How to Pass CIMA Case Study Exams: Detailed Guide to Success
This CIMA case study article shows you how to write an answer that puts you ahead of 90% of candidates, offers expert preparation tips, and increases your chances of obtaining your qualification.
%20(3).png)

1. How to Pass CIMA Case Study Exams: An Introduction to Our CIMA Exam Experience
In the last five years, we’ve helped over 10,000 CIMA students from 94 countries prepare for and pass their CIMA exams. In that time, VIVA’s tutors have seen every kind of exam answer you can imagine.
As an official CIMA tuition provider, we've seen everything; the good, the bad, and the downright baffling! But more importantly, we’ve also seen what works, and what definitely does not work.
In this article, we’ve compiled all of the key DOs and DON’Ts our CIMA tutors have gleaned from their years marking VIVA students’ CIMA mock exam answers. We see the same kinds of mistakes made over and over again. And the great news for you is — these mistakes can be very quickly rectified to help you pass your CIMA exams the first time around.
The Starting Point: What to Be Aware of before Learning More about CIMA Exam Strategies
The first step is being aware of what to avoid in CIMA exams.
This sets the foundation for you to go on refining and perfecting your approach. As in any other walk of life, perhaps the most important thing is to avoid doing foolish things rather than seeking perfection.
In the words of the very wise Charlie Munger: “It is remarkable how much long-term advantage people like us have gotten by trying to be consistently not stupid, instead of trying to be very intelligent.”
All the advice below comes directly from our CIMA case study professional tutors, who mark thousands of student scripts throughout the year.
If you wish to get your VIVA mock exam answers corrected, check out our CIMA course pages , where you can obtain professional marking services as part of the Elite Course.
2. What to Expect on Exam Day
Before we get into the meat of the matter, let’s just review what exactly you can expect to be presented with on exam day. (Feel free to skip on to the next section if you are already well acquainted with the Case Study exam format):
- You will be faced with 1 of 3 CIMA exam variants during any specific exam window (that's a total of 6 variants per pre-seen document under the 2019 CIMA syllabus)
- Each variant is broken down into timed sections (maximum of 5, minimum of 3)
- Each section will include either emails, records of conversations, schedules of information or combinations of all 3
- These give new information that leads on from the pre-seen document information
- Within each section, there will be a task or tasks for the candidate to complete (e.g. write a report, write sections of a report, write an email)
- These tasks might be embedded in the body of the email or conversation
- The task or tasks might include several different elements that pull from different pillars and competencies
- Candidates are NOT expected to perform any detailed calculations
- Each section will move through time (you cannot go back to a previous section once you move on to a subsequent section)
Of course, the best way to familiarise yourself with the real-life experience of a CIMA case study exam is to practice as many different mock exams as possible.
VIVA's OCS, MCS and SCS courses come with up to 5 different professionally prepared CIMA mock exams based on the current pre-seen, which you can practice online under timed exam conditions.
You’ll also want to review past CIMA exam variants to familiarise yourself with the different question styles that can come up.
However, there’s no substitute for timed practice based on the current pre-seen material – and that’s what you’ll get with us.

Source: Pexels
3. Reading the Question: Establishing the Case Study Exam Tasks and Requirements
One common error that our tutors' report is that students do not answer all the requirements included in the task.
In some cases, of course, this is simply down to the student not knowing how to answer the particular requirement. However, we have seen many scenarios in which students have simply missed the requirement due to not having read the question carefully enough!
Consider the sample below taken from a case study exam:

Here we have examples of what is sometimes called “triggers”. These are the places in the question where the requirements are explicitly stated.
Triggers are sometimes in the form of questions, sometimes not. Look out for phrases like “I need you to”, “I would welcome your suggestions for”, “Please draft”, and “Can you please include in your report”.
These are the sections that you really need to pay close attention to because it is there that you will be presented with the requirements.
As you can see in the screenshot above, there is one “task”, i.e., the report that you must draft. But the task has two requirements.
One is a comparison of financial performance and the other deals with the introduction of the balanced scorecard.
However, notice that each requirement contains several sub-requirements.
In the case of the first requirement, notice the “and.” You have to both “compare” and “analyse the implications.” Too many students will simply read the “Compare” part and completely pass over the analysis of the implications:

Likewise, in the case of the second requirement. There’s even more going on here, so you must pay close attention as you read through these “triggers.”
Not only do you have to offer your “suggestions for the other three quadrants,” but you also have to “explain why we have chosen the measures for each quadrant” and “ how they will influence behaviours in the company.”
All of these elements must be addressed to gain full marks.
But too often, students only pay attention to the first one or two. It is not necessarily the case that the first thing asked is the most important or even carries the most weight in terms of marks!
So, it’s essential that you carefully read these trigger sections thoroughly, making a note of each requirement as you go.

Another crucial point: answer the questions that have been asked!
This might seem obvious, but you'd be surprised how many students fail to do this. There are two main reasons students fail to answer the question asked:
(i) because they answer a question they wish they had been asked instead;
(ii) They don't read the trigger verbs carefully enough and misinterpret what is being asked.
The first reason has to do with the fact that sometimes, students will carefully prepare for particular kinds of questions during their exam preparation or practice. They will feel much more confident about some particular question types than others and will have prepared very effectively for those question types.
So strong is their hope that this kind of question will come up on exam day that when they read a question that is superficially similar, they will "shoehorn" their prepared answer into that question.
The result is an answer that is either only partly relevant or, in the worst case, utterly irrelevant to the question being asked!
So remember: don't let your hopes/preferences/strengths influence the kind of answer that you give. Allow the question to dictate what kind of answer you write.
The second reason students fail to answer the question asked is that they misinterpret a key term or phrase or forget what they were asked after they start writing. Consider the example below:

In this example, an incorrect interpretation of the question might be to think about identifying the "limitations" or "drawbacks" of TQM, instead of the "obstacles" that might be encountered when implementing TQM.
The second part of the requirement is about how to overcome those obstacles. An incorrect interpretation might be "what the benefits of TQM would be, after implementing."
You can see that these are "similar" kinds of questions, but strictly speaking, they are different. So be very careful when reading the requirements or trigger sections that you identify the key verbs and key terms so you are certain that you are answering what is being asked.
A final point: ensure that your answers are in alignment with the question asked.
This is essentially about ensuring that you do not go off task as you write your answer - that your answer tracks the requirement and corresponds to each part of the requirement.
Too often, students go off task, padding or filling out their answers with irrelevant information. While writing each new paragraph, the key is to briefly refer back to the question and quickly ask yourself: "Is this relevant and contributing to answering the question?".
This is a good "check" to ensure you align your answers with the question.
4. Good Structure and Planning Can Help You Pass Your CIMA Case Study Exam
We really can’t overemphasize the importance of proper structure for your answers when it comes to achieving the required CIMA pass rates.
There are three main reasons why structure and careful planning is essential for getting your CIMA certificate:
1. It helps to ensure you have enough points raised to obtain top marks
2. It helps to prevent the duplication of content
3. It makes life easier for the marker – more precisely, it makes it easier for the marker to see that you have indeed addressed each requirement adequately, where you have done so, and how much you have written for each requirement. Consider good structure as being key to a more positive examiner experience.
If you clearly signal where each task starts and ends with headings and sub-headings and give each relevant point a full, separate paragraph, you will be making the examiner’s life easier (a happy examiner is likely to be more generous with marks!).
The post-exam reports ALWAYS mention the importance of structure. An orderly answer indicates an orderly and clear thought process behind your answer and shows evidence of planning.
5. Our Top Tips for CIMA Case Study Exam Structure and Planning
Firstly, a common question from students is: how do I know how many paragraphs to write for each task?
Now sometimes, we get lucky and a task or requirement will say something like: "Identify five risks..." or "Give three benefits...". In such questions, it's obvious how many paragraphs there should be!
For five risks, we will write five paragraphs, one for each risk. For the three benefits, we will write three paragraphs, one for each benefit.

Unfortunately, CIMA isn't always so generous! We are not always given the specific number of points explicitly. And so the question then becomes: how do we decide on the number of paragraphs to write?
The key is to look at the percentage of marks allocated for that particular task. CIMA now includes a percentage allocation for each task.
A good rule of thumb here is that for every 10% allocated to a particular task, you add one paragraph. So if a task is worth, say, 33%, you will write approximately three paragraphs. If a task is worth 60%, you will write about six paragraphs.
Note that this is a "rule of thumb." This, of course, isn't supposed to be a perfect formula, but rather a guideline to get you started.
There may be cases where four paragraphs are sufficient for a 60% task, if the paragraphs are long and substantial enough in terms of content . Nevertheless, this is a useful general guideline.
We recommend that you plan and structure your answers before you begin writing.
However, many people lose valuable time at the beginning of each section planning their answers elaborately on the separate whiteboard provided on exam day. Instead, we recommend planning your answer within the answer box itself, not on the whiteboard or outside of the answer box.
A great way to plan on the fly is to work up a structure and fill in the gaps as you proceed. This forces you to get writing immediately, and by the time your structure is "filled out" the answer pretty much writes itself as you just go back and flesh out each heading and sub-heading:
Requirement A (this would correspond to the first requirement, so use an appropriate title, e.g., “Financial Performance”)
- Paragraph 1 (relates to first major point): idea 1, idea 2, idea 3 (If you have time, it’s worth emphasising the title of this key point by underlining it or putting it in bold, for example)
- Paragraph 2 (relates to second major point): idea 1, idea 2, idea 3
- Paragraph 3 (relates to third major point) etc etc: idea 1, idea 2, idea 3
Requirement B (this would correspond to the second requirement, e.g., "Balanced Scorecard")
…repeat as per above
TASK 2…repeat as per above
Remember, get straight to the point.
Write a 1-2 line introduction at the beginning of your answer, restating briefly what you were asked and the order in which you will address each point in the body of your answer.
Too many students waste time in their opening remarks repeating information we already know or rehearsing irrelevant information.
(You will see in VIVA’s model answers how short the introductions are -> you want to give yourself as much time and space as possible to demonstrate your knowledge and understanding! No marks are given for pleasantries).
It's also a good idea to have a "time plan" for each section of the exam. Now some students worry when they hear this: "ANOTHER PLAN!? Isn't that just going to waste even more of my time!?"
But there is no need to worry here because a time plan is really very simple - but also crucially important! You need to know roughly how much time you can spend on each task so you don't run out of time!
The first thing to do is, note the length of time allocated per section. In the case of the OCS and MCS, that will typically be 45 minutes per section (with 4 sections in total).
In the case of the SCS, it will typically be 60 minutes per section (with 3 sections in total). The next thing to note is that not all of that time will be or can be spent literally answering the question.
Because, of course, some of that time will be needed for reading the question! So it's a good idea to deduct a short amount of time from the total allocated to a section and consider this your "reading time."
A good target is around 5 or 6 minutes maximum for reading the question. The amount left over after you deduct the reading time is the amount that you can allocate to actually writing your answer.
Now, remember: writing your answer should include the planning process. So you don't need to allocate a separate portion of time to the "planning." Consider planning and writing part of the whole answering process.
So after you deduct the reading time, you now need to decide how to allocate the "answer time."
And this is a relatively simple process. Note the percentages allocated to each task/subtask. Then use that to calculate that percentage of the answer time.
So, for example, let's say you have a section of 45 minutes. You deduct 5 minutes for reading time, which leaves 40 minutes.
Let's say there are two tasks: the first one is worth 60%, and the second one is worth 40%.
60% of 40 minutes is 24 minutes. 40% of 40 minutes is 16 minutes.
And there you have it! You would have 24 minutes to write your answer for the first task, and 16 minutes to write your answer for the second task.

