Case Studies
Case study 1.
- Special school
Child’s name & age: AB, 12
Main areas of concern: Playing with private parts, poking bottom, smearing faeces, refusal to have hair cut, refusal to go to dentist

Case study 2
- Mainstream primary
Child’s name & age: CD, 5
Main areas of concern: Does not stay in seat, cannot complete work, very rough with classmates, refuses to sit for circle time, occasionally hits and bites staff
Case study 3
- Mainstream post-primary
Child’s name & age: EF, 13
Main areas of concern: Inconsistent attendance at school, increasing incidence of school refusal, minimal friendships, refusing to leave house
Case study 4
Child’s name & age: GH, 9
Main areas of concern: Biting his hand, banging his head, hitting and biting staff, high frequency of repetitive behaviours (pacing, flapping hands in front of eyes), eating non-food items
Case study 5
Child’s name & age: IJ, 16
Main areas of concern: Sensory over-responsive, Aggressive and negative outbursts during transport to and from School and in the classroom
Case study 6
Child’s name & age: KL, 13
Main areas of concern: Selective mutism in school, refusal to complete work; and to participate in class and sometimes to sit in class. Sensory over responsive, particularly with tactile and auditory input.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Autism Spectrum Disorder: A case study of Mikey
This paper describes Autism Spectrum Disorder (ASD) including diagnostic criteria, suspected causes, prevalence, comorbidities, and influences on client factors. A hypothetical case study is presented to give readers an illustration of what someone with ASD might look like. Possible treatment based on evidence and selected frame of references will be given for the hypothetical client. This paper is not all inclusive of the role of occupational therapy in the treatment of Autism Spectrum Disorder, but gives an illustrative example.
Related Papers
Jennelyn Pondang
Autism Spectrum Disorder - Recent Advances
IOSR Journals
This article aims to observe all the manifestations of the behavior of a child with Autism Spectrum Disorder (ASD), which shows deficits mainly in the communication sector. Also, the child shows repetitive and stereotypical behaviors throughout the lesson (Stasinos, 2016). Initially the paper describes the methodology followed. It then describes the child's cognitive profile and the deficits he presents. He then analyzes the intervention that was applied in order to improve the difficulties he faces and to further strengthen the skills he has already acquired. Finally, the paper presents the main conclusions as they emerged from the intervention.
Function and Disability Journal
Seyed Hassan Saneii
Melissa Vandiver Phelan
American Journal of Occupational Therapy
Renee Watling
Occupational therapy has much to offer to families of people with autism spectrum disorder (ASD). However, people outside the profession may be unaware of occupational therapy’s breadth and scope. It is our responsibility and our duty to express the full range of occupational therapy services through research, clinical practice, advocacy, and consumer education. This special issue of the American Journal of Occupational Therapy, with its focus on autism, embarks on this endeavor by highlighting research and theoretical articles that address the various aspects of occupational therapy practice that can help to fully meet the needs of people with ASD and their families.
IP International Journal of Medical Paediatrics and Oncology
Autism spectrum disorder encompasses a wide range of neurodevelopment disabilities which affect children and their families across all sections of the society both in rural and urban settings. The prevalence of autism is rising irrespective of the socioeconomic background of the children. Hence every health worker has to be aware of ways to suspect and diagnose this condition and decide the appropriate treatment. Earliest intervention in autism spectrum disorder gives better results due to neuroplasticity. This article is targeted to help Medical officers, auxiliary nurse midwifes, anganwadi workers and other peripheral health workers by providing information on basics of ASD, normal speech development, simple ways for diagnosis and treatment for the same.
The American Journal of Occupational Therapy
Objective. The purpose of this study was to examine the current practice patterns of occupational therapists experienced in working with children with autism spectrum disorders. Method. Occupational therapists experienced in providing services to 2-year-old to 12-year-old children with autism completed a mail questionnaire describing practice patterns, theoretical approaches, intervention techniques, and preferred methods of preparation for work with children with autism. Results. Of those contacted, 72 occupational therapists met the study criteria and returned completed questionnaires. Practice patterns included frequent collaboration with other professionals during assessment and intervention. Intervention services were typically provided in a one-to-one format with the most common techniques being sensory integration (99%) and positive reinforcement (93%). Theoretical approaches included sensory integration (99%), developmental (88%), and behavioral (73%). Evaluations relied hea...
The American journal of occupational therapy : official publication of the American Occupational Therapy Association
Kristie P Koenig
Evidence Connection articles provide a clinical application of systematic reviews developed in conjunction with the American Occupational Therapy Association's (AOTA's) Evidence-Based Practice Project. In this Evidence Connection article, we describe a case report of an adolescent with autism spectrum disorder. The occupational therapy assessment and treatment processes for school, home, community, and transition settings are described. Findings from the systematic reviews on this topic were published in the September/October 2015 issue of the American Journal of Occupational Therapy and in AOTA's Occupational Therapy Practice Guidelines for Individuals With Autism Spectrum Disorder. Each article in this series summarizes the evidence from the published reviews on a given topic and presents an application of the evidence to a related clinical case. Evidence Connection articles illustrate how the research evidence from the reviews can be used to inform and guide clinical ...
Javiera Poblete
Autism spectrum disorder is a term used to describe a constellation of early-appearing social communication deficits and repetitive sensory-motor behaviours associated with a strong genetic component as well as other causes. The outlook for many individuals with autism spectrum disorder today is brighter than it was 50 years ago; more people with the condition are able to speak, read, and live in the community rather than in institutions, and some will be largely free from symptoms of the disorder by adulthood. Nevertheless, most individuals will not work full-time or live independently. Genetics and neuroscience have identified intriguing patterns of risk, but without much practical benefit yet. Considerable work is still needed to understand how and when behavioural and medical treatments can be effective, and for which children, including those with substantial comorbidities. It is also important to implement what we already know and develop services for adults with autism spectrum disorder. Clinicians can make a difference by providing timely and individualised help to families navigating referrals and access to community support systems, by providing accurate information despite often unfiltered media input, and by anticipating transitions such as family changes and school entry and leaving.
RELATED PAPERS
2010 5th IEEE International Conference on Global Software Engineering
Geophysical Journal International
Goetz Bokelmann
Understanding the Earth System
Sarah Cornell
The Scottish Historical Review
Coleman Dennehy
OMBUDSMAN AKADEMİK • ÖZEL SAYI 2 /SPECIAL ISSUE 2, GAZZE
Osman Ulker
Journal of Neurochemistry
Diego Ruano
Journal of Materials Science: Materials in Electronics
Udai P Singh
Annales Universitatis Mariae Curie-Skłodowska, sectio K – Politologia
Dorota Litwin-Lewandowska
Physical Chemistry Chemical Physics
Thomas Buergi
Motricidade
Estelio H. M. Dantas
Organised Sound
Daniel TERUGGI
Scientific Reports
Gautam Talukdar
bubur bawain
The Journal of Japanese Society of Stomatognathic Function
Kazuo Okura
Turkiye Aile Hekimligi Dergisi
Meryem Gencer
Environmental Engineering Science
Wei-Ning Wang
Health education & behavior : the official publication of the Society for Public Health Education
Devan Romero
Journal for ImmunoTherapy of Cancer
David Quinn
Yuangga Kurnia yahya
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Book Appointment
- Autism Treatment
- Behavior Problems
- Mental Retardation
- Parental Guidance
- Cerebral Palsy
- Learning Disabilities
- Poor Memory
- Down Syndrome
- Sensory Processing Disorder
- Testimonials
A Case Study of Autism: Paul, 3 Years Old
- Autism Blog
- A Case Study of Autism:…
Arun was brought for consultation with Dr. A M Reddy by his parents. He was about 4 years old, the second child to the parents. Even while he was being brought into the room, we could hear his loud wailing. It took some time for the child to calm down and later we could observe that the child was very restless. He was running around the room, pulling down cushions and generally creating chaos in the room and mother was quite harried in trying to control him. He was diagnosed with ASD (Autism Spectrum Disorder).
What is ASD?
Autism or Mutinism as it was earlier known was thought primarily to affect communication skills but with more studies, it was understood that autistic children display a wide range of symptoms, hence the word “spectrum” was added to Autism disorder. Autism is a complex neurodevelopmental disorder which affects a person’s social behavior and communication skills.
Why it occurs?
The exact reason why ASD occurs is not known but many risk factors have been identified like age of the parents, poor ovulation, infections or exposures to harmful chemicals or radiation during pregnancy, thyroid, diabetes type of hormonal disorders, birth injuries, infections in childhood, vaccinations, etc.
What are its symptoms?
As its name suggests, ASD displays a myriad of symptoms but some of the common symptoms of ASD is lack of speech. While some children have no speech, in some children speech that was developed before may regress. Many of them do not prefer to mingle with children of their age group. Repetitive action, physical restlessness, inability to understand emotions, mood swings like sudden bouts of excitement, crying without any reason, are few symptoms displayed by many autistic children.

Aggressive behaviors like self-harming, head-banging, tantrum-throwing, biting/pushing others, destructiveness, can be displayed by few. Response to name call, having sustained eye contact, unable to understand commands, stereotypical actions and stimming are some of the common symptoms exhibited by many.
Coming back to the case of Arun, a detailed case history was noted down by our doctors, a summary of which is given below.
He is the second child and the age difference between both the siblings is seven years. After the first child was born, the mother developed hypothyroidism for which she was on thyroxine 50 mcg daily tablets. No history of abortions or contraceptive use was reported. Father was apparently healthy. The age of the parents was 35 and 38 years respectively during conception. She conceived naturally and pregnancy was apparently uneventful. But on deeper probing few differences were found out between both the pregnancies.
While during the first pregnancy the parents were in India, but during second there were in the United States. She was advised to continue with the same dosage of thyroxine and during 6-7 months of the pregnancy, she was given flu and T Dap vaccine. The child was born of emergency C – section as the water broke early. The birth cry was normal and seemingly the child was progressing well but after his first birthday, the child had a bout of severe gastrointestinal infection when they visited India where he was hospitalized for three days and given medicines.

