Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates

How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
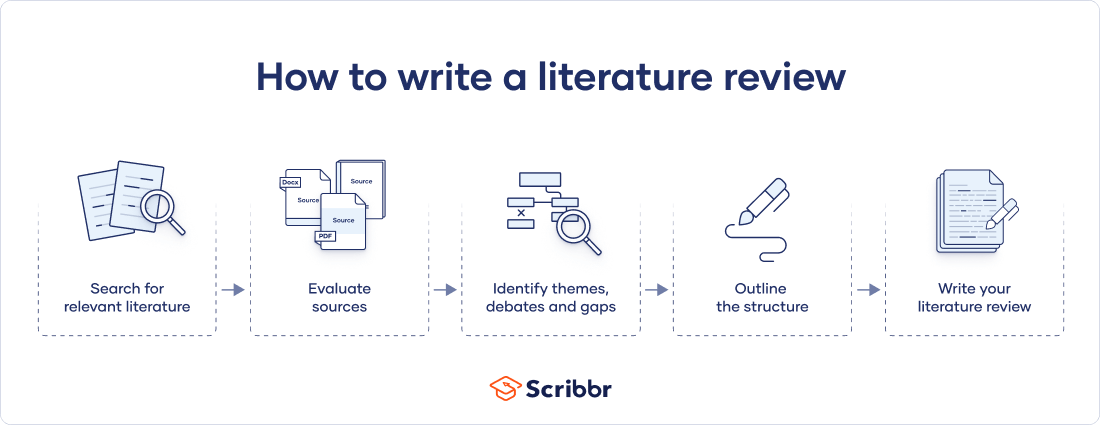
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved June 7, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, what is your plagiarism score.
Health (Nursing, Medicine, Allied Health)
- Find Articles/Databases
- Reference Resources
- Evidence Summaries & Clinical Guidelines
- Drug Information
- Health Data & Statistics
- Patient/Consumer Facing Materials
- Images and Streaming Video
- Grey Literature
- Mobile Apps & "Point of Care" Tools
- Tests & Measures This link opens in a new window
- Citing Sources
- Selecting Databases
- Framing Research Questions
- Crafting a Search
- Narrowing / Filtering a Search
- Expanding a Search
- Cited Reference Searching
- Saving Searches
- Term Glossary
- Critical Appraisal Resources
- What are Literature Reviews?
- Conducting & Reporting Systematic Reviews
- Finding Systematic Reviews
- Tutorials & Tools for Literature Reviews
- Finding Full Text
What are Systematic Reviews? (3 minutes, 24 second YouTube Video)
Systematic Literature Reviews: Steps & Resources
These steps for conducting a systematic literature review are listed below .
Also see subpages for more information about:
- The different types of literature reviews, including systematic reviews and other evidence synthesis methods
- Tools & Tutorials
Literature Review & Systematic Review Steps
- Develop a Focused Question
- Scope the Literature (Initial Search)
- Refine & Expand the Search
- Limit the Results
- Download Citations
- Abstract & Analyze
- Create Flow Diagram
- Synthesize & Report Results
1. Develop a Focused Question
Consider the PICO Format: Population/Problem, Intervention, Comparison, Outcome
Focus on defining the Population or Problem and Intervention (don't narrow by Comparison or Outcome just yet!)
"What are the effects of the Pilates method for patients with low back pain?"
Tools & Additional Resources:
- PICO Question Help
- Stillwell, Susan B., DNP, RN, CNE; Fineout-Overholt, Ellen, PhD, RN, FNAP, FAAN; Melnyk, Bernadette Mazurek, PhD, RN, CPNP/PMHNP, FNAP, FAAN; Williamson, Kathleen M., PhD, RN Evidence-Based Practice, Step by Step: Asking the Clinical Question, AJN The American Journal of Nursing : March 2010 - Volume 110 - Issue 3 - p 58-61 doi: 10.1097/01.NAJ.0000368959.11129.79
2. Scope the Literature
A "scoping search" investigates the breadth and/or depth of the initial question or may identify a gap in the literature.
Eligible studies may be located by searching in:
- Background sources (books, point-of-care tools)
- Article databases
- Trial registries
- Grey literature
- Cited references
- Reference lists
When searching, if possible, translate terms to controlled vocabulary of the database. Use text word searching when necessary.
Use Boolean operators to connect search terms:
- Combine separate concepts with AND (resulting in a narrower search)
- Connecting synonyms with OR (resulting in an expanded search)
Search: pilates AND ("low back pain" OR backache )
Video Tutorials - Translating PICO Questions into Search Queries
- Translate Your PICO Into a Search in PubMed (YouTube, Carrie Price, 5:11)
- Translate Your PICO Into a Search in CINAHL (YouTube, Carrie Price, 4:56)
3. Refine & Expand Your Search
Expand your search strategy with synonymous search terms harvested from:
- database thesauri
- reference lists
- relevant studies
Example:
(pilates OR exercise movement techniques) AND ("low back pain" OR backache* OR sciatica OR lumbago OR spondylosis)
As you develop a final, reproducible strategy for each database, save your strategies in a:
- a personal database account (e.g., MyNCBI for PubMed)
- Log in with your NYU credentials
- Open and "Make a Copy" to create your own tracker for your literature search strategies
4. Limit Your Results
Use database filters to limit your results based on your defined inclusion/exclusion criteria. In addition to relying on the databases' categorical filters, you may also need to manually screen results.
- Limit to Article type, e.g.,: "randomized controlled trial" OR multicenter study
- Limit by publication years, age groups, language, etc.
NOTE: Many databases allow you to filter to "Full Text Only". This filter is not recommended . It excludes articles if their full text is not available in that particular database (CINAHL, PubMed, etc), but if the article is relevant, it is important that you are able to read its title and abstract, regardless of 'full text' status. The full text is likely to be accessible through another source (a different database, or Interlibrary Loan).
- Filters in PubMed
- CINAHL Advanced Searching Tutorial
5. Download Citations
Selected citations and/or entire sets of search results can be downloaded from the database into a citation management tool. If you are conducting a systematic review that will require reporting according to PRISMA standards, a citation manager can help you keep track of the number of articles that came from each database, as well as the number of duplicate records.
In Zotero, you can create a Collection for the combined results set, and sub-collections for the results from each database you search. You can then use Zotero's 'Duplicate Items" function to find and merge duplicate records.

- Citation Managers - General Guide
6. Abstract and Analyze
- Migrate citations to data collection/extraction tool
- Screen Title/Abstracts for inclusion/exclusion
- Screen and appraise full text for relevance, methods,
- Resolve disagreements by consensus
Covidence is a web-based tool that enables you to work with a team to screen titles/abstracts and full text for inclusion in your review, as well as extract data from the included studies.

- Covidence Support
- Critical Appraisal Tools
- Data Extraction Tools
7. Create Flow Diagram
The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram is a visual representation of the flow of records through different phases of a systematic review. It depicts the number of records identified, included and excluded. It is best used in conjunction with the PRISMA checklist .

Example from: Stotz, S. A., McNealy, K., Begay, R. L., DeSanto, K., Manson, S. M., & Moore, K. R. (2021). Multi-level diabetes prevention and treatment interventions for Native people in the USA and Canada: A scoping review. Current Diabetes Reports, 2 (11), 46. https://doi.org/10.1007/s11892-021-01414-3
- PRISMA Flow Diagram Generator (ShinyApp.io, Haddaway et al. )
- PRISMA Diagram Templates (Word and PDF)
- Make a copy of the file to fill out the template
- Image can be downloaded as PDF, PNG, JPG, or SVG
- Covidence generates a PRISMA diagram that is automatically updated as records move through the review phases
8. Synthesize & Report Results
There are a number of reporting guideline available to guide the synthesis and reporting of results in systematic literature reviews.
It is common to organize findings in a matrix, also known as a Table of Evidence (ToE).

- Reporting Guidelines for Systematic Reviews
- Download a sample template of a health sciences review matrix (GoogleSheets)
Steps modified from:
Cook, D. A., & West, C. P. (2012). Conducting systematic reviews in medical education: a stepwise approach. Medical Education , 46 (10), 943–952.
- << Previous: Critical Appraisal Resources
- Next: What are Literature Reviews? >>
- Last Updated: May 31, 2024 10:32 AM
- URL: https://guides.nyu.edu/health
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Performing a...
Performing a literature review
- Related content
- Peer review
- Gulraj S Matharu , academic foundation doctor ,
- Christopher D Buckley , Arthritis Research UK professor of rheumatology
- 1 Institute of Biomedical Research, College of Medical and Dental Sciences, School of Immunity and Infection, University of Birmingham, UK
A necessary skill for any doctor
What causes disease, which drug is best, does this patient need surgery, and what is the prognosis? Although experience helps in answering these questions, ultimately they are best answered by evidence based medicine. But how do you assess the evidence? As a medical student, and throughout your career as a doctor, critical appraisal of published literature is an important skill to develop and refine. At medical school you will repeatedly appraise published literature and write literature reviews. These activities are commonly part of a special study module, research project for an intercalated degree, or another type of essay based assignment.
Formulating a question
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review.
Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important step because a clear question needs to be defined from the outset, which you aim to answer by doing the review. The clearer the question, the more likely it is that the answer will be clear too. It is important to have discussions with your supervisor when formulating a research question as his or her input will be invaluable. The research question must be objective and concise because it is easier to search through the evidence with a clear question. The question also needs to be feasible. What is the point in having a question for which no published evidence exists? Your supervisor’s input will ensure you are not trying to answer an unrealistic question. Finally, is the research question clinically important? There are many research questions that may be answered, but not all of them will …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident Due to planned maintenance there will be periods of time where the website may be unavailable. We apologise for any inconvenience.
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > Journals
- > BJPsych Advances
- > Volume 24 Issue 2
- > How to carry out a literature search for a systematic...

Article contents
- LEARNING OBJECTIVES
- DECLARATION OF INTEREST
Defining the clinical question
Scoping search, search strategy, sources to search, developing a search strategy, searching electronic databases, supplementary search techniques, obtaining unpublished literature, conclusions, how to carry out a literature search for a systematic review: a practical guide.
Published online by Cambridge University Press: 01 March 2018
Performing an effective literature search to obtain the best available evidence is the basis of any evidence-based discipline, in particular evidence-based medicine. However, with a vast and growing volume of published research available, searching the literature can be challenging. Even when journals are indexed in electronic databases, it can be difficult to identify all relevant studies without an effective search strategy. It is also important to search unpublished literature to reduce publication bias, which occurs from a tendency for authors and journals to preferentially publish statistically significant studies. This article is intended for clinicians and researchers who are approaching the field of evidence synthesis and would like to perform a literature search. It aims to provide advice on how to develop the search protocol and the strategy to identify the most relevant evidence for a given research or clinical question. It will also focus on how to search not only the published but also the unpublished literature using a number of online resources.
• Understand the purpose of conducting a literature search and its integral part of the literature review process
• Become aware of the range of sources that are available, including electronic databases of published data and trial registries to identify unpublished data
• Understand how to develop a search strategy and apply appropriate search terms to interrogate electronic databases or trial registries
A literature search is distinguished from, but integral to, a literature review. Literature reviews are conducted for the purpose of (a) locating information on a topic or identifying gaps in the literature for areas of future study, (b) synthesising conclusions in an area of ambiguity and (c) helping clinicians and researchers inform decision-making and practice guidelines. Literature reviews can be narrative or systematic, with narrative reviews aiming to provide a descriptive overview of selected literature, without undertaking a systematic literature search. By contrast, systematic reviews use explicit and replicable methods in order to retrieve all available literature pertaining to a specific topic to answer a defined question (Higgins Reference Higgins and Green 2011 ). Systematic reviews therefore require a priori strategies to search the literature, with predefined criteria for included and excluded studies that should be reported in full detail in a review protocol.
Performing an effective literature search to obtain the best available evidence is the basis of any evidence-based discipline, in particular evidence-based medicine (Sackett Reference Sackett 1997 ; McKeever Reference McKeever, Nguyen and Peterson 2015 ). However, with a vast and growing volume of published research available, searching the literature can be challenging. Even when journals are indexed in electronic databases, it can be difficult to identify all relevant studies without an effective search strategy (Hopewell Reference Hopewell, Clarke and Lefebvre 2007 ). In addition, unpublished data and ‘grey’ literature (informally published material such as conference abstracts) are now becoming more accessible to the public. It is important to search unpublished literature to reduce publication bias, which occurs because of a tendency for authors and journals to preferentially publish statistically significant studies (Dickersin Reference Dickersin and Min 1993 ). Efforts to locate unpublished and grey literature during the search process can help to reduce bias in the results of systematic reviews (Song Reference Song, Parekh and Hooper 2010 ). A paradigmatic example demonstrating the importance of capturing unpublished data is that of Turner et al ( Reference Turner, Matthews and Linardatos 2008 ), who showed that using only published data in their meta-analysis led to effect sizes for antidepressants that were one-third (32%) larger than effect sizes derived from combining both published and unpublished data. Such differences in findings from published and unpublished data can have real-life implications in clinical decision-making and treatment recommendation. In another relevant publication, Whittington et al ( Reference Whittington, Kendall and Fonagy 2004 ) compared the risks and benefits of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depression in children. They found that published data suggested favourable risk–benefit profiles for SSRIs in this population, but the addition of unpublished data indicated that risk outweighed treatment benefits. The relative weight of drug efficacy to side-effects can be skewed if there has been a failure to search for, or include, unpublished data.
In this guide for clinicians and researchers on how to perform a literature search we use a working example about efficacy of an intervention for bipolar disorder to demonstrate the search techniques outlined. However, the overarching methods described are purposefully broad to make them accessible to all clinicians and researchers, regardless of their research or clinical question.
The review question will guide not only the search strategy, but also the conclusions that can be drawn from the review, as these will depend on which studies or other forms of evidence are included and excluded from the literature review. A narrow question will produce a narrow and precise search, perhaps resulting in too few studies on which to base a review, or be so focused that the results are not useful in wider clinical settings. Using an overly narrow search also increases the chances of missing important studies. A broad question may produce an imprecise search, with many false-positive search results. These search results may be too heterogeneous to evaluate in one review. Therefore from the outset, choices should be made about the remit of the review, which will in turn affect the search.
A number of frameworks can be used to break the review question into concepts. One such is the PICO (population, intervention, comparator and outcome) framework, developed to answer clinical questions such as the effectiveness of a clinical intervention (Richardson Reference Richardson, Wilson and Nishikawa 1995 ). It is noteworthy that ‘outcome’ concepts of the PICO framework are less often used in a search strategy as they are less well defined in the titles and abstracts of available literature (Higgins Reference Higgins and Green 2011 ). Although PICO is widely used, it is not a suitable framework for identifying key elements of all questions in the medical field, and minor adaptations are necessary to enable the structuring of different questions. Other frameworks exist that may be more appropriate for questions about health policy and management, such as ECLIPSE (expectation, client group, location, impact, professionals, service) (Wildridge Reference Wildridge and Bell 2002 ) or SPICE (setting, perspective, intervention, comparison, evaluation) for service evaluation (Booth Reference Booth 2006 ). A detailed overview of frameworks is provided in Davies ( Reference Davies 2011 ).
Before conducting a comprehensive literature search, a scoping search of the literature using just one or two databases (such as PubMed or MEDLINE) can provide valuable information as to how much literature for a given review question already exists. A scoping search may reveal whether systematic reviews have already been undertaken for a review question. Caution should be taken, however, as systematic reviews that may appear to ask the same question may have differing inclusion and exclusion criteria for studies included in the review. In addition, not all systematic reviews are of the same quality. If the original search strategy is of poor quality methodologically, original data are likely to have been missed and the search should not simply be updated (compare, for example, Naughton et al ( Reference Naughton, Clarke and O'Leary 2014 ) and Caddy et al ( Reference Caddy, Amit and McCloud 2015 ) on ketamine for treatment-resistant depression).
The first step in conducting a literature search should be to develop a search strategy. The search strategy should define how relevant literature will be identified. It should identify sources to be searched (list of databases and trial registries) and keywords used in the literature (list of keywords). The search strategy should be documented as an integral part of the systematic review protocol. Just as the rest of a well-conducted systematic review, the search strategy used needs to be explicit and detailed such that it could reproduced using the same methodology, with exactly the same results, or updated at a later time. This not only improves the reliability and accuracy of the review, but also means that if the review is replicated, the difference in reviewers should have little effect, as they will use an identical search strategy. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was developed to standardise the reporting of systematic reviews (Moher Reference Moher, Liberati and Tetzlaff 2009 ). The PRISMA statement consists of a 27-item checklist to assess the quality of each element of a systematic review (items 6, 7 and 8 relate to the quality of literature searching) and also to guide authors when reporting their findings.
There are a number of databases that can be searched for literature, but the identification of relevant sources is dependent on the clinical or research question (different databases have different focuses, from more biology to more social science oriented) and the type of evidence that is sought (i.e. some databases report only randomised controlled trials).
• MEDLINE and Embase are the two main biomedical literature databases. MEDLINE contains more than 22 million references from more than 5600 journals worldwide. In addition, the MEDLINE In-Process & Other Non-Indexed Citations database holds references before they are published on MEDLINE. Embase has a strong coverage of drug and pharmaceutical research and provides over 30 million references from more than 8500 currently published journals, 2900 of which are not in MEDLINE. These two databases, however, are only available to either individual subscribers or through institutional access such as universities and hospitals. PubMed, developed by the National Center for Biotechnology Information of the US National Library of Medicine, provides access to a free version of MEDLINE and is accessible to researchers, clinicians and the public. PubMed comprises medical and biomedical literature indexed in MEDLINE, but provides additional access to life science journals and e-books.
In addition, there are a number of subject- and discipline-specific databases.
• PsycINFO covers a range of psychological, behavioural, social and health sciences research.
• The Cochrane Central Register of Controlled Trials (CENTRAL) hosts the most comprehensive source of randomised and quasi-randomised controlled trials. Although some of the evidence on this register is also included in Embase and MEDLINE, there are over 150 000 reports indexed from other sources, such as conference proceedings and trial registers, that would otherwise be less accessible (Dickersin Reference Dickersin, Manheimer and Wieland 2002 ).
• The Cumulative Index to Nursing and Allied Health Literature (CINAHL), British Nursing Index (BNI) and the British Nursing Database (formerly BNI with Full Text) are databases relevant to nursing, but they span literature across medical, allied health, community and health management journals.
• The Allied and Complementary Medicine Database (AMED) is a database specifically for alternative treatments in medicine.
The examples of specific databases given here are by no means exhaustive, but they are popular and likely to be used for literature searching in medicine, psychiatry and psychology. Website links for these databases are given in Box 1 , along with links to resources not mentioned above. Box 1 also provides a website link to a couple of video tutorials for searching electronic databases. Box 2 shows an example of the search sources chosen for a review of a pharmacological intervention of calcium channel antagonists in bipolar disorder, taken from a recent systematic review (Cipriani Reference Cipriani, Saunders and Attenburrow 2016a ).
BOX 1 Website links of search sources to obtain published and unpublished literature
Electronic databases
• MEDLINE/PubMed: www.ncbi.nlm.nih.gov/pubmed
• Embase: www.embase.com
• PsycINFO: www.apa.org/psycinfo
• Cochrane Central Register of Controlled Trials (CENTRAL): www.cochranelibrary.com
• Cumulative Index of Nursing and Allied Health Literature (CINAHL): www.cinahl.com
• British Nursing Index: www.bniplus.co.uk
• Allied and Complementary Medicine Database: https://www.ebsco.com/products/research-databases/amed-the-allied-and-complementary-medicine-database
Grey literature databases
• BIOSIS Previews (part of Thomson Reuters Web of Science): https://apps.webofknowledge.com
Trial registries
• ClinicalTrials.gov: www.clinicaltrials.gov
• Drugs@FDA: www.accessdata.fda.gov/scripts/cder/daf
• European Medicines Agency (EMA): www.ema.europa.eu
• World Health Organization International Clinical Trials Registry Platform (WHO ICTRP): www.who.int/ictrp
• GlaxoSmithKline Study Register: www.gsk-clinicalstudyregister.com
• Eli-Lilly clinical trial results: https://www.lilly.com/clinical-study-report-csr-synopses
Guides to further resources
• King's College London Library Services: http://libguides.kcl.ac.uk/ld.php?content_id=17678464
• Georgetown University Medical Center Dahlgren Memorial Library: https://dml.georgetown.edu/core
• University of Minnesota Biomedical Library: https://hsl.lib.umn.edu/biomed/help/nursing
Tutorial videos
• Searches in electronic databases: http://library.buffalo.edu/hsl/services/instruction/tutorials.html
• Using the Yale MeSH Analyzer tool: http://library.medicine.yale.edu/tutorials/1559
BOX 2 Example of search sources chosen for a review of calcium channel antagonists in bipolar disorder (Cipriani Reference Cipriani, Saunders and Attenburrow 2016a )
Electronic databases searched:
• MEDLINE In-Process and Other Non-Indexed Citations
For a comprehensive search of the literature it has been suggested that two or more electronic databases should be used (Suarez-Almazor Reference Suarez-Almazor, Belseck and Homik 2000 ). Suarez-Almazor and colleagues demonstrated that, in a search for controlled clinical trials (CCTs) for rheumatoid arthritis, osteoporosis and lower back pain, only 67% of available citations were found by both Embase and MEDLINE. Searching MEDLINE alone would have resulted in 25% of available CCTs being missed and searching Embase alone would have resulted in 15% of CCTs being missed. However, a balance between the sensitivity of a search (an attempt to retrieve all relevant literature in an extensive search) and the specificity of a search (an attempt to retrieve a more manageable number of relevant citations) is optimal. In addition, supplementing electronic database searches with unpublished literature searches (see ‘Obtaining unpublished literature’ below) is likely to reduce publication bias. The capacity of the individuals or review team is likely largely to determine the number of sources searched. In all cases, a clear rationale should be outlined in the review protocol for the sources chosen (the expertise of an information scientist is valuable in this process).
Important methodological considerations (such as study design) may also be included in the search strategy. Dependent on the databases and supplementary sources chosen, filters can be used to search the literature by study design (see ‘Searching electronic databases’). For instance, if the search strategy is confined to one study design term only (e.g. randomised controlled trial, RCT), only the articles labelled in this way will be selected. However, it is possible that in the database some RCTs are not labelled as such, so they will not be picked up by the filtered search. Filters can help reduce the number of references retrieved by the search, but using just one term is not 100% sensitive, especially if only one database is used (i.e. MEDLINE). It is important for systematic reviewers to know how reliable such a strategy can be and treat the results with caution.
Identifying search terms
Standardised search terms are thesaurus and indexing terms that are used by electronic databases as a convenient way to categorise articles, allowing for efficient searching. Individual database records may be assigned several different standardised search terms that describe the same or similar concepts (e.g. bipolar disorder, bipolar depression, manic–depressive psychosis, mania). This has the advantage that even if the original article did not use the standardised term, when the article is catalogued in a database it is allocated that term (Guaiana Reference Guaiana, Barbui and Cipriani 2010 ). For example, an older paper might refer to ‘manic depression’, but would be categorised under the term ‘bipolar disorder’ when catalogued in MEDLINE. These standardised search terms are called MeSH (medical subject headings) in MEDLINE and PubMed, and Emtree in Embase, and are organised in a hierarchal structure ( Fig. 1 ). In both MEDLINE and Embase an ‘explode’ command enables the database to search for a requested term, as well as specific related terms. Both narrow and broader search terms can be viewed and selected to be included in the search if appropriate to a topic. The Yale MeSH Analyzer tool ( mesh.med.yale.edu ) can be used to help identify potential terms and phrases to include in a search. It is also useful to understand why relevant articles may be missing from an initial search, as it produces a comparison grid of MeSH terms used to index each article (see Box 1 for a tutorial video link).

FIG 1 Search terms and hierarchical structure of MeSH (medical subject heading) in MEDLINE and PubMed.
In addition, MEDLINE also distinguishes between MeSH headings (MH) and publication type (PT) terms. Publication terms are less about the content of an article than about its type, specifying for example a review article, meta-analysis or RCT.
Both MeSH and Emtree have their own peculiarities, with variations in thesaurus and indexing terms. In addition, not all concepts are assigned standardised search terms, and not all databases use this method of indexing the literature. It is advisable to check the guidelines of selected databases before undertaking a search. In the absence of a MeSH heading for a particular term, free-text terms could be used.
Free-text terms are used in natural language and are not part of a database’s controlled vocabulary. Free-text terms can be used in addition to standardised search terms in order to identify as many relevant records as possible (Higgins Reference Higgins and Green 2011 ). Using free-text terms allows the reviewer to search using variations in language or spelling (e.g. hypomani* or mania* or manic* – see truncation and wildcard functions below and Fig. 2 ). A disadvantage of free-text terms is that they are only searched for in the title and abstracts of database records, and not in the full texts, meaning that when a free-text word is used only in the body of an article, it will not be retrieved in the search. Additionally, a number of specific considerations should be taken into account when selecting and using free-text terms:
• synonyms, related terms and alternative phrases (e.g. mood instability, affective instability, mood lability or emotion dysregulation)
• abbreviations or acronyms in medical and scientific research (e.g. magnetic resonance imaging or MRI)
• lay and medical terminology (e.g. high blood pressure or hypertension)
• brand and generic drug names (e.g. Prozac or fluoxetine)
• variants in spelling (e.g. UK English and American English: behaviour or behavior; paediatric or pediatric).
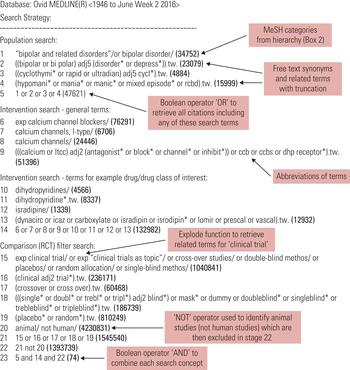
FIG 2 Example of a search strategy about bipolar disorder using MEDLINE (Cipriani Reference Cipriani, Saunders and Attenburrow 2016a ). The strategy follows the PICO framework and includes MeSH terms, free-text keywords and a number of other techniques, such as truncation, that have been outlined in this article. Numbers in bold give the number of citations retrieved by each search.
Truncation and wildcard functions can be used in most databases to capture variations in language:
• truncation allows the stem of a word that may have variant endings to be searched: for example, a search for depress* uses truncation to retrieve articles that mention both depression and depressive; truncation symbols may vary by database, but common symbols include: *, ! and #
• wild cards substitute one letter within a word to retrieve alternative spellings: for example, ‘wom?n’ would retrieve the terms ‘woman’ and ‘women’.
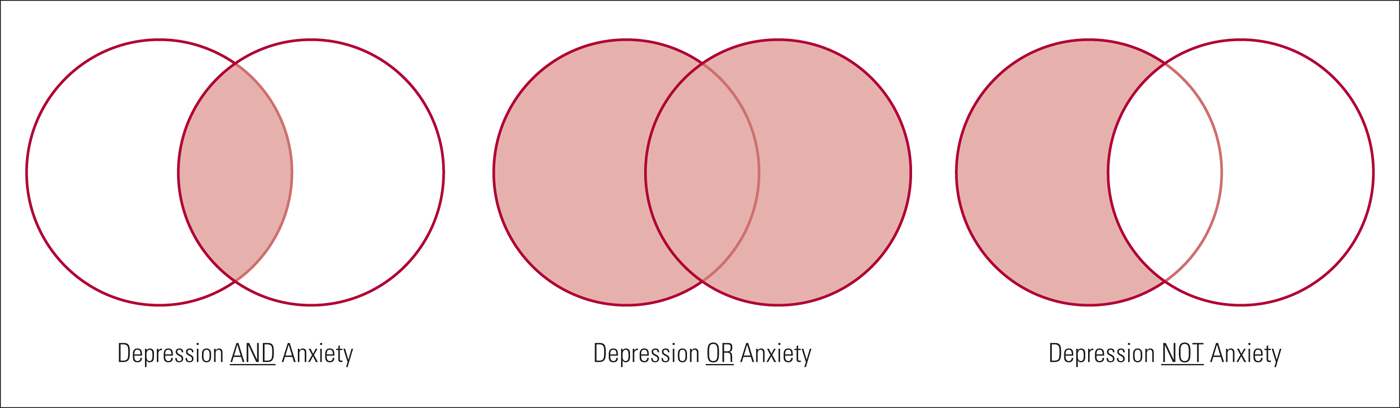
Combining search terms
Search terms should be combined in the search strategy using Boolean operators. Boolean operators allow standardised search terms and free-text terms to be combined. There are three main Boolean operators – AND, OR and NOT ( Fig. 3 ).
• OR – this operator is used to broaden a search, finding articles that contain at least one of the search terms within a concept. Sets of terms can be created for each concept, for example the population of interest: (bipolar disorder OR bipolar depression). Parentheses are used to build up search terms, with words within parentheses treated as a unit.
• AND – this can be used to join sets of concepts together, narrowing the retrieved literature to articles that contain all concepts, for example the population or condition of interest and the intervention to be evaluated: (bipolar disorder OR bipolar depression) AND calcium channel blockers. However, if at least one term from each set of concepts is not identified from the title or abstract of an article, this article will not be identified by the search strategy. It is worth mentioning here that some databases can run the search also across the full texts. For example, ScienceDirect and most publishing houses allow this kind of search, which is much more comprehensive than abstract or title searches only.
• NOT – this operator, used less often, can focus a search strategy so that it does not retrieve specific literature, for example human studies NOT animal studies. However, in certain cases the NOT operator can be too restrictive, for example if excluding male gender from a population, using ‘NOT male’ would also mean that any articles about both males and females are not obtained by the search.
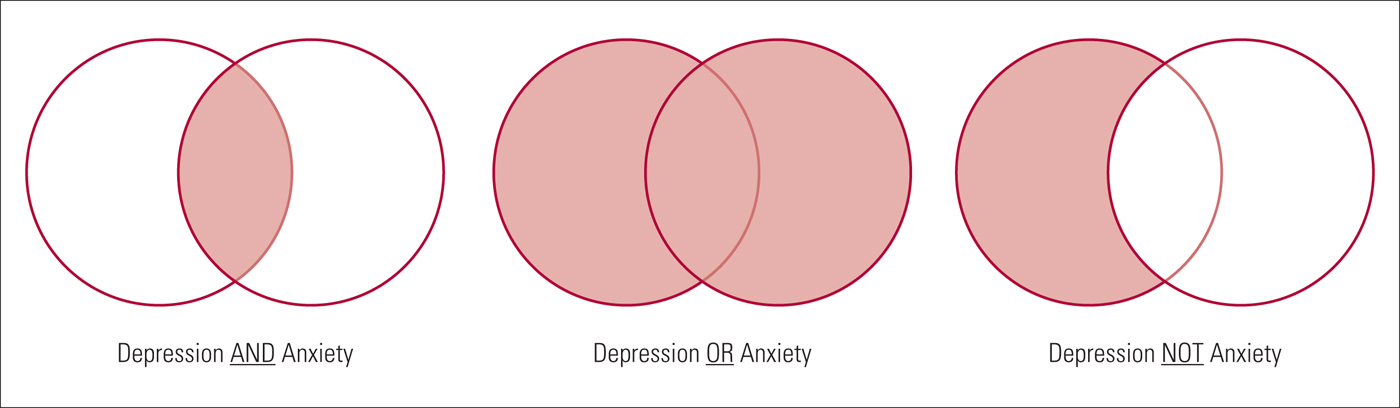
FIG 3 Example of Boolean operator concepts (the resulting search is the light red shaded area).
The conventions of each database should be checked before undertaking a literature search, as functions and operators may differ slightly between them (Cipriani Reference Cipriani, Saunders and Attenburrow 2016b ). This is particularly relevant when using limits and filters. Figure 2 shows an example search strategy incorporating many of the concepts described above. The search strategy is taken from Cipriani et al ( Reference Cipriani, Zhou and Del Giovane 2016a ), but simplified to include only one intervention.
Search filters
A number of filters exist to focus a search, including language, date and study design or study focus filters. Language filters can restrict retrieval of articles to the English language, although if language is not an inclusion criterion it should not be restricted, to avoid language bias. Date filters can be used to restrict the search to literature from a specified period, for example if an intervention was only made available after a certain date. In addition, if good systematic reviews exist that are likely to capture all relevant literature (as advised by an information specialist), date restrictions can be used to search additional literature published after the date of that included in the systematic review. In the same way, date filters can be used to update a literature search since the last time it was conducted. Reviewing the literature should be a timely process (new and potentially relevant evidence is produced constantly) and updating the search is an important step, especially if collecting evidence to inform clinical decision-making, as publications in the field of medicine are increasing at an impressive rate (Barber Reference Barber, Corsi and Furukawa 2016 ). The filters chosen will depend on the research question and nature of evidence that is sought through the literature search and the guidelines of the individual database that is used.
- Google Scholar
Google Scholar allows basic Boolean operators to be used in strings of search terms. However, the search engine does not use standardised search terms that have been tagged as in traditional databases and therefore variations of keywords should always be searched. There are advantages and disadvantages to using a web search engine such as Google Scholar. Google Scholar searches the full text of an article for keywords and also searches a wider range of sources, such as conference proceedings and books, that are not found in traditional databases, making it a good resource to search for grey literature (Haddaway Reference Haddaway, Collins and Coughlin 2015 ). In addition, Google Scholar finds articles cited by other relevant articles produced in the search. However, variable retrieval of content (due to regular updating of Google algorithms and the individual's search history and location) means that search results are not necessarily reproducible and are therefore not in keeping with replicable search methods required by systematic reviews. Google Scholar alone has not been shown to retrieve more literature than other traditional databases discussed in this article and therefore should be used in addition to other sources (Bramer Reference Bramer, Giustini and Kramer 2016 ).
Citation searching
Once the search strategy has identified relevant literature, the reference lists in these sources can be searched. This is called citation searching or backward searching, and it can be used to see where particular research topics led others. This method is particularly useful if the search identifies systematic reviews or meta-analyses of a similar topic.
Conference abstracts
Conference abstracts are considered ‘grey literature’, i.e. literature that is not formally published in journals or books (Alberani Reference Alberani, De Castro Pietrangeli and Mazza 1990 ). Scherer and colleagues found that only 52.6% of all conference abstracts go on to full publication of results, and factors associated with publication were studies that had RCT designs and the reporting of positive or significant results (Scherer Reference Scherer, Langenberg and von Elm 2007 ). Therefore, failure to search relevant grey literature might miss certain data and bias the results of a review. Although conference abstracts are not indexed in most major electronic databases, they are available in databases such as BIOSIS Previews ( Box 1 ). However, as with many unpublished studies, these data did not undergo the peer review process that is often a tool for assessing and possibly improving the quality of the publication.
Searching trial registers and pharmaceutical websites
For reviews of trial interventions, a number of trial registers exist. ClinicalTrials.gov ( clinicaltrials.gov ) provides access to information on public and privately conducted clinical trials in humans. Results for both published and unpublished studies can be found for many trials on the register, in addition to information about studies that are ongoing. Searching each trial register requires a slightly different search strategy, but many of the basic principles described above still apply. Basic searches on ClinicialTrials.gov include searching by condition, specific drugs or interventions and these can be linked using Boolean operators: for example, (bipolar disorder OR manic depressive disorder) AND lithium. As mentioned above, parentheses can be used to build up search terms. More advanced searches allow one to specify further search fields such as the status of studies, study type and age of participants. The US Food and Drug Administration (FDA) hosts a database providing information about FDA-approved drugs, therapeutic products and devices ( www.fda.gov ). The database (with open access to anyone, not only in the USA) can be searched by the drug name, its active ingredient or its approval application number and, for most drugs approved in the past 20 years or so, a review of clinical trial results (some of which remain unpublished) used as evidence in the approval process is available. The European Medicines Agency (EMA) hosts a similar register for medicines developed for use in the European Union ( www.ema.europa.eu ). An internet search will show that many other national and international trial registers exist that, depending on the review question, may be relevant search sources. The World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp ) provides access to a central database bringing a number of these national and international trial registers together. It can be searched in much the same way as ClinicalTrials.gov.
A number of pharmaceutical companies now share data from company-sponsored clinical trials. GlaxoSmithKline (GSK) is transparent in the sharing of its data from clinical studies and hosts its own clinical study register ( www.gsk-clinicalstudyregister.com ). Eli-Lilly provides clinical trial results both on its website ( www.lillytrialguide.com ) and in external registries. However, other pharmaceutical companies, such as Wyeth and Roche, divert users to clinical trial results in external registries. These registries include both published and previously unpublished studies. Searching techniques differ for each company and hand-searching through documents is often required to identify studies.
Communication with authors
Direct communication with authors of published papers could produce both additional data omitted from published studies and other unpublished studies. Contact details are usually available for the corresponding author of each paper. Although high-quality reviews do make efforts to obtain and include unpublished data, this does have potential disadvantages: the data may be incomplete and are likely not to have been peer-reviewed. It is also important to note that, although reviewers should make every effort to find unpublished data in an effort to minimise publication bias, there is still likely to remain a degree of this bias in the studies selected for a systematic review.
Developing a literature search strategy is a key part of the systematic review process, and the conclusions reached in a systematic review will depend on the quality of the evidence retrieved by the literature search. Sources should therefore be selected to minimise the possibility of bias, and supplementary search techniques should be used in addition to electronic database searching to ensure that an extensive review of the literature has been carried out. It is worth reminding that developing a search strategy should be an iterative and flexible process (Higgins Reference Higgins and Green 2011 ), and only by conducting a search oneself will one learn about the vast literature available and how best to capture it.
Acknowledgements
We thank Sarah Stockton for her help in drafting this article. Andrea Cipriani is supported by the NIHR Oxford cognitive health Clinical Research Facility.
Select the single best option for each question stem
a an explicit and replicable method used to retrieve all available literature pertaining to a specific topic to answer a defined question
b a descriptive overview of selected literature
c an initial impression of a topic which is understood more fully as a research study is conducted
d a method of gathering opinions of all clinicians or researchers in a given field
e a step-by-step process of identifying the earliest published literature through to the latest published literature.
a does not need to be specified in advance of a literature search
b does not need to be reported in a systematic literature review
c defines which sources of literature are to be searched, but not how a search is to be carried out
d defines how relevant literature will be identified and provides a basis for the search strategy
e provides a timeline for searching each electronic database or unpublished literature source.
a the Cochrane Central Register of Controlled Trials (CENTRAL)
d the Cumulative Index to Nursing and Allied Health Literature (CINAHL)
e the British Nursing Index.
a bipolar disorder OR treatment
b bipolar* OR treatment
c bipolar disorder AND treatment
d bipolar disorder NOT treatment
e (bipolar disorder) OR (treatment).
a publication bias
b funding bias
c language bias
d outcome reporting bias
e selection bias.
MCQ answers
1 a 2 d 3 b 4 c 5 a

