in ACS patients. Two main findings were identified. First, the SHR was independently associated with mid/high SYNTAX scores in ACS patients. Second, there was a U-shaped pattern in the associations, with ORs for mid/high SYNTAX scores significantly increasing when the SHR exceeded 0.875 or fell below 0.68.
CAD complexity directly reflects CAD severity, with extensive and intricate CAD consistently linked to adverse clinical outcomes in various studies [ 1 , 2 ]. Several assessment methods and scoring tools have emerged to evaluate CAD complexity. Among them, the SYNTAX score stands out as a comprehensive tool that integrates multiple scoring systems to evaluate both the extent and anatomical complexity of CAD [ 3 ]. Its value lies in two aspects. First, in predicting CAD outcomes, the BARI-2D trial showed that a mid/high SYNTAX score (≥ 23) predicted higher rates of major cardiovascular events (MACEs) [ 1 ]. Second, it guides treatment decisions: several studies have indicated that higher SYNTAX scores predict better outcomes with CABG than with PCI [ 23 , 24 , 25 ]. Current clinical guidelines for coronary artery revascularization advocate employing the SYNTAX score for evaluating CAD complexity and directing optimal revascularization strategies [ 4 , 5 ]. In essence, investigating the risk factors correlated with the SYNTAX score is highly important for both mechanistic research and the clinical diagnosis and treatment of CAD. However, current research in this area predominantly focuses on stable CAD, with relatively limited studies conducted in the ACS population.
The SHR is a metric that evaluates the ratio of stress-induced hyperglycemia to chronic glycemia using a specific formula, offering insights into the severity of critical illness [ 16 , 17 ]. Extensive literature suggests that stress hyperglycemia, caused by neurohormonal dysregulation and inflammatory responses, is common in acutely critically ill patients and acts as a marker of disease severity, including in ACS patients [ 26 , 27 ]. Stress hyperglycemia reflects ACS severity and may worsen acute cardiac pathology through various mechanisms, including inflammation activation, microcirculatory obstruction, and platelet dysfunction [ 27 , 28 , 29 ], all of which are involved in ACS progression and development based on CAD pathology and are also implicated in the progression and development of ACS based on CAD pathology [ 30 , 31 ]. In addition, as a traditional risk factor for CAD, chronic hyperglycemia can adversely impact inflammation, cellular injury, apoptosis, ischemic myocardial metabolism, endothelial function, the coagulation cascade, and platelet aggregation [ 32 ]. As CAD progresses is a chronic condition, HbA1c serves as a sensitive indicator of chronic dysglycemia and is strongly correlated with the SYNTAX score [ 13 , 33 , 34 ]. In summary, the interplay between ACS and acute and chronic hyperglycemia is complex. The integration of HbA1c and acute blood glucose levels by the SHR provides a more accurate assessment of patients’ true blood glucose status, offering a novel research perspective on CAD complexity in ACS patients [ 16 , 17 ]. Currently, there is limited literature on the relationship between the SHR and CAD complexity. Zhang Y et al. addressed this gap by assessing CAD complexity using single-vessel (SVD) and multivessel disease (MVD) [ 20 ]. This study retrospectively enrolled 987 patients who underwent CAG and revealed that the SHR was significantly correlated with the risk of MVD. Logistic regression analysis revealed that the risk of MVD was 1.939-fold greater in the T2 group and 1.860-fold greater in the T3 group than in the T1 group. Regrettably, the study had a relatively small sample size and did not explicitly specify whether the population under investigation consisted of individuals with ACS. Furthermore, it is noteworthy that within the MVD group, considerable heterogeneity in CAD complexity still existed [ 3 ]. Our study revealed an independent link between the SHR and complex coronary anatomical lesions evaluated by the SYNTAX score in ACS patients, representing a novel finding in a large-scale cohort.
To the best of our knowledge, this study may be the first to demonstrate a U-shaped correlation between the SHR and mid/high SYNTAX score in ACS patients. We consecutively enrolled ACS patients from a large prospective cohort in Asia. Our findings indicated significantly greater ORs of mid/high SYNTAX scores in the lowest and highest SHR quintiles than in the median quintile. Subsequent RCS analysis revealed a U-shaped correlation with all confounding factors adjusted. Notably, the ORs for mid/high SYNTAX scores remained relatively low in the middle three quintiles (Q2, Q3 and Q4), with no statistically significant differences between them, forming the bottom of the U-shape. The specific mechanism underlying this U-shaped association remains unclear. Indeed, there is growing evidence of this analogous U-shaped phenomenon in both short-term and long-term prognostic studies of ACS, as well as in heart failure and other critical illnesses, implying a widespread association between excessively high or low SHR and adverse effects on the human body [ 19 , 35 , 36 , 37 , 38 , 39 ]. Yang J et al. identified U-shaped associations between the SHR and the rates of major adverse cardiac and cerebrovascular events (MACCEs) as well as major adverse cardiac events (MACEs) at the 2-year follow-up in ACS patients treated with DES implantation, with the inflection point determined to be 0.78 [ 40 ]. The mechanism underlying this phenomenon may involve moderate stress-induced hyperglycemia, which is a normal physiological response and a protective mechanism during ACS [ 41 ]. Both the SYNTAX score and the SHR are related to ACS prognosis, and they may share certain causal mechanisms, thus exhibiting similar effects [ 1 , 8 ]. Our study suggested that a range of 0.68 to 0.875 may indicate a “moderate stress level”, as levels above or below this range correlate with increased CAD severity. Therefore, an SHR > 0.875 may truly indicate stress-induced hyperglycemia. This condition, coupled with higher CAD complexity, leads to more severe ACS conditions, as evidenced by the significantly increased proportion of MI in Q5 in our baseline data. In this context, higher SHR values predominantly serve as a reflection of CAD severity rather than a causative factor. Furthermore, our findings suggest that while higher SHR values reflect CAD severity, lower SHR values may contribute to increased CAD complexity as an etiological factor. This is evidenced by our observation of a greater proportion of diabetic patients with elevated HbA1c levels and lower acute blood glucose levels in both Q1 and Q5 of our baseline data. An SHR < 0.68 indicates a combination of chronic hyperglycemia (high HbA1c) and currently lower blood glucose (low acute blood glucose). Previous studies have extensively established chronic hyperglycemia as a risk factor for CAD severity [ 12 , 13 , 15 , 33 , 34 ], and additional studies have shown that oscillating glucose can have more detrimental effects than constant high glucose on endothelial function and oxidative stress, suggesting that recurrent low acute blood glucose also contributes to CAD progression [ 42 , 43 ]. Therefore, in all scenarios where the linear correlation between ABG and HbA1c deviates, the OR of the mid/high SYNTAX score increases through different mechanisms. ACS is an acute condition that requires prompt assessment and treatment planning. The U-shaped relationship between the SHR and SYNTAX can facilitate rapid and efficient assessment of disease conditions. However, there is limited research in this area, with inconsistent diagnostic thresholds. Our study also differs from previous short- and long-term prognosis studies in terms of thresholds. Future research should focus on larger-scale prospective cohort studies to determine diagnostic thresholds for the SHR and explore its predictive value for CAD complexity in ACS patients.
Furthermore, subgroup analyses generally aligned with overall population trends, except for the subgroup with DM, where their interaction exhibited a marginally significant difference (P for interaction = 0.045). Specifically, the U-shaped associations of the SHR with the mid/high SYNTAX score were attenuated in the DM subgroup. This difference primarily occurred within the SHR < 0.68 interval, where the OR trend in the DM group was weaker than that in the non-DM group, while within the SHR > 0.875 interval, both groups showed consistent OR trends. This phenomenon is somewhat consistent with the findings of a previous study. Zhang Y et al. reported that SHR (as both a continuous variable and categorized into 3 tertiles) was significantly correlated with an increased risk of MVD in the pre-DM and DM groups, but no consistent linear increase was observed in the normal glucose regulation (NGR) group [ 20 ]. However, this study seemingly did not further explore its potential nonlinear trends. Additionally, several studies have demonstrated a substantial correlation between HbA1c levels and the SYNTAX score, even among individuals without DM [ 12 , 13 , 15 , 33 , 34 ]. For example, Garg N et al. found that the HbA1c level had a linear incremental association with CAD in 1141 nondiabetic individuals, and the HbA1c level was also independently correlated with disease severity and higher SYNTAX scores. These findings could explain the U-shaped relationship observed in the non-DM subgroup in the present study. Moreover, Yan Y et al. suggested that the positive association between HbA1c and the SYNTAX score might be notably attenuated by prior medication history when included in the adjusted model [ 14 ]. Given the absence of detailed medication history data (particularly regarding antidiabetic therapy) in our study, disparities among DM and non-DM subgroups may result from unadjusted factors such as prehospital medication therapy or limitations in sample size. In other subgroups, no significant interaction was found, including the sex subgroup of particular interest. The shape of the curves for both sexes is consistent with the overall curve (Figure S1 ). Although the P for nonlinearity is 0.004 for the male subgroup and 0.521 for the female subgroup, interaction analysis suggests no significant interaction by sex (P for interaction = 0.732). We believe that the differences observed between the sex subgroups may be due to the smaller sample size.
Strengths and limitations
This study is the first to investigate the role of the SHR in assessing CAD complexity and severity using the SYNTAX score in patients with ACS. Furthermore, for the first time, we explored the nonlinear relationship between the SHR and CAD complexity, suggesting a U-shaped association between the SHR and mid/high SYNTAX score in ACS patients. However, there are limitations to our study. First, this study is based on an Asian cohort, and the extrapolation of its conclusions to other racial groups requires further validation. Second, the data were obtained from a PCI cohort, which excluded patients with low CAD complexity not requiring revascularization and those with high CAD complexity undergoing CABG, potentially introducing bias. Future research should validate our results in more comprehensive CAG cohorts. Third, we cannot completely rule out the possibility of unrecorded or unknown confounding factors, such as prehospital medication history, which may influence the associations observed in this study. Furthermore, this study discovered distinct distribution characteristics, such as gender factors in the variation of SHR. Further research can delve into this aspect to explore more directions in CAD studies.
There were U-shaped associations between the SHR and CAD complexity, as assessed by the SYNTAX score, in ACS patients, with an SHR ranging from 0.68 to 0.875 indicating a relatively lower OR for mid/high SYNTAX scores. Further studies are necessary to both evaluate the predictive value of the SHR in ACS patients and explore the underlying mechanisms of the observed U-shaped associations.
Data availability
The datasets used in the study are available from the corresponding author upon reasonable request.