Parents were worried that he seems to put everything in his mouth and his favorite items were paper, cloth, wall plaster. His demands have to be met, else he used to become very upset. Emotional connectivity towards parents was less. He would not follow simple commands and it was becoming increasingly difficult for the parents to manage him. With therapies, his eye contact improved a little and was able to follow a few simple commands but the progress was slow.
He was a picky eater and liked crunchy foods. His bowels were constipated and he was not yet toilet trained. He was given Cuprum Sulph 10 M and was kept on regular follow up.
On the next visit to Dr. A M Reddy Autism Center , the parents complained that their child developed itching on the skin but his restlessness reduced slightly. The medication was continued for about three months during which the child’s anger reduced by 30%, his eye contact improved and he was no longer constipated. His itching too reduced in the meanwhile. A second dose was repeated and about six to seven months of treatment, he started saying few words, tantrum-throwing reduced and his habit of putting everything in the mouth was gone.
The dose was repeated in 50M potency. After about a year and half of treatment, he started interactive communication, giving relevant answers to questions and was doing much better. On the advice of Dr. A M Reddy, they placed him in normal school and he is doing well.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
To read this content please select one of the options below:
Please note you do not have access to teaching notes, being diagnosed with autism in adulthood: a personal case study.
Advances in Autism
ISSN : 2056-3868
Article publication date: 19 June 2020
Issue publication date: 11 August 2021
This paper aims to report the personal experiences of an adult male diagnosed with autism at the age of 48 years.
Design/methodology/approach
A personal case study methodology was used to illustrate the journey to autism diagnosis, the experience of diagnosis and post-diagnosis support.
This case study illustrates how stress and mental health difficulties can precede autism diagnosis in adults. The personal experiences detailed highlight how an adult autism diagnosis can bring about positive change, prompting increased self-knowledge and coping skills, improved relationships and. Furthermore, it highlights how a supportive employer can make reasonable adjustments in the workplace to improve productivity of an autistic employee.
Research limitations/implications
This case study has implications for various practice issues, including post-diagnosis counselling and access to support for autistic adults nationally.
Originality/value
This paper provides an original case study highlighting the personal experiences of an adult diagnosed with autism.
- Mental health
- Autism spectrum condition
- Mental disorder
Henley, R. (2021), "Being diagnosed with autism in adulthood: a personal case study", Advances in Autism , Vol. 7 No. 3, pp. 256-261. https://doi.org/10.1108/AIA-03-2020-0018
Emerald Publishing Limited
Copyright © 2020, Emerald Publishing Limited
Related articles
We’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here
Autism Spectrum Disorder in a Child Case Study
Medical history and background information, developmental domains, works cited.
M. is a seven years old boy diagnosed with autism spectrum disorder at the age of two. He lives in Orlando, Florida with his mother and father and two other younger siblings. Patient’s physical development is within the norm; he is 43’’in tall and weighs 60 lbs. M. was born prematurely at 36 weeks through an uncomplicated vaginal delivery. Around the age of two, his parents became increasingly concerned about his lack of response to other people (Masi et al. 186).
Moreover, M. was not eager to maintain eye contact with anyone nor he shifted his gaze towards objects shown to him (Masi et al. 186). M. started primary at the age of seven and has been receiving special early childhood education. Other than ASD, M. does not suffer from any diseases or disorders safe for occasional seasonal colds and flu.
Generally, cognitive skills in children with autism vary greatly on the case to case basis (Soorya et al. 211). M. was medically recognized as a high-functioning individual on the autism spectrum; he is verbal even though he has certain struggles with language use and acquisition. At school, his education success depends on the level of engagement with the subject matter. So far, M. has discovered his inclination to numbers and simple calculations and showed the ability to retain information in his field of interest.
Communication
M.’s parents made sure that their son was involved in normal situations and never felt excluded because he was different (Gargiulo and Kilgo 160). Studies show that familial love and support help children with ASD handle their symptoms better (Woodman et al. 122). At the moment, M. is more verbal with his parents and siblings and reacts adequately to them, especially in repeated day-to-day situations. However, when put in an unfamiliar setting, M. tends to shut down and become unresponsive.
Social-emotional
Children with autism might express emotions differently as compared to their neurotypical peers (Kret and Ploeger 160). M. is handling his feelings relatively well, especially in social situations. However, his parents report cases of him having meltdowns with tears more characteristic of younger kids. The most challenging event in the social-emotional developmental domain was M.’s enrollment to primary education. When confronted with new situations, patient used to have fight-or-flight response – he became aggressive or escaped the setting altogether. As of now, parents state that his emotional health has improved.
Physical and Adaptive Development
M.’s physical development was assessed as normal – he has appropriate height and weight for his age. However, patient displays slight delays in coordination and fine motoric skills, for instance, when it comes to writing or drawing. For all the challenges, the prognosis is rather positive: the parents report improvements due to his adaptation to the school setting. In class, M. recognizes his peers, responds when talked to, and initiates contact on rare occasions. Moreover, he is less emotional about changes in daily routines even though it still takes time for him to process such information.
Gargiulo, Richard, and Jennifer L. Kilgo. An Introduction to Young Children with Special Needs: Birth through Age Eight . Nelson Education, 2010.
Kret, Mariska E., and Annemie Ploeger. “Emotion Processing Deficits: A Liability Spectrum Providing Insight into Comorbidity of Mental Disorders.” Neuroscience & Biobehavioral Reviews, vol. 52, 2015, pp. 153-171.
Masi, Anne, et al. “An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options.” Neuroscience Bulletin, vol. 33, no. 2, 2017, pp. 183-193.
Soorya, Latha V., et al. “Randomized Comparative Trial of a Social Cognitive Skills Group for Children with Autism Spectrum Disorder.” Journal of the American Academy of Child & Adolescent Psychiatry, vol. 54, no. 3, 2015, pp. 208-216.
Woodman, Ashley C., et al. “Change in Autism Symptoms and Maladaptive Behaviors in Adolescence and Adulthood: The Role of Positive Family Processes.” Journal of Autism and Developmental Disorders , vol. 45, no. 1, 2015, pp. 111-126.
- Autism Spectrum Disorder Diagnosis
- Teenagers With Autism Disorder
- Autism Spectrum Disorder
- Workshop: Understanding Child Special Concerns
- Communicative Skills' Development of Children with Problems
- Inclusive Education and Inquiry-Based Learning in Saudi Arabia
- "Emergence: Labeled Autistic" by Temple Grandin
- Special Classroom Environment and Dynamics
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2021, June 9). Autism Spectrum Disorder in a Child. https://ivypanda.com/essays/a-child-with-autism-spectrum-disorder-case-study/
"Autism Spectrum Disorder in a Child." IvyPanda , 9 June 2021, ivypanda.com/essays/a-child-with-autism-spectrum-disorder-case-study/.
IvyPanda . (2021) 'Autism Spectrum Disorder in a Child'. 9 June.
IvyPanda . 2021. "Autism Spectrum Disorder in a Child." June 9, 2021. https://ivypanda.com/essays/a-child-with-autism-spectrum-disorder-case-study/.
1. IvyPanda . "Autism Spectrum Disorder in a Child." June 9, 2021. https://ivypanda.com/essays/a-child-with-autism-spectrum-disorder-case-study/.
Bibliography
IvyPanda . "Autism Spectrum Disorder in a Child." June 9, 2021. https://ivypanda.com/essays/a-child-with-autism-spectrum-disorder-case-study/.
- Case report
- Open access
- Published: 22 September 2020
A pediatric patient with autism spectrum disorder and epilepsy using cannabinoid extracts as complementary therapy: a case report
- Juliana Andrea Ponton ORCID: orcid.org/0000-0002-7405-1797 1 ,
- Kim Smyth 2 ,
- Elias Soumbasis 1 ,
- Sergio Andres Llanos 1 ,
- Mark Lewis 1 ,
- Wilhelm August Meerholz 1 &
- Robert Lawrence Tanguay 1
Journal of Medical Case Reports volume 14 , Article number: 162 ( 2020 ) Cite this article
10k Accesses
10 Citations
59 Altmetric
Metrics details
The pharmacological treatment for autism spectrum disorders is often poorly tolerated and has traditionally targeted associated conditions, with limited benefit for the core social deficits. We describe the novel use of a cannabidiol-based extract that incidentally improved core social deficits and overall functioning in a patient with autism spectrum disorder, at a lower dose than has been previously reported in autism spectrum disorder.
Case presentation
The parents of a 15-year-old boy, of South African descent, with autism spectrum disorder, selective mutism, anxiety, and controlled epilepsy, consulted a medical cannabis physician to trial cannabis extract to replace seizure medications. Incidentally, at a very low cannabidiol-based extract dose, he experienced unanticipated positive effects on behavioral symptoms and core social deficits.
This case report provides evidence that a lower than previously reported dose of a phytocannabinoid in the form of a cannabidiol-based extract may be capable of aiding in autism spectrum disorder-related behavioral symptoms, core social communication abilities, and comorbid anxiety, sleep difficulties, and weight control. Further research is needed to elucidate the clinical role and underlying biological mechanisms of action of cannabidiol-based extract in patients with autism spectrum disorder.
Peer Review reports
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that is characterized by deficits in two major domains: restrictive, repetitive patterns of behavior, interests, or activities; and deficits in social communication and interaction [ 1 , 2 ]. ASD is associated with a higher incidence of comorbid conditions including attention deficit hyperactivity disorder, anxiety, gastrointestinal disturbances, motor impairments, and epilepsy. Symptoms appear in early childhood and vary in severity leading to a broad range of clinical manifestations [ 2 ].
The pathogenesis of ASD is not completely understood [ 3 ]. Given its complexity and diverse clinical manifestations, it is believed that the etiopathogenesis of ASD is a combination of genetic, epigenetic, neurobiological, diet, and other environmental factors [ 4 ]. Hundreds of genes ( NLGN , SHANK3 , ZNF8034A, and UNC13A) [ 5 , 6 ] have been linked to ASD, most of which are closely related to the development of the nervous system [ 1 ].
There is a myriad of theories that attempt to explain the occurrence of ASD [ 1 , 3 , 7 ], although the two most accepted are impaired synaptic transmission and disruption of neural connectivity. The endocannabinoid system (ECS) has attracted considerable attention as a potential contributor to ASD, as the development of the ECS is essential for regulating synaptic function by inhibiting the release of neurotransmitters from presynaptic neurons [ 1 ].
The management of ASD requires individualized, comprehensive treatment. Non-psychopharmacologic interventions (for example, cognitive behavioral therapy) modify disruptive behaviors and improve social communication skills with varying degrees of success. Traditional psychopharmacologic medications target specific ASD core behaviors (for example, repetitive behaviors) and associated behaviors (for example, hyperactivity, aggression, anxiety, and sleep disturbances), but do not treat core social communication deficits [ 8 , 9 ]. These medications are well known for their substantial side effects. For example, aripiprazole and risperidone, the only two medications approved by the US Food and Drug Administration (FDA) to treat irritability and agitation in ASD, frequently cause somnolence, increased appetite, and weight gain [ 10 ]. No other medication has been approved for management of behavioral and/or core ASD symptoms. Challenges with these traditional treatment approaches include barriers to access (economical, geographic), lack of efficacy, and undesirable side effects, which have led many families to seek complementary and alternative medicine (CAM) to augment or replace standard therapy [ 8 ]. One of the newest CAM options now being explored in ASD (and, in fact, the wider medical community) is cannabinoids: for example, cannabidiol-based extract (CBE), which is an extract from the cannabis plant, rich in cannabidiol (CBD) [ 11 ].
Follow-up of these patients must also be individualized as presentation of the disorder is highly variable. There are no validated questionnaires to accurately assess clinical progress, therefore, conducting an objective clinical assessment of related behavioral and core symptoms is challenging. Despite this, there are tools available for characterizing the overall functionality of patients with ASD, for example, Autism Spectrum Quotient (AQ) adults version [ 3 ].
The World Health Organization stated that CBD should not be scheduled with the International Drug Control Conventions because of growing evidence of its medicinal applications [ 12 , 13 ]. It is imperative for health care providers to understand the minutiae of how cannabinoids interact with the human body and the different forms of cannabinoids that are available for medical use (for example, synthetocannabinoids, phytocannabinoids) [ 1 ]. Delta-9-tetrahydrocannabinol (THC) and CBD are the most well-known and studied phytocannabinoids. THC is associated with the impairing psychoactive effects of cannabis, resulting in potentially undesirable side effects (dizziness, anxiety, paranoia, dependency, cognitive impairment, and so on). In contrast, CBD is only minimally psychoactive and not impairing or intoxicating at typically used doses (for example, ≥ 20 mg/kg of CBD referred in the majority of intractable seizures studies) [ 1 , 8 , 10 , 11 ].
A multitude of studies have analyzed the use of high-dose CBD extract (~ 20 mg/kg of weight per dose) in the context of intractable seizure treatment [ 14 , 15 ]. It has been reported that CBD effects are dose-dependent (for example, > 160 mg/day elicits a sedating effect and lower doses have been associated with increased wakefulness) [ 16 ]. A few case reports and observational studies have suggested the safety and efficacy of lower dose CBD, for treating behavioral symptoms in ASD [ 11 , 17 , 18 ]. In a prospective study, 188 patients with ASD were treated with lower to medium doses of phytocannabinoids (from 15 mg of CBD three times a day to 300 mg of CBD three times a day), the majority taking 1:20 CBE: 30% CBD to 1.5% THC [ 19 ]. This study found that cannabis was well tolerated, safe, and effective in relieving certain ASD symptoms. More research is needed to assess the long-term effects of CBD, as well as optimal dosing, formulation, delivery method, and so on to maximize both safety and efficacy.
This case report describes the clinical presentation of a pediatric, overweight patient with ASD, epilepsy, anxiety, insomnia, and social deficits who benefited clinically with even lower doses of CBE (4 mg of CBD and 0.2 mg of THC twice a day) compared to the ones already studied [ 19 ].
A 15-year-old boy, of South African descent, is presented with a long-standing history of social and communicative challenges dating back to early childhood, including difficulties in appropriate use of facial expressions, eye contact, and gestures to regulate social interaction (see Fig. 1 for patient’s timeline). He has a history of difficulty in establishing and maintaining relationships, although he has been able to establish some friendships. His mother notes a history of selective mutism dating back to age 3. He has areas of fixated interests and some ritualized behaviors that on assessment were below the threshold for a diagnosis of obsessive-compulsive disorder. In 2016, he was formally diagnosed as having ASD by a specialized organization in British Columbia (BC), the Interior Health Children’s Assessment Network (IHCAN), with supporting evidence from Autism Diagnostic Interview – Revised (ADI-R) and the Autism Diagnostic Observation Schedule 2 (ADOS-2). He does well academically and there are no cognitive concerns. Sometimes he shows aggressive behaviors towards his mother and other relatives.
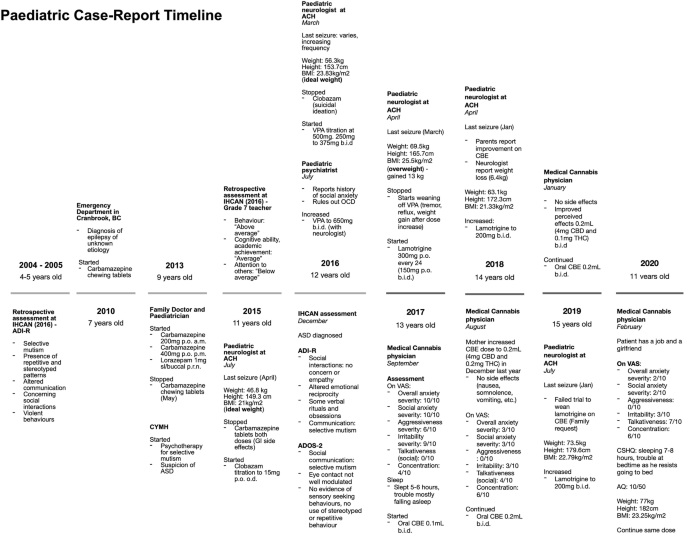
Patient’s timeline depicting important dates and events. ACH Alberta Children’s Hospital, ADI-R Autism Diagnostic Interview – Revised, ADOS-2 Autism Diagnostic Observation Schedule 2, ASD autism spectrum disorder, AQ Autism Spectrum Quotient (Adult), BC British Columbia, BMI body mass index (calculated by Du Bois method), CBD cannabidiol, CBE cannabidiol-based extract, CSHQ Children’s Sleep Habits Questionnaire (Abbreviated), CYMH Child and Youth Mental Health, IHCAN Interior Health Children’s Assessment Network, OCD obsessive-compulsive disorder, THC delta-9-tetrahydrocannabinol, upset stomach gastrointestinal side effects, VAS visual analog scale, VPA valproic acid. VAS severity for overall anxiety, social anxiety, aggressiveness and irritability, 0 = least severe, 10 = most severe. VAS for talkativeness, 0 = quiet, 10 = very talkative. VAS for focus, 0 = unfocused, 10 = focused
He was diagnosed as having epilepsy characterized by focal seizures at age 7 at an emergency department service in BC and was subsequently treated by his pediatrician and a pediatric neurologist at the Alberta Children’s Hospital (ACH). He was initially prescribed carbamazepine for seizures which was stopped in 2015 due to side effects (upset stomach), followed by clobazam (stopped in 2016 due to suicidal ideation) and valproic acid (VPA) (stopped in 2017 due to alopecia, tremor, and reflux). The latter also caused a significant weight gain of approximately 13 kg in 1 year, resulting in a calculated body mass index (BMI) with the Du Bois method of 25.5 kg/m 2 . He is currently on lamotrigine for seizures, lorazepam for breakthrough seizures, melatonin for insomnia, riboflavin, ranitidine, magnesium, and orally administered CBE 0.2 mL (4 mg of CBD and 0.1 mg twice a day). No therapy had been tried for behavioral symptoms, although his mother mentioned that VPA and lamotrigine were also prescribed for their effect on mood.
He is currently in psychotherapy at the Child and Youth Mental Health (CYMH) clinic in BC for his selective mutism and anxiety disorder diagnosed by psychiatrists in the same province. He has also had sleep difficulties since 2016. His perinatal history is unremarkable. His birth followed a full-term pregnancy and was uncomplicated except for a required caesarean section due to macrosomia (> 4000 g) and macrocephaly (his mother does not remember the measurement), and subsequent hospitalization for neonatal jaundice. No genetic syndrome was suspected; no genetic testing was ever done. He met expected neurodevelopmental milestones for his age. His mother and grandmother have a history of depression and anxiety. There is other familial history of eating disorders and alcoholism, but no history of genetic syndromes.
