An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Heart Views
- v.18(3); Jul-Sep 2017

Guidelines To Writing A Clinical Case Report
What is a clinical case report.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant literature on the topic. The case report is a rapid short communication between busy clinicians who may not have time or resources to conduct large scale research.
WHAT ARE THE REASONS FOR PUBLISHING A CASE REPORT?
The most common reasons for publishing a case are the following: 1) an unexpected association between diseases or symptoms; 2) an unexpected event in the course observing or treating a patient; 3) findings that shed new light on the possible pathogenesis of a disease or an adverse effect; 4) unique or rare features of a disease; 5) unique therapeutic approaches; variation of anatomical structures.
Most journals publish case reports that deal with one or more of the following:
- Unusual observations
- Adverse response to therapies
- Unusual combination of conditions leading to confusion
- Illustration of a new theory
- Question regarding a current theory
- Personal impact.
STRUCTURE OF A CASE REPORT[ 1 , 2 ]
Different journals have slightly different formats for case reports. It is always a good idea to read some of the target jiurnals case reports to get a general idea of the sequence and format.
In general, all case reports include the following components: an abstract, an introduction, a case, and a discussion. Some journals might require literature review.
The abstract should summarize the case, the problem it addresses, and the message it conveys. Abstracts of case studies are usually very short, preferably not more than 150 words.
Introduction
The introduction gives a brief overview of the problem that the case addresses, citing relevant literature where necessary. The introduction generally ends with a single sentence describing the patient and the basic condition that he or she is suffering from.
This section provides the details of the case in the following order:
- Patient description
- Case history
- Physical examination results
- Results of pathological tests and other investigations
- Treatment plan
- Expected outcome of the treatment plan
- Actual outcome.
The author should ensure that all the relevant details are included and unnecessary ones excluded.
This is the most important part of the case report; the part that will convince the journal that the case is publication worthy. This section should start by expanding on what has been said in the introduction, focusing on why the case is noteworthy and the problem that it addresses.
This is followed by a summary of the existing literature on the topic. (If the journal specifies a separate section on literature review, it should be added before the Discussion). This part describes the existing theories and research findings on the key issue in the patient's condition. The review should narrow down to the source of confusion or the main challenge in the case.
Finally, the case report should be connected to the existing literature, mentioning the message that the case conveys. The author should explain whether this corroborates with or detracts from current beliefs about the problem and how this evidence can add value to future clinical practice.
A case report ends with a conclusion or with summary points, depending on the journal's specified format. This section should briefly give readers the key points covered in the case report. Here, the author can give suggestions and recommendations to clinicians, teachers, or researchers. Some journals do not want a separate section for the conclusion: it can then be the concluding paragraph of the Discussion section.
Notes on patient consent
Informed consent in an ethical requirement for most studies involving humans, so before you start writing your case report, take a written consent from the patient as all journals require that you provide it at the time of manuscript submission. In case the patient is a minor, parental consent is required. For adults who are unable to consent to investigation or treatment, consent of closest family members is required.
Patient anonymity is also an important requirement. Remember not to disclose any information that might reveal the identity of the patient. You need to be particularly careful with pictures, and ensure that pictures of the affected area do not reveal the identity of the patient.

Case Reports: How to Write a Case Report
- How to Write a Case Report
- Case Report Resources
- YouTube Resources
Consensus-Based Clinical Case Reporting Guidelines

| This was developed by to correspond with key components of a case report and capture useful clinical information (including 'meaningful use' information mandated by some insurance plans). The narrative: A case report tells a story in a narrative format that includes the presenting concerns, clinical findings, diagnoses, interventions, outcomes (including adverse events), and follow-up. The narrative should include a discussion of the rationale for any conclusions and any take-away messages. |
| Title | 1 | The words "case report: should be in the title, along with area of focus |
| Keywords | 2 | Four to seven keywords - include "case report" as one of the keywords |
| Abstract | 3a | Background: What does this case report add to the medical literature? |
| 3b | Case summary: chief complaint, diagnoses, interventions, and outcomes | |
| 3c | Conclusion: What is the main "take-away" lesson from this case? | |
| Introduction | 4 | The current standard of care and contributions of this case - with references (1-2 paragraphs) |
| Timeline | 5 | Information from this case report organized into a timelines (table or figure) |
| Patient Information | 6a | De-identified demographic and other patient or client specific information |
| 6b | Chief complaint - what prompted the visit? | |
| 6c | Relevant history including past interventions and outcomes | |
| Physical Exam | 7 | Relevant physical examination findings |
| Diagnostic Assessment | 8a | Evaluations such as surveys, laboratory testing, imaging, etc. |
| 8b | Diagnostic reasoning including other diagnoses considered and challenges | |
| 8c | Consider tables or figures linking assessment, diagnoses, and interventions | |
| 8d | Prognostic characteristics where applicable | |
| Interventions | 9a | Types such as lifestyle recommendations, treatments, medications, surgery |
| 9b | Intervention administration such as dosage, frequency, and duration | |
| 9c | Note changes in intervention with explanation | |
| 9d | Other concurrent interventions | |
| Follow-up and Outcomes | 10a | Clinician assessment (and patient or client assessed outcomes when appropriate) |
| 10b | Important follow-up diagnostic evaluations | |
| 10c | Assessment of intervention adherence and tolerability, including adverse events | |
| Discussion | 11a | Strengths and limitations in your approach to the case |
| 11b | Specify how this case report informs practice or Clinical Practice Guidelines (CPG) | |
| 11c | How does this case report suggest a testable hypothesis? | |
| 11d | Conclusions and rationale | |
| Patient Perspective | 12 | When appropriate, include the assessment of the patient or client on this episode of care |
| Informed Consent | 13 | Informed consent from the person who is the subject of this case report, required by most journals |
| Additional Information | 14 | Acknowledgement section; Competing Interests; IRB approval when required |
Gagnier JJ, Riley D, Altman DG, Moher D, Sox H, Kienle GS, for the CARE group: The CARE guidelines: Consensus-based clinical case reporting guideline development. Dtsch Arztebl Int 2013; 110(37): 603-8.
Select Journals Accepting Case Reports
| The following journals are indexed in Medline and currently accepting case reports (as of 12/2/2016) either regularly or under specific circumstances. Click on the links below to view the author instructions for each journal and determine if your case meets the journal's criteria. |
Case Report Templates
The CAse REporting (CARE) team created templates in nine languages to assist clinicians, researchers, and educators with the ultimate goal of improving the completeness, transparency, and usefulness of case reports.
English , Spanish , German , Chinese , Dutch , French , Japanese , Korean , Portuguese
- Case Report Journals
A list of case report journals can be found in the pdf below. It provides information on the year launched, open-access status, reported questionable publishing practices, and whether the journal is indexed in Medline. The majority of these journals are open-access and will require a submission fee.
BMJ Case Reports
- BMJ Consent Form
The Library has an institutional fellowship with BMJ Case Reports which allows faculty, staff, and students at Weill Cornell Medicine to submit case reports without paying an individual fellowship fee. Use our fellowship code when you are ready to publish.
Please note: BMJ Case Reports, like most journals, requires a signed consent form in order for a case report to be considered for publication.
- << Previous: Home
- Next: Case Report Resources >>
- Last Updated: Aug 15, 2022 12:15 PM
- URL: https://med.cornell.libguides.com/casereports
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023
A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
26 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Writing a Case Report
This page is intended for medical students, residents or others who do not have much experience with case reports, but are planning on writing one.
What is a case report? A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are commonly of the following categories :
- Rare diseases
- Unusual presentation of disease
- Unexpected events
- Unusual combination of diseases or conditions
- Difficult or inconclusive diagnosis
- Treatment or management challenges
- Personal impact
- Observations that shed new light on a disease or condition
- Anatomical variations
It is important that you recognize what is unique or interesting about your case, and this must be described clearly in the case report.
Case reports generally take the format of :
1. Background
2. Case presentation
3. Observations and investigation
4. Diagnosis
5. Treatment
7. Discussion
Does a case report require IRB approval?
Case reports typically discuss a single patient. If this is true for your case report, then it most likely does not require IRB approval because it not considered research. If you have more than one patient, your study could qualify as a Case Series, which would require IRB review. If you have questions, you chould check your local IRB's guidelines on reviewing case reports.
Are there other rules for writing a case report?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health
While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply to any case report. This includes answering questions like: Do I need written HIPAA authorization to publish a case report? When do I need IRB review of a case report? What qualifies as a patient identifier?
How do I get started?
1. We STRONGLY encourage you to consult the CARE Guidelines, which provide guidance on writing case reports - https://www.care-statement.org/
Specifically, the checklist - https://www.care-statement.org/checklist - which explains exactly the information you should collect and include in your case report.
2. Identify a case. If you are a medical student, you may not yet have the clinical expertise to determine if a specific case is worth writing up. If so, you must seek the help of a clinician. It is common for students to ask attendings or residents if they have any interesting cases that can be used for a case report.
3. Select a journal or two to which you think you will submit the case report. Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things. Journals may also charge publication fees (see Is it free to publish? below)
4. Obtain informed consent from the patient (see " Do I have to obtain informed consent from the patient? " below). Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal.
Once you've identified the case, selected an appropriate journal(s), and considered informed consent, you can collect the required information to write the case report.
How do I write a case report?
Once you identify a case and have learned what information to include in the case report, try to find a previously published case report. Finding published case reports in a similar field will provide examples to guide you through the process of writing a case report.
One journal you can consult is BMJ Case Reports . MSU has an institutional fellowship with BMJ Case Reports which allows MSU faculty, staff and students to publish in this journal for free. See this page for a link to the journal and more information on publishing- https://lib.msu.edu/medicalwriting_publishing/
There are numerous other journals where you can find published case reports to help guide you in your writing.
Do I have to obtain informed consent from the patient?
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible). Please consider this as well.
If required, it is recommended you obtain informed consent before the case report is written.
An example of a case report consent form can be found on the BMJ Case Reports website, which you can access via the MSU library page - https://casereports.bmj.com/ . Go to "Instructions for Authors" and then "Patient Consent" to find the consent form they use. You can create a similar form to obtain consent from your patient. If you have identified a journal already, please consult their requirements and determine if they have a specific consent form they would like you to use.
Seek feedback
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before.
Selecting a journal
Aside from BMJ Case Reports mentioned above, there are many, many journals out there who publish medical case reports. Ask your mentor if they have a journal they would like to use. If you need to select on your own, here are some strategies:
1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/
a. Do a search for a topic, disease or other feature of your case report
b. When the results appear, on the left side of the page is a limiter for "article type". Case reports are an article type to which you can limit your search results. If you don't see that option on the left, click "additional filters".
c. Review the case reports that come up and see what journals they are published in.
2. Use JANE - https://jane.biosemantics.org/
3. Check with specialty societies. Many specialty societies are affiliated with one or more journal, which can be reviewed for ones that match your needs
4. Search through individual publisher journal lists. Elsevier publishes many different medical research journals, and they have a journal finder, much like JANE ( https://journalfinder.elsevier.com/ ). This is exclusive to Elsevier journals. There are many other publishers of medical journals for review, including Springer, Dove Press, BMJ, BMC, Wiley, Sage, Nature and many others.
Is it free to publish ?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option". It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. MSU-CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing
*A more thorough discussion on finding a journal, publication costs, predatory journals and other publication-related issues can be found here: https://research.chm.msu.edu/students-residents/finding-a-journal
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med . 2:38-43. doi: 10.7453/gahmj.2013.008
Riley DS, Barber MS, Kienle GS, AronsonJK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. 2017. CARE guidelines for case reports: explanation and elaboration document . J Clin Epidemiol . 89:218-234. doi: 10.1016/j.jclinepi.2017.04.026
Guidelines to writing a clinical case report. 2017. Heart Views . 18:104-105. doi: 10.4103/1995-705X.217857
Ortega-Loubon C, Culquichicon C, Correa R. The importance of writing and publishing case reports during medical education. 2017. Cureus. 9:e1964. doi: 10.7759/cureus.1964
Writing and publishing a useful and interesting case report. 2019. BMJ Case Reports. https://casereports.bmj.com/pages/wp-content/uploads/sites/69/2019/04/How-to-write-a-Case-Report-DIGITAL.pdf
Camm CF. Writing an excellent case report: EHJ Case Reports , Case of the Year 2019. 2020. European Heart Jounrnal. 41:1230-1231. https://doi.org/10.1093/eurheartj/ehaa176
*content developed by Mark Trottier, PhD
How to Write a Case Report
- First Online: 01 January 2020
Cite this chapter