7. A Quick Word on CIMA Case Study Answer Length
I'm sure you've all heard the cliche, "it's about quality, not quantity"!
Of course, there's a kernel of truth to that. But let's be realistic: you're not going to pass your exam if you write 2 lines of text, even if they're the best lines ever written by a CIMA student!
So the cliche only gets us so far. The reality is that markers consistently report that longer answers do tend to score higher marks. And that shouldn't be surprising.
Other things being equal, the longer an answer is, the more likely it will contain more points - or more detailed points.
When it comes to the OCS and MCS exams, you should be aiming for a minimum of 2 pages, but ideally, 3 pages in a typical 45-minute section would be best if you want to score well.
Each relevant point you make should get a separate paragraph and be supported by examples, reference to the pre-seen and perhaps the real-life industry if applicable, and relevant theories from the Enterprise, Performance and Financial pillars.
In the case of the SCS exam , you should aim for a minimum of 2.5 pages, but ideally, 3.5 pages + in a typical 60-minute section if you want to score well.
8. What about the Content of Your CIMA Exam Answers?
Of course, we can’t tell you exactly what to write – that depends on the questions asked on the day! But there are some fundamental rules of thumb and principles that you should bear in mind.
(i) Justify and Explain
One very simple but crucial point is the following: you have to justify all your arguments and should explain technical terms.
Now that might appear obvious. But you’d be surprised how frequently students fail to do these basic things. And our tutors believe they know why.
This is the error of assuming that the marker will already know what you are talking about. The thing is, they probably will! But that’s not the point.
The point of the exam is to demonstrate your understanding!
So even if you think your marker will probably know what you mean, you should act as if they might not. Show them that you understand, and leave no room for doubt.
A good tip here is to try to really adopt the role that you have been assigned - and correspondingly, speak to the character whom you are addressing in the scenario as if they really are that person!
That way, you are more likely to consider terms they may not fully understand and give more comprehensive explanations of your arguments and conclusions.
Consider the following passage, which is taken verbatim from a past student’s mock exam answer for the Strategic Case Study of May 2018:
“Mr. Winston, however, may not understand the online streaming industry, where consumers just want to watch movies and tv series without interruptions of advertisements in between. His presence may also de-motivate other employees looking to grow within the business. The cultures may be different and it will take him a long time to get used to the streaming business.”
The student left it at that and then moved on to the following requirement. You should be able to see clearly what is wrong here, even without knowing what question was asked.
Each of the three sentences above could (and should have) been explained. Take the first. The obvious question is: why may Mr. Winston not understand the streaming industry?
Consider then the second sentence. The obvious question here is, why might his presence de-motivate other employees?
There’s a hint when he mentions other employees have been willing to “grow within the business,” but the student still fails to make his argument explicit.
What he might be trying to say is that, given that existing employees have grown with the business and have been loyal to the company for a long time, they might feel some resentment towards an external person being given a high-ranking position – instead of hiring from within the company.
But this is not what the student wrote. And so, he lost potential marks by not spelling it out. In the case of the final sentence, there are two more key points left undeveloped: in what way exactly may the cultures be different? And why might it take Mr. Winston a long time to get used to the business?
It’s clear that these points seemed obvious to the student, but he ultimately lost marks because he did not demonstrate understanding.
What you’ll often find is that, once you begin to explain something that seems to “go without saying,” you actually think of interesting points that you hadn’t considered before or that you had forgotten. You want to give yourself as much opportunity to make as many points as possible in support of your answer.
(ii) Give Specific Examples in Terms of the Pre-seen Company
It is not enough to simply define a theory or principle or even to explain a theory or principle in the abstract.
You have to apply it as well. What does that mean?
Basically, you have to be able to say why or how a particular theory/principle/method is relevant to the specifics of the unseen and pre-seen information.
Ask yourself: How can this theory be applied to the current case? What are some concrete examples of the abstract concepts I am using here in terms of the current company?
To follow my own advice, let’s look at another example from another real student’s answer. In this case, the student is asked to give examples for each category in a cost of quality report (OCS May 2018). The student’s answer to this requirement is as follows:
“A) Examples of costs to be included in each category of the report are as below:
1. Wastage of materials when errors are found.
2. Duplication of workload when errors are found
3. Damage to morale when work has to be repeated
1. Loss of consumer confidence
2. Damage to reputation
3. Cost of replacing the product
1. Invest in better-trained staff to ensure fewer errors in production.
2. Invest in higher quality materials to ensure the material doesn't fail.
3. investment in automating processes to reduce human error
1. Inspection of raw materials on arrival
2. Inspection of completed goods before they leave the factory
Notice that this segment of the answer is quite well-structured. The student uses headings and sub-headings and orders the answer logically.
However, the problem is that the student doesn’t actually give specific examples for each category that are derived from the company in question (a luxury bag manufacturer in this case).
Instead, she gives generic examples that could come from almost any company that manufactures any product. In this case, the student would need to provide specific examples.
So instead of simply saying “wastage of materials when errors are found,” the student should give concrete examples of errors that could occur in the context of this company, a luxury bag manufacturer. What kinds of materials are likely to be wasted? What kinds of errors might be found?
These are the kinds of questions you should be asking yourself when applying a particular theory or model to the current case. The marker needs to see that you can actually use the theories and models you have learned during your objective studies in a real-world scenario and in a realistic way.
This shows you’re ready for the real world as a management accountant! Simply giving generic examples that could equally well apply to any number of companies or scenarios is not sufficient to score full marks.
(iii) Avoid List-style Answers
Another common error is that students will give their answers in the form of bullet points.
Unfortunately, this is not what markers are looking for. It might seem neat and tidy and concise to you, but to a marker, it will simply give the impression of superficial engagement. Lists also give the impression that you are rushing through the answer.
Try to write your answers in prose style. It should be conversational but professional. You are trying to engage with and guide the fictional person who has asked for your assistance. Throwing a list of bullet points without elaboration will not be acceptable!
Now that’s not to say that you can’t use bullet points to structure your answers. But this is different from simply having a list of one-liners alongside bullet points.
You may organize your sub-headings in a bullet-point style, but what follows should be in prose style, with full sentences, explanations, examples and justifications.
9. Managing Technical CIMA Questions That Appear in Exams

Many students mistakenly believe that when it comes to more technical questions involving financial statements and “the numbers,” a different approach is necessary.
Students feel they need to spend much of their time performing calculations and showing off their ability to use various formulae from their objective studies.
However, this is not the case. In fact, you are not expected to perform lengthy calculations when it comes to technical components in the case study exam. Instead, the extent to which you will be expected to demonstrate your technical capacities corresponds to the following:
You will need to be able to:
- explain how the content of a schedule/table/financial statement has been prepared
- interpret the solution from a schedule/table/financial statement
- interpret the information within the schedule/table/financial statement
- explain the accounting treatment for a certain type of transaction and the impact on the financial statements
The occasional basic calculation can be made to illustrate a point or to support your interpretation, but that interpretation should be written in prose form.
Markers do not want to see long strings of calculations and formulae without any written explanation or justification. If you do include calculations, keep them short, and focus instead on demonstrating your understanding through written means.
When it comes to a general approach to technical components, we recommend that you follow the order of operations indicated in the diagram below:

We've already dealt with structure above.
In terms of content, it's a good idea to start with the general theoretical and technical concepts/principles that you are going to be using in the requirement. You don't need to spend too long on this phase - you're not expected to give a complete, exhaustive abstract explanation of a model or theory.
Rather, give a short but jargon-free summary of the model or theory that you are making use of. You want to spend as much time on the application phase as possible. This is where you will demonstrate your deep theoretical understanding.
Remember, markers want to see you applying your knowledge as if you were really working in this company, in the specified role, and charged with the tasks outlined in the exam .
Simply stating abstract principles would not be acceptable in the real world. Nor is it acceptable in the CIMA case study exam.
Finally, and ideally, you want to move beyond the narrow application of the relevant theory to the specifics of the case.
Markers like to see students adopt a wider perspective of the business and spell out some of the broader implications of your solution to the task/requirement.
It's a good idea here to stretch out your time horizon and consider second and third-order consequences of a particular action - positive or negative.
Markers also like to see students derive conclusions and recommendations in questions where students are asked to consider advantages and disadvantages/risks and benefits of particular courses of action. This shows deeper engagement with the case and wider business awareness.
10. How to Pass CIMA: Key Takeaways for Upcoming Case Study Exams
When it comes to case study success, there are three really key components:
1. Solid theoretical knowledge relating to objective subjects
2. Intimate knowledge of the pre-seen and some familiarity with industry trends
3. Excellent exam answer technique
Too many CIMA students get hung up on revising their objective test theory in the wrong way, i.e., committing theory to memory from their CIMA objective test textbook material. The result is less flexibility in producing answers to new problems.
A much better approach is to review key theories by applying each one to the specifics of the current pre-seen document.
In this way, you kill two birds with one stone, i.e., solid theoretical knowledge and intimate knowledge of the pre-seen.
Then it's all about honing your exam technique. The reality is, our markers report over and over again that the typical student's main problem often isn't so much their lack of theoretical knowledge (although that is sometimes definitely the case), as they cannot order their thoughts, apply their knowledge, and master their timing.
When it comes to timing, there's no substitute for practice (all of VIVA's mocks can be taken in our online CIMA exam simulator under timed conditions).
If you combine practice with careful attention to the points listed above, you will likely write an exam answer that puts you ahead of 90% of CIMA students.
11. Get Ahead with VIVA Financial Tuition
It doesn't matter what kind of business career you're after, CIMA qualifications can unlock a brighter future.
Whether you aim to be a Chief Financial Officer or business owner, our CIMA exam tips and advice can get you where you want to go faster, and for a fraction of the cost of traditional tuition providers. Start your journey today and discover our range of CIMA courses .
Check out our market-leading Study Packs for the current Case Study. The packs include hours of high-quality video tuition by some of the world's leading CIMA experts, alongside mock exams modelled closely on the official CIMA exams and a whole range of support materials. You might also want to read: Working through your CIMA Case Study Course .
Like to explore how VIVA can help you with your CIMA studies?
Be the first to find out when we release new CIMA resources.
Related resources
Other resources that may be of interest to you.
- Course Finder
- Career Advice
- PQ Awards 2022
- PQ Awards 2021
- PQ Awards 2020
- PQ Magazine
- NQ Magazine
CIMA has released the November case study pass rates, and they show sitters coped well with what the examiner threw at them.
Those sitting the Management case achieved the highest pass rate, at 73%. That’s the best pass rate for this level for the whole of 2021.
Some 67% of Strategic sitters passed the final case this time around, which is slightly down on the August rate of 69%.
The Operational pass rate seems stuck, with just over half of sitters passing this time around – 52%

Stephen Flatman, Vice President, Examinations – Management Accounting, told PQ magazine:“I’m very happy to see that our students’ performance in case study exams remains strong. The efforts they are putting into their CIMA studies are clearly paying off.
“Over the past two years, our students have shown great dedication to taking their careers to the next level despite the ongoing uncertainty and disruption caused across the world by the coronavirus pandemic. After witnessing their efforts and accomplishments of the last two years, I firmly believe they have the traits employers seek, which enable CGMA designation holders to build successful careers in accounting, business and finance.
“We look forward to seeing what our students will achieve in 2022 and will continue to support them every step of their CIMA journey.”
CIMA CASE STUDY 2021 PASS RATES
Nov Aug May Feb
Operational 52% 53% 46% 60%
Management 73% 68% 69% 71%
Strategic 67% 69% 68% 67%

Copyright © 2024 PQ Publishing.
Privacy Overview

- You are here:
- Home »
- Blog »
- CIMA Exam »
CIMA Exam Results and a Look at the Grading Mechanism
- By Stephanie Ng
- / 3 COMMENTS
Eager to find out when you will get the CIMA exam results, and how to interpret the performance report?
CIMA Exam Results for Objective Tests
Since the implementation of the new format, it is the easiest and fastest to get the CIMA objective test exam results — you get a “pass” or “fall” grade immediately after the computerized exam.
After 48 hours, you will receive an email, confirming that CIMA exam results breakdown is available in the MYCIMA portal.
CIMA Exam Results Breakdown
The results are shown in a table format, listing the subject areas in the objective test.
Each subject area is marked “proficient” or “not proficient”, with more detailed comments on how you can improve.
Here is a sample showing the candidate getting a “not proficient” in Financial Reporting:
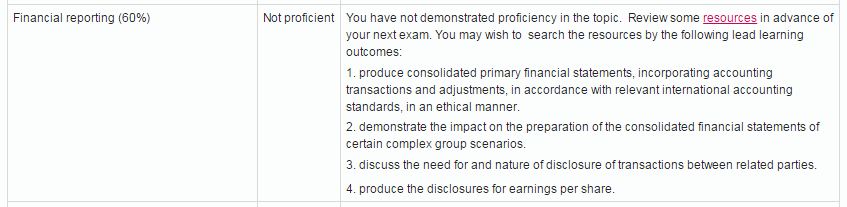
Image courtesy of thecimastudent.com
If you’d like to have a glimpse at how the full performance report looks like, check out this official video from the CIMA:
A Note on CIMA Exam Pass Mark
The CIMA states that you need a 70% accuracy to pass each objective test. At the same time, they tell you the CIMA exam pass mark is 100.
Please understand that you are given a scaled score ranging from 0 to 150. The emphasis is that this score is scaled, meaning that you cannot figure out your percentage accuracy by a straight formula. In fact, if you try to divide 100 by 150, you get 66.7% instead of 70%.
The scaling is a way for CIMA to balance the more difficult questions with the easier questions . The system weighs difficult questions slightly more, such that students who got a few more difficult questions wrong would still score the same as those getting more easier questions right.
CIMA Case Study Results
The questions in case study exams are more complex. Human graders instead of computers mark this exam, and therefore, we need to wait for approximately 5 weeks after the end of the testing window to get the results.
Similar to the objective test, the performance report is available on the MyCIMA portal, and is presented in the form of a table. We will see “Strong”, “Moderate” or “Fail” in each of the 5 competencies.
In this example, the table is showing “Moderate” under technical skills is marked “Moderate”, with details on areas of improvement:
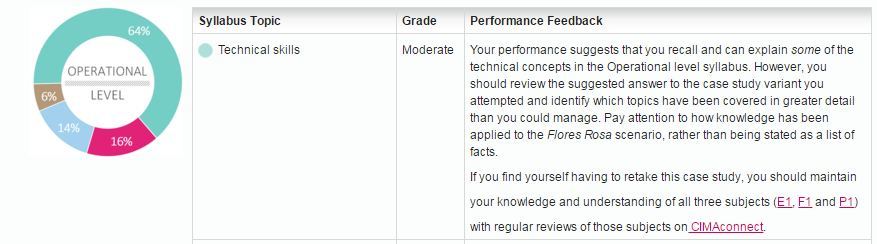
For those who would like to know the technical side of grading, here is a detailed explanation by the CIMA:
A Note on CIMA Case Study Pass Mark
The pass mark for case study is 80, out of a scaled score of 0 to 150. The candidate must also score a “Moderate” or “Strong” across all competencies in order to pass the case study exam. This is to ensure that the candidate is not weak in a particular area and that he/she is “business ready”.
Even if you pass, take a look at the performance report for rooms of improvement. This will only help you in the next CIMA case study exam.
Is This helpful?
I hope the discussion on CIMA exam grading and results is helpful in preparing psychologically for this exam. If you have any questions, please drop a note in the comment section. Thank you and good luck!
For Your Further Reading
- Frequently asked questions on the CIMA exam
- CIMA exam historical pass rates
About the Author Stephanie Ng
I am the author of How to Pass The CPA Exam (published by Wiley) and the publisher of this and several accounting professional exam prep sites.
Related Posts
Approved CIMA Exam Calculator (+ My Recommendation)
CIMA Practice Exams and Online Course: A Comparison
How Hard is the CIMA Exam? 3 Ways to Find Out the Difficulty
Popular posts
Cma exam results: cma exam release dates for 2024, how to become a cma: the cma certification process, cma requirements: how to meet the cma exam requirements & determine your eligibility, best cma exam review courses & cma exam prep 2024.
0800 048 7804 | [email protected]
About Us Testimonials Exam Dates About CIMA Quick Purchase Basket Help Contact Us studyHUB Home

CIMA Exam Dates 2024: W hen (and how) should you take your next exam?

