1.2.2 What is a systematic review?
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman 1992, Oxman 1993) . The key characteristics of a systematic review are:
a clearly stated set of objectives with pre-defined eligibility criteria for studies;
an explicit, reproducible methodology;
a systematic search that attempts to identify all studies that would meet the eligibility criteria;
an assessment of the validity of the findings of the included studies, for example through the assessment of risk of bias; and
a systematic presentation, and synthesis, of the characteristics and findings of the included studies.
Many systematic reviews contain meta-analyses. Meta-analysis is the use of statistical methods to summarize the results of independent studies (Glass 1976). By combining information from all relevant studies, meta-analyses can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review (see Chapter 9, Section 9.1.3 ). They also facilitate investigations of the consistency of evidence across studies, and the exploration of differences across studies.

Cochrane Australia
Systematic reviews explained.
Cochrane Reviews are systematic reviews of primary research in human health care and health policy, and are internationally recognised as the highest standard in evidence-based health care. They investigate the effects of interventions for prevention, treatment, and rehabilitation. They also assess the accuracy of a diagnostic test for a given condition in a specific patient group and setting. They are published online in the Cochrane Database of Systematic Reviews in the Cochrane Library .
Each systematic review addresses a clearly formulated question, for example: Can antibiotics help in alleviating the symptoms of a sore throat? All the existing primary research on a topic that meets certain criteria is searched for and collated, and then assessed using stringent guidelines, to establish whether or not there is conclusive evidence about a specific treatment. The reviews are updated as new evidence becomes available, ensuring that treatment decisions can be based on the most up-to-date and reliable evidence.
Cochrane Reviews are widely used to inform healthcare guidelines, best practice guidance in primary care and patient decision aids in shared decision making initiatives.
Cochrane Consumer Network
Cochrane and systematic reviews, about the cochrane library, systematic reviews.
- How do I know an intervention works
- What consumers can and cannot get from a review
- Levels of evidence
- Cochrane groups

Top of page
The Cochrane Library is an electronic collection of databases published on the internet and also available on CD-Rom. It is updated quarterly in an effort to add to and keep the information current. The Library is made up of a number of parts.
The Cochrane Database of Systematic Reviews (CDSR) contains the published Cochrane reviews and protocols.
The Cochrane Central Register of Controlled Trials (CENTRAL) collates references to controlled trials in health care. These healthcare trial references are entered by Cochrane groups. The main way of finding health care studies is by looking in electronic databases (such as MEDLINE, EMBASE, CINAHL) using special search terms. Other ways are by asking experts in a particular health field and through hand searching journals.
The Database of Abstracts of Reviews of Effects (DARE) is a collection of structured abstracts and bibliographic references of systematic reviews of the effects of health care. It is developed by the Centre for Research and Dissemination, University of York, UK.
Methodological reviews and articles are also presented in The Cochrane Library.
In addition, each Cochrane group (termed an entity) has a section (module) in the Library that gives information on the group’s organisation, contact details, function, reviews, and other general information.
Accessing The Cochrane Library
Abstracts of reviews are readily accessible at www.cochrane.org/reviews . In countries such as Australia, Denmark, Finland, Ireland, Latin America, Norway and UK the full reviews are freely available as the governments of these countries have subscriptions to The Cochrane Library . Consumers who live in other countries and who wish to read a full review may need to access The Cochrane Library through a university, hospital or large public library.
A Cochrane Library Users Guide is available ( https://www.cochrane.org.au/libraryguide/ ) to help you find the information you want from The Cochrane Library.
Brief summaries (plain language summaries) of Cochrane reviews are written for consumers and others to highlight the information in a review. A What’s New Digest summarises the newest reviews.
If you would like to make comments on any existing review in The Cochrane Library, you will find a special section for 'Comments and Criticisms' with the review.
If someone decides to look critically at articles that have appeared in the medical or health literature on a particular topic they are said to be ‘reviewing the literature’. The authors may review, say, all the drug treatments available for one type of heart disease. A review is very clearly defined and sets out to find what evidence there is for prescribing one particular intervention or drug in a specific health condition, often in a certain group of people.
Examples of review topics are: Single dose celecoxib for acute postoperative pain; Artichoke leaf extract for treating hypercholesterolaemia; Chocolate avoidance for preventing migraine; Etidronate for treating and preventing postmenopausal osteoporosis.
What is a systematic review?
A systematic review summarises the results of available carefully designed healthcare studies (controlled trials) and provides a high level of evidence on the effectiveness of healthcare interventions.
The review authors set about their task very methodically following, step by step, an advance plan that covers:
- the way existing studies are found;
- how the relevant studies are judged in terms of their usefulness in answering the review question;
- how the results of the separate studies are brought together to give an overall measure of effectiveness (benefits and harms) – statistical techniques used to combine the results are called meta-analysis.
What is a protocol?
A protocol is the plan or set of steps to be followed in preparing a review. A protocol for a systematic review clearly describes why the review is needed (the review question), what the review is about (the healthcare context of the review), and how the reviewer authors will go about developing the review. It details how they will seek, select as relevant, critically appraise studies, and collect and analyse data (combine data and check for significance to the healthcare situation) from the included studies.
Cochrane protocols are published in the Cochrane Database of Systematic Reviews so that people can comment on them before the actual review has been carried out.
How do I know a healthcare intervention works?
The aim of a systematic review is to thoroughly assess, by means of a set procedure, the best possible evidence about the effects of a healthcare intervention or treatment in a particular healthcare situation.
Healthcare studies are generally designed to assess the benefits, rather than the harms, of an intervention. Studies generally have a relatively short designated time period. Any possible harms of an intervention may be expected to occur less frequently and over a longer period of time than the studies cover.
The process of a review is clearly defined, before starting the actual review of the literature, to minimise associations of expectations of effects and other sources of bias. Bias is a systematic ‘error’ or mistake in the judgments and decisions made that influence the results of a study or a review. Bias differs from a ‘placebo effect’, which is where participants of a study (or assessors of the outcomes) perceive a beneficial effect, or harm, with an inactive treatment.
Synthesising evidence
The specific methods used in a review are carefully set out by The Cochrane Collaboration and are described in each review.
A Cochrane review is prepared and maintained using specific methodologies described in the Cochrane Handbook .
Systematic reviews of randomised controlled trials provide the clearest evidence for the benefits of a healthcare intervention.
This is because the best way to assess the effects of a health care treatment is to use procedures that reduce the influence of chance effects and associations of cause and effect. Individual expectations on the part of a service provider, assessor and the person receiving an intervention can all contribute to modifying observed findings from a healthcare study. Randomised controlled trials where none of these people know the exact intervention a study participant is receiving (intervention under investigation, a placebo, or a comparator) may be expected to provide the best evidence.
Comparing groups can be misleading
By assessing the health of the two comparative groups in a study after their treatments, we can tell which intervention is more successful – but only if the two groups of people were very similar before treatment began. Otherwise we might be misled. For instance, one group may become healthier not because their treatment was better but because they were younger, not so ill, at less risk of ill health before treatment began, or even self selected to a particular intervention because of a particular personality trait, for example, people who chose to take a hormone may have wanted to stay younger and be more active.
Randomised controlled trials
Randomised controlled trials are studies that are rigorously designed. People are allocated to intervention groups in a way that minimises the chances of predicting which treatment group a study participant is in. The intervention under investigation is compared against a well-known intervention or an inactive treatment (placebo). Studies are controlled so that participants have similar associated care in all ways other than the intervention. Ideally, depending on the type of intervention, the service provider is unaware of which group a participant is in and those assessing outcomes are also unaware – this is termed ’ blinding ’.
The strength of evidence for a particular intervention can be increased further by systematically looking at (reviewing) all available randomized controlled trials that have been reported relevant to a particular healthcare situation.
It is important to search thoroughly for all studies
Many people are needed to properly test an intervention. This is more than can be recruited into a single trial; it is also important to investigate the intervention in different populations. Furthermore, the technical aspects of a particular randomised controlled trial may nothave been implemented properly, for one reason or another. The effects of these shortcomings can be minimised by grouping results of a number of studies.
The results of randomised controlled trials may be published in any one of thousands of journals world wide. Indeed some studies are not published at all. In reality the studies found most easily tend to have over-optimistic results and finding reliable information about the effects of care is particularly difficult when there are negative results (the intervention is no better than placebo or another treatment). Sometimes published trials are too small to provide a conclusive result in their own right - as to whether a treatment really does work. Consequently, to find out about a healthcare intervention it is worth searching research literature thoroughly to see if the answer is already known. This may require considerable work over many months, but it will be much less work than conducting a new randomised controlled trial. This process also will not unnecessarily exclude people from effective interventions because of allocation to a placebo (or inactive treatment) group.Discussions are underway in The Cochrane Collaboration as to how qualitative studies can be used to add to the information obtained from controlled studies - those that consider outcomes measured in numerical terms (and so are termed quantitative studies). Qualitative measures include ‘quality of life’ and lifestyle changes obtained from detailed questionnaires. Qualitative studies may also use narrative interviews where participants are asked to talk about their experiences around sets of semi-structured questions and prompts to explore particular issues that information is needed on for a study.
Top of page
What consumers can, and cannot, get from systematic reviews
Systematic reviews ask a very specific research question about a particular intervention in a clearly defined group of people who have a clear health condition or problem. Reviews provide powerful information on the state of knowledge about a healthcare intervention and whether that intervention is an effective treatment of a healthcare condition.Reviews:
- cannot offer a guideline for treatment, especially if a person differs from those defined in the review. Individuals may have accompanying health problems, be in a different healthcare setting, or receive more than one intervention, for example;
- follow stringent guidelines as to what types of studies are included and how healthcare measures of effectiveness can be expressed and combined;
- may consider outcomes other than the one you are interested in and may not look at long term effects of an intervention;
- may only find studies that are limited in the healthcare setting in which they take place;
- may provide conclusions that are limited because of the question asked and/or the studies that were found.
Reviews are dependent on the availability of studies and the information these studies sought or obtained.
Healthcare studies differ dramatically in what they look for and how well they are carried out and, therefore, how much weight one canput on their conclusions. Part of the reason for performing systematic reviews is to reduce the effects of these shortcomings. Issues of conflict of interest and corporate funding of healthcare studies are also important considerations in drawing conclusions from any study.
Reviews are better suited to assess benefits rather than harms.
Well-designed healthcare studies generally set out to determine the efficacy of a healthcare intervention. Information on potential harms is less well investigated.
Carefully controlled studies take place over a limited period of time so that the researchers can account for all people who entered the study from beginning to the end of the study. Harms are generally less common than benefits and may be apparent over a different time period. This may be, for example, only in the long term so that the intervention would have to be given to more people for a long time period for adverse effects to be studied effectively.
Participants of studies are selected to reduce the risk of other problems interfering with the efficacy of an intervention. How selective thisprocess is needs to be carefully considered when assessing the relevance of a study to an individual.
Randomised controlled trials are expensive to run. They are very time consuming and multiple factors may limit how many participants are involved, the outcomes measured and the length of the trial. How many people complete the study is also very important.
Levels of evidence for healthcare interventions
The National Health and Medical Research Council of Australia (1999) defines the ‘dimensions of evidence’ using three main areas.
1. Strength of the evidence
Level of evidence: the study design used – a systematic review of all relevant randomised controlled trials is the highest level, followed by at least one randomised controlled trial, then a pseudo-randomised trial Quality of evidence: the methods used to minimise bias within a study design Statistical precision: the degree of certainty about the existence of a true effect
2. Size of effect
How much the determined intervention effect is above a ‘no apparent effect’ value for clinically relevant effects
3. Relevance of the evidence
How appropriate the outcome measure is for the healthcare problem, and its usefulness in measuring effectiveness of treatment
Using a measure of the variability of results – confidence intervals
Adapted from AD.Oxman Checklists for review articles. BMJ 1994;309:648-51
Level I. For a randomised controlled trial, the lower limit of the confidence interval (expressed as a range) for a measure of effect is still above a meaningful benefit in healthcare terms
Level II. For a randomised controlled trial, the lower limit of the confidence interval (expressed as a range) for a measure of effect is less than a meaningful beneficial effect in healthcare terms; but the point estimate of effect still shows effectiveness of the intervention
Lower levels of evidence
Level III. Measures of effectiveness are taken from non-randomised studies of groups of people where a control group has run concurrently with the group receiving the intervention being assessed
Level IV. Measures of effectiveness are taken from non-randomised studies of groups of people where intervention effects are compared with previous or historical information
Level V. Evidence is from single case studies
Confidence interval (CI):
Even studies perfectly designed and carried out may show variable results because of the play of chance. CI covers the likely range of the true effect. For example, the result of a study may be that 40 per cent (95% CI 30% to 50%) of people are helped by a treatment. That means that we can be 95 per cent certain the true effect is between 30 and 50 per cent. ( Smart Health Choices How to make informed health decisions by Judy Irwig, Les Irwig and Melissa Sweet, Allen and Unwin 1999)
Top of page
Cochrane Groups
Cochrane review groups.
Different groups exist for different health conditions: International Cochrane review groups cover important areas of health care diseases and conditions. Review groups are responsible for producing and maintaining Cochrane reviews on specific health care questions. You will see in The Cochrane Library, for example, a Cochrane Consumers and Communication Group, Cochrane Epilepsy Group, Cochrane Heart Group and a Cochrane Pregnancy and Childbirth Group.
The activities of each group (or entity in Cochrane language) are monitored and co-ordinated by one person for each group, known as the managing editor (review group co-ordinator). This person manages the day to day running of the group and is usually the contact person. The co-ordinating editor leads the group and is responsible for the quality and subject of reviews.
Each group attracts members with a variety of backgrounds, experience and expertise, who contribute to the process of developing systematic reviews. They may be doctors, nurses, researchers, health advisers, consumers and caregivers.
Cochrane Fields
Fields cover health care in a broader sense than do review groups. These may include a major section of health care such as cancer, the setting of care (e.g. primary care), the type of patient/consumer (e.g. older persons), the type of provider (e.g. nurses), or the type of intervention (e.g. vaccines). The role of fields is to facilitate the work of collaborative review groups and to ensure that Cochrane reviews appropriate to an area of interest are both relevant and accessible to service providers and consumers.
Each field works to:
- identify relevant healthcare trials and make them accessible in a specialised register;
- ensure the proper representation of its specialist area of health care in review groups;
- act as a liaison point between the entities within The Cochrane Collaboration and the specialist area of health care;
- promote the accessibility of Cochrane reviews.
The principal contact person in a field is its field co-ordinator.
Cochrane Centres
Cochrane centres provide a range of services designed to support collaborative review groups in their area and to facilitate the review process. They serve as a regional source of information about The Cochrane Collaboration, provide support to Cochrane contributors within a defined geographical area and promote access to The Cochrane Library. Each centre has a director.
The Cochrane Consumer Network (CCNet)
The Consumer Network supports consumer participation within The Cochrane Collaboration, internationally. The Network is available to any active consumer. Its mission is to enable and support consumers in contributing to the function of collaborative reviews groups and other Cochrane entities. The Network enables communication with other consumers, provides a sense of belonging within The Cochrane Collaboration, links and dissemination of information from Cochrane reviews.
© The Cochrane Collaboration Comments about this page to: [email protected]
Cochrane Switzerland
Systematic reviews.
Cochrane systematic reviews provide reliable, evidence-based information on health issues.
A systematic review is the result of a rigorous scientific process consisting of several well-defined steps, including a systematic literature search, an evaluation of the quality of each included study and a synthesis, quantified or narrative, of the results obtained. The findings summarize the evidence on the efficacy of a treatment, the risk of adverse events or the accuracy of a diagnostic test, for example. However, sometimes the authors have to acknowledge that there is a lack of rigorous scientific studies.
1. What information can be found in a Cochrane systematic review?
2. How is a systematic review produced?
3. What are the main challenges encountered when producing systematic reviews?
4. Who prepares systematic reviews at Cochrane?
5. Where can I find Cochrane systematic reviews?
6. What is the difference between Cochrane systematic reviews and other systematic reviews?
7. Systematic reviews of other study types
8. What about observational studies?
All Cochrane Systematic Reviews answer a well-defined health question, such as the efficacy and safety of a surgical procedure or drug therapy, by considering all studies conducted on this question over time that meet established quality criteria. See also " How is a systematic review produced? "
The full text is a document, often of several dozen pages, divided into several parts, the main ones being:
- A scientific abstract and a plain language summary, the latter very often translated into several languages;
- The main sections of the review (background, objectives, methods, results, discussion, conclusion);
- Tables describing the included studies (in detail), the excluded studies (with reason for exclusion) and the results of meta-analyses (if applicable);
Graphs, especially forest plots.
The elaboration of a systematic review is a rigorous scientific process consisting of several steps:
- Clearly define the question to be addressed;
- Search and identify all relevant references of clinical trials or other appropriate studies, published or unpublished, that aim to answer the review’s question;
- Assess the quality of each study using standardized tools;
- Extract and organize relevant data from the included publications and other sources of information;
- Prepare an appropriate synthesis of the extracted results. If the data permit, perform a statistical analysis called a meta-analysis, which is used to combine quantified findings from several studies into a single pooled estimate.
One of Cochrane's principles is to avoid redundancy as much as possible. To do this, the Cochrane Review Group in charge verifies that no other Cochrane review with the same question exists yet. If that’s not the case and other criteria are met, the title of the new review can be registered. For more information see the page " Become an author " on cochrane.org.
- Not all relevant studies answering the same specific health question are necessarily published, for example, when their results do not support the efficacy of a new treatment. Sometimes only part of the results are published, for example, only the outcomes with statistically significant differences. Cochrane supports the AllTrials initiative for the publication of all clinical trials and their full methods and results ( www.alltrials.net ).
- Certain publications do not describe the study methods with sufficient detail to allow for critical review and evaluation.
- Studies are often carried out under "ideal" conditions that do not account for factors compromising the efficacy of a treatment in routine care, such as patients’ co-morbidity or non-compliance with therapy.
The workload and time required to prepare and complete a Cochrane Systematic Review project is frequently underestimated, especially if the contributors are inexperienced.
Systematic reviews are conducted by health professionals or scientists, often with the ad hoc support of patients or patients' relatives. To prepare the review, the authors collaborate closely with the appropriate thematic review group (Cochrane Review Group), which ensures the editorial follow-up during the registration of the title, the writing of the protocol, the implementation of each step of the review and the publication in the Cochrane Library. The authors of Cochrane reviews are not funded by Cochrane but very often by public funds. Funding from the commercial sector is not accepted.
The Cochrane Review Groups are organised in 8 networks and ensure that the rigorous quality standards that have built Cochrane’s reputation are maintained. Both, the protocol and the full Cochrane review are peer-reviewed prior to publication in the Cochrane Library .
Systematic reviews produced by Cochrane are published in the Cochrane Library (www.cochranelibrary.com/). In addition to the Cochrane reviews, this online library also includes the CENTRAL database with references to controlled studies identified in PubMed, EMBASE and through manual searches ("hand-search"), and information about Cochrane.
Under the label "Cochrane Clinical Answers" a selection of systematic reviews of wider interest (in particular, in primary care medicine) are presented in a question-answer format with interactive tables facilitating rapid access to the results.
In Switzerland, all content of the Cochrane Library is freely accessible through a national license.
Cochrane Systematic Reviews are all built according to the same scheme, all the steps of their conduct are well described and all the choices made during the process are outlined. This transparency in the process helps readers to understand what options were taken and why they were chosen.
In addition, Cochrane Systematic Reviews are regularly updated, which is rarely the case for systematic reviews published elsewhere. These updates are performed as needed, for example, when a significant number of new studies have been published. Updating is important to ensure that the latest clinical research is taken into account.
Cochrane Systematic Reviews address questions other than the efficacy and safety of therapy. An important area is the performance and accuracy of diagnostic tests. A diagnostic test is a test, such as a laboratory test, imaging technique or clinical examination, performed on a person with a suspected disease or condition. It is used to confirm or rule out the presence of that disease or condition and should lead to a therapeutic decision (whether and which treatment to undertake). Cochrane has set standards for the development of "diagnostic reviews" and introduced this type of review as a routine process from 2008 on. One of the methods groups ( Cochrane Screening and Diagnostic Test Methods Group ) monitors the ongoing methods development and supports the author groups conducting this type of reviews.
As time goes by, other types of reviews have been admitted. At present, these reviews are still few in number and are not part of Cochrane’s routine processes. These include reviews of prognostic studies, qualitative evidence syntheses and living systematic reviews.
To assess the efficacy of a medical intervention, the results of randomized clinical trials are central to the analysis. However, they often provide little evidence of safety, especially of serious adverse events. Typically, observational studies include larger, less selected populations with longer follow-up periods. In a systematic review of interventions, it is advisable to include the results of good quality observational studies to gain a more complete picture of the benefits (effectiveness) and risks of an intervention.
Some other relevant questions can only be answered using the results of observational studies. For example, to determine the prevalence of a disease based on estimates made in different countries, a systematic review based on data from population-based studies, e.g. cross-sectional studies, might be conducted.
Jump to navigation
- Bahasa Indonesia
- Bahasa Malaysia

VIDEO: What are systematic reviews?

A systematic review attempts to identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a specific research question. Researchers conducting systematic reviews use explicit, systematic methods that are selected with a view aimed at minimizing bias, to produce more reliable findings to inform decision making.
Here is a video from Cochrane Consumers and Communication that explains what a systematic review is clearly and simply for people who may not be familiar with the concepts and terminology of systematic reviews: what they are, how researchers prepare them, and why they’re an important part of making informed decisions about health - for everyone.
Cochrane evidence provides a powerful tool to enhance your healthcare knowledge and decision making. This video from Cochrane Sweden explains a bit about how we create health evidence, including systematic reviews, and other activities of Cochrane.
- What is the difference between a Cochrane systematic review of interventions and a Cochrane diagnostic test accuracy review?
- Learn more about Cochrane and our work
- Join Cochrane
Occupational Therapy and Rehabilitation Sciences
- Defining the Research Question(s)
- Reference Resources
- Evidence Summaries & Clinical Guidelines
- Health Data & Statistics
- Patient & Consumer Facing Materials
- Images/Streaming Video
- Database Tutorials
- Crafting a Search
- Narrowing / Filtering a Search
- Expanding a Search
- Cited Reference Searching
- Find Grey Literature
- Save Your Searches
- Cite and Manage Sources
- Critical Appraisal
- Different Types of Literature Reviews
- Conducting & Reporting Systematic Reviews
- Finding Systematic Reviews
- Tutorials & Tools for Literature Reviews
- Mobile Apps for Health
Guidelines & Standards
Health sciences.
- JBI Manual for Evidence Synthesis JBI (Joanna Briggs Institute) is an international evidence-based healthcare research organization. The JBI Manual for Evidence Synthesis is meant to provide authors with a comprehensive guide to conducting JBI systematic reviews. Types of systematic reviews covered in manual include: systematic reviews of qualitative evidence, systematic reviews of effectiveness, mixed methods systematics reviews and scoping reviews, among others.
- Cochrane Handbook for Systematic Reviews of Interventions (6th Edition) The Cochrane Handbook for Systematic Reviews of Interventions is the official guide that describes in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions.
- Cochrane Training On this site, you will find interactive learning resources and pathways as well as links to webinars, courses, and handbooks produced by the Cochrane Collaboration that relate to systematic review methods. Note that select resources on this site are limited to those with an existing Cochrane account while others are publicly available.
- Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care [PDF, 1.6MB] Published by the Centre for Reviews and Dissemination, University of York, this guide outlines the methods and steps necessary to conduct a systematic review. It also addresses issues associated with reviews in specific areas, such as clinical tests, public health interventions, harm/adverse effects, economic evaluations, and how and why interventions work. Opens as PDF.
- Finding What Works in Health Care: Standards for Systematic Reviews This ebook, produced by the Institute of Medicine (2011), contains chapters on the following topics: Standards for initiating a systematic review -- Standards for finding and assessing individual studies -- Standards for synthesizing the body of evidence -- Standards for reporting systematic reviews -- Improving the quality of systematic reviews
- Methods for the Thematic Synthesis of Qualitative Research in Systematic Reviews Article abstract: There is a growing recognition of the value of synthesising qualitative research in the evidence base in order to facilitate effective and appropriate health care. In response to this, methods for undertaking these syntheses are currently being developed. Thematic analysis is a method that is often used to analyse data in primary qualitative research. This paper reports on the use of this type of analysis in systematic reviews to bring together and integrate the findings of multiple qualitative studies.
- PRESS Peer Review of Electronic Search Strategies The PRESS Guideline provides a set of recommendations concerning the information that should be used by librarians and other information specialists when they are asked to evaluate electronic search strategies developed for systematic review (SR) and health technology assessment (HTA) reports.
Social Sciences
- Systematic Reviews and Meta-Analysis This ebook, written by Littell, Corcoran, and Pillai (2008) and published by Oxford University Press, contains chapters on the following topics: Formulating a topic and developing a protocol -- Locating and screening studies -- Data extraction and study quality assessment -- Effect size metrics and pooling methods -- Assessing bias and variations in effects
- Systematic Reviews in the Social Sciences: A Practical Guide This ebook, written by Petticrew and Roberts (2006), contains chapters on the following topics: Why do we need systematic reviews? -- Starting the review : refining the question and defining the boundaries -- What sorts of studies do I include in the review? : deciding on the review's inclusion/exclusion criteria -- How to find the studies : the literature search -- How to appraise the studies : an introduction to assessing study quality -- Synthesizing the evidence -- Exploring heterogeneity and publication bias -- Disseminating the review -- Systematic reviews : urban myths and fairy tales
- Finding and Evaluating Evidence: Systematic Reviews and Evidence-Based Practice Part of the Pocket Guide to Social Work Research Method series, this ebook, written by Bronson and Davis (2012) and published by Oxford University Press, contains chapters on the following topics: Systematic reviews, evidence-based practice, and social work -- Asking the right questions, preparing a protocol, and finding the relevant research -- Critically appraising the quality and credibility of quantitative research for systematic reviews -- The art and science of managing and summarizing the available research -- Systematic reviews of qualitative research -- Assessing the quality of systematic reviews
Reporting Standards
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. PRISMA focuses on the reporting of reviews evaluating randomized trials, but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. This website includes the PRISMA statement (which outlines guidelines for reporting), the PRISMA flow diagram, and the PRISMA checklist, as well as a link to a document containing the PRISMA statement's explanation and elaboration.
Writing & Registering a Review Protocol
Writing a protocol.
A protocol is a written document that acts as an a priori plan for your evidence synthesis project. Beginning your project with a clear plan is important, even if the methods change along the way.
If your methods (e.g., search queries, inclusion/eligibility criteria) do change after you finish your protocol, you should document those changes in your final manuscript. For instance, completed Cochrane reviews often have a section titled 'Differences between protocol and review’.
Protocols generally contain sections for:
- Background literature review
- Review question
- Criteria for inclusion/exclusion of studies
- Types of studies, populations, interventions/exposures, outcome measures
- Search strategy for identification of studies
- Study selection methods
- Assessment of methodological quality (if applicable)
- Data extraction and synthesis
- Timeframe for conducting the review
For systematic reviews , PRISMA provides guidance for preparing a protocol , as does the Joanna Briggs Institute's Manual for Evidence Synthesis .
For scoping reviews , section 11.2 in the JBI Manual outlines protocol development
Registering a Protocol
Once you've written the protocol for your evidence synthesis, consider publishing or registering it. Making the protocol publicly available, through publication or registration, improves research transparency, and can help avoid unnecessary duplication of work around the same review question.
- PROSPERO PROSPERO is an international database of prospectively registered systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome. It aims to provide a comprehensive listing of systematic reviews, registered at inception, to help avoid duplication and reduce opportunity for reporting bias by enabling comparison completed review with what was planned in the protocol.
- OSF Registries Use the OSF (Open Science Framework) platform to preregister the protocol for your knowledge synthesis. OSF if a useful alternative to PROSPERO if you are not publishing a systematic review or a review of interventions with health-related outcomes. OSF is commonly used to register protocols for scoping reviews.
Publishing a Protocol
Many journals will publish a protocol for research, including systematic reviews. See the 'Information for Authors' or 'Submissions' sections of journal's websites to determine what kind of articles they publish.
Examples of Journals that Publish Protocols
- BMC Journals Many journals in BioMed Central's portfolio publish protocols for evidence syntheses. In particular, check out the journal 'Systematic Reviews'.
- JBI Evidence Synthesis The journal JBI Evidence Synthesis accepts manuscripts for evidence synthesis protocols, including systematic reviews of effects, reviews of qualitative evidence, scoping reviews and mix methods systematic reviews
- JMIR Research Protocols JMIR Research Protocols publishes protocols for systematic reviews and scoping reviews.
McGill Library. (2022). Guides: Systematic Reviews, Scoping Reviews, and Other Knowledge Syntheses: Developing the protocol . Retrieved February 4, 2022, from https://libraryguides.mcgill.ca/knowledge-syntheses/protocol
- << Previous: Different Types of Literature Reviews
- Next: Finding Systematic Reviews >>
- Last Updated: Jun 27, 2024 4:09 PM
- URL: https://guides.nyu.edu/ot
- Chamberlain University Library
- Chamberlain Library Core
Finding Types of Research
- Systematic Reviews and Meta-Analysis
On This Guide
- Evidence-Based Practice
- Quantitative and Qualitative
Find Systematic Reviews
Limit to systematic reviews, find a meta-analysis, use database meta-analysis filters, review systematic review and meta-analysis examples.
- Randomized Controlled Trials
- Observational Studies
- Literature Reviews
- Finding Research Tools This link opens in a new window
A systematic review is a type of research study where the researcher picks the topic of interest that they want to search for information about in the literature, predetermines what are the inclusion/exclusion criteria for the types of articles they are going to look at, and then analyzes those findings to draw conclusions about their question of interest.
The video below provides an overview on systematic reviews and how to find them using various sources.

When searching, you can narrow your search to systematic reviews only by adding a search term of "systematic review" to your search statement.
Example: "environmental pollution” AND “systematic review”
There are also specific databases you can use to find systematic reviews. The next section of this guide highlights databases that provide systematic reviews or filters that can help you narrow your search results to systematic reviews.
- CINAHL/Medline
- Joanna Briggs
PubMed: Systematic Review Filter
- Database - PubMed PubMed is an online source provided by the National Library of Medicine that features millions of citations for biomedical literature from Medline, life science journals, and eBooks. Citations may include links to full-text content from the Chamberlain Library, PubMed Central, and publisher websites.
To find systematic reviews in PubMed, follow the steps below.
- Go to the database using the link above.
- Enter your keyword(s) into the search box.
- Select Search .
- Select Systematic Review from the Article Type section under the my NCBI Filters section on the left-hand side of the page. The image below shows the results of a search for autism spectrum disorder and indicates the Systematic Review filter under the My NCBI Filters .

Cochrane Library: Cochrane Reviews
- Database - Cochrane Library The Cochrane Library is a collection of databases, including Cochrane Clinical Answers, Cochrane Controlled Register of Trials (CENTRAL), and Cochrane Database of Systematic Reviews, offering systematic reviews, reports of randomized and quasi-randomized controlled trials, and more.
The Cochrane Library includes the Cochrane Database of Systematic Reviews, which makes it a great place to search for systematic reviews on your topic. To search for these systematic reviews, follow the steps below.
- Log into the database using the link above.
- Enter your keyword(s) for your topic of interest into the search box in the upper right-hand corner of the page.
- Select the magnifying glass icon next to the search box to run your search.
- The results of your search are presented in different tabs under the search box. Select the Cochrane Reviews tab to find all the systematic reviews in the database related to your topic. The image below shows an example search for heart disease and highlights the Cochrane Reviews tab.

CINAHL and Medline: Systematic Review Filter
- Database - CINAHL with Full Text CINAHL with Full Text is the world’s largest database of nursing and allied health journals covering topics on nursing, biomedicine, alternative/complementary medicine, consumer health, and 17 allied health disciplines.
- Database - Medline Complete from EBSCO Medline Complete provides access to journals covering a wide range of topics in the biomedical sciences and medicine.
To find systematic reviews in CINAHL or Medline, follow the steps below.
- Select one of the database links above to open the Advanced Search page.
- Enter your keyword(s) into the search boxes.
- Move down to the Limit Your Results section. This is where you can add filters to your search to tell the database that you only want articles that fit specific criteria.
- Select Systematic Review from the Publication Type menu, as shown in the image below.

Trip Pro: Systematic Reviews Filter
- Database - Trip Pro Database Trip Pro is a clinical database that offers access to high-quality research evidence, including articles, systematic reviews, images, videos, patient information leaflets, and educational courses.
To find systematic reviews in TripPro on your topic, follow the steps below.
- Enter your keywords for your topic in the search box.
- Select the magnifying glass next to the search box to run your search.
- Select Systematic Reviews under the Filter Results section on the left-hand side of the search results. The image below shows the results of a search for heart disease and highlights the Systematic Reviews filter.

Joanna Briggs Institute: Systematic Review Filter
- Database - Joanna Briggs Institute The Joanna Briggs Institute (JBI) is the international not-for-profit research and development arm of the School of Translational Science based within the Faculty of Health Sciences at the University of Adelaide, South Australia. It provides free access to evidence-based summaries and systematic reviews.
To find systematic reviews in Joanna Briggs Institute on your topic, follow the steps below.
- Go to the database using the link above.
- Enter your search term(s) into the search boxes at the top of the page.
- Select Search .
- Once you have results for your search, select Systematic Review under Publication Type in the Filter Results section on the left-hand side of the page. The image below show the results of a search for heart disease and highlights the Systematic Review filter.

Systematic reviews and meta-analyses are often conducted together, with the latter analyzing statistical data from the articles selected for the systematic review to provide a statistical conclusion.
You can narrow your search to meta-analyses only by adding a search term of meta-analysis to your search statement.
"environmental pollution” AND “meta-analysis”
In addition, there are also filters in specific databases that allow you to limit your search results to meta-analysis studies only. In the next section of this guide, you will find information on using these databases to find meta-analyses.
PubMed: Meta-Analysis Filter
To find a meta-analysis in PubMed, follow the steps below.
- Select Meta-Analysis from the Article Type section under the My NCBI Filters section on the left-hand side of the page. The image below shows the results of a search for autism spectrum disorder and indicates the Meta-Analysis filter under the My NCBI Filters .

CINAHL or Medline: Meta-Analysis Filter
To find meta-analyses in CINAHL or Medline, follow the steps below.
- Select one of the database links above to open the Advanced Search page.
- Move down to the Limit Your Results section. This is where you can add filters to your search to tell the database that you only want articles that fit specific criteria.
- Select Meta Analysis from the Publication Type menu, as shown in the image below.