FIG 2 Example of a search strategy about bipolar disorder using MEDLINE (Cipriani 2016a). The strategy follows the PICO framework and includes MeSH terms, free-text keywords and a number of other techniques, such as truncation, that have been outlined in this article. Numbers in bold give the number of citations retrieved by each search.
This article has been cited by the following publications. This list is generated based on data provided by Crossref .
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Volume 24, Issue 2
- Lauren Z. Atkinson and Andrea Cipriani
- DOI: https://doi.org/10.1192/bja.2017.3
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.

The “PP-ICONS” approach will help you separate the clinical wheat from the chaff in mere minutes .
ROBERT J. FLAHERTY, MD
Fam Pract Manag. 2004;11(5):47-52
Keeping up with the latest advances in diagnosis and treatment is a challenge we all face as phycians. We need information that is both valid (that is, accurate and correct) and relevant to our patients and practices. While we have many sources of clinical information, such as CME lectures, textbooks, pharmaceutical advertising, pharmaceutical representatives and colleagues, we often turn to journal articles for the most current clinical information.
Unfortunately, a great deal of research reported in journal articles is poorly done, poorly analyzed or both, and thus is not valid. A great deal of research is also irrelevant to our patients and practices. Separating the clinical wheat from the chaff can take skills that many of us never were taught.
Reading the abstract is often sufficient when evaluating an article using the PP-ICONS approach.
The most relevant studies will involve outcomes that matter to patients (e.g., morbidity, mortality and cost) versus outcomes that matter to physiologists (e.g., blood pressure, blood sugar or cholesterol levels).
Ignore the relative risk reduction, as it overstates research findings and will mislead you.
The article “Making Evidence-Based Medicine Doable in Everyday Practice” in the February 2004 issue of FPM describes several organizations that can help us. These organizations, such as the Cochrane Library, Bandolier and Clinical Evidence, develop clinical questions and then review one or more journal articles to identify the best available evidence that answers the question, with a focus on the quality of the study, the validity of the results and the relevance of the findings to everyday practice. These organizations provide a very valuable service, and the number of important clinical questions that they have studied has grown steadily over the past five years. (See “Four steps to an evidence-based answer.” )
FOUR STEPS TO AN EVIDENCE-BASED ANSWER
When faced with a clinical question, follow these steps to find an evidence-based answer:
Search the Web site of one of the evidence review organizations, such as Cochrane (http://www.cochrane.org/cochrane/revabstr/mainindex.htm), Bandolier ( http://www.jr2.ox.ac.uk/bandolier ) or Clinical Evidence ( http://www.clinicalevidence.com ), described in “Making Evidence-Based Medicine Doable in Everyday Practice,” FPM, February 2004, page 51 . You can also search the TRIP+ Web site ( http://www.tripdatabase.com ), which simultaneously searches the databases of many of the review organizations. If you find a systematic review or meta-analysis by one of these organizations, you can be confident that you’ve found the best evidence available.
If you don’t find the information you need through step 1, search for meta-analyses and systematic reviews using the PubMed Web site (see the tutorial at http://www.nlm.nih.gov/bsd/pubmed_tutorial/m1001.html ). Most of the recent abstracts found on PubMed provide enough information for you to determine the validity and relevance of the findings. If needed, you can get a copy of the full article through your hospital library or the journal’s Web site.
If you cannot find a systematic review or meta-analysis on PubMed, look for a randomized controlled trial (RCT). The RCT is the “gold standard” in medical research. Case reports, cohort studies and other research methods simply are not good enough to use for making patient care decisions.
Once you find the article you need, use the PP-ICONS approach to evaluate its usefulness to your patient.
If you find a systematic review or meta-analysis done by one of these organizations, you can feel confident that you have found the current best evidence. However, these organizations have not asked all of the common clinical questions yet, and you will frequently be faced with finding the pertinent articles and determining for yourself whether they are valuable. This is where the PP-ICONS approach can help.
What is PP-ICONS?
When you find a systematic review, meta-analysis or randomized controlled trial while reading your clinical journals or searching PubMed ( http://www.ncbi.nlm.nih.gov/entrez/query.fcgi ), you need to determine whether it is valid and relevant. There are many different ways to analyze an abstract or journal article, some more rigorous than others. 1 , 2 I have found a simple but effective way to identify a valid or relevant article within a couple of minutes, ensuring that I can use or discard the conclusions with confidence. This approach works well on articles regarding treatment and prevention, and can also be used with articles on diagnosis and screening.
The most important information to look for when reviewing an article can be summarized by the acronym “PP-ICONS,” which stands for the following:
Patient or population,
Intervention,
Comparison,
Number of subjects,
Statistics.
For example, imagine that you just saw a nine-year-old patient in the office with common warts on her hands, an ideal candidate for your usual cryotherapy. Her mother had heard about treating warts with duct tape and wondered if you would recommend this treatment. You promised to call Mom back after you had a chance to investigate this rather odd treatment.
When you get a free moment, you write down your clinical question: “Is duct tape an effective treatment for warts in children?” Writing down your clinical question is useful, as it can help you clarify exactly what you are looking for. Use the PPICO parts of the acronym to help you write your clinical question; this is actually how many researchers develop their research questions.
You search Cochrane and Bandolier without success, so now you search PubMed, which returns an abstract for the following article: “Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med . 2002 Oct;156(10):971-974.”
You decide to apply PP-ICONS to this abstract (see "Abstract from PubMed" ) to determine if the information is both valid and relevant.
ABSTRACT FROM PUBMED
Using the PP-ICONS approach, physicians can evaluate the validity and relevance of clinical articles in minutes using only the abstract, such as this one, obtained free online from PubMed, http://www.ncbi. nlm.nih.gov/entrez/query.fcgi. The author uses this abstract to evaluate the use of duct tape to treat common warts.
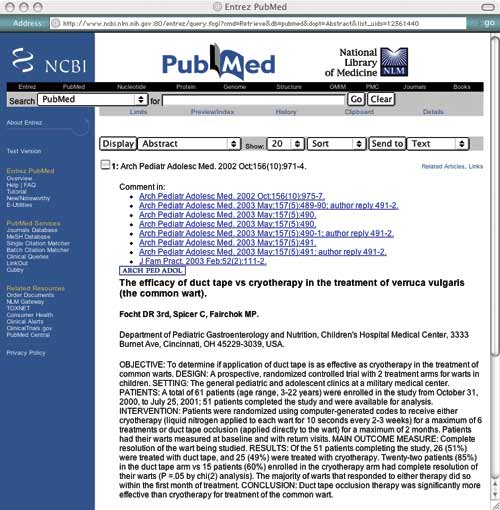
Problem. The first P in PP-ICONS is for “problem,” which refers to the clinical condition that was studied. From the abstract, it is clear that the researchers studied the same problem you are interested in, which is important since flat warts or genital warts may have responded differently. Obviously, if the problem studied were not sufficiently similar to your clinical problem, the results would not be relevant.
Patient or population. Next, consider the patient or population. Is the study group similar to your patient or practice? Are they primary care patients, for example, or are they patients who have been referred to a tertiary care center? Are they of a similar age and gender? In this case, the researchers studied children and young adults in outpatient clinics, which is similar to your patient population. If the patients in the study are not similar to your patient, for example if they are sicker, older, a different gender or more clinically complicated, the results might not be relevant.
Intervention. The intervention could be a diagnostic test or a treatment. Make sure the intervention is the same as what you are looking for. The patient’s mother was asking about duct tape for warts, so this is a relevant study.
Comparison. The comparison is what the intervention is tested against. It could be a different diagnostic test or another therapy, such as cryotherapy in this wart study. It could even be placebo or no treatment. Make sure the comparison fits your question. You usually use cryotherapy for common warts, so this is a relevant comparison.
Outcome. The outcome is particularly important. Many outcomes are “disease-oriented outcomes,” which are based on “disease-oriented evidence” (DOEs). DOEs usually reflect changes in physiologic parameters, such as blood pressure, blood sugar, cholesterol, etc. We have long assumed that improving the physiologic parameters of a disease will result in a better disease outcome, but that is not necessarily true. For instance, finasteride can improve urinary flow rate in prostatic hypertrophy, but it does not significantly change symptom scores. 3
DOEs look at the kinds of outcomes that physiologists care about. More relevant are outcomes that patients care about, often called “patient-oriented outcomes.” These are based on “patient-oriented evidence that matters” (POEMs) and look at outcomes such as morbidity, mortality and cost. Thus, when looking at a journal article, DOEs are interesting but of questionable relevance, whereas POEMs are very interesting and very relevant. In the study on the previous page, the outcome is complete resolution of the wart, which is something your patient is interested in.
Number. The number of subjects is crucial to whether accurate statistics can be generated from the data. Too few patients in a research study may not be enough to show that a difference actually exists between the intervention and comparison groups (known as the “power” of a study). Many studies are published with less than 100 subjects, which is usually inadequate to provide reliable statistics. A good rule of thumb is 400 subjects. 4 Fifty-one patients completed the wart study, which is a pretty small number to generate good statistics.
Statistics. The statistics you are interested in are few in number and easy to understand. Since statistics are frequently misused in journal articles, it is worth a few minutes to learn which to believe and which to ignore.
Relative risk reduction. It is not unusual to find a summary statement in a journal article similar to this one from an article titled “Long-Term Effects of Mammography Screening: Updated Overview of the Swedish Randomised Trials”: 5
“There were 511 breast cancer deaths in 1,864,770 women-years in the invited groups and 584 breast cancer deaths in 1,688,440 women-years in the control groups, a significant 21 percent reduction in breast cancer mortality.”
This 21-percent statistic is the relative risk reduction (RRR), which is the percent reduction in the measured outcome between the experimental and control groups. (See “Some important statistics” for more information on calculating the RRR and other statistics.) The RRR is not a good way to compare outcomes. It amplifies small differences and makes insignificant findings appear significant, and it doesn’t reflect the baseline risk of the outcome event. Nevertheless, the RRR is very popular and will be reported in nearly every journal article, perhaps because it makes weak results look good. Think of the RRR as the “reputation reviving ratio” or the “reporter’s reason for ‘riting.” Ignore the RRR. It will mislead you. In our wart treatment example, the RRR would be (85 percent - 60 percent)/60 percent x 100 = 42 percent. The RRR could thus be interpreted as showing that duct tape is 42 percent more effective than cryotherapy in treating warts.
SOME IMPORTANT STATISTICS
Absolute risk reduction (ARR): The difference between the control group’s event rate (CER) and the experimental group’s event rate (EER).
Control event rate (CER): The proportion of patients responding to placebo or other control treatment. For example, if 25 patients are in a control group and the event being studied is observed in 15 of those patients, the control event rate would be 15/25 = 0.60.
Experimental event rate (EER): The proportion of patients responding to the experimental treatment or intervention. For example, if 26 patients are in an experimental group and the event being studied is observed in 22 of those patients, the experimental event rate would be 22/26 = 0.85.
Number needed to treat (NNT): The number of patients that must be treated to prevent one adverse outcome or for one patient to benefit. The NNT is the inverse of the ARR; NNT = 1/ARR.
Relative risk reduction (RRR): The percent reduction in events in the treated group compared to the control group event rate.
| When the experimental treatment reduces the risk of a bad event: | Example: Beta-blockers to prevent deaths in high-risk patients with recent myocardial infarction: | When the experimental treatment increases the probability of a good event: | Example: Duct tape to eliminate common warts. | |
| Relative risk reduction (RRR) | CER-EER/CER | (.66 -. 50)/.66 = .24 or 24 percent | EER-CER/CER | (.85-.60)/.60 = .42 or 42 percent |
| Absolute risk reduction (ARR): | CER-EER | (.66 - .50) = .16 or 16 percent | EER-CER | .85-.60 = .25 or 25 percent |
| Number needed to treat (NNT) | 1/ARR | 1/.16 = 6 | 1/ARR | 1/.25 = 4 |
Absolute risk reduction. A better statistic is the absolute risk reduction (ARR), which is the difference in the outcome event rate between the control group and the experimental treated group. Thus, in our wart treatment example, the ARR is the outcome event rate (complete resolution of warts) for duct tape (85 percent) minus the outcome event rate for cryotherapy (60 percent) = 25 percent. Unlike the RRR, the ARR does not amplify small differences but shows the true difference between the experimental and control interventions. Using the ARR, it would be accurate to say that duct tape is 25-percent more effective than cryotherapy in treating warts.
Number needed to treat. The single most clinically useful statistic is the number needed to treat (NNT). The NNT is the number of patients who must be treated to prevent one adverse outcome. To think about it another way, the NNT is the number of patients who must be treated for one patient to benefit. (The rest who were treated obtained no benefit, although they still suffered the risks and costs of treatment.) In our wart therapy article, the NNT would tell us how many patients must be treated with the experimental treatment for one to benefit more than if he or she had been treated with the standard treatment.
Now this is a statistic that physicians and their patients can really appreciate! Furthermore, the NNT is easy to calculate, as it is simply the inverse of the ARR. For our wart treatment study, the NNT is 1/25 percent =1/0.25 = 4, meaning that 4 patients need to be treated with duct tape for one to benefit more than if treated by cryotherapy.
Wrapped up in this simple little statistic are some very important concepts. The NNT provides you with the likelihood that the test or treatment will benefit any individual patient, an impression of the baseline risk of the adverse event, and a sense of the cost to society. Thus, it gives perspective and hints at the “reasonableness” of a treatment. The value of this statistic has become appreciated in the last five years, and more journal articles are reporting it.
What is a reasonable NNT? In a perfect world, a treatment would have an NNT of 1, meaning that every patient would benefit from the treatment. Real life is not so kind (see “Examples of NNTs” ). Clearly, an NNT of 1 is great and an NNT of 1,000 is terrible. Although it is hard to come up with firm guidelines, for primary therapies I am satisfied with an NNT of 10 or less and very pleased with an NNT less than 5. Our duct tape NNT of 4 is good, particularly since the treatment is cheap, easy and painless.
EXAMPLES OF NNTS
The number needed to treat (NNT) is one of the most useful statistics for physicians and patients. It calculates the number of patients that must be treated to prevent one adverse event or for one patient to benefit. Note that NNTs for preventive interventions will usually be higher than NNTs for treatment interventions. The lower the NNT, the better.
The following examples of NNTs are borrowed from an excellent list available through the Bandolier Web site at http://www.jr2.ox.ac.uk/bandolier/band50/b50-8.html .
| Triple antibiotic therapy to eradicate | 1.1 |
| Isosorbide dinitrate for prevention of exercise-induced angina | 5 |
| Short course of antibiotics for otitis media in children | 7 |
| Statins for secondary prevention of adverse cardiovascular outcomes | 11 |
| Statins for primary prevention of adverse cardiovascular outcomes | 35 |
| Finasteride to prevent one operation for benign prostatic hyperplasia | 39 |
| Misoprostol to prevent any gastrointestinal complication in nonsteroidal anti-inflammatory drug users | 166 |
Note that NNTs for preventive interventions (e.g., the use of aspirin to prevent cardiac problems) will usually be higher than NNTs for treatment interventions (e.g., the use of duct tape to cure warts). Prevention groups contain both higher-risk and lower-risk individuals, so they produce bigger denominators, whereas treatment groups only contain diseased patients. Thus, an NNT for prevention of less than 20 might be particularly good.
When discussing a particular therapy, I explain the NNT to my patient. Since this statistical concept is easy to understand, it can help the patient be a more informed partner in therapeutic decisions.
You will soon start to see a similar statistic, the number needed to screen (NNS), which is the number of patients needed to screen for a particular disease for a given duration for one patient to benefit. 6 Although few NNSs have been calculated, they are likely to involve higher numbers, since the screening population consists of patients with and without the disease. For example, in the article on mammography screening mentioned above, the NNS was 961 for 16 years. In other words, you would need to screen 961 women for 16 years to prevent one breast cancer death.
The good news and the bad
Using PP-ICONS to assess the wart study, the problem, the patient/population, the intervention, the comparison and the outcome are all relevant to your patient. The number of subjects is on the small side, making you a little wary, but the intervention is cheap and low-risk. The statistics, particularly the NNT, are reasonable. On balance, this looks like a fair approach, so you call the patient’s mother and discuss it with her.
The PP-ICONS approach is an easy way to screen an article for validity and relevance, and the abstract often contains all of the information you need. Even the statistics can be done quickly in your head. You can apply PP-ICONS when searching for a particular article, when you come across an article in your reading, when data are presented at lectures, when a pharmaceutical representative hands you an article to support his or her pitch, and even when reading news stories describing medical breakthroughs.
Don’t be discouraged if you find that high-quality articles are rare, even in the most prestigious journals. This seems to be changing for the better, although many careers are still being built on questionable research. Nevertheless, screening articles will help you find the truth that is out there and will help you practice the best medicine. And as we become more discerning end-users of research, we might just stimulate improvements in clinical research in the process.
Miser WF. Critical appraisal of the literature. J Amer Board Fam Pract . 1999;12(4):315-333.
Guyatt GH, et al. Users’ guides to the medical literature. How to use an article about therapy or prevention. Are the results of the study valid?. JAMA . 1993;270(21):2598-2601.
Lepor H, et al. The efficacy of terazosin, finasteride or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med . 1996;335(8):533-539.
Krejcie RV, Morgan DW. Determining sample size for research activities. Educational and Psychological Measurement . 1970;30:607-610.
Nystrom L, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet . 2002;359(9310):909-919.
Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ . 1998;317:307-312.
Continue Reading
More in fpm, more in pubmed.
Copyright © 2004 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

Writing in the Health Sciences: Research and Lit Reviews
- Research and Lit Reviews
- Tables and Figures
- Citation Management
- Further Reference
What Is a Literature Review?
In simple terms, a literature review investigates the available information on a certain topic. It may be only a knowledge survey with an intentional focus. However, it is often a well-organized examination of the existing research which evaluates each resource in a systematic way. Often a lit review will involve a series of inclusion/exclusion criteria or an assessment rubric which examines the research in-depth. Below are some interesting sources to consider.

The Writing Center's Literature Reviews - UNC-Chapel Hill's writing center explains some of the key criteria involved in doing a literature review.
Literature Review vs. Systematic Review - This recent article details the difference between a literature review and a systematic review. Though the two share similar attributes, key differences are identified here.
Literature Review Steps
1. Identify a research question. For example: "Does the use of warfarin in elderly patients recovering from myocardial infarction help prevent stroke?"
2. Consider which databases might provide information for your topic. Often PubMed or CINAHL will cover a wide spectrum of biomedical issues. However, other databases and grey literature sources may specialize in certain disciplines. Embase is generally comprehensive but also specializes in pharmacological interventions.
3. Select the major subjects or ideas from your question. Focus in on the particular concepts involved in your research. Then brainstorm synonyms and related terminology for these topics.
4. Look for the preferred indexing terms for each concept in your question. This is especially important with databases such as PubMed, CINAHL, or Scopus where headings within the MeSH database or under the Emtree umbrella are present. For example, the above question's keywords such as " warfarin " or "myocardial infarction" can involve related terminology or subject headings such as "anti-coagulants" or "cardiovascular disease."
5. Build your search using boolean operators. Combine the synonyms in your database using boolean operators such as AND or OR. Sometimes it is necessary to research parts of a question rather than the whole. So you might link searches for things like the preventive effects of anti-coagulants with stroke or embolism, then AND these results with the therapy for patients with cardiovascular disease.
6. Filter and save your search results from the first database (do this for all databases). This may be a short list because of your topic's limitations, but it should be no longer than 15 articles for an initial search. Make sure your list is saved or archived and presents you with what's needed to access the full text.
7. Use the same process with the next databases on your list. But pay attention to how certain major headings may alter the terminology. "Stroke" may have a suggested term of "embolism" or even "cerebrovascular incident" depending on the database.
8. Read through the material for inclusion/exclusion . Based on your project's criteria and objective, consider which studies or reviews deserve to be included and which should be discarded. Make sure the information you have permits you to go forward.
9. Write the literature review. Begin by summarizing why your research is important and explain why your approach will help fill gaps in current knowledge. Then incorporate how the information you've selected will help you to do this. You do not need to write about all of the included research you've chosen, only the most pe rtinent.
10. Select the most relevant literature for inclusion in the body of your report. Choose the articles and data sets that are most particularly relevant to your experimental approach. Consider how you might arrange these sources in the body of your draft.
Library Books

Call #: WZ 345 G192h 2011
ISBN #: 9780763771867
This book details a practical, step-by-step method for conducting a literature review in the health sciences. Aiming to synthesize the information while also analyzing it, the Matrix Indexing System enables users to establish a structured process for tracking, organizing and integrating the knowledge within a collection.
Key Research Databases
PubMed - The premier medical database for review articles in medicine, nursing, healthcare, other related biomedical disciplines. PubMed contains over 20 million citations and can be navigated through multiple database capabilities and searching strategies.
CINAHL Ultimate - Offers comprehensive coverage of health science literature. CINAHL is particularly useful for those researching the allied disciplines of nursing, medicine, and pharmaceutical sciences.
Scopus - Database with over 12 million abstracts and citations which include peer-reviewed titles from international and Open Access journals. Also includes interactive bibliometrics and researcher profiling.
Embase - Elsevier's fully interoperable database of both Medline and Emtree-indexed articles. Embase also specializes in pharmacologic interventions.
Cochrane - Selected evidence-based medicine resources from the Cochrane Collaboration that includes peer-reviewed systematic reviews and randomized controlled trials. Access this database through OVID with TTUHSC Libraries.
DARE - Literally the Datatase of Abstracts of Reviews of Effectiveness, this collection of systematic reviews and other evidence-based research contains critical assessments from a wide variety of medical journals.
TRIP - This TRIP database is structured according to the level of evidence for its EBM content. It allows users to quickly and easily locate high-quality, accredited medical literature for clinical and research purposes.
Web of Science - Contains bibliographic articles and data from a wide variety of publications in the life sciences and other fields. Also, see this link for conducting a lit review exclusively within Web of Science.
- << Previous: Welcome
- Next: Drafting >>
- Last Updated: Sep 29, 2023 10:07 AM
- URL: https://ttuhsc.libguides.com/Writing_HealthSciences

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 February 2022
Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics
- Christoph U. Correll ORCID: orcid.org/0000-0002-7254-5646 1 , 2 , 3 ,
- Amber Martin 4 ,
- Charmi Patel 5 ,
- Carmela Benson 5 ,
- Rebecca Goulding 6 ,
- Jennifer Kern-Sliwa 5 ,
- Kruti Joshi 5 ,
- Emma Schiller 4 &
- Edward Kim ORCID: orcid.org/0000-0001-8247-6675 7
Schizophrenia volume 8 , Article number: 5 ( 2022 ) Cite this article
15k Accesses
53 Citations
14 Altmetric
Metrics details
- Schizophrenia
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature review of English-language CPGs and synthesized current recommendations for the acute and maintenance management with antipsychotics. Searches for schizophrenia CPGs were conducted in MEDLINE/Embase from 1/1/2004–12/19/2019 and in guideline websites until 06/01/2020. Of 19 CPGs, 17 (89.5%) commented on first-episode schizophrenia (FES), with all recommending antipsychotic monotherapy, but without agreement on preferred antipsychotic. Of 18 CPGs commenting on maintenance therapy, 10 (55.6%) made no recommendations on the appropriate maximum duration of maintenance therapy, noting instead individualization of care. Eighteen (94.7%) CPGs commented on long-acting injectable antipsychotics (LAIs), mainly in cases of nonadherence (77.8%), maintenance care (72.2%), or patient preference (66.7%), with 5 (27.8%) CPGs recommending LAIs for FES. For treatment-resistant schizophrenia, 15/15 CPGs recommended clozapine. Only 7/19 (38.8%) CPGs included a treatment algorithm.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