In mid-2017, his parents consulted a medical cannabis physician from Caleo Health to assess the suitability of cannabis-based medicines as adjunctive or replacement therapy for seizures. At the time, a physical examination and laboratory findings were normal. A neurologic examination was unremarkable; mental status – awake, alert, cooperative; cranial nerves – normal; motor – normal tone, bulk, strength, and reflexes in upper and lower extremities, proximal and distal, deep tendon reflexes 2+ symmetric. A skin examination was unremarkable, there were no hypopigmented macules, café-au-lait macules, neurofibromas, or axillae ephelides. A long-term (48-hour) electroencephalogram done in 2016 did not record any epileptogenic potentials; magnetic resonance imaging (MRI) showed no intracranial abnormalities and a computed tomography (CT) scan of his head was normal (2015). Laboratory results done mid-2017 were normal: complete blood count and differential, vitamin B12, creatinine, sodium, potassium, calcium, magnesium, total bilirubin, albumin, alkaline phosphatase, aspartate aminotransferase, gamma-glutamyl transferase, alanine aminotransferase, and triacylglycerol lipase. He had low ferritin (11 μg/L) but normal hemoglobin (159 g/L), due to starting a vegetarian diet, which was followed up by the family physician.
He had not had a clinical seizure in 6 months (last seizure in March 2017). Medications at the time of initiation of CBE were: lamotrigine 200 mg twice a day for seizures, lorazepam 1 mg sublingual (SL)/buccal as necessary, infrequently used for seizures, melatonin 6 mg for sleep initiation, riboflavin 400 mg administered orally once daily, magnesium 1 tablet administered orally once daily, and ranitidine 150 mg twice a day. When asked for symptom severity on a visual analog scale (VAS) (0 = least severe, 10 = most severe), his mother reported overall anxiety, social anxiety, aggressiveness, and irritability severity, at 10/10, 10/10, 6/10, and 9/10, respectively. On VAS to assess for talkativeness (0 = quiet, 10 = very talkative) in social situations, the mother reported 0/10. On VAS for concentration (0 = unfocused, 10 = very focused), 4/10 was reported. In regards to sleep, the mother stated he was sleeping approximately 5 to 6 hours, and was having trouble falling asleep. After the initial assessment, his parents gave consent to start therapy and CBE was prescribed (60 mL bottle of 1:20 CBE – 0.001% THC and 0.02% CBD), from CanniMed, with an olive oil carrier. His parents were instructed to administer 0.1 mL twice a day (2 mg CBD and 0.1 mg THC) and increase by 0.1 mL (2 mg CBD and 0.1 mg THC) per dose if no effects were shown to a maximum of 0.5 mL (10 mg CBD and 0.5 mg THC) per dose.
In December 2017, after 3 months of the CBE prescription, his mother increased the dose to 0.2 mL twice a day (4 mg CBD and 0.2 mg THC) as the family noted only mild improvements in anxiety symptoms. In August 2018, a medical cannabis follow-up was conducted. At 0.2 mL twice a day for almost 9 months, our patient’s family reported an improvement of 7 points for overall and social anxiety and irritability, and 6 points on aggressiveness on their respective VAS. Talkativeness improved by 4 points and focus by 2 points. In February 2020, another medical cannabis follow-up was conducted and positive effects were still evident at the same dose. When the mother was asked to complete the Children’s Sleep Habits Questionnaire (CSHQ) Abbreviated, she stated that he slept 7 hours and only had trouble falling asleep in his own bed as he resists going to bed. No side effects were reported (nausea/vomiting, diarrhea, headaches, euphoria, feeling high, anxiety, panic attacks, palpitations, somnolence during the day or drowsiness). Laboratory results remained normal and low ferritin was corrected. He began to initiate and reciprocate conversations with acquaintances he had previously been unable to speak to (for example, doctors, community members). He became more motivated and energetic, starting his own vegetarian diet and exercise programs, ultimately losing 6.4 kg after starting CBE for a calculated BMI of 21.33 kg/m 2 . He was able to start his first part-time job helping customers and interacting with them. He was instructed to fill out the self-administered Adult AQ which resulted in a normal score of 10 as shown in Table 1 . His mother stated he now also has a girlfriend. Recently, his mother started weaning him off CBE to go on a trip and noted an immediate change. He became more irritable and aggressive.
In discussion with their neurologist, the family decided to wean lamotrigine while remaining on CBE (0.2 mL twice a day – 4 mg CBD and 0.2 mg THC). Unfortunately, there was a recurrence of seizures and lamotrigine was titrated back to the full 200 mg twice a day dose.
Currently, our patient remains on the same medication as mentioned above, as well as low dose of CBE. He has maintained the positive effect on his behavioral symptoms, anxiety, sleep, and social deficits on CBE 1:20 ratio, 0.2 mL twice a day (4 mg CBD and 0.2 mg THC) and no side effects have been reported.
This case demonstrates the benefit of a lower than previously studied CBE dose for core social communicative and behavioral ASD symptoms, as well as improvements in co-occurring anxiety, sleep dysregulation, and weight, which led to substantial improvements in both our patient’s and his family’s quality of life and daily functioning. There was partial response at the initial dose of 0.1 mL twice a day (2 mg CBD and 0.1 mg THC) and a dramatic response at 0.2 mL twice a day (4 mg CBD and 0.2 mg THC). Seizures recurred when conventional anti-epileptic medication (lamotrigine) was weaned while on the CBE at the 0.1 mL twice a day dose (low doses), reiterating CBE at this dose did not have any significant anti-epileptic effect; lamotrigine has not been weaned while on the 0.2 mL twice a day (4 mg CBD and 0.2 mg THC) dose of CBE. Typically, significant higher CBD doses are needed for seizure control (> 20 mg/kg per day) [ 14 , 15 ].
The symptom improvement occurred within a 6-month period following the initiation of CBE treatment, during which time there were no new additions or significant alterations of/to any concurrent medications or therapies that could otherwise explain the improvements in symptomatology. It remains unclear whether the CBE directly modified the core ASD symptoms in some way, or whether the impact of CBE was secondary to its positive effects on comorbid conditions, namely anxiety and/or sleep dysregulation, which were producing or exacerbating underlying ASD behaviors. We must also consider there are limitations inherent in the method used to assess his clinical improvement, as the VAS and the AQ are not yet validated. These measures were chosen by default, as no scale is currently validated to assess clinical progress [ 3 ]. Seizures recurred at the initial 0.1 mL twice a day (2 mg CBD and 0.1 mg THC) dose of CBE. In addition to the fact that seizures were well controlled prior to starting CBE, the recurrence of seizures on the initial 0.1 mL twice a day dose of CBE, a dose at which symptoms were already starting to improve, suggests that improvements in ASD symptoms were not related to improvements in epilepsy control; the anti-seizure properties of CBD alone are unlikely to be the predominant mechanism responsible for the improvements in this patient’s ASD symptoms.
The ECS is a unique biological system that is present in the majority of body tissues. It plays an important role in cellular processes at the early stages of development [ 21 ]. The ECS is an essential regulatory system of the central nervous system that modulates both neurotransmission and synaptic plasticity. It is also involved in emotional and social functioning, and cognition [ 1 , 21 ]. There is evidence that the ECS is underdeveloped in ASD [ 1 , 22 ]. CBD may be treating core symptoms in ASD by interacting with the ECS to boost function in one way. CBD may increase the availability of the endogenous cannabinoids, anandamide (AEA), by directly inhibiting one of its degrading enzymes, that is, fatty amide acid hydrolase (FAAH) [ 1 , 23 , 24 ]. Wei et al. demonstrated that selective inhibition of FAAH in BTBR animals, increased AEA activity [ 25 ]. Further to this, a case–control study by Karhson et al . assessed AEA concentrations in ASD ( n = 59) versus controls, and found lower AEA concentrations associated with ASD [ 26 ].
High-dose CBD has been studied for seizures and has been approved by the FDA (Epidiolex) for the treatment of intractable epilepsy [ 14 , 15 , 27 , 28 ], but there remains a lack of evidence for the use of phytocannabinoids in ASD [ 11 ]. Only a few low-powered studies address the clinical efficacy of cannabinoids for such symptoms, and there are no established recommendations for its use in ASD treatment [ 5 , 6 ]. The majority of published studies for ASD either involve synthetic cannabinoids [ 11 , 17 , 18 ] or synthetic enzyme-inhibitors [ 25 , 29 ]. Only a few studies offer evidence for the use of phytocannabinoids in ASD. An observational study by Bar-Lev Schleider et al. provided valuable information on safety and efficacy, but the study design was insufficient to draw strong conclusions on standard clinical care [ 19 ]. Clinicaltrials.gov lists an ongoing randomized trial comparing different phytocannabinoid extracts in the setting of behavioral symptoms, but results are not yet available [ 30 ]. Therefore, this case report is rare as it documents observed effects of CBE in ASD-related symptoms as opposed to other forms of cannabinoids (for example, nabilone, dronabinol, and nabiximols).
From a clinical perspective, the use of CBD-based products to treat neuropsychiatric symptoms must be done only after appropriate education and informed discussion with families, including consideration of risks and benefits of CBD compared to other available treatment options, and with vigilant monitoring.
Research into the role of cannabinoids in treating ASD symptoms and associated behaviors is in its infancy. Although there is an increasing amount of evidence providing biological plausibility for the use of CBD in treating ASD [ 1 , 5 , 25 , 26 , 31 ], further research is essential to better understand the effects of phytocannabinoids on neurobiological pathways and their impact on behavior and brain function. Rigorous, controlled clinical trials are needed to further establish safety, especially long-term safety, optimal dosing, and efficacy, including further delineation of the effect of CBE on core versus associated ASD symptoms. Until sufficient, supportive evidence is found, CBE remains an unproven alternative treatment and should not replace conventional evidence-based treatments for children with autism. However, the unexpected and significant benefits of CBE in this case report highlight the urgent need and potential benefits of continuing to pursue research in this area.
While there is a lack of strong evidence to support the use of CBE in ASD, this case report provides the first insight about lower than previously reported doses of phytocannabinoids in the form of CBE, which may benefit ASD-related behavioral and core social symptoms, as well as anxiety, sleep disturbances, and weight. We encourage scientists and clinicians to pioneer placebo-controlled studies to validate the clinical efficacy of very low doses of CBE in a larger cohort.
Availability of data and materials
The dataset generated and analyzed during this case report are available in Netcare (Alberta’s public Electronic Health Record used to store patient information).
Abbreviations
Autism spectrum disorder
Endocannabinoid system
Food and Drug Administration
Complementary and alternative medicine
Cannabidiol
Computed tomography
Child and Youth Mental Health
Fatty amide acid hydrolase
Delta-9-tetrahydrocannabinol
Cannabidiol-based extract
Interior Health Children’s Assessment Network
Magnetic resonance imaging
Autism Diagnostic Interview – Revised
Autism Diagnostic Observation Schedule 2
Alberta Children’s Hospital
Valproic acid
Body mass index
British Columbia
Visual analog scale
Children’s Sleep Habits Questionnaire
Autism Spectrum Quotient
Zou M, Li D, Li L, Wu L, Sun C. Role of the endocannabinoid system in neurological disorders. Int J Dev Neurosci. 2019;76:95–102.
CAS PubMed Google Scholar
American Psychiatric Association. DSM-5 Diagnostic Classification. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013 [cited 2019 Nov 6]. Available from: https://psychiatryonline.org/doi/10.1176/appi.books.9780890425596.x00DiagnosticClassification .
Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910.
PubMed Google Scholar
Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15(9):409–16.
PubMed PubMed Central Google Scholar
Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–9.
CAS PubMed PubMed Central Google Scholar
Nguyen QA, Horn ME, Nicoll RA. Distinct roles for extracellular and intracellular domains in neuroligin function at inhibitory synapses. Elife. 2016;5:e19236.
Yenkoyan K, Grigoryan A, Fereshetyan K, Yepremyan D. Advances in understanding the pathophysiology of autism spectrum disorders. Behav Brain Res. 2017;331:92–101.
Weissman L. Autism spectrum disorder in children and adolescents: Overview of management. In: Augustyn M, Patterson MC, Torchia MM, editors. UpToDate [Internet]. Waltham (MA): UpToDate Inc.; 2019 [updated 2019 Dec 19; cited 2019 Sep 19]. Available from: https://www.uptodate.com/contents/autism-spectrum-disorder-in-children-and-adolescents-overview-of-management?search=autismspectrumdisorder&source=search_result&selectedTitle=1~146&usage_type=default&display_rank=1 .
Posey DJ, McDougle CJ. Pharmacotherapeutic management of autism. Expert Opin Pharmacother. 2001;2(4):587–600.
Goel R, Hong JS, Findling RL, Ji NY. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78–95.
Weissman L, Hodges HK. Autism spectrum disorder in children and adolescents: Complementary and alternative therapies. In: Augustyn M, Patterson MC, Torchia MM, editors. Waltham (MA): UpToDate Inc.; 2019 [updated 2020 Feb 05; cited 2019 Sep 19]. Available from: https://www.uptodate.com/contents/autism-spectrum-disorder-in-children-andadolescents-complementary-and-alternative-therapies?search=Autism spectrum disorder in children and adolescents: Complementary and alternative therapies&source= .
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–73.
World Health Organization. WHO Expert Committee on Drug Dependence: Fortieth Report [Internet]. 2018 [cited 2020 Jan 29]. Available from: https://apps.who.int/iris/bitstream/handle/10665/279948/9789241210225-eng.pdf?ua=1#:~:text=The fortieth meeting of the WHO, cannabis and its component substances .
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017;376(21):2011–20.
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N Engl J Med. 2018;378(20):1888–97.
Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr Psychiatry Rep. 2017;19(4):23.
Kurz R, Blaas K. Use of dronabinol ( delta-9-THC ) in autism: A prospective single-case-study with an early infantile autistic child. Cannabinoids. 2010;5(4):4–6.
Google Scholar
Kruger T, Christophersen E. An Open Label Study of the Use of Dronabinol (Marinol) in the Management of Treatment-Resistant Self-Injurious Behavior in 10 Retarded Adolescent Patients. J Dev Behav Pediatr. 2006;27(5):441.
Bar-Lev Schleider L, Mechoulam R, Saban N, Meiri G, Novack V. Real life Experience of Medical Cannabis Treatment in Autism: Analysis of Safety and Efficacy. Sci Rep. 2019;9(1):200.
Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17.
Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicol Teratol. 2016;58:5–14.
Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14(3):345–50.
Schwarz R, Ramer R, Hinz B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab Rev. 2018;50(1):26–53.
Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158(12):2442–51.
Wei D, Dinh D, Lee D, Li D, Anguren A, Moreno-Sanz G, et al. Enhancement of Anandamide-Mediated Endocannabinoid Signaling Corrects Autism-Related Social Impairment. Cannabis Cannabinoid Res. 2016;1(1):81–9.
Karhson DS, Krasinska KM, Dallaire JA, Libove RA, Phillips JM, Chien AS, et al. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol Autism. 2018;9(1):1–6.
Food and Drug Administration. Medication Guide - Epidiolex. In Carlsbad (CA): Greenwich Biosciences Inc.; 2018 [cited 2019 Nov 23]. p. 30. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf .
Thiele EA, Marsh ED, French JA, Mazurkiewicz MB, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–96.
Gould GG, Seillier A, Weiss G, Giuffrida A, Burke TF, Hensler JG, et al. Acetaminophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice. Prog Neuro-Psychopharmacology Biol Psychiatry. 2012;38(2):260–9.
CAS Google Scholar
ClinicalTrials.gov [internet]. United States: Bethesda (MA): US National Library of Medicine; 2000 - . Identifier NCT02956226, Cannabinoids for Behavioral Problems in Autism Spectrum Disorder: A Double Blind, Randomized, Placebo-Controlled Trial with Crossover; 2016 Nov 6 [cited 2019 Sep 19]. Available from: https://www.clinicaltrials.gov/ct2/show/study/NCT02956226?show_desc=Y#desc .
Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KL, et al. Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: Systematic review and meta-analysis of case-control and randomised controlled trials. Nutrients. 2017;9(2):1–32.
Download references
Acknowledgements
We want to thank Caleo Health and all the administrative staff. Special thanks to Michelle Andoy for supporting us.
WAM provided all the funding.
Author information
Authors and affiliations.
Caleo Health, Suite 200, 1402 8th Ave N.W., Calgary, AB, T2N 1B9, Canada
Juliana Andrea Ponton, Elias Soumbasis, Sergio Andres Llanos, Mark Lewis, Wilhelm August Meerholz & Robert Lawrence Tanguay
Alberta Children’s Hospital, 2888 Shaganappi Trail N.W., Calgary, AB, Canada
You can also search for this author in PubMed Google Scholar
Contributions
JAP conceptualization, writing – draft and final manuscript, and analyzed information. WAM and ES made substantial contributions to acquisition of data. KS contributed with analysis as well as acquisition of the data. RLT investigated, conceived, and supervised the project. KS, RLT, and ES provided critical feedback and helped shaped the analysis of the case. JAP, WAM, and SAL assisted with acquisition of data. KS, ES, ML, and RLT participated in manuscript editing. WAM and RLT participated in funding acquisition. JAP and SAL coordinated and designed graphics. All authors discussed and contributed to the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Juliana Andrea Ponton .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ponton, J.A., Smyth, K., Soumbasis, E. et al. A pediatric patient with autism spectrum disorder and epilepsy using cannabinoid extracts as complementary therapy: a case report. J Med Case Reports 14 , 162 (2020). https://doi.org/10.1186/s13256-020-02478-7
Download citation
Received : 05 April 2020
Accepted : 30 July 2020
Published : 22 September 2020
DOI : https://doi.org/10.1186/s13256-020-02478-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cannabidiol extract
- Phytocannabinoids
- Complementary treatment
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Signs and Symptoms
- Living with Autism Spectrum Disorder
- Frequently Asked Questions (FAQs)
- Data and Statistics on Autism Spectrum Disorder
- Autism Materials and Resources
- Diagnosis ASD
- Information on ASD for Healthcare Providers
- Acceptance Month Partner Toolkit
- 2023 Community Report on Autism
- Autism Data Visualization Tool
Autism Spectrum Disorder Articles
At a glance.
Below is a list of recent scientific articles on autism spectrum disorder (ASD) generated from CDC programs and activities.