- Richard Balon 2 &
- Eugene V. Beresin 3 , 4 , 5
1398 Accesses
1 Altmetric
Case reports are an important starting point for academic writing and for producing new, interesting, and educational information for the field. They usually describe a unique syndrome or disease, an unexpected relationship between relatively uncommon diseases or symptoms, unique or rare events or outcomes in describing a disease, or a unique therapeutic approach to an illness. Authors should not write a case report simply for the sake of writing. Rather, the case report must help improve understanding of a disease or improve therapy. Thus, detailed preparation is crucial, including a thorough literature search about the disease, treatment, and outcomes. A single outlying case or freak episode may unintentionally negatively influence some clinical practice. Hence, authors must remember that there is a great professional responsibility in providing a case report. Patient privacy must be preserved. All identifying information should be omitted unless essential for scientific purposes, and informed consent is often required. The structure of the case report should include the Title/Title page; Abstract (summary of the case) (only if required by the journal); Introduction (purpose, worthiness of the case, based on references); Case Description (most salient parts of the case and its outcome); Discussion/Conclusion (presents the broad view of the case, its uniqueness, and contribution to the literature); at times Patient’s Perspective; Acknowledgments; and References.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

The Historical Tradition of Case Reporting

How to Write a Case Report?
Borus JF. Writing for publication. In: Kay J, Silberman EK, Pessar L, editors. Handbook of psychiatric education and faculty development. Washington, D.C.: American Psychiatric Press; 1999. p. 57–93.
Google Scholar
Schnur D, Cheruvanki B, Mustafa R. The case report: a user-friendly educational tool for psychiatry residency programs. Acad Psychiatry. 2016;40:857–8.
Article Google Scholar
Martyn C. Case reports, case series and systematic reviews. Q J Med. 2002;95:197–8.
Article CAS Google Scholar
Akers KG. New journals for publishing medical case reports. J Med Libr Assoc. 2016;104:146–9.
Rison RA. A guide to writing case reports for the journal of medical case reports and BioMed central research notes. J Med Cas Rep. 2013;7:239. https://doi.org/10.1186/1752-1947-7-239 .
Roselli D, Otero A. The case report is far from dead. Lancet. 2002;359:84.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, the CARE Group. The CARE guidelines: consensus –based clinical case reporting guideline development. BMJ Case Rep. 2013;2013:bcr2013201554. https://doi.org/10.1136/bcr-2013-201554.
Article PubMed PubMed Central Google Scholar
Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35.
Green BN, Johnson CD. How to write a case report for publication. J Chiropr Med. 2006;5:72–82.
Iles RL, Piepho RW. Presenting and publishing case reports. J Clin Pharmacol. 1996;36:573–9.
Wright SM, Kouroukis C. Capturing zebras: what to do with a reportable case. Can Med Assoc J. 2000;163:429–31.
CAS Google Scholar
Procopio M. Publication of case reports. Br J Psychiatry. 2005;187:91.
DeBakey L, DeBakey S. The case report. I. Guidelines for preparation. Int J Cardiol. 1983;4:357–64.
Sorinola O, Loufowobi O, Coomarasamy A, Khan KS. Instructions to authors for case reporting are limited: a review of a core journal list. BMC Med Educ. 2004;4:4.
Rison RA, Shepphird JK, Kidd MR. How to choose the best journal for your case report. J Med Cas Rep. 2017;11:198. https://doi.org/10.1186/s13256-017-1351-y .
Beall J. Best practices for scholarly authors in the age of predatory journals. Ann R Coll Surg Engl. 2016;98:77–8.
Beall J. Dangerous predatory publishers threaten medical research. J Korean Med Sci. 2016;31:1511–3.
International Committee of Medical Journal Editors. Protection of patients’ rights to privacy. BMJ. 1995;311:1272.
Singer PA. Consent to publication of patient information. BMJ. 2004;329:566–8.
Levine SB, Stagno SJ. Informed consent for case reports. The ethical dilemma of right to privacy versus pedagogical freedom. J Psychother Pract Res. 2001;10:193–201.
CAS PubMed PubMed Central Google Scholar
Har-El G. Does it take a village to write a case report? Otolaryng Head Neck Surg. 1999;120:787–8.
McCarthy LH, Reilly KEH. How to write a case report. Fam Med. 2000;32:190–5.
CAS PubMed Google Scholar
Squires BP. Case reports: what editors want from authors and peer reviewers. Can Med Assoc J. 1989;141:379–80.
Chelvarajah R, Bycroft J. Writing and publishing case reports: to road to success. Acta Neurochir. 2004;146:313–6.
Cohen H. How to write a patient case report. Am J Health Syst Pharm. 2006;63:1888–92.
DeBakey L, DeBakey S. The case report. II. Style and form. Int J Cardiol. 1984;6:247–54.
Resnik PJ, Soliman S. Draftsmanship. In: Buchanan A, Norco MA, editors. The psychiatric report. Principles and practice of forensic writing. New York: Cambridge University Press; 2011. p. 81–92.
Chapter Google Scholar
Suggested Reading
Huth FJ. Writing and publishing in medicine. 3rd ed. Baltimore: Williams & Wilkins; 1999.. (previously published as How to write and publish papers in medical sciences )
Download references
Author information
Authors and affiliations.
Departments of Psychiatry and Behavioral Neurosciences and Anesthesiology, Wayne State University, and Detroit Medical Center, Detroit, MI, USA
Richard Balon
Harvard Medical School, Boston, MA, USA
Eugene V. Beresin
The Clay Center for Young Healthy Minds, Boston, MA, USA
Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Richard Balon .
Editor information
Editors and affiliations.
Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA, USA
Laura Weiss Roberts
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Balon, R., Beresin, E.V. (2020). How to Write a Case Report. In: Roberts, L. (eds) Roberts Academic Medicine Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-31957-1_30
Download citation
DOI : https://doi.org/10.1007/978-3-030-31957-1_30
Published : 01 January 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-31956-4
Online ISBN : 978-3-030-31957-1
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Get new issue alerts Get alerts
- Submit a Manuscript
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
How to Write Case Reports and Case Series
Ganesan, Prasanth
Department of Medical Oncology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Address for correspondence: Dr. Prasanth Ganesan, Medical Oncology, 3 rd Floor, SSB, Jawaharlal Institute of Postgraduate Medical Education and Research, Dhanvantari Nagar, Puducherry - 605006, India. E-mail: [email protected]
Received March 13, 2022
Received in revised form April 10, 2022
Accepted April 10, 2022
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Case reports are considered the smallest units of descriptive studies. They serve an important function in bringing out information regarding presentation, management, and/or outcomes of rare diseases. They can also be a starting point in understanding unique associations in clinical medicine and can introduce very effective treatment paradigms. Preparing the manuscript for a case report may be the first exposure to scientific writing for a budding clinician/researcher. This manuscript describes the steps of writing a case report and essential considerations when publishing these articles. Individual components of a case report and the “dos and don'ts” while preparing these components are detailed.
INTRODUCTION
A case report describes several aspects of an individual patient's presentation, investigations, management decisions, and/or outcomes. This is a type of observational study and has been described as the smallest publishable unit in medical literature.[ 1 ] A case series involves a group of patients with similar presentations or treatments. In modern medicine [ Figure 1 ], these publications are categorized as the “lowest level of evidence”.[ 2 ] However, they serve several essential functions. For example, there are rare diseases where large, randomized trials, or even observational studies may not be possible. Medical practice, in these conditions, is often guided by well-presented case reports or series. There are situations where a single case report has heralded an important therapy change.[ 3 ] Further, case reports are often a student's first exposure to manuscript writing. Hence, these serve as training for budding scholars to understand scientific writing, learn the process of manuscript submission, and receive and respond to reviewer comments. This article explains the reasons why case reports are published and provides guidance for writing such type of articles.

WHY ARE CASE REPORTS PUBLISHED?
A case report is often published to highlight the rarity of a particular presentation. However, it may be of much more value if it also informs some aspects of management. This could be in the form of rare expressions of a common disease so that clinicians who read will be aware and can consider additional possibilities and differential diagnoses when encountering similar situations. A new form of evaluation of a patient, either to facilitate the diagnostics or to improve understanding of the disease condition, may stimulate a case report. Novel treatments may be tried, and the results might be necessary to disseminate. This may be encountered either in rare diseases or conditions where treatment options are exhausted. Moreover, randomized trials report outcomes of a group and often do not inform about the individual patient. [ Table 1 ] describes a few examples of case reports/case series which have had a remarkable impact on medical practice.