The serotonin theory of depression: a systematic umbrella review of the evidence

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Introduction.
Schizophrenia is an often debilitating, chronic, and relapsing mental disorder with complex symptomology that manifests as a combination of positive, negative, and/or cognitive features 1 , 2 , 3 . Standard management of schizophrenia includes the use of antipsychotic medications to help control acute psychotic episodes 4 and prevent relapses 5 , 6 , whereas maintenance therapy is used in the long term after patients have been stabilized 7 , 8 , 9 . Two main classes of drugs—first- and second-generation antipsychotics (FGA and SGA)—are used to treat schizophrenia 10 . SGAs are favored due to the lower rates of adverse effects, such as extrapyramidal effects, tardive dyskinesia, and relapse 11 . However, pharmacologic treatment for schizophrenia is complicated because nonadherence is prevalent, and is a major risk factor for relapse 9 and poor overall outcomes 12 . The use of long-acting injectable (LAI) versions of antipsychotics aims to limit nonadherence-related relapses and poor outcomes 13 .
Patient treatment pathways and treatment choices are determined based on illness acuity/severity, past treatment response and tolerability, as well as balancing medication efficacy and adverse effect profiles in the context of patient preferences and adherence patterns 14 , 15 . Clinical practice guidelines (CPG) serve to inform clinicians with recommendations that reflect current evidence from meta-analyses of randomized controlled trials (RCTs), individual RCTs and, less so, epidemiologic studies, as well as clinical experience, with the goal of providing a framework and road-map for treatment decisions that will improve quality of care and achieve better patients outcomes. The use of clinical algorithms or other decision trees in CPGs may improve the ease of implementation of the evidence in clinical practice 16 . While CPGs are an important tool for mental health professionals, they have not been updated on a regular basis like they have been in other areas of medicine, such as in oncology. In the absence of current information, other governing bodies, healthcare systems, and hospitals have developed their own CPGs regarding the treatment of schizophrenia, and many of these have been recently updated 17 , 18 , 19 . As such, it is important to assess the latest guidelines to be aware of the changes resulting from consideration of updated evidence that informed the treatment recommendations. Since CPGs are comprehensive and include the diagnosis as well as the pharmacological and non-pharmacological management of individuals with schizophrenia, a detailed comparative review of all aspects of CPGs for schizophrenia would have been too broad a review topic. Further, despite ongoing efforts to broaden the pharmacologic tools for the treatment of schizophrenia 20 , antipsychotics remain the cornerstone of schizophrenia management 8 , 21 . Therefore, a focused review of guideline recommendations for the management of schizophrenia with antipsychotics would serve to provide clinicians with relevant information for treatment decisions.
To provide an updated overview of United States (US) national and English language international guidelines for the management of schizophrenia, we conducted a systematic literature review (SLR) to identify CPGs and synthesize current recommendations for pharmacological management with antipsychotics in the acute and maintenance phases of schizophrenia.
Systematic searches for the SLR yielded 1253 hits from the electronic literature databases. After removal of duplicate references, 1127 individual articles were screened at the title and abstract level. Of these, 58 publications were deemed eligible for screening at the full-text level, from which 19 were ultimately included in the SLR. Website searches of relevant organizations yielded 10 additional records, and an additional three records were identified by the state-by-state searches. Altogether, this process resulted in 32 records identified for inclusion in the SLR. Of the 32 sources, 19 primary CPGs, published/issued between 2004 and 2020, were selected for extraction, as illustrated in the PRISMA diagram (Fig. 1 ). While the most recent APA guideline was identified and available for download in 2020, the reference to cite in the document indicates a publication date of 2021.
SLR systematic literature review.
Of the 19 included CPGs (Table 1 ), three had an international focus (from the following organizations: International College of Neuropsychopharmacology [CINP] 22 , United Nations High Commissioner for Refugees [UNHCR] 23 , and World Federation of Societies of Biological Psychiatry [WFSBP] 24 , 25 , 26 ); seven originated from the US; 17 , 18 , 19 , 27 , 28 , 29 , 30 , 31 , 32 three were from the United Kingdom (British Association for Psychopharmacology [BAP] 33 , the National Institute for Health and Care Excellence [NICE] 34 , and the Scottish Intercollegiate Guidelines Network [SIGN] 35 ); and one guideline each was from Singapore 36 , the Polish Psychiatric Association (PPA) 37 , 38 , the Canadian Psychiatric Association (CPA) 14 , the Royal Australia/New Zealand College of Psychiatrists (RANZCP) 39 , the Association Française de Psychiatrie Biologique et de Neuropsychopharmacologie (AFPBN) from France 40 , and Italy 41 . Fourteen CPGs (74%) recommended treatment with specific antipsychotics and 18 (95%) included recommendations for the use of LAIs, while just seven included a treatment algorithm Table 2 ). The AGREE II assessment resulted in the highest score across the CPGs domains for NICE 34 followed by the American Psychiatric Association (APA) guidelines 17 . The CPA 14 , BAP 33 , and SIGN 35 CPGs also scored well across domains.
Acute therapy
Seventeen CPGs (89.5%) provided treatment recommendations for patients experiencing a first schizophrenia episode 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 41 , but the depth and focus of the information varied greatly (Supplementary Table 1 ). In some CPGs, information on treatment of a first schizophrenia episode was limited or grouped with information on treating any acute episode, such as in the CPGs from CINP 22 , AFPBN 40 , New Jersey Division of Mental Health Services (NJDMHS) 32 , the APA 17 , and the PPA 37 , 38 , while the others provided more detailed information specific to patients experiencing a first schizophrenia episode 14 , 18 , 19 , 23 , 24 , 28 , 33 , 34 , 35 , 36 , 39 , 41 . The American Association of Community Psychiatrists (AACP) Clinical Tips did not provide any information on the treatment of schizophrenia patients with a first episode 29 .
There was little agreement among CPGs regarding the preferred antipsychotic for a first schizophrenia episode. However, there was strong consensus on antipsychotic monotherapy and that lower doses are generally recommended due to better treatment response and greater adverse effect sensitivity. Some guidelines recommended SGAs over FGAs when treating a first-episode schizophrenia patient (RANZCP 39 , Texas Medication Algorithm Project [TMAP] 28 , Oregon Health Authority 19 ), one recommended starting patients on an FGA (UNHCR 23 ), and others stated specifically that there was no evidence of any difference in efficacy between FGAs and SGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , Singapore guidelines 36 ), or did not make any recommendation (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , Schizophrenia Patient Outcomes Research Team [PORT] 30 , 31 ). The BAP 33 and WFBSP 24 noted that while there was probably no difference between FGAs and SGAs in efficacy, some SGAs (olanzapine, amisulpride, and risperidone) may perform better than some FGAs. The Schizophrenia PORT recommendations noted that while there seemed to be no differences between SGAs and FGAs in short-term studies (≤12 weeks), longer studies (one to two years) suggested that SGAs may provide benefits in terms of longer times to relapse and discontinuation rates 30 , 31 . The AFPBN guidelines 40 and Florida Medicaid Program guidelines 18 , which both focus on use of LAI antipsychotics, both recommended an SGA-LAI for patients experiencing a first schizophrenia episode. A caveat in most CPGs was that physicians and their patients should discuss decisions about the choice of antipsychotic and that the choice should consider individual patient factors/preferences, risk of adverse and metabolic effects, and symptom patterns 17 , 18 , 19 , 22 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 41 .
Most CPGs recommended switching to a different monotherapy if the initial antipsychotic was not effective or not well tolerated after an adequate antipsychotic trial at an appropriate dose 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 32 , 33 , 35 , 36 , 39 . For patients initially treated with an FGA, the UNHCR recommended switching to an SGA (olanzapine or risperidone) 23 . Guidance on response to treatment varied in the measures used but typically required at least a 20% improvement in symptoms (i.e. reduction in Positive and Negative Syndrome Scale or Brief Psychiatric Rating Scale scores) from pre-treatment levels.
Several CPGs contained recommendations on the duration of antipsychotic therapy after a first schizophrenia episode. The NJDMHS guidelines 32 recommended nine to 12 months; CINP 22 recommended at least one year; CPA 14 recommended at least 18 months; WFSBP 25 , the Italian guidelines 41 , and NICE 34 recommended 1 to 2 years; and the RANZCP 39 , BAP 33 , and SIGN 35 recommended at least 2 years. The APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy.
Twelve guidelines 14 , 18 , 22 , 24 , 28 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 (63.2%) discussed the treatment of subsequent/multiple episodes of schizophrenia (i.e., following relapse). These CPGs noted that the considerations guiding the choice of antipsychotic for subsequent/multiple episodes were similar to those for a first episode, factoring in prior patient treatment response, adverse effect patterns and adherence. The CPGs also noted that response to treatment may be lower and require higher doses to achieve a response than for first-episode schizophrenia, that a different antipsychotic than used to treat the first episode may be needed, and that a switch to an LAI is an option.
Several CPGs provided recommendations for patients with specific clinical features (Supplementary Table 1 ). The most frequently discussed group of clinical features was negative symptoms, with recommendations provided in the CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , APA 17 , and NJDMHS guidelines; 32 negative symptoms were the sole focus of the guidelines from the PPA 37 , 38 . The guidelines noted that due to limited evidence in patients with predominantly negative symptoms, there was no clear benefit for any strategy, but that options included SGAs (especially amisulpride) rather than FGAs (WFSBP 24 , CINP 22 , AFPBN 40 , SIGN 35 , NJDMHS 32 , PPA 37 , 38 ), and addition of an antidepressant (WFSBP 24 , UNHCR 23 , SIGN 35 , NJDMHS 32 ) or lamotrigine (SIGN 35 ), or switching to another SGA (NJDMHS 32 ) or clozapine (NJDMHS 32 ). The PPA guidelines 37 , 38 stated that the use of clozapine or adding an antidepressant or other medication class was not supported by evidence, but recommended the SGA cariprazine for patients with predominant and persistent negative symptoms, and other SGAs for those with full-spectrum negative symptoms. However, the BAP 33 stated that no recommendations can be made for any of these strategies because of the quality and paucity of the available evidence.
Some of the CPGs also discussed treatment of other clinical features to a limited degree, including depressive symptoms (CINP 22 , UNHCR 23 , CPA 14 , APA 17 , and NJDMHS 32 ), cognitive dysfunction (CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , and NJDMHS 32 ), persistent aggression (CINP 22 , WFSBP 24 , CPA 14 , AFPBN 40 , NICE 34 , SIGN 35 , BAP 33 , and NJDMHS 32 ), and comorbid psychiatric diagnoses (CINP 22 , RANZCP 39 , BAP 33 , APA 17 , and NJDMHS 32 ).
Fifteen CPGs (78.9%) discussed treatment-resistant schizophrenia (TRS); all defined it as persistent, predominantly positive symptoms after two adequate antipsychotic trials; clozapine was the unanimous first choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 . However, the UNHCR guidelines 23 , which included recommendations for treatment of refugees, noted that clozapine is only a reasonable choice in regions where white blood cell monitoring and specialist supervision are available, otherwise, risperidone or olanzapine are alternatives if they had not been used in the previous treatment regimen.
There were few options for patients who are resistant to clozapine therapy, and evidence supporting these options was limited. The CPA guidelines 14 therefore stated that no recommendation can be given due to inadequate evidence. Other CPGs discussed options (but noted there was limited supporting evidence), such as switching to olanzapine or risperidone (WFSBP 24 , TMAP 28 ), adding a second antipsychotic to clozapine (CINP 22 , NICE 34 , TMAP 28 , BAP 33 , Florida Medicaid Program 18 , Oregon Health Authority 19 , RANZCP 39 ), adding lamotrigine or topiramate to clozapine (CINP 22 , Florida Medicaid Program 18 ), combination therapy with two non-clozapine antipsychotics (Florida Medicaid Program 18 , NJDMHS 32 ), and high-dose non-clozapine antipsychotic therapy (BAP 33 , SIGN 35 ). Electroconvulsive therapy was noted as a last resort for patients who did not respond to any pharmacologic therapy, including clozapine, by 10 CPGs 17 , 18 , 19 , 22 , 24 , 28 , 32 , 35 , 36 , 39 .
Maintenance therapy
Fifteen CPGs (78.9%) discussed maintenance therapy to various degrees via dedicated sections or statements, while three others referred only to maintenance doses by antipsychotic agent 18 , 23 , 29 without accompanying recommendations (Supplementary Table 2 ). Only the Italian guideline provided no reference or comments on maintenance treatment. The CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 recommended keeping patients on the same antipsychotic and at the same dose on which they had achieved remission. Several CPGs recommended maintenance therapy at the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , and TMAP 28 ). The CPA 14 and SIGN 35 defined the lower dose as 300–400 mg chlorpromazine equivalents or 4–6 mg risperidone equivalents, and the Singapore guidelines 36 stated that the lower dose should not be less than half the original dose. TMAP 28 stated that given the relapsing nature of schizophrenia, the maintenance dose should often be close to the original dose. While SIGN 35 recommended that patients remain on the same antipsychotic that provided remission, these guidelines also stated that maintenance with amisulpride, olanzapine, or risperidone was preferred, and that chlorpromazine and other low-potency FGAs were also suitable. The BAP 33 recommended that the current regimen be optimized before any dose reduction or switch to another antipsychotic occurs. Several CPGs recommended LAIs as an option for maintenance therapy (see next section).
Altogether, 10/18 (55.5%) CPGs made no recommendations on the appropriate duration of maintenance therapy, noting instead that each patient should be considered individually. Other CPGs made specific recommendations: Both the Both BAP 33 and SIGN 35 guidelines suggested a minimum of 2 years, the NJDMHS guidelines 32 recommended 2–3 years; the WFSBP 25 recommended 2–5 years for patients who have had one relapse and more than 5 years for those who have had multiple relapses; the RANZCP 39 and the CPA 14 recommended 2–5 years; and the CINP 22 recommended that maintenance therapy last at least 6 years for patients who have had multiple episodes. The TMAP was the only CPG to recommend that maintenance therapy be continued indefinitely 28 .
Recommendations on the use of LAIs
All CPGs except the one from Italy (94.7%) discussed the use of LAIs for patients with schizophrenia to some extent. As shown in Table 3 , among the 18 CPGs, LAIs were primarily recommended in 14 CPGs (77.8%) for patients who are non-adherent to other antipsychotic administration routes (CINP 22 , UNHCR 23 , RANZCP 39 , PPA 37 , 38 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , TMAP 28 , NJDMHS 32 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ). Twelve CPGs (66.7%) also noted that LAIs should be prescribed based on patient preference (RANZCP 39 , CPA 14 , AFPBN 40 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , Schizophrenia PORT 30 , 31 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ).
Thirteen CPGs (72.2%) recommended LAIs as maintenance therapy 18 , 19 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 . While five CPGs (27.8%), i.e., AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and the Florida Medicaid Program 18 recommended LAIs specifically for patients experiencing a first episode. While the CPA 14 did not make any recommendations regarding when LAIs should be used, they discussed recent evidence supporting their use earlier in treatment. Five guidelines (27.8%, i.e., Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ) noted that evidence around LAIs was not sufficient to support recommending their use for first-episode patients. The AFPBN guidelines 40 also stated that LAIs (SGAs as first-line and FGAs as second-line treatment) should be more frequently considered for maintenance treatment of schizophrenia. Four CPGs (22.2%, i.e., CINP 22 , UNHCR 23 , Italian guidelines 41 , PPA guidelines 37 , 38 ) did not specify when LAIs should be used. The AACP guidelines 29 , which evaluated only LAIs, recommended expanding their use beyond treatment for nonadherence, suggesting that LAIs may offer a more convenient mode of administration or potentially address other clinical and social challenges, as well as provide more consistent plasma levels.
Treatment algorithms
Only Seven CPGs (36.8%) included an algorithm as part of the treatment recommendations. These included decision trees or flow diagrams that map out initial therapy, durations for assessing response, and treatment options in cases of non-response. However, none of these guidelines defined how to measure response, a theme that also extended to guidelines that did not include treatment algorithms. Four of the seven guidelines with algorithms recommended specific antipsychotic agents, while the remaining three referred only to the antipsychotic class.
LAIs were not consistently incorporated in treatment algorithms and in six CPGs were treated as a separate category of medicine reserved for patients with adherence issues or a preference for the route of administration. The only exception was the Florida Medicaid Program 18 , which recommended offering LAIs after oral antipsychotic stabilization even to patients who are at that point adherent to oral antipsychotics.
Benefits and harms
The need to balance the efficacy and safety of antipsychotics was mentioned by all CPGs as a basic treatment paradigm.
Ten CPGs provided conclusions on benefits of antipsychotic therapy. The APA 17 and the BAP 33 guidelines stated that antipsychotic treatment can improve the positive and negative symptoms of psychosis and leads to remission of symptoms. These CPGs 17 , 33 as well as those from NICE 34 and CPA 14 stated that these treatment effects can also lead to improvements in quality of life (including quality-adjusted life years), improved functioning, and reduction in disability. The CPA 14 and APA 17 guidelines noted decreases in hospitalizations with antipsychotic therapy, and the APA guidelines 17 stated that long-term antipsychotic treatment can also reduce mortality. The UNHCR 23 and the Italian 41 guidelines noted that early intervention increased positive outcomes. The WFSBP 24 , AFPBN 40 , CPA 14 , BAP 33 , APA 17 , and NJDMHS 32 affirmed that relapse prevention is a benefit of continued/maintenance treatment.
Some CPGs (WFSBP 24 , Italian 41 , CPA 14 , and SIGN 35 ) noted that reduced risk for extrapyramidal adverse effects and treatment discontinuation were potential benefits of SGAs vs. FGAs.
The risk of adverse effects (e.g., extrapyramidal, metabolic, cardiovascular, and hormonal adverse effects, sedation, and neuroleptic malignant syndrome) was noted by all CPGs as the major potential harm of antipsychotic therapy 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 . These adverse effects are known to limit long-term treatment and adherence 24 .
This SLR of CPGs for the treatment of schizophrenia yielded 19 most updated versions of individual CPGs, published/issued between 2004 and 2020. Structuring our comparative review according to illness phase, antipsychotic type and formulation, response to antipsychotic treatment as well as benefits and harms, several areas of consistent recommendations emerged from this review (e.g., balancing risk and benefits of antipsychotics, preferring antipsychotic monotherapy; using clozapine for treatment-resistant schizophrenia). On the other hand, other recommendations regarding other areas of antipsychotic treatment were mostly consistent (e.g., maintenance antipsychotic treatment for some time), somewhat inconsistent (e.g., differences in the management of first- vs multi-episode patients, type of antipsychotic, dose of antipsychotic maintenance treatment), or even contradictory (e.g., role of LAIs in first-episode schizophrenia patients).
Consistent with RCT evidence 43 , 44 , antipsychotic monotherapy was the treatment of choice for patients with first-episode schizophrenia in all CPGs, and all guidelines stated that a different single antipsychotic should be tried if the first is ineffective or intolerable. Recommendations were similar for multi-episode patients, but factored in prior patient treatment response, adverse effect patterns, and adherence. There was also broad consensus that the side-effect profile of antipsychotics is the most important consideration when making a decision on pharmacologic treatment, also reflecting meta-analytic evidence 4 , 5 , 10 . The risk of extrapyramidal symptoms (especially with FGAs) and metabolic effects (especially with SGAs) were noted as key considerations, which are also reflected in the literature as relevant concerns 4 , 45 , 46 , including for quality of life and treatment nonadherence 47 , 48 , 49 , 50 .
Largely consistent with the comparative meta-analytic evidence regarding the acute 4 , 51 , 52 and maintenance antipsychotic treatment 5 effects of schizophrenia, the majority of CPGs stated there was no difference in efficacy between SGAs and FGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , and Singapore guidelines 36 ), or did not make any recommendations (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , and Schizophrenia PORT 30 , 31 ); three CPGs (BAP 33 , WFBSP 24 , and Schizophrenia PORT 30 , 31 ) noted that SGAs may perform better than FGAs over the long term, consistent with a meta-analysis on this topic 53 .
The 12 CPGs that discussed treatment of subsequent/multiple episodes generally agreed on the factors guiding the choices of an antipsychotic, including that the decision may be more complicated and response may be lower than with a first episode, as described before 7 , 54 , 55 , 56 .
There was little consensus regarding maintenance therapy. Some CPGs recommended the same antipsychotic and dose that achieved remission (CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 ) and others recommended the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , TMAP 28 , CPA 14 , and SIGN 35 ). This inconsistency is likely based on insufficient data as well as conflicting results in existing meta-analyses on this topic 57 , 58 , 59 .
The 15 CPGs that discussed TRS all used the same definition for this condition, consistent with recent commendations 60 , and agreed that clozapine is the primary evidence-based treatment choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , reflecting the evidence base 61 , 62 , 63 . These CPGs also agreed that there are few options well supported by evidence for patients who do not respond to clozapine, with a recent meta-analysis of RCTs showing that electroconvulsive therapy augmentation may be the most evidence-based treatment option 64 .
One key gap in the treatment recommendations was how long patients should remain on antipsychotic therapy after a first episode or during maintenance therapy. While nine of the 17 CPGs discussing treatment of a first episode provided a recommended timeframe (varying from 1 to 2 years) 14 , 22 , 24 , 32 , 33 , 34 , 35 , 39 , 41 , the APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy. Similarly, six of the 18 CPGs discussing maintenance treatment recommended a specific duration of therapy (ranging from two to six years) 14 , 22 , 25 , 32 , 39 , while as many as 10 CPGs did not point to a firm end of the maintenance treatment, instead recommending individualized decisions. The CPGs not stating a definite endpoint or period of maintenance treatment after repeated schizophrenia episodes or even after a first episode of schizophrenia, reflects the different evidence types on which the recommendation is based. The RCT evidence ends after several years of maintenance treatment vs. discontinuation supporting ongoing antipsychotic treatment; however, naturalistic database studies do not indicate any time period after which one can safely discontinue maintenance antipsychotic care, even after a first schizophrenia episode 8 , 65 . In fact, stopping antipsychotics is associated not only with a substantially increased risk of hospitalization but also mortality 65 , 66 , 67 . In this sense, not stating an endpoint for antipsychotic maintenance therapy should not be taken as an implicit statement that antipsychotics should be discontinued at any time; data suggest the contrary.
A further gap exists regarding the most appropriate treatment of negative symptoms, such as anhedonia, amotivation, asociality, affective flattening, and alogia 1 , a long-standing challenge in the management of patients with schizophrenia. Negative symptoms often persist in patients after positive symptoms have resolved, or are the presenting feature in a substantial minority of patients 22 , 35 . Negative symptoms can also be secondary to pharmacotherapy 22 , 68 . Antipsychotics have been most successful in treating positive symptoms, and while eight of the CPGs provided some information on treatment of negative symptoms, the recommendations were generally limited 17 , 22 , 23 , 24 , 32 , 33 , 35 , 40 . Negative symptom management was a focus of the PPA guidelines, but the guidelines acknowledged that supporting evidence was limited, often due to the low number of patients with predominantly negative symptoms in clinical trials 37 , 38 . The Polish guidelines are also one of the more recently developed and included the newer antipsychotic cariprazine as a first-line option, which although being a point of differentiation from the other guidelines, this recommendation was based on RCT data 69 .
Another area in which more direction is needed is on the use of LAIs. While all but one of the 19 CPGs discussed this topic, the extent of information and recommendations for LAI use varied considerably. All CPGs categorized LAIs as an option to improve adherence to therapy or based on patient preference. However, 5/18 CPGs (27.8%) recommended the use of LAI early in treatment (at first episode: AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and Florida Medicaid Program 18 ) or across the entire illness course, while five others stated there was not sufficient evidence to recommend LAIs for these patients (Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ). The role of LAIs in first-episode schizophrenia was the only point where opposing recommendations were found across CPGs. This contradictory stance was not due to the incorporation of newer data suggesting benefits of LAIs in first episode and early-phase patients with schizophrenia 70 , 71 , 72 , 73 , 74 in the CPGs recommending LAI use in first-episode patients, as CPGs recommending LAI use were published between 2005 and 2020, while those opposing LAI use were published between 2011 and 2020. Only the Florida Medicaid CPG recommended LAIs as a first step equivalent to oral antipsychotics (OAP) after initial OAP response and tolerability, independent of nonadherence or other clinical variables. This guideline was also the only CPG to fully integrate LAI use in their clinical algorithm. The remaining six CPGs that included decision tress or treatment algorithms regarded LAIs as a separate paradigm of treatment reserved for nonadherence or patients preference rather than a routine treatment option to consider. While some CPGs provided fairly detailed information on the use of LAIs (AFPBN 40 , AACP 29 , Oregon Health Authority 19 , and Florida Medicaid Program 18 ), others mentioned them only in the context of adherence issues or patient preference. Notably, definitions of and means to determine nonadherence were not reported. One reason for this wide range of recommendations regarding the placement of LAIs in the treatment algorithm and clinical situations that prompt LAI use might be due to the fact that CPGs generally favor RCT evidence over evidence from other study designs. In the case of LAIs, there was a notable dissociation between consistent meta-analytic evidence of statistically significant superiority of LAIs vs OAPs in mirror-image 75 and cohort study designs 76 and non-significant advantages in RCTs 77 . Although patients in RCTs comparing LAIs vs OAPs were less severely ill and more adherent to OAPs 77 than in clinical care and although mirror-image and cohort studies arguably have greater external validity than RCTs 78 , CPGs generally disregard evidence from other study designs when RCT evidence exits. This narrow focus can lead to disregarding important additional data. Nevertheless, a most updated meta-analysis of all 3 study designs comparing LAIs with OAPs demonstrated consistent superiority of LAIs vs OAPs for hospitalization or relapse across all 3 designs 79 , which should lead to more uniform recommendations across CPGs in the future.
Only seven CPGs included treatment algorithms or flow charts to guide LAI treatment selection for patients with schizophrenia 17 , 18 , 19 , 24 , 29 , 35 , 40 . However, there was little commonality across algorithms beyond the guidance on LAIs mentioned above, as some listed specific treatments and conditions for antipsychotic switches, while others indicated that medication choice should be based on a patient’s preferences and responses, side effects, and in some cases, cost effectiveness. Since algorithms and flow charts facilitate the reception, adoption and implementation of guidelines, future CPGs should include them as dissemination tools, but they need to reflect the data and detailed text and be sufficiently specific to be actionable.
The systematic nature in the identification, summarization, and assessment of the CPGs is a strength of this review. This process removed any potential bias associated with subjective selection of evidence, which is not reproducible. However, only CPGs published in English were included and regardless of their quality and differing timeframes of development and publication, complicating a direct comparison of consensus and disagreement. Finally, based on the focus of this SLR, we only reviewed pharmacologic management with antipsychotics. Clearly, the assessment, other pharmacologic and, especially, psychosocial interventions are important in the management of individuals with schizophrenia, but these topics that were covered to varying degrees by the evaluated CPGs were outside of the scope of this review.
Numerous guidelines have recently updated their recommendations on the pharmacological treatment of patients with schizophrenia, which we have summarized in this review. Consistent recommendations were observed across CPGs in the areas of balancing risk and benefits of antipsychotics when selecting treatment, a preference for antipsychotic monotherapy, especially for patients with a first episode of schizophrenia, and the use of clozapine for treatment-resistant schizophrenia. By contrast, there were inconsistencies with regards to recommendations on maintenance antipsychotic treatment, with differences existing on type and dose of antipsychotic, as well as the duration of therapy. However, LAIs were consistently recommended, but mainly suggested in cases of nonadherence or patient preference, despite their established efficacy in broader patient populations and clinical scenarios in clinical trials. Guidelines were sometimes contradictory, with some recommending LAI use earlier in the disease course (e.g., first episode) and others suggesting they only be reserved for later in the disease. This inconsistency was not due to lack of evidence on the efficacy of LAIs in first-episode schizophrenia or the timing of the CPG, so that other reasons might be responsible, including possibly bias and stigma associated with this route of treatment administration. Lastly, gaps existed in the guidelines for recommendations on the duration of maintenance treatment, treatment of negative symptoms, and the development/use of treatment algorithms whenever evidence is sufficient to provide a simplified summary of the data and indicate their relevance for clinical decision making, all of which should be considered in future guideline development/revisions.
The SLR followed established best methods used in systematic review research to identify and assess the available CPGs for pharmacologic treatment of schizophrenia with antipsychotics in the acute and maintenance phases 80 , 81 . The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including use of a prespecified protocol to outline methods for conducting the review. The protocol for this review was approved by all authors prior to implementation but was not submitted to an external registry.
Data sources and search algorithms
Searches were conducted by two independent investigators in the MEDLINE and Embase databases via OvidSP to identify CPGs published in English. Articles were identified using search algorithms that paired terms for schizophrenia with keywords for CPGs. Articles indexed as case reports, reviews, letters, or news were excluded from the searches. The database search was limited to CPGs published from January 1, 2004, through December 19, 2019, without limit to geographic location. In addition to the database sources, guideline body websites and state-level health departments from the US were also searched for relevant CPGs published through June 2020. A manual check of the references of recent (i.e., published in the past three years), relevant SLRs and relevant practice CPGs was conducted to supplement the above searches and ensure and the most complete CPG retrieval.
This study did not involve human subjects as only published evidence was included in the review; ethical approval from an institution was therefore not required.
Selection of CPGs for inclusion
Each title and abstract identified from the database searches was screened and selected for inclusion or exclusion in the SLR by two independent investigators based on the populations, interventions/comparators, outcomes, study design, time period, language, and geographic criteria shown in Table 4 . During both rounds of the screening process, discrepancies between the two independent reviewers were resolved through discussion, and a third investigator resolved any disagreement. Articles/documents identified by the manual search of organizational websites were screened using the same criteria. All accepted studies were required to meet all inclusion criteria and none of the exclusion criteria. Only the most recent version of organizational CPGs was included for data extraction.
Data extraction and synthesis
Information on the recommendations regarding the antipsychotic management in the acute and maintenance phases of schizophrenia and related benefits and harms was captured from the included CPGs. Each guideline was reviewed and extracted by a single researcher and the data were validated by a senior team member to ensure accuracy and completeness. Additionally, each included CPG was assessed using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool. Following extraction and validation, results were qualitatively summarized across CPGs.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of the SLR are available from the corresponding author upon request.
Correll, C. U. & Schooler, N. R. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 16 , 519–534 (2020).
Article PubMed PubMed Central Google Scholar
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1 , 15067 (2015).
Article PubMed Google Scholar
Millan, M. J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15 , 485–515 (2016).
Article CAS PubMed Google Scholar
Huhn, M. et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 , 939–951 (2019).
Article CAS PubMed PubMed Central Google Scholar
Kishimoto, T., Hagi, K., Nitta, M., Kane, J. M. & Correll, C. U. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18 , 208–224 (2019).
Leucht, S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379 , 2063–2071 (2012).
Carbon, M. & Correll, C. U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 16 , 505–524 (2014).
Correll, C. U., Rubio, J. M. & Kane, J. M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17 , 149–160 (2018).
Emsley, R., Chiliza, B., Asmal, L. & Harvey, B. H. The nature of relapse in schizophrenia. BMC Psychiatry 13 , 50 (2013).
Correll, C. U. & Kane, J. M. Ranking antipsychotics for efficacy and safety in schizophrenia. JAMA Psychiatry 77 , 225–226 (2020).
Kane, J. M. & Correll, C. U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 12 , 345–357 (2010).
Kane, J. M., Kishimoto, T. & Correll, C. U. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12 , 216–226 (2013).
Correll, C. U. et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J. Clin. Psychiatry 77 , 1–24 (2016).
Remington, G. et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 62 , 604–616 (2017).
American Psychiatric Association. Treatment recommendations for patients with schizophrenia. Am. J. Psychiatry 161 , 1–56 (2004).
Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines, Field, M. J., & Lohr, K. N. (Eds.). Clinical Practice Guidelines: Directions for a New Program. (National Academies Press (US), 1990).
American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia , 3rd edn. (2021). https://doi.org/10.1176/appi.books.9780890424841 .
Florida Medicaid Drug Therapy Management Program. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults . (2020). Available at: https://floridabhcenter.org/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf .
Mental Health Clinical Advisory Group, Oregon Health Authority. Mental Health Care Guide for Licensed Practitioners and Mental Health Professionals. (2019). Available at: https://sharedsystems.dhsoha.state.or.us/DHSForms/Served/le7548.pdf .
Krogmann, A. et al. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 24 , 38–69 (2019).
Fountoulakis, K. N. et al. The report of the joint WPA/CINP workgroup on the use and usefulness of antipsychotic medication in the treatment of schizophrenia. CNS Spectr . https://doi.org/10.1017/S1092852920001546 (2020).
Leucht, S. et al. CINP Schizophrenia Guidelines . (CINP, 2013). Available at: https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf .
Ostuzzi, G. et al. Mapping the evidence on pharmacological interventions for non-affective psychosis in humanitarian non-specialised settings: A UNHCR clinical guidance. BMC Med. 15 , 197 (2017).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 13 , 318–378 (2012).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry 14 , 2–44 (2013).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int. J. Psychiatry Clin. Pract. 21 , 82–90 (2017).
Moore, T. A. et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J. Clin. Psychiatry 68 , 1751–1762 (2007).
Texas Medication Algorithm Project (TMAP). Texas Medication Algorithm Project (TMAP) Procedural Manual . (2008). Available at: https://jpshealthnet.org/sites/default/files/inline-files/tmapalgorithmforschizophrenia.pdf .
American Association of Community Psychiatrists (AACP). Clinical Tips Series, Long Acting Antipsychotic Medications. (2017). Accessed at: https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view .
Buchanan, R. W. et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36 , 71–93 (2010).
Kreyenbuhl, J. et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr. Bull. 36 , 94–103 (2010).
New Jersey Division of Mental Health Services. Pharmacological Practice Guidelines for the Treatment of Schizophrenia . (2005). Available at: https://www.state.nj.us/humanservices/dmhs_delete/consumer/NJDMHS_Pharmacological_Practice_Guidelines762005.pdf .
Barnes, T. R. et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 34 , 3–78 (2020).
National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. (2014). Available at: http://www.nice.org.uk/guidance/cg178 .
Scottish Intercollegiate Guidelines Network (SIGN). Management of Schizophrenia: A National Clinical Guideline . (2013). Available at: https://www.sign.ac.uk/assets/sign131.pdf .
Verma, S. et al. Ministry of Health Clinical Practice Guidelines: Schizophrenia. Singap. Med. J. 52 , 521–526 (2011).
CAS Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 1. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 1. 53 , 497–524 (2019).
Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 2. 53 , 525–540 (2019).
Galletly, C. et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust. N. Z. J. Psychiatry 50 , 410–472 (2016).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13 , 340 (2013).
De Masi, S. et al. The Italian guidelines for early intervention in schizophrenia: development and conclusions. Early Intervention Psychiatry 2 , 291–302 (2008).
Article Google Scholar
Barnes, T. R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 25 , 567–620 (2011).
Correll, C. U. et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74 , 675–684 (2017).
Galling, B. et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16 , 77–89 (2017).
Pillinger, T. et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 , 64–77 (2020).
Rummel-Kluge, C. et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 38 , 167–177 (2012).
Angermeyer, M. C. & Matschinger, H. Attitude of family to neuroleptics. Psychiatr. Prax. 26 , 171–174 (1999).
CAS PubMed Google Scholar
Dibonaventura, M., Gabriel, S., Dupclay, L., Gupta, S. & Kim, E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12 , 20 (2012).
McIntyre, R. S. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J. Clin. Psychiatry 70 (Suppl 3), 5–11 (2009).
Tandon, R. et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: a worldwide, cross-sectional, web-based survey. Ann. Gen. Psychiatry 19 , 42 (2020).
Zhang, J. P. et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 16 , 1205–1218 (2013).
Zhu, Y. et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 4 , 694–705 (2017).
Kishimoto, T. et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol. Psychiatry 18 , 53–66 (2013).
Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174 , 927–942 (2017).
Samara, M. T., Nikolakopoulou, A., Salanti, G. & Leucht, S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr. Bull. 45 , 639–646 (2019).
Zhu, Y. et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 27 , 835–844 (2017).
Bogers, J. P. A. M., Hambarian, G., Michiels, M., Vermeulen, J. & de Haan, L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. A systematic review and meta-analysis. Schizophr. Bull. Open 46 , Suppl 1 S326 (2020).
Tani, H. et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology 45 , 887–901 (2020).
Uchida, H., Suzuki, T., Takeuchi, H., Arenovich, T. & Mamo, D. C. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr. Bull. 37 , 788–799 (2011).
Howes, O. D. et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174 , 216–229 (2017).
Masuda, T., Misawa, F., Takase, M., Kane, J. M. & Correll, C. U. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry 76 , 1052–1062 (2019).
Siskind, D., McCartney, L., Goldschlager, R. & Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 209 , 385–392 (2016).
Siskind, D., Siskind, V. & Kisely, S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can. J. Psychiatry 62 , 772–777 (2017).
Wang, G. et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J. Psychiatr. Res. 105 , 23–32 (2018).
Tiihonen, J., Tanskanen, A. & Taipale, H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am. J. Psychiatry 175 , 765–773 (2018).
Taipale, H. et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res . 197 , 274–280 (2018).
Taipale, H. et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 19 , 61–68 (2020).
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19 (Suppl 1), 38–52 (2014).
PubMed Google Scholar
Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 , 625–639 (2018).
Kane, J. M. et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry 77 , 1217–1224 (2020).
Schreiner, A. et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr. Res. 169 , 393–399 (2015).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72 , 822–829 (2015).
Tiihonen, J. et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 168 , 603–609 (2011).
Zhang, F. et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr. Dis. Treat. 11 , 657–668 (2015).
Kishimoto, T., Nitta, M., Borenstein, M., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J. Clin. Psychiatry 74 , 957–965 (2013).
Kishimoto, T. et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr. Bull. 44 , 603–619 (2018).
Kishimoto, T. et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr. Bull. 40 , 192–213 (2014).
Kane, J. M., Kishimoto, T. & Correll, C. U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 66 , S37–41 (2013).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8 , 387–404 (2021).
Cook, D. J., Mulrow, C. D. & Haynes, R. B. Systematic reviews: synthesis of best evidence for clinical decisions. Ann. Intern. Med. 126 , 376–380 (1997).
Higgins, J. P. T. & Green, S. Cochrane Collaboration Handbook for Systematic Reviews of Interventions . (The Cochrane Collaboration and John Wiley & Sons, Ltd, 2008).
Download references
Acknowledgements
This study received financial funding from Janssen Scientific Affairs.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
The Zucker Hillside Hospital, Department of Psychiatry, Northwell Health, Glen Oaks, NY, USA
- Christoph U. Correll
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, NY, USA
Charité Universitätsmedizin Berlin, Department of Child and Adolescent Psychiatry, Berlin, Germany
Evidera, Waltham, MA, USA
Amber Martin & Emma Schiller
Janssen Scientific Affairs, LLC, Titusville, NJ, USA
Charmi Patel, Carmela Benson, Jennifer Kern-Sliwa & Kruti Joshi
Goulding HEOR Consulting Inc., Vancouver, BC, Canada
Rebecca Goulding
Biohaven Pharmaceuticals, New Haven, CT, USA
You can also search for this author in PubMed Google Scholar
Contributions
C.C., A.M., R.G., C.P., C.B., K.J., J.K.S., E.S. and E.K. contributed to the conception and the design of the study. A.M., R.G. and E.S. conducted the literature review, including screening, and extraction of the included guidelines. All authors contributed to the interpretations of the results for the review; A.M. and C.C. drafted the manuscript and all authors revised it critically for intellectual content. All authors gave their final approval of the completed manuscript.
Corresponding author
Correspondence to Christoph U. Correll .
Ethics declarations
Competing interests.
C.C. has received personal fees from Alkermes plc, Allergan plc, Angelini Pharma, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Inc, Janssen Pharmaceutica/Johnson & Johnson, LB Pharma International BV, H Lundbeck A/S, MedAvante-ProPhase, Medscape, Neurocrine Biosciences, Noven Pharmaceuticals, Inc, Otsuka Pharmaceutical Co, Inc, Pfizer, Inc, Recordati, Rovi, Sumitomo Dainippon Pharma, Sunovion Pharmaceuticals, Inc, Supernus Pharmaceuticals, Inc, Takeda Pharmaceutical Company Limited, Teva Pharmaceuticals, Acadia Pharmaceuticals, Inc, Axsome Therapeutics, Inc, Indivior, Merck & Co, Mylan NV, MedInCell, and Karuna Therapeutics and grants from Janssen Pharmaceutica, Takeda Pharmaceutical Company Limited, Berlin Institute of Health, the National Institute of Mental Health, Patient Centered Outcomes Research Institute, and the Thrasher Foundation outside the submitted work; receiving royalties from UpToDate; and holding stock options in LB Pharma. A.M., R.G., and E.S. were all employees of Evidera at the time the study was conducted on which the manuscript was based. C.P., C.B., K.J., J.K.S., and E.K. were all employees of Janssen Scientific Affairs, who hold stock/shares, at the time the study was conducted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Correll, C.U., Martin, A., Patel, C. et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophr 8 , 5 (2022). https://doi.org/10.1038/s41537-021-00192-x
Download citation
Received : 26 February 2021
Accepted : 02 November 2021
Published : 24 February 2022
DOI : https://doi.org/10.1038/s41537-021-00192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Psychosis superspectrum ii: neurobiology, treatment, and implications.
- Roman Kotov
- William T. Carpenter
- Katherine G. Jonas
Molecular Psychiatry (2024)
Sex Differences Between Female and Male Individuals in Antipsychotic Efficacy and Adverse Effects in the Treatment of Schizophrenia
- Megan Galbally
- Karen Wynter
- Susanna Every-Palmer
CNS Drugs (2024)
Antipsychotic Discontinuation through the Lens of Epistemic Injustice
- Helene Speyer
- Lene Falgaard Eplov
Community Mental Health Journal (2024)
Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery
- Celso Arango
- Andrea Fagiolini
BMC Psychiatry (2023)
Machine learning methods to predict outcomes of pharmacological treatment in psychosis
- Lorenzo Del Fabro
- Elena Bondi
- Paolo Brambilla
Translational Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 05 June 2024
Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature
- Javier Loricera 1 na1 ,
- Toluwalase Tofade 2 na1 ,
- Diana Prieto-Peña 1 ,
- Susana Romero-Yuste 3 ,
- Eugenio de Miguel 4 ,
- Anne Riveros-Frutos 5 ,
- Iván Ferraz-Amaro 6 ,
- Eztizen Labrador 7 ,
- Olga Maiz 8 ,
- Elena Becerra 9 ,
- Javier Narváez 10 ,
- Eva Galíndez-Agirregoikoa 11 ,
- Ismael González-Fernández 12 ,
- Ana Urruticoechea-Arana 13 ,
- Ángel Ramos-Calvo 14 ,
- Fernando López-Gutiérrez 1 ,
- Santos Castañeda 15 ,
- Sebastian Unizony ORCID: orcid.org/0000-0002-8935-7473 16 na2 &
- Ricardo Blanco ORCID: orcid.org/0000-0003-2344-2285 1 na2
Arthritis Research & Therapy volume 26 , Article number: 116 ( 2024 ) Cite this article
114 Accesses
Metrics details
A substantial proportion of patients with giant cell arteritis (GCA) relapse despite standard therapy with glucocorticoids, methotrexate and tocilizumab. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway is involved in the pathogenesis of GCA and JAK inhibitors (JAKi) could be a therapeutic alternative. We evaluated the effectiveness of JAKi in relapsing GCA patients in a real-world setting and reviewed available literature.
Retrospective analysis of GCA patients treated with JAKi for relapsing disease at thirteen centers in Spain and one center in United States (01/2017-12/2022). Outcomes assessed included clinical remission, complete remission and safety. Clinical remission was defined as the absence of GCA signs and symptoms regardless of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values. Complete remission was defined as the absence of GCA signs and symptoms along with normal ESR and CRP values. A systematic literature search for other JAKi-treated GCA cases was conducted.
Thirty-five patients (86% females, mean age 72.3) with relapsing GCA received JAKi therapy (baricitinib, n = 15; tofacitinib, n = 10; upadacitinib, n = 10). Before JAKi therapy, 22 (63%) patients had received conventional synthetic immunosuppressants (e.g., methotrexate), and 30 (86%) biologics (e.g., tocilizumab). After a median (IQR) follow-up of 11 (6-15.5) months, 20 (57%) patients achieved and maintained clinical remission, 16 (46%) patients achieved and maintained complete remission, and 15 (43%) patients discontinued the initial JAKi due to relapse ( n = 11 [31%]) or serious adverse events ( n = 4 [11%]). A literature search identified another 36 JAKi-treated GCA cases with clinical improvement reported for the majority of them.
Conclusions
This real-world analysis and literature review suggest that JAKi could be effective in GCA, including in patients failing established glucocorticoid-sparing therapies such as tocilizumab and methotrexate. A phase III randomized controlled trial of upadacitinib is currently ongoing (ClinicalTrials.gov ID NCT03725202).
Introduction
Giant cell arteritis (GCA) is an inflammatory disorder of large and medium-sized arteries affecting people over 50 years [ 1 , 2 ]. It is the most common type of vasculitis in adults in Europe and North America. The disease is characterized by the granulomatous involvement of the aorta and its main branches with complications including blindness and thoracic aortic aneurysm [ 1 , 2 ].
Glucocorticoids have been the cornerstone of GCA treatment for decades at the expense of significant treatment-related toxicity and high relapse rates upon dose reduction or drug discontinuation. Other therapies, such as methotrexate, leflunomide, azathioprine, hydroxychloroquine, cyclophosphamide or tumor necrosis factor (TNF)- \(a\) inhibitors have proven to be ineffective or shown mixed results [ 3 , 4 ]. To date, tocilizumab, a monoclonal antibody against the IL-6 receptor (IL-6R), is the only medication with demonstrated efficacy in terms of remission maintenance and glucocorticoid-sparing [ 5 , 6 ]. However, up to 40% of patients receiving tocilizumab fail treatment due to disease relapse or tocilizumab-related side effects [ 6 , 7 ]. In addition, more than half of the patients responding to tocilizumab relapse upon drug discontinuation [ 6 , 8 , 9 , 10 ]. Therefore, other treatment options are greatly needed for patients with GCA.
Advances in the understanding of the pathophysiology of GCA have paved the way for several therapies that are currently under investigation [ 11 , 12 , 13 ]. In the last years, the critical role of the janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways in immune-mediated diseases has been therapeutically exploited with the development of JAK inhibitors (JAKi), small molecules that block the action of type I/II cytokines [ 14 ]. In GCA, CD4 + T-cells and macrophages, which respond to certain key mediators through the JAK/STAT system (e.g., IL-6/STAT3, granulocyte-macrophage colony-stimulating factor [GM-CSF]/STAT5, interferon [IFN]-γ/STAT1), are present in the arterial inflammatory lesions [ 14 ]. Thus, the inhibition of JAK signalling could be an effective treatment as suggested in animal models [ 14 ]. Nevertheless, the published literature on the utility of JAKi in GCA is scarce consisting in retrospective case reports and small series of patients, and a single prospective pilot study of 15 patients [ 15 , 16 , 17 , 18 , 19 , 20 ]. Therefore, additional data on this topic would be valuable.
We retrospectively assessed the outcomes of a series of patients with relapsing GCA treated with JAKi in a real-world setting. We also systematically searched the literature for other patients treated with JAKi and compared the group of patients in our series receiving baricitinib with patients that received this medication in the setting of the pilot study mentioned above [ 15 ].
Study design and patient population
We conducted an observational, retrospective analysis of patients with GCA treated with JAKi in thirteen centers in Spain and one center in United States. All patients met the 1990 American College of Rheumatology classification criteria for GCA [ 21 ], and/or had positive temporal artery biopsy or evidence suggesting vasculitis by imaging. The types of vascular imaging studies considered for the purpose of GCA diagnosis were ultrasound of the temporal arteries (i.e., halo sign), and computed tomography angiography (CTA) (i.e., diffuse, concentric mural thickening), magnetic resonance imaging/angiography (MRI/MRA) (i.e., diffuse, concentric mural thickening with or without T2 hyperintensity and/or contrast uptake), and positron emission tomography (PET) (e.g., diffuse, concentric F18 fluorodeoxyglucose [FDG] uptake) of the large arteries (e.g., aorta and main aortic branches).
Patients received JAKi at the discretion of the treating rheumatologist for disease relapsed despite the use of glucocorticoids (e.g., prednisone or methylprednisolone) and other immunosuppressants including conventional synthetic (e.g., methotrexate) and biologic (e.g., tocilizumab) immunosuppressants. A washout period corresponding to a half-life of elimination of the biologic agent was carried out between the end of biologic therapy and the start of JAKi therapy. Because this was a retrospective study of real-world data generated by a group of independent rheumatologists, there was no pre-determined criteria to select the type or dose of JAKi or the glucocorticoid tapering regimen following JAKi therapy initiation. Factors influencing providers when making those decisions may have included patient’s preference, provider’s experience and judgement, insurance authorization, cost, and safety.
Study assessments and outcomes
Effectiveness and safety outcomes were evaluated during JAKi treatment by systematically reviewing all rheumatology notes, laboratory values and vascular imaging results available in each patient’s medical record. During follow-up, patients were seen by the rheumatology providers at variable intervals, but mostly every one to six months. Data were extracted from the medical records following a specifically designed protocol. To minimize entry mistakes, all data were double-checked.
The primary outcome was clinical remission defined as the absence of signs and symptoms attributable to GCA (e.g., headaches, jaw claudication, polymyalgia rheumatica symptoms [PMR], etc.) regardless of the value of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Secondary outcomes included complete remission defined, as per the European Alliance of Associations for Rheumatology (EULAR) criteria, as the absence of signs and symptoms attributable to GCA and the normalization of the ESR and CRP values [ 22 ]. Relapse was defined as the reappearance of clinical manifestations of GCA that required treatment intensification [ 23 ]. Additional outcomes evaluated were the ability to discontinue glucocorticoids and the occurrence of adverse events.
The ESR was considered to be increased when it was higher than 20 or 25 mm/hour for men or women, respectively. A serum CRP value greater than 0.5 mg/dL was considered elevated. Anemia was defined as a hemoglobin level ≤ 11 g/dL, leukopenia as < 4000 leukocytes/µL, lymphopenia as < 1500 lymphocytes/µL, neutropenia as < 1500 neutrophils/µL, and thrombocytopenia as < 100,000 platelets/µL.
Systematic literature search
A systematic literature search of published randomized controlled trials, non-randomized trials, cohort studies, case series, and case reports was done in MEDLINE/PubMed ( https://pubmed.ncbi.nlm.nih.gov/ ) from the inception of each database to May 31, 2023.
Statistical analysis
All data were analyzed using descriptive methods. Continuous data were summarized using means, medians, standard deviations (SD), ranges and interquartile ranges (IQR) where appropriate. Categorical data were summarized as numbers and corresponding percentages. Additionally, a comparison between 15 GCA patients from a pilot study by Koster et al. [ 15 ] and the 15 GCA patients of our series treated with baricitinib was performed. Continues variables were compared using Mann-Whitney U-test and categorical variables were compared using Fisher´s exact test. Statistical significance was considered as a p-value < 0.05. The analysis was conducted using STATISTICA software (StatSoft Inc. Tulsa, OK, USA).
Ethical considerations
The study was approved by the Cantabria Clinical Research Ethics Comittee (approval number 2021.414), and was conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonization. All data extracted from the medical records were stored de-identified prior to the analysis. As per the Clinical Research Ethics Committee, this retrospective research did not require informed consent.
Baseline general features at JAKi initiation
A total of 35 patients (30 women and 5 men) with GCA who received treatment with JAKi were included. GCA was confirmed by temporal artery biopsy in 15 (62%) patients and by vascular imaging in 24 (69%) patients [Table 1 ]. Vascular ultrasonography was performed in 15 patients, observing signs of vasculitis in 7 of them. The mean (SD) age at the initiation of JAKi therapy was 72.3 (8.0) years. Overall, 15 (43%) patients received baricitinib (2–4 mg daily), 10 (29%) tofacitinib (5 mg twice a day) and 10 (29%) upadacitinib (15 mg daily). The median (IQR) time from GCA diagnosis to JAKi therapy initiation was 30 (12–48) months. Without considering concomitant glucocorticoid use, JAKi was prescribed as monotherapy in 34 (97%) patients, and combined with methotrexate in one patient. Thus, only one patient who started treatment with baricitinib maintained concurrent treatment with methotrexate at a dose of 10 mg weekly and a prednisone dose of 5 mg/day. Thirty-one patients started treatment with JAKi in combination with glucocorticoids, and three patients initiated JAKi therapy without any other drugs for the treatment of GCA.
The main clinical manifestations of the patients at the time of JAKi initiation are summarized in Table 1 . Those included headache ( n = 15 [43%]), jaw claudication ( n = 6 [17%]), visual symptoms ( n = 5 [14%]), and PMR symptoms ( n = 12 [34%]). The median (IQR) baseline serum CRP and ESR values were 0.9 (0.4–2.5) mg/dL and 28 (7–48) mm/hour. The median (IQR) baseline prednisone dose was 16.2 (8.7–30) mg/day.
Before JAKi therapy, 22 (63%) patients had received several conventional synthetic immunosuppressants such as methotrexate ( n = 22 [63%]), hydroxychloroquine ( n = 3 [9%]), and leflunomide ( n = 1 [3%]) [Table 1 ]. In addition, 30 (86%) patients had been treated with biologics including tocilizumab ( n = 26 [74%]), sarilumab ( n = 3 [9%]), abatacept ( n = 8 [23%]), adalimumab ( n = 2 [6%]), and ustekinumab ( n = 2 [6%]) [Table 1 ].
Clinical outcomes
Once on JAKi, patients were followed for a median (IQR) period of 11 (6-15.5) months with 35 patients followed for at least one month, 33 patients followed for at least three months, 28 patients followed for at least six months and 20 patients followed for at least twelve months. Most patients experienced improvement of clinical manifestations and laboratory parameters over time following JAKi therapy. Clinical remission was observed at one, three, six and twelve months in 18/35 (51%), 18/33 (54%), 17/28 (61%) and 14/20 (70%) patients, respectively [Fig. 1 A]. Complete remission was observed at one, three, six and twelve months in 15/35 (43%), 16/33 (48%), 16/28 (57%) and 13/20 (65%) patients, respectively [Fig. 1 B]. Effectiveness was similar across all JAKi [Fig. 1 A and B].