Key findings and scientific articles
Key findings.
These key findings provide brief summaries of some of CDC's latest ASD research.
Key Findings: ADDM Network Expands Surveillance to Identify Healthcare Needs and Transition Planning for Youth
Five of CDC's ADDM Network sites (Arkansas, Georgia, Maryland, Utah, and Wisconsin) began monitoring autism spectrum disorder (ASD) in 2018 among 16-year-old adolescents who were initially identified as having characteristics of ASD in 2010. (Published: February 25, 2023)
Key Findings: Study Shows Linking Statewide Data for ASD Prevalence is Effective
Linking statewide health and education data is an effective way for states to have actionable local ASD prevalence estimates when resources are limited. (Published: January 18, 2023)
Key Findings: CDC Releases First Estimates of the Number of Adults Living with Autism Spectrum Disorder in the United States
This study fills a gap in data on adults living with ASD in the United States because there is not an existing surveillance system to collect this information. (Published May 10, 2020)

CDC scientific articles
These articles are either from CDC-funded research or have at least one CDC author. These articles are listed by year of publication, with the most recent first.
- Adolescents With Autism Spectrum Disorder: Diagnostic Patterns, Co-occurring Conditions, and Transition Planning. Hughes MM, Shaw KA, Patrick ME, et al. J Adolesc Health. 2023;73(2):271-278.
- Statewide county-level autism spectrum disorder prevalence estimates—seven U.S. states, 2018. Shaw KA, Williams S, Hughes MM, et al. Ann Epidemiol. 2023;79:39-43.
- The Prevalence and Characteristics of Children With Profound Autism, 15 Sites, United States, 2000-2016. Hughes MM, Shaw KA, DiRienzo M, et al. Public Health Rep. 2023;138(6):971-980.
- Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Maenner MJ, Warren Z, Williams AR, et al. MMWR Surveill Summ. 2023;72(2):1-14. Published 2023 Mar 24. [ Easy-Read Summary ]
- Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Shaw KA, Bilder DA, McArthur D, et al. MMWR Surveill Summ. 2023;72(1):1-15. Published 2023 Mar 24. [ Easy-Read Summary ]
- Social vulnerability and prevalence of Autism Spectrum Disorder, Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP). Patrick ME, Hughes MM, Ali A, Shaw KA, Maenner MJ. Ann Epidemiol. 2023;83:47-53.e1.
- Individualized Education Programs and Transition Planning for Adolescents With Autism. Hughes MM, Kirby AV, Davis J, et al. Pediatrics. 2023;152(1):e2022060199. [ Watch Video Abstract ]
" There is no epidemic of autism. It's an epidemic of need."
Two authors provide their commentary on CDC's 2023 Community Report in an article published in ST A T News' First Opinion (March 2023).
Read the full article here.
- Toileting Resistance Among Preschool-Age Children With and Without Autism Spectrum Disorder. Wiggins LD, Nadler C, Hepburn S, Rosenberg S, Reynolds A, Zubler J. J Dev Behav Pediatr. 2022;43(4):216-223.
- Defining in Detail and Evaluating Reliability of DSM-5 Criteria for Autism Spectrum Disorder (ASD) Among Children Rice CE, Carpenter LA, Morrier MJ, et al. J Autism Dev Disord. 2022;52(12):5308-5320. [published correction appears in J Autism Dev Disord. 2022 Jan 29;:].
- Reasons for participation in a child development study: Are cases with developmental diagnoses different from controls? Bradley CB, Tapia AL, DiGuiseppi CG, et al. Paediatr Perinat Epidemiol. 2022;36(3):435-445.
- Features that best define the heterogeneity and homogeneity of autism in preschool-age children: A multisite case–control analysis replicated across two independent samples. Wiggins LD, Tian LH, Rubenstein E, et al. Autism Res. 2022;15(3):539-550.
- Progress and Disparities in Early Identification of Autism Spectrum Disorder: Autism and Developmental Disabilities Monitoring Network, 2002–2016. Shaw KA, McArthur D, Hughes MM, et al. J Am Acad Child Adolesc Psychiatry. 2022;61(7):905-914.
- Peri-Pregnancy Cannabis Use and Autism Spectrum Disorder in the Offspring: Findings from the Study to Explore Early Development. DiGuiseppi C, Crume T, Van Dyke J, et al. J Autism Dev Disord. 2022;52(11):5064-5071.
- Heterogeneity in Autism Spectrum Disorder Case-Finding Algorithms in United States Health Administrative Database Analyses. Grosse SD, Nichols P, Nyarko K, Maenner M, Danielson ML, Shea L. J Autism Dev Disord. 2022;52(9):4150-4163.
- Early identification of autism spectrum disorder among children aged 4 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2018. Shaw KA, Maenner MJ, Bakian AV, et al. MMWR Surveill Summ. 2021;70(10):1-14. Published 2021 Dec 3.
- Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. Maenner MJ, Shaw KA, Bakian AV, et al. MMWR Surveill Summ. 2021;70(11):1-16. Published 2021 Dec 3.
- Comparison of 2 Case Definitions for Ascertaining the Prevalence of Autism Spectrum Disorder Among 8-Year-Old Children. Maenner MJ, Graves SJ, Peacock G, Honein MA, Boyle CA, Dietz PM. Am J Epidemiol. 2021;190(10):2198-2207.
- Healthcare Costs of Pediatric Autism Spectrum Disorder in the United States, 2003–2015. Zuvekas SH, Grosse SD, Lavelle TA, Maenner MJ, Dietz P, Ji X. J Autism Dev Disord. 2021;51(8):2950-2958.
- Association between pica and gastrointestinal symptoms in preschoolers with and without autism spectrum disorder: Study to Explore Early Development. Fields VL, Soke GN, Reynolds A, et al. Disabil Health J. 2021;14(3):101052.
- Health Status and Health Care Use Among Adolescents Identified With and Without Autism in Early Childhood—Four US Sites, 2018–2020. Powell PS, Pazol K, Wiggins LD, et al. MMWR Morb Mortal Wkly Rep. 2021;70(17):605-611. Published 2021 Apr 30.
- Evaluation of sex differences in preschool children with and without autism spectrum disorder enrolled in the study to explore early development. Wiggins LD, Rubenstein E, Windham G, et al. Res Dev Disabil. 2021;112:103897.
- A Distinct Three-Factor Structure of Restricted and Repetitive Behaviors in an Epidemiologically Sound Sample of Preschool-Age Children with Autism Spectrum Disorder. Hiruma L, Pretzel RE, Tapia AL, et al. J Autism Dev Disord. 2021;51(10):3456-3468.
- Spending on Young Children With Autism Spectrum Disorder in Employer-Sponsored Plans, 2011–2017 Grosse SD, Ji X, Nichols P, Zuvekas SH, Rice CE, Yeargin-Allsopp M. Psychiatr Serv. 2021;72(1):16-22. [published correction appears in Psychiatr Serv. 2021 Jan 1;72(1):97].
- A Preliminary Epidemiology Study of Social (Pragmatic) Communication Disorder Relative to Autism Spectrum Disorder and Developmental Disability Without Social Communication Deficits. Ellis Weismer S, Rubenstein E, Wiggins L, Durkin MS. J Autism Dev Disord. 2021;51(8):2686-2696.
- CE: From the CDC: Understanding Autism Spectrum Disorder. Christensen D, Zubler J. Am J Nurs. 2020;120(10):30-37.
- Early Identification of Autism Spectrum Disorder Among Children Aaged 4 Years—Early Autism and Developmental Disability Monitoring Network, Six Sites, United States, 2016. Shaw KA, Maenner MJ, Baio J, et al. MMWR Surveill Summ. 2020;69(3):1-11. Published 2020 Mar 27.
- Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Maenner MJ, Shaw KA, Baio J, et al. MMWR Surveill Summ. 2020;69(4):1-12. Published 2020 Mar 27. [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Apr 24;69(16):503].
- Disparities in Documented Diagnoses of Autism Spectrum Disorder Based on Demographic, Individual, and Service Factors. Wiggins LD, Durkin M, Esler A, et al. Autism Res. 2020;13(3):464-473.
SEED Research
Researchers working on CDC's Study to Explore Early Development (SEED) have published many studies reporting on important findings related to ASD.
For more information on the methods and descriptions of the SEED study sample, SEED publications, and the evaluation of clinical and laboratory methods using SEED data, click the link below.
Featured Article | Summer 2023
Cdc seed study explores prenatal ultrasound use and risk of autism spectrum disorder.

Prenatal ultrasound use and risk of autism spectrum disorder: Findings from the case-control Study to Explore Early Development (SEED). Christensen D, Pazol K, Overwyk KJ, et al. Paediatr Perinat Epidemiol. 2023;37(6):527-535.
Study findings
Many additional studies are underway. We will provide summaries of those studies in the future.
All articles
Search CDC Stacks for articles that have been published by CDC authors within the National Center on Birth Defects and Developmental Disabilities from 1990 to present.
Feature articles and an Easy-Read Summary

Higher Autism Prevalence and COVID-19 Disruptions

Past, Present, and Future Impact of SEED
Easy-Read Summary

Autism among 4-year-old and 8-year-old Children: An Easy-Read Summary
Additional resources

