Insights > Pharmacovigilance: Literature Monitoring Best Practices

Dive Into Our Latest Blogs

Pharmacovigilance: Literature Monitoring Best Practices
By ClinChoice July 12, 2022
Safe and effective use of health products is a key objective of pharmacovigilance. Information is provided about the safety of these substances to patients, healthcare providers, and the general public as soon as possible. Pharmacovigilance includes reviewing the development, management, and introduction of pharmaceuticals. It is probably the most tightly regulated part of the pharmaceutical industry. Pharmacovigilance aims to identify, detect, assess, and report any adverse drug reaction (ADR) related to pharmaceutical products. In the United States, the European Union, and other parts of the world, regulatory requirements have emerged that have grown in diversity and nuance. These requirements include systematic monitoring and review of medical literature, including comprehensive screening of medical journals for adverse drug reactions, which remain on the rise. Having a robust pharmacovigilance system is paramount for a manufacturer, and any deficiencies can have an adverse effect on patient safety.
Literature monitoring includes published articles, articles, and reviews in indexed or non-indexed journals, any content posted anywhere online, posters and conference abstracts, etc. Holders of a Marketing Authorization (MA) must monitor global and local literature throughout the duration of that authorization, regardless of the availability of the product on the market1. Regulatory reports, clinical trial reports, literature reports, license partner reports, and spontaneous reports all serve as sources of data for deeper analysis of regulatory reporting, signal detection, and aggregate reporting. The individual safety report (ICSR) is valuable for developing risk assessments. It is incumbent upon holders of marketing authorizations to stay up-to-date on potential publications (including ahead of print articles) by reviewing widely used reference databases (e.g., Medline, Embase, Excerpta Medica) every week 2 . Adverse events that meet the criteria for the ICSR are handled per regulatory guidelines on handling and reporting adverse events. When a relevant article has been identified, it will be further screened to determine if it meets the four essential criteria for consideration for Individual case safety report (ICSR) and adverse event reporting: 1) identified source, 2) company product, 3) patient, 4) adverse event 3 . Any analysis regarding the safety profile of a product should be based on scientific and medical publications. Literature searches and monitoring are primarily intended to identify single case reports of adverse effects and to track any changes in benefit-risk profiles associated with the drug, particularly when new safety signals safety concerns arise 4 .
Literature Monitoring: An Overview of Best Practices
When the foundation is compromised, a process can result in a cascade of unintended repercussions. Therefore, an unbiased search is vital for monitoring medical literature accurately and efficiently. The growing volume of data has made it more critical to get the best results without introducing unwanted data. The literature monitoring process is usually characterized by two major challenges, which can be overcome. The first challenge is to come up with the right search strategy, and the second is to deal with duplication. Drug manufacturers must often track hundreds of drugs at once. So how can literature monitoring be accurate and valid?
Optimal Search Strategy Design and Database Selection
Regulatory authorities require marketing authorization holders to conduct medical literature surveillance at least weekly according to the GVP module VI and based on the required frequency as described by the local regulatory authorities, both for globally indexed literature databases and locally (non-indexed) literature journals 5 . When developing search strategies, it is important to consider ICSRs, aggregate reports, and any potential safety-related information. Therefore, it is essential to develop and progressively improve search strategies to limit the risk of overlooking relevant ADR information. Specifically, to retrieve all relevant records, query terms must be highly recallable and carefully crafted to retrieve maximum publications reporting any safety concerns about the product in question.
The database must be comprehensive and meet minimum standards to ensure that safety-critical signals are not missed. Pharmacovigilance searchers typically utilize at least two databases, usually three or more, because having access to multiple databases increases their recall-finding capabilities, ensuring more coverage.
Implement a search approach that balances the need for accuracy and precision. For example, 1) use several Boolean operators, 2) browse a thesaurus of terms, 3) perform proximity search, and 4) incorporate abbreviations to recall results. Using the most recent thesaurus update will ensure accuracy and compliance 6 .
As part of the local literature review, it is recommended to identify the non-indexed journals published locally and to screen those in either an online or print format depending on their availability. There are a few local regulatory agencies that recommend performing local literature searches in a few databases that are locally approved. The MAH handles any publications identified as containing information in local languages in accordance with the translation process established within the institution.
Industry best practice calls for constant review of search terms and updating them based on safety-related updates pertaining to the products. A GOLD standard data set of records is used to validate the modified search strategies. It is recommended to review your search strategy annually and make amendments as necessary 7 .
EMA hosts a robust system for medical literature monitoring. Thousands of records are added daily. It is generally the responsibility of marketing authorization holders to monitor medical literature and report individual cases of suspected adverse reactions into EudraVigilance and national safety databases 8 . They are not required to monitor or report suspected adverse reactions for active substances to EudraVigilance for substances covered by EMA’s service 9 .
Duplicate Data Management
Scientific publications and medical literature are abundant with sources and references, so it’s likely that the same publication could be indexed in multiple formats across a variety of journals, which results in duplicate findings. This creates a whole series of redundant tasks and false signals regarding drug safety. It leads to erroneous evaluations and, ultimately, compliance problems. Duplicate management processes, however, can solve this issue. Even though this is the best way of dealing with articles, it comes with a few challenges. There may be limitations to duplicate identification within the tool due to the presence of special characters, or it may be the case that the same study has been published across different journals or conference abstracts, making the process cumbersome.
It is important to search multiple databases to capture multiple publications across different journals. Keeping track of previous searches will also facilitate the identification of duplicates. To identify duplicate publications, there should not be just a focus on the article title but also the author’s name and, in some cases, the name of the study cited in the article.
According to Article 107(3) of Directive 2001/83/EC, to avoid duplicate submissions of ICSRs, the holder of a marketing authorization must submit the ICSRs that are not already assessed or monitored by EMA through the Medical Literature Monitoring (MLM) services 10 .
Service providers should use a standardized and well-established deduplication system, enabling them to confirm that they are not missing relevant references or creating duplicates inadvertently.
Along with routine literature surveillance, MAH also conducts targeted literature searches, which are searches specifically designed to answer a specific research question. When conducting signal analysis, these searches are conducted to confirm or disprove the association between the adverse event and the product.
Pharmacovigilance involves a substantial amount of literature monitoring. The process of devising a solid search strategy could be challenging but is essential. A professional with the required skills, experience, and training will ensure adverse event-related safety information is never missed. It is necessary to develop and maintain search strategies, elicit ideas from different stakeholders, and develop approved and suitable strategies for the purpose at hand. It is critical to set up a thorough process to handle and manage duplicate articles. Regularly review search strategies, and ensure the documentation is robust to ensure the finest quality results. The following points can be considered to check whether the MAH’s literature monitoring systems meet quality standards;
- A drug safety expert with experience researching literature is needed.
- Conduct risk assessments to ensure that the search criteria are robust and relevant to the objective of the literature search.
- Conduct literature searches and evaluate the results for literature per regional requirements (Global and Local).
- Monitoring and reviewing the Eudravigilance Medical Literature Monitoring (MLM) system, managed by EMA, to identify ICSRs in the literature if your product is included in the active ingredient screened by EMA.
- The search string is reviewed and updated annually to optimize results.
About the Author
Dr. Poonam Wagle; Associate Manager, Pharmacovigilance
Poonam brings expertise in literature management as a pharmacovigilance and safety expert with over eleven years of experience in the areas of ICSR, Literature, Aggregate Reports, and Signal Reviews. She has established and led several projects and programs in the field, including ICSRs, and literature monitoring, in both global and local surveillance.
ClinChoice is a leading global Contract Research Organization (CRO), with over 3400 clinical research professionals across North America, Asia, and Europe. For more than 27 years, ClinChoice has been providing high-quality contract research services to pharmaceutical, biotechnology, medical device, and consumer products clients, encompassing a broad range of services and therapeutic areas. ClinChoice offers cutting-edge, full-service solutions for Clinical Trials, Regulatory Affairs, Medical Device Safety, Toxicology, and Medical Affairs.
- 1) https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
- 2) https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-vi-management-reporting-adverse-reactions_en-0.pdf
- 3) https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf
- 4) https://database.ich.org/sites/default/files/E2D_Guideline.pdf
- 5) https://europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-iv-pharmacovigilance-audits-rev-1_en.pdf
- 6) https://www.ema.europa.eu/en/documents/other/monitoring-medical-literature-entry-relevant-information-eudravigilance-database-european-medicines_en.pdf
- 7) https://www.elsevier.com/solutions/embase-biomedical-research/coverage-and-content
- 8) https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
- 9) https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
- 10) https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
Let's Build a Healthier & Safer World, Together
Connect with ClinChoice to discover how we can support the development and commercialization of your drug or device.
- All services
- +34 91 277 10 76

- November 06, 2023
- How to perform a good Pharmacovigilance literature search strategy
The search of scientific literature in Pharmacovigilance (PV) is an activity of great importance within pharmacovigilance, as it allows the collection of valuable information on the safety and efficacy of drugs, in addition to complying with the applicable legal requirements (in Spain it includes Royal Decree 577/2013 and the European directive 2010/84/EU ) and good pharmacovigilance practices ( Module VI GVP “Guideline on good pharmacovigilance practices (GVP) Module VI- Collection, management and submission of reports of suspected adverse reactions to medicinal products ).
The first action to be taken when defining the PV search strategy is the selection of sources, which will determine which materials will be used, depending on the type of search to be performed, such as publications in local journals in each region or country, and global databases and other international publications, in the case of international searches. The differentiation between these 2 types of searches is due to the need to address drug safety from multiple perspectives and to adapt to the type of monitoring and, as we have seen above, to comply with local and global regulations.
Another important consideration is to have an updated list of the products and active ingredients of the project on which the pharmacovigilance search will be performed, creating procedures that allow the inclusion of new entries, as well as the elimination of drugs that become obsolete for bibliographic searches. Both the list of products and active ingredients, as well as the list of journals chosen for monitoring, should be reviewed annually to maintain an updated list and to ensure that the searches are relevant. In the case of journals, it is important to bear in mind that the publications should be appropriate to the objective and therapeutic area.

For pharmacovigilance searches in databases, it will be necessary to define a strategy that takes into account the creation of periodic alerts, day zero and algorithms that allow retrieving the most appropriate and relevant information. For the construction of an algorithm, it will be necessary to take into account that it must be built in a robust way, giving the expected and consistent results, i.e., reproducible and easy to update. These parameters not only guarantee the quality of the service, but also provide a fully auditable activity.
Finally, in order to ensure the quality of the searches, and again to allow traceability and quality, a “Quality Control” should be included as part of the strategy to check both the relevance and suitability of the results and the formal aspects of their recording. This is where the training of technicians, both in pharmacovigilance and in the project, becomes essential to guarantee the best service.
In short, in order to obtain valuable information on drug safety in the search for scientific literature, it is first necessary to establish an adapted strategy for PV searches that defines the needs and methods to be used to retrieve the information, and to guarantee the quality of the service through internal control systems and adequate staff training.
From Azierta part of QbD Group, we are experts in Medical Information and in Pharmacovigilance. We can help you with our solutions. Do not hesitate to contact us!
Share this post with your friends.

The search of scientific literature in Pharmacovigilance (PV) ...

