Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Case-Control Study? | Definition & Examples

What Is a Case-Control Study? | Definition & Examples
Published on February 4, 2023 by Tegan George . Revised on June 22, 2023.
A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the “case,” and those without it are the “control.”
It’s important to remember that the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
Table of contents
When to use a case-control study, examples of case-control studies, advantages and disadvantages of case-control studies, other interesting articles, frequently asked questions.
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative , and they often are in healthcare settings. Case-control studies can be used for both exploratory and explanatory research , and they are a good choice for studying research topics like disease exposure and health outcomes.
A case-control study may be a good fit for your research if it meets the following criteria.
- Data on exposure (e.g., to a chemical or a pesticide) are difficult to obtain or expensive.
- The disease associated with the exposure you’re studying has a long incubation period or is rare or under-studied (e.g., AIDS in the early 1980s).
- The population you are studying is difficult to contact for follow-up questions (e.g., asylum seekers).
Retrospective cohort studies use existing secondary research data, such as medical records or databases, to identify a group of people with a common exposure or risk factor and to observe their outcomes over time. Case-control studies conduct primary research , comparing a group of participants possessing a condition of interest to a very similar group lacking that condition in real time.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Case-control studies are common in fields like epidemiology, healthcare, and psychology.
You would then collect data on your participants’ exposure to contaminated drinking water, focusing on variables such as the source of said water and the duration of exposure, for both groups. You could then compare the two to determine if there is a relationship between drinking water contamination and the risk of developing a gastrointestinal illness. Example: Healthcare case-control study You are interested in the relationship between the dietary intake of a particular vitamin (e.g., vitamin D) and the risk of developing osteoporosis later in life. Here, the case group would be individuals who have been diagnosed with osteoporosis, while the control group would be individuals without osteoporosis.
You would then collect information on dietary intake of vitamin D for both the cases and controls and compare the two groups to determine if there is a relationship between vitamin D intake and the risk of developing osteoporosis. Example: Psychology case-control study You are studying the relationship between early-childhood stress and the likelihood of later developing post-traumatic stress disorder (PTSD). Here, the case group would be individuals who have been diagnosed with PTSD, while the control group would be individuals without PTSD.
Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages.
Advantages of case-control studies
- Case-control studies are a great choice if you have any ethical considerations about your participants that could preclude you from using a traditional experimental design .
- Case-control studies are time efficient and fairly inexpensive to conduct because they require fewer subjects than other research methods .
- If there were multiple exposures leading to a single outcome, case-control studies can incorporate that. As such, they truly shine when used to study rare outcomes or outbreaks of a particular disease .
Disadvantages of case-control studies
- Case-control studies, similarly to observational studies, run a high risk of research biases . They are particularly susceptible to observer bias , recall bias , and interviewer bias.
- In the case of very rare exposures of the outcome studied, attempting to conduct a case-control study can be very time consuming and inefficient .
- Case-control studies in general have low internal validity and are not always credible.
Case-control studies by design focus on one singular outcome. This makes them very rigid and not generalizable , as no extrapolation can be made about other outcomes like risk recurrence or future exposure threat. This leads to less satisfying results than other methodological choices.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
A case-control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group .
While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are designed to follow a group of people with a common exposure or risk factor over time and observe their outcomes.
Case-control studies, in contrast, require both a case group and a control group, as suggested by their name, and usually are used to identify risk factors for a disease by comparing cases and controls.
A case-control study differs from a cross-sectional study because case-control studies are naturally retrospective in nature, looking backward in time to identify exposures that may have occurred before the development of the disease.
On the other hand, cross-sectional studies collect data on a population at a single point in time. The goal here is to describe the characteristics of the population, such as their age, gender identity, or health status, and understand the distribution and relationships of these characteristics.
Cases and controls are selected for a case-control study based on their inherent characteristics. Participants already possessing the condition of interest form the “case,” while those without form the “control.”
Keep in mind that by definition the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
The strength of the association between an exposure and a disease in a case-control study can be measured using a few different statistical measures , such as odds ratios (ORs) and relative risk (RR).
No, case-control studies cannot establish causality as a standalone measure.
As observational studies , they can suggest associations between an exposure and a disease, but they cannot prove without a doubt that the exposure causes the disease. In particular, issues arising from timing, research biases like recall bias , and the selection of variables lead to low internal validity and the inability to determine causality.
Sources in this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
George, T. (2023, June 22). What Is a Case-Control Study? | Definition & Examples. Scribbr. Retrieved June 7, 2024, from https://www.scribbr.com/methodology/case-control-study/
Schlesselman, J. J. (1982). Case-Control Studies: Design, Conduct, Analysis (Monographs in Epidemiology and Biostatistics, 2) (Illustrated). Oxford University Press.
Is this article helpful?
Tegan George
Other students also liked, what is an observational study | guide & examples, control groups and treatment groups | uses & examples, cross-sectional study | definition, uses & examples, what is your plagiarism score.
What Is A Case Control Study?
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
A case-control study is a research method where two groups of people are compared – those with the condition (cases) and those without (controls). By looking at their past, researchers try to identify what factors might have contributed to the condition in the ‘case’ group.
Explanation
A case-control study looks at people who already have a certain condition (cases) and people who don’t (controls). By comparing these two groups, researchers try to figure out what might have caused the condition. They look into the past to find clues, like habits or experiences, that are different between the two groups.
The “cases” are the individuals with the disease or condition under study, and the “controls” are similar individuals without the disease or condition of interest.
The controls should have similar characteristics (i.e., age, sex, demographic, health status) to the cases to mitigate the effects of confounding variables .
Case-control studies identify any associations between an exposure and an outcome and help researchers form hypotheses about a particular population.
Researchers will first identify the two groups, and then look back in time to investigate which subjects in each group were exposed to the condition.
If the exposure is found more commonly in the cases than the controls, the researcher can hypothesize that the exposure may be linked to the outcome of interest.
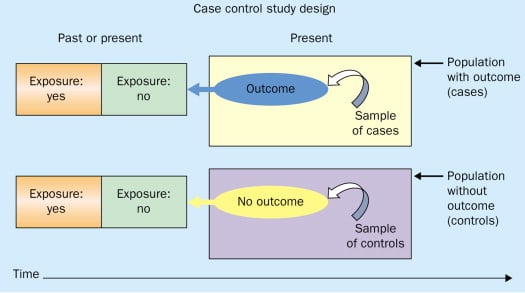
Figure: Schematic diagram of case-control study design. Kenneth F. Schulz and David A. Grimes (2002) Case-control studies: research in reverse . The Lancet Volume 359, Issue 9304, 431 – 434
Quick, inexpensive, and simple
Because these studies use already existing data and do not require any follow-up with subjects, they tend to be quicker and cheaper than other types of research. Case-control studies also do not require large sample sizes.
Beneficial for studying rare diseases
Researchers in case-control studies start with a population of people known to have the target disease instead of following a population and waiting to see who develops it. This enables researchers to identify current cases and enroll a sufficient number of patients with a particular rare disease.
Useful for preliminary research
Case-control studies are beneficial for an initial investigation of a suspected risk factor for a condition. The information obtained from cross-sectional studies then enables researchers to conduct further data analyses to explore any relationships in more depth.
Limitations
Subject to recall bias.
Participants might be unable to remember when they were exposed or omit other details that are important for the study. In addition, those with the outcome are more likely to recall and report exposures more clearly than those without the outcome.
Difficulty finding a suitable control group
It is important that the case group and the control group have almost the same characteristics, such as age, gender, demographics, and health status.
Forming an accurate control group can be challenging, so sometimes researchers enroll multiple control groups to bolster the strength of the case-control study.
Do not demonstrate causation
Case-control studies may prove an association between exposures and outcomes, but they can not demonstrate causation.
A case-control study is an observational study where researchers analyzed two groups of people (cases and controls) to look at factors associated with particular diseases or outcomes.
Below are some examples of case-control studies:
- Investigating the impact of exposure to daylight on the health of office workers (Boubekri et al., 2014).
- Comparing serum vitamin D levels in individuals who experience migraine headaches with their matched controls (Togha et al., 2018).
- Analyzing correlations between parental smoking and childhood asthma (Strachan and Cook, 1998).
- Studying the relationship between elevated concentrations of homocysteine and an increased risk of vascular diseases (Ford et al., 2002).
- Assessing the magnitude of the association between Helicobacter pylori and the incidence of gastric cancer (Helicobacter and Cancer Collaborative Group, 2001).
- Evaluating the association between breast cancer risk and saturated fat intake in postmenopausal women (Howe et al., 1990).
Frequently asked questions
1. what’s the difference between a case-control study and a cross-sectional study.
Case-control studies are different from cross-sectional studies in that case-control studies compare groups retrospectively while cross-sectional studies analyze information about a population at a specific point in time.
In cross-sectional studies , researchers are simply examining a group of participants and depicting what already exists in the population.
2. What’s the difference between a case-control study and a longitudinal study?
Case-control studies compare groups retrospectively, while longitudinal studies can compare groups either retrospectively or prospectively.
In a longitudinal study , researchers monitor a population over an extended period of time, and they can be used to study developmental shifts and understand how certain things change as we age.
In addition, case-control studies look at a single subject or a single case, whereas longitudinal studies can be conducted on a large group of subjects.
3. What’s the difference between a case-control study and a retrospective cohort study?
Case-control studies are retrospective as researchers begin with an outcome and trace backward to investigate exposure; however, they differ from retrospective cohort studies.
In a retrospective cohort study , researchers examine a group before any of the subjects have developed the disease, then examine any factors that differed between the individuals who developed the condition and those who did not.
Thus, the outcome is measured after exposure in retrospective cohort studies, whereas the outcome is measured before the exposure in case-control studies.
Boubekri, M., Cheung, I., Reid, K., Wang, C., & Zee, P. (2014). Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 10 (6), 603-611.
Ford, E. S., Smith, S. J., Stroup, D. F., Steinberg, K. K., Mueller, P. W., & Thacker, S. B. (2002). Homocyst (e) ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. International journal of epidemiology, 31 (1), 59-70.
Helicobacter and Cancer Collaborative Group. (2001). Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut, 49 (3), 347-353.
Howe, G. R., Hirohata, T., Hislop, T. G., Iscovich, J. M., Yuan, J. M., Katsouyanni, K., … & Shunzhang, Y. (1990). Dietary factors and risk of breast cancer: combined analysis of 12 case—control studies. JNCI: Journal of the National Cancer Institute, 82 (7), 561-569.
Lewallen, S., & Courtright, P. (1998). Epidemiology in practice: case-control studies. Community eye health, 11 (28), 57–58.
Strachan, D. P., & Cook, D. G. (1998). Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax, 53 (3), 204-212.
Tenny, S., Kerndt, C. C., & Hoffman, M. R. (2021). Case Control Studies. In StatPearls . StatPearls Publishing.
Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Martami, F., & Seifishahpar, M. (2018). Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case-Control Study. Headache, 58 (10), 1530-1540.
Further Information
- Schulz, K. F., & Grimes, D. A. (2002). Case-control studies: research in reverse. The Lancet, 359(9304), 431-434.
- What is a case-control study?
Related Articles
Research Methodology
Qualitative Data Coding

What Is a Focus Group?
Cross-Cultural Research Methodology In Psychology

What Is Internal Validity In Research?

Research Methodology , Statistics
What Is Face Validity In Research? Importance & How To Measure

Criterion Validity: Definition & Examples
- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
Statistics By Jim
Making statistics intuitive
Case Control Study: Definition, Benefits & Examples
By Jim Frost 2 Comments
What is a Case Control Study?
A case control study is a retrospective, observational study that compares two existing groups. Researchers form these groups based on the existence of a condition in the case group and the lack of that condition in the control group. They evaluate the differences in the histories between these two groups looking for factors that might cause a disease.

By evaluating differences in exposure to risk factors between the case and control groups, researchers can learn which factors are associated with the medical condition.
For example, medical researchers study disease X and use a case-control study design to identify risk factors. They create two groups using available medical records from hospitals. Individuals with disease X are in the case group, while those without it are in the control group. If the case group has more exposure to a risk factor than the control group, that exposure is a potential cause for disease X. However, case-control studies establish only correlation and not causation. Be aware of spurious correlations!
Case-control studies are observational studies because researchers do not control the risk factors—they only observe them. They are retrospective studies because the scientists create the case and control groups after the outcomes for the subjects (e.g., disease vs. no disease) are known.
This post explains the benefits and limitations of case-control studies, controlling confounders, and analyzing and interpreting the results. I close with an example case control study showing how to calculate and interpret the results.
Learn more about Experimental Design: Definition, Types, and Examples .
Related posts : Observational Studies Explained and Control Groups in Experiments
Benefits of a Case Control Study
A case control study is a relatively quick and simple design. They frequently use existing patient data, and the experimenters form the groups after the outcomes are known. Researchers do not conduct an experiment. Instead, they look for differences between the case and control groups that are potential risk factors for the condition. Small groups and individual facilities can conduct case-control studies, unlike other more intensive types of experiments.
Case-control studies are perfect for evaluating outbreaks and rare conditions. Researchers simply need to let a sufficient number of known cases accumulate in an established database. The alternative would be to select a large random sample and hope that the condition afflicts it eventually.
A case control study can provide rapid results during outbreaks where the researchers need quick answers. They are ideal for the preliminary investigation phase, where scientists screen potential risk factors. As such, they can point the way for more thorough, time-consuming, and expensive studies. They are especially beneficial when the current state of science knows little about the connection between risk factors and the medical condition. And when you need to identify potential risk factors quickly!
Cohort studies are another type of observational study that are similar to case-control studies, but there are some important differences. To learn more, read my post about Cohort Studies .
Limitations of a Case Control Study
Because case-control studies are observational, they cannot establish causality and provide lower quality evidence than other experimental designs, such as randomized controlled trials . Additionally, as you’ll see in the next section, this type of study is susceptible to confounding variables unless experimenters correctly match traits between the two groups.
A case-control study typically depends on health records. If the necessary data exist in sources available to the researchers, all is good. However, the investigation becomes more complicated if the data are not readily available.
Case-control studies can incorporate biases from the underlying data sources. For example, researchers frequently obtain patient data from hospital records. The population of hospital patients is likely to differ from the general population. Even the control patients are in the hospital for some reason—they likely have serious health problems. Consequently, the subjects in case-control studies are likely to differ from the general population, which reduces the generalizability of the results.
A case-control study cannot estimate incidence or prevalence rates for the disease. The data from these studies do not allow you to calculate the probability of a new person contracting the condition in a given period nor how common it is in the population. This limitation occurs because case-control studies do not use a representative sample.
Case-control studies cannot determine the time between exposure and onset of the medical condition. In fact, case-control studies cannot reliably assess each subject’s exposure to risk factors over time. Longitudinal studies, such as prospective cohort studies, can better make those types of assessment.
Related post : Causation versus Correlation in Statistics
Use Matching to Control Confounders
Because case-control studies are observational studies, they are particularly vulnerable to confounding variables and spurious correlations . A confounder correlates with both the risk factor and the outcome variable. Because observational studies don’t use random assignment to equalize confounders between the case and control groups, they can become unbalanced and affect the results.
Unfortunately, confounders can be the actual cause of the medical condition rather than the risk factor that the researchers identify. If a case-control study does not account for confounding variables, it can bias the results and make them untrustworthy.
Case-control studies typically use trait matching to control confounders. This technique involves selecting study participants for the case and control groups with similar characteristics, which helps equalize the groups for potential confounders. Equalizing confounders limits their impact on the results.
Ultimately, the goal is to create case and control groups that have equal risks for developing the condition/disease outside the risk factors the researchers are explicitly assessing. Matching facilitates valid comparisons between the two groups because the controls are similar to cases. The researchers use subject-area knowledge to identify characteristics that are critical to match.
Note that you cannot assess matching variables as potential risk factors. You’ve intentionally equalized them across the case and control groups and, consequently, they do not correlate with the condition. Hence, do not use the risk factors you want to evaluate as trait matching variables.
Learn more about confounding variables .
Statistical Analysis of a Case Control Study
Researchers frequently include two controls for each case to increase statistical power for a case-control study. Adding even more controls per case provides few statistical benefits, so studies usually do not use more than a 2:1 control to case ratio.
For statistical results, case-control studies typically produce an odds ratio for each potential risk factor. The equation below shows how to calculate an odds ratio for a case-control study.