Why Act Early if You’re Concerned about Development?
Autism Spectrum Disorder (ASD)
Autism spectrum disorder (ASD) is a developmental disability that can cause significant social, communication and behavioral challenges. CDC is committed to continuing to provide essential data on ASD and develop resources that help identify children with ASD as early as possible.
For Everyone
Health care providers, public health.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Psychiatry
- v.13(6); 2023 Jun 19
- PMC10294139
Pharmacotherapy in autism spectrum disorders, including promising older drugs warranting trials
Jessica hellings.
Department of Psychiatry, University of Missouri-Kansas City, Lee's Summit, MO 64063, United States. [email protected]
Corresponding author: Jessica Hellings, MB.BCh., MMed, Professor, Department of Psychiatry, University of Missouri-Kansas City, 300 SE Second St, Lee's Summit, MO 64063, United States. [email protected]
Available pharmacotherapies for autism spectrum disorders (ASD) are reviewed based on clinical and research experience, highlighting some older drugs with emerging evidence. Several medications show efficacy in ASD, though controlled studies in ASD are largely lacking. Only risperidone and aripiprazole have Federal Drug Administration approval in the United States. Methylphenidate (MPH) studies showed lower efficacy and tolerability for attention deficit hyperactivity disorder (ADHD) than in the typically developing (TD) population; atomoxetine demonstrated lower efficacy but comparable tolerability to TD outcomes. Guanfacine improved hyperactivity in ASD comparably to TD. Dex-troamphetamine promises greater efficacy than MPH in ASD. ADHD medications reduce impulsive aggression in youth, and may also be key for this in adults. Controlled trials of the selective serotonin reuptake inhibitors citalopram and fluoxetine demonstrated poor tolerability and lack of efficacy for repetitive behaviors. Trials of antiseizure medications in ASD remain inconclusive, however clinical trials may be warranted in severely disabled individuals showing bizarre behaviors. No identified drugs treat ASD core symptoms; oxytocin lacked efficacy. Amitriptyline and loxapine however, show promise. Loxapine at 5-10 mg daily resembled an atypical antipsychotic in positron emission tomography studies, but may be weight-sparing. Amitriptyline at approximately 1 mg/ kg/day used cautiously, shows efficacy for sleep, anxiety, impulsivity and ADHD, repetitive behaviors, and enuresis. Both drugs have promising neurotrophic properties.
Core Tip: Most prescribing in autism spectrum disorders (ASD) is off-label; only risperidone and aripiprazole are Federal Drug Administration-approved in ASD, for irritability. Atypical antipsychotics are associated with metabolic side effects. Loxapine at 5-10 mg/day resembled an atypical antipsychotic in positron emission tomography studies; preliminary studies and clinical experience in ASD suggest efficacy and a promising metabolic profile. Controlled attention deficit hyperactivity disorder (ADHD) medication trials in ASD youth include methylphenidate, atomoxetine and guanfacine. The author recommends dextroamphetamine as an important treatment option for ADHD in ASD. Amitriptyline often improves impulsive aggression, self-injury, sleep, anxiety and enuresis. This article recommends additional older drug trials in ASD: Detroamphetamine, amitriptyline, loxapine, and lamotrigine for likely seizures.
INTRODUCTION
Autism spectrum disorder (ASD) is diagnosed using criteria of significant deficits in social communication and interaction, together with at least two types of restricted and repetitive interests and behaviors (RRBs)[ 1 ]. ASD develops prenatally and during early childhood. There is no longer an age cut-off for diagnosis, though it is often evident by age 1-3 years. The prevalence of ASD has risen globally since 2000. Two separate United States studies using the 2016 National Survey of Children’s Health reported ASD prevalence of 1 in 40 children[ 2 , 3 ]. After decades there is still no definitive medication treatment for the core features of autism likely due to the heterogeneity of ASD, including various genetic causes. Recent studies with negative findings for core symptoms include oxytocin, bumetanide and selective serotonin reuptake inhibitors (SSRIs) fluoxetine and citalopram for RRBs[ 4 ]. A meta-analysis confirmed there are still no treatments with efficacy for RRBs[ 5 ].
In addition to core ASD disabilities, the majority of these individuals have other serious challenges affecting them. Approximately 30%-50% also have intellectual disability (ID)[ 6 ]. Those more severely affected for example by birth injuries may have hydrocephalus and cerebral palsy, along with varying degrees of motor paralysis. Although there is a tendency worldwide to diagnose ASD in high-functioning, milder cases, an estimated quarter of individuals with ASD have less than 20 words of expressive language and are thus minimally verbal[ 7 ]. Approximately 20%-40% of those with ASD also have epilepsy, with greater rates in the more severely affected[ 8 ], which includes minimally verbal individuals.
In addition, psychiatric illness occurs several times more commonly in those with ASD than in the general population[ 9 , 10 ]. Common presenting problems include hyperactivity, impulsive aggression, property destruction and self-injury, which are not Diagnostic and Statistical Manual-fifth edition-Text Revised (DSM-5-TR) diagnoses. A study of 1380 youth with ASD found that over two thirds (68%) manifested aggression towards a caregiver, and almost half (49%) showed aggression towards non-caregivers[ 11 ]. Psychiatrist training in the field of developmental disabilities is seriously lacking in most universities worldwide, and has marginally improved in the United States in the past 5 years[ 12 , 13 ]. Individuals with ASD and their caregivers have great difficulty identifying a provider in their geographical area who will treat them. The field still suffers from a serious lack of clinical trials to guide treatment of psychiatric comorbidity. Those providers who treat such patients must rely on the few ASD clinical trials published, experience gained by different medication trials, and extrapolation from studies in typically developing (TD) individuals.
An analysis of 33565 children with ASD, found that 35% received 2 or more psychotropic medications, while 15% received 3 or more[ 14 ]. Polypharmacy especially with antipsychotics is even greater in adults, when many non-psychiatric medications are also prescribed apart from psychotropic medications[ 15 ]. The lack of evidence base results inevitably in exposure of these individuals to repeated medication trials, an unnecessary burden of side effects, and attrition from care[ 16 ]. Individuals with ASD often have one or more comorbid DSM-5-TR diagnoses. Working DSM-5-TR diagnoses are important guides for selecting classes of medications. Diagnostic symptoms of DSM-5-TR diagnoses may be more difficult to recognize in those more severely affected, including the minimally verbal. The Diagnostic Manual of Intellectual Disabilities-2 (DM-ID2)[ 17 ] is a useful crosswalk for applying DSM-5 criteria to individuals with intellectual and developmental disorders and/or ASD. Clearly the verbal criteria for diagnoses are not used in the minimally verbal.
Only risperidone and aripiprazole are Federal Drug Administration (FDA)-approved in the United States for individuals with ASD and irritability. The few other drugs prospectively studied in randomized controlled trials (RCTs) in ASD include methylphenidate (MPH), atomoxetine (ATX), guanfacine, the SSRIs fluoxetine and citalopram, and valproic acid[ 18 ]. Metformin, arbaclofen, lovastatin, trifinetide, 5-hydroxytryptamine7 (5-HT7) agonist ligands, flavonoids, and the dietary supplement sulfurophane amongst others, are still being studied[ 4 ]. More RCTs are urgently needed for individuals with ASD/ID. While studies continue to test possible treatments for the core symptoms of ASD, even experts frequently run out of options for the many comorbidities, after many medication trials including clozapine have failed. It may also turn out that no one drug will target and treat the core symptoms in ASD, given the vast heterogeneity of genetic and other causes.
Behavior analysis and psychosocial treatments play a key role in any overall management plan, since problems due to environmental factors or maladaptive learning will not respond to medication treatments. This article highlights several available older medications, with decades of community use in the general population, that show promise in ASD. Emerging evidence about them includes preliminary observed efficacy, neurotrophic effects and apparent tolerability in low dose.
ATTENTION DEFICIT HYPERACTIVITY DISORDER: EXISTING STUDIES AND EMERGING EVIDENCE ON OTHER OLD MEDICATIONS
Symptoms of attention deficit hyperactivity disorder (ADHD) include inattention, distractibility, hyperactivity and impulsivity. ADHD in ASD is often associated with dangerous behaviors including impulsive aggression and self-injury[ 19 ]. Prior to DSM-5, ADHD was not recognized as a separate diagnosis for individuals with ASD. Since it does not manifest in all individuals with ASD but does so in a large proportion, notably 28%-68%[ 20 ] it is now included as a separate diagnosis. ADHD is increasingly identified and treated in adults with ASD; a recent study found high rates of ADHD in 63 tertiary-referred adults with ASD screened for psychiatric comorbidity, notably 68% for lifetime prevalence of ADHD[ 9 ]. Additionally, ADHD is less likely to improve after adolescence in youth with ASD than in the general population with ADHD. In the community, inattentive-type ADHD is the most common subtype found in ASD/ID, however it is often untreated.
The hyperactive-impulsive subtype of ADHD has poorer outcomes in individuals with ASD, related to the more disruptive nature of hyperactivity as well as a greater likelihood of impulsive aggression, self-injury and property destruction[ 19 ]. Affect dysregulation, the inability to properly regulate and modulate emotions, was not included in DSM-5 as a diagnostic feature of ADHD, but is emphasized in DM-ID2 as an important feature in individuals with developmental disabilities including ASD. The authors of the DSM-5 ADHD criteria later published an article emphasizing affect dysregulation as an important part of ADHD[ 21 ]. ADHD-associated mood fluctuations present an important source of impairment especially in those with developmental disabilities and ADHD. Especially in adults with ASD, the ADHD diagnosis may be overlooked, resulting in a bipolar or borderline personality disorder misdiagnosis.
ADHD medications are important for improving learning, speech and language, and executive functions including inhibitory self- control. These medications improve affect dysregulation in ASD, which often manifests as impulsive aggression when the person is frustrated. Response inhibition of affective fluctuations such as laughing or crying is impaired in ADHD, related to executive function deficits. A meta-analysis of executive function in ASD found that broad executive function deficits remain stable and do not improve across development in such individuals[ 22 ]. Obsessive compulsive disorder (OCD) is very commonly associated as well in ASD, and could complicate treatment of ADHD with stimulants since the latter may increase anxiety in a dose-related manner[ 23 ]. On the other hand, non-stimulant ADHD medications may help reduce OCD and repetitive behaviors in ASD, although studies are still needed. Medications for ADHD can be divided into stimulant and non-stimulant drug categories.
When to try stimulants in ASD?
Stimulants are more likely to show efficacy and tolerability in higher-functioning individuals with ASD who have predominantly ADHD symptoms in contrast to cases with OCD symptoms, prominent repetitive behaviors or self-injury. In the latter group, non-stimulant medications may be a more tolerable choice. Young children with ASD often begin their first ADHD medication trials when their disruptive behavior interferes with education of themselves and others in the classroom. As with TD young children with ADHD, the first drug tried is usually the stimulant MPH, in divided doses three times a day, up to 1 mg/kg/day or less; individual responses vary.
Dextroamphetamine (DEX) immediate release (ir) merits study in ASD, according to the author’s decades-long experience. DEX has double the potency and duration of action as MPH, notably 4 to 6 h. A meta-analysis comparing efficacy of stimulants in 23 controlled studies for ADHD found a modest advantage of amphetamines over MPH for treating ADHD in pediatric patients[ 24 ]. Divided doses given morning, lunch time, and a half-dose at 4 pm if needed, totaling approximately 0.5 mg/kg/day or less give good coverage, better than MPH. Overall, DEX ir produces less lunch-time appetite suppression, less anxiety and irritability than long-acting stimulants according to author experience. Despite the current low level of evidence for DEX in ASD, clinical trials are warranted, and patient trials in the office may be beneficial.
However, MPH is the only stimulant studied so far in ASD, with findings of lower tolerability and lower efficacy than in TD youth. Large studies include a multisite study by the group Research Units on Pediatric Psychopharmacology (RUPP)[ 25 ], and a Cochrane database systematic review[ 26 ]. The RUPP study of 72 children with ASD, aged 5 to 13 years, found all low doses studied were superior to placebo for hyperactivity and impulsivity. Subjects were pre-selected for ability to tolerate a test dose of MPH for a week. Total doses, each given for a week, were 0.125 mg/kg, 0.25 mg/kg, and 0.5 mg/kg and were deliberately low in order to minimize side effects. However only 49% were responders, a rate much lower than the 75% response rate in TD children. Even the greatest effect size of 0.54 was significantly lower than that for ADHD response in TD children. Side effect rates were approximately double those found in TD children, and 18% exited the study early due to intolerable side effects. These included irritability, decreased appetite, and insomnia. Parent-rated lethargy, social withdrawal, and inappropriate speech increased significantly. There are also two small RCT studies and one multisite study of MPH for ADHD in ASD. Two small RCT studies of MPH for aggression in ASD found benefit over placebo on the Aberrant Behavior Checklist-Irritability (ABC-I) subscale[ 27 - 29 ]. Intolerable side effects were common in the latter study also, including mood changes, agitation and abnormal movements.
The Cochrane systematic review[ 26 ] of MPH in children and adolescents with ASD included 4 crossover studies, totaling 113 children ages 5 to 13 years; most (83%) were boys. There was a significant benefit on teacher-rated inattention but insufficient data to perform an impulsivity-outcome meta-analysis. Treatment duration for each dose of MPH was 1 wk. High-dose MPH significantly improved hyperactivity as rated by teachers in 4 studies, 73 subjects, ( P < 0.001) low quality evidence, and parents in 2 studies, 71 subjects ( P = 0.02), low quality evidence. Ratings were on the hyperactivity subscale of the ABC. MPH clinical usefulness is also limited by its short half-life of 2-4 h.
Of the long-acting stimulants in ASD, only one small study has been published. This small study of 24 children, mean age 8.8 years, found significant benefit of MPH-extended release in ASD[ 30 ]. However this was not a representative ASD sample, since the participants’ mean IQ was 85.0 (SD = 16.8). MPH-ER may be useful and more tolerable for example in high-functioning individuals with ASD. Comparative studies of long-acting stimulants are lacking in ASD, including for irritability[ 31 ]. Long-acting stimulants were designed to take effect and wear off gradually, and to reduce side effects and rebound effects in the general population with ADHD. However, clinical observations suggest that in ASD, long-acting stimulants may have even greater side effects than immediate-release preparations, including worsened anxiety, appetite suppression, self-injury, lip-licking, nail-picking, trichotillomania, and compulsive behaviors, in a dose-dependent manner. The more severe the ASD, the more of a problem such side effects present, although studies are needed. Therefore, non-stimulant ADHD medications may be preferable in these individuals.
When to try non-stimulant ADHD medications in ASD?
As stated, non-stimulant ADHD medications are preferable to stimulants for individuals who have more severe ASD, and those who also have prominent OCD, RRBs and self-injury. These include ATX, alpha agonists and tricyclic antidepressants (TCAs). Clinical experience in ASD suggests that these medications can be added to low-dose stimulants that are partially helpful if the person is unable to tolerate stimulant dose increases due to side effects. Several clinical trials in TD individuals have found efficacy and tolerability of ATX in combination with stimulants, although such combinations are not FDA-approved[ 32 ]. A recent review compared responses between MPH, ATX and guanfacine in 9 controlled studies of 430 children with ASD[ 33 ]. MPH and ATX were superior to placebo for ADHD. Poorer response was found in more cognitively disabled individuals.
Atomoxetine (ATX)
ATX is a noradrenergic reuptake inhibitor shown to produce improvements in inhibitory control as part of executive functions. Importantly, acute ATX administration increased behavioral inhibition as measured by a stop-signal task in adult ADHD not accompanied by ASD[ 34 ] as well as in normal adults without either ADHD or ASD[ 35 ]. Author experience confirms that ATX may be a good choice for impulsive aggression in ASD including in adults and minimally verbal individuals, and for poor focus and disorganization in higher-functioning individuals. A randomized, multisite 10-wk double-blind placebo-controlled trial of ATX, with or without parent training, was performed for ADHD in 128 children aged 5 to 14 years with ASD. ATX showed greatest efficacy together with parent- training, but also the drug alone was superior to placebo[ 36 ]. Overall, tolerability was good, to a maximum dose of 1.8 mg/kg/day; mean dose was 1.4 mg/kg/day. Dosing was divided into twice-daily doses, to reduce side effects. The most common side effects were nausea, decreased appetite, early morning wakening and fatigue. Suicidal ideation and QTc changes were not found, in contrast to findings in children without ASD[ 37 ]. In addition, another acute RCT study of 97 youths with ASD treated with ATX, including open long-term follow-up, showed moderately improved ADHD symptoms and side effects similar to those found in studies of ATX in youth with ADHD but no ASD[ 38 , 39 ].
ATX trials are warranted in ADHD in adults with ASD, especially for impulsive aggression, based on author experience. The strategy is to “start low and go slow” while response is observed for, using divided doses of twice a day to improve tolerability and coverage. A recent retrospective study disputes the need for extra caution however and found similar responses to ADHD treatments in adults with ADHD and ASD to those found in a comparison group with ADHD but no ASD[ 40 ]. The therapeutic window may be narrower in minimally verbal and lower-functioning individuals with more severe degrees of ASD, according to clinical experience. Should behavioral worsening occur after an ATX dose increase, the beneficial response is usually recaptured by dose reduction.
Amitriptyline
Amitriptyline in low doses may be especially useful if used with caution, in comparison with other available non-stimulant medications, despite a lack of comparative studies. TCAs including amitriptyline are second only to stimulants in ADHD efficacy, although most evidence for their use in ADHD is from studies of the second generation TCA desipramine in youth without ASD. An advantage over stimulants according to this author’s experience is that amitriptyline may benefit ADHD, anxiety, OCD, gastrointestinal pain, headaches, enuresis and insomnia[ 15 ]. Though currently there is a low level of published evidence, prospective studies are warranted, in the author’s opinion. A retrospective chart review on amitriptyline[ 41 ] published by the author’s group examined 50 tertiary-referred children and adolescents with ASD, ADHD and high rates of aggression and self-injury, who received low dose AMI (mean dose 1.3 ± 0.6 mg/kg/day) with mean trough blood level of 114.1 ± 50.5 ng/mL. Response occurred clinically in 60% of patients at the final visit, and in 82% of patients for at least 50% of follow-up visits. Importantly, 30% had failed ATX, and 40% had failed 3 or more other ADHD medication trials. Amtriptyline was used in combination with stimulants, most often low dose DEX ir, and also low dose risperidone or aripiprazole. In the low doses used amitriptyline did not cause complaints of constipation or urinary retention. Side effects included QTc increase on routine electrocardiogram, which did not halt treatment except in 3 cases with QTc > 440, behavioral activation and worsening of aggression. Prospective, randomized controlled studies of amitriptyline in ASD are warranted.
While a 2014 Cochrane review[ 42 ] of TCAs in TD youth showed no serious adverse events associated with taking TCAs, mild increases in pulse rates and diastolic blood pressure occurred. Of note is that the overdose risk with TCAs is lower in individuals with ASD since most individuals including adults with ASD do not self-administer their medications. TCAs should not be prescribed for use in chaotic households or those with a risk of overdose by a family member.
Alpha agonists
The class of alpha-agonist drugs is FDA-approved for ADHD in TD children but not in ASD. Since these drugs may benefit tics and Tourette disorder, they are usually a first-line treatment choice in such individuals. This drug class includes guanfacine, clonidine, long-acting guanfacine (Intuniv TM) and long-acting clonidine XR (Kapvay TM). An 8-wk multisite study of extended-release guanfacine in 62 children with ASD and ADHD, mean age 8.5 years, found a significant improvement in comparison with placebo. Modal guanfacine ER dose was 3 mg/day (range 1-4 mg/day)[ 43 ]. The most common side effects were fatigue, drowsiness and decreased appetite. For subjects on guanfacine, blood pressure dropped in the initial 4 wk, but returned almost to baseline by week 8. Pulse rate also dropped but remained lower than baseline at week 8. A small study of clonidine[ 44 ] examined response of 8 male children with autistic disorder in a double-blind, placebo-controlled crossover design for ADHD symptoms. While parent-rated Conner’s questionnaire ADHD ratings improved significantly during clonidine treatment, teacher ratings were not significantly improved except for oppositional behavior. Side effects included drowsiness and decreased activity. Due to their short half-lives, the immediate-release preparations of clonidine and guanfacine should be dosed 3 times a day. Dosing is built up gradually while monitoring for dizziness, hypotension and bradycardia. Other side effects include weight gain, sedation and irritability.
Although alpha agonists improve attention, studies in otherwise TD youth with ADHD have shown their combination use with a stimulant medication produces greater attentional improvement than does either alone. Combination treatments of alpha agonists and stimulants are FDA-approved for ADHD in the non-ASD population, but not in ASD. Clinical experience suggests however that alpha agonists may be less helpful for ADHD symptoms in adults with ASD.
Thus in the author’s opinion, DEX, ATX and amitriptyline may be useful additions to treatment options for ADHD comorbid with ASD, including in adults.
EXISTING ANTIPSYCHOTIC STUDIES, AND EMERGING EVIDENCE FOR OTHER ANTIPSYCHOTICS
Antipsychotics are used to treat psychosis as well as irritability in ASD, and are classified into two classes: Atypical/novel antipsychotics and typical/classical antipsychotics. ASD core symptoms including odd, stereotyped talk on unusual restricted topics of interest are still often misdiagnosed as schizophrenia symptoms in everyday practice. Psychosis can also be confused with bizarre behavior related to subclinical seizures, in which case antiseizure medications may help. Psychotic disorders can be comorbid with ASD, including schizophrenia, delusional disorder, unspecified psychosis, or as a component of a major mood disorder such as bipolar disorder, major depressive disorder or schizoaffective disorder[ 45 ].
Typical antipsychotics
Typical antipsychotics block dopamine D2 receptors to alleviate psychosis or mania, but produce motor side effects including acute dystonias, extrapyramidal side effects (EPS), tardive dyskinesia and more rarely, neuroleptic malignant syndrome which can be fatal. Haloperidol was studied in early trials by Campbell and colleagues, in young children, but found to produce tardive withdrawal movements[ 46 , 47 ] and further studies were halted. According to clinical experience, typical antipsychotics often have a lag time to onset of response in individuals with ASD, and increasing the dose early in treatment especially of high potency antipsychotics like haloperidol may result in extremely severe EPS and dysphagia after a while, especially more severely disabled individuals, with resulting joint contractures[ 16 ]. Low potency typical antipsychotics including chlorpromazine produce hypotension, slowing, cognitive dulling and weight gain in those with developmental disabilities as well as in the general population. Thioridazine produced QTc prolongation and is no longer marketed.
The medium-potency, typical antipsychotic loxapine blocks serotonin as well as dopamine, and in low doses resembles an atypical antipsychotic in positron emission tomography (PET) studies, but with less or no weight gain[ 48 - 50 ] which will be discussed in more detail below. Atypical antipsychotics were designed to overcome these motor side effects of typical antipsychotics by a different mechanism of action, notably by blocking serotonin as well as dopamine receptors, amongst others. However an unanticipated side effect of the atypical antipsychotics turned out to be weight gain, Type II diabetes and multiple other medical side effects[ 51 ], which are more severe in those with developmental disabilities. Atypical antipsychotics also produce possible motor side effects including neuroleptic malignant syndrome and tardive dyskinesia in the general population but also in ASD.
Atypical antipsychotics
Only two antipsychotics are FDA-approved in ASD, for children ages 6 years and older with irritability, notably risperidone and aripiprazole. The RUPP multisite 8-wk risperidone RCT study of 101 children and adolescents, mean age 8.8 years, found significant efficacy of risperidone vs placebo for irritability on the Clinical Global Impressions-Improvement subscale[ 52 ], and the ABC-I subscale[ 29 ] at a mean dose of 1.8 mg/day. Effect size was 1.2. Side effects included significant weight gain, appetite increase in 73%, fatigue in 59%, and drowsiness in 49%, as well as prolactin elevation. The greatest benefits reported by parents were for self-injury and aggression. Another larger multisite RCT study of risperidone and parent training in 124 children and adolescents ages 4 through 13 found that parent training could lessen the dose of risperidone needed[ 53 ]. Risperidone doses were a mean of 2.26 mg/day or 0.071 mg/ kg in the risperidone-only group, vs 1.98 mg/day or 0.066 mg/kg ( P = 0.04, two-sided test) in the combination group of risperidone plus parent training.
Weight gain associated with risperidone treatment was marked, especially in some individuals in a double-blind crossover study performed by the author’s group, of risperidone vs placebo for challenging behaviors in participants aged 6 to 65 with ID and ASD[ 54 ]. In a subset of 19 subjects over approximately a year, weight gain was as follows: Children ( n = 5) ages 8 to 12 years gained 8.2 kg on average, adolescents ( n = 6) aged 13 to 16 years gained 8.4 kg on average, and adults gained 5.4 kg on average[ 55 ]. Prolactin elevation is greater with risperidone than with other atypical antipsychotics. Breast development, galactorrhea and amenorrhea should be monitored[ 56 ]. It is important to monitor for weight gain and metabolic syndrome abnormalities, notably hypertension, glucose elevation, midline obesity, and triglyceride elevations. These are important predisposing factors for diabetes, stroke, myocardial infarction, and cognitive dysfunction and brain abnormalities[ 55 ]. In the author’s experience, keeping risperidone doses low at or below 2 mg/day total, and splitting dosing to three times a day can help minimize weight gain. Importantly, clinical experience suggests that risperidone may be the most effective antipsychotic for self-injurious behavior.