The first half of 2024 flew by - but don't worry!
You can still write your CIMA exams this year. We've compiled a list of the remaining CIMA exam dates to assist you in getting ready. Make a note of which exam you plan to write and use that date as a marker for your preparations.
Objective Tests
Objective Tests can be taken on-demand and are available to sit all year round. You will need to complete an Objective Test for all E, P, F subjects, as well as any Certificate Level subjects, unless are on the Finance Leadership Programme.
2024 CIMA Case Study Exam Timetable
August 2024 cima exam dates.
- Exam entry opening – January 24th, 2024
- Exam entry closing – July 23rd, 2024
- Pre-seen material released – March 22nd, 2024
- Exam dates – August 7th to 9th, 2024
- Results published – September 26th, 2024
- Exam entry opening – January 31st, 2024
- Exam entry closing – July 30th, 2024
- Pre-seen material released – March 27th, 2024
- Exam dates – August 14th to 16th, 2024
- Results published – October 3rd, 2024
- Exam entry opening – February 7th, 2024
- Exam entry closing – August 6th, 2024
- Pre-seen material released – April 5th, 2024
- Exam dates – August 21st to 23rd, 2024
- Results published – October 10th, 2024

November 2024 CIMA Exam Dates
- Exam entry opening – April 24th, 2024
- Exam entry closing – October 22nd, 2024
- Pre-seen material released – September 20th, 2024
- Exam dates – November 6th to 8th, 2024
- Results published – January 2nd, 2025
- Exam entry opening – May 1st, 2024
- Exam entry closing – October 29th, 2024
- Pre-seen material released – September 27th, 2024
- Exam dates – November 13th to 15th, 2024
- Results published – January 9th, 2025
- Exam entry opening – May 8th, 2024
- Exam entry closing – November 5th, 2024
- Pre-seen material released – October 4th, 2024
- Exam dates – November 20th to 22nd, 2024
Need help registering for an exam or getting started with the CIMA qualification? We're here for that too!
Complete the form below, select 'CIMA', and one of our friendly Student Advisors will be in touch to assist you.
Email Address
Contact number
Select Institute
I would like to receive course related updates, news and promotional information from IBTC.
Please note that it can take up to 2 working days to receive an answer.
Related Articles

The first half of 2024 flew by - but don't worry! You can still write your CIMA exams this year. We've compiled a list of the remaining CIMA exam dates to assist you in getting ready. Make a note of which exam you plan to write and use that date as a marker for your preparations.

When Is The Best Time To Start FLP?
“What is the best time of year to start CIMA’s Finance Leadership Programme (FLP)?” This is one of the most common questions we receive from students when trying to plan their studies. While you can enrol in the FLP programme year-round, not all start dates are created equal. Here is why choosing the right start date can have a huge impact on your FLP subscription.

5 Tips for CIMA Case Study Exams
Preparing for the CIMA Case Study Exams can be a daunting task. As future management accountants, you need to showcase not only your knowledge of the syllabus but also your ability to apply this knowledge in real-world scenarios. Here are five essential tips that can help you excel in your upcoming CIMA case study exams.
Get started
The secret to getting ahead is getting started.
100% Secure
© 2024 International Business Training College | Level 2 B-BBEE Supplier
- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .
- My View My View
- Following Following
- Saved Saved
Wegovy users keep weight off for four years, Novo Nordisk study says
- Medium Text
HEART BENEFITS
Sign up here.
Reporting by Maggie Fick Editing by Bill Berkrot and Louise Heavens
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Thomson Reuters
Maggie is a Britain-based reporter covering the European pharmaceuticals industry with a global perspective. In 2023, Maggie's coverage of Danish drugmaker Novo Nordisk and its race to increase production of its new weight-loss drug helped the Health & Pharma team win a Reuters Journalists of the Year award in the Beat Coverage of the Year category. Since November 2023, she has also been participating in Reuters coverage related to the Israel-Hamas war. Previously based in Nairobi and Cairo for Reuters and in Lagos for the Financial Times, Maggie got her start in journalism in 2010 as a freelancer for The Associated Press in South Sudan.
Business Chevron

US FDIC needs 'fresh start' with new chair, White House official says
The White House believes the U.S. Federal Deposit Insurance Corp needs a "fresh start" with a new chair who is not part of the leadership that presided over its long-running cultural problems, a White House official told Reuters on Tuesday.

How to pass the Operational Case Study
As you may already know, Operational Case Study (OCS) is the first case study under CIMA's CGMA professional qualification and is designed to test your ability to apply the technical knowledge you’ve learnt from E1, P1 and F1.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 May 2024
CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials
- Neil H. Segal ORCID: orcid.org/0000-0002-3047-2303 1 ,
- Ignacio Melero ORCID: orcid.org/0000-0002-1360-348X 2 , 3 ,
- Victor Moreno ORCID: orcid.org/0000-0001-6099-4236 4 ,
- Neeltje Steeghs ORCID: orcid.org/0000-0003-2989-2279 5 ,
- Aurelien Marabelle ORCID: orcid.org/0000-0002-5816-3019 6 ,
- Kristoffer Rohrberg ORCID: orcid.org/0000-0002-5448-9003 7 ,
- Maria E. Rodriguez-Ruiz 2 ,
- Joseph P. Eder 8 ,
- Cathy Eng 9 ,
- Gulam A. Manji 10 ,
- Daniel Waterkamp 11 ,
- Barbara Leutgeb 12 ,
- Said Bouseida ORCID: orcid.org/0000-0003-3908-4646 12 ,
- Nick Flinn 12 ,
- Meghna Das Thakur 11 ,
- Markus C. Elze 12 ,
- Hartmut Koeppen 11 ,
- Candice Jamois 12 ,
- Meret Martin-Facklam 12 ,
- Christopher H. Lieu ORCID: orcid.org/0000-0002-4852-4642 13 ,
- Emiliano Calvo ORCID: orcid.org/0000-0003-4921-829X 14 ,
- Luis Paz-Ares 15 ,
- Josep Tabernero ORCID: orcid.org/0000-0002-2495-8139 16 &
- Guillem Argilés 16 , 17
Nature Communications volume 15 , Article number: 4091 ( 2024 ) Cite this article
2052 Accesses
2 Altmetric
Metrics details
- Antibody therapy
- Cancer immunotherapy
- Colon cancer
Cibisatamab is a bispecific antibody-based construct targeting carcinoembryonic antigen (CEA) on tumour cells and CD3 epsilon chain as a T-cell engager. Here we evaluated cibisatamab for advanced CEA-positive solid tumours in two open-label Phase 1 dose-escalation and -expansion studies: as a single agent with or without obinutuzumab in S1 (NCT02324257) and with atezolizumab in S2 (NCT02650713). Primary endpoints were safety, dose finding, and pharmacokinetics in S1; safety and dose finding in S2. Secondary endpoints were anti-tumour activity (including overall response rate, ORR) and pharmacodynamics in S1; anti-tumour activity, pharmacodynamics and pharmacokinetics in S2. S1 and S2 enrolled a total of 149 and 228 patients, respectively. Grade ≥3 cibisatamab-related adverse events occurred in 36% of S1 and 49% of S2 patients. The ORR was 4% in S1 and 7% in S2. In S2, patients with microsatellite stable colorectal carcinoma (MSS-CRC) given flat doses of cibisatamab and atezolizumab demonstrated an ORR of 14%. In S1 and S2, 40% and 52% of patients, respectively, developed persistent anti-drug antibodies (ADAs). ADA appearance could be mitigated by obinutuzumab-pretreatment, with 8% of patients having persistent ADAs. Overall, cibisatamab warrants further exploration in immunotherapy combination strategies for MSS-CRC.
Similar content being viewed by others