- Animal Health
- Audit & Certification
- Clinical Trials
- Food Supplements
- GxP Compliance & Safety
- Landing in Europe
- Medical Devices
- medical information
- Pharmacovigilance
- Regulatory Affairs
- Sin categoría
Latest posts
- Bibliometric analysis in scientific publications
- First publications of electronic medicines information (ePI)
- Life Sciences Management Program at IE Business School and Azierta part of QbD Group
- Current and Future Perspectives on Environmental Impact Assessment for Medicines: Regulation, Implementation and Pharmaceutical Sustainability
- Open access
- Published: 22 December 2022
LiSA: an assisted literature search pipeline for detecting serious adverse drug events with deep learning
- Vincent Martenot 1 ,
- Valentin Masdeu 1 ,
- Jean Cupe 1 ,
- Faustine Gehin 1 ,
- Margot Blanchon 1 ,
- Julien Dauriat 1 ,
- Alexander Horst 2 ,
- Michael Renaudin 2 ,
- Philippe Girard 2 &
- Jean-Daniel Zucker 3
BMC Medical Informatics and Decision Making volume 22 , Article number: 338 ( 2022 ) Cite this article
2973 Accesses
2 Citations
Metrics details
Introduction
Detecting safety signals attributed to a drug in scientific literature is a fundamental issue in pharmacovigilance. The constant increase in the volume of publications requires the automation of this tedious task, in order to find and extract relevant articles from the pack. This task is critical, as serious Adverse Drug Reactions (ADRs) still account for a large number of hospital admissions each year.
The aim of this study is to develop an augmented intelligence methodology for automatically identifying relevant publications mentioning an established link between a Drug and a Serious Adverse Event, according to the European Medicines Agency (EMA) definition of seriousness.
The proposed pipeline, called LiSA (for Literature Search Application), is based on three independent deep learning models supporting a precise detection of safety signals in the biomedical literature. By combining a Bidirectional Encoder Representations from Transformers (BERT) algorithms and a modular architecture, the pipeline achieves a precision of 0.81 and a recall of 0.89 at sentences level in articles extracted from PubMed (either abstract or full-text). We also measured that by using LiSA, a medical reviewer increases by a factor of 2.5 the number of relevant documents it can collect and evaluate compared to a simple keyword search. In the interest of re-usability, emphasis was placed on building a modular pipeline allowing the insertion of other NLP modules to enrich the results provided by the system, and extend it to other use cases. In addition, a lightweight visualization tool was developed to analyze and monitor safety signal results.
Conclusions
Overall, the generic pipeline and the visualization tool proposed in this article allows for efficient and accurate monitoring of serious adverse drug reactions from the literature and can easily be adapted to similar pharmacovigilance use cases. To facilitate reproducibility and benefit other research studies, we also shared a first benchmark dataset for Serious Adverse Drug Events detection.
Peer Review reports
The development of a drug is a long road that can take several years. This journey involves several requests for approval with regulatory authorities, whether to start clinical trials, to actually market the drug or to modify some of the claims. Throughout these approval processes, the regulator, that carries out a public safety mission, must ensure that no prior safety signal about the drug is known at the time or after the authorization is granted. This task requires the regulator to review and monitor both biomedical literature and surveillance reports. More specifically, medical reviewers have to identify portions of text mentioning an explicit association between a drug and a serious ADR. According to the EMA, a serious adverse event is “any untoward medical occurrence that at any dose:
results in death,
is life-threatening,
requires inpatient hospitalisation or prolongation of existing hospitalisation,
results in persistent or significant disability/incapacity, or
is a congenital anomaly/birth defect.”
It should be distinguished from what is called an Important Medical Event (IME) where the outcome might not fall into one of these 5 categories. For example, in the sentence There was one treatment-related death due to myositis in the pembrolizumab group. , the serious outcome (death) is clearly associated with the drug (pembrolizumab) through the expression (treatment-related). Conversely, in We observed Rivaroxaban-induced rash in \(60\%\) of the patients , the side effect mentioned cannot be qualified as serious. As such, it would be regarded as a safety issue by the regulator. Meanwhile, the tremendous increase of publication volume, and the number of treatments that require authorization in a limited time frame make it practically impossible for medical reviewers to review all documents exhaustively. Consequently, critical safety-related information can be missed when applying a human-only process.
Even though many publications have focused on literature review assistance [ 1 , 2 , 3 ] or on the detection of relationship between drug and ADR [ 4 , 5 , 6 , 7 , 8 ], only two have proposed approaches to tackle the detection of seriousness [ 9 , 10 ]. Meanwhile, in the first publication, the targeted documents are FAERS reports which differ from biomedical literature in terms of syntax and vocabulary. The second one, thus tested on biomedical corpus, does not provide any kind of relationship between a drug and an adverse event.
In this paper, we present LiSA (Literature Search Application), an AI-based system designed to assist medical reviewers in their market surveillance by automatically screening the biomedical literature to detect safety signals.
LiSA was designed to enable medical reviewers to monitor the publication of articles related to potential safety signals on medical treatments or medicines. More specifically, it is able to identify, filter and rank publications mentioning an established relationship between a specified drug and one or several serious Adverse Events (SAE), i.e. severe Adverse Drug Reactions (SADR). To meet these goals, we propose 4 contributions to the problem of pharmacovigilance information retrieval from open data literature:
A deep learning pipeline for the identification of serious adverse events within biomedical literature based on Pub-Med. The performance achieved is respectively of 81.1 \(\%\) in precision and 88.6 \(\%\) in recall.
A visualization tool designed to allow biomedical expert to review and monitor the results provided by the pipeline for specific drugs.
A modular pipeline built on pre-existing and independent open source models (transformers) allowing flexibility of usage for related use-cases in pharmacovigilance. This approach also provides more explainability compared to a lone neural network algorithm. The pipeline, instead of creating a new neural network algorithm with very specific outputs, is composed of independent algorithms providing intermediate outputs. These outputs are then combined to build an efficient and performing system aiming at qualifying and extracting the information corresponding to the following questions:
What are the monitored drugs and indication mentioned in the document?
What are the sentences that mention an established relationship between a drug and an AE?
What are the entities recognized as Drug or Adverse Event?
The identification of relevant documents regarding seriousness drug adverse reaction signals is then performed on the basis of this information and meta data available in the data source (Ex: date, journal, type of publication, etc...).
A benchmark dataset for seriousness classification task based on PubMed literature sentences.
After a review of related work, we describe the LiSA pipeline architecture and provide a high-level performance analysis of the proposed solution.
Related work
In most of the papers mentioned in this section, the focus is on Adverse Events (AE) detection and not on Adverse Drug Reaction (ADR), meaning that there is no specific detection of a drug associated with an adverse event. For the sake of clarity we will use, only in this part, the terms Adverse Drug Events (ADR) to indifferently designate AE or ADR.
Adverse Drug Reaction detection plays a key role in drug-safety surveillance and has motivated the creation of various monitoring systems or databases. The FAERS [ 11 ] reporting system and Medwatch [ 12 ], a medical product for safety reporting, are the current official solutions provided by the FDA. Meanwhile, these tools are only based on declarative reports and not on systematic analysis of the biomedical literature or any web-based source to identify potential ADRs. Several solutions have been proposed to perform biomedical literature monitoring in order to identify, filter and rank papers related to a specific domain or medical concept. For example, ASE [ 13 ] demonstrates the value of reference management, statistics, natural language summarizing to interactively select key papers. STELLAR [ 3 ] leverages data mining techniques to help researcher to identify, rank and recommend reference papers for a specific literature review. More recently, [ 1 ] proposed ASReview, an efficient active learning based-tool to perform systematic literature review and meta-analysis.
As per today, only a small number of literature review systems relate to adverse drug reactions detection. Among them, the PV-OWL tool [ 2 ] was built to link different databases to obtain novel safety indicators (FAERS, PubMed, social media...). The semi-automated pipeline published by [ 14 ] supports extracting ADR pairs from adverse events databases using statistical BPCNN algorithm for Natural Language Processing. Among other classical approaches commonly used in NLP, distributional semantics based on patterns of ADR co-reporting [ 15 ], Hidden Markov Models [ 16 ] or disproportionality analysis (DPA) [ 17 ] were already attempted to perform ADR detection. In 2012, Gurulingappa, Harsha et al. published an open-source reference dataset and developed a dictionary-based algorithm for extraction of adverse drug events in PubMed literature [ 18 , 19 ]. Following the significant advances in natural language processing with deep learning, more recent publications have exploited these technologies to improve safety signal detection. Several works perform ADR detection and extraction on social networks (e.g. Twitter) or on drugs review platforms like Drugs . com using deep learning techniques [ 4 , 5 , 6 , 7 , 8 ].
However, there is a lack of studies aimed at predicting the seriousness of adverse events or any other type of qualification. The seriousness of an adverse event is nevertheless critical since it will decide whether or not to trigger actions from the safety surveillance agencies. We only found two publications related to this specific topic. The first one from [ 9 ] is based on FAERS report and does not treat biomedical literature. On the contrary, the second provides a robust approach to detect, extract and categorize serious adverse events [ 10 ]. The study relies on three different deep learning algorithms for seriousness classification, seriousness categorization and seriousness annotation. Performance is evaluated on three datasets among which one is built on biomedical literature. Like the latter study from [ 10 ], which will also be used as the primary basis for performance evaluation, LiSA is capable of qualifying potential severity but differs in its ability to detect and extract adverse drug reaction entities and classify documents for display in a literature search tool interface.
The LiSA pipeline description
The architecture described in this section is the final result of a sequence of iterations aimed at improving the overall performance to maintain a satisfactory balance between precision and recall (more details are available in the “ Results and discussion ” sect.). The objective of the following steps is to identify and extract relevant information in documents (drug names, Adverse Events, association between drug and AE, seriousness,...) to be used for the final ranking and filtering of articles. The document processing pipeline is described in Fig. 1 below.
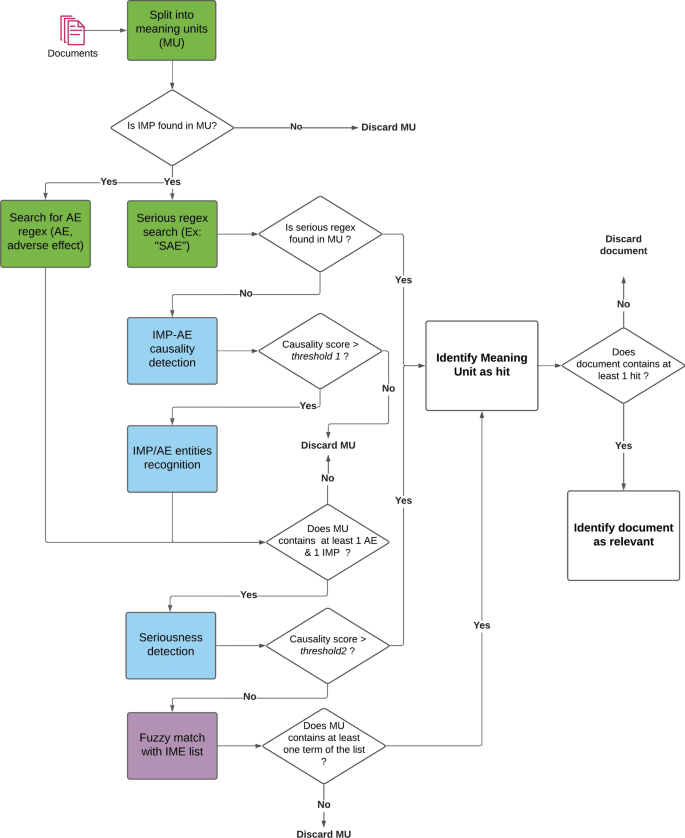
Decision diagram of the document processing pipeline. Green color boxes represent regex-based algorithms, blue color boxes represent deep learning based algorithms and purple color boxes fuzzy-matching based algorithms
Query definition and document collection
Most of the medical publications that mention adverse drug reaction are published and available through PubMed, a free archive of biomedical and life sciences journal literature and considered as a reference for biomedical publications. Some publications require a licensed access, but still provide a free version of the corresponding abstract. Therefore, PubMed was used as the main data source for literature monitoring to build the system. By construction, documents collection should be associated to a “query” which is composed of a combination search terms. A query contains a main drug, an optional second drug and optional indication. Indication should be only approved indication to avoid the case where indication and adverse events are confused. Consequently, LiSA collects all articles available on PubMed published on the last six years Footnote 1 associated to a query through the PubMed API. This timeframe was chosen as a good tradeoff between actuality of information and being sure not to miss a relevant signal that might have been reported a while ago. Only the main drug serves as keyword search to trigger the API. Other query terms (optional drug and indication) are only searched to tag the document if they are mentioned in it.
Drug and indication could be expressed under various synonyms in biomedical literature. To ensure the comprehensiveness of data collection, every drug and indication term is conjointly searched with all its synonyms based on open-source molecule and disease classification. For drug, we used the Chembl database [ 20 ], and for indications the MedDRA [ 21 ] hierarchy. Table 1 gives an example of a query definition.
Document preprocessing
This section describes the methodology applied to preprocess documents into a suitable format for deep learning algorithms described below.
Data preparation
To structure the documents, we propose a standard architecture able to accommodate any type of collected documents or data sources and adapted to natural language processing algorithms. As a matter of fact, raw documents cannot be processed directly by transformers and achieve a satisfying performance [ 22 ]. They should be split into meaning units of limited number of tokens like sentence or short paragraphs. This process, called sentence tokenization, is performed with a pre-built algorithm (on common English language) from the package nltk and adapted with specific cases found in biomedical literature.
Structured data is then formatted into 3 different tables:
Documents table: This table stores all the metadata and the full content of a document. This table contains one line per document.
Contents table: This table stores only the content of a document but split in different sections or paragraphs based on the pre-defined structuration already available in the document (e.g.: abstract, methods, results, conclusions...). The contents available in figures captions or tables was not collected.
Meaning units table: This table stores information at sentence level and is built from the contents table. A section or paragraph is split in different sentences and each sentence represents one line in this table. During the split, if a sentence is too short (between 4 to 10 words), it is concatenated with either the previous or the next one (only in case it is less than 20 words long) to reduce the risk of missing an AE-Drug relationship. These choices were applied for two reasons:
Concerning the maximum length of a meaning unit: BERT input size is limited to 512 tokens, which makes it impossible to use a whole article as input for prediction. Furthermore, it has been shown in the literature that BERT performs better on a limited number of tokens, therefore sentence as in input will be better than paragraph as in input.
Concerning the minimum length of a meaning unit: this decision was motivated by the empirical observation that in case of very short sentences, one information was actually present in the adjacent sentence. The threshold number of tokens was selected empirically and could be optimized in further work.
This generic structure has been designed to fit any type of document and serves as a basis for the visualisation tool presented in the “ Visualisation interface ” section.
Drug and indication search
The first filter applies to all meaning units found in collected documents and is based on a simple keyword search method. We use the Aho-corasick algorithm [ 23 ], an efficient dictionary-matching algorithm, to search for a drug term and associated synonyms in every meaning unit. Aho-corasick was used for its computation efficiency and because drug names have an invariant spelling in biomedical literature, there is thus no need to perform fuzzy-matching at this step. This association is then stored in the meaning units table. This step has a double objective:
First, to isolate the meaning units associated with the drug of interest (since LiSA is built to monitor serious adverse events associated with a defined drug).
Second, to reduce the number of meaning units to be used as input for the downstream deep learning modules that are more computationally intensive.
At the same time, a second keyword search is applied to identify mentions of therapeutic indications in historical documents. Unlike drug search, indication search is only performed at document level and is used to provide a clue of whether the document discusses about a drug aiming at treating a specific indication. The detection of an established relationship between a molecule and a disease is not performed in this pipeline. This task would be part of a possible improvement. The indication of interest are defined by biomedical reviewers and enriched with associated synonyms using the MedDRA hierarchy. Meanwhile, unlike drug names, indication terms are frequently composed of multiple tokens, which are not always expressed with the exact same form in the literature. For example the MedDRA indication “B-cell chronic lymphocytic leukaemia” could be found as “B-cell lymphocytic leukaemia” or “lymphocytic leukaemia of B-cell” in published articles. Therefore a simple expression search will most likely miss some expressions associated to the same indication. To overcome this problem, we built a fuzzy-matching algorithm allowing permuted and incomplete expression of an indication to be found in the text, which creates a list of expressions on the basis of a root indication. This list is composed of all permutations of the tokens contained in the root indication, with a random suppression of some of them to keep at least 2 tokens. All the expression of that list are then searched in the document, with the same Aho-corasick algorithm but allowing the presence of 20 characters between 2 consecutive tokens of the list. For example when searching for “B-cell lymphocytic leukaemia”, the expression “B-cell and C-cell lymphocytic leukaemia” will be accepted by this algorithm.
Deep learning
The three main AI modules presented in this section are the core of LiSA. They correspond to 3 different NLP tasks which are computed in parallel for all sentences containing a monitored drug (as described in the next section). Once calculated and stored in the database the different information are used to filter and qualify the hit sentences and relevant documents as depicted in Fig. 2 . Details about the different pre-trained algorithms and their respective performance are provided in Table 3 .
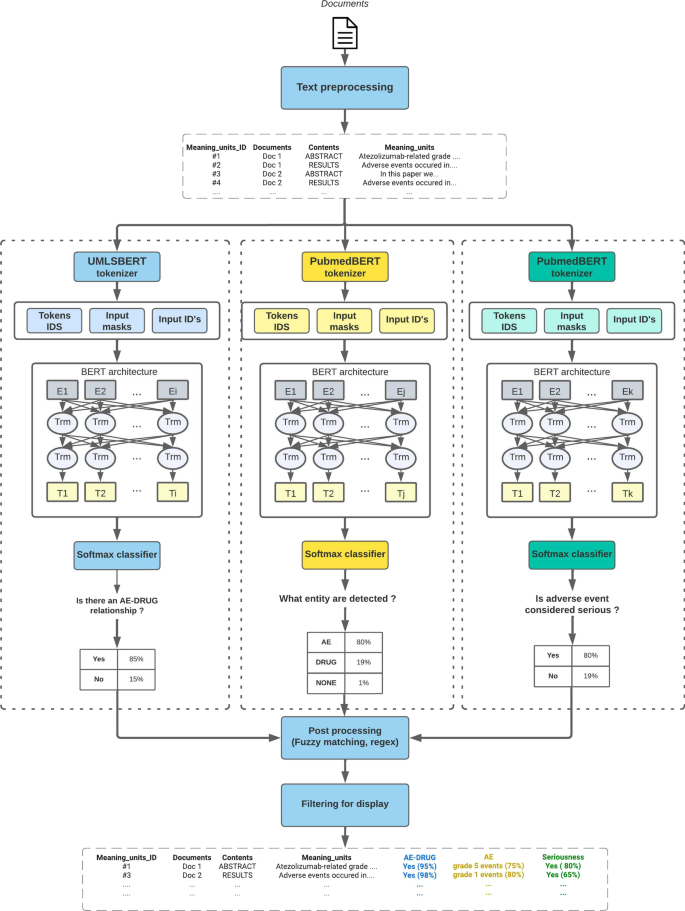
Schematized machine learning architecture of the LiSA pipeline and its three main modules. Unlike other post processing tasks, the serious regex search task is performed before deep learning inference and is not represented on the schema
Drug-AE relationship classification
To assess the association between a drug and an AE, we chose to rely on state of the art deep learning algorithms with attention-based mechanism (BERT). This family of algorithms is trained on very large corpora to build contextual embeddings and has been shown to perform extremely well in highly context-dependent prediction tasks, such as AE detection. The presence of a drug-AE causality relationship within a sentence was predicted with a two class (“has causality”, “has no causality”) sentence classifier, as defined in the ADE-Corpus-V2 dataset [ 18 ] used for training. This dataset contains more than 20 000 sentences extracted from PubMed and pre-labelled for drug-AE causality classification. In particular, the two classes are defined without prior knowledge of the entities corresponding to drug and ADRs. The ADE-Corpus-V2 dataset was split into training, validation, and testing sets with the ratio of 8:1:1 and used to fine-tune several pre-trained algorithms and to select the most accurate one.
In order to further increase prediction performance, we performed manual data augmentation based on badly predicted observations of ADE-corpus-V2. Typical treated case are sentences including a negation form, containing an unspecified adverse effect (“AEs”, “TRAEs”, “Serious adverse effects”) or related to specific lexical fields. The score threshold to predict a sentence as positive was chosen at 0.2. This value offers the highest possible recall and keep precision higher than 0.9 (threshold determination was manually performed based on a precision-recall curve) In the production version of LiSA, every meaning units predicted class and score are stored in the meaning units table.
Named Entity Recognition (NER)
LiSA is also supposed to identify the different entities found in a relevant document corresponding to a drug or an Adverse Drug Reaction. For this task, we used Named Entity Recognition (NER) pre-trained algorithms within the same family of algorithms built on BERT architecture. Using the same open source corpus, we fine-tuned and bench-marked several models for the task of identifying two different entities: drug and ADR.
The NER task was built as defined in the \(ADE-corpus-V2\) dataset [ 19 ]: find spans associated to 2 types of entities: DRUG and AE. No distinction was made between beginning, inside and outside tokens of a selected entity.
In the final pipeline, the entity detection is only applied on meaning units that successfully passed the drug-AE causality prediction with a score higher than the defined threshold (the standard threshold value 0.5 was used). This pre-filtering step was made to reduce the inference computation time. As for the previous step, detected entities and associated scores are stored in the meaning units database. The NER step was also applied after Drug-AE relationship classification since it reduces the computation time without major change in terms of performance. Inference time remains the most time-consuming task in the LiSA pipeline, which is critical for the system to be used in production.
Seriousness score prediction
According to the European Medicines Agency [ 24 ], an adverse event can be qualified of serious of the consecutive reaction to a treatment:
results in death
is life-threatening
requires inpatient hospitalisation or prolongation of existing hospitalisation
results in persistent or significant disability/incapacity
is a congenital anomaly/birth defect.
This definition clearly underlines the fact that the seriousness of an ADR is measured according to the outcome that it produces, whose expression in a document, is here again, highly context-dependent. BERT-like architecture based on contextual embeddings is once more a very promising solution. The same training framework applied in the two previous NLP tasks was applied here. We fine-tuned several pre-trained models on a sentence classification task. Unlike common ADR detection, we did not found an open access dataset to train the seriousness detection algorithm. This problem was overcome by labelling 7776 sentences extracted from PubMed in three categories: “serious”, “important medical event”, “none” (a “serious” sentence being an “important medical event” sentence with a serious outcome). The labelling process was performed by medical reviewers and based on examples extracted from positive examples of the \(ADE-corpus-V2\) dataset [ 18 ]. The third class “important medical event” was only added to have a more detailed labelled dataset for possible additional application in ADR detection. The ADR entities were not provided to the expert during annotation to force the annotator to take into account the full sentence and not only part of it (like extacted ADR) to make his decision. In addition, we performed data augmentation by semi-automatically building sentence examples to address some weaknesses of the algorithm in specific contexts or syntax (negation, cancer, etc..), that were also annotated by medical experts before being included in the training set. 917 sentences were used as a testing test and allowed to reach a performance at the state of the art. More concretely, this models yields a class and a score and is only calculated on meaning units that contains at least one drug entity and one ADR entity from the NER module.
Post-processing for performance improvement
Although the performances obtained by the previous pipeline on average matches the level reached in recent publications [ 10 , 19 ] (more details in the “ Results ” Sect.), it appeared that some specific cases were relatively badly predicted. A typical encountered issue was a random detection of non specific adverse events corresponding to expressions like “AE”, “adverse effects”, “TRAEs”,... To address those issues, different strategies were implemented in addition to the improvement of the three previous deep learning algorithms by data augmentation.
The first strategy implemented was the use of regular expressions that by themselves indicate the presence of an adverse event in sentence. A few example of these are “side/adverse event(s)/effect(s)/reaction(s)” or “(TR)AE(s)”. The same method is applied to the case of non specific serious adverse events with regular expressions such as “serious adverse event(s)/effect(s)/reaction(s)”, “grade 4/5 reaction(s)” or “SAE(s)”. This double search is applied on all meaning units containing an drug of interest since they are computationally light.
The second strategy used is specifically designed to catch serious adverse outcomes based on a list of terms built together with biomedical experts. That list contains expressions of diseases or reactions that are always associated with a serious outcome (death, hospitalization, infirmity, congenital, life-threatening). This is for example the case for “pneumonia”, “ventricular fibrillation”, “intracranial bleeding”,“teratogenic effects”. The same fuzzy-matching approaches as the one described in the previous section is applied in this case, since we are considering multiple-tokens expressions. Unlike regular expressions search, the fuzzy-matching is only applied to meaning units that were rejected by the seriousness score algorithm to optimize the computation time.
Document filtering and ranking
LiSA is built to provide a curated list of documents to the user, as well as the sentences where safety signals (called “hits”) are detected, and the recognized entities (drug and ADR). The decision process depicted in Fig. 1 is used to select and filter the documents to be finally displayed to the final user. It can appear counter-intuitive that the AE-drug relationship classification results are used before the entity recognition. This order showed the best performance and was selected after different experiments that are not detailed in this paper.
A rule-based system was also implemented to calculate a ranking score based on some information extracted from documents (sentence hit scores, number of hits per document,...). This score is then used by the user to rank the relevant papers in the visualisation interface.
Visualisation interface