Systematic Review
- Article - A systematic review of predictive models for hospital‐acquired pressure injury using machine learning Zhou, Y., Yang, X., Ma, S., Yuan, Y., & Yan, M. (2023). A systematic review of predictive models for hospital-acquired pressure injury using machine learning. Nursing open, 10(3), 1234–1246. https://doi.org/10.1002/nop2.1429
Meta-Analysis
- Article - Adequacy of health literacy and its effect on diabetes self-management: a meta-analysis. Guo, X., Zhai, X., & Hou, B. (2020). Adequacy of health literacy and its effect on diabetes self-management: A meta-analysis. Australian Journal of Primary Health, 26 (6), 458–465. https://doi.org/10.1071/PY20079
- Search Website
- Library Tech Support
- Services for Colleagues
Chamberlain College of Nursing is owned and operated by Chamberlain University LLC. In certain states, Chamberlain operates as Chamberlain College of Nursing pending state authorization for Chamberlain University.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Efficacy and Safety of Diet Therapies in Children With Autism Spectrum Disorder: A Systematic Literature Review and Meta-Analysis
Affiliations.
- 1 Graduate College of Tianjin Medical University, Tianjin, China.
- 2 Tianjin Children's Hospital (Children's Hospital of Tianjin University), Tianjin, China.
- 3 Tianjin Pediatric Research Institute, Tianjin, China.
- 4 Tianjin Key Laboratory of Birth Defects for Prevention and Treatment, Tianjin, China.
- 5 Department of Neurosurgery, Tianjin Children's Hospital, Tianjin, China.
- PMID: 35359629
- PMCID: PMC8963985
- DOI: 10.3389/fneur.2022.844117
Objective: Autism Spectrum Disorder is a neurodevelopmental disorder, with a rapid increase in recognition over the past decade. Interest in alternative therapies is growing annually, such as dietary therapies including gluten-free and/or casein-free diet, and the ketogenic diet. However, there is no consensus on the efficacy and safety of dietary therapy in children with ASD up to now. This study aimed to assess the efficacy and safety of these diet interventions for children with ASD based on a meta-analysis of global data.
Methods: Seven databases (Cochrane Library, PubMed, EMBASE, Web of Science, VIP, CNKI, and Wanfang) were searched according to the established inclusion criteria, from the inception of the databases to August 18, 2021. The Cochrane Bias risk assessment tool was intended to assess the quality of the included studies. Review Manager 5.4 software was used as an efficacy analysis tool of the included studies, taking the core autistic symptoms and scales of ASD as therapeutic efficacy evaluations.
Results: In total, 7 RCTs with 338 participants were finally obtained. All studies assessed the association between core autistic symptoms and therapeutic diet, showing a statistically significant effect (standard mean difference (SMD) of -0.51, 95% confidence interval (Cl): -0.81 to -0.21), in which two studies which followed the GFD diet reported significant reductions in social behaviors (SMD of-0.41, 95% Cl: -0.75 to -0.06), showing no correlation with the length of the interventions ( P < 0.05). Two studies were performed in KD diet suggested a significant effect in core symptoms (SMD of -0.67, 95% Cl: -1.04 to -0.31). No statistically significant changes were observed in the GFCF diet, GFD diet, cognition, communication, and stereotypical behaviors subgroups (all P > 0.05).
Conclusion: The results of a meta-analysis suggest that diet therapies can significantly ameliorate core symptoms of ASD, and GFD diets are conducive to improving social behaviors. Although the results suggest the effectiveness of dietary therapy for ASD, limited by the small sample size of RCTs, more well-designed, and high-quality clinical trials are needed to validate the above conclusions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42021277565.
Keywords: Autism Spectrum Disorders; childhood; gluten-free and casein-free diet; gluten-free diet; ketogenic diet; meta-analysis.
Copyright © 2022 Yu, Huang, Chen, Fu, Wang, Pu, Gu and Cai.
PubMed Disclaimer
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Flow diagram of selected studies.…
Flow diagram of selected studies. CNKI, China National Knowledge Infrastructure; VIP, China Science…
Risk of bias summary.
Meta-analysis results and scales for…
Meta-analysis results and scales for clinician-reported core symptoms. GARS (-2), Gilliam Autism Rating…
Meta-analysis results and scales for dietary intervention. GFCF, gluten-free and casein-free diet; GFD,…
Meta-analysis results and scales for the duration. GARS (-2), Gilliam Autism Rating Scale…
Meta-analysis results and scales for social behaviors. ATEC, Autism Treatment Evaluation Checklist; GARS-2,…
Similar articles
- A network meta-analysis of the effect of physical exercise on core symptoms in patients with autism spectrum disorders. Li L, Jia S, Wang P, Li S, Wang X, Zhu X. Li L, et al. Front Neurol. 2024 May 9;15:1360434. doi: 10.3389/fneur.2024.1360434. eCollection 2024. Front Neurol. 2024. PMID: 38784898 Free PMC article.
- A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Quan L, Xu X, Cui Y, Han H, Hendren RL, Zhao L, You X. Quan L, et al. Nutr Rev. 2022 Apr 8;80(5):1237-1246. doi: 10.1093/nutrit/nuab073. Nutr Rev. 2022. PMID: 34617108 Free PMC article.
- The Effect of a Combined Gluten- and Casein-Free Diet on Children and Adolescents with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Keller A, Rimestad ML, Friis Rohde J, Holm Petersen B, Bruun Korfitsen C, Tarp S, Briciet Lauritsen M, Händel MN. Keller A, et al. Nutrients. 2021 Jan 30;13(2):470. doi: 10.3390/nu13020470. Nutrients. 2021. PMID: 33573238 Free PMC article.
- Gluten- and casein-free diet and autism spectrum disorders in children: a systematic review. Piwowarczyk A, Horvath A, Łukasik J, Pisula E, Szajewska H. Piwowarczyk A, et al. Eur J Nutr. 2018 Mar;57(2):433-440. doi: 10.1007/s00394-017-1483-2. Epub 2017 Jun 13. Eur J Nutr. 2018. PMID: 28612113 Review.
- Parent-mediated early intervention for young children with autism spectrum disorders (ASD). Oono IP, Honey EJ, McConachie H. Oono IP, et al. Cochrane Database Syst Rev. 2013 Apr 30;(4):CD009774. doi: 10.1002/14651858.CD009774.pub2. Cochrane Database Syst Rev. 2013. PMID: 23633377 Review.
- Associations between genetically determined dietary factors and risk of autism spectrum disorder: a Mendelian randomization study. Li W, Liu C, Chen S. Li W, et al. Front Nutr. 2024 Mar 1;11:1210855. doi: 10.3389/fnut.2024.1210855. eCollection 2024. Front Nutr. 2024. PMID: 38496795 Free PMC article.
- Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance. Hung LY, Margolis KG. Hung LY, et al. Nat Rev Gastroenterol Hepatol. 2024 Mar;21(3):142-163. doi: 10.1038/s41575-023-00857-1. Epub 2023 Dec 19. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38114585 Review.
- Protocol for a randomized clinical trial comparing the efficacy of Structured Diet (SD) and Regular Therapy (RT) for adolescents with malnutrition having Autism Spectrum Disorder (ASD). Akter R, Urme NA, Hossain KMA, Hossain T, Ahammad S, Yeasmin MH, Hossain MZ, Parvin R, Hossain MS, Zahid MA. Akter R, et al. PLoS One. 2023 Nov 29;18(11):e0292326. doi: 10.1371/journal.pone.0292326. eCollection 2023. PLoS One. 2023. PMID: 38019825 Free PMC article.
- Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey. Matthews JS, Adams JB. Matthews JS, et al. J Pers Med. 2023 Sep 29;13(10):1448. doi: 10.3390/jpm13101448. J Pers Med. 2023. PMID: 37888059 Free PMC article.
- Autism Spectrum Disorder in 2023: A Challenge Still Open. Posar A, Visconti P. Posar A, et al. Turk Arch Pediatr. 2023 Nov;58(6):566-571. doi: 10.5152/TurkArchPediatr.2023.23194. Turk Arch Pediatr. 2023. PMID: 37850666 Free PMC article.
- Lord C, Bishop SL. Recent advances in autism research as reflected in DSM-5 criteria for autism spectrum disorder. Annu Rev Clin Psychol. (2015) 11:53–70. 10.1146/annurev-clinpsy-032814-112745 - DOI - PubMed
- Wiggins LD, Rice CE, Barger B, Soke GN, Lee LC, Moody E. DSM-5 criteria for autism spectrum disorder maximizes diagnostic sensitivity and specificity in preschool children. Soc Psychiatry Psychiatr Epidemiol. (2019) 54:693–701. 10.1007/s00127-019-01674-1 - DOI - PMC - PubMed
- Genovese A, Butler MG. Clinical assessment, genetics, and treatment approaches in Autism Spectrum Disorder (ASD). Int J Mol Sci. (2020) 21:4726. 10.3390/ijms21134726 - DOI - PMC - PubMed
- Kodak T, Bergmann S. Autism spectrum disorder: characteristics, associated behaviors, and early intervention. Pediatr Clin North Am. (2020) 67:525–35. 10.1016/j.pcl.2020.02.007 - DOI - PubMed
- Centers for Disease Control and Prevention . Available online at: https://www.cdc.gov/ncbddd/autism/addm.html (accessed January 11, 2022).
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Frontiers Media SA
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
SYSTEMATIC REVIEW article
The effect of supplementing with saccharomyces boulardii on bismuth quadruple therapy for eradicating helicobacter pylori : a systematic review and meta-analysis of randomized controlled trials.

- Department of Gastroenterology, Tongren People’s Hospital, Tongren, Guizhou Province, China
Background and objective: It remains uncertain if the addition of Saccharomyces boulardii ( S. boulardii ) to bismuth quadruple therapy (BQT) recommended in the current guidelines can enhance the Helicobacter pylori ( H. pylori ) eradication rate and decrease the incidence of adverse events. We therefore conducted a meta-analysis of randomized controlled trials (RCTs) to address this issue.
Methods: We performed comprehensive searches in PubMed, Embase, Web of Science, and Cochrane library databases from the inception of the databases through to November 1, 2023. A meta-analysis was conducted to determine the pooled relative risk (RR) with 95% confidence intervals (CI) using a random-effects model. We utilized the revised Cochrane Risk of Bias Tool to assess the risk of bias of included studies.
Results: A total of six RCTs (1,404 patients) included in this meta-analysis. The results of the intention-to-treat analysis showed that the combination of S. boulardii with BQT had a higher eradication rate than BQT alone (87.0% versus 83.3%), with a pooled RR of 1.05 (95% CI: 1.00–1.10, p = 0.03). In the per-protocol analysis, however, there was no statistical significance between the two groups in the eradication rate (93.7% versus 91.0%, RR = 1.03, 95% CI: 1.00–1.06, p = 0.07). The combination of S. boulardii and BQT had a significantly lower rate of overall adverse events (22% vs. 39%, RR = 0.56, 95% CI: 0.44–0.70, p < 0.00001), diarrhea (7.9% vs. 25.7%, RR = 0.29, 95% CI: 0.17–0.48, p < 0.00001), constipation (2.9% vs. 8.4%, RR = 0.35, 95% CI: 0.14–0.88, p = 0.03) and abdominal distention (4.9% vs. 12.7%, RR = 0.41, 95% CI: 0.23–0.72, p = 0.002) than BQT alone. For the assessment of risk of bias, five studies were deemed to have some concerns, while one study was judged to have a low risk.
Conclusion: Current evidence suggests that supplementation with S. boulardii in BQT may not have a major effect on the H. pylori eradication rate, but significantly reduces the incidence of overall adverse events, diarrhea, abdominal distention and constipation. Combining S. Boulardii with BQT can help alleviate symptoms, potentially improving patient adherence.
Systematic review registration: https://osf.io/n9z7c.
1 Introduction
Helicobacter pylori ( H. pylori ) is a Gram negative, spiral-shaped microbe that colonizes the stomach and has become a major public health concern, with more than half of the world’s population affected by it ( 1 , 2 ). It is a widely accepted notion that H. pylori infection is correlated with a range of gastrointestinal ailments, including chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. In the past few years, numerous studies have revealed that H. pylori infection is not only the cause of gastrointestinal issues, but could also be associated with a variety of extragastrointestinal illnesses including cardiovascular, hematological, neurological, metabolic, and skin diseases ( 3 – 5 ). By eliminating H. pylori in its initial stages, the chances of developing gastric cancer can be significantly reduced ( 6 , 7 ).
In the past, standard triple therapy (STT), which was made up of a proton pump inhibitor (PPI) and two antibiotics (amoxicillin and clarithromycin/metronidazole), was the most common approach to eradicating H. pylori . However, the growing prevalence of antibiotic resistance has complicated attempts to eradicate H. pylori , particularly with regards to clarithromycin resistance, and thus clarithromycin triple therapy may no longer be the most suitable first-line treatment ( 3 , 8 ). Currently, the bismuth quadruple therapy (BQT) for 10–14 days is first-line treatment that are recommended in several guidelines and consensus reports ( 3 , 9 – 11 ). Although BQT is an effective method for eradicating H. pylori infection, adverse events and poor compliance during the eradication process are common ( 12 , 13 ). Additionally, increasing evidence points to the fact that eradication drugs, especially antibiotics and PPI, can cause an imbalance in the gut microbiota, which has a significant impact on human health ( 14 , 15 ). Therefore, new therapies are needed.
Probiotics, as living microorganisms, have been employed extensively to treat illnesses like antibiotic-induced diarrhea, colitis, and metabolic syndrome ( 16 ). Saccharomyces boulardii ( S. boulardii ) is the only probiotic preparation derived from fungi that is used worldwide. A meta-analysis by Szajewska et al. ( 17 ) demonstrated that the supplementation of S. boulardii in the STT for H. pylori can result in a higher eradication rate and a lower incidence of side effects. The resistance to antibiotics has increased significantly, making STT no longer as effective as before. It remains uncertain if the addition of S. boulardii to BQT recommended in the current guidelines can enhance the H. pylori eradication rate and decrease the incidence of adverse events. Several randomized controlled trials (RCTs) have been conducted on this topic in recent years, yet the small sample size of each study has not allowed for any definite conclusions to be made. To evaluate the efficacy and safety of S. boulardii assisted BQT versus BQT, we therefore performed this systematic review and meta-analysis.
2 Materials and methods
This systematic review was conducted in accordance with the PRISMA 2020 statement ( 18 ). The protocol of this study was registered in the Open Science Framework. 1
2.1 Literature search
Utilizing pre-determined search terms, we performed systematic searches in PubMed, Embase, Web of Science, and the Cochrane library database, up to November 1, 2023, without any language limitations. The search terms included “ helicobacter pylori ,” “ H. pylori ,” helicobacter , “ campylobacter pylori ,” “saccharomyces boulardii,” “ S. boulardii ,” probiotics, probiotic, “bismuth.” Taking PubMed as an example, the detailed search strategy was follow: (“ helicobacter ”[MeSH Terms] OR “ helicobacter ”[tiab] OR “ helicobacter pylori ”[MeSH Terms] OR “ helicobacter pylori ”[tiab] OR “ H. pylori ”[tiab] OR “ campylobacter pylori ”[tiab]) AND (“ saccharomyces boulardii ”[tiab] OR “ S. boulardii ”[tiab] OR probiotics[MeSH] OR probiotics[tiab] OR probiotic[tiab]) AND (“bismuth”[MeSH Terms] OR “bismuth”[tiab]). The retrieval strategies for the other three electronic databases are detailed in Supplementary Table S1 . Additionally, we examined the reference lists of the evaluated studies to identify any further eligible studies.
2.2 Study selection
Two reviewers (Chen Y and Teng T) independently conducted two screenings of the study selection. They initially evaluated the title and abstract of the articles and excluded those that were unlikely to be related to the research. Then, the two reviewers examined the full-text articles and chose those that were eligible for meta-analysis.
Studies that fulfilled the following criteria were included: (1) Study design: RCTs; (2) Participants: adults patients who have not had any professional treatment for H. pylori in the past; (3) Intervention: Combined treatment of S. boulardii with BQT; (4) Comparison: the same BQT (bismuth + PPI + two antibiotics); and (5) Outcomes: H. pylori eradication rate and the incidence of adverse events (including the overall and specific adverse events). We will not consider studies on other probiotics, duplicates, Non-RCTs, animal experiments, reviews and meta-analysis, conference abstracts, letters, editorials, guidelines and consensus, and studies from which data cannot be gathered.
2.3 Data extraction and risk of bias
The data collected from the eligible studies included the name of the first author, year of publication, country of origin, sample size, diagnosis methods used for H. pylori , information regarding the intervention and control groups, and relevant data on the outcomes of interest.
We utilized the revised Cochrane Risk of Bias Tool (RoB 2.0) for randomized trials to assess the risk of bias in the included studies, encompassing the following five domains: (1) bias arising during randomization; (2) bias due to deviations from intended interventions; (3) bias from missing outcome data; (4) bias in outcome measurement; and (5) bias in reporting outcome selection. The bias risk in each category can be categorized into three levels: low risk of bias, some concerns, and high risk of bias ( 19 ). Two reviewers (Chen Y and Teng T) independently collected data and assessed the risk of bias for each study, and any disagreements were resolved through consensus.
2.4 Statistical analysis
The relative risk (RR) and 95% confidence intervals (CIs) were calculated as summary effect size following the random-effects model. To assess the heterogeneity between studies, both the I 2 statistic and the chi-square test with a p value <0.10 were employed. If the p value was <0.10, substantial heterogeneity was determined. Heterogeneity was categorized as insignificant, low, moderate, or high, depending on the I 2 values, which were 0–25%, 26–50%, 51–75%, and above 75%, respectively ( 20 ). Data for H. pylori eradication rate were analyzed using both intention-to-treat (ITT) and per-protocol (PP) analysis. The ITT analysis involved all participants who were initially assigned to the group through random selection. The PP analysis excluded patients who did not withdraw for any reason and received treatment doses below 90%. We performed pre-specified subgroup analyses by duration of BQT, dosage of S. boulardii and duration of supplementation with S. boulardii . Subgroup analyses of H. pylori eradication rate were conducted through ITT analysis. The publication bias should be investigated by funnel plot and Egger test if at least 10 studies are included in the meta-analysis ( 21 , 22 ). It was determined that a p -value of less than 0.05 was indicative of a significant publication bias. All analyses were conducted using the RevMan 5.3 software (the Cochrane Collaboration, Copenhagen, Denmark) and STATA/SE (Version 12.0, STATA Corporation, Texas, United States).
3.1 Study selection
System retrieval produced 470 records, of which 208 were duplicates, leaving 262 records. After screening titles and abstracts, 234 records were excluded, leaving 28 full-text articles to be reviewed. Ultimately, 6 RCTs (8 intervention arms) ( 23 – 28 ) were included in the meta-analysis, as illustrated in Figure 1 .
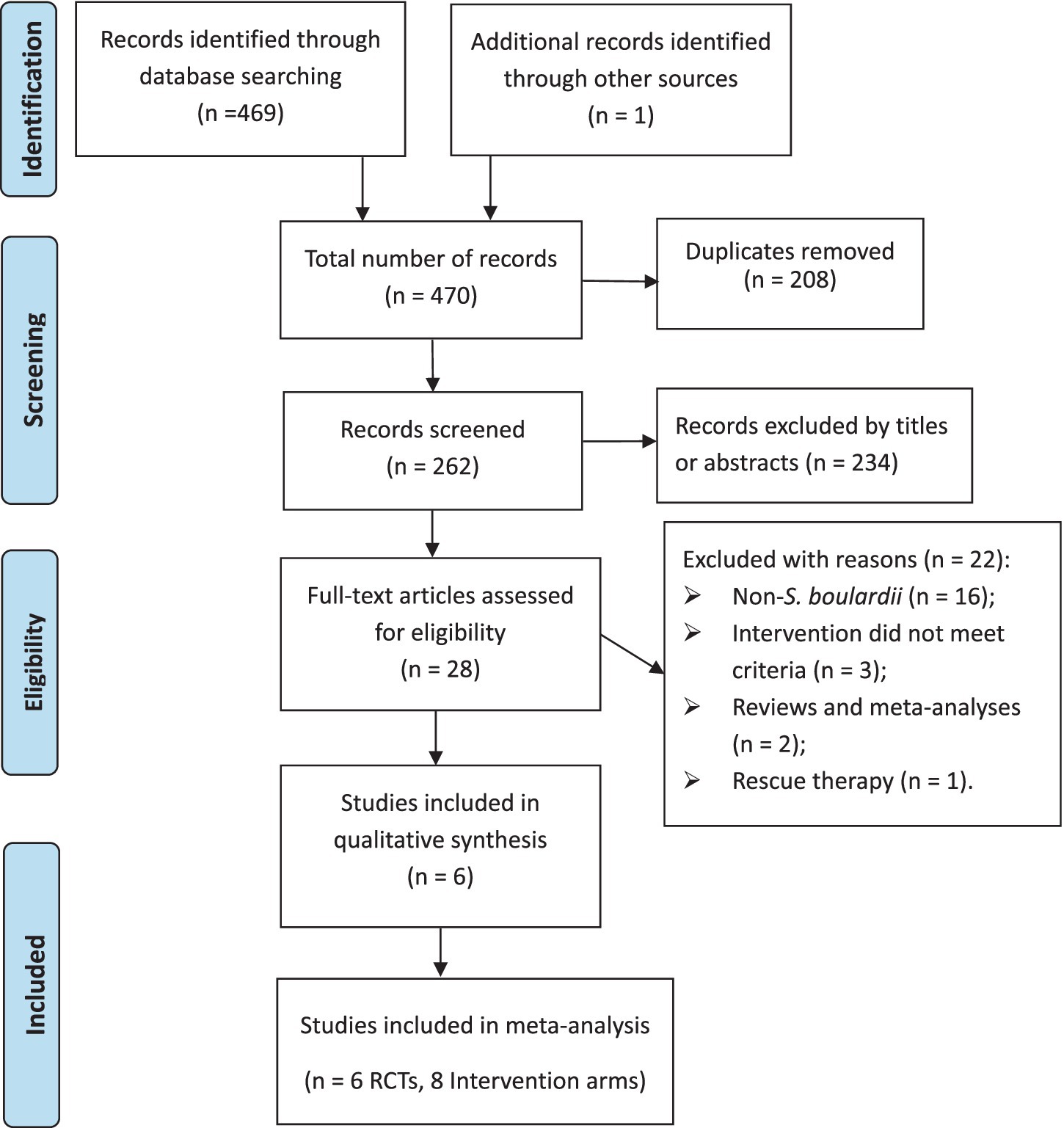
Figure 1 . PRISMA flowchart of study selection process.
3.2 Study characteristics
A total of six RCTs (involving 1,404 patients) published from 2017 to 2023 included in our meta-analysis. All studies originate from Asia, five of which are from China ( 23 – 26 , 28 ) and one from Iran ( 27 ). Of all the studies, one was a multicenter RCT ( 26 ), while the remaining five were single center RCTs. Two RCTs ( 23 , 25 ) included multiple arms. The number of participants in these RCTs varied from 104 to 348. Regarding the duration of treatment for BQT, there were three studies ( 23 – 25 ) with a duration of 10 days and the other three studies ( 26 – 28 ) with a duration of 14 days. The dosage of S. boulardii varied between studies, with one study ( 27 ) using 500 mg/day and the others 1,000 mg/day ( 23 – 26 , 28 ). The duration of S. boulardii regimen was 14 or 28 days in one study, while the other five studies only had 14 days of treatment. The major characteristics of the studies incorporated are outlined in Table 1 .
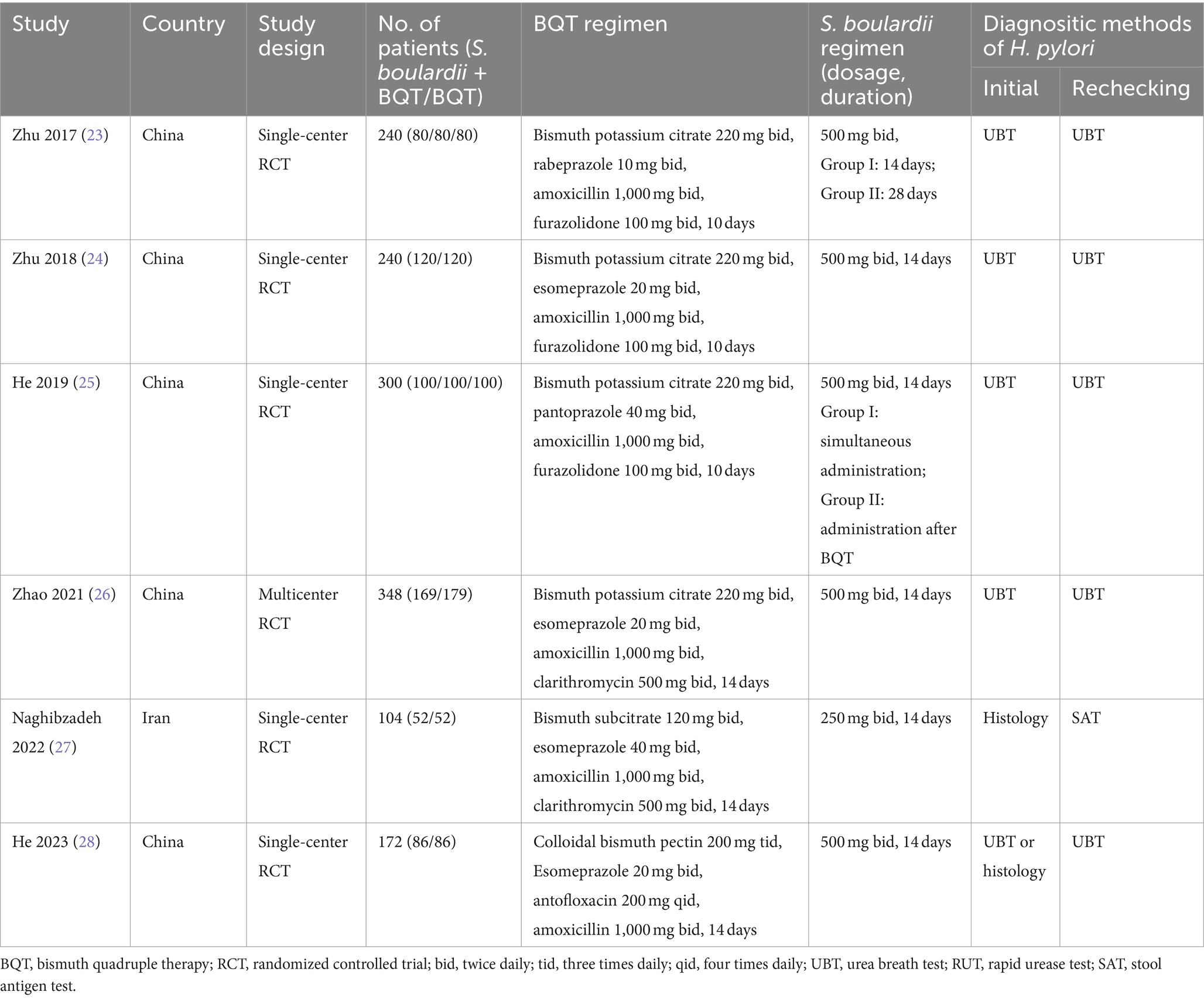
Table 1 . Main characteristics of included studies.
3.3 Risk of bias
All of the six RCTs included in the analysis exhibited a low risk of bias in terms of the randomization process, missing outcome data, measurement of the outcome, and selection of reported results. In relation to bias from deviations in the intended intervention, five studies were deemed to have some concerns, while one study was judged to have a low risk. The details of the risk of bias are presented in Supplementary Figures S1 , S2 .