A multisite RCT of aripiprazole in 218 children and adolescents with ASD, aged 6-17 years, mean age 9.3 years, found significant improvement in irritability in the aripiprazole vs the placebo group. Doses were 5, 10 or 15 mg/day in this 8-wk, parallel groups study. However, there was no protection against long-term relapse, the author agrees with this finding based on clinical practice, meaning that the efficacy may decrease over time, and increasing the dose may not recapture the initial good response. Side effects included sedation, the most common side effect leading to discontinuation, and significant weight gain[ 57 ]. Mean weight increases at week 8 were 0.3 kg for placebo, 1.3 kg for 5 mg/day, 1.3 kg for 10 mg/day and 1.5 kg for 15 mg/day groups, all P < 0.05 vs placebo. Importantly, aripiprazole in a low dose of 1 mg/day normalizes prolactin for example in an individual responding to risperidone who has elevated prolactin producing gynecomastia[ 58 ]. One small open pilot study compared olanzapine with haloperidol in children with autistic disorder[ 59 ] and one studied ziprasidone vs placebo[ 60 ] in ASD. Metformin for weight gain treatment with atypical antipsychotics was studied in a 16-wk, 4-center multisite RCT of 60 children. Metformin was associated with reductions in future weight gain, notably body mass index (BMI) z-scores decreased significantly more from baseline to week 16 than in the placebo group ( P = 0.003). However metformin did not alter lipid abnormalities, and gastrointestinal side effects identified included abdominal discomfort, abdominal pains and diarrhea[ 61 ] (in contrast to loxapine substitution discussed below).
Loxapine resembles an atypical antipsychotic at 5-10 mg/day
Loxapine shows promise clinically in adolescents and adults with ASD according to preliminary studies, and RCTs are warranted. This antipsychotic is a dibenzoxazepine tricyclic structure classified in the medium potency group of the typical antipsychotic class. Loxapine was designed in the 1980s to resemble clozapine but without the clozapine molecular component causing agranulocytosis. Loxapine has a history of extensive use in schizophrenia, usually at 40 to 80 mg/day (maximum dose of 200 mg/day) and may lack the marked weight gain and metabolic side effects of clozapine and other atypical antipsychotics[ 62 ]. A case report of a 10 year old female with autistic disorder who responded to loxapine 15 mg/day described its efficacy for treatment-resistant aggression and self-injurious behavior[ 63 ]. In low doses of 5 to 10 mg/day, loxapine resembles an atypical antipsychotic on PET brain studies, but lacks the weight gain and metabolic side effects[ 64 , 65 ]. A prospective 12-wk open trial of loxapine for irritability and aggression in 16 adolescents and adults with ASD[ 48 ], demonstrated that loxapine in low doses of 5 to 10 mg per day significantly improved irritability ratings on the ABC-I, with large pre- to post- treatment effect sizes on 4 subscales, d = 1.0-1.1. Fourteen of 16 subjects completed the study, all of whom had Clinical Global Impressions-Improvement scale ratings of Very Much Improved or Much Improved at week 12. Larger clinical trials are warranted.
A retrospective loxapine chart review, also by the author’s group, of 15 outpatient adolescents and adults with ASD and irritability, illustrates the strategy of adding loxapine 5-10 mg/day, followed by extremely gradual taper of offending antipsychotics, which reversed weight gain, metabolic syndrome and insulin resistance including diabetes[ 49 ]. All those in the series had gained weight and manifested at least one other metabolic abnormality since starting on the baseline antipsychotic. Fourteen of the subjects were being treated with atypical antipsychotics and one received chlorpromazine, prior to addition of loxapine 5 to 10 mg daily, followed by behavioral improvement and then taper of the offending antipsychotic. Final loxapine dose in 12 subjects was 5 mg/day, and 10 mg/day in 2 subjects. At the time of chart review, all but one subject (93%) were Very Much Improved or Much Improved on CGI-I. Mean weight loss after an average of 17 mo (range 7 to 26 mo) on loxapine was -5.7 kg, with BMI reduction averaging -1.9. Mean reduction in triglycerides was -33.7 mg/dL ( P = 0.03). Two subjects were tapered off metformin by their endocrinologists, and one person’s insulin for Type II diabetes was discontinued. Weight loss did not differ in those already receiving metformin at the time of loxapine add-on ( n = 4) though the numbers are small and the reader is therefore cautioned.
In a long-term outcomes chart review study, of 34 children, adolescents and adults with ASD, mean age 23.4 years (range 8 to 32 years), long-term low-dose loxapine at a mean dose of 8.9 mg/day (range 5 to 30 mg) was associated with lower rates of tardive dyskinesia and EPS than expected for a typical antipsychotic, mean treatment duration was 4.2 years[ 50 ]. Stahl[ 62 ] describes the addition of low doses of a classical antipsychotic to an atypical antipsychotic to “lead in” or “top up” the effect. Using loxapine add-on at 5-10 mg/day, the author has been able to minimize risperidone dose increases above 1.5-2 mg a day total of risperidone and this strategy appears weight-sparing.
Dysphagia and bowel obstruction associated with antipsychotics
A clinical word of caution is important regarding minimally verbal and neurologically impaired individuals treated with antipsychotics. Dysphagia is a common but often overlooked side effect of antipsychotics, predisposing to aspiration pneumonia and initiation of parenteral feeding after surgical insertion of gastrostomy tubes, which may then be life-long if the antipsychotic medications are not changed. Aspiration pneumonia is more common in those with severe developmental disabilities and minimally verbal individuals and those with cerebral palsy or quadriplegia treated with even moderate doses of antipsychotics, especially if the individual also receives concomitant cytochrome P450 2D6 (CYP2D6)-inhibiting SSRIs[ 66 ].
Substitution of the antipsychotic with other medications if needed, and gradual dose taper may allow swallowing improvement and normal eating reinstatement provided a repeat video swallow study is normal. A large study in non-psychiatric inpatients without ASD receiving antipsychotics mostly for delirium control found a significant association with aspiration pneumonia in comparison with a non-antipsychotic-exposed group[ 67 ]. The association magnitude was similar for typical and atypical antipsychotics. Also repeated ED and medical visits are commonly needed for ostomy revisions and infections. In clinical practice the problem is often magnified in individuals with spasticity by high dose anticholinergics such as baclofen or tizanidine. SSRIs that inhibit CYP2D6 may increase the effective dose of antispychotics and other medications such that small-appearing doses actually are effectively much larger. In addition, such prescribing practices often lead to severe constipation, paralytic ileus, bowel obstruction and resection in individuals with severe disabilities. The author avoids using loxapine in individuals with severe disabilities and uses low dose risperidone in divided doses instead, due to the elevated dysphagia and EPS risks.
SSRI STUDIES IN ASD; AND WHAT DRUGS MAY HELP RRBs?
Ssri studies have not demonstrated efficacy for rrbs.
While SSRIs may initially appear to help anxiety, depression and compulsive behaviors they may later worsen problems significantly and produce behavioral activation, especially in higher doses, in a dose-related manner. A 12-wk RCT study of 149 youth aged 5 to 17 years with ASD treated with citalopram, dosed up to 20 mg daily (mean dose 16 mg/day) for RRBs in ASD, was negative[ 68 ]. Overall there was no change in repetitive behavior but also significant side effects occurred. These included impulsiveness, increased energy level, hyperactivity, decreased concentration, increased RRBs, insomnia, diarrhea and skin dryness and itching.
A 14-wk RCT study of 158 youth aged 5 to 17 years with ASD, treated with fluoxetine found no differences from placebo for RRBs as rated on the Child Yale-Brown Obsessive Compulsive Scale-Pervasive Developmental Disorders version[ 69 ]. Another fluoxetine RCT was also negative; Australian investigators randomized 146 youth aged 7.5 to 18 years with ASD to fluoxetine (20 mg/day if < 40 kg or 30 mg/day if ≥ 40 kg) or placebo. Any differences favoring fluoxetine were statistically nonsignificant after variables of gender, verbal abilities and baseline differences were controlled for. There was also no significant trend toward improvement on secondary outcome measures of RRBs, irritability, anxiety or global change[ 70 ]. An older, smaller RCT study of 39 youths aged 5-16 years found that a mean dose of 9.9 mg/day of fluoxetine was superior to placebo[ 71 ], however this has not been replicated. Some individual case studies and a case series suggested fluoxetine response however[ 72 ].
SSRIs are the most commonly prescribed drugs in ASD[ 4 ], although their use is not backed by study evidence. In the author’s experience they may be helpful in high-functioning individuals with ASD for anxiety or depression. The Cochrane collaboration literature review of SSRIs in autism found no overall benefit in ASD, weighing positive and negative studies against each other[ 73 ]. In the author’s experience, non-stimulant ADHD medications rather than SSRIs can help OCD and repetitive behaviors, including ATX and amitriptyline, this is anecdotal evidence but could be worth a try in the clinic. Many times the patient is presenting on an antipsychotic already. RRBs may relate also to ADHD symptoms, notably impulsivity, as part of a common cognitive impairment of executive function (“putting the breaks on”) i.e. non-specific response inhibition[ 74 ]. These investigators found significant associations between repetitive speech and impulsive speech, between stereotyped behavior and overactivity, and between restricted preferences and impulsivity. This study further justifies the argument for studying non-stimulant ADHD medications for RRBs.
The TCA clomipramine reduced RRBs in one small study
Two TCAs typically targeting OCD, repetitive behaviors and hyperactivity, notably clomipramine and desipramine were compared with placebo in one double-blind study[ 75 ]. The investigators compared clomipramine to placebo in 12 subjects with autism using a crossover design, together with 12 different subjects completing a parallel trial of clomipramine vs desipramine. Clomipramine was superior to placebo and desipramine in reducing ratings on stereotypies, compulsive ritualized behaviors ( P < 0.05) and anger, while desipramine was no different from placebo except in reducing hyperactivity. However in the author’s experience substitution of amitriptyline for clomipramine in patients who present on clomipramine has produced greater global clinical improvements. This was an empirical observation made by the author’s team in the 1990s that appears valid still today[ 41 ].
In a small cross-over study, 5 of 18 children (28%) treated with low dose fluvoxamine responded[ 76 ]. Fluvoxamine was found to benefit RRBs, maladaptive behavior, aggression and language in a small 12-wk RCT of 30 adults with autistic disorder[ 77 ]. Treatment studies of SSRIs or other classes of agents for depression and for suicidal behavior in ASD are lacking. For anxiety disorders in general in ASD, some smaller studies suggest the efficacy of citalopram, and some were positive for buspirone. One buspirone study in ASD found worsening of aggression and self-injury[ 78 ].
Maintenance benzodiazepines are avoided as a general principle in individuals with developmental disabilities, except for insomnia and as pre-sedation for blood tests and other procedures including dental work. Downsides include disinhibition effects, cognitive slowing and impairment, clumsiness, falls and injuries associated with benzodiazepine treatment.
HOW TO APPROACH ANTI-SEIZURE MEDICATIONS?
The therapeutic behavioral effects of anti-seizure medications in ASD for use other than seizures are inconclusive, according to available evidence. An RCT by the author’s team of valproic acid for aggressive behavior in youth with ASD was negative, although some subjects appeared to benefit from it, likely related to the heterogeneity within ASD[ 79 ]. Another study found valproic acid to be beneficial for RRBs in ASD, however this finding has not been replicated. Worsening of behavior occurred in 4 of 13 cases[ 80 ]. Divalproex was effective for controlling irritability associated with fluoxetine treatment in ASD[ 81 ].
Clinical experience suggests a trial of anti-seizure medication such as valproic acid or lamotrigine (LTG) may be beneficial especially if seizures are known or suspected, and the presentation of behavior problems is bizarre or atypical. This pertains especially to minimally verbal individuals with severe ASD, who have very high rates of seizures, and those with a known history of traumatic brain injury.
For mood disorders
Apart from ADHD, bipolar disorder is another, much less common cause of impulsive aggression in ASD. A 25% lifetime prevalence for bipolar disorder vs 68% for ADHD was found in a tertiary-referred population of high-functioning adults with ASD[ 9 ]. Minimally verbal individuals may also present with bipolar-like illness however studies of this portion of the ASD spectrum are still needed. Although lithium may be helpful, anti-seizure medications are a first line of treatment for bipolar disorder in individuals with developmental disabilities.
Divalproex and carbamazepine
Mood-stabilizing anti-seizure medications including divalproex and carbamazepine are the first-line treatments for mania, mixed or rapid cycling bipolar disorder in the general population[ 82 ] as well as in individuals with developmental disabilities. Valproate/divalproex is FDA-approved for bipolar mania but not for acute bipolar depression in the general population. Divalproex can also be effective for acute mixed bipolar disorder[ 83 ]. Side effects include weight gain, polycystic ovarian syndrome, low blood platelets, alopecia, elevated liver enzymes and less often pancreatitis. In addition, divalproex can cause ASD if taken in early pregnancy[ 84 ]. Weight gain, hepatic enzymes and blood cell counts require monitoring.
Both divalproex and carbamazepine are available in extended-release formulations. Carbamazepine is weight-neutral but side effects may include nausea, vomiting, dizziness, drowsiness, dry mouth, constipation and unsteadiness. A rare but extremely serious potential side effect of carbamazepine is Stevens-Johnson syndrome, which may start as an influenza-like illness but progress to a blistering skin rash, skin peeling and death.
Lamotrigine (LTG)
LTG is the mood-stabilizing anti-seizure medication of choice for bipolar depression treatment as well as prophylaxis[ 85 ]. Apart from the vigilance needed for a serious skin rash again associated with Stevens-Johnson syndrome, and the need to start LTG slowly to try and prevent this, the longer-term profile of LTG is favorable in comparison with other anti-seizure medications. Another important use for consideration in psychiatry, according to author experience, is for suspected seizures including spells of eye-blinking, mouth movements or disorientation episodes accompanied by bizarre behavior presentations in ASD, as mentioned above.
Evidence for LTG is weaker for acute bipolar depression and rapid cycling bipolar disorder in the general population. LTG must be started extremely slowly by adding a low dose every 1 to 2 wk, and even more gradually if the individual is receiving divalproex (adding 25 mg every 2 wk), to avoid a potentially life-threatening skin rash that begins on the upper chest region. Skin rash signs include skin peeling, blistering, hives, itching and painful sores in the mouth or around the eyes. Other LTG side effects include blurred or double vision, poor motor coordination, headache, drowsiness, and difficulty thinking or speaking.
Gabapentin is an add-on anti-seizure medication often prescribed off-label in psychiatry for various indications despite negative RCTs including for bipolar disorder. Rather than acting on gamma-amino butyric acid, gabapentin likely acts on calcium channels in the brain and spinal cord, and has few drug interactions since it is renally excreted. Gabapentin add-on to valproic acid and low dose antipsychotic was helpful in an open study by the author, in adults with developmental disabilities[ 86 ]. Gabapentin in divided doses 3 times a day, totaling 900 to 1800 mg a day were effective as add-on to valproic acid and low dose antipsychotic, and also in a subset replaced lithium and thus eliminated lithium side effects. Gabapentin side effects included dizziness and clumsiness; to prevent these it was started at 100 mg daily and increased slowly by only 100-200 mg per week, although prospective RCT studies are needed.
While lithium is still used in ASD, the side effects are often worse in those with developmental disabilities, and include polydipsia and polyuria (excessive thirst, drinking and enuresis) and tremor. Acute toxicity is a medical emergency requiring dialysis and intensive care units treatment, and is a greater risk in individuals with disabilities. Vomiting, diarrhea, failure to drink fluids for any reason, and certain medications including the angiotensin-converting enzyme inhibitor losartan predispose to toxicity[ 86 ].
Insomnia is very common in ASD and should not be interpreted as mania-related illness unless accompanied by other observable mania features. Another pitfall is that loud, rapid speech and outgoing personality may not be due to bipolar disorder but an enduring personality trait with a life-long history.
Anti-seizure medication-related behavioral side effects
Importantly, several anti-seizure medications while benefitting seizures may produce adverse behavioral effects. The latter may not have been considered by the neurologist if the seizures are adequately controlled. Therefore identification of such side effects by the psychiatrist is essential. Barbiturate-based anti-seizure medications including phenobarbital and phenytoin, and ben-zodiazepine-based medications, as well as vigabitrin often worsen behavior. Such medications may lead to an ADHD-like picture of affect dysregulation, hyperactivity, restlessness, impulsive aggression and self-injury[ 87 ]. Carbamazepine, oxcarbazepine, levetiracetam and topiramate may also worsen hyperactivity, mood or psychotic symptoms or other behavior problems. LTG and divalproex may be less likely to have behavioral side effects in adults with ASD according to clinical experience.
Studies included followed a broad and thorough literature review of pharmacotherapy in ASD, in order to provide a clear overview of the topic as well as the author’s expert opinion. For a summary of key points for pharmacotherapy in ASD (Table (Table1). 1 ). Limitations of this opinion review include that aside from evidence-based guidelines, prescribing practices may be extremely variable, not only by country and region, but also by individual practitioners who may find other medications useful in ASD. The author has however attempted to provide a personal but balanced view overall. Regarding future drug treatments for core ASD symptoms it may not be possible for one drug to target and treat all of the many subtypes of ASD, given the many genetic and other causes. Of note is that while certain drugs such as ATX may not be available in all countries, amitriptyline is approved in many countries and is available in generic forms.
Key points for pharmacotherapy in autism spectrum disorders
Selective serotonin reuptake inhibitors may reduce anxiety or depression in high-functioning individuals but are unlikely to alleviate repe-titive/compulsive behaviors in autism spectrum disorders, and often cause activation and behavioral worsening. ASD: Autism spectrum disorders; ADHD: Attention deficit hyperactivity disorder.
CLINICAL PEARLS GLEANED OVER MANY DECADES OF RESEARCH AND PRACTICE TREATING ALL AGES WITH DEVELOPMENTAL DISABILITIES
Environmental and emotional causes are more likely to respond to behavioral consultation: this can be key also in treatment resistance.
It is important to emphasize that environmental and emotional causes of behavior problems will be more likely to respond to behavioral consultation and psychosocial interventions. Of late, there has been greater recognition of environmental contributors to psychiatric illness in the field in general. Abuse of all types is also more likely in vulnerable individuals such as those with developmental disabilities. Taking a detailed longitudinal history is essential, regarding likely environmental stressors such as family deaths or job losses, moves and staff changes leading to frustration and severe “protest” behavior problems including aggression, before making psychiatric diagnoses and trying medication treatments. Protest behaviors and use of aggression as communication are more likely especially if the individual has demonstrated consistently good functioning over one or more periods of time in their past. A developmental and childhood psychiatric history is also essential to understanding of presenting problems. Irritability can result from many non-psychiatric causes, including medical illness, lack of sleep, general frustration or unhappiness with a living situation. Treating just dimensional behavior problems, such as irritability or hyperactivity with single medications may be feasible for milder cases. As in other branches of medicine, if the diagnosis is wrong then the treatment will unlikely help.
Closer examination for ADHD and trying ADHD treatments pays off, including in females and adults with severe disabilities
This applies to ADHD wrongly diagnosed as bipolar disorder, since antipsychotics and mood stabilizers do not adequately treat ADHD-related impulsivity. This was a personal lesson the author learned early on in practice after specializing in treating this population. Females diagnosed with depression and recurrent suicidality may also respond to ADHD treatments, allowing for cautious taper off of antidepressants. Parents and caregivers often describe a person with ADHD person as “anxious” since they rarely sit still, and “moody” due to lack of affect regulation associated with easy crying or laughing spells.
DEX, ATX and amitriptyline are useful for ADHD comorbid with ASD
Impulsive aggression such as cussing, hitting, kicking, biting, pinching and running off may respond to one or more ADHD treatments if the ADHD history and diagnosis are elicited. Many adults already received treatment for ADHD as children but once transitioning services happens the ADHD diagnosis is overlooked. Although only studies in TD individuals are available as discussed above, a combination of low dose stimulant together with a non-stimulant ADHD medication such as ATX, amitriptyline or guanfacine may be needed. Low dose risperidone may also be used in combination with the ADHD treatments, although again only one study in the TD participants is available regarding this[ 88 ].
Two ADHD medications may be needed (stimulant and non-stimulant) possibly also together with low dose antipsychotic such as risperidone in moderate-to -severe cases with aggression. In the author’s experience, ATX is frequently clinically useful for ADHD with impulsive aggression, including in more severely disabled individuals. The tolerable doses may be lower than in higher functioning individuals, although improvements may be regained if the dose is decreased again after behavioral worsening following a dose increase occurs. More studies are warranted. Amitriptyline in low doses can be extremely helpful for cases with insomnia, headaches, gastrointestinal issues, ADHD, impulsive aggression and OCD, used with caution and watching for drug interactions. Studies are warranted of amitriptyline for RRBs in ASD according to author experience.
Are RRBs part of the ADHD spectrum, and could they respond to ADHD treatments?
The study by Burbridge and coworkers[ 74 ] leading to the concept of RRBs as related to ADHD, in other words a type of motor impulsivity, may be key to guiding future studies for RRB etiology and treatments. One study found ATX was somewhat effective for RRBs in youth with ASD, which is promising[ 39 ]. No known treatment currently exists for core ASD features, likely due to the heterogeneity and many different genetic causes. Metformin, arbaclofen, lovastatin, trifinetide, 5-HT7 agonist ligands, flavonoids, cannabidiol, cannabis and the dietary supplement sulfurophane amongst others, are still being studied[ 4 ].
Risperidone remains useful in youth with severe irritability and may be helpful for self-injury; dose at ≤ 2 mg/day in divided doses
Dosing risperidone at or below 2 mg/day given in divided doses may mitigate weight gain and metabolic side effects, though individuals vary in this regard. Another author observation is that risperidone may be the most effective medication for self-injurious behaviors including self-biting, head- banging, self-hitting and others. Weight gain and metabolic side effects require monitoring.
Loxapine at 5-10 mg/day resembles an atypical antipsychotic but likely with emerging safety evidence of a more favorable metabolic profile
Loxapine is one of the main antipsychotics now used in practice by the author and several colleagues in other regions, for adolescents and adults with ASD, related to an empirical finding made over 2 decades ago and then the preliminary published studies discussed above. Addition of 5-10 mg/day of loxapine often produces significant clinical improvement in irritability and aggression, which if needed then allows very gradual taper of other antipsychotics which have caused excessive weight gain or produced too little response. While a common practice may be to follow schizophrenia guidelines and convert a treatment-resistant person to a depo antipsychotic, hoping for improved aggression control, adding loxapine, in the author’s experience produces superior results overall. However loxapine is likely not suitable for more severely disabled individuals due to its potent dopamine blocking action that may cause dysphagia in them; low dose risperidone may be preferable in this setting. Olanzapine is another cause of dysphagia in those with more severe disabilities, according to clinical experience.
Gabapentin may be a useful add-on to divalproex and low dose antipsychotic if lithium is not a good choice for the individual patient
Published preliminary evidence on gabapentin add-on to valproate and low dose antipsychotic in ASD may be useful when lithium is not tolerated due to side effects, or if lithium toxicity has already occurred once or more. Studies are needed.
SSRIs may be helpful in higher-functioning ASD for anxiety or depression, but not for RRBs
SSRIs remain the most widely prescribed drug class in ASD in the United States overall. Recent negative studies of citalopram and fluoxetine for RRBs in youth with ASD are helpful in this clarification. In many cases, high dose SSRIs worsen OCD and agitation, while gradual SSRI taper may lead to clinical improvements. Also, in cases involving SSRIs increasing the effective antipsychotic dose due to CYP2D6 inhibition, swallowing impairment and bowel motility problems may be reversed by gradual SSRI taper and medication revisions.
Existing studies in ASD are useful guides for clinical practice, but many more are still needed. Most prescribing in individuals with developmental disabilities is of clinical necessity off-label. Some older psychotropic medications with emerging evidence may extend and improve possible successful treatment options for clinicians serving individuals of all ages with ASD and severe behavior problems. Until controlled studies of these drugs become available, cautious clinical use starting with low doses and minding drug interactions may be justified. Another important focus should be alerts regarding possible ADHD with impulsive aggression, especially in females and in adults with ASD. The older medications worth trying include, but are clearly not limited to, DEX, ATX and amitriptyline for individuals with ADHD associated with impulsive aggression.
For irritability and psychotic comorbidity in adolescents and adults with ASD, preliminary published evidence and clinical experience point to loxapine in doses of 5-10 mg/day having atypical antipsychotic properties but likely with lower metabolic risk associated. For likely seizure activity associated with bizarre behaviors that is unable to be worked up via electroencephalogram due to lack of cooperation, LTG may be considered, especially in those with severe disabilities since they have higher rates of seizures. No medications have been identified and replicated so far to treat the core symptoms of autism, including RRBs. Drugs without demonstrated benefit for core symptoms include risperidone, oxytocin, bumetanide, buspirone, citalopram, fluoxetine, fluvoxamine and N-acetyl cysteine. While SSRIs are the most commonly prescribed drugs in ASD and may help individual patients, recent RCT studies did not show significant efficacy for RRBs in ASD, but rather a significant side effect burden including behavioral activation. Clinical trials of the older drugs discussed are warranted. All medications should be used in conjunction with other multimodal therapies including behavioral consultation, and selected for the individual patient.
ACKNOWLEDGEMENTS
Thanks to Jakob Waterborg, PhD, for assistance with the table.
Conflict-of-interest statement: The author has been an investigator for Janssen Pharmaceuticals, Abbott Laboratories, Forest Laboratories, Supernus, Young Living Essential Oils, NIMH and NICHD. NICHD previously funded a risperidone program project grant with the author as principal investigator of the drug study project. The author currently has internal funding from University of Missouri-Kansas City to study amitriptyline in ASD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 28, 2022
First decision: February 21, 2023
Article in press: April 18, 2023
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Czech Republic; Nwabo Kamdje AH, Cameroon S-Editor: Fan JR L-Editor: A P-Editor: Chen YX