Visualizing and exploring the results is key to ensure user adoption. Depending of the query definition, the pipeline can return a relatively large number of documents (volume of some example queries are provided on Fig. 5 ) indeed. In order to prevent users from being overwhelmed by a mass of articles to review, and in order for them to monitor results over time, we propose a simple exploration interface built with PowerBI, a powerful and cost-effective data visualisation tool. Captures of the two main interfaces are presented on Fig. 3 , 4 . First the QUERY DEFINITION interface allows a user to create or join search queries containing one or several search criteria, as defined above. Second, the RESULTS interface displays documents found in the literature, with at least one hit mentioning a serious drug adverse reaction. On the left side, a series of filtering options (publication date, indication found, AR Frequency, Route of administration, etc...) are available to help the user refine displayed results. These filters are fed by information already extracted by the pipeline, and by results from keyword searches performed by powerQuery (PowerBI’s data preparation engine). The results can be explored at a document/sentence level (high level results) showing only information down to the sentence and document, and at a more detailed level (detailed results) which includes ADR entities detected in the text.
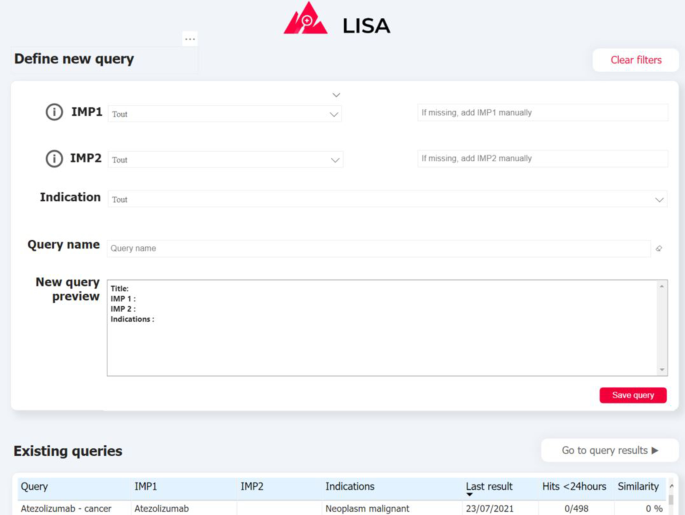
Screenshot of the “QUERY DEFINITION” tab of the interface. (1) Drug and indication dictionaries drop-down lists (2) Query preview (3) Summary of previously-created queries
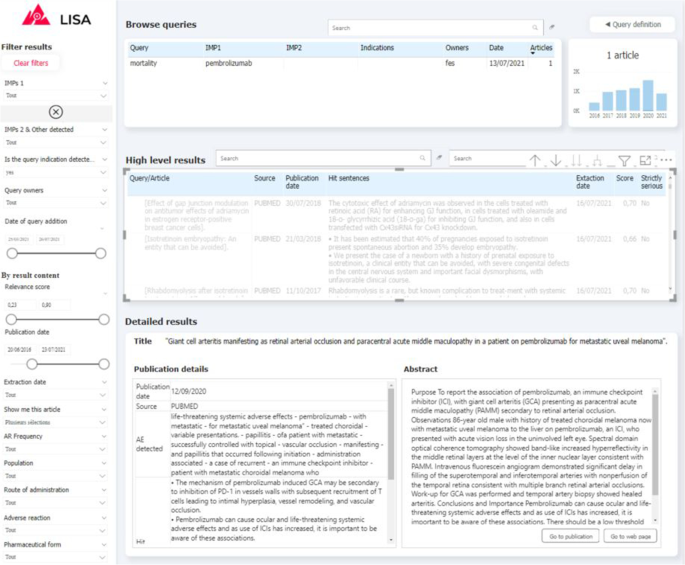
Screenshot of the “RESULTS” tab of the visualisation interface. (1) Query browser to select a set of results (2) Filtering tab to refine query results (3) High-level results table containing general information about results associated to the selected queries (4) Detailed results which provide additional information for every article selected in the high-level results table (5) Histogram of results volume of publication by year for the selected queries
Results and discussion
The following section is dedicated to:
Describing the obtained results and justify the need for the use of a new benchmark dataset for evaluating the task of serious ADR detection.
Discussing the limits of the current pipeline and pave the way for future work.
Performance assessment was performed with two strategies:
Evaluate the results based on a train/test approach on different datasets for different tasks. The performance of the tested models is displayed in Table 3 .
Evaluate the performance of LiSA from the perspective of medical reviewers (end users).
Implementation details
For individual NLP tasks evaluation, we used a specific test dataset for each task. This test set was created by selecting 10 \(\%\) of available labeled data that remained unseen by the algorithm. For AE-drug relationship classification as well as NER, we used the ADE-corpus-v2 dataset. For seriousness classification, the test set was carved out of the manually labelled dataset mentioned in subsection. Training was systematically performed with a learning rate of 3E-5, using the Adam optimizer and a batch size equal to 16. The pre-trained language model used in the evaluation are detailed in Table 2 .
Evaluation metrics
We choose to first evaluate the performance separately at task level and select the best performing algorithm according to results displayed in Table 3 . Meanwhile, a good performance of each independent algorithm does not necessary imply a good performance of the whole pipeline. This could especially be the case if the decision process that narrows down the scope of relevant sentences with successive filters becomes too restrictive. In addition to that, the performance of each independent algorithm is calculated at the meaning units level and not at the document level, which is a more representative metric for the intended use-case of LiSA. Nonetheless, performance evaluation at document level is difficult since it requires to find a sample corpus of relevant publications in the literature. That sample should have the same ratio of relevant and irrelevant documents available in PubMed. However it is almost impossible to estimate that ratio unless going through hundreds of articles for every single drug.
Instead, we propose two methods to measure the global performance of the pipeline. First, we calculate the precision and recall at sentence level only, with a sample dataset extracted from PubMed. Second, we propose to evaluate LiSA with a simple keyword search-based method to perform safety monitoring literature review.
Dataset-based performance evaluation
Sentence level evaluation of LiSA
To assess the performance at sentence level, we chose to use the classic performance metric for binary classification: precision, recall and f1-score. To calculate those metrics, we retrieved all documents associated with a list of drugs, as described in “ The LiSA pipeline description ” section. The list of selected drugs was selected to demonstrate how LiSA performs with new preparations, named with labcodes, and with established tradenames and comprise compounds for which certain signals were known to the experts in order to check whether they had been found accordingly (the list is available in “Appendix”). All documents were then fed into the LiSA pipeline to detect all positives sentences (hits) and their parent articles. The volume of documents and meaning units after every successive filter is available in Fig. 5 .
In the absence of a benchmark dataset to evaluate the performance of serious ADR detection, we created the SADR dataset with the help of medical reviewers with the following procedure. We first collected all documents freely available on Pubmed that contains a drug in the list available in “Appendix ”, and only kept the sentences that explicitly contains one of the drugs (since its absence would inevitably make the sentence irrelevant). These sentences were passed to the pipeline to get a prediction regarding the presence of a serious ADR. Then we asked medical experts to review the sentences and check whether the prediction was correct or not. In total, 1231 sentences from 988 unique documents were analyzed, among which 275 are abstracts only and 713 also provide main text. Tables and figures were not analyzed, as well as references. In that sample, LiSA reached a performance of 88.6 \(\%\) in recall, 81.1 \(\%\) in precision and 84,7 \(\%\) in F1-score. We observed better results on abstracts sentences with 89.7 \(\%\) in recall, 81.4 \(\%\) in precision and 85.3 \(\%\) in F1-score than on documents other parts (TITLE, INTRO, METHODS, RESULTS, DISCUSS, CASE, CONCL). More details is provided in Table 4 .
The achieved performance makes LiSA a state of the art system in terms of safety signal detection for the use-case considered in as much as it is closed to the performance obtained in [ 10 ]. Meanwhile, the task evaluated in this paper differs from the case of LiSA. Especially, there is no mention of a drug-AE relationship classification task. In addition there is no code available neither benchmark dataset from [ 10 ] that could have been used for direct comparison. For benchmark purpose, we provide the test dataset used to assess LiSA’s performance at sentence level, in supplementary materials.
The performance is higher for recall than for precision. This was designed on purpose, since there is a stronger need to not miss safety signals publication than achieving a higher precision. This optimization towards recall was especially enabled by the additional post processing modules described previously.
As far as the total number of collected documents and meaning units is concerned, as displayed in Fig. 5 , LiSA is able to perform a very imbalanced prediction task with a high precision. Indeed with more than 53k documents and 3.8 millions meaning units to filter, there are only \(0.2\%\) of meaning units that should be considered as relevant, for about \(10\%\) of all collected documents.
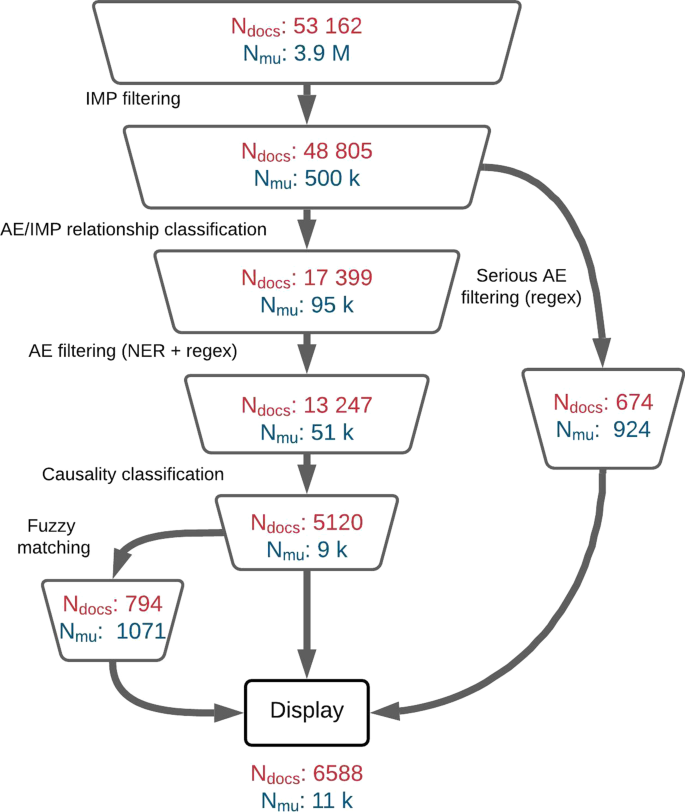
Volume of documents ( \(N_{docs}\) ) and meaning units ( \(N_{mu}\) ) after all decision steps in the LiSA pipeline. The volume corresponds to the documents collected with the drug list available in “Appendix”
Document level evaluation of LiSA
As mentioned before, evaluating the performance at document level is quite challenging. We can calculate the precision using the benchmark dataset available in “Appendix”. Over the 988 documents contained in the benchmark dataset, we found a precision of \(78.5\%\) .
Meanwhile, we are not in capacity to provide a good estimation of LiSA document recall. For that purpose, we should be able to measure to which extent the system is able to avoid missing relevant articles in the literature, which would require to label a corpus of at least a few thousand documents (which corresponds to about 80 000 sentences in total). This is an extremely time consuming task and is not immune to potential bias during the document selection phase to build the sample corpus.
User-based performance evaluation
To further assess the ability of LiSA to perform an efficient and comprehensive literature review on safety issues, we compare the results obtained by an expert medical reviewer using LiSA and using a simple keyword based search on PubMed. This type of evaluation is common in other systems for assisted literature [ 32 ].
For that purpose, we selected one drug, chosen for its relatively low number of associated papers found in the literature, making an exhaustive safety survey difficult. The goal is to compare the number of relevant articles that a manual search would yield to a LiSA-assisted search. On the one hand, a medical reviewer was asked to perform keyword search on PubMed with the expression “drug” + “serious adverse events” to review as much papers as possible within 2 h and retrieve the relevant papers and sentence hits only relatively to the presence of a serious adverse event. Some examples of queries used for this work are “sildenafil adverse events”, “emtricitacine serious adverse effects”. On the other hand, a second medical reviewer was asked to do the same literature review based on LiSA interface, within the same time frame. We also performed the same work for a drug notoriously known for its serious adverse drug effects: Azetolizumab. Due to the large number of papers mentioning serious ADRs in the literature (a few hundreds), the comparative performance between LiSA and a manual search is not significant. Time frame was limited because LiSA aims at speeding up drug monitoring process. Providing unlimited time to medical reviewer is not realistic regarding their daily work. In addition, the two reviews were performed by a different reviewers in order to ensure that the results of the second review will not be influenced by the first one if the same reviewer was doing both of them. Inter-rater Reliability between reviewers was measured on other molecules and was superior to 95
For a survey based on the drugs “Emtricitabine” and “Aflibercept”, the results achieved were as follows:
Emtricitabine:
7 articles were found with the keyword-based search
18 articles were found with the LiSA-assisted search.
Aflibercept:
8 articles were found with the keyword-based search
17 articles were found with the LiSA-assisted search.
The use of LiSA therefore makes it possible to largely increase the volume of relevant papers found during a defined search time (by a factor 2.5), especially when serious ADRs mentions are rare in the literature.
Comparison with state of the art models
The comparative analysis of pre-trained language models has shown different behaviors depending on the task:
for AE-Drug relationship, no major differences were observed between the 7 selected models. This is most probably linked to the nature itself of the task which consists in detecting an association/causality relationship. This will not depends on specific biomedical vocabulary but rather on grammatical forms used to link a drug to an adverse event. This is probably why non-biomedical models like BERT and sciBERT also obtained good results. UMLSBERT provided the best baseline in terms of F1-score and was then selected.
For Named Entity Recognition, the ability of a model to properly identify entities highly depends on the vocabulary learned by the model. On Table 3 , the F1-score levels largely hide subtle differences in performance for specific biomedical sub-domain. Especially, we observed that UMLSBERT and PubMedBERT performed better on text related to oncology where there is a subtle difference between Adverse Events and drug effects related to drug mechanisms (that could be destructive). The specific pre-training of these algorithms might explain their superiority over other models used in the benchmark. We choose PubMedBERT as the best performing model.
For seriousness classification, the vocabulary mastered by the model also highly matters. Indeed, many serious adverse events expressed with technical terms are by essence considered as serious (Stevens Johnson Syndrom, Rhabdomyolysis, Agranulocytosis...) and are better captured with specialized models like PubMedBERT, BioBERT and UMLSBERT. PubMedBERT was selected in this case.
The lack of extensive work on seriousness detection of Adverse Drug Reactions in the literature makes the comparison difficult to perform. In addition to that, the only publication [ 10 ] that tackles the problem does not provide any code implementation. Thus, apart from re-implementing the solution, there is no possibility to compare our algorithm with the one of this publication. Meanwhile, on a corpus extracted from Medical Literature, our pipeline reached a higher performance up to 0.81 in precision and 0.88 in recall (respectively compared to 0.83 and 0.82 [ 10 ]. Even if the dataset are not strictly comparable, we can conclude that our pipeline reached a state of the art performance on the specific task of seriousness classification.
Besides, the calculated overall performance of the pipeline at document level relies on a reduced number of documents (988). The statistical significance of the conclusion might be arguable since we cannot cover all the variety of semantic fields available in PubMed. Meanwhile, we believe that the global performance remains valid, especially since it is added to the already good performance achieved at sentence level, and calculated over a larger volume of examples.
Pipeline flexibility and portability
One important objective of the study was to build a system with a flexible architecture to enable the use of the pipeline on related use cases. For example, we could replace the seriousness classification by seriousness categorization (Death, Hospitalization, IME, Disability, Congenital anomaly [ 10 ]) or adverse events grade classification (Grade 1 to 5). This adjustment would of course require to train a new algorithm (for seriousness categories or adverse events grades classification) but with no impact of the 2 other modules. This is made possible by the independence of the three algorithms, them not being chained. They can then perform inference on the same type of input (a sentence containing at least one monitored drug). This approach is likely to introduce an overlap between the 3 NLP tasks that could be criticized, but allows a full flexibility in the combination of their outputs to build the required decision process.
limitations of the proposed system
A first type of limitation of our systel is related to relation extraction. Indeed, the proposed pipeline does not predict a direct relationship between an adverse event and a drug as defined, for example, in relation extraction tasks in NLP. As a matter of fact, the AE-drug relationship classifier is only trained to categorize meaning units into 2 categories “states a relationship” or “does not state a relationship”. Therefore, if two AEs and two drugs are coexisting in the same meaning unit, the pipeline is not able to separate and identify the possible multiple AE-drug relationships. Meanwhile, due to the relatively reduced length of meaning units (25 tokens on average and max 80–100 tokens) this situation remains very rare and has low impact on the performance.
Another limitation is related to the very assessment of the recall. Indeed, one of the main difficulty in assessing the performance of such systems lies in evaluating the proportion of documents existing in the literature, that are actually missed by the system. As mentioned during the results presentation, this would require the extraction of a test sample with the same distribution of relevant documents available in the literature. Unfortunately, except with a comprehensive work consisting of reviewing hundreds of articles and a strict control of bias during article selection, it is very difficult to get a correct and unbiased estimation of the recall. Instead, we chose to evaluate the recall only within relevant documents at sentence level.
In this paper, we presented the LiSA approach, a deep learning based pipeline for Adverse Drug Reaction monitoring in the biomedical literature. To our knowledge, our work is the first one to rely on a modular architecture of open-source fine-tuned models and providing access to multilevel outputs (AE/Drug relationship, AE and Drug entities, ADR Seriousness monitoring). We evaluated the performance of the system at two levels a) predictive performance based on a benchmark dataset labeled by medical reviewer and made available for future research and b) user-based performance where ADR monitoring with LiSA is compared with a semi-manual work based on keyword search on PubMed search engine. We have shown that based on LiSA user interface, a medical reviewer is able to retrieve 2.5 times more relevant documents than with a simple semi-manual search. Assisted literature monitoring with deep learning has proved to be a viable an extremely efficient approach to address the current challenges in pharmacovigilance. Future research could move toward assessing relationships across the boundaries of single units of meaning, attempting to combine the benefits of the deep learning described here with traditional language models, which would expand the application areas of the pipeline described here for other pharmacovigilance tasks.
Data Availability
The data that support the findings of this study are available from the excel file that our research group created as a supplementary material.
This number was chosen by experts as a good compromise between timeliness of information and the certainty of not missing a relevant signal that might have been reported some time ago.
Abbreviations
Natural language processing
Named entity recognition
Adverse drug reaction
Adverse reaction
Severe adverse drug reaction
Adverse effect
Adverse drug effect
Serious adverse effect
van de Schoot R, de Bruin J, Schram R, Zahedi P, de Boer J, Weijdema F, Kramer B, Huijts M, Hoogerwerf M, Ferdinands G, Harkema A, Willemsen J, Ma Y, Fang Q, Hindriks S, Tummers L, Oberski DL. An open source machine learning framework for efficient and transparent systematic reviews. Nature Machine Intelligence 2021;3(2):125–133. https://doi.org/10.1038/s42256-020-00287-7 . Bandiera_abtest: a Cc_license_type: cc_by Cg_type: Nature Research Journals Number: 2 Primary_atype: Research Publisher: Nature Publishing Group Subject_term: Computational biology and bioinformatics;Computer science;Medical research;SARS-CoV-2 Subject_term_id: computational-biology-and-bioinformatics;computer-science;medical-research;sars-cov-2
Piccinni C, Poluzzi E, Orsini M, Bergamaschi S. PV-OWL – Pharmacovigilance surveillance through semantic web-based platform for continuous and integrated monitoring of drug-related adverse effects in open data sources and social media. 2017 IEEE 3rd International Forum on Research and Technologies for Society and Industry (RTSI) (2017). https://doi.org/10.1109/RTSI.2017.8065931 .
Brisebois R, Abran A, Nadembega A, N’techobo P. An Assisted Literature Review using Machine Learning Models to Recommend a Relevant Reference Papers List, 2017;24.
Alimova I, Tutubalina E. Detecting Adverse Drug Reactions from Biomedical Texts with Neural Networks. In: Proceedings of the 57th Annual Meeting of the Association for Computational Linguistics: Student Research Workshop, 2019;415–421. Association for Computational Linguistics, Florence, Italy. https://doi.org/10.18653/v1/P19-2058 . https://www.aclweb.org/anthology/P19-2058
Fan B, Fan W, Smith C, Garner H. Adverse drug event detection and extraction from open data: a deep learning approach. Inf Process Manage. 2020;57(1): 102131. https://doi.org/10.1016/j.ipm.2019.102131 .
Article Google Scholar
Eberts M, Ulges A. Span-based Joint Entity and Relation Extraction with Transformer Pre-training. arXiv:1909.07755 [cs] 2021. https://doi.org/10.3233/FAIA200321
Ding P, Zhou X, Zhang X, Wang J, Lei Z. An attentive neural sequence labeling model for adverse drug reactions mentions extraction. IEEE Access. 2018;PP:1–1. https://doi.org/10.1109/ACCESS.2018.2882443 .
Zhang S, Dev S, Voyles J, Rao AS. Attention-Based Multi-Task Learning in Pharmacovigilance. In: 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), 2018, pp. 2324–22328. https://doi.org/10.1109/BIBM.2018.8621286 .
Yuwen L, Chen S, Zhang H. Detecting Potential Serious Adverse Drug Reactions Using Sequential Pattern Mining Method. In: 2018 IEEE 9th International Conference on Software Engineering and Service Science (ICSESS), 2018, pp. 56–59. https://doi.org/10.1109/ICSESS.2018.8663856
Routray R, Tetarenko N, Abu-Assal C, Mockute R, Assuncao B, Chen H, Bao S, Danysz K, Desai S, Cicirello S, Willis V, Alford SH, Krishnamurthy V, Mingle E. Application of augmented intelligence for pharmacovigilance case seriousness determination. Drug Saf. 2020;43(1):57–66. https://doi.org/10.1007/s40264-019-00869-4 .
Article CAS Google Scholar
FDA: FAERS (FDA Adverse Event Reporting System). [Online; Accessed 11 Dec 2021]. https://open.fda.gov/data/faers/ .
FDA: MedWatch: The FDA Safety Information and Adverse Event Reporting Program. [Online; Accessed 11 Dec 2021]. https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program .
Dunne C, Shneiderman B, Gove R, Klavans J, Dorr B. Rapid understanding of scientific paper collections: integrating statistics, text analysis, and visualization (2011).
Yeleswarapu S, Rao A, Joseph T, Saipradeep VG, Srinivasan R. A pipeline to extract drug-adverse event pairs from multiple data sources. BMC Med Inform Decis Mak. 2014;14:13. https://doi.org/10.1186/1472-6947-14-13 .
Gattepaille LM. Using the WHO database of spontaneous reports to build joint vector representations of drugs and adverse drug reactions, a promising avenue for pharmacovigilance. In: 2019 IEEE International Conference on Healthcare Informatics (ICHI), 2019:1–6. https://doi.org/10.1109/ICHI.2019.8904551 .
Sampathkumar H, Chen X-W, Luo B. Mining Adverse Drug Reactions from online healthcare forums using Hidden Markov Model. BMC Med Inform Decis Mak. 2014;14(1):91. https://doi.org/10.1186/1472-6947-14-91 .
Harpaz R, Vilar S, Dumouchel W, Salmasian H, Haerian K, Shah NH, Chase HS, Friedman C. Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions. JAMIA. 2013;20(3):413–9. https://doi.org/10.1136/amiajnl-2012-000930 .
Gurulingappa H, Rajput AM, Roberts A, Fluck J, Hofmann-Apitius M, Toldo L. Development of a benchmark corpus to support the automatic extraction of drug-related adverse effects from medical case reports. J Biomed Inform. 2012;45(5):885–92. https://doi.org/10.1016/j.jbi.2012.04.008 .
Gurulingappa H, Mateen-Rajput A, Toldo L. Extraction of potential adverse drug events from medical case reports. J Biomed Semant. 2012;3:15. https://doi.org/10.1186/2041-1480-3-15 .
ChEMBL: ChEMBL Is a Manually curated database of bioactive molecules with drug-like properties. [Online; Accessed 11 Dec 2021]. https://www.ebi.ac.uk/chembl/ .
MedDRA: Medical Dictionary for Regulatory Activities. [Online; Accessed 11 Dec 2021]. https://www.meddra.org/ .
Pappagari R, Żelasko P, Villalba J, Carmiel Y, Dehak N. Hierarchical Transformers for Long Document Classification. arXiv:1910.10781 [cs, stat] 2019. arXiv: 1910.10781
Aho AV, Corasick MJ. Efficient string matching: an aid to bibliographic search. Commun ACM. 1975;18(6):333–40. https://doi.org/10.1145/360825.360855 .
EMA: ICH E2A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. [Online; Accessed 11 Dec 2021]. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event .
Yuan Z, Zhao Z, Sun H, Li J, Wang F, Yu S. Coder: Knowledge-infused cross-lingual medical term embedding for term normalization. J Biomed Inf. 2022. https://doi.org/10.1016/j.jbi.2021.103983 .
Lee J, Yoon W, Kim S, Kim D, Kim S, So CH, Kang J. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2019;36(4):1234–40. https://doi.org/10.1093/bioinformatics/btz682 . arxiv:1901.08746 .
Peng Y, Yan S, Lu Z. Transfer learning in biomedical natural language processing: An evaluation of bert and elmo on ten benchmarking datasets. In: Proceedings of the 2019 Workshop on Biomedical Natural Language Processing (BioNLP 2019), (2019), pp. 58–65.
Beltagy I, Lo K, Cohan A. Scibert: A pretrained language model for scientific text. In: EMNLP. Association for Computational Linguistics? (2019). https://www.aclweb.org/anthology/D19-1371 .
Alsentzer E, Murphy JR, Boag W, Weng W, Jin D, Naumann T, McDermott MBA. Publicly available clinical BERT embeddings. CoRR abs/1904.03323 2019. arxiv:1904.03323 .
Devlin J, Chang M, Lee K, Toutanova K. BERT: pre-training of deep bidirectional transformers for language understanding. CoRR abs/1810.04805 (2018). arxiv:1810.04805 .
Gu Y, Tinn R, Cheng H, Lucas M, Usuyama N, Liu X, Naumann T, Gao J, Poon H. Domain-Specific Language Model Pretraining for Biomedical Natural Language Processing 2020. arxiv:2007.15779 .
Marshall IJ, Kuiper J, Wallace BC. RobotReviewer: evaluation of a system for automatically assessing bias in clinical trials. J Am Med Inform Assoc. 2016;23(1):193–201.
Download references
Acknowledgements
We would like to thank Mayra Latorre Martinez (Swissmedic) for her valuable contribution throughout the project.
The results presented here were obtained within a project funded by Swissmedic; however, this study was conducted independently without any Swissmedic funding.
Author information
Authors and affiliations.
Quinten, 8 rue Vernier, 75017, Paris, France
Vincent Martenot, Valentin Masdeu, Jean Cupe, Faustine Gehin, Margot Blanchon & Julien Dauriat
Swiss Agency for Therapeutic Products, Swissmedic, Hallerstrasse 7, 3012, Bern, Switzerland
Alexander Horst, Michael Renaudin & Philippe Girard
UMMISCO, Sorbonne University, IRD, Bondy, France
Jean-Daniel Zucker
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design: All authors, Collection and assembly of data: Vi. M., J. D., M. B., Data analysis and interpretation: Vi. M., Va. M., J. C., M. B., A. H., and J. D., Manuscript writing: Vi. M., Va. M., J. C., F. G. and J-D. Z., Manuscript correcting: all authors, Final approval of manuscript: All authors. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Vincent Martenot or Jean-Daniel Zucker .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preferred name | Synonyms used when available |
|---|---|
Fluticasone Fluticasone furoate | FLUTICASONE FUROATE/ GSK 685 698/ GSK685968/ GSK-685968/ GW685698X/ GW-685698X |
PEMBROLIZUMAB | KEYLYNK-010 COMPONENT PEMBROLIZUMAB/ LAMBROLIZUMAB/ MK-3475/ PEMBROLIZUMAB/ PEMBROLIZUMAB COMPONENT OF KEYLYNK-010/ SCH-900475 |
BAY2327949 | |
NIVOLUMAB | NIVOLUMAB/ ONO-4538/ MDX-1106/ BMS-986298/ BMS-936558 |
IPILIMUMAB | BMS-734016/ MDX-CTLA-4/ MDX-101/ MDX-CTLA4/ MDX-010 |
METAMIZOLE SODIUM | DIPYRONE/ METAMIZOLE SODIUM/ METAMIZOLE SODIUM MONOHYDRATE/ METHAMPYRONE/ NORAMIDOPYRINE METHANESULFONATE SODIUM/ NSC-73205/ SULPYRINE/ SULPYRINE HYDRATE |
IFOSFAMIDE | IFOSFAMIDE/ MJF 9325/ MJF-9325/ NSC-109724/ Z4942/ Z-4942/ Ifex/ Ifsofamide/ MITOXANA |
MK-8931 | |
Darboepoetin alfa | |
EPOETIN ALFA | |
INGENOL MEBUTATE | AGN 204332/ INGENOL MEBUTATE/ PEP005/ PEP-005 |
Cisplatin | NSC-131558/ TRANSPLATIN/ Cisplatin/ Platinol/ Platinol-AQ |
DEBIO 1143 | DEBIO 1143/ AT-406/ D-1143/ DEBIO-1143/ IAP INHIBITOR AT-406/ SM-406/ XEVINAPANT |
REMDESIVIR | GS 5734/ GS-5734/ REMDESIVIR |
RIVAROXABAN | BAY 59-7939/ BAY-59-7939/ JNJ39039039/ JNJ-39039039/ RIVAROXABAN |
Atezolizumab | ATEZOLIZUMAB/ Anti-PDL1/ Anti-PD-L1/ MPDL3280A/ MPDL-3280A/ RG7446/ RG-7446/ TECENTRIQ |
FINGOLIMOD | Fingolimod/ FINGOLIMOD/ FTY-720/ FINGOLIMOD HYDROCHLORIDE/ FTY720/ FTY-720 HYDROCHLORIDE/ TY720 HYDROCHLORIDE |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Martenot, V., Masdeu, V., Cupe, J. et al. LiSA: an assisted literature search pipeline for detecting serious adverse drug events with deep learning. BMC Med Inform Decis Mak 22 , 338 (2022). https://doi.org/10.1186/s12911-022-02085-0
Download citation
Received : 28 June 2022
Accepted : 13 December 2022
Published : 22 December 2022
DOI : https://doi.org/10.1186/s12911-022-02085-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adverse drug events
- Assisted literature review
- Deep Learning
BMC Medical Informatics and Decision Making
ISSN: 1472-6947
- General enquiries: [email protected]