Abbreviations
blood glucose
body mass index
chronic obstructive pulmonary disease
chronic total occlusion
diastolic blood pressure
estimated glomerular filtration rate
high-sensitivity C-reactive protein
high-density lipoprotein
low-density lipoprotein
left main disease
left ventricular ejection fraction
myocardial infarction
multivessel disease
non-ST-segment elevation myocardial infarction
peripheral vascular disease
systolic blood pressure
ST-segment elevation myocardial infarction
triglyceride
total cholesterol
unstable angina
Synergy between PCI with TAXUS™ and Cardiac Surgery
Ikeno F, Brooks MM, Nakagawa K, Kim M-K, Kaneda H, Mitsutake Y, et al. SYNTAX score and long-term outcomes: the BARI-2D trial. J Am Coll Cardiol. 2017;69(4):395–403.
Article PubMed Google Scholar
Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28(14):1709–16.
Sianos G, Morel M-A, Kappetein AP, Morice M-C, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2005;1(2):219–27.
Google Scholar
Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2).
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J Am Coll Cardiol. 2022;79(2):197–215.
Kundu A, Sardar P, O’Day K, Chatterjee S, Owan T, Dawn Abbott J. SYNTAX score and outcomes of Coronary Revascularization in Diabetic patients. Curr Cardiol Rep. 2018;20(5):28.
De Servi S, Crimi G, Calabrò P, Piscione F, Cattaneo M, Maffeo D et al. Relationship between diabetes, platelet reactivity, and the SYNTAX score to one-year clinical outcome in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. EuroIntervention: Journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016;12(3):312–8.
Yang C-H, Hsieh M-J, Chen C-C, Chang S-H, Wang C-Y, Lee C-H, et al. SYNTAX score: an independent predictor of long-term cardiac mortality in patients with acute ST-elevation myocardial infarction. Coron Artery Dis. 2012;23(7):445–9.
Açar B, Ozeke O, Karakurt M, Ozen Y, Özbay MB, Unal S, et al. Association of Prediabetes with higher coronary atherosclerotic burden among patients with First Diagnosed Acute Coronary Syndrome. Angiology. 2019;70(2):174–80.
Ul-Haque I, Ud Deen Z, Shafique S, Ur Rehman SI, Zaman M, Basalat ST, et al. The role of Glycated Hemoglobin A1c in determining the severity of Coronary Artery Disease in Diabetic and non-diabetic subjects in Karachi. Cureus. 2019;11(6):e4982.
PubMed PubMed Central Google Scholar
Habib S, Ullah SZ, Saghir T, Syed Muhammad A, Ud Deen Z, Naseeb K, et al. The Association between Hemoglobin A1c and the severity of coronary artery disease in non-diabetic patients with Acute Coronary Syndrome. Cureus. 2020;12(1):e6631.
Yang J, Zhou Y, Zhang T, Lin X, Ma X, Wang Z, et al. Fasting blood glucose and HbA1c correlate with severity of coronary artery disease in elective PCI patients with HbA1c 5.7% to 6.4. Angiology. 2020;71(2):167–74.
Article CAS PubMed Google Scholar
Ikeda N, Iijima R, Hara H, Moroi M, Nakamura M, Sugi K. Glycated hemoglobin is associated with the complexity of coronary artery disease, even in non-diabetic adults. J Atheroscler Thromb. 2012;19(12):1066–72.
Yan Y, Gao R, Zhang S, Gao Z, Chen A, Wang J, et al. Hemoglobin A1c and angiographic severity with coronary artery disease: a cross-sectional study. Int J Gen Med. 2022;15:1485–95.
Article CAS PubMed PubMed Central Google Scholar
Arbel Y, Zlotnik M, Halkin A, Havakuk O, Berliner S, Herz I, et al. Admission glucose, fasting glucose, HbA1c levels and the SYNTAX score in non-diabetic patients undergoing coronary angiography. Clin Res Cardiol. 2014;103(3):223–7.
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490–7.
Xu W, Yang Y-M, Zhu J, Wu S, Wang J, Zhang H, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. 2022;21(1):48.
Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with Acute Coronary Syndrome: insight from a large cohort study in Asia. Diabetes Care. 2022;45(4):947–56.
Zhang Y, Song H, Bai J, Xiu J, Wu G, Zhang L, et al. Association between the stress hyperglycemia ratio and severity of coronary artery disease under different glucose metabolic states. Cardiovasc Diabetol. 2023;22(1):29.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Article PubMed PubMed Central Google Scholar
Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–826.
Thuijs DJFM, Kappetein AP, Serruys PW, Mohr F-W, Morice M-C, Mack MJ, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet (London England). 2019;394(10206):1325–34.
Head SJ, Milojevic M, Daemen J, Ahn J-M, Boersma E, Christiansen EH, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet (London England). 2018;391(10124):939–48.
Mohr FW, Morice M-C, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet (London England). 2013;381(9867):629–38.
Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet (London England). 2009;373(9677):1798–807.
Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–10.
Jensen CJ, Eberle HC, Nassenstein K, Schlosser T, Farazandeh M, Naber CK, et al. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin Res Cardiol. 2011;100(8):649–59.
Angeli F, Reboldi G, Poltronieri C, Lazzari L, Sordi M, Garofoli M, et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. 2015;9(6):412–24.
Crea F, Libby P. Acute Coronary syndromes: the Way Forward from mechanisms to Precision Treatment. Circulation. 2017;136(12):1155–66.
Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol. 2020;17(4):229–41.
Undas A, Wiek I, Stêpien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008;31(8):1590–5.
Garg N, Moorthy N, Kapoor A, Tewari S, Kumar S, Sinha A, et al. Hemoglobin A(1c) in nondiabetic patients: an independent predictor of coronary artery disease and its severity. Mayo Clin Proc. 2014;89(7):908–16.
Basman C, Fishman SL, Avtanski D, Rashid U, Kodra A, Chen K, et al. Glycosylated hemoglobin, but not advanced glycation end products, predicts severity of coronary artery disease in patients with or without diabetes. Metabol Open. 2020;7:100050.
Karakasis P, Stalikas N, Patoulias D, Pamporis K, Karagiannidis E, Sagris M et al. Prognostic value of stress hyperglycemia ratio in patients with acute myocardial infarction: a systematic review with bayesian and frequentist meta-analysis. Trends Cardiovasc Med. 2023.
Li L, Ding L, Zheng L, Wu L, Hu Z, Liu L, et al. U-shaped association between stress hyperglycemia ratio and risk of all-cause mortality in cardiac ICU. Diabetes Metab Syndr. 2023;18(1):102932.
Zhou Y, Liu L, Huang H, Li N, He J, Yao H, et al. Stress hyperglycemia ratio and in-hospital prognosis in non-surgical patients with heart failure and type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):290.
Li L, Zhao M, Zhang Z, Zhou L, Zhang Z, Xiong Y, et al. Prognostic significance of the stress hyperglycemia ratio in critically ill patients. Cardiovasc Diabetol. 2023;22(1):275.
Zhou Q, Yang J, Wang W, Shao C, Hua X, Tang Y-D. The impact of the stress hyperglycemia ratio on mortality and rehospitalization rate in patients with acute decompensated heart failure and diabetes. Cardiovasc Diabetol. 2023;22(1):189.
Cunha FM, Carreira M, Ferreira I, Bettencourt P, Lourenço P. Low stress hyperglycemia ratio predicts worse prognosis in diabetic acute heart failure patients. Rev Port Cardiol. 2023;42(5):433–41.
Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Watanabe M, Kawai Y, Kitayama M, Akao H, Motoyama A, Wakasa M, et al. Diurnal glycemic fluctuation is associated with severity of coronary artery disease in prediabetic patients: possible role of nitrotyrosine and glyceraldehyde-derived advanced glycation end products. J Cardiol. 2017;69(4):625–31.
Download references
Acknowledgements
Thanks to home-for-researchers.com for language editing assistance.
This work was supported by the Continuous Improvement Research Project on Evidence-based Healthcare Quality Management (YLZXXZH006) and the China University Industry Research Institute Innovation Fund-Next-generation Information Technology Innovation Project (2022IT057).
Author information
Sheng Zhao, Zuoxiang Wang and Ping Qing contributed equally to this work.
Authors and Affiliations
Fuwai Hospital, National Centre for Cardiovascular Diseases, National Clinical Research Centre for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Sheng Zhao, Zuoxiang Wang, Ping Qing, Minghui Li, Xiaojin Gao, Jie Zhao & Yongjian Wu
Department of Cardiology, Beijing Aerospace General Hospital, Beijing, China
Qingrong Liu
Department of Cardiology, the Second Medical Centre, Chinese PLA General Hospital, Beijing, China
Fuwai Hospital, National Centre for Cardiovascular Diseases, National Clinical Research Centre for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, No.167 North Lishi Road, Xicheng District, Beijing, 100037, China
Xiaojin Gao, Jie Zhao & Yongjian Wu
You can also search for this author in PubMed Google Scholar
Contributions
SZ, ZW and PQ: study design, data analysis, manuscript writing and editing. ML: statistical support for the data analysis; QL and KW: data management and analysis; JZ, XG and YW: study design and manuscript revision.