Notice how this ratio takes the exposure odds in the case group and divides it by the exposure odds in the control group. Consequently, it quantifies how much higher the odds of exposure are among cases than the controls.
In general, odds ratios greater than one flag potential risk factors because they indicate that exposure was higher in the case group than in the control group. Furthermore, higher ratios signify stronger associations between exposure and the medical condition.
An odds ratio of one indicates that exposure was the same in the case and control groups. Nothing to see here!
Ratios less than one might identify protective factors.
Learn more about Understanding Ratios .
Now, let’s bring this to life with an example!
Example Odds Ratio in a Case-Control Study
The Kent County Health Department in Michigan conducted a case-control study in 2005 for a company lunch that produced an outbreak of vomiting and diarrhea. Out of multiple lunch ingredients, researchers found the following exposure rates for lettuce consumption.
| 53 | 33 | |
| 1 | 7 |
By plugging these numbers into the equation, we can calculate the odds ratio for lettuce in this case-control study.

The study determined that the odds ratio for lettuce is 11.2.
This ratio indicates that those with symptoms were 11.2 times more likely to have eaten lettuce than those without symptoms. These results raise a big red flag for contaminated lettuce being the culprit!
Learn more about Odds Ratios.
Epidemiology in Practice: Case-Control Studies (NIH)
Interpreting Results of Case-Control Studies (CDC)
Share this:

Reader Interactions
January 18, 2022 at 7:56 am
Great post, thanks for writing it!
Is it possible to test an odds ration for statistical significance?
January 18, 2022 at 7:41 pm
Hi Michael,
Thanks! And yes, you can test for significance. To learn more about that, read my post about odds ratios , where I discuss p-values and confidence intervals.
Comments and Questions Cancel reply

- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Why is biology important?
- When did science begin?
- Where was science invented?

case-control study
Our editors will review what you’ve submitted and determine whether to revise the article.
- National Center for Biotechnology Information - Case Control Studies
case-control study , in epidemiology , observational (nonexperimental) study design used to ascertain information on differences in suspected exposures and outcomes between individuals with a disease of interest (cases) and comparable individuals who do not have the disease (controls). Analysis yields an odds ratio (OR) that reflects the relative probabilities of exposure in the two populations. Case-control studies can be classified as retrospective (dealing with a past exposure) or prospective (dealing with an anticipated exposure), depending on when cases are identified in relation to the measurement of exposures. The case-control study was first used in its modern form in 1926. It grew in popularity in the 1950s following the publication of several seminal case-control studies that established a link between smoking and lung cancer .
Case-control studies are advantageous because they require smaller sample sizes and thus fewer resources and less time than other observational studies. The case-control design also is the most practical option for studying exposure related to rare diseases. That is in part because known cases can be compared with selected controls (as opposed to waiting for cases to emerge, which is required by other observational study designs) and in part because of the rare disease assumption, in which OR mathematically becomes an increasingly better approximation of relative risk as disease incidence declines. Case-control studies also are used for diseases that have long latent periods (long durations between exposure and disease manifestation) and are ideal when multiple potential risk factors are at play.
The primary challenge in designing a case-control study is the appropriate selection of cases and controls. Poor selection can result in confounding, in which correlations that are unrelated to the exposure exist between case and control subjects. Confounding in turn affects estimates of the association between disease and exposure, causing selection bias, which distorts OR figures. To overcome selection bias, controls typically are selected from the same source population as that used for the selection of cases. In addition, cases and controls may be matched by relevant characteristics. During the analysis of study data, multivariate analysis (usually logistic regression) can be used to adjust for the effect of measured confounders.
Bias in a case-control study might also result if exposures cannot be measured or recalled equally in both cases and controls. Healthy controls, for example, may not have been seen by a physician for a particular illness or may not remember the details of their illness. Choosing from a population with a disease different from the one of interest but of similar impact or incidence may minimize recall and measurement bias, since affected individuals may be more likely to recall exposures or to have had their information recorded to a level comparable to cases.
Study Design 101: Case Control Study
- Case Report
- Case Control Study
- Cohort Study
- Randomized Controlled Trial
- Practice Guideline
- Systematic Review
- Meta-Analysis
- Helpful Formulas
- Finding Specific Study Types
A study that compares patients who have a disease or outcome of interest (cases) with patients who do not have the disease or outcome (controls), and looks back retrospectively to compare how frequently the exposure to a risk factor is present in each group to determine the relationship between the risk factor and the disease.
Case control studies are observational because no intervention is attempted and no attempt is made to alter the course of the disease. The goal is to retrospectively determine the exposure to the risk factor of interest from each of the two groups of individuals: cases and controls. These studies are designed to estimate odds.
Case control studies are also known as "retrospective studies" and "case-referent studies."
- Good for studying rare conditions or diseases
- Less time needed to conduct the study because the condition or disease has already occurred
- Lets you simultaneously look at multiple risk factors
- Useful as initial studies to establish an association
- Can answer questions that could not be answered through other study designs
Disadvantages
- Retrospective studies have more problems with data quality because they rely on memory and people with a condition will be more motivated to recall risk factors (also called recall bias).
- Not good for evaluating diagnostic tests because it's already clear that the cases have the condition and the controls do not
- It can be difficult to find a suitable control group
Design pitfalls to look out for
Care should be taken to avoid confounding, which arises when an exposure and an outcome are both strongly associated with a third variable. Controls should be subjects who might have been cases in the study but are selected independent of the exposure. Cases and controls should also not be "over-matched."
Is the control group appropriate for the population? Does the study use matching or pairing appropriately to avoid the effects of a confounding variable? Does it use appropriate inclusion and exclusion criteria?
Fictitious Example
There is a suspicion that zinc oxide, the white non-absorbent sunscreen traditionally worn by lifeguards is more effective at preventing sunburns that lead to skin cancer than absorbent sunscreen lotions. A case-control study was conducted to investigate if exposure to zinc oxide is a more effective skin cancer prevention measure. The study involved comparing a group of former lifeguards that had developed cancer on their cheeks and noses (cases) to a group of lifeguards without this type of cancer (controls) and assess their prior exposure to zinc oxide or absorbent sunscreen lotions.
This study would be retrospective in that the former lifeguards would be asked to recall which type of sunscreen they used on their face and approximately how often. This could be either a matched or unmatched study, but efforts would need to be made to ensure that the former lifeguards are of the same average age, and lifeguarded for a similar number of seasons and amount of time per season.
Real-life Examples
Boubekri, M., Cheung, I., Reid, K., Wang, C., & Zee, P. (2014). Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine, 10 (6), 603-611. https://doi.org/10.5664/jcsm.3780
This pilot study explored the impact of exposure to daylight on the health of office workers (measuring well-being and sleep quality subjectively, and light exposure, activity level and sleep-wake patterns via actigraphy). Individuals with windows in their workplaces had more light exposure, longer sleep duration, and more physical activity. They also reported a better scores in the areas of vitality and role limitations due to physical problems, better sleep quality and less sleep disturbances.
Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Martami, F., & Seifishahpar, M. (2018). Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case-Control Study. Headache, 58 (10), 1530-1540. https://doi.org/10.1111/head.13423
This case-control study compared serum vitamin D levels in individuals who experience migraine headaches with their matched controls. Studied over a period of thirty days, individuals with higher levels of serum Vitamin D was associated with lower odds of migraine headache.
Related Formulas
- Odds ratio in an unmatched study
- Odds ratio in a matched study
Related Terms
A patient with the disease or outcome of interest.
Confounding
When an exposure and an outcome are both strongly associated with a third variable.
A patient who does not have the disease or outcome.
Matched Design
Each case is matched individually with a control according to certain characteristics such as age and gender. It is important to remember that the concordant pairs (pairs in which the case and control are either both exposed or both not exposed) tell us nothing about the risk of exposure separately for cases or controls.
Observed Assignment
The method of assignment of individuals to study and control groups in observational studies when the investigator does not intervene to perform the assignment.
Unmatched Design
The controls are a sample from a suitable non-affected population.
Now test yourself!
1. Case Control Studies are prospective in that they follow the cases and controls over time and observe what occurs.
a) True b) False
2. Which of the following is an advantage of Case Control Studies?
a) They can simultaneously look at multiple risk factors. b) They are useful to initially establish an association between a risk factor and a disease or outcome. c) They take less time to complete because the condition or disease has already occurred. d) b and c only e) a, b, and c
Evidence Pyramid - Navigation
- Meta- Analysis
- Case Reports
- << Previous: Case Report
- Next: Cohort Study >>

- Last Updated: Sep 25, 2023 10:59 AM
- URL: https://guides.himmelfarb.gwu.edu/studydesign101