Clinical outcomes of 35 patients with Giant Cell Arteritis after JAK inhibitor initiation. Legend: ( A ) Clinical remission; ( B ) Complete remission. JAK: Janus kinase
The median (IQR) ESR declined from 28 (7–48) mm/hour at baseline to 3 (7.2–29.2) mm/hour at last follow-up ( p < 0.001). The median (IQR) CRP concentration decreased from 0.9 (0.4–2.5) at baseline to 0.4 (0.2–2.1) mg/dL at last follow up ( p = 0.53) [Fig. 2 A and B]. The median (IQR) daily dose of prednisone decreased from 16.2 (8.7–30) at baseline to 5 (0-12.5) mg at last follow up ( p < 0.001) [Fig. 2 C]. In addition, 7 (20%) patients were able to stop glucocorticoids completely.
Laboratory abnormalities and reduction of glucocorticoid dose after JAK inhibitor initiation
Legend: (data expressed as median values; p compared with baseline). ( A ) Erythrocyte sedimentation rate (ESR); ( B ) Serum C-reactive protein (CRP); and ( C ) Glucocorticoid dose. JAK: Janus kinase. *: p < 0.05 in overall series. +: p < 0.05 in baricitinib group. #: p < 0.05 in tofacitinib group. ^: p < 0.05 in upadacitinib group
Overall, eleven (31%) patients discontinued JAKi therapy due to relapse or persistence of active disease. Of these eleven patients, five were on tofacitinib, four on baricitinib, and two on upadacitinib.
Adverse events were reported in five (14%) patients during JAKi therapy. One patient on baricitinib developed a urinary tract infection without requiring permanent JAKi discontinuation. The adverse events led to drug discontinuation in the other four patients. These cases included a 74-year-old woman on baricitinib 2 mg/day that developed significant elevation of liver enzymes, a 67-year-old woman on tofacitinib 5 mg twice a day that developed palpitations and dyspnea, a 67-year-old woman on upadacitinib 15 mg daily complicated with disseminated herpes zoster, and a 72-year-old man diagnosed with glioblastoma multiforme six months after starting upadacitinib 15 mg daily. No thromboembolism, major adverse cardiovascular events, or significant cytopenias were observed during follow-up. No cases of GCA-related permanent vision loss were reported either.
Outcomes in patients previously treated with IL-6R antagonists
Overall, 28 (80%) patients (24 women and 4 men) had previously received treatment with IL-6R blockers including tocilizumab ( n = 26) and sarilumab ( n = 3) (one patient received both). Within this group of 28 patients, IL-6R blockade therapy had been stopped due to inefficacy in 20 (71%) of them.
The JAKi prescribed to this subgroup of patients were baricitinib ( n = 10), tofacitinib ( n = 9), and upadacitinib ( n = 9). During a median (IQR) follow-up of 12 (8.7–16.2) months, 16 (57%) out of 28 patients who had previously received IL-6R blockers achieved clinical remission (baricitinib [ n = 7; 44%], tofacitinib [ n = 5; 31%], upadacitinib [ n = 4; 25%]), and 13 (46%) of them complete remission (baricitinib [ n = 6; 46%], tofacitinib [ n = 3; 23%], upadacitinib [ n = 4; 31%]), no observing differences between the three JAKi used. As expected, the ESR (median [IQR] 11 [5–39] mm/hour) and CRP (median [IQR] 0.8 mg/dL [0.3–1.9]) values at the moment of IL-6R blockade therapy discontinuation and initiation of JAKi were normal. Those values further decreased during JAKi therapy (median [IQR] ESR 10 [5.7–22] mm/hour and CRP 0.4 [0.1–1.2] at last follow up, p > 0.05 for both comparisons) At the last visit, the median (IQR) dose of prednisone decreased from 15.6 (10–30) mg/day to 5 (0.6–10) mg/day ( p < 0.001). Of 25 patients who were receiving glucocorticoids at JAKi initiation, six (24%) were able to discontinue them. The median (IQR) daily prednisone dose at last follow up was 5 [0.6–10] mg. JAKi was withdrawn because of GCA relapse in nine (32%) patients. Of these, five patients were receiving tofacitinib, two baricitinib, and 2 upadacitinib.
Comparison between patients treated with baricitinib in a prospective pilot study and this case series
The main features of the two series of patients are summarized in Supplementary Table 1 . The study by Koster et al. was a prospective, proof-of-concept trial of baricitinib (4 mg/day) that enrolled 15 GCA patients with relapsing disease, and employed a relatively rapid (15–22 weeks), structured glucocorticoid taper starting at 10 mg, 20 mg or 30 mg/day [ 15 ]. Compared to the patients included in the study by Koster et al., the 15 patients in our series treated with baricitinib had longer disease duration (median [IQR] 32 [12–48] months vs. 9 [ 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ] months; p = 0.008), less incidence of PMR symptoms at baseline (53% vs. 26%; p = 0.010), significantly higher levels of ESR and CRP at baseline ( p ≤ 0.001), and higher rate of prior immunosuppressive treatment failure at the time of starting baricitinib ( p < 0.001). As expected given the different study design (prospective clinical trial vs. retrospective real-world study), the prednisone dose at six and twelve months after baricitinib initiation was higher in the patients included in our series. All patients from the study by Koster et al. [ 15 ] who completed twelve months of treatment were able to discontinue prednisone, while in our series the median (IQR) daily prednisone dose of the eight patients that received baricitinib for 12 months was 3.7 [0.6–10.6] with only one (14%) patient completely off prednisone by that timepoint (Supplementary Table 1 ). By week 52, thirteen of the fourteen patients (93%) completing the trial prococol by Koster et al. and five of the eight patients (62%) treated with baricitinib who had reached 52 weeks of in our series were in remission.
The results from this observational study and from the literature review indicate that JAKi may be effective in a sizable proportion of GCA patients, including those that previously failed established glucocorticoid-sparing treatments such as methotrexate and tocilizumab. After a median follow up of nearly one year, approximately 60% of the patients receiving JAKi in our series achieved and maintained clinical remission. In addition, the patients in our cohort were able to significantly reduce their daily prednisone doses to a median of 5 mg and 20% of them weaned off glucocorticoids completely.
Glucocorticoids have been the cornerstone of the treatment of GCA for decades. However, relapses are common when glucocorticoid doses are tapered and adverse events from this type of medications are frequent [ 24 ]. In addition, glucocorticoids impair quality of life and are negatively regarded in the long-term by most patients with GCA [ 24 ]. Over the last several years, other drugs, such as methotrexate and TNF- \(a\) inhibitors have been trialed with controversial or disappointing results [ 3 , 4 ]. More recently, phase 2 randomized controlled trials with abatacept, mavrilimumab, and secukinumab have shown encouraging preliminary findings that await confirmation [ 11 , 12 , 13 ]. The only medication thus far with demonstrated efficacy in a phase 3 randomized controlled trial, however, is tocilizumab [ 6 ]. Nevertheless, up to one third of patients relapse while on tocilizumab and up to 10% must discontinue treatment due to adverse events [ 6 , 7 , 9 , 25 ]. Furthermore, nearly two thirds of patients experience relapses within 12–24 months after tocilizumab discontinuation [ 8 , 9 , 10 ]. The treatment landscape described above underscores the need for additional GCA therapies.
Macrophages and CD4 + cell with T helper phenotype type 1 (Th1) and 17 (Th17), which are the main immune cell effectors present in GCA lesions, respond to several cytokines through JAK/STAT pathways, and produce cytokines that in turn amplify the inflammatory response in a JAK/STAT-dependent manner creating a vicious cycle [ 26 , 27 ]. These cytokines include IL-2, IL-12, IL-23, IL-6, GM-CSF, and IFN- \(\gamma\) , among others [ 26 , 28 , 29 , 30 ]. Preclinical investigations have shown downregulation of IFN- \(\gamma\) , IL-17 and IL-21 leading to decreased CD4 + cell infiltrates, neovascularization and intimal proliferation in response to tofacitinib in a model that utilizes a human artery implant on an immunodeficient humanized mouse. Such model recapitulates GCA-like arterial inflammation upon transfusion with peripheral blood mononuclear cells from GCA patients [ 14 ].
Clinical data on the efficacy and safety of JAKi in GCA are scarce and limited to a few cases reports, one small retrospective case series, and one small prospective study (Supplementary Table 2 ) [ 15 , 16 , 17 , 18 , 19 , 20 ]. Eriksson et al. [ 16 ], evaluated the effectiveness and safety of baricitinib and tofacitinib in 15 relapsing patients with GCA and observed no further relapses, reduction in prednisone use, and improvement in CRP and ESR values during the observation period. Of note, only 20% of these patients had previously received conventional synthetic immunosuppressants and 20% had previously received biologics. Koster et al. [ 15 ]. conducted a prospective, open-label, pilot study with baricitinib 4 mg daily for 52 weeks in 15 patients with relapsing GCA. The study employed a pre-specified prednisone taper over 15–22 weeks starting between 10 and 30 mg daily. Fourteen patients completed 52 weeks of treatment and only 1 patient relapsed. The remaining 13 patients achieved and maintained disease remission and were able to discontinue prednisone as per protocol until the end of the study. Noteworthy, only 13% and 7% of the patients enrolled in the trial had previously been treated with conventional synthetic immunosuppressants and biologics, respectively, and the median disease duration of the cohort prior to JAKi therapy was 9 months.
In our series, approximately one third of the patients relapsed while on JAKi and, despite a significant reduction in the daily prednisone dose by the end of follow up, only 20% stopped prednisone completely during the observation period. Possible explanations between our results and the results from the study by Eriksson et al. [ 16 ] may include the fact that our cohort of patients could have had more refractory disease reflected by the fact that 63% failed conventional synthetic immunosuppressive agents and 86% failed biological therapy before JAKi initiation. A comparison between our study and the one by Koster et al. [ 15 ] is challenging given the markedly different study designs (i.e., retrospective versus prospective), but factors determining what seems to have been an encouraging, yet poorer response to JAKi in our series may also comprise more recalcitrant disease in our cases demonstrated by longer disease duration, and again reflected in the higher exposures to first and second line therapies before JAKi treatment.
Five of the patients treated with JAKi in our series developed adverse events that led to JAKi discontinuation in four of them. Adverse events included two infections (bacterial urinary infection and disseminated varizella-zoster virus infection), one case of liver dysfunction, and one case of glioblastoma multiforme diagnosed after six months of JAKi therapy. No cases of thromboembolism, stroke, myocardial infarction, significant cytopenias, or GCA-related permanent vision loss were observed. Adverse events reported in the study of Eriksson at al [ 16 ]. included a case of Aspergillus fumigatus infection and a case Enterococcus faecalis bacteremia. As expected for a prospective clinical trial, over 90% of patients in the study by Koster et al. [ 15 ] reported at least one adverse event during the 52 weeks of follow-up. Those adverse events included infection not requiring antibiotics ( n = 8), infection requiring antibiotics ( n = 5), nausea ( n = 6), leg swelling ( n = 2), fatigue ( n = 2), diarrhea ( n = 1), and abdominal pain ( n = 1). One patient experienced a severe adverse event consistent of transient thrombocytopenia, which was attributed to concomitant use of antivirals.
The main limitations of our study are its retrospective nature that could have introduced bias due to missing data and the relatively small sample size. In addition, incomplete documentation of data related to individual prednisone tapering courses made the calculation of cumulative prednisone dose, a key outcome measure in GCA, inaccurate and therefore not analyzable. Despite these limitations, information about key efficacy and safety events (e.g., remission, relapse, serious adverse events, and drug discontinuation) were unequivocally present in the data source, which makes our estimations reliable. Moreover, to our knowledge, this is the largest study to date evaluating outcomes of GCA patients treated with JAKi. As such, our results expand prior findings [ 15 , 16 ], which have been mostly limited to patients naïve to treatment with conventional synthetic immunosuppressants and biologics, suggesting that JAKi can be useful in patients failing those therapies as well.
In summary, in this retrospective study, JAKi treatment was associated with GCA disease control including reduction in the prednisone use in a sizable proportion of patients, most of whom had failed established glucocorticoid-sparing options including tocilizumab and methotrexate. Until the results of a large phase 3 randomized-controlled trial with upadacitinib (Clinical Trials.gov identifier NCT03725202) become available, our findings may inform clinical decision making for GCA patients in routine practice.
Data availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included in the paper.
Abbreviations
C-reactive protein
Computed tomography angiography
Erythrocyte sedimentation rate
European League Against Rheumatism
Fluorodeoxyglucose
- Giant cell arteritis
Granulocyte-macrophage colony-stimulating factor
IL-6 receptor
Interquartile range
JAK inhibitors
Janus kinase/signal transducers and activators of transcription
Magnetic resonance imaging/angiography
Positron emission tomography
Polymyalgia rheumatica
Standard deviation
T helper type 1
T helper type 17
tumor necrosis factor
Salvarani C, Cantini F, Hunder GG. Polymyalgia Rheumatica and giant-cell arteritis. Lancet. 2008;372:234–45.
Article PubMed Google Scholar
Loricera J, Blanco R, Hernández JL, et al. Use of positron emission tomography (PET) for the diagnosis of large-vessel vasculitis. Rev Esp Med Nucl Imagen Mol. 2015;34:372–7.
CAS PubMed Google Scholar
Narváez J, Estrada P, Llop D, et al. Efficacy and safety of leflunomide in the management of large vessel vasculitis: a systematic review and metaanalysis of cohort studies. Semin Arthritis Rheum. 2023;59:152166.
Loricera J, Blanco R, Hernández JL, et al. Biologic therapy in ANCA-negative vasculitis. Int Immunopharmacol. 2015;27:213–9.
Article CAS PubMed Google Scholar
Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomized, double-blind, placebo-controlled trial. Lancet. 2016;387:1921–7.
Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–28.
Unizony SH, Bao M, Han J, Luder Y, Pavlov A, Stone JH. Treatment failure in giant cell arteritis. Ann Rheum Dis. 2021;80:1467–74.
Stone JH, Aringer M, Blockmans D, et al. Long-term effect of tocilizumab in patients with giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatol. 2021;3:e328–36.
Matza MA, Dagincourt N, Mohan SV, et al. Outcomes during and after long-term tocilizumab treatment in patients with giant cell arteritis. RMD Open. 2023;9:e002923.
Article PubMed PubMed Central Google Scholar
Samec MJ, Rakholiya J, Langenfeld H, et al. Relapse risk and safety of long-term tocilizumab use among patients with giant cell arteritis: a single-enterprise cohort study. J Rheumatol. 2023;5:341–50.
Google Scholar
Venhoff N, Schmidt WA, Bergner R, et al. Safety and efficacy of secukinumab in patients with giant cell arteritis (TitAIN): a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Rheumatol. 2023;5:E341–50.
Cid MC, Unizony SH, Blockmans D, et al. Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81:653–61.
Langford CA, Cuthbertson D, Ytterberg SR, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol. 2017;69:837–45.
Article CAS PubMed PubMed Central Google Scholar
Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation. 2018;137:1934–48.
Koster MJ, Crowson CS, Giblon RE, et al. Baricitinib for relapsing giant cell arteritis: a prospective open-label 52-week pilot study. Ann Rheum Dis. 2022;81:861–67.
Eriksson P, Skoglund O, Hemgren C, Sjöwall C. Clinical experience and safety of Janus kinase inhibitors in giant cell arteritis: a retrospective case series from Sweden. Front Immunol. 2023;14:1187584.
Prigent K, Aouba A, Aide N, de Boysson H. JAK inhibitor effectiveness in giant-cell arteritis with large-vessel involvement assessed by 18F-FDG PET-CT. Clin Nucl Med. 2022;47:234–35.
Herlihy N, Curto-García N, O´Sullivan J, et al. Successful treatment of chronic neutrophilic leukaemia and associated giant cell arteritis with the combination of ruxolitinib and azacytidine [abstract]. Br J Haematol. 2019;185:71–2.
Camellino D, Dejaco C, Giusti A, et al. Baricitinib in Polymyalgia Rheumatica and giant cell arteritis: report of six cases [abstract]. Ann Rheum Dis. 2021;80:1216.
Article Google Scholar
Sanada A, Abe N, Bohgaki M, Kasahara H. Therapeutic effectiveness of upadacitinib combined with glucocorticoid on remission induction and maintenance in giant cell arteritis. Rheumatology (Oxford). 2022;61:e274–6.
Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8.
Dejaco C, Kerschbaumer A, Aletaha D et al. Treat-to-target recommendations in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis 2023; ard-2022-223429.
Maz M, Chung SA, Abril A, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021;73:1349–65.
Wilson JC, Sarsour K, Collinson N, et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): a nested case-control analysis. Semin Arthritis Rheum. 2017;46:819–27.
Miyabe C, Miyabe Y, Strle K, et al. An expanded population of pathogenic regulatory T cells in giant cell arteritis is abrogated by IL-6 blockade therapy. Ann Rheum Dis. 2017;76:898–905.
Bursi R, Cafaro G, Perricone C, et al. Contribution of Janus-Kinase/Signal Transduction Activator of Transcription Pathway in the pathogenesis of Vasculitis: a possible treatment target in the Upcoming Future. Front Pharmacol. 2021;12:635663.
Castelo-Soccio L, Kim H, Gadina M, Schwartzberg PL, Laurence A, O´Shea JJ. Protein kinases: drug targets for immunological disorders. Nat Rev Immunol. 2023;15:1–20.
Watanabe R, Berry GJ, Liang DH, Goronzy JJ, Weyand CM. Cellular signaling pathways in medium and large vessel vasculitis. Front Immunol. 2020;11:587089.
Schwartz DM, Bonelli M, Gadina M, O´Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36.
Terrades-Garcia N, Cid MC. Pathogenesis of giant-cell arteritis: how targeted therapies are influencing our understanding of the mechanisms involved. Rheumatology (Oxford). 2018;57:51–62.
Download references
Acknowledgements
We thank all the members of the different hospitals and patients included in this study.
No specific funding was received from any bodies in the public, commercial or non-for-profit sectors to carry out the work described in this article.
Author information
Javier Loricera and Toluwalase Tofade shared junior authorship
Santos Castañeda, Sebastian Unizony and Ricardo Blanco shared senior authorship.
Authors and Affiliations
Department of Rheumatology, IDIVAL, Immunopathology Group, Hospital Universitario Marqués de Valdecilla, Avda. Valdecilla s/n, Santander, ES- 39008, Spain
Javier Loricera, Diana Prieto-Peña, Fernando López-Gutiérrez & Ricardo Blanco
Neurology Department, Massachusetts General Hospital, Boston, MA, USA
Toluwalase Tofade
Department of Rheumatology, Complejo Hospitalario Universitario de Pontevedra, Pontevedra, Spain
Susana Romero-Yuste
Department of Rheumatology, Hospital Universitario La Paz, Madrid, Spain
Eugenio de Miguel
Department of Rheumatology, Hospital Universitario Germans Trias i Pujol, Badalona, Spain
Anne Riveros-Frutos
Department of Rheumatology, Complejo Hospitalario Universitario de Canarias, Santa Cruz de Tenerife, Spain
Iván Ferraz-Amaro
Department of Rheumatology, Hospital San Pedro, Logroño, Spain
Eztizen Labrador
Department of Rheumatology, Hospital Universitario de Donosti, San Sebastián, Spain
Department of Rheumatology, Hospital Universitario de Elda, Alicante, Spain
Elena Becerra
Department of Rheumatology, Hospital de Bellvitge, Barcelona, Spain
Javier Narváez
Department of Rheumatology, Hospital Universitario de Basurto, Bilbao, Spain
Eva Galíndez-Agirregoikoa
Department of Rheumatology, Complejo Asistencial Universitario de León, León, Spain
Ismael González-Fernández
Department of Rheumatology, Hospital Universitario Son Espases, Palma de Mallorca, Spain
Ana Urruticoechea-Arana
Department of Rheumatology, Complejo Hospitalario de Soria, Soria, Spain
Ángel Ramos-Calvo
Department of Rheumatology, Hospital Universitario La Princesa, IIS-Princesa, Madrid, Spain
Santos Castañeda
Vasculitis and Glomerulonephritis Center, Rheumatology, Immunology and Allergy Division, Massachusetts General Hospital, Boston, MA, 02114, USA
Sebastian Unizony
You can also search for this author in PubMed Google Scholar
Contributions
JL, TT, SC, SU and RB conceptualized, and designed the study. SU and RB were the research coordinators for the study. RB was the project manager for the study. IF-A supervised the study. JL, TT, DP-P, SR-Y, EM, AR-F, EL, OM, EB, JN, EG-A, IG-F, AU-A, AR-C, FL-G, SC and SU collected data. JL, IF-A, SC, SU and RB curated the data. JL, IF-A, SC, SU and RB analysed the data. JL, IF-A, SC, SU and RB interpreted the data. JL wrote the original manuscript. JL, TT, DP-P, SR-Y, EM, AR-F, EL, OM, EB, JN, EG-A, IG-F, AU-A, AR-C, FL-G, SC, SU and RB reviewed and edited the original manuscript. JL and RB had direct access to the data and verified the underlying raw data reported in the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.