Cancer therapy with antibodies

Antibody drug conjugate: the “biological missile” for targeted cancer therapy

Long-term outcomes following CAR T cell therapy: what we know so far
Introduction.
New treatment options aim to expand the benefit of cancer immunotherapy beyond inflamed tumours 1 . Despite the approval of immune checkpoint inhibitors in a diverse range of solid malignancies 2 , these therapies lack efficacy in the majority of patients. This is particularly true for patients with cancers that are characterized as nonimmunogenic (sometimes termed cold) 3 because they lack sufficient tumour-specific T cells, have insufficient expression of neoantigens, show defects in major histocompatibility complex antigen-presentation machinery and/or are rich in immunosuppressive factors in the tumour microenvironment 2 , 4 , 5 , 6 , 7 . Bispecific T-cell engagers were developed to redirect cytotoxic T cells to predefined tumour targets, primarily for major histocompatibility complex–independent cancer cell elimination 8 .
Cibisatamab is a T-cell bispecific antibody (TCB) that uses a 2-to-1 molecular format to target carcinoembryonic antigen (CEA) expressed on the surface of tumour cells and CD3ε on T cells 9 . A human immunoglobulin G1 (IgG1)–based TCB, cibisatamab’s heterodimeric Fc region has been disabled to avoid Fc receptor engagement and confer an extended half-life. The flexible antibody structure, with 2 CEA binding domains and 1 CD3ε binding domain, enables higher CEA avidity, for selective killing of CEA-expressing tumour cells.
CEA is a cell-surface glycoprotein that reportedly plays a role in cell adhesion, invasion and metastasis of cancer cells 10 and is overexpressed on a variety of cancers, including colorectal cancer (CRC) 11 , 12 . CEA can be released from the plasma membrane upon enzymatic disruption of the glycosyl phosphatidylinositol binding bridge 13 and constitutes a widely used measurable quantitative biomarker 14 . Importantly, the CEA binding site for cibisatamab persists on tumour cells even if the link is cleaved and released from the plasma membrane. Indeed, to avoid toxicity from on-target off-tumour binding to circulating CEA, cibisatamab was optimized to recognize an epitope that is only present in the CEA membrane-attached form 9 .
Simultaneous binding of cibisatamab to CEA and CD3ε causes T-cell activation independent of T-cell receptor specificity, leading to lymphocyte-mediated tumour cell killing, immune-stimulatory cytokine release and further release of tumour antigens. In nonclinical models, cibisatamab demonstrated the ability to increase T-cell infiltration in CEA-expressing tumours, thus converting non-inflamed programmed death-ligand 1 (PD-L1)–negative tumours into highly inflamed PD-L1–positive tumours 15 . Furthermore, combining cibisatamab with an anti–PD-L1 blocking antibody synergistically enhanced its efficacy in humanised mice 16 . In cultured human tumour organoids, redirected T-cell–mediated cytotoxicity depended on the level of CEA surface expression 17 .
Because monoclonal antibodies and related constructs may induce the development of anti-drug antibodies (ADAs), researchers have studied whether pre-treatment with obinutuzumab, a glycoengineered humanized anti-CD20 monoclonal antibody that recognizes the CD20 antigen present on B-cells, could blunt or attenuate ADA generation. In nonclinical and clinical studies, obinutuzumab induces a profound B-cell depletion, which has been shown to result in suppression of de novo antibody responses, while leaving largely intact the protective humoral memory of long-lived plasma cells 18 , 19 .
Here we collectively describe the results of two multi-institutional Phase 1 dose-escalation and -expansion studies of cibisatamab in patients with advanced CEA-positive solid tumours: S1, studying cibisatamab as monotherapy (Study BP29541; NCT02324257 20 ), and S2, studying cibisatamab combined with atezolizumab (Study WP29945; NCT02650713 21 ). We evaluated the safety of cibisatamab as a single agent as well as in combination with atezolizumab in patients with MSS-CRC, demonstrating a safety profile consistent with that of each individual agent. Coupled with preliminary efficacy results, these data suggest that this treatment approach may be worth further clinical investigation.
From 2014 to 2018, 149 and 228 patients were enrolled and treated in S1 and S2, respectively (Supplementary Figs 1 and 2 ). S1 included 125 patients with CRC (83.9%), 100 with confirmed microsatellite stable (MSS) disease (67.1%), 2 with confirmed microsatellite instability-high (MSI-H) (1.3%) disease and remaining patients unknown. All patients had metastatic disease at study entry, most with metastases to the liver ( n = 38 [25.5%]), lung ( n = 30 [20.1%]) or both ( n = 73 [49.0%]). At study entry, patients’ mean age was 60 years (range: 22-80 years), 61 (40.9%) were female. Eastern Cooperative Oncology Group performance status (ECOG PS) at baseline was 0 ( n = 82 [55.0%]) or 1 ( n = 67 [45.0%]). 147 patients (98.7%) had received at least one prior line of therapy for metastatic disease, 61 (40.9%) received prior adjuvant treatment and 90 (60.4%) received 3 or more prior lines of therapy.
S2 included 192 patients with CRC (84.2%), 187 with confirmed MSS (82%) disease, 5 with confirmed MSI-H (2.2%) disease and remaining patients unknown. All patients had metastatic disease at study entry, most with metastases to the liver ( n = 56 [24.6%]), lung ( n = 33 [14.5%]) or both ( n = 127 [55.7%]). At study entry, patients’ mean age was 57 years (range: 24-81 years), 96 (42.1%) were female. ECOG PS at baseline was 0 ( n = 132 [57.9%]) or 1 ( n = 96 [42.1%]). 227 patients (99.6%) had received at least one prior line of therapy for metastatic disease, 91 (39.9%) received prior adjuvant treatment and 140 (61.4%) received 3 or more prior lines of therapy. Detailed baseline characteristics for S1 and S2 are presented in Supplementary Table 1 .
In S1, 149 patients were treated with flat-dose levels of cibisatamab ranging from 0.052 to 600 mg and with step-up dosing cohorts, and with obinutuzumab pretreatment. In S2, 228 patients were treated at flat-dose levels of cibisatamab ranging from 5 to 300 mg and in step-up dosing cohorts in combination with atezolizumab.
All patients were included in the S1 and S2 safety- and efficacy-evaluable populations, which were defined, respectively, as all patients who received at least one dose of cibisatamab or obinutuzumab and all patients who received at least one dose of cibisatamab or atezolizumab. In S1, 65 patients who received flat-dose cibisatamab without obinutuzumab prior to treatment and 15 patients who received cibisatamab with obinutuzumab prior to treatment were evaluable for dose-limiting toxicities (DLTs). Step-up dosing cohorts were explored to assess whether the approach of starting with low-dose cibisatamab then dose-escalating would mitigate infusion-related reactions observed during late cycles. An additional 22 patients in step-up dosing cohorts were evaluable for DLTs to determine the late cycle MTD. In S2, 73 patients in Part IA (dose escalation) were evaluable, while 153 patients in Part IB (dose schedule finding) were evaluable for DLTs to determine the late cycle MTD.
Safety (primary objective)
In S1, 116 of the 149 patients (77.9%) received cibisatamab only, 27 patients (18.1%) received obinutuzumab pretreatment followed by cibisatamab and 6 patients (4.0%) received obinutuzumab only due to treatment discontinuation prior to receiving the first dose of cibisatamab. An overview of safety in S1 and S2 is provided in Table 1 . Apart from a lower incidence of both on-target/off-tumour gastrointestinal events (diarrhoea, nausea, vomiting) and infusion-related reactions (IRRs) at doses below 60 mg in S1 and doses below 80 mg in S2, no clear trends for dose-dependent adverse events (AEs) were observed. Of note, most patients developed early onset transient tumour inflammation and systemic cytokine-related effects (see below).
In S1, 6 of 149 patients (4.0%) experienced the following grade 5 events: dyspnoea, IRR and respiratory failure (all considered related to cibisatamab); sepsis (considered related to obinutuzumab); and cardio-respiratory arrest and tumour thrombosis (not considered treatment-related by the investigator). In S2, 6 of 228 patients (2.6%) experienced grade 5 events: 1 cibisatamab-related event of hypovolemic shock and 5 events that were not considered treatment related by the investigator (respiratory tract infection, urinary tract infection, disseminated intravascular coagulation, bile duct obstruction and cerebrovascular accident).
DLTs and MTD (primary objective)
In S1, the maximum tolerated dose (MTD) was defined as 400 mg for flat continuous dosing once weekly (QW) and once every 3 weeks (Q3W), based on 7 cibisatamab-related DLTs reported in 7 of the 102 DLT-evaluable patients (6.9%). Two of these 7 DLTs were grade 5 events of respiratory failure and dyspnoea. In S2, which included Part IA (dose escalation) and Part IB (dose schedule finding), a total of 17 DLTs were reported in 15 of the 226 DLT-evaluable patients (6.6%) and included a grade 5 event of hypovolemic shock. During the S2 dose-escalation phase, all 3 patients at the 300-mg dose level had serious AEs (SAEs) after their first infusion. While these SAEs were not classified as DLTs, it was decided not to escalate the dose further. Thus, the MTD for cibisatamab in combination with atezolizumab was not reached.
Frequency and severity of AEs
Cibisatamab demonstrated a dynamic safety profile, with the onset of most toxicity beginning in cycles 1 to 3 and decreasing as treatment progressed and tolerance improved. The main exceptions to this trend were fatigue, arthralgia and pruritus; these had notably later median onset times in S2 than in S1 and are known risks of atezolizumab (Fig. 1 ).
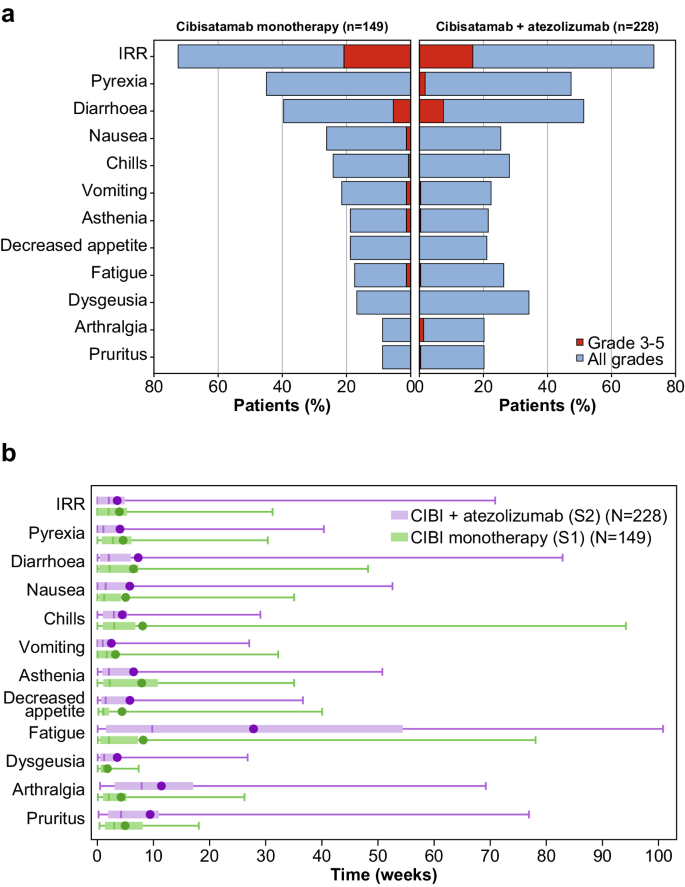
a Most frequent treatment-related adverse events (≥ 20% in either study) in S1 and S2. b Median time to onset (middle line; with means as circles, quartiles as boxes, and ranges as bars) of most common treatment-related adverse events (≥20% in either study) in S1 and S2. CIBI Cibisatamab, IRR Infusion-related reaction. Source data can be requested from the authors for academic research purposes.
AEs were predominantly associated with infusion of study treatment and the subsequent activation of immune cells, leading to local inflammation in the tumours and systemic cytokine-related effects.
In both S1 and S2, IRRs were reported in more than 70% of patients, with pyrexia, chills, vomiting, nausea and diarrhoea being the most commonly observed symptoms reported within 24 hours of the end of infusion (Fig. 1 and Supplementary Table 2 ). Grade 3 IRRs occurred in 31 of 143 S1 patients (21.7%) and 38 of 228 S2 patients (16.2%). The majority of IRRs and associated symptoms were short in duration and resolved the same day (see supplementary Tables 3 and 4 ) with protocol-recommended premedication measures and supportive care. To further mitigate the risk of IRRs and cytokine-release syndrome (CRS), corticosteroid pretreatment was given during step-up dosing (intravenous [IV] dexamethasone 10 mg or equivalent) and for patients who experienced a grade 2 IRR or CRS in the previous cycle (IV methylprednisolone 80 mg or IV dexamethasone 16 mg).
No CRS events were reported by preferred term in S1; in S2, CRS events were reported in 7 of 228 patients (3.1%). Given the overlap in signs and symptoms of IRR and CRS and the timing of the studies relative to the evolving definition and clinical management of CRS, it is clear that patients in studies S1 and S2 may have experienced CRS events that were instead reported as IRRs. To assess the incidence of CRS in these studies, a retrospective analysis was conducted to identify all events of hypoxia, hypotension or both (reported as standalone events or as reported symptoms of IRR) that occurred within 48 h of cibisatamab infusion. Patient records that indicated treatment of these events with supplemental oxygen or vasopressors and laboratory findings such as cytokine levels and ADA status were used to support the analysis.
Twenty-eight of 143 patients (19.6%) in S1 and 23 of 228 patients (10.1%) in S2 would have been captured as grade ≥ 2 CRS according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0 (in addition to the 7 patients in S2 [3.1%] for whom CRS was reported as the preferred term) (Table 2 ).
Tumour inflammation
In addition to systemic cytokine-driven toxicities, engagement of the tumour target and activation of T cells may drive a rapid expansion and infiltration of immune cells, resulting in tumour inflammation or flair, pain at the tumour site and, depending on the location of the inflamed tumour, mass effects that can impact organ function.
This phenomenon of temporary tumour enlargement was consistently observed in patients who received computed tomography (CT) scans within 48 to 96 h after drug administration and was attributed to inflammation of the tumour tissue (Fig. 2 ). In most cases, events of tumour inflammation, tumour pain and associated sequelae resolved within 2 to 3 days of administration, following management with corticosteroids and supportive care. However, severe pulmonary toxicity was observed in a small number of patients with multiple bilateral lung lesions and in patients with tumours sited near critical organ structures. Of note, one patient had respiratory failure and died following their first dose of cibisatamab 600 mg, as the result of an extrinsic tracheal/bronchial obstruction by an enlarged tumour mass. For this reason, patients with important thoracic involvement at critical sites and high lung tumour burden were excluded from ensuing trials.
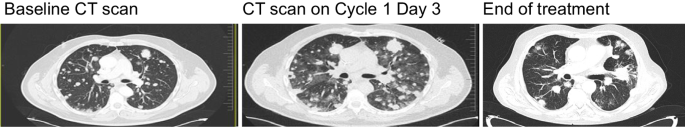
The patient received 60 mg cibisatamab on Cycle 1 Day 1 and experienced transient hypoxia and transient dyspnoea 48 h after infusion. CT Computed tomography.
Incidence of AEs in ADA-positive and -negative patients