3.4 Helicobacter pylori eradication rate
Six RCTs with 1,404 participants reported data on the H. pylori eradication rate. The results of the ITT analysis showed that the combination of S. boulardii with BQT had a higher eradication rate than BQT alone (87.0% versus 83.3%), with a pooled RR of 1.05 (95% CI: 1.00–1.10, p = 0.03) and no heterogeneity (I 2 = 0%, p = 0.95) ( Figure 2 ). In the PP analysis, the eradication rate of S. boulardii in combination with BQT was higher than BQT alone (93.7% versus 91.0%), however, there was no statistical significance between the two groups (RR = 1.03, 95% CI: 1.00–1.06, p = 0.07) ( Figure 3 ). No statistical heterogeneity was observed (I 2 = 0%, p = 0.90).
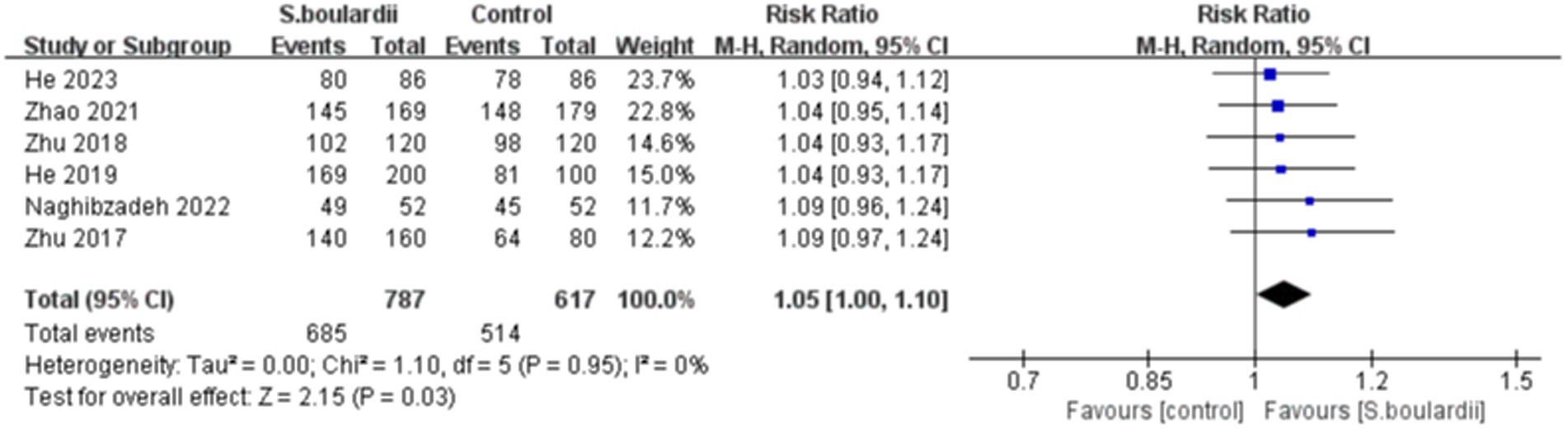
Figure 2 . Forest plot of the H. pylori eradication rate (ITT data).
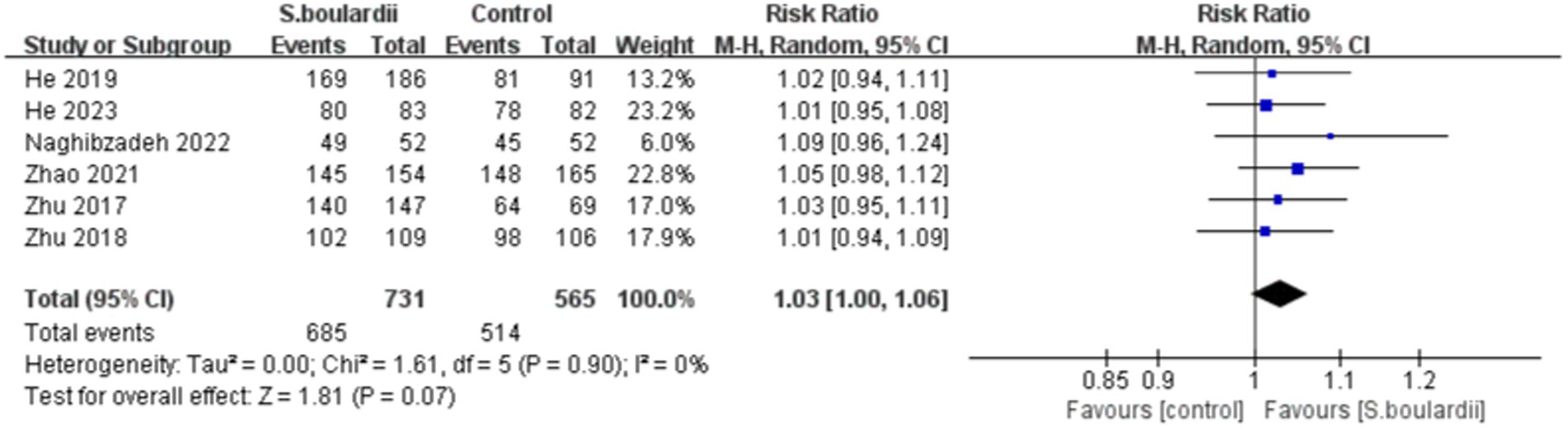
Figure 3 . Forest plot of the H. pylori eradication rate (PP data).
Based on ITT analysis data, we further conducted subgroup analyses based on duration of BQT regimen, dosages of S. boulardii , and duration of S. boulardii to explore the potential influencing factor on the overall results. Results from the subgroup analysis based on duration of BQT regimen showed that the H. pylori eradication rate was higher in the S. boulardii supplementation group in the 10-day subgroup ( n = 3 RCTs, RR = 1.06, 95% CI: 0.99–1.13, p = 0.11) and 14-day subgroup ( n = 3 RCTs, RR = 1.04, 95% CI: 0.99–1.10, p = 0.15), yet the difference was not statistically significant. Results from the subgroup analysis based on dosages of S. boulardii showed that the H. pylori eradication rate was higher in the S. boulardii supplementation group in the subgroup of 500 mg/day ( n = 1 RCT, RR = 1.09, 95% CI: 0.96–1.24, p = 0.19) and the subgroup of 1,000 mg/day ( n = 5 RCTs, RR = 1.04, 95% CI: 1.00–1.09, p = 0.07), yet the difference was not statistically significant. In a subgroup analysis based on duration of S. boulardii , the H. pylori eradication rate increased significantly in the 14-day subgroup ( n = 6 RCTs, RR = 1.05, 95% CI: 1.00–1.09, p = 0.04), but not in the 28-day subgroup ( n = 1 RCT, RR = 1.09, 95% CI: 0.95–1.25, p = 0.20). The results of subgroup analyses were summarized in Table 2 .
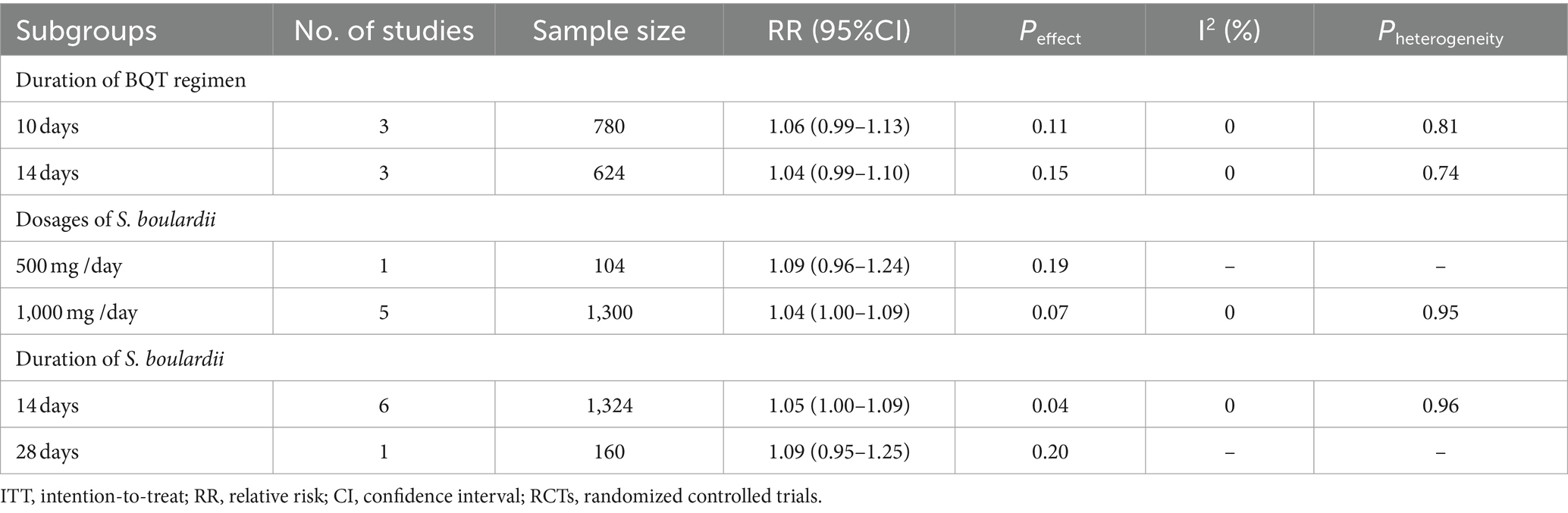
Table 2 . Results of subgroup analyses of H. pylori eradication rate (ITT data).
3.5 Adverse events
Four RCTs ( 23 – 26 ) involving a total of 1,128 participants revealed the incidence of overall adverse events. Results of the meta-analysis showed that the combination of S. boulardii and BQT had a significantly lower rate of overall adverse events than BQT alone (22% vs. 39%, RR = 0.56, 95% CI: 0.44–0.70, p < 0.00001). The heterogeneity was low (I 2 = 38, p = 0.18) ( Figure 4 ).
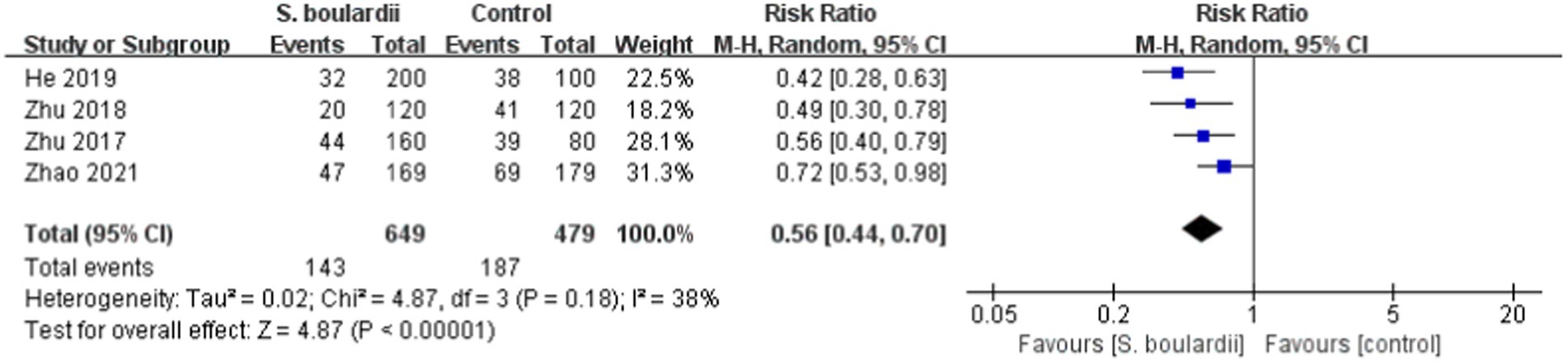
Figure 4 . Forest plot of the overall adverse events.
When it comes to specific adverse events, the results from the meta-analysis showed that those in the S. boulardii combined BQT group experienced a lower rate of diarrhea ( n = 5 RCTs, 7.9% vs. 25.7%, RR = 0.29, 95% CI: 0.17–0.48, p < 0.00001), constipation ( n = 3 RCTs, 2.9% vs. 8.4%, RR = 0.35, 95% CI: 0.14–0.88, p = 0.03) and abdominal distention ( n = 4 RCTs, 4.9% vs. 12.7%, RR = 0.41, 95% CI: 0.23–0.72, p = 0.002) than the BQT group. Nonetheless, no significant difference was seen between the two groups in regards to nausea, vomiting, abdominal pain, rash and dizzy. The results of adverse events were presented in Figures 5 , 6 .
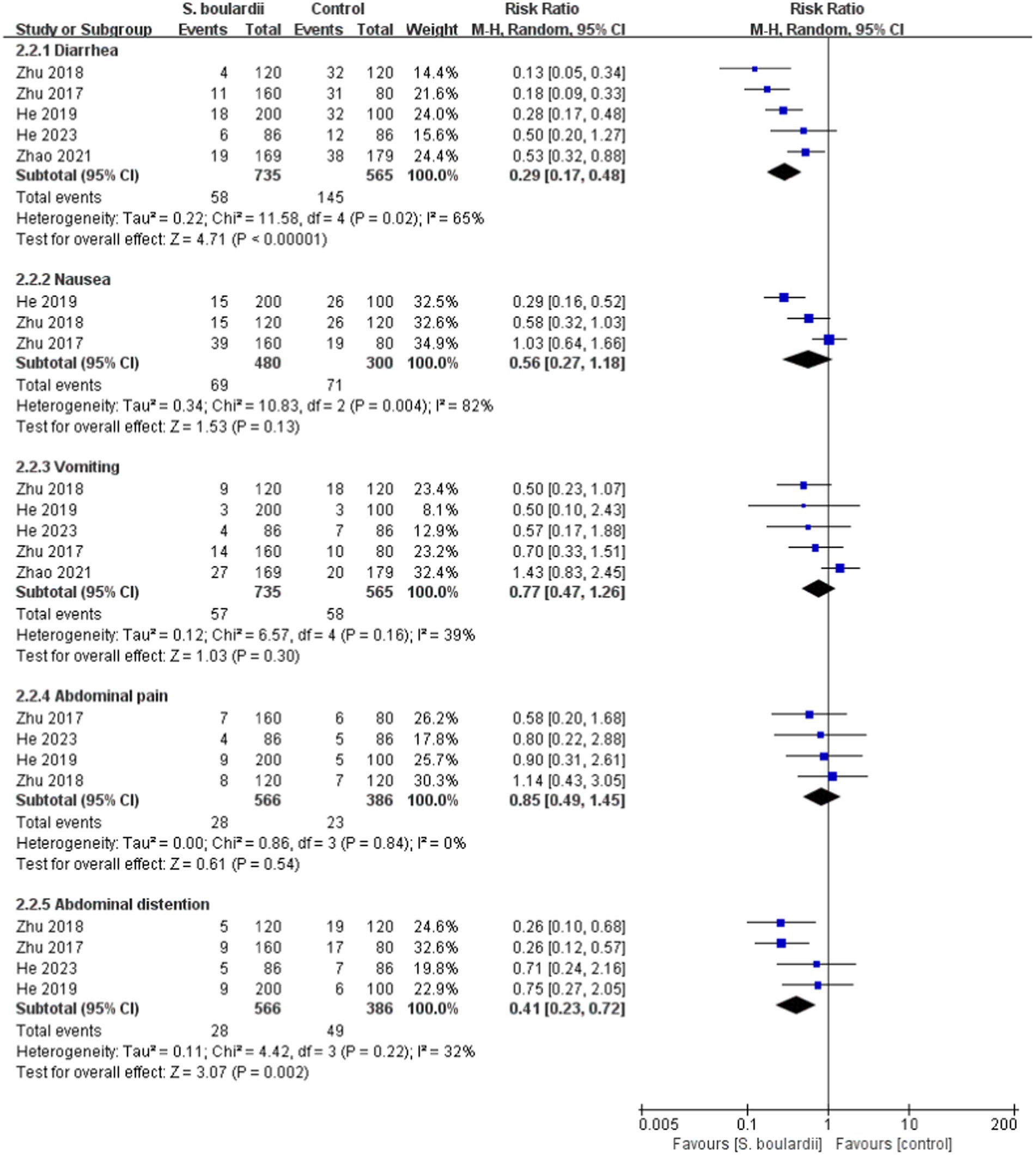
Figure 5 . Forest plot of the specific adverse events (diarrhea, nausea, vomiting, abdominal pain, and abdominal distention).
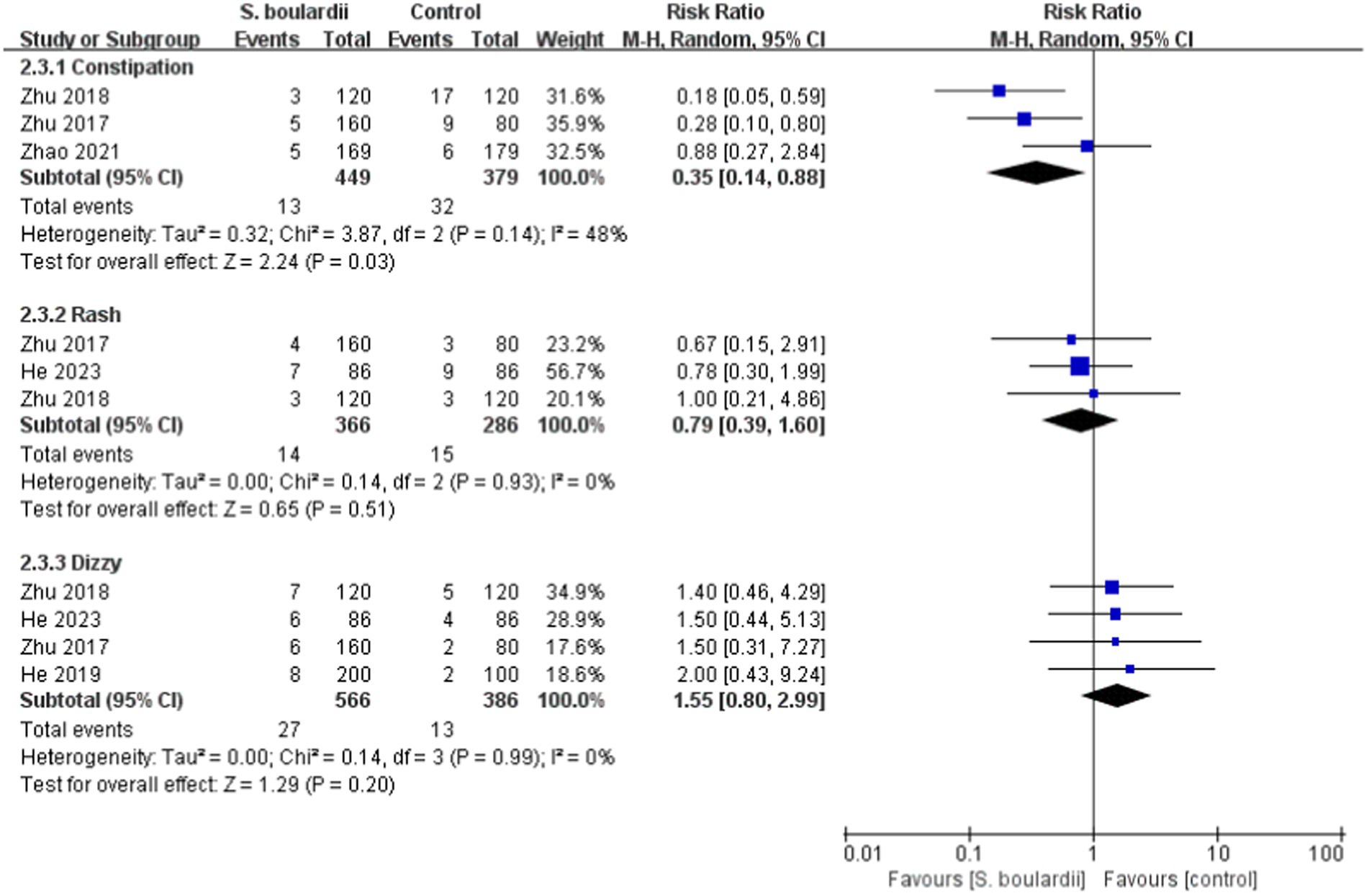
Figure 6 . Forest plot of the specific adverse events (constipation, rash, and dizzy).
4 Discussion
4.1 main findings and potential explanations.
To our knowledge, this is the first time that a meta-analysis has been conducted to explore the effects of combining S. boulardii with BQT for the eradication of H. pylori compared to BQT alone. In this meta-analysis of six RCTs with 1,404 participants, we demonstrated that, when analyzed by ITT or PP, the eradication rate of the S. boulardii -supplemented BQT group was higher than that of the single BQT group. However, in PP analysis, there was no statistically significant difference between the two groups. It is worth noting that the 95% CI for both ITT and PP analyses overlapped with the invalid line, which could be due to the sample size being too small to draw statistically significant conclusions, or the high eradication rate of BQT, making it difficult to see the effects of adding S. Boulardii . In order to explore the effect of different durations of BQT regimen, different dosages and durations of S. boulardii on the overall results, we conducted subgroup analyses. The results showed that, although the eradication rate of H. pylori in the group supplemented with S. boulardii was higher than that in the single BQT group, except for the subgroup that was treated with S. boulardii for 14 days, there was no statistically significant difference between the two groups in the other subgroups. With such a small number of studies included in these subgroups, it may be difficult to draw statistically significant conclusions. Our meta-analysis also demonstrated that taking S. boulardii can reduce the occurrence of overall adverse events, diarrhea, abdominal distention, and constipation while eradicating H. pylori .
Saccharomyces boulardii , a fungal probiotic preparation, was originally isolated from tropical fruit peels. It is stable over a wide pH range, including acidic conditions and temperature levels, as well as during contact with bile salts and gastrointestinal enzymes ( 29 ). Due to its natural properties, the fungus is impervious to the antibiotic. Furthermore, the introduction of S. boulardii CNCM I-745 cannot generate antibiotic resistance since the exchange of antibiotic resistance genes with bacteria is improbable ( 30 , 31 ). Evidence from current studies suggests that S. boulardii can successfully combat H. pylori infection both in vitro and in vivo . S. boulardii has the ability to directly inhibit H. pylori through the production of lactic acid, short-chain fatty acids, bacteritin, hydrogen peroxide, neuraminidase, and other substances ( 28 ). Furthermore, compared to other probiotic bacterial strains, S. boulardii has a much larger volume, resulting in a greater surface area and improved ability to adhere to pathogenic bacteria, thus impacting the colonization of H. pylori in the gastric mucosa ( 32 ). S. boulardii has neuraminidase activity that is specific to alpha (2–3)-linked sialic acid, and it acts by attaching itself to the adhesin of H. pylori , thereby preventing the adhesion of H. pylori in the duodenum ( 33 ). Moreover, S. boulardii can promote immunoprotection by triggering the secretion of sIgA and immunoglobulin in the gastrointestinal tract ( 34 ). Additionally, S. boulardii has an impact on the gut microbiota, thus decreasing gastrointestinal issues in patients ( 35 ), which leads to increased compliance and, as a result, a higher eradication rate of H. pylori .
4.2 Comparison with previous work
Previously, Yao et al. ( 36 ) conducted a meta-analysis of 10 RCTs and explored the effect of probiotic-supplemented BQT for the treatment of H. pylori. The results of the meta-analysis indicated that the eradication rate of the probiotic-supplemented BQT group was higher than that of the BQT group alone in both ITT (RR = 1.07, 95% CI: 1.02–1.11, p = 0.003) and PP analyses (RR = 1.04, 95% CI: 1.00–1.07, p = 0.03). In addition, probiotic supplementation was associated with a lower rate of side effects, diarrhea, and a bitter taste. Nevertheless, the meta-analysis included different probiotics, with only one study using S. Boulardii , which may lead to an inaccurate conclusion due to the strain specificity of probiotics and the fact that not all probiotics improve the H. pylori eradication rate or reduce the incidence of side effects ( 37 ). In comparison to the prior meta-analyses, our meta-analysis was more reliable due to the fact that it only focused on a particular probiotic strain ( S. Boulardii ) for consolidation.
4.3 Strengths and limitations
This meta-analysis has the major advantage of providing the latest and most comprehensive data on the impact of utilizing a single probiotic strain ( S. boulardii ) in combination with BQT to evaluate the eradication of H. pylori compared to BQT. In addition, we employed a rigorous systematic review methodology, employing a comprehensive search strategy, explicit inclusion and exclusion criteria, strict quality assessment, and strictly adhering to PRISMA statement for reporting, all of which ensured our results were transparent and reliable.
Despite this, this study still has certain limitations. First, out of all the studies we included, only one was a placebo-controlled double-blind trial, while the others did not include placebos. The blinding method and allocation were unclear, which could have an impact on our subjective outcome indicators (e.g., incidence of adverse events). Therefore, in the future, it is necessary to further conduct high-quality, placebo-controlled, double-blind trials to further verify these findings. Second, subgroup analyses only involve limited data, which can make it difficult to identify significant differences. Third, with the limited data available, it is difficult to ascertain the ideal dosage and duration of S. boulardii to achieve the desired results. Further optimization and confirmation is needed through further research. Fourth, this study did not include the classic BQT, currently recommended by international guidelines, that consists of PPI, salt of bismuth, tetracycline and metronidazole. Further exploration of this limitation is necessary in future research. Additionally, as there were less than 10 studies included, further publication bias testing was not conducted. Nevertheless, the potential for bias cannot be entirely dismissed. Finally, our meta-analysis focused on Asian populations, thus the results of this study can be applied only to the Asian population, and further research is required to determine if the findings can be extended to other populations.
5 Conclusion
Current evidence suggests that supplementation with S. boulardii in BQT may not have a major effect on the H. pylori eradication rate, but significantly reduces the incidence of overall adverse events, diarrhea, abdominal distention and constipation. Combining S. Boulardii with BQT can help alleviate symptoms, potentially improving patient adherence and offering a valuable treatment option.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author.
Author contributions
YC: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization. TT: Formal analysis, Methodology, Validation, Writing – original draft. YS: Data curation, Methodology, Validation, Writing – original draft. W-ZC: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1344702/full#supplementary-material
1. ^ https://osf.io/n9z7c
1. Shirani, M, Pakzad, R, Haddadi, MH, Akrami, S, Asadi, A, Kazemian, H, et al. The global prevalence of gastric cancer in Helicobacter pylori -infected individuals: a systematic review and meta-analysis. BMC Infect Dis . (2023) 23:543. doi: 10.1186/s12879-023-08504-5
PubMed Abstract | Crossref Full Text | Google Scholar
2. Hooi, JKY, Lai, WY, Ng, WK, Suen, MMY, Underwood, FE, Tanyingoh, D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology . (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
Crossref Full Text | Google Scholar
3. Malfertheiner, P, Megraud, F, Rokkas, T, Gisbert, JP, Liou, JM, Schulz, C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut . (2022):gutjnl-2022-327745. doi: 10.1136/gutjnl-2022-327745
4. Sun, Q, Yuan, C, Zhou, S, Lu, J, Zeng, M, Cai, X, et al. Helicobacter pylori infection: a dynamic process from diagnosis to treatment. Front Cell Infect Microbiol . (2023) 13:1257817. doi: 10.3389/fcimb.2023.1257817
5. Pellicano, R, Ianiro, G, Fagoonee, S, Settanni, CR, and Gasbarrini, A. Review: extragastric diseases and Helicobacter pylori . Helicobacter . (2020) 25:e12741. doi: 10.1111/hel.12741
6. Lee, YC, Chiang, TH, Chou, CK, Tu, YK, Liao, WC, Wu, MS, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and Meta-analysis. Gastroenterology . (2016) 150:1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028
7. Ford, AC, Forman, D, Hunt, RH, Yuan, Y, and Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ . (2014) 348:g3174. doi: 10.1136/bmj.g3174
8. Liu, WZ, Xie, Y, Lu, H, Cheng, H, Zeng, ZR, Zhou, LY, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter . (2018) 23:e12475. doi: 10.1111/hel.12475
9. Fallone, CA, Chiba, N, van Zanten, SV, Fischbach, L, Gisbert, JP, Hunt, RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology . (2016) 151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006
10. Zhou, L, Lu, H, Song, Z, Lyu, B, Chen, Y, Wang, J, et al. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J . (2022) 135:2899–910. doi: 10.1097/CM9.0000000000002546
11. Leung, WK, Cheung, KS, Sham, PCO, Tang, RSY, Loo, CK, Hsu, ASJ, et al. Consensus recommendations for the screening, diagnosis, and management of Helicobacter pylori infection in Hong Kong. Hong Kong Med J . (2023) 29:532–41. doi: 10.12809/hkmj2210321
12. Liou, JM, Fang, YJ, Chen, CC, Bair, MJ, Chang, CY, Lee, YC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori : a multicentre, open-label, randomised trial. Lancet . (2016) 388:2355–65. doi: 10.1016/S0140-6736(16)31409-X
13. Yang, J, Zhang, Y, Fan, L, Zhu, YJ, Wang, TY, Wang, XW, et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori . Am J Gastroenterol . (2019) 114:437–45. doi: 10.14309/ajg.0000000000000132
14. Ye, Q, Shao, X, Shen, R, Chen, D, and Shen, J. Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: a systematic review and meta-analysis. Helicobacter . (2020) 25:e12713. doi: 10.1111/hel.12713
15. Tao, ZH, Han, JX, and Fang, JY. Helicobacter pylori infection and eradication: exploring their impacts on the gastrointestinal microbiota. Helicobacter . (2020) 25:e12754. doi: 10.1111/hel.12754
16. He, C, Xie, Y, Zhu, Y, Zhuang, K, Huo, L, Yu, Y, et al. Probiotics modulate gastrointestinal microbiota after Helicobacter pylori eradication: a multicenter randomized double-blind placebo-controlled trial. Front Immunol . (2022) 13:1033063. doi: 10.3389/fimmu.2022.1033063
17. Szajewska, H, Horvath, A, and Kołodziej, M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther . (2015) 41:1237–45. doi: 10.1111/apt.13214
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . (2021) 372:n71. doi: 10.1136/bmj.n71
19. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . (2019) 366:l4898. doi: 10.1136/bmj.l4898
20. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ . (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Sterne, JA, and Egger, M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol . (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
22. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. eds. Cochrane handbook for systematic reviews of interventions version 6.4 (updated 2023) . Cochrane: (2023) Available at: www.training.cochrane.org/handbook .
Google Scholar
23. Zhu, XY, Du, J, Wu, J, Zhao, LW, Meng, X, and Liu, GF. Influence of Saccharomyces boulardii sachets combined with bismuth quadruple therapy for initial Helicobacter pylori eradication. Zhonghua Yi Xue Za Zhi . (2017) 97:2353–6. doi: 10.3760/cma.j.issn.0376-2491.2017.30.008
24. Zhu, XY, Du, J, Zhao, WJ, Wu, J, Zhao, LW, Meng, X, et al. Influence of two kinds of probiotics combined with bismuth quadruple therapy for Helicobacter pylori eradication. Zhonghua Yi Xue Za Zhi . (2018) 98:2246–9. doi: 10.3760/cma.j.issn.0376-2491.2018.28.007
25. He, CX, Kong, FT, Liang, F, Wang, KX, Li, H, Liu, YL, et al. Influence of different timing of Saccharomyces boulardii combined with bismuth quadruple therapy for Helicobacter pylori eradication. Zhonghua Yi Xue Za Zhi . (2019) 99:1731–4. doi: 10.3760/cma.j.issn.0376-2491.2019.22.010
26. Zhao, Y, Yang, Y, Aruna,, Xiao, J, Song, J, Huang, T, et al. Saccharomyces boulardii combined with quadruple therapy for Helicobacter pylori eradication decreased the duration and severity of diarrhea: a multi-center prospective randomized controlled trial. Front Med (Lausanne) . (2021) 8:776955. doi: 10.3389/fmed.2021.776955
27. Naghibzadeh, N, Salmani, F, Nomiri, S, and Tavakoli, T. Investigating the effect of quadruple therapy with Saccharomyces boulardii or Lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of Helicobacter pylori and treatments adverse effects: a double-blind placebo-controlled randomized clinical trial. BMC Gastroenterol . (2022) 22:107. doi: 10.1186/s12876-022-02187-z
28. He, XJ, Wang, XL, Sun, DJ, Huang, XY, Liu, G, Li, DZ, et al. The efficacy and safety of Saccharomyces boulardii in addition to antofloxacin-based bismuth quadruple therapy for Helicobacter pylori eradication: a single-center, prospective randomized-control study. Ther Adv Gastroenterol . (2023) 16:17562848221147763. doi: 10.1177/17562848221147763
29. Kaźmierczak-Siedlecka, K, Ruszkowski, J, Fic, M, Folwarski, M, and Makarewicz, W. Saccharomyces boulardii CNCM I-745: a non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr Microbiol . (2020) 77:1987–96. doi: 10.1007/s00284-020-02053-9
30. Neut, C, Mahieux, S, and Dubreuil, LJ. Antibiotic susceptibility of probiotic strains: is it reasonable to combine probiotics with antibiotics? Med Mal Infect . (2017) 47:477–83. doi: 10.1016/j.medmal.2017.07.001
31. Moré, MI, and Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis – a review. Clin Exp Gastroenterol . (2015) 8:237–55. doi: 10.2147/CEG.S85574
32. Czerucka, D, Piche, T, and Rampal, P. Review article: yeast as probiotics –Saccharomyces boulardii. Aliment Pharmacol Ther . (2007) 26:767–78. doi: 10.1111/j.1365-2036.2007.03442.x
33. Sakarya, S, and Gunay, N. Saccharomyces boulardii expresses neuraminidase activity selective for α2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS . (2014) 122:941–50. doi: 10.1111/apm.12237
34. Buts, JP, Bernasconi, P, Vaerman, JP, and Dive, C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci . (1990) 35:251–6. doi: 10.1007/BF01536771
35. Cárdenas, PA, Garcés, D, Prado-Vivar, B, Flores, N, Fornasini, M, Cohen, H, et al. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur J Clin Microbiol Infect Dis . (2020) 39:1365–72. doi: 10.1007/s10096-020-03854-3
36. Yao, G, Fan, X, and Lu, D. Efficacy and safety of probiotic-supplemented bismuth quadruple therapy for the treatment of Helicobacter pylori infection: a systematic review and meta-analysis. J Int Med Res . (2023) 51:3000605231203841. doi: 10.1177/03000605231203841
37. McFarland, LV, Huang, Y, Wang, L, and Malfertheiner, P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J . (2016) 4:546–61. doi: 10.1177/2050640615617358
Keywords: Saccharomyces boulardii , Helicobacter pylori , probiotics, bismuth quadruple therapy, systematic review, meta-analysis
Citation: Chen Y, Teng T, Su Y and Chen W-Z (2024) The effect of supplementing with Saccharomyces boulardii on bismuth quadruple therapy for eradicating Helicobacter pylori : a systematic review and meta-analysis of randomized controlled trials. Front. Med . 11:1344702. doi: 10.3389/fmed.2024.1344702
Received: 26 November 2023; Accepted: 08 April 2024; Published: 17 April 2024.
Reviewed by:
Copyright © 2024 Chen, Teng, Su and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Zhong Chen, [email protected]
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Omega-3 fatty acid supplementation on post-exercise inflammation, muscle damage, oxidative response, and sports performance in physically healthy adults—a systematic review of randomized controlled trials.