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed, case control studies, affiliations.
- 1 University of Nebraska Medical Center
- 2 Spectrum Health/Michigan State University College of Human Medicine
- PMID: 28846237
- Bookshelf ID: NBK448143
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes. The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to the case individuals but do not have the outcome of interest. The researcher then looks at historical factors to identify if some exposure(s) is/are found more commonly in the cases than the controls. If the exposure is found more commonly in the cases than in the controls, the researcher can hypothesize that the exposure may be linked to the outcome of interest.
For example, a researcher may want to look at the rare cancer Kaposi's sarcoma. The researcher would find a group of individuals with Kaposi's sarcoma (the cases) and compare them to a group of patients who are similar to the cases in most ways but do not have Kaposi's sarcoma (controls). The researcher could then ask about various exposures to see if any exposure is more common in those with Kaposi's sarcoma (the cases) than those without Kaposi's sarcoma (the controls). The researcher might find that those with Kaposi's sarcoma are more likely to have HIV, and thus conclude that HIV may be a risk factor for the development of Kaposi's sarcoma.
There are many advantages to case-control studies. First, the case-control approach allows for the study of rare diseases. If a disease occurs very infrequently, one would have to follow a large group of people for a long period of time to accrue enough incident cases to study. Such use of resources may be impractical, so a case-control study can be useful for identifying current cases and evaluating historical associated factors. For example, if a disease developed in 1 in 1000 people per year (0.001/year) then in ten years one would expect about 10 cases of a disease to exist in a group of 1000 people. If the disease is much rarer, say 1 in 1,000,0000 per year (0.0000001/year) this would require either having to follow 1,000,0000 people for ten years or 1000 people for 1000 years to accrue ten total cases. As it may be impractical to follow 1,000,000 for ten years or to wait 1000 years for recruitment, a case-control study allows for a more feasible approach.
Second, the case-control study design makes it possible to look at multiple risk factors at once. In the example above about Kaposi's sarcoma, the researcher could ask both the cases and controls about exposures to HIV, asbestos, smoking, lead, sunburns, aniline dye, alcohol, herpes, human papillomavirus, or any number of possible exposures to identify those most likely associated with Kaposi's sarcoma.
Case-control studies can also be very helpful when disease outbreaks occur, and potential links and exposures need to be identified. This study mechanism can be commonly seen in food-related disease outbreaks associated with contaminated products, or when rare diseases start to increase in frequency, as has been seen with measles in recent years.
Because of these advantages, case-control studies are commonly used as one of the first studies to build evidence of an association between exposure and an event or disease.
In a case-control study, the investigator can include unequal numbers of cases with controls such as 2:1 or 4:1 to increase the power of the study.
Disadvantages and Limitations
The most commonly cited disadvantage in case-control studies is the potential for recall bias. Recall bias in a case-control study is the increased likelihood that those with the outcome will recall and report exposures compared to those without the outcome. In other words, even if both groups had exactly the same exposures, the participants in the cases group may report the exposure more often than the controls do. Recall bias may lead to concluding that there are associations between exposure and disease that do not, in fact, exist. It is due to subjects' imperfect memories of past exposures. If people with Kaposi's sarcoma are asked about exposure and history (e.g., HIV, asbestos, smoking, lead, sunburn, aniline dye, alcohol, herpes, human papillomavirus), the individuals with the disease are more likely to think harder about these exposures and recall having some of the exposures that the healthy controls.
Case-control studies, due to their typically retrospective nature, can be used to establish a correlation between exposures and outcomes, but cannot establish causation . These studies simply attempt to find correlations between past events and the current state.
When designing a case-control study, the researcher must find an appropriate control group. Ideally, the case group (those with the outcome) and the control group (those without the outcome) will have almost the same characteristics, such as age, gender, overall health status, and other factors. The two groups should have similar histories and live in similar environments. If, for example, our cases of Kaposi's sarcoma came from across the country but our controls were only chosen from a small community in northern latitudes where people rarely go outside or get sunburns, asking about sunburn may not be a valid exposure to investigate. Similarly, if all of the cases of Kaposi's sarcoma were found to come from a small community outside a battery factory with high levels of lead in the environment, then controls from across the country with minimal lead exposure would not provide an appropriate control group. The investigator must put a great deal of effort into creating a proper control group to bolster the strength of the case-control study as well as enhance their ability to find true and valid potential correlations between exposures and disease states.
Similarly, the researcher must recognize the potential for failing to identify confounding variables or exposures, introducing the possibility of confounding bias, which occurs when a variable that is not being accounted for that has a relationship with both the exposure and outcome. This can cause us to accidentally be studying something we are not accounting for but that may be systematically different between the groups.
Copyright © 2024, StatPearls Publishing LLC.
PubMed Disclaimer
Conflict of interest statement
Disclosure: Steven Tenny declares no relevant financial relationships with ineligible companies.
Disclosure: Connor Kerndt declares no relevant financial relationships with ineligible companies.
Disclosure: Mary Hoffman declares no relevant financial relationships with ineligible companies.
- Introduction
- Issues of Concern
- Clinical Significance
- Enhancing Healthcare Team Outcomes
- Review Questions
Similar articles
- Suicidal Ideation. Harmer B, Lee S, Rizvi A, Saadabadi A. Harmer B, et al. 2024 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. 2024 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 33351435 Free Books & Documents.
- Qualitative Study. Tenny S, Brannan JM, Brannan GD. Tenny S, et al. 2022 Sep 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. 2022 Sep 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 29262162 Free Books & Documents.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- The epidemiology of classic, African, and immunosuppressed Kaposi's sarcoma. Wahman A, Melnick SL, Rhame FS, Potter JD. Wahman A, et al. Epidemiol Rev. 1991;13:178-99. doi: 10.1093/oxfordjournals.epirev.a036068. Epidemiol Rev. 1991. PMID: 1765111 Review.
- Epidemiology of Kaposi's sarcoma. Beral V. Beral V. Cancer Surv. 1991;10:5-22. Cancer Surv. 1991. PMID: 1821323 Review.
- Setia MS. Methodology Series Module 2: Case-control Studies. Indian J Dermatol. 2016 Mar-Apr;61(2):146-51. - PMC - PubMed
- Sedgwick P. Bias in observational study designs: case-control studies. BMJ. 2015 Jan 30;350:h560. - PubMed
- Groenwold RHH, van Smeden M. Efficient Sampling in Unmatched Case-Control Studies When the Total Number of Cases and Controls Is Fixed. Epidemiology. 2017 Nov;28(6):834-837. - PubMed
Publication types
- Search in PubMed
- Search in MeSH
- Add to Search
Related information
Linkout - more resources, full text sources.
- NCBI Bookshelf
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- En español – ExME
- Em português – EME
Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
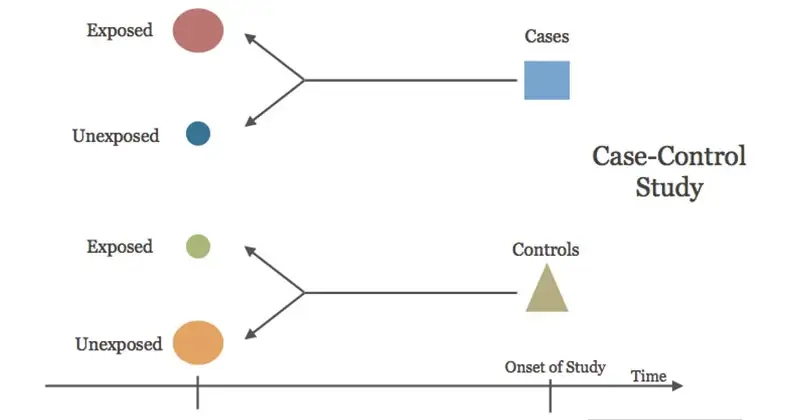
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
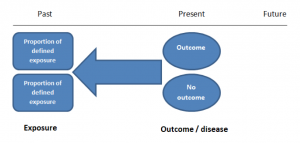
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
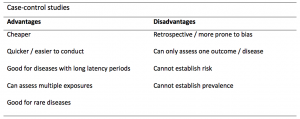
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
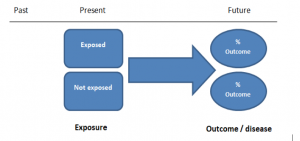
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
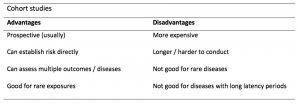
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.

Microbe Notes
Case-Control Study- Definition, Steps, Advantages, Limitations
A case-control study (also known as a case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute.
- It is designed to help determine if an exposure is associated with an outcome (i.e., disease or condition of interest). In recent years, the case-control approach has emerged as a permanent method of epidemiological investigation.
- Case-control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who have that condition/disease (the “cases”) with patients who do not have the condition/disease but are otherwise similar (the “controls”).
- In theory, the case-control study can be described simply. First, identify the cases (a group known to have the outcome) and the controls (a group known to be free of the outcome). Then, look back in time to learn which subjects in each group had the exposure(s), comparing the frequency of the exposure in the case group to the control group.
- This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is the odds ratio (OR).
Source: EBM Consult, LLC
Table of Contents
Interesting Science Videos
The Nature of Case-Control Studies
- By definition, a case-control study is always retrospective because it starts with an outcome then traces back to investigate exposures. When the subjects are enrolled in their respective groups, the outcome of each subject is already known by the investigator. This, and not the fact that the investigator usually makes use of previously collected data, is what makes case-control studies ‘retrospective’.
- The case-control study compares the prevalence of suspected causal factors between individuals with disease and controls who do not have the disease. If the prevalence of the factor is significantly different in cases than it is in controls, this factor may be associated with the disease.
- Although case-control studies can identify associations, they do not measure risk. An estimate of relative risk, however, can be derived by calculating the odds ratio.
- both exposure and outcome (disease) have occurred before the start of the study
- the study proceeds backward from effect to cause; and
- it uses a control or comparison group to support or refute an inference.
Steps Involved in Case-Control Studies
- By definition, a case-control study involves two populations – cases and controls.
- The focus is on a disease or some other health problem that has already developed.
- Case-control studies are basically comparison studies. Cases and controls must be comparable with respect to known “confounding factors” such as age, sex, occupation, social status, etc.
- The questions asked relate to personal characteristics and antecedent exposures which may be responsible for the condition studied.
- For example, one can use as “cases” the immunized children and use as “controls” un-immunized children and look for factors of interest in their past histories.
There are four basic steps in conducting a case-control study:
- Selection of cases and controls
- Measurement of exposure, and
- Analysis and interpretation
A. Selection of Cases and Controls
- The first is to identify a suitable group of cases and a group of controls.
- While the identification of cases is relatively easy, the selection of suitable controls may present difficulties.
- The definition of what constitutes a “case” is crucial to the case-control study.
- DIAGNOSTIC CRITERIA: The diagnostic criteria of the disease and the stage of the disease, if any (e.g., breast cancer Stage I) to be included in the study must be specified before the study is undertaken. Once the diagnostic criteria are established, they should not be altered or changed until the study is over.
- ELIGIBILITY CRITERIA: The second criterion is that of eligibility. A criterion customarily employed is the requirement that only newly diagnosed (incident) cases within a specified period of time are eligible than old cases or cases in advanced stages of the disease (prevalent cases).
- The cases may be drawn from hospitals or the general population.
- The cases should be fairly representative of all cases in the community.
- The controls must be free from the disease under study.
- They must be as similar to the cases as possible, except for the absence of the disease under study.
- As a rule, a comparison group is identified before a study is done, comprising of persons who have not been exposed to the disease or some other factor whose influence is being studied.
- Difficulties may arise in the selection of controls if the disease under investigation occurs in subclinical forms whose diagnosis is difficult.
- Selection of an appropriate control group is, therefore, an important prerequisite, for it is against this, we make comparisons, draw inferences and make judgments about the outcome of the investigation.
- The possible sources from which controls may be selected include hospitals, relatives, neighbors and the general population.
- If many cases are available, and a large study is contemplated, and if the cost to collect case and control is about equal, then one tends to use one control for each case. If the study group is small (say under 50) as many as 2,3, or even 4 controls can be selected for each study subject.
- To sum up, the selection of proper cases and controls is crucial to the interpretation of the results of case-control studies.
B. Matching
- The controls may differ from the cases in a number of factors such as age, sex, occupation, social status, etc.
- An important consideration is to ensure comparability between cases and controls. This involves what is known as “matching”.
- Matching is defined as the process by which we select controls in such a way that they are similar to cases with regard to certain pertinent selected variables (e.g., age) which are known to influence the outcome of the disease and which, if not adequately matched for comparability, could distort or confound the results.
- While matching it should be borne in mind that the suspected aetiological factor or the variable we wish to measure should not be matched, because, by matching, its aetiological role is eliminated in that study. The cases and controls will then become automatically alike with respect to that factor.
- There are several kinds of matching procedures such as group matching, pair matching, etc.
C. Measurement of Exposure
- Definitions and criteria about exposure (or variables which may be of aetiological importance) are just as important as those used to define cases and controls.
- Information about exposure should be obtained in precisely the same manner both for cases and controls.
- This may be obtained by interviews, by questionnaires or by studying past records of cases such as hospital records, employment records, etc.
- It is important to recognize that when case-control studies are being used to test associations, the most important factor to be considered, even more, important than the P. values obtained, is the question of “bias” or systematic error which must be ruled out.
D. Analysis
The final step is analysis, to find out:
- Exposure rates among cases and controls to suspected factor.
- Estimation of disease risk associated with exposure (Odds ratio).

Advantages of Case-Control Studies
- Relatively easy to carry out.
- Rapid and inexpensive (compared with cohort studies).
- Require comparatively few subjects.
- Particularly suitable to investigate rare diseases or diseases about which little is known. But a disease which is rare in the general population (e.g., leukemia in adolescents) may not be rare in the special exposure group (e.g. prenatal X-rays).
- No risk to subjects.
- Allows the study of several different aetiological factors (e.g., smoking, physical activity and personality characteristics in myocardial infarction).
- Risk factors can be identified. Rational prevention and control programs can be established.
- No attrition problems, because case-control studies do not require follow-up of individuals into the future.
- Ethical problems are minimal.
Limitations of Case-Control Study
- Problems of bias relies on memory or past records, the accuracy of which may be uncertain; validation of information obtained is difficult or sometimes impossible.
- Selection of an appropriate control group may be difficult.
- We cannot measure incidence, and can only estimate the relative risk.
- Do not distinguish between causes and associated factors.
- Not suited to the evaluation of therapy or prophylaxis of disease.
- Another major concern is the representativeness of cases and controls.
- A hypothesis is necessary for case-control studies. Relationships will be observed only for those factors studied.
- Case-control studies are not useful for determining the spectrum of health outcomes resulting from specific exposures, because a definition of a case is required in order to do a case-control study.
- Gordis, L. (2014). Epidemiology (Fifth edition.). Philadelphia, PA: Elsevier Saunders.
- White, F., Stallones, L., & Last, J. M. (2013). Global public health: Ecological foundations. New York, NY: Oxford University Press.
- Park, K. (n.d.). Park’s textbook of preventive and social medicine.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706071/
- https://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated/8-case-control-and-cross-sectional
- https://www.students4bestevidence.net/case-control-and-cohort-studies-overview/
About Author
Sagar Aryal
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Case–Control Study
- First Online: 13 December 2023
Cite this chapter

- Noraini Abdul Ghafar 2
556 Accesses
A case–control study is an observational study designed to determine if a risk factor is associated with an outcome of interest (disease or condition). This study design permits the researcher to determine if an exposure is associated with an outcome. First, a group with the outcome of interest (“cases”) is identified. Next, a group similar to cases (“controls”) is selected from the “study base” that yielded the cases but without the outcome of interest. Matching of cases and controls on certain characteristics ensures similarity and increases study efficiency. Historical risk factors in both groups are evaluated to determine whether some RFs occur more frequently in cases than controls. Case–control studies may establish an association between a risk factor and outcome but cannot demonstrate causation because of its retrospective nature. Compared to other study designs, case–control studies are inexpensive, quick, and allow the evaluation of several risk factors. Case–control study designs are useful for studying rare diseases, diseases with long latent periods, and for outbreak investigations. Limitations include selection bias and recall bias. Among the strategies to overcome selection bias include an appropriate sampling method, matching, using a minimum of two control groups, and drawing both cases and controls from the same population. Meanwhile, recall bias could be minimized by blinding.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Case Control Studies