Corresponding authors
Correspondence to Xiaojin Gao , Jie Zhao or Yongjian Wu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Fuwai Hospital Ethics Review Committee and strictly adhered to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13098_2024_1382_MOESM1_ESM.docx
Supplementary Material 1: Additional file 1: Table S1. The variance inflation factor (VIF) and tolerance of covariates; Additional file 1: Figure S1. Restricted cubic splines for the odds ratio of mid/high SYNTAX score in males (A) and females (B). Adjusted for age, body mass index, current smoker, DM, hypertension, dyslipidemia, previous MI, previous stroke, PVD, eGFR, TG, HDL-C, LDL-C, Lp(a), hs-CRP, uric acid, LVEF < 40%, and clinical presentation. Abbreviations as shown in Table 1 .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhao, S., Wang, Z., Qing, P. et al. Association of the stress hyperglycemia ratio with coronary artery disease complexity as assessed by the SYNTAX score in patients with acute coronary syndrome. Diabetol Metab Syndr 16 , 139 (2024). https://doi.org/10.1186/s13098-024-01382-0
Download citation
Received : 15 April 2024
Accepted : 13 June 2024
Published : 25 June 2024
DOI : https://doi.org/10.1186/s13098-024-01382-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stress hyperglycemia ratio
- Coronary artery disease
- Acute coronary syndrome
- Diabetes mellitus
Diabetology & Metabolic Syndrome
ISSN: 1758-5996
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 24 June 2024
Placebo effects in randomized trials of pharmacological and neurostimulation interventions for mental disorders: An umbrella review
- Nathan T. M. Huneke ORCID: orcid.org/0000-0001-5981-6707 1 , 2 ,
- Jay Amin ORCID: orcid.org/0000-0003-3792-0428 1 , 2 ,
- David S. Baldwin 1 , 2 , 3 ,
- Alessio Bellato 4 , 5 ,
- Valerie Brandt ORCID: orcid.org/0000-0002-3208-2659 5 , 6 ,
- Samuel R. Chamberlain 1 , 2 ,
- Christoph U. Correll ORCID: orcid.org/0000-0002-7254-5646 7 , 8 , 9 , 10 ,
- Luis Eudave 11 ,
- Matthew Garner 1 , 5 , 12 ,
- Corentin J. Gosling 5 , 13 , 14 ,
- Catherine M. Hill 1 , 15 ,
- Ruihua Hou 1 ,
- Oliver D. Howes ORCID: orcid.org/0000-0002-2928-1972 16 , 17 , 18 ,
- Konstantinos Ioannidis 1 , 2 ,
- Ole Köhler-Forsberg 19 , 20 ,
- Lucia Marzulli 21 ,
- Claire Reed ORCID: orcid.org/0000-0003-1385-4729 5 ,
- Julia M. A. Sinclair 1 ,
- Satneet Singh 2 ,
- Marco Solmi ORCID: orcid.org/0000-0003-4877-7233 5 , 22 , 23 , 24 , 25 na1 &
- Samuele Cortese ORCID: orcid.org/0000-0001-5877-8075 1 , 5 , 26 , 27 , 28 na1
Molecular Psychiatry ( 2024 ) Cite this article
666 Accesses
9 Altmetric
Metrics details
- Drug discovery
- Neuroscience
- Psychiatric disorders
There is a growing literature exploring the placebo response within specific mental disorders, but no overarching quantitative synthesis of this research has analyzed evidence across mental disorders. We carried out an umbrella review of meta-analyses of randomized controlled trials (RCTs) of biological treatments (pharmacotherapy or neurostimulation) for mental disorders. We explored whether placebo effect size differs across distinct disorders, and the correlates of increased placebo effects. Based on a pre-registered protocol, we searched Medline, PsycInfo, EMBASE, and Web of Knowledge up to 23.10.2022 for systematic reviews and/or meta-analyses reporting placebo effect sizes in psychopharmacological or neurostimulation RCTs. Twenty meta-analyses, summarising 1,691 RCTs involving 261,730 patients, were included. Placebo effect size varied, and was large in alcohol use disorder ( g = 0.90, 95% CI [0.70, 1.09]), depression ( g = 1.10, 95% CI [1.06, 1.15]), restless legs syndrome ( g = 1.41, 95% CI [1.25, 1.56]), and generalized anxiety disorder ( d = 1.85, 95% CI [1.61, 2.09]). Placebo effect size was small-to-medium in obsessive-compulsive disorder ( d = 0.32, 95% CI [0.22, 0.41]), primary insomnia ( g = 0.35, 95% CI [0.28, 0.42]), and schizophrenia spectrum disorders (standardized mean change = 0.33, 95% CI [0.22, 0.44]). Correlates of larger placebo response in multiple mental disorders included later publication year (opposite finding for ADHD), younger age, more trial sites, larger sample size, increased baseline severity, and larger active treatment effect size. Most (18 of 20) meta-analyses were judged ‘low’ quality as per AMSTAR-2. Placebo effect sizes varied substantially across mental disorders. Future research should explore the sources of this variation. We identified important gaps in the literature, with no eligible systematic reviews/meta-analyses of placebo response in stress-related disorders, eating disorders, behavioural addictions, or bipolar mania.
Similar content being viewed by others
Effects of open-label placebos in clinical trials: a systematic review and meta-analysis

A transdiagnostic meta-analysis of acute augmentations to psychological therapy
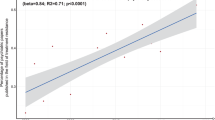
Treatment resistance in psychiatry: state of the art and new directions
Introduction.
A placebo is an ‘inactive’ substance or ‘sham’ technique that is used as a control for assessing the efficacy of an active treatment [ 1 ]. However, study participants in a placebo control group may experience considerable symptom improvements - a ‘placebo response’ [ 1 , 2 , 3 ]. Statistical artifacts or non-specific effects account for some of the placebo response. For example, many individuals seek treatment and are enrolled in clinical trials while their symptoms are at their worst. Their symptoms will gradually return to their usual severity (‘regression to the mean’), giving the appearance of a placebo response [ 4 ]. Further, it has been suggested that the placebo response is exacerbated due to unreliable ratings as well as baseline symptom severity inflation if raters are aware of severity criteria for entry to a trial [ 5 , 6 ]. Other potential sources of apparent placebo responses include sampling biases caused by the withdrawal of the least improved patients in the placebo arm, non-specific beneficial effects resulting from interactions with staff delivering the trial, environmental effects due to inpatient care during placebo-controlled trials, or other unaccounted for factors, such as dietary or exercise changes during the trial [ 7 , 8 , 9 ]. Nonetheless, there is evidence that placebo administration results in ‘true’ - or non-artefactual - placebo effects, that is, identifiable changes in biological systems [ 1 , 10 , 11 ]. For example, placebo administration is capable of causing immunosuppression [ 12 , 13 ], placebo effects in Parkinson’s disease are driven by striatal dopamine release [ 10 , 14 ], and placebo analgesia is mediated by endogenous opioid release [ 15 , 16 ]. Furthermore, there is evidence that placebo effects in depressive and anxiety disorders are correlated with altered activity in the ventral striatum, orbitofrontal cortex, rostral anterior cingulate cortex, and the default mode network [ 17 ]. The placebo effect size can be increased through the use of verbal suggestions and conditioning procedures, thus suggesting the underlying role of psychological mechanisms including learning and expectations [ 11 , 18 ].
Across age groups, treatment modalities, and diverse mental disorders, biological treatments (pharmacotherapy or neurostimulation) do reduce symptoms [ 19 , 20 , 21 , 22 ], but only a subgroup of patients experience a clinically significant symptom response or enter remission [ 23 , 24 , 25 ]. Furthermore, current medications may also have unfavourable side effects [ 23 , 26 , 27 , 28 , 29 , 30 , 31 ]. Given the high prevalence of mental disorders and their significant socioeconomic burden [ 32 , 33 , 34 ], there is a need to develop more effective and safer psychopharmacologic and neurostimulation treatments. However, in randomized-controlled trials (RCTs), the magnitude of the placebo response may be considerable, which can affect the interpretation of their results [ 35 , 36 , 37 ]. For example, in antipsychotic trials over the past 40 years, placebo response has increased while medication response has remained consistent [ 38 , 39 ]. Consequently, the trial’s ability to statistically differentiate between an active medication and a placebo is diminished [ 40 ]. Indeed, large placebo response rates have been implicated in hindering psychotropic drug development [ 41 , 42 ]. The increased placebo response can also affect larger data synthesis approaches, such as network meta-analysis, in which assumptions about placebo responses (e.g. stability over time) might affect the validity of results [ 43 ].
Improved understanding of participant, trial, and mental disorder-related factors that contribute to placebo response might allow better clinical trial design to separate active treatment from placebo effects. There is a growing body of research, including individual studies and systematic reviews/meta-analyses, examining the placebo response within specific mental disorders [ 35 ]. However, to date, no overarching synthesis of this literature, to detect any similarities or differences across mental disorders, has been published. We therefore carried out an umbrella review of meta-analyses to address this need. We aimed to assess the placebo effect size in RCTs for a range of mental disorders, whether the effect size differs across distinct mental disorders, and identify any correlates of increased placebo effect size or response rate.
The protocol for this systematic umbrella review was pre-registered on the open science framework ( https://osf.io/fxvn4/ ) and published [ 44 ]. Deviations from this protocol, and additions to it, were: eight authors were involved in record screening rather than two; we reported effect sizes pooled across age groups and analyses comparing placebo effect sizes between age groups; and we included a meta-analysis that incorporated trials of dietary supplements as well as medications in autism. For the rationale behind these decisions, see eMethods.
Eight authors (NH, AB, VB, LE, OKF, LM, CR, SS) carried out the systematic review and data extraction independently in pairs. Discrepancies were resolved through consensus or through arbitration by a third reviewer (NH or SCo). We searched, without date or language restrictions, up to 23.10.2022, Medline, PsycInfo, EMBASE + EMBASE Classic, and Web of Knowledge for systematic reviews with or without meta-analyses of RCTs of biological treatments (psychopharmacotherapy or neurostimulation) compared with a placebo or sham treatment in individuals with mental disorders diagnosed according to standardized criteria. The full search strategy is included in eMethods. We also sought systematic reviews of RCTs conducted in patients with sleep-wake disorders, since these disorders are included in the DSM-5 and their core symptoms overlap with those of mental disorders [ 45 ]. We retained systematic reviews with or without meta-analyses that reported within-group changes in symptoms in the placebo arm.
Next, to prevent duplication of data, a matrix containing all eligible systematic reviews/meta-analyses for each category of mental disorder was created. Where there were multiple eligible systematic reviews/meta-analyses for the same disorder and treatment, we preferentially included meta-analyses, and if multiple eligible meta-analyses remained, then we included the one containing the largest number of studies for the same disorder and treatment, in line with recent umbrella reviews [ 46 , 47 ].
Data were extracted by at least two among six reviewers (AB, VB, LE, OKF, CR, SS) independently in pairs via a piloted form. All extracted data were further checked by a third reviewer (NH). See eMethods for a list of extracted data.
Our primary outcome was the pre-post effect size of the placebo/sham related to the condition-specific primary symptom change for each mental disorder. Secondary outcomes included any other reported clinical outcomes in eligible reviews. We report effect sizes calculated within-group from baseline and post-treatment means by meta-analysis authors, including Cohen’s d and Hedges’ g for repeated measures, which account for both mean difference and correlation between paired observations; and standardized mean change, where the average change score is divided by standard deviation of the change scores. We interpreted the effect size in line with the suggestion by Cohen [ 48 ], i.e. small (~0.2), medium (~0.5), or large (~0.8).
In addition, we extracted data regarding potential correlates of increased placebo effect size or response rate (as defined and assessed by the authors of each meta-analysis) in each mental disorder identified through correlation analyses or meta-regression. Where available, results from multivariate analyses were preferred.