- Communications
- Energy and Utilities (E&U)
- Financial Services
- Healthcare & Life Sciences
- Manufacturing
- Media & Entertainment
- Retail and Ecommerce
- Travel & Hospitality
- Artificial Intelligence Testing
- Big Data & Analytics Testing
- Blockchain Testing
- Cloud Migration Assurance
- Security Assurance
- Internet of Things (IoT) Testing
- Mobile Testing
- Robotic Process Automation (RPA)
- 5G Assurance Services
- Accessibility Testing
- DevOps Testing
- Compatibility Testing
- Functional Testing
- Performance Testing
- Regression Testing
- Security Testing
- Test Automation
- Crowdsourced Testing
- Quality Engineering
- ERP Testing
- Salesforce Testing
- Medical Devices Testing
- Agile Testing
- Test Data Management
- Service Virtualization
- DevOps Transformation
- Testing Center of Excellence
- Test Advisory & Transformation Services
- Build Operate Transfer (BOT) Model
- Programmatic Innovation
- Agile Transformation
- Software Product Engineering
- Application Modernization
- Blockchain Engineering
- Enterprise Application Development
- User Experience Engineering
- Site Reliability Engineering
- Data Insights
- AI & ML
- Verita – Quality Engineering Dashboard
- Velocita – Test Automation Accelerators
- Praxia – Process Accelerator
- MAP – Model Assurance Platform
- CLAP – Cloud Migration Assurance Platform
- iNSta – Intelligent Scriptless Test Automation
- Cesta – Integrated Migration Solutions
- Incight – App Experience Analyzer Tool
- Zastra.ai – Active Learning Driven Annotation Platform
- Performance Engineering Lab
- Robotics Lab
- IOT and Smart Meters Lab
- Case Studies
- Digital ARMER
- Digital Dialogues – Podcast
- Digital Dialogues – Webinars
- herDIGITALstory TM
- LiQE Events
- Newsletter – Cignizine
- Tech Briefs
- Testimonials
- White Papers
- Infrastructure
- Cigniti in the News
- Press Releases
- Board of Directors
- Board Code of Conduct
- Annual Reports
- Corporate Presentation
- Quarterly Results
- Shareholding Pattern
- Announcements
- Analyst Recognitions
- Cigniti Careers
- Campus Connect
- Alumni Relations
- Life @ Cigniti
- Diversity, Equity, and Inclusion
- Corporate Social Responsibility
- Sustainability
Literature Search in Pharmacovigilance & Drug Safety: A Guide
Pharmacovigilance is a domain that works entirely on drug safety through the collection, detection, assessment, monitoring, and prevention of adverse effects associated with pharmaceutical products. It is a science that needs no introduction these days, thanks to increased vigilance in pharmaceutical drug discovery and development, as well as the introduction of new products to the market.
For Drug safety or pharmacovigilance, data will be collected from different sources to understand and assess the drug, either in its development stage while doing clinical trials or after releasing it into the larger population to understand its safety and efficacy in a wide range of populations.
Spontaneous reports, clinical trial reports, literature reports, license partner reports, and regulatory reports are major reports a company receives and processes for further analysis on regulatory reporting, signal detection, and aggregate reporting. Out of different source data, literature case reporting is one of the essential inputs, as the literature reports sometimes come with a combination of information from other report types.
This makes it more critical for companies to collect the literature information about the cases or articles involved with their drug. Literature article collection is required to assess a drug’s benefit-risk profile and comply with the regulatory requirement of not missing any reportable case within the required timelines.
Role of EMA
The EU health authority, EMA, is also responsible for monitoring several substances and selecting medical literature to help identify suspected adverse reactions to medicines authorized in the European Union. EMA’s Medical Literature Monitoring (MLM) services started on September 1, 2015. EMA maintains this information in its database called EudraVigilance. However, EMA can only monitor a certain number of substances based on information submitted to EMA’s Article 57 database by marketing authorization holders. So, the companies are responsible for monitoring their products in the global and local literature databases.
As per the EMA, marketing authorization holders are usually responsible for monitoring the medical literature on their medicines and reporting individual cases of suspected adverse reactions into EudraVigilance and national safety databases, which are not covered under the EMA’s services for that marketing authorization holder.
It was also declared that all products having marketing authorization, regardless of commercial status, should undergo literature searches. As a result, it’s reasonable to expect that literature searches begin with submitting a marketing permission application and continue throughout the authorization.
Where to search for literature articles?
Medline is a well-known database with the latest medicinal product information. Along with Medline, the other databases with the newest information on published medical literature articles or journals are Embase and Excerpta Medica. The published articles or journals and relevant abstracts from meetings and draught manuscripts should be reviewed for valid ICSRs and included in periodic safety update reports.
Literature search, monitoring, and screening
As we discussed above, the goal of literature search and monitoring is to identify individual case safety reports and any possible changes to the benefit-risk profile of the substance being monitored, particularly about detecting new safety signals or emerging safety issues.
Marketing authorization holders should perform medical literature monitoring on their products from day one of product authorization until they are active, whether the product is in the market or not. All the medical literature published globally and locally should be monitored and screened for all the adverse events reported in that literature for ICSR processing, as the medical literature is an essential source of information for identifying suspected adverse reactions to authorized medicines.
Once an article is identified as relevant to the MAH’s product, it will be screened further to determine whether it meets its four minimum criteria (identified reporter, company product, patient, and an adverse event) to consider it for further case processing and adverse event reporting. If there are any signals to consider for aggregate reporting and benefit-risk assessment, they will be shared for further review by the MAH.
How can Cigniti help you with this?
From this article, we got an idea of how important it is to monitor and screen the published literature articles and abstracts about an authorized medicinal product for an MAH. We at Cigniti have our experts to help you with literature monitoring and screening for your timely reporting of ICSR.
Our experts work with you to understand the requirements and develop a search strategy based on your global and local literature data collection requirements. We will work with you to understand the purpose of your requirement, whether it is for ICSR reporting, signal detection, or aggregate reporting, and provide you with a solution that meets the regulatory requirements while being compliant with timelines.
Schedule a discussion with our healthcare and life sciences experts to learn more about the criticality of literature search in Drug Safety and Pharmacovigilance.