ETHICAL ISSUES
If there is a possibility of patient identification from the report, it is mandatory to obtain informed consent from the patient while approval from the institutional ethics committee (IEC) may also be needed depending on institutional policies.[ 7 ] If identifying information is absent (or if suitable steps are taken to remove identifying information or hide the identity, (such as by covering the eyes), it may still be required by some journals to obtain ethics committee approval for certain types of case reports. If a case series involves retrospective chart review, “waiver-of-consent” may be sought from the ethics committee. Indian Ethical Guidelines do not separately address this issue in case reports.[ 8 ] The Committee on Publication Ethics has described best practices for journals when publishing case reports which also gives links to model consent forms.[ 9 ]
HOW TO START?
If you are a beginner and you have identified an interesting case which you want to report, the first step would be to sit with your team and discuss the aspects of the case you want to highlight in your publication.[ 10 ] Do a literature search and try to summarize available information before writing the draft. It would also be a good idea to understand which journal you are targeting; this will assist in determining the number of figures, the word limits, and ethical requirements (such as informed consent). Discussions with senior faculty about the authors and their order should also be done at this point to avoid issues later. For a beginner, it would be a good practice to present the case in the department or in an institutional scientific forum before writing up the manuscript.
COMPONENTS OF a CASE REPORT
A case report usually has the following sections: an abstract, a brief introduction, the actual description of the case, and finally, the discussion which highlights the uniqueness of the case and includes a conclusion statement. Many journals these days publish case reports only as a letter to editor; in such cases, an abstract is not usually required.
The title must be informative about the problem being reported. It may refer to the particular issue being highlighted in the report, or it may refer to the educational aspect of that particular report. Catchy titles are often used by authors to trigger interest among the readers and make them want to read the article. Authors may remember to use titles which will help people locate the article when searching the literature.
When writing a title, it may be best to avoid terms such as “case report,” “review of literature,” “unique,” “rare,” “first-report”; these do not add value to the presentation.
Introduction
This must introduce the condition and clearly state why the case report is worth reading. It may also contain a brief mention of the current status of the problem being described with supporting references.
Describing the case
The case must be presented succinctly, in a chronological order, clearly highlighting the salient aspects of the case being reported. Relevant negative findings may be provided. For example, if a case is being reported for elaborating a new type of treatment, then more attention must be given to treatment aspects (e.g., name of the drug, dosage, schedule, dose modifications, or the type of surgery, duration, and type of anesthesia) after briefly describing the presentation and diagnostics. The idea is that the reader must be able to apply the treatment in his/her practice if required.
However, if the case is being presented for diagnostic rarity/unusual clinical features/pathological aspects, then more attention must be given to these aspects. For example, if the emphasis is on tissue pathology, then the description must include details about tissue processing, types of stains, and immunohistochemistry details.
Figures and tables
Figures, as in any publication, should be self-explanatory. A properly constructed figure legend can be used for describing certain aspects of the case much better than long-winded text in the main manuscript. This will also help to reduce the word count in the main manuscript. If there are multiple figures (e.g., follow-up radiology series and response to treatment images), these can be combined as [ Figure 1 ]a, [ Figure 1 ]b, [ Figure 1c ] or [ Figure 1 ]a, [ Figure 1 ]b, [ Figure 1 ]c, [ Figure 1d ]. This will help conform to the figure number limits prescribed by the journal. While preparing the figures, one must ensure that the quality of the art/photograph is not compromised. Further, patient identifying features must be masked, unless necessary to show.
Tables are usually not part of case reports but may be used. One example is presenting the baseline investigations in a tabular format which can facilitate assimilation as well as reduce the word count. Tables are more often used in case series. The most common is a type of table where the features of all the cases included are summarized with each row referring to an individual patient. This usually works for a series of up to ten patients; beyond that, the table may become crowded and difficult to understand. Tables may also be used in the discussion section to summarize related, published reports to date.
Discussion including review
A case report may help to alter the approach to patient management in the clinic or it may even stimulate original research evaluating a new treatment. Thus, the discussion must summarize the unique aspects of the case (why is the case different?) and the essential learning points/implications (how will it change management?/What further research needs to be done?). In addition to stating the differences from existing literature, the discussion should also attempt to explain these differences.
If the condition or treatment approach being focused on is sufficiently rare, reviewing all available cases published until that point is critical. This review may be presented in a table with each case described briefly. A more nuanced study might attempt to summarize the relevant demographics and clinical details of the various cases published to date in the form of a table (e.g., median age, gender distribution, and survival outcomes).
CASE SERIES - WHAT IS DIFFERENT?
There is no formal definition as to what is case series and what would be considered a retrospective cohort study. In general, a case series comprises <10 cases; beyond that, it may be feasible to apply formal statistics and may be considered a cohort study.
Both case reports and case series are descriptive studies. Case series must have similar cases and hence the inclusion must be clearly defined. The interventions must be documented in a way that is reproducible and follow-up of each individual in the report must be available. Although formal statistical analyses are usually not a part of case series, authors may attempt to summarize baseline demographic parameters using descriptive statistics.
ABSTRACT OF a CASE REPORT
As explained earlier, a few journals do not require abstracts for case report submissions. When required, one should try to highlight the salient aspects of the case presented and the reason for the publication within the abstract word limit, which may be as short as 100–200 words. Spend time and effort in writing a good abstract as this is a portion which is usually read by the editor during manuscript screening and may have implications for whether the article progresses to the next stage of editorial processing.
REFERENCES IN a CASE REPORT
One may only cite key references in a case report or series as there is limited scope for elaborate literature search. Most journals have a limit of 10–15 references for case reports; when publishing as a letter to editor (or correspondence), the allowed reference limit may be even lower (five or less for some journals).
CHOOSING THE RIGHT JOURNAL
Many journals have recently stopped publishing case reports and series. This is often an attempt by journals to optimize their resources (space and reviewer time) to attain the highest possible impact. Although this is unfortunate, it is a reality which must be acknowledged. Nonetheless, the advent of online-only journals has led to more options for aspiring authors. Some journals accept case series, whereas others have “sister” journals created to accept case reports and other, less definitive, contributions to the literature.[ 11 ] It is an important exercise to study all available journals accepting case reports of the type being written. The case report must be tailored to the journal's requirements. Many journals may charge an article processing fee; author(s) must consider whether they are willing to pay and publish. Some of these may be predatory journals; authors must be wary of them and scrupulously avoid publishing in such journals as they can permanently stain the publication records of a researcher.
PUBLISHING THE CASE REPORT/SERIES AS a LETTER TO EDITOR/IMAGE SERIES
When the matter to be conveyed is very minimal or is being published mainly for its rarity, letters to editor may be an alternate route to publish case report data. Interesting images may be published in the form of “images” series which is now a part of many journals. The flexibility of web-based publishing also allows interesting videos to be published online.
GUIDELINES FOR CASE REPORTS
There are guidelines which help authors in the preparation and submission of case reports. The CAse REports (CARE) checklist is one such popular guideline. It provides a “checklist” and other resources for authors that can help navigate the process of writing a case report, especially when a person is doing it for the first time.[ 12 ]
AUTHORSHIP IN CASE REPORTS
Although there are no separate guidelines for authorship in “case reports,” general authorship rules follow that for any manuscript. “Gift” authorship must be avoided. All authors must have contributed to the creation of the manuscript in addition to being involved in some aspect of care of the patient being reported. Authorship order should be ideally predecided based on mutual consensus.
CONCLUSIONS
A case report is a useful starting point for one's scientific writing career. There are useful online resources which describe the steps for a newbie writer.[ 13 14 ] [ Table 2 ] summarizes the important components to follow and understand when writing case reports. Although many frontline journals have reduced their acceptance of case reports, these publications continue to serve an essential scientific and academic role.

Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Case reports; manuscript writing; case series; references
- + Favorites
- View in Gallery
Readers Of this Article Also Read
How to revise and resubmit the manuscript after a favorable peer review, glossopharyngeal neuralgia following coronavirus disease 2019 infection, polymorphous adenocarcinoma of the parotid – an uncommon site of occurrence, primary vulval mucinous adenocarcinoma of intestinal type masquerading as..., detection of rare blood group ax phenotype in blood donor.
Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.
Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
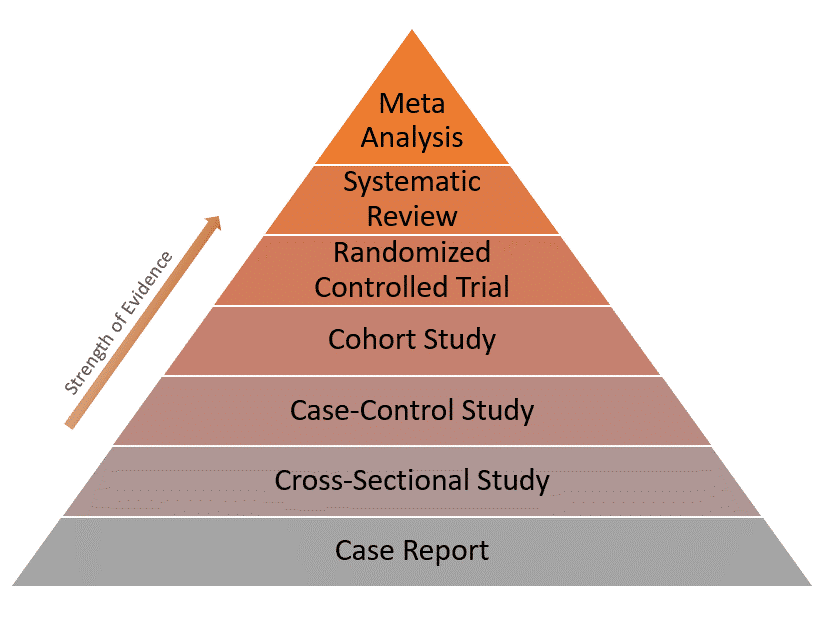
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Medical Studies
How to Write a Medical Case Study Report
Last Updated: April 18, 2024 Fact Checked
This article was medically reviewed by Mark Ziats, MD, PhD and by wikiHow staff writer, Jennifer Mueller, JD . Dr. Mark Ziats is an Internal Medicine Physician, Scientist, Entrepreneur, and the Medical Director of xBiotech. With over five years of experience, he specializes in biotechnology, genomics, and medical devices. He earned a Doctor of Medicine degree from Baylor College of Medicine, a Ph.D. in Genetics from the University of Cambridge, and a BS in Biochemistry and Chemistry from Clemson University. He also completed the INNoVATE Program in Biotechnology Entrepreneurship at The Johns Hopkins University - Carey Business School. Dr. Ziats is board certified by the American Board of Internal Medicine. There are 15 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 187,526 times.
You've encountered an interesting and unusual case on your rounds, and a colleague or supervising physician says, "Why don't you write up a case study report?" If you've never written one before, that might sound intimidating, but it's a great way to get started in medical writing. Case studies always follow a standard structure and format, so the writing is very formulaic once you get the hang of it. Read on for a step-by-step guide to writing your first case study report.
What is a case study report?

- Medical students or residents typically do the bulk of the writing of the report. If you're just starting your medical career, a case study report is a great way to get a publication under your belt. [2] X Research source

- If the patient is a minor or is incapable of giving informed consent, get consent from their parents or closest relative. [4] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- Your hospital likely has specific consent forms to use. Ask your supervising physician if you're not sure where to get one.
- Some journals also have their own consent form. Check your target journal's author or submission information to make sure. [5] X Research source
How is a case study report structured?

- Even though the introduction is the first part of a case study report, doctors typically write it last. You'll have a better idea of how to introduce your case study to readers after you've written it.
- Your abstract comes at the top, before the introduction, and provides a brief summary of the entire report. Unless your case study is published in an open-access journal, the abstract is the only part of the article many readers will see.

- Many journals offer templates and checklists you can use to make sure your case study includes everything necessary and is formatted properly—take advantage of these! Some journals, such as BMJ Case Reports , require all case studies submitted to use their templates.
Drafting Your Medical Case Study Report

- Patient description
- Chronological case history
- Physical exam results
- Results of any pathological tests, imaging, or other investigations
- Treatment plan
- Expected outcome of treatment
- Actual outcome of treatment

- Why the patient sought medical help (you can even use their own words)
- Important information that helped you settle on your diagnosis
- The results of your clinical examination, including diagnostic tests and their results, along with any helpful images
- A description of the treatment plan
- The outcome, including how and why treatment ended and how long the patient was under your care [11] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- You will need references to back up symptoms of the condition, common treatment, and the expected outcome of that common treatment.
- Use your research to paint a picture of the usual case of a patient with a similar condition—it'll help you show how unusual and different your patient's case is.
- Generally, aim for around 20 references—no fewer than 15, but no more than 25. [13] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- Close your discussion section with a summary of the lessons learned from the case and why it's significant to consider when treating similar cases in the future.
- Outline any open questions that remain. You might also provide suggestions for future research.

- In your conclusion, you might also give suggestions or recommendations to readers based on what you learned as a result of the case.
- Some journals don't want a separate conclusion section. If that's the case for one of your target journals, just move this paragraph to the end of your discussion section.
Polishing Your Report for Submission to Publishers

- Most titles are fewer than 10 words long and include the name of the disease or condition treated.
- You might also include the treatment used and whether the outcome was successful. When deciding what to include, think about the reason you wrote the case study in the first place and why you think it's important for other clinicians to read.

- Made a significant intellectual contribution to the case study report
- Was involved in the medical care of the patient reported
- Can explain and defend the data presented in the report
- Has approved the final manuscript before submission for publication

- Keep in mind that the abstract is not just going to be the first thing people read—it will often be the only thing people read. Make sure that if someone is going to walk away having only read the abstract, they'll still get the same message they would have if they read the whole thing.
- There are 2 basic types of abstract: narrative and structured. A narrative abstract is a single paragraph written in narrative prose. A structured abstract includes headings that correspond with the sections of the paper, then a brief summary of each section. Use the format preferred by your target journal.

- Look for keywords that are relevant to your field or sub-field and directly related to the content of your article, such as the name of the condition or specific treatments you used.
- Most journals allow 4-8 keywords but check the submission guidelines of your target journal to make sure.