Corresponding authors
Correspondence to Sebastian Unizony or Ricardo Blanco .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Cantabria Clinical Research Ethics Committee (approval number 2021.414), and was conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonization. All data extracted from the medical records were stored de-identified prior to the analysis. As per the Clinical Research Ethics Committee, this retrospective research did not require informed consent.
Consent for publication
Not applicable.
Competing interests
Disclosures that might be interpreted as constituting possible conflict(s) of interest for the study: Javier Loricera had consultation fees/participation in company-sponsored speaker´s bureau from Roche, AbbVie, Galápagos, Novartis, UCB Pharma, MSD, Celgene, Astra Zeneca, and Grünenthal and received support for attending meetings and/or travel from Janssen, AbbVie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Diana Prieto-Peña has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from UCB Pharma, Novartis, Amgen, Lilly, Sanofi, and Janssen, and received support for attending meetings and/or travel from Lilly, Roche, Sanofi, Pfizer and Abbvie. Susana Romero-Yuste has received grants/recents supports from Abbvie, Lilly, Pfizer, and Galápagos. Eugenio de Miguel Research funding/consulting and conferences fees from: Abbvie, Novartis, Pfizer, Roche, Janssen, Lilly, MSD, BMS, UCB, Grünenthal, and Sanofi, and received support for attending meetings and/or travel from Abbvie, Pfizer, Sanofi, and Grünenthal. Iván Ferraz-Amaro would like to acknowledge that he has received grants/research supports from Abbvie, MSD, Janssen, and Roche, as well as consultation fees from company sponsored speakers bureaus associated with Abbvie, Pfizer, Roche, Sanofi, Celgene, and MSD, and received support for attending meetings and/or travel from Abbvie, MSD, Janssen, Pfizer, Roche, Sanofi, and Celgene. Eva Galíndez-Agirregoikoa had consultation fees/participation in company-sponsored speaker´s bureau from Lilly, Janssen, Abbvie, and Amgen, and received support for attenting meetings and/or travel from Lilly, Abbvie, and Pfizer. Ismael González-Fernández had consultation fees/participation in company-sponsored speaker´s bureau from Novartis, Janssen, Grunenthal, MSD, and Theramex, and received support for attending meetings and/or travel from Janssen, and MSD. Ana Urruticoechea-Arana had received research grants, fees for consultancies or presentations and participated in medical meetings or courses from Abbvie, Galápagos, UCB, Pfizer, MSD, Novartis, GSK, Hanssen, and Amgen, and participated on a data safety monitoring board or advisory board GSK. Ángel Ramos-Calvo had consultation fees/participation in company-sponsored speaker´s bureau from Amgen, UCB, and Galápagos, and received support for attending meetings and/or travel from Pfizer. Fernando López-Gutiérrez received support for attending meetings and/or travel from Janssen, Abbvie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Santos Castañeda has received research supports from MSD, and Pfizer and had consultation fees/participation in company-sponsored speaker’s bureau from BMS, Eli-Lilly, MSD, Roche, and UCB, and received support for attending meetings and/or travel from BMS, Lilly, MSD, Roche, and UCB. Sebastian Unizony received research support from Genentech, participated in an advisory board for Abbvie, and provided consulting for Sanofi, Kiniksa, and Janssen.Ricardo Blanco received grants/research support from AbbVie, MSD, and Roche, and had consultation fees/participation in a company-sponsored speaker’s bureau from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB and MSD, and received support for attending meetings and/or travel from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB, and MSD. The following authors did not declare financial disclosure : Toluwalase Tofade, Anne Riveros-Frutos, Eztizen Labrador, Olga Maiz, Elena Becerra, Javier Narváez. The following authors did not declare financial disclosure: Toluwalase Tofade, Anne Riveros-Frutos, Eztizen Labrador, Olga Maiz, Elena Becerra, Javier Narváez.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Loricera, J., Tofade, T., Prieto-Peña, D. et al. Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature. Arthritis Res Ther 26 , 116 (2024). https://doi.org/10.1186/s13075-024-03314-9
Download citation
Received : 06 February 2024
Accepted : 19 March 2024
Published : 05 June 2024
DOI : https://doi.org/10.1186/s13075-024-03314-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Large vessel vasculitis
- Janus kinase inhibitors
- Baricitinib
- Tofacitinib
- Upadacitinib
Arthritis Research & Therapy
ISSN: 1478-6362
- General enquiries: [email protected]
Clinical presentation of Fibroepithelioma of Pinkus: systematic review of the literature
- Research Letter
- Published: 08 June 2024
- Volume 316 , article number 357 , ( 2024 )
Cite this article

- Richard W. Kim 1 ,
- Jeffery P. North 2 , 3 &
- Ruby Ghadially 3 , 4
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
No datasets were generated or analysed during the current study.
Bowen AR, LeBoit PE (2005) Fibroepithelioma of Pinkus is a fenestrated trichoblastoma. Am J Dermatopathol 27:149–154. https://doi.org/10.1097/01.dad.0000138051.71415.fe
Article PubMed Google Scholar
Longo C, Pellacani G, Tomasi A et al (2016) Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol 4:797–800. https://doi.org/10.3892/mco.2016.794
Article PubMed PubMed Central Google Scholar
Nanda JK, Marghoob N, Forero Cuevas DM et al (2021) Clinical and dermoscopic features of Fibroepithelioma of Pinkus: case series with an emphasis on hypopigmented to pink lines intersecting at acute angles. Arch Dermatol Res 313:633–640. https://doi.org/10.1007/s00403-020-02142-6
Article CAS PubMed Google Scholar
Zalaudek I, Ferrara G, Broganelli P et al (2006) Dermoscopy patterns of fibroepithelioma of pinkus. Arch Dermatol 142:1318–1322. https://doi.org/10.1001/archderm.142.10.1318
Yonan Y, Maly C, DiCaudo D et al (2019) Dermoscopic description of Fibroepithelioma of Pinkus with negative network. Dermatol Pract Concept 9:246–247. https://doi.org/10.5826/dpc.0903a23
Download references
Acknowledgements
The patients in this manuscript have given written informed consent to publication of their case details.
This article has no funding source.
Author information
Authors and affiliations.
University of California San Francisco School of Medicine, San Francisco, USA
Richard W. Kim
Department of Pathology, University of California, San Francisco, CA, USA
Jeffery P. North
Department of Dermatology, University of California San Francisco School of Medicine, 1700 Owens Street #324, San Francisco, CA, 94158, USA
Jeffery P. North & Ruby Ghadially
Epithelial Section of the UCSF Eli and Edythe Broad, Center of Regeneration Medicine and Stem Cell Research, San Francisco, CA, USA
Ruby Ghadially
You can also search for this author in PubMed Google Scholar
Contributions
RWK and RG contributed to the study conception and design. Material preparation, data collection were performed by JN, RWK, and RG. Analysis were performed by RWK and RG. The first draft of the manuscript was written by RWK and RG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ruby Ghadially .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Conflict of interest
The authors report no conflict of interest relevant to this article.
IRB approval
Reviewed and approved by UCSF IRB; approval 21-33750.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Kim, R.W., North, J.P. & Ghadially, R. Clinical presentation of Fibroepithelioma of Pinkus: systematic review of the literature. Arch Dermatol Res 316 , 357 (2024). https://doi.org/10.1007/s00403-024-02985-3
Download citation
Received : 01 April 2024
Revised : 25 April 2024
Accepted : 26 April 2024
Published : 08 June 2024
DOI : https://doi.org/10.1007/s00403-024-02985-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fibroepithelioma of pinkus
- Diagnostic accuracy
- Clinical diagnosis
- Skin cancer
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 08 June 2024
Diagnostic and prognostic relevance of plain radiographs for periprosthetic joint infections of the hip: a literature review
- Ulf Krister Hofmann 1 ,
- Georgios Eleftherakis 2 ,
- Filippo Migliorini 1 , 3 ,
- Bernd Fink 4 , 5 &
- Moritz Mederake 6
European Journal of Medical Research volume 29 , Article number: 314 ( 2024 ) Cite this article
Metrics details
Conventional radiography is regularly used to evaluate complications after total hip arthroplasty. In various recent consensus meetings, however, plain radiographs of a potentially infected hip joint have been judged as being only relevant to exclude diagnoses other than infection. Solid data on radiographic presentations of periprosthetic joint infection (PJI) are scarce. As a result, the prognostic value of radiological features in low-grade PJI remains uncertain. The present review article aims to present an overview of the available literature and to develop ideas on future perspectives to define the diagnostic possibilities of radiography in PJIs of the hip. The primary outcome of interest of this systematic review was the radiologic presentation of periprosthetic joint infections of the hip. As secondary outcome of interest served the sensitivity and specificity of the radiologic presentation of periprosthetic joint infections. Of the included articles, 26 were reviews, essays, or case reports and only 18 were clinical studies. Typical radiologic abnormalities of PJI were a periosteal reaction, a wide band of radiolucency at the cement–bone or metal–bone interface, patchy osteolysis, implant loosening, bone resorption around the implant, and transcortical sinus tracts. The frequency of their occurrence is still inadequately defined. A deeper understanding of the underlying causes and the relation between microorganisms to radiologic abnormalities can probably help clinicians in the future to diagnose a PJI. This is why further research shall focus on the radiographic features of PJI.
Introduction
Conventional radiography is regularly used to evaluate joint prostheses after implantation and during follow-up, as X-rays can detect potential abnormalities involving both the implant and the surrounding bone. Such abnormalities could be for example periprosthetic fracture, dislocation, osteolysis due to third body wear, or sinking of the shaft. One of the major complications after arthroplasty is periprosthetic joint infection (PJI). In various recent consensus meetings, however, plain radiographs of a potentially infected hip joint have been judged as being only relevant to exclude diagnoses other than infection [ 1 , 2 , 3 ]. In the consensus statement published by Romano and colleagues in 2020, for example, the diagnostic performance of conventional radiography in detecting PJI would be very low.
Furthermore, conventional radiography would show demineralization only when more than 30–50% of bone mass has been lost. Abnormalities of bone around the implant would usually be non-specific for infection. In addition, up to 50% of conventional X - ray exams would give negative results [ 1 ]. In another recent consensus statement published by Signore et al. [ 2 , 3 ], the authors state that “Regarding PJI, conventional radiography often yields normal results or may detect non-specific signs of soft-tissue swelling. Serial plain radiography has been reported to have a sensitivity of 14% and specificity of 70% in detecting implant-associated infections [ 4 ]. Radiographic signs that may reveal PJI with high specificity are gas formation and active, immature periostitis. Radiographic signs with low specificity include soft-tissue swelling, periprosthetic lucency, and component loosening. However, differentiation between septic and aseptic periprosthetic lucency and component loosening is almost impossible in conventional radiography. Also, these signs are visible only when almost 30% of the bone mass has been lost; thus, 50% of radiographs remain normal despite the presence of infection”. These consensus statements are, however, only based on three references [ 5 , 6 , 7 ].
Most of the studies to which these statements relate date to the late 80 s and early 90 s [ 5 , 7 , 8 ]. The foundations of the data referenced here are also quite weak, such as in the study from Tigges et al., [ 7 ] with 20 confirmed infected hip arthroplasties, or Lyons et al. [ 5 ] 50 painful hip arthroplasties. The largest series presented was from Thoren and Hallin in 1989 [ 8 ], where the authors analysed 102 hip revisions. Of these, however, only 47 were infected and the prostheses analysed were original Charnley prostheses which possessed a 22.225-mm head in ultra-high molecular weight polyethylene (UHMW PE) in an all-cemented technique and a metal-on PE bearing. The arthroplasty landscape has, however, largely changed since then. Today's arthroplasties often contain an uncemented cup and a ceramic head. Depending on the country the stem is often uncemented ranging from 37% in the National Joint Registry in Great Britain to 78% in the German Arthroplasty Registry in Germany [ 9 ]. Uncemented stems usually consist of titanium which altogether changes the immunogenicity of the wear particles generated. Moreover, the annual number of patients treated with arthroplasties has multiplied and as such the surgical technique and postoperative rehabilitation protocols have been optimized and largely standardized. In addition, life expectancy has increased with people in old age having multiple comorbidities in addition to joint replacement. This supposedly changes the spectrum of bacteria responsible for infections. While we are quite effective in treating acute PJIs, the successful management of low-grade infections is still a challenge. This includes reliable, sensitive and specific diagnostics of such low-grade PJIs. Nevertheless, substantial progress has been made in the diagnosis of such an event with improvements in the histopathological analysis of the periprosthetic membrane [ 10 , 11 ], the advent of PCR analyses [ 12 ], and synovial fluid analysis including the analysis of PMNs, alpha-defensin or leukocyte esterase-levels [ 13 , 14 , 15 , 16 ]. Other newer markers are presently under investigation, such as pentraxin-3 [ 17 ], calprotectin [ 18 ], or myeloperoxidase [ 19 ]. Of note, thresholds for leukocytes in low-grade PJIs have been constantly lowered over the past decades ranging now—depending on the joint—between 1000 and 2000 leukocytes/µl only [ 20 ]. Cultivation techniques for bacteria have also been more and more standardized. We can assume that the overall sensitivity and specificity have increased over the past 30 years. Similarly, the technique of acquiring radiographs has also improved with the widespread introduction of digital radiography. The digital data set allows for post-imaging optimization of each X-ray to visualize structures that were difficult to discern in traditional images intended for a good bone contrast only. Nevertheless, solid data on radiographic presentations of PJI are scarce. As a result, the prognostic value of radiological features in low-grade PJI remains uncertain. The present review article aims to present an overview of the available literature and to develop ideas on future perspectives to define the diagnostic possibilities of radiography in PJIs of the hip.
Materials and methods
Eligibility criteria.
All published articles related to the radiographic presentation of PJI of the hip were accessed. Only articles available in English, French, Spanish or German were eligible. Original studies with a level of evidence of I to IV according to the Oxford Centre of Evidence-Based Medicine [ 21 ] plus review articles and essays were considered.
Search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the 2020 PRISMA statement [ 22 ]. On July 24, 2023, PubMed and Web of Science were accessed. The following keywords were used: "periprosthetic joint infection hip radiograph"; "Prosthesis-Related Infections"[Mesh] AND "Radiography"[Mesh]) AND "Hip"[Mesh]; "Radiography"[Mesh]) AND "Hip"[Mesh]) AND "Infections"[Mesh]; "radiography" "hip arthroplasty" "infection"; "x-ray" "hip arthroplasty" "infection"; "x-ray" "periprosthetic joint infection" "hip"; "periprosthetic joint infection" "hip" "radiograph"; "heterotopic ossification" "hip arthroplasty" "infection"; "heterotopic ossification" "hip" "periprosthetic joint infection". No filters were applied.
Selection and data collection
Three authors (UKH; MM; GE) independently performed the database search. All the resulting titles were screened and, if suitable, the abstract was accessed. The full text of the abstracts, which matched the topic, was accessed. A cross-reference of the bibliography of the full-text articles was also screened for inclusion. If the full text was not accessible or available, the article was not considered for inclusion. Disagreements were debated before final inclusion into the study.
Data items and outcome of interest
The following data at baseline were extracted: author, year of publication and journal, PMID/PCMID/DOI, type of analysis performed, country of origin, main study outline, number of patients used for the relevant statements, and statements made regarding radiography and PJI of the hip. A suitable level of evidence was attributed to each study. The primary outcome of interest was the radiologic presentation of PJI of the hip. As secondary outcome of interest served the sensitivity and specificity of the described image characteristics.
Study selection
The literature search resulted in 1248 articles (Fig. 1 ). After the removal of duplicates, 1121 articles were screened. Having screened titles and abstracts, the original manuscript was accessed for 71 studies. Of these 44 finally met the inclusion criteria, the other publications were either in Chinese ( n = 3), not retrievable ( n = 10), retracted ( n = 1), were not related to the research question ( n = 6), or did not directly report radiographic parameters ( n = 7). Of the finally included articles, 26 were reviews, essays, or case reports. Only 18 publications were clinical studies with a level of evidence between IV and II.