- Copy/Paste Link Link Copied
What are the treatments for autism?
There is currently no one standard treatment for autism spectrum disorder (ASD).
But there are many ways to help minimize the symptoms and maximize abilities. People who have ASD have the best chance of using all of their abilities and skills if they receive appropriate therapies and interventions.
The most effective therapies and interventions are often different for each person. However, most people with ASD respond best to highly structured and specialized programs. 1 In some cases, treatment can greatly reduce symptoms and help people with autism with daily activities.
Research shows that early diagnosis and interventions, such as during preschool or before, are more likely to have major positive effects on symptoms and later skills. Read more about early interventions for autism.
Because there can be overlap in symptoms between ASD and other disorders, such as attention deficit hyperactivity disorder (ADHD), 2 it's important that treatment focus on a person's specific needs, rather than the diagnostic label.
Select the links for more information on each type of treatment for ASD.
- Behavioral management therapy
- Cognitive behavior therapy
- Early intervention
- Educational and school-based therapies
- Joint attention therapy
- Medication treatment
- Nutritional therapy
- Occupational therapy
- Parent-mediated therapy
- Physical therapy
- Social skills training
- Speech-language therapy
If you have a question about treatment, talk to a health care provider who specializes in caring for people with ASD. These resources have more information about treatments for autism:
- The Centers for Disease Control and Prevention describes some treatment options. http://www.cdc.gov/ncbddd/autism/treatment.html
- National Institute of Mental Health. (2011). A parent's guide to autism spectrum disorder. Retrieved March 8, 2012, from http://www.nimh.nih.gov/health/publications/a-parents-guide-to-autism-spectrum-disorder/index.shtml
- Kotte, A., Joshi, G., Fried, R., Uchida, M., Spencer, A., Woodworth, K. Y., et al. (2013). Autistic traits in children with and without ADHD. Pediatrics, 132 (3), e612–e622.
This website uses cookies to ensure you get the best experience on our website. Without cookies your experience may not be seamless.