The methodological quality of included reviews was assessed by at least two among six reviewers (AB, VB, LE, OKF, NH, CR) independently and in pairs using the AMSTAR-2 tool, a critical appraisal tool that enables reproducible assessments of the conduct of systematic reviews [ 49 ]. The methodological quality of each included review was rated as high, moderate, low, or critically low.
Our initial search identified 6,108 records. After screening titles and abstracts, we obtained and assessed 115 full-text reports (see eResults for a list of articles excluded following full-text assessment, with reasons). Of these, 20 were deemed eligible, and all were systematic reviews with meta-analysis (Fig. 1 ). In total, the 20 included meta-analyses synthesized data from 1,691 RCTs (median 55) involving 261,730 patients (median 5,365). These meta-analyses were published between 2007 and 2022 and involved individuals with the following mental disorders: major depressive disorder (MDD; n = 6) [ 50 , 51 , 52 , 53 , 54 , 55 ], anxiety disorders ( n = 4) [ 55 , 56 , 57 , 58 ], schizophrenia spectrum disorders ( n = 3) [ 38 , 59 , 60 ], alcohol use disorder (AUD; n = 1) [ 61 ], attention-deficit/hyperactivity disorder (ADHD; n = 1) [ 62 ], autism spectrum disorders ( n = 1) [ 63 ], bipolar depression ( n = 1) [ 64 ], intellectual disability ( n = 1) [ 65 ], obsessive-compulsive disorder (OCD; n = 1) [ 66 ], primary insomnia ( n = 1) [ 67 ], and restless legs syndrome (RLS; n = 1) [ 68 ].

Twenty meta-analyses were included.
The methodological quality of the included meta-analyses according to AMSTAR-2 ratings was high in two meta-analyses (ADHD and autism), low in four meta-analyses, and critically low in the remaining 14 meta-analyses (Table 1 ). The most common sources of bias that led to downgrading on the AMSTAR-2 were: no list of excluded full-text articles with reasons ( k = 14), no explicit statement that the protocol was pre-registered ( k = 14), and no assessment of the potential impact of risk of bias in individual studies on the results ( k = 13). The full reasoning behind our AMSTAR-2 ratings is included in eResults.
Our first objective was to determine placebo effect sizes across mental conditions. Data regarding within-group placebo efficacy were reported in sixteen of the included meta-analyses [ 38 , 50 , 52 , 53 , 55 , 56 , 57 , 58 , 60 , 61 , 62 , 63 , 65 , 66 , 67 , 68 ]. Placebo effect sizes for the primary outcomes ranged from 0.23 to 1.85, with a median of 0.64 (Fig. 2 ). Median heterogeneity across meta-analyses was I 2 = 72%, suggesting a generally high percentage of heterogeneity due to true variation across studies.

Dots represent placebo group effect size while triangles represent active effect size. CI confidence interval, MDD major depressive disorder, GAD generalized anxiety disorder, SAD social anxiety disorder, OCD obsessive-compulsive disorder, g Hedges’ g, d Cohen’s d, SMC standardized mean change, NR not reported.
A detailed description of each meta-analysis included for this objective is included in eResults. Here, we report a summary of these results in order of the greatest number of RCT’s and meta-analyses included per disorder. In MDD, a large within-group placebo effect was observed ( g = 1.10, 95% CI [1.06, 1.15]), although active medication had an even larger effect size ( g = 1.49, 95% CI [1.44, 1.53]) [ 50 ]. Similarly, in children and adolescents with MDD, placebo effect size was large ( g = 1.57, 95% CI [1.36, 1.78]), as was serotonergic medication effect size ( g = 1.85, 95% CI [1.70, 2.00]) [ 55 ]. In treatment-resistant MDD, the within-group placebo effect size was smaller than in non-treatment-resistant MDD ( g = 0.89, 95% CI [0.81, 0.98]) [ 52 ]. In neuromodulation trials for MDD, the effect size of sham was g = 0.80 (95% CI [0.65, 0.95]) [ 53 ]. In this meta-analysis, the effect size was larger for non-treatment-resistant ( g = 1.28, 95% CI [0.47, 2.97]) compared to treatment-resistant participants (g = 0.50 95% CI [0.03, 0.99]) [ 53 ]. In adults with anxiety disorders, placebo effect sizes varied across disorders, with a medium effect size in panic disorder ( d = 0.57, 95% CI [0.50, 0.64]) [ 56 ] and large effect sizes in generalized anxiety disorder (GAD) ( d = 1.85, 95% CI [1.61, 2.09]) and social anxiety disorder (SAD) ( d = 0.94, 95% CI [0.77, 1.12]) [ 57 ]. Other meta-analyses in children and adolescents and older adults pooled RCTs across anxiety disorders, and found large placebo effect sizes ( g = 1.03, 95% CI [0.84, 1.21] and d = 1.06, 95% CI [0.71, 1.42], respectively) [ 55 , 58 ]. In ADHD, placebo effect size was medium-to-large for clinician-rated outcomes (SMC = 0.75, 95% CI [0.67, 0.83]) [ 62 ]. There was additionally a significant negative relationship between placebo effect size and drug-placebo difference (−0.56, p < 0.01) for self-rated outcomes [ 62 ]. In schizophrenia spectrum disorders, placebo effect size was small-to-medium in antipsychotic RCTs (SMC = 0.33, 95% CI [0.22, 0.44]) [ 38 ] and medium in RCTs focusing specifically on negative symptoms ( d = 0.64, 95% CI [0.46, 0.83]) [ 60 ]. Placebo effect size in RLS was large when measured via rating scales ( g = 1.41, 95% CI [1.25, 1.56]), but small ( g = 0.02 to 0.24) in RCTs using objective outcomes [ 68 ]. In autism, placebo effect sizes were small (SMC ranged 0.23 to 0.36) [ 63 ]. Similarly, placebo effect size was small in OCD ( d = 0.32, 95% CI [0.22, 0.41]), although larger in children and adolescents ( d = 0.45, 95% CI [0.35, 0.56]) compared with adults ( d = 0.27, 95% CI [0.15, 0.38]) [ 66 ]. Placebo effect size was large in AUD ( g = 0.90, 95% CI [0.70, 1.09]) [ 61 ], small in primary insomnia ( g ranged 0.25 to 0.43) [ 67 ], and medium in intellectual disability related to genetic causes ( g = 0.47, 95% CI [0.18, 0.76]) [ 65 ].
Our second objective was to examine the correlates of increased placebo response. We included 14 meta-analyses that reported correlates of placebo effect size or response rate through correlation analysis or meta-regression [ 38 , 51 , 53 , 54 , 56 , 57 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 68 ]. The key correlates extracted from these studies are summarized in Table 2 .
Several variables were consistently identified across meta-analyses. Increased number of trial sites was a positive correlate of increased placebo response in MDD [ 51 , 54 ], schizophrenia spectrum disorders [ 59 ], and autism spectrum disorders [ 63 ]. Similarly, increased sample size was positively associated with placebo effect size in schizophrenia spectrum disorders [ 59 ], OCD [ 66 ], and panic disorder [ 56 ]. Later publication or study year was associated with greater placebo response in anxiety disorders [ 56 , 57 ], schizophrenia spectrum disorders [ 38 ], AUD [ 61 ], and OCD [ 66 ] but not in MDD [ 51 ], and with reduced placebo response in ADHD [ 62 ]. Younger age was associated with increased placebo responses in schizophrenia spectrum disorders [ 38 , 59 ] and OCD [ 66 ]. Increased baseline illness severity was associated with increased placebo response in schizophrenia spectrum disorders [ 38 ], ADHD [ 62 ], and AUD [ 61 ]. Increased trial or follow-up duration was positively associated with increased placebo response in MDD [ 51 ], but negatively associated with placebo response in schizophrenia spectrum disorders [ 38 , 60 ] and OCD [ 66 ]. Finally, the effect size of active treatment was positively associated with increased placebo response in neurostimulation trials for MDD [ 53 ], bipolar depression [ 64 ], autistic spectrum disorders [ 63 ], and ADHD [ 62 ].
There were also some variables associated with increased placebo response in single disorders only. Flexible dosing, rather than fixed dosing, was associated with increased placebo response in MDD [ 51 ]. Increased illness duration was associated with reduced placebo response in schizophrenia spectrum disorders [ 38 ]. In RCTs for negative symptoms of schizophrenia, a higher number of active treatment arms was associated with increased placebo response [ 60 ]. A number of treatment administrations was a positive correlate of increased placebo response in patients with AUD [ 61 ]. A low risk of bias in selective reporting was associated with increased placebo response in ADHD [ 62 ]. Finally, a low risk of bias in allocation concealment was associated with increased placebo response in autism [ 63 ].
To our knowledge, this is the first overarching synthesis of the literature exploring the placebo response in RCTs of biological treatments across a broad range of mental disorders. We found that placebo responses were present and detectable across mental disorders. Further, the placebo effect size across these disorders varied between small and large (see Fig. 3 ). Additionally, several variables appeared to be associated with increased placebo effect size or response rate across a number of disorders, while others were reported for individual disorders only.

CI confidence interval, MDD major depressive disorder, GAD generalized anxiety disorder, SAD social anxiety disorder, OCD obsessive-compulsive disorder, g Hedges’ g, d Cohen’s d, SMC standardized mean change.
Our umbrella review distinguishes itself from a recent publication on placebo mechanisms across medical conditions [ 69 ]. Only four systematic reviews of research in mental disorders were included in that recent review [ 69 ], none of which were eligible for inclusion in our umbrella review, as we focus specifically on RCTs in mental disorders. Thus, our current umbrella review synthesizes different literature and is complementary [ 69 ].
We found substantial variation in placebo effect sizes across mental disorders. In GAD, SAD, MDD, AUD, and RLS (for subjective outcomes), placebo effects were large (>0.9), while they were small (approximately 0.3) in OCD, primary insomnia, autism, RLS (for objective outcomes), and schizophrenia spectrum disorders. It is noteworthy that placebo effect size/response rate correlated with active treatment effect size/response rate in many disorders (MDD, bipolar depression, ADHD, and autism). Nonetheless, where reported, active treatment was always superior. This possibly suggests an underlying ‘treatment responsiveness’ of these disorders that can vary in size. Perhaps, the natural history of a disorder is an important factor in ‘responsiveness’, i.e., disorders in which there is greater natural fluctuation in severity will show larger placebo (and active treatment) effect sizes. Supporting this hypothesis, increased trial duration predicted a larger placebo effect size in MDD, a disorder in which the natural course includes improvement [ 31 , 51 , 70 ]. Conversely, in schizophrenia spectrum disorders where improvement (particularly of negative symptoms) is less likely [ 71 ], increased trial and illness duration predicted a smaller placebo effect size [ 38 , 60 ]. However, previous meta-analyses suggest that natural improvement, for example, measured via waiting list control, does not fully account for the placebo effect in depression and anxiety disorders [ 72 , 73 ]. Statistical artifact, therefore, does not seem to fully explain the variation in effect size.