1. Introduction
2. materials and methods, 2.1. search strategy, 2.2. selection criteria, 2.3. study selection, 2.4. quality assessment, 2.5. risk-of-bias assessment, 2.6. data extraction, 3.1. study selection, 3.2. quality assessment, 3.3. risk-of-bias assessment, 3.4. outcome evaluation, 3.4.1. characteristics of the sample, 3.4.2. omega-3 supplementation, 3.4.3. inflammatory markers, 3.4.4. muscle damage, 3.4.5. oxidant response, 3.4.6. sports performance, 4. discussion, 4.1. omega-3 supplementation, 4.2. inflammatory markers, 4.3. muscle damage, 4.4. oxidant response, 4.5. sports performance, 5. limitations and strengths, 6. practical applications, 7. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest, appendix a. search strategy.
| (omega-3 OR omega-3 supplementation OR Polyunsaturated fatty acids) AND (“muscle recovery”), AND (athletic performance OR improved athletic performance) AND (exercise-induced muscle damage OR muscle soreness OR muscle damage) AND (eccentric exercise) AND (inflammation OR oxidative stress) AND (benefits). Filters: Full text, Trial, in the last 10 years | 678 | |
| (“omega-3” [Title/Abstract] OR “omega-3 supplementation” [Title/Abstract] OR “Polyunsaturated fatty acids” [Title/Abstract]) AND (“muscle recovery” [Title/Abstract]), AND (“athletic performance” [Title/Abstract] OR “improved athletic performance” [Title/Abstract]) AND (“exercise-induced muscle damage” [Title/Abstract] OR “muscle soreness” [Title/Abstract] OR “muscle damage” [Title/Abstract]) AND (“eccentric exercise” [Title/Abstract]) AND (“inflammation” [Title/Abstract] OR “oxidative stress” [Title/Abstract]) AND (“benefits” [Title/Abstract]). In Title Abstract Keyword in All Text—with Publication Year from 2013 to 2024. Filters: Full text, Trial, in the last 10 years | 51 | |
| ((omega-3 OR omega-3 supplementation OR Polyunsaturated fatty acids (topic)) AND (“muscle recovery”), AND ((athletic performance OR improved athletic performance (topic)) AND ((exercise-induced muscle damage OR muscle soreness OR muscle damage(topic)) AND ((eccentric exercise (topic)) AND ((inflammation OR oxidative stress (topic)) AND ((benefits (topic)). Anywhere Publication 2013-2024, Filters: Full text, Trial, in the last 10 years | 768 |
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020 , 12 , 501. [ Google Scholar ] [ CrossRef ]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; Orazio, N.D. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018 , 11 , 46. [ Google Scholar ] [ CrossRef ]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014 , 20 , 1126–1167. [ Google Scholar ] [ CrossRef ]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016 , 2016 , 17–19. [ Google Scholar ] [ CrossRef ]
- Škrgat, S.; Korošec, P.; Kern, I.; Šilar, M.; Šelb, J.; Fležar, M.; Marčun, R. Systemic and Airway Oxidative Stress in Competitive Swimmers. Respir. Med. 2018 , 137 , 129–133. [ Google Scholar ] [ CrossRef ]
- Reid, M.B. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med. Sci. Sports Exerc. 2001 , 33 , 371–376. [ Google Scholar ] [ CrossRef ]
- Miguel, A.M.C.S.; Roche, E.; Herranz-López, M.; Miguel, M.C.S.; Mielgo-Ayuso, J.; Fernández-Lázaro, D. Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients 2024 , 16 , 1011. [ Google Scholar ] [ CrossRef ]
- Mielgo-Ayuso, J.; Fernández-Lázaro, D. Nutrition and Muscle Recovery. Nutrients 2021 , 13 , 294. [ Google Scholar ] [ CrossRef ]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction, and the future. Proc. Nutr. Soc. 2018 , 77 , 52–72. [ Google Scholar ] [ CrossRef ]
- Zaboli, G.; Igl, W.; Johansson, A.C.V.; Ameur, A.; Enroth, S.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; Meitinger, T.; et al. Genetic Adaptation of Fatty-Acid Metabolism: A Human-Specific Haplotype Increasing the Biosynthesis of Long-Chain Omega-3 and Omega-6 Fatty Acids. Am. J. Hum. Genet. 2012 , 90 , 809–820. [ Google Scholar ] [ CrossRef ]
- Black, K.E.; Witard, O.C.; Baker, D.; Healey, P.; Lewis, V.; Tavares, F.; Christensen, S.; Pease, T.; Smith, B. Adding Omega-3 Fatty Acids to a Protein-Based Supplement during Pre-Season Training Results in Reduced Muscle Soreness and the Better Maintenance of Explosive Power in Professional Rugby Union Players. Eur. J. Sport. Sci. 2018 , 18 , 1357–1367. [ Google Scholar ] [ CrossRef ]
- Mickleborough, T.D.; Lindley, M.R.; Ionescu, A.A.; Fly, A.D. Protective Effect of Fish Oil Supplementation on Exercise-Induced Bronchoconstriction in Asthma. Chest 2006 , 129 , 39–49. [ Google Scholar ] [ CrossRef ]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-Related Effects of Eicosapentaenoic Acid on Innate Immune Function in Healthy Humans: A Comparison of Young and Older Men. Am. J. Clin. Nutr. 2006 , 83 , 331–342. [ Google Scholar ] [ CrossRef ]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish Oil-Derived n-3 PUFA Therapy Increases Muscle Mass and Function in Healthy Older Adults. Am. J. Clin. Nutr. 2015 , 102 , 115–122. [ Google Scholar ] [ CrossRef ]
- Tinsley, G.M.; Gann, J.J.; Huber, S.R.; Andre, T.L.; La Bounty, P.M.; Bowden, R.G.; Gordon, P.M.; Grandjean, P.W. Effects of Fish Oil Supplementation on Postresistance Exercise Muscle Soreness. J. Diet. Suppl. 2017 , 14 , 89–100. [ Google Scholar ] [ CrossRef ]
- Lembke, P.; Capodice, J.; Hebert, K.; Swenson, T. Influence of Omega-3 (N3) Index on Performance and Wellbeing in Young Adults after Heavy Eccentric Exercise. J. Sports Sci. Med. 2014 , 3 , 151–156. [ Google Scholar ]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and Docosahexaenoic Acids-Rich Fish Oil Supplementation Attenuates Strength Loss and Limited Joint Range of Motion after Eccentric Contractions: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Eur. J. Appl. Physiol. 2016 , 116 , 1179–1188. [ Google Scholar ] [ CrossRef ]
- Camandola, S.; Leonarduzzi, G.; Musso, T.; Varesio, L.; Carini, R.; Scavazza, A.; Chiarpotto, E.; Baeuerle, P.A.; Poli, G. Nuclear Factor KB Is Activated by Arachidonic Acid but Not by Eicosapentaenoic Acid. Biochem. Biophys. Res. Commun. 1996 , 229 , 643–647. [ Google Scholar ] [ CrossRef ]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear Factor-KappaB: Its Role in Health and Disease. J. Mol. Med. 2004 , 82 , 434–448. [ Google Scholar ] [ CrossRef ]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017 , 14 , 23. [ Google Scholar ] [ CrossRef ]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 Fatty Acids Supplementation Attenuates Inflammatory Markers after Eccentric Exercise in Untrained Men. Clin. J. Sport Med. 2011 , 21 , 131–137. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gladman, S.J.; Huang, W.; Lim, S.-N.; Dyall, S.C.; Boddy, S.; Kang, J.X.; Knight, M.M.; Priestley, J.V.; Michael-Titus, A.T. Improved Outcome after Peripheral Nerve Injury in Mice with Increased Levels of Endogenous ω-3 Polyunsaturated Fatty Acids. J. Neurosci. Off. J. Soc. Neurosci. 2012 , 32 , 563–571. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patten, G.S.; Abeywardena, M.Y.; McMurchie, E.J.; Jahangiri, A. Dietary Fish Oil Increases Acetylcholine- and Eicosanoid-Induced Contractility of Isolated Rat Ileum. J. Nutr. 2002 , 132 , 2506–2513. [ Google Scholar ] [ CrossRef ]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The Effects of Ingestion of Omega-3 Fatty Acids on Perceived Pain and External Symptoms of Delayed Onset Muscle Soreness in Untrained Men. Clin. J. Sport Med. 2009 , 19 , 115–119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. 1997 , 273 , E122–E129. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mcglory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016 , 4 , e12715. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; Wells, G.D.; Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; et al. 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J. Int. Soc. Sports Nutr. 2015 , 12 , 28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sports Med. 2022 , 56 , 175–195. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007 , 15 , 508–511. [ Google Scholar ] [ CrossRef ]
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies ; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998. [ Google Scholar ]
- Moseley, A.M.; Elkins, M.R.; Van derWees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020 , 24 , 384–391. [ Google Scholar ] [ CrossRef ]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 ; Updated February 2021; Cochran: Pittsburg, PA, USA, 2021; Available online: https://training.cochrane.org/handbook (accessed on 26 May 2024).
- Tomczyk, M.; Bidzan-Wiącek, M.; Kortas, J.A.; Kochanowicz, M.; Jost, Z.; Fisk, H.L.; Calder, P.C.; Antosiewicz, J. Omega-3 fatty acid supplementation affects tryptophan metabolism during a 12-week endurance training in amateur runners: A randomized controlled trial. Sci. Rep. 2024 , 14 , 4102. [ Google Scholar ] [ CrossRef ]
- Tsuchiya, Y.; Ueda, H.; Yanagimoto, K.; Kato, A.; Ochi, E. 4-Week Eicosapentaenoic Acid-Rich Fish Oil Supplementation Partially Protects Muscular Damage Following Eccentric Contractions. J. Int. Soc. Sports Nutr. 2021 , 18 , 18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vandusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Kerksick, C.M.; Mangine, G.T.; Holmes, A.J.; Lee, M.; Endito, M.R.; et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients 2020 , 12 , 2246. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ávila-Gandía, V.; Torregrosa-García, A.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; López-Román, F.J. Re-esterified DHA improves ventilatory threshold 2 in competitive amateur cyclists. J. Int. Soc. Sports Nutr. 2020 , 17 , 51. [ Google Scholar ] [ CrossRef ]
- Barquilha, G.; Miguel, C.; Dos, M.; Caçula, K.G.; Polotow, T.G.; Vasconcellos, C.V.; Fernandes, A.; Rodrigues, L.E.; Lambertucci, R.H.; Duarte, T.; et al. Fish Oil Supplementation Improves the Repeated-Bout Effect Strength Training. Nutrients 2023 , 15 , 1708. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brook, M.S.; Din, U.; Tarum, J.; Selby, A.; Quinlan, J.; Bass, J.J.; Gharahdaghi, N.; Boereboom, C.; Abdulla, H.; Franchi, M.V.; et al. Omega-3 supplementation during unilateral resistance exercise training in older women: A within subject and double-blind placebo-controlled trial. Clin. Nutr. ESPEN 2021 , 46 , 394–404. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Heileson, J.L.; Machek, S.B.; Harris, D.R.; Tomek, S.; de Souza, L.C.; Kieffer, A.J.; Barringer, N.D.; Gallucci, A.; Forsse, J.S.; Funderburk, L.L.K. The effect of fish oil supplementation on resistance training-induced adaptations. J. Int. Soc. Sports Nutr. 2023 , 20 , 2174704. [ Google Scholar ] [ CrossRef ]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an Acute Dose of Omega-3 Fish Oil Following Exercise-Induced Muscle Damage. Eur. J. Appl. Physiol. 2017 , 117 , 575–582. [ Google Scholar ] [ CrossRef ]
- Lee, S.-R.; Directo, D.; Khamoui, A. V Fish Oil Administration Combined with Resistance Exercise Training Improves Strength, Resting Metabolic Rate, and Inflammation in Older Adults. Aging Clin. Exp. Res. 2022 , 34 , 3073–3081. [ Google Scholar ] [ CrossRef ]
- Mullins, V.A.; Graham, S.; Cummings, D.; Wood, A.; Ovando, V.; Skulas-Ray, A.C.; Polian, D.; Wang, Y.; Hernandez, G.D.; Lopez, C.M.; et al. Effects of Fish Oil on Biomarkers of Axonal Injury and Inflammation in American Football Players: A Placebo-Controlled Randomized Controlled Trial. Nutrients 2022 , 14 , 2139. [ Google Scholar ] [ CrossRef ]
- Nieman, D.C.; Gillitt, N.D.; Meaney, M.P.; Dew, D.A. No Positive Influence of Ingesting Chia Seed Oil on Human Running Performance. Nutrients 2015 , 7 , 3666–3676. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016 , 785 , 2–9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; López-Román, F.J.; Miñarro, J.; Contreras, C.; Soto-Méndez, F.; Domingo Pedrol, J.C.; Luque-Rubia, A.J. Supplementation of Re-Esterified Docosahexaenoic and Eicosapentaenoic Acids Reduce Inflammatory and Muscle Damage Markers after Exercise in Endurance Athletes: A Randomized, Controlled Crossover Trial. Nutrients 2020 , 12 , 719. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, S.; He, Q.; Shi, L.; Wu, Y. Impact of Antarctic krill oil supplementation on skeletal muscle injury recovery after resistance exercise. Eur. J. Nutr. 2023 , 62 , 1345–1356. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Urdampilleta, A.; López-Grueso, R.; Martínez-Sanz, J.M.; Mielgo-Ayuso, J. Basic biochemical, hematological and hormonal parameters for monitoring the health and nutritional status in athletes. Rev. Esp. Nutr. Hum. Diet. 2014 , 18 , 155. [ Google Scholar ] [ CrossRef ]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2021 , 18 , 9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lange, K.W.; Nakamura, Y.; Gosslau, A.M.; Li, S. Are there serious adverse effects of omega-3 polyunsaturated fatty acid supplements? J. Food Bioact. 2019 , 7 , 1–6. [ Google Scholar ] [ CrossRef ]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.R.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015 , 13 , 6977–7004. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tachtsis, B.; Camera, D.; Lacham-Kaplan, O. Potential Roles of N-3 PUFAs during Skeletal Muscle Growth and Regeneration. Nutrients 2018 , 10 , 309. [ Google Scholar ] [ CrossRef ]
- DiLorenzo, F.M.; Drager, C.J.; Rankin, J.W. Docosahexaenoic Acid Affects Markers of Inflammation and Muscle Damage after Eccentric Exercise. J. Strength Cond. Res. 2014 , 28 , 2768–2774. [ Google Scholar ] [ CrossRef ]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017 , 45 , 1105–1115. [ Google Scholar ] [ CrossRef ]
- Canals-Garzón, C.; Guisado-Barrilao, R.; Martínez-García, D.; Chirosa-Ríos, I.J.; Jerez-Mayorga, D.; Guisado-Requena, I.M. Effect of Antioxidant Supplementation on Markers of Oxidative Stress and Muscle Damage after Strength Exercise: A Systematic Review. Int. J. Environ. Res. Public Health 2022 , 19 , 1803. [ Google Scholar ] [ CrossRef ]
- Bloomer, R.J.; Larson, D.E.; Fisher-Wellman, K.H.; Galpin, A.J.; Schilling, B.K. Effect of Eicosapentaenoic and Docosahexaenoic Acid on Resting and Exercise-Induced Inflammatory and Oxidative Stress Biomarkers: A Randomized, Placebo Controlled, Cross-over Study. Lipids Health Dis. 2009 , 8 , 36. [ Google Scholar ] [ CrossRef ]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish Oil Supplementation Reduces Markers of Oxidative Stress but Not Muscle Soreness after Eccentric Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2014 , 24 , 206–214. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015 , 10 , e0139174. [ Google Scholar ] [ CrossRef ]
- Pereira Panza, V.S.; Diefenthaeler, F.; da Silva, E.L. Benefits of Dietary Phytochemical Supplementation on Eccentric Exercise-Induced Muscle Damage: Is Including Antioxidants Enough? Nutrition 2015 , 31 , 1072–1082. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Philpott, J.D.; Donnelly, C.; Walshe, I.H.; MacKinley, E.E.; Dick, J.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. Adding Fish Oil to Whey Protein, Leucine, and Carbohydrate Over a Six-Week Supplementation Period Attenuates Muscle Soreness Following Eccentric Exercise in Competitive Soccer Players. Int. J. Sport. Nutr. Exerc. Metab. 2018 , 28 , 26–36. [ Google Scholar ] [ CrossRef ]
- Chalchat, E.; Gaston, A.-F.; Charlot, K.; Peñailillo, L.; Valdés, O.; Tardo-Dino, P.-E.; Nosaka, K.; Martin, V.; Garcia-Vicencio, S.; Siracusa, J. Appropriateness of Indirect Markers of Muscle Damage Following Lower Limbs Eccentric-Biased Exercises: A Systematic Review with Meta-Analysis. PLoS ONE 2022 , 17 , e0271233. [ Google Scholar ] [ CrossRef ]
- Lv, Z.; Zhang, J.; Zhu, W. Omega-3 Polyunsaturated Fatty Acid Supplementation for Reducing Muscle Soreness after Eccentric Exercise: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2020 , 2020 , 8062017. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant Supplements and Endurance Exercise: Current Evidence and Mechanistic Insights. Redox Biol. 2020 , 35 , 101471. [ Google Scholar ] [ CrossRef ]
- Fayh, A.P.T.; Borges, K.; Cunha, G.S.; Krause, M.; Rocha, R.; de Bittencourt, P.I.H.J.; Moreira, J.C.F.; Friedman, R.; da Silva Rossato, J.; Fernandes, J.R.; et al. Effects of N-3 Fatty Acids and Exercise on Oxidative Stress Parameters in Type 2 Diabetic: A Randomized Clinical Trial. J. Int. Soc. Sports Nutr. 2018 , 15 , 18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-Week n-3 Fatty-Acid Supplementation on Oxidative Stress and Inflammation in Middle- and Long-Distance Running Athletes: A Pilot Study. J. Int. Soc. Sports Nutr. 2020 , 17 , 55. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Drobnic, F.; Storsve, A.B.; Burri, L.; Ding, Y.; Banquells, M.; Riera, J.; Björk, P.; Ferrer-Roca, V.; Domingo, J.C. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients 2021 , 13 , 4237. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill Oil Reduces Plasma Triacylglycerol Level and Improves Related Lipoprotein Particle Concentration, Fatty Acid Composition and Redox Status in Healthy Young Adults—A Pilot Study. Lipids Health Dis. 2015 , 14 , 163. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marques, C.G.; Santos, V.C.; Levada-Pires, A.C.; Jacintho, T.M.; Gorjão, R.; Pithon-Curi, T.C.; Cury-Boaventura, M.F. Effects of DHA-Rich Fish Oil Supplementation on the Lipid Profile, Markers of Muscle Damage, and Neutrophil Function in Wheelchair Basketball Athletes before and after Acute Exercise. Appl. Physiol. Nutr. Metab. 2015 , 40 , 596–604. [ Google Scholar ] [ CrossRef ]
- Atashak, S.; Sharafi, H.; Azarbayjani, M.A. Effect of Omega-3 Supplementation On The Blood Levels Of Oxidative Stress. Muscle Damage Inflamm. Markers After Acute Resist. 2013 , 45 , 22–29. [ Google Scholar ]
- De Salazar, L.; Contreras, C.; Torregrosa-García, A.; Luque-Rubia, A.J.; Ávila-Gandía, V.; Domingo, J.C.; López-Román, F.J. Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation. Antioxidants 2020 , 18 , 1145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McKinley-Barnard, S.K.; Andre, T.L.; Gann, J.J.; Hwang, P.S.; Willoughby, D.S. Effectiveness of Fish Oil Supplementation in Attenuating Exercise-Induced Muscle Damage in Women During Midfollicular and Midluteal Menstrual Phases. J. Strength Cond. Res. 2018 , 32 , 1601–1612. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Luostarinen, R.; Saldeen, T. Dietary Fish Oil Decreases Superoxide Generation by Human Neutrophils: Relation to Cyclooxygenase Pathway and Lysosomal Enzyme Release. Prostaglandins Leukot. Essent. Fatty Acids 1996 , 55 , 167–172. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fisher, M.; Levine, P.H.; Weiner, B.H.; Johnson, M.H.; Doyle, E.M.; Ellis, P.A.; Hoogasian, J.J. Dietary N-3 Fatty Acid Supplementation Reduces Superoxide Production and Chemiluminescence in a Monocyte-Enriched Preparation of Leukocytes. Am. J. Clin. Nutr. 1990 , 51 , 804–808. [ Google Scholar ] [ CrossRef ]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The Effects of Fish Oil and Isoflavones on Delayed Onset Muscle Soreness. Med. Sci. Sports Exerc. 2002 , 34 , 1605–1613. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Owen, J.B.; Butterfield, D.A. Measurement of Oxidized/Reduced Glutathione Ratio. Methods Mol. Biol. 2010 , 648 , 269–277. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Owens, D.; Twist, C.; Cobley, J.; Howatson, G.; Close, G. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019 , 19 , 71–85. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) in Muscle Damage and Function. Nutrients 2018 , 10 , 552. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ross, B.M. The emerging role of eicosapentaenoic acid as an important psychoactive natural product: Some answers but a lot more questions. Lipid Insights 2008 , 2 , 89–97. [ Google Scholar ] [ CrossRef ]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sport. Med. 2015 , 45 , 93–104. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021 , 13 , 3746. [ Google Scholar ] [ CrossRef ]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022 , 10 , 29. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Study | Items | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| [ ] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| [ ] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 14 | 87.5 | VG |
| [ ] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| [ ] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 13 | 81.3 | VG |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 15 | 93.7 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 15 | 93.7 | E |
| Study | Items | Total | % | Quality Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| [ ] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| [ ] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| [ ] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| [ ] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| random sequence generation (selection bias) | allocation concealment (selection bias) | blinding (performance bias and detection bias) participant | blinding (performance bias and detection bias) personnel | blinding (performance bias and detection bias) outcome assessor | incomplete outcome (attrition bias) | selective reporting (reporting bias) | other bias | |
| Ávila-Gandía et al., 2020 [ ] | ||||||||
| Barquilha et al., 2023 [ ] | ||||||||
| Brook et al., 2021 [ ] | ||||||||
| Heileson et al., 2023 [ ] | ||||||||
| Jakeman et al., 2017 [ ] | ||||||||
| Lee et al., 2022 [ ] | ||||||||
| Lembke et al., 2014 [ ] | ||||||||
| Mullins et al., 2022 [ ] | ||||||||
| Nieman et al., 2015 [ ] | ||||||||
| Tomczk et al., 2024 [ ] | ||||||||
| Tsuchiya et al., 2021 [ ] | ||||||||
| Tsuchiya et al., 2016 [ ] | ||||||||
| VanDusseldrorp et al., 2020 [ ] |
| Characteristics | Types | Reference |
|---|---|---|
| Amateur competitive | [ , , ] | |
| Amateur | [ ] | |
| Recreationally | [ , ] | |
| Recreationally active | [ , ] | |
| Physically active | [ , , , , ] | |
| Capsule | [ , , , , , , , ] | |
| Soft gel | [ , ] | |
| Water with seed oil | [ ] | |
| Unspecified | [ , ] | |
| High * (750 mg EPA + 50 mg DHA) Low * (150 mg EPA + 100 mg DHA) | [ ] | |
| 2 g (1400 mg: 800 mg EPA + 600 mg DHA) 4 g (2800 mg: 1600 mg EPA + 1200 mg DHA) 6 g (4200 mg: 2400 mg EPA + 1800 mg DHA) | [ ] | |
| 780 mg EPA + 606 mg DHA | [ ] | |
| 1220 mg/d (975 mg DHA + 120 mg EPA) | [ ] | |
| 2.1 g/d EPA + 0.78 g/d DHA | [ ] | |
| 2.275 g/d EPA + 1.575 g/d DHA | [ ] | |
| 2234 mg/d EPA + 930 mg/d DHA | [ ] | |
| 2.4 g/d (600 mg EPA + 260 mg DHA) | [ ] | |
| 2.7 g/day | [ ] | |
| 3.5 g/d (1 g: 407 mg/g DHA +170 mg/g EPA) | [ ] | |
| 2400 mg (1360 mg EPA + 1040 mg DHA) | [ ] | |
| 3680 mg/d (1860 mg EPA +1540 mg DHA) | [ ] | |
| 31 g ALA for the average | [ ] | |
| once a day: post-lunch morning | [ ] | |
| 30 min after meals with water | [ ] | |
| 30 min before exercise | [ ] | |
| once a day: post-exercise | [ ] | |
| 3 times/days (morning, lunch, dinner) | [ ] | |
| Unspecified | [ , , , , , , , ] | |
| 2/4/6 capsules | [ ] | |
| 3 capsules | [ , , ] | |
| 6 capsules | [ , ] | |
| 8 capsules | [ , ] | |
| 1 g (capsule)/10 kg/BM | [ ] | |
| 7 capsules | [ ] | |
| 0.43 g ALA/kg BM | [ ] | |
| Unspecified | [ ] | |
| twice separated by two weeks | [ ] | |
| 1 day | [ ] | |
| 30 days | [ ] | |
| 4 weeks | [ ] | |
| 4.5 weeks | [ ] | |
| 6 weeks | [ , ] | |
| 7.5 weeks | [ ] | |
| 8 week + 5 days | [ ] | |
| 10 weeks | [ ] | |
| 12 weeks | [ , ] | |
| 26 weeks | [ ] | |
| Endurance + functional/resistance | [ , ] | |
| Cycling test to exhaustion | [ ] | |
| Maximum eccentric extensions of the forearm or elbow | [ , , ] | |
| Plyometric jumps | [ ] | |
| Resistance exercise training | [ , , , ] | |
| Running at constant speed until exhaustion | [ ] | |
| Unspecified | [ ] |
| First Author, Year of Publication, and Country | Study Design | Participants | Intervention | Outcomes | Results | |
|---|---|---|---|---|---|---|
| Ávila-Gandía et al. [ ], 2020, Spain | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 50 ♂ Amateur cyclists competing at regional level Gn-3 n = 18 Age (mean ± SD) 35.5 ± 7.3 years Weight (mean ± SD) t 72.4 ± 4.4 kg BMI (mean ± SD) 23.83 ± 1.43 Relative VO max (mean ± SD) 48.5 ± 6.8 mL/min/kg CG n = 20 Age (mean ± SD) 36.0 ± 9.6 years Weight (mean ± SD) 71.1 ± 3.4 kg BMI (mean ± SD) 23.42 ± 1.31 Relative VO max (mean ± SD) 49.3 ± 6.1mL/min/kg Study withdrawals: 12 | Gn-3 3 soft-gels Per unit: 325 mg DHA + 40 mg EPA (Brudy plus, Brudytechnology, Barcelona, Spain) CG Sunflower oil Supplementation time: 30 days | Muscle damage Blood Lactate Physical performance Absolute VO HR MPO Relative VO RP time VO VT2 | Gn-3 vs. CG ↔ Blood Lactate ↓*Absolute VO (6’) ↓* HR ↑* MPO ↓* Relative VO (6’) ↑* RP ↑* Time ↔ VO ↑* VT2 | Gn-3 Changes from baseline ↔ Blood Lactate ↓* Absolute VO (6’) ↓* HR ↑* MPO ↓* Relative VO (6’) ↑* RP ↑* Time ↑* VO CG Changes from baseline ↔ Blood Lactate ↔ Absolute VO (6’) ↔ HR ↔ MPO ↔ Relative VO (6’) ↔ RP ↔ time ↔ VO |
| Barquilha et al. [ ], 2023, Brazil | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 21 ♂ Gn-3 n = 8 CG n = 8 Physically active Age: 20–30 years Study withdrawals: Gn-3 n = 3 CG n = 2 | Gn-3 3 capsules Per unit: 260 mg EPA + 202 mg DHA 3 times daily (Capsule Naturalis Nutricao & Farma LTDA, Sao Paulo, Brazil) Supplementation time: 6 weeks | Hematology Heme Iron Iron Hormones T/C Inflammatory biomarkers CRP IL-6 Muscle damage CK LDH Oxidative stress GSH GSSG GSH/GSSG TEAC | Gn-3 vs. CG ↔ Heme Iron ↔ Iron ↓ CRP ↓ IL-6 ↓ CK ↓ LDH ↑* GSH ↓* GSSG ↑* GSH/GSSG ↔TEAC | Gn-3 Changes from baseline ↔T/C ↓* CRP ↓* IL-6 ↓ CK ↓ LDH |
| Brook et al. [ ], 2021, United Kingdon | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 16 ♀ Recreationally active Gn-3 n = 8 ♀ Age (mean ± SD) 64.4 ± 0.8 years Height (mean ± SD) 162 ± 0.02 cm Weight (mean ± SD) 70.5 ± 2.5 kg BMI (mean ± SD) 26.6 ± 0.7 kg/m % Fat (mean ± SD) 40.8 ± 1.1% Lean Mass (mean ± SD) 39.4 ± 1.1 kg CG n = 8 ♀ Age (mean ± SD) 66.5 ± 1.4 years Height (mean ± SD) 158 ± 0.02 cm Weight (mean ± SD) 64.3 ± 1.9 kg BMI (mean ± SD) 2.8 ± 0.9 kg/m % Fat (mean ± SD) 39.1 ± 1.6% Lean Mass (mean ± SD) 37.1 ± 1.6 kg Study withdrawals: 0 | Gn-3 Per unit: 1860 mg EPA +1540 mg DHA (Minami Epacor) CG Cornoil Supplementation time: 6 weeks | Anthropometry BM Bone mass FFM LBM Muscle function ASR Calpain MAFbx MPS Myonuclei SC Ubiquitin VL Physical performance 1-RM MVC | Gn-3 vs. CG ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↔ ASR ↔ Calpain ↔ MAFbx ↔ MPS ↔ Myonuclei ↔ SC ↔ Ubiquitin ↑ 1-RM ↔ MCV ↔ MCV | Gn-3 Changes from baseline ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↑* ASR ↑ ASR ↔ Calpain ↔ MAFbx ↔ MPS ↔ MPS ↑* Myonuclei ↔ SC ↔ Ubiquitin ↑ 1-RM ↔ MCV ↔ MCV CG Changes from baseline ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↑* ASR ↔ ASR ↔ Calpain ↔ MAFbx ↑* MPS ↔ MPS ↑* Myonuclei ↔ SC ↔ Ubiquitin ↑ 1-RM ↔ MCV ↔ MCV |
| Heileson et al. [ ], 2023, United States | Randomized, single-blind, placebo-controlled, parallel-group trial | n = 28 (n = 12 ♂ and n = 16 ♀) Recreationally Trained Gn-3 n = 10 n = 5 ♂ and n = 5 ♀ Age (mean ± SD) 28.0 ± 7.4 years Height (mean ± SD) 169.7 ± 9.6 cm Weight (mean ± SD) 75.1 ± 16.0 kg BMI (mean ± SD) 25.8 ± 3.5 kg/m % Fat (mean ± SD) 23.9 ± 6.9% CG n = 11 5 ♂ and 6 ♀ Age (mean ± SD) 30.5 ± 5.7 years Height (mean ± SD) 171.8 ± 8.9 cm Weight (mean ± SD) 79.0 ± 16.0 kg BMI (mean ± SD) 26.6 ± 4.3 kg/m % Fat (mean ± SD) 24.9 ± 8.0% Study withdrawals: Gn-3 n = 4 CG n = 3 | Gn-3 7 capsules 2.275 g/d EPA + 1.575 g/d DHA (Nordic Naturals, ProOmega, Watsonville, CA, USA) CG 5 capsules 4.5 g/d (NOW, Bloomingdale, IL, USA) Supplementation time: 10 weeks | Anthropometry LBM FM BF Biochemistry DBS Physical performance absolute 1RM absolute 1RM ∆ relative 1RM ∆ relative 1RM | Gn-3 vs. CG ↔ LBM ↔ FM ↔ BF ↑* DBS ↑* absolute1RM ↔ absolute 1RM ↑* ∆ relative 1RM ↑* ∆ relative 1RM | Gn-3 Changes from baseline ↑ LBM ↓ FM ↓ BF ↑* DBS ↑ absolute 1RM ↑ absolute 1RM ↑* ∆ relative 1RM ↑* ∆ relative 1RM CG Changes from baseline ↑ LBM ↓ FM ↔ BF ↔ DBS ↑ absolute 1RM ↑ absolute 1RM ↑* ∆ relative 1RM ↑* ∆ relative 1RM |
| Jakeman et al. [ ], 2017, United Kingdom | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 27 ♂ Physically active > 3 h/week of vigorous athletic training + HIIT High Gn-3 n = 9 Age (mean ± SD) 25.5 ± 5.2 years Height (mean ± SD) 1.74 ± 0.06 m Weight (mean ± SD) 76.5 ± 12.6 kg Low Gn-3 n = 9 Age (mean ± SD) 25.6 ± 4.8 years Height (mean ± SD) 1.82 ± 0.09 m Weight (mean ± SD 80.2 ± 12.0 kg CG n = 9 Age (mean ± SD) 26.2 ± 4.2 years Height (mean ± SD) 1.78 ± 0.01 m Weight (mean ± SD 82.9 ± 12.1 kg Study withdrawals: 0 | Gn-3 1 g/capsule Dose: 1 g/10 kg BM High Gn-3 (EPA 750 mg + DHA 50 mg)/capsule Low Gn-3 (EPA 150 mg + DHA 100 mg)/ capsule CG Oil (flavour masker and gelatine) Supplementation time: 1 day | Inflammatory biomarkers IL-6 Muscle damage CK Perception markers VAS Physical performace CJ Knee extensor strength SJ | High Gn-3, Low Gn-3 vs. CG ↔ IL-6 ↔ CK ↔ VAS ↔ CJ ↔ Knee extensor strength ↑* SJ | High Gn-3, Low Gn-3 Changes from baseline ↔ IL-6 ↑* CK (24 h) ↑* VAS (24 h) ↓ (at 96 h) ↓ CJ (at 1 h) ↓* Knee extensor strength to 60° s and 180° s (1 h–96 h) ↓* SJ (at 1 h) |
| Lee et al. [ ], 2022, United States | Randomized, placebo-controlled trial | n = 28 (n = 10 ♂ and n = 18 ♀) Physically active RET-G n-3 n = 10 Age (mean ± SD) 67.1 ± 4.4 years Height (mean ± SD) 171.6 ± 9.3 cm Weight (mean ± SD) 70.8 ± 13.5 kg BMI (mean ± SD) 24.0 ± 3.2 kg/m RET n = 10 Age (mean ± SD) 66.6 ± 7.3 years Height (mean ± SD) 167.9 ± 5.7 cm Weight (mean ± SD) 66.5 ± 11.5 kg BMI (mean ± SD) 23.5 ± 3.6 kg/m CG n = 8 Age (mean ± SD) 66.5 ± 5.0 years Height (mean ± SD) 167.2 ± 10.24 cm Weight (mean ± SD) 68.9 ± 15.8 kg BMI (mean ± SD) 24.3 ± 3.4 kg/m Study withdrawals: 0 | RET- Gn-3: 3 capsules/day Per unit: 700 mg EPA + 240 mg DHA RET 3 capsules/day Safflower oil CG 3 capsules/day Safflower oil Supplementation time: 12 weeks | Inflammatory biomarkers IL-6 CRP TNF-α Metabolism TMR FAT oxidation CHO oxidation Physical Performance 1RM lat pull-dow 1RM leg-press 1RM seated row 1RM calf rise 1RM biceps curl VO VCO RER | RET-Gn-3 vs. RET vs. CG ↓* IL-6 (RET-Gn-3 vs. CG) ↓* CRP ↓* TNF-α (RET-Gn-3 vs. CG) ↔ TMR ↑* 1RM lat pull-dow (RET-Gn-3, RET) ↑* 1RM leg-press (RET-Gn-3, RET) ↑* 1RM seated row (RET-Gn-3, RET) ↑* 1RM calf rise (RET-Gn-3, RET) ↑* 1RM biceps curl (RET-Gn-3, RET) ↑* VO ↑* VCO ↓* RER | RET-Gn-3 Changes from baseline ↓* IL-6 ↓* CRP ↓ TNF-α ↑* TMR ↑* FAT oxidation ↓* CHO oxidation ↑* 1RM in lateral pull ↑* 1RM leg-press ↑* 1RM seated row ↑* 1RM calf rise ↑* 1RM biceps curl ↑* VO ↑* VCO ↓* RER RET Changes from baseline ↔ IL-6 ↔ CRP↔ TNF-α ↑* TMR↑ FAT oxidation ↓ CHO oxidation ↑* 1RM in lateral pull ↑* 1RM leg-press ↑* 1RM seated row ↑* 1RM calf rise ↑* 1RM biceps curl ↑* VO ↑* VCO ↔ RER CG Changes from baseline ↔ IL-6 ↔ CRP ↑ TNF-α ↔ TMR ↔ FAT oxidation ↔ CHO oxidation ↓* 1RM in lateral pull ↓* 1RM leg-press ↔ 1RM seated row ↓* 1RM calf rise ↓* 1RM biceps curl ↔ VO ↔ VCO ↔ RER |
| Lembke et al. [ ], 2014, United States | Randomized, Single-blind, placebo-controlled, parallel-group trial | n = 69 ♂ and ♀ Physically active Gn-3 n = 42 Age (mean ± SD) 18.6 ± 1.2 years CG n = 22 Age (mean ± SD) 18.9 ± 1.1 years Study withdrawals: 5 | Gn-3 6 capsules 2.7 g/day (KD Pharma, Bexbach, Germany) CG 6 capsules High oleic sunflower oil Supplementation time: 30 days | Inflammatory biomarkers CRP Muscle damage Blood lactate CK Perception markers VAS POMS Physical Performance ROM Torque | Gn-3 vs. CG ↓* CRP ↓* Blood lactate ↔ CK (48 -96 h) ↓* VAS (at 72, at 96 h) ↑* POMS (72 h) ↔ ROM ↔ Torque | Gn-3 Changes from baseline ↓* CRP ↔ CK ↓ VAS ↓ POMS (at 48 h and 96 h) ↑ POMS (at 48 h, and at 96 h CG) ↓ ROM ↓ Torque (until 48 h) |
| Mullins et al. [ ], 2022, United States | Randomized, double-blind, placebo-controlled, parallel-group trial | n =38 ♂ Competitive Gn-3 n =12 CG n = 17 Study withdrawals: Gn-3 n =7 CG n = 2 | Gn-3 Soft gel capsules Per unit: 1 g: 407 mg/g DHA+ 170 mg/g EPA Pharmavite (West Hills, California) CG Per capsule 713 mg/g oleic acid + 130 mg/g linoleic acid (safflower oil) Pharmavite (West Hills, California) Supplementation time: 26 weeks | Biochemistry Plasma AA Plasma DHA Plasma DPA Plasma EPA Inflammatory biomarkers IL-6 TNF-α Injury Neurofilament | Gn-3 vs.CG ↔ Plasma AA ↓* Plasma DHA ↑* Plasma DHA ↓* Plasma DPA ↑* Plasma EPA ↔ IL-6 ↔ TNF-α ↔ Neurofilament | Gn-3 Changes from baseline ↓*Plasma AA ↑*Plasma DHA ↑*Plasma DPA ↑*Plasma EPA ↔IL-6 ↔TNF-α ↑Neurofilament CG Changes from baseline ↔Plasma AA ↔Plasma DHA ↔Plasma DPA ↔Plasma EPA ↔IL-6 ↔TNF-α ↑Neurofilament |
| Nieman et al. [ ], 2015, United States | Randomized (1:1 allocation), placebo-controlled, crossover trial | n = 24 16 ♂ and 8 ♀ Competitive runners Age (mean ± SD) 38.0 ±1.7 year Height (mean ± SD) 1.72 ±0.02 m Weight (mean ± SD) 71.8 ± 3.0 kg % Fat (mean ± SD) 19.9 ±1.6 VO (mean ± SD) 47.9 ±1.6 Study withdrawals: 0 | Gn-3 0.5 L water with chia seed oil 0.43 g ALA/BM (Dole Foods California, USA), CG 0.5 L of flavored water alone Supplementation time: two occasions separated by 2 weeks | Biochemistry Plasma glucose Leukocyte Plasma ALA Hormones Cortisol Inflammatory biomarkers IL-6 IL-8 IL-10 TNF-α Muscle damage Blood lactate Perception markers RPE Physical performance HR RER VO | Gn-3 vs.CG ↓ Plasma glucose ↔ Leukocyte ↑* Plasma ALA ↑* Cortisol ↓ IL-6 ↓ IL-8 ↓ IL10 ↓TNF-α ↓ Blood lactate ↓ RPE ↔ HR ↔ RER ↓VO | Changes from baseline ↑ Plasma glucose ↑* Leukocyte ↑* Plasma ALA ↑* Cortisol ↑* IL-6 ↑* IL-8 ↑* IL10 ↑* TNF-α ↑ Blood lactate |
| Tomczyk et al. [ ], 2024, Poland | Randomized, placebo-controlled, parallel-group trial | n = 40 ♂ Endurance runners Gn-3 n = 14 Age (mean ± SD) 37 ± 3 years Height (mean ± SD) 181 ± 7 cm Weight (mean ± SD) 76 ± 11 kg HRmax (mean ± SD) 190 ± 9 beats/min CG n =12 Age (mean ± SD) 37 ± 4 years Height (mean ± SD) 180 ± 4 cm Weight (mean ± SD) 78 ± 8 kg HRmax (mean ± SD) 186 ± 9 beats/min Study withdrawals: 14 | Gn-3 2234 mg/d EPA + 930 mg/d DHA CG 4000 mg/d MCT Supplementation time: 12 weeks | Biochemistry Red blood cell DHA Plasma DHA Red blood cell EPA Plasma EPA Plasma Trp metabolites (7) Inflammatory biomarkers IL-6 Perception markers EA HT TA | Gn-3 vs. CG IL-6 Red blood cell DHA Plasma DHA Red blood cell EPA Plasma EPA Plasma Trp metabolites ↔ EA ↔ HT ↔ TA | Gn-3 Changes from baseline ↑* Red blood cell DHA ↑* Plasma DHA ↑* Red blood cell EPA ↑* Plasma EPA ↑* Plasma Trp metabolites ↔ IL-6 ↔ EA ↔ HT ↔ TA CG Changes from baseline ↔ Red blood cell DHA ↔ Plasma DHA ↔ Red blood cell EPA ↔ Plasma EPA ↔ Plasma Trp metabolites ↔ IL-6 ↔ EA ↔ HT ↔ TA |
| Tsuchiya, et al. [ ], 2021, Japan | Randomized, double-blind, placebo-controlled, parallel-group trial | n =23 ♂ Recreational Gn-3 n = 11 Age (mean ± SD) 20.2 ± 0.4 years Height (mean ± SD) 167.4 ± 5.4 cm Weight (mean ± SD) 65.0 ± 8.9 kg % Fat (mean ± SD) 17.2 ± 6.9% BMI (mean ± SD) 23.2 ± 2.9 kg/m CG n = 11 Age (mean ± SD) 19.8 ± 1.5 years Height (mean ± SD) 169.0 ± 7.8 cm Weight (mean ± SD) 65.4 ± 8.4 kg % Fat (mean ± SD) 15.7 ± 7.6% BMI (mean ± SD) 23.2 ± 3.3 kg/m Study withdrawals: 1 | Gn-3: 8 Softgel capsule of 300 mg /d Total: 2.4 g/d (600 mg EPA + 260 mg DHA) Nippon Suisan Kaisha Ltd., Tokyo, Japan CG: 8 softgel capsules of 300 mg/d corn oil Supplementation time: 4.5 weeks | Anthropometry UAC Biochemistry Blood lipids AA EPA DGLA DHA Dietary Intake Kcal CHO prot FAT Omega-3 Inflammatory biomarkers IL-6 Muscle damage CK Perception markers VAS Physical Performance Echo thickness Echo intensity MVIC ROM | Gn-3 vs. CG ↔ UAC ↑* EPA ↔ Kcal ↔ CHO ↔ prot ↔ FAT ↔ Omega-3 ↔ IL-6 ↓* CK ↔ VAS ↔ Echo thickness ↔ Echo intensity ↔ MVIC ↑* ROM (IP) | Gn-3 Changes from baseline ↑*UAC (IP) ↑* EPA (after 4 w) ↔ AA ↔ DGLA ↔ DHA ↔ Kcal ↔ CHO ↔ prot ↔ FAT ↔ Omega-3 ↔ CK ↔ IL-6 ↑* VAS (1–4 d) ↔ Echo thickness ↑ Echo intensity ↓* MVIC ↓* IP and 1 d, after ↑ = to pre |
| Tsuchiya et al. [ ], 2016, Japan | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 24 ♂ Recreational Gn-3 n = 12 Age (mean ± SD) 19.4 ± 0.7 years Height (mean ± SD) 174.4 ± 5.6 cm Weight (mean ± SD) 64.3 ± 7.7 kg % Fat (mean ± SD) 13.0 ± 3.5% CG n =12 Age (mean ± SD) 19.5 ± 0.8 years Height (mean ± SD) 174.3 ± 6.7 cm Weight (mean ± SD) 66.2 ± 8.0 kg % Fat (mean ± SD) 13.6 ± 2.8% Study withdrawals: 0 | Gn-3 8 Softgel capsule Fish oil Per unit: 300 mg EPA + 130 mg DHA (Nippon Suisan Kaisha Ltd. Tokyo) CG 8 Softgel capsule Per unit: 300 mg corn oil (Nippon Suisan Kaisha Ltd. Tokyo) Supplementation time: 8 weeks prior to exercise + 5 days after exercise | Anthropometry UAC Biochemistry AA DHA Inflammatory biomarkers IL-6 TNF-α Muscle damage CK Mb Perception markers VAS brachii VAS brachialis VAS brachioradialis Physical Performance MVC torque ROM | Gn-3 vs. CG ↔ UAC ↔ AA ↔ DHA ↓* IL-6 ↔ TNF-α ↔ CK ↔ Mb ↔ VAS brachii ↓* VAS brachialis ↔VAS brachioradialis ↑* MVC ↑* ROM | Gn-3 Changes from baseline ↔ UAC ↔ AA ↔ DHA ↔ IL-6 ↔ TNF-α ↔ CK ↔ Mb ↑ VAS brachii (at day 1–3) ↑* VAS brachialis (at day 2) ↔VAS brachioradialis ↓*MVC ↓ ROM CG Changes from baseline ↔ UAC ↔ AA ↔ DHA ↑* IL-6 (at day 3) ↔ TNF-α ↔ CK ↑* Mb ↑ VAS brachii (day 1 to day 3) ↑* VAS brachialis (day 1 to day 3) ↔ VAS brachioradialis ↓* MVC ↓* ROM (at day 3) |
| VanDusseldrorp et al. [ ], 2020, United States | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 32 (16 ♂ and 16 ♀) Physically active: 3 to 5 d/w, minimum of 3 h/w and a maximum of 8 h/w and no more than 2 h/w of aerobic exercise 2 g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.5 ± 3.3 years Height (mean ± SD) 170.9 ± 6.9 cm Weight (mean ± SD) 76.1 ± 14.2 kg % Fat (mean ± SD) 20.8 ± 4.1% 4g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.3 ± 3.0 years Height (mean ± SD) 172.9 ± 4.7 cm Weight (mean ± SD) 69.7 ± 15.9 kg % Fat (mean ± SD) 19.0 ± 6.2% 6g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.8 ± 2.8 years Height (mean ± SD) 173.8 ± 7.6 cm Weight (mean ± SD) 72.8 ± 13.5 kg % Fat (mean ± SD) 19.4 ± 6.1% CG n = 8 (4♂ and 4♀) Age (mean ± SD) 23.0 ± 3.0 years Height (mean ± SD) 173.6 ± 6.2 cm Weight (mean ± SD) 67.9 ± 10.7 kg % Fat (mean ± SD) 20.6 ± 7.2% Study withdrawals: 0 | Gn-3 Capsule Per unit: 400 mg EPA + 300 mg DHA 2 g Gn-3 2 g/d (1400 mg: 800 mg EPA + 600 mg DHA) 4 g Gn-3 4 g/d (2800 mg: 1600 mg EPA + 1200 mg DHA) 6g Gn-3 6 g/d (4200 mg: 2400 mg EPA + 1800 mg DHA) (MusclePharm, Denver, USA) CG Safflower oil (Capsule Muscle Pharm) Supplementation time: 7.5 weeks | Muscle damage CK LDH Perception markers VAS Performance MVIC VJ 40 yd Sprint | 2 g Gn-3, 4g Gn-, 6g Gn-3 vs. CG 24 h: ↓*6 g Gn-3 vs. 2 g Gn-3 48 h: ↓* 6 g Gn-3 vs. 4 g Gn-3 72 h: ↓* 6 g Gn-3 vs. CG LDH ↓* 6 g Gn-3 vs. CG (at to 72 h) ↓* 6 g Gn-3 vs. 2 g Gn-3 (at to 72 h) VAS 2 h: CG ↑* vs. 6 g Gn-3 24 h: ↓* 4 g Gn-3 vs. CG ↑* CG vs. 6 g Gn-3 48 h: ↑* CG vs. 6 g Gn-3 ↓* 6 g Gn-3 vs. 4 g Gn-3, 2 g Gn-3 72 h: ↑* CG vs. 4 g Gn-3 ↑* CG vs. 6 g Gn-3 ↔ MVIC ↓* VJ CG ↔ 40 yd Sprint | Changes from baseline ↑* CK in all group ↑* LDH in all group ↓* 40 yd Sprint VAS ↓* MVIC (until 70 h) ↓ VJ (until 48 h) ↑* in all group (24 h) ↑* CG, 2 g Gn-3, 4 g Gn-3 (48 h) |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024 , 16 , 2044. https://doi.org/10.3390/nu16132044
Fernández-Lázaro D, Arribalzaga S, Gutiérrez-Abejón E, Azarbayjani MA, Mielgo-Ayuso J, Roche E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients . 2024; 16(13):2044. https://doi.org/10.3390/nu16132044
Fernández-Lázaro, Diego, Soledad Arribalzaga, Eduardo Gutiérrez-Abejón, Mohammad Ali Azarbayjani, Juan Mielgo-Ayuso, and Enrique Roche. 2024. "Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials" Nutrients 16, no. 13: 2044. https://doi.org/10.3390/nu16132044
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Jump to navigation