- Kennedy Institute of Ethics Journal
Ethical Concerns with Applied Behavior Analysis for Autism Spectrum “Disorder”
- Daniel A. Wilkenfeld , Allison M. McCarthy
- Johns Hopkins University Press
- Volume 30, Number 1, March 2020
- 10.1353/ken.2020.0000
- View Citation
Additional Information

This paper has both theoretical and practical ambitions. The theoretical ambitions are to explore what would constitute both effective and ethical treatment of Autism Spectrum Disorder (ASD). However, the practical ambition is perhaps more important: we argue that a dominant form of Applied Behavior Analysis (ABA), which is widely taken to be far-and-away the best “treatment” for ASD, manifests systematic violations of the fundamental tenets of bioethics. Moreover, the supposed benefits of the treatment not only fail to mitigate these violations, but often exacerbate them. Warnings of the perils of ABA are not original to us—autism advocates have been ringing this bell for some years. However, their pleas have been largely unheeded, and ABA continues to be offered to and quite frequently pushed upon parents as the appropriate treatment for autistic children. Our contribution is to argue that, from a bioethical perspective, autism advocates are fully justified in their concerns—the rights of autistic children and their parents are being regularly infringed upon. Specifically, we will argue that employing ABA violates the principles of justice and nonmaleficence and, most critically, infringes on the autonomy of children and (when pushed aggressively) of parents as well.
Project MUSE Mission
Project MUSE promotes the creation and dissemination of essential humanities and social science resources through collaboration with libraries, publishers, and scholars worldwide. Forged from a partnership between a university press and a library, Project MUSE is a trusted part of the academic and scholarly community it serves.

2715 North Charles Street Baltimore, Maryland, USA 21218
+1 (410) 516-6989 [email protected]
©2024 Project MUSE. Produced by Johns Hopkins University Press in collaboration with The Sheridan Libraries.
Now and Always, The Trusted Content Your Research Requires

Built on the Johns Hopkins University Campus

IMAGES
VIDEO
COMMENTS
Children with Autism Spectrum Disorders: Three Case Studies. Speech-language pathologists play a critical role in screening, assessing, diagnosing, and treating the language and social communication disorders of individuals with autism spectrum disorders (ASD). People with ASD use a variety of communication modes including speech, facial ...
CASE STUDY EXAMPLE effective instructional strategy and parents of children with ASD have used this strategy to successfully teach requesting. Additionally, naturalistic intervention is designed to be conducted within natural routines. Next, Mrs. Dell creates a data collection system that is succinct and easy to implement
Autism Spectrum Disorders Module: Prompting CASE STUDY EXAMPLE: Least-to-Most Prompting Abby is a 22-month-old toddler with developmental delays who has been identified at high risk for ASD. A speech language pathologist and an occupational therapist each deliver early intervention services to Abby and her family in their home.
Clinical case scenarios: Autism: recognition, referral and diagnosis. September 2011 Page 5 of 60 Clinical case scenarios for professionals working with children and young people Case scenario 1: Howard - recognition, referral and diagnosis Presentation Howard is 7 years old and presents at his GP with his mother as she is
Abstract. This article aims to observe all the manifestations of the behavior of a child with Autism Spectrum Disorder (ASD), which shows deficits mainly in the communication sector. Also, the ...
ABSTRACT. Language skills as well as general cognitive skills show a considerable variation in children with autism spectrum disorder (ASD). In previous studies, at least three profiles based on these skills have been suggested; autism with language and non-verbal cognitive skills within the average/normal range (ALN), autism with language disorder (ALD) without concurrent non-verbal cognitive ...
for such educational settings which uniquely cater for pre-school children with autism spectrum disorders, working in close partnership with their parents for the best all round results. It can be challenging to have a special needs child but finding the right educational setting is probably the biggest
Western Michigan University Abstract. This paper describes Autism Spectrum Disorder (ASD) including diagnostic criteria, suspected. causes, prevalence, comorbidities, and influences on client factors. A hypothetical case study is. presented to give readers an illustration of what someone with ASD might look like.
If you would like to share your personal story, please contact us at [email protected]. Autism Spectrum Disorders (ASDs) are a group of developmental disabilities that can cause significant social, communication and behavioral challenges. CDC is working to find out how many children have ASDs, discover the risk factors, and raise awareness of the ...
Making an Autism Spectrum Disorder Diagnosis Autism Case Training: A Developmental-Behavioral Pediatrics Curriculum 4 Distribute Case Study Part I Slide 3 Case Study Part I Billy is a 3 ½ -year-old boy you are seeing for the first time in your resident practice. He was born full term following a normal pregnancy and delivery. His newborn ...
The Communication Symbolic and Behavior Scales Developmental Profile (CSBS DP; Wetherby & Prizant, 1993) was used to determine communicative competence. This norm-referenced instrument for children 6-24 months old is characterized by outstanding psychometric data (i.e., sensitivity=89.4%- 94.4%; specificity=89.4%).
This case study shows how an 8-year-old boy with autism and mild intellectual disability underwent positive psychological development in terms of play, social communication, and mentalization during a year and a half of group-based therapy using COMSI®-(COMmunication and Social Interaction). This eclectic treatment has a relational approach and is based on developmental psychology, knowledge ...
This observational study examines the one-year diagnostic stability of autism spectrum disorder diagnosis in a clinical sample of 147 children diagnosed between 18 and 48 months of age.
Anxiety, Autism, and Intellectual Disability. Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by social communication deficits and restricted and repetitive behaviors (American Psychiatric Association [APA], 2013).The United States prevalence rate of ASD in 2016 was 2.76% (Zablotsky et al., 2017).It was previously believed that the majority of individuals with ASD ...
Frontiers in Psychiatry is proud to present our Case Reports series. Our case reports aim to highlight unique cases of patients that present with an unexpected/unusual diagnosis, treatment outcome, or clinical course. Case reports provide insight into the differential diagnosis, decision-making, and clinical management of unusual cases and are a valuable educational tool.<br/><br/>This ...
The goal of the study was to evaluate Theraplay using a sample of autistic children. Eight children diagnosed with mild to moderate autism participated in a 2-week intensive Theraplay intervention.
Case study 6. Mainstream post-primary. Child's name & age: KL, 13. Main areas of concern: Selective mutism in school, refusal to complete work; and to participate in class and sometimes to sit in class. Sensory over responsive, particularly with tactile and auditory input. Read full case study.
Gabrielle Lober. 2015. This paper describes Autism Spectrum Disorder (ASD) including diagnostic criteria, suspected causes, prevalence, comorbidities, and influences on client factors. A hypothetical case study is presented to give readers an illustration of what someone with ASD might look like. Possible treatment based on evidence and ...
A Case Study of Autism:…. Arun was brought for consultation with Dr. A M Reddy by his parents. He was about 4 years old, the second child to the parents. Even while he was being brought into the room, we could hear his loud wailing. It took some time for the child to calm down and later we could observe that the child was very restless.
This case study illustrates how stress and mental health difficulties can precede autism diagnosis in adults. The personal experiences detailed highlight how an adult autism diagnosis can bring about positive change, prompting increased self-knowledge and coping skills, improved relationships and. Furthermore, it highlights how a supportive ...
M. is a seven years old boy diagnosed with autism spectrum disorder at the age of two. He lives in Orlando, Florida with his mother and father and two other younger siblings. Patient's physical development is within the norm; he is 43''in tall and weighs 60 lbs. M. was born prematurely at 36 weeks through an uncomplicated vaginal delivery.
The pharmacological treatment for autism spectrum disorders is often poorly tolerated and has traditionally targeted associated conditions, with limited benefit for the core social deficits. We describe the novel use of a cannabidiol-based extract that incidentally improved core social deficits and overall functioning in a patient with autism spectrum disorder, at a lower dose than has been ...
Abstract. Autism Spectrum Disorders (ASDs) are a group of neurodevelopmental disorders characterised by impairments in 3 domains: communication, social interaction and repetitive behaviour. It is ...
Peri-Pregnancy Cannabis Use and Autism Spectrum Disorder in the Offspring: Findings from the Study to Explore Early Development. DiGuiseppi C, Crume T, Van Dyke J, et al. J Autism Dev Disord. 2022;52 (11):5064-5071. Heterogeneity in Autism Spectrum Disorder Case-Finding Algorithms in United States Health Administrative Database Analyses.
Core Tip: Most prescribing in autism spectrum disorders (ASD) is off-label; only risperidone and aripiprazole are Federal Drug Administration-approved in ASD, for irritability. Atypical antipsychotics are associated with metabolic side effects. Loxapine at 5-10 mg/day resembled an atypical antipsychotic in positron emission tomography studies; preliminary studies and clinical experience in ASD ...
Science Update: Altered fluid channels in the brain may be linked to diagnosis of autism spectrum disorder, NIH-funded study suggests Spotlight: Looking Back on NICHD in 2023 Science Update: Pain experienced during newborn intensive care could influence preterm infants' neurodevelopment, suggests NIH-funded study
Ethical Concerns with Applied Behavior Analysis for Autism Spectrum "Disorder". This paper has both theoretical and practical ambitions. The theoretical ambitions are to explore what would constitute both effective and ethical treatment of Autism Spectrum Disorder (ASD). However, the practical ambition is perhaps more important: we argue ...