- Blur out the patient's face as well as any tattoos, birthmarks, or unrelated scars that are visible in diagnostic images.

- It's common to thank the patient, but that's up to you. Even if you don't, include a statement indicating that you have the patient's written, informed consent to publish the information.
- Read the journal's submission guidelines for a definition of what that journal considers a conflict of interest. They're generally the same, but some might be stricter than others. [22] X Research source

- If you're not familiar with the citation style used by your target journal, check online for a guide. There might also be one available at your hospital or medical school library.
- Medical librarians can also help with citation style and references if you run into something tricky—don't just wing it! Correct citation style insures that readers can access the materials you cite.

- It's also a good idea to get a beta reader who isn't a medical professional. Their comments can help you figure out where you need to clarify your points.
- Read a lot of case studies published in your target journals—it will help you internalize the tone and style that journal is looking for.
Submitting Your Report to Publishers

- Look into the background and reputation of journals before you decide to submit to them. Only seek publication from reputable journals in which articles go through a peer-review process.
- Find out what publishing fees the journals charge. Keep in mind that open-access journals tend to charge higher publishing fees. [26] X Research source
- Read each journal's submission and editorial guidelines carefully. They'll tell you exactly how to format your case study, how long each section should be, and what citation style to use. [27] X Research source
- For electronic journals that only publish case reports, try BMJ Case Reports , Journal of Medical Case Reports , or Radiology Case Reports .

- If your manuscript isn't suitable for the journal you submitted to, the journal might offer to forward it to an associated journal where it would be a better fit.
- When your manuscript is provisionally accepted, the journal will send it to other doctors for evaluation under the peer-review process.
- Most medical journals don't accept simultaneous submissions, meaning you'll have to submit to your first choice, wait for their decision, then move to the next journal on the list if they don't bite.

- Along with your revised manuscript, include a letter with your response to each of the reviewer's comments. Where you made revisions, add page numbers to indicate where the revisions are that address that reviewer's comments.
- Sometimes, doctors involved in the peer review process will indicate that the journal should reject the manuscript. If that's the case, you'll get a letter explaining why your case study report won't be published and you're free to submit it elsewhere.

- Some journals require you to have your article professionally copy-edited at your own cost while others do this in-house. The editors will let you know what you're responsible for.

- With your acceptance letter, you'll get instructions on how to make payment and how much you owe. Take note of the deadline and make sure you pay it as soon as possible to avoid publication delays.
- Some journals will publish for free, with an "open-access option" that allows you to pay a fee only if you want open access to your article. [32] X Research source

- Through the publishing agreement, you assign your copyright in the article to the journal. This allows the journal to legally publish your work. That assignment can be exclusive or non-exclusive and may only last for a specific term. Read these details carefully!
- If you published an open-access article, you don't assign the copyright to the publisher. The publishing agreement merely gives the journal the right to publish the "Version of Record." [33] X Research source
How do I find a suitable case for a report?

- A rare disease, or unusual presentation of any disease
- An unusual combination of diseases or conditions
- A difficult or inconclusive diagnosis
- Unexpected developments or responses to treatment
- Personal impact
- Observations that shed new light on the patient's disease or condition

- There might be other members of your medical team that want to help with writing. If so, use one of these brainstorming sessions to divvy up writing responsibilities in a way that makes the most sense given your relative skills and experience.
- Senior doctors might also be able to name some journals that would potentially publish your case study. [36] X Research source
Expert Q&A
You Might Also Like

- ↑ https://www.elsevier.com/connect/authors-update/the-dos-and-donts-of-writing-and-publishing-case-reports
- ↑ https://www.bmj.com/content/350/bmj.h2693
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686928/
- ↑ https://health.usf.edu/medicine/internalmedicine/im-impact/~/media/B3A3421F4C144FA090AE965C21791A3C.ashx
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6476221/
- ↑ https://www.springer.com/gp/authors-editors/authorandreviewertutorials/writing-a-journal-manuscript/title-abstract-and-keywords/10285522
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://thelancet.com/pb/assets/raw/Lancet/authors/tl-info-for-authors.pdf
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-017-1351-y
- ↑ https://guides.himmelfarb.gwu.edu/casereports
- ↑ https://casereports.bmj.com/pages/authors/
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-7-239
- ↑ https://research.chm.msu.edu/students-residents/writing-a-case-report
- ↑ https://authorservices.taylorandfrancis.com/publishing-your-research/moving-through-production/copyright-for-journal-authors/#
About This Article

Medical Disclaimer
The content of this article is not intended to be a substitute for professional medical advice, examination, diagnosis, or treatment. You should always contact your doctor or other qualified healthcare professional before starting, changing, or stopping any kind of health treatment.
Read More...
To start a medical case study report, first choose a title that clearly reflects the contents of the report. You’ll also need to list any participating authors and develop a list of keywords, as well as an abstract summarizing the report. Your report will need to include an introduction summarizing the context of the report, as well as a detailed presentation of the case. Don’t forget to include a thorough citation list and acknowledgements of anyone else who participated in the study. For more tips from our Medical co-author, including how to get your case study report published, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Sep 5, 2020
Did this article help you?

Asfia Banu Pasha
Apr 10, 2017
Jun 20, 2021
Mar 1, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life
Case report
Journal of Medical Case Reports welcomes well-described reports of cases that include the following:
- Unreported or unusual side effects or adverse interactions involving medications.
- Unexpected or unusual presentations of a disease.
- New associations or variations in disease processes.
- Presentations, diagnoses and/or management of new and emerging diseases.
- An unexpected association between diseases or symptoms.
- An unexpected event in the course of observing or treating a patient.
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect.
Case reports submitted to Journal of Medical Case Reports should make a contribution to medical knowledge and must have educational value or highlight the need for a change in clinical practice or diagnostic/prognostic approaches. The journal will not consider case reports describing preventive or therapeutic interventions, as these generally require stronger evidence.
Authors are encouraged to describe how the case report is rare or unusual as well as its educational and/or scientific merits in the covering letter that accompanies the submission of the manuscript.
Any images should protect the patient’s anonymity as far as possible. Any photos or medical imaging should not show the patient's name, medical record number, or date of birth. Images should be cropped only to show the key feature. As per journal policy, JMCR does not consider images with patient faces or patient facial features. If an image of a face must be published, this should be cropped so that only the affected area is shown.
Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Patient ethnicity must be included in the Abstract and in the Main Body under the Case Presentation section.
Reporting standards
For case reports, Journal of Medical Case Reports requires authors to follow the CARE guidelines . The CARE checklist should be provided as an additional files. Submissions received without these elements will be returned to the authors as incomplete.
The checklist will not be used as a tool for judging the suitability of manuscripts for publication in Journal of Medical Case Reports , but is intended as an aid to authors to clearly, completely, and transparently let reviewers and readers know what authors did and found. Using the CARE guideline to write the case report and completing the CARE checklist are likely to optimize the quality of reporting and make the peer review process more efficient.

Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
Title page
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review, A case report etc."
- or, for non-clinical or non-research studies: a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. The abstract must include the following separate sections:
- Background: why the case should be reported and its novelty
- Case presentation: a brief description of the patient’s clinical and demographic details, the diagnosis, any interventions and the outcomes
- Conclusions: a brief summary of the clinical impact or potential implications of the case report
Keywords
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the case report or study, its aims, a summary of the existing literature.
Case presentation
This section should include a description of the patient’s relevant demographic details, medical history, symptoms and signs, treatment or intervention, outcomes and any other significant details.
Discussion and Conclusions
This should discuss the relevant existing literature and should state clearly the main conclusions, including an explanation of their relevance or importance to the field.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
- Editorial Board
- Manuscript editing services
- Meet the Editors
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to write a case...
How to write a case report
- Related content
- Peer review
- Rahij Anwar , locum registrar in trauma and orthopaedics 1 ,
- Huma Kabir , clinical fellow in paediatrics 2 ,
- Rajesh Botchu , senior house officer in trauma and orthopaedics 3 ,
- Shah Alam Khan , assistant professor 4 ,
- Nitish Gogi , senior house officer in trauma and orthopaedics 5
- 1 Royal London Hospital, Bexleyheath, London DA6 8DR
- 2 Queen Mary's Hospital, Bexleyheath, London DA6 8DR
- 3 Maidstone Hospital, Maidstone ME16 9PQ
- 4 Department of Orthopaedics, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India
- 5 Russels Hall Hospital, Dudley DY1 2LU
Rahij Anwar and colleagues give advice on the practical details of writing case reports
Research has become an integral part of medical careers. A case report is a way of communicating information to the medical world about a rare or unreported feature, condition, complication, or intervention by publishing it in a medical journal.
When to start
Be on the look out for a case report from the start of your basic surgical or medical training. This will introduce you to the research world, and if your report is published it will be an asset to your CV. Any kind of research entails a lot of hard work and persistence. Your thought processes should be geared towards research in your postgraduate career, and you should use every opportunity you get for writing a report. So if you come across something unusual, discuss it with a consultant, particularly one who is keen on research.
Many consultants have huge amounts of material in the top drawers of their desks, waiting to be published. All they want is an enthusiastic medic who will help share their load in writing and getting it published. They are usually helpful if you ask them about this.
How to start
A senior doctor's help is a must from the beginning. He or she may know from their experience what cases are suitable for publication. Do an extensive literature search--PubMed, Medline, Ovid, Embase, and even search engines like Google will give you a vast amount of information related to the condition or feature you are after. Narrow down the search to your actual topic. If this comes up with very few search results, it means (assuming your search method is correct) that the case is rare and the report …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
Why the Pandemic Probably Started in a Lab, in 5 Key Points

By Alina Chan
Dr. Chan is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “Viral: The Search for the Origin of Covid-19.”
This article has been updated to reflect news developments.
On Monday, Dr. Anthony Fauci returned to the halls of Congress and testified before the House subcommittee investigating the Covid-19 pandemic. He was questioned about several topics related to the government’s handling of Covid-19, including how the National Institute of Allergy and Infectious Diseases, which he directed until retiring in 2022, supported risky virus work at a Chinese institute whose research may have caused the pandemic.
For more than four years, reflexive partisan politics have derailed the search for the truth about a catastrophe that has touched us all. It has been estimated that at least 25 million people around the world have died because of Covid-19, with over a million of those deaths in the United States.
Although how the pandemic started has been hotly debated, a growing volume of evidence — gleaned from public records released under the Freedom of Information Act, digital sleuthing through online databases, scientific papers analyzing the virus and its spread, and leaks from within the U.S. government — suggests that the pandemic most likely occurred because a virus escaped from a research lab in Wuhan, China. If so, it would be the most costly accident in the history of science.
Here’s what we now know:
1 The SARS-like virus that caused the pandemic emerged in Wuhan, the city where the world’s foremost research lab for SARS-like viruses is located.
- At the Wuhan Institute of Virology, a team of scientists had been hunting for SARS-like viruses for over a decade, led by Shi Zhengli.
- Their research showed that the viruses most similar to SARS‑CoV‑2, the virus that caused the pandemic, circulate in bats that live r oughly 1,000 miles away from Wuhan. Scientists from Dr. Shi’s team traveled repeatedly to Yunnan province to collect these viruses and had expanded their search to Southeast Asia. Bats in other parts of China have not been found to carry viruses that are as closely related to SARS-CoV-2.

The closest known relatives to SARS-CoV-2 were found in southwestern China and in Laos.
Large cities
Mine in Yunnan province
Cave in Laos
South China Sea

The closest known relatives to SARS-CoV-2
were found in southwestern China and in Laos.
philippines

The closest known relatives to SARS-CoV-2 were found
in southwestern China and Laos.
Sources: Sarah Temmam et al., Nature; SimpleMaps
Note: Cities shown have a population of at least 200,000.