Flowchart of the literature search
Review summary
Summarizing the statements provided in the review articles, essays, and case reports (Table 1 ), a few commonly reported traits can be made out: claimed radiographic signs of infection are periosteal reaction [ 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ], formation of lamellae [ 23 ], focal osteolysis or bone destruction [ 7 , 23 , 24 , 26 , 28 , 31 , 33 , 34 , 35 , 36 , 37 , 38 ], a wide radiolucent zone [ 23 , 32 , 36 , 39 ], signs of loosening in a previously well-fixed implant [ 24 , 27 , 28 , 32 , 34 ], heterotopic bone formation [ 28 , 40 ], mottling [ 31 ], an intracortical sinus tract [ 24 , 28 ], periprosthetic fractures [ 27 ], adjacent soft-tissue collection [ 29 , 30 , 32 ], and rapid disease progression [ 25 ]. Aseptic loosening tends to produce uniform radiolucency, whereas particle disease produces multifocal radiolucencies related to localized osteolysis. Infection can produce either of these patterns [ 41 , 42 ] with an osteolysis of > 2 mm being indicative of infection [ 41 ]. The radiographic presentation thereby seems to be a function of time, with early infections presenting without radiological features and late infections presenting with inflammatory and reactive osteoproliferative changes of the bone [ 34 , 43 ]. These signs are only present in a subpopulation of all PJI and thus have a low sensitivity while having a reasonable specificity.
Original data
Looking at the original data (Table 2 ), the numbers on which statements are based are generally low ranging from 2 to 50 with a median of 15. The discrepancies in the reported data are, however, extreme: radiographic abnormalities are visible in all cases of PJI of the hip [ 44 , 45 , 46 , 47 ]. Other authors only report very low incidences of such findings [ 48 , 49 , 50 ]. Related features are periosteal reaction [ 5 , 45 ], a radiolucent zone [ 7 , 45 ], sinking of the prosthesis or loosening [ 45 , 49 , 50 , 51 ], periprosthetic osteolysis or scalloped endosteal bone resorption [ 5 , 7 , 38 , 50 , 52 ], scalloping [ 45 ], and in cemented shafts cement mantle irregularities [ 51 ]. The first changes appear to be visible 3 months after the onset of symptoms [ 45 ]. The absence of periprosthetic osteolysis was reported to be predictive of aseptic loosening [ 52 ]. Lyons et al., reported a sensitivity of 47% and a specificity of 96% for scalloped bone resorption whereas laminated periosteal new bone had only a 25% sensitivity with, however, also a 92% specificity ( n = 16) [ 5 ].
Heterotopic bone formation can occur in PJIs [ 53 ], but it can also be present in cases of aseptic loosening or just idiopathic [ 52 ]. In a retrospective study including 168 patients, Manrique et al. [ 54 ], reported an incidence of heterotopic ossifications following surgical treatment of PJI of the hip 84% and in aseptic revision cases of only 11%. Cases after periprosthetic joint infection in that study also had significantly higher Brooker grades [ 54 ]. In a CT-based study, Isern-Kebschull et al., reported a sensitivity for periarticular ossifications of 20% (8.4–36.9), a specificity of 79% (66.3–88.1), a positive predictive value of 35 (15.4–59.2), and a negative predictive value of 63 (51.3–73.9) [ 55 ].
Regarding risk factors for infection, an interesting observation was made by Rey Fernandez et al. who described that patients with more than 60 mm soft-tissue thickness over the greater trochanter had a sevenfold higher risk of infection [ 56 ].
Only one study explicitly elaborated on the detected bacteria and described the associated radiologic findings: Barrack et al. [ 44 ], reported 5 cases infected with S. epidermidis and found periostitis (3×), focal lysis (1×), diffuse lysis with endosteal scalloping (1×) and heterotopic ossification (2×). One case with Strept. sanguis had focal lyses around the implant.
PJI is one of the most serious complications after total hip arthroplasty and poses major diagnostic challenges for clinicians. Radiological imaging, especially plain radiographs, is part of every diagnostic workup in case of suspected complications after total hip arthroplasty. There are plenty of reviews and original works dealing with the diagnostic algorithm of PJI. This review aimed to summarize and analyse the available information about the radiologic presentation of PJI and the frequency of appearance. Furthermore, we raised the question of whether the causative microorganisms differ regarding the radiologic presentation.
The first observation of this review is the lack of original data evaluating the radiologic characteristics of PJI of the hip. There are more reviews ( n = 26) repeating findings of other studies than original works ( n = 18). These reviews mainly concentrate on the diagnosis of PJI, however, they do not focus on the radiographic presentation of PJIs. As described in the section "results", the main statements regarding the radiologic presentation in the reviews are the above-mentioned radiologic pathologies. They do not report on the frequency of the radiologic findings and no correlation to causative microorganisms was analysed. Furthermore, the findings regarding the radiologic presentation of PJI of original works are often coproducts of other primary endpoints [ 5 , 44 , 46 , 48 , 50 , 51 , 53 ].
The next interesting finding of this review is the frequency of radiologic abnormalities in the case of a proven PJI of the hip. While some authors report radiographic changes in all of their PJI cases (100%) [ 44 , 45 , 47 ], other authors only presented abnormalities in 14–63% [ 48 , 49 , 50 , 57 ]. Multiple reasons could explain this wide range. Therefore, having a closer look at the original works reporting the frequencies is necessary. The first possible explanation is the lack of a standardized follow-up to classify radiologic changes in the case of PJI. Some authors present radiographs already 6 months after surgery while other authors describe X-rays taken nine years postoperatively. The given collectives are mostly very small with numbers of patients between 6 and 20. Such collectives are not big enough to give sufficient information about the frequency of the appearance of radiological abnormalities.
Furthermore, the oldest and latest works reporting frequencies are separated by more than 40 years of medical evolution [ 45 , 47 ]. The definition and the diagnostic algorithm have, however, relevantly changed since then and therefore these studies lack comparability [ 58 , 59 ]. Similarly, the technique of acquiring radiographs has also improved with the advent of digital radiography. This allows post-imaging optimization of each X-ray to visualize also structures such as soft tissues that were difficult to discern in traditional images intended for a good bone contrast only.
When using radiographs not only to exclude differential diagnoses, but also to diagnose a PJI, sensitivity and specificity are crucial. Two original works are reporting about the diagnostic value with similar results. The sensitivity could be classified as low at 20–25% while the specificity of periosteal reactions and periarticular calcifications is moderate to good (79–92%) [ 5 , 52 ]. Consequently, plain radiographs could be used to confirm PJI in the presence of characteristic radiologic abnormalities. Especially in the case of early PJI, the majority of cases of early PJI radiographs are still usually normal [ 43 ]. As a consequence, the question of how to interpret and use radiographs to diagnose late PJIs and the diagnostic value of this technique remains equivocal with the analysed literature.
Having a look at radiographs taken after septic revisions of hip arthroplasties, three original works report heterotopic ossifications. Incidences range depending on the collective analysis between 12 and 84%. Brooker grades presented were mainly between one and three [ 53 , 54 , 60 ]. Interestingly, when comparing aseptic and septic revisions, heterotopic ossifications are significantly more likely in septic cases (11 vs. 84%) and present with significantly higher Brooker grades [ 54 ]. Consequently, some kind of interaction between soft tissue and the causing microorganisms can be assumed. This assumption is highlighted by the observation of Rey Fernández et al., that there is an association between higher soft-tissue thickness and risk for PJI after primary total hip replacements. Furthermore, patients with more than 60 mm soft-tissue thickness around the major trochanter had a sevenfold higher risk of PJI [ 56 ]. Unfortunately, there are no data available considering the association between the causing microorganisms and soft-tissue changes.
Historically and as presented in the analysed reviews, characteristics that are described to recognize a PJI of the hip on a plain radiograph are periosteal reaction, a wide band of radiolucency at the cement–bone or metal–bone interface, patchy osteolysis, implant loosening, bone resorption around the implant, and transcortical sinus tracts. In cases of aseptic loosening, there is slow and progressive evolution, while in cases of infectious loosening, this loosening occurs rapidly, in a more aggressive manner and with greater bone destruction [ 7 , 61 ]. Data regarding the frequency of occurrence of the named radiologic abnormalities in cases of PJI are insufficient. Prospective or at least retrospective analyses with larger collectives are necessary to evaluate the frequency and to define the diagnostic value of the radiologic presentation for the diagnosis of PJI. Furthermore, there is a lack of data analysing the association between the causative microorganisms and the radiologic appearance. Although imaging techniques like computer tomography [ 52 , 55 , 62 ] or magnetic resonance imaging [ 63 ] have been reported to also provide additional and helpful clues concerning the presence of a PJI such as periosteal reaction, capsule oedema, and intramuscular oedema, the mainstay in diagnosing PJI to date remains plain radiographs.
Typical radiologic abnormalities of PJI are periosteal reaction, a wide band of radiolucency at the cement–bone or metal–bone interface, patchy osteolysis, implant loosening, bone resorption around the implant, and transcortical sinus tracts. The frequency of their occurrence is still inadequately defined. A deeper understanding of the underlying causes and the relation to microorganisms can probably help clinicians in the future to diagnose a PJI. This is why further research should still further evaluate the radiographic features and value in the context of PJI.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Romano CL, Petrosillo N, Argento G, Sconfienza LM, Treglia G, Alavi A, Glaudemans A, Gheysens O, Maes A, Lauri C, et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. J Clin Med. 2020;9(8):2548.
Article PubMed PubMed Central Google Scholar
Signore A, Sconfienza LM, Borens O, Glaudemans A, Cassar-Pullicino V, Trampuz A, Winkler H, Gheysens O, Vanhoenacker F, Petrosillo N, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging. 2019;46(4):971–88.
Signore A, Sconfienza LM, Borens O, Glaudemans A, Cassar-Pullicino VN, Trampuz A, Winkler H, Gheysens O, Vanhoenacker F, Petrosillo N, et al. Correction to: consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging. 2019;46(5):1203.
Rabin DN, Smith C, Kubicka RA, Rabin S, Ali A, Charters JR, Rabin H. Problem prostheses: the radiologic evaluation of total joint replacement. Radiographics. 1987;7(6):1107–27.
Article CAS PubMed Google Scholar
Lyons CW, Berquist TH, Lyons JC, Rand JA, Brown ML. Evaluation of radiographic findings in painful hip arthroplasties. Clin Orthop Relat Res. 1985;195:239–51.
Article Google Scholar
Math KR, Zaidi SF, Petchprapa C, Harwin SF. Imaging of total knee arthroplasty. Semin Musculoskelet Radiol. 2006;10(1):47–63.
Article PubMed Google Scholar
Tigges S, Stiles RG, Roberson JR. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol. 1994;163(2):377–80.
Thoren B, Hallin G. Loosening of the Charnley hip. Radiographic analysis of 102 revisions. Acta Orthop Scand. 1989;60(5):533–9.
Humez M, Kotter K, Skripitz R, Kuhn KD. Register data on cemented arthroplasty: a proof for cementless fixation? Orthopadie (Heidelb). 2023;53:163.
PubMed Google Scholar
Morawietz L, Classen RA, Schroder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59(6):591–7.
Article CAS PubMed PubMed Central Google Scholar
Krenn V, Morawietz L, Perino G, Kienapfel H, Ascherl R, Hassenpflug GJ, Thomsen M, Thomas P, Huber M, Kendoff D, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract. 2014;210(12):779–86.
Qu X, Zhai Z, Li H, Li H, Liu X, Zhu Z, Wang Y, Liu G, Dai K. PCR-based diagnosis of prosthetic joint infection. J Clin Microbiol. 2013;51(8):2742–6.
Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res. 1997;345:8–16.
Duff GP, Lachiewicz PF, Kelley SS. Aspiration of the knee joint before revision arthroplasty. Clin Orthop Relat Res. 1996;331:132–9.
Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE Jr. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229–35.
Unter Ecker N, Koniker A, Gehrke T, Salber J, Zahar A, Hentschke M, Citak M. What is the diagnostic accuracy of alpha-defensin and leukocyte esterase test in periprosthetic shoulder infection? Clin Orthop Relat Res. 2019;477(7):1712–8.
Logroscino G, Fidanza A, Giusti I, Poppa G, Troianello L, Calafiore F, Saracco M, Avigni R, Leone R, Mantovani A, et al. Pentraxin 3, a new biomarker for the diagnosis and management of PJI in primary and revision hip arthroplasty. Acta Biomed. 2023;94(S2): e2023100.
CAS PubMed Google Scholar
Xing J, Li J, Yan Z, Li Y, Liu X, He L, Xu T, Wang C, Zhao L, Jie K. Diagnostic accuracy of calprotectin in periprosthetic joint infection: a diagnostic meta-analysis. J Orthop Surg Res. 2022;17(1):11.
Ikeda S, Uchiyama K, Minegishi Y, Nakamura M, Takaso M. Evaluation of myeloperoxidase in synovial fluid as a biomarker for chronic periprosthetic joint infection. Int Orthop. 2020;44(10):1915–20.
Sendi P, Muller AM, Berbari E. Are all joints equal? Synovial fluid analysis in periprosthetic joint infection. J Bone Jt Infect. 2018;3(5):258–9.
Howick J CI, Glasziou P, Greenhalgh T, Carl Heneghan, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM levels of evidence. Oxford centre for evidence-based medicine. https://cir.nii.ac.jp/all?q=http://www.cebm.net/index.aspx?o%3D5653 . 2011. Accessed Mar 2022
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Breitenseher MJ, Mayerhöfer M, Gottsauner-Wolf F, Krestan C, Toma CD, Imhof H. Diagnostic imaging in hip prostheses. Radiologe. 2002;42(6):474–9.
Enayatollahi MA, Parvizi J. Diagnosis of infected total hip arthroplasty. Hip Int. 2015;25(4):294–300.
Enge Júnior DJ, Castro A, Fonseca E, Baptista E, Padial MB, Rosemberg LA. Main complications of hip arthroplasty: pictorial essay. Radiol Bras. 2020;53(1):56–62.
Fritz J, Lurie B, Miller TT. Imaging of hip arthroplasty. Semin Musculoskelet Radiol. 2013;17(3):316–27.
Lee KJ, Goodman SB. Identification of periprosthetic joint infection after total hip arthroplasty. J Orthop Translat. 2015;3(1):21–5.
Lüdemann CM, Schütze N, Rudert M. Diagnosis of periprosthetic hip infections. Oper Orthop Traumatol. 2015;27(3):237–50 ( quiz 251 ).
Miller TT. Imaging of hip arthroplasty. Eur J Radiol. 2012;81(12):3802–12.
Mulcahy H, Chew FS. Current concepts of hip arthroplasty for radiologists: part 2, revisions and complications. AJR Am J Roentgenol. 2012;199(3):570–80.
Mullins MF, Sutton RN, Lodwick GS. Complications of total hip replacement. A roentgen evaluation. Am J Roentgenol Radium Ther Nucl Med. 1974;121(1):55–60.
Parvizi J, Fassihi SC, Enayatollahi MA. Diagnosis of periprosthetic joint infection following hip and knee arthroplasty. Orthop Clin North Am. 2016;47(3):505–15.
Thejeel B, Endo Y. Imaging of total hip arthroplasty: part II–imaging of component dislocation, loosening, infection, and soft tissue injury. Clin Imag. 2022. https://doi.org/10.1016/j.clinimag.2022.09.011 .
Hargunani R, Madani H, Khoo M, Fotiadou A, Pressney I, Calleja M, O’Donnell P. Imaging of the painful hip arthroplasty. Can Assoc Radiol J. 2016;67(4):345–55.
Lohmann CH, Rampal S, Lohrengel M, Singh G. Imaging in peri-prosthetic assessment: an orthopaedic perspective. EFORT Open Rev. 2017;2(5):117–25.
Mushtaq N, To K, Gooding C, Khan W. Radiological imaging evaluation of the failing total hip replacement. Front Surg. 2019;6:35.
Song Y, Shao HY, Cheng X, Guo Y. First case of periprosthetic joint infection due to Clostridioides difficile in China. BMC Infect Dis. 2021;21(1):1–5.
Van Odijk J, Marchal G, Baert A, Mulier J, Verhelst M. The radiological images of complications of the total hip arthroplasty. J Belge Radiol. 1974;57(6):429–36.
Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guerin therapy. J Arthroplasty. 2007;22(5):759–62.
Kinoshita Y, Nakano S, Yoshioka S, Nakamura M, Goto T, Hamada D, Sairyo K. A rare case of extremely severe heterotopic ossification after primary total hip arthroplasty due to persistent mild periprosthetic joint infection. Case Rep Orthop. 2021;2021:8849929.
PubMed PubMed Central Google Scholar
Awan O, Chen L, Resnik CS. Imaging evaluation of complications of hip arthroplasty: review of current concepts and imaging findings. Can Assoc Radiol J. 2013;64(4):306–13.
Chang CY, Huang AJ, Palmer WE. Radiographic evaluation of hip implants. Semin Musculoskelet Radiol. 2015;19(1):12–20.
Bültmann FJ, Dihlmann W. Radiologically recognisable local complications of alloplastic hip replacement (total prosthesis). Fortschr Geb Rontgenstr Nuklearmed. 1973;119(1):101–7.
Barrack RL, Harris WH. The value of aspiration of the hip joint before revision total hip arthroplasty. J Bone Joint Surg Am. 1993;75(1):66–76.
Bergström B, Lidgren L, Lindberg L. Radiographic abnormalities caused by postoperative infection following total hip arthroplasty. Clin Orthop Relat Res. 1974;99:95–102.
Rajkumar N, Soundarrajan D, Kumar PC, Dhanasekararaja P, Rajasekaran S. Clinical and radiological outcome of acetabular reconstruction rings in complex primary and revision total hip arthroplasty. Indian J Orthop. 2021;55(5):1267–76.
Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. JBJS. 1998;80(7):961–8.
Article CAS Google Scholar
Ring PA. Total replacement of the hip joint. A review of a thousand operations. J Bone Joint Surg Br. 1974;56(1):44–58.
Schuldt A, Lehnick D, Agten CA, Beck M, Kist M, Bhure U, del Pérez Sol Lago M, Strobel K. Performance of radiography and single-photon emission computed tomography/computed tomography in painful total hip arthroplasty and impact on arthroplasty survival. Nucl Med Commun. 2020;41(9):875–82.
Sirka A, Clauss M, Tarasevicius S, Wingstrand H, Stucinskas J, Robertsson O, Emil Ochsner P, Ilchmann T. Excellent long-term results of the Müller acetabular reinforcement ring in primary total hip arthroplasty: a prospective study on radiology and survival of 321 hips with a mean follow-up of 11 years. Acta Orthop. 2016;87(2):100–5.
Tapadiya D, Walker RH, Schurman DJ. Prediction of outcome of total hip arthroplasty based on initial postoperative radiographic analysis. Matched, paired comparisons of failed versus successful femoral components. Clin Orthop Relat Res. 1984;186:5–15.
Isern-Kebschull J, Tomas X, García-Díez AI, Morata L, Moya I, Ríos J, Soriano A. Value of multidetector computed tomography for the differentiation of delayed aseptic and septic complications after total hip arthroplasty. Skelet Radiol. 2020;49(6):893–902.
Burastero G, Alessio-Mazzola M, Cavagnaro L, Chiarlone F, Carrega G, Capello AG, Lovisolo S, Felli L. Conservative two-stage revision with primary components of infected total hip arthroplasty: an analysis of survival, clinical and radiographic outcomes. PLoS ONE. 2020;15(10): e0239981.
Manrique J, Alijanipour P, Heller S, Dove M, Parvizi J. Increased risk of heterotopic ossification following revision hip arthroplasty for periprosthetic joint infection. Arch Bone Jt Surg. 2018;6(6):486–91.
Isern-Kebschull J, Tomas X, García-Díez AI, Morata L, Ríos J, Soriano A. Accuracy of computed tomography-guided joint aspiration and computed tomography findings for prediction of infected hip prosthesis. J Arthroplasty. 2019;34(8):1776–82.
Rey Fernández L, Angles Crespo F, Miguela Álvarez SM, Bernaus-Johnson MC, Bartra Ylla A, Font-Vizcarra L. Soft-tissue thickness radiographic measurement: a marker to evaluate acute periprosthetic joint infection risk in total hip replacement. J Bone Jt Infect. 2021;6(6):211–7.
Tigges S, Stiles R, Roberson J. Complications of hip arthroplasty causing periprosthetic radiolucency on plain radiographs. AJR Am J Roentgenol. 1994;162(6):1387–91.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-1314 e1302.
Villa JM, Pannu TS, Piuzzi N, Riesgo AM, Higuera CA. Evolution of diagnostic definitions for periprosthetic joint infection in total hip and knee arthroplasty. J Arthroplasty. 2020;35(3S):S9–13.
Rosteius T, Rausch V, Pätzholz S, Lotzien S, Baecker H, Schildhauer TA, Geßmann J. Incidence and risk factors for heterotopic ossification following periprosthetic joint infection of the hip. Arch Orthop Trauma Surg. 2019;139:1307–14.
Temmerman OP, Raijmakers PG, Berkhof J, Hoekstra OS, Teule GJ, Heyligers IC. Accuracy of diagnostic imaging techniques in the diagnosis of aseptic loosening of the femoral component of a hip prosthesis: a meta-analysis. J Bone Joint Surg Br. 2005;87(6):781–5.
Cyteval C, Hamm V, Sarrabere MP, Lopez FM, Maury P, Taourel P. Painful infection at the site of hip prosthesis: CT imaging. Radiology. 2002;224(2):477–83.
Galley J, Sutter R, Stern C, Filli L, Rahm S, Pfirrmann CWA. Diagnosis of periprosthetic hip joint infection using MRI with metal artifact reduction at 1.5 T. Radiology. 2020;296(1):98–108.
Khodarahmi I, Fritz J. Advanced MR imaging after total hip arthroplasty: the clinical impact. Semin Musculoskelet Radiol. 2017;21(5):616–29.
Koutserimpas C, Naoum S, Giovanoulis V, Raptis K, Alpantaki K, Dretakis K, Vrioni G, Samonis G. Fungal periprosthetic hip joint infections. Diagnostics (Basel). 2022;12(10):2341.
Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66–78.
Roth TD, Maertz NA, Parr JA, Buckwalter KA, Choplin RH. CT of the hip prosthesis: appearance of components, fixation, and complications. Radiographics. 2012;32(4):1089–107.
Itasaka T, Kawai A, Sato T, Mitani S, Inoue H. Diagnosis of infection after total hip arthroplasty. J Orthop Sci. 2001;6(4):320–6.
Gustilo RB, Pasternak HS. Revision total hip arthroplasty with titanium ingrowth prosthesis and bone grafting for failed cemented femoral component loosening. Clin Orthop Relat Res. 1988;235:111–9.
Riegler HF, Harris CM. Heterotopic bone formation after total hip arthroplasty. Clin Orthop Relat Res. 1976;117:209–16.
Google Scholar
Download references
Acknowledgements
Registration and protocol.
The present review was not registered.
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and affiliations.
Department of Orthopaedic, Trauma, and Reconstructive Surgery, RWTH University Medical Centre, Pauwelsstraße 30, 52074, Aachen, Germany
Ulf Krister Hofmann & Filippo Migliorini
Department of Orthopaedic Surgery, University Hospital of Tübingen, 72076, Tübingen, Germany
Georgios Eleftherakis
Department of Orthopedics and Trauma Surgery, Academic Hospital of Bolzano (SABES-ASDAA), Teaching Hospital of the Paracelsus Medical University, 39100, Bolzano, Italy
Filippo Migliorini
Department of Arthroplasty and Revision Arthroplasty, Orthopaedic Clinic Markgröningen GmbH, Kurt-Lindemann-Weg 10, 71706, Markgröningen, Germany
Orthopaedic Department, University-Hospital Hamburg-Eppendorf, Martinistrasse 52, 20246, Hamburg, Germany
Department of Trauma and Reconstructive Surgery, BG Klinik, University of Tübingen, 72076, Tübingen, Germany
Moritz Mederake
You can also search for this author in PubMed Google Scholar
Contributions
UH: conception and design, drafting (original and revision); FM: supervision, drafting (revision); GF: literature search, study selection and data extraction, risk of bias assessment; MM: literature search, study selection and data extraction; BF: supervision, drafting (revision). All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Correspondence to Ulf Krister Hofmann .
Ethics declarations
Ethics approval and consent to participate.
This study complies with ethical standards.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have any competing interests for this article.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hofmann, U.K., Eleftherakis, G., Migliorini, F. et al. Diagnostic and prognostic relevance of plain radiographs for periprosthetic joint infections of the hip: a literature review. Eur J Med Res 29 , 314 (2024). https://doi.org/10.1186/s40001-024-01891-8
Download citation
Received : 19 December 2023
Accepted : 20 May 2024
Published : 08 June 2024
DOI : https://doi.org/10.1186/s40001-024-01891-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Radiography
- Periprosthetic joint infection
- Hip arthroplasty
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
research@BSPH
The School’s research endeavors aim to improve the public’s health in the U.S. and throughout the world.
- Funding Opportunities and Support
- Faculty Innovation Award Winners
Conducting Research That Addresses Public Health Issues Worldwide
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research ( research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice. Research along this entire spectrum represents a fundamental mission of the Johns Hopkins Bloomberg School of Public Health.
From laboratories at Baltimore’s Wolfe Street building, to Bangladesh maternity wards in densely packed neighborhoods, to field studies in rural Botswana, Bloomberg School faculty lead research that directly addresses the most critical public health issues worldwide. Research spans from molecules to societies and relies on methodologies as diverse as bench science and epidemiology. That research is translated into impact, from discovering ways to eliminate malaria, increase healthy behavior, reduce the toll of chronic disease, improve the health of mothers and infants, or change the biology of aging.
120+ countries
engaged in research activity by BSPH faculty and teams.
of all federal grants and contracts awarded to schools of public health are awarded to BSPH.
citations on publications where BSPH was listed in the authors' affiliation in 2019-2023.
publications where BSPH was listed in the authors' affiliation in 2019-2023.
Departments
Our 10 departments offer faculty and students the flexibility to focus on a variety of public health disciplines
Centers and Institutes Directory
Our 80+ Centers and Institutes provide a unique combination of breadth and depth, and rich opportunities for collaboration
Institutional Review Board (IRB)
The Institutional Review Board (IRB) oversees two IRBs registered with the U.S. Office of Human Research Protections, IRB X and IRB FC, which meet weekly to review human subjects research applications for Bloomberg School faculty and students
Generosity helps our community think outside the traditional boundaries of public health, working across disciplines and industries, to translate research into innovative health interventions and practices
Introducing the research@BSPH Ecosystem
The research@BSPH ecosystem aims to foster an interdependent sense of community among faculty researchers, their research teams, administration, and staff that leverages knowledge and develops shared responses to challenges. The ultimate goal is to work collectively to reduce administrative and bureaucratic barriers related to conducting experiments, recruiting participants, analyzing data, hiring staff, and more, so that faculty can focus on their core academic pursuits.
Research at the Bloomberg School is a team sport.
In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral fellows, students, and committed staff are united in our collaborative, collegial, and entrepreneurial approach to problem solving. T he Bloomberg School ensures that our research is accomplished according to the highest ethical standards and complies with all regulatory requirements. In addition to our institutional review board (IRB) which provides oversight for human subjects research, basic science studies employee techniques to ensure the reproducibility of research.
Research@BSPH in the News
Four bloomberg school faculty elected to national academy of medicine.
Considered one of the highest honors in the fields of health and medicine, NAM membership recognizes outstanding professional achievements and commitment to service.
The Maryland Maternal Health Innovation Program Grant Renewed with Johns Hopkins
Lerner center for public health advocacy announces inaugural sommer klag advocacy impact award winners.
Bloomberg School faculty Nadia Akseer and Cass Crifasi selected winners at Advocacy Impact Awards Pitch Competition
- Open access
- Published: 29 September 2022
Determinants of prescribing decisions for off-patent biological medicines in Belgium: a qualitative study
- Yannick Vandenplas 1 ,
- Steven Simoens 1 ,
- Philippe Van Wilder 2 ,
- Arnold G. Vulto 1 , 3 ,
- Florian Turk 4 &
- Isabelle Huys 1
BMC Health Services Research volume 22 , Article number: 1211 ( 2022 ) Cite this article
1383 Accesses
2 Citations
2 Altmetric
Metrics details
A competitive market for off-patent biologicals leads to more affordable and high-quality healthcare. In recent years, Belgium has been characterized by its low use of biosimilars and by its shifts from off-patent biologicals toward new alternative therapies. Yet, the prescribing decisions involved in these observations are poorly understood. This study aims to better understand prescribing choices among Belgian physicians in the ambulatory care setting.
This study consisted of two phases. First, a scoping literature review to identify determinants of prescribing choices was conducted. Scientific databases (Embase and PubMed) were searched until 4 November 2021. Second, the nominal group technique (NGT) was employed during focus group discussions with Belgian physicians to consider and validate these determinants for off-patent biologicals in the Belgian context. The qualitative data resulting from the literature review and focus group discussions were analyzed using the thematic framework method.
Fifty-three scientific articles that discussed elements that determine prescribing choices were identified. Out of these, 17 determinants of prescribing choices were found. These were divided into five categories: (1) product-related, (2) physicians’ personal, (3) healthcare system-related, (4) patient-related, and (5) determinants related to the pharmaceutical company or brand. Nineteen Belgian physicians from different therapeutic areas that regularly prescribe biologicals then participated in focus group discussions. Using the NGT, the group discussions revealed that prescribing choices for off-patent biologicals are determined by a complex set of elements. Clinical data, geographical region, working environment, pharmaceutical marketing, patient profile, clinical guidelines, and preference of key opinion leaders (KOL) were considered most influential. Physicians indicated that the importance of these determinants differs depending on product classes or therapeutic domain.
Conclusions
Multiple elements determine the choice of an off-patent biological or biosimilar product. The importance of each of these determinants varies depending on the context in which the prescribing choice is made. To increase the prescription of best-value biologicals in the Belgian ambulatory care, a set of synergistic measures is required including information for healthcare providers (HCP) and patients, prescribing feedback, prescribing targets, tangible incentives, KOL involvement, guidelines regarding pharmaceutical promotion, and regular revision of reimbursement modalities.
Peer Review reports
Governments have increasingly experienced difficulties in keeping their healthcare budgets under control over the past few decades. This problem has become even more pressing as there is a growing number of challenges faced by healthcare systems worldwide, such as ageing populations, the emergence of expensive innovative therapies like precision medicine, and the recent COVID-19 pandemic [ 1 ]. Belgium is no exception and faces these challenges as well. Belgian healthcare spending has increased annually and is now among the highest in the European Union [ 2 ]. A significant portion of healthcare spending is on pharmaceuticals [ 3 ]. The most recent data show that pharmaceuticals represent 18% of the total Belgian healthcare budget, with an overall pharmaceutical budget of approximately 5 billion euros in 2021 [ 4 , 5 , 6 ]. Moreover, the pharmaceutical budget in Belgium has risen rapidly in recent years, with a growth of 10.7% in 2021. This situation is no longer sustainable given the limited resources available in a country where healthcare is mainly publicly funded. The national health insurer (the National Institute of Health and Disability Insurance or NIHDI) also identified this problem during their recent budget discussions [ 4 , 7 ].
Therefore, there is a clear need to develop strategies to maintain the financial sustainability of the Belgian healthcare system. One of these strategies should focus on the rational and cost-effective use of medicines, more specifically of biological medicines. Biological medicines or biologicals are medicines produced by living organisms, such as bacteria or animal cells [ 8 ]. They have been successful in treating several life-threatening or chronic disorders such as cancer, diabetes, and immune mediated inflammatory disorders. Because of a costly development and manufacturing process, biological medicines are generally more expensive compared to traditional chemical molecules [ 9 ]. Together with their widespread use, this results in biologicals representing a high cost to national healthcare systems. To date, biologicals represent about 30% of all pharmaceutical spending across Europe [ 5 ]. The expected advent of a large number of new biological therapies in the coming years will probably result in a further increase of this share [ 1 ]. After the expiry of market exclusivities of biologicals and the market entry of similar versions of authorized biologicals (i.e., biosimilars), off-patent biologicals obtain a more favorable cost-effectiveness profile [ 10 ]. Yet, in order to benefit from these more cost-effective medicines, a sustainable off-patent biologicals market is required. A sustainable market refers to a situation in which both off-patent biological and biosimilar products can coexist. It therefore not only strives towards a competitive market, but also towards economic viability for pharmaceutical industry [ 11 ]. However, it has become clear that the Belgian market is not sustainable [ 12 , 13 ]. Significant shifts from off-patent biologicals towards new therapeutic classes (such as interleukin (IL) inhibitors, Janus Kinase (JAK) inhibitors, etc.) and the dominant market position of originator biologicals that lost their market exclusivities (and thus a low use of biosimilars) are the main elements supporting this observation [ 12 , 13 , 14 ]. Biosimilars (including etanercept, adalimumab, and insulin glargine) have been part of Belgium’s ambulatory care since 2016. Yet, their market shares have only increased slowly over the past few years and remain very limited [ 12 , 13 ]. This is especially so in the ambulatory segment of the market where the choice for a particular biological is made by the prescribing physician and is not subject to tender decisions [ 12 , 13 ].
Due to substantial mandatory price reductions for both biosimilars and reference biological products, price differences are limited and both generate savings for the Belgian healthcare system. Therefore, the concept of best-value biologicals was introduced, which recognizes that both originator biologicals and biosimilars can contribute to a more sustainable healthcare system [ 13 , 15 ]. The Belgian government supports the concept of best-value biologicals and introduced several initiatives to encourage the use of cost-effective biologicals in the past years [ 12 ]. In addition, given the very low biosimilar market shares in Belgium’s ambulatory care, the Belgian government has aimed to specifically increase the use of biosimilars as well [ 4 , 12 , 16 , 17 ]. However, these initiatives have not been successful to date, underlining a poor understanding of what drives prescribing decisions within the off-patent biologicals market [ 12 , 13 ]. As a result, it is of particular interest to better understand what elements determine the choice between different molecules, as well as between a reference biological and its biosimilar(s). In order to design policy interventions to stimulate physicians to prescribe cost-effective medicines in the future, the underlying drivers behind prescribing decisions have to be clearly understood [ 18 , 19 ].
This study aimed to identify elements that determine the prescription choice for off-patent biologicals in the Belgian ambulatory setting. In doing so, we must not lose sight of the current reality and set the goal of looking beyond the choice between reference products and their biosimilar(s). As described earlier, when choosing a biological product, new molecules or follow-on products are also considered. Ultimately, a better understanding of prescribing choices can be used to inform Belgian policymakers when they are designing interventions to stimulate the prescription of best-value biological medicines or biosimilars.
Since there currently is no published evidence on the kinds of elements that determine the prescription of off-patent biological medicines, a two-step qualitative approach was used to identify these determinants. During the first step, an overview was made of elements that generally determine prescribing behavior based on a scoping literature review of scientific databases. This served to inform the next step where these identified elements were discussed in focus groups with Belgian physicians. An overview of the consecutive steps of the used methodological approach is provided in Fig. 1 .
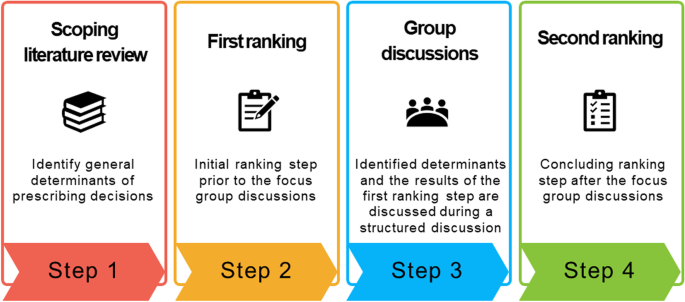
Overview of the methodological approach of this study
Scoping literature review
A scoping review of the literature in Embase and PubMed (MEDLINE) was conducted using a predefined search strategy. Scoping literature reviews aim to identify and map the existing evidence related to a certain topic of interest in a systematic way [ 20 , 21 ]. Full-text, peer-reviewed, English articles that were published between 2008 and 2021 were eligible for inclusion. Both original research articles and literature reviews were considered. Only articles that described determinants of prescribing decisions were included. Search terms were therefore related to ‘prescribing’, ‘behavior’, and ‘physician’. These search terms were adjusted according to the respective scientific database. Initially, the literature was searched up to March 2021 but was subsequently updated to 4 November 2021. The full literature search protocol can be found in the Supplementary Material .
All identified articles were imported into Mendeley software and duplicates were removed. The remaining articles were then screened by one reviewer (YV) based on title and abstract using Rayyan software [ 22 ]. Subsequent screening based on the full text of the selected articles was conducted by the same researcher (YV), and the remaining articles were included in the qualitative analysis. Peer-reviewed articles describing determinants that influence prescribing choices were analyzed qualitatively using the thematic framework method described by Gale et al. [ 23 ]. This process included mostly inductive coding as the researchers did not know in advance which determinants were involved in prescribing decisions. During the analysis of the first articles by two researchers (YV and IH), similar codes were identified as determinants of prescribing behavior and were grouped together to form a coding tree (i.e., thematic framework). The codes of the developed framework were agreed upon by the two researchers who coded the first articles. Subsequently, the remaining articles were analyzed by placing relevant passages under the developed thematic framework. In case new themes or codes were found, these were added to the existing framework. In the last phase, the results were summarized in a framework matrix and interpreted by the researchers.
Focus group discussions using the nominal group technique (NGT)
After general determinants of prescribing decisions were identified from the scoping literature review, the purpose of this study was to further examine these determinants in relation to off-patent biological medicines in the Belgian context. The results of the scoping literature review were therefore used as a starting point. Each of the identified determinants were discussed separately during focus group discussions and were scored quantitatively by the participants according to their relative importance. The nominal group technique (NGT) was used to achieve the latter. The NGT is a method to establish structured group discussions or interactions, which encourages all participants to provide their insights and to react to these insights [ 24 ]. It was chosen since the NGT allows for structured group discussions with the purpose to achieve an agreement related to a particular topic of interest. The NGT was considered appropriate for this research question as the technique also allows for ranking of priorities and the generation of new ideas [ 24 ].
The NGT consists of four distinct parts as defined by Delbecq and Van de Ven: (i) silent generation, (ii) round robin (i.e., the moderator asks each participant to share a single idea with the group), (iii) clarification, and (iv) ranking [ 24 , 25 ]. However, the NGT is a highly adaptable method depending on the specific research question and desired outcomes [ 24 ]. This allows researchers to replace or skip certain steps. For example, in this study, silent generation was omitted and replaced by a comprehensive literature review. Based on the ideas identified (in this case, determinants of prescribing behavior), the participants performed an initial ranking step prior to the group discussions. During the ranking exercise, each participant was asked to score the importance of each of the identified determinants on a five-point Likert scale in the following way: 1 = very low impact, 2 = low impact, 3 = some impact, 4 = high impact, 5 = very high impact. Average ranking values were calculated per determinant. In addition, participants were asked if there were other determinants influencing prescribing decisions regarding off-patent biologicals or if certain elements were irrelevant in this regard. After the initial ranking step, the traditional steps of the NGT were followed. As a result, the NGT consisted of the following steps:
First grading step prior to the group discussion, based on the list of determinants of prescribing decisions resulting from the scoping literature review
Presentation of the aggregated results of the first grading step to the participants (i.e., average ranking values per determinant)
Group discussion where each participant was asked to comment on the identified determinants of prescribing decisions and the results of the first grading step
Second grading step where participants were asked to rank the identified determinants again
Concluding step where each participant could share any final thoughts or remarks.
Usually, group discussions take place face-to-face, but the COVID-19 pandemic situation did not allow for physical interactions at the time when the study was conducted. Therefore, all the group discussions took place remotely via Microsoft Teams. The focus group discussions were anticipated to last between 90 and 120 minutes. All focus groups were moderated by the same researchers (YV and IH), who have experience with conducting qualitative research.
Participant selection and recruitment
Participants were Belgian specialist physicians working in therapeutic domains where off-patent biologicals and biosimilars are being prescribed in the ambulatory care setting. These products are listed in the Supplementary Material and mainly include tumor necrosis factor (TNF)-alpha inhibitors and insulin analogues. Therefore, eligible participating physicians were endocrinologists, dermatologists, gastroenterologists, and rheumatologists. The physicians were required to have a good knowledge of biologics and experience with prescribing both originator biologicals and biosimilars. The physicians were selected based on purposeful sampling, that is the identification and selection of individuals or groups who are especially knowledgeable about the topic of interest [ 26 ]. Participants were contacted via e-mail. Their contact details were obtained via publicly available information or through the network of the research unit where this study was conducted.
NGT group discussions usually involve two to fourteen participants, with an ideal number of seven participants [ 24 ]. To ensure a good balance between input coming from a variety of participants and giving each participant sufficient time to speak, the researchers aimed to have a minimum of four and a maximum of ten participants per group. The researchers ensured that a variety of backgrounds (i.e., region, working environment, therapeutic area) were present in each of the focus groups. Differences in language (i.e., Dutch or French) were also considered. Therefore, participants were asked to speak English during the focus groups. However, if necessary, participants could express themselves in their native language and the moderators acted as translators. Eligible participants were invited via e-mail and provided with all relevant study information and the informed consent form (Cfr. Supplementary Material ). All participants were required to provide written informed consent prior via e-mail to the start of the study. The study was approved by the Ethics Committee Research of UZ/KU Leuven (S65328).
Data analysis
The group discussions were audio recorded and transcribed verbatim manually. The transcripts were analyzed according to the thematic framework with NVivo software (Release 1.3), using a combination of inductive and deductive coding [ 23 ]. The deductive coding was based on the findings of the first step of this study (i.e., the scoping literature review). The transcripts were analyzed by categorizing the relevant transcript sections to the thematic framework that was developed during the first step of this study. Additional codes that arose during the group discussions were added inductively to the coding tree. The group discussions also served to detect subcodes under the existing themes that were identified through the scoping literature review. Next, the results of the coding process were charted into a framework matrix and interpreted by the researchers. Ultimately, the results were narratively described, and illustrative quotes supporting the findings were extracted.
To guarantee the confidentiality of all participants, the recordings and transcripts were pseudonymized by removing all names and references towards the identifiable person (Cfr. Supplementary Material ). These were replaced by a code randomly assigned to each participant per focus group, of which only the researchers have the code key to identify each participant.
The NGT also generates a small amount of quantitative data in addition to qualitative data. These are the result of the first and second ranking step (i.e., the five-point Likert scale) and are reported as an average score per determinant. The results of the first ranking step were presented to the participants, while the average scores per determinant of the second ranking were reported as the final result of this study (Cfr. Table 3 ). Prior to the study, a mean cutoff ranking score of 3.5 was defined by the researchers to identify the predominant determinants. The cutoff score was based on previous similar research, as well as on the perceptions of the NGT participants [ 27 ]. Finally, all average scores per determinant were ranked from high to low (Table 3 ).
As discussed above, a scoping literature review was conducted prior to the focus group discussions in order to identify elements that drive prescribing decisions in general. These identified determinants were used as a starting point for the focus group discussions with Belgian physicians.
The predefined search strategy identified a total of 2587 records. After the removal of duplicates and screening of the title and abstract, 48 articles remained. Further screening of the full-text articles led to 10 articles being excluded. The literature search was updated at the time the researchers finalized the analysis, by again using the same search strategy to look for newly published articles. This resulted in an additional 15 records that were included in the qualitative analysis. Therefore, 53 articles were included in the final qualitative analysis. An overview of the literature search process, including the flow chart of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 28 ], is included in the Supplementary Material .
Five main categories of determinants that drive prescribing choices were identified: (1) product-related, (2) physicians’ personal, (3) healthcare system-related, (4) patient-related, and (5) determinants related to the pharmaceutical company or brand. Each of these main categories was subdivided into specific determinants. An overview of all elements determining prescribing decisions with a brief explanation is provided in Table 1 . A detailed overview of the different determinants of prescribing choices identified in each article can be found in the Supplementary Material , along with a summary of the key characteristics of each study (i.e., title, author, year, location, study objectives, design, and sample size).
Demographics of participants
Nineteen physicians participated in the focus group discussions. Most participants were working in Flanders ( n = 11), while the rest worked either in Wallonia ( n = 6) or Brussels ( n = 2). They included rheumatologists ( n = 7), gastroenterologists ( n = 6), dermatologists ( n = 3), and endocrinologists ( n = 3). All age groups were represented in this study, except for the youngest group of 18 to 29-year-olds. This was expected given the age at which physician specialists graduate in Belgium. Most of the physicians were working in an academic hospital setting ( n = 10). A summary of the demographic characteristics of all participants can be found in Table 2 .
The results of the focus group discussions are presented below for each of the 17 determinants identified from the scoping literature review. An overview of all these elements, along with their average ranking after the second round, is summarized in Table 3 .
Product-related
Clinical data.
Physicians stated that the need for clinical data regarding biosimilars has evolved over the years. In the past, Belgian physicians had insufficient knowledge about the clinical development of biosimilars, which influenced their prescription practices. Until recently, uncertainty existed among Belgian physicians about the clinical differences when transitioning from an original biological to a biosimilar. These doubts are less prevalent nowadays due to an increase of positive experiences with biosimilars. It was agreed that when choosing between a biosimilar and its originator biological, doubts regarding reduced efficacy or safety are becoming less of an issue in Belgium. According to the participating physicians, the concept of extrapolation of indication does not pose an issue anymore for most Belgian physicians.
However, the situation differs when choosing between products with different mechanisms of action (MOA). In this case, the clinical data were considered important. For example, among the dermatologists, IL-17/23 inhibitors are considered clinically superior to existing off-patent alternatives (i.e., TNF-alpha inhibitors). This is one of the main reasons why existing off-patent biologicals are rarely prescribed for the treatment of new patients with psoriasis. This importance was also reflected in the average ranking score of 3.6 (Cfr. Table 3 ).
Physicians agreed that cost should be an important determinant, given the rising pharmaceutical expenditure in Belgium over the last few years. However, despite the important price differences between off-patent and competing patented products, it was believed that this only mattered for a small number of physicians when choosing a particular biological. The main reason for this would be that Belgian physicians are not sufficiently aware of the cost of medicines. Participants mentioned that there is a general lack of awareness about increasing pharmaceutical costs and the importance of a financially sustainable healthcare system. Moreover, the other determinants discussed in this article were believed to outweigh the impact of price considerations, according to the participating physicians.
According to the participants, cost considerations become an almost irrelevant determinant when choosing between an originator biological and its biosimilar(s) because of the limited price differences in the ambulatory care.
Overall, it was concluded that the cost of biological products has a limited influence on prescribing decisions in Belgium.
Administration
Physicians agreed that the administration modalities play a role for certain therapeutic areas. However, the influence of this depends greatly on the product or on whether different administration routes, devices, or frequencies are available. Off-patent biologicals in the ambulatory setting are always subcutaneously (SC) administered. Hence, the route of administration was considered to be unimportant. However, for certain therapeutic groups, oral (non-biological) alternatives are also available (e.g., JAK inhibitors). The oral administration route was thought to be one of the reasons for the current increase of JAK inhibitors being prescribed in Belgium, especially for patients with rheumatoid arthritis.
For most off-patent SC products, there are products available with different injection devices, frequencies of administration, and injection comfort. Physicians agreed that these differences, if present, play a role in the choice of a particular off-patent biological, although they are mostly related to patient comfort and patient preferences (see patient-related). Physicians concluded that administration modalities are certainly taken into consideration but should not be overestimated when looking at the overall picture.
Notwithstanding the success of certain new alternatives (such as IL-17/23 inhibitors, patented TNF-alpha inhibitors, JAK inhibitors, etc.) compared to off-patent biologicals, the mere fact that these are new products was believed to only play a small part in prescribing choices. The other elements discussed in this study are more likely to be causing the observed shift in prescribing behavior.
Physicians’ personal
Working environment.
Regarding the working environment of prescribers, the main point raised during the focus group discussions was that Belgian physicians working in hospitals or private practices differ in their access to information and certain support services. In academic hospitals, physicians would often have more access to information and are visited more frequently by pharmaceutical representatives. In addition, physicians in academic hospitals often already have experience with new MOA products through research studies, which would lead to a faster prescription uptake of these products. Differences in support services between individual hospitals, however, was considered to be even more influential by the participating physicians. Also, participants expressed that academic hospitals often have access to a larger nursing staff or more hospital beds. As a result, when pharmaceutical companies offer value-added services as a tool to promote their products to prescribers, this would influence their prescribing decisions to a lesser extent than for physicians working in general hospitals. Participants indicated that services provided by the pharmaceutical industry will have a lower impact on physicians working in private practices, which is particularly common among Belgian dermatologists and rheumatologists.
In general, physicians noted the importance of the prescriber’s working environment, but this difference was mostly linked to other determinants, such as pharmaceutical promotion, prescribing habits, and the influence of KOLs.
Several physicians pointed out some demonstrable differences between Belgian regions. For example, in rheumatology the transition from existing off-patent TNF inhibitors to JAK inhibitors is more prominent in Wallonia. However, the participants could not provide a particular reason for this. In addition, it was also indicated that there is a difference in the use of SC-administered biosimilars between Flanders and Wallonia, with higher SC biosimilar prescription rates in Flanders. It was argued that such differences may be due to different pharmaceutical marketing approaches and prescribing habits in both regions.
Prescribing habits
Prescribing habits were described by physicians as one of the most important determinants of prescribing choices. It was confirmed across all specialties that physicians tend to stick with products they have experience with. As a result, originator biologicals are often preferred over biosimilars in Belgium as prescribers have had limited experiences with biosimilars in the ambulatory care to date. An associated fear of loss of clinical efficacy was believed to be the main underlying reason for this therapeutic inertia when making prescribing choices. However, participants mentioned that prescribing habits tend to be more influencial when choosing between reference products and biosimilars compared to when new MOA products are being considered. For the latter, physicians had the impression that the abovementioned therapeutic inertia plays less of a role as moves to new MOAs are common practice in the Belgian ambulatory care.
Participating physicians agreed that the age of the prescribing physician plays little or no role in their prescribing choices. However, it was mentioned several times that older physicians, who have also known a time without the abundance of therapeutic options, are probably more inclined to prescribe the products that have been on the market for a longer period (i.e., off-patent products). Moreover, it was argued that younger physicians will have accumulated less experience with the more mature biologicals and are more likely to transition to new MOAs for this reason.
Healthcare system-related
Preference of key opinion leaders (kol).
The preference of KOLs was regarded as a major determining element when making prescribing choices for off-patent biological medicines. Physicians indicated that when KOLs express a particular preference regarding biological medicines, this generally has an impact on the prescribing choices of their peers. However, the preferences of KOLs were said to be mostly shown for specific MOAs, and not for reference biologicals and their biosimilars. According to the participants, this might have resulted in the shift that has been seen from off-patent biologicals towards new MOA products. KOLs have been less outspoken about their preferences towards biosimilars. Physicians indicated that it is precisely because KOLs give little attention to biosimilars, fewer biosimilars are being prescribed.
Clinical guidelines
Regarding the impact of clinical guidelines as a determinant for choosing a biological, all physicians emphasized that there is a substantial difference between choosing a particular molecule versus an originator biological or biosimilar. Belgian guidelines are generally scarce, and so European guidelines are seen as impactful. Clinical guidelines were thought to be a substantial determinant when choosing between different MOAs and thus between different off-patent (or patented) biologicals. However, it was indicated that clinical guidelines usually do not influence the decision when choosing between an originator biological and its biosimilar. The main reason for this is that both at the Belgian and the European level, this choice is often not explicitly incorporated in the clinical guidelines.
Time pressure
There was agreement among the participating physicians that it takes time to sufficiently explain to patients a transition from an original biological to a biosimilar. It was indicated that the limited length of a consultation does not allow for enough time to provide an explanation of a possible switch to a biosimilar. However, it was emphasized that time constraints are a commonly used excuse not to prescribe biosimilars. Physicians from the various therapeutic areas admitted that time pressure only plays a minor role when choosing between an originator biological or biosimilar medicine. In reality, there are other more predominant elements that determine this choice.
Across the different therapeutic areas, there was an agreement that transitioning between different molecules was regarded as common practice without the concerns of time constraints. Hence, there is no reason to assume that this should be much different when transitioning to a biosimilar. Therefore, even if more explanation is required when transitioning to a biosimilar, this should not be a valid reason to avoid prescribing biosimilars.
Financial incentives
During the group discussions, it emerged that financial incentives can be an important determinant of prescribing choices for off-patent biologicals. It was pointed out that the right incentives to increase the use of biosimilars may have an important impact, although these are currently absent in Belgium. The financial incentive introduced in 2019 to promote biosimilar prescription was cited as an example of the negligible impact of individual financial incentives. An incentive that supports the quality of care could have a much larger impact according to the participants.
Moreover, the impact of an incentive to promote biosimilar prescription would differ between hospitals. As indicated earlier, the amount of support varies from hospital to hospital. Therefore, the potential impact of supporting incentives from the government is partly dependent on this. Physicians working in private practices indicated that financial incentives, whether on an individual basis or at the hospital level, have a minor impact on their prescribing choices.
Patient-related
Patient profile.
Participating physicians agreed that the patient profile plays little or no role in the choice of an originator biological versus its biosimilar. When it comes to choosing between different molecules or MOAs, the patient profile does play a role. Comorbidities, age, lifestyle, socio-professional context, and comedication were mentioned as relevant examples in this context. Physicians indicated that these determinants, together with the administration modality, can largely determine medication adherence. For example, a less frequent administration would be more appropriate for an active or young patient. Participants also indicated that these aspects are often mentioned in clinical guidelines. Therefore, the importance patient profile is also linked to the availability of clinical guidelines.
Patient preference
Physicians indicated that the choice of a biological is only marginally influenced by the patient as most patients have limited preferences. If patients do have a preference, which is mostly for a particular injection device or administration route, it was mentioned that this obviously has a significant impact on the prescribing choice. Physicians confirmed that apart from a small group of patients, most patients generally have no preference regarding a biosimilar or originator biological.
Knowledge or perception of patients
Patient knowledge or perception about a particular type of medicine only has a minor effect on prescribing choices, as patients typically have a limited knowledge about biologicals. Physicians agreed that it may play a role with a small number of patients who have a rather negative perception towards biosimilar medicines. Nonetheless, if strong negative preconceptions about a particular medicine exists, this could have a significant impact on prescribing choices since these negative preconceptions may induce nocebo effects (i.e., the induction or exacerbation of symptoms through negative expectations about the therapy). Endocrinologists indicated that a resistance to taking insulin therapy can be an important barrier for patients with type 2 diabetes, as there is often an underlying perception of failure and shame when being treated with insulin.
Related to the pharmaceutical company or brand
Pharmaceutical marketing and promotion.
The impact of promotion and marketing activities from the pharmaceutical industry was considered by all participating physicians to be very large. It was striking that the focus group participants mentioned that although its impact is undoubtably significant, physicians often deny this impact. The influence of everything related to pharmaceutical marketing was rated as especially high when it comes to prescribing an originator biological or its biosimilar(s). It was postulated that this is because physicians usually know that the originator biological and its biosimilar(s) are clinically equivalent products. Therefore, physicians acknowledged that marketing and promotion become more important determinants in this context, compared to when choosing between different molecules or MOAs where clinical differences might come into play.
Based on the suggestions provided during the focus group discussions, this category was divided into three subcategories: (a) value-added services, (b) visits by pharmaceutical companies, and (c) sponsorship or grants.
Value-added services
When choosing between an original biological or a biosimilar, the services that a company offers to physicians or hospitals were considered the most important determinant within the category of marketing and promotion. Examples cited by participating physicians included patient support programs, therapeutic drug monitoring (TDM), and nursing support. Companies offering such services are preferred when choosing between an original biological and its biosimilar(s). Usually, companies that market the originator biological offer these services already before biosimilars become available. Physicians, therefore, questioned why they would transition to a biosimilar if prescribers and patients would lose this important support. However, it was also indicated that physicians generally do not find this a transparent and correct system, and therefore, government incentives to support biosimilar prescribing remain important. As elaborated earlier, it became clear during the group discussions that the impact of these services also depends in part on the support already in place in the hospital where the prescriber works.
Visits by pharmaceutical companies
Physicians agreed that one should not underestimate the impact of representatives from the pharmaceutical industry. Consciously or unconsciously, prescribers will be influenced by this when choosing an off-patent biological. A good personal contact with the prescriber would certainly have an impact. Physicians pointed out that this was a reason why prescribers do not transition to a biosimilar, but also why they do transition more quickly to another MOA. After all, the alternative MOA products are often marketed by the same company as the originator biological, and thus, by the same pharmaceutical representative. The information provided by representatives during visits was also considered to have a great effect. The physicians indicated that they usually have little time to inform themselves about the latest products, and as a result, they are sometimes limited to the information provided to them by industry representatives.
Sponsorships or grants
Many pharmaceutical companies provide additional sponsorships to prescribers to conduct research with their pharmaceutical product. This was also mentioned as a common practice for biological medicines, both with innovator and off-patent biologicals. Physicians indicated that such sponsorships certainly increase the likelihood of prescribing these products. This may occur because the prescriber would like to give something back to the company. Also, the fact that the physician has already some experience with the product increases the likelihood of future prescriptions.
Reputation of the pharmaceutical company or brand
Participating physicians mentioned that the reputation and trust in the pharmaceutical company is an element that matters when choosing a medicine, but is certainly of less importance than promotional activities. When deciding between an original biological and a biosimilar, certain companies have a less favorable reputation among Belgian physicians than others. Participating physicians appear to prefer companies that also invest in drug research and development, rather than companies that purely produce or market biosimilars. Participants also indicated that prescribers are more likely to prefer biologicals produced by companies that have experience with producing biological products. Both elements were viewed by participants as reasons why biosimilars are less often considered in prescribing decisions. In general, it was assumed that reputation has an impact on prescribing behavior especially when choosing between products with the same MOA, such as between an originator biological and it biosimilar(s).
Key study findings
This study identified five main categories of elements that may determine prescribing choices for off-patent biologicals in Belgium. These five categories were the result of a scoping literature review that looked for determinants of prescribing choices in general: (1) product-related, (2) physicians’ personal, (3) healthcare system-related, (4) patient-related, and (5) determinants related to the pharmaceutical company or brand. Each of these was subdivided into several subcategories, leading to a total of seventeen determinants. In the second phase of this study, focus group discussions using the NGT with Belgian physicians were conducted to validate these determinants for prescribing off-patent biologicals in Belgium. From this, seven elements emerged as predominant in this setting: clinical data, region, working environment, marketing and promotion, patient profile, clinical guidelines, and preference of KOLs.
One of the most discussed topics was the influence of pharmaceutical promotion. Physicians generally agreed during the group discussions that the influence of pharmaceutical promotion is substantial. Several reviews on the influence of pharmaceutical promotion have concluded as well that its impact on prescribing decisions is considerable [ 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ]. This study confirms these findings also for off-patent biological medicines.
The other much debated element was the impact of the medication cost. Although not strongly reflected in the ranking scores, participating physicians appeared to agree that too little attention is paid to medication cost among Belgian prescribers. This was also demonstrated in a previous study of Belgian prescribing practices where shifts towards more expensive molecules rather than off-patent biologicals were observed [ 13 ].
It is clear that prescription choices are complex and determined by a wide range of elements [ 36 ]. This was illustrated by the considerable number of determinants that were identified from the literature, and the fact that almost all were considered important to some degree by the Belgian participants in the context of off-patent biological prescribing.
Avenues for further research
This study, in addition to the existing knowledge [ 12 , 13 , 14 ], made an important step towards informing Belgian policymakers to create tailored measures for a more sustainable market for off-patent biologicals and biosimilars in Belgium. Nonetheless, in the future, a more thorough and quantitative examination of the determinants of prescribing behavior would be interesting. A quantitative approach with a behavioral model is therefore advisable. This qualitative study could serve as a base for creating such a behavioral model and for thoroughly investigating prescribing behavior in the future.
Opportunities for policymakers
Investments should be made to continuously increase the awareness of physicians about the importance of cost-effective prescribing. In this regard, several opportunities for policymakers were listed in Table 4 based on the identified determinants of prescribing decisions for off-patent biologicals in Belgium.
Benefit-sharing incentives appear to have an influence on prescribing choices, although they will need to be introduced in the form of healthcare support within hospital departments and not as individual financial incentives at the level of the prescriber [ 37 ]. This study made clear that these needs differ between types of hospitals, regions, and therapeutic areas. Therefore, a certain amount of freedom on how hospital departments choose to deploy these incentives within their working environment is appropriate. More restrictive measures such as prescription targets (on biosimilars or on best-value biologicals) are helpful but should not be used as a standalone measure [ 27 , 38 , 39 ]. Invariably, prescribing targets should be accompanied by information, benefit-sharing initiatives, and other measures to empower physicians in their prescribing choices [ 40 ].
In an ideal world, resources are infinite and governments can provide all patients with access to the medicine with the best clinical outcomes or therapeutic value. However, in reality, budgets are limited and therefore the use of cost-effective therapies is essential [ 41 ]. In this way, clinical outcomes are maximized and as many patients as possible can be treated with the limited financial resources available [ 42 , 43 ]. For this reason, clinical guidelines increasingly focus on the importance of cost-effectiveness in medicine selection [ 44 , 45 , 46 , 47 ]. The Belgian reimbursement committee usually adopts these clinical guidelines in their reimbursement modalities. However, in the specific case of JAK inhibitors versus TNF-alpha inhibitors in rheumatology, these have not yet been included in the reimbursement modalities. As a result, physicians are free to choose the various second-line products that are in competition with off-patent biologicals, regardless of the significant differences in treatment costs that have emerged after biosimilar market entry. The Belgian reimbursement committee should, therefore, periodically revise reimbursement modalities for off-patent biologicals as soon as they lose their market exclusivity or when biosimilars enter the market [ 10 , 13 ]. This may result in bringing more cost-effective products into the treatment line sooner or at an earlier disease stage [ 48 ].
As mentioned above, pharmaceutical promotion may be an important determinant of prescribing decisions. Despite the existing regulations (i.e., Article 10 of the Belgian Law on Pharmaceutical products of March 1964 and Directive 2001/83/EC on Medicines for human use) and code of ethics (i.e., Mdeon) at the Belgian and European level [ 49 , 50 , 51 , 52 ], more guidelines on transparency and authorized promotion are desirable. Belgian physicians indicated that there is a need for more detailed transparency regarding what support physicians receive from pharmaceutical companies. This was also previously proposed by Medaxes, the umbrella association for the Belgian generic and biosimilar industry [ 53 ]. Moreover, in particular for value-added services and patient support programs, guidelines are advised on which services are allowed (and which are prohibited).
Given the rapidly evolving off-patent biologics landscape with new biological medicines losing their market exclusivities, new second-generation products, and new therapeutic classes coming to the market, it is important that policymakers act proactively. Policy measures should therefore be tailored to the product-specific context and implemented prior to the changing market environment when biosimilars enter the market.
Study strengths and limitations
There have been no peer-reviewed research articles published that have examined prescribing choices for off-patent biologicals to date. Therefore, as far as the authors know, this study is the first to do so in an academic setting. By starting from elements found in the existing scientific literature and evaluating these further in the context of biological medicines with various Belgian physicians during focus group discussions, a comprehensive picture was obtained. The NGT was appropriate for this purpose as qualitative insights from the group discussions could be combined with a quantitative aspect to estimate the relative impact of each determinant in prescribing decisions.
This study sought to identify which aspects influence prescribing choices through a combination of a literature review and focus group discussions. The scoping literature review aimed to identify general determinants of prescribing choices from the scientific literature. Because of the broad search strategy and the fact that only one independent investigator performed the screening, relevant articles may have been missed (i.e., selection bias). However, the purpose of this scoping review was not to provide a complete overview of all articles describing determinants of prescribing behavior. Instead, the researchers aimed to give an overall picture of the existing literature on this topic, in order to allow for further exploration in a second step during focus group discussions.
A significant part of human decisions happens unconsciously, and people do not consciously make specific trade-offs all the time. This aspect is challenging to map through focus group discussions. In addition, we should also consider possible bias in the results because physicians may be more likely to name elements that should drive prescribing choices rather than those that actually drive their choices. However, this was avoided as much as possible by clearly informing the participating physicians about the research aims.
A disadvantage of the chosen qualitative method is that it is more difficult to draw conclusions about the entire population due to the relatively small sample. This selection bias is inherent to the purposeful sampling method [ 54 ]. Also, the majority of participants were active in academic hospitals. Considering the differences between academic hospitals and private practices or general hospitals, this may also have led to a certain bias in the results. Lastly, a generalization of the obtained conclusions to a wider geographical scope other than Belgium would not be appropriate.
As is inherent in qualitative research, personal biases and opinions may influence the interpretation of the research findings [ 55 ]. However, through close consultation with the researchers involved and a standardized protocol for each step of this study, the probabilities of this were kept to a minimum. In this way, reflexivity was embedded in the research process.
Prescribing is inextricably linked to human choices and behavior, and thus should be understood in this context. Product-, patient-, healthcare system-, personal-, and pharmaceutical marketing-related elements play a role in prescribing decisions, underlining the complexity of prescribing choices. To increase the prescribing of best-value biologicals in the future, policy measures should be tailored to the product-specific context and implemented prior to the changing market environment when biosimilars enter the market.
Availability of data and materials
The data generated or analyzed related to the literature review of this study are included in this published article and its supplementary information files. The datasets generated and/or analyzed during the focus group discussions are not publicly available due to privacy constraints but are available from the corresponding author on reasonable request.
Abbreviations
Nominal Group Technique
Key Opinion Leader
National Institute for Health and Disability Insurance
Interleukin
Janus Kinase
Tumor Necrosis Factor
Mechanism of Action
Subcutaneous
Therapeutic Drug Monitoring
IQVIA. The global use of medicines 2022: outlook to 2026. 2021. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicines-2022/global-use-of-medicines-2022-outlook-to-2026-12-21-forweb.pdf . Accessed 16 Dec 2021.
Google Scholar
OECD. Health spending (indicator). 2022. https://data.oecd.org/healthres/health-spending.htm#indicator-chart . Accessed 5 May 2022.
OECD. Pharmaceutical spending (indicator). 2022. https://data.oecd.org/healthres/pharmaceutical-spending.htm#indicator-chart . Accessed 3 May 2022.
National Institute for Health and Disability Insurance (NIHDI). Budget 2021 – Begrotingsvoorstel van het Verzekeringscomité. 2020. https://www.riziv.fgov.be/SiteCollectionDocuments/RIZIV_ARGV_2020_065.pdf . Accessed 16 Dec 2020.
IQVIA. The Impact of Biosimilar Competition in Europe. 2020. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe . Accessed 16 Jan 2021.
OECD. Health at a Glance. 2021. https://www.oecd-ilibrary.org/docserver/ae3016b9-en.pdf?expires=1639586364&id=id&accname=guest&checksum=9754877E2D983E7EA02C3901126787D3 . Accessed 15 Dec 2021.
National Institute for Health and Disability Insurance (NIHDI). Budget 2022 – Begrotingsvoorstel van het Verzekeringscomité. 2022. https://www.riziv.fgov.be/SiteCollectionDocuments/voorstel_budget_2022_verzekeringscomite.pdf . Accessed 15 Dec 2021.
European Medicines Agency (EMA). Biosimilars in the EU: Information guide for healthcare professionals. 2017. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf . Accessed 12 Apr 2020.
Cornes P, Bennett D. Biosimilars. Oxford: Health Press Limited; 2018.
Simoens S, Vulto AG. A health economic guide to market access of biosimilars. Expert Opin Biol Ther. 2021;21:9–17.
Article PubMed Google Scholar
Simoens S, Huys I. Emerging insights into European Markets of Biologics, Including Biosimilars. Pharmaceuticals. 2022;15:615.
Article PubMed PubMed Central Google Scholar
Moorkens E, Vulto AG, Huys I, Vulto AG. Biosimilars in Belgium : a proposal for a more competitive market. Acta Clin Belg. 2020;76:1–12.
Vandenplas Y, Simoens S, Van Wilder P, Vulto AG, Huys I. Off-patent biological and biosimilar medicines in Belgium: a market landscape analysis. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.644187 .
Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. Pharmacoeconomics. 2014;32:681–91.
HSE Medicines Management Programme. Best-Value Biological Medicines: Tumour Necrosis Factor-α Inhibitors on the High Tech Drug Scheme. 2019. https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-medicines/best-value-biological-medicines/mmp report bvb medicines tnf alpha inhibitors may 2019.pdf. Accessed 3 Dec 2021.
National Institute for Health and Disability Insurance (NIHDI). Convenant “Doorstart voor biosimilaire geneesmiddelen in Begië.” 2016. https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/geneesmiddel-voorschrijven/Paginas/biosimilaire-geneesmiddelen.aspx . Accessed 19 Mar 2020.
National Institute for Health and Disability Insurance (NIHDI). Biosimilaire geneesmiddelen: Incentive voor het voorschrijven van biosimilaire geneesmiddelen buiten het ziekenhuis. 2019. https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/geneesmiddel-voorschrijven/Paginas/biosimilaire-geneesmiddelen-buiten-ziekenhuis.aspx#.XUlIX-gzaUk . Accessed 4 Dec 2019.
Matjasko JL, Cawley JH, Baker-Goering MM, Yokum DV. Applying behavioral economics to public health policy: illustrative examples and promising directions. Am J Prev Med. 2016;50:S13–9.
Connelly LB, Birch S. Sustainability of publicly funded health care systems: what does Behavioural economics offer? Pharmacoeconomics. 2020;38:1289–95.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Paré G, Trudel M-C, Jaana M, Kitsiou S. Synthesizing information systems knowledge: a typology of literature reviews. Inf Manag. 2015;52:183–99.
Article Google Scholar
Qatar computing research institute. Rayyan. 2022. https://www.rayyan.ai/ . Accessed 5 Aug 2022.
Gale NK, Heath G, Cameron E. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;7:117.
McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–62.
PubMed PubMed Central Google Scholar
Lago PP, Beruvides MG, Jian J-Y, Canto AM, Sandoval A, Taraban R. Structuring group decision making in a web-based environment by using the nominal group technique. Comput Ind Eng. 2007;52:277–95.
Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K, et al. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2016;42:533–44.
Barbier L, Simoens S, Declerck P, Vulto AG, Huys I. Biosimilar use and switching in Belgium: avenues for integrated policy making. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.821616/ .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Hulscher MEJL, Grol RPTM, van der Meer JWM. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10:167–75.
Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41:203–12.
Article CAS PubMed Google Scholar
Rose J, Crosbie M, Stewart A. A qualitative literature review exploring the drivers influencing antibiotic over-prescribing by GPs in primary care and recommendations to reduce unnecessary prescribing. Perspect Public Health. 2021;141:19–27.
Davari M, Khorasani E, Tigabu BM. Factors influencing prescribing decisions of physicians: a review. Ethiop J Health Sci. 2018;28:795–804.
Md Rezal RS, Hassali MA, Alrasheedy AA, Saleem F, Md Yusof FA, Godman B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti-Infect Ther. 2015;13:665–80.
Machowska A, Lundborg CS, Stålsby LC. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health. 2019;16:27.
Alowi M, Kani Y. Impact of pharmaceutical companies’ promotional tools on physicians’ prescription patterns: a systematic review. J Appl Pharm. 2018;10.
Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 2014;14:469.
Barcina Lacosta T, Vulto AG, Turcu-Stiolica A, Huys I, Simoens S. Qualitative analysis of the design and implementation of benefit-sharing programs for biologics across Europe. BioDrugs. 2022;36:217–29.
Remuzat C, Dorey J, Cristeau O, Ionescu D, Radiere G, Toumi M, et al. Key drivers for market penetration of biosimilars in Europe. J Mark access Heal policy. 2017;5:1272308.
Moorkens E, Jonker-Exler C, Huys I, Declerck P, Simoens S, Vulto AG. Overcoming barriers to the market access of biosimilars in the European union: The case of biosimilar monoclonal antibodies. Front Pharmacol. 2016;7 JUN:1–9.
Barbier L, Simoens S, Vulto AG, Huys I. European stakeholder learnings regarding Biosimilars: part II—improving biosimilar use in clinical practice. BioDrugs. 2020;34:797–808.
Sculpher M. Evaluating the cost-effectiveness of interventions designed to increase the utilization of evidence-based guidelines. Fam Pract. 2000;17 suppl_1:S26–31.
Chalkidou K, Glassman A, Marten R, Vega J, Teerawattananon Y, Tritasavit N, et al. Priority-setting for achieving universal health coverage. Bull World Health Organ. 2016;94:462.
Bilinski A, Neumann P, Cohen J, Thorat T, McDaniel K, Salomon JA. When cost-effective interventions are unaffordable: integrating cost-effectiveness and budget impact in priority setting for global health programs. PLoS Med. 2017;14:e1002397.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:1–15.
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohn's Colitis. 2020;14:4–22.
Gossec L, Smolen JS, Ramiro S, De Wit M, Cutolo M, Dougados M, et al. European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510.
Davies MJ, Alessio DAD, Fradkin J, Kernan WN, Mathieu C, Management of hyperglycaemia in type 2 diabetes. 2018 A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2018;61:2461–98.
Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about Price! BioDrugs. 2020. https://doi.org/10.1007/s40259-019-00395-w .
European Parliament. Directive 2001/83/EC On the Community Code relating to Medicinal Products for Human Use.
Ministerie van Volksgezondheid en Leefmilieu. 25 maart 1964 - Wet op de geneesmiddelen. 1964.
MDeon. Code voor Deontologie: Deontologisch Gezondheidsplatform 2018. https://www.mdeon.be/wp-content/uploads/2020/10/Code-Mdeon-NL-01.10.2020.pdf . Accessed 8 Aug 2022.
Federal Agency for Medicines and Health Products (FAMHP). Reclame, premies, voordelen, monsters. 2021. https://www.fagg-afmps.be/nl/MENSELIJK_gebruik/geneesmiddelen/geneesmiddelen/goed_gebruik_geneesmiddel/reclame . Accessed 8 Aug 2022.
Medaxes. Medaxes Memorandum 2019. 2019.
Sharma G. Pros and cons of different sampling techniques. Int J Appl Res. 2017;3:749–52.
Guillemin M, Gillam L. Ethics, reflexivity, and “ethically important moments” in research. Qual Inq. 2004;10:261–80.
Download references
Acknowledgements
The authors would like to express their appreciation to the Pharmaceutical Policy Department of NIHDI for their financial support in this research project. In addition, the authors would like to thank all participating physicians who shared their expertise during the focus group discussions.
This manuscript is supported and funded by KU Leuven and the Belgian National Institute for Health and Disability Insurance (NIHDI).
Author information
Authors and affiliations.
Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium, Germany
Yannick Vandenplas, Steven Simoens, Arnold G. Vulto & Isabelle Huys
Ecole de Santé Publique, Université Libre de Bruxelles (ULB), Brussels, Belgium, Germany
Philippe Van Wilder
Hospital Pharmacy, Erasmus University Medical Center, Rotterdam, the Netherlands
Arnold G. Vulto
Unversity of Paderborn, Paderborn, Germany
Florian Turk
You can also search for this author in PubMed Google Scholar
Contributions
IH, FT, AGV, SS, and YV developed the idea for the study and were involved in its design. YV conducted the data collection and drafted the initial version of the manuscript. IH, FT, AGV, SS, and PVW critically reviewed the manuscript. All authors have read and approved the final manuscript.
Authors’ information
YV works as a PhD researcher in the Department of Clinical Pharmacology and Pharmacotherapy at KU Leuven (Belgium) and is involved in a research project entitled ‘Policy for best-value biological medicines in Belgium’. This research project is supported by the Belgian national health insurer, RIZIV-INAMI.
SS is a professor in Health Economics in the Department of Clinical Pharmacology and Pharmacotherapy at KU Leuven (Belgium).
PVW is a professor in Health Economics at the Ecole de Santé Publique of ULB (Belgium).
AGV is a professor of Hospital Pharmacy & Practical Therapeutics at the department of Clinical Pharmacology and Pharmacotherapy at KU Leuven (Belgium) and Erasmus MC in Rotterdam (the Netherlands).
FT is a professor at the University of Paderborn (Germany), with profound expertise in behavioral sciences and biosimilar policymaking.
IH is a professor in Regulatory Sciences in the Department of Clinical Pharmacology and Pharmacotherapy at KU Leuven (Belgium).
Corresponding author
Correspondence to Yannick Vandenplas .
Ethics declarations
Ethics approval and consent to participate.
The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations (including the Declaration of Helsinki). This study was approved by the Ethics Committee Research of UZ/KU Leuven (S65328). Written informed consent was obtained from all participants prior to the study.
Consent for publication
Not applicable.
Competing interests
This research project is funded by the Belgian National Institute for Health and Disability Insurance (NIHDI). NIHDI was not involved in the design of the study and collection, analysis, interpretation of data, nor in writing the manuscript.
SS, IH and AGV founded the KU Leuven Fund for Market Analysis of Biologics and Biosimilars following Loss of Exclusivity (MABEL). SS was involved in a stakeholder roundtable on biologics and biosimilars sponsored by Amgen, Pfizer, and MSD; he has participated in advisory board meetings for Pfizer, Sandoz, and Amgen; he has contributed to studies on biologics and biosimilars for Hospira (together with AGV and IH), Celltrion, Mundipharma, and Pfizer; and he has had speaking engagements for Amgen, Celltrion, and Sandoz. AGV is involved in consulting, advisory work, and speaking engagements for several companies, including AbbVie, Accord, Amgen, Biogen, EGA, Effik Benelux, Pfizer/Hospira, Mundipharma, Roche, and Sandoz. PVW acted as a healthcare consultant to public and private organizations, including pharmaceutical companies and their professional associations. FT acts as an advisor to, and is a consultant for, several pharmaceutical organizations and has represented pharmaceutical organizations in professional associations.
All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vandenplas, Y., Simoens, S., Van Wilder, P. et al. Determinants of prescribing decisions for off-patent biological medicines in Belgium: a qualitative study. BMC Health Serv Res 22 , 1211 (2022). https://doi.org/10.1186/s12913-022-08591-1
Download citation
Received : 15 June 2022
Accepted : 21 September 2022
Published : 29 September 2022
DOI : https://doi.org/10.1186/s12913-022-08591-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biological medicine
- Prescription
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
APA Sample Paper