Cochrane Training
Chapter 4: searching for and selecting studies.
Carol Lefebvre, Julie Glanville, Simon Briscoe, Robin Featherstone, Anne Littlewood, Maria-Inti Metzendorf, Anna Noel-Storr, Robin Paynter, Tamara Rader, James Thomas, L. Susan Wieland; on behalf of the Cochrane Information Retrieval Methods Group
Key Points:
- Review authors should work closely, from the start of the protocol, with an experienced medical/healthcare librarian or information specialist.
- Studies (not reports of studies) are included in Cochrane Reviews but identifying reports of studies is currently the most convenient approach to identifying the majority of studies and obtaining information about them and their results.
- The Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE, together with Embase (if access to Embase is available to the review team), should be searched for all Cochrane Reviews.
- Additionally, for all Cochrane Reviews, the Specialized Register(s) of the relevant Cochrane Review Group(s) should be searched, either internally within the Review Group or via CENTRAL.
- Trials registers should be searched for all Cochrane Reviews and other sources such as regulatory agencies and clinical study reports (CSRs) are increasingly important for identifying study results.
- Searches should aim for high sensitivity, which may result in relatively low precision.
- Search strategies should avoid using too many different search concepts but a wide variety of search terms should be combined with OR within each included concept.
- Both free-text and subject headings (e.g. Medical Subject Headings (MeSH) and Emtree) should be used.
- Published, highly sensitive, validated search filters to identify randomized trials should be considered, such as the Cochrane Highly Sensitive Search Strategies for identifying randomized trials in MEDLINE, Embase and CINAHL (but do not apply these randomized trial or human filters in CENTRAL).
Cite this chapter as: Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Metzendorf M-I, Noel-Storr A, Paynter R, Rader T, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
4.1 Introduction
Cochrane Reviews take a systematic and comprehensive approach to identifying studies that meet the eligibility criteria for the review. This chapter outlines some general issues in searching for and selecting studies. It describes the main sources of potential studies; and discusses how to plan the search process, design and carry out search strategies, manage references found during the search process, correctly document the search process and select studies from the search results.
This chapter aims to provide review authors with background information on all aspects of searching for and selecting studies so that they can better understand the search and selection processes. All authors of systematic reviews should, however, identify an experienced medical/healthcare librarian or information specialist to collaborate with on the search process. The chapter also aims to provide advice and guidance for medical/healthcare librarians and information specialists (within and beyond Cochrane) involved in the search process to identify studies for inclusion in systematic reviews.
This chapter focuses on searching for randomized trials. Many of the search principles discussed, however, will also apply to other study designs. Considerations for searching for non-randomized studies are discussed in Chapter 24 (see also Chapter 19 when these are specifically for adverse effects). Other discussion of searching for specific types of evidence appears in chapters dedicated to these types of evidence, such as Chapter 17 on intervention complexity, Chapter 20 on economic evidence and Chapter 21 on qualitative evidence.
An online Technical Supplement to this chapter provides more detail on searching methods.
4.2 General issues
4.2.1 role of the information specialist/librarian.
Medical/healthcare librarians and information specialists have an integral role in the production of Cochrane Reviews. There is increasing evidence of the involvement of information specialists in systematic reviews ( Spencer and Eldredge 2018 , Ross-White 2021 , Brunskill and Hanneke 2022 , Lê et al 2023 ) and evidence to support the improvement in the quality of various aspects of the search process ( Koffel 2015 , Rethlefsen et al 2015 , Meert et al 2016 , Metzendorf 2016 , Aamodt et al 2019 , Hameed et al 2020 , Schellinger et al 2021 , Ghezzi-Kopel et al 2022 , Ramirez et al 2022 ).
Many Cochrane Review Groups (CRGs) employ an information specialist to collaborate with authors on the search process. The range of services, however, offered by CRGs and/or their information specialists varies according to the resources available. Cochrane Review authors should, therefore, contact their CRG or the Central Editorial Service at the earliest stage to find out what advice and level of contribution is available to them. Authors conducting their own searches should seek advice from their Cochrane Information Specialist not only on which sources to search, but also with respect to the exact strategies to be run (see Section 4.4). If the CRG does not provide this service or employ an information specialist, we recommend that review authors seek guidance from a medical/healthcare librarian or information specialist, preferably one with experience in identifying studies for systematic reviews.
Cochrane Information Specialists are responsible for working with authors in searching for studies for inclusion in their reviews, and for keeping up to date with Cochrane methodological developments in information retrieval ( Cochrane Information Specialist Support Team 2021a ). Some Cochrane Information Specialists maintain a Specialized Register for their CRG, containing reports of trials relating to the group’s scope. Within the limits of licensing restrictions, the content of these group registers is shared with users worldwide via the Cochrane Central Register of Controlled Trials (CENTRAL), part of the Cochrane Library (see Section 4.3.3 ).
Most CRGs collaborate with authors in study identification from the early planning stage to the final write-up of the review and any updates. This may include some or all of the following:
- advising authors on which databases and other sources to search;
- designing, or providing guidance on designing, search strategies for the main bibliographic databases and trials registers;
- running searches in databases and trials registers available to the information specialist;
- saving and collating search results, and sharing them with authors in appropriate formats;
- advising authors on how to run searches in other sources and how to download results;
- drafting, or working with authors in drafting, the search methods sections of a Cochrane protocol, review and/or update;
- ensuring that Cochrane protocols, reviews and updates meet the requirements set out in the Methodological Expectations of Cochrane Intervention Reviews (MECIR) relating to searching activities for reviews;
- organizing translations, or at least data extraction, of study reports where required to enable authors to assess these reports for inclusion/exclusion in their reviews;
- obtaining copies of trial reports for review teams when required (within copyright legislation);
- providing advice to and collaborate with author teams on the use of reference management tools and other software used in review production, including review production tools such as Covidence, EPPI-Reviewer and RevMan; and
- checking and formatting the references to included and/or excluded studies in line with the Cochrane Style Manua l.
The Cochrane Information Specialists’ Handbook contains further information about how Cochrane Information Specialists can collaborate with authors ( Cochrane Information Specialist Support Team 2021b ).
4.2.2 Minimizing bias
Systematic reviews require a thorough, objective and reproducible search of a range of sources to identify as many eligible studies as possible (within resource limits). This is a major factor distinguishing systematic reviews from traditional narrative reviews, which helps to minimize bias and achieve more reliable estimates of effects and uncertainties. A search of MEDLINE alone is not considered adequate. Research evidence indicates that not all known published randomized trials are available in MEDLINE and that even if relevant records are in MEDLINE, it can be difficult to retrieve them (see Section 4.3.1.2 ).
Searching beyond MEDLINE is important not only for ensuring that as many relevant studies as possible are identified, but also to minimize selection bias for those that are found. Relying exclusively on a MEDLINE search may retrieve a set of reports unrepresentative of all reports that would have been identified through a wider or more extensive search of several sources.
Time and budget restraints require the review team to balance the thoroughness of the search with efficiency in the use of time and funds. The best way of achieving this balance is to be aware of, and try to minimize, the biases such as publication bias and language bias that can result from restricting searches in different ways (see Chapter 8 and Chapter 13 for further guidance on assessing these biases). Unlike for tasks such as study selection or data extraction, it is not considered necessary (or even desirable) for two people to conduct independent searches in parallel. It is strongly recommended, however, that all search strategies should be peer reviewed, before being run, by a suitably qualified and experienced medical/healthcare librarian or information specialist (see Section 4.4.8 ).
4.2.3 Studies versus reports of studies
Systematic reviews have studies as the primary units of interest and analysis. A single study may have more than one report about it (or record for it), and each of these reports or other records may contribute useful information for the review (see Section 4.6.1 ). For most of the sources listed in Section 4.3 , the search process will retrieve individual reports of studies, so that multiple reports of the same study will need to be identified and associated with each other manually by the review authors. There is, however, an increasing number of study-based sources, which link multiple records of the same study together, such as the Cochrane Register of Studies and the Specialized Registers of a number of CRGs (see online Technical Supplement ), and some other trials registers, regulatory and industry sources. Processes and software to select and group publications by study are discussed in Section 4.6 .
4.2.4 Copyright and licensing
All review authors and others involved in Cochrane should adhere to copyright legislation and the terms of database licensing agreements. With respect to searching for studies, this refers in particular to adhering to the terms and conditions of use when searching databases and other sources and downloading records, as well as adhering to copyright legislation when obtaining copies of publications. Review authors should seek guidance on this from a medical/healthcare librarian or information specialist, as copyright legislation varies across jurisdictions and licensing agreements vary across organizations.
4.3 Sources to search
The sections that follow refer to sources to search for studies for inclusion in intervention reviews, irrespective of the intervention. For more detailed discussion of specific issues around searching for medical devices, please refer to this recent method note ( Cooper et al 2022b ).
4.3.1 Bibliographic databases
4.3.1.1 introduction to bibliographic databases.
For further details on this topic, please refer to Section 1.1. of the online Technical Supplement and its subsections.
The search for studies for a Cochrane Review should be as extensive as possible in order to reduce the risk of reporting bias and to identify as much relevant evidence as possible (see MECIR Box 4.3.a ). Searches of health-related bibliographic databases are generally the most efficient way to identify an initial set of relevant reports of studies ( EUnetHTA JA3WP6B2-2 Authoring Team 2019 ). Database selection should be guided by the review topic ( Suarez-Almazor et al 2000 , Stevinson and Lawlor 2004 , Lorenzetti et al 2014 ). Searching two or more databases lowers the risk of missing eligible studies ( Ewald et al 2022 ). Especially when topics are specialized, cross-disciplinary, or involve emerging technologies ( Rice et al 2016 ), additional databases may need to be identified and searched ( Wallace et al 1997 , Stevinson and Lawlor 2004 , Frandsen et al 2019a ).
MECIR Box 4.3.a Relevant expectations for conduct of intervention reviews
| Planning the search ( ) | |
|
| Searches should be motivated directly by the eligibility criteria for the review, and it is important that all types of eligible studies are considered when planning the search. If searches are restricted by publication status or by language of publication, there is a possibility of publication bias, or language bias (whereby the language of publication is selected in a way that depends on the findings of the study), or both. Removing language restrictions in English language databases is not a good substitute for searching non-English language journals and databases. |
| Searching general bibliographic databases and CENTRAL ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. The minimum databases to be covered are the CRG’s Specialized Register (if it exists and was designed to support reviews in this way), CENTRAL, MEDLINE and Embase (if Embase is available to either the CRG or the review author). Expertise may be required to avoid unnecessary duplication of effort. Some, but not all, reports of eligible studies from MEDLINE, Embase and the CRGs’ Specialized Registers are already included in CENTRAL. |
The three bibliographic databases generally considered to be the most important sources to search for reports of trials are CENTRAL ( Noel-Storr et al 2020 ), MEDLINE ( Halladay et al 2015 , Sampson et al 2016 ) and Embase ( Woods and Trewheellar 1998 , Sampson et al 2003 , Bai et al 2007 ). These databases are described in more detail in Sections 4.3.1.2 and 4.3.1.3 and in the online Technical Supplement . For Cochrane Reviews, CENTRAL, MEDLINE and Embase (if access to Embase is available to the review team) should be searched (see MECIR Box 4.3.a ). These searches may be undertaken specifically for the review, or indirectly by searching the CRG’s Specialized Register.
Some bibliographic databases, such as MEDLINE and Embase, include abstracts for the majority of recent records. A key advantage of such databases is that they can be searched electronically both for words in the title or abstract and by using the standardized indexing terms, or controlled vocabulary, assigned to each record (see Section 4.3.1.2 and 4.4.4 ). In addition to MEDLINE and Embase, Cochrane has developed a database of reports of randomized trials called the Cochrane Central Register of Controlled Trials (CENTRAL), which is published within the Cochrane Library (see Section 4.3.1.3 ).
Bibliographic databases are available to individuals for a fee (by subscription or on a ‘pay-as-you-go’ basis) or free at the point of use. They may be available through national provisions, site-wide licences at institutions such as universities or hospitals, through professional organizations as part of their membership packages or free-of-charge on the internet. Some international initiatives provide free or low-cost online access to databases (and full-text journals) over the internet. The Health InterNetwork Access to Research Initiative ( HI NARI ) programme, set up by the World Health Organization (WHO) together with major publishers and now part of the Research4Life programme (R4L), provides access to a wide range of databases including the Cochrane Library for healthcare professionals in local, not-for-profit institutions in more than 120 countries, areas and territories. The International Network for the Availability of Scientific Publications ( INASP ) also provides access to a wide range of databases (and journals) including the Cochrane Library. Electronic Information for Libraries ( EIFL ) is a similar initiative based on library consortia to support affordable licensing of journals and other sources in more than 50 developing and transition countries in Africa, Asia, Europe and Latin America.
The online Technical Supplement provides more detailed information about how to search these sources and other databases. The accompanying Appendix provides a list of general healthcare databases by region and healthcare databases by subject area. Further evidence-based information about sources to search can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
4.3.1.2 MEDLINE and Embase
Cochrane Reviews of interventions should include a search of MEDLINE (see MECIR Box 4.3.a ). MEDLINE (as of February 2023) contains approximately 30 million references to journal articles in biomedicine and health from 1946 onwards. More than 5000 journals in about 40 languages are indexed for MEDLINE ( US National Library of Medicine 2021 ).
PubMed provides access to a free version of MEDLINE that also includes up-to-date citations not yet indexed for MEDLINE ( US National Library of Medicine 2023 ). Additionally, PubMed includes records from journals that are not indexed for MEDLINE and records considered ‘out-of-scope’ from journals that are partially indexed for MEDLINE ( US National Library of Medicine 2020 ). Further details about MEDLINE, PubMed and PubMed Central and how they differ are available ( US National Library of Medicine 2018 ).
MEDLINE is also available on subscription from a number of other database vendors, such as EBSCO, Ovid, ProQuest and STN. Access is usually ‘free at-the-point-of-use’ to members of the institutions paying the subscriptions (e.g. hospitals and universities). Ovid MEDLINE (segment name ‘MEDALL’) covers all of the available content and metadata in PubMed with a delay of one working day (except during the annual reload, at the end of each year, when Ovid MEDLINE will not match the PubMed baseline). Aside from the MEDLINE records, Ovid includes all content types available in PubMed including: Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations.
When searching MEDLINE via service providers or interfaces other than Ovid or PubMed, we recommend verification of the exact coverage of the database in relation to PubMed, where no explicit information on this is readily available. Note that MEDLINE (i.e. PubMed) is searched regularly by Cochrane for reports of trials. These records are included in CENTRAL (see online Technical Supplement ).
Cochrane Reviews of interventions should include a search of Embase (if access to Embase is available to the review team) (see MECIR Box 4.3.a ). Embase (as of February 2023) contains more than 40 million records from 1947 onwards, including records from more than 8000 currently published journals from approximately 100 countries ( Elsevier 2023c , Elsevier 2023b ). Embase now includes all MEDLINE records, thus, technically, allowing both databases to be searched simultaneously. Further details on the implications of this for searching are available in the online Technical Supplement . There are more than 10 million records in Embase from approximately 3000 journals that are not indexed in MEDLINE ( Elsevier 2023b ). Embase Classic provides access to almost two million records digitized from the Excerpta Medica print journals (the original print indexes from which Embase was created) from 1947 to 1973 ( Elsevier 2022 ). Embase Classic is only available as an add-on to an Embase subscription. Embase also includes pre-print articles from medRxiv and bioRxiv ( Elsevier 2023a ); see Embase Release Notes November 2021 .
Embase is only available by subscription, either directly via Elsevier (as Embase.com) or from other database vendors such as Ovid, ProQuest or STN. It is mandatory for Cochrane intervention reviews to include a search of Embase if access is available to the review team (see MECIR Box 4.3.a ). Note that Embase is searched regularly by Cochrane for reports of trials. These records are included in CENTRAL (see online Technical Supplement ).
The online Technical Supplement provides guidance on how to search MEDLINE and Embase for reports of trials. The actual degree of reference overlap between MEDLINE and Embase varies widely according to the topic, but studies comparing searches of the two databases have generally concluded that a comprehensive search requires that both databases be searched ( Lefebvre et al 2008 , Bramer et al 2016 ) (see MECIR Box 4.3.a ).
Conversely, two studies examined different samples of Cochrane Reviews and identified the databases from which the included studies of these reviews originated ( Halladay et al 2015 , Hartling et al 2016 ). Halladay showed that the majority of included studies could be identified via PubMed (range 75% to 92%) and Hartling showed that the majority of included studies could be identified by using a combination of two databases, but the two databases were different in each case. Both studies, one across all healthcare areas ( Halladay et al 2015 ) and the other on child health ( Hartling et al 2016 ), report a minimal extent to which the inclusion of studies not indexed in PubMed altered the meta-analyses. PubMed coverage across systematic review topics has been further evaluated in a recent study based on a comprehensive sample of Cochrane Reviews. It provides further evidence of PubMed’s relatively high coverage (range 68% to 73%), with an emphasis that it is markedly variable across and within specialties ( Frandsen et al 2019b , Metzendorf and Featherstone 2019 ). A study evaluating abbreviated literature searches suggested that a combination of MEDLINE and CENTRAL was sufficient to find the majority of available RCTs on clinical interventions. The authors concluded that, if decision-makers are willing to accept less certainty and a small risk for opposite conclusions, some abbreviated searches are viable options for rapid evidence syntheses. They also concluded, however, that decisions demanding high certainty require comprehensive searches ( Nussbaumer-Streit et al 2018 ). Uncertainty remains about the circumstances under which it is most important to search multiple databases. Comprehensive searches of multiple databases may be more important for public health interventions ( Levay et al 2022 ) and for study designs other than RCTs. The current recommendation of searching multiple databases, therefore, needs to be evaluated further, in order to confirm under which circumstances comprehensive searches of multiple databases are warranted.
4.3.1.3 The Cochrane Central Register of Controlled Trials (CENTRAL)
Since its inception, the Cochrane Central Register of Controlled Trials (CENTRAL) has been recognized as the most comprehensive source of reports of randomized trials ( Egger and Smith 1998 ). A more recent study reconfirmed the high sensitivity of CENTRAL in identifying randomized controlled trials ( Noel-Storr et al 2020 ). CENTRAL is published as part of the Cochrane Library and is updated monthly. As of July 2023, CENTRAL contains more than 2,000,000 records of reports of trials/trials registry records potentially eligible for inclusion in Cochrane Reviews, by far the majority of which are randomized trials ( Noel-Storr et al 2020 ).
Many of the records in CENTRAL have been identified through systematic searches of MEDLINE, Embase, CINAHL Plus, the Australasian Medical Index, KoreaMed, ClinicalTrials.gov and the trial records available through the WHO International Clinical Trials Registry Portal (see online Technical Supplement ). CENTRAL, however, also includes citations to reports of randomized trials that are not included in MEDLINE, Embase or other bibliographic databases; citations published in many languages; and citations that are available only in conference proceedings or other sources that are difficult to access. It also includes records from trials registers and trials results registers beyond ClinicalTrials.gov and the WHO portal.
Additional records have been added to CENTRAL by Cochrane Information Specialists, who have conducted comprehensive searches to populate CRG Specialized Registers in their field. These Specialized Registers are included in CENTRAL and some CRGs continue to maintain them. Where a Specialized Register is available, for which sufficiently comprehensive searching has been conducted and kept up to date, a search of the Specialized Register may be conducted instead of separately searching CENTRAL, MEDLINE and Embase for a specific review. In these cases, the search will be more precise, but an equivalent number of included studies will be identified with lower numbers of records to screen. There will, however, be a time-lag between records appearing in databases such as MEDLINE or Embase and their inclusion in a Specialized Register.
CENTRAL is available through the Cochrane Library. Many review authors have full access free-of-charge at the point-of-use through national provisions and other similar arrangements, or as part of a paid subscription to the Cochrane Library. All Cochrane Information Specialists have full access to CENTRAL.
The online Technical Supplement provides information on what is in CENTRAL from MEDLINE, Embase and other sources, as well as guidance on searching CENTRAL.
4.3.1.4 Other bibliographic databases
For further details on this topic, please refer to Section 1.1 of the online Technical Supplement and its subsections.
Many countries and regions produce bibliographic databases that focus on the literature produced in those regions and which often include journals and other literature not indexed elsewhere. There are also subject-specific bibliographic databases, such as AMED (allied and complementary medicine), CINAHL (nursing and allied health) and APA PsycInfo (psychology and psychiatry). It is highly desirable that searches be conducted of appropriate national, regional and subject specific bibliographic databases (see MECIR Box 4.3.b ). Further details are provided in the online Technical Supplement .
Citation indexes are bibliographic databases that record instances where a particular reference is cited, in addition to the standard bibliographic content. Citation indexes can be used to identify studies that are similar to a study report of interest, as it is probable that other reports citing or cited by a study will contain similar or related content. Further details are provided in the online Technical Supplement .
MECIR Box 4.3.b Relevant expectations for conduct of intervention reviews
| Searching specialist bibliographic databases ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. Databases relevant to the review topic should be covered (e.g. CINAHL for nursing-related topics, APA PsycInfo for psychological interventions), and regional databases (e.g. LILACS) should be considered. |
4.3.2 Ongoing studies and unpublished data sources
For further details on this topic, please refer to Section 1.2 of the online Technical Supplement and its subsections.
Initiatives to provide access to ongoing studies and unpublished data constitute a fast-moving field ( Isojarvi et al 2018 ). Review authors should therefore consult a medical/healthcare librarian or information specialist for current advice.
It is important to identify ongoing studies, so that when a review is updated these can be assessed for possible inclusion. Awareness of the existence of a possibly relevant ongoing study and its expected completion date might affect not only decisions with respect to when to update a specific review, but also when to aim to complete a review. Information about possibly relevant ongoing studies should be included in the review in the ‘Characteristics of ongoing studies’ table.
Even when studies are completed or terminated, some are never published. An association between ‘statistically significant’ results and publication has been documented across a number of studies, as summarized in Chapter 13 . Finding out about unpublished studies, and including their results in a systematic review when eligible and appropriate ( Cook et al 1993 ), is important for minimizing bias. Several studies and other articles addressing issues around identifying unpublished studies have been published ( Easterbrook et al 1991 , Weber et al 1998 , Manheimer and Anderson 2002 , MacLean et al 2003 , Lee et al 2008 , Chan 2012 , Bero 2013 , Schroll et al 2013 , Chapman et al 2014 , Kreis et al 2014 , Scherer et al 2015 , Hwang et al 2016 , Lampert et al 2016 ).
There is no easy and reliable single way to obtain information about studies that have been completed or terminated but never published. There have, however, been several important initiatives resulting in better access to studies and their results from sources other than the main bibliographic databases and journals. These include the further development of trials registers and trials results registers (see Section 4.3.3 ), and improved access to regulatory agency sources and clinical study reports (CSRs), which are the very detailed reports prepared by industry for regulatory approval (see Section 4.3.4 ). A study ( Halfpenny et al 2016 ) assessed the value and usability for systematic reviews and network meta-analyses of data from trials registers, CSRs and regulatory authorities, and concluded that data from these sources have the potential to influence systematic review results. Two earlier studies showed that a considerably higher proportion of CSRs prepared for regulatory approval of drugs provided complete information on study methods and results than did trials register records or journal publications ( Wieseler et al 2012 ) and that conventional, publicly available sources (European Public Assessment Reports, journal publications, and trials register records) provide insufficient information on new drugs, especially on patient relevant outcomes in approved subpopulations ( Köhler et al 2015 ).
An annotated bibliography of published studies addressing searching for unpublished studies and obtaining access to unpublished data is also available ( Arber et al 2013 ). One particular study focused on the contribution of unpublished studies, including dissertations, and studies in languages other than English, to the results of meta-analyses in reviews relevant to children ( Hartling et al 2017 ). They found that, in their sample, unpublished studies and studies in languages other than English rarely had any impact on the results and conclusions of the review. They did, however, concede that inclusion of these study types may have an impact in situations where there are few relevant studies, or where there are ‘questionable vested interests’ in the published literature.
Asking researchers for information about completed or terminated but never published studies has not always been found to be fruitful ( Hetherington et al 1989 , Horton 1997 ) though some researchers have reported that this is an important method for retrieving studies for systematic reviews ( Royle and Milne 2003 , Greenhalgh and Peacock 2005 , Reveiz et al 2006 ). Correspondence can be an important source of information about unpublished studies. It is highly desirable for authors of Cochrane Reviews of interventions to contact relevant individuals and organizations for information about unpublished or ongoing studies (see MECIR Box 4.3.c ). Letters of request for information can be used to identify completed or terminated but unpublished studies. One way of doing this is to send a comprehensive list of relevant articles and study records along with the eligibility criteria for the review to the first author of reports of included studies, asking if they know of any additional studies (ongoing, completed or terminated; published or unpublished) that might be relevant. This approach may be especially useful in areas where there are few trials or a limited number of active research groups. It may also be desirable to send the same letter to other experts and pharmaceutical companies or others with an interest in the area. Some review teams set up websites for systematic review projects, listing the studies identified to date and inviting submission of information on studies not already listed. A Cochrane Methodology Review examined studies assessing methods for obtaining unpublished data and concluded that those carrying out systematic reviews should continue to contact authors for missing data and that email contact was more successful than other methods ( Young and Hopewell 2011 ). A study assessed the value of contacting trial authors and concluded that data supplied by authors modified the outcomes of some systematic reviews, but this was poorly reported in the reviews ( Meursinge Reynders et al 2019 ). A further case study evaluated the effectiveness, efficiency, cost and value of contacting study authors in a systematic review and concluded that this was cost-effective in terms of time taken and costs in carrying out this work compared with unique data identified from the authors’ replies ( Cooper et al 2019 ). Another case study of a Cochrane Methodology Review reported that making contact with clinical trials units and trial methodologists provided data for six of the 38 RCTs included in the review, which had not been identified through other search methods ( Brueton et al 2017 ).
A further study reported successful outcomes of a digital media strategy to obtain unpublished data from trial authors by contacting them using a combination of email, ResearchGate and LinkedIn ( Godard-Sebillotte et al 2018 ). A study assessed the value of requesting information from drug manufacturers for systematic reviews and concluded that this helped to reduce reporting and publication bias and helped to fill important gaps, sometimes leading to new or altered conclusions, primarily where no other evidence existed ( McDonagh et al 2018 ).
MECIR Box 4.3.c Relevant expectations for conduct of intervention reviews
| Searching by contacting relevant individuals and organizations ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. It is important to identify ongoing studies, so that these can be assessed for possible inclusion when a review is updated. |
The RIAT (Restoring Invisible and Abandoned Trials) initiative ( Doshi et al 2013 ) aims to address the problems outlined above by offering a methodology that allows others to re-publish mis-reported and to publish unreported trials. Anyone who can access the trial data and document trial abandonment can use this methodology. The RIAT Support Centre offers free-of-charge support and competitive funding to researchers interested in this approach. It has also been suggested that legislation such as Freedom of Information Acts (FOIAs) in various countries might be used to gain access to information about unpublished trials ( Bennett and Jull 2003 , MacLean et al 2003 ). In the US, the FOIA is frequently used to obtain information about the US Department of Health and Human Services (HHS) and its agencies. It has been suggested, in light of growing costs, minimal fees collected and lengthy processing times, that HHS agencies’ FOIA programmes might be made more efficient through greater proactive record disclosure ( Egilman et al 2019 ).
4.3.3 Trials registers and trials results registers
For further details on this topic, please refer to Section 1.2.1 of the online Technical Supplement .
Cochrane Reviews of interventions should search relevant trials registers and repositories of results (see MECIR Box 4.3.d ). A recent audit by Cochrane investigators showed that the majority of Cochrane Reviews do comply with this standard ( Berber et al 2019 ). It is important to note that trials registers are an important source of information about completed, terminated, and ongoing trials, and an increasingly important source of results for completed and terminated trials, especially those whose results have not been published ( Fain et al 2018 , Zarin et al 2019 , Nelson et al 2023 ). Studies have suggested that trials registers are an important source for identifying additional randomized trials ( Baudard et al 2017 , Banno et al 2020 , Alqaidoom et al 2023 ) and also for identifying additional or discrepant data for published studies ( Chen et al 2022 , Paladin and Pranić 2022 ). Although there are many other trials registers and related resources such as portals, those that are considered to be the most important for searching to identify studies for a systematic review are ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) portal ( Pansieri et al 2017 ). Research has shown that even though ClinicalTrials.gov is included in the WHO ICTRP Search Portal, not all ClinicalTrials.gov records could be successfully retrieved via searches of the ICTRP Search Portal ( Glanville et al 2014 , Hausner 2014 , Knelangen et al 2018 ). The extent to which this might still be the case with the new ICTRP interface released in its final version in June 2021 and the changes being made under the ClinicalTrials.gov Modernization programme remains to be ascertained. Therefore, the current guidance that it is not sufficient to search the ICTRP alone still stands, pending further research. A recent study reviewed the search interfaces of the EU Clinical Trials Register (EUCTR), ClinicalTrials.gov and the WHO ICTRP and offers further insights into how to search these resources ( Cooper et al 2021a ), as does another recent article ( Hunter et al 2022 ). Guidance for searching these and other trials registers is provided in the online Technical Supplement .
In addition to Cochrane, other organizations also advocate searching trials registers and/or related portals. These include the Agency for Healthcare Research and Quality (AHRQ) in the US, the European Network for Health Technology Assessment (EUnetHTA), the Institute for Quality and Efficiency in Health Care (IQWiG) in Germany, the Institute of Medicine in the US, and the National Institute of Health and Care Excellence (NICE) in the UK ( Institute of Medicine 2011 , Agency for Healthcare Research and Quality 2014 , National Institute for Health and Care Excellence (NICE) 2014 , EUnetHTA JA3WP6B2-2 Authoring Team 2019 , Institute for Quality and Efficiency in Health Care 2022 ).
There has been an increasing recognition by funders, governments, institutions and investigators of the importance of registering trials at inception and providing access to trial results. Despite perceptions and even assertions to the contrary, however, there is no global, universal, legal requirement to register clinical trials at inception or at any other stage in the process, and no global, universal, legal requirement to publish trial results. The situation is, however, improving and some countries and organizations have introduced such legislation ( Viergever and Li 2015 ) together with plans for sanctions for non-compliance ( Grabenstein 2023 ).
Efforts have been made by a number of organizations, including organizations representing the pharmaceutical industry and individual pharmaceutical companies, to begin to provide central access to ongoing trials and in some cases trial results on completion, either on a national or international basis. An audit of pharmaceutical companies’ policies on access to trial data, results and methods, however, showed that the commitments made by companies to transparency of trials were highly variable ( Goldacre et al 2017 ). Increasingly, as already noted, trials registers such as ClinicalTrials.gov also contain the results of completed and terminated trials where these have been posted by the investigator(s), not just simply listings of the details of the trial and/or details of associated publications.
MECIR Box 4.3.d Relevant expectations for conduct of intervention reviews
| Searching trials registers ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. Although ClinicalTrials.gov is included as one of the registers within the WHO ICTRP portal, it is recommended that both ClinicalTrials.gov and the ICTRP portal are searched separately due to additional features in ClinicalTrials.gov. |
4.3.4 Regulatory agency sources and clinical study reports
For further details on this topic, please refer to Section 1.2.2 of the online Technical Supplement .
A number of organizations, including Cochrane, recommend searching regulatory agency sources for trial information including clinical study reports. Clinical study reports (CSRs) are the reports of clinical trials providing detailed information, submitted in support of marketing authorization applications, on the methods and results or outcomes of clinical trials. The organizations which recommend searching these sources include the Agency for Healthcare Research and Quality (AHRQ) in the US, the Institute for Quality and Efficiency in Health Care (IQWiG) in Germany, and the Institute of Medicine in the US ( Institute of Medicine 2011 , Agency for Healthcare Research and Quality 2014 , Institute for Quality and Efficiency in Health Care 2022 ). Relevant regulatory agency sources for CSRs or related information include the Australian Therapeutic Goods Administration (TGA), the European Medicines Agency (EMA), Health Canada, the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and the US Food and Drug Administration (FDA). Details of these are provided in the online Technical Supplement . In addition to providing access to CSRs, regulatory agencies are also a source of trials-register-like records, for example the EMA with respect to the EU Clinical Trials Register, the recently launched European Clinical Trials Information Service/the EU Clinical Trials database, and The European Database on Medical Devices (EUDAMED) (under development); and the FDA with respect to Drugs@FDA and medical devices information from the FDA. These resources are all covered in the online Technical Supplement .
A study ( Jefferson et al 2018 ) that looked at use of regulatory documents in Cochrane Reviews, found that understanding within the Cochrane community was limited and that guidance and support would be required if review authors were to engage with regulatory documents as a source of evidence. Specifically, guidance on how to use data from regulatory sources is needed. For more information about identifying CSRs, see the online Technical Supplement . Further guidance on collecting data from CSRs is provided in Chapter 5, Section 5.5.6 .
4.3.5 Other sources
For further details on this topic, please refer to Section 1 of the online Technical Supplement and its subsections.
The term ‘grey literature’ is often used to refer to reports published outside of traditional commercial publishing. In particular, review authors consider searching sources such as dissertations and conference abstracts (see MECIR Box 4.3.e ).
Review authors may also consider searching the internet, handsearching journals and searching full texts of journals electronically where available (see online Technical Supplement for details). They should examine previous reviews on the same topic and check reference lists of included studies and relevant systematic reviews (see MECIR Box 4.3.e ).
MECIR Box 4.3.e Relevant expectations for conduct of intervention reviews
| Searching for grey literature ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. |
| Searching within other reviews ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. |
| Searching reference lists ( ) | |
|
| Searches for studies should be as extensive as possible in order to reduce the risk of publication bias and to identify as much relevant evidence as possible. |
4.4 Designing search strategies
4.4.1 introduction to search strategies.
This section highlights some of the issues to consider when designing search strategies. Designing search strategies can be complex and the section does not fully address the many complexities in this area. Review teams will benefit from the skills and expertise of a medical/healthcare librarian or information specialist. Many of the issues highlighted relate to both the subject aspects of the search (e.g. the PICO elements) and to the study design (e.g. randomized trials). For a search to be robust, both aspects require attention to be sure that relevant records are not missed.
Issues to consider in planning a search include:
- the nature or type of the intervention(s) being assessed;
- the complexity of the review question and the need to consider additional conceptual frameworks (see Chapter 3 and Chapter 17 );
- the time period when any evaluations of the interventions may have taken place (as specified in the review protocol) (see Section 4.4.5 );
- any geographic considerations, such as the need to search the African Index Medicus for studies relating to African populations or the Chinese literature for studies in Chinese herbal medicine (see online Technical Supplement );
- whether the review is limited to randomized trials or other study designs are eligible (see Chapter 24 );
- whether a validated methodological search filter (for specific study designs) is available (see Section 4.4.7 );
- whether unpublished data are to be sought specifically, see Sections 4.3.2 , 4.3.3 and 4.3.4 ; and
- whether the review has specific eligibility criteria around study design to address adverse effects (see Chapter 19 ), economic evidence (see Chapter 20 ) or qualitative evidence (see Chapter 21 ), in which case searches to address these criteria should be undertaken (see MECIR Box 4.4.a ).
Further evidence-based information about designing search strategies can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
MECIR Box 4.4.a Relevant expectations for conduct of intervention reviews
| Searching for different types of evidence ( ) | |
|
| Sometimes a review will address questions about adverse effects, economic issues or qualitative research using a different set of eligibility criteria from the main (effectiveness) component. In such situations, the searches for evidence must be suitable to identify relevant study designs for these questions. Different searches may need to be conducted for different types of evidence. |
4.4.2 Structure of a search strategy
The starting point for developing a search strategy is to consider the main concepts being examined in a review. This is often referred to as PICO – that is Patient (or Participant or Population or Problem), Intervention, Comparison and Outcomes ( Richardson et al 1995 ): see also Chapter 2 and Chapter 3 for guidance on developing and refining PICO definitions that will be operationalized in the search strategy. Examples are provided in the appendices to the Cochrane Information Specialists’ Handbook ( Cochrane Information Specialist Support Team 2021d ). For a Cochrane Review, the review objective should provide the PICO concepts, and the eligibility criteria for studies to be included will further assist in the selection of appropriate subject headings and text words for the search strategy.
The structure of search strategies in bibliographic databases should be informed by the main concepts of the review (see Chapter 3 ), using appropriate elements from PICO and the study design (see MECIR Box 4.4.b ). It is usually unnecessary, however, and may even be undesirable, to search on every aspect of the review’s clinical question ( Frandsen et al 2020 ). Although a research question may specify particular comparators or outcomes, these concepts may not be well described in the title or abstract of an article and are often not well indexed with controlled vocabulary terms. Therefore, when searching general databases, such as MEDLINE, a search strategy will typically have three sets of terms: (i) terms to search for the health condition of interest, i.e. the population; (ii) terms to search for the intervention(s) evaluated; and (iii) terms to search for the types of study design to be included. Typically, a broad set of search terms will be gathered for each concept and combined with the OR Boolean operator to achieve sensitivity within concepts. The results for each concept are then combined using the AND Boolean operator, to ensure each concept is represented in the final search results.
It is important to consider the structure of the search strategy on a question-by-question basis. In some cases it is possible and reasonable to search for the comparator, for example if the comparator is explicitly placebo; in other cases the outcomes may be particularly well defined and consistently reported in abstracts. The advice on whether or not to search for outcomes for adverse effects differs from the advice given above (see Chapter 19 ).
MECIR Box 4.4.b Relevant expectations for conduct of intervention reviews
| : Structuring search strategies for bibliographic databases ( ) | |
|
| Inappropriate or inadequate search strategies may fail to identify records that are included in bibliographic databases. Expertise may need to be sought, in particular from the CRG’s Information Specialist. The structure of a search strategy should be based on the main concepts being examined in a review. In general databases, such as MEDLINE, a search strategy to identify studies for a Cochrane Review will typically have three sets of terms: (i) terms to search for the health condition of interest, i.e. the population; (ii) terms to search for the intervention(s) evaluated; and (iii) terms to search for the types of study design to be included (typically a ‘filter’ for randomized trials). There are exceptions, however. For instance, for reviews of complex interventions, it may be necessary to search only for the population or the intervention. Within each concept, terms are joined together with the Boolean ‘OR’ operator, and the concepts are combined with the Boolean ‘AND’ operator. The ‘NOT’ operator should be avoided where possible to avoid the danger of inadvertently removing records that are relevant from the search set. |
Some search strategies may not easily divide into the structure suggested, particularly for reviews addressing complex or unknown interventions, or diagnostic tests ( Huang et al 2006 , Irvin and Hayden 2006 , Petticrew and Roberts 2006 , Booth 2016a , Spijker et al 2023 ) or using specific approaches such as realist reviews which may require iterative searches and multiple search strategies ( Booth et al 2020 ). Cochrane Reviews of public health interventions and of qualitative data may adopt very different search approaches to those described here ( Lorenc et al 2014 , Booth 2016a ) (see Chapter 17 on intervention complexity, and Chapter 21 on qualitative evidence). The term ‘tailored approach’ has been suggested for searches for complex topics or review methods which may not adopt a PICO structure ( Cooper et al 2022a ). Some options to explore for these situations include:
- consider whether a non-PICO question breakdown may be helpful. A list of question structures is available at https://docplayer.net/160814951-Alternative-question-structures-for-different-types-of-systematic-review.html ( Liu et al 2021 );
- use a single concept such as searching for the intervention alone ( European Food Safety Authority 2010 );
- break a concept into two or more subconcepts;
- use a multi-stranded or multi-faceted approach that uses a series of searches, with different combinations of concepts, to capture a complex research question ( Lefebvre et al 2013 );
- use a variety of different search approaches to compensate for when a specific concept is difficult to define ( Shemilt et al 2014 );
- use iterative searches ( Bravata et al 2005 , Zwakman et al 2018 , Booth et al 2020 );
- use citation searching on key articles in addition to a database search ( Haddaway et al 2015 , Hinde and Spackman 2015 ) (see online Technical Supplement ); and
- consider machine learning options whereby broad, sensitive searches are conducted and the search results are categorized or prioritized within machine learning software ( Muller et al 2021 , Shemilt et al 2021 , Cawley 2022 , Shemilt et al 2022 , Stansfield et al 2022 ).
In the process of updating reviews, the PICO elements and any impact on search structure should also be reviewed and updated if necessary ( Bendersky et al 2022 ).
4.4.3 Sensitivity versus precision
Searches for systematic reviews aim to be as extensive as possible in order to ensure that as many of the relevant studies as possible are included in the review. It is, however, necessary to strike a balance between striving for comprehensiveness and maintaining relevance when developing a search strategy.
The properties of searches are often quantified using ‘sensitivity’ (also called ‘recall’) and ‘precision’ (see Table 4.4.a ). Sensitivity is defined as the number of relevant reports identified divided by the total number of relevant reports in the resource. Precision is defined as the number of relevant reports identified divided by the total number of reports identified. Increasing the comprehensiveness (or sensitivity) of a search will reduce its precision and will usually retrieve more non-relevant reports.
Searches for Cochrane Reviews should seek to maximize sensitivity whilst striving for reasonable precision (see MECIR Box 4.4.b ). Article abstracts identified through a database search can usually be screened very quickly to ascertain potential relevance. At a conservatively estimated reading rate of one or two abstracts per minute, the results of a database search can be screened at the rate of 60–120 per hour (or approximately 500–1000 over an 8-hour period), so the high yield and low precision associated with systematic review searching may not be as daunting as it might at first appear in comparison with the total time to be invested in the review.
Table 4.4.a Sensitivity and precision of a search
|
|
| |
|
| Relevant reports retrieved (a) | Relevant reports not retrieved (b) |
|
| Irrelevant reports retrieved (c) | Irrelevant reports not retrieved (d) |
| Sensitivity: fraction of relevant reports retrieved from all relevant reports (a/(a+b)) Precision: fraction of relevant reports retrieved from all reports retrieved (a/(a+c)) | ||
4.4.4 Controlled vocabulary and text words
For further details on this topic, please refer to Section 3.2 of the online Technical Supplement and its subsections.
MEDLINE and Embase (and many other databases) can be searched using a combination of two retrieval approaches. One is based on text words, that is terms occurring in the title, abstract or other relevant fields available in the database. The other is based on standardized subject terms assigned to the references either by indexers (specialists who appraise the articles and describe their topics by assigning terms from a specific thesaurus or controlled vocabulary) or automatically using automated indexing approaches. Searches for Cochrane Reviews should use an appropriate combination of these two approaches, i.e. text words and controlled vocabulary (see MECIR Box 4.4.c ). Approaches for identifying text words and controlled vocabulary to combine appropriately within a search strategy, including text mining approaches, are presented in the online Technical Supplement .
MECIR Box 4.4.c Relevant expectations for conduct of intervention reviews
| Developing search strategies for bibliographic databases ( ) | |
|
| Inappropriate or inadequate search strategies may fail to identify records that are included in bibliographic databases. Search strategies need to be customized for each database. It is important that MeSH terms are ‘exploded’ wherever appropriate, in order not to miss relevant articles. The same principle applies to Emtree when searching Embase and also to a number of other databases. The controlled vocabulary search terms for MEDLINE and Embase are not identical, and neither is the approach to indexing. In order to be as comprehensive as possible, it is necessary to include a wide range of free-text terms for each of the concepts selected. This might include the use of truncation and wildcards. Developing a search strategy is an iterative process in which the terms that are used are modified, based on what has already been retrieved. |
4.4.5 Language, date and document format restrictions
Searches should capture as many studies as possible that meet the eligibility criteria, ensuring that relevant time periods and sources are covered and not restricted by language or publication status (see MECIR Box 4.3.a ). Review authors should justify the use of any restrictions in the search strategy on publication date and publication format (see MECIR Box 4.4.d ).
To reduce the risk of introducing bias, searches should not be restricted by language. By including all languages, conservative search strategies avoid the risk of excluding study records with missing, inconsistent, or incorrect values in their metadata ( Aali and Shokraneh 2021 ). It has also been argued that, when language restrictions are justified, these should not be imposed by limiting the search but by including language as an eligibility criterion during study selection ( Pieper and Puljak 2021 ). Recommendations for rapid reviews searches to limit publication language to English and add other languages only when justified ( Garritty et al 2021 ) are supported by evidence that excluding non-English studies does not change the conclusions of most systematic reviews ( Morrison et al 2012 , Jiao et al 2013 , Hartling et al 2017 , Nussbaumer-Streit et al 2020 , Dobrescu et al 2021 ). However, exceptions that non-English studies do influence review findings have been observed for complementary and alternative medicine ( Moher et al 2003 , Pham et al 2005 , Wu et al 2013 ), psychiatry, rheumatology and orthopaedics ( Egger et al 2003 ).
Studies have identified a risk of introducing bias by including lower quality, non-English language trials in systematic reviews ( Jüni et al 2002 , Egger et al 2003 ), but similar evaluations found only minor quality differences between reports of English and non-English language trials ( Moher et al 2003 ). Additionally, when searches are limited to English or to databases containing only English-language articles, there is a risk that eligible studies may be missed from countries where a particular intervention of interest is more common (e.g. traditional Chinese medicines) ( Pilkington et al 2005 , Morrison et al 2012 ). Searches restricted to English-language databases or trials registers may also fail to retrieve all eligible study records of drug interventions (indexing bias) or those reporting negative results ( Jia et al 2020 ). For further discussion of these issues see Chapter 13 .
Particularly when resources and time are available, the inclusion of non-English studies in systematic reviews is recommended to minimize the risk of language or indexing bias ( Egger et al 1997 , Pilkington et al 2005 , Morrison et al 2012 ). Consequently, Cochrane author teams should plan at the protocol stage not to restrict the search by language (see MECIR Box 4.3.a ) and to consider searching non-English language databases and trials registers. If warranted, author teams should engage a medical/healthcare librarian or information specialist who is capable of designing and executing search strategies in these sources.
If a Cochrane Review team requires help with translation of or data extraction from non-English language reports of studies, they should seek assistance to do so (this is a common task for which volunteer assistance can be sought via Cochrane Exchange (previously known as Cochrane’s TaskExchange platform), accessible to both Cochrane and non-Cochrane review teams. Where it is not possible to extract the relevant information and data from non-English language reports, readers should be informed of the existence of other possibly relevant reports by adding such reports to ‘studies awaiting classification’ rather than ‘excluded studies’. This information should be reflected in the PRISMA flow diagram (or, if there is no flow diagram, then in the text of the review) as ‘studies awaiting classification’.
Date limits may be used to focus searches ( Cooper et al 2018a ) as long as the restriction is reported and justified ( Egger et al 2003 ) (see MECIR Box 4.4.d ). Further use of a supportive narrative may help explain why a particular date restriction was applied ( Craven and Levay 2011 , Cooper et al 2018b ). For example, a database date restriction of 1989-current for a review of nurse-led community training of epinephrine autoinjectors is justified because this is the approval date of the first device ( Center for Drug Evaluation and Research 1989 ). A date limit may be safely applied in this case as any references published before this date would not meet the review’s selection criteria.
While evidence supports that arbitrary date restrictions (e.g. last 20 years) have little impact on the results for rapid reviews of diagnostic test accuracy studies ( Furuya-Kanamori et al 2023 ), this approach is not recommended for Cochrane systematic reviews of interventions and should be avoided.
Caution should be exercised when designing database search strategies with date restrictions. Information specialists should be aware of the various date fields available from database providers (e.g. create date, entry date, last update date, publication date) as well as the coverage dates of the datafiles searched. It may be necessary to search additional sources or datafiles to ensure adequate coverage of the date period of interest for the review. To account for inconsistent publication dates in database records (e.g. a record for an electronic version of a publication may have an earlier publication date than the print version), search strategies should be restricted to a wider date range than the period of interest for the review.
As any information about an eligible study may contain valuable details for analysis, document format restrictions should not be applied to systematic review searches. For example, excluding letters is not recommended because letters may contain important additional information relating to an earlier trial report or new information about a trial not reported elsewhere ( Iansavichene et al 2008 ). In addition, articles indexed as ‘Comments’ should not be routinely excluded without further examination as these may contain early warnings of possible future retraction (see Section 4.4.6 ).
As with comments and letters, preprints (versions of scientific articles that precede formal peer review and publication in a journal) should also be considered a potentially relevant source of study evidence, particularly for emerging topics where little evidence exists. Recent and widespread availability of preprints has resulted from an urgent demand for emerging evidence during the COVID-19 pandemic ( Gianola et al 2020 , Kirkham et al 2020 , Callaway 2021 , Fraser et al 2021 ). A recent study ( Zeraatkar et al 2022 ) indicated that there was no compelling evidence that preprints provide results that are inconsistent with published papers. As study data are often reported in multiple publications and may be reported differently in each ( Oikonomidi et al 2020 ), efforts to identify all reports for eligible studies, regardless of publication format, are necessary to support subsequent stages of the review process to select, assess and analyse complete study data. However, practical problems have been observed with regard to including preprints in systematic or rapid reviews, such as limited search features or download capabilities of preprint archives ( Hoy 2020 , Brietzke et al 2023 ), and challenges incorporating different versions of preprints and their final publications ( Clyne et al 2021 ).
Similarly, no limits should be applied at the search stage of the systematic review process to exclude articles published in so-called ‘predatory journals’. The direction of bias associated with non-inclusion of studies published in predatory journals depends on whether they are publishing valid studies with null results or studies whose results are biased towards finding an effect (see Chapter 7 ) ( Boulos et al 2022 ).
Further evidence-based information about language, date and other limits can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
MECIR Box 4.4.d Relevant expectations for conduct of intervention reviews
| Restricting database searches ( ) | |
|
| Date restrictions in the search should only be used when there are date restrictions in the eligibility criteria for studies. They should be applied only if it is known that relevant studies could only have been reported during a specific time period, for example if the intervention was only available after a certain time point. Searches for updates to reviews might naturally be restricted by date of entry into the database (rather than date of publication) to avoid duplication of effort. Publication format restrictions (e.g. exclusion of letters) should generally not be used in Cochrane Reviews, since any information about an eligible study may be of value. |
4.4.6 Identifying fraudulent studies, other retracted publications, errata and comments
For further details on this topic, please refer to Section 3.9 of the online Technical Supplement .
When considering the eligibility of studies for inclusion in a Cochrane Review, it is important to be aware that publications for some studies may have been found to contain errors or to be fraudulent or may, for other reasons, have been corrected, retracted or had expressions of concern associated with them since publication. Review authors should examine any relevant retraction notices and errata for information ( MECIR Box 4.4.e ). This applies both to ‘new’ studies identified for inclusion in a review and to studies that are already included in a review when the review is updated. For review updates, it is important to search MEDLINE and Embase for the latest version of the citations to the records for the (previously) included studies, in case the publications have since been corrected or retracted.
Errata are published to correct unintended errors (accepted as errors by the author(s)) that do not invalidate the conclusions of the article. Retractions are defined by the Committee on Publication Ethics (COPE) Council’s retraction guidelines ( Committee on Publication Ethics (COPE) Council 2019 ) as “… a mechanism for correcting the literature and alerting readers to articles that contain such seriously flawed or erroneous content or data that their findings and conclusions cannot be relied upon. Unreliable content or data may result from honest error, naïve mistakes, or research misconduct.” A recent study has shown that scientific misconduct was the most common reason for retractions, specifically duplication, plagiarism and fabrication of data ( Gaudino et al 2021 ). Comments are published under a range of circumstances including when errors are suggested by others and also for early concerns regarding suspected misconduct. The US National Library of Medicine, in its MeSH Scope Note , defines expression of concern as: “A notification about the integrity of a published article that is typically written by an editor and should be labelled prominently in the item title”.
Including data from studies that are fraudulent or studies that include errors can have an impact on the overall estimates in systematic reviews. There is an increasing awareness of the importance of not including retracted studies or those with significant errata in systematic reviews and how best to avoid this ( Royle and Waugh 2004 , Wright and McDaid 2011 , Decullier et al 2014 ). A recent study, however, showed that even when review authors suspect research misconduct, including data falsification, in the trials that they are considering including in their systematic reviews, they do not always report it ( Elia et al 2016 ). Details of how to identify fraudulent studies, other retracted publications, errata, comments and expressions of concern are described in the online Technical Supplement . Cochrane holds a policy for managing potentially problematic studies and provides associated implementation guidance .
MECIR Box 4.4.e Relevant expectations for conduct of intervention reviews
| Examining errata ( ) | |
|
| Some studies may have been found to be fraudulent or may have been retracted since publication for other reasons. Errata can reveal important limitations, or even fatal flaws, in included studies. All of these may lead to the potential exclusion of a study from a review or meta-analysis. Care should be taken to ensure that this information is retrieved in all database searches by downloading the appropriate fields, together with the citation data. |
4.4.7 Search filters
For further details on this topic, please refer to Section 3.6 of the online Technical Supplement and its subsections.
Search filters are search strategies that are designed to retrieve specific types of records, such as those of a particular methodological design. When searching for randomized trials in humans, a validated filter should be used to identify studies with the appropriate design (see MECIR Box 4.4.f ). Filters to identify randomized trials for CENTRAL have been developed specifically for databases such as MEDLINE, Embase and CINAHL Plus. CENTRAL, however, aims to contain only reports with study designs possibly relevant for inclusion in Cochrane Reviews, so searches of CENTRAL should not use a trials ‘filter’ or be limited to human studies. The Cochrane MEDLINE and Embase highly sensitive filters are provided here for ease of access; there are also launch links for them on the ISSG Search Filter Resource website ( https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/home/rcts ). The online Technical Supplement has other filters including the balanced sensitivity and precision filters for MEDLINE.
Box 4.4.a Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity-maximizing version (2008 revision); PubMed format
PubMed search syntax (for Box 4.4.a above):
Box 4.4.b Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity-maximizing version (2023 revision); Ovid format
Ovid search syntax (for Box 4.4.b above):
Box 4.4.c Cochrane Highly Sensitive Search Strategy for identifying randomized trials in Embase (2023 revision); Embase.com format (adapted from Glanville et al (2019) )
Embase.com search syntax (for Box 4.4.c above):
Box 4.4.d Cochrane Highly Sensitive Search Strategy for identifying randomized trials in Embase (2023 revision); Ovid format (adapted from Glanville et al (2019) )
Ovid search syntax (for Box 4.4.d above):
The InterTASC Information Specialists’ Sub-Group Search Filter Resource offers a collection of search filters , focusing predominantly on methodological search filters and providing critical appraisals of some of these filters. The site includes, amongst others, filters for identifying systematic reviews, randomized and non-randomized studies and qualitative research in a range of databases and across a range of service providers ( Glanville et al 2006 ).
Increasingly, search filters for selected study designs (classifiers) such as randomized controlled trials are built into systematic review software such as EPPI-Reviewer ( https://eppi.ioe.ac.uk/cms/er4/Features/tabid/3396/Default.aspx ) and RobotReviewer ( https://www.robotreviewer.net/ ), or can be designed within systematic review software such as DistillerSR (https://www.distillersr.com/products/modules ( Marshall et al 2023 ). When using study design filters available within systematic review software, ensure that the search strategies used to identify studies from databases do not also contain study design filters. A systematic review of ten varied studies that used supervised machine learning to identify “high-quality clinical articles” reported that machine learning, largely using gold standard sets of records from the ACP Journal Club, achieved a best balance sensitivity of 95% with 86% precision in one study ( Abdelkader et al 2021 ). Other studies had higher sensitivity but lower precision. The authors noted that this is a fast-moving field, although it is hampered by the lack of large reference sets of known relevant RCTs.
Search filter performance is discussed further in a detailed report ( Lefebvre et al 2017 ). For further discussion around the design and use of search filters, see the online Technical Supplement. Further evidence-based information about search filters can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
MECIR Box 4.4.f Relevant expectations for conduct of intervention reviews
| : Using search filters ( ) | |
|
| Inappropriate or inadequate search strategies may fail to identify records that are included in bibliographic databases. Search filters should be used with caution. They should be assessed not only for the reliability of their development and reported performance, but also for their current accuracy, relevance and effectiveness given the frequent interface and indexing changes affecting databases. |
4.4.8 Peer review of search strategies
It is strongly recommended that search strategies be peer reviewed before the searches are run. Peer review of search strategies is increasingly recognized as a necessary step in designing and executing high-quality search strategies to identify studies for possible inclusion in systematic reviews ( Folb et al 2020 , Neilson 2021 ). As discussed elsewhere ( Lefebvre and Duffy 2021 ), the following organizations and documents advocate peer review of searches: The Agency for Healthcare Research and Quality (AHRQ) in the US, the Centre for Reviews and Dissemination in the UK, the European Network for Health Technology Assessment (EUnetHTA), the Institute for Quality and Efficiency in Health Care (IQWiG) in Germany, the Institute of Medicine in the US, the National Institute of Health and Care Excellence (NICE) in the UK, the Preferred Reporting Items for Systematic reviews and Meta-Analyses – Extension for Searches (PRISMA-S Extension) and the PRISMA 2020 statement and explanation and elaboration documents ( Centre for Reviews and Dissemination 2009 , Institute of Medicine 2011 , Agency for Healthcare Research and Quality 2014 , National Institute for Health and Care Excellence (NICE) 2014 , EUnetHTA JA3WP6B2-2 Authoring Team 2019 , Page et al 2021a , Page et al 2021b , Rethlefsen et al 2021 , Institute for Quality and Efficiency in Health Care 2022 ). Additionally, the Campbell Collaboration includes an item on peer review of search strategies in its Information Retrieval Methods Group Checklist in its searching guidance ( Kugley et al 2017 ).
Studies have shown that errors occur in the search strategies underpinning systematic reviews and that search strategies are not always conducted or reported to a high standard ( Sampson and McGowan 2006 , Mullins et al 2014 , Layton 2017 , Salvador-Olivan et al 2019 , Masterson and Martinez-Silveira 2022 , Ramirez et al 2022 ). This has also been shown to be the case within some Cochrane Reviews ( Franco et al 2018 , Price et al 2022 ). The PRISMA-S Extension states that authors “should strongly consider having the search strategy peer reviewed by an experienced searcher, informational specialist, or librarian” ( Rethlefsen et al 2021 ) and encourages authors to consider using the Peer Review of Electronic Search Strategies (PRESS) Guideline Statement and Checklist ( McGowan et al 2016b ). Research has shown that peer review using a specially designed checklist can improve the quality of searches both in systematic reviews ( Relevo and Paynter 2012 , Spry et al 2013 ) and in rapid reviews ( Spry et al 2013 , Spry and Mierzwinski-Urban 2018 ). An evidence-based checklist such as the PRESS Evidence-Based Checklist should be used to assess which elements are important in peer review of electronic search strategies ( McGowan et al 2016a , McGowan et al 2016b ). The PRESS Checklist covers not only the technical accuracy of the strategy (line numbers, spellings, etc.), but also whether the search strategy addresses all relevant aspects of the protocol and has interpreted the research question appropriately.
It is recommended that authors provide information on the search strategy development and peer review processes. The PRISMA 2020 explanation and elaboration article and the PRISMA-S Extension provide guidance on how and where authors should describe the processes used to develop and validate or peer review the search strategy ( Page et al 2021a , Rethlefsen et al 2021 ). For example, the PRISMA 2020 explanation and elaboration article ( Page et al 2021a ) states that “the description of the search strategy development process might include details of the approaches used to identify keywords, synonyms, or subject indexing terms used in the search strategies or any processes used to validate or peer review the search strategies”, and “if the search strategy was peer reviewed, report the peer review process used and specify any tool used, such as the Peer Review of Electronic Search Strategies (PRESS) checklist” ( McGowan et al 2016b ). In the example for Item 7 (Search strategy) of the PRISMA 2020 checklist, the authors propose using the following statement: “The strategy was developed by an information specialist and the final strategies were peer reviewed by an experienced information specialist within our team”( Page et al 2021b ). The PRISMA-S Extension ( Rethlefsen et al 2021 ) advocates that the use of peer review be reported and described in the methods section, and proposes the following statement in Item 14 (Peer review: Describe any search peer review process): “The strategies were peer reviewed by another senior information specialist prior to execution using the PRESS Checklist” ( McGowan et al 2016b ). For Cochrane Reviews, the names, credentials, and institutions of the peer reviewers of the search strategies should be noted in the review (with their permission) in the Acknowledgments section.
Further evidence-based information about peer reviewing search strategies can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
4.4.9 Alerts
Alerts, also called literature surveillance services, ‘push’ services or SDIs (selective dissemination of information), are an excellent method of staying up to date with the medical literature currently being published and with other resources such as trials register resources, as a supplement to designing and running specific searches for specific reviews. In practice, alerts are based on a previously developed search strategy, which is saved in a personal account on the database platform (e.g. ‘My EBSCOhost – search alerts’ on EBSCO, ‘My Searches & Alerts’ on Ovid and ‘MyNCBI – saved searches’ on PubMed). These saved strategies filter the content as the database (or other resource) is being updated with new information. The account owner is notified (usually via email) when new or updated publications or records meeting their specified search parameters are added to the database (or other resource). In the case of PubMed, the alert can be set up to be delivered weekly or monthly, or in real-time and can comprise email or RSS feeds.
For review authors, alerts are a useful tool to help monitor their review topic area after the original search has been conducted. By following an alert, authors can become aware of a new study that might meet the review’s eligibility criteria, and decide either to include it in the review immediately or to mention it in the review’s ‘studies awaiting classification’ section, for inclusion during the next review update (see online Chapter IV ). Authors should consider setting up alerts so that the review can be as current as possible at the time of publication.
Another way of attempting to stay current with the literature as it emerges is by using alerts based on journal tables of contents (TOCs). These usually cannot be specifically tailored to the information needs in the same way as search strategies developed to cover a specific topic. They can, however, be a good way of trying to keep up to date on a more general level by monitoring what is currently being published in journals of interest. Many journals, even those that are available by subscription only, offer TOC alert services free of charge. In addition, a number of publishers and organizations offer TOC services (see online Technical Supplement ). Use of TOCs is not proposed as a single alternative to the various other methods of study identification necessary for undertaking systematic reviews, rather as a supplementary method (See also Chapter 22 , Sections 22.2 and 22.3 for a discussion of new technologies to support evidence surveillance in the context of ‘living’ systematic reviews).
As mentioned above, alerts should also be considered for sources beyond databases and journal TOCs, such as trials register resources and regulatory information.
4.4.10 Timing of searches
The published review should be as up to date as possible. Searches for all the relevant sources (databases, trials registers, etc.) should be rerun prior to publication, if the initial search date is more than 12 months (preferably six months) from the intended publication date (see MECIR Box 4.4.g ). The results should also be screened to identify potentially eligible studies. Ideally, the studies should be incorporated fully in the review. If not, then the potentially eligible studies will need to be reported as references under ‘studies awaiting classification’ (or under ‘ongoing studies’ if they are not yet completed).
MECIR Box 4.4.g Relevant expectations for conduct of intervention reviews
| Rerunning searches ( ) | |
|
| The published review should be as up to date as possible. The search must be rerun close to publication, if the initial search date is more than 12 months (preferably six months) from the intended publication date, and the results screened for potentially eligible studies. Ideally, the studies should be incorporated fully in the review. If not, then the potentially eligible studies will need to be reported, at a minimum as a reference under ‘Studies awaiting classification’ (or ‘Ongoing studies’ if they have not yet completed). |
| Incorporating findings from rerun searches ( ) | |
|
| The published review should be as up to date as possible. After the rerun of the search, the decision whether to incorporate any new studies fully into the review will need to be balanced against the delay in publication. |
4.4.11 When to stop searching