There are hundreds of large cities in China and Southeast Asia.

There are hundreds of large cities in China
and Southeast Asia.

The pandemic started roughly 1,000 miles away, in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away,
in Wuhan, home to the world’s foremost SARS-like virus research lab.

The pandemic started roughly 1,000 miles away, in Wuhan,
home to the world’s foremost SARS-like virus research lab.
- Even at hot spots where these viruses exist naturally near the cave bats of southwestern China and Southeast Asia, the scientists argued, as recently as 2019 , that bat coronavirus spillover into humans is rare .
- When the Covid-19 outbreak was detected, Dr. Shi initially wondered if the novel coronavirus had come from her laboratory , saying she had never expected such an outbreak to occur in Wuhan.
- The SARS‑CoV‑2 virus is exceptionally contagious and can jump from species to species like wildfire . Yet it left no known trace of infection at its source or anywhere along what would have been a thousand-mile journey before emerging in Wuhan.
2 The year before the outbreak, the Wuhan institute, working with U.S. partners, had proposed creating viruses with SARS‑CoV‑2’s defining feature.
- Dr. Shi’s group was fascinated by how coronaviruses jump from species to species. To find viruses, they took samples from bats and other animals , as well as from sick people living near animals carrying these viruses or associated with the wildlife trade. Much of this work was conducted in partnership with the EcoHealth Alliance, a U.S.-based scientific organization that, since 2002, has been awarded over $80 million in federal funding to research the risks of emerging infectious diseases.
- The laboratory pursued risky research that resulted in viruses becoming more infectious : Coronaviruses were grown from samples from infected animals and genetically reconstructed and recombined to create new viruses unknown in nature. These new viruses were passed through cells from bats, pigs, primates and humans and were used to infect civets and humanized mice (mice modified with human genes). In essence, this process forced these viruses to adapt to new host species, and the viruses with mutations that allowed them to thrive emerged as victors.
- By 2019, Dr. Shi’s group had published a database describing more than 22,000 collected wildlife samples. But external access was shut off in the fall of 2019, and the database was not shared with American collaborators even after the pandemic started , when such a rich virus collection would have been most useful in tracking the origin of SARS‑CoV‑2. It remains unclear whether the Wuhan institute possessed a precursor of the pandemic virus.
- In 2021, The Intercept published a leaked 2018 grant proposal for a research project named Defuse , which had been written as a collaboration between EcoHealth, the Wuhan institute and Ralph Baric at the University of North Carolina, who had been on the cutting edge of coronavirus research for years. The proposal described plans to create viruses strikingly similar to SARS‑CoV‑2.
- Coronaviruses bear their name because their surface is studded with protein spikes, like a spiky crown, which they use to enter animal cells. T he Defuse project proposed to search for and create SARS-like viruses carrying spikes with a unique feature: a furin cleavage site — the same feature that enhances SARS‑CoV‑2’s infectiousness in humans, making it capable of causing a pandemic. Defuse was never funded by the United States . However, in his testimony on Monday, Dr. Fauci explained that the Wuhan institute would not need to rely on U.S. funding to pursue research independently.

The Wuhan lab ran risky experiments to learn about how SARS-like viruses might infect humans.
1. Collect SARS-like viruses from bats and other wild animals, as well as from people exposed to them.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of human cells.

2. Identify high-risk viruses by screening for spike proteins that facilitate infection of
human cells.

In Defuse, the scientists proposed to add a furin cleavage site to the spike protein.
3. Create new coronaviruses by inserting spike proteins or other features that could make the viruses more infectious in humans.

4. Infect human cells, civets and humanized mice with the new coronaviruses, to determine how dangerous they might be.

- While it’s possible that the furin cleavage site could have evolved naturally (as seen in some distantly related coronaviruses), out of the hundreds of SARS-like viruses cataloged by scientists, SARS‑CoV‑2 is the only one known to possess a furin cleavage site in its spike. And the genetic data suggest that the virus had only recently gained the furin cleavage site before it started the pandemic.
- Ultimately, a never-before-seen SARS-like virus with a newly introduced furin cleavage site, matching the description in the Wuhan institute’s Defuse proposal, caused an outbreak in Wuhan less than two years after the proposal was drafted.
- When the Wuhan scientists published their seminal paper about Covid-19 as the pandemic roared to life in 2020, they did not mention the virus’s furin cleavage site — a feature they should have been on the lookout for, according to their own grant proposal, and a feature quickly recognized by other scientists.
- Worse still, as the pandemic raged, their American collaborators failed to publicly reveal the existence of the Defuse proposal. The president of EcoHealth, Peter Daszak, recently admitted to Congress that he doesn’t know about virus samples collected by the Wuhan institute after 2015 and never asked the lab’s scientists if they had started the work described in Defuse. In May, citing failures in EcoHealth’s monitoring of risky experiments conducted at the Wuhan lab, the Biden administration suspended all federal funding for the organization and Dr. Daszak, and initiated proceedings to bar them from receiving future grants. In his testimony on Monday, Dr. Fauci said that he supported the decision to suspend and bar EcoHealth.
- Separately, Dr. Baric described the competitive dynamic between his research group and the institute when he told Congress that the Wuhan scientists would probably not have shared their most interesting newly discovered viruses with him . Documents and email correspondence between the institute and Dr. Baric are still being withheld from the public while their release is fiercely contested in litigation.
- In the end, American partners very likely knew of only a fraction of the research done in Wuhan. According to U.S. intelligence sources, some of the institute’s virus research was classified or conducted with or on behalf of the Chinese military . In the congressional hearing on Monday, Dr. Fauci repeatedly acknowledged the lack of visibility into experiments conducted at the Wuhan institute, saying, “None of us can know everything that’s going on in China, or in Wuhan, or what have you. And that’s the reason why — I say today, and I’ve said at the T.I.,” referring to his transcribed interview with the subcommittee, “I keep an open mind as to what the origin is.”
3 The Wuhan lab pursued this type of work under low biosafety conditions that could not have contained an airborne virus as infectious as SARS‑CoV‑2.
- Labs working with live viruses generally operate at one of four biosafety levels (known in ascending order of stringency as BSL-1, 2, 3 and 4) that describe the work practices that are considered sufficiently safe depending on the characteristics of each pathogen. The Wuhan institute’s scientists worked with SARS-like viruses under inappropriately low biosafety conditions .

In the United States, virologists generally use stricter Biosafety Level 3 protocols when working with SARS-like viruses.
Biosafety cabinets prevent
viral particles from escaping.
Viral particles
Personal respirators provide
a second layer of defense against breathing in the virus.
DIRECT CONTACT
Gloves prevent skin contact.
Disposable wraparound
gowns cover much of the rest of the body.

Personal respirators provide a second layer of defense against breathing in the virus.
Disposable wraparound gowns
cover much of the rest of the body.
Note: Biosafety levels are not internationally standardized, and some countries use more permissive protocols than others.

The Wuhan lab had been regularly working with SARS-like viruses under Biosafety Level 2 conditions, which could not prevent a highly infectious virus like SARS-CoV-2 from escaping.
Some work is done in the open air, and masks are not required.
Less protective equipment provides more opportunities
for contamination.

Some work is done in the open air,
and masks are not required.
Less protective equipment provides more opportunities for contamination.
- In one experiment, Dr. Shi’s group genetically engineered an unexpectedly deadly SARS-like virus (not closely related to SARS‑CoV‑2) that exhibited a 10,000-fold increase in the quantity of virus in the lungs and brains of humanized mice . Wuhan institute scientists handled these live viruses at low biosafet y levels , including BSL-2.
- Even the much more stringent containment at BSL-3 cannot fully prevent SARS‑CoV‑2 from escaping . Two years into the pandemic, the virus infected a scientist in a BSL-3 laboratory in Taiwan, which was, at the time, a zero-Covid country. The scientist had been vaccinated and was tested only after losing the sense of smell. By then, more than 100 close contacts had been exposed. Human error is a source of exposure even at the highest biosafety levels , and the risks are much greater for scientists working with infectious pathogens at low biosafety.
- An early draft of the Defuse proposal stated that the Wuhan lab would do their virus work at BSL-2 to make it “highly cost-effective.” Dr. Baric added a note to the draft highlighting the importance of using BSL-3 to contain SARS-like viruses that could infect human cells, writing that “U.S. researchers will likely freak out.” Years later, after SARS‑CoV‑2 had killed millions, Dr. Baric wrote to Dr. Daszak : “I have no doubt that they followed state determined rules and did the work under BSL-2. Yes China has the right to set their own policy. You believe this was appropriate containment if you want but don’t expect me to believe it. Moreover, don’t insult my intelligence by trying to feed me this load of BS.”
- SARS‑CoV‑2 is a stealthy virus that transmits effectively through the air, causes a range of symptoms similar to those of other common respiratory diseases and can be spread by infected people before symptoms even appear. If the virus had escaped from a BSL-2 laboratory in 2019, the leak most likely would have gone undetected until too late.
- One alarming detail — leaked to The Wall Street Journal and confirmed by current and former U.S. government officials — is that scientists on Dr. Shi’s team fell ill with Covid-like symptoms in the fall of 2019 . One of the scientists had been named in the Defuse proposal as the person in charge of virus discovery work. The scientists denied having been sick .
4 The hypothesis that Covid-19 came from an animal at the Huanan Seafood Market in Wuhan is not supported by strong evidence.
- In December 2019, Chinese investigators assumed the outbreak had started at a centrally located market frequented by thousands of visitors daily. This bias in their search for early cases meant that cases unlinked to or located far away from the market would very likely have been missed. To make things worse, the Chinese authorities blocked the reporting of early cases not linked to the market and, claiming biosafety precautions, ordered the destruction of patient samples on January 3, 2020, making it nearly impossible to see the complete picture of the earliest Covid-19 cases. Information about dozens of early cases from November and December 2019 remains inaccessible.
- A pair of papers published in Science in 2022 made the best case for SARS‑CoV‑2 having emerged naturally from human-animal contact at the Wuhan market by focusing on a map of the early cases and asserting that the virus had jumped from animals into humans twice at the market in 2019. More recently, the two papers have been countered by other virologists and scientists who convincingly demonstrate that the available market evidence does not distinguish between a human superspreader event and a natural spillover at the market.
- Furthermore, the existing genetic and early case data show that all known Covid-19 cases probably stem from a single introduction of SARS‑CoV‑2 into people, and the outbreak at the Wuhan market probably happened after the virus had already been circulating in humans.

An analysis of SARS-CoV-2’s evolutionary tree shows how the virus evolved as it started to spread through humans.
SARS-COV-2 Viruses closest
to bat coronaviruses
more mutations

Source: Lv et al., Virus Evolution (2024) , as reproduced by Jesse Bloom

The viruses that infected people linked to the market were most likely not the earliest form of the virus that started the pandemic.

- Not a single infected animal has ever been confirmed at the market or in its supply chain. Without good evidence that the pandemic started at the Huanan Seafood Market, the fact that the virus emerged in Wuhan points squarely at its unique SARS-like virus laboratory.
5 Key evidence that would be expected if the virus had emerged from the wildlife trade is still missing.

In previous outbreaks of coronaviruses, scientists were able to demonstrate natural origin by collecting multiple pieces of evidence linking infected humans to infected animals.
Infected animals
Earliest known
cases exposed to
live animals
Antibody evidence
of animals and
animal traders having
been infected
Ancestral variants
of the virus found in
Documented trade
of host animals
between the area
where bats carry
closely related viruses
and the outbreak site

Infected animals found
Earliest known cases exposed to live animals
Antibody evidence of animals and animal
traders having been infected
Ancestral variants of the virus found in animals
Documented trade of host animals
between the area where bats carry closely
related viruses and the outbreak site

For SARS-CoV-2, these same key pieces of evidence are still missing , more than four years after the virus emerged.