Level III Evidence: A Case-Control Study

Epidemiological Studies
Lewallen S, Courtright P. Epidemiology in practice: case-control studies. Community Eye Health. 1998;11(28):57–8. PMID: 17492047; PMCID: PMC1706071
CAS PubMed PubMed Central Google Scholar
dos Santos Silva I. 1999. Chapter 9: case control studies in cancer epidemiology: principles and methods ISBN-13 978–92–832-0405-3.
Google Scholar
Breslow NE. Statistics in epidemiology: the case-control study. J Am Stat Assoc. 1996;91(433):14–28. https://doi.org/10.1080/01621459.1996.10476660 . PMID: 12155399
Article CAS PubMed Google Scholar
Setia MS. Methodology series module 2: case control studies. Indian J Dermatol. 2016;61:146–51.
Article PubMed PubMed Central Google Scholar
Mitra AK. Investigating the types of epidemiologic studies. In: Epidemiology for dummies. 1st ed. Hoboken, New Jersey, United States: Wiley; 2023.
Critchley J. Epidemiology for the uninitiated, 5th ed. J Epidemiol Community Health. 2004;58
Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I Principles Am J Epidemiol. 1992;135(9):1019–28. https://doi.org/10.1093/oxfordjournals.aje.a116396 . PMID: 1595688
Hennessy S, Bilker WB, Berlin JA, Strom BL. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol. 1999;149(2):195–7. https://doi.org/10.1093/oxfordjournals.aje.a009786 . Erratum in: Am J Epidemiol 1999 Mar 1;149(5):489. PMID: 9921965
Sedgwick P. Case-control studies: advantages and disadvantages. BMJ. 2014;3(348):f7707. https://doi.org/10.1136/bmj.f7707 . PMID: 31419845
Article Google Scholar
Sedgwick P. Nested case-control studies: advantages and disadvantages. BMJ (online). 2014;348:g1532. https://doi.org/10.1136/bmj.g1532 .
Sedgwick P. Nested case-control studies. BMJ. 2010;(340):c2582. https://doi.org/10.1136/bmj.c2582 . PMID: 20484347
Ernster. Nested case control studies. Prev Med. 1994;23:587–90.
Langholz B, Clayton D. Sampling strategies in nested case-control studies. Environ Health Perspect. 1994;102 Suppl 8(Suppl 8):47–51. https://doi.org/10.1289/ehp.94102s847 . PMID: 7851330; PMCID: PMC1566552
Tan MM, Ho WK, Yoon SY, Mariapun S, Hasan SN, Lee DS, Hassan T, Lee SY, Phuah SY, Sivanandan K, Ng PP, Rajaram N, Jaganathan M, Jamaris S, Islam T, Rahmat K, Fadzli F, Vijayananthan A, Rajadurai P, See MH, Thong MK, Mohd Taib NA, Yip CH, Teo SH. A case-control study of breast cancer risk factors in 7,663 women in Malaysia. PLoS One. 2018;13(9):e0203469. https://doi.org/10.1371/journal.pone.0203469 . PMID: 30216346; PMCID: PMC6138391
Article CAS PubMed PubMed Central Google Scholar
Ganesh B, Sushama S, Monika S, Suvarna P. A case-control study of risk factors for lung cancer in Mumbai. India Asian Pac J Cancer Prev. 2011;12(2):357–62. PMID: 21545194
CAS PubMed Google Scholar
Xi C, Luo M, Wang T, Wang Y, Wang S, Guo L, Lu C. Association between maternal lifestyle factors and low birth weight in preterm and term births: a case-control study. Reprod Health. 2020;17(1):93. https://doi.org/10.1186/s12978-020-00932-9 .
Shimeles E, Enquselassie F, Aseffa A, Tilahun M, Mekonen A, Wondimagegn G, Hailu T. Risk factors for tuberculosis: a case-control study in Addis Ababa, Ethiopia. PLoS One. 2019;14(4):e0214235. https://doi.org/10.1371/journal.pone.0214235 . PMID: 30939169; PMCID: PMC6445425
Kalra A. The odds ratio: principles and applications. J Pract Cardiovasc Sci. 2016;2:49–51.
Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010;19(3):227–9. Erratum in: J Can Acad Child Adolesc Psychiatry. 2015 Winter;24(1):58. PMID: 20842279; PMCID: PMC2938757
PubMed PubMed Central Google Scholar
Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20:54–60.
Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–42. https://doi.org/10.1097/PRS.0b013e3181f44abc . PMID: 20697313; PMCID: PMC2998589
Download references
Author information
Authors and affiliations.
School of Health Sciences, Universiti Sains Malaysia (Health Campus), Kubang Kerian, Kelantan, Malaysia
Noraini Abdul Ghafar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Noraini Abdul Ghafar .
Editor information
Editors and affiliations.
Department of Epidemiology and Biostatistics, Jackson State University, Jackson, MS, USA
Amal K. Mitra
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Ghafar, N.A. (2024). Case–Control Study. In: Mitra, A.K. (eds) Statistical Approaches for Epidemiology. Springer, Cham. https://doi.org/10.1007/978-3-031-41784-9_3
Download citation
DOI : https://doi.org/10.1007/978-3-031-41784-9_3
Published : 13 December 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-41783-2
Online ISBN : 978-3-031-41784-9
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Quantitative study designs: Case Control
Quantitative study designs.
- Introduction
- Cohort Studies
- Randomised Controlled Trial
Case Control
- Cross-Sectional Studies
- Study Designs Home
In a Case-Control study there are two groups of people: one has a health issue (Case group), and this group is “matched” to a Control group without the health issue based on characteristics like age, gender, occupation. In this study type, we can look back in the patient’s histories to look for exposure to risk factors that are common to the Case group, but not the Control group. It was a case-control study that demonstrated a link between carcinoma of the lung and smoking tobacco . These studies estimate the odds between the exposure and the health outcome, however they cannot prove causality. Case-Control studies might also be referred to as retrospective or case-referent studies.
Stages of a Case-Control study
This diagram represents taking both the case (disease) and the control (no disease) groups and looking back at their histories to determine their exposure to possible contributing factors. The researchers then determine the likelihood of those factors contributing to the disease.
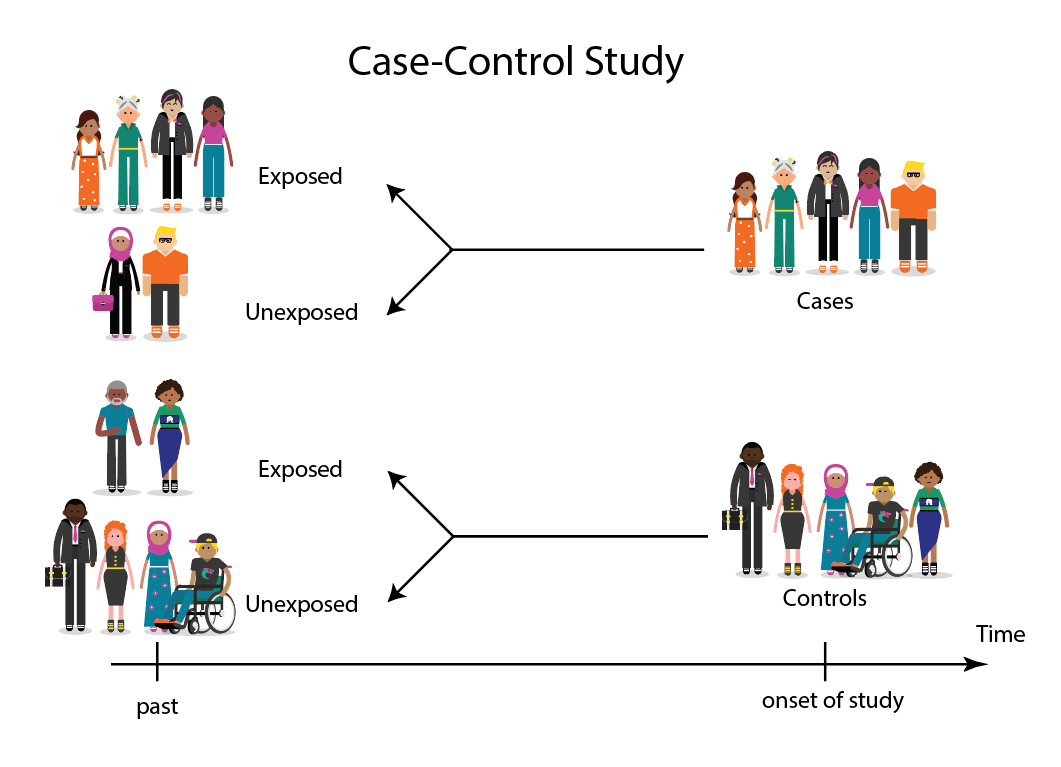
(FOR ACCESSIBILITY: A case control study is likely to show that most, but not all exposed people end up with the health issue, and some unexposed people may also develop the health issue)
Which Clinical Questions does Case-Control best answer?
Case-Control studies are best used for Prognosis questions.
For example: Do anticholinergic drugs increase the risk of dementia in later life? (See BMJ Case-Control study Anticholinergic drugs and risk of dementia: case-control study )
What are the advantages and disadvantages to consider when using Case-Control?
* Confounding occurs when the elements of the study design invalidate the result. It is usually unintentional. It is important to avoid confounding, which can happen in a few ways within Case-Control studies. This explains why it is lower in the hierarchy of evidence, superior only to Case Studies.
What does a strong Case-Control study look like?
A strong study will have:
- Well-matched controls, similar background without being so similar that they are likely to end up with the same health issue (this can be easier said than done since the risk factors are unknown).
- Detailed medical histories are available, reducing the emphasis on a patient’s unreliable recall of their potential exposures.
What are the pitfalls to look for?
- Poorly matched or over-matched controls. Poorly matched means that not enough factors are similar between the Case and Control. E.g. age, gender, geography. Over-matched conversely means that so many things match (age, occupation, geography, health habits) that in all likelihood the Control group will also end up with the same health issue! Either of these situations could cause the study to become ineffective.
- Selection bias: Selection of Controls is biased. E.g. All Controls are in the hospital, so they’re likely already sick, they’re not a true sample of the wider population.
- Cases include persons showing early symptoms who never ended up having the illness.
Critical appraisal tools
To assist with critically appraising case control studies there are some tools / checklists you can use.
CASP - Case Control Checklist
JBI – Critical appraisal checklist for case control studies
CEBMA – Centre for Evidence Based Management – Critical appraisal questions (focus on leadership and management)
STROBE - Observational Studies checklists includes Case control
SIGN - Case-Control Studies Checklist
Real World Examples
Smoking and carcinoma of the lung; preliminary report
- Doll, R., & Hill, A. B. (1950). Smoking and carcinoma of the lung; preliminary report. British Medical Journal , 2 (4682), 739–748. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2038856/
- Key Case-Control study linking tobacco smoking with lung cancer
- Notes a marked increase in incidence of Lung Cancer disproportionate to population growth.
- 20 London Hospitals contributed current Cases of lung, stomach, colon and rectum cancer via admissions, house-physician and radiotherapy diagnosis, non-cancer Controls were selected at each hospital of the same-sex and within 5 year age group of each.
- 1732 Cases and 743 Controls were interviewed for social class, gender, age, exposure to urban pollution, occupation and smoking habits.
- It was found that continued smoking from a younger age and smoking a greater number of cigarettes correlated with incidence of lung cancer.
Anticholinergic drugs and risk of dementia: case-control study
- Richardson, K., Fox, C., Maidment, I., Steel, N., Loke, Y. K., Arthur, A., . . . Savva, G. M. (2018). Anticholinergic drugs and risk of dementia: case-control study. BMJ , 361, k1315. Retrieved from http://www.bmj.com/content/361/bmj.k1315.abstract .
- A recent study linking the duration and level of exposure to Anticholinergic drugs and subsequent onset of dementia.
- Anticholinergic Cognitive Burden (ACB) was estimated in various drugs, the higher the exposure (measured as the ACB score) the greater likeliness of onset of dementia later in life.
- Antidepressant, urological, and antiparkinson drugs with an ACB score of 3 increased the risk of dementia. Gastrointestinal drugs with an ACB score of 3 were not strongly linked with onset of dementia.
- Tricyclic antidepressants such as Amitriptyline have an ACB score of 3 and are an example of a common area of concern.
Omega-3 deficiency associated with perinatal depression: Case-Control study
- Rees, A.-M., Austin, M.-P., Owen, C., & Parker, G. (2009). Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Research , 166(2), 254-259. Retrieved from http://www.sciencedirect.com/science/article/pii/S0165178107004398 .
- During pregnancy women lose Omega-3 polyunsaturated fatty acids to the developing foetus.
- There is a known link between Omgea-3 depletion and depression
- Sixteen depressed and 22 non-depressed women were recruited during their third trimester
- High levels of Omega-3 were associated with significantly lower levels of depression.
- Women with low levels of Omega-3 were six times more likely to be depressed during pregnancy.
References and Further Reading
Doll, R., & Hill, A. B. (1950). Smoking and carcinoma of the lung; preliminary report. British Medical Journal, 2(4682), 739–748. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2038856/
Greenhalgh, Trisha. How to Read a Paper: the Basics of Evidence-Based Medicine, John Wiley & Sons, Incorporated, 2014. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/deakin/detail.action?docID=1642418 .
Himmelfarb Health Sciences Library. (2019). Study Design 101: Case-Control Study. Retrieved from https://himmelfarb.gwu.edu/tutorials/studydesign101/casecontrols.cfm
Hoffmann, T., Bennett, S., & Del Mar, C. (2017). Evidence-Based Practice Across the Health Professions (Third edition. ed.): Elsevier.
Lewallen, S., & Courtright, P. (1998). Epidemiology in practice: case-control studies. Community Eye Health, 11(28), 57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706071/
Pelham, B. W. a., & Blanton, H. (2013). Conducting research in psychology : measuring the weight of smoke /Brett W. Pelham, Hart Blanton (Fourth edition. ed.): Wadsworth Cengage Learning.
Rees, A.-M., Austin, M.-P., Owen, C., & Parker, G. (2009). Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Research, 166(2), 254-259. Retrieved from http://www.sciencedirect.com/science/article/pii/S0165178107004398
Richardson, K., Fox, C., Maidment, I., Steel, N., Loke, Y. K., Arthur, A., … Savva, G. M. (2018). Anticholinergic drugs and risk of dementia: case-control study. BMJ, 361, k1315. Retrieved from http://www.bmj.com/content/361/bmj.k1315.abstract
Statistics How To. (2019). Case-Control Study: Definition, Real Life Examples. Retrieved from https://www.statisticshowto.com/case-control-study/
- << Previous: Randomised Controlled Trial
- Next: Cross-Sectional Studies >>
- Last Updated: May 15, 2024 11:37 AM
- URL: https://deakin.libguides.com/quantitative-study-designs
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Observational Studies: Cohort and Case-Control Studies
Jae w. song.
1 Research Fellow, Section of Plastic Surgery, Department of Surgery The University of Michigan Health System; Ann Arbor, MI
Kevin C. Chung
2 Professor of Surgery, Section of Plastic Surgery, Department of Surgery The University of Michigan Health System; Ann Arbor, MI
Observational studies are an important category of study designs. To address some investigative questions in plastic surgery, randomized controlled trials are not always indicated or ethical to conduct. Instead, observational studies may be the next best method to address these types of questions. Well-designed observational studies have been shown to provide results similar to randomized controlled trials, challenging the belief that observational studies are second-rate. Cohort studies and case-control studies are two primary types of observational studies that aid in evaluating associations between diseases and exposures. In this review article, we describe these study designs, methodological issues, and provide examples from the plastic surgery literature.
Because of the innovative nature of the specialty, plastic surgeons are frequently confronted with a spectrum of clinical questions by patients who inquire about “best practices.” It is thus essential that plastic surgeons know how to critically appraise the literature to understand and practice evidence-based medicine (EBM) and also contribute to the effort by carrying out high-quality investigations. 1 Well-designed randomized controlled trials (RCTs) have held the pre-eminent position in the hierarchy of EBM as level I evidence ( Table 1 ). However, RCT methodology, which was first developed for drug trials, can be difficult to conduct for surgical investigations. 3 Instead, well-designed observational studies, recognized as level II or III evidence, can play an important role in deriving evidence for plastic surgery. Results from observational studies are often criticized for being vulnerable to influences by unpredictable confounding factors. However, recent work has challenged this notion, showing comparable results between observational studies and RCTs. 4 , 5 Observational studies can also complement RCTs in hypothesis generation, establishing questions for future RCTs, and defining clinical conditions.
Levels of Evidence Based Medicine
| Level of Evidence | Qualifying Studies |
|---|---|
| I | High-quality, multicenter or single-center, randomized controlled trial with adequate power; or systematic review of these studies |
| II | Lesser quality, randomized controlled trial; prospective cohort study; or systematic review of these studies |
| III | Retrospective comparative study; case-control study; or systematic review of these studies |
| IV | Case-series |
| V | Expert opinion; case report or clinical example; or evidence based on physiology, bench research, or “first principles” |
From REF 1 .
Observational studies fall under the category of analytic study designs and are further sub-classified as observational or experimental study designs ( Figure 1 ). The goal of analytic studies is to identify and evaluate causes or risk factors of diseases or health-related events. The differentiating characteristic between observational and experimental study designs is that in the latter, the presence or absence of undergoing an intervention defines the groups. By contrast, in an observational study, the investigator does not intervene and rather simply “observes” and assesses the strength of the relationship between an exposure and disease variable. 6 Three types of observational studies include cohort studies, case-control studies, and cross-sectional studies ( Figure 1 ). Case-control and cohort studies offer specific advantages by measuring disease occurrence and its association with an exposure by offering a temporal dimension (i.e. prospective or retrospective study design). Cross-sectional studies, also known as prevalence studies, examine the data on disease and exposure at one particular time point ( Figure 2 ). 6 Because the temporal relationship between disease occurrence and exposure cannot be established, cross-sectional studies cannot assess the cause and effect relationship. In this review, we will primarily discuss cohort and case-control study designs and related methodologic issues.