Developing a search within a database is often an iterative and exploratory process. It involves exploring trade-offs between search terms and assessing their overall impact on the sensitivity and precision of the search. It is often difficult to decide in a scientific or objective way when a search is complete and search strategy development can stop. The ability to decide when to stop typically develops through experience of developing many strategies. Suggestions for stopping rules have been made around the retrieval of new records, for example to stop if adding in a series of new terms to a database search strategy yields no new relevant records, or if precision falls below a particular cut-off point ( Chilcott et al 2003 ). Stopping might also be appropriate when the removal of terms or concepts results in missing relevant records. Another consideration is the amount of evidence that has already accrued: in topics where evidence is scarce, authors might need to be more cautious about deciding when to stop searching. Although methods have been described to assist with deciding when to stop developing the search, there has been little formal evaluation of the approaches ( Booth 2010 , Arber and Wood 2021 ).
Research has demonstrated that searches within databases may miss relevant studies and that careful attention should be paid to achieving a sensitive search strategy since there may always be room for improvement ( Matthews et al 1999 , Savoie et al 2003 , Booth 2016b ). At a basic level, investigation is needed as to whether a strategy is performing adequately. One simple test is to check whether the search is finding the publications that have been recommended as key publications or that have been included in other similar reviews ( Cooper et al 2018c , EUnetHTA JA3WP6B2-2 Authoring Team 2019 ). It is not enough, however, for the strategy to find only those records, otherwise this might be a sign that the strategy is biased towards known studies and other relevant records might be being missed. In addition, citation searches (see online Technical Supplement Section 1.1.4 ) and reference checking (see online Technical Supplement Section 1.3.4 ) are useful checks of strategy performance. If those additional methods are finding documents that the searches have already retrieved, but that the team did not necessarily know about in advance, then this is one sign that the strategy might be performing adequately. Also, an evidence-based checklist such as the PRESS Evidence-Based Checklist ( McGowan et al 2016b ) should be used to assess whether the search strategy is adequate (see Section 4.4.8 ). If some of the PRESS dimensions seem to be missing without adequate explanation or arouse concerns, then the search may not yet be complete.
Statistical techniques can be used to assess performance, such as capture-recapture ( Spoor et al 1996 , Ferrante di Ruffano et al 2012 ), also known as capture-mark-recapture ( Kastner et al 2009 , Lane et al 2013 ), or the relative recall technique ( Sampson et al 2006 , Sampson and McGowan 2011 ). Kastner suggests the capture-mark-recapture technique merits further investigation since it could be used to estimate the number of studies in a literature prospectively and to determine where to stop searches once suitable cut-off levels have been identified. Kastner’s approach involves searching databases, conducting record selection, calculating capture-mark-recapture and then making decisions about whether further searches are necessary. This would entail potentially an iterative search and selection process. Capture-recapture needs results from at least two searches to estimate the number of missed studies. Further investigation of published prospective techniques seems warranted to learn more about the potential benefits.
Relative recall ( Sampson et al 2006 , Sampson and McGowan 2011 ) requires a range of searches to have been conducted so that the relevant studies have been built up by a set of sensitive searches. The performance of the individual searches can then be assessed in each individual database by determining how many of the studies that were deemed eligible for the evidence synthesis and were indexed within a database, can be found by the database search used to populate the synthesis. If a search in a database did not perform well and missed many studies, then that search strategy is likely to have been suboptimal. If the search strategy found most of the studies that were available to be found in the database, then it was likely to have been a sensitive strategy. Assessments of precision could also be made, but these mostly inform future search approaches since they cannot affect the searches and record assessment already undertaken. Relative recall may be most useful at the end of the search process since it relies on the achievement of several searches to make judgements about the overall performance of strategies.
In evidence synthesis involving qualitative data, searching is often more organic and intertwined with the analysis such that the searching stops when new information ceases to be identified ( Booth 2016a ). The reasons for stopping need to be documented and it is suggested that explanations or justifications for stopping may centre around saturation ( Booth 2016a ). Further information on searches for qualitative evidence can be found in Chapter 21 .
In searches for complex topics or in those types of review where there are different approaches to the completeness of study identification, exhaustive structured searches of bibliographic databases may not be the highest priority and so the issue of knowing when to stop may take on a different focus ( Cooper et al 2018c , Cooper et al 2022a ).
4.5 Documenting and reporting the search process
Review authors should document the search process in enough detail to ensure that it can be reported correctly in the review (see MECIR Box 4.5.a ). The searches of all resources should be reproducible to the extent that this is possible. By documenting the search process, we refer to internal record-keeping, which is distinct from reporting the search process in the review (discussed in online Chapter III ).
MECIR Box 4.5.a Relevant expectations for conduct of intervention reviews
| Documenting the search process ( ) | |
|
| The search process (including the sources searched, when, by whom, and using which terms) needs to be documented in enough detail throughout the process to ensure that it can be reported correctly in the review, to the extent that all the searches of all the databases are reproducible. |
Medical/healthcare librarians and information specialists involved with the review should draft, or at least comment on, the search strategy sections of the review prior to publication.
Suboptimal reporting of systematic review search activities and methods has been observed ( Sampson et al 2008 , Roundtree et al 2009 , Niederstadt and Droste 2010 ). Research has also shown a lack of compliance with guidance in the Handbook with respect to search strategy description in published Cochrane Reviews ( Sampson and McGowan 2006 , Yoshii et al 2009 , Franco et al 2018 ). The lack of consensus regarding optimal reporting has been a challenge with respect to the values of transparency and reproducibility. The PRISMA-Search (PRISMA-S) Extension ( Rethlefsen et al 2021 ), an extension to the PRISMA Statement ( Page et al 2021a , Page et al 2021b ), addresses the reporting of search strategies in systematic reviews. PRISMA-S (together with the major revision of PRISMA itself) provides enough detail and specific examples for systematic review authors to report search methods and information sources in a clear, reproducible way. In Box 2 of the PRISMA 2020 guidance under “Noteworthy changes to the PRISMA 2009 statement” the guidance has been strengthened to stipulate: “Modification of the ‘Search’ item to recommend authors present full search strategies for all databases, registers and websites searched, not just at least one database (see item #7)”. This brings the PRISMA 2020 guidance more into line with Cochrane standards for reporting of search strategies. Further PRISMA-related guidance has been published relating to tracking records and completion of the PRISMA flow diagram ( Rethlefsen and Page 2022 ).
There is also a recommendation in the PRISMA 2020 guidance (see item 27) that “authors state whether data used in the review are publicly available and if so, where they can be accessed” ( Page et al 2021b ). These recommendations may influence record keeping practices of searchers.
It is recommended that review authors seek guidance from a medical/healthcare librarian or information specialist at the earliest opportunity with respect to documenting the search process ( Rethlefsen et al 2015 , Meert et al 2016 ). For Cochrane Reviews, the bibliographic database search strategies should all be copied and pasted into the search strategies supplementary material exactly as run and in full, together with the search set numbers and the total number of records retrieved by each search strategy. The same process is also good practice for searches of trials registers and other sources, where the interface used, such as introductory or advanced, should also be specified. Creating a report of the search process can be accomplished through methodical documentation of the steps taken by the searcher. This need not be onerous if suitable record keeping is performed during the process of the search, but it can be nearly impossible to recreate post hoc. Many database interfaces have facilities for search strategies to be saved online or to be emailed; an offline copy in text format should also be saved. For some databases, taking and saving a screenshot of the search may be the most practical approach ( Rader et al 2014 ).
Documenting the searching of sources other than databases, including the search terms used, is also required if searches are to be reproducible ( Atkinson et al 2015 , Chow 2015 , Witkowski and Aldhouse 2015 , Booth et al 2020 ).
Details about contacting experts or manufacturers, searching reference lists, scanning websites, and decisions about search iterations can be produced as an appendix in the final document and used for future updates. The purpose of search documentation is transparency, internal assessment, and reference for any future update. It is important to plan how to record searching of sources other than databases since some activities (contacting experts, reference list searching, and forward citation searching) will occur later on in the review process after the database results have been screened ( Rader et al 2014 ). The searcher should record any correspondence on key decisions and report a summary of this correspondence alongside the search strategy in a search narrative. The narrative describes the major decisions that shaped the strategy and can give a peer reviewer an insight into the rationale for the search approach ( Craven and Levay 2011 ). A worked example of a search narrative is available ( Cooper et al 2018b ).
It is particularly important to save locally or file print copies of any information found on the internet, such as information about ongoing and/or unpublished trials, as this information may no longer be accessible at the time the review is written. Local copies should be stored in a structured way to allow retrieval when needed. There are also web-based tools which archive webpage content for future reference, such as WebCite ( Eysenbach and Trudel 2005 ). The results of web searches will not be reproducible to the same extent as bibliographic database searches because web content and search engine algorithms frequently change, and search results can differ between users due to a general move towards localization and personalization ( Cooper et al 2021b ). It is still important, however, to document the search process to ensure that the methods used can be transparently reported ( Briscoe 2018 ). In cases where a search engine retrieves more results than it is practical to screen in full (it is rarely practical to search thousands of web results, as the precision of web searches is likely to be relatively low), the number of results that are documented and reported should be the number that were screened rather than the total number ( Dellavalle et al 2003 , Bramer 2016 ).
Decisions should be documented for all records identified by the search. Details of the flow of studies from the number(s) of references identified in the search to the number of studies included in the review will need to be reported in the final review, ideally using a flow diagram such as that proposed in the PRISMA guidance (see online Chapter III ); these can be generated using software including Covidence, DistillerSR, EPPI-Reviewer, the METAGEAR package for R, the PRISMA Flow Diagram Generator, and RevMan. A table of ‘Characteristics of excluded studies’ will also need to be presented (see Section 4.6.5 ). Numbers of records are sufficient for exclusions based on initial screening of titles and abstracts. Broad categorizations are sufficient for records classed as potentially eligible during an initial screen of the full text. Authors will need to decide for each review when to map records to studies (if multiple records refer to one study). The flow diagram records initially the total number of records retrieved from various sources, then the total number of studies to which these records relate. Review authors need to match the various records to the various studies in order to complete the flow diagram correctly. Lists of included and excluded studies must be based on studies rather than records (see also Section 4.6.1 ).
Further evidence-based information about documenting and reporting the search process can be found on the SuRe Info portal , which is updated twice per year ( Isojarvi and Glanville 2021 ).
4.6 Selecting studies
4.6.1 studies (not reports) as the unit of interest.
A Cochrane Review is a review of studies that meet pre-specified eligibility criteria. Since each study may have been reported in several articles, abstracts or other reports, an extensive search for studies for the review may identify many reports for each potentially relevant study. Two distinct processes are therefore required to determine which studies can be included in the review. One is to link together multiple reports of the same study; and the other is to use the information available in the various reports to determine which studies are eligible for inclusion. Although sometimes there is a single report for each study, it should never be assumed that this is the case.
As well as the studies that inform the systematic review, other studies will also be identified and these should be recorded or tagged as they are encountered, so that they can be listed in the relevant tables in the review (see Section 4.6.3 ).
4.6.2 Identifying multiple reports from the same study
Duplicate publication can introduce substantial biases if studies are inadvertently included more than once in a meta-analysis ( Tramèr et al 1997 ). Duplicate publication can take various forms, ranging from identical manuscripts to reports describing different outcomes of the study or results at different time points ( von Elm et al 2004 ). The number of participants may differ in the different publications. It can be difficult to detect duplicate publication and some ‘detective work’ by the review authors may be required.
Some of the most useful criteria for comparing reports are:
- trial identification numbers (e.g. ClinicalTrials.gov Identifier (NCT number); ISRCTN; Universal Trial Number (UTN) (assigned by the ICTRP); other identifiers such as those from the sponsor) ( Liu et al 2022 , Smalheiser and Holt 2022 );
- author names (most duplicate reports have one or more authors in common, although this is not always the case);
- location and setting (particularly if institutions, such as hospitals, are named);
- specific details of the interventions (e.g. dose, frequency);
- numbers of participants and baseline data; and
- date and duration of the study (which can also clarify whether different sample sizes are due to different periods of recruitment).
Where uncertainties remain after considering these and other factors, it may be necessary to correspond with the authors of the reports and/or the principal investigators of the studies for unpublished data.
Multiple reports of the same study should be collated, so that each study, rather than each report, is the unit of interest in the review (see MECIR Box 4.6.a ). Review authors will need to choose and justify which report (the primary report) to use as a source for study results, particularly if two reports include conflicting results. They should not discard other (secondary) reports, since they may contain additional outcome measures and valuable information about the design and conduct of the study.
MECIR Box 4.6.a Relevant expectations for conduct of intervention reviews
| Collating multiple reports ( ) | |
|
| It is wrong to consider multiple reports of the same study as if they are multiple studies. Secondary reports of a study should not be discarded, however, since they may contain valuable information about the design and conduct. Review authors must choose and justify which report to use as a source for study results. |
4.6.3 A typical process for selecting studies
A typical process for selecting studies for inclusion in a review is as follows (the planned process should be detailed in the protocol for the review):
- Merge search results from different sources using reference management software, and remove duplicate records of the same report (i.e. records reporting the same journal title, volume and page numbers).
- Examine titles and abstracts to remove obviously irrelevant reports (authors should generally be over-inclusive at this stage).
- Retrieve the full text of the potentially relevant reports.
- Link together multiple reports of the same study (see Section 4.6.2 ).
- Examine full-text reports for compliance of studies with eligibility criteria.
- Correspond with investigato rs , where appropriate, to clarify study eligibility (it may be appropriate to request further information, such as missing methods information or results, at the same time). If study data remain incomplete/unobtainable those studies should be tagged/recorded as incomplete, and should be listed in the table of ‘Characteristics of studies awaiting classification’ in the review.
- Make final decisions on study inclusion and proceed to data collection.
- Tag or recor d (i) any ongoing studies, so that they can be added to the ongoing studies table, and (ii) any completed or terminated studies (including any that are presumed to be completed based on the information available) but not yet reported, so that they can be added to either the included studies table or the table of studies awaiting classification, depending on whether the study clearly meets the review’s eligibility criteria.
Note that studies should not be omitted from a review solely on the basis of measured outcome data not being reported (see MECIR Box 4.6.b and Chapter 13 ).
MECIR Box 4.6.b Relevant expectations for conduct of intervention reviews
| Excluding studies without useable data ( ) | |
|
| Systematic reviews typically should seek to include all relevant participants who have been included in eligible study designs of the relevant interventions and had the outcomes of interest measured. Reviews must not exclude studies solely on the basis of reporting of the outcome data, since this may introduce bias due to selective outcome reporting and risk undermining the systematic review process. While such studies cannot be included in meta-analyses, the implications of their omission should be considered. Note that studies may legitimately be excluded because outcomes were not measured. Furthermore, issues may be different for adverse effects outcomes, since the pool of studies may be much larger and it can be difficult to assess whether such outcomes were measured. |
4.6.4 Implementation of the selection process
Decisions about which studies to include in a review are among the most influential decisions that are made in the review process and they involve judgement. Use (at least) two people working independently to determine whether each study meets the eligibility criteria. Ideally, screening of titles and abstracts to remove irrelevant reports should also be done in duplicate by two people working independently (although it is acceptable that this initial screening of titles and abstracts is undertaken by only one person). It is essential, however, that two people working independently are used to make a final determination as to whether each study considered possibly eligible after title/abstract screening meets the eligibility criteria based on the full text of the study report(s) (see MECIR Box 4.6.c ).
MECIR Box 4.6.c Relevant expectations for conduct of intervention reviews
| Making inclusion decisions ( ) | |
|
| Duplicating the study selection process reduces both the risk of making mistakes and the possibility that selection is influenced by a single person’s biases. The inclusion decisions should be based on the full texts of potentially eligible studies when possible, usually after an initial screen of titles and abstracts. It is desirable, but not mandatory, that two people undertake this initial screening, working independently. |
It has been shown that using at least two authors may reduce the possibility that relevant reports will be discarded ( Edwards et al 2002 , Waffenschmidt et al 2019 , Gartlehner et al 2020 ) although other case reports have suggested single screening approaches may be adequate ( Doust et al 2005 , Shemilt et al 2016 ). Opportunities for screening efficiencies seem likely to become available through promising developments in single human screening in combination with machine learning approaches ( O'Mara-Eves et al 2015 ).
Experts in a particular area frequently have pre-formed opinions that can bias their assessment of both the relevance and validity of articles ( Cooper and Ribble 1989 , Oxman and Guyatt 1993 ). Thus, while it is important that at least one author is knowledgeable in the area under review, it may be an advantage to have a second author who is not a content expert.
Disagreements about whether a study should be included can generally be resolved by discussion. Often the cause of disagreement is a simple oversight on the part of one of the review authors. When the disagreement is due to a difference in interpretation, this may require arbitration by another person. Occasionally, it will not be possible to resolve disagreements about whether to include a study without additional information. In these cases, authors may choose to categorize the study in their review as one that is awaiting assessment until the additional information is obtained from the study authors or investigators.
A single failed eligibility criterion is sufficient for a study to be excluded from a review. In practice, therefore, eligibility criteria for each study should be assessed in order of importance, so that the first ‘no’ response can be used as the primary reason for exclusion of the study, and the remaining criteria need not be assessed. The eligibility criteria order may be different in different reviews and they do not always need to be the same.
For most reviews it will be worthwhile to pilot test the eligibility criteria on a sample of reports (say six to eight articles, including ones that are thought to be definitely eligible, definitely not eligible and doubtful). The pilot test can be used to refine and clarify the eligibility criteria, train the people who will be applying them and ensure that the criteria can be applied consistently by more than one person.
For Cochrane Reviews the selection process must be documented in sufficient detail to be able to complete a flow diagram and a table of ‘Characteristics of excluded studies’ (see MECIR Box 4.6.d ). During the selection process it is crucial to keep track of the number of references and subsequently the number of studies so that a flow diagram can be constructed. The decisions and reasons for exclusion can be tracked using reference management software, a simple document or spreadsheet, or using specialist systematic review software (see Section 4.6.6.1 ).
MECIR Box 4.6.d Relevant expectations for conduct of intervention reviews
| Documenting decisions about records identified ( ) | |
|
| Decisions should be documented for all records identified by the search. Numbers of records are sufficient for exclusions based on initial screening of titles and abstracts. Broad categorizations are sufficient for records classed as potentially eligible during an initial screen. Studies listed in the table of ‘Characteristics of excluded studies’ should be those that a user might reasonably expect to find in the review. At least one explicit reason for their exclusion must be documented. Authors will need to decide for each review when to map records to studies (if multiple records refer to one study). Lists of included and excluded studies must be based on studies rather than records. |
4.6.5 Selecting ‘excluded studies’
A Cochrane Review includes a list of excluded studies called ‘Characteristics of excluded studies’, detailing the specific reason for exclusion for any studies that a reader might plausibly expect to see among the included studies. This covers all studies that may, on the surface, appear to meet the eligibility criteria but which, on further inspection, do not. It also covers those that do not meet all of the criteria but are well known and likely to be thought relevant by some readers. By listing such studies as excluded and giving the primary reason for exclusion, the review authors can show that consideration has been given to these studies. The list of excluded studies should be as brief as possible. It should not list all of the reports that were identified by an extensive search. It should not list studies that obviously do not fulfil the eligibility criteria for the review, such as ‘Types of studies’, ‘Types of participants’, and ‘Types of interventions’. In particular, it should not list studies that are obviously not randomized if the review includes only randomized trials. Based on a sample, undertaken in 2017/2018 by one of the authors (JT), of approximately 60% of the intervention reviews in the Cochrane Library which included randomized trials only, the average number of studies listed in the ‘excluded studies’ table was 30.
4.6.6 Software support for selecting studies
An extensive search for eligible studies in a systematic review can often identify thousands of records that need to be manually screened. Selecting studies from within these records can be a particularly time-consuming, laborious and logistically challenging aspect of conducting a systematic review. These and other challenges have led to the development of various software tools (and approaches for using ‘generic’ tools) that offer support for the selection process.
Broadly, software to support selecting studies can be classified as:
- systems that support the study selection process, typically involving multiple reviewers (see Section 4.6.6.1 ); and
- tools and techniques based on text mining and/or machine learning, which aim to semi- or fully-automate the selection process (see Section 4.6.6.2 ).
Software to support the selection process, along with other stages of a systematic review, including text mining tools, can be identified using the Systematic Review Toolbox . The Systematic Review Toolbox is a community driven, web-based catalogue of tools that provide support for systematic reviews ( Marshall and Brereton 2015 ).
4.6.6.1 Software for managing the selection process
Managing the selection process can be challenging, particularly in a large-scale systematic review that involves multiple reviewers. Basic productivity tools can help (such as word processors, spreadsheets, and reference management software), and several purpose-built systems that support multiple concurrent users are also available that offer support for the study selection process. Software for managing the selection process can be identified using the Systematic Review Toolbox mentioned above.
Compatibility with other software tools used in the review process (such as RevMan) may be a consideration when selecting a tool to support study selection. Covidence and EPPI-Reviewer are Cochrane-preferred tools, and are likely to have the strongest integration with RevMan. Should specialist software not be available, Bramer and colleagues have reported a method for using the widely available software EndNote X7 for managing the screening process ( Bramer et al 2017 ).
4.6.6.2 Automating the selection process
Research into automating the study selection process through artificial intelligence (‘AI’), machine learning and text mining has received considerable attention over recent years, resulting in the development of numerous tools and techniques for reviewers to consider. The use of automated tools has the potential to reduce the workload involved with selecting studies significantly ( Thomas et al 2017 ). For example, research suggests that adopting automation can reduce the need for manual screening by at least 30% and possibly more than 90%, although sometimes at the cost of up to a 5% reduction in sensitivity ( O'Mara-Eves et al 2015 ). This section discusses these tools in three main areas: a) those that operate across reviews; b) those that use crowdsourcing to reduce reviewer workload; and c) those that operate within individual reviews.
a) The first class of tool is machine learning models (or ‘classifiers’) that can be built where sufficient data are available. Of particular practical use to Cochrane Review authors is a classifier (the ‘RCT Classifier’) that can identify reports of randomized trials based on titles and abstracts. The classifier is highly accurate because it is built on a large dataset of hundreds of thousands of records screened by Cochrane Crowd , Cochrane’s citizen science platform, where contributors help to identify and describe health research ( Marshall et al 2018 , Noel-Storr et al 2021a , Thomas et al 2021 ). Guidance on using the RCT Classifier in Cochrane Reviews, for example to exclude studies already flagged as not being randomized trials, or to access Cochrane Crowd to assist with screening, is available from the Cochrane Information Specialists’ Handbook ( Cochrane Information Specialist Support Team 2021c ).
An emerging suite of tools built on ‘Large Language Models’ (LLMs) such as ChatGPT may soon offer practical advantages in automating study selection and other parts of the review process. These models promise the possibility of carrying out ‘zero shot learning’, where records can be classified automatically without the need for any specific training. Such approaches may offer substantial benefits, though, at the time of writing in mid-2023, no sufficiently large and valid evaluations are available.
b) Cochrane has also implemented a screening workflow called Screen4Me ( Noel-Storr et al 2021b ). This workflow incorporates the use of the RCT Classifier and Cochrane Crowd, to identify the RCTs found in authors’ search results. Cochrane author teams conducting intervention reviews that incorporate RCTs can access this workflow via the Cochrane Register of Studies. Author teams wishing to use the Screen4Me workflow should liaise directly with their CRG. To date (July 2023), Screen4Me has been used in more than 200 Cochrane intervention reviews. Workload reduction in terms of screening burden varies depending on the prevalence of RCTs in the domain area and the sensitivity of the searches conducted. A recent internal, as yet (July 2023) unpublished, evaluation by one of the authors (AN-S) showed a mean reduction in screening workload of 53% (range 26% to 84%). More information on Screen4Me can be found in the Cochrane Information Specialists’ Handbook ( Cochrane Information Specialist Support Team 2021c ).
c) In addition to learning from large datasets such as those generated by Cochrane Crowd, it is also possible for machine learning models to learn how to apply eligibility criteria within individual reviews. This approach uses a process called ‘active learning’ and it is able to semi-automate study selection by continuously promoting records most likely to be relevant to the top of the results list ( O'Mara-Eves et al 2015 ). It is difficult for authors to determine in advance when it is safe to stop screening and allow some records to be eliminated automatically without manual assessment. Recent work has suggested that this barrier is not insurmountable, and that it is possible to estimate how many relevant records remain to be found based on the sample already screened ( Sneyd and Stevenson 2019 , Callaghan and Muller-Hansen 2020 , Li and Kanoulas 2020 ). The automatic elimination of records using this approach has not been recommended for use in Cochrane Reviews at the time of writing in mid-2023, since more work is needed to develop and validate safe ‘stopping rules’. This active learning process can still be useful, however, since by prioritizing records for screening in order of relevance, it enables authors to identify the studies that are most likely to be included much earlier in the screening process than would otherwise be possible.
Recent developments in this class of tools have seen increased support for keeping living systematic reviews (see also Chapter 22 ) up to date. Work is still ongoing, but the COVID-19 pandemic lent an urgency to tool development in order to maintain surveillance on the rapidly evolving evidence base ( Thomas 2021 ). Evidence surveillance in living systematic reviews may be a fruitful use case for automation, because of the availability of data on which the machine can ‘learn’. Two case studies found that automation to support living reviews in COVID-19 were both accurate and saved manual effort ( Shemilt et al 2021 , Marshall et al 2023 ).
Finally, tools are available that use natural language processing to highlight sentences and key phrases automatically (e.g. PICO elements, trial characteristics, details of randomization) to support the reviewer whilst screening ( Tsafnat et al 2014 ).
4.7 Chapter information
Authors: Carol Lefebvre, Julie Glanville, Simon Briscoe, Robin Featherstone, Anne Littlewood, Maria-Inti Metzendorf, Anna Noel-Storr, Robin Paynter, Tamara Rader, James Thomas, L. Susan Wieland; on behalf of the Cochrane Information Retrieval Methods Group
Acknowledgements: This chapter has been developed from sections of previous editions of the Handbook co-authored since 1995 by Kay Dickersin, Julie Glanville, Kristen Larson, Carol Lefebvre, Eric Manheimer, Chris Marshall and Farhad Shokraneh. Many of the sources listed in this chapter and the accompanying online Technical Supplement have been brought to our attention by a variety of people over the years and we should like to acknowledge this. We should like to acknowledge: Ruth Foxlee, (formerly) Information Specialist, Cochrane Editorial Unit; Miranda Cumpston, (formerly) Head of Learning & Support, Cochrane Central Executive; Colleen Finley, Product Manager, John Wiley and Sons, for checking sections relating to searching the Cochrane Library; the (UK) National Institute for Health and Care Excellence and the German Institute for Quality and Efficiency in Health Care (IQWiG) for support in identifying some of the references; the (US) Agency for Healthcare Research and Quality (AHRQ) Effective Healthcare Program Scientific Resource Center for their previous Article Alert service; Tianjing Li, Co-Convenor, Comparing Multiple Interventions Methods Group, for text and references that formed the basis of the re-drafting of parts of Section 4.6 Selecting studies; Lesley Gillespie, Cochrane author and former Editor and Trials Search Co-ordinator of the Cochrane Bone, Joint and Muscle Trauma Group, for copy-editing an early draft; the Cochrane Information Specialists’ Executive, the previous Cochrane Information Specialists’ Support Team, Cochrane Information Specialists and members of the Cochrane Information Retrieval Methods Group for comments on drafts; Su Golder, Co-Convenor, Adverse Effects Methods Group and Steve McDonald, Co-Director, Cochrane Australia for peer review of earlier versions.
4.8 References
Aali G, Shokraneh F. No limitations to language, date, publication type, and publication status in search step of systematic reviews. J Clin Epidemiol 2021; 133: 165-167.
Aamodt M, Huurdeman H, Strømme H. Librarian co-authored systematic reviews are associated with lower risk of bias compared to systematic reviews with acknowledgement of librarians or no participation by librarians. Evidence Based Library and Information Practice 2019; 14: 103-127.
Abdelkader W, Navarro T, Parrish R, Cotoi C, Germini F, Iorio A, Haynes RB, Lokker C. Machine learning approaches to retrieve high-quality, clinically relevant evidence from the biomedical literature: systematic review. JMIR Medical Informatics 2021; 9: e30401.
Agency for Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews: AHRQ publication no. 10(14)-EHC063-EF. 2014. https://effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview .
Alqaidoom Z, Nguyen PY, Awadh M, Page MJ. Impact of searching clinical trials registers in systematic reviews of pharmaceutical and non-pharmaceutical interventions: Reanalysis of meta-analyses. Research Synthesis Methods 2023; 14: 52-67.
Arber M, Cikalo M, Glanville J, Lefebvre C, Varley D, Wood H. Annotated bibliography of published studies addressing searching for unpublished studies and obtaining access to unpublished data. 2013. https://methods.cochrane.org/sites/methods.cochrane.org.irmg/files/public/uploads/Annotatedbibliographtifyingunpublishedstudies.pdf .
Arber M, Wood H, Miller P. Search strategy development. Last updated 29 March 2023. 2023. In: SuRe Info: Summarized Research in Information Retrieval for HTA Updated 4 April 2023 [Internet]. Available from: https://www.sure-info.org//search-strategy-development .
Atkinson KM, Koenka AC, Sanchez CE, Moshontz H, Cooper H. Reporting standards for literature searches and report inclusion criteria: making research syntheses more transparent and easy to replicate. Research Synthesis Methods 2015; 6: 87-95.
Bai Y, Gao J, Zou D, Li Z. Is MEDLINE alone enough for a meta-analysis? Alimentary Pharmacology and Therapeutics 2007; 26: 125-126; author reply 126.
Banno M, Tsujimoto Y, Kataoka Y. Using the Cochrane Central Register of Controlled Trials to identify clinical trial registration is insufficient: a cross-sectional study. BMC Medical Research Methodology 2020; 20: 200.
Baudard M, Yavchitz A, Ravaud P, Perrodeau E, Boutron I. Impact of searching clinical trial registries in systematic reviews of pharmaceutical treatments: methodological systematic review and reanalysis of meta-analyses. BMJ 2017; 356: j448.
Bendersky J, Auladell-Rispau A, Urrutia G, Rojas-Reyes MX. Methods for developing and reporting living evidence synthesis. J Clin Epidemiol 2022; 152: 89-100.
Bennett DA, Jull A. FDA: untapped source of unpublished trials. Lancet 2003; 361: 1402-1403.
Berber S, Tan-Koay AG, Opiyo N, Dwan K, Glanville JM, Lasserson TJ, Willson ML. A cross-sectional audit showed that most Cochrane intervention reviews searched trial registers. J Clin Epidemiol 2019; 113: 86-91.
Booth A. How much searching is enough? Comprehensive versus optimal retrieval for technology assessments. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2010; 26: 431-435.
Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Systematic reviews 2016a; 5: 74.
Booth A. Over 85% of included studies in systematic reviews are on MEDLINE. J Clin Epidemiol 2016b; 79: 165-166.
Booth A, Briscoe S, Wright JM. The "realist search": A systematic scoping review of current practice and reporting. Research Synthesis Methods 2020; 11: 14-35.
Boulos L, Rothfus M, Goudreau A, Manley A. A descriptive study found low prevalence of presumed predatory publications in a subset of Cochrane reviews. J Clin Epidemiol 2022; 152: 316-325.
Bramer WM, Giustini D, Kramer BM. Comparing the coverage, recall, and precision of searches for 120 systematic reviews in Embase, MEDLINE, and Google Scholar: a prospective study. Systematic Reviews 2016; 5: 39.
Bramer WM. Variation in number of hits for complex searches in Google Scholar. Journal of the Medical Library Association 2016; 104: 143-145.
Bramer WM, Milic J, Mast F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. Journal of the Medical Library Association 2017; 105: 84-87.
Bravata DM, McDonald KM, Shojania KG, Sundaram V, Owens DK. Challenges in systematic reviews: synthesis of topics related to the delivery, organization, and financing of health care. Ann Intern Med 2005; 142: 1056-1065.
Brietzke E, Gomes FA, Gerchman F, Freire RCR. Should systematic reviews and meta-analysis include data from preprints? Trends in Psychiatry and Psychotherapy 2023; 45: 1-2.
Briscoe S. A review of the reporting of web searching to identify studies for Cochrane systematic reviews. Research Synthesis Methods 2018; 9: 89-99.
Brueton V, Tierney JF, Stenning S, Rait G. Identifying additional studies for a systematic review of retention strategies in randomised controlled trials: making contact with trials units and trial methodologists. Systematic reviews 2017; 6: 167.
Brunskill A, Hanneke R. The case of the disappearing librarians: analyzing documentation of librarians' contributions to systematic reviews. Journal of the Medical Library Association 2022; 110: 409-418.
Callaghan MW, Muller-Hansen F. Statistical stopping criteria for automated screening in systematic reviews. Systematic reviews 2020; 9: 273.
Callaway J. Librarian Reserve Corps: Literature indexing and metadata enhancement (LIME) observations from a year in the field. Journal of Health Information and Libraries Australasia 2021; 2: 35-45.
Cawley MA. Using Machine Learning to Locate Evidence More Efficiently: New Roles for Academic Research Librarians. 2022. In: Handbook of Research on Academic Libraries as Partners in Data Science Ecosystems [Internet]. Hershey, PA: IGI Global; [144-168]. Available from: https://www.igi-global.com/chapter/using-machine-learning-to-locate-evidence-more-efficiently/302752 .
Center for Drug Evaluation and Research. Approval package for EpiPen Auto Injector and EpiPen Jr. Auto Injector. 1989. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019430Orig1s001.pdf .
Centre for Reviews and Dissemination. Systematic Reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2009.
Chan AW. Out of sight but not out of mind: how to search for unpublished clinical trial evidence. BMJ 2012; 344: d8013.
Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ 2014; 349: g6870.
Chen KY, Borglund EM, Postema EC, Dunn AG, Bourgeois FT. Reporting of clinical trial safety results in ClinicalTrials.gov for FDA-approved drugs: A cross-sectional analysis. Clinical Trials 2022; 19: 442-451.
Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P. The role of modelling in prioritising and planning clinical trials. HEALTH TECHNOLOGY ASSESSMENT 2003; 7: iii, 1-125.
Chow TK. Electronic search strategies should be repeatable. European Journal of Pain 2015; 19: 1562-1563.
Clyne B, Walsh KA, O'Murchu E, Sharp MK, Comber L, KK OB, Smith SM, Harrington P, O'Neill M, Teljeur C, Ryan M. Using preprints in evidence synthesis: Commentary on experience during the COVID-19 pandemic. J Clin Epidemiol 2021; 138: 203-210.
Cochrane Information Specialist Support Team. Section 1: Role of a Cochrane Information Specialist. 2021a. In: Cochrane Information Specialists' Handbook [Internet]. Available from: https://training.cochrane.org/resource/cochrane-information-specialists-handbook .
Cochrane Information Specialist Support Team. Section 6: Author support. 2021b. In: Cochrane Information Specialists' Handbook [Internet]. Available from: https://training.cochrane.org/resource/cochrane-information-specialists-handbook .
Cochrane Information Specialist Support Team. Section 3: The Cochrane Register of Studies (CRS). 2021c. In: Cochrane Information Specialists' Handbook [Internet]. Available from: https://training.cochrane.org/resource/cochrane-information-specialists-handbook .
Cochrane Information Specialist Support Team. Appendices. 2021d. In: Cochrane Information Specialists' Handbook [Internet]. Available from: https://training.cochrane.org/resource/cochrane-information-specialists-handbook .
Cochrane Style Manual Working Group. Cochrane Style Manual (accessed 11 April 2023). https://community.cochrane.org/style-manual .
Committee on Publication Ethics (COPE) Council. COPE Guidelines: Retraction guidelines. Version 2: November. 2019. https://publicationethics.org/files/retraction-guidelines.pdf .
Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, McIlroy W, Oxman AD. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993; 269: 2749-2753.
Cooper C, Booth A, Varley-Campbell J, Britten N, Garside R. Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies. BMC Medical Research Methodology 2018a; 18: 85.
Cooper C, Dawson S, Peters J, Varley-Campbell J, Cockcroft E, Hendon J, Churchill R. Revisiting the need for a literature search narrative: A brief methodological note. Research Synthesis Methods 2018b; 9: 361-365.
Cooper C, Varley-Campbell J, Booth A, Britten N, Garside R. Systematic review identifies six metrics and one method for assessing literature search effectiveness but no consensus on appropriate use. J Clin Epidemiol 2018c; 99: 53-63.
Cooper C, Bou JT, Varley-Campbell J. Evaluating the effectiveness, efficiency, cost and value of contacting study authors in a systematic review: a case study and worked example. BMC Medical Research Methodology 2019; 19: 45.
Cooper C, Court R, Kotas E, Schauberger U. A technical review of three clinical trials register resources indicates where improvements to the search interfaces are needed. Research Synthesis Methods 2021a; 12: 384-393.
Cooper C, Lorenc T, Schauberger U. What you see depends on where you sit: The effect of geographical location on web-searching for systematic reviews: A case study. Research Synthesis Methods 2021b; 12: 557-570.
Cooper C, Booth A, Husk K, Lovell R, Frost J, Schauberger U, Britten N, Garside R. A tailored approach: A model for literature searching in complex systematic reviews. Journal of information science 2022a; 0.
Cooper C, Dawson S, Lefebvre C. Searching for medical devices - Practical guidance. Research Synthesis Methods 2022b; 13: 144-154.
Cooper H, Ribble RG. Influences on the outcome of literature searches for integrative research reviews. Science Communication 1989; 10: 179-201.
Craven J, Levay P. Recording database searches for systematic reviews - What is the value of adding a narrative to peer-review checklists? A case study of NICE interventional procedures guidance. Evidence Based Library and Information Practice 2011; 6: 72-87.
Decullier E, Huot L, Maisonneuve H. What time-lag for a retraction search on PubMed? BMC Research Notes 2014; 7: 395.
Dellavalle RP, Hester EJ, Heilig LF, Drake AL, Kuntzman JW, Graber M, Schilling LM. Information science. Going, going, gone: lost Internet references. Science 2003; 302: 787-788.
Dobrescu AI, Nussbaumer-Streit B, Klerings I, Wagner G, Persad E, Sommer I, Herkner H, Gartlehner G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: a systematic review. J Clin Epidemiol 2021; 137: 209-217.
Doshi P, Dickersin K, Healy D, Vedula SS, Jefferson T. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ 2013; 346: f2865.
Doust JA, Pietrzak E, Sanders S, Glasziou PP. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. J Clin Epidemiol 2005; 58: 444-449.
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991; 337: 867-872.
Edwards P, Clarke M, DiGuiseppi C, Pratap S, Roberts I, Wentz R. Identification of randomized controlled trials in systematic reviews: accuracy and reliability of screening records. Statistics in Medicine 2002; 21: 1635-1640.
Egger M, Zellweger Z, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997; 350: 326-329.
Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998; 316: 61-66.
Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. HEALTH TECHNOLOGY ASSESSMENT 2003; 7: 1-76.
Egilman AC, Wallach JD, Morten CJ, Lurie P, Ross JS. Systematic overview of Freedom of Information Act requests to the Department of Health and Human Services from 2008 to 2017. Research Integrity and Peer Review 2019; 4: 26.
Elia N, von Elm E, Chatagner A, Popping DM, Tramèr MR. How do authors of systematic reviews deal with research malpractice and misconduct in original studies? A cross-sectional analysis of systematic reviews and survey of their authors. BMJ Open 2016; 6: e010442.
Elsevier. Embase Classic Fact Sheet; 2022. https://www.elsevier.com/solutions/embase-biomedical-research/learn-and-support .
Elsevier. Embase 2022-23 Developments;. 2023a. https://www.elsevier.com/solutions/embase-biomedical-research/learn-and-support .
Elsevier. Embase content coverage; 2023b. https://www.elsevier.com/solutions/embase-biomedical-research/coverage-and-content .
Elsevier. Embase resources; 2023c. https://www.elsevier.com/solutions/embase-biomedical-research/learn-and-support .
EUnetHTA JA3WP6B2-2 Authoring Team. Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness. Diemen (The Netherlands): EUnetHTA; 2019. https://www.eunethta.eu/ .
European Food Safety Authority. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010; 8: 1637.
Ewald H, Klerings I, Wagner G, Heise TL, Stratil JM, Lhachimi SK, Hemkens LG, Gartlehner G, Armijo-Olivo S, Nussbaumer-Streit B. Searching two or more databases decreased the risk of missing relevant studies: a metaresearch study. J Clin Epidemiol 2022; 149: 154-164.
Eysenbach G, Trudel M. Going, going, still there: using the WebCite service to permanently archive cited web pages. Journal of Medical Internet Research 2005; 7: e60.
Fain KM, Rajakannan T, Tse T, Williams RJ, Zarin DA. Results reporting for trials with the same sponsor, drug, and condition in ClinicalTrials.gov and peer-reviewed publications. JAMA Internal Medicine 2018; 178: 990-992.
Fanelli D, Wong J, Moher D. What difference might retractions make? An estimate of the potential epistemic cost of retractions on meta-analyses. Accountability in Research 2022; 29: 442-459.
Ferrante di Ruffano L, Davenport C, Eisinga A, Hyde C, Deeks JJ. A capture-recapture analysis demonstrated that randomized controlled trials evaluating the impact of diagnostic tests on patient outcomes are rare. J Clin Epidemiol 2012; 65: 282-287.
Folb BL, Klem ML, Youk AO, Dahm JJ, He M, Ketchum AM, Wessel CB, Hartman LM. Continuing education for systematic reviews: a prospective longitudinal assessment of a workshop for librarians. Journal of the Medical Library Association 2020; 108: 36-46.
Franco JVA, Garrote VL, Escobar Liquitay CM, Vietto V. Identification of problems in search strategies in Cochrane Reviews. Research Synthesis Methods 2018; 9: 408-416.
Frandsen TF, Eriksen MB, Hammer DMG, Christensen JB. PubMed coverage varied across specialties and over time: a large-scale study of included studies in Cochrane reviews. J Clin Epidemiol 2019a; 112: 59-66.
Frandsen TF, Gildberg FA, Tingleff EB. Searching for qualitative health research required several databases and alternative search strategies: a study of coverage in bibliographic databases. J Clin Epidemiol 2019b; 114: 118-124.
Frandsen TF, Bruun Nielsen MF, Lindhardt CL, Eriksen MB. Using the full PICO model as a search tool for systematic reviews resulted in lower recall for some PICO elements. J Clin Epidemiol 2020; 127: 69-75.
Fraser N, Brierley L, Dey G, Polka JK, Palfy M, Nanni F, Coates JA. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biology 2021; 19: e3000959.
Furuya-Kanamori L, Lin L, Kostoulas P, Clark J, Xu C. Limits in the search date for rapid reviews of diagnostic test accuracy studies. Research Synthesis Methods 2023; 14: 173-179.
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, Affengruber L, Stevens A. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 2021; 130: 13-22.
Gartlehner G, Affengruber L, Titscher V, Noel-Storr A, Dooley G, Ballarini N, Konig F. Single-reviewer abstract screening missed 13 percent of relevant studies: a crowd-based, randomized controlled trial. J Clin Epidemiol 2020; 121: 20-28.
Gaudino M, Robinson NB, Audisio K, Rahouma M, Benedetto U, Kurlansky P, Fremes SE. Trends and characteristics of retracted articles in the biomedical literature, 1971 to 2020. JAMA Internal Medicine 2021; 181: 1118-1121.
Ghezzi-Kopel K, Ault J, Chimwaza G, Diekmann F, Eldermire E, Gathoni N, Kelly J, Kinengyere AA, Kocher M, Lwoga ET, Page J, Young S, Porciello J. Making the case for librarian expertise to support evidence synthesis for the sustainable development goals. Research Synthesis Methods 2022; 13: 77-87.
Gianola S, Jesus TS, Bargeri S, Castellini G. Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic. PLoS One 2020; 15: e0240123.
Glanville J, Lefebvre C, Manson P, Robinson S, Brbre I, Woods L, (editors). ISSG Search Filter Resource [Internet]. York (UK): The InterTASC Information Specialists' Sub-Group 2006 updated [May 2023;Cited 20 July 2023]. https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/home .
Glanville J, Foxlee R, Wisniewski S, Noel-Storr A, Edwards M, Dooley G. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Information and Libraries Journal 2019; 36: 264-277.
Glanville JM, Duffy S, McCool R, Varley D. Searching ClinicalTrials.gov and the International Clinical Trials Registry Platform to inform systematic reviews: what are the optimal search approaches? Journal of the Medical Library Association 2014; 102: 177-183.
Godard-Sebillotte C, Le Berre M, Karunananthan S, Hong QN, Vedel I. A digital media strategy to obtain unpublished data for a systematic review yields a very high author response rate. J Clin Epidemiol 2018; 104: 141-143.
Goldacre B, Lane S, Mahtani KR, Heneghan C, Onakpoya I, Bushfield I, Smeeth L. Pharmaceutical companies' policies on access to trial data, results, and methods: audit study. BMJ 2017; 358: j3334.
Grabenstein D. The state of global clinical trial disclosure: what noncompliance penalties are in place, and how they are enforced [White paper]. 2023. https://pharmaintelligence.informa.com/en-US/resources/product-content/the-state-of-clinical-trial-disclosure .
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005; 331: 1064-1065.
Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS One 2015; 10: e0138237.
Halfpenny NJ, Quigley JM, Thompson JC, Scott DA. Value and usability of unpublished data sources for systematic reviews and network meta-analyses. Evidence-based Medicine 2016; 21: 208-213.
Halladay CW, Trikalinos TA, Schmid IT, Schmid CH, Dahabreh IJ. Using data sources beyond PubMed has a modest impact on the results of systematic reviews of therapeutic interventions. J Clin Epidemiol 2015; 68: 1076-1084.
Hameed I, Demetres M, Tam DY, Rahouma M, Khan FM, Wright DN, Mages K, DeRosa AP, Baltich Nelson B, Pain K, Delgado D, Girardi LN, Fremes SE, Gaudino M. An assessment of the quality of current clinical meta-analyses. BMC Medical Research Methodology 2020; 20: 105.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. The contribution of databases to the results of systematic reviews: a cross-sectional study. BMC Medical Research Methodology 2016; 16: 127.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Medical Research Methodology 2017; 17: 64.
Hausner E. Problems encountered with ICTRP Search Portal. Comment on: van Enst WA, Scholten RJPM, Hooft L. Identification of additional trials in prospective trial registers for Cochrane Systematic Reviews. PLoS One 2014; 7: e42812.
Hetherington J, Dickersin K, Chalmers I, Meinert CL. Retrospective and prospective identification of unpublished controlled trials: lessons from a survey of obstetricians and pediatricians. Pediatrics 1989; 84: 374-380.
Hinde S, Spackman E. Bidirectional citation searching to completion: an exploration of literature searching methods. Pharmacoeconomics 2015; 33: 5-11.
Horton R. Medical editors trial amnesty. Lancet 1997; 350: 756.
Hoy MB. Rise of the Rxivs: How preprint servers are changing the publishing process. Medical Reference Services Quarterly 2020; 39: 84-89.
Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annual Symposium Proceedings/AMIA Symposium 2006: 359-363.
Hunter KE, Webster AC, Page MJ, Willson M, McDonald S, Berber S, Skeers P, Tan-Koay AG, Parkhill A, Seidler AL. Searching clinical trials registers: guide for systematic reviewers. BMJ 2022; 377: e068791.
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Internal Medicine 2016; 176: 1826-1833.
Iansavichene AE, Sampson M, McGowan J, Ajiferuke IS. Should systematic reviewers search for randomized, controlled trials published as letters? Ann Intern Med 2008; 148: 714-715.
Institute for Quality and Efficiency in Health Care. IQWiG general methods: Version 6.1. Cologne; 2022. https://www.iqwig.de/methoden/general-methods_version-6-1.pdf .
Institute of Medicine. Finding what works in health care: Standards for systematic reviews. Washington, DC: The National Academies Press; 2011. http://books.nap.edu/openbook.php?record_id=13059 .
Irvin E, Hayden J. Developing and testing an optimal search strategy for identifying studies of prognosis [Poster] 14th Cochrane Colloquium, Dublin, Ireland; October 23-26 2006. https://abstracts.cochrane.org/2006-dublin/developing-and-testing-optimal-search-strategy-identifying-studies-prognosis .
Isojarvi J, Wood H, Lefebvre C, Glanville J. Challenges of identifying unpublished data from clinical trials: getting the best out of clinical trials registers and other novel sources. Research Synthesis Methods 2018: 561-578.
Isojarvi J, Glanville J. Evidence-based searching for health technology assessment: keeping up to date with SuRe Info. International Journal of Technology Assessment in Health Care 2021; 37: e51.
Jefferson T, Doshi P, Boutron I, Golder S, Heneghan C, Hodkinson A, Jones M, Lefebvre C, Stewart LA. When to include clinical study reports and regulatory documents in systematic reviews. BMJ Evidence-Based Medicine 2018; 23: 210-217.
Jia Y, Huang D, Wen J, Wang Y, Rosman L, Chen Q, Robinson KA, Gagnier JJ, Ehrhardt S, Celentano DD. Assessment of language and indexing biases among chinese-sponsored randomized clinical trials. JAMA Network Open 2020; 3: e205894.
Jiao S, Tsutani K, Haga N. Review of Cochrane reviews on acupuncture: how Chinese resources contribute to Cochrane reviews. Journal of Alternative and Complementary Medicine 2013; 19: 613-621.
Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. International journal of epidemiology 2002; 31: 115-123.
Kastner M, Straus SE, McKibbon KA, Goldsmith CH. The capture-mark-recapture technique can be used as a stopping rule when searching in systematic reviews. J Clin Epidemiol 2009; 62: 149-157.
Kirkham JJ, Penfold NC, Murphy F, Boutron I, Ioannidis JP, Polka J, Moher D. Systematic examination of preprint platforms for use in the medical and biomedical sciences setting. BMJ Open 2020; 10: e041849.
Knelangen M, Hausner E, Metzendorf M-I, Sturtz S, Waffenschmidt S. Trial registry searches for randomized controlled trials of new drugs required registry-specific adaptation to achieve adequate sensitivity. J Clin Epidemiol 2018; 94: 69-75.
Koffel JB. Use of recommended search strategies in systematic reviews and the impact of librarian involvement: a cross-sectional survey of recent authors. PLoS One 2015; 10: e0125931.
Köhler M, Haag S, Biester K, Brockhaus AC, McGauran N, Grouven U, Kölsch H, Seay U, Hörn H, Moritz G, Staeck K, Wieseler B. Information on new drugs at market entry: retrospective analysis of health technology assessment reports versus regulatory reports, journal publications, and registry reports. BMJ 2015; 350: h796.
Kreis J, Panteli D, Busse R. How health technology assessment agencies address the issue of unpublished data. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2014; 30: 34-43.
Kugley S, Wade A, Thomas J, Mahood Q, Jørgensen AMK, Hammerstrom K, Sathe N. Searching for studies: a guide to information retrieval for Campbell systematic reviews. Campbell Collaboration; 2017. Version 1.1 https://onlinelibrary.wiley.com/doi/10.4073/cmg.2016.1 .
Lampert A, Hoffmann GF, Ries M. Ten years after the International Committee of Medical Journal Editors' clinical trial registration initiative, one quarter of phase 3 pediatric epilepsy clinical trials still remain unpublished: a cross sectional analysis. PLoS One 2016; 11: e0144973.
Lane D, Dykeman J, Ferri M, Goldsmith CH, Stelfox HT. Capture-mark-recapture as a tool for estimating the number of articles available for systematic reviews in critical care medicine. Journal of Critical Care 2013; 28: 469-475.
Layton D. A critical review of search strategies used in recent systematic reviews published in selected prosthodontic and implant-related journals: Are systematic reviews actually systematic? International Journal of Prosthodontics 2017; 30: 13-21.
Lê M-L, Neilson CJ, Winkler J. [Preprint]. Benchmarking Librarian Support of Systematic Reviews in the Sciences, Humanities, and Social Sciences 2023 [11 July 2023]. Available from: https://osf.io/v7m9y/ .
Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Medicine 2008; 5: e191.
Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world-wide--the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerg Themes Epidemiol 2008; 5: 13.
Lefebvre C, Glanville J, Wieland LS, Coles B, Weightman AL. Methodological developments in searching for studies for systematic reviews: past, present and future? Systematic reviews 2013; 2: 78.
Lefebvre C, Glanville J, Beale S, Boachie C, Duffy S, Fraser C, Harbour J, McCool R, Smith L. Assessing the performance of methodological search filters to improve the efficiency of evidence information retrieval: five literature reviews and a qualitative study. HEALTH TECHNOLOGY ASSESSMENT 2017; 21: 1-148.
Lefebvre C, Duffy S. Peer review of searches for studies for health technology assessments, systematic reviews, and other evidence syntheses. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2021; 37: e64.
Levay P, Heath A, Tuvey D. Efficient searching for NICE public health guidelines: Would using fewer sources still find the evidence? Research Synthesis Methods 2022; 13: 760-789.
Li D, Kanoulas E. When to Stop Reviewing in Technology-Assisted Reviews: Sampling from an Adaptive Distribution to Estimate Residual Relevant Documents. ACM Transactions on Information Systems 2020; 38: Article 41.
Liu S, Bourgeois FT, Dunn AG. Identifying unreported links between ClinicalTrials.gov trial registrations and their published results. Research Synthesis Methods 2022; 13: 342-352.
Liu X, Anstey J, Li R, Sarabu C, Sono R, Butte AJ. Rethinking PICO in the machine learning era: ML-PICO. Applied Clinical Informatics 2021; 12: 407-416.
Lorenc T, Tyner EF, Petticrew M, Duffy S, Martineau FP, Phillips G, Lock K. Cultures of evidence across policy sectors: systematic review of qualitative evidence. European Journal of Public Health 2014; 24: 1041-1047.
Lorenzetti DL, Topfer LA, Dennett L, Clement F. Value of databases other than MEDLINE for rapid health technology assessments. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2014; 30: 173-178.
MacLean CH, Morton SC, Ofman JJ, Roth EA, Shekelle PG, Southern California Evidence-Based Practice Center. How useful are unpublished data from the Food and Drug Administration in meta-analysis? J Clin Epidemiol 2003; 56: 44-51.
Manheimer E, Anderson D. Survey of public information about ongoing clinical trials funded by industry: evaluation of completeness and accessibility. BMJ 2002; 325: 528-531.
Marshall C, Brereton P. Systematic review toolbox: a catalogue of tools to support systematic reviews. Proceedings of the 19th International Conference on Evaluation and Assessment in Software Engineering (EASE) 2015: Article no. 23.
Marshall I, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018; 9: 602-614.
Marshall IJ, Trikalinos TA, Soboczenski F, Yun HS, Kell G, Marshall R, Wallace BC. In a pilot study, automated real-time systematic review updates were feasible, accurate, and work-saving. J Clin Epidemiol 2023; 153: 26-33.
Masterson D, Martinez-Silveira MS. Aplicação do Peer Review of Electronic Search Strategies para avaliação da qualidade das estratégias de busca das revisões sistemáticas [Application of Peer Review of Electronic Search Strategies (PRESS) to assess the quality of systematic reviews search strategies]. Em Questão 2022; 28: 117865.
Matthews EJ, Edwards AG, Barker J, Bloor M, Covey J, Hood K, Pill R, Russell I, Stott N, Wilkinson C. Efficient literature searching in diffuse topics: lessons from a systematic review of research on communicating risk to patients in primary care. Health Libraries Review 1999; 16: 112-120.
McDonagh MS, Thakurta S, Peterson K. A study of the value of requesting information from drug manufacturers for systematic reviews; 9 years of experience from the drug effectiveness review project. Systematic reviews 2018; 7: 172.
McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E) Ottawa: CADTH; 2016a. https://www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_2016.pdf .
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016b; 75: 40-46.
Meert D, Torabi N, Costella J. Impact of librarians on reporting of the literature searching component of pediatric systematic reviews. Journal of the Medical Library Association 2016; 104: 267-277.
Metzendorf M-I. Why medical information specialists should routinely form part of teams producing high quality systematic reviews – a Cochrane perspective. Journal of the European Association for Health Information and Libraries 2016; 12: 6-9.
Metzendorf MI, Featherstone RM. Frandsen et al. provide insights to PubMed coverage across all Cochrane Review Groups, but more in-depth analyses would further help inform database choices. J Clin Epidemiol 2019; 116: 135-136.
Meursinge Reynders R, Ladu L, Di Girolamo N. Contacting of authors modified crucial outcomes of systematic reviews but was poorly reported, not systematic, and produced conflicting results. J Clin Epidemiol 2019; 115: 64-76.
Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. HEALTH TECHNOLOGY ASSESSMENT 2003; 7: 1-90.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2012; 28: 138-144.
Muller AE, Ames H, Himmels J, Jardim PJ, Nguyen L, Rose C, Van de Velde S. Implementation of machine learning in evidence syntheses in the Cluster for Reviews and Health Technology Assessments: Final report 2020-2021. Oslo; 2021.
Mullins MM, DeLuca JB, Crepaz N, Lyles CM. Reporting quality of search methods in systematic reviews of HIV behavioral interventions (2000–2010): are the searches clearly explained, systematic and reproducible? Research Synthesis Methods 2014; 5: 116-130.
National Institute for Health and Care Excellence (NICE). Identifying the evidence: literature searching and evidence submission. 2014. In: Developing NICE guidelines: the manual (Last updated: 18 January 2022) Process and Methods Guides 20 [Internet]. Available from: https://www.nice.org.uk/process/pmg20/chapter/identifying-the-evidence-literature-searching-and-evidence-submission .
Neilson CJ. Adoption of peer review of literature search strategies in knowledge synthesis from 2009 to 2018: An overview. Health Information & Libraries Journal 2021; 38: 160-171.
Nelson JT, Tse T, Puplampu-Dove Y, Golfinopoulos E, Zarin DA. Comparison of availability of trial results in ClinicalTrials.gov and PubMed by data source and funder type. JAMA 2023; 329: 1404-1406.
Niederstadt C, Droste S. Reporting and presenting information retrieval processes: the need for optimizing common practice in health technology assessment. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2010; 26: 450-457.
Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, Wisniewski S, McDonald S, Murano M, Glanville J, Foxlee R, Beecher D, Ware J, Thomas J. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. J Clin Epidemiol 2021a; 133: 130-139.
Noel-Storr A, Dooley G, Affengruber L, Gartlehner G. Citation screening using crowdsourcing and machine learning produced accurate results: Evaluation of Cochrane's modified Screen4Me service. J Clin Epidemiol 2021b; 130: 23-31.
Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, Featherstone R, Foxlee R. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: A retrospective analysis. J Clin Epidemiol 2020; 127: 142-150.
Nussbaumer-Streit B, Klerings I, Wagner G, Heise TL, Dobrescu AI, Armijo-Olivo S, Stratil JM, Persad E, Lhachimi SK, Van Noord MG, Mittermayr T, Zeeb H, Hemkens L, Gartlehner G. Abbreviated literature searches were viable alternatives to comprehensive searches: a meta-epidemiological study. J Clin Epidemiol 2018; 102: 1-11.
Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, Kamel C, Affengruber L, King VJ, Gartlehner G. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol 2020; 118: 42-54.
O'Mara-Eves A, Thomas J, McNaught J, Miwa M, Ananiadou S. Using text mining for study identification in systematic reviews: a systematic review of current approaches. Systematic Reviews 2015; 4: 5. (Erratum in: Systematic Reviews 2015; 2014: 2059).
Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, Covid-NMA Consortium. Changes in evidence for studies assessing interventions for COVID-19 reported in preprints: meta-research study. BMC Medicine 2020; 18: 402.
Oxman AD, Guyatt GH. The science of reviewing research. Annals of the New York Academy of Sciences 1993; 703: 125-133; discussion 133-134.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021a; 372: n160.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021b; 372: n71.
Paladin I, Pranić SM. Reporting of the safety from allergic rhinitis trials registered on ClinicalTrials.gov and in publications: An observational study. BMC Medical Research Methodology 2022; 22: 262.
Pansieri C, Pandolfini C, Bonati M. Clinical trial registries: more international, converging efforts are needed. Trials 2017; 18: 86.
Petticrew M, Roberts H. Systematic Reviews in the Social Sciences: a Practical Guide. Oxford (UK): Blackwell; 2006.
Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol 2005; 58: 769-776.
Pieper D, Puljak L. Language restrictions in systematic reviews should not be imposed in the search strategy but in the eligibility criteria if necessary. J Clin Epidemiol 2021; 132: 146-147.
Pilkington K, Boshnakova A, Clarke M, Richardson J. "No language restrictions" in database searches: what does this really mean? Journal of Alternative and Complementary Medicine 2005; 11: 205-207.
Price C, Saragossi J, Tran C, Lyon J. [Preprint]. Evaluating the consistency and quality of search strategies and methodology in Cochrane Urology Group Systematic Reviews2022 15 August 2023. Available from: https://osf.io/28u6z .
Rader T, Mann M, Stansfield C, Cooper C, Sampson M. Methods for documenting systematic review searches: a discussion of common issues. Research Synthesis Methods 2014; 5: 98-115.
Ramirez D, Foster MJ, Kogut A, Xiao D. Adherence to systematic review standards: Impact of librarian involvement in Campbell Collaboration's education reviews. The Journal of Academic Librarianship 2022; 48: 102567.
Relevo R, Paynter R. Peer Review of Search Strategies. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. https://www.ncbi.nlm.nih.gov/books/NBK98353/ .
Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ. Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol 2015; 68: 617-626.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S. Group. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Systematic reviews 2021; 10: 39.
Rethlefsen ML, Page MJ. PRISMA 2020 and PRISMA-S: common questions on tracking records and the flow diagram. Journal of the Medical Library Association 2022; 110: 253-257.
Reveiz L, Cardona AF, Ospina EG, de Agular S. An e-mail survey identified unpublished studies for systematic reviews. J Clin Epidemiol 2006; 59: 755-758.
Rice DB, Kloda LA, Levis B, Qi B, Kingsland E, Thombs BD. Are MEDLINE searches sufficient for systematic reviews and meta-analyses of the diagnostic accuracy of depression screening tools? A review of meta-analyses. Journal of Psychosomatic Research 2016; 87: 7-13.
Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP Journal Club 1995; 123: A12-13.
Ross-White A. An environmental scan of librarian involvement in systematic reviews at Queen's University: 2020 update. Journal of the Canadian Health Libraries Association / Journal de l'Association des bibliothèques de la santé du Canada 2021; 42: 110-117.
Roundtree AK, Kallen MA, Lopez-Olivo MA, Kimmel B, Skidmore B, Ortiz Z, Cox V, Suarez-Almazor ME. Poor reporting of search strategy and conflict of interest in over 250 narrative and systematic reviews of two biologic agents in arthritis: a systematic review. J Clin Epidemiol 2009; 62: 128-137.
Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE 2003; 19: 591-603.
Royle P, Waugh N. Should systematic reviews include searches for published errata? Health Information & Libraries Journal 2004; 21: 14-20.
Salvador-Olivan JA, Marco-Cuenca G, Arquero-Aviles R. Errors in search strategies used in systematic reviews and their effects on information retrieval. Journal of the Medical Library Association 2019; 107: 210-221.
Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, St John PD, Viola R, Raina P. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol 2003; 56: 943-955.
Sampson M, McGowan J. Errors in search strategies were identified by type and frequency. J Clin Epidemiol 2006; 59: 1057-1063.
Sampson M, Zhang L, Morrison A, Barrowman NJ, Clifford TJ, Platt RW, Klassen TP, Moher D. An alternative to the hand searching gold standard: Validating methodological search filters using relative recall. BMC Medical Research Methodology 2006; 6: 33.
Sampson M, McGowan J, Tetzlaff J, Cogo E, Moher D. No consensus exists on search reporting methods for systematic reviews. J Clin Epidemiol 2008; 61: 748-754.
Sampson M, McGowan J. Inquisitio validus Index Medicus: A simple method of validating MEDLINE systematic review searches. Research Synthesis Methods 2011; 2: 103-109.
Sampson M, de Bruijn B, Urquhart C, Shojania K. Complementary approaches to searching MEDLINE may be sufficient for updating systematic reviews. J Clin Epidemiol 2016; 78: 108-115.
Savoie I, Helmer D, Green CJ, Kazanjian A. Beyond Medline: reducing bias through extended systematic review search. Int J Technol Assess Health Care 2003; 19: 168-178.
Schellinger J, Sewell K, Bloss JE, Ebron T, Forbes C. The effect of librarian involvement on the quality of systematic reviews in dental medicine. PLoS One 2021; 16: e0256833.
Scherer RW, Ugarte-Gil C, Schmucker C, Meerpohl JJ. Authors report lack of time as main reason for unpublished research presented at biomedical conferences: a systematic review. J Clin Epidemiol 2015; 68: 803-810.
Schroll JB, Bero L, Gøtzsche PC. Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ 2013; 346: f2231.
Shemilt I, Simon A, Hollands GJ, Marteau TM, Ogilvie D, O'Mara-Eves A, Kelly MP, Thomas J. Pinpointing needles in giant haystacks: use of text mining to reduce impractical screening workload in extremely large scoping reviews. Research Synthesis Methods 2014; 5: 31-49.
Shemilt I, Khan N, Park S, Thomas J. Use of cost-effectiveness analysis to compare the efficiency of study identification methods in systematic reviews. Systematic reviews 2016; 5: 140.
Shemilt I, Arno A, Thomas J, Lorenc T, Khouja C, Raine G, Sutcliffe K, Preethy DS, Kwan I, Wright K, Sowden A. Cost-effectiveness of Microsoft Academic Graph with machine learning for automated study identification in a living map of coronavirus disease 2019 (COVID-19) research [version 1; peer review: 2 approved with reservations]2021; (6):[210 p.].
Shemilt I, Noel-Storr A, Thomas J, Featherstone R, Mavergames C. Machine learning reduced workload for the Cochrane COVID-19 Study Register: development and evaluation of the Cochrane COVID-19 Study Classifier. Syst Rev 2022; 11: 15.
Smalheiser NR, Holt AW. A web-based tool for automatically linking clinical trials to their publications. Journal of the American Medical Informatics Association 2022; 29: 822-830.
Sneyd A, Stevenson M. Modelling stopping criteria for search results using Poisson processes. 2019. In: Proceedings of the 2019 Conference on Empirical Methods in Natural Language Processing and the 9th International Joint Conference on Natural Language Processing (EMNLP-IJCNLP) [Internet]. [3484–3489]. Available from: https://aclanthology.org/D19-1351 .
Spencer AJ, Eldredge JD. Roles for librarians in systematic reviews: a scoping review. Journal of the Medical Library Association 2018; 106: 46-56.
Spijker R, Dinnes J, Glanville J, Eisinga A. Chapter 6: Searching for and selecting studies. 2023. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 20 (updated July 2023) [Internet]. Cochrane. Available from: https://training.cochrane.org/handbook-diagnostic-test-accuracy/current .
Spoor P, Airey M, Bennett C, Greensill J, Williams R. Use of the capture-recapture technique to evaluate the completeness of systematic literature searches. BMJ 1996; 313: 342-343.
Spry C, Mierzwinski-Urban M, Rabb D. Peer review of literature search strategies: does it make a difference? 21st Cochrane Colloquium; 2013; Quebec City, Canada. https://abstracts.cochrane.org/2013-quebec-city/peer-review-literature-search-strategies-does-it-make-difference .
Spry C, Mierzwinski-Urban M. The impact of the peer review of literature search strategies in support of rapid review reports. Research Synthesis Methods 2018; 9: 521-526.
Stansfield C, Stokes G, Thomas J. Applying machine classifiers to update searches: Analysis from two case studies. Research Synthesis Methods 2022; 13: 121-133.
Stevinson C, Lawlor DA. Searching multiple databases for systematic reviews: added value or diminishing returns? Complementary Therapies in Medicine 2004; 12: 228-232.
Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Controlled Clinical Trials 2000; 21: 476-487.
Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C, Glasziou P, Shemilt I, Synnot A, Turner T, Elliott J, Living Systematic Review N. Living systematic reviews: 2. Combining human and machine effort. J Clin Epidemiol 2017; 91: 31-37.
Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, Marshall IJ. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. J Clin Epidemiol 2021; 133: 140-151.
Thomas J. Evidence surveillance during the COVID-19 pandemic using automation and crowdsourcing. Journal of EAHIL 2021; 17: 5-6.
Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ 1997; 315: 635-640.
Tsafnat G, Glasziou P, Choong MK, Dunn A, Galgani F, Coiera E. Systematic review automation technologies. Systematic reviews 2014; 3: 74.
US National Library of Medicine. MEDLINE, PubMed, and PMC (PubMed Central): How are they different? (Last Reviewed: Oct 25, 2022). 2018. https://www.nlm.nih.gov/bsd/difference.html .
US National Library of Medicine. MEDLINE: Overview (Last reviewed: May 2, 2022). 2021. https://www.nlm.nih.gov/medline/medline_overview.html .
US National Library of Medicine. PubMed Overview (Last update: August 15, 2023). 2023. https://pubmed.ncbi.nlm.nih.gov/about/ .
Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open 2015; 5: e008932.
von Elm E, Poglia G, Walder B, Tramèr MR. Different patterns of duplicate publication: an analysis of articles used in systematic reviews. JAMA 2004; 291: 974-980.
Waffenschmidt S, Knelangen M, Sieben W, Buhn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Medical Research Methodology 2019; 19: 132.
Wallace S, Daly C, Campbell M, Cody J, Grant A, Vale L, Donaldson C, Khan I, Lawrence P, MacLeod A. After MEDLINE? Dividend from other potential sources of randomised controlled trials. Second International Conference Scientific Basis of Health Services & Fifth Annual Cochrane Colloquium; 1997; Amsterdam, The Netherlands. https://abstracts.cochrane.org/1997-amsterdam/after-medline-dividend-other-potential-sources-randomised-controlled-trials .
Weber EJ, Callaham ML, Wears RL, Barton C, Young G. Unpublished research from a medical specialty meeting: why investigators fail to publish. JAMA 1998; 280: 257-259.
Wieseler B, Kerekes MF, Vervoelgyi V, McGauran N, Kaiser T. Impact of document type on reporting quality of clinical drug trials: a comparison of registry reports, clinical study reports, and journal publications. BMJ 2012; 344: d8141.
Witkowski MA, Aldhouse N. Transparency and reproducibility of supplementary search methods in NICE single technology appraisal manufacturer submissions. Value in Health 2015; 18: A721-722.
Wolfe N, Gotzsche PC, Bero L. Strategies for obtaining unpublished drug trial data: a qualitative interview study. Systematic reviews 2013; 2: 31.
Woods D, Trewheellar K. Medline and Embase complement each other in literature searches. BMJ 1998; 316: 1166.
Wright K, McDaid C. Reporting of article retractions in bibliographic databases and online journals. Journal of the Medical Library Association 2011; 99: 164-167.
Wu XY, Tang JL, Mao C, Yuan JQ, Qin Y, Chung VC. Systematic reviews and meta-analyses of traditional Chinese medicine must search Chinese databases to reduce language bias. Evidence-Based Complementary and Alternative Medicine 2013; 2013: 812179.
Yoshii A, Plaut DA, McGraw KA, Anderson MJ, Wellik KE. Analysis of the reporting of search strategies in Cochrane systematic reviews. Journal of the Medical Library Association 2009; 97: 21-29.
Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews 2011; 11: MR000027.
Zarin DA, Fain KM, Dobbins HD, Tse T, Williams RJ. 10-Year update on study results submitted to ClinicalTrials.gov. New England Journal of Medicine 2019; 381: 1966-1974.
Zeraatkar D, Pitre T, Leung G, Cusano E, Agarwal A, Khalid F, Escamilla Z, Cooper MA, Ghadimi M, Wang Y, Verdugo-Paiva F, Rada G, Kum E, Qasim A, Bartoszko JJ, Siemieniuk RAC, Patel C, Guyatt G, Brignardello-Petersen R. Consistency of covid-19 trial preprints with published reports and impact for decision making: retrospective review. BMJ Medicine 2022; 1: e000309.
Zwakman M, Verberne LM, Kars MC, Hooft L, van Delden JJM, Spijker R. Introducing PALETTE: an iterative method for conducting a literature search for a review in palliative care. BMC Palliative Care 2018; 17: 82.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
Our site uses cookies to improve your experience. You can find out more about our use of cookies in About Cookies, including instructions on how to turn off cookies if you wish to do so. By continuing to browse this site you agree to us using cookies as described in About Cookies .
The Cochrane Library
Trusted evidence. Informed decisions. Better health.
Scolaris Search Portlet Scolaris Search Portlet
Scolaris language selector scolaris language selector.
| Select your preferred language for Cochrane Reviews and other content. Sections without translation will be in English. | ||
| Select your preferred language for the Cochrane Library website. | ||
Scolaris Content Language Banner Portlet Scolaris Content Language Banner Portlet
Scolaris-toc-portlet scolaris-toc-portlet, cochrane database of systematic reviews: all issues, browse issues.
- 2024 - Current issue
2021, Issue 2
Withdrawn protocols.