Cibisatamab induced the formation of ADAs in 50% to 70% of patients. Figure 3 shows the combined incidence from S1 and S2 of selected AEs and associated symptoms related to IRR and CRS in ADA-positive and ADA-negative patients by infusion (first 7 infusions shown).
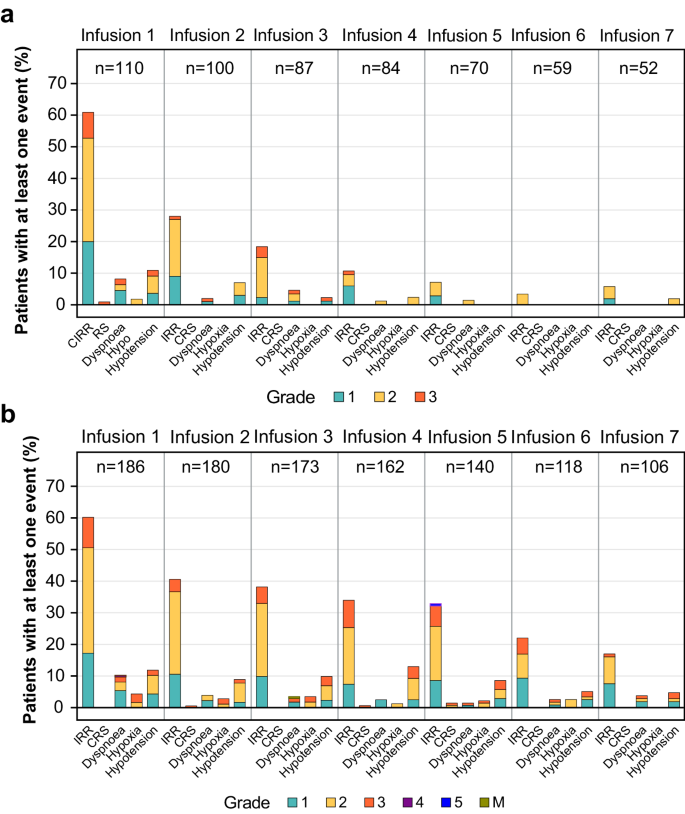
Data for ( a ) ADA-negative and ( b ) ADA-positive patients are shown by infusion for patients receiving 40–600 mg of cibisatamab (flat-dose and step-up dosing cohorts) in S1 and S2. ADA, Anti-drug antibody; CRS, Cytokine-release syndrome; IRR, Infusion-related reaction; M Grade information not provided by site. Source data can be requested from the authors for academic research purposes.
In both studies, IRRs and associated symptoms were more frequent and of higher severity in ADA-positive patients than in ADA-negative patients (Fig. 3 ). IRRs of grade 3 or higher were also more persistent in later cycles in ADA-positive patients than in ADA-negative patients. Irrespective of a patient’s ADA status, most IRRs were observed following the first infusion (median time to ADA onset in S1 was 22 days; in S2, 16 days).
Pharmacokinetics and Immunogenicity (primary objective in S1; secondary objective in S2)
A two-compartment pharmacokinetic (PK) model with first-order linear elimination well described the cibisatamab serum concentration time course in ADA-negative patients. In S1 and S2, the median clearance values were 0.063 L/h and 0.048 L/h and the volume of distribution at steady state values were 8.25 L and 8.31 L, respectively.
Clearance did not depend on dose and exposure and was similar after administration of cibisatamab alone or in combination with atezolizumab. After 100 mg Q3W, the maximum concentration and area under the time-concentration curve of the first dosing interval was 26.7 μg/mL (13.8–47.9) and 756 μg×h/mL (266–2398), respectively (median, 5th-95th percentile, n = 20). In ADA-negative patients, pharmacokinetics were time-independent. The cibisatamab clearance is approximately 7-fold to 8-fold higher than a typical IgG1 antibody in humans 22 .
The incidence of ADA development was high (50% in S1 in patients not pretreated with obinutuzumab; 71% in S2; Table 3 ). The drug tolerance of the ADA assay used in S2 was significantly better than that of the assay used in S1; therefore, the incidence of ADAs is not directly comparable. The median time to ADA onset was 22 days (range, 6–146 days) and 16 days (range, 7–260 days) in studies S1 and S2, respectively. Higher ADA titers were associated with a larger impact on loss of cibisatamab exposure, suggesting that ADAs are neutralizing, i.e., directed to epitopes in the CEA- or the CD3-binding regions of cibisatamab, or both, or increasing drug clearance from plasma.
After obinutuzumab pretreatment, 11 of 26 patients (42%) were ADA positive. Nine of 11 patients (82%) had transient ADAs, and in the remaining 2 patients later time points were unavailable (the last available ADA sample was early—at 3 and 9 weeks after the first dose of cibisatamab). The maximum observed ADA titer in obinutuzumab-pretreated patients was 1:270, while ADA titers were in general higher in patients who were not pretreated with obinutuzumab, with a maximal observed titer of 1:196,830 (study S1). In the obinutuzumab-pretreated population, cibisatamab exposure was sustained in ADA-positive patients, and the cibisatamab concentration levels were similar to those in ADA-negative patients (Supplementary Fig. 3 ).
In ADA-negative patients, PK was time-independent, i.e., serum exposure was maintained after multiple doses. In persistent ADA-positive patients, however, ADA-mediated, time-dependent PK was observed with reduced or no detectable exposure at end of infusion. Median time to onset of complete loss of exposure (exposure not detectable at end of infusion) was 35 days (range, 21–111 days) and 63 days (range, 21–189 days) in studies S1 and S2, respectively. Step-up dosing to high doses could not overcome the negative impact of ADAs on active cibisatamab exposure.
Efficacy (secondary objectives)
In S1, all patients ( n = 149) had measurable disease at baseline and were evaluable for efficacy. Six patients (4.0%, 90% CI: 1.8, 7.8) achieved a confirmed partial response (PR), including 3 of 46 patients (6.5%) enrolled in the step-up cohorts, 2 of 27 patients (7.4%) enrolled in the cohorts with obinutuzumab pretreatment and 1 of 65 patients (1.5%) enrolled in the flat dose cohorts. Five of 6 PRs occurred in patients with MSS-CRC and one in a patient with pancreatic cancer. The median duration of response was 6.5 months (90% CI: 3.9, 7.4). Forty patients (26.8%) had a best overall response of stable disease (SD), 74 patients (49.7%) had a best overall response of progressive disease (PD) and 29 patients (19.5%) had missing or non-evaluable best overall responses across all dose cohorts. Response results for the cibisatamab monotherapy cohorts are summarized in Supplementary Tables 5 and 6 .
In S2, all enrolled patients ( n = 228) had measurable disease at baseline and were evaluable for efficacy. Across all dose levels, schedules and tumour types, the investigator-assessed overall response rate (ORR) was 6.6% (90% CI: 4.1, 9.9); 34.6% of patients (79/228) achieved a SD and 47.8% (109/228) showed PD as best overall response and a disease control rate (DCR) of 41.2% (90% CI: 35.8, 46.9). Eleven percent of the patients were non-evaluable for response.
In Part IA ( n = 75), the DCR was 27.8% (5/18) in the patients who were enrolled in the dose-escalation cohorts ranging from 5 to 40 mg. No objective response was observed. In the dose-escalation cohorts that ranged from 80 to 160 mg, the DCR was 61.5% (8/13) and one patient had a PR (7.7%, 90% CI: 0.4, 31.6). Based on these early efficacy findings, the 160-mg cohort was expanded to recruit an additional 41 patients. In addition, Part IB was opened to test the randomized flat dose with 100 mg QW vs 100 mg Q3W and to include several step-up cohorts (see the CONSORT diagrams in Supplementary Figs. 1 , 2 ).
Table 4 summarizes the efficacy results in the flat-dose 100- to 160-mg MSS-CRC cohorts, the step-up dose MSS-CRC cohorts and all enrolled patients with MSS-CRC. Figure 4 shows the spider plot of change in target lesion of the patients with MSS-CRR who were treated in the randomized 100-mg cohorts. Efficacy based on objective response appeared to be similar in patients with MSS-CRC who were treated with flat doses of cibisatamab 100 mg QW, 100 mg Q3W or 160 mg QW in combination with atezolizumab, and efficacy seemed to be greater in these patients receiving flat doses than in those who received step-up dosing.
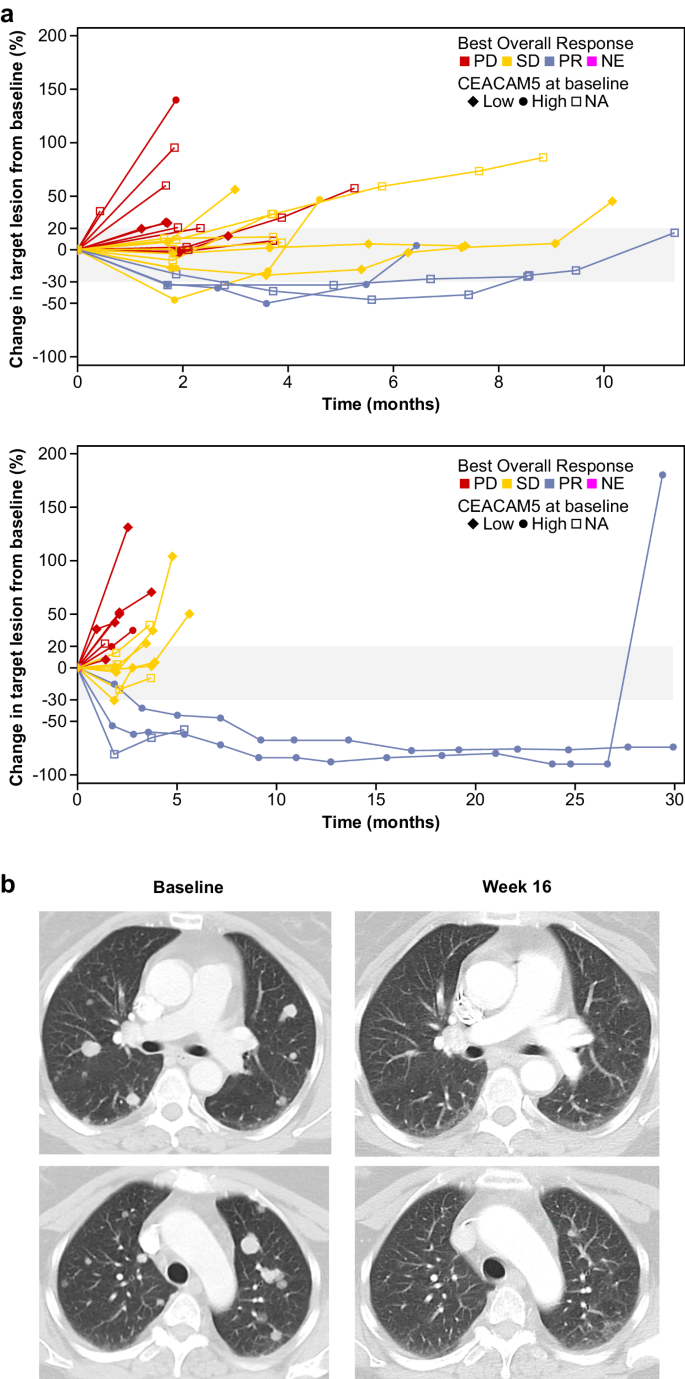
a Spider plot of change in sum of target lesions from baseline according to Response Evaluation Criteria in Solid Tumors version 1.1 in patients with MSS-CRC receiving cibisatamab flat dose 100 mg once weekly (top, n = 20), and 100 mg once every 3 weeks (bottom, n = 19) plus atezolizumab in S2. b Computerised tomography scan images from a RECIST-confirmed partial responder with MSS-CRC taken at baseline and week 16 after receiving cibisatamab 160 mg QW and atezolizumab 1200 mg Q3W. CEACAM5, Carcinoembryonic antigen-related cell adhesion molecule 5; NA Not applicable, NE Not evaluable, PD Progressive disease, PR Partial response, SD Stable disease. Source data can be requested from the authors for academic research purposes.
Patients with several other diagnoses received cibisatamab in combination with atezolizumab, including patients with pancreatic ( n = 17), gastric ( n = 12), non-small cell lung ( n = 3), breast ( n = 2) and bile duct ( n = 2) cancer. The efficacy results are summarised in Supplementary Table 7 .
Of the enrolled patients in S2, 127 had CEA-related cell adhesion molecule 5 ( CEACAM5 ) messenger RNA expression data available; 105 of these patients had MSS-CRC and 45 were in the 100‑ to 160-mg dose cohorts (Fig. 5A ). Thirteen patients were classified as CEACAM5 -high and 32 patients were CEACAM5 -low, according to a normalised RNA-seq cutoff of 1500 reads per kilobase of transcript per million reads mapped (RPKM). Importantly, all 4 of the tested patients who had a confirmed PR were CEACAM5 -high.
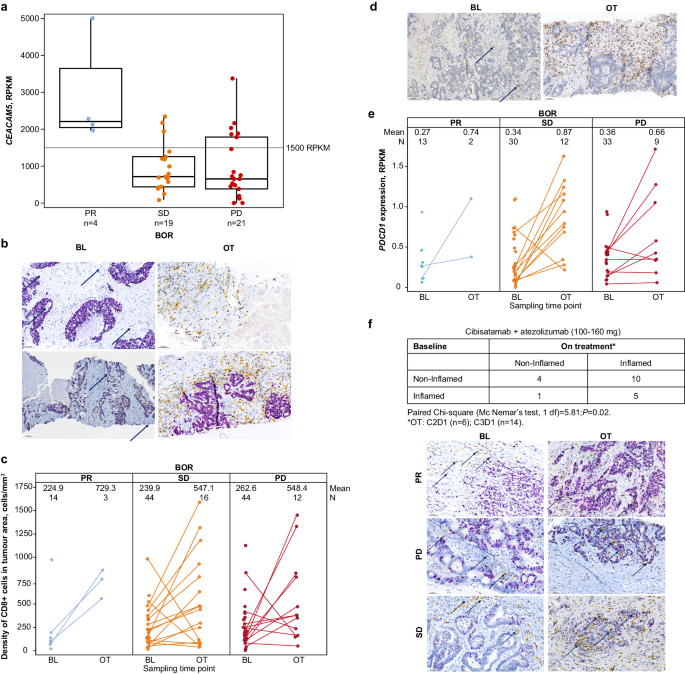
a CEACAM5 level vs best overall response according to Response Evaluation Criteria in Solid Tumors version 1.1 in the 100–160 mg cibisatamab cohort (MSS-CRC population) including all patients who received at least one dose of any study treatment (medians shown as middle lines, quartiles as boxes, and ranges as bars). b – f Refer to changes between baseline and on-treatment samples from patients with MSS-CRC in S2 who received doses ≥ 100 mg cibisatamab, by best overall response. b Dual chromogenic IHC for CD8 (yellow) and Ki67 (purple nuclear signal, outlining most tumour cells) for a patient with partial response (upper panel) and a patient with stable disease (lower panel). Scale bar represents 88.5 μm (top left), 77.3 μm (top right), 84.4 μm (bottom left) and 73.0 μm (bottom right). c Changes in CD8 + T cells between baseline and on-treatment tissue were quantified with a KI57/CD8 duplex assay. d Single chromogenic IHC for PD-1 at baseline (left) and on-treatment (right). Scale bar represents 76.7 μm (left) and 77.4 μm (right). e Changes in PDCD1 gene expression between baseline and on-treatment tissue were quantified by RNAseq. f Cibisatamab induces tissue pharmacodynamic changes. IHC for CD8 (yellow) and Ki67 (purple, outlining most tumour cells). The immune-excluded immunophenotype is shown on the left (baseline sample) and the immune-inflamed immunophenotype is shown on the right (on-treatment sample). Scale bar represents 65.4 μm (top left), 60.2 μm (top right), 64.4 μm (middle left), 52.8 μm (middle right), 64.5 μm (bottom left), and 61.0 μm (bottom right). In panels a , c , e , blue colour refers to PR, orange to SD, and red to PD. The N or n refers to individual patients. In subplots ( c , e ) dots for the same patient are connected by a line. The exploratory test in subplot f is two-sided. Atezo Atezolizumab; BL Baseline tissue; BOR Best overall response; CEACAM5 Carcinoembryonic antigen-related cell adhesion molecule 5; cibi Cibisatamab OT On-treatment tissue; PD Progressive disease; PR Partial response; RPKM Reads per kilobase of transcript per million reads mapped; SD Stable disease.
All key analyses were repeated disaggregated by sex with selected results presented in Supplementary Table 8 . No substantial differences between the sexes were found.
Exploratory biomarker results
The immunohistochemistry (IHC) data from the 100- to 160-mg QW or Q3W cohorts show that in the majority of patients (80%, n = 31), cibisatamab in combination with atezolizumab induces an increase in intra-tumoural T-cell infiltration (comparing the C2D1 and C3D1 on-treatment time points to baseline levels). On-treatment PDCD1 levels ( PDCD1 encodes programmed death ligand-1 [PD-L1]) were similarly higher than baseline levels (Fig. 5B–E presents the CD8 and PD-1 IHC images and data that were quantified). Furthermore, cibisatamab treatment frequently triggered the relocalization of the CD8 + T cells from the stroma to the tumour beds, resulting in the conversion of immune-excluded tumours (in which the CD8 T cells are primarily in the tumour stroma) to inflamed tumours (in which the CD8 T cells are infiltrating the tumour nests) (Fig. 5F ). In this limited data set, these biomarkers only reflect pharmacodynamic changes and are not predictive of response.
Cibisatamab is designed to allow for activation of T cells regardless of their antigen specificity. This was confirmed by RNAseq analysis on tissue collected at baseline and on treatment ( n = 23 in the cibisatamab-atezolizumab 100-mg cohorts). As expected, T effector (Teff) gene expression ( CD8A, GZMA, GZMB, IFNG, EOMES, PRF1, CXCL9, CXCL10, TBX21) increased on treatment (supporting the CD8 and PD-L1 data). However, no changes in antigen presentation genes ( TAPBP, TAP1, TAP2, PSMB8, PSMB9 ) were seen, supporting the theorized synthetic immunity mechanism of action of TCBs, whereby conceivably no antigen-specific T cells are necessary for activity (Fig. 6 ). Interestingly, it does seem that in the Teff gene signature, the change was greater in the patients with PR and SD than in those with PD (mean changes: patients with PR, 1.5; SD, 1.3; PD, 0.7). However, this trend needs further validation. More minimal changes in peripheral CD8 and CD4 cells were seen by response in patients receiving cibisatamab (Supplementary Fig. 4 ).
These findings demonstrate increased T-effector gene signatures, with no changes in antigen presentation genes, and support the theorised synthetic immunity mechanism of action of cibisatamab targeting tumour-associated CEA, without the requirement for antigen-specific T cells and acquired immunity for activity. Medians are shown as middle lines, quartiles as boxes, and ranges as bars. N refers to individual patients with the same patients shown for antigen-processing and T-effector. Blue colour refers to PR, orange to SD, and red to PD. Antigen-processing gene set: TAPBP, TAP1, TAP2, PSMB8, PSMB9 . T-effector gene set: CD8A, GZMA, GZMB, IFNG, EOMES, PRF1, CXCL9, CXCL10, TBX21 . BL Baseline tissue; OT On-treatment tissue; PD Progressive disease; PR Partial response; SD Stable disease.
Dose selection
Multiple dose levels and schedules for cibisatamab monotherapy (with or without obinutuzumab pretreatment) and cibisatamab in combination with atezolizumab have been tested in studies S1 and S2 in approaches intended to improve safety and efficacy. The flat-dosing regimens of cibisatamab (100 mg QW, 100 mg Q3W, and 160 mg QW) in combination with atezolizumab seemed to have a more favorable benefit-risk profile than step-up dosing regimens that started at 40 mg and escalated to the 1200-mg dose. In S2, the safety profile and clinical efficacy of cibisatamab appeared to be similar with both the randomized 100-mg dose administered QW and Q3W and the 160-mg dose administered QW in S2.