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Note: This page reflects the latest version of the APA Publication Manual (i.e., APA 7), which released in October 2019. The equivalent resource for the older APA 6 style can be found here .
Media Files: APA Sample Student Paper , APA Sample Professional Paper
This resource is enhanced by Acrobat PDF files. Download the free Acrobat Reader
Note: The APA Publication Manual, 7 th Edition specifies different formatting conventions for student and professional papers (i.e., papers written for credit in a course and papers intended for scholarly publication). These differences mostly extend to the title page and running head. Crucially, citation practices do not differ between the two styles of paper.
However, for your convenience, we have provided two versions of our APA 7 sample paper below: one in student style and one in professional style.
Note: For accessibility purposes, we have used "Track Changes" to make comments along the margins of these samples. Those authored by [AF] denote explanations of formatting and [AWC] denote directions for writing and citing in APA 7.
APA 7 Student Paper:
Apa 7 professional paper:.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Grad Med Educ
- v.8(3); 2016 Jul
The Literature Review: A Foundation for High-Quality Medical Education Research
a These are subscription resources. Researchers should check with their librarian to determine their access rights.
Despite a surge in published scholarship in medical education 1 and rapid growth in journals that publish educational research, manuscript acceptance rates continue to fall. 2 Failure to conduct a thorough, accurate, and up-to-date literature review identifying an important problem and placing the study in context is consistently identified as one of the top reasons for rejection. 3 , 4 The purpose of this editorial is to provide a road map and practical recommendations for planning a literature review. By understanding the goals of a literature review and following a few basic processes, authors can enhance both the quality of their educational research and the likelihood of publication in the Journal of Graduate Medical Education ( JGME ) and in other journals.
The Literature Review Defined
In medical education, no organization has articulated a formal definition of a literature review for a research paper; thus, a literature review can take a number of forms. Depending on the type of article, target journal, and specific topic, these forms will vary in methodology, rigor, and depth. Several organizations have published guidelines for conducting an intensive literature search intended for formal systematic reviews, both broadly (eg, PRISMA) 5 and within medical education, 6 and there are excellent commentaries to guide authors of systematic reviews. 7 , 8
- A literature review forms the basis for high-quality medical education research and helps maximize relevance, originality, generalizability, and impact.
- A literature review provides context, informs methodology, maximizes innovation, avoids duplicative research, and ensures that professional standards are met.
- Literature reviews take time, are iterative, and should continue throughout the research process.
- Researchers should maximize the use of human resources (librarians, colleagues), search tools (databases/search engines), and existing literature (related articles).
- Keeping organized is critical.
Such work is outside the scope of this article, which focuses on literature reviews to inform reports of original medical education research. We define such a literature review as a synthetic review and summary of what is known and unknown regarding the topic of a scholarly body of work, including the current work's place within the existing knowledge . While this type of literature review may not require the intensive search processes mandated by systematic reviews, it merits a thoughtful and rigorous approach.
Purpose and Importance of the Literature Review
An understanding of the current literature is critical for all phases of a research study. Lingard 9 recently invoked the “journal-as-conversation” metaphor as a way of understanding how one's research fits into the larger medical education conversation. As she described it: “Imagine yourself joining a conversation at a social event. After you hang about eavesdropping to get the drift of what's being said (the conversational equivalent of the literature review), you join the conversation with a contribution that signals your shared interest in the topic, your knowledge of what's already been said, and your intention.” 9
The literature review helps any researcher “join the conversation” by providing context, informing methodology, identifying innovation, minimizing duplicative research, and ensuring that professional standards are met. Understanding the current literature also promotes scholarship, as proposed by Boyer, 10 by contributing to 5 of the 6 standards by which scholarly work should be evaluated. 11 Specifically, the review helps the researcher (1) articulate clear goals, (2) show evidence of adequate preparation, (3) select appropriate methods, (4) communicate relevant results, and (5) engage in reflective critique.
Failure to conduct a high-quality literature review is associated with several problems identified in the medical education literature, including studies that are repetitive, not grounded in theory, methodologically weak, and fail to expand knowledge beyond a single setting. 12 Indeed, medical education scholars complain that many studies repeat work already published and contribute little new knowledge—a likely cause of which is failure to conduct a proper literature review. 3 , 4
Likewise, studies that lack theoretical grounding or a conceptual framework make study design and interpretation difficult. 13 When theory is used in medical education studies, it is often invoked at a superficial level. As Norman 14 noted, when theory is used appropriately, it helps articulate variables that might be linked together and why, and it allows the researcher to make hypotheses and define a study's context and scope. Ultimately, a proper literature review is a first critical step toward identifying relevant conceptual frameworks.
Another problem is that many medical education studies are methodologically weak. 12 Good research requires trained investigators who can articulate relevant research questions, operationally define variables of interest, and choose the best method for specific research questions. Conducting a proper literature review helps both novice and experienced researchers select rigorous research methodologies.
Finally, many studies in medical education are “one-offs,” that is, single studies undertaken because the opportunity presented itself locally. Such studies frequently are not oriented toward progressive knowledge building and generalization to other settings. A firm grasp of the literature can encourage a programmatic approach to research.
Approaching the Literature Review
Considering these issues, journals have a responsibility to demand from authors a thoughtful synthesis of their study's position within the field, and it is the authors' responsibility to provide such a synthesis, based on a literature review. The aforementioned purposes of the literature review mandate that the review occurs throughout all phases of a study, from conception and design, to implementation and analysis, to manuscript preparation and submission.
Planning the literature review requires understanding of journal requirements, which vary greatly by journal ( table 1 ). Authors are advised to take note of common problems with reporting results of the literature review. Table 2 lists the most common problems that we have encountered as authors, reviewers, and editors.
Sample of Journals' Author Instructions for Literature Reviews Conducted as Part of Original Research Article a