Non-specific treatment mechanisms are likely an additional source of the observed placebo effect. For example, those with treatment-resistant illness might have reduced expectations regarding treatment. This assumption is supported by the subgroup analysis reported by Razza and colleagues showing sham neuromodulation efficacy reduced as the number of previous failed antidepressant trials increased [ 53 ]. Another factor to consider is the outcome measure chosen. For example, the placebo effect size in panic disorder was smaller when calculated with objective or self-report measures compared with clinician-rated measures [ 56 ]. A similar finding was reported in ADHD trials [ 62 ]. Why placebo effect sizes would differ with clinician-rated versus self-rated scales is unclear. This might result from ‘demand characteristics’ (i.e., cues that suggest to a patient how they ‘should’ respond), or unblinding of the rater, or a combination of the two [ 74 , 75 ].
Several correlates of increased placebo response were reported in included meta-analyses. These included a larger sample size, more study sites, a later publication year (but with an opposite finding for ADHD), younger age, and increased baseline illness severity. This might reflect changes in clinical trial methods over time, the potential for increased ‘noise’ in the data with larger samples or more study sites, and, more speculatively, variables associated with increased volatility in symptoms [ 39 , 51 , 76 ]. A more extensive discussion regarding the potential reasons these variables might correlate with, or predict, placebo response is included in the eDiscussion. Although some correlates of increased placebo response were identified, perhaps more pertinently, it is unknown whether these also predict the separation between active treatment and placebo in most mental disorders. Three included meta-analyses did show that as placebo response increases, the likelihood of drug-placebo separation decreases [ 38 , 62 , 64 ]. This suggests correlates of placebo effect size are also correlates of trial success or failure, but this hypothesis needs explicit testing. In addition, few of the meta-analyses we included explored whether correlates of placebo response differed from correlates of active treatment response. For example, in clinical trials for gambling disorder, response to active treatment was predicted by weeks spent in the trial and by baseline severity, while response to placebo was predicted by baseline depressive and anxiety symptoms [ 77 ]. Furthermore, there is evidence that industry sponsorship is a specific correlate of reduced drug-placebo separation in schizophrenia spectrum disorders [ 78 ]. The largest meta-analysis that we included (conducted by Scott et al. [ 50 ]) did not explore correlates of increased placebo response through meta-regression analysis; rather, it was designed specifically to assess the impact of the use of placebo run-in periods in antidepressant trials. The authors found that use of a placebo run-in was associated with reduced placebo response. However, this effect did not enhance sensitivity to detect medication efficacy versus control groups, as trials with placebo run-in periods were also associated with a reduced medication response. Similar effects of placebo run-in were seen in univariate (but not multivariable) models in ADHD, where placebo run-in reduced placebo effect size in youth, but did not affect drug vs placebo difference [ 62 ]. Further work should be undertaken to ascertain whether trial-level correlates (including the use of placebo run-in) differentially explain active treatment or placebo response and whether controlling for these can improve drug-placebo separation.
Our results should be considered in the light of several possible limitations. First, as in any umbrella review, we were limited by the quality of the meta-analyses we included. Our AMSTAR-2 ratings suggest that confidence in the conclusions of most included meta-analyses should be critically low or low. Indeed, several meta-analyses did not assess for publication bias or for bias in included RCTs. This is relevant, as the risk of bias in selective reporting was highlighted as potentially being associated with placebo effect size in ADHD [ 62 ], and might therefore be relevant in other mental disorders. Second, our results are potentially vulnerable to biases or unmeasured confounders present in the included meta-analyses. Third, we attempted to prevent overlap and duplication of information by including only the meta-analyses with the most information. This might, however, have resulted in some data not being included in our synthesis. Fourth, an exploration of the potential clinical relevance of the placebo effect sizes reported here was outside the scope of the current review but should be considered an important question for future research. Finally, the meta-analyses we included encompassed RCTs with different levels of blinding (double-blind, single-blind). Although the majority of trials were likely double-blind, it is possible that different levels of blinding could have influenced placebo effect sizes through effects on expectations. Future analyses of placebo effects and their correlates should either focus on double-blind trials or compare results across levels of blinding. Related to this, the included meta-analyses pooled phase 2 and phase 3 trials (the latter of which will usually follow positive phase 2 trials), which might result in different expectation biases. Therefore, placebo effects should be compared between phase 2 and phase 3 trials in the future.
In this umbrella review, we found placebo effect sizes varied substantially across mental disorders. The sources of this variation remain unknown and require further study. Some variables were correlates of increased placebo response across mental disorders, including larger sample size, higher number of study sites, later publication year (opposite for ADHD), younger age, and increased baseline illness severity. There was also evidence that clinician-rated outcomes were associated with larger placebo effect sizes than self-rated or objective outcomes. We additionally identified important gaps in the literature, with no eligible systematic reviews identified in stress-related disorders, eating disorders, behavioural addictions, or bipolar mania. In relation to these disorders, some analyses have been published but they have not been included in systematic reviews/meta-analyses (e.g. analyses of individual patient data pooled across RCTs in acute mania [ 79 ] or gambling disorder [ 77 , 80 ]) and therefore were not eligible for inclusion here. We also focused on placebo response in RCTs of pharmacotherapies and neurostimulation interventions for mental disorders. We did not include placebo effects in psychosocial interventions, but such an analysis would also be valuable. Future studies should address these gaps in the literature and furthermore should compare findings in placebo arms with active treatment arms, both regarding treatment effect size and its correlates. Gaining additional insights into the placebo response may improve our ability to separate active treatment effects from placebo effects, thus paving the way for potentially effective new treatments for mental disorders.
Data availability
The datasets generated during and/or analysed during the current study are available in the Open Science Framework repository, https://osf.io/fxvn4/ .
Evers AWM, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, et al. Implications of placebo and nocebo effects for clinical practice: Expert Consensus. Psychother Psychosom. 2018;87:204–10.
Article PubMed Google Scholar
McQueen D, Cohen S, John-Smith PS, Rampes H. Rethinking placebo in psychiatry: the range of placebo effects. Adv Psychiatr Treat. 2013;19:162–70.
Article Google Scholar
Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–6.
Article CAS PubMed Google Scholar
Harris I. When the placebo effect is not an effect. Acta Orthop. 2021;92:501–2.
Article PubMed PubMed Central Google Scholar
Landin R, DeBrota DJ, DeVries TA, Potter WZ, Demitrack MA. The impact of Restrictive Entry Criterion during the placebo lead-in period. Biometrics. 2000;56:271–8.
Jones BDM, Razza LB, Weissman CR, Karbi J, Vine T, Mulsant LS, et al. Magnitude of the Placebo response across treatment modalities used for treatment-resistant depression in adults: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2125531.
Miller FG, Rosenstein DL. The nature and power of the placebo effect. J Clin Epidemiol. 2006;59:331–5.
Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the Placebo effect: an affective appraisal account. Annu Rev Clin Psychol. 2017;13:73–98.
Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ. 1995;311:551–3.
Article CAS PubMed PubMed Central Google Scholar
De La Fuente-Fernandez R. Expectation and Dopamine release: mechanism of the Placebo effect in Parkinson’s disease. Science. 2001;293:1164–6.
Benedetti F, Carlino E, Pollo A. How Placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–54.
Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–73.
Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, et al. Placebo effects on the immune response in humans: the role of learning and expectation. PloS One. 2012;7:e49477.
Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ, et al. Effects of expectation on placebo-induced Dopamine release in Parkinson disease. Arch Gen Psychiatry. 2010;67:857–65.
Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–94.
Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–15.
Huneke NTM, Aslan IH, Fagan H, Phillips N, Tanna R, Cortese S, et al. Functional neuroimaging correlates of placebo response in patients with depressive or anxiety disorders: A systematic review. Int J Neuropsychopharmacol. 2022;25:433–47.
Vase L, Riley JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–52.
Solmi M, Croatto G, Piva G, Rosson S, Fusar-Poli P, Rubio JM, et al. Efficacy and acceptability of psychosocial interventions in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. Mol Psychiatry. 2023;28:354–68.
Monteleone AM, Pellegrino F, Croatto G, Carfagno M, Hilbert A, Treasure J, et al. Treatment of eating disorders: A systematic meta-review of meta-analyses and network meta-analyses. Neurosci Biobehav Rev. 2022;142:104857.
Rosson S, de Filippis R, Croatto G, Collantoni E, Pallottino S, Guinart D, et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: An umbrella review. Neurosci Biobehav Rev. 2022;139:104743.
Correll CU, Cortese S, Croatto G, Monaco F, Krinitski D, Arrondo G, et al. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: an umbrella review. World Psychiatry. 2021;20:244–75.
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45.
Stone MB, Yaseen ZS, Miller BJ, Richardville K, Kalaria SN, Kirsch I. Response to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the US Food and Drug Administration: individual participant data analysis. BMJ. 2022;378:e067606.
Hendriks SM, Spijker J, Licht CMM, Hardeveld F, de Graaf R, Batelaan NM, et al. Long-term disability in anxiety disorders. BMC Psychiatry. 2016;16:248.
Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry. 2019;76:1241–55.
Croatto G, Vancampfort D, Miola A, Olivola M, Fiedorowicz JG, Firth J, et al. The impact of pharmacological and non-pharmacological interventions on physical health outcomes in people with mood disorders across the lifespan: An umbrella review of the evidence from randomised controlled trials. Mol Psychiatry. 2023;28:369–90.
Papola D, Ostuzzi G, Gastaldon C, Morgano GP, Dragioti E, Carvalho AF, et al. Antipsychotic use and risk of life-threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand. 2019;140:227–43.
Linden M. How to define, find and classify side effects in psychotherapy: from unwanted events to adverse treatment reactions. Clin Psychol Psychother. 2013;20:286–96.
Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment – pharmacological mechanisms. Pharmacol Ther. 2010;125:169–79.
Cuijpers P, Karyotaki E, Weitz E, Andersson G, Hollon SD, van Straten A. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: A meta-analysis. J Affect Disord. 2014;159:118–26.
Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The global economic burden of noncommunicable diseases. PGDA Work Pap. (2012).
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1575–86.
Huneke NTM, van der Wee N, Garner M, Baldwin DS. Why we need more research into the placebo response in psychiatry. Psychol Med. 2020;50:2317–23.