For SARS-CoV-2, these same key pieces of evidence are still missing ,
more than four years after the virus emerged.
- Despite the intense search trained on the animal trade and people linked to the market, investigators have not reported finding any animals infected with SARS‑CoV‑2 that had not been infected by humans. Yet, infected animal sources and other connective pieces of evidence were found for the earlier SARS and MERS outbreaks as quickly as within a few days, despite the less advanced viral forensic technologies of two decades ago.
- Even though Wuhan is the home base of virus hunters with world-leading expertise in tracking novel SARS-like viruses, investigators have either failed to collect or report key evidence that would be expected if Covid-19 emerged from the wildlife trade . For example, investigators have not determined that the earliest known cases had exposure to intermediate host animals before falling ill. No antibody evidence shows that animal traders in Wuhan are regularly exposed to SARS-like viruses, as would be expected in such situations.
- With today’s technology, scientists can detect how respiratory viruses — including SARS, MERS and the flu — circulate in animals while making repeated attempts to jump across species . Thankfully, these variants usually fail to transmit well after crossing over to a new species and tend to die off after a small number of infections. In contrast, virologists and other scientists agree that SARS‑CoV‑2 required little to no adaptation to spread rapidly in humans and other animals . The virus appears to have succeeded in causing a pandemic upon its only detected jump into humans.
The pandemic could have been caused by any of hundreds of virus species, at any of tens of thousands of wildlife markets, in any of thousands of cities, and in any year. But it was a SARS-like coronavirus with a unique furin cleavage site that emerged in Wuhan, less than two years after scientists, sometimes working under inadequate biosafety conditions, proposed collecting and creating viruses of that same design.
While several natural spillover scenarios remain plausible, and we still don’t know enough about the full extent of virus research conducted at the Wuhan institute by Dr. Shi’s team and other researchers, a laboratory accident is the most parsimonious explanation of how the pandemic began.
Given what we now know, investigators should follow their strongest leads and subpoena all exchanges between the Wuhan scientists and their international partners, including unpublished research proposals, manuscripts, data and commercial orders. In particular, exchanges from 2018 and 2019 — the critical two years before the emergence of Covid-19 — are very likely to be illuminating (and require no cooperation from the Chinese government to acquire), yet they remain beyond the public’s view more than four years after the pandemic began.
Whether the pandemic started on a lab bench or in a market stall, it is undeniable that U.S. federal funding helped to build an unprecedented collection of SARS-like viruses at the Wuhan institute, as well as contributing to research that enhanced them . Advocates and funders of the institute’s research, including Dr. Fauci, should cooperate with the investigation to help identify and close the loopholes that allowed such dangerous work to occur. The world must not continue to bear the intolerable risks of research with the potential to cause pandemics .
A successful investigation of the pandemic’s root cause would have the power to break a decades-long scientific impasse on pathogen research safety, determining how governments will spend billions of dollars to prevent future pandemics. A credible investigation would also deter future acts of negligence and deceit by demonstrating that it is indeed possible to be held accountable for causing a viral pandemic. Last but not least, people of all nations need to see their leaders — and especially, their scientists — heading the charge to find out what caused this world-shaking event. Restoring public trust in science and government leadership requires it.
A thorough investigation by the U.S. government could unearth more evidence while spurring whistleblowers to find their courage and seek their moment of opportunity. It would also show the world that U.S. leaders and scientists are not afraid of what the truth behind the pandemic may be.
More on how the pandemic may have started

Where Did the Coronavirus Come From? What We Already Know Is Troubling.
Even if the coronavirus did not emerge from a lab, the groundwork for a potential disaster had been laid for years, and learning its lessons is essential to preventing others.
By Zeynep Tufekci

Why Does Bad Science on Covid’s Origin Get Hyped?
If the raccoon dog was a smoking gun, it fired blanks.
By David Wallace-Wells

A Plea for Making Virus Research Safer
A way forward for lab safety.
By Jesse Bloom
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Alina Chan ( @ayjchan ) is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of “ Viral : The Search for the Origin of Covid-19.” She was a member of the Pathogens Project , which the Bulletin of the Atomic Scientists organized to generate new thinking on responsible, high-risk pathogen research.
- Share full article
Advertisement
For authors
What we publish, how to submit.
- BMJ Case Reports Author Guide
Case report templates
Author statements, reviewer guidance, editorial policy, patient consent and confidentiality, peer review, competing interests, what will it cost, open access, rapid responses.
- flowcharts that show clinical course time lines
- illustrative diagrams that facilitate the interpretation of clinical images
- graphs of results
- management algorithms
- referenced guidelines
- assess the efficacy or effectiveness of new interventions, new drugs, unlicensed substances, or lifestyle changes
- describe drug efficacy, drug interactions or adverse drug effects in patients enrolled in ongoing clinical trials
- describe single-instance, off-label or experimental use of an existing drug or a combination of drugs used for a new clinical indication or the results of phase 2 clinical trials
- have been previously submitted to a preprint server as there are patient confidentiality concerns
- have more than one case (case series). If we feel that an article is strengthened by the inclusion of more than one case, we may consider the article provided it includes no more than three patients. Please contact the editor-in-chief before submitting a case series
- the learning outcomes should be important and novel
- there should be a detailed and balanced review of relevant up-to-date literature
- include diagrams, flowcharts and algorithms that you have drawn so that each case may be used as a textbook case
- there should be comprehensive and critical appraisal of relevant global health literature
- include published public health and epidemiological data
- include an in-depth understanding of the anthropological background of the case
- Videos are published under the same copyright terms as the associated article
- The content and focus of the video must relate directly to the case report
- If audio narration is used, please, ensure that this is clear
- Annotate and label essential structures in videos
- Do not add background music or colourful animation
- Use the compression parameters that video sharing sites use. Often these are standard options in your editing software. A comprehensive guide is available from Vimeo
- Do not show any identifiable features of living patients and/or identifiable personal details in the foreground or background
- Clinical Case Report reviewer guidance >>
- Global Health Case Report reviewer guidance >>
- Images in/Videos reviewer guidance >>
- be involved in the clinical care of the patient
- give final approval of the manuscript
- be responsible for drafting of the text, sourcing and editing of clinical images, results of clinical investigations, drawing of original diagrams and management algorithms, and critical revision for important intellectual content
- agree to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
- authors are required to declare in the author statement that they are the patient in the case report, this statement will appear in the case report
- we do not publish case reports where the patient is the sole author or the patient is a relative of the author
- the report will require careful anonymisation - patients with concerns about anonymity are advised not to co-author manuscripts but to add their patient perspective instead
- we require consent from everyone whose medical information is disclosed in the manuscript (e.g., parents, siblings, etc)
- signature of the consent form should be after the patient has seen and approved the manuscript
- if the manuscript is substantially changed as a result of revisions, the authors should confirm that the patient has seen and approved the final manuscript
- patients should be made aware that published online content may be picked up by non-BMJ or non-medical media
- after publication of a case report, should authors wish to submit a second manuscript describing the progress of the same patient, up to date informed consent will be needed with a new consent form signed by the patient
- anonymise all details of patients in the text, tables, figures, figure legends and within the patient perspective section
- unless clinically relevant, ethnicity and occupation should not be included
- when describing family history in the case report use “first degree relative” or ”second degree relative” for parents or siblings or grandparents or cousins
- exclude specific ages, instead use “early”/”mid”/”late” “20s”, “30s”, “40s”….
- childhood age ranges include preterm neonatal, term neonatal (birth – 27 days), infancy (28 days – 12 months), toddler (13 months – 2 years), early childhood (2 – 5 years), middle childhood (6 – 11 years), early adolescence (12 – 18 years) and late adolescence (19 – 21 years)

Common Abbreviations in Medical Notes
- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE , Medicine , Surgery , Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Table of Contents
Suggest an improvement
- Hidden Post Title
- Hidden Post URL
- Hidden Post ID
- Type of issue * N/A Fix spelling/grammar issue Add or fix a link Add or fix an image Add more detail Improve the quality of the writing Fix a factual error
- Please provide as much detail as possible * You don't need to tell us which article this feedback relates to, as we automatically capture that information for you.
- Your Email (optional) This allows us to get in touch for more details if required.
- Which organ is responsible for pumping blood around the body? * Enter a five letter word in lowercase
- Email This field is for validation purposes and should be left unchanged.
Introduction
Abbreviations and acronyms are commonly used in medical notes . If you are unfamiliar with common abbreviations, it can make understanding medical notes challenging. We’ve curated a list of medical abbreviations/acronyms to help you understand entries in the medical notes.
Remember, using ambiguous abbreviations increases the risk of miscommunication. Your hospital may have an approved list of abbreviations.
Structure of the medical history
| Presenting complaint | |
| History of presenting complaint | |
| Past medical history | |
| Systems review | |
| Drug history | |
| Family history | |
| Social history | |
| Last menstrual period | |
| History of | |
| With | |
| No/none Chest pain = No chest pain) | |
| Activities of daily living |
- Number of days = number of days/7 (e.g. 3/7 = 3 days)
- Number of weeks = number of weeks/52 (e.g. 4/52 = 4 weeks)
- Number of hours = Xº (e.g. 8º = 8 hours)
Medical conditions and procedures
While care should always be taken when using acronyms in notes, it is particularly important for medical conditions, as the same acronym can mean different conditions depending on the specialty.
| Abdominal aortic aneurysm | |
| Coronary artery bypass graft | |
| Coronary artery disease | |
| Gastro-oesophageal reflux disease | |
| Irritable bowel syndrome | |
| Oesophagogastro duodenoscopy | |
| Open reduction internal fixation | |
| Peripheral arterial disease | |
Medications
| Once daily | |
| Twice daily | |
| Three times daily | |
| Four times daily | |
| As required | |
| Immediately | |
| Over the counter | |
| – Every morning | |
| – Every night | |
Examination
| On examination | |
| Respiratory rate | |
| Oxygen saturation | |
| Room air | |
| Heart sounds 1 and 2 heard, with no added sounds | |
| Bowel sounds | |
| Right/left upper limb | |
| Right/left lower limb | |
| Right/left upper quadrant | |
| Right/left lower quadrant | |
| Right iliac fossa/left iliac fossa | |
| Cranial nerve | |
| Pupils equal and reactive to light | |
| Full range of movement | |
| Nothing abnormal detected/discovered | |
| Soft, non-tender | |
| Straight leg raise | |
| Positive / negative | |
| Present | |
| Present significantly | |
| Present in excess |
Investigations
| Antinuclear antibody | |
| Anti-neutrophil cytoplasmic antibody | |
| (short for ‘Boehringer Mannheim’ – a manufacturer of blood glucose test strips) | |
| (for blood sugar) capillary blood gas | |
| CT angiography | |
| CT scan of the thorax/chest, abdomen and pelvis | |
| Catheter specimen of urine | |
| Electroencephalogram | |
| Erythrocyte sedimentation rate | |
| Group and save | |
| Magnetic resonance imaging | |
| Magnetic resonance cholangiopancreatography | |
| Mid-stream urine sample | |
| Oral glucose tolerance test | |
| Ultrasound/ultrasound scan | |
| Crossmatch |
Other abbreviations
| Adult therapeutic dose > | |
| Approved mental health professional | |
| Asked to see patient | |
| Autologous/allogenic stem cell transplant | |
| Brought in by ambulance | |
| Bowels not open(ed) | |
| Community psychiatric nurse | |
| Differential diagnoses | |
| Did not attend | |
| Diarrhoea & vomiting | |
| Estimated date of discharge | |
| Examination under anaesthesia | |
| Face arms speech test | |
| Fresh frozen plasma | |
| Human albumin solution | |
| Healthcare assistant | |
| Intravenous infusion | |
| Investigations | |
| Lasting power of attorney | |
| Multidisciplinary team | |
| Medically fit for discharge | |
| Montreal cognitive assessment | |
| Methicillin-resistant Staphylococcus aureus | |
| Nil by mouth | |
| National early warning score | |
| Next of kin | |
| Occupational therapist / occupational therapy | |
| Power of attorney | |
| Package of care | |
| Plaster of paris | |
| Physiotherapist/physiotherapy | |
| Passed urine | |
| Return of spontaneous circulation | |
| Speech (and) language therapist | |
| Shortness of breath (on exertion) | |
| To come in | |
| To-take-out/To-take-away | |
| Venous thromboembolism | |
| Fracture | |
| ΔΔ | Used to indicate differential diagnoses |
| Δ | Used to indicate the current diagnosis |
Suggest an abbreviation
[email protected].
Or use the live chat function.