Intervention
Qualitative.
The clinical implications of SARS‐CoV‐2 infection are highly variable. Some people with SARS‐CoV‐2 infection remain asymptomatic, whilst the infection can cause mild to moderate COVID‐19 and COVID‐19 pneumonia in others. This can lead to some people requiring intensive care support and, in some case…
Xpert MTB/RIF and Xpert MTB/RIF Ultra (Xpert Ultra) are World Health Organization (WHO)‐recommended rapid tests that simultaneously detect tuberculosis and rifampicin resistance in people with signs and symptoms of tuberculosis. This review builds on our recent extensive Cochrane Review of Xpert MTB…
Dementia is a syndrome that comprises many differing pathologies, including Alzheimer's disease dementia (ADD), vascular dementia (VaD) and frontotemporal dementia (FTD). People may benefit from knowing the type of dementia they live with, as this could inform prognosis and may allow for tailored tr…
To support patient‐centred care, healthcare organisations increasingly offer patients access to data stored in the institutional electronic health record (EHR). Objectives Primary objective 1. To assess the effects of providing adult patients with access to electronic health records (EHRs) alo…
Bacterial folliculitis and boils are globally prevalent bacterial infections involving inflammation of the hair follicle and the perifollicular tissue. Some folliculitis may resolve spontaneously, but others may progress to boils without treatment. Boils, also known as furuncles, involve adjacent ti…
Belimumab, the first biologic approved for the treatment of systemic lupus erythematosus (SLE), has been shown to reduce autoantibody levels in people with SLE and help control disease activity. Objectives To assess the benefits and harms of belimumab (alone or in combination) in systematic lupu…
Breast cancer is one of the most common cancers among women. Surgical removal of the cancer is the mainstay of treatment; however, tumour handling during surgery can cause microscopic dissemination of tumour cells and disease recurrence. The body's hormonal response to surgery (stress response) and …
Diet plays a major role in the aetiology of cardiovascular disease (CVD) and as a modifiable risk factor is the focus of many prevention strategies. Recently vegan diets have gained popularity and there is a need to synthesise existing clinical trial evidence for their potential in CVD prevention. …
The most frequent indications for tooth extractions, generally performed by general dental practitioners, are dental caries and periodontal infections. Systemic antibiotics may be prescribed to patients undergoing extractions to prevent complications due to infection. This is an update of a review f…
- Conclusions changed
Fractures of the patella (kneecap) account for around 1% of all human fractures. The treatment of these fractures can be surgical or conservative (such as immobilisation with a cast or brace). There are many different surgical and conservative interventions for treating fractures of the patella in a…
Pressure ulcers (PUs) are injuries to the skin and underlying tissues that occur most commonly over bony prominences, such as the hips and heels as a result of pressure and shear forces. PUs cause pain, discomfort, longer hospital stays, and decreased quality of life. They are also very costly to tr…
Increased physical activity has been recommended as an important lifestyle modification for the prevention and control of hypertension. Walking is a low‐cost form of physical activity and one which most people can do. Studies testing the effect of walking on blood pressure have revealed inconsistent…
Coronary heart disease is the leading cause of mortality worldwide with approximately 7.4 million deaths each year. People with established coronary heart disease have a high risk of subsequent cardiovascular events including myocardial infarction, stroke, and cardiovascular death. Antibiotics might…
Heavy menstrual bleeding (HMB) is common in otherwise healthy women of reproductive age, and can affect physical health and quality of life. Surgery is usually a second‐line treatment of HMB. Endometrial resection/ablation (EA/ER) to remove or ablate the endometrium is less invasive than hysterectom…
In‐hospital growth of preterm infants remains a challenge in clinical practice. The high nutrient demands of preterm infants often lead to growth faltering. For preterm infants who cannot be fed maternal or donor breast milk or may require supplementation, preterm formulas with fat in the form of me…
Vascular cognitive impairment (VCI) describes a broad spectrum of cognitive impairments caused by cerebrovascular disease, ranging from mild cognitive impairment to dementia. There are currently no pharmacological treatments recommended for improving either cognition or function in people with VCI. …
The strain on public resources to meet the healthcare needs of populations through publicly‐provided health insurance programmes is increasing and many governments turn to private health insurance (PHI) to ease the pressure on government budgets. With the goal of improving access to basic health car…
Dental professionals are well placed to help their patients stop using tobacco products. Large proportions of the population visit the dentist regularly. In addition, the adverse effects of tobacco use on oral health provide a context that dental professionals can use to motivate a quit attempt. O…
Transient tachypnea of the newborn (TTN) is caused by delayed clearance of lung fluid at birth. TTN typically appears within the first two hours of life in term and late preterm neonates and is characterized by tachypnea and signs of respiratory distress. Although it is usually a self‐limited condit…
Extracranial carotid artery stenosis is the major cause of stroke, which can lead to disability and mortality. Carotid endarterectomy (CEA) with carotid patch angioplasty is the most popular technique for reducing the risk of stroke. Patch material may be made from an autologous vein, bovine pericar…
Infantile nystagmus syndrome (INS) is a type of eye movement disorder that can negatively impact vision. Currently, INS cannot be cured, but its effects can potentially be treated pharmacologically, optically, or surgically. This review focuses on the surgical interventions for INS. Despite the rang…
The prevalence of type 2 diabetes is increased in individuals with mental disorders. Much of the burden of disease falls on the populations of low‐ and middle‐income countries (LMICs). Objectives To assess the effects of pharmacological, behaviour change, and organisational interventions versus …
People with chronic kidney disease (CKD) requiring dialysis are at a particularly high risk of cardiovascular death and morbidity. Several clinical studies suggested that aldosterone antagonists would be a promising treatment option for people undergoing dialysis. However, the clinical efficacy and …
Symptoms of autism spectrum disorder (ASD) have been associated, in part, with the dysfunction of N‐methyl‐D‐aspartate (NMDA) glutamate receptors at excitatory synapses and glutamate abnormalities. Medications related to glutamatergic neurotransmission, such as D‐cycloserine ‐ which is a partial ago…
Depression is one of the most common morbidities of the postnatal period. It has been associated with adverse outcomes for women, children, the wider family and society as a whole. Treatment is with psychosocial interventions or antidepressant medication, or both. The aim of this review is to evalua…
The coronavirus disease 2019 (COVID‐19) pandemic has resulted in substantial mortality. Some specialists proposed chloroquine (CQ) and hydroxychloroquine (HCQ) for treating or preventing the disease. The efficacy and safety of these drugs have been assessed in randomized controlled trials. Objecti…
Lower urinary tract symptoms (LUTS) due to benign prostatic obstruction (BPO) represent one of the most common clinical complaints in men. Alpha‐blockers are widely used as first‐line therapy for men with LUTS secondary to BPO, but up to one third of men report no improvement in their LUTS after tak…
The leading causes of mortality globally in children younger than five years of age (under‐fives), and particularly in the regions of sub‐Saharan Africa (SSA) and Southern Asia, in 2018 were infectious diseases, including pneumonia (15%), diarrhoea (8%), malaria (5%) and newborn sepsis (7%) (UNICEF …
Functional Abdominal Pain Disorders (FAPDs) present a considerable burden to paediatric patients, impacting quality of life, school attendance and causing higher rates of anxiety and depression disorders. There are no international guidelines for the management of this condition. A previous Cochrane…
Communication is a common element in all medical consultations, affecting a range of outcomes for doctors and patients. The increasing demand for medical students to be trained to communicate effectively has seen the emergence of interpersonal communication skills as core graduate competencies in me…
Chronic suppurative otitis media (CSOM), sometimes referred to as chronic otitis media (COM), is a chronic inflammation and often polymicrobial infection (involving more than one micro‐organism) of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tym…
All major guidelines for antihypertensive therapy recommend weight loss. Dietary interventions that aim to reduce body weight might therefore be a useful intervention to reduce blood pressure and adverse cardiovascular events associated with hypertension. Objectives Primary objectives To asses…
Diabetic foot ulceration (DFU) can be defined as a full‐thickness wound below the ankle and is a major complication of diabetes mellitus. Despite best practice, many wounds fail to heal, and when they do, the risk of recurrence of DFU remains high. Beliefs about personal control, or influence, on ul…
Epithelial ovarian cancer presents at an advanced stage in the majority of women. These women require surgery and chemotherapy for optimal treatment. Conventional treatment has been to perform surgery first and then give chemotherapy. However, there may be advantages to using chemotherapy before sur…
Transient tachypnea of the newborn is characterized by tachypnea and signs of respiratory distress. Transient tachypnea typically appears within the first two hours of life in term and late preterm newborns. Although transient tachypnea of the newborn is usually a self‐limited condition, it is assoc…
Eczema and food allergy are common health conditions that usually begin in early childhood and often occur together in the same people. They can be associated with an impaired skin barrier in early infancy. It is unclear whether trying to prevent or reverse an impaired skin barrier soon after birth …
Glaucoma is the leading cause of irreversible blindness. Minimally invasive surgical techniques, such as ab interno trabecular bypass surgery, have been introduced to prevent glaucoma from progressing. Objectives In light of the potential benefits for people with open‐angle glaucoma and the wi…
In vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatments conventionally consist of a fresh embryo transfer, possibly followed by one or more cryopreserved embryo transfers in subsequent cycles. An alternative option is to freeze all suitable embryos and transfer cryopreserv…
Chronic suppurative otitis media (CSOM) is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane. The predominant symptoms of CSOM are ear discharge and hearing loss. Systemic antibiotics are a com…
Commercial video games are a vastly popular form of recreational activity. Whilst concerns persist regarding possible negative effects of video games, they have been suggested to provide cognitive benefits to users. They are also frequently employed as control interventions in comparisons of more co…
Coenzyme Q10, or ubiquinone, is a non‐prescription nutritional supplement. It is a fat‐soluble molecule that acts as an electron carrier in mitochondria, and as a coenzyme for mitochondrial enzymes. Coenzyme Q10 deficiency may be associated with a multitude of diseases, including heart failure. The …
Interventions by specialist breast cancer nurses (SBCNs) aim to support women and help them cope with the impact of the disease on their quality of life. Objectives To assess the effects of individual interventions carried out by SBCNs on indicators of quality of life, anxiety, depression, and p…
Dementia is a progressive syndrome characterised by deterioration in memory, thinking and behaviour, and by impaired ability to perform daily activities. Two classes of drug ‐ cholinesterase inhibitors (donepezil, galantamine and rivastigmine) and memantine ‐ are widely licensed for dementia due to …
Interstitial lung disease (ILD) is characterised by reduced functional capacity, dyspnoea and exercise‐induced hypoxia. Pulmonary rehabilitation is often used to improve symptoms, health‐related quality of life and functional status in other chronic lung conditions. There is accumulating evidence fo…
Cervical artery dissection (CeAD) is a pathological bleed or tear, or both, in the wall of the carotid or vertebral arteries as they course through the neck, and is a leading cause of stroke in young people. Objectives To assess the effectiveness of surgical and radiological interventions versus…
Critical care telemedicine (CCT) has long been advocated for enabling access to scarce critical care expertise in geographically‐distant areas. Additional advantages of CCT include the potential for reduced variability in treatment and care through clinical decision support enabled by the analysis o…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effects (benefits and harms) of interventions to treat olfactory dysfunction in people with COVID‐19 infection. A secondary objective is to maintain the currency of the evidence, using a living syst…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effectiveness of silicone gel sheeting for the treatment of keloid scars compared with standard care or other therapies.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effectiveness and safety of the application of autologous platelet‐rich plasma (aPRP) in ART cycles.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To determine the effects of higher versus standard doses of caffeine on mortality and major neurodevelopmental disability in preterm infants with (or at risk of) apnea, and preterm infants peri‐extubation. To…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effects of urethral bulking injections for treating stress urinary incontinence in women; and summarise the principal findings of relevant economic evaluations.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: The main objective is to assess the benefits and harms of rituximab compared to placebo or another DMT for people with multiple sclerosis. Specific comparisons include: rituximab compared with placebo or other …
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effects of phototherapy regimen (e.g. NB‐UVB, BB‐UVB, PUVA, UVA1) for people with atopic eczema.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: The objective of this review is to assess the effects of various preoperative fasting regimens (i.e. the duration of fasting from liquids and solids and the type and volume of permitted intake) on perioperative c…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To compare the efficacy and tolerability of antiepileptic drugs (AEDs) taken as add‐on treatment for drug‐resistant, focal‐onset epilepsy, and to generate a clinically useful ranking of available AEDs.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To compare the effectiveness and safety of minimally invasive surgery (either robotic or laparoscopic) with laparotomy (open surgery) for women with FIGO 2014 stage III‐IV epithelial ovarian cancer, fallopian‐t…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the efficacy and safety of donor‐based FMT versus control for the treatment of rCDI in immunocompetent participants.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To compare effects of different physical interventions in adult participants with Parkinson's disease on the improvement of motor functions and QoL and the reduction of adverse events. To generate a clinically me…
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To investigate the efficacy, tolerability, acceptability, and safety of brexpiprazole as monotherapy or adjunct treatment in the acute‐ and long‐term treatment of major depression.
The protocol is out of date and does not meet the current methodological standards of Cochrane. Withdrawn by Cochrane Colorectal group, currently no plans to revise/republish.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the efficacy, safety, and tolerability of ozanimod for people with RRMS.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the efficacy and safety of electromagnetic‐guided placement of nasoenteral feeding tubes compared to endoscopic placement.
This is a protocol for a Cochrane Review (intervention). The objectives are as follows: To assess the effectiveness of healthy eating interventions delivered in ECEC settings for improving child dietary intake in children aged six years or under, relative to usual care or no intervention. Secondary …
23 February 2021. The protocol was withdrawn because it is out of date and does not meet the current methodological standards of Cochrane.
23 February 2021 The protocol was withdrawn because it is out of date and does not meet the current methodological standards of Cochrane.
The protocol is out of date and does not meet the current methological standards of Cochrane. Withdrawn by Cochrane Colorectal group, current no plans to revise/republish.
The protocol is out of date and does not meet the current methodological standards of Cochrane.
This protocol for a Cochrane review has been withdrawn. The protocol is out of date and does not meet the current methodological standards of Cochrane. Withdrawn by Cochrane Colorectal Group. Currently no plans to revise/republish the protocol.
This protocol has been withdrawn by the Cochrane Skin Managing Editor as slow progress was made by the author team on the review and they decided not to continue.
This protocol has been withdrawn by the Cochrane Skin Managing Editor as slow progress was made by the author team on the review and the title has been awarded to a new team.
Sign In Sign In
Scolaris content display scolaris content display.
Search for your institution's name below to login via Shibboleth
Previously accessed institutions
If you have a Wiley Online Library institutional username and password, enter them here.