Anusha Chowdary is a Senior Business Analyst and Domain expert for Healthcare with 10 years of experience in working with Healthcare and Healthcare IT. She is an experienced professional and subject matter expert in handling Drug safety applications. Her expertise includes thorough knowledge of health care and pharmaceutical regulations, with an educational background in Pharmacy.
Leave a Reply
Your email address will not be published. Required fields are marked *
Related Posts

Healthcare in 2021 will be all about digital health & virtual care
By Cigniti Technologies
Listen on the go! 2020 or ‘the year of pandemic’ has been remarkable in terms of the scale and the... read more

4 Reasons Why Quality Assurance in Healthcare is Important
By Subir Saha
Listen on the go! In the healthcare industry, the need of the hour is to make informed decisions at the... read more

Ensuring viability of the digital twin technology in Healthcare
Listen on the go! In a typical episode of House M.D., a patient comes in with an unusual and puzzling condition,... read more
Pharmacovigilance Literature Search
Our Pharmacovigilance Literature Search Service offers a reliable and efficient solution for accessing, collecting, and interpreting safety-related information from the scientific literature to support pharmaceutical companies, regulatory agencies, and healthcare organizations in promoting patient safety in the pharmaceutical industry.
With a team of pharmacovigilance experts, we ensure thorough coverage of published studies, literature cases, and other emergency safety issues. We empower pharmaceutical companies to maintain proactive safety surveillance, comply with regulatory requirements, and make evidence-based decisions to evaluate the safety profile of their medicinal products.
Customized Search Strategies: We develop tailored search strategies based on your specific requirements, including target drugs, adverse events, patient populations, and therapeutic areas of interest.
Our approach ensures that we capture relevant literature while minimizing noise and irrelevant results.
Our literature search covers a wide range of resources, including international and local resources. Additionally, we can include customized resources to meet the local regulatory requirements.
Timely Delivery: We understand the importance of timely access to safety information. Our efficient workflow and dedicated team ensure that search results are delivered promptly, allowing you to stay informed and make timely decisions.
Cost-effective Solution : Outsourcing pharmacovigilance literature searches to our experienced team offers a cost-effective solution compared to in-house resource allocation and ensures high-quality results delivered in a timely manner.
Our Pharmacovigilance Literature Search Service is an essential component of a comprehensive drug safety and pharmacovigilance program. By leveraging our expertise and resources, you can ensure that your literature monitoring activities are efficient, effective, and fully compliant with regulatory expectations. Contact us today to learn more about how we can support your pharmacovigilance needs.
Our Pharmacovigilance Literature Search Service is tailored to meet the needs of pharmaceutical companies, biotech firms, and other organizations involved in drug safety monitoring and surveillance.
Contact us today to learn more about how we can support your pharmacovigilance system with our literature search service.

Copyright © 2024 Insight CRO - All Rights Reserved.
- CTD Training
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Pharmacol Ther

A New Era in Pharmacovigilance: Toward Real‐World Data and Digital Monitoring
Adam lavertu.
1 Biomedical Informatics Training Program, Stanford University, Stanford California, USA
Bianca Vora
2 Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, San Francisco California, USA
Kathleen M. Giacomini
Russ altman.
3 Department of Bioengineering, Stanford University, Stanford California, USA
4 Departments of Biomedical Data Science, Genetics, and Medicine, Stanford University, Stanford California, USA
Stefano Rensi
Adverse drug reactions (ADRs) are a major concern for patients, clinicians, and regulatory agencies. The discovery of serious ADRs leading to substantial morbidity and mortality has resulted in mandatory phase IV clinical trials, black box warnings, and withdrawal of drugs from the market. Real‐world data, data collected during routine clinical care, is being adopted by innovators, regulators, payors, and providers to inform decision making throughout the product life cycle. We outline several different approaches to modern pharmacovigilance, including spontaneous reporting databases, electronic health record monitoring and research frameworks, social media surveillance, and the use of digital devices. Some of these platforms are well‐established while others are still emerging or experimental. We highlight both the potential opportunity, as well as the existing challenges within these pharmacovigilance systems that have already begun to impact the drug development process, as well as the landscape of postmarket drug safety monitoring. Further research and investment into different and complementary pharmacovigilance systems is needed to ensure the continued safety of pharmacotherapy.
The safety of a drug continues to be monitored after approval and marketing in an ongoing process of pharmacovigilance. 1 This postmarket drug safety monitoring is especially important with regard to adverse drug reactions (ADRs) that are rare, only occurring in certain subgroups, and/or only develop after long‐term drug exposure. In some cases, serious ADRs are not recognized until long after a drug has been approved for market, as seen in the case of thalidomide where its use in pregnant women led to congenital malformations. Accordingly, the importance of postmarket monitoring is highlighted by the finding that one‐third of newly identified safety issues in the postmarketing period are added to the warnings and precautions section of the label, the second highest tier of severity, indicating the serious nature of newly identified ADRs. 2
The passage of the 21st Century Cures Act has modernized clinical trials and requires the evaluation of the potential use of real‐world data (RWD), data collected during routine clinical care in the form of electronic health records (EHRs), medical billing, and other data generating activities in the regulatory decision making and approval process. Real‐world evidence (RWE) is the evidence of the potential benefits of the medical product in a clinical setting derived from RWD. Results from various study designs and analyses, both prospective and retrospective, that use RWD are accepted as RWE. The US Food and Drug Administration (FDA) guidance on RWE describes several contexts in which it can be used during the product life cycle, such as proving an unmet medical need, substituting for a control group, as supporting evidence for a label expansion, and as a part of postmarketing studies. The multiple emergency use authorizations granted to drugs during the coronavirus disease 2019 (COVID‐19) pandemic highlights a situation where postmarket pharmacovigilance becomes pivotal to maintaining long‐term patient safety. Collectively, the legislative acts and regulatory practices have led to an increased reliance on postmarket pharmacovigilance to inform drug safety. Innovation in pharmacovigilance is needed to address these challenges and complement clinical trials by improving the sensitivity and specificity of ADR detection and streamlining the process of refining RWD into RWE that supports regulatory decision making.
ESTABLISHED PHARMACOVIGILANCE SYSTEMS
Published case reports have been circulated among physicians since the late 1960s and continue to serve an important role in pharmacovigilance. They are typically rich in information because physicians are trained in the rigorous evaluation of medical histories, drug exposures, and outcomes; additionally, peer review provides a form of quality control. However, case reports are fundamentally anecdotal data points, and as such cannot support conclusions in broader populations. The digitization of written media and advent of databases and search engines make it possible to collect, store, and rapidly retrieve relevant and comprehensive case series, but the data are unstructured text, which is not suitable for rigorous quantitative analysis. Despite these limitations, case reports published in journals are useful for generating hypotheses, and pharmacovigilance studies often start with a search of the relevant case literature.
Medwatch has been the principal means of collecting and analyzing information about ADRs since 1993 and is used by the FDA to collect information on both small molecule drugs and biologics. Data are collected using standardized individual case safety reports forms, which are submitted physically or electronically to the FDA Adverse Event Reporting System (FAERS). The aggregate data are then mined for safety signals, which generate hypotheses for further investigation. FAERS has successfully identified previously unreported ADRs, with FAERS data contributing to more than 50% of all postmarket safety‐related label changes. 3 Table 1 lists a selection of additional pharmacovigilance studies in which FAERS or other ADR databases have played a prominent role. In addition to FAERS, the FDA has event reporting systems for (1) foods, dietary supplements, and cosmetics, (2) medical devices, and (3) vaccines, via CAERS, MAUDE, and VAERS, respectively.
Select examples of successful pharmacovigilance studies in which ADR and RWD database studies played a prominent role
| Drug(s) | Effect(s) | Source(s) | Citation |
|---|---|---|---|
| Acetaminophen | Liver injury | HER | |
| Agomelatine | Liver injury | Lit review | |
| Gabapentin, pregabalin | Liver injury, hematological disorders | ADR database | |
| Apixaban | Liver injury | Case report, ADR database | |
| Ketoconazole | Liver injury | Lit review, ADR database | |
| Methadone | Arrhythmia | Lit review, ADR database | |
| Ranolazine | Seizure | Sentinel | |
| Levetiracetam, phenytoin | Angioedema | OHDSI | |
| Citalopram | Arrhythmia | EHR | |
| Hydroxyzine | Arrhythmia | Lit review, ADR database |
ADR, adverse drug reaction; EHR, electronic health record; OHDSI, Open Health Data Science Informatics; RWD, real‐world data.
However, FAERS case reports as a source of data are limited by incompleteness, bias, and inconsistency. Prescribing decisions are often influenced by factors that affect clinical outcomes, such as comorbidities, insurance, and access to primary care, information that is not available in the publicly available FAERS data. The Institute for Safe Medical Practices found that over half of the reports in FAERS were missing basic information, such as age, gender, exposure date, and outcome. Additionally, FAERS does not measure the total number of exposures in the population, so there is no “denominator” to estimate the frequency of adverse events. Although adverse events are generally under‐reported, stimulated reporting driven by news, social media, and advertising can increase reporting rates for certain drugs. Incorrect hypotheses generated from erroneous or incomplete adverse event report data can be costly, with false‐positives resulting in resources wasted on unnecessary studies and false‐negatives leading to harm to patients.
EMERGING PHARMACOVIGILANCE SYSTEMS
Another component of the data revolution within health care has been the adoption of information technology by the health insurance industry and the adoption of EHRs by healthcare systems as a result of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act. Insurance claims capture prescription and medical diagnoses across healthcare providers, with the caveat that they do not directly measure outcomes. EHRs contain rich information, such as clinical notes, images, and laboratory test values; however, they are often locked within institutional silos on systems that are unique for each provider institution and suffer from bias related to their primary purpose, a clinical and legal record ( Figure 1 ).

Overview of pharmacovigilance methods at varying stages of development. Established (green, left), emerging (yellow, middle), and experimental (red, right) pharmacovigilance data sources and systems are presented. Examples of methodological areas that are currently used and under active development for the analysis of these different data types are included in the bottom box. FAERS, FDA Adverse Event Reporting System.
The Sentinel initiative extends the pharmacovigilance capabilities of the FDA by leveraging EHR systems and insurance claims data in distributed data networks of partner institutions. 4 The Sentinel system is used to study specific drug‐event outcomes and, more recently, is being used to generate drug safety signals. Analyses can be submitted to the partner network and run independently at each site and results can then be combined to provide comprehensive safety profiles. The integration of these various data sources has allowed for a more comprehensive and synergistic pipeline and capabilities. A general workflow is presented in the top row of Figure 2 . Sentinel required the development and implementation of a common data model and data quality assurance standards to ensure interoperability of data and reliability of analytical findings. Current efforts have been primarily focused on billing and claims data. Several new data partnership networks and consortia have emerged, such as PedsNet and the Open Health Data Science Informatics (OHDSI) network, that are improving and extending the governance, interoperability, and data stewardship frameworks pioneered by Sentinel. For example, the OHDSI network has adopted the OMOP’s Common Data Model for standardizing identifiers for diseases, procedures, drugs, and other components of a patient’s health record and has created a network of hospitals standardized to this data model. This enables an analysis designed at one member institution to be quickly replicated in other healthcare systems within the OHDSI network with minimal need to readjust the analysis. For instance, an analysis designed at Stanford could be run at hospitals in Israel, South Korea, and Australia, quickly finding support for or discrepancies in the findings of a single institution. Patient Centered Outcome Research Institute (PCORI) is establishing data networks, as well as procedures for evaluating and ensuring the relevance and reliability of data. The FDA is piloting demonstration cases for the use of RWE in regulatory decision making.