Other pages
- Product Bundles 🎉
- Join the Team 🙌
- Institutional Licence 📚
- OSCE Station Creator Tool 🩺
- Create and Share Flashcards 🗂️
- OSCE Group Chat 💬
- Newsletter 📰
- Advertise With Us
Join the community

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
ALERT: Reports of a $600 payment increase in June are FALSE: NO COLA increase will occur UNTIL January 2025.
Appeal a decision we made
If you don't agree with a decision we made, follow the process to request a change.
You have four opportunities to appeal our decision
You may not have to go through all the appeal levels. To start, ask us to reconsider a decision we made. Continue to move through the process if you disagree with the decisions.
Request reconsideration
Start by asking us to reconsider a decision we made.
Hearing with a judge
Request a hearing with an administrative law judge if you don't agree with our response to your request for reconsideration.
Review of hearing decision
Request a review with the Appeals Council if you don't agree with the decision made by the judge in your hearing.
File federal district court action
File a federal district court action with the U.S. District Court if you don't agree with the response from the Appeals Council.
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
COVID-19 Vaccine: What You Need to Know
The COVID-19 vaccine is very good at preventing serious illness, hospitalization and death. Because the virus that causes COVID-19 continues to change, vaccines are updated to help fight the disease. It is important to check the Centers for Disease Control and Prevention (CDC) COVID-19 vaccine information for the latest details. (Posted 11/22/23)
What is the COVID-19 vaccine?
The COVID-19 vaccine lessens the severity of COVID-19 by teaching the immune system to recognize and fight the virus that causes the disease.
For fall/winter 2023–2024, the updated COVID-19 vaccine is based on the XBB.1.5 variant. The updated vaccine is made by Pfizer-BioNTech, Moderna and Novavax. This season, only one shot of the vaccine is needed for most people, and there are no boosters. (People who are immunocompromised or ages 6 months to 4 years may need more than one 2023–2024 vaccine.)
How is the 2023–2024 COVID-19 vaccine different from previous COVID-19 vaccines?
The 2023–2024 COVID-19 vaccine targets XBB.1.5, a subvariant of Omicron. While none of the variants currently circulating are exact matches to the vaccine, they are all closely related to the XBB.1.5 strain. Studies show that the updated vaccine is effective against the variants currently causing the majority of COVID-19 cases in the U.S.
Who should get a COVID-19 vaccine?
Because the 2023–2024 vaccine is effective for recent strains of COVID-19, it is recommended that everyone stay up to date with this vaccine. Previous vaccines or boosters were not developed to target the more recent strains. For 2023–2024, the CDC recommends:
- Everyone age 5 and older receive one shot of the updated vaccine.
- Children ages 6 months to 4 years may need more than one shot to be up to date.
- People who are moderately or severely immunocompromised may need more than one shot.
You can review the full recommendations on the CDC’s Stay Up to Date with COVID-19 Vaccines webpage . Be sure to talk to your primary care doctor or pediatrician if you are unsure about vaccine recommendations.
What are the side effects of the COVID-19 vaccine?
Side effects vary and may last one to three days. Common side effects are:
- Soreness at the injection site
COVID-19 Vaccine and Pregnancy
COVID-19 vaccines approved by the Food and Drug Administration (FDA) are safe and recommended for people who are pregnant or lactating, as well as for those r intending to become pregnant.
People who are pregnant or were recently pregnant are at a greater risk for severe COVID-19. Having a severe case of COVID-19 while pregnant is linked to a higher risk of pre-term birth and stillbirth and might increase the risk of other pregnancy complications.
What should parents know about the COVID-19 vaccine and children?
The CDC recommends the 2023–2024 vaccine for adolescents and teenagers ages 12 and older, and for children ages 6 months through 11 years.
- Children age 5 and older need one shot of the updated vaccine.
Children are less likely to become seriously ill from COVID-19 than adults, although serious illness can happen. Speak with your pediatrician if you have questions about having your child vaccinated.
If I recently had COVID-19, do I need a 2023–2024 vaccine?
If you recently had COVID-19, the CDC recommends waiting about three months before getting this updated vaccine. If you encounter the virus again, having the updated vaccine will:
- Lessen your risk of severe disease that could require hospitalization
- Reduce the chance that you infect someone else with COVID-19
- Help keep you protected from currently circulating COVID-19 variants
How long should I wait to get this vaccine if I recently had an earlier version of a COVID-19 vaccine or booster?
People age 5 years and older should wait at least two months after getting the last dose of any COVID-19 vaccine before receiving the 2023–2024 vaccine, according to CDC guidance .
Is natural immunity better than a vaccine?
Natural immunity is the antibody protection your body creates against a germ once you’ve been infected with it. Natural immunity to the virus that causes COVID-19 is no better than vaccine-acquired immunity, and it comes with far greater risks. Studies show that natural immunity to the virus weakens over time and does so faster than immunity provided by COVID-19 vaccination.
Do I need a COVID-19 booster?
The 2023–2024 vaccine is a one-shot vaccine for most people, and there is no booster this season. (People who are immunocompromised or ages 6 months to 4 years may need more than one 2023–2024 vaccine.)
The FDA calls this an updated vaccine (not a “booster” like previous shots) because it builds a new immune response to variants that are currently circulating. This change reflects the current approach of treating COVID-19 similarly to the flu, with preventive measures such as an annual vaccination.
When should I get a COVID-19 vaccine?
Like the flu and other respiratory diseases, COVID-19 tends to be more active in the fall and winter, so getting a vaccine in the fall is recommended.
How quickly does the COVID-19 vaccine become effective?
It usually takes about two weeks for the vaccine to become effective. The CDC website provides more information on how the COVID-19 vaccines work .
How long does the COVID-19 vaccine last?
Studies suggest that COVID-19 vaccines are most effective during the first three months after vaccination.
Is it safe to get a flu and COVID-19 vaccine at the same time?
Yes, it safe to get both shots at the same time. Keep in mind that each has similar side effects and you may experience side effects from both.
Is the COVID-19 vaccine safe?
Yes. COVID-19 vaccines approved by the FDA meet rigorous testing criteria and are safe and effective at preventing serious illness, hospitalization and death. Millions of people have received the vaccines, and the CDC continues to monitor their safety and effectiveness as well as rare adverse events.
Where can I get a COVID-19 vaccine?
The COVID-19 vaccine is available at pharmacies. See vaccines.gov to find a convenient location.

Trauma Team Puts an Athlete Back in the Saddle

Patient Safety Infographic
Coronavirus: Younger Adults Are at Risk, Too
Related Topics
- Infectious Diseases
‘Great Attrition’ or ‘Great Attraction’? The choice is yours
More than 19 million US workers— and counting—have quit their jobs since April 2021, a record pace disrupting businesses everywhere. Companies are struggling to address the problem, and many will continue to struggle for one simple reason: they don’t really understand why their employees are leaving in the first place. Rather than take the time to investigate the true causes of attrition, many companies are jumping to well-intentioned quick fixes that fall flat: for example, they’re bumping up pay or financial perks, like offering “thank you” bonuses without making any effort to strengthen the relational ties people have with their colleagues and their employers. The result? Rather than sensing appreciation, employees sense a transaction. This transactional relationship reminds them that their real needs aren’t being met.
If the past 18 months have taught us anything, it’s that employees crave investment in the human aspects of work. Employees are tired, and many are grieving . They want a renewed and revised sense of purpose in their work. They want social and interpersonal connections with their colleagues and managers. They want to feel a sense of shared identity. Yes, they want pay, benefits, and perks, but more than that they want to feel valued by their organizations and managers. They want meaningful—though not necessarily in-person— interactions , not just transactions.
By not understanding what their employees are running from, and what they might gravitate to, company leaders are putting their very businesses at risk. Moreover, because many employers are handling the situation similarly—failing to invest in a more fulfilling employee experience and failing to meet new demands for autonomy and flexibility at work—some employees are deliberately choosing to withdraw entirely from traditional forms of full-time employment.
About the research
To better understand what’s driving voluntary attrition in the labor market, we conducted separate surveys of employers and of employees in Australia, Canada, Singapore, the United Kingdom, and the United States. Both surveys spanned multiple industries. The employee survey included 5,774 people of working age; the employer survey, 250 managers specializing in talent (for instance, chief talent officers). These managers were evenly split between large organizations (with more than $1 billion in revenues) and midsize ones (with revenues from $50 million to $1 billion).
In this article, we highlight new McKinsey research into the nature and characteristics of the Great Attrition—or what many are calling the Great Resignation—and what’s driving it (see sidebar, “About the research”). The bottom line: the Great Attrition is happening, it’s widespread and likely to persist—if not accelerate—and many companies don’t understand what’s really going on, despite their best efforts. These companies are making ineffective moves based on faulty assumptions.
It doesn’t have to be this way. If companies make a concerted effort to better understand why employees are leaving and take meaningful action to retain them , the Great Attrition could become the Great Attraction. By seizing this unique moment, companies could gain an edge in the race to attract, develop, and retain the talent they need to create a thriving postpandemic organization .

Pop quiz: Can you turn attrition into attraction?
We’ve assembled a hypothetical team that you lead. Can you retain them, or will you lose them to the Great Attrition? Forty percent of workers are considering leaving their current jobs.
But this won’t be easy, because it requires companies and their leaders to truly understand their employees. It requires leaders to develop a much deeper empathy for what employees are going through and to pair that empathy with the compassion—and determination—to act and change. Only then can employers properly reexamine the wants and needs of their employees—together with those employees—and begin to provide the flexibility, connectivity, and sense of unity and purpose that people crave.
Along the way, many senior executives will be challenged to reimagine how they lead. The skills that made leaders effective before the COVID-19 pandemic—strong coaching, mentoring, creating strong teams—are just table stakes for the challenge of the months and years ahead.
By not understanding what their employees are running from, and what they might gravitate to, company leaders are putting their very businesses at risk.
The Great Attrition is happening—and will probably continue
Executives who think that employee attrition is easing—or is limited to particular industries—are misguided. Forty percent of the employees in our survey said they are at least somewhat likely to quit in the next three to six months. Eighteen percent of the respondents said their intentions range from likely to almost certain. These findings held across all five countries we surveyed (Australia, Canada, Singapore, the United Kingdom, and the United States) and were broadly consistent across industries (Exhibit 1). Businesses in the leisure and hospitality industry are the most at risk for losing employees, but many healthcare and white-collar workers say they also plan to quit. Even among educators—the employees least likely to say they may quit—almost one-third reported that they are at least somewhat likely to do so.
Furthermore, these trends may persist. Fifty-three percent of the employers said that they are experiencing greater voluntary turnover than they had in previous years, and 64 percent expect the problem to continue—or worsen—over the next six months (Exhibit 2).
Attrition could get worse, since employees are willing to quit without a job lined up
Among the employees in our survey, 36 percent who had quit in the past six months did so without having a new job in hand (Exhibit 3). This is yet another way the Great Attrition (or what many call the Great Resignation) differs fundamentally from previous downturn-and-recovery cycles—and another sign that employers may be out of touch with just how hard the past 18 months have been for their workers.
Employees in the United States were the most likely to say they had left their old jobs without a new one (40 percent). At the industry level, 42 percent of healthcare and social-assistance workers who quit did so without having a new job—a reminder of the pandemic’s toll on frontline workers. One-quarter of white-collar employees who quit said they had done so without having a job lined up, a finding that held across income levels.
This trend not only is poised to continue but could get much worse. Among employees who said they are at least somewhat likely to leave their jobs in the next three to six months, almost two-thirds added that they would do so without lining up new jobs.