VIDEO
COMMENTS
Browse by Topic. Browse Cochrane Reviews, Protocols and Clinical Answers. The Cochrane Library is a collection of high-quality, independent evidence to inform healthcare decision-making, including the Cochrane Database of Systematic Reviews and the CENTRAL register of controlled trials.
The Cochrane Database of Systematic Reviews ( CDSR) is the leading database for systematic reviews in health care. The CDSR includes Cochrane Reviews (systematic reviews) and protocols for Cochrane Reviews as well as editorials and supplements. The CDSR (ISSN 1469-493X) is owned and produced by Cochrane, a global, independent network of ...
Systematic reviews address a need for health decision makers to be able to access high quality, relevant, accessible and up-to-date information. Systematic reviews aim to minimize bias through the use of pre-specified research questions and methods that are documented in protocols, and by basing their findings on reliable research.
Many Cochrane Reviews measure benefits and harms by collecting data from more than one trial, and combining them to generate an average result. This aims to provide a more precise estimate of the effects of an intervention and to reduce uncertainty. Not every review in the Cochrane Database of Systematic Reviews contains a meta-analysis.
Cochrane Handbook for Systematic Reviews of Interventions. Version 6.4, 2023. Senior Editors: Julian Higgins 1, James Thomas 2. Associate Editors: Jacqueline Chandler 3, Miranda Cumpston 4,5, Tianjing Li 6, Matthew Page 4, Vivian Welch 7. Part 1: About Cochrane Reviews.
About the Handbook. The Cochrane Handbook for Systematic Reviews of Interventions is the official guide that describes in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions.All authors should consult the Handbook for guidance on the methods used in Cochrane systematic reviews.The Handbook includes guidance on the standard ...
What are systematic reviews? Watch on. Cochrane evidence, including our systematic reviews, provides a powerful tool to enhance your healthcare knowledge and decision making. This video from Cochrane Sweden explains a bit about how we create health evidence and what Cochrane does. About Cochrane.
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman ...
Cochrane Reviews are systematic reviews of primary research in human health care and health policy, and are internationally recognised as the highest standard in evidence-based health care. They investigate the effects of interventions for prevention, treatment, and rehabilitation. They also assess the accuracy of a diagnostic test for a given ...
The Cochrane Library is an electronic collection of databases published on the internet and also available on CD-Rom. It is updated quarterly in an effort to add to and keep the information current. The Library is made up of a number of parts. The Cochrane Database of Systematic Reviews (CDSR) contains the published Cochrane reviews and protocols.
The approach described is the revised guidance based on our review of current literature relevant to LSRs and consultation with a range of stakeholders, including the LSR Network, which includes members within Cochrane and beyond. ... First two living systematic reviews now live on Cochrane Library! (8 September 2017) LSRs: On the road with ...
The new edition of the Handbook is divided into four parts. The first section (available only online) addresses issues specific to working with Cochrane. The second describes the core methods applicable to systematic reviews of interventions, from framing the question through to interpreting the results. The third and fourth parts address ...
Cochrane Database of Systematic Reviews: all issues. Jessica A Schults, Tricia Kleidon, Karina Charles, Emily Rebecca Young, Amanda J Ullman. Isabelle Ethier, Ashik Hayat, Juan Pei, Carmel M Hawley, David W Johnson, Ross S Francis, Germaine Wong, Jonathan C Craig, Andrea K Viecelli, Yeoungjee Cho, Htay Htay, Samantha Ng, Saskia Leibowitz.
Cochrane systematic reviews provide reliable, evidence-based information on health issues. A systematic review is the result of a rigorous scientific process consisting of several well-defined steps, including a systematic literature search, an evaluation of the quality of each included study and a synthesis, quantified or narrative, of the ...
Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. ... or relevant literature and terminology understood by decision makers; where outcomes are likely to be inconsistently labelled and described, listing examples may convey the scope of the domain;
The new focused review format uses a slightly different structure from the previous review format. In the focused review format:the main article now includes only the main content of the review, with everything else moved to supplementary materials;in-text citations are now numbers in square brackets;some subheadings are different.For guidance on the content, structure, and format of all types ...
The approach described in this guidance is based on: literature relevant to living systematic reviews, including a series of papers published in the Journal of Clinical Epidemiology on behalf of the Living Evidence Network (4-7) Evidence Network members)experiences of teams involved in the pi.
VIDEO: What are systematic reviews? A systematic review attempts to identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a specific research question. Researchers conducting systematic reviews use explicit, systematic methods that are selected with a view aimed at minimizing bias ...
The Cochrane Handbook for Systematic Reviews of Interventions is the official guide that describes in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions. ... Background literature review; Review question; Criteria for inclusion/exclusion of studies; Types of studies ...
A systematic review is a type of research study where the researcher picks the topic of interest that they want to search for information about in the literature, predetermines what are the inclusion/exclusion criteria for the types of articles they are going to look at, and then analyzes those findings to draw conclusions about their question of interest.
Efficacy and Safety of Diet Therapies in Children With Autism Spectrum Disorder: A Systematic Literature Review and Meta-Analysis Front Neurol. 2022 Mar 14 ... from the inception of the databases to August 18, 2021. The Cochrane Bias risk assessment tool was intended to assess the quality of the included studies. Review Manager 5.4 software was ...
This module will teach you to: Recognize features of systematic reviews as a research design. Recognize the importance of using rigorous methods to conduct a systematic review. Identify the types of review questions. Identify the elements of a well-defined review question. Understand the steps in a systematic review.
This review summarized the efficacy of Internet-based self-help treatments for treating mental health problems. • Twenty-five studies were included in this systematic review. • Internet-based self-help interventions were found to be effective on mental health disorders compared to control groups.
To evaluate the efficacy and safety of S. boulardii assisted BQT versus BQT, we therefore performed this systematic review and meta-analysis. 2 Materials and methods. This systematic review was conducted in accordance with the PRISMA 2020 statement . The protocol of this study was registered in the Open Science Framework. 1. 2.1 Literature search
Cochrane Database of Systematic Reviews: all issues. Browse issues. 2024 - Current issue; 2023 ; 2022 ; 2021 . Issue 12 ; Issue 11 ; Issue 10 ; Issue 9 ; Issue 8 ; Issue 7 ; Issue 6 ; Issue 5 ; Issue 4 ; ... Cochrane Review language. Select your preferred language for Cochrane Reviews and other content. Sections without translation will be in ...
A systematic review. 2022. 11. Lefebrve C, Glanville J, Briscoe S, Littlewood A, al e. Chapter 4: Searching for and selecting studies Cochrane Handbook for Systematic Reviews of Intervention 6.2 ed; 2021. 12. Page MJ, Higgins JP, Sterne JA. 13. McArthur A, Klugárová J, Yan H, Florescu S. Innovations in the systematic review of text and opinion.
V.2.1 Definition of a Cochrane Overview #section--2-1. Cochrane Overviews of Reviews (Cochrane Overviews) use explicit and systematic methods to search for and identify multiple systematic reviews on related research questions in the same topic area for the purpose of extracting and analysing their results across important outcomes.
Omega-3 is a family of n-3 polyunsaturated fatty acids (PUFAs), which have been used to treat a wide variety of chronic diseases, due mainly to their antioxidant and anti-inflammatory properties, among others. In this context, omega-3 could be post-exercise recovery agent and sports supplement that could improve performance by preserving and promoting skeletal muscle mass and strength. No ...
Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane, 2023. ... Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta ...
Maeve M Kelleher a, Suzie Cro a, Victoria Cornelius, Karin C Lodrup Carlsen, Håvard O Skjerven, Eva M Rehbinder, Adrian J Lowe, Eishika Dissanayake, Naoki Shimojo a, Kaori Yonezawa, Yukihiro Ohya, Kiwako Yamamoto-Hanada, Kumiko Morita, Emma Axon, Christian Surber, Michael Cork, Alison Cooke, Lien Tran, Eleanor Van Vogt, Jochen Schmitt, Stephan Weidinger, Danielle McClanahan, Eric Simpson ...