General pharmacovigilance workflows for emerging and experimental systems. EHR based pharmacovigilance workflow is shown in the purple top row. A mobile device‐based pharmacovigilance workflow is shown in the orange middle row. The social media‐based pharmacovigilance workflow is shown in the blue bottom row. These data can then be used separately or in combination to perform pharmacovigilance research and analysis. API, application programming interface; EHR, electronic health record.
An example of a new drug approval that relied on RWE, is Avelumab, a monoclonal antibody directed against programmed death ligand 1. Avelumab was approved based on a single arm, phase II trial where historical controls were identified from EHRs and were used to characterize the natural history of the disease. 5 Additionally, Aspirin Dosing: A Patient‐Centric Trial Assessing Benefits and Long‐Term Effectiveness (ADAPTABLE), a clinical trial evaluating the optimal dose of aspirin in patients with atherosclerotic cardiovascular disease, has utilized PCORnet EHRs and claims data at multiple stages of their study, from identifying patients who meet the inclusion/exclusion criteria to capturing primary and secondary study end points. 6 The ADAPTABLE trial represents the first randomized trial within PCORnet and, as such, has also developed new methodologies to take advantage of the data with the PCORnet data infrastructure.
The primary purpose of EHRs is to inform clinical decisions and/or support administrative functions (i.e., documentation to support billing). As a result, issues such as human/coding errors or bias may affect how information is captured prior to analysis. Additionally, the fractionalized nature of the US healthcare system makes it difficult to track patients across different healthcare systems resulting in incomplete data entries.
Clinical definitions, terminology, and note‐taking style vary between and within healthcare systems, making the extraction and transformation of clinical information to standardized elements, such as SNOMED codes, technically difficult. The challenging nature of clinical note processing has resulted in the majority of analyses to date primarily focusing on the billing related International Classification of Disease 10 codes. Last, unpredictability about patient compliance (i.e., even if a prescription is written does not mean the patient will pick it up) limits the use and extension of these data. These represent major obstacles to widespread pharmacovigilance using EHRs and future work will need to overcome these issues before the benefits of EHR data can be fully realized.
EXPERIMENTAL PHARMACOVIGILANCE SYSTEMS
Although Sentinel, PCORI, and OHDSI have greatly improved pharmacovigilance efforts, they rely on a constrained set of information within the healthcare system, that is, information in the EHR or in billing and claims data. 7 Outside the healthcare system, data from social media represent another key opportunity for pharmacovigilance. Social media data contains various data streams, potentially enabling us to identify patterns in behavior, environment, drug use, drug‐drug interactions, and ADRs. A general workflow for pharmacovigilance in social media data is presented in the bottom row of Figure 2 . The broad usage of social media by the public yields a massive dataset that is continuously growing and has huge potential for generating public health benefits. Individual experiences with a particular drug are often posted directly to social media. These testimonials can be found on both general platforms like Twitter and Reddit, as well as health‐oriented websites, such as AskaPatient.com, drugs.com, and iodine.com. Social media data often contain information critical to postmarket pharmacovigilance, such as individual experiences of ADRs, information about environmental factors, reports of pill diversions, and polypharmacy (both recreational and prescribed) that is often missed by other postmarketing surveillance systems.
There has been progress in developing new methods for postmarketing surveillance in social media data through the use of statistical models, machine learning, and deep neural network architectures. The annual Social Media Mining for Health Applications (SMM4H) workshop has resulted in algorithms capable of identifying drug mentions with high precision and recall, even in situations where these mentions are informal slang terms or misspelled drug names. However, high performance of ADRs continues to present a challenge as text descriptions of a particular ADR might vary greatly in written language, for instance “stomach” may be expressed as “stomach ache,” “stomach pain,” “abdominal pain,” “tummy ache,” etc. Additionally, classifying a particular tweet for first‐person vs. secondary reports of medication ingestion presents another challenge and has also been featured as challenges for the community with varying levels of success. Ideally, these efforts will culminate in systems capable of actively monitoring social media data and generating real‐time statistics relevant to pharmacovigilance efforts.
Although social media can provide a large volume of easily accessible data, the nature of social media presents several challenges for the extraction of signals related to pharmacovigilance. The first set of these challenges are that: (1) very few social media posts are relevant to pharmacovigilance, ~ 0.2% of tweets mention a medication 8 ; (2) information is represented in unstructured text; (3) drugs and medical conditions are often misspelled, abbreviated, or discussed using slang 9 ; (4) mentions of medical events may not be firsthand accounts; and (5) social media reports will contain false‐positives, but often provide less information than clinical case reports and so the reliable identification of true drug side effects from these data will be difficult. Recent work, as mentioned above, indicates that many of these problems may be overcome in the near future. Once these systems can produce robust ADR event statistics, further work may extend their functionality through analysis of the individual testimonies found within social media data. Social media data often contains lifestyle information like exercise patterns, eating habits, socio‐economic issues, and/or drug abuse behavior that will be missing from the EHR for the foreseeable future. For example, systems may find indications of relative quality of life improvements given a particular medication, patient preferences, or capture additional demographic information that could be key to protecting at risk populations, such as pregnant women and children.
In a demonstration of the value of general social media, recent efforts using Twitter have focused on vulnerable populations, such as pregnant women, that are often excluded from clinical trials, and, as a result, drug safety is not typically established in these groups in the premarket space. Although there are methods to gather this information postapproval, such as pregnancy registries, these databases are often constrained by issues, such as attrition, cost, and patient compliance. A recent study using data from Twitter accounts of pregnant women observed a higher medication intake in women who reported birth defects. 10 Similarly, another study developed a natural language processing method to identify tweets by users whose child had a birth defect. 11 These preliminary studies demonstrate how social media, such as Twitter, might help supplement existing resources, especially in vulnerable populations. Thus, it represents an exciting source of potentially complementary information for postmarket pharmacovigilance efforts.
A recent effort questioned the overall value proposition of social media data, citing the low prevalence of posts relevant to pharmacovigilance and low coverage for many drugs. 12 The analysis compared ADR signals from social media to Vigibase report statistics, focusing on the FDA drug labeling changes or “validated” safety signals, where there is evidence the drug has a causal relationship with the ADR. However, Vigibase report statistics may not be an appropriate evaluation baseline because the FDA labeling changes and/or the “validated” safety signal may have resulted from signals within the spontaneous reporting systems, likely inflating the baseline performance. Additionally, this evaluation effort did not adequately address the noisy nature of social media drug reports, failing to include drug misspellings or slang terms in their search queries, potentially missing a substantial number of reports. 9 It is likely that more advanced report identification methods would increase the value of social media data. The overall lack of social media discussions surrounding some drugs will continue to pose a challenge. Although the authors did not recommend the use of general social media data for pharmacovigilance, they indicated that social media generated in the context of a drug or health‐oriented platform (e.g., drugs.com) vs. a general platform (e.g., Twitter) may still hold value.
Beyond the technical challenges of working with social media data, its pseudonymous, open, and ephemeral nature creates new challenges in ethics, law, and reproducibility that must be navigated. Many platforms limit the sharing of data collected from their users and require that content be deleted upon user request. Social media posts experience high deletion rates with more than 40% of posts from one study being deleted from the platform after the study was published. 13 Researchers must preserve their own copies of data used for a particular study to ensure reproducibility. The publishing of the contents of social media posts in scientific journals may disclose potentially sensitive information about users, such as illicit drug use or mental health issues. Researchers must balance between making research reproducible and the ethical concerns of the risk of making research datasets freely available, which might increase the risk of abuse.
Mobile devices are a recent innovation in capturing information about ADRs, again providing another avenue of data collection in an uncontrolled setting. A general workflow for pharmacovigilance using mobile devices is presented in the middle row of Figure 2 . MyHeart Counts is used to do a 6‐minute walk test, which can be done daily in an in‐home setting. MedWatcher was a mobile application version of the FDA 3500 form for medical devices and is currently undergoing implementation in the European Union. Hugo platform for postmarket surveillance is under development at the Yale‐Mayo Center of Excellence in Regulatory Science and Innovation, Yale‐Mayo, which can collect electronic patient‐reported outcomes outside of the hospital. 14 Next steps include interfacing with connected devices to measure end points; however, the strides made in this more recent area of pharmacovigilance are very promising.
These are two modalities among many that researchers are investigating as potential new means of pharmacovigilance. Through the FDA funded Centers of Excellence in Regulatory Science and Innovation (CERSI), other databases and methodologies are being studied as potential pharmacovigilance systems, for examples see https://pharm.ucsf.edu/cersi/research .
Clearly, the development of these massive sources of data for future pharmacovigilance efforts creates an opportunity for capitalizing on recent advances in deep learning and anomaly detection. A continuously learning artificial intelligence system could not only learn to integrate these heterogeneous data sources for real‐time ADR detection, but could help identify potential cases and interface with members of the pharmacotherapy community to gather more information when needed. The field of pharmacovigilance is rapidly evolving, however, the resources we have highlighted are only part of the solution; the FDA and National Institutes of Health (NIH) will need to continue their funding of research that focuses on how to effectively analyze these data streams. Ideally, funding mechanisms will ensure interdisciplinary teams of experts from epidemiology, sociology, statistics, and computer science among others. Collaborative interdisciplinary efforts will ensure both institutional buy‐in as well as methodological rigor. Ultimately, the combination of various data sources and expertise will result in safer and more effective pharmacotherapy for everyone.
A.L. is supported by the National Science Foundation Graduate Research Fellowship, DGE – 1656518. B.V. is supported by an Oak Ridge Institute for Science and Education (ORISE) Fellowship, and is the recipient of an Achievement Rewards for College Scientists (ARCS) Scholarship. R.B.A. is supported by NIH GM102365, HG010615, and the Chan‐Zuckerberg Biohub. This work was partially supported by Grant Number U01FD004979/U01FD005978 from the FDA, which supports the UCSF‐Stanford Center of Excellence in Regulatory Sciences and Innovation.
Conflict of Interest
The authors declare no conflict of interest.
As Deputy Editor‐in‐Chief of Clinical Pharmacology & Therapeutics , Kathleen M. Giacomini was not involved in the review or decision process for this paper.
ACKNOWLEDGMENTS
The authors would like to thank the reviewers for their excellent feedback during the review process and the editorial board for their understanding during the COVID‐19 pandemic. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Health and Human Services (HHS) or the FDA.
Contributor Information
Russ Altman, Email: [email protected] .
Stefano Rensi, Email: ude.drofnats@isners .
- Submit RFI/RFP
Literature Monitoring in Pharmacovigilance
Incorporating data from medical and scientific literature is a paramount aspect of patient safety. This wealth of knowledge often constitutes a substantial part of the safety profile of medicinal products. From providing insights on potential side effects to shedding light on different patient experiences, this literature contributes significantly to how we understand and navigate the safety of medical products.
Regulatory bodies hold medicinal products’ marketing authorization holders (MAHs) to a high standard. One key aspect of their responsibility is performing regular scientific literature searches, including unpublished manuscripts and abstracts presented at medical or scientific conferences.
To underscore the importance of literature monitoring in pharmacovigilance, consider that properly conducted searches can lead to important discoveries, timely interventions, and overall improved product safety. On the other hand, inadequate searches can result in missed opportunities, overlooked risks, and in some cases, significant patient harm.
Types of Literature Searches
Literature searches generally fall into two categories. First, exhaustive searches cast a wide net across various databases and resources, capturing a broad spectrum of available literature on a given subject. Second, selective searches focus on specific databases or key publications, often targeting high-impact literature.
Exhaustive Searches
Exhaustive searches, or systematic reviews, aim to include all available evidence on a specific research question. This involves extensive searching of several databases, websites, and sometimes even manual searching of journals and books to ensure no relevant study is overlooked.
The exhaustive search is labor-intensive and requires a clear understanding of the topic and complex search strategies. However, the end result is a comprehensive overview of the literature, including studies of all levels of quality and size. This approach is often used when performing meta-analyses, where the results of several studies are combined to provide a more definitive answer to a research question.

Selective Searches
On the other hand, selective searches are more focused and usually target specific databases or key publications. Instead of casting a wide net, selective searches aim at catching ‘big fish’, which refers to high-impact literature that has the potential to change practices or policies.
This method requires knowledge about the topic and the ability to discern the importance of different pieces of literature. The targeted databases or journals often have high impact factors, meaning their published works are frequently cited in other research. While less comprehensive than exhaustive searches, selective searches are quicker and more feasible in certain circumstances, especially when time is limited.
The choice between the two depends on the research question, the project’s scope, and the available resources.
Global vs. Local Literature Searches
When conducting research, you might come across the terms ‘global literature search’ and ‘local literature search.’ Understanding the differences between these two methods can significantly influence the depth and breadth of the findings of a research project. Let’s dive into the characteristics that distinguish global and local literature searches.
Global Literature Searches
Global literature searches are broad and comprehensive. They seek to capture the full scope of existing research on a topic, regardless of the geographical origin of the data or the research institution. Global searches often employ multiple databases and resources, not limited to any specific country or region.
A global literature search intends to gather information from diverse sources, giving researchers an extensive understanding of the subject matter. This approach offers several benefits, including a broad perspective on the topic, a wide range of data, and the inclusion of different cultural, geographical, and socioeconomic contexts. However, it may require more time and resources due to the vast amount of information that needs to be reviewed and analyzed.
Local Literature Searches
Local literature searches, on the other hand, are more focused and region-specific. They center on information from a particular area, country, or group of countries. Researchers may use region-specific databases, local libraries, government reports, and documents from local institutions in a local literature search.
This search type is beneficial when the research question pertains to a particular region or population. Considering local conditions, policies, and cultural practices can provide more context-specific information. However, a local literature search may offer a more limited perspective and overlook globally relevant findings.
In conclusion, choosing between a global or local literature search depends on your research objectives. Both have merits and can be used effectively to gather relevant information. Sometimes, combining both may be the best approach to ensure a comprehensive literature review.
Literature Search Strategies
One does not simply dive into literature searches; it requires a strategy. An effective literature search strategy is one that is constructed to ensure the optimum retrieval of the most relevant articles. This includes identifying the right keywords, knowing the databases to target, and being able to discern the value and relevance of different pieces of literature.
Embarking on a literature search is a bit like setting off on a treasure hunt. In this case, the treasure is the relevant, high-quality literature on your subject of interest. And, like any good treasure hunt, success lies in having a solid strategy. Here’s a closer look at the components of an effective literature search strategy.
Identifying the Right Keywords
Your search for literature starts with the right keywords. Keywords are the terms that best represent the core concepts of your topic. Brainstorming a comprehensive list of keywords covering various aspects of your subject is essential. Using a combination of broad and narrow terms can be beneficial. Moreover, consider synonyms, alternative spellings, acronyms, and both your keywords’ singular and plural forms.
Using Boolean Operators
Boolean operators—AND, OR, and NOT—are the glue that holds your search together. They allow you to combine or exclude keywords in a way that focuses your search. For example, using AND narrows your search (e.g., “diabetes AND children”), using OR broadens it (e.g., “children OR adolescents”), and using NOT helps eliminate irrelevant results (e.g., “diabetes NOT type 2”).
Knowing the Databases to Target
Different databases host different types of literature. For example, PubMed is excellent for biomedical literature, while IEEE Xplore is better suited for engineering and technology literature. Knowing the appropriate databases to target for your subject matter can significantly increase the relevancy of your search results.
Understanding Search Filters
Most databases provide options to filter your search results. Filters can be applied based on the publication date, language, study type, and more. Utilizing these filters can help streamline your search results, making finding the most relevant literature easier.
Appraising the Value and Relevance of Literature
Finally, having retrieved a list of literature, it’s important to appraise each article for its value and relevance to your research question. Look at the credibility of the authors, the methodology used, and whether the article’s conclusions are supported by the data presented.
In summary, a well-constructed literature search strategy is like a roadmap guiding you to the most relevant and valuable literature for your research. Developing can take time and patience, but the rewards are well worth the effort.
Implications of Inadequate Literature Searches
The importance of thorough literature searches becomes clear when we consider the implications of inadequate ones. Inadequate searches can lead to a lack of crucial information, causing potential risks to patient safety, regulatory repercussions, and even damaging the reputation of the MAH.
When to Start Literature Searches
The ideal time to start literature searches is early in product development. This allows for the early identification and management of potential risks. However, these searches must continue post-launch to stay abreast of the latest research and findings related to the product.
Regulatory Requirements for Literature Monitoring In Pharmacovigilance
In the realm of patient safety and medicinal products, regulatory requirements play a vital role. MAHs are expected to abide by specific protocols, including regularly and thoroughly searching the scientific literature. Compliance with these requirements is not only necessary for maintaining their licensing but also for ensuring patient safety and the overall integrity of the medical product.
Guidance from FDA and EMA on Literature Monitoring In Pharmacovigilance
Both the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) provide guidance on literature monitoring in the realm of pharmacovigilance.
The FDA’s guidance on the subject can be found in various documents provided by the agency. These documents provide comprehensive information on how to conduct effective and thorough literature searches. A crucial resource is the FDA’s Guidance for Industry, which provides detailed instructions and recommendations on good pharmacovigilance practices, including literature monitoring.
FDA Guidance for Industry
Meanwhile, the EMA also provides extensive guidance on literature monitoring. Their Good Pharmacovigilance Practices (GVP) guidelines, particularly Module VI on the management and reporting of adverse reactions to medicinal products, outlines the responsibilities of MAHs in regards to conducting literature searches. This module addresses the scope and frequency of literature searches, the criteria for selecting data sources, and how to report findings.
EMA Good Pharmacovigilance Practices (GVP)
These guidelines provided by regulatory bodies are not just recommendations; they form a part of the regulatory requirements for MAHs. Therefore, understanding and adhering to them is crucial in ensuring compliance and maintaining the safety profile of medicinal products.
Always remember to review these documents regularly, as the regulatory landscape evolves, and guidelines can be updated. This helps to ensure that your literature monitoring activities align with the most recent regulatory requirements and best practices.
Effective and comprehensive literature searches form a cornerstone of patient safety data. They allow MAHs and regulatory bodies to gather crucial information about medicinal products, offering valuable insights that contribute to their safety profiles. The importance of conducting these searches early and regularly, and the potential consequences of inadequate searches, underscore the need for rigorous and strategic literature review processes.
Start A Conversation
Are you considering pharmacovigilance outsourcing for your organization? Navigating the pharmacovigilance can be daunting, but you don’t have to do it alone. Our team of experienced professionals is ready to provide the support and expertise necessary to ensure your pharmacovigilance operations are efficient, compliant, and effective.
Don’t hesitate – reach out today for a personalized pharmacovigilance consultation. We look forward to partnering with you to enhance patient safety and streamline your operations.
Literature monitoring of Pharmacovigilance in realities of war in Ukraine
Feb 6, 2024 | Pharmacovigilance
It is fascinating to observe how the realities of war have affected the way in which the literature monitoring of Pharmacovigilance is carried out in Ukraine. The need for continuous monitoring of drug safety in a country at war, and the ability to adapt to the...
Pharmacovigilance & CSV: System Upgrades
Oct 9, 2023 | Pharmacovigilance
Explore the intersection of Pharmacovigilance & CSV during system upgrades. Ensure drug safety while optimizing computer system validation.
CSV & Risk Management in Pharmacovigilance
Sep 1, 2023 | Pharmacovigilance
CSV & Risk Management Pharmacovigilance stands as a guardian in drug development and monitoring, ensuring that medicines remain safe and effective throughout their lifecycle. But behind this sentinel lies a complex web of systems, processes, and regulations, each...
Big Enough To Cover All Your Needs. Small Enough To Care.
Clinical research, regulatory affairs, pharmacovigilance, medical information, clinical trials, pv system & qppv, post-authorization, we value your privacy., privacy overview.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

- ICSR Case Processing
Literature Search
- Aggregate Reporting
- Signal Management
- Risk Management
Global and local literature monitoring for adverse event identification across the drug life cycle
Marketing Authorization Holders (MAH) are required to monitor the scientific literature of their products on a periodic basis. Global and local literature search is performed to identify the adverse reactions and for reporting to health authorities.
APCER conducts the literature searches for products under investigational stage (in clinical trial phase), in pre-authorization stages, and post-marketing phase to meet the regulatory requirements of its clients.
Our Literature Search services portfolio includes:
- Global literature screening
- End-to-end literature search and review
- Defining search strategy
- Expertise in handling various literature search databases such as Embase, PubMed, and customer-specified databases
- Medical literature monitoring
- Local literature screening as per local regulations
- Procurement and review of full text articles where applicable
- Translation of literature articles by certified translators
How can we work together for better health?
Explore more.