Analytic Study Designs. Adapted with permission from Joseph Eisenberg, Ph.D.

Temporal Design of Observational Studies: Cross-sectional studies are known as prevalence studies and do not have an inherent temporal dimension. These studies evaluate subjects at one point in time, the present time. By contrast, cohort studies can be either retrospective (latin derived prefix, “retro” meaning “back, behind”) or prospective (greek derived prefix, “pro” meaning “before, in front of”). Retrospective studies “look back” in time contrasting with prospective studies, which “look ahead” to examine causal associations. Case-control study designs are also retrospective and assess the history of the subject for the presence or absence of an exposure.
COHORT STUDY
The term “cohort” is derived from the Latin word cohors . Roman legions were composed of ten cohorts. During battle each cohort, or military unit, consisting of a specific number of warriors and commanding centurions, were traceable. The word “cohort” has been adopted into epidemiology to define a set of people followed over a period of time. W.H. Frost, an epidemiologist from the early 1900s, was the first to use the word “cohort” in his 1935 publication assessing age-specific mortality rates and tuberculosis. 7 The modern epidemiological definition of the word now means a “group of people with defined characteristics who are followed up to determine incidence of, or mortality from, some specific disease, all causes of death, or some other outcome.” 7
Study Design
A well-designed cohort study can provide powerful results. In a cohort study, an outcome or disease-free study population is first identified by the exposure or event of interest and followed in time until the disease or outcome of interest occurs ( Figure 3A ). Because exposure is identified before the outcome, cohort studies have a temporal framework to assess causality and thus have the potential to provide the strongest scientific evidence. 8 Advantages and disadvantages of a cohort study are listed in Table 2 . 2 , 9 Cohort studies are particularly advantageous for examining rare exposures because subjects are selected by their exposure status. Additionally, the investigator can examine multiple outcomes simultaneously. Disadvantages include the need for a large sample size and the potentially long follow-up duration of the study design resulting in a costly endeavor.

Cohort and Case-Control Study Designs
Advantages and Disadvantages of the Cohort Study
| Gather data regarding sequence of events; can assess causality |
| Examine multiple outcomes for a given exposure |
| Good for investigating rare exposures |
| Can calculate rates of disease in exposed and unexposed individuals over time (e.g. incidence, relative risk) |
| Large numbers of subjects are required to study rare exposures |
| Susceptible to selection bias |
| May be expensive to conduct |
| May require long durations for follow-up |
| Maintaining follow-up may be difficult |
| Susceptible to loss to follow-up or withdrawals |
| Susceptible to recall bias or information bias |
| Less control over variables |
Cohort studies can be prospective or retrospective ( Figure 2 ). Prospective studies are carried out from the present time into the future. Because prospective studies are designed with specific data collection methods, it has the advantage of being tailored to collect specific exposure data and may be more complete. The disadvantage of a prospective cohort study may be the long follow-up period while waiting for events or diseases to occur. Thus, this study design is inefficient for investigating diseases with long latency periods and is vulnerable to a high loss to follow-up rate. Although prospective cohort studies are invaluable as exemplified by the landmark Framingham Heart Study, started in 1948 and still ongoing, 10 in the plastic surgery literature this study design is generally seen to be inefficient and impractical. Instead, retrospective cohort studies are better indicated given the timeliness and inexpensive nature of the study design.
Retrospective cohort studies, also known as historical cohort studies, are carried out at the present time and look to the past to examine medical events or outcomes. In other words, a cohort of subjects selected based on exposure status is chosen at the present time, and outcome data (i.e. disease status, event status), which was measured in the past, are reconstructed for analysis. The primary disadvantage of this study design is the limited control the investigator has over data collection. The existing data may be incomplete, inaccurate, or inconsistently measured between subjects. 2 However, because of the immediate availability of the data, this study design is comparatively less costly and shorter than prospective cohort studies. For example, Spear and colleagues examined the effect of obesity and complication rates after undergoing the pedicled TRAM flap reconstruction by retrospectively reviewing 224 pedicled TRAM flaps in 200 patients over a 10-year period. 11 In this example, subjects who underwent the pedicled TRAM flap reconstruction were selected and categorized into cohorts by their exposure status: normal/underweight, overweight, or obese. The outcomes of interest were various flap and donor site complications. The findings revealed that obese patients had a significantly higher incidence of donor site complications, multiple flap complications, and partial flap necrosis than normal or overweight patients. An advantage of the retrospective study design analysis is the immediate access to the data. A disadvantage is the limited control over the data collection because data was gathered retrospectively over 10-years; for example, a limitation reported by the authors is that mastectomy flap necrosis was not uniformly recorded for all subjects. 11
An important distinction lies between cohort studies and case-series. The distinguishing feature between these two types of studies is the presence of a control, or unexposed, group. Contrasting with epidemiological cohort studies, case-series are descriptive studies following one small group of subjects. In essence, they are extensions of case reports. Usually the cases are obtained from the authors' experiences, generally involve a small number of patients, and more importantly, lack a control group. 12 There is often confusion in designating studies as “cohort studies” when only one group of subjects is examined. Yet, unless a second comparative group serving as a control is present, these studies are defined as case-series. The next step in strengthening an observation from a case-series is selecting appropriate control groups to conduct a cohort or case-control study, the latter which is discussed in the following section about case-control studies. 9
Methodological Issues
Selection of subjects in cohort studies.
The hallmark of a cohort study is defining the selected group of subjects by exposure status at the start of the investigation. A critical characteristic of subject selection is to have both the exposed and unexposed groups be selected from the same source population ( Figure 4 ). 9 Subjects who are not at risk for developing the outcome should be excluded from the study. The source population is determined by practical considerations, such as sampling. Subjects may be effectively sampled from the hospital, be members of a community, or from a doctor's individual practice. A subset of these subjects will be eligible for the study.

Levels of Subject Selection. Adapted from Ref 9 .
Attrition Bias (Loss to follow-up)
Because prospective cohort studies may require long follow-up periods, it is important to minimize loss to follow-up. Loss to follow-up is a situation in which the investigator loses contact with the subject, resulting in missing data. If too many subjects are loss to follow-up, the internal validity of the study is reduced. A general rule of thumb requires that the loss to follow-up rate not exceed 20% of the sample. 6 Any systematic differences related to the outcome or exposure of risk factors between those who drop out and those who stay in the study must be examined, if possible, by comparing individuals who remain in the study and those who were loss to follow-up or dropped out. It is therefore important to select subjects who can be followed for the entire duration of the cohort study. Methods to minimize loss to follow-up are listed in Table 3 .
Methods to Minimize Loss to Follow-Up
| Exclude subjects likely to be lost |
| Planning to move |
| Non-committal |
| Obtain information to allow future tracking |
| Collect subject's contact information (e.g. mailing addresses, telephone numbers, and email addresses) |
| Collect social security and/or Medicare numbers |
| Maintain periodic contact |
| By telephone: may require calls during the weekends and/or evenings |
| By mail: repeated mailings by e-mail or with stamped, self-addressed return envelopes |
| Other: newsletters or token gifts with study logo |
Adapted from REF 2 .
CASE-CONTROL STUDIES
Case-control studies were historically borne out of interest in disease etiology. The conceptual basis of the case-control study is similar to taking a history and physical; the diseased patient is questioned and examined, and elements from this history taking are knitted together to reveal characteristics or factors that predisposed the patient to the disease. In fact, the practice of interviewing patients about behaviors and conditions preceding illness dates back to the Hippocratic writings of the 4 th century B.C. 7
Reasons of practicality and feasibility inherent in the study design typically dictate whether a cohort study or case-control study is appropriate. This study design was first recognized in Janet Lane-Claypon's study of breast cancer in 1926, revealing the finding that low fertility rate raises the risk of breast cancer. 13 , 14 In the ensuing decades, case-control study methodology crystallized with the landmark publication linking smoking and lung cancer in the 1950s. 15 Since that time, retrospective case-control studies have become more prominent in the biomedical literature with more rigorous methodological advances in design, execution, and analysis.
Case-control studies identify subjects by outcome status at the outset of the investigation. Outcomes of interest may be whether the subject has undergone a specific type of surgery, experienced a complication, or is diagnosed with a disease ( Figure 3B ). Once outcome status is identified and subjects are categorized as cases, controls (subjects without the outcome but from the same source population) are selected. Data about exposure to a risk factor or several risk factors are then collected retrospectively, typically by interview, abstraction from records, or survey. Case-control studies are well suited to investigate rare outcomes or outcomes with a long latency period because subjects are selected from the outset by their outcome status. Thus in comparison to cohort studies, case-control studies are quick, relatively inexpensive to implement, require comparatively fewer subjects, and allow for multiple exposures or risk factors to be assessed for one outcome ( Table 4 ). 2 , 9
Advantages and Disadvantages of the Case-Control Study
| Good for examining rare outcomes or outcomes with long latency |
| Relatively quick to conduct |
| Relatively inexpensive |
| Requires comparatively few subjects |
| Existing records can be used |
| Multiple exposures or risk factors can be examined |
| Susceptible to recall bias or information bias |
| Difficult to validate information |
| Control of extraneous variables may be incomplete |
| Selection of an appropriate comparison group may be difficult |
| Rates of disease in exposed and unexposed individuals cannot be determined |
An example of a case-control investigation is by Zhang and colleagues who examined the association of environmental and genetic factors associated with rare congenital microtia, 16 which has an estimated prevalence of 0.83 to 17.4 in 10,000. 17 They selected 121 congenital microtia cases based on clinical phenotype, and 152 unaffected controls, matched by age and sex in the same hospital and same period. Controls were of Hans Chinese origin from Jiangsu, China, the same area from where the cases were selected. This allowed both the controls and cases to have the same genetic background, important to note given the investigated association between genetic factors and congenital microtia. To examine environmental factors, a questionnaire was administered to the mothers of both cases and controls. The authors concluded that adverse maternal health was among the main risk factors for congenital microtia, specifically maternal disease during pregnancy (OR 5.89, 95% CI 2.36-14.72), maternal toxicity exposure during pregnancy (OR 4.76, 95% CI 1.66-13.68), and resident area, such as living near industries associated with air pollution (OR 7.00, 95% CI 2.09-23.47). 16 A case-control study design is most efficient for this investigation, given the rarity of the disease outcome. Because congenital microtia is thought to have multifactorial causes, an additional advantage of the case-control study design in this example is the ability to examine multiple exposures and risk factors.
Selection of Cases
Sampling in a case-control study design begins with selecting the cases. In a case-control study, it is imperative that the investigator has explicitly defined inclusion and exclusion criteria prior to the selection of cases. For example, if the outcome is having a disease, specific diagnostic criteria, disease subtype, stage of disease, or degree of severity should be defined. Such criteria ensure that all the cases are homogenous. Second, cases may be selected from a variety of sources, including hospital patients, clinic patients, or community subjects. Many communities maintain registries of patients with certain diseases and can serve as a valuable source of cases. However, despite the methodologic convenience of this method, validity issues may arise. For example, if cases are selected from one hospital, identified risk factors may be unique to that single hospital. This methodological choice may weaken the generalizability of the study findings. Another example is choosing cases from the hospital versus the community; most likely cases from the hospital sample will represent a more severe form of the disease than those in the community. 2 Finally, it is also important to select cases that are representative of cases in the target population to strengthen the study's external validity ( Figure 4 ). Potential reasons why cases from the original target population eventually filter through and are available as cases (study participants) for a case-control study are illustrated in Figure 5 .