In a longitudinal mixed-effect nonlinear model that included patients with MSS-CRC who were enrolled in S2 and had been exposed to flat doses, doses of ≥ 80 mg cibisatamab led to a higher tumour shrinkage rate and to a trend toward lower growth rates.
Owing to the high affinity of cibisatamab to CEA and consistent with cibisatamab positron emission tomography (PET) imaging studies in tumour-bearing mice 23 and a clinical imaging study with a labeled molecule ( 89 Zr CEA-IL2v) that binds to the same CEA epitope as cibisatamab 24 , tumour retention is predicted to be considerably longer than that based on the serum PK half-life, which further supported the Q3W schedule. In addition, the 100-mg Q3W schedule will be more convenient for patients and will allow for a longer recovery period between cibisatamab administrations. Thus, 100 mg Q3W cibisatamab in combination with 1200 mg Q3W atezolizumab has been selected as the recommended Phase 2 dose (RP2D) and schedule.
There remains a significant unmet clinical need to optimize immunotherapeutic strategies for patients with cancer, in particular MSS-CRC. T-cell redirecting therapies using bispecific antibodies present a unique opportunity to engage T cells and induce potent anti-tumour immunity. Cibisatamab is the first T-cell bispecific antibody with a 2-to-1 format, optimized for safety and efficacy, directly binding CEA on tumour cells and CD3 on T cells, regardless of antigen specificity, resulting in increased T-cell infiltration, T-cell activation and tumour-cell killing 15 .
The S1 and S2 studies were performed in order to evaluate the safety, optimal dose and schedule, and preliminary efficacy of cibisatamab alone and in combination with atezolizumab. The safety profile of cibisatamab was dominated by IRRs, diarrhoea and tumour inflammatory events. Grade 3 and 4 toxicities were mostly IRRs, diarrhoea, transaminitis and anaemia. We observed that most IRRs and CRSs typically occurred following the first cibisatamab infusion and likely mediated by on-tumour/on-target effects. Later-cycle IRR and CRSs were more pronounced in ADA-positive patients, potentially due to ADA-mediated crosslinking of cibisatamab-bound CD3 leading to peripheral T-cell activation. The tumour inflammation and flare events occurred early and may be mediated by the mechanism of action of cibisatamab at the tumour, leading to an influx of inflammatory cells, thereby indicating an on-tumour/on-target effect. In contrast, the observed gastrointestinal toxicity likely represents an off-tumour/on-target effect of cibisatamab, targeting normal colonic mucosa, which is known to express high levels of CEACAM5 .
The MTD for cibisatamab monotherapy was determined as a flat dose of 400 mg QW and Q3W. Safety of cibisatamab in combination with atezolizumab was consistent with the known safety profiles of the individual drugs, with no further signals reported. The MTD for cibisatamab in combination with atezolizumab was not formally determined since during the dose-escalation phase, all 3 patients at the 300-mg dose level had a serious AE after the first infusion (chills, hypoxia, diarrhoea) and it was decided not to escalate the dose further. Cibisatamab exposure was similar after administration as a single agent or in combination with atezolizumab. However, administration of cibisatamab was frequently associated with a high incidence of ADAs, leading to reduced exposure or to exposure below the limit of quantification in a significant number of patients. Cibisatamab exposure was sustained in obinutuzumab-pretreated patients, with PK profiles similar to those in ADA‑negative patients, demonstrating the usefulness of B-cell depletion to reduce the formation of ADAs.
Preliminary data of anti-tumour activity with cibisatamab monotherapy and in combination with atezolizumab indicate these treatments resulted in clinical efficacy, with a few confirmed responses in patients with CEA-positive tumours across cohorts. Efficacy based on objective response appeared to be best in patients with MSS-CRC who were treated with flat doses of cibisatamab 100 mg QW, 100 mg Q3W or 160 mg QW in combination with atezolizumab. Additionally, efficacy seemed to be further enhanced in patients with high CEACAM5 expression. However, small sample sizes must be acknowledged as a limitation of these results, and the studies were not designed to evaluate efficacy.
The biomarker data show that cibisatamab treatment triggered the relocalization of CD8 T cells from the periphery and/or the expansion of pre-existing CD8 T cells within the tumour, as well as increased PDCD1 levels, leading to an inflamed tumour phenotype. The biomarker data also support the mechanisms of action of cibisatamab, in that the drug can elicit anti-tumour responses by cross-linking T cells to tumour cells and mediate polyclonal T-cell expansion that is independent of T-cell receptor specificity; evidenced by an increase in activated T cells in on-treatment tumour samples but no change in antigen presentation machinery. However, these pharmacodynamic changes did not correlate with response.
Cibisatamab is the first T-cell bispecific antibody consisting of a 2-to-1 format, optimised for safety and efficacy, directly binding tumour cells via CEA and T cells via CD3; resulting in increased T-cell infiltration, T-cell activation and tumour-cell killing. The preliminary efficacy observed with cibisatamab in combination with atezolizumab in patients with MSS-CRC warrants further exploration. Study CO40939 is an ongoing Phase 1b study that enrolled patients with advanced MSS-CRC with high CEACAM5 expression treated with cibisatamab at the RP2D in combination with atezolizumab after pretreatment with obinutuzumab (NCT03866239). Furthermore, a study of cibisatamab combined with a similar bispecific construct targeting CD137/4-1BB and fibroblast activation protein α is ongoing (NCT04826003).
Study design and ethics
S1 (BP29541, first-in-human study) was an open-label, multi-center, multi-cohort dose-escalation Phase 1 study designed to evaluate the safety, pharmacokinetics and therapeutic activity of cibisatamab (CEA-TCB) as a single agent, with or without obinutuzumab pretreatment. S2 (WP29945) was an open‑label, multi‑center, multi-cohort dose-escalation and dose schedule–finding Phase 1b clinical study of cibisatamab in combination with atezolizumab. Both studies were designed and conducted in compliance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation, and the study design and conduct complied with all relevant regulations regarding the use of human study participants. Written informed consent was obtained from each patient before the initiation of study procedures. Both study protocols (available in Supplementary Information) were approved by the institutional review boards or independent ethics committee at each study site. For study S1, the initial protocol was first approved by clinical research ethics committee at Vall d’Hebron Hospital (Barcelona, Spain) on December 12, 2014, for study conduct at Vall d’Hebron University Hospital (Barcelona, Spain) and the University of Navarra Hospital (Navarra, Spain). For study S2, the clinical research ethics committee of the Government of Navarra Department of Health (Navarra, Spain) was first to approve the initial protocol on December 15, 2015, for study conduct at the same sites as for S1. Additional study sites in North America and Europe were opened thereafter. S1 and S2 were first authorised by The Spanish Agency for Medicine and Health Products on December 18, 2014, and December 28, 2015, respectively, with additional health authority authorizations occurring through 2017. The S1 study record was first submitted to ClinicalTrials.gov on December 12, 2014 ( https://clinicaltrials.gov/study/NCT02324257 ); patients in S1 were enrolled between December 2014 and May 2018. The S2 study record was first submitted to ClinicalTrials.gov on January 6, 2016 ( https://clinicaltrials.gov/study/NCT02650713 ); patients in S2 were enrolled between January 2016 and May 2018.
In both studies, eligible patients aged 18 years or older were enrolled from 17 (S1) and 23 (S2) academic hospitals in 6 (S1) and 7 (S2) countries between 2014 and 2018 with sponsorship by F. Hoffmann-La Roche Ltd. Patients were required to have locally advanced or metastatic solid tumours after progression on a standard therapy, be intolerant of and/or non-amenable to the standard-of-care therapies and have at least one tumour lesion of accessible non-critical location to biopsy, adequate organ function, radiologically measurable and clinically evaluable disease (per Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST 1.1]), and an ECOG PS 0 or 1. Patients with select non-CRC tumours had tumour CEA expression (≥ 20% of tumour cells expressing moderate or high intensity CEA expression—IHC2+ and IHC3 + ) assessed locally in Europe and centrally in North America. Patients were excluded if they had ongoing or recent autoimmune disease, had undergone allogeneic bone marrow or solid-organ transplantation, or had concurrent cancer. A full list of inclusion and exclusion criteria is provided in the Supplementary Methods.
Study drug administration and dose escalation
Because the human CEA binder of cibisatamab does not cross-react with cynomolgus monkey CEA and CEA is absent in rodents, the minimal anticipated biological effect level (MABEL) approach was used to define the starting dose for the first-in-human study S1. Results from in vitro studies on human cells using the most sensitive test systems and assay conditions yielded a MABEL dose for cibisatamab of 0.052 mg, which was the starting dose for this study 25 .
In S1, patients were enrolled in single-patient ascending cohorts with flat-dose levels (i.e., same dose at each infusion) starting from 0.052 mg and up to 2.5 mg cibisatamab QW. In Part II of S1, patients were enrolled in multiple patient cohorts with flat-dose levels starting from 2.5 to 600 mg cibisatamab administered QW or Q3W (with or without obinutuzumab pretreatment) or step-up dosing regimen with QW and Q3W schedules (first dose of 40 mg up to 1200 mg, if tolerated). As preliminary data suggested that cibisatamab ADAs decreased the cibisatamab serum exposure (see Pharmacokinetics and Immunogenicity), new patient cohorts were created to include obinutuzumab pretreatment, to assess whether obinutuzumab could attenuate the development of ADAs. Step-up dosing cohorts were also added, to assess whether, by increasing the cibisatamab dose, the negative impact of cibisatamab ADAs on exposure could be overcome and thereby optimize efficacy. Obinutuzumab was administered as pretreatment either on Day ‒13 at a dose of 2000 mg or on two consecutive days, Day ‒13 and Day ‒12, at a dose of 1000 mg prior to treatment with cibisatamab on Cycle 1 Day 1.
In S2, patients were enrolled first into Part IA (dose-escalation), in which they received flat-dose cibisatamab between 5 and 300 mg QW, and then into Part IB (dose- and schedule-finding), in which patients with MSS-CRC were randomized to cibisatamab 100 mg QW or 100 mg Q3W (Cohort A). Several step-up cohorts were added with a cibisatamab starting dose of 40 mg and stepwise dose increase QW during the first 3 administrations and Q3W thereafter (Cohorts C1 and C2: patients with MSS-CRC who received a maximum cibisatamab dose of 150 mg and 600 mg, respectively; Cohort B1: patients MSS or MSI-high CRC who received a maximum cibisatamab dose of 1200 mg) The study also included step-up safety cohorts enrolling patients with indications other than CRC, including breast, gastric and pancreatic cancer, who received a maximum cibisatamab dose of 600 mg.
In both studies, a 24-h hospital stay was required following administration of study drugs at Cycle 1 Day 1 and also where a patient had experienced a grade ≥ 2 event of IRR/CRS at the previous administration. Patients were allowed to remain on therapy until loss of clinical benefit, PD, unacceptable toxicities, loss of cibisatamab exposure or withdrawal of consent. Investigators had the option to adjust the dose and/or the schedule of cibisatamab to allow patients who could potentially benefit from cibisatamab to remain on the study drug.
Tumour response assessments
Tumour response was measured according to RECIST 1.1 and assessed by the investigators in S1 and by the investigators and independent review in S2.
Tumour assessment was performed once during screening. The first assessment after the start of treatment was performed at 8 weeks, and assessments were performed every 8 weeks thereafter for the first year and every 12 weeks thereafter until PD or treatment discontinuation.
In both studies, the safety assessments were done at each visit and included vital signs, physical examinations, electrocardiograms, and laboratory tests. AEs were graded according to the NCI CTCAE v4.03, and CRS AEs were evaluated using NCI CTCAE v5.0. DLTs were defined as prespecified AEs that in S1 were related to cibisatamab and in S2 were related to cibisatamab or/and atezolizumab and in both cases had occurred during the DLT period (21 days following Cycle 1 Day 1) in patients enrolled during the dose-escalation part and during the step-up dose-escalation part of each study. Reported causality of the investigational drug was based on the judgment of the treating physicians. After each patient cohort (minimum of 3 patients) had been completed (i.e., the last patient enrolled in the cohort had reached Day 21), the Sponsor organized teleconferences with the investigators to discuss the safety and tolerability of cibisatamab (with or without obinutuzumab pretreatment) and in combination with atezolizumab and to determine the dose level for the next cohort. The next dose levels were recommended using a modified continual reassessment method with dose escalation overdose control (mCRM with EWOC) design (see Statistical Considerations) during the dose-escalation phase and discussed by the Sponsor and investigators. In addition, the clinical judgment of the Sponsor and investigators was also used in the dose-selection process.