PSUSA procedures and implementation of the outcome of a PSUSA procedure

Designing a Safety and Risk Management System for Cellular Therapies

Scaling up to address safety challenges for an Oncology product

Outsourcing Pharmacovigilance: Benefits of a Specialized Safety Partner

End-to-end pharmacovigilance support for a UK-based pharmaceutical company

Enabling global expansion by establishing an integrated PV system
Importance of engaging a safety partner early in the drug life cycle.
Upload Document ( Optional ) (Format Supported : PDF, DOC or DOCX | Max File Size : 1 MB)
Please check here to indicate your consent to receive catalogues, service offerings and marketing related emails from APCER Life Sciences.
Please note that by submitting this form, you consent to your personal data being processed in alignment with our Privacy Policy . APCER is committed to ensuring the security and protection of your personal information. Your personal data will be processed for purpose of facilitating your request and may be used for sending you additional marketing and business development-related information about APCER Life Sciences, its affiliates and our services. You can withdraw your consent by writing to us at [email protected] .
- Have any questions?
- (+1) 609 455 1600
- [email protected]
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Pharmacovigilance: Literature Monitoring Best Practices
Safe and effective use of health products is a key objective of pharmacovigilance. Information is provided about the safety of these substances to patients, healthcare providers, and the general public as soon as possible. Pharmacovigilance includes reviewing the development, management, and introduction of pharmaceuticals. It is probably the most tightly regulated part of the pharmaceutical industry. Pharmacovigilance aims to identify, detect, assess, and report any adverse drug reaction (ADR) related to pharmaceutical products. In the United States, the European Union, and other parts of the world, regulatory requirements have emerged that have grown in diversity and nuance. These requirements include systematic monitoring and review of medical literature, including comprehensive screening of medical journals for adverse drug reactions, which remain on the rise. Having a robust pharmacovigilance system is paramount for a manufacturer, and any deficiencies can have an adverse effect on patient safety.
Literature monitoring includes published articles, articles, and reviews in indexed or non-indexed journals, any content posted anywhere online, posters and conference abstracts, etc. Holders of a Marketing Authorization (MA) must monitor global and local literature throughout the duration of that authorization, regardless of the availability of the product on the market 1 . Regulatory reports, clinical trial reports, literature reports, license partner reports, and spontaneous reports all serve as sources of data for deeper analysis of regulatory reporting, signal detection, and aggregate reporting. The individual safety report (ICSR) is valuable for developing risk assessments. It is incumbent upon holders of marketing authorizations to stay up-to-date on potential publications (including ahead of print articles) by reviewing widely used reference databases (e.g., Medline, Embase, Excerpta Medica) every week 2 . Adverse events that meet the criteria for the ICSR are handled per regulatory guidelines on handling and reporting adverse events. When a relevant article has been identified, it will be further screened to determine if it meets the four essential criteria for consideration for Individual case safety report (ICSR) and adverse event reporting: 1) identified source, 2) company product, 3) patient, 4) adverse event 3 . Any analysis regarding the safety profile of a product should be based on scientific and medical publications. Literature searches and monitoring are primarily intended to identify single case reports of adverse effects and to track any changes in benefit-risk profiles associated with the drug, particularly when new safety signals safety concerns arise 4 .
Literature Monitoring: An Overview of Best Practices
When the foundation is compromised, a process can result in a cascade of unintended repercussions. Therefore, an unbiased search is vital for monitoring medical literature accurately and efficiently. The growing volume of data has made it more critical to get the best results without introducing unwanted data. The literature monitoring process is usually characterized by two major challenges, which can be overcome. The first challenge is to come up with the right search strategy, and the second is to deal with duplication. Drug manufacturers must often track hundreds of drugs at once. So how can literature monitoring be accurate and valid?
Optimal Search Strategy Design and Database Selection
Regulatory authorities require marketing authorization holders to conduct medical literature surveillance at least weekly according to the GVP module VI and based on the required frequency as described by the local regulatory authorities, both for globally indexed literature databases and locally (non-indexed) literature journals5. When developing search strategies, it is important to consider ICSRs, aggregate reports, and any potential safety-related information. Therefore, it is essential to develop and progressively improve search strategies to limit the risk of overlooking relevant ADR information. Specifically, to retrieve all relevant records, query terms must be highly recallable and carefully crafted to retrieve maximum publications reporting any safety concerns about the product in question.
The database must be comprehensive and meet minimum standards to ensure that safety-critical signals are not missed. Pharmacovigilance searchers typically utilize at least two databases, usually three or more, because having access to multiple databases increases their recall-finding capabilities, ensuring more coverage.
Implement a search approach that balances the need for accuracy and precision. For example, 1) use several Boolean operators, 2) browse a thesaurus of terms, 3) perform proximity search, and 4) incorporate abbreviations to recall results. Using the most recent thesaurus update will ensure accuracy and compliance 6 .
As part of the local literature review, it is recommended to identify the non-indexed journals published locally and to screen those in either an online or print format depending on their availability. There are a few local regulatory agencies that recommend performing local literature searches in a few databases that are locally approved. The MAH handles any publications identified as containing information in local languages in accordance with the translation process established within the institution.
Industry best practice calls for constant review of search terms and updating them based on safety-related updates pertaining to the products. A GOLD standard data set of records is used to validate the modified search strategies. It is recommended to review your search strategy annually and make amendments as necessary 7 .
EMA hosts a robust system for medical literature monitoring. Thousands of records are added daily. It is generally the responsibility of marketing authorization holders to monitor medical literature and report individual cases of suspected adverse reactions into EudraVigilance and national safety databases 8 . They are not required to monitor or report suspected adverse reactions for active substances to EudraVigilance for substances covered by EMA’s service 9 .
Duplicate Data Management
Scientific publications and medical literature are abundant with sources and references, so it’s likely that the same publication could be indexed in multiple formats across a variety of journals, which results in duplicate findings. This creates a whole series of redundant tasks and false signals regarding drug safety. It leads to erroneous evaluations and, ultimately, compliance problems. Duplicate management processes, however, can solve this issue. Even though this is the best way of dealing with articles, it comes with a few challenges. There may be limitations to duplicate identification within the tool due to the presence of special characters, or it may be the case that the same study has been published across different journals or conference abstracts, making the process cumbersome.
It is important to search multiple databases to capture multiple publications across different journals. Keeping track of previous searches will also facilitate the identification of duplicates. To identify duplicate publications, there should not be just a focus on the article title but also the author’s name and, in some cases, the name of the study cited in the article.
According to Article 107(3) of Directive 2001/83/EC, to avoid duplicate submissions of ICSRs, the holder of a marketing authorization must submit the ICSRs that are not already assessed or monitored by EMA through the Medical Literature Monitoring (MLM) services 10 .
Service providers should use a standardized and well-established deduplication system, enabling them to confirm that they are not missing relevant references or creating duplicates inadvertently.
Along with routine literature surveillance, MAH also conducts targeted literature searches, which are searches specifically designed to answer a specific research question. When conducting signal analysis, these searches are conducted to confirm or disprove the association between the adverse event and the product.
Pharmacovigilance involves a substantial amount of literature monitoring. The process of devising a solid search strategy could be challenging but is essential. A professional with the required skills, experience, and training will ensure adverse event-related safety information is never missed. It is necessary to develop and maintain search strategies, elicit ideas from different stakeholders, and develop approved and suitable strategies for the purpose at hand. It is critical to set up a thorough process to handle and manage duplicate articles. Regularly review search strategies, and ensure the documentation is robust to ensure the finest quality results. The following points can be considered to check whether the MAH’s literature monitoring systems meet quality standards;
- A drug safety expert with experience researching literature is needed.
- Conduct risk assessments to ensure that the search criteria are robust and relevant to the objective of the literature search.
- Conduct literature searches and evaluate the results for literature per regional requirements (Global and Local).
- Monitoring and reviewing the Eudravigilance Medical Literature Monitoring (MLM) system, managed by EMA, to identify ICSRs in the literature if your product is included in the active ingredient screened by EMA.
- The search string is reviewed and updated annually to optimize results.
ClinChoice is a leading global Contract Research Organization (CRO), with over 3400 clinical research professionals across North America, Asia, and Europe. For more than 27 years, ClinChoice has been providing high-quality contract research services to pharmaceutical, biotechnology, medical device, and consumer products clients, encompassing a broad range of services and therapeutic areas. ClinChoice offers cutting-edge, full-service solutions for Clinical Trials, Regulatory Affairs, Medical Device Safety, Toxicology, and Medical Affairs.
- https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
- https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-vi-management-reporting-adverse-reactions_en-0.pdf
- https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf
- https://database.ich.org/sites/default/files/E2D_Guideline.pdf
- https://europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-iv-pharmacovigilance-audits-rev-1_en.pdf
- https://www.ema.europa.eu/en/documents/other/monitoring-medical-literature-entry-relevant-information-eudravigilance-database-european-medicines_en.pdf
- https://www.elsevier.com/solutions/embase-biomedical-research/coverage-and-content
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Description
[Free White Paper]
Pharmacovigilance (PV) Literature Searches
A Whitepaper on optimizing search strategies for patient safety
Information obtained from medical and scientific literature is an important source of patient safety data and can contribute enormously to the safety profile of a medicinal product. It is a regulatory requirement for the marketing authorization holder (MAH) of a medicinal product to perform regular searches of the scientific literature, including any unpublished manuscripts and abstracts presented at medical or scientific conferences.
Within this whitepaper we will explore;
- The different types of literature searches
- Regulatory requirements
- Literature search strategy and how these should be constructed in such a way to ensure the optimum retrieval of the most appropriate articles
- The implications of inadequate literature searches
- When to start literature searches
- Examples of inspection findings

Download this paper
© 2024 Quanticate
Performing Literature Searches to Meet Vigilance Requirements
10 january 2022

Performing literature searches to meet vigilance requirements
As the Good pharmacovigilance practices (GVP) module VI states “medical literature is a significant source of information” for marketing authorization holders and has to be taken into consideration. Marketing authorization holders are expected to perform international and local literature searches to identify pharmacovigilance (PV) cases and to detect safety signals to establish a product’s benefit-risk profile. ProductLife Group can help with all these activities.
International literature
With a background in literature searches and training in PV, the information and documentation officers (IDOs) of the literature Line of Business (LoB) are best qualified to perform international searches. In order to answer the GVP recommendations, searches are performed weekly in one or two widely used reference databases: AdisInsight Safety , and PubMed . Databases are selected in collaboration with the client, based on the product portfolio and whether the international nonproprietary name (INN), in other words the generic name for a pharmaceutical substance, is followed in the medical literature monitoring service provided by the European Medicines Agency (EMA).
Searches are performed based only on the INN and the searched period, unless the client specifically asks for a search strategy that includes pharmacovigilance keywords. All potential PV cases are identified, as well as special situations according to the GVP definitions. Abstracts are recorded and sent to the client once the search is finalized. Based on the contract and technical agreement, IDOs can also pre-analyze the abstracts according to the GVP inclusion/exclusion criteria. Results are sent on a weekly basis, whether abstracts were identified or not.
Regular interaction with the client is the key to meeting PV requirements and the IDOs are fully aware of the importance of being responsive and reachable. The IDOs also work in collaboration with the case management team and, if PLG is in charge of this activity, all information flows seamlessly between the two lines of business.
Local literature
Local literature monitoring is carried out on a range of journals and websites suggested by our teams, then chosen by mutual agreement with the client according to the therapeutic areas of the client’s portfolio. Journals followed are not indexed in the international literature databases such as Embase and PubMed. The list of journals is updated regularly, depending on portfolio changes.
Monitoring is carried out in France by IDOs, and in other countries by local safety officers (LSOs) or local PLG staff native speakers of the relevant language. The central team of the literature LoB coordinates the activity so each article identified is sent to the IDOs who either forward search results to the client or, where relevant, to the PLG case management team.
Depending on what is agreed with the client, the selection of articles can be limited to PV cases and special situations, any article with safety information, or any article mentioning a client’s products. The central team of the literature LoB also ensures weekly or monthly reporting to the client.
Our teams are in constant communication across all countries in Europe to provide a comprehensive and multilingual local literature offering.
Literature for safety reports
The literature search for the safety reports is an important task within medical writing. While the writing of the safety reports is managed by the medical writing LoB, a dedicated central team is responsible for performing the literature searches.
The IDOs interact with the experts of the medical writing Lob to agree on the research period, search strings based on the type of reports and a list of keywords to ensure the search encompasses any relevant areas, as well as deadlines.
International searches are caried out either in the AdisInsight database and/or PUBMED to find any article related to safety, benefits-risks, efficacy, as well as other relevant vigilance searches depending on the type of report.
Most searches are performed with the molecule name and the relevant period using specific keywords. The more accurate and detailed the keywords, the more targeted and relevant the search will be.
Read more on how ProductLife Group may support you in managing your literature search
Register to our news and events
Go to our Events to register Go to our News to get insights

Unfortunately we don't fully support your browser. If you have the option to, please upgrade to a newer version or use Mozilla Firefox , Microsoft Edge , Google Chrome , or Safari 14 or newer. If you are unable to, and need support, please send us your feedback .
We'd appreciate your feedback. Tell us what you think! opens in new tab/window
Embase is the medical research database for high-quality, comprehensive evidence
Regulators around the world recognize Embase as a source for medical literature. Life science experts rely on Embase to find relevant and current results based on Emtree indexing of full-text content and dedicated search terms.

Related products
Pharmapendium.
The risk assessment tool for translational safety, drug efficacy and DMPK
Expertly curated chemical data and insights for better decision making
ScienceDirect
Elsevier's premiere platform of peer-reviewed scholarly literature
Conduct systematic literature reviews
Embase guides you in using the PICO method in a search. And you can quickly formulate an advanced query to explore deeply indexed content. Your results feed into a strong systematic review that helps you to:
Access relevant information from trusted sources
Confidently publish original research
Tap into a multitude of topics and deeply connected records

Screen for adverse drug events
Embase empowers your pharmacovigilance search strategy for high-recall, high-precision drug safety monitoring.
Use smart tools to ensure you don’t miss important information for compliant pharmacovigilance
Track drug-disease relations and drug-drug interactions
Recognized by regulatory bodies as a source for discovering adverse events

Comply with medical device regulations
Embase is recommended by the EMA for meeting the stricter requirements of MDR and IVDR.
Capture mentions of medical devices with dedicated device terms, subheadings and synonym suggestions from Emtree
Collect data for clinical evaluations, as well as post-market surveillance
Quickly create precise and high-recall queries
What makes Embase a complete biomedical resource?
Millions of records updated daily, full-text indexing with the emtree thesaurus, search wizards and templates, advanced search, alerts, download and search history tools, historical records from 1947 to 1973 with embase classic, what access options are available for embase, annual subscription.
Unlimited access for organizations
For government, institutions, corporate agencies, contract research organizations and consultancies
30-Day access
One user gets 30-day access to Embase.com (limited to four per year). Includes five email alerts.
For individuals, small and medium size businesses. Excludes contract research organizations and consultancies.
7-Day access
One user gets seven-day access to Embase.com (limited to four per year).
"Performing a systematic search of peer-reviewed and gray literature using Embase is generally accepted as a best practice for evidence-based medicine."