Levels of Case Selection. Adapted from Ref 2 .
Selection of Controls
Selecting the appropriate group of controls can be one of the most demanding aspects of a case-control study. An important principle is that the distribution of exposure should be the same among cases and controls; in other words, both cases and controls should stem from the same source population. The investigator may also consider the control group to be an at-risk population, with the potential to develop the outcome. Because the validity of the study depends upon the comparability of these two groups, cases and controls should otherwise meet the same inclusion criteria in the study.
A case-control study design that exemplifies this methodological feature is by Chung and colleagues, who examined maternal cigarette smoking during pregnancy and the risk of newborns developing cleft lip/palate. 18 A salient feature of this study is the use of the 1996 U.S. Natality database, a population database, from which both cases and controls were selected. This database provides a large sample size to assess newborn development of cleft lip/palate (outcome), which has a reported incidence of 1 in 1000 live births, 19 and also enabled the investigators to choose controls (i.e., healthy newborns) that were generalizable to the general population to strengthen the study's external validity. A significant relationship with maternal cigarette smoking and cleft lip/palate in the newborn was reported in this study (adjusted OR 1.34, 95% CI 1.36-1.76). 18
Matching is a method used in an attempt to ensure comparability between cases and controls and reduces variability and systematic differences due to background variables that are not of interest to the investigator. 8 Each case is typically individually paired with a control subject with respect to the background variables. The exposure to the risk factor of interest is then compared between the cases and the controls. This matching strategy is called individual matching. Age, sex, and race are often used to match cases and controls because they are typically strong confounders of disease. 20 Confounders are variables associated with the risk factor and may potentially be a cause of the outcome. 8 Table 5 lists several advantages and disadvantages with a matching design.
Advantages and Disadvantages for Using a Matching Strategy
| Advantages | Disadvantages |
|---|---|
| Eliminate influence of measurable confounders (e.g. age, sex) | May be time-consuming and expensive |
| Eliminate influence of confounders that are difficult to measure | Decision to match and confounding variables to match upon are decided at the outset of the study |
| May be a sampling convenience, making it easier to select the controls in a case-control study | Matched variables cannot be examined in the study |
| May improve study efficiency (i.e. smaller sample size) | Requires a matched analysis |
| Vulnerable to overmatching: when matching variable has some relationship with the outcome |
Multiple Controls
Investigations examining rare outcomes may have a limited number of cases to select from, whereas the source population from which controls can be selected is much larger. In such scenarios, the study may be able to provide more information if multiple controls per case are selected. This method increases the “statistical power” of the investigation by increasing the sample size. The precision of the findings may improve by having up to about three or four controls per case. 21 - 23
Bias in Case-Control Studies
Evaluating exposure status can be the Achilles heel of case-control studies. Because information about exposure is typically collected by self-report, interview, or from recorded information, it is susceptible to recall bias, interviewer bias, or will rely on the completeness or accuracy of recorded information, respectively. These biases decrease the internal validity of the investigation and should be carefully addressed and reduced in the study design. Recall bias occurs when a differential response between cases and controls occurs. The common scenario is when a subject with disease (case) will unconsciously recall and report an exposure with better clarity due to the disease experience. Interviewer bias occurs when the interviewer asks leading questions or has an inconsistent interview approach between cases and controls. A good study design will implement a standardized interview in a non-judgemental atmosphere with well-trained interviewers to reduce interviewer bias. 9
The STROBE Statement: The Strengthening the Reporting of Observational Studies in Epidemiology Statement
In 2004, the first meeting of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) group took place in Bristol, UK. 24 The aim of the group was to establish guidelines on reporting observational research to improve the transparency of the methods, thereby facilitating the critical appraisal of a study's findings. A well-designed but poorly reported study is disadvantaged in contributing to the literature because the results and generalizability of the findings may be difficult to assess. Thus a 22-item checklist was generated to enhance the reporting of observational studies across disciplines. 25 , 26 This checklist is also located at the following website: www.strobe-statement.org . This statement is applicable to cohort studies, case-control studies, and cross-sectional studies. In fact, 18 of the checklist items are common to all three types of observational studies, and 4 items are specific to each of the 3 specific study designs. In an effort to provide specific guidance to go along with this checklist, an “explanation and elaboration” article was published for users to better appreciate each item on the checklist. 27 Plastic surgery investigators should peruse this checklist prior to designing their study and when they are writing up the report for publication. In fact, some journals now require authors to follow the STROBE Statement. A list of participating journals can be found on this website: http://www.strobe-statement.org./index.php?id=strobe-endorsement .
Due to the limitations in carrying out RCTs in surgical investigations, observational studies are becoming more popular to investigate the relationship between exposures, such as risk factors or surgical interventions, and outcomes, such as disease states or complications. Recognizing that well-designed observational studies can provide valid results is important among the plastic surgery community, so that investigators can both critically appraise and appropriately design observational studies to address important clinical research questions. The investigator planning an observational study can certainly use the STROBE statement as a tool to outline key features of a study as well as coming back to it again at the end to enhance transparency in methodology reporting.
Acknowledgments
Supported in part by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to Dr. Kevin C. Chung).
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.

Case-Control Studies
- 1
- | 2
- | 3
- | 4
- | 5
- | 6
- | 7
- | 8

When is a Case-Control Study Desirable?
The des case-control study.

E pi_Tools.XLSX
All Modules
Given the greater efficiency of case-control studies, they are particularly advantageous in the following situations:
- When the disease or outcome being studied is rare.
- When the disease or outcome has a long induction and latent period (i.e., a long time between exposure and the eventual causal manifestation of disease).
- When exposure data is difficult or expensive to obtain.
- When the study population is dynamic.
- When little is known about the risk factors for the disease, case-control studies provide a way of testing associations with multiple potential risk factors. (This isn't really a unique advantage to case-control studies, however, since cohort studies can also assess multiple exposures.)
Another advantage of their greater efficiency, of course, is that they are less time-consuming and much less costly than prospective cohort studies.
A classic example of the efficiency of the case-control approach is the study (Herbst et al.: N. Engl. J. Med. Herbst et al. (1971;284:878-81) that linked in-utero exposure to diethylstilbesterol (DES) with subsequent development of vaginal cancer 15-22 years later. In the late 1960s, physicians at MGH identified a very unusual cancer cluster. Eight young woman between the ages of 15-22 were found to have cancer of the vagina, an uncommon cancer even in elderly women. The cluster of cases in young women was initially reported as a case series, but there were no strong hypotheses about the cause.
In retrospect, the cause was in-utero exposure to DES. After World War II, DES started being prescribed for women who were having troubles with a pregnancy -- if there were signs suggesting the possibility of a miscarriage, DES was frequently prescribed. It has been estimated that between 1945-1950 DES was prescribed for about 20% of all pregnancies in the Boston area. Thus, the unborn fetus was exposed to DES in utero, and in a very small percentage of cases this resulted in development of vaginal cancer when the child was 15-22 years old (a very long latent period). There were several reasons why a case-control study was the only feasible way to identify this association: the disease was extremely rare (even in subjects who had been exposed to DES), there was a very long latent period between exposure and development of disease, and initially they had no idea what was responsible, so there were many possible exposures to consider.
In this situation, a case-control study was the only reasonable approach to identify the causative agent. Given how uncommon the outcome was, even a large prospective study would have been unlikely to have more than one or two cases, even after 15-20 years of follow-up. Similarly, a retrospective cohort study might have been successful in enrolling a large number of subjects, but the outcome of interest was so uncommon that few, if any, subjects would have had it. In contrast, a case-control study was conducted in which eight known cases and 32 age-matched controls provided information on many potential exposures. This strategy ultimately allowed the investigators to identify a highly significant association between the mother's treatment with DES during pregnancy and the eventual development of adenocarcinoma of the vagina in their daughters (in-utero at the time of exposure) 15 to 22 years later.
For more information see the DES Fact Sheet from the National Cancer Institute .
An excellent summary of this landmark study and the long-range effects of DES can be found in a Perspective article in the New England Journal of Medicine. A cohort of both mothers who took DES and their children (daughters and sons) was later formed to look for more common outcomes. Members of the faculty at BUSPH are on the team of investigators that follow this cohort for a variety of outcomes, particularly reproductive consequences and other cancers.
return to top | previous page | next page
Content ©2016. All Rights Reserved. Date last modified: June 7, 2016. Wayne W. LaMorte, MD, PhD, MPH
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Long COVID or Post-COVID Conditions
Some people who have been infected with the virus that causes COVID-19 can experience long-term effects from their infection, known as Long COVID or Post-COVID Conditions (PCC). Long COVID is broadly defined as signs, symptoms, and conditions that continue or develop after acute COVID-19 infection. This definition of Long COVID was developed by the Department of Health and Human Services (HHS) in collaboration with CDC and other partners.
People call Long COVID by many names, including Post-COVID Conditions, long-haul COVID, post-acute COVID-19, long-term effects of COVID, and chronic COVID. The term post-acute sequelae of SARS CoV-2 infection (PASC) is also used to refer to a subset of Long COVID.
What You Need to Know
- Long COVID is a real illness and can result in chronic conditions that require comprehensive care. There are resources available .
- Long COVID can include a wide range of ongoing health problems; these conditions can last weeks, months, or years.
- Long COVID occurs more often in people who had severe COVID-19 illness, but anyone who has been infected with the virus that causes COVID-19 can experience it.
- People who are not vaccinated against COVID-19 and become infected may have a higher risk of developing Long COVID compared to people who have been vaccinated.
- People can be reinfected with SARS-CoV-2, the virus that causes COVID-19, multiple times. Each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing Long COVID.
- While most people with Long COVID have evidence of infection or COVID-19 illness, in some cases, a person with Long COVID may not have tested positive for the virus or known they were infected.
- CDC and partners are working to understand more about who experiences Long COVID and why, including whether groups disproportionately impacted by COVID-19 are at higher risk.
In July 2021, Long COVID was added as a recognized condition that could result in a disability under the Americans with Disabilities Act (ADA). Learn more: Guidance on “Long COVID” as a Disability Under the ADA .
About Long COVID
Long COVID is a wide range of new, returning, or ongoing health problems that people experience after being infected with the virus that causes COVID-19. Most people with COVID-19 get better within a few days to a few weeks after infection, so at least 4 weeks after infection is the start of when Long COVID could first be identified. Anyone who was infected can experience Long COVID. Most people with Long COVID experienced symptoms days after first learning they had COVID-19, but some people who later experienced Long COVID did not know when they got infected.
There is no test that determines if your symptoms or condition is due to COVID-19. Long COVID is not one illness. Your healthcare provider considers a diagnosis of Long COVID based on your health history, including if you had a diagnosis of COVID-19 either by a positive test or by symptoms or exposure, as well as based on a health examination.
Science behind Long COVID
RECOVER: Researching COVID to Enhance Recovery
People with Long COVID may experience many symptoms.
People with Long COVID can have a wide range of symptoms that can last weeks, months, or even years after infection. Sometimes the symptoms can even go away and come back again. For some people, Long COVID can last weeks, months, or years after COVID-19 illness and can sometimes result in disability.
Long COVID may not affect everyone the same way. People with Long COVID may experience health problems from different types and combinations of symptoms that may emerge, persist, resolve, and reemerge over different lengths of time. Though most patients’ symptoms slowly improve with time, speaking with your healthcare provider about the symptoms you are experiencing after having COVID-19 could help determine if you might have Long COVID.
People who experience Long COVID most commonly report:
General symptoms ( Not a Comprehensive List)
- Tiredness or fatigue that interferes with daily life
- Symptoms that get worse after physical or mental effort (also known as “ post-exertional malaise ”)
Respiratory and heart symptoms
- Difficulty breathing or shortness of breath
- Fast-beating or pounding heart (also known as heart palpitations)
Neurological symptoms
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”)
- Sleep problems
- Dizziness when you stand up (lightheadedness)
- Pins-and-needles feelings
- Change in smell or taste
- Depression or anxiety
Digestive symptoms
- Stomach pain
Other symptoms
- Joint or muscle pain
- Changes in menstrual cycles
Symptoms that are hard to explain and manage
Some people with Long COVID have symptoms that are not explained by tests or easy to manage.
People with Long COVID may develop or continue to have symptoms that are hard to explain and manage. Clinical evaluations and results of routine blood tests, chest X-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and other poorly understood chronic illnesses that may occur after other infections. People with these unexplained symptoms may be misunderstood by their healthcare providers, which can result in a delay in diagnosis and receiving the appropriate care or treatment.
Review these tips to help prepare for a healthcare provider appointment for Long COVID.
Health conditions
Some people experience new health conditions after COVID-19 illness.
Some people, especially those who had severe COVID-19, experience multiorgan effects or autoimmune conditions with symptoms lasting weeks, months, or even years after COVID-19 illness. Multi-organ effects can involve many body systems, including the heart, lung, kidney, skin, and brain. As a result of these effects, people who have had COVID-19 may be more likely to develop new health conditions such as diabetes, heart conditions, blood clots, or neurological conditions compared with people who have not had COVID-19.
People experiencing any severe illness may develop health problems
People experiencing any severe illness, hospitalization, or treatment may develop problems such as post-intensive care syndrome (PICS).
PICS refers to the health effects that may begin when a person is in an intensive care unit (ICU), and which may persist after a person returns home. These effects can include muscle weakness, problems with thinking and judgment, and symptoms of post-traumatic stress disorder (PTSD), a long-term reaction to a very stressful event. While PICS is not specific to infection with SARS-CoV-2, it may occur and contribute to the person’s experience of Long COVID. For people who experience PICS following a COVID-19 diagnosis, it is difficult to determine whether these health problems are caused by a severe illness, the virus itself, or a combination of both.
People More Likely to Develop Long COVID
Some people may be more at risk for developing Long COVID.
Researchers are working to understand which people or groups of people are more likely to have Long COVID, and why. Studies have shown that some groups of people may be affected more by Long COVID. These are examples and not a comprehensive list of people or groups who might be more at risk than other groups for developing Long COVID:
- People who have experienced more severe COVID-19 illness, especially those who were hospitalized or needed intensive care.
- People who had underlying health conditions prior to COVID-19.
- People who did not get a COVID-19 vaccine.
Health Inequities May Affect Populations at Risk for Long COVID
Some people are at increased risk of getting sick from COVID-19 because of where they live or work, or because they can’t get health care. Health inequities may put some people from racial or ethnic minority groups and some people with disabilities at greater risk for developing Long COVID. Scientists are researching some of those factors that may place these communities at higher risk of getting infected or developing Long COVID.
Preventing Long COVID
The best way to prevent Long COVID is to protect yourself and others from becoming infected. For people who are eligible, CDC recommends staying up to date on COVID-19 vaccination , along with improving ventilation, getting tested for COVID-19 if needed, and seeking treatment for COVID-19 if eligible. Additional preventative measures include avoiding close contact with people who have a confirmed or suspected COVID-19 illness and washing hands or using alcohol-based hand sanitizer.
Research suggests that people who get a COVID-19 infection after vaccination are less likely to report Long COVID, compared to people who are unvaccinated.
CDC, other federal agencies, and non-federal partners are working to identify further measures to lessen a person’s risk of developing Long COVID. Learn more about protecting yourself and others from COVID-19 .
Living with Long COVID
Living with Long COVID can be hard, especially when there are no immediate answers or solutions.
People experiencing Long COVID can seek care from a healthcare provider to come up with a personal medical management plan that can help improve their symptoms and quality of life. Review these tips to help prepare for a healthcare provider appointment for Long COVID. In addition, there are many support groups being organized that can help patients and their caregivers.
Although Long COVID appears to be less common in children and adolescents than in adults, long-term effects after COVID-19 do occur in children and adolescents .
Talk to your doctor if you think you or your child has Long COVID. Learn more: Tips for Talking to Your Healthcare Provider about Post-COVID Conditions
Data for Long COVID
Studies are in progress to better understand Long COVID and how many people experience them.
CDC is using multiple approaches to estimate how many people experience Long COVID. Each approach can provide a piece of the puzzle to give us a better picture of who is experiencing Long COVID. For example, some studies look for the presence of Long COVID based on self-reported symptoms, while others collect symptoms and conditions recorded in medical records. Some studies focus only on people who have been hospitalized, while others include people who were not hospitalized. The estimates for how many people experience Long COVID can be quite different depending on who was included in the study, as well as how and when the study collected information. Estimates of the proportion of people who had COVID-19 that go on to experience Long COVID can vary.
CDC posts data on Long COVID and provides analyses, the most recent of which can be found on the U.S. Census Bureau’s Household Pulse Survey .
CDC and other federal agencies, as well as academic institutions and research organizations, are working to learn more about the short- and long-term health effects associated with COVID-19 , who gets them and why.
Scientists are also learning more about how new variants could potentially affect Long COVID. We are still learning to what extent certain groups are at higher risk, and if different groups of people tend to experience different types of Long COVID. CDC has several studies that will help us better understand Long COVID and how healthcare providers can treat or support patients with these long-term effects. CDC will continue to share information with healthcare providers to help them evaluate and manage these conditions.
CDC is working to:
- Better identify the most frequent symptoms and diagnoses experienced by patients with Long COVID.
- Better understand how many people are affected by Long COVID, and how often people who are infected with COVID-19 develop Long COVID
- Better understand risk factors and protective factors, including which groups might be more at risk, and if different groups experience different symptoms.
- Help understand how Long COVID limit or restrict people’s daily activity.
- Help identify groups that have been more affected by Long COVID, lack access to care and treatment for Long COVID, or experience stigma.
- Better understand the role vaccination plays in preventing Long COVID.
- Collaborate with professional medical groups to develop and offer clinical guidance and other educational materials for healthcare providers, patients, and the public.
Related Pages
- Caring for People with Post-COVID Conditions
- Preparing for Appointments for Post-COVID Conditions
- Researching COVID to Enhance Recovery
- Guidance on “Long COVID” as a Disability Under the ADA
For Healthcare Professionals
- Post-COVID Conditions: Healthcare Providers
Search for and find historical COVID-19 pages and files. Please note the content on these pages and files is no longer being updated and may be out of date.
- Visit archive.cdc.gov for a historical snapshot of the COVID-19 website, capturing the end of the Federal Public Health Emergency on June 28, 2023.
- Visit the dynamic COVID-19 collection to search the COVID-19 website as far back as July 30, 2021.
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Get notified in your email when a new post is published to this blog
The Old New Thing