Common Problem Areas for Reporting Literature Reviews in the Context of Scholarly Articles

Locating and Organizing the Literature
Three resources may facilitate identifying relevant literature: human resources, search tools, and related literature. As the process requires time, it is important to begin searching for literature early in the process (ie, the study design phase). Identifying and understanding relevant studies will increase the likelihood of designing a relevant, adaptable, generalizable, and novel study that is based on educational or learning theory and can maximize impact.
Human Resources
A medical librarian can help translate research interests into an effective search strategy, familiarize researchers with available information resources, provide information on organizing information, and introduce strategies for keeping current with emerging research. Often, librarians are also aware of research across their institutions and may be able to connect researchers with similar interests. Reaching out to colleagues for suggestions may help researchers quickly locate resources that would not otherwise be on their radar.
During this process, researchers will likely identify other researchers writing on aspects of their topic. Researchers should consider searching for the publications of these relevant researchers (see table 3 for search strategies). Additionally, institutional websites may include curriculum vitae of such relevant faculty with access to their entire publication record, including difficult to locate publications, such as book chapters, dissertations, and technical reports.
Strategies for Finding Related Researcher Publications in Databases and Search Engines

Search Tools and Related Literature
Researchers will locate the majority of needed information using databases and search engines. Excellent resources are available to guide researchers in the mechanics of literature searches. 15 , 16
Because medical education research draws on a variety of disciplines, researchers should include search tools with coverage beyond medicine (eg, psychology, nursing, education, and anthropology) and that cover several publication types, such as reports, standards, conference abstracts, and book chapters (see the box for several information resources). Many search tools include options for viewing citations of selected articles. Examining cited references provides additional articles for review and a sense of the influence of the selected article on its field.
Box Information Resources
- Web of Science a
- Education Resource Information Center (ERIC)
- Cumulative Index of Nursing & Allied Health (CINAHL) a
- Google Scholar
Once relevant articles are located, it is useful to mine those articles for additional citations. One strategy is to examine references of key articles, especially review articles, for relevant citations.
Getting Organized
As the aforementioned resources will likely provide a tremendous amount of information, organization is crucial. Researchers should determine which details are most important to their study (eg, participants, setting, methods, and outcomes) and generate a strategy for keeping those details organized and accessible. Increasingly, researchers utilize digital tools, such as Evernote, to capture such information, which enables accessibility across digital workspaces and search capabilities. Use of citation managers can also be helpful as they store citations and, in some cases, can generate bibliographies ( table 4 ).
Citation Managers

Knowing When to Say When
Researchers often ask how to know when they have located enough citations. Unfortunately, there is no magic or ideal number of citations to collect. One strategy for checking coverage of the literature is to inspect references of relevant articles. As researchers review references they will start noticing a repetition of the same articles with few new articles appearing. This can indicate that the researcher has covered the literature base on a particular topic.
Putting It All Together
In preparing to write a research paper, it is important to consider which citations to include and how they will inform the introduction and discussion sections. The “Instructions to Authors” for the targeted journal will often provide guidance on structuring the literature review (or introduction) and the number of total citations permitted for each article category. Reviewing articles of similar type published in the targeted journal can also provide guidance regarding structure and average lengths of the introduction and discussion sections.
When selecting references for the introduction consider those that illustrate core background theoretical and methodological concepts, as well as recent relevant studies. The introduction should be brief and present references not as a laundry list or narrative of available literature, but rather as a synthesized summary to provide context for the current study and to identify the gap in the literature that the study intends to fill. For the discussion, citations should be thoughtfully selected to compare and contrast the present study's findings with the current literature and to indicate how the present study moves the field forward.
To facilitate writing a literature review, journals are increasingly providing helpful features to guide authors. For example, the resources available through JGME include several articles on writing. 17 The journal Perspectives on Medical Education recently launched “The Writer's Craft,” which is intended to help medical educators improve their writing. Additionally, many institutions have writing centers that provide web-based materials on writing a literature review, and some even have writing coaches.
The literature review is a vital part of medical education research and should occur throughout the research process to help researchers design a strong study and effectively communicate study results and importance. To achieve these goals, researchers are advised to plan and execute the literature review carefully. The guidance in this editorial provides considerations and recommendations that may improve the quality of literature reviews.
Clinical evaluation of platelet-rich plasma therapy for osteonecrosis of the femoral head: A systematic review and meta-analysis
- Guo, Guimei
- Ouyang, Wensi
- Wang, Guochen
- Zhao, Wenhai
- Zhao, Changwei
Objective This meta-analysis aims to assess the efficacy and safety of platelet-rich plasma (PRP) for osteonecrosis of the femoral head (ONFH). Methods We comprehensively searched randomized controlled trials in PubMed, Web of Science, EMBASE, the Cochrane Central Register of Controlled Trials, Chinese National Knowledge Infrastructure, China Science and Technology Journal Database, WanFang, and Chinese BioMedical Literature Database from inception until October 25, 2024. The literature on the clinical efficacy of autologous PRP for ONFH was collated. According to the inclusion and exclusion criteria, the literature was screened, quality evaluated and the data was extracted. Meta-analysis was carried out with the software Review Manager 5.4.1 software and Stata 17.0 software. In addition, potential publication bias was detected by the funnel plot test and Egger's test. The GRADE system was used to evaluate the quality of evidence for outcome indicators. Results Fourteen studies involving 909 patients were included in this study. Compared with non-PRP, PRP exhibited significant improvements in the Harris hip score (HHS) at 3 months (MD = 3.58, 95% Cl: 1.59 to 5.58, P = 0.0004), 6 months (MD = 6.19, 95% Cl: 3.96 to 8.41, P < 0.00001), 12 months (MD = 4.73, 95% Cl: 3.24 to 6.22, P < 0.00001), ≥ 24 months (MD = 6.83, 95% Cl: 2.09 to 11.59, P = 0.0003), and the last follow-up (MD = 6.57, 95% Cl: 4.81 to 8.33, P < 0.00001). The PRP also showed improvement in HHS compared to baseline than the non-PRP at 3 months (MD = 3.60, 95% Cl: 1.26 to 5.94, P = 0.003), 6 months (MD = 6.17, 95% Cl: 3.74 to 8.61, P < 0.00001), 12 months (MD = 5.35, 95% Cl: 3.44 to 7.25, P < 0.00001), ≥ 24 months (MD = 8.19, 95% Cl: 3.76 to 12.62, P = 0.0003), and the last follow-up (MD = 6.94, 95% Cl: 5.09 to 8.78, P < 0.00001). The change in visual analog scale (VAS) score 3 months post intervention (MD = -0.33, 95% Cl: -0.52 to -0.13, P = 0.001), 6 months (MD = -0.69, 95% Cl: -0.90 to -0.48, P < 0.00001), 12 months (MD = -0.75, 95% Cl: -1.05 to -0.46, P < 0.00001), ≥ 24 months (MD = -1.05, 95% Cl: -1.20 to -0.89, P < 0.00001), and the last follow-up (MD = -0.75, 95% Cl: -0.97 to -0.54, P < 0.00001). The PRP also showed a decrease in VAS score compared to baseline than the non-PRP at 3 months (MD = -0.29, 95% Cl: -0.41 to -0.17, P = 0.003), 6 months (MD = -0.63, 95% Cl: -0.96 to -0.30, P = 0.0002), 12 months (MD = -0.78, 95% Cl: -1.22 to -0.33, P = 0.0006), ≥ 24 months (MD = -1.11, 95% Cl: -1.27 to -0.96, P < 0.00001), and the last follow-up (MD = -0.74, 95% Cl: -1.05 to -0.43, P < 0.00001). Additionally, it was found that the PRP group had the advantages in the following aspects: collapse rate of the femoral head (RR = 0.33, 95% Cl: 0.17 to 0.62, P = 0.0006), rate of conversion to total hip arthroplasty (RR = 0.37, 95% Cl: 0.18 to 0.74, P = 0.005), and overall complications (RR = 0.33, 95% Cl: 0.13 to 0.83, P = 0.02). The GRADE evidence evaluation showed overall complication as very low quality and other indicators as low quality. Conclusion There is limited evidence showing benefit of PRP therapy for treatment of ONFH patients, and most of this evidence is of low quality. Caution should therefore be exercised in interpreting these results. It is recommended that future research involve a greater number of high-quality studies to validate the aforementioned conclusions. Systematic review registration https://www.crd.york.ac.uk/prospero/ #recordDetails, CRD42023463031.

IMAGES
VIDEO
COMMENTS
Systematic Review A summary of the clinical literature. A systematic review is a critical assessment and evaluation of all research studies that address a particular clinical issue. The researchers use an organized method of locating, assembling, and evaluating a body of literature on a particular topic using a set of specific criteria.
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... review of the available literature, identifying a ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
As mentioned previously, there are a number of existing guidelines for literature reviews. Depending on the methodology needed to achieve the purpose of the review, all types can be helpful and appropriate to reach a specific goal (for examples, please see Table 1).These approaches can be qualitative, quantitative, or have a mixed design depending on the phase of the review.
Introduction. Literature review is an essential feature of academic research. Fundamentally, knowledge advancement must be built on prior existing work. To push the knowledge frontier, we must know where the frontier is. By reviewing relevant literature, we understand the breadth and depth of the existing body of work and identify gaps to explore.
This article is a practical guide to conducting data analysis in general literature reviews. The general literature review is a synthesis and analysis of published research on a relevant clinical issue, and is a common format for academic theses at the bachelor's and master's levels in nursing, physiotherapy, occupational therapy, public health and other related fields.
9.3. Types of Review Articles and Brief Illustrations. EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic.
Literature Review A literature review is a survey of scholarly sources that provides an overview of a particular topic. Literature reviews are a collection of the most relevant and significant publications regarding that topic in order to provide a comprehensive look at what has been said on the topic and by whom.
Literature search. Fink has defined research literature review as a "systematic, explicit and reproducible method for identifying, evaluating, and synthesizing the existing body of completed and recorded work produced by researchers, scholars and practitioners."[]Review of research literature can be summarized into a seven step process: (i) Selecting research questions/purpose of the ...
2. Scope the Literature. A "scoping search" investigates the breadth and/or depth of the initial question or may identify a gap in the literature. Eligible studies may be located by searching in: Background sources (books, point-of-care tools) Article databases; Trial registries; Grey literature; Cited references; Reference lists
A literature review is defined as "a critical analysis of a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles." (The Writing Center University of Winconsin-Madison 2022) A literature review is an integrated analysis, not just a summary of scholarly work on a specific topic.
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review. Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important ...
A literature search is distinguished from, but integral to, a literature review. Literature reviews are conducted for the purpose of (a) locating information on a topic or identifying gaps in the literature for areas of future study, (b) synthesising conclusions in an area of ambiguity and (c) helping clinicians and researchers inform decision-making and practice guidelines.
State how the literature search and reference selection were done. Use several sources of evidence-based reviews on the topic. Rate the level of evidence for key recommendations in the text ...
Reading the abstract is often sufficient when evaluating an article using the PP-ICONS approach. The most relevant studies will involve outcomes that matter to patients (e.g., morbidity, mortality ...
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
PubMed - The premier medical database for review articles in medicine, nursing, healthcare, other related biomedical disciplines. PubMed contains over 20 million citations and can be navigated through multiple database capabilities and searching strategies. CINAHL Ultimate - Offers comprehensive coverage of health science literature. CINAHL is particularly useful for those researching the ...
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature ...
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications .For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively .Given such mountains of papers, scientists cannot be expected to examine in detail every ...
The purpose of this guide is to provide information and resources for clinical psychology students engaged in writing a literature review.
A literature search identified another 36 JAKi-treated GCA cases with clinical improvement reported for the majority of them. This real-world analysis and literature review suggest that JAKi could be effective in GCA, including in patients failing established glucocorticoid-sparing therapies such as tocilizumab and methotrexate.
Here, we add 11 clinical photos to the 42 found in the literature, compare our cases with previous reports, and demonstrate diversity of clinical presentations. We conducted a retrospective analysis of FeP at University of California, San Francisco Dermatopathology 1/1/2010-12/31/2021.
The present review article aims to present an overview of the available literature and to develop ideas on future perspectives to define the diagnostic possibilities of radiography in PJIs of the hip. The primary outcome of interest of this systematic review was the radiologic presentation of periprosthetic joint infections of the hip.
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research (research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice.Research along this entire spectrum represents a fundamental mission of the Johns Hopkins ...
A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question.
Scoping literature review. A scoping review of the literature in Embase and PubMed (MEDLINE) was conducted using a predefined search strategy. Scoping literature reviews aim to identify and map the existing evidence related to a certain topic of interest in a systematic way [20, 21].Full-text, peer-reviewed, English articles that were published between 2008 and 2021 were eligible for inclusion.
Media Files: APA Sample Student Paper , APA Sample Professional Paper This resource is enhanced by Acrobat PDF files. Download the free Acrobat Reader. Note: The APA Publication Manual, 7 th Edition specifies different formatting conventions for student and professional papers (i.e., papers written for credit in a course and papers intended for scholarly publication).
Purpose and Importance of the Literature Review. An understanding of the current literature is critical for all phases of a research study. Lingard 9 recently invoked the "journal-as-conversation" metaphor as a way of understanding how one's research fits into the larger medical education conversation. As she described it: "Imagine yourself joining a conversation at a social event.
The literature on the clinical efficacy of autologous PRP for ONFH was collated. According to the inclusion and exclusion criteria, the literature was screened, quality evaluated and the data was extracted. Meta-analysis was carried out with the software Review Manager 5.4.1 software and Stata 17.0 software.