Pharmacokinetic and immunogenicity assessment
Serum PK and ADA samples were regularly collected. Validated bifunctional PK assays with a lower limit of quantification of 0.925 ng/mL (S1) and 2 ng/mL (S2) was used to determine cibisatamab serum concentrations. Anti‑cibisatamab antibodies were analyzed in a three‑tiered approach including screening, confirmation and titration assay using a validated method (bridging enzyme-linked immunosorbent assay). Patients were categorized being ADA-negative or ADA-positive as recommended by Shankar et al. 26 . Non-linear mixed effect modeling with software NONMEM version 7.4.3 (Icon Development Solutions, Ellicott City, Maryland) was used to analyze the dose-concentration-time data of cibisatamab.
Pharmacodynamics and biomarker assessments
In S1 and S2, baseline and on-treatment mandatory paired biopsies were collected from all patients treated at dose levels >5 mg, except for patients with non-small cell lung cancer, for whom there was no accessible lesion. On-treatment tumour biopsy samples were assessed centrally by IHC for increases in intratumoural CD8 T cells (using PanCK-CD8 assay, Clone SP239 and Clone AE1/AE3/PCK26, at HistoGeneX), and PD-L1 (PD-L1 Clone SP263 at Ventana) expression on tumour cells and T cells. In addition, RNA sequencing was also performed on these matched-pair tissue samples (at Q2 Solutions) to assess changes at the gene level. Formalin-fixed paraffin-embedded (FFPE) tumour tissue was macrodissected to maximize tumour area by using hematoxylin and eosin as a guide. RNA extraction was conducted using High Pure FFPET RNA Isolation Kit (Roche) and assessed by Qubit and Agilent Bioanalyzer for quantity and quality. The prepared libraries were sequenced using the Illumina sequencing method. Whole blood, serum and plasma samples were regularly collected and processed centrally to analyse the number, activation and differentiation of immune cells by flow cytometry, to analyse soluble CEA as a disease-monitoring marker, to analyse cytokines and to assess relationships with dose levels, efficacy ADA status or AEs such as an IRR, or a CRS.
Additionally, we performed a retrospective subgroup analysis of efficacy by MSS-CRC status and of baseline CEACAM5 gene expression in fresh baseline FFPE tumour tissue. Due to the limited dynamic range of the CEA IHC assay (using Cell Marque CEA31 monoclonal antibody, Catalog No. 236M-96, at Ventana), we analysed CEACAM5 expression using RNA sequencing. Subgroup analyses of efficacy endpoints were performed based on a dichotomy of CEACAM5 high (≥ 1500 RPKM) and low (< 1500 RPKM); 1500 RPKM was selected as the candidate biomarker cutoff based on the preliminary data, shown in Fig. 5A , resulting from the subgroup analyses.
Study objectives
In S1, the primary objectives were to assess the safety of cibisatamab with or without obinutuzumab pretreatment in each cohort, as measured by the incidence of AEs and DLTs, to determine the MTD and/or the RP2D and schedule, to establish the pharmacokinetics of cibisatamab as monotherapy with or without obinutuzumab pretreatment and to assess the effect of obinutuzumab pretreatment on the rate and time of onset of ADA against cibisatamab. Secondary objectives were to evaluate preliminary anti-tumour activity of cibisatamab with or without obinutuzumab pretreatment and to describe the preliminary pharmacodynamic effects for cibisatamab with or without obinutuzumab pretreatment in mandatory paired tumour biopsies and paired blood samples.
In S2, the primary objectives were to establish the preliminary safety and tolerability profile, to determine the MTD in Cycle 1 and in later cycles, and to identify a RP2D of cibisatamab in combination with atezolizumab. Secondary objectives were to evaluate preliminary anti-tumour activity of cibisatamab in combination with atezolizumab and to describe the preliminary pharmacodynamic effects in mandatory paired tumour biopsies and paired blood samples.
Statistical considerations
Studies S1 and S2 were analysed separately. The safety, efficacy, PK and pharmacodynamic analysis populations consisted of all patients who received at least one dose of any study drug. For efficacy analyses, at least one post-baseline measurement (such as a tumour assessment) was required for inclusion in the analysis.
To minimize the number of patients treated below therapeutically relevant doses, Part I of S1 consisted of single-patient cohorts for each dose level. In Part II of S1 and Part IA of S2, multiple patients were enrolled per dose level, and a mCRM with EWOC was used to determine the MTD; at least 3 patients per dose level were required for the mCRM. Dose-escalation decisions were made by the Sponsor and the participating investigators after the review of all collected relevant safety information. We also studied step-up dosing and QW vs Q3W schedules in S1 and S2.
Efficacy was reported descriptively. Binary endpoints, including ORR and DCR, were summarized using relative frequencies and 90% CI. Time-to-event endpoints, including progression-free survival, overall survival and duration of response, were summarized using Kaplan-Meier methodology as well as median time-to-event endpoints with 90% CI. Response endpoints were defined according to RECIST 1.1. Rave EDC (Medidata, New York, NY, USA) was used for capturing and managing clinical data. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses of safety and efficacy data.
Analyses of biomarkers were post hoc and exploratory, with a focus on hypothesis generation. The Kruskal-Wallis rank sum test was used to assess the relationship between categorical and numerical variables, and the McNemar test was used to assess the relationship between two categorical variables.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Due to the informed consent provided by patients during trial enrolment, as well as applicable data protection laws in some countries, source data, including RNA sequencing data, cannot be shared publicly. However, qualified researchers can request it. Requests to access the data from the trials described in the current manuscript can be submitted through: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/ . Requested data are available for 12 months from the approval of the request. For -up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing . Anonymised records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
The remaining data are available within the Article and its Supplementary Information.
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541 , 321–330 (2017).
Article ADS CAS PubMed Google Scholar
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359 , 1350–1355 (2018).
Article ADS CAS PubMed PubMed Central Google Scholar
Ochoa de Olza, M., Navarro Rodrigo, B., Zimmermann, S. & Coukos, G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 21 , e419–e430 (2020).
Article CAS PubMed Google Scholar
Zappasodi, R., Wolchok, J. D. & Merghoub, T. Strategies for Predicting Response to Checkpoint Inhibitors. Curr. Hematol. Malig. Rep. 13 , 383–395 (2018).
Article PubMed PubMed Central Google Scholar
Darvin, P., Toor, S. M., Sasidharan Nair, V. & Elkord, E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med 50 , 1–11 (2018).
Article PubMed Google Scholar
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19 , 133–150 (2019).
Article CAS PubMed PubMed Central Google Scholar
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351 , 1463–1469 (2016).
Huehls, A. M., Coupet, T. A. & Sentman, C. L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 93 , 290–296 (2015).
Bacac, M. et al. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin. Cancer Res 22 , 3286–3297 (2016).
Beauchemin, N. & Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 32 , 643–671 (2013).
Hammarstrom, S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 9 , 67–81 (1999).
Tiernan, J. P. et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 108 , 662–667 (2013).
Hefta, S. A., Hefta, L. J., Lee, T. D., Paxton, R. J. & Shively, J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc. Natl Acad. Sci. USA 85 , 4648–4652 (1988).
Shimada, H., Noie, T., Ohashi, M., Oba, K. & Takahashi, Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 17 , 26–33 (2014).
Bacac, M., Klein, C. & Umana, P. CEA TCB: A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology 5 , e1203498 (2016).
Sam, J. et al. Combination of T-Cell Bispecific Antibodies With PD-L1 Checkpoint Inhibition Elicits Superior Anti-Tumor Activity. Front. Oncol. 10 , 575737 (2020).
Teijeira, A. et al. Three-dimensional colon cancer organoids model the response to CEA-CD3 T-cell engagers. Theranostics 12 , 1373–1387 (2022).
Cartron, G. et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 124 , 2196–2202 (2014).
Salles, G. et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 119 , 5126–5132 (2012).
ClinicalTrials.gov. A study of RO6958688 in participants with locally advanced and/or metastatic carcinoembryonic antigen positive solid tumors, < https://clinicaltrials.gov/ct2/show/NCT02324257 > (2022).
ClinicalTrials.gov. A study of the safety, pharmacokinetics, and therapeutic activity of RO6958688 in combination with atezolizumab in participants with locally advanced and/or metastatic carcinoembryonic antigen (CEA)-positive solid tumors, < https://clinicaltrials.gov/ct2/show/NCT02650713 > (2022).
Bai, S. et al. A guide to rational dosing of monoclonal antibodies. Clin. Pharmacokinet. 51 , 119–135 (2012).
Lehmann, S. et al. In Vivo Fluorescence Imaging of the Activity of CEA TCB, a Novel T-Cell Bispecific Antibody, Reveals Highly Specific Tumor Targeting and Fast Induction of T-Cell-Mediated Tumor Killing. Clin. Cancer Res 22 , 4417–4427 (2016).
Tabernero, J., Homicsko, K. & JH., S. Clinical evidence of intra-tumoral immune activation and tumor targeting with RG2813, a CEA-targeted engineered IL-2 immunocytokine. Eur. J. Cancer 51 , S104–S105 (2015).
Dudal, S. et al. Application of a MABEL Approach for a T-Cell-Bispecific Monoclonal Antibody: CEA TCB. J. Immunother. 39 , 279–289 (2016).
Shankar, G. et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 16 , 658–673 (2014).
Download references
Acknowledgements
The authors would like to thank the patients who participated in the trial, the patients’ families, and the investigators and staff at all clinical study sites. This study was sponsored by F. Hoffmann-La Roche, Ltd. The sponsor played role in study design, data collection, data analysis and interpretation, and writing of the manuscript, in collaboration with the authors. Editorial assistance for this manuscript was provided by Jessica Bessler, PhD, CMPP of Nucleus Global, an Inizio Company, and funded by F. Hoffmann-La Roche, Ltd.
Author information
Authors and affiliations.
Memorial Sloan Kettering Cancer Center, New York, NY, United States; Weill Cornell Medical College, New York, NY, USA
Neil H. Segal
Clínica Universidad de Navarra and CIMA University of Navarra, Navarra, Spain
Ignacio Melero & Maria E. Rodriguez-Ruiz
CIBERONC, Instituto de Salud Carlso III, Madrid, Spain
Ignacio Melero
Hospital Fundación Jiménez Díaz, Madrid, Spain
Victor Moreno
Netherlands Cancer Institute, Amsterdam, Netherlands
Neeltje Steeghs
Gustave Roussy, Université Paris-Saclay, Villejuif, France
Aurelien Marabelle
Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark
Kristoffer Rohrberg
Yale University Cancer Center, New Haven, CT, USA
Joseph P. Eder
Vanderbilt Ingram Cancer Center, Nashville, TN, USA
Columbia University, New York, NY, USA
Gulam A. Manji
Genentech, Inc., South San Francisco, CA, USA
Daniel Waterkamp, Meghna Das Thakur & Hartmut Koeppen
F. Hoffmann-La Roche, Ltd, Basel, Switzerland
Barbara Leutgeb, Said Bouseida, Nick Flinn, Markus C. Elze, Candice Jamois & Meret Martin-Facklam
University of Colorado, Aurora, Denver, CO, USA
Christopher H. Lieu
START Madrid-CIOCC, Centro Integral Oncológico Clara Campal, Madrid, Spain
Emiliano Calvo
University Hospital 12 de Octubre, Madrid, Spain
Luis Paz-Ares
Vall d’Hebron Hospital Campus and Institute of Oncology (VHIO), Barcelona, Spain
Josep Tabernero & Guillem Argilés
Departament de Cirurgia, Universitat Autònoma de Barcelona, Barcelona, Spain
Guillem Argilés
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Neil H. Segal, Ignacio Melero, Victor Moreno, Neeltje Steeghs, Aurelien Marabelle, Daniel Waterkamp, Barbara Leutgeb, Said Bouseida, Nicholas Flinn, Markus Elze, Christopher Lieu, Emiliano Calvo, Josep Tabernero, Guillem Argilés Methodology: Neil H. Segal, Maria E. Rodriguez-Ruiz, Daniel Waterkamp, Barbara Leutgeb, Said Bouseida, Nicholas Flinn, Meghna Das Thakur, Hartmut Koeppen, Christopher Lieu, Josep Tabernero, Guillem Argilés Validation: Neil H. Segal, Cathy Eng, Markus Elze, Candice Jamois, Christopher Lieu, Emiliano Calvo Formal analysis: Neil H. Segal, Said Bouseida, Nicholas Flinn, Markus Elze, Candice Jamois, Christopher Lieu, Emiliano Calvo, Josep Tabernero Investigation: Neil H. Segal, Victor Moreno, Aurelien Marabelle, Neeltje Steeghs, Kristoffer Staal Rohrberg, Maria E. Rodriguez-Ruiz, Cathy Eng, Gulam Abbas Manji, Barbara Leutgeb, Said Bouseida, Christopher Lieu, Luis Paz-Ares, Josep Tabernero Resources: Neil H. Segal, Neeltje Steeghs, Kristoffer Staal Rohrberg, Joseph Paul Eder, Luis Paz-Ares, Josep Tabernero, Guillem Argilés Data curation: Neil H. Segal, N. Steeghs, Kristoffer Staal Rohrberg, Maria E. Rodriguez-Ruiz, Joseph Paul Eder, Gulam Abbas Manji, Said Bouseida, Nicholas Flinn, Candice Jamois, Christopher Lieu, Luis Paz-Ares, Josep Tabernero, Guillem Argilés Writing-original draft: Neil H. Segal, Ignacio Melero, Neeltje Steeghs, Daniel Waterkamp, Barbara Leutgeb, Said Bouseida, Nicholas Flinn, Meghna Das Thakur, Markus Elze, Hartmut Koeppen, Candice Jamois, Meret Martin-Facklam, Christopher Lieu, Emiliano Calvo Writing-review and editing: Neil H. Segal, Ignacio Melero, Victor Moreno, Aurelien Marabelle, Neeltje Steeghs, Kristoffer Staal Rohrberg, Maria E. Rodriguez-Ruiz, Cathy Eng, Gulam Abbas Manji, Daniel Waterkamp, Barbara Leutgeb, Said Bouseida, Nicholas Flinn, Meghna Das Thakur, Markus Elze, Hartmut Koeppen, Candice Jamois,,Meret Martin-Facklam, Christopher Lieu, Emiliano Calvo, Luis Paz-Ares, Josep Tabernero, Guillem Argilés Visualization: Neil H. Segal, Victor Moreno, Christopher Lieu, Supervision: Neil H. Segal, Aurelien Marabelle, Kristoffer Staal Rohrberg, Joseph Paul Eder, Markus Elze, Emiliano Calvo, Luis Paz-Ares, Josep Tabernero, Guillem Argilés.