What’s the deal with std::type_identity ?
Can INI files be Unicode? Yes, they can, but it has to be your idea
How 16-bit windows cached ini files for performance, why does globallock max out at 255 locks, more on harmful overuse of std:: move, a graphical depiction of the steps in building a c++ executable, with xaml and packaging, a graphical depiction of the steps in building a c++ executable, enhanced for classic win32, a graphical depiction of the steps in building a c++ executable, basics, how can i force a dll to register itself if it won’t respond to regsvr32 , is there any difference between stringfromiid and stringfromclsid .

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
How to circumvent and beat the ransomware in android operating system—a case study of locker.cbtr.

1. Introduction
- Our approach is to provide the ransomware mitigation solution once it hits the victim, while most of the works in this area deal with detection or prevention against ransomware.
- We provide a procedure for recovering the infected mobile device based on the analysis and impersonation of the communication between the ransomware instance of the terminal and its C&C server.
- We accomplish the reconstruction of the communication patterns and exchanged data formats between the ransomware instance and its C&C server.
- We provide a method to dynamically replace the IP address of the original C&C server with our own. This leads to impersonating the ransomware C&C server and gaining full control over the ransomware instance of the infected mobile device.
- We develop a C&C server implementation that may communicate with the ransomware instance and order it to decrypt the file system and unlock the terminal screen after a ransomware attack.
2. Defense from Ransomware: Analysis of Existing Solutions
2.1. methods for detecting ransomware, 2.2. methods for preventing ransomware, 2.3. methods for mitigating ransomware, 3. a method to mitigate the ransomware—the case study of locker.cbtr, 3.1. overview of locker.cbtr, 3.2. methodology and materials.
- Enjarify—a tool similar to “dex2jar”. It converts Dalvik bytecode to Java bytecode so that further analysis can be based on Java decompilers, e.g., CFR, Fernflower.
- Apktool—it is an open-source software that allows the conversion of application resources such as binary XML into plain text, as well as decompiling the executable code of the Dalvik machine into an intermediate format called smali.
- ByteCodeViewer—it is a multifunctional software that also serves as a decompiler, editor, and even debugger. The main advantage of this tool is the ability to divide the view of the decompiled class into many parts and use different decompilers on each part. It allows us to compare the performance and effectiveness of different decompilers and choose the best one in a given case. Currently supported decompilers are Procyon, CFR, JD-GUI, FernFlower, Krakatau, and JADX. Besides that, it also allows the display of the content of Smali files or Java bytecode.
- Frida—a set of tools for dynamic code manipulation that allows for injecting fragments of JavaScript code or own libraries into native applications on Windows, Mac OS, iOS, and Android. More specifically, this tool provides the ability to analyze the behavior of the program at runtime by injecting code into the target process.
3.3. Analysis of Locker.CB!tr Ransomware Operation
3.4. details of defeating locker.cbtr ransomware.
- name—a name of the device model (Android in the case shown in Figure 8 );
- imei—International Mobile Equipment Identifier (with a value of 358240051111110 in the case shown in Figure 8 );
- client_version—the number of client (ransomware) version (with a value of 1.03 in the case shown in Figure 8 );
- id—an identifier of the device (with a value of 90f1efbc800cc949 in the case shown in Figure 8 );
- android_version—the version of Android OS on the infected terminal (with a value of 5.0.2 in the case shown in Figure 8 );
- phone_number—a telephone number of the terminal (with a value of +15555215554 encoded as %2B15555215554 in the case shown in Figure 8 ).
4. Testing and Discussing the Effectiveness of the Proposed Solution
4.1. validation tests, 4.2. effectiveness of the proposed solution.
- The usage of asymmetric cryptographic key pairs generated on a C&C server side to encrypt the file system on the victim’s device: This is a good way to eliminate potential interference by an infected person or a security researcher who will try to decrypt the compromised files. Encryption with a locally stored public key is a good solution for the ransomware creator because a possible finding of the public key in the decompiled code does not impact the risk level of discovering the private key, which is necessary to decrypt the files. The only downside to this approach is that it requires an Internet connection. Otherwise, the private key needed to decrypt the files will not reach the infected device.
- The implementation of communication at the TCP socket layer instead of using HTTP protocol: HTTP protocol is standardized and commonly known, so the arrangement and methods of encoding the transferred elements are known, e.g., the way of transferring parameters in GET requests using application/x-www-form-urlencoded encoding. Therefore, after intercepting the communication between the ransomware and its C&C server, it is easier to analyze the captured HTTP messages and understand the format and data transfer methods, thus accessing the transferred content. In the case of data transfer via a pure TCP connection, a ransomware creator independently determines the format, the location, and the method of encoding the data. Additionally, they can use encryption for all these data except the TCP header. The essence of this approach is that it is very difficult to access the content of the intercepted messages because the method of locating individual elements and their encoding (i.e., the meaning of individual bytes and the determination of the size and boundaries of individual elements) is not generally known and must be discovered, which may significantly complicate countering ransomware attacks. Such protection does not prevent the reproduction of the communication pattern between the ransomware and its C&C server, but it makes it significantly more difficult.
- Encrypting the communication between the ransomware on the infected terminal and its C&C server: Instead of standard cryptographic protocols, one can consider the usage of one’s own encryption algorithm, e.g., the dynamic xor function of all incoming and outgoing bytes. The advantage of this solution would be the key changes over time after each use and the lack of knowledge of the algorithm itself, which would make it significantly more difficult to take control of the ransomware. If the communication was encrypted using standard cryptographic protocols, the static keys could be discovered by a security researcher after successfully decompiling the code. In addition to the decompilation, recovering dynamic keys requires recreating functions that change the dynamic key, and these could be well obfuscated.
- The use of obfuscation not only makes the decompilation process more difficult but also works against antiviruses that mostly rely on scanning known signatures.
5. Conclusions
Author contributions, data availability statement, conflicts of interest.
| ADB | Android Debug Bridge |
| AES | Advanced Encryption Standard |
| API | Application Programming Interface |
| APK | Android Package Kit |
| AVD | Android Virtual Device |
| C&C | Command and Control |
| DGAs | Domain Generation Algorithms |
| DEX | Dalvik Executable |
| FSM | Finite State Machine |
| HTTP | HyperText Transfer Protocol |
| IDE | Integrated Development Environment |
| JAR | Java ARchive |
| JSON | JavaScript Object Notation |
| MFA | Multi-Factor Authentication |
| MIMEs | Multipurpose Internet Mail Extensions |
| ML | Machine Learning |
| OS | Operating System |
| SBOM | Software Bill of Materials |
| SD card | Secure Digital card |
| SMS | Short Message Service |
| TCP | Transmission Control Protocol |
| URL | Uniform Resource Locator |
| XML | Extended Markup Language |
- Richardson, R.; North, M. Ransomware: Evolution, mitigation and prevention. Int. Manag. Rev. 2017 , 13 , 10–21. [ Google Scholar ]
- Meland, P.H.; Bayoumy, Y.F.F.; Sindre, G. The Ransomware-as-a-Service economy within the darknet. Comput. Secur. 2020 , 92 , 101762. [ Google Scholar ] [ CrossRef ]
- Reshmi, T.R. Information security breaches due to ransomware attacks—A systematic literature review. Int. J. Inf. Manag. Data Insights 2021 , 1 , 100013. [ Google Scholar ] [ CrossRef ]
- Beaman, C.; Barkworth, A.; Akande, T.D.; Hakak, S.; Khan, M.K. Ransomware: Recent advances, analysis, challenges and future research directions. Comput. Secur. 2021 , 111 , 102490. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Taheri, L.; Kadir, A.F.A.; Lashkari, A.H. Extensible android malware detection and family classification using network-flows and API-calls. In Proceedings of the International Carnahan Conference on Security Technology, Chennai, India, 1–3 October 2019; pp. 1–8. [ Google Scholar ]
- Ko, J.; Jo, J.; Kim, D.; Choi, S.; Kwak, J. Real time android ransomware detection by analyzed android applications. In Proceedings of the International Conference on Electronics, Information, and Communication, Auckland, New Zealand, 22–25 January 2019; pp. 1–5. [ Google Scholar ]
- Andronio, N.; Zanero, S.; Maggi, F. HelDroid: Dissecting and detecting mobile ransomware. In Proceedings of the International Symposium on Recent Advances in Intrusion Detection, Kyoto, Japan, 2–4 November 2015; pp. 382–404. [ Google Scholar ]
- Abdullah, Z.; Muhadi, F.W.; Saudi, M.M.; Hamid, I.R.A.; Foozy, C.F.M. Android Ransomware Detection Based on Dynamic Obtained Features ; Ghazali, R., Nawi, N., Deris, M., Abawajy, J., Eds.; Recent Advances on Soft Computing and Data Mining. SCDM 2020. Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2019; Volume 978. [ Google Scholar ] [ CrossRef ]
- Scalas, M.; Maiorca, D.; Mercaldo, F.; Visaggio, C.A.; Martinelli, F.; Giacinto, G. On the effectiveness of system API-related information for Android ransomware detection. Comput. Secur. 2019 , 86 , 168–182. [ Google Scholar ] [ CrossRef ]
- Mercaldo, F.; Nardone, V.; Santone, A.; Visaggio, C.A. Ransomware steals your phone. Formal methods rescue it. In Proceedings of the International Conference on Formal Techniques for Distributed Objects, Components, and Systems, Heraklion, Greece, 6–9 June 2016; pp. 212–221. [ Google Scholar ]
- Milner, R. Communication and Concurrency ; PHI Series in Computer Science; Prentice Hall: Upper Saddle River, NJ, USA, 1989. [ Google Scholar ]
- Ramesh, G.; Menen, A. Automated dynamic approach for detecting ransomware using finite-state machine. Decis. Support Syst. 2020 , 138 , 113400. [ Google Scholar ] [ CrossRef ]
- Continella, A.; Guagnelli, A.; Zingaro, G.; De Pasquale, G.; Barenghi, A.; Zanero, S.; Maggi, F. ShieldFS: A self-healing, ransomware-aware filesystem. In Proceedings of the 32nd Annual Conference on Computer Security Applications (ACSAC ’16), Los Angeles, CA USA, 5–8 December 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 336–347. [ Google Scholar ] [ CrossRef ]
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.antivirus (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.avast.android.mobilesecurity (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.bitdefender.security (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.symantec.mobilesecurity (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.trendmicro.tmmspersonal (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=ransomware.defender (accessed on 23 May 2024).
- Google Play. Available online: https://play.google.com/store/apps/details?id=com.checkpoint.zonealarm.mobilesecurity (accessed on 23 May 2024).
- Virustotal. Available online: https://www.virustotal.com (accessed on 18 May 2024).
- Kolodenker, E.; Koch, W.; Stringhini, G.; Egele, M. PayBreak: Defense against cryptographic ransomware. In Proceedings of the 2017 ACM on Asia Conference on Computer and Communications Security, Abu Dhabi, United Arab Emirates, 2–6 April 2017; ACM: New York, NY, USA; pp. 599–611. [ Google Scholar ]
- Cabaj, K.; Mazurczyk, W. Using software-defined networking for ransomware mitigation: The case of cryptowall. IEEE Netw. 2016 , 30 , 14–20. [ Google Scholar ] [ CrossRef ]
- Suarez-Tangil, G.; Dash, S.K.; Ahmadi, M.; Kinder, J.; Giacinto, G.; Cavallaro, L. Droidsieve: Fast and accurate classification of obfuscated android malware. In Proceedings of the Seventh ACM on Conference on Data and Application Security and Privacy, Scottsdale, AZ, USA, 22–24 March 2017; ACM: New York, NY, USA, 2017; pp. 309–320. [ Google Scholar ]
- Fayi, S.Y. What Petya/NotPetya ransomware is and what its remidiations are. In Information Technology-New Generations ; Springer: Cham, Switzerland, 2018; pp. 93–100. [ Google Scholar ]