Huneke NTM. Is superiority to placebo the most appropriate measure of efficacy in trials of novel psychotropic medications? Eur Neuropsychopharmacol. 2022;62:7–9.
Khan A, Brown WA. Antidepressants versus placebo in major depression: An overview. World Psychiatry. 2015;14:294–300.
Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170:1335–44.
Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–42.
Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204.
Correll CU, Solmi M, Cortese S, Fava M, Højlund M, Kraemer HC, et al. The future of psychopharmacology: a critical appraisal of ongoing phase 2/3 trials, and of some current trends aiming to de-risk trial programmes of novel agents. World Psychiatry. 2023;22:48–74.
Stahl SM, Greenberg GD. Placebo response rate is ruining drug development in psychiatry: why is this happening and what can we do about it? Acta Psychiatr Scand. 2019;139:105–7.
Nikolakopoulou A, Chaimani A, Furukawa TA, Papakonstantinou T, Rücker G, Schwarzer G. When does the placebo effect have an impact on network meta-analysis results? BMJ Evid-Based Med. 2023. https://doi.org/10.1136/bmjebm-2022-112197 .
Huneke NTM, Amin J, Baldwin DS, Chamberlain SR, Correll CU, Garner M, et al. Placebo effects in mental health disorders: protocol for an umbrella review. BMJ Open. 2023;13:e073946.
Gauld C, Lopez R, Morin CM, Maquet J, Mcgonigal A, Geoffroy P-A, et al. Why do sleep disorders belong to mental disorder classifications? A network analysis of the “Sleep-Wake Disorders” section of the DSM-5. J Psychiatr Res. 2021;142:153–9.
Köhler-Forsberg O, Stiglbauer V, Brasanac J, Chae WR, Wagener F, Zimbalski K, et al. Efficacy and safety of antidepressants in patients with comorbid depression and medical diseases: an umbrella systematic review and meta-Analysis. JAMA Psychiatry. 2023. https://doi.org/10.1001/jamapsychiatry.2023.2983 .
Belbasis L, Bellou V, Ioannidis JPA. Conducting umbrella reviews. BMJ Med. 2022;1:e000071.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; (1988).
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Scott AJ, Sharpe L, Quinn V, Colagiuri B. Association of Single-blind Placebo Run-in Periods With the Placebo Response in Randomized Clinical Trials of Antidepressants: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:42.
Furukawa TA, Cipriani A, Atkinson LZ, Leucht S, Ogawa Y, Takeshima N, et al. Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies. Lancet Psychiatry. 2016;3:1059–66.
Scott F, Hampsey E, Gnanapragasam S, Carter B, Marwood L, Taylor RW, et al. Systematic review and meta-analysis of augmentation and combination treatments for early-stage treatment-resistant depression. J Psychopharmacol. 2023;37:268–78.
Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:105–13.
Meister R, Abbas M, Antel J, Peters T, Pan Y, Bingel U, et al. Placebo response rates and potential modifiers in double-blind randomized controlled trials of second and newer generation antidepressants for major depressive disorder in children and adolescents: a systematic review and meta-regression analysis. Eur Child Adolesc Psychiatry. 2020;29:253–73.
Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective Serotonin reuptake inhibitors, Serotonin-Norepinephrine Reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. Jama Psychiatry. 2017;74:1011–20.
Ahmadzad-Asl M, Davoudi F, Mohamadi S, Hadi F, Nejadghaderi SA, Mirbehbahani SH, et al. Systematic review and meta-analysis of the placebo effect in panic disorder: Implications for research and clinical practice. Aust N Z J Psychiatry. 2022;56:1130–41.
Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183–92.
Pinquart M, Duberstein PR. Treatment of anxiety disorders in older adults: a meta-analytic comparison of behavioral and pharmacological interventions. Am J Geriatr Psychiatry. 2007;15:639–51.
Leucht S, Chaimani A, Leucht C, Huhn M, Mavridis D, Helfer B, et al. 60 years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Meta-regression of predictors of placebo response. Schizophr Res. 2018;201:315–23.
Czobor P, Kakuszi B, Bitter I. Placebo response in trials of negative symptoms in Schizophrenia: A critical reassessment of the evidence. Schizophr Bull. 2022;48:1228–40.
Del Re AC, Maisel N, Blodgett J, Wilbourne P, Finney J. Placebo group improvement in trials of pharmacotherapies for alcohol use disorders: a multivariate meta-analysis examining change over time. J Clin Psychopharmacol. 2013;33:649.
Faraone SV, Newcorn JH, Cipriani A, Brandeis D, Kaiser A, Hohmann S, et al. Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: a systematic review and meta-analysis. Mol Psychiatry. 2022;27:212–9.
Siafis S, Çıray O, Schneider-Thoma J, Bighelli I, Krause M, Rodolico A, et al. Placebo response in pharmacological and dietary supplement trials of autism spectrum disorder (ASD): systematic review and meta-regression analysis. Mol Autism. 2020;11:66.
Iovieno N, Nierenberg AA, Parkin SR, Hyung Kim DJ, Walker RS, Fava M, et al. Relationship between placebo response rate and clinical trial outcome in bipolar depression. J Psychiatr Res. 2016;74:38–44.
Curie A, Yang K, Kirsch I, Gollub RL, des Portes V, Kaptchuk TJ, et al. Placebo responses in genetically determined intellectual disability: a meta-analysis. PloS One. 2015;10:e0133316.
Mohamadi S, Ahmadzad-Asl M, Nejadghaderi SA, Jabbarinejad R, Mirbehbahani SH, Sinyor M, et al. Systematic review and meta-analysis of the placebo effect and its correlates in obsessive compulsive disorder. Can J Psychiatry. 2023;68:479–94.
Winkler A, Rief W. Effect of placebo conditions on polysomnographic parameters in primary insomnia: a meta-analysis. Sleep. 2015;38:925–31.
PubMed PubMed Central Google Scholar
Silva MA, Duarte GS, Camara R, Rodrigues FB, Fernandes RM, Abreu D, et al. Placebo and nocebo responses in restless legs syndrome: A systematic review and meta-analysis. Neurology. 2017;88:2216–24.
Frisaldi E, Shaibani A, Benedetti F, Pagnini F. Placebo and nocebo effects and mechanisms associated with pharmacological interventions: an umbrella review. BMJ Open. 2023;13:e077243.
Cuijpers P, Stringaris A, Wolpert M. Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry. 2020;7:925–7.
Bromet EJ, Fennig S. Epidemiology and natural history of schizophrenia. Biol Psychiatry. 1999;46:871–81.
Rutherford BR, Mori S, Sneed JR, Pimontel MA, Roose SP. Contribution of spontaneous improvement to placebo response in depression: A meta-analytic review. J Psychiatr Res. 2012;46:697–702.
Fernández-López R, Riquelme-Gallego B, Bueno-Cavanillas A, Khan KS. Influence of placebo effect in mental disorders research: A systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e13762.
Goodwin GM, Croal M, Marwood L, Malievskaia E. Unblinding and demand characteristics in the treatment of depression. J Affect Disord. 2023;328:1–5.
Coles NA, Gaertner L, Frohlich B, Larsen JT, Basnight-Brown DM. Fact or artifact? Demand characteristics and participants’ beliefs can moderate, but do not fully account for, the effects of facial feedback on emotional experience. J Pers Soc Psychol. 2023;124:287–310.
Weimer K, Colloca L, Enck P. Placebo eff ects in psychiatry: mediators and moderators. Lancet Psychiatry. 2015;2:246–57.
Huneke NTM, Chamberlain SR, Baldwin DS, Grant JE. Diverse predictors of treatment response to active medication and placebo in gambling disorder. J Psychiatr Res. 2021;144:96–101.
Leucht S, Chaimani A, Mavridis D, Leucht C, Huhn M, Helfer B, et al. Disconnection of drug-response and placebo-response in acute-phase antipsychotic drug trials on schizophrenia? Meta-regression analysis. Neuropsychopharmacology. 2019;44:1955–66.
Welten CCM, Koeter MWJ, Wohlfarth T, Storosum JG, van den Brink W, Gispen-de Wied CC, et al. Placebo response in antipsychotic trials of patients with acute mania. Eur Neuropsychopharmacol. 2015;25:1018–26.
Grant JE, Chamberlain SR. The placebo effect and its clinical associations in gambling disorder. Ann Clin Psychiatry. 2017;29:167.
Download references
Acknowledgements
Dr Nathan TM Huneke is an NIHR Academic Clinical Lecturer. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, NHS, or the UK Department of Health and Social Care. For the purpose of open access, the author has applied a Creative Commons Attribution License (CC BY) to any Author Accepted Manuscript version arising from this submission.
Author contributors
NTMH, JA, DSB, SRC, CUC, MG, CMH, RH, ODH, JMAS, MS, and SCo conceptualized the study. NTMH, AB, VB, LE, CJG, OKF, LM, CR, SS, and SCo contributed to data collection, data curation, or data analysis. NTMH, MS, and SCo wrote the first draft of the manuscript. All authors had access to the raw data. All authors reviewed and edited the manuscript and had final responsibility for the decision to submit it for publication.
Author information
These authors contributed equally: Marco Solmi, Samuele Cortese.