A McKinsey Live event on 'The Great Attrition or the Great Attraction: What will it be for you?'
Otherwise satisfied employees may also be tempted to quit as their options expand
CEOs may be tempted to take solace in the fact that 60 percent of the employees in our survey said they were not at all likely to quit in the next three to six months. But employers shouldn’t consider this 60 percent “safe” from the prospect of attrition either. Options are increasing, and with more and more employers offering remote-work choices for hard-to-source talent, these employees could change their intentions.
Consider a few significant findings. Among employees who said they were not at all likely to quit, 65 percent reported that a primary reason to stay in their job was that they liked where they lived. But among survey respondents who took new jobs in new cities during the past six months, almost 90 percent didn’t have to relocate (Exhibit 4), because so many more companies are allowing remote work. Having more “location agnostic” positions to choose from could prompt otherwise satisfied employees to start second-guessing their commitment to the companies where they now work, particularly if executives mishandle the transition to a hybrid-work environment —or stubbornly fail to offer one at all.
Employers can’t fix what they don’t understand
To stem the tide of the Great Attrition (or what many call the Great Resignation), senior executives must understand why employees are leaving. Many are struggling to do so. For example, when employers were asked why their people had quit, they cited compensation, work–life balance, and poor physical and emotional health. These issues did matter to employees—just not as much as employers thought they did. By contrast, the top three factors employees cited as reasons for quitting were that they didn’t feel valued by their organizations (54 percent) or their managers (52 percent) or because they didn’t feel a sense of belonging at work (51 percent). Notably, employees who classified themselves as non-White or multiracial were more likely than their White counterparts to say they had left because they didn’t feel they belonged at their companies—a worrying reminder of the inequities facing Black employees and other minority groups.
Exhibit 5 shows where the disconnect between employers and employees was most acute. It highlights how employees were far more likely to prioritize relational factors, whereas employers were more likely to focus on transactional ones.
Employees were far more likely to prioritize relational factors, whereas employers were more likely to focus on transactional ones.
Start turning attrition into attraction
Our research underscores the many ways the pandemic has irrevocably changed what people expect from work. The landscape will continue to change as companies try out new hybrid-work approaches. If you’re a CEO or a member of a top team, your best move now is to hit “pause” and take the time to think through your next moves. A heavy-handed back-to-the-office policy or other mandates delivered from on high—no matter how well intentioned—are likely to backfire.
But don’t think through your next moves in a vacuum; include your employees in the process. Look to them to help shape the plan and solutions. Our research suggests that executives aren’t listening to their people nearly enough. Don’t be one of these executives.
As you take stock, ask the following questions:
Do we shelter toxic leaders? Executives who don’t make their people feel valued can drive them from companies, with or without a new job in hand. If you don’t have leaders who motivate and inspire their teams and lead with compassion, you need them—desperately.
Do we have the right people in the right places (especially managers)? Many employers in our survey reported having the right people but not necessarily in the right places. When it comes to managers, this problem can be particularly damaging, especially in hybrid environments, where new leadership skills are required. Training and capability building will be crucial for managers and executives who didn’t come from hybrid or virtual environments—in other words, for everyone from the C-suite to the front line.
How strong was our culture before the pandemic? If you’re like many executives we know, you see a return to the office as a way to address lingering culture and connectivity concerns. Or you prefer a full return to the office because you miss it yourself (a case of “absence makes the heart grow fonder”). You should remember that although the needs of your employees have changed, your culture may not have kept up, and any prior organizational weaknesses are now magnified. Employees will have little tolerance for a return to a status quo they didn’t like before.
Is our work environment transactional? If your only response to attrition is to raise compensation, you’re unwittingly telling your people that your relationship with them is transactional and that their only reason to stay with you is a paycheck. Your very best people will always have a better cash offer somewhere else. You want to solve the problems of the whole person (not just their bank accounts) as well as the whole organization.
Are our benefits aligned with employee priorities? Free parking or entertainment-related perks are probably not top of mind for employees right now. Among survey respondents who had left their jobs, 45 percent cited the need to take care of family as an influential factor in their decision. A similar proportion of people who are thinking of quitting cited the demands of family care. Expanding childcare, nursing services, or other home- and family-focused benefits could help keep such employees from leaving and show that you value them as whole people. Patagonia, long the standard-bearer for progressive workplace policies, retains nearly 100 percent of its new moms with on-site childcare and other benefits for parents.
Employees want career paths and development opportunities. Can we provide it? Employees are looking for jobs with better, stronger career trajectories. They desire both recognition and development. Smart companies find ways to reward people by promoting them not only into new roles but also into additional levels within their existing ones. This is one way companies can more quickly reward and recognize people for good work. Waffle House famously offers three levels for grill positions—which at other companies is just one role. Entry-level cooks are “grill operators,” more experienced cooks “master grill operators,” and the best cooks are known as “rock star grill operators,” or more colloquially as “Elvis on the grill.”
How are we building a sense of community? Remote work is no panacea, but neither is a full on-site return. In-person connectivity continues to have massive benefits for your organization. But it will require considerable management attention to get right as health and safety concerns continue to evolve, particularly because employees’ needs and expectations have changed. For example, employees with unvaccinated young children may feel unsafe at large in-person gatherings. One organization took an inclusive approach by sending out themed “staycation” packages: a movie night with popcorn and a gift card; a game night with family-oriented games, chips, and salsa; and a “virtual spa day” complete with face masks, tea, and chocolate. The company created a Slack channel for posting photos and stories, encouraging employees to share these experiences. Another organization encouraged connectivity among employees by offering coffee gift cards to those who signed up to participate in one-on-one “coffee chats” with employees they didn’t know—a perk that improved connectivity and helped people expand their networks.
If you lead a large team or a company, remember this: the Great Attrition is real, will continue, and may get worse before it gets better. Yet this unique moment also represents a big opportunity. To seize it, take a step back, listen, learn, and make the changes employees want—starting with a focus on the relational aspects of work that people have missed the most. By understanding why they are leaving and by acting thoughtfully, you may just be able to turn the Great Attrition into the Great Attraction.
Aaron De Smet is a senior partner in McKinsey’s New Jersey office, Bonnie Dowling is an associate partner in the Denver office, Marino Mugayar-Baldocchi is a research science specialist in the New York office, and Bill Schaninger is a senior partner in the Philadelphia office.
The authors wish to thank Bryan Hancock, Randy Lim, David Mendelsohn, Mihir Mysore, and Nicolette Rainone for their contributions to this article and its underlying research.
This article was edited by Tom Fleming, an executive editor in the Chicago office.
Explore a career with us
Related articles.

Help your employees find purpose—or watch them leave

Your organization is grieving—here’s how you can help

It’s time for leaders to get real about hybrid

IMAGES
VIDEO
COMMENTS
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Learn how to turn an interesting case into an educational report with this guide from two doctors. It covers topics such as consent, information gathering, writing up, title, background, and conclusion.
Learn from two BMJ Case Reports editors how to choose the right patient, message, and journal for your case report. Find out how to get published and avoid common pitfalls in medical writing.
Follow the same steps as for a Case Report. Template for writing a Case Series Title: Choose an informative title and, if need be, sub-title; always include "Case Series" so that the. reader knows immediately what study type you have chosen. Authors: Check the Instructions to Authors of the journal you are submitting to for formatting ...
This checklist was developed by CARE to correspond with key components of a case report and capture useful clinical information (including 'meaningful use' information mandated by some insurance plans).. The narrative: A case report tells a story in a narrative format that includes the presenting concerns, clinical findings, diagnoses, interventions, outcomes (including adverse events), and ...
Title of case. You do not need to include "a case report" in the title - you may be cryptic if you wish. Summary. This will be freely available online. Up to 150 words summarising the case presentation and outcome. We need a good flavour of the case - emphasise the learning points. Background.
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow the diagnostic pathway. For example - Patient X is a 10 ...
Research Medical Library. Education Hub. Quick Help Videos and Guides. Writing. Clinical Case Reports. Learn how to identifiy an appropriate case, conduct a literature search, identify a target journal, and write a clinical case report.
Submit Your Case: The Sky Is the Limit. As a last message, to all of the young authors who are trying to get their clinical cases published, remember that the "sky is the limit"—be creative, find a good mentor, and write up an interesting case. Once your manuscript is accepted, it is just the beginning of your academic career.
A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
It is best to write the actual report in one stretch if possible, including as much detail as you think is relevant. You can always edit the discussion and trim down the article at a later stage. Below is the general format adopted for most case reports. Introduction. Summarise your case report in a sentence. Mention how rare this condition is ...
There are various categories or types of case reports (these may include a single case report, two cases report, or case series). The two main types are the regular, clinically oriented case report, published in a full case report format (see below) or as a letter to the editor, and the educational case report, which includes a broader description, discussion by an expert or multiple experts ...
can introduce very effective treatment paradigms. Preparing the manuscript for a case report may be the first exposure to scientific writing for a budding clinician/researcher. This manuscript describes the steps of writing a case report and essential considerations when publishing these articles. Individual components of a case report and the "dos and don'ts" while preparing these ...
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
5. Complete your introduction and conclusion after you've written the body. Since these sections summarize key points of your case study, it's best to write them last. The introduction gives a brief overview of the basic condition of your patient and the problem you'll address in your case study.
For case reports, Journal of Medical Case Reports requires authors to follow the CARE guidelines. The CARE checklist should be provided as an additional files. Submissions received without these elements will be returned to the authors as incomplete. The checklist will not be used as a tool for judging the suitability of manuscripts for ...
Rahij Anwar and colleagues give advice on the practical details of writing case reports Research has become an integral part of medical careers. A case report is a way of communicating information to the medical world about a rare or unreported feature, condition, complication, or intervention by publishing it in a medical journal. Be on the look out for a case report from the start of your ...
Dr. Chan is a molecular biologist at the Broad Institute of M.I.T. and Harvard, and a co-author of "Viral: The Search for the Origin of Covid-19." This article has been updated to reflect news ...
All submissions to BMJ Case Reports require the completion of author statements. Case reports should have a maximum of four authors. One of the co-authors should be the lead clinician responsible for the patient's care and responsible for the integrity of the content of the case report, signed informed consent and author statements accompanying each manuscript.
We've curated a list of medical abbreviations/acronyms to help you understand entries in the medical notes. Remember, using ambiguous abbreviations increases the risk of miscommunication. Your hospital may have an approved list of abbreviations. History Structure of the medical history
Video. Home. Live
You may not have to go through all the appeal levels. To start, ask us to reconsider a decision we made. Continue to move through the process if you disagree with the decisions.
People who are pregnant or were recently pregnant are at a greater risk for severe COVID-19. Having a severe case of COVID-19 while pregnant is linked to a higher risk of pre-term birth and stillbirth and might increase the risk of other pregnancy complications. What should parents know about the COVID-19 vaccine and children?
To better understand what's driving voluntary attrition in the labor market, we conducted separate surveys of employers and of employees in Australia, Canada, Singapore, the United Kingdom, and the United States. Both surveys spanned multiple industries. The employee survey included 5,774 people of working age; the employer survey, 250 managers specializing in talent (for instance, chief ...