George R. Khachatryan
Frequently asked questions
Which regulatory agencies recommend or reference embase.
Embase is recommended and/or referenced by:
EMA - Guideline on Good Pharmacovigilance Practices (GVP)
European Union Medical Device Regulation (EU MDR)
Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2
China’s NMPA Guideline for the Collection and Reporting of Adverse Drug Reactions
WHO Handbook for Guideline Development
UK's NICE Interim Clinical Guideline Surveillance Process and Methods Guide
JBI Manuals for Evidence Synthesis and Evidence Implementation
Brazilian Ministry of Health
How does Embase compare with MEDLINE?
Embase has:
99% of journals in MEDLINE plus more than 3,000 additional journals
More than 99,000 preferred terms, including MeSH terms
More than 15,000 conferences
509,372 synonyms, ~479,000 more than MeSH
Granular indexing for devices and drugs
Pharmacovigilance Literature Screening
– recognized also as medical literature monitoring (mlm), surveillance or search –
Now, fully automated for global and local literature searches with Tepsivo Literature >

Why tepsivo?
Tepsivo Platform with its literature monitoring software module manages global and local literature review around all world the world from one place. Major benefit is in removing all unnecessary admin overhead heavily pushing down total costs, all in line with our value-based philosophy.
Reach out to us to learn more >
✓ automated reporting, ✓ full audit trail of actions, ✓ no admin overhead, ✓ lowest cost possible, ✓ 100% legally compliant.
For simplicity, we follow the usual split between global and literature screening. Global literature monitoring meaning the weekly search in databases such as PubMed or Embase and Local literature screenin g to mean the searches in non-indexed journals published locally around the world.
Global Literature Screening
Global literature screening is one of the key pharmacovigilance obligations you have. Depending on what your portfolio is, it can either be one of the easiest tasks or one of the most time-consuming ones. Either way, Tepsivo literature monitoring service can fully cover your regulatory duties.
Do you have one or a handful of products on the market?
First things first. Let us check if your substance isn’t already monitored by EMA’s Medical Literature Monitoring service. If yes, then there is no reason to duplicate the effort. To its credit, EMA has gone in the right direction by centralizing the screening in one place for many substances.
If your substance isn’t monitored centrally by EMA, you will need someone to screen the databases weekly and track any relevant results. That’s what we can easily do. Thanks to our automated Tepsivo Platform, it’s very easy for us to track and report any outputs of the screening, further dropping down the total price.
Do you have a large portfolio of products?
If you represent a generic company with a large portfolio, the global literature screening may in fact become a colossal cost for you. Here is where you need to automate.
We’ve spent a lot of time going through the best possible options how to optimize literature monitoring and decrease the associated time effort and cost. Reach out to us to discuss your situation and we’ll prepare the most fitting solution for you. Most likely a combination of an AI-powered search tool and our inhouse Tepsivo Platform, automating both the screening time as well as the reporting side of the process.
Let’s talk!
Are you all caught up with your reading all over the world?
With Tepsivo global PV network covering over 150 countries , you can be assured to comply with the literature monitoring requirements from EMA and regulatory authorities around the world. Based on your drug and commercial status in your respective markets, we will prepare your literature screening strategy for each country individually.
Our literature screeners will use their PV and relevant language knowledge to define the right keywords and, especially, to select the most relevant journals. To ensure you don’t miss any potentially relevant information, the journal selection is crucial for a good literature monitoring strategy, and it’s best done by specialists with thorough knowledge of the medical environment in their relevant countries.
What makes us special?
Our pragmatism and belief in efficiency in contrast to greed.
It is our view that local literature screening in non-indexed journals is an outdated requirement, and it shouldn’t really exist in today’s world , at least not in the form it is required now. That is, if we believe that pharmacovigilance process effectiveness should be a measurement of quality.
If you’re present globally, local literature screening will constitute a large part of your PV budget. We’ve been making arguments to the EMA to remove this requirement but to no avail. It’s here to stay and local literature screening will remain expensive for pharmaceutical companies.
So if EMA and legislators are not taking action, what can we do?

We can automate the process and heavily bring down your total costs.

Our unique Tepsivo Literature searches through thousands of sources based on keywords and sends auto-translated abstracts to one central place that is Tepsivo Platform. All with 0 people trapped doing mundane activities.
Plus, thanks to Tepsivo Platform , we removed the traditionally heavy work in reporting/tracking/reconciling that usually adds so much extra time effort to an already time intense activity. Especially in global operations.
For traditional pharmacovigilance providers and CROs, local literature monitoring is a financially lucrative activity. But we’re not greedy, we don’t believe that it belongs, in its current form, to the field and it certainly doesn’t meet the criteria of what we see as value-based healthcare. That is what we developed Tepsivo Literature, saving time and money on meaningless tasks
Want to learn more?
List of countries
Sub-saharan africa, let’s have a chat.
Whatever you needs are, we look forward to getting in touch with you. Feel free to drop us a message and we will contact you right away.
Tepsivo Oy | Urho Kekkosen katu, 4-6 E, 00 100, Helsinki, Finland | VAT number FI31367614 | [email protected] | +358 402 204 698 | Privacy policy
- Privacy Overview
- Strictly Necessary Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
- +91 9566299022
- +1-972-502-9262
- [email protected]

Global and Local Literature Search Screening
- Clinical Trial Audit & Monitoring
- Clinical Study Design
- Global Regulatory & Clinical Writing
- Clinical Biostatistics
- Clinical Trial Patient Recruitment
- Regulatory Affairs
- Clinical Data Management
- Post-Market Surveillance
- Clinical Technology & Process
- Healthcare Analytics
- Health Data Collection
Screening published medical and scientific literature regarding medical devices, and medicinal products is a mandatory requirement for marketing authorization holder (MAH) or License holders. MAHs need to regularly monitor the literature for suspected Adverse Drug Reactions (ADRs) and other information, including potential drug interaction, misuse, off-label use, and class effects.
Pepgra offers pharmacovigilance Literature review search services as part of the drug safety and efficacy services. Our medical regulatory staff have extensive experience in searching for articles from multiple databases published and can comprehensively manage your literature screening requirement in a cost-effective manner in conjunction with writing periodic safety reports. Our literature specialist team of Pepgra has 15+ years of experience in global pharma, with a focus on literature. We work closely with customers on one-on-engagement model and develop the search strategy methodology to ensure that criteria are robust and an unbiased approach. We perform the literature search be for aggregate reports, or benefit-risk analyses or for signal evaluation or ongoing screening as required by local and regional requirement. Our weekly search is not only limited to the identification of individual case safety reports but also detection of safety issues. We can conduct literature screening as per your requirement in local territories.
Our pharmacovigilance researchers are aware of the standard pharmacovigilance guidelines and regulations, including 21 CFR part 314.80 and 600.80 and basics of clinical development of a drug. In addition, our reports will be prepared based on ICH E2C (R2) PBRER and other reports (PFSB/SD 0917/2; PFSB/SD 0216/2; PFSB/SD 033 /9). Our writers also aware of data protection /privacy regulations, and work under stringent timelines in tandem with multiple stakeholders who might have different opinions, to arrive at a suitable consensus in a timely manner.
Our local representatives perform the review of specific local-non-indexed scientific and medical journals for the identification and processing of ICSRs.
Our Comprehensive Literature Review Screening Solutions
- Identification of Studies : Our experts conduct searches through validated databases including Embase, Medline or other local country-specific publications (e.g. JDream Databases for Japan) and screen abstracts for identification of potential ICSRs. We notify immediately of new safety information from screening.
- Regular Reviewing of local (non-indexed) Journals and documentation of all serious, unexpected adverse reactions (AR) and non-serious adverse reactions reported in the scientific literature. Even if there are no ARs, an ASR will be prepared and shared. In case reports present evidence of any serious, suspected adverse reactions, it would be forwarded receipt immediately.
- Literature Review Protocol identifying the elements, including the background, objectives, and methods for identification, selection and collection of the relevant publication to address literature review questions. Full published articles will be shared as per the order in which they have been used. Full quality checked report.
- Abstracts screening for identification of potential new and significant safety findings for inclusion in PSURs. The following information will be collected, as appropriate.
- Pregnancy outcome with no adverse events.
- Compassionate supply named patient use
- Asymptomatic overdose, abuse or misuse
- Off-label use, class effects
- Drug/food interaction, the suspected transmission of an infection’s agent
- Use in pediatric, elderly or organ impaired population
- Any other important non-clinical safety results. Ordering selected full publications for evaluation of ICSRs or safety issues

Biostatistical capabilities
White Paper
Clinical evaluations.

Medical writing
Pepgra has done plethora of work in the area of clinical trial audits and monitoring for top pharmaceutical companies. Our CRAs will ensure a thorough review of data, frequent the sites, and perform interim analysis. All tasks in compliance to ethics committee and regulatory standards such as Schedule Y, study protocol, ICH GCP and the other regulations.
We deliver study designs balanced to meet your business needs and expectations with the current scientific understanding and all regulatory requirements considered.
Allow us to help propel your product forward.
Pepgra CRAs did a fabulous job of frequenting the clinical trial sites at different times during the course of study. Apart from their technical know-how, they also had a great affinity with our site team members and finally documented pivotal research findings in the monitoring report which was an eye-opener for us. I would strongly recommend Pepgra as the CRO of choice.
— Barry Stein, VP of a leading medical device manufacturer.
We’ll scale
Up as your needs grow..
No compromising on integrity and quality. Our processes are well defined and flexible to ramp up as per your requirements.
Partnering with
You till the project end..
We come with you all the way. From design to market support
Pepgra CRO Offerings
"Changing global regulatory system, globalization of clinical trials, increased consumer expectations, infrastructural and culture issues, and various diagnostic requirements should never hamper your research and development programs. With our support..."
Download brochure on our CRO offerings (PDF).
SEE WHAT PEPGRA CRO CAN DO FOR YOU
FILL OUT THE BRIEF FORM BELOW TO GET IN TOUCH.

- Privacy Overview
- Strictly Necessary Cookies
- 3rd Party Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!

Pharmacovigilance Guide: Assist researchers in drug pharmacovigilance on the Ovid Platform
Welcome to this tutorial were we aim to assist researchers undertake drug Pharmacovigilance on the Ovid Platform
Definition of Pharmacovigilance
Adopted from the WHO definition for pharmacovigilance
- WHO defines pharmacovigilance as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem”.
- Reference - http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/
What is the aim of the OvidPV Tool?
- The OvidPV Tool has been designed to meet the needs of those professionals in Pharmacovigilance requiring a structured process that integrates industry benchmarks.
- OvidPV allows these users to quickly build a comprehensive and guided pharmacovigilance literature search in 5 easy steps!
- The Ovid Platform’s excellent workflow and tools will keep users informed of the latest information and developments in EMBASE .
Video Tutorials

What is Ovid?

OvidPV Tool

Introduction to Embase

Advanced techniques on Embase
Ovid is the world's leading information search, discovery, and management solution providing professionals in science, medicine, and healthcare all over the world with a single online destination for seamlessly accessing and working with premium online journals, books, and databases from the world's leading publishers.
Advanced Techniques on Embase

COMMENTS
The European Medicines Agency (EMA) is responsible for monitoring a number of substances and selected medical literature, to help identify suspected adverse reactions to medicines authorised in the European Union (EU). EMA also enters relevant information into the EudraVigilance database. Human Regulatory and procedural guidance Pharmacovigilance.
GLOBAL AND EUROPEAN PHARMACOVIGILANCE SERVICE PROVIDER. Literature Monitoring (MLM) ServiceMedical monitoring of literature and entry into Eudravigilance by the EMA of individual cases of Adverse Drug Reactions (ADRs) identified in the literature has been fully. perational since 01 September 2015. The monitoring includes active substances ...
The search string is reviewed and updated annually to optimize results. About the Author. Dr. Poonam Wagle; Associate Manager, Pharmacovigilance. Poonam brings expertise in literature management as a pharmacovigilance and safety expert with over eleven years of experience in the areas of ICSR, Literature, Aggregate Reports, and Signal Reviews.
The search of scientific literature in Pharmacovigilance (PV) is an activity of great importance within pharmacovigilance, as it allows the collection of valuable information on the safety and efficacy of drugs, in addition to complying with the applicable legal requirements (in Spain it includes Royal Decree 577/2013 and the European directive 2010/84/EU) and good pharmacovigilance practices ...
Guideline on good pharmacovigilance practices (GVP) Module VI - Collection, management and submission of reports of suspected ... - Updated guidance on the management of ICSRs described in the medical literature; ... Contracting out literature search services ..... 97 VI.App.2.10. Electronic submission of copies of articles on suspected ...
2.3. Search of scientific and medical literature that the Agency is monitoring GVP Module VI. 5 describes the principles for d atabase searches. For the substance groups outlined in chapter 2.1. the following applies: • A daily search of the biomedical reference database as described in chapter 2.2. Daily refers to
Pharmacovigilance in the Center for Drug Evaluation and Research (CDER) March 26, 2019 Kim Swank, PharmD. Division of Pharmacovigilance. ... literature search. Identify a safety signal.
Detecting safety signals attributed to a drug in scientific literature is a fundamental issue in pharmacovigilance. The constant increase in the volume of publications requires the automation of this tedious task, in order to find and extract relevant articles from the pack. This task is critical, as serious Adverse Drug Reactions (ADRs) still account for a large number of hospital admissions ...
As we discussed above, the goal of literature search and monitoring is to identify individual case safety reports and any possible changes to the benefit-risk profile of the substance being monitored, particularly about detecting new safety signals or emerging safety issues. Marketing authorization holders should perform medical literature ...
Keywords: Pharmacovigilance, software design, user-computer interface, data mining, translational research. Go to: 1. Background and Significance. Literature reports are highly relevant to the detection of drug-adverse event (ADE) safety 'signals' for drugs and biologic products [ 1 ]. The published literature complements the safety ...
Our Pharmacovigilance Literature Search Service offers a reliable and efficient solution for accessing, collecting, and interpreting safety-related information from the scientific literature to support pharmaceutical companies, regulatory agencies, and healthcare organizations in promoting patient safety in the pharmaceutical industry.
Despite these limitations, case reports published in journals are useful for generating hypotheses, and pharmacovigilance studies often start with a search of the relevant case literature. Medwatch has been the principal means of collecting and analyzing information about ADRs since 1993 and is used by the FDA to collect information on both ...
To underscore the importance of literature monitoring in pharmacovigilance, consider that properly conducted searches can lead to important discoveries, timely interventions, and overall improved product safety. On the other hand, inadequate searches can result in missed opportunities, overlooked risks, and in some cases, significant patient harm.
Global and local literature search is performed to identify the adverse reactions and for reporting to health authorities. APCER conducts the literature searches for products under investigational stage (in clinical trial phase), in pre-authorization stages, and post-marketing phase to meet the regulatory requirements of its clients.
Pharmacovigilance involves a substantial amount of literature monitoring. The process of devising a solid search strategy could be challenging but is essential. A professional with the required skills, experience, and training will ensure adverse event-related safety information is never missed.
When constructing a literature search strategy, it is important to define the purpose of the search. For example, a literature search designed purely to identify Individual Case Safety Reports (ICSRs) may not be suitable or broad enough to identify any articles that would be required for aggregate reports, such as the Development Safety Update ...
A Whitepaper on pharmacovigilance literature searches and optmizing search strategies for patient safety +44 (0)1462 440 084 ... Literature search strategy and how these should be constructed in such a way to ensure the optimum retrieval of the most appropriate articles;
As the Good pharmacovigilance practices (GVP) module VI states "medical literature is a significant source of information" for marketing authorization holders and has to be taken into consideration. Marketing authorization holders are expected to perform international and local literature searches to identify pharmacovigilance (PV) cases ...
Embase is the medical research database for high-quality, comprehensive evidence. Regulators around the world recognize Embase as a source for medical literature. Life science experts rely on Embase to find relevant and current results based on Emtree indexing of full-text content and dedicated search terms. Contact us.
Pharmacovigilance Literature Screening - recognized also as medical literature monitoring (mlm), surveillance or search - ... Most likely a combination of an AI-powered search tool and our inhouse Tepsivo Platform, automating both the screening time as well as the reporting side of the process.
Literature report is any adverse drug reactions reported in. 1. Published abstracts or. 2. Articles in medical/scientific journals. 3. Unpublished manuscripts involving case reports. 4. Important safety findings or clinical studies including posters, letters to the editors, and associated communication from scientific meetings.
Global and Local Literature Search Screening ... Our pharmacovigilance researchers are aware of the standard pharmacovigilance guidelines and regulations, including 21 CFR part 314.80 and 600.80 and basics of clinical development of a drug. In addition, our reports will be prepared based on ICH E2C (R2) PBRER and other reports (PFSB/SD 0917/2 ...
- OvidPV allows these users to quickly build a comprehensive and guided pharmacovigilance literature search in 5 easy steps! - The Ovid Platform's ... Ovid is the world's leading information search, discovery, and management solution providing professionals in science, medicine, and healthcare all over the world with a single online ...