Authors and Affiliations
Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, UK
Nathan T. M. Huneke, Jay Amin, David S. Baldwin, Samuel R. Chamberlain, Matthew Garner, Catherine M. Hill, Ruihua Hou, Konstantinos Ioannidis, Julia M. A. Sinclair & Samuele Cortese
Southern Health NHS Foundation Trust, Southampton, UK
Nathan T. M. Huneke, Jay Amin, David S. Baldwin, Samuel R. Chamberlain, Konstantinos Ioannidis & Satneet Singh
University Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
David S. Baldwin
School of Psychology, University of Nottingham Malaysia, Semenyih, Malaysia
Alessio Bellato
Centre for Innovation in Mental Health, School of Psychology, Faculty of Environmental and Life Sciences, University of Southampton, Southampton, UK
Alessio Bellato, Valerie Brandt, Matthew Garner, Corentin J. Gosling, Claire Reed, Marco Solmi & Samuele Cortese
Clinic of Psychiatry, Social Psychiatry and Psychotherapy, Hannover Medical School, Hanover, Germany
Valerie Brandt
Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin Berlin, Berlin, Germany
Christoph U. Correll
Department of Psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, NY, USA
Department of Psychiatry and Molecular Medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA
Center for Psychiatric Neuroscience, Feinstein Institute for Medical Research, Manhasset, NY, USA
Faculty of Education and Psychology, University of Navarra, Pamplona, Spain
Luis Eudave
School of Psychology, Faculty of Environmental and Life Sciences, University of Southampton, Southampton, UK
Matthew Garner
Université Paris Nanterre, DysCo Lab, F-92000, Nanterre, France
Corentin J. Gosling
Université de Paris, Laboratoire de Psychopathologie et Processus de Santé, F-92100, Boulogne-Billancourt, France
Department of Sleep Medicine, Southampton Children’s Hospital, Southampton, UK
Catherine M. Hill
Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK
Oliver D. Howes
H Lundbeck A/s, Iveco House, Watford, UK
Institute of Clinical Sciences (ICS), Faculty of Medicine, Imperial College London, London, UK
Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Ole Köhler-Forsberg
Psychosis Research Unit, Aarhus University Hospital–Psychiatry, Aarhus, Denmark
Department of Translational Biomedicine and Neuroscience (DIBRAIN), University of Studies of Bari “Aldo Moro”, Bari, Italy
Lucia Marzulli
Department of Psychiatry, University of Ottawa, Ottawa, ON, Canada
Marco Solmi
Department of Mental Health, Ottawa Hospital, Ottawa, ON, Canada
Ottawa Hospital Research Institute (OHRI) Clinical Epidemiology Program, University of Ottawa, Ottawa, ON, Canada
School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
Solent NHS Trust, Southampton, UK
Samuele Cortese
DiMePRe-J-Department of Precision and Regenerative Medicine-Jonic Area, University “Aldo Moro”, Bari, Italy
Hassenfeld Children’s Hospital at NYU Langone, New York University Child Study Center, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nathan T. M. Huneke .
Ethics declarations
Competing interests.
DSB is President of the British Association for Psychopharmacology, Editor of the Human Psychopharmacology journal (for which he receives an editor’s honorarium), and has received royalties from UpToDate. CMH has acted on an expert advisory board for Neurim Pharmaceuticals. ODH is a part-time employee and stockholder of Lundbeck A/s. He has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche and Viatris/Mylan. ODH has a patent for the use of dopaminergic imaging. All other authors declare no competing interests. MS has received honoraria/has been a consultant for Angelini, Lundbeck, and Otsuka. SCo has received honoraria from non-profit associations (BAP, ACAMH, CADDRA) for educational activities and an honorarium from Medice. KI has received honoraria from Elsevier for editorial work. SRC receives honoraria from Elsevier for associate editor roles at comprehensive psychiatry and NBR journals. CUC has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Tolmar, Vertex, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41380_2024_2638_moesm1_esm.docx.
PLACEBO EFFECTS IN RANDOMIZED TRIALS OF PHARMACOLOGICAL AND NEUROSTIMULATION INTERVENTIONS FOR MENTAL DISORDERS: AN UMBRELLA REVIEW SUPPLEMENTARY APPENDIX
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Huneke, N.T.M., Amin, J., Baldwin, D.S. et al. Placebo effects in randomized trials of pharmacological and neurostimulation interventions for mental disorders: An umbrella review. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02638-x
Download citation
Received : 01 February 2024
Revised : 17 June 2024
Accepted : 19 June 2024
Published : 24 June 2024
DOI : https://doi.org/10.1038/s41380-024-02638-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Isolated cerebellar stroke in a paediatric patient with typical haemolytic uraemic syndrome: a case report and literature review
- Case Report
- Open access
- Published: 26 June 2024
Cite this article
You have full access to this open access article

- Manuela Lo Bianco ORCID: orcid.org/0000-0002-5413-419X 1 , 2 ,
- Sergio Rinella ORCID: orcid.org/0000-0002-9368-7088 3 , 4 ,
- Felice D’Arco ORCID: orcid.org/0000-0001-7396-591X 5 ,
- Evangelia Ioannidou ORCID: orcid.org/0000-0003-0422-1727 6 &
- Marios Kaliakatsos ORCID: orcid.org/0000-0001-6299-9866 7
110 Accesses
1 Altmetric
Explore all metrics
Haemolytic Uraemic Syndrome (HUS) is a rare medical condition characterised by microangiopathic haemolytic anaemia, thrombocytopenia, and acute kidney injury. Neurological complications are documented but rarely involve the cerebellum. We present a unique case of a 23-month-old male with HUS triggered by Escherichia coli -O157 ( E.coli- O157) infection leading to an isolated cerebellar stroke.
The patient initially presented with fever, bloody stools, and seizures. Confirmation of E.coli- O157 infection was obtained, and MRI revealed an isolated cerebellar stroke. Treatment included supportive care, anticoagulation for a right atrial thrombus, with gradual improvement observed.
This case highlights the unusual occurrence of isolated cerebellar stroke in HUS patients, emphasising the importance of promptly recognizing manifestations of the central nervous system and the necessity for a multidisciplinary approach. Finally, a comprehensive literature review was conducted to identify cases of HUS patients with cerebellar involvement.
Similar content being viewed by others
Nephrotic-range proteinuria and central nervous involvement in typical hemolytic uremic syndrome: a case report

Remote cerebellar hemorrhage following repeated lumbar punctures

Haemolytic uraemic syndrome - a rare case report of bloody diarrhoea in adults
Avoid common mistakes on your manuscript.
Introduction
Haemolytic Uraemic Syndrome (HUS) is a rare medical condition characterised by microangiopathic haemolytic anaemia, thrombocytopenia, and acute kidney injury. Neurological complications occur in approximately 10–50% of cases and have been well documented [ 1 ]. However, cerebellar stroke involvement is an exceptionally uncommon presentation with very limited reported cases. We present a unique case of a 23-month-old male who developed an isolated cerebellar stroke as a complication of HUS triggered by Escherichia Coli (E.Coli) O157 infection. This case highlights the rarity and complexity of such cases, emphasising the importance of early recognition and multidisciplinary care.
Case report
A previously healthy full-term 23-month-old male, born by vaginal delivery, presented to the local hospital with a 5-day history of pyrexia and bloody loose stools. The child was up to date with immunisation and had no history of travel. Initial diagnostic concerns included intussusception, therefore he was transferred to a tertiary paediatric surgical unit, where this diagnosis was excluded, and the patient discharged. A stool sample confirmed the presence of E.Coli- O157, and an ongoing E.Coli- O157 outbreak was reported at his nursery. Twenty-four hours after discharge, he re-presented to the local Emergency Department following a generalised tonic–clonic (GTC) seizure. He was also noted to have oligo-anuria. He also experienced clusters of GTC seizures, which persisted despite the administration of two doses of benzodiazepines. His blood gas revealed metabolic acidosis with marked hyponatraemia and his blood test confirmed mild anaemia, deranged kidney function, elevated liver enzymes, and a markedly elevated C-reactive protein. Continuous veno-venous hemofiltration, hypertonic (3%) saline and diuretics were administered in view of acute kidney injury with metabolic acidosis.
The patient was urgently intubated for neuroprotection and a brain computed tomography scan was performed to exclude intracranial bleeding which was negative.
Subsequently, he was transferred to the Paediatric Intensive Care Unit at Great Ormond Street Hospital for Children, London, UK, with suspected of HUS. At the time of arrival the patient was neurologically unassessable as was intubated and paralysed.
He underwent a cardiac echo which showed right internal jugular and right atrial (RA) thrombus so he was placed on a heparin infusion. There was no evidence of right to left communication in the heart. His magnetic resonance imaging (MRI) and angiography brain scan revealed an acute left posterior-inferior cerebellar artery (PICA) stroke, showing diffusion restriction, and a reduction in the flow signal of left PICA (Fig. 1 ). His electroencephalogram was suggestive of diffuse encephalopathic process.

MRI brain scan showing left PICA acute ischemic stroke. The ischaemic area is hyperintense on axial T2 weighted images (A), shows diffusion restriction on axial diffusion weighted images (B) and apparent coefficient diffusion maps (C) and is hyperintense in coronal FLAIR (D). Magnetic resonance angiography, 3D maximum intensity projection (MIP) and axial MIP (E and F) show presence of the right PICA only (arrow) without visualisation of the left one
A diagnosis of isolated cerebellar stroke secondary to HUS was made. The patient was treated conservatively and showed gradual clinical improvement, with resolution of seizures, improvement of renal function and urine output. A repeat cardiac echo was unremarkable with no evident thrombus and the child was discharged home three weeks after his initial presentation, on low molecular weight heparin and amlodipine, with close monitoring of factor Xa. At the time of discharge and at review one month later the patient was neurologically normal.
This case report delves into the rare occurrence of isolated cerebellar stroke in a paediatric patient with typical HUS triggered by E.Coli -O157 infection.
Shiga toxin, produced by disease-causing strains of bacteria (e.g. E.Coli- O157), plays a pivotal role in organ damage. Once released, the toxin is absorbed into the bloodstream through the gastrointestinal tract and binds to globotriaosylceramide (Gb3) on the surface of vascular endothelial cells [ 2 ]. This binding triggers a cascade of pathophysiological events by promoting inflammation, inducing the expression of cytokines and chemokines, and eliciting a ribotoxic stress response. Consequently, endothelial cell damage leads to the formation of microthrombi, activation of the alternative complement pathway, and injury to target organs, especially kidney and brain.
Damage to the CNS primarily contributes to mortality in individuals with acute HUS. This brain damage is probably due to the occurrence of microvascular damage in susceptible brain regions, such as brainstem and basal ganglia. Notably, Gb3 has been detected in various types of neurons in murine models, including the cerebellum [ 3 ]. Cerebellum is characterised by a unique neurovascular architecture whose main feeders are the superior cerebellar artery, the anterior inferior cerebellar artery, and the posterior inferior cerebellar artery (PICA). The cerebellar vasculature, while intricate, is less susceptible to thrombosis compared to the densely vascularized basal ganglia. However, when a cerebellar stroke occurs, it predominantly manifests at the level of the PICA, with a 40% prevalence [ 4 ].
Hence, the most reasonable explanation for the occurrence of cerebellar stroke in the present case could be the underlying vascular changes due to endothelial injury/inflammation in the left PICA territory. However, the occurrence of isolated cerebellar involvement remains extremely rare and the pathogenesis unclear.
Lee et al. [ 5 ] reported that isolated cerebellar stroke could originate when cardioembolism occurs in the context of a specific angulation of the PICA. In fact, in the majority of cases, strokes involving the PICA are attributed to cardioembolism [ 4 ]. Our patient developed a RA thrombus as a consequence of HUS, which could represent a possible factor bringing to the isolated cerebellar stroke via distal emboli. Nevertheless, there were no other emboli present and two cardiac echos showed no evidence of intra-atrial communication.