- Caviglione, L. Trends and challenges in network covert channels countermeasures. Appl. Sci. 2021 , 11 , 1641. [ Google Scholar ] [ CrossRef ]
- Shah, A.; Rathod, D.M.; Mehta, Y. A comparative study of Covert Channel attacks in Android with different parameters and detection tools. Int. J. Electron. Secur. Digit. Forensics 2024 , 16 , 304–316. [ Google Scholar ] [ CrossRef ]
- Hafiz, N.; Briliyant, O.; Priambodo, D.; Hasbi, M.; Siswanti, S. Remote Penetration Testing with Telegram Bot. J. RESTI (Rekayasa Sist. dan Teknol. Informasi) 2023 , 7 , 705–714. [ Google Scholar ] [ CrossRef ]
- Yuste, J.; Pastrana, S. Avaddon ransomware: An in-depth analysis and decryption of infected systems. Comput. Secur. 2021 , 109 , 102388. [ Google Scholar ] [ CrossRef ]
- Bajpai, P.; Enbody, R. Attacking Key Management in Ransomware. IT Prof. 2020 , 22 , 21–27. [ Google Scholar ] [ CrossRef ]
- Kim, G.; Kang, S.; Baek, S.; Kim, K.; Kim, J. A Method for Decrypting Data Infected with Rhysida Ransomware. arXiv 2024 , arXiv:2402.06440. [ Google Scholar ] [ CrossRef ]
- Martín, A.; Hernandez-Castro, J.; Camacho, D. An in-depth study of the jisut family of Android ransomware. IEEE Access 2018 , 6 , 57205–57218. [ Google Scholar ] [ CrossRef ]
- Fraud Risk Suite—ThreatFabric. Available online: https://www.threatfabric.com/blogs/blackrock_the_trojan_that_wanted_to_get_them_all (accessed on 23 May 2024).
- BleepingComputer: Cybersecurity, Technology News and Support. Available online: https://www.bleepingcomputer.com/news/security/android-malware-escobar-steals-your-google-authenticator-mfa-codes/ (accessed on 23 May 2024).
- Fraud Risk Suite—ThreatFabric. Available online: https://www.threatfabric.com/blogs/sova-new-trojan-with-fowl-intentions (accessed on 23 May 2024).
- Cleafy: Online Fraud Management and Prevention Solution. Available online: https://www.cleafy.com/cleafy-labs/nexus-a-new-android-botnet (accessed on 23 May 2024).
- Kharraz, A.; Robertson, W.; Balzarotti, D.; Bilge, L.; Kirda, E. Cutting the gordian knot: A look under the hood of ransomware attacks. In Proceedings of the International Conference on Detection of Intrusions and Malware, and Vulnerability Assessment, Milan, Italy, 9–10 July 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–24. [ Google Scholar ]
- Threat Encyclopedia. Available online: https://www.fortiguard.com/encyclopedia/virus/6733993 (accessed on 27 April 2024).
- Oz, H.; Aris, A.; Levi, A.; Uluagac, S. A Survey on Ransomware: Evolution, Taxonomy, and Defense Solutions. ACM Comput. Surv. 2022 , 54 , 1–37. [ Google Scholar ] [ CrossRef ]
- Aresu, M.; Ariu, D.; Ahmadi, M.; Maiorca, D.; Giacinto, G. Clustering android malware families by http traffic. In Proceedings of the 2015 10th International Conference on Malicious and Unwanted Software (MALWARE), Fajardo, PR, USA, 20–22 October 2015; pp. 128–135. [ Google Scholar ] [ CrossRef ]
- Android Developers. Available online: https://developer.android.com/studio/command-line/adb (accessed on 27 April 2024).
- Fan, W.; Zhao, L.; Wang, J.; Chen, Y.; Wu, F.; Liu, Y. FamDroid: Learning-Based Android Malware Family Classification Using Static Analysis. arXiv 2021 , arXiv:2101.03965. [ Google Scholar ] [ CrossRef ]
- Lim, B. Android Tapjacking Vulnerability. arXiv 2015 , arXiv:1507.08694. [ Google Scholar ] [ CrossRef ]
- Gómez Hernández, J.A.; García Teodoro, P.; Magán Carrión, R.; Rodríguez Gómez, R. Crypto-Ransomware: A Revision of the State of the Art, Advances and Challenges. Electronics 2023 , 12 , 4494. [ Google Scholar ] [ CrossRef ]
- McIntosh, T.; Kayes, A.S.M.; Chen, Y.P.P.; Ng, A.; Watters, P. Ransomware Mitigation in the Modern Era: A Comprehensive Review, Research Challenges, and Future Directions. ACM Comput. Surv. 2021 , 54 , 1–36. [ Google Scholar ] [ CrossRef ]
| Specific URL Element | Name of Implementing Class | Meaning |
|---|---|---|
| /eaction/ | CommandConfirmRequest | Confirmation of the previous command completion |
| /gac/ | CommandRequest | Request for the next command |
| /sc/ | ContactsRequest | List of contacts read from the infected terminal |
| /pha/ | DeviceDataRequest | Information about the infected terminal |
| /cpm/ | PaymentRequest | Information about the payment (ransom) |
| /scs/ | ReceivedSmsRequest | Information about the SMS captured at the infected terminal (content and delivery information) |
| /ssms/ | SmsRequest | List of SMSes read from the infected terminal |
| /gfsf/ | SmsToSendRequest | Request for data to send an SMS from the infected terminal (SMS destination address and content) |
| /gt | UserAddressRequest | Request for information about the country, city, and IP address of the infected terminal |
| /logsms/ | SuccessCountRequest | Information about the number of SMSes successfully sent from the infected terminal |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Drabent, K.; Janowski, R.; Mongay Batalla, J. How to Circumvent and Beat the Ransomware in Android Operating System—A Case Study of Locker.CB!tr. Electronics 2024 , 13 , 2212. https://doi.org/10.3390/electronics13112212
Drabent K, Janowski R, Mongay Batalla J. How to Circumvent and Beat the Ransomware in Android Operating System—A Case Study of Locker.CB!tr. Electronics . 2024; 13(11):2212. https://doi.org/10.3390/electronics13112212
Drabent, Kornel, Robert Janowski, and Jordi Mongay Batalla. 2024. "How to Circumvent and Beat the Ransomware in Android Operating System—A Case Study of Locker.CB!tr" Electronics 13, no. 11: 2212. https://doi.org/10.3390/electronics13112212
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
A case-control study (also known as case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. Case-control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who have the condition with patients who do not have ...
Revised on June 22, 2023. A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the "case," and those without it are the "control.".
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes.[1] The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to the case individuals but do not have the ...
Examples. A case-control study is an observational study where researchers analyzed two groups of people (cases and controls) to look at factors associated with particular diseases or outcomes. Below are some examples of case-control studies: Investigating the impact of exposure to daylight on the health of office workers (Boubekri et al., 2014).
A case control study is a retrospective, observational study that compares two existing groups. Researchers form these groups based on the existence of a condition in the case group and the lack of that condition in the control group. They evaluate the differences in the histories between these two groups looking for factors that might cause a ...
Introduction. A case-control study is designed to help determine if an exposure is associated with an outcome (i.e., disease or condition of interest). In theory, the case-control study can be described simply. First, identify the cases (a group known to have the outcome) and the controls (a group known to be free of the outcome).
General Overview of Case-Control Studies. In observational studies, also called epidemiologic studies, the primary objective is to discover and quantify an association between exposures and the outcome of interest, in hopes of drawing causal inference. Observational studies can have a retrospective study design, a prospective design, a cross ...
Compared with prospective cohort studies, which involve observing a cohort of subjects with variable levels of the exposure of interest over time to relate the occurrence of the outcome of interest to the exposure, case-control studies start with case subjects and control subjects (ie, the outcome of interest is known) and look back retrospectively at the subjects' exposures to find an ...
Abstract. Case-control studies are observational studies in which cases are subjects who have a characteristic of interest, such as a clinical diagnosis, and controls are (usually) matched subjects who do not have that characteristic. After cases and controls are identified, researchers "look back" to determine what past events (exposures ...
case-control study, in epidemiology, observational (nonexperimental) study design used to ascertain information on differences in suspected exposures and outcomes between individuals with a disease of interest (cases) and comparable individuals who do not have the disease (controls). Analysis yields an odds ratio (OR) that reflects the relative probabilities of exposure in the two populations.
Case control studies are also known as "retrospective studies" and "case-referent studies." Advantages. Good for studying rare conditions or diseases; ... A case-control study was conducted to investigate if exposure to zinc oxide is a more effective skin cancer prevention measure. The study involved comparing a group of former lifeguards that ...
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes. The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to ...
Case-control studies. Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups.
A case-control study (also known as a case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. It is designed to help determine if an exposure is associated with an outcome (i.e., disease or condition of interest).
Case-Control Study. In a case-control study (also known as a case-referent study), two groups of individuals are selected for study, of which one has the disease whose causation is to be studied (the cases) and the other does not (the controls). In the context of the chemical industry, the aim of a case-control study is to evaluate the ...
However, a special type of case-control study, known as nested case-control study (discussed later in this chapter), is a hybrid design (partly prospective and partly retrospective). In traditional case-control study, cases and controls are selected at the starting point of the study - cases having the disease of interest, and control ...
"Case-control studies are best understood by considering as the starting point a source population, which represents a hypothetical study population in which a cohort study might have been conducted.The source population is the population that gives rise to the cases included in the study. If a cohort study were undertaken, we would define the exposed and unexposed cohorts (or several cohorts ...
Case Control. In a Case-Control study there are two groups of people: one has a health issue (Case group), and this group is "matched" to a Control group without the health issue based on characteristics like age, gender, occupation. ... There is a known link between Omgea-3 depletion and depression;
Cohort studies and case-control studies are two primary types of observational studies that aid in evaluating associations between diseases and exposures. In this review article, we describe these study designs, methodological issues, and provide examples from the plastic surgery literature. Keywords: observational studies, case-control study ...
Case-control studies are particularly useful for studying the cause of an outcome that is rare and for studying the effects of prolonged exposure. For example, a case-control study could be used ...
In a case-crossover study, each case serves as its own control because at the beginning of the study and prior to the onset of the acute event the individual belongs to the control group. For each person, there is a period during which the person was a case, called a "case window," and a period associated with not being a case, known as the ...
When the study population is dynamic. When little is known about the risk factors for the disease, case-control studies provide a way of testing associations with multiple potential risk factors. (This isn't really a unique advantage to case-control studies, however, since cohort studies can also assess multiple exposures.)
Case control studies are also known as "retrospective studies" and "case-referent studies." Advantages Good for studying rare conditions or diseases ... A case-control study was conducted to investigate if exposure to zinc oxide is a more effective skin cancer prevention measure. The study involved comparing a group of former lifeguards that ...
Some people who have been infected with the virus that causes COVID-19 can experience long-term effects from their infection, known as Long COVID or Post-COVID Conditions (PCC). Long COVID is broadly defined as signs, symptoms, and conditions that continue or develop after acute COVID-19 infection. This definition of Long COVID was developed by ...
Get notified in your email when a new post is published to this blog
Ransomware is one of the most extended cyberattacks. It consists of encrypting a user's files or locking the smartphone in order to blackmail a victim. The attacking software is ordered on the infected device from the attacker's remote server, known as command and control. In this work, we propose a method to recover from a Locker.CB!tr ransomware attack after it has infected and hit a ...