Corresponding author
Correspondence to Neil H. Segal .
Ethics declarations
Competing interests.
NHS reports research funding from Regeneron, Immunocore, Puretech, AstraZeneca, BMS, Merck, Pfizer, Roche/Genentech; served as an consultant/advisory board for Immunocore, PsiOxus, Roche/Genentech, Boehringer Ingelheim, Revitope, ABL Bio, Novartis, GSK, Astra Zeneca, Numab. IM reports grants from Roche, Bristol Myers, AstraZeneca, Genmab, Highlight therapeutics; consultancy fees from Roche, Bristol Myers, AstraZeneca, Genmab, Highlights therapeutics, Numab, F-Star, Catalym, Pharmamamar, Biolinerx, Gossamer, Bright peak, Pieris, Alligator, Pierre FabreVM reports consulting fees from: Roche, Bayer, BMS, Janssen and Basilea; Principal Investigator – Institutional Funding: AbbVie, AceaBio, Adaptimmune, ADC Therapeutics, Aduro, Agenus, Amcure, Amgen, Astellas, AstraZeneca Bayer Beigene BioInvent International AB, BMS, Boehringer, Boheringer, Boston, Celgene, Daichii Sankyo, DEBIOPHARM,Eisai, e-Terapeutics, Exelisis, Forma Therapeutics, Genmab, GSK, Harpoon, Hutchison, Immutep, Incyte, Inovio, Iovance, Janssen, Kyowa Kirin, Lilly, Loxo, MedSir, Menarini, Merck, Merus, Millennium, MSD, Nanobiotix, Nektar, Novartis, Odonate Therapeutics, Pfizer, Pharma Mar, PharmaMar, Principia, PsiOxus, Puma, Regeneron, Rigontec, Roche, Sanofi, Sierra Oncology, Synthon, Taiho, Takeda, Tesaro, Transgene, Turning Point Therapeutics, Upshersmith.. NS reports provided consultation or attended advisory boards for Boehringer Ingelheim, Ellipses Pharma; received research grants for the institute from AB Science, Abbvie, Actuate Therapeutics, ADCtherapeutics, Amgen, Array, Ascendis Pharma, Astex, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, Celgene, CellCentric, Cresecendo, Cytovation, Deciphera, Eli Lilly, Exelixis, Genentech, Genmab, Gilead, GlaxoSmithKline, Incyte, InteRNA, Janssen/Johnson&Johnson, Kinate, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Novartis, Numab, Pfizer, Pierre Fabre, Regeneron, Roche, Sanofi, Seattle Genetics, Servier, Taiho, Takeda (outside the submitted work). AM over the last 5 years received honoraria and travel expense coverage for participation to Roche and Genentech Scientific Advisory Boards although not in direct relationship with the content of the manuscript. KSR reports compensation for conduction of clinical trial from Lilly, Roche/Genentech, Bristol-Myers Squibb, Symphogen, Pfizer, Novartis, Bayer, Alligator Bioscience, Incyte, Genmab, Puma Biotechnology, Orion Clinical, Bioinvent, Monta Bioscience, AstraZeneca; Speaker fees from Bayer, Amgen; Travel and conference reimbursement from AstraZeneca. MERR reports receiving research funding from Roche and Highlight Therapeutics. She also has received speaker’s bureau honoraria from BMS and ROCHE. JE has nothing to disclose. CE reports research support for this trial provided to Vanderbilt-Ingram Cancer Center. GAM reports research funding from Regeneron, BioLineRx, Merck, Roche/Genentech; served on the advisory for CEND Pharmaceuticals, Roche/Genentech; owns stock in CEND Pharmaceuticals. DW is an employee of F. Hoffmann-La Roche. BL has nothing to disclose. SB owns stock in Roche. NF is an employee of F. Hoffmann-La Roche. MDT is an employee of F. Hoffmann-La Roche. ME is an employee of and owns stock in F. Hoffmann-La Roche AG. HK is an employee of Genentech, Inc. and owns stock in F. Hoffmann-La Roche, Ltd. CJ is an employee of and owns stock in F. Hoffmann-La Roche, Ltd. MMF reports Roche profit participation certificate. CL has nothing to disclose. EC reports payment or honoraria from HM Hospitales Group; research funding form START; leadership role at start; consulting for Nanobiotix, Janssen-Cilag, Roche/Genentech, TargImmune Therapeutics, Bristol-Myers Squibb, Amunix, Adcendo, Anaveon, AstraZeneca/MedImmune, Chugai Pharma, MonTa, MDS Oncology, Nouscom, Novartis, Oncology, PharmaMar; employee of START, HM Hospitales Group; owns in START and Oncoart Associate; serves as president and found of foundation INTHEOS (Investigational Therapeutics in Oncological Sciences). LPA reports payment or honoraria from AstraZeneca, Janssen, Merck, Mirati; consulting fees from Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, bayer, BMS, Mirati, GSK, Janssen, Takeda; research grants from MSD, AstraZeneca, Pfizer, BMS, member of board of directros for Altum Sequencing and Genomica; principle investigator for Alkermes, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, IO Biotech, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Pharmamar, Roche, Sanofi, Takeda, and Tesaro. JT reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho,Tessa Therapeutics and TheraMyc. And also educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER); declares institutional financial interest in form of financial support for clinical trials or contracted research for Amgen Inc, Array Biopharma Inc,AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK. GA reports travel support from Amgen; speaker fees from Amgen, serves as medical advisor for Gadeta BV.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Segal, N.H., Melero, I., Moreno, V. et al. CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials. Nat Commun 15 , 4091 (2024). https://doi.org/10.1038/s41467-024-48479-8
Download citation
Received : 30 January 2023
Accepted : 02 May 2024
Published : 15 May 2024
DOI : https://doi.org/10.1038/s41467-024-48479-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
View key dates to help you plan your studies Plan your CIMA's CGMA® exams. On-demand tests are available all year. There are four windows a year when you can sit the Case Study Exams (February, May, August, and November). Within each window, exams will be available for three days, from Wednesday through Friday.
Exam Resource. What do my exam results mean. Further reading. Exam Technique. Case Study support 1 - preparing for the Case Study exam. Exam Technique. Case Study support 2 - planning a good answer. Exam Technique. Case Study support 3 - developing a fuller answer.
This document contains the full post exam supporting materials for the May and August 2021 management case study and CIMA gateway exam containing: pre-seen material. exam variants. suggested solutions. examiners report. marking guidance. Open PDF. Published 22/10/21. The CGMA Study Hub keeps you on track to achieve your personal study goals.
The purpose of the case study exam. CIMA describes the case study exam as a "role simulation". It requires candidates to "respond to authentic work-based activities presented during the examination, drawing together learning from each of the three subjects to provide solutions to the issues and challenges presented".
CIMA now includes a percentage allocation for each task. A good rule of thumb here is that for every 10% allocated to a particular task, you add one paragraph. So if a task is worth, say, 33%, you will write approximately three paragraphs. If a task is worth 60%, you will write about six paragraphs.
7 resources. The CGMA Study Hub keeps you on track to achieve your personal study goals.
Results for CIMA case study take four to five weeks to come through as they are human-marked. It's a notoriously difficult exam, but we're proud that Kaplan's CIMA pass rates consistently exceed the national average. In November 2021 we had the highest achievers at all three levels of case study.
The case study is TOTALLY different to any CIMA exams you've sat before, so you need a new way of doing things. This definitive guide will answer all of your burning questions about the CIMA case study exams, giving you the BEST chance at success. 1. The case study exams: the basics.
04/02/2022. CIMA has released the November case study pass rates, and they show sitters coped well with what the examiner threw at them. Those sitting the Management case achieved the highest pass rate, at 73%. That's the best pass rate for this level for the whole of 2021. Some 67% of Strategic sitters passed the final case this time around ...
Steve Flatman (CIMA Director of Examinations) and Peter Stewart (CIMA Director of Learning) explain how Case Study exam results are issued, how to interpret ...
If you're going to be sitting the operational or management case study exam, you need to learn ALL about the exam format and how it's different from the obje...
After your exam. What's next? Our guarantee. Our guarantee. Every purchase you make from the AICPA & CIMA is safe and secure. We also guarantee 100% customer satisfaction on most of our products. If you're not satisfied with your purchase, please contact us. Our refund policy. Our refund policy.
A Note on CIMA Case Study Pass Mark. The pass mark for case study is 80, out of a scaled score of 0 to 150. The candidate must also score a "Moderate" or "Strong" across all competencies in order to pass the case study exam. This is to ensure that the candidate is not weak in a particular area and that he/she is "business ready".
Follow these 7 simple steps to avoid the panic and clear the path ahead to enjoy case study exam success. 1. Follow a Focused Study Plan. Starting your study is often the hardest step to take.In our experience, following a plan increases productivity, cuts time-wasting, tracks progress and allows for personal planning around the study to ...
Booking your CIMA case study exam. The rules are slightly different for the three case study exams. ... Results published: 28 Mar 2024: 4 Apr 2024: 11 Apr 2024: CIMA February 2024 case study exams. Operational: Exam entry open: 2 Aug 2023: Exam entry closed: 23 Jan 2024: Pre-seen material available:
OCS November 2023 and February 2024 post exam materials. This document contains the full post exam supporting materials for the November 2023 and February 2024 Operational Case Study exams. This includes: pre-seen material. exam variants. suggested solutions. examiners report. marking guidance. Open PDF.
Certificate in Business Accounting, CIMA's CGMA® Professional Qualification Objective Test and Case Study Exam pass rates
For information about results visit the CIMA website. The three objective tests within the Management Level can be sat in any order. You must pass (or be exempt from) each of these objective tests before you can move onto the case study exam. When you have passed the case study exam you can move onto the Strategic Level. CIMA membership:
For information about results visit the CIMA website. The three objective tests within the Operational Level can be sat in any order. You must pass (or be exempt from) each of these objective tests before you can move onto the case study exam. When you have passed the case study exam you can move onto the Management Level. CIMA membership:
I've started revising the pre-seen even before my course for the case study starts this month but I'm so nervous and I'm worried I'm just going to not be able to explain myself well enough for the examiner... 1. kiosfade. • 3 yr. ago. I'm waiting for my scs result which is on 15th July. I've applied for annual leave for that day.
This site is brought to you by the Association of International Certified Professional Accountants, the global voice of the accounting and finance profession, founded by the American Institute of CPAs and The Chartered Institute of Management Accountants.
Advertisement Feature. CGMA candidate exam FAQs. CIMA's CGMA exams are available in many countries you can choose to schedule at a test centre or online. More information on test centre exams in your country is available at Pearson Vue. Advertisement Feature.
2024 CIMA Case Study Exam Timetable August 2024 CIMA Exam Dates Operational Level. Exam entry opening - January 24th, 2024 ... Results published - September 26th, 2024; Management Level. Exam entry opening - January 31st, 2024; Exam entry closing - July 30th, 2024; Pre-seen material released - March 27th, 2024; Exam dates - August ...
How to pass the Management Case Study. With the next set of exams coming up we thought it would be great to give you an overview of the management case study, also known as CGMA gateway. The aim of CIMA's CGMA Professional Management case study is to apply the knowledge you have learnt across the whole management level. The CGMA Study Hub keeps ...
May 6, 2024. Scientists are proposing a new way of understanding the genetics of Alzheimer's that would mean that up to a fifth of patients would be considered to have a genetically caused form ...
Patients taking Novo Nordisk's Wegovy obesity treatment maintained an average of 10% weight loss after four years, potentially boosting the drugmaker's case to insurers and governments to cover ...
As you may already know, Operational Case Study (OCS) is the first case study under CIMA's CGMA professional qualification and is designed to test your ability to apply the technical knowledge you've learnt from E1, P1 and F1. The CGMA Study Hub keeps you on track to achieve your personal study goals.
The urban texture is the physical manifestation of the urban form's evolution. In the rapid process of urbanization, protecting and reshaping the urban texture has become an essential means to sustain the overall form and vitality of cities. Previous studies in this field have primarily relied on image analysis or typological methods, lacking a quantitative approach to identify and analyze ...
Results from in vitro studies on human cells using the most sensitive test systems and assay conditions yielded a MABEL dose for cibisatamab of 0.052 mg, which was the starting dose for this study 25.