A comprehensive literature review was conducted to identify cases of HUS patients with cerebellar involvement. Our search revealed only six cases in which the cerebellum along with other brain areas was implicated in haemorrhagic/ischaemic events associated with HUS (Table 1 ) [ 6 , 7 , 8 , 9 , 10 ].
Only one case involved a 5-year-old patient with typical HUS who initially exhibited an isolated cerebellar ischemic stroke [ 8 ]. However, HUS was not associated with E.Coli- O157 and the report lacked any MRI images for review or mention of the territorial involvement. Unlike our isolated cerebellar presentation, she experienced subsequent haemorrhages in additional cerebral areas.
In conclusion, we report a rare case of an isolated cerebellar stroke in a child with typical HUS triggered by E.Coli- O157 infection, suggesting the underlying endothelial injury and/or inflammation in cerebellar vessels can be part of the spectrum of CNS manifestations in patients with HUS.
Finally, effective management of acute HUS necessitates a multidisciplinary approach involving paediatricians, nephrologists, neurologists, haematologists, and psychologists, as early recognition and treatment is crucial for a good outcome.
Data availability
The data used in this study are available from the corresponding author upon request.
Boyer O, Niaudet P (2022) Hemolytic-Uremic Syndrome in Children. Pediatr Clin North Am 69:1181–1197. https://doi.org/10.1016/j.pcl.2022.07.006
Article PubMed Google Scholar
Joseph A, Cointe A, Mariani Kurkdjian P et al (2020) Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins 12:67. https://doi.org/10.3390/toxins12020067
Article CAS PubMed PubMed Central Google Scholar
Obata F, Tohyama K, Bonev AD et al (2008) Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J Infect Dis 198:1398–1406. https://doi.org/10.1086/591911
Article CAS PubMed Google Scholar
Miao H-L, Zhang D-Y, Wang T et al (2020) Clinical Importance of the Posterior Inferior Cerebellar Artery: A Review of the Literature. Int J Med Sci 17:3005–3019. https://doi.org/10.7150/ijms.49137
Article PubMed PubMed Central Google Scholar
Lee SH, Cha JH, Jung IE et al (2019) Relationship between the Angle of the Posterior Inferior Cerebellar Artery and Cardioembolic Stroke. J Stroke Cerebrovasc Dis 28:693–698. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.11.007
Gawlitza M, Hoffmann K-T, Lobsien D (2015) Mild Encephalitis/Encephalopathy with Reversible Splenial and Cerebellar Lesions (MERS Type II) in a Patient with Hemolytic Uremic Syndrome (HUS). J Neuroimaging 25:145–146
Steinborn M, Leiz S, Rüdisser K et al (2004) CT and MRI in haemolytic uraemic syndrome with central nervous system involvement: Distribution of lesions and prognostic value of imaging findings. Pediatr Radiol 34:805–810
Mewasingh LD, Kadhim H, Christophe C et al (2003) Nonsurgical cerebellar mutism (anarthria) in two children. Pediatr Neurol 28:59–63
Nakamura H, Takaba H, Inoue T et al (2003) MRI findings of hemolytic uremic syndrome with encephalopathy: Widespread symmetrical distribution. J Neuroimaging 13:75–78
Hager A, Staudt M, Klare B et al (1999) Hemolytic-uremic syndrome with involvement of basal ganglia and cerebellum. Neuropediatrics 30:210–213
Download references
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. No funds, grants, or other support was received for this study.
Author information
Authors and affiliations.
Postgraduate Training Program in Pediatrics, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Manuela Lo Bianco
Department of Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, University College London, London, UK
Department of Educational Sciences, University of Catania, Catania, Italy
Sergio Rinella
Department of Neuroradiology, Great Ormond Street Hospital for Children, London, UK
Felice D’Arco
Paediatric Specialty Trainee in Paediatric Neurology, Great Ormond Street Hospital for Children, London, UK
Evangelia Ioannidou
Department of Neurology, Great Ormond Street Hospital for Children, London, UK
Marios Kaliakatsos
You can also search for this author in PubMed Google Scholar
Contributions
MK and FD contributed to the study conception and design. Material preparation, data collection and analysis were performed by MLB, SR and EI. The first draft of the manuscript was written by MLB, SR and EI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sergio Rinella .
Ethics declarations
Ethics approval.
In accordance with international ethical guidelines for research involving minors and the publication of paediatric case reports, this study did not require formal ethical approval. Special care was taken to protect the privacy and confidentiality of the information of the paediatric patients involved. No identifiable personal data were included in the manuscript.
Informed consent
In compliance with the ethical principles on research involving minors, written informed consent was obtained from the parents of the minor for the publication of this case report and related information. This consent covers the use of clinical data and any associated images with the utmost confidentiality and without compromising the anonymity of the patient.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Lo Bianco, M., Rinella, S., D’Arco, F. et al. Isolated cerebellar stroke in a paediatric patient with typical haemolytic uraemic syndrome: a case report and literature review. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03407-x
Download citation
Received : 16 February 2024
Accepted : 09 June 2024
Published : 26 June 2024
DOI : https://doi.org/10.1007/s00234-024-03407-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 1 Cerebellum
- 2 Posterior-Inferior Cerebellar Artery
- 5 Case report
- 6 Ischaemic injury
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased.
Abstract. The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth.
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls ...
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls ...
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls ...
Research on triple X syndrome may yield more insight into brain and behaviour relations, developmental psychopathology, auditory-processing disorders, EEG disorders, personality and psychotic disorders, etc. The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX ...
Article Title: Triple X syndrome: a review of the literature Authors: Otter, Schrander-Stumpel, and Curfs Date of Publication: July 1, 2009 "Triple X syndrome is a syndrome with a high level of variety in the physical and behavioural phenotype. Triple X syndrome is not rare, but it is often undiagnosed. Notwithstanding the relatively high prevalence of triple X
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47, XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased.
Triple X syndrome: A review of the literature. Eur J Hum Genet. 2010; 18:265-271. [PMC free article] [Google Scholar] Ratcliffe S, Butler G, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth Defects Orig Artic Ser. 1990; 26:1-44.
Triple X syndrome (TXS) is a genetic syndrome first described back in 1959 in an infertile woman. 1 TXS is characterised by a 47, XXX karyotype and has an estimated incidence of 1 in 1000 newborn girls. 2 The phenotype associated with TXS is variable, but is generally mild; therefore, estimates suggest that only 16% of cases are clinically diagnosed. 3 Individuals with TXS typically present ...
Background. Knowledge with respect to the increased prevalence of psychiatric disorders in adult women diagnosed with triple X syndrome (TXS) is scarce [Reference Freilinger, Kliegel, Hanig, Oehl-Jaschkowitz, Henn and Meyer 1].Psychiatric disorders and psychological complaints, as well as the modifier impaired social functioning, are hardly studied in this group.
Introduction. Triple X syndrome (trisomy X, 47,XXX), first described by Jacobs 1959, is a sex chromosomal aneuploidy condition with female phenotype[].The incidence of the syndrome is estimated at 10.7 per 100000 live born girls[].Its symptoms vary very widely, including tall stature, hypertelorism, epicanthal folds, clinodactyly, congenital heart disease, genitourinary and some other ...
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased.
The cases with SCA were investigated in a prospective study design for 20 years Triple X syndrome: a review M Otter et al 266 Table 1 Growth and development in triple X syndrome according to the results of unbiased studies Adolescents and (young) Physical growth Motor development Children o6 years of age15,19 School children16,20 Teenage ...
The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls have a lower mean birth weight and a smaller head circumference. Triple X diagnosis was not suspected at birth. The maternal age seems to be increased.
Abstract. Background: Triple X syndrome (47,XXX or trisomy X) is a relatively frequent cytogenetic condition with a large variety of physical and behavioural phenotypes. Method: Two adult patients with a triple X karyotype are described. Results: Their karyotype was unknown until some years ago. What these patients have in common is that they were diagnosed with a broader autism phenotype ...
Triple X syndrome or Trisomy X Triple X syndrome (Trisomy X) is a genetic condition that only affects females. Girls and women with triple X syndrome have an extra X chromosome. ... The guide also refers to Triple X syndrome: a review of the literature by a psychiatrist, Dr Maarten Otter and his
Women with triple X syndrome (TXS) have an extra X chromosome. TXS appeared to be associated with psychiatric disorders in biased or underpowered studies. ... Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010; 18:265-71. doi: 10.1038/ejhg.2009.109. [PMC free article] [Google Scholar] [11] Berglund A, Stochholm K, Gravholt ...
To highlight the clinical features and diagnosis of triple X syndrome, we present a rare phenotype of the syndrome. Case presentation: A 5.9 year-old girl was admitted to our hospital because of short stature. Both her height and weight were below the 3 (rd) percentile compared to the normal peers. She was found with mild motor and speech delay.
In this Review, the authors summarize the literature around immune cancer cell STING signaling and how it is finely balanced between pro-tumorigenic and anti-tumorigenic functions.
Europe PMC is an archive of life sciences journal literature.
Background. Triple X syndrome is a sex chromosomal aneuploidy condition characterized by tall stature, microcephaly, hypertelorism, congenital abnormalities, and motor and language delays. It is mainly derived from maternal nondisjunctional errors during meiosis. To highlight the clinical features and diagnosis of triple X syndrome, we present ...
Triple X syndrome: a review of the literature The developmental and clinical aspects in the literature on triple X syndrome are reviewed. Prenatal diagnosis depends on karyotyping. The incidence is 1 of 1000 females. At birth, 47,XXX girls have a lower mean birth weight and a smaller head
Mounting evidence supports a significant correlation between the stress hyperglycemia ratio (SHR) and both short- and long-term prognoses in patients with acute coronary syndrome (ACS). Nevertheless, research examining the association between the SHR and the complexity of coronary artery disease (CAD) is scarce. Therefore, this study aimed to explore the association between the SHR and CAD ...
There is a growing literature exploring the placebo response within specific mental disorders, but no overarching quantitative synthesis of this research has analyzed evidence across mental disorders.
Haemolytic Uraemic Syndrome (HUS) is a rare medical condition characterised by microangiopathic haemolytic anaemia, thrombocytopenia, and acute kidney injury. Neurological complications are documented but rarely involve the cerebellum. We present a unique case of a 23-month-old male with HUS triggered by Escherichia coli-O157 (E.coli-O157) infection leading to an isolated cerebellar stroke.The ...
Triple X syndrome (trisomy X, 47,XXX), first described by Jacobs 1959, is a sex chromosomal aneuploidy condition with female phenotype[1]. The incidence of the syndrome is estimated at 10.7 per ...